Abstract
Objective
We aimed to investigate the cost-effectiveness of tislelizumab plus chemotherapy compared to chemotherapy alone as a first-line treatment for advanced or metastatic oesophageal squamous cell carcinoma (OSCC).
Methods
A partitioned survival model was developed to evaluate the cost-effectiveness of tislelizumab plus chemotherapy versus chemotherapy alone in patients with advanced or metastatic OSCC over a 10-year lifetime horizon from the perspective of the Chinese healthcare system. Costs and utilities were derived from the drug procurement platform and published literature. The model outcomes comprised of costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratio (ICER). One-way and probabilistic sensitivity analyses were conducted to address uncertainty and ensure the robustness of the model.
Results
Tislelizumab plus chemotherapy yielded an additional 0.337 QALYs and incremental costs of $7,117.007 compared with placebo plus chemotherapy, generating an ICER of $21,116.75 per QALY, which was between 1 time ($12,674.89/QALY) and 3 times GDP ($38,024.67/QALY) per capita. In one-way sensitivity analysis, the ICER is most affected by the cost of oxaliplatin, paclitaxel and tislelizumab. In the probabilistic sensitivity analysis, when the willingness-to-pay threshold was set as 1 or 3 times GDP per capita, the probability of tislelizumab plus chemotherapy being cost-effective was 1% and 100%, respectively.
Conclusion
Tislelizumab plus chemotherapy was probably cost-effective compared with chemotherapy alone as the first-line treatment for advanced or metastatic OSCC in China.
Introduction
Oesophageal cancer is a prevalent malignant tumor of the digestive system worldwide, ranking seventh in incidence (604,000 new cases) and sixth in overall mortality (544,000 deaths) [1]. Oesophageal squamous cell carcinoma (OSCC) is the most common histologic type of oesophageal cancers, accounting for 85.79% of all cases in China [2,3]. The disease burden of oesophageal cancer is particularly significant in China [1,4], the disability-adjusted life years (DALYs) caused by oesophageal cancer ranks fourth among all cancers [5]. Advanced OSCC has a poor prognosis, with a 5-year survival rate of approximately 15–25% [6]. The efficacy of conventional chemotherapy regimens for advanced OSCC is limited, with less than one year of overall survival for patients treated with first-line cisplatin in combination with paclitaxel or fluorouracil regimens [6–8].
With the recent advancements in immune checkpoint inhibitors (ICIs), there are now more treatment options available for advanced or metastatic OSCC [7,9–11]. It has been demonstrated that adding programmed death-1 (PD-1) inhibitors to standard chemotherapy can improve survival rates while maintaining manageable toxicity profiles [12–17]. According to the results of the RATIONALE-306 clinical trial, Tislelizumab, a PD-1 inhibitor, has shown superior survival benefits when used in combination with chemotherapy compared to chemotherapy alone in patients with advanced or metastatic OSCC (Median OS 17.2 vs. 10.6 months; stratified hazard ratio 0.66) [18]. And tislelizumab plus chemotherapy was recommended as the first-line treatment for advanced or metastatic OSCC by the Chinese Society of Clinical Oncology (CSCO) guideline [19].
While tislelizumab demonstrates clinical efficacy in treating advanced or metastatic OSCC, its economic burden cannot be overlooked. This study aims to compare the cost-effectiveness of tislelizumab plus chemotherapy versus chemotherapy alone as first-line treatment for advanced or metastatic OSCC from the perspective of the Chinese healthcare system.
Materials and methods
Population and treatments
The target population and treatment regimens in this study were consistent with those of the RATIONALE-306 trial (NCT03783442). Patients aged 18 years or older with unresectable, locally advanced, recurrent or metastatic OSCC (regardless of PD-L1 expression), Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, and measurable or evaluable disease according to Response Evaluation Criteria in Solid Tumors (version 1.1) were enrolled. Included patients were randomly assigned in a 1:1 ratio to receive tislelizumab 200 mg or placebo administered intravenously every three weeks on day one, in combination with a chemotherapy doublet selected by the investigator. The doublet regimen included a platinum agent (cisplatin 60–80 mg/m² intravenously on day one or oxaliplatin 130 mg/m² intravenously on day one) combine with fluorouracil (750–800 mg/m² intravenously on days one to five) or capecitabine (1000 mg/m² orally twice daily on days one to fourteen), or paclitaxel (175mg/m² intravenously on day one).
Model structure
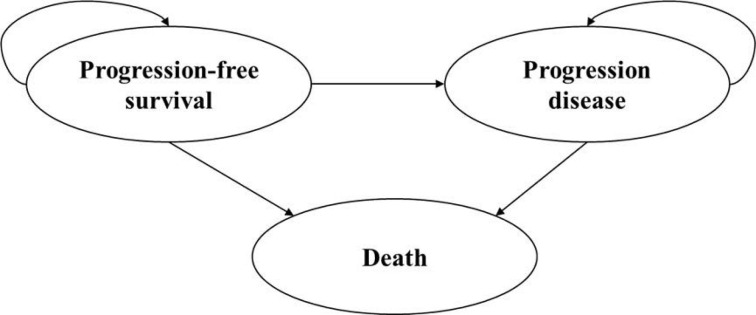
A partitioned survival model was developed in Microsoft Excel to assess the cost-effectiveness of tislelizumab plus chemotherapy compared to chemotherapy alone in patients with advanced or metastatic OSCC, from the perspective of the Chinese healthcare system. The model simulated three health states: progression-free survival (PFS), disease progression (PD), and death; it is assumed that all patients enter the model in PFS state, each patient can transition from one state to another or remain unchanged within each cycle, the PD state cannot revert to the PFS state, and both the PFS state and PD state may transition to the death (Fig 1). The proportion of survival patients in each cycle (3-week cycle) was estimated by the area under the OS curve, and the proportion survived in PFS state was estimated by the area under the PFS curve. The proportion survived in PD state was estimated by the difference between the OS and PFS curves [20]. The proportion of patients in each health state can be directly derived from the Kaplan-Meier (K-M) curves in RATIONALE-306 trial. The cycle length was set to 3 weeks, while the time horizon was extrapolated to 10 years. The model outcomes included cost, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratio (ICER). Both costs and effectiveness are discounted at 5% annually as recommended by China guidelines for pharmacoeconomic evaluations [21]. The willingness-to-pay (WTP) threshold was set at 1or 3 times Chinese GDP per capita in 2022 as recommended by the World Health Organization (WHO) [22]. If ICER<1 time GDP per capita, the intervention strategy is totally cost-effective; if ICER is between 1 time and 3 times GDP per capita, the intervention strategy is probably cost-effective; if ICER > 3 times GDP per capita, the intervention strategy is considered not cost-effective.
Fig 1. Three main health states of partitioned survival model.
Clinical data and survival estimates
Safety and efficacy data were obtained from the results of the RATIONALE-306 trial. Firstly, K-M curves reported in the RATIONALE-306 trial were digitized using WebPlotDigitizer (https://apps.automeris.io/wpd/index.zh_CN.html). Subsequently, parametric distributions including exponential, gompertz, weibull, log-logistic and log-normal were fitted to the extracted data through the “gee” package in R software. The optimal distribution was determined by evaluating Akaike’s information criterion (AIC) and Bayesian information criterion (BIC). Both OS and PFS data were best fit with the log-logistic distribution, as evidenced by AIC, BIC.
The original and reconstructed K-M curves are presented in S1 Fig. Validation plots and survival distributions can be found in S2 and S3 Figs, respectively. Estimated parameters and goodness of fit for each survival model are reported in S1 Table.
Cost and utility estimates
Only direct medical costs were considered in the model, including costs of drug acquisition, laboratory tests and radiological examinations, routine follow-up, the management of adverse events (AEs) and terminal care. All costs were converted into United States dollars with an exchange rate of 1 US dollar = 7.05 Chinese yuan (average exchange rate in 2023). The drug prices were derived from drug procurement platform (med.ybj.zj.gov.cn). To determine chemotherapy dosages and expenses, we assumed an average height of 165cm, weight of 65kg, and body surface area (BSA) of 1.72m2 [8]. Our model only involved severe adverse events rated as grade≥3 that were reported in at least 5% of patients in the RATIONALE-306 trial. The subsequent treatment after tislelizumab plus chemotherapy or chemotherapy alone assumes single-agent chemotherapy (e.g. docetaxel, paclitaxel or irinotecan) as recommended in the CSCO and NCCN guidelines [10,19]. Other costs were derived from previously published literature and have been recalculated using the average exchange rates for 2023 [8,23–25].
The health state utility values utilized in our model were obtained from previously published literature since quality of life for patients in the RATIONALE-306 trial was unavailable. Specifically, the utility value for progression-free survival (PFS) and disease progression (PD) in patients with advanced or metastatic OSCC were determined to be 0.75 and 0.60, respectively [23].
All the cost-related parameters and utility-related parameters were shown in Table 1.
Table 1. Model input parameters.
| Parameter | Base case | Range | Distribution | Source | |
|---|---|---|---|---|---|
| Low | High | ||||
| Costs input ($) | |||||
| Tislelizumab (100mg) | 195.39 | 156.31 | 234.47 | Gamma | drug procurement platform |
| Cisplatin (100mg) | 8.61 | 3.75 | 22.41 | Gamma | |
| Oxaliplatin (50mg) | 23.99 | 6.64 | 297.87 | Gamma | |
| 5-fluorouracil (250mg) | 20.71 | 12.38 | 21.13 | Gamma | |
| Capecitabine (500mg) | 0.76 | 0.27 | 3.90 | Gamma | |
| Paclitaxel (30mg) | 15.34 | 7.89 | 99.26 | Gamma | |
| Docetaxel(20mg) | 7.52 | 4.26 | 129.08 | Gamma | |
| Irinotecan(40mg) | 69.24 | 7.94 | 87.30 | Gamma | |
| Laboratory tests and radiological examinations per cycle | 284.69 | 227.75 | 341.63 | Gamma | [26] |
| Routine follow-up cost per cycle | 29.51 | 23.61 | 35.41 | Gamma | [27] |
| Terminal care in end-of-life | 762.72 | 610.18 | 915.26 | Gamma | [27] |
| Management cost of Anaemia | 263.27 | 210.62 | 315.92 | Gamma | [25] |
| Management cost of decreased white blood cell count | 243.39 | 194.71 | 292.07 | Gamma | [24] |
| Management cost of decreased neutrophil count | 228.51 | 182.81 | 274.21 | Gamma | [25] |
| Management cost of neutropaenia | 228.51 | 182.81 | 274.21 | Gamma | [25] |
| Management cost of hypokalaemia | 7.82 | 6.26 | 9.38 | Gamma | [23] |
| Management cost of hyponatraemia | 11.54 | 9.23 | 13.85 | Gamma | [23] |
| Risk of adverse events in tislelizumab plus chemotherapy group (grade ≥3) | |||||
| Anaemia | 0.15 | 0.12 | 0.18 | Beta | [18] |
| Decreased white blood cell count | 0.11 | 0.09 | 0.13 | Beta | [18] |
| Decreased neutrophil count | 0.31 | 0.25 | 0.37 | Beta | [18] |
| Neutropaenia | 0.07 | 0.06 | 0.08 | Beta | [18] |
| Hypokalaemia | 0.06 | 0.05 | 0.07 | Beta | [18] |
| Hyponatraemia | 0.07 | 0.06 | 0.08 | Beta | [18] |
| Risk of adverse events in placebo plus chemotherapy group (grade ≥3) | |||||
| Anaemia | 0.13 | 0.10 | 0.16 | Beta | [18] |
| Decreased white blood cell count | 0.16 | 0.13 | 0.19 | Beta | [18] |
| Decreased neutrophil count | 0.33 | 0.26 | 0.40 | Beta | [18] |
| Neutropaenia | 0.10 | 0.08 | 0.12 | Beta | [18] |
| Hypokalaemia | 0.03 | 0.02 | 0.04 | Beta | [18] |
| Hyponatraemia | 0.03 | 0.02 | 0.04 | Beta | [18] |
| Health utility | |||||
| Utility of PFS | 0.75 | 0.60 | 0.90 | Beta | [8] |
| Utility of PD | 0.60 | 0.48 | 0.72 | Beta | [8] |
| Disutility due to AEs | |||||
| Anemia | 0.07 | 0.06 | 0.08 | Beta | [28] |
| Decreased white blood cell count | 0.20 | 0.16 | 0.24 | Beta | [28] |
| Decreased neutrophil count | 0.09 | 0.06 | 0.11 | Beta | [29] |
| Neutropenia | 0.20 | 0.16 | 0.24 | Beta | [8] |
| Hypokalaemia | 0.03 | 0.02 | 0.04 | Beta | [8] |
| Hyponatraemia | 0.03 | 0.02 | 0.04 | Beta | [8] |
| Discount rate | 0.05 | 0.00 | 0.08 | Beta | [30] |
| Body surface area (m 2 ) | 1.72 | 1.38 | 2.06 | Gamma | [8] |
PFS, progression-free survival; PD, progressed disease.
Sensitivity analysis
One-way deterministic sensitivity analysis (DSA) and probabilistic sensitivity analysis (PSA) were performed to assess the robustness of the model. In DSA, drug prices were adjusted within the range of the purchase price, the cost of tislelizumab was subject to a ± 20% adjustment range because there was only one purchase price, the discount rate varied from 0 to 8% [30], and ranges for other parameters were assumed to be reasonable ranges (±20%) referring to previous publications since 95% confidence intervals (CI) was not available [8,23]. The impact of each input parameter on the model results was shown in the tornado diagram. We performed 1,000 Monte Carlo simulations to assess the stability in model results when all parameters randomly varied simultaneously within the preset distribution [31]. The results of PSA were presented in the form of scatter plots and cost-effectiveness acceptability (CEAC) curves. The ranges and distributions of input parameters were shown in Table 1.
Results
Base-case analysis
The results of the base-case analysis were shown in Table 2. Over a 10-year horizon, tislelizumab plus chemotherapy yielded an additional 0.337 QALYs and incremental costs of $7,117.007 compared with placebo plus chemotherapy, generating an ICER of $21,116.75 per QALY, which was between 1 time ($12,674.89/QALY) and 3 times GDP ($38,024.67/QALY) per capita. The base-case result indicated that tislelizumab plus chemotherapy treatment was probably cost-effective as the first-line treatment for advanced or metastatic OSCC in China.
Table 2. Base-case results.
| Cost ($) | QALYs | Incremental cost | Incremental QALYs | ICER | |
|---|---|---|---|---|---|
| Tislelizumab plus chemotherapy | 106,466.407 | 1.334 | 7,117.007 | 0.337 | 21,116.75 |
| Placebo plus chemotherapy | 99,349.400 | 0.997 | - | - | - |
QALYs, quality-adjusted life years; ICER, incremental cost-effectiveness ratio.
Sensitivity analysis
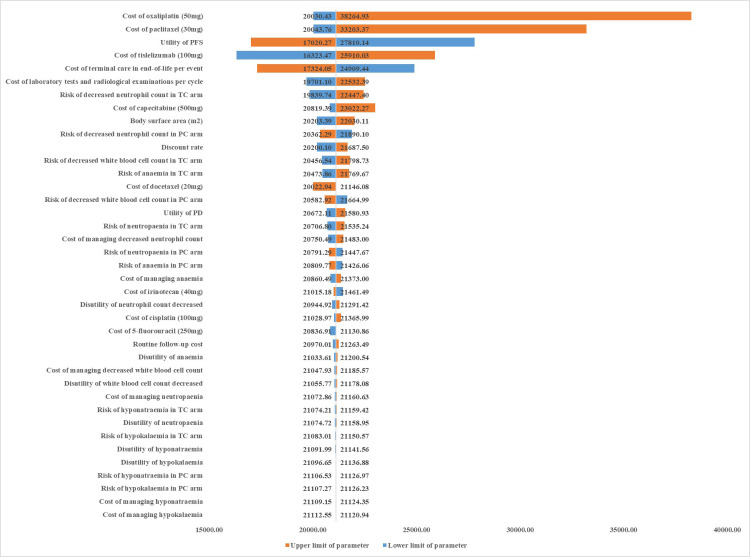
The impact of variations in each of parameters on the ICER has been presented in tornado diagrams (Fig 2). The cost of oxaliplatin has the greatest impact on the ICER followed by costs of paclitaxel and tislelizumab. Among all the parameters, the price variation of oxaliplatin was the only factor that could be the reason why tislelizumab combination chemotherapy was not cost-effective, and when the price of oxaliplatin was at the upper limit ($297.87/50mg), the ICER of tislelizumab combination chemotherapy was $38,264.93/QALY, which was higher than 3 times the GDP per capita ($38,024.67/QALY).
Fig 2. Tornado diagram of one-way sensitivity analyses of tislelizumab plus chemotherapy vs chemotherapy alone.
PFS, progression-free survival; PD, progression disease.
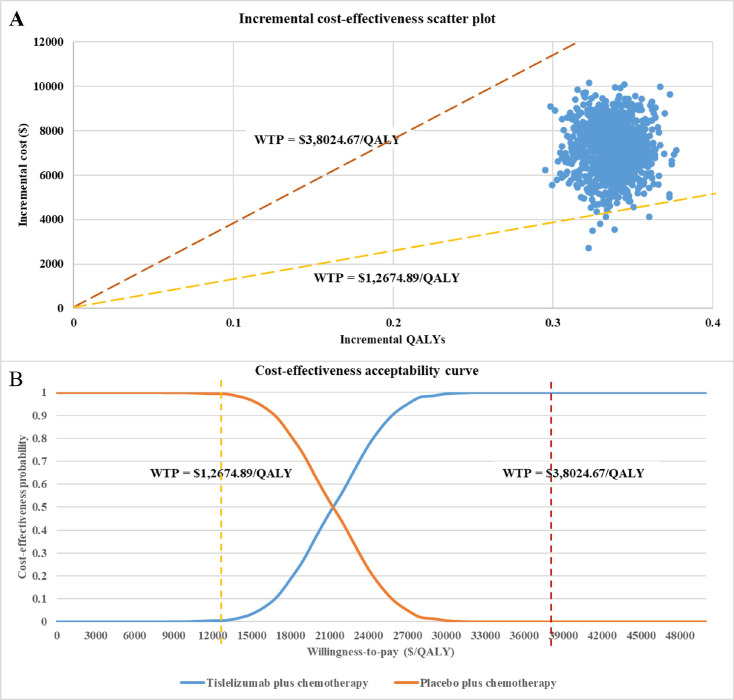
As the results of 1,000 Monte Carlo simulations shown in Fig 3A, a small fraction of the scatter points falls in the fourth quadrant, implying that compared to chemotherapy alone, tislelizumab in combination with chemotherapy is a dominant option. The remaining scatter points of ICER which fall in the first quadrant were all distributed below the line of the WTP threshold which was set as 3 times Chinese GDP per capita ($38,024.67/QALY), but only a tiny fraction of them distributed below the willing-to-pay threshold line of 1 time Chinese GDP per capita ($12,674.89 per QALY). According to the results of the CEAC shown in Fig 3B, the probability of tislelizumab plus chemotherapy treatment being cost-effective as the first-line treatment for advanced or metastatic OSCC in China was 1% and 100%, when the WTP threshold was set as 1 or 3 times GDP per capita, respectively.
Fig 3. Probabilistic sensitivity analysis.
(A) Incremental cost-effectiveness scatter plot; (B) Cost-effectiveness acceptability curve.
Discussion
The cost-effectiveness of tislelizumab as a first-line treatment for patients with advanced or metastatic OSCC was assessed from the perspective of the Chinese healthcare system in this study. The results showed that the ICER of tislelizumab chemotherapy plus chemotherapy compared with placebo plus chemotherapy was $21,116.75/QALY, which was between 1 time ($12,510.66/QALY) and 3 times GDP ($38,024.67/QALY) per capita. Our study demonstrated that tislelizumab and chemotherapy combination may be a cost-effective treatment strategy considering the current state of Chinese economy. The results provide data for decision-making in treatment of patients with OSCC, and serve as a reference for medical insurance access.
The CSCO guideline recommend seven PD-1 inhibitors for the first-line treatment of OSCC. The economics of PD-1 inhibitors for OSCC treatment are also receiving increasing attention. Several cost-effective results of PD-1 inhibitors combined with chemotherapy in OSCC treatment have been published [8,23,31–40]. A cost-effectiveness analysis was conducted to compare different PD-1 inhibitors in combination with chemotherapy for the first-line treatment of advanced OSCC in China [8]. Moreover, two studies investigated the cost-effectiveness of tislelizumab in the first-line treatment of oesophageal squamous carcinoma, which were similar to the design of our study [41,42]. Lu S. et al.’ study [42] used the Markov model, while our study used the partitioned survival model. The study of Zhou C. et al. [41] used a partitioned survival model, but there were some limitations in the methodology. Firstly, the chemotherapy regimen of the first-line treatment combined with tislelizumab only included cisplatin plus paclitaxel, which was inconsistent with the design of the RATIONALE-306 clinical trial. In the clinical trial, the chemotherapy regimen for the first-line treatment was platinum (cisplatin/oxaliplatin) plus fluoropyrimidine (5-fluorouracil/capecitabine) or paclitaxel [18]. It is necessary to include oxaliplatin in model simulation since oxaliplatin is preferred over cisplatin due to its lower toxicity according to the NCCN Guideline [10]. Furthermore, fluoropyrimidine also occupied a crucial position in the clinical practice in treating oesophageal cancer patients in China. Our study addressed this issue by selecting the initial treatment regimen based on the clinical trial by including oxaliplatin and fluorouracil. Secondly, in Zhou C. et al’ study, the subsequent treatment regimen assumed cisplatin plus paclitaxel, which is not recommended as a second-line treatment in either the CSCO or the NCCN guidelines [10,19]. Our study optimised the assumption by selecting a second-line regimen based on the recommendations of both guidelines, ultimately choosing a single-agent chemotherapy (e.g. docetaxel, paclitaxel or irinotecan).
In the one-way sensitivity analysis, the prices of oxaliplatin, paclitaxel, and tislelizumab were identified as the top three parameters that affect the results of the partitioned survival model. The great impact of the prices of oxaliplatin and paclitaxel on the results might due to the wide range of prices between generic and original drugs. The low price for tislelizumab resulted in the tislelizumab combination strategy being cost-effective and advantageous in treatment of patients with OSCC. During the Chinese national drug pricing negotiations in 2021 and 2022, the price of tislelizumab was reduced by 80% and 33%, respectively. Chinese national drug pricing negotiations is an effective way to lower the drug prices and make the innovative drug more cost-effective. The drugs negotiated during the 2023 agreement period benefited over 210 million patients and result in a reduction of over 200 billion yuan in financial burden for patients. This study also confirms the importance of China’s national drug pricing negotiation policy, which is significant in enhancing drug supply and reducing the medical burden, the further reduction of the price of tislelizumab is a major measure to improve its economy.
There are several limitations in the study. First, our cost-effectiveness analysis was based on the RATIONALE-306 clinical trial, where treatment options and costs were as close to the trial design as possible and were not as complex and variable as real-world clinical treatments. Second, since the RATIONALE-306 trial did not publish data for quality of life, the utilities in this analysis were derived from previously published literature, which may not reflect the true health status of the patients treated. Third, the fitting extrapolation method based on the parameter distribution of the survival curves used in the study tends to ignored the short observation period of clinical study and failed to identify non-disease-related deaths, which would make the extrapolated survival curve inconsistent with reality.
Conclusions
From the perspective of the Chinese healthcare system, tislelizumab plus chemotherapy was probably cost-effective compared with chemotherapy alone as the first-line treatment for advanced or metastatic OSCC in China. Our results could provide new data for decision-making in treatment of patients with OSCC and bring more confidence to the implementation of drug price negotiation policy in China. However, real-world studies are still necessary to validate the efficacy, safety and economics of tislelizumab compared with other alternative strategies.
Supporting information
(A) Original overall survival curves from the RATIONALE-306 trial. (B) Reconstructed overall survival curves. (C) Original progression free survival curves from the RATIONALE-306 trial. (D) Reconstructed progression free survival curves.
(TIF)
(TIF)
(TIF)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Projects of Medical and Health Technology Program in Zhejiang Province (2022KY090), Zhejiang Pharmaceutical Society's Clinical Comprehensive Evaluation of Drugs Special Research Funding Project (2022ZYYL11), Project of Health Economics and Health Technology Assessment from Zhejiang Pharmaceutical Association (2022ZYJ18), Medical Health Science and Technology Project of Zhejiang province (2023KY608). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. All authors have read and approved the revised manuscript.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. Epub 2021/02/05. doi: 10.3322/caac.21660 . [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69(9):1564–71. Epub 2020/07/02. doi: 10.1136/gutjnl-2020-321600 . [DOI] [PubMed] [Google Scholar]
- 3.Chen R, Zheng R, Zhang S, Wang S, Sun K, Zeng H, et al. Patterns and trends in esophageal cancer incidence and mortality in China: An analysis based on cancer registry data. Journal of the National Cancer Center. 2023;3(1):21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond). 2021;41(10):1037–48. Epub 2021/07/22. doi: 10.1002/cac2.12197 ; PubMed Central PMCID: PMC8504144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–58. Epub 2019/06/30. doi: 10.1016/S0140-6736(19)30427-1 ; PubMed Central PMCID: PMC6891889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu Y, Wang W, Wang F. Clinical benefits of PD-1 inhibitors in specific subgroups of patients with advanced esophageal squamous cell carcinoma: a systematic review and meta-analysis of phase 3 randomized clinical trials. Front Immunol. 2023;14:1171671. Epub 2023/05/19. doi: 10.3389/fimmu.2023.1171671 ; PubMed Central PMCID: PMC10185849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah MA, Kennedy EB, Alarcon-Rozas AE, Alcindor T, Bartley AN, Malowany AB, et al. Immunotherapy and Targeted Therapy for Advanced Gastroesophageal Cancer: ASCO Guideline. J Clin Oncol. 2023;41(7):1470–91. Epub 2023/01/06. doi: 10.1200/JCO.22.02331 . [DOI] [PubMed] [Google Scholar]
- 8.Liu S, Dou L, Li S. Cost-effectiveness analysis of PD-1 inhibitors combined with chemotherapy as first-line therapy for advanced esophageal squamous-cell carcinoma in China. Front Pharmacol. 2023;14:1055727. Epub 2023/03/21. doi: 10.3389/fphar.2023.1055727 ; PubMed Central PMCID: PMC10017726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obermannova R, Alsina M, Cervantes A, Leong T, Lordick F, Nilsson M, et al. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):992–1004. Epub 2022/08/02. doi: 10.1016/j.annonc.2022.07.003 . [DOI] [PubMed] [Google Scholar]
- 10.Ajani JA, D’Amico TA, Bentrem DJ, Cooke D, Corvera C, Das P, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21(4):393–422. Epub 2023/04/05. doi: 10.6004/jnccn.2023.0019 . [DOI] [PubMed] [Google Scholar]
- 11.Muro K, Lordick F, Tsushima T, Pentheroudakis G, Baba E, Lu Z, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic oesophageal cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30(1):34–43. Epub 2018/11/27. doi: 10.1093/annonc/mdy498 . [DOI] [PubMed] [Google Scholar]
- 12.Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med. 2022;386(5):449–62. Epub 2022/02/03. doi: 10.1056/NEJMoa2111380 . [DOI] [PubMed] [Google Scholar]
- 13.Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ. 2022;377:e068714. Epub 2022/04/21. doi: 10.1136/bmj-2021-068714 ; PubMed Central PMCID: PMC9016493 at www.icmje.org/coi_disclosure.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, et al. Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA. 2021;326(10):916–25. Epub 2021/09/15. doi: 10.1001/jama.2021.12836 ; PubMed Central PMCID: PMC8441593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng J, et al. Toripalimab plus chemotherapy in treatment-naive, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell. 2022;40(3):277–88 e3. Epub 2022/03/05. doi: 10.1016/j.ccell.2022.02.007 . [DOI] [PubMed] [Google Scholar]
- 16.Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398(10302):759–71. Epub 2021/08/30. doi: 10.1016/S0140-6736(21)01234-4 . [DOI] [PubMed] [Google Scholar]
- 17.Song Y, Zhang B, Xin D, Kou X, Tan Z, Zhang S, et al. First-line serplulimab or placebo plus chemotherapy in PD-L1-positive esophageal squamous cell carcinoma: a randomized, double-blind phase 3 trial. Nat Med. 2023;29(2):473–82. Epub 2023/02/03. doi: 10.1038/s41591-022-02179-2 ; PubMed Central PMCID: PMC9941045 has served a consulting or advisory role for Merck Sharp & Dohme Oncology and Roche. All other authors declare no competing interests. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J, Kato K, Raymond E, Hubner RA, Shu Y, Pan Y, et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line treatment for advanced or metastatic oesophageal squamous cell carcinoma (RATIONALE-306): a global, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2023;24(5):483–95. Epub 2023/04/21. doi: 10.1016/S1470-2045(23)00108-0 . [DOI] [PubMed] [Google Scholar]
- 19.Chinese Society of Clinical Oncology (CSCO). CSCO guidelines: esophageal cancer. Beijing: People’s Medical Publishing House. 2023.
- 20.Su D, Wu B, Shi L. Cost-effectiveness of Atezolizumab Plus Bevacizumab vs Sorafenib as First-Line Treatment of Unresectable Hepatocellular Carcinoma. JAMA Netw Open. 2021;4(2):e210037. Epub 2021/02/25. doi: 10.1001/jamanetworkopen.2021.0037 ; PubMed Central PMCID: PMC7905498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.G. L, S. H, J. W, J. W. China guidelines for pharmacoeconomic evaluations (2020). Beijing: China Market Press. 2020.
- 22.Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93(2):118–24. Epub 2015/04/18. doi: 10.2471/BLT.14.138206 ; PubMed Central PMCID: PMC4339959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Wang L, Chen L, Ding Y, Zhang Q, Shu Y. Cost-effectiveness of sintilimab plus chemotherapy versus chemotherapy alone as first-line treatment of locally advanced or metastatic oesophageal squamous cell carcinoma. Front Immunol. 2023;14:1092385. Epub 2023/02/10. doi: 10.3389/fimmu.2023.1092385 ; PubMed Central PMCID: PMC9899904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai Y, Xu Y, Wu B. Cost-Effectiveness and Budget Impact Analysis of Apatinib for Advanced Metastatic Gastric Cancer from the Perspective of Health Insurance System. Gastroenterol Res Pract. 2017;2017:2816737. Epub 20171126. doi: 10.1155/2017/2816737 ; PubMed Central PMCID: PMC5727640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu B, Dong B, Xu Y, Zhang Q, Shen J, Chen H, Xue W. Economic evaluation of first-line treatments for metastatic renal cell carcinoma: a cost-effectiveness analysis in a health resource-limited setting. PLoS One. 2012;7(3):e32530. Epub 20120308. doi: 10.1371/journal.pone.0032530 ; PubMed Central PMCID: PMC3297611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q, Wu P, He X, Ding Y, Shu Y. Cost-Effectiveness Analysis of Camrelizumab vs. Placebo Added to Chemotherapy as First-Line Therapy for Advanced or Metastatic Esophageal Squamous Cell Carcinoma in China. Front Oncol. 2021;11:790373. Epub 20211201. doi: 10.3389/fonc.2021.790373 ; PubMed Central PMCID: PMC8671697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu B, Li T, Cai J, Xu Y, Zhao G. Cost-effectiveness analysis of adjuvant chemotherapies in patients presenting with gastric cancer after D2 gastrectomy. BMC Cancer. 2014;14:984. Epub 20141219. doi: 10.1186/1471-2407-14-984 ; PubMed Central PMCID: PMC4301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non-small cell lung cancer: An international study. Asia Pac J Clin Oncol. 2017;13(5):e195–e203. Epub 2016/03/19. doi: 10.1111/ajco.12477 . [DOI] [PubMed] [Google Scholar]
- 29.Zheng Z, Lin J, Zhu H, Cai H. Cost-Effectiveness Analysis of Pembrolizumab Plus Chemotherapy vs. Chemotherapy Alone as First-Line Treatment in Patients With Esophageal Squamous Cell Carcinoma and PD-L1 CPS of 10 or More. Front Public Health. 2022;10:893387. Epub 2022/07/02. doi: 10.3389/fpubh.2022.893387 ; PubMed Central PMCID: PMC9237361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yue X, Li Y, Wu J, Guo JJ. Current Development and Practice of Pharmacoeconomic Evaluation Guidelines for Universal Health Coverage in China. Value Health Reg Issues. 2021;24:1–5. Epub 2020/12/23. doi: 10.1016/j.vhri.2020.07.580 . [DOI] [PubMed] [Google Scholar]
- 31.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD, Force I-SMGRPT. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making. 2012;32(5):722–32. Epub 2012/09/20. doi: 10.1177/0272989X12458348 . [DOI] [PubMed] [Google Scholar]
- 32.Shi F, He Z, Su H, Wang L, Han S. Economic evaluation of tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma in China. Front Pharmacol. 2022;13:961347. Epub 2022/12/06. doi: 10.3389/fphar.2022.961347 ; PubMed Central PMCID: PMC9708733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu J, Ye Z, Xu Z, Hao Z, Wang Y. Cost-effectiveness analysis of pembrolizumab vs. chemotherapy as second-line treatment for advanced esophageal carcinoma in the United States. Front Public Health. 2022;10:941738. Epub 2022/12/27. doi: 10.3389/fpubh.2022.941738 ; PubMed Central PMCID: PMC9780281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin YT, Liu TX, Chen J, Wang C, Chen Y. Cost-Effectiveness of Nivolumab Immunotherapy vs. Paclitaxel or Docetaxel Chemotherapy as Second-Line Therapy in Advanced Esophageal Squamous Cell Carcinoma in China. Front Public Health. 2022;10:923619. Epub 2022/07/19. doi: 10.3389/fpubh.2022.923619 ; PubMed Central PMCID: PMC9277084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhan M, Xu T, Zheng H, He Z. Cost-Effectiveness Analysis of Pembrolizumab in Patients With Advanced Esophageal Cancer Based on the KEYNOTE-181 Study. Front Public Health. 2022;10:790225. Epub 2022/03/22. doi: 10.3389/fpubh.2022.790225 ; PubMed Central PMCID: PMC8924414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu S, Jiang N, Dou L, Li S. Cost-effectiveness analysis of serplulimab plus chemotherapy in the first-line treatment for PD-L1-positive esophageal squamous cell carcinoma in China. Front Immunol. 2023;14:1172242. Epub 2023/05/22. doi: 10.3389/fimmu.2023.1172242 ; PubMed Central PMCID: PMC10192749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gong J, Shang J, Su D, Qian X, Liu G, Sun Z. Cost-effectiveness of camrelizumab plus chemotherapy versus chemotherapy alone as first-line therapy in advanced or metastatic esophageal squamous cell carcinoma. Expert Rev Pharmacoecon Outcomes Res. 2023;23(6):709–17. Epub 2023/05/16. doi: 10.1080/14737167.2023.2214732 . [DOI] [PubMed] [Google Scholar]
- 38.Zheng Z, Fang L, Cai H, Zhu H. Cost-effectiveness analysis of serplulimab as first-line treatment for advanced esophageal squamous cell carcinoma. Immunotherapy. 2023;15(13):1045–55. Epub 2023/07/04. doi: 10.2217/imt-2023-0059 . [DOI] [PubMed] [Google Scholar]
- 39.Zheng Z, Fang L, Cai H, Zhu H. Economic evaluation of toripalimab plus chemotherapy compared with chemotherapy as first-line treatment for advanced esophageal squamous cell carcinoma in China. Expert Rev Pharmacoecon Outcomes Res. 2023;23(6):683–90. Epub 2023/04/22. doi: 10.1080/14737167.2023.2206570 . [DOI] [PubMed] [Google Scholar]
- 40.Xu K, Wu H, Zhou C, Bao Y, Yu M, Zhang L, Li X. Cost-effectiveness of toripalimab plus chemotherapy for advanced esophageal squamous cell carcinoma. Int J Clin Pharm. 2023;45(3):641–9. Epub 2023/02/18. doi: 10.1007/s11096-023-01540-w . [DOI] [PubMed] [Google Scholar]
- 41.Zhou C, Wei J, Xu K, Lin Y, Zhang L, Li X. Cost-Effectiveness Analysis of Tislelizumab Plus Chemotherapy as First-Line Treatment for Advanced or Metastatic Esophageal Squamous Cell Carcinoma in China. Risk Manag Healthc Policy. 2023;16:2447–58. Epub 20231115. doi: 10.2147/RMHP.S436750 ; PubMed Central PMCID: PMC10657759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu S, Lou Y, Rong Y, Huang Z, Lin X, Chen J, Luo K. Tislelizumab Plus Chemotherapy Versus Placebo Plus Chemotherapy as First-Line Treatment for Chinese Patients with Advanced Esophageal Squamous Cell Carcinoma: A Cost-Effectiveness Analysis. Clin Drug Investig. 2023;43(8):643–52. Epub 20230805. doi: 10.1007/s40261-023-01295-2 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Original overall survival curves from the RATIONALE-306 trial. (B) Reconstructed overall survival curves. (C) Original progression free survival curves from the RATIONALE-306 trial. (D) Reconstructed progression free survival curves.
(TIF)
(TIF)
(TIF)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.