Abstract
Depression is a prevalent, heterogeneous, and debilitating disorder that often emerges in adolescence, and there is a need to better understand vulnerability processes to inform more targeted intervention efforts. Psychophysiological methods, like event-related potentials (ERPs), can offer unique insights into cognitive and emotional processes underlying depression vulnerability. I review my and others’ research examining ERP measures of reward responsiveness in youth depression and present a conceptual model of the development of low reward responsiveness, its role in depression vulnerability, and potential windows for targeted intervention. There is evidence that a blunted reward positivity (RewP) is observable in children at risk for depression, appears to be shaped in part by early social experiences, and predicts later depressive symptoms in combination with other risk factors like stress exposure. Further, a component consistent with RewP is reliably elicited in response to social acceptance feedback in computerized peer interaction tasks and demonstrates unique associations with social contextual factors and depressive symptoms, supporting the utility of developing psychophysiological tasks that may better capture youths’ real-world experiences and social risk processes. In addition, I address the translational implications of clinical psychophysiological research and describe a series of studies showing that a reduced RewP predicts greater reductions in depressive symptoms with treatment but is not modifiable by current treatments like cognitive behavior therapy. Finally, I describe our preliminary efforts to develop a positive emotion-focused intervention for offspring of depressed mothers, informed by the RewP literature, and describe future directions for translating psychophysiological research to intervention and prevention.
1. Introduction
Depression is a major and urgent public health concern worldwide. Depressive disorders, which are characterized by periods of sad mood, loss of interest or pleasure, or irritability, are prevalent, debilitating, and a leading cause of disability (Ferrari et al., 2013). Developmentally, rates of depression increase dramatically beginning around age 14, with estimates of up to 25% of youth experiencing a depressive episode by young adulthood (Hankin et al., 1998; Kessler et al., 2001). Even more concerningly, rates of depression in adolescents have increased over the past decade (Twenge et al., 2018), which is further compounded by a two-fold increase in the prevalence of youth depressive symptoms during the COVID-19 pandemic (Racine et al., 2021). Early intervention is of paramount importance as youth depression is associated with profound difficulties across the lifespan, including high rates of recurrence, comorbid psychopathologies, functional impairments across domains, and elevated risk of suicidal thoughts and behaviors (Copeland et al., 2020; Harrington et al., 1994; McLeod et al., 2016). There is a critical need to understand developmental pathways to depression, identify youth at greatest risk, and intervene early to reduce the burden of depression on youth and families.
Further emphasizing the substantial burden of depression, even interventions with the strongest evidence bases do not work for all youth with depression (March et al., 2004), with many families facing major barriers in finding effective and accessible treatments. The limitations of our existing treatments are attributable to a broad range of factors, including that depression is highly heterogeneous, presenting in hundreds of different symptom combinations and shaped by a complex interplay of biological, cognitive, affective, social, and environmental factors (Lynch et al., 2020). As such, we are unlikely to discover a treatment that works for all adolescents with depression. Instead, we need interventions to target more specific underlying vulnerabilities. To do this, we need to further develop more objective tools, like psychophysiological measures, that can be applied to characterize specific risk processes, allowing for targeted intervention earlier in development to promote mental health. In this review, I outline my and others’ research applying psychophysiological measures, particularly event-related potential (ERP) measures of reward responsiveness and social feedback processing, to clarify understanding of depression vulnerability and translate findings to prevention and treatment.
2. Brief Overview of Depression Vulnerability in Youth
Vulnerabilities for depression are trait-like, internal processes that explain underlying mechanisms contributing to the disorder (Ingram & Luxton, 2005). Understanding depression vulnerability is critical for recognizing youth at greatest risk before symptoms emerge and—given heterogeneity of the disorder—to identify individual-level mechanisms to target with personalized prevention. Just as depression can manifest as many different symptom profiles, there are many possible pathways to youth depression and a range of processes across development that interact to shape risk and resilience. Nonetheless, several key risk processes have emerged in the literature to date. First, depression is known to run in families, with offspring of depressed parents approximately three times more likely to develop depression compared to offspring of nondepressed parents (Weissman et al., 2016). In addition, cognitive factors, including negative attention biases and negative interpretation biases, play a role in the development of depression (Platt et al., 2017). Certain temperament styles, such as high negative emotionality and low positive emotionality, are associated with increased depression risk (Lonigan et al., 2003). In addition to these internal individual differences, depression risk is impacted by contextual factors and environmental experiences. For example, exposure to stressful life events, particularly interpersonal stressors, plays a key role in the development to depression for many people (Hammen, 2009). Further, parenting styles that are characterized by low warmth and high rejection, overcontrol, hostility, or withdrawal are associated with depression in youth (McLeod et al., 2007). Finally, over the past two decades, research on depression vulnerability has extended to affective neuroscience methods, including ERPs derived from the electroencephalogram (EEG) and functional magnetic resonance imaging (fMRI), with growing evidence that neural measures can be applied to better understand alterations in emotionality that precede the development of depressive symptoms (Kujawa & Burkhouse, 2017).
3. Applications of ERPs to Depression Vulnerability Research
EEG/ERP methods, discovered in the early 20th century, are well-established, objective approaches to characterizing emotional and cognitive processes at the neural level and across development (Kujawa & Brooker, 2022). These methods are particularly promising in that they benefit from a long history and large existing literature on the most rigorous methods, while also offering flexibility and accessibility for innovative applications and extensions. In line with the National Institute of Mental Health (NIMH)’s Research Domain Criteria (RDoC) initiative which emphasizes the study of core domains across levels of analysis (Sanislow, 2020), EEG/ERP methods offer unique insights into neurophysiological processes, which can be integrated with other “units”, including other physiological, circuit, behavioral, and self-report measures. EEG/ERP methods have been applied to offer some of the earliest insights into the developing brain and early emerging neural markers of depression risk. Specifically, early studies on the neural basis of depression risk used EEG to measure asymmetrical patterns of activation in electrodes over frontal regions of the brain, finding evidence that offspring of mothers with depression exhibit greater relative right vs. left frontal activity—thought to index withdrawal tendencies and negative emotions—beginning in early infancy (Jones et al., 1997). This area of research has grown in recent years, extending to studies across childhood and adolescence and to task-based EEG measures, including ERPs, which are well-suited to characterize the temporal dynamics of emotional and cognitive processing at the millisecond level, and to the development of innovative task designs that aim to capture neural processes as they might manifest in youth’s daily lives. I was first introduced to EEG/ERP methods as a post-baccalaureate research assistant working with Dr. Koraly Pérez-Edgar and have spent the past 15 years applying these methods to better understand depression risk and intervention. EEG/ERPs show promise for characterizing a range of RDoC constructs and depression vulnerabilities, including processing of negative emotional stimuli (Dickey et al., 2021) and emotion regulation abilities (Gupta et al., 2022), but I focus this review on reward responsiveness—a key component of RDoC’s positive valence systems—and connections with social processes. A simplified conceptual model of the development of low reward responsiveness, associations with depression risk, and potential windows for intervention is presented in Figure 1.
Figure 1.

Simplified conceptual model showing factors that shape the development of low reward responsiveness by middle to late childhood, which predisposes to later risk for depressive symptoms in combination with other risk factors, and highlighting potential time windows for preventive interventions.
3.1. ERP Measures of Reward Responsiveness
Low levels of reinforcers in the environment and difficulties adjusting behavior to obtain rewards have long been considered key factors in the etiology and treatment of depression (MacPhillamy & Lewinsohn, 1974). Affective neuroscience has advanced this highly influential work by elucidating neural systems that underlie reward responsiveness, and, critically, revealing that alterations in these systems are apparent in those at risk for depression, preceding the onset of the disorder (Keren et al., 2018; Kujawa & Burkhouse, 2017). In RDoC, reward responsiveness includes brain and behavioral responses in anticipation of a possible reinforcer and following the receipt of a reward (National Institute of Mental Health, 2023). ERPs are well suited to characterize the temporal dynamics of reward responsiveness and can be applied to examine multiple stages of processing (Novak & Foti, 2015; Pegg, Jeong, et al., 2021), including responses to cues indicating potential rewards (e.g., cue-P3), anticipation of possible rewards (e.g., stimulus preceding negativity), and responses to reward feedback (e.g., reward positivity [RewP], feedback P3).
The RewP component (also referred to as the feedback negativity [FN] or feedback-related negativity [FRN]) is the most studied reward-related ERP in depression vulnerability research (Keren et al., 2018; Kujawa & Burkhouse, 2017) and the focus of this review. Example trials from two tasks we commonly use to elicit the RewP in response to reward feedback in youth are presented in Figure 2. It is important to note, however, that other tasks are better suited to characterize other stages of reward processing, like the monetary incentive delay task which offers unique insights into anticipatory processing (Novak & Foti, 2015). The RewP peaks approximately 250–350 ms after feedback onset over frontocentral sites as an enhanced positivity to reward or positive feedback compared to neutral or negative feedback (Figure 3). The RewP is thought to reflect reinforcement learning processes and is sensitive to manipulations that influence the value of rewards, including delay (Cherniawsky & Holroyd, 2013), certainty (Muir et al., 2021), and perceived control (Harmon-Jones et al., 2020; Mühlberger et al., 2017). Studies leveraging both EEG and neuroimaging methods indicate correlations between the RewP and activation of reward-related brain regions, including ventral striatum and medial prefrontal cortex (Becker et al., 2014; Carlson et al., 2011; Foti et al., 2014). Particularly relevant for developmental and psychopathology research, the RewP is reliably elicited across childhood and adolescence (Kujawa et al., 2018). Further, although the RewP is most studied in monetary reward or performance feedback tasks, my lab and others have consistently shown that it can be reliably elicited in computerized social interaction and evaluation tasks (Babinski et al., 2019; Ethridge et al., 2017; Funkhouser et al., 2020; Kujawa, Arfer, et al., 2014; Kujawa et al., 2017; Pegg et al., 2022; Pegg, Ethridge, Shields, et al., 2019; Rappaport et al., 2019), which may enhance ecological validity and the personal salience of feedback, with the potential to improve understanding of depression vulnerability.
Figure 2.
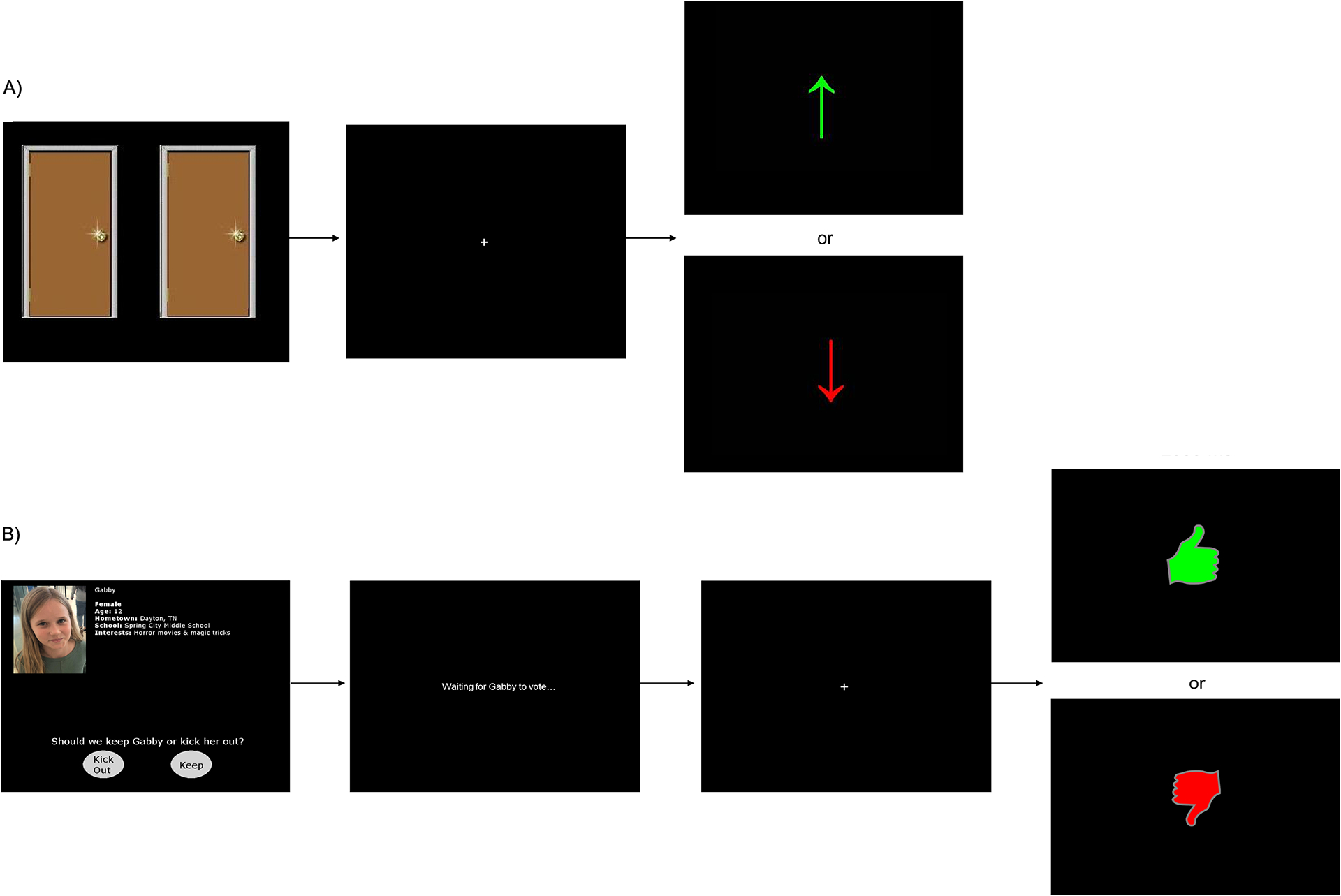
Example trials from ERP tasks we use to examine reward responsiveness in children and adolescents. A) A guessing reward task, referred to as the Doors Task (Proudfit, 2015), commonly used to elicit neural responses to monetary rewards across development. B) Example voting and feedback trials from the Island Getaway computerized peer interaction task used to elicit neutral responses to social rewards (Kujawa, Arfer, et al., 2014).
Figure 3.

Neural responses to monetary and social rewards in a sample of adolescents aged 14 to 17 years old. A) ERP waveforms at Cz (negative up, mastoid reference) in response to monetary reward and loss feedback, and scalp distribution depicting responses to monetary rewards vs. losses 250–350 ms after feedback onset. B) ERP waveforms at Cz (negative up, mastoid reference) in response to social reward (peer acceptance) and rejection feedback, and scalp distribution depicting responses to social acceptance vs. rejection 250–350 ms after feedback onset. Both types of feedback elicit a RewP-like component, beginning around 250 ms after feedback onset and presenting as an enhanced positivity for positive vs. negative feedback. Responses to social feedback appear more broadly distributed over parietal sites due in part to other overlapping components like P3.
3.2. Low Reward Responsiveness and Depression Development
Supporting associations outlined in Figure 1, we and others have observed a prospective association between a blunted RewP to monetary reward feedback in middle to late childhood or adolescence and depressive symptoms assessed 1.5 to 3 years later (Bress et al., 2013, 2015; Kujawa, Hajcak, et al., 2019; Nelson et al., 2016). For example, in my work on the Stony Brook Temperament Study with Dan Klein and Greg Hajcak, we showed that a reduced RewP at age 9 predicted greater depressive symptoms at age 12, accounting for depressive symptoms presenting earlier in development (Kujawa, Hajcak, et al., 2019). Further, the RewP accounted for unique variance in symptom outcomes beyond self-reported reward responsiveness, supporting the utility of integrating multiple levels of analysis into depression research. Importantly, similar patterns have also been observed for ventral striatum activation assessed by fMRI, such that blunted activation in anticipation of rewards predicts the development of depression in adolescents (Stringaris et al., 2015). Together, these findings suggest that low reward responsiveness at the neural level could reflect a vulnerability that is apparent at least by middle to late childhood and predisposes to the later development of depression in combination with other risk factors.
Despite the promise of these findings and relative consistency across samples and studies, there are several caveats. First, associations between RewP and depression or depression risk factors (e.g., parental depression) in youth are generally small to medium in effect sizes (e.g., Cohen’s d range .26 to .50; for reviews, Burkhouse & Kujawa, 2023; Kujawa & Burkhouse, 2017). In general, links between physiological or neural measures and clinical symptoms tend to be relatively modest due in part to the lack of shared method variance (Patrick et al., 2013). A single measure of a single risk process is unlikely to account for large amounts of variance alone, and instead, there is a need to integrate multiple interacting factors and combinations of measures in predicting depression outcomes. Consistent with this, a reduced RewP appears to be a stronger vulnerability for depression among children of mothers with depression histories than relatively low risk children of mothers with no history of depression (Kujawa, Hajcak, et al., 2019). In addition, there is considerable support for vulnerability-stress models where a reduced RewP moderates the effects of stressors on depression (Burani et al., 2019; Goldstein et al., 2019; Pegg, Ethridge, Shields, et al., 2019), and we and others have been interested in the development of novel methods to combine multiple indicators of positive valence systems function to increase the variance accounted for in depression (Hill et al., 2023).
Second, it is important to note that in some studies, we and others have shown non-significant cross-sectional associations between a blunted RewP and depressive symptoms or diagnoses (Ait Oumeziane & Foti, 2016; Hill et al., 2023; Kujawa, Hajcak, et al., 2019; Novak & Foti, 2015; Pegg, Arfer, et al., 2021). There are several possible explanations for variability across studies, including sample characteristics, task parameters, and study design. For example, there is some evidence that associations in adult samples may be less consistent than those observed earlier in development (Keren et al., 2018). Given relatively modest effect sizes, smaller sample studies may also be underpowered to detect significant effects. Further, associations may differ for tasks in which feedback is more clearly linked to behavioral performance (e.g., monetary incentive delay) as opposed to tasks where control over outcomes is more ambiguous (e.g., guessing reward tasks like Doors). It is surprising that longitudinal associations may be more consistently observed than cross-sectional associations at least in youth, although this pattern may be generally consistent with vulnerability models. That is, a reduced RewP may indicate greater trait-like predispositions to develop depression, but reward responsiveness as measured by the RewP may not fluctuate with depressive symptom levels at specific time points.
4. Developmental Trajectories to Low Reward Responsiveness
Assuming low reward responsiveness as measured by the RewP reflects an underlying vulnerability for the emergence of depression, how does low reward responsiveness develop and what factors could be targeted to promote healthy reward-related brain function? Stress exposure is widely considered to be a key factor shaping the developing reward system, although evidence of direct effects of stressors on the RewP is mixed (Kujawa et al., 2020), likely due in part to challenges with stress assessment, including identifying what types of stressors and developmental timings are most relevant. In general, associations are more commonly observed for acute laboratory stressors, rather than naturalistic stressors (Pegg & Kujawa, 2024). In addition, individual differences in the RewP appear to partly reflect manifestations of temperament traits like positive emotionality (Kujawa, Proudfit, Kessel, et al., 2015), to run in families (Weinberg et al., 2015), to be more apparent in offspring of mothers with depression (but not anxiety) histories (Kujawa, Proudfit, et al., 2014), and to be shaped in part by early parenting (Kujawa, Proudfit, Laptook, et al., 2015).
Although a growing literature is offering insight into how reward responsiveness develops, many questions remain about what a relatively reduced RewP means about an individual. It is possible that distinct emotional and cognitive processes might impact the magnitude of the RewP depending on the context. For example, there is evidence that the extent to which a person believes they have control over the outcome can impact RewP magnitude (Mühlberger et al., 2017), and a blunted RewP in depression may be due in part to a lack of perceived control (Chang et al., 2020). Further, we have found distinct associations between specific aspects of behavioral activation system function and the RewP, such that self-reported determination in pursuing goals was associated with an enhanced RewP but urges for new rewards and excitement was associated with a blunted RewP in a computerized monetary reward task (Pegg, Jeong, et al., 2021). It is also possible that people could exhibit individual differences in developmental trajectories of the RewP (e.g., chronically low, stress reactive) that impact depression risk, and further longitudinal research is needed to investigate these possibilities (Kujawa et al., 2020).
5. The Role of Social Contexts in Reward Responsiveness
Research on the RewP in depression vulnerability relies predominantly on monetary reward tasks, which are assumed to be a proxy for responses to naturalistic reinforcers, but conceptual models of depression commonly emphasize social dynamics (Davey et al., 2008; Forbes & Dahl, 2012; Hammen, 2009). Depression is often characterized by impairments in social functioning, including low social motivation, social anhedonia, and difficulties with social communication and perception (Kupferberg et al., 2016), which may be driven in part by alterations in neurobiological processes underlying social reward responsiveness. Further, interpersonal stress that disrupts social reinforcers in the environment is one of the most established predictors of depression (Hammen, 2009) and appears to be particularly pronounced among those with pre-existing tendencies towards low reward responsiveness (Pegg et al., 2019). In addition, personality theories of depression posit that people differ in the value placed on social relationships vs. achievement or independence, and individual differences in these traits shape responses to related types of stressors (Clark et al., 1992). This suggests that monetary reward tasks alone may not capture neural responses relevant to depression vulnerability for all people and contexts. Instead, assessing reward function across domains could lead to improved understanding of heterogeneity in pathways to depression and more accurate prediction of the onset and course of depression.
Reward-related brain function in social contexts is also thought to partly drive the dramatically increased prevalence of depression in adolescence, a developmental period characterized by increasing motivational value of peer relationships (Davey et al., 2008; Nelson et al., 2016). In humans, evidence using monetary reward tasks indicates that adolescence is characterized by heightened reward responsiveness in the ventral striatum and protracted development of regulatory regions like the prefrontal cortex (Davey et al., 2008; Silk et al., 2012). Animal models further support heightened social reward responsiveness in adolescents, with adolescent rodents exhibiting greater selection of and more sustained dopamine responses to social interaction compared to adults (Foulkes & Blakemore, 2016). The combination of distinct trajectories of subcortical and cortical brain development, along with increasing salience and valuation of peer relationships may contribute to increasing rates of depression in adolescence, particularly when the availability of social reinforcers is disrupted by interpersonal stressors. Together, these lines of research raise questions about how to capture social reward-related processes with EEG/ERP and the clinical utility of measuring reward responses across multiple domains.
5.1. Is There a “Social RewP”?
Compared to monetary rewards, social rewards are more challenging to manipulate and deliver in a realistic and motivationally salient manner, balancing internal validity and reliability with ecological validity for measuring processes in the way they might manifest outside of the lab. As a graduate student, I developed a task with my collaborator Kodi Arfer to elicit a RewP in the context of a computerized perceived interaction with peers (see Figure 2; Kujawa, Arfer, et al., 2014). Participants set up a profile with their photograph and basic information and then play an interactive game with perceived age-matched peers, with the storyline that they are “traveling” along the Hawaiian Islands and must eliminate one player at each island (in reality, all responses from “peers” are computerized and participants are debriefed following completion of the study). In each round, participants vote to reject or accept peers and receive a combination of acceptance (green thumbs up) and rejection (red thumbs down) feedback in return, presumably based on peer perceptions of the participant’s personal information, appearance, and voting behaviors throughout the task. One player is voted out each round, and the participant and remaining peers move on to the next round, which begins with answering another personal question (e.g., “Who’s your favorite singer or musical group?”) and reviewing peer responses to facilitate the gradual exchange of information when getting to know a new person. The participant ultimately makes it to the Big Island with a group of perceived peers but receives equal proportions of each type of feedback across the task, allowing us to reliably measure social feedback-related ERPs and avoid confounds due to differences in the probability of each type of feedback.
Our research and that of our collaborators indicates that this peer interaction task reliably elicits a component consistent with the RewP that is enhanced in response to acceptance relative to rejection and neutral feedback (Babinski et al., 2019; Ethridge et al., 2017; Funkhouser et al., 2020; Kujawa, Arfer, et al., 2014; Kujawa et al., 2017; Pegg et al., 2022; Pegg, Ethridge, Shields, et al., 2019; Rappaport et al., 2019), although it presents somewhat later than that observed in monetary reward tasks and the topography appears more broadly distributed, extending over parietal sites (Figure 3). Using temporospatial principal component analysis, we find that this RewP component is consistently elicited in both adolescent and young adult samples and peaks at frontocentral sites similar to the RewP in monetary reward tasks (Ethridge et al., 2017; Kujawa et al., 2017; Pegg et al., 2022). But ERP responses to social feedback are quite complex, and the RewP overlaps in close proximity with components consistent with P2 and P3, which show distinct modulations based on feedback valence (Pegg et al., 2022). Further, in young adults, the relative difference in the RewP magnitude between acceptance and rejection feedback appears attenuated compared to monetary feedback (Ethridge et al., 2017). This could be because the behaviors which presumably impact outcomes are much more complex in the social interaction task than typical monetary reward tasks, and social acceptance feedback is more abstract than monetary rewards which are actually administered following task completion. Importantly, RewP residuals for each reward type (partialing out variance accounted for by responses to negative feedback) tend to be moderately correlated (Pegg, Arfer, et al., 2021), indicating there is some convergence, but they seem to be capturing unique aspects of reward responsiveness, both with potential relevance for depression.
5.2. Social and Monetary Reward Responsiveness in Depression
Consistent with conceptual links between social processing and depression, our work consistently indicates that this “social RewP” offers unique insights into depression vulnerability. For example, we have shown that never-depressed offspring of mothers with depression show a blunted RewP to social feedback (Freeman et al., 2022), and the RewP to social acceptance feedback appears to be shaped in part by prior social experiences and to moderate the effects of interpersonal stress on depression. For example, experiences of peer victimization are associated with a blunted RewP to social feedback in adolescents and young adults (Rappaport et al., 2019), and social stressors are more strongly associated with depressive symptoms in emerging adults who also exhibit a blunted RewP to social acceptance feedback (Pegg, Ethridge, Shields, et al., 2019). Importantly, the magnitude of the RewP to social acceptance feedback predicts adjustments in social behavior across the task (Weinberg et al., 2021), supporting the idea that this component may reflect reinforcement learning processes in social contexts.
We have also been interested in integrating both social and monetary reward tasks into the same studies to test the extent to which patterns of associations generalize across tasks and reward domains or exhibit specificity in their associations. In general, this work supports the utility of extending reward responsiveness to the social domain in terms of offering unique information about depression. For example, in a cross-sectional sample of emerging adults, the combination of high rejection sensitivity and blunted reward responsiveness was associated with greater depressive symptoms—but only when examined in the social and not monetary reward domain (Pegg, Arfer, et al., 2021). In addition, to explore the possibility that RewP is shaped in part by social experiences, we tested the combined associations of adolescent depressive symptoms and parent-adolescent conflict (as a proxy for less positive and rewarding relationships) on the RewP in social and monetary reward tasks (Hill et al., 2023). The combination of elevated depressive symptoms and greater conflict were associated with the most blunted reward response, but this pattern was observed only in the social and not monetary domain.
We are currently working on new iterations of this peer interaction task to address limitations of the initial design, including its reliance on binary sex and gender, and creating versions that can be applied across development, including earlier childhood. There are certainly limitations to this task. For example, it is possible that some participants do not find the task realistic, and we are revising the task to be more similar to a social media platform and working on methods to assess individual differences in believability to consider in analyses. Further, given the inherent complexity of social interactions, the task design is necessarily quite distinct from commonly used monetary reward tasks and the links between participant responses and feedback are more complicated than a typical reinforcement learning paradigm, but presumably feedback is based on a broad range of participant behaviors, including their profiles, poll responses, and voting patterns across the task. Finally, peer acceptance feedback is only one type of social reward, and there is a need to create novel tasks to elicit other social reward processes (e.g., development of romantic relationships, attachment between caregivers and children) and examine associations with depression. Despite these limitations, our work to date shows utility in developing affective neuroscience tasks that may more directly tap into the real-world experiences that we think are most relevant in shaping adolescent depression risk. There is a continued need for studies leveraging multiple tasks and measures to examine the unique predictive validity of each, test the specificity of these associations, and develop innovative methods for integrating information obtained across measures to improve prediction of depression.
6. Implications for Treatment and Prevention
I started this review with statistics on the prevalence of depression and the tremendous burden it places on individuals, families, and society more broadly. This is because, as much as I value psychophysiological methods, my professional identity is as a clinical psychologist, and I have seen firsthand the suffering depression causes and the limitations of available treatments and access to these treatments. These problems are complex and require interdisciplinary groups of experts working at different angles. My personal goal is to begin to close the gap between affective neuroscience and clinical practice by empirically testing what methods like EEG/ERP can tell us about intervention and prevention. Below, I briefly describe this work to date, which is still in the very early stages, and outline directions for future research.
6.1. Reward Responsiveness as a Predictor of Treatment Outcomes
I first began research examining ERPs as predictors of treatment outcome as a clinical intern and postdoctoral fellow with Luan Phan. In our first study, my collaborator Katie Burkhouse and I initially expected that a reduced RewP might predict poorer outcomes following cognitive behavioral therapy (CBT). Yet, to our surprise, in a study of adults with anxiety and depression, a relatively blunted RewP pre-treatment predicted more improvement and greater reductions in depressive symptoms with CBT (Burkhouse et al., 2016). We have since replicated this pattern in children with anxiety disorders treated with CBT or selective serotonin reuptake inhibitors (Kujawa, Burkhouse, et al., 2019), and more recently in my own lab, in a sample of adolescents with clinical depression treated with group CBT (Dickey et al., 2023). This suggests that youth with blunted reward responsiveness may benefit most from these treatment approaches, whereas those with internalizing disorders but intact reward responsiveness may need treatment to address other contributing factors (e.g., interpersonal therapy). Importantly, on average, the RewP does not appear to increase following CBT or SSRI treatment, although relative increases in RewP correlate with improvement in symptoms (Burkhouse et al., 2016), suggesting that there may be clinical utility in enhancing the RewP. But if standard treatments for depression do not consistently enhance the RewP, is it even possible to modify the RewP with interventions? My Ph.D. student Samantha Pegg and I tested this through an analogue study where we randomized emerging adults to a brief motivational manipulation or a standard reward task instruction and found that prompts to increase the personal salience of rewards and motivation to win enhanced the RewP and improved behavioral performance (Pegg & Kujawa, 2020). This suggests that it may be possible to enhance the RewP with intervention, but many questions remain about which skills are most important for modifying the RewP and when across development these interventions are the most effective.
6.2. Translating Reward Responsiveness to Prevention
To begin to address these questions and translate evidence of a reduced RewP in depression vulnerability to intervention, Katie Burkhouse and I developed a novel neuroscience- and RDoC-informed preventive intervention for depression called Family Promoting Positive Emotions (FPPE). FPPE is a positive emotion-focused preventive intervention aimed to increase reward responsiveness in 8- to 12-year-old children who are at high risk for depression due to maternal history. Given the key role of social contexts in shaping positive emotions and rewards, the intervention is dyadic and focuses on teaching cognitive and behavioral skills to both mothers and children to enhance positivity in the family environment, including recognizing positive emotions; increasing positive family activities; attending to, savoring, anticipating, and taking credit for the positive; and reflecting on gratitude and love for self and others. The format and content of the intervention was informed in part by cognitive behavioral preventive interventions for depression in families (Compas et al., 2009, 2015) and positive affect treatment for internalizing disorders in adults (Craske et al., 2019). In a preliminary randomized trial, we found that FPPE was associated with increases in child-reported daily positive affect, and compared to written information psychoeducation, led to greater reductions in parent-rated symptoms of child depression (Burkhouse et al., 2023).
We recently received a grant from NIMH that will allow us to test whether this intervention enhances the RewP in response to monetary and social feedback, and if so, whether increases in reward responsiveness correspond with lower depressive symptoms across time in children. In this way, we aim to intervene at the relatively early window highlighted in Figure 1 by trying to enhance reward responsiveness in children with other risk factors for depression (i.e., maternal history of depression). Interventions to target emotional processes measured by ERPs in childhood before symptoms emerge is only one possible application and one of many possible development time windows. For example, ERP measures could also be used along with clinical factors to identify young adolescents at greatest risk for depression who could benefit from more established depression preventions to mitigate the effects of moderating factors, like stressors, on the development of depressive symptoms. The larger goal of our work is to develop brief preventive interventions with well-defined targets and mechanisms that could be compiled to meet the needs of individual children and families to promote healthy social and emotional development and reduce risk for depression and associated forms of psychopathology. With continued extension to large samples, refinement of methods, advances in technology, and development of norms and clinical cut-offs, I envision that psychophysiological methods like EEG/ERP could one day be applied as clinical assessment tools, complementing information from behavioral, self-, and informant-reports, to identify vulnerabilities for depression early in development and implement personalized interventions to mitigate risk processes and reduce the burden of depression on youth and families.
7. Conclusions
Psychophysiological methods offer unique advantages in elucidating vulnerability factors for depression, identifying youth at greatest risk, and informing core emotional and cognitive processes to target with intervention. A particularly exciting body of evidence is emerging for reward responsiveness, with converging evidence across methods showing that low reward-related brain function prospectively predicts the later development of depression. Despite the promise of this work, I have argued here for the need to refine methods of capturing reward responsiveness in the social domain, given strong conceptual links with the development of depression and to capture neural processes that may be more directly relevant to youth’s daily experiences. Indeed, our work to date provides initial support for this approach, in that a component consistent with RewP is reliably elicited in response to peer acceptance vs. rejection feedback and shows distinct associations with social experiences and depressive symptoms beyond those observed with monetary RewP. There is a need to better leverage the wealth of information provided by ERPs in future clinical neuroscience research by integrating multiple reward domains, as well as multiple components reflecting distinct stages of reward processing, like anticipation and feedback processing.
In addition, I have reviewed our attempts so far to integrate EEG/ERP measures of reward responsiveness into intervention research and new efforts to enhance the RewP in children at risk for depression through a positive emotion-focused dyadic intervention. It is important to note, however, that many challenges and questions remain about how exactly psychophysiology can inform intervention and prevention. One possibility is the development of novel interventions to target emotional processes measured by ERPs before symptoms emerge. In addition, EEG/ERPs may show utility—in combination with clinical measures—in identifying youth at greatest risk of depression who could benefit most from preventive interventions for depression. Given the major burden of depression and associated forms of psychopathology, there is a critical need to refine early intervention and prevention efforts. As I move from early to mid-career, questions of how to leverage psychophysiological methods in this way are ones that I find particularly motivating and rewarding and look forward to continuing to pursue.
Acknowledgements
Like the key role that social processes play in reward processing and mental health, social connections and relationships have been critical to my development as a clinical scientist and psychophysiologist. In particular, I would like to thank my mentors: Jennifer Mailloux who first introduced me to research, Koraly Pérez-Edgar who taught me what an ERP is and has been a source of support for me through every career stage, Dan Klein who taught me how to be a clinical scientist and continues to be an amazingly supportive mentor, Greg Hajcak for strong training in ERP methods, and Luan Phan for introducing me to intervention research. My brilliant graduate school friends and ERP experts Anna Weinberg, Annmarie MacNamara, and Dan Foti have been critical supports for me and taught me so much about clinical psychophysiology and how to communicate the value of this work. I am also grateful for many amazing collaborators and friends, particularly Katie Burkhouse, Kate Humphreys, Dara Babinski, and Alex Bettis. Their time, feedback, and ideas have been invaluable in shaping the direction of my research. My department and college at Vanderbilt have been incredibly generous with resources to support this work, and I am particularly thankful for my colleagues and mentors David Cole, Bruce Compas, Bethany Rittle-Johnson, and Sarah Brown-Schmidt. Thank you to Kodi Arfer for programming and contributing to the design of our social interaction task, and Anh Dao for believing in its potential and taking it in new directions. Finally, thank you to members of the Mood, Emotion, & Development lab, whose hard work has made all this possible, particularly Sam Pegg, Emili Cárdenas, Lindsay Dickey, Lisa Venanzi, Yinru Long, Resh Gupta, Kaylin Hill, Anh Dao, Maya Jackson, Haley Green, Elizabeth Estes, Alex Argiros, Maddie Politte-Corn, Irena Kesselring, and many wonderful undergraduate RAs.
My research on reward responsiveness has been supported by National Institute of Mental Health grants R61MH131751, R01MH130364, and R01MH131950, Brain and Behavior Research Foundation Young Investigator Grants, including a Katherine Deschner Family Young Investigator Grant, a Klingenstein Third Generation Foundation Fellowship, the John and Polly Sparks Early Career Grant from the American Psychological Foundation, and Vanderbilt Peabody Small Grants.
Footnotes
Conflict of Interest
None
Data Availability Statement
No data are available for this review paper.
References
- Ait Oumeziane B, & Foti D (2016). Reward-related neural dysfunction across depression and impulsivity: A dimensional approach. Psychophysiology. 10.1111/psyp.12672 [DOI] [PubMed] [Google Scholar]
- Babinski DE, Kujawa A, Kessel EMM, Arfer KBB, & Klein DNN (2019). Sensitivity to peer feedback in young adolescents with symptoms of ADHD: Examination of neurophysiological and self-report measures. Journal of Abnormal Child Psychology, 47(4). https://doi.org/0.1007/s10802-018-0470-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MPI, Nitsch AM, Miltner WHR, & Straube T (2014). A single-trial estimation of the feedback-related negativity and its relation to BOLD responses in a time-estimation task. The Journal of Neuroscience, 34(8), 3005–3012. 10.1523/JNEUROSCI.3684-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress JN, Foti D, Kotov R, Klein DN, & Hajcak G (2013). Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology, 50(1), 74–81. 10.1111/j.1469-8986.2012.01485.x [DOI] [PubMed] [Google Scholar]
- Bress JN, Meyer A, & Proudfit GH (2015). The stability of the feedback negativity and its relationship with depression during childhood and adolescence. Development and Psychopathology, 17, 1285–1294. 10.1017/S0954579414001400 [DOI] [PubMed] [Google Scholar]
- Burani K, Klawohn J, Levinson AR, Klein DN, Nelson BD, & Hajcak G (2019). Neural response to rewards, stress and sleep interact to prospectively predict depressive symptoms in adolescent girls. Journal of Clinical Child & Adolescent Psychology, 1–10. 10.1080/15374416.2019.1630834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Dao A, Argiros A, Granros M, Cárdenas EF, Dickey L, Feurer C, Hill KH, Pegg S, Venanzi L, & Kujawa A (2023). Targeting positive valence systems function in children of mothers with depressive symptoms: A pilot randomized trial of an RDoC-informed preventive intervention. Behaviour Research and Therapy, 168. 10.1016/j.brat.2023.104384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, & Kujawa A (2023). Annual Research Review: Emotion processing in offspring of mothers with depression diagnoses–a systematic review of neural and physiological research. Journal of Child Psychology and Psychiatry, 64(4), 583–607. 10.1111/jcpp.13734 [DOI] [PubMed] [Google Scholar]
- Burkhouse KL, Kujawa A, Kennedy AE, Shankman SA, Langenecker SA, Phan KL, & Klumpp H (2016). Neural reactivity to reward as a predictor of cognitive behavioral therapy response in anxiety and depression. Depression and Anxiety, 33(4), 281–288. 10.1002/da.22482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, & Hajcak G (2011). Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. Neuroimage, 57(4), 1608–1616. 10.1016/j.neuroimage.2011.05.037 [DOI] [PubMed] [Google Scholar]
- Chang Y, Wang Y, Mei S, Yi W, & Zheng Y (2020). Blunted neural effects of perceived control on reward feedback in major depressive disorder. Journal of Affective Disorders, 276, 112–118. 10.1016/j.jad.2020.06.071 [DOI] [PubMed] [Google Scholar]
- Cherniawsky AS, & Holroyd CB (2013). High temporal discounters overvalue immediate rewards rather than undervalue future rewards: An event-related brain potential study. Cognitive, Affective and Behavioral Neuroscience, 13(1), 36–45. 10.3758/s13415-012-0122-x [DOI] [PubMed] [Google Scholar]
- Clark DA, Beck AT, & Brown GK (1992). Sociotropy, autonomy, and life event perceptions in dysphoric and nondysphoric individuals. Cognitive Therapy and Research, 16(6), 635–652. 10.1007/BF01175404 [DOI] [Google Scholar]
- Compas BE, Forehand R, Keller G, Champion JE, Rakow A, Reeslund KL, McKee L, Fear JM, Colletti CJM, & Hardcastle E (2009). Randomized controlled trial of a family cognitive-behavioral preventive intervention for children of depressed parents. Journal of Consulting and Clinical Psychology, 77(6), 1007. 10.1037/a0016930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas BE, Forehand R, Thigpen J, Hardcastle E, Garai E, McKee L, Keller G, Dunbar JP, Watson KH, Rakow A, Bettis A, Reising M, Cole D, & Sterba S (2015). Efficacy and moderators of a family group cognitive-behavioral preventive intervention for children of parents with depression. Journal of Consulting and Clinical Psychology, 83(3), 541–553. 10.1037/a0039053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Alaie I, Jonsson U, & Shanahan L (2020). Associations of childhood and adolescent depression with adult psychiatric and functional outcomes. Journal of the American Academy of Child & Adolescent Psychiatry. 10.1016/j.jaac.2020.07.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Meuret AE, Ritz T, Treanor M, Dour H, & Rosenfield D (2019). Positive affect treatment for depression and anxiety: A randomized clinical trial for a core feature of anhedonia. Journal of Consulting and Clinical Psychology, 87(5), 457. https://doi.org/ 10.1037/ccp0000396 [DOI] [PubMed] [Google Scholar]
- Davey CG, Yücel M, & Allen NB (2008). The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neuroscience and Biobehavioral Reviews, 32(1), 1–19. 10.1016/j.neubiorev.2007.04.016 [DOI] [PubMed] [Google Scholar]
- Dickey L, Pegg S, Cárdenas EF, Green H, Dao A, Waxmonsky J, Pérez-Edgar K, & Kujawa A (2023). Neural predictors of improvement with cognitive behavioral therapy for adolescent depression: An examination of reward responsiveness and emotion regulation. Research on Child and Adolescent Psychopathology. 10.1007/s10802-023-01054-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey L, West M, Pegg S, Green H, & Kujawa A (2021). Neurophysiological responses to interpersonal emotional images prospectively predict the impact of COVID-19 pandemic–related stress on internalizing symptoms. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 10.1016/j.bpsc.2021.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethridge P, Kujawa A, Dirks MA, Arfer KB, Kessel EM, Klein DN, & Weinberg A (2017). Neural responses to social and monetary reward in early adolescence and emerging adulthood. Psychophysiology, 54, 1786–1799. 10.1111/psyp.12957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJL, Vos T, & Whiteford HA (2013). Burden of depressive disorders by country, sex, age, and year: Findings from the Global Burden of Disease Study 2010. PLoS Medicine, 10(11). 10.1371/journal.pmed.1001547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, & Dahl RE (2012). Research review: Altered reward function in adolescent depression: What, when and how? Journal of Child Psychology and Psychiatry, 53(1), 3–15. 10.1111/j.1469-7610.2011.02477.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Carlson JM, Sauder CL, & Proudfit GH (2014). Reward dysfunction in major depression: Multimodal neuroimaging evidence for refining the melancholic phenotype. NeuroImage, 101, 50–58. 10.1016/j.neuroimage.2014.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes L, & Blakemore S-J (2016). Is there heightened sensitivity to social reward in adolescence? Current Opinion in Neurobiology, 40, 81–85. 10.1016/j.conb.2016.06.016 [DOI] [PubMed] [Google Scholar]
- Freeman C, Ethridge P, Banica I, Sandre A, Dirks MA, Kujawa A, & Weinberg A (2022). Neural response to rewarding social feedback in never-depressed adolescent girls and their mothers with remitted depression: Associations with multiple risk indices. Journal of Psychopathology and Clinical Science, 131(2), 141. 10.1037/abn0000728 [DOI] [PubMed] [Google Scholar]
- Funkhouser CJ, Auerbach RP, Kujawa A, Morelli SA, Phan KL, & Shankman SA (2020). Social feedback valence differentially modulates the reward positivity, P300, and late positive potential. Journal of Psychophysiology, 34(4). 10.1027/0269-8803/a000253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BL, Kessel EM, Kujawa A, Finsaas MCMC, Davila J, Hajcak G, & Klein DN (2019). Stressful life events moderate the effect of neural reward responsiveness in childhood on depressive symptoms in adolescence. Psychological Medicine, 50(9). 10.1017/S0033291719001557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RS, Dickey L, & Kujawa A (2022). Neural markers of emotion regulation difficulties moderate effects of COVID‐19 stressors on adolescent depression. Depression and Anxiety, 39(6), 515–523. 10.1002/da.23268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C (2009). Adolescent depression: Stressful interpersonal contexts and risk for recurrence. Current Directions in Psychological Science, 18(4), 200–204. 10.1111/j.1467-8721.2009.01636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, & Angell KE (1998). Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology, 107(1), 128–140. 10.1037/0021-843X.107.1.128 [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Willoughby C, Paul K, & Harmon-Jones C (2020). The effect of perceived effort and perceived control on reward valuation: Using the reward positivity to test a dissonance theory prediction. Biological Psychology, 154, 107910. 10.1016/j.biopsycho.2020.107910 [DOI] [PubMed] [Google Scholar]
- Harrington R, Bredenkamp D, Groothues C, Rutter M, Fudge H, & Pickles A (1994). Adult outcomes of childhood and adolescent depression. III Links with suicidal behaviours. Journal of Child Psychology and Psychiatry, 35(7), 1309–1319. 10.1111/j.1469-7610.1994.tb01236.x [DOI] [PubMed] [Google Scholar]
- Hill KE, Dickey L, Pegg S, Dao A, Arfer KN, & Kujawa A (2023). Associations between parental conflict and social and monetary reward responsiveness in adolescents with clinical depression. Research on Child and Adolescent Psychopathology, 51, 119–131. 10.1007/s10802-022-00949-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KE, Pegg S, Dao A, Boldwyn E, Dickey L, Venanzi L, Argiros A, & Kujawa A (2023). Characterizing positive and negative valence systems function in adolescent depression: An RDoC-informed approach integrating multiple neural measures. Journal of Mood and Anxiety Disorders, 3, 100025. 10.1016/j.xjmad.2023.100025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RE, & Luxton DD (2005). Vulnerability-stress models. In Hankin BL & Abela JRZ (Eds.), Development of psychopathology: A vulnerability-stress perspective. (pp. 32–46). Sage Publications, Inc. [Google Scholar]
- Jones NA, Field T, Fox NA, Lundy B, & Davalos M (1997). EEG activation in 1-month-old infants of depressed mothers. Development and Psychopathology, 9(3), 491–505. 10.1017/s0954579497001260 [DOI] [PubMed] [Google Scholar]
- Keren H, Callaghan GO, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, Pan PM, Meffert L, Kaiser A, Wolke S, Pine DS, & Stringaris A (2018). Reward processing in depression: A conceptual and meta-analytic review across fMRI and EEG studies. American Journal of Psychiatry, 19, 1–10. 10.1176/appi.ajp.2018.17101124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Merikangas KR, Ries Merikangas K, Merikangas KR, Ries Merikangas K, Merikangas KR, & Ries Merikangas K (2001). Mood disorders in children and adolescents: An epidemiologic perspective. Biological Psychiatry, 49(12), 1002–1014. 10.1016/s0006-3223(01)01129-5 [DOI] [PubMed] [Google Scholar]
- Kujawa A, Arfer KB, Klein DN, & Proudfit GH (2014). Electrocortical reactivity to social feedback in youth: A pilot study of the Island Getaway task. Developmental Cognitive Neuroscience, 10, 140–147. 10.1016/j.dcn.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, & Brooker RJ (2022). Methods and metrics for EEG/ERP assessment of emotion and cognition in young children. Developmental Psychobiology, 64(6), e22284. 10.1002/dev.22284 [DOI] [PubMed] [Google Scholar]
- Kujawa A, & Burkhouse KL (2017). Vulnerability to depression in youth: Advances from affective neuroscience. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(1), 28–37. 10.1016/j.bpsc.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Burkhouse KL, Karich SR, Fitzgerald KD, Monk CS, & Phan KL (2019). Reduced reward responsiveness predicts change in depressive symptoms in anxious children and adolescents following treatment. Journal of Child and Adolescent Psychopharmacology, 29(5), 378–385. 10.1089/cap.2018.0172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Carroll A, Mumper E, Mukherjee D, Kessel EM, Olino T, Hajcak G, & Klein DN (2018). A longitudinal examination of event-related potentials sensitive to monetary reward and loss feedback from late childhood to middle adolescence. International Journal of Psychophysiology, 132, 323–330. 10.1016/j.ijpsycho.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, & Klein DN (2019). Reduced reward responsiveness moderates the effect of maternal depression on depressive symptoms in offspring: evidence across levels of analysis. Journal of Child Psychology and Psychiatry, 60(1), 82–90. 10.1111/jcpp.12944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Kessel EM, Carroll A, Arfer KB, & Klein DN (2017). Social processing in early adolescence: Associations between neurophysiological, self-report, and behavioral measures. Biological Psychology, 128, 55–62. 10.1016/j.biopsycho.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Klein DN, Pegg S, & Weinberg A (2020). Developmental trajectories to reduced activation of positive valence systems: A review of biological and environmental contributions. Developmental Cognitive Neuroscience, 43, 100791. 10.1016/j.dcn.2020.100791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Proudfit GH, Kessel EM, Dyson M, Olino T, & Klein DN (2015). Neural reactivity to monetary rewards and losses in childhood: Longitudinal and concurrent associations with observed and self-reported positive emotionality. Biological Psychology, 104, 41–47. 10.1016/j.biopsycho.2014.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Proudfit GH, & Klein DN (2014). Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. Journal of Abnormal Psychology, 123(2), 287–297. 10.1037/a0036285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Proudfit GH, Laptook R, & Klein DN (2015). Early parenting moderates the association between parental depression and neural reactivity to rewards and losses in offspring. Clinical Psychological Science, 3(4), 503–515. 10.1177/2167702614542464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferberg A, Bicks L, & Hasler G (2016). Social functioning in major depressive disorder. Neuroscience and Biobehavioral Reviews, 69, 313–332. 10.1016/j.neubiorev.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Lonigan CJ, Phillips BM, & Hooe ES (2003). Relations of positive and negative affectivity to anxiety and depression in children: evidence from a latent variable longitudinal study. Journal of Consulting and Clinical Psychology, 71(3), 465. 10.1037/0022-006X.71.3.465 [DOI] [PubMed] [Google Scholar]
- Lynch CJ, Gunning FM, & Liston C (2020). Causes and consequences of diagnostic heterogeneity in depression: paths to discovering novel biological depression subtypes. Biological Psychiatry, 88(1), 83–94. 10.1016/j.biopsych.2020.01.012 [DOI] [PubMed] [Google Scholar]
- MacPhillamy DJ, & Lewinsohn PM (1974). Depression as a function of levels of desired and obtained pleasure. Journal of Abnormal Psychology, 83(6), 651. 10.1037/h0037467 [DOI] [PubMed] [Google Scholar]
- March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, Burns B, Domino M, McNulty S, Vitiello B, & Severe J (2004). Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA: The Journal of the American Medical Association, 292(7), 807–820. 10.1001/jama.292.7.807 [DOI] [PubMed] [Google Scholar]
- McLeod BD, Weisz JR, & Wood JJ (2007). Examining the association between parenting and childhood depression: A meta-analysis. Clinical Psychology Review, 27(8), 986–1003. 10.1016/j.cpr.2007.03.001 [DOI] [PubMed] [Google Scholar]
- McLeod GFH, Horwood LJ, & Fergusson DM (2016). Adolescent depression, adult mental health and psychosocial outcomes at 30 and 35 years. Psychological Medicine, 46(7), 1401–1412. 10.1017/S0033291715002950 [DOI] [PubMed] [Google Scholar]
- Mühlberger C, Angus DJ, Jonas E, Harmon‐Jones C, & Harmon‐Jones E (2017). Perceived control increases the reward positivity and stimulus preceding negativity. Psychophysiology, 54(2), 310–322. 10.1111/psyp.12786 [DOI] [PubMed] [Google Scholar]
- Muir AM, Eberhard AC, Walker MS, Bennion A, South M, & Larson MJ (2021). Dissociating the effect of reward uncertainty and timing uncertainty on neural indices of reward prediction errors: A reward positivity (RewP) event-related potential (ERP) study. Biological Psychology, 108121. 10.1016/j.biopsycho.2021.108121 [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health. (2023). RDoC Matrix. http://www.nimh.nih.gov/research-priorities/rdoc/constructs/rdoc-matrix.shtml
- Nelson BD, Perlman G, Klein DN, Kotov R, & Hajcak G (2016). Blunted neural response to rewards prospectively predicts the development of depression in adolescent girls. The American Journal of Psychiatry, 173(12), 1223–1230. 10.1176/appi.ajp.2016.15121524 [DOI] [PubMed] [Google Scholar]
- Nelson EE, Jarcho JM, & Guyer AE (2016). Social re-orientation and brain development: An expanded and updated view. Developmental Cognitive Neuroscience, 17, 118–127. 10.1016/j.dcn.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak KD, & Foti D (2015). Teasing apart the anticipatory and consummatory processing of monetary incentives: An event-related potential study of reward dynamics. Psychophysiology, 52(11), 1470–1482. 10.1111/psyp.12504 [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, & Kramer MD (2013). A construct-network approach to bridging diagnostic and physiological domains: Application to assessment of externalizing psychopathology. Journal of Abnormal Psychology, 122(3), 902. 10.1037/a0032807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg S, Arfer KB, & Kujawa A (2021). Altered reward responsiveness and depressive symptoms: An examination of social and monetary reward domains and interactions with rejection sensitivity. Journal of Affective Disorders, 282, 717–725. 10.1016/j.jad.2020.12.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg S, Ethridge P, Shields GS, Slavich GM, Weinberg A, & Kujawa A (2019). Blunted social reward responsiveness moderates the effect of lifetime social stress exposure on depressive symptoms. Frontiers in Behavioral Neuroscience, 13. 10.3389/fnbeh.2019.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg S, Jeong HJ, Foti D, & Kujawa A (2021). Differentiating stages of reward responsiveness: Neurophysiological measures and associations with facets of the behavioral activation system. Psychophysiology, 58, e13764. 10.1111/psyp.13764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg S, & Kujawa A (2020). The effects of a brief motivation manipulation on reward responsiveness: A multi-method study with implications for depression. International Journal of Psychophysiology, 150, 100–107. 10.1016/j.ijpsycho.2020.02.004 [DOI] [PubMed] [Google Scholar]
- Pegg S, & Kujawa A (2024). The effects of stress on reward responsiveness: A systematic review and preliminary meta-analysis of the event-related potential literature. Cognitive, Affective, and Behavioral Neuroscience. 10.3758/s13415-023-01143-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg S, Lytle MN, Arfer KB, & Kujawa A (2022). The time course of reactivity to social acceptance and rejection feedback: An examination of event‐related potentials and behavioral measures in a peer interaction task. Psychophysiology, 59(7), e14007. 10.1111/psyp.14007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt B, Waters AM, Schulte-Koerne G, Engelmann L, & Salemink E (2017). A review of cognitive biases in youth depression: attention, interpretation and memory. Cognition and Emotion, 31(3), 462–483. 10.1080/02699931.2015.1127215 [DOI] [PubMed] [Google Scholar]
- Proudfit GH (2015). The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology, 52(4), 449–459. 10.1111/psyp.12370 [DOI] [PubMed] [Google Scholar]
- Racine N, McArthur BA, Cooke JE, Eirich R, Zhu J, & Madigan S (2021). Global prevalence of depressive and anxiety symptoms in children and adolescents during COVID-19: a meta-analysis. JAMA Pediatrics, 175(11), 1142–1150. 10.1001/jamapediatrics.2021.2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport BI, Hennefield L, Kujawa A, Arfer KB, Kelly D, Kappenman ES, Luby JL, & Barch DM (2019). Peer victimization and dysfunctional reward processing: ERP and behavioral responses to social and monetary rewards. Frontiers in Behavioral Neuroscience, 13, 120. 10.3389/fnbeh.2019.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanislow CA (2020). RDoC at 10: changing the discourse for psychopathology. World Psychiatry : Official Journal of the World Psychiatric Association (WPA), 19(3), 311–312. 10.1002/wps.20800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Davis S, McMakin DL, Dahl RE, & Forbes EE (2012). Why do anxious children become depressed teenagers? The role of social evaluative threat and reward processing. Psychological Medicine, 42, 2095–2107. https://doi.org/ 10.1017/S0033291712000207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Belil PVR, Artiges E, Lemaitre H, Gollier-Briant F, Wolke S, Vulser H, Miranda R, Penttilä J, Struve M, Fadai T, Kappel V, Grimmer Y, Goodman R, Poustka L, Conrod P, Cattrell A, Banaschewski T, Bokde ALW, … Consortium, I. (2015). The brain’s response to reward anticipation and depression in adolescence: Dimensionality, specificity, and longitudinal predictions in a community-based sample. American Journal of Psychiatry, 172(12), 1215–1223. 10.1176/appi.ajp.2015.14101298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twenge JM, Joiner TE, Rogers ML, & Martin GN (2018). Increases in depressive symptoms, suicide-related outcomes, and suicide rates among U.S. adolescents after 2010 and links to increased new media screen time. Clinical Psychological Science, 6(1), 3–17. 10.1177/2167702617723376 [DOI] [Google Scholar]
- Weinberg A, Ethridge P, Pegg S, Freeman C, Kujawa A, & Dirks MA (2021). Neural responses to social acceptance predict behavioral adjustments following peer feedback in the context of a real‐time social interaction task. Psychophysiology, 58(3), e13748. 10.1111/psyp.13748 [DOI] [PubMed] [Google Scholar]
- Weinberg A, Liu H, Hajcak G, & Shankman SA (2015). Blunted neural response to rewards as a vulnerability factor for depression: Results from a family study. Journal of Abnormal Psychology, 124(4), 878–889. 10.1037/abn0000081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Wickramaratne P, Gameroff MJ, Warner V, Pilowsky D, Kohad RG, Verdeli H, Skipper J, & Talati A (2016). Offspring of depressed parents: 30 years later. American Journal of Psychiatry, 173(10), 1024–1032. 10.1176/appi.ajp.2016.15101327 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data are available for this review paper.


