Abstract
Background:
Stromal tumor-infiltrating lymphocytes (sTILs) are associated with pathologic complete response (pCR) and long-term outcomes for triple-negative breast cancer (TNBC) in the setting of anthracycline-based chemotherapy. Impact of sTILs on refining outcomes beyond prognostic information provided by pCR in anthracycline-free neoadjuvant chemotherapy (NAC) is not known.
Patients & Methods:
This is a pooled analysis of two studies where patients with stage I (T>1cm)–III TNBC received carboplatin (AUC 6) plus docetaxel (75 mg/m2) (CbD) NAC. sTILs were evaluated centrally on pre-treatment H&E slides using standard criteria. Cox regression analysis was used to examine effect of variables on event-free survival (EFS) and overall survival (OS).
Results:
Among 474 patients, 44% had node-positive disease. Median sTILs were 5% (range 1%–95%), and 32% of patients had ≥30% sTILs. pCR rate was 51%. On multivariable analysis, T stage (OR=2.08, p=0.007), nodal status (OR=1.64, p=0.035), and sTILs (OR=1.10, p=0.011) were associated with pCR. On multivariate analysis, nodal status (HR=0.46, p=0.008), pCR (HR=0.20, p<0.001), and sTILs (HR=0.95, p=0.049) were associated with OS. At 30% cut-point, sTILs stratified outcomes in stage III disease, with 5-year OS 86% vs 57% in ≥30% vs <30% sTILs (HR=0.29, p=0.014), and numeric trend in stage II, with 5-year OS 93% vs 89% in ≥30% vs <30% sTILs (HR=0.55, p=0.179). Among stage II-III patients with pCR, EFS was better in those with ≥30% sTILs (HR=0.16, p=0.047).
Conclusions:
sTILs density was an independent predictor of OS beyond clinicopathologic features and pathologic response in TNBC patients treated with anthracycline-free CbD chemotherapy. Notably, sTILs density stratified outcomes beyond TNM stage and pathologic response. These findings highlight role of sTILs in patient selection and stratification for neo/adjuvant escalation and de-escalation strategies.
Clinical trial registration:
Keywords: triple-negative breast cancer, tumor-infiltrating lymphocytes, neoadjuvant chemotherapy, outcome stratification
INTRODUCTION
Compared to other breast cancer subtypes, triple-negative breast cancer (TNBC) is associated with inferior long-term outcomes.1,2 Systemic multiagent chemotherapy improves long-term outcomes and is recommended for TNBC patients with stage I (T>1cm)–III disease, with most patients in the current era being treated with neoadjuvant chemotherapy.3,4 Anthracyclines and cyclophosphamide (AC) have typically constituted the chemotherapy backbone of multiagent regimens. Given the late risks of secondary leukemia/myelodysplastic syndrome and cardiomyopathy associated with AC, interest in anthracycline-free chemotherapy regimens has been gaining momentum among patient and physician communities.5–7
Carboplatin plus taxane regimens have demonstrated very good efficacy with a favorable toxicity profile in TNBC. Neoadjuvant carboplatin plus taxane regimens yield pathologic complete response (pCR) rates of 45–55% in TNBC, and patients achieving pCR with these regimens demonstrate excellent 3-year outcomes without adjuvant anthracycline.8–11 In a randomized study, six cycles of adjuvant carboplatin plus paclitaxel showed superior disease-free survival compared to an anthracycline plus taxane regimen (CEF-T).12 Carboplatin plus docetaxel is listed in NCCN guidelines as an “other recommended” neoadjuvant chemotherapy (NAC).4
The importance of immune response and tumor microenvironment for prognosis and treatment response is now being increasingly appreciated across many cancer types. Stromal tumor-infiltrating lymphocytes (sTILs) are one such measure of immune response and tumor immunogenicity. TNBC is the most immunogenic among breast cancer subtypes, as evidenced by high sTILs and programmed death-ligand 1 (PD-L1) expression in the tumor microenvironment.13,14
A pooled analysis of nine adjuvant clinical trials with over 2000 patients with TNBC demonstrated that sTILs are prognostic for invasive disease-free survival (iDFS) and overall survival (OS) in patients treated with adjuvant anthracycline-based chemotherapy.15 In a follow-up of this pooled analysis, Loi et al have reported that at a cut-point of 30%, sTILs can up- and downstage traditional anatomic AJCC stage groups.16 Numerous studies have reported a positive association between pre-treatment sTILs and pCR with anthracycline-based NAC.11,17–19 However, the prognostic role of sTILs on long-term outcomes beyond the impact of known prognostic variables like pathologic response in the setting of NAC has not yet been demonstrated in TNBC.
This study aimed to investigate the impact of sTILs density on outcomes independent of clinicopathologic variables in TNBC patients treated with an anthracycline-free NAC regimen of carboplatin and docetaxel (CbD).
PATIENTS AND METHODS
Patient Population
The study population is a pooled analysis of patients with stage I–III TNBC treated with a NAC regimen of CbD in two prospective studies. All procedures were performed in compliance with relevant laws and institutional guidelines. Each study was approved by the institutional ethics committee and conducted according to the guidelines of the Declaration of Helsinki, and all participants provided written informed consent. Details of the two cohorts and study population have been previously published.20 A brief description of the study population is provided below. See Supplementary Table 1 for representativeness of study participants.
University of Kansas (KU) study:
Patients with clinical stage I (T≥1cm)–III TNBC were enrolled in a multisite study (NCT02302742). Triple negativity was defined as estrogen receptor (ER) and progesterone receptor (PgR) immunohistochemical nuclear (IHC) staining ≤10% and HER2 negativity per current ASCO/CAP guidelines.21,22 From 2011 to 2021, 186 enrolled patients received neoadjuvant CbD.
Spanish study:
Patients with clinical stage I-III TNBC were enrolled on a multicenter non-randomized study (NCT01560663; MMJ-CAR-2014-01 and MMJ-CAR-2018-01). See Supplement for list of participating institutions. Triple negativity was defined as ER and PgR IHC <1% and HER2 IHC of 0/1+ or FISH ratio <2.0 if IHC 2+/not performed.21 From 2013–2019, 299 enrolled patients received neoadjuvant CbD.
Study Procedures
NAC regimen for both studies was carboplatin (AUC 6) + docetaxel (75 mg/m2) given every 21 days for 6 cycles. All patients received myeloid growth factor support according to the guidelines of each institution. In patients with clinically suspicious axillary lymph node/s, histological confirmation by biopsy or fine-needle aspiration was encouraged. Following NAC, all patients underwent breast and axillary surgery per standard practice. Extent of axillary surgery, subsequent irradiation, and postoperative adjuvant chemotherapy were determined by the treating physician. Patients were followed for recurrence and survival status. BRCA1/2 germline testing was done as clinically indicated.
Pathologic Evaluation
pCR was defined as the absence of residual invasive disease in the breast and axilla with or without ductal carcinoma in situ (ypT0/isN0). Pathologic response was determined locally. Residual cancer burden (RCB) was calculated using the classification by Symmans et al.23
Tumor-infiltrating lymphocytes
Central determination of sTILs density was assessed for both studies according to the previously described and published International Immuno-Oncology Biomarker Working Group guidelines24 utilizing pre-treatment core needle biopsy hematoxylin and eosin (H&E)-stained section by a single pathologist (R.S.) who was blinded to outcome information. sTILs density was reported as a percentage estimate in increments of 5%.
Statistical Methods
Event-free survival (EFS) was defined as the time from diagnosis to first recurrence (invasive ipsilateral breast, invasive local/regional, or distant) or to breast cancer-related death or breast cancer treatment-related death.25 Overall survival (OS) was defined as time from diagnosis to death as a result of any cause. Confidence intervals (CI) for the proportion of patients with pCR and RCB classes were calculated according to the exact binomial test. Logistic regression was used to examine the effect of variables on attainment of pathologic response. Survival curves were assessed by the Kaplan-Meier method and compared by log-rank tests. Cox regression modeling was used for univariate and multivariable analysis of factors associated with survival. Prespecified analysis used sTILs as a continuous variable and at a cut-point of 30%. All reported p-values and CIs are from two-sided tests. All analyses were conducted using SPSS Statistics version 27.
Data Availability
Any requests for anonymized trial data or supporting material will be reviewed on a case-by-case basis. Only requests that have scientifically and methodologically sound proposals will be considered, and the usage of the shared trial data or supporting material will be limited to the approved proposal. The final decision as to whether data or supporting material might be shared and the exact data or supporting material to be shared will be made between the trial team and the principal investigators. Proposals should be directed to the corresponding authors.
RESULTS
Study Cohort
This pooled analysis included 474 patients (see CONSORT diagram, Supplementary Figure 1). sTILs density was unavailable for N=86 due to unavailability/inadequacy of pre-treatment tumor sample. Baseline disease characteristics, EFS, and OS were comparable between the subset with available sTILs and the entire study cohort (Supplementary Tables 2 and 3). Supplementary Table 4 shows baseline disease characteristics of the KU and Spanish cohorts. Compared with the KU cohort, patients in the Spanish cohort had more disease burden as reflected by larger tumors, higher rate of node positivity, and higher TNM stage; given these differences, study cohort was included as a variable in all univariable and multivariable analysis for pCR and survival.
Patient Characteristics
Median age was 52 years, 15% were Hispanic, 8% were black, and 45% had clinically node-positive disease. 13% had a deleterious germline BRCA1/2 mutation. A small percentage (4%, all from KU cohort) had ER/PgR expression 1%–10% (Table 1).
Table 1.
Patient characteristics
| Characteristic – N (%) | All patients (N=474) | |
|---|---|---|
|
| ||
| Age at diagnosis, years – median (range) | 52 (29–80) | |
| Race a | White | 426 (90%) |
| Black | 39 (8%) | |
| Other | 9 (2%) | |
| Ethnicity b | Non-Hispanic | 402 (85%) |
| Hispanic | 69 (15%) | |
| Menopausal status c | Pre | 221 (47%) |
| Post | 251 (53%) | |
| Histological grade d | 1 | 6 (1%) |
| 2 | 107 (24%) | |
| 3 | 341 (75%) | |
| T stage e | 1 | 85 (18%) |
| 2 | 271 (57%) | |
| 3 | 76 (16%) | |
| 4 | 42 (9%) | |
| Lymph node status e,f | Negative | 261 (55%) |
| Positive | 210 (45%) | |
| TNM stage e | I | 62 (13%) |
| II | 299 (62%) | |
| III | 119 (25%) | |
| ER/PgR | ER and PgR <1% | 453 (96%) |
| ER and/or PgR 1–10% | 21 (4%) | |
| Germline BRCA1/2 mutation | Yes | 63 (13%) |
| No | 371 (78%) | |
| Unknown | 40 (8%) | |
| sTILs, % – median (range) g | 5 (1–95) | |
| sTILs g | <30% | 263 (68%) |
| ≥30% | 125 (32%) | |
| Surgery type h | Lumpectomy | 218 (46%) |
| Mastectomy | 252 (54%) | |
| pCR i | Yes | 240 (51%) |
| No | 232 (49%) | |
| RCB j | 0 | 240 (52%) |
| I | 50 (11%) | |
| II | 128 (28%) | |
| III | 40 (9%) | |
| Adjuvant radiotherapy | Yes | 358 (76%) |
| No | 116 (24%) | |
| Adjuvant chemotherapy in patients with residual disease (N=232) k | 118 (51%) | |
| Anthracycline-based | 104 (88%) | |
| Capecitabine | 9 (8%) | |
| Other | 5 (4%) | |
Race is not known for n=1 patient
Ethnicity is not known for n=3 patients
Menopausal status is not known for n=2 patients
Histological grade is not available for n=20 patients
T, N, and TNM stages are from pre-treatment clinical staging
Lymph node status is not available for n=3 patients
sTILs score is not available for n=86 patients
Surgery type information is not available for n=4 patients
pCR information is not available for n=2 patients
RCB status is not available for n=16 patients
Adjuvant chemotherapy information is not available for n=1 patient with residual disease
Abbreviations: ER, estrogen receptor; pCR, pathologic complete response; PgR, progesterone receptor; RCB, residual cancer burden; sTILs, stromal tumor-infiltrating lymphocytes
Pathologic Response
pCR and RCB 0+I rates were 51% (240/472) (95% CI: 46%–55%) and 63% (290/458) (95% CI: 59%–68%), respectively (Supplementary Figure 2). On multivariate analysis, lower T stage (OR=2.08, p=0.007), node-negative status (OR=1.64, p=0.035), and higher sTILs (OR=1.10 for every 5% increase, p=0.011) were associated with higher likelihood of pCR (Table 2). sTILs were associated with pCR both on a continuous scale and at cut-point of 30%. 32% had ≥30% sTILs, and pCR was 62% and 46% in those with ≥30% vs <30% sTILs, respectively (OR=1.96, p=0.002).
Table 2.
Factors associated with pathologic complete response
| Univariable analysis | pCR |
|||
|---|---|---|---|---|
| Frequency | OR (95% CI) | p | ||
|
| ||||
| Cohort | KU | 57% | 1.51 (1.04–2.19) | 0.031 |
| Spanish | 47% | 1 | ||
| Age at diagnosis | < median | 57% | 1.63 (1.12–2.32) | 0.010 |
| ≥ median | 45% | 1 | ||
| Race | Non-White | 58% | 1.39 (0.76–2.55) | 0.282 |
| White | 50% | 1 | ||
| Menopausal status | Pre | 56% | 1.49 (1.03–2.14) | 0.032 |
| Post | 46% | 1 | ||
| Histological grade | 1–2 | 43% | 0.62 (0.40–0.95) | 0.029 |
| 3 | 54% | 1 | ||
| T stage | T1–2 | 56% | 2.19 (1.42–3.36) | <0.001 |
| T3–4 | 36% | 1 | ||
| Lymph node status | Negative | 57% | 1.81 (1.25–2.61) | 0.002 |
| Positive | 43% | 1 | ||
| ER/PgR | ER and PgR <1% | 50% | 0.52 (0.20–1.27) | 0.145 |
| ER and/or PgR 1–10% | 67% | 1 | ||
| Germline BRCA1/2 mutation | Yes | 57% | 1.34 (0.78–2.30) | 0.287 |
| No | 50% | 1 | ||
| sTILs | ≥ 30% | 62% | 1.96 (1.27–3.04) | 0.002 |
| < 30% | 46% | 1 | ||
| Every 5% absolute increase | - | 1.06 (1.02–1.09) | 0.001 | |
|
| ||||
| Multivariable analysis | pCR |
|||
| OR (95% CI) | p | |||
|
| ||||
| Cohort (KU vs Spanish) | 1.02 (0.64–1.64) | 0.925 | ||
| Age at diagnosis (< median vs ≥ median) | 2.00 (0.96–4.16) | 0.065 | ||
| Menopausal status (pre vs post) | 0.88 (0.42–1.83) | 0.725 | ||
| Histological grade (1–2 vs 3) | 0.78 (0.47–1.32) | 0.361 | ||
| T stage (T1–2 vs T3–4) | 2.08 (1.22–3.54) | 0.007 | ||
| Lymph node status (negative vs positive) | 1.64 (1.03–2.60) | 0.035 | ||
| sTILs (every 5% absolute increase) | 1.10 (1.02–1.18) | 0.011 | ||
T stage and lymph node status are from pre-treatment clinical staging
Menopausal status is not known for n=2 patients
Histological grade is not available for n=20 patients
Lymph node status is not available for n=3 patients
Germline BRCA1/2 status is not available for n=40 patients
sTILs score is not available for n=86 patients
pCR is not known for n=2 patients
Variables that showed association with pCR at p<0.05 in univariable analysis were included in the multivariable model (variables included: cohort, age at diagnosis, menopausal status, histological grade, T stage, lymph node status, and sTILs).
Abbreviations: CI, confidence interval; ER, estrogen receptor; OR, odds ratio; pCR, pathologic complete response; PgR, progesterone receptor; sTILs, stromal tumor-infiltrating lymphocytes
Survival outcomes
At a median follow-up of 58 months (range: 5–163 months), there were 89 EFS events (distant N=74, local/regional N=14, second primary breast cancer N=1) and 87 deaths, with estimated 5-year EFS and OS of 81% (95% CI:77%–85%) and 85% (95% CI: 81%–88%), respectively (Supplementary Figure 3). Patients achieving pCR demonstrated significantly better EFS and OS compared to patients without pCR; estimated 5-year EFS was 94% (95% CI: 91%–97%) and 67% (95% CI: 61%–74%), respectively (HR=0.16; 95% CI: 0.09–0.28, p<0.001), and 5-year OS was 97% (95% CI: 94%–99%) and 73% (95% CI: 67%–79%), respectively (HR=0.16; 95% CI: 0.09–0.30, p<0.001) for patients with and without pCR (Supplementary Figure 3). 5-year EFS/OS for patients with RCB I, II, and III were 88%/93%, 70%/75%, and 35%/44%, respectively (Supplementary Figure 3).
sTILs density was prognostic for EFS and OS both as a continuous marker and at a 30% cut-point (Table 3). 5-year EFS was 89% and 76% in patients with ≥30% and <30% sTILs (HR=0.46, 95% CI: 0.26–0.83, p=0.008) and 5-year OS was 91% and 81% in patients with ≥30% and <30% sTILs, respectively (HR=0.43, 95% CI: 0.22–0.82, p=0.008) (Figure 1). Impact of sTILs on survival was analyzed within AJCC anatomical TNM stage groups. At a cut-point of 30%, sTILs density up- and down-staged anatomic TNM III and showed a numeric trend for up- and down-staging TNM stage II (Figure 1, Table 4). For stage III, 5-year EFS was 83% vs 55% for patients with ≥30% and <30% sTILs, respectively (HR=0.32, 95% CI: 0.12–0.83, p=0.013). For stage II, 5-year EFS was 91% vs 82% for patients with ≥30% and <30% sTILs, respectively (HR=0.63, 95% CI: 0.29–1.38, p=0.244). OS by sTILs in each TNM stage showed findings similar to EFS. A small number of patients (N=62) had stage I disease. Excellent EFS (5-year EFS >94%) and OS (5-year OS >93%) was noted in stage I in both ≥30% and <30% sTILs categories, with only two EFS events and one OS event in patients with stage I disease (Figure 1, Table 4).
Table 3.
Factors associated with survival
| Univariable analysis | EFS |
OS |
|||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||
|
| |||||
| Cohort | KU | 0.63 (0.39–0.99) | 0.047 | 0.75 (0.46–1.22) | 0.246 |
| Spanish | 1 | 1 | |||
| Age at diagnosis | < median | 0.86 (0.57–1.31) | 0.483 | 0.89 (0.56–1.40) | 0.599 |
| ≥ median | 1 | 1 | |||
| Race | Non-White | 0.80 (0.37–1.72) | 0.562 | 0.79 (0.34–1.82) | 0.579 |
| White | 1 | 1 | |||
| Menopausal status | Pre | 0.91 (0.60–1.39) | 0.666 | 1.06 (0.67–1.66) | 0.816 |
| Post | 1 | 1 | |||
| Histological grade | 1–2 | 1.49 (0.95–2.34) | 0.085 | 1.38 (0.84–2.27) | 0.197 |
| 3 | 1 | 1 | |||
| T stage | T1–2 | 0.36 (0.24–0.55) | <0.001 | 0.33 (0.21–0.53) | <0.001 |
| T3–4 | 1 | 1 | |||
| Lymph node status | Negative | 0.34 (0.22–0.54) | <0.001 | 0.36 (0.22–0.59) | <0.001 |
| Positive | 1 | 1 | |||
| Germline BRCA1/2 mutation | Yes | 0.91 (0.48–1.73) | 0.782 | 0.89 (0.44–1.79) | 0.743 |
| No | 1 | 1 | |||
| sTILs | ≥ 30% | 0.46 (0.26–0.83) | 0.010 | 0.43 (0.22–0.82) | 0.010 |
| < 30% | 1 | 1 | |||
| Every 5% absolute increase | 0.94 (0.90–0.98) | 0.007 | 0.93 (0.89–0.98) | 0.008 | |
| pCR | Yes | 0.16 (0.09–0.28) | <0.001 | 0.16 (0.09–0.30) | <0.001 |
| No | 1 | 1 | |||
|
| |||||
| Multivariable analysis | EFS |
OS |
|||
| HR (95% CI) | p | HR (95% CI) | p | ||
|
| |||||
| Cohort (KU vs Spanish) | 0.98 (0.57–1.70) | 0.946 | - | - | |
| T stage (T1–2 vs T3–4) | 0.59 (0.36–0.96) | 0.032 | 0.59 (0.34–1.01) | 0.055 | |
| Lymph node status (negative vs positive) | 0.43 (0.26–0.73) | 0.002 | 0.46 (0.26–0.82) | 0.008 | |
| pCR (yes vs no) | 0.20 (0.11–0.37) | <0.001 | 0.20 (0.10–0.39) | <0.001 | |
| sTILs (every 5% absolute increase) | 0.92 (0.83–1.01) | 0.081 | 0.95 (0.90–1.00) | 0.049 | |
T stage and lymph node status are from pre-treatment clinical staging
Menopausal status is not known for n=2 patients
Histological grade is not available for n=20 patients
Lymph node status is not available for n=3 patients
Germline BRCA1/2 status is not available for n=40 patients
sTILs score is not available for n=86 patients
pCR is not known for n=2 patients
Variables that showed association with EFS or OS at p<0.05 in univariable analysis were included in the multivariable model (variables included: cohort, T stage, lymph node status, sTILs, and pCR).
Abbreviations: CI, confidence interval; EFS, event-free survival; HR, hazard ratio; pCR, pathologic complete response, OS, overall survival; sTILs, stromal tumor-infiltrating lymphocytes
Figure 1:
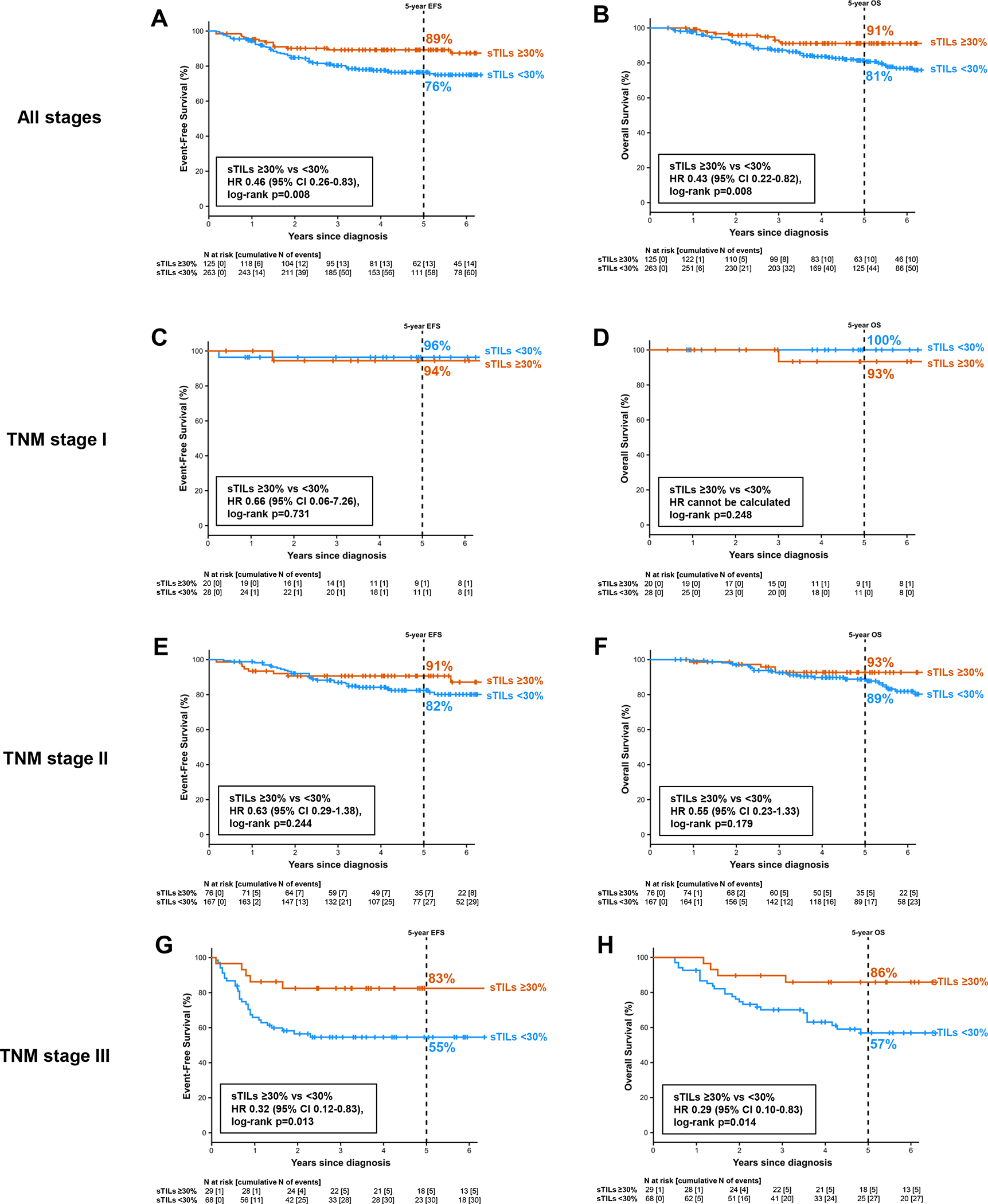
Survival by sTILs. (A) Event-free survival in all stages. (B) Overall survival in all stages. (C) Event-free survival in TNM stage I. (D) Overall survival in TNM stage I. (E) Event-free survival in TNM stage II. (F) Overall survival in TNM stage II. (G) Event-free survival in TNM stage III. (H) Overall survival in TNM stage III.
Table 4.
Survival by sTILs within TNM stages
| EFS |
OS |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Events n/N | 5-year % (95% CI) | HR (95% CI) | p* | Events n/N | 5-year % (95% CI) | HR (95% CI) | p* | ||
|
|
|
||||||||
| All | sTILs ≥30% | 13/125 | 89% (84%–95%) | 0.46 (0.26–0.83) | 0.008 | 10/125 | 91% (86%–96%) | 0.43 (0.22–0.82) | 0.008 |
| sTILs <30% | 58/263 | 76% (71%–82%) | 1 | 44/263 | 81% (76%–86%) | 1 | |||
| TNM stage I | sTILs ≥30% | 1/20 | 94% (84%–100%) | 0.66 (0.06–7.26) | 0.731 | 1/20 | 93% (81%–100%) | cannot be calculated | 0.248 |
| sTILs <30% | 1/28 | 96% (90%–100%) | 1 | 0/28 | 100% | ||||
| TNM stage II | sTILs ≥30% | 7/76 | 91% (84%–97%) | 0.63 (0.29–1.38) | 0.244 | 5/76 | 93% (86%–99%) | 0.55 (0.23–1.33) | 0.179 |
| sTILs <30% | 27/167 | 82% (76%–88%) | 1 | 17/167 | 89% (84%–94%) | 1 | |||
| TNM stage III | sTILs ≥30% | 5/29 | 83% (69%–96%) | 0.32 (0.12–0.83) | 0.013 | 4/29 | 86% (73%–99%) | 0.29 (0.10–0.83) | 0.014 |
| sTILs <30% | 30/68 | 55% (42%–67%) | 1 | 27/68 | 57% (44%–69%) | 1 | |||
Log-rank p
TNM stage is from pre-treatment clinical staging
Abbreviations: CI, confidence interval; EFS, event-free survival; HR, hazard ratio; OS, overall survival; sTILs, stromal tumor-infiltrating lymphocytes
Impact of sTILs on survival was analyzed within pCR and residual disease (RD) categories in patients with stage II and III disease (stage I was not included in this analysis given excellent outcomes regardless of sTILs, small number of patients, and very low event rate). Among patients with pCR, EFS was better in those with ≥30% sTILs, with 5-year EFS 98% vs 90% (HR=0.16, 95% CI: 0.02–1.26, p=0.047) and 5-year OS of 98% vs 95% (HR=0.19, 95% CI: 0.02–1.53, p=0.083) in ≥30% and <30% sTILs subgroups, respectively (Figure 2). Among patients with RD numerically better outcomes were noted in those with ≥30% sTILs, with 5-year EFS of 74% vs 62% (HR=0.73, 95% CI: 0.39–1.38, p=0.329) and OS of 79% vs 68% (HR=0.61, 95% CI: 0.30–1.26, p=0.180) in ≥30% and <30% sTILs subgroups, respectively (Figure 2).
Figure 2:
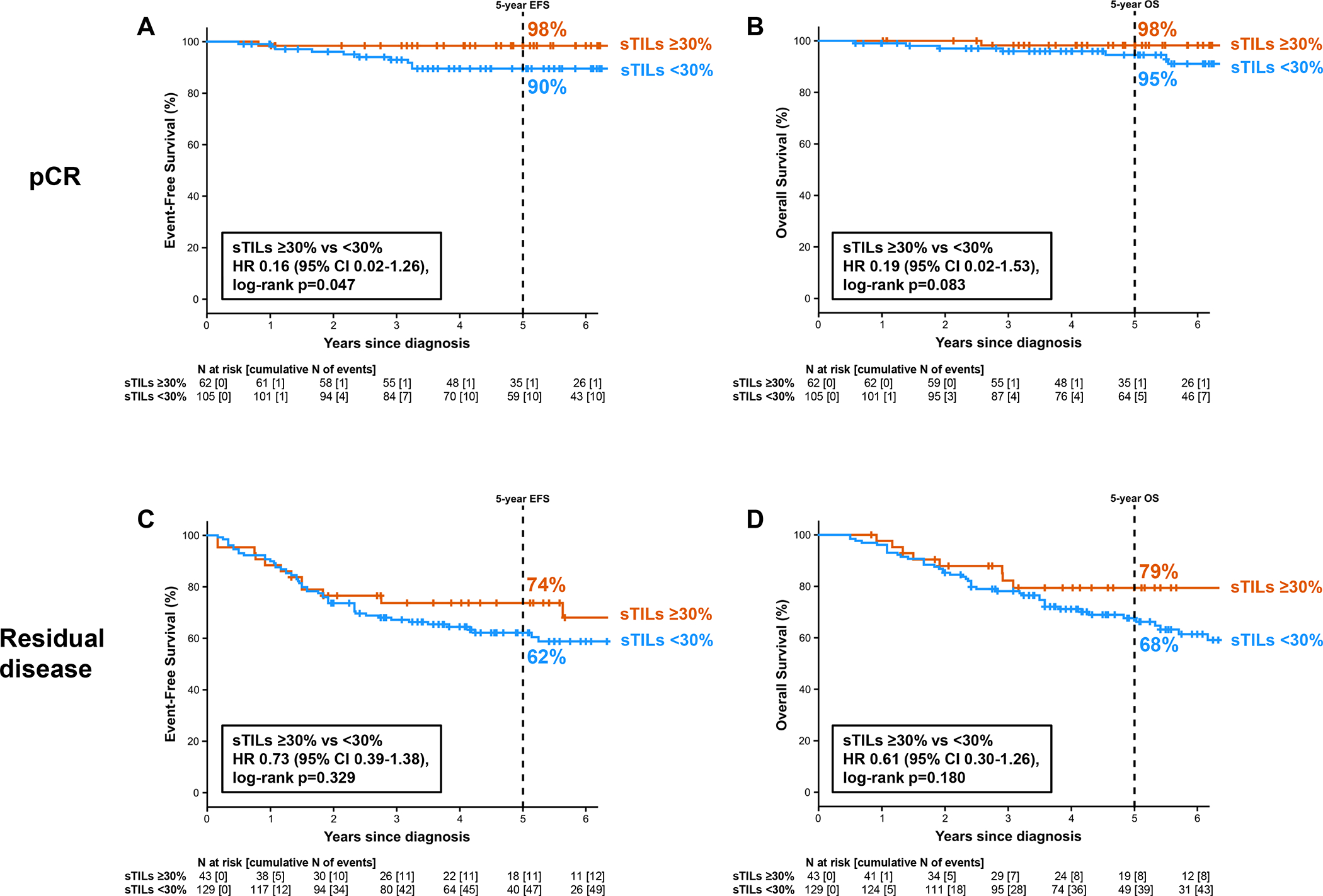
Survival by sTILs and pathologic response in TNM stage II-III. (A) Event-free survival in patients with pathologic complete response. (B) Overall survival in patients with pathologic complete response. (C) Event-free survival in patients with residual disease. (D) Overall survival in patients with residual disease.
On univariate analysis, baseline node-negative status, lower baseline T stage, pCR, and higher sTILs were all predictors of better EFS and OS, whereas age, BRCA1/2 mutation status, and histological grade did not impact EFS and OS (Table 3). On multivariate analysis that included T stage, nodal status, and pCR, sTILs continued to be significantly associated with OS (HR=0.95, p=0.049 for every 5% absolute increase) and showed trend for association with EFS (HR=0.92, p=0.081) (Table 3).
Adjuvant chemotherapy use and survival
51% (118/232) of patients with residual disease received adjuvant chemotherapy, with the majority (88%) receiving anthracycline-based regimens (Table 1). Adjuvant chemotherapy use did not significantly impact EFS or OS among patients with residual disease (Supplementary Figure 4). Impact of adjuvant chemotherapy in patients with pCR was not evaluated as only 10/240 patients with pCR received adjuvant chemotherapy.
Adverse events
Adverse event data for this patient population have been previously reported.20 91% of patients completed all 6 cycles of treatment. 9% discontinued treatment (toxicity 4.0%, patient/physician choice 3.3%, progressive disease 1.7%).
DISCUSSION
In this study, we demonstrate that the anthracycline-free NAC regimen of carboplatin plus docetaxel yields a high pathologic response rate translating to encouraging 5-year EFS and OS. sTILs density was an independent predictor of pCR and long-term outcomes in our study. A previous German Breast Cancer Group (GBG) pooled analysis of anthracycline-based neoadjuvant trials has demonstrated sTILs to be associated with pathologic response and long-term outcomes in patients with TNBC.17 Robustness of pathologic response as a surrogate for long-term outcomes in TNBC is well established.2,26,27 Given the strong association of sTILs with pCR, we investigated whether sTILs were independently associated with long-term outcomes beyond the prognostic information yielded by pCR. Indeed, in our study sTILs provided prognostic information for OS that was complementary to pCR. To our knowledge this is the first study to show sTILs to be independent predictors of survival when accounting for pCR. Our findings contrast with the GBG pooled analysis, where sTILs were no longer associated with long-term outcomes in multivariable analysis upon inclusion of pathologic response as a parameter. Notably, anthracycline-based chemotherapy was employed in the six trials included in the GBG analysis, whereas all patients in our study received platinum-taxane NAC. The type of NAC might explain differences in observations between our study and the GBG analysis. DNA-damaging chemotherapy agents like platinum compounds have been shown to induce immunogenic cell death, and immune biomarkers including sTILs have been associated with preferential response to neoadjuvant carboplatin.19,28
In line with previous reports, the association of sTILs with pCR, EFS, and OS in our study was linear. These findings support the notion that sTILs are a continuous measure of tumor-immune cell interaction. While continuous analysis of a biomarker may be biologically meaningful, categorization into risk groups is often needed for clinical application. Accordingly, clinically meaningful cutoffs have been investigated in prior studies. In a pooled analysis of anthracycline-based adjuvant chemotherapy trials, 5-year iDFS and OS were significantly different for patients with ≥30% vs <30% sTILs (5-year iDFS 69% vs 79% and 5-year OS 77% vs 87%).15 In follow-up of this pooled analysis, at a cut-point of 30%, sTILs up- and down-staged anatomical AJCC stage groups.16 Our findings in the setting of anthracycline-free neoadjuvant chemotherapy are similar, with sTILs significantly stratifying survival outcomes in stage III and showing a trend in stage II, at the 30% cutoff. 32% of patients in our study had ≥30% sTILs, which is consistent with previous reports (30% of patients in the adjuvant pooled analysis had ≥30% sTILs).15 Interestingly, in our analysis, patients with stage II disease and ≥30% sTILs appeared to have excellent OS (5-year OS 93%). KEYNOTE-522 has established an intense five-drug, six-month neoadjuvant chemoimmunotherapy regimen with adjuvant pembrolizumab as standard for stage II-III TNBC.29,30 Given the 5-year OS of 93% with anthracycline-free NAC alone (without immunotherapy) in stage II patients with ≥30% sTILs, the incremental benefit of adding immunotherapy in these patients may be questionable. Alternatively, if immunotherapy is used in the high-sTILs subgroup, perhaps chemotherapy can be de-escalated. Future trials should explore these important optimization questions. To that end, an ongoing neoadjuvant trial is evaluating 12 weeks of taxane-platinum chemoimmunotherapy in high-sTILs TNBC (NeoTRACT, NCT05645380).
This carboplatin plus docetaxel NAC regimen has also been combined with pembrolizumab in a phase II trial of 120 patients (NeoPACT, NCT03639948). NeoPACT reported a very encouraging pCR rate of 58% with 3-year EFS and OS of 86% and 89%, respectively.31 These findings suggest that 3–4-drug anthracycline-based polychemotherapy may not be needed when immunotherapy is part of the neoadjuvant treatment regimen. Accordingly, an ongoing large, randomized phase III trial SWOG S2212 (NCT05929768, SCARLET: Shorter Anthracycline-free Chemoimmunotherapy Adapted to pathological Response in Early TNBC), is comparing the NeoPACT regimen to the KEYNOTE-522 regimen in stage II-III TNBC. S2212 will also prospectively evaluate the predictive role of sTILs and other immune markers in the setting of anthracycline-based and anthracycline-free chemoimmunotherapy, thus adding valuable information regarding clinical utility of sTILs. In our study, where all patients received NAC, we noted excellent outcomes for T1cN0 disease regardless of sTILs levels. Retrospective studies show excellent outcomes without chemotherapy for subgroups with T1N0 TNBC and high sTILs.32,33 sTILs may be able to identify patients with T1c disease who need either none or very abbreviated systemic chemotherapy. Efforts are underway to design prospective studies for this patient population.
sTILs are quantified using standard H&E slides, and the International Immuno-Oncology Biomarker Working Group guidelines provide standardization guidance for sTILs quantification in breast cancer (www.tilsinbreastcancer.org). To ensure reproducibility of sTILs assessment, we followed these guidelines for sTILs assessment in our study. At a cut-point of 30%, trained pathologists have a high pairwise reporting concordance rate of up to 0.93 for sTILs reporting.34,35 This concordance rate compares favorably to the reproducibility of other biomarkers (e.g. Ki-67 and PD-L1) and morphological features (e.g. histological grade) commonly used in breast cancer.36 Taken together, these data support analytical and clinical validity of sTILs as a biomarker. sTILs are recognized as a prognostic factor by the 2019 St Gallen Consensus, and ESMO clinical practice guidelines for early breast cancer state that sTILs scoring may be used to add information about patient prognosis but should not be used to make treatment decisions.3,37 The value of sTILs in predicting preferential sensitivity to neoadjuvant chemoimmunotherapy vs polychemotherapy vs specific chemotherapeutic agents is not yet well established. Thus, sTILs quantification does not currently aid in selecting specific chemotherapeutic or immunotherapy agents. However, employing a biomarker such as sTILs for selection of immune enrichment, high pCR rate, and excellent long-term outcomes is a very promising approach for investigation of de-escalation and escalation strategies.
A novel finding from our study is the impact of sTILs in further refining outcomes in patients with pCR. 5-year EFS was 90% in patients with pCR and <30% sTILs compared to 98% in those with ≥30% sTILs. Patients with pCR have low recurrence risk, thus it is not surprising that the number of events in the pCR group in our study was low. These findings should be confirmed in other studies. Adjuvant pembrolizumab has now become part of standard adjuvant therapy for TNBC regardless of pathologic response. However, the magnitude of benefit from adjuvant pembrolizumab in the setting of pCR achieved with neoadjuvant chemoimmunotherapy is not clear. Perhaps this approach may only be of value in terms of benefit-risk ratio in patients with pCR and low (<30%) pre-treatment sTILs. An ongoing randomized phase 3 trial (Alliance A012103) is evaluating the role of adjuvant pembrolizumab in patients who achieve pCR with chemoimmunotherapy, and will address this important clinical question. In our study, baseline sTILs also numerically impacted outcomes in RD, although this difference was not statistically significant. This observation strengthens the argument for stratification based on sTILs in RD trials that are assessing systemic therapy escalation using novel agents like antibody-drug conjugates. Previous studies have also shown that amount of sTILs in residual tumor tissue after neoadjuvant chemotherapy is prognostic for long-term outcomes in TNBC (reference).38 It is possible that in patients with RD, sTILs quantification in residual tumor is a more powerful predictor of outcomes compared to pre-treatment sTILs quantification. Future studies should study this important question.
In our study, 51% of patients with RD received adjuvant chemotherapy which was mainly anthracycline-based; however, adjuvant chemotherapy use did not impact outcomes in patients with RD. These findings highlight the need for and support the ongoing investigations of novel therapies in RD. Previous studies have demonstrated that prognosis in patients with RD may be refined by sTILs quantification in residual tissue.38,39 Other biomarkers like circulating tumor DNA are also known to be prognostic in RD.40,41 sTILs quantification on RD tissue from our study is ongoing. Ultimately, the combination of amount of residual disease (i.e., RCB) and biomarkers may be a more precise way of identifying those at highest risk of relapse.
Our study does have limitations. Given the enrollment period, the NAC regimen did not include immunotherapy which is now considered standard of care for stage II-III TNBC. However, having immunotherapy-free regimen provided the opportunity to report excellent outcomes with chemotherapy alone in some subgroups (T1cN0 and stage II with high sTILs). The decision to combine the two patient cohorts for the purpose of reporting outcomes and sTILs was made after each of these cohorts had already started enrolling, which could be considered a limitation. However, this report includes over 450 TNBC patients treated across three different continents, and sTILs were uniformly read by one pathologist for all patients. Due to lack of tumor tissue, sTILs were not available for about 18% of the study population; however, baseline characteristics and outcomes were comparable between the subset with available sTILs and the entire study cohort. Although more than half of patients with RD received adjuvant chemotherapy, there was very limited use of adjuvant capecitabine. This is likely due to the enrollment years of the two studies and variable penetrance of adjuvant capecitabine in clinical practice during that time.
Our combined analysis represents the largest cohort to date of non-anthracycline NAC in TNBC. We report excellent long-term outcomes which are comparable to those with conventional anthracycline plus platinum-based regimens.42,43 Furthermore, sTILs density was an independent predictor of pCR and long-term outcomes and added prognostic information complementary to that provided by pathologic response. These results highlight the role of sTILs in patient selection and stratification for neo/adjuvant escalation and de-escalation strategies.
Supplementary Material
Statement of translational relevance.
Among 474 patients treated with neoadjuvant docetaxel + carboplatin in this study, sTILs density was an independent predictor of overall and event-free survival after adjusting for pathologic response and known clinical prognostic factors. At a 30% cut-point, sTILs density significantly stratified survival in the pathologic complete response subgroup and in TNM stage III disease. These findings of association of sTILs density with survival in the setting of anthracycline-free chemotherapy inform patient selection and stratification for neo/adjuvant escalation and de-escalation strategies.
Financial Support
This work was supported by The University of Kansas Cancer Center (KUCC); the National Cancer Institute Cancer Center Support Grant to KUCC [P30 CA168524] (Biospecimen Repository Core Facility); The Kansas Institute of Precision Medicine COBRE [P20 GM130423]; Instituto de Salud Carlos III (ISCIII) [PI15/00117, PI18/01775, PI22/01346]; and co-funded by the European Union. The report was drafted by the corresponding author and was reviewed and approved by all authors. The funding sources of the study had no role in the design of the study, collection, analysis, or interpretation of the data, or in the writing of this report.
Footnotes
Potential conflicts of interest: MM reports research funding from PUMA; consulting/advisory fees from Roche, Novartis, AstraZeneca, Daiichi-Sankyo, Seagen, Lilly, and Sanofi; speaker’s honoraria from Seagen, Lilly, AstraZeneca, Pfizer, Daiichi-Sankyo, and Roche; role as chairman for GEICAM; and member of the Board of Directors for TRIO. RS reports research funding from Roche, Puma, Merck, and BMS; consulting or advisory role for BMS, Roche, Owkin, AstraZeneca, Daiichi Sankyo, and Case45; and travel and conferences fees from Roche, Merck, BMS, Daiichii Sankyo, and AstraZeneca. IE reports speakers’ fees from Roche, Teva, Novartis, Pfizer, and Lilly; and advisory role for Lilly and AstraZeneca. APO reports honoraria, consulting/advisory role, and travel fees from AstraZeneca, Daiichi Sankyo, Novartis, Pfizer, Puma Biotechnology, Seattle Genetics, and Stemline. LEN reports advisory role for Myriad. CB reports speaker’s honoraria from Roche, Novartis, Lilly, MSD, AstraZeneca, Daiichi Sankyo, and GSK; and travel grants from Roche, Novartis, and Pfizer. YJ reports consultant/advisory role fees from Novartis, Pfizer, Roche, and AstraZeneca; speaker’s honoraria from Roche, Novartis, Lilly, and AstraZeneca; and travel and training grants from Roche, Novartis, Pfizer, and Lilly. JAGS reports consulting/advisory role for Lilly, Novartis, AstraZeneca, Daiichi Sankyo, Stemline Menarini, Pierre Fabre, Seagen, Gilead, Adium, and Exact Sciences. FM reports research funding from Pfizer; consulting/advisory role for Pfizer, Novartis, Seagen, AstraZeneca, Daiichi Sankyo, Gilead, Roche, and Exact Sciences; and travel expenses from Pfizer and Pierre Fabre. TM reports consulting/advisory board fees from AstraZeneca, Novartis, Roche, and GSK; and travel grants from Novartis and AstraZeneca. AKG reports research funding to the institution from VITRAC Therapeutics and Predicine; consulting/advisory role for Sinochips Diagnostics; honoraria from Biovica; and stock options for Clara Biotech and EXOKĒRYX. SLT reports speaker’s bureau fees from Lilly; and consulting/advisory role fees from AstraZeneca, Novartis, Roche, Pfizer, Pierre Fabre, Lilly, Seagen, Daiichi Sankyo Europe GmbH, Gilead Sciences, MDS, GSK, and Veracyte. PS reports research funding to the institution from Novartis, Bristol Myers Squibb, Merck, and Gilead; consulting/advisory board participation for Merck, Pfizer, Gilead, GSK, Sanofi, Exact Life sciences, SeaGen, Novartis, and AstraZeneca; stock ownership in Amgen, Johnson & Johnson/Janssen, and Sanofi; and royalties from UpToDate. All remaining authors declare no potential conflicts of interest.
Previous presentation: This work was presented in part at the 2023 San Antonio Breast Cancer Symposium.
References
- 1.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. Aug 1 2007;13(15 Pt 1):4429–34. doi: 10.1158/1078-0432.ccr-06-3045 [DOI] [PubMed] [Google Scholar]
- 2.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. Mar 10 2008;26(8):1275–81. doi: 10.1200/jco.2007.14.4147 [DOI] [PubMed] [Google Scholar]
- 3.Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. Oct 1 2019;30(10):1674. doi: 10.1093/annonc/mdz189 [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: breast cancer. Version 4.2023. 2023. March 23, 2023. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf [DOI] [PubMed]
- 5.Wolff AC, Blackford AL, Visvanathan K, et al. Risk of marrow neoplasms after adjuvant breast cancer therapy: the national comprehensive cancer network experience. J Clin Oncol. Feb 1 2015;33(4):340–8. doi: 10.1200/jco.2013.54.6119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khouri MG, Douglas PS, Mackey JR, et al. Cancer therapy-induced cardiac toxicity in early breast cancer: addressing the unresolved issues. Circulation. December 4, 2012. 2012;126(23):2749–2763. doi: 10.1161/circulationaha.112.100560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan TC, Neilan TG, Francis S, Plana JC, Scherrer-Crosbie M. Anthracycline-induced cardiomyopathy in adults. Compr Physiol. Jul 1 2015;5(3):1517–40. doi: 10.1002/cphy.c140059 [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, Lopez-Tarruella S, Garcia-Saenz JA, et al. Pathological response and survival in triple-negative breast cancer following neoadjuvant carboplatin plus docetaxel. Clin Cancer Res. Jul 30 2018;24(23):5820–5829. doi: 10.1158/1078-0432.CCR-18-0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gluz O, Nitz U, Liedtke C, et al. Comparison of neoadjuvant nab-paclitaxel+carboplatin vs nab-paclitaxel+gemcitabine in triple-negative breast cancer: randomized WSG-ADAPT-TN trial results. J Natl Cancer Inst. Dec 8 2018;110(6)doi: 10.1093/jnci/djx258 [DOI] [PubMed] [Google Scholar]
- 10.Gluz O, Nitz U, Kolberg-Liedtke C, et al. De-escalated neoadjuvant chemotherapy in early triple-negative breast cancer (TNBC): impact of molecular markers and final survival analysis of the WSG-ADAPT-TN trial. Clin Cancer Res. 2022; [DOI] [PubMed] [Google Scholar]
- 11.Sharma P, Kimler BF, O’Dea A, et al. Randomized Phase II Trial of Anthracycline-free and Anthracycline-containing Neoadjuvant Carboplatin Chemotherapy Regimens in Stage I-III Triple-negative Breast Cancer (NeoSTOP). Clin Cancer Res. Feb 15 2021;27(4):975–982. doi: 10.1158/1078-0432.CCR-20-3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu KD, Ye FG, He M, et al. Effect of Adjuvant Paclitaxel and Carboplatin on Survival in Women With Triple-Negative Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. Sep 1 2020;6(9):1390–1396. doi: 10.1001/jamaoncol.2020.2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali HR, Provenzano E, Dawson SJ, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. Aug 2014;25(8):1536–43. doi: 10.1093/annonc/mdu191 [DOI] [PubMed] [Google Scholar]
- 14.Luen S, Virassamy B, Savas P, Salgado R, Loi S. The genomic landscape of breast cancer and its interaction with host immunity. Breast. Oct 2016;29:241–50. doi: 10.1016/j.breast.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 15.Loi S, Drubay D, Adams S, et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. Jan 16 2019:JCO1801010. doi: 10.1200/JCO.18.01010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loi S, Salgado R, Adams S, et al. Tumor infiltrating lymphocyte stratification of prognostic staging of early-stage triple negative breast cancer. NPJ Breast Cancer. Jan 11 2022;8(1):3. doi: 10.1038/s41523-021-00362-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. Jan 2018;19(1):40–50. doi: 10.1016/S1470-2045(17)30904-X [DOI] [PubMed] [Google Scholar]
- 18.Fanucci KA, Bai Y, Pelekanou V, et al. Image analysis-based tumor infiltrating lymphocytes measurement predicts breast cancer pathologic complete response in SWOG S0800 neoadjuvant chemotherapy trial. NPJ Breast Cancer. May 13 2023;9(1):38. doi: 10.1038/s41523-023-00535-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denkert C, von Minckwitz G, Brase JC, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2–positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33(9):983–91. [DOI] [PubMed] [Google Scholar]
- 20.Sharma P, Lopez-Tarruella S, Garcia-Saenz JA, et al. Efficacy of neoadjuvant carboplatin plus docetaxel in triple negative breast cancer: combined analysis of two cohorts. Clin Cancer Res. Feb 1 2017;23(3):649–657. doi: 10.1158/1078-0432.ccr-16-0162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolff AC, Hammond MEH, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. November 1, 2013. 2013;31(31):3997–4013. doi: 10.1200/jco.2013.50.9984 [DOI] [PubMed] [Google Scholar]
- 22.Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. July 10 2018;36(20):2105–2122. doi: 10.1200/JCO.2018.77.8738 [DOI] [PubMed] [Google Scholar]
- 23.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. Oct 1 2007;25(28):4414–22. doi: 10.1200/JCO.2007.10.6823 [DOI] [PubMed] [Google Scholar]
- 24.Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. Feb 2015;26(2):259–71. doi: 10.1093/annonc/mdu450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. May 20 2007;25(15):2127–32. doi: 10.1200/JCO.2006.10.3523 [DOI] [PubMed] [Google Scholar]
- 26.Cortazar P, Geyer CE. Pathological complete response in neoadjuvant treatment of breast cancer. Ann Surg Oncol. May 2015;22(5):1441–6. doi: 10.1245/s10434-015-4404-8 [DOI] [PubMed] [Google Scholar]
- 27.I-SPY2 Trial Consortium. Association of event-free and distant recurrence-free survival with individual-level pathologic complete response in neoadjuvant treatment of stages 2 and 3 breast cancer: three-year follow-up analysis for the I-SPY2 adaptively randomized clinical trial. JAMA Oncol. Jul 23 2020;doi: 10.1001/jamaoncol.2020.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hato SV, Khong A, de Vries IJ, Lesterhuis WJ. Molecular pathways: the immunogenic effects of platinum-based chemotherapeutics. Clin Cancer Res. Jun 1 2014;20(11):2831–7. doi: 10.1158/1078-0432.CCR-13-3141 [DOI] [PubMed] [Google Scholar]
- 29.Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. Feb 27 2020;382(9):810–821. doi: 10.1056/NEJMoa1910549 [DOI] [PubMed] [Google Scholar]
- 30.Schmid P, Cortes J, Dent R, et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N Engl J Med. Feb 10 2022;386(6):556–567. doi: 10.1056/NEJMoa2112651 [DOI] [PubMed] [Google Scholar]
- 31.Sharma P, Stecklein SR, Yoder R, et al. Clinical and Biomarker Findings of Neoadjuvant Pembrolizumab and Carboplatin Plus Docetaxel in Triple-Negative Breast Cancer: NeoPACT Phase 2 Clinical Trial. JAMA Oncol. Nov 22 2023;doi: 10.1001/jamaoncol.2023.5033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Jong VMT, Wang Y, Ter Hoeve ND, et al. Prognostic Value of Stromal Tumor-Infiltrating Lymphocytes in Young, Node-Negative, Triple-Negative Breast Cancer Patients Who Did Not Receive (neo)Adjuvant Systemic Therapy. J Clin Oncol. Jul 20 2022;40(21):2361–2374. doi: 10.1200/JCO.21.01536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park JH, Jonas SF, Bataillon G, et al. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann Oncol. Dec 1 2019;30(12):1941–1949. doi: 10.1093/annonc/mdz395 [DOI] [PubMed] [Google Scholar]
- 34.Denkert C, Wienert S, Poterie A, et al. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: results of the ring studies of the international immuno-oncology biomarker working group. Mod Pathol. Oct 2016;29(10):1155–64. doi: 10.1038/modpathol.2016.109 [DOI] [PubMed] [Google Scholar]
- 35.Kos Z, Roblin E, Kim RS, et al. Pitfalls in assessing stromal tumor infiltrating lymphocytes (sTILs) in breast cancer. NPJ Breast Cancer. 2020;6:17. doi: 10.1038/s41523-020-0156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nielsen TO, Leung SCY, Rimm DL, et al. Assessment of Ki67 in Breast Cancer: Updated Recommendations From the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst. Jul 1 2021;113(7):808–819. doi: 10.1093/jnci/djaa201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burstein HJ, Curigliano G, Loibl S, et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. Oct 1 2019;30(10):1541–1557. doi: 10.1093/annonc/mdz235 [DOI] [PubMed] [Google Scholar]
- 38.Luen SJ, Salgado R, Dieci MV, et al. Prognostic implications of residual disease tumor-infiltrating lymphocytes and residual cancer burden in triple-negative breast cancer patients after neoadjuvant chemotherapy. Ann Oncol. Feb 1 2019;30(2):236–242. doi: 10.1093/annonc/mdy547 [DOI] [PubMed] [Google Scholar]
- 39.Dieci MV, Criscitiello C, Goubar A, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol. 2014;25(3):611–618. doi: 10.1093/annonc/mdt556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stecklein SR, Kimler BF, Yoder R, et al. ctDNA and residual cancer burden are prognostic in triple-negative breast cancer patients with residual disease. NPJ Breast Cancer. Mar 6 2023;9(1):10. doi: 10.1038/s41523-023-00512-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radovich M, Jiang G, Hancock BA, et al. Association of circulating tumor DNA and circulating tumor cells after neoadjuvant chemotherapy with disease recurrence in patients with triple-negative breast cancer: preplanned secondary analysis of the BRE12–158 randomized clinical trial. JAMA Oncol. Sep 1 2020;6(9):1410–1415. doi: 10.1001/jamaoncol.2020.2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geyer CE, Sikov WM, Huober J, et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Ann Oncol. Apr 2022;33(4):384–394. doi: 10.1016/j.annonc.2022.01.009 [DOI] [PubMed] [Google Scholar]
- 43.Shepherd JH, Ballman K, Polley MC, et al. CALGB 40603 (Alliance): Long-Term Outcomes and Genomic Correlates of Response and Survival After Neoadjuvant Chemotherapy With or Without Carboplatin and Bevacizumab in Triple-Negative Breast Cancer. J Clin Oncol. Apr 20 2022;40(12):1323–1334. doi: 10.1200/JCO.21.01506 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any requests for anonymized trial data or supporting material will be reviewed on a case-by-case basis. Only requests that have scientifically and methodologically sound proposals will be considered, and the usage of the shared trial data or supporting material will be limited to the approved proposal. The final decision as to whether data or supporting material might be shared and the exact data or supporting material to be shared will be made between the trial team and the principal investigators. Proposals should be directed to the corresponding authors.


