Abstract
Objective
Integrating clinical research into routine clinical care workflows within electronic health record systems (EHRs) can be challenging, expensive, and labor-intensive. This case study presents a large-scale clinical research project conducted entirely within a commercial EHR during the COVID-19 pandemic.
Case Report
The UCSD and UCSDH COVID-19 NeutraliZing Antibody Project (ZAP) aimed to evaluate antibody levels to SARS-CoV-2 virus in a large population at an academic medical center and examine the association between antibody levels and subsequent infection diagnosis.
Results
The project rapidly and successfully enrolled and consented over 2000 participants, integrating the research trial with standing COVID-19 testing operations, staff, lab, and mobile applications. EHR-integration increased enrollment, ease of scheduling, survey distribution, and return of research results at a low cost by utilizing existing resources.
Conclusion
The case study highlights the potential benefits of EHR-integrated clinical research, expanding their reach across multiple health systems and facilitating rapid learning during a global health crisis.
Keywords: EHR-integration, clinical informatics, clinical research, interoperability, COVID-19 antibodies
Introduction
Integrating clinical research into routine clinical care workflows within the same electronic health system tends to be challenging, as well as expensive and labor intensive.1 Historically, clinical and research endeavors fundamentally differ in how encounters are managed, how data is collected and managed, and the degree of procedural rigor, including decisional flexibility at the point of care and revenue capture. In the past, this has led to the natural evolving development of separate clinical and research information systems, even though using a single system would most benefit the participant and the healthcare delivery system conducting the research. However, as healthcare embraces continuous improvement through learning healthcare systems (LHSs), clinical research and clinical care activities are being conducted in parallel to a greater extent and, in many cases, overlap. Now with a shared goal—learning from clinical interventions—health systems’ electronic health records (EHRs) are increasingly being utilized for research purposes, primarily for data aggregation, information exchange, and large clinical trials,1–4 though processes remain limited and often fragmented.5
The global COVID-19 pandemic created unprecedented challenges to health systems at large, and has forced creativity and flexibility around both learning and work environments. There is an immense amount of unknown information, and yet the speed at which new information is generated is rapid, in many instances outstripping our ability to analyze and interpret it. To adapt to this environment, utilizing a highly reliable LHS’s resources, particularly informatics resources, efficiently and effectively becomes vital.6–11 It is here where integration and alignment of research trial and clinical activities through the EHR are not only novel and invaluable, but also necessary.
The Agency for Healthcare Research and Quality (AHRQ) defines LHSs as those in which internal data and experience are systematically integrated with external evidence to facilitate data-driven intervention.6,12 A key characteristic of LHSs is the use of the “Deming Cycle,” a derivation of the scientific method.13 Contemporary EHRs are evolving to better support this cycle in LHSs. We posited that research that aligns with evidence-based evaluations commonly done in LHS could be conducted at scale with speed using a modern commercial EHR.
We present our experience leveraging an existing commercial EHR to conduct a large-scale clinical research project quickly during the COVID-19 pandemic. The aim of this specific project was to investigate population-level immunological response to COVID-19 by integrating clinical data on diagnostic testing and vaccination and research-based quantification of antibodies against multiple SARS-CoV-2 variants. Speed was of the essence during the pandemic, as it was important to understand the level of immunity in the population from both viral infection and vaccination, as well as the utility of the antibody assays utilized for the study. The primary goal of this case report is to share this novel experience of integrating research participant recruitment and follow-up within existing routine clinical workflows in the EHR.
Methods
The University of California San Diego (UCSD) and University of California San Diego Health (UCSDH) COVID-19 NeutraliZing Antibody Project (ZAP) is an ongoing project that began in January 2022 aiming to evaluate the immune response to SARS-CoV-2 virus in the population of a large academic institution, and examine whether antibody levels (from either vaccination or infection) are associated with subsequent diagnosis of SARS-CoV-2 infection. The project is open to all staff and students at UCSD and UCSDH, who were regularly testing for SARS-CoV-2 infection from October 2020 to July 2022 according to institutional and state guidelines. This project represented an LHS activity; however, quality improvement (QI) activities can sometimes involve risk (minimal in this case) and the protocol was submitted and approved by the Institutional Review Board. In addition, this report aligns with the Consolidation Framework for Implementation Research (CFIR).
Given the societal emphasis on social distancing during respective COVID-19 surges, recruitment for this project needed to be conducted primarily electronically—specific methods included emails that contained links and QR (Quick Response) codes to the scheduling platform, UCSD mobile application announcements, and flyers with QR codes that were posted in clinical and educational sites where staff and students had in-person activities. Participants self-scheduled research visits linked to their existing medical records using the patient-facing portal for the UCSDH EHR (Epic MyChart, Verona, WI). As a result of the EHR integration of the scheduling platform, participants had the ability to complete an “eCheck-in” process in Epic MyChart prior to their study visits, which included a study questionnaire and electronic informed study consent14 and HIPAA (Health Insurance Portability and Accountability Act) authorization (eConsent) in addition to relevant ambulatory items as necessary. This eCheck-in process could be completed remotely from any computer or mobile device with internet access.
Operationally, the in-person fingerstick blood spot testing was primarily integrated into existing vaccination and/or COVID-19 testing sites at UCSD and UCSDH. At the testing sites, participants utilize their UCSD mobile application scanner to capture barcodes on the blood spot collection cards (the same method used for on-site COVID-19 staff self-testing), which sends linked barcode/participant identification pairings to the lab performing the serology and neutralizing antibody testing. A secure laboratory information system (LIS) accessions and tracks the study samples, and intakes and stores serology and neutralizing antibody results, which are then returned securely to participants back in the patient-facing EHR, along with a link to the ZAP project website, which contains additional information for contextualizing individual results.
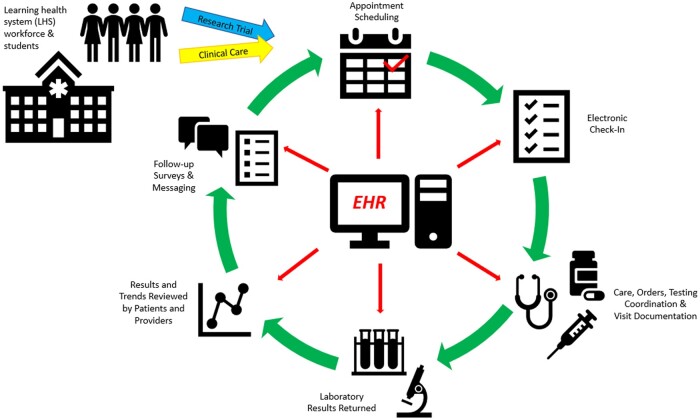
Participants are sent follow-up study questionnaires through the EHR patient portal 30 and 90 days after their initial study visit, which can be completed on the MyChart mobile or desktop applications. Participants are also invited for return visits to track results longitudinally. Given the project’s full integration with the EHR, individual and group reports are able to be pulled directly from the EHR’s data warehouse and analyzed using the research team’s analytic programs of choice (Figure 1).
Figure 1.
Alignment of clinical care and research trials through the electronic health record (EHR) at a learning healthcare system.
Results
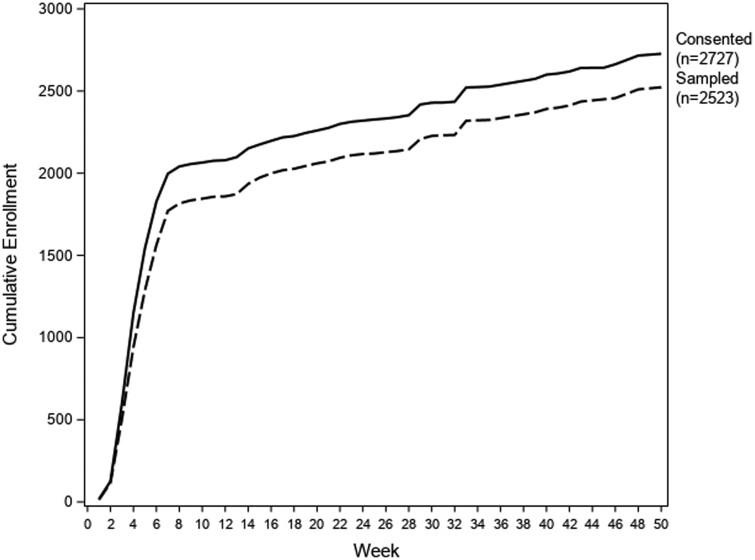
By the end of 2022, over 2500 UCSD and UCSDH staff and students had participated in the project. There were 2523 initial visits and 652 repeat visits (participants were allowed to present for multiple research visits). Figure 2 shows cumulative consent and sample collections over time; we note that intensive recruitment was focused on the first 2 months of the study period. Participant demographics are outlined in Table 1. More than 90% of those who enrolled and consented for the study completed at least one visit, including completion of the pre-visit questionnaire and submission of a fingerstick blood sample. Follow-up survey response rate was 70.1% at 30-day post initial visit, and 48.5% at 90-day post initial visit.
Figure 2.
ZAP enrollment over time. Solid line representing participants who consented to the study, and dotted line representing participants who consented and from whom samples were collected and recorded.
Table 1.
ZAP participant demographics.
| Participant information (consented N = 2727) | ||
|---|---|---|
| Demographics | ||
| Age (years) | ||
| Mean (SD) | 38.3 | 12.8 |
| Median (Min, Max) | 37 | 18 80 |
| Sex (n, %) | ||
| Male | 867 | 31.8% |
| Female | 1852 | 67.9% |
| Unknown | 8 | 0.3% |
| Ethnicity (n, %) | ||
| Hispanic | 473 | 17.4% |
| Non-Hispanic | 2095 | 76.8% |
| Unknown | 159 | 5.8% |
| Race (n, %) | ||
| White | 1503 | 55.1% |
| Black | 65 | 2.4% |
| Asian | 703 | 25.8% |
| Native American/Alaska Native | 17 | 0.6% |
| Native Hawaiian/Pacific Islander | 32 | 1.2% |
| Other/Mixed | 283 | 10.4% |
| Unknown | 124 | 4.5% |
|
| ||
| Prior exposure status | ||
|
| ||
| Vaccinated, full (n, %)a | 2678 | 98.2% |
|
| ||
| Study visits | ||
|
| ||
| Initial visitsb | 2523 | 92.5% |
| Repeat visits (total) | 652 | – |
| Unique follow-up visitsc | 601 | 22.0% |
|
| ||
| Survey rates | ||
|
| ||
| Baseline | 2583 | 94.7% |
| 30 days | 1912 | 70.1% |
| 90 days | 1322 | 48.5% |
An additional 8 participants were partially vaccinated (1 dose of either Pfizer or Moderna).
Number sampled; does not include participants who arrived for a visit but did not scan a sample.
Number of participants with at least 1 follow-up sample.
Discussion
This project is one of the first published reports describing a clinical research project that is integrated into an existing commercial EHR as a single centralized study platform and conducted at a quaternary healthcare system. In addition to commercial EHR integration, the research project also leveraged existing COVID-19 testing and vaccination operations, including clinical and laboratory staff, the UCSD mobile application, and a clinical LIS. This utilization of existing resources, including EHR integration, allowed for increased enrollment during a global pandemic, at low cost. During this process, many valuable lessons were learned.
The implementation of electronic health record systems for clinical operations, though met with some criticisms, has been overall beneficial for record-keeping and searchability, health information exchange,15,16 patient safety,17,18 and patient engagement.19 Research systems, including traditional clinical trial management systems (CTMSs), were initially developed separately because of their many workflow differences, data rigor differences and different goals at the time. More recently however, given the benefits of the EHR as outlined, it seems logical that integrating clinical research projects using available EHRs might reap these same benefits as a result of increased interoperability.
The Veterans Affairs Healthcare System (VA) through the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC) has conducted several integrated “Point of Care Clinical Trials” (POCCT) using the EHR.20–22 In these trials, clinicians are prompted in real time during clinical care patient encounters by a clinical decision support system to consider enrolling their patient.20 Unlike the MAVERIC POCCT trials, which utilized a bespoke EHR developed for and restricted to the VA Healthcare System, ZAP used a commercial vendor, which has the valuable potential for expansion across multiple health systems.
ZAP was conducted in a unique environment at an increased speed necessary to understand the COVID-19 pandemic as it progressed. As an established “rapid learning” healthcare system,6 UCSDH was able to support this kind of speed, particularly in this instance related to an infectious disease that infects both healthcare and non-healthcare personnel the same. As a result, the research team demonstrated that a large-scale research trial can be performed using existing information ecosystems, without negatively impacting or interfering with other clinical processes.
It is important to note that the complete functionality does not yet exist in this vendor’s EHR to support a fully interventional randomized clinical trial (RCT) involving possible blinding of treatment. However, the system supports several research-focused functions, such as research specific data collection in electronic case report forms, documenting adverse events/severe adverse events (AE/SAE), and external trial monitoring, albeit these are nascent in their functional evolution. This case study highlights the potential benefits of EHR-integrated clinical research for a subset of research trials or projects that can be embedded into standard clinical workflows, facilitating rapid learning during a global health crisis.23
The sample size of trial participants in other clinical trials conducted to correlate COVID-19 antibody assays with vaccine protection ranges from 338 to 1575, at the highest.24–28 The ZAP project was able to accrue higher levels of participants at relatively low cost as the result of EHR-integration. The ease of study enrollment using the electronic platform reduced friction substantially. The primary cost of patient acquisition was labor, though this was mitigated by integrating with existing self-testing workflows. Therefore, ZAP highlights the demonstration that one can use Office of the National Coordinator for Health Information Technology (ONC)-certified (commercial) EHRs to very rapidly conduct research in which the cohort, the workflows, and the intervention are optimally aligned with the capabilities of existing commercial EHRs.
This high and rapid enrollment was also feasible in part because the study targeted a student and employee population, including both healthcare and non-healthcare personnel. Importantly, at the study institution, both the employee health and student health systems are EHR-integrated with 100% enrollment in the EHR patient-facing portal.29,30 We view this as a unique cohort of clinical research participants—relatively motivated and healthy people who participate in educational and/or employment activities every day, are highly knowledgeable and engaged with their healthcare and the UCSDH system, are generally comfortable with technology and mobile applications, underwent regular COVID-19 testing during the pandemic, and were mostly (98.2%) fully vaccinated. Although the study population did not represent the full patient population of the study institution, it was an ideal population for proof-of-concept of an EHR-integrated research project, and to understand the relative contributions of COVID-19 vaccination and infection on immune response.
An LHS is not just a place to render care. A highly reliable LHS is a forward-thinking healthcare system,6,31 learning from not only our patients but from ourselves (institutional employees and students). This project is one example of this, and led to important insights into the implementation of a fully EHR-integrated clinical research project that utilizes existing institutional operations.
Though many successes were identified from this project, there were also several obstacles faced by the both the investigators and participants. While end-to-end integration of electronic systems from recruitment and enrollment, through sample and data collection, to reporting of results, streamlined record-keeping and standardization, parts of the process, such as eCheck-in, were surprisingly difficult for some participants to complete efficiently. Additionally, there were ethical issues to consider given the participant population, including how to establish an LHS by learning from its own staff and students while preserving and protecting personnel autonomy. It is important to note that individual results were released to the participant only, and were not made available to supervisors or professors. Lastly, this project was conducted at a single institution with strong existing digital health infrastructure (though with multiple campuses), which may impact generalizability of the study results.
Our vision for the future of learning workflow integration into the EHR is bright. The EHR can not only house and make relatively available large amounts of clinical data for observational research, but it can also significantly lower the oftentimes prohibitive costs of prospective clinical research trials. One group has previously described a “green button function” that leverages aggregate EHR data for real-time personalized comparative effectiveness informational support at the point of care.32 With this approach, a successful learning health care system could enhance clinical patient care and research to help guide providers and patients toward informed health decisions at the bedside in the present and in the future.
Contributor Information
Nicole H Goldhaber, Department of Surgery, University of California San Diego Health, La Jolla, CA 92037, United States.
Marni B Jacobs, Department of Obstetrics, Gynecology and Reproductive Services, University of California San Diego Health, La Jolla, CA 92037, United States.
Louise C Laurent, Department of Obstetrics, Gynecology and Reproductive Services, University of California San Diego Health, La Jolla, CA 92037, United States.
Rob Knight, Department of Pediatrics, University of California San Diego Health, La Jolla, CA 92037, United States; Department of Computer Science and Engineering, Center for Microbiome Innovation, University of California San Diego, La Jolla, CA 92037, United States; Department of Bioengineering, Center for Microbiome Innovation, University of California San Diego, La Jolla, CA 92037, United States.
Wenhong Zhu, Information Services, University of California San Diego Health, La Jolla, CA 92037, United States.
Dean Pham, Information Services, University of California San Diego Health, La Jolla, CA 92037, United States.
Allen Tran, Information Services, University of California San Diego Health, La Jolla, CA 92037, United States.
Sandip P Patel, Division of Oncology, Department of Medicine, University of San Diego Health, La Jolla, CA 92037, United States.
Michael Hogarth, Division of Biomedical Informatics, Department of Medicine, University of San Diego Health, La Jolla, CA 92037, United States.
Christopher A Longhurst, Department of Pediatrics, University of California San Diego Health, La Jolla, CA 92037, United States; Division of Biomedical Informatics, Department of Medicine, University of San Diego Health, La Jolla, CA 92037, United States.
Author contributions
Nicole H. Goldhaber (Conception, Design, Data acquisition/interpretation, Drafting, Final approval), Marni B. Jacobs (Conception, Design, Data acquisition/analysis/interpretation, Review, Final approval), Louise C. Laurent (Conception, Design, Data interpretation, Review, Final approval), Rob Knight (Conception, Design, Review, Final approval), Wenhong Zhu (Design, Data analysis, Review, Final approval), Dean Pham (Design, Data acquisition, Review, Final approval), Allen Tran (Design, Data acquisition, Review, Final approval), Sandip P. Patel (Conception, Design, Review, Final approval), Michael Hogarth (Conception, Design, Data interpretation, Review, Final approval), and Christopher A. Longhurs (Conception, Design, Data interpretation, Review, Final approval)
Funding
This work was supported internally by UCSD Chancellor Khosla and UCSDH CEO Maysent.
Conflicts of interest
The authors have no competing interests to declare.
Data availability
The data underlying this article may be shared on reasonable request to the corresponding author.
References
- 1. Coorevits P, Sundgren M, Klein GO, et al. Electronic health records: new opportunities for clinical research. J Intern Med. 2013;274(6):547-560. [DOI] [PubMed] [Google Scholar]
- 2. Richesson RL, Horvath MM, Rusincovitch SA.. Clinical research informatics and electronic health record data. Yearb Med Inform. 2014;9(1):215-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bruland P, Doods J, Brix T, Dugas M, Storck M.. Connecting healthcare and clinical research: workflow optimizations through seamless integration of the EHR, pseudonymization services and EDC systems. Int J Med Inform. 2018;119:103-108. [DOI] [PubMed] [Google Scholar]
- 4. Daniel C, Kalra D; Section Editors for the IMIA Yearbook Section on Clinical Research Informatics. Clinical research informatics. Yearb Med Inform. 2020;29(1):203-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holmes JH, Beinlich J, Boland MR, et al. Why is the electronic health record so challenging for research and clinical care? Methods Inf Med. 2021;60(1-02):32-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Payne PRO, Wilcox AB, Embi PJ, Longhurst CA.. Better together: integrating biomedical informatics and healthcare IT operations to create a learning health system during the COVID-19 pandemic. Learn Health Syst. 2022;6(2):e10309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reeves JJ, Hollandsworth HM, Torriani FJ, et al. Rapid response to COVID-19: health informatics support for outbreak management in an academic health system. J Am Med Inform Assoc. 2020;27(6):853-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kannampallil TG, Foraker RE, Lai AM, Woeltje KF, Payne PRO.. When past is not a prologue: adapting informatics practice during a pandemic. J Am Med Inform Assoc. 2020;27(7):1142-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dixon BE, Grannis SJ, McAndrews C, et al. Leveraging data visualization and a statewide health information exchange to support COVID-19 surveillance and response: application of public health informatics. J Am Med Inform Assoc. 2021;28(7):1363-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zeng ML, Hong Y, Clunis J, He S, Coladangelo LP.. Implications of knowledge organization systems for health information exchange and communication during the COVID-19 pandemic. Data Inf Manag. 2022;4(3):148-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bartlett AH, Makhni S, Ruokis S, et al. Use of clinical pathways integrated into the electronic health record to address the COVID-19 pandemic. Infect Control Hosp Epidemiol. 2022;44(2):260-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. About Learning Health Systems. Content last reviewed May 2019. Agency for Healthcare Research and Quality, Rockville, MD. https://www.ahrq.gov/learning-health-systems/about.html
- 13. Taylor MJ, McNicholas C, Nicolay C, Darzi A, Bell D, Reed JE.. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23(4):290-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buckley MT, O'Shea MR, Kundu S, et al. Digitalizing the clinical research informed consent process: assessing the participant experience in comparison with traditional paper-based methods. JCO Oncol Pract. 2022;19(3):e355-e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Downing NL, Adler-Milstein J, Palma JP, et al. ; Northern California HIE Collaborative. Health information exchange policies of 11 diverse health systems and the associated impact on volume of exchange. J Am Med Inform Assoc. 2016;24(1):113-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hersh WR, Totten AM, Eden KB, et al. Outcomes from health information exchange: systematic review and future research needs. JMIR Med Inform. 2015;3(4):e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. King J, Patel V, Jamoom EW, Furukawa MF.. Clinical benefits of electronic health record use: National findings. Health Serv Res. 2014;49(1 Pt 2):392-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Middleton B, Dente MA, Hashmat B, et al. ; American Medical Informatics Association. Enhancing patient safety and quality of care by improving the usability of electronic health record systems: recommendations from AMIA. J Am Med Inform Assoc. 2013;20(e1):e2-e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tai-Seale M, Rosen R, Ruo B, et al. Implementation of patient engagement tools in electronic health records to enhance patient-centered communication: protocol for feasibility evaluation and preliminary results. JMIR Res Protoc. 2021;10(8):e30431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gruber WH, Powell AC, Torous JB.. The power of capturing and using information at the point of care. Healthc (Amst). 2017;5(3):86-88. [DOI] [PubMed] [Google Scholar]
- 21. Fiore LD, Brophy M, Ferguson RE, et al. A point-of-care clinical trial comparing insulin administered using a sliding scale versus a weight-based regimen. Clin Trials. 2011;8(2):183-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim JP, Roberts LW.. The transition to precision psychiatry and pragmatic inquiry methods in academic psychiatry: the example of point-of-care clinical trials. Acad Psychiatry. 2017;42(4):529-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gilbert PB, Donis RO, Koup RA, Fong Y, Plotkin SA, Follmann D.. A COVID-19 milestone attained—a correlate of protection for vaccines. N Engl J Med. 2022;387(24):2203-2206. [DOI] [PubMed] [Google Scholar]
- 24. Gilbert PB, Montefiori DC, McDermott AB, United States Government (USG)/CoVPN Biostatistics Team, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375(6576):43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fong Y, McDermott AB, Benkeser D, et al. Immune correlates analysis of the ENSEMBLE single Ad26.COV2.S dose vaccine efficacy clinical trial. Nat Microbiol. 2022;7(12):1996-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fong Y, Huang Y, Benkeser D, et al. Immune correlates analysis of the PREVENT-19 COVID-19 vaccine efficacy clinical trial. Nat Commun. 2023;14(1):1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feng S, Phillips DJ, White T, et al. ; Oxford COVID Vaccine Trial Group. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27(11):2032-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Havervall S, Marking U, Svensson J, et al. Anti-spike mucosal IgA protection against SARS-CoV-2 omicron infection. N Engl J Med. 2022;387(14):1333-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reeves JJ, San Miguel SJ, Juarez R, et al. Bringing student health and well-being onto a health system EHR: the benefits of integration in the COVID-19 era. J Am College Health. 2020;70(7):1968-1974. [DOI] [PubMed] [Google Scholar]
- 30. Isakari M, Sanchez A, Conic R, et al. Benefits and challenges of transitioning occupational health to an enterprise electronic health record. J Occup Environ Med. 2023;65(7):615-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. El-Kareh R, Brenner DA, Longhurst CA.. Developing a highly-reliable learning health system. Learn Health Syst. 2022;7(3):e10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Longhurst CA, Harrington RA, Shah NH.. A ‘Green Button’ for using aggregate patient data at the point of care. Health Aff (Millwood). 2014;33(7):1229-1235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article may be shared on reasonable request to the corresponding author.