Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
nab-Sirolimus is approved in the United States for the treatment of metastatic or locally advanced malignant perivascular epithelioid cell tumor (PEComa) on the basis of the primary analysis results of the phase II Advanced Malignant Perivascular Epithelioid Cell Tumors (AMPECT) trial (ClinicalTrials.gov identifier: NCT02494570). Results from the primary analysis were previously published; however, the median duration of response (mDOR) had not been reached at that time. Here, 3 years after the primary analysis, we report final efficacy and safety data (data cutoff: April 29, 2022). At study completion, the confirmed overall response rate (by independent radiologist review using RECIST v1.1) was 38.7% (95% CI, 21.8 to 57.8), with an additional converted confirmed complete response (n = 2). Median progression-free survival remained the same at 10.6 months (95% CI, 5.5 to 41.2). The mDOR was reached at 39.7 months (95% CI, 6.5 to not reached [NR]), and the median overall survival at completion was 53.1 months (95% CI, 22.2 to NR). The most common treatment-related adverse events (TRAEs) were stomatitis (82.4%) and fatigue and rash (each 61.8%). No new or unexpected adverse events occurred, and no grade ≥4 TRAEs were reported. These results highlight the long-term clinical benefit of nab-sirolimus in patients with advanced malignant PEComa, with a DOR of >3 years.
INTRODUCTION
Malignant perivascular epithelioid cell tumors (PEComas) are an ultrarare, aggressive soft tissue sarcoma in which cytotoxic chemotherapy regimens have shown modest benefit.1 nab-Sirolimus is a nanoparticle, albumin-bound, intravenously administered mTOR inhibitor approved in the United States for the treatment of patients with locally advanced, unresectable, or metastatic malignant PEComas.2 In the phase II Advanced Malignant Perivascular Epithelioid Cell Tumors (AMPECT) trial (ClinicalTrials.gov identifier: NCT02494570), the predefined primary analysis was conducted when all patients were treated for at least 6 months (data cutoff [DCO]: May 22, 2019). The confirmed overall response rate (ORR), as assessed by independent radiologists, was 38.7% (12 of 31 patients; 95% CI, 21.8 to 57.8), all partial responses.
At the 1.5-year follow-up after the primary analysis date (November 23, 2020; 2 years after the last patient started treatment), seven of 12 responders had ongoing treatment, and the median duration of response (DOR) had not been reached after a median follow-up for response of 2.5 years (DOR range, 5.6-47.2+ months).3 The median progression-free survival (PFS) was 10.6 months (95% CI, 5.5 months to not reached [NR]), and the median overall survival (OS) was 40.8 months (95% CI, 22.2 to NR). Most treatment-related adverse events (TRAEs) were grade 1 or 2 and were manageable for long-term treatment; no deaths related to nab-sirolimus occurred. Herein, we report the final analysis (DCO: April 29, 2022), including the median DOR, at 3.5 years after the last patient initiated treatment.
METHODS
AMPECT was an open-label, phase II, multicenter, registrational trial in adults (18 years and older). Trial design details and outcome measures have been previously published.3
Eligible patients had histologically confirmed diagnosis of malignant PEComa, an Eastern Cooperative Group performance status score of ≤1, locally advanced inoperable or metastatic disease, and no previous mTOR inhibitor use. Patients received nab-sirolimus 100 mg/m2 by intravenous infusion once weekly for 2 weeks, on days 1 and 8, of a 21-day cycle until disease progression, unacceptable toxicity, patient preference, or transfer to commercially available drug after US Food and Drug Administration (FDA) approval. The primary end point was confirmed ORR by 6 months on the basis of independent radiographic review using RECIST v1.1. Secondary end points included DOR, PFS at 6 months, median PFS, median OS, and safety and tolerability. Exploratory end points included assessment of tumor biomarkers using next-generation sequencing and immunohistochemistry and their relationship to clinical outcomes.
The study was approved by independent institutional review boards of each participating site and was conducted in accordance with the ethics principles of the Declaration of Helsinki and with Good Clinical Practice guidelines defined by the International Conference on Harmonization. All patients provided written informed consent.
RESULTS
Baseline patient demographics and clinical characteristics are summarized in Table 1.
TABLE 1.
Baseline Patient Demographics and Clinical Characteristics
| Parameter | All Treated Patients (N = 34) | ||
|---|---|---|---|
| Age, years, median (range) | 60 (27-78) | ||
| ≥65 years, No. (%) | 15 (44.1) | ||
| Sex, female, No. (%) | 28 (82.4) | ||
| Race, No. (%) | |||
| White | 24 (70.6) | ||
| Black | 3 (8.8) | ||
| Asian | 3 (8.8) | ||
| Pacific Islander/Hawaiian | 1 (2.9) | ||
| Other/unknown | 1 (2.9) | ||
| Not reported | 2 (5.9) | ||
| ECOG PS | |||
| 0 | 26 (76.5) | ||
| 1 | 8 (23.5) | ||
| Disease status, No. (%) | |||
| Metastatic | 29 (85.3) | ||
| Locally advanced, inoperable | 5 (14.7) | ||
| Previous systemic therapy for advanced PEComa, No. (%)a | 4 (11.8) | ||
| Primary tumor location, No. (%) | |||
| Uterus | 8 (23.5) | ||
| Pelvis, extrauterine | 6 (17.6) | ||
| Retroperitoneum | 6 (17.6) | ||
| Lung | 4 (11.8) | ||
| Kidney | 4 (11.8) | ||
| Aorta | 1 (2.9) | ||
| Brain | 1 (2.9) | ||
| Liver | 1 (2.9) | ||
| Muscle | 1 (2.9) | ||
| Ovary | 1 (2.9) | ||
| Small bowel | 1 (2.9) | ||
| Site of metastatic disease, No. (%)b | |||
| Lung, thoracicc | 21 (72.4) | ||
| Abdomend | 8 (27.6) | ||
| Pelvis | 7 (24.1) | ||
| Liver | 6 (20.7) | ||
| Colon | 4 (13.8) | ||
| Retroperitoneum | 3 (10.3) | ||
| Bone, unspecified | 2 (6.9) | ||
| Spleen | 2 (6.9) | ||
| Kidney | 1 (3.4) | ||
| Ovary | 1 (3.4) | ||
| No. of metastatic sites, No. (%) | |||
| 1 | 11 (37.9) | ||
| 2 | 9 (31.0) | ||
| 3 | 7 (24.1) | ||
| >3 | 2 (6.9) | ||
NOTE. Reused from the study by Wagner et al3 licensed under CC BY 4.0.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; PEComa, perivascular epithelioid cell tumor.
Includes systemic treatment for advanced disease (docetaxel, doxorubicin, gemcitabine, ifosfamide, and olaratumab; excludes neoadjuvant and adjuvant therapy).
n = 29.
Includes lymph nodes (hilar and precarinal).
Includes omentum, perigastric area, mesenteric root, peritoneum, and serosa.
Efficacy
In the final analysis, 3 of 34 patients were receiving treatment with nab-sirolimus. The mean treatment duration for all patients was 14.5 months (range, 0.3-65.2). Reasons for discontinuing treatment were disease progression (n = 20, 58.8%), withdrawal of consent (n = 6, 17.6%), adverse event (n = 2, 5.9%), and initiation of new cancer therapy, death, and other (no reason recorded; n = 1 each, 2.9%).
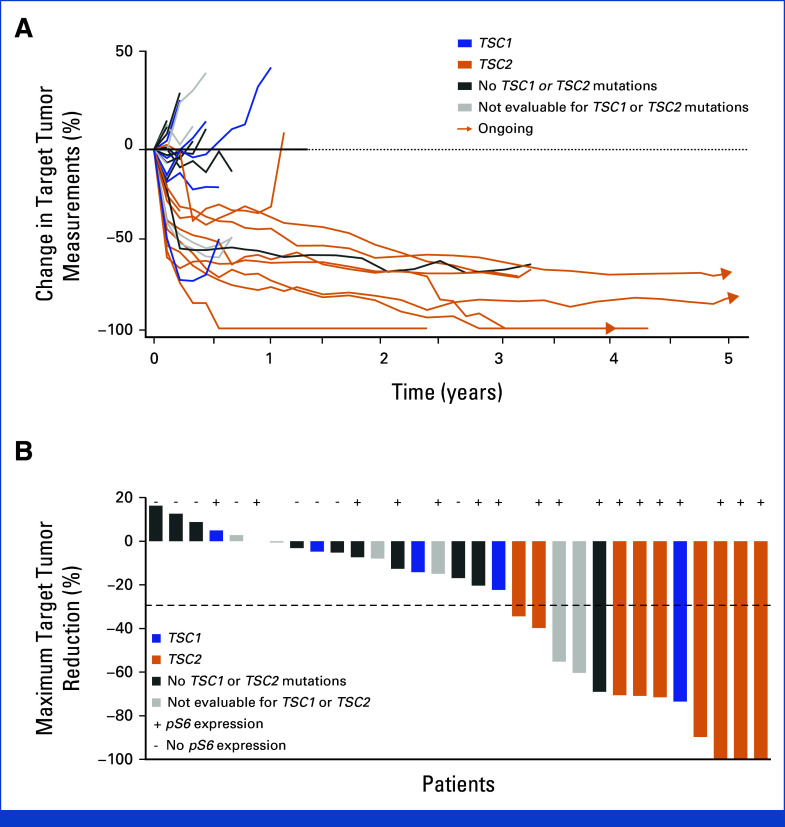
In the efficacy-evaluable population (n = 31), the primary end point of confirmed ORR by RECIST v1.1 remained at 38.7% (95% CI, 21.8 to 57.8; Table 2). By study completion, 2 of 31 (6.5%) patients achieved a confirmed complete response, both of which had a TSC2-inactivating alteration (one frameshift, one homozygous deletion), at 11 and 34 months from the start of therapy, despite having a dose reduction or interruption (Table 2). The median DOR was 39.7 months (95% CI, 6.5 to NR; Table 2, Fig 1A), ranging from 5.6 to 60.8 months. Stable disease was the best response in 16 (51.6%) patients and progressive disease in three (9.7%) patients (Table 2). Disease control rate remained at 71% (95% CI, 52.0 to 85.8; Table 2). The median PFS was 10.6 months (95% CI, 5.5 to 41.2; Table 2, Fig 1A), and the median OS was 53.1 months (95% CI, 22.2 to NR). Two patients initially ineligible for surgery of their locally advanced tumor subsequently had resection of disease after treatment with nab-sirolimus and remained in long-term survival follow-up for 3.5 years and 5.5 years.
TABLE 2.
Summary of Key Efficacy End Points in Final Analysis on the Basis of RECIST Assessments
| Efficacy End Point | All Efficacy-Evaluable Patients (n = 31) | Patients With Alterations in TSC1 (n = 5)a | Patients With Alterations in TSC2 (n = 9)a |
|---|---|---|---|
| Overall response rate, No. (%) | 12 (38.7) | — | — |
| 95% CI | 21.8 to 57.8 | — | — |
| Best overall response (confirmed), No. (%) | |||
| Complete response | 2 (6.5) | 0 | 2 (22.2) |
| Partial response | 10 (32.3) | 1 (20.0) | 6 (66.7) |
| Stable disease | 16 (51.6) | 3 (60.0) | 1 (11.1) |
| Progressive disease | 3 (9.7) | 1 (20.0) | 0 |
| Disease control rate,b No. (%) | 22 (71.0) | — | — |
| 95% CI | 52.0 to 85.8 | — | — |
| Median DOR, months | 39.7 | 5.6 | 51.7 |
| 95% CI | 6.5 to NR | NE | 6.5 to NR |
| Median PFS, months | 10.6 | 5.5 | 53.1 |
| 95% CI | 5.5 to 41.2 | 1.4 to NR | 10.6 to NR |
| Median OS, monthsc | 53.1 | 31.6 | NR |
| 95% CI | 22.2 to NR | 3.8 to NR | 53.1 to NR |
NOTE. Percentages are rounded and are based on the number of patients with positive, negative, or unevaluable biomarker status at screening.
Abbreviations: DOR, duration of response; NE, not evaluable; NR, not reached; OS, overall survival; PFS, progression-free survival.
Of the 25 patients with tissue sufficient for next-generation sequencing, 14 patients had inactivating alterations in TSC1 or TSC2.
Percentage of patients with a confirmed objective response or with stable disease of ≥12-week duration.
The median time from first dose to last contact during OS follow-up was 22.0 months (range, 1-66).
FIG 1.
Tumor responses to nab-sirolimus in patients with PEComa per RECIST v1.1. (A) Spider plot showing changes in the sum of target tumor measurements over time. Arrowheads indicate patients who were still on treatment at the time of the 3.5-year follow-up. (B) Waterfall plot of the best overall change from baseline in the sum of target tumor measurements, evaluated at the 3.5-year follow-up. PEComa, perivascular epithelioid cell tumor.
Observed outcomes from exploratory biomarker analyses of response were consistent with previously reported responses for patients with inactivating alterations in TSC1 (n = 5) or TSC2 (n = 9) and with expression of pS6, a downstream marker of mTOR activation (Table 2 and Fig 1B).
Safety
The safety-evaluable population consisted of 34 patients treated with at least one dose of nab-sirolimus. There was no change in the overall safety profile during long-term follow-up (Appendix Table A1, online only). The most common TRAEs were stomatitis (28 of 34 patients [82.4%]) and fatigue and rash (21 of 34 patients [61.8%] each). Of the 56 stomatitis events that occurred among 28 patients, 91.0% (51 of 56) resolved at the time of data cutoff. Stomatitis was most commonly managed with steroid mouthwash; 68% of all patients received stomatologic preparations (most frequently magic mouthwash, sucralfate, nystatin, and dexamethasone).
Overall, the median dose intensity was 60 mg/m2/week, representing 90% of the planned protocol maximum dose intensity of 66.6 mg/m2/week, with a dosing schedule of 100 mg/m2 given once weekly for 2 weeks, on days 1 and 8, of a 21-day cycle. In the final analysis, 14 of 34 (41.2%) patients had at least one dose reduction (n = 13 because of TRAEs; n = 1 because of physician decision); 3 of the 14 patients received two dose reductions during the study, and most (76.5%) dose reductions occurred within the first 3 months of treatment (Appendix Fig A1). The most common TRAEs associated with dose reductions were pneumonitis (4 of 13 patients [31%]) and stomatitis (3 of 13 patients [23%]; Appendix Table A2). Of 22 noninfectious pneumonitis events that occurred in 7 of 34 (20.6%) patients, all were grade 1 or 2, 21 (95.5%) events were resolved, and no events led to treatment discontinuation.
Five of 14 patients with a dose reduction had a confirmed response either before (3 of 5 patients) or after (2 of 5 patients) their first dose reduction (Appendix Fig A1). All five responders maintained their response for 6.1-37.3 months after the first dose reduction (Appendix Fig A1). None of the 14 patients who received a dose reduction discontinued nab-sirolimus because of a TRAE. At study closure, no additional discontinuations because of TRAEs were reported, and no new treatment-related serious safety signals were identified.
DISCUSSION
This long-term follow-up analysis of nab-sirolimus for patients with malignant PEComa showed a clinically meaningful overall response rate, median duration of response of more than 3 years, and durable disease control and survival, reflecting the clinical benefit of nab-sirolimus in the treatment of this aggressive, ultrarare sarcoma. Although responses were most commonly seen in patients with tumors harboring TSC2 mutations, responses and prolonged disease control were also observed in patients with differing tumor genotypes, suggesting that mTOR inhibition is effective for a broader patient population with malignant PEComa. The sample size in this single-arm phase II study was too small to draw definitive conclusions about the exploratory analysis of tumor genotypes; however, preliminary efficacy signals observed among patients with tumors with select biomarkers warrant further evaluation.
There were no new safety signals at study closure, which reaffirms the manageable long-term safety profile of nab-sirolimus, consistent with the mTOR inhibitor class. Taken together, the durable clinical benefit and acceptable safety profile support the use of nab-sirolimus as the first FDA-approved therapy for patients with advanced malignant PEComa.2
ACKNOWLEDGMENT
We thank all the patients and their families who participated in this study. The authors thank Cynthia D. Gioiello, PharmD, and Stephen Bublitz, ELS, of MedVal Scientific Information Services, LLC, and Andrea Humphries, PhD, CMPP, of Twist Medical, LLC for medical writing and editorial assistance, which were funded by Aadi Bioscience, Inc. This manuscript was prepared according to the International Society for Medical Publication Professionals' “Good Publication Practice for Communicating Company-Sponsored Medical Research: GPP3.”
APPENDIX
FIG A1.
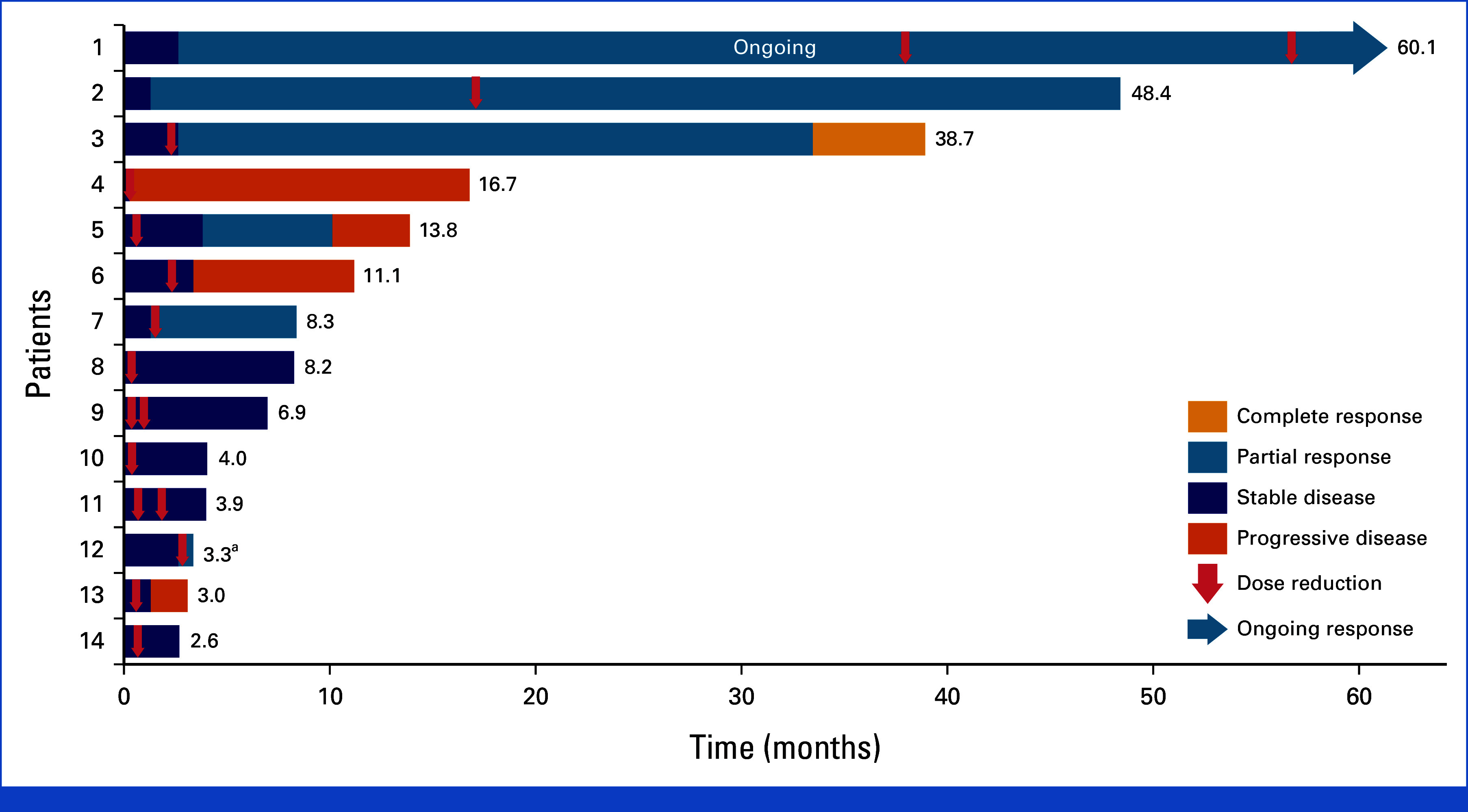
Best overall response among patients with at least one dose reduction (n = 14). Best overall responses are based on independent radiologist review; however, treatment decisions were made by the treating investigator on the basis of radiology review and clinical symptoms. aThe patient had unconfirmed PR and discontinued therapy because of an AE without a confirmatory scan. AE, adverse event; PR, partial response.
TABLE A1.
Any-Grade TRAEs Occurring in ≥25% of Patients (N = 34)
| TRAE | Any Grade, No. (%) | Grade 3, No. (%)a |
|---|---|---|
| Hematologic TRAEs | ||
| Anemiab | 18 (52.9) | 5 (14.7) |
| Thrombocytopeniab | 12 (35.3) | 1 (2.9) |
| Nonhematologic TRAEs | ||
| Stomatitisb | 28 (82.4) | 6 (17.6) |
| Fatigue | 21 (61.8) | 1 (2.9) |
| Rashb | 21 (61.8) | 0 |
| Nausea | 16 (47.1) | 0 |
| Diarrheab | 14 (41.2) | 1 (2.9) |
| Hyperglycemiab | 14 (41.2) | 3 (8.8) |
| Weight decreased | 14 (41.2) | 0 |
| Hypertriglyceridemiab | 12 (35.3) | 1 (2.9) |
| Edemab | 12 (35.3) | 1 (2.9) |
| Appetite decreased | 12 (35.3) | 0 |
| Hypercholesterolemiab | 11 (32.4) | 0 |
| Headache | 11 (32.4) | 0 |
| Dysgeusiab | 10 (29.4) | 0 |
| Pruritus | 9 (26.5) | 0 |
| ALT increasedb | 9 (26.5) | 1 (2.9) |
| Vomiting | 9 (26.5) | 1 (2.9) |
Abbreviation: TRAE, treatment-related adverse event.
Additional grade 3 TRAEs reported in ≥1 patient were abdominal pain, AST increased, lymphopenia, pancytopenia (n = 1 each), and dehydration and hypokalemia (n = 2 each). No grade 4 or 5 TRAEs were reported.
Reported on the basis of groupings of preferred terms defined by standardized queries in the Medical Dictionary for Regulatory Activities v24.0.
TABLE A2.
Any-Grade TRAEs Leading to Dose Reduction
| TRAE | Patient (N = 34), No. (%) |
|---|---|
| Any TRAE leading to dose reduction | 13 (38.2) |
| Pneumonitis | 4 (11.8) |
| Stomatitis | 3 (8.8) |
| Abdominal pain | 1 (2.9) |
| Acute coronary syndrome | 1 (2.9) |
| Dehydration | 1 (2.9) |
| Fatigue | 1 (2.9) |
| Hyperglycemia | 1 (2.9) |
| Increased ALT | 1 (2.9) |
| Increased AST | 1 (2.9) |
| Increased creatinine | 1 (2.9) |
| Thrombocytopenia | 1 (2.9) |
| Weight decreased | 1 (2.9) |
Abbreviation: TRAE, treatment-related adverse event.
Andrew J. Wagner
Consulting or Advisory Role: Lilly, Daiichi Sankyo, Deciphera, Mundipharma, Cogent Biosciences, Boehringer Ingelheim, AADi, BioAtla, SERVIER, InhibRx
Research Funding: Lilly (Inst), Plexxikon (Inst), Daiichi Sankyo (Inst), Karyopharm Therapeutics (Inst), Deciphera (Inst), Foghorn Therapeutics (Inst), AADi (Inst), Rain Therapeutics (Inst), Cogent Biosciences (Inst)
Vinod Ravi
Stock and Other Ownership Interests: TRACON Pharma, Merck, AstraZeneca, Pfizer, Moderna Therapeutics
Honoraria: UpToDate
Consulting or Advisory Role: Daiichi Sankyo, Gerson Lehrman Group, Guidepoint Inc, AADi
Research Funding: Novartis (Inst), TRACON Pharma (Inst), AADi (Inst), Athenex (Inst)
Patents, Royalties, Other Intellectual Property: Author Royalties from Uptodate
Travel, Accommodations, Expenses: Daiichi Sankyo
Richard F. Riedel
Employment: Limbguard
Leadership: Limbguard
Stock and Other Ownership Interests: Limbguard
Consulting or Advisory Role: Daiichi Sankyo, NanoCarrier, Deciphera, SpringWorks Therapeutics, Blueprint Medicines, AADi, GlaxoSmithKline, Adaptimmune, Boehringer Ingelheim
Research Funding: TRACON Pharma (Inst), AADi (Inst), Arog (Inst), Daiichi Sankyo (Inst), NanoCarrier (Inst), GlaxoSmithKline (Inst), SpringWorks Therapeutics (Inst), Blueprint Medicines (Inst), Epizyme (Inst), Philogen (Inst), Ayala Pharmaceuticals (Inst), Trillium Therapeutics (Inst), Deciphera (Inst), PTC Therapeutics (Inst), Cogent Biosciences (Inst), Oncternal Therapeutics (Inst), BioAtla (Inst), InhibRx (Inst)
Patents, Royalties, Other Intellectual Property: PandoNet—Limbguard
Travel, Accommodations, Expenses: Daiichi Sankyo, NanoCarrier, SpringWorks Therapeutics
Kristen Ganjoo
Consulting or Advisory Role: Foundation Medicine, Deciphera, Adaptimmune, Boehringer Ingelheim, Boehringer Ingelheim
Brian A. Van Tine
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Leadership: Polaris
Honoraria: Iterion Therapeutics, Inc, Total Health Conference
Consulting or Advisory Role: Epizyme, Daiihi Sankyo, Bayer, Deciphera, ADRx, Ayala Pharmaceuticals, Intellisphere, PTC Therapeutics, Boehringer Ingelheim, EcoR1 Capital, Deciphera Pharmaceuticals, Advenchen Laboratories, Putnam Associates, Salarius Pharmaceuticals, Inc, Boxer Capital LLC, Acuta Capital Partners, LLC, Aadi, Race Oncology, Hinge Bio, Inc, Kronos Bio, Inc, PTC Therapeutics, Agenus, Regeneron, Curis
Research Funding: Pfizer, Merck, TRACON Pharma, GlaxoSmithKline, Polaris
Patents, Royalties, Other Intellectual Property: Patent on the use of ME1 as a biomarker, Patent on ALEXT3102, Accuronix Therapeutics—Licensing agreement. Sigma-2 Receptor Ligands and Therapeutic uses thereof (006766), Modular Platform for Targeted Therapeutic Delivery (006755), Sigma-2 Receptor Ligand Drug Conjugates as Antitumor Compounds, Methods of synthesis and Uses Thereof (014229)
Expert Testimony: Health Advances
Travel, Accommodations, Expenses: Adaptimmune, Advenchen Laboratories, Kronos Bio, Inc
Rashmi Chugh
Consulting or Advisory Role: Deciphera, Jazz Pharmaceuticals, InhibRx, SpringWorks Therapeutics
Research Funding: Epizyme (Inst), AADi (Inst), Advenchen Laboratories (Inst), SpringWorks Therapeutics (Inst), GlaxoSmithKline (Inst), Qilu Puget Sound Biotherapeutics (Inst), AstraZeneca (Inst), Janssen (Inst), Ayala Pharmaceuticals (Inst), Cogent Biosciences (Inst), InhibRx (Inst), Cornerstone Pharmaceuticals (Inst), Pfizer (Inst), PTC Therapeutics (Inst), Kronos Bio (Inst), Astex Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Wolters Kluwer
Lee Cranmer
Research Funding: AADi (Inst), Advenchen Laboratories (Inst), Lilly (Inst), Exelixis (Inst), Iterion Therapeutics (Inst), Philogen (Inst), Merck (Inst), TRACON Pharma (Inst), Avacta Life Sciences (Inst), Salarius Pharmaceuticals (Inst), InhibRx (Inst), Salarius Pharmaceuticals (Inst), Boehringer Ingelheim (Inst)
Travel, Accommodations, Expenses: AADi
Erlinda M. Gordon
Stock and Other Ownership Interests: Counterpoint Biomedica, Delta Next-Gene
Research Funding: Jazz Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Co-inventor of patents on targeting pharmaceutical agents to injured tissues
Travel, Accommodations, Expenses: Immix BioPharma
Jason L. Hornick
Consulting or Advisory Role: AADi, TRACON Pharma, Adaptimmune, Leica Biosystems
Li Ding
Employment: AADi
Stock and Other Ownership Interests: AADi
Anita N. Schmid
Employment: AADi
Stock and Other Ownership Interests: AADi
Willis H. Navarro
Employment: AADi, Atara Biotherapeutics
Stock and Other Ownership Interests: Atara Biotherapeutics, Pfizer, bluebird bio, Novo Nordisk, Moderna Therapeutics, AADi
Consulting or Advisory Role: Imago Pharma
David J. Kwiatkowski
Consulting or Advisory Role: Genentech/Roche, AADI, Slingshot Insights, Guidepoint Global, Bridgebio, William Blair
Research Funding: AADi, Revolution Medicines, Genentech/Roche
Mark A. Dickson
Research Funding: Lilly (Inst), AADi (Inst), Sumitomo Dainippon Pharma Oncology (Inst)
No other potential conflicts of interest were reported.
SUPPORT
Supported by Aadi Bioscience. This study was funded in part by US Food and Drug Administration Office of Orphan Products Development (OOPD) Grant No. R01FD005749.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
The data used for the analyses in this manuscript are available on request from the corresponding author.
AUTHOR CONTRIBUTIONS
Conception and design: Andrew J. Wagner, Vinod Ravi, Kristen Ganjoo, Anita N. Schmid, Mark A. Dickson
Provision of study materials or patients: Andrew J. Wagner, Vinod Ravi, Richard F. Riedel, Kristen Ganjoo, Brian A. Van Tine, Rashmi Chugh, Lee Cranmer, Erlinda M. Gordon, Mark A. Dickson
Collection and assembly of data: Andrew J. Wagner, Vinod Ravi, Richard F. Riedel, Kristen Ganjoo, Brian A. Van Tine, Rashmi Chugh, Lee Cranmer, Jason L. Hornick, Heng Du, Anita N. Schmid, Mark A. Dickson, Li Ding, David J. Kwiatkowski
Data analysis and interpretation: Andrew J. Wagner, Vinod Ravi, Richard F. Riedel, Kristen Ganjoo, Brian A. Van Tine, Rashmi Chugh, Lee Cranmer, Erlinda M. Gordon, Jason L. Hornick, Li Ding, Anita N. Schmid, Willis H. Navarro, David J. Kwiatkowski, Mark A. Dickson, Li Ding
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Trial of nab-Sirolimus in Patients With Advanced Malignant Perivascular Epithelioid Cell Tumors (AMPECT): Long-Term Efficacy and Safety Update
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Andrew J. Wagner
Consulting or Advisory Role: Lilly, Daiichi Sankyo, Deciphera, Mundipharma, Cogent Biosciences, Boehringer Ingelheim, AADi, BioAtla, SERVIER, InhibRx
Research Funding: Lilly (Inst), Plexxikon (Inst), Daiichi Sankyo (Inst), Karyopharm Therapeutics (Inst), Deciphera (Inst), Foghorn Therapeutics (Inst), AADi (Inst), Rain Therapeutics (Inst), Cogent Biosciences (Inst)
Vinod Ravi
Stock and Other Ownership Interests: TRACON Pharma, Merck, AstraZeneca, Pfizer, Moderna Therapeutics
Honoraria: UpToDate
Consulting or Advisory Role: Daiichi Sankyo, Gerson Lehrman Group, Guidepoint Inc, AADi
Research Funding: Novartis (Inst), TRACON Pharma (Inst), AADi (Inst), Athenex (Inst)
Patents, Royalties, Other Intellectual Property: Author Royalties from Uptodate
Travel, Accommodations, Expenses: Daiichi Sankyo
Richard F. Riedel
Employment: Limbguard
Leadership: Limbguard
Stock and Other Ownership Interests: Limbguard
Consulting or Advisory Role: Daiichi Sankyo, NanoCarrier, Deciphera, SpringWorks Therapeutics, Blueprint Medicines, AADi, GlaxoSmithKline, Adaptimmune, Boehringer Ingelheim
Research Funding: TRACON Pharma (Inst), AADi (Inst), Arog (Inst), Daiichi Sankyo (Inst), NanoCarrier (Inst), GlaxoSmithKline (Inst), SpringWorks Therapeutics (Inst), Blueprint Medicines (Inst), Epizyme (Inst), Philogen (Inst), Ayala Pharmaceuticals (Inst), Trillium Therapeutics (Inst), Deciphera (Inst), PTC Therapeutics (Inst), Cogent Biosciences (Inst), Oncternal Therapeutics (Inst), BioAtla (Inst), InhibRx (Inst)
Patents, Royalties, Other Intellectual Property: PandoNet—Limbguard
Travel, Accommodations, Expenses: Daiichi Sankyo, NanoCarrier, SpringWorks Therapeutics
Kristen Ganjoo
Consulting or Advisory Role: Foundation Medicine, Deciphera, Adaptimmune, Boehringer Ingelheim, Boehringer Ingelheim
Brian A. Van Tine
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Leadership: Polaris
Honoraria: Iterion Therapeutics, Inc, Total Health Conference
Consulting or Advisory Role: Epizyme, Daiihi Sankyo, Bayer, Deciphera, ADRx, Ayala Pharmaceuticals, Intellisphere, PTC Therapeutics, Boehringer Ingelheim, EcoR1 Capital, Deciphera Pharmaceuticals, Advenchen Laboratories, Putnam Associates, Salarius Pharmaceuticals, Inc, Boxer Capital LLC, Acuta Capital Partners, LLC, Aadi, Race Oncology, Hinge Bio, Inc, Kronos Bio, Inc, PTC Therapeutics, Agenus, Regeneron, Curis
Research Funding: Pfizer, Merck, TRACON Pharma, GlaxoSmithKline, Polaris
Patents, Royalties, Other Intellectual Property: Patent on the use of ME1 as a biomarker, Patent on ALEXT3102, Accuronix Therapeutics—Licensing agreement. Sigma-2 Receptor Ligands and Therapeutic uses thereof (006766), Modular Platform for Targeted Therapeutic Delivery (006755), Sigma-2 Receptor Ligand Drug Conjugates as Antitumor Compounds, Methods of synthesis and Uses Thereof (014229)
Expert Testimony: Health Advances
Travel, Accommodations, Expenses: Adaptimmune, Advenchen Laboratories, Kronos Bio, Inc
Rashmi Chugh
Consulting or Advisory Role: Deciphera, Jazz Pharmaceuticals, InhibRx, SpringWorks Therapeutics
Research Funding: Epizyme (Inst), AADi (Inst), Advenchen Laboratories (Inst), SpringWorks Therapeutics (Inst), GlaxoSmithKline (Inst), Qilu Puget Sound Biotherapeutics (Inst), AstraZeneca (Inst), Janssen (Inst), Ayala Pharmaceuticals (Inst), Cogent Biosciences (Inst), InhibRx (Inst), Cornerstone Pharmaceuticals (Inst), Pfizer (Inst), PTC Therapeutics (Inst), Kronos Bio (Inst), Astex Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Wolters Kluwer
Lee Cranmer
Research Funding: AADi (Inst), Advenchen Laboratories (Inst), Lilly (Inst), Exelixis (Inst), Iterion Therapeutics (Inst), Philogen (Inst), Merck (Inst), TRACON Pharma (Inst), Avacta Life Sciences (Inst), Salarius Pharmaceuticals (Inst), InhibRx (Inst), Salarius Pharmaceuticals (Inst), Boehringer Ingelheim (Inst)
Travel, Accommodations, Expenses: AADi
Erlinda M. Gordon
Stock and Other Ownership Interests: Counterpoint Biomedica, Delta Next-Gene
Research Funding: Jazz Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Co-inventor of patents on targeting pharmaceutical agents to injured tissues
Travel, Accommodations, Expenses: Immix BioPharma
Jason L. Hornick
Consulting or Advisory Role: AADi, TRACON Pharma, Adaptimmune, Leica Biosystems
Li Ding
Employment: AADi
Stock and Other Ownership Interests: AADi
Anita N. Schmid
Employment: AADi
Stock and Other Ownership Interests: AADi
Willis H. Navarro
Employment: AADi, Atara Biotherapeutics
Stock and Other Ownership Interests: Atara Biotherapeutics, Pfizer, bluebird bio, Novo Nordisk, Moderna Therapeutics, AADi
Consulting or Advisory Role: Imago Pharma
David J. Kwiatkowski
Consulting or Advisory Role: Genentech/Roche, AADI, Slingshot Insights, Guidepoint Global, Bridgebio, William Blair
Research Funding: AADi, Revolution Medicines, Genentech/Roche
Mark A. Dickson
Research Funding: Lilly (Inst), AADi (Inst), Sumitomo Dainippon Pharma Oncology (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. Sanfilippo R, Jones RL, Blay JY, et al. Role of chemotherapy, VEGFR inhibitors, and mTOR inhibitors in advanced perivascular epithelioid cell tumors (PEComas) Clin Cancer Res. 2019;25:5295–5300. doi: 10.1158/1078-0432.CCR-19-0288. [DOI] [PubMed] [Google Scholar]
- 2.Aadi Bioscience . FYARRO (Sirolimus Protein-Bound Particles for Injectable Suspension) (Albumin-bound), for Intravenous Use [Prescribing Information] Pacific Palisades, CA: Aadi Bioscience; 2021. [Google Scholar]
- 3. Wagner AJ, Ravi V, Riedel RF, et al. nab-Sirolimus for patients with malignant perivascular epithelioid cell tumors. J Clin Oncol. 2021;39:3660–3670. doi: 10.1200/JCO.21.01728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for the analyses in this manuscript are available on request from the corresponding author.