Abstract
Background
Pancreatic cancer is an aggressive cancer. Resection of the cancer is the only treatment with the potential to achieve long‐term survival. However, a third of patients with pancreatic cancer have locally advanced cancer involving adjacent structures such as blood vessels which are not usually removed because of fear of increased complications after surgery. Such patients often receive palliative treatment. Resection of the pancreas along with the involved vessels is an alternative to palliative treatment for patients with locally advanced pancreatic cancer.
Objectives
To compare the benefits and harms of surgical resection versus palliative treatment in patients with locally advanced pancreatic cancer.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 12), MEDLINE, EMBASE, Science Citation Index Expanded, and trial registers until February 2014.
Selection criteria
We included randomised controlled trials comparing pancreatic resection versus palliative treatments for patients with locally advanced pancreatic cancer (irrespective of language or publication status).
Data collection and analysis
Two authors independently assessed trials for inclusion and independently extracted the data. We analysed the data with both the fixed‐effect and random‐effects models using Review Manager (RevMan). We calculated the hazard ratio (HR), risk ratio (RR) or mean difference (MD) with 95% confidence intervals (CI) based on an intention‐to‐treat analysis.
Main results
We identified two trials comparing pancreatic resection versus other treatments for patients with locally advanced pancreatic cancer. Ninety eight patients were randomised to pancreatic resection (n = 47) or palliative treatment (n = 51) in the two trials included in this review. Both trials were at high risk of bias. Both trials included patients who had locally advanced pancreatic cancer which involved the serosa anteriorly or retroperitoneum posteriorly or involved the blood vessels. Such pancreatic cancers would be considered generally unresectable. One trial included patients with pancreatic cancer in different locations of the pancreas including the head, neck and body (n = 42). The patients allocated to the pancreatic resection group underwent partial pancreatic resection (pancreatoduodenectomy with lymph node clearance or distal pancreatic resection with lymph node clearance) in this trial; the control group received palliative treatment with chemoradiotherapy. In the other trial, only patients with cancer in the head or neck of the pancreas were included (n = 56). The patients allocated to the pancreatic resection group underwent en bloc total pancreatectomy with splenectomy and vascular reconstruction in this trial; the control group underwent palliative bypass surgery with chemoimmunotherapy. The pancreatic resection group had lower mortality than the palliative treatment group (HR 0.38; 95% CI 0.25 to 0.58, very low quality evidence). Both trials followed the survivors up to at least five years. There were no survivors at two years in the palliative treatment group in either trial. Approximately 40% of the patients who underwent pancreatic resection were alive in the pancreatic resection group at the end of three years. This difference in survival was statistically significant (RR 22.68; 95% CI 3.15 to 163.22). The difference persisted at five years of follow‐up (RR 8.65; 95% CI 1.12 to 66.89). Neither trial reported severe adverse events but it is likely that a significant proportion of patients suffered from severe adverse events in both groups. The overall peri‐operative mortality in the resection group in the two trials was 2.5%. None of the trials reported quality of life. The estimated difference in the length of total hospital stay (which included all admissions of the patient related to the treatment) between the two groups was imprecise (MD ‐23.00 days; 95% CI ‐59.05 to 13.05, very low quality evidence). The total treatment costs were significantly lower in the pancreatic resection group than the palliative treatment group (MD ‐10.70 thousand USD; 95% CI ‐14.11 to ‐7.29, very low quality evidence).
Authors' conclusions
There is very low quality evidence that pancreatic resection increases survival and decreases costs compared to palliative treatments for selected patients with locally advanced pancreatic cancer and venous involvement. When sufficient expertise is available, pancreatic resection could be considered for selected patients with locally advanced pancreatic cancer who are willing to accept the potentially increased morbidity associated with the procedure. Further randomised controlled trials are necessary to increase confidence in the estimate of effect and to assess the quality of life of patients and the cost‐effectiveness of pancreatic resection versus palliative treatment for locally advanced pancreatic cancer.
Keywords: Humans; Chemoradiotherapy, Adjuvant; Immunotherapy; Immunotherapy/methods; Lymph Node Excision; Palliative Care; Palliative Care/methods; Pancreas; Pancreas/blood supply; Pancreas/surgery; Pancreatectomy; Pancreatectomy/methods; Pancreatic Neoplasms; Pancreatic Neoplasms/blood supply; Pancreatic Neoplasms/mortality; Pancreatic Neoplasms/therapy; Pancreaticoduodenectomy; Pancreaticoduodenectomy/methods; Randomized Controlled Trials as Topic; Splenectomy; Survival Rate
Plain language summary
Surgical removal of part of the pancreas and other tissues versus other treatments for patients with pancreatic cancer which invades the surrounding structures
Background
The pancreas is an organ in the abdomen which secretes digestive juices for the digestion of food. It also harbours the insulin secreting cells which maintain the blood sugar levels. Pancreatic cancer is an aggressive cancer. Surgery to remove the cancer improves survival. However, a third of patients with pancreatic cancer have locally advanced cancer involving major blood vessels which are not usually removed because of the fear of increased complications after surgery. Such patients receive palliative treatment. Resection (removing part of an organ) of the pancreas has been suggested as an alternative to palliative treatment for patients with locally advanced pancreatic cancer. However, in this group of patients the benefits and harms of surgical resection versus other treatments are not clear. We set out to answer this question by performing a thorough search of the literature for studies which compared surgical removal with palliative treatments. We included only randomised controlled trials, studies which, if designed appropriately, can help avoid arriving at wrong conclusions. We searched the literature for all studies reported until December 2013. Two authors independently assessed the trials for inclusion and independently extracted data to minimise errors.
Characteristics of studies
We identified two trials comparing surgical removal of the pancreas versus other treatments for patients with locally advanced pancreatic cancer. Ninety eight patients were included in these two trials. Forty seven patients received surgery while the remaining patients received palliative treatment. The choice of who received surgery and who received other treatments was decided by a method similar to the toss of a fair coin.
Main results
Approximately 97% of patients who underwent cancer removal surgery survived the surgery in the two trials. The patients who received surgery were twice as likely to live as those who received other treatments. The survivors were followed until at least five years. There were no survivors at two years in the palliative treatment group while approximately 40% of the patients who underwent surgical removal were alive at the end of three years. This difference in survival was statistically significant. The studies did not report the complications related to surgery although it is likely that a significant proportion of patients suffered from complications in both groups. None of the trials reported quality of life. There was no evidence of any difference in the length of total hospital stay (which included all admissions of a patient related to the treatment) between the two groups. The total treatment costs were significantly lower in the surgical removal group (by about USD 10,000) than in the palliative treatment group in the trial conducted in Japan. There was no information about the costs in the other trial, which was conducted in Greece.
Quality of evidence
Overall, the trials were at high risk of bias (that is, there is a potential to arrive at wrong conclusions). This was because it was not clear how the randomisation was performed, whether the people assessing the outcomes were aware of the group to which the participants belonged, and whether all participants were included in the analysis. The overall quality of evidence was very low as the trials were at high risk of bias and there were few trials to assess whether only studies with negative results were published.
Conclusions
There is very low quality evidence that surgical resection increases survival and decreases costs compared to palliative treatments for patients with locally advanced pancreatic cancer with involvement of veins. In selected patients pancreatic resection could be considered for patients with locally advanced pancreatic cancer who are willing to accept the potentially increased complications associated with the surgical procedure and when sufficient expertise is available.
Future research
Further randomised controlled trials are necessary to obtain more precise results and to assess the quality of life of patients and the value for money of surgical removal versus other treatments for locally advanced pancreatic cancer.
Summary of findings
Summary of findings for the main comparison. Pancreatic resection versus palliative treatment for locally advanced pancreatic cancer.
| Pancreatic resection versus palliative treatment for locally advanced pancreatic cancer | ||||||
| Patient or population: patients with locally advanced pancreatic cancer Settings: tertiary care Intervention: pancreatic resection Comparison: palliative treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Palliative treatment | Pancreatic resection | |||||
| Overall mortality Follow‐up: mean 3 years | 990 per 1000 | 826 per 1000 (684 to 931) | HR 0.38 (0.25 to 0.58) | 98 (2 studies) | ⊕⊝⊝⊝ very low1,2 | |
| Length of hospital stay | The mean length of hospital stay in the control groups was 124 days | The mean length of hospital stay in the intervention groups was 23 lower (59.05 lower to 13.05 higher) | 42 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | ||
| Costs | The mean costs in the control groups was 28.2 thousand US dollars | The mean costs in the intervention groups was 10.7 lower (14.11 to 7.29 lower) | 42 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| *All patients in the control group were dead within 2 years. A mortality proportion of 99% was used for illustration purposes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HR: Hazard ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The trial(s) was/were at high risk of bias and were downgraded 2 points. 2 There were few trials to assess publication bias and were downgraded 1 point. 3 Overlaps 0 and minimal clinically important difference. There were fewer than 400 patients in both groups. The quality of the evidence was downgraded 2 points.
Background
Description of the condition
Adenocarcinoma of the pancreas is the most common malignancy of the exocrine pancreas. It is the 10th most common cancer in the US, the fifth most common cause of cancer‐related mortality in the east, and the fourth most common cause of cancer‐related mortality in the west (Parkin 2001; Parkin 2005; Yamamoto 1998). In 2008, there were 280,000 new patients diagnosed with pancreatic cancer and 265,000 deaths due to pancreatic cancer globally (GLOBOCAN 2008). There is global variation in the incidence of pancreatic cancers with an age‐standardised annual incidence rate of 9.8 per 100,000 population in the more developed regions and an age‐standardised annual incidence rate of 3.5 per 100,000 population in the less developed regions (GLOBOCAN 2008). A similar trend is noted in the age‐standardised annual mortality rates of 9.3 per 100,000 population in the more developed regions and an age‐standardised annual mortality rate of 3.2 per 100,000 population in the less developed regions as a result of pancreatic cancer (GLOBOCAN 2008). Pancreatic adenocarcinoma has a poor prognosis for many reasons. It is a biologically aggressive cancer that is relatively resistant to chemotherapy and radiotherapy and has a high rate of local and systemic recurrence (Abrams 2009; Ghaneh 2007; Orr 2010). Surgical resection remains the only treatment with the potential for long‐term survival and cure. However, about half the patients have metastatic disease at presentation and a third have locally advanced unresectable disease, leaving only about 10% to 20% of patients suitable for resection (Tucker 2008). Attempted complete resection entails a major resection because of the anatomical location, and hence there is considerable morbidity and mortality. The median survival for metastatic pancreatic cancer is three to five months, while that of locally advanced cancer is six to 10 months (Alderson 2005). Recently, combination chemotherapy using oxaliplatin, irinotecan, leucovorin, and fluorouracil (FOLFIRINOX) has been reported to result in a median survival of about 11 months in metastatic pancreatic cancer (Conroy 2011). The overall five‐year survival after radical resection ranges from 7% to 25% (Cameron 1993; Livingston 1991; Niederhuber 1995; Nitecki 1995; Orr 2010; Trede 1990), with a median survival of 11 to 15 months (Alderson 2005). With adjuvant chemotherapy, the median survival after radical resection varies between 14 and 24 months (Liao 2013).
There is consensus on the primary management strategy in early as well as metastatic pancreatic cancer. In early pancreatic cancer, surgical resection remains the primary treatment of choice in patients likely to withstand major surgery. In metastatic pancreatic cancer, patients receive palliative treatment. However, the optimal strategy in the subgroup of patients with locally advanced pancreatic cancer who are potentially resectable but carry a high risk of being positive at the margin is controversial. This subset of disease has been termed borderline resectable pancreatic cancer. These tumours have one or more of the following characteristics as defined in the consensus conference report on resectable and borderline resectable pancreatic cancer (Abrams 2009):
tumour‐associated deformity of the superior mesenteric vein (SMV) or portal vein (PV);
abutment of the SMV or PV more than 180 degrees;
short segment occlusion of SMV or PV amenable to resection and reconstruction;
short segment involvement of the hepatic artery or its branches amenable to resection and reconstruction;
abutment of superior mesenteric artery less than 180 degrees.
The treatment options available for such patients are radical surgical resection and palliative treatment, which includes chemotherapy, radiotherapy, immunotherapy, best supportive care, surgical or endoscopic palliation of symptoms, or a combination of these (Cardenes 2011; Hirooka 2009; Recchia 2009; Sanders 2010; Scott 2009; Sultana 2007; Tachezy 2011). Neoadjuvant chemotherapy may improve the resectability of the cancer and survival in patients with locally advanced pancreatic cancer (Heinemann 2013).
Description of the intervention
Surgical resection involves removal of part of the pancreas along with any vessels infiltrated by cancer (SMV or PV or superior mesenteric artery or hepatic artery) and reconstruction of the superior mesenteric vein, portal vein and arteries (Bockhorn 2011; Chu 2010).
How the intervention might work
The intervention works by removing the parts of the body affected by cancer.
Why it is important to do this review
While radical surgical resection might remove the cancer locally, cancer may have disseminated because of involvement of major vessels. In addition, pancreatic resection with vascular reconstruction is associated with increased surgical mortality and morbidity compared with patients who undergo pancreatic resection without vascular reconstruction (Bockhorn 2011). A systematic review of surgical resection for locally advanced cancer revealed that surgery for locally advanced pancreatic cancer results in better survival than palliative treatments even in those requiring arterial reconstruction (Mollberg 2011). However, this was based on evidence from cohort studies and there may have been selection bias, that is, patients undergoing surgery might have less disease than those undergoing palliative treatments, as the authors of the systematic review acknowledge (Mollberg 2011). Randomised clinical trials do not suffer from selection bias since the purpose of randomisation is to ensure that similar participants receive both types of treatments (Higgins 2011). There has been no systematic reviews of randomised clinical trials on whether surgical resection is better than palliative treatments in the management of locally advanced pancreatic cancer.
Objectives
To compare the benefits and harms of surgical resection versus palliative treatment in patients with locally advanced pancreatic cancer.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials irrespective of blinding, language, publication status, date of publication, or sample size were included. We excluded quasi‐randomised studies.
Types of participants
Patients with locally advanced pancreatic cancer irrespective of arterial or venous involvement. We accepted any definition used by the author to define patients as having locally advanced pancreatic cancer. Since the consensus criteria for the definition of locally advanced pancreatic cancer have been defined only recently (Abrams 2009), we expected that different definitions would have been used in the studies. We assessed carefully whether the authors' definitions incorporated at least some of the elements of the consensus definition. We were fully aware that there may be issues with external validity and generalisability of the information to the current definition of locally advanced pancreatic cancer and considered such issues carefully before coming to conclusions.
Types of interventions
Attempted complete resection surgery (irrespective of whether arterial or venous reconstruction was performed) versus any palliative treatment.
Types of outcome measures
Primary outcomes
Overall survival
Other severe adverse events, defined as any event that would increase mortality; is life‐threatening; requires inpatient hospitalisation; results in a persistent or significant disability; or any important medical event that might have jeopardised the patient or required intervention to prevent it (ICH‐GCP 1996)
Quality of life
Secondary outcomes
Total hospital stay
Costs
Search methods for identification of studies
Electronic searches
We searched:
the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 1, 2014), see Appendix 1;
MEDLINE (1948 to February 2014), see Appendix 2;
EMBASE (1974 to February 2014), see Appendix 3;
Science Citation Index Expanded (Royle 2003) (1945 to February 2014), see Appendix 4.
Searching other resources
We searched the references of the identified trials to identify further relevant trials. We searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (http://apps.who.int/trialsearch/). The platform includes the ISRCTN register and US National Institutes of Health (NIH) ClinicalTrials.gov register among other registers.
Data collection and analysis
We performed the systematic review following the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors (KG and SK) identified the trials for inclusion independently of each other. We have listed the excluded studies with the reasons for the exclusion. Any differences were resolved through discussion.
Data extraction and management
Both review authors (KG and SK) independently extracted the following data.
Year and language of publication.
Country.
Year of conduct of the trial.
Inclusion and exclusion criteria.
Definition of locally advanced pancreatic cancer used by the study authors.
Sample size.
Details about intervention and control.
Outcomes (described above).
Risk of bias (described below).
Any unclear or missing information was sought by contacting the authors of the individual trials. If there was any doubt whether the trials shared the same patients, completely or partially (by identifying common authors and centres), we contacted the authors of the trials to clarify whether the trial report had been duplicated. We resolved any differences in opinion through discussion.
Assessment of risk of bias in included studies
We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). According to empirical evidence (Kjaergard 2001; Lundh 2012; Moher 1998; Schulz 1995; Wood 2008), the risks of bias of the trials were assessed based on the following bias risk domains.
Sequence generation
Low risk of bias (the methods used were either adequate (e.g. computer‐generated random numbers, table of random numbers) or unlikely to introduce confounding).
Uncertain risk of bias (there was insufficient information to assess whether the method used was likely to introduce confounding).
High risk of bias (the method used (e.g. quasi‐randomised studies) was improper and likely to introduce confounding). Such studies were excluded.
Allocation concealment
Low risk of bias (the method used (e.g. central allocation) was unlikely to induce bias on the final observed effect).
Uncertain risk of bias (there was insufficient information to assess whether the method used was likely to induce bias on the estimate of effect).
High risk of bias (the method used (e.g. open random allocation schedule) was likely to induce bias on the final observed effect).
Blinding of participants and personnel
Low risk of bias (blinding was performed adequately, or the outcome measurement was not likely to be influenced by lack of blinding).
Uncertain risk of bias (there was insufficient information to assess whether the type of blinding used was likely to induce bias on the estimate of effect).
High risk of bias (no blinding or incomplete blinding, and the outcome or the outcome measurement was likely to be influenced by lack of blinding).
We feel that blinding of participants is unethical if the patients in the control group did not receive routine palliative surgery. Blinding of personnel is impossible. We assessed the trials to be at high risk of bias for outcomes such as severe adverse events other than mortality, quality of life, hospital stay and costs.
Blinding of outcome assessors
Low risk of bias (blinding was performed adequately, or the outcome measurement was not likely to be influenced by lack of blinding).
Uncertain risk of bias (there was insufficient information to assess whether the type of blinding used was likely to induce bias on the estimate of effect).
High risk of bias (no blinding or incomplete blinding, and the outcome or the outcome measurement was likely to be influenced by lack of blinding).
Incomplete outcome data
Low risk of bias (the underlying reasons for missingness are unlikely to make treatment effects depart from plausible values, or proper methods have been employed to handle missing data).
Uncertain risk of bias (there was insufficient information to assess whether the missing data mechanism in combination with the method used to handle missing data were likely to induce bias on the estimate of effect).
High risk of bias (the crude estimate of effects (e.g. complete case estimate) was clearly biased due to the underlying reasons for missingness, and the methods used to handle missing data are unsatisfactory).
Selective outcome reporting
Low risk of bias (the trial protocol was available and all of the trial's pre‐specified outcomes that are of interest in the review have been reported, or similar; if the trial protocol was not available, all the primary outcomes in this review that were likely to be measured in such trials are reported).
Uncertain risk of bias (there was insufficient information to assess whether the magnitude and direction of the observed effect was related to selective outcome reporting).
High risk of bias (not all of the trial's pre‐specified primary outcomes were reported, or similar; or the primary outcomes in this review that were likely to be measured in such trials were not reported).
Vested interest bias
Low risk of bias (if the trial was conducted according to a publicly available protocol or if it was conducted by a party without any vested interests in the outcome of the trial).
Uncertain risk of bias (if the trial protocol was not available and if it was not clear if the trial was conducted by a party with vested interest in the outcome of the trial).
High risk of bias (if the trial was not conducted according to a publicly available protocol; or if a protocol was not available, the trial was conducted by a party with vested interests in the outcome of the trial).
Differential expertise bias
While the palliative treatments for locally advanced pancreatic cancer have been available for a long time and even new palliative treatments have a short learning curve, surgery for locally advanced pancreatic cancer requires additional surgical skills. So, we assessed differential expertise bias as follows.
Low risk of bias (if the surgery was performed by surgeons with prior experience in resection of locally advanced pancreatic cancers).
Uncertain risk of bias (if the prior experience of surgeons performing the surgery was not known).
High risk of bias (if it appears that the surgeons were inexperienced in performing surgery for locally advanced pancreatic cancer).
We considered trials that were classified as low risk of bias in all the above domains as low bias‐risk trials.
Measures of treatment effect
For dichotomous variables, we calculated the risk ratio (RR) with 95% confidence interval (CI). For continuous variables, we calculated the mean difference (MD) with 95% CI for outcomes such as hospital stay and planned to calculate the standardised mean difference (SMD) with 95% CI for quality of life (where different scales might be used). For time‐to‐event outcomes such as survival, we calculated the hazard ratio (HR) with 95% CI.
Unit of analysis issues
The unit of analysis was individual patients who had locally advanced pancreatic cancer.
Dealing with missing data
We performed an intention‐to‐treat analysis (Newell 1992) whenever possible. We planned to impute data for binary outcomes using various scenarios such as good outcome analysis, bad outcome analysis, best‐case scenario and worst‐case scenario (Gurusamy 2009).
For continuous outcomes, we planned to use available‐case analysis. We planned to impute the standard deviation from P values according to the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we planned to use the median for the meta‐analysis when the mean was not available. If it was not possible to calculate the standard deviation from the P value or the CIs, we planned to impute the standard deviation as the highest standard deviation in the other trials included under that outcome, fully recognising that this form of imputation will decrease the weight of the study for calculation of MDs and bias the effect estimate to no effect in the case of SMD (Higgins 2011).
For time‐to‐event outcomes, if the HR and 95% CI were not reported, we obtained the logarithm of the HR (ln(HR)) and the standard error (SE) of ln(HR) according to the methods described by Parmar 1998.
Assessment of heterogeneity
We explored heterogeneity by the Chi² test with significance set at a P value of 0.10, and measured the quantity of heterogeneity by the I² statistic (Higgins 2002). We used overlapping of CIs on the forest plot to determine heterogeneity.
Assessment of reporting biases
We planned to use visual asymmetry of a funnel plot to explore reporting bias in the presence of at least 10 trials (Egger 1997; Macaskill 2001). We planned to perform linear regression as described by Egger 1997 to determine the funnel plot asymmetry. Selective reporting was also considered as evidence for reporting bias.
Data synthesis
We performed the meta‐analyses using the software package Review Manager 5 (RevMan 2011) and followed the recommendations of The Cochrane Collaboration (Higgins 2011). We used both the random‐effects model (DerSimonian 1986) and fixed‐effect model (DeMets 1987) in meta‐analyses. In the case of a discrepancy between the two models, resulting in a change of conclusions, we planned to report both results. However, since this was not the case we have reported the results of the fixed‐effect model. We used the generic inverse method to combine the HRs for time‐to‐event outcomes.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses:
trials with low risk of bias compared to trials with high risk of bias;
different palliative treatments.
We planned to use the test for subgroup differences to identify the differences between subgroups.
Sensitivity analysis
We planned to perform a sensitivity analysis by imputing data for binary outcomes using various scenarios such as good outcome analysis, bad outcome analysis, best‐case scenario and worst‐case scenario (Gurusamy 2009). For binary outcomes, RR calculations do not include trials in which no events occurred in either group, whereas risk difference calculations do. We planned to report the risk difference if the results using this association measure resulted in a change of conclusions. We planned to perform a sensitivity analysis by excluding the trials in which the mean and the standard deviation were imputed.
Results
Description of studies
Results of the search
We identified a total of 3342 references through the electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (n = 137), MEDLINE (n = 916), EMBASE (n = 1431), Science Citation Index Expanded (n = 819), and WHO ICTRP (n = 39). We have shown the flow of references in Figure 1. We excluded 856 duplicates and 679 clearly irrelevant references through reading titles and abstracts. Seven references were retrieved for further assessment. No references were identified through scanning reference lists of the identified randomised trials. Of the seven references, we excluded two because of the reasons listed in the table 'Characteristics of excluded studies'. In total, five publications describing two randomised trials fulfilled the inclusion criteria.
1.
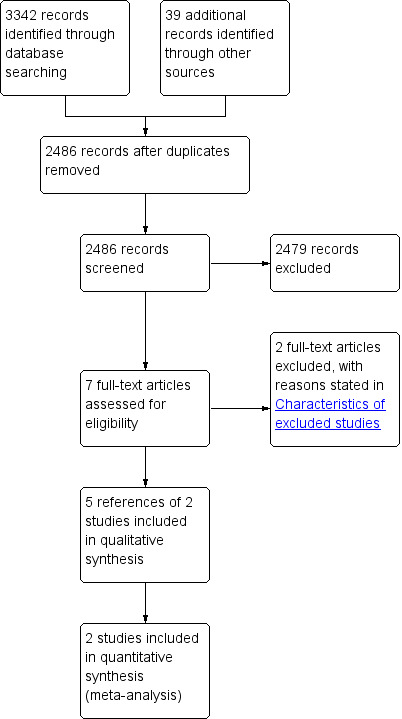
Study flow diagram.
Included studies
A total of 98 patients were randomised to pancreatic resection (n = 47) or palliative treatment (n = 51) in the two trials included in this review (Doi 2008; Lygidakis 2004). The mean ages of the patients in the two trials were 64 years and 66 years respectively, in Doi 2008 and Lygidakis 2004. The proportion of females was 35.7% in the only trial that reported the gender of the patients (Doi 2008).
Of the two trials that were included in this review, both trials included patients who had locally advanced pancreatic cancer which involved the serosa anteriorly or retroperitoneum posteriorly or involved the blood vessels (Doi 2008; Lygidakis 2004). Such pancreatic cancers would generally be considered unresectable. However, it was not clear whether all the patients included in these two trials met the criteria for the locally advanced pancreatic cancer definition which was defined in 2009 (Abrams 2009). One trial included patients with pancreatic cancer in different locations of the pancreas including the head, neck and body (Doi 2008). The patients in the resection group underwent partial pancreatic resection (pancreatoduodenectomy or distal pancreatic resection with lymph node clearance) in this trial (Doi 2008). The control group received palliative treatment with chemoradiotherapy in this trial (Doi 2008). In the other trial, only patients with cancer of the head or neck of the pancreas were included (Lygidakis 2004). The patients in the resection group underwent en bloc total pancreatectomy with splenectomy and vascular reconstruction in this trial (Lygidakis 2004). The control group received palliative bypass surgery with chemoimmunotherapy in this trial (Lygidakis 2004).
The outcomes reported in the trials are shown in Characteristics of included studies.
Excluded studies
The two excluded studies did not meet the inclusion criteria (Characteristics of excluded studies).
Risk of bias in included studies
Both trials were at high risk of bias (Figure 2).
2.
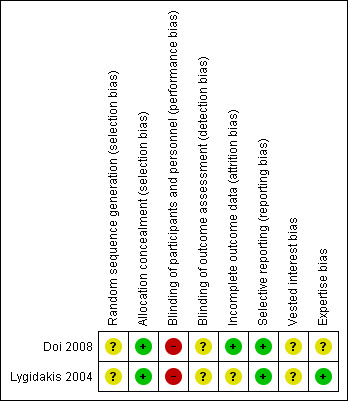
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Both trials had unclear sequence generation and low risk of bias in terms of allocation concealment (Doi 2008; Lygidakis 2004).
Blinding
Blinding of the healthcare providers was impossible. So, the trials were at high risk of bias for all outcomes other than mortality. No information on blinding of outcome assessors was available, in both trials (Doi 2008; Lygidakis 2004).
Incomplete outcome data
One trial was at low risk of bias as there were no post‐randomisation dropouts. It was not clear whether there were any post‐randomisation dropouts in the other trial.
Selective reporting
Both trials reported survival and hence were at low risk of bias (Doi 2008; Lygidakis 2004).
Other potential sources of bias
The source of funding was not clear in either trial (Doi 2008; Lygidakis 2004). The experience of the surgeons was also not clear in one trial (Doi 2008). The surgeries were performed by an experienced surgeon in the other trial (Lygidakis 2004).
Effects of interventions
See: Table 1
The results are summarised in the Table 1.
Primary outcomes
Survival
Both trials reported the peri‐operative and overall mortality (Doi 2008; Lygidakis 2004). One patient died post‐operatively in the pancreatic resection group in one trial (Lygidakis 2004). This patient was included in the calculation of the overall mortality and in the survival at different time points. There was no peri‐operative mortality in the pancreatic resection group in the other trial (Doi 2008). The patients belonging to the pancreatic resection group had lower mortality than those belonging to the palliative treatment group (HR 0.38; 95% CI 0.25 to 0.58) (Analysis 1.1). Both trials followed survivors until at least five years. One trial reported the three‐year survival in both groups (Doi 2008) and the other trial reported the two‐year, three‐year, four‐year and five‐year survival in both groups (Lygidakis 2004). We were able to obtain the one‐year, two‐year, four‐year, and five‐year survival from the survival graph available in the trial that reported only the three‐year survival (Doi 2008). There were no survivors at two years in the palliative treatment group in either trial. Approximately 40% of patients in the resection group were alive at three years and 15% were alive at five years. The survival was significantly higher in the resection group at one year, two years, three years, four years, and five years (Analysis 1.2).
1.1. Analysis.
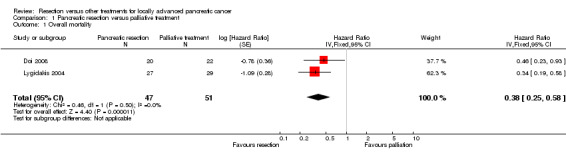
Comparison 1 Pancreatic resection versus palliative treatment, Outcome 1 Overall mortality.
1.2. Analysis.
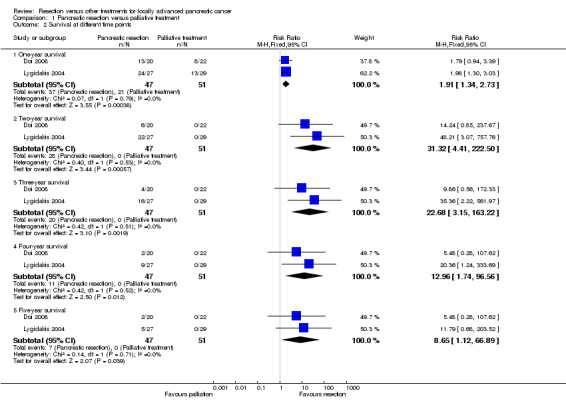
Comparison 1 Pancreatic resection versus palliative treatment, Outcome 2 Survival at different time points.
Other severe adverse events
Neither trial reported severe adverse events. It was likely that a significant proportion of patients suffered from severe adverse events in both groups.
Quality of life
None of the trials reported quality of life.
Secondary outcomes
Total hospital stay
One trial reported the length of total hospital stay, which included all admissions of a patient related to the treatment and its complications (Doi 2008). There was no significant difference in the length of total hospital stay between the two groups (MD ‐23.00 days; 95% CI ‐59.05 to 13.05) (Analysis 1.3).
1.3. Analysis.
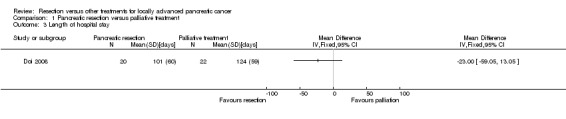
Comparison 1 Pancreatic resection versus palliative treatment, Outcome 3 Length of hospital stay.
Costs
One trial reported the total costs of the treatment (Doi 2008). The total costs were significantly lower in the pancreatic resection group than the palliative treatment group (MD ‐10.70 thousand USD; 95% CI ‐14.11 to ‐7.29) (Analysis 1.4).
1.4. Analysis.
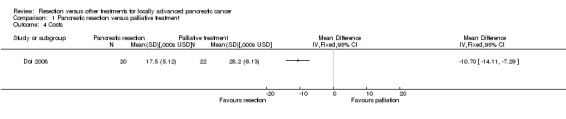
Comparison 1 Pancreatic resection versus palliative treatment, Outcome 4 Costs.
Other information
Variation in the effect measures and model used
There was no change in the results using the random‐effects model. The conclusions of the review did not change for any of the binary outcomes other than five‐year survival by using risk differences rather than risk ratios. Using risk ratio, there was no significant difference between the groups (RR 11.79; 95% CI 0.68 to 203.52). Using risk difference, a higher proportion of patients survived in the pancreatic resection group than the palliative treatment group (RD 0.19; 95% CI 0.03 to 0.34).
Subgroup analysis
Subgroup analysis was not performed because of the few trials in this review.
Sensitivity analysis
Sensitvity analysis was not performed since there were no post‐randomisation dropouts in one trial (Doi 2008) and the number of post‐randomisation dropouts was not mentioned in the other trial (Lygidakis 2004). We did not impute the mean or the standard deviation for the continuous outcomes.
Reporting bias
We did not explore reporting bias by using the funnel plot because of few trials in this review.
Discussion
Summary of main results
This review has shown that in selected patients, pancreatic resection increases the survival in patients with locally advanced pancreatic cancers when compared to palliative treatments. There were no survivors in the palliative treatment group beyond two years in either trial (Doi 2008; Lygidakis 2004). On the other hand, a significant proportion of patients (approximately 40% of patients) were alive at three years in the resection group. However, resection is more extensive and is likely to be a more morbid procedure than routine pancreaticoduodenectomy or distal pancreatic resection. The post‐operative mortality after routine pancreatic resection is 4% to 5% (Diener 2011; Gurusamy 2012) and approximately 30% of patients develop complication after pancreatic surgery (Gurusamy 2012). In addition, pancreatic resection with lymph node dissection or total pancreatectomy or pancreatic resection with vascular reconstruction is likely to be more morbid than routine pancreatic operations. For example, the short‐term mortality after pancreatic resection with vascular reconstruction is generally considered to be around 4% to 12% (Mollberg 2011; Siriwardana 2006; Zhou 2012) and approximately 17% to 100% of patients develop complications after pancreatic resection with vascular reconstruction (Mollberg 2011; Zhou 2012). The trials included in this review did not report the morbidity after the procedure. There was only one death in the 47 patients who underwent pancreatic resection for locally advanced pancreatic cancer in this review. However, it must be noted that the overall mortality and morbidity after pancreatic resection for locally advanced pancreatic cancer will be at least similar to that of routine pancreatic surgery but is more likely to be higher than routine pancreatic resection. This is likely to affect the quality of life of patients, at least in the short term. However, one must not forget that chemotherapy and radiotherapy, or a combination of the two, are also likely to affect the quality of life. While prophylactic gastrojejunostomy does not appear to improve the survival or quality of life of patients with unresectable periampullary cancer (Gurusamy 2010), the proportion of patients who developed gastric outlet obstruction was lower in the group that underwent prophylactic gastrojejunostomy. Thus the pancreatic resection, which is a more radical procedure than prophylactic gastrojejunostomy, is likely to be beneficial in decreasing the proportion of patients with gastric outlet obstruction. Assessing the quality of life will help in answering these questions.
The lack of information on the morbidity of the pancreatic resection for patients with locally advanced pancreatic resection should not distract us from noting that the survival is significantly better after pancreatic resection than palliative treatment. On average, the survival in patients undergoing pancreatic resection was twice as long as for those who underwent palliative treatment. It must be noted that none of the patients who underwent palliative treatment survived beyond two years, which is to be expected in an aggressive cancer such as pancreatic cancer.
The average total hospital stay, which included the hospital stay related to all episodes of treatment and its complications, was not significantly different between the two groups. However, we cannot be sure whether this is because of effect (true negative results) or lack of evidence of effect (that is false negative results). The total costs of treatment including its complications were lower in the pancreatic resection group than the palliative chemoradiotherapy group in the only trial that reported this outcome (Doi 2008). Thus, it appears that pancreatic resection for locally advanced pancreatic cancer improves survival in selected patients and decreases the costs related to the treatment of these patients.
Overall completeness and applicability of evidence
This review is applicable only to patients with locally advanced pancreatic cancer not invading the superior mesenteric artery and without peritoneal or distant metastases. The patients were randomised only after excluding invasion of the superior mesenteric artery and distant metastases. It is not clear whether all the patients met the consensus criteria for borderline resectable pancreatic cancer (Abrams 2009) but the inclusion criteria in the trials appear to exclude patients who will be offered pancreatic resection routinely.
It must be noted that the trials were conducted at tertiary centres. Although the experience of the surgeons was not reported in one of the trials (Doi 2008), it is likely that these surgeons were experienced in the procedures. The surgeries were performed by an experienced surgeon in the other trial (Lygidakis 2004). The findings of this review are applicable only in centres with sufficient expertise in performing pancreatic resections.
Although new palliative treatments such as FOLFIRINOX combination therapy and gemcitabine are available for palliative treatment of pancreatic cancer, two‐year survivors are rare with even the most recent drugs used for palliative treatment of pancreatic cancer (Conroy 2011; Nakai 2012). So, this evidence is applicable even when the improvement in palliative treatment is taken into account.
Quality of the evidence
The quality of the evidence is very low but one must note that this is the best available evidence on this topic. The search of trial registers shows that there are no current ongoing trials on this topic.
Potential biases in the review process
The review includes periods when it was not mandatory to register clinical trials, and for publication in journals. We were not able to assess publication bias because of the few trials included in this review. For these reasons we cannot rule out the possibility of an unpublished trial which does not show any benefit in pancreatic resection. However, one has to be pragmatic and realise that such trials may not exist or even if they exist are unlikely to be found unless the researcher reports the trial.
Agreements and disagreements with other studies or reviews
This is the first systematic review of randomised clinical trials on this topic. We agree with the trial authors that pancreatic resection may increase survival compared to palliative treatments. We also agree with the systematic review of cohort studies on pancreatic resection with vascular reconstruction, which suggested that patients undergoing pancreatic resection with venous reconstruction have better survival than those undergoing palliative treatment (Mollberg 2011). Although that systematic review also concluded that patients undergoing pancreatic resection with arterial reconstruction have better survival than those undergoing palliative treatment (Mollberg 2011), we are unable to comment on this issue since the trials included in this review did not include patients who had arterial involvement or underwent arterial reconstruction.
Authors' conclusions
Implications for practice.
There is very low quality evidence that pancreatic resection appears to increase survival and decrease costs compared to palliative treatments for selected patients with locally advanced pancreatic cancer with venous involvement. When sufficient expertise is available, pancreatic resection could be considered for selected patients with locally advanced pancreatic cancer who are willing to accept the potentially increased morbidity associated with the procedure.
Implications for research.
Further randomised controlled trials are necessary to increase confidence in the estimate of effect and to assess the quality of life of patients and the cost‐effectiveness of pancreatic resection versus palliative treatment for locally advanced pancreatic cancer.
Acknowledgements
We would like to thanks the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group and its peer reviewers.
This project was funded by the National Institute for Health Research. Disclaimer of the Department of Health: 'The views and opinions expressed in the review are those of the authors and do not necessarily reflect those of the National Institute for Health Research (NIHR), National Health Services (NHS), or the Department of Health'.
Appendices
Appendix 1. CENTRAL search strategy
#1 (randomized controlled trial):pt #2 (controlled clinical trial):pt #3 (randomized):ab #4 (placebo):ab #5 MeSH descriptor Drug Therapy, this term only #6 (randomly):ab #7 (trial):ab #8 (groups):ab #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) #10 (pancreas):ti,ab #11 (pancrea*):ti,ab #12 MeSH descriptor Pancreas, this term only #13 MeSH descriptor Pancreas, Exocrine, this term only #14 MeSH descriptor Pancreatic Ducts, this term only #15 (#10 OR #11 OR #12 OR #13 OR #14) #16 MeSH descriptor Carcinoma, this term only #17 MeSH descriptor Adenocarcinoma, this term only #18 MeSH descriptor Carcinoma, Ductal, this term only #19 (cancer) #20 (cancer*) #21 MeSH descriptor Neoplasms explode all trees #22 (tumo*) #23 (#16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22) #24 (#15 AND #23) #25 MeSH descriptor Surgical Procedures, Operative explode all trees #26 MeSH descriptor Surgery explode all trees #27 (surger* OR operatio* OR operative therap*) #28 (resection*):kw #29 (#25 OR #26 OR #27 OR #28) #30 (#24 AND #29) #31 MeSH descriptor Pancreatectomy explode all trees #32 MeSH descriptor Pancreaticojejunostomy explode all trees #33 MeSH descriptor Pancreaticoduodenectomy explode all trees #34 (pancreaticoduodenectom*):ti,ab #35 (pancreatectomy):ab,ti #36 (pancreaticojejunostomy):ab,ti #37 (duodenopancreatectom*):ab,ti #38 (pancreaticogastrostomy):ab,ti #39 (advanced):ab,ti #40 (unresectable):ab,ti #41 (borderline resectable):ab,ti #42 (inoperable):ab,ti #43 (#30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38) #44 (#39 OR #40 OR #41 OR #42) #45 (#9 AND #43 AND #44)
Appendix 2. MEDLINE search strategy
#1 ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT (humans[mh] AND animals[mh])) #2 Pancreatic Neoplasms [MeSH] OR pancreas cancer [tiab] OR pancreas cancers [tiab] OR pancreas neoplasm [tiab] OR pancreas neoplasms [tiab] OR pancreas carcinoma [tiab] OR pancreas tumor [tiab] OR pancreas tumors [tiab] OR pancreas tumour [tiab] OR pancreas tumours [tiab] #3 Surgical Procedures, Operative[MeSH] OR Surgery[MeSH] OR operation OR operations[tiab] OR operative therapy [tiab] OR operative therapies [tiab] OR surgery[tiab] OR surgeries [tiab] OR resection[tiab] OR resections [tiab] #4 #2 and #3 #5 Pancreatectomy[MeSH] OR Pancreaticojejunostomy[MeSH] OR Pancreaticoduodenectomy[MeSH] OR pancreaticoduodenectomy [tiab] OR pancreatectomy [tiab] OR pancreaticojejunostomy [tiab] OR pancreaticogastrostomy [tiab] OR pancreaticoduodenectomies [tiab] OR duodenopancreatectomy [tiab] OR duodenopancreatectomies [tiab] #6 #4 or #5 #7 advanced[tiab] OR unresectable[tiab] OR borderline resectable[tiab] OR inoperable[tiab] #8 #1 AND #6 AND #7
Appendix 3. EMBASE search strategy
1 randomized controlled trial/ 2 controlled clinical trial/ 3 randomized.ab. 4 placebo.ab. 5 drug therapy/ 6 randomly.ab. 7 trial.ab. 8 groups.ab. 9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10 animals/ 11 humans/ 12 11 and 10 13 10 not 12 14 9 not 13 15 pancreas.ab,ti. 16 pancrea$.ab,ti. 17 pancreas/ or pancreas, exocrine/ or pancreatic ducts/ 18 16 or 17 or 15 19 carcinoma/ or adenocarcinoma/ or carcinoma, ductal/ 20 cancer.tw. 21 cancer$.tw. 22 exp neoplasms/ 23 tumo$.tw. 24 22 or 21 or 23 or 19 or 20 25 18 and 24 26 exp Surgical Procedures, Operative/ 27 exp Surgery/ 28 surger$.tw. 29 operatio$.tw. 30 operative therap$.tw. 31 resection$.mp. 32 27 or 28 or 30 or 26 or 31 or 29 33 25 and 32 34 exp Pancreatectomy/ 35 exp Pancreaticojejunostomy/ 36 exp Pancreaticoduodenectomy/ 37 pancreaticoduodenectom$.ab,ti. 38 pancreatectomy.ab,ti. 39 pancreaticojejunostomy.ab,ti. 40 pancreaticogastrostomy.ab,ti. 41 duodenopancreatectom$.ab,ti. 42 35 or 33 or 39 or 40 or 36 or 41 or 38 or 34 or 37 43 exp advanced cancer/ 44 exp inoperable cancer/ 45 advanced.ab,ti. 46 unresectable.ab,ti. 47 borderline resectable.ti,ab. 48 inoperable.ti,ab. 49 46 or 45 or 43 or 44 or 48 or 47 50 42 and 49 51 50 and 14
Appendix 4. Science Citation Index Expanded
# 1 TS=(randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR drug therapy OR randomly OR trial OR groups) # 2 TS=(Pancreatic Neoplasms OR pancreas cancer OR pancreas cancers OR pancreas neoplasm OR pancreas neoplasms OR pancreas carcinoma OR pancreas tumor OR pancreas tumors OR pancreas tumour OR pancreas tumours) # 3 TS=(Operative Surgical Procedures OR Surgery OR operation OR operations OR operative therapy OR operative therapies OR surgery OR surgeries OR resection OR resections) # 4 #3 AND #2 # 5 TS=(Pancreatectomy OR Pancreaticojejunostomy OR Pancreaticoduodenectomy OR pancreaticoduodenectomy OR pancreatectomy OR pancreaticojejunostomy OR pancreaticogastrostomy OR pancreaticoduodenectomies OR duodenopancreatectomy OR duodenopancreatectomies)
# 6 #5 OR #4 # 7 TS=(advanced OR unresectable OR borderline resectable OR inoperable)
# 8 #7 AND #6 AND #1
Appendix 5. 5 WHO ICTRP
pancreatic in title AND (advanced OR unresectable OR borderline resectable OR inoperable) in condition
Data and analyses
Comparison 1. Pancreatic resection versus palliative treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall mortality | 2 | 98 | Hazard Ratio (Fixed, 95% CI) | 0.38 [0.25, 0.58] |
| 2 Survival at different time points | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 One‐year survival | 2 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.34, 2.73] |
| 2.2 Two‐year survival | 2 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 31.32 [4.41, 222.50] |
| 2.3 Three‐year survival | 2 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 22.68 [3.15, 163.22] |
| 2.4 Four‐year survival | 2 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.96 [1.74, 96.56] |
| 2.5 Five‐year survival | 2 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.65 [1.12, 66.89] |
| 3 Length of hospital stay | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Costs | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Doi 2008.
| Methods | Randomised clinical trial | |
| Participants | Country: Japan. Number randomised: 42 Post‐randomisation dropouts: 0 (0%) Revised sample size: 42 Average age: 64 years Females: 15 (35.7%) Inclusion criteria 1. Age between 20 and 75 years, with a performance status (PS) of 0 to 2 2. Tumor invasion of either the serosal (anterior) or retroperitoneal (posterior) surface of the pancreas, or extension into the intrapancreatic portal vein without complete obstruction; defined as S2, RP2, or PV2 according to the JCS 3. No involvement of adjacent organs, apart from the transverse mesocolon, duodenum, and common bile duct 4. No invasion of the superior mesenteric artery, the common hepatic artery, or the peripancreatic nerve plexuses (A0 and PL0) 5. No para‐aortic lymph node metastasis (N0 or N1) 6. Greatest diameter of the tumour within the range of 2 to 6 cm (TS2 or TS3) 7. No liver metastasis or peritoneal seeding (H0 and P0) Exclusion criteria 1. History of radiation therapy or chemotherapy 2. Idiosyncrasy to drugs including contrast media 3. Coexistence of serious cardiovascular, pulmonary, renal or hepatic diseases 4. Concurrent active neoplasm 5. Any other condition that authors considered could preclude the trial | |
| Interventions | Participants were randomly assigned to two groups Group 1: surgical resection (n = 20) Further details: Patients underwent pancreatoduodenectomy (PD) or distal pancreatectomy for resection of the main pancreatic cancer, with dissection of the Group 1 regional lymph nodes or more according to the JCS (Japanese Cancer Society). At least a half‐circle of the plexus of the root of the superior mesenteric artery was resected Group 2: palliative treatment (n = 20) Further details: Within 1 week, the patient received X‐ray irradiation. Radiation therapy was delivered as a single course, to a total radiation dose of 5040 cGy, in 28 fractions at 180 cGy over 5.5 weeks. Chemotherapy included infusion of 5‐fluorouracil (5‐FU) during radiation therapy. After finishing the regimen, these patients were given an intravenous weekly infusion of 5‐fluorouracil (5‐FU), usually starting within 1 week and at least within 4 weeks of completion of the radiochemotherapy. Surgeon in charge was permitted to perform anastomotic surgery such as gastrojejunostomy or biliodigestive anastomosis | |
| Outcomes | The outcomes reported were survival, hospital stay and costs | |
| Notes | Attempts were made to contact the authors in January 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Low risk | Quote: "Patient was randomized by a telephone call to the central office" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: It is unethical to blind the patients and impossible to blind the healthcare providers |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no post‐randomisation dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: Survival was reported |
| Vested interest bias | Unclear risk | Comment: This information was not available |
| Expertise bias | Unclear risk | Comment: This information was not available |
Lygidakis 2004.
| Methods | Randomised clinical trial | |
| Participants | Country: Greece Number randomised: 56 Post‐randomisation dropouts: not stated Revised sample size: 56 Average age: 66 years Females: not stated Inclusion criteria 1. Carcinoma of the head of the pancreas with regional vascular involvement of the superior mesenteric or the portal vein 2. Tumour at least 5 cm in diameter 3. Extension of the tumour on to the neck of the pancreas Exclusion criteria 1. Underwent a standard Whipple's procedure 2. Had distant metastases 3. Vascular invasion could not be documented during dissection | |
| Interventions | Participants were randomly assigned to two groups Group 1: surgical resection (n = 27) Further details: Patients underwent en bloc splenopancreatectomy which involved total pancreatectomy, splenectomy, and end‐to‐end portal venous mesenteric repair Group 2: palliative treatment (n = 29) Further details: Palliative bypass surgery with chemoimmunoradiotherapy. Chemotherapy included a 5‐day course which included gemcitabine, carboplatin, mitoxantrone hydrochloride, mitomycin, fluorouracil and folinic acid. Immunotherapy involved interleukin‐2. Adjuvant chemoimmunotherapy was carried out every 2 months for the first year, every 4 months for the 2nd and 3rd years and every 6 months for the 4th and 5th years | |
| Outcomes | The outcome reported was survival | |
| Notes | Attempts were made to contact the authors in January 2013. The author replied about the experience of the surgeons | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomly divided into two groups, A and B, by the anaesthetist, according to the chosen sealed envelope in the operating theatre" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: It is impossible to blind the healthcare providers |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available |
| Selective reporting (reporting bias) | Low risk | Comment: Survival was reported |
| Vested interest bias | Unclear risk | Comment: This information was not available |
| Expertise bias | Low risk | Comment: The pancreatic surgery was performed by experienced surgeons (implied in author's replies) |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Lygidakis 1995 | Not a randomised controlled trial |
| Lygidakis 1998 | Not locally advanced pancreatic cancer |
Contributions of authors
KS Gurusamy wrote the review, assessed the trials for inclusion and extracted data on included trials at the review stage.
S Kumar contributed to the background, independently assessed the trials for inclusion and extracted data on included trials at the review stage.
BR Davidson and G Fusai were involved in the design of the review and critically commented on the review, and provided advice for improving the review.
Sources of support
Internal sources
None, Other.
External sources
-
National Institute for Health Research (NIHR), UK.
NIHR is the health research wing of the UK Government. It part funds Dr K Gurusamy's salary and funds all the materials needed for the preparation of this review.
Declarations of interest
None
New
References
References to studies included in this review
Doi 2008 {published data only}
- Doi R. The final results of a randomized controlled trial on surgery versus radiochemotherapy for pancreatic cancer. Pancreatology 2010;10:35. [Google Scholar]
- Doi R, Imamura M, Hosotani R, Imaizumi T, Hatori T, Takasaki K, et al. Surgery versus radiochemotherapy for resectable locally invasive pancreatic cancer: final results of a randomized multi‐institutional trial. Surgery Today 2008;38(11):1021‐8. [DOI] [PubMed] [Google Scholar]
- Imamura M, Doi R. Treatment of locally advanced pancreatic cancer: should we resect when resectable?. Pancreas 2004;28(3):293‐5. [DOI] [PubMed] [Google Scholar]
- Imamura M, Doi R, Imaizumi T, Funakoshi A, Wakasugi H, Sunamura M, et al. A randomized multicenter trial comparing resection and radiochemotherapy for resectable locally invasive pancreatic cancer. Surgery 2004;136(5):1003‐11. [DOI] [PubMed] [Google Scholar]
Lygidakis 2004 {published data only}
- Lygidakis NJ, Singh G, Bardaxoglou E, Dedemadi G, Sgourakis G, Nestoridis J, et al. Mono‐bloc total spleno‐pancreaticoduodenectomy for pancreatic head carcinoma with portal‐mesenteric venous invasion. A prospective randomized study. Hepatogastroenterology 2004;51(56):427‐33. [PubMed] [Google Scholar]
References to studies excluded from this review
Lygidakis 1995 {published data only}
- Lygidakis NJ, Papadopoulou P. Pancreatic head carcinoma: Is pancreatic resection indicated for patients with stage III pancreatic duct cancer?. Hepatogastroenterology 1995;42(5):587‐96. [PubMed] [Google Scholar]
Lygidakis 1998 {published data only}
- Lygidakis NJ, Berberabe AE, Spentzouris N, Dedemadi G, Kalligas T, Loukas G, et al. A prospective randomized study using adjuvant locoregional chemoimmunotherapy in combination with surgery for pancreatic carcinoma. Hepatogastroenterology 1998;45(24):2376‐81. [PubMed] [Google Scholar]
Additional references
Abrams 2009
- Abrams RA, Lowy AM, O'Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Annals of Surgical Oncology 2009;16(7):1751‐6. [DOI] [PubMed] [Google Scholar]
Alderson 2005
- Alderson D, Johnson C, Neoptolemos J, Ainley C, Bennett M, Campbell F, et al. Guidelines for the management of patients with pancreatic cancer periampullary and ampullary carcinomas. Gut 2005;54 Suppl 5:v1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bockhorn 2011
- Bockhorn M, Burdelski C, Bogoevski D, Sgourakis G, Yekebas EF, Izbicki JR. Arterial en bloc resection for pancreatic carcinoma. The British Journal of Surgery 2011;98(1):86‐92. [DOI] [PubMed] [Google Scholar]
Cameron 1993
- Cameron JL, Pitt HA, Yeo CJ, Lillemoe KD, Kaufman HS, Coleman J. One hundred and forty‐five consecutive pancreaticoduodenectomies without mortality. Annals of Surgery 1993;217(5):430‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cardenes 2011
- Cardenes HR, Moore AM, Johnson CS, Yu M, Helft P, Chiorean EG, et al. A phase II study of gemcitabine in combination with radiation therapy in patients with localized, unresectable, pancreatic cancer: a Hoosier Oncology Group study. American Journal of Clinical Oncology 2011;34(5):460‐5. [DOI] [PubMed] [Google Scholar]
Chu 2010
- Chu CK, Farnell MB, Nguyen JH, Stauffer JA, Kooby DA, Sclabas GM, et al. Prosthetic graft reconstruction after portal vein resection in pancreaticoduodenectomy: a multicenter analysis. Journal of the American College of Surgeons 2010;211(3):316‐24. [DOI] [PubMed] [Google Scholar]
Conroy 2011
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. The New England Journal of Medicine 2011;364(19):1817‐25. [DOI: 10.1056/NEJMoa1011923] [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Diener 2011
- Diener MK, Fitzmaurice C, Schwarzer G, Seiler CM, Antes G, Knaebel HP, et al. Pylorus‐preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD006053.pub4] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghaneh 2007
- Ghaneh P, Costello E, Neoptolemos JP. Biology and management of pancreatic cancer. Gut 2007;56(8):1134‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
GLOBOCAN 2008
- International Agency for Research on Cancer. GLOBOCAN 2008, 2010. http://globocan.iarc.fr/ (accessed 12 October 2012).
Gurusamy 2009
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. The British Journal of Surgery 2009;96(4):342‐9. [DOI] [PubMed] [Google Scholar]
Gurusamy 2010
- Gurusamy KS, Kumar S, Davidson BR. Prophylactic gastrojejunostomy for unresectable periampullary carcinoma. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD008533.pub2] [DOI] [PubMed] [Google Scholar]
Gurusamy 2012
- Gurusamy KS, Koti R, Fusai G, Davidson BR. Somatostatin analogues for pancreatic surgery. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD008370.pub2] [DOI] [PubMed] [Google Scholar]
Heinemann 2013
- Heinemann V, Haas M, Boeck S. Neoadjuvant treatment of borderline resectable and non‐resectable pancreatic cancer. Annals of Oncology 2013;24(10):2484‐92. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirooka 2009
- Hirooka Y, Itoh A, Kawashima H, Hara K, Nonogaki K, Kasugai T, et al. A combination therapy of gemcitabine with immunotherapy for patients with inoperable locally advanced pancreatic cancer. Pancreas 2009;38(3):e69‐74. [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1996
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Code of Federal Regulation & ICH Guidelines. Media: Parexel Barnett, 1996. [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Liao 2013
- Liao WC, Chien KL, Lin YL, Wu MS, Lin JT, Wang HP, et al. Adjuvant treatments for resected pancreatic adenocarcinoma: a systematic review and network meta‐analysis. Lancet Oncology 2013;14(11):1095‐103. [DOI] [PubMed] [Google Scholar]
Livingston 1991
- Livingston EH, Welton ML, Reber HA. Surgical treatment of pancreatic cancer. The United States experience. International Journal of Pancreatology 1991;9:153‐7. [DOI] [PubMed] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Mollberg 2011
- Mollberg N, Rahbari NN, Koch M, Hartwig W, Hoeger Y, Buchler MW, et al. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta‐analysis. Annals of Surgery 2011;254(6):882‐93. [DOI] [PubMed] [Google Scholar]
Nakai 2012
- Nakai Y, Isayama H, Sasaki T, Sasahira N, Tsujino T, Toda N, et al. A multicentre randomised phase II trial of gemcitabine alone vs gemcitabine and S‐1 combination therapy in advanced pancreatic cancer: GEMSAP study. British Journal of Cancer 2012;106(12):1934‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
Niederhuber 1995
- Niederhuber JE, Brennan MF, Menck HR. The National Cancer Data Base report on pancreatic cancer. Cancer 1995;76(9):1671‐7. [DOI] [PubMed] [Google Scholar]
Nitecki 1995
- Nitecki SS, Sarr MG, Colby TV, Heerden JA. Long‐term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving?. Annals of Surgery 1995;221(1):59‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Orr 2010
- Orr RK. Outcomes in pancreatic cancer surgery. Surgical Clinics of North America 2010;90(2):219‐34. [DOI] [PubMed] [Google Scholar]
Parkin 2001
- Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. European Journal of Cancer 2001;37 Suppl 8:S4‐66. [DOI] [PubMed] [Google Scholar]
Parkin 2005
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: A Cancer Journal for Clinicians 2005;55(2):74‐108. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Recchia 2009
- Recchia F, Sica G, Candeloro G, Bisegna R, Bratta M, Bonfili P, et al. Chemoradioimmunotherapy in locally advanced pancreatic and biliary tree adenocarcinoma: a multicenter phase ii study. Pancreas 2009;38(6):e163‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Sanders 2010
- Sanders MK, Moser AJ, Khalid A, Fasanella KE, Zeh HJ, Burton S, et al. EUS‐guided fiducial placement for stereotactic body radiotherapy in locally advanced and recurrent pancreatic cancer. Gastrointestinal Endoscopy 2010;71(7):1178‐84. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Scott 2009
- Scott EN, Garcea G, Doucas H, Steward WP, Dennison AR, Berry DP. Surgical bypass vs. endoscopic stenting for pancreatic ductal adenocarcinoma. HPB Surgery 2009;11(2):118‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siriwardana 2006
- Siriwardana HP, Siriwardena AK. Systematic review of outcome of synchronous portal‐superior mesenteric vein resection during pancreatectomy for cancer. The British Journal of Surgery 2006;93(6):662‐73. [DOI] [PubMed] [Google Scholar]
Sultana 2007
- Sultana A, Smith CT, Cunningham D, Starling N, Neoptolemos JP, Ghaneh P. Meta‐analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. Journal of Clinical Oncology 2007;25(18):2607‐15. [DOI] [PubMed] [Google Scholar]
Tachezy 2011
- Tachezy M, Bockhorn M, Gebauer F, Vashist YK, Kaifi JT, Izbicki JR. Bypass surgery versus intentionally incomplete resection in palliation of pancreatic cancer: is resection the lesser evil?. Journal of Gastrointestinal Surgery 2011;15(5):829‐35. [DOI] [PubMed] [Google Scholar]
Trede 1990
- Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Annals of Surgery 1990;211(4):447‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tucker 2008
- Tucker ON, Rela M. Controversies in the management of borderline resectable proximal pancreatic adenocarcinoma with vascular involvement. HPB Surgery 2008;2008:839503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamamoto 1998
- Yamamoto M, Ohashi O, Saitoh Y. Japan Pancreatic Cancer Registry: current status. Pancreas 1998;16(3):238‐42. [DOI] [PubMed] [Google Scholar]
Zhou 2012
- Zhou Y, Zhang Z, Liu Y, Li B, Xu D. Pancreatectomy combined with superior mesenteric vein‐portal vein resection for pancreatic cancer: a meta‐analysis. World Journal of Surgery 2012;36(4):884‐91. [DOI] [PubMed] [Google Scholar]


