Abstract
Introduction
Hepatocellular carcinoma (HCC) is the sixth most commonly diagnosed cancer and the third leading cause of cancer death worldwide. While there has been rapid evolution in the treatment paradigm of HCC across the past decade, the extent to which these newly approved therapies are utilized in clinical practice in the real world is, however, unknown. The INSIGHT study was an investigator-initiated, multi-site longitudinal cohort study conducted to reflect real-world epidemiology and clinical practice in Asia-Pacific in the immediate 7-year period after the conclusion of the BRIDGE study.
Methods
Data were collected both retrospectively (planned 30% of the total cohort size) and prospectively (planned 70%) from January 2013 to December 2019 from eligible patients newly diagnosed with HCC from 33 participating sites across 9 Asia-Pacific countries.
Results
A total of 2,533 newly diagnosed HCC patients (1,052 in retrospective cohort and 1,481 in prospective cohort) were enrolled. The most common risk factor was hepatitis B in all countries except Japan, Australia, and New Zealand, where the prevalence of hepatitis C and diabetes were more common. The top three comorbidities reported in the INSIGHT study include cirrhosis, hypertension, and diabetes. We observe high heterogeneity in the first-line treatment recorded across countries and across disease stages, which significantly affects survival outcomes. Stratification by factors such as etiologies, tumor characteristics, the presence of extrahepatic metastases or macrovascular invasion, and the use of subsequent lines of treatment were performed.
Conclusion
The INSIGHT study describes a wide spectrum of clinical management practices in HCC, where patient demographics, differential costs, and patient access to therapies may lead to wide geographical variations through the patient’s treatment cycle, from diagnosis to clinical outcome. The high heterogeneity in patient outcomes demonstrates the need for more robust and clinical management strategies to be designed and adopted to bring about better patient outcomes.
Keywords: Hepatocellular carcinoma, Liver cancer, Epidemiology, Asia-Pacific
Introduction
Hepatocellular carcinoma (HCC) is the sixth most commonly diagnosed cancer and the third leading cause of cancer death worldwide [1]. In 2020, there were approximately 906,000 new cases and 830,000 deaths from HCC [2], and Asia shoulders a disproportionate 80% of the global disease burden [3]. HCC remains highly lethal, with a mortality-to-incidence ratio of 0.92, the highest for any solid tumor [4]. The prevalence of HCC within Asia, however, is highly heterogenous, with the highest rates in Eastern and Northern Asia [1], mostly as a result of the high prevalence of chronic viral hepatitis infection in the region.
There has been rapid evolution in the treatment paradigm of HCC across the past decade, especially in the utility of systemic therapy. The SHARP trial (2008) was significant as it led to FDA approval of sorafenib as the first systemic therapy for patients with advanced HCC. In the 15 years since, a number of positive phase III trials of systemic therapy have led to FDA approvals for second-line therapy following the failure of sorafenib, e.g., regorafenib [5], cabozantinib [6], ramucirumab [7], and pembrolizumab [8]. In 2018, lenvatinib was found to be non-inferior to sorafenib in a phase III trial [9]. More recently, positive phase III trials have been reported for new immunotherapeutic drugs as first-line treatment for advanced HCC, such as the combination of atezolizumab plus bevacizumab in 2021 and tremelimumab plus durvalumab in 2022 [10–12]. Online supplementary Table 1 (for all online suppl. material, see https://doi.org/10.1159/000534513) shows landmark phase III trials in the evolving landscape of systemic therapies in HCC, as well as ongoing trials that could yield promising results for the future.
The success of the LEGACY study led to approval of Yttrium-90 microspheres (SIRT-Y90) as monotherapy for solitary HCC of up to 8 cm by the US FDA in 2021 [13, 14], and radioembolization has been added as a curative monotherapy modality to existing ablative modalities, radiofrequency ablation (RFA), surgical resection, and transplantation [15, 16] in early stage HCC. There have also been recent attempts to combine loco-regional therapies (trans-arterial chemoembolization [TACE] and trans-arterial radioembolization [TARE]) with systemic therapies [17–19], and hypotheses that TARE may have a synergistic effect when used with immunotherapy have led to ongoing clinical trials [20, 21]. Considerations to extend the indications of surgical resection to intermediate HCC have also been suggested [22]. In early 2023, we saw the announcement of the first positive adjuvant therapy trial in HCC, the pivotal IMBrave050 study, with the combination of atezolizumab plus bevacizumab following surgery [23].
The extent to which these newly approved therapies are utilized in clinical practice in the real world is, however, unknown. While the Barcelona Clinic Liver Cancer (BCLC) system is commonly used to compare outcomes of therapy in HCC, its use to guide therapy is by no means universal [24]. Regional professional guidelines, including those from China [25], Taiwan [26], Hong Kong [27], Korea [28], Japan [29], Australia [30], and Singapore [15, 31], have been established in attempts to guide therapeutic decisions, but their concordance and impact on real world practices are uncertain. Real-world data are however important to guide future public health strategies and therapeutic decisions and also the implementation of more holistic national guidelines at the country level. Such real-world data on clinical management practices in HCC are, however, sparse and limited. Data on HCC are mostly provided by clinical trial reports which are not reflective of real-world practice as they include highly specific and narrow groups of patients and treatment outcomes or by case series from specialized tertiary centers. Furthermore, much existing epidemiological real-world data are often country-specific, which are not representative of the region as a whole [32, 33].
The BRIDGE study published in 2015 was a notable attempt to meet this data gap. This was the first multi-regional, large-scale study to document the HCC patient experience from diagnosis to death, and data were retrospectively collected from the 7-year period of 2005–2012 [34]. Patients were recruited from Europe, North America, and 4 Asian countries: China, Taiwan, South Korea, and Japan, of which China contributed 72.2% of the total Asia-Pacific patient cohort. Additionally, sorafenib was the only approved systemic therapy during the BRIDGE period as the study concluded before maturation of the aforementioned clinical trials which significantly expanded the number of systemic therapies available for HCC. Thus, there is a compelling need for a study to capture real-world data on HCC, especially for the Asia-Pacific region, in the period following the BRIDGE study.
The INSIGHT (“Insight into Real-World Practice of Management of HCC in Asia-Pacific”) study (ClinicalTrials.gov Identifier: NCT03233360) was an investigator-initiated multi-site longitudinal cohort study [35], conducted under the auspices of the Asia-Pacific Hepatocellular Carcinoma (AHCC) Trials Group, that aimed to provide detailed information on the HCC patient journey from patients from several representative Asia-Pacific countries, namely China, Taiwan, Japan, Hong Kong, South Korea, Singapore, Australia, New Zealand, and Thailand. The aim of the registry was to collect data that reflect real-world epidemiology and clinical practice in Asia-Pacific in the immediate 7-year period after the conclusion of the BRIDGE study. This study also aims to show changes in the HCC demographics and clinical management over time in the Asia-Pacific since the BRIDGE study. Parameters of comparison include patient demographics, patterns of diagnosis, clinical characteristics, and treatment patterns. All the comparisons were made with reference to the BCLC staging system for uniformity in the comparison of patient characteristics and treatment outcomes.
Materials and Methods
Study Design
The INSIGHT study was initiated by the National Cancer Centre Singapore, in collaboration with the AHCC Trials Group, the Singapore Clinical Research Institute (SCRI), and IQVIA Solutions Asia Pte. Ltd. The AHCC Trials Group is an academic collaborative research network involving more than 50 healthcare institutions from 17 Asia-Pacific countries with a common goal of seeking efficacious treatments for HCC and engaging in research for patients with HCC [36]. The list of participating sites is presented in online supplementary Table 2. A steering committee, comprised of multidisciplinary experts (surgical oncologist, medical oncologist, radiation oncologist, interventional radiologist, hepatologist, and medical statistician) from participating Asia-Pacific countries with extensive research experience in HCC, was formed to provide scientific oversight and inputs to the planning and conduct of the study.
Patient Selection
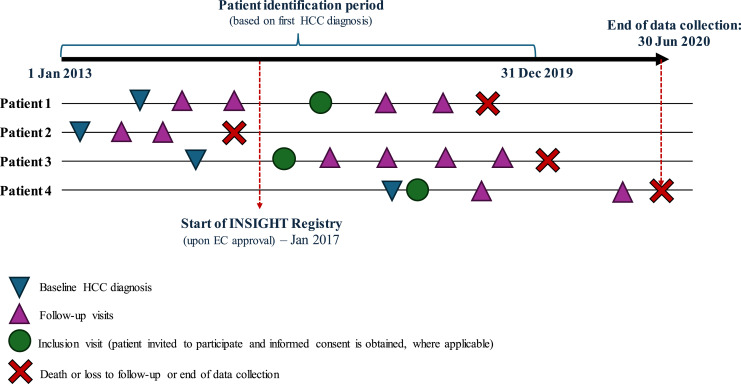
Inclusion criteria were patients aged 21 years or older, newly diagnosed with HCC between January 1, 2013, and December 31, 2019, using one or more of the following diagnostic criteria: (1) American Association for the Study of Liver Disease (AASLD) imaging criteria [37]; (2) Asian-Pacific Association for the Study of the Liver (APASL) imaging criteria [37]; (3) histology/cytology; (4) space-occupying lesion in the liver and a serum alpha-fetoprotein level of >400 ng/mL in a patient with chronic viral hepatitis or cirrhosis from any cause [38]. Approximately 30% of the sample size was identified from January 2013 up to December 2016 retrospectively (referred now onward as “retrospective cohort”), and 70% was identified from January 2017 up to December 2019 prospectively (referred now onward as “prospective cohort”), with an even distribution of consecutively diagnosed patients within the different years and months [35]. This ratio aimed to represent the most up-to-date clinical management of patients with greater emphasis on the patients diagnosed in the prospective cohort. Figure 1 illustrates the study design of the INSIGHT study.
Fig. 1.
Study design of the INSIGHT study.
All sites in the study had approval from their local institutional review boards (IRB) to conduct the study in accordance with a common protocol. Potentially eligible patients attending routine clinic visits were approached by their treating physicians and invited to participate in the study. Prior to data collection, patient informed consent was obtained as per institutional requirements. Each site was assigned unique ranges of subject codes prior to the study, and recruited patients were then assigned a unique 6-digit identifier sequentially. All aspects of treatment decisions and clinical management of patients were conducted in accordance with local clinical practice and at the discretion of the treating physician. The observation period for each patient began from the point of HCC diagnosis up to the end of the registry (June 30, 2020) or death or loss to follow-up (whichever occur first).
Study Sites
Principal investigators of the sites include physicians from a range of specialties that routinely manage patients in the local clinical setting and include specialists in medical oncology, hepatology, surgery, or internal medicine based at tertiary care centers.
Data Collection
For the prospective cohort, study data collection was aligned with patients’ routine visits to the clinic (on a 3–6 month basis), and for the retrospective cohort, it was collected from the respective institution’s medical records. Key data collected included patient demographics, HCC risk factors, socio-economic factors, clinical characteristics (disease staging, etiology, tumor burden, liver function, serum biomarkers, and comorbidities), HCC-directed therapy, and outcomes. Data on healthcare resource use related to HCC were also collected (e.g., physician visits, types and dates of assessments, direct and indirect costs) from sites where the IRB allowed such data to be collected.
Statistical Methods and Analysis
All eligible patients enrolled in the study were included in the analysis population. The patients’ demographics, clinical characteristics, disease etiology, and comorbidities at baseline were reported as aggregate data for each country. Univariate and multiple Cox proportional hazards regression models including a subset of covariates like etiologies and treatment modalities were explored (online suppl. Table 3). Number and percentage of patients were reported for categorical variables, and descriptive statistics (mean and standard deviation) were reported for numerical variables. Treatment modalities were reported using the number and percentage of patients for each country by BCLC staging. The overall survival (OS) of patients by country was presented using Kaplan-Meier plots and bubble plots by BCLC staging and in the overall population. The bubble sizes are proportionate to the sample sizes in each country. The median overall survival (mOS) with 95% confidence intervals was reported for each country by BCLC stage. Homogeneity of the patient population between retrospective and prospective cohorts was also evaluated and presented in online supplementary Table 4.
All analyses were conducted in SAS version 9.4. Figures 2–4 were produced using the R package ggplot2, versions 4.1.3 and 4.2.0 [39].
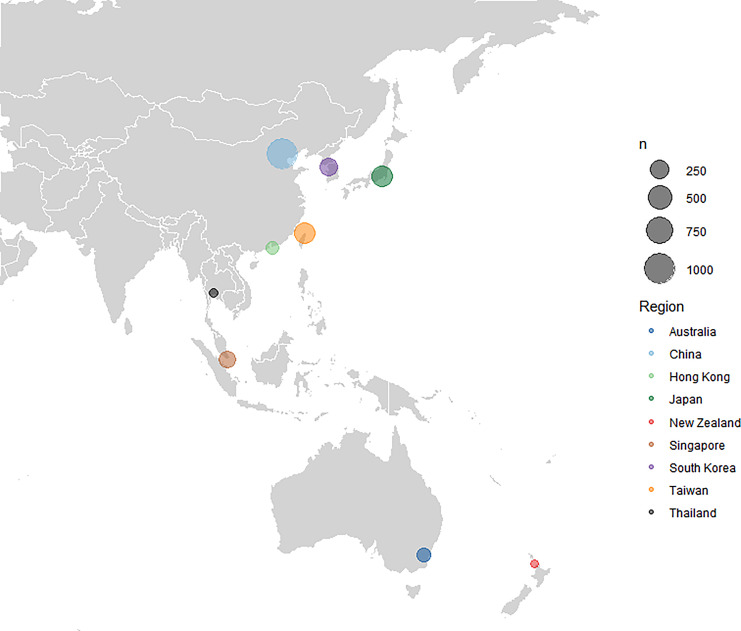
Fig. 2.
Distribution of recruited patients by country.
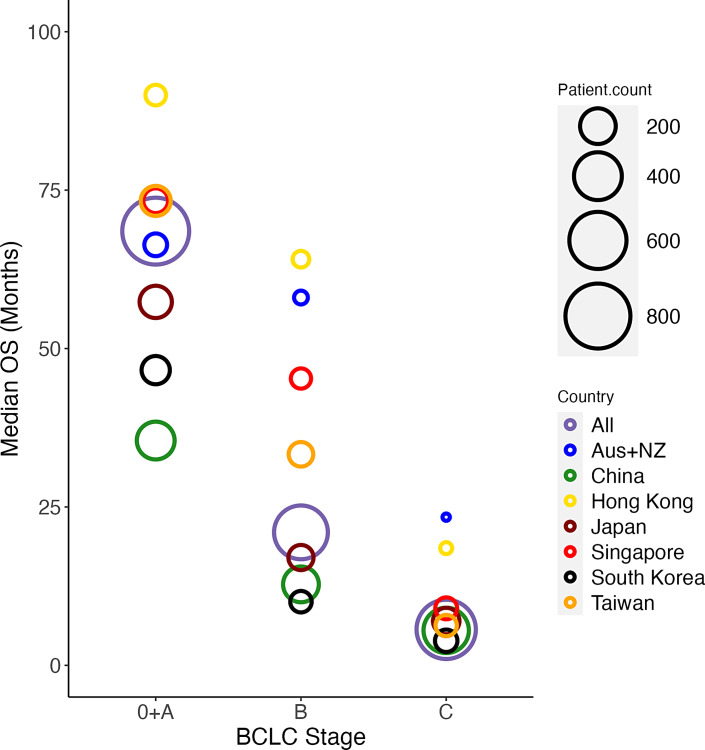
Fig. 4.
Bubble plot of mOS at the country level stratified by BCLC staging. Bubble size indicates relative cohort size.
Data from the BRIDGE study for the 4 Asia countries (China, Taiwan, South Korea, and Japan) in the study were extracted from Park et al. [34] for patient characteristics, treatment modality, and overall survival (hereafter referred to as the “AP-BRIDGE cohort”) and used as comparators to the INSIGHT study. Where some of the treatment-related data were not explicitly available from the summary tables, it was extracted from the corresponding graphs with the accuracy of nearest percent. Otherwise, graphs were scaled until 10 percent on the ordinate corresponded to 1 cm in actual dimension. The points were then read to the nearest 1-mm division using a ruler [40].
Results
Patient Demographics
At the point of data lock of the INSIGHT study on December 31, 2019, a total of 2,533 newly diagnosed HCC patients (1,052 in retrospective cohort and 1,481 in prospective cohort) were recruited from 33 institutions in nine countries in the Asia-Pacific. This proportion differed from the original planned recruitment owing to different recruitment paces at sites, hence recruiting more retrospective patients than planned. This included 1,078 patients from China, 374 patients from Taiwan, 347 from Japan, 94 from Hong Kong, 239 from South Korea, 189 from Singapore, 114 from Australia, 48 from New Zealand, and 50 from Thailand (shown in Fig. 2). Table 1 illustrates the clinical characteristics at the point of HCC diagnosis stratified by country. As a comparison, an overwhelming 48.2% of the BRIDGE cohort was recruited from China, followed by 8.8% from Taiwan, 6.8% from South Korea, and 3.0% from Japan [34].
Table 1.
Baseline clinical characteristics between countries
| All (N = 2,533) | China (N = 1,078) | Taiwan (N = 374) | Japan (N = 347) | South Korea (N = 239) | Singapore (N = 189) | Australia (N = 114) | Hong Kong (N = 94) | New Zealand (N = 48) | Thailand (N = 50) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, years, mean (SD) | 59.6 (12.7) | 53.4 (11.5) | 63.2 (11.2) | 71.1 (10.9) | 60.3 (12.1) | 64.4 (11.0) | 60.8 (8.8) | 60.5 (9.8) | 64.3 (9.9) | 59.8 (8.6) |
| Men, n (%) | 2,115 (83.5) | 966 (89.6) | 283 (75.7) | 283 (81.6) | 182 (76.2) | 159 (84.1) | 93 (81.6) | 83 (88.3) | 35 (72.9) | 31 (62.0) |
| Ethnicity, n (%) | N = 2,533 | N = 1,078 | N = 374 | N = 347 | N = 239 | N = 189 | N = 114 | N = 94 | N = 48 | N = 50 |
| Chinese | 1,697 (67.0) | 1,076 (99.8) | 372 (99.5) | 3 (<1) | 1 (<1) | 146 (77.2) | 3 (2.6) | 94 (100.0) | 2 (4.2) | 0 |
| Japanese | 343 (13.5) | 0 | 0 | 343 (98.8) | 0 | 0 | 0 | 0 | 0 | 0 |
| Korean | 241 (9.5) | 0 | 0 | 1 (<1) | 237 (99.2) | 0 | 1 (<1) | 0 | 2 (4.2) | 0 |
| Caucasian | 120 (4.7) | 0 | 2 (<1) | 0 | 0 | 2 (1.1) | 87 (76.3) | 0 | 29 (60.4) | 0 |
| Other | 65 (2.6) | 0 | 0 | 0 | 1 (<1) | 30 (15.9) | 21 (18.4) | 0 | 13 (27.1) | 0 |
| Thai | 50 (2.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 50 (100.0) |
| Indian | 12 (<1) | 2 (<1) | 0 | 0 | 0 | 6 (3.2) | 2 (1.8) | 0 | 2 (4.2) | 0 |
| Malay | 5 (<1) | 0 | 0 | 0 | 0 | 5 (2.6) | 0 | 0 | 0 | 0 |
| Alcohol consumption, n (%) | N = 2,247 | N = 1,020 | N = 346 | N = 258 | N = 195 | N = 181 | N = 107 | N = 66 | N = 40 | N = 34 |
| Never | 1,338 (53.3) | 654 (60.8) | 294 (78.6) | 128 (37.0) | 74 (32.7) | 104 (55.6) | 27 (23.7) | 29 (30.9) | 4 (8.3) | 24 (53.3) |
| Occasionally/regularly | 909 (36.2) | 366 (34.0) | 52 (13.9) | 130 (37.6) | 121 (53.5) | 77 (41.2) | 80 (70.2) | 37 (39.4) | 36 (75.0) | 10 (22.2) |
| HCC risk factors, n (%) | N = 2,531 | N = 1,078 | N = 373 | N = 347 | N = 239 | N = 189 | N = 114 | N = 93 | N = 48 | N = 50 |
| HBV | 1,639 (64.8) | 984 (91.3) | 232 (62.2) | 57 (16.4) | 154 (64.4) | 87 (46.0) | 17 (14.9) | 77 (82.8) | 7 (14.6) | 24 (48.0) |
| HCV | 385 (15.2) | 18 (1.7) | 116 (31.1) | 94 (27.1) | 24 (10.0) | 24 (12.7) | 65 (57.0) | 6 (6.5) | 19 (39.6) | 19 (38.0) |
| Diabetes | 282 (11.1) | 19 (1.8) | 53 (14.2) | 90 (25.9) | 25 (10.5) | 51 (27.0) | 36 (31.6) | 3 (3.2) | 4 (8.3) | 1 (2.0) |
| ALD | 256 (10.1) | 29 (2.7) | 16 (4.3) | 70 (20.2) | 48 (20.1) | 15 (7.9) | 52 (45.6) | 7 (7.5) | 16 (33.3) | 3 (6.0) |
| NASH/NAFLD | 90 (3.5) | 2 (<1) | 7 (1.9) | 20 (17.4) | 1 (<1) | 24 (12.7) | 25 (21.9) | 1 (1.1) | 9 (18.8) | 1 (2.0) |
| Comorbidity, n (%) | N = 2,532 | N = 1,078 | N = 374 | N = 347 | N = 239 | N = 189 | N = 114 | N = 94 | N = 48 | N = 50 |
| Cirrhosis | 932 (36.8) | 301 (27.9) | 155 (41.4) | 98 (28.2) | 104 (43.7) | 82 (43.4) | 104 (91.2) | 29 (30.9) | 19 (39.6) | 40 (80.0) |
| Hypertension | 755 (29.8) | 143 (13.3) | 149 (39.8) | 155 (44.7) | 88 (37.0) | 111 (58.7) | 36 (31.6) | 32 (34.0) | 19 (39.6) | 22 (44.0) |
| Diabetes | 529 (20.9) | 59 (5.5) | 115 (30.7) | 126 (36.3) | 64 (26.9) | 78 (41.3) | 40 (35.1) | 17 (18.1) | 14 (29.2) | 16 (32.0) |
| Hyperlipidemia | 216 (8.5) | 21 (1.9) | 19 (5.1) | 58 (16.7) | 9 (3.8) | 77 (40.7) | 8 (7.0) | 5 (5.3) | 9 (18.8) | 10 (20.0) |
| Liver dysfunction | 165 (6.5) | 97 (9.0) | 15 (4.0) | 10 (2.9) | 17 (7.1) | 6 (3.2) | 15 (13.2) | 0 | 2 (4.2) | 3 (6.0) |
| None | 640 (25.3) | 417 (38.7) | 81 (21.7) | 35 (10.1) | 48 (20.2) | 14 (7.4) | 0 | 30 (31.9) | 12 (25.0) | 3 (6.0) |
| BCLC stages, n (%) | N = 2,067 | N = 803 | N = 267 | N = 347 | N = 238 | N = 189 | N = 43 | N = 92 | N = 48 | N = 40 |
| 0 | 181 (8.8) | 11 (1.4) | 42 (15.7) | 37 (10.7) | 44 (18.5) | 3 (1.6) | 12 (27.9) | 13 (14.1) | 10 (20.8) | 9 (22.5) |
| A | 672 (32.5) | 219 (27.3) | 89 (33.3) | 120 (34.6) | 69 (29.0) | 78 (41.3) | 18 (41.9) | 37 (40.2) | 25 (52.1) | 17 (42.5) |
| B | 523 (25.3) | 201 (25.0) | 79 (29.6) | 84 (24.2) | 56 (23.5) | 48 (25.4) | 10 (23.3) | 29 (31.5) | 8 (16.7) | 8 (20.0) |
| C | 652 (31.5) | 353 (44.0) | 55 (20.6) | 96 (27.7) | 63 (26.5) | 59 (31.2) | 3 (7.0) | 13 (14.1) | 5 (10.4) | 5 (12.5) |
| D | 39 (1.9) | 19 (2.4) | 2 (<1) | 10 (2.9) | 6 (2.5) | 1 (<1) | 0 | 0 | 0 | 1 (2.5) |
| Child Pugh class, n (%) | N = 1,493 | N = 694 | N = 249 | N = 220 | N = 137 | N = 128 | N = 43 | N = 0 | N = 7 | N = 15 |
| A | 1,178 (78.9) | 503 (72.5) | 213 (85.5) | 190 (86.4) | 117 (85.4) | 100 (78.1) | 36 (83.7) | 0 | 4 (57.1) | 15 (100.0) |
| B | 296 (19.8) | 181 (26.1) | 33 (13.3) | 29 (13.2) | 17 (12.4) | 27 (21.1) | 7 (16.3) | 0 | 2 (28.6) | 0 |
| C | 19 (1.3) | 10 (1.4) | 3 (1.2) | 1 (<1) | 3 (2.2) | 1 (<1) | 0 | 0 | 1 (14.3) | 0 |
| Mode of first HCC diagnosis, n (%) | N = 2,533 | N = 1,078 | N = 374 | N = 347 | N = 239 | N = 189 | N = 114 | N = 94 | N = 48 | N = 50 |
| Radiological | 1,949 (76.9) | 660 (61.2) | 255 (68.2) | 328 (94.5) | 235 (98.3) | 172 (91.0) | 113 (99.1) | 93 (98.9) | 45 (93.8) | 48 (96.0) |
| Histopathological | 554 (21.9) | 390 (36.2) | 112 (29.9) | 17 (4.9) | 7 (2.9) | 16 (8.5) | 2 (1.8) | 4 (4.3) | 3 (6.3) | 3 (6.0) |
| Cytological | 212 (8.4) | 191 (17.7) | 9 (2.4) | 7 (2.0) | 5 (2.1) | 0 | 0 | 0 | 0 | 0 |
| ECOG status, n (%) | N = 1,834 | N = 660 | N = 216 | N = 347 | N = 239 | N = 142 | N = 44 | N = 90 | N = 48 | N = 48 |
| 0 | 1,195 (65.2) | 252 (38.2) | 192 (88.9) | 267 (76.9) | 168 (70.3) | 117 (82.4) | 41 (93.2) | 78 (86.7) | 39 (81.3) | 41 (85.4) |
| 1 | 566 (30.9) | 379 (57.4) | 18 (8.3) | 65 (18.7) | 54 (22.6) | 23 (16.2) | 3 (6.8) | 10 (11.1) | 7 (14.6) | 7 (14.6) |
| >1 | 73 (4.0) | 29 (4.4) | 6 (2.8) | 15 (4.3) | 17 (7.1) | 2 (1.4) | 0 | 2 (2.2) | 2 (4.2) | 0 |
| Tumor diameter | N = 1,211 | N = 382 | N = 239 | N = 210 | N = 122 | N = 141 | N = 101 | N = 0 | N = 1 | N = 15 |
| Median | 35.0 | 50.0 | 28.0 | 31.0 | 30.0 | 40.0 | 23.0 | – | 40.0 | 14.0 |
| Presence of MVI, n (%) | N = 2,345 | N = 1,003 | N = 335 | N = 345 | N = 178 | N = 187 | N = 110 | N = 89 | N = 48 | N = 50 |
| Yes | 660 (28.1) | 431 (43.0) | 40 (11.9) | 90 (26.1) | 32 (18.0) | 38 (20.3) | 3 (2.7) | 18 (20.2) | 4 (8.3) | 4 (8.0) |
| Presence of distant metastasis, n (%) | N = 2,345 | N = 1,003 | N = 335 | N = 345 | N = 178 | N = 187 | N = 110 | N = 89 | N = 48 | N = 50 |
| Yes | 190 (8.1) | 90 (9.0) | 19 (5.7) | 35 (10.1) | 19 (10.7) | 17 (9.1) | 1 (<1) | 6 (6.7) | 1 (2.1) | 2 (4.0) |
HBV, hepatitis B virus; HCV, hepatitis C virus; ALD, alcoholic liver disease; NASH/NAFLD, non-alcoholic steatosis/non-alcoholic fatty liver disease.
The AP-BRIDGE and INSIGHT studies had mean ages at diagnosis at 55 years old and 60 years old, respectively. Japan had the oldest diagnosed patients in both the AP-BRIDGE (mean 69 years) and INSIGHT (mean 71 years). Patients from China in both studies made up the youngest population, with a mean age at diagnosis of 52 years old and 53 years old in the AP-BRIDGE and INSIGHT studies, respectively.
Gender, which is a known risk factor [41], showed a highly skewed imbalance in all countries with the proportion of men being more than threefold compared to women. Overall, 83.5% of the cohort was men, with China (89.6%), Hong Kong (88.3%), and Singapore (84.1%) having the most skewed gender distribution. Similar observation was made for the AP-BRIDGE study, with 83.1% male representation.
Ethnicity was largely homogenous within each country of the INSIGHT study. The dominant ethnic group in countries like China (99.8%), Taiwan (99.5%), Singapore (77.2%), and Hong Kong (100%) was Chinese and Caucasians in Australia and New Zealand. Within countries like Japan, Korea, and Thailand, it was distinctly represented by 98.8% Japanese, 99.2% Korean, and 100% Thai. The AP-BRIDGE did not report ethnicity information in the study.
Etiology and Comorbidity
The most common risk factor for HCC was hepatitis B in China (91.3%), Taiwan (62.2%), South Korea (64.4%), Hong Kong (82.8%), Thailand (48.0%), and Singapore (46.0%). Distinct etiological differences were observed in Japan, Australia, and New Zealand, where hepatitis C and diabetes were more prevalent. Most recorded etiologies were consistent between the two studies, with a high prevalence of hepatitis B in China (77.0% in AP-BRIDGE), Taiwan (62.5% in AP-BRIDGE), and South Korea (75.4% in AP-BRIDGE) [34]. About 26.0% of the Japanese patients in the INSIGHT study had diabetes, but the corresponding information was not reported in the BRIDGE study.
The top five comorbidities reported in the INSIGHT study were cirrhosis (36.8%), hypertension (29.8%), diabetes (20.9%), hyperlipidemia (8.5%), and liver dysfunction (6.5%), where a single patient may present with one or more comorbidities. Overall, cirrhosis was the most common comorbidity in all countries except Japan, Singapore, and Hong Kong. Hypertension was the highest reported comorbidity in Singapore and Hong Kong and hepatitis C in Japan [42, 43]. Reported alcohol consumption was the highest in Australia and New Zealand in the INSIGHT study of more than 70%, followed by South Korea at 53.5%. The South Korean representation in the AP-BRIDGE was comparable, at 67%. In contrast, we see a vast difference in alcohol consumption for the Japanese population, at 2% in AP-BRIDGE and 37.6% in INSIGHT. However, while in the INSIGHT study, significant alcohol consumption is defined as 14 units per week, this was not formally defined in the BRIDGE study. Information on comorbidities was not reported in the BRIDGE study paper.
Clinical Status at Diagnosis and Mode of Diagnosis
Of the 2,533 patients recruited in the INSIGHT study, BCLC information at baseline was available for 2,077 patients. Most patients were diagnosed at BCLC A (32.5%) or C (31.5%), followed by BCLC B (25.3%), and only a minority were diagnosed at late stage BCLC D (1.9%). Of patients diagnosed at BCLC A, the highest proportion was observed in Singapore (41.3%) and New Zealand (52.1%), and the lowest in China (27.3%). In China, most patients were diagnosed at BCLC C (44%). South Korea has a relatively uniform distribution of patients across BCLC staging (A 29.0%, B 23.5%, and C 26.5%). Across the countries, most patients had an Eastern Cooperative Oncology Group (ECOG) performance status grade of 0 or 1 (96.1%) at diagnosis.
The AP-BRIDGE cohort was mostly diagnosed at BCLC C (47.3%), followed by BCLC A (34.3%) and BCLC B (10.2%). Consistent with the INSIGHT study, most patients from Taiwan (55.4% in BRIDGE vs. 33.3% in INSIGHT) and Japan (47.6% vs. 34.6%) were diagnosed with BCLC A, while mainland Chinese patients mostly presented at the late stage (BCLC C 55.5% in BRIDGE vs. 44.0% in INSIGHT). Distinct differences in the distribution of the BCLC stages at point of HCC diagnosis are observed for the South Korean population between AP-BRIDGE and INSIGHT. The majority of South Korean patients in the BRIDGE study were diagnosed at BCLC C (52.5%), while the INSIGHT study had only 26.5% of patients diagnosed at this stage and similar spread of patients across other BCLC stages in INSIGHT (BCLC 0 18.5%, BCLC A 29.0%, and BCLC B 23.5%).
Across all countries, the most common mode of diagnosis for HCC was radiological imaging by computerized tomography (CT) or magnetic resonance imaging (MRI) (76.9%) using AASLD/APASL criteria, followed by histopathology (21.9%). The countries with the highest radiological diagnoses were Australia, Hong Kong, New Zealand, and Thailand (each >90%), whereas China (61.2%) and Taiwan (68.2%) had a lower percentage of patients diagnosed with radiological imaging compared to the mean of 77% from all countries. In general, histopathology was not widely used for diagnosis apart from China and Taiwan.
Median tumor diameter at diagnosis ranged from 14 mm to 50 mm, with the largest median tumor size recorded in Chinese patients and the smallest in Thailand patients. Similarly, in AP-BRIDGE, Chinese patients had the largest tumors at 67 mm. The largest absolute difference observed between the AP-BRIDGE and INSIGHT cohorts was in South Korea (44 mm in AP-BRIDGE and 30 mm in INSIGHT). The highest incidence of macrovascular invasion (MVI) was recorded in China during both the AP-BRIDGE and the INSIGHT periods. Compared to the AP-BRIDGE, there was an increase in presence of MVI in China and Japan at 20% and 16.1%, respectively.
First-Recorded Treatment Modalities
First-line treatment modalities stratified by BCLC staging are described in Table 2. The results are highly heterogeneous across countries. Patients who received treatment were found to have significantly better OS compared to patients who did not receive any treatment across all stages of the disease (p < 0.05). Overall, 82.5% of the patients diagnosed at BCLC stages 0 and A received ablative/surgical treatment modalities such as surgical resection, radiofrequency ablation, and transplant as first-line treatment. Compared with the BRIDGE study, there was a 19.2% increase in the use of surgical resection with an accompanied 11.5% decrease in TACE.
Table 2.
The first-line treatment modalities between countries
| All (N = 2,533) | China (N = 1,078) | Taiwan (N = 374) | Japan (N = 347) | South Korea (N = 239) | Singapore (N = 189) | Australia and New Zealand (N = 162) | Hong Kong (N = 94) | Thailand (N = 50) | |
|---|---|---|---|---|---|---|---|---|---|
| Stage 0 and A, n (%) | N = 825 | N = 224 | N = 130 | N = 148 | N = 110 | N = 74 | N = 66 | N = 47 | N = 26 |
| Ablative/surgical therapy | 681 (82.5) | 191 (85.3) | 119 (91.5) | 134 (90.5) | 83 (75.5) | 50 (67.6) | 45 (68.2) | 41 (87.2) | 19 (73.1) |
| Resection | 506 (61.3) | 180 (80.4) | 59 (45.4) | 106 (71.6) | 76 (69.1) | 41 (55.4) | 15 (22.7) | 27 (57.4) | 11 (42.3) |
| RFA | 172 (20.8) | 11 (4.9) | 59 (45.4) | 28 (18.9) | 5 (4.5) | 9 (12.2) | 30 (45.5) | 14 (29.8) | 8 (30.8) |
| Transplant | 3 (<1) | 0 | 1 (<1) | 0 | 2 (1.8) | 0 | 0 | 0 | 0 |
| Loco-regional therapy | 192 (23.3) | 37 (16.5) | 14 (10.8) | 21 (14.2) | 30 (27.3) | 24 (32.4) | 25 (37.9) | 28 (59.6) | 12 (46.2) |
| TACE | 155 (18.8) | 37 (16.5) | 12 (9.2) | 20 (13.5) | 26 (23.6) | 8 (10.8) | 23 (34.8) | 16 (34.0) | 12 (46.2) |
| Radiation therapy | 37 (4.5) | 0 | 2 (1.5) | 1 (<1) | 4 (3.6) | 16 (21.6) | 2 (3.0) | 12 (25.5) | 0 |
| Received more than 1 line of therapy | 280 (33.9) | 90 (40.2) | 49 (37.7) | 32 (21.6) | 23 (20.9) | 36 (48.6) | 24 (36.4) | 17 (36.2) | 9 (34.6) |
| Stage B, n (%) | N = 494 | N = 184 | N = 81 | N = 79 | N = 49 | N = 47 | N = 19 | N = 27 | N = 8 |
| Resection | 190 (38.5) | 84 (45.7) | 35 (43.2) | 36 (45.6) | 12 (24.5) | 25 (53.2) | 1 (5.3) | 10 (37.0) | 0 |
| Loco-regional therapy | 265 (53.6) | 98 (53.3) | 43 (53.1) | 32 (40.5) | 34 (69.4) | 10 (21.3) | 18 (94.7) | 22 (81.5) | 8 (100) |
| RFA | 29 (5.9) | 4 (2.2) | 7 (8.6) | 2 (2.5) | 2 (4.1) | 4 (8.5) | 3 (15.8) | 5 (18.5) | 2 (25.0) |
| TACE | 236 (47.8) | 94 (51.1) | 36 (44.4) | 30 (38.0) | 32 (65.3) | 6 (12.8) | 15 (78.9) | 17 (63.0) | 6 (75.0) |
| Radiation therapy | 41 (8.3) | 1 (<1) | 2 (2.5) | 3 (3.8) | 4 (8.2) | 27 (57.4) | 1 (5.3) | 3 (11.1) | 0 |
| Systemic therapy | 40 (8.1) | 5 (2.7) | 2 (2.5) | 10 (12.7) | 5 (10.2) | 4 (8.5) | 2 (10.5) | 12 (44.4) | 0 |
| Received more than 1 line of therapy | 208 (42.1) | 66 (35.9) | 43 (53.1) | 31 (39.2) | 13 (26.5) | 34 (72.3) | 6 (31.6) | 14 (51.9) | 1 (12.5) |
| Stage C, n (%) | N = 657 | N = 354 | N = 56 | N = 95 | N = 68 | N = 58 | N = 8 | N = 13 | N = 5 |
| Resection | 164 (25.0) | 106 (29.9) | 11 (19.6) | 30 (31.6) | 10 (14.7) | 1 (1.7) | 2 (25.0) | 4 (30.8) | 0 |
| Loco-regional Therapy | 242 (36.8) | 129 (36.4) | 19 (33.9) | 32 (33.7) | 36 (52.9) | 18 (31.0) | 2 (25.0) | 2 (15.4) | 4 (80.0) |
| TACE | 201 (30.6) | 124 (35.0) | 13 (23.2) | 30 (31.6) | 27 (39.7) | 2 (3.4) | 0 | 2 (15.4) | 3 (60.0) |
| Radiation therapy | 41 (6.2) | 5 (1.4) | 6 (10.7) | 2 (2.1) | 9 (13.2) | 16 (27.6) | 2 (25.0) | 0 | 1 (20.0) |
| Systemic therapy | 132 (20.1) | 13 (3.7) | 27 (48.2) | 16 (16.8) | 26 (38.2) | 29 (50.0) | 5 (62.5) | 12 (92.3) | 4 (80.0) |
| No treatment | 157 (23.9) | 110 (31.1) | 2 (3.6) | 20 (21.1) | 9 (13.2) | 13 (22.4) | 2 (25.0) | 1 (7.7) | 0 |
| Received more than 1 line of therapy | 155 (23.6) | 74 (20.9) | 14 (25.0) | 31 (32.6) | 17 (25.0) | 12 (20.7) | 3 (37.5) | 3 (23.1) | 1 (20.0) |
| Stage D, n (%) | N = 41 | N = 19 | N = 2 | N = 11 | N = 6 | N = 2 | N = 0 | N = 0 | N = 1 |
| Resection | 5 (12.2) | 3 (15.8) | 0 | 2 (18.2) | 0 | 0 | 0 | 0 | 0 |
| TACE | 3 (7.3) | 2 (10.5) | 0 | 0 | 1 (16.7) | 0 | 0 | 0 | 0 |
| No treatment | 29 (70.7) | 14 (73.7) | 1 (50) | 8 (72.7) | 4 (66.7) | 1 (50) | 0 | 0 | 1 (100) |
| Received more than 1 line of therapy | 3 (7.3) | 1 (5.3) | 0 | 2 (18.2) | 0 | 0 | 0 | 0 | 0 |
RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
In the BCLC B subpopulation, 53.6% of patients were treated first using loco-regional therapies like TACE, followed by 38.5% of patients receiving resection. There is similar preference for the use of loco-regional therapy and surgical resection in countries like China, Taiwan, and Japan. A strong preference for the use of loco-regional therapy over resection in this subpopulation was observed in South Korea, Hong Kong, Australia, and New Zealand, which is evidenced by a 4.3% overall increase in the use of other loco-regional therapies such as radiation therapy compared with the BRIDGE cohort.
For BCLC C patients, only 20.1% of the patients received systemic therapy as first-line therapy, whereas 36.8% of patients received loco-regional therapy, with another 25% of patients receiving surgical resection. This proportion is highly divergent from that captured in the BRIDGE cohort, where a vast majority of 47.2% received TACE and a 4% minority received sorafenib (see Discussion). At the country level, the preferred first-line therapy is highly divergent. Countries like Taiwan (48.2%), Singapore (50%), and Hong Kong (92.3%) showed high preference for the use of systemic therapies for BCLC C patients. Majority of patients in China, South Korea, and Japan received loco-regional therapy, usually TACE. Notably, 31.1% of patients were provided the best supportive care in China [13, 44]. A small minority of 41 patients (1.61%) were diagnosed at BCLC D.
Subsequent lines of treatment were provided for 33.9% of BCLC 0 and A patients, 42.1% of BCLC B patients and 23.6% of BCLC C patients. This was seen especially for patients from Singapore and Taiwan across all BCLC stages, which potentially explains better survival outcomes in these countries (Fig. 3).
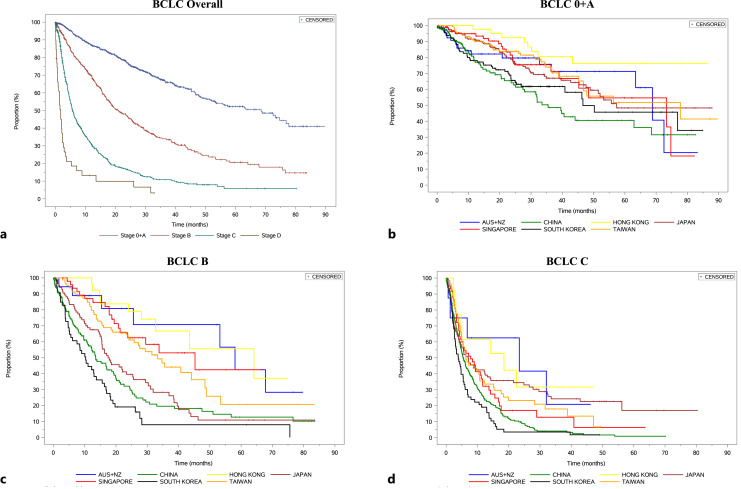
Fig. 3.
Kaplan-Meier analysis of median overall survival across BCLC stage (a), for BCLC 0-A across countries (b), for BCLC B across countries (c), for BCLC C across countries (d).
Survival Analyses
OS from the first HCC diagnosis by BCLC stage is shown in Figure 3 stratified by country. From INSIGHT, the median OS (mOS) was 68.53 months (BCLC 0 and A), 20.99 months (BCLC B), 5.68 months (BCLC C), and 1.81 months (BCLC D). At the country level, mOS differed greatly. Patients from Australia and New Zealand, Taiwan, Singapore, and Hong Kong consistently fared better than the other countries, regardless of BCLC staging. While univariate analyses suggest that patients with non-viral origins tend to fare worse than patients of viral origins, this is no longer significant upon multivariate analysis. Treatment modality, however, remains a significant prognostic factor for OS with multivariate analysis (online suppl. Table 3; online suppl. Fig. 1). Figure 4 is a bubble plot showing the mOS at the country level stratified by BCLC staging, with the bubble size indicating relative cohort size.
For BCLC 0 and A patients, mOS was the shortest in China at 35.48 months and the best in Hong Kong (mOS not met). South Korea and Japan fared worse compared to mOS for the whole study (68.53 months), while countries like Taiwan and Singapore had generally longer mOS. Multivariate analysis has shown that patients who receive surgery have superior outcomes (p < 0.05) compared to those who received loco-regional therapies and palliative care with a mOS of 78.9 months compared to 56.5 months and 5.9 months, respectively.
BCLC B patients recorded the longest mOS in Hong Kong at 64.10 months (see Discussion) and the shortest in South Korea at 10.05 months. Countries like China and Japan recorded mOS less than the median of the cohort (20.99 months), while patients from Taiwan, Singapore, Australia, and New Zealand performed better. Using treatment with loco-regional therapy as the reference (mOS of 19.7 months), BCLC B patients who received surgery fared better with a mOS of 29.9 months, while patients who received systemic therapy (11.1 months) and palliative care (2.83 months) fared worse. The ALBI scoring profiles of those who received surgery, loco-regional therapy, and systemic therapy were similar (grade 2: 68.28%, 64.15%, 65.96%, respectively; online suppl. Table 5).
In BCLC C patients, the mOS was highest in the Australia and New Zealand subpopulation at 23.39 months and lowest in South Korea at 3.88 months. Apart from Hong Kong and Australia/New Zealand, all other countries had mOS within the range of 5.52–7.85 months. Within BCLC C, it is notable that patients who received surgery had better mOS of 16 months compared to those who received loco-regional therapy (7.9 months), systemic therapy (5.6 months), and palliative care (2.7 months). 169 BCLC C patients (25.9%) had presence of either extrahepatic metastases or MVI upon HCC diagnosis. Patients who received surgical resection with extrahepatic metastases (9.5%) had a mOS of 10.6 months compared to those with MVI only (45.4%) of 26.1 months and those with BCLC C with neither extrahepatic metastases nor MVI (45.4%) of 34.9 months (online suppl. Fig. 2) (see Discussion).
Discussion
The INSIGHT study describes an Asia-Pacific observational cohort of patients diagnosed with HCC between 2013 and 2019 and provided both retrospective and prospectively collected data with high granularity in the patient journey, including sequential lines of treatment choices and outcomes. These data are attributable for each BCLC stage in each country and serve as a rich source of primary data to document and evaluate real-world clinical practice in the management of HCC. To enhance the value of the study, we compared data from this study to data from the BRIDGE study in the preceding 7-year period to enable a head-to-head comparison for the 4 countries (Japan, Taiwan, South Korea, and China), where data from both BRIDGE and INSIGHT were available and relevant. This allowed us to observe any changes in HCC treatment approaches during the period of 2005–2019.
In age, gender, and etiology, the distribution between the AP-BRIDGE and INSIGHT studies is largely consistent. Differences between the cohort were observed in comorbidities, alcohol consumption, tumor characteristics, stage at diagnosis, and first-recorded treatment modalities as described above. With respect to the BCLC stages at HCC diagnosis, we observe a decrease in late stage diagnosis from 47.3% in AP-BRIDGE to 31.5% in INSIGHT. At the country level, this is observed particularly in South Korea and China. In South Korea, the proportion of BCLC C patients at diagnosis was 53.0% in the BRIDGE study but 26.5% in INSIGHT, showing a sharp decrease in the proportion of late stage diagnosis recorded in the second 7-year period. This decrease is also validated by data from the Korean Nationwide Cancer Registry (N = 1,558) which showed that in 2015, there were 39.0% of newly diagnosed BCLC C patients, a figure that is intermediary to the periods of BRIDGE and INSIGHT [45]. In addition, the majority of INSIGHT patients from Taiwan (33.3%), Japan (34.6%), and Singapore (41.3%) were diagnosed early, at BCLC stage A. This could be reflective of effectiveness and success of surveillance programs, influencing the trends of diagnosis of HCC in those countries [33, 46].
There have been recent changes in the epidemiology of HCC, and major risk factors for the development of HCC have been in transition [1]. The prevalence of HBV and HCV has been declining due to universal vaccination (for HBV) and the introduction of anti-viral medications (for HCV), while the prevalence of excess body weight and type 2 diabetes mellitus (T2DM) is on the rise in many regions. Data from the BRIDGE and the INSIGHT showed that there was a slight decrease in prevalence of HBV in HCC patients from 72.5% in AP-BRIDGE to 64.8% in INSIGHT. The INSIGHT, however, also showed that an alarming proportion of 20.9% of patients reported T2DM as a comorbidity, reflecting the rising prevalence of metabolic liver disease. There is increasing evidence that T2DM and HCC are closely linked due to their association with obesity and impaired insulin sensitivity. The concomitant increase in the prevalence of non-alcoholic fatty liver disease (NAFLD) and metabolic liver diseases should be mitigated through amendments in management guidelines based on real-world epidemiology [47].
Preferred first-line treatment options were different in AP-BRIDGE and INSIGHT. These treatment variations can be partly attributed to the number and selection of sites in the studies [34]. The AP-BRIDGE only included single sites from Japan, South Korea, and Taiwan, while INSIGHT included a total of 5, 8, and 5 sites across the 3 countries, respectively. In the AP-BRIDGE, the singular sites selected in those 3 countries were led by principal investigators who were hepatologists, whereas INSIGHT included principal investigators with a range of specialties, including surgeons, medical oncologist, and interventional radiologists, and hence covers a wider range of patients across the disease spectrum, which may explain the varied treatment utility. In South Korea, resection was reported as the preferred first-line treatment in INSIGHT over TACE in AP-BRIDGE [45]. The vast difference in the utility of TACE, for example, reflects the inclusion of a range of specialty sites (surgery, hepatology, medical oncology) in our study.
Within INSIGHT, additional analysis on the contribution of ethnicity, etiology, and first-line treatment options was conducted to investigate if they impact the mOS as observed (Table 1). Although Chinese was the dominant ethnic group in 4 countries, country-level variation in mOS was independent of ethnicity. In addition, multivariate analysis shows that etiology was not a significant factor in influencing survival outcomes. Instead, the observed heterogeneity in mOS recorded is attributable to the preferred first-line treatment options offered to the patients. The preferred first-line treatment utilized in the INSIGHT is consistent with the recommendations of the latest BCLC guidelines published in 2022, except for BCLC C patients [24]. Patients with early HCC (BCLC 0 and A) largely received curative treatment like resection and ablation in all countries (82.5%). We observed high heterogeneity in the largest measurable single tumor size across countries, ranging from a median of 14 mm in Thailand to 40.5 mm in China, potentially influencing mOS. Most BCLC B patients receive loco-regional treatment like TACE and Y90 (53.6%), but a significant proportion receives surgery (38.5%). Although our data showed that patients who received surgery performed better than those who received loco-regional therapies, the superior mOS observed in Taiwan, Singapore, and Hong Kong could also be attributed to the use of multiple lines of treatment in those countries, which reflects the importance of multidisciplinary care to provide better outcomes for patients [15].
Within the BCLC C stage, we see strong country-level variation in treatment, which is also a distinct change from the BRIDGE study. While the BRIDGE study recorded low utility of sorafenib as the first-recorded treatment (less than 5% in China and Taiwan, 10% in South Korea, and less than 10% in Japan), the INSIGHT shows the preferential use of systemic therapies as the first-line treatment for advanced stage patients in Taiwan, Singapore, and Hong Kong (countries and territories previously not included in the BRIDGE). This could be owing to increasing drug confidence and the market entry of sorafenib into various countries. Furthermore, state subsidies contribute greatly to the use of systemic therapies as well. In South Korea, where sorafenib has become reimbursable, we observe a corresponding increase in the utility of systemic therapy in the country [48, 49]. Although survival analysis by first-line treatment showed that patients who received resection and loco-regional therapies had better outcomes, very few of these patients had extrahepatic metastases (10% in liver resection and 3.3% in loco-regional therapy), which could therefore skew the results (online suppl. Fig. 2). This highlights the heterogeneity of the BCLC C group, which encompasses patients with either MVI or extrahepatic metastases (or both) or neither, which have different impacts on mOS [50]. In addition, as lenvatinib was shown to be non-inferior to sorafenib as the first-line treatment in unresectable HCC only from 2018 onward [51], which was toward the end of data collection in the INSIGHT study, these two studies, AP-BRIDGE and INSIGHT, may not be sufficient to fully capture the evolution of systemic therapy in Asia-Pacific. With the recent positive results from pivotal trials such as the IMBRAVE150 (with mOS of 19.2 months), IMBrave050, and HIMALAYA studies, the use of systemic therapy in HCC will continue to evolve, and the data from INSIGHT will continue to serve as relevant comparators in the coming decade for the implementation of new drugs that are approved for use [52]. This suggests a need for future registries to be conducted in order to accurately reflect the changing practices of HCC management.
First-line treatment preference was also influenced heavily by changes in national treatment guidelines. There was a high preference for the use of resection (46.5%) and TACE (21.7%) in Japan recorded during the INSIGHT period. This preference is consistent with the findings from the Report of the 22nd Nationwide Follow-up Survey of Primary Liver Cancer in Japan (2012–2013, N = 19,209) [53], where 40.3% and 23.9% of the patients received resection and TACE, respectively [53]. In Singapore, there is significant use of radiation therapy for BCLC B (57.4%) and BCLC C (50%) patients as first-line therapy in addition to sorafenib, consistent with the guidelines published in 2021 [15, 54]. Further studies on the extent of the effect of regional guidelines in influencing reimbursement strategies may shed light on the practice patterns reported in this study.
The primary strengths of the INSIGHT study are the large patient population with a strict Asia-Pacific focus (where the disease is endemic) and the capture of longitudinal real-world clinical practice data [3] across 9 countries that comprise both emerging and developed countries. As with every cohort study, however, the INSIGHT also has its limitations. Since the data for the retrospective part of the study is abstracted from medical records, the robustness of data depends on the thoroughness of each site’s interpretation and documentation of medical history, treatment, and response. Since each country has its preferred staging system, such as the HKLC and JIS, not every site utilizes the BCLC staging in clinical practice; this leads to investigators’ interpretation and judgment to stage patients to the BCLC staging. Missing data and loss to follow-up are additional limitations of cohort studies, although substantial efforts were made to limit the amount of missing data. In addition, like the BRIDGE, the INSIGHT is limited by potential site selection bias which limits the generalizability of results. The selected sites are tertiary referral centers, which are expected to provide the highest standard of care available in each country. In an attempt to maximize generalization of results across countries, multiple sites with investigators of different specialties were selected in order to be more representative of the country as a whole.
In conclusion, the INSIGHT study describes a wide spectrum of clinical management practices in HCC, where patient demographics, differential costs, and patient access to therapies may lead to wide geographical variations throughout the patient’s journey, from diagnosis to clinical outcome. The high heterogeneity in patient outcomes reveals the need for more robust and clinical management strategies to be designed and adopted. As demonstrated by comparing data from INSIGHT with AP-BRIDGE, the continued collection of real-world clinical practice data will aid the understanding of the enormity of the disease burden and epidemiology of HCC in Asia-Pacific. This influences future prevention strategies, the monitoring of at-risk populations, implementation of more holistic national guidelines, and means to improve access to healthcare [55]. These will contribute to the long-term goal of reducing the burden of HCC in the Asia-Pacific region [56].
Acknowledgments
The authors would like to acknowledge those below as participating investigators and institutions. Edmund Tse, Royal Adelaide Hospital; Simone Strasser, Royal Prince Alfred Hospital; Li Lequn, Guangxi Medical University Cancer Centre; Jiangtao Li, Second Affliated Hospital Zhejiang University School of Medicine; Fan Jia, Zhongshan Hospital, Fudan University; Zhu Xu, Beijing Cancer Hospital; Yuxian Bai, Harbin Medical University Cancer Hospital; Qin Shu-Kui, Nanjing Bayi Hospital; Thomas Yau, Queen Mary Hospital; Masatoshi Kudo, Kindai University Hospital; Junji Furuse, Kyorin University School of Medicine; Kazuaki Shimada, National Cancer Centre Japan; Kiyoshi Hasegawa, University of Tokyo; Nobuyuki Takemura, National Centre for Global Health and Medicine; Adam Bartlett, Auckland City Hospital; Pierce Chow, National Cancer Centre Singapore; Kieron Lim, Tan Poh Seng, Dan Yock Young, National University Hospital Singapore; Brian Goh, Singapore General Hospital; Hyun-Ki Yoon, Asan Medical Centre; Kim Yun Hwan, Cho Sungbum, Korea University Anam Hospital; Joon Hyeok Lee, Samsung Medical; Ho-Seong Han, Seoul National University Hospital; Yang Jin-Mo, St Vincent’s Hospital; Choi Jong Young, St. Mary’s Hospital; Hee-Jung Wang, Ajou University Hospital, Do Young Kim, Severance Hospital, Yonsei University College of Medicine; Peng Cheng Yuan, China Medical University Hospital; Yee Chao, Taipei Veterans General Hospital; Tsung-Hui Hu, KS-Chang Gung Memorial Hospital; Pin-Nan Cheng, National Cheng Kung University Hospital; Chien-Hung Chen, National Taiwan University Hospital; Rawisak Chanwat, National Cancer Institute Thailand; Supot Nimanong, Siriraj Hospital, Mahidol University. The authors would also like to thank Neo Shuen Kai and Sekar Karthik for biostatistical support.
Statement of Ethics
This study protocol was reviewed and approved by individual Independent Ethics Committee (IEC) or Institutional Review Board (IRB) within each country, according to local regulations.Individual site approval numbers are as follows: 201701604B0C601, CMUH106-REC3-105(CR-3), A-ER-106-219, 201707013RSB, 2017-10-002AC for Taiwan, 2016/2914 for Singapore, 192_2017RB_IN522, Si 673/2017 for Thailand, AHREC 000066 for New Zealand, 2018-0258, AJIRB-MED-SUR-17-476, 2018AN0054, KC18OCGI0045, B-1802/451-301, SMC 2019-01-056-006, VC18OCGI0025, 2017-3080-015 for Korea, 31-011, H28-102-06, NCGM-G-003057-00, 2018-216, 11465-(3) for Japan, UW 18-613 for Hong Kong, HREC/17/RPAH/180 for Australia, 2016YJZ50-GZ02, KS2018 (34), 2017-101, 81YY-ZLLL-16-1602, E2021001039, B2017-001 for China. More information about the regulatory approvals is available upon request to the corresponding author. Prior to data collection, informed consent will be obtained from all participants as per local requirements in each region/country. Before enrollment in the registry, the participating physician or an authorized member of the participating site personnel must explain to potential participant of their involvement in the registry and data protection. Participants were informed that their participation in the registry is voluntary and that they may withdraw consent for data collection at any time. They were informed that choosing not to participate in this registry will not affect the standard of care the patient will receive. The patient was given sufficient time to read the informed consent form (in a language that the patient can read and understand) and will be given the opportunity to ask questions. This effort will be documented in the patient’s case notes. After explanation and before entry into the registry, consent was appropriately recorded by means of the patient’s personally dated signature.
Conflict of Interest Statement
P.K.H.C. received honoraria from La Hoffman-Roche and Sirtex Medical; received research funding from Sirtex Medical, Ipsen, IQVIA, New B Innovation, AMiLi, Perspectum, MiRXES, and La Hoffman-Roche; and holds a consultancy or advisory role in Sirtex Medical, Ipsen, BMS, Oncosil, Bayer, New B Innovation, MSD, Guerbet, Roche, AUM Bioscience, L.E.K. Consulting, AstraZeneca, Eisai, Genentech, IQVIA, and Abbott. S.I.S. received honoraria from Eisai, Roche Products, AstraZeneca, Gilead Sciences, Pfizer, Chiesi, Bayer, Sirtex Medical, AbbVie, Norgine, and MSD. M.K. received honoraria from Chugai, Eisai, Eli Lilly, and Takeda; received research funding from Otsuka, Taiho, Chugai, GE Healthcare, Eisai, AbbVie, and EA Pharma; and holds a consultancy role in Chugai, Roche, Eisai, and AstraZeneca. All other authors have no conflicts of interest to declare.
Funding Sources
This investigator-initiated study was conducted by the Asia-Pacific Hepatocellular Carcinoma (AHCC) Trials Group, with financial support from the SingHealth Duke-NUS Programme Grant Award and IQVIA Solutions.
Author Contributions
Conceptualization, supervision, funding acquisition, and writing – review and editing: P.K.H.C. Data curation, investigation, and methodology: P.K.H.C., L.L., C.-H.C., J.H.L., M.K., R.C., S.I.S., and M.G. Resources, software, and formal analysis: M.G., Y.M.P., Y.Z., and L.S. Project administration: P.K.H.C. and Y.K.S. Visualization: M.C.C., Y.K.S., M.G., Y.M.P., Y.Z., and L.S. Writing – original draft: Y.K.S. and M.C.C. (contributed equally). Approval of final manuscript: all authors.
Funding Statement
This investigator-initiated study was conducted by the Asia-Pacific Hepatocellular Carcinoma (AHCC) Trials Group, with financial support from the SingHealth Duke-NUS Programme Grant Award and IQVIA Solutions.
Data Availability Statement
The authors declare that the main data supporting the results in this study are available within the paper and its Supplementary Information. The analyzed datasets generated during the study are available upon reasonable request to the corresponding author.
Supplementary Material
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021 May;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2. Zhang CH, Cheng Y, Zhang S, Fan J, Gao Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. 2022 Aug;42(9):2029–41. [DOI] [PubMed] [Google Scholar]
- 3. Jafri W, Kamran M. Hepatocellular carcinoma in Asia: a challenging situation. Euroasian J Hepatogastroenterol. 2019;9(1):27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022 Dec;77(6):1598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017 Jan 7;389:56–66. [DOI] [PubMed] [Google Scholar]
- 6. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. REACH-2: a randomized, double-blind, placebo-controlled phase 3 study of ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated baseline alpha-fetoprotein (AFP) following first-line sorafenib. J Clin Oncol. 2018;36(15 Suppl):4003. [Google Scholar]
- 8. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018 Jul;19(7):940–52. [DOI] [PubMed] [Google Scholar]
- 9. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. Jul 24 2008;359(4):378–90. [DOI] [PubMed] [Google Scholar]
- 10. Yamashita T, Kudo M, Ikeda K, Izumi N, Tateishi R, Ikeda M, et al. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol. 2020 Jan;55(1):113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–905. [DOI] [PubMed] [Google Scholar]
- 12. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1(8):EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 13. Viveiros P, Riaz A, Lewandowski RJ, Mahalingam D. Current state of liver-directed therapies and combinatory approaches with systemic therapy in hepatocellular carcinoma (HCC). Cancers. 2019 Jul 31;11(8):1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang Y, Si T. Yttrium-90 transarterial radioembolization versus conventional transarterial chemoembolization for patients with hepatocellular carcinoma: a systematic review and meta-analysis. Cancer Biol Med. 2018 Aug;15(3):299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chew XH, Sultana R, Mathew EN, Ng DCE, Lo RHG, Toh HC, et al. Real-world data on clinical outcomes of patients with liver cancer: a prospective validation of the national cancer centre Singapore consensus guidelines for the management of hepatocellular carcinoma. Liver Cancer. 2021 Jun;10(3):224–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salem R, Johnson GE, Kim E, Riaz A, Bishay V, Boucher E, et al. Yttrium-90 radioembolization for the treatment of solitary, unresectable HCC: the LEGACY study. Hepatology. 2021 Nov;74(5):2342–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011 Sep;47(14):2117–27. [DOI] [PubMed] [Google Scholar]
- 18. Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016 May;64(5):1090–8. [DOI] [PubMed] [Google Scholar]
- 19. Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017 Aug;2(8):565–75. [DOI] [PubMed] [Google Scholar]
- 20. Chew V, Lee YH, Pan L, Nasir NJM, Lim CJ, Chua C, et al. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut. 2019;68(2):335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rivoltini L, Bhoori S, Camisaschi C, Bergamaschi L, Lalli L, Frati P, et al. Y90-radioembolisation in hepatocellular carcinoma induces immune responses calling for early treatment with multiple checkpoint blockers. Gut. 2023;72(2):406–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wee IJY, Moe FNN, Sultana R, Ang RWT, Quek PPS, Goh BKP, et al. Extending surgical resection for hepatocellular carcinoma beyond Barcelona clinic for liver cancer (BCLC) stage A: a novel application of the modified BCLC staging system. J Hepatocell Carcinoma. 2022;9:839–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Genentech . “Genentech’s tecentriq plus avastin is the first treatment combination to reduce the risk of cancer returning in people with certain types of early-stage liver cancer in a phase III trial,”ed. 2023. [Google Scholar]
- 24. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022 Mar;76(3):681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020 Aug;9(4):452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Surveillance group; Diagnosis group; Staging group; Surgery group; Local ablation group; TACE/TARE/HAI group, et al. Management consensus guideline for hepatocellular carcinoma: 2016 updated by the taiwan liver cancer association and the gastroenterological society of taiwan. J Formos Med Assoc. 2018 May;117(5):381–403. [DOI] [PubMed] [Google Scholar]
- 27. Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014 Jun;146(7);1691–700.e3. [DOI] [PubMed] [Google Scholar]
- 28. Korean Liver Cancer AssociationNational Cancer Center . 2018 Korean liver cancer association-national cancer center Korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver. 2019 May 15;13(3):227–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of hepatocellular carcinoma in Japan: JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021 Jun;10(3):181–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lubel JS, Strasser SI, Thompson AJ, Cowie BC, MacLachlan J, Allard NL, et al. Australian consensus recommendations for the management of hepatitis B. Med J Aust. 2022 May 16;216(9):478–86. [DOI] [PubMed] [Google Scholar]
- 31. Chow PK, Choo SP, Ng DC, Lo RH, Wang ML, Toh HC, et al. National cancer centre Singapore consensus guidelines for hepatocellular carcinoma. Liver Cancer. 2016 Apr;5(2):97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goh KL, Razlan H, Hartono JL, Qua CS, Yoong BK, Koh PS, et al. Liver cancer in Malaysia: epidemiology and clinical presentation in a multiracial Asian population. J Dig Dis. 2015 Mar;16(3):152–8. [DOI] [PubMed] [Google Scholar]
- 33. Goh GB, Li JW, Chang PE, Chow KY, Tan CK. Deciphering the epidemiology of hepatocellular carcinoma through the passage of time: a study of 1,401 patients across 3 decades. Hepatol Commun. 2017 Aug;1(6):564–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. Sep 2015;35(9):2155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pierce Chow M; National Cancer Centre, Singapore . Hepatocellular Carcinoma Registry in Asia: The INSIGHT Registry (INSIGHT) [Online]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03233360.
- 36. Kong NH, Chow PK. Conducting randomised controlled trials across countries with disparate levels of socio-economic development: the experience of the Asia-Pacific Hepatocellular Carcinoma Trials Group. Contemp Clin Trials. 2013 Nov;36(2):682–6. [DOI] [PubMed] [Google Scholar]
- 37. Tan CH, Low SC, Thng CH. APASL and AASLD consensus guidelines on imaging diagnosis of hepatocellular carcinoma: a review. Int J Hepatol. 2011;2011:519783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bialecki ES, Di Bisceglie AM. Diagnosis of hepatocellular carcinoma. HPB. 2005;7(1):26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wickham H. gplot2: elegant graphics for data analysis. New York: Springer-Verlag. Available from: https://ggplot2.tidyverse.org. [Google Scholar]
- 40. Lim KC, Chow PK, Allen JC, Siddiqui FJ, Chan ES, Tan SB. Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria. Br J Surg. 2012 Dec;99(12):1622–9. [DOI] [PubMed] [Google Scholar]
- 41. Wu EM, Wong LL, Hernandez BY, Ji JF, Jia W, Kwee SA, et al. Gender differences in hepatocellular cancer: disparities in nonalcoholic fatty liver disease/steatohepatitis and liver transplantation. Hepatoma Res. 2018;4:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mu XM, Wang W, Jiang YY, Feng J. Patterns of comorbidity in hepatocellular carcinoma: a network perspective. Int J Environ Res Public Health. 2020 Apr 29;17(9):3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tarao K, Nozaki A, Ikeda T, Sato A, Komatsu H, Komatsu T, et al. Real impact of liver cirrhosis on the development of hepatocellular carcinoma in various liver diseases-meta-analytic assessment. Cancer Med. 2019 Mar;8(3):1054–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018 Aug;68(2):723–50. [DOI] [PubMed] [Google Scholar]
- 45. Yoon JS, Lee HA, Kim HY, Sinn DH, Lee DH, Hong SK, et al. Hepatocellular carcinoma in Korea: an analysis of the 2015 Korean nationwide cancer registry. J Liver Cancer. 2021;21(1):58–68. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46. Kwon JW, Tchoe HJ, Lee J, Suh JK, Lee JH, Shin S. The impact of national surveillance for liver cancer: results from real-world setting in Korea. Gut Liver. 2020 Jan 15;14(1):108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rawla P, Sunkara T, Muralidharan P, Raj JP. Update in global trends and aetiology of hepatocellular carcinoma. Contemp Oncol. 2018;22(3):141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cancer drug list [Online]. 2022. Available from: https://www.moh.gov.sg/home/our-healthcare-system/medishield-life/what-is-medishield-life/what-medishield-life-benefits/cancer-drug-list. [Google Scholar]
- 49. Kim BK, Kim DY, Han K-H, Seong J. Changes in real-life practice for hepatocellular carcinoma patients in the Republic of Korea over a 12-year period: a nationwide random sample study. PLoS One. 2019;14(10):e0223678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sinn DH, Cho JY, Gwak GY, Paik YH, Choi MS, Lee JH, et al. Different survival of Barcelona clinic liver cancer stage C hepatocellular carcinoma patients by the extent of portal vein invasion and the type of extrahepatic spread. PLoS One. 2015;10(4):e0124434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018 Mar 24;391(10126):1163–73. [DOI] [PubMed] [Google Scholar]
- 52. Liu Z, Liu X, Liang J, Liu Y, Hou X, Zhang M, et al. Immunotherapy for hepatocellular carcinoma: current status and future prospects. Front Immunol. 2021;12:765101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kudo M, Izumi N, Kokudo N, Sakamoto M, Shiina S, Takayama T, et al. Report of the 22nd nationwide follow-up Survey of primary liver cancer in Japan (2012–2013). Hepatol Res. 2022;52(1):5–66. [DOI] [PubMed] [Google Scholar]
- 54. Salem R, Gabr A, Riaz A, Mora R, Ali R, Abecassis M, et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology. 2018 Oct;68(4):1429–40. [DOI] [PubMed] [Google Scholar]
- 55. Wong MCS, Huang JLW, George J, Huang J, Leung C, Eslam M, et al. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol. 2019 Jan;16(1):57–73. [DOI] [PubMed] [Google Scholar]
- 56. Sarin SK, Kumar M, Eslam M, George J, Al Mahtab M, Akbar SMF, et al. Liver diseases in the asia-pacific region: a lancet gastroenterology & hepatology commission. Lancet Gastroenterol Hepatol. Feb 2020;5(2):167–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the main data supporting the results in this study are available within the paper and its Supplementary Information. The analyzed datasets generated during the study are available upon reasonable request to the corresponding author.