Abstract
PURPOSE
Allogeneic hematopoietic cell transplantation (HCT) improves outcomes for patients with AML harboring an internal tandem duplication mutation of FLT3 (FLT3-ITD) AML. These patients are routinely treated with a FLT3 inhibitor after HCT, but there is limited evidence to support this. Accordingly, we conducted a randomized trial of post-HCT maintenance with the FLT3 inhibitor gilteritinib (ClinicalTrials.gov identifier: NCT02997202) to determine if all such patients benefit or if detection of measurable residual disease (MRD) could identify those who might benefit.
METHODS
Adults with FLT3-ITD AML in first remission underwent HCT and were randomly assigned to placebo or 120 mg once daily gilteritinib for 24 months after HCT. The primary end point was relapse-free survival (RFS). Secondary end points included overall survival (OS) and the effect of MRD pre- and post-HCT on RFS and OS.
RESULTS
Three hundred fifty-six participants were randomly assigned post-HCT to receive gilteritinib or placebo. Although RFS was higher in the gilteritinib arm, the difference was not statistically significant (hazard ratio [HR], 0.679 [95% CI, 0.459 to 1.005]; two-sided P = .0518). However, 50.5% of participants had MRD detectable pre- or post-HCT, and, in a prespecified subgroup analysis, gilteritinib was beneficial in this population (HR, 0.515 [95% CI, 0.316 to 0.838]; P = .0065). Those without detectable MRD showed no benefit (HR, 1.213 [95% CI, 0.616 to 2.387]; P = .575).
CONCLUSION
Although the overall improvement in RFS was not statistically significant, RFS was higher for participants with detectable FLT3-ITD MRD pre- or post-HCT who received gilteritinib treatment. To our knowledge, these data are among the first to support the effectiveness of MRD-based post-HCT therapy.
INTRODUCTION
AML is stratified into different molecular subtypes to guide therapy.1 Internal tandem duplication mutations of FLT3 (FLT3-ITD) are common in AML and confer an increased relapse risk.2 Allogeneic hematopoietic stem cell transplantation (HCT) in first remission is considered the standard of care for these patients when feasible.1,3
CONTEXT
Key Objective
To determine if all patients with internal tandem duplication mutation of FLT3 (FLT3-ITD) AML undergoing allogeneic hematopoietic stem-cell transplantation (HCT) benefit from post-HCT maintenance with the FLT3 inhibitor gilteritinib or if benefit is restricted to those patients who have FLT3-ITD measurable residual disease (MRD) at the time of HCT.
Knowledge Generated
Patients with AML with FLT3-ITD MRD detectable in the peri-HCT period benefit from post-HCT gilteritinib, whereas those without detectable MRD do not. These prospective results establish FLT3-ITD mutations as essential markers of MRD and illustrate how molecular MRD can be used to guide the therapy of patients with AML undergoing HCT.
Relevance (C.F. Craddock)
-
Post-transplant gilteritinib maintenance represents a significant therapeutic advance in patients allografted for FLT3-ITD AML who have evidence of peri-transplant MRD. MRD-negative patients derive no benefit from gilteritinib maintenance but instead may be exposed to unnecessary toxicity.*
*Relevance section written by JCO Associate Editor Charles F. Craddock, MD.
Guidelines from the National Comprehensive Cancer Network recommend post-HCT maintenance with FLT3 inhibitors to reduce the risk of relapse4 on the basis of results from small randomized trials of sorafenib and midostaurin.5-8 However, this practice is controversial9 as patients in these trials were not treated with FLT3 inhibitors pre-HCT (the current standard practice) and two of the trials6,8 were nonblinded and allowed only myeloablative conditioning (MAC). Treatment with FLT3 inhibitors can be toxic and often needs to be interrupted or halted because of adverse events (AEs).8,10-13 For patients treated with current induction standards for FLT3-ITD AML undergoing HCT in first remission, the question remains if the benefits of maintenance with FLT3 inhibition outweigh the risks of toxicity. Despite the risk of post-HCT relapse, at least half of patients with FLT3-ITD AML transplanted in first remission are cured without further treatment,4 which means that many patients treated with post-HCT FLT3 inhibition are subjected to an unnecessary therapy.
The presence of measurable residual disease (MRD) pre- or post-HCT is highly predictive of outcomes.14-17 Because of their apparent instability during the course of the disease, FLT3-ITD mutations have not historically been regarded as useful markers of MRD, but recent data suggest otherwise.18-20 Highly sensitive assays using sequential polymerase chain reaction (PCR) and next-generation sequencing (NGS) detect low levels of FLT3-ITD mutations in patients in remission, and retrospective studies suggest that the presence of these mutations correlates with relapse.18,19,21,22
Gilteritinib is a potent, well-tolerated oral FLT3 inhibitor approved as monotherapy for relapsed or refractory FLT3-mutated AML.23 The randomized, double-blinded, placebo-controlled Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 1506 (MORPHO) trial was designed to determine (1) if post-HCT maintenance with gilteritinib provided benefit for patients with FLT3-ITD AML in first remission undergoing HCT and (2) if FLT3-ITD MRD detection could be used to identify the patients who benefit.
METHODS
Patients
Eligible patients were adults with FLT3-ITD AML (diagnosed with local mutation testing) who were in continuous first remission achieved with not more than two cycles of intensive therapy (with or without a FLT3 inhibitor and including any investigational regimens) and intended to undergo allogeneic HCT after induction and any consolidation within 1 year of achieving remission. Any donor source, conditioning regimen, and graft-versus-host disease (GVHD) prophylaxis were permitted.
Trial Design and Treatment
Participants were registered before HCT, and a bone marrow (BM) aspirate was obtained to confirm remission and for MRD analysis. Once engrafted (defined by absolute neutrophil count ≥500/mm3, platelet count ≥20,000/mm3, and platelet transfusion–independent) and provided that they were free of grade II-IV GVHD (and requiring not more than 0.5 mg/kg prednisone per day), participants were randomly assigned between days 30 and 90 after HCT to placebo or 120 mg per day gilteritinib for 24 months. Immediately before random assignment, a second BM aspirate was obtained to confirm ongoing remission and for MRD analysis. Random assignment was double-blinded at a ratio of 1:1 between the treatment arm and the placebo arm using permuted blocks of random sizes, stratified by conditioning regimen intensity (myeloablative v reduced intensity/nonmyeloablative), time from transplantation to random assignment (30-60 v 61-90 days), and the presence of FLT3-ITD MRD at a level of 1 × 10−4 or greater (present v absent/indeterminate) on the basis of the pre-HCT BM aspirate.
MRD Assay
The first 2 mL of any study marrow aspirate was reserved for MRD analysis. For the MRD assay,21 700 ng of genomic DNA was amplified by 25 cycles of PCR using primers flanking exons 14 and 15 of FLT3 and the amplicons were analyzed by NGS. The limit of blank (LOB) was two variant reads, and the lower limit of detection was estimated to be the FLT3-ITD variant allele frequency of 5 × 10−5. However, any level of FLT3-ITD mutation (minimum of three variant reads) above the LOB (quantified as low as 1 × 10−6), irrespective of whether it was the same mutation reported at diagnosis, was considered detectable MRD. The pre-HCT level used for stratification was 1 × 10−4 or higher. Investigators were blinded to the results of MRD analyses.
End Points and Assessments
The primary end point was relapse-free survival (RFS) as assessed by a blinded end point review committee (BERC), measured from the time of random assignment to either morphological relapse or death, using the intention-to-treat (ITT) population. Morphological relapse was defined as BM blasts 5% or higher, any circulating blasts, or any extramedullary blast foci as per published criteria.24 Overall survival (OS) was a key secondary objective. Other secondary objectives included nonrelapse mortality (NRM) and examining the effect of MRD on RFS and OS in the gilteritinib and placebo arms and the effect of gilteritinib versus placebo separately in patients with and without MRD. Additional details on end points and assessments are provided in the Data Supplement (Appendix, online only).
Trial Conduct and Oversight
This trial was conducted in accordance with the Declaration of Helsinki. Institutional review boards at each site approved the trial protocol, and all investigators obtained informed consent from each participant or each participant's guardian. The trial was funded by grant Nos. U10HL069294 and U24HL138660 to the BMT CTN from the National Heart Lung and Blood Institute (NHLBI) and the National Cancer Institute and by Astellas Pharma Global Development, Inc. The trial was designed by the BMT CTN and approved by the NHLBI and Astellas. The Emmes Company monitored North American sites, and Parexel monitored non–North American sites. All investigators and the industry sponsor were blinded to outcomes. Data collection and monitoring procedures are provided in the Data Supplement (Appendix). The investigators had full access to the data at study closure. The study cochairs (M.J.L. and Y.-B. C.) reviewed the data and wrote the manuscript with editorial input from coauthors and without assistance from nonauthors.
Statistical Analysis
The sample size was based on estimates of RFS in the control group of 67% at 1 year, 59% at 2 years, and 55% at 3 years derived from Center for International Blood and Marrow Transplant Research data on participants with FLT3-ITD mutation transplanted in first remission. A total of 122 events would provide 85% power to detect a hazard ratio (HR) of 0.57 (corresponding to a 15% difference in 2-year RFS) with a two-sided significance level of 0.05. The analysis was scheduled for when 122 events were observed or 2.5 years after the last patient was randomly assigned, whichever came first. The primary end point of RFS was summarized using Kaplan-Meier curves and compared between arms using stratified log-rank tests, with the random assignment factors used as stratification variables. A stratified Cox proportional hazards model was used to provide HR estimates and CIs. To maintain the overall two-sided type I error rate at 0.05, formal significance testing of OS using a gatekeeping approach was to be conducted if the RFS comparison was statistically significant. Otherwise, OS analysis would be considered exploratory. OS was analyzed in the ITT population in the same manner as RFS. Competing risk end points (relapse, NRM, acute GVHD [aGVHD], chronic GVHD [cGVHD], eradication or detection of MRD) were summarized using the cumulative incidence function and compared between arms using Gray's test, with subdistribution HRs obtained using the Fine-Gray model. Prespecified subgroup analyses of MRD status were conducted using interaction testing between treatment and subgroup, and forest plots of the treatment effect within subgroups were drawn. No formal multiplicity adjustment for secondary end points or subgroup analyses was used.
RESULTS
Participants
Between August 17, 2017, and July 8, 2020, 620 patients at 122 centers in 16 countries were screened for eligibility, 488 participants were registered, and 356 were randomly assigned, 178 in each arm (Fig 1). The last participant finished treatment in July 2022. The primary analysis is based on a data cutoff on January 7, 2023 (2.5 years after the last participant was randomly assigned). Of 488 participants registered, 132 (27%) participants were not randomly assigned for the following reasons: 68 (51.5%) failed to meet random assignment criteria (including GVHD and failure to engraft); 26 (19.7%) for patient/physician decision; 16 (12.1%) for early death; 10 (7.6%) for relapse; and 12 (9.1%) for other reasons. The safety analysis set (SAF) comprised 355 participants (178 in the gilteritinib arm and 177 in the placebo arm) who took at least one dose of study drug (one participant randomly assigned to placebo received gilteritinib, and one participant randomly assigned to gilteritinib did not take study drug). The most common reasons for early discontinuation were an AE in the gilteritinib arm (17.4%) and relapse (23%) in the placebo arm (Fig 1).
FIG 1.
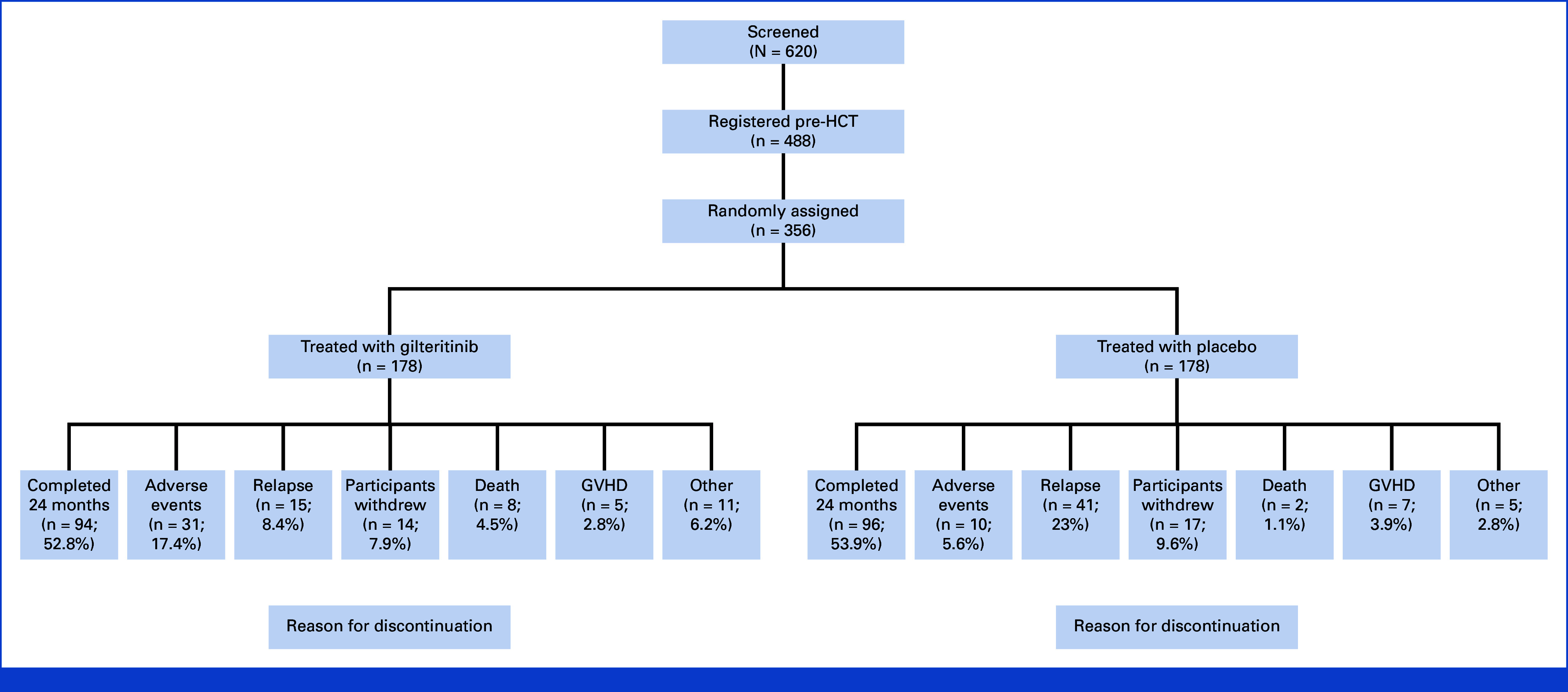
Screening, registration, random assignment, and reasons for discontinuing study treatment. GVHD, graft-versus-host disease; HCT, hematopoietic cell transplantation.
Participant characteristics are displayed in Table 1. There were more than 30 unique conditioning regimens used worldwide. NPM1 mutations were reported in 34.6% of participants. Information on other comutations or FLT3-ITD allelic ratio was not available, and so classification according to the European LeukemiaNet 2022 system was not possible.1 Marrow aspirates for MRD analysis were available from 350 of 356 (98%) participants pre-HCT and 347 of 356 (97.5%) post-HCT (before random assignment). MRD was detected at the stratification level (1 × 10−4 or higher) in 75 of 356 (21.1%) participants and at a level of 1 × 10−6 or higher in 164 of 356 (46.1%) pre-HCT. Post-HCT, MRD was detected at a level of 1 × 10−6 or higher in 71 of 356 (19.9%), including 16 (4.5%) participants with detectable MRD post-HCT but not pre-HCT. Therefore, a total of 180 ([164 + 16 of 356]; 50.6%) participants had detectable MRD in the peri-HCT period.
TABLE 1.
Participant Characteristics at Baseline (ITT population)
| Parameter | Gilteritinib (n = 178) | Placebo (n = 178) |
|---|---|---|
| Age, years, median (range) | 53 (20-78) | 53 (18-76) |
| Sex, No. (%) | ||
| Male | 91 (51.1) | 92 (51.7) |
| Female | 87 (48.9) | 86 (48.3) |
| Race, No. (%) | ||
| White | 114 (64) | 106 (59.6) |
| African American | 6 (3.4) | 3 (1.7) |
| Asian | 47 (26.4) | 56 (31.5) |
| Other/missing | 11 (6.2) | 13 (7.3) |
| Geographic, No. (%) | ||
| North America | 77 (43.3) | 77 (43.3) |
| Europe | 49 (27.5) | 43 (24.2) |
| Asia/Pacific | 52 (29.2) | 58 (32.6) |
| Genetic results at AML diagnosis, No. (%) | ||
| Favorable karyotype | 9 (5.1) | 4 (2.2) |
| Intermediate karyotype | 119 (66.9) | 90 (50.6) |
| Adverse karyotype | 7 (3.9) | 7 (3.9) |
| Unknown | 29 (16.3) | 51 (28.7) |
| Other | 14 (7.9) | 26 (14.6) |
| FLT3 inhibitor pre-HCT, No. (%) | 110 (61.8) | 103 (57.9) |
| HCT-specific comorbidity index, No. (%) | ||
| 0 | 79 (44.4) | 70 (39.3) |
| 1-2 | 49 (27.5) | 51 (28.7) |
| 3+ | 49 (27.5) | 57 (32) |
| Conditioning regimen intensity, No. (%) | ||
| MAC | 106 (59.6) | 107 (60.1) |
| RIC/nonmyeloablative | 72 (40.4) | 71 (39.9) |
| Stem-cell donor, No. (%) | ||
| Matched sibling | 55 (30.9) | 48 (27) |
| Haploidentical | 22 (12.4) | 38 (21.3) |
| Matched unrelated | 71 (39.9) | 65 (36.5) |
| Mismatched unrelated | 15 (8.4) | 17 (9.6) |
| Cord blood | 11 (6.2) | 8 (4.5) |
| Stem-cell source, No. (%) | ||
| Peripheral blood | 140 (78.7) | 140 (78.7) |
| Marrow | 27 (15.2) | 30 (16.9) |
| Cord blood | 11 (6.2) | 8 (4.5) |
| GVHD prophylaxis, No. (%) | ||
| Calcineurin inhibitor + methotrexate | 98 (55.1) | 96 (53.9) |
| Calcineurin inhibitor + mycophenolate mofetil | 43 (24.2) | 51 (28.7) |
| Other | 37 (20.8) | 30 (16.9) |
| Missing | 0 (0) | 1 (0.6) |
| Time from HCT to random assignment, No. (%) | ||
| 30-60 days | 95 (53.4) | 97 (54.5) |
| 61-90 days | 83 (46.6) | 81 (45.5) |
| MRD, No. (%) | ||
| Pre-HCT MRD ≥ 10−4 | 39 (21.9) | 36 (20.2) |
| Pre-HCT MRD ≥ 10−6 | 82 (46.1) | 82 (46.1) |
| Pre- or post-HCT MRD ≥ 10−6 | 89 (50) | 91 (51.1) |
Abbreviations: GVHD, graft-versus-host disease; HCT, hematopoietic cell transplantation; ITT, intention-to-treat; MAC, myeloablative conditioning; MRD, measurable residual disease; RIC, reduced-intensity conditioning.
Efficacy
Among the 270 participants who survived at data cutoff, the median follow-up was 43.8 months. A total of 103 RFS events (by BERC) were observed in the primary analysis, which led to an approximate reduction in power to 78.6% instead of 85.0%. Longer follow-up would not have increased the number of events measurably because of very low event rates beyond 2 years post-HCT. While there was improved RFS in the gilteritinib arm compared with that in the placebo arm (Fig 2A), the difference did not meet the predetermined threshold for significance (HR, 0.679 [95% CI, 0.459 to 1.005]; two-sided P = .0518). The 2-year RFS rate by BERC (95% CI) was 77.2% (CI, 70.1 to 82.8) for participants receiving gilteritinib and 69.9% (CI, 62.4 to 76.2) for those receiving placebo. OS (Fig 2B) was analyzed by ITT in the primary analysis (which included a total of 86 deaths) and did not show a statistically significant difference (HR, 0.846 in favor of gilteritinib [95% CI, 0.554 to 1.293]; two-sided P = .4394). The incidence of relapse was lower and NRM was higher in the gilteritinib arm compared with the placebo arm (Figs 2C and 2D). Of 47 participants who relapsed in the placebo arm, 20 (42.6%) were treated with a FLT3 inhibitor (gilteritinib-13, quizartinib-4, sorafenib-3) after relapse. The cumulative incidence of relapse by geographic region is displayed in the Data Supplement (Fig 1).
FIG 2.
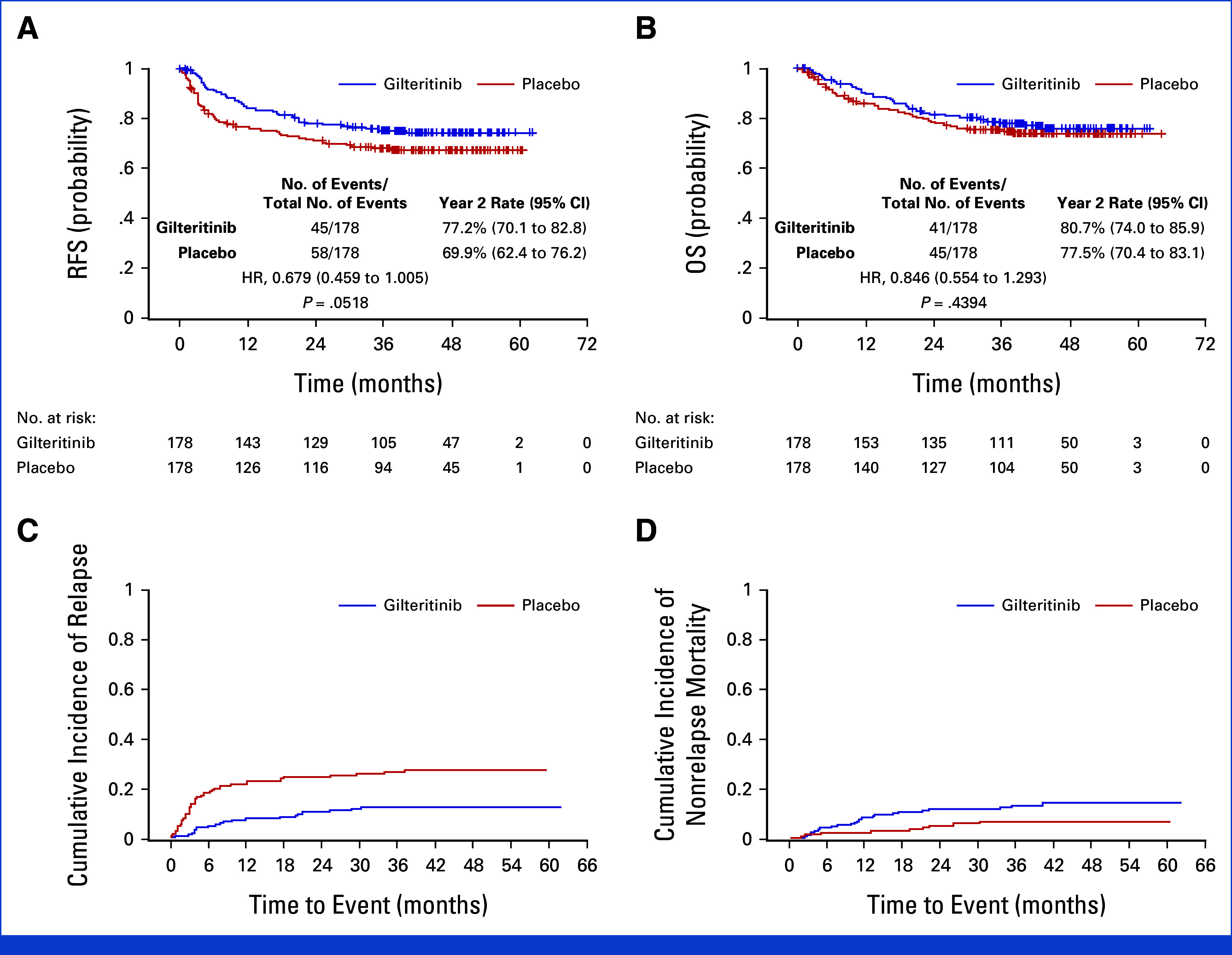
Survival, relapse, and nonrelapse mortality (ITT population). (A) Relapse-free survival, (B) overall survival for the gilteritinib and placebo groups, (C) cumulative incidence of relapse for the gilteritinib group versus placebo group, and (D) cumulative incidence of nonrelapse mortality (defined as death without documentation of morphological relapse). HR, hazard ratio; ITT, intention-to-treat; OS, overall survival; RFS, relapse-free survival.
MRD at a level of 1 × 10−6 or greater was associated with decreased RFS and OS (Figs 3A and 3B) irrespective of the treatment arm. Subgroup analysis of RFS and OS performed on MRD and other prespecified subgroups is displayed in the Data Supplement (Figs 2 and 3). Participants with detectable MRD pre- or post-HCT had a significantly improved RFS if they were on gilteritinib compared with the placebo arm, whereas MRD-negative participants in both arms had similar RFS (Figs 3C and 3D). This was the case for participants with detectable MRD pre-HCT (P = .0105), post-HCT (P = .0143), or either pre- or post-HCT (P = .0065). Similarly, participants with pre- or post-HCT MRD at a level of 1 × 10−6 or greater had improved OS when treated with gilteritinib (Data Supplement, Fig 4) although this did not reach statistical significance (P = .0731).
FIG 3.
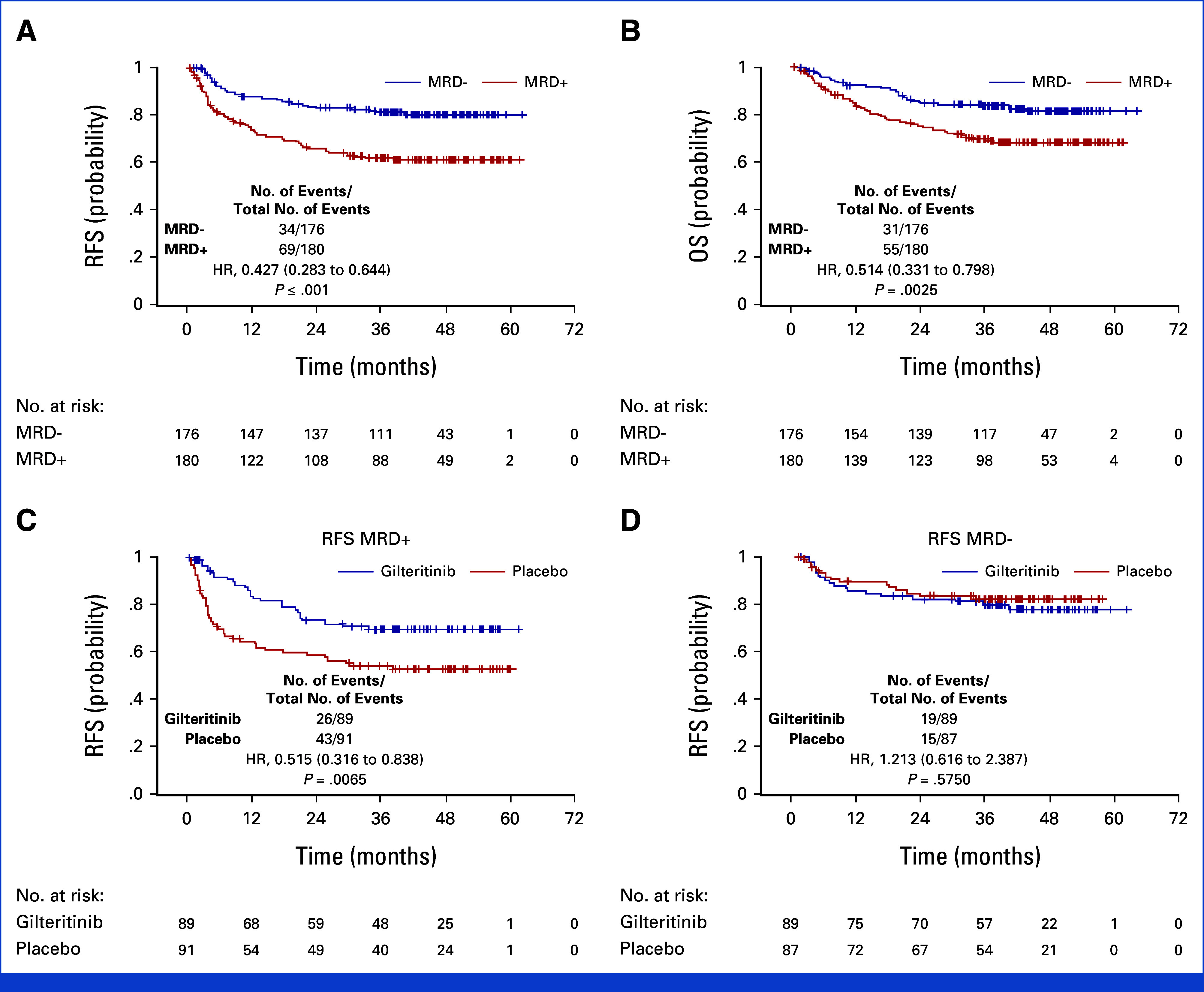
The impact of measurable residual disease on relapse-free survival (ITT population). (A) Relapse-free survival, (B) overall survival for all participants irrespective of the treatment arm according to whether any (eg, FLT3-ITD variant allele frequency of 1 × 10−6 or above) MRD was detectable peri-HCT, (C) relapse-free survival in participants with any (eg, FLT3-ITD variant allele frequency of 1 × 10−6 or above) detectable peri-HCT MRD according to the treatment arm, and (D) relapse-free survival in participants with no detectable peri-HCT MRD, according to the treatment arm. FLT3-ITD, internal tandem duplication mutation of FLT3; HCT, hematopoietic cell transplantation; HR, hazard ratio; ITT, intention-to-treat; MRD, measurable residual disease; OS, overall survival; RFS, relapse-free survival.
For participants who received a FLT3 inhibitor pre-HCT (60%), gilteritinib conferred a RFS benefit compared with placebo (HR, 0.598; P = .0436) although there was no difference between those who did and did not receive pre-HCT FLT3 inhibition in the rate of detectable pre-HCT MRD (48.3% v 52.1%). Participants who received MAC had improved OS compared with those who received reduced-intensity conditioning (RIC) (HR, 0.529; P = .0027), irrespective of MRD status (Data Supplement, Fig 5). The effect of gilteritinib versus placebo in participants receiving MAC and RIC separately is shown in the Data Supplement (Fig 6).
Subgroup analysis revealed differences in outcomes according to the geographic region. Gilteritinib was beneficial in North America, was of minimal benefit in Asia/rest of world (ROW), and had a mildly negative effect in Europe (Fig 4). However, there were distinct geographic differences in study populations and practice patterns, such as the time from diagnosis to HCT, number of induction and consolidation courses, pre-HCT FLT3 inhibitor use, conditioning regimen, and concomitant azole use (Data Supplement, Table 1).
FIG 4.
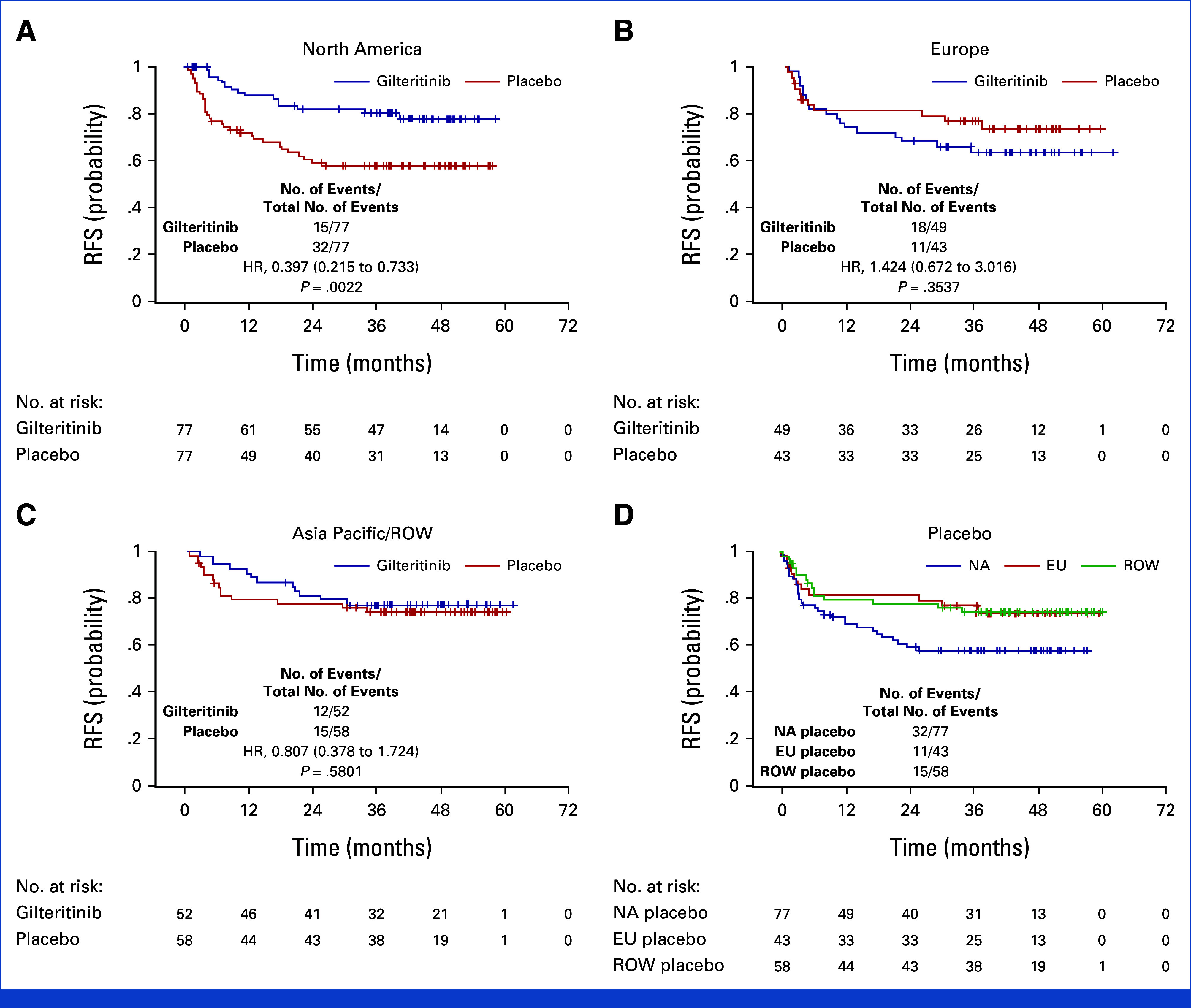
Relapse-free survival by treatment arm according to the geographic region: (A) North America (United States and Canada), (B) Europe (Greece, Belgium, France, Spain, Italy, United Kingdom, Denmark, Poland, Germany), (C) Asia Pacific and ROW (Japan, Korea, Taiwan, Australia, New Zealand), and (D) Relapse-free survival of placebo arms only from the three regions (NA, EU, and ROW). EU, Europe; HR, hazard ratio; NA, North America; ROW, rest of world.
Safety
The SAF consisted of 178 gilteritinib and 177 placebo participants. In the gilteritinib arm, 94 of 178 (52.8%) participants completed 24 months of maintenance compared with 96 of 178 (53.9%) on placebo. Treatment-emergent grade II-IV aGVHD occurred in 33 of 178 (18.5%) participants on gilteritinib versus 36 of 177 (20.3%) on placebo (P = .6157), whereas treatment-emergent cGVHD occurred in 93 of 178 (52.2%) on gilteritinib versus 75 of 177 (42.4%) on placebo (P = .181).
Treatment-emergent AEs (TEAEs) ≥grade 3 occurred in 146 of 178 (82%) participants on gilteritinib compared with 94 of 177 (53.1%) on placebo. Both treatment-emergent myelosuppression and infection were more common in the gilteritinib arm compared with placebo, and myelosuppression was the most common reason for early withdrawal from study treatment. Table 2 lists grade 3 or greater TEAEs occurring in 5% or more of participants, and TEAEs leading to drug interruption, dose reduction, or withdrawal from treatment are summarized in the Data Supplement (Table 2). TEAEs leading to drug discontinuation by geographic region are displayed in the Data Supplement (Table 3).
TABLE 2.
Grade 3 or Greater Treatment-Emergent Adverse Events Occurring in 5% or More of Participants (SAF population)
| Adverse Event | Gilteritinib (n = 178) | Placebo (n = 177) | Total (n = 355) |
|---|---|---|---|
| No. of Patients (%) | |||
| Hematologic | |||
| Neutrophil count decreased | 64 (36) | 23 (13) | 87 (24.5) |
| Platelet count decreased | 38 (21.3) | 20 (11.3) | 58 (16.3) |
| Anemia | 17 (9.6) | 14 (7.9) | 31 (8.7) |
| WBC count decreased | 18 (10.1) | 3 (1.7) | 21 (5.9) |
| Nonhematologic | |||
| ALT increased | 11 (6.2) | 8 (4.5) | 19 (5.4) |
| AST increased | 11 (6.2) | 6 (3.4) | 17 (4.8) |
| Hypertension | 11 (6.2) | 6 (3.4) | 17 (4.8) |
| Creatine phosphokinase elevation | 14 (7.9) | 1 (0.6) | 15 (4.2) |
Abbreviation: SAF, safety analysis set.
Because of a previously noted association between azole use, gilteritinib trough levels, and myelosuppression,25 we examined gilteritinib pharmacokinetics using plasma collected at regular intervals. A total of 67.8% of participants were treated with concomitant azoles (fluconazole, itraconazole, posaconazole, voriconazole, and isavuconazonium), with considerable geographic variation. Concomitant azole use was associated with higher median gilteritinib concentrations, but there was wide interparticipant variability (Data Supplement, Fig 7A). Concomitant azole use was more common outside of North America (Data Supplement, Fig 7B).
DISCUSSION
These data show that the improvement in RFS conferred by gilteritinib over placebo did not reach the predetermined level of significance. However, in secondary analysis, consistent with the pretrial hypothesis, participants with FLT3-ITD AML who undergo HCT in first remission with peri-HCT detectable FLT3-ITD MRD benefit from post-HCT gilteritinib. By contrast, participants in deep remissions did not benefit from maintenance gilteritinib and were therefore exposed unnecessarily to its potential toxicity.
Our data suggest that FLT3 inhibition during induction and/or consolidation may select for participants who are more likely to benefit from post-HCT FLT3 inhibition, which was somewhat unexpected. It is possible that in many cases, pre-HCT FLT3 inhibition serves to control, but not eliminate, FLT3-driven AML clones, and continuous inhibition is necessary until an allogeneic effect can eradicate the disease. In the absence of FLT3 inhibition during induction, many participants with these FLT3-driven clones presumably relapse before HCT.
Although FLT3-ITD mutations detected by standard PCR have generally been considered unreliable markers of MRD,26 recent studies have established the value of PCR-NGS FLT3-ITD MRD.18-20 Using that assay (currently available in the United States),21 we found a high correlation between detection of a FLT3-ITD mutation (at any level) and benefit from a drug specifically targeting that mutation. A post hoc analysis of a recent study using a similar MRD assay suggested that a level of 10−4 was an important survival discriminator, but this was postinduction rather than peri-HCT.20 Our prospective findings establish FLT3-ITD mutations as reliable and actionable markers of MRD in the peri-HCT setting.
The principal toxicity observed in this study was myelosuppression, a known effect of potent FLT3 inhibitors.23,27 The mechanism is likely inhibition of wild-type FLT3 on multipotent progenitor cells.28 A study of gilteritinib combined with intensive chemotherapy reported an association between higher gilteritinib plasma concentrations and concomitant azole use and myelosuppression.25 Azole use was much more common outside North America, and given that myelosuppression led to drug interruption, reduction, or withdrawal, variations in azole use might have contributed to the geographic variation in efficacy we observed.
A single cause of the observed regional differences was not identified in efficacy end points. Participants in the placebo arm in North America, in contrast to those in Europe or Asia/ROW, displayed a 2-year RFS very close to the 59% that was predicted from Center for International Blood and Marrow Transplant Research data used in the statistical analysis plan (Fig 4D). In contrast to the other participants, most North American participants received FLT3 inhibitors pre-HCT and, in general, were bridged more rapidly to HCT (Data Supplement, Table 1). FLT3-ITD AML is a molecularly heterogeneous disease, with responsiveness to FLT3 inhibition clearly influenced by comutations.29,30 It is possible that, outside of North America, patients with disease in which FLT3 was a more prominent driver were less likely to remain in remission long enough to enroll on this study because of lack of FLT3 inhibition, a longer time from diagnosis to transplant, or both. These differences might have selected for a different patient population in North America, one more likely to benefit from post-HCT FLT3 inhibition. At the 110 different centers on this study, the variation in number and intensity of induction and consolidation regimens, azole use, availability of FLT3 inhibitors, time to transplantation, conditioning regimens, and GVHD prophylaxis platforms all were reflections of local clinical practice. They might have contributed to such regional differences, but no single practice or group of practices explaining the differences could be identified in multivariate regression models.
We conducted this study to challenge the assumption that all patients with FLT3-ITD AML worldwide, regardless of those variations, should receive a FLT3 inhibitor post-HCT, and our results have indeed invalidated that assumption. In summary, we found that post-HCT maintenance with gilteritinib does confer a benefit for patients with FLT3-ITD AML, but only for those with peri-HCT FLT3-ITD MRD. At the same time, we have validated the utility of FLT3-ITD mutations as useful markers of MRD with clear implications for intervention. These findings are practice-changing, and further study of the data from this trial is likely to yield more insights into the biology and management of this disease.
ACKNOWLEDGMENT
The BMT-CTN 1506/MORPHO Study Investigators are presented in Appendix Table A1 (online only).
APPENDIX
TABLE A1.
BMT-CTN 1506/MORPHO Study Investigators
| Investigator | Institution |
|---|---|
| Ed Agura | Baylor University Research Institute |
| Jessica Altman | Northwestern Medicine |
| Achiles Anagnostopoulos | General Hospital of Thessaloniki “G. Papanikolaou” |
| Sarah Anand | University of Michigan |
| Andrew Artz | University of Chicago |
| Walter Aulitzky | Robert-Bosch-Krankenhaus GmbH |
| Sophia Balderman | Roswell Park Cancer Institute |
| Karen Ballen | University of Virginia |
| Michael Becker | University of Rochester Medical Center |
| Yves Beguin | CHU de Liege |
| Leanne Berkahn | Auckland Hospital |
| Zwi Berneman | UZ Antwerpen |
| Vijaya Bhatt | University of Nebraska Medical Center |
| Ian Bilmon | Westmead Hospital |
| Francesca Bonifazi | A.O.di Bologna Policl.S.Orsola |
| Adrienne Briggs | Cancer Transplant Institute at Virginia G. Piper Cancer Center |
| Benedetto Bruno | Universita di Torino |
| Claudio Brunstein | University of Minnesota |
| Michael Byrne | Vanderbilt University Medical Center |
| Jenny Byrne | Nottingham City Hospital |
| Monica Cabrero | Hospital Universitario de Salamanca |
| Roberto Cairoli | Ospedale Metropolitano Niguarda |
| George Carrum | Baylor College of Medicine |
| Jan Cerny | University of Massachusetts Memorial Medical Center |
| Yi-Bin Chen | Massachusetts General Hospital |
| June-Won Cheong | Severance Hospital in Yonsei University Health System |
| Fabio Ciceri | Ospedale San Raffaele |
| Mercedes Colorado | H.U.Marq.Valdecilla |
| Rachel Cook | Oregon Health & Science University |
| Daniel Couriel | University of Utah, Huntsman Cancer Institute |
| Charles Craddock | Queen Elizabeth Hospital Birmingham |
| Lloyd Damon | University of California, San Francisco |
| Abhinav Deol | Karmanos Cancer Institute |
| Yohan Desbrosses | Hopital Jean Minjoz |
| Steve Devine | Ohio State University Hospital |
| Carmela Di Grazia | Diparitmento di Malattie Infettive, IRCCS San Martino IST |
| Antonio Di Stasi | University of Alabama at Birmingham |
| Ajoy Dias | Beth Israel Deaconess Medical Center |
| Kathy Dorritie | University of Pittsburgh Cancer Institute |
| James Essell | Oncology Hematology Care, Inc |
| Tetsuya Eto | KKR Hamanomachi Hospital |
| Sherif Farag | Indiana University |
| Edouard Forcade | Hopital Haut Leveque |
| Olga Frankfurt | Northwestern Medicine |
| Shinichiro Fujiwara | Jichi Medical University Hospital |
| Takahiro Fukuda | National Cancer Center Hospital |
| Kentaro Fukushima | Osaka University Hospital |
| Sabine Furst | Institut Paoli-Calmettes |
| Tatsunori Goto | Japanese Red Cross Aichi Medical Center Nagoya Daiichi Hospital |
| Aric Hall | University of Wisconsin Hospital & Clinics |
| Shunsuke Hatta | National Hospital Organization Sendai Medical Center |
| Yosr Hicheri | Hopital Saint-Eloi |
| Mitchell Horwitz | Duke University Health System |
| Hsin-An Hou | National Taiwan University Hospital |
| Jonathan How | McGill University Health Centre |
| Dianna Howard | Wake Forest Baptist Health |
| Wei-Hsun (Blake) Hsu | Christchurch Clinical Studies Trust Ltd |
| Anne Huynh | I.U.C.T-O |
| David Irvine | Beatson West of Scotland Cancer Centre |
| Takayuki Ishikawa | Kobe City Medical Center General Hospital |
| Katarzyna Jamieson | University of North Carolina Chapel Hill |
| Wieslaw Jedrzejczak | MTZ Clinical Research Sp. z o.o. |
| Yogesh Jethava | Indiana Blood and Marrow Transplant |
| Antonio Jimenez | University of Miami University of Miami Hospital and Clinics |
| Chul Won Jung | Samsung Medical Center |
| Junya Kanda | Kyoto University Hospital |
| Dimitrios Karakasis | Evangelismos Hospital |
| Jun Kato | Keio University Hospital |
| Natasha Kekre | Ottawa Hospital |
| Nandita Khera | Mayo Clinic—Phoenix, AZ |
| Hee-Je Kim | Seoul St Mary's Hospital |
| Andreas Klein | Tufts Medical Center |
| Guido Kobbe | Universitätsklinikum Düsseldorf, Klinik für Nephrologie |
| Brian Kornblit | Rigshospitalet |
| Vamsi Kota | Augusta University, Georgia Regents University |
| Silvy Lachance | Maisonneuve-Rosemont, Université de Montréal |
| Brian Leber | Hamilton Health Sciences/Juravinski Cancer Centre |
| Catherine Lee | University of Utah, Huntsman Cancer Institute |
| Je Hwan Lee | Asan Medical Center |
| Mark J. Levis | Johns Hopkins University |
| Tung-Liang Lin | Chang Gung Medical Foundation-Linkou Branch |
| Mark Litzow | Mayo Clinic—Rochester |
| Ta-Chih Liu | Kaohsiung Medical University Hospital |
| Maurizio Martelli | Università degli Studi di Firenze |
| Carmen Martinez | Hospital Clinic de Barcelona |
| Kenichi Matsuoka | Okayama University Hospital |
| John McCarty | Virginia Commonwealth University, Massey Cancer Center |
| Lourdes Mendez | Beth Israel Deaconess Medical Center |
| Fotios Michelis | Princess Margaret Cancer Centre |
| Jan-Henrik Mikesch | Universitatsklinikum Muenster |
| Shin Mineishi | Penn State Hershey Medical Center |
| Asmita Mishra | H. Lee Moffitt Cancer Center |
| Mohamad Mohty | Hopital Saint-Antoine |
| Ine Moors | UZ Gent |
| Gabriela Motyckova | LDS Hospital, Intermountain BMT |
| Lutz Mueller | Universitatsklinik und Poliklinik fuer Innere Medizin IV |
| Lori Muffly | Stanford University |
| Yuho Najima | Tokyo Metropolitan Komagome Hospital |
| Hirohisa Nakamae | Osaka Metropolitan University Hospital |
| Nobuaki Nakano | Imamura Bun-in Hospital |
| Sunita Nathan | Rush University Medical Center |
| Emma Nicholson | Royal Marsden NHS Foundation |
| Maxim Norkin | University of Florida |
| Yoshiaki Ogawa | Tokai University Hospital |
| Gitte Olesen | Aarhus University Hospital |
| Olalekan Oluwole | Vanderbilt University Medical Center |
| Masahiro Onozawa | Hokkaido University Hospital |
| Jeremy Pantin | Augusta University, Georgia Regents University |
| Esperanza B. Papadopoulos | Memorial Sloan-Kettering Cancer Center |
| Kristjan Paulson | CancerCare Manitoba |
| Lucy Pemberton | Dunedin Hospital |
| Travis Perera | Wellington Hospital |
| Alexander E. Perl | University of Pennsylvania |
| Beata Piatkowska-Jakubas | Szpital Uniwersytecki w Krakowie |
| Xavier Poire | Cliniques Universitaires Saint-Luc |
| Rachel Protheroe | University Hospitals Bristol NHS Foundation Trust |
| Alessandro Rambaldi | Ospedale Papa Giovanni XXIII |
| David Ritchie | Royal Melbourne Hospital |
| Kelly Ross | West Virginia University Medicine |
| Marie-Therese Rubio | CHRU Brabois—Service Hématologie et Medecine Interne |
| Stella Santarone | Ospedale Civile Santo Spirito |
| Jaime Sanz Caballer | H. U. Politecnico La Fe |
| Masashi Sawa | Anjo Kosei Hospital |
| Dale Schaar | Rutgers Cancer Institute |
| Christoph Scheid | Medical University of Cologne |
| Jeffrey Schriber | Cancer Transplant Institute at Virginia G. Piper Cancer Center |
| Stuart Seropian | Yale University |
| Nilay Shah | West Virginia University Medicine |
| Nirav Shah | Medical College of Wisconsin |
| Tsiporah Shore | NYP/Weill Cornell Medical Center |
| Jorge Sierra Gil | Hospital de la Santa Creu i Sant Pau |
| Anurag Singh | The University of Kansas Health System |
| Ronald Sobecks | Cleveland Clinic Foundation |
| Gerard Socie | Hopital Saint Louis |
| Robert Soiffer | Dana Farber Cancer Institute |
| Melhem Solh | Northside Hospital |
| Kellie Sprague | Tufts Medical Center |
| Alexandros Spyridonidis | University General Hospital of Patras |
| Matthias Stelljes | Universitatsklinikum Muenster |
| Patrick Stiff | Loyola University Medical Center |
| Robert Stuart | Medical University of South Carolina |
| Masatsugu Tanaka | Kanagawa Cancer Center |
| Anand Tandra | Indiana Blood and Marrow Transplant |
| Eleni Tholouli | Central Manchester University Hospital NHS Foundation Trust |
| Xavier Thomas | Centre Hospitalier Lyon Sud |
| Kirsty Thomson | University College London Hospital NHS Foundation Trust |
| Mario Tiribelli | Azienda Ospedaliero-Universitaria di Udine |
| Benjamin Tomlinson | University Hospitals Cleveland Medical Center |
| Panagiotis Tsirigotis | University Hospital Attikon |
| Dimitrios Tzachanis | University of California San Diego |
| Naoyuki Uchida | KKR Toranomon Hospital |
| Masumi Ueda | Fred Hutchinson Cancer Research Center |
| Celalettin Ustun | University of Minnesota |
| Geoffrey L. Uy | Washington University in St Louis |
| David Valcarcel Ferreiras | Hospital Universitario Vall D'Hebron |
| Sumithra Vasu | Ohio State University Hospital |
| Eva Wagner | Johannes-Gutenberg-Universitat, Universitätsklinik Mainz |
| Edmund K. Waller | Emory University |
| Anne-Marie Watson | Liverpool Hospital |
| Daniel Weisdorf | University of Minnesota |
| John R. Wingard | University of Florida |
| Christine Wolschke | Universitatsklinikum Hamburg-Eppendorf |
| Tomasz Wrobel | Uniw Szpital Kliniczny im Jana Mikulicza-Radeckieg we Wrocla |
| Ibrahim Yakoub-Agha | CHRU de Lille |
| Takuji Yamauchi | Kyushu University Hospital |
| Jean Yared | University of Maryland Medical Center |
| Su-Peng Yeh | China Medical University Hospital |
| Sung-Soo Yoon | Seoul National University Hospital |
| Satoshi Yoshihara | Hyogo College of Medicine, College Hospital |
Mark J. Levis
Consulting or Advisory Role: Daiichi Sankyo, Amgen, Fujifilm, Astellas Pharma, Menarini, Bristol Myers Squibb, AbbVie/Genentech, GlaxoSmithKline, Jazz Pharmaceuticals
Research Funding: Astellas Pharma (Inst), Fujifilm (Inst)
Travel, Accommodations, Expenses: Astellas Pharma
Mehdi Hamadani
Honoraria: Celgene
Consulting or Advisory Role: Incyte, ADC Therapeutics, Puma Biotechnology, Verastem, Kite/Gilead, MorphoSys, Omeros, Novartis, Gamida Cell, Seagen, Genmab, Myeloid Therapeutics, BeiGene, AstraZeneca, Sanofi, Bristol Myers Squibb/Celgene, CRISPR Therapeutics, Caribou Biosciences, AbbVie, Genentech
Speakers' Bureau: Genzyme, AstraZeneca, BeiGene, ADC Therapeutics, Kite/Gilead
Research Funding: Takeda, Spectrum Pharmaceuticals, Otsuka, Astellas Pharma, Genzyme
Mark Litzow
Honoraria: BeiGene Shanghai, Amgen
Speakers' Bureau: BeiGene Shanghai, Amgen
Research Funding: Amgen, Astellas Pharma, Actinium Pharmaceuticals, Syndax
Travel, Accommodations, Expenses: BeiGene Shanghai, Amgen
Other Relationship: Biosight
John R. Wingard
Consulting or Advisory Role: Shire, Celgene, Cidara Therapeutics, F2G, ORCA Therapeutics
Esperanza B. Papadopoulos
Employment: Biogen, Exelixis, Regulus Therapeutics, Graviton Bioscience Corp, EpiKast
Leadership: Biogen, Exelixis, Regulus Therapeutics
Stock and Other Ownership Interests: Biogen, Exelixis, Regulus Therapeutics, Apellis Pharmaceuticals, Leap Therapeutics, Actio Biosciences Inc
Consulting or Advisory Role: Actio Biosciences
Research Funding: AbbVie
Travel, Accommodations, Expenses: Biogen, Exelixis, Regulus Therapeutics
Alexander E. Perl
Honoraria: Astellas Pharma, Daiichi Sankyo
Consulting or Advisory Role: Astellas Pharma, Actinium Pharmaceuticals, Daiichi Sankyo, AbbVie, FORMA Therapeutics, Sumitomo Dainippon, Celgene/Bristol Myers Squibb, Syndax, Genentech, BerGenBio, Immunogen, Foghorn Therapeutics, Rigel, Curis
Research Funding: Astellas Pharma (Inst), Bayer (Inst), Daiichi Sankyo (Inst), Fujifilm (Inst), AbbVie (Inst), Syndax (Inst)
Travel, Accommodations, Expenses: Daiichi Sankyo
Robert Soiffer
Leadership: Kiadis Pharma, Be the Match/NMDP
Consulting or Advisory Role: Juno Therapeutics, Gilead Sciences, Rheos Medicines, Cugene, Jazz Pharmaceuticals, Precision Biosciences, Takeda, Jasper Therapeutics, Alexion Pharmaceuticals, Neovii, Vor Biopharma, Smart Immune, Bluesphere Bio
Expert Testimony: Pfizer
Travel, Accommodations, Expenses: Gilead Sciences
Celalettin Ustun
Employment: Takeda, Blueprint Medicines
Honoraria: Novartis, Blueprint Medicines
Speakers' Bureau: Novartis
Geoffrey L. Uy
Consulting or Advisory Role: Jazz Pharmaceuticals
Edmund K. Waller
Leadership: Cambium Medical Technologies, Cambium Oncology
Stock and Other Ownership Interests: Cambium Medical Technologies, Cambium Oncology, Cerus, Chimerix
Honoraria: Novartis, Verastem, Kite, a Gilead Company, Pharmacyclics, Karyopharm Therapeutics, Sanofi, Janssen Oncology
Consulting or Advisory Role: Novartis, Verastem, Pharmacyclics, Karyopharm Therapeutics, Partners Healthcare, Kite, a Gilead Company, Cambium Medical Technologies, Alimera Sciences, Sanofi
Research Funding: Novartis, Amgen, Juno Therapeutics, Verastem, Partners Healthcare, Sanofi
Patents, Royalties, Other Intellectual Property: Receive Royalties from patent on preparing platelet lysate that has been licensed to Cambium Medical Technologies
Travel, Accommodations, Expenses: Janssen Oncology
Sumithra Vasu
Consulting or Advisory Role: Omeros, Johnson and Johnson
Research Funding: Sanofi (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/725618https://openpaymentsdata.cms.gov/physician/725618
Melhem Solh
Speakers' Bureau: Bristol Myers Squibb, Amgen, Seagen, GlaxoSmithKline
Research Funding: Partner Therapeutics
Asmita Mishra
Research Funding: Novartis
Lori Muffly
Stock and Other Ownership Interests: Corvus Pharmaceuticals
Honoraria: UpToDate
Consulting or Advisory Role: Amgen, Medexus Pharmaceuticals, Astellas Pharma, Kite, a Gilead Company, CTI BioPharma Corp
Research Funding: Adaptive Biotechnologies, Astellas Pharma, Jasper Therapeutics, Kite, a Gilead Company, Bristol Myers Squibb
Hee-Je Kim
Honoraria: AbbVie, AML-Hub, BMS, Hando, Novartis, Aston Sci, Amgen, Takeda, Green-Cross, AIM BioSciences, Astellas Pharma, Jazz Pharmaceuticals, Janssen, LG Chemical, Pfizer, ViGen Cell, Ingenium, Sanofi, Meiji Pharm, MSD
Consulting or Advisory Role: Jazz Pharmaceuticals, Novartis, AbbVie, Astellas Pharma, MSD, BMS, Takeda, Sanofi, Handok, AML-Hub
Speakers' Bureau: Jazz Pharmaceuticals, Takeda, Novartis
Jan-Henrik Mikesch
Honoraria: Pfizer, Novartis, Jazz Pharmaceuticals, BeiGene, BMS GmbH & Co. KG, Celgene, Laboratoires Delbert, Daiichi Sankyo Europe GmbH, Servier
Consulting or Advisory Role: Pfizer, Daiichi Sankyo Deutschland GmbH
Travel, Accommodations, Expenses: Daiichi Sankyo Deutschland GmbH, Celgene, Kite, a Gilead Company
Yuho Najima
Consulting or Advisory Role: Daiichi Sankyo/UCB Japan, Astellas Pharma
Speakers' Bureau: Astellas Pharma, Daiichi Sankyo/UCB Japan, AbbVie, Amgen, Bristol Myers Squibb Japan, Chugai Pharma, CSL Behring, Jannssen Pharma, Kyowa, Nippon Shinyaku, Novartis, Otsuka, Sumitomo Pharma Oncology, Takeda, MSD, JCR Pharmaceuticals
Masahiro Onozawa
Honoraria: Astellas Pharma
Speakers' Bureau: Astellas Pharma, Daiichi Sankyo, Otsuka, Novartis
Andrew H. Wei
Honoraria: Amgen, Servier, Novartis, Celgene, AbbVie/Genentech, Pfizer, Janssen Oncology, Astellas Pharma, Macrogenics, AstraZeneca, Gilead/Forty Seven, Stemline Therapeutics, BeiGene
Consulting or Advisory Role: Servier, Novartis, Amgen, AbbVie/Genentech, Celgene, Macrogenics, Pfizer, Astellas Pharma, AstraZeneca, Janssen, Stemline Therapeutics, BeiGene
Speakers' Bureau: AbbVie/Genentech, Novartis, Celgene/Bristol Myers Squibb, Astex Pharmaceuticals, Servier
Research Funding: Novartis (Inst), Celgene (Inst), AbbVie (Inst), AstraZeneca (Inst), Servier (Inst), Amgen (Inst), Roche (Inst)
Patents, Royalties, Other Intellectual Property: A.H.W. is a current employee of the Walter and Eliza Hall Institute, which receives milestone and royalty payments related to venetoclax, and is eligible for benefits related to these payments. A.H.W. receives payments from WEHI related to venetoclax
Guido Marcucci
Stock and Other Ownership Interests: Ostentus Therapeutics, Inc
Honoraria: Novartis, AbbVie
Speakers’ Bureau: Novartis, AbbVie
Nahla Hasabou
Employment: Astellas Pharma
Research Funding: Astellas Pharma (Inst)
David Delgado
Employment: Astellas Pharma
Matt Rosales
Employment: Astellas Pharma
Stock and Other Ownership Interests: Astellas Pharma
Research Funding: Astellas Pharma
Travel, Accommodations, Expenses: Astellas Pharma
Jason Hill
Employment: Astellas Pharma
Stock and Other Ownership Interests: Ligacept, LLC
Stanley C. Gill
Employment: Astellas Pharma
Rishita Nuthethi
Employment: Astellas Pharma
Steven M. Devine
Leadership: National Marrow Donor Program
Mary M. Horowitz
Consulting or Advisory Role: Medac (Inst)
Research Funding: Jazz Pharmaceuticals (Inst), Novartis (Inst), Sanofi (Inst), Astellas Pharma (Inst), Xenikos (Inst), Gamida Cell (Inst)
Yi-Bin Chen
Leadership: ImmunoFree
Stock and Other Ownership Interests: ImmunoFree
Consulting or Advisory Role: Magenta Therapeutics, Incyte, Novo Nordisk, Editas Medicine, Alexion Pharmaceuticals, Astellas Pharma, Takeda, Pharmacosmos, Vor Biopharma
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at European Hematology Association Annual Meeting, Frankfurt, Germany, June 8-11, 2023.
SUPPORT
Supported by grant Nos. U10HL069294 and U24HL138660 to the Blood and Marrow Transplant Clinical Trials Network from the National Heart, Lung and Blood Institute and the National Cancer Institute, and funding from Astellas Pharma Global Development Inc.
CLINICAL TRIAL INFORMATION
NCT02997202 (MORPHO)
See accompanying Editorial, p. 1731
Contributor Information
Collaborators: Ed Agura, Jessica Altman, Achiles Anagnostopoulos, Sarah Anand, Andrew Artz, Walter Aulitzky, Sophia Balderman, Karen Ballen, Michael Becker, Yves Beguin, Leanne Berkahn, Zwi Berneman, Vijaya Bhatt, Ian Bilmon, Francesca Bonifazi, Adrienne Briggs, Benedetto Bruno, Claudio Brunstein, Michael Byrne, Jenny Byrne, Monica Cabrero, Roberto Cairoli, George Carrum, Jan Cerny, Yi-Bin Chen, June-Won Cheong, Fabio Ciceri, Mercedes Colorado, Rachel Cook, Daniel Couriel, Charles Craddock, Lloyd Damon, Abhinav Deol, Yohan Desbrosses, Steve Devine, Carmela Di Grazia, Antonio Di Stasi, Ajoy Dias, Kathy Dorritie, James Essell, Tetsuya Eto, Sherif Farag, Edouard Forcade, Olga Frankfurt, Shinichiro Fujiwara, Takahiro Fukuda, Kentaro Fukushima, Sabine Furst, Tatsunori Goto, Aric Hall, Shunsuke Hatta, Yosr Hicheri, Mitchell Horwitz, Hsin-An Hou, Jonathan How, Dianna Howard, Wei-Hsun (Blake) Hsu, Anne Huynh, David Irvine, Takayuki Ishikawa, Katarzyna Jamieson, Wieslaw Jedrzejczak, Yogesh Jethava, Antonio Jimenez, Chul Won Jung, Junya Kanda, Dimitrios Karakasis, Jun Kato, Natasha Kekre, Nandita Khera, Hee-Je Kim, Andreas Klein, Guido Kobbe, Brian Kornblit, Vamsi Kota, Silvy Lachance, Brian Leber, Catherine Lee, Je Hwan Lee, Mark J. Levis, Tung-Liang Lin, Mark Litzow, Ta-Chih Liu, Maurizio Martelli, Carmen Martinez, Kenichi Matsuoka, John McCarty, Lourdes Mendez, Fotios Michelis, Jan-Henrik Mikesch, Shin Mineishi, Asmita Mishra, Mohamad Mohty, Ine Moors, Gabriela Motyckova, Lutz Mueller, Lori Muffly, Yuho Najima, Hirohisa Nakamae, Nobuaki Nakano, Sunita Nathan, Emma Nicholson, Maxim Norkin, Yoshiaki Ogawa, Gitte Olesen, Olalekan Oluwole, Masahiro Onozawa, Jeremy Pantin, Esperanza B. Papadopoulos, Kristjan Paulson, Lucy Pemberton, Travis Perera, Alexander E. Perl, Beata Piatkowska-Jakubas, Xavier Poire, Rachel Protheroe, Alessandro Rambaldi, David Ritchie, Kelly Ross, Marie-Therese Rubio, Stella Santarone, Jaime Sanz Caballer, Masashi Sawa, Dale Schaar, Christoph Scheid, Jeffrey Schriber, Stuart Seropian, Nilay Shah, Nirav Shah, Tsiporah Shore, Jorge Sierra Gil, Anurag Singh, Ronald Sobecks, Gerard Socie, Robert Soiffer, Melhem Solh, Kellie Sprague, Alexandros Spyridonidis, Matthias Stelljes, Patrick Stiff, Robert Stuart, Masatsugu Tanaka, Anand Tandra, Eleni Tholouli, Xavier Thomas, Kirsty Thomson, Mario Tiribelli, Benjamin Tomlinson, Panagiotis Tsirigotis, Dimitrios Tzachanis, Naoyuki Uchida, Masumi Ueda, Celalettin Ustun, Geoffrey L. Uy, David Valcarcel Ferreiras, Sumithra Vasu, Eva Wagner, Edmund K. Waller, Anne-Marie Watson, Daniel Weisdorf, John R. Wingard, Christine Wolschke, Tomasz Wrobel, Ibrahim Yakoub-Agha, Takuji Yamauchi, Jean Yared, Su-Peng Yeh, Sung-Soo Yoon, and Satoshi Yoshihara
DATA SHARING STATEMENT
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
AUTHOR CONTRIBUTIONS
Conception and design: Mark J. Levis, Mehdi Hamadani, Brent Logan, Richard J. Jones, Anurag K. Singh, Alexander E. Perl, Robert Soiffer, Edmund K. Waller, Guido Marcucci, Nahla Hasabou, Matt Rosales, Jason Hill, Mary M. Horowitz, Yi-Bin Chen
Administrative support: Jan-Henrik Mikesch, Guido Marcucci, Heather Wittsack, Mary M. Horowitz
Provision of study materials or patients: Richard J. Jones, Mark Litzow, John R. Wingard, Alexander E. Perl, Robert Soiffer, Geoffrey L. Uy, Edmund K. Waller, Sumithra Vasu, Hee-Je Kim, Jan-Henrik Mikesch, Masahiro Onozawa, Kirsty Thomson, Guido Marcucci, Yi-Bin Chen
Collection and assembly of data: Mark J. Levis, Mehdi Hamadani, Anurag K. Singh, Mark Litzow, John R. Wingard, Alexander E. Perl, Robert Soiffer, Geoffrey L. Uy, Edmund K. Waller, Sumithra Vasu, Hee-Je Kim, Jan-Henrik Mikesch, Yuho Najima, Masahiro Onozawa, Kirsty Thomson, Guido Marcucci, David Delgado, Matt Rosales, Jason Hill, Denise King, Heather Wittsack, Mary M. Horowitz, Yi-Bin Chen
Data analysis and interpretation: Mark J. Levis, Mehdi Hamadani, Brent Logan, Anurag K. Singh, Mark Litzow, John R. Wingard, Esperanza B. Papadopoulos, Alexander E. Perl, Robert Soiffer, Celalettin Ustun, Masumi Ueda Oshima, Geoffrey L. Uy, Edmund K. Waller, Sumithra Vasu, Melhem Solh, Asmita Mishra, Lori Muffly, Hee-Je Kim, Jan-Henrik Mikesch, Masahiro Onozawa, Arnon Nagler, Andrew H. Wei, Guido Marcucci, Nancy L. Geller, Nahla Hasabou, David Delgado, Matt Rosales, Jason Hill, Stanley C. Gill, Rishita Nuthethi, Steven M. Devine, Mary M. Horowitz, Yi-Bin Chen
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Gilteritinib as Post-Transplant Maintenance for AML With Internal Tandem Duplication Mutation of FLT3
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Mark J. Levis
Consulting or Advisory Role: Daiichi Sankyo, Amgen, Fujifilm, Astellas Pharma, Menarini, Bristol Myers Squibb, AbbVie/Genentech, GlaxoSmithKline, Jazz Pharmaceuticals
Research Funding: Astellas Pharma (Inst), Fujifilm (Inst)
Travel, Accommodations, Expenses: Astellas Pharma
Mehdi Hamadani
Honoraria: Celgene
Consulting or Advisory Role: Incyte, ADC Therapeutics, Puma Biotechnology, Verastem, Kite/Gilead, MorphoSys, Omeros, Novartis, Gamida Cell, Seagen, Genmab, Myeloid Therapeutics, BeiGene, AstraZeneca, Sanofi, Bristol Myers Squibb/Celgene, CRISPR Therapeutics, Caribou Biosciences, AbbVie, Genentech
Speakers' Bureau: Genzyme, AstraZeneca, BeiGene, ADC Therapeutics, Kite/Gilead
Research Funding: Takeda, Spectrum Pharmaceuticals, Otsuka, Astellas Pharma, Genzyme
Mark Litzow
Honoraria: BeiGene Shanghai, Amgen
Speakers' Bureau: BeiGene Shanghai, Amgen
Research Funding: Amgen, Astellas Pharma, Actinium Pharmaceuticals, Syndax
Travel, Accommodations, Expenses: BeiGene Shanghai, Amgen
Other Relationship: Biosight
John R. Wingard
Consulting or Advisory Role: Shire, Celgene, Cidara Therapeutics, F2G, ORCA Therapeutics
Esperanza B. Papadopoulos
Employment: Biogen, Exelixis, Regulus Therapeutics, Graviton Bioscience Corp, EpiKast
Leadership: Biogen, Exelixis, Regulus Therapeutics
Stock and Other Ownership Interests: Biogen, Exelixis, Regulus Therapeutics, Apellis Pharmaceuticals, Leap Therapeutics, Actio Biosciences Inc
Consulting or Advisory Role: Actio Biosciences
Research Funding: AbbVie
Travel, Accommodations, Expenses: Biogen, Exelixis, Regulus Therapeutics
Alexander E. Perl
Honoraria: Astellas Pharma, Daiichi Sankyo
Consulting or Advisory Role: Astellas Pharma, Actinium Pharmaceuticals, Daiichi Sankyo, AbbVie, FORMA Therapeutics, Sumitomo Dainippon, Celgene/Bristol Myers Squibb, Syndax, Genentech, BerGenBio, Immunogen, Foghorn Therapeutics, Rigel, Curis
Research Funding: Astellas Pharma (Inst), Bayer (Inst), Daiichi Sankyo (Inst), Fujifilm (Inst), AbbVie (Inst), Syndax (Inst)
Travel, Accommodations, Expenses: Daiichi Sankyo
Robert Soiffer
Leadership: Kiadis Pharma, Be the Match/NMDP
Consulting or Advisory Role: Juno Therapeutics, Gilead Sciences, Rheos Medicines, Cugene, Jazz Pharmaceuticals, Precision Biosciences, Takeda, Jasper Therapeutics, Alexion Pharmaceuticals, Neovii, Vor Biopharma, Smart Immune, Bluesphere Bio
Expert Testimony: Pfizer
Travel, Accommodations, Expenses: Gilead Sciences
Celalettin Ustun
Employment: Takeda, Blueprint Medicines
Honoraria: Novartis, Blueprint Medicines
Speakers' Bureau: Novartis
Geoffrey L. Uy
Consulting or Advisory Role: Jazz Pharmaceuticals
Edmund K. Waller
Leadership: Cambium Medical Technologies, Cambium Oncology
Stock and Other Ownership Interests: Cambium Medical Technologies, Cambium Oncology, Cerus, Chimerix
Honoraria: Novartis, Verastem, Kite, a Gilead Company, Pharmacyclics, Karyopharm Therapeutics, Sanofi, Janssen Oncology
Consulting or Advisory Role: Novartis, Verastem, Pharmacyclics, Karyopharm Therapeutics, Partners Healthcare, Kite, a Gilead Company, Cambium Medical Technologies, Alimera Sciences, Sanofi
Research Funding: Novartis, Amgen, Juno Therapeutics, Verastem, Partners Healthcare, Sanofi
Patents, Royalties, Other Intellectual Property: Receive Royalties from patent on preparing platelet lysate that has been licensed to Cambium Medical Technologies
Travel, Accommodations, Expenses: Janssen Oncology
Sumithra Vasu
Consulting or Advisory Role: Omeros, Johnson and Johnson
Research Funding: Sanofi (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/725618https://openpaymentsdata.cms.gov/physician/725618
Melhem Solh
Speakers' Bureau: Bristol Myers Squibb, Amgen, Seagen, GlaxoSmithKline
Research Funding: Partner Therapeutics
Asmita Mishra
Research Funding: Novartis
Lori Muffly
Stock and Other Ownership Interests: Corvus Pharmaceuticals
Honoraria: UpToDate
Consulting or Advisory Role: Amgen, Medexus Pharmaceuticals, Astellas Pharma, Kite, a Gilead Company, CTI BioPharma Corp
Research Funding: Adaptive Biotechnologies, Astellas Pharma, Jasper Therapeutics, Kite, a Gilead Company, Bristol Myers Squibb
Hee-Je Kim
Honoraria: AbbVie, AML-Hub, BMS, Hando, Novartis, Aston Sci, Amgen, Takeda, Green-Cross, AIM BioSciences, Astellas Pharma, Jazz Pharmaceuticals, Janssen, LG Chemical, Pfizer, ViGen Cell, Ingenium, Sanofi, Meiji Pharm, MSD
Consulting or Advisory Role: Jazz Pharmaceuticals, Novartis, AbbVie, Astellas Pharma, MSD, BMS, Takeda, Sanofi, Handok, AML-Hub
Speakers' Bureau: Jazz Pharmaceuticals, Takeda, Novartis
Jan-Henrik Mikesch
Honoraria: Pfizer, Novartis, Jazz Pharmaceuticals, BeiGene, BMS GmbH & Co. KG, Celgene, Laboratoires Delbert, Daiichi Sankyo Europe GmbH, Servier
Consulting or Advisory Role: Pfizer, Daiichi Sankyo Deutschland GmbH
Travel, Accommodations, Expenses: Daiichi Sankyo Deutschland GmbH, Celgene, Kite, a Gilead Company
Yuho Najima
Consulting or Advisory Role: Daiichi Sankyo/UCB Japan, Astellas Pharma
Speakers' Bureau: Astellas Pharma, Daiichi Sankyo/UCB Japan, AbbVie, Amgen, Bristol Myers Squibb Japan, Chugai Pharma, CSL Behring, Jannssen Pharma, Kyowa, Nippon Shinyaku, Novartis, Otsuka, Sumitomo Pharma Oncology, Takeda, MSD, JCR Pharmaceuticals
Masahiro Onozawa
Honoraria: Astellas Pharma
Speakers' Bureau: Astellas Pharma, Daiichi Sankyo, Otsuka, Novartis
Andrew H. Wei
Honoraria: Amgen, Servier, Novartis, Celgene, AbbVie/Genentech, Pfizer, Janssen Oncology, Astellas Pharma, Macrogenics, AstraZeneca, Gilead/Forty Seven, Stemline Therapeutics, BeiGene
Consulting or Advisory Role: Servier, Novartis, Amgen, AbbVie/Genentech, Celgene, Macrogenics, Pfizer, Astellas Pharma, AstraZeneca, Janssen, Stemline Therapeutics, BeiGene
Speakers' Bureau: AbbVie/Genentech, Novartis, Celgene/Bristol Myers Squibb, Astex Pharmaceuticals, Servier
Research Funding: Novartis (Inst), Celgene (Inst), AbbVie (Inst), AstraZeneca (Inst), Servier (Inst), Amgen (Inst), Roche (Inst)
Patents, Royalties, Other Intellectual Property: A.H.W. is a current employee of the Walter and Eliza Hall Institute, which receives milestone and royalty payments related to venetoclax, and is eligible for benefits related to these payments. A.H.W. receives payments from WEHI related to venetoclax
Guido Marcucci
Stock and Other Ownership Interests: Ostentus Therapeutics, Inc
Honoraria: Novartis, AbbVie
Speakers’ Bureau: Novartis, AbbVie
Nahla Hasabou
Employment: Astellas Pharma
Research Funding: Astellas Pharma (Inst)
David Delgado
Employment: Astellas Pharma
Matt Rosales
Employment: Astellas Pharma
Stock and Other Ownership Interests: Astellas Pharma
Research Funding: Astellas Pharma
Travel, Accommodations, Expenses: Astellas Pharma
Jason Hill
Employment: Astellas Pharma
Stock and Other Ownership Interests: Ligacept, LLC
Stanley C. Gill
Employment: Astellas Pharma
Rishita Nuthethi
Employment: Astellas Pharma
Steven M. Devine
Leadership: National Marrow Donor Program
Mary M. Horowitz
Consulting or Advisory Role: Medac (Inst)
Research Funding: Jazz Pharmaceuticals (Inst), Novartis (Inst), Sanofi (Inst), Astellas Pharma (Inst), Xenikos (Inst), Gamida Cell (Inst)
Yi-Bin Chen
Leadership: ImmunoFree
Stock and Other Ownership Interests: ImmunoFree
Consulting or Advisory Role: Magenta Therapeutics, Incyte, Novo Nordisk, Editas Medicine, Alexion Pharmaceuticals, Astellas Pharma, Takeda, Pharmacosmos, Vor Biopharma
No other potential conflicts of interest were reported.
REFERENCES
- 1. Dohner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–1377. doi: 10.1182/blood.2022016867. [DOI] [PubMed] [Google Scholar]
- 2. Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17:1738–1752. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- 3. Tallman MS, Wang ES, Altman JK, et al. Acute myeloid leukemia, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:721–749. doi: 10.6004/jnccn.2019.0028. [DOI] [PubMed] [Google Scholar]
- 4. Deol A, Sengsayadeth S, Ahn KW, et al. Does FLT3 mutation impact survival after hematopoietic stem cell transplantation for acute myeloid leukemia? Cancer. 2016;122:3005–3014. doi: 10.1002/cncr.30140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NCCN Guidelines: NCCN Clinical Practice Guidelines in Oncology for Guideline for AML 2023. http://NCCN.org [Google Scholar]
- 6. Xuan L, Wang Y, Huang F, et al. Sorafenib maintenance in patients with FLT3-ITD acute myeloid leukaemia undergoing allogeneic haematopoietic stem-cell transplantation: An open-label, multicentre, randomised phase 3 trial. Lancet Oncol. 2020;21:1201–1212. doi: 10.1016/S1470-2045(20)30455-1. [DOI] [PubMed] [Google Scholar]
- 7. Burchert A, Bug G, Fritz LV, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN) J Clin Oncol. 2020;38:2993–3002. doi: 10.1200/JCO.19.03345. [DOI] [PubMed] [Google Scholar]
- 8. Maziarz RT, Levis M, Patnaik MM, et al. Midostaurin after allogeneic stem cell transplant in patients with FLT3-internal tandem duplication-positive acute myeloid leukemia. Bone Marrow Transpl. 2021;56:1180–1189. doi: 10.1038/s41409-020-01153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levis MJ, Chen YB, Hamadani M, et al. FLT3 inhibitor maintenance after allogeneic transplantation: Is a placebo-controlled, randomized trial ethical? J Clin Oncol. 2019;37:1604–1607. doi: 10.1200/JCO.19.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen YB, Li S, Lane AA, et al. Phase I trial of maintenance sorafenib after allogeneic hematopoietic stem cell transplantation for fms-like tyrosine kinase 3 internal tandem duplication acute myeloid leukemia. Biol Blood Marrow Transpl. 2014;20:2042–2048. doi: 10.1016/j.bbmt.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pratz KW, Rudek MA, Smith BD, et al. A prospective study of peritransplant sorafenib for patients with FLT3-ITD acute myeloid leukemia undergoing allogeneic transplantation. Biol Blood Marrow Transpl. 2020;26:300–306. doi: 10.1016/j.bbmt.2019.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dohner H, Weber D, Krzykalla J, et al. Midostaurin plus intensive chemotherapy for younger and older patients with AML and FLT3 internal tandem duplications. Blood Adv. 2022;6:5345–5355. doi: 10.1182/bloodadvances.2022007223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morin S, Giannotti F, Mamez AC, et al. Real-world experience of sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for FLT3-ITD AML reveals high rates of toxicity-related treatment interruption. Front Oncol. 2023;13:1095870. doi: 10.3389/fonc.2023.1095870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dillon LW, Gui G, Page KM, et al. DNA sequencing to detect residual disease in adults with acute myeloid leukemia prior to hematopoietic cell transplant. JAMA. 2023;329:745–755. doi: 10.1001/jama.2023.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Walter RB, Buckley SA, Pagel JM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. 2013;122:1813–1821. doi: 10.1182/blood-2013-06-506725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou Y, Othus M, Araki D, et al. Pre- and post-transplant quantification of measurable (“minimal”) residual disease via multiparameter flow cytometry in adult acute myeloid leukemia. Leukemia. 2016;30:1456–1464. doi: 10.1038/leu.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hourigan CS, Dillon LW, Gui G, et al. Impact of conditioning intensity of allogeneic transplantation for acute myeloid leukemia with genomic evidence of residual disease. J Clin Oncol. 2020;38:1273–1283. doi: 10.1200/JCO.19.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loo S, Dillon R, Ivey A, et al. Pretransplant FLT3-ITD MRD assessed by high-sensitivity PCR-NGS determines posttransplant clinical outcome. Blood. 2022;140:2407–2411. doi: 10.1182/blood.2022016567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grob T, Sanders MA, Vonk CM, et al. Prognostic value of FLT3-internal tandem duplication residual disease in acute myeloid leukemia. J Clin Oncol. 2023;41:756–765. doi: 10.1200/JCO.22.00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Erba HP, Montesinos P, Kim HJ, et al. Quizartinib plus chemotherapy in newly diagnosed patients with FLT3-internal-tandem-duplication-positive acute myeloid leukaemia (QuANTUM-First): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401:1571–1583. doi: 10.1016/S0140-6736(23)00464-6. [DOI] [PubMed] [Google Scholar]
- 21. Levis MJ, Perl AE, Altman JK, et al. A next-generation sequencing-based assay for minimal residual disease assessment in AML patients with FLT3-ITD mutations. Blood Adv. 2018;2:825–831. doi: 10.1182/bloodadvances.2018015925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blatte TJ, Schmalbrock LK, Skambraks S, et al. Vol. 33. Leukemia: 2019. getITD for FLT3-ITD-based MRD monitoring in AML; pp. 2535–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381:1728–1740. doi: 10.1056/NEJMoa1902688. [DOI] [PubMed] [Google Scholar]
- 24. Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 25.Pratz KW, Cherry M, Altman JK, et al. Gilteritinib in combination with induction and consolidation chemotherapy and as maintenance therapy: A phase IB study in patients with newly diagnosed AML J Clin Oncol 41426-42462023 [DOI] [PubMed] [Google Scholar]
- 26. Heuser M, Freeman SD, Ossenkoppele GJ, et al. 2021 Update on MRD in acute myeloid leukemia: A consensus document from the European LeukemiaNet MRD Working Party. Blood. 2021;138:2753–2767. doi: 10.1182/blood.2021013626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cortes JE, Khaled S, Martinelli G, et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): A multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2019;20:984–997. doi: 10.1016/S1470-2045(19)30150-0. [DOI] [PubMed] [Google Scholar]
- 28. Beaudin AE, Boyer SW, Forsberg EC. Flk2/Flt3 promotes both myeloid and lymphoid development by expanding non-self-renewing multipotent hematopoietic progenitor cells. Exp Hematol. 2014;42:218–229.e4. doi: 10.1016/j.exphem.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dohner K, Thiede C, Jahn N, et al. Impact of NPM1/FLT3-ITD genotypes defined by the 2017 European LeukemiaNet in patients with acute myeloid leukemia. Blood. 2020;135:371–380. doi: 10.1182/blood.2019002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith CC, Levis MJ, Perl AE, et al. Molecular profile of FLT3-mutated relapsed/refractory patients with AML in the phase 3 ADMIRAL study of gilteritinib. Blood Adv. 2022;6:2144–2155. doi: 10.1182/bloodadvances.2021006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.


