Abstract
PURPOSE
The OlympiA randomized phase III trial compared 1 year of olaparib (OL) or placebo (PL) as adjuvant therapy in patients with germline BRCA1/2, high-risk human epidermal growth factor receptor 2–negative early breast cancer after completing (neo)adjuvant chemotherapy ([N]ACT), surgery, and radiotherapy. The patient-reported outcome primary hypothesis was that OL-treated patients may experience greater fatigue during treatment.
METHODS
Data were collected before random assignment, and at 6, 12, 18, and 24 months. The primary end point was fatigue, measured with the Functional Assessment of Chronic Illness Therapy-Fatigue scale. Secondary end points, assessed with the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire, Core 30 item, included nausea and vomiting (NV), diarrhea, and multiple functional domains. Scores were compared between treatment groups using mixed model for repeated measures. Two-sided P values <.05 were statistically significant for the primary end point. All secondary end points were descriptive.
RESULTS
One thousand five hundred and thirty-eight patients (NACT: 746, ACT: 792) contributed to the analysis. Fatigue severity was statistically significantly greater for OL versus PL, but not clinically meaningfully different by prespecified criteria (≥3 points) at 6 months (diff OL v PL: NACT: –1.3 [95% CI, –2.4 to –0.2]; P = .022; ACT: –1.3 [95% CI, –2.3 to –0.2]; P = .017) and 12 months (NACT: –1.6 [95% CI, –2.8 to –0.3]; P = .017; ACT: –1.3 [95% CI, –2.4 to –0.2]; P = .025). There were no significant differences in fatigue severity between treatment groups at 18 and 24 months. NV severity was worse in patients treated with OL compared with PL at 6 months (NACT: 6.0 [95% CI, 4.1 to 8.0]; ACT: 5.3 [95% CI, 3.4 to 7.2]) and 12 months (NACT: 6.4 [95% CI, 4.4 to 8.3]; ACT: 4.5 [95% CI, 2.8 to 6.1]). During treatment, there were some clinically meaningful differences between groups for other symptoms but not for function subscales or global health status.
CONCLUSION
Treatment-emergent symptoms from OL were limited, generally resolving after treatment ended. OL- and PL-treated patients had similar functional scores, slowly improving during the 24 months after (N)ACT and there was no clinically meaningful persistence of fatigue severity in OL-treated patients.
Patient-reported outcomes in the OlympiA trial show that olaparib was well tolerated compared with placebo.
INTRODUCTION
OlympiA, a randomized, double-blind, parallel group, placebo (PL)-controlled, multi-center phase III study, compared 1 year of olaparib (OL) with PL as adjuvant therapy in patients with germline pathogenic or likely pathogenic variants in BRCA1 or BRCA2 (gBRCA1/2pv) and high-risk, human epidermal growth factor receptor 2 (HER2)–negative early breast cancer (EBC), after completing definitive local treatment and (neo)adjuvant chemotherapy [(N)ACT].1 Invasive disease-free survival (IDFS) was the primary outcome of the OlympiA trial. Patient-reported outcomes (PROs) were included among the secondary objectives, with a primary focus on fatigue, as well as other symptoms and health-related quality of life (HRQOL). Informed by previous research in patients with EBC who experienced substantial fatigue associated with ACT,2-4 we focused on the potential for OL to impair recovery from postchemotherapy fatigue and to delay improvements in HRQOL after chemotherapy. Observational studies have documented persistent fatigue in 25%-30% of EBC survivors,4 but with variable patterns of resolution over time.5-7 Would OL adjuvant treatment after standard [N]ACT delay fatigue resolution compared with PL? The PL-controlled trial provided an important opportunity to control for expected recovery in symptoms and HRQOL after intensive [N]ACT.
CONTEXT
Key Objectives
The OlympiA trial demonstrated significant and clinically meaningful improvement in invasive disease-free survival and overall survival, comparing 1 year of adjuvant olaparib (OL) versus placebo (PL) in patients with germline pathogenic or likely pathogenic variants in BRCA1/BRCA2 and high-risk, human epidermal growth factor receptor 2–negative early breast cancer. This paper reports on results of the patient-reported outcomes (PROs) study, a secondary trial objective.
Knowledge Generated
Primary outcomes of the PRO study focused on whether adjuvant OL increased the likelihood of significantly greater fatigue severity during 12 months of treatment after (neo)adjuvant chemotherapy and whether there would be resolution of fatigue during the post-treatment year. Additional symptoms and health-related quality of life were also explored. There was no clinically meaningful increase in fatigue with OL versus PL; only nausea and vomiting were mildly increased by OL.
Relevance (K.D. Miller)
-
Shared decision making in the adjuvant setting requires balancing benefits and risks. PROs from the OlympiA trial complement physician-documented toxicity and suggest minimal impact on quality of life when OL was added to adjuvant therapy in patients with high risk of recurrence.*
*Relevance section written by JCO Senior Deputy Editor Kathy D. Miller, MD.
METHODS
OlympiA Study Design and Rationale for the PRO Selection
Patients in OlympiA were randomly assigned (1:1) to 1 year of either oral OL 300 mg twice a day or matching PL. Random assignment was stratified by hormone receptor status (estrogen receptor–positive or progesterone receptor–positive/both negative), previous chemotherapy (NACT/ACT), and previous platinum use for EBC (yes/no). Patients with triple-negative EBC who had received NACT were required to have residual invasive cancer in breast or axillary nodes, and those who had received ACT had to have either a primary tumor ≥2 cm or positive axillary nodes. Patients with hormone receptor–positive/HER2-negative EBC treated with initial surgery were required to have four or more positive axillary nodes and those who had received NACT had to have a clinical and pathologic staging, as well as estrogen-receptor status and nuclear grade, plus post-treatment pathologic staging score of ≥3.1 PRO data collection was planned in all enrolled patients.
Physical disruption and treatment-associated symptoms are common at the end of ACT treatment for EBC.8,9 Recovery may take a year or more, with symptoms persisting beyond improvements in HRQOL.4,10-14 Thus, in studies designed to capture PROs during treatment, consideration should be given to assessment of symptoms and relevant HRQOL domains. Selection of the OlympiA PRO questionnaires was also guided by earlier studies of OL.15,16
PRO Measures and Assessment
The Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) scale17 was selected to measure treatment-related fatigue, as a reliable and validated questionnaire, available in multiple languages. It is a 13-item questionnaire that assesses self-reported fatigue and its impact upon daily activities and function. Each item is scaled 0-4 and a composite score is determined by summing the individual item scores. The composite score ranges from 0 to 52, with higher scores indicating less fatigue. Cancer-specific HRQOL was assessed with the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire, Core 30 item (EORTC QLQ-C30),18 to track recovery in key domains of HRQOL during adjuvant therapy with OL or PL, as well as in the post-treatment year. The EORTC QLQ-C30 also contains a symptom checklist that facilitated assessment of potential treatment-emergent GI symptoms (nausea and vomiting [NV], diarrhea) associated with OL. All EORTC QLQ-C30 scales and single-item measures range in score from 0 to 100. For symptoms, higher scores indicate worse severity, and for functional scales, higher scores indicate better functioning.
PRO questionnaires were administered on paper at baseline and every 6 months until 24 months after random assignment. No PRO assessments were expected after disease recurrence, diagnosis of a second primary cancer, or consent withdrawal. Patients who discontinued study drug for other reasons were expected to continue with assessments. Missing data forms were completed by the institutional staff when a questionnaire was not completed for a given assessment.
PRO Hypotheses
The primary PRO study hypothesis was that patients receiving OL may experience greater fatigue severity during treatment than those receiving PL, as measured by the FACIT-Fatigue scale at 6 and 12 months after random assignment. Secondary hypotheses were that there would be (1) no difference in fatigue after discontinuation of study treatment as measured at 18 and 24 months, (2) no difference in HRQOL over duration of the PRO study as measured by the Global Health Status/Quality of Life (GHQ) score and other EORTC QLQ-C30 functional subscales, and (3) patients receiving OL may experience greater GI symptom (NV, diarrhea) severity during treatment than those receiving PL as measured at 6 and 12 months after random assignment, but no difference expected by 24 months.
Statistical Analyses
A mixed model for repeated-measures (MMRM) analysis was used to examine the primary and secondary end point scores. The change from baseline in each individual score was compared between the treatment groups in the model that included treatment, time and treatment-by-time interaction, corresponding baseline score, and baseline score-by-time interaction. Per the statistical analysis plan, treatment-by-time interaction was to remain in the model regardless of statistical significance. The primary hypothesis was evaluated by fitting the MMRM for the 6- and 12-month assessments. All secondary hypotheses were performed by the MMRM analyses of all postbaseline scores. Analyses of EORTC QLQ-C30 functional scales were planned with particular interest in the Emotional and Physical scales. The least-squares means of the change of individual scores from baseline are presented unless specified otherwise.
Because responses to questionnaires may be influenced by differences between country/language categories,19 a subgroup MMRM analysis was planned for FACIT-Fatigue to assess consistency of treatment effect across geographic regions predefined as Asia Pacific and South Africa, Europe, North America, and South America.
On the basis of published literature, a 3-point difference for the FACIT-Fatigue score was prespecified as a clinically meaningful difference.20 With the planned sample size, we estimated having 93% and 96% statistical power for NACT and ACT strata, respectively, to detect a declared difference between the two treatment groups. For the EORTC QLQ-C30 scores, differences of 5-10 points were considered of small magnitude and differences of 10-20 points were considered of moderate magnitude when interpreting the results of secondary analyses.21
In secondary analyses of the FACIT-Fatigue scores, adjustments for previous treatment exposures (radiotherapy [XRT], type of chemotherapy, and surgery) and the investigation of the presence of treatment-by-hormone receptor status interaction were planned. NACT and ACT strata were analyzed separately, as possible differences in PROs were expected because of differences in timing of previous chemotherapy relative to baseline assessment. Two-sided P values <.05 were considered statistically significant for the primary PRO end point. All P values presented for the secondary PRO end points are considered descriptive. No adjustments for multiplicity were planned, as per protocol.
Although the protocol requested baseline PRO data collection in all enrolled patients, the PRO study analysis included only patients who initiated protocol treatment, had at least one evaluable baseline score, and at least one follow-up assessment. Distribution of patient and demographic characteristics for those with only baseline PRO data and those who were in the PRO study were compared with the distribution of the characteristics in the complete OlympiA population by means of the chi-square goodness-of-fit tests.
The questionnaire status completion and the reasons for missing assessments were tabulated. Adherence rates were defined as the proportion of the submitted questionnaires relative to the expected ones and were evaluated using a MMRM logistic regression. Sensitivity analyses of the FACIT-Fatigue score were performed by also using scores from the assessments completed outside of the collection windows (±4 weeks around the 6- and 12-month time points, and ± 6 weeks around the 18- and 24- months time points).
The trial was conducted in accordance with the amended Declaration of Helsinki, and the protocol was approved by the institutional review board at each participating center. All patients provided written informed consent for the treatment trial and PRO study; however, those in the United States were required to provide specific consent for the PRO study. All analyses are based on the July 12, 2021, data cutoff.
RESULTS
Patient Characteristics
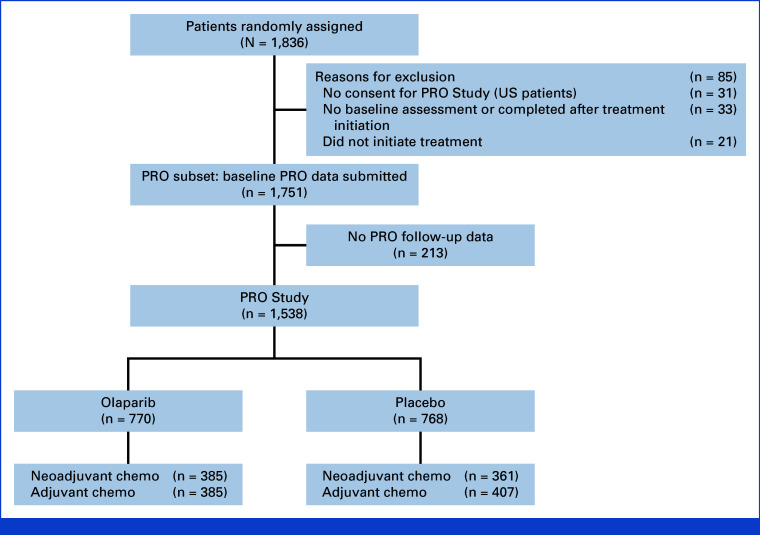
Among 1,836 patients randomly assigned in OlympiA, 1,751 (NACT: 875, ACT: 876) completed a baseline PRO questionnaire (Fig 1). Among patients completing the baseline PROs, no follow-up PRO assessment was available for 213. Therefore, 1,538 patients (NACT: 746 [OL: 385, PL: 361], ACT: 792 [OL: 385, PL: 407]) were included in the PRO study primary analyses. Characteristics are described in Table 1. There was a slight difference in age distribution between OL and PL for the ACT subgroup, with a higher percentage of younger patients on OL. Patient and tumor characteristics for patients with baseline PRO (Data Supplement, Table S1 [online only]) and the PRO study sample were similar to the OlympiA intention-to-treat population.
FIG 1.
CONSORT diagram: OlympiA PRO Study. PRO, patient-reported outcomes.
TABLE 1.
Patient and Tumor Characteristics by Chemotherapy Subgroup: OlympiA PRO Study
| Characteristic | Neoadjuvant Chemotherapy, No. (%) | Adjuvant Chemotherapy, No. (%) | ||||
|---|---|---|---|---|---|---|
| OL (n = 385) | PL (n = 361) | Total (N = 746) | OL (n = 385) | PL (n = 407) | Total (N = 792) | |
| Age groups at random assignment, years | ||||||
| <30 | 24 (6.2) | 21 (5.8) | 45 (6.0) | 16 (4.2) | 26 (6.4) | 42 (5.3) |
| 30-39 | 137 (35.6) | 141 (39.1) | 278 (37.3) | 145 (37.7) | 110 (27.0) | 255 (32.2) |
| 40-49 | 119 (30.9) | 114 (31.6) | 233 (31.2) | 142 (36.9) | 153 (37.6) | 295 (37.2) |
| 50-59 | 87 (22.6) | 62 (17.2) | 149 (20.0) | 52 (13.5) | 81 (19.9) | 133 (16.8) |
| 60-69 | 16 (4.2) | 21 (5.8) | 37 (5.0) | 25 (6.5) | 35 (8.6) | 60 (7.6) |
| ≥70 | 2 (0.5) | 2 (0.6) | 4 (0.5) | 5 (1.3) | 2 (0.5) | 7 (0.9) |
| Sex | ||||||
| Female | 384 (99.7) | 360 (99.7) | 744 (99.7) | 384 (99.7) | 404 (99.3) | 788 (99.5) |
| Male | 1 (0.3) | 1 (0.3) | 2 (0.3) | 1 (0.3) | 3 (0.7) | 4 (0.5) |
| Race | ||||||
| American Indian or Alaska Native | 1 (0.3) | 1 (0.3) | 2 (0.3) | 2 (0.5) | 0 (0.0) | 2 (0.3) |
| Asian | 77 (20.0) | 85 (23.5) | 162 (21.7) | 159 (41.3) | 169 (41.5) | 328 (41.4) |
| Black or African American | 7 (1.8) | 10 (2.8) | 17 (2.3) | 4 (1.0) | 11 (2.7) | 15 (1.9) |
| Native Hawaiian or Other Pacific Islander | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| White | 297 (77.1) | 261 (72.3) | 558 (74.8) | 214 (55.6) | 221 (54.3) | 435 (54.9) |
| Other | 1 (0.3) | 2 (0.6) | 3 (0.4) | 1 (0.3) | 3 (0.7) | 4 (0.5) |
| Missing | 2 (0.5) | 2 (0.6) | 4 (0.5) | 4 (1.0) | 3 (0.7) | 7 (0.9) |
| Ethnic origin | ||||||
| Hispanic or Latino | 11 (2.9) | 8 (2.2) | 19 (2.5) | 15 (3.9) | 9 (2.2) | 24 (3.0) |
| Non-Hispanic or Latino | 342 (88.8) | 322 (89.2) | 664 (89.0) | 341 (88.6) | 365 (89.7) | 706 (89.1) |
| Not known, not recorded, or refused | 32 (8.3) | 31 (8.6) | 63 (8.4) | 29 (7.5) | 33 (8.1) | 62 (7.8) |
| Jewish/Ashkenazi descent | ||||||
| Yes, of Ashkenazi Jewish descent | 22 (5.7) | 14 (3.9) | 36 (4.8) | 11 (2.9) | 12 (2.9) | 23 (2.9) |
| No, not of Ashkenazi Jewish descent | 363 (94.3) | 346 (95.8) | 709 (95.0) | 374 (97.1) | 394 (96.8) | 768 (97.0) |
| Missing | 0 (0.0) | 1 (0.3) | 1 (0.1) | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Previous platinum therapy | ||||||
| Yes | 132 (34.3) | 138 (38.2) | 270 (36.2) | 68 (17.7) | 74 (18.2) | 142 (17.9) |
| No | 253 (65.7) | 223 (61.8) | 476 (63.8) | 317 (82.3) | 333 (81.8) | 650 (82.1) |
| Type of previous chemotherapy | ||||||
| Anthracycline | 2 (0.5) | 2 (0.6) | 4 (0.5) | 4 (1.0) | 8 (2.0) | 12 (1.5) |
| Taxane | 8 (2.1) | 8 (2.2) | 16 (2.1) | 13 (3.4) | 21 (5.2) | 34 (4.3) |
| Anthracycline and taxane | 375 (97.4) | 351 (97.2) | 726 (97.3) | 368 (95.6) | 377 (92.6) | 745 (94.1) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Surgery type | ||||||
| Conservative surgery | 90 (23.4) | 99 (27.4) | 189 (25.3) | 176 (45.7) | 171 (42.0) | 347 (43.8) |
| Nonconservative surgery | 295 (76.6) | 262 (72.6) | 557 (74.7) | 209 (54.3) | 234 (57.5) | 443 (55.9) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.5) | 2 (0.3) |
| Radiation | ||||||
| Yes | 288 (74.8) | 260 (72.0) | 548 (73.5) | 247 (64.2) | 273 (67.1) | 520 (65.7) |
| No | 97 (25.2) | 101 (28.0) | 198 (26.5) | 138 (35.8) | 134 (32.9) | 272 (34.3) |
| Hormone receptor status | ||||||
| ER-positive and/or PgR-positive/HER2-negative | 81 (21.0) | 69 (19.1) | 150 (20.1) | 45 (11.7) | 56 (13.8) | 101 (12.8) |
| TNBC | 304 (79.0) | 292 (80.9) | 596 (79.9) | 340 (88.3) | 351 (86.2) | 691 (87.2) |
| Centrally confirmed BRCA gene name | ||||||
| BRCA1 | 250 (64.9) | 237 (65.7) | 487 (65.3) | 227 (59.0) | 225 (55.3) | 452 (57.1) |
| BRCA2 | 110 (28.6) | 94 (26.0) | 204 (27.3) | 80 (20.8) | 86 (21.1) | 166 (21.0) |
| BRCA1 and BRCA2 | 6 (1.6) | 17 (4.7) | 23 (3.1) | 15 (3.9) | 14 (3.4) | 29 (3.7) |
| Missinga | 19 (4.9) | 13 (3.6) | 32 (4.3) | 63 (16.4) | 82 (20.1) | 145 (18.3) |
| Geographic region | ||||||
| North America | 51 (13.2) | 38 (10.5) | 89 (11.9) | 33 (8.6) | 51 (12.5) | 84 (10.6) |
| South America | 4 (1.0) | 2 (0.6) | 6 (0.8) | 9 (2.3) | 7 (1.7) | 16 (2.0) |
| Europe | 233 (60.5) | 217 (60.1) | 450 (60.3) | 162 (42.1) | 154 (37.8) | 316 (39.9) |
| Asia Pacific and South Africa | 97 (25.2) | 104 (28.8) | 201 (26.9) | 181 (47.0) | 195 (47.9) | 376 (47.5) |
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; OL, olaparib; PgR, progesterone receptor; PL, placebo; PRO, patient-reported outcomes; TNBC, triple-negative breast cancer.
Most missing are due to central Myriad testing not done/not available in China.
FACIT-Fatigue
Baseline FACIT-Fatigue scores were somewhat worse in patients enrolled in OlympiA compared with the average FACIT-Fatigue score reported in healthy women (42.7 ± 8.9),22 with no difference between OL and PL (Data Supplement, Table S2).
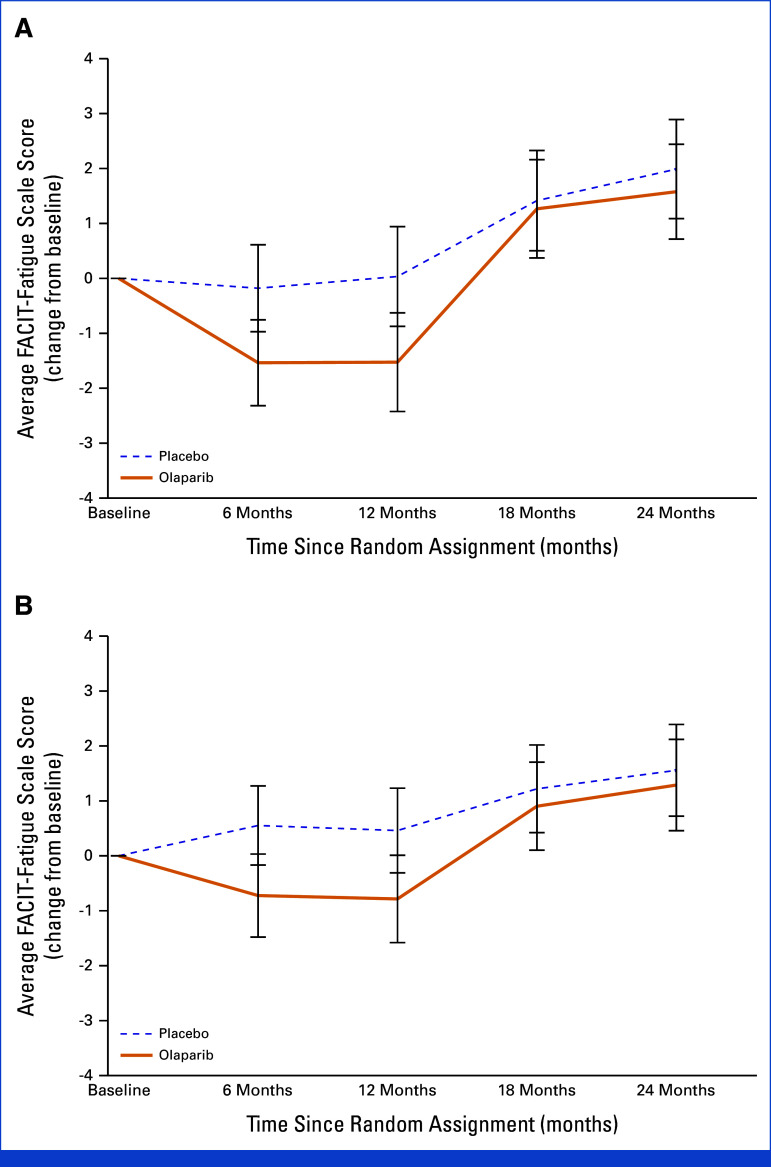
On the basis of the primary analysis, fatigue severity was statistically significantly greater in patients treated with OL than PL at 6 months (diff OL v PL: NACT: –1.3 [95% CI, –2.4 to –0.2]; P = .022; ACT: –1.3 [–2.3 to –0.2]; P = .017) and 12 months (NACT: –1.6 [–2.8 to –0.3]; P = .017; ACT: –1.3 [–2.4 to –0.2]; P = .025); however, differences did not meet the 3-point prespecified criterion for clinical meaningfulness. At 18 and 24 months, OL and PL scores were similar (Table 2; Fig 2).
TABLE 2.
FACIT-Fatigue and EORTC QLQ-C30 NV and Diarrhea Symptom Scores Over Time by Treatment and Chemotherapy Subgroup: OlympiA PRO Study
| Symptom | Time Point | Neoadjuvant Chemotherapy | Adjuvant Chemotherapy | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OL, Mean (95% CI) | PL, Mean (95% CI) | Difference, Mean (95% CI) | P | OL, Mean (95% CI) | PL, Mean (95% CI) | Difference, Mean (95% CI) | P | ||
| FACIT-Fatigue | Baseline | 39.6 (38.6 to 40.7) | 40.0 (39.0 to 40.9) | –0.3 (–1.7 to 1.1) | .635 | 40.9 (40.0 to 41.8) | 40.8 (39.9 to 41.6) | 0.1 (–1.1 to 1.4) | .822 |
| 6 months | 38.5 (37.8 to 39.3) | 39.9 (39.1 to 40.7) | –1.4 (–2.5 to –0.2) | .017 | 40.3 (39.6 to 41.1) | 41.6 (40.9 to 42.3) | –1.3 (–2.3 to –0.2) | .017 | |
| 12 months | 38.6 (37.7 to 39.5) | 40.1 (39.2 to 41.0) | –1.6 (–2.8 to –0.3) | .017 | 40.3 (39.5 to 41.0) | 41.5 (40.7 to 42.3) | –1.2 (–2.4 to –0.1) | .028 | |
| 18 months | 41.3 (40.5 to 42.2) | 41.5 (40.6 to 42.4) | –0.1 (–1.4 to 1.1) | .819 | 41.9 (41.1 to 42.7) | 42.3 (41.5 to 43.1) | –0.3 (–1.4 to 0.8) | .582 | |
| 24 months | 41.7 (40.8 to 42.5) | 42.1 (41.2 to 43.0) | –0.4 (–1.7 to 0.8) | .518 | 42.3 (41.5 to 43.2) | 42.6 (41.8 to 43.4) | –0.3 (–1.4 to 0.9) | .655 | |
| EORTC QLQ-C30 NV | Baseline | 2.9 (2.0 to 3.7) | 3.4 (2.3 to 4.5) | –0.5 (–1.9 to 0.8) | .442 | 3.0 (2.1 to 3.9) | 3.4 (2.4 to 4.3) | –0.3 (–1.6 to 1.0) | .621 |
| 6 months | 10.6 (9.2 to 12.0) | 4.5 (3.1 to 5.9) | 6.0 (4.1 to 8.0) | <.001 | 9.9 (8.6 to 11.3) | 4.6 (3.3 to 5.9) | 5.3 (3.4 to 7.2) | <.001 | |
| 12 months | 10.3 (8.9 to 11.6) | 3.9 (2.5 to 5.3) | 6.4 (4.4 to 8.3) | <.001 | 8.5 (7.3 to 9.7) | 4.0 (2.8 to 5.2) | 4.5 (2.8 to 6.1) | <.001 | |
| 18 months | 3.6 (2.6 to 4.6) | 3.0 (1.9 to 4.0) | 0.7 (–0.7 to 2.1) | .346 | 3.5 (2.4 to 4.6) | 4.0 (2.9 to 5.0) | –0.5 (–2.0 to 1.0) | .532 | |
| 24 months | 4.6 (3.5 to 5.7) | 2.3 (1.1 to 3.5) | 2.3 (0.7 to 3.9) | .006 | 3.0 (1.9 to 4.1) | 3.4 (2.3 to 4.5) | –0.4 (–2.0 to 1.2) | .613 | |
| EORTC QLQ-C30 diarrhea | Baseline | 5.6 (4.1 to 7.1) | 5.8 (4.2 to 7.4) | –0.2 (–2.4 to 2.0) | .854 | 6.0 (4.5 to 7.5) | 6.1 (4.7 to 7.6) | –0.2 (–2.3 to 2.0) | .888 |
| 6 months | 7.4 (5.7 to 9.1) | 7.1 (5.4 to 8.8) | 0.3 (–2.1 to 2.7) | .787 | 6.2 (4.4 to 7.9) | 7.8 (6.2 to 9.5) | –1.7 (–4.1 to 0.7) | .175 | |
| 12 months | 9.5 (7.5 to 11.6) | 7.7 (5.6 to 9.7) | 1.8 (–1.1 to 4.7) | .213 | 7.6 (6.0 to 9.2) | 7.4 (5.8 to 9.0) | 0.2 (–2.1 to 2.4) | .884 | |
| 18 months | 8.3 (6.3 to 10.4) | 6.9 (4.8 to 9.0) | 1.4 (–1.5 to 4.3) | .339 | 5.7 (4.2 to 7.3) | 5.8 (4.2 to 7.4) | –0.1 (–2.3 to 2.1) | .957 | |
| 24 months | 6.1 (4.3 to 7.9) | 5.3 (3.5 to 7.2) | 0.8 (–1.8 to 3.3) | .562 | 4.5 (3.1 to 6.0) | 5.5 (4.0 to 6.9) | –1.0 (–3.0 to 1.1) | .360 | |
NOTE. FACIT-Fatigue score ranges from 0 to 52, with higher scores indicating less fatigue. EORTC QLQ-C30 symptom scale scores range from 0 to 100, with higher scores indicating worse symptom severity. Difference is the value for OL minus PL. Adjusted least-square mean scores, 95% CI, and P values for all time points after baseline are obtained from mixed model for repeated-measures analysis of all postbaseline scores. The model includes treatment, time and treatment-by-time interaction, corresponding baseline score, and the baseline-score-by-time interaction. The comparison at baseline is based on the t-test. P values are not adjusted for multiplicity.
Abbreviations: EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life questionnaire, Core 30 item; FACIT-Fatigue, Functional Assessment of Chronic Illness Therapy-Fatigue; NV, nausea and vomiting; OL, olaparib; PL, placebo; PRO, patient-reported outcomes.
FIG 2.
FACIT-Fatigue score change from baseline over time by treatment group for patients (A) who have completed neoadjuvant chemotherapy and (B) who have completed adjuvant chemotherapy: OlympiA PRO Study. FACIT-Fatigue score ranges from 0-52 with higher score indicating less fatigue. FACIT-Fatigue, Functional Assessment of Chronic Illness Therapy-Fatigue; PRO, patient-reported outcomes.
When adjustments for treatment exposures (XRT, type of chemotherapy, platinum therapy, and type of breast surgery) were considered, only previous XRT was identified as a key covariate for the ACT subgroup. On average, patients who did not receive previous XRT had less fatigue severity (diff no XRT v XRT: 1.4 [95% CI, 0.5 to 2.3]; P = .003). No key covariates were identified in the NACT subgroup. The least-square means obtained from the adjusted model were not clinically meaningfully different from the unadjusted least-square means (not presented). There was no difference in treatment effect on fatigue severity by hormone receptor status.
Differences by Geographical Region
Patient and tumor characteristics by geographical region are presented in the Data Supplement (Table S3). The comparison of fatigue severity between OL and PL was performed by predefined geographical regions (Data Supplement, Tables S4 and S5). In general, results were similar to the overall comparison but may not be reliable in smaller subgroups.
EORTC QLQ-C30
At baseline, there was no difference in the EORTC QLQ-C30 scores between OL and PL groups (Data Supplement, Table S2). Patients in the ACT subgroup had slightly better scores on the GHQ and functional scales than patients in the NACT subgroup. Patients' baseline GHQ scores on average were 10 points lower and baseline functional scales scores were 5 points lower than pretreatment scores previously reported as reference values for the EORTC scales in patients with EBC.23
There were no clinically meaningful differences between the OL and PL groups over time for the GHQ, Physical, or Emotional scales. Some improvements in functioning over time were demonstrated in both groups (Data Supplement, Tables S6 and S7; Fig 3). The difference in the change of GHQ score at 24 months between NACT patients treated with OL and PL was not clinically meaningful (–3.3 [–6.5 to –0.1]; P = .041). Nonclinically meaningful differences between OL and PL were detected in the ACT subgroup for the GHQ score at 6 months (–2.7 [–5.1 to –0.4]; P = .022) and 12 months (–2.5 [–5.0 to –0.1]; P = .042) and for the Physical functioning scale score at 12 months (–1.7 [–3.3 to –0.2]; P = .027).
FIG 3.
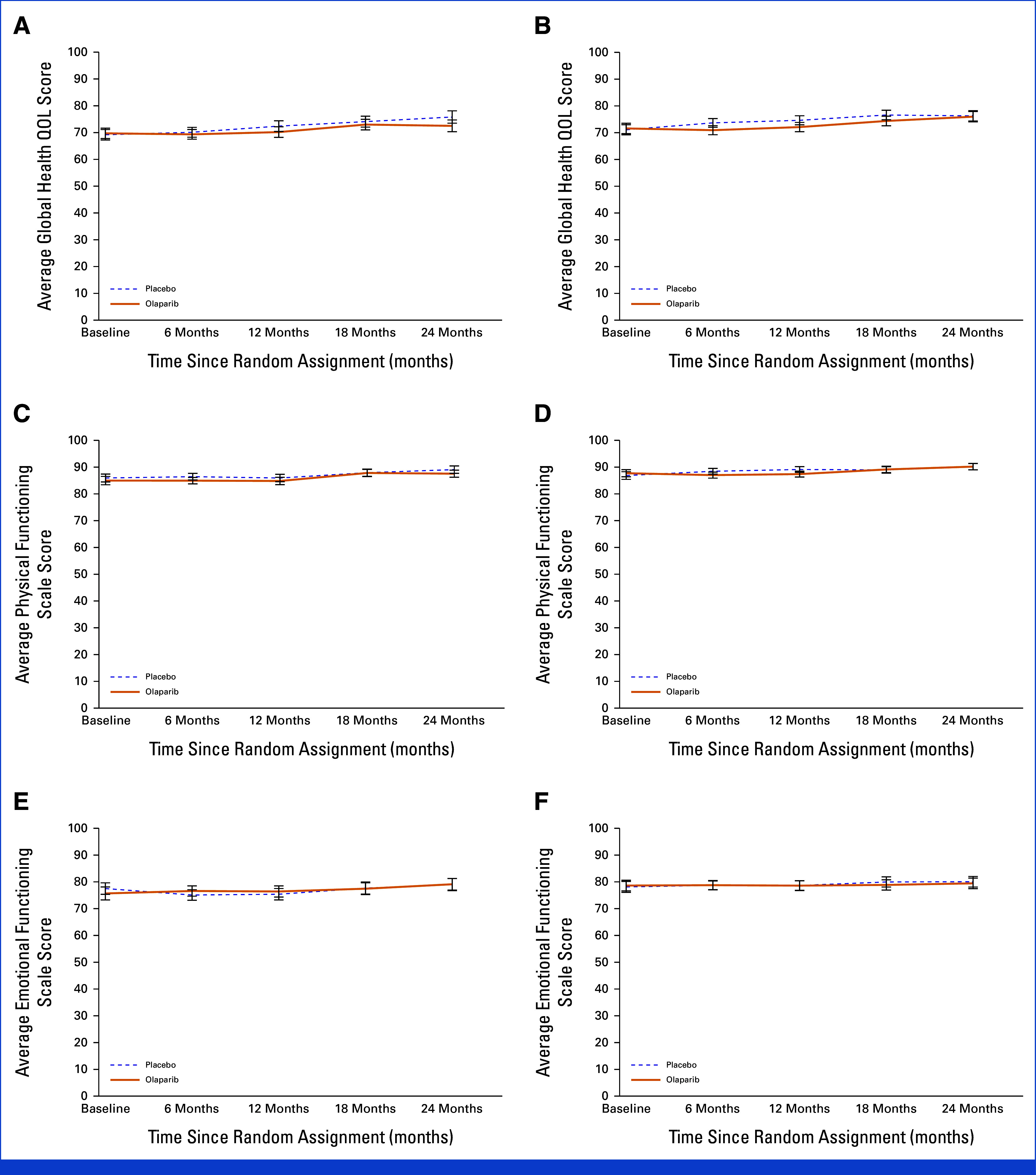
EORTC QLQ-C30 scores over time by treatment and chemotherapy subgroup for Global Health Status ((A) neoadjuvant chemotherapy, (B) adjuvant chemotherapy), Physical scale ((C) neoadjuvant chemotherapy, (D) adjuvant chemotherapy), and Emotional scale ((E) neoadjuvant chemotherapy, (F) adjuvant chemotherapy): OlympiA PRO Study. EORTC QLQ-C30 Global Health Status/QOL Score, Physical and Emotional subscale scores range from 0-100, higher score indicates better quality of life, or functioning. EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire, Core 30 item; PRO, patient-reported outcomes; QOL, quality of life.
NV symptom severity difference was worse in patients treated with OL than PL at 6 months (NACT: 6.0 [4.1 to 8.0]; P < .001; ACT: 5.3 [3.4 to 7.2]; P < .001) and 12 months (NACT: 6.4 [4.4 to 8.3]; P < .001; ACT: 4.5 [2.8 to 6.1]; P < .001). Scores were clinically meaningful at 6 months in both NACT and ACT patients (small difference, 5-10 points) but only clinically meaningful at 12 months in the NACT group. There were no differences in NV severity at 18 months for either chemotherapy group or at 24 months for the ACT group. A small difference in NV symptom severity between OL and PL was detected at 24 months for the NACT group (2.3 [0.7 to 3.9]; P = .006). No difference in the severity of diarrhea symptoms between OL and PL was observed over time (Table 2; Fig 4).
FIG 4.
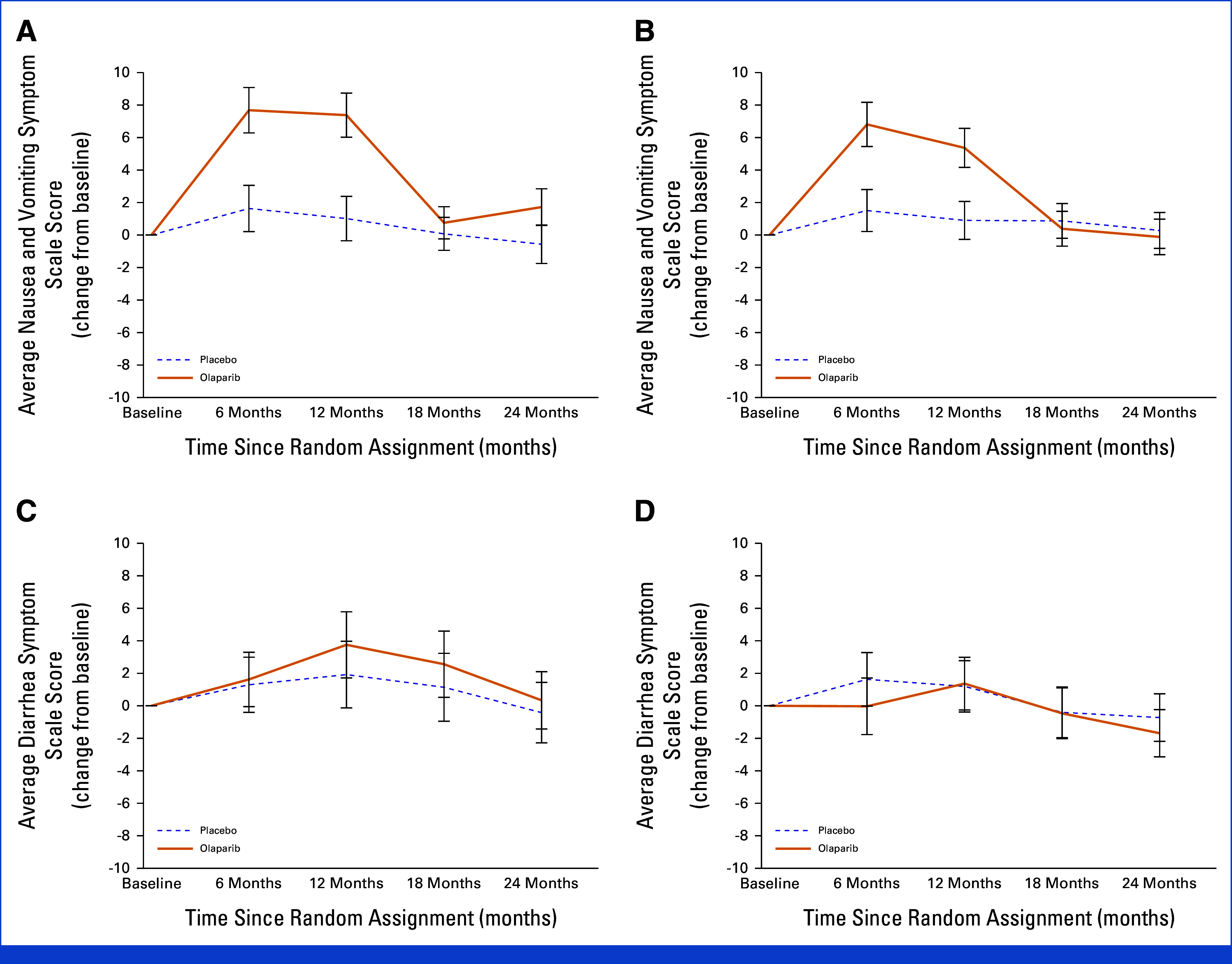
EORTC QLQ-C30 scores change from baseline over time by treatment and chemotherapy subgroup for nausea and vomiting symptom ((A) neoadjuvant chemotherapy, (B) adjuvant chemotherapy) and diarrhea symptom ((C) neoadjuvant chemotherapy, (D) adjuvant chemotherapy): OlympiA PRO Study. EORTC QLQ-C30 Nausea and vomiting and Diarrhea symptoms scale scores range from 0-100, higher score indicate worse symptom. EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire, Core 30 item; PRO, patient-reported outcomes.
Other functional scales and symptoms were analyzed (Data Supplement, Tables S6 and S7). For the NACT group, the Role, Cognitive, and Social Functioning scales were comparable between OL and PL groups over the 24 months, with meaningful improvements in Role and Social Functioning from baseline to 24 months independent of treatment arm (Data Supplement, Table S6). For the ACT group, the OL and PL groups were comparable over the 24 months for the Role, Cognitive, and Social Functioning scales, with clinically meaningful improvement in Social Functioning from baseline to 24 months independent of treatment arm (Data Supplement, Table S7).
Additional symptoms assessed included pain, fatigue, dyspnea, insomnia, appetite loss, constipation, and financial difficulties (Data Supplement, Tables S6 and S7). For both the (N)ACT groups, OL and PL were comparable in pain, insomnia, or financial difficulties during 24 months. For all patients, financial difficulties improved meaningfully during the 24 months, with the change from baseline to 24 months ranging from 7.7 to 10 points for all treatment and chemotherapy groups. By contrast, patients treated with OL compared with PL reported clinically significantly greater increase in appetite loss while on treatment, which resolved at the 18- and 24-month assessments. Fatigue symptom severity was also increased during OL therapy as measured on this scale, consistent with the FACIT-Fatigue primary end point, and similarly, the magnitude of difference between the two arms was not clinically meaningful. Constipation severity was worse in the OL arm at 6 months (P = .014) for the NACT group and at 12 months (P = .004) for the ACT group. Dyspnea was worse in severity at 12 months (P = .002) and 24 months (P = .049) in the OL arm for the NACT group only (Data Supplement, Table S6) but neither met the criterion for a clinically meaningful difference of at least 5 points.
Missing Data
The questionnaire adherence rates ranged from 97% at the 6-month assessment to 69% at the 24-month assessment (Data Supplement, Table S8). There was no evidence that the reasons for nonadherence were related to patients' health status.
Lower adherence rates were observed at later time points and for patients enrolled in Europe or North America compared with Asia Pacific and South Africa regions. Because of the small numbers of patients enrolled from South America, no reliable conclusions regarding adherence rates for this region could be drawn (data not shown). ACT patients treated with OL had lower adherence rates than patients treated with PL at the 6-month time point (OL, 91.2%; PL, 96.6%), with no differences detected at later time points (P value for time-point-by-treatment interaction = .005).
For a number of patients (Data Supplement, Table S8), questionnaires were completed outside the collection windows, and therefore, were not included in the primary analyses. As part of the sensitivity analyses, assessments completed outside of collection windows were also included. The adherence rates increased to 79% for the 24-month time point as the lowest and 98% for the 6-month time point as the highest. Adherence rates of patients treated with OL or PL were comparable. A sensitivity analysis of the FACIT-Fatigue score was performed by including scores from assessments outside the collection windows and produced similar results (Data Supplement, Table S9).
DISCUSSION
The OlympiA trial demonstrated the efficacy of OL in improving IDFS, distant disease-free survival, and overall survival in a large international sample of high-risk patients with EBC and gBRCA1/2pv.1,24 The HRQOL data support the favorable tolerability of OL in patients who had previously received intensive standard (N)ACT, surgery, XRT, and hormonal therapy when indicated.
The PRO Study primary outcome found no evidence of clinically meaningful increased fatigue severity in patients receiving OL compared with PL during drug administration or in the subsequent follow-up year-off trial therapy. Fatigue assessments were not affected by covariate adjustments. In addition, mean fatigue levels did not change from baseline to 12 months in the PL group, likely reflecting slowed recovery from more intensive EBC treatments in this high-risk population. Thus, OL did not meaningfully contribute to fatigue in this setting.
There was a small clinically meaningful difference in NV reported by patients during OL therapy, which resolved by 18 and 24 months of follow-up. The study Protocol had detailed recommendations for management of NV during treatment; thus, the results reported here reflect implementation of these management strategies and should be followed in clinical practice when OL is prescribed. It is also possible that a PRO assessment shortly after treatment initiation might have found meaningful differences in fatigue and NV that were addressed by dose reductions or other interventions.
There were no differences in diarrhea severity between patients in the two treatment arms during the entire study. Additional exploratory examination of other symptoms identified clinically meaningful increases in appetite loss during OL administration, which resolved after treatment. These findings are consistent with the clinically reported adverse events in the primary OlympiA trial report1 but reflect the patients' own assessments.
With the large patient sample in the PRO study, we identified some small differences between treatment groups, which did not translate into clinically meaningful differences in symptoms, nor did they affect global QOL, Physical, or Emotional functioning. However, we note that the self-reported HRQOL functioning scales of the EORTC QLQ-C30 demonstrated very small improvements over 2 years of observation, confirming the overall burden of therapy in EBC noted in the literature25 and the long-term potential impact of (N)ACT on HRQOL.13,26,27
The primary random assignment between OL and PL achieved excellent balance in baseline PRO data, providing confidence in interpretation of changes over time, without evidence that demographic or treatment covariates influenced outcomes. However, the number of patients with hormone receptor–positive EBC included in the trial was small, limiting meaningful evaluation of this subgroup of patients. We found that there were predictable differences in EBC treatment patterns between the (N)ACT groups, including differences in surgery (eg, conservative surgery used more often with ACT), chemotherapy drugs (eg, platinum used more frequently with NACT), and younger patients more likely to receive NACT (Data Supplement, Table S1). Furthermore, there were differences in treatment patterns by geographic region; for example, NACT and platinum therapy were more likely to be used in Europe and North America than the rest of the world (Data Supplement, Table S3). Nevertheless, these variables were balanced between treatment arms, reflecting the large sample size and careful stratification, indicating that study findings are relevant for an international population of patients meeting the trial eligibility criteria.
ACKNOWLEDGMENT
The authors thank the patients and their families, the staff members of the trial partners (Breast International Group, NRG Oncology, Frontier Science Foundation, AstraZeneca, Merck & Co, Inc [Rahway, NJ], and the National Cancer Institute), and the current and former members of the trial committees. The authors also thank Wendy L. Rea, BA, Editorial Associate, for assistance with preparation and submission of the manuscript, who is an employee of NSABP Foundation, and was not compensated beyond her normal salary for this work. Finally, the authors acknowledge the many contributions of Bella Kaufman, MD, who served as co-chair of the OlympiA trial during the design, implementation, accrual, follow-up, and initial analyses of the trial until her death on May 13, 2021.
PRIOR PRESENTATION
Presented at the San Antonio Breast Cancer Symposium, San Antonio, TX, December 7-10, 2021.
SUPPORT
Supported by the National Institutes of Health grant numbers: U10CA-180868, UG1CA-189867, and U10CA-180822, with funding and provision of olaparib and placebo by AstraZeneca as part of an alliance between AstraZeneca and Merck and Co, Inc. This work was conducted as a collaborative partnership among the Breast International Group, NRG Oncology, Frontier Science Foundation, and AstraZeneca/Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, with funding and provision of olaparib and placebo by AstraZeneca as part of an alliance between AstraZeneca and Merck and Co, Inc.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Individual participant data that underlie the results reported in this article, after deidentification, will be made available to investigators whose proposed use of the data has been approved by the OlympiA Steering Committee. The data provided will be limited to that required to answer the objectives of the proposal and in line with the informed consent form and country-level legislation. Proposals can be submitted once an end point defined in the protocol has been reached, the corresponding analysis performed, and the data related to the end point released through the first public presentation (retention period will be mentioned in the data transfer agreement [DTA]). Proposals should be directed to olympiaproposals@frontier-science.co.uk. To gain access, data requestors will need to sign a DTA.
AUTHOR CONTRIBUTIONS
Conception and design: Patricia A. Ganz, Hanna Bandos, Suzette Delaloge, Masakazu Toi, Seock-Ah Im, Chuan-gui Song, Fernando Henao-Carrasco, Susan M. Domchek, Priya Rastogi, Anitra Fielding, Richard D. Gelber, Charles E. Geyer, Andrew N.J. Tutt
Provision of study materials or patients: Sherko Kuemmel, Suzette Delaloge, Etienne Brain, Hideko Yamauchi, Seock-Ah Im, Hong Zheng, Tomasz Sarosiek, Priyanka Sharma, Cuizhi Geng, Peifen Fu, Kerstin Rhiem, Heike Frauchiger-Heuer, Daphné t'Kint de Roodenbeke, Annabel Goodwin, Camille Chakiba-Brugère, Michael Friedlander, Keun Seok Lee, Toshimi Takano, Frances Valdes-Albini, Charles Bane, Edward C. McCarron, Judy Garber
Collection and assembly of data: Suzette Delaloge, Masakazu Toi, Hideko Yamauchi, Eduardo-M. de Dueñas, Seock-Ah Im, Chuan-gui Song, Hong Zheng, Tomasz Sarosiek, Priyanka Sharma, Cuizhi Geng, Peifen Fu, Kerstin Rhiem, Heike Frauchiger-Heuer, Daphné t'Kint de Roodenbeke, Ning Liao, Camille Chakiba-Brugère, Michael Friedlander, Toshimi Takano, Fernando Henao-Carrasco, Shamsuddin Virani, Frances Valdes-Albini, Susan M. Domchek, Charles Bane, Edward C. McCarron, Monica Mita, Elsemieke D. Scheepers, Charles E. Geyer, Andrew N.J. Tutt
Data analysis and interpretation: Patricia A. Ganz, Hanna Bandos, Sue Friedman, Sherko Kuemmel, Suzette Delaloge, Etienne Brain, Hideko Yamauchi, Eduardo-M. de Dueñas, Anne Armstrong, Seock-Ah Im, Chuan-gui Song, Pauline Wimberger, Annabel Goodwin, Keun Seok Lee, Sylvie Giacchetti, Fernando Henao-Carrasco, Shamsuddin Virani, Susan M. Domchek, Monica Mita, Giovanna Rossi, Priya Rastogi, Anitra Fielding, Richard D. Gelber, David Cameron, Judy Garber, Charles E. Geyer, Andrew N.J. Tutt
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Patient-Reported Outcomes in OlympiA: A Phase III, Randomized, Placebo-Controlled Trial of Adjuvant Olaparib in gBRCA1/2 Mutations and High-Risk Human Epidermal Growth Factor Receptor 2–Negative Early Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No other potential conflicts of interest were reported.
REFERENCES
- 1. Tutt ANJ, Garber JE, Kaufman B, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384:2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Donovan KA, Jacobsen PB, Andrykowski MA, et al. Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J Pain Symptom Manage. 2004;28:373–380. doi: 10.1016/j.jpainsymman.2004.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andrykowski MA, Schmidt JE, Salsman JM, et al. Use of a case definition approach to identify cancer-related fatigue in women undergoing adjuvant therapy for breast cancer. J Clin Oncol. 2005;23:6613–6622. doi: 10.1200/JCO.2005.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bower JE, Ganz PA, Desmond KA, et al. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 5. Bower JE, Wiley J, Petersen L, et al. Fatigue after breast cancer treatment: Biobehavioral predictors of fatigue trajectories. Health Psychol. 2018;37:1025–1034. doi: 10.1037/hea0000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bower JE, Ganz PA, Irwin MR, et al. Do all patients with cancer experience fatigue? A longitudinal study of fatigue trajectories in women with breast cancer. Cancer. 2021;127:1334–1344. doi: 10.1002/cncr.33327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vaz-Luis I, Di Meglio A, Havas J, et al. Long-term longitudinal patterns of patient-reported fatigue after breast cancer: A group-based trajectory analysis. J Clin Oncol. 2022;40:2148–2162. doi: 10.1200/JCO.21.01958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ganz PA, Land SR, Geyer CE, et al. Menstrual history and quality-of-life outcomes in women with node-positive breast cancer treated with adjuvant therapy on the NSABP B-30 trial. J Clin Oncol. 2011;29:1110–1116. doi: 10.1200/JCO.2010.29.7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ganz PA, Kwan L, Stanton AL, et al. Quality of life at the end of primary treatment of breast cancer: First results from the moving beyond cancer randomized trial. J Natl Cancer Inst. 2004;96:376–387. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- 10. Ganz PA, Kwan L, Stanton AL, et al. Physical and psychosocial recovery in the year after primary treatment of breast cancer. J Clin Oncol. 2011;29:1101–1109. doi: 10.1200/JCO.2010.28.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ganz PA, Rowland JH, Desmond K, et al. Life after breast cancer: Understanding women's health-related quality of life and sexual functioning. J Clin Oncol. 1998;16:501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 12. Ganz PA, Rowland JH, Meyerowitz BE, et al. Impact of different adjuvant therapy strategies on quality of life in breast cancer survivors. Recent Results Cancer Res. 1998;152:396–411. doi: 10.1007/978-3-642-45769-2_38. [DOI] [PubMed] [Google Scholar]
- 13. Ganz PA, Desmond KA, Leedham B, et al. Quality of life in long-term, disease-free survivors of breast cancer: A follow-up study. J Natl Cancer Inst. 2002;94:39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 14. Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: A longitudinal investigation. Cancer. 2006;106:751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 15. Gelmon KA, Tischkowitz M, Mackay H, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: A phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12:852–861. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 16. Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 17. Yellen SB, Cella DF, Webster K, et al. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 18. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 19. Bernhard J, Zahrieh D, Castiglione-Gertsch M, et al. Adjuvant chemotherapy followed by goserelin compared with either modality alone: The impact on amenorrhea, hot flashes, and quality of life in premenopausal patients—The International Breast Cancer Study Group Trial VIII. J Clin Oncol. 2007;25:263–270. doi: 10.1200/JCO.2005.04.5393. [DOI] [PubMed] [Google Scholar]
- 20. Cella D, Eton DT, Lai J-S, et al. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) Anemia and Fatigue Scales. J Pain Symptom Manage. 2002;24:547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 21. Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 22. Cella D, Lai JS, Chang CH, et al. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94:528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 23. Mierzynska J, Taye M, Pe M, et al. Reference values for the EORTC QLQ-C30 in early and metastatic breast cancer. Eur J Cancer. 2020;125:69–82. doi: 10.1016/j.ejca.2019.10.031. [DOI] [PubMed] [Google Scholar]
- 24. Geyer CE, Garber JE, Gelber RD, et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high risk, early breast cancer. Ann Oncol. 2022;33:1250–1268. doi: 10.1016/j.annonc.2022.09.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Di Meglio A, Havas J, Gbenou AS, et al. Dynamics of long-term patient-reported quality of life and health behaviors after adjuvant breast cancer chemotherapy. J Clin Oncol. 2022;40:3190–3204. doi: 10.1200/JCO.21.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bandos H, Melnikow J, Rivera DR, et al. Long-term peripheral neuropathy in breast cancer patients treated with adjuvant chemotherapy: NRG Oncology/NSABP B-30. J Natl Cancer Inst. 2018;110:djx162. doi: 10.1093/jnci/djx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rivera DR, Ganz PA, Weyrich MS, et al. Chemotherapy-associated peripheral neuropathy in patients with early-stage breast cancer: A systematic review. J Natl Cancer Inst. 2018;110:djx140. doi: 10.1093/jnci/djx140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual participant data that underlie the results reported in this article, after deidentification, will be made available to investigators whose proposed use of the data has been approved by the OlympiA Steering Committee. The data provided will be limited to that required to answer the objectives of the proposal and in line with the informed consent form and country-level legislation. Proposals can be submitted once an end point defined in the protocol has been reached, the corresponding analysis performed, and the data related to the end point released through the first public presentation (retention period will be mentioned in the data transfer agreement [DTA]). Proposals should be directed to olympiaproposals@frontier-science.co.uk. To gain access, data requestors will need to sign a DTA.