Abstract
AIM:
The “2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation” provides recommendations to guide clinicians in the treatment of patients with atrial fibrillation.
METHODS:
A comprehensive literature search was conducted from May 12, 2022, to November 3, 2022, encompassing studies, reviews, and other evidence conducted on human subjects that were published in English from PubMed, EMBASE, the Cochrane Library, the Agency for Healthcare Research and Quality, and other selected databases relevant to this guideline. Additional relevant studies, published through November 2022, during the guideline writing process, were also considered by the writing committee and added to the evidence tables, where appropriate.
STRUCTURE:
Atrial fibrillation is the most sustained common arrhythmia, and its incidence and prevalence are increasing in the United States and globally. Recommendations from the “2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation” and the “2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation” have been updated with new evidence to guide clinicians. In addition, new recommendations addressing atrial fibrillation and thromboembolic risk assessment, anticoagulation, left atrial appendage occlusion, atrial fibrillation catheter or surgical ablation, and risk factor modification and atrial fibrillation prevention have been developed.
Keywords: AHA Scientific Statements, acute coronary syndrome, alcohol, anticoagulants, anticoagulation agents, antiplatelet agents, apixaban, atrial fibrillation, atrial flutter, cardioversion, catheter ablation, coronary artery disease, coronary heart disease, dabigatran, edoxaban, exercise, heart failure, hypertension, idarucizumab, left atrial appendage occlusion, myocardial infarction, obesity, percutaneous coronary intervention, pulmonary vein isolation, risk factors, rivaroxaban, sleep apnea, stents, stroke, surgical ablation, thromboembolism, warfarin
PREAMBLE
Since 1980, the American College of Cardiology (ACC) and American Heart Association (AHA) have translated scientific evidence into clinical practice guidelines with recommendations to improve cardiovascular health. These guidelines, which are based on systematic methods to evaluate and classify evidence, provide a foundation for the delivery of quality cardiovascular care. The ACC and AHA sponsor the development and publication of clinical practice guidelines without commercial support, and members volunteer their time to the writing and review efforts. Guidelines are the official policy of the ACC and AHA. For some guidelines, the ACC and AHA collaborate with other organizations.
Intended Use
Clinical practice guidelines provide recommendations applicable to patients with or at risk of developing cardiovascular disease. The focus is on medical practice in the United States, but these guidelines are relevant to patients throughout the world. Although guidelines may be used to inform regulatory or payer decisions, the intent is to improve quality of care and align with patients’ interests. Guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances and should not replace clinical judgment.
Clinical Implementation
Management, in accordance with guideline recommendations, is effective only when followed by both practitioners and patients. Adherence to recommendations can be enhanced by shared decision-making between clinicians and patients, with patient engagement in selecting interventions on the basis of individual values, preferences, and associated conditions and comorbidities.
Methodology and Modernization
The ACC/AHA Joint Committee on Clinical Practice Guidelines (Joint Committee) continuously reviews, updates, and modifies guideline methodology on the basis of published standards from organizations, including the Institute of Medicine,1,2 and on the basis of internal reevaluation. Similarly, presentation and delivery of guidelines are reevaluated and modified in response to evolving technologies and other factors to optimally facilitate dissemination of information to health care professionals at the point of care.
Numerous modifications to the guidelines have been implemented to make them shorter and enhance “user friendliness.” Guidelines are written and presented in a modular, “knowledge chunk” format, in which each chunk includes a table of recommendations, a brief synopsis, recommendation-specific supportive text and, when appropriate, flow diagrams or additional tables. Hyperlinked references are provided for each modular knowledge chunk to facilitate quick access and review.
In recognition of the importance of cost–value considerations, in certain guidelines, when appropriate and feasible, an analysis of value for a drug, device, or intervention may be performed in accordance with the ACC/AHA methodology.3
To ensure that guideline recommendations remain current, new data will be reviewed on an ongoing basis by the writing committee and staff. Going forward, targeted sections/knowledge chunks will be revised dynamically after publication and timely peer review of potentially practice-changing science. The previous designations of “full revision” and “focused update” will be phased out. For additional information and policies on guideline development, readers may consult the ACC/AHA guideline methodology manual4 and other methodology articles.5–7
Selection of Writing Committee Members
The Joint Committee strives to ensure that the guideline writing committee contains requisite content expertise and is representative of the broader cardiovascular community by selection of experts across a spectrum of backgrounds, representing different geographic regions, sexes, races, ethnicities, intellectual perspectives/biases, and clinical practice settings. Organizations and professional societies with related interests and expertise are invited to participate as partners or collaborators.
Relationships With Industry and Other Entities
The ACC and AHA have rigorous policies and methods to ensure that documents are developed without bias or improper influence. The complete policy on relationships with industry and other entities (RWI) can be found online. Appendix 1 of the guideline lists writing committee members’ comprehensive and relevant RWI; for the purposes of full transparency, comprehensive and relevant disclosure information for the Joint Committee is also available online.
Evidence Review and Evidence Review Committees
In developing recommendations, the writing committee uses evidence-based methodologies that are based on all available data.4,5 Literature searches focus on randomized controlled trials (RCTs) but also include registries, nonrandomized comparative and descriptive studies, case series, cohort studies, systematic reviews, and expert opinion. Only key references are cited.
An independent evidence review committee is commissioned when there are ≥1 questions deemed of utmost clinical importance and merit formal systematic review to determine which patients are most likely to benefit from a drug, device, or treatment strategy, and to what degree. Criteria for commissioning an evidence review committee and formal systematic review include absence of a current authoritative systematic review, feasibility of defining the benefit and risk in a time frame consistent with the writing of a guideline, relevance to a substantial number of patients, and likelihood that the findings can be translated into actionable recommendations. Evidence review committee members may include methodologists, epidemiologists, clinicians, and biostatisticians. Recommendations developed by the writing committee on the basis of the systematic review are marked “SR”.
Guideline-Directed Management and Therapy
The term guideline-directed management and therapy (GDMT) encompasses clinical evaluation, diagnostic testing, and both pharmacological and procedural treatments. For these and all recommended drug treatment regimens, the reader should confirm dosage with product insert material and evaluate for contraindications and interactions. Recommendations are limited to drugs, devices, and treatments approved for clinical use in the United States.
Joshua A. Beckman, MD, MS, FAHA, FACC Chair, ACC/AHA Joint Committee on Clinical Practice Guidelines
1. INTRODUCTION
1.1. Methodology and Evidence Review
The recommendations listed in this guideline are, whenever possible, evidence based. An initial extensive evidence review, which included literature derived from research involving human subjects, published in English, and indexed in MEDLINE (through PubMed), EMBASE, the Cochrane Library, the Agency for Healthcare Research and Quality, and other selected databases relevant to this guideline, was conducted from May 2022 to November 2022. Key search words included but were not limited to the following: atrial fibrillation; pregnancy; heart defects, congenital; smoking; cardiomyopathy, hypertrophic; alcohol; caffeine; sleep; diet; fitness; obesity; anticoagulants; diabetes; rhythm monitoring; heart failure; genetics; heart valve diseases; rate control; catheter ablation; social determinants of health; chronic kidney disease; sinus rhythm; chronic coronary syndromes; left atrial appendage occlusion; left atrial appendage exclusion; cardiac surgical procedures; amiodarone; electrical cardioversion; thromboembolism; rhythm control; Wolff-Parkinson-White syndrome. Additional relevant studies, published through November 2022 during the guideline writing process, were also considered by the writing committee and added to the evidence tables when appropriate. The final evidence tables are included in the Online Data Supplement and summarize the evidence used by the writing committee to formulate recommendations. References selected and published in the present document are representative and not all-inclusive.
1.2. Organization of the Writing Committee
The writing committee consisted of cardiologists, cardiac electrophysiologists, surgeons, pharmacists, and patient representatives/lay stakeholders. The writing committee included representatives from the ACC and AHA, ACCP, and HRS. Appendix 1 of the current document lists writing committee members’ comprehensive and relevant RWI.
1.3. Document Review and Approval
The Joint Committee appointed a peer review committee to review the document. The peer review committee comprised individuals nominated by the ACC, AHA, and the collaborating organizations. Reviewers’ RWI information was distributed to the writing committee and is published in this document (Appendix 2).
This document was approved for publication by the governing bodies of the ACC and the AHA and was endorsed by ACCP and HRS.
1.4. Scope of the Guideline
In developing the “2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation” (2023 atrial fibrillation guideline), the writing committee reviewed previously published guidelines. Table 1 contains a list of these publications deemed pertinent to this writing effort and is intended for use as a resource, thus obviating the need to repeat existing guideline recommendations.
Table 1.
Associated ACC/AHA Guidelines
| Title | Organization | Publication Year (Reference) |
|---|---|---|
| Guidelines | ||
| Atrial fibrillation | AHA/ACC/HRS | 20191
* 20142 |
| Atrial fibrillation | ESC/EACTS/EHRA | 20213 |
| Atrial fibrillation | CCS | 20204 |
| Management of adults with congenital heart disease | AHA/ACC | 20185 |
| Diagnosis and treatment of patients with hypertrophic cardiomyopathy | AHA/ACC | 20206 |
| Management of patients with valvular heart disease | ACC/AHA | 20217 |
| Coronary artery revascularization | ACC/AHA/AATS/STS/SCAI | 20218 |
| Evaluation and diagnosis of chest pain | AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR | 20219 |
| Prevention of stroke in patients with stroke and transient ischemic attack | AHA/ASA | 202110 |
| Management of heart failure | AHA/ACC/HFSA | 202211 |
| Management of arrhythmias in pregnancy | HRS | 202312 |
AATS indicates American Association for Thoracic Surgery; ACC, American College of Cardiology; AF, atrial fibrillation; AHA, American Heart Association; ASA, American Stroke Association; ASE, American Society of Echocardiography; CCS, Canadian Cardiovascular Society; CHEST, American College of Chest Physicians; EACTS, European Association for Cardio Thoracic Surgery; EHRA, European Heart Rhythm Association; ESC, European Society of Cardiology; HFSA, Heart Failure Society of America; HRS, Heart Rhythm Society; SAEM, Society for Academic Emergency Medicine; SCAI, Society for Cardiovascular Angiography and Interventions; SCCT, Society of Cardiovascular Computed Tomography; SCMR, Society for Cardiovascular Magnetic Resonance; and STS, Society of Thoracic Surgery.
1.5. Class of Recommendations and Level of Evidence
The Class of Recommendation (COR) indicates the strength of recommendation and encompasses the estimated magnitude and certainty of benefit in proportion to risk. The Level of Evidence (LOE) rates the quality of scientific evidence supporting the intervention on the basis of the type, quantity, and consistency of data from clinical trials and other sources (Table 2).
Table 2.
Applying American College of Cardiology/American Heart Association Class of Recommendation and Level of Evidence to Clinical Strategies, Interventions, Treatments, or Diagnostic Testing in Patient Care* (Updated May 2019)
| CLASS (STRENGTH) OF RECOMMENDATION | |
| CLASS 1 (STRONG) | Benefit >>> Risk |
|
Suggested phrases for writing recommendations: • Is recommended • Is indicated/useful/effective/beneficial • Should be performed/administered/other • Comparative-Effectiveness Phrases†: – Treatment/strategy A is recommended/indicated in preference to treatment B – Treatment A should be chosen over treatment B | |
| CLASS 2a (MODERATE) | Benefit >> Risk |
|
Suggested phrases for writing recommendations: • Is reasonable • Can be useful/effective/beneficial • Comparative-Effectiveness Phrases†: – Treatment/strategy A is probably recommended/indicated in preference to treatment B – It is reasonable to choose treatment A over treatment B | |
| CLASS 2b (WEAK) | Benefit ≥ Risk |
|
Suggested phrases for writing recommendations: • May/might be reasonable • May/might be considered • Usefulness/effectiveness is unknown/unclear/uncertain or not well-established | |
| CLASS 3: No Benefit (MODERATE) (Generally, LOE A or B use only) | Benefit = Risk |
|
Suggested phrases for writing recommendations: • Is not recommended • Is not indicated/useful/effective/beneficial • Should not be performed/administered/other | |
| Class 3: Harm (STRONG) | Risk > Benefit |
|
Suggested phrases for writing recommendations: • Potentially harmful • Causes harm • Associated with excess morbidity/mortallty • Should not be performed/administered/other | |
| LEVEL (QUALITY) OF EVIDENCE ‡ | |
| LEVEL A | |
| • High-quality evidence‡ from more than 1 RCT • Meta-analyses of high-quality RCTs • One or more RCTs corroborated by high-quality registry studies | |
| LEVEL B-R | (Randomized) |
| • Moderate-quality evidence‡ from 1 or more RCTs • Meta-analyses of moderate-quality RCTs | |
| LEVEL B-NR | (Nonrandomized) |
| • Moderate-quality evidence‡ from 1 or more well-designed, well-executed nonrandomized studies, observational studies, or registry studies • Meta-analyses of such studies | |
| LEVEL C-LD | (Limited Data) |
| • Randomized or nonrandomized observational or registry studies with limitations of design or execution • Meta-analyses of such studies • Physiological or mechanistic studies In human subjects | |
| LEVEL C EO | (Expert Opinion) |
| • Consensus of expert opinion based on clinical experience | |
COR and LOE are determined Independently (any COR may be paired with any LOE).
A recommendation with LOE C does not imply that the recommendation is weak, Many important clinical questions addressed in guidelines do not lend themselves to clinical trials. Although RCTs are unavailable, there may be a very clear clinical consensus that a particular test or therapy is useful or effective
The outcome or result of the intervention should he specified (an improved clinical outcome or increased diagnostic accuracy or incremental prognostic information).
For comparative-effectiveness recommendatiens (COR 1 and 2a; LOE A and B only), studies that support the use of comparator verbs should involve direct comparisons of the treatments or strategies being evaluated.
The method of assessing quality is evolving, including the application of standardized, widely-used, and preferably validated evidence grading tools, and for systematic reviews, the incorporation of an Evidence Review Committee.
COR indicates Class of Recommendation; EO, expert opinion; LD, limited data; LOE, Level of Evidence; NR, nonrandomized; R, randomized; and RCT, randomized controlled trial.
2. BACKGROUND AND PATHOPHYSIOLOGY
2.1. Epidemiology
Atrial fibrillation (AF) is the most sustained common arrhythmia, and its incidence and prevalence are increasing in the United States and globally (Figures 1 to 3).1,2 The increasing burden is multifactorial; causes include the aging of the population, rising tide of obesity, increasing detection, and increasing survival with AF and other forms of cardiovascular disease (CVD). The estimated global prevalence was 50 million in 2020.2,3 Although the prevalence of undiagnosed AF in the community is unknown, using back-calculation methodology, investigators have estimated that, in 2015, about 11% (591 000 cases) of the >5.6 million AF cases in the United States were undiagnosed.4
Figure 1. Temporal Trends in Counts and Age-Standardized Rates of AF-Prevalent Cases by Social Demographic Index Quintile for Both Sexes Combined, 1990–2017.
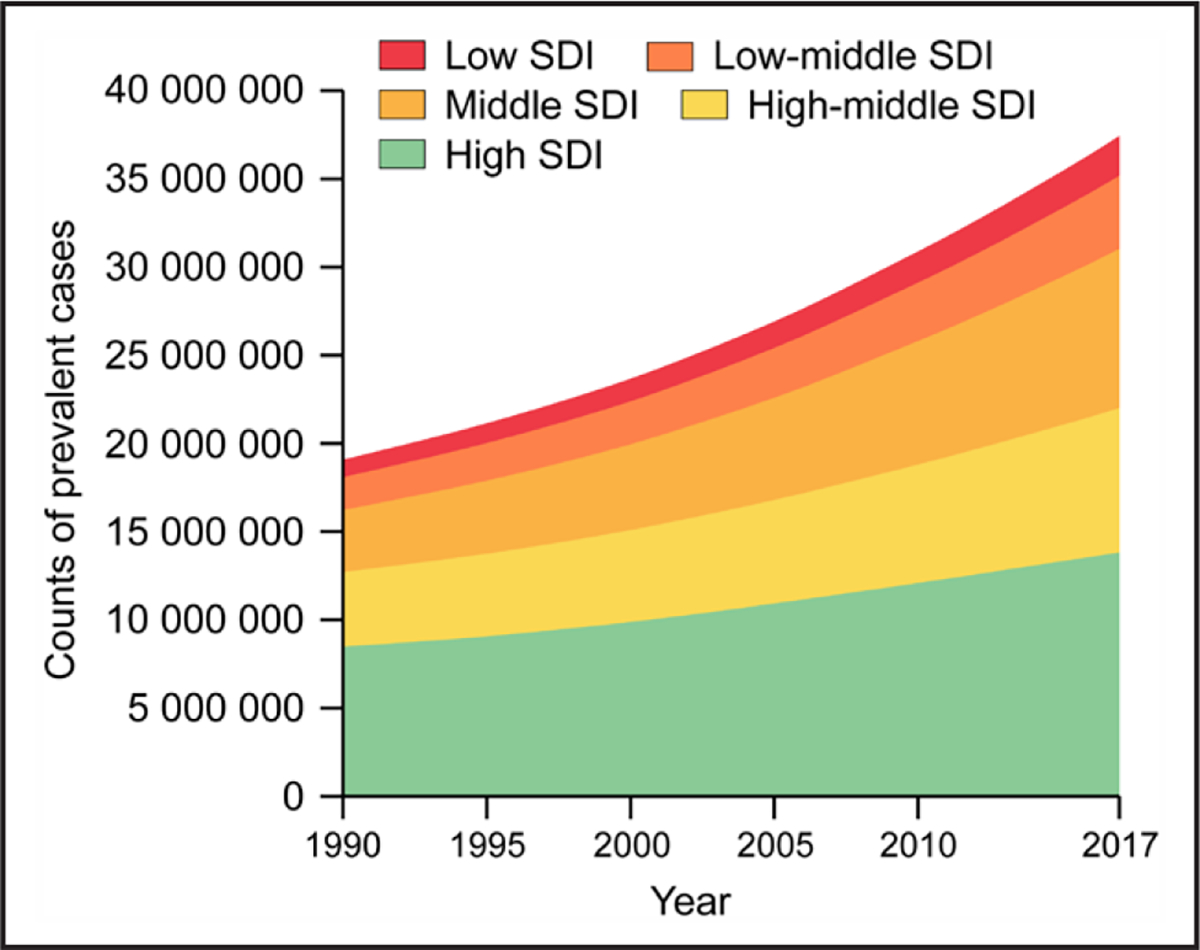
Trends in counts of AF-prevalent cases by SDI quintile, 1990–2017. SDI was made up of the geometric mean of 3 common indicators: the lag distributed income per capita, mean educational achievement for those aged ≥15 y, and total fertility rate <25 y. SDI ranged from 0 to 1, where 0 represents the theoretical minimum level of development, whereas 1 represents the theoretical maximum level of development. Modified from Dai et al7 by permission of Oxford University Press on behalf of the European Society of Cardiology. Copyright 2020 Oxford University Press. AF indicates atrial fibrillation; and SDI, Social Demographic Index.
Figure 3. Age-Standardized Global Prevalence Rates of AF and Atrial Flutter per 100 000, Both Sexes, 2020.
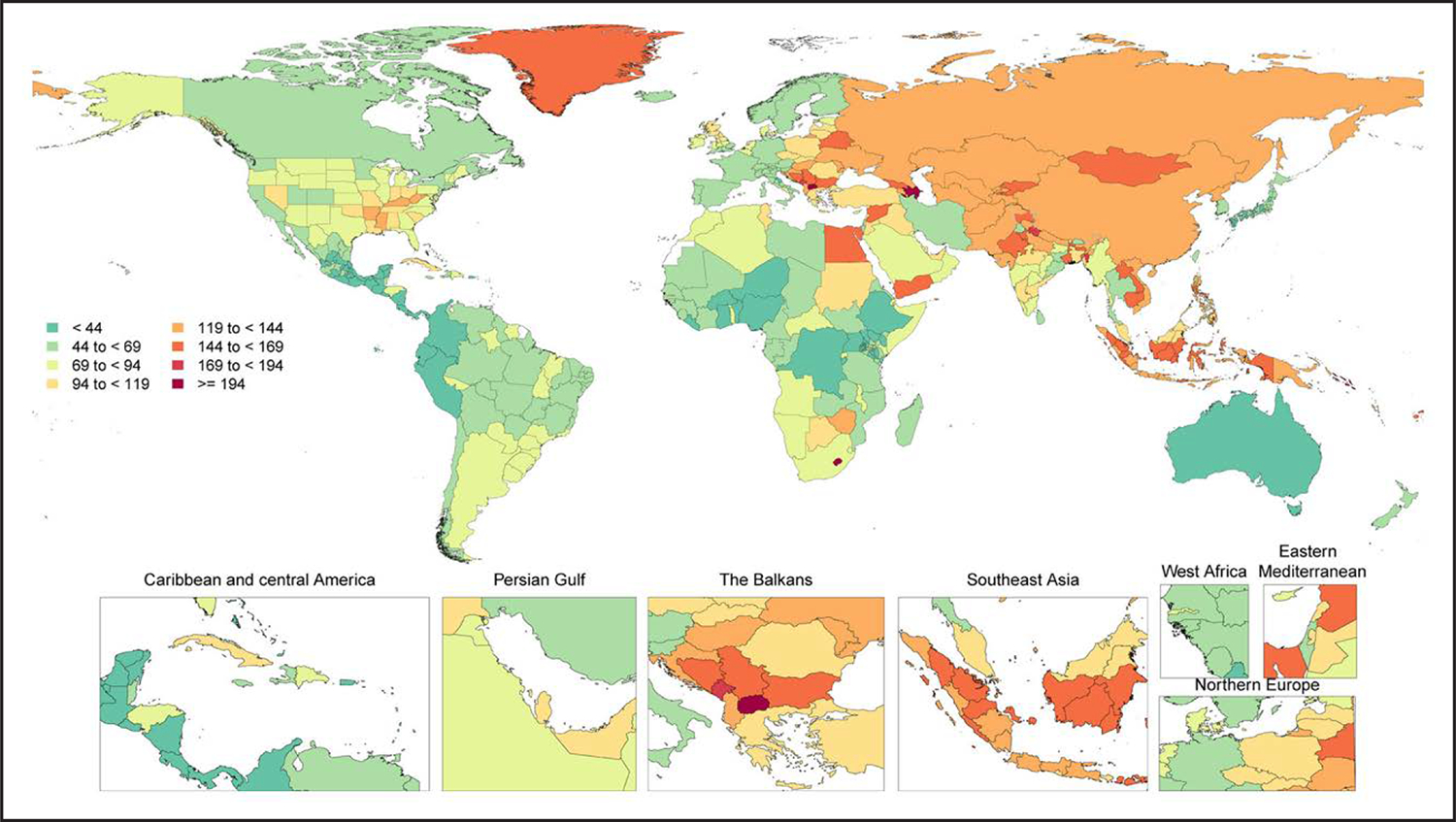
During each annual GBD Study cycle, population health estimates are produced for the full-time series. Improvements in statistical and geospatial modeling methods and the addition of new data sources may lead to changes in past results across GBD Study cycles.
Modified with permission from Tsao et al.2 Copyright 2023 American Heart Association, Inc. Source: Institute for Health Metrics Evaluation. Used with permission. All rights reserved. AF indicates atrial fibrillation; and GBD, Global Burden of Disease.
AF is associated with higher health care utilization and costs.2 Using US data from Optum (an administrative claims database for commercially insured [United Healthcare] patients in the United States), compared with patients without AF, patients with incident AF had an increased risk of inpatient visits and more cardiovascular-related emergency department visits (relative risk [RR], 2.41 [95% CI, 2.35–2.47]).5 AF is costly. Examining Optum data, individuals with AF have annual health care costs of $63 031, which is $27 896 more than individuals without AF.5 Investigators examining public and private health insurer data estimated that in US dollars in 2016, AF accounted for $28.4 billion (95% CI, $24.6 billion–$33.8 billion) in health care spending.6
2.1.1. Prevalence, Incidence, Morbidity, and Mortality
AF prevalence in the United States was estimated to be 5.2 million in 2010, with an expectation to rise to 12.1 million in 2030.1 Corresponding estimates for US incidence was 1.2 million cases in 2010, with an expectation to rise to 2.6 million cases in 2030.1 The rate of AF diagnosis varies by education, income,2 clinical,3,4 and genetic3 factors. Overall lifetime risk is about 30% to 40% in White individuals,2–4 about 20% in African American individuals,2 and about 15% in Chinese5 individuals.
AF is associated with a 1.5- to 2-fold increased risk of death6,7; studies suggest that the mortality risk may be higher in women than in men.6 In meta-analyses, AF is also associated with increased risk of multiple adverse outcomes, including a 2.4-fold risk of stroke,7 1.5-fold risk of cognitive impairment or dementia,8 1.5-fold risk of myocardial infarction (MI),9 2-fold risk of sudden cardiac death,10 5-fold risk of heart failure (HF),7 1.6-fold risk of chronic kidney disease (CKD),7 and 1.3-fold risk of peripheral artery disease (PAD).7 In Medicare beneficiaries, the most frequent outcome in the 5 years after AF diagnosis was death (19.5% at 1 year; 48.8% at 5 years)11; the next most common diagnosis was HF (13.7%), followed by new-onset stroke (7.1%), gastrointestinal hemorrhage (5.7%), and MI (3.9%).11
2.1.2. Risk Factors and Associated Heart Disease
In Table 3, we present the evidence for the most widely reported and validated factors for AF from single studies, meta-analyses, and Mendelian randomization studies. Risk factors include demographic, anthropometric, and cardiovascular risk factors, CVD, noncardiac conditions, biomarkers (eg, electrocardiographic, imaging, circulating), and genetic markers.1 Models predicting risk of AF onset are presented in Section 4.1 (“Risk Stratification and Population Screening”). Most studies of AF risk factors and outcomes have been reported from high-income countries and in individuals of European ancestry.
Table 3.
Risk Factors for Diagnosed AF
| Condition | Study Type | Effect on Risk of AF | Summary Risk of Incident AF | Effect of LRFM |
|---|---|---|---|---|
| Risk Factors | ||||
| Advancing age | SR/MA | Age per 5 y: ↑ risk (HR, 1.43–1.66)2,3 | ↑ Risk | N/A |
| MR | Accelerated epigenetic age by MR: no association4 | |||
| Smoking | Single study | Current smoking: ↑ risk (9.8%)5 | ↑ Risk | N/A |
| SR/MA | Smoking: ↑ risk (HR, 1.21–1.43)2,6 | |||
| MR | Smoking initiation: ↑ risk (OR, 1.11)7 | |||
| Physical activity | SR/MA | Sedentary lifestyle: ↑ risk (OR, 2.47)8 Guideline-recommended physical activity: ↓ risk (HR, 0.94)9 Elite athletes vs nonathletes: ↑ risk (OR, 2.46)10 |
U curve: Sedentary lifestyle and elite/extreme exercise: ↑ risk | Exercise: ↓ AF burden, recurrence, symptoms; ↑ quality of life, functional capacity11–16 |
| Alcohol | Single studies | Risk of AF episode within 4 h of 1 drink: ↑ risk (OR, 2.02)17 Greater access to alcohol law: ↑ risk18 |
↑ Risk | Randomized abstinence: ↑ AF recurrence and burden19 N-of-1 studies of alcohol avoidance: ↓ near-term AF20 Alcohol avoidance or reduction as part of a comprehensive LRFM program: ↓ AF burden, symptoms, progression of AF21–24 |
| SR/MA | Dose response (#drinks/d): ↑ risk (RR) 1: 1.08; 2: 1.17; 3: 1.33; 4: 1.36; 5: 1.4725 | |||
| MR | Genetically predicted heavy alcohol consumption (>35 U/wk for women and >50 U/wk for men): ↑ risk (OR, 1.11)7 | |||
| Adiposity markers: weight, BMI, obesity | Single study | Obesity: population attributable fraction 12.7%–16.9%5,26 | ↑ Risk | Weight loss in overweight or obese patients with AF as part of a comprehensive LRFM program: ↓ AF symptoms, burden, recurrence, progression21–24 Bariatric surgery in class III obesity: associated with reversal of AF type, ↑ sinus rhythm postablation27–29 Weight loss in long-lasting persistent AF and obesity: ↔30 |
| SR/MA | BMI: RR, 1.28 per 5-unit ↑ in BMI31 Weight:2 HR, 1.12 per 15 kg ↑ |
|||
| MR | Obesity3 Birthweight: 1.26 per SD ↑32 Childhood BMI (OR, 1.18)32 BMI 1.31 per unit BMI33 |
|||
| Height | MA | Height per 10 cm: ↑ risk (HR, 1.28)2 | ↑ Risk | N/A |
| SR/MA | Increasing height: ↑ risk3 | |||
| MR | Increasing height: ↑ risk (OR per unit, 1.33)33 | |||
| Hypertension and BP | Single studies | Elevated BP: ↑ risk, population attributable fraction, 21.6%5 Presence of hypertension treatment: ↑ risk (HR, 1.35–1.68), incidence 9.8%–19.5%; both AF and SBP decreased over time26 |
Hypertension: ↑ risk SBP: ↑ risk DBP: ↑↓↔ risk |
Renal denervation: ↓ AF postablation34 Mineralocorticoid receptor antagonists: ↓ AF burden35 BP control postablation: ↔36 Intensive BP control to SBP <120 mm Hg in patients with hypertension at high risk for CVD: ↓ AF risk37 BP control as part of a comprehensive LRFM program: ↓ AF burden21–24,38 |
| MA | BP: SBP: ↑ risk (HR per 20 mm Hg, 1.22); DBP per 10 mm Hg ↓ risk (HR, 0.90); use of BP medications ↑ risk (HR, 1.42)2 |
|||
| SR/MA | Hypertension: ↑ risk3 | |||
| MR | SBP33,39 ↑ risk; DBP mixed results ↔↑ risk39,40; pulse pressure ↑ risk40 | |||
| Resting heart rate | SR/MA | Resting heart rate: J-shaped relationship with incident AF. Lowest risk at 68–80 bpm; <70 bpm (RR, 1.09 per 10 bpm ↓); >70 bpm (RR, per 10 bpm ↑ RR 1.06)41 | Slow heart rate: ↑↓ variable risk Higher heart rate: ↑↓ variable risk |
N/A |
| MR | Heart rate: <65 bpm slower (HR ↑ risk); heart rate per 5 bpm ↑, 0.8242 | |||
| Diabetes | Single study | Diabetes: ↑ risk, population attributable fraction 3.1%5 Diabetes: ↑ risk, population attributable fraction ↑ over time 3.2%–5.9%26 |
↑ Risk | Optimal glycemic control preablation may ↓ AF recurrence postablation43 |
| MA | Diabetes: ↑ risk (HR, 1.27 [95% CI, 1.10–1.46])2 | |||
| SR/MA | Diabetes: ↑ risk (RR, 1.28, excluding large outlying study)44 Pre-diabetes: ↑ risk (RR, 1.20)44 Blood glucose; ↑ risk (RR per 20 mg/dL ↑, 1.11)44 |
|||
| Cardiovascular disease | ||||
| HF or CAD | Single study | HF or CAD: population attributable fraction 5.4%5 | ↑ Risk | N/A |
| HF | Single studies | HF: ↑ risk but population attributable fraction ↑ d over time 7.8%–1.4%26 Bidirectional relation between AF and HF45 |
↑ Risk | N/A |
| MA | History of HF: ↑ risk (HR, 2.02)2 | |||
| MR | Genetically predicted HF: ↑ risk (OR, 1.86)46 | |||
| CAD | Single study | MI: Population attributable fraction 3.6%26 | ↑ Risk | N/A |
| MA | History of MI: HR, 1.642 | |||
| MR | Genetically predicted CAD: OR, 1.1833 | |||
| VHD | Single studies | Significant heart murmur: ↑ risk (HR, 2.38)47 Significant heart murmur (any diastolic and grade ≥3/6 systolic murmur): ↑ risk, population attributable fraction 21.9% ↓ d over time to 3.1%26 |
↑ Risk | N/A |
| MR | Genetically predicted risk of AF in individuals of European ancestry: associated with VHD with rheumatic fever (OR, 1.26) and nonrheumatic VHD (OR, 1.27)48 | |||
| Cardiac surgery | Single study | Multicenter validated risk prediction model: ↑ risk AF after CABG49 | ↑ Risk | Prophylactic amiodarone, beta blockers: ↓↔ postop AF50–54 Posterior left pericardiotomy during CABG, aortic valve, ascending aortic aneurysm surgery: ↓ postop AF55,56 |
| SR/MA | Postop AF incidence: 23.7%–25.5%56 of cardiac surgery patients57 | |||
| Other conditions | ||||
| CKD | SR/MA | CKD: ↑ risk (HR, 1.47)58 | ↑↔ Risk | N/A |
| MR | Bidirectional relation between CKD and AF59 AF causal for CKD; CKD not causal for AF60 |
|||
| Obstructive sleep apnea | SR/MA | OSA: ↑ risk (OR, 1.71), with potential dose response relation by severity61 | ↑ Risk | Observational studies of SDB treatment: ↓ AF burden62–67 Small RCTs of SDB treatment: ↔68–70 |
| MR | Genetically predicted OSA: ↑ risk (OR, 1.21)71 | |||
| Thyroid disease | SR/MA | Clinical hyperthyroidism: ↑ risk (RR, 2.35)72 | ↑ Risk | |
| MR | Hyperthyroidism: ↑ risk (OR, 1.31)73 | |||
| Sepsis | Single study | Severe sepsis: ↑ risk (OR, 6.82)74; Medicare population75 | ↑ Risk | N/A |
| SR/MA | Sepsis severity: ↑ risk76 | |||
| Markers on ECG | ||||
| PR interval | SR/MA | Prolonged PR: ↑ risk (RR, 1.45)77 | Prolonged PR: ↓ risk PR interval polygenic risk score: ↓ risk PR interval risk SNPs: variable ↑↓ risk |
N/A |
| MR | Polygenic risk score PR interval prolongation: ↓ AF risk (OR, 0.95; P=4.30×10−8) with some variants associated with ↑ and some with ↓ AF risk78 | |||
| LVH | Single study | ECG LVH: Population attributable fraction 10.4% ↓ d over time to 1.8%26 | ↑ Risk | N/A |
| SR/MA | LVH: ↑ risk (RR, 1.46)79 | |||
| Biomarkers | ||||
| Natriuretic peptides | MA | BNP: ↑ risk (HR per 1-SD ln-BNP 1.66)80 | ↑↔ Risk | N/A |
| MR | Natriuretic peptides not associated81 | |||
| Inflammatory markers | SR/MA | CRP: ↑ risk (SMD, 0.95)82 IL-6: ↑ risk (SMD, 0.89)82 TNF-α: ↑ risk (SMD, 2.20)82 |
CRP IL-6, TNF-α, DUSP13, FKBP7, Spondin-1: ↑ risk IL-6R, TNFS12: ↓ risk |
N/A |
| MR | DUSP13, FKBP7, Spondin-1 ↑ risk33 IL-6R, TNFS12 ↓ risk33 |
|||
| Lp(a) | SR/MA | Lp(a): HR, 1.03; only 39% of Lp(a) risk mediated via ASCVD83 | ↑ Risk | N/A |
| MR | Genetically predicted ↑ Lp(a): ↑ risk (HR per 23 mg/dL genetically predicted ↑ Lp(a), 1.04)83 | |||
| Imaging markers | ||||
| LA size or function | Single studies | LA anterior-posterior dimension: ↑ risk (HR per 5 mm ↑, 1.39)84 End diastolic LA volume (min): ↑ risk (HR, 1.12)85 LA emptying fraction: ↑ risk (HR, 1.03)85 |
↑ LA size, emptying fraction: ↑ risk | Surgical LA reduction in conjunction with cardiac surgery or surgical AF ablation in patients with persistent AF may ↑ rates of sinus rhythm86–89 |
| MR | Genetic susceptibility to AF (independent measure) is associated with ↑indexed LA size and ↓ LA ejection fraction (dependent measures)90 | |||
| LV wall thickness | Single study | LV posterior wall thickness: ↑ risk (HR per 4-mm ↑, 1.28)84 | ↑ Risk | N/A |
| SR/MA | LVH: ↑ risk (RR, 1.46)79 | |||
| Social determinants of health | ||||
| Education | Single studies | Higher education: ↑ lifetime risk of AF (US-based ARIC study)91 Higher education in young individuals: ↓ risk of AF diagnosis (Danish study)92 |
Variable ↑↓ risk | N/A |
| MR | AF risk related but largely mediated via BMI (57.5%), type 2 diabetes (9.8%), SBP (18.7%), and smoking (7.1%)93 | |||
| Income | Single studies | Higher income: ↑ lifetime risk of AF (US-based ARIC study)91 Higher income in young individuals: ↓ risk of AF diagnosis (Danish study)92 |
Variable ↑↓ risk | N/A |
| SES | Single studies | Cumulative socioeconomic disadvantage: ↑ risk (HR, 1.57)94 Individual’s poorest areas: 12% ↑ d risk95 |
Low SES: ↑↔ risk | N/A |
| SR/MA | Heterogeneous results96 | |||
| Genetics | ||||
| Family history/heritability | Single studies | Family history of AF: ↑ risk97–99 | ↑ Risk | N/A |
| MR | Proportion heritability explained by loci in European ancestry analysis, 42%100 | |||
| GWAS | MA | Number of AF risk loci ↑s with ↑ number of subjects studied. In 2018, 97–111 loci explained ~11%–42% of the heritability of AF in individuals of European ancestry100,101 | ↑ Risk | N/A |
Population attributable fraction: the proportional disease incidence in the population that is estimated to be due to the risk factor. Statistically significant associations reported, unless otherwise indicated.
↓ indicates decreased; ↑, increased; ↔ no significant change in risk; AF, atrial fibrillation; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; BNP, brain naturiuretic peptide; BP, blood pressure; CABG, coronary artery bypass graft surgery; CAD, coronary artery disease; CI, confidence interval; CKD, chronic kidney disease; DBP, diastolic blood pressure; ECG, electrocardiogram; GWAS, genome-wide association study; HF, heart failure; HR, hazard ratio; LA, left atrial; LRFM, lifestyle and risk factor modification; LV, left ventricular; LVH, left ventricular hypertrophy; MA, meta-analysis; MR, Mendelian randomization; N/A, not available/applicable; OR odds ratio; RR, relative risk; OSA, obstructive sleep apnea; SMD, standardized mean difference; SBP, systolic blood pressure; SES, socioeconomic status; SR, systematic review; and VHD, valvular heart disease.
2.2. Atrial Arrhythmia Classification and Definitions
2.2.1. AF Classification
The previous classification of AF, which was based only on arrhythmia duration, although useful, tended to emphasize AF once it was diagnosed and focused mainly on therapeutic interventions. The new proposed classification using stages aims to correct the deficiencies of the previous classification by recognizing AF as a progressive disease that requires different strategies at the different stages, from prevention to screening, to rate and rhythm control therapies. The different stages better define AF as a progressive disease and highlight the need to address it at the earliest stages, especially emphasizing the importance of prevention, risk factor management, and timing for screening in those patients at the highest risk. The stages are not mutually exclusive (eg, risk factors should be managed through multiple stages) (Figure 4).
Figure 4. AF Stages: Evolution of Atrial Arrhythmia Progression.
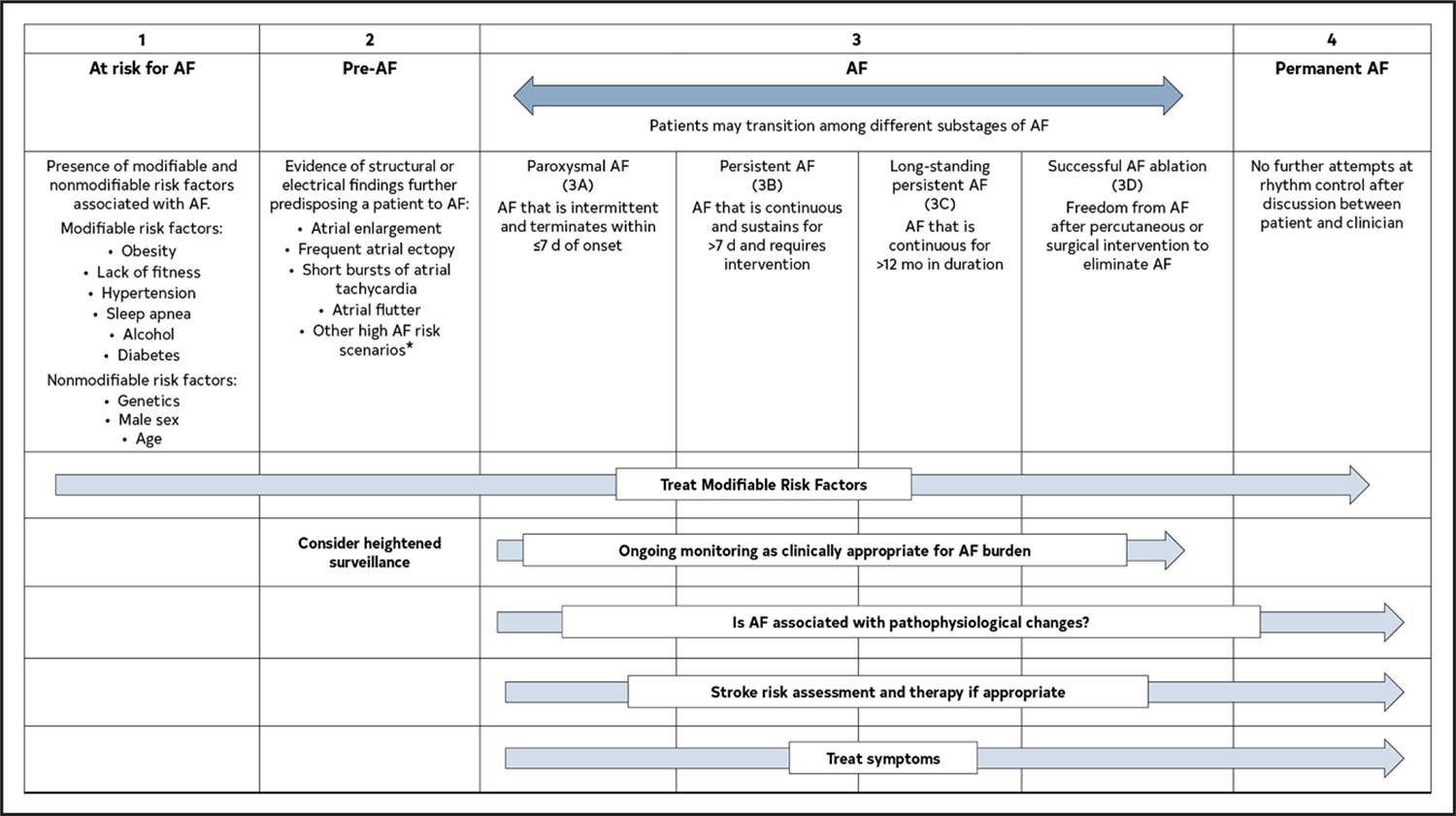
*Heart failure, valve disease, coronary artery disease, hypertrophic cardiomyopathy, neuromuscular disorders, thyroid disease. Original figure created by the 2023 Atrial Fibrillation Guideline Writing Committee. AF indicates atrial fibrillation.
AF is the most common arrhythmia in the world and accounts for significant morbidity and mortality. Over the past decade, evidence has consistently shown that the best treatment of atrial fibrillation requires multiple stakeholders committed to providing comprehensive patient-centered care. In addition, as emphasized in this guideline, AF should be thought of in a more holistic sense over an individual patient’s lifetime.
The foundation of optimal AF management is the treatment of risk factors and implementing lifestyle changes to decrease the likelihood of developing AF (Figure 5). Once AF develops, patient care should focus on assessing the risk of stroke and implementing any necessary treatment, continued optimization of all modifiable risk factors, and managing potential symptoms of AF, with an initial focus on evaluating and minimizing AF burden. However, as outlined in this guideline, access to all aspects of health care to all patients is necessary for any true improvement to be realized.
Figure 5. Pillars for AF Management.
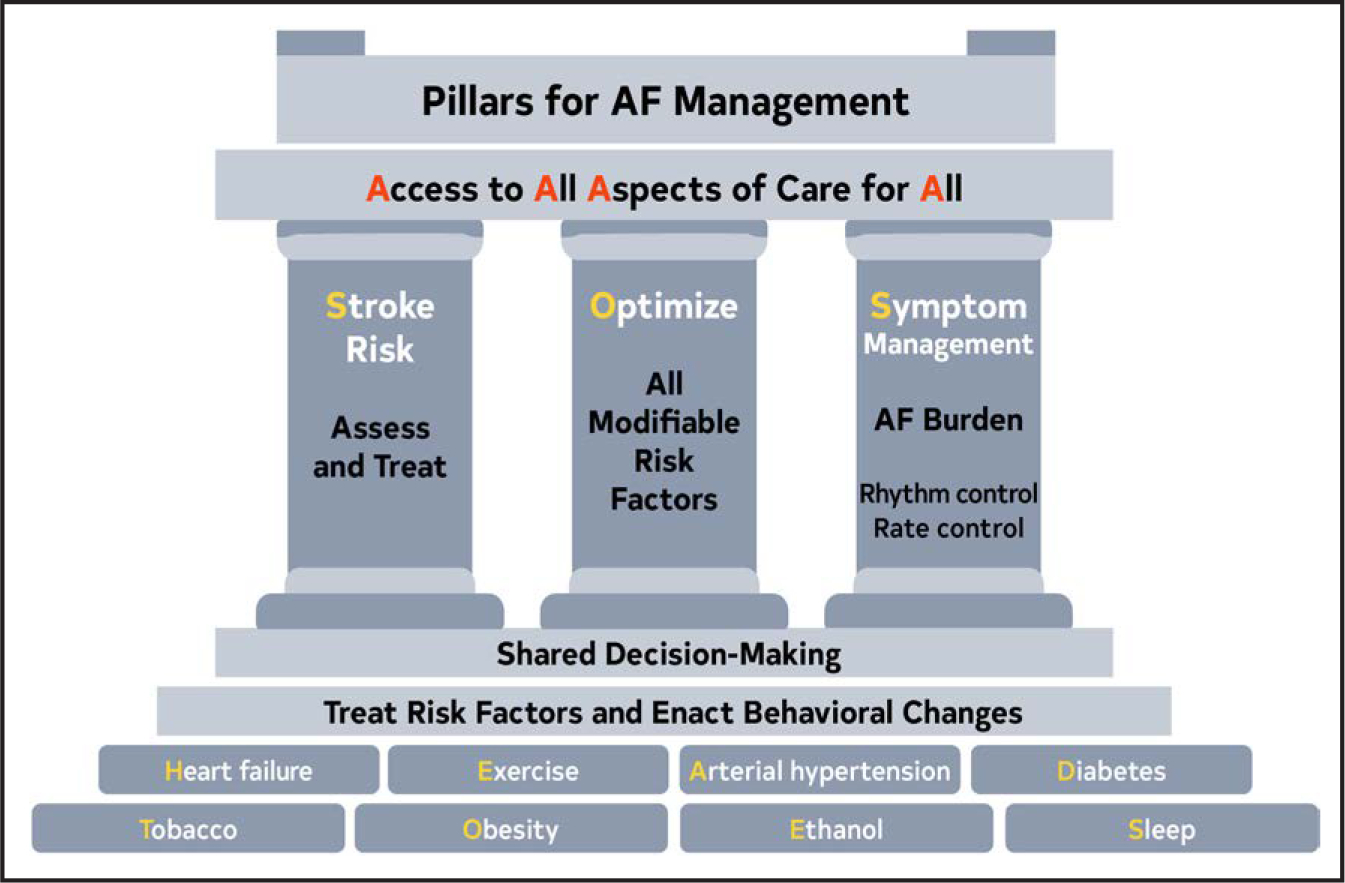
AF indicates atrial fibrillation.
When AF develops, holistic and optimal care of the patient at risk for AF, or who has developed AF, can be simply modeled using a building. The foundation of care is treatment of comorbidities and risk factors and implementing behavioral change in all individuals to decrease the likelihood of developing AF and reducing its burden (Screening for all risk factors from HEAD 2 TOES). Once AF develops, there are 3 important care processes that must be specifically addressed with all patients and aligned with their goals of therapy: Stroke risk assessment and treatment, if appropriate, Optimizing all modifiable risk factors, and Symptom management using rate- and rhythm-control strategies that consider AF burden in the context of an individual patient’s needs (SOS). The overarching principle for AF management is Access to All Aspects of care to All (4 As).
2.2.2. Associated Arrhythmias
Other atrial arrhythmias are often encountered in patients with AF.
Atrial Tachycardia (AT):
It is a regular atrial rhythm at a constant rate of >100 beats per minute (bpm) with discrete P waves and atrial activation sequences originating outside of the sinus node.1 The mechanism can be automaticity, triggered activity, or a microreentry circuit. Focal ATs arise from a single discrete site within the left or right atrium, in contrast to macroreentrant atrial arrhythmias and AF, which involve multiple sites or larger circuits. In multifocal AT, the atrial activation sequence and P-wave morphology vary.
Atrial Flutter (AFL) and Macroreentrant AT:
They occur in many of the same situations as AF. Typical AFL, also known as “typical AFL” or “cavotricuspid isthmus (CTI)-dependent AFL,”2 involves a macroreentrant circuit around the tricuspid annulus traversing the CTI on the right side of the heart (Figure 6). This is the arrhythmia associated with the classic electrocardiogram (ECG) finding of sawtooth flutter waves in the inferior leads when the circuit goes in the counterclockwise direction. The same circuit in the clockwise direction is called “reverse typical AFL.” If the flutter involves a different circuit than tricuspid valve/isthmus, then it is called “atypical” AFL, which is also known as “noncavotricuspid isthmus–dependent macroreentrant AT.”2 AFL was previously classified as either type I or type II. That terminology is no longer used.
Figure 6. Types of Atrial Flutter and Macroreentrant Atrial Tachycardia.
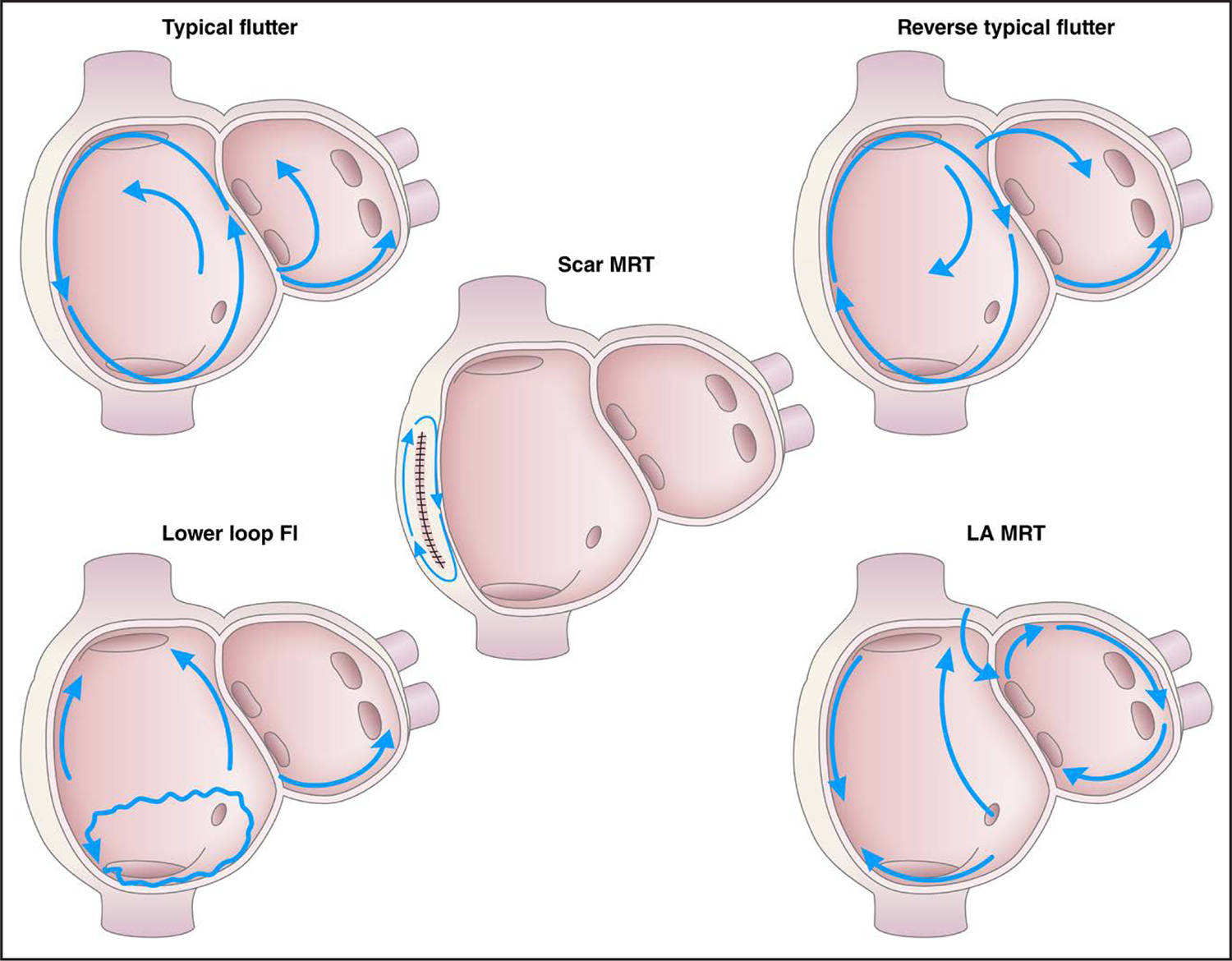
The typical, reverse typical, and the lower-loop flutter all have the low right atrial isthmus incorporated in the flutter circuit. Other macroreentrant flutters include scar-mediated reentrant tachycardia and left mitral isthmus flutter. Modified with permission from Wellens et al.3 Copyright 2002 American Heart Association, Inc. Fl indicates flutter; LA, left atrium; and MRT, macroreentrent.
2.3. Mechanisms and Pathophysiology
AF is a chaotic, rapid (300–500 bpm), and irregular atrial rhythm. Although normal rhythms are conducted through the atria in smooth waves initiated by the sinoatrial node, AF is the result of either electrophysiological abnormalities that underlie impulse generation and/or structural abnormalities of cellular connections that typically facilitate rapid and uniform impulse conduction. AF often stems from waves of electrical activity originating from ectopic action potentials most commonly generated in the pulmonary veins (PVs) of the left atrium (LA),1 or in response to reentrant activity promoted by heterogeneous conduction due to interstitial fibrosis.2 Atrial ectopy can generate runs of tachycardia, while persistent AF requires a substrate that is either sufficiently large or conduction that is sufficiently heterogeneous to enable reentrant activity to persist. The electrical abnormalities evident on the ECG during AF likely represent a shared phenotype of a condition with many distinct etiologies (genetic, environmental, and metabolic) (Figure 7).
Figure 7. Mechanisms and Pathways Leading to AF.
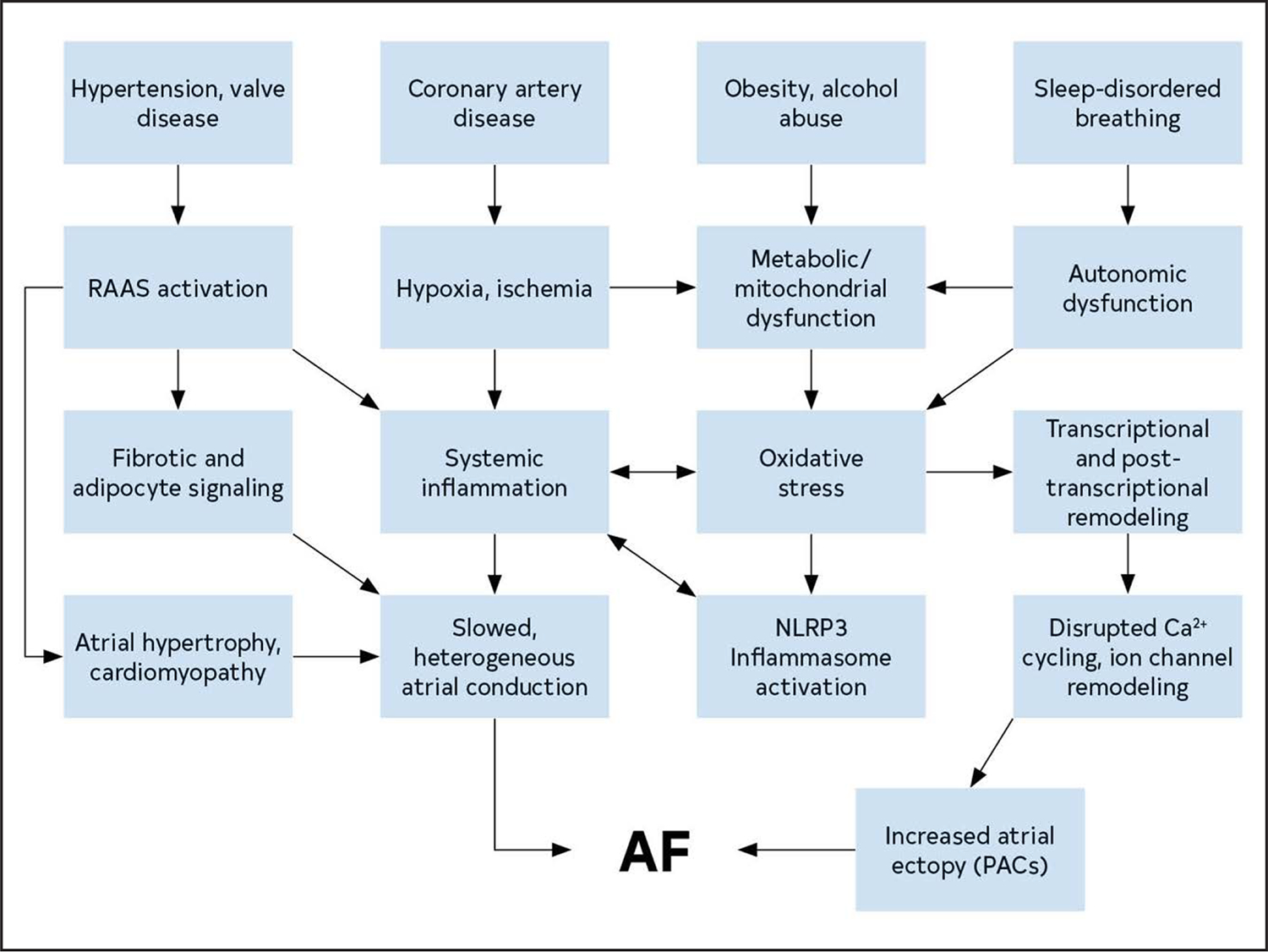
The pathways that contribute to the development of AF create a substrate for reentry and provide triggers that can initiate arrhythmic activity. AF indicates atrial fibrillation; PAC, premature atrial contraction; NLRP3, NOD-, LRR- and pyrin domain-containing protein 3; and RAAS, renin-angiotensin-aldosterone system.
2.3.1. Electrophysiological Mechanisms and Electrical Remodeling
The observation by Haissaguerre et al1 that ectopic firing from PVs triggers AF revolutionized treatments for AF. PV features that increase vulnerability for initiating ectopy include a higher resting membrane potential, stretch-activated channels,2 and pattern of cross myofiber orientation.
Electrical remodeling, which can contribute to and result from AF, includes perturbations that culminate in abnormal Ca2+ handling and shortened, proarrhythmic triangulated action potentials (eg, from decreased L-type Ca2+ current3 and increase in IK1).4 Heterogeneity in IK1 between left and right atria further promotes arrhythmogenicity.5 Downregulation of connexin results in decreased gap junctions, leading to slow heterogeneous atrial conduction velocity and repolarization,6 promoting regional functional conduction block that can support reentry. Connexin remodeling can be due to genetic7 or acquired factors, such as inflammatory state,8,9 older age, or sleep-disordered breathing (SDB).10
Calcium mishandling from remodeling increases calcium load in the sarcoplasmic reticulum and dysfunction of ryanodine receptors, which regulate intracellular calcium release. Remodeling in AF of sarcoplasmic reticulum calcium ATPase underlies sequestration of intracellular calcium between beats, and/or altered regulation of sarcoplasmic reticulum calcium ATPase by phospholamban and sarcolipin.11 Increased intracellular sodium from calcium-calmodulin II-induced increased late sodium current or cardiac glycosides can also increase sarcoplasmic reticulum calcium through the sodium-calcium exchanger.12,13 Several upstream mechanisms (eg, oxidative stress, inflammatory signaling) promote calcium-calmodulin II-activation. CaMKII-mediated and hyperphosphorylation of ryanodine receptor 2 promotes spontaneous diastolic Ca2+ leak by increasing ryanodine receptor 2 channel open probability, leading to higher intracellular Ca2+ levels and the milieu for delayed afterdepolarizations, the most likely trigger for AF initiation.14 Electrogenic action of sodium-calcium exchanger drives afterdepolarizations, and expression of sodium-calcium exchanger is increased in HF and AF. Action potential alternans related to Ca2+ mishandling can be noted to precede AF onset and increases with age.
2.3.1.1. Triggers of AF
The atria of patients with AF tend to have both shorter effective refractory periods and slower conduction, which enhances dispersion of repolarization and favor reentry. This substrate is sensitive to AF initiation, frequently after premature atrial contractions (PACs).1 PAC burden is associated with development of AF.2 Larger LA volume, increased NT-proBNP levels, and impaired LA emptying are associated with increased PAC burden.3 In a study of 100 patients undergoing PV isolation, PACs induced 41 episodes of AF in 22 patients, with most episodes originating in the PVs.4 Earlier studies also reported an LA gradient of background potassium currents, resulting in shorter LA than right atrial effective refractory period in patients with AF.5 Mapping studies of AF electrograms in canine models documented a left-right gradient of high frequency sources (drivers), with the highest frequency regions located near the PVs in the LA.6
2.3.2. Atrial Structural Abnormalities, Remodeling, and Atrial Myopathy
Atrial cardiomyopathy has been identified as “any complex of structural, architectural, contractile or electrophysiological changes affecting the atria with the potential to produce clinically relevant manifestations.”1 Atrial cardiomyopathy is common, associated with aging and other comorbidities with metabolic or hemodynamic stress, and often leads to or results from AF. Structural and electrical remodeling promote AF and include interstitial changes, increased myofibroblast activity, and collagen deposition, fibrofatty deposits, altered ion channel expression, calcium signaling and contractility, and inflammatory infiltrates.1 Myeloperoxidase, an oxidase in neutrophils and macrophages, is associated with fibroblast activation, interstitial fibrosis, and inducibility of AF in a mouse model.2 Prothrombotic changes are often evident in the LA, including increased endocardial expression of von Willebrand factor, vascular cell adhesion molecule-1 and monocyte chemoattractant protein-1 changes, which may increase risk of thrombus formation and stroke.1
Experimental hemodynamic overload, such as reversible aortopulmonary artery shunting promotes atrial dilation, atrial myocyte hypertrophy, interstitial fibrosis, alterations in extracellular matrix composition and vascular dysfunction, and increased vulnerability to atrial ectopy and AF inducibility.3 Electrical remodeling similar to that seen during AF is observed, with characteristic loss of calcium currents. Shunt closure reverses electrophysiological changes.3 Clinically, the hemodynamic structural changes are difficult or impossible to reverse.
2.3.2.1. Upstream Pathways
Upstream pathways include inflammatory, oxidative stress, fibrosis, calcium handling, genetic, metabolic, obesity, and other mechanisms implicated in increasing susceptibility to or progression of AF. The renin-angiotensin-aldosterone system (RAAS), oxidative stress, inflammatory signaling, and calcium overload are discussed here and in Section 2.3.1 (“Electrophysiological Mechanisms and Electrical Remodeling”). Upstream therapies are discussed in Section 8.3.4 (“Upstream Therapy”).
The RAAS regulates blood pressure (BP) and is activated in hypertension and obesity. RAAS activation promotes vascular smooth muscle constriction (increasing BP), activates fibroblasts (increasing atrial interstitial collagen), and increases reactive oxygen species in the sympathetic nervous system. BP and weight control, including treatment with angiotensin-converting enzyme (ACE) inhibitors or angiotensin-II receptor blockers, attenuate pathologic changes.
Oxidative stress is associated with: (1) activation of calcium-dependent calmodulin kinase II1,2 and jun kinase 2,3 which promotes ryanodine receptor phosphorylation and leads to spontaneous calcium release4; (2) increased late sodium current, INa,L, and lower calcium current amplitude; (3) formation of reactive lipid products (isolevuglandins) in hypertension5 and AF6; (4) activation of the redox-sensitive transcription factor nuclear factor-kappa B and the NLRP3 inflammasome7; (5) increase in mitochondrial and metabolic stress; (6) inflammatory changes; and (7) myofilament protein degradation, impairing atrial contractility, which may increase thromboembolism risk.8
Systemic inflammatory activation, first documented with AF after cardiac surgery,9 has also been associated with nonsurgical AF.10 NLRP3 knockdown prevented AF inducibility in a mouse model, implicating the NLRP3 inflammasome in AF pathophysiology.11 NLRP3-blocking drugs are in development.
2.3.2.2. Persistence of AF
In general, persistence of AF reflects the substrate for AF. A suitable substrate for AF has a wavelength (wavelength = refractory period × conduction velocity) that is shorter than the dimensions of the tissue, with heterogenous conduction velocity and/or repolarization duration. Thus, an individual with large, fibrotic, and/or fatty atria is more likely to have persistent AF than one with a normal-sized atria with little interstitial fibrosis or adipose infiltration. Electrophysiologic remodeling is typically a response to persistent AF rather than the trigger.
2.3.3. Role of the Autonomic Nervous System
The autonomic nervous system (ANS) has an important role as trigger and substrate (Figure 8).
Figure 8. Contemporary Summary of the Role of the ANS in AF.
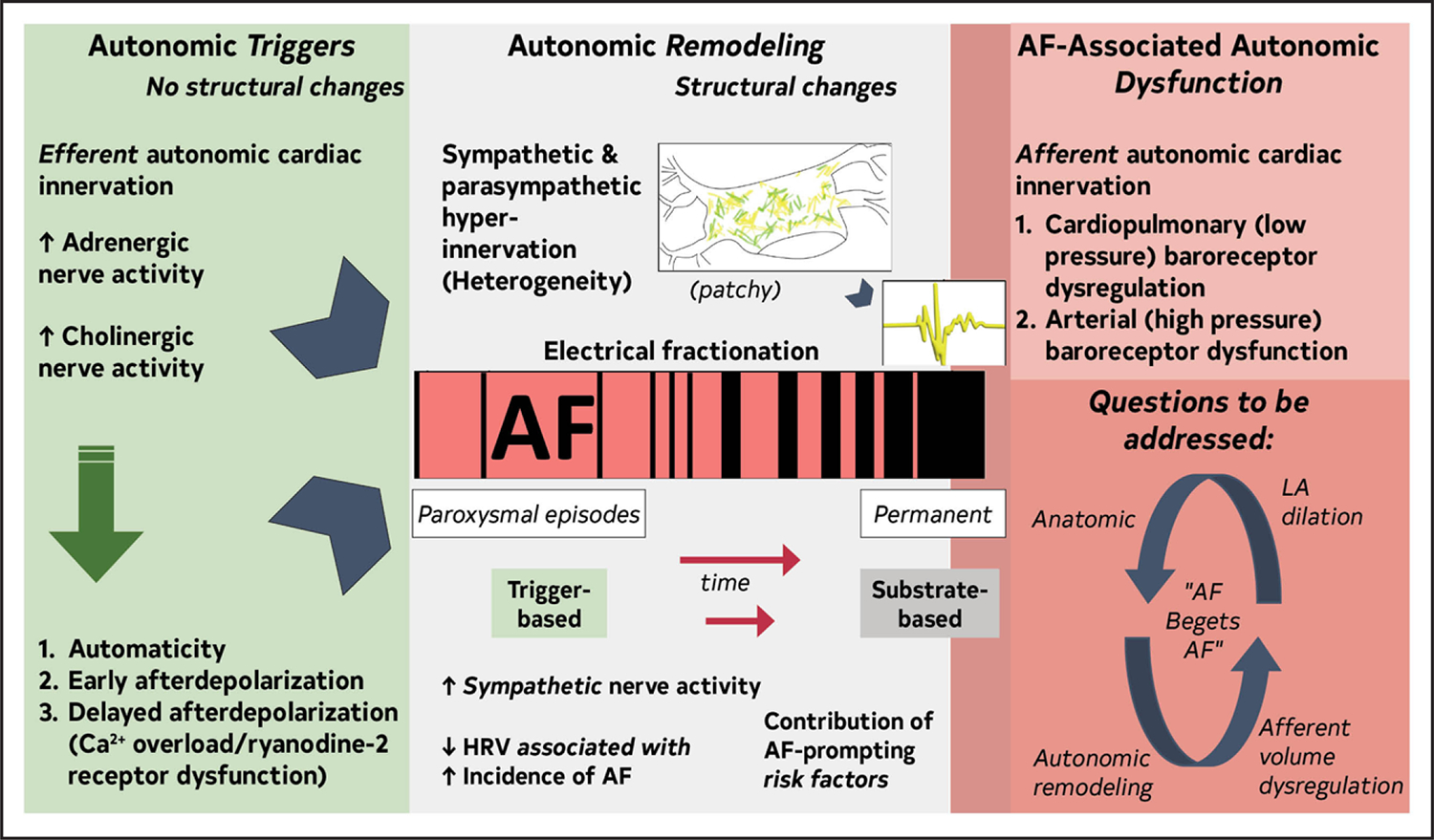
Original figure created by the 2023 Atrial Fibrillation Guideline Writing Committee. AF indicates atrial fibrillation; ANS, autonomic nervous system; HRV, heart rate variability; and LA, left atrium.
ANS triggers AF
The ANS as AF trigger is detailed in several reviews.1–6 Sympathetic efferent stimulation releases noradrenaline, stimulating G-coupled β-adrenergic receptors, enhancing L-type calcium channels, and increasing inward current (automaticity/early afterdepolarization). Delayed afterdepolarization occurs via calcium overload and ryanodine-2 receptor dysfunction. Parasympathetic stimulation shortens atrial effective refractory period by increasing IKACh (acetyl-choline receptor mediated inward rectifying potassium channel) activity. Atrial effective refractory period heterogeneity follows the pattern of autonomic innervation. Sympathetic and parasympathetic activity, alone or combined, can trigger AF.
ANS maintains AF
Atrial sympathetic and parasympathetic hyperinnervation and spatial heterogeneity, coupled with electrical fractionation and altered atrial electrophysiology, contribute to substrate.7–10 Modifiable AF risk factors promote ANS dysfunction.3 AF produces autonomic afferent reflex deficiencies elicited by decreased cardiac volume11,12 and increases sympathetic activity.13 Afferent abnormalities disrupt blood volume and pressure homeostasis. Similar abnormalities to AF were identified in HF.14 Afferent ANS dysfunction could link autonomic with anatomic remodeling (atrial dilatation15), contributing to AF self-perpetuation.
2.4. Genetics
Both common forms and familial AF are heritable.1–3 Multiple recent genome-wide association studies have documented >100 loci specific for AF.4,5 Numerous AF loci appear consistent across multiethnic groups,5 with some population variation.6,7 With large genome-wide genotyped cohorts such as the UK Biobank and the US National Heart, Lung, and Blood Institute’s (NHLBI’s) Trans Omics for Precision Medicine, the genetic architecture of AF is now emerging. A UK Biobank study identified TTN loss of function variants in 0.44% of participants, 14% of whom had AF.8 In a Trans Omics for Precision Medicine study of nearly 1300 participants <66 years of age with AF, 10.1% harbored a disease-associated genetic variant in genes associated with inherited cardiomyopathy or arrhythmia syndromes (most common were TTN, MYH7, MYH6, LMNA, and KCNQ1), and 62.8% had variants of undetermined significance. Disease-associated variants were more prevalent at younger age of AF onset, 16.8% in those <30 years.9 A smaller study of persons of Hispanic or African American descent reported 7% of persons with AF onset at ≤66 years of age harbored rare likely pathogenic or pathogenic sequence variants, mostly in myocardial structural proteins and ion channels.10
2.5. Addressing Health Inequities and Barriers to AF Management
Recommendation to Address Health Inequities and Barriers to AF Management
Referenced studies that support the recommendation are summarized in the Online Data Supplement.
| COR | LOE | Recommendation |
|---|---|---|
| 1 | B-NR | 1. Patients with AF, regardless of sex1 and gender diversity, race and ethnicity,2 or adverse social determinants of health (SDOH),3,4* should be equitably offered guideline-directed stroke risk reduction therapies as well as rate or rhythm control strategies and LRFM as indicated to improve quality of life (QOL) and prevent adverse outcomes. |
Synopsis
Inequities in AF care and outcomes in individuals who are women, from underrepresented racial and ethnic groups (UREGs),2 or who have adverse SDOH have been documented.3,4 Sex differences in AF treatment have been described with respect to anticoagulation6,7 and rhythm control therapy approaches.7–9 Racial and ethnic differences in clinical presentation, management, and prognosis, including stroke, HF, and death, in patients with AF are widely reported.2,10
To avoid guidelines having the unintended consequence of widening inequities in clinical care and outcomes in individuals with AF, it is essential to longitudinally measure the receipt of AF GDMT and outcomes at the clinical practice and health system levels stratified by specific populations who have historically experienced inequitable care. If inequities are identified, barriers to GDMT should be eliminated. Data are needed to assess the impact of addressing SDOH in patients with AF on process measures, health care utilization, costs, and clinical outcomes.11 In other health contexts, there are observational and randomized data12 that screening and addressing SDOH leads to improved medication adherence,13 risk factor control,14 and clinical outcomes.15
Recommendation-Specific Supportive Text
Despite the elevated risk of stroke in women and several UREGs, many are less likely to be treated with stroke risk reduction therapies.6,7 Although women and individuals from UREGs with AF are more symptomatic and report worse QOL than their counterparts, they also are less likely to be referred to an electrophysiologist7 and receive catheter ablation.2,7–9,16 Women are referred for ablation later in the disease course and at older ages than men.7,17,18 These differences or delays in therapy may result in worse outcome given early rhythm control of AF improves outcomes1,19 in select patients. In addition, in the Catheter Ablation versus Antiarrhythmic Drugs in AF trial, individuals from UREGs treated with catheter ablation had a 72% relative reduction in the all-cause mortality rate.20 Therefore, ensuring timely and equitable referral of women, individuals from UREG, and those with adverse SDOH for rhythm control therapy is important. In patients with AF, indicators of lower socioeconomic status were associated with lower oral anticoagulation rates,21 lower rate of adherence during direct oral anticoagulant (DOAC) initiation,22 specialty care,21,23 and less use of cardioversion4,21 and catheter ablation.4,21,24 Indicators of socioeconomic disadvantages, such as increased risk of hospitalization,25 stroke,4,21 HF,4,21,26 and death, were also associated with complications in patients with AF.4,21,27
3. SHARED DECISION-MAKING (SDM) IN AF MANAGEMENT
Recommendation for SDM in AF Management
Referenced studies that support the recommendation are summarized in the Online Data Supplement.
Synopsis
There are wide variations in how SDM is implemented in clinical care settings.5,6 Decision aids may provide standardization of SDM approaches for better informing patients about stroke reduction therapies and improve patient-reported measures but to date have not consistently been developed with recommended frameworks, have rarely been tested in systemically disadvantaged populations (low health literacy, UREGs, low socioeconomic status, rural geography, older adults), and have had variable impact on adherence and clinical outcomes.1–8 Ongoing work will measure health and digital literacy and strengthen the evidence for the impact of decision aids on decisional quality, adherence to treatment, and health outcomes.9
Symptom severity strongly correlates with QOL; thus, minimizing symptoms is an essential component of patient-centered AF management decisions. Rhythm control strategies improve QOL, particularly when maintenance of sinus rhythm or low AF burden is achieved.10 Notably, few SDM decision aids are focused on rate or rhythm control treatment options, and few have measured QOL as an outcome.3,5
Recommendation-Specific Supportive Text
Recently, 2 comprehensive reviews of decision aids for stroke reduction therapies were conducted to determine the impact of these tools on patient-reported measures of decisional quality, while considering other important outcomes including oral anticoagulant (OAC) uptake, medication adherence, and the effect on bleeding and stroke.3,4 Most decision aids focused on patient-reported measures, and few underwent rigorous pilot testing or correlated the aid with clinical outcomes, such as stroke and bleeding. Decision aids consistently demonstrate improvements in patient knowledge. The pooled analysis by Song et al noted lower decisional conflict using decision aids and enhanced OAC uptake (risk ratio, 1.03 [95% CI, 1.01–1.05]).4 Decision aids have historically shown marginal improvement in 3-month measures of adherence, and the 2 largest randomized trials to date showed no improvement in adherence between decision aids and usual care at 1 year.1,2 There is a paucity of data on the impact of decision aids on stroke, thromboembolic events, or bleeding, and when assessed the benefit has been minimal or neutral.4,11 Despite the US Centers for Medicaid & Medicare Services coverage decision requirement for SDM for percutaneous left atrial appendage occlusion (LAAO), only 1 tool was identified that incorporated this option (Table 5).5
Table 5.
Publicly Available Decision Aids
| Agency | Website | Focus Area |
|---|---|---|
| American College of Cardiology Colorado Program for Patient Centered Decisions | https://patientdecisionaid.org/icd/atrial-fibrillation/ | Stroke risk reduction therapies |
| Anticoagulation Choice Decision Aid | https://anticoagulationdecisionaid.mayoclinic.org/ | Stroke risk reduction therapies |
| Ottawa Hospital Research Institute Developer Healthwise | https://decisionaid.ohri.ca/AZlist.html | AF ablation Stroke risk reduction |
| Stanford | https://afibguide.com/ | Stroke risk reduction therapies |
4. CLINICAL EVALUATION
4.1. Risk Stratification and Population Screening
There are >20 risk prediction models for incident AF in the community.1 The most widely replicated risk prediction model for newly diagnosed AF is CHARGE-AF (Cohorts for Heart and Aging Research in Genomic Epidemiology model for atrial fibrillation; Table 6),2 while the C2HEST score (coronary artery disease or chronic obstructive pulmonary disease [1 point each]; hypertension [1 point]; elderly [age ≥75 years, 2 points]; systolic HF [2 points]; thyroid disease [hyperthyroidism, 1 point]) was derived and validated in Asian cohorts (Table 7).3,4
Table 6.
CHARGE-AF Risk Score for Detecting Incident AF*
| Variable (X) | Estimated β Coefficient (SE) | HR (95% CI) |
|---|---|---|
| Age (per 5-y increment) | 0.508 (0.022) | 1.66 (1.59–1.74) |
| White race | 0.465 (0.093) | 1.59 (1.33–1.91) |
| Height (per 10-cm increment) | 0.248 (0.036) | 1.28 (1.19–1.38) |
| Weight (per 15-kg increment) | 0.115 (0.033) | 1.12 (1.05–1.20) |
| Systolic BP (per 20-mm Hg increment) | 0.197 (0.033) | 1.22 (1.14–1.30) |
| Diastolic BP (per 10-mm Hg increment) | −0.101 (0.032) | 0.90 (0.85–0.96) |
| Smoking (current versus former/never) | 0.359 (0.063) | 1.42 (1.25–1.60) |
| Diabetes (yes) | 0.237 (0.073) | 1.27 (1.64–2.48) |
| Myocardial infarction (yes) | 0.496 (0.089) | 1.64 (1.38–1.96) |
Table 6 does not encompass all complications.
Five-year risk is given by: 1 − 0.9718412736exp(ΣβX − 124411305), where β is the regression coefficient (column 2) and X is the level of each variable risk factor.2
AF indicates atrial fibrillation; BP, blood pressure; CHARGE-AF, Cohorts for Heart and Aging Research in Genomic Epidemiology model for atrial fibrillation; HR, hazard ratio; and SE, standard error.
Table 7.
C2HEST Risk Score for Detecting Incident AF*
| Acronym | Risk Factor | Points |
|---|---|---|
| C2 | CAD/COPD | 1–2 |
| H | Hypertension | 1 |
| E | Elderly (age ≥75 y) | 2 |
| S | Systolic heart failure | 2 |
| T | Thyroid disease (hyperthyroidism) | 1 |
Total points 0–8. For the C2HEST score, the C statistic was 0.749, with 95% CI of 0.729–0.769.10 The incident rate of AF increased significantly with higher C2HEST scores.
AF indicates atrial fibrillation; CAD, coronary artery disease; C2HEST, coronary artery disease or chronic obstructive pulmonary disease [1 point each]; hypertension [1 point]; elderly [age ≥75 y, 2 points]; systolic HF [2 points]; thyroid disease [hyperthyroidism, 1 point]; and COPD, chronic obstructive pulmonary disease.
Screening for AF has been investigated, mostly in patients >65 years of age, using various protocols that include both 1-time electrocardiographic recordings, recurring intermittent ECGs (including consumer-based devices), or continuous electrocardiographic external monitors. Most screening trials have shown higher AF detection using intermittent or continuous electrocardiographic recordings5 and higher AF detection in patients with higher predicted risk for AF.6 A recent study also showed that an AI algorithm able to risk-stratify a relatively uniform population (eg, older adults at risk for stroke) to detect undiagnosed AF during short-term cardiac monitoring was associated with increased AF.7 Conversely, mass population screening with a smartwatch app only rarely detected a new diagnosis of AF.8 Ultimately, for risk stratification models and screening programs to be useful, they would need to improve outcomes and be cost-effective.9 It is not yet established that patients at high risk of developing AF by a validated risk score benefit from screening and interventions to improve rates of ischemic stroke, systemic embolism, and survival.
4.2. Basic Evaluation
4.2.1. Basic Clinical Evaluation
Recommendations for Basic Clinical Evaluation
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with newly diagnosed AF, a transthoracic echocardiogram1–4 to assess cardiac structure, laboratory testing to include a complete blood count, metabolic panel, and thyroid function,5–7 and when clinical suspicion exists, targeted testing to assess for other medical conditions associated with AF are recommended to determine stroke and bleeding risk factors, as well as underlying conditions that will guide further management. |
| 3: No benefit | B-NR | 2. In patients with newly diagnosed AF, protocolized testing for ischemia, acute coronary syndrome (ACS), and pulmonary embolism (PE) should not routinely be performed to assess the etiology of AF unless there are additional signs or symptoms to indicate those disorders.8–10 |
Synopsis
The initial clinical evaluation of the patient with newly diagnosed or suspected AF should be focused on confirming the diagnosis and identifying relevant clinical factors that will impact management. A targeted history and physical examination should be performed at the initial assessment and repeated during periodic follow-up, especially given the evolving risk of thromboembolism and the cadence of symptoms in response to therapy (see Section 11, “Future Research Needs”). An ECG can assess other electrical abnormalities, including possible substrates such as Wolff-Parkinson-White (WPW) syndrome, coexisting atrial arrhythmias, as well as abnormalities that may affect decision-making in pharmacological management (eg, bradycardia, QT duration). Basic laboratory tests should be performed to determine if other clinically relevant disorders are present and impact on management, particularly with respect to stroke and bleeding risk. A transthoracic echocardiogram provides information on chamber size, thickness, function, and the presence of valvular pathology. Additional testing, including multimodality advanced imaging and further ambulatory electrocardiographic monitoring, may be pursued based on the results of these initial evaluations. AF itself does not increase the likelihood of myocardial ischemia, ACS or PE, and therefore routine testing for these disorders in the absence of signs or symptoms is of no benefit.
Recommendation-Specific Supportive Text
A transthoracic echocardiogram is essential to evaluate chamber size and function, valve function, and right ventricular (RV) pressure. Left ventricular ejection fraction (LVEF) impacts decisions for antiarrhythmic drug therapy and whether to prioritize other rhythm control therapies, including catheter ablation. Additionally, strain imaging may suggest an underlying infiltrative cardiomyopathy, such as amyloidosis.1 Echocardiography also provides information on LA size and function. Altered LA compliance is known to be associated with AF11 and progression toward persistent-type AF.2 In a meta-analysis, AF recurrence after ablation was associated with a lower LA strain,3 while LA volume was a stronger predictor of recurrence after ablation than the characterization of AF as paroxysmal or persistent.4 Laboratory testing can detect other medical conditions that are associated with AF and would impact therapeutic decision-making, such as CKD,5 liver dysfunction,6 and hyperthyroidism.7,12 Laboratory testing may also reveal electrolyte abnormalities, including from medications such as diuretics. Laboratory testing is also needed to determine stroke risk and bleeding risk factors, which will guide management decisions. When clinical suspicion exists, additional testing might be needed to evaluate for potentially related conditions, such as significant valvular disease.
The presence of AF itself should not prompt routine protocolized testing for myocardial ischemia, ACS, or PE, in the absence of signs or symptoms to suggest those diseases. A retrospective analysis of asymptomatic patients with AF compared with age- and sex-matched controls that were referred for myocardial stress imaging found no difference in mean summed stress score or rate of abnormal studies.8 A retrospective analysis of 1700 asymptomatic AF patients (no chest pain or dyspnea) found that 4.6% had >5% ischemic myocardium, and the yield to detect ischemia that resulted in revascularization was only 0.4%.9 Among patients suspected of PE, a retrospective analysis showed that the presence of AF did not increase the probability of PE.10 Certainly, this would not preclude from evaluating patients with signs and/or symptoms of ischemia and PE.
4.2.2. Rhythm Monitoring Tools and Methods
Recommendations for Rhythm Monitoring Tools and Methods
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. Among individuals without a known history of AF, it is recommended that an initial AF diagnosis be made by a clinician using visual interpretation of the electrocardiographic signals, regardless of the type of rhythm or monitoring device.1–5 |
| 1 | B-NR | 2. In patients with an intracardiac rhythm device capable of a diagnosis of AF, such as from an atrial pacemaker lead, a diagnosis of AF should only be made after it is visually confirmed by reviewing intracardiac tracings to exclude signal artifacts and other arrhythmias.6–9 |
| 2a | B-R | 3. For patients who have had a systemic thromboembolic event without a known history of AF and in whom maximum sensitivity to detect AF is sought, an implantable cardiac monitor is reasonable.10,11 |
| 2a | B-NR | 4. Among patients with a diagnosis of AF, it is reasonable to infer AF frequency, duration, and burden using automated algorithms available from electrocardiographic monitors, implantable cardiac monitors, and cardiac rhythm devices with an atrial lead, recognizing that periodic review can be required to exclude other arrythmias.1,4,5,12 |
| 2a | B-R | 5. Among patients with AF in whom cardiac monitoring is advised, it is reasonable to recommend use of a consumer-accessible electrocardiographic device that provides a high-quality tracing to detect recurrences.13 |
Synopsis
Monitoring options for AF include a standard 12-lead ECG, continuously recording or loop-recording electrocardiographic monitors using separate electrodes or as patches, implantable cardiac monitors (sometimes referred to as implantable loop recorders), cardiac rhythm management devices with an atrial lead (eg, pacemakers and defibrillators), handheld ECGs, and smartwatches. Photoplethysmography has been used to infer AF from irregular pulse patterns using a variety of devices, predominately smartphone cameras14 and smartwatches.15–18 Electrocardiographic monitors often deploy automated algorithms for AF detection, but due to variable accuracy,1–5 the initial diagnosis should rely on a health care professional’s examination of the electrocardiographic tracing. Although photoplethysmography monitors may alert individuals to obtain an electrocardiographic tracing, it is not sufficiently reliable to establish an AF diagnosis.14–18 AF detected from an atrial lead has been validated versus surface ECGs6,7 and independently predicts stroke.8,9 RCTs have demonstrated that implantable cardiac monitors exhibit the highest sensitivity in detecting AF compared with external ambulatory monitors, likely related to the longer duration of monitoring.10,11 Automated algorithms to analyze electrocardiographic devices have generally been found to be sufficiently reliable to infer the frequency, duration, and burden of AF among those with an AF diagnosis.1,4,5,12 A randomized trial showed that a handheld electrocardiographic monitor resulted in earlier detection of recurrent AF.13
Recommendation-Specific Supportive Text
Although automated algorithms in various devices are generally reliable, health care professional overread of electrocardiographic tracings remains necessary given the imperfect test characteristics of those algorithms.1–5 Similarly, while algorithms utilizing photoplethysmography signals (derived using smartphones or smartwatches) to infer irregular heart rates can discriminate AF from normal sinus rhythm, these are not sufficiently reliable to establish an AF diagnosis.14–18
Cardiac rhythm devices with an atrial lead have been shown to detect AF validated against conventional surface electrocardiographic tracings.6–8 In addition, both the presence and duration of AF detected solely by these devices predict stroke in a manner that would be expected of AF.8,9 It is still essential that the intracardiac tracings are reviewed for confirmation because false-positives are possible. The duration of AF that mandates intervention with anticoagulation will be discussed in Section 6.4.1 (“Oral Anticoagulation for Device-Detected Atrial High-Rate Episodes Among Patients Without a Previous Diagnosis of AF”).
The more frequent and longer monitoring for AF is deployed, the greater the sensitivity in detecting AF.10,19 Randomized trials, predominately among cryptogenic stroke patients, have revealed that implantable cardiac monitors exhibit the highest sensitivity in detecting AF in view of extended monitoring periods compared with external monitors.10,11
It is often not feasible to manually review all electrocardiographic strips from various monitoring devices, either due to inaccessibility or time and resource constraints on health care professionals. Although variability in accuracy across different devices may be present, the validity demonstrated in automated algorithms is generally sufficient to infer frequency, duration, and burden of AF using electrocardiographic devices such as continuously wearable monitors, implantable cardiac monitors, and cardiac rhythm devices with an atrial lead.1,4,5,12
Cardiac monitoring may be advised to AF patients for various reasons, such as for detecting recurrences, screening, or response to therapy. Among patients with AF who are undergoing cardioversion or AF ablation, a single-center, randomized trial demonstrated that use of a self-administered handheld ECG resulted in earlier detection of recurrent AF13 and possibly improvement in survey-determined AF-related QOL20 compared with usual care.
5. LIFESTYLE AND RISK FACTOR MODIFICATION (LRFM) FOR AF MANAGEMENT
5.1. Primary Prevention
Recommendation for Primary Prevention
Referenced studies that support the recommendation are summarized in the Online Data Supplement.
Synopsis
The clinical, family history, and genetic risk factors for AF are well established (Table 3), and risk prediction models (Section 4.1, “Risk Stratification and Population Screening”) for AF have been reported and replicated.7–9 Multiple reports have established that maintenance of optimal risk factors and ideal cardiovascular health are associated with substantially reduced risk of AF10,11 onset and complications (Section 5, “Lifestyle and Risk Factor Modification [LRFM] for AF Management”). To reduce risk of AF onset, individuals in the general population, particularly those at increased risk of AF, should receive comprehensive integrated LRFM, including maintenance of ideal weight and weight loss if overweight or obese1,12; pursue a physically active lifestyle,2 particularly if sedentary; receive smoking cessation counseling and/or medications4; moderate (≤1 standard alcoholic drink/day) or abstain from alcohol and avoidance of binge drinking3; control diabetes5; and control BP in accordance with GDMT.6,13 There is also an association of cannabis, cocaine, methamphetamine, or opiate use with increased incidence of AF.14
Recommendation-Specific Supportive Text
Most cardiovascular risk factors are associated with increased risk of new-onset AF. Observational studies have demonstrated that obesity and physical inactivity each independently increase the risk of newly diagnosed AF.1,2,12,15–20 However, caution should be considered in pursuing years of regular, high-volume (≥3 h/day) high-intensity endurance training given observational data linking it with increased AF risk21–23 in men and similar “J” curve risk curve observed for high or vigorous activity in both men and women in another study.24 Alcohol consumption enhances the risk of AF in a fairly linear fashion, with clear evidence that binge drinking heightens the risk.3,25–29 Uncertainty persists regarding harms or benefits of no more than 1 regular drink per day.28,29 Self-reported,4 biomarker-verified,30 and genetically predicted31 smoking is associated with increased risk, and smoking cessation is associated with decreased risk of incident AF.4 The presence of either type 1 or type 2 diabetes increases AF risk,5,32 with evidence that worse glucose control correlates with a higher probability of developing AF.33 Hypertension is the risk factor with the highest attributable risk for AF10; intensive BP control lowers the risk of incident AF in observational and randomized data.6 Effective strategies to manage risk factors and prevent CVD have been reported elsewhere.12,13
5.2. Secondary Prevention: Management of Comorbidities and Risk Factors
5.2.1. Weight Loss in Individuals Who Are Overweight or Obese
Recommendation for Weight Loss in Individuals Who Are Overweight or Obese
Referenced studies that support the recommendation are summarized in the Online Data Supplement.
Synopsis
Obesity is associated with the development and progression of AF.5 It results in direct changes to the atrial myocardium forming the substrate for AF.6–8 In addition, obesity is also associated with several comorbidities that have been independently associated with the development of AF.9,10 Obesity has a significant adverse impact on attempts to maintain sinus rhythm, with each 5-unit increase in BMI being associated with a 10% and 13% greater risk of postoperative and postablation AF, respectively.5,11,12 Management of weight is important in the prevention and treatment of AF.
Recommendation-Specific Supportive Text
In an RCT in overweight and obese individuals with BMI >27 kg/m2 and AF, weight loss, as part of a comprehensive LRFM program, was associated with reduction in arrhythmia symptoms, recurrence, and burden.1 Observational studies demonstrated graded responses commensurate with the degree of weight loss, with achievement of at least 10% weight loss associated with greater maintenance of sinus rhythm,2 improved ablation outcomes,3 and reversal of AF type.4 In observational studies, bariatric surgery in Class III obese individuals (BMI ≥40 kg/m2) with AF was associated with improved sinus rhythm maintenance after catheter ablation13,14 and reversal of AF type.15 The greater number of risk factors managed associated with likelihood of maintaining sinus rhythm.16 However, a small observational study in individuals with obesity (BMI ≥30 kg/m2) with long-lasting persistent AF observed no difference in symptoms or sinus rhythm maintenance despite significant weight loss, suggesting that there may be extreme substrates in which a weight loss strategy may not be effective.17
Structured programs with regular review of progress facilitate achievement of weight loss and appear essential, as demonstrated by inability to reduce AF burden in a small RCT that achieved only 4.5% weight loss in the intervention arm.
5.2.2. Physical Fitness
Recommendation for Physical Fitness
Referenced studies that support the recommendation are summarized in the Online Data Supplement.
| COR | LOE | Recommendation |
|---|---|---|
| 1 | B-R | 1. In individuals with AF,* moderate-to-vigorous exercise training to a target of 210 minutes per week is recommended to reduce AF symptoms1–3 and burden,2,3 increase maintenance of sinus rhythm,3–5 increase functional capacity, and improve QOL.3,5,6 |
In patients without AF related to excessive exercise training.
Synopsis
Randomized trials provide evidence that prescribed aerobic exercise interventions may reduce arrhythmia burden in those with nonpermanent AF2 and improve functional capacity and health-related QOL in both permanent1,6 and nonpermanent AF.2 In the ACTIVE-AF (An Exercise and Physical Activity Program in Patients With Atrial Fibrillation) study, an exercise intervention combining home and supervised aerobic exercise over 6 months resulted in greater freedom from arrhythmia recurrence, reduced burden, and improved QOL.3
Recommendation-Specific Supportive Text
In patients with nonpermanent AF, aerobic exercise training may contribute to a reduction in AF burden and improve maintenance of sinus rhythm.2–5 Aerobic exercise interventions reduce severity of AF symptoms, increase functional capacity, and improve health-related QOL among patients with both nonpermanent and permanent AF.1,3,6 Initiation of or continuing regular exercise after development of AF was associated with a lower risk of HF and mortality in a population-based cohort study.7 Exercise training should be moderate- to vigorous-intensity aerobic exercise, with a target of 210 minutes per week, and should be prescribed to reduce the frequency and duration of AF episodes, while improving cardiorespiratory fitness and symptom severity.3 Exercise prescription may be further modified to patient comorbidities, such as obesity, hypertension, and diabetes.4 Caution should be applied to ensure adequate ventricular rate control during exercise training and absence of atrial myopathy related to excessive exercise training. To date, there is little evidence that high-intensity aerobic exercise may be favorable over moderate-intensity activities,8 and extreme levels of exercise have been associated with higher incidence of AF.9,10 Exercise training may be delivered as a stand-alone intervention or as a component of multidisciplinary cardiac rehabilitation. Exercise-based cardiac rehabilitation improves QOL and functional capacity among patients with AF undergoing ablation,11,12 although available studies have not been adequately powered to assess AF-specific outcomes, such as arrhythmia recurrence.13 There is mixed evidence for a reduction in hospitalization or all-cause mortality with exercise training or exercise-based cardiac rehabilitation.12,14,15
5.2.3. Smoking Cessation
Recommendation for Smoking Cessation
Referenced studies that support the recommendation are summarized in the Online Data Supplement.
Synopsis
Observational data support that cigarette smoking in individuals with AF is associated with worse cardiovascular outcomes and death and that individuals with AF who quit smoking are less likely to develop stroke or die. Despite the benefits of smoking cessation, individuals with AF are less likely to receive smoking cessation management than counterparts without AF.7,8 Patients with AF should be strongly advised to quit cigarette smoking9 and should receive GDMT for tobacco cessation, including behavioral interventions10,11 and pharmacotherapy.2
Recommendation-Specific Supportive Text
1. In individuals with AF, cigarette smoking was associated with poorer outcomes. For individuals who have undergone AF catheter ablation, current cigarette smoking was associated with increased risk for AF recurrence.12,13 Cigarette smoking was associated with less time in therapeutic range for patients on warfarin.14 Although some heterogeneity exists, the preponderance of studies have reported that individuals with AF who smoked cigarettes had increased risks of stroke,3,4 HF,5 hospitalization,6 and death.6,15 Of concern, studies have reported that individuals with AF (versus those without) were less likely to receive smoking cessation interventions.7,8 Observational studies suggest that compared with current smokers, individuals with AF who quit smoking after AF diagnosis were less likely to experience CVD,3 stroke,3,16 and all-cause mortality.16
5.2.4. Alcohol Consumption
Recommendation for Alcohol Consumption
Referenced studies that support the recommendation are summarized in the Online Data Supplement.
Synopsis
Among patients with a diagnosis of AF, randomized trials have demonstrated a reduction in AF burden on assignment to abstinence,1 that alcohol acutely changes human electrophysiology in a fashion that renders the atria more prone to fibrillate,2 and suggest that avoiding alcohol can reduce the risk of a near-term AF event.3
Recommendation-Specific Supportive Text
Among patients with AF, a case-crossover study revealed a higher risk of a discrete AF episode hours after objectively confirmed alcohol consumption.4 In the context of a structured comprehensive management of risk factors, alcohol abstinence1 or reduction to ≤3 standard drinks per week has been demonstrated to reduce AF symptoms,5 AF burden,6,7 and progression of AF from paroxysmal to persistent.8
5.2.5. Caffeine Consumption
Recommendation for Caffeine Consumption
Referenced studies that support the recommendation are summarized in the Online Data Supplement.
Synopsis
A randomized N-of-1 trial,2 as well as longitudinal, observational studies, have generally found that caffeine consumed within normal limits is either associated with no heightened risk3–6 or a reduced risk of incident AF.3,5,7–9 Patients often report caffeine as a trigger of AF,10 although this has not been supported by objective data.
Recommendation-Specific Supportive Text
A randomized trial of caffeine failed to show any difference in postoperative AF risk,1 and paroxysmal AF patients experienced no detectable difference in AF episodes when exposing themselves to versus avoiding caffeine in randomized N-of-1 trials.2 Longitudinal, observational studies have generally found that caffeine consumed in usual amounts is either associated with no heightened risk3–6 or a reduced risk of incident AF.3,5,7–9Also, Mendelian randomization studies examining caffeine metabolism-related genetic variants as instrumental-variable surrogates of caffeine consumption have not shown either a harmful or protective effect related to incident AF.8,11 Several case reports have described a relationship between excessive consumption of caffeine (involving overdoses or highly caffeinated energy drinks)12,13 and AF in young, healthy individuals. Individuals should not begin or increase their caffeine consumption with the intent of reducing their AF risk. Patients often report that caffeine triggers their AF,10,14 although this has neither been supported by nor extensively studied in an objective manner. Current studies cannot exclude the possibility of individual-level idiosyncratic relationships between caffeine and AF. It is also possible that caffeine exacerbates symptoms of AF, or causes similar symptoms of palpitations, or enhances heart rhythm awareness.
5.2.6. Diet and Dietary Supplementation
Promoting a healthy diet is an effective strategy for prevention of cardiovascular disease. The evidence pertaining to the prevention of AF using dietary supplements is inconsistent, complicated by substantial misclassification and difficulties controlling for potential confounding factors associated with dietary interventions.
Several studies have evaluated the role of omega-3 fatty acids and AF, demonstrating an inverse relationship between plasma omega-3 polyunsaturated fatty acid levels and prevalent AF.1,2 However, with supplementation there has been no effect or a potential for greater AF.3–7 Although vitamin D supplementation is not useful on a population basis,8,9 its use in the perioperative period in deficient individuals reduces AF.10 Similarly, ascorbic acid has been beneficial in reducing postoperative AF in some studies, although not uniformly.11
The importance of weight management on AF symptoms, burden, and recurrence is increasingly recognized,12,13 leading to various dietary interventions. Although evidence on diets is still evolving, analyses of the ARIC (Atherosclerosis Risk in Communities) study caution using low-carbohydrate diets, which were found to increase the risk of incident AF, regardless of the type of protein or fat used to replace carbohydrate.14
5.2.7. Diabetes
AF and diabetes are associated with increased risk of cardiovascular mortality and sudden death.1 In patients with diabetes, vascular mortality was lower in patients treated with DOACs compared with warfarin.2 In patients with AF and diabetes undergoing catheter ablation, optimal glycemic control preablation may lessen the risk of AF recurrence postablation.3
5.2.8. Treatment of Hypertension
Recommendation for the Treatment of Hypertension
Referenced studies that support the recommendation are summarized in the Online Data Supplement.
Synopsis
Renal denervation and mineralocorticoid receptor antagonists (MRAs) have been associated with decreased AF burden in clinical trials.1,2 The only randomized trial singularly targeting BP control as a mechanism to reduce the recurrence of AF8 showed no significant difference in the primary outcome of recurrent symptomatic atrial arrhythmia beyond 3 months’ postablation among the 2 groups. However, clinical trials of integrated comprehensive LRFM programs in conjunction with rhythm control therapies for AF have resulted in longer arrhythmia-free survival and decreased AF burden.3,5,9 Among patients with AF, treating elevated BP to guideline-directed goals reduces major cardiovascular events.9
Recommendation-Specific Supportive Text
In patients with AF, treatment of hypertension should aim for current BP guidelines to reduce stroke, bleeding, and other adverse outcomes.5,10 An RCT of patients with paroxysmal AF and hypertension noted fewer recurrences among participants treated with renal denervation and pulmonary vein isolation (PVI) compared with PVI alone.2 Randomized studies of mineralocorticoid receptor antagonists for hypertension have been shown to reduce AF burden, and ACE inhibitors and angiotensin II receptor blockers have been associated with lower AF incidence in secondary analyses of RCTs (see Section 8.3.4, “Upstream Therapy”). In addition, several integrated LRFM programs that have resulted in a decrease in BP have been associated with a decreased recurrence of AF.3,11The SMAC (Substrate Modification With Aggressive Blood Pressure Control) AF trial randomized hypertensive patients scheduled for AF ablation to standard or aggressive BP treatment with a systolic BP target of 120 or 140 mm Hg.8 No significant difference was shown in recurrent symptomatic atrial arrhythmia beyond 3 months postablation (median follow-up, 14 months), and this is the only RCT to date singularly targeting BP control as a mechanism to reduce AF recurrence.8 Post hoc analyses of DOAC clinical trials to reduce the risk of stroke in patients with AF have consistently found lower rates of stroke in patients with controlled BP.6,7
5.2.9. Sleep
Recommendation for Sleep
Referenced studies that support the recommendation are summarized in the Online Data Supplement.
Synopsis
SDB is a risk factor for the development of incident AF, and more severe SDB acutely increases the risk of a discrete AF episode.14–18 Independent of SDB, poor sleep quality is associated with an increased risk of developing incident AF.19–21 When formal testing for SDB is used, the disease is frequently observed in >20% of patients with an AF diagnosis.1–5 Observational studies suggest that treatment of SDB may reduce the risk of AF recurrence and AF burden, but RCTs have not been adequately powered to reveal a relationship between treating SDB and reduced AF.6–13
Recommendation-Specific Supportive Text
The prevalence of SDB is remarkably and consistently high in patients with AF (generally substantially >20% and sometimes >50% regardless of the type of AF population assessed), is often undetected, and, as conventional symptoms of SDB may be absent, may require formal study to establish a diagnosis of SDB.1–5,11 Multiple observational studies have described fewer recurrences of AF and a reduction in AF burden, including after cardioversion and catheter ablation procedures, among those undergoing treatment for SDB compared with those not treated for their SDB.6–11,22 However, small trials randomizing patients with AF and SDB treatment versus usual care have not shown significant differences in AF burden or AF recurrence, including after cardioversion or PVI ablation,12,13,22 although studies tended to exclude patients who might benefit most from SDB treatment (eg, those who had more symptomatic sleep apnea [Epworth Sleepiness Scale score >10 or 15], severe cardiovascular disease [LVEF <40% or 45%], or severe obesity).
5.2.10. Comprehensive Care
Recommendations for Comprehensive Care
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | A | 1. Patients with AF should receive comprehensive care addressing guideline-directed LRFM, AF symptoms, risk of stroke, and other associated medical conditions to reduce AF burden, progression, or consequences.1–3 |
| 2a | B-R | 2. In patients with AF, use of clinical care pathways, such as nurse-led AF clinics, is reasonable to promote comprehensive, team-based care and to enhance adherence to evidence-based therapies for AF and associated conditions.4–6 |
Synopsis
Almost all patients with AF have multiple conditions that either increase AF risk or are exacerbated by AF. Patients with AF are also at risk of developing thromboembolism, stroke, and HF, so a comprehensive approach tailored to the needs of the individual patient should improve outcomes. Randomized trials have shown that interventions aimed at AF risk factors, such as alcohol use, overweight, and HF, reduce AF burden. Clinical care pathways and algorithms for AF management, including LRFM, have shown promise in randomized trials through coordinating care and facilitating comprehensive AF management.
Although comprehensive care of the multiple conditions associated with AF is logical, it is unclear whether integrated care by a multidisciplinary team leads to better outcomes than comprehensive care by a single clinician applying an evidence-based AF clinical care algorithm or pathway. Nevertheless, coordination of care among multiple clinicians should also improve care, even though it has not been evaluated rigorously.7
Recommendation-Specific Supportive Text
Randomized trials have shown the efficacy of many individual components of patient-centered care for AF, as discussed earlier in this section. Several randomized and nonrandomized studies have utilized comprehensive programs for patients with AF.2,8,9 The RACE 3 (Rate Control versus Electrical cardioversion for persistent atrial fibrillation) trial3 found that multifaceted treatment for patients with AF and early HF (with mineralocorticoid receptor antagonists, statins, ACE inhibitors and/or angiotensin receptor blockers [ARBs], and cardiac rehabilitation) improved maintenance of sinus rhythm.
Various clinical care pathways, algorithms, and electronic clinical decision support systems for the care of patients with AF have been tested in clinical trials, with mixed results. Care provided by specialty nurses using a clinical support system aimed at enhancing adherence to guideline-directed therapies improved cardiovascular outcomes in a single center study4 but not in the subsequent multicenter RACE 4 trial, where results were associated with experience of the center, although adherence to guideline-based recommendations was still greatly improved.10 In a cluster randomized trial of elderly patients with AF where integrated care intervention consisting of (a) quarterly AF check-ups by trained nurses in primary care, also focusing on comorbidities, (b) monitoring of anticoagulation therapy in primary care, and finally (c) easy-access availability of consultations from cardiologists and anticoagulation clinics, the patients assigned to integrated care experienced a 45% reduction in all-cause mortality when compared with usual care.11 In another study (SAFETY [Standard versus Atrial Fibrillation specific management study]), a posthospital discharge management program specific to AF was associated with proportionately more days alive and out of hospital but not prolonged event-free survival relative to standard management.12 On the other hand, data on technology-based clinical decision support systems are mixed, with a reduction in stroke and thromboembolism in the mAF-App 2 (Mobile Health Technology for Improved Screening and Optimized Integrated Care in Atrial Fibrillation) trial5 and improved anticoagulation use in the CDS-AF (clinical decision support tool for stroke prevention) trial,6 but no significant effect on primary outcomes in 3 other randomized trials.13–15 Educational interventions for both providers and patients can improve use of anticoagulation.16
6. PREVENTION OF THROMBOEMBOLISM
6.1. Risk Stratification Schemes
Recommendations for Risk Stratification Schemes
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. Patients with AF should be evaluated for their annual risk of thromboembolic events using a validated clinical risk score, such as CHA2DS2-VASc.1–4 |
| 1 | B-NR | 2. Patients with AF should be evaluated for factors that specifically indicate a higher risk of bleeding, such as previous bleeding and use of drugs that increase bleeding risk, in order to identify possible inteiventions to prevent bleeding on anticoagulation.5–7 |
| 2a | C-LD | 3. Patients with AF at intermediate annual risk of thromboembolic events by risk scores (eg, equivalent to CHA2DS2-VASc score of 1 in men or 2 in women), who remain uncertain about the benefit of anticoagulation, can benefit from consideration of factors that might modify their risk of stroke to help inform the decision.* |
| 3: No Benefit | B-NR | 4. In patients who are deemed at high risk for stroke, bleeding risk scores should not be used in isolation to determine eligibility for oral anticoagulation but instead to identify and modify bleeding risk factors and to inform medical decision-making.8–10 |
Factors may include AF burden or other features in Table 3.
Synopsis
Patients with AF have an increased risk of stroke that varies widely among individuals. Several risk scores based on clinical factors have been developed.1–3 Risk scores should be evaluated using accepted criteria11: their ability to discriminate between high- and low-risk individuals (eg, as assessed by the c-index), their accurate calibration to actual risk levels, and their validation in independent populations. A patient’s absolute risk of stroke is central to recommendations about anticoagulation and can be characterized as low (~<1%/y), intermediate (~1 to ~2%/y), and high (~>2%/y). Currently used risk scores discriminate moderately well between patients at higher and lower risk yet can greatly overestimate or underestimate absolute risk levels4 in different populations. Current risk scores may be inaccurate because they omit other factors that alter risk of stroke, especially characteristics of AF12–14; consequently, risk scores should be calibrated against the actual annual rates of stroke in the target population to assure an accurate, unbiased risk prediction. Newer risk scores may modestly improve risk discrimination (c-index) compared with CHA2DS2-VASc and may offer potential advantages in specific populations (Table 8). Online calculators are available for the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation),1 CHA2DS2-VASc,2 and GARFIELD-AF3 (Global Anticoagulant Registry in the Field-Atrial Fibrillation) risk scores.
Table 8.
Three Validated Risk Models for Stroke
| Risk Factor | CHA2DS2-VASc2 | ATRIA1 | GARFIELD3 |
|---|---|---|---|
| Age ≥85 y | 6 | 0.98 | |
| Age ≥75 y | 2 | 5 | 0.59 |
| Age 65–74 y | 1 | 3 | 0.20 |
| Female sex | 1 | 1 | |
| Hypertension | 1 | 0.16 | |
| Renal disease | 1 | 0.35 | |
| Diabetes | 1 | 1 | 0.21 |
| Current smoking | 0.48 | ||
| Congestive heart failure | 1 | 1 | 0.23 |
| Previous stroke or TIA | 2 | 2–8* | 0.80 |
| Vascular disease | 1 | 0.20 | |
| Dementia | 0.51 | ||
| Previous bleeding | 0.30 | ||
| Proteinuria | 1 | ||
| Low risk score | 0 | 0–5 | 0–0.89 |
| Intermediate risk score | 1 | 6 | 0.90–1.59 |
| High risk score | ≥2 | 7–15 | ≥1.60 |
| C-index (11) | 0.63 | 0.66 | - |
| C-index (13) | 0.67 | - | 0.71 |
8 points if age <65 y; 4 points if age 65–74 y; 2 points if age 75–84 y; and 3 points if ≥85 y.
ATRIA indicates Anticoagulation and Risk Factors in Atrial Fibrillation: anemia, renal disease, elderly (age ≥75 y), any previous bleeding, hypertension; CHA2DS2-VASc, indicates congestive heart failure, hypertension, age ≥75 y (doubled), diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism (doubled), vascular disease, age 65 to 74 y, sex category; GARFIELD-AF, Global Anticoagulant Registry in the Field-Atrial Fibrillation; and TIA, transient ischemic attack.
Anticoagulation increases the risk of bleeding, so patients with AF are generally evaluated for bleeding risk as part of SDM about anticoagulation. Currently used bleeding risk scores—HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio [INR], elderly [age ≥65 years], drugs/alcohol concomitantly]),5 HEMORR2HAGES (hepatic or renal disease, ethanol abuse, malignancy, older age [≥75 years], reduced platelet count or function, re-bleeding risk, hypertension [uncontrolled], anemia, genetic factors, excessive fall risk, stroke),6 and ATRIA (anemia, renal disease, elderly [age ≥75 years], any prior bleeding, hypertension)7—discriminate poorly between patients with and without bleeding15 and include many nonspecific factors that predict an increased risk of stroke as well as an increased risk of bleeding (eg, age, hypertension, renal disease, and previous stroke). Assessment of factors that specifically predict an increased risk of bleeding without predicting an increased risk of stroke is more helpful when balancing risks and benefits of anticoagulation.
Recommendation-Specific Supportive Text 1.
Several risk scores based on clinical factors have been developed1–3; in general, risk discrimination is improved by including more predictors (eg, CHA2DS2-VASc improved on the original CHADS22 [congestive heart failure, hypertension, age >75 years, diabetes, stroke/transient ischemia attack/thromboembolism] by adding additional risk factors and age categories). However, the absolute risk associated with any particular score level varies widely across populations; among 15 cohort studies, patients with a CHA2DS2-VASc score of 2 had annual rates of stroke that ranged from low to high: <1% in 4 cohorts, 1% to 2% in 6 cohorts, and >2% in 5 cohorts,4 although higher scores were associated with higher stroke risk in each cohort (Figure 9). The CHA2DS2-VASc score is considered the most validated score, most therapies have used that score to prove efficacy, and thus CHA2DS2-VASc is generally the preferred score.
Figure 9. Rates of Stroke by Stroke Risk Score Levels in Different Cohorts.
Overall stroke rate in atrial fibrillation cohorts in order of descending stroke rate (events per 100 person-years). Reproduced with permission from Quinn et al.4 Copyright 2017 American Heart Association, Inc.
Yet, despite its extensive use, CHA2DS2-VASc has shown suboptimal performance in selected populations, such as those with renal disease, prompting the creation of other scores. Newer risk scores, such as the ATRIA1,16,17 and GARFIELD-AF3,18 scores, modestly improve risk discrimination (c-index) compared with CHA2DS2-VASc, but their calibration and risk reclassification performance has not been as rigorously evaluated. A recent meta-analysis reported that 19 risk scores, 329 external validations, and 76 risk score updates have been conducted to predict ischemic stroke in patients with AF.19 Potential differences of the most studied risk scores are listed in Table 9. Some potential advantages of other scores, for example, the GARFIELD-AF score, includes mortality and bleeding risk to facilitate discussion with patients in a more comprehensive way. Also, when uncertainty exists, a score such as GARFIELD-AF adds additional variables to consider, such as smoking status, renal disease, and dementia. This guideline does not intend to preclude the future development of more accurate scores for stratifying patients.
Table 9.
Some Best Known Published Clinical Scores With Potential Advantages
| Year of Publication, Score Name | Score Components | Potential Advantages | No. of Validation Studies19 | Hyperlink to Online Score Calculator, if Available |
|---|---|---|---|---|
| 2001 CHADS225 | CHF, hypertension, age (≥65 y is 1 point, ≥75 y is 2 points), diabetes, stroke/TIA (2 points) | CHADS2 was superior to existing risk classification schemes AFI scheme: C-statistic, 0.68 (0.65–0.71) SPAF-III scheme: C-statistic, 0.74 (0.71–0.76) CHADS2 score: C-statistic, 0.82 (0.80–0.84) |
46 | https://www.mdcalc.com/calc/40/chads2-score-atrial-fibrillation-stroke-risk |
| 2010 CHA2DS2-VASc2 | CHF, hypertension, age ≥75 y, diabetes, stroke or TIA, vascular disease, age 65–74 y, female sex | Most commonly used and studied, superior to CHADS2 score. C-statistic, 0.606 (0.513–0.699) for CHA2DS2-VASc score vs 0.561 (0.450–0.672) for CHADS2 score Improved compared with original CHADS2 score |
82 | https://www.mdcalc.com/calc/801/cha2ds2-vasc-score-atrial-fibrillation-stroke-risk#next-steps |
| 2013 ATRIA1 | Age (65–74 y is 3 points, 75–84 y is 5 points, ≥85 y is 6 points), hypertension, diabetes, CHF, proteinuria, GFR <45 mL/min/1.73 m2, sex | Includes more age categories, renal function, and proteinuria More patients were classified as low or high risk but not as well tested in general. | 11 | https://www.mdcalc.com/calc/1842/atria-stroke-risk-score |
| 2017 GARFIELD-AF3 | Web-based, uses routinely collected clinical data, and includes a total of 16 questions | Web-based tool for predicting stroke and mortality, includes the effect of the different anticoagulants, bleeding risk and mortality to facilitate shared decision-making on the potential benefits/risks of anticoagulation | 4 | https://af.garfieldregistry.org/garfield-af-risk-calculator |
| 2016 MCHA2DS2-VASc26 | Expanded lower threshold for age to 50 y (1 point for age 50–74 y) | Validated in Asian cohort Can further identify Asian AF patients who may derive benefits from stroke prevention. In 1 study, MCHA2DS2-VASc was superior to CHA2DS2-VASc C-statistics = 0.708 (0.703–0.712) vs 0.689 (0.684–0.694) |
1 |
ATRIA indicates Anticoagulation and Risk Factors in Atrial Fibrillation: anemia, renal disease, elderly (age ≥75 y), any previous bleeding, hypertension; CHADS2, congestive heart failure, hypertension, age >75 y, diabetes, stroke/transient ischemia attack/thromboembolism; CHA2DS2-VASc, indicates congestive heart failure, hypertension, age ≥75 y (doubled), diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism (doubled), vascular disease, age 65 to 74 y, sex category; CHF, congestive heart failure; GARFIELD-AF, Global Anticoagulant Registry in the Field-Atrial Fibrillation; GFR, glomerular filtration rate; SPAF-III, stroke prevention atrial fibrillation, and TIA, transient ischemic attack.
Clinical decisions surrounding stroke prevention therapy in patients with AF must balance the risks of ischemic stroke, the risks of bleeding with treatment, net clinical benefit, and patient preferences. The most studied bleeding risk scores (HAS-BLED,5 HEMORR2HAGES,6 and ATRIA7) discriminate poorly between patients with and without bleeding20 (c-index 0.58 to 0.59 in a French nationwide study21). These scores are problematic to use in clinical decision-making because they incorporate several clinical factors that increase the risks of both stroke and bleeding (eg, age, hypertension, renal disease, and previous stroke), which makes it difficult to balance the benefits and risks of anticoagulation because the same risk factors also predict higher risk of stroke. Consideration of factors that specifically indicate a higher risk of bleeding without predicting higher risk of stroke (eg, previous bleeding, anemia, and certain medications) may better inform decision-making about the balance of benefits and harms expected from anticoagulation. Assessment of risk factors specific for bleeding may suggest interventions to reduce bleeding risk, such as discontinuing antiplatelet medications or nonsteroidal anti-inflammatory medications or the use of LAAO devices.10
The decision to treat with OACs for stroke prevention in patients with a CHA2DS2-VASc of 1 (CHA2DS2-VASc of 2 in women) may at times require additional discussion with patients as the strength or recommendation is less robust (2a), and often patients might be more reluctant. Also, the 1-point-concept of risk estimation in the subgroup of patients with a CHA2DS2-VASc score of 1 has shown to be simplistic because the magnitude of risk for each factor is heterogeneous and because data have shown a wide range of risk depending on the studied cohort.22 Thus, as part of SDM discussions with patients, other factors, such as AF burden, can be considered when interpreting a stroke risk score.12–14,23 Additional factors, such as degree of hypertension control, can also affect the risk of stroke; in the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial, a single elevated BP measurement during the study was associated with a 50% increased risk of stroke.23,24 Additional patient-specific risk factors, such as certain biomarkers (eg, proBNP), LA or left atrial appendage (LAA) function and anatomy, or electrocardiographic features, among others, have been demonstrated to influence stroke risk, yet it is unclear how to incorporate them into clinical practice.12 Antithrombotic treatment with DOACs seems to offer a superior net-benefit with regard to prevention of thromboembolic events and the risk of major bleeding compared with vitamin K antagonists (VKAs). Consideration of these additional factors may inform decision-making for patients with an intermediate risk of stroke who remain uncertain on deciding anticoagulation.
Unless an absolute contraindication to anticoagulation is present, bleeding risk scores have limitation in clinical decision-making because the most commonly used scores (HAS-BLED,5 HEMORR2HAGES,6 and ATRIA7) are based on several clinical factors that indicate higher risks of both stroke and bleeding, and patients with higher risk of bleeding also tend to have a higher risk of stroke. Furthermore, a bleeding risk score cannot be interpreted in isolation because it does not assess the net clinical benefit of anticoagulation or balance the risk of bleeding against the risk of stroke. Population-based studies suggest that the benefits of stroke prevention with oral anticoagulation generally outweigh the risks of bleeding, even in patients determined to be at high risk for bleeding.8,9 Decision-making about oral anticoagulation should be based on consideration of both benefits and harms, not by using bleeding risk scores in isolation, and the best utility of these scores may be identifying potential modifying risk factors.8,9
6.2. Risk-Based Selection of Oral
Anticoagulation: Balancing Risks and Benefits
Recommendations for Risk-Based Selection of Oral Anticoagulation: Balancing Risks and Benefits
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-R | 1. In patients diagnosed with AF who have an estimated annual risk of stroke or thromboembolic events ≥2%, selection of therapy to reduce the risk of stroke should be based on the risk of thromboembolism, regardless of whether the AF pattern is paroxysmal, persistent, long-standing persistent, or permanent.1–3 |
| 1 | B-NR | 2. In patients with AF at risk for stroke, reevaluation of the need for and choice of stroke risk reduction therapy at periodic intervals is recommended to reassess stroke and bleeding risk, net clinical benefit, and proper dosing.4,5 |
Synopsis
Prevention of stroke is important in patients with AF to maximize survival, health, and QOL. Selection of stroke risk reduction therapy should be guided by the patient’s risk of stroke, risks of bleeding with therapy, and their individual preferences. When considering stroke prevention therapy, the risk of stroke should inform the decision regardless of the pattern of AF (paroxysmal, persistent, long-standing persistent, or permanent). All decisions regarding stroke prevention therapy should be periodically reassessed since a patient’s risk, eligibility, and preferences can change over time.
Recommendation-Specific Supportive Text
Although the risk of stroke and systemic embolism and all-cause mortality are increased in persons with more persistent forms of AF, selection of stroke prevention therapy should be based on the risk of stroke and not the pattern of AF. Treatment effects with oral anticoagulation are consistent across AF patterns (paroxysmal, persistent, long-standing persistent, or permanent) in trials of stroke prevention therapy.1–3
AF is a lifelong condition and, thus, patient characteristics, risk factors, and net clinical benefit can and often will change over time.4 In long-term follow-up, stroke risk increases due to age and accumulation of other risk factors.6 Physiologic factors that impact stroke prevention therapy also change over time and can have important implications for proper medication dosing and patient safety.5 Typically, periodic assessment should be performed once a year but might need to be performed more frequently in the context of changes in clinical status, such as reduction in renal function or development of additional risk factors.
6.3. Oral Anticoagulants
Vitamin K Antagonists
Since the 1950s, warfarin was used as a first-line therapy until DOACs came into practice. A narrow therapeutic window based on international normalized ratios (INRs), frequent monitoring, more frequent drug interactions (mainly through CYP2C9), dietary restrictions, and low clinical safety profile affected the routine use of warfarin in practice. Because of affordability issues of DOACs for some patients with AF, warfarin is still an appropriate OAC due to its lower cost for patients who cannot afford DOACs. About 21% of patients with nonvalvular AF were still receiving warfarin, while the rest received DOACs in the first quarter of 2017.1 Warfarin remains the first-line therapy in patients with AF and moderate-severe rheumatic mitral stenosis or mechanical heart valves. Clinical studies show that the target INR is between 2 and 3, and risk of bleeding becomes mostly apparent when INR exceeds 4. OAC use in special populations will be discussed in separate sections.
Direct oral anticoagulants
DOACs were developed to address the disadvantages of warfarin and are currently recommended as the first-line therapy over warfarin in patients with AF (except moderate to severe mitral stenosis or mechanical heart valve recipients) in this guideline. All 4 pivotal clinical trials comparing individual DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) with warfarin showed superiority or noninferiority to warfarin for the prevention of stroke or systemic embolism in patients with AF except for moderate to severe mitral stenosis or mechanical heart valve.2–5 They also showed significantly lower risks of major bleeding in the apixaban, dabigatran 110 mg twice daily group, and edoxaban 30 mg or 60 mg daily dose groups compared with the warfarin group or nonsignificant differences in major bleeding between dabigatran 150 mg twice daily group or the rivaroxaban group and warfarin. All DOAC groups showed significantly lower risks of intracranial hemorrhage (ICH) compared with warfarin. Gastrointestinal bleeding risks were significantly higher in the dabigatran 150 mg twice daily, edoxaban 60 mg once daily, and rivaroxaban groups compared with the warfarin group. However, the apixaban group did not significantly increase the risk of gastrointestinal bleeding compared with the warfarin group.
6.3.1. Antithrombotic Therapy
Recommendations for Antithrombotic Therapy
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | A | 1. For patients with AF and an estimated annual thromboembolic risk of ≥2% per year (eg, CHA2DS2-VASc score of ≥2 in men and ≥3 in women), anticoagulation is recommended to prevent stroke and systemic thromboembolism.1–7 |
| 1 | A | 2. In patients with AF who do not have a history of moderate to severe rheumatic mitral stenosis or a mechanical heart valve, and who are candidates for anticoagulation, DOACs are recommended over warfarin to reduce the risk of mortality, stroke, systemic embolism, and ICH.1–7 |
| 2a | A | 3. For patients with AF and an estimated annual thromboembolic risk of ≥1% but <2% per year (equivalent to CHA2DS2-VASc score of 1 in men and 2 in women), anticoagulation is reasonable to prevent stroke and systemic thromboembolism.1,3 |
| 3: Harm | B-R | 4. In patients with AF who are candidates for anticoagulation and without an indication for antiplatelet therapy, aspirin either alone or in combination with clopidogrel as an alternative to anticoagulation is not recommended to reduce stroke risk.8,9 |
| 3: No Benefit | B-NR | 5. In patients with AF without risk factors for stroke, aspirin monotherapy for prevention of thromboembolic events is of no benefit.10,11 |
Synopsis
A high risk for stroke or systemic embolism is about 2% per year, and all the DOAC trials (Re-LY [Randomized Evaluation of Long-Term Anticoagulation Therapy]; ROCKET AF [Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation]; ARISTOTLE; and ENGAGE AF-TIMI 48 [Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation – Thrombolysis in Myocardial Infarction 48]) included patients with this level of risk.1–4 Patients at intermediate risk (1%-2%/y) can also benefit from anticoagulation, and the RE-LY1 and ARISTOTLE3 trials included this population. Stroke risk scores applied to cohorts give different stroke rates, and therefore any score should be viewed as only an estimate of true risk; in addition some scores used stroke, while others used thromboembolic events. Nonetheless, it is practical to use a validated risk score, such as CHA2DS2-VASc, ATRIA, or GARFIELD-AF. Future research may yield improved risk scores that refine how to incorporate risk modifiers, such as female sex12 and other parameters such as AF burden. Anticoagulation has also been shown to be superior to antiplatelet therapy to reduce stroke risk.8,9 Recommendations for antithrombotic selection in valvular heart disease (VHD) are provided in Section 6.8.5 (“AF in VHD”) and for AFL in Section 6.8.6 (Figure 10).
Figure 10. Antithrombotic Options in Patients With AF.
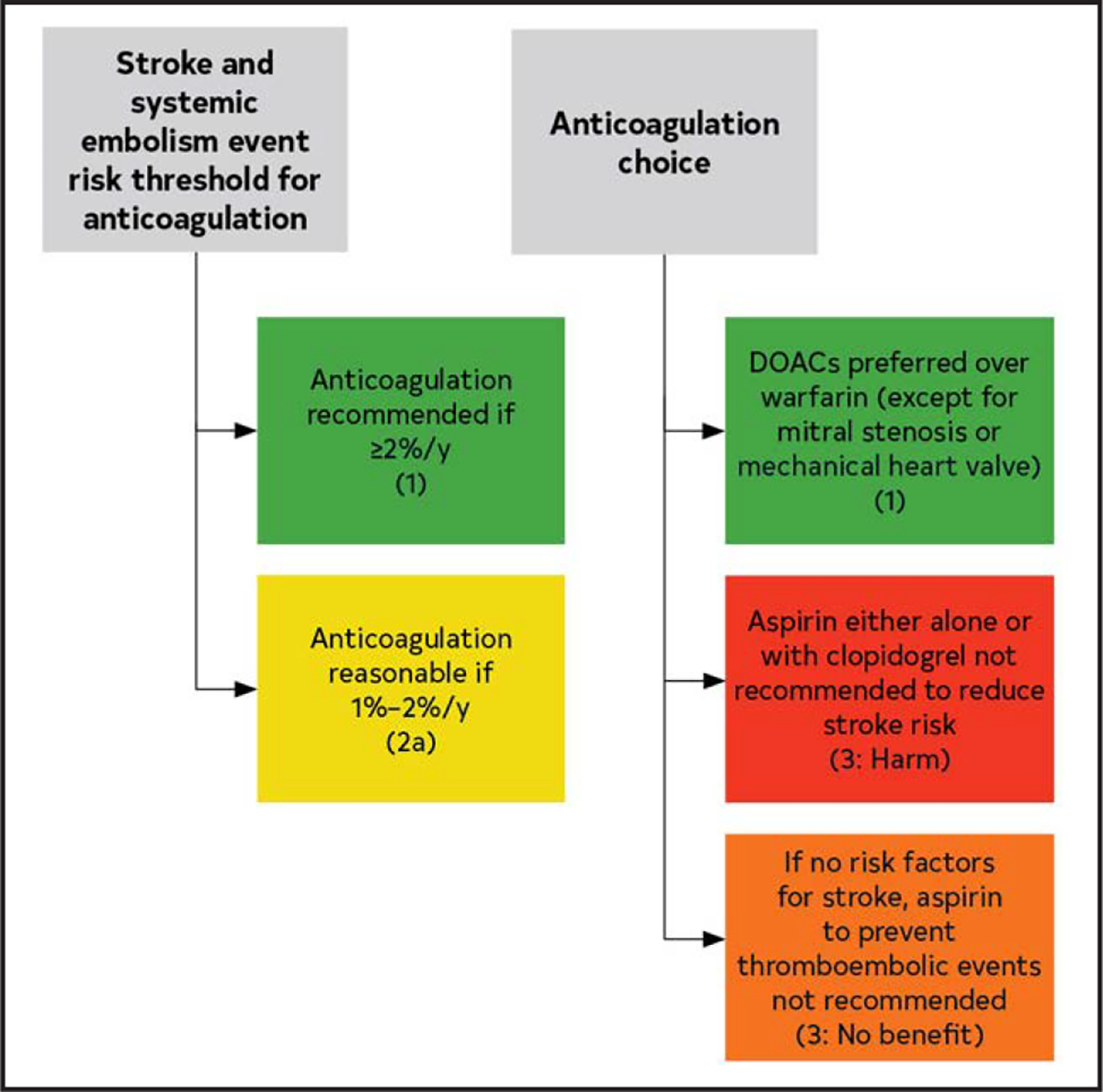
Colors correspond to Table 2. AF indicates atrial fibrillation; and DOAC, direct oral anticoagulant.
Recommendation-Specific Supportive Text
Randomized trials published in the 1990s established the superiority of anticoagulation, at that time limited to warfarin, to reduce stroke.11 Since that time, management has advanced considerably, and the randomized trials that compared DOACs with warfarin1–4 are more relevant to current antithrombotic management. In these trials1–4 and metanalyses,5–7 DOACs were favored for lower risk of stroke, systemic embolism, and ICH.1–7 These trials reported the CHADS2 score. The recently studied ATRIA and Swedish cohorts13,14 reported lower stroke rates, by CHADS2 score, compared with that previously reported.15 A CHADS2 score of 1 gave a stroke rate of 1.20 in ATRIA14 and 2.4 in a Swedish cohort.13 A CHADS2 score of 2 gave a stroke rate of 2.59 in ATRIA14 and 3.5 in the Swedish cohort.13 Therefore, the stroke risk of patients in the rivaroxaban2 and edoxaban4 trials was likely >2% given a required minimum CHADS2 score of 2, and lower in the apixaban3 and dabigatran1 trials that included scores of 0 and 1. A Markov state transition decision model16 concluded that anticoagulation was preferred for a stroke rate of 1.7%. Therefore, a stroke and systemic embolism risk threshold of 2% is likely to yield a benefit that far exceeds risk. In some conditions, such as hypertrophic cardiomyopathy, the risk of stroke is high enough independent of risk score to indicate anticoagulation.17
In the randomized DOAC trials, all DOACs achieved noninferiority1–4 and in 2 trials (for dabigatran, RE-LY, and for apixaban, ARISTOTLE) were superior to warfarin. With warfarin, regular assessment of the INR is necessary to maintain a therapeutic value. In the RCTs, the mean time in therapeutic range was only 55% in ROCKET AF2 and highest at 66% in ARISTOTLE.3 In a meta-analysis,6 DOACs, compared with warfarin, had a relative risk of stroke of 0.81 (95% CI, 0.73–0.91; P<0.0001), a relative risk of mortality of 0.90 (95% CI, 0.85–0.95; P=0.0003), relative risk of ICH of 0.48 (95% CI, 0.39–0.59; P<0.0001), and increased relative risk of bleeding of 1.25 (95% CI, 1.01–1.55; P=0.043). Although patients with moderate to severe mitral stenosis or mechanical valve were excluded from DOAC trials (and subsequently shown they may have worse outcomes with DOACS), other forms of VHD were allowed, such as aortic stenosis or regurgitation or mitral regurgitation. Bioprosthetic valves and valve repair were allowed in the edoxaban (ENGAGE AF) and apixaban (ARISTOTLE) trials, and valve repair in the rivaroxaban (ROCKET AF) trial. A systematic review18 of patients with VHD (other than mitral stenosis or mechanical valve) concluded that DOACs were safe.
As the DOAC trials1–4 demonstrated improved safety compared with warfarin, the threshold of using a DOAC might be different than for warfarin.16 A Markov decision model found the tipping point for warfarin to be at 1.7%/year stroke risk.16 Considering the improved ICH and mortality risk of DOACs compared with warfarin in meta-analyses of the DOAC trials,5–7 it is appropriate to designate a lower stroke risk threshold if a DOAC is utilized. The dabigatran1 and apixaban3 trials included lower risk patients and about one-third of patients had a CHADS2 score of 0 or 1. This likely corresponds to an estimated annual stroke risk of about 1%, considering more recent cohorts.13,14 This is further supported by meta-analyses that showed a consistent benefit of DOACs across a broad range of vulnerable patients, including as classified by CHADS2 scores.6,7
A meta-analysis of AF trials from the 1990s and early 2000s found that while antiplatelet therapy (APT), most commonly aspirin, reduced stroke and systemic embolism compared with placebo, APT was inferior to warfarin in patients with AF.11 Aspirin was studied compared to apixaban in the AVERROES trial (Apixaban Versus ASA to Prevent Stroke In AF Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment)9 for patients unsuitable for VKAs; this trial was stopped early due to the benefit of apixaban over aspirin to prevent stroke or systemic embolism, while major bleeding was similar between the 2 arms. The combination of clopidogrel and aspirin was compared to VKAs in ACTIVEW (Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events),8 and this trial was stopped prematurely due to the superiority of anticoagulation with VKAs to prevent stroke, non–central nervous system systemic embolus, MI, or vascular death. Unless there is an indication for antiplatelet therapy, such as coronary artery disease (CAD) or vascular disease, patients with AF should not be prescribed antiplatelet therapy to reduce stroke risk.
Aspirin therapy was compared with no treatment in patients with low stroke risk in a multicenter RCT in Japan, the Japan Atrial Fibrillation Stroke Trial.10 This trial was stopped early when an interim analysis showed that aspirin gave a marginally higher risk of major bleeding and was unlikely to prevent primary or secondary endpoints. The primary endpoint was noncardiovascular death, ICH, major bleeding, and peripheral embolization. A meta-analysis of 7 trials that studied aspirin versus placebo found that aspirin reduced stroke by 19%, which did not reach statistical significance with a CI that included 0 (−1% to 35%). Four of these were primary prevention trials and showed an absolute risk reduction with aspirin of 0.8% per year, with a number needed to treat of 125.11 Although aspirin has not been studied in patients without any risk factors for stroke, patients without stroke risk may derive no benefit from aspirin therapy. In fact, 1 study that analyzed stroke mechanisms in the SPAF (Stroke Prevention in Atrial Fibrillation) I-III trials demonstrated that aspirin did not decrease the rate of cardioembolic stroke and that AF patients at highest risk for stroke are those with the highest rates of cardioembolic stroke and have the greatest reduction in stroke with anticoagulants.19 Thus, modest reduction in stroke observed in some trials may have been related to noncardioembolic stroke in patients with additional risk factors.
6.3.1.1. Considerations in Managing Anticoagulants
Recommendations for Considerations in Managing Anticoagulants
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. For patients with AF receiving DOACs, optimal management of drug interactions is recommended for those receiving concomitant therapy with interacting drugs, especially CYP3A4 and/or p-glycoprotein inhibitors or inducers (Table 13).1–7 |
| 1 | B-R | 2. For patients with AF receiving warfarin,* a target INR between 2 and 3 is recommended, as well as optimal management of drug-drug interactions, consistency in vitamin K dietary intake, and routine INR monitoring to improve time in therapeutic range and to minimize risks of preventable thromboembolism or major bleeding.8–10 |
| 3: Harm | B-NR | 3. For patients with AF, nonevidence-based doses of DOACs should be avoided to minimize risks of preventable thromboembolism or major bleeding and to improve survival.11,12 |
Excludes patients with mechanical valves.
Table 13.
OACs Pharmacokinetic Characteristics and Dosing
| Class | VKA | Direct Thrombin Inhibitor | Factor Xa Inhibitor | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Name | Warfarin | Dabigatran | Rivaroxaban | Apixaban | Edoxaban | ||||
| Metabolism | S-isomer: CYP2C9 R-isomer: CYP1A2, CYP2C19, CYP3A4 |
Minimal | CYP3A4/5 | CYP3A4 | Minimal CYP3A4 | ||||
| P-glycoprotein substrate | No | Yes | Yes | Yes | Yes | ||||
| Excretion | 0% renal; very little warfarin excreted unchanged in urine | 80% renal | 66% renal, 28% feces | 27% renal, 73% biliary and intestinal | 50% renal, 50% liver and biliary/intestinal | ||||
| Half-life | 20–60 h | 12–17 h | 5–9 h | 12 h | 10–14 h | ||||
| Renal dosing adjustment based on actual body weight | N/A | CrCl >30 mL/min | 150 mg twice daily | CrCl >50 mL/min | 20 mg daily with the biggest meal* | 5 mg twice daily | CrCl >50–≤95 mL/min | 60 mg once daily | |
| CrCl 15–30 mL/min | 75 mg twice daily | CrCl 15–50 mL/min | 15 mg daily with the biggest meal* | If any 2 of the following: age ≥80y, body weight ≤60 kg, SCr ≥1.5 mg/dL | 2.5 mg twice daily | CrCl 15–50 mL/min | 30 mg once daily | ||
| Drug interaction management based on concomitant therapy of CYP3A4 inhibitors/p-glycoprotein inhibitors | Adjust dose based on INR trends | CrCl 30–50 mL/min with concomitant use of dronedarone or systemic ketoconazole: 75 mg twice daily CrCl <30 mL/min: avoid dabigatran use concomitantly with dronedarone or systemic ketoconazole |
Avoid rivaroxaban use with concomitant therapy of combined p-glycoprotein and strong CYP3A4 inhibitors (eg, systemic ketoconazole and ritonavir) No dose adjustment required with clarithromycin Avoid rivaroxaban use in patients with CrCl 15–<80 mL/min receiving combined p-glycoprotein and moderate CYP3A4 inhibitors (eg, erythromycin) |
In patients receiving apixaban 5 mg twice daily, reduce dose to 2.5 mg twice daily when combined p-glycoprotein and strong CYP3A4 inhibitors (eg, itraconazole, systemic ketoconazole, ritonavir) are used concomitantly If patients already receiving apixaban 2.5 mg twice daily, avoid apixaban use if combined p-glycoprotein and strong CYP3A4 inhibitors are concomitantly used |
No dose adjustment is required | ||||
| Drug interaction management based on concomitant therapy of p-glycoprotein/CYP3A4 inducers (eg, carbamazepine, phenytoin, rifampin, St. John’s wort) | Adjust dose based on INR trends | Avoid use | Avoid use | Avoid use | Avoid use with rifampin. No study evaluated the effect of other p-glycoprotein/CYP3A4 inducers on edoxaban drug levels | ||||
| Appropriate use based on liver function (Child-Pugh score)† Child-Pugh A (mild) |
Not mentioned in the labeling | No dose adjustment needed | No dose adjustment needed | No dose adjustment needed | No dose adjustment needed | ||||
| Child-Pugh B (moderate) | Use with caution | Avoid use | Use with caution | Use with caution | |||||
| Child-Pugh C (severe) | Avoid use | Avoid use | Avoid use | Avoid use | |||||
Information obtained from manufacturer package inserts.13,14,20–22 Adapted with permission from pgs. 28–31 of Kido et al.23 Copyright 2021 American College of Clinical Pharmacy.
The effect of food (high-fat, high-calorie meal) on bioavailability for 10- and 20-mg tablet was evaluated in 24 subjects under fed and fasting conditions. After a single oral 20-mg dose, area under the curve was increased by 39%, and Cmax was increased by 76% under fed condition, but area under the cuive and Cmax were similar between fasting and fed conditions.19
Child-Pugh scoring: the severity of liver disease, primarily cirrhosis. Child-Pugh A (mild): 5 to 6 points; Child-Pugh B (moderate): 7 to 9 points; Child-Pugh C (severe): 10 to 15 points. The score is based on the 5 variables: encephalopathy (none=1 point, grade 1 and 2=2 points, grade 3 and 4=3 points); ascites (none=1 point, slight=2 points, moderate=3 points); total bilirubin (<2 mg/mL=1 point, 2–3 mg/mL=2 points, >3 mg/mL=3 points); albumin (>3.5 mg/mL=1 point, 2.8–3.5 mg/mL=2 points, <2.8 mg/mL=3 points); INR (<1.7=1 point, 1.7–2.2=2 points, >2.2=3 points).
CrCl indicates creatinine clearance; INR, international normalized ratio; OAC, oral anticoagulant; Scr, serum creatinine; and VKA, vitamin K antagonist.
Synopsis
Multiple factors need to be considered to select an optimal OAC in patients with AF. Efficacy, safety, insurance coverage, renal/hepatic function, drug interaction screening, medication adherence, and patient preferences are the major factors for consideration. Dosing of DOACs in patients with AF should be selected based on age, renal function, weight, and concomitant medications. The hepatic function also needs to be evaluated for the appropriateness of DOACs. Characteristics and dosing of OACs are summarized in Table 13. Although DOAC drug interactions occur in practice less frequently than with warfarin, clinically significant drug interactions through CYP3A4 and/or p-glycoprotein should be carefully evaluated. Strong CYP3A4 and/or p-glycoprotein inhibitors such as ketoconazole, itraconazole, and ritonavir may significantly increase DOAC plasma levels and the risk of bleeding, while strong CYP3A4 and/or p-glycoprotein inducers such as rifampin, phenytoin, phenobarbital, primidone, carbamazepine, or St. John’s wort may lower DOAC plasma levels and increase the risks of stroke or systemic embolism. The routine measurement of DOAC plasma concentrations is not indicated in practice due to the lack of well-established therapeutic ranges in the literature. DOAC levels may be indicated when clinicians assess DOAC adherence for potentially noncompliant patients, quantify residual anticoagulation levels before emergency invasive procedures/surgeries, or evaluate the absorption of DOAC after bariatric surgery. Regular monitoring is recommended to optimize indications, ensure appropriate dosing, and avoid adverse effects. Suggested laboratory monitoring is summarized in Figure 11.
Figure 11. DOAC Laboratory Monitoring.
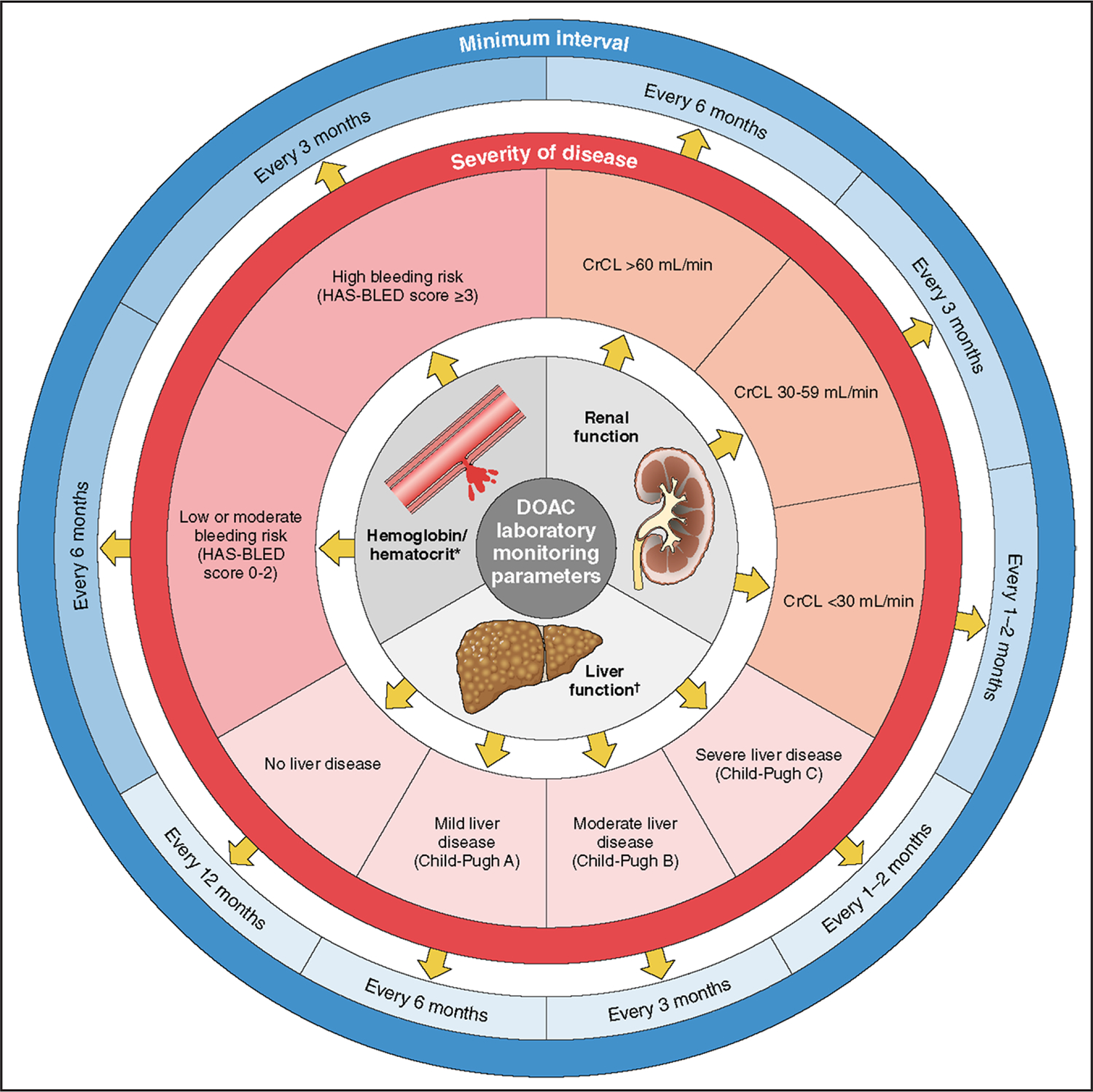
*HAS-BLED scoring (low risk=score 0, moderate risk=score 1–2, high risk=score ≥3): uncontrolled hypertension (systolic blood pressure >160 mm Hg)=1 point; abnormal renal (serum creatinine >2.26 mg/dL, dialysis, or kidney transplant) or hepatic function (bilirubin >2 times upper limit normal, alanine aminotransferase/aspartate aminotransferase/alkaline phosphatase >3 times upper limit normal, or cirrhosis)=1 or 2 points; stroke (hemorrhagic or ischemic)=1 point; bleeding history or predisposition=1 point; labile INR (time in therapeutic range <60%)=1 point; elderly age ≥65 years=1 point; drugs (antiplatelet agents or nonsteroidal anti-inflammatory drugs) or excessive alcohol intake (8 units/week)=1 or 2 points. †Child-Pugh scoring: the severity of liver disease, primarily cirrhosis in patients with documented liver disease. Child-Pugh A (mild): 5 to 6 points; Child-Pugh B (moderate): 7 to 9 points; Child-Pugh C (severe): 10 to 15 points. The score is based on the 5 variables: encephalopathy (none=1 point, grade 1 and 2=2 points, grade 3 and 4=3 points); ascites (none=1 point, slight=2 points, moderate=3 points); total bilirubin (<2 mg/mL=1 point, 2–3 mg/mL=2 points, >3 mg/mL=3 points); albumin (>3.5 mg/mL=1 point, 2.8–3.5 mg/mL=2 points, <2.8 mg/mL=3 points); INR (<1.7=1 point, 1.7–2.2=2 points, >2.2=3 points). Original figure created by the 2023 Atrial Fibrillation Guideline Writing Committee. CrCL indicates creatinine clearance based on actual body weight; DOAC, direct oral anticoagulant; and INR, international normalized ratio.
If warfarin is selected over DOACs, achievement of higher time in therapeutic range (eg, ≥70%), and thorough consideration of drug-drug interactions, vitamin K food intake advice, and patient education on adherence to dosing instructions are important to reduce adverse effects.
Recommendation-Specific Supportive Text
Rivaroxaban is contraindicated for patients receiving ketoconazole or ritonavir, because ketoconazole or ritonavir coadministration significantly increased rivaroxaban plasma levels by 158% or 153%, respectively.1 Apixaban also requires dose adjustment to 2.5 mg twice daily for patients receiving apixaban 5 mg twice daily when ketoconazole, itraconazole, or ritonavir is started.13 Apixaban plasma level was significantly increased by 99% when ketoconazole was coadministered in healthy subjects.2 The concomitant therapy of dronedarone or ketoconazole for patients with creatinine clearance (CrCl) 30 to 50 mL/min receiving dabigatran requires dose adjustment to dabigatran 75 mg twice daily because it produced comparable dabigatran exposure in patients with CrCl 30 to 50 mL/min receiving the concomitant therapy to patients with CrCl 15 to 29 mL/min receiving only dabigatran.14 A retrospective study revealed that the probability of patients with subreference DOAC levels was significantly higher in patients receiving antiepileptic drugs compared to those not receiving any CYP3A4 inducers.4 The claim-based retrospective cohort study showed that CYP-inducing antiepileptic drug use was significantly associated with an 86% increase in thromboembolic and ischemic adverse events in patients receiving DOACs.6 A prospective multicenter cohort study corroborated that the patients receiving concomitant therapy of DOACs and antiepileptic drugs developed a higher rate of stroke/TIA/systemic embolism (5.7% patient-year).7
Warfarin remains the preferred agent in patients with AF receiving CYP3A4/p-glycoprotein–inducing agents, or moderate-severe mitral stenosis or mechanical heart valve. Also, warfarin may be selected over DOACs in patients with AF due to higher cost or intolerances of DOACs. The optimal INR control, with a therapeutic INR goal of 2 to 3, needs to be achieved through the appropriate drug-drug interaction management, vitamin K dietary education, and routine INR monitoring. A systematic review of 47 studies found that time in therapeutic INR range showed negative correlation with risks of thromboembolism (R, −0.59; P=0.01) and major bleeding (R, −0.59; P=0.002).8 An RCT revealed that a weekly vitamin K dietary modification significantly achieved a therapeutic INR more frequently than a conventional group (74 versus 58%; P=0.04) at 90 days after randomization.9 A meta-analysis of 11 RCTs and 61 observational studies for warfarin drug interactions found that the concomitant use of APT (odds ratio [OR], 1.74 [95% CI, 1.56–1.94]), nonsteroidal anti-inflammatory drugs (OR, 1.83 [95% CI, 1.29–2.59]), selective serotonin reuptake inhibitors (OR, 1.62 [95% CI, 1.42–1.85]), or antimicrobial agents (OR, 1.63 [95% CI, 1.45–1.83]) was significantly associated with higher risks of clinically relevant bleeding.10
A significant number of patients with nonvalvular AF are receiving DOAC off-label doses not compliant with labeling.15–18 A meta-analysis of cohort studies showed that inappropriately lower DOAC doses are significantly associated with higher stroke or systemic embolism risks compared with the standard labeled doses (OR, 1.21 [95% CI, 1.02–1.43]; P=0.03), but no significant differences in bleeding risks were observed (OR, 1.03 [95% CI, 0.92–1.15]; P=0.62).11 Of note, 9 of 16 included studies were performed in Asia, while 4 studies in the United States, 2 studies in Europe, and 1 worldwide study were included. Another meta-analysis of observational studies found that inappropriately higher DOAC doses were significantly associated with higher risks of ischemic stroke/systemic embolism (hazard ratio [HR], 1.26 [95% CI, 1.11–1.43]; P=0.003) or major bleeding (HR, 1.30 [95% CI, 1.04–1.62]; P=0.025) compared with the standard doses.12 In this study, underdosing was also associated with a higher risk of net clinical outcome (HR, 1.19 [95% CI, 1.04–1.40]; P=0.04) and all-cause death (HR, 1.24 [95% CI, 1.04–1.48]; P=0.02). Off-label dosing should be avoided to optimize the efficacy and safety of DOACs.
6.4. Silent AF and Stroke of Undetermined Cause
Recommendation for Silent AF and Stroke of Undetermined Cause
Referenced studies that support the recommendation are summarized in the Online Data Supplement.
| COR | LOE | Recommendation |
|---|---|---|
| 2a | B-R | 1. In patients with stroke or TIA of undetermined cause, initial cardiac monitoring and, if needed, extended monitoring with an implantable loop recorder are reasonable to improve detection of AF.1 |
Synopsis
Approximately 25% to 30% of ischemic strokes remain cryptogenic after standard stroke evaluation.2,3 A large proportion of these events are presumed to be related to occult AF, particularly those of embolic appearance.4,5 APT is the recommended treatment of choice for patients with cryptogenic stroke, including embolic stroke of undetermined source.6,7 For patients with AF in general, however, anticoagulation has been shown to be superior to APT for stroke risk reduction.8 Thus, detection of occult AF after stroke may have significant therapeutic implications. Randomized trials have shown longer durations of cardiac monitoring result in higher rates of AF detection after stroke.1,9,10 However, data are limited regarding the effects of extended monitoring on risk reduction of recurrent stroke or poststroke mortality. For recommendations on anticoagulation and risk factors, refer to Section 6.4.1 (“Oral Anticoagulants”).
Recommendation-Specific Supportive Text
Growing evidence supports the use of extended cardiac monitoring for the identification of occult AF in patients with cryptogenic stroke.11,12 In the EMBRACE (30 Day Event Monitoring Belt for Recording Atrial Fibrillation After a Cerebral Ischemic Event) trial, which randomized 572 patients ≥55 years of age with recent cryptogenic stroke or TIA to either 30-day external loop recorder or conventional 24-hour Holter monitoring, extended monitoring was associated with higher rates of AF detection at 90 days.1The CRYSTAL-AF (Cryptogenic Stroke and Underlying AF) trial randomized 441 patients ≥40 years of age with recent cryptogenic stroke or TIA to cardiac monitoring with either insertable loop recorder or conventional follow-up ECGs. Implantable recorder was superior in detecting AF at 6 months (8.9% versus 1.4%), 12 months (12.4% versus 2.0%), and 3 years (30% versus 3%).9 In FIND-AF (Future Innovations in Novel Detection of Atrial Fibrillation), which compared repeated sets of 10-day Holter monitoring (at baseline, 3-month, and 6-month timepoints) to conventional 24-Holter in patients ≥60 years of age with recent stroke, higher rates of detection were associated with repeated monitoring (14% versus 5%; absolute difference, 9.0% [95% CI, 3.4–14.5]; P=0.002).10 Finally, the PER DIEM (Post-Embolic Rhythm Detection with Implantable vs External Monitoring) RCT also showed a significantly greater proportion of patients with AF detected at 1 year with prolonged monitoring.13 Additional studies are needed, however, to determine whether extended cardiac monitoring improves long-term poststroke outcomes.
6.4.1. Oral Anticoagulation for Device-Detected Atrial High-Rate Episodes Among Patients Without a Previous Diagnosis of AF
Recommendations for Oral Anticoagulation for Device-Detected Atrial High-Rate Episodes Among Patients Without a Previous Diagnosis of AF Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-NR | 1. For patients with a device-detected atrial high-rate episode (AHRE) lasting ≥24 hours1 and with a CHA2DS2-VASc score ≥2 or equivalent stroke risk,2 it is reasonable to initiate oral anticoagulation3 within a SDM framework that considers episode duration and individual patient risk. |
| 2b | B-NR | 2. For patients with a device-detected AHRE lasting between 5 minutes and 24 hours and with a CHA2DS2-VASc score ≥3 or equivalent stroke risk,2 it may be reasonable to initiate anticoagulation within a SDM framework that considers episode duration and individual patient risk. |
| 3: No Benefit | B-NR | 3. Patients with a device-detected AHRE lasting <5 minutes and without another indication for oral anticoagulation should not receive oral anticoagulation.4,5 |
Synopsis
AHREs detected by a cardiovascular implantable electronic device are associated with a stroke risk lower than that of clinical AF6 that varies according to the episode duration1 and CHA2DS2-VASc score.2 Clinician confirmation of the duration and nature of the longest atrial high-rate episode is recommended.1 Episodes lasting ≥24 hours are associated with a significant risk of stroke or systemic embolism1,7 that may be reduced with oral anticoagulation.3 By contrast, short episodes, commonly defined as <5 minutes, are not associated with clinical events.4,5 Patients with episodes of intermediate duration and with an elevated stroke risk may benefit from oral anticoagulation.2 There is an interaction between AHRE duration and CHA2DS2-VASc score,2 suggesting both may be used to guide oral anticoagulation candidacy (Figure 12). Of note, the threshold for anticoagulation is higher as in device-detected AF the risk of stroke may be lower than in clinical AF.
Figure 12. Consideration of Oral Anticoagulation for Device-Detected AHREs According to Patient Stroke Risk by CHA2DS2-VASc Score and Episode Duration.
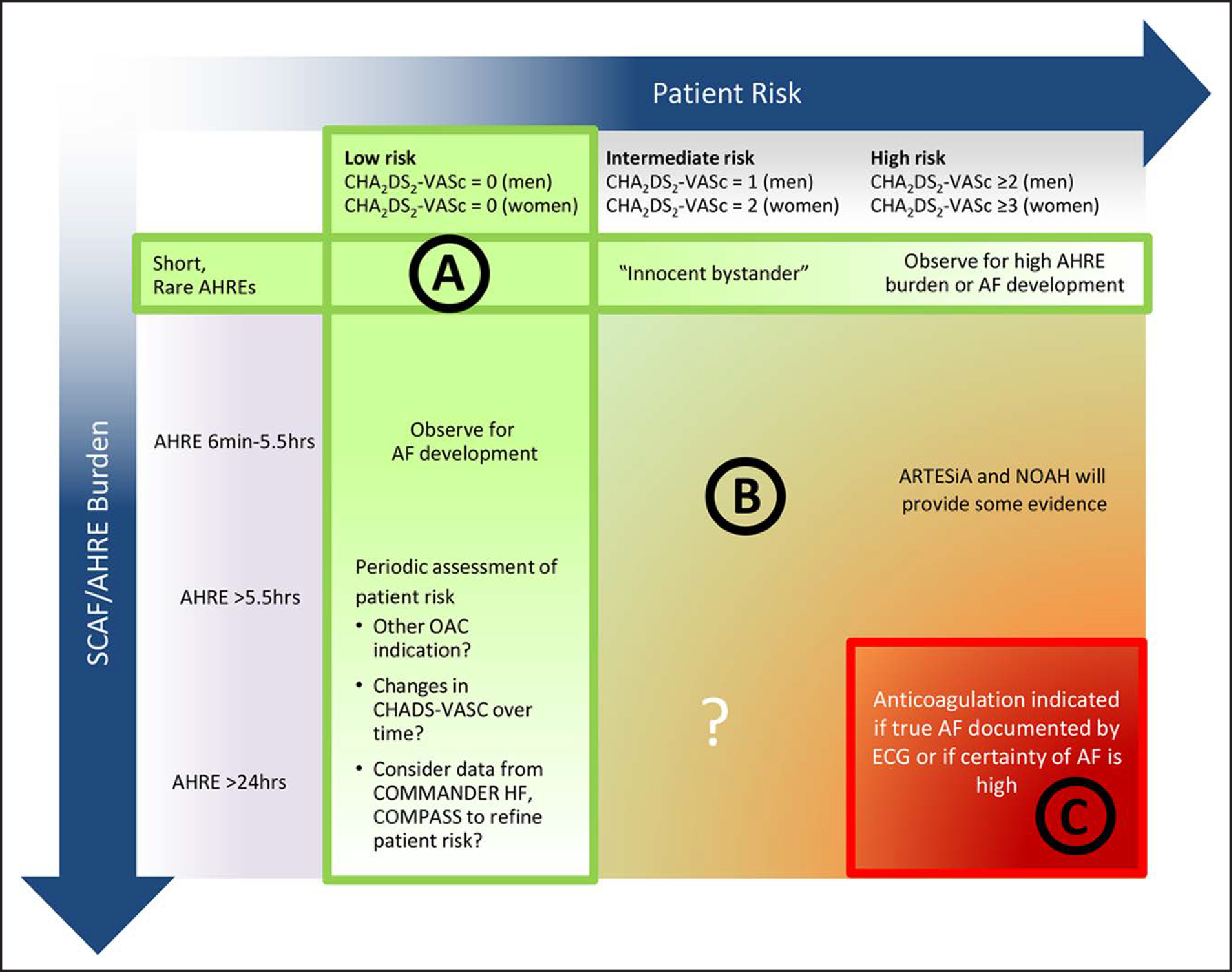
A potential approach to patients with SCAF could consider both patient risk (as gauged by the CHA2DS2-VASc score) and SCAF burden/duration. Circle A indicates patients at low risk or with short and infrequent AHREs do not require anticoagulation; Circle B, patients with intermediate risk and AHREs lasting >6 min to 24 h are an uncertain population but are currently under study in 2 prospective randomized controlled trials; and Circle C, patients at high risk with longer episodes could be considered reasonable candidates for anticoagulation, although the precise threshold for SCAF duration remains uncertain. Reproduced with permission from Noseworthy et al.13 Copyright 2019 American Heart Association, Inc. Modified from Freedman et al.14 Copyright 2017 Springer Nature Limited. AF indicates atrial fibrillation; AHRE, atrial high-rate episode; ARTESiA, Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Subclinical Atrial Fibrillation trial; COMMANDER HF, A Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction, or Stroke in Participants With Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure; COMPASS, Cardiovascular Outcomes for People Using Anticoagulation Strategies; ECG, electrocardiogram; NOAH, Non–Vitamin K Antagonist Oral Anticoagulants in Patients With Atrial High Rate Episodes Trial; OAC, oral anticoagulation; and SCAF, subclinical atrial fibrillation. Female sex is treated as a modifier in the computation of the CHA2DS2-VASc score.
Recommendation-Specific Supportive Text
AHREs lasting ≥24 hours are associated with an elevated risk of stroke that varies according to CHA2DS2-VASc score. AHRE burden is most commonly defined according to the duration of the longest-detected episode, though cumulative duration has also been examined. In a secondary analysis of the ASSERT (Atrial Fibrillation Reduction Atrial Pacing Trial), adjudicated AHREs >24 hours was associated with an increased risk of subsequent stroke or systemic embolism (adjusted HR, 3.24 [95% CI, 1.51–6.95]; P=0.003).1 Similar findings are seen in observational studies in which most AHREs were not adjudicated,7 though clinician confirmation is preferred. In an observational study linking administrative claims data2 to a device database in which continuous rhythm monitoring data were available but not adjudicated, the risk of stroke among patents with subclinical AF >23.5 hours and a sex-modified CHA2DS2-VASc score ≥2 not taking anticoagulation exceeded 1% per year, a threshold that may be appropriate for use of non-VKAs, with a still higher stroke risk with increasing CHA2DS2-VASc score ≥3.8 In a retrospective cohort study of patients with subclinical AF >24 hours with an average CHA2DS2-VASc score of 4.2±1.4, treatment of subclinical AF >24 hours with OAC was associated with a reduced stroke risk (HR, 0.28 [95% CI, 0.10–0.81]; P=0.02).2,3
Although the risk of stroke among patients with an AHRE of intermediate duration is lower than that among patients with an AHRE ≥24 hours, the precise stroke risk is uncertain and may vary according to the CHA2DS2-VASc score. In ASSERT, AHREs lasting >6 minutes to 24 hours were not associated with stroke or systemic embolism compared with patients without AHREs.1 The CHA2DS2-VASc score of this subpopulation was 2.2±1.0 (mean±SD). In the aforementioned study linking administrative claims data to a device database in which continuous rhythm monitoring data were available, the risk of stroke and systemic embolism off anticoagulation exceeded 1% among patients with a sex-modified CHA2DS2-VASc score of 3 to 4 and exceeded 2% among patients with a sex-modified CHA2DS2-VASc score ≥5.2
Brief episodes of subclinical AF are at low risk of clinical events. In RATE (Registry of Atrial Tachycardia and Atrial Fibrillation), patients with clinical events such as hospitalization, death, or stroke were more likely than those without to have AHREs with either onset or offset of an adjudicated, single ECG (31.9% versus 22.1% among patients with pacemakers and 28.7% and 20.2% among patients with implantable cardioverter-defibrillators [ICDs]).4 AHREs >5 minutes are highly correlated with AF and AFL, whereas shorter episodes often represent other tachyarrhythmias.9 To remove most episodes of oversensing, AHREs lasting ≤5 minutes were excluded from analysis of MOST (Mode Selection Trial).10 A similar threshold was used in several subsequent key studies to exclude spurious events.1,3,5,11,12
6.5. Nonpharmacological Stroke Prevention
Although oral anticoagulation is the standard of care to reduce the risk of ischemic stroke in patients with AF, it is contraindicated in some patients due to an excess risk of major bleeding. Surgical and percutaneous techniques to occlude the LAA have been developed to reduce the risk of ischemic stroke. These techniques have the potential to obviate the need for or supplement long-term oral anticoagulation in select patients. Surgical LAAO is a particularly important consideration in patients with AF who undergo cardiac surgery.
6.5.1. Percutaneous Approaches to Occlude the LAA
Recommendations for Percutaneous Approaches to Occlude the LAA
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-NR | 1. In patients with AF, a moderate to high risk of stroke (CHA2DS2-VASc score ≥2), and a contraindication (Table 14) to long-term oral anticoagulation due to a nonreversible cause, percutaneous LAAO (pLAAO) is reasonable.1–4 |
| 2b | B-R | 2. In patients with AF and a moderate to high risk of stroke and a high risk of major bleeding on oral anticoagulation, pLAAO may be a reasonable alternative to oral anticoagulation based on patient preference, with careful consideration of procedural risk and with the understanding that the evidence for oral anticoagulation is more extensive.1–3,5,6 |
Table 14.
Situations in Which Long-Term Anticoagulation Is Contraindicated and Situations When It Remains Reasonable
| Long-Term Anticoagulation Contraindicated | Long-Term Anticoagulation Is Still Reasonable |
|---|---|
| Severe bleeding due to a nonreversible cause involving the gastrointestinal, pulmonary, or genitourinary systems Spontaneous intracranial/intraspinal bleeding due to a nonreversible cause Serious bleeding related to recurrent falls when cause of falls is not felt to be treatable |
Bleeding involving the gastrointestinal, pulmonary, or genitourinary systems that is treatable Bleeding related to isolated trauma Bleeding related to procedural complications |
Synopsis
A large body of evidence supports the use of OACs to reduce the risk of ischemic stroke in patients with AF; however, OACs may be contraindicated in some patients (Table 14). pLAAO devices are designed to prevent embolization of LAA thrombi and potentially obviate the need for OAC for stroke risk reduction. RCTs have demonstrated pLAAO to be noninferior to warfarin and DOACs for stroke and systemic embolism with a reduced risk of major bleeding.1–4 A prospective nonrandomized study has shown that patients deemed unsuitable for OAC who undergo pLAAO implant have a lower-than-expected risk of ischemic stroke.5 Prospective registries of pLAAO device implant in patients who are not on long-term OAC have shown a high rate of procedural success, a low risk of major procedure-related complications, and a low rate of ischemic stroke in follow-up.6,7 Based on these findings, pLAAO is reasonable in patients with nonvalvular AF who have a contraindication to long-term OAC due to a nonreversible cause. Additionally, pLAAO may be reasonable as an alternative to OAC based on patient preference after careful consideration of procedural risk and with the understanding that there is a much greater body of evidence supporting OAC in this population in general.
Recommendation-Specific Supportive Text
pLAAO has been evaluated in patients with AF and a contraindication to OAC. The ASAP (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology) study was a nonrandomized trial evaluating the performance of the Watchman device in 150 patients with AF who were deemed ineligible for OAC with warfarin.5 The rate of ischemic stroke was lower in patients who received the Watchman device than would be expected based on the CHADS2 scores of the cohort (2.3% versus 7.3%). The EWOLUTION (Evaluating Real-Life Clinical Outcomes in Atrial Fibrillation Patients Receiving the WATCHMAN Left Atrial Appendage Closure Technology) prospective registry showed a high rate of Watchman device procedural success (98.5%) with a low ischemic stroke risk (1.1%). This low ischemic stroke was demonstrated despite most of the patients (73%) not using oral anticoagulation periprocedurally.6 The PINNACLE FLX (Protection Against Embolism for Nonvalvular AF Patients: Investigational Device Evaluation of the Watchman FLX LAA Closure Technology) prospective registry of the next-generation Watchman FLX device showed a high rate of procedural success (98.8%) and a low rate (0.5%) of the primary safety endpoint (death, ischemic stroke, systemic embolism, procedure-related events requiring open cardiac surgery or major endovascular intervention).7 Registry data have shown substantial variation in postprocedure antithrombotic regimens in real-world practice compared with protocols in the pivotal RCTs; the effect this has on long-term outcomes has not been clearly established.8 ASAPTOO (Assessment of the Watchman Device in Patients Unsuitable for Oral Anticoagulation) is an ongoing RCT evaluating the safety and efficacy of LAAO in patients with AF who are deemed ineligible for oral anticoagulation.9
PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation) was a noninferiority RCT comparing pLAAO with the Watchman device with warfarin in patients with AF at an increased risk for stroke. pLAAO was noninferior to warfarin for both primary composite efficacy (stroke, systemic embolism, and cardiovascular/unexplained death) and safety (procedure-related events and major bleeding) endpoints.1 pLAAO was superior to warfarin in terms of cardiovascular mortality (HR, 0.40, 95% CI, 0.21–0.75; P=0.005) and all-cause mortality (HR, 0.66, 95% CI, 0.45–0.98; P=0.004). The PREVAIL (Watchman Left Atrial Appendage Closure Device in Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy) study was a follow-up RCT that compared the Watchman device with warfarin in a similar patient population.2 pLAAO did not achieve noninferiority for the first primary composite efficacy endpoint (stroke, systemic embolism, and cardiovascular/unexplained death) but was limited in statistical power because of unexpectedly low event rates. pLAAO was statistically noninferior to warfarin for nonprocedure-related strokes. Higher implant success and lower procedure-related safety event rates were observed in PREVAIL than PROTECT AF. A 5-year patient-level meta-analysis of these trials demonstrated similar efficacy of pLAAO for stroke prevention and reduced rates of major bleeding compared with warfarin.3 PRAGUE-17 (Left Atrial Appendage Closure vs Novel Anticoagulation Agents in Atrial Fibrillation) was an RCT that compared pLAAO with DOACs in patients with nonvalvular AF.4 pLAAO was noninferior to DOACs for the combined safety and efficacy composite endpoint (stroke, TIA, systemic embolism, cardiovascular death, major or nonmajor clinically relevant bleeding, or procedure-/device-related complications).
6.5.2. Cardiac Surgery—LAA Exclusion/Excision
Recommendations for Cardiac Surgery–LAA Exclusion/Excision
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | A | 1. In patients with AF undergoing cardiac surgery with a CHA2DS2-VASc score ≥2 or equivalent stroke risk, surgical LAA exclusion, in addition to continued anticoagulation, is indicated to reduce the risk of stroke and systemic embolism.1–3 |
| 1 | A | 2. In patients with AF undergoing cardiac surgery and LAA exclusion, a surgical technique resulting in absence of flow across the suture line and a stump of <1 cm as determined by intraoperative transesophageal echocardiography should be used.1,4,5 |
| 2b | A | 3. In patients with AF undergoing cardiac surgery with CHA2DS2-VASc score ≥2 or equivalent stroke risk, the benefit of surgical LAA exclusion in the absence of continued anticoagulation to reduce the risk of stroke and systemic embolism is uncertain.1–3 |
Synopsis
Surgical left atrial appendage occlusion (S-LAAO) for reduction in the risk of recurrent arterial thromboemboli was first reported in 1949.6 After decades of relative clinical equipoise,7,8 accumulating evidence,2,8–10 culminating in the large RCT LAAOS III (Left Atrial Appendage Occlusion Study),1 supports a benefit of S-LAAO in patients with AF who undergo2,9 coronary artery bypass graft surgery (CABG) or valve surgeries. A 2019 meta-analysis before the publication of LAAOS III reviewed 22 studies, including 280 585 patients of whom 36 686 underwent S-LAAO during cardiac surgery. S-LAAO showed an association with a 29% lower risk of stroke or thromboembolism. The all-cause mortality rate at 2 years was lower in patients who underwent S-LAAO. No benefit was found in the subgroup of studies that included patients without preoperative AF.9 These favorable findings were validated by the 4770-subject LAAOS III, which confirmed S-LAAO in addition to OAC provides a 33% reduction in risk of stroke and systemic embolism.1
LAAOS III enrolled only patients with preexisting AF; evidence of the effectiveness of S-LAAO in patients without AF is inconsistent and suggests the benefit of adjunctive S-LAAO is unclear in patients without preoperative AF.7,9 The results of ongoing RCTs in this population are awaited.
Recommendation-Specific Supportive Text
-
LAAOS III was performed with 4770 patients with AF and a CHA2DS2-VASc score ≥2 undergoing cardiac surgery randomized to S-LAAO or no S-LAAO. Intraoperative transesophageal echocardiogram (TEE) was recommended to assess successful closure using an endorsed technique. Oral anticoagulation was continued postoperatively. After a mean follow-up of 3.8 years, the primary endpoint of stroke or systemic embolism occurred in 4.8% of the S-LAAO group and 7.0% of the no S-LAAO group (HR, 0.67 [95% CI, 0.53–0.85]; P=0.001). Mortality rate, cross-clamp and bypass time, chest tube output, and bleeding were not different between groups. More than 75% of subjects in both groups received OAC. These data indicate S-LAAO provides additional benefit to oral anticoagulation without increasing risk of adverse events.
These findings cannot be extrapolated to patients without AF or patients who are not candidates for OAC. Most subjects underwent CABG or valve surgery, and the mean CHA2DS2-VASc score was 4.2. Surgical AF ablation was performed in one-third of both groups. Patients undergoing mechanical valve surgery, transplant, off-pump bypass, left ventricular assistive device surgery, and congenital heart disease (CHD) cases were excluded. A subsequent meta-analysis of 5 RCTs and 22 observational studies including 540 111 patients showed a significant decrease in stroke and thromboembolism with S-LAAO.11 The postoperative mortality rate did not differ but, in follow-up, was reduced in the S-LAAO group after 2 years. No difference in major bleeding, all-cause rehospitalizations, or cross-clamp time was observed.11
Substantial heterogeneity in S-LAAO methods exists in the literature, and not all techniques effectively exclude the LAA.4 An increased risk of stroke and thromboembolism is associated with incomplete S-LAAO5,12; however, many studies do not report assessment of occlusion success.9 This inconsistency has likely contributed to the variability in outcome.7,9 LAAOS III permitted 4 techniques: amputation and closure (which was promoted as the preferred technique), used in 56%; stapler closure, used in 11%; double-layer linear closure (if confirmed by TEE), used in 14%; or an approved LAAO device, used in 15%. Other techniques were approved on a case-by-case basis (4%). Intraoperative TEE was recommended to assess successful closure, defined as absence of flow across the suture line and a stump of <1 cm. If the initial closure was unsuccessful, additional suturing was performed for repair. Contemporary data show closure success of >95% using a surgical clip device.13 In the LAAOS series, the mean cross-clamp and bypass times were similar, and perioperative bleeding was not increased in the S-LAAO group.1,4 These data support the importance of complete S-LAAO in achieving beneficial outcomes.
In LAAOS III, S-LAAO reduced stroke and thromboembolism by 33% in addition to OAC. LAAOS III was not designed to resolve whether oral anticoagulation can be safely stopped after S-LAAO; at hospital discharge, >80% of subjects in both groups were receiving OAC, with >75% on OAC at the 3-year visit.1 A systematic review and meta-analysis focusing on the effects of anticoagulation found no statistically significant difference in stroke rates of S-LAAO/no S-LAAO subjects on OAC versus off OAC, with high heterogeneity noted for both ischemic events and mortality,14 and findings are subject to confounding by indication and the potentially beneficial effects of surgical AF ablation. The LAACS (Left Atrial Appendage Closure with Surgery) study of 187 subjects undergoing CABG, valve surgery, or both who were randomized to S-LAAO versus no S-LAAO showed a reduction in the risk of stroke in the S-LAAO group, independent of anticoagulation status.15 These results must be interpreted with caution, however, because the study was not powered to demonstrate a reduction in stroke, and substantial crossover occurred.
6.6. Active Bleeding on Anticoagulant Therapy and Reversal Drugs
Recommendations for Active Bleeding on Anticoagulant Therapy and Reversal Drugs
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with AF receiving dabigatran who develop life-threatening bleeding, treatment with idarucizumab is recommended to rapidly reverse dabigatran’s anticoagulation effect.1–3 |
| 2a | C-LD | 2. In patients with AF receiving dabigatran who develop life-threatening bleeding, treatment with activated prothrombin complex concentrate (PCC) is reasonable to reverse dabigatran’s anticoagulation effect if idarucizumab is unavailable.4,5 |
| 1 | B-NR * | 3. In patients with AF receiving factor Xa inhibitors who develop life-threatening bleeding, treatment with either andexanet alfa (apixaban or rivaroxaban,* edoxaban†) or 4-factor prothrombin complex concentrate† is recommended to rapidly reverse factor Xa inhibitor’s anticoagulation effect.6,7 |
| C-LD † | ||
| 1 | A | 4. In patients with AF receiving warfarin who develop life-threatening bleeding, treatment with 4-factor prothrombin complex concentrate (if available) in addition to intravenous vitamin K is recommended to rapidly achieve INR correction over fresh frozen plasma and intravenous vitamin K treatment.8–10 |
| 2b | B-NR | 5. In patients with AF who develop major gastrointestinal bleeding, resumption of oral anticoagulation therapy may be reasonable alter correction of reversible causes of bleeding and reassessment of its long-term benefits and risks with a multidisciplinary team approach during SDM with patients.11,12 |
B-NR LOE applies to data on apixaban or rivaroxaban.
C-LD LOE applies to data on edoxaban.
Synopsis
About 2% to 4% of patients who receive OACs experience major bleeding and require intervention.13 Activated charcoal may be administered up to 6 to 8 hours after the last dose of an OAC.14,15 Hemodialysis may be also considered to eliminate dabigatran but may be challenging and impractical due to coagulopathy and hemodynamic instability.16 The proportion of emergency department visits for bleeding from OACs due to DOACs increased from 2.3% in 2011 to 37.9% in 2017 because of the increased use of DOACs over the past decade.17 Some patients may require reversal agents to achieve rapid hemostasis. Failure to achieve effective hemostasis is associated with a >3 times higher risk of death.18 Idarucizumab for dabigatran-induced major bleeding and andexanet alfa for apixaban or rivaroxaban associated major bleeding are US Food and Drug Administration (FDA) approved. Four-factor PCC for DOAC-induced major bleeding was compared with andexanet alfa in retrospective cohort studies, but the comparison has never been investigated in clinical trials.7 For VKA, if life-threatening bleeding cannot be managed with supportive measures, the rapid reversal treatment with 4-factor PCC is preferred over fresh frozen plasma.8–10 Table 15 summarizes reversal agents for OACs. Table 16 describes bleeding events attributable to DOACs in pivotal clinical trials.
Table 15.
Reversal Agents for Oral Anticoagulants
| Idarucizumab | Andexanet alfa | 4-Factor PCC | Activated PCC | |
|---|---|---|---|---|
| Class | Humanized monoclonal antibody fragment binding to dabigatran and neutralizing anticoagulation effects | A recombinant modified human factor Xa protein binding and sequestering the factor Xa inhibitors | PCC: coagulation factors II, VII, IX, and X Anticoagulation proteins C and S |
Nonactivated factors II, IX, and X Activated VII |
| FDA indications | Reversal of dabigatran effects For emergency surgery/urgent procedures Life-threatening or uncontrolled bleeding |
Reversal of apixaban or rivaroxaban For life-threatening or uncontrolled bleeding |
The urgent reversal for acute major bleeding or need for an urgent surgery/invasive procedure in patients receiving VKAs | Control and prevention of bleeding episodes, perioperative management, prophylaxis to prevent or reduce bleeding frequency in patients with hemophilia A and B |
| Off-label indications | N/A | Edoxaban-associated life-threatening bleeding | Reversal of factor Xa inhibitors in patients requiring urgent procedure or with life-threatening bleeding | Dabigatran-associated life-threatening bleeding |
| Dosing | 5-g (2 separate vials of 2.5 g/vial) intravenous infusion over 5 min. Additional 5 g may be given if reappearance of bleeding with elevated coagulation parameters have been observed or patients require second emergency surgery/procedure and elevated coagulation parameters | Low-dose regimen: 400-mg bolus at a target rate of 30 mg/min followed by 4 mg/min for up to 120 min High-dose regimen: 800-mg bolus at a target rate of 30 mg/min followed by 8 mg/min for up to 120 min The recommended dosing is based on apixaban or rivaroxaban, dose, and time since the patient’s last dose of apixaban or rivaroxaban |
Warfarin reversal based on pretreatment INR (units of factor IX): 1. INR 2–<4: 25 units/kg |(up to 2500 units) 2. INR 4–6: 35 units/kg (up to 3500 units) 3. INR >6: 50 units/kg (up to 5000 units) Oral factor Xa inhibitors: 2000 units once or 25 to 50 units/kg |
Dabigatran-associated life-threatening bleeding: 50 units/kg once |
| Onset | Within 5 min | Within 2 min | Within 10 min | Within 30 min |
| Duration | 12–24 h | 2 h | 8 h | 12 h |
| Monitoring | Coagulation parameters (aPTT, diluted thrombin time, or ecarin clotting time) between 12 and 24 h to assess redistribution of dabigatran from peripheral to plasma | Current commercial anti-Xa activity assays are unsuitable for measuring factor Xa activities after andexanet alfa use | Warfarin reversal: Repeat INR within 30 min after the administration | N/A |
| Others | Risk of serious reactions (hypoglycemia, hypophosphatemia, metabolic acidosis, increase in uric acid, acute liver failure) in patients with hereditary fructose intolerance (due to sorbitol excipient 4 g in each 5 g of idarucizumab) No procoagulant effect based on endogenous thrombin potential |
No FDA indication for other factor Xa inhibitors other than apixaban or rivaroxaban Andexanet alfa may interfere with the anticoagulation effect of heparin US black box warning: Serious and life-threatening adverse events (arterial and venous thromboembolism, myocardial infarction, ischemic stroke, cardiac arrest, sudden deaths) |
May not be indicated for patients with thromboembolic events in the previous 3 mo It includes heparin Administer intravenous vitamin K 10 mg over 10–20 min in addition to 4-factor PCC |
It does not include heparin Coagulation parameters do not correlate with the drug’s efficacy Not effective to reverse factor Xa inhibitors |
Information in table was obtained from manufacturer package inserts.
aPTT indicates activated partial thromboplastin time; FDA, US Food and Drug Administration; PCC, prothrombin complex concentrate; and VKA, vitamin K antagonists.
Table 16.
Bleeding Events (Percentage Per Year) in DOAC Pivotal Clinical Trials
| Bleeding Event | RE-LY (n=18 113) | ARISTOTLE (n=18 201) | ENGAGE AF-TIMI 48 (n=21 105) | ROCKET AF (n=14 264) |
|---|---|---|---|---|
| Major bleeding | Dabigatran 150 mg 3.11% (RR, 0.93 [95% CI, 0.81–1.07]; P=0.31) Dabigatran 110 mg 2.71% (RR, 0.80 [95% CI, 0.69–0.93]; P=0.003) Warfarin 3.36% |
Apixaban 2.13% (HR, 0.69 [95% CI, 0.60–0.80]; P<0.001) Warfarin 3.09% |
Edoxaban 60 mg 2.75% vs warfarin 3.43% (HR, 0.80 [95% CI, 0.71–0.91]; P<0.001) Edoxaban 30 mg 1.61% vs warfarin 3.43% (HR, 0.47 [95% CI, 0.41–0.55]; P<0.001) |
Rivaroxaban 3.6% vs warfarin 3.4% (HR, 1.04 [95% CI, 0.90–1.20]; P=0.58) |
| Gastrointestinal bleeding | Dabigatran 150 mg 1.51% vs warfarin 1.02% (RR, 1.50 [95% CI, 1.19–1.89]; P<0.001) Dabigatran 110 mg 1.12% vs warfarin 1.02% (RR, 1.10 [95% CI, 0.86–1.41]; P=0.43) |
Apixaban 0.76% (HR, 0.89 [95% CI, 0.70–1.15]; P=0.37) Warfarin 0.86% |
Edoxaban 60 mg 1.51% (HR, 1.23 [95% CI, 1.02–1.50]; P=0.03) Edoxaban 30 mg 0.82% (HR, 0.67 [95% CI, 0.53–0.83]; P<0.001) Warfarin 1.23% |
Rivaroxaban 3.2% (HR not reported; P<0.001) Warfarin 2.2% |
| Intracranial bleeding | Dabigatran 150 mg 0.30% (RR, 0.40 [95% CI, 0.27–0.60]; P<0.001) Dabigatran 110 mg 0.23% (RR, 0.31 [95% CI, 0.20–0.47]; P<0.001) Warfarin 0.74% |
Apixaban 0.33% (HR, 0.42 [95% CI, 0.30–0.58]; P<0.001) Warfarin 0.80% |
Edoxaban 60 mg 0.39% (HR, 0.47 [95% CI, 0.34–0.63]; P<0.001) Edoxaban 30 mg 0.26% (HR, 0.30 [95% CI, 0.21–0.43]; P<0.001) Warfarin 0.85% |
Rivaroxaban 0.80% (HR, 0.67 [95% CI, 0.47–0.93]; P=0.02) Warfarin 1.20% |
Adapted with permission from Kido et al.22 Copyright 2021 American College of Clinical Pharmacy.
ARISTOTLE indicates Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; DOAC, direct anticoagulant; ENGAGE AF-TIMI 48, Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation – Thrombolysis in Myocardial Infarction 48; HR indicates hazard ratio; RE-LY, Randomized Evaluation of Long-Term Anticoagulation Therapy; ROCKET AF, Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation; and RR, relative risk.
Recommendation-Supportive Text
The RE-VERSE AD (Reversal of Dabigatran Anticoagulant Effect With Idarucizumab) study was a multicenter, prospective cohort study evaluating the use of idarucizumab in patients receiving dabigatran for the management of uncontrolled or life-threatening bleeding or in patients requiring urgent surgery or invasive procedure.1 In patients with uncontrolled or life-threatening bleeding, idarucizumab reversed the dabigatran anticoagulation effect in 100% of patients, and 67.7% had bleeding cessation. Thrombosis events after the reversal agent use was 4.7% within 30 days. However, this study did not have a control group. The post hoc analysis of the RE-VERSE AD study in patients with gastrointestinal bleeding found that 97.5% of patients achieved complete reversal of dabigatran, and bleeding cessation occurred in 76.2% of patients.2 Another post hoc analysis of the same study showed that idarucizumab was effective at reversing dabigatran’s anticoagulation effect, regardless of baseline renal function.3
Compared with idarucizumab, the data on PCC for dabigatran-associated bleeding are very limited. A prospective multicenter cohort study compared the use of activated PCC in 14 patients with dabigatran-associated major bleeding compared with historical matched cases.4 The study was prematurely stopped due to the availability of idarucizumab in the market. The effectiveness of activated PCC was assessed based on the assessment guide rating from good, moderate, and poor. Nine patients (64%) were rated as good, 5 patients (36%) as moderate, and none as poor. No thromboembolic events were found. A randomized, placebo-controlled crossover trial in 12 healthy subjects evaluated the reversal effect of 4-factor PCC on rivaroxaban or dabigatran’s effect.5 It showed 4-factor PCC completely reversed rivaroxaban’s effect based the prothrombin time, but it did not reverse dabigatran’s effect based on the coagulation tests (aPTT, ecarin clotting time, and thrombin time). Four-factor PCC may not be appropriate for dabigatran-associated major bleeding management until further investigation is performed in prospective studies.
The ANNEXA 4 (Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of Factor Xa Inhibitors) trial evaluated the use of andexanet alfa in adult patients with acute major bleeding (safety population, n=254; efficacy population, n=254) receiving factor Xa inhibitors (apixaban 55%, rivaroxaban 36%, edoxaban 3%, and enoxaparin 6%).6 It found that after the bolus administration, the median anti-factor Xa activity decreased by 89%. Twelve hours after the andexanet infusion, clinical hemostasis was adjudicated as excellent or good in 37 of 47 patients in the efficacy analysis (79% [95% CI, 64%-89%]); however, thrombotic events occurred in 12 of 67 patients (18%) during the 30-day follow-up. Currently, andexanet alfa is FDA-approved for only apixaban- or rivaroxaban-associated life-threatening or uncontrolled bleeding, because only limited data are available for other factor Xa inhibitors. For 4-factor PCC, a meta-analysis of 22 observational studies compared andexanet alfa (n=438) with PCC (n=1278) in patients with acute bleeding associated with oral factor Xa inhibitors.7 It showed that mean hemostatic effectiveness for andexanet alfa was 82% at 12 hours and 71% at 24 hours versus 88% at 12 hours and 76% at 24 hours for PCC. Mean 30-day venous thromboembolism rate was 5.0% in the andexanet alfa group versus 1.9% in the PCC group. However, the meta-analysis had more significant methodological issues than the ANNEXA 4 trial. Thus, the level of evidence for the 4-factor PCC use was downgraded.
A meta-analysis of 13 studies (5 randomized trials and 8 observational studies) compared 4-factor PCC with fresh frozen plasma for warfarin-associated bleeding or urgent surgery/procedure.8 Four-factor PCC use was significantly associated with lower risk of all-cause mortality (25.1% versus 28.8%; OR, 0.56 [95% CI, 0.37–0.84]), higher probability to achieve INR correction (60.6% versus 12.8%; OR, 10.8 [95% CI, 6.12–19.07]), shortened time to INR correction (−6.5 h [95% CI, −9.75 h to −3.24 h]), and lower risk of volume overload (2.1% versus 8.8%; OR, 0.27 [95% CI, 0.13–0.58]) compared with fresh frozen plasma. No significant difference in thromboembolic events (4.2% versus 4.8%; OR, 0.91 [95% CI, 0.44–1.89]; P=0.81) was found.
A meta-analysis of 10 cohort studies compared anticoagulation resumption (n=2080) versus discontinuation (n=2296) after gastrointestinal bleeding in patients receiving OACs for AF, venous thromboembolism, or prosthetic valve.11 Restarting anticoagulation was significantly associated with higher risks of recurrent gastrointestinal bleeding (10.1% versus 5.3%) but also with lower risks of thromboembolic events (6.3% versus 10.6%) and a reduction in all-cause mortality (21.3% versus 31%). Another meta-analysis of 7 observational studies in patients with AF found no significant difference in stroke rates between resumption and discontinuation groups (9.1% versus 8.3%; OR, 0.75 [95% CI, 0.37–1.51]).12 Resumption of anticoagulation therapy was associated with significant reduction in any thromboembolic events (8.0% versus 12.2%; OR, 0.54 [95% CI, 0.43–0.68]) and all-cause mortality (10.8% absolute risk reduction; OR, 0.38 [95% CI, 0.24–0.60]), at the expense of recurrent major bleeding (10.2% versus 5.0%). One study found the optimal timing of resuming a VKA after upper gastrointestinal bleeding was between 3 and 6 weeks, while other studies revealed that resuming a VKA 1 week after gastrointestinal bleeding is the optimal timing.19–21 There is a paucity of data evaluating the timing of DOACs after gastrointestinal bleeding.
6.6.1. Management of Patients With AF and ICH
Recommendations for Management of Patients With AF and ICH
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-LD | 1. In patients with AF and conditions associated with very high risk of thromboembolic events (>5%/year), such as rheumatic heart disease or a mechanical heart valve, early (1–2 weeks) resumption of anticoagulation after ICH is reasonable to reduce the risk of thromboembolic events.1 |
| 2b | C-LD | 2. In patients with AF and ICH, delayed (4–8 weeks) resumption of anticoagulation may be considered to balance the risks of thromboembolic and hemorrhagic complications after careful risk benefit assessment.2–5 |
| 2b | B-NR | 3. In patients with AF and conditions associated with high risk of recurrent ICH (eg, cerebral amyloid angiopathy) anticoagulation-sparing strategies (eg, LAAO) may be considered to reduce the risk of recurrent hemorrhage.6,7 |
Synopsis
ICH is a term that refers to bleeding within any of the compartments of the cranial vault, including intraparenchymal (or intracerebral), subarachnoid, subdural, and epidural hemorrhage. In clinical practice, the different forms of ICH are often classified by mechanism and most broadly as traumatic and nontraumatic or spontaneous ICHs (Figure 14). In patients with AF and conditions associated with high-risk for thromboembolic complications (eg, mechanical valve, rheumatic valvular disease), anticoagulation is often resumed early after ICH, regardless of mechanism. For other patients with AF, decisions regarding if (and when) to resume anticoagulation after ICH requires more careful risk-benefit assessment of patient-specific clinical factors. Patients with traumatic etiologies generally have lower long-term risk of recurrent hemorrhage, and anticoagulation is generally considered safe to resume. Nontraumatic/spontaneous etiologies (eg, hypertensive parenchymal hemorrhage, hemorrhagic transformation after ischemic stroke, cerebral amyloid angiopathy-associated hemorrhage, chronic subdural hemorrhage) are generally associated with higher risk of recurrence. Clinical decision-making regarding the appropriateness of resumption in these patients is based largely on individual risk-benefit calculations given the lack of randomized data assessing safety and long-term outcomes. For patients with ICH deemed at high risk of recurrence, LAA closure may be a viable alternative to anticoagulation, although data on efficacy and safety are lacking in the ICH population.
Figure 14. Forms of ICH, Classified by Mechanism.
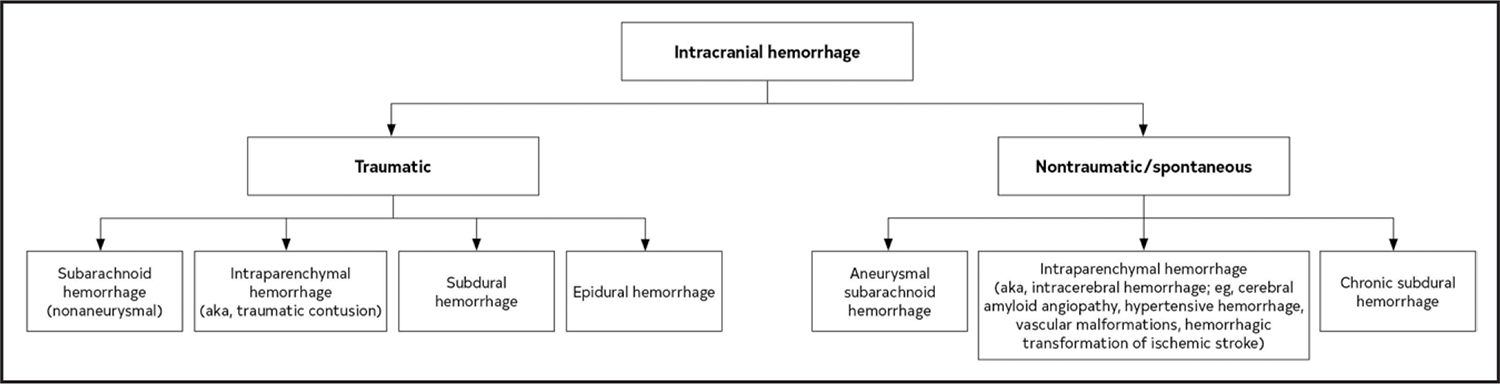
ICH indicates intracranial hemorrhage.
Recommendation-Specific Supportive Text
Rheumatic valvular disease is associated with a >5% per year risk of embolic events.8 In patients with mechanical heart valves, the rate can range from 4% to 23% per year depending on the type and position of the prosthetic.9–11 These rates are even higher in patients with concurrent AF but are significantly reduced with the use of VKAs. Data are limited that explore the safety and timing of anticoagulation resumption in patients with ICH and high-risk valvular disease. In 1 observational analysis of 137 patients with ICH and mechanical heart valves, resuming anticoagulation within 2 weeks from index ICH was associated with increased hemorrhagic complications (HR, 7.06 [95% CI, 2.33–21.37]).1 However, in balancing the risk of hemorrhagic with thrombotic complications, the optimal window of resumption was found to be between 1 and 2 weeks post-ICH after weighing individual patient factors (Table 17).
Anticoagulation is associated with up to 25% of ICHs and is associated with worse functional outcomes and higher mortality.12–15 After ICH, anticoagulation is generally held in the acute setting. Decisions regarding the appropriateness and timing of resumption are complex given the limited amount of randomized data addressing risks and benefits in this population. Previous observational reports evaluating risk of recurrent ICH, thromboembolism, and mortality in patients with nonvalvular AF have suggested possible reduction in thromboembolic risk and mortality with resumption of anticoagulation but are limited by discordant patient populations and study designs, predominant use of VKAs, and potential selection biases.16–22 In a meta-analysis of 2452 patients with AF and ICH, resumption of OACs was associated with lower risk of ischemic stroke (RR, 0.46 [95% CI, 0.29–0.72]) and no difference in risk of recurrent ICH compared with patients in whom anticoagulation was not resumed2 (Table 17). However, data are limited on the optimal timing for resumption. In an analysis of 2619 ICH survivors with AF, a composite net benefit minimizing risk of bleeding and thromboembolic complications occurred when anticoagulation was resumed at 7 to 8 weeks after ICH.3 In a retrospective study that included 1752 patients with OAC-related ICH across 3 Danish registries, anticoagulation resumed a median of 34 days was associated with an adjusted HR of ischemic stroke, systemic embolism, and all-cause mortality of 0.55 (95% CI, 0.39–0.78) compared with no anticoagulation.4 In another meta-analysis of 8 observational studies, resuming OAC was associated with reduced risk of ischemic stroke without increased risk of recurrent ICH at a median time to resumption of 10 to 39 days5 (Table 17). Of note, DOACs have been associated with lower rates of major bleeding complications compared with VKAs.23
Anticoagulation is used for stroke risk reduction in patients with AF at risk of thromboembolism. For some patients, however, risk of recurrent ICH may be prohibitively high with anticoagulation. Cerebral amyloid angiopathy is the most common cause of lobar hemorrhage in older adults and is associated with annual ICH recurrence risk that ranges from 5% in patients with isolated microhemorrhages to 26.9% in patients with associated cortical superficial siderosis.24 Thus, in patients with AF and cerebral amyloid angiopathy-related ICH, alternative strategies for secondary stroke prevention are often pursued. The LAA is considered the most common site for thrombus formation in patients with AF.25,26 Two RCTs—PROTECT AF study and PREVAIL study—compared rates of stroke and mortality after LAA closure versus VKA in patients with AF.6,7 In a meta-analysis examining 5-year outcomes in the 2 RCTs, LAA closure was associated with significant reductions in hemorrhagic stroke, disabling and fatal stroke, and all-cause mortality, with comparable rates of stroke and systemic embolism compared with warfarin.27 Of note, both studies excluded patients who were not candidates for anticoagulation. Additionally, newer treatment options such as epicardial LAA clipping are emerging for stroke prevention without need for postprocedure APT.28,29
Table 17.
Risk Factors for Thromboembolic Complications and Recurrent ICH
| Factors Associated With High Risk of Thromboembolism | Factors Associated With High Risk of Recurrent ICH |
|---|---|
| Mechanical heart valve | Suspected cerebral amyloid angiopathy |
| Rheumatic valve disease | Lobar IPH |
| Previous history of stroke/thromboembolism | Older age |
| Hypercoagulable state (eg, active malignancy, genetic thrombophilia) | >10 cerebral microbleeds on MRI |
| High CHA2DS2-VASc score (>5) | Disseminated cortical superficial siderosis on MRI |
| Poorly controlled hypertension | |
| Previous history of spontaneous ICH | |
| Genetic/acquired coagulopathy | |
| Untreated symptomatic vascular malformation or aneurysm |
CHA2DS2-VASc indicates congestive heart failure, hypertension, age ≥75 y (doubled), diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism (doubled), vascular disease, age 65 to 74 y, sex category; ICH, intracranial hemorrhage; IPH, intraparenchymal hemorrhage; and MRI, magnetic resonance imaging.
6.7. Periprocedural Management
Recommendations for Periprocedural Management
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-R * | 1. In patients with AF (excluding those with recent stroke or TIA, or a mechanical valve) and on oral anticoagulation with either warfarin* or DOAC† who are scheduled to undergo an invasive procedure or surgery, temporary cessation of oral anticoagulation without bridging anticoagulation is recommended.1–4 |
| B-NR † | ||
| 1 | A | 2. In patients with AF on warfarin anticoagulation and an annual predicted risk of thromboembolism of ≥5% undergoing pacemaker or defibrillator implantation or generator change, continued anticoagulation is recommended in preference to interruption of warfarin and bridging anticoagulation with heparin to reduce the risk of pocket hematoma.5–7 |
| 2a | A | 3. In patients with AF with CHA2DS2-VASc score ≥2 or equivalent risk of stroke, on DOAC anticoagulation and undergoing pacemaker or defibrillator implantation or generator change, either uninterrupted or interrupted DOAC is reasonable.8–10 |
| 1 | B-NR | 4. In patients with AF on DOAC and scheduled to undergo an invasive procedure or surgery that cannot be performed safely on uninterrupted anticoagulation, the timing of interruption of DOAC should be guided by the specific agent, renal function, and the bleeding risk of the procedure (Table 18).4,11,12 |
| 2a | B-NR | 5. In patients with AF on DOAC that has been interrupted for an invasive procedure or surgery, in general, resumption of anticoagulation the day after low bleeding risk surgery and between the evening of the second day and the evening of the third day after high bleeding risk surgery is reasonable, as long as hemostasis has been achieved and further bleeding is not anticipated.4 |
| 3: Harm | B-R | 6. In patients with AF on warfarin anticoagulation, who are undergoing surgeries or procedures for which they are holding warfarin, except in patients with mechanical valve or recent stroke or TIA, bridging anticoagulation with low-molecular-weight heparin should not be administered.1,3,13–15 |
B-R LOE applies to the data on warfarin.
B-NR LOE applies to the data on DOAC.
Table 18.
Timing of Discontinuation of OACs in Patients With AF Scheduled to Undergo an Invasive Procedure or Surgery in Whom Anticoagulation Is to Be Interrupted
| Anticoagulant | Low Bleeding Risk Procedure | High Bleeding Risk Procedure |
|---|---|---|
| Apixaban (CrCl >25 mL/min)* | 1 d† | 2 d |
| Dabigatran (CrCl >50 mL/min) | 1 d | 2 d |
| Dabigatran (CrCl 30–50 mL/min) | 2 d | 4 d |
| Edoxaban (CrCl >15 mL/min) | 1 d | 2 d |
| Rivaroxaban (CrCl >30 mL/min) | 1 d | 2 d |
| Warfarin | 5 d for a target INR <1.5 2–3 d for a target INR <2 |
5 d |
For patients on DOAC with creatinine clearance lower than the values in the table, few clinical data exist. Consider holding for an additional 1 to 3 days, especially for high bleeding risk procedures.
The number of days is the number of full days before the day of surgery in which the patient does not take any dose of anticoagulant. The drug is also not taken the day of surgery. For example, in the case of holding a twice daily drug for 1 day, if the drug is taken at 8 pm, and surgery is at 8 am, at the time of surgery, it will be 36 hours since the last dose was taken.
AF indicates atrial fibrillation; CrCl, creatinine clearance; DOAC, direct oral anticoagulation; INR, international normalized ratio; and OAC, oral anticoagulant.
Synopsis
Periprocedural anticoagulation management is driven by 2 competing aims: to hold the anticoagulant agent for the shortest possible time to minimize the risk of thromboembolism and to ensure coagulation parameters are as close to normal as possible at the time of the procedure to facilitate hemostasis and avoid intra- and postoperative bleeding. Considerations include the bleeding risk of the procedure, consequences of bleeding should it occur, patient-specific risk factors, and thrombotic risk while off anticoagulation. Invasive procedures and surgeries have been divided into high and low bleeding risks in several different classifications.16 Generally, endoscopic, dental extraction, many ophthalmologic procedures, and percutaneous vascular access, such as cardiac catheterization, are considered to be of low bleeding risk. Higher bleeding risk surgeries include intra-abdominal, pelvic, orthopedic, neurosurgical, cardiac, and transvenous lead extraction procedures. Very high bleeding risk procedures include neuraxial anesthesia and spinal surgery. Available data do not suggest high rates of bleeding from these procedures, although bleeding could cause significant consequences.17 Patient-specific factors include abnormal liver and kidney function, bleeding or clotting disorders, concomitant antiplatelet or nonsteroidal anti-inflammatory use, alcohol, and anemia. Recent bleeding or thromboembolism may increase the risk for recurrent events. To put thrombotic risk in perspective, the incidence of thromboembolism while anticoagulation was temporarily held has generally been <1%.3,18
Recommendation-Specific Supportive Text
To mitigate thromboembolic risk during interruption of warfarin therapy for a procedure or surgery, “bridging” anticoagulation can be administered. However, observational studies consistently showed an increased risk of bleeding without a difference in thromboembolic risk with this strategy.3 The BRIDGE (Bridging Anticoagulation in Patients who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery) trial included patients with a mean CHADS2 score of 2.3 (range, 1–6); 61.7% of patients had a CHADS2 score <3.1 In this study, discussed in more depth below, interruption of warfarin without bridging anticoagulation was superior to bridging.1 In a meta-analysis of 6 observational studies reporting on patients not at high thromboembolic risk receiving prophylactic dose or no bridging had an overall thromboembolic event rate of 0.6% (11 of 1702). 3 A similar RCT has not been done in the DOAC era, given the ease of brief interruption of DOAC therapy; however, observational studies have shown similar results.2 The PAUSE (Perioperative Anticoagulation Use for Surgery Evaluation) cohort study, discussed below, enrolling patients with a mean CHADS2 score of 2.1 (range, 1–6) and CHA2DS2-VASc score of 3.5 (range, 1–9), found a low incidence of thromboembolic events when DOAC was briefly interrupted without bridging.4 This suggests that in patients at low-to-moderate thromboembolic risk, interruption of anticoagulation, without bridging, may provide a low risk of bleeding with an acceptable risk of stroke/TIA. Patients with mechanical valves, and recent stroke/TIA, or other high-risk markers, were not included in most studies and may still benefit from bridging anticoagulation: in these scenarios, management should be individualized.
Evidence has accrued that periprocedural unfractionated or low-molecular-weight heparin bridging has been associated with an increased risk of pocket hematoma after pacemaker or ICD procedures.19 BRUISE-CONTROL (Bridge or Continue Coumadin for Device Surgery Randomized Controlled Trial) randomized patients with AF (88%) or a mechanical valve with an estimated annual stroke risk of ≥5%, and undergoing elective device surgery, to continued warfarin versus warfarin interruption with bridging anticoagulation. The incidence of clinically significant pocket hematoma was >4 times higher in the bridging group.5 Several other procedures have been performed on uninterrupted anticoagulation.20 Further study is needed to define which procedures, in which patients, can safely be performed with uninterrupted anticoagulation. Lead extraction procedures are generally considered high bleeding risk and therefore should be treated as such (high bleeding risk procedures in Table 18) and warfarin interrupted, with occasional exceptions.
Two RCTs and several observational studies have shown little or no difference in the risk of pocket hematoma and thromboembolic events in patients with AF anticoagulated with DOACs and undergoing device surgery.8–10 Therefore, brief interruption of DOAC therapy (low bleeding risk procedures in Table 18 may be preferable for many, while uninterrupted DOAC may be preferred for those at particularly high thromboembolic risk. Lead extraction procedures are generally considered high bleeding risk and therefore should be treated as such (high bleeding risk procedures in Table 18) and DOAC interrupted, with occasional exceptions.
To achieve the goals of reducing or eliminating anticoagulant effect at the time of surgery, while also minimizing the time the patient spends without anticoagulation to prevent thromboembolism, the duration of cessation of anticoagulation should take into account both the pharmacokinetics and pharmacodynamics of the agent, the patient’s renal function, and the bleeding risk of the procedure. Direct measurement of DOAC levels is unavailable in clinical practice, but levels <30 ng/mL, which correspond to the expected plasma DOAC concentration reached after 3 half-lives, when most of the drug (87.5% of the Cmax) has been eliminated, is reached in two-thirds of patients 24 to 48 hours after the last drug intake.11 Similar results were observed for apixaban.12 A drug “hold” of 48 hours in clinical practice typically means 2 full days without drug intake, in addition to the day of the procedure, leading to a longer interval between last drug intake and procedure start longer than 48 hours, usually an additional 12 hours. Meta-analysis of the reports of periprocedural events from the pivotal trials of each of the 4 available DOACs suggested similar outcomes in patients on DOAC or warfarin, with reduced major bleeding during an uninterrupted strategy with DOACs.21 The PAUSE protocol was studied in patients on apixaban, dabigatran, and rivaroxaban, and resulted in low rates of major bleeding (0.9%-1.85%) and thromboembolism (<1%). A high rate (>90%) of minimal anticoagulant level (<50 ng/mL) was achieved. Similar results have been observed with edoxaban in clinical practice.22 Different protocols have not been directly compared. Table 18 presents recommended DOAC interruption times based on agent, renal function, and the bleeding risk of the procedure.
The timing of resumption of interrupted anticoagulation after surgery is a complex decision that should integrate patient and surgical characteristics. Warfarin, which takes several days (usually 3–5) to become therapeutic, can often be restarted the evening of the procedure. Postoperative bridging anticoagulation has not been shown to be generally beneficial.1,13 In the PAUSE study, DOACs were started on the first postoperative day for low bleeding risk procedures and between the evening of the second and third postoperative days for high bleeding risk procedures. This resulted in a low risk of major bleeding.4 Input from the proceduralist should always be incorporated into decisions.
Several studies have compared a strategy of bridging anticoagulation with a comparator of no bridging in patients on anticoagulants who were scheduled to undergo an invasive procedure or surgery. The BRIDGE trial randomized 1 884 patients with AF or AFL to bridging anticoagulation before and after the procedure with dalteparin versus placebo.1 Almost 90% of patients were undergoing low bleeding risk procedures. No bridging was superior to bridging with respect to major bleeding and was noninferior with respect to thromboembolism, although the observed rate (0.4%) was lower than anticipated (1.0%). PERIOP2 (A Double Blind Randomized Control Trial of Post-Operative Low Molecular Weight Heparin Bridging Therapy Versus Placebo Bridging Therapy for Patients Who Are at High Risk for Arterial Thromboembolism) randomized patients, about two-thirds of whom were undergoing high bleeding risk procedures, and 79% of whom had AF, to postoperative bridging with dalteparin or placebo until the INR was >1.9. All patients received preoperative bridging. Bleeding and thromboembolism outcomes were similar.13 These studies also excluded patients with recent bleeding or thromboembolism, and those undergoing spinal and neurological surgery, and in these cases, management should be individualized and may include bridging anticoagulation.1,13 A small randomized trial found that dental extractions could be performed on uninterrupted warfarin without bridging.14 A large multicenter registry also did not find a difference in thromboembolism, but an increase in bleeding, in bridged patients.15 Patients with mechanical valves should be managed as outlined in the “2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease.”23
6.8. Anticoagulation in Specific Populations
6.8.1. AF Complicating ACS or Percutaneous Coronary Intervention (PCI)
Recommendations for AF Complicating ACS or PCI
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | A | 1. In patients with AF and an increased risk for stroke who undergo PCI, DOACs are preferred over VKAs in combination with APT to reduce the risk of clinically relevant bleeding.1–5 |
| 1 | A | 2. In most patients with AF who take oral anticoagulation and undergo PCI, early discontinuation of aspirin (1–4 wk) and continuation of dual antithrombotic therapy with OAC and a P2Y12 inhibitor is preferred over triple therapy (OAC, P2Y12 inhibitor, and aspirin) to reduce the risk of clinically relevant bleeding.1–4,6 |
Synopsis
Encountering a patient with AF at an increased risk for stroke who undergoes PCI for stable CAD or ACS is a common clinical scenario. The addition of APT to OAC after PCI introduces a risk for clinically significant bleeding. RCTs have compared the safety and efficacy of various antithrombotic regimens after PCI is performed in patients with AF. They have consistently shown that DOACs, when used in conjunction with APT, are associated with a lower risk of clinically relevant bleeding compared to VKAs.1–5 They have also shown that dual therapy with an OAC and single APT with a P2Y12 inhibitor significantly lowers the risk of clinically relevant bleeding compared with triple therapy (oral anticoagulation, P2Y12 inhibitor, and aspirin) without substantially increasing risk of major adverse cardiovascular events.1–4,6 Meta-analyses of these trials have shown a slightly increased risk of stent thrombosis with dual therapy compared to triple therapy.5 Thus, the risk of stent thrombosis must be weighed against the risk of bleeding in determining the antithrombotic regimen after PCI. This is particularly true in patients with a relatively high perceived risk of stent thrombosis (complex revascularization, multivessel PCI, previous history of stent thrombosis).
Recommendation-Specific Supportive Text
Post-PCI antithrombotic regimens comparing DOACs with VKAs in combination with APT in patients with AF who do not have a specific indication for VKAs (eg, mechanical valve) have consistently shown a reduction in clinically relevant bleeding.1,2,4 A meta-analysis of 3 RCTs comparing DOACs with VKAs after PCI, PIONEER AF-PCI (Open-Label, Randomized, Controlled, Multicenter Study Exploring Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects with Atrial Fibrillation who Undergo Percutaneous Coronary Intervention) (rivaroxaban), RE-DUAL PCI trial (Randomized Evaluation of Dual Antithrombotic Therapy with Dabigatran versus Triple Therapy with Warfarin in Patients with Nonvalvular Atrial Fibrillation Undergoing Percutaneous Coronary Intervention) (dabigatran), and AUGUSTUS (Open-Label, 2×2 Factorial, Randomized, Controlled Clinical Trial to Evaluate the Safety of Apixaban vs Vitamin K Antagonist and Aspirin vs Aspirin Placebo in Patients With Atrial Fibrillation and Acute Coronary Syndrome and/or Percutaneous Coronary Intervention) (apixaban), showed a 42% relative risk reduction for major bleeding in DOAC-based regimens compared with VKA-based regimens (OR, 0.577 [95% CI, 0.477–0.698]; P<0.001).5 There was no statistically significant difference in the risk of ischemic events between groups. The ENTRUST-AF PCI (Edoxaban Treatment vs Vitamin K Antagonist in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention) trial showed that edoxaban-based regimens were noninferior to VKA-based regimens in terms of clinically relevant bleeding after PCI.3 An important limitation of the aforementioned data is that most studies compared DOACs monotherapy against VKA plus aspirin, the only VKA-based dual antithrombotic therapy (DAT) regimen was available in AUGUSTUS trial, thus providing limited information in comparison to DOAC-based DAT regimens available in all trials.5
RCTs have supported DAT with OAC and a P2Y12 inhibitor over triple therapy with OAC, P2Y12 inhibitor, and aspirin to reduce the risk of clinically relevant bleeding in patients with AF after PCI, including WOEST (What Is the Optimal Antiplatelet and Anticoagulant Therapy in Patients with Oral Anticoagulation and Coronary Stenting) (VKA), PIONEER AF-PCI (rivaroxaban), RE-DUAL (dabigatran), and AUGUSTUS (apixaban).1,2,4,6 A meta-analysis of PIONEER AF-PCI, RE-DUAL, and AUGUSTUS demonstrated a 40% relative risk reduction for International Society on Thrombosis and Haemostasis major bleeding in dual therapy regimens compared with triple therapy (OR, 0.598 [95% CI, 0.491–0.727]; P<0.001).5 Patients in the intervention group received varying duration of triple therapy with aspirin after PCI up to 7 days. This meta-analysis showed that DAT with DOAC and P2Y12 inhibitor was associated with an increased risk of stent thrombosis (OR, 1.672 [95% CI, 1.022–2.733]; P=0.041) compared with triple therapy.5 This finding was mainly driven by outcomes in the 110-mg dosing group of dabigatran in the RE-DUAL trial. The number needed to treat for International Society on Thrombosis and Haemostasis major bleeding (n=42) was substantially lower than the number needed to harm for stent thrombosis (n=223) with DAT compared with triple therapy.5 Based on these findings, extended triple therapy up to 30 days after PCI is a consideration when the perceived risk of stent thrombosis is high (eg, complex revascularization, multivessel PCI, previous history of stent thrombosis).
6.8.2. Chronic Coronary Disease (CCD)
Recommendation for CCD
Referenced studies that support the recommendation are summarized in the Online Data Supplement.
| COR | LOE | Recommendation |
|---|---|---|
| 1 | B-R | 1. In patients with AF and CCD (beyond 1 year after revascularization or CAD not requiring coronary revascularization) without history of stent thrombosis, oral anticoagulation monotherapy is recommended over the combination therapy of OAC and single APT (aspirin or P2Y12 inhibitor) to decrease the risk of major bleeding.1–3 |
Synopsis
APT is the first-line therapy to prevent recurrent cardiovascular events in patients with CAD, while anticoagulation therapy is recommended to prevent stroke or systemic embolism in patients with AF at increased risk of thromboembolism. Minimal evidence was available until recently if a combination therapy of APT in addition to OAC therapy provides additional value to prevent cardiovascular events in patients with AF and concomitant CCD (at least 12 months after revascularization or CAD not requiring coronary revascularization) compared with oral anticoagulation monotherapy. A recent landmark clinical trial showed that rivaroxaban monotherapy significantly decreased the primary composite outcome (stroke, systemic embolism, MI, unstable angina requiring revascularization, or death from any cause) and decreased the risk of major bleeding compared with the combination therapy.1 The subgroup analysis of the previously mentioned clinical trial in patients with AF only after coronary stenting and another clinical trial corroborated the main findings in a similar population.2,3
Recommendation-Specific Supportive Text
An RCT compared the rivaroxaban monotherapy (n=1107) with the combination of rivaroxaban and a single APT (n=1108) in patients with AF and CCD or CAD not requiring revascularization.1 The primary efficacy outcome was the composite of stroke, systemic embolism, MI, unstable angina requiring revascularization, or death from any cause. Rivaroxaban monotherapy was noninferior to dual therapy for the primary outcome (4.14% versus 5.75%/patient-year; HR, 0.72 [95% CI, 0.55–0.95]) and was superior for primary safety endpoint of major bleeding (1.62% versus 2.76%/patient-year; HR, 0.59 [95% CI, 0.39–0.89]) compared with combination therapy. Another clinical trial also showed the composite outcome of death from all causes, MI, stroke, or systemic embolism (15.7% versus 13.6%; HR, 1.16 [95% CI, 0.79–1.72]; P=0.20 for noninferiority); the major bleeding risk in the oral anticoagulation monotherapy group was numerically lower than the combination therapy group (7.8% versus 10.4%; HR, 0.73 [95% CI, 0.44–1.20]).2 However, this trial was terminated prematurely because of slow enrollment and did not have enough power for noninferiority; thus, it was inconclusive. Of note, both clinical trials excluded patients with history of stent thrombosis. Further investigation of oral anticoagulation therapy in patients with a history of stent thrombosis is needed.
6.8.3. Peripheral Artery Disease (PAD)
Recommendation for PAD
Referenced studies that support the recommendation are summarized in the Online Data Supplement.
Synopsis
Antiplatelet monotherapy is the standard of care for patients with symptomatic PAD and reasonable in asymptomatic PAD with ankle-brachial index ≤0.90 to reduce the risk of MI, stroke, and vascular death.6 The 2016 AHA/ACC PAD guidelines recommend against anticoagulation for ischemic event risk reduction in the general PAD population (COR 3; LOE A) due to increased risk of bleeding without significant reduction in ischemic events compared with antiplatelet monotherapy,6,7 without making specific recommendations for patients with comorbid PAD and AF. Conversely, anticoagulation is recommended for patients with AF at intermediate-to-high risk of stroke due to the inferiority of APT to prevent stroke and systemic thromboembolism in this population. Subgroup analysis of landmark DOAC clinical trials demonstrate comparable efficacy and safety of DOACs compared with warfarin among patients with AF with and without PAD.2–4 Clinical trial evidence is lacking to guide decisions regarding DAT in the setting of concomitant AF and PAD (whether stable PAD or after lower extremity revascularization). Observational studies of patients with concomitant AF and PAD treated with OAC monotherapy compared with dual therapy (OAC plus antiplatelet) found an increased risk of bleeding on dual therapy without reduction in major adverse cardiac events.5,8 However, an important limitation of these studies was the lack of PAD-specific outcomes such as acute or critical limb ischemia, revascularization, or amputation as primary or secondary outcomes.5,8
Recommendation-Specific Supportive Text
1. In the WAVE (Warfarin Antiplatelet Vascular Evaluation) trial of patients with PAD but without AF, dual therapy (antiplatelet plus warfarin) did not reduce the primary outcome of MI, stroke, cardiovascular death (RR, 0.92 [95% CI, 0.73–1.16]; P=0.48) compared with monotherapy (antiplatelet alone), and dual therapy increased the risk of life-threatening bleeding (RR, 3.41 [95% CI, 1.84–6.35]; P<0.001).7 Meta-analysis of 3 RCT subgroup analyses (ENGAGE AF-TIMI 48, ARISTOTLE, ROCKET AF; total N=2 564) compared safety and efficacy outcomes between warfarin and DOACs in patients with concomitant AF and PAD.1–4 The meta-analysis demonstrated similar efficacy for DOACs compared with warfarin, including stroke and systemic embolism (RR, 0.93 [95% CI, 0.61–1.42]; P=0.73), all-cause death (RR, 0.91 [95% CI, 0.70–1.19]; P=0.50), and MI (RR, 1.10 [95% CI, 0.64–1.90]; P=0.74), without a statistically significant difference in the risk of major bleeding (RR, 1.12 [95% CI, 0.70–1.81]; P=0.63) or ICH (RR, 0.54 [95% CI, 0.16–1.85]; P=0.33).1 In ORBIT-AF II (The Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II),5 among AF patients with concomitant vascular disease (CAD or PAD), major adverse cardiac or neurological events and bleeding events were compared between patients on OAC monotherapy versus dual therapy (OAC plus APT). Major adverse cardiac or neurological events (HR, 1.5 [95% CI, 1.00–2.25]) did not differ significantly between mono- and dual therapy, while dual therapy was associated with an increased risk of bleeding (HR, 2.27 [95% CI, 1.38–3.73]; P=0.001).5
6.8.4. Chronic Kidney Disease (CKD)/Kidney Failure
Recommendations for CKD/Kidney Failure
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-R | 1. For patients with AF at elevated risk for stroke and CKD stage 3, treatment with warfarin or, preferably, evidence-based doses of direct thrombin or factor Xa inhibitors (Table 19) is recommended to reduce the risk of stroke.1–3 |
| 2a | B-NR | 2. For patients with AF at elevated risk for stroke and CKD stage 4, treatment with warfarin or labeled doses of DOACs is reasonable to reduce the risk of stroke.4,5 |
| 2b | B-NR | 3. For patients with AF at elevated risk for stroke and who have end-stage CKD (CrCl <15 mL/min) or are on dialysis, it might be reasonable to prescribe warfarin (INR 2.0–3.0) or an evidence-based dose of apixaban for oral anticoagulation to reduce the risk of stroke.6,7 |
Table 19.
Recommended Doses of Currently Approved DOACs According to Renal Function
| DOAC | CrCl (mL/min) | ||||
|---|---|---|---|---|---|
| >95 | 51–95 | 31–50 | 15–30 | <15 or on dialysis | |
| Apixaban | 5 or 2.5 mg twice daily* | 5 or 2.5 mg twice daily* | 5 or 2.5 mg twice daily* | 5 or 2.5 mg twice daily* | 5 or 2.5 mg twice daily* |
| Dabigatran | 150 mg twice daily | 150 mg twice daily | 150 mg twice daily | 75 mg twice daily | Contraindicated |
| Edoxaban | Contraindicated | 60 mg once daily | 30 mg once daily | 30 mg once daily | Contraindicated |
| Rivaroxaban | 20 mg once daily | 20 mg once daily | 15 mg once daily | 15 mg once daily | 15 mg once daily† |
Note that other, nonrenal considerations such as drug interactions may also apply. The gray area indicates doses not studied in the pivotal clinical trials of these agents.
If at least 2 of the following are present: serum creatinine ≥1.5 mg/dL, age ≥80 y, or body weight ≤60 kg, the recommended dose is 2.5 mg twice daily. The ARISTOTLE trial excluded patients with either a creatinine of >2.5 mg/dL or a calculated CrCl <25 mL/min.
Rivaroxaban is not recommended for other indications in patients with a CrCl <15 mL/min, but such a recommendation is not made for the AF indication. However, pharmacokinetic data are limited.
AF indicates atrial fibrillation; ARISTOTLE, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; CrCl, creatinine clearance; and DOAC, direct oral anticoagulant.
Synopsis
CKD is a risk factor for stroke in patients with AF, independent of other risk factors.8 The prevalence of AF among patients with renal failure on dialysis is high, including asymptomatic AF.9 Despite compelling evidence in the general population, conflicting data exist as to whether AF is a risk factor for stroke in patients on dialysis.10,11 Potential explanations for this discrepancy include competing risks of stroke and mortality, a high prevalence of undiagnosed AF in cohorts thought not to have AF, and the confounding effect of anticoagulation thrice weekly during hemodialysis.12 VKAs are limited by a markedly reduced time in therapeutic range in patients with severe CKD, including those on dialysis,13 and association with calciphylaxis.14 The pivotal DOAC trials excluded patients on dialysis, and recommendations for dosing are based on pharmacokinetic data only. Early experience with dabigatran and rivaroxaban in this population suggested an increased risk of bleeding relative to warfarin, driven by the higher dose of each.15 Only dabigatran is substantially removed by dialysis.16 A small RCT and a retrospective population-based cohort study found a lower risk of thromboembolism with rivaroxaban versus warfarin,17,18 but these findings await confirmation in larger studies. Limited data on LAAO exist in this group of patients, and prospective comparison to anticoagulation or no therapy is awaited.19
Recommendation-Specific Supportive Text
Early trials demonstrating the role of warfarin anticoagulation in preventing stroke did not enroll or separately report outcomes in patients with CKD. An analysis of the SPAF III trial showed that dose-adjusted warfarin, compared with fixed low-dose warfarin plus aspirin, reduced the risk of stroke with a similar magnitude to patients with normal kidney function.3 In the AVERROES trial of apixaban versus aspirin in patients deemed unsuitable for warfarin, apixaban showed similar efficacy in patients with an estimated glomerular filtration rate (eGFR) of 25 to 50 mL/min as those with an eGFR >50 mL/min.20 Patients with CKD stage 3, which includes those with an eGFR of 30 to 60 mL/min, were enrolled in the pivotal trials of the DOACs and showed similar results compared with warfarin as seen in patients with normal kidney function.2
Evidence on the use of anticoagulation in patients with AF and stage 4 CKD (eGFR, 15–30 mL/min/1.73 m2) is largely observational. A prospective, multicenter cohort study of survivors of MI with AF found that warfarin prescription at discharge associated with a reduced risk of death, readmission due to MI, ischemic stroke, and bleeding.5 An analysis of patients with CrCl 25 to 30 mL/min at the time of enrollment in the ARISTOTLE trial found numerically fewer stroke and major bleeding events with apixaban than warfarin.4 Because CrCl overestimates eGFR, a proportion of the patients enrolled in the pivotal clinical trials of DOACs, which used CrCl cut-offs for enrollment, had CKD stage 4 as assessed by eGFR in current clinical practice.
The role of anticoagulation in patients with AF and severe CKD, including those on dialysis, is controversial.6,7,21,22 Pending the results of RCTs comparing warfarin with no treatment, and warfarin with DOACs, SDM incorporating risks of bleeding and thromboembolism is encouraged. The pivotal clinical trials of apixaban excluded patients with CrCl <25 mL/min.23,24 Subsequently, the FDA-approved labeling was extended to include those with end-stage renal failure, including those on hemodialysis.25 A subsequent pharmacokinetic study found that drug levels with apixaban 2.5 mg twice daily approximate those seen with 5 mg twice daily in patients with normal renal function, suggesting 2.5 mg is a more appropriate dose in such patients.26 A large retrospective cohort study of Medicare beneficiaries found no difference in thromboembolism, but less major bleeding, in patients treated with apixaban versus warfarin.27 Another analysis found an increased risk of thromboembolism and of fatal or intracranial bleeding with apixaban 5 mg twice daily versus no anticoagulation but not with apixaban 2.5 mg twice daily.28 Two small trials of apixaban versus warfarin in patients with AF on hemodialysis could not show differences in safety or efficacy outcomes but significantly more bleeding than stroke events.29,30
6.8.5. AF in VHD
Recommendations for AF in VHD
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-R | 1. In patients with rheumatic mitral stenosis or mitral stenosis of moderate or greater severity and history of AF, long-term anticoagulation with warfarin is recommended over DOACs, independent of the CHA2DS2-VASc score to prevent cardiovascular events, including stroke or death.1 |
| 1 | B-NR | 2. In patients with AF and valve disease other than moderate or greater mitral stenosis or a mechanical heart valve, DOACs are recommended over VKAs.2–8 |
Synopsis
At least one-third of patients with AF have some degree of VHD. Patients with AF and VHD have a higher prevalence of stroke and systemic thromboembolism than those patients with AF without VHD.9 Patients with VHD and AF should be evaluated for risk of thromboembolic events and treated with oral anticoagulation if they are at high risk or if they have significant mitral stenosis or a mechanical heart valve. VKAs are the anticoagulation drugs of choice for patients with rheumatic mitral stenosis, mechanical heart valves, and new-onset AF within 3 months after mechanical aortic or mitral valve surgery.10
Recommendation-Specific Supportive Text
Historical data have reported that in patients with mitral stenosis and AF, stroke risk was nearly 18-fold higher than an age-, sex-, and hypertension-matched population without AF.11 More recently, stroke or systemic embolism rates ranged between 0.4 and 4 per 100 patient-years among anticoagulated patients and were highest in those with previous embolism. Warfarin is generally prescribed, but it has been questioned whether a DOAC may be an alternative. A retrospective cohort study from Korea examined 2230 individuals with mitral stenosis and AF who were prescribed either warfarin or a DOAC, using propensity matching on 10 clinical variables. Thromboembolic events in the DOAC group were 2.22% per year compared with 4.19% per year in the warfarin group (HR, 0.28 [95% CI, 0.18–0.45]).12 However, in the INVICTUS (The Investigation of Rheumatic AF Treatment Using VKAs, Rivaroxaban or Aspirin Studies) RCT,1 patients with AF, rheumatic heart disease, and mitral stenosis had a mean survival time to a primary outcome event of stroke, systemic embolism, MI, or death from vascular or unknown cause of 1 675 days if treated with a VKA compared with 1 599 days if with rivaroxaban (difference, −76 days [95% CI, −121 to −31]; P<0.001).
Although patients with moderate to severe mitral stenosis or mechanical valve were excluded from DOAC trials, other forms of VHD were allowed, such as aortic stenosis or mitral regurgitation. Bioprosthetic valves and valve repair were allowed in the edoxaban (ENGAGE AF) and apixaban (ARISTOTLE) trials, and valve repair in the rivaroxaban (ROCKET AF) trial. A systematic review13 of patients with VHD (other than moderate to severe mitral stenosis or mechanical valve) concluded that DOACs were safe. A meta-analysis confirmed that DOACs decreased the risk of stroke/systemic embolism compared with warfarin in patients with (HR, 0.70 [95% CI, 0.58–0.86]) and without VHD (HR, 0.84 [95% CI, 0.75–0.95]).14 However, for patients with mechanical heart valves,15 the RE-ALIGN (Randomized, Phase II Study to Evaluate the Safety and Pharmacokinetics of Oral Dabigatran Etexilate in Patients After Heart Valve Replacement)10 trial was halted during phase II after enrolling 252 subjects due to increased thromboembolism and bleeding in the dabigatran arm. PROACT Xa (A Trial to Determine if Participants with an On-X Aortic Valve Can be Maintained Safely on Apixaban) was also terminated early due to higher thromboembolic events with apixaban in patients who were >3 months after mechanical On-X aortic valves.
6.8.6. Anticoagulation of Typical AFL
Recommendations for Anticoagulation of Typical AFL*
Referenced studies that support the recommendations are summarized in Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. For patients with AFL, anticoagulant therapy is recommended according to the same risk profile used for AF.1–3 |
| 1 | C-LD | 2. In patients with AFL who undergo successful cardioversion or ablation resulting in restoration of sinus rhythm, anticoagulation should be continued for at least 4 weeks postprocedure.1–4 |
| 1 | A | 3. Patients with typical AFL who have undergone successful CTI ablation and have had AF previously detected before AFL ablation should receive ongoing oral anticoagulation postablation as indicated for AF.5,6 |
| 1 | B-NR | 4. Patients with typical AFL who have undergone successful CTI ablation and are deemed to be at high thromboembolic risk, without any known previous history of AF, should receive close follow-up and arrhythmia monitoring to detect silent AF if they are not receiving ongoing anticoagulation in view of significant risk of AF.7–9 |
| 2b | B-NR | 5. In patients with typical AFL who have undergone successful CTI ablation without any known previous history of AF who are at high risk for development of AF (eg, LA enlargement, inducible AF, chronic obstructive pulmonary disease [COPD], HF), it may be reasonable to prescribe long-term anticoagulation if thromboembolic risk assessment suggests high risk (>2% annual risk) for stroke.5,10–13 |
This section refers to typical right-sided (CTI-dependent) AFL. Left-sided AFLs or atrial tachycardias (ATs) that develop after ablation of AF should be anticoagulated and managed in a manner similar to AF. “Typical” AFL is defined as either typical counterclockwise AFL when the macroreentrant circuit is dependent on the CTI using the isthmus from the patient’s right to left or typical clockwise AFL when the macroreentrant circuit is dependent on the CTI and uses this isthmus from the patient’s left to right. “Atypical” AFL is not dependent on the CTI and may arise from a macroreentrant circuit in the LA, such as perimitral or LA roof flutter or could be dependent on scar from previous ablation or surgery.
Synopsis
AFL is a common atrial arrhythmia with a reported overall incidence of 88 per 100 000 person-years, and the incidence increases with age.14 AFL is 2.5 times more common in men than in women, and it is significantly more likely to occur in patients with underlying HF or COPD.14
Because of the high success rate and low recurrence rate of typical AFL after CTI ablation, catheter ablation is often used as a first-line treatment. The high effectiveness of CTI ablation may decrease thromboembolic risk so that many physicians may elect to discontinue oral anticoagulation >1 month after ablation in the absence of previously detected AF. However, the high incidence of new onset AF at some time after CTI ablation places this practice in question. Because AF may be subclinical or asymptomatic, patients could be at significant risk of thromboembolic events after ablation of typical AFL depending on thromboembolic risk factors.
Catheter ablation of non–CTI-dependent AFL is technically more difficult, and “atypical” AFL or AT often occurs after AF ablation. These arrhythmias should be anticoagulated and managed in a manner similar to AF. Recommendations in this section refer to treatment of “typical” AFL.
Recommendation-Specific Supportive Text
AFL is associated with increased thromboembolic risk, but the exact risk and benefit of oral anticoagulation are difficult to ascertain as AFL and AF often coexist. In a population-based retrospective cohort study of patients with typical AFL and no history of AF, stroke occurred in 4.1% of patients with AFL compared with 1.2% of a general population-matched cohort (P<0.001).1 However, no large, randomized trials specifically address thromboembolic risks and benefits of anticoagulation in patients who have only AFL.
In patients referred for ablation of typical AFL without adequate previous appropriate anticoagulation, and no previous history of AF, TEE showed evidence for LA thrombus or dense spontaneous echocardiographic contrast in 3%.2 A systematic review identified thromboembolic event rates of 0% to 6% with cardioversion, with the highest incidence of thromboembolic events occurring 1 to 2 days postcardioversion of AFL.3 Echocardiographic studies reported LA thrombus in 0% to 38% and spontaneous echocardiographic contrast in 21% to 28% of cases.3 In 1 ablation study, thromboembolic events occurred in 13.9% of patients who did not receive anticoagulation.3 Observational studies report an elevated risk of stroke with AFL compared with a control group (RR, 1.4 [95% CI, 1.35–1.46]) and elevated mortality risk (risk ratio, 1.9 [95% CI, 1.2–3.1]) with long-term follow-up.3
The incidence of embolism after cardioversion of AFL is similar to that of AF (0.72% versus 0.46%; P=not significant).4 Atrial stunning has been noted on TEE in patients undergoing cardioversion of AF, with partial atrial recovery 15 to 30 days and full recovery 30 to 90 days postcardioversion.15 LAA stunning also occurs in patients with AFL, although it appears to occur at a lesser degree than what is seen with AF.16 Although limited data are available for AFL, 1 study demonstrated more forceful mechanical LAA contraction and absence of spontaneous echocardiographic contrast 2 weeks after ablation of persistent AFL, suggesting earlier recovery.17 Because LA and LAA stunning are thought to contribute to thrombus formation and thromboembolic events after cardioversion, continued anticoagulation is recommended early after conversion to sinus rhythm.
In a meta-analysis, the subsequent incidence of AF after ablation of CTI-dependent AFL was 34% over 14 months. However, the incidence of AF after flutter ablation is significantly higher in patients with a history of AF before ablation than those without AF before ablation (53% versus 23% after 16–18 months; P<0.05).5 The incidence of AF continues to increase over time in both patients with and without previous AF.5 Oral anticoagulation reduces risk of stroke or systemic thromboembolism in the setting of nonvalvular AF.6 Because AF is not necessarily reduced after ablation of AFL, continued anticoagulation is recommended based on thromboembolic risk assessment. The acute success rate for catheter ablation typical AFL is reported to be 92% with a single procedure and 97% with multiple procedures in a meta-analysis.18 However, the subsequent incidence of AF occurring after ablation of CTI-dependent AFL is reported to be 16% to 82%,5,10,11 with a higher incidence in patients with a history of AF before ablation than those without (53% versus 23% after 16–18 months; P<0.05).5 In 1 study, 82% of patients who underwent typical AFL ablation experienced new-onset AF during long-term follow-up (mean, 39 months).11
Because of the high rate of occurrence of new-onset AF after CTI ablation, close follow-up and monitoring are recommended. In patients undergoing AFL ablation, the detection of AF in patients without previous AF significantly increased with more frequent monitoring and/or longer duration of follow-up.7 This is especially important in patients at high risk of stroke or thromboembolism (>2%/year). Intermittent monitoring may be performed with ambulatory monitors or wearable devices. Alternatively, implantable devices can provide more prolonged and continuous monitoring. Implantable cardiac monitors have been used in multiple settings to detect AF, including after AF ablation or in patients with cryptogenic stroke. Implantable cardiac monitors have also been used for surveillance after AF ablation.9
Factors that predict AF after flutter ablation include LA enlargement,19–21 inducible AF at the time of flutter ablation,22,23 interatrial conduction time,24 prolonged HV interval,25 COPD,26 obstructive sleep apnea,20 and HATCH (hypertension, age ≥75 years, TIA or stroke, COPD, heart failure) score.21 One study that reported new-onset AF in 38% of patients after AFL ablation demonstrated a thromboembolic event rate of 10% during a mean follow-up of 5 years.12 In a Danish registry of patients undergoing flutter ablation, 5% of patients suffered a stroke, and 10% died during a mean follow-up of 4 years.13
Thus, patients who undergo successful flutter ablation are not free from thromboembolic complications during long-term follow-up, likely due to a high rate of development of new-onset AF.
7. RATE CONTROL
7.1. Broad Considerations for Rate Control
Recommendations for Broad Considerations for Rate Control
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with AF, SDM with the patient is recommended to discuss rhythm- versus rate-control strategies (taking into consideration clinical presentation, comorbidity burden, medication profile, and patient preferences), discuss therapeutic options, and for assessing long-term benefits.1–3 |
| 2a | B-R | 2. In patients with AF without HF who are candidates for select rate-control strategies, heart rate target should be guided by underlying patient symptoms, in general aiming at a resting heart rate of <100 to 110 bpm.2,4–6 |
Synopsis
Rate control is a suitable strategy for many patients with AF.1,3 A solitary randomized trial has evaluated the optimal heart rate among patients with AF. In the RACE II study (Rate Control Efficacy in Permanent Atrial Fibrillation: A Comparison Between Lenient Versus Strict Rate Control II), 614 patients with permanent AF were randomly assigned to either lenient rate control (resting heart rate <110 bpm) or strict rate control (heart rate, <80 bpm). A difference was not seen regarding either the primary composite outcome of death from cardiovascular causes, hospitalization for HF, stroke, systemic embolism, bleeding, and life-threatening arrhythmic events (absolute difference of −2.0 percentage points [90% CI, −7.6 to 3.5; P<0.001] for the noninferiority margin)2 or the secondary outcome of QOL7 at 3 years. These data suggest heart rate control intensity is not a central determinant of clinical outcomes. However, interpretation is limited by a difference of only 10 bpm between study groups attributable to the proportion of patients assigned to strict control who did not achieve target rate control (32.6%; n=98/301) and because 78% of lenient rate control participants having heart rate <100 bpm.8 In addition, because patients with HF were underrepresented, whether the results can be extrapolated to those with HF is unknown. Nonrandomized data focused on target heart rate among outpatients,4,9,10 as well as those with HF,5,11–16 are divergent.
Recommendation-Specific Supportive Text
Rate- and rhythm-control strategies have comparable clinical outcomes in many patients with AF. In the AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) study, rate control was comparable to cardioversion and antiarrhythmic drugs regarding survival (HR, 1.15 [95% CI, 0.99–1.34]; P=0.08).1 In the HOT CAFE (How to Treat Chronic Atrial Fibrillation) trial, rate control was comparable to cardioversion and use of antiarrhythmic drugs with respect to a composite endpoint of all-cause mortality, number of thromboembolic events, or major bleeding (OR, 1.98 [95% CI, 0.28–22.3]; P>0.71).6 In a meta-analysis of randomized trials and observational studies comparing rhythm with rate control, management strategies were comparable with respect to all-cause (OR, 1.34 [95% CI, 0.89–2.02]) and cardiac mortality (OR, 0.96 [95% CI, 0.77–1.20]) as well as stroke (OR, 0.99 [95% CI, 0.76–1.30]).3 Although selection of a rhythm-control therapy within a year of AF diagnosis may be considered to reduce the risk of adverse cardiovascular outcomes,17 early rate control may still be appropriate as a function of clinical presentation, comorbidity burden, medication profile, and patient preferences. In this context, providers should partner with their patients to select a value-concordant approach within an SDM framework.
In RACE II, rate-control intensity (target resting heart rate, <110 bpm versus <80 bpm) did not influence cardiovascular morbidity or mortality.6,18 Rate control (AFFIRM: goal heart rate, ≤80 bpm at rest and ≤110 bpm during a 6-minute walk test; HOT CAFE: 70–90 bpm at rest and ≤140 bpm with moderate exercise) has been shown to be comparable to rhythm control in a meta-analysis of 5 trials, yet some limitation of the studies include that most patients had well-tolerated persistent AF or high risk of recurrence, no long-term follow-up, and should not be extrapolated to patients with paroxysmal AF, highly symptomatic, or patients with HF.1 Observational data among outpatients and those with HF are conflicting.4 In the ORBIT-AF trial, increasing heart rate was associated with higher all-cause mortality9 and incident HF.10 Whether a lenient rate-control strategy may be safely and effectively used in patients with heart failure with preserved ejection fraction (HFpEF)5,14 or reduced fraction11–13,15,16 has also shown conflicting results. Other populations that may benefit from a low heart rate goal include those with rate-related cardiac dysfunction,19 ICDs,20 cardiac resynchronization therapy,21 and tachycardia-bradycardia form of sick sinus syndrome22 (Table 20).
Table 20.
Clinical Presentations and Objectives of Heart Rate Control
| Presentation | Objective |
|---|---|
| Symptomatic AF | To reduce symptoms |
| Tachycardia-induced cardiomyopathy | To improve heart function or reduce the risk of recurrent cardiomyopathy19 |
| ICD use | To reduce risk of inappropriate shock20 |
| Cardiac resynchronization therapy use | To enhance biventricular pacing, likelihood of myocardial recovery, and/or preservation of function21 |
| Tachycardia-bradycardia form of sick sinus syndrome among those with a pacemaker | To reduce the risk of hospitalization22 |
AF indicates atrial fibrillation; and ICD, implantable cardioverter-defibrillator.
7.2. Specific Pharmacological Agents for Rate Control
The overall goals for rate control in patients with both acute and chronic AF with a rapid ventricular response center on control of symptoms and the risk of developing LV systolic dysfunction. In general, nondihydropyridine calcium channel blockers (diltiazem and verapamil) and beta blockers are the standard of care in rate-controlling AF. Nondihydropyridine calcium channel blockers slow conduction through the atrioventricular node and have negative inotropic and chronotropic effects. These agents are useful in ventricular rate control in the absence of preexcitation. They provide reasonable rate control and also improve AF-related symptoms compared with beta blockers.1,2 Beta blockers also slow conduction through the atrioventricular node by blocking beta-1 receptors. Digoxin is a time-honored medication for patients with AF particularly among patients with HFrEF due to its positive inotropic and vagotonic effects. Limited data exist directly comparing rate-control agents, especially in the setting of long-term rate control. Selection of specific agents should consider patient-specific characteristics and response. This section will outline the principles of treatment for acute and long-term rate control.
7.2.1. Acute Rate Control
Recommendations for Acute Rate Control
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-R | 1. In patients with AF with rapid ventricular response who are hemodynamically stable, beta blockers or nondihydropyridine calcium channel blockers (verapamil, diltiazem; provided that EF >40%) are recommended for acute rate control (Figure 17).1–4 |
| 2a | B-R | 2. In patients with AF with rapid ventricular response in whom beta blockers and nondihydropyridine calcium channel blockers are ineffective or contraindicated, digoxin can be considered for acute rate control, either alone or in combination with the aforementioned agents.5–9 |
| 2a | A | 3. In patients with AF with rapid ventricular response, the addition of intravenous magnesium to standard rate-control measures is reasonable to achieve and maintain rate control.10,11 |
| 2b | B-NR | 4. In patients with AF with rapid ventricular response who are critically ill and/or in decompensated HF in whom beta blockers and nondihydropyridine calcium channel blockers are ineffective or contraindicated, intravenous amiodarone may be considered for acute rate control.*12,13 |
| 3: Harm | B-NR | 5. In patients with AF with rapid ventricular response and known moderate or severe LV systolic dysfunction with or without decompensated HF, intravenous nondihydropyridine calcium channel blockers should not be administered.14,15 |
Consider the risk of cardioversion and stroke when using amiodarone as a rate-control agent.
Figure 17. Acute Rate Control in AF With RVR.
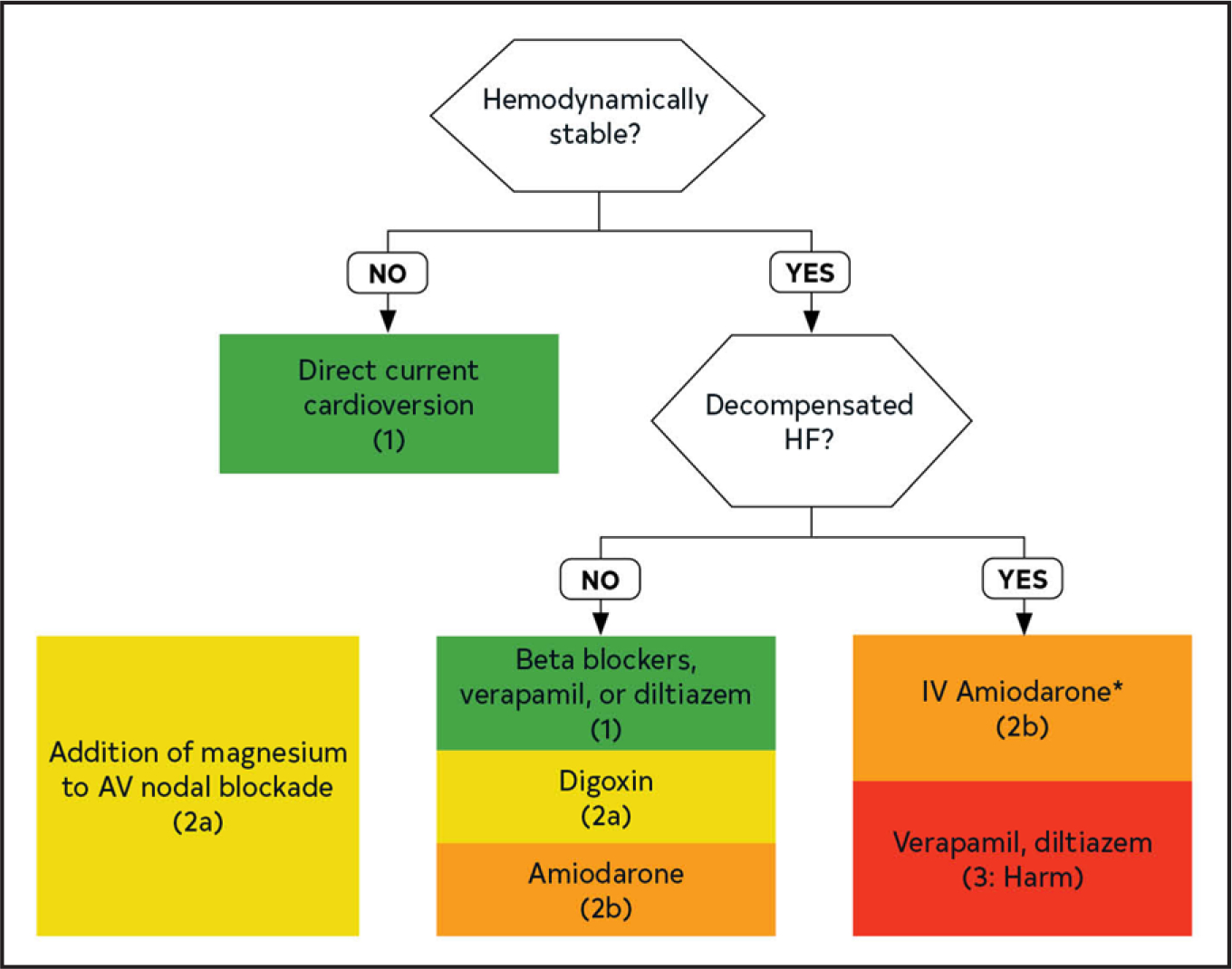
*Contraindicated in patients with moderate-severe LV dysfunction regardless of decompensated HF. Colors correspond to Table 2. AF indicates atrial fibrillation; AV, atrioventricular; HF, heart failure; LV, left ventricular; and RVR, rapid ventricular response.
Synopsis
Controlling the ventricular rate in AF with a rapid ventricular response in the acute setting can vary effectiveness for control of symptoms. It can also present challenges, given some agents may or may not be preferable in the context of underlying comorbidities given the myriad of contributing factors. For patients requiring intravenous rate-control agents, hypotension and/or the presence of decompensated HF may limit use of otherwise efficacious agents. In the acute setting, recognizing patients needing emergency cardioversion is important.
Recommendation-Specific Supportive Text
A randomized, double-blind, placebo-controlled trial investigated the safety and efficacy of continuous intravenous diltiazem infusion for 24-hour heart rate control during AF and AFL. Seventeen of the 23 patients (74%) receiving diltiazem infusion and none of the 21 with placebo infusion maintained a therapeutic response for 24 hours. Over 24 hours, patients receiving diltiazem infusion lost response significantly more slowly than did those receiving placebo infusion. In another RCT, patients were randomly assigned in 1:1:1 ratio to receive intravenous diltiazem, digoxin, or amiodarone for ventricular rate control. At 24 hours, rate control was achieved in 119 of 150 patients (79%). The time to rate control was significantly shorter among patients in the diltiazem group, with the percentage of patients who achieved rate control being higher in the diltiazem group (90%) than the digoxin group (74%) and the amiodarone group (74%). A randomized, parallel, open-label study aimed to investigate esmolol versus verapamil in the acute treatment of AF or AFL. The heart rate significantly declined with esmolol and verapamil. Fifty percent of esmolol-treated patients with new onset of arrhythmias converted to sinus rhythm, whereas only 12% of those who received verapamil converted. Mild hypotension was observed in both treatment groups.
The use of intravenous digoxin has been shown to be effective in controlling the rate of rapid AF compared with placebo in 1 multicenter RCT.5 However, other agents may be safer and more effective in achieving acute rate control. Intravenous diltiazem was more effective than intravenous digoxin in 2 small RCTs.6,7 One trial performed at an emergency department at an academic center randomized 150 patients with AF without major comorbidities and a ventricular rate >120 bpm in a 1:1:1 ratio to intravenous diltiazem, digoxin, and amiodarone.6 The time to reduce the heart rate <90 bpm was significantly shorter in the diltiazem group compared with either digoxin or amiodarone, with fewer symptoms and shorter hospital lengths of stay. Another RCT of 30 patients randomized patients with AF and a rapid ventricular response to either intravenous diltiazem, digoxin, or a combination of both agents. Treatment with intravenous diltiazem significantly decreased heart rate within 5 minutes versus 3 hours with intravenous digoxin.7 Intravenous diltiazem reduced heart rate more rapidly than intravenous digoxin in a double-blinded RCT in 40 patients with rapid AF after CABG, with similar control at 12 to 24 hours. In another RCT of 84 patients in rapid AF who presented to the emergency department, intravenous amiodarone resulted in faster control of the ventricular response compared with intravenous digoxin.8 Finally, 52 patients with rapid AF were randomized to receive either an intravenous combination of diltiazem and digoxin or intravenous diltiazem alone, with a more rapid and durable response to the combination.9
In addition to atrioventricular nodal blockers and antiarrhythmics, intravenous magnesium has been investigated for rate controlling rapid AF. 10,11 The mechanism likely stems from blockade of slow inward calcium channels in the sinoatrial and atrioventricular node, thereby slowing the heart rate and prolonging atrioventricular conduction velocity, respectively.16 Its low adverse effect profile and minimal toxicity make it a favorable option, often as an adjunct to conventional therapy (ie, atrioventricular nodal blockers). A meta-analysis including 6 RCTs (n=745 patients) investigated the effectiveness of intravenous magnesium in rate and rhythm control of rapid AF when administered in combination with standard rate-control methods (including beta blockers, diltiazem, verapamil, digoxin), as well as placebo. In the pooled analysis, intravenous magnesium was superior in achieving rate control (63% versus 40%; OR, 2.49 [95% CI, 1.80–3.45]) and modestly effective in rhythm conversion to sinus (21% versus 14%; OR, 1.75 [95% CI, 1.08–2.84]) compared with standard rate-control methods. Subgroup analysis showed the superiority of a lower dose (≤5 g) (24% versus 13%; OR, 2.10 [95% CI, 1.22–3.61]) compared with the higher dose (>5 g) (16% versus 13%; OR, 1.23 [95% CI, 0.65–2.32]) in rhythm control when compared with placebo.10
Intravenous amiodarone has been shown to be effective in controlling ventricular rates in patients who are critically ill. In a retrospective study of 38 patients admitted to the intensive care unit, intravenous amiodarone was associated with a statistically significant decrease in heart rate without decrease in BP compared with intravenous diltiazem and digoxin.12 One study of 60 critically ill patients, predominantly with AF, with heart rate consistently >120 bpm randomized patients to 1 of 3 intravenous treatment regimens: diltiazem in a 25-mg bolus followed by a continuous infusion of 20 mg/h for 24 hours; amiodarone in a 300-mg bolus; and amiodarone in a 300-mg bolus followed by 45 mg/h for 24 hours.13 A >30% rate reduction within 4 hours was not statistically different in either treatment arm, with less effective control more frequently observed in those receiving a bolus of amiodarone alone; thus overall, diltiazem allowed for significantly better heart rate control over 24 hours, but it also caused a significantly higher incidence of hypotension requiring discontinuation of the drug.
Limited data exist regarding the use of nondihydropyridine calcium channel blockers in patients with HFrEF, due to the presumed limitation of negative inotropic effects. Two retrospective analyses highlight potential for increased morbidity when administered in this population. In a retrospective analysis of patients hospitalized with AF with rapid ventricular rate (RVR), diltiazem was associated with an increased risk of acute kidney injury within 48 hours of initiation of diltiazem in those patients with LVEF ≤50%, compared with those with normal EF (10% versus 3.6%; P=0.002).14 An additional retrospective analysis compared patients with HFrEF receiving either intravenous metoprolol or diltiazem. There was a higher incidence of worsening HF symptoms (increased oxygen requirement within 4 hours or initiation of inotropic support with 48 hours) in those patients receiving diltiazem (33% versus 15%; P=0.019). Neither analysis noted an increase in the in-hospital mortality rate, need for a higher level of care, or hypotension.
7.2.2. Long-Term Rate Control
Recommendations for Long-Term Rate Control
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with AF, beta blockers or nondihydropyridine calcium channel blockers (diltiazem, verapamil) are recommended for long-term rate control with the choice of agent according to underlying substrate and comorbid conditions.1,2 |
| 2a | B-NR | 2. For patients with AF in whom measuring serum digoxin levels is indicated, it is reasonable to target levels <1.2 ng/mL.3–6 |
| 2a | B-R | 3. In patients with AF and HF symptoms, digoxin is reasonable for long-term rate control in combination with other rate-controlling agents, or as monotherapy if other agents are not preferred, not tolerated, or contraindicated.7–9 |
| 3: Harm | C-LD | 4. In patients with AF and LVEF <40%, nondihydropyridine calcium channel–blocking drugs should not be administered given their potential to exacerbate HF.10,11 |
| 3: Harm | B-R | 5. In patients with permanent AF who have risk factors for cardiovascular events, dronedarone should not be used for long-term rate control.12 |
Synopsis
Both nondihydropyridine calcium channel blockers (verapamil and diltiazem) and beta blockers are effective for long-term rate control. These agents are useful in ventricular rate control in the absence of preexcitation. Digoxin may be useful in patients with limited tolerability to other agents, or as adjunct therapy in patients with a difficult to control ventricular rate. Limited data exist comparing various rate control agents, especially in the setting of chronic rate control. Selection of specific agents should consider patient-specific characteristics (ie, HFrEF, reactive airway disease) and response (Figure 18).
Figure 18. AF Long-Term Rate Control.
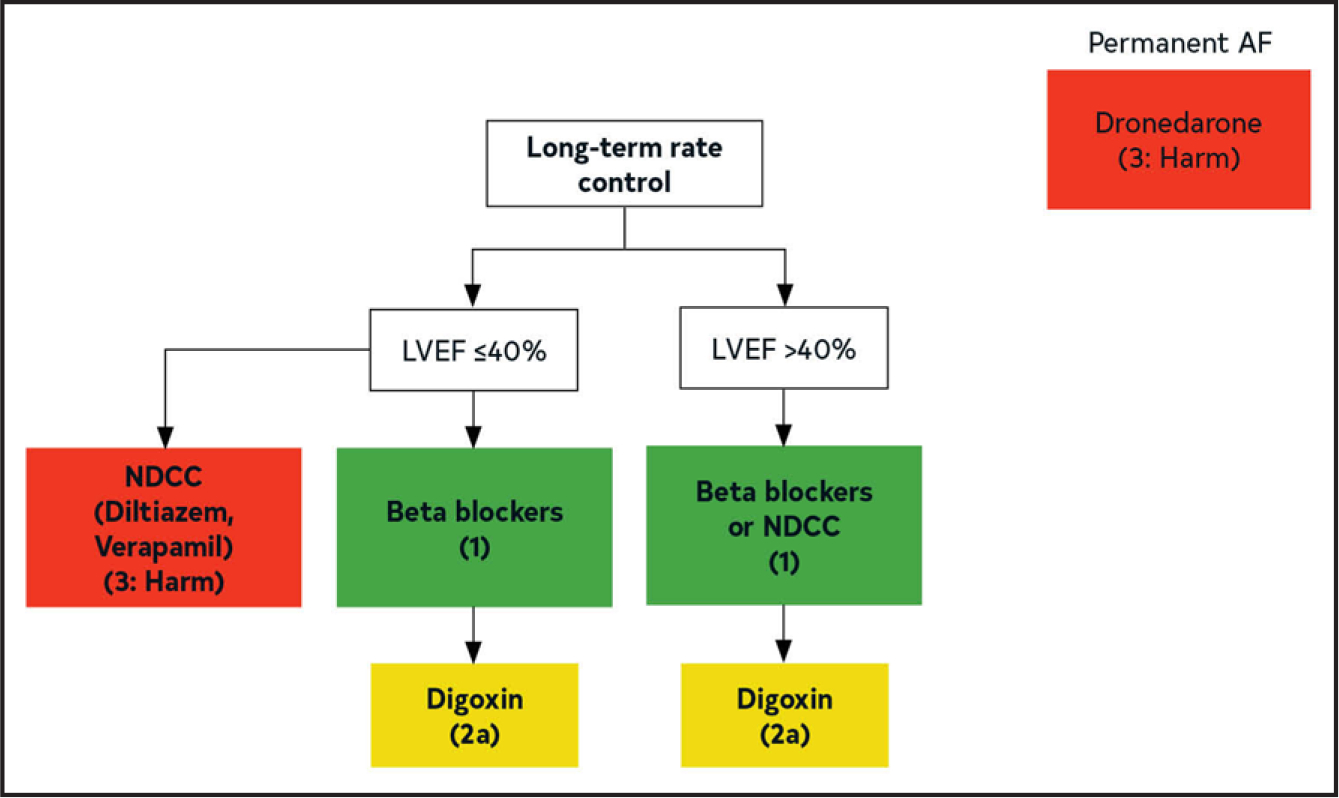
Colors correspond to Table 2. AF indicates atrial fibrillation; LVEF, left ventricular ejection fraction; and NDCC, nondihydropyridine calcium channel blocker.
Recommendation-Specific Supportive Text
One prospective, randomized, investigator-blinded, crossover study compared the effect of 4 rate-reducing once-daily drug regimens on the ventricular heart rate and arrhythmia-related symptoms in patients with permanent AF. The 24-hour mean heart rate was 96±12 bpm at baseline (no treatment), 75±10 bpm with diltiazem, 81±11 bpm with verapamil, 82±11 bpm with metoprolol, and 84±11 bpm with carvedilol. All drugs significantly reduced the heart rate compared with baseline.13 In a retrospective cohort study, investigators used a database from 2 urban emergency departments to identify consecutive patients with emergency department discharge diagnoses of AF. A total of 259 consecutive patients were enrolled, with 100 receiving calcium channel blockers and 159 receiving beta blockers. Baseline demographics and comorbidities were similar. Twenty-seven percent of patients taking beta blockers were admitted, 31.0% of patients taking calcium channel blockers were admitted, and there were no significant differences in emergency department length of stay, adverse events, or 7- or 30-day emergency department revisits. A follow-up analysis of the AFFIRM study examined the difference in all hospitalization and all-cause mortality in participants treated with a single rate-control agent at baseline. No difference was found among those participants receiving beta blockers, nondihydropyridine calcium channel blockers, or digoxin.2
Seminal work investigating digoxin toxicity was based on a small series of patients, where toxicity was determined by electrocardiographic abnormalities.14 Toxicity was seen when serum digoxin concentrations exceeded 2.0 ng/mL, and almost certainly at levels >3.0 ng/mL. Based on this small study, the narrow therapeutic range of digoxin is usually cited as 0.8 to 2.0 ng/mL, with substantial variation across institutional laboratories and published references.15,16 However, 3 post hoc analyses of the DIG (Digitalis Investigation Group) trial suggested that safe use of digoxin is seen at lower serum concentrations.4–6 Serum digoxin concentrations of 0.5 to 0.9 ng/mL were associated with significantly lower all-cause mortality rates and hospitalizations compared with concentrations ≥1.0 ng/mL.3 Another analysis suggested that serum digoxin concentrations >1.2 ng/mL may be harmful, particularly in women.7 A post hoc analysis comparing patients with AF taking digoxin with propensity score-matched controls suggested that patients with serum digoxin concentrations <0.9 ng/mL had no increased risk of death, concentrations of 0.9 to 1.1 ng/mL had a nonsignificant increased risk of death, and serum digoxin concentrations ≥1.2 ng/mL were associated with a significant (56%) increased risk of death.4 Serum digoxin concentrations of 0.5 to 0.8 ng/mL seem safest in terms of benefit without adverse effects in patients with HFrEF.17
Digoxin may have added efficacy as rate control in conjunction with beta blockers in patients with AF. A small RCT of 47 patients with persistent AF and concomitant HFrEF compared the effects of digoxin alone, carvedilol alone, and their combination.10 Combination therapy was significantly associated with better rate control and symptom relief than monotherapy with either agent. The RATE-AF trial randomized 160 patients with persistent AF and concomitant HFrEF to digoxin and bisoprolol.11 No significant differences in the primary endpoint of QOL were noted at 6 months with either agent. Several secondary outcomes, including N-terminal pro-brain natriuretic peptide level, were significantly lower in the digoxin group, without a significant difference in resting heart rate between the 2 groups at 12 months. Adverse effects were less common in the digoxin group. A large meta-analysis of 75 studies and approximately 620 000 patients found a significantly increased risk of death in digoxin users overall but, when limited to 7 RCTs (~8400 patients), no difference in mortality rate was observed between digoxin or placebo—of note, these 7 RCTs in the subanalysis were in patients with concomitant HF.9 A post hoc sensitivity analysis assessing the impact of digoxin on mortality in patients with HF and concomitant AF found no impact on overall mortality in patients taking digoxin, most with HFrEF.
MDPIT (Multicenter Diltiazem Postinfarction Trial) demonstrated an association of worsening HF in patients with a recent MI, with LV dysfunction randomized to diltiazem. Among those with a baseline EF of <40%, late HF appeared in significantly more patients receiving diltiazem compared with placebo (21% versus 12%; P=0.004). Life table analysis in patients with an EF of <40% confirmed significantly more frequent late HF in those taking diltiazem. In addition, the diltiazem-associated rise in the frequency of late congestive heart failure (CHF) was progressively greater with increasingly severe decrements in baseline EF.10
The safety and efficacy of dronedarone, originally designed as an antiarrhythmic to maintain sinus rhythm in patients with AF, was addressed in several seminal trials.18–20 PALLAS (Palbociclib Collaborative Adjuvant Study) was designed to investigate whether dronedarone would reduce major vascular events or hospitalizations in patients with permanent AF. The benefits of dronedarone in patients with permanent AF were initially suggested from the ERATO (Efficacy and Safety of Dronedarone for the Control of ventricular rate during atrial fibrillation) trial, which showed a significant reduction in the ventricular response for patients with paroxysmal and permanent AF in the 24-hour setting, which were carried forward in a 6-month follow-up.12 PALLAS randomized approximately 3200 patients with permanent AF at high risk for adverse events. The trial was terminated early, because of the strong signal for harm in 43 patients receiving dronedarone versus 19 receiving placebo, namely death (13/21 due to arrhythmia), stroke, and hospitalization for HF.12
7.3. Atrioventricular Nodal Ablation (AVNA)
Recommendations for AVNA
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In patients with AF and a persistently rapid ventricular response who undergo AVNA, initial pacemaker lower rate programming should be 80 to 90 bpm to reduce the risk of sudden death.1,2 |
| 2a | B-R | 2. In patients with AF and uncontrolled rapid ventricular response refractory to rate-control medications (who are not candidates for or in whom rhythm control has been unsuccessful), AVNA can be useful to improve symptoms and QOL.3–6 |
| 1 | B-NR | 3. In patients with AF who are planned to undergo AVNA, implantation of a pacemaker before the ablation (ie, before or same day of ablation) is recommended to ensure adequacy of the pacing leads before performing ablation.7–9 |
| 2b | C-LD | 4. In patients with AF with normal EF undergoing AVNA, conduction system pacing of the His bundle10–13 or left bundle area12,13 may be reasonable. |
Synopsis
AVNA provides ventricular rate control effectively and without medications yet creates dependence on pacing.3 Consideration of the consequences of lifelong pacemaker implantation, particularly with respect to age and comorbidities, is central to decision-making regarding benefit.
Small RCTs of AVNA and right ventricular pacing (RVP) have shown improvements in symptoms and QOL compared with medical rate control alone but found no significant differences in EF or other measures of cardiac performance.3,14 Progression to persistent AF is described in many patients with previously paroxysmal AF after AVNA.15,16 Antiarrhythmic drugs reduce the transition to permanent AF but do not improve QOL or echocardiographic parameters, and patients treated with antiarrhythmic drugs have more episodes of HF and hospitalizations.17
Early concerns regarding AVNA included risks for device-related complications, sudden cardiac death, and worsened HF. Deaths due to malignant arrhythmias have been nearly eradicated by programming higher lower-rate pacing in the early postprocedure period.1,2,18 The risks of HF may be ameliorated by non-RVP strategies, but data are limited in patients who do not have HF before AVNA.19
Overall, long-term data on outcomes after AVNA are limited, and no evidence supports AVNA as first-line therapy.9
Recommendation-Specific Supportive Text
Early observational studies showed a concerning incidence of sudden death after AVNA in 3% to 7% of patients.20,21 This immediate postprocedural risk results from ventricular fibrillation (VF) predominantly due to bradycardia, QT prolongation, and heterogeneity of repolarization. Initiation of VF is often pause dependent or occurs during a relatively slow pacing rate.18 Deaths due to malignant arrhythmias have been minimized by current protocols specifying higher lower-rate pacing in patients after AVNA in the early postprocedure period.1,2,18 Subsequent lower rate adjustment is performed over the course of several weeks.
AVNA and pacemaker implantation provide safe and effective rate control in the fraction of patients with AF who cannot be effectively rate controlled with medical therapy3,4,22 and in whom sinus rhythm has failed or is not deemed to be effective. A meta-analysis of 6 small RCTs of studies comparing AVNA and pacemaker versus medical therapy or medical therapy with pacemaker including 323 subjects with paroxysmal or persistent AF showed improvement in symptoms and QOL in the AVNA group.6 No differences in survival, stroke, hospitalization, EF, or exercise capacity were found. There was no effect of AVNA on all-cause mortality in a pooled analysis.6 Nonrandomized studies have shown improvement in a range of clinical outcomes5 in addition to data showing improved EF in patients with suspected tachycardia-related cardiomyopathy after AVNA.23 These favorable findings in nonrandomized studies are likely subject to the placebo effect of device implantation. Early and late complication rates are not inconsequential, specifically in young patients in view of the risk of pacemaker-mediated cardiomyopathy, and long-term follow-up data are scant.9
Although few studies concerning AVNA and pacing report complication rates for the pacemaker implant, the overall risk of lead dislodgement or failure is approximately 2%, and many operators perform both AVNA and pacemaker implant during the same procedure.9,13,24 Practice patterns vary, however, and a European survey indicated up to 80% of operators will choose to perform AVNA 1 to 3 months after pacemaker implantation to reduce risk of adverse outcome due to early lead dislodgement.25 In the prospective FOLLOWPACE registry from 23 centers in the Netherlands, lead dislodgment was the most frequent lead-related complication, occurring in 3.3% of patients within the first 2 months of implant.7 Relevant to the older age population usually undergoing AVNA, a pooled patient-level analysis of 4814 subjects from CTOPP (Canadian Trial of Physiologic Pacing), UKPACE (United Kingdom pacing and cardiovascular events), and Danish pacing trials—designed to compare pacemaker implantation complication rates in age <75 years versus age ≥75 years—found a higher risk of early complications in those age ≥75 years (5% versus 3%), driven by a higher rate of lead dislodgement (2%) and pneumothorax (1.6%).8 If AVNA and pacing are performed concomitantly, ideally, leads should be placed first to ensure adequate lead function.
HF after AVNA is attributed to the deleterious effects of RVP,26 and risk is correlated with baseline cardiac function.9 The PAVE (Post AV Nodal Ablation Evaluation) trial compared outcomes in patient with persistent AF and uncontrolled rates undergoing AVNA and RVP with AVNA and biventricular pacing (BiVP) and found an improvement in 6-minute walk test and EF, but benefit was predominantly seen in subjects with EF <45% or New York Heart Association (NYHA) class II to III HF at baseline.27 Not all patients appear to be at high risk of HF after AVNA and RVP.28 Because the benefit is less and the risk of complications is higher in BiVP compared with conventional pacing, RVP is advised in patients with preserved EF undergoing AVNA.19
Conduction system pacing of the His bundle or left bundle area offers promise to reduce the risk of RVP-induced cardiomyopathy and improve outcomes. Early studies have justifiably focused on patients with HF undergoing AVNA, and limited data are available in patients with preserved EF and no HF diagnosis.27 His bundle pacing with AVNA has been shown to be feasible and associated with improvements in EF in patients with EF <40%, and NYHA class, albeit with elevated pacing thresholds.10,11 Left bundle area pacing may deliver the advantages of His bundle pacing with reduced risk of elevated pacing thresholds or lead dislodgement.12 Early studies have shown stable EF with conduction system pacing and AVNA in follow-up, and improvement in patients with EF <50%.12,24
8. RHYTHM CONTROL
8.1. Goals of Therapy With Rhythm Control
Recommendations for Goals of Therapy With Rhythm Control
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-R | 1. In patients with reduced LV function and persistent (or high burden) AF, a trial of rhythm control should be recommended to evaluate whether AF is contributing to the reduced LV function.1–6 |
| 2a | B-R | 2. In patients with symptomatic AF, rhythm control can be useful to improve symptoms.7–11 |
| 2a | B-R | 3. In patients with a recent diagnosis of AF (<1 year), rhythm control can be useful to reduce hospitalizations, stroke, and mortality.12–14 |
| 2a | B-R | 4. In patients with AF and HF, rhythm control can be useful for improving symptoms and improving outcomes, such as mortality and hospitalizations for HF and ischemia.15–19 |
| 2a | B-NR | 5. In patients with AF, rhythm-control strategies can be useful to reduce the likelihood of AF progression.20–27 |
| 2b | C-LD | 6. In patients with AF where symptoms associated with AF are uncertain, a trial of rhythm control (eg, cardioversion or pharmacological therapy) may be useful to determine what if any symptoms are attributable to AF.28–32 |
| 2b | B-NR | 7. In patients with AF, rhythm-control strategies may be useful to reduce the likelihood of development of dementia or worsening cardiac structural abnormalities.33–45 |
Synopsis
When caring for a patient with AF, deciding between rhythm and rate control is important but critical to acknowledge that the decision is nuanced, can evolve over time, and that the 2 strategies are necessarily not mutually exclusive depending on the definition used. For example, in EAST-AFNET 4 (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial), which randomized patients with recently identified AF to rhythm- or rate-control strategies, in those patients randomized to the rhythm control arm, initially 87% were treated with antiarrhythmic drugs and 8% with AF ablation; but, at 2 years, 35% were not on antiarrhythmic drugs or had received AF ablation.12 Similarly, although the decision on whether to use rhythm control is often based on symptoms, in a post hoc analysis of the same trial, benefits in the composite outcome of death, stroke, or cardiac hospitalization was observed in all patients regardless of whether symptoms were present or not.30 Data from EAST-AFNET 4 and large registries have consistently demonstrated the importance of monitoring patients for increased AF burden once AF has been identified and that rhythm-control therapies are more likely to be successful when implemented early when AF burden begins to increase.13,14,30,46,47 Finally, for all patients with AF, continued long-term management of modifiable risk factors is essential.48
Recommendation-Specific Supportive Text
AF and reduced LV function are often recognized in the same patient, with the combination being associated with worse prognosis when compared with patients with either HF or AF alone.1,49 Several studies have demonstrated improved LV function in patients with AF after restoration of sinus rhythm.2–6 The incidence of AF or other atrial arrhythmias as an important contributor to reduced LVEF is unknown and likely dependent on arrhythmia burden, ventricular rates, and other factors.4–6 However, in patients who present with both AF and reduced LV function or develop reduced LV function after an initial diagnosis of AF, where no identifiable cause for the reduced LV function is observed, AF should initially be assumed as a very possible cause until proven otherwise. Although AF as a cause for reduced LV function is usually considered in the setting of rapid ventricular rates, improved LV function with rhythm control has been reported in the setting of AF with relatively well controlled heart rates.2,3
Rhythm and rate control can improve symptoms in AF.7,8,50,51 Multiple studies have shown improved QOL with rhythm control.7–9,50,51 Continued symptoms due to AF are common with rate control. In the RACE II study, lenient rate control had similar primary outcomes (a combination of cardiovascular death, hospitalization for HF, and several other endpoints) compared to strict rate control, but at the end of the follow-up period (>2 years), 46% of patients in both groups had continued symptoms associated with AF.10 Although a different population, symptoms due to recurrent AF were identified in 56 of 303 (18%) patients with newly identified AF undergoing rhythm control with either AADs or catheter ablation.11
EAST-AFNET 4 randomized 2733 patients with early onset AF (<1 year) and other risk factors for stroke to rhythm control or rate-control strategies.12 Rhythm control was associated with a 25% reduction in the combined endpoint of mortality rate, stroke, and hospitalizations due to HF or ACS. In 2 observational studies, 1 from claims data and another from a large national database, when compared with rate control, early initiation of rhythm control (<1 year) in patients with AF was associated with 15% and 19% reductions in the combined endpoint of cardiovascular death, ischemic stroke, or hospitalization for ischemia or HF, respectively.13,14 However, these recent findings must be balanced against studies that have reported increased emergency department visits and health care resource utilization associated with rhythm control.52–54
In the AF-CHF (Atrial Fibrillation and Congestive Heart Failure) trial, rhythm control did not affect cardiovascular mortality but was associated with improved QOL.15,55 Contemporary trials in patients with AF and HF, regardless of LV function, and often using catheter ablation as an important therapy, have reported trends or statistically significant improvement in clinical outcomes associated with rhythm control when compared to rate control.16,17,49,56–58 In an analysis of patients with HF in EAST-AFNET 4, rhythm control was associated with a significant reduction in the composite primary endpoint of cardiovascular death, stroke, or hospitalization due to HF or acute ischemic syndrome (HR, 0.74 [95% CI, 0.56–0.97]; P=0.03).16 Similarly, in the CABANA (Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation) trial, catheter ablation-based rhythm control was associated with a 46% reduction in the mortality rate in patients with HF compared with medical therapy.19 Although catheter ablation is more effective in maintaining sinus rhythm than antiarrhythmic drugs, maintenance of sinus rhythm was higher in the AF-CHF trial. In this study, amiodarone was the preferential strategy for rhythm control (82% of patients) compared with the ablation-based CASTLE-AF (Catheter Ablation versus Standard Conventional Therapy in Patients with Left Ventricular Dysfunction and Atrial Fibrillation) trial (sinus rhythm at study completion: AF-CHF: 75% versus CASTLE-AF: 66%), and additionally the benefit of rhythm control in patients with HF is likely greatest in those patients with recently identified AF.15–18,49
AF commonly, but not always, progresses overtime to higher burdens and becomes more sustained.20,59 Large registry studies have reported that AF progression is more commonly observed with a rate-control strategy compared to a rhythm-control strategy over a 1- to 2-year period, although the absolute magnitude and difference has varied. For example, in 2 registry studies, progression to a more sustained form of AF burden was observed in 26% to 28% of patients undergoing a rate-control strategy compared with 6% to 11% of patients treated with a rhythm-control strategy while, in an analysis of the ORBIT AF trial, although rhythm control was associated with a lower likelihood of progression, AF progression was much more common regardless of strategy (66% for rate control and 56% for rhythm control) likely due to older patients in this cohort.21–23 AF progression is a complex process, and although the choice between rhythm-control and rate-control strategy has an impact, many other factors such as age, presence or absence of HF or other comorbidities, LA size, heart rate, and modifiable risk factors are also important.21–27
The reported prevalence of asymptomatic AF varies from 10% to 40% depending on study cohort.28 Asymptomatic and symptomatic patients with AF have similar risks for death and stroke.28,29 In a post hoc analysis of EAST-AFNET 4, similar magnitudes of benefit were observed in symptomatic and asymptomatic patients for reducing the combined endpoint of cardiovascular death, stroke, and hospitalization for HF.30 Nevertheless, the evidence base for the benefits of a rhythm-control trial in asymptomatic patients with sinus rhythm is limited. Improved energy with return to sinus rhythm was noted in 1 observational study of 13 asymptomatic patients with persistent AF.31 In another study that included 18 asymptomatic patients, at 1-month follow-up, no patients had improvement in symptoms, although sinus rhythm was maintained in only 35% of patients.32 In 2 small studies that focused on the impact of catheter ablation in asymptomatic patients, maintenance of sinus rhythm was associated with improved QOL indices in all patients, but 24% to 34% of patients developed symptoms mainly due to AT after the ablation procedure.53,54
AF is associated with increased cognitive impairment, progressive increase in AF burden, and structural changes in the heart.33,34 Nonrandomized studies from registries or post hoc analyses of randomized trials have reported that rhythm control is associated with a reduction in the incidence of dementia and development of HF.36–38 Furthermore, AF can also be an etiologic reason for mitral regurgitation or tricuspid regurgitation.39–45 In 1 study of 53 patients referred for AF ablation with moderate mitral regurgitation or worse, maintenance of sinus rhythm at follow-up was associated with significant decreases in LA size, mitral annular dilatation, and mitral regurgitation.39 In another study, 70% of patients undergoing ablation for AF who maintained sinus rhythm at follow-up had improvement in mitral regurgitation.40 Similar results have been found in tricuspid valve function as assessed by echocardiography.42–45
8.2. Electrical and Pharmacological Cardioversion
Cardioversion to restore sinus rhythm is a mode of acute rhythm control. Cardioversion can be achieved electrically or pharmacologically. Electrical cardioversion, given rapidity and efficacy, is the treatment of choice for patients with hemodynamic instability attributable to AF. Acute rhythm control with cardioversion should also be considered for patients with hemodynamically stable AF intolerant of atrioventricular dyssynchrony, loss of atrial kick, or unable to achieve adequate rate control. In patients with hemodynamically stable AF, both electrical cardioversion and pharmacological cardioversion are acceptable, safe, and efficacious methods for acute rhythm control.1–3 Electrical cardioversion is more effective than pharmacological cardioversion alone4,5 but involves the trade-off of anesthesia or sedation.6 Thromboembolic risks and considerations for anticoagulation apply to both pharmacological cardioversion and electrical cardioversion.7,8
8.2.1. Prevention of Thromboembolism in the Setting of Cardioversion
Recommendations for Prevention of Thromboembolism in the Setting of Cardioversion
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-R | 1. In patients with AF duration of ≥48 hours, a 3-week duration of uninterrupted therapeutic anticoagulation or imaging evaluation to exclude intracardiac thrombus is recommended before elective cardioversion.1 |
| 1 | B-NR | 2. In patients with AF undergoing cardioversion, therapeutic anticoagulation should be established before cardioversion and continued for at least 4 weeks afterwards without interruption to prevent thromboembolism.2–7 |
| 1 | C-LD | 3. In patients with AF in whom cardioversion is deferred due to LAA thrombus detected on precardioversion imaging, therapeutic anticoagulation should be instituted for at least 3 to 6 weeks, after which imaging should be repeated before cardioversion.1,8 |
| 2b | B-NR | 4. In patients with AF and previous LAAO who are not on anticoagulation, imaging evaluation to assess the adequacy of LAAO and exclude device-related thrombosis before cardioversion may be reasonable.9–19 |
| 2b | C-LD | 5. In patients with AF and previous LAAO with residual leak, pericardioversion anticoagulation may be considered and continued thereafter.9–11,20 |
| 2b | C-LD | 6. In patients with reported AF duration of <48 hours (not in the setting of cardiac surgery) and who are not on anticoagulation, precardioversion imaging to exclude intracardiac thrombus may be considered ins those who are at elevated thromboembolic risk (CHA2DS2-VASc score ≥2 or equivalent).21,22 |
| 2b | C-LD | 7. In patients with low thromboembolic risks (CHA2DS2-VASc 0–1 or equivalent) and AF duration of <12 hours, the benefit of precardioversion imaging or pericardioversion anticoagulation is uncertain given the low incidence of pericardioversion thromboembolic events in this population.21,22 |
Synopsis
Thromboembolic risks and considerations of anticoagulation apply to both pharmacological cardioversion and electrical cardioversion.23,24 Thromboembolic risks in the setting of acute cardioversion relate to preexisting thrombus, change in atrial mechanical function with restoration of sinus rhythm, atrial stunning postcardioversion, and transient prothrombotic state.2,25 DOACs are alternatives to VKAs as thromboprophylaxis in the setting of cardioversion.26–29 Given additional favorable attributes of rapid time to therapeutic efficacy, reliability of maintenance of therapeutic efficacy, and ease of continuation, DOACs may be preferentially considered over VKAs for patients with AF planned for cardioversion barring contraindications to DOAC therapy. Precardioversion imaging is prudent for patients with AF who have previously undergone LAAO and are not on anticoagulation.9,10,14–16,19 Thromboembolic risks for patients with <48 hours of AF were not homogenous but, rather, varied by a patient’s risk profile for thromboembolic complications (as defined by risk scores in specific studies) and duration of AF.21,22 More nuanced consideration of a patient’s thromboembolic risks in the setting of cardioversion for AF may integrate a patient’s substrate for atrial myopathy and risks for thromboembolism instead of strict dependence on the previously used 48-hour duration threshold.
Recommendation-Specific Supportive Text
Early experience with both pharmacological and then electrical cardioversion of AF led to the observation that thromboembolism was less frequent in patients who were chronically anticoagulated than those who were not.30 This led to an empiric recommendation for 3 weeks of anticoagulation before cardioversion in the prospective, randomized, multicenter ACUTE (Assessment of Cardioversion Using Tranesophageal Echocardiography) study of patients with >2 days’ duration of AF.31 Precardioversion anticoagulation of at least 3 weeks may be accomplished via either therapeutic anticoagulation with VKAs or DOACs.26–28 Cardiac computed tomography, particularly with delayed contrast-enhanced image acquisition protocol, has emerged as an alternate imaging modality to exclude intracardiac thrombus.32–34
Rationales for establishment of therapeutic anticoagulation before cardioversion and continuation for the subsequent 4 weeks after cardioversion for AF are driven by elevated thromboembolic risks,6,7,35 high early recurrence of AF,36 and observation of atrial dysfunction2–5 during this period. Thromboembolic risks are elevated around the time of cardioversion, especially within the 30 days after cardioversion.6,7 The observation of spontaneous echocardiographic contrast in association with atrial dysfunction on TEE after cardioversion raised mechanistic concerns for atrial stunning in the setting of cardioversion,2,3 with recovery of mechanical atrial systole over the ensuing 1 month.4,5 Rapidity of recovery of mechanical atrial systole may be influenced by clinical variables,37 duration of the antecedent AF episode leading to cardioversion,5 and the extent of comorbid conditions as risks factors for atrial myopathy.38
In patients with AF, detection of intracardiac thrombus should prompt cancellation of planned cardioversion and institution of therapeutic anticoagulation in anticoagulant-naïve patients. Detection of intracardiac thrombus in patients already on anticoagulation should prompt assessment of compliance, dosing appropriateness, drug absorption, and drug interactions. In patients with thrombus detected despite already being anticoagulated, potential reasons should be investigated, such as nonadherence and drug interaction, which may cause subtherapeutic levels. Switching to an alternate anticoagulant can be considered, although the benefit is uncertain. In anticoagulant-naïve patients, 61.2% had resolution of thrombus on follow-up TEE performed 3 to 12 weeks after initiation of VKA in the CLOT-AF (Retrospective Registry Providing Baseline Data on the Outcome of Left Atrial [LA] or LA Appendage [LAA] Thrombus in Patients With Nonvalvular Atrial Fibrillation [AF] or Atrial Flutter After Standard of Care [SoC] Anticoagulant Therapy) study, and 41.5% had resolution of thrombus on follow-up TEE performed 6 weeks after therapy with rivaroxaban in the X-TRA (Exploring the Efficacy of Once Daily Oral Rivaroxaban for Treatment of Thrombus in Left Atrial/Left Atrial Appendage in Subjects With Nonvalvular Atrial Fibrillation or Atrial Flutter) study.8 Repeat imaging (TEE or computed tomography32–34) is generally pursued after at least 3 to 6 weeks of therapeutic anticoagulation to assess for resolution of intracardiac thrombus before reconsidering cardioversion.1,8
In the subset of patients who have undergone surgical or pLAAO procedures, inadequate occlusion may be seen acutely during the procedure or delayed after the procedure.9–14 Inadequate LAAO has been associated with elevated risks for thromboembolism.9–11 Device-related thrombosis, reported in patients with pLAAO devices,15–19 has also been associated with elevated stroke/systemic embolism.15–18 Imaging with TEE or cardiac computed tomography can assess adequacy of LAAO, residual leak, and device-related thrombosis.39–41 In the setting of transesophageal echocardiographic imaging before electrical cardioversion, device-related thrombus was detected in 2.7% (4 of 148 patients) of a small multicenter retrospective analysis of patients with Watchman LAAO device.20 These 4 patients were treated with oral anticoagulation and after 6 to 8 weeks, on confirmation of thrombus resolution by repeat TEE, underwent successful electrical cardioversion.20
Data are limited to guide pericardioversion anticoagulation in patients with LAAO. In the broader (not limited to cardioversion) context, residual leak ≤5 mm by computed tomography after LAAO by surgical ligation has been associated with particularly elevated stroke/system embolism risks (especially in the absence of anticoagulation)9 and similarly for residual leak <5 mm by TEE post-Lariat.10 A large analysis that stratified National Cardiovascular Data Registry LAAO registry Watchman 2.5 device patients by transesophageal echocardiographic observation of residual leak (none, ≤5 mm, >5 mm) at 45 days, found ≤5 mm residual leak to be common (13 258 of 51 333 patients; 25.8%) and associated with modestly higher incidence of thromboembolic events over 1-year follow-up compared with no leak.11 OAC use was more frequent in patients with large (>5 mm) residual leak.11 Data specific to the setting of cardioversion are further limited. The largest to date is a retrospective multicenter study of 148 Watchman LAAO device patients who underwent electrical cardioversion after precardioversion exclusion of intracardiac thrombus or large residual leak (≥5 mm in this study) by TEE.20 No thromboembolic events were observed within 6 weeks of cardioversion, in recipients or nonrecipients of pericardioversion anticoagulation.20 However, detection of thromboembolic events may be limited by study’s sample size. Pericardioversion antithrombotic regimen was at the discretion of the treating physicians,20 and the relationship of anticoagulation with presence/absence of small residual leak was not reported. Considerations for anticoagulation may also vary based on specific surgical or pLAAO procedures and devices.
The safety of cardioversion without further assessment or previous anticoagulation in patients with AF duration of <48 hours has been challenged. In addition to concerns for underestimation of actual duration of episode and burden due to potential for asymptomatic occurrence of AF, emerging data demonstrate that thromboembolic risks in patients with <48 hours of AF were not homogenously low. Time to cardioversion >12 hours has been reported as an independent predictor for thromboembolic complications.21 A single-center observational study of patients undergoing cardioversion for AF of <48 hours duration did not observe thromboembolic events in patients with CHA2DS2-VASc score of 0 or 1 or patients with postoperative AF but noted differential thromboembolic rates in CHA2DS2-VASc score ≥2.42 Larger studies similarly demonstrated that among patients with <48 hours of AF, postcardioversion thromboembolic risks increased with increasing CHA2DS2-VASc score,22,43 especially in those with CHA2DS2-VASc score ≥2 in the absence of anticoagulation.22
Retrospective observational data from the FinCV (Finnish CardioVersion) study noted low incidence of 30-day pericardioversion thromboembolic event rate of 0.4% in patients with AF with CHA2DS2-VASc of 0 to 122 or 0.3% in patients with duration of <12 hours of AF.21 Other retrospective studies have similarly demonstrated low pericardioversion thromboembolic risks in patients with CHA2DS2-VASc score of 0 to 1.42 The combination of CHA2DS2-VASc of 0 to 1 in conjunction with duration of <12 hours of AF may identify a population at particularly low risk for pericardioversion thromboembolism.
8.2.2. Electrical Cardioversion
Recommendations for Electrical Cardioversion
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In patients with hemodynamic instability attributable to AF, immediate electrical cardioversion should be performed to restore sinus rhythm.1 |
| 1 | B-R | 2. In patients with AF who are hemodynamically stable, electrical cardioversion can be performed as initial rhythm-control strategy or after unsuccessful pharmacological cardioversion.2 |
| 1 | C-LD | 3. In patients with AF undergoing electrical cardioversion, energy delivery should be confirmed to be synchronized to the QRS to reduce the risk of inducing VF.3 |
| 2a | B-R | 4. For patients with AF undergoing elective electrical cardioversion, the use of biphasic energy of at least 200 J as initial energy can be beneficial to improve success of initial electrical shock.4,5 |
| 2a | B-NR | 5. In patients with AF undergoing elective cardioversion, with longer duration of AF or unsuccessful initial shock, optimization of electrode vector, use of higher energy, and pretreatment with antiarrhythmic drugs can facilitate success of electrical cardioversion.5–9 |
| 2b | C-LD | 6. In patients with obesity and AF, use of manual pressure augmentation and/or further escalation of electrical energy may be beneficial to improve success of electrical cardioversion.10 |
Synopsis
Electrical cardioversion is a strategy for acute rhythm control. Compared with pharmacological cardioversion alone, electrical cardioversion with synchronized direct current cardioversion is more rapid and efficacious for restoring sinus rhythm from AF.1,11,12 Given rapidity and efficacy, electrical cardioversion is the treatment of choice for patients with hemodynamically unstable AF.
In hemodynamically stable patients with AF planned for acute rhythm control, electrical cardioversion can be performed as an initial strategy or after unsuccessful pharmacological cardioversion.2,6,13,14 Electrical cardioversion is favored over pharmacological cardioversion when the patient is able to tolerate sedation, desires more immediate rhythm conversion, or has failed or not met candidacy for pharmacological cardioversion.
Although electrical cardioversion may achieve acute restoration of sinus rhythm, durability of sinus rhythm may be influenced by ongoing acute conditions, LA size, duration of the history of AF and/or specific episode, and comorbidities.14 In patients with a longer duration of AF, immediate recurrence of AF, a previous unsuccessful electrical cardioversion, or a desire to employ all reasonable efforts to avoid an AF recurrence, pretreatment antiarrhythmic drugs and subsequent continuation to facilitate success of acute cardioversion and promote maintenance of sinus rhythm postcardioversion6,8,9 may be pursued in the context of SDM with the patient. Optimization of energy delivery, electrode vector, and reduction of transthoracic impedance with manual pressure augmentation may also improve success of electrical cardioversion.5–10
Recommendation-Specific Supportive Text
In patients with hemodynamically unstable AF, immediate electrical cardioversion with synchronized direct current cardioversion is the treatment of choice. Direct data on emergency cardioversion of hemodynamically unstable patients is limited because of the emergency circumstances of the clinical context. Data on direct current cardioversion of AF in hemodynamically stable patients demonstrate high success rate of restoring sinus rhythm with direct current cardioversion.1,11,15–18 Electrical cardioversion is rapid. Electrical cardioversion alone is more effective than pharmacological cardioversion alone.1,11,12
Goals of rhythm control are summarized in Section 8.1 (“Goals of Therapy With Rhythm Control”). Real-world data support both electrical cardioversion and pharmacological cardioversion as acceptable, safe, and efficacious methods for acute rhythm control.6,13,14 Electrical cardioversion is more effective than pharmacological cardioversion alone1,11 but involves the trade-off of requiring sedation.19 In patients with hemodynamically stable AF of recent onset, the RAFF2 (Electrical versus pharmacological cardioversion for emergency department patients with acute atrial fibrillation) study demonstrated high success of restoration of sinus rhythm with either an upfront electrical cardioversion strategy or a stepwise strategy with initial pharmacological cardioversion and then direct current cardioversion if the pharmacological intervention did not restore sinus rhythm.2 In patients with unsuccessful pharmacological cardioversion, instead of switching to another antiarrhythmic drug (and with concerns for potentiation of adverse effects such as QT prolongation or bradycardia), electrical cardioversion should be pursued as next step for acute rhythm control. Therefore, in patients with hemodynamically stable AF, electrical cardioversion is favored over pharmacological cardioversion when the patient is able to tolerate sedation, desires more immediate rhythm conversion, or has failed or not met candidacy for pharmacological cardioversion. Monitoring and preparedness during and after electrical cardioversion include monitoring for rare chronotropic, hemodynamic, or thromboembolic complications, and the effects of anesthesia administered during electrical cardioversion.6,13,14,20–22
Unsynchronized or inappropriately synchronized cardioversion can induce VF if the shock energy is delivered during the vulnerable period, the inscription of the T wave.3,23–25 Therefore, electrical cardioversion for AF should be synchronized with the QRS complex. The synchronization feature needs to be turned on or confirmed that it is on; in patients who require multiple shocks, the synchronization feature must be manually activated and/or confirmed before each shock attempt. Visual confirmation of synchronization to QRS is important to detect inappropriate synchronization and ensure appropriate synchronization.
An earlier small, multicenter, prospective randomized study demonstrated higher efficacy of initial electrical cardioversion with a biphasic waveform instead of monophasic waveform.4 A more contemporary single-centered, randomized study of 279 patients who were randomized to synchronized direct current cardioversion with biphasic waveform with either upfront maximum-fixed energy or strategy of low-escalating energy found higher first shock success with maximum-fixed energy (75% compared with 34% for low-escalating energy group).5 In the contemporary RAFF2 study of patients with hemodynamically stable AF of recent onset, the upfront electrical cardioversion group with initial biphasic 200 J shock (and up to 3 consecutive shocks allowed, with higher energy permitted for subsequent shocks) had conversion to sinus rhythm in 176 of 192 (92%) patients in this upfront electrical cardioversion shock-only group.2
A previous study of patients with persistent AF (median AF duration, 5 months) undergoing direct current cardioversion with escalation of energy found the anterior-posterior orientation of the electrode vector more successful at restoring sinus rhythm than an anterior-lateral vector,7 while a subsequent study of patients with recently diagnosed AF (≥3 hours and <7 days) undergoing direct current cardioversion using biphasic upfront 200 J showed similar success rates with electrode vectors in either anterior-posterior or anterior-lateral orientation.2 Taken together, when energy output is optimized as biphasic and maximal output and the AF is of recent onset, either vector orientation may be reasonable, but for patients with longer duration of AF, the anterior-posterior orientation of electrode vector may be favorable.2 Manual pressure augmentation26 or administration of antiarrhythmic drugs as pretreatment (Section 8.2.3, “Pharmacological Cardioversion”)6,8,9 may also facilitate electrical cardioversion.
Increased body weight has been associated with reduced success of electrical cardioversion.27 In a small randomized clinical study, use of paddles, manual pressure augmentation (using electrically silent objects as insulator), and further escalation of electrical energy improved success of electrical cardioversion of obese patients with AF,10 and the benefits of these maneuvers may mechanistically relate to overcoming increased transthoracic impedance. Other alternatives in refractory cases include the use of 2 defibrillators simultaneously, effectively doubling the delivered energy.
8.2.3. Pharmacological Cardioversion
Recommendations for Pharmacological Cardioversion
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | C-LD | 1. For patients with AF, pharmacological cardioversion is reasonable as an alternative to electrical cardioversion for those who are hemodynamically stable or in situations when electrical cardioversion is preferred but cannot be performed.1 |
| 2a | A | 2. For patients with AF, ibutilide2,3 is reasonable for pharmacological cardioversion for patients without depressed LV function (LVEF <40%). |
| 2a | A | 3. For patients with AF, intravenous amiodarone is reasonable for pharmacological cardioversion, although time to conversion is generally longer than with other agents (8–12 hours).4–8 |
| 2a | A | 4. For patients with recurrent AF occurring outside the setting of a hospital, the “pill-in-the-pocket” (PITP) approach with a single oral dose of tlecainide9–11 or propafenone,10–14 with a concomitant atrioventricular nodal blocking agent,15 is reasonable for pharmacological cardioversion if previously tested in a monitored setting.16–18 |
| 2b | B-R | 5. For patients with AF, use of intravenous procainamide may be considered for pharmacological cardioversion when other intravenous agents are contraindicated or not preferred.19 |
Synopsis
Pharmacological cardioversion is indicated for patients with new-onset or persistent AF that is hemodynamically stable, or in rare instances when electrical cardioversion is desired but contraindicated. Ibutilide works rapidly for pharmacological cardioversion of AF but is associated with QT interval prolongation and torsades de pointes, particularly in patients with HFrEF.2,3,20,21 Data support intravenous amiodarone for pharmacological cardioversion,4–8 although intravenous amiodarone requires a longer time for AF conversion than ibutilide. Evidence from randomized studies support the efficacy of flecainide9–11 and propafenone10–14 for pharmacological cardioversion, and these drugs are reasonable for administration via the PITP approach for AF occurring outside of the hospital.16–18 Intravenous procainamide is more effective than placebo for pharmacological conversion of AF19 but is less effective than ibutilide.22,23 Dofetilide,24,25 oral amiodarone,26–28 and oral sotalol27,28 have been shown to be effective for conversion of AF to sinus rhythm but require several days for efficacy and therefore are not practical choices for acute pharmacological cardioversion. Data do not support intravenous sotalol for pharmacological cardioversion of AF.29,30
Recommendation-Specific Supportive Text
No studies compare the efficacy of electrical cardioversion with that of pharmacological cardioversion in patients with AF who are hemodynamically unstable. However, in a retrospective, propensity score-matched analysis of 374 hemodynamically stable patients with AF who presented to an emergency department, the incidence of successful cardioversion was higher in the direct current cardioversion group (78.2%) than in those who underwent pharmacological conversion (59.2%) or those for whom a “wait-and-watch” approach (37.9%) was used (P<0.001).1 Compared with the “wait-and-watch” strategy, the ORs for conversion to sinus rhythm for direct current cardioversion and pharmacological cardioversion were 6.00 (95% CI, 3.38–10.66) and 2.47 (1.45–4.20), respectively (P<0.001). Due to the potential greater efficacy of direct current cardioversion compared with that of pharmacological cardioversion, and due to the need for rapid successful conversion to sinus rhythm, immediate electrical cardioversion is generally the favored option, yet some patients may not be candidates (eg, cannot undergo anesthesia), for whom pharmacological options are available.31
Randomized, double-blind placebo-controlled studies have established the efficacy of ibutilide2,3 for conversion of AF to sinus rhythm, with AF conversion rates of about 30% (compared with 2% in placebo groups).2,3 The efficacy of ibutilide is greater for AFL (conversion rates, 38%-63%) than for AF.2,3 Ibutilide terminates AF rapidly, with most patients converting to sinus rhythm within 30 to 90 minutes (Table 22). Ibutilide is associated with a risk of QT interval prolongation and torsades de pointes. The incidence of torsades de pointes associated with ibutilide is higher in patients with reduced LVEF than in those with normal LVEF,2,3 and the risk seems particularly high in patients with severely depressed LVEF.20 HFrEF is an independent risk factor for ibutilide-associated torsades de pointes.3,21 Therefore, ibutilide is best avoided in patients with LVEF ≤40%. Of note, studies have shown that a magnesium infusion immediately before administration of ibutilide may mitigate the risk of torsades de pointes and excessive QT prolongation.32,33
Multiple randomized studies have found intravenous amiodarone to be effective for conversion of AF to sinus rhythm.4–7 A meta-analysis of randomized, placebo-controlled studies reported relative risks of sinus rhythm associated with intravenous amiodarone at 6 to 8 hours and at 24 hours of 1.23 (P=0.022) and 1.44 (P<0.001), respectively.8 However, pharmacological cardioversion of AF with intravenous amiodarone requires 8 to 12 hours, compared with a much shorter response time with ibutilide (Table 22).
Single oral doses of flecainide9–11 and propafenone10–14 are effective for conversion of AF to sinus rhythm, with 3- to 4-hour conversion rates of 58% to 68% for flecainide (compared with 18%-29% for placebo) and 45% to 57% for propafenone (compared with 17%-29% for placebo) (Table 22). A beta blocker or nondihydropyridine calcium channel blocker is generally administered at least 30 minutes before a dose of flecainide or propafenone to prevent 1:1 atrioventricular conduction during AFL.15 The PITP strategy was studied in 268 patients with stable AF of recent onset who presented to the emergency department.16 Patients received single-dose oral flecainide or propafenone, and those who were successfully treated (n=210) were discharged with a plan for the PITP approach. In the 15±5-month follow-up period, single-dose flecainide or propafenone was successful in 94% of episodes. However, the incidence of adverse effects is not insignificant (6%-17%).16–18 In view of this, and because in most studies, most patients received the first dose in the hospital before taking it as an outpatient, the PITP strategy should only be used for highly selected patients and after it first has been observed to be safe and effective in an inpatient setting.17,18,34
Intravenous procainamide was shown to be more effective than placebo for conversion of AF to sinus rhythm (conversion rates at 1 hour, 69% versus 38%; P=0.012) in a randomized, double-blind study of 114 patients.19 However, intravenous procainamide is less effective than ibutilide for conversion of AF to sinus rhythm (conversion rates, 14% versus 76%; P=0.001).22 The efficacy of procainamide for conversion of AF to sinus rhythm was similarly inferior to that of ibutilide in an analysis of pooled data.23 In addition, intravenous procainamide is associated with a relatively high incidence of clinically significant hypotension (5%-12%)19,23 and can exacerbate HFrEF.
Table 22.
Drugs for Pharmacological Conversion of AF to Sinus Rhythm
| Drug | Route of Administration | Loading Dose | Maintenance Dose | Approximate Time to Conversion to Sinus Rhythm | Primary Route(s) of Elimination | Elimination Half-Life | Major Adverse Effects |
|---|---|---|---|---|---|---|---|
| Amiodarone | IV | 5–7 mg/kg or 300 mg* | 1200–3000 mg via continuous infusion over 24 h | 8–12 h | Liver metabolism Biliary excretion |
9–36 d | Bradycardia Hypotension QT prolongation Phlebitis TdP |
| Flecainide | Oral† | 200 mg if <70 kg, 300 mg if >70 kg, single dose | N/A | 3–8 h | Liver (70%) Kidney (30%)‡ |
12–27 h | Atrial flutter AV block Dizziness Dyspnea Exacerbation of HFrEF Headache Nausea QT prolongation VT Visual disturbances |
| Ibutilide | IV | ≥60 kg: 1 mg over 10 min <60 kg: 0.01 mg/kg over 10 min If arrhythmia does not terminate within 10 min after the end of the first infusion, may administer a second dose, equal to the first dose. |
N/A | 30–90 min | Liver | 2–12 h | Nonsustained VT QT prolongation TdP |
| Procainamide | IV | 1 g over 30 min | 2 mg/min continuous infusion over 1 h | 30–60 min | Liver (16–33%) Kidney (50–65%)‡ |
3–4 h (parent) 7 h (NAPA) |
Agranulocytosis AV block Exacerbation of HFrEF Hypotension Neutropenia QT prolongation Rash Thrombocytopenia TdP |
| Propafenone | Oral | 450 mg if <70 kg, 600 mg if >70 kg, single dose | N/A | 3–8 h | Liver | 9 h | Atrial flutter AV block Dizziness Dyspnea Exacerbation of HFrEF Nausea Taste disturbances VT Visual disturbances |
Some studies have administered intravenous amiodarone for 24 h followed by oral administration.
Flecainide is available in an intravenous dosage form in Europe.
Percentage of a dose excreted unchanged in urine.
AV indicates atrioventricular; AF, atrial fibrillation; HFrEF, heart failure with reduced ejection fraction; IV, intravenous; N/A, not applicable; NAPA, N-acetylprocainamide; TdP, torsades de pointes; and VT, ventricular tachycardia.
8.3. Antiarrhythmic Drugs for Maintenance of Sinus Rhythm
8.3.1. Specific Drug Therapy for Long-Term Maintenance of Sinus Rhythm
Recommendations for Specific Drug Therapy for Long-Term Maintenance of Sinus Rhythm
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | A * | 1. For patients with AF and HFrEF (≤40%), therapy with dofetilide*1 or amiodaronet2 is reasonable for long-term maintenance of sinus rhythm. |
| B-NR † | ||
| 2a | A | 2. For patients with AF and no previous MI, or known or suspected significant structural heart disease, or ventricular scar or fibrosis, use of tlecainide3–5 or propafenone5–12 is reasonable for long-term maintenance of sinus rhythm. |
| 2a | A | 3. For patients with AF without recent decompensated HF or severe LV dysfunction, use of dronedarone5,13–15 is reasonable for long-term maintenance of sinus rhythm. |
| 2a | A | 4. For patients with AF without significant baseline QT interval prolongation or uncorrected hypokalemia or hypomagnesemia, use of dofetilide1,5,16 5–7,10,17,18 is reasonable for long-term maintenance of sinus rhythm, with proper dose selection based on kidney function and close monitoring of the QT interval, serum potassium and magnesium concentrations, and kidney function. |
| 2a | A | 5. For patients with AF and normal LV function, use of low-dose amiodarone (100–200 mg/d) is reasonable for long-term maintenance of sinus rhy thm2,5,17–22 but, in view of its adverse effect profile,5,23,24 should be reserved for patients in whom other rhythm control strategies are ineffective, not preferred, or contraindicated. |
| 2b | A | 6. For patients with AF without significant baseline QT interval prolongation, hypokalemia, hypomagnesemia, or bradycardia, use of sotalol5–7,10,17,18 may be considered for long-term maintenance of sinus rhythm, with proper dose selection based on kidney function and close monitoring of the QT interval, heart rate, serum potassium and magnesium concentrations, and kidney function. |
| 3: Harm | B-R | 7. In patients with previous MI and/or significant structural heart disease, including HFrEF (LVEF ≤40%), flecainide and propafenone25 should not be administered due to the risk of worsening HF, potential proarrhythmia, and increased mortality.26,27 |
| 3: Harm | B-R | 8. For patients with AF, dronedarone should not be administered for maintenance of sinus rhythm to those with NYHA class III and IV HF or patients who have had an episode of decompensated HF in the past 4 weeks, due to the risk of increased early mortality associated with worsening HF.28 |
A LOE applies to data on dofetilide.
B-NR LOE applies to data on amiodarone.
Synopsis
Antiarrhythmic drugs are reasonable for long-term maintenance of sinus rhythm for patients with AF who are not candidates for, or decline, catheter ablation or who prefer antiarrhythmic therapy. Flecainide3–5 and propafenone5–11 are options for maintenance of sinus rhythm in patients with no previous history of MI or significant structural heart disease.26,27 Dronedarone is an option for maintenance of sinus rhythm in patients without recent decompensated HF or severe LV dysfunction.5,13–15 Dofetilide1,5,16 and sota lol5–7,10,17,18 are effective for maintenance of sinus rhythm but are associated with torsades de pointes and require QT interval monitoring. Sotalol is best avoided in patients with HFrEF, because most patients are already taking a beta blocker, and the addition of sotalol is unlikely to be well-tolerated. A Cochrane database meta-analysis of randomized studies5 reported sotalol to be associated with an increase in all-cause mortality (RR, 2.23 [95% CI, 1.03–4.81]). Low-dose amiodarone is more effective than sotalol and Class IC agents for maintenance of sinus rhythm; but, in view of its adverse effects and multiple drug interactions, is best reserved for patients for whom other antiarrhythmic drugsare ineffective, not preferred, or contraindicated. However, amiodarone and dofetilide are options for maintenance of sinus rhythm for patients with HFrEF, as they are effective,1,2 and most other drugs are contraindicated.
Recommendation-Specific Supportive Text
In subanalyses of randomized trials, dofetilide1 and amiodarone2 have been shown to be effective for maintenance of sinus rhythm in patients with AF who have HF. Most antiarrhythmic agents, except amiodarone and dofetilide, are contraindicated in patients with HFrEF due to worsening of HF and/or increased mortality. Sotalol is best avoided in most patients with HFrEF, because most patients with HFrEFare already taking a beta blocker for mortality reduction, and the addition of a second beta blocker (sotalol) is unlikely to be well-tolerated. Therefore, although amiodarone is associated with a wide range of adverse effects and many clinically important drug interactions, amiodarone is often used as a first-line agent for maintenance of sinus rhythm in patients with AF and HFrEF. Dofetilide is also an option for this indication. Patients undergoing initiation or reloading of dofetilide29,30 should be admitted for at least 3 days to a health care facility that can provide calculations of CrCl, continuous electrocardiographic monitoring, and availability of cardiac resuscitation.
Randomized, controlled studies and a comprehensive Cochrane database analysis have established the efficacy of flecainide3–5 and propafenone5–11 for maintenance of sinus rhythm in patients with AF. In the Flec-SL (Short-term versus long-term antiarrhythmic drug treatment after cardioversion of atrial fibrillation) trial,4 flecainide prevented episodes of paroxysmal AF in 31% of patients over 4 months compared with 9% in the placebo group (0.013). Sustained-release propafenone 425, 325, and 225 mg twice daily extended the median time to the occurrence of AF, AFL, or supraventricular tachycardia (SVT) compared with placebo (>300, 291, and 112 days versus 41 days, P<0.001, for all propafenone doses versus placebo).8 Patients with previous MI or significant structural heart disease (scar or fibrosis) should not take flecainide of propafenone,26,27 and individuals taking flecainide or propafenone should be concomitantly taking an atrioventricular nodal blocking agent to reduce the risk of 1:1 AFL.12
Randomized, controlled studies13–15 and a comprehensive Cochrane database analysis5 have established the efficacy of dronedarone for maintenance of sinus rhythm in patients with AF. The combined analysis (n=1237) of EURIDIS (European Trial in Atrial Fibrillation or Flutter Patients Receiving Dronedarone for the Maintenance of Sinus Rhythm) and ADONIS (American-Australian-African Trial with Dronedarone in Atrial Fibrillation or Flutter Patients for the Maintenance of Sinus Rhythm) reported longer median times to AF recurrence in dronedarone groups versus placebo (EURIDIS: 96 versus 41 days; P=0.001; ADONIS 158 versus 59 days; P=0.002).14
Randomized, controlled studies and a comprehensive Cochrane database analysis5 have established the efficacy of dofetilide for maintenance of sinus rhythm in patients with AF. In the SAFIRE-D (Symptomatic Atrial Fibrillation Investigative Research on Dofetilide) study,1,16 the probability of remaining in sinus rhythm at 1 year was higher in patients receiving dofetilide 500 μg twice daily compared with placebo (0.58 versus 0.25; P<0.001). Patients undergoing initiation or reloading of dofetilide29,30 should be admitted for at least 3 days to a health care facility that can provide calculations of CrCl, continuous electrocardiographic monitoring, and cardiac resuscitation.
Multiple randomized, controlled studies have established the efficacy of amiodarone for maintenance of sinus rhythm in AF and have shown amiodarone to be superior to other antiarrhythmic agents. In SAFE-T (Sotalol Amiodarone Atrial Fibrillation Efficacy Trial), amiodarone was superior to sotalol and placebo with respect to median time to recurrence of AF (487 versus 74 versus 6 days, respectively; P<0.001, for amiodarone versus placebo and amiodarone versus sotalol).17 In CTAF (Canadian Trial of Atrial Fibrillation), AF recurrence rates at 16 months were 35% in the amiodarone group and 63% in those randomized to combined propafenone or sotalol (P<0.001).19 However, the adverse effects profile of amiodarone is onerous. Amiodarone is associated with a wide range of adverse effects, including pulmonary fibrosis, hypo- or hyperthyroidism, elevated transaminases, and more rarely hepatotoxicity, photosensitivity, changes in skin pigmentation, peripheral neuropathy, sinus bradycardia, QT interval prolongation and torsades de pointes, corneal microdeposits, and rarely optic neuropathy.24 In addition, amiodarone is associated with many clinically relevant drug interactions.31 Therefore, amiodarone is best reserved for patients who do not respond to other recommended antiarrhythmic agents or for whom other antiarrhythmic drugs are contraindicated.
Randomized, controlled studies and a comprehensive Cochrane database analysis5 have established the efficacy of sotalol6,7,10,17,18 for maintenance of sinus rhythm in patients with AF. In SAFE-T, the median time to AF recurrence was 74 days in the sotalol group, compared with 6 days for placebo (P<0.001).16 A Cochrane database meta-analysis of 5 randomized studies totaling 1882 patients with AF5 reported sotalol to be associated with an increase in all-cause mortality (RR, 2.23 [95% CI, 1.03–4.81]). However, this study included patients with advanced HF, and sotalol may still have a role in patients with preserved heart function. Patients undergoing initiation or reloading of oral sotalol should be admitted for at least 3 days to a health care facility that can provide calculations of CrCl, continuous electrocardiographic monitoring, and cardiac resuscitation. A recent FDA-approved intravenous sotalol formulation can be used to evaluate the drug safety and tolerability within 6 hours and thus may reduce cost and length of stay, although the data are limited to a small sample size and very selected population.32
In the randomized, double-blind, placebo-controlled CAST (Cardiac Arrhythmia Suppression Trial),26,27 the Vaughan Williams class IC antiarrhythmic agents flecainide and encainide were associated with an increased mortality rate in patients with recent MI; most patients also had LVEF <50%.26 In the continuation of the study (CAST-II), the class IC agent moricizine also increased the mortality rate within the first 14 days of treatment.33 In CASH (Cardiac Arrest Study Hamburg),34 propafenone was associated with worse outcomes and increased mortality in a population of cardiac arrest survivors, most of whom had structural heart disease. Thus, IC agents are best avoided in patients with AF who have a previous MI or significant structural heart disease, including HFrEF, due to the risk of worsening HF and increased mortality.
ANDROMEDA (Antiarrhythmic Trial with Dronedarone in Moderate to Severe CHF Evaluating Morbidity Decrease)28 was a randomized, double-blind placebo-controlled study that tested the hypothesis that dronedarone would reduce the rate of hospitalization attributable to HF and possibly reduce mortality by reducing the incidence of arrhythmic death. The study enrolled patients who were hospitalized with new or worsening HF and who had at least 1 episode of NYHA class III or IV HF (shortness of breath on minimal exertion or at rest). More than 40% of the patients had NYHA class II HF at baseline. The investigators reported that, contrary to the study’s hypothesis, the mortality rate was higher in the dronedarone group compared with that in the placebo group (8.1% versus 3.8%; HR, 2.13 [95% CI, 1.07–4.25]; P=0.03). The higher mortality rate was principally associated with worsening HF.
8.3.2. Inpatient Initiation of Antiarrhythmic Agents
Recommendations for Inpatient Initiation of Antiarrhythmic Agents
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | A | 1. Patients with AF who are initiating, increasing the dose of, or reinitiating dofetilide therapy should be admitted for a minimum of 3 days to a facility that can provide continuous electrocardiographic monitoring, calculations of CrCl, and cardiac resuscitation, given the potential for proarrhythmia.1–7 |
| 2a | B-R | 2. In patients with AF, it is reasonable to initiate sotalol therapy in a facility that can provide continuous electrocardiographic monitoring, calculations of CrCl, and cardiac resuscitation, given the potential for proarrhythmia and bradycardia.4,8–10 |
| 2a | B-NR | 3. In patients with AF who are initiating PITP dosing of flecainide and propafenone with concomitant atrioventricular nodal blocking drugs, it is reasonable to receive the first dose in a facility that can provide continuous electrocardiographic monitoring, given the potential for proarrhythmia.9–13 |
Synopsis
Pharmacological rhythm control is an alternative to catheter ablation in appropriately selected patients. However, many antiarrhythmic agents have a paradoxic risk of proarrhythmia and require close monitoring on initiation. Dofetilide and sotalol confer a relatively high risk of torsades de pointes given they prolong the QT interval, and this can occur early during initiation. As such, patients need to be admitted and closely monitored when starting or reinitiating dofetilide, as well as for increasing the dosage. Many practitioners choose to initiate sotalol in an inpatient setting given it can cause torsades de pointes; furthermore, unlike dofetilide, it can also cause bradyarrhythmia. Amiodarone, flecainide, and propafenone can be started in the outpatient setting. Because PITP with class IC (with concomitant atrioventricular nodal blockers) can cause brady- or tachyarrhythmias, the first attempt during an acute episode of AF may be performed in a monitored environment if a high dose is used.
Recommendation-Specific Supportive Text
Dofetilide, a class III antiarrhythmic drug, is a selective IKr blocker that also blocks late Na+ current (INaL) and poses a high risk for torsades de pointes. The SAFIRE-D (Symptomatic Atrial Fibrillation Investigative Research on Dofetilide) study investigated dofetilide in cardioverting and maintaining sinus rhythm in 325 patients with AF or AFL who underwent a minimum 3-day hospitalization.1 Of 241 patients randomized to dofetilide, 10 patients had QTc prolongation within the first 3 days, with 2 episodes of torsades de pointes degenerating to VF. In a substudy of the AFFIRM trial, 1 of 12 patients on dofetilide experienced torsades de pointes.3 One retrospective cohort of 378 patients found no significant differences in the incidence of torsades de pointes during inpatient initiation of dofetilide and sotalol (1.3% versus 1.2%).4 One systematic review found a 1% to 10% incidence of torsades de pointes in patients taking dofetilide, highest in patients with HFrEF and taking higher than recommended (or not renally adjusted) doses.14 In 2 randomized RCTs from the DIAMOND (Management of Hyperkalemia in Subjects Receiving Renin-angiotensin-aldosterone System Inhibitor Medications for Heart Failure with Reduced Ejection Fraction) population, in which all patients were hospitalized for at least 3 days after dofetilide was initiated, torsades de pointes occurred in 25 of 762 patients (3.3%)5 and 7 of 749 patients (0.9%),7 respectively, with most occurring within the first 3 days of dosing. In the DIAMOND AF substudy of these trials,6 torsades de pointes occurred in 4 dofetilide-treated patients with AF (1.6%).
Sotalol is a class III antiarrhythmic with beta-blocking properties. Its action potential prolongation (due to the IKr-blocking properties of the d isomer in concert with INaL blockade) predisposes patients to torsades de pointes. One meta-analysis demonstrated that sotalol, compared with placebo or no treatment, was associated with a significantly higher all-cause mortality rate (RR, 2.23; 1882 patients in 5 RCTs) and proarrhythmia (RR, 3.55; 2989 patients in 12 RCTs).18 The SAFE-T trial randomized 665 patients to amiodarone, sotalol, or placebo, all initiated in the outpatient setting (sotalol at 80 mg twice daily for the first week and 160 mg twice daily subsequently).15 Fifteen deaths occurred in the sotalol group, 8 of which were sudden, with a nonsignificant mortality ratio of 1.8 compared with placebo. In 1 retrospective cohort of 120 patients undergoing sotalol initiation as an inpatient, 2 of 7 had torsades de pointes; 20 patients developed significant bradyarrhythmias necessitating dose reductions, and 3 patients required permanent pacing.8 Intravenous sotalol was introduced in the United States in 2015 and reaches therapeutic levels rapidly and with easier dose titration to QTc levels and clinical response. As such, it may be an option for more expedient inpatient initiation (obviating the need for a 3-day initiation period).16
The PITP strategy—a high dose of a class IC agent (flecainide 200–300 mg or propafenone 450–600 mg, with an atrioventricular nodal blocker)—can be considered for patients who experience infrequent episodes of symptomatic AF and do not prefer chronic antiarrhythmic therapy. Class IC agents are generally administered with concomitantly atrioventricular nodal blocking agents to mitigate risk of rapidly conducting AFL. Because of the risk of adverse effects and proarrhythmia with high-dose IC agents, inpatient initiation of PITP has been studied. In 1 trial evaluating the safety of outpatient PITP therapy after initiation as an inpatient, 58 of 268 (22%) patients experienced transient hypotension, 1:1 and 2:1 AFL, and symptomatic bradycardia.9 In a prospective study of 43 patients who presented to the emergency department for initiation of PITP,10 sinus rhythm was restored in 30 patients; 13 patients remained in AF, and/or had symptomatic hypotension, converted to rapid AFL requiring cardioversion, or experienced a syncopal conversion pause. In a retrospective cohort13 study of 273 patients initiating PITP (62% inpatient), 7 patients experienced significant adverse events, including syncope, symptomatic bradycardia/hypotension, and organization to 1:1 AFL—2 of 7 of these patients initiated PITP as an inpatient, and 2 patients required permanent pacemakers for bradycardia.
8.3.3. Antiarrhythmic Drug Follow-Up
Antiarrhythmic drugs are associated with important adverse effects, and monitoring is recommended to assess their efficacy for maintaining sinus rhythm and for prevention and/or early detection of adverse events.
Amiodarone
Recommendations for follow-up monitoring for patients taking oral amiodarone are provided in Table 24. Amiodarone contains 37% iodine by weight and is therefore associated with thyroid abnormalities in 2% to 24% of patients.1 In 1 study, the median time to onset of amiodarone-induced hypothyroidism was 183 days, with a median onset of hyperthyroidism of 720 days.2 Oral amiodarone may also provoke elevations in hepatic transaminases and, rarely, hepatotoxicity.3
Table 24.
Recommended Monitoring for Patients Taking Oral Amiodarone
| Adverse Effect | Baseline Testing | Initial Follow-Up Testing | Additional Follow-Up Testing |
|---|---|---|---|
| Hypo- or hyperthyroidism | TSH (T4 and T3 if TSH abnormal) | 3–6 mo | Every 6 mo |
| Hepatotoxicity | AST, ALT | 3–6 mo | Every 6 mo |
| QT interval prolongation | ECG | Annually | – |
| Interstitial lung disease | Chest x-ray: Recommended CT chest: Not recommended |
Chest x-ray: Unexplained cough or dyspnea or other signs/symptoms suspicious for interstitial lung disease | CT chest: As indicated to follow-up ongoing symptoms or chest x-ray findings |
| Corneal microdeposits (epithelial keratopathy) | Not recommended | Development of visual abnormalities, which may indicate optic neuropathy | – |
| Dermatologic (blue-gray skin discoloration), photosensitivity | Not recommended | Physical examination annually | Development of skin discoloration, severe sunburn |
| Neurological | Not recommended | Physical examination annually | Development of peripheral neuropathy or other neurological abnormalities |
ALT indicates alanine transaminase; AST, aspartate transaminase; CT, computed tomography; ECG, electrocardiogram; TSH, thyroid-stimulating hormone; and TdP torsades de pointes.
Amiodarone may cause pulmonary toxicity, most commonly in the form of interstitial lung disease or hypersensitivity syndrome,4 in 1% to 2% of patients and is fatal in approximately 10% of cases. Consequently, a chest x-ray is recommended at baseline and when there is a clinical suspicion for pulmonary toxicity.4,5 Pulmonary function testing, including diffusing capacity for carbon monoxide, can reveal relatively early changes in pulmonary function that may be attributable to amiodarone-associated pulmonary toxicity; however, the sensitivity of the test for routine screening of drug-induced lung toxicity in general is uncertain.4–7
In patients who develop unexplained cough and dyspnea while taking amiodarone, a chest CT scan can be helpful for diagnosis.5
Corneal microdeposits (epithelial keratopathy) are common, but visual abnormalities and light sensitivity are rare.8 Therefore, an ophthalmologic examination is reasonable only if visual abnormalities develop. Amiodarone has also been associated with neurological toxicity, particularly peripheral neuropathy.
Although less common than class III antiarrhythmic agents, oral amiodarone may cause torsades de pointes9; 1 analysis estimated the incidence to be 0.7%.10
Other Antiarrhythmic Drugs
Recommendations for follow-up of other antiarrhythmic agents used for management of AF are provided in Table 25.
Table 25.
Recommended Monitoring for Patients Taking Other Antiarrhythmic Drugs
| Drug | Baseline Testing | Follow-Up Testing | Additional Follow-Up Testing |
|---|---|---|---|
| Dofetilide | 12-lead ECG* Continuous electrocardiographic monitoring during 3-d hospitalization for dofetilide initiation Serum potassium and magnesium concentration Serum creatinine for estimation of CrCl |
In 3–6 mo: 1 2-lead ECG* Serum potassium and magnesium concentration Serum creatinine for estimation of CrCl |
Every 3–6 mo (more frequently for patients concomitantly taking other QT interval-prolonging drugs or with changing kidney function: 1 2-lead ECG* Serum potassium and magnesium concentration Serum creatinine for estimation of CrCl |
| Dronedarone | 12-lead ECG* AST† ALT† |
Within first 6 mo: AST† ALT† |
– |
| Ibutilide | 12-lead ECG* Determination of serum potassium and magnesium concentrations and correction of hypokalemia and/or hypomagnesemia is recommended before initiation of the infusion |
Continuous electrocardiographic monitoring for assessment of QTc interval duration is recommended for at least 4 h after infusion or until the QTc has returned to baseline to minimize the risk of ibutilide-associated TdP | – |
| Procainamide | 12-lead ECG* BP |
Electrocardiographic monitoring for assessment of rhythm, QRS width and QTc interval is recommended during the infusion to minimize the risk of procainamide-associated ventricular proarrhythmia, including TdP BP monitoring is recommended during the infusion to detect clinically relevant hypotension | – |
| Sotalol | 12-lead ECG* Continuous electrocardiographic monitoring during 3-d hospitalization for sotalol initiation Serum potassium and magnesium concentration Serum creatinine for estimation of CrCl |
In 3–6 mo: 12-lead ECG* Serum potassium and magnesium concentration Serum creatinine for estimation of CrCl |
Every 3–6 mo (more frequently for patients concomitantly taking other QT interval-prolonging drugs or with changing kidney function: 12-lead ECG* Serum potassium and magnesium concentration Serum creatinine for estimation of CrCl |
Assess rhythm and calculate QTc.
To facilitate early detection of potential dronedarone-associated hepatotoxicity.
ALT indicates alanine transaminase; AST, aspartame transaminase; BP, blood pressure; ECG, electrocardiogram; CrCl, creatinine clearance; and TdP, torsades de pointes.
Dofetilide
Dofetilide is a potent inhibitor of IKr, augments INa-L, and is associated with torsades de pointes.9 The incidence of torsades de pointes associated with dofetilide ranges from 0.9% to 3.3%.11,12 However, the incidence can be substantially higher if the dofetilide dose is not properly adjusted for kidney function. Patients undergoing initiation or reloading of dofetilide are generally admitted for at least 3 days to a health care facility that can provide calculations of CrCl, continuous electrocardiographic monitoring, and cardiac resuscitation. After discharge on a stable dose of these drugs, a 12-lead ECG, and levels for serum magnesium, potassium, and creatinine are recommended every 3 to 6 months for assessment of QTc interval duration, electrolyte balance, and renal function, and more frequently for patients concomitantly taking other QT interval-prolonging drugs or with changing kidney function, to minimize the risk of drug-associated torsades de pointes.
Dronedarone
Rare cases of severe liver injury have been reported in association with dronedarone. These cases have occurred 4.5 to 6 months after initiation of dronedarone therapy, although 1 case of hepatotoxicity was reported as early as 2 days after initiation of dronedarone (and was ultimately fatal a few days later),13 although a severe case of dronedarone-associated toxic hepatitis occurred 9 months after initiation of therapy.14
Ibutilide
Ibutilide may prolong the QT interval and cause torsades de pointes15–22 and therefore continuous electrocardiographic monitoring is recommended during infusion and for 4 hours after completion of ibutilide infusion. Ibutilide is associated with nonsustained ventricular tachycardia (VT), QT interval prolongation, and torsades de pointes. The incidence of nonsustained VT has been reported to be as high as 8.3%.23 Torsades de pointes occurs in approximately 2% to 7% of patients,15–22 but the incidence increases substantially in patients with depressed LVEF.22 Most reported cases of ibutilide-associated nonsustained VT and torsades de pointes have occurred within 30 minutes after the last dose; torsades de pointes has rarely been reported as late as 2.5 hours after an ibutilide infusion.22 QTc intervals generally return to baseline within 2 to 3 hours after a dose.
The risk of drug-induced torsades de pointes is higher in patients with hypokalemia and/or hypomagnesemia. Hypokalemia has been reported to be present in 17% to 70% of patients who have developed drug-induced torsades de pointes.24–27 Similarly, hypomagnesemia has been reported to be a contributing factor to numerous published cases of drug-induced torsades de pointes.27–29 Therefore, maintenance of serum potassium and magnesium concentrations within the normal range is important before administration of ibutilide, and magnesium supplementation may mitigate the risk of torsades de pointes.
Procainamide
Intravenous procainamide is associated with hypotension in 5% to 12% of patients when used for management of AF,30,31 and BP monitoring is recommended. Intravenous procainamide is also associated with widening of the QRS complex and prolongation of the QT interval and may provoke proarrhythmia in the form of monomorphic VT32 and torsades de pointes.33
Sotalol
Sotalol is a potent inhibitor of IKr, augments INa-L, and is associated with torsades de pointes, with an incidence ranging from 0.4% to 2.3%.34,35 Patients undergoing initiation or dose escalation of sotalol are often admitted for at least 3 days to a health care facility that can provide calculations of CrCl, continuous electrocardiographic monitoring, and cardiac resuscitation. A new intravenous form of sotalol was recently FDA approved, which may obviate the need for 3-day admission. After discharge on a stable dose of these drugs, a 12-lead ECG, and levels of serum magnesium, potassium, and creatinine are recommended every 3 to 6 months for assessment of QTc interval duration, electrolyte balance, and renal function, and more frequently for patients concomitantly taking other QT interval-prolonging drugs or with changing kidney function, to minimize the risk of drug-associated torsades de pointes.
8.3.4. Upstream Therapy
Pharmacological treatments targeting upstream pathways have included glucocorticoids, ACE inhibitors, ARBs, aldosterone antagonists, statins, omega-3 polyunsaturated fatty acids, antioxidants, and sodium-glucose cotransporter 2 inhibitors (see Section 5, “Lifestyle and Risk Factor Modification for AF Management”). Targeting inflammation, a randomized study of glucocorticoids reduced recurrent AF after first occurrence of persistent AF, but adverse effects inhibit long-term steroid use.1–9 Statins reduced postoperative AF in small RCTs.10–14 However, an adequately powered placebo-controlled trial of rosuvastatin did not reduce postoperative AF.15 Statins do not prevent AF in other cardiovascular settings.16 RCTs and a meta-analysis provide limited though consistent support for the antioxidant ascorbic acid for postoperative AF.17 Targeting fibrosis, small or secondary studies of RCTs reported lower new AF with ACE inhibitors or ARBs.2–9 However, larger RCTs targeting AF failed to reduce recurrent AF.18–20 RCTs showed that MRAs reduced new-onset atrial arrhythmias in patients with HFrEF along with improvement of other cardiovascular outcomes.21,22 In patients with type 2 diabetes, HF, or CKD, sodium-glucose cotransporter 2 inhibitors appear to prevent new AF.23–25 In contrast, omega-3 fatty acids do not appear to reduce AF and, in 1 large study, was associated with higher occurrence of AF.26–31 With only limited or inconsistent data, no recommendations are made for use of these upstream therapies for prevention of AF.
8.4. AF Catheter Ablation
Recommendations for AF Catheter Ablation
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | A | 1. In patients with symptomatic AF in whom antiarrhythmic drugs have been ineffective, contraindicated, not tolerated or not preferred, and continued rhythm control is desired, catheter ablation is useful to improve symptoms.1–10 |
| 1 | A | 2. In selected patients (generally younger with few comorbidities) with symptomatic paroxysmal AF in whom rhythm control is desired, catheter ablation is useful as first-line therapy to improve symptoms and reduce progression to persistent AF.11–16 |
| 1 | A | 3. In patients with symptomatic or clinically significant AFL, catheter ablation is useful for improving symptoms.17–19 |
| 2a | B-NR | 4. In patients who are undergoing ablation for AF, ablation of additional clinically significant supraventricular arrhythmias can be useful to reduce the likelihood of future arrhythmia.17,18,20–27 |
| 2a | B-R | 5. In patients (other than younger with few comorbidities) with symptomatic paroxysmal or persistent AF who are being managed with a rhythm-control strategy, catheter ablation as first-line therapy can be useful to improve symptoms.11–13,28 |
| Cost Value Statement: Intermediate | B-R | 6. Catheter ablation for symptomatic AF provides intermediate economic value compared with antiarrhythmic drug therapy.29,30 |
| 2b | B-NR | 7 In selected* patients with asymptomatic or minimally symptomatic AF, catheter ablation may be useful for reducing progression of AF and its associated complications.31–39 |
Younger patients with few comorbidities and a moderate to high burden of AF or persistent AF and AFL.
Synopsis
Catheter ablation has become an established therapy for AF because of multiple RCTs and evidence from large registries and continues to evolve as new technologies are developed. Previous professional society documents have provided different recommendations for catheter ablation dependent on whether AF was persistent or paroxysmal.25,40 More recent information has shown that ablation for AF is more effective than antiarrhythmic drugs for both persistent and paroxysmal AF and that earlier implementation of rhythm control strategies is an important factor for improving AF ablation success rat es.4,35,41–44 As with all strategies for rhythm control of AF, impact on a patient’s goals of care and QOL should be the focus. For example, significantly reducing the frequency and duration of AF episodes but not eliminating all future episodes of AF may represent a clinically important improvement. Although RCTs have mainly used younger patients (<70 years of age) who also experience the largest benefits, observational studies have reported improvement in QOL with catheter ablation in older patients.3–6,8,45–47
Recommendation-Specific Supportive Text
In patients who have not responded to an antiarrhythmic drug due to a high burden of recurrent AF or adverse effects from the medication, RCTs have consistently demonstrated lower risk for recurrent symptomatic AF after ablation when compared with using another antiarrhythmic medication.1–10 As an example, in STOP-AF, patients who had failed ≥1 antiarrhythmic drug (approximately 70% and 30% for 1 or 2 failed drugs, respectively) were randomized to either another antiarrhythmic drug or catheter ablation. At 1 year follow-up, catheter ablation was associated with a treatment success rate of 70% compared with 7% in the drug arm.10 Similarly, in the Thermo-cool (NAVISTAR THERMOCOOL Catheter for the Radiofrequency Ablation of Symptomatic Paroxysmal Atrial Fibrillation) trial, patients with paroxysmal AF who had failed 1 antiarrhythmic medication were randomized to catheter ablation or another antiarrhythmic drug. After 9 months, 66% of patients in the catheter ablation group were free from recurrent arrhythmia compared with 16% in the antiarrhythmic drug group.8 Finally, most recently, in the CABANA trial, 80% of patients were on an antiarrhythmic medication and thought to be candidates for AF ablation and were randomized to catheter ablation or continued antiarrhythmic therapy. Catheter ablation was associated with a nearly 50% reduction in recurrent AF (HR, 0.52 [95% CI, 0.45–0.60]; P<0.001).4,28
In selected patients with paroxysmal AF, ablation is a suitable first-line option. Several initial RCTs suggested a decrease in recurrent AF or AF burden with catheter ablation when compared with antiarrhythmic drugs.16 More recent trials have shown a significant reduction in recurrent AF with catheter ablation compared with antiarrhythmic drugs. In a follow-up report of 1 study, after 3-year follow-up, catheter ablation continued to be associated with a significant decrease in recurrent atrial tachyarrhythmias when compared with antiarrhythmic drugs (HR, 0.51 [95% CI, 0.38–0.67]).14 More importantly, episodes of persistent AF developed in only 1.9% of patients randomized to catheter ablation compared with 7.4% of patients in the antiarrhythmic drug arm (HR, 0.25 [95% CI, 0.13–0.70]).14 All of the studies that have evaluated catheter ablation as an initial strategy for rhythm control in patients with paroxysmal AF, although fairly standard exclusion criteria were used, enrolled relatively young patients (average age approximately 60 years) who had relatively few comorbidities (if present, mainly hypertension).16
AFL is most commonly due to the critical isthmus formed by the inferior vena cava and the tricuspid valve. More rarely, in patients who have not undergone previous ablation procedures atypical AFL or focal ATs can be observed. Catheter ablation of typical AFL is effective and relatively low risk.17–19 In an older meta-analysis of AFL ablation studies, AFL ablation was associated with an acute success rate of 90% and a complication rate of 2.6%.18 The occurrence of AF after AFL ablation was 34%, with a recurrence rate of 23% in those patients without a history of AF compared with 53% in patients with a history of AF. By 5 years, AF developed in 60% to 70% of patients regardless of whether the patient had a history of AF before the AFL ablation. Little evidence is available in these studies for the clinical significance of AF after AFL ablation.
AF can be associated with other atrial arrhythmias, particularly AFL.20–22 During ablation for AF, ablation of previously documented or inducible sustained SVT or AFL is useful to reduce the likelihood of recurrent arrhythmias.22–25 Although ablation alone for AF in patients with both AF and AFL will reduce the likelihood of AFL, and ablation targeting an inducible SVT in a patient with AF may reduce future AF, in both cases the likelihood of recurrent arrhythmias is high, although the actual recurrence rate will depend on the specific population.17,18,25–27,48,49 Conversely, prophylactic catheter ablation of the CTI in patients without documented or inducible AFL likely has minimal benefit.50 Alternatively, in 1 study, cryoballoon PVI as first-line treatment for AFL is equally effective compared with standard CTI ablation for preventing recurrence of atrial arrhythmia and better at preventing new-onset AF.51
Several randomized trials have compared catheter ablation to AADs as first-line therapy for AF.11–13 In the MANTRA-AF study, ablation and antiarrhythmic drugs as first-line therapy for paroxysmal AF was evaluated. Although no differences in AF were identified during the first 18 months, at 24-month follow-up, ablation was associated with a lower AF burden (ablation, 9% versus antiarrhythmic drugs, 18%; P=0.004).11 Subsequent studies have demonstrated similar results: In the EARLY-AF (Early Aggressive Invasive Intervention for Atrial Fibrillation) study, at 1-year follow-up, recurrent atrial arrhythmias were identified in 43% of the ablation group and 68% of the antiarrhythmic drug group (P<0.001), and in the STOP AF First (Cryoballoon Catheter Ablation in an Antiarrhythmic Drug Naive Paroxysmal Atrial Fibrillation) study, recurrent atrial arrhythmias were identified in 25% of the ablation group and 55% of the antiarrhythmic drug group (P<0.001).12,13 Procedural complications were observed in 2% to 5% of patients in all 3 studies.11–13 Although AF burden was significantly reduced with ablation compared with antiarrhythmic drug therapy in EARLY-AF, AF burden was low with either strategy (percentage of time in AF: ablation, 0.6% versus antiarrhythmic drugs, 3.9%) and, in all 3 trials, the average age was ≤60 years.11–13 Although these trials enrolled patients with paroxysmal AF, recent studies have consistently found that catheter ablation is more commonly used and also effective in patients with persistent AF, particularly if of relatively recent onset (<1 year).28
Catheter ablation for AF is a costly procedure yet appears to provide value by improving the symptoms of AF and patient QOL. Some of the initially higher costs of the ablation procedure may be offset by reductions in subsequent cardiovascular hospitalizations. Formal economic analyses have been performed alongside 2 randomized controlled clinical trials. Both trials showed higher costs and improved QOL during follow-up, with incremental cost-effectiveness ratios of $58 000 and €51 000 per quality adjusted life-year added, values within the intermediate value range (between $50 000 and $150 000 per quality29,30,52–55 adjusted life-year added) by ACC/AHA criteria. Models of the cost-effectiveness of catheter ablation have reported more favorable results, but their results generally depended on assumptions that ablation reduced mortality, or ischemic stroke, or both, which have not been proven.
In most patients, continued untreated AF will lead to progression and higher AF burden associated with worse clinical outcomes.31–39 In patients with subclinical AF <24 hours, progression to overt AF or episodes >24 hours occurs at a rate of 8.8% per year, and those patients who develop AF are more likely to be hospitalized for HF.56 Several observational studies have reported AF progression may be improved by using a catheter-based ablation rhythm-control strategy.33–35,37,38 As an example, in 1 randomized trial that was stopped prematurely, in 255 patients with paroxysmal AF randomized to catheter ablation or antiarrhythmic medication, freedom from persistent AF or AT was significantly higher with catheter ablation (ablation: 2.4%, versus antiarrhythmic medication, 17.5%, 1-sided P=0.0009).57 However, serious adverse events associated with the ablation were observed in 8% of patients.57 Despite the potential risks associated with catheter ablation, in a recent analysis of a large registry that specifically evaluated asymptomatic patients, rhythm control was associated with an improvement in the composite outcome of cardiac death, ischemic stroke, and hospitalization for HF.58 The impact was largest in those patients who underwent catheter ablation for rhythm control, particularly in those with paroxysmal AF, LA diameters ≤50 mm,58 or higher stroke risk (CHA2DS2-VASc score ≥3). In a post hoc analysis of EAST-AFNET 4, the magnitude of benefit from a rhythm-control strategy for reducing the primary composite outcome of cardiovascular death, stroke, or hospitalizations for HF or ACS was independent of symptom status, and catheter ablation for rhythm control was used in approximately 20% of patients, whether symptomatic or not.59
8.4.1. Patient Selection
Patient selection is the first step and a critically important step in deciding candidacy for catheter ablation.1–4 Younger patients are likely to derive greater long-term benefit, including delaying AF progression. However, clinical trials have demonstrated improved cardiovascular outcomes with rhythm control, even with median ages in the 70s.5 Patients with minimal atrial enlargement have the best outcomes,6 whereas increased myocardial fibrosis7,8 and more persistent forms of AF are associated with higher rates of recurrence after ablation. Time from diagnosis to ablation is also associated with improved outcomes after ablation.9,10 Although the likelihood of recurrence of AF is 1 factor that should be considered, it is difficult to predict,11 and certain patients may derive even greater benefits from catheter ablation, such as in patients with HFrEF, who have been shown to have improved functional status, LV function, and cardiovascular outcomes.12–14
In some patients, ablation should be avoided as it is unlikely to be successful due to overwhelming substrate or ongoing physiologic processes that either strongly perpetuate the risk of AF or make maintenance of sinus rhythm unlikely. These situations include but are not limited to advanced infiltrative cardiomyopathies like amyloid, severe mitral stenosis or regurgitation, and cor pulmonale.
8.4.2. Techniques and Technologies for AF Catheter Ablation
Recommendations for Techniques and Technologies for AF Catheter Ablation
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | A | 1. In patients undergoing ablation for AF, PVI is recommended as the primary lesion set for all patients unless a different specific trigger is identified.1–7 |
| 2b | B-R | 2. In patients undergoing ablation for AF, the value of other endpoints beyond PVI such as noninducibility and ablation of additional anatomic ablation targets (eg, posterior wall sites, low voltage areas, complex fractionated electrograms, rotors) is uncertain.8–18 |
Synopsis
Catheter ablation is now established as an effective option for rhythm control in patients with AF and AFL.1–7,16 However, in patients with AF, additional ablation targets beyond isolation of PVI as a routine strategy have not reduced AF recurrence or burden in RCTs.8–18 Although achieving durable pulmonary vein ablation in patients with AF has been associated with less recurrent arrhythmias, other endpoints, such as termination of F and noninducibility, have not been associated with improved outcomes.19–28 Significant complications of AF ablation include stroke and TIAs, pericardial effusion, and vascular complications.16 The most serious complication that occurs after the ablation procedure is development of an esophageal atrial fistula, although other complications, such as PV stenosis can occur.8–18
Recommendation-Specific Supportive Text
Multiple randomized studies have shown that PVI is more effective than medical therapy for reducing AF burden.1–7 A recent meta-analysis of 6 RCTs found that strategies that included PVI were associated with a 50% reduction in the development of recurrent AF when compared with strategies that did not include PVI.7
Studies have evaluated the use of different endpoints immediately after catheter ablation for predicting durable PVI and the likelihood of future arrhythmias with mixed results.19–28 In the largest randomized study to date, techniques such as waiting for 30 minutes, using adenosine triphosphate to facilitate PV reconnection, or the combination of waiting and using adenosine were not superior to no testing for predicting durable pulmonary vein isolation or reduction in future arrhythmias. Although noninducibility remains important in determining whether ablation for paroxysmal SVT has been successful, noninducibility after AF ablation or termination of AF with ablation have had mixed results for predicting the likelihood of future recurrence.19–29
Multiple ablation targets beyond PVI have been evaluated in randomized controlled and observational trials, including the LAA, posterior wall, the ligament of Marshall, or atrial scar.8–18 Results have been mixed, and no strategy has emerged that is broadly applicable in all patients, likely because the individual mechanisms for AF vary from patient to patient.8–18 Targeting large areas of atrial tissue may have potential negative consequences, including a higher likelihood of complications such as atrioesophageal fistula, poor LA mechanics, atypical flutters, and increased risk for stroke.30–35
8.4.3. Management of Recurrent AF After Catheter Ablation
Recommendations for Management of Recurrent AF After Catheter Ablation
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients with recurrent symptomatic AF after catheter ablation, repeat catheter ablation or antiarrhythmic drug therapy is useful to improve symptoms and freedom from AF.1–11 |
| 2a | A | 2. In some patients who have undergone catheter ablation of AF, short-term antiarrhythmic drug therapy after ablation can be useful to reduce early recurrences of atrial arrhythmia and hospitalization.12–16 |
Synopsis
Recurrences of AF are common after a first ablation procedure, occurring in 30% to 40% of patients in contemporary clinical trials.1,2 In general US practice, 11% of patients undergoing de novo ablation have a repeat ablation by 1 year.3 Patients often experience improved symptoms and QOL even when they experience recurrence after catheter ablation.4 Nonetheless, recurrent atrial arrhythmia that leads to symptoms or LV dysfunction requires treatment.
Recommendation-Specific Supportive Text
In general, successful suppression of AF improves with additional or multiple ablation procedures.5–7 The absolute limit to the number of ablations a patient can undergo is unknown. For example, it is possible that a patient with previous PVI and repeat ablations with substrate modification may benefit from ablation of PV to LA reconnections, or ablation of additional triggers. However, repeat ablations carry increased risks of adverse effects. These risks are rare but include PV stenosis and stiff LA syndrome.8,9,17
Many of the randomized clinical trials, which have demonstrated superiority of catheter ablation over antiarrhythmic drug therapy, included repeat ablation procedures as part of their prospective study designs.1,10,11,18 Randomized data also suggest that repeat ablation is superior to antiarrhythmic drug therapy for the treatment of recurrent AF after first ablation.19 Although no large direct head-to-head trials are available of repeat catheter ablation versus no repeat catheter ablation, the aggregate data from randomized trials suggest that repeat ablation improves arrhythmia suppression and symptom control. Limited numbers of randomized trials can guide the optimal approach to repeat catheter ablation (beyond reisolation of the PVs).12,13
Although randomized data suggest that repeat ablation is superior to antiarrhythmic drug therapy for the treatment of recurrent AF after first ablation,19 recurrences of AF after catheter ablation can also be successfully treated with antiarrhythmic drug therapy even when these same medications may have been ineffective before ablation.3,14 Antiarrhythmic drugs have also been associated with decreased recurrent atrial arrhythmias in those who have undergone previous ablation.14 Other studies have shown that use of previously ineffective antiarrhythmic therapy can be effective after ablation.15 Optimal rhythm control often requires a combined approach of catheter ablation and adjunctive antiarrhythmic drug therapy, especially in patients with long-standing persistent AF or patients with advanced atrial myopathies.
Several clinical trials have demonstrated the efficacy and safety of short-term (3–6 months postablation) antiarrhythmic therapy to prevent recurrences of AF, symptoms, cardioversion, and hospitalization after ablation.16,20,21 Moreover, continued use of antiarrhythmic drugs beyond the blanking period (3 months) may reduce the risk of recurrent atrial arrhythmias out to 1 year.15 Meta-analysis of randomized trials evaluating the use of short-term antiarrhythmic drug therapy have determined that it significantly decreases the risk of early, but not late, recurrence.22,23 Observational data from nationwide practice data also suggest that antiarrhythmic therapy after ablation can reduce hospital readmissions by 37% in the short term (90 days) after ablation.24 Although the evidence base for short-term antiarrhythmic drug therapy after ablation is robust, the decision to use short-term antiarrhythmic drug therapy should be based on the patient’s risk and informed by SDM.
8.4.4. Anticoagulation Therapy Before and After Catheter Ablation
Recommendations for Anticoagulation Therapy Before and After Catheter Ablation
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients on warfarin who are undergoing catheter ablation of AF, catheter ablation should be performed on uninterrupted therapeutic anticoagulation with a goal INR of 2.0 to 3.0.1 |
| 1 | A | 2. In patients on a DOAC who are undergoing catheter ablation of AF, catheter ablation should be performed with either continuous or minimally interrupted oral anticoagulation.2–10 |
| 1 | B-NR | 3. In patients who have undergone catheter ablation of AF, oral anticoagulation should be continued for at least 3 months after the procedure with a longer duration determined by underlying risk.11 |
| 1 | B-NR | 4. In patients who have undergone catheter ablation of AF, continuation of longer-term oral anticoagulation should be dictated according to the patients’ stroke risk (eg, CHA2DS2-VASc score ≥2).11–17 |
Synopsis
Catheter ablation has been demonstrated in randomized clinical trials to reduce arrhythmia burden improve QOL and, in select populations, it has been shown to improve cardiovascular outcomes. Complications after catheter ablation are infrequent, yet the risk of thromboembolic events is increased after ablation. The risk of stroke in the first 30 days after ablation is 0.8%.18 Thus, oral anticoagulation before, during, and after catheter ablation procedures is paramount.
Recommendation-Specific Supportive Text
All patients undergoing catheter ablation require intraprocedural intravenous anticoagulation with heparin or direct thrombin inhibitors in those with heparin allergies. Management of oral anticoagulation during these procedures is also critically important. In those patients undergoing catheter ablation of AF who are taking warfarin for their oral anticoagulation, evidence from observational studies and randomized trial data suggest that optimal efficacy and safety is achieved with uninterrupted warfarin. Patients undergoing catheter ablation of AF who have subtherapeutic INRs or interrupted warfarin have more evidence of silent cerebral ischemic events on brain magnetic resonance imaging after the procedure.1 In the COMPARE (Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation [AF] Patients Undergoing Catheter Ablation) trial, interruption of warfarin was highly associated with periprocedural thromboembolic events (OR, 13 [95% CI, 3.1–55.6]; P<0.001).1 Patients treated with uninterrupted warfarin had lower rates of thromboembolic events (5% versus 0.25%; P<0.001) and lower rates of periprocedural bleeding compared with those in whom warfarin was stopped or interrupted.
Although uninterrupted warfarin is superior to interrupted warfarin among persons undergoing catheter ablation of AF, meta-analyses have demonstrated lower risks of major bleeding with uninterrupted direct oral anticoagulation compared with uninterrupted vitamin K antagonism.2 Many randomized trials have been completed that compare uninterrupted DOACs versus uninterrupted VKAs, including trials of apixaban,3,4 dabigatran,5 edoxaban,6 and rivaroxaban.7 In aggregate, these trials have demonstrated the noninferiority of uninterrupted direct-acting oral anticoagulation compared with uninterrupted vitamin K antagonism. Several randomized trials also compare minimally interrupted direct-acting oral anticoagulation versus uninterrupted direct-acting oral anticoagulation8 or uninterrupted vitamin K antagonism.9 These clinical trials and meta-analyses suggest that outcomes with minimally interrupted direct-acting oral anticoagulation are not different compared with continuous anticoagulation.10
The risk of thromboembolic and stroke events increases after ablation due to vascular and cardiac instrumentation, release of tissue factor, and myocardial injury. Most randomized clinical trials of catheter ablation that demonstrate a low risk of periprocedural stroke mandated oral anticoagulation for a minimum of 2 months after ablation to reduce the risk of stroke, regardless of CHA2DS2-VASc score.19
Greater burden or time in AF is associated with higher rates of stroke.12–14 Accordingly, reducing exposure to AF via catheter ablation might be expected to reduce the risk of stroke. Although some observational studies have identified lower stroke risk after catheter ablation,15,16 randomized clinical trials of catheter ablation have not demonstrated reductions in stroke.11,17 Observational data regarding the risk of stroke after catheter ablation of AF are mixed and vary across study design and methods. For example, in an analysis of 4 050 patients undergoing catheter ablation with a CHA2DS2-VASc score ≥2 in Denmark, the risk after ablation was low and comparable in those who did (0.93/100 patient years) and did not (0.97/100 patient years) discontinue anticoagulation >3 months after ablation.20 In contrast, a study of 6 866 patients who underwent catheter ablation of AF in US clinical practice found that the risk of stroke was increased in patients with a CHA2DS2-VASc score ≥2 who discontinued oral anticoagulation after 3 months (HR, 2.48 [95% CI, 1.11–5.52]; P<0.05).21 In this same study, no increased risk was observed that was associated with discontinuation after 3 months in those with a CHA2DS2-VASc score of 0 to 1. Ongoing randomized trials are investigating whether oral anticoagulation can be safely discontinued in patients who have no significant arrhythmia recurrences after catheter ablation. Finally, the OPTION (Comparison of Anticoagulation With Left Atrial Appendage Closure After AF Ablation) trial (NCT03795298) will test whether LAAO is noninferior to continued oral anticoagulation after catheter ablation of AF.22 Given (1) the absence of randomized data that directly address this question, (2) the absence of consistent data demonstrating a lower risk of stroke after ablation, and (3) increased rates of stroke among persons with a CHA2DS2-VASc score ≥2 who discontinue oral anticoagulation after 3 months, persons who have undergone catheter ablation AF should continue guideline-directed oral anticoagulation beyond 3 months based on their stroke risk, such as those with moderate-high risk of stroke.
8.4.5. Complications After AF Catheter Ablation
Complications of AF catheter ablation occur in approximately 5% of patients, with most being vascular (Table 26).1–4 However, several life-threatening complications may occur. Complications are more common with annual operator procedures <25 patients and hospital procedures <50 patients.1 Some studies demonstrate a higher incidence of complications in women,1 but the CABANA RCT did not.5 The most severe complication is an LA to esophageal fistula, which is often fatal but quite rare. Another life-threatening complication is pericardial tamponade, but this can usually be treated with prompt pericardiocentesis. Clinically significant strokes and TIAs are observed in <1% of patients.1–4 Deaths during ablations are typically from tamponade but may occur with strokes.1–4
Table 26.
Complications After AF Catheter Ablation
| Complication | Frequency of Complication1–4 | Timing of Complication | Signs and Symptoms | Diagnosis | Treatment |
|---|---|---|---|---|---|
| LA-esophageal fistula | 0.2% | 1–4 wk | Chest pain, pain with swallowing, fever, stroke symptoms | CT scan of chest | Surgery |
| Cardiac perforation with tamponade | 0.4%–1.5% | During procedure | Hypotension | Echocardiography | Pericardiocentesis |
| CVA/TIA | 0.1%–1.0% | During procedure and up to 1 wk | Neurological findings | MRI or CT scan | Anticoagulate when safe |
| PV stenosis | 0.1%–0.8% | Months | Dyspnea, hemoptysis | MRI or CT scan | Stent |
| Phrenic nerve paralysis | 0.2%–0.4% | During procedure | Dyspnea | Fluoroscopy | Time |
| Vascular access complications | 1%–7% | During procedure and up to 1 mo | Pain, swelling at access site | Ultrasound or CT scan | Observation |
| Vascular access complications requiring surgery | 0.1%–0.3% | During procedure and up to 1 mo | Pain and swelling at access site | Ultrasound or CT scan | Surgery |
| Death | 0.1%–0.4% | During procedure | |||
| Pneumonia | 0.4%–1.0% | Days | Cough, fever | Chest x-ray | Antibiotics |
AF indicates atrial fibrillation; CT, computed tomography; CVA, cerebrovascular accident; LA, left atrial; PV, pulmonary vein; TIA, transient ischemic attack; and MRI, magnetic resonance imaging.
Phrenic and vagal nerve paralysis are seen with cryoablation but are less common in radiofrequency ablation.2 Early reports of PV stenosis were largely due to ablation inside the veins; with antral ablation this is rarely seen. The most common complications include bruising and bleeding at the vascular access site. Less common vascular complications include damage to the arterial or venous system or an atrioventricular fistula requiring surgery.
8.5. Role of Pacemakers and ICDs for the Prevention and Treatment of AF
Recommendations for the Role of Pacemakers and ICDs for the Prevention and Treatment of AF
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | A | 1. In patients with bradycardia requiring cardiac-implanted electronic devices who have normal atrioventricular conduction, device selection and programming strategies to maintain atrioventricular synchrony and minimize ventricular pacing should be used to reduce the incidence and progression of AF.1–6 |
| 2b | B-NR | 2. In selected patients with a pacemaker and symptomatic atrial tachyarrhythmias, antitachycardia atrial pacing and ventricular pacing minimization may be useful for reducing symptoms.7–13 |
| 2b | C-LD | 3. In patients with AF who require significant ventricular pacing, conduction system pacing may be useful to reduce progression of AF.14,15 |
| 3: No benefit | B-R | 4. In patients with AF, specialized atrial pacing algorithms designed to suppress AF are not useful for reducing the incidence or slowing the progression of AF.12,16–18 |
Synopsis
In patients with pacemakers, programming strategies to reduce RV pacing reduce the incidence of AF.1–6 Because AF is observed in patients with symptomatic sinus node dysfunction who require permanent pacemakersfor rate support, pacing algorithms were designed 20 to 30 years ago to suppress atrial ectopy but, unfortunately, no algorithm was able to successfully reduce AF in RCTs.12,16–19 More recently, antitachycardia pacing algorithms have been designed to identify and terminate atrial arrhythmias and have been moderately effective in patients with arrhythmias associated with more organized atrial activation due to reentry.7–13
Recommendation-Specific Supportive Text
Several randomized and nonrandomized studies have shown that in patients who require cardiac-implanted electronic devices for sinus node dysfunction who have intact atrioventricular conduction, devices that maintain atrioventricular synchrony reduce the incidence and progression of AF.1–6 Although maintenance of atrioventricular synchrony is important, post hoc analysis of MOST and a meta-analysis have demonstrated that the amount of ventricular pacing is an important contributor to the development of AF.6,20 Algorithms designed specifically to reduce ventricular pacing reduced the incidence of AF in individual studies, but meta-analyses of RCTs suggests that the benefit may extend only to those patients with very infrequent pacing (<10%).19,21–23 In the only randomized study that solely evaluated patients with atrioventricular block (and >70 years of age) who required permanent pacing, dual-chamber pacing did not reduce the incidence of AF when compared with single-chamber ventricular pacing.24
Several studies have reported that specialized antitachycardia pacing algorithms can terminate atrial tachyarrhythmias but have not reduced AF burden.7–9 However, in a post hoc analysis of a subset of patients who had high success rate of antitachycardia pacing (≥60%), antitachycardia pacing algorithms reduced the overall AF/AT burden from 2.5 hours/day to 0.68 hours/day (P<0.01).10 A more recent study (MINERVA [Minimize Right Ventricular Pacing to Prevent Atrial Fibrillation and Heart Failure]) found a modestly effective pacing algorithm that monitors for periods of regularization of atrial activity to initiate pacing algorithms reduced progression to persistent AF.11,12 This algorithm may be more effective in patients who have undergone previous AF ablation.13
Early nonrandomized evidence suggests that His bundle and left bundle pacing may reduce AF incidence and progression when compared with RV pacing in patients with high burden ventricular pacing.14,15 In 1 study, left bundle branch area pacing reduced the incidence of new AF when compared with RV pacing, but this benefit was only evident in those patients with ventricular pacing >20%.14 However, a meta-analysis that has evaluated all published evidence has only demonstrated a trend toward reduced AF. In 1 study, His bundle pacing reduced the likelihood of development of persistent AF in patients with sinus node dysfunction and a PR interval >180 ms.25 There is indirect evidence for the potential benefit of the ventricular depolarization pattern for reducing AF because an algorithm that automatically adjusts pacing parameters based on intrinsic conduction has been associated with a decrease in longer AF episodes (>48 hours) when compared with conventional programming in a retrospective analysis.26–28
Several studies have evaluated atrial pacing algorithms to reduce AF.12,16–19 Pacing algorithms to minimize ventricular pacing in ATTEST (Atrial Therapy Efficacy and Safety Trial), a combination of algorithms designed to suppress AF and also provide antitachycardia pacing did not suppress atrial arrhythmias in patients with paroxysmal AF (AF frequency 1.3 episodes/month when “on” compared with 1.2 episodes/month when “off”).8 Despite optimistic results in some studies,12 a meta-analysis found no benefit with atrial pacing algorithms for reducing the incidence of AF.19
8.6. Surgical Ablation
Recommendations for Surgical Ablation
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-R | 1. For patients with AF who are undergoing cardiac surgery, concomitant surgical ablation can be beneficial to reduce the risk of recurrent AF.1–3 |
| 2a | B-NR | 2. In patients undergoing surgical ablation, anticoagulation therapy is reasonable for at least 3 months after the procedure to reduce the risk of stroke or systemic embolism.2–4 |
| 2b | B-R | 3. For patients with symptomatic, persistent AF refractory to antiarrhythmic drug therapy a hybrid epicardial and endocardial ablation might be reasonable to reduce the risk of recurrent atrial arrhythmia.5–7 |
Synopsis
Among patients with AF or AFL, concomitant surgical ablation at the time of cardiac surgery has been shown to reduce the risk of recurrent atrial arrhythmia. However, it is associated with an increased risk of renal dysfunction and pacemaker placement. Among patients with symptomatic, persistent AF, a hybrid procedure combining epicardial and endocardial ablation has been shown to reduce the burden of atrial arrhythmia.
Recommendation-Specific Supportive Text
The original atrial maze procedure consisted of a biatrial lesion set derived from a “cut-and-sew” technical approach.8 Similar lesion sets delivered by cryoenergy or radiofrequency were subsequently developed. Surgical ablation concomitant with cardiac surgery currently takes place in approximately 1 in 5 patients with previous AF, most commonly at the time of a mitral valve procedure but also during aortic and tricuspid procedures or CABG.9 In a Cochrane review of 22 clinical trials conducted between 1998 and 2013, there was evidence that surgical ablation is associated with increased freedom from AF, AFL, or AT (RR, 2.04 [95% CI, 1.63–2.55]). However, risk of pacemaker placement was increased (RR, 1.69 [95% CI, 1.12–2.54]).1,2 A subsequent trial of patients undergoing mitral valve surgery with AF similarly found that ablation leads to increased freedom from AF at 1 year (63.2% versus 29.4%; P<0.001) but with increased risk of pacemaker placement (21.5 versus 8.1 per 100 patient-years; P=0.01).3 Observational data suggest surgical ablation is associated with increased survival as well as risk of pacemaker placement and renal dysfunction.10–14
Two components of Virchow’s triad—endothelial injury and stasis of blood flow—can lead to a prothrombogenic milieu in the context of surgical ablation. The former is a consequence of suture lines or ablation lesions, whether they result from radiofrequency or cryoenergy. The latter can occur with postcardioversion atrial stunning and/or atrial manipulation and consequent loss of mechanical function. Accordingly, trial protocols for several key studies of surgical ablation include the use of oral anticoagulation for at least 3 months after intervention.2,3 Nonrandomized data suggest that stroke or systemic embolism in the first 3 months after catheter ablation is uncommon, but the risk increases with discontinuation of oral anticoagulation, especially in high-risk patients.4 Patients who undergo surgical ablation may be at similar risk, and thus oral anticoagulation, whether it be with warfarin or a DOAC15 because appropriate accounting for concomitant indications such as a mechanical heart valve or mitral stenosis, should be initiated regardless of stroke risk when adequate surgical hemostasis has been achieved.
In a randomized trial of 149 patients with symptomatic and refractory AF, compared with catheter ablation, a hybrid procedure consisting of closed-chest, epicardial ablation combined with and followed by endocardial ablation led to increased freedom atrial arrhythmias, including AF, AFL, and AT at 12 months (67.7% versus 50.0%; P=0.036) at the expense of an increased risk of major adverse events such as stroke, bleeding, or pericardial effusion (0.0% versus 7.8%; P=0.0525).16 The ablation set of the hybrid procedure focused on surgical ablation of the LA posterior wall and PVI, while endocardial ablation completed PVI and addressed remaining gaps. Other observational studies of hybrid procedures with limited sizes show comparable rates of AF-free survival and safety.5–7
9. MANAGEMENT OF PATIENTS WITH HF
9.1. General Considerations for AF and HF
AF and HF frequently coexist, and either can predispose to the development of the other. Shared risk factors (eg, age, obesity, sedentary lifestyle, and hypertension) may contribute, as may atrial remodeling and diastolic dysfunction.1–3 The prevalence of AF is higher in patients with heart failure with preserved ejection fraction compared with HFrEF.4 In a recent study of the Framingham cohort, among individuals with new AF, more than one-third (37%) had HF.5 Conversely, among individuals with new HF, more than half (57%) had AF. A meta-analysis, including 53 969 patients from 9 observational and 7 randomized trials, concluded that after adjusting for confounding factors, AF was associated with an increased total mortality, irrespective of LV systolic dysfunction, with an OR of 1.40 (95% CI, 1.32–1.48) in randomized trials and an OR of 1.14 (95% CI, 1.03–1.26) in observational studies.6 The risk of mortality is higher when AF is incident after a diagnosis of HF as compared with prevalent AF, and in patients with HFrEF compared with heart failure with preserved ejection fraction.4,7–9
Special considerations for the management of patients with HF and AF are discussed in the next section on management of AF in HF, as well as in Section 7.2 (“Specific Pharmacological Agents for Rate Control”) and Section 8.2 (“Electrical and Pharmacological Cardioversion”).
9.2. Management of AF in Patients With HF
Recommendations for Management of AF in Patients With HF* Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In patients who present with a new diagnosis of HFrEF and AF, arrhythmia-induced cardiomyopathy should be suspected, and an early and aggressive approach to AF rhythm control is recommended.1,2 |
| 1 | A | 2. In appropriate patients with AF and HFrEF who are on GDMT, and with reasonable expectation of procedural benefit (Figure 24), catheter ablation is beneficial to improve symptoms, QOL, ventricular function, and cardiovascular outcomes.3–13 |
| 2a | B-NR | 3. In appropriate patients with symptomatic AF and HFpEF with reasonable expectation of benefit, catheter ablation can be useful to improve symptoms and improve QOL.14,15 |
| 2a | B-R | 4. In patients with AF and HF, digoxin is reasonable for rate control, in combination with other rate-controlling agents or as monotherapy if other agents are not tolerated.16,17 |
| 2a | B-NR | 5. In patients with AF and HF with rapid ventricular rates in whom beta blockers or calcium channel blockers are contraindicated or ineffective, intravenous amiodarone is reasonable for acute rate control.†18,19 |
| 2a | B-R | 6. In patients with AF, HFrEF (LVEF <50%), and refractory rapid ventricular response who are not candidates for or in whom rhythm control has failed, AVNA and biventricular pacing therapy can be useful to improve symptoms, QOL, and EF.20–23 |
| 2a | B-NR | 7 In patients with AF, HF, and implanted biventricular pacing therapy in whom an effective pacing percentage cannot be achieved with pharmacological therapy, AVNA can be beneficial to improve functional class,24,25 reduce the risk of ICD shock,26 and improve survival.24,25 |
| 2a | B-NR | 8. In patients with AF-induced cardiomyopathy who have recovered LV function, long-term surveillance can be beneficial to detect recurrent AF in view of the high risk of recurrence of arrhythmia-induced cardiomyopathy.27,28 |
| 2b | B-NR | 9. In patients with suspected AF-induced cardiomyopathy or refractory HF symptoms undergoing pharmacological rate-control therapy for AF, a stricter rate-control strategy (target heart rate <80 bpm at rest and <110 bpm during moderate exercise) may be reasonable.29–31 |
| 2b | C-LD | 10. In patients with AF and HFrEF who undergo AVNA, conduction system pacing of the His bundle or left bundle branch area may be reasonable as an alternative to biventricular pacing to improve symptoms, QOL, and LV function.32–35 |
| 3: Harm | B-R | 11. In patients with AF and known LVEF <40%, nondihydropyridine calcium channel-blocking drugs should not be administered because of their potential to exacerbate HF.36 |
| 3: Harm | B-R | 12. For patients with AF, dronedarone should not be administered for maintenance of sinus rhythm to those with NYHA class III and IV HF or patients who have had an episode of decompensated HF in the past 4 weeks, due to the risk of increased early mortality associated with worsening HF.37 |
Please see other recommendations on anticoagulation in AF (Section 8.4.4, “Anticoagulation Therapy Before and After Catheter Ablation”), rate control in HF (Section 7, “Rate Control”), and agents for pharmacological cardioversion (Section 7.2, “Specific Pharmacological Agents for Rate Control”) and maintenance of sinus rhythm (Section 8.3.1, “Specific Drug Therapy for Long-Term Maintenance of Sinus Rhythm”).
Consider the risk of cardioversion and stroke when using amiodarone as a rate-control agent.
Figure 24. Management of Patients With HF and AF.
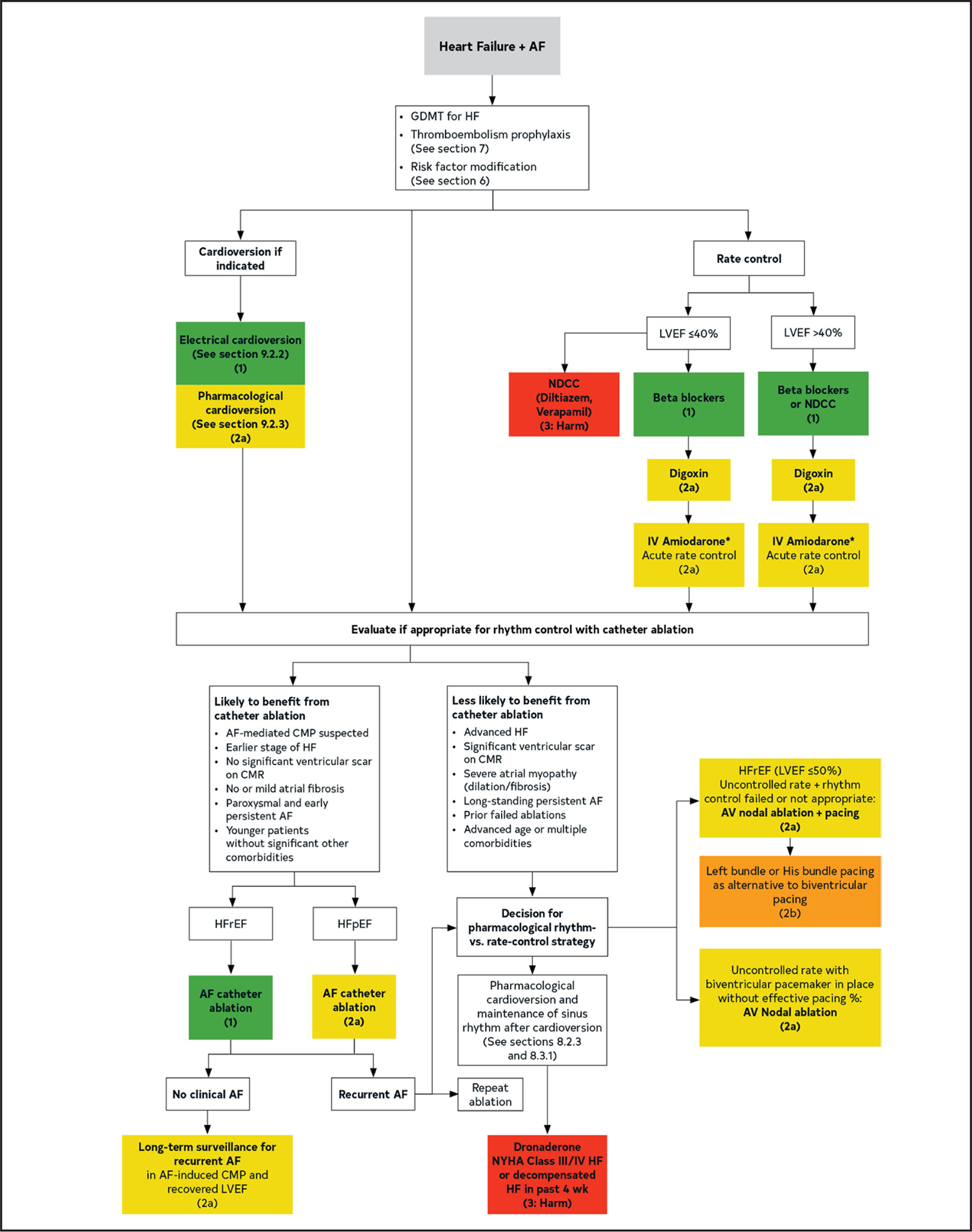
Colors correspond to Table 2. AF indicates atrial fibrillation; AV, atrioventricular; CMP, cardiomyopathy; CMR, cardiac magnetic resonance; GDMT, guideline-directed medical therapy; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IV, intravenous; LVEF, left ventricular ejection fraction; NDCC, nondihydropyridine calcium channel blocker; and NYHA, New York Heart Association.
Synopsis
AF is the most common cause of arrhythmia-induced cardiomyopathy,27,38 a condition where persistent AF, with or without rapid ventricular rates, can lead to LV dysfunction and HF that can be partially or completely reversed with adequate arrhythmia control.39 AF can either be the only reason for cardiomyopathy (AF-induced) or can exacerbate LV dysfunction and HF in a patient with concomitant structural heart disease (AF-mediated).38,39 Medical therapies for rate control (Section 7) or rhythm control (Section 8) of AF have special considerations in patients with HF based on differential safety. Routine pharmacological rhythm compared with rate control has not demonstrated benefit on clinical outcomes in patients with HFrEF,29,40 although a recent subgroup analysis of patients with HF (most heart failure with preserved ejection fraction) demonstrated benefit of early rhythm control compared to rate control on clinical outcomes.1 Several trials have compared AF catheter ablation with medical therapy in patients with HFrEF. Although earlier trials were small and focused on endpoints such as improvement in LVEF, QOL, and 6-minute walk, 2 of the largest trials have shown a benefit in reducing mortality rate and HF hospitalization.3–13 Patients with AF and HF may undergo AVNA for rate control refractory to medical therapy.20,21 Similar to findings in patients who undergo pacemaker implantation for conduction system disease, patients with HF appear to be more likely to develop or have worsened cardiomyopathy related to RV pacing dyssynchrony41,42 and have improved outcomes with biventricular or conduction system pacing.23,32,33,35
Recommendation-Specific Supportive Text
It can be difficult to determine the extent of the contribution of AF to cardiomyopathy and new onset HFrEF. Allowing AF to persist long term, regardless of reasonable rate control, may result in worsening HF and cardiomyopathy. An early and aggressive approach to rhythm control can reduce AF burden, resulting in favorable ventricular remodeling and halting of any occult arrhythmia-induced cardiomyopathy. In a prespecified subanalysis of 798 HF patients (NYHA class II/III or LVEF <50%) in EAST-AFNET (HFpEF, 442; midrange, 211; HFrEF [<40%], 132), early rhythm control significantly improved the composite outcome of death, stroke, or hospitalization for worsening of HF or for ACS (early rhythm control, 94/396; 5.7/100 patient-years) versus usual care (130/402; 7.9/100 patient-years; HR, 0.74 [95% CI, 0.56–0.97]; P=0.03), and this was not altered by HF status. Safety outcomes in each group were comparable. However, only 17% of the HF population had LVEF <40%.1 In a post hoc analysis of the CASTLE-AF trial, an AF burden <50% at 6 months postcatheter ablation was associated with significant improvement in the mortality rate and HF hospitalization in patients with AF and HFrEF.2
Multiple RCTs in HFrEF have shown that in patients with symptoms due to AF, ablation improves symptoms and QOL compared with a rate control or an antiarrhythmic drug strategy.3–13 In each of 3 meta-analyses, QOL was improved.3,10,13 Although in the past a trial of antiarrhythmic drugs was advised before pursuing catheter ablation, current data support ablation for AF as first-line therapy even before antiarrhythmic drugs; often patients require both. Data on LVEF, hospitalizations, and mortality rates are less robust, but nearly every larger RCT does show some benefit in these outcomes (Table 27). Characteristics that may identify patients with a higher likelihood of success or failure with AF catheter ablation are listed in Figure 24.43
Data for AF catheter ablation are far less robust for HFpEF. The largest analysis for this group of patients was a subanalysis of the CABANA trial of patients with HF; in this subgroup analysis, 778 patients (35% of the study population) enrolled in the study who had NYHA class II HF experienced improvement in survival, freedom from AF recurrence, and improved QOL compared with the medical therapy group.14 Most of these had HFpEF. In this subanalysis, catheter ablation was associated with a reduction in the mortality rate and HF. A meta-analysis on catheter ablation in patients with HFpEF that included 7 studies and 764 patients showed the ablation to be safe and effective and associated with lower hospitalizations and the mortality rate.15
A small RCT in patients with persistent AF and HF (most HFrEF) compared the effects of digoxin, carvedilol, and the combination.16 Combination therapy was associated with better rate control and symptom relief than monotherapy. The RATE-AF trial randomized 160 patients (≥60 years of age) with persistent AF and HF (81% with LVEF ≥50%) to digoxin or bisoprolol.17 No significant difference was noted in the primary endpoint of QOL at 6 months. Secondary outcomes, including natriuretic peptide levels, were significantly lower in the digoxin group, without a significant difference in resting heart rate at 12 months. Adverse effects were less common in the digoxin group. Observational and post hoc analyses have suggested an excess mortality with digoxin in patients with AF; however, residual confounding due to the use of digoxin in sicker patients may have a role.44 In patients with HF on digoxin chronically, post hoc analyses have suggested increased mortality rates and hospitalization with serum digoxin levels ≥1 ng/mL; levels <0.8 ng/mL in HF were associated with the lowest mortality rate, although the study was for therapy of HF and not AF.45 In the DAAF (Digitalis in Acute Atrial Fibrillation) trial of 239 patients (12% with HF), intravenous digoxin led to a significant decrease in heart rate at 2 hours compared with placebo.
In trials of critically ill patients, some of which included a smaller proportion of patients with HF, intravenous amiodarone has been shown to be effective in controlling ventricular rates.18,19,46 In a randomized trial of 60 such patients (13 with HF), significant rate control was achieved with both intravenous diltiazem and intravenous amiodarone. However, intravenous diltiazem was associated with a significantly higher frequency of hypotension requiring discontinuation.19 In another retrospective study of 38 patients admitted to the intensive care unit (9 with HF), intravenous amiodarone was associated with a statistically significant decrease in ventricular rate without decrease in BP (and in some cases improved hemodynamics) compared with intravenous diltiazem and digoxin.18 Intravenous beta blockers may also cause hypotension and may not be tolerated in patients with HF and hemodynamic instability. Although less frequent, intravenous amiodarone can also be associated with hypotension, specifically with the intravenous bolus. The possibility of conversion to sinus rhythm with intravenous amiodarone and associated potential for thromboembolism, especially in patients with longer-standing AF not on anticoagulation, should factor into the risk-benefit decision when intravenous amiodarone is chosen for rate control.
Small RCTs of patients with medically refractory AF and HF show that patients who undergo AVNA and conventional pacemaker implantation are less symptomatic and have improvements in QOL and exercise tolerance compared with subjects who are managed with medical rate control.20–22,47 A meta-analysis comparing outcomes with AVNA or medications showed, in 2 studies analyzing patients with reduced EF, a modest, yet significant +4% increase in EF in patients undergoing AVNA and RV pacing, while patients with normal EF at baseline did not show a significant change.48 A meta-analysis of 93 studies including 11 343 patients who underwent AVNA with either RV pacing or biventricular pacing showed periprocedural complications of arrhythmia, HF, and lead dislodgement in 2% to 2.5%,49 few long-term data, and worse outcomes in younger patients with reduced EF and RV pacing.49 Overall, these data showed improved mortality in patients with HF who are treated with cardiac resynchronization therapy (CRT), at the risk of increased device-related complications.49 The APAF-CRT (Ablate and Pace in Atrial Fibrillation plus Cardiac Resynchronization Therapy) mortality trial of 133 subjects with AF, HF, and narrow QRS was stopped early due to a HR of 0.26 for all-cause mortality, also suggesting that when AVNA is performed in patients with HF, CRT is preferable to conventional RV pacing.23 Data are accumulating regarding similarly improved outcomes with conduction system pacing compared with conventional RV pacing in this population.32–35
Conduction of AF may hinder delivery of biventricular pacing therapy (CRT) and worsen outcomes.25 Initial clinical trials of CRT included low numbers of patients with AF,50 and observational analyses suggested a reduced benefit of CRT in patients with AF due to ineffective biventricular pacing.51,52 A systematic review to evaluate the impact of AVNA in patients with AF and CRT found AVNA was associated with significant reductions in all-cause mortality (RR, 0.42 [95% CI, 0.26–0.68]), cardiovascular mortality (RR, 0.44 [95% CI, 0.24–0.81]), and improvement in NYHA class (RR, −0.52 [95% CI, −0.87 to −0.17]).24 Confirmatory evidence of the importance of adequate biventricular pacing percentage is provided by an analysis of remote monitoring data showing a strong association between increasing percentages of biventricular pacing and reduced mortality, with the greatest magnitude reduction at >98% pacing.53 In a systematic review and meta-analysis of 31 studies in 83 571 individuals, CRT alone did not improve the mortality rate in patients with AF, but the all-cause mortality rate was lower in the AVNA group.25 Many patients with CRT devices also have combined defibrillator therapy. In a pooled analysis of 664 patients with AF and CRT in whom 282 underwent AVNA, a significant reduction in appropriate and inappropriate ICD shocks and a decrease in all-cause hospitalizations was associated with AVNA.26
In an observational study of 24 patients with AF-induced cardiomyopathy and NYHA class III/IV HF, the median time from onset of arrhythmia to cardiomyopathy and HF was 4.2 years. Aggressive rate/rhythm control resulted in significant LVEF recovery in all patients within 6 months. Five of 24 patients had recurrent AF, and all had a rapid decline in LVEF within 6 months after arrhythmia recurrence.27 In an observational cohort of 69 patients with AF and LVEF <40% who underwent catheter ablation, patients who maintained sinus rhythm (65%) had complete LV function recovery and maintained normal LVEF over 28±11 months of follow-up, compared with those who had recurrent AF/AT.28 Long-term surveillance to detect recurrent AF can be beneficial and can be accomplished by various modalities, including wearable devices, smart watches, random monitoring (Holter, event, mobile telemetry), and implantable loop recorders.
In patients with arrhythmia-induced cardiomyopathy and HFrEF, the ideal target heart rate for rate control remains uncertain. However, because of the potential negative impact of rapid ventricular rates on HF and cardiomyopathy, a stricter rate-control strategy may be reasonable. In the AF-CHF trial, which compared rate control versus rhythm control using antiarrhythmic therapy in AF and HFrEF, the target rate control was defined as ventricular rate <80 bpm during resting 12-lead electrocardiography and <110 bpm during a 6-minute walk test.29 In the RACE II trial, 614 patients with permanent AF were randomized to a heart rate <80 bpm at rest and <110 bpm during moderate exercise or lenient control (resting heart rate, <110 bpm), with results showing similar outcomes in each arm. However, RACE II trial patients were rate controlled at enrollment (baseline heart rate, 96 bpm), and only 15% of the study cohort had an LVEF <40%, plus very few patients had heart rates >100 bpm, even in the lenient rate control gorup.54 In a crossover study of 20 patients with persistent AF and LVEF <40%, patients were given escalating doses of metoprolol (average dose, 121 mg) to get target heart rate <70 bpm. After 3 months, average resting heart rate decreased from 94±14 bpm to 85±12 bpm, but no significant differences in QOL on functional status were noted.31 Strict rate control is also advisable in patients with ICDs and CRT (See Table 20 in Section 7.1, “Broad Considerations for Rate Control”).55,56
In small observational series of patients undergoing AVNA and His bundle pacing, postprocedural increase in EF and improved functional class has been demonstrated, at the expense of increased pacing thresholds in some patients.32,35 A single-center study comparing outcomes after AVNA with His bundle pacing and left bundle area pacing in 105 patients showed a higher rate of recurrent AVN conduction in the His bundle pacing group and longer fluoroscopy time.33 Acute threshold rise, exit block, and elevated long-term thresholds were also seen in the His bundle pacing group but not in left bundle area pacingin follow-up limited to 10.5 months. Both strategies were associated with a significantly improved EF in patients with EF <50% at baseline (N=16; 37%±7.6% versus 46%±13%; P=0.02).33 An observational comparative study of patients undergoing AVNA, of whom 113 had RV (56) or BiV (57) pacing and 110 had conduction system pacing (84 His bundle pacing and 46 left bundle area pacing) showed increased EF in conduction system pacing compared with the mixed RV/BivP group (46.5%±14.2% to 51.9%±11.2%; P=0.02) in conduction system pacing and 36.4%±16.1% to 39.5%±16% (P=0.04) in RV/BiVP.34 A combined endpoint of time to death or HF hospitalization was reduced in the conduction system pacing group in >2 years of follow-up, but extrapolation is limited due to the observational and selected nature of the population and because the RV/BiVP group had lower EF and wider QRS at baseline.34
The nondihydropyridine calcium channel block-ers—diltiazem and verapamil—are negative inotropic agents and may not be tolerated in patients with HFrEF. In the acute setting, limited data from 2 retrospective analyses suggest the potential for increased morbidity with the use of diltiazem in patients with HFrEF, including worsening HF, need for inotropic support, or acute kidney injury.57,58 In the chronic setting, diltiazem had no impact on mortality in a trial of patients with nonischemic cardiomyopathy.59 However, in the multicenter randomized MDPIT of patients postacute MI, HF and cardiac events (cardiac death or nonfatal reinfarction) were more frequent in the patients with LVEF <40% or pulmonary congestion who were randomized to diltiazem. Among those with a baseline EF of <40%, late HF appeared in 12% receiving placebo and 21% receiving diltiazem (P=0.004). Life table analysis in patients with an EF of <40% confirmed more frequent late HF in those taking diltiazem (P=0.0017). In addition, the diltiazem-associated increase in the frequency of late HF was progressively greater with decreasing baseline EF.36 This increase in late HF was not seen in patients with pulmonary congestion at baseline but an EF of ≥40%, suggesting the association of diltiazem-related late HF was present only in those with systolic LV dysfunction.
The ANDROMEDA37 study was a randomized, double-blind placebo-controlled study that tested the hypothesis that dronedarone would reduce the rate of hospitalization due to HF and possibly reduce mortality by reducing the incidence of arrhythmic death. The study enrolled patients who were hospitalized with new or worsening HF and who had at least 1 episode of NYHA class III or IV HF (shortness of breath on minimal exertion or at rest). More than 40% of the patients had NYHA class II HF at baseline. The investigators reported that, contrary to the study’s hypothesis, mortality was higher in the dronedarone group compared to that in the placebo group (8.1% versus 3.8%; HR, 2.13 [95% CI, 1.07–4.25]; P=0.03). The excess mortality was principally associated with worsening HF.
Table 27.
Randomized Trials of Rhythm Control in HF
| Study/Author (y) | No. Pts. | Inclusion | Exclusion | Intervention | Primary Outcome | Death and Hospitalization | Death | Hospital izations | Reduction in AF | LVEF | QOL | 6MWT | Peak VO2 Max | BNP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Roy (2008)60 | 1376 | LVEF <35%, CHF | AAD (primarily amiodarone) vs rate control | Cardiovascular mortality was no different between rhythm vs rate control | No change | No difference | No difference | |||||||
| MacDonald (2011)8 | 41 | Persistent AF; LVEF <35%, CHF II-IV | PAF; QRS >150 | RF to medical therapy | Similar increase in CMR LVEF | No difference | Improved with RF | Improved | No change | No change | ||||
| ARC-HF: Jones (2013)6 | 52 | Persistent AF; LVEF <35%, CHF | RF to medical therapy | Improvement in peak VO2 with RF | No change | No difference | No difference | No change | Improved with RF | No change | Improved | |||
| CAMTAF (2014)5 | 50 | Persistent AF; LVEF <50%; CHF | RF to medical therapy | LVEF significantly improved with RF | No change | No difference | Improved | Improved with RF | Improved | |||||
| AATAC (2016)4 | 203 | Persistent AF; LVEF <40%, CHF II-III | RF to amiodarone | At 24 mo, RF patients more likely to be in NSR | Improvement with RF | Improved | Improved | Improved | Improved with ablation | Improved | ||||
| CAMERA MRI (2017)12 | 66 | Persistent AF; LVEF <45%, CHF II-III; idiopathic CM | RF to medical therapy | Improved LVEF with RF | Improved | No change | No change | Improved | ||||||
| CASTLE-AF (2018)9 | 363 | PAF or persistent AF; LVEF <36%, CHF II-IV and ICD | RF to medical therapy | Composite of death and hospitalization lower with RF | Improvement with RF | Improved | Improved | Improved | ||||||
| AMICA (2019)7 | 140 | Persistent AF; LVEF <36% | RF to medical therapy | No difference in change in LVEF | No change | No change | No change | No change | ||||||
| CABANA substudy (2021)14 | 778 | Clinical HF (largely HFpEF) | RF to medical therapy | Decrease in composite of MACE | Improved | Improved with RF | Improved with RF | |||||||
| RAFT-AF (2022)11 | 411 | ≥4 PAF/y or persistent AF, NYHA class II or III HF, elevated pro-BNP | RF to medical therapy | No difference in change in mortality/HF | No difference in change in mortality/HF | No change | No change | Improved with RF | Improved with RF | Improved with RF | Improved with RF | Improved with RF | ||
| Meta-analysis-Turagam (2019)13 | 775 | RF to medical therapy | Improved | Reduced | Improved | Improved | Improved | Improved | ||||||
| Meta-analysis-Chen (2020)3 | 1112 | RF to medical therapy | Improved | Reduced | Improved with RF | Improved | Improved | |||||||
| Meta-analysis-Pan (2021)10 | 775 | RF to medical therapy | Improved | Reduced | Improved | Improved | Improved |
AAD indicates antiarrhythmic drug; AATAC, Ablation vs Amiodarone for Treatment of Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted ICD/CRTD; AF, atrial fibrillation; AMICA, Atrial Fibrillation Management in Congestive Heart Failure With Ablation; ARC-HF, A Randomised Trial to Assess Catheter Ablation Versus Rate Control in the Management of Persistent Atrial Fibrillation in Chronic Heart Failure; BNP, brain natriuretic peptide; CABANA, Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation; CAMERA MRI, Catheter Ablation versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction-an MRI-Guided Multi-centre Randomised Controlled Trial; CAMTAF, Catheter Ablation Versus Medical Treatment of AF in Heart Failure; CASTLE-AF, Catheter Ablation versus Standard Conventional Therapy in Patients with Left Ventricular Dysfunction and Atrial Fibrillation; CHF, congestive heart failure; CM, cardiomyopathy; CMR, cardiac magnetic resonance: HF, heart failure; HFpEF, heart failure with persistent ejection fraction; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events; NSR, normal sinus rhythm; NYHA, New York Heart Association; PAF, paroxysmal atrial fibrillation; QOL, quality of life; RAFT-AF, Rhythm Control–Catheter Ablation With or Without Anti-arrhythmic Drug Control of Maintaining Sinus Rhythm Versus Rate Control With Medical Therapy and/or Atrio-ventricular Junction Ablation and Pacemaker Treatment for Atrial Fibrillation; RF, radiofrequency; VO2 max, maximal oxygen consumption; and 6MWT, 6-minute walk test.
10. AF AND SPECIFIC PATIENT GROUPS
10.1. Management of Early Onset AF, Including Genetic Testing
Recommendations for Management of Early Onset AF, Including Genetic Testing
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 2b | B-NR | 1. In patients with an onset of unexplained AF before 30 years of age, electrophysiological study to evaluate and treat reentrant supraventricular tachyarrhythmias with a targeted ablation may be reasonable because of the high prevalence of reentrant arrhythmias in this group.1–3 |
| 2b | B-NR | 2. In patents with an onset of AF before 45 years of age without obvious risk factors for AF, referral for genetic counseling, genetic testing for rare pathogenic variants, and surveillance for cardiomyopathy or arrhythmia syndromes may be reasonable.4,5 |
Synopsis
Young patients are less likely to develop AF or associated strokes compared with older populations. Consumer-driven use of wearable devices is increasing and can lead to the diagnosis of AF at a young age.6 In those <30 years of age, reentrant SVTs (atrioventricular nodal reentrant tachycardia and atrioventricular reentrant tachycardia) are found in about 25% of patients.1–3 Targeted ablation of the reentrant tachycardia is followed by resolution of AF episodes in most of these young patients. However, additional factors need to be taken into consideration in young patients who present with AF. Growing evidence suggests that, in addition to risk factors for AF present in older populations, young patients who develop AF may also harbor susceptibility for inherited ion channel and cardiomyopathic disorders,4 even in those with normal echocardiograms.5 In addition to the standard workup for newly diagnosed AF, genetic testing for rare pathogenic variation, advanced imaging modalities, and surveillance screening could detect otherwise occult cardiomyopathy.
Recommendation-Specific Supportive Text
Two retrospective observational cohort studies included patients with onset of AF before the age of 21 years2 and 30 years1 who underwent electrophysiological study to assess for reentrant SVTs. The former study found atrioventricular node reentry tachycardia or atrioventricular reentry tachycardia in 39% of patients and the latter in 24%. In both studies, after targeted ablation of the reentry tachycardia, patients were observed for recurrence of AF for a median of about 1.5 years. The former study reported no recurrences of AF, and the latter study reported that 86% remained free of atrial arrhythmias. In an additional prospective cohort study of patients referred for AF ablation who had inducible atrioventricular node reentry tachycardia, 12 of 13 patients who underwent slow pathway ablation without PVI had no recurrence of AF after a mean follow-up of 21 months.3
A prospective observational cohort study in patients with AF diagnosed before the age of 66 years and who had sequencing of genes typically found in ion channelopathy and cardiomyopathy panels reported that among 1293 patients studied, 10% (131) were found to have a disease-associated variant, primarily in cardiomyopathy-related genes.4 In 1 study of 23 unrelated patients with onset of AF at <45 years of age who had a normal echocardiogram and no other readily identifiable causes of AF, 24% had a pathogenic or likely pathogenic variant identified. Most of these were also in cardiomyopathy-related genes.5
10.2. Athletes
Recommendation for Athletes
Referenced studies that support the recommendation are summarized in the Online Data Supplement.
Synopsis
Engaging in moderate levels of exercise has several cardiovascular benefits and, in some studies, has been associated with a lower incidence of AF.3,4 However, high-volume endurance athleticism, defined here as exercise of >45 metabolic equivalent-hours per week, has been associated with a higher prevalence of AF,5 particularly in young athletes.6 The mechanisms behind this association are not well established but may be related to atrial myopathy from exercise-induced stretch or perhaps inflamation.7,8 No studies have prospectively studied the effects of detraining on AF burden, but some have proposed that moderation of exercise may be considered, although it is unclear whether changes are reversible.9 Nevertheless, athletes will often choose to continue sport participation, and aggressive rhythm control strategies are often sought. Because many of these patients are young, medications are often not tolerated or preferred. PVI has been shown to be safe and effective in this population without resulting in a significant reduction in exercise capacity1 and has been shown to significantly improve QOL.2
Recommendation-Specific Supportive Text
In a prospective cohort study of 144 athletes, AF ablation with PVI, including multiple procedures, resulted in freedom from AF at 1 year in 86% of athletes with paroxysmal AF and 68% of athletes with persistent AF over a median follow-up of 3 years.1 Athletes underwent cardiopulmonary exercise stress testing before and after ablation and, although the sinus rate increased on average 10 bpm after ablation (which may bother some athletes and thus should be discussed before the procedure), no significant changes were observed in maximum metabolic equivalents on exercise treadmill testing. In a retrospective cohort study of 133 competitive athletes who underwent catheter ablation for AF with 10-year follow-up, 83% of athletes did not have recurrent events and had significantly improved QOL scores.2
10.3. Management Considerations in Patients With AF and Obesity
Obesity is a strong risk factor for AF.1 In the Framingham Study, overweight and obesity increased the risk of incident AF, with a 4% increase in AF risk per unit increase in BMI in men and women. Adjusted HRs for AF associated with obesity were 1.52 (95% CI, 1.09–2.13; P=0.02) and 1.46 (95% CI, 1.03–2.07; P=0.03) for men and women, respectively, compared with individuals with normal BMI. In that study, the excess risk of AF associated with obesity appeared to be mediated through LA dilatation.2 Other studies suggest that the risk of AF with obesity is mediated through epicardial and abdominal fat, or structural changes in the atria.1,3 Obesity is also associated with other comorbidities, including hypertension, HF, and sleep apnea, which themselves predispose to an increased incidence of AF. Further, higher BMI and obesity are associated with an increase in the burden of AF, including progression from paroxysmal to permanent AF, and an increased risk of AF recurrence after AF ablation.4 Special considerations for the management of AF in patients with obesity include the role of weight loss in the primary and secondary prevention of AF (Section 5, “Lifestyle and Risk Factor Modification for AF Management”), as well as anticoagulation in patients with severe or class III obesity in this section and in Section 10.4 (“Anticoagulation Considerations in Patients With Class III Obesity”).
10.4. Anticoagulation Considerations in Patients With Class III Obesity
Recommendations for Anticoagulation Considerations in Patients With Class III Obesity
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-NR | 1. In patients with AF and class III obesity (BMI ≥ 40 kg/m2), DOACs are reasonable to choose over warfarin for stroke risk reduction.1–5 |
| 2b | C-LD | 2. In patients with AF who have undergone bariatric surgery, warfarin may be reasonable to choose over DOACs for stroke risk reduction in view of concerns about DOAC drug absorption.6,7 |
Synopsis
Patients with class III obesity (BMI ≥40 kg/m2) are underrepresented in the major clinical trials evaluating the efficacy and safety of DOACs in patients with AF. Post hoc analyses of major DOAC trials in AF and large observational studies have shown comparable efficacy and safety of DOACs compared with warfarin across weight groups, including patients with class III obesity.1–5 Thus, DOACs are preferred over warfarin for stroke risk prevention in patients with class III obesity and AF. Little evidence is available that evaluates the pharmacokinetics, efficacy, and safety of DOACs in patients with AF who have undergone bariatric surgery. Thus, warfarin may be reasonable to choose over DOACs to reduce stroke risk in this population until further data become available.
Recommendation-Specific Supportive Text
Post hoc analyses of the ARISTOTLE (apixaban versus warfarin), ROCKET AF (rivaroxaban versus warfarin), and ENGAGE AF-TIMI 48 (edoxaban versus warfarin) demonstrated consistent efficacy (stroke and systemic embolism) and safety (major bleeding) of DOACs compared with warfarin across weight groups in patients with AF.3–5 A meta-analysis including these trials and RE-LY (dabigatran versus warfarin) again showed similar efficacy and safety of DOACs compared with warfarin, although the outcome differences were attenuated in obese patients.2 A large retrospective observational study including 36 094 patients compared risk of ischemic stroke, significant bleeding, and mortality in patients receiving a DOAC or warfarin, stratified by BMI group.1 For all 3 endpoints, better outcomes were observed with DOACs across BMI groups. In patients with a BMI ≥40 kg/m2, a relative risk reduction of 25% for ischemic stroke (adjusted HR, 0.75 [95% CI, 0.64–0.87]; P<0.001), 57% for significant bleeding (adjusted HR, 0.43 [95% CI, 0.20–0.94]; P<0.001), and 34% for all-cause mortality (adjusted HR, 0.66 [95% CI, 0.56–0.77]; P<0.001) was observed with DOACs compared with warfarin.
Although case reports exist that demonstrate expected factor Xa trough levels in patients taking a DOAC who undergo bariatric surgery, a meta-analysis of cohort studies, case series, and case reports has shown that 42% of patients who have undergone bariatric surgery had peak drug levels below the expected range.6,7 Patients who have undergone bariatric surgery are not well represented in any of the major trials comparing DOACs with warfarin, and few observational data support DOAC use in this population. Warfarin’s therapeutic effect can be routinely monitored. Thus, in patients with AF who have undergone bariatric surgery, warfarin may be a reasonable choice over DOACs for stroke risk reduction at this time.
10.5. AF and VHD
VHD and AF commonly coexist. AF independently predicts the risk of cerebrovascular events among patients with valve disease.1 This risk may be addressed with the use of oral anticoagulation, percutaneous and surgical LAAO, and surgical ablation as described in Sections 6.5.1 (“Percutaneous Approaches to Occlude the LAA”), 6.5.2 (“Cardiac Surgery—LAA Exclusion/Excision”), and 6.8.5 (“AF in VHD”).
10.6. WPW and Preexcitation Syndromes
Recommendations for WPW and Preexcitation Syndromes
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. Patients with AF with rapid anterograde conduction (preexcited AF), and hemodynamic instability should be treated with electrical cardioversion.1,2 |
| 1 | B-NR | 2. For patients with AF with rapid anterograde conduction (preexcited AF), catheter ablation of accessory pathways (APs) is recommended.3–7 |
| 1 | C-LD | 3. In patients with AF with rapid anterograde conduction (preexcited AF) and hemodynamic stability, pharmacological cardioversion with intravenous ibutilide or intravenous procainamide is recommended as an alternative to elective cardioversion.1,8,9 |
| 3: Harm | B-NR | 4. For patients with AF with anterograde accessory pathway conduction (preexcited AF), pharmacological agents that block atrioventricular nodal conduction (verapamil, diltiazem, amiodarone, digoxin, adenosine, or beta blockers) are contraindicated due to risk of precipitating VF or hemodynamic deterioration.10–14 |
Synopsis
WPW and preexcitation syndromes refer to the presence of extra-atrioventricular nodal connection(s) between atrium and ventricle; such APs are comprised of atrial tissue.15 AF occurs in approximately 15% of persons with WPW and although the mechanism(s) remain unclear, the faster conduction properties of atrial tissue compared with the atrioventricular node allow for rapid rates in preexcited AF.15–17 The risk of preexcited AF is higher for those with multiple pathways and short antegrade pathway refractory period (<250 ms), which allows rapid conduction and predisposes to VF and sudden death.1,18,19 Moreover, AF in young persons without evidence of comorbid conditions may be associated with an AP, and pathway ablation is definitive therapy for AF and SVT in these persons.1 For those who cannot undergo ablation, preventive pharmacological therapy is directed to slowing pathway conduction.20,21
Recommendation-Specific Supportive Text
Preexcited AF can conduct rapidly over the pathway and lead to VF. During an acute episode of preexcited AF with rapid ventricular response and hemodynamic instability, electrical cardioversion must be used to restore sinus rhythm.1,2
Pathway ablation has well-established efficacy and safety for those with preexcited AF for prevention of VF.1 Multiple large-patient series point to a high success rate (93%-95%) balanced with low risk of major short- and long-term complications.3–7 Electrophysiological study and AP ablation can be offered as first-line therapy.
Pharmacological treatments that increase the refractory period of atrial tissue (ie, ibutilide or procainamide) can slow AP conduction and terminate AF. Hemodynamically stable patients presenting with preexcited AF with or without rapid ventricular rates can be pharmacologically managed with such agents.8,9 In 1 study involving 22 patients, ibutilide terminated AF in 21 patients and prolonged refractoriness of the atrioventricular node, His-Purkinje system, and AP.9
AP conduction can accelerate with atrioventricular nodal block. Administration of pharmacological agents that block atrioventricular nodal conduction can increase the risk of VF. The use of atrioventricular nodal blocking agents for patients with preexcited AF are potentially harmful due to the risk of accelerated pathway conduction and associated VF.1,10–12 One small series that reviewed intravenous amiodarone for this indication raised significant concerns in view of several published reports on instances of VF precipitated by amiodarone.13 In 1 study of patients with WPW who received verapamil during electrophysiological study, verapamil decreased the shortest RR interval between preexcited ventricular complexes during AF, and 2 patients deteriorated and required cardioversion.14 Data are very limited and observational, but because other safer options are readily available, atrioventricular nodal agents are best avoided in this setting.
10.7. Hypertrophic Cardiomyopathy
The “AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy” was published in December 2020, and this guideline included recommendations on AF.1 Data on AF in patients with HCM published since the 2020 hypertrophic cardiomyopathy guideline were reviewed but did not result in any substantive changes in the recommendations from 2020.2–6 AF is highly prevalent in HCM and increases the risk of stroke. The CHA2DS2-VASc score should not apply to patients with HCM, because they have roughly an equivalent risk to a CHADs-VASc of 3. Data on the use of DOACs in patients with HCM suggest these to be acceptable alternatives to warfarin. When a rhythm-control strategy is needed, several antiarrhythmic drugs have been shown to be safe and effective, allowing for individualization according to underlying substrate and patient preference. Catheter ablation is also an important option, although more confirmatory studies have shown AF ablation is of lower success compared with patients without HCM.7 For specifics, please see the “2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy.”1
10.8. Adult Congenital Heart Disease (ACHD)
Recommendations for ACHD
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In adults with congenital heart disease and AF, it is recommended to evaluate for and treat precipitating factors and reversible causes of AF, recognizing that residual hemodynamic sequelae may contribute to the occurrence of the arrhythmia.1,2 |
| 1 | C-LD | 2. In adults with AF and moderate or complex congenital heart disease, electrophysiological procedures should be performed by operators with expertise in ACHD procedures and in collaboration with an ACHD cardiologist, ideally in specialized centers, when available.3–5 |
| 1 | C-LD | 3. In adults with congenital heart disease and symptomatic or hemodynamically significant paroxysmal or persistent AF, an initial strategy of rhythm control is recommended regardless of lesion severity as AF in this population is often poorly tolerated.6 |
| 2a | B-NR | 4. In symptomatic patients with simple congenital heart disease with antiarrhythmic drug–refractory AF, it is reasonable to choose ablation over long-term antiarrhythmic therapies.4,7 |
| 2b | C-LD | 5. In adults with congenital heart disease with AF undergoing PVI, it may be reasonable to include an ablative strategy in the right atrium directed at reentrant arrhythmia secondary to atriotomy scars and the CTI.8,9 |
| 2b | C-LD | 6. In adults with AF and moderate or severe forms of congenital heart disease, particularly those with low-flow states such as Fontan circulation, blind-ending cardiac chambers, and cyanosis, it may be reasonable to treat with anticoagulation independent of conventional risk score to reduce risk of thromboembolic events.10 |
Synopsis
Atrial arrhythmias are a leading cause of morbidity in adults with congenital heart disease, and AF surpasses intra-atrial reentrant tachycardia in patients with CHD >50 years of age.11 Risk factors for development of AF in patients with ACHD are similar to those for the general population; however, the presence of an atrial septal defect is an independent risk factor. Adults may be classified as having simple, moderate, or complex forms of CHD, based on a classification proposed in the “2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease.”12 Choosing pharmacological therapy for treatment of patients with ACHD who have AF should consider factors such as coexisting sinus node dysfunction or atrioventricular block, as well as the ventricular function and other comorbidities. Ablation strategies established in normal hearts can be applied to patients with simple forms of CHD; however, caution must be taken in those patients with moderate or complex forms of ACHD.13 Knowledge of the underlying congenital anatomy is key, because access to the atrial chambers may require specialized approaches. In those patients with moderate or complex forms of CHD, electrophysiological procedures should be performed by operators with expertise in CHD and in collaboration with an ACHD cardiologist.3
Recommendation-Specific Supportive Text
Adults with CHD often have hemodynamic sequelae of childhood repair, and any new onset arrhythmia, including AF, should prompt an evaluation for treatable and reversible causes. Studies have shown that AF is correlated with RA dilation, thus necessitating addressing the underlying substrate in addition to treating the arrhythmia.1,2
Patients with ACHD who are undergoing invasive electrophysiological procedures in specialized ACHD centers generally have better outcomes, including survival, than those managed in other care settings.3–5 Special attention is required to ensure appropriate periprocedural care, including identification of procedure-related risk factors and availability of ancillary imaging.12
Adults with simple forms of CHD and AF are generally managed according to standard published guidelines. In those patients with moderate or complex forms of CHD, choice of antiarrhythmic therapy should be individualized.6 Most antiarrhythmic drugs are associated with proarrhythmic effects and increased mortality in ACHD patients.14 Rhythm control is generally preferred over rate control in patients with ACHD with moderate or complex disease who may tolerate AF poorly. Class I antiarrhythmic drugs must be avoided in patients with ACHD with CAD or significant subpulmonary or systemic ventricular dysfunction.14–16 Amiodarone is an effective antiarrhythmic agent for maintaining sinus rhythm in patients with ACHD; however, long-term therapy is limited by adverse effects, particularly amiodarone-induced thyrotoxicosis, which is common in women with CHD, cyanotic CHD, and those with Fontan physiology.17 Amiodarone should also be avoided in patients with ACHD who have a BMI <21 kg/m2 due to the increased incidence of thyrotoxicosis in these patients.18 Dofetilide may be a viable alternative to amiodarone in this complex population.19 Extrapolating data from noncongenital populations, dronedarone is best avoided in patients with ACHD who have moderate or complex disease or significant ventricular dysfunction due to the increased risk of HF, stroke, and death.20,21
Data on ablative strategies for AF in patients with ACHD are limited; however, when performed by experienced centers, AF ablation in patients with simple forms of CHD is safe.4,13 Knowledge of the underlying congenital anatomy is key, as access to the atrial chambers may require different approaches, including transseptal puncture. Patients with ACHD who have persistent AF have lower success rates for ablation, and multiple procedures may be required.22 There are insufficient data on the role, safety, and benefit of AF ablation in complex forms of CHD.
PVI has a lower success rate in patients with ACHD than in patients with structurally normal hearts. The operator should understand the variations in systemic and pulmonary venous anatomy, such as a left superior vena cava, which may be a trigger for AF.9 Due to related pathology in the right atrium of patients with repaired complex ACHD, additional ablative therapies directed at atriotomy scars and the CTI may be necessary. Cryoballoon ablation is a safe option for patients with simple or moderate forms of CHD and AF.23,24
All patients with CHD should be considered for some form of thromboembolic prophylaxis. Select groups of patients with CHD are at higher risks for thromboembolic events, such as cyanotic lesions, those with low-flow states (Fontan physiology), and those with blind-ending cardiac chambers. The prevalence of cerebrovascular accidents in patients with ACHD is estimated to be 10- to 100-fold higher than age-matched controls, and thromboembolic events account for at least 4% of all-cause mortality in patients with CHD.25 Increased complexity of CHD is associated with thromboembolic events, but CHA2DS2 and CHA2DS2-VASc scores are not predictive of thromboembolic risk in patients with ACHD; therefore, therapies must be individualized.10 Limited data are available for the use of DOACs in patients with ACHD, but in a survey, they appeared to be safe and effective.26
10.9. Prevention and Treatment of AF After Cardiac Surgery
10.9.1. Prevention of AF After Cardiac Surgery
Recommendations for Prevention of AF After Cardiac Surgery
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-R | 1. In patients undergoing cardiac surgery who are at high risk for postoperative AF, it is reasonable to administer short-term prophylactic beta blockers or amiodarone to reduce the incidence of postoperative AF.1–5 |
| 2a | B-R | 2. In patients undergoing CABG, aortic valve, or ascending aortic aneurysm operations, it is reasonable to perform concomitant posterior left pericardiotomy to reduce the incidence of postoperative AF.6,7 |
Synopsis
New-onset AF after cardiac surgery is common and has been associated with increased risks of late mortality and stroke.8 Trials of prophylactic amiodarone and beta blockers have demonstrated effectiveness reducing the occurrence of new AF, but not in all studies. Building off previous studies, the PALACS (Effect of Posterior Pericardiotomy on the Incidence of Atrial Fibrillation After Cardiac Surgery) randomized trial studied the use of posterior left pericardiotomy7 and found significant reduction of AF in the treated group (17% versus 32% no intervention group; P=0.0007; OR, 0.44). Colchicine has shown uncertain benefit based on the results of more recent studies compared with earlier encouraging trials. Drug adverse effects result in frequent drug discontinuation.9–11
Recommendation-Specific Supportive Text
Preoperative prophylaxis with beta blockers or amiodarone has shown mixed benefit, and studies are difficult to compare because of variable medication combinations.1–5 One double-blind RCT of 601 patients listed for nonemergency CABG surgery and/or valve replacement/repair surgery showed a significant reduction of AF with amiodarone.
A meta-analysis and RCT showed that the incidence of postoperative AF was significantly lower in patients treated with posterior left pericardiotomy during their cardiac procedure; however, the studies did not include patients undergoing mitral or tricuspid procedures.6,7
10.9.2. Treatment of AF After Cardiac Surgery
Recommendations for Treatment of AF After Cardiac Surgery
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | A * | 1. In postoperative cardiac surgery patients, beta blockers* are recommended to achieve rate control for AF1–4 unless contraindicated or ineffective in which case a nondihydropyridine calcium channel blocker† is recommended.5 |
| B-Rt | ||
| 1 | B-R | 2. In hemodynamically stable cardiac surgery patients with postoperative AF, rate-control (target heart rate, <100 bpm) and/or rhythm-control medications are recommended as initial therapy, with the choice of strategy according to patient symptoms, hemodynamic consequences of the arrhythmia, and physician preference.5,6 |
| 1 | B-R | 3. In patients who develop poorly tolerated AF after cardiac surgery, direct current cardioversion in combination with antiarrhythmic drug therapy is recommended, with consideration of imaging to rule out left appendage thrombus before cardioversion in those patients in whom AF has been present >48 hours and who have not been on anticoagulation.5,6 |
| 2a | B-NR | 4. In patients who develop postoperative AF after cardiac surgery, it is reasonable to administer anticoagulation when deemed safe in regard to surgical bleeding for 60 days after surgery unless complications develop and to reevaluate the need for longer term anticoagulation at that time.5,7 |
| 2a | C-LD | 5. In patients who develop AF after cardiac surgery and who are treated with rate-control strategy, at 30- to 60-day follow-up it is reasonable to perform rhythm assessment and, if AF does not revert to sinus rhythm spontaneously, consider cardioversion after an adequate duration of anticoagulation.5,6 |
A LOE applies to the data on beta blockers.
B-R LOE applies to the data on nondihydropyridine calcium channel blockers.
Synopsis
For new-onset AF after cardiac surgery, rate control is typically managed with beta blockers, or calcium channel blockers.1–4 A randomized trial showed no significant clinical differences comparing a strategy of rate-control versus rhythm-control medications for patients who are hemodynamically stable.5 For those with poorly controlled AF, cardioversion is reasonable, but for those with >48 hours of AF, imaging of the LAA (even if surgically closed) should be considered.5,6 The use of cardioversion and imaging in postoperative patients is a judgment call considering the patient’s clinical condition. For patients who develop postoperative AF, including during follow-up postdischarge, rhythm assessment, and possible cardioversion for the small number who are still in AF, should be considered.5,6 Colchicine had been shown to be effective, but more recent studies did not confirm this, and gastrointestinal adverse effects may be significant.8–10
Recommendation-Specific Supportive Text
The use of beta blockers to control heart rate is well documented.1–4 Calcium channel blockers are an option for rate control when beta blockers are ineffective.5
The use of rate control or rhythm control are both acceptable options in hemodynamically stable patients because neither has a distinct clinical advantage over the other based on a randomized study that included 529 patients. In this study, patients in the rhythm-control group received amiodarone.5,6
Cardioversion of patients with sustained postoperative AF is regularly performed, especially for those patients with hemodynamically poorly tolerated AF. Cardioversion is routinely combined with pharmacological therapy, especially amiodarone.5,6
A review of 8 observational studies that included 15 335 patients found a protective impact on the mortality rate at 5 years but no differences in thromboembolic events in patients with postoperative AF treated with anticoagulants.7 In general, the duration of anticoagulation is a minimum of 60 days; in the randomized trial of rate control versus rhythm control, if patients remained in AF or had recurrent AF 48 hours after randomization, anticoagulation was recommended to be continued for 60 days, unless complications occurred. Patients should be evaluated before discontinuation to determine underlying rhythm, especially those at high risk for stroke.
The need for a rhythm-control strategy should be evaluated postdischarge. Patients who tolerate rate control can be followed and often convert spontaneously after 6 to 8 weeks. A net advantage of rate control is earlier discharge in hemodynamically stable patients. Those who do not convert can be managed accordingly with rhythm-control strategy.5,6
10.10. Acute Medical Illness or Surgery (Including AF in Critical Care)
Recommendations for Acute Medical Illness or Surgery (Including AF in Critical Care)
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. Patients with AF who are identified in the setting of acute medical illness or surgery should be counseled about the significant risk of recurrent AF after the acute illness is resolved.1–6 |
| 2a | B-NR | 2. In patients with AF who are identified in the setting of acute medical illness or surgery, outpatient follow-up for thromboembolic risk stratification and decision-making on OAC initiation or continuation, as well as AF surveillance, can be beneficial given a high risk of AF recurrence.4–9 |
| 2b | B-NR | 3. In patients with AF who are identified in the setting of critical illness due to sepsis, the benefits of anticoagulation during critical illness for stroke prevention are uncertain.10,11 |
Synopsis
AF identified in the setting of hospitalization for acute noncardiac illness (acute AF), including patients who are critically ill, may represent new-onset AF that has been detected and treated for the first time. Incidence ranges from 1% to 46% in medical illness,1,12–14 with 6% to 22% in severe sepsis,15 and 3% to 16% after noncardiac surgery.16 AF can be an incidental finding during low-risk procedures but can occur associated with a range of conditions, including critical illness or sepsis. A combination of underlying arrhythmogenic substrate and triggers related to the acute illness likely contribute to acute AF. Acute AF can be paroxysmal or persistent, with or without symptoms, and is associated with higher risk of AF recurrence.1–3 Acute AF is also associated with prolonged hospitalization13,14 as well as increased morbidity12,15 and mortality.13,15 Management of acute AF is directed toward detection and treatment of potential triggers, optimizing hemodynamics, rate and/or rhythm control, and reducing in-hospital and long-term risk of stroke. Rate- or rhythm-control strategy should be individualized, balancing the impact of rapid rates and atrioventricular dyssynchrony on hemodynamics with ability to tolerate treatment. Decisions regarding initiating anticoagulation should be based on risk stratification of patient substrate and comorbidities, and the timing should take into consideration bleeding risks and the complexity of the acute illness.
Recommendation-Specific Supportive Text
In patients who develop or are discovered to have AF in the setting of acute medical illness and noncardiac surgery, recurrent AF was noted in 42% to 68%1,2 and 39%,3 respectively, during 5-year follow-up. Regardless of presence of an initial precipitant, recurrent AF was associated with increased risks of HF (HR, 2.74 [95% CI, 2.39–3.15]; P<0.001), stroke (HR, 1.57 [95% CI, 1.30–1.90]; P<0.001), and mortality (HR, 2.96 [95% CI, 2.70–3.24]; P<0.001).2
In 10 723 patients with newly diagnosed AF (67.9±–9.9 years, 41% women) identified from a longitudinal database, 19% of patients had an acute AF precipitant, the most common being cardiac surgery (22%), pneumonia (20%), and noncardiac surgery (15%). AF recurrence at 5 years was 41% among those with a precipitant versus 52% in those without one (adjusted HR, 0.75 [95% CI, 0.69–0.81]; P<0.001) (Figure 27).3 Regardless of a precipitant, recurrent AF was associated with increased risk of HF (HR, 2.74 [95% CI, 2.39–3.15]; P<0.001), stroke (HR, 1.57 [95% CI, 1.30–1.90]; P<0.001), and death (HR, 2.96 [95% CI, 2.70–3.24]; P<0.001). In patients with acute AF after cardiac surgery, observational studies show that those who underwent continuous rhythm monitoring had a higher detection of AF.4–6 No randomized clinical trial has specifically compared different monitoring strategies for outpatient follow-up of patients with acute AF. However, parallels could be drawn from studies like the CRYSTAL-AF8 and SEARCH-AF (Post-Surgical Enhanced Monitoring for Cardiac Arrhythmias and Atrial Fibrillation)9 trials, which showed increased sensitivity for AF detection with long-term monitoring. Close outpatient follow-up with consideration of heart rhythm monitoring and thromboembolic risk stratification is important considering the high risk of AF recurrence in these patients, especially in those who underwent noncardiac surgery and with risk factors for stroke in whom the AF is likely to recur (Figure 28). The optimal frequency, duration, and type of rhythm monitoring for patients with acute AF remain unclear and need further study.
In a retrospective cohort study of 38 582 patients with AF and sepsis admitted to US hospitals from July 1, 2010, to June 30, 2013, 13 611 patients (35.3%) received parenteral anticoagulation for AF. The CHA2DS2-VASc score was a poor predictor of acute stroke risk, and parenteral anticoagulation did not reduce stroke risk (1.3% versus 1.4%; RR, 0.94 [95% CI, 0.77–1.15]) and was associated with an increased risk of clinically significant bleeding (8.6% versus 7.2%; RR, 1.21 [95% CI, 1.10–1.32]). Risk of stroke did not differ whether patients had preexisting AF and new onset AF.10 In a retrospective cohort study of 102 patients ≥65 years of age who were hospitalized with sepsis and with new-onset AF, 28% received a prescription for OACs within 30 days of admission. Over 3-year follow-up, no significant association was noted between anticoagulation and a lower incidence of ischemic stroke (OR, 1.98 [95% CI, 0.29–13.47]) or bleeding (OR, 0.96 [95% CI, 0.29–3.21]).11 Gaps in knowledge exist, and further research is needed to better understand the benefits and risks of acute and long-term anticoagulation in critically ill patients with acute AF.
Figure 27. Unadjusted Cumulative Risk of AF Recurrence.
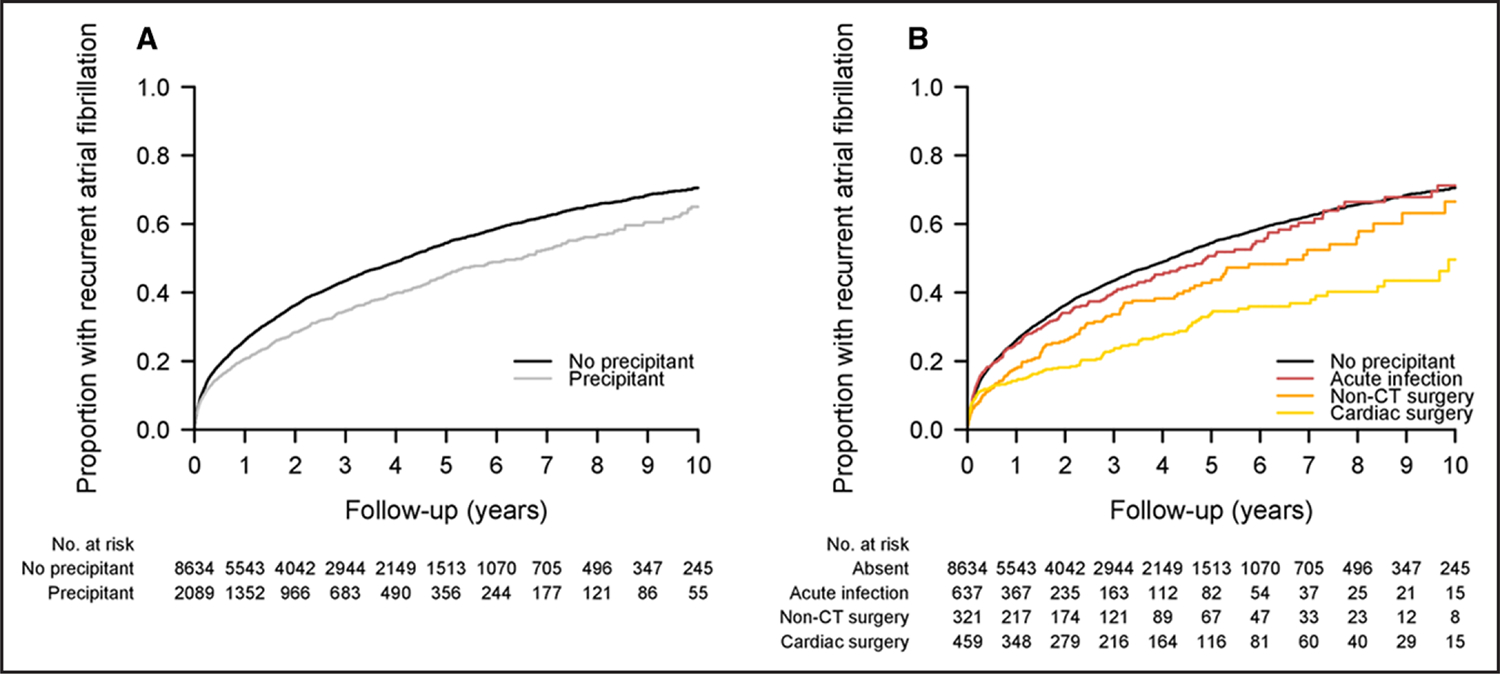
Unadjusted curves displaying cumulative risk of recurrent AF, generated using Kaplan-Meier method. (A) Overall risk of recurrent AF among individuals with and without acute precipitants. (B) Overall risk of recurrent AF among individuals with infection, cardiac surgery, and noncardiothoracic surgery compared with no precipitant. These 3 precipitants were selected for display because the risk of recurrent AF was significantly lower compared with the referent group without precipitants in multivariable adjusted models. Individuals with other AF precipitants were excluded from this plot for clarity. Reproduced with permission from Wang et al.3 Copyright 2020 American Heart Association, Inc. AF indicates atrial fibrillation; and CT, cardiothoracic.
Figure 28. Acute Medical or Surgical Illness.
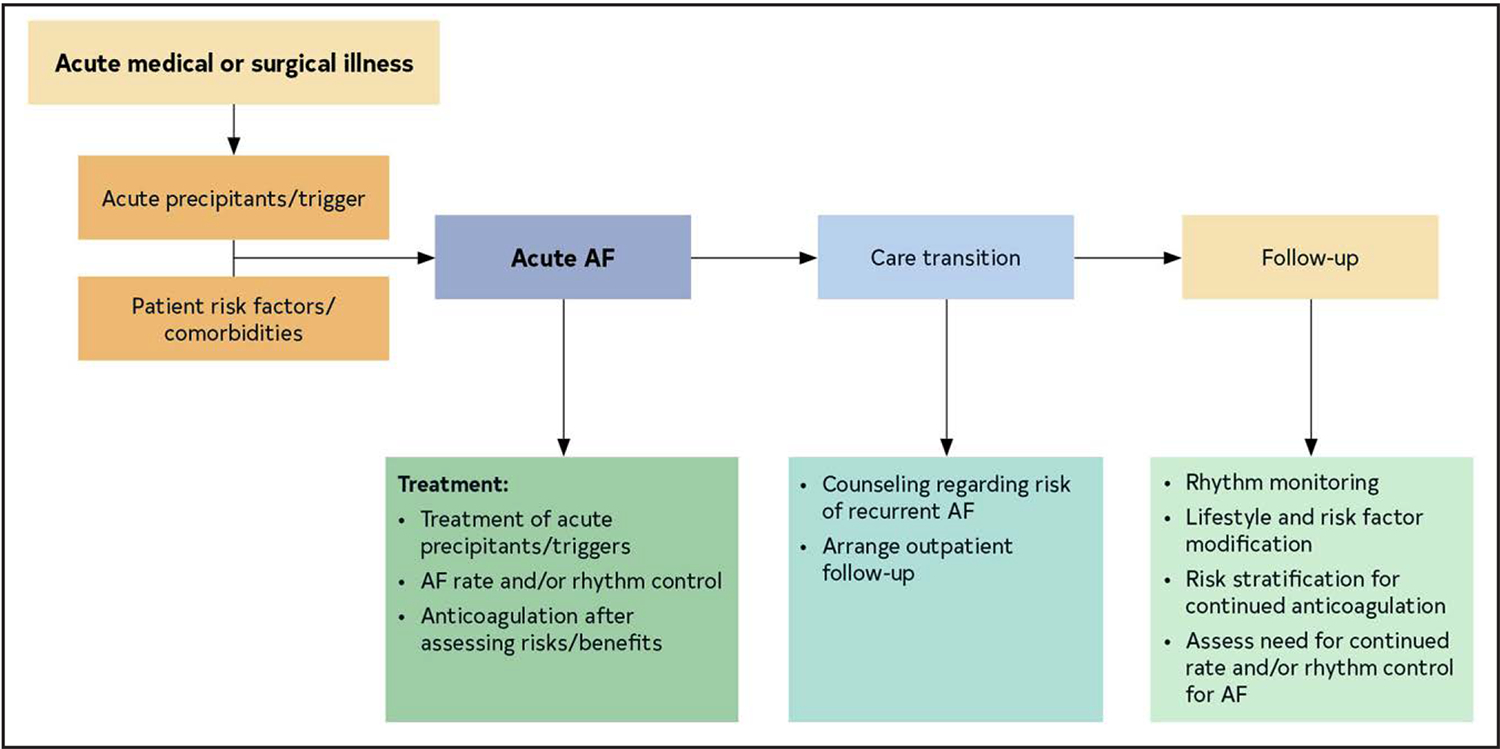
Adapted with permission from Chyou et al. 17 Copyright 2023 American Heart Association, Inc. AF indicates atrial fibrillation.
10.11. Hyperthyroidism
Recommendation for Hyperthyroidism
Referenced studies that support the recommendation are summarized in the Online Data Supplement.
Synopsis
Hyperthyroidism, defined as elevated blood levels of thyroid hormone with reduced levels of thyroid-stimulating hormone, is relatively common and may not lead to overt symptoms of thyrotoxicosis.3,4 Patients with hyperthyroidism are at increased risk of developing AF,5 which resolves in most cases after the restoration of the euthyroid state with effective antithyroid treatment.6,7 Excess thyroid hormone increases sensitivity to catecholamines,3,8 and beta blockers reduce symptoms and tachycardia in patients with hyperthyroidism.9–12 Nonselective beta blockers may reduce the systemic symptoms of hyperthyroidism a bit more than beta-1–selective agents do, and propranolol reduces the peripheral conversion of T4 to T3.10,13 Although it is uncertain whether hyperthyroidism is an independent risk factor for stroke, many patients have other risk factors that elevate stroke risk, and anticoagulation reduces risk of stroke to a similar extent among patients with and without hyperthyroidism.1,2,14 Restoration of sinus rhythm is unlikely until a euthyroid state has been achieved15 and, in 1 study, spontaneous reversion to sinus rhythm occurred at a median of 1 to 3 weeks after thyroid hormone levels returned to normal.6
Recommendation-Specific Supportive Text
Patients with hyperthyroidism often have laboratory findings that suggest hypercoagulability,16 but it is uncertain whether this translates into a higher risk of stroke.1,17 Nevertheless, patients with hyperthyroidism are commonly at elevated risk of stroke due to other clinical factors, so anticoagulation should be considered using the framework in Section 6.3.1 (“Antithrombotic Therapy”). A Danish national registry study found that patients with thyrotoxicosis and AF had a lower overall risk of thromboembolic events but a similar reduction in the risk of thromboembolic events from anticoagulation compared with patients who did not have a secondary cause of AF.5 In a large, randomized trial of apixaban versus warfarin, patients with a history of hyperthyroidism or hypothyroidism had risk of stroke similar to that of patients with no history of thyroid disease, and the effectiveness of apixaban in reducing stroke was consistent across subgroups with and without a history of thyroid disease.16
10.12. Pulmonary Disease
Recommendations for Pulmonary Disease
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-R | 1. In patients with AF and COPD, it is reasonable to use cardioselective beta blockers for rate control of AF, especially where other indications exist (eg, MI and HF).1–3 |
| 2a | B-NR | 2. In patients with pulmonary hypertension (PH) with pulmonary vascular disease and AF or AFL, a rhythm-control strategy is reasonable to improve functional status and potentially prolong survival.4–12 |
Synopsis
Pulmonary disease is commonly associated with arrhythmias. AF is common in COPD and is associated with an increased risk of mortality and bleeding.13 The combination of AF and asthma is less common, and beta blockers are generally avoided to prevent bronchospasm. A bidirectional relationship is seen between AF and pulmonary embolus (PE), although it is little studied. The risk of PE is increased in patients with AF.14 AF may also complicate acute PE, where it is a marker of increased risk of mortality.15 Whether AF in the setting of PE should influence the intensity or duration of anticoagulation, or is associated with future AF recurrence, is unknown. AF and other supraventricular arrhythmias are important causes of decompensation in patients with PH, which is especially well described in World Health Organization groups 1 and 4. A successful rhythm-control strategy is associated with better outcomes in these patients.
Recommendation-Specific Supportive Text
AF is common in patients with COPD, with a prevalence of 11% in a large European registry.16 Furthermore, patients with COPD often have other concomitant heart disease. Because beta-2 agonists can be used for the treatment of COPD, there has been a reluctance to prescribe beta blockers in case these would antagonize beta-2 agonists or precipitate bronchospasm.17 However, a systematic review and meta-analysis of observational studies found that beta-blocker use, for diverse indications including HF and CAD, was associated with a significantly reduced risk of mortality in patients with COPD.1 Another meta-analysis of RCTs assessing the effect of cardioselective beta blockers on respiratory function and symptoms did not find any change in these parameters or in response to beta-2 agonists.2 An RCT of patients with advanced COPD at risk of severe exacerbation, but without an indication for beta-blocker treatment, found no difference in the time to first exacerbation but did find an increased risk of severe exacerbation.3 Therefore, although other agents can be used for rate control of AF (Section 7, “Rate Control”), beta blockers, especially cardioselective beta blockers, for treatment of AF need not be avoided when indicated, especially in patients with less than severe COPD. However, beta blockers can exacerbate reactive airway disease and are generally avoided in that setting, such as in patients with asthma.
Atrial arrhythmias are common in PH with pulmonary vascular disease (PHPVD; hemodynamically defined as PH with increased pulmonary vascular resistance and normal left heart filling pressures, typical of World Health Organization group 1 but also of groups 3 and 4). Right atrial contraction accounts for a significantly greater proportion of total right heart function in patients with PHPVD than in normal subjects,18 and the onset of atrial arrhythmia is frequently associated with clinical deterioration.4–12 Restoration of sinus rhythm results in improvement, whereas persistent arrhythmia or recurrence is associated with mortality.4,8,9 Pharmacological rhythm control and cardioversion have both been used, although negative inotropy may limit antiarrhythmic drug therapy. Catheter ablation of typical AFL has been well described in patients with PHPVD, with acceptable results and safety, as well as improvement in clinical and hemodynamic parameters.5,7,12 LA ablation has also been described, although the possibility of creating a right-to-left shunt should always be considered when planning transseptal puncture. Anesthetic management is also complex, and these patients are best cared for by centers with extensive clinical expertise in these conditions.
10.13. Pregnancy
Recommendations for Pregnancy
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | B-NR | 1. In pregnant patients with AF, DCCV is safe to the patient and fetus and should be performed in the same manner as in patients who are not pregnant.1 |
| 2b | C-LD | 2. In pregnant individuals with structurally normal hearts and hemodynamically stable AF, pharmacological cardioversion with agents with history of safe use in pregnancy, such as intravenous procainamide, may be considered.1,2 |
| 2a | C-LD | 3. In pregnant individuals with AF and without structural heart disease, antiarrhythmic agents with history of safe use in pregnancy (eg, flecainide and sotalol) are reasonable for maintenance of sinus rhythm.1,2 |
| 2a | B-NR | 4. In pregnant individuals with persistent AF, rate-control agents with a record of safety in pregnancy, such as beta blockers (eg, propranolol or metoprolol) and digoxin, either alone or in combination with beta blockers, are reasonable as first-line agents.1,2 |
| 2b | C-LD | 5. Pregnant individuals with AF and elevated risk of stroke may be considered for anticoagulation with the recognition that no anticoagulation strategy is completely safe for both the mother and fetus, and an SDM discussion should take place regarding risks to both mother and fetus (Table 28).3 |
Table 28.
Anticoagulation Strategies During Pregnancy
| Antenatal Options | ||||
|---|---|---|---|---|
| Method 1 | Method 2 | Method 3 | Alternative Method 4 | |
| First trimester | Warfarin ≤5 mg | LMWH | UFH | LMWH |
| Second trimester | Warfarin | Warfarin | Warfarin | LMWH |
| Third trimester | Warfarin | Warfarin | Warfarin | LMWH |
| Delivery Planning | ||
|---|---|---|
| Method 1 | Method 2 | |
| 1 wk before | Discontinue warfarin → continuous IV U FH | Dose-adjusted LMWH |
| 36 h before | Continuous IV UFH | Switch to continuous IV UFH |
| 4–6 h before | Stop IV heparin | Stop IV heparin |
Adapted with permission from Otto et al.15 Copyright 2021 American Heart Association, Inc., and American College of Cardiology Foundation.
IV indicates intravenous; LMWH, low-molecular-weight heparin; and UFH, unfractionated heparin.
Synopsis
The incidence of atrial arrhythmias is rising in pregnancy, and data from the national inpatient sample suggest that this is largely driven by increasing incidence of AF, with a reported frequency of 27 per 100 000 hospitalizations.4 The incidence of AF/AFL in pregnant patients with heart disease is 1.3%, with a peak occurrence between 23 and 30 weeks gestation.5,6 Hospitalizations during pregnancy for arrhythmias are associated with an increased risk of maternal and fetal adverse outcomes. AF during pregnancy is associated with increased maternal mortality (OR, 13.13 [95% CI, 7.77–22.21]; P<0.0001).9
Atrial arrhythmias may manifest for the first time during pregnancy; however, AF is usually associated with underlying structural or CHD. Factors associated with an increased incidence of AF in pregnant individuals include advanced maternal age,6 African American race, lower socioeconomic status, and left-sided obstructive lesions.4,5 Obesity is associated with the development of hypertensive disorders of pregnancy, which is also a known risk factor for AF.7 Another potential mechanism for development of AF in pregnancy is the cardiogastric interaction that occurs with elevated progesterone levels, leading to gastroesophageal reflux disease.8
In patients deemed to be at high risk of stroke, recommendations for anticoagulation in pregnancy are extrapolated from experience in managing VHD. DOACs are contraindicated during pregnancy and during breastfeeding because of a lack of data to support their safety and efficacy in this patient population.
Recommendation-Specific Supportive Text
Rapid atrioventricular conduction may have serious hemodynamic consequences for both mother and fetus.9 DCCV of AF in pregnant individuals is safe for both mother and fetus and should be performed within 48 hours of presentation to minimize risk of stroke.1 Appropriate sedation should be provided to the pregnant individuals before DCCV. Fetal monitoring is generally used during and directly after DCCV.
Antiarrhythmic drugs are used in pregnancy for both maternal and fetal conditions; therefore, their mechanisms and adverse effect profile must be tolerable to both mother and fetus. These medications should be avoided if possible during the first trimester, and initiation of the drug should be attempted at the lowest dose with involvement of an electrophysiologist or cardiologist skilled in the management of arrhythmias in pregnancy.10 Intravenous ibutilide has been used effectively in pregnant women with refractory AF, although published reports included a very small number of patients. In a pregnant patient presenting with AF and manifest preexcitation, intravenous procainamide is also an option.11
In pregnant individuals with structurally normal hearts and AF, rhythm-control strategies may include agents with a long record of safety (eg, flecainide and sotalol) with appropriate monitoring that includes surveillance ECGs.1,12 Amiodarone is generally avoided and reserved for life-threatening arrhythmias because of potential toxicities to the fetus, including goiter, neurodevelopmental abnormalities, bradycardia, delayed growth, and premature birth.10,12
In pregnant individuals with persistent AF, rate-control strategies may include beta blockers with long record of safety in pregnancy (propranolol, metoprolol) or digoxin. Atenolol is generally avoided in pregnancy due to concerns about intrauterine growth retardation. Serum digoxin levels may be unreliable in pregnancy as digoxin-like immunoreactive substances are increased in pregnant individuals and react with antibodies against digoxin, interfering with the radioassay used for detection of digoxin serum levels.13
Tools available to predict stroke risk in AF have not been validated in pregnancy. In the rare case of AF with no risk factors or signs of heart disease, anticoagulation may not be necessary.1,14 SDM should be used when considering anticoagulation during pregnancy (Table 28). Some patients may be deemed at high risk for stroke, such as those with mitral stenosis, for which recommendations for anticoagulation in pregnancy are extrapolated from experience in managing VHD. Warfarin may be administered in the first trimester providing the dose needed to achieve a therapeutic INR is ≤5 mg. If the dose of warfarin is >5 mg/day, low-molecular-weight heparin should be administered throughout the first trimester and then transitioned to warfarin for the remainder of the antenatal period until 36 hours before a planned delivery, at which time unfractionated heparin is started.15 Vaginal delivery should be advised for most women, and VKAs are generally held to mitigate risk of bleeding compliciations.13 Use of novel DOACs is not recommended during pregnancy in view of lack of evidence about safety.16
10.14. Cardio-Oncology and Anticoagulation Considerations
Recommendations for Cardio-Oncology and Anticoagulation Considerations
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 1 | C-LD | 1. In patients with cancer and AF, multidisciplinary communication including cardiology, oncology and other clinicians, and SDM with the patient is recommended to optimize cancer and AF treatment and to reduce the risk of drug-drug interactions, QTc prolongation, proarrhythmia, bleeding, and thromboembolism.1–3 |
| 2a | C-LD | 2. In patients who are to be initiated on cancer therapies associated with an increased risk of developing AF, increased vigilance for incident AF and treatment of contributing factors is reasonable to decrease morbidity.1,4–6 |
| 2a | B-NR | 3. In most patients with AF and cancer (remote history or receiving active cancer treatment), DOACs are reasonable to choose over VKAs for stroke risk reduction.7,8 |
Synopsis
Patients with cancer have an increased risk of AF. A Danish nationwide study observed an incident AF rate of 17.4 per 1000 person-years compared with 3.7 per 1000 person-years in patients without cancer.9 A meta-analysis of observational studies demonstrated the risk of AF to be ~47% higher in patients with cancer compared with patients without cancer.5,10 The increased risk of AF is likely related to increased age, shared risk factors between cancer and CVD (eg, obesity and systemic inflammation), coexistent comorbidities (eg, hypertension and HF), cardiac involvement by the tumor, as well as certain medical therapeutics (Table 29), oncologic surgery, especially thoracic, and thoracic radiation therapy.11,12 New-onset AF in patients with cancer is associated with a higher risk of HF, thromboembolism, bleeding, and mortality.13–15 Notably, Bruton’s tyrosine kinase (BTK) inhibitors, such as ibrutinib, which are used to treat chronic lymphocytic leukemia and lymphoproliferative malignancies and often used for an extended period of time, are associated with a higher risk of AF due to off-target cardiac effects.16 In 1 meta-analysis, the risk of AF with use of ibrutinib was 4 times higher compared with that without use of ibrutinib.17
Table 29.
Medical Cancer Therapy Associated With Increased Risk of AF (>1%)
| Frequency Reported in Clinical Trials and Observational Studies |
|||
|---|---|---|---|
| Cancer Therapy | Common: Incidence 1%–10% | Frequent: >10% | Comments |
|
| |||
| Anthracyclines | AF may be a secondary result of anthracycline cardiotoxicity; studies in different populations demonstrate variable risk of AF | ||
| Doxorubicin, epirubicin, idarubicin, mitoxantrone | X | ||
|
| |||
| Antimetabolites | |||
| Clofarabine combined with cytarabine | X | ||
| 5FU | X | ||
| Cepecitabine | X | ||
| Gemcitabine | X | ||
|
| |||
| Alkylating agents | *Stem cell transplantation is associated with an increased risk of AF,28,29 and the risk may be higher with melphalan-associated regimens. | ||
| Cyclophosphamide | X | ||
| Melphalan + stem cell transplantation | X* | ||
|
| |||
| Immunomodulatory drugs | Given rates reported from patients with multiple myeloma, AF due to underlying cardiac AL amyloid may contribute | ||
| Lenalidomide | X | ||
| Interleukin-2 | X | ||
|
| |||
| TKIs | †Reported AF rates with ibrutinib have varied across trials (4%–18%),6,30 partly related to varying duration of follow-up and patient factors. Second-generation BTKis have more selective BTK activity and are associated with a lower incidence of AF than ibrutinib.31 Based on FDA adverse event reporting system4 |
||
| Ibrutinib (BTKi) | X† | X† | |
| Acalbrutinib (second-generation BTKi) | X | ||
| Zanubrutinib (second-generation BTKi) | X | ||
| Ponatinib (BCR-ABL TKI) and other TKIs (eg, trametinib, osimertinib, nilotinib, ribociclib) | X | ||
| VEGF inhibitor | |||
| Sorafenib in combination with 5FU | X | ||
| BRAF inhibitor | |||
| Vemurafenib | X | ||
|
| |||
| CAR T-cell therapy | |||
| Tisagenlecleucel | X | ||
| Axicabtagene ciloleucel | X | ||
|
| |||
| Monoclonal antibodies | |||
| Rituximab | X | ||
Table developed by 2023 Atrial Fibrillation Guideline Writing Committee. Data extracted from Buza et al32 and Fradley et al.33
AF indicates atrial fibrillation; BTKi, Bruton’s kinase inhibitor; CAR, chimeric antigen receptor; FDA, US Food and Drug Administration; 5FU, 5 fluorouracil; and TKI, tyrosine kinase inhibitor.
Although patients with cancer are underrepresented in the major trials comparing DOACs to VKAs, large observational studies and meta-analyses of studies of patients with cancer and AF have demonstrated similar or lower rates of ischemic stroke and bleeding in patients taking DOACs compared with warfarin.7,8 Patients with AF and cancer are at an increased risk for major bleeding.18 They also are commonly on antineoplastic agents, which may have important drug interactions with OACs.19 Thus, these factors should be carefully considered in determining indication and choice of OAC.
Recommendation-Specific Supportive Text
In patients with cancer who develop AF in the presence of cancer therapeutics associated with an increased risk of incident AF (Table 29), the first approach usually consists of optimizing AF control while continuing cancer therapy. However, if AF control cannot be achieved, decisions regarding dose reduction of the cancer therapy or even stoppage can only be made in concert with the oncology team and SDM with the patient. Drug-drug interactions between cancer, antiarrhythmic, rate-control, and anticoagulant therapies are common and require close attention.1 For example, in the case of BTK inhibitors, CYP3A inhibitors such as amiodarone, dronedarone, verapamil, and diltiazem are usually avoided, due to the associated risk of elevation in ibrutinib levels.1 Further, ibrutinib can raise levels of digoxin, a P-glycoprotein substrate.1 Therefore, beta blockers are considered the best option for rate control in BTK inhibitor-associated AF. If additional agents are needed for AF control, any concomitant reduction in dose of ibrutinib is guided by oncology.1 Further, QTc prolongation, which is more common in patients with cancer due to several cancer therapies, antiemetics, antibiotics, and electrolyte derangements, can be further exacerbated by QT-prolonging antiarrhythmic agents.3 Structured screening by pharmacists for potential drug-drug interactions could provide benefit.2
Certain medical cancer therapies can increase the risk of incident AF (Table 29). Patients with cancer and AF are older and have higher rates of hypertension, MI, and HF compared with those who do not develop AF.13–15 The development of perioperative AF has been strongly associated with perioperative complications.20 In general, patients with cancer and CVD may be undertreated with GDMT or less often referred to specialists for cardiovascular care.21 Optimization of cardiovascular conditions such as hypertension and HF may decrease the risk of incident AF, AF recurrence, and of major adverse cardiovascular events, including stroke.22–25 Therefore, in patients without a history of AF, or those with previous history of AF, who are to be started on cancer therapies associated with a higher risk of AF, evaluation and optimization of cardiovascular risk factors or disease by a cardiologist could be beneficial in decreasing incident and recurrent AF and associated cardiovascular events, although no data are available specifically in patients with cancer. Furthermore, elevated natriuretic peptides or a history of previous AF can identify patients at higher risk of AF after major thoracic cancer surgery.26,27
No large RCTs are available that compare DOACs to warfarin in patients with AF and cancer. A meta-analysis of post-hoc analyses of RCTs and retrospective observational studies that compared DOACs with warfarin in patients with AF and cancer (n=229 221) demonstrated a lower risk of stroke or systemic embolism (RR, 0.65 [95% CI, 0.52–0.81]; P=0.001) and major bleeding (RR, 0.68 [95% CI, 0.50–0.92]; P=0.01) with DOACs.8 This meta-analysis included post hoc analyses of patients with cancer and AF from the ROCKET AF (rivaroxaban versus warfarin), ENGAGE TIMI-48 (edoxaban versus warfarin), and ARISTOTLE (apixaban versus warfarin) trials. These findings were consistent in patients defined as having active cancer, although definitions of active cancer varied by study. An additional large retrospective observation study comparing DOACs to warfarin in patients with active cancer demonstrated a lower risk of the composite endpoint of ischemic stroke/intracranial bleeding in patients taking DOACs compared with VKA (adjusted HR, 0.65 [95% CI, 0.48–0.88]).7 Although most of the data in this patient population came from retrospective observational studies that may be prone to selection bias, findings are largely consistent with post hoc analyses from RCTs and the major RCTs comparing DOACs with VKA in general. Special consideration should be given to patient bleeding risk and antineoplastic drug interactions in this patient population (Table 30).18,19
Table 30.
Special Considerations for Anticoagulation in Patients With AF on Active Cancer Treatment
| Increased bleeding risk | High bleeding risk estimators (eg, HAS-BLED) Thrombocytopenia (platelet <50 000/uL) Intracranial malignancy Gastrointestinal malignancy History of major bleeding Severe kidney dysfunction (eGFR <30 mL/min/1.73 m2) |
| Drug interactions | P-glycoprotein inducers or inhibitors CYP3A4 inducers or inhibitors |
AF indicates atrial fibrillation; eGFR, estimated glomerular filtration rate; and HAS-BLED, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio (INR), elderly (age ≥65 years), drugs/alcohol concomitantly).
10.15. CKD and Kidney Failure
CKD and kidney failure (formerly end-stage renal disease) share common risk factors with AF, such as hypertension and diabetes, and unsurprisingly, CKD and AF are associated in a disproportionate manner. In population studies, baseline serum creatinine, eGFR, and proteinuria were strongly associated with incident of AF during follow-up.1,2 AF at baseline also predicted new renal dysfunction or proteinuria, suggesting a bidirectional relationship between CKD and AF.2 In an insurance database of 206 229 adults with confirmed CKD, over a mean follow-up of 5 years, there was a 67% increased rate of kidney failure among patients with CKD who developed incident AF compared with those without AF, even after adjustment for baseline factors.3 Data on the management of AF in patients with CKD is limited because the major trials of rate control, rhythm control, and catheter ablation have generally not reported eGFR or CKD as a baseline variable or excluded such patients. Antiarrhythmic drug doses are adjusted based on pharmacokinetic data and clinical experience, with amiodarone being the only drug that does not require dose adjustment in patients with CKD or those receiving dialysis. Catheter ablation is feasible, although particular attention must be paid to fluid balance when using irrigated radiofrequency catheters. Anticoagulation in patients with CKD and AF is covered in Section 6.8.4 (“Chronic Kidney Disease [CKD]/Kidney Failure”).
10.16. Anticoagulation Use in Patients With Liver Disease
Recommendations for Anticoagulation Use in Patients With Liver Disease
Referenced studies that support the recommendations are summarized in the Online Data Supplement.
| COR | LOE | Recommendations |
|---|---|---|
| 2a | B-NR | 1. For patients with AF who are at increased risk of systemic thromboembolism and mild or moderate liver disease (Child-Pugh* class A or B), OAC therapy is reasonable in the absence of clinically significant liver disease–induced coagulopathy or thrombocytopenia.1–7 |
| 2a | B-NR | 2. For patients with AF who are at increased risk of systemic thromboembolism and mild or moderate liver disease (Child-Pugh class A or B) and who are deemed to be candidates for anticoagulation, it is reasonable to prescribe DOACs (Child-Pugh class A: any DOAC; Child-Pugh class B: apixaban, dabigatran, or edoxaban) over warfarin.1,7–11 |
| 3: Harm | C-LD | 3. For patients with AF and moderate liver disease (Child-Pugh class B) at increased risk of systemic thromboembolism, rivaroxaban is contraindicated due to the potentially increased risk of bleeding.12 |
Child-Pugh scoring: the severity of liver disease, primarily cirrhosis in patients with diagnosed liver disease. Child-Pugh A (mild): 5–6 points; Child-Pugh B (moderate): 7–9 points; Child-Pugh C (severe): 10–15 points. The score is based on the 5 variables: encephalopathy (none=1 point, grade 1 and 2=2 points, grade 3 and 4=3 points); ascites (none=1 point, slight=2 points, moderate=3 points); total bilirubin (<2 mg/mL=1 point, 2–3 mg/mL=2 points, >3 mg/mL=3 points); albumin (>3.5 mg/mL=1 point, 2.8–3.5 mg/mL=2 points, <2.8 mg/mL=3 points); INR (<1.7=1 point, INR 1.7–2.2=2 points, INR >2.2=3 points).
Synopsis
No RCT has evaluated the use of OACs in patients with AF and liver disease. Three observational studies and 1 meta-analysis of observational studies have compared OAC therapy with no OAC therapy in patients with AF and active liver disease or a history of liver disease.1–4 In addition, 3 observational studies have compared OAC therapy in patients with liver disease to those without liver disease.5–7 In general, anticoagulation has been associated with lower risks of ischemic stroke or thromboembolism but was also associated with a significantly higher risk of major bleeding or ICH compared with no oral anticoagulation therapy in patients with AF and liver disease. Oral factor Xa inhibitors undergo hepatic metabolism, and impaired liver function may increase their plasma concentrations and the risk of bleeding. Pivotal clinical trials for DOACs excluded patients with active liver disease. Four postmarketing retrospective studies and 2 meta-analyses have evaluated the comparison between DOACs and VKAs in this population, and they showed DOACs were associated with a lower risk of major bleeding or ICH.1,7–11 Limited data exist on the safety of DOAC in severe liver disease. The uses of DOACs based on a Child-Pugh Score are summarized in Table 13 (Section 6.3.1.1. “Considerations in Managing Anticoagulation”).
Recommendation-Specific Supportive Text
A meta-analysis showed that VKA use was significantly associated with lower risks of ischemic stroke or thromboembolism but was also associated with significantly higher risks of major bleeding or ICH in patients with AF and liver disease compared with no oral anticoagulation therapy.7 A large-scale retrospective study also corroborated that OAC use was significantly associated with a lower risk of stroke or all-cause mortality in patients with AF and cirrhosis.3 VKA use in the liver fibrosis group was associated with a higher risk of the composite cardiovascular events and major bleeding than in the nonliver fibrosis group.4 However, no significant difference in the composite cardiovascular events and major bleeding between the 2 groups receiving DOACs was found. The efficacy and safety of VKAs or DOACs were not affected by nonalcohol fatty liver disease status.5 The subgroup analysis of the meta-analysis found that the risk of major bleeding was not affected by the presence of esophageal varices.13
A meta-analysis found no significant difference in the risk of ischemic stroke and thromboembolism between DOACs and VKAs (OR, 0.82 [95% CI, 0.361.88]; P=0.64) in patients with almost exclusively mildto-moderate liver disease (Child-Pugh class A-B), but DOAC use was associated with lower risks of major bleeding (OR, 0.54 [95% CI, 0.38–0.75]; P=0.0003) and ICH (OR, 0.35 [95% CI, 0.23–0.53]; P<0.0001) compared with the warfarin group.7 Another meta-analysis showed that, in patients with exclusively mild-to-moderate cirrhosis (Child-Pugh score A-B), DOAC use was associated with lower risks of ischemic stroke (HR, 0.62 [95% CI, 0.10–0.90]), major bleeding (HR, 0.64 [95% CI, 0.57–0.72]), or ICH (HR, 0.49 [95% CI, 0.40–0.59]) compared with warfarin.11 DOACs are reasonable oral anticoagulation agents in patients with AF and mild-to-moderate liver disease (Child-Pugh class A-B). However, data on DOACs are lacking on patients with AF and severe liver disease (Child-Pugh class C).
Rivaroxaban is currently avoided in patients with moderate liver disease (Child-Pugh class B). Rivaroxaban AUC significantly increased by 2.27-fold after a single dose of rivaroxaban 10 mg in patients with moderate liver disease compared with healthy subjects.12 Although the FDA labeling recommends avoiding edoxaban in patients with moderate liver disease due to the concern of underlying coagulopathy, the edoxaban AUC was not significantly increased after a single dose of edoxaban 15 mg in patients with moderate liver disease compared with healthy subjects.14 Apixaban AUC was not significantly increased after a single dose of apixaban 5 mg in subjects with mild and moderate liver disease (Child-Pugh class A and B) compared with healthy subjects.15 Dabigatran AUC was also not significantly different after a single dose of dabigatran 150 mg in patients with moderate liver disease (Child-Pugh class B) compared with that in healthy subjects.16
11. FUTURE RESEARCH NEEDS
Enormous progress and important advances have been made in recent years on the understanding of mechanisms and on the prevention and management of AF, including substantial progress since the 2014 guideline, as well as the 2019 focused update. Although the writing committee has tried to make significant progress in this current guideline to address many of the gaps in the clinical management of AF, important gaps in knowledge remain and are listed below.
AF as a disease continuum: AF must be seen as a disease continuum, yet historically the emphasis has been on rhythm management. More evidence is needed on how to best improve in other aspects of AF care, such as prevention, modification of risk factors, and how to incorporate holistic approaches to AF management into daily clinical practice.
Downstream consequences: The downstream consequences of AF over the long term must be better studied and defined. Most studies generally focus on short-term effects of therapy, usually 6 to 12 months. Many long-term consequences of AF remain poorly characterized, such as dementia and valvular insufficiency.
Better goal and outcome definition: Historically, ablation failure has been defined as having 30 seconds of AF identified during a follow-up period. However, that number might be too simplistic because AF burden may be a more important metric and because the 30-second metric might not reflect significant burden reduction that may be observed in many patients. Patient-oriented outcomes would also be more important, such as QOL.
Role of risk modifiers in AF stroke prevention: Great variation exists in risk of stroke depending on nonbinary or dynamic risk factors. Yet it is unclear how to best manage patients accordingly. For example, how should risk of stroke be assessed in a person with hypertension in whom the BP is very well controlled? Or, perhaps, how do we incorporate AF burden on the risk calculation?
Individualization of AF and stroke risk: Although some studies have demonstrated an effective role of biomarkers in predicting AF and stroke, how to incorporate these findings into clinical practice remains elusive. Similarly, how to incorporate other clinical markers of atrial cardiopathy into clinical practice must be better defined, as well as clarification as to how these nontraditional risk factors help better stratify patient risk.
Incorporating other stroke risk scores: The ubiquitous nature and simplicity of the CHA2DS2-VASc score have diminished with consideration of other scores of potential utility, better c-statistics, and with potential advantages in particular populations. Additional studies are needed to better take advantage of scores for specific groups, scores that could potentially be incorporated into the electronic medical record, and perhaps some with the potential benefit of better discriminating the true low-risk patients.
Surgical exclusion and LAAO: Increasingly available evidence has demonstrated the benefit of surgical LAA exclusion for stroke prevention, but the most scientifically rigorous trials included patients who received concomitant anticoagulation. More evidence is needed on the magnitude of benefit of LAA exclusion in preventing stroke in patients who have contraindications to anticoagulation and how to best manage those patients around rhythm-control interventions, such as cardioversion. Clinicians would also like to see additional data on pLAAO, particularly peri-implant stroke management and longer-term outcomes in patients in whom periprocedure anticoagulation is not possible.
Subclinical AF: What magnitude of AF burden mandates stroke prevention therapy in patients with subclinical AF must be better defined. Certainly, large general risk categories have been identified, and general guidelines exist, but extensive practice variations remain, and more precise recommendations for the community are needed. In addition, the role and impact on outcomes of AF screening in general and poststroke should be better defined.
Use and applicability of consumer-based wearable heart monitoring devices: These devices are now widespread and are used to diagnose and monitor response to therapy in patients with AF. Validation on the accuracy of the most common available technologies is needed. How to best use these devices in practice, including for AF screening, must be better defined.
Standardization of ablation procedures: Great practice variation exists on how AF ablation procedures are performed, either as first or repeat procedures. Large registries and more data are required to better define standards of care in this field. Many interventions, such as extra ablation lines, are still performed despite limited data demonstrating efficacy, whereas the best approaches to persistent AF and repeat ablation are poorly defined.
Candidates for ablation: We must better identify clinical markers to better identify when catheter ablation is unlikely to benefit patients and define specific criteria for candidacy for first time and repeat procedures.
Artificial intelligence for AF management: Artificial intelligence could potentially be used to better tailor therapy to the individual patient, taking into consideration numerous factors that may better select candidates for therapeutic approaches, such as anticoagulation versus LAAO, rhythm versus rate control, catheter ablation versus medical therapy, modification of risk factors, genetics, and others.
Strategies for anticoagulation: Ongoing clinical trials are assessing different strategies for oral anticoagulation in persons who have undergone catheter ablation, including the OCEAN (Optimal AntiCoagulation for Enhanced-Risk Patients Post-Catheter Ablation for Atrial Fibrillation) trial (NCT02168829)1 and ODIn-AF (Prevention of Silent Cerebral Thromboembolism by Oral Anticoagulation With Dabigatran After Pulmonary Vein Isolation for Atrial Fibrillation) (NCT02067182)2 trial, because it is unclear in whom anticoagulation can be discontinued after successful ablation. Several studies also have investigated the use of intermittent or continuous rhythm monitoring to guide oral anticoagulation in persons with AF, including those who have undergone ablation.3,4 These so-called “PITP” or “electrocardiographic-guided” approaches are investigational. Ongoing clinical trials like REACT-AF (Rhythm Evaluation for Anticoagulation Therapy for Atrial Fibrillation) will help determine whether they are suitable for clinical practice. Also, anticoagulation/DOAC use in pregnancy needs to be investigated because it is currently contraindicated in view of lack of data on safety.
Sleep: Among patients with AF and SDB, the impact of treatment of SDB on maintenance of sinus rhythm and on general cardiovascular outcomes remains uncertain. Most studies have been observational, which introduces confounding factors. Once diagnosed, the adherence to treatment for SDB is also suboptimal.
SDM and decision aids: Decision aids are perceived as important tools to better inform patients about options for AF management; yet, it is unclear if those tools improve clinical outcomes. Most decision aids developed for AF focus on anticoagulation; there is a paucity of validated decision aids, including for AF rhythm management using antiarrhythmic drugs or ablation.
Genetic testing: The use and applicability of consumer-based or targeted genetic testing for AF remains uncertain. Polygenic risk scores can indicate higher risk for AF, but the use of genetic testing to impact clinical surveillance, management, and clinical outcomes remains uncertain.
Race, ethnicity, gender, and sex differences: In sexual- and gender-diverse individuals, additional research is recommended to identify the incidence and outcomes of AF as well as the impact of gender-affirming therapies on arrhythmia incidence and outcomes. Additional data across racial and ethnic groups should also be encouraged.
Standardized measures: For patients with AF, a standardized measure might be useful to assess symptoms and/or QOL and impact of management strategies.
SDOH: The measurement and impact of SDOH in AF is underutilized and understudied.
Supplementary Material
Figure 2. Prevalence of AF Among Medicare Beneficiaries, 1993–2007.

(A) In the overall cohort, (B) by age group, (C) by sex, and (D) by race. The dashed lines in panel A represent 95% CIs. Reproduced with permission from Piccini et al.8 Copyright 2012 American Heart Association, Inc. AF indicates atrial fibrillation.
Figure 13. Active Bleeding Associated With Oral Anticoagulant.
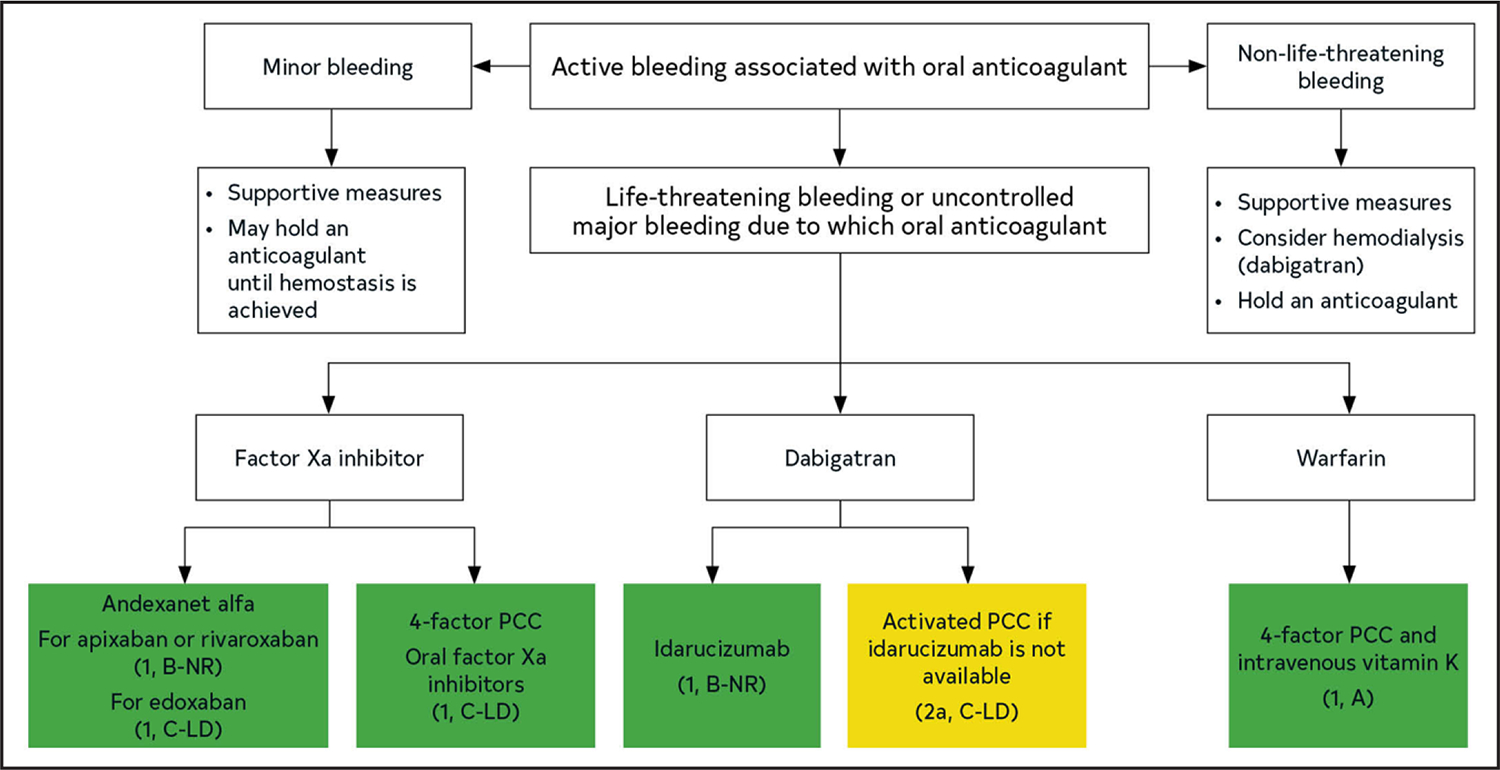
Colors correspond to Table 2. PCC indicates prothrombin complex concentrate.
Figure 15. Flowchart: Management of Periprocedural Anticoagulation in Patients With AF.
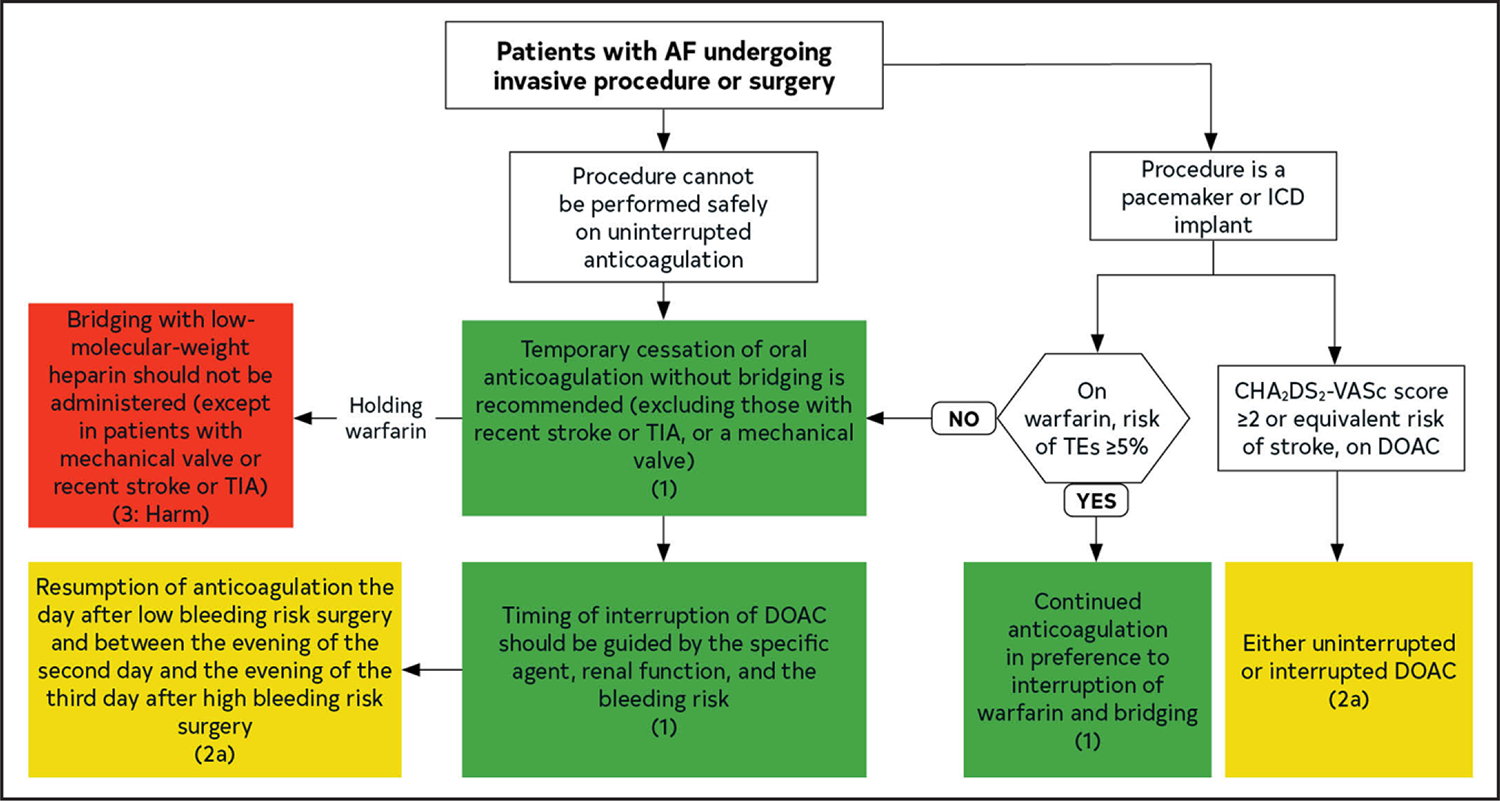
Colors correspond to Table 2. AF indicates atrial fibrillation; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 y (doubled), diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism (doubled), vascular disease, age 65 to 74 y, sex category; DOAC, direct oral anticoagulant; ICD, implantable cardioverter-defibrillator; TE, thromboembolism; and TIA, transient ischemic attack.
Figure 16. Anticoagulation for Typical (CTI-Dependent) AFL.
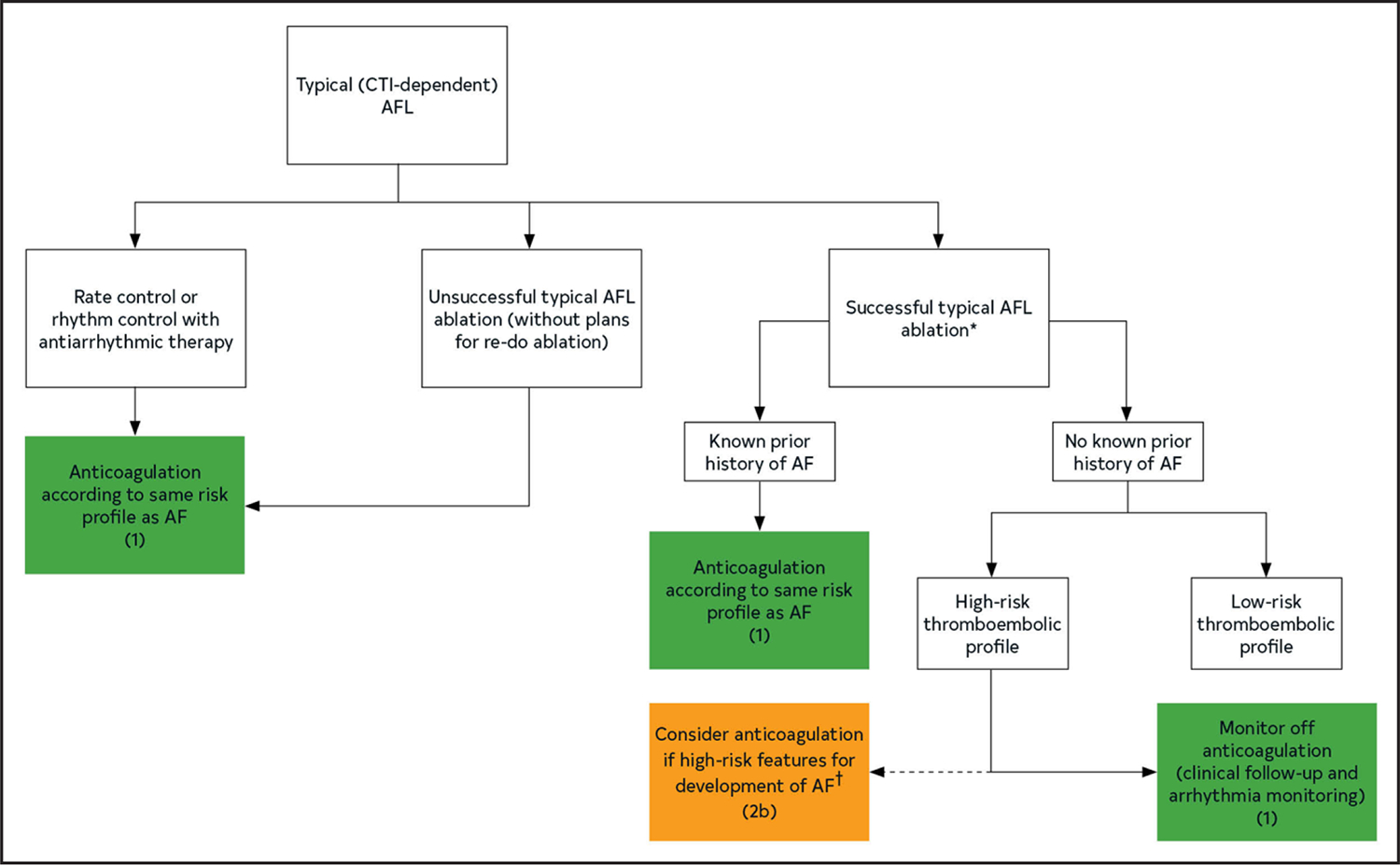
*Intraprocedural documentation of bidirectional block. †For example, left atrial enlargement, inducible AF, COPD, concomitant heart failure. Colors correspond to Table 2. AF indicates atrial fibrillation; AFL, atrial flutter; COPD, chronic obstructive pulmonary disease; and CTI, cavotricuspid isthmus.
Figure 19. Patient and Clinical Considerations for Choosing Between Rhythm Control and Rate Control.
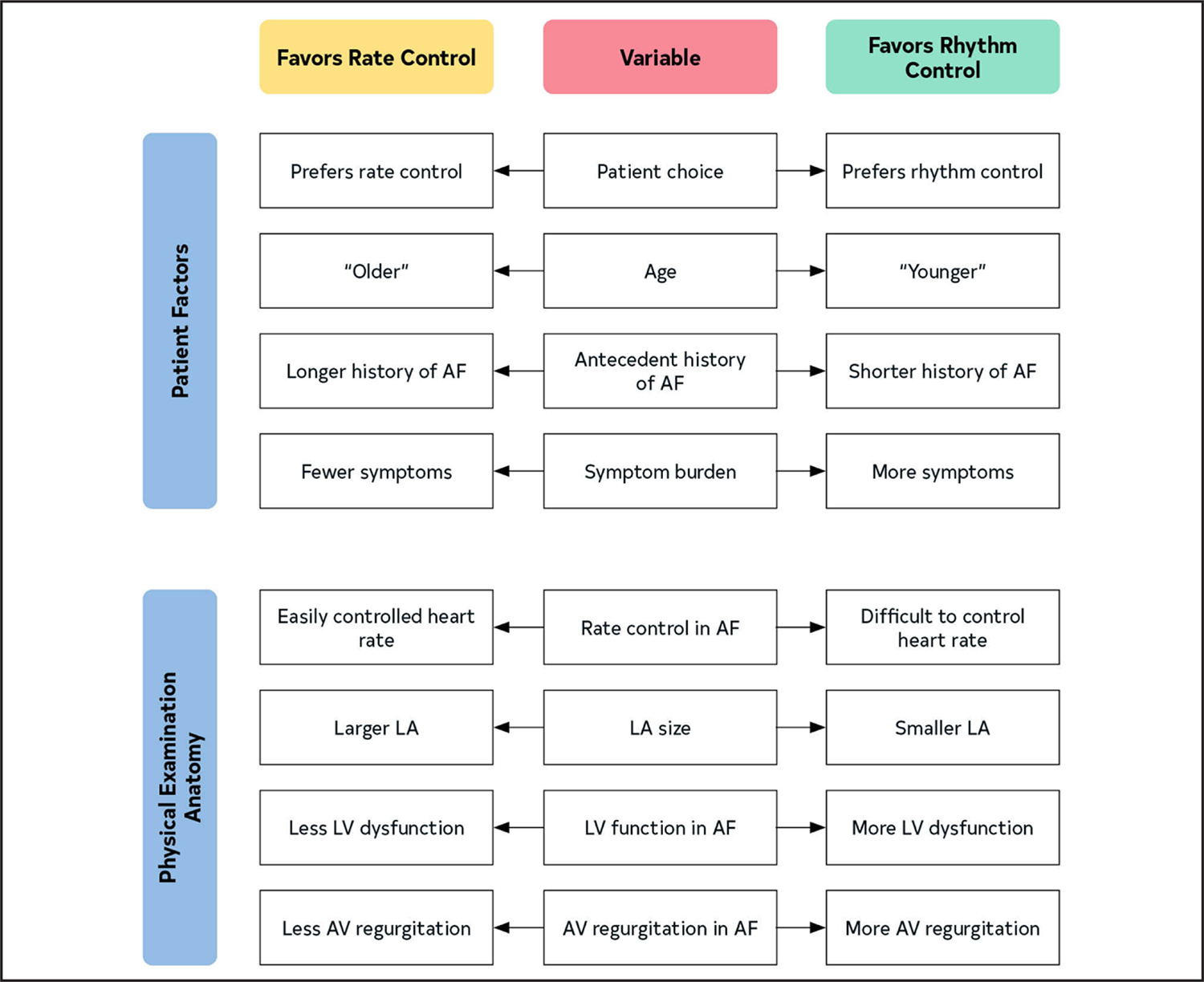
Patient and clinical considerations for deciding between rhythm- and rate-control strategies in a patient with a high burden of AF. AF indicates atrial fibrillation; AV, atrioventricular; LA, left atrium; and LV, left ventricular.
Figure 20. Flowchart for Treatment Choices When Required to Decrease AF Burden.
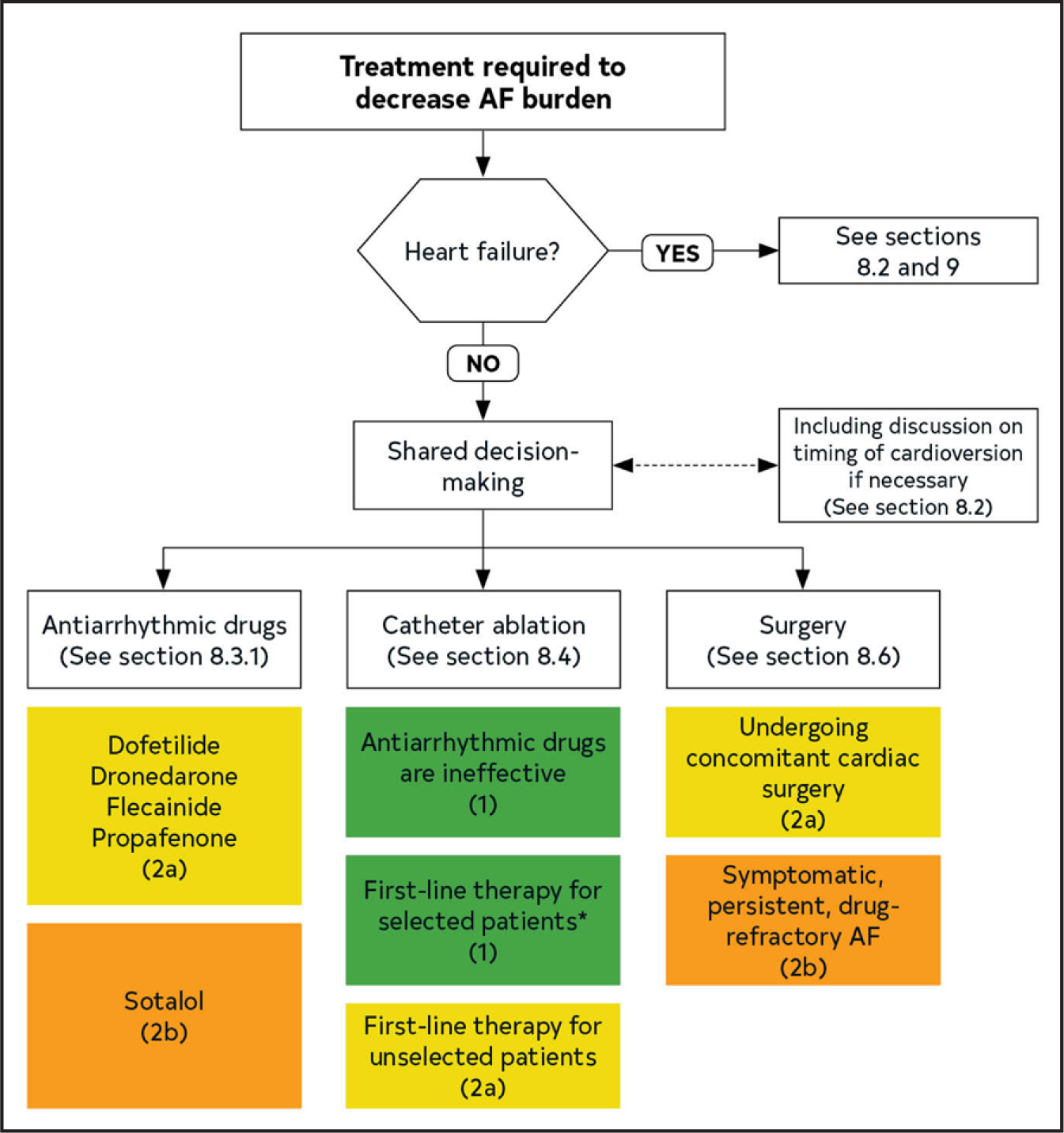
*Younger with few comorbidities. Colors correspond to Table 2. AF indicates atrial fibrillation.
Figure 21. Patients With Hemodynamically Stable AF Planned for Cardioversion.
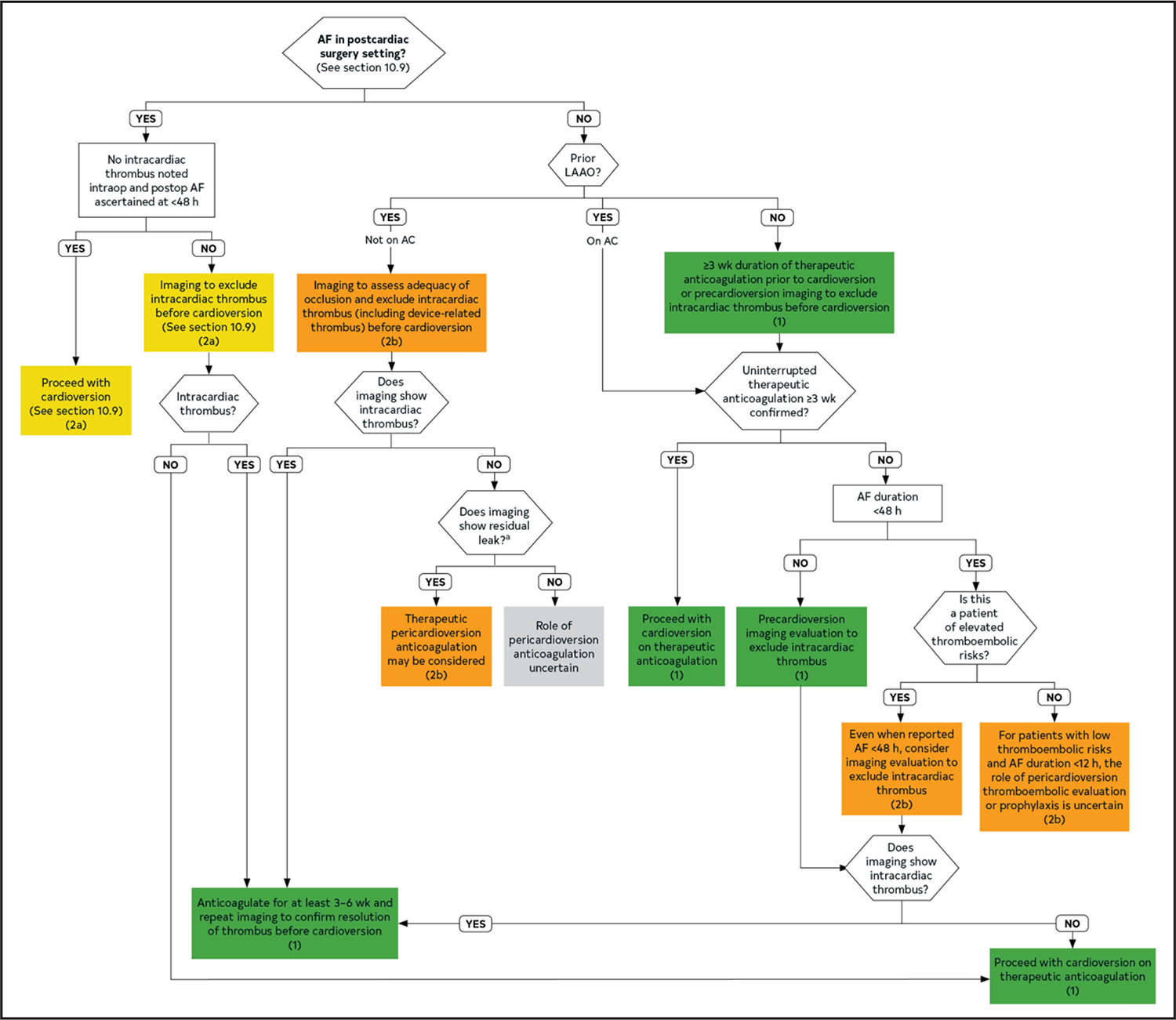
Colors correspond to Table 2. AC indicates anticoagulation; AF, atrial fibrillation; and LAAO, left atrial appendage occlusion.
Figure 22. Treatment Algorithm for Pharmacological Conversion of AF to Sinus Rhythm.
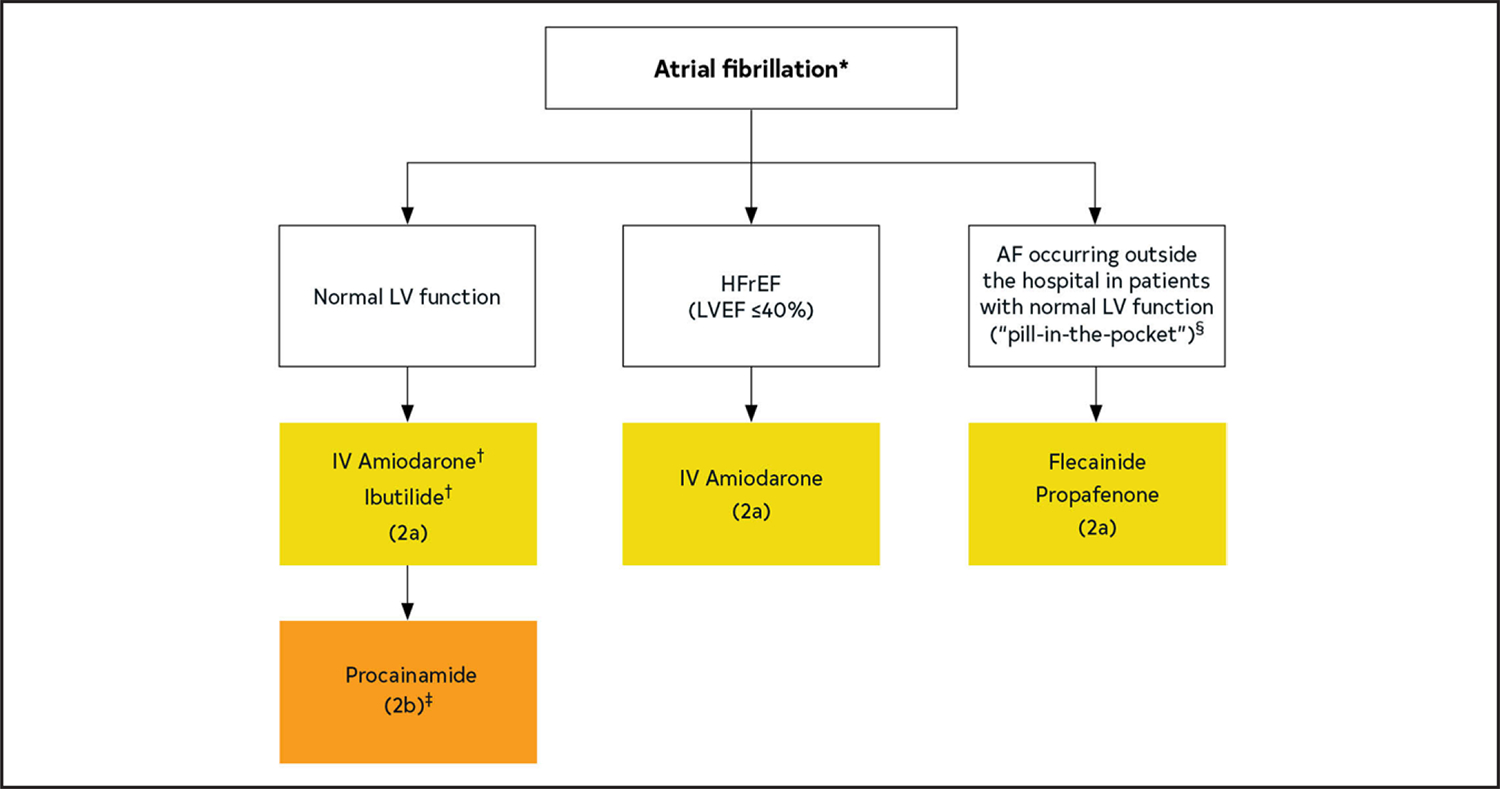
*In the absence of preexcitation. †First dose administered in a facility that can provide continuous electrocardiographic monitoring and cardiac resuscitation because of the potential for proarrhythmia or postconversion bradycardia. ‡IV amiodarone requires several hours for efficacy; ibutilide is generally effective in 30 to 90 min but carries a higher risk of QT interval prolongation and torsades de pointes. §Recommend avoidance of IV procainamide for patients initially treated with amiodarone or ibutilide to avoid excessive QT interval prolongation and torsades de pointes. Rather, procainamide may be considered for patients for whom amiodarone and ibutilide are not considered optimal as first-line drugs. Colors correspond to Table 2. AF indicates atrial fibrillation; HFrEF, heart failure with reduced ejection fraction; IV, intravenous; LV, left ventricular; LVEF, left ventricular ejection fraction.
Figure 23. Treatment Algorithm for Drug Therapy for Maintenance of Sinus Rhythm.
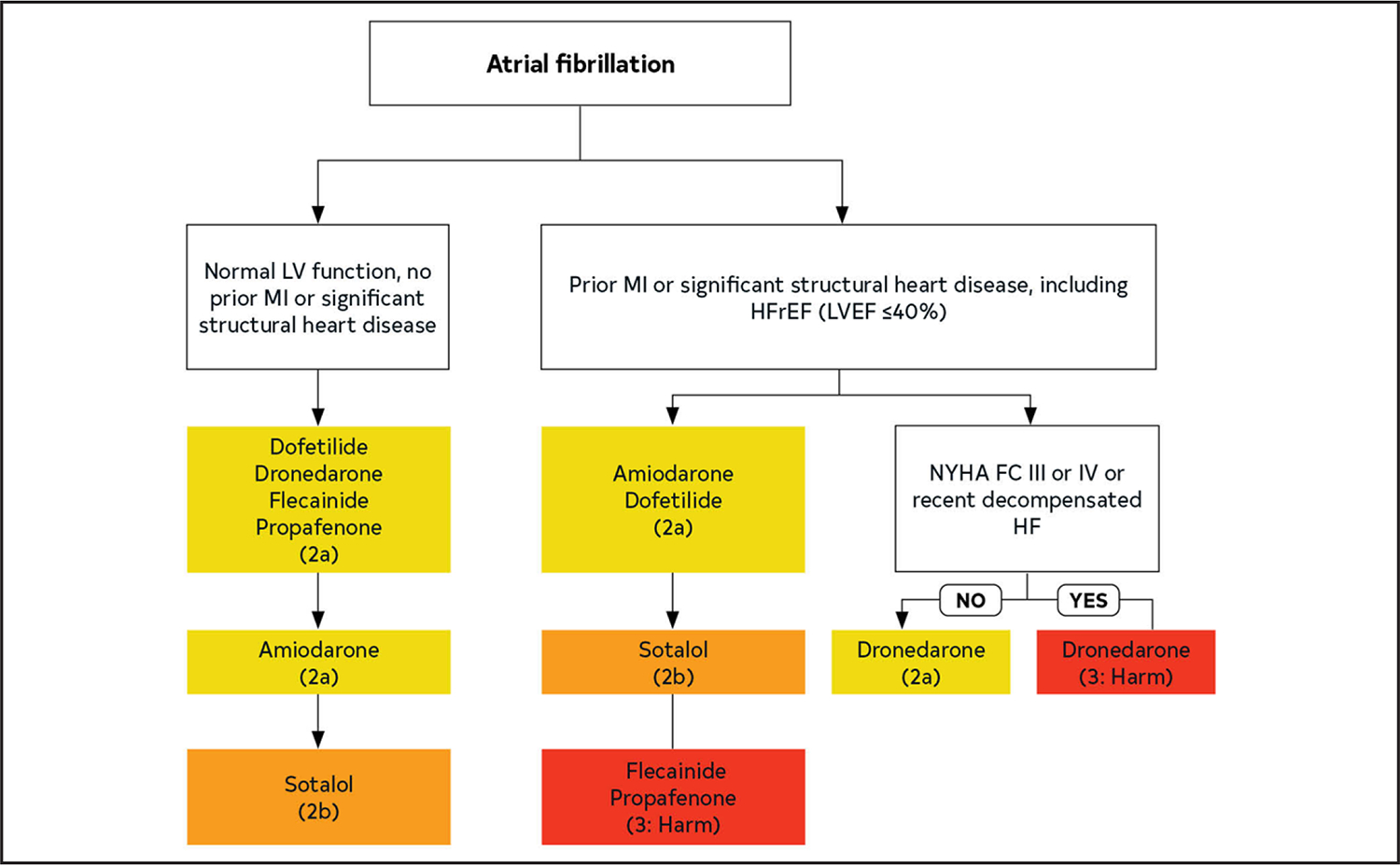
In each box, drugs are listed in alphabetical order. Significant structural heart disease with scar or fibrosis. Colors correspond to Table 2. HFrEF indicates heart failure with reduced ejection fraction; HF, heart failure; IV, intravenous; LV, left ventricular; LVEF, left ventricular ejection fraction; MI, myocardial infarction; and NYHA FC, New York Heart Association functional class.
Figure 25. Prevention of AF After Cardiac Surgery.
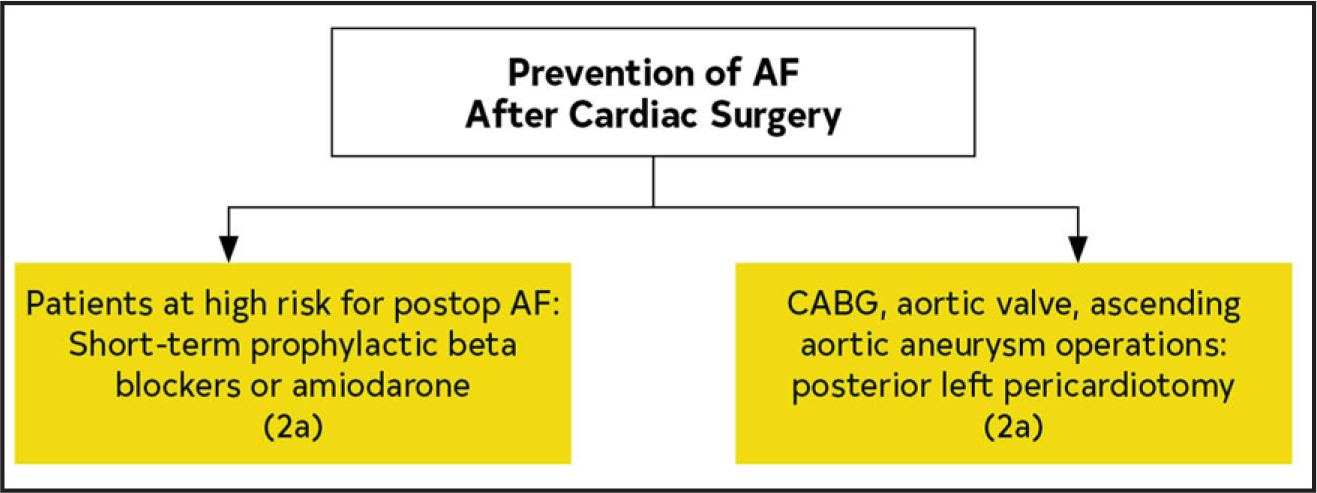
Colors correspond to Table 2. AF indicates atrial fibrillation; and CABG, coronary artery bypass graft.
Figure 26. Treatment of AF After Cardiac Surgery.

Colors correspond to Table 2. AF indicates atrial fibrillation; and HR, heart rate.
Table 4.
Definitions
| Term | Definition |
|---|---|
| AF | A supraventricular tachyarrhythmia with uncoordinated atrial activation and ineffective atrial contraction |
| Electrocardiographic characteristics include (1) irregular R-R intervals (when atrioventricular conduction is present), (2) absence of distinct P waves, and (3) irregular atrial activity also known as fibrillatory waves. AF can be documented by, for example, 12-lead ECG, rhythm strips, wearables, intracardiac electrograms, but will always require visual confirmation that the diagnosis is accurate. | |
| Clinical AF | With the increasing availability of wearable devices and other continuous monitoring technologies, the distinction between clinical and subclinical AF has become increasingly blurred, thus the writing committee felt the term clinical AF has become less useful. Yet, the term was kept because most of the evidence from randomized trials that have led to guideline recommendations for the treatment of AF refer to “clinical AF.” These trials required electrocardiographic documentation of the arrhythmia for inclusion and most patients presented for clinical evaluation and/or therapy of the arrhythmia. |
| Subclinical AF | Subclinical AF refers to this arrhythmia identified in individuals who do not have symptoms attributable to AF and in whom there are no previous ECGs documenting AF This includes AF identified by implanted devices (pacemakers, defibrillators, or implantable loop recorders) or wearable monitors |
| Atrial high-rate episodes | These are defined as atrial events exceeding the programmed detection rate limit set by the device. These are recorded by implanted devices but require visual inspection to confirm AF and exclude other atrial arrhythmias, artifact or oversensing. |
| AF burden | AF burden encompasses both frequency and duration and refers to the amount of AF that an individual has. AF burden has been defined differently across studies. For the purpose of this guideline, AF burden will be defined as the durations of an an episode or as a percentage of AF duration during the monitoring period depending on how it was defined in the individual studies. |
| First detected AF | The first documentation of AF, regardless of previous symptoms |
| Paroxysmal AF | AF that is intermittent and terminates within ≤7 d of onset |
| Persistent AF | AF that is continuous and sustains for >7 d and requires intervention. Of note, patients with persistent AF who, with therapy, become paroxysmal should still be defined as persistent as this reflects their original pattern and is more useful to predict outcomes and define substrate. |
| Long-standing persistent AF | AF that is continuous for >12 mo in duration |
| Permanent AF | A term that is used when the patient and clinician make a joint decision to stop further attempts to restore and/or maintain sinus rhythm Acceptance of AF represents a therapeutic decision and does not represent an inherent pathophysiological attribute of AF |
| Terms considered obsolete | |
| Chronic AF | This historical term has had variable definitions and should be abandoned. It has been replaced by the “paroxysmal,” “persistent,” “longstanding persistent,” and “permanent” terminology. |
| Valvular and nonvalvular AF | The distinction between “valvular” and “nonvalvular “AF remains a matter of debate. Their definitions may be confusing. Recent trials comparing vitamin K antagonists with non-vitamin K antagonist oral anticoagulants in AF were performed among patients with so-called “nonvalvular” AF. These trials have all allowed native valvular heart disease other than mitral stenosis (mostly moderate and severe) and prosthetic heart valves to be included. We should no longer consider the classification of AF as “valvular” or “nonvalvular” for the purpose of defining the etiology of AF, since the term was specific for eligibility of stroke risk reduction therapies. Valvular and nonvalvular terminology should be abandoned. |
| Lone AF | This term has been used in the past to identify AF in younger patients without structural heart disease who are at a lower risk for thromboembolism. This term does not enhance patient care, is not currently used, and should be abandoned. |
AF indicates atrial fibrillation.
Table 10.
Risk Factor Definitions for CHA2DS2-VASc Score as in the Original Article2
| C | Heart Failure | The presence of signs and symptoms of either right (elevated central venous pressure, hepatomegaly, dependent edema) or left ventricular failure (exertional dyspnea, cough, fatigue, orthopnea, paroxysmal nocturnal dyspnea, cardiac enlargement, rales, gallop rhythm, pulmonary venous congestion) or both, confirmed by noninvasive or invasive measurements demonstrating objective evidence of cardiac dysfunction |
| H | Hypertension | A resting blood pressure >140 mm Hg systolic and/or >90 mm Hg diastolic on at least 2 occasions or current antihypertensive pharmacological treatment |
| A2 | Age, additional risk/point | Age ≥75 y |
| D | Diabetes | Fasting plasma glucose level ≥70 mmol/L (126 mg/dL) or treatment with hypoglycemic agent and/or insulin |
| S2 | Thromboembolism | Either an ischemic stroke, transient ischemic attack, peripheral embolism, or pulmonary embolism |
| V | Vascular Disease | Coronary artery disease (prior myocardial infarction, angina pectoris, percutaneous coronary intervention, or coronary artery bypass surgery) or peripheral vascular disease (the presence of any of the following: intermittent claudication, previous surgery or percutaneous intervention on the abdominal aorta or the lower extremity vessels, abdominal or thoracic vascular surgery, arterial and venous thrombosis) |
| A | Age standard risk/weight | Age 65–74 y |
| Sc | Sex Category | Female sex |
Modified with permission from Lip et al.2 Copyright 2010, with permission from Elsevier.
Table 11.
Additional Risk Factors That Increase Risk of Stroke Not Included in CHA2DS2-VASc
| Higher AF burden/Long duration |
| Persistent/permanent AF versus paroxysmal |
| Obesity (BMI, ≥30 kg/m2) |
| HCM |
| Poorly controlled hypertension |
| eGFR (<45 mL/h) |
| Proteinuria (>150 mg/24 h or equivalent) |
| Enlarged LA volume (≥73 mL) or diameter (≥4.7 cm) |
AF indicates atrial fibrillation; BMI, body mass index; eGFR, estimated glomerular filtration rate; HCM, hypertrophic cardiomyopathy; and LA, left atrium.
Table 12.
Thromboembolic Event Rates by Point Score for ATRIA, CHADS2, and CHA2DS2-VASc Risk Scores*
| ATRIA | CHADS2† | CHA2DS2-VASc‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Points | Events | Person-Years | Rate per 100 Person-Years | Events | Person-Years | Rate per 100 Person-Years | Events | Person-Years | Rate per 100 Person-Years |
| 0 | 2 | 2652 | 0.08 | 22 | 6126 | 0.36 | 1 | 2493 | 0.04 |
| 1 | 12 | 2819 | 0.43 | 121 | 10 084 | 1.20 | 21 | 3806 | 0.55 |
| 2 | 14 | 1419 | 0.99 | 253 | 9757 | 2.59 | 46 | 5560 | 0.83 |
| 3 | 13 | 1780 | 0.73 | 178 | 4782 | 3.72 | 121 | 7305 | 1.66 |
| 4 | 19 | 2960 | 0.64 | 81 | 1309 | 6.19 | 193 | 6898 | 2.80 |
| 5 | 36 | 3614 | 0.99 | 19 | 450 | 4.23 | 175 | 4057 | 4.31 |
| 6 | 83 | 4346 | 1.91 | 11 | 101 | 10.84 | 85 | 1783 | 4.77 |
| 7 | 119 | 4768 | 2.50 | – | – | – | 24 | 498 | 4.82 |
| 8 | 151 | 3913 | 3.86 | – | – | – | 14 | 179 | 782 |
| 9 | 104 | 2400 | 4.33 | – | – | – | 5 | 30 | 16.62 |
| 10 | 75 | 1181 | 6.35 | – | – | – | – | – | – |
| 11 | 31 | 501 | 6.18 | – | – | – | – | – | – |
| 12 | 20 | 183 | 10.95 | – | – | – | – | – | – |
| 13 | 4 | 53 | 752 | – | – | – | – | – | – |
| 14 | 2 | 12 | 16.36 | – | – | – | – | – | – |
| 15 | 0 | 7 | 0 | – | – | – | – | – | – |
| All | 685 | 32 609 | 2.10 | – | – | – | – | – | – |
Reproduced with permission from Singer et al.1 Copyright 2013 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley-Blackwell. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Black lines identify thresholds for low-, moderate-, and high-risk categories for the 3 stroke risk point scores using published cut points.8,9
The CHADS2 score assigns points as follows: 1 point each for the presence of congestive heart failure, hypertension, age ≥75 y, and diabetes mellitus and 2 points for history of stroke/transient ischemic attack.
The CHA2DS2-VASc score assigns points as follows: 1 point each for congestive heart failure/left ventricular dysfunction, hypertension, diabetes mellitus, vascular disease, age 65 to 74 y, and female sex, and 2 points each for age ≥75 y and stroke/transient ischemic attack/thromboembolism.
ATRIA indicates Anticoagulation and Risk Factors in Atrial Fibrillation: anemia, renal disease, elderly (age ≥75 y), any previous bleeding, hypertension; CHADS2, congestive heart failure, hypertension, age >75 y, diabetes, stroke/transient ischemia attack/thromboembolism; and CHA2DS2-VASc, indicates congestive heart failure, hypertension, age ≥75 y (doubled), diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism (doubled), vascular disease, age 65 to 74 y, sex category.
Table 21.
Pharmacological Agents for Rate Control in Patients With AF
| Intravenous Administration | Oral Maintenance Dose | Elimination Half-Life | Notes | |
|---|---|---|---|---|
| Beta blockers | ||||
| Metoprolol tartrate | 2.5–5 mg bolus over 2 min; up to 3 doses | 25–200 mg, twice daily | 3–4 h | |
| Metoprolol succinate | N/A | 50–400 mg daily or twice daily in divided doses | 3–7 h | |
| Atenolol | N/A | 25–100 mg daily | 6–7 h | Renally eliminated |
| Bisoprolol | N/A | 2.5–10 mg daily | 9–12 h | |
| Carvedilol | N/A | 3.125–25 mg, twice daily | 7–10 h | |
| Esmolol | 500 μg/kg bolus over 1 min; then 50–300 μg/kg/min | N/A | 9 min | |
| Nadolol | N/A | 10–240 mg daily | 20–24 h | |
| Propranolol | 1 mg over 1 min; repeat as needed every 2 min; up to 3 doses | 10–40 mg, 3–4 times daily | IV: 2.4 h Oral: 3–6 h ER: 8–20 h |
|
| Nondihydropyridine calcium channel blockers | ||||
| Diltiazem | 0.25 mg/kg (actual body weight) IV over 2 min May repeat 0.35 mg/kg over 2 min; then 5–15 mg/h continuous infusion | 120–360 mg daily (ER) | IV: 3–5 h Oral immediate release: 3–4.5 h ER: 4–9.5 h |
Avoid in HFrEF |
| Verapamil | 5–10 mg over ≥2 min (may repeat twice); then 5 mg/h continuous infusion (max 20 mg/h) | 180–480 mg daily (ER) | IV: 6–8 h Oral: 2–7 h ER: 12–17 h |
Avoid in HFrEF |
| Digitalis glycoside | ||||
| Digoxin | 0.25–0.5 mg over several min; repeat doses of 0.25 mg every 6 h (maximum 1.5 mg/24 h) | 0.0625–0.25 mg daily | 1–2 d | Renally eliminated Increased mortality at plasma concentrations exceeding 1.2 ng/mL |
| Other | ||||
| Amiodarone | 150–300 mg IV over 1 h, then 10–50 mg/h over 24 h | 100–200 mg daily (generally IV form used for rate control) | IV: 9–36 d Oral: 26–107 d |
Loading dose 6–10 g administered over 2–4 wk; can combine IV and oral dosing to complete |
AF indicates atrial fibrillation; ER, extended release; HFrEF, heart failure with reduced ejection fraction; IV, intravenous; and N/A, not applicable.
Table 23.
Specific Drug Therapy for Maintenance of Sinus Rhythm in Patients With AF
| Drug | Loading Dose | Maintenance Dose | Primary Route(s) of Elimination | Elimination Half-Life | Mechanism of Action | Major Adverse Effects | Important Pharmacokinetic Drug Interactions |
|---|---|---|---|---|---|---|---|
| Amiodarone | Total loading dose 6–10 g, given 400–800 mg daily in 2–4 divided doses for 1–4 wk | 200 mg once daily | Liver metabolism Biliary excretion |
14–59 d | lnhibits IKr, IKs, INa, IKur, Ito, ICa-L, IKAch Noncompetitive beta blocker | AV block Bradycardia Corneal microdeposits Elevation in transaminases Hepatotoxicity Hyperthyroidism Hypothyroidism Nausea QT prolongation Peripheral neuropathy Photosensitivity Pulmonary fibrosis Skin pigmentation (blue-gray) TdP |
Moderate* inhibitor of CYP2C9, weak† inhibitor of CYP2D6 Some inhibition of CYP3A lncreases plasma concentrations of warfarin, lovastatin,‡ simvastatin,§ cyclosporine lnhibits p-gp lncreases plasma concentrations of digoxin |
| Dofetilide | N/A | CrCl >60 mL/min: 500 μg twice daily CrCl 40–60 mL/min: 250 μg twice daily CrCl 20–40 mL/min: 125 μg twice daily CrCl <20 mL/min: Contraindicated |
Kidney | 10 h | Inhibits IKr and aigments late INa | QT prolongation TdP |
Dofetilide is renally excreted via the renal cation transport system. These drugs inhibit renal cation transport, increase plasma dofetilide concentrations, and are contraindicated in patients taking dofetilide: Cimetidine Dolitegravir Ketoconazole Megestrol Prochlorperazine Trimethoprim (alone or in combination with sulfamethoxazole) Verapamil ln addition, hydrochlorothiazide (alone or in combination with triamterene) increases plasma dofetilide concentrations and should not be coadministered with dofetilide |
| Dronedarone | N/A | 400 mg twice daily | Liver metabolism | 13–19 h | lnhibits IKr IKs, INa, IKur, Ito, ICa-L, IKAch Noncompetitive beta blocker | Abdominal pain Asthenia Bradycardia Diarrhea Nausea and vomiting QT prolongation Rash TdP |
Dronedarone is a substrate for CYP3A and is a moderate inhibitor of CYP3A and CYP2D6 Dronedarone is also a substrate for, and inhibitor of, p-gp Dronedarone may increase plasma concentrations of: Dabigatran Digoxin Simvastatin∥ Sirolimus Tacrolimus Warfarin These drugs may increase plasma dronedarone concentrations: Grapefruit juice These drugs may decrease plasma dronedarone concentrations: CYP3A inducers including St. John’s wort, rifampin, and phenytoin |
| Flecainide | N/A | 50–300 mg/d PO divided q 8–12 h | Liver (70%) Kidney (30%)¶ |
12–27 h | Inhibits INa | Atrial flutter AV block Dizziness Dyspnea Exacerbation of HFrEF Headache Nausea QT prolongation VT Visual disturbances |
Flecainide is a substrate for CYP2D6 These drugs may increase plasma flecainide concentrations: Amiodarone Duloxetine Fluoxetine Paroxetine |
| Propafenone | N/A | 150–300 mg PO q 8 h, ER 225–425 PO q 12 h | Liver | 9 h | Inhibits INa | Atrial flutter Bradycardia AV block Dizziness Dyspnea Exacerbation of HFrEF Nausea Taste disturbances VT Visual disturbances |
Propafenone is a substrate for CYP2D6 These drugs may increase plasma propafenone concentrations: Fluoxetine Paroxetine Propafenone may increase plasma digoxin concentrations Propafenone may increase plasma warfarin concentrations |
| Sotalol | CrCl >60 mL/min: 40–80 mg twice daily for 3 d CrCl: 40–60 mL/min: 80 mg once daily for 3 d CrCl <40 mL/min: Contraindicated |
CrCl >60 mL/min: 80–160 mg twice daily CrCl: 40–60 mL/min: 80–160 mg once daily CrCl <40 mL/min: Contraindicated |
Kidney | 12 h | Inhibits IKr Beta blocker d-Sotalol augments late INa | AV block Bradycardia Bronchospasm Diarrhea Exacerbation of HFrEF Fatigue Nausea and vomiting QT prolongation TdP |
None |
Moderate inhibitor: Causes a 2-fold to <5-fold increase in AUC or a 50% to 80% decrease in clearance.
Mild inhibitor: Causes a ≥1.25-fold but <2-fold increase in AUC or a 20% to 50% decrease in clearance.
Lovastatin doses should not exceed 40 mg daily in patients taking amiodarone.
Simvastatin doses should not exceed 20 mg daily in patients taking amiodarone.
Simvastatin doses should not exceed 10 mg daily in patients taking dronedarone.
Percentage of a dose excreted unchanged in urine.
AF indicates atrial fibrillation; AUC, area under the plasma concentration versus time curve; AV, atrioventricular; CrCl, creatinine clearance; CYP cytochrome P-450; ER, extended release; HFrEF, heart failure with reduced ejection fraction; IV, intravenous; N/A, not applicable; NAPA, N-acetylprocainamide; p-gp, p-glycoprotein; PO, orally; TdP, torsades de pointes; and VT, ventricular tachycardia.
TOP 10 TAKE-HOME MESSAGES.
Stages of atrial fibrillation (AF): The previous classification of AF, which was based only on arrhythmia duration, although useful, tended to emphasize therapeutic interventions. The new proposed classification, using stages, recognizes AF as a disease continuum that requires a variety of strategies at the different stages, from prevention, lifestyle and risk factor modification, screening, and therapy.
AF risk factor modification and prevention: This guideline recognizes lifestyle and risk factor modification as a pillar of AF management to prevent onset, progression, and adverse outcomes. The guideline emphasizes risk factor management throughout the disease continuum and offers more prescriptive recommendations, accordingly, including management of obesity, weight loss, physical activity, smoking cessation, alcohol moderation, hypertension, and other comorbidities.
Flexibility in using clinical risk scores and expanding beyond CHA2DS2-VASc for prediction of stroke and systemic embolism: Recommendations for anticoagulation are now made based on yearly thromboembolic event risk using a validated clinical risk score, such as CHA2DS2-VASc. However, patients at an intermediate annual risk score who remain uncertain about the benefit of anticoagulation can benefit from consideration of other risk variables to help inform the decision, or the use of other clinical risk scores to improve prediction, facilitate shared decision making, and incorporate into the electronic medical record.
Consideration of stroke risk modifiers: Patients with AF at intermediate to low (<2%) annual risk of ischemic stroke can benefit from consideration of factors that might modify their risk of stroke, such as the characteristics of their AF (eg, burden), nonmodifiable risk factors (sex), and other dynamic or modifiable factors (blood pressure control) that may inform shared decision-making discussions.
Early rhythm control: With the emergence of new and consistent evidence, this guideline emphasizes the importance of early and continued management of patients with AF that should focus on maintaining sinus rhythm and minimizing AF burden.
Catheter ablation of AF receives a Class 1 indication as first-line therapy in selected patients: Recent randomized studies have demonstrated the superiority of catheter ablation over drug therapy for rhythm control in appropriately selected patients. In view of the most recent evidence, we upgraded the Class of Recommendation.
Catheter ablation of AF in appropriate patients with heart failure with reduced ejection fraction receives a Class 1 indication: Recent randomized studies have demonstrated the superiority of catheter ablation over drug therapy for rhythm control in patients with heart failure and reduced ejection failure. In view of the data, we upgraded the Class of Recommendation for this population of patients.
Recommendations have been updated for device-detected AF: In view of recent studies, more prescriptive recommendations are provided for patients with device-detected AF that consider the interaction between episode duration and the patienťs underlying risk for thromboembolism. This includes considerations for patients with AF detected via implantable devices and wearables.
Left atrial appendage occlusion devices receive higher level Class of Recommendation: In view of additional data on safety and efficacy of left atrial appendage occlusion devices, the Class of Recommendation has been upgraded to 2a compared with the 2019 AF Focused Update for use of these devices in patients with long-term contraindications to anticoagulation.
Recommendations are made for patients with AF identified during medical illness or surgery (precipitants): Emphasis is made on the risk of recurrent AF after AF is discovered during noncardiac illness or other precipitants, such as surgery.
Future Research Needs.
Verma A, Ha ACT, Kirchhof P, et al. The Optimal Anti-Coagulation for Enhanced-Risk Patients Post-Catheter Ablation for Atrial Fibrillation (OCEAN) trial. Am Heart J. 2018;197:124–132.
Schrickel JW, Linhart M, Bänsch D, et al. Rationale and design of the ODIn-AF Trial: randomized evaluation of the prevention of silent cerebral thromboembolism by oral anticoagulation with dabigatran after pulmonary vein isolation for atrial fibrillation. Clin Res Cardiol. 2016;105:95–105.
Riley MP, Zado E, Hutchinson MD, et al. Risk of stroke or transient ischemic attack after atrial fibrillation ablation with oral anticoagulant use guided by ECG monitoring and pulse assessment. J Cardiovasc Electrophysiol. 2014;25:591–596.
Passman R, Leong-Sit P, Andrei AC, et al. Targeted anticoagulation for atrial fibrillation guided by continuous rhythm assessment with an insertable cardiac monitor: the Rhythm Evaluation for Anticoagulation With Continuous Monitoring (REACTCOM). Pilot Study. J Cardiovasc Electrophysiol. 2016;27:264–270.
Abbreviations
- ARB
angiotensin receptor blocker
- AT
atrial tachycardia
- AVNA
atrioventricular nodal ablation
- BiVP
biventricular pacing
- BMI
body mass index
- BP
blood pressure
- bpm
beats per minute
- BTK
Bruton’s tyrosine kinase
- CABG
coronary artery bypass graft surgery
- CAD
coronary artery disease
- CCD
chronic coronary disease
- ACE
angiotensin-converting enzyme
- ACHD
adult congenital heart disease
- ACS
acute coronary syndrome
- AF
atrial fibrillation
- AFL
atrial flutter
- AHRE
atrial high-rate episodes
- ANS
autonomic nervous system
- AP
accessory pathway
- APT
antiplatelet therapy
- CHADS2
congestive heart failure, hypertension, age >75 years, diabetes mellitus, stroke/transient ischemia attack/thromboembolism
- CHA2DS2VASc
congestive heart failure, hypertension, age ≥75 years (doubled), diabetes mellitus, prior stroke or transient ischemic attack or thromboembolism (doubled), vascular disease, age 65 to 74 years, sex category
- CHD
congenital heart disease
- CHF
congestive heart failure
- CKD
chronic kidney disease
- COPD
chronic obstructive pulmonary disease
- COR
Class of Recommendation
- CrCl
creatinine clearance
- CRT
cardiac resynchronization therapy
- CTI
cavotricuspid isthmus
- CVD
cardiovascular disease
- DAT
dual antithrombotic therapy
- DOAC
direct oral anticoagulant
- ECG
Electrocardiogram
- EF
ejection fraction
- eGFR
estimated glomerular filtration rate
- FDA
US Food and Drug Administration
- GDMT
guideline-directed management and therapy
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HR
hazard ratio
- ICD
implantable cardioverter-defibrillator
- ICH
intracranial hemorrhage
- LA
left atrium
- LAA
left atrial appendage
- LAAO
left atrial appendage occlusion
- LOE
Level of Evidence
- LRFM
lifestyle risk factor modification
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- MI
myocardial infarction
- MRA
mineralocorticoid receptor antagonist
- NYHA
New York Heart Association
- OAC
oral anticoagulant
- PAC
premature atrial contraction
- PAD
peripheral artery disease
- PCI
percutaneous coronary intervention
- PCC
prothrombin complex concentrate
- PE
pulmonary embolism
- PH
pulmonary hypertension
- PHPVD
pulmonary hypertension with pulmonary vascular disease
- PITP
pill-in-the-pocket
- pLAAO
percutaneous left atrial appendage occlusion
- PV
pulmonary veins
- PVI
pulmonary vein isolation
- QOL
quality of life
- RAAS
renin-angiotensin-aldosterone system
- RCT
randomized controlled trial
- RV
right ventricular
- RVP
right ventricular pacing
- SDB
sleep-disordered breathing
- SDOH
social determinants of health
- SDM
shared decision-making
- S-LAAO
surgical removal of the left atrial appendage occlusion
- SVT
supraventricular tachycardia
- TEE
transesophageal echocardiogram
- TIA
transient ischemic attack
- UREG
underrepresented racial and ethnic groups
- VF
ventricular fibrillation
- VHD
valvular heart disease
- VKA
vitamin K antagonist
- VT
ventricular tachycardia
- WPW
Wolff-Parkinson-White
Appendix 1. Author Relationships With Industry and Other Entities—2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation
| Committee Member | Employment | Consultant | Speakers Bureau | Ownership/Partnership/Principal | Personal Research | Institutional, Organizational, or Other Financial Benefit | Expert Witness |
|---|---|---|---|---|---|---|---|
| José A. Joglar, Chair | UT Southwestern Medical Center– Professor and Vice Chair for Clinical Affairs at Parkland, Department of Internal Medicine Elizabeth Thaxton and Ellis Batten Page Professorship in Cardiac Electrophysiology Research Director of Cardiac Electrophysiology Fellowship Program | None | None | None | None | None | None |
| Mina K. Chung, Vice Chair | Cleveland Clinic, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University–Cardiac Pacing and Electrophysiology Department of Cardiovascular Medicine, Heart and Vascular Institute Department of Cardiovascular & Metabolic Sciences, Lerner Research Institute | NOT RELEVANT • ABIM* • Geisinger Health Systems |
None | None | NOT RELEVANT • AHA* • NIH* |
NOT RELEVANT • Abbott‡ • AHA* • Biosense Webster‡ • Boston Scientific‡ • Elsevier • FNRA • Hamilton Health Sciences/PHRI‡ • HRS† • Medtronic‡ • Myocardial Solutions‡ • UptoDate |
None |
| Anastasia L. Armbruster | St. Louis College of Pharmacy AT UHSP–Associate Professor, Pharmacy Practice | None | RELEVANT • AstraZeneca |
None | None | None | None |
| Emelia J. Benjamin | Boston University Schools of Medicine and Public Health, The Framingham Heart Study–Associate Provost for Faculty Development, Boston University Medical Campus Vice Chair, Faculty Development and Diversity, Department of Medicine Cardiologist, Boston Medical Center, Boston University Medical Group | None | None | None | NOT RELEVANT • AHA* • Cardia (DSMB)† • NIH* |
NOT RELEVANT • Circulation† • European Heart Journal† • Nature Reviews Cardiology† |
None |
| Janice Y. Chyou | Icahn School of Medicine at Mount Sinai–Assistant Clinical Professor, Cardiac Electrophysiology, Cardiology Heart and Heart Rhythm Care | None | None | None | None | NOT RELEVANT • AHA† • HRS† • McGraw Hill |
None |
| Edmond M. Cronin | Temple University Hospital–Main Campus–Temple Heart & Vascular Institute | RELEVANT • Medtronic |
None | None | None | NOT RELEVANT • Biosense Webster§ • Janssen Pharmaceuticals§ • Medtronic§ |
None |
| Anita Deswal | The University of Texas MD Anderson Cancer Center–Chair, Department of Cardiology Ting Tsung and Wei Fong Chao Distinguished Chair Professor of Medicine |
None | None | None | None | NOT RELEVANT • ACC* • CPRIT* • NIH |
None |
| Lee Eckhardt | University of Wisconsin-Madison–Associate Professor of Medicine Gary and Marie Weiner Professor in Cardiovascular Medicine Research Cellular and Molecular Arrhythmia Research Program Division of Cardiovascular Medicine |
None | None | None | None | None | None |
| Zachary D. Goldberger | University of Wisconsin-Madison School of Medicine and Public Health Division of Cardiovascular Medicine/Electrophysiology–Associate Professor of Medicine Program Director, Cardiovascular Disease Fellowship Associate Program Director, Clinical Cardiac Electrophysiology Fellowship |
None | None | None | None | None | None |
| Rakesh Gopinathannair | University of Louisville–Associate Professor of Medicine (Adjunct) University of Missouri-Columbia–Cardiac EP Lab Director, Kansas City Heart Rhythm Institute EP Medical Director, Research Medical Center Professor of Medicine |
RELEVANT • Abbott* • Biosense Webster • Biotronik • Boston Scientific* • Johnson & Johnson • Pfizer* • Sanofi-Aventis • Zoll† NOT RELEVANT • Academy for Continued Healthcare Learning |
RELEVANT • Pfizer† • Zoll† |
None | None | NOT RELEVANT • AHA • Atricure • Geisinger Health System • HRS • Janssen Pharmaceuticals • Kowa Pharmaceuticals • Medtronic • Methodist Health System • Novartis • Pacemate† • Philips |
None |
| Bulent Gorenek | Eskisehir Osmangazi University School of Medicine–Cardiovascular Diseases, Arrhythmias in Intensive Cardiac Care Unit. Professor in Cardiology Vice Director, Cardiology Department | RELEVANT • AstraZeneca • Bayer • Daiichi Sankyo • Roche NOT RELEVANT • Sandoz |
None | None | None | None | None |
| Paul L. Hess | University of Colorado School of Medicine Seattle-Denver Center of Innovation Rocky Mountain Regional VA Medical Center–Associate Professor of Medicine |
None | None | None | None | NOT RELEVANT • CPC Clinical Research* • VA CSP |
None |
| Mark Hlatky | Stanford University School of Medicine–Professor of Health Policy, and of Medicine (Cardiovascular Medicine), and, by courtesy, of Epidemiology and Population Health | RELEVANT • Boehringer Ingelheim NOT RELEVANT • BCBS Center for Effectiveness Evaluation* |
None | None | None | NOT RELEVANT • George Institute |
None |
| Gail Hogan | Patient Representative | None | None | None | None | NOT RELEVANT • Ohio State University Wexner Medical Center |
None |
| Chinwe Ibeh | Columbia University CUIMC/Neurological Institute of New York–Assistant Professor of Neurology Department of Neurology Division of Stroke and Cerebrovascular Disease |
None | None | None | None | None | None |
| Julia H. Indik | University of Arizona College of Medicine–Professor of Medicine Director, Cardiovascular Disease Fellowship Program Division of Cardiology/Sarver Heart Center |
None | None | None | None | None | None |
| Kazuhiko Kido | West Virginia University–Cardiology Pharmacy Specialist, Advanced Heart Failure and Pulmonary Hypertension Clinical Assistant Professor of Clinical Pharmacy | None | None | None | None | None | None |
| Fred Kusumoto | Mayo Clinic Jacksonville | None | None | None | NOT RELEVANT • EP Research Foundation (TAP-CHF Trial) |
NOT RELEVANT • Biotronik§ |
None |
| Mark S. Link | UT Southwestern Medical Center, Dallas–Professor of Medicine. Director, Cardiac Electrophysiology. Laurence and Susan Hirsch/Centex Distinguished Chair in Heart Disease Department of Internal Medicine, Division of Cardiology |
None | None | None | None | NOT RELEVANT • Circulation* • Journal Watch Cardiology • UpToDate* |
None |
| Kathleen T. Linta | Patient Representative | None | None | None | None | None | None |
| Gregory M. Marcus | University of California, San Francisco–Professor of Medicine in Residence Associate Chief of Cardiology for Research Endowed Professor of Atrial Fibrillation Research |
NOT RELEVANT • InCarda* RELEVANT • Johnson & Johnson |
None | None | NOT RELEVANT • Baylis* |
NOT RELEVANT • InCarda* |
None |
| Patrick M. McCarthy | Northwestern Medicine–Executive Director, Bluhm Cardiovascular Institute Vice President, Northwestern Medical Group Chief, Division of Cardiac Surgery Heller-Sacks Professor of Surgery |
RELEVANT • Abbott • Atricure • Edwards Lifesciences* • Medtronic |
None | None | RELEVANT • Abbott† |
NOT RELEVANT • Egnite • Siemens RELEVANT • Edwards Lifesciences* |
None |
| Nimesh Patel | UT Southwestern Medical Center– Assistant Professor of Medicine, Clinical Cardiac Electrophysiology | None | None | None | None | NOT RELEVANT • Abbvie§ • Novo Nordisk§ |
None |
| Kristen K. Patton | University of Washington–Professor of Medicine | None | None | None | None | NOT RELEVANT • ACGME† • AHA† • HRS† • JAMA • US FDA CDRH* |
None |
| Marco V. Perez | Stanford University–Associate Professor-University Med Line Department of Medicine, Med/Cardiovascular | RELEVANT • Biotronik* • Boehringer Ingelheim* • Boston Scientific* • Bristol Myers Squibb • Johnson & Johnson |
None | NOT RELEVANT • Feather Health* |
NOT RELEVANT • Apple* • NIH* |
None | NOT RELEVANT Defendant, Digital health tools case, 2021 |
| Jonathan P. Piccini | Duke University School of Medicine– Department of Medicine | RELEVANT • Abbott* • Abbvie • ARCA Biopharma • Biotronik • Bristol Myers Squibb* • Electrophysiology Frontiers* • Itamar Medical • Medtronic* • Milestone Medical • Philips* • Recor • Sanofi-Aventis* NOT RELEVANT • UpToDate* |
None | None | RELEVANT • Abbott* • Bayer* • Boston Scientific* • iRhythm* • Philips* NOT RELEVANT • AAMI* • Acutus Medical, Inc.§ • AHA* • Atricure§ • Element Science (DSMB)* • LivaNova (CEAC)* • LSI Solutions§ |
NOT RELEVANT • LivaNova (CEAC)* |
None |
| Andrea M. Russo | Cooper University–Professor of Medicine Director, Electrophysiology and Arrhythmia Services Director of Research, Cooper Heart Institute Program Director, Clinical Cardiac Electrophysiology Fellowship |
RELEVANT • Abbott • Atricure • Biosense Webster • Biotonik • Boston Scientific • Bristol Myers Squibb • Medtronic • Pfizer • Sanofi NOT RELEVANT • Medical Device Business Services, Inc. • Pacemate |
None | None | None | NOT RELEVANT • ABIM • Boston Scientific‡ • Kestra‡ • MediLynx‡ • UpToDate |
None |
| Prashanthan Sanders | The University of Adelaide Royal Adelaide Hospital–Director, Centre for Heart Rhythm Disorders Director, Cardiac Electrophysiology & Pacing NHMRC Practitioner Fellow |
None | None | None | RELEVANT • Abbott* • BD* • Boston Scientific* • Medtronic* |
RELEVANT • Abbott • Boston Scientific • CathRX • Medtronic NOT RELEVANT • Pacemate |
None |
| Megan M. Streur | University of Washington–Assistant Professor and Endowed Faculty Fellow for Cardiovascular Disease Prevention Department of Biobehavioral Nursing and Health Informatics Adjunct Assistant Professor, Cardiac Electrophysiology Department of Medicine |
None | None | None | NOT RELEVANT • NIH/NINR* |
None | None |
| Kevin L. Thomas | Duke University School of Medicine– Professor of Medicine Vice Dean for Diversity, Equity and Inclusion Member in the Duke Clinical Research Institute |
RELEVANT • Biosense Webster • Johnson & Johnson |
None | None | None | NOT RELEVANT • Community Health Coalition† |
None |
| Sabrina Times§ | American Heart Association/American College of Cardiology Science and Health Advisor, Guidelines | None | None | None | None | None | None |
| James E. Tisdale | Purdue University College of Pharmacy–Professor Indiana University School of Medicine–Adjunct Professor | None | None | None | NOT RELEVANT • AHRQ* • AHA* • NIH* |
NOT RELEVANT • American Society for Health-Systems Pharmacists • AZCert† • Crediblemeds. org† • Nova Southeastern University • Pharmacotherapy Journal • Pharmacotherapy Publications, Inc. † |
None |
| Anne Marie Valente | Boston Children’s Hospital, Brigham and Women’s Hospital–Co-Director, Pregnancy and Cardiovascular Disease Program Brigham and Women’s Hospital Associate Professor, Medicine and Pediatrics Harvard Medical School Section Chief, Boston Adult Congenital Heart Disease (BACH) and Pulmonary Hypertension Program |
NOT RELEVANT • Elsevier* |
None | None | None | NOT RELEVANT • Elsevier* • Journal of the ACC* • United Therapeutics§ |
None |
| David R. Van Wagoner | Cleveland Clinic, ND-50–Department of Cardiovascular & Metabolic Sciences | None | None | None | NOT RELEVANT • AHA* • NIH/NHLBI* |
None | None |
This table represents all relationships of committee members with industry and other entities that were reported by authors, including those not deemed to be relevant to this document, at the time this document was under development. The table does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest represents ownership of ≥5% of the voting stock or share of the business entity, or ownership of ≥$5000 of the fair market value of the business entity; or if funds received by the person from the business entity exceed 5% of the person’s gross income for the previous year. Relationships that exist with no financial benefit are also included for the purpose of transparency. Relationships in this table are modest unless otherwise noted. Please refer to https://www.acc.org/guidelines/about-guidelines-and-clinical-documents/relationships-with-industry-policy for definitions of disclosure categories or additional information about the ACC/AHA Disclosure Policy for Writing Committees.
Significant relationship.
No financial benefit.
This disclosure was entered under the Clinical Trial Enroller category in the ACC’s disclosure system. To appear in this category, the author acknowledges that there is no direct or institutional relationship with the trial sponsor as defined in the (ACCF or AHA/ACC) Disclosure Policy for Writing Committees.
Sabrina Times is an AHA/ACC joint staff member and acts as the Science and Health Advisor for the “2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation.” No relevant relationships to report. Nonvoting author on measures and not included/counted in the RWI balance for this writing committee.
AAMI indicates Association for the Advancement of Medical Instrumentation; ABIM, American Board of Internal Medicine; ACC, American College of Cardiology; ACGME, Accreditation Council for Graduate Medical Education; AHA, American Heart Association; AHRQ, Agency for Health Research and Quality; BCBS, Blue Cross Blue Shield; BD, Becton Dickinson; CDRH, Center for Devices and Radiological Health; CEAC, Clinical Event Adjudication Committee; CPRIT, Cancer Prevention and Research Institute of Texas; CUIMC, Columbia University Imng Medical Center; DSMB, data and safety monitoring board; EP, electrophysiology; FDA, US Food and Drug Administration; FNRA, French National Research Agency; HRS, Heart Rhythm Society; JAMA, Journal of the American Medical Association; NIH, National Institutes of Health; NINR, National Institute of Nursing Research; NHLBI, National Heart, Lung, and Blood Institute; NHMRC, National Health and Medical Research Council; PHRI, Population Health Research Institute; UHSP, University of Health Science and Pharmacy in St. Louis; UT, University of Texas; and VA CSP, Veterans Affairs Cooperative Studies Program.
Appendix 2. Reviewer Relationships With Industry and Other Entities (Comprehensive)—2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation
| Reviewer | Representation | Employment | Consultant | Speakers Bureau | Ownership/Partnership/Principal | Personal Research | Institutional, Organizational, or Other Financial Benefit | Expert Witness |
|---|---|---|---|---|---|---|---|---|
| Andrew D. Krahn | AHA/ACC Atrial Fibrillation Peer Review Committee Chair | University of British Columbia | None | None | • CCS* • HRS* |
None | None | None |
| Jason G. Andrade | AHA/ACC Atrial Fibrillation Peer Review Committee | University of Montreal | • Biosense Webster • Medtronic |
• Bayer • Bristol Myers Squibb/Pfizer Alliance • Servier |
None | • Medtronic† | None | None |
| Craig J. Beavers | AHA/ACC Atrial Fibrillation Peer Review Committee, representing ACCP | University of Kentucky College of Pharmacy | None | None | None | None | None | None |
| James M. Bischoff | AHA/ACC Atrial Fibrillation Peer Review Committee, Patient Representative | Superintendent, Retired | None | None | None | None | None | None |
| T. Jared Bunch | AHA/ACC Atrial Fibrillation Peer Review Committee, representing HRS | University of Utah School of Medicine | None | None | None | • Altathera† • Boehringer Ingelheim† |
• Abbott‡ • Altathera‡ • Cardiva‡ • St. Jude’s Medical‡ |
None |
| Kristen Bova Campbell | AHA/ACC Atrial Fibrillation Peer Review Committee | Duke University | • Wolters Kluwer | None | None | None | None | None |
| Lin Yee Chen | AHA/ACC Atrial Fibrillation Peer Review Committee | University of Minnesota School of Medicine | None | None | None | None | • NIH† | None |
| Robin Dharia | AHA/ACC Atrial Fibrillation Peer Review Committee | Thomas Jefferson University | None | None | None | None | • NIH‡ | None |
| Michael P. Dorsch | AHA/ACC Atrial Fibrillation Peer Review Committee, representing JCPM | University of Michigan | None | None | None | None | None | None |
| Edward P. Gerstenfeld | AHA/ACC Atrial Fibrillation Peer Review Committee | University of California San Francisco | • Biosense Webster† • Medtronic • St. Jude Medical |
None | None | • Abbott (DSMB)† • Adagio* • Boston Scientific* • Thermedical (DSMB)† |
• Boston Scientific* | None |
| Aubrey E. Jones | AHA/ACC Atrial Fibrillation Peer Review Committee | University of Utah College of Pharmacy | None | None | None | • AHRQ† • NIH-NHLBI† |
None | None |
| Stephanie Dwyer Kaluzna | AHA/ACC Atrial Fibrillation Peer Review Committee | University of Illinois Chicago College of Pharmacy | None | None | None | None | None | None |
| Luke Masha | AHA/ACC Atrial Fibrillation Peer Review Committee | Oregon Health & Science University | None | None | None | None | • Ancora‡ • Endotronix‡ |
None |
| Isabelle Nault | AHA/ACC Atrial Fibrillation Peer Review Committee | Quebec Heart and Lung Institute | • Bristol Myers Squibb • Pfizer |
• Bayer† • Biosense Webster • Medtronic • Servier† |
None | None | None | None |
| Peter A. Noseworthy | AHA/ACC Atrial Fibrillation Peer Review Committee | Mayo Clinic | None | None | None | None | • Anumana* • Milestone‡ |
None |
| Cara N. Pellegrini | AHA/ACC Atrial Fibrillation Peer Review Committee | University of California San Francisco | • Abbott • Biosense Webster • Cook Medical • Medtronic |
None | None | None | None | None |
| Stylianos E. Tzeis | AHA/ACC Atrial Fibrillation Peer Review Committee | Mitera Hospital | • Pfizer | None | None | None | • Bayer‡ | None |
| Annabelle Santos Volgman | AHA/ACC Atrial Fibrillation Peer Review Committee | Rush University Medical Center | • Bristol Myers Squibb Foundation DCICDP • Janssen Pharmaceuticals • Merck • Pfizer • Sanofi Aventis† |
None | None | • NIH† | • Apple* • Novartis‡ |
None |
| Emily P. Zeitler | AHA/ACC Atrial Fibrillation Peer Review Committee | Dartmouth Hitchock Medical Center | • Abbott • Biosense Webster • Medtronic |
None | None | • Boston Scientific† • Sanofi Aventis* |
None | None |
This table represents all reviewers’ relationships with industry and other entities that were reported at the time of peer review, including those not deemed to be relevant to this document, at the time this document was under review. The table does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest represents ownership of ≥5% of the voting stock or share of the business entity, or ownership of ≥$5 000 of the fair market value of the business entity; or if funds received by the person from the business entity exceed 5% of the person’s gross income for the previous year. Relationships that exist with no financial benefit are also included for the purpose of transparency. Relationships in this table are modest unless otherwise noted. Please refer to http://www.acc.org/guidelines/about-guidelines-and-clinical-documents/relationships-with-industry-policy for definitions of disclosure categories or additional information about the ACC/AHA Disclosure Policy for Writing Committees.
No financial benefit.
Significant relationship.
This disclosure was entered under the Clinical Trial Enroller category in the ACC’s disclosure system. To appear in this category, the author acknowledges that there is no direct or institutional relationship with the trial sponsor as defined in the (ACCF or AHA/ACC) Disclosure Policy for Writing Committees.
ACC indicates American College of Cardiology; ACCF, American College of Cardiology Foundation; AACP, American Association of Colleges of Pharmacy; AHA, American Heart Association; AHRQ, Agency for Healthcare Research and Quality; CCS, Canadian Cardiovascular Society; DCICDP, Diverse Clinical Investigator Career Development Program; DSMB; data and safety monitoring board; HRS, Heart Rhythm Society; JCPM, ACC/AHA Joint Committee on Performance Measures, NHLBI, National Heart, Lung, and Blood Institute; and NIH, National Institutes of Health.
Footnotes
PEER REVIEW COMMITTEE MEMBERS
Andrew D. Krahn, MD, FHRS, Chair; Jason G. Andrade, MD, FHRS; Craig J. Beavers, PharmD, FACC, FAHA, FCCP, BCCP, BCPS (AQ-Cardiology), CACP; James M. Bischoff; T. Jared Bunch, MD, FACC, FHRS; Kristen Bova Campbell, PharmD, FACC; Lin Yee Chen, MD, MS, FAHA, FHRS; Robin Dharia, MD, FAHA; Michael P. Dorsch, PharmD, MS, FACC, FAHA; Edward P. Gerstenfeld, MD, MS, FACC, FHRS; Aubrey E. Jones, PharmD, MSCI; Stephanie Dwyer Kaluzna, PharmD; Luke Masha, MD, MPH; Isabelle Nault, MD; Peter A. Noseworthy, MD, FHRS; Cara N. Pellegrini, MD, FACC, FHRS; Stylianos E. Tzeis, MD; Annabelle Santos Volgman, MD, FACC, FAHA; Emily P. Zeitler, MD, MHS, FACC
ACC/AHA JOINT COMMITTEE ON CLINICAL PRACTICE GUIDELINES
Joshua A. Beckman, MD, MS, FAHA, FACC, Chair; Catherine M. Otto, MD, FACC, FAHA, Chair-Elect; Anastasia L. Armbruster, PharmD, FACC; Leslie L. Davis, PhD, ANP-BC, FACC, FAHA; Lisa de las Fuentes, MD, MS, FAHA; Anita Deswal, MD, MPH, FACC, FAHA; Victor A. Ferrari, MD, FACC, FAHA; Adrian F. Hernandez, MD, FAHA; Heather M. Johnson, MD, MS, FAHA, FACC; W. Schuyler Jones, MD, FACC; Prateeti Khazanie, MD, MPH; Michelle M. Kittleson, MD, PhD, FACC; Debabrata Mukherjee, MD, FACC, FAHA; Latha Palaniappan, MD, MS, FAHA, FACC; Tanveer Rab, MD, FACC; Jacqueline E. Tamis-Holland, MD, FACC, FAHA; Y. Joseph Woo, MD, FACC, FAHA; Boback Ziaeian, MD, PhD, FAHA
PRESIDENTS AND STAFF
American College of Cardiology
B. Hadley Wilson, MD, FACC, President
Cathleen C. Gates, Chief Executive Officer
Richard J. Kovacs, MD, MACC, Chief Medical Officer
Mindy J. Saraco, MHA, Director, Clinical Policy and Guidelines
Grace D. Ronan, Senior Production and Operations Manager, Clinical Policy Publications
Leah Patterson, Project Manager, Clinical Content Development
American Heart Association/American College of Cardiology
Thomas S.D. Getchius, National Senior Director, Guidelines
Abdul R. Abdullah, MD, Director, Guideline Science and Methodology
American Heart Association
Joseph C. Wu, MD, PhD, FAHA, President
Nancy Brown, Chief Executive Officer
Mariell Jessup, MD, FAHA, Chief Science and Medical Officer
Nicole Aiello Sapio, EdD, Executive Vice President, Office of Science Strategies and Operations
Radhika Rajgopal Singh, PhD, Senior Vice President, Office of Science and Medicine
Prashant Nedungadi, BPharm, PhD, Vice President, Science and Medicine, Clinical Guidelines
Anne Leonard, MPH, BSN, RN, FAHA, National Senior Director, Science and Medicine
Jody Hundley, Senior Production and Operations Manager, Scientific Publications, Office of Science Operations
ARTICLE INFORMATION
This document was approved by the American College of Cardiology Clinical Policy Approval Committee and the American Heart Association Science Advisory and Coordinating Committee in July 2023, and the American College of Cardiology Science and Quality Committee and the American Heart Association Executive Committee in August 2023.
Supplemental materials are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIR.0000000000001193
This article has been copublished in the Journal of the American College of Cardiology.
Copies: This document is available on the websites of the American College of Cardiology (www.acc.org) and the American Heart Association (professional.heart. org). A copy of the document is also available at https://professional.heart.org/statements by selecting the “Guidelines & Statements” button. To purchase additional reprints, call 215-356-2721 or email Meredith.Edelman@wolterskluwer.com.
The expert peer review of AHA-commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit https://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu near the top of the webpage, then click “Publication Development.”
Permissions: Multiple copies, modification, alteration, enhancement, and distribution of this document are not permitted without the express permission of the American Heart Association. Instructions for obtaining permission are located at https://www.heart.org/permissions. A link to the “Copyright Permissions Request Form” appears in the second paragraph (https://www.heart.org/en/about-us/statements-and-policies/copyright-request-form).
Developed in Collaboration With and Endorsed by the American College of Clinical Pharmacy and the Heart Rhythm Society
Contributor Information
Writing Committee Members:
José A. Joglar, Mina K. Chung, Anastasia L. Armbruster, Emelia J. Benjamin, Janice Y. Chyou, Edmond M. Cronin, Anita Deswal, Lee L. Eckhardt, Zachary D. Goldberger, Rakesh Gopinathannair, Bulent Gorenek, Paul L. Hess, Mark Hlatky, Gail Hogan, Chinwe Ibeh, Julia H. Indik, Kazuhiko Kido, Fred Kusumoto, Mark S. Link, Kathleen T. Linta, Gregory M. Marcus, Patrick M. McCarthy, Nimesh Patel, Kristen K. Patton, Marco V. Perez, Jonathan P. Piccini, Andrea M. Russo, Prashanthan Sanders, Megan M. Streur, Kevin L. Thomas, Sabrina Times, James E. Tisdale, Anne Marie Valente, and David R. Van Wagoner
REFERENCES
PREAMBLE
- 1.Committee on Standards for Developing Trustworthy Clinical Practice Guidelines, Institute of Medicine (US). Clinical Practice Guidelines We Can Trust. National Academies Press; 2011. [Google Scholar]
- 2.Committee on Standards for Systematic Reviews of Comparative Effectiveness Research, Institute of Medicine (US). Finding What Works in Healthcare: Standards for Systematic Reviews. National Academies Press; 2011. [PubMed] [Google Scholar]
- 3.Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129:2329–2345. [DOI] [PubMed] [Google Scholar]
- 4.ACCF/AHA Task Force on Practice Guidelines. Methodology Manual and Policies From the ACCF/AHA Task Force on Practice Guidelines. American College of Cardiology and American Heart Association. 2010. Accessed September 23, 2019. https://www.acc.org/Guidelines/About-Guidelines-and-Clinical-Documents/Methodology and https://professional.heart.org/-/media/phd-files/guidelines-and-statements/methodology_manual_and_policies_ucm_319826.pdf. [Google Scholar]
- 5.Halperin JL, Levine GN, Al-Khatib SM, et al. Further evolution of the ACC/AHA clinical practice guideline recommendation classification system: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2016;133:1426–1428. [DOI] [PubMed] [Google Scholar]
- 6.Arnett DK, Goodman RA, Halperin JL, et al. AHA/ACC/HHS strategies to enhance application of clinical practice guidelines in patients with cardiovascular disease and comorbid conditions: from the American Heart Association, American College of Cardiology, and US Department of Health and Human Services. Circulation. 2014;130:1662–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine GN, O’Gara PT, Beckman JA, et al. Recent innovations, modifications, and evolution of ACC/AHA clinical practice guidelines: an update for our constituencies: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e879–e886. [DOI] [PubMed] [Google Scholar]
1.4. Scope of the Guideline
- 1.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 3.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation Developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 4.Andrade JG, Aguilar M, Atzema C, et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol. 2020;36:1847–1948. [DOI] [PubMed] [Google Scholar]
- 5.Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e698–e800. [DOI] [PubMed] [Google Scholar]
- 6.Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020;142:e558–e631. [DOI] [PubMed] [Google Scholar]
- 7.Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72–e227. [DOI] [PubMed] [Google Scholar]
- 8.Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e18–e114. [DOI] [PubMed] [Google Scholar]
- 9.Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144:e368–e454. [DOI] [PubMed] [Google Scholar]
- 10.Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52:e364–e467. [DOI] [PubMed] [Google Scholar]
- 11.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–e1032. [DOI] [PubMed] [Google Scholar]
- 12.Joglar JA, Kapa S, Saarel EV, et al. 2023 HRS expert consensus statement on the management of arrhythmias during pregnancy. Heart Rhythm. 2023; doi: 10.1016/j.hrthm.2023.05.01 [DOI] [PubMed] [Google Scholar]
2.1. Epidemiology
- 1.Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation. 2023;147:e93–e621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 study. Circulation. 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turakhia MP, Guo JD, Keshishian A, et al. Contemporary prevalence estimates of undiagnosed and diagnosed atrial fibrillation in the United States. Clin Cardiol. 2023;46:484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deshmukh A, Iglesias M, Khanna R, et al. Healthcare utilization and costs associated with a diagnosis of incident atrial fibrillation. Heart Rhythm O2. 2022;3:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dieleman JL, Cao J, Chapin A, et al. US health care spending by payer and health condition, 1996–2016. JAMA. 2020;323:863–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai H, Zhang Q, Much AA, et al. Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990–2017: results from the Global Burden of Disease Study 2017. Eur Heart J Qual Care Clin Outcomes. 2021;7:574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 19932007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
2.1.1. Prevalence, Incidence, Morbidity, and Mortality
- 1.Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the US adult population. Am J Cardiol. 2013;112:1142–1147. [DOI] [PubMed] [Google Scholar]
- 2.Mou L, Norby FL, Chen LY, et al. Lifetime risk of atrial fibrillation by race and socioeconomic status: ARIC study (Atherosclerosis Risk in Communities). Circ Arrhythm Electrophysiol. 2018;11:e006350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weng LC, Preis SR, Hulme OL, et al. Genetic predisposition, clinical risk factor burden, and lifetime risk of atrial fibrillation. Circulation. 2018;137:1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staerk L, Wang B, Preis SR, et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the Framingham Heart Study. BMJ. 2018;361:k1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chao TF, Liu CJ, Tuan TC, et al. Lifetime risks, projected numbers, and adverse outcomes in Asian patients with atrial fibrillation: a report from the Taiwan Nationwide AF Cohort Study. Chest. 2018;153:453–466. [DOI] [PubMed] [Google Scholar]
- 6.Emdin CA, Wong CX, Hsiao AJ, et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ. 2016;532:h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Odutayo A, Wong CX, Hsiao AJ, et al. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. 2016;354:i4482. [DOI] [PubMed] [Google Scholar]
- 8.Papanastasiou CA, Theochari CA, Zareifopoulos N, et al. Atrial fibrillation is associated with cognitive impairment, all-cause dementia, vascular dementia, and Alzheimer's disease: a systematic review and meta-analysis. J Gen Intern Med. 2021;36:3122–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruddox V, Sandven I, Munkhaugen J, et al. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: a systematic review and meta-analysis. Eur J Prev Cardiol. 2017;24:1555–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rattanawong P, Upala S, Riangwiwat T, et al. Atrial fibrillation is associated with sudden cardiac death: a systematic review and meta-analysis. J Interv Card Electrophysiol. 2018;51:91–104. [DOI] [PubMed] [Google Scholar]
- 11.Piccini JP, Hammill BG, Sinner MF, et al. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J. 2014;35:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
2.1.2. Risk Factors and Associated Heart Disease
- 1.Belbasis L, Mavrogiannis MC, Emfietzoglou M, et al. Environmental factors, serum biomarkers and risk of atrial fibrillation: an exposure-wide umbrella review of meta-analyses. Eur J Epidemiol. 2020;35:223–239. [DOI] [PubMed] [Google Scholar]
- 2.Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allan V, Honarbakhsh S, Casas JP, et al. Are cardiovascular risk factors also associated with the incidence of atrial fibrillation? A systematic review and field synopsis of 23 factors in 32 population-based cohorts of 20 million participants. Thromb Haemost. 2017;117:837–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts JD, Vittinghoff E, Lu AT, et al. Epigenetic age and the risk of incident atrial fibrillation. Circulation. 2021;144:1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huxley RR, Lopez FL, Folsom AR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aune D, Schlesinger S, Norat T, et al. Tobacco smoking and the risk of atrial fibrillation: a systematic review and meta-analysis of prospective studies. Eur J Prev Cardiol. 2018;25:1437–1451. [DOI] [PubMed] [Google Scholar]
- 7.Lu Y, Guo Y, Lin H, et al. Genetically determined tobacco and alcohol use and risk of atrial fibrillation. BMC Med Genomics. 2021;14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohanty S, Mohanty P, Tamaki M, et al. Differential association of exercise intensity with risk of atrial fibrillation in men and women: evidence from a meta-analysis. J Cardiovasc Electrophysiol. 2016;27:1021–1029. [DOI] [PubMed] [Google Scholar]
- 9.Mishima RS, Verdicchio CV, Noubiap JJ, et al. Self-reported physical activity and atrial fibrillation risk: a systematic review and meta-analysis. Heart Rhythm. 2021;18:520–528. [DOI] [PubMed] [Google Scholar]
- 10.Newman W, Parry-Williams G, Wiles J, et al. Risk of atrial fibrillation in athletes: a systematic review and meta-analysis. Br J Sports Med. 2021;55:1233–1238. [DOI] [PubMed] [Google Scholar]
- 11.Hegbom F, Stavem K, Sire S, et al. Effects of short-term exercise training on symptoms and quality of life in patients with chronic atrial fibrillation. Int J Cardiol. 2007;116:86–92. [DOI] [PubMed] [Google Scholar]
- 12.Malmo V, Nes BM, Amundsen BH, et al. Aerobic interval training reduces the burden of atrial fibrillation in the short term: a randomized trial. Circulation. 2016;133:466–473. [DOI] [PubMed] [Google Scholar]
- 13.Elliott AD, Verdicchio CV, Mahajan R, et al. An exercise and physical activity program in patients with atrial fibrillation: the ACTIVE-AF randomized controlled trial. JACC Clin Electrophysiol. 2023;9:455–465. [DOI] [PubMed] [Google Scholar]
- 14.Pathak RK, Elliott A, Middeldorp ME, et al. Impact of CARDIOrespiratory FITness on arrhythmia recurrence in obese individuals with atrial fibrillation: the CARDIO-FIT study. J Am Coll Cardiol. 2015;66:985–996. [DOI] [PubMed] [Google Scholar]
- 15.Oesterle A, Giancaterino S, Van Noord MG, et al. Effects of supervised exercise training on atrial fibrillation: a meta-analysis of randomized controlled trials. J Cardiopulm Rehabil Prev. 2022;42:258–265. [DOI] [PubMed] [Google Scholar]
- 16.Osbak PS, Mourier M, Kjaer A, et al. A randomized study of the effects of exercise training on patients with atrial fibrillation. Am Heart J. 2011;162:1080–1087. [DOI] [PubMed] [Google Scholar]
- 17.Marcus GM, Vittinghoff E, Whitman IR, et al. Acute consumption of alcohol and discrete atrial fibrillation events. Ann Intern Med. 2021;174:1503–1509. [DOI] [PubMed] [Google Scholar]
- 18.Dukes JW, Dewland TA, Vittinghoff E, et al. Access to alcohol and heart disease among patients in hospital: observational cohort study using differences in alcohol sales laws. BMJ. 2016;353:i2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382:20–28. [DOI] [PubMed] [Google Scholar]
- 20.Marcus GM, Modrow MF, Schmid CH, et al. Individualized studies of triggers of paroxysmal atrial fibrillation: the I-STOP-AFib randomized clinical trial. JAMA Cardiol. 2022;7:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310:2050–2060. [DOI] [PubMed] [Google Scholar]
- 22.Pathak RK, Middeldorp ME, Lau DH, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231. [DOI] [PubMed] [Google Scholar]
- 23.Pathak RK, Middeldorp ME, Meredith M, et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol. 2015;65:2159–2169. [DOI] [PubMed] [Google Scholar]
- 24.Middeldorp ME, Pathak RK, Meredith M, et al. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. Europace. 2018;20:1929–1935. [DOI] [PubMed] [Google Scholar]
- 25.Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol. 2014;64:281–289. [DOI] [PubMed] [Google Scholar]
- 26.Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donnellan E, Wazni O, Kanj M, et al. Outcomes of atrial fibrillation ablation in morbidly obese patients following bariatric surgery compared with a nonobese cohort. Circ Arrhythm Electrophysiol. 2019;12:e007598. [DOI] [PubMed] [Google Scholar]
- 28.Donnellan E, Wazni OM, Kanj M, et al. Association between pre-ablation bariatric surgery and atrial fibrillation recurrence in morbidly obese patients undergoing atrial fibrillation ablation. Europace. 2019;21:1476–1483. [DOI] [PubMed] [Google Scholar]
- 29.Donnellan E, Wazni OM, Elshazly M, et al. Impact of bariatric surgery on atrial fibrillation type. Circ Arrhythm Electrophysiol. 2020;13:e007626. [DOI] [PubMed] [Google Scholar]
- 30.Mohanty S, Mohanty P, Natale V, et al. Impact of weight loss on ablation outcome in obese patients with longstanding persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2018;29:246–253. [DOI] [PubMed] [Google Scholar]
- 31.Aune D, Sen A, Schlesinger S, et al. Body mass index, abdominal fatness, fat mass and the risk of atrial fibrillation: a systematic review and dose-response meta-analysis of prospective studies. Eur J Epidemiol. 2017;32:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y, Zha L, Pan S. The risk of atrial fibrillation increases with earlier onset of obesity: a Mendelian randomization study. Int J Med Sci. 2022;19:1388–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Q, Richardson TG, Sanderson E, et al. A phenome-wide bidirectional Mendelian randomization analysis of atrial fibrillation. Int J Epidemiol. 2022;51:1153–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinberg JS, Shabanov V, Ponomarev D, et al. Effect of renal denervation and catheter ablation vs catheter ablation alone on atrial fibrillation recurrence among patients with paroxysmal atrial fibrillation and hypertension: the ERADICATE-AF randomized clinical trial. JAMA. 2020;323:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neefs J, van den Berg NW, Limpens J, et al. Aldosterone pathway blockade to prevent atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol. 2017;231:155–161. [DOI] [PubMed] [Google Scholar]
- 36.Parkash R, Wells GA, Sapp JL, et al. Effect of aggressive blood pressure control on the recurrence of atrial fibrillation after catheter ablation: a randomized, open-label clinical trial (SMAC-AF [Substrate Modification With Aggressive Blood Pressure Control]). Circulation. 2017;135:1788–1798. [DOI] [PubMed] [Google Scholar]
- 37.Soliman EZ, Rahman AF, Zhang ZM, et al. Effect of intensive blood pressure lowering on the risk of atrial fibrillation. Hypertension. 2020;75:1491–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinho-Gomes AC, Azevedo L, Copland E, et al. Blood pressure-lowering treatment for the prevention of cardiovascular events in patients with atrial fibrillation: an individual participant data meta-analysis. PLoS Med. 2021;18:e1003599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le NN, Tran TQB, Lip S, et al. Unravelling the distinct effects of systolic and diastolic blood pressure using Mendelian randomisation. Genes (Basel). 2022;13:1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hyman MC, Levin MG, Gill D, et al. Genetically predicted blood pressure and risk of atrial fibrillation. Hypertension. 2021;77:376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Guo N, Zhu W, et al. Resting heart rate and the risk of atrial fibrillation. Int Heart J. 2019;60:805–811. [DOI] [PubMed] [Google Scholar]
- 42.Siland JE, Geelhoed B, Roselli C, et al. Resting heart rate and incident atrial fibrillation: a stratified Mendelian randomization in the AFGen consortium. PLoS One. 2022;17:e0268768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donnellan E, Aagaard P, Kanj M, et al. Association between pre-ablation glycemic control and outcomes among patients with diabetes undergoing atrial fibrillation ablation. JACC Clin Electrophysiol. 2019;5:897–903. [DOI] [PubMed] [Google Scholar]
- 44.Aune D, Feng T, Schlesinger S, et al. Diabetes mellitus, blood glucose and the risk of atrial fibrillation: a systematic review and meta-analysis of cohort studies. J Diabetes Complications. 2018;32:501–511. [DOI] [PubMed] [Google Scholar]
- 45.Santhanakrishnan R, Wang N, Larson MG, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwok MK, Schooling CM. Mendelian randomization study on atrial fibrillation and cardiovascular disease subtypes. Sci Rep. 2021;11:18682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao J, Bai Y, Ji H. Genetically predicted atrial fibrillation and valvular heart disease: a two-sample Mendelian randomization study. Front Cardiovasc Med. 2022;9:845734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–1729. [DOI] [PubMed] [Google Scholar]
- 50.Filardo G, da Graca B, Sass DM, et al. Preoperative β-blockers as a coronary surgery quality metric: the lack of evidence of efficacy. Ann Thorac Surg. 2020;109:1150–1158. [DOI] [PubMed] [Google Scholar]
- 51.Gillinov AM, Bagiella E, Moskowitz AJ, et al. Rate control versus rhythm control for atrial fibrillation after cardiac surgery. N Engl J Med. 2016;374:1911–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mehaffey JH, Hawkins RB, Byler M, et al. Amiodarone protocol provides cost-effective reduction in postoperative atrial fibrillation. Ann Thorac Surg. 2018;105:1697–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guarnieri T Intravenous antiarrhythmic regimens with focus on amiodarone for prophylaxis of atrial fibrillation after open heart surgery. Am J Cardiol. 1999;84:152r–155r. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell LB, Exner DV, Wyse DG, et al. Prophylactic oral amiodarone for the prevention of arrhythmias that begin early after revascularization, valve replacement, or repair: PAPABEAR: a randomized controlled trial. JAMA. 2005;294:3093–3100. [DOI] [PubMed] [Google Scholar]
- 55.Gaudino M, Sanna T, Ballman KV, et al. Posterior left pericardiotomy for the prevention of atrial fibrillation after cardiac surgery: an adaptive, singlecentre, single-blind, randomised, controlled trial. Lancet. 2021;398:2075–2083. [DOI] [PubMed] [Google Scholar]
- 56.Eikelboom R, Sanjanwala R, Le ML, et al. Postoperative atrial fibrillation after cardiac surgery: a systematic review and meta-analysis. Ann Thorac Surg. 2021;111:544–554. [DOI] [PubMed] [Google Scholar]
- 57.Woldendorp K, Farag J, Khadra S, et al. Postoperative atrial fibrillation after cardiac surgery: a meta-analysis. Ann Thorac Surg. 2021;112:2084–2093. [DOI] [PubMed] [Google Scholar]
- 58.Shang W, Li L, Huang S, et al. Chronic kidney disease and the risk of new-onset atrial fibrillation: a meta-analysis of prospective cohort studies. PLoS One. 2016;11:e0155581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geurts S, van der Burgh AC, Bos MM, et al. Disentangling the association between kidney function and atrial fibrillation: a bidirectional Mendelian randomization study. Int J Cardiol. 2022;355:15–22. [DOI] [PubMed] [Google Scholar]
- 60.Park S, Lee S, Kim Y, et al. Atrial fibrillation and kidney function: a bidirectional Mendelian randomization study. Eur Heart J. 2021;42:2816–2823. [DOI] [PubMed] [Google Scholar]
- 61.Zhang D, Ma Y, Xu J, et al. Association between obstructive sleep apnea (OSA) and atrial fibrillation (AF): a dose-response meta-analysis. Medicine (Baltim). 2022;101:e29443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li X, Zhou X, Xu X, et al. Effects of continuous positive airway pressure treatment in obstructive sleep apnea patients with atrial fibrillation: a meta-analysis. Medicine (Baltim). 2021;100:e25438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Y, Ning Y, Wen W, et al. CPAP is associated with decreased risk of AF recurrence in patients with OSA, especially those younger and slimmer: a meta-analysis. J Interv Card Electrophysiol. 2020;58:369–379. [DOI] [PubMed] [Google Scholar]
- 64.Congrete S, Bintvihok M, Thongprayoon C, et al. Effect of obstructive sleep apnea and its treatment of atrial fibrillation recurrence after radiofrequency catheter ablation: a meta-analysis. J Evid Based Med. 2018;11:145–151. [DOI] [PubMed] [Google Scholar]
- 65.Deng F, Raza A, Guo J. Treating obstructive sleep apnea with continuous positive airway pressure reduces risk of recurrent atrial fibrillation after catheter ablation: a meta-analysis. Sleep Med. 2018;46:5–11. [DOI] [PubMed] [Google Scholar]
- 66.Qureshi WT, Nasir UB, Alqalyoobi S, et al. Meta-analysis of continuous positive airway pressure as a therapy of atrial fibrillation in obstructive sleep apnea. Am J Cardiol. 2015;116:1767–1773. [DOI] [PubMed] [Google Scholar]
- 67.Szymanski FM, Filipiak KJ, Platek AE, et al. Presence and severity of obstructive sleep apnea and remote outcomes of atrial fibrillation ablationsa long-term prospective, cross-sectional cohort study. Sleep Breath. 2015;19:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caples SM, Mansukhani MP, Friedman PA, et al. The impact of continuous positive airway pressure treatment on the recurrence of atrial fibrillation post cardioversion: a randomized controlled trial. Int J Cardiol. 2019;278:133–136. [DOI] [PubMed] [Google Scholar]
- 69.Traaen GM, Aakeroy L, Hunt TE, et al. Effect of continuous positive airway pressure on arrhythmia in atrial fibrillation and sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2021;204:573–582. [DOI] [PubMed] [Google Scholar]
- 70.Hunt TE, Traaen GM, Aakeroy L, et al. Effect of continuous positive airway pressure therapy on recurrence of atrial fibrillation after pulmonary vein isolation in patients with obstructive sleep apnea: a randomized controlled trial. Heart Rhythm. 2022;19:1433–1441. [DOI] [PubMed] [Google Scholar]
- 71.Chen L, Sun X, He Y, et al. Obstructive sleep apnea and atrial fibrillation: insights from a bidirectional Mendelian randomization study. BMC Med Genomics. 2022;15:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang M, Yang S, Ge G, et al. Effects of thyroid dysfunction and the thyroid-stimulating hormone levels on the risk of atrial fibrillation: a systematic review and dose-response meta-analysis from cohort studies. Endocr Pract. 2022;28:822–831. [DOI] [PubMed] [Google Scholar]
- 73.Ellervik C, Roselli C, Christophersen IE, et al. Assessment of the relationship between genetic determinants of thyroid function and atrial fibrillation: a Mendelian randomization study. JAMA Cardiol. 2019;4:144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walkey AJ, Wiener RS, Ghobrial JM, et al. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306:2248–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walkey AJ, Greiner MA, Heckbert SR, et al. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165:949–955 e943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bosch NA, Cohen DM, Walkey AJ. Risk factors for new-onset atrial fibrillation in patients with sepsis: a systematic review and meta-analysis. Crit Care Med. 2019;47:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kwok CS, Rashid M, Beynon R, et al. Prolonged PR interval, first-degree heart block and adverse cardiovascular outcomes: a systematic review and meta-analysis. Heart. 2016;102:672–680. [DOI] [PubMed] [Google Scholar]
- 78.Ntalla I, Weng LC, Cartwright JH, et al. Multi-ancestry GWAS of the electrocardiographic PR interval identifies 202 loci underlying cardiac conduction. Nat Commun. 2020;11:2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiang H, Xue Y, Chen Z, et al. The association between left ventricular hypertrophy and the occurrence and prognosis of atrial fibrillation: a meta-analysis. Front Cardiovasc Med. 2021;8:639993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sinner MF, Stepas KA, Moser CB, et al. B-type natriuretic peptide and C-reactive protein in the prediction of atrial fibrillation risk: the CHARGE-AF Consortium of community-based cohort studies. Europace. 2014;16:1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Geelhoed B, Borschel CS, Niiranen T, et al. Assessment of causality of natriuretic peptides and atrial fibrillation and heart failure: a Mendelian randomization study in the FINRISK cohort. Europace. 2020;22:1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu N, Xu B, Xiang Y, et al. Association of inflammatory factors with occurrence and recurrence of atrial fibrillation: a meta-analysis. Int J Cardiol. 2013;169:62–72. [DOI] [PubMed] [Google Scholar]
- 83.Mohammadi-Shemirani P, Chong M, Narula S, et al. Elevated lipoprotein(a) and risk of atrial fibrillation: an observational and Mendelian randomization study. J Am Coll Cardiol. 2022;79:1579–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vaziri SM, Larson MG, Benjamin EJ, et al. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724–730. [DOI] [PubMed] [Google Scholar]
- 85.Olsen FJ, Mogelvang R, Jensen GB, et al. Relationship between left atrial functional measures and incident atrial fibrillation in the general population: the Copenhagen City Heart Study. JACC Cardiovasc Imaging. 2019;12:981–989. [DOI] [PubMed] [Google Scholar]
- 86.Scherer M, Therapidis P, Miskovic A, et al. Left atrial size reduction improves the sinus rhythm conversion rate after radiofrequency ablation for continuous atrial fibrillation in patients undergoing concomitant cardiac surgery. Thorac Cardiovasc Surg. 2006;54:34–38. [DOI] [PubMed] [Google Scholar]
- 87.Scherer M, Dzemali O, Aybek T, et al. Impact of left atrial size reduction on chronic atrial fibrillation in mitral valve surgery. J Heart Valve Dis. 2003;12:469–474. [PubMed] [Google Scholar]
- 88.Scherer M, Therapidis P, Wittlinger T, et al. Impact of left atrial size reduction and endocardial radiofrequency ablation on continuous atrial fibrillation in patients undergoing concomitant cardiac surgery: three-year results. J Heart Valve Dis. 2007;16:126–131. [PubMed] [Google Scholar]
- 89.Joshibayev S, Bolatbekov B. Early and long-term outcomes and quality of life after concomitant mitral valve surgery, left atrial size reduction, and radiofrequency surgical ablation of atrial fibrillation. Anatol J Cardiol. 2016;16:797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van de Vegte YJ, Siland JE, Rienstra M, et al. Atrial fibrillation and left atrial size and function: a Mendelian randomization study. Sci Rep. 2021;11:8431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mou L, Norby FL, Chen LY, et al. Lifetime risk of atrial fibrillation by race and socioeconomic status: ARIC study (Atherosclerosis Risk in Communities). Circ Arrhythm Electrophysiol. 2018;11:e006350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lunde ED, Joensen AM, Lundbye-Christensen S, et al. Socioeconomic position and risk of atrial fibrillation: a nationwide Danish cohort study. J Epidemiol Community Health. 2020;74:7–13. [DOI] [PubMed] [Google Scholar]
- 93.Liu Y, Liu C, Liu Q. Education and atrial fibrillation: Mendelian randomization study. Glob Heart. 2022;17:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bonaccio M, Di Castelnuovo A, Costanzo S, et al. Life course socioeconomic status and risk of hospitalization for heart failure or atrial fibrillation in the Moli-Sani study cohort. Am J Epidemiol. 2021;kwab046. [DOI] [PubMed] [Google Scholar]
- 95.Chung SC, Sofat R, Acosta-Mena D, et al. Atrial fibrillation epidemiology, disparity and healthcare contacts: a population-wide study of 56 million individuals. Lancet Reg Health Eur. 2021;7:100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lunde ED, Nielsen PB, Riahi S, et al. Associations between socioeconomic status, atrial fibrillation, and outcomes: a systematic review. Expert Rev Cardiovasc Ther. 2018;16:857–873. [DOI] [PubMed] [Google Scholar]
- 97.Alzahrani Z, Ornelas-Loredo A, Darbar SD, et al. Association between family history and early-onset atrial fibrillation across racial and ethnic groups. JAMA Netw Open. 2018;1:e182497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arnar DO, Thorvaldsson S, Manolio TA, et al. Familial aggregation of atrial fibrillation in Iceland. Eur Heart J. 2006;27:708–712. [DOI] [PubMed] [Google Scholar]
- 99.Lubitz SA, Yin X, Fontes JD, et al. Association between familial atrial fibrillation and risk of new-onset atrial fibrillation. JAMA. 2010;304:2263–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roselli C, Chaffin MD, Weng LC, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet. 2018;50:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nielsen JB, Thorolfsdottir RB, Fritsche LG, et al. Biobank-driven genom-ic discovery yields new insight into atrial fibrillation biology. Nat Genet. 2018;50:1234–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
2.2.2. Associated Arrhythmias
- 1.Saoudi N, Cosio F, Waldo A, et al. A classification of atrial flutter and regular atrial tachycardia according to electrophysiological mechanisms and anatomical bases; a statement from a Joint Expert Group from The Working Group of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 2001;22:1162–1182. [DOI] [PubMed] [Google Scholar]
- 2.Brugada J, Katritsis DG, Arbelo E, et al. 2019 ESC guidelines for the management of patients with supraventricular tachycardia. Eur Heart J. 2020;41:655–720. [DOI] [PubMed] [Google Scholar]
- 3.Wellens HJ. Contemporary management of atrial flutter. Circulation. 2002;106:649–652. [DOI] [PubMed] [Google Scholar]
2.3. Mechanisms and Pathophysiology
- 1.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 2.Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230–246. [DOI] [PubMed] [Google Scholar]
2.3.1. Electrophysiological Mechanisms and Electrical Remodeling
- 1.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 2.Egorov YV, Rosenshtraukh LV, Glukhov AV. Arrhythmogenic Interaction Between Sympathetic Tone and Mechanical Stretch in Rat Pulmonary Vein Myocardium. Front Physiol. 2020;11:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Wagoner DR, Pond AL, Lamorgese M, et al. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–436. [DOI] [PubMed] [Google Scholar]
- 4.Van Wagoner DR, Pond AL, McCarthy PM, et al. Outward K+ current densities and Kv15 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80:772–781. [DOI] [PubMed] [Google Scholar]
- 5.Voigt N, Trausch A, Knaut M, et al. Left-to-right atrial inward rectifier potassium current gradients in patients with paroxysmal versus chronic atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gemel J, Levy AE, Simon AR, et al. Connexin40 abnormalities and atrial fibrillation in the human heart. J Mol Cell Cardiol. 2014;76:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wirka RC, Gore S, Van Wagoner DR, et al. A common connexin-40 gene promoter variant affects connexin-40 expression in human atria and is associated with atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazzerini PE, Laghi-Pasini F, Acampa M, et al. Systemic Inflammation Rapidly Induces Reversible Atrial Electrical Remodeling: The Role of Interleukin-6-Mediated Changes in Connexin Expression. J Am Heart Assoc. 2019;8:e011006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan J, Kong W, Zhang Q, et al. c-Jun N-terminal kinase activation contributes to reduced connexin43 and development of atrial arrhythmias. Cardiovasc Res. 2013;97:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwasaki YK, Kato T, Xiong F, et al. Atrial fibrillation promotion with long-term repetitive obstructive sleep apnea in a rat model. J Am Coll Cardiol. 2014;64:2013–2023. [DOI] [PubMed] [Google Scholar]
- 11.Landstrom AP, Dobrev D, Wehrens XHT. Calcium Signaling and Cardiac Arrhythmias. Circ Res. 2017;120:1969–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greer-Short A, Musa H, Alsina KM, et al. Calmodulin kinase II regulates atrial myocyte late sodium current, calcium handling, and atrial arrhythmia. Heart Rhythm. 2020;17:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voigt N, Li N, Wang Q, et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Chen X, Dobrev D, et al. The crosstalk between cardiomyocyte calcium and inflammasome signaling pathways in atrial fibrillation. Pflugers Arch. 2021;473:389–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
2.3.1.1. Triggers of AF
- 1.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 2.Dewland TA, Vittinghoff E, Mandyam MC, et al. Atrial ectopy as a predictor of incident atrial fibrillation: a cohort study. Ann Intern Med. 2013;159:721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heckbert SR, Jensen PN, Austin TR, et al. Associations of left atrial function and structure with supraventricular ectopy: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2021;10:e018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howell SJ, Dukes JW, Vittinghoff E, et al. Premature atrial contraction location and atrial fibrillation inducibility. Circ Arrhythm Electrophysiol. 2023;16:e011623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voigt N, Trausch A, Knaut M, et al. Left-to-right atrial inward rectifier potassium current gradients in patients with paroxysmal versus chronic atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryu K, Sahadevan J, Khrestian CM, et al. Frequency analysis of atrial electrograms identifies conduction pathways from the left to the right atrium during atrial fibrillation-studies in two canine models. J Cardiovasc Electrophysiol. 2009;20:667–674. [DOI] [PubMed] [Google Scholar]
2.3.2. Atrial Structural Abnormalities, Remodeling, and Atrial Myopathy
- 1.Goette A, Kalman JM, Aguinaga L, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Heart Rhythm. 2017;14:e3–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudolph V, Andrie RP, Rudolph TK, et al. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat Med. 2010;16:470–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deroubaix E, Folliguet T, Rucker-Martin C, et al. Moderate and chronic hemodynamic overload of sheep atria induces reversible cellular electrophysiologic abnormalities and atrial vulnerability. J Am Coll Cardiol. 2004;44:1918–1926. [DOI] [PubMed] [Google Scholar]
2.3.2.1. Upstream Pathways
- 1.Purohit A, Rokita AG, Guan X, et al. Oxidized Ca(2+)/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation. 2013;128:1748–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mesubi OO, Rokita AG, Abrol N, et al. Oxidized CaMKII and O-GlcNAcylation cause increased atrial fibrillation in diabetic mice by distinct mechanisms. J Clin Invest. 2021;131:e95747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan J, Zhao W, Thomson JK, et al. Stress signaling JNK2 crosstalk with CaMKII underlies enhanced atrial arrhythmogenesis. Circ Res. 2018;122:821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan J, Bare DJ, DeSantiago J, et al. JNK2, a newly-identified SERCA2 enhancer, augments an arrhythmic [Ca(2+)]SR leak-load relationship. Circ Res. 2021;128:455–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med. 2018;215:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prinsen JK, Kannankeril PJ, Sidorova TN, et al. Highly reactive isolevuglandins promote atrial fibrillation caused by hypertension. JACC Basic Transl Sci. 2020;5:602–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobrev D, Dudley SC. Oxidative stress: a bystander or a causal contributor to atrial remodelling and fibrillation? Cardiovasc Res. 2021;117:2291–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet. 2009;373:155–166. [DOI] [PubMed] [Google Scholar]
- 9.Chung MK. Cardiac surgery: postoperative arrhythmias. Crit Care Med. 2000;28:N136–N144. [DOI] [PubMed] [Google Scholar]
- 10.Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. [DOI] [PubMed] [Google Scholar]
- 11.Yao C, Veleva T, Scott L Jr, et al. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation. 2018;138:2227–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
2.3.3. Role of the Autonomic Nervous System
- 1.Chen PS, Chen LS, Fishbein MC, et al. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114:1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linz D, Elliott AD, Hohl M, et al. Role of autonomic nervous system in atrial fibrillation. Int J Cardiol. 2019;287:181–188. [DOI] [PubMed] [Google Scholar]
- 3.Malik V, Mishima R, A DE, et al. The “road” to atrial fibrillation: the role of the cardiac autonomic nervous system. J Atr Fibrillation. 2020;13:2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lau DH, Schotten U, Mahajan R, et al. Novel mechanisms in the pathogenesis of atrial fibrillation: practical applications. Eur Heart J. 2016;37:1573–1581. [DOI] [PubMed] [Google Scholar]
- 5.Schotten U, Verheule S, Kirchhof P, et al. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91:265–325. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki YK, Nishida K, Kato T, et al. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264–2274. [DOI] [PubMed] [Google Scholar]
- 7.Jayachandran JV, Sih HJ, Winkle W, et al. Atrial fibrillation produced by prolonged rapid atrial pacing is associated with heterogeneous changes in atrial sympathetic innervation. Circulation. 2000;101:1185–1191. [DOI] [PubMed] [Google Scholar]
- 8.Chang CM, Wu TJ, Zhou S, et al. Nerve sprouting and sympathetic hyperinnervation in a canine model of atrial fibrillation produced by prolonged right atrial pacing. Circulation. 2001;103:22–25. [DOI] [PubMed] [Google Scholar]
- 9.Yu Y, Wei C, Liu L, et al. Atrial fibrillation increases sympathetic and parasympathetic neurons in the intrinsic cardiac nervous system. Pacing Clin Electrophysiol. 2014;37:1462–1469. [DOI] [PubMed] [Google Scholar]
- 10.Gussak G, Pfenniger A, Wren L, et al. Region-specific parasympathetic nerve remodeling in the left atrium contributes to creation of a vulnerable substrate for atrial fibrillation. JCI Insight. 2019;4:e130532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malik V, Elliott AD, Thomas G, et al. Autonomic afferent dysregulation in atrial fibrillation. JACC Clin Electrophysiol. 2022;8:152–164. [DOI] [PubMed] [Google Scholar]
- 12.Malik V, McKitrick DJ, Lau DH, et al. Clinical evidence of autonomic dysfunction due to atrial fibrillation: implications for rhythm control strategy. J Interv Card Electrophysiol. 2019;54:299–307. [DOI] [PubMed] [Google Scholar]
- 13.Wasmund SL, Li JM, Page RL, et al. Effect of atrial fibrillation and an irregular ventricular response on sympathetic nerve activity in human subjects. Circulation. 2003;107:2011–2015. [DOI] [PubMed] [Google Scholar]
- 14.Mohanty PK, Arrowood JA, Ellenbogen KA, et al. Neurohumoral and hemodynamic effects of lower body negative pressure in patients with congestive heart failure. Am Heart J. 1989;118:78–85. [DOI] [PubMed] [Google Scholar]
- 15.De With RR, Marcos EG, Dudink E, et al. Atrial fibrillation progression risk factors and associated cardiovascular outcome in well-phenotyped patients: data from the AF-RISK study. Europace. 2020;22:352–360. [DOI] [PubMed] [Google Scholar]
2.4. Genetics
- 1.Lubitz SA, Yin X, Fontes JD, et al. Association between familial atrial fibrillation and risk of new-onset atrial fibrillation. JAMA. 2010;304:2263–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brugada R, Tapscott T, Czernuszewicz GZ, et al. Identification of a genetic locus for familial atrial fibrillation. N Engl J Med. 1997;336:905–911. [DOI] [PubMed] [Google Scholar]
- 3.Chen YH, Xu SJ, Bendahhou S, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. [DOI] [PubMed] [Google Scholar]
- 4.Weng LC, Choi SH, Klarin D, et al. Heritability of atrial fibrillation. Circ Cardiovasc Genet. 2017;10:e001838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roselli C, Chaffin MD, Weng LC, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet. 2018;50:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Low SK, Takahashi A, Ebana Y, et al. Identification of six new genetic loci associated with atrial fibrillation in the Japanese population. Nat Genet. 2017;49:953–958. [DOI] [PubMed] [Google Scholar]
- 7.Lee JY, Kim TH, Yang PS, et al. Korean atrial fibrillation network genome-wide association study for early-onset atrial fibrillation identifies novel susceptibility loci. Eur Heart J. 2017;38:2586–2594. [DOI] [PubMed] [Google Scholar]
- 8.Choi SH, Jurgens SJ, Weng LC, et al. Monogenic and polygenic contributions to atrial fibrillation risk: results from a national biobank. Circ Res. 2020;126:200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoneda ZT, Anderson KC, Quintana JA, et al. Early-onset atrial fibrillation and the prevalence of rare variants in cardiomyopathy and arrhythmia genes. JAMA Cardiol. 2021;6:1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalazan B, Mol D, Darbar FA, et al. Association of rare genetic variants and early-onset atrial fibrillation in ethnic minority individuals. JAMA Cardiol. 2021;6:811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
2.5. Addressing Health Inequities and Barriers to AF Management
- 1.Kim D, Yang PS, You SC, et al. Treatment timing and the effects of rhythm control strategy in patients with atrial fibrillation: nationwide cohort study. BMJ. 2021;373:n991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamirisa KP, Al-Khatib SM, Mohanty S, et al. Racial and ethnic differences in the management of atrial fibrillation. CJC Open. 2021;3:S137–S148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lunde ED, Nielsen PB, Riahi S, et al. Associations between socioeconomic status, atrial fibrillation, and outcomes: a systematic review. Expert Rev Cardiovasc Ther. 2018;16:857–873. [DOI] [PubMed] [Google Scholar]
- 4.Hagengaard L, Andersen MP, Polcwiartek C, et al. Socioeconomic differences in outcomes after hospital admission for atrial fibrillation or flutter. Eur Heart J Qual Care Clin Outcomes. 2021;7:295–303. [DOI] [PubMed] [Google Scholar]
- 5.Harrington RA, Califf RM, Balamurugan A, et al. Call to action: rural health: a presidential advisory from the American Heart Association and American Stroke Association. Circulation. 2020;141:e615–e644. [DOI] [PubMed] [Google Scholar]
- 6.Thompson LE, Maddox TM, Lei L, et al. Sex differences in the use of oral anticoagulants for atrial fibrillation: a report from the National Cardiovascular Data Registry (NCDR(®)) PINNACLE registry. J Am Heart Assoc. 2017;6:e005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhave PD, Lu X, Girotra S, et al. Race- and sex-related differences in care for patients newly diagnosed with atrial fibrillation. Heart Rhythm. 2015;12:1406–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avgil Tsadok M, Gagnon J, Joza J, et al. Temporal trends and sex differences in pulmonary vein isolation for patients with atrial fibrillation. Heart Rhythm. 2015;12:1979–1986. [DOI] [PubMed] [Google Scholar]
- 9.Patel N, Deshmukh A, Thakkar B, et al. Gender, race, and health insurance status in patients undergoing catheter ablation for atrial fibrillation. Am J Cardiol. 2016;117:1117–1126. [DOI] [PubMed] [Google Scholar]
- 10.Ugowe FE, Jackson LR 2nd, Thomas KL. Racial and ethnic differences in the prevalence, management, and outcomes in patients with atrial fibrillation: a systematic review. Heart Rhythm. 2018;15:1337–1345. [DOI] [PubMed] [Google Scholar]
- 11.Benjamin EJ, Thomas KL, Go AS, et al. Transforming atrial fibrillation research to integrate social determinants of health: a National Heart, Lung, and Blood Institute Workshop Report. JAMA Cardiol. 2023;8:182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottlieb LM, Wing H, Adler NE. A systematic review of interventions on patients' social and economic needs. Am J Prev Med. 2017;53:719–729. [DOI] [PubMed] [Google Scholar]
- 13.White-Williams C, Rossi LP, Bittner VA, et al. Addressing social determinants of health in the care of patients with heart failure: a scientific statement from the American Heart Association. Circulation. 2020;141:e841–e863. [DOI] [PubMed] [Google Scholar]
- 14.Davidson KW, Kemper AR, Doubeni CA, et al. Developing primary care-based recommendations for social determinants of health: methods of the US Preventive Services Task Force. Ann Intern Med. 2020;173:461–467. [DOI] [PubMed] [Google Scholar]
- 15.Davidson KW, Krist AH, Tseng CW, et al. Incorporation of social risk in US Preventive Services Task Force recommendations and identification of key challenges for primary care. JAMA. 2021;326:1410–1415. [DOI] [PubMed] [Google Scholar]
- 16.Volgman AS, Benjamin EJ, Curtis AB, et al. Women and atrial fibrillation. J Cardiovasc Electrophysiol. 2021;32:2793–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russo AM, Zeitler EP, Giczewska A, et al. Association between sex and treatment outcomes of atrial fibrillation ablation versus drug therapy: results from the CABANA trial. Circulation. 2021;143:661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell ML, Larson J, Farid T, et al. Sex-based differences in procedural complications associated with atrial fibrillation catheter ablation: a systematic review and meta-analysis. J Cardiovasc Electrophysiol. 2020;31:3176–3186. [DOI] [PubMed] [Google Scholar]
- 19.Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–1316. [DOI] [PubMed] [Google Scholar]
- 20.Thomas KL, Al-Khalidi HR, Silverstein AP, et al. Ablation versus drug therapy for atrial fibrillation in racial and ethnic minorities. J Am Coll Cardiol. 2021;78:126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdel-Qadir H, Akioyamen LE, Fang J, et al. Association of neighborhood-level material deprivation with atrial fibrillation care in a single-payer health care system: a population-based cohort study. Circulation. 2022;146:159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teppo K, Jaakkola J, Biancari F, et al. Association of income and educational levels with adherence to direct oral anticoagulant therapy in patients with incident atrial fibrillation: a Finnish nationwide cohort study. Pharmacol Res Perspect. 2022;10:e00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Neal WT, Sandesara PB, Claxton JS, et al. Influence of sociodemographic factors and provider specialty on anticoagulation prescription fill patterns and outcomes in atrial fibrillation. Am J Cardiol. 2018;122:388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eberly LA, Garg L, Yang L, et al. Racial/ethnic and socioeconomic disparities in management of incident paroxysmal atrial fibrillation. JAMA Netw Open. 2021;4:e210247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tertulien T, Chen Y, Althouse AD, et al. Association of income and educational attainment in hospitalization events in atrial fibrillation. Am J Prev Cardiol. 2021;7:100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaRosa AR, Claxton J, O'Neal WT, et al. Association of household income and adverse outcomes in patients with atrial fibrillation. Heart. 2020;106:1679–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doshi R, Al-Khafaji JF, Dave M, et al. Comparison of baseline characteristics and in-hospital outcomes in medicaid versus private insurance hospitalizations for atrial fibrillation. Am J Cardiol. 2019;123:776–781. [DOI] [PubMed] [Google Scholar]
3. Shared Decision-Making (SDM) in AF Management
- 1.Kunneman M, Branda ME, Hargraves IG, et al. Assessment of shared decision-making for stroke prevention in patients with atrial fibrillation: a randomized clinical trial. JAMA Intern Med. 2020;180:1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang PJ, Lu Y, Mahaffey KW, et al. A randomized clinical trial to evaluate an atrial fibrillation stroke prevention shared decision-making pathway. J Am Heart Assoc. 2022;12:e8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres Roldan VD, Brand-McCarthy SR, Ponce OJ, et al. Shared decision making tools for people facing stroke prevention strategies in atrial fibrillation: a systematic review and environmental scan. Med Decis Making. 2021;41:540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song D, Zhou J, Fan T, et al. Decision aids for shared decision-making and appropriate anticoagulation therapy in patients with atrial fibrillation: a systematic review and meta-analysis. Eur J Cardiovasc Nurs. 2022;21:97–106. [DOI] [PubMed] [Google Scholar]
- 5.Chung MK, Fagerlin A, Wang PJ, et al. Shared decision making in cardiac electrophysiology procedures and arrhythmia management. Circ Arrhythm Electrophysiol. 2021;14:e007958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yen RW, Smith J, Engel J, et al. A systematic review and meta-analysis of patient decision aids for socially disadvantaged populations: update from the International Patient Decision Aid Standards (IDPAS). Med Decis Making. 2021;41:870–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noseworthy PA, Branda ME, Kunneman M, et al. Effect of shared decision-making for stroke prevention on treatment adherence and safety outcomes in patients with atrial fibrillation: a randomized clinical trial. J Am Heart Assoc. 2022;11:e023048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aronis KN, Edgar B, Lin W, et al. Health literacy and atrial fibrillation: relevance and future directions for patient-centred care. Eur Cardiol. 2017;12:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones AE, McCarty MM, Brito JP, et al. Randomized evaluation of decision support interventions for atrial fibrillation: rationale and design of the RED-AF study. Am Heart J. 2022;248:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mark DB, Anstrom KJ, Sheng S, et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunneman M, Branda ME, Hargraves IG, et al. Assessment of shared decision-making for stroke prevention in patients with atrial fibrillation: a randomized clinical trial. JAMA Intern Med. 2020;180:1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
4.1. Risk Stratification and Population Screening
- 1.Himmelreich JCL, Veelers L, Lucassen WAM, et al. Prediction models for atrial fibrillation applicable in the community: a systematic review and meta-analysis. Europace. 2020;22:684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li YG, Pastori D, Farcomeni A, et al. A simple clinical risk score (C2HEST) for predicting incident atrial fibrillation in asian subjects: derivation in 471 446 Chinese subjects, with internal validation and external application in 451 199 Korean subjects. Chest. 2019;155:510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu WS, Lin CL. Prediction of new-onset atrial fibrillation for general population in Asia: a comparison of C2HEST and HATCH scores. Int J Cardiol. 2020;313:60–63. [DOI] [PubMed] [Google Scholar]
- 5.Biersteker TE, Schalij MJ, Treskes RW. Impact of mobile health devices for the detection of atrial fibrillation: systematic review. JMIR Mhealth Uhealth. 2021;9:e26161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin JY, Larson J, Schoenberg J, et al. Serial 7-day electrocardiogram patch screening for AF in high-risk older women by the CHARGE-AF score. JACC Clin Electrophysiol. 2022;8:1523–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noseworthy PA, Attia ZI, Behnken EM, et al. Artificial intelligence-guided screening for atrial fibrillation using electrocardiogram during sinus rhythm: a prospective non-randomised interventional trial. Lancet. 2022;400:1206–1212. [DOI] [PubMed] [Google Scholar]
- 8.Perez MV, Mahaffey KW, Hedlin H, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Pastori D, Guo Y, et al. Risk factors for new-onset atrial fibrillation: a focus on Asian populations. Int J Cardiol. 2018;261:92–98. [DOI] [PubMed] [Google Scholar]
4.2.1. Basic Clinical Evaluation
- 1.Koyama J, Ray-Sequin PA, Falk RH. Longitudinal myocardial function assessed by tissue velocity, strain, and strain rate tissue Doppler echocardiography in patients with AL (primary) cardiac amyloidosis. Circulation. 2003;107:2446–2452. [DOI] [PubMed] [Google Scholar]
- 2.Reddy YNV, Obokata M, Verbrugge FH, et al. Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Am Coll Cardiol. 2020;76:1051–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma XX, Boldt LH, Zhang YL, et al. Clinical relevance of left atrial strain to predict recurrence of atrial fibrillation after catheter ablation: a meta-analysis. Echocardiogr. 2016;33:724–733. [DOI] [PubMed] [Google Scholar]
- 4.Costa FM, Ferreira AM, Oliveira S, et al. Left atrial volume is more important than the type of atrial fibrillation in predicting the long-term success of catheter ablation. Int J Cardiol. 2015;184:56–61. [DOI] [PubMed] [Google Scholar]
- 5.Shang W, Li L, Huang S, et al. Chronic kidney disease and the risk of new-onset atrial fibrillation: a meta-analysis of prospective cohort studies. PLoS One. 2016;11:e0155581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minhas AM, Usman MS, Khan MS, et al. Link between non-alcoholic fatty liver disease and atrial fibrillation: a systematic review and meta-analysis. Cureus. 2017;9:e1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frost L, Vestergaard P, Mosekilde L. Hyperthyroidism and risk of atrial fibrillation or flutter: a population-based study. Arch Intern Med. 2004;164:1675–1678. [DOI] [PubMed] [Google Scholar]
- 8.Askew JW, Miller TD, Hodge DO, et al. The value of myocardial perfusion single-photon emission computed tomography in screening asymptomatic patients with atrial fibrillation for coronary artery disease. J Am Coll Cardiol. 2007;50:1080–1085. [DOI] [PubMed] [Google Scholar]
- 9.Cremer PC, Mentias A, Newton D, et al. Low yield of myocardial perfusion imaging in asymptomatic patients with atrial fibrillation. JAMA Intern Med. 2015;175:1854–1855. [DOI] [PubMed] [Google Scholar]
- 10.Gex G, Gerstel E, Righini M, et al. Is atrial fibrillation associated with pulmonary embolism? J Thromb Haemost. 2012;10:347–351. [DOI] [PubMed] [Google Scholar]
- 11.Delgado V, Di Biase L, Leung M, et al. Structure and function of the left atrium and left atrial appendage: AF and stroke implications. J Am Coll Cardiol. 2017;70:3157–3172. [DOI] [PubMed] [Google Scholar]
- 12.Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725–1735. [DOI] [PubMed] [Google Scholar]
4.2.2. Rhythm Monitoring Tools and Methods
- 1.Yang TY, Huang L, Malwade S, et al. Diagnostic accuracy of ambulatory devices in detecting atrial fibrillation: systematic review and meta-analysis. JMIR Mhealth Uhealth. 2021;9:e26167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prasitlumkum N, Cheungpasitporn W, Chokesuwattanaskul A, et al. Diagnostic accuracy of smart gadgets/wearable devices in detecting atrial fibrillation: a systematic review and meta-analysis. Arch Cardiovasc Dis. 2021;114:4–16. [DOI] [PubMed] [Google Scholar]
- 3.Wong KC, Klimis H, Lowres N, et al. Diagnostic accuracy of handheld electrocardiogram devices in detecting atrial fibrillation in adults in community versus hospital settings: a systematic review and meta-analysis. Heart. 2020;106:1211–1217. [DOI] [PubMed] [Google Scholar]
- 4.Kapa S, Epstein AE, Callans DJ, et al. Assessing arrhythmia burden after catheter ablation of atrial fibrillation using an implantable loop recorder: the ABACUS study. J Cardiovasc Electrophysiol. 2013;24:875–881. [DOI] [PubMed] [Google Scholar]
- 5.Podd SJ, Sugihara C, Furniss SS, et al. Are implantable cardiac monitors the 'gold standard' for atrial fibrillation detection? A prospective randomized trial comparing atrial fibrillation monitoring using implantable cardiac monitors and DDDRP permanent pacemakers in post atrial fibrillation ablation patients. Europace. 2016;18:1000–1005. [DOI] [PubMed] [Google Scholar]
- 6.de Voogt WG, van Hemel NM, van de Bos AA, et al. Verification of pacemaker automatic mode switching for the detection of atrial fibrillation and atrial tachycardia with Holter recording. Europace. 2006;8:950–961. [DOI] [PubMed] [Google Scholar]
- 7.Eysenck W, Freemantle N, Sulke N. A randomized trial evaluating the accuracy of AF detection by four external ambulatory ECG monitors compared to permanent pacemaker AF detection. J Interv Card Electrophysiol. 2020;57:361–369. [DOI] [PubMed] [Google Scholar]
- 8.Vitolo M, Imberti JF, Maisano A, et al. Device-detected atrial high rate episodes and the risk of stroke/thrombo-embolism and atrial fibrillation incidence: a systematic review and meta-analysis. Eur J Intern Med. 2021;92:100–106. [DOI] [PubMed] [Google Scholar]
- 9.Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. [DOI] [PubMed] [Google Scholar]
- 10.Buck BH, Hill MD, Quinn FR, et al. Effect of implantable vs prolonged external electrocardiographic monitoring on atrial fibrillation detection in patients with ischemic stroke: the PER DIEM randomized clinical trial. JAMA. 2021;325:2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernstein RA, Kamel H, Granger CB, et al. Effect of long-term continuous cardiac monitoring vs usual care on detection of atrial fibrillation in patients with stroke attributed to large- or small-vessel disease: the STROKE-AF randomized clinical trial. JAMA. 2021;325:2169–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hannun AY, Rajpurkar P, Haghpanahi M, et al. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med. 2019;25:65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldenthal IL, Sciacca RR, Riga T, et al. Recurrent atrial fibrillation/flutter detection after ablation or cardioversion using the AliveCor KardiaMobile device: iHEART results. J Cardiovasc Electrophysiol. 2019;30:2220–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill S, Bunting KV, Sartini C, et al. Smartphone detection of atrial fibrillation using photoplethysmography: a systematic review and meta-analysis. Heart. 2022;108:1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tison GH, Sanchez JM, Ballinger B, et al. Passive detection of atrial fibrillation using a commercially available smartwatch. JAMA Cardiol. 2018;3:409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez MV, Mahaffey KW, Hedlin H, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Y, Wang H, Zhang H, et al. Mobile Photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol. 2019;74:2365–2375. [DOI] [PubMed] [Google Scholar]
- 18.Avram R, Ramsis M, Cristal AD, et al. Validation of an algorithm for continuous monitoring of atrial fibrillation using a consumer smartwatch. Heart Rhythm. 2021;18:1482–1490. [DOI] [PubMed] [Google Scholar]
- 19.Dussault C, Toeg H, Nathan M, et al. Electrocardiographic monitoring for detecting atrial fibrillation after ischemic stroke or transient ischemic attack: systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2015;8:263–269. [DOI] [PubMed] [Google Scholar]
- 20.Caceres BA, Hickey KT, Bakken SB, et al. Mobile electrocardiogram monitoring and health-related quality of life in patients with atrial fibrillation: findings from the iPhone Helping Evaluate Atrial Fibrillation Rhythm Through Technology (iHEART) study. J Cardiovasc Nurs. 2020;35:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
5.1. Primary Prevention
- 1.Tedrow UB, Conen D, Ridker PM, et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women's health study). J Am Coll Cardiol. 2010;55:2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elliott AD, Linz D, Mishima R, et al. Association between physical activity and risk of incident arrhythmias in 402 406 individuals: evidence from the UK Biobank cohort. Eur Heart J. 2020;41:1479–1486. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher C, Hendriks JML, Elliott AD, et al. Alcohol and incident atrial fibrillation - a systematic review and meta-analysis. Int J Cardiol. 2017;246:46–52. [DOI] [PubMed] [Google Scholar]
- 4.Aune D, Schlesinger S, Norat T, et al. Tobacco smoking and the risk of atrial fibrillation: a systematic review and meta-analysis of prospective studies. Eur J Prev Cardiol. 2018;25:1437–1451. [DOI] [PubMed] [Google Scholar]
- 5.Aune D, Feng T, Schlesinger S, et al. Diabetes mellitus, blood glucose and the risk of atrial fibrillation: a systematic review and meta-analysis of cohort studies. J Diabetes Complications. 2018;32:501–511. [DOI] [PubMed] [Google Scholar]
- 6.Soliman EZ, Rahman AF, Zhang ZM, et al. Effect of intensive blood pressure lowering on the risk of atrial fibrillation. Hypertension. 2020;75:1491–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Himmelreich JCL, Veelers L, Lucassen WAM, et al. Prediction models for atrial fibrillation applicable in the community: a systematic review and meta-analysis. Europace. 2020;22:684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roselli C, Chaffin MD, Weng LC, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet. 2018;50:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huxley RR, Lopez FL, Folsom AR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg PK, O'Neal WT, Ogunsua A, et al. Usefulness of the American Heart Association's Life Simple 7 to predict the risk of atrial fibrillation (from the REasons for Geographic And Racial Differences in Stroke [REGARDS] study). Am J Cardiol. 2018;121:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Benedetto L, Michels G, Luben R, et al. Individual and combined impact of lifestyle factors on atrial fibrillation in apparently healthy men and women: the EPIC-Norfolk prospective population study. Eur J Prev Cardiol. 2018;25:1374–1383. [DOI] [PubMed] [Google Scholar]
- 13.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin AL, Nah G, Tang JJ, et al. Cannabis, cocaine, methamphetamine, and opiates increase the risk of incident atrial fibrillation. Eur Heart J. 2022;43:4933–4942. [DOI] [PubMed] [Google Scholar]
- 15.Mishima RS, Verdicchio CV, Noubiap JJ, et al. Self-reported physical activity and atrial fibrillation risk: a systematic review and meta-analysis. Heart Rhythm. 2021;18:520–528. [DOI] [PubMed] [Google Scholar]
- 16.Jin MN, Yang PS, Song C, et al. Physical activity and risk of atrial fibrillation: a nationwide cohort study in general population. Sci Rep. 2019;9:13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khurshid S, Weng LC, Al-Alusi MA, et al. Accelerometer-derived physical activity and risk of atrial fibrillation. Eur Heart J. 2021;42:2472–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tikkanen E, Gustafsson S, Ingelsson E. Associations of fitness, physical activity, strength, and genetic risk with cardiovascular disease: longitudinal analyses in the UK Biobank study. Circulation. 2018;137:2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qureshi WT, Alirhayim Z, Blaha MJ, et al. Cardiorespiratory fitness and risk of incident atrial fibrillation results from the Henry Ford exercise testing (FIT) project. Circulation. 2015;131:1827–1834. [DOI] [PubMed] [Google Scholar]
- 20.Khan H, Kella D, Rauramaa R, et al. Cardiorespiratory fitness and atrial fibrillation: a population-based follow-up study. Heart Rhythm. 2015;12:1424–1430. [DOI] [PubMed] [Google Scholar]
- 21.Andersen K, Farahmand B, Ahlbom A, et al. Risk of arrhythmias in 52 755 long-distance cross-country skiers: a cohort study. Eur Heart J. 2013;34:3624–3631. [DOI] [PubMed] [Google Scholar]
- 22.Baldesberger S, Bauersfeld U, Candinas R, et al. Sinus node disease and arrhythmias in the long-term follow-up of former professional cyclists. Eur Heart J. 2008;29:71–78. [DOI] [PubMed] [Google Scholar]
- 23.Newman W, Parry-Williams G, Wiles J, et al. Risk of atrial fibrillation in athletes: a systematic review and meta-analysis. Br J Sports Med. 2021;55:1233–1238. [DOI] [PubMed] [Google Scholar]
- 24.Morseth B, Graff-Iversen S, Jacobsen BK, et al. Physical activity, resting heart rate, and atrial fibrillation: the Tromsø study. Eur Heart J. 2016;37:2307–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcus GM, Vittinghoff E, Whitman IR, et al. Acute consumption of alcohol and discrete atrial fibrillation events. Ann Intern Med. 2021;174:1503–1509. [DOI] [PubMed] [Google Scholar]
- 26.Voskoboinik A, McDonald C, Chieng D, et al. Acute electrical, autonomic and structural effects of binge drinking: insights into the 'holiday heart syndrome'. Int J Cardiol. 2021;331:100–105. [DOI] [PubMed] [Google Scholar]
- 27.Whitman IR, Agarwal V, Nah G, et al. Alcohol abuse and cardiac disease. J Am Coll Cardiol. 2017;69:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Csengeri D, Sprunker NA, Di Castelnuovo A, et al. Alcohol consumption, cardiac biomarkers, and risk of atrial fibrillation and adverse outcomes. Eur Heart J. 2021;42:1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tu SJ, Gallagher C, Elliott AD, et al. Risk Thresholds for total and beverage-specific alcohol consumption and incident atrial fibrillation. JACC Clin Electrophysiol. 2021;7:1561–1569. [DOI] [PubMed] [Google Scholar]
- 30.Zuo H, Nygard O, Vollset SE, et al. Smoking, plasma cotinine and risk of atrial fibrillation: the Hordaland Health Study. J Intern Med. 2018;283:73–82. [DOI] [PubMed] [Google Scholar]
- 31.Lu Y, Guo Y, Lin H, et al. Genetically determined tobacco and alcohol use and risk of atrial fibrillation. BMC Med Genomics. 2021;14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsson SC, Wallin A, Hakansson N, et al. Type 1 and type 2 diabetes mellitus and incidence of seven cardiovascular diseases. Int J Cardiol. 2018;262:66–70. [DOI] [PubMed] [Google Scholar]
- 33.Qi W, Zhang N, Korantzopoulos P, et al. Serum glycated hemoglobin level as a predictor of atrial fibrillation: a systematic review with meta-analysis and meta-regression. PLoS One. 2017;12:e0170955. [DOI] [PMC free article] [PubMed] [Google Scholar]
5.2.1. Weight Loss in Individuals Who Are Overweight or Obese
- 1.Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310:2050–2060. [DOI] [PubMed] [Google Scholar]
- 2.Pathak RK, Middeldorp ME, Meredith M, et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol. 2015;65:2159–2169. [DOI] [PubMed] [Google Scholar]
- 3.Pathak RK, Middeldorp ME, Lau DH, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231. [DOI] [PubMed] [Google Scholar]
- 4.Middeldorp ME, Pathak RK, Meredith M, et al. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. Europace. 2018;20:1929–1935. [DOI] [PubMed] [Google Scholar]
- 5.Wong CX, Sullivan T, Sun MT, et al. Obesity and the risk of incident, postoperative, and post-ablation atrial fibrillation: a meta-analysis of 626 603 individuals in 51 studies. JACC: Clin Electrophysiol. 2015;1:139–152. [DOI] [PubMed] [Google Scholar]
- 6.Abed HS, Samuel CS, Lau DH, et al. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm. 2013;10:90–100. [DOI] [PubMed] [Google Scholar]
- 7.Mahajan R, Lau DH, Brooks AG, et al. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol. 2015;66:1–11. [DOI] [PubMed] [Google Scholar]
- 8.Mahajan R, Lau DH, Brooks AG, et al. Atrial fibrillation and obesity: reverse remodeling of atrial substrate with weight reduction. JACC Clin Electrophysiol. 2021;7:630–641. [DOI] [PubMed] [Google Scholar]
- 9.Grundvold I, Bodegard J, Nilsson PM, et al. Body weight and risk of atrial fibrillation in 7 169 patients with newly diagnosed type 2 diabetes; an observational study. Cardiovasc Diabetol. 2015;14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Benedetto L, Michels G, Luben R, et al. Individual and combined impact of lifestyle factors on atrial fibrillation in apparently healthy men and women: the EPIC-Norfolk prospective population study. Eur J Prev Cardiol. 2018;25:1374–1383. [DOI] [PubMed] [Google Scholar]
- 11.Providência R, Adrag ão P, de Asmundis C, et al. Impact of body mass index on the outcomes of catheter ablation of atrial fibrillation: a European observational multicenter study. J Am Heart Assoc. 2019;8:e012253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glover BM, Hong KL, Dagres N, et al. Impact of body mass index on the outcome of catheter ablation of atrial fibrillation. Heart. 2019;105:244–250. [DOI] [PubMed] [Google Scholar]
- 13.Donnellan E, Wazni O, Kanj M, et al. Outcomes of atrial fibrillation ablation in morbidly obese patients following bariatric surgery compared with a nonobese cohort. Circ Arrhythm Electrophysiol. 2019;12:e007598. [DOI] [PubMed] [Google Scholar]
- 14.Donnellan E, Wazni OM, Kanj M, et al. Association between pre-ablation bariatric surgery and atrial fibrillation recurrence in morbidly obese patients undergoing atrial fibrillation ablation. Europace. 2019;21:1476–1483. [DOI] [PubMed] [Google Scholar]
- 15.Donnellan E, Wazni OM, Elshazly M, et al. Impact of bariatric surgery on atrial fibrillation type. Circ Arrhythm Electrophysiol. 2020;13:e007626. [DOI] [PubMed] [Google Scholar]
- 16.Donnellan E, Wazni OM, Kanj M, et al. Impact of risk-factor modification on arrhythmia recurrence among morbidly obese patients undergoing atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2020;31:1979–1986. [DOI] [PubMed] [Google Scholar]
- 17.Mohanty S, Mohanty P, Natale V, et al. Impact of weight loss on ablation outcome in obese patients with longstanding persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2018;29:246–253. [DOI] [PubMed] [Google Scholar]
5.2.2. Physical Fitness
- 1.Hegbom F, Stavem K, Sire S, et al. Effects of short-term exercise training on symptoms and quality of life in patients with chronic atrial fibrillation. Int J Cardiol. 2007;116:86–92. [DOI] [PubMed] [Google Scholar]
- 2.Malmo V, Nes BM, Amundsen BH, et al. Aerobic interval training reduces the burden of atrial fibrillation in the short term: a randomized trial. Circulation. 2016;133:466–473. [DOI] [PubMed] [Google Scholar]
- 3.Elliott AD, Verdicchio CV, Mahajan R, et al. An exercise and physical activity program in patients with atrial fibrillation: the ACTIVE-AF randomized controlled trial. JACC Clin Electrophysiol. 2023;9:455–465. [DOI] [PubMed] [Google Scholar]
- 4.Pathak RK, Elliott A, Middeldorp ME, et al. Impact of CARDIOrespiratory FITness on arrhythmia recurrence in obese individuals with atrial fibrillation: the CARDIO-FIT study. J Am Coll Cardiol. 2015;66:985–996. [DOI] [PubMed] [Google Scholar]
- 5.Oesterle A, Giancaterino S, Van Noord MG, et al. Effects of supervised exercise training on atrial fibrillation: a meta-analysis of randomized controlled trials. J Cardiopulm Rehabil Prev. 2022;42:258–265. [DOI] [PubMed] [Google Scholar]
- 6.Osbak PS, Mourier M, Kjaer A, et al. A randomized study of the effects of exercise training on patients with atrial fibrillation. Am Heart J. 2011;162:1080–1087. [DOI] [PubMed] [Google Scholar]
- 7.Ahn HJ, Lee SR, Choi EK, et al. Association between exercise habits and stroke, heart failure, and mortality in Korean patients with incident atrial fibrillation: a nationwide population-based cohort study. PLoS Med. 2021;18:e1003659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skielboe AK, Bandholm TQ, Hakmann S, et al. Cardiovascular exercise and burden of arrhythmia in patients with atrial fibrillation - a randomized controlled trial. PLoS One. 2017;12:e0170060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdulla J, Nielsen JR. Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. Europace. 2009;11:1156–1159. [DOI] [PubMed] [Google Scholar]
- 10.Andersen K, Farahmand B, Ahlbom A, et al. Risk of arrhythmias in 52 755 long-distance cross-country skiers: a cohort study. Eur Heart J. 2013;34:3624–3631. [DOI] [PubMed] [Google Scholar]
- 11.Smart NA, King N, Lambert JD, et al. Exercise-based cardiac rehabilitation improves exercise capacity and health-related quality of life in people with atrial fibrillation: a systematic review and meta-analysis of randomised and non-randomised trials. Open Heart. 2018;5:e000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Risom SS, Zwisler AD, Rasmussen TB, et al. Cardiac rehabilitation versus usual care for patients treated with catheter ablation for atrial fibrillation: results of the randomized CopenHeartRFA trial. Am Heart J. 2016;181:120–129. [DOI] [PubMed] [Google Scholar]
- 13.Kato M, Ogano M, Mori Y, et al. Exercise-based cardiac rehabilitation for patients with catheter ablation for persistent atrial fibrillation: a randomized controlled clinical trial. Eur J Prev Cardiol. 2019;26:1931–1940. [DOI] [PubMed] [Google Scholar]
- 14.Luo N, Merrill P, Parikh KS, et al. Exercise Training in patients with chronic heart failure and atrial fibrillation. J Am Coll Cardiol. 2017;69:1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckley BJR, Harrison SL, Fazio-Eynullayeva E, et al. Exercise-based cardiac rehabilitation and all-cause mortality among patients with atrial fibrillation. J Am Heart Assoc. 2021;10:e020804. [DOI] [PMC free article] [PubMed] [Google Scholar]
5.2.3. Smoking Cessation
- 1.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patnode CD, Henderson JT, Thompson JH, et al. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the US Preventive Services Task Force. Ann Intern Med. 2015;163:608–621. [DOI] [PubMed] [Google Scholar]
- 3.Choi S, Chang J, Kim K, et al. Association of smoking cessation after atrial fibrillation diagnosis on the risk of cardiovascular disease: a cohort study of South Korean men. BMC Public Health. 2020;20:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benz AP, Healey JS, Chin A, et al. Stroke risk prediction in patients with atrial fibrillation with and without rheumatic heart disease. Cardiovasc Res. 2022;118:295–304. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee NA, Chae CU, Kim E, et al. Modifiable risk factors for incident heart failure in atrial fibrillation. JACC Heart Fail. 2017;5:552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamberlain AM, Alonso A, Gersh BJ, et al. Multimorbidity and the risk of hospitalization and death in atrial fibrillation: a population-based study. Am Heart J. 2017;185:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sardana M, Tang Y, Magnani JW, et al. Provider-level variation in smoking cessation assistance provided in the cardiology clinics: insights from the NCDR PINNACLE registry. J Am Heart Assoc. 2019;8:e011412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Y, Britton J, Hubbard R, et al. Who receives prescriptions for smoking cessation medications? An association rule mining analysis using a large primary care database. Tob Control. 2013;22:274–279. [DOI] [PubMed] [Google Scholar]
- 9.Stead LF, Buitrago D, Preciado N, et al. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2013;5:CD000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann-Boyce J, Livingstone-Banks J, Ordonez-Mena JM, et al. Behavioural interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2021;1:CD013229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suissa K, Lariviere J, Eisenberg MJ, et al. Efficacy and safety of smoking cessation interventions in patients with cardiovascular disease: a network meta-analysis of randomized controlled trials. Circ Cardiovasc Qual Outcomes. 2017;10:e002458. [DOI] [PubMed] [Google Scholar]
- 12.Buiatti A, Kaess B, Reents T, et al. Catheter ablation for “lone” atrial fibrillation: efficacy and predictors of recurrence. J Cardiovasc Electrophysiol. 2016;27:536–541. [DOI] [PubMed] [Google Scholar]
- 13.Cheng WH, Lo LW, Lin YJ, et al. Cigarette smoking causes a worse long-term outcome in persistent atrial fibrillation following catheter ablation. J Cardiovasc Electrophysiol. 2018;29:699–706. [DOI] [PubMed] [Google Scholar]
- 14.Apostolakis S, Sullivan RM, Olshansky B, et al. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: the SAMe-TT(2)R(2) score. Chest. 2013;144:1555–1563. [DOI] [PubMed] [Google Scholar]
- 15.Albertsen IE, Rasmussen LH, Lane DA, et al. The impact of smoking on thromboembolism and mortality in patients with incident atrial fibrillation: insights from the Danish Diet, Cancer, and Health study. Chest. 2014;145:559–566. [DOI] [PubMed] [Google Scholar]
- 16.Lee SR, Choi EK, Jung JH, et al. Smoking cessation after diagnosis of new-onset atrial fibrillation and the risk of stroke and death. J Clin Med. 2021;10:2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
5.2.4. Alcohol Consumption
- 1.Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382:20–28. [DOI] [PubMed] [Google Scholar]
- 2.Marcus GM, Dukes JW, Vittinghoff E, et al. A randomized, double-blind, placebo-controlled trial of intravenous alcohol to assess changes in atrial electrophysiology. JACC Clin Electrophysiol. 2021;7:662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus GM, Modrow MF, Schmid CH, et al. Individualized studies of triggers of paroxysmal atrial fibrillation: the I-STOP-AFib randomized clinical trial. JAMA Cardiol. 2022;7:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcus GM, Vittinghoff E, Whitman IR, et al. Acute consumption of alcohol and discrete atrial fibrillation events. Ann Intern Med. 2021;174:1503–1509. [DOI] [PubMed] [Google Scholar]
- 5.Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310:2050–2060. [DOI] [PubMed] [Google Scholar]
- 6.Pathak RK, Middeldorp ME, Lau DH, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231. [DOI] [PubMed] [Google Scholar]
- 7.Pathak RK, Middeldorp ME, Meredith M, et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol. 2015;65:2159–2169. [DOI] [PubMed] [Google Scholar]
- 8.Middeldorp ME, Pathak RK, Meredith M, et al. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. Europace. 2018;20:1929–1935. [DOI] [PubMed] [Google Scholar]
5.2.5. Caffeine Consumption
- 1.Lagier D, Nee L, Guieu R, et al. Peri-operative oral caffeine does not prevent postoperative atrial fibrillation after heart valve surgery with cardiopulmonary bypass: a randomised controlled clinical trial. Eur J Anaesthesiol. 2018;35:911–918. [DOI] [PubMed] [Google Scholar]
- 2.Marcus GM, Modrow MF, Schmid CH, et al. Individualized Studies of triggers of paroxysmal atrial fibrillation: the I-STOP-AFib randomized clinical trial. JAMA Cardiol. 2022;7:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caldeira D, Martins C, Alves LB, et al. Caffeine does not increase the risk of atrial fibrillation: a systematic review and meta-analysis of observational studies. Heart. 2013;99:1383–1389. [DOI] [PubMed] [Google Scholar]
- 4.Conen D, Chiuve SE, Everett BM, et al. Caffeine consumption and incident atrial fibrillation in women. Am J Clin Nutr. 2010;92:509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazal P, Gea A, Navarro AM, et al. Caffeinated coffee consumption and risk of atrial fibrillation in two Spanish cohorts. Eur J Prev Cardiol. 2021;28:648–657. [DOI] [PubMed] [Google Scholar]
- 6.Larsson SC, Drca N, Jensen-Urstad M, et al. Coffee consumption is not associated with increased risk of atrial fibrillation: results from two prospective cohorts and a meta-analysis. BMC Med. 2015;13:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng M, Hu Z, Lu X, et al. Caffeine intake and atrial fibrillation incidence: dose response meta-analysis of prospective cohort studies. Can J Cardiol. 2014;30:448–454. [DOI] [PubMed] [Google Scholar]
- 8.Kim EJ, Hoffmann TJ, Nah G, et al. Coffee consumption and incident tachyarrhythmias: reported behavior, Mendelian randomization, and their interactions. JAMA Intern Med. 2021;181:1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bodar V, Chen J, Gaziano JM, et al. Coffee consumption and risk of atrial fibrillation in the physicians' health study. J Am Heart Assoc. 2019;8:e011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groh CA, Faulkner M, Getabecha S, et al. Patient-reported triggers of paroxysmal atrial fibrillation. Heart Rhythm. 2019;16:996–1002. [DOI] [PubMed] [Google Scholar]
- 11.Yuan S, Larsson SC. No association between coffee consumption and risk of atrial fibrillation: a Mendelian randomization study. Nutr Metab Cardiovasc Dis. 2019;29:1185–1188. [DOI] [PubMed] [Google Scholar]
- 12.Mattioli AV, Pennella S, Farinetti A, et al. Energy drinks and atrial fibrillation in young adults. Clin Nutr. 2018;37:1073–1074. [DOI] [PubMed] [Google Scholar]
- 13.Di Rocco JR, During A, Morelli PJ, et al. Atrial fibrillation in healthy adolescents after highly caffeinated beverage consumption: two case reports. J Med Case Rep. 2011;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattioli AV, Bonatti S, Zennaro M, et al. Effect of coffee consumption, lifestyle and acute life stress in the development of acute lone atrial fibrillation. J Cardiovasc Med (Hagerstown). 2008;9:794–798. [DOI] [PubMed] [Google Scholar]
5.2.6. Diet and Dietary Supplementation
- 1.Aleksova A, Masson S, Maggioni AP, et al. n-3 polyunsaturated fatty acids and atrial fibrillation in patients with chronic heart failure: the GISSI-HF trial. Eur J Heart Fail. 2013;15:1289–1295. [DOI] [PubMed] [Google Scholar]
- 2.Metcalf RG, Skuladottir GV, Indridason OS, et al. U-shaped relationship between tissue docosahexaenoic acid and atrial fibrillation following cardiac surgery. Eur J Clin Nutr. 2014;68:114–118. [DOI] [PubMed] [Google Scholar]
- 3.Gencer B, Djousse L, Al-Ramady OT, et al. Effect of long-term marine omega-3 fatty acids supplementation on the risk of atrial fibrillation in randomized controlled trials of cardiovascular outcomes: a systematic review and meta-analysis. Circulation. 2021;144:1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darghosian L, Free M, Li J, et al. Effect of omega-three polyunsaturated fatty acids on inflammation, oxidative stress, and recurrence of atrial fibrillation. Am J Cardiol. 2015;115:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowey PR, Reiffel JA, Ellenbogen KA, et al. Efficacy and safety of prescription omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: a randomized controlled trial. JAMA. 2010;304:2363–2372. [DOI] [PubMed] [Google Scholar]
- 6.Nigam A, Talajic M, Roy D, et al. Fish oil for the reduction of atrial fibrillation recurrence, inflammation, and oxidative stress. J Am Coll Cardiol. 2014;64:1441–1448. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Sutherland F, Stevenson I, et al. Effects of long-term ω-3 polyunsaturated fatty acid supplementation on paroxysmal atrial tachyarrhythmia burden in patients with implanted pacemakers: results from a prospective randomised study. Int J Cardiol. 2013;168:3812–3817. [DOI] [PubMed] [Google Scholar]
- 8.Albert CM, Cook NR, Pester J, et al. Effect of marine omega-3 fatty acid and vitamin D supplementation on incident atrial fibrillation: a randomized clinical trial. JAMA. 2021;325:1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang WL, Yang J, Yang J, et al. Vitamin D and new-onset atrial fibrillation: a meta-analysis of randomized controlled trials. Hellenic J Cardiol. 2018;59:72–77. [DOI] [PubMed] [Google Scholar]
- 10.Kara H, Yasim A. Effects of high-dose vitamin D supplementation on the occurrence of post-operative atrial fibrillation after coronary artery bypass grafting: randomized controlled trial. Gen Thorac Cardiovasc Surg. 2020;68:477–484. [DOI] [PubMed] [Google Scholar]
- 11.Shi R, Li ZH, Chen D, et al. Sole and combined vitamin C supplementation can prevent postoperative atrial fibrillation after cardiac surgery: a systematic review and meta-analysis of randomized controlled trials. Clin Cardiol. 2018;41:871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310:2050–2060. [DOI] [PubMed] [Google Scholar]
- 13.Pathak RK, Middeldorp ME, Meredith M, et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol. 2015;65:2159–2169. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S, Zhuang X, Lin X, et al. Low-carbohydrate diets and risk of incident atrial fibrillation: a prospective cohort study. J Am Heart Assoc. 2019;8:e011955. [DOI] [PMC free article] [PubMed] [Google Scholar]
5.2.7. Diabetes
- 1.Echouffo-Tcheugui JB, Shrader P, Thomas L, et al. Care patterns and outcomes in atrial fibrillation patients with and without diabetes: ORBIT-AF registry. J Am Coll Cardiol. 2017;70:1325–1335. [DOI] [PubMed] [Google Scholar]
- 2.Patti G, Di Gioia G, Cavallari I, et al. Safety and efficacy of nonvitamin K antagonist oral anticoagulants versus warfarin in diabetic patients with atrial fibrillation: a study-level meta-analysis of phase III randomized trials. Diabetes Metab Res Rev. 2017;33:e2876. [DOI] [PubMed] [Google Scholar]
- 3.Donnellan E, Aagaard P, Kanj M, et al. Association between pre-ablation glycemic control and outcomes among patients with diabetes undergoing atrial fibrillation ablation. JACC Clin Electrophysiol. 2019;5:897–903. [DOI] [PubMed] [Google Scholar]
5.2.8. Treatment of Hypertension
- 1.Neefs J, van den Berg NW, Limpens J, et al. Aldosterone pathway blockade to prevent atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol. 2017;231:155–161. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg JS, Shabanov V, Ponomarev D, et al. Effect of renal denervation and catheter ablation vs catheter ablation alone on atrial fibrillation recurrence among patients with paroxysmal atrial fibrillation and hypertension: the ERADICATE-AF randomized clinical trial. JAMA. 2020;323:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pathak RK, Middeldorp ME, Lau DH, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231. [DOI] [PubMed] [Google Scholar]
- 4.Rienstra M, Hobbelt AH, Alings M, et al. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J. 2018;39:2987–2996. [DOI] [PubMed] [Google Scholar]
- 5.Pinho-Gomes AC, Azevedo L, Copland E, et al. Blood pressure-lowering treatment for the prevention of cardiovascular events in patients with atrial fibrillation: an individual participant data meta-analysis. PLoS Med. 2021;18:e1003599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pokorney SD, Piccini JP, Stevens SR, et al. Cause of death and predictors of all-cause mortality in anticoagulated patients with nonvalvular atrial fibrillation: data from ROCKET AF. J Am Heart Assoc. 2016;5:e002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao MP, Halvorsen S, Wojdyla D, et al. Blood pressure control and risk of stroke or systemic embolism in patients with atrial fibrillation: results from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. J Am Heart Assoc. 2015;4:e002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parkash R, Wells GA, Sapp JL, et al. Effect of aggressive blood pressure control on the recurrence of atrial fibrillation after catheter ablation: a randomized, open-label clinical trial (SMAC-AF [Substrate Modification With Aggressive Blood Pressure Control]). Circulation. 2017;135:1788–1798. [DOI] [PubMed] [Google Scholar]
- 9.Soliman EZ, Rahman AF, Zhang ZM, et al. Effect of intensive blood pressure lowering on the risk of atrial fibrillation. Hypertension. 2020;75:1491–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim TH, Yang PS, Yu HT, et al. Effect of hypertension duration and blood pressure level on ischaemic stroke risk in atrial fibrillation: nationwide data covering the entire Korean population. Eur Heart J. 2019;40:809–819. [DOI] [PubMed] [Google Scholar]
- 11.Pathak RK, Middeldorp ME, Meredith M, et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol. 2015;65:2159–2169. [DOI] [PubMed] [Google Scholar]
5.2.9. Sleep
- 1.Kadhim K, Middeldorp ME, Elliott AD, et al. Prevalence and assessment of sleep-disordered breathing in patients with atrial fibrillation: a systematic review and meta-analysis. Can J Cardiol. 2021;37:1846–1856. [DOI] [PubMed] [Google Scholar]
- 2.Mohammadieh AM, Sutherland K, Kanagaratnam LB, et al. Clinical screening tools for obstructive sleep apnea in a population with atrial fibrillation: a diagnostic accuracy trial. J Clin Sleep Med. 2021;17:1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linz D, Brooks AG, Elliott AD, et al. Variability of sleep apnea severity and risk of atrial fibrillation: the VARIOSA-AF study. JACC Clin Electrophysiol. 2019;5:692–701. [DOI] [PubMed] [Google Scholar]
- 4.Abumuamar AM, Dorian P, Newman D, et al. The prevalence of obstructive sleep apnea in patients with atrial fibrillation. Clin Cardiol. 2018;41:601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platek AE, Szymanski FM, Filipiak KJ, et al. Stratification of cardiovascular risk in patients with atrial fibrillation and obstructive sleep apnea-validity of the 2MACE score. Sleep Breath. 2017;21:601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Zhou X, Xu X, et al. Effects of continuous positive airway pressure treatment in obstructive sleep apnea patients with atrial fibrillation: a meta-analysis. Medicine (Baltim). 2021;100:e25438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y, Ning Y, Wen W, et al. CPAP is associated with decreased risk of AF recurrence in patients with OSA, especially those younger and slimmer: a meta-analysis. J Interv Card Electrophysiol. 2020;58:369–379. [DOI] [PubMed] [Google Scholar]
- 8.Congrete S, Bintvihok M, Thongprayoon C, et al. Effect of obstructive sleep apnea and its treatment of atrial fibrillation recurrence after radiofrequency catheter ablation: a meta-analysis. J Evid Based Med. 2018;11:145–151. [DOI] [PubMed] [Google Scholar]
- 9.Deng F, Raza A, Guo J. Treating obstructive sleep apnea with continuous positive airway pressure reduces risk of recurrent atrial fibrillation after catheter ablation: a meta-analysis. Sleep Med. 2018;46:5–11. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi WT, Nasir UB, Alqalyoobi S, et al. Meta-analysis of continuous positive airway pressure as a therapy of atrial fibrillation in obstructive sleep apnea. Am J Cardiol. 2015;116:1767–1773. [DOI] [PubMed] [Google Scholar]
- 11.Szymanski FM, Filipiak KJ, Platek AE, et al. Presence and severity of obstructive sleep apnea and remote outcomes of atrial fibrillation ablations - a long-term prospective, cross-sectional cohort study. Sleep Breath. 2015;19:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caples SM, Mansukhani MP, Friedman PA, et al. The impact of continuous positive airway pressure treatment on the recurrence of atrial fibrillation post cardioversion: a randomized controlled trial. Int J Cardiol. 2019;278:133–136. [DOI] [PubMed] [Google Scholar]
- 13.Traaen GM, Aakeroy L, Hunt TE, et al. Effect of continuous positive airway pressure on arrhythmia in atrial fibrillation and sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2021;204:573–582. [DOI] [PubMed] [Google Scholar]
- 14.Marti-Almor J, Marques P, Jesel L, et al. Incidence of sleep apnea and association with atrial fibrillation in an unselected pacemaker population: results of the observational RESPIRE study. Heart Rhythm. 2020;17:195–202. [DOI] [PubMed] [Google Scholar]
- 15.Yeung C, Drew D, Hammond S, et al. Extended cardiac monitoring in patients with severe sleep apnea and no history of atrial fibrillation (the Reveal XT-SA study). Am J Cardiol. 2018;122:1885–1889. [DOI] [PubMed] [Google Scholar]
- 16.Zhao E, Chen S, Du Y, et al. Association between sleep apnea hypopnea syndrome and the risk of atrial fibrillation: a meta-analysis of cohort study. Biomed Res Int. 2018;2018:5215868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.May AM, Blackwell T, Stone PH, et al. Central sleep-disordered breathing predicts incident atrial fibrillation in older men. Am J Respir Crit Care Med. 2016;193:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chao TF, Liu CJ, Chen SJ, et al. Incidence and risk of atrial fibrillation in sleep-disordered breathing without coexistent systemic disease. Circ J. 2014;78:2182–2187. [DOI] [PubMed] [Google Scholar]
- 19.Christensen MA, Dixit S, Dewland TA, et al. Sleep characteristics that predict atrial fibrillation. Heart Rhythm. 2018;15:1289–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Genuardi MV, Ogilvie RP, Saand AR, et al. Association of short sleep duration and atrial fibrillation. Chest. 2019;156:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itoh H, Kaneko H, Fujiu K, et al. Risk factors and lifestyles in the development of atrial fibrillation among individuals aged 20–39 years. Am J Cardiol. 2021;155:40–44. [DOI] [PubMed] [Google Scholar]
- 22.Hunt TE, Traaen GM, Aakeroy L, et al. Effect of continuous positive airway pressure therapy on recurrence of atrial fibrillation after pulmonary vein isolation in patients with obstructive sleep apnea: a randomized controlled trial. Heart Rhythm. 2022;19:1433–1441. [DOI] [PubMed] [Google Scholar]
5.2.10. Comprehensive Care
- 1.Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382:20–28. [DOI] [PubMed] [Google Scholar]
- 2.Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310:2050–2060. [DOI] [PubMed] [Google Scholar]
- 3.Rienstra M, Hobbelt AH, Alings M, et al. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J. 2018;39:2987–2996. [DOI] [PubMed] [Google Scholar]
- 4.Hendriks JM, de Wit R, Crijns HJ, et al. Nurse-led care vs usual care for patients with atrial fibrillation: results of a randomized trial of integrated chronic care vs routine clinical care in ambulatory patients with atrial fibrillation. Eur Heart J. 2012;33:2692–2699. [DOI] [PubMed] [Google Scholar]
- 5.Guo Y, Lane DA, Wang L, et al. Mobile health technology to improve care for patients with atrial fibrillation. J Am Coll Cardiol. 2020;75:1523–1534. [DOI] [PubMed] [Google Scholar]
- 6.Karlsson LO, Nilsson S, Bång M, et al. A clinical decision support tool for improving adherence to guidelines on anticoagulant therapy in patients with atrial fibrillation at risk of stroke: a cluster-randomized trial in a Swedish primary care setting (the CDS-AF study). PLoS Med. 2018;15:e1002528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccini JP Sr, Allred J, Bunch TJ, et al. Rationale, considerations, and goals for atrial fibrillation centers of excellence: a Heart Rhythm Society perspective. Heart Rhythm. 2020;17:1804–1832. [DOI] [PubMed] [Google Scholar]
- 8.Younis A, Shaviv E, Nof E, et al. The role and outcome of cardiac rehabilitation program in patients with atrial fibrillation. Clin Cardiol. 2018;41:1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angaran P, Mariano Z, Dragan V, et al. The Atrial Fibrillation Therapies after ER visit: Outpatient Care for Patients with Acute AF - the AFTER3 study. J Atr Fibrillation. 2015;7:1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wijtvliet E, Tieleman RG, van Gelder IC, et al. Nurse-led vs usual-care for atrial fibrillation. Eur Heart J. 2020;41:634–641. [DOI] [PubMed] [Google Scholar]
- 11.van den Dries CJ, van Doorn S, Rutten FH, et al. Integrated management of atrial fibrillation in primary care: results of the ALL-IN cluster randomized trial. Eur Heart J. 2020;41:2836–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart S, Ball J, Horowitz JD, et al. Standard versus atrial fibrillation-specific management strategy (SAFETY) to reduce recurrent admission and prolong survival: pragmatic, multicentre, randomised controlled trial. Lancet. 2015;385:775–784. [DOI] [PubMed] [Google Scholar]
- 13.Willis TA, Collinson M, Glidewell L, et al. An adaptable implementation package targeting evidence-based indicators in primary care: a pragmatic cluster-randomised evaluation. PLoS Med. 2020;17:e1003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox JL, Parkash R, Foster GA, et al. Integrated Management Program Advancing Community Treatment of Atrial Fibrillation (IMPACT-AF): a cluster randomized trial of a computerized clinical decision support tool. Am Heart J. 2020;224:35–46. [DOI] [PubMed] [Google Scholar]
- 15.Arts DL, Abu-Hanna A, Medlock SK, et al. Effectiveness and usage of a decision support system to improve stroke prevention in general practice: a cluster randomized controlled trial. PLoS One. 2017;12:e0170974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vinereanu D, Lopes RD, Bahit MC, et al. A multifaceted intervention to improve treatment with oral anticoagulants in atrial fibrillation (IMPACT-AF): an international, cluster-randomised trial. Lancet. 2017;390:1737–1746. [DOI] [PubMed] [Google Scholar]
6.1. Risk Stratification Schemes
- 1.Singer DE, Chang Y, Borowsky LH, et al. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J Am Heart Assoc. 2013;2:e000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 3.Fox KAA, Lucas JE, Pieper KS, et al. Improved risk stratification of patients with atrial fibrillation: an integrated GARFIELD-AF tool for the prediction of mortality, stroke and bleed in patients with and without anticoagulation. BMJ Open. 2017;7:e017157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn GR, Severdija ON, Chang Y, et al. Wide variation in reported rates of stroke across cohorts of patients with atrial fibrillation. Circulation. 2017;135:208–219. [DOI] [PubMed] [Google Scholar]
- 5.Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 6.Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151:713–719. [DOI] [PubMed] [Google Scholar]
- 7.Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125:2298–2307. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg BA, Ballew NG, Greiner MA, et al. Ischemic and bleeding outcomes in patients with atrial fibrillation and contraindications to oral anticoagulation. JACC Clin Electrophysiol. 2019;5:1384–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chao TF, Lip GYH, Lin YJ, et al. Incident risk factors and major bleeding in patients with atrial fibrillation treated with oral anticoagulants: a comparison of baseline, follow-up and delta HAS-BLED scores with an approach focused on modifiable bleeding risk factors. Thromb Haemost. 2018;118:768–777. [DOI] [PubMed] [Google Scholar]
- 11.Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alkhouli M, Friedman PA. Ischemic stroke risk in patients with nonval-vular atrial fibrillation: JACC review topic of the week. J Am Coll Cardiol. 2019;74:3050–3065. [DOI] [PubMed] [Google Scholar]
- 13.Takabayashi K, Hamatani Y, Yamashita Y, et al. Incidence of stroke or systemic embolism in paroxysmal versus sustained atrial fibrillation: the Fushimi Atrial Fibrillation Registry. Stroke. 2015;46:3354–3361. [DOI] [PubMed] [Google Scholar]
- 14.Steinberg BA, Hellkamp AS, Lokhnygina Y, et al. Higher risk of death and stroke in patients with persistent vs paroxysmal atrial fibrillation: results from the ROCKET-AF Trial. Eur Heart J. 2015;36:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao X, Cai X, Yang Y, et al. Diagnostic accuracy of the HAS-BLED bleeding score in VKA- or DOAC-treated patients with atrial fibrillation: a systematic review and meta-analysis. Front Cardiovasc Med. 2021;8:757087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu W, Fu L, Ding Y, et al. Meta-analysis of ATRIA versus CHA(2)DS(2)-VASc for predicting stroke and thromboembolism in patients with atrial fibrillation. Int J Cardiol. 2017;227:436–442. [DOI] [PubMed] [Google Scholar]
- 17.van den Ham HA, Klungel OH, Singer DE, et al. Comparative performance of ATRIA, CHADS2, and CHA2DS2-VASc risk scores predicting stroke in patients with atrial fibrillation: results from a national primary care database. J Am Coll Cardiol. 2015;66:1851–1859. [DOI] [PubMed] [Google Scholar]
- 18.Dalgaard F, Pieper K, Verheugt F, et al. GARFIELD-AF model for prediction of stroke and major bleeding in atrial fibrillation: a Danish nationwide validation study. BMJ Open. 2019;9:e033283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Endt VHW, Milders J, Penning de Vries BBL, et al. Comprehensive comparison of stroke risk score performance: a systematic review and meta-analysis among 6 267 728 patients with atrial fibrillation. Europace. 2022;24:1739–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang G, Xie Q, Ma L, et al. Accuracy of HAS-BLED and other bleeding risk assessment tools in predicting major bleeding events in atrial fibrillation: a network meta-analysis. J Thromb Haemost. 2020;18:791–801. [DOI] [PubMed] [Google Scholar]
- 21.Fauchier L, Chaize G, Gaudin AF, et al. Predictive ability of HAS-BLED, HEMORR2HAGES, and ATRIA bleeding risk scores in patients with atrial fibrillation A French nationwide cross-sectional study. Int J Cardiol. 2016;217:85–91. [DOI] [PubMed] [Google Scholar]
- 22.Shah SJ, Eckman MH, Aspberg S, et al. Effect of variation in published stroke rates on the net clinical benefit of anticoagulation for atrial fibrillation. Ann Intern Med. 2018;169:517–527. [DOI] [PubMed] [Google Scholar]
- 23.Go AS, Reynolds K, Yang J, et al. Association of burden of atrial fibrillation with risk of ischemic stroke in adults with paroxysmal atrial fibrillation: the KP-RHYTHM study. JAMA Cardiol. 2018;3:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao MP, Halvorsen S, Wojdyla D, et al. Blood pressure control and risk of stroke or systemic embolism in patients with atrial fibrillation: results from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. J Am Heart Assoc. 2015;4:e002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 26.Chao TF, Lip GY, Liu CJ, et al. Validation of a modified CHA2DS2-VASc score for stroke risk stratification in Asian patients with atrial fibrillation: a nationwide cohort study. Stroke. 2016;47:2462–2469. [DOI] [PubMed] [Google Scholar]
6.2. Risk-Based Selection of Oral Anticoagulation: Balancing Risks and Benefits
- 1.Al-Khatib SM, Thomas L, Wallentin L, et al. Outcomes of apixaban vs warfarin by type and duration of atrial fibrillation: results from the ARISTOTLE trial. Eur Heart J. 2013;34:2464–2471. [DOI] [PubMed] [Google Scholar]
- 2.Link MS, Giugliano RP, Ruff CT, et al. Stroke and mortality risk in patients with various patterns of atrial fibrillation: results from the ENGAGE AF-TIMI 48 Trial (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48). Circ Arrhythm Electrophysiol. 2017;10:e004267. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg BA, Hellkamp AS, Lokhnygina Y, et al. Higher risk of death and stroke in patients with persistent vs paroxysmal atrial fibrillation: results from the ROCKET-AF Trial. Eur Heart J. 2015;36:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chao TF, Lip GYH, Lin YJ, et al. Incident risk factors and major bleeding in patients with atrial fibrillation treated with oral anticoagulants: a comparison of baseline, follow-up and delta HAS-BLED scores with an approach focused on modifiable bleeding risk factors. Thromb Haemost. 2018;118:768–777. [DOI] [PubMed] [Google Scholar]
- 5.Minhas AS, Jiang Q, Gu X, et al. Renal function in atrial fibrillation patients switched from warfarin to a direct oral anticoagulant. J Thromb Thrombolysis. 2016;42:566–572. [DOI] [PubMed] [Google Scholar]
- 6.Yoon M, Yang PS, Jang E, et al. Dynamic changes of CHA2DS2-VASc score and the risk of ischaemic stroke in Asian patients with atrial fibrillation: a nationwide cohort study. Thromb Haemost. 2018;118:1296–1304. [DOI] [PubMed] [Google Scholar]
6.3. Oral Anticoagulants
- 1.Zhu J, Alexander GC, Nazarian S, et al. Trends and variation in oral anticoagulant choice in patients with atrial fibrillation, 2010–2017. Pharmacotherapy. 2018;38:907–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 3.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 4.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 5.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
6.3.1. Antithrombotic Therapy
- 1.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 2.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 3.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 4.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Lopez JA, Sterne JAC, Thom HHZ, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. Bmj. 2017;359:j5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 7.Carnicelli AP, Hong H, Connolly SJ, et al. Direct oral anticoagulants versus warfarin in patients with atrial fibrillation: patient-level network meta-analyses of randomized clinical trials with interaction testing by age and sex. Circulation. 2022;145:242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Investigators AWGA, Connolly S, Pogue J, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–1912. [DOI] [PubMed] [Google Scholar]
- 9.Connolly SJ, Camm AJ, Halperin JL, et al. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med. 2011;365:2268–2276. [DOI] [PubMed] [Google Scholar]
- 10.Sato H, Ishikawa K, Kitabatake A, et al. Low-dose aspirin for prevention of stroke in low-risk patients with atrial fibrillation: Japan Atrial Fibrillation Stroke Trial. Stroke. 2006;37:447–451. [DOI] [PubMed] [Google Scholar]
- 11.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen PB, Skjoth F, Overvad TF, et al. Female sex is a risk modifier rather than a risk factor for stroke in atrial fibrillation: should we use a CHA2DS2-VA score rather than CHA2DS2-VASc? Circulation. 2018;137:832–840. [DOI] [PubMed] [Google Scholar]
- 13.Aspberg S, Chang Y, Atterman A, et al. Comparison of the ATRIA, CHADS2, and CHA2DS2-VASc stroke risk scores in predicting ischaemic stroke in a large Swedish cohort of patients with atrial fibrillation. Eur Heart J. 2016;37:3203–3210. [DOI] [PubMed] [Google Scholar]
- 14.Singer DE, Chang Y, Borowsky LH, et al. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J Am Heart Assoc. 2013;2:e000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 16.Eckman MH, Singer DE, Rosand J, et al. Moving the tipping point: the decision to anticoagulate patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2011;4:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020;142:e558–e631. [DOI] [PubMed] [Google Scholar]
- 18.Brokmeier H, Kido K. Off-label use for direct oral anticoagulants: valvular atrial fibrillation, heart failure, left ventricular thrombus, superficial vein thrombosis, pulmonary hypertension-a systematic review. Ann Pharmacother. 2021;55:995–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart RG, Pearce LA, Miller VT, et al. Cardioembolic vs noncardioembolic strokes in atrial fibrillation: frequency and effect of antithrombotic agents in the stroke prevention in atrial fibrillation studies. Cerebrovasc Dis. 2000;10:39–43. [DOI] [PubMed] [Google Scholar]
6.3.1.1. Considerations in Managing Anticoagulants
- 1.Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol. 2013;76:455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frost CE, Byon W, Song Y, et al. Effect of ketoconazole and diltiazem on the pharmacokinetics of apixaban, an oral direct factor Xa inhibitor. Br J Clin Pharmacol. 2015;79:838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parasrampuria DA, Mendell J, Shi M, et al. Edoxaban drug-drug interactions with ketoconazole, erythromycin, and cyclosporine. Br J Clin Pharmacol. 2016;82:1591–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perlman A, Goldstein R, Choshen Cohen L, et al. Effect of enzyme-inducing antiseizure medications on the risk of sub-therapeutic concentrations of direct oral anticoagulants: a retrospective cohort study. CNS Drugs. 2021;35:305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vakkalagadda B, Frost C, Byon W, et al. Effect of rifampin on the pharmacokinetics of apixaban, an oral direct inhibitor of factor Xa. Am J Cardiovasc Drugs. 2016;16:119–127. [DOI] [PubMed] [Google Scholar]
- 6.Perlman A, Wanounou M, Goldstein R, et al. Ischemic and thrombotic events associated with concomitant Xa-inhibiting direct oral anticoagulants and antiepileptic drugs: analysis of the FDA Adverse Event Reporting System (FAERS). CNS Drugs. 2019;33:1223–1228. [DOI] [PubMed] [Google Scholar]
- 7.Giustozzi M, Mazzetti M, Paciaroni M, et al. Concomitant use of direct oral anticoagulants and antiepileptic drugs: a prospective cohort study in patients with atrial fibrillation. Clin Drug Investig. 2021;41:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan Y, Heneghan C, Perera R, et al. Anticoagulation control and prediction of adverse events in patients with atrial fibrillation: a systematic review. Circ Cardiovasc Qual Outcomes. 2008;1:84–91. [DOI] [PubMed] [Google Scholar]
- 9.De Assis MC, Rabelo ER, Ávila CW, et al. Improved oral anticoagulation after a dietary vitamin K-guided strategy: a randomized controlled trial. Circulation. 2009;120:1115–1122. [DOI] [PubMed] [Google Scholar]
- 10.Wang M, Zeraatkar D, Obeda M, et al. Drug-drug interactions with warfarin: a systematic review and meta-analysis. Br J Clin Pharmacol. 2021;87:4051–4100. [DOI] [PubMed] [Google Scholar]
- 11.Kido K, Shimizu M, Shiga T, et al. Meta-analysis comparing inappropriately low dose versus standard dose of direct oral anticoagulants in patients with atrial fibrillation. J Am Pharm Assoc (2003). 2022;62:487–495.e482. [DOI] [PubMed] [Google Scholar]
- 12.Zhang XL, Zhang XW, Wang TY, et al. Off-label under- and overdosing of direct oral anticoagulants in patients with atrial fibrillation: a meta-analysis. Circ Cardiovasc Qual Outcomes. 2021;14:e007971. [DOI] [PubMed] [Google Scholar]
- 13.Eliquis [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2021. [Google Scholar]
- 14.Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2021. [Google Scholar]
- 15.Sugrue A, Sanborn D, Amin M, et al. Inappropriate dosing of direct oral anticoagulants in patients with atrial fibrillation. Am J Cardiol. 2021;144:52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato T, Aizawa Y, Fuse K, et al. The comparison of inappropriate-low-doses use among 4 direct oral anticoagulants in patients with atrial fibrillation: from the database of a single-center registry. J Stroke Cerebrovasc Dis. 2018;27:3280–3288. [DOI] [PubMed] [Google Scholar]
- 17.Yu HT, Yang PS, Jang E, et al. Label adherence of direct oral anticoagulants dosing and clinical outcomes in patients with atrial fibrillation. J Am Heart Assoc. 2020;9:e014177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camm AJ, Cools F, Virdone S, et al. Mortality in patients with atrial fibrillation receiving nonrecommended doses of direct oral anticoagulants. J Am Coll Cardiol. 2020;76:1425–1436. [DOI] [PubMed] [Google Scholar]
- 19.Stampfuss J, Kubitza D, Becka M, et al. The effect of food on the absorption and pharmacokinetics of rivaroxaban. Int J Clin Pharmacol Ther. 2013;51:549–561. [DOI] [PubMed] [Google Scholar]
- 20.Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2023. [Google Scholar]
- 21.Savaysa [package insert]. Basking Ridge, NJ: Daiichi Sankyo, Inc; 2021. [Google Scholar]
- 22.Coumadin [package insert]. Princeton, NJ: Bristol-Myers Squibb; 2012. [Google Scholar]
- 23.Kido K Atrial fibrillation/atrial flutter: stroke prevention. In: CardSAP 2021; Book 2. Arrhythmias and Thrombosis. American College of Clinical Pharmacy; 2021:32–33. [Google Scholar]
6.4. Silent AF and Stroke of Undetermined Cause
- 1.Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014;370:2467–2477. [DOI] [PubMed] [Google Scholar]
- 2.Saver JL. CLINICAL PRACTICE Cryptogenic Stroke. N Engl J Med. 2016;374:2065–2074. [DOI] [PubMed] [Google Scholar]
- 3.Ibeh C, Elkind MSV. Stroke prevention after cryptogenic stroke. Curr Cardiol Rep. 2021;23:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melis F, Guido M, Amellone C, et al. Prevalence and predictors of atrial fibrillation in patients with embolic stroke of undetermined source: a real-life single-center retrospective study. Neurol Sci. 2021;42:3707–3714. [DOI] [PubMed] [Google Scholar]
- 5.Victor CU, Carolina PE, Jorge TR, et al. Incidence and predictive factors of hidden atrial fibrillation detected by implantable loop recorder after an embolic stroke of undetermined source. J Atr Fibrillation. 2018;11:2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hart RG, Connolly SJ, Mundl H. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;379:987. [DOI] [PubMed] [Google Scholar]
- 7.Diener HC, Sacco RL, Easton JD, et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med. 2019;380:1906–1917. [DOI] [PubMed] [Google Scholar]
- 8.Stroke Prevention in Atrial Fibrillation Study. Final results. Circulation. 1991;84:527–539. [DOI] [PubMed] [Google Scholar]
- 9.Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486. [DOI] [PubMed] [Google Scholar]
- 10.Wachter R, Gröschel K, Gelbrich G, et al. Holter-electrocardiogram-monitoring in patients with acute ischaemic stroke (Find-AF(RANDOMISED)): an open-label randomised controlled trial. Lancet Neurol. 2017;16:282–290. [DOI] [PubMed] [Google Scholar]
- 11.Sposato LA, Cipriano LE, Saposnik G, et al. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2015;14:377–387. [DOI] [PubMed] [Google Scholar]
- 12.Brambatti M, Connolly SJ, Gold MR, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–2099. [DOI] [PubMed] [Google Scholar]
- 13.Buck BH, Hill MD, Quinn FR, et al. Effect of implantable vs prolonged external electrocardiographic monitoring on atrial fibrillation detection in patients with ischemic stroke: the per diem randomized clinical trial. JAMA. 2021;325:2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
6.4.1. Oral Anticoagulation for Device-Detected Atrial High-Rate Episodes Among Patients Without a Previous Diagnosis of AF
- 1.Van Gelder IC, Healey JS, Crijns H, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38:1339–1344. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan RM, Koehler J, Ziegler PD, et al. Stroke risk as a function of atrial fibrillation duration and CHA2DS2-VASc score. Circulation. 2019;140:1639–1646. [DOI] [PubMed] [Google Scholar]
- 3.Perino AC, Fan J, Askari M, et al. Practice variation in anticoagulation prescription and outcomes after device-detected atrial fibrillation. Circulation. 2019;139:2502–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swiryn S, Orlov MV, Benditt DG, et al. Clinical implications of brief device-detected atrial tachyarrhythmias in a cardiac rhythm management device population: results from the registry of atrial tachycardia and atrial fibrillation episodes. Circulation. 2016;134:1130–1140. [DOI] [PubMed] [Google Scholar]
- 5.Boriani G, Glotzer TV, Santini M, et al. Device-detected atrial fibrillation and risk for stroke: an analysis of >10 000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J. 2014;35:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahajan R, Perera T, Elliott AD, et al. Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J. 2018;39:1407–1415. [DOI] [PubMed] [Google Scholar]
- 7.Park YJ, Kim JS, Park KM, et al. Subclinical atrial fibrillation burden and adverse clinical outcomes in patients with permanent pacemakers. Stroke. 2021;52:1299–1308. [DOI] [PubMed] [Google Scholar]
- 8.Eckman MH, Singer DE, Rosand J, et al. Moving the tipping point: the decision to anticoagulate patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2011;4:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollak WM, Simmons JD, Interian A Jr, et al. Clinical utility of intraatrial pacemaker stored electrograms to diagnose atrial fibrillation and flutter. Pacing Clin Electrophysiol. 2001;24:424–429. [DOI] [PubMed] [Google Scholar]
- 10.Glotzer TV, Hellkamp AS, Zimmerman J, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003;107:1614–1619. [DOI] [PubMed] [Google Scholar]
- 11.Capucci A, Santini M, Padeletti L, et al. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol. 2005;46:1913–1920. [DOI] [PubMed] [Google Scholar]
- 12.Botto GL, Padeletti L, Santini M, et al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009;20:241–248. [DOI] [PubMed] [Google Scholar]
- 13.Noseworthy PA, Kaufman ES, Chen LY, et al. Subclinical and device-detected atrial fibrillation: pondering the knowledge gap: a scientific statement from the American Heart Association. Circulation. 2019;140:e944–e963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freedman B, Boriani G, Glotzer TV, et al. Management of atrial high-rate episodes detected by cardiac implanted electronic devices. Nat Rev Cardiol. 2017;14:701–714. [DOI] [PubMed] [Google Scholar]
6.5.1. Percutaneous Approaches to Occlude the LAA
- 1.Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988–1998. [DOI] [PubMed] [Google Scholar]
- 2.Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. [DOI] [PubMed] [Google Scholar]
- 3.Reddy VY, Doshi SK, Kar S, et al. 5-Year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70:2964–2975. [DOI] [PubMed] [Google Scholar]
- 4.Osmancik P, Herman D, Neuzil P, et al. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol. 2020;75:3122–3135. [DOI] [PubMed] [Google Scholar]
- 5.Reddy VY, Mobius-Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol. 2013;61:2551–2556. [DOI] [PubMed] [Google Scholar]
- 6.Boersma LV, Ince H, Kische S, et al. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1-year follow-up outcome data of the EWOLUTION trial. Heart Rhythm. 2017;14:1302–1308. [DOI] [PubMed] [Google Scholar]
- 7.Kar S, Doshi SK, Sadhu A, et al. Primary outcome evaluation of a next-generation left atrial appendage closure device: results from the PINNACLE FLX trial. Circulation. 2021;143:1754–1762. [DOI] [PubMed] [Google Scholar]
- 8.Freeman JV, Higgins AY, Wang Y, et al. Antithrombotic therapy after left atrial appendage occlusion in patients with atrial fibrillation. J Am Coll Cardiol. 2022;79:1785–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmes DR, Reddy VY, Buchbinder M, et al. The assessment of the Watchman device in patients unsuitable for oral anticoagulation (ASAP-TOO) trial. Am Heart J. 2017;189:68–74. [DOI] [PubMed] [Google Scholar]
6.5.2. Cardiac Surgery—LAA Exclusion/Excision
- 1.Whitlock RP, Belley-Cote EP, Paparella D, et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med. 2021;384:2081–2091. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DJ, Piccini JP, Wang T, et al. Association between left atrial appendage occlusion and readmission for thromboembolism among patients with atrial fibrillation undergoing concomitant cardiac surgery. JAMA. 2018;319:365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martín Gutiérrez E, Castaño M, Gualis J, et al. Beneficial effect of left atrial appendage closure during cardiac surgery: a meta-analysis of 280 585 patients. Eur J Cardiothorac Surg. 2020;57:252–262. [DOI] [PubMed] [Google Scholar]
- 4.Healey JS, Crystal E, Lamy A, et al. Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J. 2005;150:288–293. [DOI] [PubMed] [Google Scholar]
- 5.Aryana A, Singh SK, Singh SM, et al. Association between incomplete surgical ligation of left atrial appendage and stroke and systemic embolization. Heart Rhythm. 2015;12:1431–1437. [DOI] [PubMed] [Google Scholar]
- 6.Madden JL. Resection of the left auricular appendix; a prophylaxis for recurrent arterial emboli. JAMA. 1949;140:769–772. [DOI] [PubMed] [Google Scholar]
- 7.Melduni RM, Schaff HV, Lee HC, et al. Impact of left atrial appendage closure during cardiac surgery on the occurrence of early postoperative atrial fibrillation, stroke, and mortality. Circulation. 2017;135:366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elbadawi A, Ogunbayo GO, Elgendy IY, et al. Impact of left atrial appendage exclusion on cardiovascular outcomes in patients with atrial fibrillation undergoing coronary artery bypass grafting (from the National Inpatient Sample Database). Am J Cardiol. 2017;120:953–958. [DOI] [PubMed] [Google Scholar]
- 9.Gutiérrez EM, Castaño M, Gualis J, et al. Beneficial effect of left atrial appendage closure during cardiac surgery: a meta-analysis of 280 585 patients. Eur J Cardiothorac Surg. 2020;57:252–262. [DOI] [PubMed] [Google Scholar]
- 10.Elbadawi A, Olorunfemi O, Ogunbayo GO, et al. Cardiovascular outcomes with surgical left atrial appendage exclusion in patients with atrial fibrillation who underwent valvular heart surgery (from the National Inpatient Sample Database). Am J Cardiol. 2017;119:2056–2060. [DOI] [PubMed] [Google Scholar]
- 11.Prasad RM, Saleh Y, Al-Abcha A, et al. Left atrial appendage closure during cardiac surgery for atrial fibrillation: a meta-analysis. Cardiovasc Revasc Med. 2022;40:26–36. [DOI] [PubMed] [Google Scholar]
- 12.Rashid HN, Layland J. Modification of the left atrial appendage and its role in stroke risk reduction with non-valvular atrial fibrillation. Int J Cardiol Heart Vasc. 2021;32:100688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toale C, Fitzmaurice GJ, Eaton D, et al. Outcomes of left atrial appendage occlusion using the AtriClip device: a systematic review. Interact Cardiovasc Thorac Surg. 2019;29:655–662. [DOI] [PubMed] [Google Scholar]
- 14.Nso N, Nassar M, Zirkiyeva M, et al. Outcomes of cardiac surgery with left atrial appendage occlusion versus no occlusion, direct oral anticoagulants, and vitamin K antagonists: a systematic review with meta-analysis. Int J Cardiol Heart Vasc. 2022;40:100998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park-Hansen J, Holme SJV, Irmukhamedov A, et al. Adding left atrial appendage closure to open heart surgery provides protection from ischemic brain injury six years after surgery independently of atrial fibrillation history: the LAACS randomized study. J Cardiothorac Surg. 2018;13:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
6.6. Active Bleeding on Anticoagulant Therapy and Reversal Drugs
- 1.Pollack CV Jr, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal - full cohort analysis. N Engl J Med. 2017;377:431–441. [DOI] [PubMed] [Google Scholar]
- 2.Van der Wall SJ, Lopes RD, Aisenberg J, et al. Idarucizumab for dabigatran reversal in the management of patients with gastrointestinal bleeding. Circulation. 2019;139:748–756. [DOI] [PubMed] [Google Scholar]
- 3.Eikelboom JW, van Ryn J, Reilly P, et al. Dabigatran reversal with idarucizumab in patients with renal impairment. J Am Coll Cardiol. 2019;74:1760–1768. [DOI] [PubMed] [Google Scholar]
- 4.Schulman S, Ritchie B, Nahirniak S, et al. Reversal of dabigatran-associated major bleeding with activated prothrombin concentrate: A prospective cohort study. Thromb Res. 2017;152:44–48. [DOI] [PubMed] [Google Scholar]
- 5.Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124:1573–1579. [DOI] [PubMed] [Google Scholar]
- 6.Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380:1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nederpelt CJ, Naar L, Krijnen P, et al. Andexanet alfa or prothrombin complex concentrate for factor Xa inhibitor reversal in acute major bleeding: a systematic review and meta-analysis. Crit Care Med. 2021;49:e1025–e1036. [DOI] [PubMed] [Google Scholar]
- 8.Chai-Adisaksopha C, Hillis C, Siegal DM, et al. Prothrombin complex concentrates versus fresh frozen plasma for warfarin reversal. A systematic review and meta-analysis. Thromb Haemost. 2016;116:879–890. [DOI] [PubMed] [Google Scholar]
- 9.Sarode R, Milling TJ Jr, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation. 2013;128:1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein JN, Refaai MA, Milling TJ Jr, et al. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial. Lancet. 2015;385:2077–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tapaskar N, Pang A, Werner DA, et al. Resuming anticoagulation following hospitalization for gastrointestinal bleeding is associated with reduced thromboembolic events and improved mortality: results from a systematic review and meta-analysis. Dig Dis Sci. 2021;66:554–566. [DOI] [PubMed] [Google Scholar]
- 12.Proietti M, Romiti GF, Romanazzi I, et al. Restarting oral anticoagulant therapy after major bleeding in atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol. 2018;261:84–91. [DOI] [PubMed] [Google Scholar]
- 13.Siegal DM. What we have learned about direct oral anticoagulant reversal. Hematology Am Soc Hematol Educ Program. 2019;2019:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Mondal S, Wang J, et al. Effect of activated charcoal on apixaban pharmacokinetics in healthy subjects. Am J Cardiovasc Drugs. 2014;14:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ollier E, Hodin S, Lanoiselee J, et al. Effect of activated charcoal on rivaroxaban complex absorption. Clin Pharmacokinet. 2017;56:793–801. [DOI] [PubMed] [Google Scholar]
- 16.Chai-Adisaksopha C, Hillis C, Lim W, et al. Hemodialysis for the treatment of dabigatran-associated bleeding: a case report and systematic review. J Thromb Haemost. 2015;13:1790–1798. [DOI] [PubMed] [Google Scholar]
- 17.Geller AI, Shehab N, Lovegrove MC, et al. Emergency visits for oral anticoagulant bleeding. J Gen Intern Med. 2020;35:371–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gómez-Outes A, Alcubilla P, Calvo-Rojas G, et al. Meta-analysis of reversal agents for severe bleeding associated with direct oral anticoagulants. J Am Coll Cardiol. 2021;77:2987–3001. [DOI] [PubMed] [Google Scholar]
- 19.Majeed A, Wallvik N, Eriksson J, et al. Optimal timing of vitamin K antagonist resumption after upper gastrointestinal bleeding. A risk modelling analysis. Thromb Haemost. 2017;117:491–499. [DOI] [PubMed] [Google Scholar]
- 20.Qureshi W, Mittal C, Patsias I, et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol. 2014;113:662–668. [DOI] [PubMed] [Google Scholar]
- 21.Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 2012;172:1484–1491. [DOI] [PubMed] [Google Scholar]
- 22.Kido K Atrial fibrillation/atrial flutter: stroke prevention. In: CardSAP 2021; Book 2. Arrhythmias and Thrombosis. American College of Clinical Pharmacy; 2021:32–33. [Google Scholar]
6.6.1. Management of Patients With AF and ICH
- 1.Kuramatsu JB, Sembill JA, Gerner ST, et al. Management of therapeutic anticoagulation in patients with intracerebral haemorrhage and mechanical heart valves. Eur Heart J. 2018;39:1709–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korompoki E, Filippidis FT, Nielsen PB, et al. Long-term antithrombotic treatment in intracranial hemorrhage survivors with atrial fibrillation. Neurology. 2017;89:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pennlert J, Overholser R, Asplund K, et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2017;48:314–320. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen PB, Larsen TB, Skjoth F, et al. Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality, and bleeding: a nationwide cohort study. Circulation. 2015;132:517–525. [DOI] [PubMed] [Google Scholar]
- 5.Murthy SB, Gupta A, Merkler AE, et al. Restarting anticoagulant therapy after intracranial hemorrhage: a systematic review and meta-analysis. Stroke. 2017;48:1594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–542. [DOI] [PubMed] [Google Scholar]
- 7.Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. [DOI] [PubMed] [Google Scholar]
- 8.Szekely P Systemic embolism and anticoagulant prophylaxis in rheumatic heart disease. Br Med J. 1964;1:1209–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannegieter SC, Rosendaal FR, Briet E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994;89:635–641. [DOI] [PubMed] [Google Scholar]
- 10.Baudet EM, Puel V, McBride JT, et al. Long-term results of valve replacement with the St Jude Medical prosthesis. J Thorac Cardiovasc Surg. 1995;109:858–870. [DOI] [PubMed] [Google Scholar]
- 11.Salem DN, Stein PD, Al-Ahmad A, et al. Antithrombotic therapy in valvular heart disease--native and prosthetic: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:457S–482S. [DOI] [PubMed] [Google Scholar]
- 12.Schols AM, Schreuder FH, van Raak EP, et al. Incidence of oral anticoagulant-associated intracerebral hemorrhage in the Netherlands. Stroke. 2014;45:268–270. [DOI] [PubMed] [Google Scholar]
- 13.Seiffge DJ, Goeldlin MB, Tatlisumak T, et al. Meta-analysis of haematoma volume, haematoma expansion and mortality in intracerebral haemorrhage associated with oral anticoagulant use. J Neurol. 2019;266:3126–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaherty ML, Haverbusch M, Sekar P, et al. Location and outcome of anticoagulant-associated intracerebral hemorrhage. Neurocrit Care. 2006;5:197–201. [DOI] [PubMed] [Google Scholar]
- 15.Flibotte JJ, Hagan N, O'Donnell J, et al. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63:1059–1064. [DOI] [PubMed] [Google Scholar]
- 16.Poli L, Grassi M, Zedde M, et al. Anticoagulants resumption after warfarinrelated intracerebral haemorrhage: the Multicenter Study on Cerebral Hemorrhage in Italy (MUCH-Italy). Thromb Haemost. 2018;118:572–580. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen-Kudsk JE, Johnsen SP, Wester P, et al. Left atrial appendage occlusion versus standard medical care in patients with atrial fibrillation and intracerebral haemorrhage: a propensity score-matched follow-up study. EuroIntervention. 2017;13:371–378. [DOI] [PubMed] [Google Scholar]
- 18.Ottosen TP, Grijota M, Hansen ML, et al. Use of antithrombotic therapy and long-term clinical outcome among patients surviving intracerebral hemorrhage. Stroke. 2016;47:1837–1843. [DOI] [PubMed] [Google Scholar]
- 19.Chao TF, Liu CJ, Liao JN, et al. Use of Oral anticoagulants for stroke prevention in patients with atrial fibrillation who have a history of intracranial hemorrhage. Circulation. 2016;133:1540–1547. [DOI] [PubMed] [Google Scholar]
- 20.Park YA, Uhm JS, Pak HN, et al. Anticoagulation therapy in atrial fibrillation after intracranial hemorrhage. Heart Rhythm. 2016;13:1794–1802. [DOI] [PubMed] [Google Scholar]
- 21.Kuramatsu JB, Gerner ST, Schellinger PD, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA. 2015;313:824–836. [DOI] [PubMed] [Google Scholar]
- 22.Biffi A, Kuramatsu JB, Leasure A, et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol. 2017;82:755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Y, Lip GY, Apostolakis S. Bleeding risk assessment and management in atrial fibrillation patients. Key messages for clinical practice from the European Heart Rhythm Association position statement. Pol Arch Med Wewn. 2012;122:235–242. [PubMed] [Google Scholar]
- 24.Charidimou A, Boulouis G, Xiong L, et al. Cortical superficial siderosis and first-ever cerebral hemorrhage in cerebral amyloid angiopathy. Neurology. 2017;88:1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoddard MF, Dawkins PR, Prince CR, et al. Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: a transesophageal echocardiographic study. J Am Coll Cardiol. 1995;25:452–459. [DOI] [PubMed] [Google Scholar]
- 26.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–759. [DOI] [PubMed] [Google Scholar]
- 27.Reddy VY, Doshi SK, Kar S, et al. 5-Year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70:2964–2975. [DOI] [PubMed] [Google Scholar]
- 28.Branzoli S, Marini M, Guarracini F, et al. Epicardial standalone left atrial appendage clipping for prevention of ischemic stroke in patients with atrial fibrillation contraindicated for oral anticaogulation. J Cardiovasc Electrophysiol. 2020;31:2187–2191. [DOI] [PubMed] [Google Scholar]
- 29.Antaki T, Michaelman J, McGroarty J. Robotics-assisted epicardial left atrial appendage clip exclusion. JTCVS Tech. 2021;9:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
6.7. Periprocedural Management
- 1.Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becerra AF, Cornavaca MT, Revigliono JI, et al. Perioperative management of vitamin K antagonists in patients with low thromboembolic risk undergoing elective surgery: a prospective experience. Med Clin (Barc). 2017;149:281–286. [DOI] [PubMed] [Google Scholar]
- 3.Siegal D, Yudin J, Kaatz S, et al. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126:1630–1639. [DOI] [PubMed] [Google Scholar]
- 4.Douketis JD, Spyropoulos AC, Duncan J, et al. Perioperative management of patients with atrial fibrillation receiving a direct oral anticoagulant. JAMA Intern Med. 2019;179:1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birnie DH, Healey JS, Wells GA, et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med. 2013;368:2084–2093. [DOI] [PubMed] [Google Scholar]
- 6.Cheng A, Nazarian S, Brinker JA, et al. Continuation of warfarin during pacemaker or implantable cardioverter-defibrillator implantation: a randomized clinical trial. Heart Rhythm. 2011;8:536–540. [DOI] [PubMed] [Google Scholar]
- 7.Tolosana JM, Berne P, Mont L, et al. Preparation for pacemaker or implantable cardiac defibrillator implants in patients with high risk of thrombo-embolic events: oral anticoagulation or bridging with intravenous heparin? A prospective randomized trial. Eur Heart J. 2009;30:1880–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendoza PA, Narula S, McIntyre WF, et al. Continued versus interrupted direct oral anticoagulation for cardiac electronic device implantation: a systematic review. Pacing Clin Electrophysiol. 2020;43:1373–1381. [DOI] [PubMed] [Google Scholar]
- 9.Birnie DH, Healey JS, Wells GA, et al. Continued vs interrupted direct oral anticoagulants at the time of device surgery, in patients with moderate to high risk of arterial thrombo-embolic events (BRUISE CONTROL-2). Eur Heart J. 2018;39:3973–3979. [DOI] [PubMed] [Google Scholar]
- 10.Ricciardi D, Creta A, Colaiori I, et al. Interrupted versus uninterrupted novel oral anticoagulant peri-implantation of cardiac device: a single-center randomized prospective pilot trial. Pacing Clin Electrophysiol. 2018;41:1476–1480. [DOI] [PubMed] [Google Scholar]
- 11.Godier A, Martin AC, Leblanc I, et al. Peri-procedural management of dabigatran and rivaroxaban: duration of anticoagulant discontinuation and drug concentrations. Thromb Res. 2015;136:763–768. [DOI] [PubMed] [Google Scholar]
- 12.Merli GJ, Kraft WK, Eraso LH, et al. Apixaban Discontinuation for Invasive Or major Surgical procedures (ADIOS): a prospective cohort study. Vasc Med. 2022;27:269–276. [DOI] [PubMed] [Google Scholar]
- 13.Kovacs MJ, Wells PS, Anderson DR, et al. Postoperative low molecular weight heparin bridging treatment for patients at high risk of arterial thromboembolism (PERIOP2): double blind randomised controlled trial. Bmj. 2021;373:n1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bajkin BV, Popovic SL, Selakovic SD. Randomized, prospective trial comparing bridging therapy using low-molecular-weight heparin with maintenance of oral anticoagulation during extraction of teeth. J Oral Maxillofac Surg. 2009;67:990–995. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg BA, Peterson ED, Kim S, et al. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131:488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol. 2017;69:871–898. [DOI] [PubMed] [Google Scholar]
- 17.Rosencher N, Llau JV, Mueck W, et al. Incidence of neuraxial haematoma after total hip or knee surgery: RECORD programme (rivaroxaban vs enoxaparin). Acta Anaesthesiol Scand. 2013;57:565–572. [DOI] [PubMed] [Google Scholar]
- 18.Garcia DA, Regan S, Henault LE, et al. Risk of thromboembolism with short-term interruption of warfarin therapy. Arch Intern Med. 2008;168:63–69. [DOI] [PubMed] [Google Scholar]
- 19.Michaud GF, Pelosi F Jr, Noble MD, et al. A randomized trial comparing heparin initiation 6 h or 24 h after pacemaker or defibrillator implantation. J Am Coll Cardiol. 2000;35:1915–1918. [DOI] [PubMed] [Google Scholar]
- 20.Beyer-Westendorf J, Gelbricht V, Forster K, et al. Peri-interventional management of novel oral anticoagulants in daily care: results from the prospective Dresden NOAC registry. Eur Heart J. 2014;35:1888–1896. [DOI] [PubMed] [Google Scholar]
- 21.Nazha B, Pandya B, Cohen J, et al. Periprocedural outcomes of direct oral anticoagulants versus warfarin in nonvalvular atrial fibrillation. Circulation. 2018;138:1402–1411. [DOI] [PubMed] [Google Scholar]
- 22.Colonna P, von Heymann C, Santamaria A, et al. Routine clinical practice in the periprocedural management of edoxaban therapy is associated with low risk of bleeding and thromboembolic complications: the prospective, observational, and multinational EMIT-AF/VTE study. Clin Cardiol. 2020;43:769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72–e227. [DOI] [PubMed] [Google Scholar]
6.8.1. AF Complicating ACS or PCI
- 1.Lopes RD, Heizer G, Aronson R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380:1509–1524. [DOI] [PubMed] [Google Scholar]
- 2.Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513–1524. [DOI] [PubMed] [Google Scholar]
- 3.Vranckx P, Valgimigli M, Eckardt L, et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. 2019;394:1335–1343. [DOI] [PubMed] [Google Scholar]
- 4.Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–2434. [DOI] [PubMed] [Google Scholar]
- 5.Potpara TS, Mujovic N, Proietti M, et al. Revisiting the effects of omitting aspirin in combined antithrombotic therapies for atrial fibrillation and acute coronary syndromes or percutaneous coronary interventions: meta-analysis of pooled data from the PIONEER AF-PCI, RE-DUAL PCI, and AUGUSTUS trials. Europace. 2020;22:33–46. [DOI] [PubMed] [Google Scholar]
- 6.Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381:1107–1115. [DOI] [PubMed] [Google Scholar]
6.8.2. Chronic Coronary Disease (CCD)
- 1.Yasuda S, Kaikita K, Akao M, et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med. 2019;381:1103–1113. [DOI] [PubMed] [Google Scholar]
- 2.Matsumura-Nakano Y, Shizuta S, Komasa A, et al. Open-label randomized trial comparing oral anticoagulation with and without single antiplatelet therapy in patients with atrial fibrillation and stable coronary artery disease beyond 1 year after coronary stent implantation. Circulation. 2019;139:604–616. [DOI] [PubMed] [Google Scholar]
- 3.Matoba T, Yasuda S, Kaikita K, et al. Rivaroxaban monotherapy in patients with atrial fibrillation after coronary stenting: insights from the AFIRE trial. JACC Cardiovasc Interv. 2021;14:2330–2340. [DOI] [PubMed] [Google Scholar]
6.8.3. Peripheral Artery Disease (PAD)
- 1.Zhang H, Xue Z, Yi D, et al. Non-vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation with coronary or peripheral artery disease. Int Heart J. 2020;61:231–238. [DOI] [PubMed] [Google Scholar]
- 2.Jones WS, Hellkamp AS, Halperin J, et al. Efficacy and safety of rivaroxaban compared with warfarin in patients with peripheral artery disease and non-valvular atrial fibrillation: insights from ROCKET AF. Eur Heart J. 2014;35:242–249. [DOI] [PubMed] [Google Scholar]
- 3.Hu PT, Lopes RD, Stevens SR, et al. Efficacy and safety of apixaban compared with warfarin in patients with atrial fibrillation and peripheral artery disease: insights from the ARISTOTLE trial. J Am Heart Assoc. 2017;6:e004699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonaca MP, Antman EM, Cunningham JW, et al. Ischaemic and bleeding risk in atrial fibrillation with and without peripheral artery disease and efficacy and safety of full- and half-dose edoxaban vs warfarin: insights from ENGAGE AF-TIMI 48. Eur Heart J Cardiovasc Pharmacother. 2022;8:695–706. [DOI] [PubMed] [Google Scholar]
- 5.Inohara T, Shrader P, Pieper K, et al. Treatment of atrial fibrillation with concomitant coronary or peripheral artery disease: results from the outcomes registry for better informed treatment of atrial fibrillation II. Am Heart J. 2019;213:81–90. [DOI] [PubMed] [Google Scholar]
- 6.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e726–e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anand S, Yusuf S, et al. ; The Warfarin Antiplatelet Vascular Evaluation Trial Investigators. Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. N Engl J Med. 2007;357:217–227. [DOI] [PubMed] [Google Scholar]
- 8.Lamberts M, Lip GY, Ruwald MH, et al. Antithrombotic treatment in patients with heart failure and associated atrial fibrillation and vascular disease: a nationwide cohort study. J Am Coll Cardiol. 2014;63:2689–2698. [DOI] [PubMed] [Google Scholar]
6.8.4. Chronic Kidney Disease (CKD)/Kidney Failure
- 1.Kimachi M, Furukawa TA, Kimachi K, et al. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev. 2017;11:Cd011373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ha JT, Neuen BL, Cheng LP, et al. Benefits and harms of oral anticoagulant therapy in chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. 2019;171:181–189. [DOI] [PubMed] [Google Scholar]
- 3.Hart RG, Pearce LA, Asinger RW, et al. Warfarin in atrial fibrillation patients with moderate chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:2599–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanifer JW, Pokorney SD, Chertow GM, et al. Apixaban versus warfarin in patients with atrial fibrillation and advanced chronic kidney disease. Circulation. 2020;141:1384–1392. [DOI] [PubMed] [Google Scholar]
- 5.Carrero JJ, Evans M, Szummer K, et al. Warfarin, kidney dysfunction, and outcomes following acute myocardial infarction in patients with atrial fibrillation. JAMA. 2014;311:919–928. [DOI] [PubMed] [Google Scholar]
- 6.Bonde AN, Lip GY, Kamper AL, et al. Net clinical benefit of antithrombotic therapy in patients with atrial fibrillation and chronic kidney disease: a nationwide observational cohort study. J Am Coll Cardiol. 2014;64:2471–2482. [DOI] [PubMed] [Google Scholar]
- 7.Randhawa MS, Vishwanath R, Rai MP, et al. Association between use of warfarin for atrial fibrillation and outcomes among patients with end-stage renal disease: a systematic review and meta-analysis. JAMA Netw Open. 2020;3:e202175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Go AS, Fang MC, Udaltsova N, et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 2009;119:1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts PR, Stromberg K, Johnson LC, et al. A systematic review of the incidence of arrhythmias in hemodialysis patients undergoing long-term monitoring with implantable loop recorders. Kidney Int Rep. 2021;6:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmerman D, Sood MM, Rigatto C, et al. Systematic review and meta-analysis of incidence, prevalence and outcomes of atrial fibrillation in patients on dialysis. Nephrol Dial Transplant. 2012;27:3816–3822. [DOI] [PubMed] [Google Scholar]
- 11.Murray AM, Seliger S, Lakshminarayan K, et al. Incidence of stroke before and after dialysis initiation in older patients. J Am Soc Nephrol. 2013;24:1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Vriese AS, Heine G. Anticoagulation management in haemodialysis patients with atrial fibrillation: evidence and opinion. Nephrol Dial Transplant. 2022;37:2072–2079. [DOI] [PubMed] [Google Scholar]
- 13.Yang F, Hellyer JA, Than C, et al. Warfarin utilisation and anticoagulation control in patients with atrial fibrillation and chronic kidney disease. Heart. 2017;103:818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi M, Takamatsu I, Kanno Y, et al. A case-control study of calciphylaxis in Japanese end-stage renal disease patients. Nephrol Dial Transplant. 2012;27:1580–1584. [DOI] [PubMed] [Google Scholar]
- 15.Chan KE, Edelman ER, Wenger JB, et al. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation. 2015;131:972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stangier J, Rathgen K, Stahle H, et al. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet. 2010;49:259–268. [DOI] [PubMed] [Google Scholar]
- 17.De Vriese AS, Caluwé R, Van Der Meersch H, et al. Safety and efficacy of vitamin K antagonists versus rivaroxaban in hemodialysis patients with atrial fibrillation: a multicenter randomized controlled trial. J Am Soc Nephrol. 2021;32:1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin YC, Chen BL, Shih CM, et al. Effectiveness and safety of rivaroxaban versus warfarin in Taiwanese patients with end-stage renal disease and nonvalvular atrial fibrillation: a real-world nationwide cohort study. PLoS One. 2021;16:e0249940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genovesi S, Porcu L, Slaviero G, et al. Outcomes on safety and efficacy of left atrial appendage occlusion in end stage renal disease patients undergoing dialysis. J Nephrol. 2021;34:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eikelboom JW, Connolly SJ, Gao P, et al. Stroke risk and efficacy of apixaban in atrial fibrillation patients with moderate chronic kidney disease. J Stroke Cerebrovasc Dis. 2012;21:429–435. [DOI] [PubMed] [Google Scholar]
- 21.Winkelmayer WC, Liu J, Setoguchi S, et al. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6:2662–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welander F, Renlund H, Dimeny E, et al. Efficacy and safety of warfarin in patients with non-valvular atrial fibrillation and CKD G3-G5D. Clin Kidney J. 2022;15:1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. [DOI] [PubMed] [Google Scholar]
- 24.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Tirucherai G, Marbury TC, et al. Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end-stage renal disease on hemodialysis. J Clin Pharmacol. 2016;56:628–636. [DOI] [PubMed] [Google Scholar]
- 26.Mavrakanas TA, Samer CF, Nessim SJ, et al. Apixaban pharmacokinetics at steady state in hemodialysis patients. J Am Soc Nephrol. 2017;28:2241–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siontis KC, Zhang X, Eckard A, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. 2018;138:1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mavrakanas TA, Garlo K, Charytan DM. Apixaban versus no anticoagulation in patients undergoing long-term dialysis with incident atrial fibrillation. Clin J Am Soc Nephrol. 2020;15:1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reinecke H, Engelbertz C, Bauersachs R, et al. A randomized controlled trial comparing apixaban to the vitamin K-antagonist phenprocoumon in patients on chronic hemodialysis: the AXADIA-AFNET 8 study. Circulation. 2022;147:296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pokorney SD, Chertow GM, Al-Khalidi HR, et al. Apixaban for patients with atrial fibrillation on hemodialysis: a multicenter randomized controlled trial. Circulation. 2022;146:1735–1745. [DOI] [PubMed] [Google Scholar]
6.8.5. AF in VHD
- 1.Connolly SJ, Karthikeyan G, Ntsekhe M, et al. Rivaroxaban in rheumatic heart disease-associated atrial fibrillation. N Engl J Med. 2022;387:978–988. [DOI] [PubMed] [Google Scholar]
- 2.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 4.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 5.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 6.López-López JA, Sterne JAC, Thom HHZ, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ. 2017;359:j5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carnicelli AP, Hong H, Connolly SJ, et al. Direct oral anticoagulants versus warfarin in patients with atrial fibrillation: patient-level network meta-analyses of randomized clinical trials with interaction testing by age and sex. Circulation. 2022;145:242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 9.Lip GYH, Jensen M, Melgaard L, et al. Stroke and bleeding risk scores in patients with atrial fibrillation and valvular heart disease: evaluating 'valvular heart disease' in a nationwide cohort study. Europace. 2019;21:33–40. [DOI] [PubMed] [Google Scholar]
- 10.Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214. [DOI] [PubMed] [Google Scholar]
- 11.Wolf PA, Dawber TR, Thomas HE Jr, et al. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology. 1978;28:973–977. [DOI] [PubMed] [Google Scholar]
- 12.Kim JY, Kim SH, Myong JP, et al. Outcomes of direct oral anticoagulants in patients with mitral stenosis. J Am Coll Cardiol. 2019;73:1123–1131. [DOI] [PubMed] [Google Scholar]
- 13.Brokmeier H, Kido K. Off-label use for direct oral anticoagulants: valvular atrial fibrillation, heart failure, left ventricular thrombus, superficial vein thrombosis, pulmonary hypertension-a systematic review. Ann Pharmacother. 2021;55:995–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siontis KC, Yao X, Gersh BJ, et al. Direct oral anticoagulants in patients with atrial fibrillation and valvular heart disease other than significant mitral stenosis and mechanical valves: a meta-analysis. Circulation. 2017;135:714–716. [DOI] [PubMed] [Google Scholar]
- 15.Jawitz OK, Wang TY, Lopes RD, et al. Rationale and design of PROACT Xa: a randomized, multicenter, open-label, clinical trial to evaluate the efficacy and safety of apixaban versus warfarin in patients with a mechanical On-X Aortic Heart Valve. Am Heart J. 2020;227:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
6.8.6. Anticoagulation of Typical AFL
- 1.Gula LJ, Redfearn DP, Jenkyn KB, et al. Elevated incidence of atrial fibrillation and stroke in patients with atrial flutter-a population-based study. Can J Cardiol. 2018;34:774–783. [DOI] [PubMed] [Google Scholar]
- 2.Gronefeld GC, Wegener F, Israel CW, et al. Thromboembolic risk of patients referred for radiofrequency catheter ablation of typical atrial flutter without prior appropriate anticoagulation therapy. Pacing Clin Electrophysiol. 2003;26:323–327. [DOI] [PubMed] [Google Scholar]
- 3.Vadmann H, Nielsen PB, Hjortshøj SP, et al. Atrial flutter and thromboembolic risk: a systematic review. Heart. 2015;101:1446–1455. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher MM, Hennessy BJ, Edvardsson N, et al. Embolic complications of direct current cardioversion of atrial arrhythmias: association with low intensity of anticoagulation at the time of cardioversion. J Am Coll Cardiol. 2002;40:926–933. [DOI] [PubMed] [Google Scholar]
- 5.Pérez FJ, Schubert CM, Parvez B, et al. Long-term outcomes after catheter ablation of cavo-tricuspid isthmus dependent atrial flutter: a meta-analysis. Circ Arrhyth Electrophysiol. 2009;2:393–401. [DOI] [PubMed] [Google Scholar]
- 6.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 7.Maskoun W, Pino MI, Ayoub K, et al. Incidence of atrial fibrillation after atrial flutter ablation. JACC Clin Electrophysiol. 2016;2:682–690. [DOI] [PubMed] [Google Scholar]
- 8.Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486. [DOI] [PubMed] [Google Scholar]
- 9.Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384:305–315. [DOI] [PubMed] [Google Scholar]
- 10.Haghjoo M, Salem N, Rafati M, et al. Predictors of the atrial fibrillation following catheter ablation of typical atrial flutter. Res Cardiovasc Med. 2013;2:90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis K, Wazni O, Marrouche N, et al. Incidence of atrial fibrillation post-cavotricuspid isthmus ablation in patients with typical atrial flutter: left-atrial size as an independent predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2007;18:799–802. [DOI] [PubMed] [Google Scholar]
- 12.Exposito V, Rodriguez-Entem F, Gonzalez-Enriquez S, et al. Stroke and systemic embolism after successful ablation of typical atrial flutter. Clin Cardiol. 2016;39:347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giehm-Reese M, Johansen MN, Kronborg MB, et al. Discontinuation of oral anticoagulation and risk of stroke and death after ablation for typical atrial flutter: a nation-wide Danish cohort study. Int J Cardiol. 2021;333:110–116. [DOI] [PubMed] [Google Scholar]
- 14.Granada J, Uribe W, Chyou PH, et al. Incidence and predictors of atrial flutter in the general population. J Am Coll Cardiol. 2000;36:2242–2246. [DOI] [PubMed] [Google Scholar]
- 15.Ammar AS, Elsherbiny I, El-Dosouky II, et al. Left atrial and left atrial appendage functional recovery after cardioversion in patients with recent atrial fibrillation: serial echocardiographic study. Cardiol J. 2015;22:699–707. [DOI] [PubMed] [Google Scholar]
- 16.Grimm RA, Stewart WJ, Arheart K, et al. Left atrial appendage “stunning” after electrical cardioversion of atrial flutter: an attenuated response compared with atrial fibrillation as the mechanism for lower susceptibility to thromboembolic events. J Am Coll Cardiol. 1997;29:582–589. [DOI] [PubMed] [Google Scholar]
- 17.Takami M, Suzuki M, Sugi K, et al. Time course for resolution of left atrial appendage stunning after catheter ablation of chronic atrial flutter. J Am Coll Cardiol. 2003;41:2207–2211. [DOI] [PubMed] [Google Scholar]
- 18.Spector P, Reynolds MR, Calkins H, et al. Meta-analysis of ablation of atrial flutter and supraventricular tachycardia. Am J Cardiol. 2009;104:671–677. [DOI] [PubMed] [Google Scholar]
- 19.Celikyurt U, Knecht S, Kuehne M, et al. Incidence of new-onset atrial fibrillation after cavotricuspid isthmus ablation for atrial flutter. Europace. 2017;19:1776–1780. [DOI] [PubMed] [Google Scholar]
- 20.Voight J, Akkaya M, Somasundaram P, et al. Risk of new-onset atrial fibrillation and stroke after radiofrequency ablation of isolated, typical atrial flutter. Heart Rhythm. 2014;11:1884–1889. [DOI] [PubMed] [Google Scholar]
- 21.Chen K, Bai R, Deng W, et al. HATCH score in the prediction of new-onset atrial fibrillation after catheter ablation of typical atrial flutter. Heart Rhythm. 2015;12:1483–1489. [DOI] [PubMed] [Google Scholar]
- 22.Romero J, Diaz JC, Di Biase L, et al. Atrial fibrillation inducibility during cavotricuspid isthmus-dependent atrial flutter ablation as a predictor of clinical atrial fibrillation A meta-analysis. J Interv Card Electrophysiol. 2017;48:307–315. [DOI] [PubMed] [Google Scholar]
- 23.Joza J, Filion KB, Eberg M, et al. Prognostic value of atrial fibrillation inducibility after right atrial flutter ablation. Heart Rhythm. 2014;11:1870–1876. [DOI] [PubMed] [Google Scholar]
- 24.Henmi R, Ejima K, Shoda M, et al. Interatrial conduction time can predict new-onset atrial fibrillation after radiofrequency ablation of isolated, typical atrial flutter. J Cardiovasc Electrophysiol. 2016;27:1293–1297. [DOI] [PubMed] [Google Scholar]
- 25.Valles E, Marti-Almor J, Grau N, et al. Influence of PACE score and conduction disturbances in the incidence of early new onset atrial fibrillation after typical atrial flutter ablation. J Cardiol. 2022;79:417–422. [DOI] [PubMed] [Google Scholar]
- 26.Seara JG, Roubin SR, Gude Sampedro F, et al. Risk of atrial fibrillation, stroke, and death after radiofrequency catheter ablation of typical atrial flutter. Clin Res Cardiol. 2014;103:543–552. [DOI] [PubMed] [Google Scholar]
7.1. Broad Considerations for Rate Control
- 1.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. [DOI] [PubMed] [Google Scholar]
- 2.Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. [DOI] [PubMed] [Google Scholar]
- 3.Al-Khatib SM, Allen LaPointe NM, Chatterjee R, et al. Rate- and rhythm-control therapies in patients with atrial fibrillation: a systematic review. Ann Intern Med. 2014;160:760–773. [DOI] [PubMed] [Google Scholar]
- 4.Moschovitis G, Johnson LSB, Blum S, et al. Heart rate and adverse outcomes in patients with prevalent atrial fibrillation. Open Heart. 2021;8:e001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song S, Ko JS, Lee HA, et al. Clinical implications of heart rate control in heart failure with atrial fibrillation: Multi-Center Prospective Observation Registry (CODE-AF Registry). Front Cardiovasc Med. 2022;9:787869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Opolski G, Torbicki A, Kosior DA, et al. Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the results of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) Study. Chest. 2004;126:476–486. [DOI] [PubMed] [Google Scholar]
- 7.Groenveld HF, Crijns HJ, Van den Berg MP, et al. The effect of rate control on quality of life in patients with permanent atrial fibrillation: data from the RACE II (Rate Control Efficacy in Permanent Atrial Fibrillation II) study. J Am Coll Cardiol. 2011;58:1795–1803. [DOI] [PubMed] [Google Scholar]
- 8.Groenveld HF, Tijssen JG, Crijns HJ, et al. Rate control efficacy in permanent atrial fibrillation: successful and failed strict rate control against a background of lenient rate control: data from RACE II (Rate Control Efficacy in Permanent Atrial Fibrillation). J Am Coll Cardiol. 2013;61:741–748. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg BA, Kim S, Thomas L, et al. Increased heart rate is associated with higher mortality in patients with atrial fibrillation (AF): results from the Outcomes Registry for Better Informed Treatment of AF (ORBIT-AF). J Am Heart Assoc. 2015;4:e002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandey A, Kim S, Moore C, et al. Predictors and prognostic implications of incident heart failure in patients with prevalent atrial fibrillation. JACC Heart Fail. 2017;5:44–52. [DOI] [PubMed] [Google Scholar]
- 11.Cullington D, Goode KM, Zhang J, et al. Is heart rate important for patients with heart failure in atrial fibrillation? JACC Heart Fail. 2014;2:213–220. [DOI] [PubMed] [Google Scholar]
- 12.Li SJ, Sartipy U, Lund LH, et al. Prognostic significance of resting heart rate and use of beta-blockers in atrial fibrillation and sinus rhythm in patients with heart failure and reduced ejection fraction: findings from the Swedish Heart Failure Registry. Circ Heart Fail. 2015;8:871–879. [DOI] [PubMed] [Google Scholar]
- 13.Mulder BA, Damman K, Van Veldhuisen DJ, et al. Heart rate and outcome in heart failure with reduced ejection fraction: differences between atrial fibrillation and sinus rhythm-a CIBIS II analysis. Clin Cardiol. 2017;40:740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sartipy U, Savarese G, Dahlstrom U, et al. Association of heart rate with mortality in sinus rhythm and atrial fibrillation in heart failure with preserved ejection fraction. Eur J Heart Fail. 2019;21:471–479. [DOI] [PubMed] [Google Scholar]
- 15.Hess PL, Sheng S, Matsouaka R, et al. Strict versus lenient versus poor rate control among patients with atrial fibrillation and heart failure (from the Get With The Guidelines - Heart Failure Program). Am J Cardiol. 2020;125:894–900. [DOI] [PubMed] [Google Scholar]
- 16.Docherty KF, Shen L, Castagno D, et al. Relationship between heart rate and outcomes in patients in sinus rhythm or atrial fibrillation with heart failure and reduced ejection fraction. Eur J Heart Fail. 2020;22:528–538. [DOI] [PubMed] [Google Scholar]
- 17.Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–1316. [DOI] [PubMed] [Google Scholar]
- 18.Van Gelder IC, Groenveld HF, Crijns HJGM, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. [DOI] [PubMed] [Google Scholar]
- 19.Nerheim P, Birger-Botkin S, Piracha L, et al. Heart failure and sudden death in patients with tachycardia-induced cardiomyopathy and recurrent tachycardia. Circulation. 2004;110:247–252. [DOI] [PubMed] [Google Scholar]
- 20.van Gelder IC, Phan HM, Wilkoff BL, et al. Prognostic significance of atrial arrhythmias in a primary prevention ICD population. Pacing Clin Electrophysiol. 2011;34:1070–1079. [DOI] [PubMed] [Google Scholar]
- 21.Boriani G, Gasparini M, Landolina M, et al. Incidence and clinical relevance of uncontrolled ventricular rate during atrial fibrillation in heart failure patients treated with cardiac resynchronization therapy. Eur J Heart Fail. 2011;13:868–876. [DOI] [PubMed] [Google Scholar]
- 22.Boriani G, Padeletti L, Santini M, et al. Rate control in patients with pacemaker affected by brady-tachy form of sick sinus syndrome. Am Heart J. 2007;154:193–200. [DOI] [PubMed] [Google Scholar]
7.2. Specific Pharmacological Agents for Rate Control
- 1.Ulimoen SR, Enger S, Carlson J, et al. Comparison of four single-drug regimens on ventricular rate and arrhythmia-related symptoms in patients with permanent atrial fibrillation. Am J Cardiol. 2013;111:225–230. [DOI] [PubMed] [Google Scholar]
- 2.Nikolaidou T, Channer KS. Chronic atrial fibrillation: a systematic review of medical heart rate control management. Postgrad Med J. 2009;85:303–312. [DOI] [PubMed] [Google Scholar]
7.2.1. Acute Rate Control
- 1.Platia EV, Michelson EL, Porterfield JK, et al. Esmolol versus verapamil in the acute treatment of atrial fibrillation or atrial flutter. Am J Cardiol. 1989;63:925–929. [DOI] [PubMed] [Google Scholar]
- 2.Elkayam U Calcium channel blockers in heart failure. Cardiology. 1998;89(suppl 1):38–46. [DOI] [PubMed] [Google Scholar]
- 3.Lan Q, Wu F, Han B, et al. Intravenous diltiazem versus metoprolol for atrial fibrillation with rapid ventricular rate: a meta-analysis. Am J Emerg Med. 2022;51:248–256. [DOI] [PubMed] [Google Scholar]
- 4.Fromm C, Suau SJ, Cohen V, et al. Diltiazem vs metoprolol in the management of atrial fibrillation or flutter with rapid ventricular rate in the emergency department. J Emerg Med. 2015;49:175–182. [DOI] [PubMed] [Google Scholar]
- 5.Intravenous digoxin in acute atrial fibrillation. Results of a randomized, placebo-controlled multicentre trial in 239 patients. The Digitalis in Acute Atrial Fibrillation (DAAF) Trial Group. Eur Heart J. 1997;18:649–654. [DOI] [PubMed] [Google Scholar]
- 6.Siu CW, Lau CP, Lee WL, et al. Intravenous diltiazem is superior to intravenous amiodarone or digoxin for achieving ventricular rate control in patients with acute uncomplicated atrial fibrillation. Crit Care Med. 2009;37:2174–2179; quiz 2180. [DOI] [PubMed] [Google Scholar]
- 7.Schreck DM, Rivera AR, Tricarico VJ. Emergency management of atrial fibrillation and flutter: intravenous diltiazem versus intravenous digoxin. Ann Emerg Med. 1997;29:135–140. [DOI] [PubMed] [Google Scholar]
- 8.Shojaee M, Feizi B, Miri R, et al. Intravenous amiodarone versus digoxin in atrial fibrillation rate control; a clinical trial. Emergency. 2017;5:e29. [PMC free article] [PubMed] [Google Scholar]
- 9.Wattanasuwan N, Khan IA, Mehta NJ, et al. Acute ventricular rate control in atrial fibrillation: IV combination of diltiazem and digoxin vs IV diltiazem alone. Chest. 2001;119:502–506. [DOI] [PubMed] [Google Scholar]
- 10.Ramesh T, Lee PYK, Mitta M, et al. Intravenous magnesium in the management of rapid atrial fibrillation: a systematic review and meta-analysis. J Cardiol. 2021;78:375–381. [DOI] [PubMed] [Google Scholar]
- 11.Ho KM, Sheridan DJ, Paterson T. Use of intravenous magnesium to treat acute onset atrial fibrillation: a meta-analysis. Heart. 2007;93:1433–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clemo HF, Wood MA, Gilligan DM, et al. Intravenous amiodarone for acute heart rate control in the critically ill patient with atrial tachyarrhythmias. Am J Cardiol. 1998;81:594–598. [DOI] [PubMed] [Google Scholar]
- 13.Delle Karth G, Geppert A, Neunteufl T, et al. Amiodarone versus diltiazem for rate control in critically ill patients with atrial tachyarrhythmias. Crit Care Med. 2001;29:1149–1153. [DOI] [PubMed] [Google Scholar]
- 14.Jandali MB. Safety of intravenous diltiazem in reduced ejection fraction heart failure with rapid atrial fibrillation. Clin Drug Investig. 2018;38:503–508. [DOI] [PubMed] [Google Scholar]
- 15.Hasbrouck M, Nguyen TT. Acute management of atrial fibrillation in congestive heart failure with reduced ejection fraction in the emergency department. Am J Emerg Med. 2022;58:39–42. [DOI] [PubMed] [Google Scholar]
- 16.DiCarlo LA Jr, Morady F, de Buitleir M, et al. Effects of magnesium sulfate on cardiac conduction and refractoriness in humans. J Am Coll Cardiol. 1986;7:1356–1362. [DOI] [PubMed] [Google Scholar]
7.2.2. Long-Term Rate Control
- 1.Scheuermeyer FX, Grafstein E, Stenstrom R, et al. Safety and efficiency of calcium channel blockers versus beta-blockers for rate control in patients with atrial fibrillation and no acute underlying medical illness. Acad Emerg Med. 2013;20:222–230. [DOI] [PubMed] [Google Scholar]
- 2.Zaman N, Naccarelli G, Foy A. A comparison of rate control agents for the treatment of atrial fibrillation: follow-up investigation of the AFFIRM study. J Cardiovasc Pharmacol Ther. 2021;26:328–334. [DOI] [PubMed] [Google Scholar]
- 3.Lopes RD, Rordorf R, De Ferrari GM, et al. Digoxin and mortality in patients with atrial fibrillation. J Am Coll Cardiol. 2018;71:1063–1074. [DOI] [PubMed] [Google Scholar]
- 4.Rathore SS, Curtis JP, Wang Y, et al. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289:871–878. [DOI] [PubMed] [Google Scholar]
- 5.Adams KF Jr, Patterson JH, Gattis WA, et al. Relationship of serum digoxin concentration to mortality and morbidity in women in the digitalis investigation group trial: a retrospective analysis. J Am Coll Cardiol. 2005;46:497–504. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed A, Gambassi G, Weaver MT, et al. Effects of discontinuation of digoxin versus continuation at low serum digoxin concentrations in chronic heart failure. Am J Cardiol. 2007;100:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khand AU, Rankin AC, Martin W, et al. Carvedilol alone or in combination with digoxin for the management of atrial fibrillation in patients with heart failure? J Am Coll Cardiol. 2003;42:1944–1951. [DOI] [PubMed] [Google Scholar]
- 8.Kotecha D, Bunting KV, Gill SK, et al. Effect of digoxin vs bisoprolol for heart rate control in atrial fibrillation on patient-reported quality of life: the RATE-AF randomized clinical trial. JAMA. 2020;324:2497–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziff OJ, Lane DA, Samra M, et al. Safety and efficacy of digoxin: systematic review and meta-analysis of observational and controlled trial data. BMJ. 2015;351:h4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein RE, Boccuzzi SJ, Cruess D, et al. Diltiazem increases late-onset congestive heart failure in postinfarction patients with early reduction in ejection fraction The Adverse Experience Committee; and the Multicenter Diltiazem Postinfarction Research Group. Circulation. 1991;83:52–60. [DOI] [PubMed] [Google Scholar]
- 11.Multicenter Diltiazem Postinfarction Trial Research Group. The effect of diltiazem on mortality and reinfarction after myocardial infarction. N Engl J Med. 1988;319:385–392. [DOI] [PubMed] [Google Scholar]
- 12.Connolly SJ, Camm AJ, Halperin JL, et al. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med. 2011;365:2268–2276. [DOI] [PubMed] [Google Scholar]
- 13.Ulimoen SR, Enger S, Carlson J, et al. Comparison of four single-drug regimens on ventricular rate and arrhythmia-related symptoms in patients with permanent atrial fibrillation. Am J Cardiol. 2013;111:225–230. [DOI] [PubMed] [Google Scholar]
- 14.Nikolaidou T, Channer KS. Chronic atrial fibrillation: a systematic review of medical heart rate control management. Postgrad Med J. 2009;85:303–312. [DOI] [PubMed] [Google Scholar]
- 15.Smith TW, Butler VP Jr, Haber E. Determination of therapeutic and toxic serum digoxin concentrations by radioimmunoassay. N Engl J Med. 1969;281:1212–1216. [DOI] [PubMed] [Google Scholar]
- 16.Hauptman PJ, McCann P, Romero JM, et al. Reference laboratory values for digoxin following publication of Digitalis Investigation Group (DIG) trial data. JAMA Intern Med. 2013;173:1552–1554. [DOI] [PubMed] [Google Scholar]
- 17.Goldberger ZD, Goldberger AL. Therapeutic ranges of serum digoxin concentrations in patients with heart failure. Am J Cardiol. 2012;109:1818–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kober L, Torp-Pedersen C, McMurray JJ, et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–2687. [DOI] [PubMed] [Google Scholar]
- 19.Hohnloser S, Crijns H, van Eickels M, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. Rev Port Cardiol. 2009;28:345–347. [DOI] [PubMed] [Google Scholar]
- 20.Davy JM, Herold M, Hoglund C, et al. Dronedarone for the control of ventricular rate in permanent atrial fibrillation: the Efficacy and safety of dRonedArone for the cOntrol of ventricular rate during atrial fibrillation (ERATO) study. Am Heart J. 2008;156:527.e521–527.e529. [DOI] [PubMed] [Google Scholar]
7.3. Atrioventricular Nodal Ablation (AVNA)
- 1.Geelen P, Brugada J, Andries E, et al. Ventricular fibrillation and sudden death after radiofrequency catheter ablation of the atrioventricular junction. Pacing Clin Electrophysiol. 1997;20:343–348. [DOI] [PubMed] [Google Scholar]
- 2.Wang RX, Lee HC, Hodge DO, et al. Effect of pacing method on risk of sudden death after atrioventricular node ablation and pacemaker implantation in patients with atrial fibrillation. Heart Rhythm. 2013;10:696–701. [DOI] [PubMed] [Google Scholar]
- 3.Brignole M, Menozzi C, Gianfranchi L, et al. Assessment of atrioventricular junction ablation and VVIR pacemaker versus pharmacological treatment in patients with heart failure and chronic atrial fibrillation: a randomized, controlled study. Circulation. 1998;98:953–960. [DOI] [PubMed] [Google Scholar]
- 4.Lim KT, Davis MJ, Powell A, et al. Ablate and pace strategy for atrial fibrillation: long-term outcome of AIRCRAFT trial. Europace. 2007;9:498–505. [DOI] [PubMed] [Google Scholar]
- 5.Wood MA, Brown-Mahoney C, Kay GN, et al. Clinical outcomes after ablation and pacing therapy for atrial fibrillation: a meta-analysis. Circulation. 2000;101:1138–1144. [DOI] [PubMed] [Google Scholar]
- 6.Bradley DJ, Shen WK. Atrioventricular junction ablation combined with either right ventricular pacing or cardiac resynchronization therapy for atrial fibrillation: the need for large-scale randomized trials. Heart Rhythm. 2007;4:224–232. [DOI] [PubMed] [Google Scholar]
- 7.Udo EO, Zuithoff NP, van Hemel NM, et al. Incidence and predictors of short- and long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm. 2012;9:728–735. [DOI] [PubMed] [Google Scholar]
- 8.Armaganijan LV, Toff WD, Nielsen JC, et al. Are elderly patients at increased risk of complications following pacemaker implantation? A meta-analysis of randomized trials. Pacing Clin Electrophysiol. 2012;35:131–134. [DOI] [PubMed] [Google Scholar]
- 9.Baudo M, D'Ancona G, Trinca F, et al. Atrioventricular node ablation and pacing for atrial tachyarrhythmias: a meta-analysis of postoperative outcomes. Int J Cardiol. 2022;363:80–86. [DOI] [PubMed] [Google Scholar]
- 10.Vijayaraman P, Subzposh FA, Naperkowski A. Atrioventricular node ablation and His bundle pacing. Europace. 2017;19:iv10–iv16. [DOI] [PubMed] [Google Scholar]
- 11.Morina-Vazquez P, Moraleda-Salas MT, Arce-Leon A, et al. Effectiveness and safety of AV node ablation after His bundle pacing in patients with uncontrolled atrial arrhythmias. Pacing Clin Electrophysiol. 2021;44:1004–1009. [DOI] [PubMed] [Google Scholar]
- 12.Pillai A, Kolominsky J, Koneru JN, et al. Atrioventricular junction ablation in patients with conduction system pacing leads: a comparison of His-bundle vs left bundle branch area pacing leads. Heart Rhythm. 2022;19:1116–1123. [DOI] [PubMed] [Google Scholar]
- 13.Vijayaraman P, Mathew AJ, Naperkowski A, et al. Conduction system pacing versus conventional pacing in patients undergoing atrioventricular node ablation: nonrandomized, on-treatment comparison. Heart Rhythm O2. 2022;3:368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brignole M, Gianfranchi L, Menozzi C, et al. Atrioventricular junction ablation and pacemaker therapy versus drug treatment in atrial fibrillation. Cardiol Rev. 1998;15:49–53. [Google Scholar]
- 15.Gillis AM, Connolly SJ, Lacombe P, et al. Randomized crossover comparison of DDDR versus VDD pacing after atrioventricular junction ablation for prevention of atrial fibrillation. Circulation. 2000;102:736–741. [DOI] [PubMed] [Google Scholar]
- 16.D H, Novak P, O S. Survival of dual chamber pacing post atrioventricular nodal ablation in patients with atrial fibrillation. Heart Rhythm. 2009;6:S406–S407. [Google Scholar]
- 17.Brignole M, Menozzi C, Gasparini M, et al. An evaluation of the strategy of maintenance of sinus rhythm by antiarrhythmic drug therapy after ablation and pacing therapy in patients with paroxysmal atrial fibrillation. Eur Heart J. 2002;23:892–900. [DOI] [PubMed] [Google Scholar]
- 18.Nowinski K, Gadler F, Jensen-Urstad M, et al. Transient proarrhythmic state following atrioventricular junctional radiofrequency ablation. Pacing Clin Electrophysiol. 2002;25:291–299. [DOI] [PubMed] [Google Scholar]
- 19.Slotwiner DJ, Raitt MH, Del-Carpio Munoz F, et al. Impact of physiologic pacing versus right ventricular pacing among patients with left ventricular ejection fraction greater than 35%: a systematic review for the 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;140:e483–e503. [DOI] [PubMed] [Google Scholar]
- 20.Olgin JE, Scheinman MM. Comparison of high energy direct current and radiofrequency catheter ablation of the atrioventricular junction. J Am Coll Cardiol. 1993;21:557–564. [DOI] [PubMed] [Google Scholar]
- 21.Kay GN, Ellenbogen KA, Giudici M, et al. The Ablate and Pace Trial: a prospective study of catheter ablation of the AV conduction system and permanent pacemaker implantation for treatment of atrial fibrillation APT Investigators. J Interv Card Electrophysiol. 1998;2:121–135. [DOI] [PubMed] [Google Scholar]
- 22.Weerasooriya R, Davis M, Powell A, et al. The Australian Intervention Randomized Control of Rate in Atrial Fibrillation Trial (AIRCRAFT). J Am Coll Cardiol. 2003;41:1697–1702. [DOI] [PubMed] [Google Scholar]
- 23.Redfield MM, Kay GN, Jenkins LS, et al. Tachycardia-related cardiomyopathy: a common cause of ventricular dysfunction in patients with atrial fibrillation referred for atrioventricular ablation. Mayo Clin Proc. 2000;75:790–795. [DOI] [PubMed] [Google Scholar]
- 24.Vijayaraman P, Hashimova N, Mathew AJ, et al. Simultaneous conduction system pacing and atrioventricular node ablation via axillary vs femoral access. Heart Rhythm. 2022;19:1019–1021. [DOI] [PubMed] [Google Scholar]
- 25.Bongiorni MG, Proclemer A, Dobreanu D, et al. Preferred tools and techniques for implantation of cardiac electronic devices in Europe: results of the European Heart Rhythm Association survey. Europace. 2013;15:1664–1668. [DOI] [PubMed] [Google Scholar]
- 26.Vernooy K, Dijkman B, Cheriex EC, et al. Ventricular remodeling during long-term right ventricular pacing following His bundle ablation. Am J Cardiol. 2006;97:1223–1227. [DOI] [PubMed] [Google Scholar]
- 27.Doshi RN, Daoud EG, Fellows C, et al. Left ventricular-based cardiac stimulation post AV nodal ablation evaluation (the PAVE study). J Cardiovasc Electrophysiol. 2005;16:1160–1165. [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Hodge D, Jahangir A, et al. Preserved left ventricular ejection fraction following atrioventricular junction ablation and pacing for atrial fibrillation. J Cardiovasc Electrophysiol. 2008;19:19–27. [DOI] [PubMed] [Google Scholar]
8.1. Goals of Therapy With Rhythm Control
- 1.Santhanakrishnan R, Wang N, Larson MG, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu LF, Jais P, Sanders P, et al. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351:2373–2383. [DOI] [PubMed] [Google Scholar]
- 3.Prabhu S, Costello BT, Taylor AJ, et al. Regression of diffuse ventricular fibrosis following restoration of sinus rhythm with catheter ablation in patients with atrial fibrillation and systolic dysfunction: a substudy of the CAMERA MRI trial. JACC Clin Electrophysiol. 2018;4:999–1007. [DOI] [PubMed] [Google Scholar]
- 4.Stronati G, Guerra F, Urbinati A, et al. Tachycardiomyopathy in patients without underlying structural heart disease. J Clin Med. 2019;8:1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeong YH, Choi KJ, Song JM, et al. Diagnostic approach and treatment strategy in tachycardia-induced cardiomyopathy. Clin Cardiol. 2008;31:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcusohn E, Postnikov M, Kobo O, et al. Factors associated with left ventricular function recovery in patients with atrial fibrillation related cardiomyopathy. Isr Med Assoc J. 2022;24:101–106. [PubMed] [Google Scholar]
- 7.Cooper HA, Bloomfield DA, Bush DE, et al. Relation between achieved heart rate and outcomes in patients with atrial fibrillation (from the Atrial Fibrillation Follow-up Investigation of Rhythm Management [AFFIRM] Study). Am J Cardiol. 2004;93:1247–1253. [DOI] [PubMed] [Google Scholar]
- 8.Miura K, Ikemura N, Kimura T, et al. Treatment strategies and subsequent changes in the patient-reported quality-of-life among elderly patients with atrial fibrillation. Am Heart J. 2020;222:83–92. [DOI] [PubMed] [Google Scholar]
- 9.Kosior DA, Szulc M, Rosiak M, et al. Functional status with rhythm- versus rate-control strategy for persistent atrial fibrillation. Pol Arch Intern Med. 2018;128:658–666. [DOI] [PubMed] [Google Scholar]
- 10.Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. [DOI] [PubMed] [Google Scholar]
- 11.Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384:305–315. [DOI] [PubMed] [Google Scholar]
- 12.Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–1316. [DOI] [PubMed] [Google Scholar]
- 13.Kim D, Yang PS, You SC, et al. Treatment timing and the effects of rhythm control strategy in patients with atrial fibrillation: nationwide cohort study. BMJ. 2021;373:n991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickow J, Kirchhof P, Van Houten HK, et al. Generalizability of the EAST-AFNET 4 Trial: assessing outcomes of early rhythm-control therapy in patients with atrial fibrillation. J Am Heart Assoc. 2022;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. [DOI] [PubMed] [Google Scholar]
- 16.Rillig A, Magnussen C, Ozga AK, et al. Early rhythm control therapy in patients with atrial fibrillation and heart failure. Circulation. 2021;144:845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 18.Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637–1644. [DOI] [PubMed] [Google Scholar]
- 19.Packer DL, Piccini JP, Monahan KH, et al. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation. 2021;143:1377–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veasey RA, Sugihara C, Sandhu K, et al. The natural history of atrial fibrillation in patients with permanent pacemakers: is atrial fibrillation a progressive disease? J Interv Card Electrophysiol. 2015;44:23–30. [DOI] [PubMed] [Google Scholar]
- 21.Holmqvist F, Kim S, Steinberg BA, et al. Heart rate is associated with progression of atrial fibrillation, independent of rhythm. Heart. 2015;101:894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang YY, Qiu C, Davis PJ, et al. Predictors of progression of recently diagnosed atrial fibrillation in REgistry on Cardiac Rhythm DisORDers Assessing the Control of Atrial Fibrillation (RecordAF)-United States cohort. Am J Cardiol. 2013;112:79–84. [DOI] [PubMed] [Google Scholar]
- 23.De Vos CB, Breithardt G, Camm AJ, et al. Progression of atrial fibrillation in the REgistry on Cardiac rhythm disORDers assessing the control of Atrial Fibrillation cohort: clinical correlates and the effect of rhythm-control therapy. Am Heart J. 2012;163:887–893. [DOI] [PubMed] [Google Scholar]
- 24.Blum S, Aeschbacher S, Meyre P, et al. Incidence and predictors of atrial fibrillation progression. J Am Heart Assoc. 2019;8:e012554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pathak RK, Middeldorp ME, Meredith M, et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol. 2015;65:2159–2169. [DOI] [PubMed] [Google Scholar]
- 26.Koldenhof T, Wijtvliet PEPJ, Pluymaekers NAHA, et al. Rate control drugs differ in the prevention of progression of atrial fibrillation. EP: Europace. 2022;24:384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang WY, Du X, Fawzy AM, et al. Associations of atrial fibrillation progression with clinical risk factors and clinical prognosis: a report from the Chinese Atrial Fibrillation Registry study. J Cardiovasc Electrophysiol. 2021;32:333–341. [DOI] [PubMed] [Google Scholar]
- 28.Kalman JM, Sanders P, Rosso R, et al. Should we perform catheter ablation for asymptomatic atrial fibrillation? Circulation. 2017;136:490–499. [DOI] [PubMed] [Google Scholar]
- 29.Sgreccia D, Manicardi M, Malavasi VL, et al. Comparing outcomes in asymptomatic and symptomatic atrial fibrillation: a systematic review and meta-analysis of 81 462 patients. J Clin Med. 2021;10:3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willems S, Borof K, Brandes A, et al. Systematic, early rhythm control strategy for atrial fibrillation in patients with or without symptoms: the EAST-AFNET 4 trial. Eur Heart J. 2022;43:1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganapathy AV, Monjazeb S, Ganapathy KS, et al. “Asymptomatic” persistent or permanent atrial fibrillation: a misnomer in selected patients. Int J Cardiol. 2015;185:112–113. [DOI] [PubMed] [Google Scholar]
- 32.Hermans ANL, Pluymaekers N, Lankveld TAR, et al. Clinical utility of rhythm control by electrical cardioversion to assess the association between selfreported symptoms and rhythm status in patients with persistent atrial fibrillation. Int J Cardiol Heart Vasc. 2021;36:100870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senoo K, Suzuki S, Otsuka T, et al. Progression to the persistent form in asymptomatic paroxysmal atrial fibrillation. Circ J. 2014;78:1121–1126. [DOI] [PubMed] [Google Scholar]
- 34.Disertori M, Lombardi F, Barlera S, et al. Clinical characteristics of patients with asymptomatic recurrences of atrial fibrillation in the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico-Atrial Fibrillation (GISSI-AF) trial. Am Heart J. 2011;162:382–389. [DOI] [PubMed] [Google Scholar]
- 35.Bodagh N, Yap R, Kotadia I, et al. Impact of catheter ablation versus medical therapy on cognitive function in atrial fibrillation: a systematic review. J Interv Card Electrophysiol. 2022;65:271–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim D, Yang PS, You SC, et al. Association of rhythm control with incident dementia among patients with atrial fibrillation: a nationwide population-based cohort study. Age Ageing. 2022;51:1–9. [DOI] [PubMed] [Google Scholar]
- 37.Bunch TJ, Crandall BG, Weiss JP, et al. Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22:839–845. [DOI] [PubMed] [Google Scholar]
- 38.Slee A, Saksena S. Impact of initial heart failure emergence on clinical outcomes of atrial fibrillation patients in the AFFIRM trial. Am Heart J. 2020;220:1–11. [DOI] [PubMed] [Google Scholar]
- 39.Gertz ZM, Raina A, Saghy L, et al. Evidence of atrial functional mitral regurgitation due to atrial fibrillation: reversal with arrhythmia control. J Am Coll Cardiol. 2011;58:1474–1481. [DOI] [PubMed] [Google Scholar]
- 40.Reddy ST, Belden W, Doyle M, et al. Mitral regurgitation recovery and atrial reverse remodeling following pulmonary vein isolation procedure in patients with atrial fibrillation: a clinical observation proof-of-concept cardiac MRI study. J Interv Card Electrophysiol. 2013;37:307–315. [DOI] [PubMed] [Google Scholar]
- 41.Abe Y, Takahashi Y, Shibata T. Looking into the mechanistic link between mitral regurgitation and atrial fibrillation. Cardiol Clin. 2021;39:281–288. [DOI] [PubMed] [Google Scholar]
- 42.Soulat-Dufour L, Lang S, Addetia K, et al. Restoring sinus rhythm reverses cardiac remodeling and reduces valvular regurgitation in patients with atrial fibrillation. J Am Coll Cardiol. 2022;79:951–961. [DOI] [PubMed] [Google Scholar]
- 43.Markman TM, Plappert T, De Feria Alsina A, et al. Improvement in tricuspid regurgitation following catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2020;31:2883–2888. [DOI] [PubMed] [Google Scholar]
- 44.Pype L, Embrechts L, Cornez B, et al. Long-term effect of atrial fibrillation on the evolution of mitral and tricuspid valve regurgitation. Acta Cardiol. 2020;75:639–647. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Han J, Li Y, et al. Impact of Surgical ablation of atrial fibrillation on the progression of tricuspid regurgitation and right-sided heart remodeling after mitral-valve surgery: a propensity-score matching analysis. J Am Heart Assoc. 2016;5:e004213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin PL, Huang CC, Wu YJ, et al. Relations between baseline burden, maximum duration, and relative reduction of atrial fibrillation: insights from continuous monitoring in rhythm control. J Cardiovasc Electrophysiol. 2019;30:178–182. [DOI] [PubMed] [Google Scholar]
- 47.Chew DS, Jones KA, Loring Z, et al. Diagnosis-to-ablation time predicts recurrent atrial fibrillation and rehospitalization following catheter ablation. Heart Rhythm O2. 2022;3:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141:e750–e772. [DOI] [PubMed] [Google Scholar]
- 49.Gopinathannair R, Chen LY, Chung MK, et al. Managing atrial fibrillation in patients with heart failure and reduced ejection fraction: a scientific statement from the American Heart Association. Circ Arrhythm Electrophysiol. 2021;14:e000078. [DOI] [PubMed] [Google Scholar]
- 50.Hagens VE, Ranchor AV, Van Sonderen E, et al. Effect of rate or rhythm control on quality of life in persistent atrial fibrillation. Results from the Rate Control Versus Electrical Cardioversion (RACE) Study. J Am Coll Cardiol. 2004;43:241–247. [DOI] [PubMed] [Google Scholar]
- 51.Ha AC, Breithardt G, Camm AJ, et al. Health-related quality of life in patients with atrial fibrillation treated with rhythm control versus rate control: insights from a prospective international registry (Registry on Cardiac Rhythm Disorders Assessing the Control of Atrial Fibrillation: RECORD-AF). Circ Cardiovasc Qual Outcomes. 2014;7:896–904. [DOI] [PubMed] [Google Scholar]
- 52.Govindapillai A, Cox JL, Thabane L, et al. Rhythm control vs rate control in a contemporary ambulatory atrial fibrillation cohort: post hoc analysis of the IMPACT-AF trial. CJC Open. 2022;4:551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu L, Lu Y, Zheng L, et al. Comparison of radiofrequency catheter ablation between asymptomatic and symptomatic persistent atrial fibrillation: a propensity score matched analysis. J Cardiovasc Electrophysiol. 2016;27:531–535. [DOI] [PubMed] [Google Scholar]
- 54.Mohanty S, Santangeli P, Mohanty P, et al. Catheter ablation of asymptomatic longstanding persistent atrial fibrillation: impact on quality of life, exercise performance, arrhythmia perception, and arrhythmia-free survival. J Cardiovasc Electrophysiol. 2014;25:1057–1064. [DOI] [PubMed] [Google Scholar]
- 55.Suman-Horduna I, Roy D, Frasure-Smith N, et al. Quality of life and functional capacity in patients with atrial fibrillation and congestive heart failure. J Am Coll Cardiol. 2013;61:455–460. [DOI] [PubMed] [Google Scholar]
- 56.Hsu LF, Jaïs P, Sanders P, et al. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351:2373–2383. [DOI] [PubMed] [Google Scholar]
- 57.Parkash R, Wells GA, Rouleau J, et al. Randomized ablation-based rhythm-control versus rate-control trial in patients with heart failure and atrial fibrillation: results from the RAFT-AF trial. Circulation. 2022;145:1693–1704. [DOI] [PubMed] [Google Scholar]
- 58.Chiou WR, Lin PL, Huang CC, et al. Rhythm control without catheter ablation may have benefits beyond stroke prevention in rivaroxaban-treated non-permanent atrial fibrillation. Sci Rep. 2022;12:3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
8.2. Electrical and Pharmacologic Cardioversion
- 1.Crijns HJ, Weijs B, Fairley AM, et al. Contemporary real life cardioversion of atrial fibrillation: Results from the multinational RHYTHM-AF study. Int J Cardiol. 2014;172:588–594. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez-Madrid A, Svendsen JH, Lip GY, et al. Cardioversion for atrial fibrillation in current European practice: results of the European Heart Rhythm Association survey. Europace. 2013;15:915–918. [DOI] [PubMed] [Google Scholar]
- 3.Pisters R, Nieuwlaat R, Prins MH, et al. Clinical correlates of immediate success and outcome at 1-year follow-up of real-world cardioversion of atrial fibrillation: the Euro Heart Survey. Europace. 2012;14:666–674. [DOI] [PubMed] [Google Scholar]
- 4.Stiell IG, Clement CM, Brison RJ, et al. Variation in management of recent-onset atrial fibrillation and flutter among academic hospital emergency departments. Ann Emerg Med. 2011;57:13–21. [DOI] [PubMed] [Google Scholar]
- 5.Dankner R, Shahar A, Novikov I, et al. Treatment of stable atrial fibrillation in the emergency department: a population-based comparison of electrical direct-current versus pharmacological cardioversion or conservative management. Cardiology. 2009;112:270–278. [DOI] [PubMed] [Google Scholar]
- 6.Brandes A, Crijns H, Rienstra M, et al. Cardioversion of atrial fibrillation and atrial flutter revisited: current evidence and practical guidance for a common procedure. Europace. 2020;22:1149–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lip GY, Gitt AK, Le Heuzey JY, et al. Overtreatment and undertreatment with anticoagulation in relation to cardioversion of atrial fibrillation (the RHYTHM-AF study). Am J Cardiol. 2014;113:480–484. [DOI] [PubMed] [Google Scholar]
- 8.Airaksinen KE, Grönberg T, Nuotio I, et al. Thromboembolic complications after cardioversion of acute atrial fibrillation: the FinCV (Finnish CardioVersion) study. J Am Coll Cardiol. 2013;62:1187–1192. [DOI] [PubMed] [Google Scholar]
8.2.1. Prevention of Thromboembolism in the Setting of Cardioversion
- 1.Klein AL, Grimm RA, Murray RD, et al. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med. 2001;344:1411–1420. [DOI] [PubMed] [Google Scholar]
- 2.Fatkin D, Kuchar DL, Thorburn CW, et al. Transesophageal echocardiography before and during direct current cardioversion of atrial fibrillation: evidence for “atrial stunning” as a mechanism of thromboembolic complications. J Am Coll Cardiol. 1994;23:307–316. [DOI] [PubMed] [Google Scholar]
- 3.Grimm RA, Stewart WJ, Maloney JD, et al. Impact of electrical cardioversion for atrial fibrillation on left atrial appendage function and spontaneous echo contrast: characterization by simultaneous transesophageal echocardiography. J Am Coll Cardiol. 1993;22:1359–1366. [DOI] [PubMed] [Google Scholar]
- 4.Manning WJ, Leeman DE, Gotch PJ, et al. Pulsed Doppler evaluation of atrial mechanical function after electrical cardioversion of atrial fibrillation. J Am Coll Cardiol. 1989;13:617–623. [DOI] [PubMed] [Google Scholar]
- 5.Manning WJ, Silverman DI, Katz SE, et al. Impaired left atrial mechanical function after cardioversion: relation to the duration of atrial fibrillation. J Am Coll Cardiol. 1994;23:1535–1540. [DOI] [PubMed] [Google Scholar]
- 6.Berger M, Schweitzer P. Timing of thromboembolic events after electrical cardioversion of atrial fibrillation or flutter: a retrospective analysis. Am J Cardiol. 1998;82:1545–1547. A1548. [DOI] [PubMed] [Google Scholar]
- 7.McIntyre WF, Connolly SJ, Wang J, et al. Thromboembolic events around the time of cardioversion for atrial fibrillation in patients receiving antiplatelet treatment in the ACTIVE trials. Eur Heart J. 2019;40:3026–3032. [DOI] [PubMed] [Google Scholar]
- 8.Lip GY, Hammerstingl C, Marin F, et al. Left atrial thrombus resolution in atrial fibrillation or flutter: results of a prospective study with rivaroxaban (X-TRA) and a retrospective observational registry providing baseline data (CLOT-AF). Am Heart J. 2016;178:126–134. [DOI] [PubMed] [Google Scholar]
- 9.Aryana A, Singh SK, Singh SM, et al. Association between incomplete surgical ligation of left atrial appendage and stroke and systemic embolization. Heart Rhythm. 2015;12:1431–1437. [DOI] [PubMed] [Google Scholar]
- 10.Mohanty S, Gianni C, Trivedi C, et al. Risk of thromboembolic events after percutaneous left atrial appendage ligation in patients with atrial fibrillation: long-term results of a multicenter study. Heart Rhythm. 2020;17:175–181. [DOI] [PubMed] [Google Scholar]
- 11.Alkhouli M, Du C, Killu A, et al. Clinical impact of residual leaks following left atrial appendage occlusion: insights from the NCDR LAAO registry. JACC Clin Electrophysiol. 2022;8:766–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viles-Gonzalez JF, Kar S, Douglas P, et al. The clinical impact of incomplete left atrial appendage closure with the Watchman Device in patients with atrial fibrillation: a PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) substudy. J Am Coll Cardiol. 2012;59:923–929. [DOI] [PubMed] [Google Scholar]
- 13.Boersma LV, Ince H, Kische S, et al. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1-Year follow-up outcome data of the EWOLUTION trial. Heart Rhythm. 2017;14:1302–1308. [DOI] [PubMed] [Google Scholar]
- 14.Afzal MR, Gabriels JK, Jackson GG, et al. Temporal changes and clinical implications of delayed peridevice leak following left atrial appendage closure. JACC Clin Electrophysiol. 2022;8:15–25. [DOI] [PubMed] [Google Scholar]
- 15.Fauchier L, Cinaud A, Brigadeau F, et al. Device-related thrombosis after percutaneous left atrial appendage occlusion for atrial fibrillation. J Am Coll Cardiol. 2018;71:1528–1536. [DOI] [PubMed] [Google Scholar]
- 16.Alkhouli M, Busu T, Shah K, et al. Incidence and clinical impact of device-related thrombus following percutaneous left atrial appendage occlusion: a meta-analysis. JACC: Clinical Electrophysiology. 2018;4:1629–1637. [DOI] [PubMed] [Google Scholar]
- 17.Dukkipati SR, Kar S, Holmes DR, et al. Device-related thrombus after left atrial appendage closure: incidence, predictors, and outcomes. Circulation. 2018;138:874–885. [DOI] [PubMed] [Google Scholar]
- 18.Simard T, Jung RG, Lehenbauer K, et al. Predictors of device-related thrombus following percutaneous left atrial appendage occlusion. J Am Coll Cardiol. 2021;78:297–313. [DOI] [PubMed] [Google Scholar]
- 19.Kubo S, Mizutani Y, Meemook K, et al. Incidence, characteristics, and clinical course of device-related thrombus after Watchman left atrial appendage occlusion device implantation in atrial fibrillation patients. JACC Clin Electrophysiol. 2017;3:1380–1386. [DOI] [PubMed] [Google Scholar]
- 20.Sharma SP, Turagam MK, Gopinathannair R, et al. Direct current cardioversion of atrial fibrillation in patients with left atrial appendage occlusion devices. J Am Coll Cardiol. 2019;74:2267–2274. [DOI] [PubMed] [Google Scholar]
- 21.Nuotio I, Hartikainen JE, Gronberg T, et al. Time to cardioversion for acute atrial fibrillation and thromboembolic complications. JAMA. 2014;312:647–649. [DOI] [PubMed] [Google Scholar]
- 22.Grönberg T, Hartikainen JE, Nuotio I, et al. Anticoagulation, CHA2DS2VASc score, and thromboembolic risk of cardioversion of acute atrial fibrillation (from the FinCV study). Am J Cardiol. 2016;117:1294–1298. [DOI] [PubMed] [Google Scholar]
- 23.Lip GY, Gitt AK, Le Heuzey JY, et al. Overtreatment and undertreatment with anticoagulation in relation to cardioversion of atrial fibrillation (the RHYTHM-AF study). Am J Cardiol. 2014;113:480–484. [DOI] [PubMed] [Google Scholar]
- 24.Airaksinen KE, Grönberg T, Nuotio I, et al. Thromboembolic complications after cardioversion of acute atrial fibrillation: the FinCV (Finnish CardioVersion) study. J Am Coll Cardiol. 2013;62:1187–1192. [DOI] [PubMed] [Google Scholar]
- 25.Lip GY. Cardioversion of atrial fibrillation. Postgrad Med J. 1995;71:457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cappato R, Ezekowitz MD, Klein AL, et al. Rivaroxaban vs vitamin K antagonists for cardioversion in atrial fibrillation. Eur Heart J. 2014;35:3346–3355. [DOI] [PubMed] [Google Scholar]
- 27.Ezekowitz MD, Pollack CV Jr, Halperin JL, et al. Apixaban compared to heparin/vitamin K antagonist in patients with atrial fibrillation scheduled for cardioversion: the EMANATE trial. Eur Heart J. 2018;39:2959–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goette A, Merino JL, Ezekowitz MD, et al. Edoxaban versus enoxaparin-warfarin in patients undergoing cardioversion of atrial fibrillation (ENSURE-AF): a randomised, open-label, phase 3b trial. Lancet. 2016;388:1995–2003. [DOI] [PubMed] [Google Scholar]
- 29.Kotecha D, Pollack CV Jr, De Caterina R, et al. Direct oral anticoagulants halve thromboembolic events after cardioversion of AF compared with warfarin. J Am Coll Cardiol. 2018;72:1984–1986. [DOI] [PubMed] [Google Scholar]
- 30.Lown B Electrical reversion of cardiac arrhythmias. Br Heart J. 1967;29:469–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeSilva RA, Graboys TB, Podrid PJ, et al. Cardioversion and defibrillation. Am Heart J. 1980;100:881–895. [DOI] [PubMed] [Google Scholar]
- 32.Spagnolo P, Giglio M, Di Marco D, et al. Diagnosis of left atrial appendage thrombus in patients with atrial fibrillation: delayed contrast-enhanced cardiac CT. Eur Radiol. 2021;31:1236–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romero J, Husain SA, Kelesidis I, et al. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta-analysis. Circ Cardiovasc Imaging. 2013;6:185–194. [DOI] [PubMed] [Google Scholar]
- 34.Pathan F, Hecht H, Narula J, et al. Roles of transesophageal echocardiography and cardiac computed tomography for evaluation of left atrial thrombus and associated pathology: a review and critical analysis. JACC Cardiovasc Imaging. 2018;11:616–627. [DOI] [PubMed] [Google Scholar]
- 35.Black IW, Fatkin D, Sagar KB, et al. Exclusion of atrial thrombus by transesophageal echocardiography does not preclude embolism after cardioversion of atrial fibrillation. A multicenter study. Circulation. 1994;89:2509–2513. [DOI] [PubMed] [Google Scholar]
- 36.Tieleman RG, Van Gelder IC, Crijns HJ, et al. Early recurrences of atrial fibrillation after electrical cardioversion: a result of fibrillation-induced electrical remodeling of the atria? J Am Coll Cardiol. 1998;31:167–173. [DOI] [PubMed] [Google Scholar]
- 37.Harjai KJ, Mobarek SK, Cheirif J, et al. Clinical variables affecting recovery of left atrial mechanical function after cardioversion from atrial fibrillation. J Am Coll Cardiol. 1997;30:481–486. [DOI] [PubMed] [Google Scholar]
- 38.Spartera M, Stracquadanio A, Pessoa-Amorim G, et al. The impact of atrial fibrillation and stroke risk factors on left atrial blood flow characteristics. Eur Heart J Cardiovasc Imaging. 2021;23:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korsholm K, Jensen JM, Nørgaard BL, et al. Detection of device-related thrombosis following left atrial appendage occlusion: a comparison between cardiac computed tomography and transesophageal echocardiography. Circ Cardiovasc Interv. 2019;12:e008112. [DOI] [PubMed] [Google Scholar]
- 40.Berte B, Jost CA, Maurer D, et al. Long-term follow-up after left atrial appendage occlusion with comparison of transesophageal echocardiography versus computed tomography to guide medical therapy and data about postclosure cardioversion. J Cardiovasc Electrophysiol. 2017;28:1140–1150. [DOI] [PubMed] [Google Scholar]
- 41.Saw J, Fahmy P, DeJong P, et al. Cardiac CT angiography for device surveillance after endovascular left atrial appendage closure. Eur Heart J Cardiovasc Imaging. 2015;16:1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garg A, Khunger M, Seicean S, et al. Incidence of thromboembolic complications within 30 days of electrical cardioversion performed within 48 hours of atrial fibrillation onset. JACC Clin Electrophysiol. 2016;2:487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong BM, Perry JJ, Cheng W, et al. Thromboembolic events following cardioversion of acute atrial fibrillation and flutter: a systematic review and meta-analysis. CJEM. 2021;23:500–511. [DOI] [PubMed] [Google Scholar]
8.2.2. Electrical Cardioversion
- 1.Dankner R, Shahar A, Novikov I, et al. Treatment of stable atrial fibrillation in the emergency department: a population-based comparison of electrical direct-current versus pharmacological cardioversion or conservative management. Cardiology. 2009;112:270–278. [DOI] [PubMed] [Google Scholar]
- 2.Stiell IG, Sivilotti MLA, Taljaard M, et al. Electrical versus pharmacological cardioversion for emergency department patients with acute atrial fibrillation (RAFF2): a partial factorial randomised trial. Lancet. 2020;395:339–349. [DOI] [PubMed] [Google Scholar]
- 3.Lown B Electrical reversion of cardiac arrhythmias. Br Heart J. 1967;29:469–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mittal S, Ayati S, Stein KM, et al. Transthoracic cardioversion of atrial fibrillation: comparison of rectilinear biphasic versus damped sine wave monophasic shocks. Circulation. 2000;101:1282–1287. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt AS, Lauridsen KG, Torp P, et al. Maximum-fixed energy shocks for cardioverting atrial fibrillation. Eur Heart J. 2020;41:626–631. [DOI] [PubMed] [Google Scholar]
- 6.Crijns HJ, Weijs B, Fairley AM, et al. Contemporary real life cardioversion of atrial fibrillation: results from the multinational RHYTHM-AF study. Int J Cardiol. 2014;172:588–594. [DOI] [PubMed] [Google Scholar]
- 7.Kirchhof P, Eckardt L, Loh P, et al. Anterior-posterior versus anterior-lateral electrode positions for external cardioversion of atrial fibrillation: a randomised trial. Lancet. 2002;360:1275–1279. [DOI] [PubMed] [Google Scholar]
- 8.Um KJ, McIntyre WF, Healey JS, et al. Pre- and post-treatment with amiodarone for elective electrical cardioversion of atrial fibrillation: a systematic review and meta-analysis. Europace. 2019;21:856–863. [DOI] [PubMed] [Google Scholar]
- 9.Oral H, Souza JJ, Michaud GF, et al. Facilitating transthoracic cardioversion of atrial fibrillation with ibutilide pretreatment. N Engl J Med. 1999;340:1849–1854. [DOI] [PubMed] [Google Scholar]
- 10.Voskoboinik A, Moskovitch J, Plunkett G, et al. Cardioversion of atrial fibrillation in obese patients: results from the Cardioversion-BMI randomized controlled trial. J Cardiovasc Electrophysiol. 2019;30:155–161. [DOI] [PubMed] [Google Scholar]
- 11.Stiell IG, Clement CM, Brison RJ, et al. Variation in management of recent-onset atrial fibrillation and flutter among academic hospital emergency departments. Ann Emerg Med. 2011;57:13–21. [DOI] [PubMed] [Google Scholar]
- 12.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation. Developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez-Madrid A, Svendsen JH, Lip GY, et al. Cardioversion for atrial fibrillation in current European practice: results of the European Heart Rhythm Association survey. Europace. 2013;15:915–918. [DOI] [PubMed] [Google Scholar]
- 14.Pisters R, Nieuwlaat R, Prins MH, et al. Clinical correlates of immediate success and outcome at 1-year follow-up of real-world cardioversion of atrial fibrillation: the Euro Heart Survey. Europace. 2012;14:666–674. [DOI] [PubMed] [Google Scholar]
- 15.Burton JH, Vinson DR, Drummond K, et al. Electrical cardioversion of emergency department patients with atrial fibrillation. Ann Emerg Med. 2004;44:20–30. [DOI] [PubMed] [Google Scholar]
- 16.Cristoni L, Tampieri A, Mucci F, et al. Cardioversion of acute atrial fibrillation in the short observation unit: comparison of a protocol focused on electrical cardioversion with simple antiarrhythmic treatment. Emerg Med J. 2011;28:932–937. [DOI] [PubMed] [Google Scholar]
- 17.Jacoby JL, Cesta M, Heller MB, et al. Synchronized emergency department cardioversion of atrial dysrhythmias saves time, money and resources. J Emerg Med. 2005;28:27–30. [DOI] [PubMed] [Google Scholar]
- 18.Lo GK, Fatovich DM, Haig AD. Biphasic cardioversion of acute atrial fibrillation in the emergency department. Emerg Med J. 2006;23:51–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandes A, Crijns H, Rienstra M, et al. Cardioversion of atrial fibrillation and atrial flutter revisited: current evidence and practical guidance for a common procedure. Europace. 2020;22:1149–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gronberg T, Nuotio I, Nikkinen M, et al. Arrhythmic complications after electrical cardioversion of acute atrial fibrillation: the FinCV study. Europace. 2013;15:1432–1435. [DOI] [PubMed] [Google Scholar]
- 21.Botkin SB, Dhanekula LS, Olshansky B. Outpatient cardioversion of atrial arrhythmias: efficacy, safety, and costs. Am Heart J. 2003;145:233–238. [DOI] [PubMed] [Google Scholar]
- 22.Lewis SR, Nicholson A, Reed SS, et al. Anaesthetic and sedative agents used for electrical cardioversion. Cochrane Database Syst Rev. 2015;3:CD010824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebrahimi R, Rubin SA. Electrical cardioversion resulting in death from synchronization failure. Am J Cardiol. 1994;74:100–102. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda S, An Y, Yanagisawa M, et al. Iatrogenic ventricular fibrillation caused by inappropriately synchronized cardioversion in a patient with pre-excited atrial fibrillation: a case report. J Cardiol Cases. 2021;23:31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufmann MR, McKillop MS, Burkart TA, et al. Iatrogenic ventricular fibrillation after direct-current cardioversion of preexcited atrial fibrillation caused by inadvertent T-wave synchronization. Tex Heart Inst J. 2018;45:39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Squara F, Elbaum C, Garret G, et al. Active compression versus standard anterior-posterior defibrillation for external cardioversion of atrial fibrillation: a prospective randomized study. Heart Rhythm. 2021;18:360–365. [DOI] [PubMed] [Google Scholar]
- 27.Lévy S, Lauribe P, Dolla E, et al. A randomized comparison of external and internal cardioversion of chronic atrial fibrillation. Circulation. 1992;86:1415–1420. [DOI] [PubMed] [Google Scholar]
8.2.3. Pharmacological Cardioversion
- 1.Dankner R, Shahar A, Novikov I, et al. Treatment of stable atrial fibrillation in the emergency department: a population-based comparison of electrical direct-current versus pharmacological cardioversion or conservative management. Cardiology. 2009;112:270–278. [DOI] [PubMed] [Google Scholar]
- 2.Stambler BS, Wood MA, Ellenbogen KA, et al. Efficacy and safety of repeated intravenous doses of ibutilide for rapid conversion of atrial flutter or fibrillation Ibutilide Repeat Dose Study Investigators. Circulation. 1996;94:1613–1621. [DOI] [PubMed] [Google Scholar]
- 3.Ellenbogen KA, Stambler BS, Wood MA, et al. Efficacy of intravenous ibutilide for rapid termination of atrial fibrillation and atrial flutter: a dose-response study. J Am Coll Cardiol. 1996;28:130–136. [DOI] [PubMed] [Google Scholar]
- 4.Hou ZY, Chang MS, Chen CY, et al. Acute treatment of recent-onset atrial fibrillation and flutter with a tailored dosing regimen of intravenous amiodarone A randomized, digoxin-controlled study. Eur Heart J. 1995;16:521–528. [DOI] [PubMed] [Google Scholar]
- 5.Kochiadakis GE, Igoumenidis NE, Simantirakis EN, et al. Intravenous propafenone versus intravenous amiodarone in the management of atrial fibrillation of recent onset: a placebo-controlled study. Pacing Clin Electrophysiol. 1998;21:2475–2479. [DOI] [PubMed] [Google Scholar]
- 6.Cotter G, Blatt A, Kaluski E, et al. Conversion of recent onset paroxysmal atrial fibrillation to normal sinus rhythm: the effect of no treatment and high-dose amiodarone A randomized, placebo-controlled study. Eur Heart J. 1999;20:1833–1842. [DOI] [PubMed] [Google Scholar]
- 7.Vardas PE, Kochiadakis GE, Igoumenidis NE, et al. Amiodarone as a first-choice drug for restoring sinus rhythm in patients with atrial fibrillation: a randomized, controlled study. Chest. 2000;117:1538–1545. [DOI] [PubMed] [Google Scholar]
- 8.Chevalier P, Durand-Dubief A, Burri H, et al. Amiodarone versus placebo and class Ic drugs for cardioversion of recent-onset atrial fibrillation: a meta-analysis. J Am Coll Cardiol. 2003;41:255–262. [DOI] [PubMed] [Google Scholar]
- 9.Capucci A, Lenzi T, Boriani G, et al. Effectiveness of loading oral flecainide for converting recent-onset atrial fibrillation to sinus rhythm in patients without organic heart disease or with only systemic hypertension. Am J Cardiol. 1992;70:69–72. [DOI] [PubMed] [Google Scholar]
- 10.Capucci A, Boriani G, Botto GL, et al. Conversion of recent-onset atrial fibrillation by a single oral loading dose of propafenone or flecainide. Am J Cardiol. 1994;74:503–505. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim OA, Belley-Côté EP, Um KJ, et al. Single-dose oral anti-arrhythmic drugs for cardioversion of recent-onset atrial fibrillation: a systematic review and network meta-analysis of randomized controlled trials. Europace. 2021;23:1200–1210. [DOI] [PubMed] [Google Scholar]
- 12.Botto GL, Capucci A, Bonini W, et al. Conversion of recent onset atrial fibrillation to sinus rhythm using a single oral loading dose of propafenone: comparison of two regimens. Int J Cardiol. 1997;58:55–61. [DOI] [PubMed] [Google Scholar]
- 13.Boriani G, Biffi M, Capucci A, et al. Oral propafenone to convert recent-onset atrial fibrillation in patients with and without underlying heart disease: a randomized, controlled trial. Ann Intern Med. 1997;126:621–625. [DOI] [PubMed] [Google Scholar]
- 14.Azpitarte J, Alvarez M, Baún O, et al. Value of single oral loading dose of propafenone in converting recent-onset atrial fibrillation. Results of a randomized, double-blind, controlled study. Eur Heart J. 1997;18:1649–1654. [DOI] [PubMed] [Google Scholar]
- 15.Brembilla-Perrot B, Houriez P, Beurrier D, et al. Predictors of atrial flutter with 1:1 conduction in patients treated with class I antiarrhythmic drugs for atrial tachyarrhythmias. Int J Cardiol. 2001;80:7–15. [DOI] [PubMed] [Google Scholar]
- 16.Alboni P, Botto GL, Baldi N, et al. Outpatient treatment of recent-onset atrial fibrillation with the “pill-in-the-pocket” approach. N Engl J Med. 2004;351:2384–2391. [DOI] [PubMed] [Google Scholar]
- 17.Alboni P, Botto GL, Boriani G, et al. Intravenous administration of flecainide or propafenone in patients with recent-onset atrial fibrillation does not predict adverse effects during 'pill-in-the-pockeť treatment. Heart. 2010;96:546–549. [DOI] [PubMed] [Google Scholar]
- 18.Andrade JG, MacGillivray J, Macle L, et al. Clinical effectiveness of a systematic “pill-in-the-pocket” approach for the management of paroxysmal atrial fibrillation. Heart Rhythm. 2018;15:9–16. [DOI] [PubMed] [Google Scholar]
- 19.Kochiadakis GE, Igoumenidis NE, Solomou MC, et al. Conversion of atrial fibrillation to sinus rhythm using acute intravenous procainamide infusion. Cardiovasc Drugs Ther. 1998;12:75–81. [DOI] [PubMed] [Google Scholar]
- 20.Oral H, Souza JJ, Michaud GF, et al. Facilitating transthoracic cardioversion of atrial fibrillation with ibutilide pretreatment. N Engl J Med. 1999;340:1849–1854. [DOI] [PubMed] [Google Scholar]
- 21.Kowey PR, VanderLugt JT, Luderer JR. Safety and risk/benefit analysis of ibutilide for acute conversion of atrial fibrillation/flutter. Am J Cardiol. 1996;78:46–52. [DOI] [PubMed] [Google Scholar]
- 22.Volgman AS, Carberry PA, Stambler B, et al. Conversion efficacy and safety of intravenous ibutilide compared with intravenous procainamide in patients with atrial flutter or fibrillation. J Am Coll Cardiol. 1998;31:1414–1419. [DOI] [PubMed] [Google Scholar]
- 23.Stambler BS, Wood MA, Ellenbogen KA. Antiarrhythmic actions of intravenous ibutilide compared with procainamide during human atrial flutter and fibrillation: electrophysiological determinants of enhanced conversion efficacy. Circulation. 1997;96:4298–4306. [DOI] [PubMed] [Google Scholar]
- 24.Singh S, Zoble RG, Yellen L, et al. Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter: the Symptomatic Atrial Fibrillation Investigative Research on Dofetilide (SAFIRE-D) study. Circulation. 2000;102:2385–2390. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen OD, Bagger H, Keller N, et al. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation. 2001;104:292–296. [DOI] [PubMed] [Google Scholar]
- 26.Deedwania PC, Singh BN, Ellenbogen K, et al. Spontaneous conversion and maintenance of sinus rhythm by amiodarone in patients with heart failure and atrial fibrillation: observations from the veterans affairs congestive heart failure survival trial of antiarrhythmic therapy (CHF-STAT). The Department of Veterans Affairs CHF-STAT Investigators. Circulation. 1998;98:2574–2579. [DOI] [PubMed] [Google Scholar]
- 27.Singh BN, Singh SN, Reda DJ, et al. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. 2005;352:1861–1872. [DOI] [PubMed] [Google Scholar]
- 28.Vijayalakshmi K, Whittaker VJ, Sutton A, et al. A randomized trial of prophylactic antiarrhythmic agents (amiodarone and sotalol) in patients with atrial fibrillation for whom direct current cardioversion is planned. Am Heart J. 2006;151:863.e861–863.e866. [DOI] [PubMed] [Google Scholar]
- 29.Sung RJ, Tan HL, Karagounis L, et al. Intravenous sotalol for the termination of supraventricular tachycardia and atrial fibrillation and flutter: a multicenter, randomized, double-blind, placebo-controlled study. Sotalol Multicenter Study Group. Am Heart J. 1995;129:739–748. [DOI] [PubMed] [Google Scholar]
- 30.Vos MA, Golitsyn SR, Stangl K, et al. Superiority of ibutilide (a new class III agent) over DL-sotalol in converting atrial flutter and atrial fibrillation. Heart. 1998;79:568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Gelder IC, Tuinenburg AE, Schoonderwoerd BS, et al. Pharmacologic versus direct-current electrical cardioversion of atrial flutter and fibrillation. Am J Cardiol. 1999;84:147r–151r. [DOI] [PubMed] [Google Scholar]
- 32.Caron MF, Kluger J, Tsikouris JP, et al. Effects of intravenous magnesium sulfate on the QT interval in patients receiving ibutilide. Pharmacotherapy. 2003;23:296–300. [DOI] [PubMed] [Google Scholar]
- 33.Kalus JS, Spencer AP, Tsikouris JP, et al. Impact of prophylactic iv magnesium on the efficacy of ibutilide for conversion of atrial fibrillation or flutter. Am J Health Syst Pharm. 2003;60:2308–2312. [DOI] [PubMed] [Google Scholar]
- 34.Markman TM, Jarrah AA, Tian Y, et al. Safety of pill-in-the-pocket class 1C antiarrhythmic drugs for atrial fibrillation. JACC: Clin Electrophysiol 2022;8:1515–1520. [DOI] [PubMed] [Google Scholar]
8.3.1. Specific Drug Therapy for Long-Term Maintenance of Sinus Rhythm
- 1.Pedersen OD, Bagger H, Keller N, et al. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish Investigations of Arrhythmia and Mortality on Dofetilide (DIAMOND) substudy. Circulation. 2001;104:292–296. [DOI] [PubMed] [Google Scholar]
- 2.Deedwania PC, Singh BN, Ellenbogen K, et al. Spontaneous conversion and maintenance of sinus rhythm by amiodarone in patients with heart failure and atrial fibrillation: observations from the Veterans Affairs Congestive Heart Failure Survival Trial of Antiarrhythmic Therapy (CHF-STAT). The Department of Veterans Affairs CHF-STAT Investigators. Circulation. 1998;98:2574–2579. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JL, Gilbert EM, Alpert BL, et al. Prevention of symptomatic recurrences of paroxysmal atrial fibrillation in patients initially tolerating antiarrhythmic therapy. A multicenter, double-blind, crossover study of flecainide and placebo with transtelephonic monitoring. Flecainide Supraventricular Tachycardia Study Group. Circulation. 1989;80:1557–1570. [DOI] [PubMed] [Google Scholar]
- 4.Kirchhof P, Andresen D, Bosch R, et al. Short-term versus long-term antiarrhythmic drug treatment after cardioversion of atrial fibrillation (Flec-SL): a prospective, randomised, open-label, blinded endpoint assessment trial. Lancet. 2012;380:238–246. [DOI] [PubMed] [Google Scholar]
- 5.Valembois L, Audureau E, Takeda A, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2019;9:Cd005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reimold SC, Cantillon CO, Friedman PL, et al. Propafenone versus sotalol for suppression of recurrent symptomatic atrial fibrillation. Am J Cardiol. 1993;71:558–563. [DOI] [PubMed] [Google Scholar]
- 7.Bellandi F, Simonetti I, Leoncini M, et al. Long-term efficacy and safety of propafenone and sotalol for the maintenance of sinus rhythm after conversion of recurrent symptomatic atrial fibrillation. Am J Cardiol. 2001;88:640–645. [DOI] [PubMed] [Google Scholar]
- 8.Pritchett EL, Page RL, Carlson M, et al. Efficacy and safety of sustainedrelease propafenone (propafenone SR) for patients with atrial fibrillation. Am J Cardiol. 2003;92:941–946. [DOI] [PubMed] [Google Scholar]
- 9.Dogan A, Ergene O, Nazli C, et al. Efficacy of propafenone for maintaining sinus rhythm in patients with recent onset or persistent atrial fibrillation after conversion: a randomized, placebo-controlled study. Acta Cardiol. 2004;59:255–261. [DOI] [PubMed] [Google Scholar]
- 10.Kochiadakis GE, Igoumenidis NE, Hamilos ME, et al. Sotalol versus propafenone for long-term maintenance of normal sinus rhythm in patients with recurrent symptomatic atrial fibrillation. Am J Cardiol. 2004;94:1563–1566. [DOI] [PubMed] [Google Scholar]
- 11.Meinertz T, Lip GY, Lombardi F, et al. Efficacy and safety of propafenone sustained release in the prophylaxis of symptomatic paroxysmal atrial fibrillation (The European Rythmol/Rytmonorm Atrial Fibrillation Trial [ERAFT] Study). Am J Cardiol. 2002;90:1300–1306. [DOI] [PubMed] [Google Scholar]
- 12.Brembilla-Perrot B, Houriez P, Beurrier D, et al. Predictors of atrial flutter with 1:1 conduction in patients treated with class I antiarrhythmic drugs for atrial tachyarrhythmias. Int J Cardiol. 2001;80:7–15. [DOI] [PubMed] [Google Scholar]
- 13.Touboul P, Brugada J, Capucci A, et al. Dronedarone for prevention of atrial fibrillation: a dose-ranging study. Eur Heart J. 2003;24:1481–1487. [DOI] [PubMed] [Google Scholar]
- 14.Singh BN, Connolly SJ, Crijns HJ, et al. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med. 2007;357:987–999. [DOI] [PubMed] [Google Scholar]
- 15.Hohnloser SH, Crijns HJ, van Eickels M, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360:668–678. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Zoble RG, Yellen L, et al. Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter: the Symptomatic Atrial Fibrillation Investigative Research on Dofetilide (SAFIRE-D) study. Circulation. 2000;102:2385–2390. [DOI] [PubMed] [Google Scholar]
- 17.Singh BN, Singh SN, Reda DJ, et al. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. 2005;352:1861–1872. [DOI] [PubMed] [Google Scholar]
- 18.Kochiadakis GE, Igoumenidis NE, Marketou ME, et al. Low dose amiodarone and sotalol in the treatment of recurrent, symptomatic atrial fibrillation: a comparative, placebo controlled study. Heart. 2000;84:251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy D, Talajic M, Dorian P, et al. Amiodarone to prevent recurrence of atrial fibrillation Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med. 2000;342:913–920. [DOI] [PubMed] [Google Scholar]
- 20.Galperin J, Elizari MV, Chiale PA, et al. Efficacy of amiodarone for the termination of chronic atrial fibrillation and maintenance of normal sinus rhythm: a prospective, multicenter, randomized, controlled, double blind trial. J Cardiovasc Pharmacol Ther. 2001;6:341–350. [DOI] [PubMed] [Google Scholar]
- 21.Greene HL, Waldo AL, Corley SD, et al. Maintenance of sinus rhythm in patients with atrial fibrillation: an AFFIRM substudy of the first antiarrhythmic drug. J Am Coll Cardiol. 2003;42:20–29. [DOI] [PubMed] [Google Scholar]
- 22.Le Heuzey JY, De Ferrari GM, Radzik D, et al. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol. 2010;21:597–605. [DOI] [PubMed] [Google Scholar]
- 23.Epstein AE, Olshansky B, Naccarelli GV, et al. Practical management guide for clinicians who treat patients with amiodarone. Am J Med. 2016;129:468–475. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton DS, Nandkeolyar S, Lan H, et al. Amiodarone: a comprehensive guide for clinicians. Am J Cardiovasc Drugs. 2020;20:549–558. [DOI] [PubMed] [Google Scholar]
- 25.Kuck KH, Cappato R, Siebels J, et al. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH). Circulation. 2000;102:748–754. [DOI] [PubMed] [Google Scholar]
- 26.Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406–412. [DOI] [PubMed] [Google Scholar]
- 27.Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. [DOI] [PubMed] [Google Scholar]
- 28.Kober L, Torp-Pedersen C, McMurray JJ, et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–2687. [DOI] [PubMed] [Google Scholar]
- 29.Abraham JM, Saliba WI, Vekstein C, et al. Safety of oral dofetilide for rhythm control of atrial fibrillation and atrial flutter. Circ Arrhythm Electrophysiol. 2015;8:772–776. [DOI] [PubMed] [Google Scholar]
- 30.Cho JH, Youn SJ, Moore JC, et al. Safety of oral dofetilide reloading for treatment of atrial arrhythmias. Circ Arrhythm Electrophysiol. 2017;10:e005333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mar PL, Horbal P, Chung MK, et al. Drug interactions affecting antiarrhythmic drug use. Circ Arrhythm Electrophysiol. 2022;15:e007955. [DOI] [PubMed] [Google Scholar]
- 32.Lakkireddy D, Ahmed A, Atkins D, et al. Feasibility and safety of intravenous sotalol loading in adult patients with atrial fibrillation (DASH-AF). JACC Clin Electrophysiol. 2023;9:555–564. [DOI] [PubMed] [Google Scholar]
- 33.Cardiac Arrhythmia Suppression Trial III. Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. N Engl J Med. 1992;327:227–233. [DOI] [PubMed] [Google Scholar]
- 34.Siebels J, Cappato R, Rüppel R, et al. Preliminary results of the Cardiac Arrest Study Hamburg (CASH). CASH Investigators. Am J Cardiol. 1993;72:109f–113f. [DOI] [PubMed] [Google Scholar]
8.3.2. Inpatient Initiation of Antiarrhythmic Agents
- 1.Singh S, Zoble RG, Yellen L, et al. Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter: the Symptomatic Atrial Fibrillation Investigative Research on Dofetilide (SAFIRE-D) study. Circulation. 2000;102:2385–2390. [DOI] [PubMed] [Google Scholar]
- 2.Abraham JM, Saliba WI, Vekstein C, et al. Safety of oral dofetilide for rhythm control of atrial fibrillation and atrial flutter. Circ Arrhythm Electrophysiol. 2015;8:772–776. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman ES, Zimmermann PA, Wang T, et al. Risk of proarrhythmic events in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study: a multivariate analysis. J Am Coll Cardiol. 2004;44:1276–1282. [DOI] [PubMed] [Google Scholar]
- 4.Yarlagadda B, Vuddanda V, Dar T, et al. Safety and efficacy of inpatient initiation of dofetilide versus sotalol for atrial fibrillation. J Atr Fibrillation. 2017;10:1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torp-Pedersen C, Møller M, Bloch-Thomsen PE, et al. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. N Engl J Med. 1999;341:857–865. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen OD, Bagger H, Keller N, et al. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation. 2001;104:292–296. [DOI] [PubMed] [Google Scholar]
- 7.Kober L, Bloch Thomsen PE, Moller M, et al. Effect of dofetilide in patients with recent myocardial infarction and left-ventricular dysfunction: a randomised trial. Lancet. 2000;356:2052–2058. [DOI] [PubMed] [Google Scholar]
- 8.Chung MK, Schweikert RA, Wilkoff BL, et al. Is hospital admission for initiation of antiarrhythmic therapy with sotalol for atrial arrhythmias required? Yield of in-hospital monitoring and prediction of risk for significant arrhythmia complications. J Am Coll Cardiol. 1998;32:169–176. [DOI] [PubMed] [Google Scholar]
- 9.Alboni P, Botto GL, Baldi N, et al. Outpatient treatment of recent-onset atrial fibrillation with the “pill-in-the-pocket” approach. N Engl J Med. 2004;351:2384–2391. [DOI] [PubMed] [Google Scholar]
- 10.Andrade JG, MacGillivray J, Macle L, et al. Clinical effectiveness of a systematic “pill-in-the-pocket” approach for the management of paroxysmal atrial fibrillation. Heart Rhythm. 2018;15:9–16. [DOI] [PubMed] [Google Scholar]
- 11.Boriani G, Biffi M, Capucci A, et al. Conversion of recent-onset atrial fibrillation to sinus rhythm: effects of different drug protocols. Pacing Clin Electrophysiol. 1998;21:2470–2474. [DOI] [PubMed] [Google Scholar]
- 12.Azpitarte J, Alvarez M, Baún O, et al. Value of single oral loading dose of propafenone in converting recent-onset atrial fibrillation Results of a randomized, double-blind, controlled study. Eur Heart J. 1997;18:1649–1654. [DOI] [PubMed] [Google Scholar]
- 13.Markman TM, Jarrah AA, Tian Y, et al. Safety of pill-in-the-pocket class 1C antiarrhythmic drugs for atrial fibrillation. JACC: Clin Electrophysiol 2022;8:1515–1520. [DOI] [PubMed] [Google Scholar]
- 14.Tisdale JE, Chung MK, Campbell KB, et al. Drug-induced arrhythmias: a scientific statement from the American Heart Association. Circulation. 2020;142:e214–e233. [DOI] [PubMed] [Google Scholar]
- 15.Singh BN, Singh SN, Reda DJ, et al. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. 2005;352:1861–1872. [DOI] [PubMed] [Google Scholar]
- 16.Batul SA, Gopinathannair R. Intravenous sotalol - reintroducing a forgotten agent to the electrophysiology therapeutic arsenal. J Atr Fibrillation. 2017;9:1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
8.3.3. Antiarrhythmic Drug Follow-Up
- 1.Ylli D, Wartofsky L, Burman KD. Evaluation and treatment of amiodarone-induced thyroid disorders. J Clin Endocrinol Metab. 2021;106:226–236. [DOI] [PubMed] [Google Scholar]
- 2.Kinoshita S, Hosomi K, Yokoyama S, et al. Time-to-onset analysis of amiodarone-associated thyroid dysfunction. J Clin Pharm Ther. 2020;45:65–71. [DOI] [PubMed] [Google Scholar]
- 3.Flaharty KK, Chase SL, Yaghsezian HM, et al. Hepatotoxicity associated with amiodarone therapy. Pharmacotherapy. 1989;9:39–44. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton D Sr, Nandkeolyar S, Lan H, et al. Amiodarone: a comprehensive guide for clinicians. Am J Cardiovasc Drugs. 2020;20:549–558. [DOI] [PubMed] [Google Scholar]
- 5.Goldschlager N, Epstein AE, Naccarelli GV, et al. A practical guide for clinicians who treat patients with amiodarone: 2007. Heart Rhythm. 2007;4:1250–1259. [DOI] [PubMed] [Google Scholar]
- 6.Gleadhill IC, Wise RA, Schonfeld SA, et al. Serial lung function testing in patients treated with amiodarone: a prospective study. Am J Med. 1989;86:4–10. [DOI] [PubMed] [Google Scholar]
- 7.Bui A, Han S, Alexander M, et al. Pulmonary function testing for the early detection of drug-induced lung disease: a systematic review in adults treated with drugs associated with pulmonary toxicity. Intern Med J. 2020;50:1311–1325. [DOI] [PubMed] [Google Scholar]
- 8.Passman RS, Bennett CL, Purpura JM, et al. Amiodarone-associated optic neuropathy: a critical review. Am J Med. 2012;125:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tisdale JE, Chung MK, Campbell KB, et al. Drug-induced arrhythmias: a scientific statement from the American Heart Association. Circulation. 2020;142:e214–e233. [DOI] [PubMed] [Google Scholar]
- 10.Hohnloser SH, Klingenheben T, Singh BN. Amiodarone-associated pro-arrhythmic effects. A review with special reference to torsade de pointes tachycardia. Ann Intern Med. 1994;121:529–535. [DOI] [PubMed] [Google Scholar]
- 11.Singh S, Zoble RG, Yellen L, et al. Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter: the Symptomatic Atrial Fibrillation Investigative Research on Dofetilide (SAFIRE-D) study. Circulation. 2000;102:2385–2390. [DOI] [PubMed] [Google Scholar]
- 12.Torp-Pedersen C, Møller M, Bloch-Thomsen PE, et al. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. N Engl J Med. 1999;341:857–865. [DOI] [PubMed] [Google Scholar]
- 13.Rizkallah J, Kuriachan V, Brent Mitchell L. The use of dronedarone for recurrent ventricular tachycardia: a case report and review of the literature. BMC Res Notes. 2016;9:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahn S, Zollner G, Lackner C, et al. Severe toxic hepatitis associated with dronedarone. Curr Drug Saf. 2013;8:201–202. [DOI] [PubMed] [Google Scholar]
- 15.VanderLugt JT, Mattioni T, Denker S, et al. Efficacy and safety of ibutilide fumarate for the conversion of atrial arrhythmias after cardiac surgery. Circulation. 1999;100:369–375. [DOI] [PubMed] [Google Scholar]
- 16.Ellenbogen KA, Stambler BS, Wood MA, et al. Efficacy of intravenous ibutilide for rapid termination of atrial fibrillation and atrial flutter: a dose-response study. J Am Coll Cardiol. 1996;28:130–136. [DOI] [PubMed] [Google Scholar]
- 17.Stambler BS, Beckman KJ, Kadish AH, et al. Acute hemodynamic effects of intravenous ibutilide in patients with or without reduced left ventricular function. Am J Cardiol. 1997;80:458–463. [DOI] [PubMed] [Google Scholar]
- 18.Wood MA, Stambler BS, Ellenbogen KA, et al. Suppression of inducible ventricular tachycardia by ibutilide in patients with coronary artery disease Ibutilide Investigators. Am Heart J. 1998;135:1048–1054. [DOI] [PubMed] [Google Scholar]
- 19.Abi-Mansour P, Carberry PA, McCowan RJ, et al. Conversion efficacy and safety of repeated doses of ibutilide in patients with atrial flutter and atrial fibrillation. Am Heart J. 1998;136:632–642. [DOI] [PubMed] [Google Scholar]
- 20.Gowda RM, Punukollu G, Khan IA, et al. Ibutilide for pharmacological cardioversion of atrial fibrillation and flutter: impact of race on efficacy and safety. Am J Ther. 2003;10:259–263. [DOI] [PubMed] [Google Scholar]
- 21.Stambler BS, Wood MA, Ellenbogen KA, et al. Efficacy and safety of repeated intravenous doses of ibutilide for rapid conversion of atrial flutter or fibrillation Ibutilide Repeat Dose Study Investigators. Circulation. 1996;94:1613–1621. [DOI] [PubMed] [Google Scholar]
- 22.Kay GN, Plumb VJ, Arciniegas JG, et al. Torsade de pointes: the long-short initiating sequence and other clinical features: observations in 32 patients. J Am Coll Cardiol. 1983;2:806–817. [DOI] [PubMed] [Google Scholar]
- 23.Vardas PE, Kochiadakis GE, Igoumenidis NE, et al. Amiodarone as a first-choice drug for restoring sinus rhythm in patients with atrial fibrillation: a randomized, controlled study. Chest. 2000;117:1538–1545. [DOI] [PubMed] [Google Scholar]
- 24.McKibbin JK, Pocock WA, Barlow JB, et al. Sotalol, hypokalaemia, syncope, and torsade de pointes. Br Heart J. 1984;51:157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roden DM, Woosley RL, Primm RK. Incidence and clinical features of the quinidine-associated long QT syndrome: implications for patient care. Am Heart J. 1986;111:1088–1093. [DOI] [PubMed] [Google Scholar]
- 26.Zeltser D, Justo D, Halkin A, et al. Torsade de pointes due to noncardiac drugs: most patients have easily identifiable risk factors. Medicine (Baltim). 2003;82:282–290. [DOI] [PubMed] [Google Scholar]
- 27.Marsepoil T, Blin F, Hardy F, et al. [Torsades de pointes and hypomagnesemia]. Ann Fr Anesth Reanim. 1985;4:524–526. [DOI] [PubMed] [Google Scholar]
- 28.Gysel M, Vieweg WV, Hasnain M, et al. Torsades de pointes following clarithromycin treatment. Expert Rev Cardiovasc Ther. 2013;11:1485–1493. [DOI] [PubMed] [Google Scholar]
- 29.Overbey AN, Austin A, Seidensticker DF, et al. Overdrive pacing in a patient with incessant torsades de pointes. BMJ Case Rep. 2013;2013:bcr2013200146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kochiadakis GE, Igoumenidis NE, Solomou MC, et al. Conversion of atrial fibrillation to sinus rhythm using acute intravenous procainamide infusion. Cardiovasc Drugs Ther. 1998;12:75–81. [DOI] [PubMed] [Google Scholar]
- 31.Stambler BS, Wood MA, Ellenbogen KA. Antiarrhythmic actions of intravenous ibutilide compared with procainamide during human atrial flutter and fibrillation: electrophysiological determinants of enhanced conversion efficacy. Circulation. 1997;96:4298–4306. [DOI] [PubMed] [Google Scholar]
- 32.Bhandari AK, Au PK, Rahimtoola SH. Procainamide induced sustained monomorphic ventricular tachycardia in a patient with benign premature ventricular complexes. Can J Cardiol. 1986;2:6–9. [PubMed] [Google Scholar]
- 33.Singh BN, Kehoe R, Woosley RL, et al. Multicenter trial of sotalol compared with procainamide in the suppression of inducible ventricular tachycardia: a double-blind, randomized parallel evaluation. Sotalol Multicenter Study Group. Am Heart J. 1995;129:87–97. [DOI] [PubMed] [Google Scholar]
- 34.Singh BN, Singh SN, Reda DJ, et al. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. 2005;352:1861–1872. [DOI] [PubMed] [Google Scholar]
- 35.Fetsch T, Bauer P, Engberding R, et al. Prevention of atrial fibrillation after cardioversion: results of the PAFAC trial. Eur Heart J. 2004;25:1385–1394. [DOI] [PubMed] [Google Scholar]
8.3.4. Upstream Therapy
- 1.Dernellis J, Panaretou M. Relationship between C-reactive protein concentrations during glucocorticoid therapy and recurrent atrial fibrillation. Eur Heart J. 2004;25:1100–1107. [DOI] [PubMed] [Google Scholar]
- 2.Schneider MP, Hua TA, Böhm M, et al. Prevention of atrial fibrillation by renin-angiotensin system inhibition a meta-analysis. J Am Coll Cardiol. 2010;55:2299–2307. [DOI] [PubMed] [Google Scholar]
- 3.Healey JS, Baranchuk A, Crystal E, et al. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis. J Am Coll Cardiol. 2005;45:1832–1839. [DOI] [PubMed] [Google Scholar]
- 4.Ducharme A, Swedberg K, Pfeffer MA, et al. Prevention of atrial fibrillation in patients with symptomatic chronic heart failure by candesartan in the Candesartan in Heart failure: assessment of Reduction in Mortality and morbidity (CHARM) program. Am Heart J. 2006;151:985–991. [DOI] [PubMed] [Google Scholar]
- 5.McMurray JJ, Young JB, Dunlap ME, et al. Relationship of dose of background angiotensin-converting enzyme inhibitor to the benefits of candesartan in the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM)-Added trial. Am Heart J. 2006;151:985–991. [DOI] [PubMed] [Google Scholar]
- 6.Wachtell K, Lehto M, Gerdts E, et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End Point Reduction in Hypertension (LIFE) study. J Am Coll Cardiol. 2005;45:712–719. [DOI] [PubMed] [Google Scholar]
- 7.Anand K, Mooss AN, Hee TT, et al. Meta-analysis: inhibition of renin-angiotensin system prevents new-onset atrial fibrillation. Am Heart J. 2006;152:217–222. [DOI] [PubMed] [Google Scholar]
- 8.Okin PM, Wachtell K, Devereux RB, et al. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in patients with hypertension. JAMA. 2006;296:1242–1248. [DOI] [PubMed] [Google Scholar]
- 9.Olsson LG, Swedberg K, Ducharme A, et al. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction results from the candesartan in heart failure-assessment of reduction in mortality and morbidity (CHARM) program. J Am Coll Cardiol. 2006;47:1997–2004. [DOI] [PubMed] [Google Scholar]
- 10.Liakopoulos OJ, Choi YH, Kuhn EW, et al. Statins for prevention of atrial fibrillation after cardiac surgery: a systematic literature review. J Thorac Cardiovasc Surg. 2009;138:678–686 e671. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn EW, Liakopoulos OJ, Stange S, et al. Preoperative statin therapy in cardiac surgery: a meta-analysis of 90 000 patients. Eur J Cardiothorac Surg. 2014;45:17–26. discussion 26. [DOI] [PubMed] [Google Scholar]
- 12.Patti G, Chello M, Candura D, et al. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA-3 (Atorvastatin for Reduction of MYocardial Dysrhythmia After cardiac surgery) study. Circulation. 2006;114:1455–1461. [DOI] [PubMed] [Google Scholar]
- 13.Kourliouros A, De Souza A, Roberts N, et al. Dose-related effect of statins on atrial fibrillation after cardiac surgery. Ann Thorac Surg. 2008;85:1515–1520. [DOI] [PubMed] [Google Scholar]
- 14.Saso S, Vecht JA, Rao C, et al. Statin therapy may influence the incidence of postoperative atrial fibrillation: what is the evidence? Tex Heart Inst J. 2009;36:521–529. [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Z, Jayaram R, Jiang L, et al. Perioperative rosuvastatin in cardiac surgery. N Engl J Med. 2016;374:1744–1753. [DOI] [PubMed] [Google Scholar]
- 16.Rahimi K, Emberson J, McGale P, et al. Effect of statins on atrial fibrillation: collaborative meta-analysis of published and unpublished evidence from randomised controlled trials. BMJ. 2011;342:d1250. [DOI] [PubMed] [Google Scholar]
- 17.Hemilä H, Suonsyrjä T. Vitamin C for preventing atrial fibrillation in high risk patients: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2017;17:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delise P, Bertocchi F, Maiocchi G, et al. Valsartan for prevention of recurrent atrial fibrillation. N Engl J Med. 2009;360:1606–1617. [DOI] [PubMed] [Google Scholar]
- 19.Yusuf S, Healey JS, Pogue J, et al. Irbesartan in patients with atrial fibrillation. N Engl J Med. 2011;364:928–938. [DOI] [PubMed] [Google Scholar]
- 20.Goette A, Schön N, Kirchhof P, et al. Angiotensin II-antagonist in paroxysmal atrial fibrillation (ANTIPAF) trial. Circ Arrhythm Electrophysiol. 2012;5:43–51. [DOI] [PubMed] [Google Scholar]
- 21.Swedberg K, Zannad F, McMurray JJ, et al. Eplerenone and atrial fibrillation in mild systolic heart failure: results from the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) study. J Am Coll Cardiol. 2012;59:1598–1603. [DOI] [PubMed] [Google Scholar]
- 22.Neefs J, van den Berg NW, Limpens J, et al. Aldosterone pathway blockade to prevent atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol. 2017;231:155–161. [DOI] [PubMed] [Google Scholar]
- 23.Zheng RJ, Wang Y, Tang JN, et al. Association of SGLT2 inhibitors with risk of atrial fibrillation and stroke in patients with and without type 2 diabetes: a systemic review and meta-analysis of randomized controlled trials. J Cardiovasc Pharmacol. 2022;79:e145–e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okunrintemi V, Mishriky BM, Powell JR, et al. Sodium-glucose co-transporter-2 inhibitors and atrial fibrillation in the cardiovascular and renal outcome trials. Diabetes Obes Metab. 2021;23:276–280. [DOI] [PubMed] [Google Scholar]
- 25.Li WJ, Chen XQ, Xu LL, et al. SGLT2 inhibitors and atrial fibrillation in type 2 diabetes: a systematic review with meta-analysis of 16 randomized controlled trials. Cardiovasc Diabetol. 2020;19:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bianconi L, Cal L, Mennuni M, et al. N-3 Polyunsaturated fatty acids for the prevention of arrhythmia recurrence after electrical cardioversion of chronic persistent atrial fibrillation: a randomized, double-blind, multicentre study. Europace. 2011;13:174–181. [DOI] [PubMed] [Google Scholar]
- 27.Kowey PR, Reiffel JA, Ellenbogen KA, et al. Efficacy and safety of prescription omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: a randomized controlled trial. JAMA. 2010;304:2363–2372. [DOI] [PubMed] [Google Scholar]
- 28.Mozaffarian D, Marchioli R, Macchia A, et al. Fish oil and postoperative atrial fibrillation: the Omega-3 Fatty Acids for Prevention of Post-operative Atrial Fibrillation (OPERA) randomized trial. JAMA. 2012;308:2001–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macchia A, Grancelli H, Varini S, et al. Omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: results of the FORWARD (Randomized Trial to Assess Efficacy of PUFA for the Maintenance of Sinus Rhythm in Persistent Atrial Fibrillation) trial. J Am Coll Cardiol. 2013;61:463–468. [DOI] [PubMed] [Google Scholar]
- 30.Gencer B, Djousse L, Al-Ramady OT, et al. Effect of long-term marine −3 fatty acids supplementation on the risk of atrial fibrillation in randomized controlled trials of cardiovascular outcomes: a systematic review and meta-analysis. Circulation. 2021;144:1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savelieva I, Kourliouros A, Camm J. Primary and secondary prevention of atrial fibrillation with statins and polyunsaturated fatty acids: Review of evidence and clinical relevance. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:207–219. [DOI] [PubMed] [Google Scholar]
8.4. AF Catheter Ablation
- 1.Krittayaphong R, Raungrattanaamporn O, Bhuripanyo K, et al. A randomized clinical trial of the efficacy of radiofrequency catheter ablation and amiodarone in the treatment of symptomatic atrial fibrillation. J Med Assoc Thai. 2003;86(suppl 1):S8–S16. [PubMed] [Google Scholar]
- 2.Stabile G, Bertaglia E, Senatore G, et al. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation for the Cure of Atrial Fibrillation Study). Eur Heart J. 2006;27:216–221. [DOI] [PubMed] [Google Scholar]
- 3.Pappone C, Augello G, Sala S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol. 2006;48:2340–2347. [DOI] [PubMed] [Google Scholar]
- 4.Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oral H, Pappone C, Chugh A, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354:934–941. [DOI] [PubMed] [Google Scholar]
- 6.Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2498–2505. [DOI] [PubMed] [Google Scholar]
- 7.Forleo GB, Mantica M, De Luca L, et al. Catheter ablation of atrial fibrillation in patients with diabetes mellitus type 2: results from a randomized study comparing pulmonary vein isolation versus antiarrhythmic drug therapy. J Cardiovasc Electrophysiol. 2009;20:22–28. [DOI] [PubMed] [Google Scholar]
- 8.Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–340. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald MR, Connelly DT, Hawkins NM, et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart. 2011;97:740–747. [DOI] [PubMed] [Google Scholar]
- 10.Packer DL, Kowal RC, Wheelan KR, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61:1713–1723. [DOI] [PubMed] [Google Scholar]
- 11.Cosedis Nielsen J, Johannessen A, Raatikainen P, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587–1595. [DOI] [PubMed] [Google Scholar]
- 12.Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384:305–315. [DOI] [PubMed] [Google Scholar]
- 13.Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384:316–324. [DOI] [PubMed] [Google Scholar]
- 14.Andrade JG, Deyell MW, Macle L, et al. Progression of atrial fibrillation after cryoablation or drug therapy. N Engl J Med. 2023;388:105–116. [DOI] [PubMed] [Google Scholar]
- 15.Morillo CA, Verma A, Connolly SJ, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA. 2014;311:692–700. [DOI] [PubMed] [Google Scholar]
- 16.Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293:2634–2640. [DOI] [PubMed] [Google Scholar]
- 17.Wazni O, Marrouche NF, Martin DO, et al. Randomized study comparing combined pulmonary vein-left atrial junction disconnection and cavotricuspid isthmus ablation versus pulmonary vein-left atrial junction disconnection alone in patients presenting with typical atrial flutter and atrial fibrillation. Circulation. 2003;108:2479–2483. [DOI] [PubMed] [Google Scholar]
- 18.Pérez FJ, Schubert CM, Parvez B, et al. Long-term outcomes after catheter ablation of cavo-tricuspid isthmus dependent atrial flutter: a meta-analysis. Circ Arrhythm Electrophysiol. 2009;2:393–401. [DOI] [PubMed] [Google Scholar]
- 19.Patel NJ, Deshmukh A, Pau D, et al. Contemporary utilization and safety outcomes of catheter ablation of atrial flutter in the United States: analysis of 89 638 procedures. Heart Rhythm. 2016;13:1317–1325. [DOI] [PubMed] [Google Scholar]
- 20.Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter mechanisms and clinical implications. J Am Coll Cardiol. 2008;51:779–786. [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Tai CT, Hsieh MH, et al. Predictors of non-pulmonary vein ectopic beats initiating paroxysmal atrial fibrillation: implication for catheter ablation. J Am Coll Cardiol. 2005;46:1054–1059. [DOI] [PubMed] [Google Scholar]
- 22.Di Biase L, Burkhardt JD, Mohanty P, et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation. 2010;122:109–118. [DOI] [PubMed] [Google Scholar]
- 23.Katritsis DG, Giazitzoglou E, Wood MA, et al. Inducible supraventricular tachycardias in patients referred for catheter ablation of atrial fibrillation. Europace. 2007;9:785–789. [DOI] [PubMed] [Google Scholar]
- 24.Miyamoto KJ, Tsuchihashi K, Uno K, et al. Studies on the prevalence of complicated atrial arrhythmias, flutter, and fibrillation in patients with reciprocating supraventricular tachycardia before and after successful catheter ablation. Pacing Clin Electrophysiol. 2001;24:969–978. [DOI] [PubMed] [Google Scholar]
- 25.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yalin K, Ikitimur B, Aksu T, et al. Catheter ablation for atrial fibrillation in patients </=30 years of age. Am J Cardiol. 2022;166:53–57. [DOI] [PubMed] [Google Scholar]
- 27.El Assaad I, Hammond BH, Kost LD, et al. Management and outcomes of atrial fibrillation in 241 healthy children and young adults: revisiting “lone” atrial fibrillation-a multi-institutional PACES collaborative study. Heart Rhythm. 2021;18:1815–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mont L, Bisbal F, Hernandez-Madrid A, et al. Catheter ablation vs antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study). Eur Heart J. 2014;35:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chew DS, Li Y, Cowper PA, et al. Cost-effectiveness of catheter ablation versus antiarrhythmic drug therapy in atrial fibrillation: the CABANA randomized clinical trial. Circulation. 2022;146:535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aronsson M, Walfridsson H, Janzon M, et al. The cost-effectiveness of radiofrequency catheter ablation as first-line treatment for paroxysmal atrial fibrillation: results from a MANTRA-PAF substudy. Europace. 2015;17:48–55. [DOI] [PubMed] [Google Scholar]
- 31.Disertori M, Lombardi F, Barlera S, et al. Clinical characteristics of patients with asymptomatic recurrences of atrial fibrillation in the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico-Atrial Fibrillation (GISSI-AF) trial. Am Heart J. 2011;162:382–389. [DOI] [PubMed] [Google Scholar]
- 32.Senoo K, Suzuki S, Otsuka T, et al. Progression to the persistent form in asymptomatic paroxysmal atrial fibrillation. Circ J. 2014;78:1121–1126. [DOI] [PubMed] [Google Scholar]
- 33.Padfield GJ, Steinberg C, Swampillai J, et al. Progression of paroxysmal to persistent atrial fibrillation: 10-year follow-up in the Canadian Registry of Atrial Fibrillation. Heart Rhythm. 2017;14:801–807. [DOI] [PubMed] [Google Scholar]
- 34.Ogawa H, An Y, Ikeda S, et al. Progression From paroxysmal to sustained atrial fibrillation is associated with increased adverse events. Stroke. 2018;49:2301–2308. [DOI] [PubMed] [Google Scholar]
- 35.Yang WY, Du X, Fawzy AM, et al. Associations of atrial fibrillation progression with clinical risk factors and clinical prognosis: a report from the Chinese Atrial Fibrillation Registry study. J Cardiovasc Electrophysiol. 2021;32:333–341. [DOI] [PubMed] [Google Scholar]
- 36.R BS, Pecen L, Engler D, et al. Atrial fibrillation patterns are associated with arrhythmia progression and clinical outcomes. Heart. 2018;104:1608–1614. [DOI] [PubMed] [Google Scholar]
- 37.Liao YC, Liao JN, Lo LW, et al. Left atrial size and left ventricular end-systolic dimension predict the progression of paroxysmal atrial fibrillation after catheter ablation. J Cardiovasc Electrophysiol. 2017;28:23–30. [DOI] [PubMed] [Google Scholar]
- 38.Walters TE, Nisbet A, Morris GM, et al. Progression of atrial remodeling in patients with high-burden atrial fibrillation: implications for early ablative intervention. Heart Rhythm. 2016;13:331–339. [DOI] [PubMed] [Google Scholar]
- 39.Holmqvist F, Kim S, Steinberg BA, et al. Heart rate is associated with progression of atrial fibrillation, independent of rhythm. Heart. 2015;101:894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 41.Kuck K-H, Lebedev DS, Mikhaylov EN, et al. Catheter ablation or medical therapy to delay progression of atrial fibrillation: the randomized controlled atrial fibrillation progression trial (ATTEST). EP: Europace. 2021;23:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monahan KH, Bunch TJ, Mark DB, et al. Influence of atrial fibrillation type on outcomes of ablation vs drug therapy: results from CABANA. Europace. 2022;24:1430–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nyong J, Amit G, Adler AJ, et al. Efficacy and safety of ablation for people with non-paroxysmal atrial fibrillation. Cochrane Database Syst Rev. 2016;11:Cd012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedman DJ, Field ME, Rahman M, et al. Catheter ablation and healthcare utilization and cost among patients with paroxysmal versus persistent atrial fibrillation. Heart Rhythm O2. 2021;2:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bahnson TD, Giczewska A, Mark DB, et al. Association between age and outcomes of catheter ablation versus medical therapy for atrial fibrillation: results from the CABANA trial. Circulation. 2022;145:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mark DB, Anstrom KJ, Sheng S, et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miura K, Ikemura N, Kimura T, et al. Treatment strategies and subsequent changes in the patient-reported quality-of-life among elderly patients with atrial fibrillation. Am Heart J. 2020;222:83–92. [DOI] [PubMed] [Google Scholar]
- 48.Delise P, Gianfranchi L, Paparella N, et al. Clinical usefulness of slow pathway ablation in patients with both paroxysmal atrioventricular nodal reentrant tachycardia and atrial fibrillation. Am J Cardiol. 1997;79:1421–1423. [DOI] [PubMed] [Google Scholar]
- 49.Weiss R, Knight BP, Bahu M, et al. Long-term follow-up after radiofrequency ablation of paroxysmal supraventricular tachycardia in patients with tachycardia-induced atrial fibrillation. Am J Cardiol. 1997;80:1609–1610. [DOI] [PubMed] [Google Scholar]
- 50.Waranugraha Y, Rizal A, Rohman MS, et al. Prophylactic cavotricuspid isthmus ablation in atrial fibrillation without documented typical atrial flutter: a systematic review and meta-analysis. Arrhythm Electrophysiol Rev. 2022;11:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta D, Ding WY, Calvert P, et al. Cryoballoon pulmonary vein isolation as first-line treatment for typical atrial flutter. Heart. 2023;109:364–371. [DOI] [PubMed] [Google Scholar]
- 52.Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129:2329–2345. [DOI] [PubMed] [Google Scholar]
- 53.Gao L, Moodie M. Modelling the lifetime cost-effectiveness of catheter ablation for atrial fibrillation with heart failure. BMJ Open. 2019;9:e031033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chew DS, Loring Z, Anand J, et al. Economic evaluation of catheter ablation of atrial fibrillation in patients with heart failure with reduced ejection fraction. Circ Cardiovasc Qual Outcomes. 2020;13:E007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lau D, Sandhu RK, Andrade JG, et al. Cost-utility of catheter ablation for atrial fibrillation in patients with heart failure: an economic evaluation. J Am Heart Assoc. 2021;10:e019599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong JA, Conen D, Van Gelder IC, et al. Progression of device-detected subclinical atrial fibrillation and the risk of heart failure. J Am Coll Cardiol. 2018;71:2603–2611. [DOI] [PubMed] [Google Scholar]
- 57.Navarrete A, Conte F, Moran M, et al. Ablation of atrial fibrillation at the time of cavotricuspid isthmus ablation in patients with atrial flutter without documented atrial fibrillation derives a better long-term benefit. J Cardiovasc Electrophysiol. 2011;22:34–38. [DOI] [PubMed] [Google Scholar]
- 58.Kim JY, Park HS, Park HW, et al. Clinical outcomes of rhythm control strategies for asymptomatic atrial fibrillation according to the quality-of-life score: the CODE-AF (Comparison Study of Drugs for Symptom Control and Complication Prevention of Atrial Fibrillation) registry. J Am Heart Assoc. 2022;11:e025956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Willems S, Borof K, Brandes A, et al. Systematic, early rhythm control strategy for atrial fibrillation in patients with or without symptoms: the EAST-AFNET 4 trial. Eur Heart J. 2022;43:1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
8.4.1. Patient Selection
- 1.Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade JG, Turgeon RD, Macle L, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. Eur Cardiol. 2022;17:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calkins H, Reynolds MR, Spector P, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009;2:349–361. [DOI] [PubMed] [Google Scholar]
- 4.Blomstrom-Lundqvist C, Gizurarson S, Schwieler J, et al. Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation: the CAPTAF randomized clinical trial. JAMA. 2019;321:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–1316. [DOI] [PubMed] [Google Scholar]
- 6.Njoku A, Kannabhiran M, Arora R, et al. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: a meta-analysis. Europace. 2018;20:33–42. [DOI] [PubMed] [Google Scholar]
- 7.McLellan AJ, Ling LH, Azzopardi S, et al. Diffuse ventricular fibrosis measured by T(1) mapping on cardiac MRI predicts success of catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:834–840. [DOI] [PubMed] [Google Scholar]
- 8.Marrouche NF, Wilber D, Hindricks G, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311:498–506. [DOI] [PubMed] [Google Scholar]
- 9.Chew DS, Jones KA, Loring Z, et al. Diagnosis-to-ablation time predicts recurrent atrial fibrillation and rehospitalization following catheter ablation. Heart Rhythm O2. 2022;3:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chew DS, Black-Maier E, Loring Z, et al. Diagnosis-to-ablation time and recurrence of atrial fibrillation following catheter ablation: a systematic review and meta-analysis of observational studies. Circ Arrhythm Electrophysiol. 2020;13:e008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black-Maier E, Parish A, Steinberg BA, et al. Predicting atrial fibrillation recurrence after ablation in patients with heart failure: validity of the APPLE and CAAP-AF risk scoring systems. Pacing Clin Electrophysiol. 2019;42:1440–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637–1644. [DOI] [PubMed] [Google Scholar]
- 13.Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 14.Turagam MK, Garg J, Whang W, et al. Catheter ablation of atrial fibrillation in patients with heart failure: a meta-analysis of randomized controlled trials. Ann Intern Med. 2019;170:41–50. [DOI] [PubMed] [Google Scholar]
8.4.2. Techniques and Technologies for AF Catheter Ablation
- 1.Atienza F, Almendral J, Ormaetxe JM, et al. Comparison of radiofrequency catheter ablation of drivers and circumferential pulmonary vein isolation in atrial fibrillation: a noninferiority randomized multicenter RADAR-AF trial. J Am Coll Cardiol. 2014;64:2455–2467. [DOI] [PubMed] [Google Scholar]
- 2.Chen M, Yang B, Chen H, et al. Randomized comparison between pulmonary vein antral isolation versus complex fractionated electrogram ablation for paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22:973–981. [DOI] [PubMed] [Google Scholar]
- 3.Di Biase L, Elayi CS, Fahmy TS, et al. Atrial fibrillation ablation strategies for paroxysmal patients: randomized comparison between different techniques. Circ Arrhythm Electrophysiol. 2009;2:113–119. [DOI] [PubMed] [Google Scholar]
- 4.Katritsis DG, Pokushalov E, Romanov A, et al. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol. 2013;62:2318–2325. [DOI] [PubMed] [Google Scholar]
- 5.Mamchur SE, Mamchur IN, Khomenko EA, et al. 'Electrical exclusion' of a critical myocardial mass by extended pulmonary vein antrum isolation for persistent atrial fibrillation treatment. Interv Med Appl Sci. 2014;6:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verma A, Mantovan R, Macle L, et al. Substrate and Trigger Ablation for Reduction of Atrial Fibrillation (STAR AF): a randomized, multicentre, international trial. Eur Heart J. 2010;31:1344–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sau A, Howard JP, Al-Aidarous S, et al. Meta-analysis of randomized controlled trials of atrial fibrillation ablation with pulmonary vein isolation versus without. JACC Clin Electrophysiol. 2019;5:968–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrouche NF, Wazni O, McGann C, et al. Effect of MRI-guided fibrosis ablation vs conventional catheter ablation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the DECAAF II randomized clinical trial. JAMA. 2022;327:2296–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gianni C, Mohanty S, Di Biase L, et al. Acute and early outcomes of focal impulse and rotor modulation (FIRM)-guided rotors-only ablation in patients with nonparoxysmal atrial fibrillation. Heart Rhythm. 2016;13:830–835. [DOI] [PubMed] [Google Scholar]
- 10.Narayan SM, Baykaner T, Clopton P, et al. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: extended follow-up of the CONFIRM trial (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation). J Am Coll Cardiol. 2014;63:1761–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tilz RR, Lenz C, Sommer P, et al. Focal impulse and rotor modulation ablation vs pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation: results from the FIRMAP AF study. Europace. 2021;23:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandler B, Kim MY, Sikkel MB, et al. Targeting the ectopy-triggering ganglionated plexuses without pulmonary vein isolation prevents atrial fibrillation. J Cardiovasc Electrophysiol. 2021;32:235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kircher S, Arya A, Altmann D, et al. Individually tailored vs standardized substrate modification during radiofrequency catheter ablation for atrial fibrillation: a randomized study. EP: Europace. 2018;20:1766–1775. [DOI] [PubMed] [Google Scholar]
- 14.Lee KN, Roh SY, Baek YS, et al. Long-term clinical comparison of procedural end points after pulmonary vein isolation in paroxysmal atrial fibrillation: elimination of nonpulmonary vein triggers versus noninducibility. Circ Arrhythm Electrophysiol. 2018;11:e005019. [DOI] [PubMed] [Google Scholar]
- 15.Di Biase L, Burkhardt JD, Mohanty P, et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation. 2010;122:109–118. [DOI] [PubMed] [Google Scholar]
- 16.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo L, Frankel DS, Lin A, et al. PRECAF randomized controlled trial. Circ Arrhythm Electrophysiol. 2021;14:e008993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang XH, Liu X, Sun YM, et al. Pulmonary vein isolation combined with superior vena cava isolation for atrial fibrillation ablation: a prospective randomized study. Europace. 2008;10:600–605. [DOI] [PubMed] [Google Scholar]
- 19.Darma A, Daneschnejad SS, Gaspar T, et al. Role of inducibility and its dynamic change in the outcome of catheter ablation of atrial fibrillation: a single center prospective study. J Cardiovasc Electrophysiol. 2020;31:705–711. [DOI] [PubMed] [Google Scholar]
- 20.Fiala M, Bulkova V, Sknouril L, et al. Sinus rhythm restoration and arrhythmia noninducibility are major predictors of arrhythmia-free outcome after ablation for long-standing persistent atrial fibrillation: a prospective study. Heart Rhythm. 2015;12:687–698. [DOI] [PubMed] [Google Scholar]
- 21.Santangeli P, Zado ES, Garcia FC, et al. Lack of prognostic value of atrial arrhythmia inducibility and change in inducibility status after catheter ablation of atrial fibrillation. Heart Rhythm. 2018;15:660–665. [DOI] [PubMed] [Google Scholar]
- 22.Richter B, Gwechenberger M, Filzmoser P, et al. Is inducibility of atrial fibrillation after radio frequency ablation really a relevant prognostic factor? Eur Heart J. 2006;27:2553–2559. [DOI] [PubMed] [Google Scholar]
- 23.Tohoku S, Fukunaga M, Nagashima M, et al. Clinical impact of eliminating nonpulmonary vein triggers of atrial fibrillation and nonpulmonary vein premature atrial contractions at initial ablation for persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2021;32:224–234. [DOI] [PubMed] [Google Scholar]
- 24.Jiang R, Chen M, Fan J, et al. Efficacy of ablation index-guided pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2022;45:1186–1193. [DOI] [PubMed] [Google Scholar]
- 25.Kawai S, Mukai Y, Inoue S, et al. Predictive value of the induction test with atrial burst pacing with regard to long-term recurrence after ablation in persistent atrial fibrillation. J Arrhythm. 2019;35:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng LJ, Shi L, Tian Y, et al. Pace capture and adenosine triphosphate provocation are complementary rather than mutually exclusive methods to ensure durable pulmonary vein isolation. J Cardiovasc Electrophysiol. 2019;30:815–823. [DOI] [PubMed] [Google Scholar]
- 27.Choi Y, Kim SH, Kim JY, et al. Utility of acute arrhythmia termination as an ablation endpoint for induced atrial tachyarrhythmia after complete pulmonary vein isolation during catheter ablation for persistent atrial fibrillation. J Interv Card Electrophysiol. 2019;54:25–34. [DOI] [PubMed] [Google Scholar]
- 28.McLellan AJA, Prabhu S, Voskoboinik A, et al. Isolation of the posterior left atrium for patients with persistent atrial fibrillation: routine adenosine challenge for dormant posterior left atrial conduction improves long-term outcome. Europace. 2017;19:1958–1966. [DOI] [PubMed] [Google Scholar]
- 29.Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e506–e574. [DOI] [PubMed] [Google Scholar]
- 30.Di Biase L, Mohanty S, Trivedi C, et al. Stroke risk in patients with atrial fibrillation undergoing electrical isolation of the left atrial appendage. J Am Coll Cardiol. 2019;74:1019–1028. [DOI] [PubMed] [Google Scholar]
- 31.Park JW, Yu HT, Kim TH, et al. Atrial fibrillation catheter ablation increases the left atrial pressure. Circ Arrhythm Electrophysiol. 2019;12:e007073. [DOI] [PubMed] [Google Scholar]
- 32.Gibson DN, Di Biase L, Mohanty P, et al. Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm. 2011;8:1364–1371. [DOI] [PubMed] [Google Scholar]
- 33.Kim YG, Shim J, Kim DH, et al. Characteristics of atrial fibrillation patients suffering atrioesophageal fistula after radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2018;29:1343–1351. [DOI] [PubMed] [Google Scholar]
- 34.Muller P, Dietrich JW, Halbfass P, et al. Higher incidence of esophageal lesions after ablation of atrial fibrillation related to the use of esophageal temperature probes. Heart Rhythm. 2015;12:1464–1469. [DOI] [PubMed] [Google Scholar]
- 35.Mohanty S, Trivedi C, Della Rocca DG, et al. Thromboembolic risk in atrial fibrillation patients with left atrial scar post-extensive ablation: a single-center experience. JACC Clin Electrophysiol. 2021;7:308–318. [DOI] [PubMed] [Google Scholar]
8.4.3. Management of Recurrent AF After Catheter Ablation
- 1.Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuck KH, Fürnkranz A, Chun KR, et al. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: reintervention, rehospitalization, and quality-of-life outcomes in the FIRE AND ICE trial. Eur Heart J. 2016;37:2858–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piccini JP, Sinner MF, Greiner MA, et al. Outcomes of Medicare beneficiaries undergoing catheter ablation for atrial fibrillation. Circulation. 2012;126:2200–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wokhlu A, Monahan KH, Hodge DO, et al. Long-term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. J Am Coll Cardiol. 2010;55:2308–2316. [DOI] [PubMed] [Google Scholar]
- 5.Calkins H, Reynolds MR, Spector P, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009;2:349–361. [DOI] [PubMed] [Google Scholar]
- 6.Ganesan AN, Shipp NJ, Brooks AG, et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2:e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhargava M, Di Biase L, Mohanty P, et al. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: results from a multicenter study. Heart Rhythm. 2009;6:1403–1412. [DOI] [PubMed] [Google Scholar]
- 8.Reddy YNV, El Sabbagh A, Packer D, et al. Evaluation of shortness of breath after atrial fibrillation ablation-is there a stiff left atrium? Heart Rhythm. 2018;15:930–935. [DOI] [PubMed] [Google Scholar]
- 9.Szegedi N, Szeplaki G, Herczeg S, et al. Repeat procedure is a new independent predictor of complications of atrial fibrillation ablation. Europace. 2019;21:732–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blomstrom-Lundqvist C, Gizurarson S, Schwieler J, et al. Effect of Catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation: the CAPTAF randomized clinical trial. JAMA. 2019;321:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Packer DL, Kowal RC, Wheelan KR, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61:1713–1723. [DOI] [PubMed] [Google Scholar]
- 12.Pokushalov E, Romanov A, Artyomenko S, et al. Cryoballoon versus radiofrequency for pulmonary vein re-isolation after a failed initial ablation procedure in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2013;24:274–279. [DOI] [PubMed] [Google Scholar]
- 13.Kim D, Yu HT, Kim TH, et al. Electrical posterior box isolation in repeat ablation for atrial fibrillation: a prospective randomized clinical study. JACC Clin Electrophysiol. 2022;8:582–592. [DOI] [PubMed] [Google Scholar]
- 14.Vamos M, Calkins H, Kowey PR, et al. Efficacy and safety of dronedarone in patients with a prior ablation for atrial fibrillation/flutter: insights from the ATHENA study. Clin Cardiol. 2020;43:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duytschaever M, Demolder A, Phlips T, et al. PulmOnary vein isolation With vs without continued antiarrhythmic Drug trEatment in subjects with Recurrent Atrial Fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J. 2018;39:1429–1437. [DOI] [PubMed] [Google Scholar]
- 16.Leong-Sit P, Roux JF, Zado E, et al. Antiarrhythmics after ablation of atrial fibrillation (5A Study): six-month follow-up study. Circ Arrhythm Electrophysiol. 2011;4:11–14. [DOI] [PubMed] [Google Scholar]
- 17.Schoene K, Sepehri Shamloo A, Sommer P, et al. Natural course of acquired pulmonary vein stenosis after radiofrequency ablation for atrial fibrillationis routine follow-up imaging indicated or not? J Cardiovasc Electrophysiol. 2019;30:1786–1791. [DOI] [PubMed] [Google Scholar]
- 18.Jais P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2498–2505. [DOI] [PubMed] [Google Scholar]
- 19.Pokushalov E, Romanov A, De Melis M, et al. Progression of atrial fibrillation after a failed initial ablation procedure in patients with paroxysmal atrial fibrillation: a randomized comparison of drug therapy versus reablation. Circ Arrhythm Electrophysiol. 2013;6:754–760. [DOI] [PubMed] [Google Scholar]
- 20.Roux JF, Zado E, Callans DJ, et al. Antiarrhythmics after ablation of atrial fibrillation (5A study). Circulation. 2009;120:1036–1040. [DOI] [PubMed] [Google Scholar]
- 21.Kaitani K, Inoue K, Kobori A, et al. Efficacy of antiarrhythmic drugs short-term use after catheter ablation for atrial fibrillation (EAST-AF) trial. Eur Heart J. 2016;37:610–618. [DOI] [PubMed] [Google Scholar]
- 22.Chen W, Liu H, Ling Z, et al. Efficacy of short-term antiarrhythmic drugs use after catheter ablation of atrial fibrillation-a systematic review with meta-analyses and trial sequential analyses of randomized controlled trials. PLoS One. 2016;11:e0156121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu B, Peng F, Tang W, et al. Short-term antiarrhythmic drugs after catheter ablation for atrial fibrillation: a meta-analysis of randomized controlled trials. Ann Pharmacother. 2016;50:697–705. [DOI] [PubMed] [Google Scholar]
- 24.Noseworthy PA, Van Houten HK, Sangaralingham LR, et al. Effect of antiarrhythmic drug initiation on readmission after catheter ablation for atrial fibrillation. JACC Clin Electrophysiol. 2015;1:238–244. [DOI] [PubMed] [Google Scholar]
8.4.4. Anticoagulation Therapy Before and After Catheter Ablation
- 1.Di Biase L, Gaita F, Toso E, et al. Does periprocedural anticoagulation management of atrial fibrillation affect the prevalence of silent thromboembolic lesion detected by diffusion cerebral magnetic resonance imaging in patients undergoing radiofrequency atrial fibrillation ablation with open irrigated catheters? Results from a prospective multicenter study. Heart Rhythm. 2014;11:791–798. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso R, Knijnik L, Bhonsale A, et al. An updated meta-analysis of novel oral anticoagulants versus vitamin K antagonists for uninterrupted anticoagulation in atrial fibrillation catheter ablation. Heart Rhythm. 2018;15:107–115. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P, Haeusler KG, Blank B, et al. Apixaban in patients at risk of stroke undergoing atrial fibrillation ablation. Eur Heart J. 2018;39:2942–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuwahara T, Abe M, Yamaki M, et al. Apixaban versus warfarin for the prevention of periprocedural cerebral thromboembolism in atrial fibrillation ablation: multicenter prospective randomized study. J Cardiovasc Electrophysiol. 2016;27:549–554. [DOI] [PubMed] [Google Scholar]
- 5.Calkins H, Willems S, Gerstenfeld EP, et al. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med. 2017;376:1627–1636. [DOI] [PubMed] [Google Scholar]
- 6.Hohnloser SH, Camm J, Cappato R, et al. Uninterrupted edoxaban vs vitamin K antagonists for ablation of atrial fibrillation: the ELIMINATE-AF trial. Eur Heart J. 2019;40:3013–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cappato R, Marchlinski FE, Hohnloser SH, et al. Uninterrupted rivaroxaban vs uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J. 2015;36:1805–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reynolds MR, Allison JS, Natale A, et al. A prospective randomized trial of apixaban dosing during atrial fibrillation ablation: the AEIOU trial. JACC Clin Electrophysiol. 2018;4:580–588. [DOI] [PubMed] [Google Scholar]
- 9.Nogami A, Harada T, Sekiguchi Y, et al. Safety and efficacy of minimally interrupted dabigatran vs uninterrupted warfarin therapy in adults undergoing atrial fibrillation catheter ablation: a randomized clinical trial. JAMA Netw Open. 2019;2:e191994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Vugt SPG, Westra SW, Volleberg R, et al. Meta-analysis of controlled studies on minimally interrupted vs continuous use of non-vitamin K antagonist oral anticoagulants in catheter ablation for atrial fibrillation. Europace. 2021;23:1961–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinberg BA, Hellkamp AS, Lokhnygina Y, et al. Higher risk of death and stroke in patients with persistent vs paroxysmal atrial fibrillation: results from the ROCKET-AF Trial. Eur Heart J. 2015;36:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Khatib SM, Thomas L, Wallentin L, et al. Outcomes of apixaban vs warfarin by type and duration of atrial fibrillation: results from the ARISTOTLE trial. Eur Heart J. 2013;34:2464–2471. [DOI] [PubMed] [Google Scholar]
- 14.Uittenbogaart SB, Lucassen WAM, van Etten-Jamaludin FS, et al. Burden of atrial high-rate episodes and risk of stroke: a systematic review. Europace. 2018;20:1420–1427. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds MR, Gunnarsson CL, Hunter TD, et al. Health outcomes with catheter ablation or antiarrhythmic drug therapy in atrial fibrillation: results of a propensity-matched analysis. Circ Cardiovasc Qual Outcomes. 2012;5:171–181. [DOI] [PubMed] [Google Scholar]
- 16.Friberg L, Tabrizi F, Englund A. Catheter ablation for atrial fibrillation is associated with lower incidence of stroke and death: data from Swedish health registries. Eur Heart J. 2016;37:2478–2487. [DOI] [PubMed] [Google Scholar]
- 17.Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 18.Piccini JP, Sinner MF, Greiner MA, et al. Outcomes of Medicare beneficiaries undergoing catheter ablation for atrial fibrillation. Circulation. 2012;126:2200–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karasoy D, Gislason GH, Hansen J, et al. Oral anticoagulation therapy after radiofrequency ablation of atrial fibrillation and the risk of thromboembolism and serious bleeding: long-term follow-up in nationwide cohort of Denmark. Eur Heart J. 2015;36:307–314a. [DOI] [PubMed] [Google Scholar]
- 21.Noseworthy PA, Yao X, Deshmukh AJ, et al. Patterns of anticoagulation use and cardioembolic risk after catheter ablation for atrial fibrillation. J Am Heart Assoc. 2015;4:e002597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wazni OM, Boersma L, Healey JS, et al. Comparison of anticoagulation with left atrial appendage closure after atrial fibrillation ablation: rationale and design of the OPTION randomized trial. Am Heart J. 2022;251:35–42. [DOI] [PubMed] [Google Scholar]
8.4.5. Complications After AF Catheter Ablation
- 1.Deshmukh A, Patel NJ, Pant S, et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93 801 procedures. Circulation. 2013;128:2104–2112. [DOI] [PubMed] [Google Scholar]
- 2.Steinbeck G, Sinner MF, Lutz M, et al. Incidence of complications related to catheter ablation of atrial fibrillation and atrial flutter: a nationwide in-hospital analysis of administrative data for Germany in 2014. Eur Heart J. 2018;39:4020–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loring Z, Holmes DN, Matsouaka RA, et al. Procedural patterns and safety of atrial fibrillation ablation: findings from Get With The Guidelines-Atrial Fibrillation. Circ Arrhythm Electrophysiol. 2020;13:e007944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell ML, Larson J, Farid T, et al. Sex-based differences in procedural complications associated with atrial fibrillation catheter ablation: a systematic review and meta-analysis. J Cardiovasc Electrophysiol. 2020;31:3176–3186. [DOI] [PubMed] [Google Scholar]
- 5.Russo AM, Zeitler EP, Giczewska A, et al. Association between sex and treatment outcomes of atrial fibrillation ablation versus drug therapy: results from the CABANA trial. Circulation. 2021;143:661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
8.5. Role of Pacemakers and ICDs for the Prevention and Treatment of AF
- 1.Stambler BS, Ellenbogen KA, Orav EJ, et al. Predictors and clinical impact of atrial fibrillation after pacemaker implantation in elderly patients treated with dual chamber versus ventricular pacing. Pacing Clin Electrophysiol. 2003;26:2000–2007. [DOI] [PubMed] [Google Scholar]
- 2.Skanes AC, Krahn AD, Yee R, et al. Progression to chronic atrial fibrillation after pacing: the Canadian Trial of Physiologic Pacing. CTOPP Investigators. J Am Coll Cardiol. 2001;38:167–172. [DOI] [PubMed] [Google Scholar]
- 3.Hesselson AB, Parsonnet V, Bernstein AD, et al. Deleterious effects of long-term single-chamber ventricular pacing in patients with sick sinus syndrome: the hidden benefits of dual-chamber pacing. J Am Coll Cardiol. 1992;19:1542–1549. [DOI] [PubMed] [Google Scholar]
- 4.Andersen HR, Nielsen JC, Thomsen PE, et al. Long-term follow-up of patients from a randomised trial of atrial versus ventricular pacing for sicksinus syndrome. Lancet. 1997;350:1210–1216. [DOI] [PubMed] [Google Scholar]
- 5.Lamas GA, Lee KL, Sweeney MO, et al. Ventricular pacing or dual-chamber pacing for sinus node dysfunction. N Engl J Med. 2002;346:1854–1862. [DOI] [PubMed] [Google Scholar]
- 6.Elkayam LU, Koehler JL, Sheldon TJ, et al. The influence of atrial and ventricular pacing on the incidence of atrial fibrillation: a meta-analysis. Pacing Clin Electrophysiol. 2011;34:1593–1599. [DOI] [PubMed] [Google Scholar]
- 7.Gillis AM, Unterberg-Buchwald C, Schmidinger H, et al. Safety and efficacy of advanced atrial pacing therapies for atrial tachyarrhythmias in patients with a new implantable dual chamber cardioverter-defibrillator. J Am Coll Cardiol. 2002;40:1653–1659. [DOI] [PubMed] [Google Scholar]
- 8.Lee MA, Weachter R, Pollak S, et al. The effect of atrial pacing therapies on atrial tachyarrhythmia burden and frequency: results of a randomized trial in patients with bradycardia and atrial tachyarrhythmias. J Am Coll Cardiol. 2003;41:1926–1932. [DOI] [PubMed] [Google Scholar]
- 9.Israel CW, Hügl B, Unterberg C, et al. Pace-termination and pacing for prevention of atrial tachyarrhythmias: results from a multicenter study with an implantable device for atrial therapy. J Cardiovasc Electrophysiol. 2001;12:1121–1128. [DOI] [PubMed] [Google Scholar]
- 10.Gillis AM, Koehler J, Morck M, et al. High atrial antitachycardia pacing therapy efficacy is associated with a reduction in atrial tachyarrhythmia burden in a subset of patients with sinus node dysfunction and paroxysmal atrial fibrillation. Heart Rhythm. 2005;2:791–796. [DOI] [PubMed] [Google Scholar]
- 11.Padeletti L, Purerfellner H, Mont L, et al. New-generation atrial antitachycardia pacing (Reactive ATP) is associated with reduced risk of persistent or permanent atrial fibrillation in patients with bradycardia: results from the MINERVA randomized multicenter international trial. Heart Rhythm. 2015;12:1717–1725. [DOI] [PubMed] [Google Scholar]
- 12.Boriani G, Tukkie R, Manolis AS, et al. Atrial antitachycardia pacing and managed ventricular pacing in bradycardia patients with paroxysmal or persistent atrial tachyarrhythmias: the MINERVA randomized multicentre international trial. Eur Heart J. 2014;35:2352–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagomi T, Inden Y, Yanagisawa S, et al. Characteristics of successful reactive atrial-based antitachycardia pacing in patients with cardiac implantable electronic devices: history of catheter ablation of atrial fibrillation as a predictor of high treatment efficacy. J Cardiovasc Electrophysiol. 2022;33:1515–1528. [DOI] [PubMed] [Google Scholar]
- 14.Zhu H, Li X, Wang Z, et al. New-onset atrial fibrillation following left bundle branch area pacing vs right ventricular pacing: a two-centre prospective cohort study. Europace. 2022;25:121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravi V, Beer D, Pietrasik GM, et al. Development of new-onset or progressive atrial fibrillation in patients with permanent His bundle pacing versus right ventricular pacing: results from the RUSH HBP registry. J Am Heart Assoc. 2020;9:e018478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson MD, Ip J, Messenger J, et al. A new pacemaker algorithm for the treatment of atrial fibrillation: results of the Atrial Dynamic Overdrive Pacing Trial (ADOPT). J Am Coll Cardiol. 2003;42:627–633. [DOI] [PubMed] [Google Scholar]
- 17.Hohnloser SH, Healey JS, Gold MR, et al. Atrial overdrive pacing to prevent atrial fibrillation: insights from ASSERT. Heart Rhythm. 2012;9:1667–1673. [DOI] [PubMed] [Google Scholar]
- 18.Camm AJ, Sulke N, Edvardsson N, et al. Conventional and dedicated atrial overdrive pacing for the prevention of paroxysmal atrial fibrillation: the AFTherapy study. Europace. 2007;9:1110–1118. [DOI] [PubMed] [Google Scholar]
- 19.Munawar DA, Mahajan R, Agbaedeng TA, et al. Implication of ventricular pacing burden and atrial pacing therapies on the progression of atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Heart Rhythm. 2019;16:1204–1214. [DOI] [PubMed] [Google Scholar]
- 20.Sweeney MO, Hellkamp AS, Ellenbogen KA, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932–2937. [DOI] [PubMed] [Google Scholar]
- 21.Sweeney MO, Bank AJ, Nsah E, et al. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med. 2007;357:1000–1008. [DOI] [PubMed] [Google Scholar]
- 22.Ricci RP, Botto GL, Benezet JM, et al. Association between ventricular pacing and persistent atrial fibrillation in patients indicated to elective pacemaker replacement: results of the Prefer for Elective Replacement MVP (PreFER MVP) randomized study. Heart Rhythm. 2015;12:2239–2246. [DOI] [PubMed] [Google Scholar]
- 23.Shurrab M, Healey JS, Haj-Yahia S, et al. Reduction in unnecessary ventricular pacing fails to affect hard clinical outcomes in patients with preserved left ventricular function: a meta-analysis. Europace. 2017;19:282–288. [DOI] [PubMed] [Google Scholar]
- 24.Toff WD, Camm AJ, Skehan JD, et al. Single-chamber versus dual-chamber pacing for high-grade atrioventricular block. N Engl J Med. 2005;353:145–155. [DOI] [PubMed] [Google Scholar]
- 25.Pastore G, Marcantoni L, Lanza D, et al. Occurrence of persistent atrial fibrillation during pacing for sinus node disease: the influence of His bundle pacing versus managed ventricular pacing. J Cardiovasc Electrophysiol. 2021;32:110–116. [DOI] [PubMed] [Google Scholar]
- 26.Singh JP, Cha YM, Lunati M, et al. Real-world behavior of CRT pacing using the AdaptivCRT algorithm on patient outcomes: effect on mortality and atrial fibrillation incidence. J Cardiovasc Electrophysiol. 2020;31:825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu JC, Birnie D, Stadler RW, et al. Adaptive cardiac resynchronization therapy is associated with decreased risk of incident atrial fibrillation compared to standard biventricular pacing: a real-world analysis of 37 450 patients followed by remote monitoring. Heart Rhythm. 2019;16:983–989. [DOI] [PubMed] [Google Scholar]
- 28.Gasparini M, Birnie D, Lemke B, et al. Adaptive cardiac resynchronization therapy reduces atrial fibrillation incidence in heart failure patients with prolonged AV conduction: the adaptive CRT randomized trial. Circ Arrhythm Electrophysiol. 2019;12:e007260. [DOI] [PubMed] [Google Scholar]
8.6. Surgical Ablation
- 1.Huffman MD, Malaisrie SC, Karmali KN. Concomitant atrial fibrillation surgery for people undergoing cardiac surgery. JAMA Cardiol. 2017;2:334–335. [DOI] [PubMed] [Google Scholar]
- 2.Huffman MD, Karmali KN, Berendsen MA, et al. Concomitant atrial fibrillation surgery for people undergoing cardiac surgery. Cochrane Database Syst Rev. 2016;8:CD011814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillinov M, Moskowitz AJ, Argenziano M. Surgical ablation for atrial fibrillation. N Engl J Med. 2015;373:484. [DOI] [PubMed] [Google Scholar]
- 4.Noseworthy PA, Yao X, Deshmukh AJ, et al. Patterns of anticoagulation use and cardioembolic risk after catheter ablation for atrial fibrillation. J Am Heart Assoc. 2015;4:e002597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kress DC, Erickson L, Choudhuri I, et al. Comparative effectiveness of hybrid ablation versus endocardial catheter ablation alone in patients with persistent atrial fibrillation. JACC Clin Electrophysiol. 2017;3:341–349. [DOI] [PubMed] [Google Scholar]
- 6.Mannakkara NN, Porter B, Child N, et al. Convergent ablation for persistent atrial fibrillation: outcomes from a single-centre real-world experience. Eur J Cardiothorac Surg. 2022;63:ezac515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gersak B, Zembala MO, Muller D, et al. European experience of the convergent atrial fibrillation procedure: multicenter outcomes in consecutive patients. J Thorac Cardiovasc Surg. 2014;147:1411–1416. [DOI] [PubMed] [Google Scholar]
- 8.Cox JL, Schuessler RB, D'Agostino HJ Jr, et al. The surgical treatment of atrial fibrillation III Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991;101:569–583. [PubMed] [Google Scholar]
- 9.McCarthy PM, Davidson CJ, Kruse J, et al. Prevalence of atrial fibrillation before cardiac surgery and factors associated with concomitant ablation. J Thorac Cardiovasc Surg. 2020;159:2245–2253 e2215. [DOI] [PubMed] [Google Scholar]
- 10.Musharbash FN, Schill MR, Sinn LA, et al. Performance of the Cox-maze IV procedure is associated with improved long-term survival in patients with atrial fibrillation undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2018;155:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iribarne A, DiScipio AW, McCullough JN, et al. Surgical atrial fibrillation ablation improves long-term survival: a multicenter analysis. Ann Thorac Surg. 2019;107:135–142. [DOI] [PubMed] [Google Scholar]
- 12.Suwalski P, Kowalewski M, Jasinski M, et al. Survival after surgical ablation for atrial fibrillation in mitral valve surgery: analysis from the Polish National Registry of Cardiac Surgery Procedures (KROK). J Thorac Cardiovasc Surg. 2019;157:1007–1018.e1004. [DOI] [PubMed] [Google Scholar]
- 13.Malaisrie SC, McCarthy PM, Kruse J, et al. Ablation of atrial fibrillation during coronary artery bypass grafting: late outcomes in a Medicare population. J Thorac Cardiovasc Surg. 2021;161:1251–1261 e1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badhwar V, Rankin JS, Ad N, et al. Surgical ablation of atrial fibrillation in the United States: trends and propensity matched outcomes. Ann Thorac Surg. 2017;104:493–500. [DOI] [PubMed] [Google Scholar]
- 15.Eitel C, Koch J, Sommer P, et al. Novel oral anticoagulants in a real-world cohort of patients undergoing catheter ablation of atrial fibrillation. Europace. 2013;15:1587–1593. [DOI] [PubMed] [Google Scholar]
- 16.DeLurgio DB, Crossen KJ, Gill J, et al. Hybrid convergent procedure for the treatment of persistent and long-standing persistent atrial fibrillation: results of CONVERGE clinical trial. Circ Arrhythm Electrophysiol. 2020;13:e009288. [DOI] [PubMed] [Google Scholar]
9.1. General Considerations for AF and HF
- 1.Melenovsky V, Hwang SJ, Redfield MM, et al. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8:295–303. [DOI] [PubMed] [Google Scholar]
- 2.Tsang TS, Gersh BJ, Appleton CP, et al. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol. 2002;40:1636–1644. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg MA, Gottdiener JS, Heckbert SR, et al. Echocardiographic diastolic parameters and risk of atrial fibrillation: the Cardiovascular Health Study. Eur Heart J. 2012;33:904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ariyaratnam JP, Lau DH, Sanders P, et al. Atrial fibrillation and heart failure: epidemiology, pathophysiology, prognosis, and management. Card Electrophysiol Clin. 2021;13:47–62. [DOI] [PubMed] [Google Scholar]
- 5.Santhanakrishnan R, Wang N, Larson MG, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mamas MA, Caldwell JC, Chacko S, et al. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur J Heart Fail. 2009;11:676–683. [DOI] [PubMed] [Google Scholar]
- 7.Sartipy U, Dahlstrom U, Fu M, et al. Atrial fibrillation in heart failure with preserved, mid-range, and reduced ejection fraction. JACC Heart Fail. 2017;5:565–574. [DOI] [PubMed] [Google Scholar]
- 8.Kotecha D, Chudasama R, Lane DA, et al. Atrial fibrillation and heart failure due to reduced versus preserved ejection fraction: a systematic review and meta-analysis of death and adverse outcomes. Int J Cardiol. 2016;203:660–666. [DOI] [PubMed] [Google Scholar]
- 9.Zafrir B, Lund LH, Laroche C, et al. Prognostic implications of atrial fibrillation in heart failure with reduced, mid-range, and preserved ejection fraction: a report from 14 964 patients in the European Society of Cardiology Heart Failure Long-Term Registry. Eur Heart J. 2018;39:4277–4284. [DOI] [PubMed] [Google Scholar]
9.2. Management of AF in Patients With HF
- 1.Rillig A, Magnussen C, Ozga AK, et al. Early rhythm control therapy in patients with atrial fibrillation and heart failure. Circulation. 2021;144:845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brachmann J, Sohns C, Andresen D, et al. Atrial fibrillation burden and clinical outcomes in heart failure: the CASTLE-AF trial. JACC Clin Electrophysiol. 2021;7:594–603. [DOI] [PubMed] [Google Scholar]
- 3.Chen S, Pürerfellner H, Meyer C, et al. Rhythm control for patients with atrial fibrillation complicated with heart failure in the contemporary era of catheter ablation: a stratified pooled analysis of randomized data. Eur Heart J. 2020;41:2863–2873. [DOI] [PubMed] [Google Scholar]
- 4.Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637–1644. [DOI] [PubMed] [Google Scholar]
- 5.Hunter RJ, Berriman TJ, Diab I, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol. 2014;7:31–38. [DOI] [PubMed] [Google Scholar]
- 6.Jones DG, Haldar SK, Hussain W, et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol. 2013;61:1894–1903. [DOI] [PubMed] [Google Scholar]
- 7.Kuck KH, Merkely B, Zahn R, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA trial. Circ Arrhythm Electrophysiol. 2019;12:e007731. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald MR, Connelly DT, Hawkins NM, et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart. 2011;97:740–747. [DOI] [PubMed] [Google Scholar]
- 9.Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 10.Pan KL, Wu YL, Lee M, et al. Catheter ablation compared with medical therapy for atrial fibrillation with heart failure: a systematic review and meta-analysis of randomized controlled trials. Int J Med Sci. 2021;18:1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parkash R, Wells GA, Rouleau J, et al. Randomized ablation-based rhythm-control versus rate-control trial in patients with heart failure and atrial fibrillation: results from the RAFT-AF trial. Circulation. 2022;145:1693–1704. [DOI] [PubMed] [Google Scholar]
- 12.Prabhu S, Taylor AJ, Costello BT, et al. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA-MRI study. J Am Coll Cardiol. 2017;70:1949–1961. [DOI] [PubMed] [Google Scholar]
- 13.Turagam MK, Garg J, Whang W, et al. Catheter ablation of atrial fibrillation in patients with heart failure: a meta-analysis of randomized controlled trials. Ann Intern Med. 2019;170:41–50. [DOI] [PubMed] [Google Scholar]
- 14.Packer DL, Piccini JP, Monahan KH, et al. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation. 2021;143:1377–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siddiqui MU, Junarta J, Riley JM, et al. Catheter ablation in patients with atrial fibrillation and heart failure with preserved ejection fraction: a systematic review and meta-analysis. J Arrhythm. 2022;38:981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khand AU, Rankin AC, Martin W, et al. Carvedilol alone or in combination with digoxin for the management of atrial fibrillation in patients with heart failure? J Am Coll Cardiol. 2003;42:1944–1951. [DOI] [PubMed] [Google Scholar]
- 17.Kotecha D, Bunting KV, Gill SK, et al. Effect of digoxin vs bisoprolol for heart rate control in atrial fibrillation on patient-reported quality of life: the RATE-AF randomized clinical trial. JAMA. 2020;324:2497–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clemo HF, Wood MA, Gilligan DM, et al. Intravenous amiodarone for acute heart rate control in the critically ill patient with atrial tachyarrhythmias. Am J Cardiol. 1998;81:594–598. [DOI] [PubMed] [Google Scholar]
- 19.Delle Karth G, Geppert A, Neunteufl T, et al. Amiodarone versus diltiazem for rate control in critically ill patients with atrial tachyarrhythmias. Crit Care Med. 2001;29:1149–1153. [DOI] [PubMed] [Google Scholar]
- 20.Brignole M, Menozzi C, Gianfranchi L, et al. Assessment of atrioventricular junction ablation and VVIR pacemaker versus pharmacological treatment in patients with heart failure and chronic atrial fibrillation: a randomized, controlled study. Circulation. 1998;98:953–960. [DOI] [PubMed] [Google Scholar]
- 21.Kay GN, Ellenbogen KA, Giudici M, et al. The Ablate and Pace Trial: a prospective study of catheter ablation of the AV conduction system and permanent pacemaker implantation for treatment of atrial fibrillation APT Investigators. J Interv Card Electrophysiol. 1998;2:121–135. [DOI] [PubMed] [Google Scholar]
- 22.Weerasooriya R, Davis M, Powell A, et al. The Australian Intervention Randomized Control of Rate in Atrial Fibrillation Trial (AIRCRAFT). J Am Coll Cardiol. 2003;41:1697–1702. [DOI] [PubMed] [Google Scholar]
- 23.Brignole M, Pentimalli F, Palmisano P, et al. AV junction ablation and cardiac resynchronization for patients with permanent atrial fibrillation and narrow QRS: the APAF-CRT mortality trial. Eur Heart J. 2021;42:4731–4739. [DOI] [PubMed] [Google Scholar]
- 24.Ganesan AN, Brooks AG, Roberts-Thomson KC, et al. Role of AV nodal ablation in cardiac resynchronization in patients with coexistent atrial fibrillation and heart failure: a systematic review. J Am Coll Cardiol. 2012;59:719–726. [DOI] [PubMed] [Google Scholar]
- 25.Mustafa U, Atkins J, Mina G, et al. Outcomes of cardiac resynchronisation therapy in patients with heart failure with atrial fibrillation: a systematic review and meta-analysis of observational studies. Open Heart. 2019;6:e000937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gasparini M, Kloppe A, Lunati M, et al. Atrioventricular junction ablation in patients with atrial fibrillation treated with cardiac resynchronization therapy: positive impact on ventricular arrhythmias, implantable cardioverter-defibrillator therapies and hospitalizations. Eur J Heart Fail. 2018;20:1472–1481. [DOI] [PubMed] [Google Scholar]
- 27.Nerheim P, Birger-Botkin S, Piracha L, et al. Heart failure and sudden death in patients with tachycardia-induced cardiomyopathy and recurrent tachycardia. Circulation. 2004;110:247–252. [DOI] [PubMed] [Google Scholar]
- 28.Nedios S, Sommer P, Dagres N, et al. Long-term follow-up after atrial fibrillation ablation in patients with impaired left ventricular systolic function: the importance of rhythm and rate control. Heart Rhythm. 2014;11:344–351. [DOI] [PubMed] [Google Scholar]
- 29.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. [DOI] [PubMed] [Google Scholar]
- 30.Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. [DOI] [PubMed] [Google Scholar]
- 31.Silvet H, Hawkins LA, Jacobson AK. Heart rate control in patients with chronic atrial fibrillation and heart failure. Congest Heart Fail. 2013;19:25–28. [DOI] [PubMed] [Google Scholar]
- 32.Morina-Vazquez P, Moraleda-Salas MT, Arce-Leon A, et al. Effectiveness and safety of AV node ablation after His bundle pacing in patients with uncontrolled atrial arrhythmias. Pacing Clin Electrophysiol. 2021;44:1004–1009. [DOI] [PubMed] [Google Scholar]
- 33.Pillai A, Kolominsky J, Koneru JN, et al. Atrioventricular junction ablation in patients with conduction system pacing leads: a comparison of His-bundle vs left bundle branch area pacing leads. Heart Rhythm. 2022;19:1116–1123. [DOI] [PubMed] [Google Scholar]
- 34.Vijayaraman P, Mathew AJ, Naperkowski A, et al. Conduction system pacing versus conventional pacing in patients undergoing atrioventricular node ablation: nonrandomized, on-treatment comparison. Heart Rhythm O2. 2022;3:368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vijayaraman P, Subzposh FA, Naperkowski A. Atrioventricular node ablation and His bundle pacing. Europace. 2017;19:iv10–iv16. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein RE, Boccuzzi SJ, Cruess D, et al. Diltiazem increases late-onset congestive heart failure in postinfarction patients with early reduction in ejection fraction. The Adverse Experience Committee; and the Multicenter Diltiazem Postinfarction Research Group. Circulation. 1991;83:52–60. [DOI] [PubMed] [Google Scholar]
- 37.Kober L, Torp-Pedersen C, McMurray JJ, et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–2687. [DOI] [PubMed] [Google Scholar]
- 38.Calo L, De Ruvo E, Sette A, et al. Tachycardia-induced cardiomyopathy: mechanisms of heart failure and clinical implications. J Cardiovasc Med (Hagerstown). 2007;8:138–143. [DOI] [PubMed] [Google Scholar]
- 39.Gopinathannair R, Etheridge SP, Marchlinski FE, et al. Arrhythmia-induced cardiomyopathies: mechanisms, recognition, and management. J Am Coll Cardiol. 2015;66:1714–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shelton RJ, Clark AL, Goode K, et al. A randomised, controlled study of rate versus rhythm control in patients with chronic atrial fibrillation and heart failure: (CAFE-II Study). Heart. 2009;95:924–930. [DOI] [PubMed] [Google Scholar]
- 41.Vernooy K, Dijkman B, Cheriex EC, et al. Ventricular remodeling during long-term right ventricular pacing following His bundle ablation. Am J Cardiol. 2006;97:1223–1227. [DOI] [PubMed] [Google Scholar]
- 42.Tops LF, Schalij MJ, Holman ER, et al. Right ventricular pacing can induce ventricular dyssynchrony in patients with atrial fibrillation after atrioventricular node ablation. J Am Coll Cardiol. 2006;48:1642–1648. [DOI] [PubMed] [Google Scholar]
- 43.Gopinathannair R, Chen LY, Chung MK, et al. Managing atrial fibrillation in patients with heart failure and reduced ejection fraction: a scientific statement from the American Heart Association. Circ Arrhythm Electrophysiol. 2021;14:e000078. [DOI] [PubMed] [Google Scholar]
- 44.Ziff OJ, Lane DA, Samra M, et al. Safety and efficacy of digoxin: systematic review and meta-analysis of observational and controlled trial data. BMJ. 2015;351:h4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rathore SS, Curtis JP, Wang Y, et al. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA. 2003;289:871–878. [DOI] [PubMed] [Google Scholar]
- 46.Hou ZY, Chang MS, Chen CY, et al. Acute treatment of recent-onset atrial fibrillation and flutter with a tailored dosing regimen of intravenous amiodarone A randomized, digoxin-controlled study. Eur Heart J. 1995;16:521–528. [DOI] [PubMed] [Google Scholar]
- 47.Nakagawa H, Jackman WM, Scherlag BJ, et al. Pulmonary vein isolation during atrial fibrillation: insight into the mechanism of pulmonary vein firing. J Cardiovasc Electrophysiol. 2003;14:261–262. [DOI] [PubMed] [Google Scholar]
- 48.Chatterjee NA, Upadhyay GA, Ellenbogen KA, et al. Atrioventricular nodal ablation in atrial fibrillation: a meta-analysis of biventricular vs right ventricular pacing mode. Eur J Heart Fail. 2012;14:661–667. [DOI] [PubMed] [Google Scholar]
- 49.Baudo M, D'Ancona G, Trinca F, et al. Atrioventricular node ablation and pacing for atrial tachyarrhythmias: a meta-analysis of postoperative outcomes. Int J Cardiol. 2022;363:80–86. [DOI] [PubMed] [Google Scholar]
- 50.Linde C, Leclercq C, Rex S, et al. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol. 2002;40:111–118. [DOI] [PubMed] [Google Scholar]
- 51.Upadhyay GA, Choudhry NK, Auricchio A, et al. Cardiac resynchronization in patients with atrial fibrillation: a meta-analysis of prospective cohort studies. J Am Coll Cardiol. 2008;52:1239–1246. [DOI] [PubMed] [Google Scholar]
- 52.Wilton SB, Leung AA, Ghali WA, et al. Outcomes of cardiac resynchronization therapy in patients with versus those without atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm. 2011;8:1088–1094. [DOI] [PubMed] [Google Scholar]
- 53.Hayes DL, Boehmer JP, Day JD, et al. Cardiac resynchronization therapy and the relationship of percent biventricular pacing to symptoms and survival. Heart Rhythm. 2011;8:1469–1475. [DOI] [PubMed] [Google Scholar]
- 54.Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. [DOI] [PubMed] [Google Scholar]
- 55.van Gelder IC, Phan HM, Wilkoff BL, et al. Prognostic significance of atrial arrhythmias in a primary prevention ICD population. Pacing Clin Electrophysiol. 2011;34:1070–1079. [DOI] [PubMed] [Google Scholar]
- 56.Boriani G, Gasparini M, Landolina M, et al. Incidence and clinical relevance of uncontrolled ventricular rate during atrial fibrillation in heart failure patients treated with cardiac resynchronization therapy. Eur J Heart Fail. 2011;13:868–876. [DOI] [PubMed] [Google Scholar]
- 57.Jandali MB. Safety of intravenous diltiazem in reduced ejection fraction heart failure with rapid atrial fibrillation. Clin Drug Investig. 2018;38:503–508. [DOI] [PubMed] [Google Scholar]
- 58.Hasbrouck M, Nguyen TT. Acute management of atrial fibrillation in congestive heart failure with reduced ejection fraction in the emergency department. Am J Emerg Med. 2022;58:39–42. [DOI] [PubMed] [Google Scholar]
- 59.Figulla HR, Gietzen F, Zeymer U, et al. Diltiazem improves cardiac function and exercise capacity in patients with idiopathic dilated cardiomyopathy Results of the Diltiazem in Dilated Cardiomyopathy Trial. Circulation. 1996;94:346–352. [DOI] [PubMed] [Google Scholar]
- 60.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. [DOI] [PubMed] [Google Scholar]
10.1. Management of Early Onset AF, Including Genetic Testing
- 1.Yalin K, Ikitimur B, Aksu T, et al. Catheter ablation for atrial fibrillation in patients </=30 years of age. Am J Cardiol. 2022;166:53–57. [DOI] [PubMed] [Google Scholar]
- 2.Ceresnak SR, Liberman L, Silver ES, et al. Lone atrial fibrillation in the young - perhaps not so “lone”? J Pediatr. 2013;162:827–831. [DOI] [PubMed] [Google Scholar]
- 3.Sauer WH, Alonso C, Zado E, et al. Atrioventricular nodal reentrant tachycardia in patients referred for atrial fibrillation ablation: response to ablation that incorporates slow-pathway modification. Circulation. 2006;114:191–195. [DOI] [PubMed] [Google Scholar]
- 4.Yoneda ZT, Anderson KC, Quintana JA, et al. Early-onset atrial fibrillation and the prevalence of rare variants in cardiomyopathy and arrhythmia genes. JAMA Cardiol. 2021;6:1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodyer WR, Dunn K, Caleshu C, et al. Broad genetic testing in a clinical setting uncovers a high prevalence of titin loss-of-function variants in very early onset atrial fibrillation. Circ Genom Precis Med. 2019;12:e002713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez MV, Mahaffey KW, Hedlin H, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
10.2. Athletes
- 1.Mandsager KT, Phelan DM, Diab M, et al. Outcomes of pulmonary vein isolation in athletes. JACC Clin Electrophysiol. 2020;6:1265–1274. [DOI] [PubMed] [Google Scholar]
- 2.Toso E, Gagliardi M, Peyracchia M, et al. Long-term efficacy and impact on quality of life of atrial fibrillation catheter ablation in competitive athletes. J Sports Med Phys Fitness. 2022;62:1266–1271. [DOI] [PubMed] [Google Scholar]
- 3.Azarbal F, Stefanick ML, Salmoirago-Blotcher E, et al. Obesity, physical activity, and their interaction in incident atrial fibrillation in postmenopausal women. J Am Heart Assoc. 2014;3:e001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Furberg CD, Psaty BM, et al. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2008;118:800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman W, Parry-Williams G, Wiles J, et al. Risk of atrial fibrillation in athletes: a systematic review and meta-analysis. Br J Sports Med. 2021;55:1233–1238. [DOI] [PubMed] [Google Scholar]
- 6.Ayinde H, Schweizer ML, Crabb V, et al. Age modifies the risk of atrial fibrillation among athletes: a systematic literature review and meta-analysis. Int J Cardiol Heart Vasc. 2018;18:25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trivedi SJ, Claessen G, Stefani L, et al. Differing mechanisms of atrial fibrillation in athletes and non-athletes: alterations in atrial structure and function. Eur Heart J Cardiovasc Imaging. 2020;21:1374–1383. [DOI] [PubMed] [Google Scholar]
- 8.La Gerche A, Inder WJ, Roberts TJ, et al. Relationship between inflammatory cytokines and indices of cardiac dysfunction following intense endurance exercise. PLoS One. 2015;10:e0130031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estes NAM 3rd, Madias C. Atrial fibrillation in athletes: a lesson in the virtue of moderation. JACC Clin Electrophysiol. 2017;3:921–928. [DOI] [PubMed] [Google Scholar]
10.3. Management Considerations in Patients With AF and Obesity
- 1.Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141:e750–e772. [DOI] [PubMed] [Google Scholar]
- 2.Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. [DOI] [PubMed] [Google Scholar]
- 3.Wong CX, Sun MT, Odutayo A, et al. Associations of Epicardial, abdominal, and overall adiposity with atrial fibrillation. Circ Arrhythm Electrophysiol. 2016;9:e004378. [DOI] [PubMed] [Google Scholar]
- 4.Wong CX, Sullivan T, Sun MT, et al. Obesity and the risk of incident, postoperative, and post-ablation atrial fibrillation: a meta-analysis of 626 603 individuals in 51 studies. JACC Clin Electrophysiol. 2015;1:139–152. [DOI] [PubMed] [Google Scholar]
10.4. Anticoagulation Considerations in Patients With Class III Obesity
- 1.Barakat AF, Jain S, Masri A, et al. Outcomes of direct oral anticoagulants in atrial fibrillation patients across different body mass index categories. JACC Clin Electrophysiol. 2021;7:649–658. [DOI] [PubMed] [Google Scholar]
- 2.Malik AH, Yandrapalli S, Shetty S, et al. Impact of weight on the efficacy and safety of direct-acting oral anticoagulants in patients with non-valvular atrial fibrillation: a meta-analysis. Europace. 2020;22:361–367. [DOI] [PubMed] [Google Scholar]
- 3.Hohnloser SH, Fudim M, Alexander JH, et al. Efficacy and safety of apixaban versus warfarin in patients with atrial fibrillation and extremes in body weight. Circulation. 2019;139:2292–2300. [DOI] [PubMed] [Google Scholar]
- 4.Boriani G, Ruff CT, Kuder JF, et al. Relationship between body mass index and outcomes in patients with atrial fibrillation treated with edoxaban or warfarin in the ENGAGE AF-TIMI 48 trial. Eur Heart J. 2019;40:1541–1550. [DOI] [PubMed] [Google Scholar]
- 5.Balla SR, Cyr DD, Lokhnygina Y, et al. Relation of risk of stroke in patients with atrial fibrillation to body mass index (from patients treated with rivaroxaban and warfarin in the rivaroxaban once daily oral direct factor Xa inhibition compared with vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation Trial). Am J Cardiol. 2017;119:1989–1996. [DOI] [PubMed] [Google Scholar]
- 6.Mahlmann A, Gehrisch S, Beyer-Westendorf J. Pharmacokinetics of rivaroxaban after bariatric surgery: a case report. J Thromb Thrombolysis. 2013;36:533–535. [DOI] [PubMed] [Google Scholar]
- 7.Leong R, Chu DK, Crowther MA, et al. Direct oral anticoagulants after bariatric surgery-what is the evidence? J Thromb Haemost. 2022;20:1988–2000. [DOI] [PubMed] [Google Scholar]
10.5. AF and VHD
- 1.Petty GW, Khandheria BK, Whisnant JP, et al. Predictors of cerebrovascular events and death among patients with valvular heart disease: a population-based study. Stroke. 2000;31:2628–2635. [DOI] [PubMed] [Google Scholar]
10.6. WPW and Preexcitation Syndromes
- 1.Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e506–e574. [DOI] [PubMed] [Google Scholar]
- 2.Roth A, Elkayam I, Shapira I, et al. Effectiveness of prehospital synchronous direct-current cardioversion for supraventricular tachyarrhythmias causing unstable hemodynamic states. Am J Cardiol. 2003;91:489–491. [DOI] [PubMed] [Google Scholar]
- 3.Pappone C, Vicedomini G, Manguso F, et al. Wolff-Parkinson-White syndrome in the era of catheter ablation: insights from a registry study of 2169 patients. Circulation. 2014;130:811–819. [DOI] [PubMed] [Google Scholar]
- 4.Kugler JD, Danford DA, Houston K, et al. Radiofrequency catheter ablation for paroxysmal supraventricular tachycardia in children and adolescents without structural heart disease Pediatric EP Society, Radiofrequency Catheter Ablation Registry. Am J Cardiol. 1997;80:1438–1443. [DOI] [PubMed] [Google Scholar]
- 5.Calkins H, Langberg J, Sousa J, et al. Radiofrequency catheter ablation of accessory atrioventricular connections in 250 patients Abbreviated therapeutic approach to Wolff-Parkinson-White syndrome. Circulation. 1992;85:1337–1346. [DOI] [PubMed] [Google Scholar]
- 6.Jackman WM, Wang XZ, Friday KJ, et al. Catheter ablation of accessory atrioventricular pathways (Wolff-Parkinson-White syndrome) by radiofrequency current. N Engl J Med. 1991;324:1605–1611. [DOI] [PubMed] [Google Scholar]
- 7.Belhassen B, Rogowski O, Glick A, et al. Radiofrequency ablation of accessory pathways: a 14 year experience at the Tel Aviv Medical Center in 508 patients. Isr Med Assoc J. 2007;9:265–270. [PubMed] [Google Scholar]
- 8.Sellers TD Jr, Campbell RW, Bashore TM, et al. Effects of procainamide and quinidine sulfate in the Wolff-Parkinson-White syndrome. Circulation. 1977;55:15–22. [DOI] [PubMed] [Google Scholar]
- 9.Glatter KA, Dorostkar PC, Yang Y, et al. Electrophysiological effects of ibutilide in patients with accessory pathways. Circulation. 2001;104:1933–1939. [DOI] [PubMed] [Google Scholar]
- 10.Boriani G, Biffi M, Frabetti L, et al. Ventricular fibrillation after intravenous amiodarone in Wolff-Parkinson-White syndrome with atrial fibrillation. Am Heart J. 1996;131:1214–1216. [DOI] [PubMed] [Google Scholar]
- 11.Kim RJ, Gerling BR, Kono AT, et al. Precipitation of ventricular fibrillation by intravenous diltiazem and metoprolol in a young patient with occult Wolff-Parkinson-White syndrome. Pacing Clin Electrophysiol. 2008;31:776–779. [DOI] [PubMed] [Google Scholar]
- 12.Sellers TD Jr, Bashore TM, Gallagher JJ. Digitalis in the pre-excitation syndrome Analysis during atrial fibrillation. Circulation. 1977;56:260–267. [DOI] [PubMed] [Google Scholar]
- 13.Simonian SM, Lotfipour S, Wall C, et al. Challenging the superiority of amiodarone for rate control in Wolff-Parkinson-White and atrial fibrillation. Intern Emerg Med. 2010;5:421–426. [DOI] [PubMed] [Google Scholar]
- 14.Gulamhusein S, Ko P, Carruthers SG, et al. Acceleration of the ventricular response during atrial fibrillation in the Wolff-Parkinson-White syndrome after verapamil. Circulation. 1982;65:348–354. [DOI] [PubMed] [Google Scholar]
- 15.Munger TM, Packer DL, Hammill SC, et al. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953–1989. Circulation. 1993;87:866–873. [DOI] [PubMed] [Google Scholar]
- 16.Fukatani M, Tanigawa M, Mori M, et al. Prediction of a fatal atrial fibrillation in patients with asymptomatic Wolff-Parkinson-White pattern. Jpn Circ J. 1990;54:1331–1339. [DOI] [PubMed] [Google Scholar]
- 17.Pietersen AH, Andersen ED, Sandoe E. Atrial fibrillation in the Wolff-Parkinson-White syndrome. Am J Cardiol. 1992;70:38A–43A. [DOI] [PubMed] [Google Scholar]
- 18.Klein GJ, Bashore TM, Sellers TD, et al. Ventricular fibrillation in the WolffParkinson-White syndrome. N Engl J Med. 1979;301:1080–1085. [DOI] [PubMed] [Google Scholar]
- 19.Pappone C, Santinelli V, Manguso F, et al. A randomized study of prophylactic catheter ablation in asymptomatic patients with the Wolff-ParkinsonWhite syndrome. N Engl J Med. 2003;349:1803–1811. [DOI] [PubMed] [Google Scholar]
- 20.A randomized, placebo-controlled trial of propafenone in the prophylaxis of paroxysmal supraventricular tachycardia and paroxysmal atrial fibrillation. UK Propafenone PSVT Study Group. Circulation. 1995;92:2550–2557. [PubMed] [Google Scholar]
- 21.Chimienti M, Cullen MT Jr, Casadei G. Safety of flecainide versus propafenone for the long-term management of symptomatic paroxysmal supraventricular tachyarrhythmias Report from the Flecainide and Propafenone Italian Study (FAPIS) Group. Eur Heart J. 1995;16:1943–1951. [DOI] [PubMed] [Google Scholar]
10.7. Hypertrophic Cardiomyopathy (HCM)
- 1.Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020;142:e558–e631. [DOI] [PubMed] [Google Scholar]
- 2.Guttmann OP, Rahman MS, O'Mahony C, et al. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: systematic review. Heart. 2014;100:465–472. [DOI] [PubMed] [Google Scholar]
- 3.Kubo T, Baba Y, Ochi Y, et al. Clinical significance of new-onset atrial fibrillation in patients with hypertrophic cardiomyopathy. ESC Heart Fail. 2021;8:5022–5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fauchier L, Bisson A, Bodin A, et al. Ischemic stroke in patients with hypertrophic cardiomyopathy according to presence or absence of atrial fibrillation. Stroke. 2022;53:497–504. [DOI] [PubMed] [Google Scholar]
- 5.Alphonse P, Virk S, Collins J, et al. Prognostic impact of atrial fibrillation in hypertrophic cardiomyopathy: a systematic review. Clin Res Cardiol. 2021;110:544–554. [DOI] [PubMed] [Google Scholar]
- 6.Hsu JC, Huang YT, Lin LY. Stroke risk in hypertrophic cardiomyopathy patients with atrial fibrillation: a nationwide database study. Aging (Albany NY). 2020;12:24219–24227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rozen G, Elbaz-Greener G, Marai I, et al. Utilization and complications of catheter ablation for atrial fibrillation in patients with hypertrophic cardiomyopathy. J Am Heart Assoc. 2020;9:e015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
10.8. Adult Congenital Heart Disease (ACHD)
- 1.Escudero C, Khairy P, Sanatani S. Electrophysiologic considerations in congenital heart disease and their relationship to heart failure. Can J Cardiol. 2013;29:821–829. [DOI] [PubMed] [Google Scholar]
- 2.Miyazaki A, Negishi J, Hayama Y, et al. Etiology of atrial fibrillation in patients with complex congenital heart disease - for a better treatment strategy. J Cardiol. 2020;76:438–445. [DOI] [PubMed] [Google Scholar]
- 3.Mylotte D, Pilote L, Ionescu-Ittu R, et al. Specialized adult congenital heart disease care: the impact of policy on mortality. Circulation. 2014;129:1804–1812. [DOI] [PubMed] [Google Scholar]
- 4.Liang JJ, Frankel DS, Parikh V, et al. Safety and outcomes of catheter ablation for atrial fibrillation in adults with congenital heart disease: a multicenter registry study. Heart Rhythm. 2019;16:846–852. [DOI] [PubMed] [Google Scholar]
- 5.Fischer AJ, Enders D, Wasmer K, et al. Impact of specialized electrophysiological care on the outcome of catheter ablation for supraventricular tachycardias in adults with congenital heart disease: independent risk factors and gender aspects. Heart Rhythm. 2021;18:1852–1859. [DOI] [PubMed] [Google Scholar]
- 6.Koyak Z, Kroon B, de Groot JR, et al. Efficacy of antiarrhythmic drugs in adults with congenital heart disease and supraventricular tachycardias. Am J Cardiol. 2013;112:1461–1467. [DOI] [PubMed] [Google Scholar]
- 7.Guarguagli S, Kempny A, Cazzoli I, et al. Efficacy of catheter ablation for atrial fibrillation in patients with congenital heart disease. Europace. 2019;21:1334–1344. [DOI] [PubMed] [Google Scholar]
- 8.Philip F, Muhammad KI, Agarwal S, et al. Pulmonary vein isolation for the treatment of drug-refractory atrial fibrillation in adults with congenital heart disease. Congenit Heart Dis. 2012;7:392–399. [DOI] [PubMed] [Google Scholar]
- 9.Refaat MM, Ballout J, Mansour M. Ablation of atrial fibrillation in patients with congenital heart disease. Arrhythm Electrophysiol Rev. 2017;6:191–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khairy P, Aboulhosn J, Broberg CS, et al. Thromboprophylaxis for atrial arrhythmias in congenital heart disease: a multicenter study. Int J Cardiol. 2016;223:729–735. [DOI] [PubMed] [Google Scholar]
- 11.Labombarda F, Hamilton R, Shohoudi A, et al. Increasing prevalence of atrial fibrillation and permanent atrial arrhythmias in congenital heart disease. J Am Coll Cardiol. 2017;70:857–865. [DOI] [PubMed] [Google Scholar]
- 12.Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e698–e800. [DOI] [PubMed] [Google Scholar]
- 13.Sohns C, Nurnberg JH, Hebe J, et al. Catheter ablation for atrial fibrillation in adults with congenital heart disease: lessons learned from more than 10 years following a sequential ablation approach. JACC Clin Electrophysiol. 2018;4:733–743. [DOI] [PubMed] [Google Scholar]
- 14.Khairy P, Van Hare GF, Balaji S, et al. PACES/HRS expert consensus statement on the recognition and management of arrhythmias in adult congenital heart disease Developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Heart Rhythm. 2014;11:e102–e165. [DOI] [PubMed] [Google Scholar]
- 15.Fish FA, Gillette PC, Benson DW Jr Proarrhythmia, cardiac arrest and death in young patients receiving encainide and flecainide The Pediatric Electrophysiology Group. J Am Coll Cardiol. 1991;18:356–365. [DOI] [PubMed] [Google Scholar]
- 16.Epstein AE, Hallstrom AP, Rogers WJ, et al. Mortality following ventricular arrhythmia suppression by encainide, flecainide, and moricizine after myocardial infarction The original design concept of the Cardiac Arrhythmia Suppression Trial (CAST). JAMA. 1993;270:2451–2455. [PubMed] [Google Scholar]
- 17.Thorne SA, Barnes I, Cullinan P, et al. Amiodarone-associated thyroid dysfunction: risk factors in adults with congenital heart disease. Circulation. 1999;100:149–154. [DOI] [PubMed] [Google Scholar]
- 18.Stan MN, Ammash NM, Warnes CA, et al. Body mass index and the development of amiodarone-induced thyrotoxicosis in adults with congenital heart disease--a cohort study. Int J Cardiol. 2013;167:821–826. [DOI] [PubMed] [Google Scholar]
- 19.El-Assaad I, Al-Kindi SG, Abraham J, et al. Use of dofetilide in adult patients with atrial arrhythmias and congenital heart disease: a PACES collaborative study. Heart Rhythm. 2016;13:2034–2039. [DOI] [PubMed] [Google Scholar]
- 20.Connolly SJ, Camm AJ, Halperin JL, et al. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med. 2011;365:2268–2276. [DOI] [PubMed] [Google Scholar]
- 21.Kober L, Torp-Pedersen C, McMurray JJ, et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–2687. [DOI] [PubMed] [Google Scholar]
- 22.Kottmaier M, Baur A, Lund S, et al. Atrial fibrillation ablation in adults with congenital heart disease on uninterrupted oral anticoagulation is safe and efficient. Clin Res Cardiol. 2020;109:904–910. [DOI] [PubMed] [Google Scholar]
- 23.Abadir S, Waldmann V, Dyrda K, et al. Feasibility and safety of cryoballoon ablation for atrial fibrillation in patients with congenital heart disease. World J Cardiol. 2019;11:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bessiere F, Mondesert B, Chaix MA, et al. Arrhythmias in adults with congenital heart disease and heart failure. Heart Rhythm O2. 2021;2:744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karsenty C, Zhao A, Marijon E, et al. Risk of thromboembolic complications in adult congenital heart disease: a literature review. Arch Cardiovasc Dis. 2018;111:613–620. [DOI] [PubMed] [Google Scholar]
- 26.Yang H, Bouma BJ, Dimopoulos K, et al. Non-vitamin K antagonist oral anticoagulants (NOACs) for thromboembolic prevention, are they safe in congenital heart disease? Results of a worldwide study. Int J Cardiol. 2020;299:123–130. [DOI] [PubMed] [Google Scholar]
10.9.1. Prevention of AF After Cardiac Surgery
- 1.Filardo G, da Graca B, Sass DM, et al. Preoperative β-blockers as a coronary surgery quality metric: the lack of evidence of efficacy. Ann Thorac Surg. 2020;109:1150–1158. [DOI] [PubMed] [Google Scholar]
- 2.Gillinov AM, Bagiella E, Moskowitz AJ, et al. Rate control versus rhythm control for atrial fibrillation after cardiac surgery. N Engl J Med. 2016;374:1911–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehaffey JH, Hawkins RB, Byler M, et al. Amiodarone protocol provides cost-effective reduction in postoperative atrial fibrillation. Ann Thorac Surg. 2018;105:1697–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guarnieri T Intravenous antiarrhythmic regimens with focus on amiodarone for prophylaxis of atrial fibrillation after open heart surgery. Am J Cardiol. 1999;84:152r–155r. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell LB, Exner DV, Wyse DG, et al. Prophylactic oral amiodarone for the prevention of arrhythmias that begin early after revascularization, valve replacement, or repair: PAPABEAR: a randomized controlled trial. JAMA. 2005;294:3093–3100. [DOI] [PubMed] [Google Scholar]
- 6.Arsenault KA, Yusuf AM, Crystal E, et al. Interventions for preventing postoperative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2013;2013:Cd003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaudino M, Sanna T, Ballman KV, et al. Posterior left pericardiotomy for the prevention of atrial fibrillation after cardiac surgery: an adaptive, singlecentre, single-blind, randomised, controlled trial. Lancet. 2021;398:2075–2083. [DOI] [PubMed] [Google Scholar]
- 8.Eikelboom R, Sanjanwala R, Le ML, et al. Postoperative atrial fibrillation after cardiac surgery: a systematic review and meta-analysis. Ann Thorac Surg. 2021;111:544–554. [DOI] [PubMed] [Google Scholar]
- 9.Imazio M, Brucato A, Ferrazzi P, et al. Colchicine reduces postoperative atrial fibrillation: results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) atrial fibrillation substudy. Circulation. 2011;124:2290–2295. [DOI] [PubMed] [Google Scholar]
- 10.Imazio M, Brucato A, Ferrazzi P, et al. Colchicine for prevention of postpericardiotomy syndrome and postoperative atrial fibrillation: the COPPS-2 randomized clinical trial. JAMA. 2014;312:1016–1023. [DOI] [PubMed] [Google Scholar]
- 11.Shvartz V, Le T, Kryukov Y, et al. Colchicine for prevention of atrial fibrillation after cardiac surgery in the early postoperative period. J Clin Med. 2022;11:1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
10.9.2. Treatment of AF After Cardiac Surgery
- 1.Filardo G, da Graca B, Sass DM, et al. Preoperative β-blockers as a coronary surgery quality metric: the lack of evidence of efficacy. Ann Thorac Surg. 2020;109:1150–1158. [DOI] [PubMed] [Google Scholar]
- 2.Arsenault KA, Yusuf AM, Crystal E, et al. Interventions for preventing postoperative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2013;2013:Cd003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkman W, Herbert MA, O'Brien S, et al. Preoperative beta-blocker use in coronary artery bypass grafting surgery: national database analysis. JAMA Intern Med. 2014;174:1320–1327. [DOI] [PubMed] [Google Scholar]
- 4.Kohsaka S, Miyata H, Motomura N, et al. Effects of preoperative beta-blocker use on clinical outcomes after coronary artery bypass grafting: a report from the Japanese Cardiovascular Surgery Database. Anesthesiology. 2016;124:45–55. [DOI] [PubMed] [Google Scholar]
- 5.Gillinov AM, Bagiella E, Moskowitz AJ, et al. Rate control versus rhythm control for atrial fibrillation after cardiac surgery. N Engl J Med. 2016;374:1911–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rezk M, Taha A, Nielsen SJ, et al. Clinical course of postoperative atrial fibrillation after cardiac surgery and long-term outcome. Ann Thorac Surg. 2022;114:2209–2215. [DOI] [PubMed] [Google Scholar]
- 7.Fragao-Marques M, Teixeira F, Mancio J, et al. Impact of oral anticoagulation therapy on postoperative atrial fibrillation outcomes: a systematic review and meta-analysis. Thromb J. 2021;19:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imazio M, Brucato A, Ferrazzi P, et al. Colchicine reduces postoperative atrial fibrillation: results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) atrial fibrillation substudy. Circulation. 2011;124:2290–2295. [DOI] [PubMed] [Google Scholar]
- 9.Imazio M, Brucato A, Ferrazzi P, et al. Colchicine for prevention of postpericardiotomy syndrome and postoperative atrial fibrillation: the COPPS-2 randomized clinical trial. JAMA. 2014;312:1016–1023. [DOI] [PubMed] [Google Scholar]
- 10.Shvartz V, Le T, Kryukov Y, et al. Colchicine for prevention of atrial fibrillation after cardiac surgery in the early postoperative period. J Clin Med. 2022;11:1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
10.10. Acute Medical Illness or Surgery (Including AF in Critical Care)
- 1.McIntyre WF, Um KJ, Cheung CC, et al. Atrial fibrillation detected initially during acute medical illness: a systematic review. Eur Heart J. 2019;8:130–141. [DOI] [PubMed] [Google Scholar]
- 2.Lubitz SA, Yin X, Rienstra M, et al. Long-term outcomes of secondary atrial fibrillation in the community: the Framingham Heart Study. Circulation. 2015;131:1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang EY, Hulme OL, Khurshid S, et al. Initial precipitants and recurrence of atrial fibrillation. Circ Arrhythm Electrophysiol. 2020;13:e007716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdelmoneim SS, Rosenberg E, Meykler M, et al. The incidence and natural progression of new-onset postoperative atrial fibrillation. JACC Clin Electrophysiol. 2021;7:1134–1144. [DOI] [PubMed] [Google Scholar]
- 5.Charitos EI, Herrmann FEM, Ziegler PD. Atrial fibrillation recurrence and spontaneous conversion to sinus rhythm after cardiac surgery: insights from 426 patients with continuous rhythm monitoring. J Cardiovasc Electrophysiol. 2021;32:2171–2178. [DOI] [PubMed] [Google Scholar]
- 6.El-Chami MF, Merchant FM, Smith P, et al. Management of new-onset postoperative atrial fibrillation utilizing insertable cardiac monitor technology to observe recurrence of AF (MONITOR-AF). Pacing Clin Electrophysiol. 2016;39:1083–1089. [DOI] [PubMed] [Google Scholar]
- 7.Perino AC, Fan J, Schmitt SK, et al. Treating specialty and outcomes in newly diagnosed atrial fibrillation: from the TREAT-AF study. J Am Coll Cardiol. 2017;70:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and atrial fibrillation. N Engl J Med. 2014;371:1261. [DOI] [PubMed] [Google Scholar]
- 9.Ha ACT, Verma S, Mazer CD, et al. Effect of continuous electrocardiogram monitoring on detection of undiagnosed atrial fibrillation after hospitalization for cardiac surgery: a randomized clinical trial. JAMA Netw Open. 2021;4:e2121867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walkey AJ, Quinn EK, Winter MR, et al. Practice patterns and outcomes associated with use of anticoagulation among patients with atrial fibrillation during sepsis. JAMA Cardiol. 2016;1:682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quon MJ, Behlouli H, Pilote L. Anticoagulant use and risk of ischemic stroke and bleeding in patients with secondary atrial fibrillation associated with acute coronary syndromes, acute pulmonary disease, or sepsis. JACC Clin Electrophysiol. 2018;4:386–393. [DOI] [PubMed] [Google Scholar]
- 12.Massera D, Wang D, Vorchheimer DA, et al. Increased risk of stroke and mortality following new-onset atrial fibrillation during hospitalization. Europace. 2017;19:929–936. [DOI] [PubMed] [Google Scholar]
- 13.Klein Klouwenberg PM, Frencken JF, Kuipers S, et al. Incidence, predictors, and outcomes of new-onset atrial fibrillation in critically ill patients with sepsis a cohort study. Am J Respir Crit Care Med. 2017;195:205–211. [DOI] [PubMed] [Google Scholar]
- 14.Kuipers S, Klein Klouwenberg PM, Cremer OL. Incidence, risk factors and outcomes of new-onset atrial fibrillation in patients with sepsis: a systematic review. Crit Care. 2014;18:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walkey AJ, Wiener RS, Ghobrial JM, et al. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306:2248–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McIntyre WF, Vadakken ME, Rai AS, et al. Incidence and recurrence of new-onset atrial fibrillation detected during hospitalization for noncardiac surgery: a systematic review and meta-analysis. Can J Anaesth. 2021;68:1045–1056. [DOI] [PubMed] [Google Scholar]
- 17.Chyou JY, Barkoudah E, Dukes JW, et al. Atrial Fibrillation occurring during acute hospitalization: a scientific statement from the American Heart Association. Circulation. 2023;147:e676–e698. [DOI] [PubMed] [Google Scholar]
10.11. Hyperthyroidism
- 1.Gundlund A, Kumler T, Bonde AN, et al. Comparative thromboembolic risk in atrial fibrillation with and without a secondary precipitant-Danish nationwide cohort study. BMJ Open. 2019;9:e028468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tng EL, Tiong YS, Aung AT, et al. Efficacy and safety of anticoagulation in thyrotoxic atrial fibrillation: a systematic review and meta-analysis. Endocr Connect. 2022;11:e220166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jabbar A, Pingitore A, Pearce SH, et al. Thyroid hormones and cardiovascular disease. Nat Rev Cardiol. 2017;14:39–55. [DOI] [PubMed] [Google Scholar]
- 4.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489–499. [DOI] [PubMed] [Google Scholar]
- 5.Frost L, Vestergaard P, Mosekilde L. Hyperthyroidism and risk of atrial fibrillation or flutter: a population-based study. Arch Intern Med. 2004;164:1675–1678. [DOI] [PubMed] [Google Scholar]
- 6.Nakazawa HK, Sakurai K, Hamada N, et al. Management of atrial fibrillation in the post-thyrotoxic state. Am J Med. 1982;72:903–906. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu T, Koide S, Noh JY, et al. Hyperthyroidism and the management of atrial fibrillation. Thyroid. 2002;12:489–493. [DOI] [PubMed] [Google Scholar]
- 8.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–509. [DOI] [PubMed] [Google Scholar]
- 9.Tagami T, Yambe Y, Tanaka T, et al. Short-term effects of beta-adrenergic antagonists and methimazole in new-onset thyrotoxicosis caused by Graves' disease. Intern Med. 2012;51:2285–2290. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson OR, Karlberg BE, Kagedal B, et al. Non-selective and selective beta1-adrenoceptor blocking agents in the treatment of hyperthyroidism. Acta Med Scand. 1979;206:21–25. [DOI] [PubMed] [Google Scholar]
- 11.McDevitt DG, Nelson JK. Comparative trial of atenolol and propranolol in hyperthyroidism. Br J Clin Pharmacol. 1978;6:233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansson S, Lie-Karlsen K, Stenqvist O, et al. Oxygen consumption in patients with hyperthyroidism before and after treatment with beta-blockade versus thyrostatic treatment: a prospective randomized study. Ann Surg. 2001;233:60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feely J, Peden N. Use of beta-adrenoceptor blocking drugs in hyperthyroidism. Drugs. 1984;27:425–446. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein SA, Green J, Huber K, et al. Characteristics and outcomes of atrial fibrillation in patients with thyroid disease (from the ARISTOTLE trial). Am J Cardiol. 2019;124:1406–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ari H, Gürdogan M, Erdogan E, et al. Short-term outcome of early electrical cardioversion for atrial fibrillation in hyperthyroid versus euthyroid patients. Cardiol J. 2012;19:53–60. [DOI] [PubMed] [Google Scholar]
- 16.Razvi S, Jabbar A, Pingitore A, et al. Thyroid hormones and cardiovascular function and diseases. J Am Coll Cardiol. 2018;71:1781–1796. [DOI] [PubMed] [Google Scholar]
- 17.Bruere H, Fauchier L, Bernard Brunet A, et al. History of thyroid disorders in relation to clinical outcomes in atrial fibrillation. Am J Med. 2015;128:30–37. [DOI] [PubMed] [Google Scholar]
10.12. Pulmonary Disease
- 1.Du Q, Sun Y, Ding N, et al. Beta-blockers reduced the risk of mortality and exacerbation in patients with COPD: a meta-analysis of observational studies. PLoS One. 2014;9:e113048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salpeter S, Ormiston T, Salpeter E. Cardioselective beta-blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;4:CD003566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dransfield MT, Voelker H, Bhatt SP, et al. Metoprolol for the prevention of acute exacerbations of COPD. N Engl J Med. 2019;381:2304–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tongers J, Schwerdtfeger B, Klein G, et al. Incidence and clinical relevance of supraventricular tachyarrhythmias in pulmonary hypertension. Am Heart J. 2007;153:127–132. [DOI] [PubMed] [Google Scholar]
- 5.Showkathali R, Tayebjee MH, Grapsa J, et al. Right atrial flutter isthmus ablation is feasible and results in acute clinical improvement in patients with persistent atrial flutter and severe pulmonary arterial hypertension. Int J Cardiol. 2011;149:279–280. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-Cano MJ, Gonzalez-Mansilla A, Escribano P, et al. Clinical implications of supraventricular arrhythmias in patients with severe pulmonary arterial hypertension. Int J Cardiol. 2011;146:105–106. [DOI] [PubMed] [Google Scholar]
- 7.Bradfield J, Shapiro S, Finch W, et al. Catheter ablation of typical atrial flutter in severe pulmonary hypertension. J Cardiovasc Electrophysiol. 2012;23:1185–1190. [DOI] [PubMed] [Google Scholar]
- 8.Olsson KM, Nickel NP, Tongers J, et al. Atrial flutter and fibrillation in patients with pulmonary hypertension. Int J Cardiol. 2013;167:2300–2305. [DOI] [PubMed] [Google Scholar]
- 9.Wen L, Sun ML, An P, et al. Frequency of supraventricular arrhythmias in patients with idiopathic pulmonary arterial hypertension. Am J Cardiol. 2014;114:1420–1425. [DOI] [PubMed] [Google Scholar]
- 10.Cannillo M, Grosso Marra W, Gili S, et al. Supraventricular arrhythmias in patients with pulmonary arterial hypertension. Am J Cardiol. 2015;116:1883–1889. [DOI] [PubMed] [Google Scholar]
- 11.Smith B, Genuardi MV, Koczo A, et al. Atrial arrhythmias are associated with increased mortality in pulmonary arterial hypertension. Pulm Circ. 2018;8:2045894018790316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamada H, Kaneyama J, Inoue YY, et al. Long term prognosis in patients with pulmonary hypertension undergoing catheter ablation for supraventricular tachycardia. Sci Rep. 2021;11:16176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye J, Yao P, Shi X, et al. A systematic literature review and meta-analysis on the impact of COPD on atrial fibrillation patient outcome. Heart Lung. 2022;51:67–74. [DOI] [PubMed] [Google Scholar]
- 14.Bikdeli B, Abou Ziki MD, Lip GYH. Pulmonary embolism and atrial fibrillation: two sides of the same coin? A systematic review. Semin Thromb Hemost. 2017;43:849–863. [DOI] [PubMed] [Google Scholar]
- 15.Noubiap JJ, Nyaga UF, Middeldorp ME, et al. Frequency and prognostic significance of atrial fibrillation in acute pulmonary embolism: a pooled analysis. Respir Med. 2022;199:106862. [DOI] [PubMed] [Google Scholar]
- 16.Proietti M, Laroche C, Drozd M, et al. Impact of chronic obstructive pulmonary disease on prognosis in atrial fibrillation: a report from the EURObservational Research Programme Pilot Survey on Atrial Fibrillation (EORP-AF) General Registry. Am Heart J. 2016;181:83–91. [DOI] [PubMed] [Google Scholar]
- 17.Baker JG, Wilcox RG. Beta-lockers, heart disease and COPD: current controversies and uncertainties. Thorax. 2017;72:271–276. [DOI] [PubMed] [Google Scholar]
- 18.Sivak JA, Raina A, Forfia PR. Assessment of the physiologic contribution of right atrial function to total right heart function in patients with and without pulmonary arterial hypertension. Pulm Circ. 2016;6:322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
10.13. Pregnancy
- 1.Georgiopoulos G, Tsiachris D, Kordalis A, et al. Pharmacotherapeutic strategies for atrial fibrillation in pregnancy. Expert Opin Pharmacother. 2019;20:1625–1636. [DOI] [PubMed] [Google Scholar]
- 2.Tamirisa KP, Elkayam U, Briller JE, et al. Arrhythmias in pregnancy. JACC Clin Electrophysiol. 2022;8:120–135. [DOI] [PubMed] [Google Scholar]
- 3.van Hagen IM, Roos-Hesselink JW, Ruys TP, et al. Pregnancy in women with a mechanical heart valve: data of the European Society of Cardiology Registry of Pregnancy and Cardiac Disease (ROPAC). Circulation. 2015;132:132–142. [DOI] [PubMed] [Google Scholar]
- 4.Vaidya VR, Arora S, Patel N, et al. Burden of arrhythmia in pregnancy. Circulation. 2017;135:619–621. [DOI] [PubMed] [Google Scholar]
- 5.Salam AM, Ertekin E, van Hagen IM, et al. Atrial fibrillation or flutter during pregnancy in patients with structural heart disease: data from the ROPAC (Registry on Pregnancy and Cardiac Disease). JACC Clin Electrophysiol. 2015;1:284–292. [DOI] [PubMed] [Google Scholar]
- 6.Lee MS, Chen W, Zhang Z, et al. Atrial fibrillation and atrial flutter in pregnant women-a population-based study. J Am Heart Assoc. 2016;5:e003182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scantlebury DC, Kattah AG, Weissgerber TL, et al. Impact of a history of hypertension in pregnancy on later diagnosis of atrial fibrillation. J Am Heart Assoc. 2018;7:e007584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Body C, Christie JA. Gastrointestinal diseases in pregnancy: nausea, vomiting, hyperemesis gravidarum, gastroesophageal reflux disease, constipation, and diarrhea. Gastroenterol Clin North Am. 2016;45:267–283. [DOI] [PubMed] [Google Scholar]
- 9.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation Developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 10.Halpern DG, Weinberg CR, Pinnelas R, et al. Use of medication for cardiovascular disease during pregnancy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:457–476. [DOI] [PubMed] [Google Scholar]
- 11.Enriquez AD, Economy KE, Tedrow UB. Contemporary management of arrhythmias during pregnancy. Circ Arrhythm Electrophysiol. 2014;7:961–967. [DOI] [PubMed] [Google Scholar]
- 12.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165–3241. [DOI] [PubMed] [Google Scholar]
- 13.Yalaz M, Levent E, Olukman M, et al. Role of digoxin-like immunoreactive substance in the pathogenesis of transient tachypnea of newborn. Biomed Res Int. 2013;2013:704763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anugu VR, Nalluri N, Asti D, et al. New-onset lone atrial fibrillation in pregnancy. Ther Adv Cardiovasc Dis. 2016;10:274–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72–e227. [DOI] [PubMed] [Google Scholar]
- 16.Hoeltzenbein M, Beck E, Meixner K, et al. Pregnancy outcome after exposure to the novel oral anticoagulant rivaroxaban in women at suspected risk for thromboembolic events: a case series from the German Embryotox Pharmacovigilance Centre. Clin Res Cardiol. 2016;105:117–126. [DOI] [PubMed] [Google Scholar]
10.14. Cardio-Oncology and Anticoagulation Considerations
- 1.Beavers CJ, Rodgers JE, Bagnola AJ, et al. Cardio-oncology drug interactions: a scientific statement from the American Heart Association. Circulation. 2022;145:e811–e838. [DOI] [PubMed] [Google Scholar]
- 2.van Leeuwen RWF, Jansman FGA, van den Bemt P, et al. Drug-drug interactions in patients treated for cancer: a prospective study on clinical interventions. Ann Oncol. 2015;26:992–997. [DOI] [PubMed] [Google Scholar]
- 3.Kim PY, Irizarry-Caro JA, Ramesh T, et al. How to diagnose and manage QT prolongation in cancer patients. JACC CardioOncol. 2021;3:145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye JZ, Hansen FB, Mills RW, et al. Oncotherapeutic protein kinase inhibitors associated with pro-arrhythmic liability. JACC CardioOncol. 2021;3:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Neal WT, Lakoski SG, Qureshi W, et al. Relation between cancer and atrial fibrillation (from the REasons for Geographic And Racial Differences in Stroke Study). Am J Cardiol. 2015;115:1090–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain P, Zhao S, Lee HJ, et al. Ibrutinib With rituximab in first-line treatment of older patients with mantle cell lymphoma. J Clin Oncol. 2022;40:202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atterman A, Friberg L, Asplund K, et al. Net benefit of oral anticoagulants in patients with atrial fibrillation and active cancer: a nationwide cohort study. Europace. 2020;22:58–65. [DOI] [PubMed] [Google Scholar]
- 8.Mariani MV, Magnocavallo M, Straito M, et al. Direct oral anticoagulants versus vitamin K antagonists in patients with atrial fibrillation and cancer a meta-analysis. J Thromb Thrombolysis. 2021;51:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jakobsen CB, Lamberts M, Carlson N, et al. Incidence of atrial fibrillation in different major cancer subtypes: a nationwide population-based 12 year follow up study. BMC Cancer. 2019;19:1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan M, Zhang Z, Tse G, et al. Association of cancer and the risk of developing atrial fibrillation: a systematic review and meta-analysis. Cardiol Res Pract. 2019;2019:8985273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farmakis D, Parissis J, Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol. 2014;63:945–953. [DOI] [PubMed] [Google Scholar]
- 12.Schizas D, Kosmopoulos M, Giannopoulos S, et al. Meta-analysis of risk factors and complications associated with atrial fibrillation after oesophagectomy. Br J Surg. 2019;106:534–547. [DOI] [PubMed] [Google Scholar]
- 13.Hu YF, Liu CJ, Chang PM, et al. Incident thromboembolism and heart failure associated with new-onset atrial fibrillation in cancer patients. Int J Cardiol. 2013;165:355–357. [DOI] [PubMed] [Google Scholar]
- 14.Murtaza M, Baig MMA, Ahmed J, et al. Higher mortality associated with new-onset atrial fibrillation in cancer patients: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9:867002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guha A, Fradley MG, Dent SF, et al. Incidence, risk factors, and mortality of atrial fibrillation in breast cancer: a SEER-Medicare analysis. Eur Heart J. 2022;43:300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao L, Salem JE, Clauss S, et al. Ibrutinib-mediated atrial fibrillation attributable to inhibition of C-terminal Src kinase. Circulation. 2020;142:2443–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caldeira D, Alves D, Costa J, et al. Ibrutinib increases the risk of hypertension and atrial fibrillation: systematic review and meta-analysis. PLoS One. 2019;14:e0211228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melloni C, Shrader P, Carver J, et al. Management and outcomes of patients with atrial fibrillation and a history of cancer: the ORBIT-AF registry. Eur Heart J Qual Care Clin Outcomes. 2017;3:192–197. [DOI] [PubMed] [Google Scholar]
- 19.Peixoto de Miranda EJF, Takahashi T, Iwamoto F, et al. Drug-drug interactions of 257 antineoplastic and supportive care agents with 7 anticoagulants: a comprehensive review of interactions and mechanisms. Clin Appl Thromb Hemost. 2020;26:1076029620936325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higuchi S, Kabeya Y, Matsushita K, et al. Incidence and complications of perioperative atrial fibrillation after non-cardiac surgery for malignancy. PLoS One. 2019;14:e0216239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Kindi SG, Oliveira GH. Prevalence of preexisting cardiovascular disease in patients with different types of cancer: the unmet need for onco-cardiology. Mayo Clin Proc. 2016;91:81–83. [DOI] [PubMed] [Google Scholar]
- 22.Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141:e750–e772. [DOI] [PubMed] [Google Scholar]
- 23.Rienstra M, Hobbelt AH, Alings M, et al. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J. 2018;39:2987–2996. [DOI] [PubMed] [Google Scholar]
- 24.Verdecchia P, Staessen JA, Angeli F, et al. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009;374:525–533. [DOI] [PubMed] [Google Scholar]
- 25.Rao MP, Halvorsen S, Wojdyla D, et al. Blood pressure control and risk of stroke or systemic embolism in patients with atrial fibrillation: results from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) Trial. J Am Heart Assoc. 2015;4:e002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toufektzian L, Zisis C, Balaka C, et al. Effectiveness of brain natriuretic peptide in predicting postoperative atrial fibrillation in patients undergoing non-cardiac thoracic surgery. Interact Cardiovasc Thorac Surg. 2015;20:654–657. [DOI] [PubMed] [Google Scholar]
- 27.Amar D, Zhang H, Tan KS, et al. A brain natriuretic peptide-based prediction model for atrial fibrillation after thoracic surgery: development and internal validation. J Thorac Cardiovasc Surg. 2019;157:2493–2499 e2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang EK, Chanson D, Teh JB, et al. Atrial fibrillation in patients undergoing allogeneic hematopoietic cell transplantation. J Clin Oncol. 2021;39:902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemu M, Zimmerman A, Kalra D, et al. Pretransplant cardiac evaluation using novel technology. J Clin Med. 2019;8:690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganatra S, Sharma A, Shah S, et al. Ibrutinib-associated atrial fibrillation. JACC Clin Electrophysiol. 2018;4:1491–1500. [DOI] [PubMed] [Google Scholar]
- 31.Abushukair H, Syaj S, Ababneh O, et al. First- versus second-generation Bruton tyrosine kinase inhibitors in Waldenstrom's macroglobulinemia: a systematic review and meta-analysis. Am J Hematol. 2022;97:942–950. [DOI] [PubMed] [Google Scholar]
- 32.Buza V, Rajagopalan B, Curtis AB. Cancer treatment-induced arrhythmias: focus on chemotherapy and targeted therapies. Circ Arrhythm Electrophysiol. 2017;10:e005443. [DOI] [PubMed] [Google Scholar]
- 33.Fradley MG, Beckie TM, Brown SA, et al. Recognition, prevention, and management of arrhythmias and autonomic disorders in cardio-oncology: a scientific statement from the American Heart Association. Circulation. 2021;144:e41–e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
10.15. CKD and Kidney Failure
- 1.Alonso A, Lopez FL, Matsushita K, et al. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:2946–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watanabe H, Watanabe T, Sasaki S, et al. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. Am Heart J. 2009;158:629–636. [DOI] [PubMed] [Google Scholar]
- 3.Bansal N, Fan D, Hsu CY, et al. Incident atrial fibrillation and risk of end-stage renal disease in adults with chronic kidney disease. Circulation. 2013;127:569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
10.16. Anticoagulation Use in Patients With Liver Disease
- 1.Qamar A, Antman EM, Ruff CT, et al. Edoxaban versus warfarin in patients with atrial fibrillation and history of liver disease. J Am Coll Cardiol. 2019;74:179–189. [DOI] [PubMed] [Google Scholar]
- 2.Serper M, Weinberg EM, Cohen JB, et al. Mortality and hepatic decompensation in patients with cirrhosis and atrial fibrillation treated with anticoagulation. Hepatology. 2021;73:219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Proietti M, Marzona I, Vannini T, et al. Impact of liver disease on oral anticoagulant prescription and major adverse events in patients with atrial fibrillation: analysis from a population-based cohort study. Eur Heart J Cardiovasc Pharmacother. 2021;7:f84–f92. [DOI] [PubMed] [Google Scholar]
- 4.Pastori D, Lip GYH, Farcomeni A, et al. Incidence of bleeding in patients with atrial fibrillation and advanced liver fibrosis on treatment with vitamin K or non-vitamin K antagonist oral anticoagulants. Int J Cardiol. 2018;264:58–63. [DOI] [PubMed] [Google Scholar]
- 5.Pastori D, Sciacqua A, Marcucci R, et al. Prevalence and impact of nonalcoholic fatty liver disease in atrial fibrillation. Mayo Clin Proc. 2020;95:513–520. [DOI] [PubMed] [Google Scholar]
- 6.Kuo L, Chao TF, Liu CJ, et al. Liver cirrhosis in patients with atrial fibrillation: would oral anticoagulation have a net clinical benefit for stroke prevention? J Am Heart Assoc. 2017;6:e005307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S, Purerfellner H, Meyer C, et al. Anticoagulation in atrial fibrillation and liver disease: a pooled-analysis of >20 000 patients. Eur Heart J Cardiovasc Pharmacother. 2022;8:336–345. [DOI] [PubMed] [Google Scholar]
- 8.Lee SR, Lee HJ, Choi EK, et al. Direct oral anticoagulants in patients with atrial fibrillation and liver disease. J Am Coll Cardiol. 2019;73:3295–3308. [DOI] [PubMed] [Google Scholar]
- 9.Lee HF, Chan YH, Chang SH, et al. Effectiveness and safety of non-vitamin K antagonist oral anticoagulant and warfarin in cirrhotic patients with non-valvular atrial fibrillation. J Am Heart Assoc. 2019;8:e011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang CL, Wu VC, Kuo CF, et al. Efficacy and safety of non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients with impaired liver function: a retrospective cohort study. J Am Heart Assoc. 2018;7:e009263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee ZY, Suah BH, Teo YH, et al. Comparison of the efficacy and safety of direct oral anticoagulants and vitamin k antagonists in patients with atrial fibrillation and concomitant liver cirrhosis: a systematic review and meta-analysis. Am J Cardiovasc Drugs. 2022;22:157–165. [DOI] [PubMed] [Google Scholar]
- 12.Kubitza D, Roth A, Becka M, et al. Effect of hepatic impairment on the pharmacokinetics and pharmacodynamics of a single dose of rivaroxaban, an oral, direct Factor Xa inhibitor. Br J Clin Pharmacol. 2013;76:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menichelli D, Ronca V, Di Rocco A, et al. Direct oral anticoagulants and advanced liver disease: a systematic review and meta-analysis. Eur J Clin Invest. 2021;51:e13397. [DOI] [PubMed] [Google Scholar]
- 14.Mendell J, Johnson L, Chen S. An open-label, phase 1 study to evaluate the effects of hepatic impairment on edoxaban pharmacokinetics and pharmacodynamics. J Clin Pharmacol. 2015;55:1395–1405. [DOI] [PubMed] [Google Scholar]
- 15.Frost CE, Ly V, Garonzik SM. Apixaban Pharmacokinetics and pharmacodynamics in subjects with mild or moderate hepatic impairment. Drugs R D. 2021;21:375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stangier J, Stähle H, Rathgen K, et al. Pharmacokinetics and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor, with coadministration of digoxin. J Clin Pharmacol. 2012;52:243–250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


