Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical trial updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
We present the final prespecified overall survival (OS) analysis of the open-label, phase III CLEAR study in treatment-naïve patients with advanced renal cell carcinoma (aRCC). With an additional follow-up of 23 months from the primary analysis, we report results from the lenvatinib plus pembrolizumab versus sunitinib comparison of CLEAR. Treatment-naïve patients with aRCC were randomly assigned to receive lenvatinib (20 mg orally once daily in 21-day cycles) plus pembrolizumab (200 mg intravenously once every 3 weeks) or sunitinib (50 mg orally once daily [4 weeks on/2 weeks off]). At this data cutoff date (July 31, 2022), the OS hazard ratio (HR) was 0.79 (95% CI, 0.63 to 0.99). The median OS (95% CI) was 53.7 months (95% CI, 48.7 to not estimable [NE]) with lenvatinib plus pembrolizumab versus 54.3 months (95% CI, 40.9 to NE) with sunitinib; 36-month OS rates (95% CI) were 66.4% (95% CI, 61.1 to 71.2) and 60.2% (95% CI, 54.6 to 65.2), respectively. The median progression-free survival (95% CI) was 23.9 months (95% CI, 20.8 to 27.7) with lenvatinib plus pembrolizumab and 9.2 months (95% CI, 6.0 to 11.0) with sunitinib (HR, 0.47 [95% CI, 0.38 to 0.57]). Objective response rate also favored the combination over sunitinib (71.3% v 36.7%; relative risk 1.94 [95% CI, 1.67 to 2.26]). Treatment-emergent adverse events occurred in >90% of patients who received either treatment. In conclusion, lenvatinib plus pembrolizumab achieved consistent, durable benefit with a manageable safety profile in treatment-naïve patients with aRCC.
Final OS analysis of CLEAR confirms treatment benefit of LEN + PEMBRO versus sunitinib in 1L advanced RCC
INTRODUCTION
At the primary analysis point of the phase III open-label, CLEAR study (Study 307/KEYNOTE-581), with a median survival follow-up of 26.6 months, lenvatinib plus pembrolizumab showed superior efficacy versus sunitinib in the first-line treatment of patients with advanced renal cell carcinoma (aRCC).1 Lenvatinib plus pembrolizumab showed significant improvements versus sunitinib in progression-free survival (PFS: final analysis; hazard ratio [HR], 0.39 [95% CI, 0.32 to 0.49]; P < .001) and overall survival (OS: interim analysis; HR, 0.66 [95% CI, 0.49 to 0.88]; P = .005). The objective response rate (ORR) was also improved with the combination versus sunitinib (relative risk, 1.97 [95% CI, 1.69 to 2.29]), and the safety profile was consistent with the known safety profiles of each monotherapy.1-3 An update with an additional follow-up of 7 months supported these efficacy results.4
Here, we present the results of the final prespecified OS analysis (median survival follow-up, about 4 years) for the lenvatinib plus pembrolizumab versus sunitinib comparison from CLEAR, along with updated PFS, PFS on the next line of therapy (PFS2), ORR, exposure, and safety results.
METHODS
The CLEAR study design was described previously1 (Data Supplement, online only). The protocol and related documents were approved by institutional review boards or independent ethics committees. All patients provided written informed consent.
This final prespecified OS analysis (data cutoff: July 31, 2022), with an additional follow-up of 23 months beyond the primary analysis (data cutoff: August 28, 2020), was triggered by approximately 304 OS events in the two groups. Analyses presented are descriptive and noninferential; P values are nominal. HR and two-sided 95% CI were estimated using a stratified Cox proportional hazards model with Efron's method for ties, stratified by region and Memorial Sloan Kettering Cancer Center (MSKCC) prognostic groups. Median OS and OS rate with two-sided 95% CIs were calculated using Kaplan-Meier product-limit estimates. A post hoc two-stage estimation method, adjusting for the impact of subsequent anticancer medications on OS, was applied separately (Data Supplement).
Updated PFS, ORR, and duration of response (DOR) were determined by independent imaging review (IIR), and PFS2 by investigator review, both per RECIST version 1.1 (RECIST v1.1). Methodologies used in all prespecified analyses here were identical to those used in the primary analysis.1 Efficacy was assessed in all randomly assigned patients regardless of the treatment received; safety was assessed in all patients who received at least one dose of study drug.
RESULTS
Disposition and Baseline Characteristics
At this data cutoff date (July 31, 2022), 58 (16.3%) patients in the lenvatinib plus pembrolizumab group and 24 (6.7%) patients in the sunitinib group remained on study treatment (Data Supplement, Fig S1). Most patients in this study belonged to the intermediate (MSKCC/International Metastatic Renal Cell Carcinoma Database Consortium [IMDC]) risk groups (Table 1). In the lenvatinib plus pembrolizumab group, approximately 34% of patients completed the full 35 cycles of pembrolizumab treatment and continued lenvatinib monotherapy.
TABLE 1.
Summary of Baseline Characteristics of Patients in the CLEAR Study and Updated ORR by IIR per RECIST v1.1
| Parametera | Lenvatinib + Pembrolizumab (n = 355) | Sunitinib (n = 357) |
|---|---|---|
| Age, years, median (range) | 64 (34-88) | 61 (29-82) |
| Geographic region, No. (%) | ||
| Western Europe or North America | 198 (55.8) | 199 (55.7) |
| Rest of the world | 157 (44.2) | 158 (44.3) |
| MSKCC prognostic risk group,b No. (%) | ||
| Favorable | 96 (27.0) | 97 (27.2) |
| Intermediate | 227 (63.9) | 228 (63.9) |
| Poor | 32 (9.0) | 32 (9.0) |
| IMDC risk group,c No. (%) | ||
| Favorable | 110 (31.0) | 124 (34.7) |
| Intermediate | 210 (59.2) | 192 (53.8) |
| Poor | 33 (9.3) | 37 (10.4) |
| Sarcomatoid features, No. (%) | 28 (7.9) | 21 (5.9) |
| PD-L1 expression,d No. (%) | ||
| ≥1 | 107 (30.1) | 119 (33.3) |
| <1 | 112 (31.5) | 103 (28.9) |
| Not available | 136 (38.3) | 135 (37.8) |
| Previous nephrectomy, No. (%) | 262 (73.8) | 275 (77.0) |
| Updated efficacy analysis | ||
| Best overall response, No. (%) | ||
| CR | 65 (18.3) | 17 (4.8) |
| Near CRe | 59 (16.6) | 25 (7.0) |
| PR | 188 (53.0) | 114 (31.9) |
| Stable disease | 67 (18.9) | 134 (37.5) |
| Progressive disease | 19 (5.4) | 50 (14.0) |
| Unknown/not evaluable | 16 (4.5) | 42 (11.8) |
| ORR (CR + PR), No. (%) | 253 (71.3) | 131 (36.7) |
| 95% CIf | 66.6 to 76.0 | 31.7 to 41.7 |
| Difference, % (95% CI)f | 34.6 (27.7 to 41.4) | |
| OR (95% CI)g | 4.31 (3.14 to 5.92) | |
| Relative risk (95% CI)g | 1.94 (1.67 to 2.26) | |
| Nominal P value | <.0001 | |
| Time to first objective response (months) | ||
| Patients with objective response, No. | 253 | 131 |
| Mean (standard deviation) | 3.38 (2.897) | 3.71 (3.963) |
| Median | 1.94 | 1.97 |
| Q1, Q3 | 1.87, 3.75 | 1.87, 3.75 |
| Minimum, maximum | 1.41, 22.60 | 1.61, 34.96 |
Abbreviations: CR, complete response; DOR, duration of response; HR, hazard ratio; IHC, immunohistochemistry; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; IxRS, interactive voice/web response system; MSKCC, Memorial Sloan Kettering Cancer Center; NE, not estimable; NR, not reached; OR, odds ratio; ORR, objective response rate; OS, overall survival; PFS; progression-free survival; PR, partial response.
Percentages may not total 100 because of rounding. One patient in the lenvatinib plus pembrolizumab group had carcinoma without a clear-cell component. Patients randomly assigned to receive lenvatinib plus pembrolizumab received lenvatinib at a starting dose of 20 mg orally once daily in 21-day cycles plus pembrolizumab at a dose of 200 mg intravenously once every 3 weeks (on day 1 of each 21-day cycle). Patients randomly assigned to receive sunitinib received a starting dose of 50 mg orally once every day (4 weeks on/2 weeks off).
An MSKCC score of 0 indicates favorable risk, a score of 1 or 2 intermediate risk, and a score of 3 or higher poor risk.
An IMDC score of 0 indicates favorable risk, a score of 1 or 2 intermediate risk, and a score of 3 to 6 poor risk.
PD-L1 expression was assessed using the PD-L1 IHC 22C3 pharmDx assay (Agilent Technologies, Santa Clara, CA) and reported as the combined positive score, defined as the number of PD-L1–staining cells (tumor cells, lymphocytes, and macrophages) divided by the total number of viable tumor cells, then multiplied by 100.
Partial response with a ≥75% change from baseline in sum of target lesion diameters.
95% CI is constructed using the method of normal approximation.
OR and relative risk are calculated using the Cochran-Mantel-Haenszel method, stratified by IxRS stratification factors.
OS
At the median OS follow-up time (IQR) of 49.8 months (IQR, 41.4-53.1) in the lenvatinib plus pembrolizumab group and 49.4 months (IQR, 41.6-52.8) in the sunitinib group, 308 OS events had occurred (lenvatinib plus pembrolizumab, 149; sunitinib, 159). With an HR of 0.79 (95% CI, 0.63 to 0.99; nominal P value = .0424), the median OS (95% CI) was 53.7 months (95% CI, 48.7 to not estimable [NE]) in the lenvatinib plus pembrolizumab group versus 54.3 months (95% CI, 40.9 to NE) in the sunitinib group (Fig 1A); OS rates at 36 months (95% CI) were 66.4% (95% CI, 61.1 to 71.2) and 60.2% (95% CI, 54.6 to 65.2), respectively. In patients who completed 35 cycles of pembrolizumab and continued lenvatinib monotherapy, the OS rate at 36 months was 94.2% (95% CI, 88.2 to 97.2; Data Supplement, Fig S2).
FIG 1.
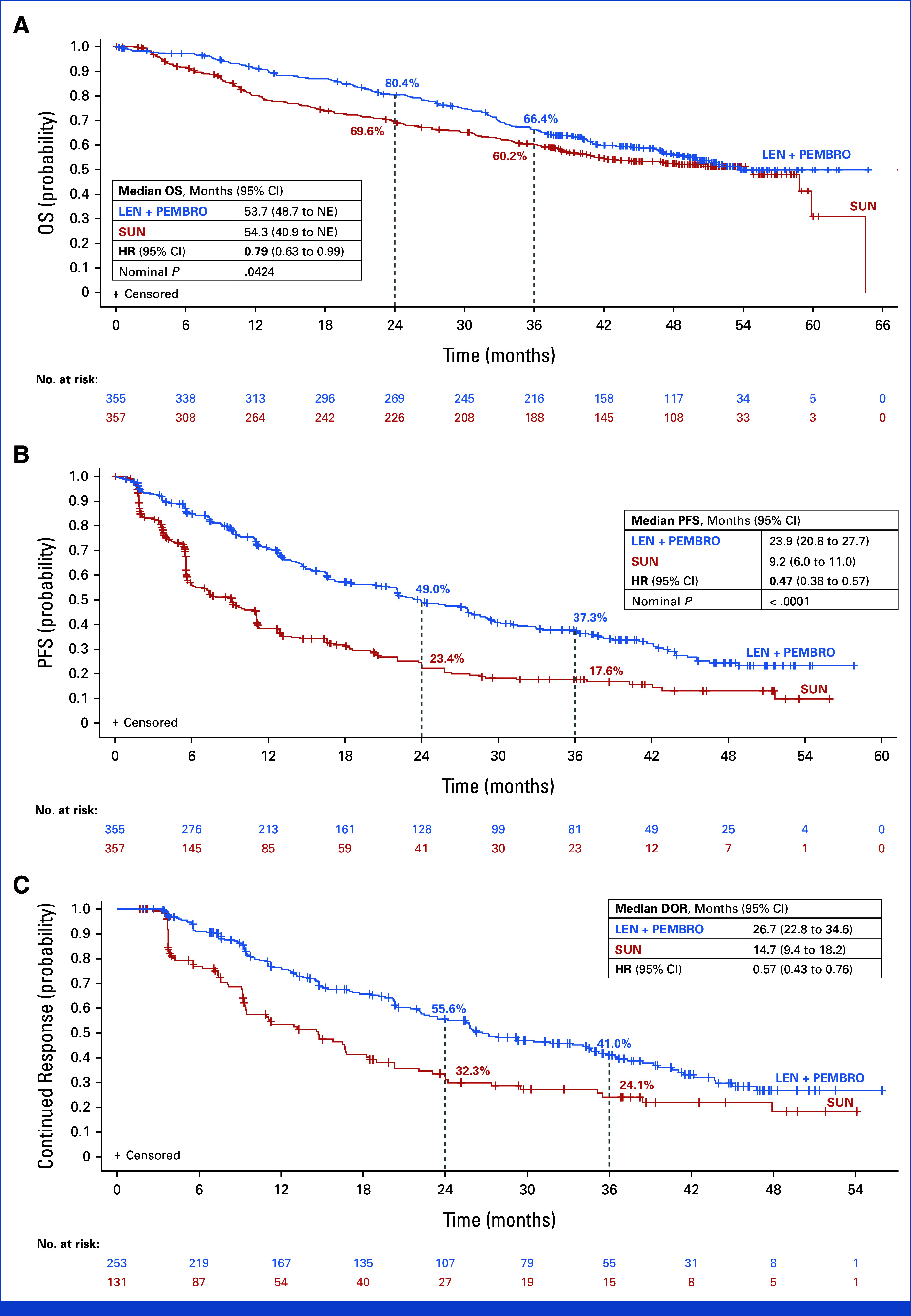
Kaplan-Meier plots of final analysis of (A) OS (unadjusted), (B) PFS by IIR per RECIST v1.1, and (C) duration of response by IIR per RECIST v1.1. The 95% CIs are estimated using a generalized Brookmeyer and Crowley method. HR is based on a Cox proportional hazards model including the treatment group as a factor; the Efron method was used for ties and stratified by geographic region (region 1: Western Europe and North America; region 2: Rest of the world) and MSKCC prognostic groups (favorable, intermediate, and poor risk) in IxRS. CR, complete response; DOR, duration of response; HR, hazard ratio; IIR, independent imaging review; IxRS, interactive voice/web response system; LEN, lenvatinib; MSKCC, Memorial Sloan Kettering Cancer Center; NE, not estimable; OS, overall survival; PEMBRO, pembrolizumab; PFS, progression-free survival; PR, partial response; SUN, sunitinib.
Fewer patients in the lenvatinib plus pembrolizumab group (51.0%) received subsequent anticancer medications compared with those in the sunitinib group (68.9%); the proportion of patients who received PD-1/PD-L1 inhibitors as subsequent therapy in the lenvatinib plus pembrolizumab group was more than three times lower than that in the sunitinib group (15.8% v 54.6%, respectively; Data Supplement, Table S1). A two-stage estimation was used to assess the impact of subsequent anticancer therapies, which are known confounders of OS estimates5 (Data Supplement). The adjusted OS HR for lenvatinib plus pembrolizumab versus sunitinib was 0.55 (95% CI, 0.44 to 0.69; Data Supplement, Fig S3A).
Lenvatinib plus pembrolizumab improved OS compared with sunitinib in both the intermediate- plus poor-risk IMDC (HR, 0.74 [95% CI, 0.57 to 0.96]; Fig 2A) and MSKCC (HR, 0.77 [95% CI, 0.60 to 0.99]) subgroups. OS was similar in the favorable-risk group; however, there were a low number of events in this subgroup and these analyses were not powered to detect such differences (Fig 2A; Data Supplement, Figs S4A and S5).
FIG 2.
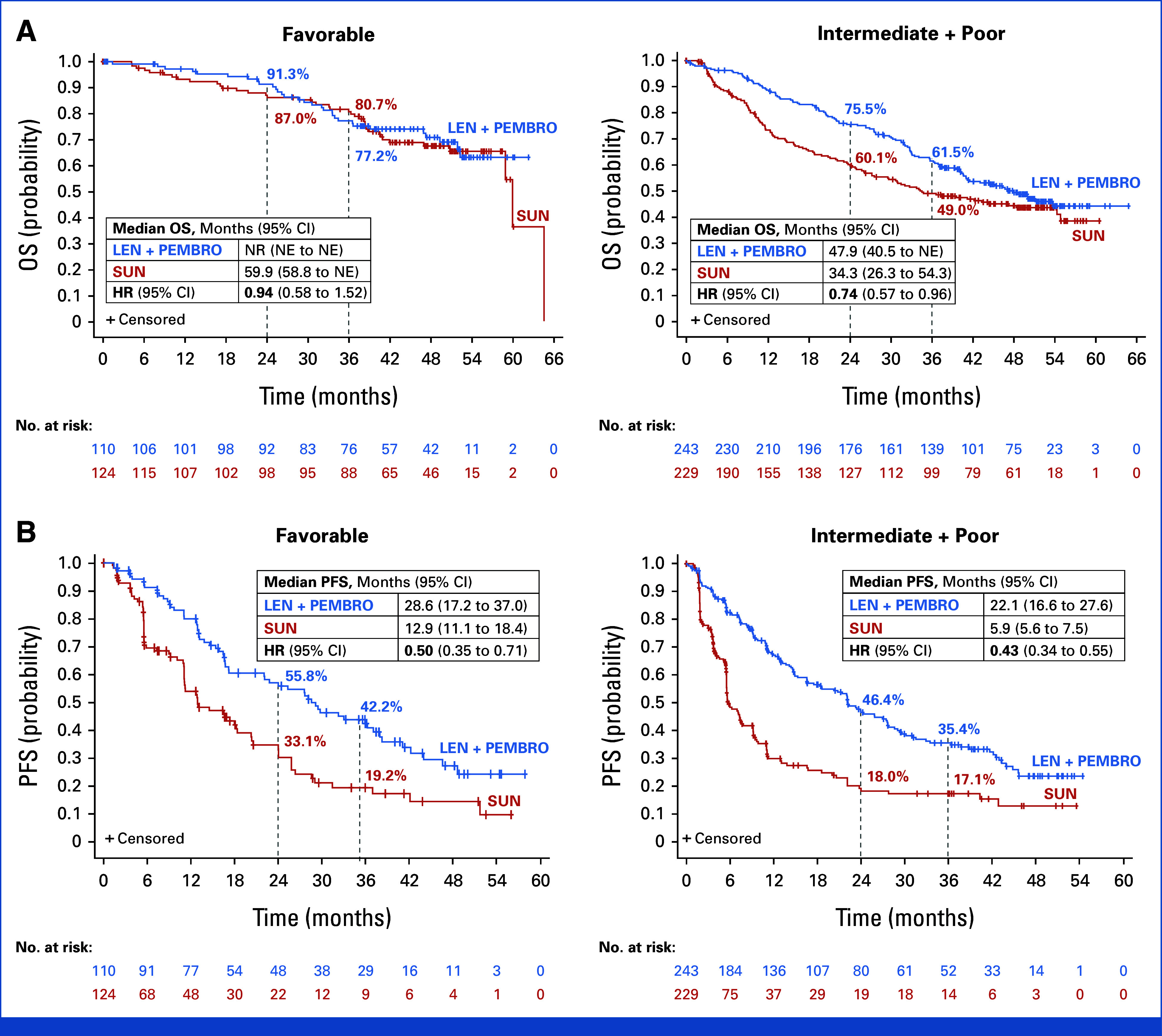
Kaplan-Meier plots of (A) final OS and (B) PFS analysis in favorable-risk and intermediate- plus poor-risk IMDC subgroups. PFS was determined by independent imaging review per RECIST v1.1. The IMDC risk group was not a stratification factor, and relevant data were derived programmatically. Medians were estimated using the Kaplan-Meier method, and 95% CIs were estimated using a generalized Brookmeyer and Crowley method. HR is based on a Cox proportional hazards model including the treatment group as a factor; the Efron method was used for ties. Stratification factors were geographic region (region 1: Western Europe and North America, region 2: Rest of the world) and MSKCC prognostic groups (favorable, intermediate, and poor risk) in IxRS. HR, hazard ratio; IMDC, International Metastatic Renal Cell Carcinoma Database Consortium; IxRS, interactive web/voice-response system; LEN, lenvatinib; MSKCC, Memorial Sloan Kettering Cancer Center; NE, not estimable; NR, not reached; OS, overall survival; PEMBRO, pembrolizumab; PFS, progression-free survival; SUN, sunitinib.
PFS
PFS events occurred in 207 patients in the lenvatinib plus pembrolizumab group and 214 patients in the sunitinib group. The median follow-up time (IQR) for PFS was 39.2 months (IQR, 22.1-48.5) with lenvatinib plus pembrolizumab and 20.6 months (IQR, 5.5-41.2) with sunitinib. The median PFS (95% CI) was 23.9 months (95% CI, 20.8 to 27.7) with lenvatinib plus pembrolizumab and 9.2 months (95% CI, 6.0 to 11.0) with sunitinib (HR, 0.47 [95% CI, 0.38 to 0.57]; nominal P value < .0001; Fig 1B). PFS favored lenvatinib plus pembrolizumab over sunitinib across all IMDC and MSKCC risk subgroups (Fig 2B; Data Supplement, Figs S4B and S6).
The median PFS2 (95% CI) was 43.3 months (95% CI, 37.2 to 50.4) with lenvatinib plus pembrolizumab and 25.9 months (95% CI, 21.3 to 32.0) with sunitinib (HR, 0.63 [95% CI, 0.51 to 0.77]; Data Supplement, Fig S3B).
ORR
The ORR (95% CI) was 71.3% (95% CI, 66.6 to 76.0) with lenvatinib plus pembrolizumab and 36.7% (95% CI, 31.7 to 41.7) with sunitinib (relative risk, 1.94 [95% CI, 1.67 to 2.26]; nominal P value < .0001; Table 1). Complete responses were achieved by 65 patients (18.3%) in the lenvatinib plus pembrolizumab group and 17 patients (4.8%) in the sunitinib group. A best overall response of progressive disease was reported in 19 patients (5.4%) in the lenvatinib plus pembrolizumab group and 50 patients (14.0%) in the sunitinib group. In responders, the median DOR (95% CI) was 26.7 months (95% CI, 22.8 to 34.6) with lenvatinib plus pembrolizumab and 14.7 months (95% CI, 9.4 to 18.2) with sunitinib (Fig 1C).
Exposure and Safety
The median overall duration of treatment (range) in the safety analysis population was 22.6 months (range, 0.1-62.1) in patients who received lenvatinib plus pembrolizumab (352 patients) and 7.8 months (range, 0.1-57.5) in patients who received sunitinib (340 patients).
In both groups, most patients had treatment-emergent adverse events (TEAEs; Data Supplement, Table S2). Grade ≥3 TEAEs occurred in 84.9% of patients treated with lenvatinib plus pembrolizumab and 74.7% of patients treated with sunitinib. Diarrhea was the most common TEAE, and hypertension was the most common grade ≥3 TEAE across treatment groups (Data Supplement, Table S2). Fatal TEAEs occurred in 16 (4.5%) patients treated with lenvatinib plus pembrolizumab and 12 (3.5%) patients treated with sunitinib (Data Supplement, Table S3); fatal treatment-related adverse events occurred in 4 (1.1%) and 1 (0.3%) patient(s) in respective groups (Data Supplement, Table S4).
DISCUSSION
In the final prespecified OS analysis, with a median OS follow-up time of about 4 years, lenvatinib plus pembrolizumab continued to show clinically meaningful efficacy compared with sunitinib as a first-line treatment for aRCC. The OS HR of 0.79 (95% CI, 0.63 to 0.99) represents a 21% reduction in the risk of death. The 36-month OS rate of 66.4% with lenvatinib plus pembrolizumab versus 60.2% with sunitinib highlights the sustained benefit with the combination treatment over sunitinib.
PFS, ORR, and response duration benefits for lenvatinib plus pembrolizumab versus sunitinib were maintained with long-term follow-up and highlight the magnitude and durability of response for this treatment combination. The ORR (71.3%) and percentage of patients with a complete response (18.3%) with lenvatinib plus pembrolizumab treatment are notable.
Safety results were consistent with those from the primary analysis and with the established safety profiles of each monotherapy and of the combination in patients with other solid tumors.2,3,6-8 In conclusion, lenvatinib plus pembrolizumab achieved consistent, durable benefit with a manageable safety profile in treatment-naïve patients with aRCC.
ACKNOWLEDGMENT
We would like to thank all the patients, their families, and/or caregivers, as well as the investigators and their teams who participated in the CLEAR study. We would like to thank the late Dr Anil Kapoor for his contributions to this study. Patients treated at the Memorial Sloan Kettering Cancer Center were supported in part by Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748). We would also like to thank Rodolfo Perini (Merck & Co, Inc, Rahway, NJ) and Corina Ductus (Eisai Inc) for their contributions to this work.
CLEAR Trial Investigators are listed in Appendix Table A1 (online only).
APPENDIX
TABLE A1.
CLEAR Study List of Countries, Sites, and Investigators
| Country | Site Name | Principal Investigator |
|---|---|---|
| United States | Memorial Sloan Kettering Cancer Center | Robert J. Motzer |
| Dana-Farber Cancer Institute | Toni Choueiri | |
| Texas Oncology, P.A. | Thomas Hutson | |
| GU Research Network | Luke Nordquist | |
| SCRI – Tennessee Oncology | David Spigel | |
| University of Miami | Jaime Merchan | |
| Roswell Park Comprehensive Cancer Center | Saby George | |
| Stanford School of Medicine | Sandhya Srinivas | |
| Providence Portland Medical Center | Brendan Curti | |
| Centricity Research IRT – DBA IACT Health Columbus Regional Research Institute | Andrew Pippas | |
| Karmanos Cancer Center | Elisabeth Heath | |
| Healthcare Research Network III, LLC | Subramanya Rao | |
| Medical University of South Carolina (MUSC) | Theodore Gourdin | |
| Cotton-O'Neil Clinical Research Center, Hematology and Oncology | Mehmood Hashmi | |
| Duly Health and Care | Nafisa Burhani | |
| Weill Cornell Medical College New York Presbyterian Hospital | Ana Molina | |
| Boca Raton Community Hospital | Alan Koletsky | |
| Temple University Hospital | Robert Alter | |
| AdventHealth | Carlos Alemany | |
| Montefiore Medical Center PRIME | Benjamin Gartrell | |
| Mount Sinai Medical Center | Mike Cusnir | |
| Cancer Center of Middle Georgia | Harsha Vyas | |
| Health Midwest Ventures Group, Inc d/b/a HCA MidAmerica Division, LLC | Stephanie Graff | |
| Cooper Research Institute | Christian Squillante | |
| Mid Ohio Oncology Hematology, Inc | Mark Knapp | |
| Florida Cancer Specialists North | Ivor Percent | |
| Florida Cancer Specialists North | Vijay Patel | |
| Florida Cancer Specialists East | Daniel Spitz | |
| Mission Hospital | Cameron Harkness | |
| Ochsner Clinic Foundation | Marc Matrana | |
| Wenatchee Valley Hospital & Clinics | Lindsay Overton | |
| Texas Oncology | Stephen Richey | |
| Texas Oncology, P.A. – Tyler | Donald Richards | |
| Texas Oncology McAllen | Habib Ghaddar | |
| Illinois Cancer Specialists | Robert Galamaga | |
| Nebraska Cancer Specialists | Ralph Hauke | |
| Associates in Oncology & Hematology, PC | Joseph Haggerty | |
| Broome Oncology, LLC | Ronald Harris | |
| Oncology/Hematology Care Clinical Trials, LLC | Mark Johns | |
| Minnesota Oncology Hematology, P.A. | Samith Kochuparambil | |
| Canada | BC Cancer – Vancouver | Christian Kollmannsberger |
| St Joseph's Healthcare Hamilton | Bobby Shayegan | |
| The Ottawa Hospital Cancer Center | Christina Canil | |
| London Health Sciences Center (LHSC) – Victoria Hospital | Eric Winquist | |
| Centre de santé et de services sociaux Champlain-Charles-Le Moyne | Catherine Sperlich | |
| Sunnybrook Research Institute | Georg Bjarnason | |
| Cross Cancer Institute | Naveen Basappa | |
| Austria | Ordensklinikum Linz GmbH Elisabethinen | Wolfgang Loidl |
| Medizinische Universität Innsbruck | Wolfgang Horninger | |
| AKH – Medizinische Universität Wien | Manuela Schmidinger | |
| Belgium | CHU UCL Namur | Lionel D'Hondt |
| ZNA Middelheim | Dirk Schrijvers | |
| GZA Ziekenhuizen | Annemie Rutten | |
| Onze Lieve Vrouw Ziekenhuis | Peter Schatteman | |
| Imeldaziekenhuis | Wim Wynendaele | |
| ETC Jessa Ziekenhuis | Daisy Luyten | |
| Institut Jules Bordet | Spyridon Sideris | |
| CHU de Liège | Christine Gennigens | |
| Czech Republic | Fakultni nemocnice Olomouc | Bohuslav Melichar |
| Fakultni nemocnice u sv. Anny v Brne | Jana Katolicka | |
| Masarykuv onkologicky ustav | Jiri Tomasek | |
| Fakultni nemocnice v Motole | Jana Prausova | |
| Fakultni Thomayerova nemocnice | Tomas Buchler | |
| Fakultni nemocnice Bulovka | Petra Holeckova | |
| France | CHU Strasbourg – Nouvel Hôpital Civil | Philippe Barthelemy |
| Institut du Cancer de Montpellier | Diego Tosi | |
| Groupe Hospitalier Pitie-Salpetriere | Baptiste Abbar | |
| Centre Leon Berard | Sylvie Negrier | |
| Hôpital Européen Georges Pompidou | Stephane Oudard | |
| Clinique Victor Hugo – Centre Jean Bernard | Eric Voog | |
| Centre Georges François Leclerc | Sylvie Zanetta | |
| ICO – Site Paul Papin | Frederic Rolland | |
| Germany | Universitaetsklinikum Tuebingen | Jens Bedke |
| Universitaetsklinikum des Saarlandes | Stefan Siemer | |
| Universitaetsklinikum Carl Gustav Carus TU Dresden | Manfred Wirth | |
| Klinikum Stuttgart-Katharinenhospital | Jan Schleicher | |
| Charité – Campus Charité Mitte | Maria De Santis | |
| Universitaetsklinikum Frankfurt Goethe-Universitaet | Lothar Bergmann | |
| LMU-Campus Grosshadern | Michael Staehler | |
| Medizinische Hochschule Hannover | Philipp Ivanyi | |
| Praxis f. Haematologie und Onkologie Koblenz | Christoph Lutz | |
| Universitaetsklinikum Hamburg-Eppendorf | Gunhild Von Amsberg | |
| Universitaetsklinikum Muenster | Martin Boegemann | |
| Universitaetsmedizin Greifswald | Uwe Zimmermann | |
| Ireland | Adelaide and Meath Hospital Incorp The National Children's Hospital | Raymond McDermott |
| Cork University Hospital | Richard Bambury | |
| University Hospital Galway | Paul Donnellan | |
| Beaumont Hospital | Oscar Breathnach | |
| Israel | Shamir Medical Center (Assaf Harofe) | Raya Leibowitz-Amit |
| Sapir Medical Center, Meir Hospital | Olesya Goldman | |
| Rambam Health Care Campus | Avivit Peer | |
| Tel Aviv Sourasky Medical Center | David Sarid | |
| Hadassah University Hospital – Ein Kerem | Hovav Nechushtan | |
| Chaim Sheba Medical Center | Raanan Berger | |
| Rabin MC | Victoria Neiman | |
| Italy | Azienda Ospedaliera San Camillo Forlanini | Fabio Calabro |
| Fondazione IRCCS Policlinico San Matteo | Paolo Pedrazzoli | |
| IRCCS Opedale Policlinico San Martino-IST | Francesco Boccardo | |
| Ospedale San Donato – ASL 8 Arezzo | Alketa Hamzaj | |
| Azienda Ospedaliera di Rilievo Nazionale A. Cardarelli | Ferdinando Riccardi | |
| IRCCS Istituto Scientifico Romagnolo Per Lo Studio Dei Tumori “Dino Amadori”—IRST | Ugo De Giorgi | |
| Istituto Nazionale Tumori Fondazione G. Pascale | Sandro Pignata | |
| Azienda Ospedaliera Santa Maria Degli Angeli | Sandra Santarossa | |
| Azienda Ospedaliera Universitaria Policlinico Sant'Orsola Malpighi IRCCS | Francesco Massari | |
| Università Campus Bio-Medico di Roma | Giuseppe Tonini | |
| Presidio Ospedaliero Vito Fazzi | Caterina Accettura | |
| Azienda Unità Sanitaria Locale- Ravenna | Francesco Carrozza | |
| A.O.U. Policlinico di Modena | Roberto Sabbatini | |
| Fondazione IRCCS Istituto Nazionale dei Tumori | Elena Verzoni | |
| I.R.C.S.S Fondazione Maugeri | Elisa Biscaldi | |
| The Netherlands | UMC Utrecht | Britt Suelmann |
| Amsterdam UMC, Locatie VUMC | Alfonsus van den Eertwegh | |
| Antoni van Leeuwenhoek | Hans van Thienen | |
| Poland | Instytut MSF Sp. o.o | Ewa Kalinka |
| Uniwersyteckie Centrum Kliniczne | Jacek Jassem | |
| SPWSZ w Szczecinie im. Marii Sklodowskiej-Curie | Violetta Sulzyc-Bielicka | |
| Dr n med. Slawomir Mandziuk Specjalistyczna Praktyka Lekarska | Slawomir Mandziuk | |
| Russian Federation | FSBSI “Russian Oncological Scientific Center n.a. N.N. Blokhin” | Sergei Tjulandin |
| FSBI “National Medical Research Radiological Center” of the MoH of the RF | Oleg Karyakin | |
| FBHI Privolzhskiy District Medical Center FMBA of Russia | Anna Alyasova | |
| FSBI “Moscow scientific research oncology institute n.a. P.A. Gertsen” of MoH of RF | Boris Alekseev | |
| Medicinskiy gorod | Alexander Zyrianov | |
| FSBSI “Russian Oncological Scientific Center n.a. N.N. Blokhin” | Vsevolod Matveev | |
| BHI of Omsk region “Clinical Oncology Dispensary” | Evgeny Kopyltsov | |
| SBHI of Novosibirsk region “Novosibirsk Regional Oncological Dispensary” | Vadim Kozlov | |
| Spain | Hospital General Universitario Gregorio Marañon | Jose Angel Arranz Arija |
| Hospital San Pedro de Alcantara | Pablo Borrega Garcia | |
| Instituto Valenciano de Oncologia IVO | Miguel Angel Climent Duran | |
| Hospital Universitario Virgen del Rocio | Begona Perez Valderrama | |
| Hospital Universitario Central de Asturias | Emilio Esteban Gonzalez | |
| ICO l'Hospitalet – Hospital Duran i Reynals | Francisco Javier Garcia del Muro Solans | |
| Hospital Universitario HM Madrid Sanchinarro | Jesus Garcia-Donas Jimenez | |
| Hospital Universitario Ramon y Cajal | Teresa Alonso Gordoa | |
| Hospital de la Santa Creu i Sant Pau | Jose Pablo Maroto Rey | |
| Hospital Clinic de Barcelona | Begoña Mellado Gonzalez | |
| Hospital Universitario Reina Sofia | Maria Jose Mendez Vidal | |
| Hospital Universitario Clinico San Carlos | Javier Puente Vazquez | |
| Hospital Universitari Vall d'Hebron | Cristina Suarez Rodriguez | |
| MD Anderson Cancer Center | Enrique Grande Pulido | |
| Complejo Asistencial Universitario de Burgos | Guillermo Crespo | |
| Hospital Universitario Lucus Augusti | Natalia Fernandez Nuñez | |
| Hospital Universitario Marques de Valdecilla | Ignacio Duran Martinez | |
| Switzerland | Inselspital – Universitaetsspital Bern | Joerg Beyer |
| Kantonsspital Winterthur | Natalie Fischer | |
| United Kingdom | Beatson West of Scotland Cancer Centre | Hilary Glen |
| Velindre Cancer Centre | Ricky Frazer | |
| The Christie Hospital | Jennifer Allison | |
| Royal Free Hospital | Thomas Powles | |
| Western General Hospital | Jahangeer Malik | |
| St James's University Hospital | Christy Ralph | |
| Guy's Hospital | Sarah Rudman | |
| Royal Bournemouth Hospital | Thomas Geldart | |
| Greece | General Hospital of Athens “Alexandra” | Aristotelis Bamias |
| Interbalkan Hospital of Thessaloniki | Sofia Baka | |
| Metropolitan General Hospital | Vassilios Georgoulias | |
| Euromedica General Clinic of Thessaloniki | Konstantinos Papazisis | |
| University Hospital of Patra | Haralabos Kalofonos | |
| General Hospital Papageorgiou | Eleni Timotheadou | |
| Republic of Korea | Seoul National University Bundang Hospital | Seok-Soo Byun |
| Asan Medical Center | Bumjin Lim | |
| SEVERANCE HOSPITAL, YONSEI UNIVERSITY | Sun Young Rha | |
| Samsung Medical Center | Seong Il Seo | |
| National Cancer Center | Jinsoo Chung | |
| Seoul National University Hospital | Miso Kim | |
| The Catholic University of Korea, Seoul St Mary's Hospital | Sung-Hoo Hong | |
| Asan Medical Center | Jae Lyun Lee | |
| Samsung Medical Center | Se Hoon Park | |
| Kyungpook National University Chilgok Hospital | Tae Gyun Kwon | |
| Australia | Box Hill Hospital | Ian Davis |
| Sunshine Hospital | Shirley Wong | |
| Royal Hobart Hospital | Ian Byard | |
| Austin Health | Andrew Weickhardt | |
| Macquarie University Hospital | Howard Gurney | |
| Icon Cancer Centre Chermside | Jeffrey Goh | |
| Japan | Hokkaido University Hospital | Takahiro Osawa |
| Sapporo Medical University Hospital | Naoya Masumori | |
| Hirosaki University Hospital | Shingo Hatakeyama | |
| Akita University Hospital | Mitsuru Saito | |
| Niigata University Medical & Dental Hospital | Yoshihiko Tomita | |
| Toranomon Hospital | Yuji Miura | |
| Juntendo University Hospital | Masayoshi Nagata | |
| Nippon Medical School Hospital | Go Kimura | |
| Keio University Hospital | Mototsugu Oya | |
| Tokyo Women's Medical University Hospital | Toshio Takagi | |
| Kyorin University Hospital | Yu Nakamura | |
| Yokohama City University Hospital | Hisashi Hasumi | |
| Kitasato University Hospital | Masatsugu Iwamura | |
| Chiba University Hospital | Akira Komiya | |
| Chiba Cancer Center | Atsushi Komaru | |
| Saitama Medical University International Medical Center | Masafumi Oyama | |
| Nagoya University Hospital | Yoshihisa Matsukawa | |
| Aichi Cancer Center Hospital | Norihito Soga | |
| Osaka Metropolitan University Hospital | Minoru Kato | |
| Kindai University Hospital | Masahiro Nozawa | |
| Nara Medical University Hospital | Makito Miyake | |
| Kobe University Hospital | Yuzo Nakano | |
| Okayama University Hospital | Kohei Edamura | |
| Hiroshima University Hospital | Nobuyuki Hinata | |
| Kagawa University Hospital | Homare Okazoe | |
| Tokushima University Hospital | Masayuki Takahashi | |
| Kyushu University Hospital | Masatoshi Eto | |
| Nagasaki University Hospital | Kojiro Oba | |
| Kanagawa Cancer Center | Takeshi Kishida | |
| University Hospital, Kyoto Prefectural University of Medicine | Osamu Ukimura |
Robert J. Motzer
Consulting or Advisory Role: Eisai, Exelixis, Merck, Genentech/Roche, Incyte, Pfizer, AstraZeneca, EMD Serono, Aveo, Takeda
Research Funding: Pfizer (Inst), Bristol Myers Squibb (Inst), Eisai (Inst), Novartis (Inst), Genentech/Roche (Inst), Exelixis (Inst), Merck (Inst), Aveo (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb
Camillo Porta
Consulting or Advisory Role: Angelini, AstraZeneca, Bristol Myers Squibb, Eisai, Ipsen, MSD
Speakers' Bureau: Bristol Myers Squibb, Ipsen, MSD
Masatoshi Eto
Consulting or Advisory Role: Eisai, Pfizer, Takeda, Merck, MSD, Chugai Pharma
Speakers' Bureau: MSD, Merck, AstraZeneca, Eisai, Ono Pharmaceutical, Takeda, Bristol Myers Squibb, Astellas Pharma, Pfizer, Janssen
Research Funding: Takeda
Thomas Powles
Honoraria: AstraZeneca, Eisai, Merck, Novartis, Pfizer, Roche, Astellas Pharma, BMS GmbH & Co KG, Exelixis, Incyte, Ipsen, Seagen, Merck Serono, Johnson & Johnson/Janssen, MashupMD
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Ipsen, Pfizer, Novartis, Seagen, Roche, Exelixis, MSD, Merck Serono, Astellas Pharma, Johnson & Johnson, Eisai, MashupMD, Merck, Incyte
Research Funding: AstraZeneca, Roche, Bristol Myers Squibb, Exelixis, Ipsen, MSD, Novartis, Pfizer, Seagen, Merck Serono, Astellas Pharma, Johnson & Johnson, Eisai
Travel, Accommodations, Expenses: Pfizer, MSD, AstraZeneca, Roche, Ipsen
Viktor Grünwald
Employment: University Hospital Essen
Stock and Other Ownership Interests: MSD, Bristol Myers Squibb, AstraZeneca, Seagen, Genmab
Honoraria: Bristol Myers Squibb, Pfizer, Ipsen, Eisai, MSD Oncology, Merck Serono, AstraZeneca, EUSA Pharma, Janssen-Cilag, Advanced Accelerator Applications/Novartis, Apogepha, Nanobiotix, Ono Pharmaceutical, Astellas Pharma, Amgen
Consulting or Advisory Role: Bristol Myers Squibb, Pfizer, Novartis, MSD Oncology, Ipsen, Janssen-Cilag, Onkowissen, Cor2Ed, Eisai, Debiopharm Group, PCI Biotech, Gilead Sciences, Cureteq, Oncorena, Synthekine
Research Funding: Amgen (Inst), MSD Oncology (Inst), BMS (Inst), Seagen (Inst), Ipsen (Inst), Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Pfizer, AstraZeneca, Janssen, Merck Serono
Thomas E. Hutson
Employment: Texas Oncology
Honoraria: Pfizer, Astellas Pharma, Bristol Myers Squibb, Exelixis, Eisai, Novartis, Johnson & Johnson, Bayer/Onyx
Consulting or Advisory Role: Bayer/Onyx, Pfizer, Novartis, Astellas Pharma, Johnson & Johnson, Bristol Myers Squibb, Eisai, Exelixis
Speakers' Bureau: Pfizer, Johnson & Johnson, Eisai, Exelixis, Astellas Pharma, Bristol Myers Squibb
Research Funding: Pfizer (Inst), Johnson & Johnson (Inst), Exelixis (Inst), Eisai (Inst), Bristol Myers Squibb (Inst)
Boris Alekseev
Honoraria: AstraZeneca, Astellas Pharma, Eisai, Janssen, Bayer, MSD, Merck, Pfizer, Roche, Bristol Myers Squibb
Consulting or Advisory Role: AstraZeneca, Astellas Pharma, Bayer, Bristol Myers Squibb, Janssen, Merck, Pfizer, MSD, Roche, Eisai
Speakers' Bureau: Janssen, Astellas Pharma, Pfizer, AstraZeneca, Bayer, Merck, Bristol Myers Squibb, MSD, Eisai, Roche
Research Funding: AstraZeneca, Merck, Bayer, Astellas Pharma, Janssen, Bristol Myers Squibb, Pfizer, ICON Clinical Research, Eisai, MSD, Roche
Travel, Accommodations, Expenses: AstraZeneca, Astellas Pharma, Bayer, Bristol Myers Squibb, Janssen, MSD, Pfizer, Sanofi
Sun Young Rha
Consulting or Advisory Role: MSD Oncology, Daiichi Sankyo, Eisai, LG Chem, Eutilex, Astellas Pharma, indivumed, AstraZeneca, Ono Pharmaceutical, Amgen
Speakers' Bureau: Lilly, Eisai, MSD Oncology, BMS/Ono, Amgen, Daiichi Sankyo/UCB Japan, AstraZeneca
Research Funding: MSD Oncology, Bristol Myers Squibb, Eisai, Roche/Genentech, ASLAN Pharmaceuticals, Sillajen, Bayer, Daichii Sankyo, Lilly, AstraZeneca, BeiGene, Zymeworks, Astellas Pharma, Indivumed, Amgen (Inst)
Jaime Merchan
Consulting or Advisory Role: Merck
Research Funding: Corvus Pharmaceuticals (Inst), Genentech/Roche (Inst), Tizona Therapeutics, Inc (Inst), Tocagen (Inst), Vyriad (Inst), Sillajen (Inst), Replimune (Inst), Peloton Therapeutics (Inst), Eisai (Inst), Seattle Genetics/Astellas (Inst), Merck (Inst), Rubius Therapeutics (Inst), BioNTech (Inst), Trishula Therapeutics (Inst), Exelixis (Inst), IMUGENE (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Jeffrey C. Goh
Stock and Other Ownership Interests: Immutep, ICON Cancer Care
Honoraria: MSD Oncology, AstraZeneca
Consulting or Advisory Role: GlaxoSmithKline, MSD, BMS, Janssen Oncology
Speakers' Bureau: Ipsen, Janssen, AstraZeneca/MedImmune, MSD Oncology, Pfizer/EMD Serono, Eisai
Travel, Accommodations, Expenses: Pfizer/EMD Serono, Bayer
Aly-Khan A. Lalani
Honoraria: Pfizer, Roche/Genentech, Merck, Novartis, Astellas Pharma, Bayer, Bristol Myers Squibb, Eisai, Ipsen
Consulting or Advisory Role: Eisai, Merck, Ipsen, Pfizer, Roche/Genentech, Bristol Myers Squibb, AbbVie, Janssen, Bayer, Astellas Pharma
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), Roche (Inst), Ipsen (Inst), EMD Serono (Inst), BioCanRx (Inst)
Ugo De Giorgi
Consulting or Advisory Role: Pfizer, Janssen, Astellas Pharma, Bristol Myers Squibb, Bayer, Ipsen, MSD, PharmaMar, Novartis, Dompé Farmaceutici, Clovis Oncology, AstraZeneca, Eisai, Amgen, the healthcare business of Merck KGaA, Darmstadt, Germany, Merck & Co, Kenilworth, NJ
Research Funding: Sanofi (Inst), AstraZeneca (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Ipsen, Pfizer, AstraZeneca/Daiichi Sankyo
Bohuslav Melichar
Honoraria: Roche, Pfizer, Bristol Myers Squibb, Astellas Pharma, Novartis, MSD, Merck Serono, AstraZeneca, Eisai, Lilly
Consulting or Advisory Role: Roche, Pfizer, Bristol Myers Squibb, Astellas Pharma, Novartis, MSD, Merck Serono, AstraZeneca, Eisai, Lilly
Travel, Accommodations, Expenses: Bristol Myers Squibb, Merck Serono, AstraZeneca, MSD
Howard Gurney
Consulting or Advisory Role: Bristol Myers Squibb, Ipsen, Merck Sharp & Dohme, AstraZeneca, Janssen-Cilag, Pfizer, Roche, Merck Serono, Astellas Pharma
Speakers' Bureau: Merck Serono, AstraZeneca
María José Méndez-Vidal
Honoraria: Astellas Pharma, Roche, Bristol Myers Squibb, Ipsen
Consulting or Advisory Role: Janssen-Cilag, Pfizer, Astellas Pharma, Bristol Myers Squibb, Novartis, Roche, Ipsen, EUSA Pharma, Merck
Travel, Accommodations, Expenses: Janssen-Cilag, Pfizer, Astellas Pharma, Bristol Myers Squibb, Roche
Sergei Tjulandin
Stock and Other Ownership Interests: RosPharmTech
Consulting or Advisory Role: AstraZeneca, BioCad, Novartis, Servier
Speakers' Bureau: AstraZeneca, Novartis, R-Pharm
Research Funding: Merck (Inst), Eisai (Inst), AbbVie (Inst), Novartis (Inst), Bristol Myers Squibb Foundation (Inst), Servier
Teresa Alonso Gordoa
Consulting or Advisory Role: Ipsen, Pfizer, Bristol Myers Squibb, Janssen-Cilag, Astellas Pharma, Bayer, Eisai, Advanced Accelerator Applications/Novartis, Lilly, MSD Oncology
Research Funding: Ipsen, Janssen Oncology
Travel, Accommodations, Expenses: Janssen Oncology
Anna Alyasova
Research Funding: Eisai (Inst)
Eric Winquist
Consulting or Advisory Role: Merck, Bayer, Eisai, Roche, Ipsen, EMD Serono
Research Funding: Roche/Genentech (Inst), Merck (Inst)
Pablo Maroto
Consulting or Advisory Role: Astellas Pharma, Ipsen, BMS, Merck/Pfizer, Bayer, Janssen, Bayer
Travel, Accommodations, Expenses: Merck/Pfizer, Bayer
Miso Kim
Honoraria: Astellas Pharma, Yuhan, Novartis, Merck
Consulting or Advisory Role: Ipsen, Bristol Myers Squibb/Ono Pharmaceutical, Eisai, Yuhan, Pfizer, MSD, Roche, Janssen, Astellas Pharma, Bayer, Bayer, Merck, Boryung
Avivit Peer
Honoraria: MSD, BMS, Astellas Pharma, Janssen Oncology, Bayer, Exelixis, Medison, Roche, AstraZeneca, Merck Serono
Consulting or Advisory Role: Janssen-Cilag, Astellas Pharma, Pfizer, Novartis, Bayer, MSD Oncology, BMS, Eisai, Takeda, AstraZeneca, Merck Serono
Travel, Accommodations, Expenses: Pfizer, Astellas Pharma, BMS, MSD
Other Relationship: AstraZeneca
Giuseppe Procopio
Consulting or Advisory Role: Bayer, Bristol Myers Squibb (BMS), Janssen, Novartis, Pfizer, Ipsen, Merck Sharp & Dohme, AstraZeneca, Eisai, MSD Oncology, Roche
Research Funding: Astellas Pharma, Ipsen, Janssen Oncology
Toshio Takagi
Honoraria: Ezai Co, Ltd
Shirley Wong
Consulting or Advisory Role: Pfizer, Merk Sharpe & Dome
Jens Bedke
Consulting or Advisory Role: Bristol Myers Squibb, Eisai, EUSA Pharma, Ipsen, MSD Oncology, Roche, Pfizer, Merck KGaA
Speakers' Bureau: MSD Oncology, Bristol Myers Squibb, Merck KGaA, Pfizer, Ipsen, Astellas Pharma, Apogepha
Research Funding: Bristol Myers Squibb (Inst), Astellas Pharma (Inst), Ipsen (Inst), MSD Oncology (Inst), Novartis (Inst), Roche (Inst), Exelixis (Inst), Pfizer (Inst), SeaGen (Inst)
Manuela Schmidinger
Honoraria: BMS, Ipsen, MSD Oncology, EISAI, EUSA Pharma, Merck Sharp & Dohme, Janssen Oncology, AstraZeneca
Consulting or Advisory Role: BMS, Ipsen, MSD Oncology, EUSA Pharma, Eisai, AstraZeneca
Research Funding: IPSEN (Inst)
Travel, Accommodations, Expenses: BMS, Ipsen
Karla Rodriguez-Lopez
Employment: Merck
Stock and Other Ownership Interests: Merck
Honoraria: Merck
Research Funding: Merck (Inst)
Travel, Accommodations, Expenses: Merck
Joseph Burgents
Employment: Merck
Stock and Other Ownership Interests: Merck
Chinyere E. Okpara
Employment: Eisai
Jodi McKenzie
Employment: Eisai
Toni K. Choueiri
Stock and Other Ownership Interests: Precede Bio, Osel, Tempest Therapeutics, Pionyr, Curesponse, Inndura, Primium
Honoraria: HiberCell, Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol Myers Squibb, Roche/Genentech, Eisai, Foundation Medicine, AstraZeneca, Exelixis, Prometheus, Ipsen, Sanofi/Aventis, Peloton Therapeutics, UpToDate, NCCN, Michael J. Hennessy Associates, Analysis Group, Clinical Care Options, PlatformQ Health, Navinata Health, Harborside Press, ASCO, The New England Journal of Medicine, Lancet Oncology, EMD Serono, Lilly, Tempest Therapeutics, Arcus Biosciences, Arcus Biosciences, Alkermes, Gilead Sciences, Scholar Rock, Janssen Oncology, Precede Bio, Aravive, Infinity Pharmaceuticals, ESMO, NiKang Therapeutics, Kanaph Therapeutics, Gilead Sciences
Consulting or Advisory Role: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol Myers Squibb, Roche/Genentech, Eisai, Foundation Medicine, AstraZeneca, Exelixis, Prometheus, Ipsen, Sanofi/Aventis, Peloton Therapeutics, UpToDate, NCCN, Michael J. Hennessy Associates, Analysis Group, Clinical Care Options, PlatformQ Health, Navinata Health, Harborside Press, ASCO, The New England Journal of Medicine, Lancet Oncology, EMD Serono, Lilly, Tempest Therapeutics, Arcus Biosciences, alkermes, Gilead Sciences, Scholar Rock, Janssen Oncology, Precede Bio, Aravive, Infinity Pharmaceuticals, ESMO, NiKang Therapeutics, Kanaph Therapeutics, Gilead Sciences
Research Funding: Pfizer (Inst), Novartis (Inst), Merck (Inst), Exelixis (Inst), TRACON Pharma (Inst), GlaxoSmithKline (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Peloton Therapeutics (Inst), Roche/Genentech (Inst), Agensys (Inst), Eisai (Inst), Takeda (Inst), Ipsen (Inst), Seattle Genetics/Astellas (Inst), Bayer (Inst), Roche (Inst), Calithera Biosciences (Inst), NiKang Therapeutics (Inst), Arcus Biosciences (Inst), AVEO (Inst)
Patents, Royalties, Other Intellectual Property: International Patent Application No. PCT/US2018/058430, titled “Biomarkers of Clinical Response and Benefit to Immune Checkpoint Inhibitor Therapy” (Inst), International Patent Application No. PCT/US2018/12209, titled “PBRM1 Biomarkers Predictive of Anti-Immune Checkpoint Response” (Inst), ctDNA technologies
Travel, Accommodations, Expenses: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol Myers Squibb, Roche/Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Exelixis, Prometheus, alligent, Ipsen, Corvus Pharmaceuticals, Lpath, Alexion Pharmaceuticals, Sanofi/Aventis, UpToDate, Peloton Therapeutics, NCCN, Michael J. Hennessy Associates, Analysis Group, Kidney Cancer Association, Clinical Care Options, PlatformQ Health, Harborside Press, Navinata Health, The New England Journal of Medicine, Lancet Oncology, EMD Serono, HERON, Lilly, ESMO
Other Relationship: Medical writing and editorial assistance support may have been funded by Communications companies funded by pharmaceutical companies such as ClinicalThinking, Health Interactions, Envision Pharma Group, Fishawack Group of Companies, Parexel
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the ASCO Annual Meeting, Chicago, IL, June 2-6, 2023; and the ANZUP Annual Scientific Meeting, Victoria, Australia, July 9-11, 2023.
SUPPORT
Supported by Eisai Inc, Nutley, NJ, and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ. Medical writing support was provided by Swati Khare, PhD, and Irene Minkina, PhD, of Oxford PharmaGenesis Inc, Newtown, PA, and was funded by the sponsors.
CLINICAL TRIAL INFORMATION
Contributor Information
Collaborators: Robert J. Motzer, Toni Choueiri, Thomas Hutson, Luke Nordquist, David Spigel, Jaime Merchan, Saby George, Sandhya Srinivas, Brendan Curti, Andrew Pippas, Elisabeth Heath, Subramanya Rao, Theodore Gourdin, Mehmood Hashmi, Nafisa Burhani, Ana Molina, Alan Koletsky, Robert Alter, Carlos Alemany, Benjamin Gartrell, Mike Cusnir, Harsha Vyas, Stephanie Graff, Christian Squillante, Mark Knapp, Ivor Percent, Vijay Patel, Daniel Spitz, Cameron Harkness, Marc Matrana, Lindsay Overton, Stephen Richey, Donald Richards, Habib Ghaddar, Robert Galamaga, Ralph Hauke, Joseph Haggerty, Ronald Harris, Mark Johns, Samith Kochuparambil, Christian Kollmannsberger, Bobby Shayegan, Christina Canil, Eric Winquist, Catherine Sperlich, Georg Bjarnason, Naveen Basappa, Wolfgang Loidl, Wolfgang Horninger, Manuela Schmidinger, Lionel D'Hondt, Dirk Schrijvers, Annemie Rutten, Peter Schatteman, Wim Wynendaele, Daisy Luyten, Spyridon Sideris, Christine Gennigens, Bohuslav Melichar, Jana Katolicka, Jiri Tomasek, Jana Prausova, Tomas Buchler, Petra Holeckova, Philippe Barthelemy, Diego Tosi, Baptiste Abbar, Sylvie Negrier, Stephane Oudard, Eric Voog, Sylvie Zanetta, Frederic Rolland, Jens Bedke, Stefan Siemer, Manfred Wirth, Jan Schleicher, Maria De Santis, Lothar Bergmann, Michael Staehler, Philipp Ivanyi, Christoph Lutz, Gunhild Von Amsberg, Martin Boegemann, Uwe Zimmermann, Raymond McDermott, Richard Bambury, Paul Donnellan, Oscar Breathnach, Raya Leibowitz-Amit, Olesya Goldman, Avivit Peer, David Sarid, Hovav Nechushtan, Raanan Berger, Victoria Neiman, Fabio Calabro, Paolo Pedrazzoli, Francesco Boccardo, Alketa Hamzaj, Ferdinando Riccardi, Ugo De Giorgi, Sandro Pignata, Sandra Santarossa, Francesco Massari, Giuseppe Tonini, Caterina Accettura, Francesco Carrozza, Roberto Sabbatini, Elena Verzoni, Elisa Biscaldi, Britt Suelmann, Alfonsus van den Eertwegh, Hans van Thienen, Ewa Kalinka, Jacek Jassem, Violetta Sulzyc-Bielicka, Slawomir Mandziuk, Sergei Tjulandin, Oleg Karyakin, Anna Alyasova, Boris Alekseev, Alexander Zyrianov, Vsevolod Matveev, Evgeny Kopyltsov, Vadim Kozlov, Jose Angel Arranz Arija, Pablo Borrega Garcia, Miguel Angel Climent Duran, Begona Perez Valderrama, Emilio Esteban Gonzalez, Francisco Javier Garcia del Muro Solans, Jesus Garcia-Donas Jimenez, Teresa Alonso Gordoa, Jose Pablo Maroto Rey, Begoña Mellado Gonzalez, Maria Jose Mendez Vidal, Javier Puente Vazquez, Cristina Suarez Rodriguez, Enrique Grande Pulido, Guillermo Crespo, Natalia Fernandez Nuñez, Ignacio Duran Martinez, Joerg Beyer, Natalie Fischer, Hilary Glen, Ricky Frazer, Jennifer Allison, Thomas Powles, Jahangeer Malik, Christy Ralph, Sarah Rudman, Thomas Geldart, Aristotelis Bamias, Sofia Baka, Vassilios Georgoulias, Konstantinos Papazisis, Haralabos Kalofonos, Eleni Timotheadou, Seok-Soo Byun, Bumjin Lim, Sun Young Rha, Seong Il Seo, Jinsoo Chung, Miso Kim, Sung-Hoo Hong, Jae Lyun Lee, Se Hoon Park, Tae Gyun Kwon, Ian Davis, Shirley Wong, Ian Byard, Andrew Weickhardt, Howard Gurney, Jeffrey Goh, Takahiro Osawa, Naoya Masumori, Shingo Hatakeyama, Mitsuru Saito, Yoshihiko Tomita, Yuji Miura, Masayoshi Nagata, Go Kimura, Mototsugu Oya, Toshio Takagi, Yu Nakamura, Hisashi Hasumi, Masatsugu Iwamura, Akira Komiya, Atsushi Komaru, Masafumi Oyama, Yoshihisa Matsukawa, Norihito Soga, Minoru Kato, Masahiro Nozawa, Makito Miyake, Yuzo Nakano, Kohei Edamura, Nobuyuki Hinata, Homare Okazoe, Masayuki Takahashi, Masatoshi Eto, Kojiro Oba, Takeshi Kishida, and Osamu Ukimura
AUTHOR CONTRIBUTIONS
Conception and design: Robert J. Motzer, Thomas Powles, Viktor Grünwald, Evgeny Kopyltsov, Vadim Kozlov, Manuela Schmidinger, Cixin He, Jodi McKenzie, Toni K. Choueiri
Financial support: Vadim Kozlov, Shirley Wong
Administrative support: Vadim Kozlov, Karla Rodriguez-Lopez
Provision of study materials or patients: Masatoshi Eto, Viktor Grünwald, Thomas E. Hutson, Jaime Merchan, Jeffrey C. Goh, Ugo De Giorgi, Bohuslav Melichar, Howard Gurney, María José Méndez-Vidal, Evgeny Kopyltsov, Teresa Alonso Gordoa, Vadim Kozlov, Anna Alyasova, Eric Winquist, Pablo Maroto, Miso Kim, Avivit Peer, Giuseppe Procopio, Manuela Schmidinger, Toni K. Choueiri
Collection and assembly of data: Robert J. Motzer, Camillo Porta, Masatoshi Eto, Thomas Powles, Viktor Grünwald, Thomas E. Hutson, Boris Alekseev, Sun Young Rha, Jeffrey C. Goh, Ugo De Giorgi, Bohuslav Melichar, Sung-Hoo Hong, Howard Gurney, María José Méndez-Vidal, Sergei Tjulandin, Vadim Kozlov, Anna Alyasova, Eric Winquist, Pablo Maroto, Miso Kim, Avivit Peer, Giuseppe Procopio, Toshio Takagi, Jens Bedke, Manuela Schmidinger, Cixin He, Chinyere E. Okpara, Jodi McKenzie
Data analysis and interpretation: Robert J. Motzer, Thomas Powles, Viktor Grünwald, Thomas E. Hutson, Boris Alekseev, Sun Young Rha, Jaime Merchan, Jeffrey C. Goh, Aly-Khan A. Lalani, Bohuslav Melichar, Sung-Hoo Hong, Evgeny Kopyltsov, Sergei Tjulandin, Teresa Alonso Gordoa, Vadim Kozlov, Eric Winquist, Pablo Maroto, Shirley Wong, Jens Bedke, Manuela Schmidinger, Karla Rodriguez-Lopez, Joseph Burgents, Cixin He, Chinyere E. Okpara, Jodi McKenzie, Toni K. Choueiri
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Lenvatinib Plus Pembrolizumab Versus Sunitinib in First-Line Treatment of Advanced Renal Cell Carcinoma: Final Prespecified Overall Survival Analysis of CLEAR, a Phase III Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Robert J. Motzer
Consulting or Advisory Role: Eisai, Exelixis, Merck, Genentech/Roche, Incyte, Pfizer, AstraZeneca, EMD Serono, Aveo, Takeda
Research Funding: Pfizer (Inst), Bristol Myers Squibb (Inst), Eisai (Inst), Novartis (Inst), Genentech/Roche (Inst), Exelixis (Inst), Merck (Inst), Aveo (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb
Camillo Porta
Consulting or Advisory Role: Angelini, AstraZeneca, Bristol Myers Squibb, Eisai, Ipsen, MSD
Speakers' Bureau: Bristol Myers Squibb, Ipsen, MSD
Masatoshi Eto
Consulting or Advisory Role: Eisai, Pfizer, Takeda, Merck, MSD, Chugai Pharma
Speakers' Bureau: MSD, Merck, AstraZeneca, Eisai, Ono Pharmaceutical, Takeda, Bristol Myers Squibb, Astellas Pharma, Pfizer, Janssen
Research Funding: Takeda
Thomas Powles
Honoraria: AstraZeneca, Eisai, Merck, Novartis, Pfizer, Roche, Astellas Pharma, BMS GmbH & Co KG, Exelixis, Incyte, Ipsen, Seagen, Merck Serono, Johnson & Johnson/Janssen, MashupMD
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Ipsen, Pfizer, Novartis, Seagen, Roche, Exelixis, MSD, Merck Serono, Astellas Pharma, Johnson & Johnson, Eisai, MashupMD, Merck, Incyte
Research Funding: AstraZeneca, Roche, Bristol Myers Squibb, Exelixis, Ipsen, MSD, Novartis, Pfizer, Seagen, Merck Serono, Astellas Pharma, Johnson & Johnson, Eisai
Travel, Accommodations, Expenses: Pfizer, MSD, AstraZeneca, Roche, Ipsen
Viktor Grünwald
Employment: University Hospital Essen
Stock and Other Ownership Interests: MSD, Bristol Myers Squibb, AstraZeneca, Seagen, Genmab
Honoraria: Bristol Myers Squibb, Pfizer, Ipsen, Eisai, MSD Oncology, Merck Serono, AstraZeneca, EUSA Pharma, Janssen-Cilag, Advanced Accelerator Applications/Novartis, Apogepha, Nanobiotix, Ono Pharmaceutical, Astellas Pharma, Amgen
Consulting or Advisory Role: Bristol Myers Squibb, Pfizer, Novartis, MSD Oncology, Ipsen, Janssen-Cilag, Onkowissen, Cor2Ed, Eisai, Debiopharm Group, PCI Biotech, Gilead Sciences, Cureteq, Oncorena, Synthekine
Research Funding: Amgen (Inst), MSD Oncology (Inst), BMS (Inst), Seagen (Inst), Ipsen (Inst), Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Pfizer, AstraZeneca, Janssen, Merck Serono
Thomas E. Hutson
Employment: Texas Oncology
Honoraria: Pfizer, Astellas Pharma, Bristol Myers Squibb, Exelixis, Eisai, Novartis, Johnson & Johnson, Bayer/Onyx
Consulting or Advisory Role: Bayer/Onyx, Pfizer, Novartis, Astellas Pharma, Johnson & Johnson, Bristol Myers Squibb, Eisai, Exelixis
Speakers' Bureau: Pfizer, Johnson & Johnson, Eisai, Exelixis, Astellas Pharma, Bristol Myers Squibb
Research Funding: Pfizer (Inst), Johnson & Johnson (Inst), Exelixis (Inst), Eisai (Inst), Bristol Myers Squibb (Inst)
Boris Alekseev
Honoraria: AstraZeneca, Astellas Pharma, Eisai, Janssen, Bayer, MSD, Merck, Pfizer, Roche, Bristol Myers Squibb
Consulting or Advisory Role: AstraZeneca, Astellas Pharma, Bayer, Bristol Myers Squibb, Janssen, Merck, Pfizer, MSD, Roche, Eisai
Speakers' Bureau: Janssen, Astellas Pharma, Pfizer, AstraZeneca, Bayer, Merck, Bristol Myers Squibb, MSD, Eisai, Roche
Research Funding: AstraZeneca, Merck, Bayer, Astellas Pharma, Janssen, Bristol Myers Squibb, Pfizer, ICON Clinical Research, Eisai, MSD, Roche
Travel, Accommodations, Expenses: AstraZeneca, Astellas Pharma, Bayer, Bristol Myers Squibb, Janssen, MSD, Pfizer, Sanofi
Sun Young Rha
Consulting or Advisory Role: MSD Oncology, Daiichi Sankyo, Eisai, LG Chem, Eutilex, Astellas Pharma, indivumed, AstraZeneca, Ono Pharmaceutical, Amgen
Speakers' Bureau: Lilly, Eisai, MSD Oncology, BMS/Ono, Amgen, Daiichi Sankyo/UCB Japan, AstraZeneca
Research Funding: MSD Oncology, Bristol Myers Squibb, Eisai, Roche/Genentech, ASLAN Pharmaceuticals, Sillajen, Bayer, Daichii Sankyo, Lilly, AstraZeneca, BeiGene, Zymeworks, Astellas Pharma, Indivumed, Amgen (Inst)
Jaime Merchan
Consulting or Advisory Role: Merck
Research Funding: Corvus Pharmaceuticals (Inst), Genentech/Roche (Inst), Tizona Therapeutics, Inc (Inst), Tocagen (Inst), Vyriad (Inst), Sillajen (Inst), Replimune (Inst), Peloton Therapeutics (Inst), Eisai (Inst), Seattle Genetics/Astellas (Inst), Merck (Inst), Rubius Therapeutics (Inst), BioNTech (Inst), Trishula Therapeutics (Inst), Exelixis (Inst), IMUGENE (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Jeffrey C. Goh
Stock and Other Ownership Interests: Immutep, ICON Cancer Care
Honoraria: MSD Oncology, AstraZeneca
Consulting or Advisory Role: GlaxoSmithKline, MSD, BMS, Janssen Oncology
Speakers' Bureau: Ipsen, Janssen, AstraZeneca/MedImmune, MSD Oncology, Pfizer/EMD Serono, Eisai
Travel, Accommodations, Expenses: Pfizer/EMD Serono, Bayer
Aly-Khan A. Lalani
Honoraria: Pfizer, Roche/Genentech, Merck, Novartis, Astellas Pharma, Bayer, Bristol Myers Squibb, Eisai, Ipsen
Consulting or Advisory Role: Eisai, Merck, Ipsen, Pfizer, Roche/Genentech, Bristol Myers Squibb, AbbVie, Janssen, Bayer, Astellas Pharma
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), Roche (Inst), Ipsen (Inst), EMD Serono (Inst), BioCanRx (Inst)
Ugo De Giorgi
Consulting or Advisory Role: Pfizer, Janssen, Astellas Pharma, Bristol Myers Squibb, Bayer, Ipsen, MSD, PharmaMar, Novartis, Dompé Farmaceutici, Clovis Oncology, AstraZeneca, Eisai, Amgen, the healthcare business of Merck KGaA, Darmstadt, Germany, Merck & Co, Kenilworth, NJ
Research Funding: Sanofi (Inst), AstraZeneca (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Ipsen, Pfizer, AstraZeneca/Daiichi Sankyo
Bohuslav Melichar
Honoraria: Roche, Pfizer, Bristol Myers Squibb, Astellas Pharma, Novartis, MSD, Merck Serono, AstraZeneca, Eisai, Lilly
Consulting or Advisory Role: Roche, Pfizer, Bristol Myers Squibb, Astellas Pharma, Novartis, MSD, Merck Serono, AstraZeneca, Eisai, Lilly
Travel, Accommodations, Expenses: Bristol Myers Squibb, Merck Serono, AstraZeneca, MSD
Howard Gurney
Consulting or Advisory Role: Bristol Myers Squibb, Ipsen, Merck Sharp & Dohme, AstraZeneca, Janssen-Cilag, Pfizer, Roche, Merck Serono, Astellas Pharma
Speakers' Bureau: Merck Serono, AstraZeneca
María José Méndez-Vidal
Honoraria: Astellas Pharma, Roche, Bristol Myers Squibb, Ipsen
Consulting or Advisory Role: Janssen-Cilag, Pfizer, Astellas Pharma, Bristol Myers Squibb, Novartis, Roche, Ipsen, EUSA Pharma, Merck
Travel, Accommodations, Expenses: Janssen-Cilag, Pfizer, Astellas Pharma, Bristol Myers Squibb, Roche
Sergei Tjulandin
Stock and Other Ownership Interests: RosPharmTech
Consulting or Advisory Role: AstraZeneca, BioCad, Novartis, Servier
Speakers' Bureau: AstraZeneca, Novartis, R-Pharm
Research Funding: Merck (Inst), Eisai (Inst), AbbVie (Inst), Novartis (Inst), Bristol Myers Squibb Foundation (Inst), Servier
Teresa Alonso Gordoa
Consulting or Advisory Role: Ipsen, Pfizer, Bristol Myers Squibb, Janssen-Cilag, Astellas Pharma, Bayer, Eisai, Advanced Accelerator Applications/Novartis, Lilly, MSD Oncology
Research Funding: Ipsen, Janssen Oncology
Travel, Accommodations, Expenses: Janssen Oncology
Anna Alyasova
Research Funding: Eisai (Inst)
Eric Winquist
Consulting or Advisory Role: Merck, Bayer, Eisai, Roche, Ipsen, EMD Serono
Research Funding: Roche/Genentech (Inst), Merck (Inst)
Pablo Maroto
Consulting or Advisory Role: Astellas Pharma, Ipsen, BMS, Merck/Pfizer, Bayer, Janssen, Bayer
Travel, Accommodations, Expenses: Merck/Pfizer, Bayer
Miso Kim
Honoraria: Astellas Pharma, Yuhan, Novartis, Merck
Consulting or Advisory Role: Ipsen, Bristol Myers Squibb/Ono Pharmaceutical, Eisai, Yuhan, Pfizer, MSD, Roche, Janssen, Astellas Pharma, Bayer, Bayer, Merck, Boryung
Avivit Peer
Honoraria: MSD, BMS, Astellas Pharma, Janssen Oncology, Bayer, Exelixis, Medison, Roche, AstraZeneca, Merck Serono
Consulting or Advisory Role: Janssen-Cilag, Astellas Pharma, Pfizer, Novartis, Bayer, MSD Oncology, BMS, Eisai, Takeda, AstraZeneca, Merck Serono
Travel, Accommodations, Expenses: Pfizer, Astellas Pharma, BMS, MSD
Other Relationship: AstraZeneca
Giuseppe Procopio
Consulting or Advisory Role: Bayer, Bristol Myers Squibb (BMS), Janssen, Novartis, Pfizer, Ipsen, Merck Sharp & Dohme, AstraZeneca, Eisai, MSD Oncology, Roche
Research Funding: Astellas Pharma, Ipsen, Janssen Oncology
Toshio Takagi
Honoraria: Ezai Co, Ltd
Shirley Wong
Consulting or Advisory Role: Pfizer, Merk Sharpe & Dome
Jens Bedke
Consulting or Advisory Role: Bristol Myers Squibb, Eisai, EUSA Pharma, Ipsen, MSD Oncology, Roche, Pfizer, Merck KGaA
Speakers' Bureau: MSD Oncology, Bristol Myers Squibb, Merck KGaA, Pfizer, Ipsen, Astellas Pharma, Apogepha
Research Funding: Bristol Myers Squibb (Inst), Astellas Pharma (Inst), Ipsen (Inst), MSD Oncology (Inst), Novartis (Inst), Roche (Inst), Exelixis (Inst), Pfizer (Inst), SeaGen (Inst)
Manuela Schmidinger
Honoraria: BMS, Ipsen, MSD Oncology, EISAI, EUSA Pharma, Merck Sharp & Dohme, Janssen Oncology, AstraZeneca
Consulting or Advisory Role: BMS, Ipsen, MSD Oncology, EUSA Pharma, Eisai, AstraZeneca
Research Funding: IPSEN (Inst)
Travel, Accommodations, Expenses: BMS, Ipsen
Karla Rodriguez-Lopez
Employment: Merck
Stock and Other Ownership Interests: Merck
Honoraria: Merck
Research Funding: Merck (Inst)
Travel, Accommodations, Expenses: Merck
Joseph Burgents
Employment: Merck
Stock and Other Ownership Interests: Merck
Chinyere E. Okpara
Employment: Eisai
Jodi McKenzie
Employment: Eisai
Toni K. Choueiri
Stock and Other Ownership Interests: Precede Bio, Osel, Tempest Therapeutics, Pionyr, Curesponse, Inndura, Primium
Honoraria: HiberCell, Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol Myers Squibb, Roche/Genentech, Eisai, Foundation Medicine, AstraZeneca, Exelixis, Prometheus, Ipsen, Sanofi/Aventis, Peloton Therapeutics, UpToDate, NCCN, Michael J. Hennessy Associates, Analysis Group, Clinical Care Options, PlatformQ Health, Navinata Health, Harborside Press, ASCO, The New England Journal of Medicine, Lancet Oncology, EMD Serono, Lilly, Tempest Therapeutics, Arcus Biosciences, Arcus Biosciences, Alkermes, Gilead Sciences, Scholar Rock, Janssen Oncology, Precede Bio, Aravive, Infinity Pharmaceuticals, ESMO, NiKang Therapeutics, Kanaph Therapeutics, Gilead Sciences
Consulting or Advisory Role: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol Myers Squibb, Roche/Genentech, Eisai, Foundation Medicine, AstraZeneca, Exelixis, Prometheus, Ipsen, Sanofi/Aventis, Peloton Therapeutics, UpToDate, NCCN, Michael J. Hennessy Associates, Analysis Group, Clinical Care Options, PlatformQ Health, Navinata Health, Harborside Press, ASCO, The New England Journal of Medicine, Lancet Oncology, EMD Serono, Lilly, Tempest Therapeutics, Arcus Biosciences, alkermes, Gilead Sciences, Scholar Rock, Janssen Oncology, Precede Bio, Aravive, Infinity Pharmaceuticals, ESMO, NiKang Therapeutics, Kanaph Therapeutics, Gilead Sciences
Research Funding: Pfizer (Inst), Novartis (Inst), Merck (Inst), Exelixis (Inst), TRACON Pharma (Inst), GlaxoSmithKline (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Peloton Therapeutics (Inst), Roche/Genentech (Inst), Agensys (Inst), Eisai (Inst), Takeda (Inst), Ipsen (Inst), Seattle Genetics/Astellas (Inst), Bayer (Inst), Roche (Inst), Calithera Biosciences (Inst), NiKang Therapeutics (Inst), Arcus Biosciences (Inst), AVEO (Inst)
Patents, Royalties, Other Intellectual Property: International Patent Application No. PCT/US2018/058430, titled “Biomarkers of Clinical Response and Benefit to Immune Checkpoint Inhibitor Therapy” (Inst), International Patent Application No. PCT/US2018/12209, titled “PBRM1 Biomarkers Predictive of Anti-Immune Checkpoint Response” (Inst), ctDNA technologies
Travel, Accommodations, Expenses: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol Myers Squibb, Roche/Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Exelixis, Prometheus, alligent, Ipsen, Corvus Pharmaceuticals, Lpath, Alexion Pharmaceuticals, Sanofi/Aventis, UpToDate, Peloton Therapeutics, NCCN, Michael J. Hennessy Associates, Analysis Group, Kidney Cancer Association, Clinical Care Options, PlatformQ Health, Harborside Press, Navinata Health, The New England Journal of Medicine, Lancet Oncology, EMD Serono, HERON, Lilly, ESMO
Other Relationship: Medical writing and editorial assistance support may have been funded by Communications companies funded by pharmaceutical companies such as ClinicalThinking, Health Interactions, Envision Pharma Group, Fishawack Group of Companies, Parexel
No other potential conflicts of interest were reported.
REFERENCES
- 1. Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384:1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 2.Lenvima® (lenvatinib) [prescribing information] Nutley, NJ: Eisai; 2023. [Google Scholar]
- 3.Keytruda® (pembrolizumab) [prescribing information] Rahway, NJ: Merck Sharp & Dohme LLC; 2023. [Google Scholar]
- 4. Choueiri TK, Eto M, Motzer R, et al. Lenvatinib plus pembrolizumab versus sunitinib as first-line treatment of patients with advanced renal cell carcinoma (CLEAR): Extended follow-up from the phase 3, randomised, open-label study. Lancet Oncol. 2023;24:228–238. doi: 10.1016/S1470-2045(23)00049-9. [DOI] [PubMed] [Google Scholar]
- 5. Latimer NR. Treatment switching in oncology trials and the acceptability of adjustment methods. Expert Rev Pharmacoecon Outcomes Res. 2015;15:561–564. doi: 10.1586/14737167.2015.1037835. [DOI] [PubMed] [Google Scholar]
- 6. Makker V, Colombo N, Casado Herráez A, et al. Lenvatinib plus pembrolizumab in previously treated advanced endometrial cancer: Updated efficacy and safety from the randomized phase III study 309/KEYNOTE-775. J Clin Oncol. 2023;41:2904–2910. doi: 10.1200/JCO.22.02152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Makker V, Colombo N, Casado Herráez A, et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med. 2022;386:437–448. doi: 10.1056/NEJMoa2108330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taylor MH, Lee CH, Makker V, et al. Phase IB/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors. J Clin Oncol. 2020;38:1154–1163. doi: 10.1200/JCO.19.01598. [DOI] [PMC free article] [PubMed] [Google Scholar]


