Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
Pembrolizumab adjuvant therapy was shown to significantly improve recurrence-free survival (RFS) and distant metastasis-free survival (DMFS) in patients with resected stage IIB or IIC melanoma in earlier analyses of the randomized, double-blind, phase III KEYNOTE-716 study (ClinicalTrials.gov identifier: NCT03553836). We report results of the protocol-specified final analysis of DMFS for KEYNOTE-716. Overall, 976 patients were randomly allocated to pembrolizumab (n = 487) or placebo (n = 489). As of January 4, 2023, median follow-up was 39.4 months (range, 26.0-51.4 months). The median DMFS was not reached in either treatment group, and the estimated 36-month DMFS was 84.4% for pembrolizumab and 74.7% for placebo (hazard ratio [HR], 0.59 [95% CI, 0.44 to 0.79]). The median RFS was not reached in either treatment group, and the estimated 36-month RFS was 76.2% for pembrolizumab and 63.4% for placebo (HR, 0.62 [95% CI, 0.49 to 0.79]). DMFS and RFS results were consistent across most prespecified subgroups, including stage IIB and stage IIC melanoma. The safety profile of pembrolizumab was manageable and consistent with previous reports. These results continue to support the use of pembrolizumab adjuvant therapy in patients with resected stage IIB or IIC melanoma.
Updated results from KEYNOTE-716 support adjuvant pembrolizumab for resected stage IIB/C melanoma
INTRODUCTION
In the randomized, double-blind, phase III KEYNOTE-716 study in patients with resected stage IIB or IIC melanoma, pembrolizumab as adjuvant therapy significantly improved recurrence-free survival (RFS) at the first interim analysis (hazard ratio [HR], 0.65 [95% CI, 0.46 to 0.92]; P = .0066)1 and distant metastasis-free survival (DMFS) at the third interim analysis (HR, 0.64 [95% CI, 0.47 to 0.88]; P = .0029)2 compared with placebo. These results led to the approval of pembrolizumab as adjuvant therapy in adult and pediatric patients with stage IIB or IIC melanoma by numerous regulatory authorities, including the US Food and Drug Administration and European Medicines Agency.3,4 We report findings from the protocol-specified fourth interim analysis of KEYNOTE-716, including final DMFS and updated RFS results.
METHODS
Study Design and Patients
The design of KEYNOTE-716 (ClinicalTrials.gov identifier: NCT03553836) has been described previously.1 Eligible patients were age 12 years and older with newly diagnosed, resected, histologically confirmed, stage IIB (T3b or T4a) or IIC (T4b) cutaneous melanoma without regional lymph node involvement confirmed pathologically by sentinel lymph node biopsy, as defined by the American Joint Committee on Cancer 2017 classification, 8th edition. Patients were required to have an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 and no previous treatment for melanoma beyond complete resection. Patients were randomly assigned in a 1:1 ratio to pembrolizumab 200 mg (2 mg/kg up to 200 mg in pediatric patients) or placebo intravenously once every 3 weeks for 17 cycles or until disease recurrence, unacceptable toxicity, or withdrawal of consent. Randomization was stratified by T category (T3b, T4a, or T4b) for adults, with a separate stratum for patients age 12-17 years. The protocol and all amendments were approved by the appropriate institutional review board or ethics committee at each institution. All patients provided written informed consent.
End Points and Statistical Analysis
This fourth interim analysis was based on a target of 195 DMFS events. The primary end point was investigator-assessed RFS. Secondary end points included investigator-assessed DMFS and safety and tolerability. Efficacy was assessed in all randomly allocated patients (intention-to-treat [ITT] population). Safety was assessed in all patients who received ≥1 dose of study treatment. RFS and DMFS were estimated using the Kaplan-Meier method. A stratified Cox proportional hazards model with the Efron method of handling ties was used to assess the magnitude of treatment difference between groups, with HRs and 95% CIs with treatment as a covariate. There was no formal hypothesis testing because statistical significance criteria for RFS and DMFS were met at previous analyses. Prespecified subgroups included T category (T3b v T4a v T4b), age (<65 v ≥65 years), sex (male v female), race (White v non-White), ECOG PS (0 v 1), and geographic region (United States v non–United States). Post hoc analysis of RFS and DMFS by disease stage was also conducted. HRs and 95% CIs for subgroups were estimated using an unstratified Cox proportional hazards model.
RESULTS
Patients
A total of 976 patients were randomly allocated to pembrolizumab (n = 487) or placebo (n = 489). Baseline characteristics were generally balanced between treatment groups (Table 1). The median time from random assignment to data cutoff (January 4, 2023) was 39.4 months (range, 26.0-51.4).
TABLE 1.
Baseline Demographics and Clinical Characteristics of the Intention-to-Treat Population
| Characteristic | Pembrolizumab (n = 487) | Placebo (n = 489) |
|---|---|---|
| Age, years, median (range) | 60 (16-84) | 61 (17-87) |
| <65 | 303 (62.2) | 295 (60.3) |
| ≥65 | 184 (37.8) | 194 (39.7) |
| Sex | ||
| Male | 300 (61.6) | 289 (59.1) |
| Female | 187 (38.4) | 200 (40.9) |
| Race | ||
| White | 435 (89.3) | 439 (89.8) |
| Other | 10 (2.1) | 5 (1.0) |
| Missing | 42 (8.6) | 45 (9.2) |
| ECOG status | ||
| 0 | 454 (93.2) | 452 (92.4) |
| 1 | 32 (6.6) | 35 (7.2) |
| 2 | 0 | 1 (0.2) |
| Missing | 1 (0.2) | 1 (0.2) |
| Geographic region | ||
| United States | 95 (19.5) | 80 (16.4) |
| Not United States | 392 (80.5) | 409 (83.6) |
| T stagea | ||
| T3a | 2 (0.4) | 0 |
| T3b | 200 (41.1) | 201 (41.1) |
| T4a | 113 (23.2) | 116 (23.7) |
| T4b | 172 (35.3) | 172 (35.2) |
| Disease stagea | ||
| IIA | 1 (0.2) | 0 |
| IIB | 309 (63.4) | 316 (64.6) |
| IIC | 171 (35.1) | 169 (34.6) |
| IIIC | 4 (0.8) | 1 (0.2) |
| IV | 0 | 2 (0.4) |
| Missing | 2 (0.4) | 1 (0.2) |
NOTE. Data are No. (%) unless otherwise specified.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; T, tumor.
Patients not meeting inclusion criteria after random assignment were recorded as protocol deviations; however, these patients were still included in the intention-to-treat population.
Efficacy
The median DMFS in the ITT population was not reached (NR) in either group (HR, 0.59 [95% CI, 0.44 to 0.79]; Fig 1A). The estimated 36-month DMFS rate was 84.4% for pembrolizumab and 74.7% for placebo. In patients with stage IIB disease, the median DMFS was NR in both groups and the 36-month DMFS rate was 86.7% for pembrolizumab and 78.9% for placebo (HR, 0.62 [95% CI, 0.42 to 0.92]; Fig 1B). In patients with stage IIC disease, the median DMFS was NR in both groups and the 36-month DMFS rate was 80.9% for pembrolizumab and 68.1% for placebo groups, respectively (HR, 0.57 [95% CI, 0.36 to 0.88]; Fig 1C). DMFS across prespecified subgroups is shown in Figure 1D.
FIG 1.
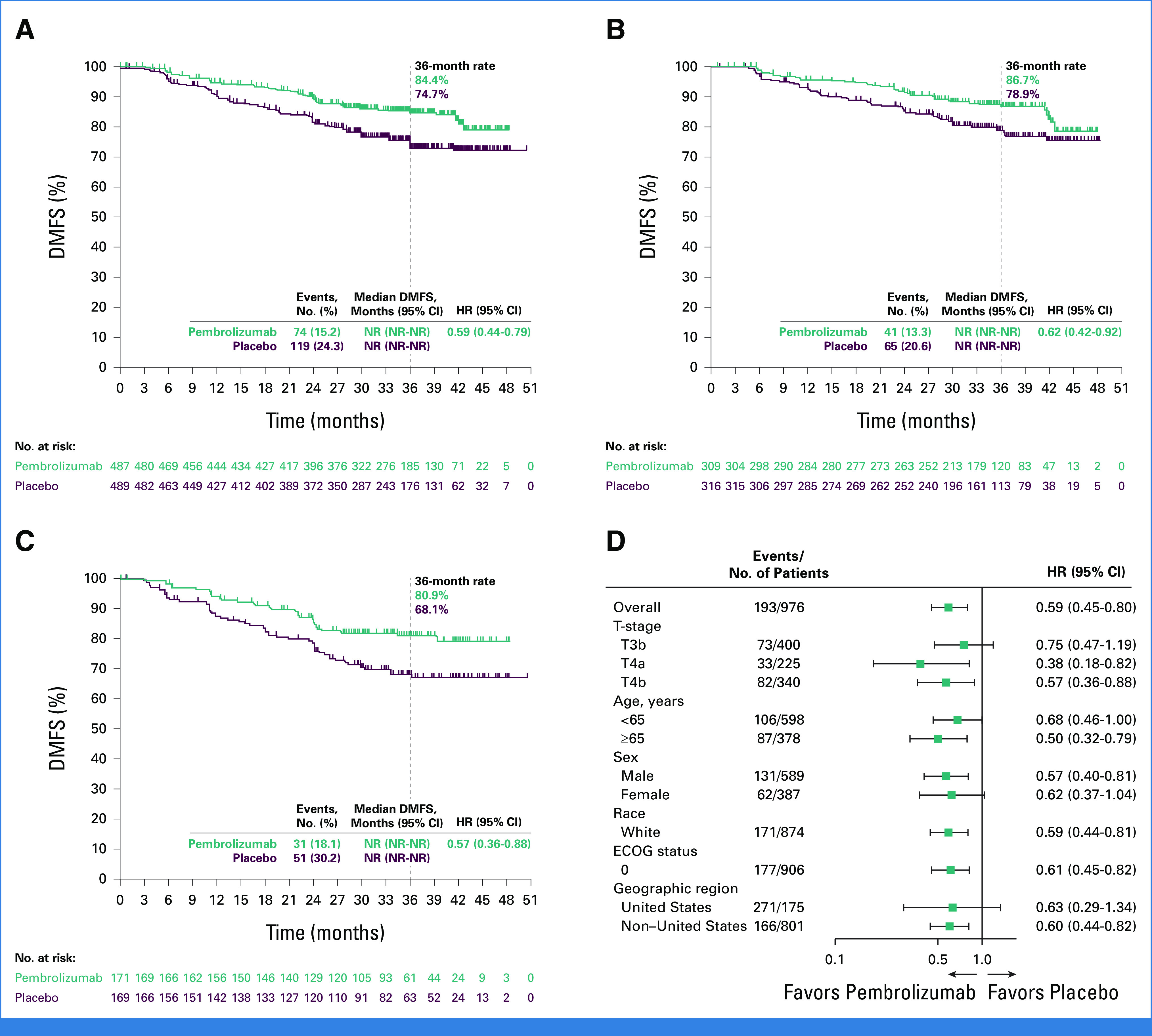
Kaplan-Meier estimates of DMFS (A) in the ITT population, (B) in patients with stage IIB melanoma, and (C) in patients with stage IIC melanoma. (D) Forest plot of key subgroups. DMFS, distant metastasis-free survival; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; ITT, intention-to-treat; NR, not reached; T, tumor.
The median RFS in the ITT population was NR in both groups (Fig 2A). The estimated 36-month RFS rate was 76.2% for pembrolizumab and 63.4% for placebo (HR, 0.62 [95% CI, 0.49 to 0.79]). In patients with stage IIB disease, the median RFS was NR in both groups and the 36-month RFS rate was 79.7% for pembrolizumab and 66.5% for placebo (HR, 0.58 [95% CI, 0.43 to 0.79]; Fig 2B). In patients with stage IIC disease, the median RFS was NR in both groups and the 36-month RFS rate was 71.4% for pembrolizumab and 58.0% for placebo (HR, 0.65 [95% CI, 0.45 to 0.94]; Fig 2C). RFS across prespecified subgroups is shown in Figure 2D.
FIG 2.
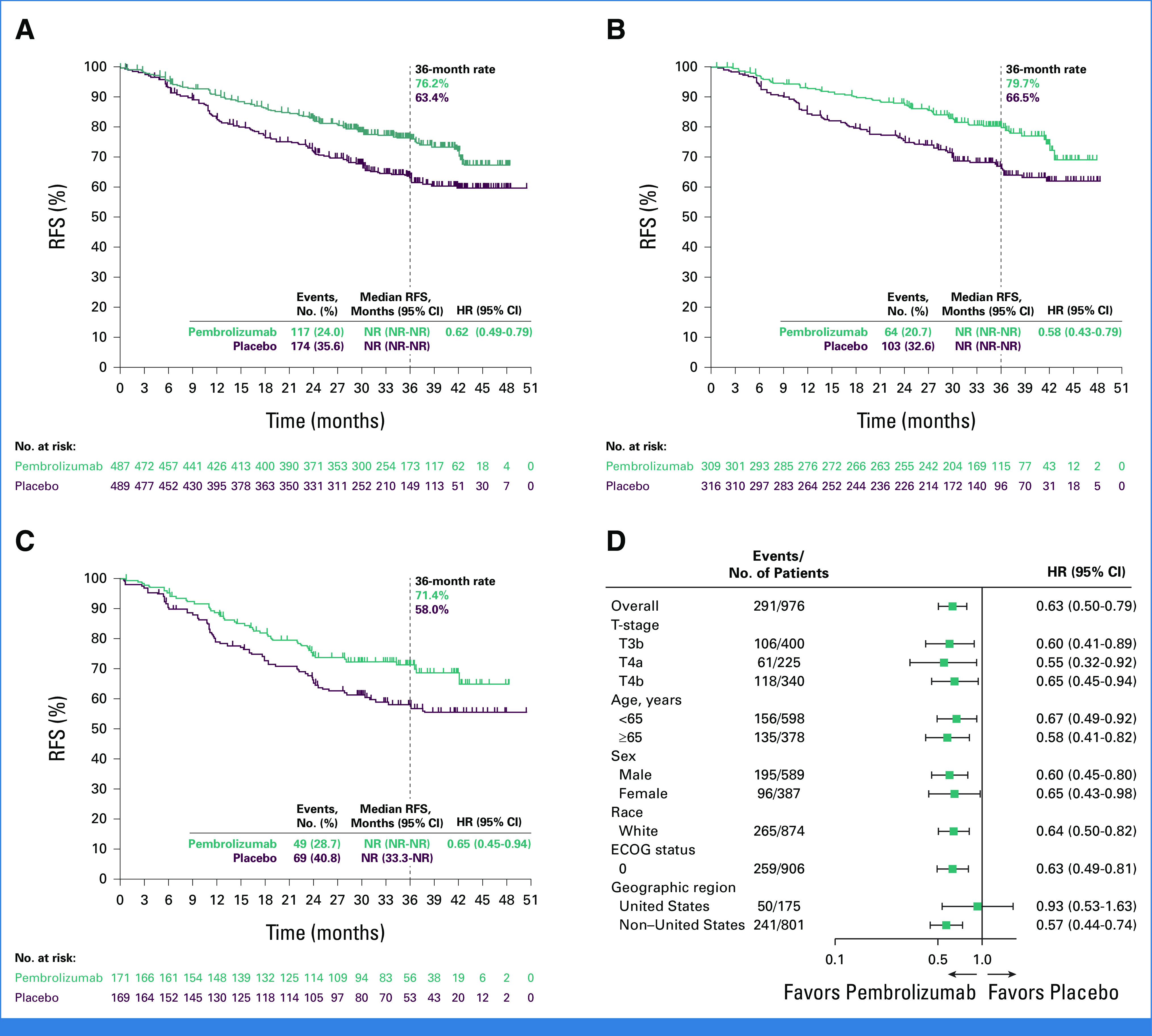
Kaplan-Meier estimates of RFS (A) in the ITT population, (B) in patients with stage IIB melanoma, and (C) in patients with stage IIC melanoma, and (D) RFS in key subgroups. ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; ITT, intention-to-treat; NR, not reached; RFS, recurrence-free survival; T, tumor.
Safety
Overall, 483 patients in the pembrolizumab group and 486 in the placebo group received ≥1 dose of study treatment. Treatment-related adverse events (TRAEs) occurred in 82.6% of patients in the pembrolizumab group (grade 3/4, 17.2%) and 63.6% in the placebo group (grade 3/4, 5.1%; Appendix Table A1, online only). TRAEs led to treatment discontinuation in 15.9% and 2.5% of patients in the pembrolizumab and placebo groups, respectively. No patients died because of TRAEs. Immune-mediated AEs and infusion reactions occurred in 37.9% of patients in the pembrolizumab group (grade 3/4, 11.0%) and 9.5% in the placebo group (grade 3/4, 1.2%; Appendix Table A2).
DISCUSSION
In this protocol-specified fourth interim and final DMFS analysis of KEYNOTE-716, pembrolizumab adjuvant therapy continued to demonstrate a DMFS and an RFS benefit compared with placebo in patients with resected stage IIB or IIC melanoma. After an additional 12 months of follow-up, the DMFS benefit previously reported at the third interim analysis was sustained,2 with pembrolizumab providing a reduction in the risk of distant metastasis compared with placebo. The DMFS benefit was also consistent across prespecified subgroups, including stage IIB and IIC melanoma. The RFS benefit previously observed with pembrolizumab was also sustained, with pembrolizumab reducing the risk of recurrence or death in the ITT population, in patients with stage IIB or stage IIC melanoma, and in most subgroups.1,2 The HRs for DMFS and RFS in the current analysis were also consistent with previous reports, indicating that the benefit observed with pembrolizumab is durable.1,2 For both DMFS and RFS, a continued separation of the Kaplan-Meier curves was observed over time and appeared to be widening for DMFS. The safety results also support previous studies showing pembrolizumab has a manageable safety profile.1,2,5 Overall survival results will be reported at the fifth interim analysis.
Patients with stage IIB and IIC melanoma have a similar prognosis as that for patients with stage III melanoma and have a similar or greater risk of recurrence than patients with stage IIIA and stage IIIB melanoma.6-8 Pembrolizumab is the first systemic adjuvant therapy to be approved for use in patients with stage II melanoma, and, to our knowledge, KEYNOTE-716 is the only study with long-term follow-up data available. Other studies investigating adjuvant treatments include the phase III CheckMate-76K study. In a prespecified interim analysis of patients with resected stage IIB or IIC melanoma enrolled in CheckMate-76K, adjuvant nivolumab improved RFS (HR, 0.42 [95% CI, 0.30 to 0.59]; P < .0001) and DMFS (HR, 0.47 [95% CI, 0.30 to 0.72]) compared with placebo, which confirmed the benefit of adjuvant therapy with an anti–PD-1 agent in the population.9 Additional studies underway include the phase III COLOMBUS-AD study, which is being conducted to investigate adjuvant encorafenib plus binimetinib versus placebo in resected stage IIB or IIC BRAFV600-mutated melanoma.10 Adjuvant pembrolizumab treatment for patients with resected high-risk stage IIB-IV melanoma is also being investigated as coformulation with vibostolimab in the phase III KEYVIBE-010 study11 and in combination with the individualized neoantigen therapy V940 in the phase III V940-001 study.
In the final DMFS analysis of KEYNOTE-716, pembrolizumab continued to demonstrate manageable safety and a clinically meaningful DMFS and RFS benefit compared with placebo, supporting the use of pembrolizumab adjuvant therapy in patients with resected stage IIB or IIC melanoma.
ACKNOWLEDGMENT
The authors thank the patients and their families and caregivers for participating in the study, along with all investigators and site personnel. The authors thank Scot Ebbinghaus (Merck & Co, Inc, Rahway, NJ), Nageatte Ibrahim (Merck & Co, Inc, Rahway, NJ), and James Anderson for critical review of the manuscript. Medical writing and/or editorial assistance was provided by Jemimah Walker, PhD, and Rob Steger, PhD, of ApotheCom (Yardley, PA). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ.
APPENDIX
TABLE A1.
TRAEs in the As-Treated Population
| TRAE With Incidence ≥5% | Pembrolizumab (n = 483) | Placebo (n = 486) | ||
|---|---|---|---|---|
| Any Grade, No. (%) | Grade 3-4,a No. (%) | Any Grade, No. (%) | Grade 3-4,a No. (%) | |
| Any | 399 (82.6) | 83 (17.2) | 309 (63.6) | 25 (5.1) |
| Pruritus | 119 (24.6) | 3 (0.6) | 52 (10.7) | 0 (0.0) |
| Fatigue | 104 (21.5) | 1 (0.2) | 93 (19.1) | 1 (0.2) |
| Diarrhea | 90 (18.6) | 5 (1.0) | 56 (11.5) | 1 (0.2) |
| Arthralgia | 79 (16.4) | 1 (0.2) | 39 (8.0) | 0 (0.0) |
| Rash | 78 (16.1) | 7 (1.4) | 34 (7.0) | 1 (0.2) |
| Hypothyroidism | 77 (15.9) | 0 (0.0) | 13 (2.7) | 0 (0.0) |
| Hyperthyroidism | 49 (10.1) | 1 (0.2) | 3 (0.6) | 0 (0.0) |
| Asthenia | 47 (9.7) | 1 (0.2) | 40 (8.2) | 0 (0.0) |
| ALT level increased | 39 (8.1) | 4 (0.8) | 22 (4.5) | 1 (0.2) |
| Nausea | 37 (7.7) | 0 (0.0) | 33 (6.8) | 0 (0.0) |
| Rash maculopapular | 36 (7.5) | 2 (0.4) | 9 (1.9) | 0 (0.0) |
| Myalgia | 32 (6.6) | 2 (0.4) | 16 (3.3) | 0 (0.0) |
| AST level increased | 31 (6.4) | 1 (0.2) | 11 (2.3) | 1 (0.2) |
Abbreviation: TRAE, treatment-related adverse event.
There were no grade 5 TRAEs.
TABLE A2.
Immune-Mediated Adverse Events and Infusion Reactions
| Event | Pembrolizumab (n = 483) | Placebo (n = 486) | ||
|---|---|---|---|---|
| Any Grade, No. (%) | Grade 3-4,a No. (%) | Any Grade, No. (%) | Grade 3-4,a No. (%) | |
| Any | 183 (37.9) | 53 (11) | 46 (9.5) | 6 (1.2) |
| Adrenal insufficiency | 13 (2.7) | 5 (1.0) | 0 (0.0) | 0 (0.0) |
| Arthritis | 2 (0.4) | 1 (0.2) | 2 (0.4) | 0 (0.0) |
| Colitis | 20 (4.1) | 8 (1.7) | 5 (1.0) | 0 (0.0) |
| Hepatitis | 11 (2.3) | 9 (1.9) | 3 (0.6) | 2 (0.4) |
| Hyperthyroidism | 51 (10.6) | 1 (0.2) | 3 (0.6) | 0 (0.0) |
| Hypophysitis | 12 (2.5) | 3 (0.6) | 0 (0.0) | 0 (0.0) |
| Hypothyroidism | 83 (17.2) | 0 (0.0) | 18 (3.7) | 0 (0.0) |
| Infusion reactions | 3 (0.6) | 0 (0.0) | 7 (1.4) | 0 (0.0) |
| Myasthenic syndrome | 2 (0.4) | 2 (0.4) | 0 (0.0) | 0 (0.0) |
| Myelitis | 1 (0.2) | 1 (0.2) | 0 (0.0) | 0 (0.0) |
| Myocarditis | 1 (0.2) | 0 (0.0) | 1 (0.2) | 1 (0.2) |
| Myositis | 6 (1.2) | 3 (0.6) | 1 (0.2) | 0 (0.0) |
| Nephritis | 7 (1.4) | 3 (0.6) | 0 (0.0) | 0 (0.0) |
| Pancreatitis | 2 (0.4) | 2 (0.4) | 0 (0.0) | 0 (0.0) |
| Sarcoidosis | 5 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Severe skin reactionsb | 15 (3.1) | 14 (2.9) | 3 (0.6) | 3 (0.6) |
| Thyroiditis | 8 (1.7) | 0 (0.0) | 2 (0.4) | 0 (0.0) |
| Type 1 diabetes mellitus | 2 (0.4) | 2 (0.4) | 0 (0.0) | 0 (0.0) |
| Uveitis | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
There were no grade 5 immune-mediated adverse events.
Includes bullous dermatitis, erythema multiforme, pemphigoid, pruritus, rash, maculopapular rash, pruritic rash, and pustular rash.
Jason J. Luke
Stock and Other Ownership Interests: Actym Therapeutics, Mavu Pharmaceutical, Pyxis, Alphamab, Tempest Therapeutics, Kanaph Therapeutics, Onc.AI, Arch Oncology, STipe Therapeutics, NeoTX
Consulting or Advisory Role: Bristol Myers Squibb, MSD, EMD Serono, Novartis, 7 Hills Pharma, Janssen, Reflexion Medical, Tempest Therapeutics, Alphamab, Abbvie, Bayer, Incyte, Partner Therapeutics, Synlogic, Werewolf Therapeutics, Ribon Therapeutics, Checkmate Pharmaceuticals, CStone Pharmaceuticals, Nektar, Regeneron, Rubius Therapeutics, Tesaro, Xilio Therapeutics, Xencor, Alnylam, Crown Bioscience, Flame Biosciences, Genentech, Kadmon, KSQ Therapeutics, Immunocore, Inzen Therapeutics, Pfizer, Silicon Therapeutics, TRex Bio, Bright Peak Therapeutics, Onc.AI, STipe Therapeutics, Codiak Biosciences, Day One Therapeutics, Endeavor BioMedicines, Gilead Sciences, Hotspot Therapeutics, SERVIER, STINGthera, Synthekine
Research Funding: MSD (Inst), Bristol Myers Squibb (Inst), Incyte (Inst), Corvus Pharmaceuticals (Inst), Abbvie (Inst), Macrogenics (Inst), Xencor (Inst), Array BioPharma (Inst), Agios (Inst), Astellas Pharma (Inst), EMD Serono (Inst), Immatics (Inst), Kadmon (Inst), Moderna Therapeutics (Inst), Nektar (Inst), Spring bank (Inst), Trishula Therapeutics (Inst), KAHR Medical (Inst), Fstar (Inst), Genmab (Inst), Ikena Oncology (Inst), Numab (Inst), Replimune (Inst), Rubius Therapeutics (Inst), Synlogic (Inst), Takeda (Inst), Tizona Therapeutics, Inc (Inst), BioNTech (Inst), Scholar Rock (Inst), NextCure (Inst)
Patents, Royalties, Other Intellectual Property: Serial No. 15/612,657 (Cancer Immunotherapy), Serial No. PCT/US18/36052 (Microbiome Biomarkers for Anti-PD-1/PD-L1 Responsiveness: Diagnostic, Prognostic and Therapeutic Uses Thereof)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Array BioPharma, EMD Serono, Janssen, MSD, Novartis, Reflexion Medical, Mersana, Pyxis, Xilio Therapeutics
Paolo A. Ascierto
Consulting or Advisory Role: Bristol Myers Squibb, Roche/Genentech, MSD, Novartis, Merck Serono, Pierre Fabre, AstraZeneca, Sun Pharma, Sanofi, Idera, Ultimovacs, Sandoz, Immunocore, 4SC, Italfarmaco, Nektar, Boehringer Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Pfizer, OncoSec, Nouscom, Lunaphore Technologies, Seagen, ITeos Therapeutics, Medicenna, Bio-AI Health, ValoTx, Replimune, Bayer, Erasca, Inc, Philogen, BioNTech SE, Anaveon
Research Funding: Bristol Myers Squibb (Inst), Roche/Genentech (Inst), Sanofi (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Pfizer, Bio-AI Health, Replimune, MSD Oncology, Pierre Fabre
Muhammad A. Khattak
Travel, Accommodations, Expenses: Pierre Fabre
Luis de la Cruz Merino
Consulting or Advisory Role: Roche (Inst), MSD Oncology, Bristol Myers Squibb, Gilead Sciences, AstraZeneca, Incyte
Research Funding: Roche (Inst), Celgene (Inst)
Travel, Accommodations, Expenses: Roche
Michele Del Vecchio
Consulting or Advisory Role: Novartis, Bristol Myers Squibb, MSD, Pierre Fabre, Immunocore
Piotr Rutkowski
Honoraria: Bristol Myers Squibb, MSD, Novartis, Pfizer, Pierre Fabre, Sanofi, MSD
Consulting or Advisory Role: Novartis, Blueprint Medicines, Bristol Myers Squibb, Pierre Fabre, MSD, Amgen, Philogen, AstraZeneca
Speakers' Bureau: Pfizer, Novartis, Pierre Fabre
Research Funding: Novartis (Inst), Roche (Inst), Bristol Myers Squibb (Inst), Pierre Fabre (Inst)
Travel, Accommodations, Expenses: Orphan Europe, Pierre Fabre
Francesco Spagnolo
Honoraria: Bristol Myers Squibb, Novartis, MSD, Pierre Fabre, Sanofi, Merck Serono, Sun Pharma
Consulting or Advisory Role: Novartis, MSD, Philogen, Sun Pharma, Pierre Fabre
Travel, Accommodations, Expenses: Bristol Myers Squibb
Jacek Mackiewicz
Consulting or Advisory Role: Bristol Myers Squibb, MSD
Travel, Accommodations, Expenses: Bristol Myers Squibb, MSD, Pierre Fabre
Other Relationship: Bristol Myers Squibb, MSD, Novartis
Vanna Chiarion-Sileni
Consulting or Advisory Role: Pierre Fabre, MSD
Travel, Accommodations, Expenses: Pierre Fabre
John M. Kirkwood
Honoraria: Bristol Myers Squibb
Consulting or Advisory Role: Harbor BioMed, Scopus BioPharma, Pfizer, AXIO Research, Immunocore, Natera, DermTech, Ankyra Therapeutics, Becker Pharmaceutical Consulting, Fenix Group International, IQVIA, MSD, Replimune, SR One Capital Management, Iovance Biotherapeutics, Checkmate Pharmaceuticals, OncoSec, OncoCyte, Cancer Network, Takeda, Applied Clinical Intelligence, PATHAI, Magnolia Innovation, iOnctura, Jazz Pharmaceuticals, Regeneron, Cancer Study Group, Istari Oncology, CytomX Therapeutics, Lytix Biopharma, PyrOjas Corporation, Bristol Myers Squibb, Amgen, Valar Labs
Research Funding: Amgen (Inst), Bristol Myers Squibb (Inst), Checkmate Pharmaceuticals (Inst), Immunocore (Inst), Iovance Biotherapeutics (Inst), Novartis (Inst), Immvira (Inst), Harbor BioMed (Inst), Takeda (Inst), Verastem (Inst), Lion Biotechnologies (Inst)
Travel, Accommodations, Expenses: Checkmate Pharmaceuticals, Bristol Myers Squibb, Regeneron, Ankyra Therapeutics, Iovance Biotherapeutics
Caroline Robert
Stock and Other Ownership Interests: RiboNexus
Consulting or Advisory Role: Bristol Myers Squibb, Roche, Novartis, Pierre Fabre, MSD, Sanofi, AstraZeneca, Pfizer, Sun Pharma
Research Funding: Novartis (Inst), Phio Pharmaceuticals (Inst)
Jean-Jacques Grob
Consulting or Advisory Role: BMS, MSD Oncology, Roche/Genentech, Novartis, Amgen, Pierre Fabre, Sun Pharma, Merck KGaA, Sanofi, Roche, Philogen, Ultimovacs
Speakers' Bureau: Novartis, Pierre Fabre
Travel, Accommodations, Expenses: BMS, MSD Oncology, Novartis, Pierre Fabre
Dirk Schadendorf
Honoraria: Roche/Genentech, Novartis, Bristol Myers Squibb, MSD, Immunocore, Merck Serono, Pfizer, Pierre Fabre, Philogen, Regeneron, 4SC, Sanofi/Regeneron, NeraCare GmbH, Sun Pharma, InflarxGmbH, Ultimovacs, Daiichi Sankyo Japan, LabCorp, Replimune, Agenus, AstraZeneca, Erasca, Inc, immatics, Novigenix, Pamgene, Seagen
Consulting or Advisory Role: Roche/Genentech, Novartis, Bristol Myers Squibb, MSD, Pierre Fabre, Sanofi/Regeneron, Agenus, AstraZeneca, Daiichi Sankyo, Erasca, Inc, immatics, Immunocore, NeraCare GmbH, Replimune
Speakers' Bureau: Bristol Myers Squibb, MSD, Novartis, Pierre Fabre, Sanofi/Regeneron, Merck KGaA
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), Roche (Inst), MSD Oncology (Inst), Array BioPharma/Pfizer (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, Bristol Myers Squibb, Merck Serono, Novartis, MSD, Pierre Fabre, Sanofi/Regeneron
Matteo S. Carlino
Honoraria: Bristol Myers Squibb, MSD, Novartis
Consulting or Advisory Role: Bristol Myers Squibb, MSD, Amgen, Novartis, Pierre Fabre, Roche, IDEAYA Biosciences, Sanofi, Merck Serono, Regeneron, QBiotics, Nektar, Eisai, OncoSec
Xi Lawrence Xu
Employment: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc
Stock and Other Ownership Interests: Merck & Co, Inc
Mizuho Fukunaga-Kalabis
Employment: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc
Stock and Other Ownership Interests: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc
Clemens Krepler
Employment: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc
Stock and Other Ownership Interests: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc
Alexander M.M. Eggermont
Stock and Other Ownership Interests: Skyline Diagnostics, IO Biotech, Sairopa
Honoraria: Ellipses Pharma, MSD, Pfizer, Sellas Life Sciences, Skyline Diagnostics, BIOINVENT, IO Biotech, BioNTech, Agenus, MSD, Sairopa, Brenus Pharma, IQVIA, TigaTx, Trained Therapeutix Discovery, CatalYm, Scorpion Therapeutics, ISA Pharmaceuticals, GenOway, Pierre Fabre, BioNTech, GlaxoSmithKline
Consulting or Advisory Role: Ellipses Pharma, ISA Pharmaceuticals, MSD, Pfizer, Sellas Life Sciences, Skyline Diagnostics, BIOINVENT, CatalYm, Agenus, IO Biotech, MSD, Sairopa, TigaTx, Trained Therapeutix Discovery, IQVIA, Brenus Pharma, Scorpion Therapeutics, Pierre Fabre, GenOway, BioNTech, BioNTech, GlaxoSmithKline
Speakers' Bureau: MSD, Bristol Myers Squibb
Georgina V. Long
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: BMS, Pierre Fabre
Consulting or Advisory Role: Agenus, Amgen, Array BioPharma, Boehringer Ingelheim, Bristol Myers Squibb, Evaxion Biotech, Hexal, Highlight Therapeutics, Innovent Biologics, MSD, Novartis, OncoSec, PHMR, Pierre Fabre, Provectus, QBiotics, Regeneron, AstraZeneca
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the 2023 ASCO Annual Meeting, Chicago, IL, June 2-6, 2023.
SUPPORT
Supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ.
CLINICAL TRIAL INFORMATION
NCT03553836 (KEYNOTE-716)
DATA SHARING STATEMENT
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
AUTHOR CONTRIBUTIONS
Conception and design: Jason J. Luke, John M. Kirkwood, Matteo S. Carlino, Muhammad A. Khattak, Alexander M.M. Eggermont
Provision of study materials or patients: All authors
Collection and assembly of data: Jason J. Luke, Muhammad A. Khattak, Luis de la Cruz Merino, Michele Del Vecchio, Piotr Rutkowski, Francesco Spagnolo, Jacek Mackiewicz, Vanna Chiarion-Sileni, John M. Kirkwood, Caroline Robert, Jean-Jacques Grob, Federica de Galitiis, Dirk Schadendorf, Matteo S. Carlino, Mizuho Fukunaga-Kalabis, Clemens Krepler, Georgina V. Long
Data analysis and interpretation: Jason J. Luke, Alexander M.M. Eggermont, Caroline Robert, Dirk Schadendorf, Georgina V. Long, Luis de la Cruz Merino, Matteo S. Carlino, Michele Del Vecchio, Paolo A. Ascierto, Xi Lawrence Wu, Muhammad A. Khattak, Mizuho Fukunaga-Kalabis, Clemens Krepler, Jean-Jacques Grob, Vanna Chiarion-Sileni, John M. Kirkwood
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pembrolizumab Versus Placebo as Adjuvant Therapy in Resected Stage IIB or IIC Melanoma: Final Analysis of Distant Metastasis-Free Survival in the Phase III KEYNOTE-716 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jason J. Luke
Stock and Other Ownership Interests: Actym Therapeutics, Mavu Pharmaceutical, Pyxis, Alphamab, Tempest Therapeutics, Kanaph Therapeutics, Onc.AI, Arch Oncology, STipe Therapeutics, NeoTX
Consulting or Advisory Role: Bristol Myers Squibb, MSD, EMD Serono, Novartis, 7 Hills Pharma, Janssen, Reflexion Medical, Tempest Therapeutics, Alphamab, Abbvie, Bayer, Incyte, Partner Therapeutics, Synlogic, Werewolf Therapeutics, Ribon Therapeutics, Checkmate Pharmaceuticals, CStone Pharmaceuticals, Nektar, Regeneron, Rubius Therapeutics, Tesaro, Xilio Therapeutics, Xencor, Alnylam, Crown Bioscience, Flame Biosciences, Genentech, Kadmon, KSQ Therapeutics, Immunocore, Inzen Therapeutics, Pfizer, Silicon Therapeutics, TRex Bio, Bright Peak Therapeutics, Onc.AI, STipe Therapeutics, Codiak Biosciences, Day One Therapeutics, Endeavor BioMedicines, Gilead Sciences, Hotspot Therapeutics, SERVIER, STINGthera, Synthekine
Research Funding: MSD (Inst), Bristol Myers Squibb (Inst), Incyte (Inst), Corvus Pharmaceuticals (Inst), Abbvie (Inst), Macrogenics (Inst), Xencor (Inst), Array BioPharma (Inst), Agios (Inst), Astellas Pharma (Inst), EMD Serono (Inst), Immatics (Inst), Kadmon (Inst), Moderna Therapeutics (Inst), Nektar (Inst), Spring bank (Inst), Trishula Therapeutics (Inst), KAHR Medical (Inst), Fstar (Inst), Genmab (Inst), Ikena Oncology (Inst), Numab (Inst), Replimune (Inst), Rubius Therapeutics (Inst), Synlogic (Inst), Takeda (Inst), Tizona Therapeutics, Inc (Inst), BioNTech (Inst), Scholar Rock (Inst), NextCure (Inst)
Patents, Royalties, Other Intellectual Property: Serial No. 15/612,657 (Cancer Immunotherapy), Serial No. PCT/US18/36052 (Microbiome Biomarkers for Anti-PD-1/PD-L1 Responsiveness: Diagnostic, Prognostic and Therapeutic Uses Thereof)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Array BioPharma, EMD Serono, Janssen, MSD, Novartis, Reflexion Medical, Mersana, Pyxis, Xilio Therapeutics
Paolo A. Ascierto
Consulting or Advisory Role: Bristol Myers Squibb, Roche/Genentech, MSD, Novartis, Merck Serono, Pierre Fabre, AstraZeneca, Sun Pharma, Sanofi, Idera, Ultimovacs, Sandoz, Immunocore, 4SC, Italfarmaco, Nektar, Boehringer Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Pfizer, OncoSec, Nouscom, Lunaphore Technologies, Seagen, ITeos Therapeutics, Medicenna, Bio-AI Health, ValoTx, Replimune, Bayer, Erasca, Inc, Philogen, BioNTech SE, Anaveon
Research Funding: Bristol Myers Squibb (Inst), Roche/Genentech (Inst), Sanofi (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Pfizer, Bio-AI Health, Replimune, MSD Oncology, Pierre Fabre
Muhammad A. Khattak
Travel, Accommodations, Expenses: Pierre Fabre
Luis de la Cruz Merino
Consulting or Advisory Role: Roche (Inst), MSD Oncology, Bristol Myers Squibb, Gilead Sciences, AstraZeneca, Incyte
Research Funding: Roche (Inst), Celgene (Inst)
Travel, Accommodations, Expenses: Roche
Michele Del Vecchio
Consulting or Advisory Role: Novartis, Bristol Myers Squibb, MSD, Pierre Fabre, Immunocore
Piotr Rutkowski
Honoraria: Bristol Myers Squibb, MSD, Novartis, Pfizer, Pierre Fabre, Sanofi, MSD
Consulting or Advisory Role: Novartis, Blueprint Medicines, Bristol Myers Squibb, Pierre Fabre, MSD, Amgen, Philogen, AstraZeneca
Speakers' Bureau: Pfizer, Novartis, Pierre Fabre
Research Funding: Novartis (Inst), Roche (Inst), Bristol Myers Squibb (Inst), Pierre Fabre (Inst)
Travel, Accommodations, Expenses: Orphan Europe, Pierre Fabre
Francesco Spagnolo
Honoraria: Bristol Myers Squibb, Novartis, MSD, Pierre Fabre, Sanofi, Merck Serono, Sun Pharma
Consulting or Advisory Role: Novartis, MSD, Philogen, Sun Pharma, Pierre Fabre
Travel, Accommodations, Expenses: Bristol Myers Squibb
Jacek Mackiewicz
Consulting or Advisory Role: Bristol Myers Squibb, MSD
Travel, Accommodations, Expenses: Bristol Myers Squibb, MSD, Pierre Fabre
Other Relationship: Bristol Myers Squibb, MSD, Novartis
Vanna Chiarion-Sileni
Consulting or Advisory Role: Pierre Fabre, MSD
Travel, Accommodations, Expenses: Pierre Fabre
John M. Kirkwood
Honoraria: Bristol Myers Squibb
Consulting or Advisory Role: Harbor BioMed, Scopus BioPharma, Pfizer, AXIO Research, Immunocore, Natera, DermTech, Ankyra Therapeutics, Becker Pharmaceutical Consulting, Fenix Group International, IQVIA, MSD, Replimune, SR One Capital Management, Iovance Biotherapeutics, Checkmate Pharmaceuticals, OncoSec, OncoCyte, Cancer Network, Takeda, Applied Clinical Intelligence, PATHAI, Magnolia Innovation, iOnctura, Jazz Pharmaceuticals, Regeneron, Cancer Study Group, Istari Oncology, CytomX Therapeutics, Lytix Biopharma, PyrOjas Corporation, Bristol Myers Squibb, Amgen, Valar Labs
Research Funding: Amgen (Inst), Bristol Myers Squibb (Inst), Checkmate Pharmaceuticals (Inst), Immunocore (Inst), Iovance Biotherapeutics (Inst), Novartis (Inst), Immvira (Inst), Harbor BioMed (Inst), Takeda (Inst), Verastem (Inst), Lion Biotechnologies (Inst)
Travel, Accommodations, Expenses: Checkmate Pharmaceuticals, Bristol Myers Squibb, Regeneron, Ankyra Therapeutics, Iovance Biotherapeutics
Caroline Robert
Stock and Other Ownership Interests: RiboNexus
Consulting or Advisory Role: Bristol Myers Squibb, Roche, Novartis, Pierre Fabre, MSD, Sanofi, AstraZeneca, Pfizer, Sun Pharma
Research Funding: Novartis (Inst), Phio Pharmaceuticals (Inst)
Jean-Jacques Grob
Consulting or Advisory Role: BMS, MSD Oncology, Roche/Genentech, Novartis, Amgen, Pierre Fabre, Sun Pharma, Merck KGaA, Sanofi, Roche, Philogen, Ultimovacs
Speakers' Bureau: Novartis, Pierre Fabre
Travel, Accommodations, Expenses: BMS, MSD Oncology, Novartis, Pierre Fabre
Dirk Schadendorf
Honoraria: Roche/Genentech, Novartis, Bristol Myers Squibb, MSD, Immunocore, Merck Serono, Pfizer, Pierre Fabre, Philogen, Regeneron, 4SC, Sanofi/Regeneron, NeraCare GmbH, Sun Pharma, InflarxGmbH, Ultimovacs, Daiichi Sankyo Japan, LabCorp, Replimune, Agenus, AstraZeneca, Erasca, Inc, immatics, Novigenix, Pamgene, Seagen
Consulting or Advisory Role: Roche/Genentech, Novartis, Bristol Myers Squibb, MSD, Pierre Fabre, Sanofi/Regeneron, Agenus, AstraZeneca, Daiichi Sankyo, Erasca, Inc, immatics, Immunocore, NeraCare GmbH, Replimune
Speakers' Bureau: Bristol Myers Squibb, MSD, Novartis, Pierre Fabre, Sanofi/Regeneron, Merck KGaA
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), Roche (Inst), MSD Oncology (Inst), Array BioPharma/Pfizer (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, Bristol Myers Squibb, Merck Serono, Novartis, MSD, Pierre Fabre, Sanofi/Regeneron
Matteo S. Carlino
Honoraria: Bristol Myers Squibb, MSD, Novartis
Consulting or Advisory Role: Bristol Myers Squibb, MSD, Amgen, Novartis, Pierre Fabre, Roche, IDEAYA Biosciences, Sanofi, Merck Serono, Regeneron, QBiotics, Nektar, Eisai, OncoSec
Xi Lawrence Xu
Employment: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc
Stock and Other Ownership Interests: Merck & Co, Inc
Mizuho Fukunaga-Kalabis
Employment: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc
Stock and Other Ownership Interests: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc
Clemens Krepler
Employment: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc
Stock and Other Ownership Interests: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc
Alexander M.M. Eggermont
Stock and Other Ownership Interests: Skyline Diagnostics, IO Biotech, Sairopa
Honoraria: Ellipses Pharma, MSD, Pfizer, Sellas Life Sciences, Skyline Diagnostics, BIOINVENT, IO Biotech, BioNTech, Agenus, MSD, Sairopa, Brenus Pharma, IQVIA, TigaTx, Trained Therapeutix Discovery, CatalYm, Scorpion Therapeutics, ISA Pharmaceuticals, GenOway, Pierre Fabre, BioNTech, GlaxoSmithKline
Consulting or Advisory Role: Ellipses Pharma, ISA Pharmaceuticals, MSD, Pfizer, Sellas Life Sciences, Skyline Diagnostics, BIOINVENT, CatalYm, Agenus, IO Biotech, MSD, Sairopa, TigaTx, Trained Therapeutix Discovery, IQVIA, Brenus Pharma, Scorpion Therapeutics, Pierre Fabre, GenOway, BioNTech, BioNTech, GlaxoSmithKline
Speakers' Bureau: MSD, Bristol Myers Squibb
Georgina V. Long
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: BMS, Pierre Fabre
Consulting or Advisory Role: Agenus, Amgen, Array BioPharma, Boehringer Ingelheim, Bristol Myers Squibb, Evaxion Biotech, Hexal, Highlight Therapeutics, Innovent Biologics, MSD, Novartis, OncoSec, PHMR, Pierre Fabre, Provectus, QBiotics, Regeneron, AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
- 1. Luke JJ, Rutkowski P, Queirolo P, et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): A randomised, double-blind, phase 3 trial. Lancet. 2022;399:1718–1729. doi: 10.1016/S0140-6736(22)00562-1. [DOI] [PubMed] [Google Scholar]
- 2. Long GV, Luke JJ, Khattak MA, et al. Pembrolizumab versus placebo as adjuvant therapy in resected stage IIB or IIC melanoma (KEYNOTE-716): Distant metastasis-free survival results of a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 2022;23:1378–1388. doi: 10.1016/S1470-2045(22)00559-9. [DOI] [PubMed] [Google Scholar]
- 3.KEYTRUDA (Pembrolizumab) Injection, for Intravenous Use [Package Insert] Rahway, NJ: Merck & Co; 2023. [Google Scholar]
- 4.KEYTRUDA 25 mg/mL Concentrate for Solution for Infusion (Summary of Product Characteristics) Haarlem, the Netherlands: Merck Sharp & Dohme B.V.; 2023. [Google Scholar]
- 5. Robert C, Hwu WJ, Hamid O, et al. Long-term safety of pembrolizumab monotherapy and relationship with clinical outcome: A landmark analysis in patients with advanced melanoma. Eur J Cancer. 2021;144:182–191. doi: 10.1016/j.ejca.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:472–492. doi: 10.3322/caac.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garbe C, Keim U, Amaral T, et al. Prognosis of patients with primary melanoma stage I and II according to American Joint Committee on Cancer version 8 validated in two independent cohorts: Implications for adjuvant treatment. J Clin Oncol. 2022;40:3741–3749. doi: 10.1200/JCO.22.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garbe C, Keim U, Suciu S, et al. Prognosis of patients with stage III melanoma according to American Joint Committee on Cancer version 8: A reassessment on the basis of 3 independent stage III melanoma cohorts. J Clin Oncol. 2020;38:2543–2551. doi: 10.1200/JCO.19.03034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Long GV, Del Vecchio M, Weber J, et al. Adjuvant therapy with nivolumab versus placebo in patients with resected stage IIB/C melanoma (CheckMate 76K) SKIN J Cutan Med. 2023;7:s163. [Google Scholar]
- 10. van Akkooi ACJ, Hauschild A, Long GV, et al. 464TiP Phase III study of adjuvant encorafenib plus binimetinib vs placebo in fully resected stage IIB/C BRAFV600-mutated melanoma: COLUMBUS-AD study design. Ann Oncol. 2022;33:S1617–S1618. [Google Scholar]
- 11. Long GV, Eggermont AM, Gershenwald JE, et al. KEYVIBE-010: Adjuvant coformulated vibostolimab with pembrolizumab versus adjuvant pembrolizumab in patients with high-risk stage II-IV melanoma. J Clin Oncol. 2023;41 suppl 16; abstr TPS9611. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.


