Abstract
Over the past two decades, control efforts have halved malaria cases globally, yet burdens remain high in much of Africa and elimination has not been achieved even where extreme reductions have been sustained, such as in South Africa1,2. Studies seeking to understand the paradoxical persistence of malaria in areas where surface water is absent for 3–8 months of the year, suggested that certain Anopheles mosquitoes employ long-distance migration3. Here, we confirmed this hypothesis by aerial sampling of mosquitoes 40–290 m above ground, providing the first evidence of windborne migration of African malaria vectors, and consequently the pathogens they transmit. Ten species, including the primary malaria vector Anopheles coluzzii, were identified among 235 anophelines captured during 617 nocturnal aerial collections in the Sahel of Mali. Importantly, females accounted for >80% of all mosquitoes collected. Of these, 90% had taken a blood meal before their migration, implying that pathogens will be transported long distances by migrating females. The likelihood of capturing Anopheles species increased with altitude and during the wet seasons, but variation between years and localities was minimal. Simulated trajectories of mosquito flights indicated mean nightly displacements of up to 300 km for 9-hour flight durations. Annually, the estimated numbers of mosquitoes at altitude crossing a 100-km line perpendicular to the winds included 81,000 An. gambiae s.s., 6 million An. coluzzii, and 44 million An. squamosus. These results provide compelling evidence that millions of previously blood-fed, malaria vectors frequently migrate over hundreds of kilometers, and thus almost certainly spread malaria over such distances. Malaria elimination success may, therefore, depend on whether sources of migrant vectors can be identified and controlled.
In Africa, malaria spans the humid equatorial forest to the semi-arid zones in the north and south. In regions where surface water, essential for larval development, is absent during the 3–8 month dry season, mosquito densities and disease transmission drop dramatically3-8. Yet, shortly after the first rain, vector populations surge6 and transmission recommences. Recent studies suggest that Sahelian Anopheles coluzzii survives the long dry season by aestivation (dormancy)3,6,9-10, whereas An. gambiae s.s. (hereafter, An. gambiae), and An. arabiensis re-establish populations by migration from distant locations where larval sites are perennial3. However, direct evidence, including the capture of aestivating adults in their shelters or the recapture of marked-mosquitoes hundreds of kilometers from their release sites, remains elusive.
Mosquito dispersal, hereafter referred to as migration11, has been extensively studied because it directly impacts disease transmission, the spread of adaptations (e.g., insecticide resistance), and control strategies, such as insecticide barriers12,13. Although tracking mosquitoes over large scales has seldom been attempted12,13, the prevailing view is that the dispersal of malaria mosquitoes does not exceed 5 km12-15 and long-range movements16-19 represent “accidental events” of minimal epidemiological importance12. Nonetheless, the prediction of long-distance migration of anophelines in the Sahel prompted us to question this dogma. Our study is the first to systematically sample insects migrating at high altitude over multiple seasons in Africa to determine if malaria vectors engage in wind-assisted movements, and if so, assess the epidemiological relevance by addressing the following questions: what species are involved? how frequently and at what heights do they fly? how many mosquitoes migrate and how likely are they to carry Plasmodium? Then, using simulations, we estimate how far mosquitoes may have travelled and from where.
During 617 aerial sampling nights, we caught 461,100 insects at heights between 40–290 m agl, in four villages in the Sahel of Mali, West Africa (ED Fig. 1), including 2,748 mosquitoes, of which 235 were anophelines (Table 1). These mosquitoes belonged to 10 species: Anopheles coluzzii, An. gambiae, An. pharoensis, An. coustani, An. squamosus, An. rufipes, An. namibiensis and three distinct but currently undetermined Anopheles (Table 1). The first two are the primary malaria vectors in Africa, with the next four of secondary importance20. Mosquitoes were not among the 564 insects captured on 508 control nets (Table 1, and Methods), confirming that these Anopheles were intercepted at altitude rather than near the ground during deployment. The maximum anophelines/night was three, indicating that migration occurred over many nights. Consistent with Poisson distributions, the values of the variance to mean ratio were all near one (Table 1, Supplementary Discussion). Unless otherwise specified, quantitative results presented hereafter refer to the five most abundant species, represented by >20 individuals (Table 1).
Table 1.
Summary of mosquitoes collected in aerial samples on standard and control panels (2013-2015)
| Standard Panelsa (N=1,894) | Control Panelsb (N=508) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxa | Total Captured |
Mean Panel Density |
L95%CL Poissonc |
U95%CL Poissonc |
Max/ Panel |
Nightly Presence (%) |
Var/Mean ratio |
% Female (n) |
% Post Blood Feedd (n) |
% Infectede (n) |
% Anthro pophilyh |
Total Captured |
Mean Panel Density |
Max/ Panel |
| An. squamosus | 100 | 0.053 | 0.042 | 0.063 | 3 | 11.02 | 1.37 | 76.0 (96) | 93.2 (73) | 0 (73) | 41.1 (17) | 0 | 0 | 0 |
| An. pharoensis | 40 | 0.021 | 0.015 | 0.028 | 2 | 6.00 | 1.08 | 82.5 (40) | 100 (33) | 0 (33) | 33.3 (6) | 0 | 0 | 0 |
| An. coustani | 30 | 0.016 | 0.01 | 0.022 | 2 | 4.38 | 1.05 | 88.9 (27) | 87.5 (24) | 0 (24) | 14.3 (7) | 0 | 0 | 0 |
| An. rufipes | 24 | 0.013 | 0.008 | 0.018 | 2 | 3.24 | 1.16 | 80 (20) | 93.8 (16) | 0 (16) | 0 (4) | 0 | 0 | 0 |
| An. coluzzii | 23 | 0.012 | 0.007 | 0.017 | 2 | 3.08 | 1.16 | 95.5 (22) | 90.5 (21) | 0 (21) | 100 (1) | 0 | 0 | 0 |
| An. (Ano.) sp. Mali 1 | 2 | 0.001 | 0 | 0.003 | 1 | 0.32 | 1 | 100 (2) | 100 (2) | 0 (2) | nd | 0 | 0 | 0 |
| An. gambiae s.s. | 1 | 0.0005 | 0 | 0.002 | 1 | 0.16 | 1 | 100 (1) | 100 (1) | 0 (1) | nd | 0 | 0 | 0 |
| An. sp. nr concolor g | 1 | 0.0005 | 0 | 0.002 | 1 | 0.16 | 1 | 0 (1) | naf | na | na | 0 | 0 | 0 |
| An. sp. Mali 2 | 1 | 0.0005 | 0 | 0.002 | 1 | 0.16 | 1 | 100 (1) | 100 (1) | 0 (1) | nd | 0 | 0 | 0 |
| An. namibiensis | 1 | 0.0005 | 0 | 0.002 | 1 | 0.16 | 1 | 100 (1) | 100 (1) | 0 (1) | nd | 0 | 0 | 0 |
| Anopheles unidentified | 12 | 0.006 | 0.003 | 0.01 | 1 | 1.78 | 0.99 | 33.3 (6) | 100 (2) | 0 (2) | nd | 0 | 0 | 0 |
| Culicinae | 2340 | 1.236 | 1.185 | 1.286 | 22 | 58.19 | 4.83 | 86.4 (1866) | 96.7 (1629) | nd | nd | 0 | 0 | 0 |
| Culicid unidentified | 173 | 0.091 | 0.078 | 0.105 | 8 | 17.18 | 1.92 | 62.9 (116) | 91.8 (73) | nd | nd | 0 | 0 | 0 |
| Total Culicidae | 2748 | 1.451 | 1.397 | 1.505 | 23 | 64.18 | 4.92 | 84.5 (1876) | 96.2 (1804) | nd | nd | 0 | 0 | 0 |
| Total Insects | 461100 | 243.58 | 242.88 | 244.29 | 2601 | 100 | 314.75 | ndf | nd | na | na | 564 | 1.110 | 31 |
Nightly aerial sampling using sticky nets (panels, usually 3/balloon) launched and retrieved at 17:00 and 07:00, respectively. Nets were raised to set altitudes between 40 and 290 m above ground (see Methods).
Control panels were raised to 40 −120 m agl and immediately retrieved during the launch and retrieval of the standard panels to estimate the number of insects captured during the ascent and descent (see Methods).
Estimated using the normal approximation of the Poisson distribution. Low negative values < −0.0001, when a single mosquito/taxon were captured, were rounded to zero.
Only a few bloodfed and half-gravid females (see text for percentages) were pooled with gravids to reflect those which were evidently exposed to at least one blood meal. In these mosquito species blood feeding is required for egg development as indicated by the gravid state. Unfed mosquitoes consisted of the rest.
Infection with human Plasmodium species was tested as described in the Methods.
na and nd denote not applicable and not determined, respectively.
This species was identified based on male genitalia
Identified via PCR (see Methods) with additional confirmations by sequencing. Nonhuman hosts include cow, goat, and possibly unknown rodents.
Females outnumbered males by >4:1 (Table 1). Critically, with 87.5% fully gravid, 0.7% semi-gravid, and 2.9% blood-fed, >90% of the anopheline females had taken a blood meal prior to their high-altitude flights (Table 1), suggesting likely exposure to malaria and other pathogens. Although 31% of bloodmeals came from humans, no Plasmodium-infected mosquitoes were detected amongst the 23 An. gambiae s.l. or the 174 secondary vectors (Table 1). Considering typical rates of Plasmodium infections in primary (1–5%) and secondary (0.1–1%) vectors5,21-23, our results probably reflect the small sample size, with likelihood for zero infected mosquitoes being >30% and >18% (assuming the highest rates in each range), in the primary and secondary vectors, respectively (Supplementary Discussion). Hence, unless infection reduces migratory capacity or migrants are resistant to parasites (there is no evidence for either), Plasmodium and other pathogens are almost certainly transported by windborne mosquitoes that may infect people post-migration.
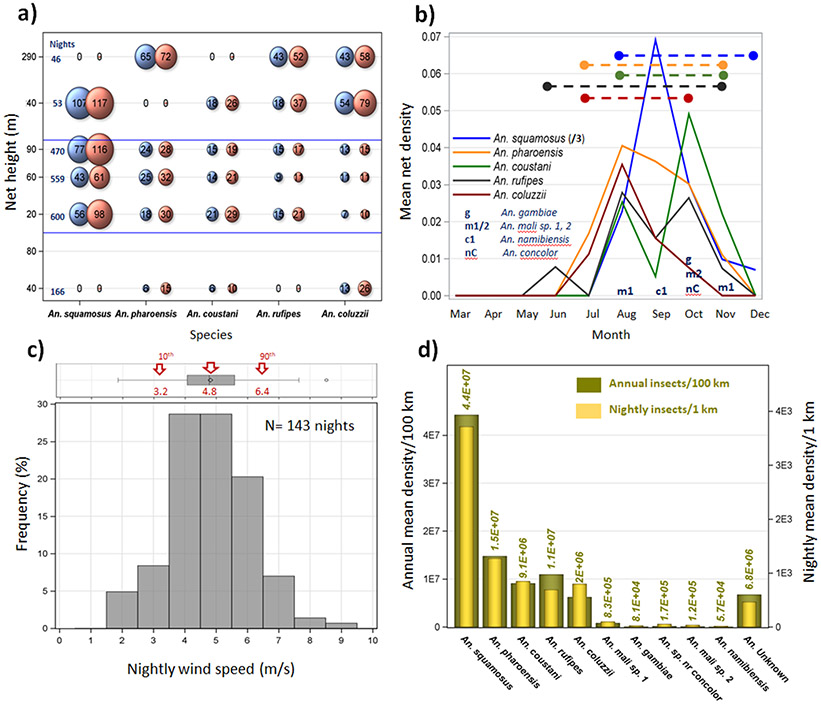
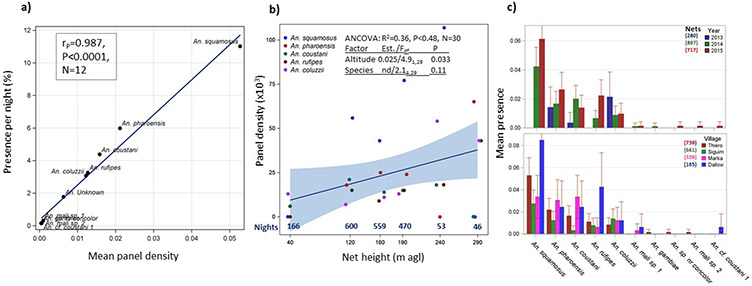
Mosquitoes were intercepted between 40 and 290 m agl (Fig. 1a). Overall panel and aerial density increased with altitude, with a significant effect across species on mean panel density (P<0.037, F1/24=4.9, ED Fig. 2b), suggesting that anopheline migration also occurs >290 m agl. The similar species distributions across years and locations (ED Fig. 2c; non-significant effects of year and location, ED Table 1), combined with its marked seasonality (aerial mosquito captures occurred between July–November, peaking between August–October, Fig. 1b, ED Table 1), all attest to the regularity of windborne migration of Anopheles mosquitoes.
Figure 1. Flight altitude, seasonality, wind speed, and abundance of migratory anopheline species.
a) The relationship of altitude (panel height) and panel- (blue) and aerial- (orange, mosquitoes/106 m3 of air) density for the five most common anopheline species (Table 1). Bubble size is proportional to density (x 103 is shown in the bubble), thus no bubble is shown with zero value. The number of sampling nights (Nights) per panel height is shown on the left. b) Monthly panel density (N=1,894 panels) for the five most common species (Table 1. Note: values of An. squamosus were divided by three to preserve scale) overlaid by the length of migration period (dashed lines). Sampling month of species collected once or twice is shown by letters. c) Distribution of mean nightly wind speed at flight height in nights with one or more anopheline collected. Wind speed data were taken from ERA5 database after matching panel height to the nearest vertical layer (Methods). Corresponding box-whisker plot (top) shows the median, mean, quartiles and extreme values overlaid by arrows indicating the mean, 10 and 90, percentiles (red). d) The number of mosquitoes per species crossing at altitude (50–250 m agl) imaginary lines perpendicular to wind (see legend). Migrants per night per 1 km (right Y axis) are superimposed on the annual number per 100 km line (left Y axis, Main text).
Using mean aerial densities and wind speeds at altitude (4.8 m/s, Fig. 1c), and conservatively assuming mosquitoes fly in a layer between 50 and 250 m agl (see above), we estimated the nightly expected numbers of migrants crossing a 1-km line perpendicular to the wind direction. Nightly estimates ranged between 27 (An. gambiae) and 3,719 (An. squamosus, Fig. 1d). When interpolated over a 100-km line joining our sampling sites (ED Figs. 1a, 2c), annual migrations exceeded 80,000 An. gambiae, 6.25 million An. coluzzii, and 44 million An. squamosus in that region alone (Fig 1d). Thus, windborne migration in the Sahel occurs on a massive scale.
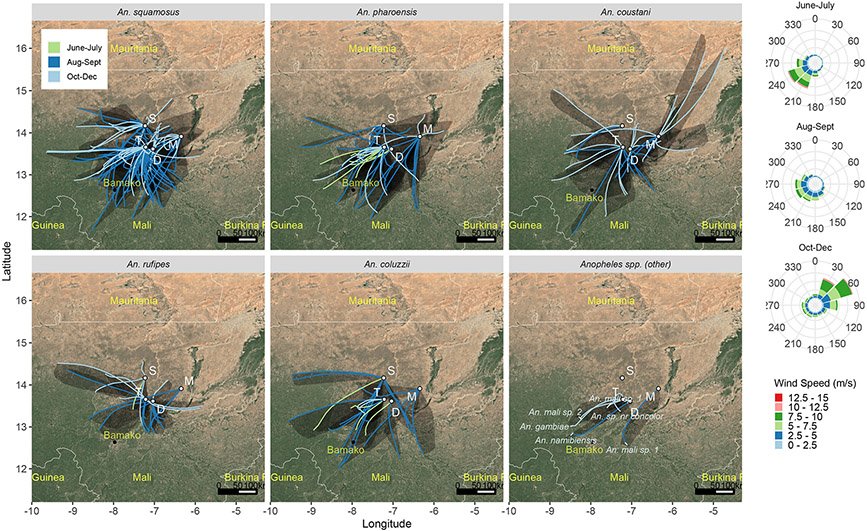
For each mosquito capture, flight trajectories for two- and nine-hour flight durations were estimated using HYSPLIT24 (using the most accurate assimilated meteorological data available: ERA5), assuming that mosquitoes ascend by their own flight but are passively carried by the wind at altitude (Methods). The mean nightly displacements were 30 and 120 km (maxima 70 and 295 km), respectively (Table 2, Fig. 2). Notably, maximal 9-hour nightly flight displacements ranged between 257–295 km for all anophelines with sample size >20 (Table 2). These backwards trajectories exhibited a south-westerly origin (Rayleigh test; mean bearing=212°, r=0.54, P<0.0001, Table 2), corresponding to the prevailing winds during peak migration (August–September, Fig. 2). Trajectories of most species originated from a broad arc (>90 degrees, Fig. 2), suggesting migrants emanated from multiple sites across a large region. Migration from this direction fits with the presence of high-density populations due to perennial larval sites and earlier population growth following the monsoon rains. The back-trajectories with a strong northerly component, observed during the sparsely sampled period of October–December (Fig. 2) might indicate southward “return flights”, on the Harmattan winds prevailing during this season.
Table 2.
Summary of displacement distance (straight-line) and source direction based on 2 and 9 hour flight trajectories of mosquitoes produced using HYSPLIT (see Methods and Figure 2).
| Trajectories: 2-hour flight | Trajectories: 9-hour flight | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxa | Trajectories Na |
Displace mean |
Displace 95%CLM |
Displace min-max |
Trajectories Na |
Displace mean |
Displace 95%CLM |
Displace min-max |
Hourly Disp. meanc |
Actual Hourly Disp. Meand |
mean Bearing Finale |
Rf [bearing] |
P[R] |
| An. squamosus | 1100 | 27.7 | 27-29 | 2-68 | 400 | 109.1 | 103-115 | 4-265 | 13.3 | 12.1 | 213 | 0.516 | 0.0000 |
| An. pharoensis | 440 | 31.1 | 30-33 | 1-65 | 160 | 125.3 | 116-134 | 24-260 | 14.7 | 13.9 | 214 | 0.660 | 0.0000 |
| An. coustani | 330 | 28.5 | 27-30 | 2-60 | 120 | 125.8 | 114-138 | 16-295 | 14.5 | 14.0 | 199 | 0.270 | 0.0802 |
| An. rufipes | 264 | 26.1 | 24-28 | 2-70 | 96 | 109.2 | 97-121 | 24-257 | 12.5 | 12.1 | 199 | 0.454 | 0.0003 |
| An. coluzzii | 253 | 38.6 | 37-41 | 3-69 | 92 | 154.1 | 140-168 | 47-270 | 17.3 | 17.1 | 217 | 0.815 | 0.0000 |
| An. sp. Mali 1 | 22 | 20 | 14-26 | 6-52 | 8 | 94.3 | 52-136 | 51-172 | 10.2 | 10.5 | 223 | 0.947 | 0.0000 |
| An. gambiae s.s. | 11 | 33.5 | NDb | NDb | 4 | 131.1 | NDb | NDb | 15.9 | 14.6 | 254 | NDb | NDb |
| An. sp. nr concolor | 11 | 17.2 | NDb | NDb | 4 | 48.2 | NDb | NDb | 8.4 | 5.4 | 184 | NDb | NDb |
| An. sp. Mali 2 | 11 | 29.9 | NDb | NDb | 4 | 104.4 | NDb | NDb | 13.1 | 11.6 | 234 | NDb | NDb |
| An. namibiensis | 11 | 40.1 | NDb | NDb | 4 | 149.3 | NDb | NDb | 16.7 | 16.6 | 241 | NDb | NDb |
| Anopheline Overall | 2453 | 29.4 | 28.8-30.0 | 1-70.4 | 892 | 118.8 | 115-123 | 4-295 | 14.1 | 13.2 | 212 | 0.540 | 0.0000 |
The number of unique nightly trajectories assumes all possible nightly interception times, given flight duration and flight start and end between 18:00 and 06:00, respectively. Thus, for each night with a captured mosquito there were eleven unique 2-hour-flight trajectories and four 9-hour-flight trajectories.
Not determined for species with a single specimen captured.
Hourly displacement between successive 1-hour points along the 9-hour trajectory.
Effective hourly displacement computed by as the quotient of the total 9-hour trajectory displacement by 9.
The mean bearing (angle) between the interception point (zero) and the final point of the 9-hour trajectory computed from the North.
A measure of angular dispersion which varies from 0 (uniform dispersion from all directions) to 1 ( a single angle where all points align to.
Figure 2. Backward flight trajectories for each anopheles capture event.
Backward nine-hour trajectories were estimated by HYSPLIT (Table 2) and overlaid on a map showing parts of Mali and neighboring countries (Map data: Google, Landsat / Copernicus 2019). Each line represents one of 4 simulated trajectories of one (or more) mosquitoes intercepted at that location and night; The area encompassed by the four trajectories is shadowed. Migration season is shown by different line color. Anopheles species is indicated above each panel. The seasonal wind rose diagrams reflecting wind conditions at 180 m agl averaged from 2013 to 2015 are shown at the right.
Contrary to the conventional view that dispersal of African anophelines is <5 km12,14,15,25, our results provide compelling evidence that primary and secondary malaria vectors regularly engage in windborne migration spanning tens to hundreds of kilometers per night. With massive numbers of females that had taken at least one blood-meal, this migration probably involves human Plasmodium among other pathogens. Separate outbreaks of malaria in Egypt and Israel have been attributed to An. pharoensis traveling >280 km16. Assuming, a conservative22,26, 1% infection rate in migrating females of An. coluzzii, An. gambiae, An. coustani, and An. pharoensis and 0.1% in the remaining anophelines (excluding the unknown An. sp. Mali 1 and 2, Supplementary Discussion), > 286,000 infected migrant mosquitoes are expected to annually cross a 100-km line perpendicular to the wind at altitude. Accordingly, An. pharoensis, An. coustani, and An. coluzzii, contributed 41%, 25%, and 17%, respectively, to the malaria transmission by infected windborne mosquitoes. Although these estimates are coarse, this suggests that migratory secondary vectors could be a major infection source and should be included in studies of transmission as well as in control programs.
Anopheles coluzzii was more common than An. gambiae among the migrants, contrary to initial predictions3 based on data suggesting that it aestivates locally and thus may not require migration to recolonize the Sahel. Indeed, migration occurs from the end of July to October, well after the surge of Sahelian An. coluzzii following the first rain (May–June)3,6. The northward and southward oscillations of the Intertropical Convergence Zone during the wet season continually create better mosquito resource-patches with the rains. Additionally, wet-season droughts endanger local mosquito populations every decade or two27. Thus, selection pressures to track fresh-water resources by riding the winds that bring rain28 may explain why Sahelian residents such as Oedaleus senegalensis grasshoppers and An. coluzzii have a mixed strategy of migration29 and local dormancy. Anopheles gambiae, which presumably recolonizes the Sahel every wet season is relatively rare in Sahelian villages3, and thus only one specimen was captured by our nets. It may migrate on fewer nights and constitute a smaller fraction of windborne migrants (Supplementary Discussion).
In areas approaching elimination, malaria cases without travel history are presumed to represent indigenous transmission. We propose that a substantial fraction of such cases, especially those that occur within ~300 km from high malaria transmission areas, arise from the bites of exogenous-windborne-infected mosquitoes. For example, north-eastern South Africa has the highest incidence of persistent malaria in the country with many cases not associated with human travel, which are concentrated in an arc extending over ~150 km from the borders with Zimbabwe and Mozambique, where transmission is still high. This area includes the Kruger National Park where roads are scarce and vehicular transport of infected mosquitoes30 may be hampered. Testing the correlation of such infection events with corresponding winds will help to assess this hypothesis. If confirmed, incorporation of disease control efforts in source populations to minimize or block migration are likely to be an essential element of the elimination strategy.
Methods
Study area
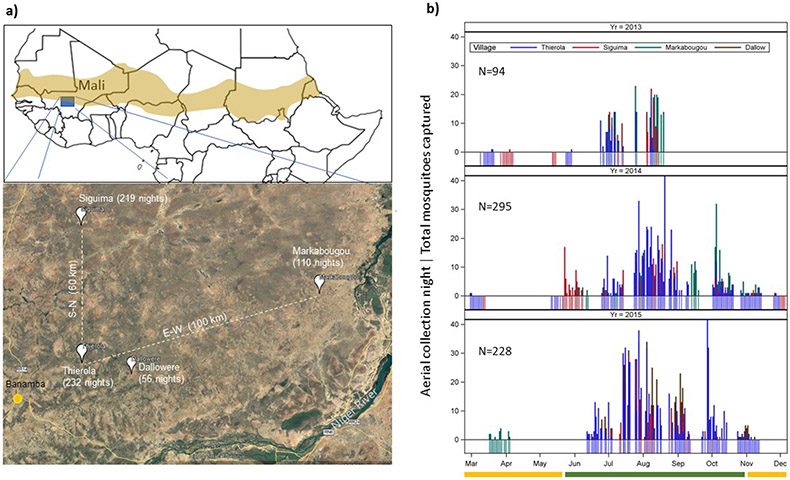
Aerial sampling stations were located in four Sahelian villages in Mali (Fig. S1): Thierola (13.6586, −7.2147) from March 2013 to November 2015, Siguima (14.1676, −7.2279) from March 2013 to October 2015; Markabougou (13.9144, −6.3438) from June 2013 to April 2015; and Dallowere (13.6158, −7.0369) from July 2015 to November 2015. This study area has been described in detail previously3,6,9,11,32-34. Briefly, the region is rural, characterized by scattered villages with traditional mud-brick houses, surrounded by fields. A single growing season (June–October) allows the farming of millet, sorghum, maize, and peanuts, as well as subsistence vegetable gardens. Over 90% of the annual rains fall during this season (~550mm). Cattle, sheep, and goats graze in the savannah that consists of grasses, shrubs, and scattered trees. The rains form small puddles and larger seasonal ponds that usually are totally dry by the end of November. From November until May, rainfall is absent or negligible (total precipitation < 50mm), and by December water is available only in deep wells.
Aerial sampling and specimen processing
Aerial sampling stations were placed ~0.5 km from the nearest house of the village in open areas away from large trees. The method of aerial insect collection was adapted from a study on high-altitude mating flights in ants35. Rectangular 3 x 1m nets (3m2), cut from a roll of tulle netting (mesh: 8 holes/cm; hole diameter of 1.2 mm), were sewn to form four narrow sleeves 1m apart along the net (ED Fig. 3). A 1m carbon rod was inserted into each sleeve and glued to the net using Duco Cement Glue (Devcon, FL, ED Fig 3). Three nets were spread over each other on a clean large wooden table topped by a 3.5 x 1.5m plywood and coated with a thin film of insect glue (Tanglefoot, Tropical Formula, Contech Enterprises Inc., BC) by rolling a PVC pipe smeared with this glue over them, while applying moderate pressure downward. The pipe was held at each end (from each side of the long table) by two persons and repeatedly rolled (and smeared) until a uniform thin layer of glue coated the net (but did not block its holes). After coating, the sticky nets were immediately rolled individually, and kept in two tightly secured plastic bags indoors, to avoid accidental contact with insects prior to setup.
Prior to the launch, polyurethane balloons (3m in diameter; Mobile Airship & Blimps, Canada, or Lighter than Air, FL, USA), were inflated to full capacity with balloon-grade helium (>98.5%) and topped up to ensure full capacity as needed, usually every 1–3 days based on the balloon condition (ED Fig. 3). Typically, balloons were launched over ~10 consecutive nights per month. The balloon was kept stationary at ~200 m agl by a cord (AmSteel®Blue, synthetic rope sling, Southwest Ocean Services, TX) secured to a 1m3 cement block inserted under the ground. The cord then went through a horizontal manually-rotating drum made of a garden-hose reel used for reeling it. A larger 3.3 m diameter balloon (Lighter than Air, FL) was used between July and September 2015, and launched to ~300 m agl.
A team of five trained technicians operated each aerial sampling station. During the launch of a balloon, one team member held the cord under the balloon with heavy-duty gloves and manually controlled its ascent and descent, another controlled the reel, while the other three added or removed the sticky nets to and from their specified positions on the cord. The nets were attached to Velcro panels previously placed on the cord at desirable positions and spaced to fit each of the matching Velcro pieces on the four carbon rods (ED Fig. 3). A knot was made below the top-most Velcro and above the bottom-most Velcro, ensuring that the nets would remain stretched even in strong winds (rather than slip on the cord). Additionally, the team secured the balloons over a “landing patch,” padded by tires covered by a tarpaulin. The balloon was secured to the ground through its main cord by a central hook, at the middle of the landing patch, and by a large tarpaulin that covered it from the top and secured to the ground using 14 large stakes. Team members inspected the nets upon launch to verify that they were free of insects. Upon retrieval of the balloon, the team worked in reverse order and immediately rolled each sticky net (hereafter, called a panel) and placed it in clean labeled plastic bags, inserted in another bag, each tightened with a cord until inspection.
Each balloon typically carried three sticky nets. Initially, they were suspended at 40, 120, and 160 m agl, but from August 2013, the typical altitude was set to 90, 120, 190 m agl. When the larger balloon was deployed in the Thierola station (August–September 2015), two additional nets were added at 240 and 290 m agl. Balloons were launched approximately 1 hour before sunset (~17:00) and retrieved 1 hour after sunrise (~07:30), the following morning. To control for insects trapped near the ground as the nets were raised and lowered, control nets were raised up to 40 m agl and immediately retrieved (between September and November 2014 the control nets were raised to 120 m agl) during the launch and retrieval operations. The control nets spent 5 minutes in the air (up to 10 minutes when raised to 120 m). Once retrieved they were processed as other nets. Following panel retrieval, inspection for insects was conducted between 09:00 and 11:30 in a dedicated clean area. The panel was stretched between two posts and scanned for mosquitoes, which were counted, removed using forceps, and preserved in 80% ethanol before all other insects were similarly processed and placed in other tubes. Depending on their condition, the sticky panels were sometimes reused the subsequent night.
Species identification
Glue attached to the insects was washed off with 100% chloroform. The mosquitoes were gently agitated (<30 sec) to loosen them from one another. Individual mosquitoes were transferred into consecutive wells filled with 85% ethanol. Using a dissecting scope, the samples were morphologically sorted by mosquito subfamily (Anophelinae, Culicinae), and tentative identifications to Anopheles species /species group undertaken. All An. gambiae s.l. visually classified (and two identified based on molecular barcode analysis, see below), were identified to species based on fragment-size differentiation after amplification of the nuclear ITS2 region and digestion of the product36. Validation was carried out in LSTM (DW’s laboratory) where each specimen was washed with 500μL heptane followed by two further washes with ethanol. DNA was then extracted using the Nexttec (Biotechnologie, GmbH) DNA isolation kit according to manufacturer’s instructions. Species identification using a standard PCR method, including all primers37 with products visualized on 2% agarose gel. Anopheles gambiae s.l. samples were further identified to species by SINE insertion polymorphism38. In cases where no species-specific bands were detected using the first method, approximately 800 bp region of the mtDNA cytochrome oxidase I genes was amplified using the primers C1_J_2183 and TL2_N_301439. PCR products were purified using the QIAquick PCR-Purification kit (QIAgen) and sequenced in both directions using the original PCR primers by MacroGen Inc. (Amsterdam, Netherlands). Sequences were aligned using CodonCode Aligner (CodonCode Corporation, Dedham, MA) and compared to existing sequences in GenBank to identify species. All other Anopheles mosquitoes were identified by the retrospective correlation of DNA barcodes, with morphologically–verified reference barcodes compiled by Walter Reed Biosystematics Unit and the Mosquito Barcoding Initiative in Y-ML’s lab. Head-thorax portions of all samples were separated and used for DNA extraction using the Autogen® automated DNA extraction protocol. MtDNA COI barcodes were amplified using the universal LCO1490 and HCO2198 barcoding primers40, and amplified, cleaned and bi-directionally sequenced according to previously detailed conditions41. All DNA barcodes generated from this study are available under the project “MALAN – Windborne Anopheles migrants in Mali” on the Barcode of Life Database (www.boldsystems.org) and in GenBank under accession numbers MK585944–MK586043. Plasmodium infection status was determined following previously described protocol42 using DNA extracts from the whole body for An. coluzzii and, for all other specimens, for thorax and head (n=190) as well as separated abdomens (n=156) extracted and tested individually using published protocols43,44. Due to the nature of the collections, all body parts were not available for each specimen, accounting for the discrepancy in numbers. Bloodmeal identification was carried out following published protocol45.
Data Analysis
Although aerial collections started in April 2012, protocol optimization and standardization took most of that year, and data included in the present analysis covers only the period March 2013–November 2015. Nights when operations were interrupted by storms or strong winds (e.g., the balloon was retrieved during darkness) were also excluded.
The total number of mosquitoes per panel represents ‘net density’ of each species. Aerial density was estimated based on the species’ panel density and total air volume that passed through that net that night, i.e.,
Net surface area was 3 m2. Wind speed data were obtained from the atmospheric re-analyses of the global climate, ERA5. Hourly data available at 31 km surface resolution with multiple vertical levels including ground, 2, 10, 32, 55, 85, 115 180, 215, 255, and 300 m agl. Overnight records (18:00 through 06:00) for the nearest grid center were used to calculate the nightly direction and mean wind speed at each village: Siguima, Markabougou and Thierola. Dallowere, located 25 km south of Thierola, was included in the same grid cell of Thierola. The mean nightly wind speed at panel height was estimated based on the nearest available altitude layer.
To evaluate clustering in mosquito panel density and the effects of season, panel height, year and locality, mixed linear models with either Poisson or negative binomial error distributions were implemented by proc GLIMMIX46. The clustering at the levels of the panel and the night of sampling were evaluated as random effects as was the case for the year of sampling and locality. These models accommodate counts as non-negative integer values. The ratio of the Pearson χ2 to the degrees of freedom was used to assess overall “goodness of fit” of the model, with values of >2 indicating a poor fit. The significance of the scale parameter estimating k of the negative binomial distribution was used to choose between Poisson and negative binomial models. Sequential model fitting was used, starting with random factors before adding fixed effects. Lower Bayesian Information Criterion (BIC) values and the significance of the underlying factors were also used to select the best fitting model of each species.
The magnitude of windborne migration was expressed as the expected minimum number of migrants per species crossing an imaginary line of 1 km perpendicular to the wind at altitude. This commonly used measure of abundance assumes that the insects fly in a layer that is 1 km wide and does not require knowledge of the distance or time the insects fly to or from the interception point47-49. We used the mean wind speed at altitude (4.8 m/s, see below) and assumed that mosquitoes fly in a layer depth of 200 m between 50 and 250 m agl, conservatively reflecting that mosquitoes were captured between 40-290 m (see below). Accordingly, this nightly migration intensity was computed as the product of the mean aerial density across the year (conservatively including periods when no migrants were captured) by the volume of air passing over the reference line during the night. The corresponding annual index was estimated by multiplying the nightly index by the period of windborne migration estimated from the difference between the first and last day and month a species was captured over the three years. Species that were captured once were assumed to migrate during a single month. The annual number of migrants per species crossing a line of 100 km was used because of the similar species composition across our sampling sites spanning 100 km (Fig. S1a and see below).
Like most insects in their size range48,50,51, the flight speed of mosquitoes does not typically exceed 1 m/s52,53. Because winds at panel altitude attain speeds considerably higher than the mosquito’s own speed, flight direction and speed are governed by the wind47,48 and thus, flight trajectory can be simulated based on the prevailing winds during the night of capture at the relevant locations and altitudes as has been done previously54-56. Accordingly, backward flight trajectories of mosquitoes were simulated using HYSPLIT: Hybrid Single-Particle Lagrangian Integrated Trajectory model25 based on ERA5 meteorological reanalysis data. Data available in ERA5 present the highest spatial and temporal resolution available for that region. Comparisons with the lower spatial and temporal resolution data available from the MERRA2 reanalysis data57 and the Global Data Assimilation System available at 0.5 degree spatial resolution showed good agreement in trajectory direction and overall distance (not shown). Trajectories of each captured mosquito were simulated starting at its capture location, altitude, and all multiple interception (full) hours during the night of the collection. Because anophelines are nocturnal, we conservatively assumed that flights started at or after 18:00 and ended by 06:00 the following morning and computed trajectories for every hour that allowed for a total of two or nine h flight. For example, to complete 9 hours flight by 06:00, a mosquito could have started at 18:00, 19:00, 20:00, or 21:00. Total flight duration of tethered female An. gambiae s.l. and An. atroparvus reached or exceeded 10 hours with average speed of 1 km/h52 in accord with other studies53,58,59. Likewise, An. vagus and An. hyrcanus caught 150 m agl after midnight over India would have been migrating for >6 hours, assuming they took off around dusk20. Thus, we conservatively assumed that windborne long-distance migrant anopheline mosquitoes fly between two and nine hours per night although longer duration is possible. Each trajectory consisted of the global positions of the mosquitoes at hourly intervals from the interception time. In addition to plotting trajectories60-67, the linear distance from the interception site and the azimuth (angle between interception site and mosquito simulated position from the North, projected on a plane) were computed for all trajectories. To evaluate distance range and dominant directions of flight, the mean and 95% CI of the distance and azimuth (as a circular statistic) were computed for the two- and nine-hours flight trajectories. The dispersion of individual angles (azimuths) around the mean was measured by the mean circular resultant length ‘r’, which can vary from 0 to 1, with higher values indicating tighter clustering around the mean. Rayleigh’s test was used to test that there was no mean direction, as when the angles form a uniform distribution over a circle68.
Extended Data
Extended Data Figure 1. Study area and aerial sampling effort.
a) Map of the study area with aerial sampling villages and the number of sampling nights per village under a schematic map of Africa showing the Sahel region (source: Wikimedia Commons, https://pt.m.wikipedia.org/wiki/Ficheiro:Sahel_Map-Africa_rough.png). b) Nightly sampling effort by year. Fringe under zero indicates the sampling nights (by village) and needles denote the total number of mosquitoes per night regardless of the number of panels per night. Dry and wet seasons are indicated by yellow and green in the ruler under the X-axis.
Extended Data Figure 2. Regularity of migratory flights, flight altitude, and variability among years and localities in species aerial presence.
a) Relationship between mosquito presence (fraction of positive nights) and mean panel density to evaluate if appearance can be accounted by overall abundance rather than by unique migratory nights. b) The relationship between panel height and mean mosquitoes density/panel (x103, regression line with shading denotes 95% CI) showing mean panel density by species. Inset summarizes the covariance analysis (ANCOVA), underlying this regression, which includes the species and panel height. Number of nights per panel altitude is given in blue along the X axis (see Figure 1a). c) Variation in mosquito presence (fraction of positive nights) by species between years (top) and villages (bottom) with their 95% CI. Sampling effort expressed as the number of panels per year/village is shown adjacent to the legend.
Extended Data Figure 3. A photo showing a tethered sticky panel setup and attachment.
A sticky panel (3x1m net) on a test helium balloon (lower volume/capacity), showing attachment of net covered with glue to the cord tethering the balloon to the ground. Note the four carbon poles and Velcro attachment points (see text for details). A close-up of the attachment of the panel to the cord and preparing to launch a standard 3 m balloon.
Extended Data Table 1. Variation in the rate of mosquito capture between years, localities and heights above ground.
Variation in mosquito capture rate between years, localities, and heights above ground (GLIMMIX models of random and fixed variables, total number of panels was 1,894).
| Dependent: Panel Density | Parameter | A. squamosus | A. pharoensis | A. coustani | A. rufipes | A. coluzzii |
|---|---|---|---|---|---|---|
| Random vars only: Poisson | Pearson χ2/df (BIC) | 1.13 (793.5) | 1.04 (394.4) | 0.90 (306.52) | 1.11 (260.4) | 1.16 (252.8) |
| Random vars only: Negative Binomial | Pearson χ2/df, Scalea (BIC) | 0.83, 5.98*** (756.2) | 0.97, 3.84ne (391.4) | 0.87, 2.09ns (306.7) | 0.99, 10.6ne (254.5) | 0.98, 15ns (246.7) |
| intercept[mean] (SD) | −4.06ns (1.23) | −3.9** (0.226) | −4.4* (0.63) | −4.7*** (0) | −4.4** (0.23) | |
| Year (SD) | 3.24ns (4.36) | 0ns (0.06) | 0.09ns (0.31) | 0.55ns (0.56) | 0ne | |
| Localityb (SD) | 0.075ns (0.116) | 0.04ns (0.15) | 0.73ns (3.19) | 0ne | 0ne | |
| Random vars only: Poisson | Nightc (SD) | 4.02** (1.42) | 1.78* (0.99) | 6.57ns (7.3) | 29.0* (16.8) | 32.0* (17.9) |
| Random vars only: Neg. Bin. | Nightc (SD), scale | 3.9** (1.5), 0.74ns | 1.6ns (1.1), 0.34ns | 0.5ne (ne), 0ne | 30.1* (17.5), 0.7ns | 33.5* (18.7), 0.76ns |
| Fixed and random: Poisson | Pearson χ2/df (BIC) | 0.37 (700) | 0.6 (403) | 0.2 (308) | 0.09 (258) | 0.08 (243) |
| Night | 1.4** (0.0) | 0.78ns (0.8) | 1.9* (1.1) | 14.0ns(13.3) | 21.9ns (15.2) | |
| Periodd | Aug-Oct* | Aug-Oct* | Aug-Octns | Aug-Octns | Aug-Oct*** | |
| Panel height (m) | 0.001*** (0) | 0.003*** (0) | −0.007*** (0) | 0.001*** (0) | 0.014* (0.006) | |
| Dependent: Aerial Density | Pearson χ2/df (BIC) | 0.42 (938) | 0.41 (503) | 0.2 (378) | 0.1 (304) | 0.09 (283) |
| Fixed and random: Poisson | Night | 2.9*** (0.8) | 2.6* (1.2) | 5.2ns (3.9) | 26.8* (16.0) | 31.5* (17.6) |
| Periodd | Aug-Octns | Aug-Oct* | Aug-Octns | Aug-Octns | Aug-Oct*** | |
| Panel height (m) | −0.003*** (0) | −0.002*** (0) | −0.008* (0.004) | −0.001*** (0) | 0.01* (0.005) |
For negative bionomial scale parameter estimates the k parameter of this distribution.
The effects locality was estimated considering only 3 locations after pooling Dallowere and Thierola which are only 20 km apart (see Methods).
The significance of clustering by night (across locations) estimated as the only random effect (using subject statement) after finding insignificant variance components of Year and Location.
Periods included: March-May, June-July, August-October, and November-December. The period of highest panel density is shown with its statistical significance.
Panel heights: 40, 120 (90-120), 160, 190, and 250, (220-290) m agl due to small sample sizes (nights) of certain altitudes.
refer to significance probability of 0.001, 0.01 and 0.05, >0.05, and to parameters that could not be estimated, respectively.
Supplementary Material
Acknowledgements
We thank the residents of Thierola, Siguima, Markabougou, and Dallowere for their consent to work near their homes and for their wonderful assistance and hospitality. Thanks to Dr. Moussa Keita, Mr. Boubacar Coulibaly, and Ousmane Kone for their valuable technical assistance with field and laboratory operations. We thank Dr. Gary Fritz for consultation on the aerial sampling method using sticky panels; Drs. Dick Sakai, Sekou F Traore, Jennifer Anderson, and Thomas Wellems, Ms. Margie Sullivan, and Mr. Samuel Moretz for logistical support, Drs. Frank Collins and Neil Lobo (Notre Dame University, USA) for support to initiate the aerial sampling project. We thank Drs. Jose’ MC Ribeiro and Alvaro Molina-Cruz for reading earlier versions of this manuscript and providing us with helpful suggestions and Drs. Alice Crawford and Fong (Fantine) Ngan, (NOAA/Air Resources Laboratory and CICS, the University of Maryland) for conversions of the MERRA2 and ERA5 datafiles to HYSPLIT format. This study was primarily supported the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Rothamsted Research received grant-aided support from the United Kingdom Biotechnology and Biological Sciences Research Council (BBSRC). Y-ML & RM are supported by the U.S. Army. Views expressed here are those of the authors, and in no way reflect the opinions of the U.S. Army or the U.S. Department of Defense. The USDA is an equal opportunity provider and employer. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA.
Footnotes
Competing Interests: All authors declare no competing financial interests.
Data and Code Availability
1. Data on anopheline capture, identification, sex, and gonotrophic status are available from www.boldsystems.org (Project code: MALAN) and in Genbank (MK585944–MK586043).
2. SAS code used for statistical analyses (and data manipulations) and 9-hour backward trajectories data for each mosquito capture event based on HYSPLIT are available from TL upon request.
3. Plotting trajectories (code available at https://github.com/benkraj/anopheles-migration)
References (Main Text)
- 1.WHO ∣ World malaria report 2017. WHO; (2018). [Google Scholar]
- 2.Gething PW et al. Mapping Plasmodium falciparum mortality in Africa between 1990 and 2015. N Engl J Med (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dao A. et al. Signatures of aestivation and migration in Sahelian malaria mosquito populations. Nature 516, 387–390 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontenille D. et al. High annual and seasonal variations in malaria transmission by anophelines and vector species composition in Dielmo, a holoendemic area in Senegal. Am J Trop Med Hyg 56, 247–253 (1997). [DOI] [PubMed] [Google Scholar]
- 5.Fontenille D. et al. Four years’ entomological study of the transmission of seasonal malaria in Senegal and the bionomics of Anopheles gambiae and A. arabiensis. Trans R Soc Trop Med Hyg 91, 647–652 (1997). [DOI] [PubMed] [Google Scholar]
- 6.Lehmann T. et al. Aestivation of the African Malaria Mosquito, Anopheles gambiae in the Sahel. Am. J. Trop. Med. Hyg 83, 601–606 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simard F, Lehmann T, Lemasson JJ, Diatta M & Fontenille D Persistence of Anopheles arabiensis during the severe dry season conditions in Senegal: an indirect approach using microsatellite loci. Insect Mol.Biol 9, 467–479 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Omer SM & Cloudsley-Thompson JL Dry season biology of Anopheles gambiae Giles in the Sudan. Nature 217, 879–880 (1968). [Google Scholar]
- 9.Mamai W. et al. Monitoring dry season persistence of Anopheles gambiae s.l. populations in a contained semi-field system in southwestern Burkina Faso, West Africa. J Med Entomol 53, 130–138 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Yaro AS et al. Dry season reproductive depression of Anopheles gambiae in the Sahel. J. Insect Physiol 58, 1050–1059 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman JW, Reynolds DR & Wilson K Long-range seasonal migration in insects: Mechanisms, evolutionary drivers and ecological consequences. Ecology Letters 18, 287–302 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Service MW Mosquito (Diptera: Culicidae) dispersal - the long and the short of it. J. Med. Entomol 34, 579–588 (1997). [DOI] [PubMed] [Google Scholar]
- 13.Service MW Mosquito Ecology Field Sampling Methods. (Elsevier Applied Science, 1993). [Google Scholar]
- 14.Costantini C. et al. Density, survival and dispersal of Anopheles gambiae complex mosquitoes in a west African Sudan savanna village. Med.Vet.Entomol 10, 203–219 (1996). [DOI] [PubMed] [Google Scholar]
- 15.Toure YT et al. Mark-release-recapture experiments with Anopheles gambiae s.l. in Banambani Village, Mali, to determine population size and structure. Med.Vet.Entomol 12, 74–83 (1998). [DOI] [PubMed] [Google Scholar]
- 16.Garrett-Jones C. The possibility of active long-distance migrations by Anopheles pharoensis Theobald. Bull. World Health Organ 27, 299–302 (1962). [PMC free article] [PubMed] [Google Scholar]
- 17.Sellers RF Weather, host and vector--their interplay in the spread of insect-borne animal virus diseases. J. Hyg. (Lond) 85, 65–102 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glick PA The distribution of insects, spieders, and mites in the air. United States Department of Agriculture, Technical Bulletin 673, (1939). [Google Scholar]
- 19.Reynolds DR et al. Atmospheric transport of mosquitoes in northeast India. Med. Vet. Entomol 10, 185–186 (1996). [DOI] [PubMed] [Google Scholar]
- 20.Kyalo D. et al. A geo-coded inventory of anophelines in the Afrotropical Region south of the Sahara: 1898-2016. Welcome Open Res. 2, 57- (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beier JC et al. Characterization of malaria transmission by Anopheles (Diptera: Culicidae) in western Kenya in preparation for malaria vaccine trials. J Med Entomol 27, 570–577 (1990). [DOI] [PubMed] [Google Scholar]
- 22.Antonio-Nkondjio C. et al. Complexity of the Malaria Vectorial System in Cameroon: Contribution of Secondary Vectors to Malaria Transmission. J. Med. Entomol 43, (2006). [DOI] [PubMed] [Google Scholar]
- 23.Toure YT et al. Perennial transmission of malaria by the Anopheles gambiae complex in a north Sudan Savanna area of Mali. Med Vet Entomol 10, 197–199 (1996). [DOI] [PubMed] [Google Scholar]
- 24.Stein AF et al. NOAA’s HYSPLIT Atmospheric transport and dispersion modeling system. Bull. Am. Meteorol. Soc 96, 2059–2077 (2015). [Google Scholar]
- 25.Verdonschot PFM & Besse-Lototskaya AA Flight distance of mosquitoes (Culicidae): A metadata analysis to support the management of barrier zones around rewetted and newly constructed wetlands. Limnologica 45, 69–79 (2014). [Google Scholar]
- 26.Hay SI, Rogers DJ, Toomer JF & Snow RW Annual Plasmodium falciparum entomological inoculation rates (EIR) across Africa: literature survey, Internet access and review. Trans. R. Soc. Trop. Med. Hyg 94, 113–27 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholson SE The West African Sahel: A review of recent studies on the rainfall regime and its interannual variability. ISRN Meteorol. 2013, 32 (2013). [Google Scholar]
- 28.Wilson K. in Insect migration: Tracking resources through space and time (eds. Drake VA & Gatehouse AG) 243–264 (Cambridge University Press, 1995). [Google Scholar]
- 29.Pedgley DE, Reynolds DR & Tatchell GM in Insect Migration: Tracking resources through space and time (eds. Drake VA & Gatehouse AG) 3–30 (Cambridge University Press, 1995). [Google Scholar]
- 30.Frean J, Brooke B, Thomas J & Blumberg L Odyssean malaria outbreaks in Gauteng Province, South Africa, 2007 - 2013. SAMJ South African Med. J. 104, 335–338 (2014). [DOI] [PubMed] [Google Scholar]
References (Methods and Extended Data)
- 32.Lehmann T. et al. Tracing the origin of the early wet-season Anopheles coluzzii in the Sahel. Evol. Appl 10, 704–717 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehmann T. et al. Seasonal Variation in Spatial Distributions of Anopheles gambiae in a Sahelian Village: Evidence for Aestivation. J. Med. Entomol 51, 27–38 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huestis DL et al. Seasonal variation in metabolic rate, flight activity and body size of Anopheles gambiae in the Sahel. J Exp Biol 215, 2013–2021 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fritz GN, Fritz AH & Vander Meer RK Sampling high-altitude and stratified mating flights of red imported fire ant. J Med Entomol 48, 508–512 (2011). [DOI] [PubMed] [Google Scholar]
- 36.Fanello C, Santolamazza F & della Torre A Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med Vet Entomol 16, 461–464 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Scott JA, Brogdon WG & Collins FH Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am.J.Trop.Med.Hyg 49, 520–529 (1993). [DOI] [PubMed] [Google Scholar]
- 38.Santolamazza F. et al. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar. J 7, 163 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon C. et al. Evolution, Weighting, and Phylogenetic Utility of Mitochondrial Gene Sequences and a Compilation of Conserved Polymerase Chain Reaction Primers. Ann. Entomol. Soc. Am 87, 651–701 (1994). [Google Scholar]
- 40.Folmer O, Black M, Hoeh W, Lutz R & Vrijenhoek R DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol 3, 294–9 (1994). [PubMed] [Google Scholar]
- 41.Linton Y-M et al. Mosquitoes of eastern Amazonian Ecuador: biodiversity, bionomics and barcodes. Mem. Inst. Oswaldo Cruz 108 Suppl 1, 100–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bass C. et al. PCR-based detection of Plasmodium in Anopheles mosquitoes: a comparison of a new high-throughput assay with existing methods. Malar. J 7, 177 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demas A. et al. Applied Genomics: Data Mining Reveals Species-Specific Malaria Diagnostic Targets More Sensitive than 18S rRNA. J. Clin. Microbiol 49, 2411–2418 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steenkeste N. et al. Towards high-throughput molecular detection of Plasmodium: new approaches and molecular markers. Malar. J 8, 86 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kent RJ & Norris DE Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. Am. J. Trop. Med. Hyg 73, 336–42 (2005). [PMC free article] [PubMed] [Google Scholar]
- 46.SAS Inc., I. SAS for Windows Version 9.3. (2011).
- 47.Hu G. et al. Mass seasonal bioflows of high-flying insect migrants. Science (80-. ). 354, 1584–1587 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Drake VA & Reynolds DR Radar entomology : observing insect flight and migration. (CABI International., 2012). [Google Scholar]
- 49.Reynolds D, Chapman J & Stewart A Windborne migration of Auchenorrhyncha (Hemiptera) over Britain. Eur. J. Entomol 114, 554–564 (2017). [Google Scholar]
- 50.Taylor LR Insect migration, flight periodicity and the Boundary Layer. J. Anim. Ecol 43, 225–238 (1974). [Google Scholar]
- 51.Chapman JW, Drake VA & Reynolds DR Recent Insights from Radar Studies of Insect Flight. Annu. Rev. Entomol. Vol 56 56, 337–356 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Kaufmann C & Briegel H Flight performance of the malaria vectors Anopheles gambiae and Anopheles atroparvus. J. vector Ecol 29, 140–153 (2004). [PubMed] [Google Scholar]
- 53.Snow WF Field estimates of the flight speed of some West African mosquitoes. Ann. Trop. Med. Parasitol 74, 239–242 (1980). [DOI] [PubMed] [Google Scholar]
- 54.Eagles D, Walker PJ, Zalucki MP & Durr PA Modelling spatio-temporal patterns of long-distance Culicoides dispersal into northern Australia. Prev. Vet. Med 110, 312–322 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Stefanescu C, Alarcón M & Àvila A Migration of the painted lady butterfly, Vanessa cardui, to north-eastern Spain is aided by African wind currents. J. Anim. Ecol 76, 888–898 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Klausner Z, Fattal E & Klement E Using synoptic systems’ typical wind trajectories for the analysis of potential atmospheric long-distance dispersal of lumpy skin disease virus. Transbound. Emerg. Dis 64, 398–410 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Gelaro R. et al. The Modern-Era Retrospective Analysis for Research and Applications, Version 2 (MERRA-2). J. Clim 30, 5419–5454 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pedgley DE Windborne pests and diseases: Meteorology of airborne organisms. (Ellis Horwood Ltd., 1982). [Google Scholar]
- 59.Gillies MT & Wilkes TJ Field experiments with a wind tunnel on the flight speed of some west African mosquitoes (Diptera: Culicidae). Bull. Entomol. Res 71, 65 (1981). [Google Scholar]
- 60.Kahle D & Wickham H ggmap: Spatial Visualization with ggplot2. R J. 5, 144–161 (2013). [Google Scholar]
- 61.Hijmans RJ geosphere: Spherical Trigonometry. (2017). [Google Scholar]
- 62.Slowikowski K. ggrepel: Automatically Position Non-Overlapping Text Labels with ‘ggplot2’. (2018). [Google Scholar]
- 63.Santos Baquero O. ggsn: North Symbols and Scale Bars for Maps Created with ‘ggplot2’ or ‘ggmap’. (2019). [Google Scholar]
- 64.Arnold JB ggthemes: Extra Themes, Scales and Geoms for ‘ggplot2’. (2019). [Google Scholar]
- 65.Grolemund G & Wickham H Dates and Times Made Easy with {lubridate}. J. Stat. Softw 40, 1–25 (2011). [Google Scholar]
- 66.RStudio Team. RStudio: Integrated Development Environment for R. (2015).
- 67.R Core Team. R: A Language and Environment for Statistical Computing. (2016).
- 68.Fisher NI Statistical Analysis of Circular Data. (Cambridge University Press, 1993). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.