Abstract
Purpose of Review
This study is to highlight the incidence of corneal pseudomicrocysts in FDA-approved antibody–drug conjugates (ADCs), and success of preventive therapies for pseudomicrocysts and related ocular surface adverse events (AEs).
Recent Findings
ADCs are an emerging class of selective cancer therapies that consist of a potent cytotoxin connected to a monoclonal antibody (mAb) that targets antigens expressed on malignant cells. Currently, there are 11 FDA-approved ADCs with over 164 in clinical trials. Various AEs have been attributed to ADCs, including ocular surface AEs (keratitis/keratopathy, dry eye, conjunctivitis, blurred vision, corneal pseudomicrocysts). While the severity and prevalence of ADC-induced ocular surface AEs are well reported, the reporting of corneal pseudomicrocysts is limited, complicating the development of therapies to prevent or treat ADC-related ocular surface toxicity.
Summary
Three of 11 FDA-approved ADCs have been implicated with corneal pseudomicrocysts, with incidence ranging from 41 to 100% of patients. Of the six ADCs that reported ocular surface AEs, only three had ocular substudies to investigate the benefit of preventive therapies including topical steroids, vasoconstrictors, and preservative-free lubricants. Current preventive therapies demonstrate limited efficacy at mitigating pseudomicrocysts and other ocular surface AEs.
Keywords: Antibody–drug conjugates, Corneal pseudomicrocysts, Microcyst-like epithelial changes, Ocular surface adverse events, Ocular surface epithelium, Cornea, Conjunctiva
Introduction
Antibody–drug conjugates (ADCs) comprise a growing category of targeted cancer therapy [1••, 2••]. The first ADC was approved in 2000 for acute myeloid leukemia (gemtuzumab ozogamicin, Mylotarg) [3]. Today, there are 11 FDA-approved ADCs on the market designed to treat hematological malignancies and solid tumors, with an additional 164 in the clinical trial pipeline [4–7]. In concept, ADCs offer increased selectivity towards cancer cells while minimizing the systemic and off-target toxicities accompanied by traditional chemotherapies [8, 9]. Despite the designed selectivity of ADCs, adverse events (AEs) are still common in various tissues, including the eye [9]. The most prevalent forms of ADC-related ocular surface AEs are keratitis/keratopathy, dry eye, conjunctivitis, blurred vision, and corneal pseudomicrocysts, previously known as microcyst-like epithelial changes (MECs) [10]. This review focuses on the prevalence and medical management of corneal pseudomicrocysts in addition to other ocular surface AEs.
An ADC is composed of a monoclonal antibody (mAb) that is fused to a highly potent cytotoxic payload by a chemical linker (Fig. 1). mAbs bind to an antigen expressed on tumor cells enabling the efficient delivery of the cytotoxic payload to the tumor [11]. Each component of an ADC has the potential to play a role in toxicity [11–13].
Fig. 1.
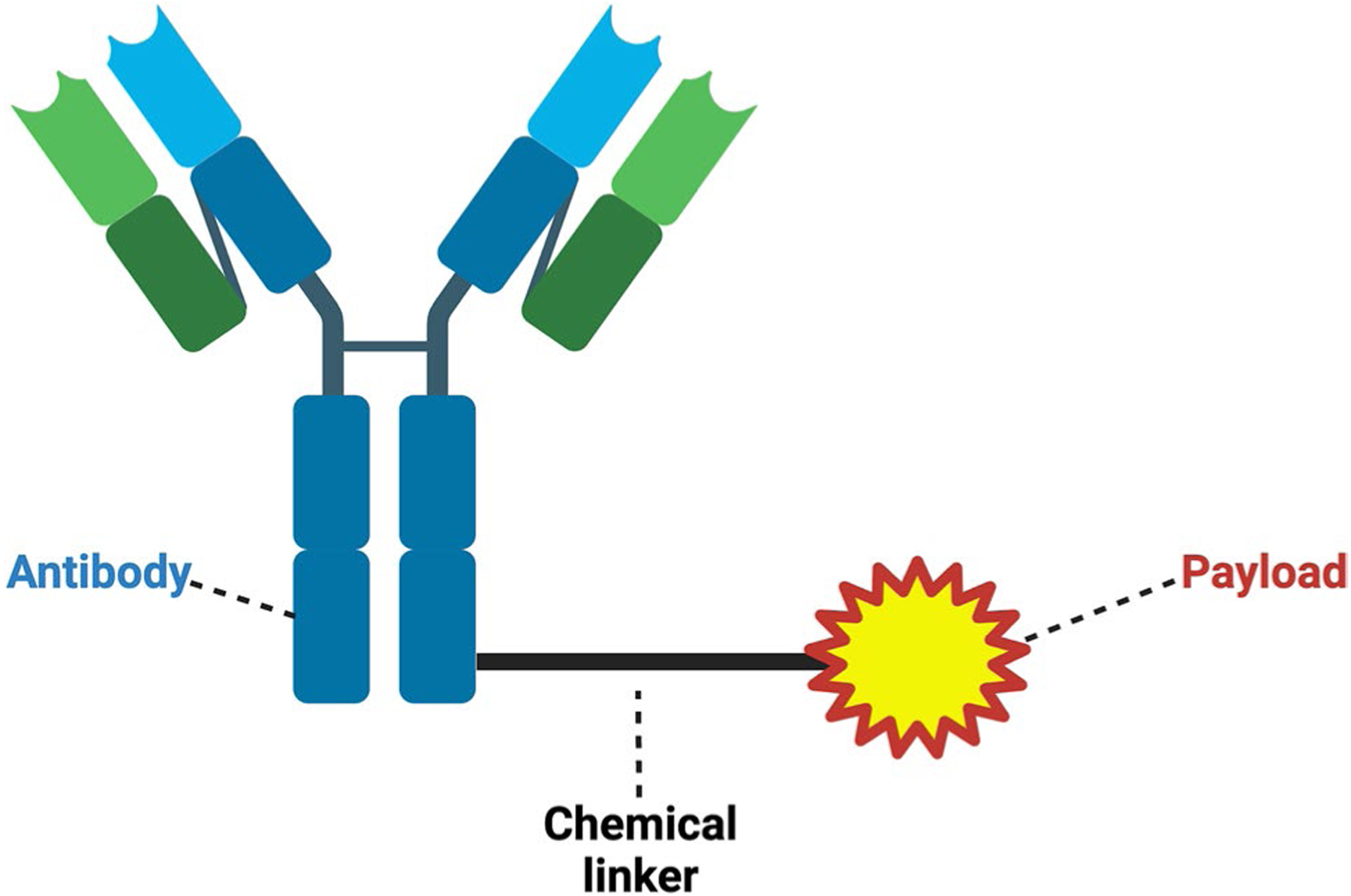
Structure of ADCs. There are three well-defined components: antibody, chemical linker, and cytotoxic payload. Of the current FDA-approved ADCs, all have IgG1 or IgG4 mAbs. Payloads, which either damage DNA or disrupt microtubule formation during mitosis, are linked to the mAb via a chemical linker, which are defined as cleavable or non-cleavable. Created with BioRender.com
ADC treatment is typically administered every 1 to 4 weeks via 30-min intravenous infusion [14•, 15]. The success of ADCs can be hindered by resistance to ADCs or severe systemic AEs across the body that necessitates discontinuation of therapy [1••]. Resistance to ADCs may be related to the rate of ADC internalization, changes to antigen expression on the target cell, or resistance to the payload [16].
Ocular surface AEs are clinically relevant due to their ability to interrupt ADC treatment. One study evaluating the safety profile of belantamab mafodotin (Blenrep) found that 72% of patients developed corneal pseudomicrocysts. These pseudomicrocysts caused dose delays in 47% of patients and dose reductions in 25%. Corneal pseudomicrocysts necessitated that 3% of patients discontinue ADC infusions [17••]. These dose modifications highlight the importance of corneal pseudomicrocyst mitigation.
Corneal Pseudomicrocysts
Corneal pseudomicrocysts are microcyst-like structures located in the corneal epithelium’s basal layer [10]. Corneal histology suggests that these represent intracytoplastic inclusions within pre-apoptotic or apoptotic epithelial cells [18]. Corneal pseudomicrocysts initially emerge bilaterally around the limbus in a ring-like pattern and migrate toward the central cornea with subsequent drug infusions (Fig. 2) [10, 17••, 19, 20•]. Anterior segment optical coherence tomography (AS-OCT) and in vivo confocal microscopy (IVCM) can identify these structures as hyperreflective tiny circles. Notably, true cystic structures would be hyporeflective [20•, 21]. Patients with corneal pseudomicrocysts commonly report symptoms such as blurred vision, dry eye, irritation, tearing, and photophobia. However, some patients with corneal pseudomicrocysts can be asymptomatic [17••, 20•]. Peripherally located corneal pseudomicrocysts cause relative central corneal flattening and induce a hyperopic shift, while centrally located cysts cause relative peripheral flattening, leading to a myopic shift [22•].
Fig. 2.
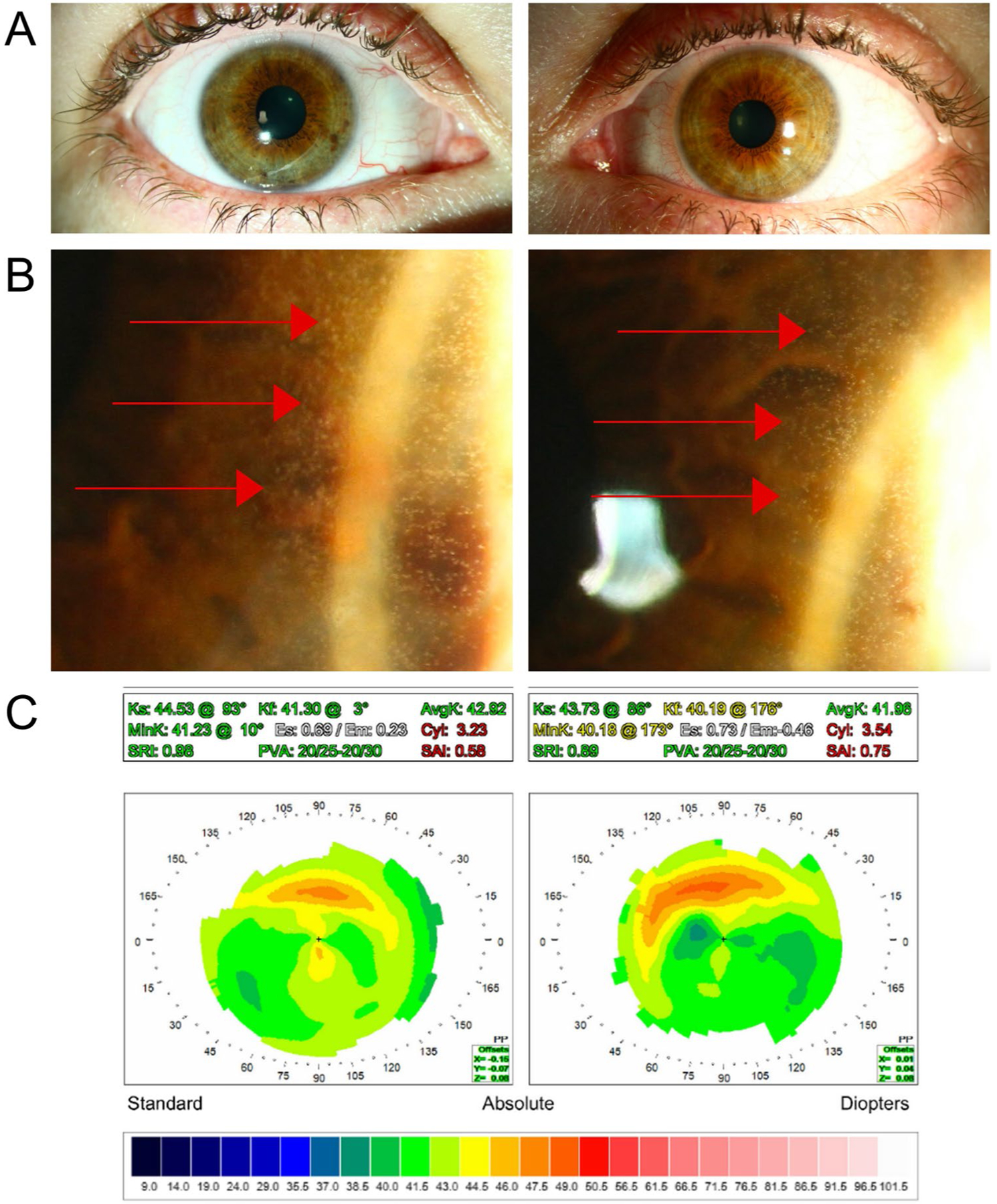
Manifestation of corneal pseudomicrocysts. A Examination of the ocular surface with diffuse illumination appears normal. B High magnification sclerotic scatter show numerous peripheral corneal pseudomicrocysts. C Corneal topography demonstrates peripheral ring-like steepening corresponding to the area of corneal pseudomicrocysts
Patients receiving ADC treatment may develop corneal pseudomicrocysts as early as 3 weeks after the start of infusions [22•, 23]. These corneal changes are reversible upon the discontinuation of treatment within a matter of weeks to months (2–32 weeks) [17••, 21, 22•, 24–26]. In our experience, some patients take up to 9 months for complete resolution of corneal pseudomicrocysts. However, resumption of ADC treatment can cause a rapid reappearance of corneal pseudomicrocysts that may take longer to resolve after ADC treatment stops [19]. The success of topical steroids, vasoconstrictors, and preservative-free artificial tears (PF-ATs) as prophylaxis or treatment for corneal pseudomicrocysts varies [17••, 27•, 28]. Dose reductions, delays, or discontinuations are currently the only known efficacious strategy for mitigation [2••, 10, 27•, 29, 30].
Literature Review
Corneal pseudomicrocysts is the accepted current terminology for the cyst-like corneal changes induced by ADCs. However, the nomenclature for corneal pseudomicrocysts is inconsistent in the literature. This review specifically evaluates corneal pseudomicrocysts reported in the literature and summarizes preventive therapies. Publications were searched for on two databases: PubMed and Drugs@ FDA. Search terms included individual FDA-approved ADC names (including belantamab mafodotin) and various terms used interchangeably to characterize corneal pseudomicrocysts. These terms include the following: pseudomicrocysts, microcyst-like epithelial changes (MECs), microcyst epithelial keratopathies (MEKs), cornea cysts, corneal epithelial deposits, corneal epithelial microcysts, keratopathy, microcyst corneal lesions, and intra-epithelial opacities.
Results
Table 1 summarizes the incidence and prevalence of the ocular surface AEs associated with ADC treatment. There are 12 ADCs listed, 11 of which are FDA-approved and used in clinical practice as of January 2024. Additional ADCs have received FDA-approval but have been removed from distribution for safety or economic reasons. Belantamab mafodotin (Blenrep) was previously FDA-approved but then withdrawn from the US market after failing to meet certain FDA requirements during phase III trials. Of the various ocular surface AEs, a special focus is given to corneal pseudomicrocysts. Three (25%) of the ADCs listed in Table 1 induced corneal pseudomicrocysts, and six (50%) were reported to induce other ocular surface AEs, of which dry eye was the most commonly reported (6, 100%). Keratopathy/keratitis was the second most common ocular surface AE (3, 50%) followed by conjunctivitis (2, 33%). Of the six ADCs with ocular surface AEs, only three (belantamab mafodotin, mirvetuximab soravtansine, tisotumab vedotin) had ocular substudies that evaluated the efficacy of preventive therapies for ocular surface AEs. Of these substudies, only one demonstrated that topical steroids could mitigate ADC-induced corneal pseudomicrocysts. Another found that a regimen of vasoconstrictors, topical steroids, and PF-ATs reduced the rate of conjunctivitis and ocular AEs.
Table 1.
Ocular Surface Toxicities in FDA-Approved Antibody–Drug-Conjugates (ADCs)
| Drug Name | Indication | Antibody (Ocular Surface Expression) | Payload (Mechanism) | Prevalence of corneal pseudomicrocysts | Other Ocular Surface AEs | Ocular Surface Preventive Therapies | Efficacy of Ocular Surface Preventive Therapies | References |
|---|---|---|---|---|---|---|---|---|
| Belantamab mafodotin (Blenrep)* | Relapsed or refractory multiple myeloma | BCMA (Not expressed) | MMAF (Microtubule disruption) | 68–78% |
|
|
|
[17••, 23, 26, 30, 33, 44–46] |
| Mirvetuximab soravtansine (Elahere) | Epithelial ovarian, fallopian tube, or primary peritoneal cancer | FRα (Conjunctiva) | DM4 (Microtubule disruption) | 41–100% |
|
|
|
[28,33,38,46,47] |
| Trastuzumab emtansine (Kadcyla) | HER2 + breast cancer | HER2 (Cornea & conjunctiva) | DM1 (Microtubule disruption) | 100% |
|
Not reported | Not reported | [33, 46, 48, 49] |
| Tisotumab vedotin (Tivdak) | Recurrent or metastatic cervical cancer | Tissue factor (Cornea & conjunctiva) | MMAE (Microtubule disruption) | Not reported |
|
|
|
[14•, 33, 46, 50–52] |
| Enfortumab vedotin (Padcev) | Urothelial bladder cancer | Nectin4 (Cornea & conjunctiva) | MMAE (Microtubule disruption) | Not reported |
|
|
Not reported | [8, 33, 46, 53] |
| Trastuzumab deruxtecan (Enhertu) | HER2 + breast cancer | HER2 (Cornea & conjunctiva) | Camptothecin (DNA damage) | Not reported |
|
Not reported | Not reported | [33, 46, 54, 55] |
| Gemtuzumab ozogamicin (Mylotarg) | Newly diagnosed, relapsed, or refractory acute myeloid leukemia | CD33 (Conjunctiva) | Calicheamicin (DNA damage) | NA | NA | NA | NA | [33, 46, 56] |
| Brentuximab vedotin (Adcetris) | Lymphoma | CD30 (Not expressed) | MMAE (Microtubule disruption) | NA | NA | NA | NA | [33, 46, 57] |
| Inotuzumab ozogamicin (Besponsa) | Relapsed or refractory B cell acute lymphoblastic leukemia | CD22 (Conjunctiva) | Calicheamicin (DNA damage) | NA | NA | NA | NA | [33, 46, 58] |
| Polatuzumab vedotin (Polivy) | Relapsed or refractory diffuse large B cell lymphoma | CD79b (Conjunctiva) | MMAE (Microtubule disruption) | NA | NA | NA | NA | [33, 46, 59] |
| Sacituzumab govitecan (Trodelvy) | Metastatic triplenegative breast cancer; urothelial bladder cancer | TROP2 (Cornea & conjunctiva) | Camptothecin (DNA damage) | NA | NA | NA | NA | [33, 46, 60] |
| Loncastuximab tesirine (Zynlonta) | Large B cell lymphoma | CD19 (Cornea) | PBD dimer (DNA damage) | NA | NA | NA | NA | (33, 46, 61) |
Withdrawn from US market after failure to meet FDA requirements from DREAMM-3 phase III confirmatory trial
Mechanism of ADC-Induced Ocular Toxicity
Characteristics unique to both the ocular surface (cornea and conjunctiva) and ADCs are suspected to play a role in toxicity of ADCs [31, 32]. The ocular surface is likely susceptible to ADC-related toxicity due to a rapidly regenerating population of limbal stem cells, diversity of cell surface receptors, and rich blood supply [10, 11]. ADCs’ structure may contribute to toxicity due to linker instability or expression of the target antigen in ocular tissues [11]. Notably, of the three ADCs that induce corneal pseudomicrocysts, one has a target antigen that is expressed in both the cornea and conjunctiva (HER2), one has a target antigen expressed solely in the conjunctiva (FRα), and one has a target antigen that is not expressed on the ocular surface (BCMA) [33].
Off-target toxicity of ADCs on the ocular surface can be attributed to various mechanisms (Fig. 3). Macropinocytosis, a form of non-specific endocytosis known as “cell drinking,” facilitates the internalization of ADCs or the deconjugated payload [10, 31]. Fc and C-type lectin receptors enable endocytosis in a receptor specific manner [10, 31]. Internalization of an ADC in normal tissue can lead to the premature release of the cytotoxin due to linker instability or linker degradation by normal metabolism in the cell [23]. Additionally, the payload may passively diffuse into a cell due to non-charged, membrane permeable residues on the payload [34]. Another mechanism is the “bystander effect” where after an ADC is internalized and degraded by a cell, a fraction of the payload can release from the dead cell into the extracellular space and kill neighboring cells, regardless of their target antigen expression (35).
Fig. 3.
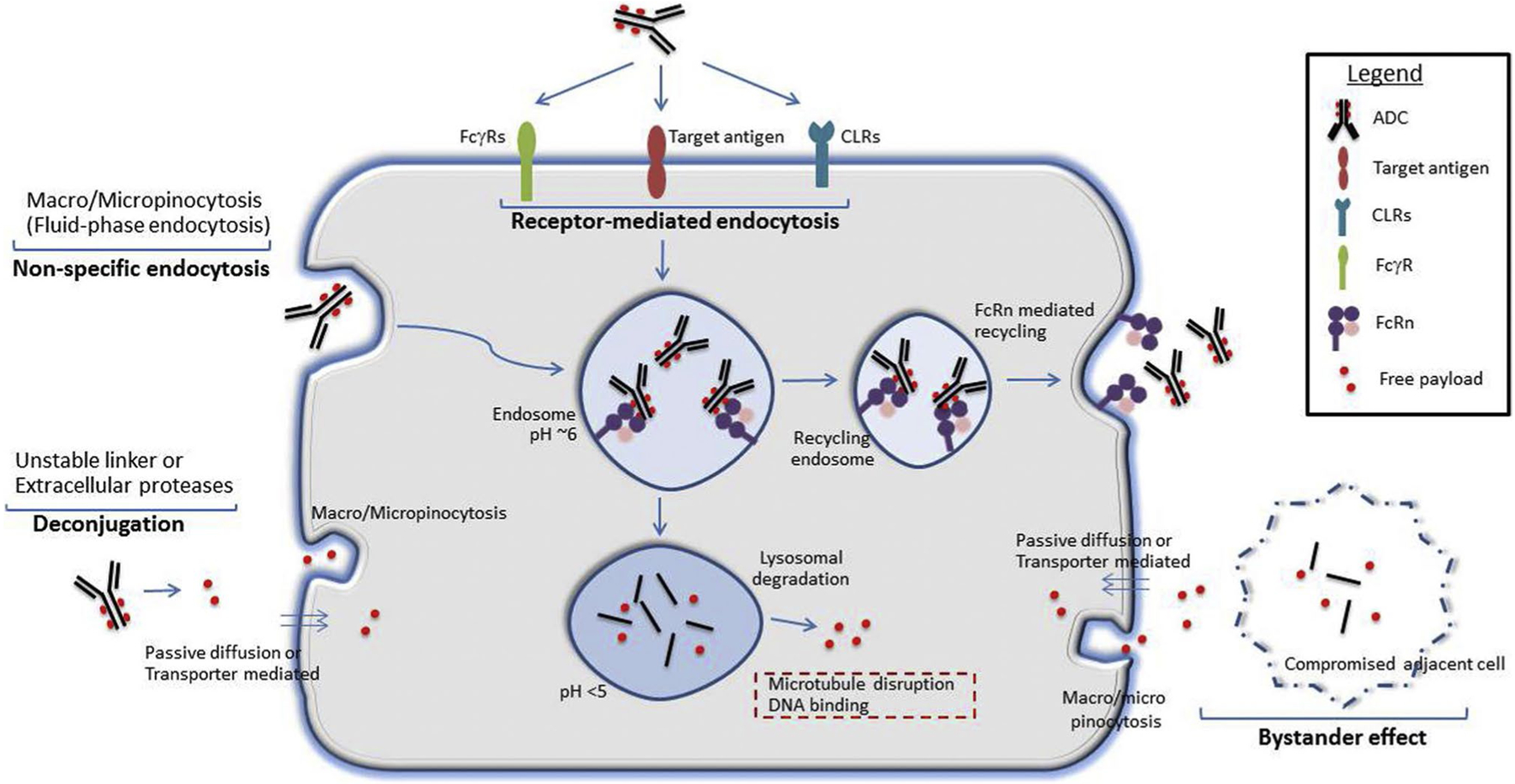
Potential mechanisms for ADC-induced ocular surface toxicity (reprinted with permission from Elsevier, originally published in Mahalingaiah et al. Potential mechanisms of target-independent uptake and toxicity of antibody–drug conjugates. Pharmacol Ther. 2019)
Drug-antibody ratio (DAR), or drug loading, is another significant player in ADC toxicity. Each antibody is attached to a certain number of payloads; FDA-approved ADCs range between DAR 2 and 8 [1••]. Mouse studies have demonstrated that a higher DAR has a lower therapeutic index [36]. In monkey studies, a higher DAR was associated with an earlier onset and a higher incidence of corneal toxicity [37•].
Novel Preventive Therapies
Several preventive therapies have been investigated to mitigate ADC-induced corneal toxicity to varying success.
Topical steroid eye drops have been used with various ADCs because they are hypothesized to slow down limbal stem cell regeneration and, in theory, make the cornea less susceptible to toxicity [28]. Outcomes from steroid eye drops have ranged from no benefit to complete clearance of corneal pseudomicrocysts [28, 30, 38]. Variation in response to steroids can likely be attributed to biological variability in the patient population and differing mechanisms of toxicity [2••]. In our experience, topical steroid eye drops do not address the underlying corneal pseudomicrocysts but may alleviate some eye pain symptoms. Vasoconstricting eye drops and cold compresses were used to mitigate ocular AEs during infusions of Tisotumab vedotin (Tivdak) by reducing drug uptake in the cornea. Although vasoconstrictors reduced the rate of conjunctivitis, their effect on corneal pseudomicrocysts has not been reported [39]. An ocular substudy on the ADC depatuxizumab mafodotin (ABT-414), an EGFR mAb conjugated to the tubulin inhibitor monomethyl auristatin F (MMAF) via a stable maleimidocaproyl link, found no difference in the prevalence of ocular surface AEs in eyes treated with topical steroids with and without vasoconstrictors. This study also evaluated the use of a bandage contact lens for ocular surface AEs and observed no protective benefit, with nearly half the patients progressing to develop grade 3 ocular surface AEs [40]. PF-ATs can provide relief for certain ocular surface AEs, such as dry eye, but have no preventive effect for the pseudomicrocysts [2••]. Therapies like antihistamines are used to alleviate ocular AEs such as conjunctivitis, but no data has been reported on their ability to mitigate corneal pseudomicrocysts [20•].
Zhao et al. [41] investigated macropinocytosis as a potential culprit of ADC-induced ocular toxicity. Specifically, they studied cell proliferation in human corneal epithelial cell (HCEC) culture exposed to modified versions of ADCs and macropinocytosis inhibitors. ADCs were modified to alter positive charges and hydrophobic residues by attaching polyethylene glycol, polyglutamate residues, or by mutating amino acids on the ADC. In a separate experiment, cells were exposed to unmodified ADCs with and without macropinocytosis inhibitors. In both conditions, cell viability of HCECs increased when treated with macropinocytosis inhibitors or modified ADCs.
Calm Water Therapeutics (Rochester, NY) demonstrated in a pilot study the ability of polylysine-graft-polyethylene glycol (PLL-g-PEG) to inhibit the uptake of ADCs by HCECs in vitro. Specifically, PLL-g-PEG dose dependently decreased ADC uptake in HCECs exposed to rituximab-MMAF, an ADC in development. PLL-g-PEG is proposed to create electrostatic interference between ADCs and off-target cell receptors to mitigate ocular surface toxicities [42]. Future studies are needed to investigate PLL-g-PEG in patients.
Loberg et al. [37•] assessed the corneal toxicity in monkeys caused by depatuxizumab mafodotin (ABT-414). They administered depatuxizumab, the anti-EGFR mAb that is the antibody component of the ADC, systemically and topically via eye drops. Their rationale was to saturate the binding of depatuxizumab to EGFR to inhibit the ADC (Depatuxizumab mafodotin) binding EGFR expressed in the cornea. They also investigated the success of topical preventive therapies, such as vasoconstrictors, tear stimulants/anti-inflammatories, and lubrication with antioxidants, none of which were successful.
Kreps et al. [43] reported the use of 20% autologous serum tear eye drops 6 times daily in one patient being treated with trastuzumab emtansine (Kadcyla) who had developed corneal pseudomicrocysts. Despite ongoing ADC treatment for 14 months, the patient had no worsening of the corneal pseudomicrocysts while using 20% autologous serum tear eye drops.
In our experience, scleral lenses offer an effective solution to improve the eye pain and blurry vision associated with corneal pseudomicrocysts. However, scleral lenses are logistically challenging to utilize given the significant time needed for lens fitting, steep learning curve to place and remove the lenses, and high out-of-pocket expense to patients.
Conclusion
ADCs are a promising and increasingly utilized targeted cancer therapy that are associated with corneal pseudomicrocysts and various ocular surface AEs. As a result of this increasing popularity of ADCs, there is a need to standardize the reporting and treatment of corneal pseudomicrocysts induced by ADCs. Current preventive therapies for corneal pseudomicrocysts (e.g., topical steroids, vasoconstrictors, PF-ATs) have limited efficacy, but corneal pseudomicrocysts are reversible with modification of ADC therapy, including dose delay, dose reduction, and drug cessation. There is a need for unambiguous ocular surface AE grading scales to ensure accurate and timely detection of ADC-related AEs. This will facilitate clear communication between eye care providers and oncologists to prevent and mitigate ocular surface toxicity through ADC dose modifications. Future research on ADCs is needed to elucidate the mechanisms of ocular surface toxicity and to explore novel therapeutic approaches.
Funding
This study was supported by K08 EY033859 from the National Eye Institute, Career Development Award from the Research to Prevent Blindness, and grant from the All May See Foundation to Dr. Pasricha. This work was made possible in part by the UCSF Vision Core Grant (P30 EY002162) and Research to Prevent Blindness Unrestricted Grant to the University of California San Francisco, Department of Ophthalmology.
Footnotes
Conflict of Interest N.D.P. serves on the US Medical Keratopathy Advisory Board for Sanofi.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.••.Dumontet C, Reichert JM, Senter PD, Lambert JM, Beck A. Antibody-drug conjugates come of age in oncology. Nat Rev Drug Discov. 2023;22(8):641–61. [DOI] [PubMed] [Google Scholar]; Reviews the role of each ADC component in ocular surface toxicity.
- 2.••.Jaffry M, Choudhry H, Aftab OM, Dastjerdi MH. Antibody-drug conjugates and ocular toxicity. J Ocul Pharmacol Ther. 2023;39(10):675–91. [DOI] [PubMed] [Google Scholar]; Highlights the potential mechanisms of ADC ocular surface toxicity and preventive therapies for FDA-approved ADCs as well as those in development.
- 3.Naito K, Takeshita A, Shigeno K, Nakamura S, Fujisawa S, Shinjo K, et al. Calicheamicin-conjugated humanized anti-CD33 monoclonal antibody (gemtuzumab zogamicin, CMA-676) shows cytocidal effect on CD33-positive leukemia cell lines, but is inactive on P-glycoprotein-expressing sublines. Leukemia. 2000;14(8):1436–43. [DOI] [PubMed] [Google Scholar]
- 4.Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Trans-duct Target Ther. 2022;7(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gogia P, Ashraf H, Bhasin S, Xu Y. Antibody-drug conjugates: a review of approved drugs and their clinical level of evidence. Cancers (Basel). 2023;15(15)3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maecker H, Jonnalagadda V, Bhakta S, Jammalamadaka V, Junutula JR. Exploration of the antibody-drug conjugate clinical landscape. MAbs. 2023;15(1):2229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maiti R, Patel B, Patel N, Patel M, Patel A, Dhanesha N. Antibody drug conjugates as targeted cancer therapy: past development, present challenges and future opportunities. Arch Pharm Res. 2023;46(5):361–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raheem F, Alsuhebany N, Hickey Zacholski E, Paulic N, Sandler A, Uk N, et al. Ocular toxicities associated with antibody drug conjugates and immunotherapy in oncology: clinical presentation, pathogenesis, and management strategies. Expert Opin Drug Saf. 2023;22(10):921–8. [DOI] [PubMed] [Google Scholar]
- 9.Zhou L, Wei X. Ocular immune-related adverse events associated with immune checkpoint inhibitors in lung cancer. Front Immunol. 2021;12:701951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eaton JS, Miller PE, Mannis MJ, Murphy CJ. Ocular adverse events associated with antibody-drug conjugates in human clinical trials. J Ocul Pharmacol Ther. 2015;31(10):589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donaghy H Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibody-drug conjugates. MAbs. 2016;8(4):659–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beck A, Goetsch L, Dumontet C, Corvaïa N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16(5):315–37. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Burris H, Wang JS, Barroilhet L, Gutierrez M, Wang Y, et al. An open-label phase I dose-escalation study of the safety and pharmacokinetics of DMUC4064A in patients with platinum-resistant ovarian cancer. Gynecol Oncol. 2021;163(3):473–80. [DOI] [PubMed] [Google Scholar]
- 14.•.Kim SK, Ursell P, Coleman RL, Monk BJ, Vergote I. Mitigation and management strategies for ocular events associated with tisotumab vedotin. Gynecol Oncol. 2022;165(2):385–92. [DOI] [PubMed] [Google Scholar]; Comprehenisve overview of Tisotumab vedotin (Tivdak) ocular AEs, preventive therapies, and their outcomes.
- 15.Liao MZ, Lu D, Kagedal M, Miles D, Samineni D, Liu SN, et al. Model-informed therapeutic dose optimization strategies for antibody-drug conjugates in oncology: what can we learn from us food and drug administration-approved antibody-drug conjugates? Clin Pharmacol Ther. 2021;110(5):1216–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shastry M, Gupta A, Chandarlapaty S, Young M, Powles T, Hamilton E. Rise of antibody-drug conjugates: the present and future. Am Soc Clin Oncol Educ Book. 2023;43:e390094. [DOI] [PubMed] [Google Scholar]
- 17.••.Farooq AV, DegliEsposti S, Popat R, Thulasi P, Lonial S, Nooka AK, et al. Corneal epithelial findings in patients with multiple myeloma treated with antibody-drug conjugate belantamab mafodotin in the pivotal, randomized, DREAMM-2 study. Ophthalmol Ther. 2020;9(4):889–911. [DOI] [PMC free article] [PubMed] [Google Scholar]; Analyzes findings of the Belantamab mafodotin (Blenrep) ocular substudy.
- 18.Lee BA, Lee MS, Maltry AC, Hou JH. Clinical and histological characterization of toxic keratopathy from depatuxizumab mafodotin (ABT-414), an antibody-drug conjugate. Cornea. 2021;40(9):1197–200. [DOI] [PubMed] [Google Scholar]
- 19.Bausell RB, Soleimani A, Vinnett A, Baroni MD, Staub SA, Binion K, et al. Corneal changes after belantamab mafodotin in multiple myeloma patients. Eye contact lens. 2021;47(6):362–5. [DOI] [PubMed] [Google Scholar]
- 20.•.Mickevicius T, Pink AE, Bhogal M, O’Brart D, Robbie SJ. Dupilumab-induced, tralokinumab-induced, and belantamab mafodotin-induced adverse ocular events-incidence, etiology, and management. Cornea. 2023;42(4):507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reviews the methods for detection of corneal pseudomicrocysts, their clinical presentation, and potential preventive therapies for Belantamab mafodotin (Blenrep).
- 21.Rousseau A, Michot JM, Labetoulle M. Belantamab mafotodin-induced epithelial keratopathy masquerading myopic surgery. Ophthalmology. 2020;127(12):1626. [DOI] [PubMed] [Google Scholar]
- 22.•.Canestraro J, Hultcrantz M, Modi S, Hamlin PA, Shoushtari AN, Konner JA, et al. Refractive shifts and changes in corneal curvature associated with antibody-drug conjugates. Cornea. 2022;41(6):792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]; Investigates the progression of refractive changes that occur with ADC treatment.
- 23.Wahab A, Rafae A, Mushtaq K, Masood A, Ehsan H, Khakwani M, et al. Ocular toxicity of belantamab mafodotin, an oncological perspective of management in relapsed and refractory multiple myeloma. Front Oncol. 2021;11:678634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marquant K, Quinquenel A, Arndt C, Denoyer A. Corneal in vivo confocal microscopy to detect belantamab mafodotin-induced ocular toxicity early and adjust the dose accordingly: a case report. J Hematol Oncol. 2021;14(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumiya W, Karaca I, Ghoraba H, Akhavanrezayat A, Mobasserian A, Hassan M, et al. Structural changes of corneal epithelium in belantamab-associated superficial keratopathy using anterior segment optical coherence tomography. Am J Ophthalmol Case Rep. 2021;23:101133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trudel S, Lendvai N, Popat R, Voorhees PM, Reeves B, Libby EN, et al. Antibody-drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: an update on safety and efficacy from dose expansion phase I study. Blood Cancer J. 2019;9(4):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.•.Hendershot A, Slabaugh M, Riaz KM, Moore KN, O’Malley DM, Matulonis U, et al. Strategies for prevention and management of ocular events occurring with mirvetuximab soravtansine. Gynecol Oncol Rep. 2023;47:101155. [DOI] [PMC free article] [PubMed] [Google Scholar]; Analyzes the Mirvetuximab soravtansine (Elahere) ocular substudy.
- 28.Matulonis UA, Birrer MJ, O’Malley DM, Moore KN, Konner J, Gilbert L, et al. Evaluation of prophylactic corticosteroid eye drop use in the management of corneal abnormalities induced by the antibody-drug conjugate mirvetuximab soravtansine. Clin Cancer Res. 2019;25(6):1727–36. [DOI] [PubMed] [Google Scholar]
- 29.Gan HK, Reardon DA, Lassman AB, Merrell R, van den Bent M, Butowski N, et al. Safety, pharmacokinetics, and antitumor response of depatuxizumab mafodotin as monotherapy or in combination with temozolomide in patients with glioblastoma. Neuro Oncol. 2018;20(6):838–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lonial S, Lee HC, Badros A, Trudel S, Nooka AK, Chari A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21(2):207–21. [DOI] [PubMed] [Google Scholar]
- 31.Mahalingaiah PK, Ciurlionis R, Durbin KR, Yeager RL, Philip BK, Bawa B, et al. Potential mechanisms of target-independent uptake and toxicity of antibody-drug conjugates. Pharmacol Ther. 2019;200:110–25. [DOI] [PubMed] [Google Scholar]
- 32.Raizman MB, Hamrah P, Holland EJ, Kim T, Mah FS, Rapuano CJ, et al. Drug-induced corneal epithelial changes. Surv Ophthalmol. 2017;62(3):286–301. [DOI] [PubMed] [Google Scholar]
- 33.Wolf J, Boneva S, Schlecht A, Lapp T, Auw-Haedrich C, Lagreze W, et al. The Human Eye Transcriptome Atlas: a searchable comparative transcriptome database for healthy and diseased human eye tissue. Genomics. 2022;114(2):110286. [DOI] [PubMed] [Google Scholar]
- 34.Erickson H, Wilhelm S, Widdison W, et al. Evaluation of the cytotoxic potencies of the major maytansinoid metabolites of antibody maytansinoid conjugates detected in vitro and in preclinical mouse models. AACR Meeting Abstracts. 2008;2150. [Google Scholar]
- 35.Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer. 2017;117(12):1736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, Cerveny CG, et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res. 2004;10(20):7063–70. [DOI] [PubMed] [Google Scholar]
- 37.•.Loberg LI, Henriques TA, Johnson JK, Miller PE, Ralston SL. Characterization and potential mitigation of corneal effects in nonclinical toxicology studies in animals administered depatuxizumab mafodotin. J Ocul Pharmacol Ther. 2022;38(7):471–80. [DOI] [PubMed] [Google Scholar]; Animal study investigation of potential ADC-related ocular surface toxicity mechanisms and novel preventive therapies.
- 38.Corbelli E, Miserocchi E, Marchese A, Giuffrè C, Berchicci L, Sacconi R, et al. Ocular toxicity of mirvetuximab. Cornea. 2019;38(2):229–32. [DOI] [PubMed] [Google Scholar]
- 39.Arn CR, Halla KJ, Gill S. Tisotumab vedotin safety and tolerability in clinical practice: managing adverse events. J Adv Pract Oncol. 2023;14(2):139–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vize CJ, Kim SK, Matthews T, Macsai M, Merrell R, Hsu S, et al. A Phase 3b study for management of ocular side effects in patients with epidermal growth factor receptor-amplified glioblastoma receiving depatuxizumab mafodotin. Ophthalmic Res. 2023;66(1):1030–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao H, Atkinson J, Gulesserian S, Zeng Z, Nater J, Ou J, et al. Modulation of macropinocytosis-mediated internalization decreases ocular toxicity of antibody-drug conjugates. Cancer Res. 2018;78(8):2115–26. [DOI] [PubMed] [Google Scholar]
- 42.Kleinman D, Hakkarainen JJ, Ghosh AK, Ogle SD, Sill K, Mendelsohn BA, et al. PLL-g-PEG inhibits antibody-drug conjugate uptake into human corneal epithelial cells in vitro. Investigative Ophthalmology & Visual Science. 2022;63(7):3239 – A0274. [Google Scholar]
- 43.Kreps EO, Derveaux T, Denys H. Corneal changes in trastuzumab emtansine treatment. Clin Breast Cancer. 2018;18(4):e427–9. [DOI] [PubMed] [Google Scholar]
- 44.Blenrep [package insert]. Research Triangle Park (NC): Glaxo-SmithKline; 2020. [Google Scholar]
- 45.Popat R, Warcel D, O’Nions J, Cowley A, Smith S, Tucker WR, et al. Characterization of response and corneal events with extended follow-up after belantamab mafodotin (GSK2857916) monotherapy for patients with relapsed multiple myeloma: a case series from the first-time-in-human clinical trial. Haematologica. 2020;105(5):e261–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tong JTW, Harris PWR, Brimble MA, Kavianinia I. An insight into FDA approved antibody-drug conjugates for cancer therapy. Molecules. 2021;26(19):5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elahere [package insert]. Waltham (MA): ImmunoGen, Inc.; 2022. [Google Scholar]
- 48.Kadcyla [package insert]. South San Francisco (CA): Genentech, Inc.; 2019 [Google Scholar]
- 49.Deklerck E, Denys H, Kreps EO. Corneal features in trastuzumab emtansine treatment: not a rare occurrence. Breast Cancer Res Treat. 2019;175(2):525–30. [DOI] [PubMed] [Google Scholar]
- 50.de Bono JS, Concin N, Hong DS, Thistlethwaite FC, Machiels JP, Arkenau HT, et al. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): a first-in-human, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(3):383–93. [DOI] [PubMed] [Google Scholar]
- 51.Tivdak [package insert]. Bothell (WA): Seagen Inc.; 2021 [Google Scholar]
- 52.Coleman RL, Lorusso D, Gennigens C, Gonzalez-Martin A, Randall L, Cibula D, et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22(5):609–19. [DOI] [PubMed] [Google Scholar]
- 53.Padcev [package insert]. Northbrook (IL): Astellas Pharma US, Inc..; 2021 [Google Scholar]
- 54.Enhertu [package insert]. Basking Ridge (NJ): Dalichi Sanyo Inc.; 2019 [Google Scholar]
- 55.Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mylotarg [package insert]. Philadelphia (PA): Wyeth Pharmaceuticals Inc.; 2017 [Google Scholar]
- 57.Adcetris [package insert]. Bothell (WA): Seattle Genetics; 2014 [Google Scholar]
- 58.Besponsa [package insert]. Philadelphia (PA): Wyeth Pharmaceuticals Inc.; 2017 [Google Scholar]
- 59.Polivy [package insert]. South San Francisco (CA): Genentech, Inc.; 2019 [Google Scholar]
- 60.Trodelvy [package inserts]. Morris Plains (NJ): Immunomedics Inc.; 2020 [Google Scholar]
- 61.Zylonta [package insert]. Murray Hill (NJ): ADC Therapeutics America; 2021 [Google Scholar]


