Abstract
PURPOSE
In the treatment of non–small-cell lung cancer (NSCLC) with a driver mutation, the role of anti–PD-(L)1 antibody after tyrosine kinase inhibitor (TKI) remains unclear. This randomized, open-label, multicenter, phase III study evaluates the efficacy of atezolizumab plus bevacizumab, paclitaxel, and carboplatin (ABCP ) in EGFR- or ALK-mutated NSCLC that progressed before TKI therapy.
MATERIALS AND METHODS
We compared the clinical efficacy of ABCP followed by maintenance therapy with atezolizumab plus bevacizumab with pemetrexed plus carboplatin or cisplatin (PC) followed by pemetrexed maintenance. The primary end point was progression-free survival (PFS).
RESULTS
A total of 228 patients with activating EGFR mutation (n = 215) or ALK translocation (n = 13) were enrolled from 16 sites in the Republic of Korea and randomly assigned at 2:1 ratio to either ABCP (n = 154) or PC arm (n = 74). The median follow-up duration was 26.1 months (95% CI, 24.7 to 28.2). Objective response rates (69.5% v 41.9%, P < .001) and median PFS (8.48 v 5.62 months, hazard ratio [HR], 0.62 [95% CI, 0.45 to 0.86]; P = .004) were significantly better in the ABCP than PC arm. PFS benefit increased as PD-L1 expression increased, with an HR of 0.47, 0.41, and 0.24 for PD-L1 ≥1%, ≥10%, and ≥50%, respectively. Overall survival was similar between ABCP and PC arm (20.63 v 20.27 months, HR, 1.01 [95% CI, 0.69 to 1.46]; P = .975). The safety profile of the ABCP arm was comparable with that previously reported, with no additional safety signals, but higher rates of treatment-related adverse events were observed compared with the PC arm.
CONCLUSION
To our knowledge, this study is the first randomized phase III study to demonstrate the clinical benefit of anti–PD-L1 antibody in combination with bevacizumab and chemotherapy in patients with EGFR- or ALK-mutated NSCLC who have progressed on relevant targeted therapy.
INTRODUCTION
A tyrosine kinase inhibitor (TKI) is the current first-line treatment for metastatic non–small-cell lung cancer (NSCLC) with activating EGFR mutation or ALK translocation.1 Osimertinib, a third-generation EGFR TKI, is the preferred first-line therapy for patients with activating EGFR mutation or with acquired Thr790Met mutations who have progressed after first- or second-generation TKI.2,3 Similarly, a number of ALK TKIs are preferred as the first-line treatment for patients with ALK translocation.4-6 Despite a high initial response and prolonged progression-free survival (PFS) up to years, almost all patients experience acquired resistance to TKIs.
CONTEXT
Key Objective
Chemoimmunotherapy combined with antiangiogenic agent can improve clinical outcomes in EGFR- or ALK-positive patients who progressed on targeted agents.
Knowledge Generated
Atezolizumab plus bevacizumab/paclitaxel/carboplatin significantly improved progression-free survival compared with pemetrexed/platinum combination with 38% risk reduction and showed a higher response rate (69.5% v 41.9%).
Relevance (T.E. Stinchcombe)
-
This phase III trial demonstrated improved objective response rate and longer PFS with the four drug combination compared to platinum-pemetrexed, but with a higher rate of grade 3 or greater treatment related adverse events.*
*Relevance section written by JCO Associate Editor Thomas E. Stinchcombe, MD.
Patients treated with anti–PD-(L)1 immune checkpoint inhibitors (ICIs), with or without chemotherapy, have demonstrated superior survival in NSCLC without a driver mutation.7,8 In contrast, a meta-analysis revealed no clinical benefit of ICI monotherapy in patients with EGFR-mutated NSCLC.9 The CheckMate-722 trial, which compared nivolumab plus platinum-doublet chemotherapy with platinum-doublet chemotherapy alone in patients who have progressed on prior EGFR TKI treatment, failed to demonstrate PFS and overall survival (OS) benefits.10 Another randomized phase III study, KEYNOTE-789, demonstrated a trend toward improved PFS and OS with pembrolizumab plus chemotherapy compared with the chemotherapy alone but failed to show statistical significance. As a consequence, platinum-based chemotherapy is the standard of care for patients with EGFR TKI failure.11
Bevacizumab, the vascular endothelial growth factor (VEGF) monoclonal antibody, may enhance the activity of chemoimmunotherapy by increasing lymphocyte trafficking to the tumor microenvironment and reversing VEGF-mediated immunosuppression.12,13 In addition, indirect effects on the EGFR pathway by VEGF inhibition were observed, showing PFS benefit.14,15 The subgroup analysis of the phase III IMpower150 study revealed the improved efficacy of anti–PD-L1 antibody in combination with antiangiogenic therapy, particularly in EGFR-mutated NSCLC.16-18 In the sensitizing EGFR-mutant subgroup, atezolizumab plus bevacizumab/carboplatin/paclitaxel (ABCP) was superior to bevacizumab plus carboplatin and paclitaxel in terms of PFS and OS. Given the exploratory nature of subgroup analysis and small proportion of patients with EGFR-mutated NSCLC (approximately 10% of the total population), a phase III randomized study is required for confirmation.
The purpose of this study was to evaluate the efficacy and safety of ABCP on the basis of the IMpower150 regimen in patients with sensitizing EGFR- or ALK-mutated NSCLC who had progressed on prior TKI treatment.
MATERIALS AND METHODS
Study Design and Treatment
This study is a phase III, multicenter, open-label, randomized trial conducted at 16 hospitals across the Republic of Korea. Patients were randomly assigned to either ABCP arm or pemetrexed plus carboplatin or cisplatin (PC arm) in a 2:1 ratio. Eligible patients were stratified on the basis of the mutation type (EGFR mutation v ALK translocation) and the presence of brain metastases (yes v no). Before random assignment, the investigator determined whether the induction treatment would be administered in four or six cycles every 21 days for both arms. After the induction phase, patients continued to receive maintenance therapy with atezolizumab plus bevacizumab in the ABCP arm and pemetrexed in the PC arm every 21 days until the disease progressed or an event of unacceptable toxicities occurred. If a clinical benefit was anticipated, continued use of atezolizumab beyond disease progression was allowed. No crossover to atezolizumab was permitted. The recruitment with Thr790Met was capped up to 30%.
The following were administered: in the ABCP arm, atezolizumab (1,200 mg) at a fixed dose, bevacizumab (15 mg/kg), paclitaxel (175 mg/body-surface area), and carboplatin at an area under the concentration-time curve (AUC, 5 or 5.5 mg/mL/min) once every 21 days and in the PC arm, pemetrexed (500 mg/m2), carboplatin (AUC 5.0 mg/mL/min), or cisplatin (70 mg/m2) once every 21 days.
This study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki. All the patients provided written informed consent before the screening. The protocol was approved by the institutional review board of Samsung Medical Center (IRB no. 2019-03-027) and participating centers (ClinicalTrials.gov identifier: NCT03991403).
Participants
Patients diagnosed with stage IV NSCLC with sensitizing EGFR mutation or ALK translocation were included in the study. All the patients experienced disease progression or intolerance to one or more EGFR or ALK TKIs. If the patient was identified to have an acquired Thr790Met mutation after the failure of a first- or second-generation EGFR TKI, the patient had to be treated with the third-generation EGFR TKI before enrollment. Patients with at least one measurable lesion, defined by RECIST,19 v1.1, an Eastern Cooperative Oncology Group (ECOG) baseline performance status score of 0 or 1, and asymptomatic brain metastases were allowed to participate (refer to the Protocol for detailed inclusion and exclusion criteria).
End Points and Assessment
The primary end point was investigator-assessed PFS according to the RECIST criteria. The secondary end points included OS, objective response rate (ORR), duration of response, PFS rates at 1 and 2 years, and OS rates at 1 and 2 years. Imaging assessments were scheduled every 6 weeks for the first year, every 9 weeks for the second year, and every 12 weeks thereafter. Patients with a PFS event who missed two or more scheduled assessments immediately before the PFS event were censored at the last tumor assessment before the missed visits. The reduction in tumor volume was calculated by comparing the sum of the target lesion at the baseline and the time point when the number is the smallest per RECIST criteria.
Exploratory Biomarker Analysis
For the exploratory analysis, the expression value of PD-L1 by SP263 and SP142, which tested from either the archival or rebiopsied tissue at the time of TKI resistance, was used. Initial PD-L1 expression results were collected using reported data from each study site. For those without available outcomes, SP142 and SP263 immunohistochemistry assays (Ventana Medical System, Tucson, AZ) were performed at a central laboratory in Samsung Medical Center (Korea). To assess the immune phenotype, we analyzed the spatial distribution of tumor-infiltrating lymphocytes (TILs) using Lunit SCOPE IO powered by artificial intelligence.20 A cutoff of 20% was used to compare the two arms (refer to the Data Supplement, supplementary methods [online only]).
Statistical Analysis
The clinical efficacy and safety were evaluated in modified intention-to-treat (ITT) patients, defined as patients who received at least one study treatment and at least one postbaseline safety assessment from the date of first patient random assignment to the study cutoff date. Using the stratified Cox regression model, hazard ratios (HRs) and 95% CI were calculated. Survival differences between the treatments were estimated using the stratified log-rank test. P value < .05 is considered statistically significant (details included in the Data Supplement, supplementary methods).
A total of 228 patients were required for this study considering 80% power, 5% two-sided type I error, an expected HR of 0.67 (median PFS of 9 months in ABCP arm v 6 months in PC arm), 24 months of enrollment with 10 months of follow-up period, 10% of expected drop-out, and a 2:1 enrollment ratio between ABCP and PC arm. Time for the main statistical analyses was planned either when 193 PFS events occur or 12 months after the last patient was randomly assigned, whichever comes first. SAS software (v9.4, SAS Institute, Cary, NC) and R (version 4.2.2) were used for the analysis. The detailed statistical analysis plan is provided as a separate document.
RESULTS
Baseline Demographics
The date for random assignment of the first and the last patient was August 27, 2019, and March 11, 2022, respectively. The data cutoff for this analysis was March 31, 2023.
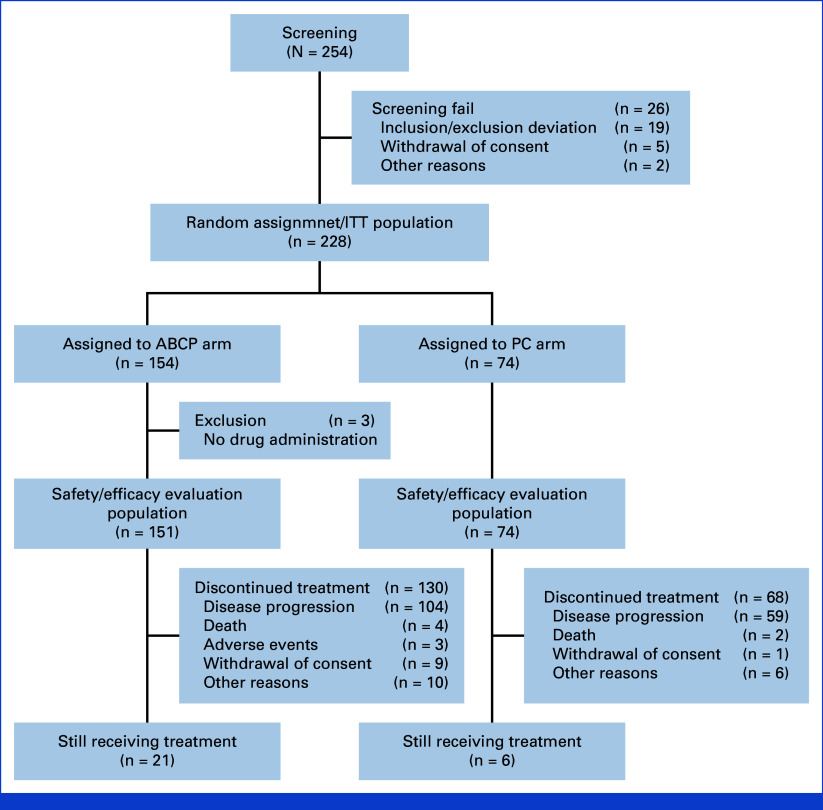
Among the randomly assigned patients (n = 228), excluding three patients from the ABCP arm who did not receive treatment after random assignment, a total of 225 patients who received treatment (ABCP arm: n = 151; PC arm: n = 74) were analyzed for clinical efficacy and safety profile (Fig 1). Table 1 shows that baseline characteristics were evenly distributed between arms. Most patients were female (56.1%) and never-smokers (62.7%). Brain metastasis was present in 42.7% of patients. The majority of patients had previously failed EGFR TKI therapy (95.5% in the ABCP and 91.9% PC arms). The subsequent treatment information in patients after this study is summarized in Appendix Table A1 (online only).
FIG 1.
CONSORT diagram. ABCP, atezolizumab plus bevacizumab, paclitaxel and carboplatin; ITT, intention-to-treat; PC, pemetrexed plus carboplatin or cisplatin.
TABLE 1.
Baseline Demographics of the Intention-to-Treat Population
| Characteristic | ABCP Arm (n = 154) | PC Arm (n = 74) | P |
|---|---|---|---|
| Age, years, median (range) | 63 (36-83) | 61 (31-90) | .444 |
| Age group, years, No. (%) | |||
| <65 | 87 (56.5) | 46 (62.2) | .416 |
| ≥65 | 67 (43.5) | 28 (37.8) | |
| Male, No. (%) | 66 (42.9) | 34 (46.0) | .660 |
| Female, No. (%) | 88 (57.1) | 40 (54.1) | |
| History of smoking use | |||
| Never | 97 (63.0) | 46 (62.2) | .904 |
| Ex or current | 57 (37.0) | 28 (37.8) | |
| ECOG PS score, No. (%) | |||
| 0 | 15 (9.7) | 10 (13.5) | .393 |
| 1 | 139 (90.3) | 64 (86.5) | |
| Histology, No. (%) | |||
| Adenocarcinoma | 153 (99.3) | 74 (100) | .532 |
| Pleomorphic carcinoma | 1 (0.7) | 0 | |
| Driver mutation status, No. (%) | |||
| EGFR mutation positive | 147 (95.5) | 68 (91.9) | .360 |
| ALK translocation | 7 (4.6) | 6 (8.1) | |
| EGFR mutations status detail, No. (%) | |||
| Deletion 19 | 70 (47.6) | 42 (61.8) | .148 |
| L858R | 75 (51.0) | 25 (36.8) | |
| Other | 2 (1.4)a | 1 (1.5)b | |
| Acquired T790M mutationsc, No. (%) | 51 (34.7) | 20 (29.4) | .444 |
| Brain metastases | 67 (43.5) | 31 (41.9) | .818 |
| Previous EGFR TKI treatment | |||
| First-/second-generation EGFR TKI | 84 (57.1) | 39 (57.4) | .445 |
| Third-generation EGFR TKI after first-/second-generation EGFR TKI | 51 (34.7) | 20 (29.4) | |
| Third-generation EGFR TKI as first-line treatment | 12 (8.2) | 9 (13.2) | |
| Previous ALK treatment | |||
| Exposed to one ALK TKI | 6 (3.9) | 4 (5.4) | .559 |
| Exposed to two or more ALK TKsI | 1 (14.3) | 2 (2.7) | |
| PD-L1 SP142 expression, No. (%)d | |||
| SP142 TC0 and IC0 | 72 (84.7) | 41 (93.2) | .166 |
| SP142 TC1/2/3 or IC1/2/3 | 13 (15.3) | 3 (6.8) | |
| PD-L1 SP263 expression, No. (%)d | |||
| SP263 PD-L1 <1% | 55 (59.8) | 24 (49.0) | .219 |
| SP263 PD-L1 ≥1% | 37 (40.2) | 25 (51.0) | |
| SP263 PD-L1 ≥10% | 25 (27.2) | 11 (22.4) | .540 |
| SP263 PD-L1 ≥50% | 15 (16.3) | 8 (16.3) | .997 |
| Lunit SCOPE analysis | 99 (64.3) | 49 (66.2) | .775 |
Abbreviations: ABCP, atezolizumab plus bevacizumab, paclitaxel, and carboplatin; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; IC, immune cell; PC, pemetrexed plus carboplatin or cisplatin; TC, tumor cell; TKI, tyrosine kinase inhibitor.
Other from the ABCP arm includes G719X & S768I, L861Q mutation.
Other from the PC arm include G719X & S768I.
T790M mutation as acquired resistance mechanism after prior first- or second-generation EGFR TKI.
Percent calculated on the basis of the patients available for each PD-L1 test within each arm.
Clinical Efficacy and Survival Analysis
The median duration of follow-up for study population was 26.1 months (24.7-28.2). The ORR in the ABCP arm was significantly higher than in the PC arm (69.5% v 41.9%, P < .001; Fig 2A). One patient (0.7%) with complete response and 104 patients (68.9%) with partial response (PR) were identified in the ABCP arm, whereas 31 patients (41.9%) with PR were identified in the PC arm (Appendix Table A2).
FIG 2.
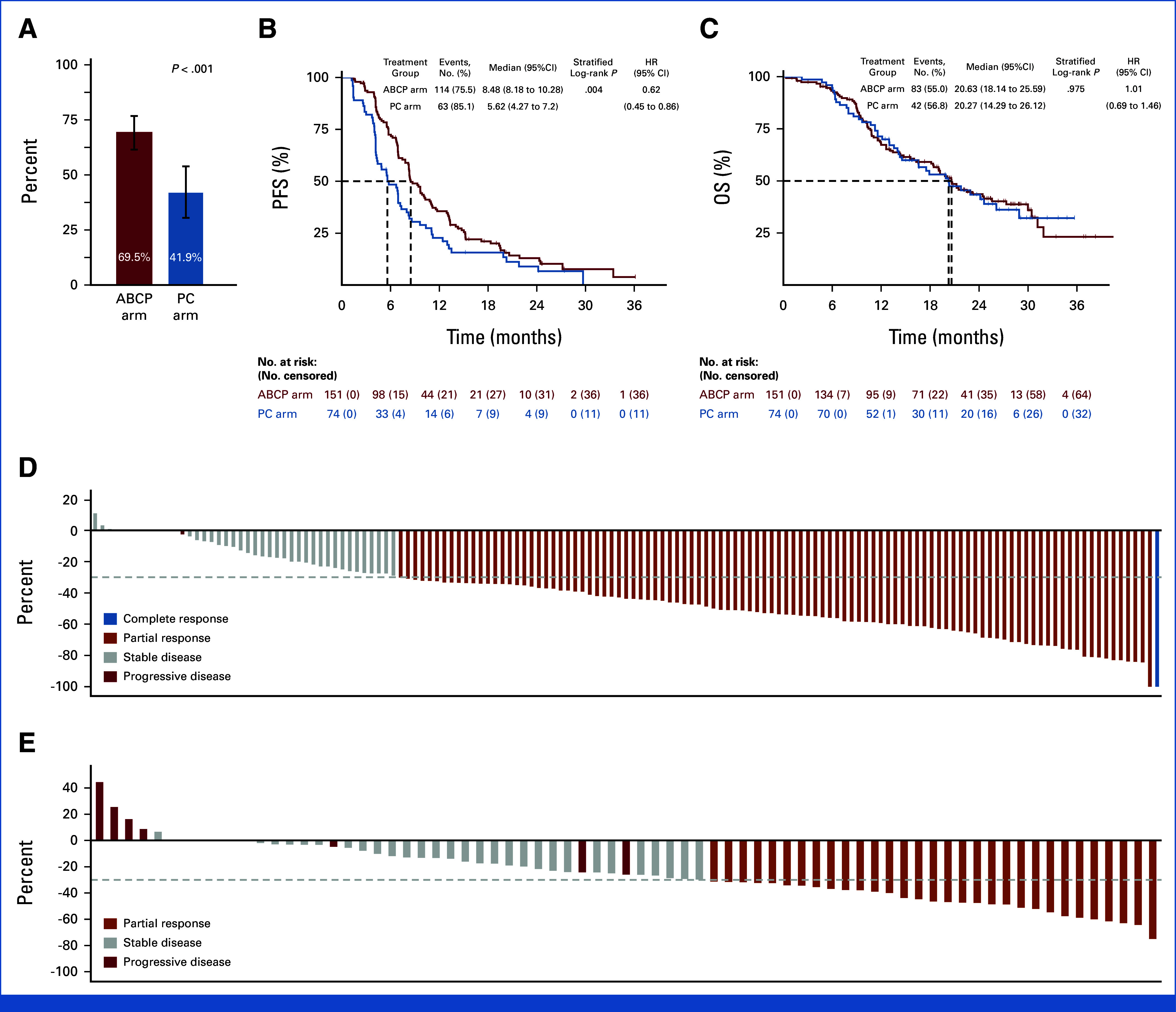
PFS, OS, and depth or response in the efficacy/safety evaluation population. (A) Objective response rate. (B) Kaplan-Meier curves for investigator-assessed progression-free survival and (C) OS; HR and P values were calculated using a stratified log-rank test using stratification factor (EGFR v ALK) and presence of brain metastases (yes v no). Waterfall plot for tumor response as per RECIST from the (D) ABCP arm (E) and PC arm. ABCP, atezolizumab plus bevacizumab, paclitaxel, and carboplatin; HR, hazard ratio; OS, overall survival; PC, pemetrexed plus carboplatin or cisplatin; PFS, progression-free survival; TKI, tyrosine kinase inhibitor.
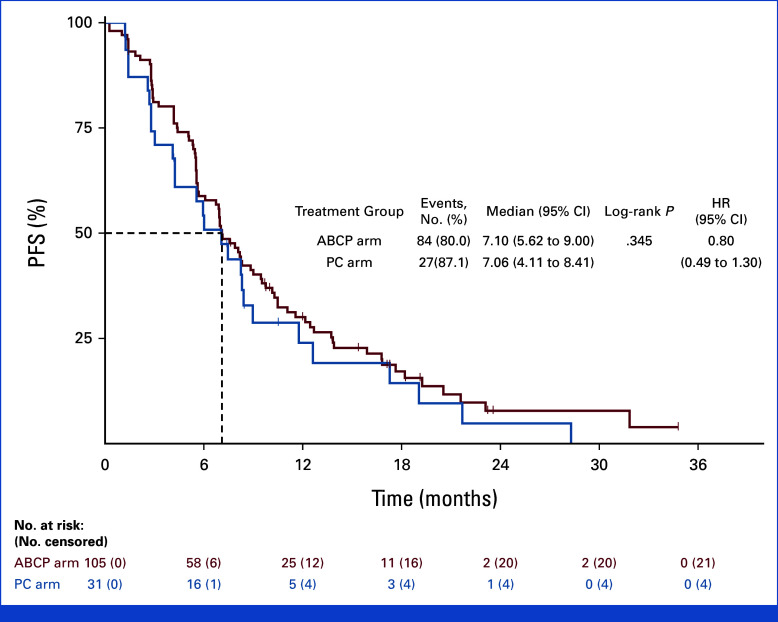
PFS events were observed in 114 patients (75.5%) in the ABCP and 63 patients (85.1%) in the PC arms. Among the censored patients, seven patients in the ABCP arm and two patients in the PC arm are censored as the date of the latest image evaluation because of missing two consecutive imaging schedules. Median PFS was significantly longer in the ABCP arm (8.48 months v 5.62 months), with an HR of 0.62 (95% CI, 0.45 to 0.86; P = .004; Fig 2B). The duration of response was 7.10 (95% CI, 5.62 to 9.00) and 7.06 (95% CI, 4.11 to 8.41) months in ABCP and PC arms, respectively (HR, 0.80 [95% CI, 0.49 to 1.30]; P = .345; Appendix Fig A1). The PFS rate at 6 months was 0.72 (95% CI, 0.64 to 0.79) versus 0.48 (95% CI, 0.36 to 0.59; P = .163) and 0.36 at 12 months (95% CI, 0.28 to 0.44) versus 0.23 (95% CI, 0.14 to 0.33; P = .012), in the ABCP and PC arms, respectively (Appendix Table A3). OS events were observed in 83 patients (55.0%) in the ABCP arm and 42 patients (56.8%) in the PC arm. The median OS was 20.63 (95% CI, 18.14 to 25.69) months in the ABCP arm and 20.27 (95% CI, 14.29 to 26.12) in the PC arm (HR, 1.01 [95% CI, 0.69 to 1.46; P = .975; Fig 2C). A significant reduction in the sum of target lesions per RECIST was observed in the ABCP arm compared with the PC arm (median 43.8% v 26.0%, P < .001; Figs 2D and 2E).
Subgroup Analysis
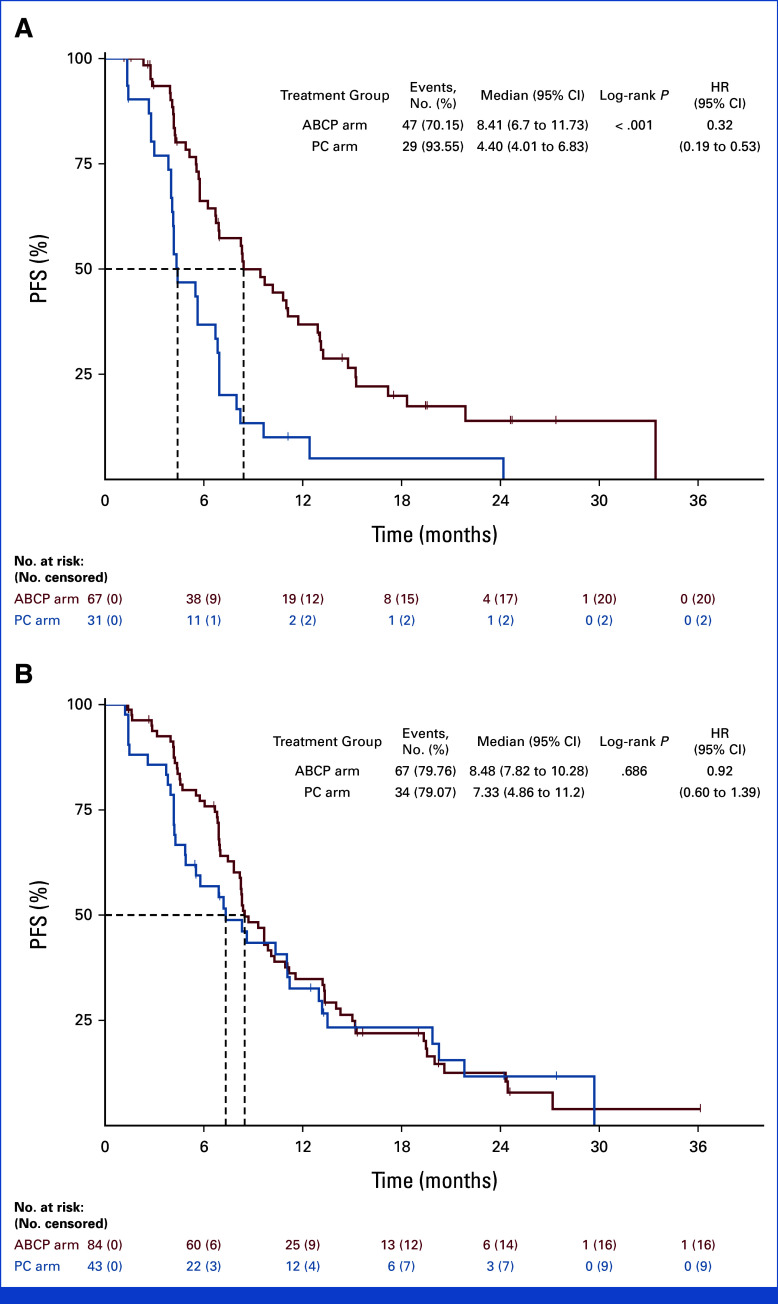
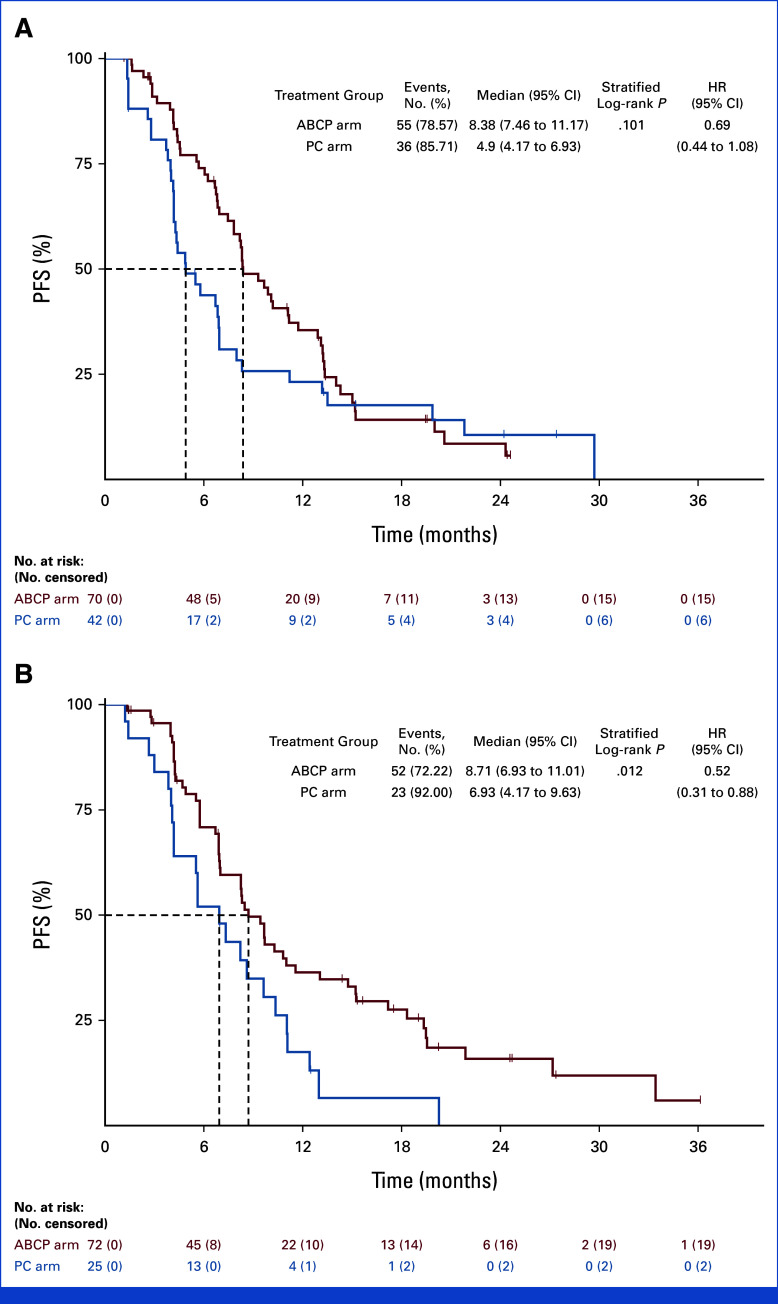
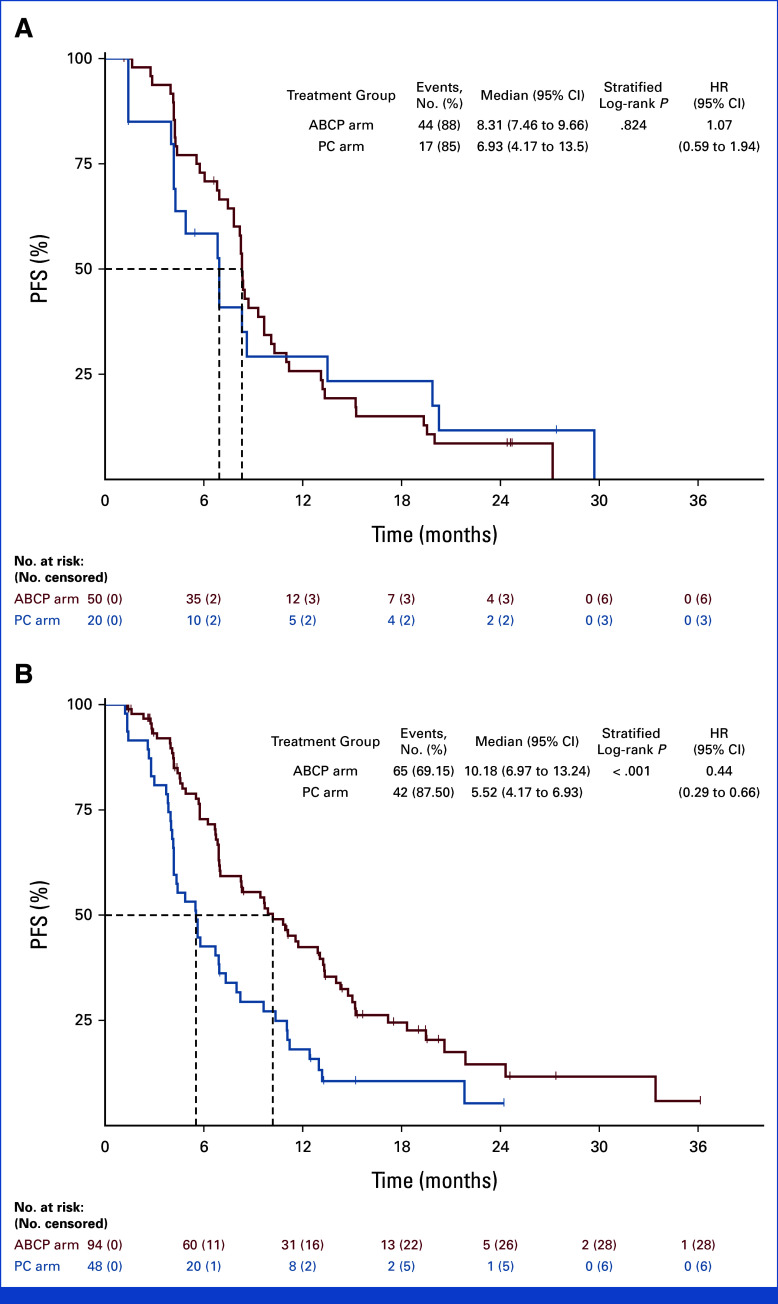
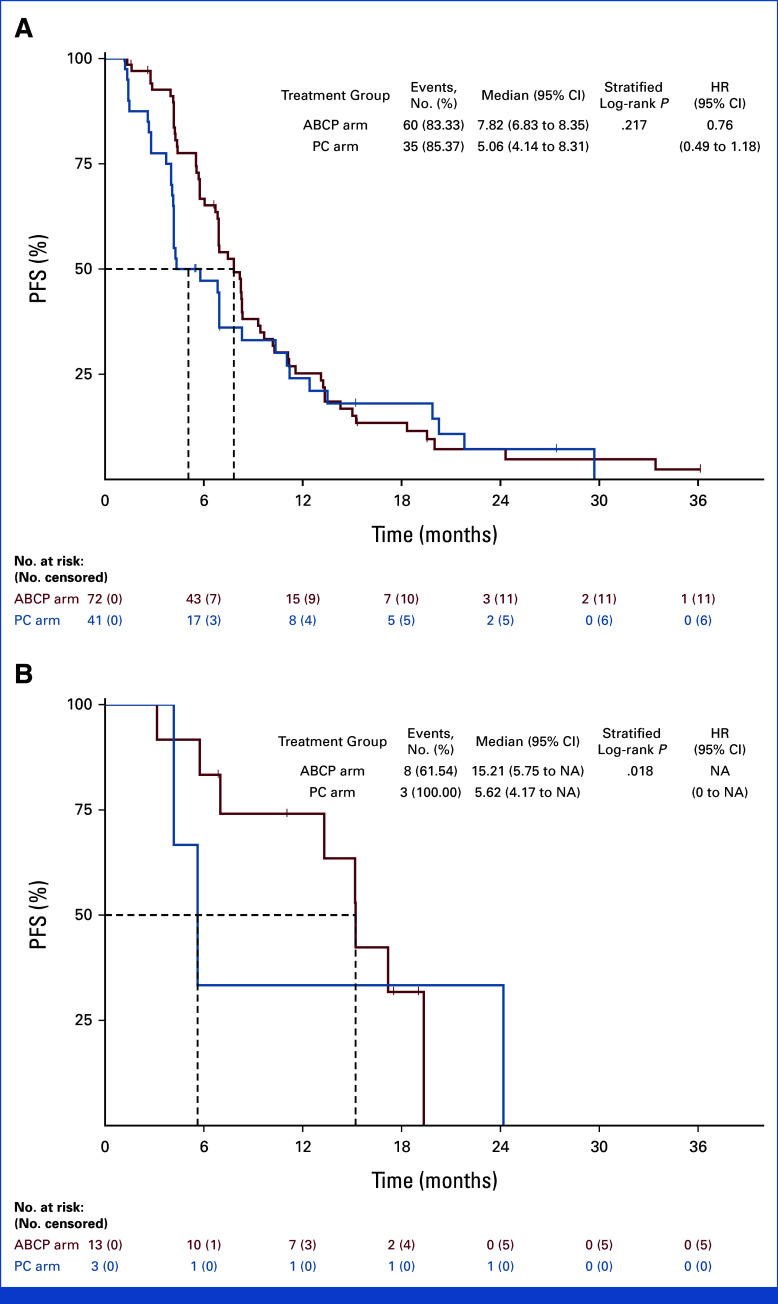
Subgroup analysis was conducted on the basis of the PFS (Fig 3). In the subgroup of patients with brain metastases at the time of study enrollment, PFS was significantly longer in the ABCP arm than the PC arm (8.41 months v 4.40 months, HR, 0.32; [95% CI, 0.19 to 0.53; P < .001]; Appendix Fig A2). In contrast, no difference in PFS was observed between ABCP and PC arms in the subgroup without brain metastases (8.48 months v 7.33 months, HR, 0.92 [95% CI, 0.60 to 1.39]; P = .686). In EGFR deletion 19 subgroup, there was no significant difference in PFS (HR, 0.69 [95% CI, 0.44 to 1.08]; P = .101). However, favorable PFS was observed in the EGFR Leu858Arg subgroup (HR, 0.52 [95% CI, 0.31 to 0.88]; P = .012; Appendix Fig A3). For those with acquired EGFR Thr790Met, there was no difference in PFS between groups (HR, 1.07 [95% CI, 0.59 to 1.94]; P = .824), whereas favorable PFS was observed in the subgroup without EGFR Thr790Met mutation (HR, 0.44 [95% CI, 0.26 to 0.66]; P < .001; Appendix Fig A4). There was no difference between subgroups in terms of OS (Appendix Fig A5).
FIG 3.
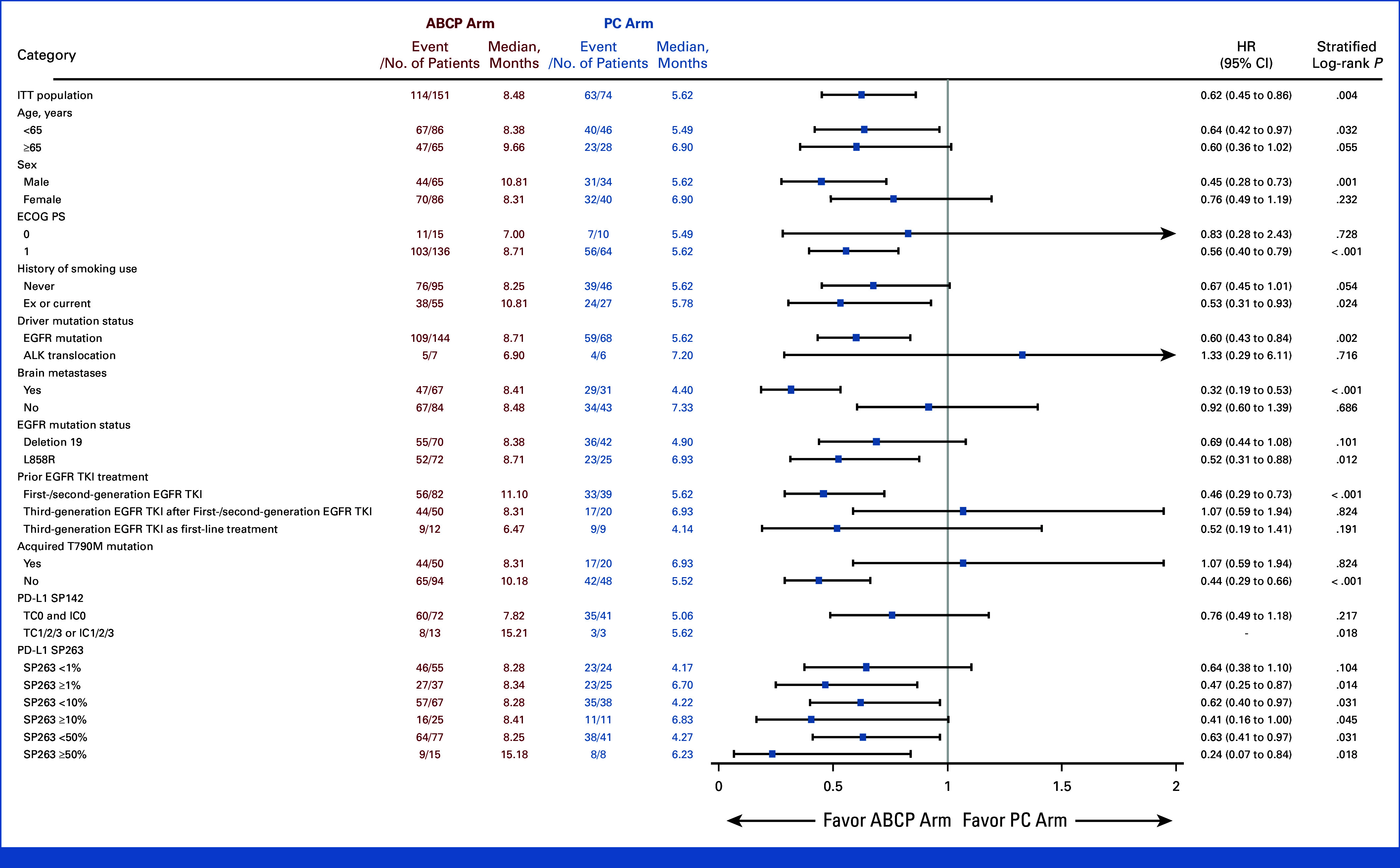
Forest plot on the basis of PFS. ABCP, atezolizumab plus bevacizumab, paclitaxel, and carboplatin; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; ITT, intention-to-treat; PC, pemetrexed plus carboplatin or cisplatin; PFS, progression-free survival.
Safety Analysis
The median number of ABCP treatment cycle during the induction phase was four (range, 1-6). As maintenance therapy, the median number of cycle was 12 for atezolizumab (range, 5-53) and 8 for bevacizumab (range, 1-42; Appendix Table A4). For PC treatment, pemetrexed (range, 2-6), cisplatin (range, 2-4), and carboplatin (range, 2-6) each had a median of four cycles of therapy during the induction phase. Pemetrexed maintenance therapy was administered for a median of 10 cycles (range, 5-43; Appendix Table A5).
Any TRAEs were observed in 96.7% of the ABCP arm and 75.7% of the PC arm (Table 2). Incidences of grade 3 or higher TRAEs were 35.1% in the ABCP arm and 14.9% in the PC arm. In the ABCP arm, peripheral neuropathy, alopecia, and myalgia were the most prevalent TRAEs. In the ABCP arm, 82 patients (54.3%) required either treatment interruption or dose modification, and two patients (1.3%) discontinued treatment permanently, which was due to grade 2 thrombocytopenia and grade 4 cerebral infarction. In the ABCP arm, there were three treatment-relate deaths owing to pneumonia (n = 2) and cerebral embolic infarction (n = 1). In the PC arm, 10 patients (13.5%) required treatment interruption or dose modification; however, no patient discontinued treatment because of the TRAEs. Detailed incidence of adverse events observed during the induction and maintenance phase is shown in Appendix Table A6. In the ABCP arm, immune-related adverse events of grade 1 or 2 was observed in 70 patients (46.3%), grade 3 in 10 patients (6.6%), and grade 4 in two patients (1.3%; Appendix Table A7).
TABLE 2.
Overview of Safety Profile and Incidence of Treatment-Related Adverse Events (≥10%)
| Category | ABCP Arm (n = 151), No. (%) | PC Arm (n = 74), No. (%) | ||||
|---|---|---|---|---|---|---|
| Any grade | Grade 3≤ | Any grade | Grade 3≤ | |||
| Any treatment-emergent adverse events | 149 (98.7) | 61 (40.4) | 62 (83.8) | 16 (21.6) | ||
| Any TRAEs | 146 (96.7) | 53 (35.1) | 56 (75.7) | 11 (14.9) | ||
| Any serious adverse event | 33 (21.9) | 24 (15.9) | 1 (1.4) | — | ||
| TRAEs leading to dose interruption or modification | 82 (54.3) | 22 (14.6) | 10 (13.5) | 4 (5.4) | ||
| TRAEs leading to permanent discontinuationa | 2 (1.3) | 1 (0.7) | — | — | ||
| TRAEs leading to deathb | 3 (2.0) | 3 (2.0) | — | — | ||
| ABCP Arm (n = 151) | PC Arm (n = 74) | |||||
| Event | Grade 1-2 | Grade 3 | Grade 4 | Grade 1-2 | Grade 3 | Grade 4 |
| Peripheral neuropathy | 105 (69.5) | 4 (2.6) | — | 8 (10.8) | — | — |
| Alopecia | 90 (59.6) | — | — | 2 (2.7) | — | — |
| Myalgia | 39 (25.8) | 2 (1.3) | 1 (0.7) | 2 (2.7) | — | — |
| Hypertension | 27 (17.9) | 3 (2.0) | — | 1 (1.4) | — | — |
| Rash | 25 (16.6) | 5 (3.3) | — | 4 (5.4) | — | — |
| Decreased appetite | 23 (15.2) | — | — | 7 (9.5) | 1 (1.4) | — |
| Fatigue | 17 (11.3) | 4 (2.6) | — | 7 (9.5) | 1 (1.4) | — |
| Pruritus | 21 (13.9) | — | — | 5 (6.8) | — | — |
| Anemia | 14 (9.3) | 5 (3.3) | — | 11 (14.9) | 2 (2.7) | — |
| Hypothyroidism | 19 (12.6) | — | — | — | — | — |
| Proteinuria | 17 (11.3) | 2 (1.3) | — | — | — | — |
| Stomatitis | 17 (11.3) | 1 (0.7) | — | 1 (1.4) | — | — |
| Neutropenia | 7 (4.6) | 8 (5.3) | 2 (1.3) | 4 (5.4) | 1 (1.4) | 1 (1.4) |
| AST/ALT increased | 12 (7.9) | 1 (0.7) | — | 8 (10.8) | 1 (1.4) | — |
| Nausea | 11 (7.3) | 1 (0.7) | — | 16 (21.6) | 1 (1.4) | — |
NOTE. No grade 5 in TRAEs with higher or equal to 10%.
Abbreviations: ABCP, atezolizumab plus bevacizumab, paclitaxel and carboplatin; PC, pemetrexed plus carboplatin or cisplatin; TRAEs, treatment-related adverse events.
From the ABCP arm because of grade 2 thrombocytopenia and grade 4 cerebral infarction.
From the ABCP arm: pneumonia (n = 2); cerebral embolic infarction (n = 1).
Exploratory Biomarker Analysis
Subgroup analysis was conducted using PD-L1 SP263 (n = 141) and SP142 (n = 129) expression profiles as exploratory biomarkers. For PD-L1 SP263 <1% subgroup, there was no difference in PFS between ABCP and PC arms (8.28 months v 4.17 months, HR, 0.64 [95% CI, 0.38 to 1.10]; P = .104; Fig 4A), whereas significant prolongation of PFS was observed in PD-L1 ≥1% (8.34 months v 6.70 months, HR, 0.47 [95% CI, 0.25 to 0.87]; P = .014; Fig 4B). PFS benefit was greater in the ABCP arm with a higher cutoff of PD-L1 SP 263 ≥10% (HR, 0.41, P = .045) and PD-L1 SP263 ≥50% (HR, 0.24, P = .018; Figs 4C and 4D). Only 13 (15.3%) in the ABCP arm and three (6.8%) in the PC arm had PD-L1 SP142 TC1/2/3 or IC 1/2/3 demonstrating a prolonged PFS (15.21 months v 5.62 months, HR, not available, P = .018). In the PD-L1 SP 142–negative subgroup, there was no difference between arms in PFS (P = .217; Appendix Fig A6).
FIG 4.
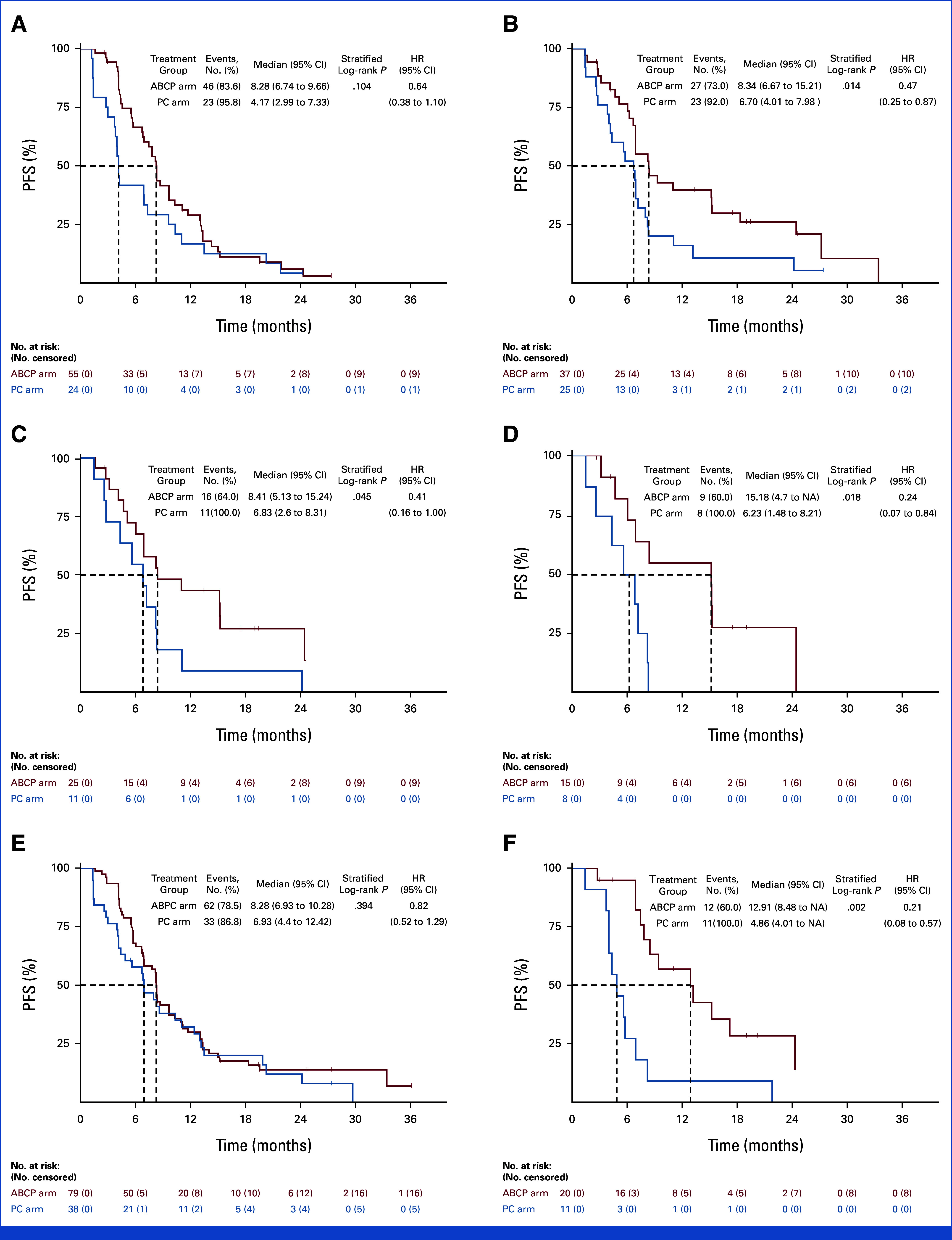
Exploratory analysis on the basis of the PD-L1 SP263 expression and Lunit SCOPE. Kaplan-Meier curves for PFS of patients with PD-L1 SP263 (A) <1%, (B) ≥1%, (C) ≥10%, (D) ≥50%, and Lunit SCOPE based inflamed score (E) <20% and (F) ≥20%. ABCP, atezolizumab plus bevacizumab, paclitaxel, and carboplatin; HR, hazard ratio; NA, not available; PC, pemetrexed plus carboplatin or cisplatin; PFS, progression-free survival.
On the basis of the distribution and density of TILs in the tumor bed, the inflamed score was calculated the spatial distribution of TILs using artificial intelligence-powered Lunit SCOPE IO.20 When 20% of the inflamed score was used as a cutoff, the proportion of high inflamed scores in patients with PD-L1 assessed by SP263 at <1%, 1%-49%, and ≥50% was 19.0% (11 of 58), 14.8% (4 of 27), and 37.5% (6 of 16), respectively. The PFS benefit was not observed in the ABCP arm compared with the PC arm (HR, 0.82, P = .394) in patients with inflamed score below 20% (Fig 4E). In contrast, for patients with ≥20% of the inflamed score, the ABCP arm significantly prolonged PFS compared with the PC arm (12.91 months v 4.86 months, HR, 0.21 [95% CI, 0.08 to 0.57]; P = .002, interaction P = .037; Fig 4F). A notable interaction indicating the PFS benefit of ABCP relative to PC, on the basis of the dichotomous classification using the inflamed score, was evident within the threshold range (11.5%-20.3%; Appendix Fig A7).
DISCUSSION
This randomized phase III study demonstrated a statistically significant prolongation in PFS with atezolizumab in combination with bevacizumab, carboplatin, and paclitaxel versus pemetrexed and cisplatin or carboplatin in patients with EGFR-mutated or ALK-translocated NSCLC who had failed prior TKI treatment. The relative risk reduction of PFS was 38%. The ORR was also significantly higher, and more profound deep response was observed in the ABCP arm compared with the PC arm. These results are consistent with the subgroup analysis of the IMpower150 study. To our knowledge, this is the first study to confirm the ABCP regimen investigated in IMpower150 clinical trial,16,18 which demonstrated improved clinical efficacy compared with platinum-based chemotherapy alone in this study population. Especially, preplanned subgroup analysis showed a significant difference in PFS according to EGFR mutation type showing significant benefit in subgroup with EGFR Leu858Arg and subgroup without acquired EGFR Thr790Met mutation. Given the poor clinical outcomes associated with Leu858Arg relative to exon 19 deletion, this combinational approach could be a reasonable treatment option for patients with Leu858Arg. Although only 10% of the study population were included in the analysis, in patients treated with third-generation EGFR TKI as first-line therapy, the PFS benefit was more prominent in the ABCP arm compared with the PC arm. Given that third-generation EGFR TKI is currently the preferred first-line therapy and no standard of care exists after progression other than platinum-doublet, this regimen should be further investigated in this patient population. Intriguingly, more than 40% of patients had brain metastasis in both arms, which is higher than in previous studies, and patients with brain metastasis had greater PFS benefit with the ABCP regimen compared with the PC arm which might be related to the efficient delivery of immune checkpoint inhibitor and chemotherapies to the brain by applying bevacizumab. Among the subgroups, owing to the small number of patients with ALK translocation in this study, there is a limitation to determine the clinical significance in ALK-mutated patients. In this study, the OS between the ABCP and PC arm were similar despite the PFS benefit. Although no clear explanation can be raised, one of the potential reasons might be related to the high incidence of subsequent poststudy treatment rate in both arms. Regarding the safety profile, incidences of grade 3 or higher TRAE were higher in the ABCP arm compared with those in the PC arms, which was mostly related to cytotoxic chemotherapy. However, some of the TRAEs associated with bevacizumab, such as hypertension or proteinuria or atezolizumab were of grade 1 or 2 but were manageable with provision of appropriate supportive care.
Although the control arm in the IMpower150 study was different from our study, where bevacizumab or atezolizumab with paclitaxel and carboplatin was investigated,16,18 prolongation of PFS of 3.3 months with ABCP was observed, which is similar to our findings. Furthermore, the ORR of ABCP regimen is 28.7% and 35.0% higher than the BCP or ACP regimen, respectively, where similar results were observed in this study. In China, a recent phase III study of sintilimab plus IBI305 (bevacizumab biosimilar) plus pemetrexed/cisplatin versus sintilimab plus chemotherapy versus chemotherapy alone (ORIENT-31 study) demonstrated significant improvement in PFS with a four-drug regimen.21,22 In contrast to our study, the OREINT-31 study used pemetrexed plus cisplatin as cytotoxic chemotherapy. It remains unclear whether the different chemotherapeutic regimens have a significant impact on clinical efficacy. In contrast, two global randomized studies (CheckMate-722 and KEYNOTE-789) comparing chemotherapy plus ICI versus chemotherapy alone in EGFR-mutated NSCLC who had progressed on EGFR TKI failed to demonstrate any clinical benefit, suggesting the role of antiangiogenic agent combined with ICI in EGFR-mutated NSCLC.10,11
Although not clearly understood, low tumor mutation burden, low PD-L1 expression, and decreased TILs, which might be related to the relatively high VEGF expression in EGFR-mutated NSCLC, may explain why EGFR-mutated NSCLC responds poorly to ICI.23,24 Hence, it is possible to hypothesize that combining VEGF inhibition with atezolizumab may increase antigen-specific T-cell migration to the tumor and further enhance T-cell–mediated cytotoxic activity.25 In alignment with this hypothesis, we performed exploratory biomarker analysis. First, using different cutoffs for PD-L1 SP263 expression, we found that the magnitude of PFS benefit increased with PD-L1 high expression, with an HR of 0.47 for PD-L1 ≥1%, 0.41 for PD-L1 ≥10%, and 0.24 for PD-L1 ≥50%. Similar trends were observed in CheckMate-722 and KEYNOTE-789, where PFS benefit in PD-L1 ≥50% with an HR of 0.64 (95% CI, 0.36 to 1.15) and OS benefit in PD-L1 ≥1% with an HR of 0.77 (95% CI, 0.58-1.02) were noted, respectively.10,11 Simultaneously, patients with highly inflamed tumors, as measured by the density of TIL, responded favorably to the ABCP regimen, indicating the essential role of bevacizumab in increasing the sensitivity of atezolizumab by reversing the immunosuppressive tumor microenvironment enriched with TILs in EGFR-mutated NSCLC.26,27 Further biomarker research is required to identify which patients will benefit from these agents.
This study has several limitations to consider. Analysis was conducted in a modified ITT patient, which might be a limitation as our approach departs from intention-to-treat principle. In addition, nine patients were censored as the date of the latest image evaluation because of missing two consecutive image evaluations but showing consistent results on overall outcomes from subsequent sensitivity analysis (data not shown). Patients were recruited from a single country consisting of Asian ethnicity. The majority of patients were treated with first- or second-generation EGFR TKI (53.9%) or third-generation EGFR TKI for Thr790Met mutation (31.1%), which is different from the current standard of care which recommends third-generation EGFR TKI as first-line therapy. In addition, the treatment regimen in the control arm was different from that of the IMpower150 study. However, pemetrexed based platinum combination has been widely used not only in clinical practice but also in clinical trials.10,11,22 Finally, the absence of OS benefit is observed in the current analysis, which needs longer follow-up.
In conclusion, the addition atezolizumab and bevacizumab to chemotherapy showed a significant improvement in PFS and ORR in patients with an activating EGFR mutation or ALK translocation who had failed prior TKI treatment. Although long-term follow-up data are needed to confirm the OS benefit, this regimen offers superior clinical benefit to pemetrexed-platinum doublets as a standard of care and should be considered as the feasible option for subsequent treatment after EGFR or ALK TKI resistance.
ACKNOWLEDGMENT
This study was operated by the Samsung Medical Center Academic-Clinical Research Organization Team (Kyung Ui Hwang, Raejin Lee, Seoha Kim) Central laboratory at Samsung Medical Center (Mi Soon Kim, Kyoung Young Lee, You-Kyung Lee, Jiae Koh, Bo Mi Ku).
APPENDIX
FIG A1.
Kaplan-Meier curves for duration of response. ABCP, atezolizumab plus bevacizumab, paclitaxel, and carboplatin; HR, hazard ratio; PC, pemetrexed plus carboplatin or cisplatin; PFS, progression-free survival.
FIG A2.
PFS on the basis of the presence of brain metastases (A) Kaplan-Meier curves for patients with brain metastases and (B) without brain metastases. ABCP, atezolizumab plus bevacizumab, paclitaxel, and carboplatin; HR, hazard ratio; PC, pemetrexed plus carboplatin or cisplatin; PFS, progression-free survival.
FIG A3.
PFS on the basis of the initial EGFR mutation status. Kaplan-Meier curves for patients (A) with EGFR deletion 19 and (B) EGFR Leu858Arg mutation. ABCP, atezolizumab plus bevacizumab, paclitaxel, and carboplatin; HR, hazard ratio; PC, pemetrexed plus carboplatin or cisplatin; PFS, progression-free survival.
FIG A4.
PFS on the basis of acquired EGFR Thr790Met mutation status in EGFR-mutated population. (A) Kaplan-Meier curves for patients with acquired EGFR Thr790Met mutation and (B) without acquired EGFR Thr790Met mutation. PFS, progression-free survival.
FIG A5.
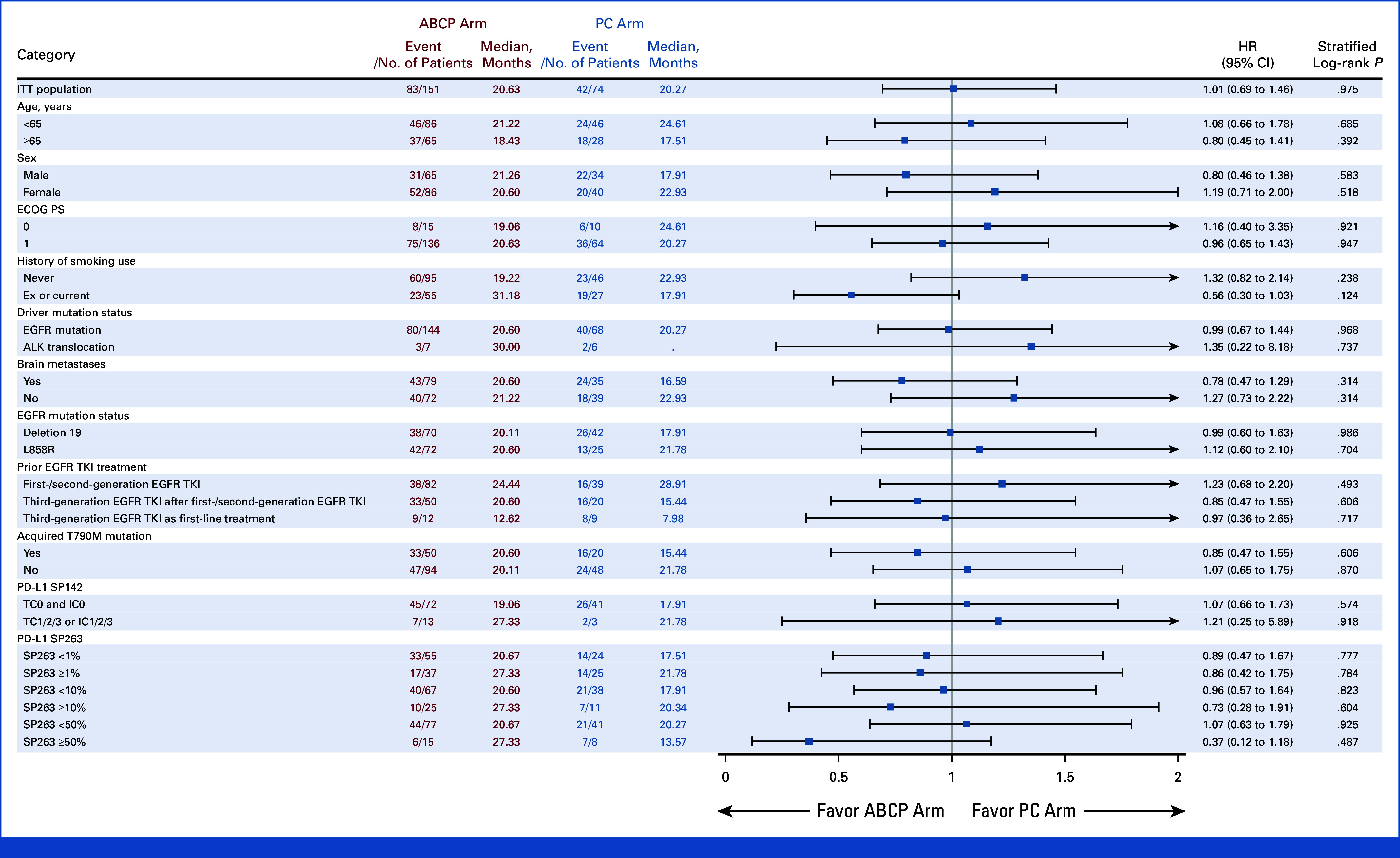
Forest plot of OS. ABCP, atezolizumab plus bevacizumab, paclitaxel, and carboplatin; ECOG PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; ITT, intention-to-treat; OS, overall survival; PC, pemetrexed plus carboplatin or cisplatin; PFS, progression-free survival; TKI, tyrosine kinase inhibitor.
FIG A6.
Exploratory analysis on the basis of PD-L1 SP142 expression profile. Kaplan-Meier curves for patients with (A) TC0 and IC0 and (B) TC1/2/3 or IC1/2/3. ABCP, atezolizumab plus bevacizumab, paclitaxel, and carboplatin; HR, hazard ratio; PC, pemetrexed plus carboplatin or cisplatin; PFS, progression-free survival.
FIG A7.
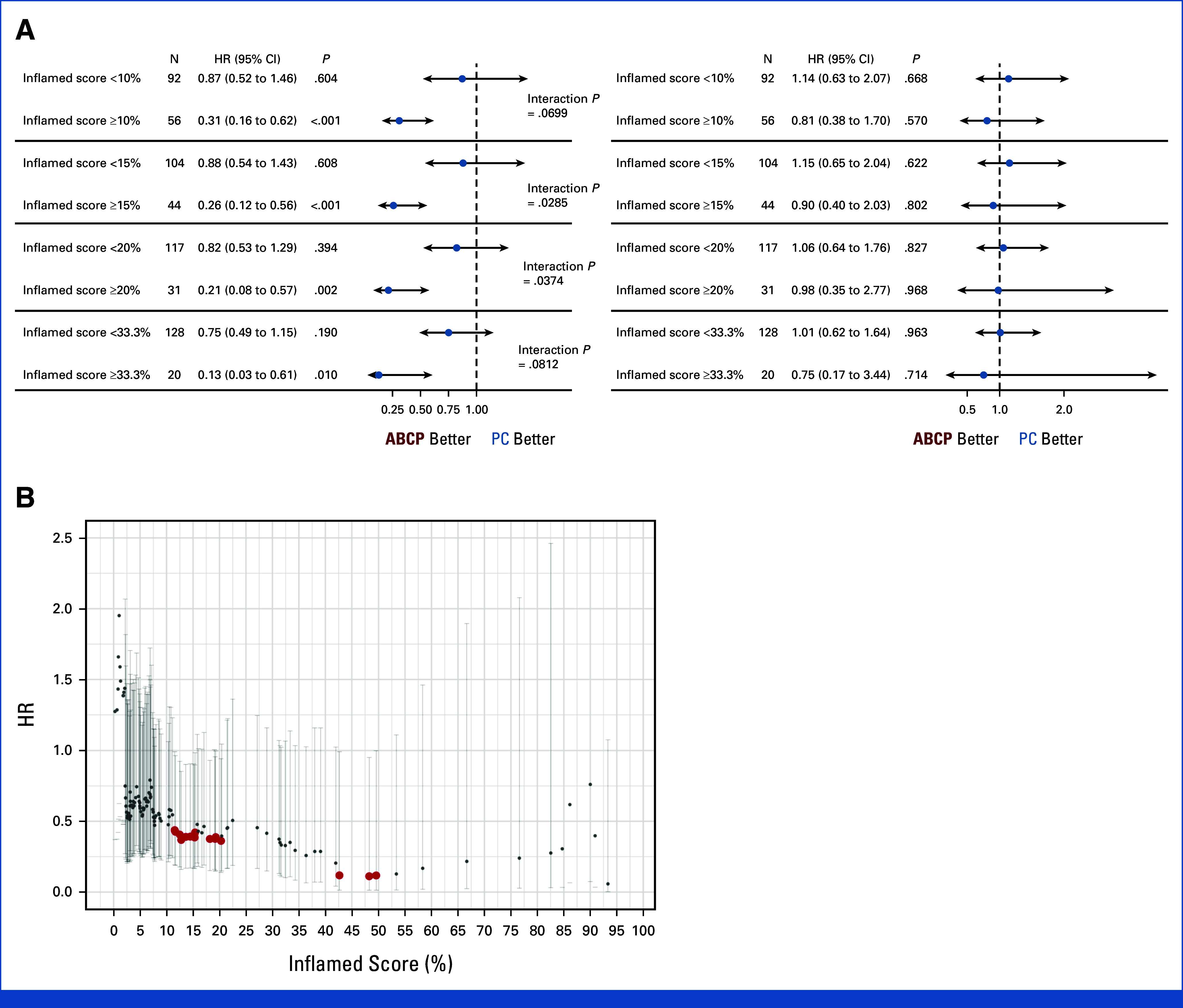
(A) Forest plot of PFS (left) and OS (right) in each arm of ABCP and PC for inflamed score cutoff of 10%, 15%, 20%, and 33.3%. The cox proportional hazard model was used to calculate HR (dot) and 95% CI (error bar). (B) HR of PFS between ABCP and PC for all analyzable inflamed score cutoffs. The cox proportional hazard model was used to calculate hazard ratio (dot) and 95% CI (error bar). The interaction P for each inflamed score cutoff was displayed (red, P < .05; black, P ≥ .05). ABCP, atezolizumab plus bevacizumab, paclitaxel, and carboplatin; HR, hazard ratio; OS, overall survival; PC, pemetrexed plus carboplatin or cisplatin; PFS, progression-free survival.
TABLE A1.
Subsequent Cancer-Related Treatment
| Category | ABCP Arm (n = 94), No. (%) | PC Arm (n = 46), No. (%) |
|---|---|---|
| Antiangiogenic agent | 4 (4.3) | 4 (8.7) |
| Antibody-drug conjugated agent | 6 (6.4) | 5 (10.9) |
| Cytotoxic chemotherapy | 42 (44.7) | 24 (52.2) |
| Immunotherapy | 1 (1.1) | 12 (26.1) |
| Other EGFR target agent | — | 3 (6.5) |
| Other tyrosine kinase inhibitor | 19 (20.2) | 13 (28.3) |
Abbreviations: ABCP, atezolizumab plus bevacizumab, paclitaxel, and carboplatin; PC, pemetrexed plus carboplatin or cisplatin.
TABLE A2.
Overall Response Rate
| Category | ABCP Arm (n = 151) | PC Arm (n = 74) |
|---|---|---|
| Best overall response, No. (%) | ||
| Complete response | 1 (0.7) | — |
| Partial response | 104 (68.9) | 31 (41.9) |
| Stable disease | 41 (26.5) | 35 (47.3) |
| Progressive disease | 1 (0.7) | 8 (10.8) |
| Not evaluable | 4 (2.7) | 1 (1.4) |
| Objective response rate, % (95% CI) | 69.5 (61.5 to 76.8) | 41.9 (30.5 to 53.9) |
| Disease control rate, % (95% CI) | 96.7 (92.4 to 98.9) | 87.8 (78.2 to 94.3) |
NOTE. Not evaluable patients had no image assessment after baseline treatment.
Abbreviations: ABCP, atezolizumab plus bevacizumab, paclitaxel, and carboplatin; PC, pemetrexed plus carboplatin or cisplatin.
TABLE A3.
Landmark Survival Analysis
| Category | ABCP Arm (n = 151) | PC Arm (n = 74) | P |
|---|---|---|---|
| PFS | |||
| 6-month PFS rate (95% CI) | 0.72 (0.64 to 0.79) | 0.48 (0.36 to 0.59) | .163 |
| 1-year PFS rate (95% CI) | 0.36 (0.28 to 0.44) | 0.23 (0.14 to 0.33) | .012 |
| 2-year PFS rate (95% CI) | 0.13 (0.07 to 0.20) | 0.09 (0.03 to 0.18) | .006 |
| OS | |||
| 6-month OS rate (95% CI) | 0.93 (0.88 to 0.96) | 0.95 (0.86 to 0.98) | .986 |
| 1-year OS rate (95% CI) | 0.67 (0.59 to 0.74) | 0.71 (0.59 to 0.80) | .455 |
| 2-year OS rate (95% CI) | 0.44 (0.35 to 0.52) | 0.43 (0.31 to 0.55) | .955 |
Abbreviations: ABCP, atezolizumab plus bevacizumab, paclitaxel, and carboplatin; PC, pemetrexed plus carboplatin or cisplatin; PFS, progression-free survival; OS, overall survival.
TABLE A4.
Treatment Intensity and Compliance of the Atezolizumab Plus Bevacizumab, Paclitaxel, and Carboplatin Arm
| Category | Induction Phase | |||||
|---|---|---|---|---|---|---|
| No. | Mean | SD | Median | Min | Max | |
| Atezolizumab | ||||||
| No. of cycles | 151 | 3.81 | 0.72 | 4.00 | 1.00 | 6.00 |
| Relative dose intensity, % | 151 | 96.96 | 7.10 | 100 | 56.38 | 107.69 |
| Temporally interruption | 0 | |||||
| Bevacizumab | ||||||
| No. of cycles | 151 | 3.75 | 0.76 | 4.00 | 1.00 | 6.00 |
| Relative dose intensity, % | 151 | 96.66 | 8.96 | 99.64 | 55.31 | 109.30 |
| Temporally interruption | 7 | |||||
| Paclitaxel | ||||||
| No. of cycles | 151 | 3.78 | 0.74 | 4.00 | 1.00 | 6.00 |
| Relative dose intensity, % | 151 | 90.12 | 11.87 | 92.75 | 48.72 | 114.64 |
| Dose reduction | 86 | |||||
| Temporally interruption | 3 | |||||
| Carboplatin | ||||||
| No. of cycles | 151 | 3.80 | 0.72 | 4.00 | 1.00 | 6.00 |
| Actual dose intensity, mg/week | 151 | 164.25 | 42.54 | 162.79 | 76.84 | 267.58 |
| Dose reduction | 91 | |||||
| Temporally interruption | 1 | |||||
| Maintenance Phase | ||||||
| Category | No. | Mean | SD | Median | Min | Max |
| Atezolizumab | ||||||
| No. of cycles | 131 | 16.34 | 10.69 | 12.00 | 5.00 | 53.00 |
| Relative dose intensity, % | 131 | 98.48 | 3.49 | 100 | 73.47 | 102.44 |
| Temporally interruption | 0 | |||||
| Bevacizumaba | ||||||
| No. of cycles | 126 | 11.56 | 9.18 | 8.00 | 1.00 | 42.00 |
| Relative dose intensity, % | 126 | 95.85 | 11.20 | 97.85 | 0.00 | 110.85 |
| Temporally interruption | 41 | |||||
NOTE. One patient received six cycles of induction treatment.
Abbreviation: SD, standard deviation.
Five patients were treated with atezolizumab alone (three patients because of the adverse events and two patients on the basis of the investigator's decision).
TABLE A5.
Treatment Intensity and Compliance of Pemetrexed Plus Carboplatin or Cisplatin Arm
| Category | Induction Phase | |||||
|---|---|---|---|---|---|---|
| No. | Mean | SD | Median | Min | Max | |
| Pemetrexed | ||||||
| No. of cycles | 74 | 3.80 | 0.76 | 4.00 | 2.00 | 6.00 |
| Relative dose intensity, % | 74 | 97.28 | 8.15 | 99.62 | 52.55 | 108.89 |
| Dose reduction | 3 | |||||
| Temporally interruption | 0 | |||||
| Cisplatin | ||||||
| No. of cycles | 8 | 3.50 | 0.76 | 4.00 | 2.00 | 4.00 |
| Relative dose intensity, % | 8 | 79.43 | 22.43 | 83.68 | 40.99 | 101.35 |
| Dose reduction | 2 | |||||
| Temporally interruption | 1 | |||||
| Carboplatin | ||||||
| No. of cycles | 66 | 3.80 | 0.77 | 4.00 | 2.00 | 6.00 |
| Actual dose intensity, mg/week | 66 | 190.60 | 48.83 | 187.57 | 94.04 | 296.67 |
| Dose reduction | 13 | |||||
| Temporally interruption | 0 | |||||
| Maintenance Phase | ||||||
| Category | No. | Mean | SD | Median | Min | Max |
| Pemetrexed | ||||||
| No. of cycles | 58 | 12.91 | 8.49 | 10.00 | 5.00 | 43.00 |
| Relative dose intensity, % | 58 | 96.17 | 10.00 | 99.06 | 56.68 | 121.73 |
| Dose reduction | 5 | |||||
| Temporally interruption | 0 | |||||
NOTE. Two patients received six cycles of induction pemetrexed/carboplatin.
Abbreviation: SD, standard deviation.
TABLE A6.
Incidence of Adverse Events On the Basis of the Treatment Phase
| Event | Induction Phase | |||||
|---|---|---|---|---|---|---|
| ABCP Arm (n = 151) | PC Arm (n = 74) | |||||
| Grade 1-2 | Grade 3 | Grade 4 | Grade 1-2 | Grade 3 | Grade 4 | |
| Peripheral neuropathy | 100 (66.2) | 4 (2.6) | — | 7 (9.5) | — | — |
| Alopecia | 90 (59.6) | — | — | 2 (2.7) | — | — |
| Myalgia | 34 (22.5) | 2 (1.3) | 1 (0.7) | 2 (2.7) | — | — |
| Hypertension | 9 (6.0) | — | — | — | — | — |
| Rash | 19 (12.6) | 5 (3.3) | — | 3 (4.1) | — | — |
| Decreased appetite | 17 (11.3) | — | — | 4 (5.4) | — | — |
| Fatigue | 13 (8.6) | 4 (2.6) | — | 6 (8.1) | 1 (1.4) | — |
| Pruritus | 15 (9.9) | — | — | — | — | — |
| Anemia | 14 (9.3) | 4 (2.6) | — | 8 (10.8) | 1 (1.4) | — |
| Hypothyroidism | — | — | — | — | — | — |
| Proteinuria | 2 (1.3) | — | — | — | — | — |
| Stomatitis | 12 (7.9) | 1 (0.7) | — | — | — | — |
| Neutropenia | 5 (3.3) | 8 (5.3) | 2 (1.3) | 3 (4.1) | — | 1 (1.4) |
| AST/ALT increased | 9 (6.0) | — | — | 4 (5.4) | 1 (1.4) | — |
| Nausea | 8 (5.3) | 1 (0.7) | — | 13 (17.6) | 1 (1.4) | — |
| Maintenance Phase | ||||||
| ABCP Arm (n = 151) | PC Arm (n = 74) | |||||
| Event | Grade 1-2 | Grade 3 | Grade 4 | Grade 1-2 | Grade 3 | Grade 4 |
| Peripheral neuropathy | 5 (3.3) | — | — | 1 (1.4) | — | — |
| Alopecia | — | — | — | — | — | — |
| Myalgia | 5 (3.3) | — | — | — | — | — |
| Hypertension | 18 (11.9) | 3 (2.0) | — | 1 (1.4) | — | — |
| Rash | 6 (4) | — | — | 1 (1.4) | — | — |
| Decreased appetite | 6 (4) | — | — | 3 (4.1) | 1 (1.4) | — |
| Fatigue | 4 (2.6) | — | — | 1 (1.4) | — | — |
| Pruritus | 6 (4) | — | — | 5 (6.8) | — | — |
| Anemia | — | 1 (0.7) | — | 3 (4.1) | 1 (1.4) | — |
| Hypothyroidism | 19 (12.6) | — | — | — | — | — |
| Proteinuria | 15 (9.9) | 2 (1.3) | — | — | — | — |
| Stomatitis | 5 (3.3) | — | — | 1 (1.4) | — | — |
| Neutropenia | 2 (1.3) | — | — | 1 (1.4) | 1 (1.4) | — |
| AST/ALT increased | 3 (2.0) | 1 (0.7) | — | 4 (5.4) | — | — |
| Nausea | 3 (2.0) | — | — | 3 (4.1) | — | — |
Abbreviations: ABCP, atezolizumab plus bevacizumab, paclitaxel, and carboplatin; PC, pemetrexed plus carboplatin or cisplatin.
TABLE A7.
Incidence of Immune-Mediated Adverse Event
| Event | ABCP Arm (n = 151) | PC Arm (n = 74) | ||||
|---|---|---|---|---|---|---|
| Grade 1-2 | Grade 3 | Grade 4 | Grade 1-2 | Grade 3 | Grade 4 | |
| Total | 70 (46.3) | 10 (6.6) | 2 (1.3) | 15 (20.3) | 2 (2.7) | — |
| Adrenal insufficiency | 1 (0.7) | 2 (1.3) | — | — | — | — |
| Amylase/lipase increased | 1 (0.7) | — | — | — | — | — |
| Arthralgia | 9 (6.0) | — | — | — | — | — |
| AST/ALT increased | 12 (7.9) | 1 (0.7) | — | 8 (10.8) | 1 (1.4) | — |
| Fatigue | 17 (11.3) | 4 (2.6) | — | 7 (9.5) | 1 (1.4) | — |
| Flushing | 4 (2.6) | — | — | — | — | — |
| Hypersensitivity | — | 1 (0.7) | — | — | — | — |
| Hypoglycemia | — | — | 1 (0.7) | — | — | — |
| Hypothyroidism | 19 (12.6) | — | — | — | — | — |
| Thrombocytopenia | 1 (0.7) | — | — | — | — | — |
| Myalgia | 39 (25.8) | 2 (1.3) | 1 (0.7) | 2 (2.7) | — | — |
| Pneumonitis | 2 (1.3) | — | — | — | — | — |
| Thyroid function abnormal | 2 (1.3) | — | — | — | — | — |
| Urticaria | 4 (2.6) | — | — | — | — | — |
Abbreviations: ABCP, atezolizumab plus bevacizumab, paclitaxel, and carboplatin; PC, pemetrexed plus carboplatin or cisplatin.
Tae Min Kim
Consulting or Advisory Role: AstraZeneca/MedImmune, Janssen Oncology, Novartis, Takeda, Yuhan, Regeneron, Samsung Bioepis, Amgen, Daiichi Sankyo/Astra Zeneca, inno.N
Speakers' Bureau: Takeda, Janssen Research & Development, IMBdx
Uncompensated Relationships: AstraZeneca/MedImmune, Novartis, Boryung, Roche/Genentech
Ji-Youn Han
Stock and Other Ownership Interests: Yuhan
Honoraria: AstraZeneca, Takeda, Novartis, Merck, Janssen, Pfizer, Yuhan
Consulting or Advisory Role: Bristol Myers Squibb, Novartis, Pfizer, Merck, ABION, J Ints Bio, Takeda, Janssen, Lantern Pharma
Research Funding: Roche, Pfizer, Ono Pharmaceutical, Takeda
Byoung Yong Shim
Consulting or Advisory Role: Takeda, AstraZeneca, Pfizer, J Ints Bio, Guardant Health, Bion
Research Funding: Yuhan
Yun-Gyoo Lee
Honoraria: Pfizer, Yuhan, Boehringer Ingelheim, MSD
Consulting or Advisory Role: Ono Pharmaceutical, Yuhan, Astellas Scientific and Medical Affairs Inc, Guardant Health
Ki Hyeong Lee
Consulting or Advisory Role: Bristol Myers Squibb, MSD, AstraZeneca, Pfizer, Lilly
Research Funding: Merck
Chan-Young Ock
Employment: Lunit
Leadership: Lunit
Stock and Other Ownership Interests: Lunit, Y-Biologics
Consulting or Advisory Role: Medpacto, Y-Biologics, Idience, Selecxine, Genome & Company, PIN therapeutics
Jaebong Lee
Consulting or Advisory Role: Seoul CRO (Inst)
Hyun-Ae Jung
Consulting or Advisory Role: Yuhan, Guardant Health, AIMEDBIO
Research Funding: Yuhan
Se-Hoon Lee
Honoraria: AstraZeneca/MedImmune, Roche, Merck, Lilly, Amgen
Consulting or Advisory Role: AstraZeneca, Roche, Merck, Pfizer, Lilly, BMS/Ono, Takeda, Janssen, IMBdx
Research Funding: Merck, AstraZeneca, Lunit
Travel, Accommodations, Expenses: Novartis
Jin Seok Ahn
Honoraria: Pfizer, Roche, BC World Pharmaceutical, Yuhan, Hanmi, Novartis, JW Pharmaceutical, Amgen, Boehringer Ingelheim, Menarini, Kyowa Kirin, AstraZeneca, Bayer, Lilly, Takeda, Boryung, Samyang
Consulting or Advisory Role: Bayer, Yooyoung Pharmaceutical Co, Ltd, Pharmbio Korea, Guardant Health, Yuhan, ImmuneOncia, Therapex, Daiichi Sankyo Korea, Roche
Myung-Ju Ahn
Honoraria: AstraZeneca, Lilly, MSD, Takeda, Amgen, Merck Serono, Yuhan
Consulting or Advisory Role: AstraZeneca, Lilly, MSD, Takeda, Alpha pharmaceutical, Amgen, Merck Serono, Pfizer, Yuhan, Arcus Ventures
Research Funding: Yuhan
No other potential conflicts of interest were reported.
See accompanying Oncology Grand Rounds, p. 1215
DISCLAIMER
The source of funding had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
SUPPORT
Supported by Roche by providing atezolizumab, bevacizumab, and funds for this study.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
The deidentified data generated in this study are available upon appropriate request to the corresponding author.
AUTHOR CONTRIBUTIONS
Conception and design: Sehhoon Park, Myung-Ju Ahn
Provision of study materials or patients: Sehhoon Park, Tae Min Kim, Ji-Youn Han, Gyeong-Won Lee, Byoung Yong Shim, Yun-Gyoo Lee, Sang-We Kim, Il Hwan Kim, Suee Lee, Yu Jung Kim, Ji Hyun Park, Sang-Gon Park, Ki Hyeong Lee, Eun Joo Kang, Ju Won Kim, Seong-Hoon Shin, Hyun-Ae Jung, Jong-Mu Sun, Se-Hoon Lee, Jin Seok Ahn, Myung-Ju Ahn
Collection and assembly of data: All authors
Data analysis and interpretation: Sehhoon Park, Chan-Young Ock, Byung-Ho Nam, Jaebong Lee, Myung-Ju Ahn
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase III, Randomized Study of Atezolizumab Plus Bevacizumab and Chemotherapy in Patients With EGFR- or ALK-Mutated Non–Small-Cell Lung Cancer (ATTLAS, KCSG-LU19-04)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Tae Min Kim
Consulting or Advisory Role: AstraZeneca/MedImmune, Janssen Oncology, Novartis, Takeda, Yuhan, Regeneron, Samsung Bioepis, Amgen, Daiichi Sankyo/Astra Zeneca, inno.N
Speakers' Bureau: Takeda, Janssen Research & Development, IMBdx
Uncompensated Relationships: AstraZeneca/MedImmune, Novartis, Boryung, Roche/Genentech
Ji-Youn Han
Stock and Other Ownership Interests: Yuhan
Honoraria: AstraZeneca, Takeda, Novartis, Merck, Janssen, Pfizer, Yuhan
Consulting or Advisory Role: Bristol Myers Squibb, Novartis, Pfizer, Merck, ABION, J Ints Bio, Takeda, Janssen, Lantern Pharma
Research Funding: Roche, Pfizer, Ono Pharmaceutical, Takeda
Byoung Yong Shim
Consulting or Advisory Role: Takeda, AstraZeneca, Pfizer, J Ints Bio, Guardant Health, Bion
Research Funding: Yuhan
Yun-Gyoo Lee
Honoraria: Pfizer, Yuhan, Boehringer Ingelheim, MSD
Consulting or Advisory Role: Ono Pharmaceutical, Yuhan, Astellas Scientific and Medical Affairs Inc, Guardant Health
Ki Hyeong Lee
Consulting or Advisory Role: Bristol Myers Squibb, MSD, AstraZeneca, Pfizer, Lilly
Research Funding: Merck
Chan-Young Ock
Employment: Lunit
Leadership: Lunit
Stock and Other Ownership Interests: Lunit, Y-Biologics
Consulting or Advisory Role: Medpacto, Y-Biologics, Idience, Selecxine, Genome & Company, PIN therapeutics
Jaebong Lee
Consulting or Advisory Role: Seoul CRO (Inst)
Hyun-Ae Jung
Consulting or Advisory Role: Yuhan, Guardant Health, AIMEDBIO
Research Funding: Yuhan
Se-Hoon Lee
Honoraria: AstraZeneca/MedImmune, Roche, Merck, Lilly, Amgen
Consulting or Advisory Role: AstraZeneca, Roche, Merck, Pfizer, Lilly, BMS/Ono, Takeda, Janssen, IMBdx
Research Funding: Merck, AstraZeneca, Lunit
Travel, Accommodations, Expenses: Novartis
Jin Seok Ahn
Honoraria: Pfizer, Roche, BC World Pharmaceutical, Yuhan, Hanmi, Novartis, JW Pharmaceutical, Amgen, Boehringer Ingelheim, Menarini, Kyowa Kirin, AstraZeneca, Bayer, Lilly, Takeda, Boryung, Samyang
Consulting or Advisory Role: Bayer, Yooyoung Pharmaceutical Co, Ltd, Pharmbio Korea, Guardant Health, Yuhan, ImmuneOncia, Therapex, Daiichi Sankyo Korea, Roche
Myung-Ju Ahn
Honoraria: AstraZeneca, Lilly, MSD, Takeda, Amgen, Merck Serono, Yuhan
Consulting or Advisory Role: AstraZeneca, Lilly, MSD, Takeda, Alpha pharmaceutical, Amgen, Merck Serono, Pfizer, Yuhan, Arcus Ventures
Research Funding: Yuhan
No other potential conflicts of interest were reported.
REFERENCES
- 1. Singh N, Temin S, Baker S, et al. Therapy for stage IV non–small-cell lung cancer with driver alterations: ASCO living guideline. J Clin Oncol. 2022;40:3310–3322. doi: 10.1200/JCO.22.00824. [DOI] [PubMed] [Google Scholar]
- 2. Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with Osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 3. Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer. N Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shaw AT, Bauer TM, De Marinis F, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383:2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 5. Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non–small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 6. Camidge DR, Kim HR, Ahn M-J, et al. Brigatinib versus crizotinib in ALK-positive non–small-cell lung cancer. N Engl J Med. 2018;379:2027–2039. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 7. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol. 2021;39:2339–2349. doi: 10.1200/JCO.21.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garassino MC, Gadgeel S, Speranza G, et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non–small-cell lung cancer: 5-Year outcomes from the phase 3 KEYNOTE-189 study. J Clin Oncol. 2023;41:1992–1998. doi: 10.1200/JCO.22.01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR- mutated non–small cell lung cancer—a meta-analysis. J Thorac Oncol. 2017;12:403–407. doi: 10.1016/j.jtho.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 10. Mok T, Nakagawa K, Park K, et al. LBA8 Nivolumab (NIVO)+ chemotherapy (chemo) vs chemo in patients (pts) with EGFR-mutated metastatic non-small cell lung cancer (mNSCLC) with disease progression after EGFR tyrosine kinase inhibitors (TKIs) in CheckMate 722. Ann Oncol. 2022;33:S1561–S1562. [Google Scholar]
- 11. Yang JC-H, Lee DH, Lee J-S, et al. Pemetrexed and platinum with or without pembrolizumab for tyrosine kinase inhibitor (TKI)-resistant, EGFR-mutant, metastatic nonsquamous NSCLC: Phase 3 KEYNOTE-789 study. J Clin Oncol. 2023;41(suppl 17; abstr LBA9000) doi: 10.1200/JCO.23.02747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen S, Mellman I, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 13. Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52:117–124. doi: 10.1016/j.semcancer.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 14. Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:1655–1669. doi: 10.1016/S1470-2045(19)30634-5. [DOI] [PubMed] [Google Scholar]
- 15. Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): Interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20:625–635. doi: 10.1016/S1470-2045(19)30035-X. [DOI] [PubMed] [Google Scholar]
- 16. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 17. Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7:387–401. doi: 10.1016/S2213-2600(19)30084-0. [DOI] [PubMed] [Google Scholar]
- 18. Nogami N, Barlesi F, Socinski MA, et al. IMpower150 final exploratory analyses for atezolizumab plus bevacizumab and chemotherapy in key NSCLC patient subgroups with EGFR mutations or metastases in the liver or brain. J Thorac Oncol. 2022;17:309–323. doi: 10.1016/j.jtho.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 19. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20. Park S, Ock C-Y, Kim H, et al. Artificial intelligence–powered spatial analysis of tumor-infiltrating lymphocytes as complementary biomarker for immune checkpoint inhibition in non–small-cell lung cancer. J Clin Oncol. 2022;40:1916–1928. doi: 10.1200/JCO.21.02010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu S, Wu L, Jian H, et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): First interim results from a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2022;23:1167–1179. doi: 10.1016/S1470-2045(22)00382-5. [DOI] [PubMed] [Google Scholar]
- 22. Lu S, Wu L, Jian H, et al. Sintilimab plus chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer with disease progression after EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): Second interim analysis from a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med. 2023;11:624–636. doi: 10.1016/S2213-2600(23)00135-2. [DOI] [PubMed] [Google Scholar]
- 23. Hung MS, Chen I, Lin PY, et al. Epidermal growth factor receptor mutation enhances expression of vascular endothelial growth factor in lung cancer. Oncol Lett. 2016;12:4598–4604. doi: 10.3892/ol.2016.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma T, Jiao J, Huo R, et al. PD-L1 expression, tumor mutational burden, and immune cell infiltration in non-small cell lung cancer patients with epithelial growth factor receptor mutations. Front Oncol. 2022;12:922899. doi: 10.3389/fonc.2022.922899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wallin JJ, Bendell JC, Funke R, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun. 2016;7:12624–12628. doi: 10.1038/ncomms12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52:117–124. doi: 10.1016/j.semcancer.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 27. Chen DS, Hurwitz H. Combinations of bevacizumab with cancer immunotherapy. Cancer J. 2018;24:193–204. doi: 10.1097/PPO.0000000000000327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The deidentified data generated in this study are available upon appropriate request to the corresponding author.