Abstract
BACKGROUND:
Hypertension is a highly prevalent cardiovascular disease risk factor that may be related to inflammation. Whether adverse levels of specific inflammatory cytokines relate to hypertension is unknown. The present study sought to determine whether higher levels of IL (interleukin)-1β, IL-6, TNF (tumor necrosis factor)-α, IFN (interferon)-γ, IL-17A, and CRP (C-reactive protein) are associated with a greater risk of incident hypertension.
METHODS:
The REGARDS study (Reasons for Geographic and Racial Difference in Stroke) is a prospective cohort study that recruited 30 239 community-dwelling Black and White adults from the contiguous United States in 2003 to 2007 (visit 1), with follow-up 9 years later in 2013 to 2016 (visit 2). We included participants without prevalent hypertension who attended follow-up 9 years later and had available laboratory measures and covariates of interest. Poisson regression estimated the risk ratio of incident hypertension by level of inflammatory biomarkers.
RESULTS:
Among 1866 included participants (mean [SD] aged of 62 [8] years, 25% Black participants, 55% women), 36% developed hypertension. In fully adjusted models comparing the third to first tertile of each biomarker, there was a greater risk of incident hypertension for higher IL-1β among White (1.24 [95% CI, 1.01–1.53]) but not Black participants (1.01 [95% CI, 0.83–1.23]) and higher TNF-α (1.20 [95% CI, 1.02–1.41]) and IFN-γ (1.22 [95% CI, 1.04–1.42]) among all participants. There was no increased risk with IL-6, IL-17A, or CRP.
CONCLUSIONS:
Higher levels of IL-1β, TNF-α, and IFN-γ, representing distinct inflammatory pathways, are elevated in advance of hypertension development. Whether modifying these cytokines will reduce incident hypertension is unknown.
Keywords: biomarkers, C-reactive protein, cytokines, hypertension, inflammation, prospective studies
NOVELTY AND RELEVANCE.
What Is New?
Elevations in certain inflammatory cytokines are associated with greater hypertension risk at 9 years.
What Is Relevant?
Higher IL (interleukin)-1β in White participants and higher TNF (tumor necrosis factor)-α and IFN (interferon)-γ in all participants were associated with a higher risk of developing hypertension 9 years later, independent of other factors.
Clinical/Pathophysiological Implications?
Among adults without hypertension, certain cytokines may identify those at the greatest risk for hypertension development.
See Editorial, pp 1254–1256
Hypertension is a preventable and modifiable cardiovascular disease risk factor.1 To prevent hypertension, understanding its pathogenesis is necessary. While the pathogenesis remains elusive,2 higher levels of the inflammatory biomarker CRP (C-reactive protein) are associated with a risk of incident hypertension.3–5 Many risk factors for the development of hypertension are also associated with adverse levels of CRP, such as obesity, low physical activity, poor diet, and smoking.6–12 CRP is a nonspecific inflammatory biomarker that cannot provide insight into which immune pathways might be involved in the pathogenesis of hypertension. Preclinical research has implicated IL (interleukin)-1β, IL-6, TNF (tumor necrosis factor)-α, IFN (interferon)-γ, and IL-17A in the onset of hypertension, further supporting the role of inflammation in the onset of hypertension.13–15 Investigation of these cytokines in prospective studies of hypertension risk is lacking. Since these inflammatory cytokines represent distinct, targetable inflammatory pathways, determining their associations with the onset of hypertension is critical to advance knowledge of the origins of hypertension.
In a subset of a large cohort study of Black and White US adults, we studied the cytokine-related risk of developing incident hypertension over 9 years. We hypothesized that higher levels of IL-1β, IL-6, TNF-α, IFN-γ, and IL-17A are associated with a greater risk of incident hypertension. As a comparator, we estimated the risk of incident hypertension by level of CRP.
METHODS
Data Availability
The REGARDS study (Reasons for Geographic and Racial Difference in Stroke) data and code are available to investigators upon approval of the REGARDS Manuscript Proposal Form. REGARDS policies and additional details are available at https://www.uab.edu/soph/regardsstudy/researchers.
REGARDS and Biomarkers as Mediators of Racial Disparities in Risk Factors Subcohort
REGARDS enrolled 30 239 Black and White adults from the 48 contiguous United States in 2003 to 2007 (visit 1).16 Exclusion criteria included race other than Black or White, Hispanic/Latino/Latina ethnicity, residence in a nursing facility, or active cancer. Visit 2 occurred in 2013 to 2016. Sociodemographics, medical history, anthropometrics, blood pressure (BP), and fasting blood and urine samples were obtained at both visits. BioMedioR (Biomarkers as Mediators of Racial Disparities in Risk Factors) is a sex-race–stratified subcohort of 4400 participants who participated in both visits.17 Additional details on REGARDS and BioMedioR appear in the Supplemental Material.17–25
Laboratory Methods
Levels of inflammatory cytokines and CRP (hereafter denoted as inflammatory biomarkers) were measured in visit 1 samples. Additional details are given in the Supplemental Material.
Covariates of Interest
Sociodemographics were self-reported. Body mass index was calculated as weight in kilograms divided by height in meters squared. The waist circumference was measured in centimeters. Exercise was the frequency of intense physical activity sufficient to cause sweating (none, 1–3× per week, or ≥4× per week).26 Alcohol use was tabulated as none, moderate (0–7 per week for women, 0–14 per week for men), or heavy (≥8 per week for women and ≥15 per week for men).27 Smoking was current, prior (not currently smoking and ≥100 cigarettes in a lifetime), or never (not currently smoking and <100 cigarettes in a lifetime). Diabetes was self-reported diabetes, use of diabetes medications, or serum glucose ≥126 mg/dL if fasting or ≥200 mg/dL if not fasting. Diet used the REGARDS Life’s Simple 7–adapted dietary measure, defined using a food frequency questionnaire, and was ranked as poor (0–1 component), intermediate (2–3 components), or ideal (4–5 components).28,29 This questionnaire was not completed by 28% of REGARDS participants. As the majority of participants had a poor diet, missing dietary scores were assigned a value of poor, as in prior studies.30 This strategy was used rather than multiple imputation, as the missingness of dietary data was not at random.
Hypertension Definitions
Hypertension was defined as (1) self-reported use of BP-lowering medications or (2) in-home visit–measured BP at or above a given threshold. The primary analysis used the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure hypertension threshold of 140/90 mm Hg.31 A secondary analysis used the American College of Cardiology/American Heart Association-led 2017 hypertension threshold of 130/80 mm Hg.32 Prevalent hypertension was defined as hypertension at visit 1. Incident hypertension was the outcome of interest and was defined as hypertension at visit 2 among those without prevalent hypertension.
Study Population
Among BioMedioR participants, we excluded those with prevalent hypertension or those missing any model covariates (other than diet as described above). This group was termed the provisional analytical population. For each individual inflammatory biomarker analysis, a final analytical sample was obtained after the exclusion of participants missing that specific inflammatory biomarker.
Statistical Analysis
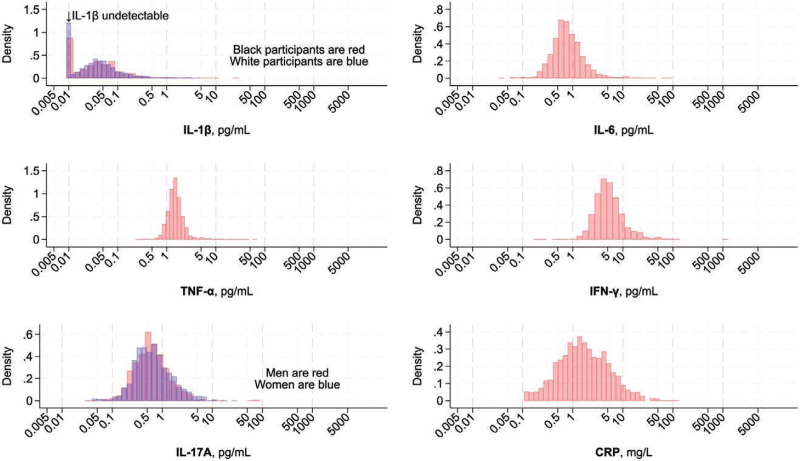
Sampling weights were used to reflect the demographic composition of the REGARDS study participants to account for the BioMedioR race-sex–stratified sampling.17 Baseline sociodemographics, covariates, medical problems, and inflammatory biomarker tertiles were stratified by incident hypertension status. Unweighted histograms visualized the distribution of these laboratory values. IL-1β was undetectable in 25% of participants. The lowest detectable IL-1β level was 0.0092 pg/mL, so for visualization in histograms, when defining tertiles, and in generating restricted cubic splines, undetectable IL-1β was assigned a value of 0.0090 pg/mL.
To understand the risk of hypertension by level of inflammatory biomarkers, Poisson regression with robust variance estimation estimated the relative risk for the higher tertile of each inflammatory biomarker in 4 models.33 The base model was unadjusted. Model 1 included age, sex, race, and systolic BP (SBP). Model 2 was model 1 plus education and income. Model 3 was model 1 plus body mass index and waist circumference. Model 4 was model 1 plus exercise, alcohol use, smoking, prevalent diabetes, and diet. Model 5 was models 1 through 4. Baseline SBP was considered an intermediate of the relationship between inflammation and incident hypertension and was included to account for differences in risk of incident hypertension for participants with lower SBP versus high-normal SBP at visit 1. Restricted cubic splines using Harrell method visualized the relative risk of incident hypertension by the higher level of each cytokine relative to the median in the unadjusted model and model 5.34 Inflammatory cytokines below the 0.5th and above the 99.5th percentiles were censored in each model except IL-1β, which did not censor the lower range since those with undetectable levels comprised the lowest level of the IL-1β range, as is described in the preceding paragraph.
We hypothesized that there would be sex and race differences in the association between inflammatory biomarkers and hypertension, so we tested for interaction with sex and race. We considered a 2-tailed P<0.05 to be statistically significant for all comparisons except for interactions, which used P<0.10 as we consider the cost of a type II error substantial. Models were race or sex stratified for any observed race×inflammatory or sex×inflammatory biomarker interactions.
Analyses used Stata, version 18.0 (StataCorp, College Station, TX).
RESULTS
Study Population
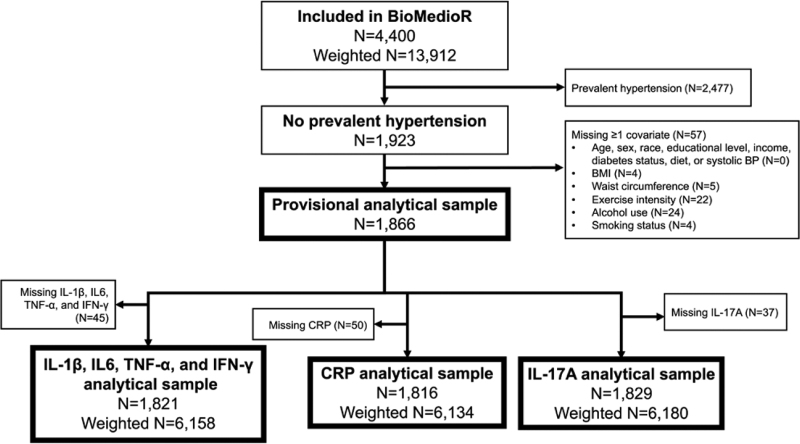
Of the 4400 BioMedioR participants (13 912 with weighting), 56% were excluded for prevalent hypertension (with weighting: 53% overall, 44% of White participants, and 68% of Black participants; Figure 1). The provisional analytical sample included 1866 participants (6307 with weighting). An additional 2% were excluded from the cytokine analytical samples and 3% from the CRP analytical sample due to missing assays. Ninety-six percent of the provisional analytical sample had all inflammatory biomarkers available, as is shown in Figure S2.
Figure 1.
Inclusion flowchart. Counts are unweighted except where specified. Missing dietary scores were assigned a value of poor and are not considered missing in this flowchart. BioMedioR indicates Biomarkers as Mediators of Racial Disparities in Risk Factors; BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; IFN, interferon; IL, interleukin; and TNF, tumor necrosis factor.
The mean (SD) age of the provisional analytical sample was 62 (8) years; 55% were women, and 25% were of Black race. As shown in the Table, 36% of the provisional analytical sample developed hypertension. There was a greater proportion of Black participants and a higher mean age, body mass index, and waist circumference among those with incident hypertension. The median follow-up was 9 years.
Table.
Baseline Characteristics by Incident Hypertension Status in the Provisional Analytical Sample
Distribution of Cytokines
The distributions of inflammatory biomarkers appear in Figure 2. IL-1β was undetectable in 25% of participants. The distributions of the other inflammatory biomarkers approximated a normal distribution after log transformation.
Figure 2.
Distribution of cytokines and CRP (C-reactive protein) at baseline. Density histograms. IL (interleukin)-1β was undetectable in 24.7% of the sample (25.9% of Black and 24.4% of White participants) and was assigned a value of 0.0090 for purpose of this histogram. IL-1β was race stratified because of an IL-1β–race interaction on incident hypertension. IL-17A is sex-stratified because of an IL-17A–sex interaction on incident hypertension. CRP indicates C-reactive protein; IFN, interferon; and TNF, tumor necrosis factor.
Proportion With Incident Hypertension
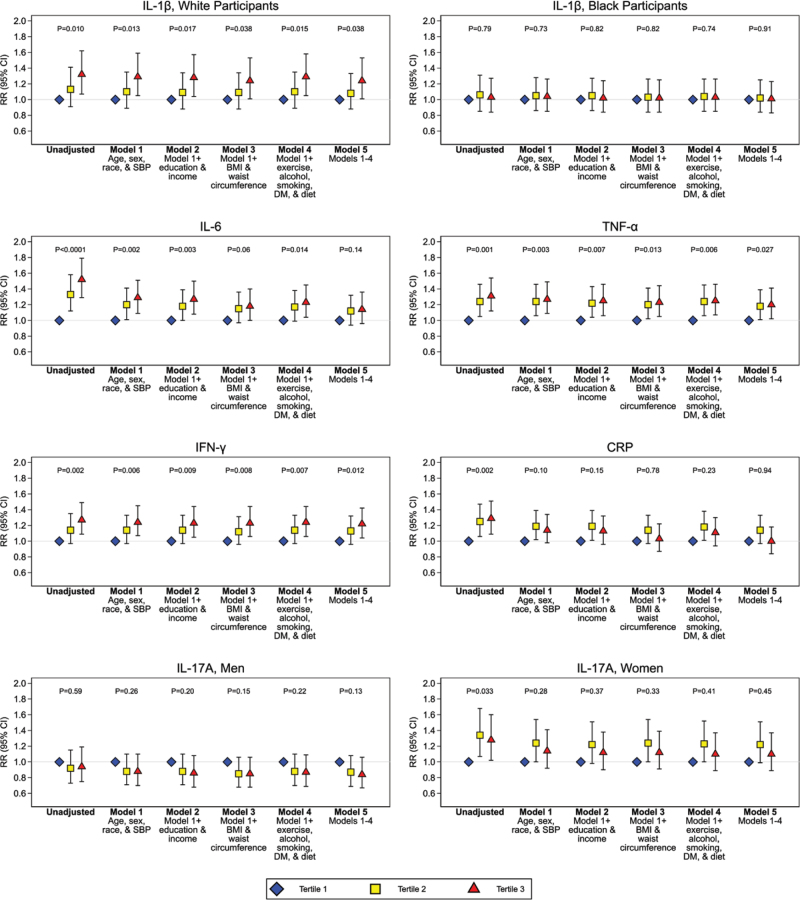
In the provisional analytical sample with weighting applied, 35% developed hypertension. This occurred in 32% of White participants, 47% of Black participants, 35% of men, and 36% of women. These proportions were similar for the other analytical samples. As shown in the Table, there was a greater hypertension incidence with higher IL-1β in White participants, IL-6 in all, TNF-α in all, IFN-γ in all, IL-17A in women, and CRP in all. There was no clear pattern for a greater burden of incident hypertension by higher IL-1β in Black participants or IL-17A in men.
Risk of Incident Hypertension by Inflammatory Biomarkers
In model 1, there was a significant race×IL-1β interaction on incident hypertension (P=0.083) and a significant sex×IL-17A interaction on incident hypertension (P=0.051). There were no other significant race×inflammatory or sex×inflammatory biomarker interactions.
In the unadjusted models, higher levels of IL-1β in White participants, IL-17A in women, and IL-6, TNF-α, IFN-γ, and CRP in all participants were associated with a greater risk of incident hypertension relative to the lowest levels (Figure 3). This association persisted for IL-1β in White participants and TNF-α and IFN-γ among all participants after adjustment for all covariates (model 5). In all participants, the associations for IL-6 and CRP were attenuated after adjustment for anthropometrics. In women, the association with IL-17A was attenuated after adjustment for age and race.
Figure 3.
Relative risk (RR) of incident hypertension by level of cytokines and CRP (C-reactive protein). RR is relative risk of incident hypertension in each model. P value represents a linear P test across tertiles. The base model is unadjusted. Model 1 is adjusted for age, sex, race, and systolic blood pressure (SBP). Model 2 adds education and income to model 1. Model 3 adds body mass index (BMI) and waist circumference to model 1. Model 4 adds exercise intensity, alcohol use, smoking status, prevalent diabetes, and diet to model 1. Model 5 combines all covariates in models 1 to 4. DM indicates diabetes mellitus; IFN, interferon; IL, interleukin; and TNF, tumor necrosis factor.
Restricted cubic splines demonstrated increasing risk across the continuum of baseline levels of IL-6 (Figure S5). There appeared to be lower incident hypertension risk for those with inflammatory biomarkers below the median for TNF-α, IFN-γ, and IL-17A in women and CRP in all. In contrast, men with IL-17A below the median had a higher risk of incident hypertension. There were no clear nonlinear associations of greater IL-1β and the risk of incident hypertension for Black or White participants.
Secondary Analysis, Using the American College of Cardiology/American Heart Association 2017 Hypertension Threshold of 130/80 mm Hg
Results from the secondary analyses appear in Table S1 and Figures S1 through S5. The provisional analytical sample was 700 participants smaller (n=1166), owing to a greater proportion with prevalent hypertension. In this group with weighting applied, 42% developed incident hypertension (40% White participants, 51% Black participants, 44% men, and 40% women). There was no longer a statistically significant IL-1β×race or IL-17A×sex interaction on incident hypertension, but given the primary analysis findings, analyses were still stratified by race (IL-1β) and sex (IL-17A). There was a significant CRP×race interaction (model 1, P=0.086). We opted not to stratify CRP by race, given the primary analysis findings. For IL-1β among White participants, there was no clear association between higher levels and incident hypertension, though the directionality of the association was the same. The associations between higher IL-6, TNF-α, and IFN-γ and the risk of incident hypertension in the unadjusted model were similar to the primary analysis; however, only higher IFN-γ was associated with greater hypertension risk in the fully adjusted model 5. Higher IL-17A was not associated with greater hypertension risk in men or women.
DISCUSSION
In a prospective cohort study of ≈2000 Black and White adult participants without prevalent hypertension, higher levels of TNF-α and IFN-γ, along with IL-1β in White participants, were associated with incident hypertension when adjusting for confounders. Observed associations for IL-17A among women and IL-6 and CRP among all participants were attenuated after accounting for confounders. Findings suggest that hypertension evolves partly through proinflammatory pathways and that interruption of these could hold promise for prevention.
Preclinical studies have implicated adverse inflammation of the brain, kidney, vasculature, and heart in the origin of hypertension, and animal models that inhibit T-cell or macrophage activation prevent the development of hypertension.15 These studies have highlighted the role of specific, drug-targetable pathways in the onset of hypertension. CRP, previously the most widely studied inflammation marker in hypertension epidemiology studies, is a nonspecific inflammatory biomarker and does not provide insight into which specific pathways might be activated in advance of hypertension onset. Despite promising preclinical discoveries, there has been little translation of specific inflammatory cytokines into population studies. The best-studied cytokine is IL-6, and its association with incident hypertension was conflicting in 3 separate cohorts.4,35,36
These findings extend preclinical discoveries and demonstrate associations between IL-6, TNF-α, IFN-γ, and CRP in all participants, IL-1β in White but not Black participants, IL-17A in women but not men, and a greater risk of incident hypertension development in unadjusted models. IL-6 is released by multiple tissues, including adipose tissue and adipose tissue macrophages.37–39 Relative to subcutaneous adipose tissue, visceral adipose tissue might release a larger proportion of IL-6.40 Individuals with obesity may have higher IL-6 owing to more adipose tissue and adipose tissue macrophages. Weight loss through caloric restriction lowers IL-6 levels.41 Weight loss also lowers BP.7 The present findings confirm other findings that higher IL-6 is associated with a greater risk of hypertension and that some of this association is attenuated upon adjusting for measures of adiposity.4,35,36 The directionality of the association between measures of adiposity, IL-6, and hypertension cannot be determined from these findings. Furthermore, use of the anti-IL-6 agent tocilizumab is associated with the development of hypertension in ≥5% of patients with autoimmune diseases.42 The effects of anti-IL-6 on BP among adults without autoimmune diseases are unclear. Statins lower IL-6 and CRP, and 1 trial reported BP lowering among adults without hypertension who were randomized to statin therapy.43,44 Further work is needed to understand the BP effects of IL-6 lowering with pharmaceuticals or behavior changes.
Macrophages are associated with adiposity and are a major source of TNF-α.45 The observed association between higher TNF-α and greater risk of incident hypertension was consistent with a prior case-control study of TNF-r2 (TNF receptor 2) as a surrogate marker for TNF-α in postmenopausal women.36 Our findings confirm this association in a prospective cohort study of men and women. A post hoc analysis of disease-modifying therapies among individuals with recent-onset rheumatoid arthritis noted lower BP among individuals randomized to the anti-TNF-α medication infliximab.46 Whether modifying TNF-α also lowers BP among individuals without rheumatoid arthritis is unclear.
We are unaware of prospective studies investigating the risk of hypertension by level of IFN-γ. In a study of circulating immune cell subsets with repeat SBP measures averaged >10 years of follow-up, higher levels of IFN-γ–producing natural killer cells, but not T helper type 1 (Th1) or type 1 CD8+ (Tc1) cells, were associated with higher SBP.47 The anti-IFN-γ agent emapalumab causes hypertension in 40% of patients with hemophagocytic lymphohistiocytosis.48 The role of IFN-γ modification among individuals without hemophagocytic lymphohistiocytosis is unclear. Of the inflammatory biomarkers in the present study, IFN-γ is unique in that it was associated with both the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (140/90 mm Hg) and the American College of Cardiology/American Heart Association 2017 threshold (130/80 mm Hg) incident hypertension. It is reasonable to consider the American College of Cardiology/American Heart Association 2017 hypertension threshold as an earlier phenotype, and perhaps IFN-γ elevation might occur earlier in the inflammatory pathways that ultimately lead to hypertension development. Alternatively, it is possible that IFN-γ represents a highly potent risk factor for hypertension development and that its elevation could lead to the earliest changes before hypertension development.
The observation that higher IL-1β is associated with greater hypertension risk in White but not Black participants is consistent with a prior case-control study of postmenopausal women.36 Race is primarily a social construct, and there is little evidence for biological differences between race groups.49,50 Black US adults develop hypertension earlier in life than other race groups,51 and it is possible that Black adults at risk for hypertension might experience IL-1β elevation earlier in life, possibly driving that earlier onset of hypertension and subsequent exclusion from this analysis of adults with a mean age of 62 years. This also might help explain the observation that IL-1β lowering with canakinumab in CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcome Study) did not prevent hypertension development.52 Participants in CANTOS had elevated CRP and a myocardial infarction in the prior 30 days and likely had adverse inflammation for many years before their cardiac event that qualified them for the trial. Other cohorts with a younger age of enrollment and >2 years of visits may opt to measure these inflammatory biomarkers at several visits to determine the temporality of elevation relative to hypertension development and specifically assess for early markers of adverse inflammation.
We were surprised that the association of IL-17A with hypertension risk differed by sex. Specifically, the restricted cubic splines depict a higher risk of hypertension with lower IL-17A in men. This association was the opposite in women, with lower levels predicting a lower risk of hypertension. It is possible that some of these differences might relate to endogenous hormones. Prior animal model studies of arthritis found estrogen modulated IL-17–producing T cells (ie, γδ T cells, IL-17–producing T helper cells).53,54 One study investigated whether γδ T cells relate to BP elevation.55 These cells were found to mediate angiotensin II–induced BP elevation in mouse models, and higher levels of γδ T cells were associated with higher SBP in humans, leading authors to hypothesize that these cells might be involved in hypertension development. Recently, in a study in the MESA (Multi-Ethnic Study of Atherosclerosis), higher levels of γδ T cells, but not Th17 cells, were associated with higher SBP, although interactions by sex were not observed.47 It is possible that the quantitative level and activity of these cells differ between sexes, which might lead to differing patterns in immune activation. For example, the differentiation of macrophages varies by exposure to IL-17,56 and it is possible that macrophages from men with low IL-17A levels may have a differential response to other cytokines. Furthermore, estrogen levels lower BP in both men and women.57–59 It is possible that men with lower IL-17A levels have higher estrogen levels from other sources; however, a direct link with hypertension remains unclear.
Taken together, the current findings support an association between distinct inflammatory cytokines and a greater risk of incident hypertension, whether defined using a threshold of 140/90 or 130/80 mm Hg. These observations are important in advancing prevention discoveries in hypertension since these cytokines represent therapeutically actionable targets. However, it is unclear whether these markers represent a causal pathway of hypertension, or whether they are markers of other drivers of BP elevation that are proinflammatory. Furthermore, the race difference in the association for IL-1β warrants additional investigation, as Black US adults experience a disproportionate burden of hypertension and its complications.60 It is unclear whether distinct pathways of inflammation might explain some of the excess burden of hypertension among Black adults. Finally, specific cytokines do not vary in isolation. The attenuation of IL-6 and CRP when accounting for measures of adiposity suggests that these measures may be most relevant in hypertension phenotypes related to high body mass index and waist circumference. Identifying specific phenotypes associated with higher levels of these inflammation biomarkers is an important next step in understanding hypertension cause.
Our study is strengthened by its size, rich phenotyping of the cohort, high-quality assessment of hypertension, and high retention. This study is limited by the disproportionate number of Black adults excluded for prevalent hypertension and 25% of participants with IL-1β below the detectable limit owing to its low circulating concentration.
In summary, in a prospective cohort study of Black and White adults followed prospectively for 9 years, higher IL-1β in White participants and higher TNF-α and IFN-γ in all participants were associated with a higher risk of incident hypertension when controlling for confounders.
PERSPECTIVES
In the current study, higher IL-1β in White participants and higher TNF-α and IFN-γ in all participants were associated with a higher risk of developing hypertension 9 years later, independent of other factors. Cytokine lowering with pharmacological and behavioral interventions may one day prevent hypertension development among people with adverse levels of these cytokines.
ARTICLE INFORMATION
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the REGARDS study (Reasons for Geographic and Racial Difference in Stroke) for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at https://www.uab.edu/soph/regardsstudy/. The authors appreciate the support and guidance of investigators from the Study Design and Molecular Epidemiology Core of the Vermont Center for Cardiovascular and Brain Health.
Sources of Funding
This study is supported by cooperative agreement U01 NS041588 cofunded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIA. Representatives of the NINDS were involved in the review of the manuscript but were not directly involved in the collection, management, analysis, or interpretation of the data. This study was also supported by the Bloomfield Early Career Professorship in Cardiovascular Research and the Cardiovascular Research Institute of Vermont. Funding was provided by P20 GM135007 from the National Institute of General Medical Sciences of the National Institutes of Health.
Disclosures
None.
Supplemental Material
Figures S1–S5
Table S1
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BioMedioR
- Biomarkers as Mediators of Racial Disparities in Risk Factors
- BP
- blood pressure
- CANTOS
- Canakinumab Anti-Inflammatory Thrombosis Outcome Study
- CRP
- C-reactive protein
- IFN
- interferon
- IL
- interleukin
- MESA
- Multi-Ethnic Study of Atherosclerosis
- REGARDS study
- Reasons for Geographic and Racial Difference in Stroke
- SBP
- systolic blood pressure
- TNF-r2
- tumor necrosis factor receptor 2
- TNF
- tumor necrosis factor
For Sources of Funding and Disclosures, see page 1252.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.123.22714.
REFERENCES
- 1.Martin SS, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Barone Gibbs B, Beaton AZ, Boehme AK, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. 2024 heart disease and stroke statistics: a report of US and global data from the American Heart Association. Circulation. 2024;149:e347–e913. doi: 10.1161/CIR.0000000000001209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oparil S, Acelajado MC, Bakris GL, Berlowitz DR, Cífková R, Dominiczak AF, Grassi G, Jordan J, Poulter NR, Rodgers A, et al. Hypertension. Nat Rev Dis Primers. 2018;4:18014. doi: 10.1038/nrdp.2018.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290:2945–2951. doi: 10.1001/jama.290.22.2945 [DOI] [PubMed] [Google Scholar]
- 4.Lakoski SG, Cushman M, Siscovick DS, Blumenthal RS, Palmas W, Burke G, Herrington DM. The relationship between inflammation, obesity and risk for hypertension in the Multi-Ethnic Study of Atherosclerosis (MESA). J Hum Hypertens. 2011;25:73–79. doi: 10.1038/jhh.2010.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plante TB, Long DL, Guo B, Howard G, Carson AP, Howard VJ, Judd SE, Jenny NS, Zakai NA, Cushman M. C-reactive protein and incident hypertension in Black and White Americans in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort study. Am J Hypertens. 2021;34:698–706. doi: 10.1093/ajh/hpaa215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selvin E, Paynter NP, Erlinger TP. The effect of weight loss on C-reactive protein: a systematic review. Arch Intern Med. 2007;167:31–39. doi: 10.1001/archinte.167.1.31 [DOI] [PubMed] [Google Scholar]
- 7.He J, Whelton PK, Appel LJ, Charleston J, Klag MJ. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension. 2000;35:544–549. doi: 10.1161/01.hyp.35.2.544 [DOI] [PubMed] [Google Scholar]
- 8.Dickinson HO, Mason JM, Nicolson DJ, Campbell F, Beyer FR, Cook JV, Williams B, Ford GA. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens. 2006;24:215–233. doi: 10.1097/01.hjh.0000199800.72563.26 [DOI] [PubMed] [Google Scholar]
- 9.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM; American Heart Association. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296–308. doi: 10.1161/01.HYP.0000202568.01167.B6 [DOI] [PubMed] [Google Scholar]
- 10.Soltani S, Chitsazi MJ, Salehi-Abargouei A. The effect of dietary approaches to stop hypertension (DASH) on serum inflammatory markers: a systematic review and meta-analysis of randomized trials. Clin Nutr. 2018;37:542–550. doi: 10.1016/j.clnu.2017.02.018 [DOI] [PubMed] [Google Scholar]
- 11.Bowman TS, Gaziano JM, Buring JE, Sesso HD. A prospective study of cigarette smoking and risk of incident hypertension in women. J Am Coll Cardiol. 2007;50:2085–2092. doi: 10.1016/j.jacc.2007.08.017 [DOI] [PubMed] [Google Scholar]
- 12.Janzon E, Hedblad B, Berglund G, Engström G. Changes in blood pressure and body weight following smoking cessation in women. J Intern Med. 2004;255:266–272. doi: 10.1046/j.1365-2796.2003.01293.x [DOI] [PubMed] [Google Scholar]
- 13.Angeli F, Reboldi G, Verdecchia P. The link between inflammation and hypertension: unmasking mediators. Am J Hypertens. 2021;34:683–685. doi: 10.1093/ajh/hpab034 [DOI] [PubMed] [Google Scholar]
- 14.Caillon A, Schiffrin EL. Role of inflammation and immunity in hypertension: recent epidemiological, laboratory, and clinical evidence. Curr Hypertens Rep. 2016;18:21. doi: 10.1007/s11906-016-0628-7 [DOI] [PubMed] [Google Scholar]
- 15.Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med. 2018;215:21–33. doi: 10.1084/jem.20171773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The Reasons for Geographic and Racial Differences in Stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678 [DOI] [PubMed] [Google Scholar]
- 17.Long DL, Guo B, McClure LA, Jaeger BC, Tison SE, Howard G, Judd SE, Howard VJ, Plante TB, Zakai NA, et al. Biomarkers as Mediators of racial disparities in risk factors (BioMedioR): rationale, study design, and statistical considerations. Ann Epidemiol. 2022;66:13–19. doi: 10.1016/j.annepidem.2021.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard VJ, Woolson RF, Egan BM, Nicholas JS, Adams RJ, Howard G, Lackland DT. Prevalence of hypertension by duration and age at exposure to the stroke belt. J Am Soc Hypertens. 2010;4:32–41. doi: 10.1016/j.jash.2010.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillett SR, Boyle RH, Zakai NA, McClure LA, Jenny NS, Cushman M. Validating laboratory results in a national observational cohort study without field centers: the Reasons for Geographic and Racial Differences in Stroke cohort. Clin Biochem. 2014;47:243–246. doi: 10.1016/j.clinbiochem.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long DL, Howard G, Long DM, Judd S, Manly JJ, McClure LA, Wadley VG, Safford MM, Katz R, Glymour MM. An investigation of selection bias in estimating racial disparity in stroke risk factors. Am J Epidemiol. 2019;188:587–597. doi: 10.1093/aje/kwy253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forman JP, Rimm EB, Stampfer MJ, Curhan GC. Folate intake and the risk of incident hypertension among US women. JAMA. 2005;293:320–329. doi: 10.1001/jama.293.3.320 [DOI] [PubMed] [Google Scholar]
- 22.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, Deal JA, McKhann GM, Mosley TH, Sharrett AR, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol. 2017;74:1246–1254. doi: 10.1001/jamaneurol.2017.1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MSD MULTI-SPOT Assay System. Proinflammatory panel 1 (human) manufacturer insert. MSD; 2018. https://www.mesoscale.com/~/media/files/product%20inserts/proinflammatory%20panel%201%20human%20insert.pdf [Google Scholar]
- 25.MSD MULTI-SPOT Assay System. TH17 panel 1 (human) kits manufacturer insert. 2019. https://www.mesoscale.com/~/media/files/product%20inserts/v-plex-th17-panel-1-human-product-insert.pdf
- 26.Washburn RA, Goldfield SR, Smith KW, McKinlay JB. The validity of self-reported exercise-induced sweating as a measure of physical activity. Am J Epidemiol. 1990;132:107–113. doi: 10.1093/oxfordjournals.aje.a115622 [DOI] [PubMed] [Google Scholar]
- 27.U.S. Department of Health and Human Services, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism. Helping Patients Who Drink Too Much, A Clinician's Guide, Updated 2005 Edition. 2007. NIH publication no. 07–3769.
- 28.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. ; American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 29.Kulshreshtha A, Vaccarino V, Judd SE, Howard VJ, McClellan WM, Muntner P, Hong Y, Safford MM, Goyal A, Cushman M. Life’s Simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke. 2013;44:1909–1914. doi: 10.1161/STROKEAHA.111.000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plante TB, Koh I, Judd SE, Howard G, Howard VJ, Zakai NA, Booth JN, Safford MM, Muntner P, Cushman M. Life’s Simple 7 and incident hypertension: the REGARDS study. J Am Heart Assoc. 2020;9:e016482. doi: 10.1161/JAHA.120.016482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, et al. ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 32.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71:1269–1324. doi: 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 33.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 34.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Springer; 2015. [Google Scholar]
- 35.Sesso HD, Wang L, Buring JE, Ridker PM, Gaziano JM. Comparison of interleukin-6 and C-reactive protein for the risk of developing hypertension in women. Hypertension. 2007;49:304–310. doi: 10.1161/01.HYP.0000252664.24294.ff [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Manson JE, Gaziano JM, Liu S, Cochrane B, Cook NR, Ridker PM, Rifai N, Sesso HD. Circulating inflammatory and endothelial markers and risk of hypertension in White and Black postmenopausal women. Clin Chem. 2011;57:729–736. doi: 10.1373/clinchem.2010.156794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450 [DOI] [PubMed] [Google Scholar]
- 38.Eder K, Baffy N, Falus A, Fulop AK. The major inflammatory mediator interleukin-6 and obesity. Inflamm Res. 2009;58:727–736. doi: 10.1007/s00011-009-0060-4 [DOI] [PubMed] [Google Scholar]
- 39.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336 [DOI] [PubMed] [Google Scholar]
- 41.Nicklas BJ, Ambrosius W, Messier SP, Miller GD, Penninx BWJH, Loeser RF, Palla S, Bleecker E, Pahor M. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004;79:544–551. doi: 10.1093/ajcn/79.4.544 [DOI] [PubMed] [Google Scholar]
- 42.Genentech USA, Inc. Actemra prescribing information. 2022. https://www.gene.com/download/pdf/actemra_prescribing.pdf
- 43.Milajerdi A, Sadeghi A, Mousavi SM, Larijani B, Esmaillzadeh A. Influence of statins on circulating inflammatory cytokines in patients with abnormal glucose homeostasis: a meta-analysis of data from randomized controlled trials. Clin Ther. 2020;42:e13–e31. doi: 10.1016/j.clinthera.2019.12.009 [DOI] [PubMed] [Google Scholar]
- 44.Golomb BA, Dimsdale JE, White HL, Ritchie JB, Criqui MH. Reduction in blood pressure with statins: results from the UCSD Statin Study, a randomized trial. Arch Intern Med. 2008;168:721–727. doi: 10.1001/archinte.168.7.721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sethi JK, Hotamisligil GS. Metabolic messengers: tumour necrosis factor. Nat Metab. 2021;3:1302–1312. doi: 10.1038/s42255-021-00470-z [DOI] [PubMed] [Google Scholar]
- 46.Klarenbeek NB, van der Kooij SM, Huizinga TJW, Goekoop-Ruiterman YPM, Hulsmans HMJ, van Krugten MV, Speyer I, de Vries-Bouwstra JK, Kerstens PJSM, Huizinga TWJ, et al. Blood pressure changes in patients with recent-onset rheumatoid arthritis treated with four different treatment strategies: a post hoc analysis from the BeSt trial. Ann Rheum Dis. 2010;69:1342–1345. doi: 10.1136/ard.2009.124180 [DOI] [PubMed] [Google Scholar]
- 47.Delaney JAC, Olson NC, Sitlani CM, Fohner AE, Huber SA, Landay AL, Heckbert SR, Tracy RP, Psaty BM, Feinstein M, et al. Natural killer cells, gamma delta T cells and classical monocytes are associated with systolic blood pressure in the Multi-Ethnic Study of Atherosclerosis (MESA). BMC Cardiovasc Disord. 2021;21:45. doi: 10.1186/s12872-021-01857-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Locatelli F, Jordan MB, Allen C, Cesaro S, Rizzari C, Rao A, Degar B, Garrington TP, Sevilla J, Putti MC, et al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N Engl J Med. 2020;382:1811–1822. doi: 10.1056/NEJMoa1911326 [DOI] [PubMed] [Google Scholar]
- 49.Goodman AH. Why genes don’t count (for racial differences in health). Am J Public Health. 2000;90:1699–1702. doi: 10.2105/ajph.90.11.1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hogarth RA. The myth of innate racial differences between White and Black people’s bodies: lessons from the 1793 yellow fever epidemic in Philadelphia, Pennsylvania. Am J Public Health. 2019;109:1339–1341. doi: 10.2105/AJPH.2019.305245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation. 2023;147:e93–e621. doi: 10.1161/CIR.0000000000001123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rothman AM, MacFadyen J, Thuren T, Webb A, Harrison DG, Guzik TJ, Libby P, Glynn RJ, Ridker PM. Effects of interleukin-1β inhibition on blood pressure, incident hypertension, and residual inflammatory risk: a secondary analysis of CANTOS. Hypertension. 2020;75:477–482. doi: 10.1161/HYPERTENSIONAHA.119.13642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andersson A, Grahnemo L, Engdahl C, Stubelius A, Lagerquist MK, Carlsten H, Islander U. IL-17-producing γδT cells are regulated by estrogen during development of experimental arthritis. Clin Immunol. 2015;161:324–332. doi: 10.1016/j.clim.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 54.Andersson A, Stubelius A, Karlsson MN, Engdahl C, Erlandsson M, Grahnemo L, Lagerquist MK, Islander U. Estrogen regulates T helper 17 phenotype and localization in experimental autoimmune arthritis. Arthritis Res Ther. 2015;17:32. doi: 10.1186/s13075-015-0548-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caillon A, Mian MOR, Fraulob-Aquino JC, Huo KG, Barhoumi T, Ouerd S, Sinnaeve PR, Paradis P, Schiffrin EL. γδ T cells mediate angiotensin II-induced hypertension and vascular injury. Circulation. 2017;135:2155–2162. doi: 10.1161/CIRCULATIONAHA.116.027058 [DOI] [PubMed] [Google Scholar]
- 56.de la Paz Sánchez-Martínez M, Blanco-Favela F, Mora-Ruiz MD, Chávez-Rueda AK, Bernabe-García M, Chávez-Sánchez L. IL-17-differentiated macrophages secrete pro-inflammatory cytokines in response to oxidized low-density lipoprotein. Lipids Health Dis. 2017;16:196. doi: 10.1186/s12944-017-0588-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ashraf MS, Vongpatanasin W. Estrogen and hypertension. Curr Hypertens Rep. 2006;8:368–376. doi: 10.1007/s11906-006-0080-1 [DOI] [PubMed] [Google Scholar]
- 58.Komesaroff PA, Fullerton M, Esler MD, Dart A, Jennings G, Sudhir K. Low-dose estrogen supplementation improves vascular function in hypogonadal men. Hypertension. 2001;38:1011–1016. doi: 10.1161/hy1101.095006 [DOI] [PubMed] [Google Scholar]
- 59.Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA. Estrogens in male physiology. Physiol Rev. 2017;97:995–1043. doi: 10.1152/physrev.00018.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The REGARDS study (Reasons for Geographic and Racial Difference in Stroke) data and code are available to investigators upon approval of the REGARDS Manuscript Proposal Form. REGARDS policies and additional details are available at https://www.uab.edu/soph/regardsstudy/researchers.