Abstract
Based on the human immunodeficiency virus type 1 (HIV-1) gag gene, subgenomic reporter constructs have been established allowing the contributions of different cis-acting elements to the Rev dependency of late HIV-1 gene products to be determined. Modification of intragenic regulatory elements achieved by adapting the codon usage of the complete gene to highly expressed mammalian genes resulted in constitutive nuclear export allowing high levels of Gag expression independent from the Rev/Rev-responsive element system and irrespective of the absence or presence of the isolated major splice donor. Leptomycin B inhibitor studies revealed that the RNAs derived from the codon-optimized gag gene lacking AU-rich inhibitory elements are directed to a distinct, CRM1-independent, nuclear export pathway.
Late human immunodeficiency virus type 1 (HIV-1) gene expression depends on cis-acting elements and the interaction of Rev with its cognate RNA recognition site, the Rev responsive element (RRE) (reviewed in reference 25). Nuclear retention of late HIV-1 unspliced and singly spliced mRNAs in the absence of Rev has been explained in many reports by inefficient splicing of the viral transcripts (4, 15, 16, 20, 26). Furthermore, the binding of U1 small nuclear RNP to an upstream splice donor site seemed to be required for Rev-dependent Env expression (18), whereas efficient splicing achieved by positioning a functional intron upstream of the env gene yielded Rev-independent expression (14). Experiments employing Rev-dependent β-globin reporter constructs suggested that inefficient splicing is essentially required for nuclear retention, which in turn represents a prerequisite for timely regulated Rev-dependent RNA export (4, 20). In view of the fact that many HIV-1 splice sites are suboptimal (22), it was speculated that Rev promotes the export of late HIV-1 RNAs entrapped within the splicing machinery (4, 13, 15).
However, the Rev-mediated nuclear export process seems not to be directly related to splicing, as shown by the observation of Fischer and colleagues that Rev can also export RNAs retained in the nucleus for entirely unrelated reasons, such as U-rich U6 RNAs (10). Accordingly, env mRNA has been reported to remain Rev dependent also in the absence of any functional splice sites (21). It has been postulated that these RNAs contain cis-active inhibitory sequences (INS) within their coding regions negatively regulating their expression (19, 21, 22, 31). Fusion of proposed INS-containing fragments to a chloramphenicol acetyltransferase gene reporter resulted in decreased expression and Rev responsiveness (6, 28, 31). Consequently, low-level gene expression of gag and pol open reading frames in the absence of Rev was overcome by clustered point mutations within the wobble positions of the coding DNA sequence (29, 30).
The scope of this study was to determine, based on a subgenomic Rev-dependent gag reporter construct, the critical contribution of proposed INS elements within the gag coding region and the 5′ untranslated region (UTR) including the major splice donor (SD) on Rev/RRE dependency, nuclear RNA stability, and export of HIV-1 Gag-encoding transcripts. For that purpose a series of subgenomic gag gene reporter constructs, in which cis-acting sites were either deleted or substantially modified, were established.
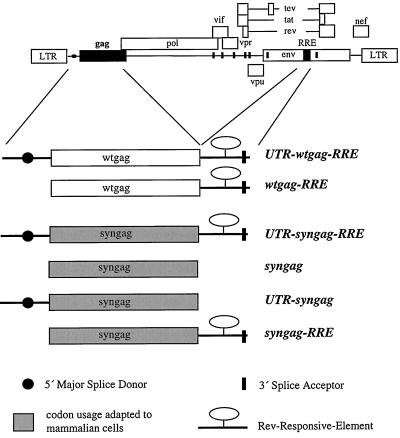
Although the precise character of the postulated INS elements still remains to be defined, they seemed to have a noticeably high AT content. In order to eliminate proposed repressor elements without having particular knowledge of their nature, we designed a synthetic gag reading frame on the basis of the Pr55gag amino acid sequence employing a codon usage occurring most frequently in highly expressed mammalian genes (2). By this procedure we introduced more than 400 substitutions homogeneously distributed throughout the complete gag gene, thereby reducing the AT content of the wild-type gag gene from 55.9% down to only 33.9%. Almost every wobble position within the wild-type coding region was changed to a G or C, resulting in a diverse nucleotide composition and decreased AT content without alterations within the encoded protein. The synthetic gag gene was constructed by a stepwise PCR amplification of overlapping 60-nucleotide (nt)-long oligonucleotides, encoding the entire Pr55gag polyprotein (of the HX10 proviral clone [27]). A comparison of synthetic gag (syngag) and wild-type gag (wtgag) coding sequences is shown in Fig. 1. Mimicking the situation of genomic RNAs, a 103-bp UTR carrying the highly functional HIV-1 major SD was fused upstream (UTR-syngag and UTR-wtgag) and an 861-nt fragment known to carry the RRE was fused downstream to Gag-encoding reading frames (syngag-RRE, UTR-syngag-RRE, wtgag-RRE, and UTR-wtgag-RRE). UTR-wtgag-RRE accommodates, in addition to the RRE, the most 3′-located splice acceptor site within the HIV-1 genome, which is known to be used very inefficiently, a property suggested to contribute to timely regulated gene expression (8, 23, 33). All synthetic gag gene derivatives and RRE-containing wild-type gag sequences were cloned into the pcDNA 3.1 (+) expression vector (Invitrogen, Leek, The Netherlands) under the transcriptional control of the immediate-early promoter-enhancer of cytomegalovirus. A schematic representation of all expression constructs is summarized in Fig. 2.
FIG. 1.
Nucleotide sequence alignment of the codon-optimized (syngag) Gag-encoding reading frame and the wild-type gag (wtgag) gene. The syngag coding sequence was adapted to a codon usage occurring in highly expressed mammalian genes and aligned to the corresponding wild-type sequence. Sequence identity between the synthetic and wild-type genes is indicated by vertical lines.
FIG. 2.
Schematic representation of wild-type and synthetic gag-containing expression plasmids. syngag and wtgag reading frames were fused to the cis-acting sequences of the 5′ UTR and RRE. The positions of the Gag-encoding region and the RRE within the HIV-1 genome are shown at the top.
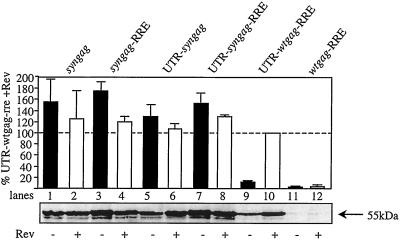
To evaluate the critical contribution of inhibitory elements to the presence of the major SD (which is present in all HIV-1 transcripts), Gag expression from the wild-type gag gene reporter was compared to synthetic gag gene-driven expression, in the presence and absence of UTR, RRE, and Rev. Cells were transfected using the calcium phosphate coprecipitation technique (12), harvested 48 h later, washed two times in phosphate-buffered saline PBS and then further analyzed. Expression within cell lysates was assayed by immunoblotting using a p24-specific antibody (35). The concentration of Pr55gag was determined by capture enzyme-linked immunosorbent assay (ELISA) (DuPont, Boston, Mass.) and quantified by a calibration curve using different concentrations of purified Pr55gag (34). High-level expression of Pr55gag, ranging from 3.5 to 6.5 ng/μg of total cellular protein, was achieved after transfection of various syngag-containing plasmids into mammalian cells (Fig. 3, lanes 1 to 8). It is noteworthy that expression levels from the optimized gag gene were not dependent on or substantially altered by the introduction of the Rev/RRE system (Fig. 3, lanes 2 to 4) and were not influenced by the presence of the UTR and the major SD (Fig. 3, lanes 5 to 8). By contrast, expression of the wild-type gag gene-derived product essentially depended on the presence of RRE, Rev, and the 5′ UTR including the major SD (Fig. 3, lanes 9 to 12), confirming previous observations made by several groups that Rev-dependent expression of late HIV-1 gene products is influenced by splice site usage (4, 13, 15, 18, 20). Pr55gag expression levels from codon-adapted genes exceeded those produced by the Rev-dependent wild-type gag reporter by 1.5- to 2-fold. Based on these results we conclude that the Rev responsiveness of HIV-1 late gene expression critically depends on appropriate wild-type codon usage.
FIG. 3.
Human H1299 lung carcinoma cells were transiently transfected with the indicated reporter constructs, which were either based on the wtgag or syngag gene. Rev responsiveness was determined by mock cotransfection (−) or cotransfection of a Rev expression plasmid (+). Expression of the gag reporter was determined by Western blot analysis of cell lysates and quantified by a Gag-specific capture ELISA (DuPont). Levels of Gag production were expressed as the percentage of Gag protein obtained after cotransfection of UTR-wtgag-RRE with Rev. The indicated values each represent the means of four independent transfection experiments. Standard deviations of the mean are indicated.
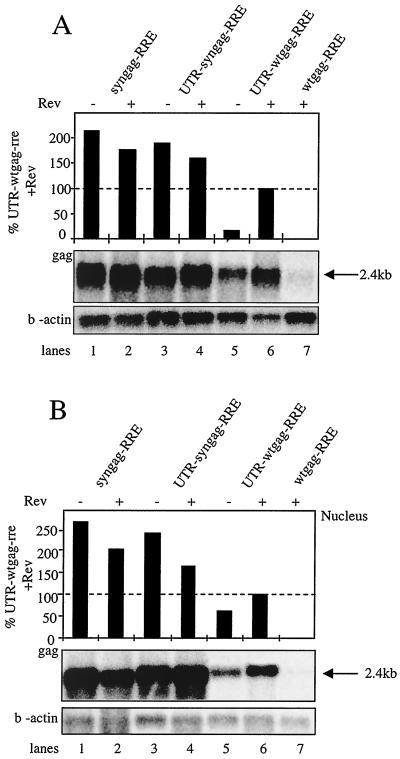
Transfected cells were partially lysed, nuclei were separated from the cytoplasm by centrifugation, and RNA was prepared from the fractions using the RNAeasy kit of Qiagen (Hilden, Germany). Northern blot analysis of nuclear and cytoplasmic fractions using radiolabeled riboprobes confirmed previous observations by others (7, 18, 32) in showing that in the absence of the SD the wild-type transcripts are targeted into a intranuclear degradative pathway (Fig. 4B, lane 7). The addition of the 5′ UTR and SD to wild-type gag (UTR-wtgag-RRE) led to a nuclear accumulation of Gag-encoding messages (Fig. 4B, lane 5) that were translocated into the cytoplasm in the presence of Rev (Fig. 4, lanes 6). By contrast syngag-encoded RNAs exhibiting marked differences in wobble positions and calculated RNA secondary structure (not shown) were readily detected both within the nucleus (Fig. 4B, lanes 1 to 4) and cytoplasm (Fig. 4A, lanes 1 to 4). It is noteworthy that nuclear and cytoplasmic levels of syngag transcripts were not influenced by the Rev-RRE interaction (Fig. 4, lanes 2 and 4) or by the presence of the 5′ UTR and SD (Fig. 4, lanes 1 and 3). The levels of nuclear syngag RNAs exceeded those of UTR-wtgag-RRE by more than twofold (Fig. 4B; compare lanes 1 to 4 with lane 5). Cotransfection of Rev seemed to increase levels of nuclear wtgag transcripts (Fig. 4B; compare lanes 5 and 6) by approximately twofold. In accordance with the expression data, cytoplasmic levels of syngag mRNA exceeded those achieved by UTR-wtgag-RRE and Rev by about 50 to 70% (Fig. 4A; compare lanes 1 to 4 with lane 6), by five- to eightfold in absence of Rev (Fig. 4A; compare lanes 1 to 4 with lane 5), and by several orders of magnitude in the absence of the 5′ UTR (Fig. 4A; compare lanes 1 to 4 with lane 7). Taken together, this clearly demonstrates that the elimination of proposed inhibitory sequence elements by a consequent codon usage adaptation transforms Gag-encoding transcripts into an RNA species with altered characteristics, rendering nuclear export and Gag expression completely independent from the presence of the SD and Rev/RRE interactions.
FIG. 4.
Northern blot analysis of cytoplasmic and nuclear RNA. H1299 cells were transfected with the indicated constructs and harvested 48 h posttransfection. Rev responsiveness was tested by cotransfection of a Rev expression plasmid (+) or empty vector (−). Cells were partially lysed, and nuclei were separated from the cytoplasm. RNA was prepared from the cytoplasmic fraction (A) and subjected, together with RNA purified from the nuclei (B), to Northern blot analysis. Gag-encoding transcripts and β-actin RNAs were detected by a radiolabeled RRE antisense riboprobe and a β-actin-specific DNA probe, respectively. The positions and calculated lengths of the Gag-encoding RNAs are indicated at the right. Intensities of the gag- and β-actin-specific signals were quantified by a PhosphorImager. Bars in panel A represent the relative amounts of specifically detected gag transcripts following normalization to the β-actin control.
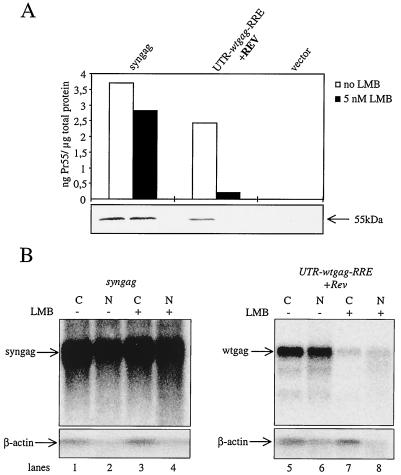
To determine whether or not the observed differences in the RNA phenotypes can be correlated to different nuclear export pathways, the influence of leptomycin B (LMB) on the nuclear export of the different RNA species was investigated. LMB has been shown recently to effectively inhibit Rev function due to its ability to directly interfere with CRM1 (exportin1)/Ran-GTP-mediated nuclear export (17, 24, 36). Culture media of cells transfected with syngag alone and with UTR-wtgag-RRE with Rev were supplemented with 5 nM LMB 24 h prior to harvesting cells. As demonstrated in Fig. 5A, Rev-dependent expression of Gag in UTR-wtgag-RRE-transfected cells was extremely sensitive to LMB treatment, resulting in significantly decreased levels of Gag expression (>90%), confirming previous results showing that LMB efficiently blocks the expression of HIV-1 late gene products within nanomolar concentrations (3, 9, 36). For comparison, syngag-driven Gag expression was decreased only by a very minor extent (10 to 20%). Northern blot analysis showing the subcellular distribution of Gag-encoding mRNAs revealed that LMB remarkably decreased cytoplasmic levels of wild-type RNAs (Fig. 5B; compare lanes 5 and 7), confirming previous LMB inhibitor studies (1, 3, 11, 36). However, we also consistently observed reduced levels of nuclear wild-type gag transcripts (Fig. 5B; compare lanes 6 and 8). This observation is in agreement with recent data on the Rev function of related lentiviruses (24), suggesting that disruption of CRM1 function causes unspliced Rev-dependent RNAs to be unstable in the nucleus, even in the presence of Rev and RRE. We speculate that Rev liberates INS-containing RNAs from the protecting splicing machinery, thereby dragging them into a intranuclear compartment where they are susceptible to degradation if they are not exported by CRM1 (due to LMB inhibition). In sharp contrast to the wild-type INS-containing RNAs, syngag-derived transcripts were readily detected in the nucleus and were shown to be exported constitutively to the cytoplasm whether or not LMB was present (Fig. 5B, lanes 1 to 4). We therefore conclude that, by altering codon usage and thereby eliminating proposed INS elements, Gag-encoding transcripts are targeted to a different, CRM1-independent nuclear export pathway. This implies that the targeting of the Gag-encoding RNAs to the Rev/CRM1 export pathway is critically determined by the sequence composition of the wtgag gene.
FIG. 5.
Influence of codon usage within the gag gene on LMB sensitivity and nuclear export pathway. (A) Influence of LMB on Gag expression from the indicated reporter constructs. H1299 cells were transfected by either syngag or UTR-wtgag-RRE in combination with Rev, each in the absence or presence of 5 nM LMB. Cells were harvested 48 h posttransfection. Synthesized Gag protein was determined from cell lysates by Western blot analysis (bottom) and quantified by a capture ELISA (top). The Pr55gag polyprotein is indicated at the right. (B) Influence of LMB on subcellular distribution of Gag-encoding RNA. H1299 cells were transfected with the indicated constructs and cultivated with (+) or without (−) LMB (5 nM). Nuclear (N) and cytoplasmic (C) levels of Gag-encoding RNA were determined by Northern blot analysis. Gag-encoding transcripts were detected by radiolabeled riboprobes specifically matching the syngag or the wtgag RNAs. β-Actin RNAs were detected by a radiolabeled β-actin-specific DNA probe.
Besides inefficient splice site usage, nuclear retention of RNAs encoding late HIV-1 proteins such as Gag was related to the INS elements localized within the Gag precursor (21, 29–31). These repressor elements have been proposed on the basis of silent point mutations that have been introduced into selected codons of a long terminal repeat promoted Gag expression construct (29, 30). However, when RRE was fused to the partly mutated gag construct and Rev was added in trans, the expression of Gag could be still significantly increased by 5.5-fold, indicating that viral regulation was at least only partly overcome by this strategy (29, 30). In contrast, our study revealed that the consequent codon usage adaptation of the entire HIV-1 gag gene involving more than 400 nucleotide substitutions allowed high-level gag expression that could not be further increased by introducing the Rev/RRE system. This obvious difference from the above-mentioned earlier studies may be explained by the low frequency and the clustering of mutations that were introduced into the p17 (28 substitutions) and p24 (56 positions) coding regions by Pavlakis and colleagues.
Thus, the construction of a codon-optimized gag gene enabled us to truly eliminate so-called AU-rich repressor sequences. We therefore strongly suggest that the overall AU content of the gag RNA, rather than previously proposed INS elements, contributes to stability and nuclear retention of wild-type gag RNAs. This assumption is in accordance with several publications correlating instability of certain cellular mRNAs with their AU content or the presence of AU-rich elements (reviewed in reference 5). Furthermore, we were able to show that increased levels of expression achieved after codon optimization of our gag reporter is due to nuclear stability and constitutive nuclear export of its transcripts rather than increased translational efficiency. Consequently, different nuclear export pathways of wild-type and codon-optimized gag RNAs could be demonstrated by the CRM1-independent export of the optimized transcripts.
Moreover, neither the addition of the 5′ UTR, including the major SD, nor the supplementation of the assay system with RRE and Rev significantly influenced the levels of Gag expression from the synthetic reporter construct. Accordingly, a cis-acting function of an isolated 5′ SD, which has been reported previously in several studies employing different INS-containing wild-type HIV sequences (4, 14, 20), was found only in combination with the wtgag gene reporter, not with the synthetic gag gene lacking AU-rich sequence elements. These data strongly support the hypothesis that, in addition to inefficient splicing, AU-rich sequence elements are required to render late HIV-1 transcripts Rev responsive (20, 29–31).
Acknowledgments
We thank B. Wolff for generously providing LMB.
This work was supported by BMBF grant no. 01KI9765/3 to R.W.
REFERENCES
- 1.Askjaer P, Jensen T H, Nilsson J, Englmeier L, Kjems J. The specificity of the CRM1-Rev nuclear export signal interaction is mediated by RanGTP. J Biol Chem. 1998;273:33414–33422. doi: 10.1074/jbc.273.50.33414. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 2. 1994. pp. A1.8–A1.9. [Google Scholar]
- 3.Bogerd H P, Echarri A, Ross T M, Cullen B R. Inhibition of human immunodeficiency virus Rev and human T-cell leukemia virus Rex function, but not Mason-Pfizer monkey virus constitutive transport element activity, by a mutant human nucleoporin targeted to Crm1. J Virol. 1998;72:8627–8635. doi: 10.1128/jvi.72.11.8627-8635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang D D, Sharp P A. Regulation by HIV Rev depends upon recognition of splice sites. Cell. 1989;59:789–795. doi: 10.1016/0092-8674(89)90602-8. [DOI] [PubMed] [Google Scholar]
- 5.Chen C Y, Shyu A B. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci. 1995;20:465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- 6.Cochrane A W, Jones K S, Beidas S, Dillon P J, Skalka A M, Rosen C A. Identification and characterization of intragenic sequences which repress human immunodeficiency virus structural gene expression. J Virol. 1991;65:5305–5313. doi: 10.1128/jvi.65.10.5305-5313.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui Y, Iwakuma T, Chang L J. Contributions of viral splice sites and cis regulatory elements to lentivirus vector function. J Virol. 1999;73:6171–6176. doi: 10.1128/jvi.73.7.6171-6176.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dyhr-Mikkelsen H, Kjems J. Inefficient spliceosome assembly and abnormal branch site selection in splicing of an HIV-1 transcript in vitro. J Biol Chem. 1995;270:24060–24066. doi: 10.1074/jbc.270.41.24060. [DOI] [PubMed] [Google Scholar]
- 9.Fischer U, Huber J, Boelens W C, Mattaj I W, Luhrmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 10.Fischer U, Pollard V W, Luhrmann R, Teufel M, Michael M W, Dreyfuss G, Malim M H. Rev-mediated nuclear export of RNA is dominant over nuclear retention and is coupled to the Ran-GTPase cycle. Nucleic Acids Res. 1999;27:4128–4134. doi: 10.1093/nar/27.21.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 12.Graham F L, Eb A J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 13.Hammarskjold M L, Heimer J, Hammarskjold B, Sangwan I, Albert L, Rekosh D. Regulation of human immunodeficiency virus env expression by the rev gene product. J Virol. 1989;63:1959–1966. doi: 10.1128/jvi.63.5.1959-1966.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammarskjold M L, Li H, Rekosh D, Prasad S. Human immunodeficiency virus env expression becomes Rev independent if the env region is not defined as an intron. J Virol. 1994;68:951–958. doi: 10.1128/jvi.68.2.951-958.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kjems J, Frankel A D, Sharp P A. Specific regulation of mRNA splicing in vitro by a peptide from HIV-1 Rev. Cell. 1991;67:169–178. doi: 10.1016/0092-8674(91)90580-r. [DOI] [PubMed] [Google Scholar]
- 16.Kjems J, Sharp P A. The basic domain of Rev from human immunodeficiency virus type 1 specifically blocks the entry of U4/U6.U5 small nuclear ribonucleoprotein in spliceosome assembly. J Virol. 1993;67:4769–4776. doi: 10.1128/jvi.67.8.4769-4776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudo N, Wolff B, Sekimoto T, Schreiner E P, Yoneda Y, Yanagida M, Horinouchi S, Yoshida M. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp Cell Res. 1998;242:540–547. doi: 10.1006/excr.1998.4136. [DOI] [PubMed] [Google Scholar]
- 18.Lu X B, Heimer J, Rekosh D, Hammarskjold M L. U1 small nuclear RNA plays a direct role in the formation of a rev-regulated human immunodeficiency virus env mRNA that remains unspliced. Proc Natl Acad Sci USA. 1990;87:7598–7602. doi: 10.1073/pnas.87.19.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maldarelli F, Martin M A, Strebel K. Identification of posttranscriptionally active inhibitory sequences in human immunodeficiency virus type 1 RNA: novel level of gene regulation. J Virol. 1991;65:5732–5743. doi: 10.1128/jvi.65.11.5732-5743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikaelian I, Krieg M, Gait M J, Karn J. Interactions of INS (CRS) elements and the splicing machinery regulate the production of Rev-responsive mRNAs. J Mol Biol. 1996;257:246–264. doi: 10.1006/jmbi.1996.0160. [DOI] [PubMed] [Google Scholar]
- 21.Nasioulas G, Zolotukhin A S, Tabernero C, Solomin L, Cunningham C P, Pavlakis G N, Felber B K. Elements distinct from human immunodeficiency virus type 1 splice sites are responsible for the Rev dependence of env mRNA. J Virol. 1994;68:2986–2993. doi: 10.1128/jvi.68.5.2986-2993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsen H S, Cochrane A W, Rosen C. Interaction of cellular factors with intragenic cis-acting repressive sequences within the HIV genome. Virology. 1992;191:709–715. doi: 10.1016/0042-6822(92)90246-l. [DOI] [PubMed] [Google Scholar]
- 23.O'Reilly M M, McNally M T, Beemon K L. Two strong 5′ splice sites and competing, suboptimal 3′ splice sites involved in alternative splicing of human immunodeficiency virus type 1 RNA. Virology. 1995;213:373–385. doi: 10.1006/viro.1995.0010. [DOI] [PubMed] [Google Scholar]
- 24.Otero G C, Harris M E, Donello J E, Hope T J. Leptomycin B inhibits equine infectious anemia virus Rev and feline immunodeficiency virus Rev function but not the function of the hepatitis B virus posttranscriptional regulatory element. J Virol. 1998;72:7593–7597. doi: 10.1128/jvi.72.9.7593-7597.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollard V W, Malim M H. The HIV-1 Rev protein. Annu Rev Microbiol. 1998;52:491–532. doi: 10.1146/annurev.micro.52.1.491. [DOI] [PubMed] [Google Scholar]
- 26.Powell D M, Amaral M C, Wu J Y, Maniatis T, Greene W C. HIV Rev-dependent binding of SF2/ASF to the Rev response element: possible role in Rev-mediated inhibition of HIV RNA splicing. Proc Natl Acad Sci USA. 1997;94:973–978. doi: 10.1073/pnas.94.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ratner L, Fisher A, Jagodzinski L L, Mitsuya H, Liou R S, Gallo R C, Wong-Staal F. Complete nucleotide sequences of functional clones of the AIDS virus. AIDS Res Hum Retrovir. 1987;3:57–69. doi: 10.1089/aid.1987.3.57. [DOI] [PubMed] [Google Scholar]
- 28.Rosen C A, Terwilliger E, Dayton A, Sodroski J G, Haseltine W A. Intragenic cis-acting art gene-responsive sequences of the human immunodeficiency virus. Proc Natl Acad Sci USA. 1988;85:2071–2075. doi: 10.1073/pnas.85.7.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider R, Campbell M, Nasioulas G, Felber B K, Pavlakis G N. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J Virol. 1997;71:4892–4903. doi: 10.1128/jvi.71.7.4892-4903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz S, Campbell M, Nasioulas G, Harrison J, Felber B K, Pavlakis G N. Mutational inactivation of an inhibitory sequence in human immunodeficiency virus type 1 results in Rev-independent gag expression. J Virol. 1992;66:7176–7182. doi: 10.1128/jvi.66.12.7176-7182.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz S, Felber B K, Pavlakis G N. Distinct RNA sequences in the gag region of human immunodeficiency virus type 1 decrease RNA stability and inhibit expression in the absence of Rev protein. J Virol. 1992;66:150–159. doi: 10.1128/jvi.66.1.150-159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seguin B, Staffa A, Cochrane A. Control of human immunodeficiency virus type 1 RNA metabolism: role of splice sites and intron sequences in unspliced viral RNA subcellular distribution. J Virol. 1998;72:9503–9513. doi: 10.1128/jvi.72.12.9503-9513.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staffa A, Cochrane A. The tat/rev intron of human immunodeficiency virus type 1 is inefficiently spliced because of suboptimal signals in the 3′ splice site. J Virol. 1994;68:3071–3079. doi: 10.1128/jvi.68.5.3071-3079.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner R, Deml L, Fliessbach H, Wanner G, Wolf H. Assembly and extracellular release of chimeric HIV-1 Pr55gag retrovirus-like particles. Virology. 1994;200:162–175. doi: 10.1006/viro.1994.1175. [DOI] [PubMed] [Google Scholar]
- 35.Wolf H, Modrow S, Soutschek E, Motz M, Grunow R, Döbl H. Production, mapping and biological characterisation of monoclonal antibodies to the core protein (p24) of the human immunodeficiency virus type 1. AIFO (AIDS-Forsch) 1990;1:24–29. [Google Scholar]
- 36.Wolff B, Sanglier J J, Wang Y. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem Biol. 1997;4:139–147. doi: 10.1016/s1074-5521(97)90257-x. [DOI] [PubMed] [Google Scholar]