Abstract
PURPOSE
The multicenter, open-label, randomized phase 2 NCI-9944 study (NCT02595892) demonstrated that addition of ATR inhibitor (ATRi) berzosertib to gemcitabine increased progression-free survival (PFS) compared to gemcitabine alone (hazard ratio [HR]=0.57, one-sided log-rank P = .044, which met the one-sided significance level of 0.1 used for sample size calculation).
METHODS
We report here the final overall survival (OS) analysis and biomarker correlations (ATM expression by immunohistochemistry, mutational signature 3 and a genomic biomarker of replication stress) along with post-hoc exploratory analyses to adjust for crossover from gemcitabine to gemcitabine/berzosertib.
RESULTS
At the data cutoff of January 27, 2023 (>30 months of additional follow-up from the primary analysis), median OS was 59.4 weeks with gemcitabine/berzosertib versus 43.0 weeks with gemcitabine alone (HR 0.79, 90% CI 0.52 to 1.2, one-sided log-rank P = .18). An OS benefit with addition of berzosertib to gemcitabine was suggested in patients stratified into the platinum-free interval ≤3 months (N = 26) subgroup (HR, 0.48, 90% CI 0.22 to 1.01, one-sided log-rank P =.04) and in patients with ATM-negative/low (N = 24) tumors (HR, 0.50, 90% CI 0.23 to 1.08, one-sided log-rank P = .06).
CONCLUSION
The results of this follow-up analysis continue to support the promise of combined gemcitabine/ATRi therapy in platinum resistant ovarian cancer, an active area of investigation with several ongoing clinical trials.
INTRODUCTION
Management of platinum-resistant ovarian cancer (PROC) remains a significant unmet medical need.1 Weekly (once weekly, either 2 weeks on/1 week off or 3 weeks on/1 week off) gemcitabine has demonstrated similar activity as pegylated liposomal doxorubicin in two randomized phase III studies in PROC and constitutes a standard chemotherapy regimen in this setting.2–4 Given that gemcitabine induces replication stress (RS) and dependence on the ATR-mediated RS response, and on the basis of preclinical studies showing synergistic antitumor activity for the combination of gemcitabine and the ATR inhibitor (ATRi) berzosertib, a randomized phase II (RP2) study of gemcitabine/berzosertib versus gemcitabine alone was conducted in patients with recurrent, platinum-resistant high-grade serous ovarian cancer (HGSOC), a histologic subtype that is enriched for genomic alterations associated with increased RS.5–7 This trial demonstrated that addition of berzosertib to gemcitabine increased progression-free survival (PFS) compared with gemcitabine alone (hazard ratio [HR], 0.57; one-sided log-rank P = .044, which met the one-sided significance level of 0.1 used for sample size calculation).7 Furthermore, a candidate biomarker of response was identified whereby only patients with RS-low tumors (defined as harboring no genomic RS alterations related to loss of retinoblastoma pathway regulation and/or oncogene-induced RS) exhibited improved PFS with addition of berzosertib to gemcitabine as opposed to patients with RS-high tumors (ie, tumors harboring ≥one of these genomic alterations).8 Here, we report the final overall survival (OS) analysis and biomarker correlations along with post hoc exploratory analyses to adjust for crossover from gemcitabine to gemcitabine/berzosertib.
METHODS
In this multicenter, open-label, RP2 trial, sponsored by the National Cancer Institute (NCI-9944 study), patients with platinum-resistant HGSOC and unlimited previous lines of cytotoxic therapy in the platinum-sensitive setting but not more than one line of cytotoxic therapy in the platinum-resistant setting were randomly assigned 1:1 to gemcitabine/berzosertib versus gemcitabine alone. Inclusion/exclusion criteria and procedures for this study have been previously reported.7 Random assignment was stratified on the basis of platinum-free interval (PFI), PFI ≤3 months versus >3 months. Crossover from gemcitabine to gemcitabine/berzosertib was allowed on disease progression by RECIST 1.1 at the discretion of the investigators. The clinical trial was designed to have 80% power to detect an improvement of median PFS (primary end point) from 15 weeks with gemcitabine alone to 27.3 weeks with gemcitabine plus berzosertib (HR, 0.55) at a one-sided alpha level of 0.1. OS was a secondary end point, and planned exploratory correlative studies included assessment of DNA repair pathway deficiencies and RS alterations by targeted gene sequencing and/or immunohistochemistry (IHC). The clinical trial was approved by the NCI Central Institutional Review Board and the US Food and Drug Administration (ClinicalTrials.gov identifier: NCT02595892); all patients provided written informed consent.
The treatment effects on OS were compared with the intent-to-treat (ITT) log-rank test (one-sided), and HRs were estimated by Cox models with robust standard errors used for 90% CIs. Patients who did not die at the data cutoff date were censored at the date of last contact. Without accounting for selective crossover from the gemcitabine arm to the gemcitabine/berzosertib arm on disease progression, the ITT can lead to underestimation of OS benefit if the addition of berzosertib is effective. To adjust for crossover, we performed some commonly used post hoc analyses: excluding crossover, censored at crossover (CXO), time-varying covariate (TVC), and inverse probability of censor weighting (IPCW). For patients who crossed over, the treatment variable was changed at their treatment switch times (defined as 1 week after disease progression) using the TVC method and their OS times were censored at the treatment switch times using the CXO and IPCW approaches. The IPCW method uses a weighted Cox model to correct for selection bias from this informative censoring at crossover. For patients assigned to gemcitabine, the stabilized time-varying weights were calculated using Cox models for treatment switch times adjusted for baseline Eastern Cooperative Oncology Group status and BRCA mutation status as well as time-dependent progressive disease status using the ipwtm function in R package ipw.9 For patients assigned to gemcitabine/berzosertib, the weights were set to one throughout follow-up. All reported P values were one-sided, and P values < .1 were considered significant for the OS analyses (as with the PFS analyses). The analysis used R version 4.2.2 (The R Foundation for Statistical Computing).
Three biomarkers were correlated with OS: RS status, ATM expression, and mutational signature 3 (Sig3).8 Tumors were defined as RS-high versus RS-low. RS-high tumors were defined as having ≥one of the following alterations associated with RS: RB1 loss, CDKN2A loss, CCNE1 amplification, KRAS amplification, MYC/MYCL1 amplification, ERBB2 amplification, and NF1 mutations; RS-low tumors had none of these alterations. The selection of genes included as RS alterations was based on alterations that are known to be prevalent and drivers in HGSOC on the basis of large-scale genomic studies such as the ovarian cancer The Cancer Genome Atlas data set and known to be associated with increased RS.5,6,10 ATM expression was evaluated by IHC and was considered negative if there was no nuclear ATM staining in the tumor cells (while stromal cells were positive), low if nuclear ATM staining was present in <50% of tumor cells, and positive if ≥50% of tumor cells exhibited nuclear ATM staining. Mutational Sig3 is characterized by a high number of larger deletions (up to 50 bp) with overlapping microhomology at breakpoint junctions. Sig3 has been proposed as a biomarker of homologous re-combination repair (HRR) deficiency, reflecting the fact that deficient HRR leads to dependence on alternative error-prone DNA repair mechanisms such as microhomology-mediated end joining, which uses microhomology at rearrangement junctions to rejoin and repair DNA double-strand breaks.11–13 The presence of Sig3 was detected using a previously developed and validated computational tool called Signature Multivariate Analysis on the OncoPanel sequencing data as previously described.14
RESULTS
Patients and Treatments
Seventy patients were randomly assigned to treatment with gemcitabine (36 patients) or gemcitabine/berzosertib (34 patients); 15 (41.7%) patients from the gemcitabine arm crossed over to gemcitabine/berzosertib on disease progression by RECIST 1.1 (Fig 1). Data cutoff occurred on January 27, 2023 (>30 months of additional follow-up from the primary analysis). The median (IQR) follow-up was 53.2 (25.6–106) weeks in the gemcitabine/berzosertib arm and 43 (23.2–78.4) weeks in the gemcitabine-alone arm, and as of the clinical cutoff date, there were 31/34 and 34/36 deaths/patients observed in the gemcitabine/berzosertib and gemcitabine-alone arms, respectively.
FIG 1.
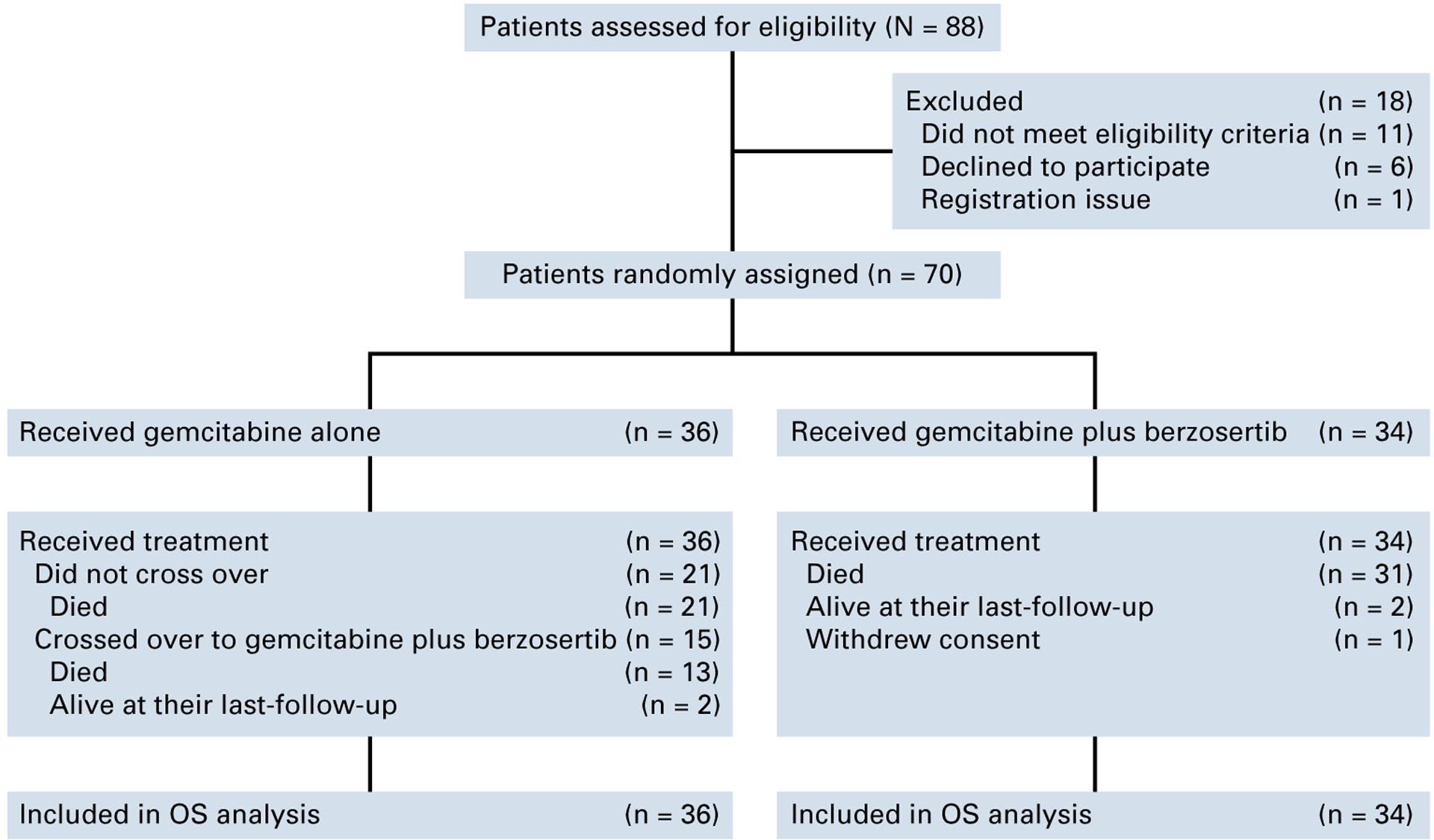
CONSORT diagram of the study. OS, overall survival.
Baseline characteristics and retrospective biomarker designations (RS biomarker, ATM expression by IHC and the presence of mutational Sig311,14) were balanced between the treatment groups (Table 1). Biomarker characteristics were also well balanced between patients who did or did not cross over (Appendix Table A1).
TABLE 1.
Baseline and Biomarker Characteristics
| Clinical Characteristic | Gemcitabine Alone (n = 36), No. (%) | Gemcitabine + M6620 (n = 34), No. (%) |
|---|---|---|
| Race | ||
| White | 34 (94) | 30 (88) |
| Asian | 0 (0) | 2 (6) |
| Unknown | 2 (6) | 2 (6) |
| ECOG | ||
| 0 | 22 (61) | 20 (59) |
| 1 | 14 (39) | 14 (41) |
| Platinum-freea interval, months | ||
| ≤3 | 13 (36) | 13 (38) |
| >3 to <6 | 23 (64) | 21 (62) |
| Previous total lines | ||
| <3 | 28 (78) | 23 (68) |
| ≥3 | 8 (22) | 11 (32) |
| Previous PARP inhibitor | ||
| Yes | 7 (19) | 11 (32) |
| No | 29 (81) | 23 (68) |
| Previous antiangiogenic therapy | ||
| Yes | 9 (25) | 10 (29) |
| No | 27 (75) | 24 (71) |
| BRCA mutation status | ||
| Yes | 5 (14) | 6 (18) |
| No | 25 (69) | 21 (62) |
| Unknown | 6 (17) | 7 (21) |
| RS biomarkerb | ||
| RS-low | 17 (47) | 13 (38) |
| RS-high | 13 (36) | 14 (41) |
| Unknown | 6 (17) | 7 (21) |
| Mutational signature 3 | ||
| Negative | 18 (50) | 16 (47) |
| Positive | 12 (33) | 11 (32) |
| Unknown | 6 (17) | 7 (21) |
| ATM expression by IHCc | ||
| Negative/low | 11 (31) | 13 (38) |
| Positive | 20 (56) | 16 (47) |
| Unknown | 5 (14) | 5 (15) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; IHC, immunohistochemistry; PARP, poly (ADP-ribose) polymerase; RS, replication stress.
Stratification factor.
RS-high tumors had ≥one of the following alterations: RB1 loss, CDKN2A loss, CCNE1 amplification, KRAS amplification, MYC/MYCL1 amplification, ERBB2 amplification, and NF1 mutations; RS-low had none of these alterations.
ATM expression was considered negative if there was no nuclear ATM staining in the tumor cells, low if nuclear ATM staining was present in <50% of tumor cells, and positive if ≥50% of tumor cells exhibited nuclear ATM staining.
OS Analysis, Biomarker Correlations, and Crossover Effect
The median OS in the ITT population was 59.4 (90% CI, 33.7 to 86.6) weeks in the gemcitabine/berzosertib group versus 43.0 (90% CI, 34.4 to 67.9) weeks in the gemcitabine-alone group (HR, 0.79 [90% CI, 0.52 to 1.2]; one-sided P = .18; Fig 2A). However, when the patients who crossed over from gemcitabine to gemcitabine/berzosertib were excluded from the analysis, an OS benefit was suggested with gemcitabine/berzosertib (HR, 0.60 [90% CI, 0.38 to 0.94]; one-sided P = .04; Fig 2B; a one-sided P value < .1 was considered significant for the OS analyses as with the PFS analyses). In patients stratified into the PFI ≤3 months subgroup (n = 26), improved OS was suggested with gemcitabine/berzosertib both in the ITT population (HR, 0.48 [90% CI, 0.22 to 1.01]; one-sided P = .04; Fig 2C) and when the patients who crossed over were excluded from the analysis HR, 0.26 (90% CI, 0.11 to 0.62; one-sided P = .009; Fig 2D). No OS benefit was observed in patients stratified into the PFI >3 months subgroup (n = 44) in both the ITT population and when patients who crossed over were excluded (Figs 2E and 2F).
FIG 2.
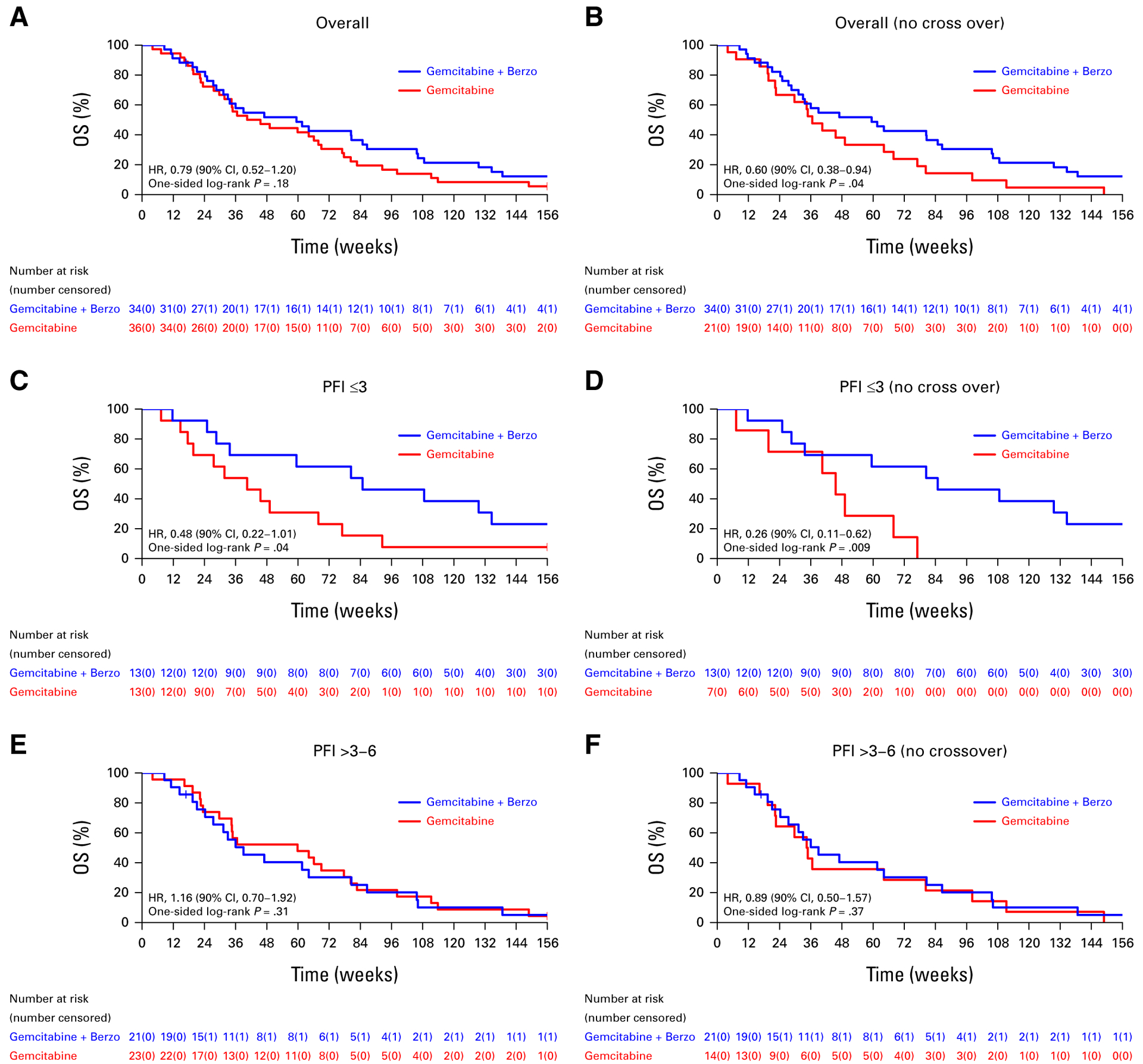
OS (A) of all patients who initiated protocol therapy and (B) after excluding patients who crossed over to gemcitabine/berzosertib, (C) of all patients with a PFI of 3 months or less who initiated protocol therapy and (D) after excluding patients who crossed over to gemcitabine/berzosertib, and (E) of all patients with a PFI of more than 3 months to <6 months who initiated protocol therapy and (F) after excluding patients who crossed over to gemcitabine/berzosertib. HR, hazard ratio; OS, overall survival; PFI, platinum-free interval.
Correlations of OS with the RS, ATM, and Sig3 biomarkers are presented in Figures 3–5, respectively. In patients with RS-low (n = 30) tumors (where a PFS advantage was previously observed with gemcitabine/berzosertib over gemcitabine alone), there was no OS benefit in the ITT population (HR, 0.77 [90% CI, 0.40 to 1.49; one-sided P = .25; Fig 3A); however, an OS benefit was suggested in the RS-low sub-group when patients who crossed over were excluded from the analysis (HR, 0.39 [90% CI, 0.19 to 0.82]; one-sided P = .03; Fig 3B). No OS benefit was observed in patients with RS-high tumors (n = 27) in both the ITT population and when patients who crossed over were excluded (Figs 3C and 3D). In patients with ATM-negative/ATM-low (n = 24) tumors by IHC, improved OS was suggested with gemcitabine/berzosertib both in the ITT population (HR, 0.50 [90% CI, 0.23 to 1.08]; one-sided P = .06) and when the patients who crossed over were excluded from the analysis (HR, 0.32 [90% CI, 0.14 to 0.73]; one-sided P = .02; Figs 4A and 4B). No OS benefit was observed in ATM-positive (n = 36) tumors (Figs 4C and 4D). Finally, no OS benefit was observed in the signature-3–positive or signature-3–negative populations, regardless of whether patients who crossed over were included (Fig 5).
FIG 3.
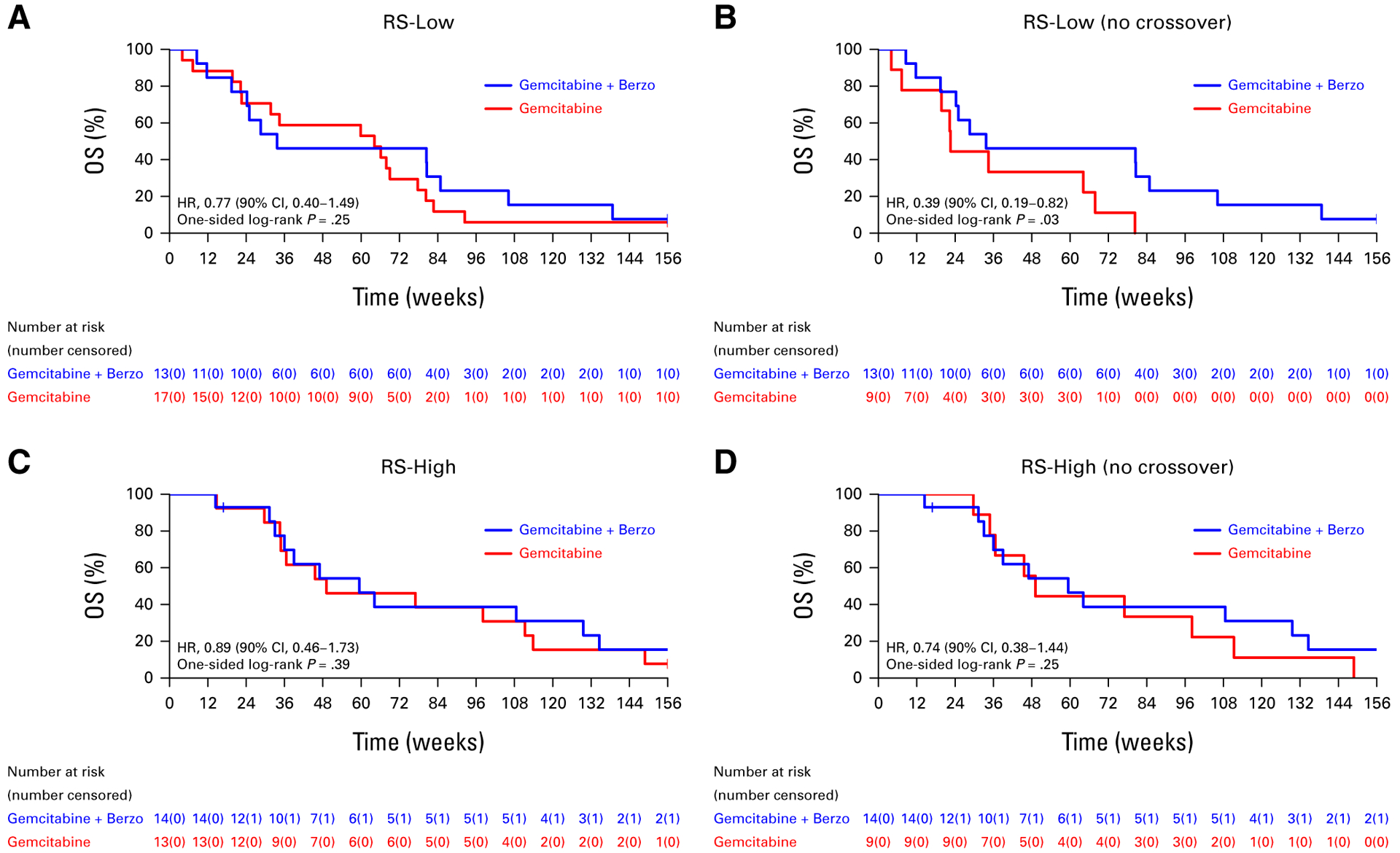
OS (A) of all patients with RS-low tumors who initiated protocol therapy and (B) after excluding patients who crossed over to gemcitabine/berzosertib and (C) of all patients with RS-high tumors who initiated protocol therapy and (D) after excluding patients who crossed over to gemcitabine/berzosertib. HR, hazard ratio; OS, overall survival; RS, replication stress.
FIG 5.
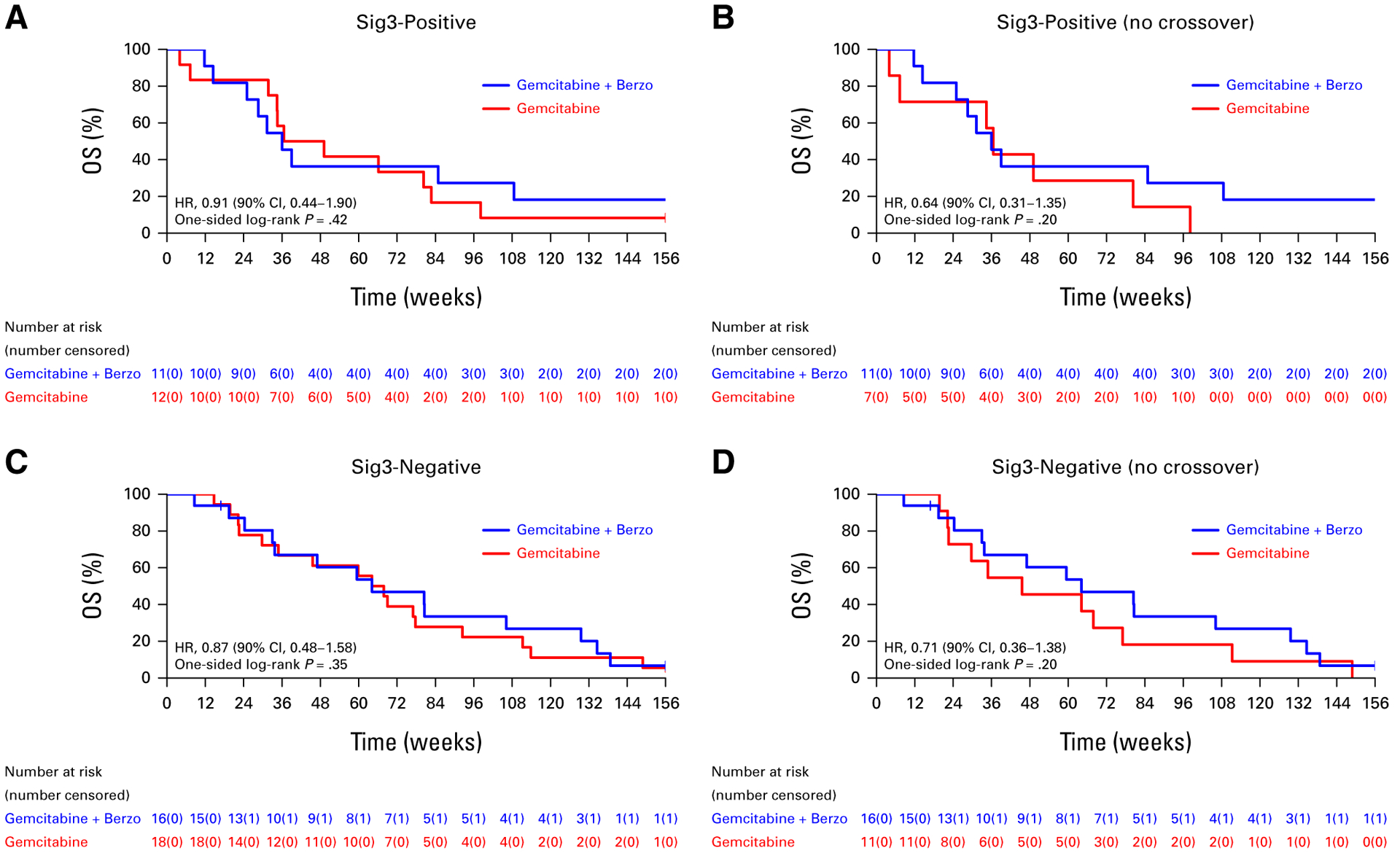
OS (A) of all patients with Sig3-positive tumors who initiated protocol therapy and (B) after excluding patients who crossed over to gemcitabine/berzosertib and (C) of all patients with Sig3-negative tumors who initiated protocol therapy and (D) after excluding patients who crossed over to gemcitabine/berzosertib. HR, hazard ratio; OS, overall survival; Sig3, signature 3.
FIG 4.
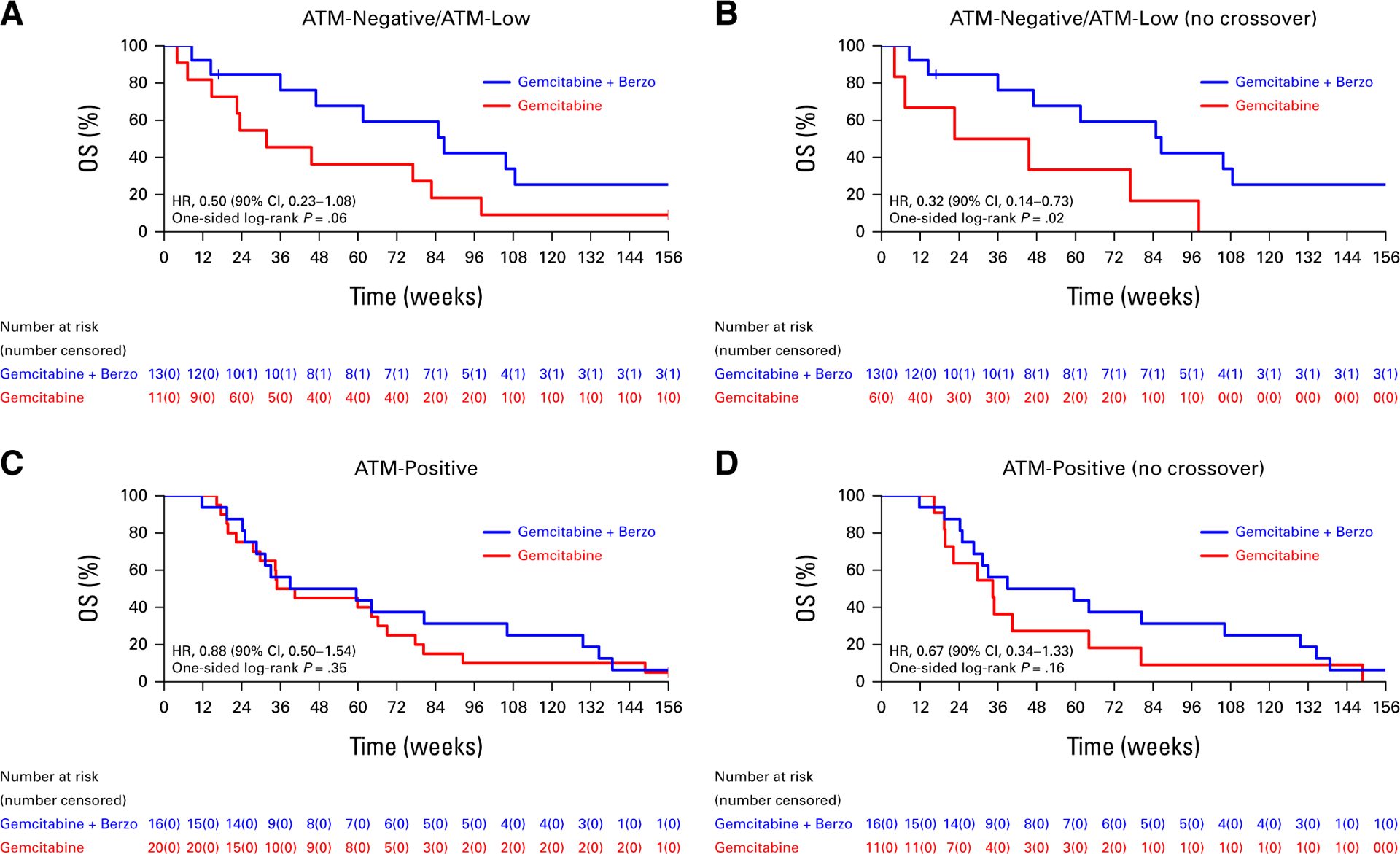
OS (A) of all patients with ATM-negative/ATM-low tumors who initiated protocol therapy and (B) after excluding patients who crossed over to gemcitabine/berzosertib and (C) of all patients with ATM-positive tumors who initiated protocol therapy and (D) after excluding patients who crossed over to gemcitabine/berzosertib. HR, hazard ratio; OS, overall survival.
We had previously observed that patients with RS-high tumors treated with gemcitabine alone exhibited significantly better PFS compared with patients with RS-low tumors (HR, 0.38 [90% CI, 0.17 to 0.86]). Here, no OS benefit was observed in patients with RS-high (n = 13) tumors treated with gemcitabine compared with patients with RS-low (n = 17) tumors (HR, 0.66 [90% CI, 0.34 to 1.29]; one-sided P = .14; Appendix Fig A1A), but when crossover patients were excluded, patients with RS-high tumors treated with gemcitabine alone exhibited better OS compared with patients with RS-low tumors (HR, 0.35 [90% CI, 0.16 to 0.77]; one-sided P = .02; Appendix Fig A1B).
Finally, Table 2 presents the summary of the statistical analyses of OS (HR of gemcitabine/berzosertib v gemcitabine alone) in the overall population, the PFI ≤3 months, the ATM-negative/ATM-low, and RS-low subgroups, including additional post hoc analyses to adjust for the treatment HRs in the presence of crossover, that is, censoring at crossover, TVC for treatment exposure, and IPCW.
TABLE 2.
Summary of Statistical Analysis of OS
| Population | Methodology | OS Hazard Ratio (90% CI) Gemcitabine/Berzosertib v Gemcitabine Alone |
|---|---|---|
| Overall | ITT | 0.79 (0.52 to 1.20) |
| Exclude crossover | 0.60 (0.38 to 0.94) | |
| CXO | 0.73 (0.47 to 1.15) | |
| TVC | 0.81 (0.55 to 1.20) | |
| IPCW | 0.58 (0.37 to 0.89) | |
| PFI ≤3 months | ITT | 0.48 (0.22 to 1.01) |
| Exclude crossover | 0.26 (0.11 to 0.62) | |
| CXO | 0.33 (0.13 to 0.83) | |
| TVC | 0.59 (0.29 to 1.21) | |
| IPCW | 0.29 (0.12 to 0.70) | |
| ATM-negative/ATM-low | ITT | 0.50 (0.23 to 1.08) |
| Exclude crossover | 0.32 (0.14 to 0.73) | |
| CXO | 0.43 (0.19 to 0.96) | |
| TVC | 0.60 (0.29 to 1.22) | |
| IPCW | 0.34 (0.16 to 0.72) | |
| RS-low | ITT | 0.77 (0.40 to 1.49) |
| Exclude crossover | 0.39 (0.19 to 0.82) | |
| CXO | 0.60 (0.27 to 1.33) | |
| TVC | 0.57 (0.31 to 1.08) | |
| IPCW | 0.49 (0.23 to 1.04) |
Abbreviations: CXO, censored at crossover; IPCW, inverse probability of censor weighting; ITT, intent-to-treat; OS, overall survival; PFI, platinum-free interval; RS, replication stress; TVC, time-varying covariate.
DISCUSSION
This follow-up analysis demonstrated that the PFS benefit previously observed with addition of berzosertib to gemcitabine did not translate into an OS benefit. Although the study was not powered for the secondary end point of OS, the lack of correlation between OS and PFS in the overall population may be partly related to the fact that 41.7% of patients treated with gemcitabine alone crossed over to gemcitabine/berzosertib on disease progression (determined by RECIST 1.1), which was allowed by the study protocol at the discretion of the investigators. To adjust for this selective crossover, we used several methodologies that have been previously reported in oncology studies (Table 2), recognizing that all these methods have limitations and that no single approach is methodologically superior or more precise.15,16 Several of these post hoc exploratory analyses demonstrated more favorable OS HRs for gemcitabine/berzosertib.
Unlike the overall population, an OS benefit was suggested among patients stratified into the PFI ≤3 months subgroup (HR, 0.48 including patients who crossed over), which correlated with the significant PFS benefit (HR, 0.29) observed in this subgroup in the primary analysis. This finding is clinically relevant as the 3-month PFI cutoff, which was also used in the stratified random assignment of the AURELIA study,17 was recently recommended at the sixth Gynecologic Cancer InterGroup ovarian cancer consensus conference as the cutoff for defining platinum-resistant disease, that is, for selecting patients for a next line of therapy that excludes platinum.18 These patients are enriched for more clinically and biologically aggressive tumors, and their management remains an unmet medical need.
We previously observed that both ATM-negative/ATM-low and ATM-positive tumors exhibited a PFS benefit from addition of berzosertib to gemcitabine (PFS HRs 0.39 and 0.45 respectively).8 Here, unlike PFS, an OS benefit from addition of berzosertib to gemcitabine was suggested only in the ATM-negative/ATM-low tumors, which is consistent with the well-described synthetic lethal interaction between ATM deficiency and ATR inhibition19; of note, the OS benefit in patients with ATM-negative/ATM-low tumors was observed even when crossover patients were included in the analysis. These observations may reflect the fact that early administration of the ATRi berzosertib (as opposed to administration at the time of crossover) may be more important for ATM-negative/ATM-low than ATM-positive tumors and/or may reflect the fact that administration of gemcitabine/berzosertib may affect subsequent therapies more favorably in ATM-negative/ATM-low than in ATM-positive tumors.
Consistent with our previous observation that patients with RS-high tumors had significantly better PFS (HR, 0.38) on gemcitabine monotherapy (compared with patients with RS-low tumors), this follow-up analysis suggested an OS benefit for patients with RS-high tumors treated with gemcitabine monotherapy after excluding patients who crossed over to gemcitabine/berzosertib (Appendix Fig A1B). The improved outcome of RS-high tumors with gemcitabine monotherapy is not inconsistent with the mechanism of action of gemcitabine, which increases RS (via incorporation of gemcitabine nucleotides into the DNA and by inhibition of ribonucleotide reductase),20,21 potentially rendering RS-high tumors more likely respond to this agent. Conversely, patients with RS-low tumors (who respond poorly to gemcitabine alone) appear to derive benefit from the addition of berzosertib to gemcitabine, in terms of both PFS (HR, 0.34, reported previously) and OS (after adjusting for the crossover effect) as suggested in this follow-up analysis (Table 2).
Taken together, the results of this follow-up analysis continue to support the promise of ATRi therapy in combination with gemcitabine in PROC. Although clinical development of berzosertib has been discontinued (in favor of the oral ATRi M1774), this is a very active area of investigation with several clinical trials currently evaluating gemcitabine in combination with other ATRi s including ceralasertib (Clinical-Trials.gov identifier: NCT03669601), camonsertib (ClinicalTrials.gov identifier: NCT04497116), elimusertib (ClinicalTrials.gov identifier: NCT04616534), and ART0380 (ClinicalTrials.gov identifier: NCT04657068).
CONTEXT.
Key Objective
The key objective of this follow-up analysis was to evaluate whether addition of ATR inhibitor (ATRi) berzosertib to gemcitabine improves overall survival (OS) in platinum-resistant ovarian cancer (PROC).
Knowledge Generated
This follow-up analysis demonstrated that the progression-free survival benefit previously observed with addition of berzosertib to gemcitabine did not translate into a significant OS benefit. However, an OS benefit with addition of berzosertib to gemcitabine was observed in patients stratified into the platinum-free interval ≤3 months subgroup and in patients with ATM-negative/ATM-low tumors (assessed by immunohistochemistry).
Relevance
The results of this follow-up analysis continue to support the promise of combined gemcitabine/ATRi therapy in PROC, an active area of investigation with several ongoing clinical trials.
APPENDIX
FIG A1.
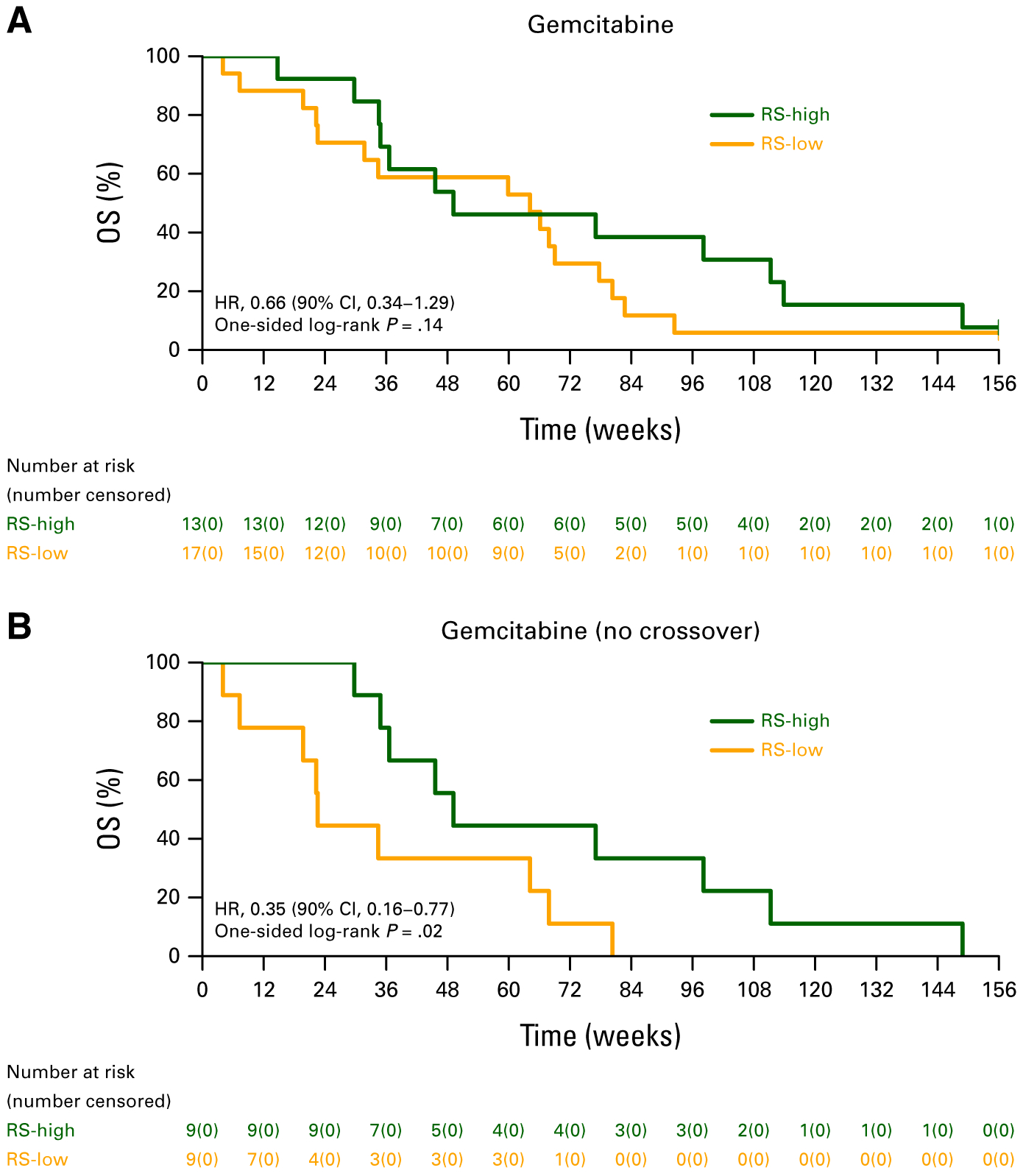
OS in the gemcitabine arm by RS-low versus RS-high status: (A) all patients who initiated protocol therapy and (B) after excluding patients who crossed over to gemcitabine/berzosertib. HR, hazard ratio; OS, overall survival; RS, replication stress.
TABLE A1.
Biomarker Designations and Crossover
| Biomarker Characteristic | Gemcitabine Alone (n = 36), No. (%) | Did Not Cross Over, No. (%) | Crossed Over, No. (%) | P |
|---|---|---|---|---|
| RS biomarker | ||||
| RS-low | 17 (47) | 9 (53) | 8 (47) | |
| RS-high | 13 (36) | 9 (69) | 4 (31) | |
| Unknown | 6 (17) | |||
| Mutational signature 3 | ||||
| Negative | 18 (50) | 11 (61) | 7 (39) | |
| Positive | 12 (33) | 7 (58) | 5 (42) | |
| Unknown | 6 (17) | |||
| ATM expression by IHC | ||||
| Negative/low | 11 (31) | 6 (55) | 5 (45) | |
| Positive | 20 (56) | 11 (55) | 9 (45) | |
| Unknown | 5 (14) |
Abbreviations: IHC, immunohistochemistry; RS, replication stress.
Footnotes
PRIOR PRESENTATION
Presented in part at the ASCO Annual Meeting in Chicago, IL, June 2–6, 2023.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Panagiotis A. Konstantinopoulos
Consulting or Advisory Role: Merck, Vertex, AstraZeneca, Pfizer/EMD Serono, Tesaro, Bayer, Alkermes, Repare Therapeutics, Kadmon, Mersana, Novartis, AADi, Artios, Immunogen
Research Funding: Pfizer (Inst), Lilly (Inst), Tesaro (Inst), Merck Serono (Inst), AstraZeneca (Inst), Merck (Inst), Bayer (Inst), Bristol Myers Squibb/Sanofi (Inst), Novartis (Inst)
Elizabeth K. Lee
Honoraria: AADi
Consulting or Advisory Role: AADi
Research Funding: Repare Therapeutics (Inst), Seagen (Inst), Merck (Inst), AADi (Inst), KSQ Therapeutics (Inst)
Doga Gulhan
Patents, Royalties, Other Intellectual Property: A provisional patent application is being drafted regarding an algorithm developed by the author for which a coversheet provisional has been filed on September 24, 2018, titled “Computational method to identify mutational signatures from sequencing data” (Inst)
Andrea E. Wahner Hendrickson
Consulting or Advisory Role: Oxcia
Research Funding: ProLynx (Inst), Amgen (Inst), Torl Biotherapeutics, Elucida Oncology
Bose Kochupurakkal
Employment: Dana-Farber Cancer Institute, Evelo Biosciences
Stock and Other Ownership Interests: Evelo Biosciences, Amgen
Research Funding: Tango Therapeutics (Inst), Merck Serono (Inst), Bristol Myers Squibb (Inst), Moderna Therapeutics (Inst)
David L. Kolin
Stock and Other Ownership Interests: Pfizer, Novartis, UnitedHealthcare, Abbott Laboratories, Alcon, Viatris, Becton Dickinson
Joyce F. Liu
Consulting or Advisory Role: Clovis Oncology, Genentech/Roche, GlaxoSmithKline, Regeneron, AstraZeneca, Eisai, Bristol Myers Squibb, Daiichi Sankyo, Zentalis
Research Funding: Genentech/Roche (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), CytomX Therapeutics (Inst), Regeneron (Inst), Clovis Oncology (Inst), 2X Oncology (Inst), Vigeo Therapeutics (Inst), Aravive (Inst), Arch Oncology (Inst), Zentalis (Inst), GlaxoSmithKline (Inst), IMPAC Medical Systems (Inst)
Richard T. Penson
Honoraria: AstraZeneca, Genentech/Roche, Mersana, Sutro Biopharma, Vascular Biogenics, Novocure, Immunogen, GlaxoSmithKline, Merck
Consulting or Advisory Role: AstraZeneca, Care4ward, Genentech, Merck, Mersana, Vascular Biogenics, Sutro Biopharma, Immunogen, Novocure, GlaxoSmithKline
Research Funding: AstraZeneca (Inst), Genentech (Inst), Regeneron (Inst), Tesaro (Inst), Vascular Biogenics (Inst)
Patents, Royalties, Other Intellectual Property: BMJ, Blackwell Publishing, UpToDate, Wolters Kluwer
Other Relationship: AstraZeneca, Roche
Elizabeth H. Stover
Patents, Royalties, Other Intellectual Property: Patent pending
Dipanjan Chowdhury
Consulting or Advisory Role: Sky Hawk Therapeutics
Patents, Royalties, Other Intellectual Property: Licensed IP on detection of ovarian cancer using circulating microRNAs to Aspira Biotech
Alan D. D’Andrea
Stock and Other Ownership Interests: Cyteir, IMPAC Medical Systems, PrimeFour Therapeutics
Consulting or Advisory Role: Cyteir, IMPAC Medical Systems, Pfizer, Tango Therapeutics, Blacksmith/Lightstone Ventures, Bristol Myers Squibb, PrimeFour Therapeutics, Zentalis Pharmaceuticals/Zeno Management, Deerfield Management Company, Faze Medicines, Servier Bio-Innovation LLC, Covant Therapeutics/Roivant
Research Funding: Merck Serono, Bristol Myers Squibb, Moderna Therapeutics, Tango Therapeutics
Travel, Accommodations, Expenses: IDEAYA Biosciences
Anniina Färkkilä
Consulting or Advisory Role: GlaxoSmithKline
Speakers’ Bureau: AstraZeneca
Research Funding: GlaxoSmithKline (Inst)
Geoffrey I. Shapiro
Consulting or Advisory Role: Merck Serono, Bicycle Therapeutics, Xinthera
Research Funding: Pfizer (Inst), Lilly (Inst), Merck Serono (Inst), Merck (Inst), Tango Therapeutics (Inst), Bristol Myers Squibb/Medarex (Inst)
Patents, Royalties, Other Intellectual Property: Patent #: 9872874 Title: Dosage regimen for sapacitabine and seliciclib Issue Date: 1/23/2018, Patent # 10,934,593 B2 Compositions and methods for predicting response and resistance to CDK4/6 inhibition
Ursula A. Matulonis
Honoraria: Alkermes, Symphogen
Consulting or Advisory Role: Merck, NextCure, Blueprint Medicines, GlaxoSmithKline, Agenus, Boehringer Ingelheim, Curelab Oncology, Allarity Therapeutics, Immunogen, Eisai, ProfoundBio, GlaxoSmithKline, Tango Therapeutics, Lilly
Speakers’ Bureau: Med Learning Group
Research Funding: Merck, Novartis, Tesaro, Syndax, Immunogen, Mersana, Leap Therapeutics, Fujifilm, SQZ Biotech
Travel, Accommodations, Expenses: AstraZeneca, Immunogen
No other potential conflicts of interest were reported.
DATA SHARING STATEMENT
Data generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Matulonis UA, Sood AK, Fallowfield L, et al. : Ovarian cancer. Nat Rev Dis Primers 2:16061, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrandina G, Ludovisi M, Lorusso D, et al. : Phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in progressive or recurrent ovarian cancer. J Clin Oncol 26:890–896, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Kurzeder C, Bover I, Marme F, et al. : Double-blind, placebo-controlled, randomized phase III trial evaluating pertuzumab combined with chemotherapy for low tumor human epidermal growth factor receptor 3 mRNA-expressing platinum-resistant ovarian cancer (PENELOPE). J Clin Oncol 34:2516–2525, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Mutch DG, Orlando M, Goss T, et al. : Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol 25: 2811–2818, 2007 [DOI] [PubMed] [Google Scholar]
- 5.The Cancer Genome Atlas Research Network: Integrated genomic analyses of ovarian carcinoma. Nature 474:609–615, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.da Costa A, Chowdhury D, Shapiro GI, et al. : Targeting replication stress in cancer therapy. Nat Rev Drug Discov 22:38–58, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konstantinopoulos PA, Cheng SC, Wahner Hendrickson AE, et al. : Berzosertib plus gemcitabine versus gemcitabine alone in platinum-resistant high-grade serous ovarian cancer: A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol 21:957–968, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konstantinopoulos PA, da Costa A, Gulhan D, et al. : A replication stress biomarker is associated with response to gemcitabine versus combined gemcitabine and ATR inhibitor therapy in ovarian cancer. Nat Commun 12:5574, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Wal WM, Geskus RB: ipw: An R package for inverse probability weighting. J Stat Softw 43:1–23, 2011 [Google Scholar]
- 10.Konstantinopoulos PA, Matulonis UA: Clinical and translational advances in ovarian cancer therapy. Nat Cancer 4:1239–1257, 2023 [DOI] [PubMed] [Google Scholar]
- 11.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. : Signatures of mutational processes in human cancer. Nature 500:415–421, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceccaldi R, Liu JC, Amunugama R, et al. : Homologous-recombination-deficient tumours are dependent on Polu-mediated repair. Nature 518:258–262, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farkkila A, Gulhan DC, Casado J, et al. : Immunogenomic profiling determines responses to combined PARP and PD-1 inhibition in ovarian cancer. Nat Commun 11:1459, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulhan DC, Lee JJ, Melloni GEM, et al. : Detecting the mutational signature of homologous recombination deficiency in clinical samples. Nat Genet 51:912–919, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Watkins C, Huang X, Latimer N, et al. : Adjusting overall survival for treatment switches: Commonly used methods and practical application. Pharm Stat 12:348–357, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Colleoni M, Giobbie-Hurder A, Regan MM, et al. : Analyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1–98 study. J Clin Oncol 29:1117–1124, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pujade-Lauraine E, Hilpert F, Weber B, et al. : Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol 32:1302–1308, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Vergote I, Gonzalez-Martin A, Lorusso D, et al. : Clinical research in ovarian cancer: Consensus recommendations from the Gynecologic Cancer InterGroup. Lancet Oncol 23:e374–e384, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reaper PM, Griffiths MR, Long JM, et al. : Selective killing of ATM- or p53-deficient cancer cells through inhibition of ATR. Nat Chem Biol 7:428–430, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Ge Y, Wang T, et al. : Inhibition of ATR potentiates the cytotoxic effect of gemcitabine on pancreatic cancer cells through enhancement of DNA damage and abrogation of ribonucleotide reductase induction by gemcitabine. Oncol Rep 37:3377–3386, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fordham SE, Blair HJ, Elstob CJ, et al. : Inhibition of ATR acutely sensitizes acute myeloid leukemia cells to nucleoside analogs that target ribonucleotide reductase. Blood Adv 2:1157–1169, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


