Summary.
What is already known about this topic?
Mycobacterium abscessus is a difficult-to-treat nontuberculous mycobacterium; various extrapulmonary infections have been reported associated with medical tourism.
What is added by this report?
In 2022, three patients developed extrapulmonary M. abscessus infections after receiving embryonic stem cell injections in three cities in Mexico. Isolates from two patients were identified as M. abscessus subspecies massiliense of a single clone, distinct from known dominant circulating clones (DCCs), by whole genome sequencing.
What are the implications for public health practice?
Because these isolates were clonal, distinct from known DCCs, and derived from distant cities, a common infected source associated with embryonic stem cell injections is suspected. Vigilance for similar cases and guidance for persons considering medical tourism are advised.
Mycobacterium abscessus is an intrinsically drug-resistant, rapidly growing, nontuberculous mycobacterium; extrapulmonary infections have been reported in association with medical tourism (1). During November–December 2022, two Colorado hospitals (hospitals A and B) treated patient A, a Colorado woman aged 30–39 years, for M. abscessus meningitis. In October 2022, she had received intrathecal donor embryonic stem cell injections in Baja California, Mexico to treat multiple sclerosis and subsequently experienced headaches and fevers, consistent with meningitis. Her cerebrospinal fluid revealed neutrophilic pleocytosis and grew M. abscessus in culture at hospital A. Hospital A’s physicians consulted hospital B’s infectious diseases (ID) physicians to co-manage this patient (2).
In spring 2023, hospital B’s ID physicians identified two additional patients with M. abscessus infections acquired after receiving stem cell injections performed at different clinics in Mexico. The first of these, patient B, an Arizona man aged 60–69 years, developed a right elbow osteoarticular infection after receiving donor embryonic stem cell injections for psoriatic arthritis at a Baja California, Mexico clinic different from the one that treated patient A, in April 2022. The second, patient C, a Colorado man aged 60–69 years, developed bilateral knee infections after receiving donor embryonic stem cell injections in both knees for osteoarthritis at a clinic in Guadalajara, Mexico, in October 2022.
Investigation and Outcomes
Hospital B’s ID physicians requested the isolates but were only able to obtain the original isolates from patients A and B. Whole genome sequencing (WGS) and phylogenetic analysis, performed at hospital B (3), revealed that the isolates were clonal M. abscessus subspecies massiliense, with only one single nucleotide polymorphism (SNP) difference between the two isolate core genomes* and were distinct from the most prevalent characterized dominant circulating clones (3) (Figure). These isolates were regrown from their original subcultures to repeat WGS, and the one SNP difference was confirmed. These patients had their stem cell injections performed at clinics 167 miles (269 km) apart in Baja California, Mexico. As of March 28, 2024, treatment is ongoing for all three patients.
FIGURE.
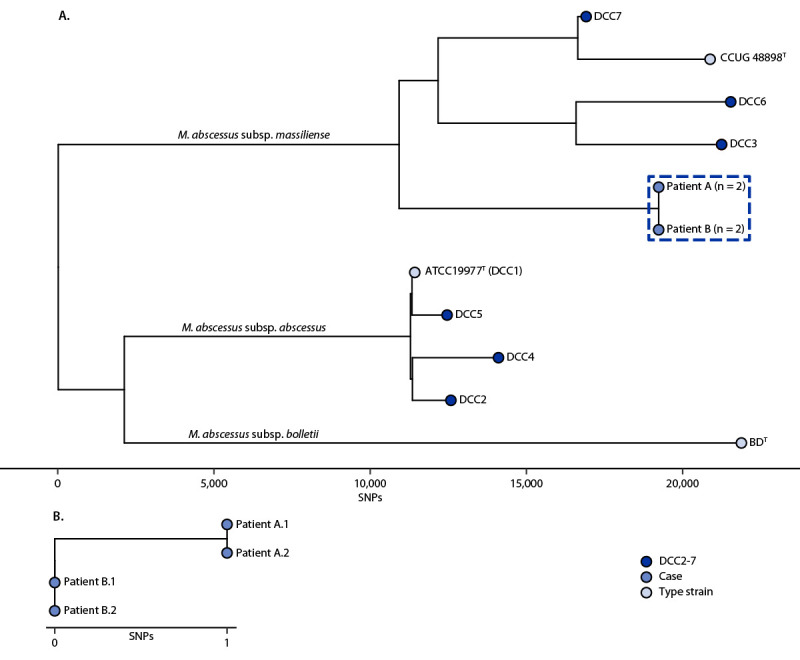
Mycobacterium abscessus whole genome phylogeny*,† of dominant circulating clones 1–7 and isolates from patients A§ and B¶ (A) and genomic similarity between the first and second whole genome sequencing single nucleotide polymorphisms for isolates from patients A§ and B¶ (B) associated with receipt of stem cell treatment in Mexico — Arizona and Colorado, 2022
Abbreviations: ATCC = American type culture collection; BD = Becton Dickinson; DCC = dominant circulating clone; SNP = single nucleotide polymorphism; WGS = whole genome sequencing.
* WGS was conducted twice to confirm the SNP difference between isolates from patients A and B.
† Superscripted T (T) is the standard designation for a type strain of a taxon.
§ Patient A.1 = isolate from patient A, first WGS result; patient A.2 = isolate from patient A, second WGS result.
¶ Patient B.1 = isolate from patient B, first WGS result; patient B.2 = isolate from patient B, second WGS result.
The patients’ treating physicians informed their state public health departments of their findings. The Colorado Department of Public Health and Environment (CDPHE) interviewed patients A and C, and the Arizona Department of Public Health interviewed patient B. CDPHE searched for similar cases within Colorado and other states and consulted with CDC; as of March 28, 2024, no additional cases have been identified. This activity was reviewed by CDC, deemed not research, and was conducted consistent with applicable federal law and CDC policy.†
Preliminary Conclusions and Actions
Given that the isolates identified from patients treated at different, distant clinics represent a single clone, the physicians and CDPHE suspect a common infected source (potentially the product, reagents, or equipment used) for patients A and B. CDPHE’s attempts to identify the product or gather details about its administration have been unsuccessful to date. CDPHE attempted to contact clinics that performed the stem cell injections, but received no response. A collaborative process with Mexican health authorities was initiated and is ongoing; however, no new substantial cases have yet been identified.
Next steps include 1) performing WGS on the organism isolated from patient C from newly acquired specimens; 2) sharing genomic information from the WGS analysis with the National Center for Biotechnology Information to ensure that comparisons can be made with additional cases; and 3) conducting prospective case finding. Historically, stem cell treatments have been linked to bacterial infections (4), and procedure-related infection risks associated with medical tourism are known (1,5). Providers and public health agencies need to be aware of the risk for M. abscessus infections from stem cell treatments for indications not approved by the Food and Drug Administration and maintain vigilance for similar cases (5). They also are advised to provide guidance for persons considering medical tourism (5).
Acknowledgments
Salika M. Shakir, ARUP Laboratories; Katrina M. Byrd, CDC.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Reeti Khare reports uncompensated participation on a Clinical Laboratory Standards Institute Antimycobacterial Working Group and that her laboratory does contract work for Insmed, Inc., RedHill Biopharma, Paratek Pharmaceuticals, AN2 Therapeutics, Spero Therapeutics, and MannKind Corporation. David E. Griffith reports receipt of consulting fees from Insmed, Inc., AN2 Therapeutics, and Paratek Pharmaceuticals; payments or honoraria from Insmed, Inc.; and participation on data safety monitoring boards for NOMAB: Nebulised Nitric Oxide for Mycobacterium abscessus pulmonary disease and MannKind Corporation: Clofzimine inhalation suspension for pulmonary nontuberculous mycobacterial disease. Charles L. Daley reports institutional support from AN2 Therapeutics, Bugworks, Insmed, Inc., Juvabis, Paratek Pharmaceuticals, the Cystic Fibrosis Foundation, the Food and Drug Administration, the Patient-Centered Outcomes Research Institute, and the National Institutes of Health; receipt of consulting fees from Genentech and Pfizer; participation on data safety monitoring boards for Otsuka, the Bill and Melinda Gates Foundation, and Eli Lilly; and participation on advisory boards for AN2 Therapeutics, Astrazeneca, Hyfe, Insmed, Inc., MannKind Corporation, Matinas Biopharma, Nob Hill Therapeutics, Paratek Pharmaceuticals, Spero Therapeutics, and Zambon. Minh-Vu H. Nguyen reports receipt of an honorarium from the Oregon Health and Science University (OHSU) for a presentation at Nontuberculous Mycobacteria Research Consortium (NTMRC) 2023; support for travel to IDWeek 2023 and American Thoracic Society 2023 from National Jewish Health; and support for travel to NTMRC 2023 from OHSU. No other potential conflicts of interest were disclosed.
Footnotes
WGS for isolates from patients A and B have been deposited in the National Center for Biotechnology Information BioProject, CDC HAI-Seq Nontuberculous mycobacterium (542112).
45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.
References
- 1.Schnabel D, Esposito DH, Gaines J, et al. ; RGM Outbreak Investigation Team. Multistate US outbreak of rapidly growing mycobacterial infections associated with medical tourism to the Dominican Republic, 2013–2014. Emerg Infect Dis 2016;22:1340–7. 10.3201/eid2208.151938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf AB, Money KM, Chandnani A, et al. Mycobacterium abscessus meningitis associated with stem cell treatment during medical tourism. Emerg Infect Dis 2023;29:1655–8. 10.3201/eid2908.230317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davidson RM, Hasan NA, Epperson LE, et al. Population genomics of Mycobacterium abscessus from U.S. cystic fibrosis care centers. Ann Am Thorac Soc 2021;18:1960–9. 10.1513/AnnalsATS.202009-1214OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartnett KP, Powell KM, Rankin D, et al. Investigation of bacterial infections among patients treated with umbilical cord blood-derived products marketed as stem cell therapies. JAMA Netw Open 2021;4:e2128615. 10.1001/jamanetworkopen.2021.28615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC. Travelers’ health. Medical tourism: travel to another country for medical care. Atlanta, GA: US Department of Health and Human Services, CDC; 2023. https://wwwnc.cdc.gov/travel/page/medical-tourism


