Abstract
Although cancer initiation and progression are generally associated with the accumulation of somatic mutations1,2, substantial epigenomic alterations underlie many aspects of tumorigenesis and cancer susceptibility3–6, suggesting that genetic mechanisms might not be the only drivers of malignant transformation7. However, whether purely non-genetic mechanisms are sufficient to initiate tumorigenesis irrespective of mutations has been unknown. Here, we show that a transient perturbation of transcriptional silencing mediated by Polycomb group proteins is sufficient to induce an irreversible switch to a cancer cell fate in Drosophila. This is linked to the irreversible derepression of genes that can drive tumorigenesis, including members of the JAK–STAT signalling pathway and zfh1, the fly homologue of the ZEB1 oncogene, whose aberrant activation is required for Polycomb perturbation-induced tumorigenesis. These data show that a reversible depletion of Polycomb proteins can induce cancer in the absence of driver mutations, suggesting that tumours can emerge through epigenetic dysregulation leading to inheritance of altered cell fates.
Subject terms: Epigenetic memory, Gene silencing
A transient perturbation of transcriptional silencing mediated by Polycomb proteins is sufficient to induce an epigenetic cancer cell fate in Drosophila in the absence of driver mutations.
Main
Genetic, epigenetic and environmental inputs are deeply intertwined, making it difficult to disentangle their respective contributions to cell fate decisions8,9, and epigenetic reprogramming is a major contributor to tumour plasticity and adaptation10,11. Over recent decades, large-scale projects expanded the known repertoire of cancer-associated genetic mutations affecting epigenetic factors12,13, including chromatin remodellers and modifiers, which regulate histone marks14,15, DNA methylation16, micro-RNAs17 and 3D-genome folding18, corroborating the role of epigenetic aberrations in the aetiology of haematological and solid malignancies19,20. Indeed, epigenetic modifications are used as biomarkers and are targeted by epi-drugs in cancer therapy21. Tumorigenesis is therefore associated with genetic as well as epigenetic determinants22–25. The fact that several hallmarks of human cancer24,26 may be acquired through epigenome dysregulation suggests that epigenetic alterations play causal roles in cancer4,27,28 and in metastatic progression29–33. In some paediatric cancers, such as posterior fossa ependymoma, low numbers of mutations were detected, consistent with the possibility that epigenetic changes may drive tumorigenesis30. These observations suggest that cancer is not solely a consequence of DNA mutations34,35, but whether purely non-genetic reprogramming mechanisms are sufficient to initiate tumorigenesis remains an open question. Polycomb group (PcG) proteins are epigenetic factors forming two main classes of complexes called Polycomb Repressive Complex 1 and 2 (PRC1 and PRC2, respectively), which are highly conserved from fly to human and play a critical role in cellular memory by repressing developmental genes throughout development36. PcG dysregulation leads to cell fate changes37, developmental transformations and is associated with cancer38. PRC2 deposits the H3K27me3 repressive mark, whereas PRC1, which contains the PH, PC, PSC and the SCE subunits in flies, is responsible for H2AK118Ub deposition36. Contrasting with the redundancy found in mammals36, most PcG components are encoded by a single gene in Drosophila, making this system more tractable for functional studies39.
Epigenetic perturbations initiate tumours
Null mutations or constant RNAi (RNA interference) knock-down (KD) targeting both ph homologues (ph-p and ph-d, which we refer to as ph for simplicity) can induce growth defects, loss of differentiation and cell overproliferation40–43. To test whether a transient epigenetic perturbation might initiate an irreversible change in cell fate, we set up a thermosensitive ph-RNAi system enabling the reversible KD of ph in the developing larval eye imaginal disc (ED) (Fig. 1a,b and Extended Data Fig. 1a–d). The PH protein is depleted in 24 h at 29 °C and is restored within 48 h of recovery at 18 °C (Extended Data Fig. 1e).
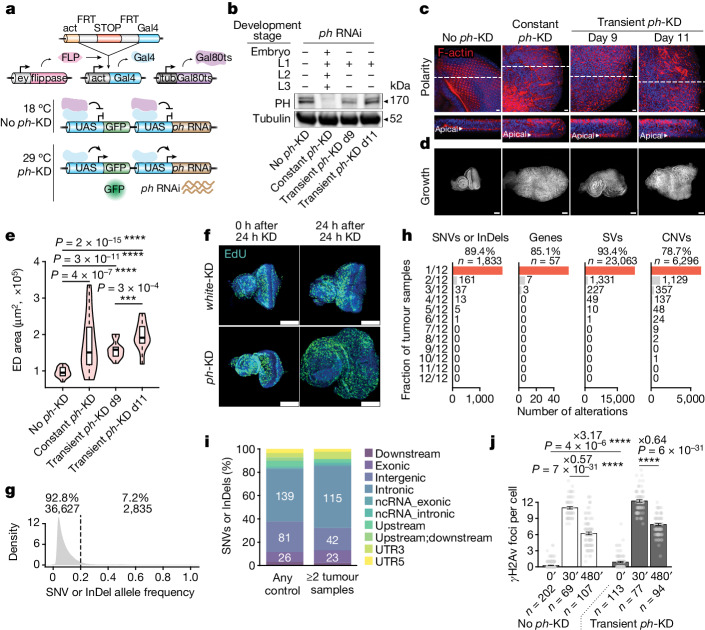
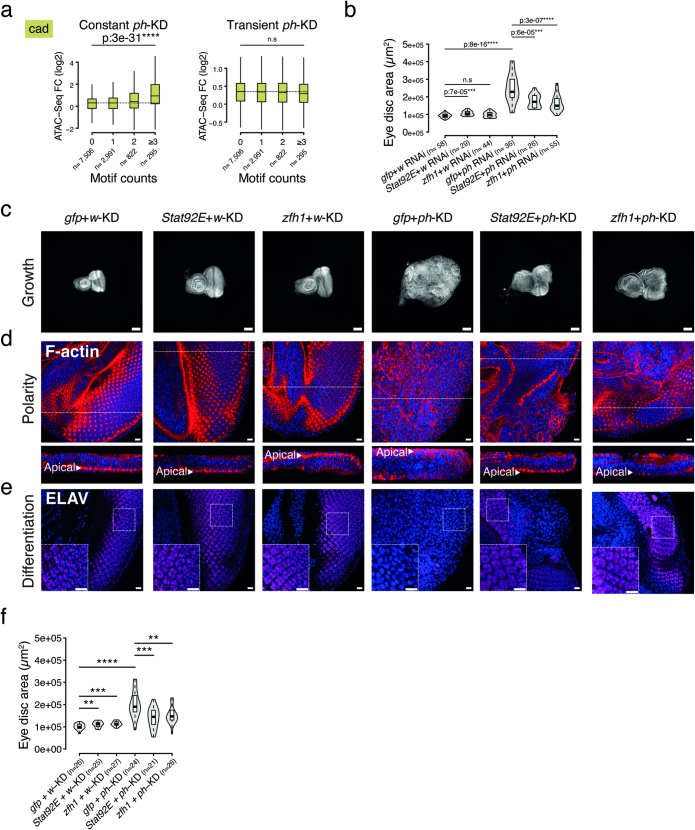
Fig. 1. Transient PRC1 depletion is sufficient to initiate tumours.
a, Scheme depicting the conditional ph-KD system (Methods). b, Western blot analysis of PH protein concentrations in the EDs of L3 larvae subjected to no ph-KD (control), constant or transient ph-KD at L1 stage. c, Representative confocal images of F-actin staining (red) showing a polarized epithelium with apical F-actin (xz cross-sections at the bottom) in no ph-KD (control, left), whereas polarity is disrupted on constant or transient ph-KD EDs (dissected at L3 stage). DNA is stained with DAPI (blue). d,e, DAPI staining (d) is used to measure ED areas (e) under no ph-KD (control), constant or transient ph-KD conditions (n = 30 EDs per condition; two-sided Wilcoxon test: ***P < 1 × 10−3, ****P < 1 × 10−5; box plots show the median (line), upper and lower quartiles (box) ±1.5× interquartile range (whiskers); outliers are not shown). f, EdU staining (green) imaged at 0 h (left) and 24 h (right) after 24 h of w-KD (control, top) or ph-KD (bottom). g, Distribution of somatic SNVs or InDel allele frequencies detected in all samples. h, Number of tumour samples in which each SNVs or InDels, gene with deleterious SNVs or InDels, structural variants (SVs) and CNVs were found. i, Feature distribution of SNVs or InDels found in any of the control samples (no ph-KD, left bar) or shared between at least two tumour samples (right bar). j, Number of γH2Av foci per cell before (0 min; indicated as 0′) and after (30 and 480 min, indicated as 30′ and 480′) exposure to 5 Gy irradiation in control (no ph-KD, left) or transient ph-KD EDs (right). Individual data points are shown in grey and bars correspond to the mean ± standard error (whiskers). Two-sided t-test ****P < 1 × 10−5. Scale bars, 10 μm (c), 100 μm (d,f).
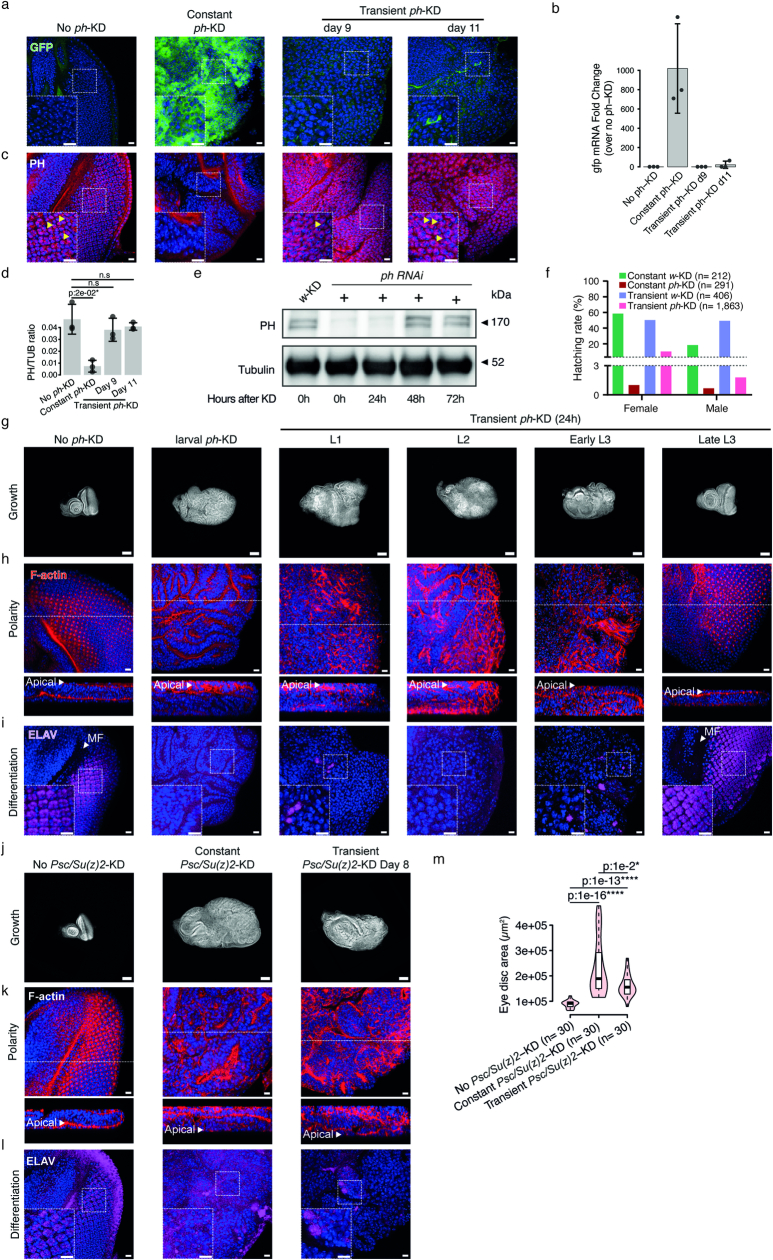
Extended Data Fig. 1. Transient PRC1 depletion generates neoplastic tumours that persist after PH protein recovery.
a- GFP staining (in green) used as a readout of the conditional ph knockdown (ph-KD) system described in Fig. 1a after no ph-KD (control), constant or transient ph-KD. The tissues were counterstained with DAPI (blue). Two independent experiments were performed with similar results. b- gfp mRNA fold change RT-qPCR measurement after no ph-KD (control), constant or transient ph-KD. Bars correspond to the mean ± standard deviation (whiskers) inferred from three biological replicates (grey dots). c- PH immunostaining (in red) after no ph-KD (control), constant or transient ph-KD. The tissues were counterstained with DAPI (blue). Two independent experiments were performed with similar results. d- Quantification of the western blot illustrated in Fig. 1b and of two other biological replicates. Bars correspond to the mean ratio ±standard deviation (whiskers) of the signal of PH over that of TUBULIN (PH/TUB) calculated from three biological replicates (grey dots). Two-sided unpaired t.test: *pval < 0.05, ns = pval > 0.05 (not significant). Error bars represent the standard error of the mean for three biological replicates. Dunnet’s test: ns = not significant, **pval < 0.01. e- Western blot showing the PH protein after early L3 EDs were subjected to 24 h of white-KD (w-KD, control) or ph-KD followed by 0 h, 24 h, 48 h and 72 h of recovery at 18 °C (see bottom axis). This time course illustrates acute depletion and allows visualization of the kinetics of PH recovery after ph-KD. f- Hatching rate after constant or transient ph-KD. g-i- DAPI (in grey, g), F-actin (in red, h) and ELAV (in magenta, i) stainings of L3 EDs after no ph-KD (control), ph-KD throughout the three larval stages (L1, L2, L3) or transient (24 h) ph-KD during the first (L1), second (L2), early (Early L3) or late (Late L3) of the L3 stage, respectively. DAPI staining is used to assess ED growth, F-actin for apico-basal polarity, and the neuronal marker ELAV for differentiation. Note that late L3 tissues look normal immediately after the end of the ph-KD. Nevertheless, their cells are reprogrammed into a malignant state, as indicated by the fact that allografts of these tissues induce tumours, as shown in Extended Data Fig. 7i, j. Two independent experiments were performed with similar results. Scale bars: 10 μm (a, c, h, i), 100 μm (g). j-l- DAPI (in gray, j), F-actin (in red, k) and ELAV (in magenta, l) stainings of EDs after no Psc/Suz(2)-KD (control, left), constant (middle) or transient Psc/Suz(2)-KD (right), respectively. DAPI staining is used to assess growth, F-actin for apico-basal polarity, and the neuronal marker ELAV for differentiation. Two independent experiments were performed with similar results. m- ED sizes quantified as overall area of DAPI staining after no Psc/Suz(2)-KD (control), constant or transient Psc/Suz(2)-KD conditions. n = 30 for each condition. Two-sided Wilcoxon test: *pval < 5e-2, ****pval<1e-5. Box plots show the median (line), upper and lower quartiles (box) ±1.5x interquartile range (whiskers), outliers are not shown. Scale bars: 100 μm (j), 10 μm (k, l).
As expected, on constant PH depletion throughout development, 100% of EDs collected at the third larval stage (L3) are transformed into tumours (Fig. 1c,d and Methods), resulting in reduced viability (Extended Data Fig. 1f). A transient 24 h depletion of PH at the L1 stage, during which the ED starts developing, is also sufficient to trigger tumour formation in L3 EDs, characterized by overgrowth, loss of apico-basal cell polarity and of the ELAV differentiation marker (Fig. 1c–e and Extended Data Fig. 1g–i). These tumours show normal concentrations of PH protein in L3 EDs, both at day 9 (transient ph-KD d9) and day 11 (transient ph-KD d11) after egg laying (AEL) (Fig. 1b and Extended Data Fig. 1c,d). EDs continue to grow after PH recovery (Fig. 1e) and cannot differentiate (Extended Data Fig. 1i), suggesting that the tumour state is stable and maintained independently of its epigenetic trigger. Likewise, PH depletion at L2 or early L3 stage induces tumours (Extended Data Fig. 1g–i), suggesting that PRC1 is required throughout development to prevent tumorigenesis. Transient depletion of PSC-SU(Z)2, another core PRC1 subunit for which null mutations drive neoplastic transformation44, is also sufficient to induce tumorigenesis (Extended Data Fig. 1j–m).
Transient PH depletion induces tumours with 100% penetrance within 2 days, as illustrated by the early L3 PH depletion experiment (Extended Data Fig. 1g–i). To assess whether such tumours may arise from a clonal subpopulation of cells, we performed EdU (5-ethynyl-2′-deoxyuridine) staining after 24 h ph-KD in early L3 EDs (Fig. 1f and Supplementary Videos 1 and 2). Aberrant replication was observed throughout the tissue within 24 h, indicating that most or all cells undergo malignant transformation. For DNA mutations to drive these tumours, they should simultaneously occur in many cells to trigger overproliferation in the whole tissue. Given the low frequency of deleterious mutations per cell generation (about 1.2 per genome45) and the limited number of genes that can act as cancer drivers in Drosophila46, this scenario seemed unlikely. Nevertheless, we sequenced whole cancer genomes by collecting eggs from several independent crosses of mated females and subjecting them to transient KD, constant KD or no ph-KD (control condition), before sequencing their genomic DNA (gDNA). In total, we sequenced four independent control samples as well as 12 independent tumour samples (Methods). When using batch-matched control tissues (no ph-KD) to identify single nucleotide variants (SNV) or small insertions and deletions (InDels)46, we found that 68.1% of the identified variants are present in only one of the samples and that 7 out of 12 tumour samples contained fewer SNVs or InDels than at least one of the control samples (Extended Data Fig. 2a), ruling out that PH depletion induces a massive increase in mutation rates and consistent with previous data47. Moreover, 92.8% of the identified SNVs or InDels had an allele frequency below 0.2, precluding them from driving whole-tissue tumours (Fig. 1g). Regarding SNVs or InDels with an allele frequency higher than 0.2, none of them was shared among the 12 tumour samples (Fig. 1h). Instead, 89% were found in only one sample and the 217 variants shared between at least two tumours had similar feature distributions compared to the variants found in control samples, without bias towards exons (Fig. 1i). No genes contained deleterious SNVs or InDels in all tumour samples, and similar results were found when considering structural variants or copy number variations (CNVs) (Fig. 1h and Methods). Together, these results argue strongly against the presence of recurrent driver mutations in these tumours.
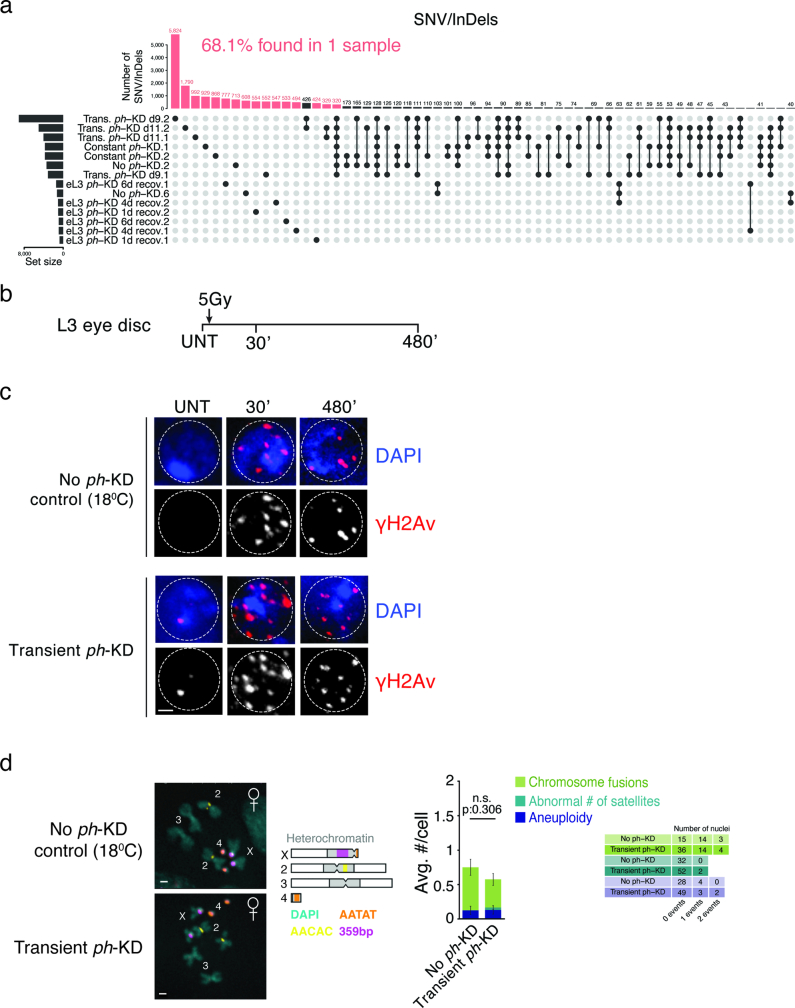
Extended Data Fig. 2. ph-KD does not induce the accumulation of mutations or aneuploidy.
a- SNV/InDels overlaps between all sequenced gDNA samples. Each vertical bar corresponds to an intersection (corresponding samples are shown below) and horizontal bars (bottom left) indicate the total number of SNV/InDels found in each sample. Only intersections containing ≥40 SNV/InDels are shown and SNV/InDels that are specific to one sample are shown in orange (68.1% of all SNV/InDels detected). b- Schematic view of the repair kinetic experiments. γH2Av foci were counted before or 30 min and 480 min after ionizing radiation (IR). c- Representative γH2Av staining in no ph-KD (control, top) and transient ph-KD EDs (bottom). Nuclei were counterstained with DAPI (in blue). d- Representative karyotypes (left) and quantification of chromosome abnormalities in EDs after no ph-KD (control, top) and transient ph-KD (bottom). The schematic representation shows the position of the satellites stained by FISH. Abnormalities were quantified from two biological replicates per condition (bar plot on the right, n = 32 for No ph-KD and n = 53 for ph-KD karyotypes). Bars correspond to the mean number of aberrations per cell ±standard error (whiskers). Two-sided t.test: ns = pval>0.05 (not significant). For each type of type of abnormality (see colour legend), the number of counted events are shown on the right (tables). Scale bars = 1 μm (c, d).
To test whether transient PH depletion could induce genome instability, we counted the number of phospho-H2AvD foci (γH2Av) per cell in control (no ph-KD) and transient ph-KD tumours before and during a time course after irradiation. Despite a slightly higher number of foci before irradiation, probably due to the higher fraction of cells engaged in DNA replication, tumour and control samples showed a similar decrease in the number of γH2Av foci between 30 and 480 minutes after irradiation (Fig. 1j and Extended Data Fig. 2b,c), suggesting that these tumours can efficiently repair DNA breaks to prevent the accumulation of mutations. Finally, karyotype analysis of the tumours collected on transient ph-KD did not show significant differences in chromosomal rearrangements compared to control samples (Extended Data Fig. 2d).
In summary, transient depletion of PRC1 components is sufficient to switch cells into a neoplastic state that is maintained even after normal PcG protein concentrations are re-established. As the same genotype can generate both a normal phenotype or a tumour depending on a transient gene regulatory modification in the absence of DNA driver mutations, we defined these tumours as epigenetically initiated cancers (EICs).
JAK–STAT signalling activation in EICs
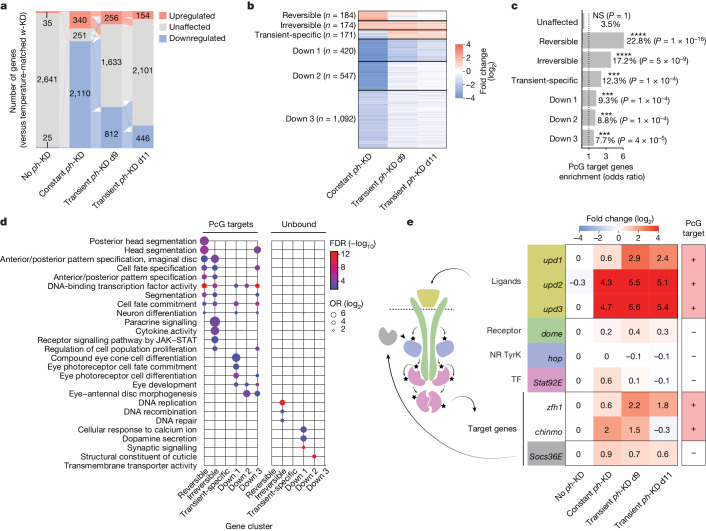
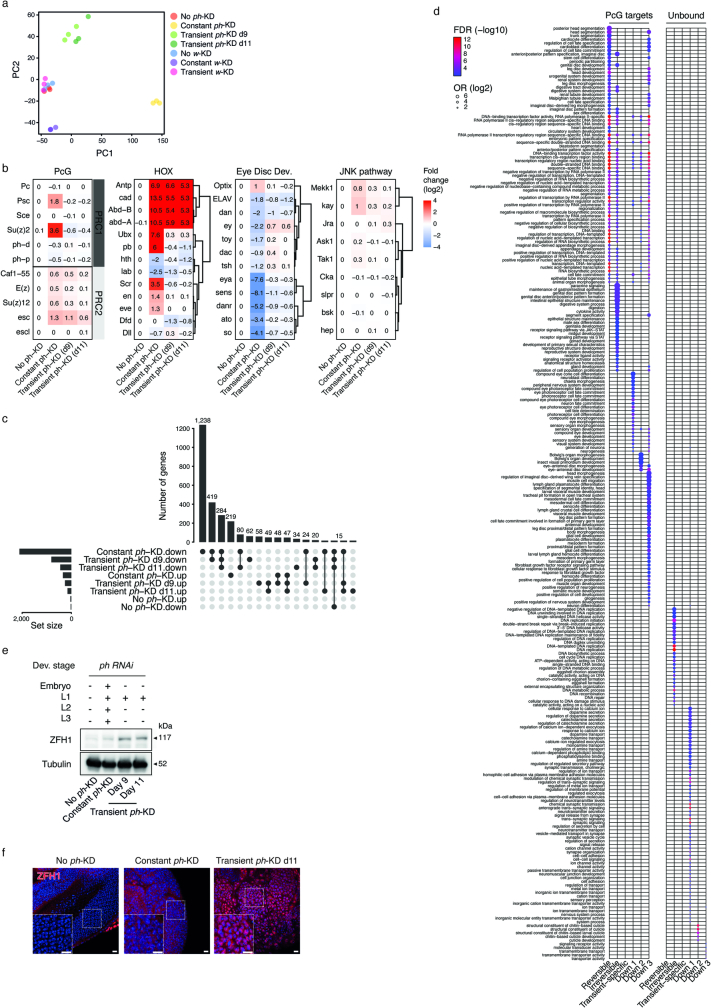
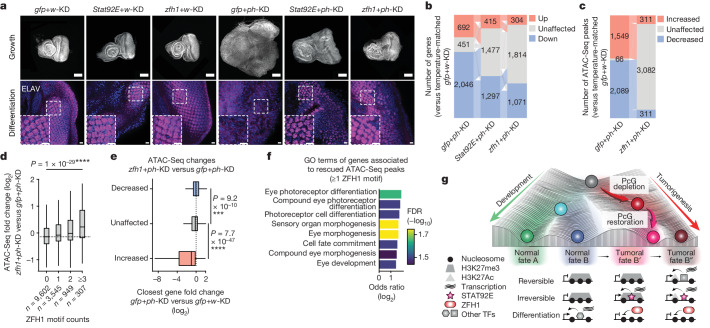
We compared the transcriptomes of the control condition (no ph-KD), transient and constant ph-KD tumours to temperature-matched controls, generated with a similar RNAi system targeting the white (w) gene, which is dispensable for normal eye development (differential transcriptome analyses are available in Supplementary Table 1). As expected, the ph-RNAi and the w-RNAi lines are hardly distinguishable at 18 °C, as well as in the transient w-KD condition (Fig. 2a and Extended Data Fig. 3a). Consistent with our previous work41,42, constant ph-KD is associated with the upregulation of 340 genes—including canonical PcG targets such as Hox and developmental transcription factor genes—and the down-regulation of 2,110 genes, including most key regulators of ED development (Fig. 2a and Extended Data Fig. 3b). Only a subset of these genes was also differentially expressed in transient ph-KD at d9 AEL (256 and 812, respectively), and even less at later d11 AEL (154 and 446, respectively), suggesting a progressive yet incomplete rescue of the transcriptome (Fig. 2a and Extended Data Fig. 3a–c). Therefore, most (75%) of the transcriptional defects observed on constant ph-KD can be restored on reinstating normal levels of PH.
Fig. 2. EICs show irreversible transcriptional changes.
a, Alluvial plot showing differentially expressed genes after no ph-KD (control), constant and transient ph-KD. Transitions between upregulated (orange), unaffected (grey) and downregulated (blue) states are indicated by thin lines of the same respective colours. b, Clustering of differentially expressed genes after constant or transient ph-KD. c, Over-representation of direct PcG target genes (defined as more than or equal to 50% of the gene body overlapping a H3K27me3 repressive domain in control condition). One-sided Fisher’s exact test P values were corrected for multiple testing using FDR: ***FDR < 1 × 10−3, ****FDR < 1 × 10−5; NS, P > 0.05. d, Representative Gene Ontology terms enriched for each gene cluster, further stratified as being direct PcG targets (left) or not (right). The full chart is available in Extended Data Fig. 3d. e, Transcriptional fold changes of genes involved in the JAK–STAT signalling pathway on ph-KD. Direct PcG targets (+) are indicated in the right column.
Extended Data Fig. 3. Transcriptional defects after constant or transient ph-KD include induction of ZFH1.
a- Principal component analysis (PCA) of normalized RNA-Seq read counts for different conditions. Each dot corresponds to one biological replicate. A close distance between samples reflects their similarity, showing that the control samples (no w-KD, no ph-KD) and the transient w-KD are very similar. b- Transcriptional fold changes after no ph-KD (control), constant or transient ph-KD (see x-axis) of the PcG core components, Hox genes (canonical targets of PcG repression), key genes that regulate ED development and the JNK pathway core members. c- Overlaps of differentially expressed genes between indicated RNA-Seq samples. Each vertical bar corresponds to an intersection (corresponding samples are shown below) and horizontal bars (bottom left) indicate the total number of differentially expressed genes in each sample. d- GO terms enriched for each gene cluster, then stratified as being direct PcG targets ( ≥ 50% of the gene body overlaps a H3K27me3 repressive domain) in control condition (left) or not (right). e- Western blot showing ZFH1 levels in EDs after no ph-KD (control), constant or transient ph-KD. Three independent experiments were performed with similar results. f- ZFH1 immunostaining (in red) after no ph-KD (control), constant or transient ph-KD. Tissues were counterstained with DAPI (in blue). Two independent experiments were performed with similar results. Scale bars: 10 μm.
Hierarchical clustering of differentially expressed genes identified three clusters that are upregulated in at least one condition, and three downregulated clusters (Fig. 2b; clustering results available in Supplementary Table 2 and Methods). The upregulated clusters show stronger and significant over-representation of PcG target genes covered with H3K27me3 in control EDs (Fig. 2c). This suggests that their upregulation is a direct consequence of compromised PcG repression, although they retain distinct patterns. The ‘reversible’ cluster includes canonical PcG target genes such as en, eve, Ubx and Scr, that are upregulated on constant ph-KD but recover control levels of expression after transient ph-KD, precluding them from being required for the maintenance of EICs (Fig. 2b and Extended Data Fig. 3b). The same is true for ‘transient-specific’ genes, whose upregulation is dispensable for tumour growth after constant ph-KD.
The ‘irreversible’ cluster is of particular interest, as it contains a high fraction of PcG target genes that remain upregulated despite PH restoration and therefore represents candidate genes involved in the development of EICs (Fig. 2b,c). Whereas PcG target genes from the reversible and irreversible clusters share ontologies associated with developmental transcription factors, irreversible genes show specific enrichments for paracrine signalling and cytokine activity (Fig. 2d and Extended Data Fig. 3d), including the JAK–STAT ligands (upd1, upd2, upd3), which were shown to be associated with various tumours, including those depending on PcG mutations43,44,48 (Fig. 2e). In addition, chinmo and zfh1 are direct PcG targets that have been described to act downstream of the JAK–STAT pathway49 and are accordingly upregulated on PH depletion (Fig. 2e). The transcriptional repressor ZFH1 is of particular interest, because it remains upregulated at d11 AEL, is known to be involved in self-renewal and tumour growth50–52 and is conserved in mammals, in which its homologue ZEB1 can induce epithelial-to-mesenchymal transition53. Consistent with its transcriptional upregulation, ZFH1 protein is increased on constant PH depletion and even more on transient PH depletion (Extended Data Fig. 3e,f), suggesting that it might support the development of EICs.
Finally, we noted that irreversible genes that are not PcG targets are enriched for Gene Ontology (GO) terms related to DNA replication and repair (Fig. 2d and Extended Data Fig. 3d), suggesting that their upregulation may be a consequence of the proliferation of tumour cells. Together, these results indicate that EICs are driven by a restricted set of irreversibly upregulated genes, including major members of the JAK–STAT signalling pathway, rather than by the vast pleiotropic dysregulation of cancer genes that is observed on constant PH depletion. Therefore, we sought to investigate why this subset of genes remains irreversibly upregulated after restoration of normal PH levels and to test whether they are required for the development of EICs. For simplicity, unless explicitly stated, further investigations of transient ph-KD EDs were conducted on tissues collected at d11 AEL after a 24 h KD at the L1 stage, representing the condition with the smallest number of differentially expressed genes.
Chromatin analysis at irreversible genes
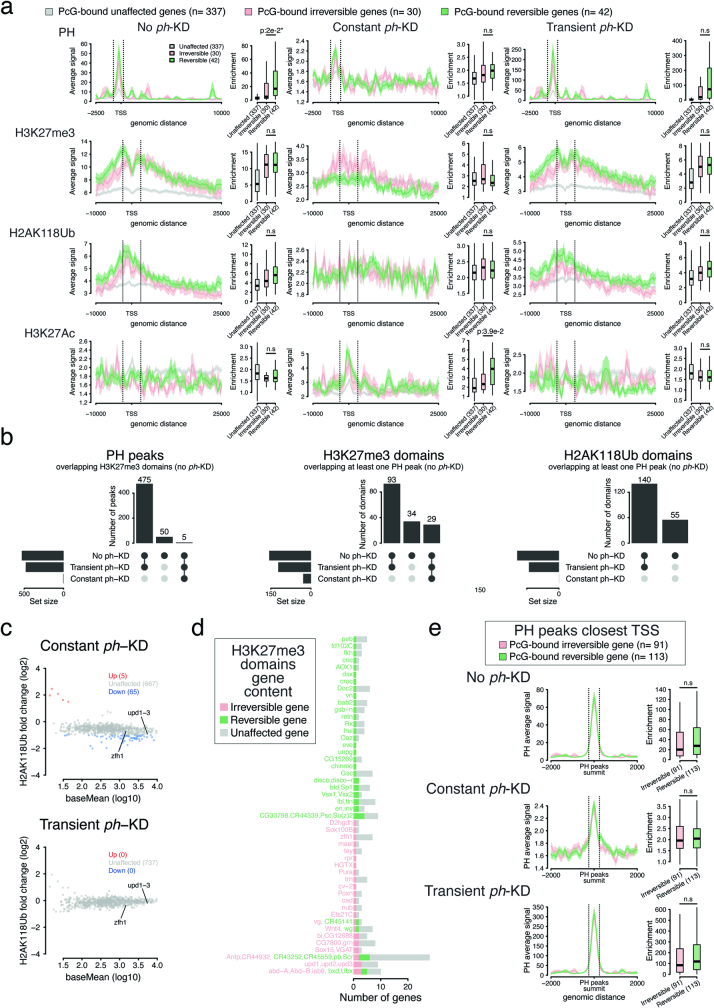
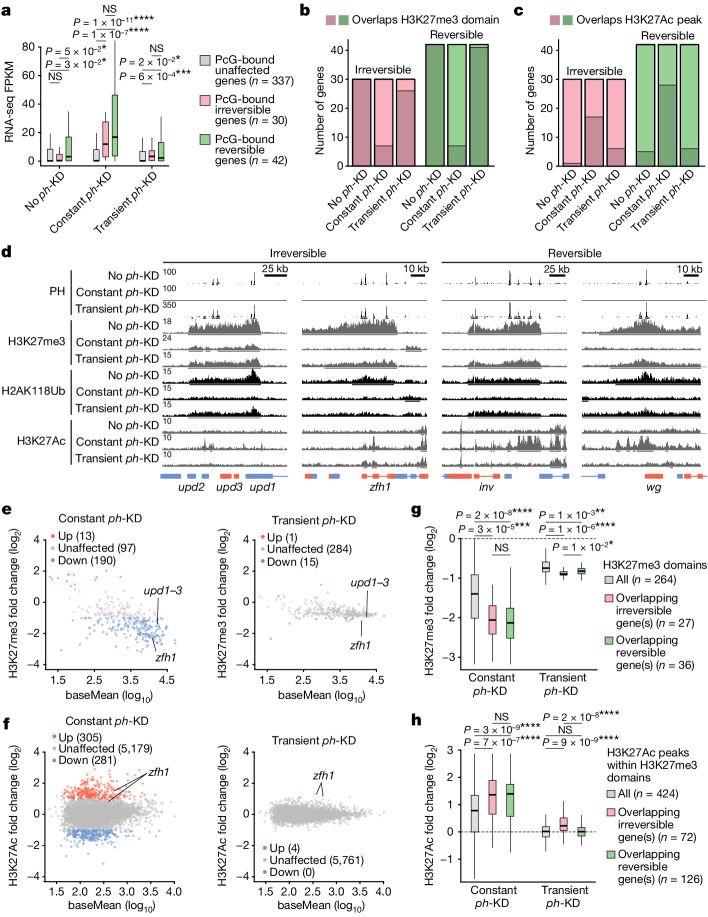
To identify their unique chromatin features, we focused on irreversible (n = 30) and reversible (n = 42) genes that are direct PcG targets and are covered with the H3K27me3 repressive mark in control EDs (for a full list of PcG target genes, see Supplementary Table 2). Both groups show similar H3K27me3 levels in control tissues (Extended Data Fig. 4a), where they are transcribed at similarly low levels (Fig. 3a). They are also induced at comparable levels on constant ph-KD, ruling out the possibility that weaker PcG repression and/or higher transcriptional levels are the reason for irreversible genes being unable to recover normal transcription after transient ph-KD (Fig. 3a).
Extended Data Fig. 4. The PcG epigenetic landscape is globally re-established after transient ph-KD.
a- PH ChIP-Seq (top row), H3K27me3 (2nd row), H2AK118Ub (3rd row) and H3K27Ac (bottom row) CUT&RUN average tracks, anchored at the TSS of the PcG-bound irreversible (in pink), reversible (in green) and unaffected genes (in gray) after no ph-KD (control, left), constant (middle) or transient ph-KD (right). For each condition, the average signal is shown (solid line) ± standard error (shaded area). The distance to the TSS is shown on the x-axis. The signal was quantified at the regions highlighted by dashed lines (see corresponding boxplots on the right). Box plots show the median (line), upper and lower quartiles (box) ±1.5x interquartile range (whiskers), outliers are not shown. Two-sided Wilcoxon test: *pval < 0.05; n.s = pval > 0.05 (not significant). Although PH binding is significantly stronger at TSSs of reversible genes in control conditions, this small difference is not reflected in significant changes in the H3K27me3 and H2AK118Ub repressive marks. Binding is strongly reduced in constant depletion but it is restored after a transient depletion. b- The PH peaks, the H3K27me3 (PRC2-deposited repressive mark) and H2AK118Ub (PRC1-deposited repressive mark) domains overlaps are shown, after no ph-KD (control), constant or transient ph-KD. Each vertical bar corresponds to an intersection (the corresponding conditions are shown below) and the horizontal bars (bottom left) indicate the total number of peaks/domains detected in each sample. To avoid weak and noisy peaks/domains, we focused on domains containing at least one PH peak and on PH peaks overlapping H3K27me3 domains in control sample. c- Differential analysis of H2AK118Ub domains that show unaffected (gray), decreased (blue) or increased (orange) enrichment upon constant (top) or transient ph-KD (bottom). d- Each bar corresponds to an H3K27me3 domain containing at least one irreversible (pink) or reversible (green) gene. For each domain, the number of irreversible (pink), reversible (green) and unaffected genes (gray) are shown. Generally, domains containing reversible genes do not contain irreversible ones and vice versa. e- Average PH ChIP-Seq signal around PH peak summits (x-axis) after no ph-KD (top), constant (middle) or transient ph-KD (top). PH peaks were stratified based on the closest TSS with a maximum of 25 kb distance. Peaks assigned to irreversible and reversible peaks are shown in pink and in green, respectively. For each condition, the average signal is shown (solid line) ± standard error (shaded area). The distance to the TSS is shown on the x-axis. The signal was quantified at the regions highlighted by dashed lines (see corresponding boxplots on the right). Box plots show the median (line), upper and lower quartiles (box) ±1.5x interquartile range (whiskers), outliers are not shown. Two-sided Wilcoxon test: n.s = pval > 0.05 (not significant).
Fig. 3. PcG repressive landscape is restored after transient ph-KD.
a, Fragments per kilobase of transcript per million mapped reads (FPKM) of irreversible (pink), reversible (green) and unaffected (grey) genes that are direct PcG targets. Two-sided Wilcoxon test: *P < 5 × 10−2, ***P< 1 × 10−3, ****P < 1 × 10−5, NS, P > 0.05. Box plots show the median (line), upper and lower quartiles (box) ±1.5× interquartile range (whiskers), outliers are not shown. b, Number of irreversible (pink) and reversible (green) genes overlapping an H3K27me3 domain (more than or equal to 50% of the gene body) after no ph-KD (control), constant or transient ph-KD. c, Number of irreversible (pink) and reversible (green) genes overlapping at least one H3K27Ac peak (in the gene body or up to 2.5 kb upstream of the TSS) after no ph-KD (control), constant or transient ph-KD. d, Screenshot of PH ChIP–seq, H3K27me3, H2AK118Ub and H3K27Ac CUT&RUNs tracks at representative irreversible (left) or reversible (right) loci under the indicated conditions (left). e,f, For H3K27me3 domains (e) and H3K27Ac peaks (f), fold changes are shown as a function of their average-normalized counts across all samples (baseMean) for constant (left) or transient (right) ph-KD conditions. Significant changes are highlighted using a colour code (colour legend). g, The H3K27me3 fold changes (between constant or transient ph-KD and no ph-KD conditions) at H3K27me3 domains that are found in the control sample (no ph-KD) and overlap irreversible (pink) or reversible (green) genes. All H3K27me3 domains are shown for reference (grey). Two-sided Wilcoxon test: *P < 5 × 10−2, **P < 1 × 10−2, ***P < 1 × 10−3, ****P < 1 × 10−5, NS, P > 0.05. Box plots show the median (line), upper and lower quartiles (box) ±1.5× interquartile range (whiskers), outliers are not shown. h, The H3K27Ac fold changes at H3K27Ac peaks overlapping the H3K27me3 domains found in control sample (no ph-KD) and overlapping the irreversible (pink) or reversible (green) genes. All H3K27Ac peaks overlapping control H3K27me3 domains are shown for reference (grey). Two-sided Wilcoxon test: ****P <1 × 10−5, NS. P > 0.05. Box plots show the median (line), upper and lower quartiles (box) ±1.5× interquartile range (whiskers), outliers are not shown.
We then explored the possibility that chromatin might not be correctly re-established at irreversible genes in EICs, by performing chromatin immunoprecipitation combined with sequencing (ChIP–seq) for PH and CUT&RUN for several histone marks after no ph-KD (control), constant and transient ph-KD. Whereas most reversible and irreversible genes lost the H3K27me3 repressive mark on constant ph-KD, H3K27me3 domains were notably recovered after transient ph-KD (Fig. 3b,d). Most H3K27me3 domains and overlapping PH peaks are erased on constant PH depletion, but are recovered after transient depletion (Extended Data Fig. 4b). The same applies to the H2AK118Ub repressive mark deposited by PRC1 (Fig. 3d and Extended Data Fig. 4b). H3K27me3 loss on constant ph-KD is accompanied by a reciprocal gain of H3K27Ac peaks, its activating counterpart, at both reversible and irreversible genes (Fig. 3c,d). Nevertheless, both groups show similar H3K27me3 and H3K27Ac levels after transient ph-KD, suggesting that comparable chromatin landscapes may promote distinct transcriptional outcomes (Extended Data Fig. 4a). Inspection of individual loci showed that recovery of chromatin composition is similar at the level of reversible and irreversible genes, as evidenced by the upd locus, which does not contain H3K27Ac peaks after transient ph-KD although it is irreversibly upregulated (Figs. 2e and 3d).
Nevertheless, we noted some exceptions, such as the zhf1 gene that retains low but significantly higher levels of H3K27Ac compared to control tissues on transient depletion of PH (Fig. 3d), suggesting that a fraction of irreversible loci might retain small quantitative differences. Differential analyses indicated that most H3K27me3 domains showed a steep decrease on constant ph-KD but overall recovered to normal levels under transient conditions (Fig. 3e). Similar trends were found at H3K27Ac peaks and H2AK118Ub domains, whereby transient ph-KD showed weaker and fewer significant differences compared to constant ph-KD (Fig. 3f and Extended Data Fig. 4c, respectively). This approach again identified the zfh1 locus as an outlier showing significantly increased H3K27Ac peaks after transient ph-KD (Fig. 3f). To precisely assess whether small differences in terms of H3K27me3 or H3K27Ac fold changes would be predictive of irreversible transcriptional changes, we classified H3K27me3 domains based on whether they contain irreversible or reversible genes and interestingly found that genes from the two groups are usually found in different domains (Extended Data Fig. 4d). Domains overlapping irreversible versus reversible genes showed small differences in H3K27me3 or H3K27Ac fold changes (Fig. 3g,h), which are unlikely to explain the clear-cut difference between reversible and irreversible genes. Therefore, irreversible transcriptional changes drive tumorigenesis despite the re-establishment of an essentially normal chromatin landscape at PcG target genes.
Heritable chromatin accessibility changes
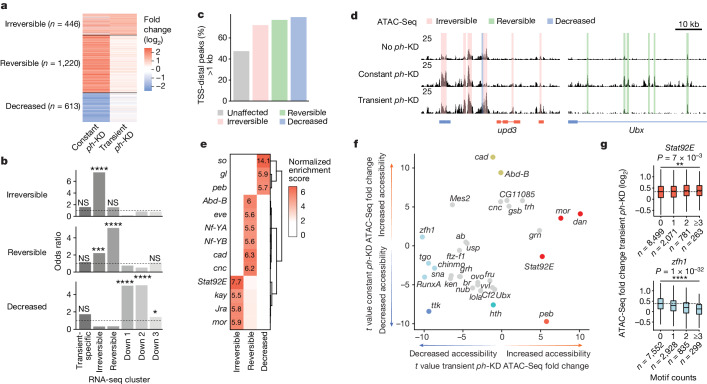
The analysis of PH binding levels at PH peaks located ±25 kb from the transcription start sites (TSS) of reversible (n = 113) or irreversible (n = 91) genes revealed no significant differences either in control EDs (no ph-KD) or after transient ph-KD (Extended Data Fig. 4e). This is consistent with the levels of H3K27me3 and H2AK118Ub repressive marks, which are also similar (Extended Data Fig. 4a). We therefore wondered whether the irreversible transcriptional changes found in EICs might be due to the binding of specific transcription factors to specific chromatin targets on ph-KD, preventing re-repression on restoration of PH. In this scenario, one would expect the opening of specific sites at irreversible gene loci. To test this hypothesis, we performed ATAC-Seq in control EDs (no ph-KD) or after constant or transient ph-KD, and found 1,220 reversible peaks showing a stark increase in accessibility after constant PH depletion but returning to normal levels after transient KD (Fig. 4a). By contrast, 446 ATAC-Seq peaks increased accessibility both on constant as well as on transient PH depletion (Fig. 4a). We named these ATAC-Seq regions irreversible peaks (clusters are fully available in Supplementary Table 3).
Fig. 4. Chromatin accessibility changes underlie reversible and irreversible transcriptional changes.
a, Clustering of ATAC-Seq peaks showing significant changes after constant or transient ph-KD. b, Over-representation of genes associated with irreversible (top), reversible (middle) or decreased (bottom) ATAC-Seq peaks, for each of the six RNA-seq clusters defined in Fig. 2b. One-sided Fisher’s exact test P values were corrected for multiple testing using FDR: *FDR < 5 × 10−2, ***FDR < 1 × 10−3, ****FDR < 1 × 10−5, NS, P > 0.05. Exact FDR values: 2 × 10−1, 4 × 10−23, 1 × 10−1, 1 × 100, 1 × 100, 1 × 100 (irreversible); 4 × 10−1, 2 × 10−5, 2 × 10−34, 1 × 100, 1 × 100, 3 × 10−1 (reversible), 8 × 10−2, 1 × 100, 1 × 100, 2 × 10−21, 5 × 10−32, 1 × 10−2 (decreased). c, Fraction of TSS-distal peaks per cluster (greater than 1 kb). d, Screenshot of ATAC-Seq tracks after no ph-KD (control, top), constant (middle) or transient (bottom) ph-KD, at the irreversibly upregulated upd3 gene (left) and the reversibly upregulated Ubx gene (right). e, Normalized enrichment scores of DNA binding motifs found at each cluster of ATAC-Seq peaks (±250 bp, x axis). f, Linear model t values of DNA binding motifs associated with increased (positive t values) or decreased (negative t values) accessibility after transient (x axis) or constant ph-KD (y axis). Only motifs with a significant P < 1 × 10−5 in at least one of the two linear models are shown. g, Fold changes at ATAC-Seq peaks (y axis) on transient ph-KD, as a function of the number of Stat92E (left, in orange) or zfh1 (right, in blue) motifs that they contain (x axis). Two-sided Wilcoxon test: **P < 1 × 10−2, ****P < 1 × 10−5. Box plots show the median (line), upper and lower quartiles (box) ±1.5× interquartile range (whiskers), outliers are not shown.
To assess whether reversible and irreversible peaks correlate with transcriptional changes, we assigned them to the closest TSS (±25 kb, Methods). Reversible and irreversible ATAC-Seq peaks were significantly associated with the reversible and irreversible genes identified by RNA sequencing (RNA-seq) analysis in Fig. 2b, respectively (Fig. 4b). This suggests that a substantial fraction of these peaks might correspond to enhancer elements that activate the transcription of cognate TSSs from a distance. Consistently, roughly 70% of reversible and irreversible peaks are found more than 1 kb away from the closest TSS (Fig. 4c,d). For example, the upd3 gene is irreversibly upregulated after transient ph-KD and is surrounded by several promoter-distal irreversible ATAC-Seq peaks, whereas the reversible gene Ubx shows reversible ATAC-Seq peaks that can be observed only on constant ph-KD (Fig. 4d). In parallel, 604 peaks show reduced accessibility and are associated with downregulated genes (Figs. 2b and 4a,b).
To understand which transcription factors might cause these differences in accessibility, we searched for DNA binding motifs in ATAC-Seq peaks. Reversible and irreversible peaks show distinct motif signatures (Fig. 4e). Reversible peaks are enriched for Abd-B, cad and eve motifs, three different PcG canonical targets involved in antero-posterior patterning that are strongly upregulated after constant ph-KD compared to transient ph-KD (Extended Data Fig. 3b). By contrast, irreversible peaks are enriched for Jra and kay motifs, the Drosophila homologues of AP-1, which are the main transcription factors of the oncogenic JNK signalling pathway54. Furthermore, they were strongly and specifically enriched for Stat92E motifs, the key effector of the JAK–STAT pathway55. Finally, decreased peaks are enriched in glass (gl) and sine oculis (so) motifs, two key regulators of eye development that are irreversibly downregulated (the down 1 cluster in Fig. 2b and Extended Data Fig. 3b). This latter point indicates that the activation of the retinal determination gene network is compromised in the absence of PcG, consistent with our previous work42.
These results indicate that the Abd-B, cad and eve genes are responsible for the pleiotropic transcriptional defects observed on constant PH depletion, but are unlikely to be required for the progression of EICs. On the other hand, recruitment of AP-1 and STAT92E at irreversible peaks could maintain irreversible genes in an active state, potentially by maintaining open chromatin at their cis-regulatory regions. To tackle this latter point, we sought to predict ATAC-Seq changes using transcription factor motif counts (Methods). cad and Abd-B motifs are associated with increased accessibility after constant PH depletion but not in a transient condition (Fig. 4f and Extended Data Fig. 5a), suggesting that their effect on chromatin and transcription is dispensable for the growth of EICs. Conversely, STAT92E and ZFH1 motifs were among the best predictors of increased and decreased accessibility after transient ph-KD, respectively (Fig. 4f,g).
Extended Data Fig. 5. Analysis of transcription factors in EICs shows that transient Stat92E-KD and zfh1-KD are sufficient to substantially rescue transient ph-KD neoplastic signatures.
a- Fold changes at ATAC-Seq peaks (y-axis) upon constant (left) or transient (right) ph-KD, as a function of the number of caudal (cad) motifs they contain (x-axis). Box plots show the median (line), upper and lower quartiles (box) ±1.5x interquartile range (whiskers), outliers are not shown. Two-sided Wilcoxon test: ****pval < 1e-5, n.s = pval>0.05 (not significant). b- ED areas upon constant depletion of STAT92E and ZFH1, alone or in addition to PH depletion. Areas are measured using DAPI-stained tissues (number of measured EDs is reported in brackets). Box plots show the median (line), upper and lower quartiles (box) ±1.5x interquartile range (whiskers), outliers are not shown. c-e DAPI (gray, c), F-actin (red, d) and ELAV (magenta, e) stainings of EDs after transient gfp-KD (control), Stat92E-KD and zfh1-KD in the presence of a concomitant, transient depletion of white (w-KD, control, first three columns) or ph (ph-KD, last three columns). DAPI staining was used to assess growth, F-actin staining was used for apico-basal polarity and the neuronal marker ELAV for differentiation. Two independent experiments were performed with similar results. f- ED sizes quantified as overall DAPI staining area for different conditions, showing that transient Stat92E-KD or zfh1-KD decreased ED overgrowth associated with transient ph-KD. Box plots show the median (line), upper and lower quartiles (box) ±1.5x interquartile range (whiskers), outliers are not shown. Two-sided Wilcoxon test: **pval < 1e-2, ***pval < 1e-3, ****pval < 1e-5. Scale bars: 100 μm (c), 10 μm (d, e).
Tumorigenesis requires STAT92E and ZFH1
To assess whether the STAT92E activator and the ZFH1 repressor are necessary for the development of EICs, we set up dual RNAi systems allowing the depletion of each of the two factors in combination with white or ph. As a control, we combined gfp (green fluorescent protein) and white-RNAi (gfp + w-KD), which had no impact on ED growth or differentiation, whereas gfp+ph-KD induced tumours as expected (Fig. 5a and Extended Data Fig. 5b). Both on constant and on transient depletion, Stat92E and zfh1-KD alone had no visible effect. However, when combined with ph-KD, they both significantly reduced ph-dependent tumour growth and partially restored cell polarity and photoreceptor differentiation (Fig. 5a and Extended Data Fig. 5b–f), indicating that they are both bona fide drivers of the tumour phenotype. These rescues are also associated with an overall rescue of constant gfp + ph-KD transcriptomes, with 50% of differentially expressed genes returning to control levels on gfp+zfh1-KD (Fig. 5b). Consistent with previous studies showing that zfh1 is a target of STAT92E (ref. 50), the zfh1 gene returned to control levels in Stat92E+ph-KD (differential analyses are available in Supplementary Table 4). Thus, ZFH1 seems to play a master role in shaping the tumour transcriptome.
Fig. 5. Tumour development requires STAT92E and ZFH1.
a, DAPI (top, in grey) and neuronal differentiation marker ELAV (bottom, in magenta) stainings of EDs after constant KD of the following components: gfp+w, Stat92E+w, zfh1+w, gfp+ph, Stat92E+ph and zfh1+ph (top labels). Two independent biological replicates were performed with similar results. Scale bars: 100 μm (DAPI), 10 μm (ELAV). b, Number of differentially expressed genes after gfp+ph-KD (tumours), Stat92E+ph-KD and zfh1+ph-KD. Transitions between upregulated (orange), unaffected (grey) and downregulated (blue) states are indicated by thin lines of the same respective colours. c, Number of ATAC-Seq peaks showing significant accessibility changes after gfp+ph-KD or zfh1+ph-KD. Transitions between increased (orange), unaffected (grey) and decreased (blue) states are indicated by thin lines of the same respective colours. d, Fold changes at ATAC-Seq peaks between zfh1+ph-KD and gfp+ph-KD, depending on the number of ZFH1 motifs they contain (x axis). Two-sided Wilcoxon test, ****P < 1 × 10−5. Box plots show the median (line), upper and lower quartiles (box) ±1.5× interquartile range (whiskers), outliers are not shown. e, RNA-seq fold changes on gfp+ph-KD (x axis) of genes associated with ATAC-Seq peaks that are decreased (in blue), unaffected (in grey) or increased (in orange) after zfh1+ph-KD compared to gfp+ph-KD (y axis). Two-sided Wilcoxon test: ****P < 1 × 10−5. Box plots show the median (line), upper and lower quartiles (box) ±1.5× interquartile range (whiskers), outliers are not shown. f, Top enriched Gene Ontology (GO) terms for genes associated with ATAC-Seq peaks containing at least one ZFH1 motif and showing significantly increased accessibility after zfh1+ph-KD compared to gfp+ph-KD. g, Schematic illustration showing that PcG depletion triggers an epigenetic switch to a cancer fate. Resulting cancers persist after the PcG protein is restored, and their maintenance is associated with stable transcriptional changes supported by the STAT92E activator and the ZFH1 repressor.
Therefore, we sought to investigate its impact on chromatin by performing comparative ATAC-Seq experiments in gfp+ph-KD, gfp+zfh1-KD and zfh1+ph-KD. Consistent with our previous result showing that zfh1 motifs are associated with decreased accessibility in tumours compared to control tissues (Fig. 4g), zfh1-KD in combination with ph-KD was found to be associated with the reopening of roughly 1,700 peaks showing decreased accessibility in gfp+ph-KD tumours (Fig. 5c). Moreover, zfh1 motif counts are predictive of an increase in ATAC-Seq signal between gfp+ph-KD and zfh1+ph-KD tissues (Fig. 5d). These results indicate that zfh1 represses transcription by reducing the accessibility of a subset of regulatory elements. Thus, we classified ATAC-Seq peaks based on their fold change between gfp+ph-KD and zfh1+ph-KD and assigned them to the closest TSS (±25 kb). Peaks with increased accessibility on zfh1+ph-KD were associated with genes that were aberrantly downregulated on gfp+ph-KD (Fig. 5e) and are involved in eye development and differentiation (Fig. 5f), reminiscent of the genes identified in the Down 1 RNA-seq cluster (Fig. 2b).
Altogether, these results indicate a multistep model (Fig. 5g) in which transient disruption of PcG-mediated silencing irreversibly activates the JAK–STAT pathway, which induces cell proliferation as well as the zfh1 gene. In turn, ZFH1 represses genes required for ED development, thereby preventing cell differentiation in EICs.
EICs are autonomous immortal tumours
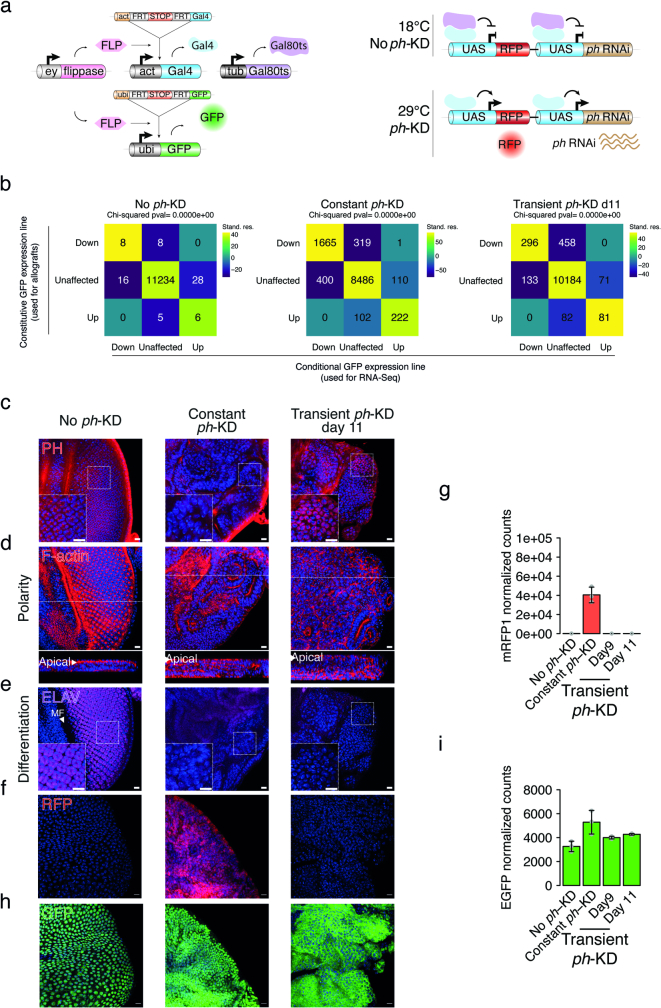
Most EIC-bearing larvae die after day 11 AEL, preventing the study of tumour development over time. To circumvent this limitation, allografts of imaginal disc tissue into the abdomen of adult Drosophila hosts are commonly used to assess the tumorigenic potential of a tissue, and we previously showed that ph mutant EDs continuously grow until they eventually kill the host43. To be able to track transplanted EICs, we developed a variant of our thermosensitive system that constitutively expresses GFP in the eye, whereas an upstream activation sequence-red fluorescent protein (UAS-RFP) cassette can be used as a reporter of continuing ph-KD (Extended Data Fig. 6a,f–i). This system induces EICs with similar penetrance, morphological and transcriptional defects, showing that EICs can be obtained in different genetic backgrounds (Extended Data Fig. 6b–e). The differential analyses of the corresponding transcriptomes are available in Supplementary Table 5. We then performed allografts using this line (Extended Data Fig. 7), keeping host flies at a restrictive temperature after transplant (18 °C) to preclude activation of ph-RNAi in transplanted tissues.
Extended Data Fig. 6. Description and validation of the conditional genetic tool allowing long-term tracking of cells subjected to constant or transient ph-KD.
a- Schematic overview of the thermosensitive ph-RNAi genetic system used in allograft experiments. Unlike the system described in Fig. 1a (conditional GFP expression), this one ubiquitously expresses GFP under the control of the Ubi-p63E promoter (constitutive GFP expression), while RFP expression is a readout of ongoing RNAi KD. b- Comparison of differentially expressed genes after no ph-KD (control), constant or transient ph-KD between the two genetic systems, allowing either the conditional (x-axis) or the constitutive (y-axis) expression of GFP. For each intersection, the corresponding number of genes is indicated (see numbers). Similarity between the two systems was assessed using chi-squared tests (see p.values on top) and chi-squared standardized residuals are shown using heat maps’ colour code. The more an intersection exceeds the size that would be expected by chance, the higher the standard residuals. c-f- PH (in red, c), F-actin (in red, d), ELAV (in magenta, e) and RFP (in red, f) stainings of EDs after no ph-KD (control, left), constant (middle) or transient ph-KD (right), respectively. PH staining was used to visualize PH loss and recovery under constant and transient conditions. F-actin is used to analyse apico-basal polarity, the neuronal marker ELAV for differentiation and RFP as a conditional marker for induction of the RNAi system. The tissues were counterstained with DAPI (blue). Two independent experiments were performed with similar results. g- Normalized read counts of mRFP1 mRNAs after no ph-KD (control), constant or transient ph-KD, showing that transcriptional expression of RFP occurs at constant 29 °C exposure but returns to basal levels after transient ph-KD. For each condition, mean normalized read counts ±standard deviation (whiskers) were inferred from three biological replicates of RNA-Seq (grey dots). h- GFP staining (in green) after no ph-KD (control), constant or transient ph-KD. GFP is constitutively expressed after transient ph-KD. The tissues were counterstained with DAPI (in blue). i- Normalized read counts of GFP mRNAs after no ph-KD (control), constant or transient ph-KD, showing that GFP expression is irreversibly induced after transient ph-KD. For each condition, mean normalized read counts ±standard deviation (whiskers) were inferred from three biological replicates of RNA-Seq (grey dots). Scale bars: 10 μm (c, d, e, f, h).
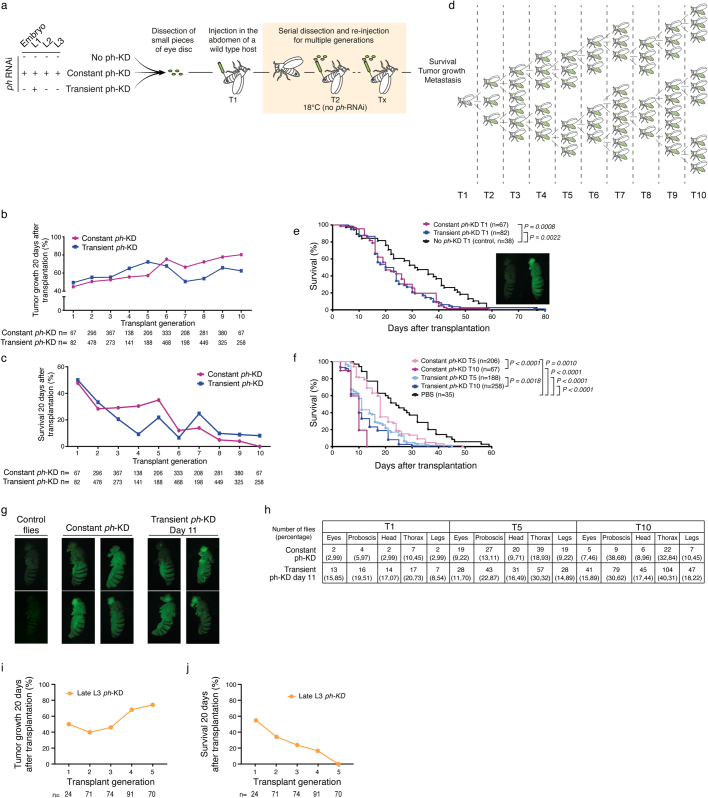
Extended Data Fig. 7. Comparative analysis of tumour growth by serial transplantation of constant and transient ph-KD EICs.
a- Schematic overview of the experimental allograft workflow. Flies of the same genotype were subjected to no ph-KD (control, 18 °C), constant ph-KD (29 °C) or transient ph-KD (24h ph-KD at 29 °C during L1 stage). L3 EDs expressing constitutive GFP were dissected from donor larvae and repeatedly allografted into the abdomen of host flies for 10 consecutive rounds until T10 of transplantation (T10≈ 3 months). All allograft experiments were performed at 18 °C to avoid ph-RNAi expression after transplantation. b-c- Tumour growth measured as the percentage of flies showing tumour progression 20 days after transplantation (b) or surviving 20 days after each allograft (c) constant (purple) or transient (blue) ph-KD tumours for 10 rounds of transplantation (x-axis). d- Tree representation of the allograft assay. A primary ED tumour derived from constant or transient ph-KD is dissected from L3 donor larvae and repeatedly allografted into the abdomen of a female host maintained at 18 °C to prevent re-expression of-ph-RNAi. Each injected fly is monitored every two days. When the host fly abdomen is completely filled with GFP positive cells, the host is dissected and the tumour cells are injected again into multiple hosts. The procedure was repeated until the tenth generation (T10). e- Host lifespan (x-axis) after the first transplantation (T1) of control (no ph-KD, in black), constant (in purple) or transient (in blue) ph-KD tumours. Statistical significance was assessed using log-rank test. f- Host lifespan (x-axis) after the fifth (T5) and the tenth (T10) rounds of transplantations of constant (T5 in pink, T10 in purple) or transient (T5 in light blue, T10 in blue) ph-KD tumours. Since control (no ph-KD) tissues do not grow and cannot be serially transplanted, PBS injections were used as control (in black). Statistical significance was assessed using log-rank test. g- Flies injected with dissected grafts after no ph-KD (control), constant or transient ph-KD. Only primary tumours generated by constant or transient ph-KD can invade the abdomen and surrounding tissues but not EDs resulting from no ph-KD (control) conditions. h- In order to score the frequency of metastases, the injected flies were monitored twice a week and the appearance of metastases in the thorax, head, proboscis, eyes and legs were noted for each generation. Values in the table represent the number and percentage (in brackets) of flies with metastases after the 1st, 5th or 10th round of transplantation (T1, T5 and T10, respectively) of constant or transient ph-KD tumours. i-j- Tumour growth measured as the percentage of flies showing tumour progression 20 days after transplantation (d) and host fly survival 20 days after allograft of late L3 ph-KD (e) for 5 consecutive rounds of transplantation.
Constant ph-KD primary tumours grew in a high fraction of the injected host flies within 20 days of transplantation (Extended Data Fig. 7a–c). Transient ph-KD primary EICs behaved similarly, indicating that their overgrowth results from an autonomous, stably acquired state (Extended Data Fig. 7a–c). To measure tumour growth over time, we set up a scheme allowing us to trace the tumour of origin (Extended Data Fig. 7d). Tumours derived from both constant or transient PH depletion maintained their ability to expand in host flies more than ten rounds of transplantation. Tumour growth penetrance, defined as the percentage of host flies bearing GFP-positive cells 20 days after transplantation, increased over generations of transplantation (Extended Data Fig. 7b), whereas the survival of host flies decreased (Extended Data Fig. 7c,e,f). Furthermore, tumours metastasized to regions and organs far from the injection site, with increasing penetrance with the number of transplants (Extended Data Fig. 7g,h). Finally, allografts originating from tissues injected after a transient ph-KD at the late L3 stage also gave rise to tumours of increasing penetrance over the number of transplantations (Extended Data Fig. 7i,j).
Together, these results indicate that the tumorigenic potential of EICs is maintained autonomously, increases over time and can propagate months after ph-RNAi has been removed. This progression might suggest that EICs acquire secondary modifications, either epigenetic or genetic, that increase their aggressiveness over time.
Discussion
It is difficult to discriminate among genetic, environmental and cell-intrinsic epigenetic contributions to tumorigenesis33. The system described here shows that on transient depletion of PRC1 subunits cells undergo neoplastic transformation (Fig. 5g and Extended Data Fig. 8), associated with the irreversible activation of genes including key JAK–STAT pathway members that sustain cell growth, proliferation, loss of cell polarity, cell migration and cytokine activity. One main difference between these irreversibly activated genes and reversible PcG target genes is the presence of different sets of transcription factor binding motifs in their vicinity. We posit that, even if PRC1 is wiped out from both classes of genes on depletion, the preferential binding of JAK–STAT related transcription factors in the vicinity of irreversible genes might specifically foster their transcription after transient perturbation of PcG, dampening their re-repression and inducing a self-sustaining aberrant cell state (Extended Data Fig. 8). One of these JAK–STAT targets, zfh1 plays an important role by blocking cell differentiation. Altogether, this cascade of events results in a self-sustaining mechanism that drives tumorigenesis even after recovery of normal PcG protein concentrations and in the wake of the rescue of their chromatin function at most of the PcG binding sites.
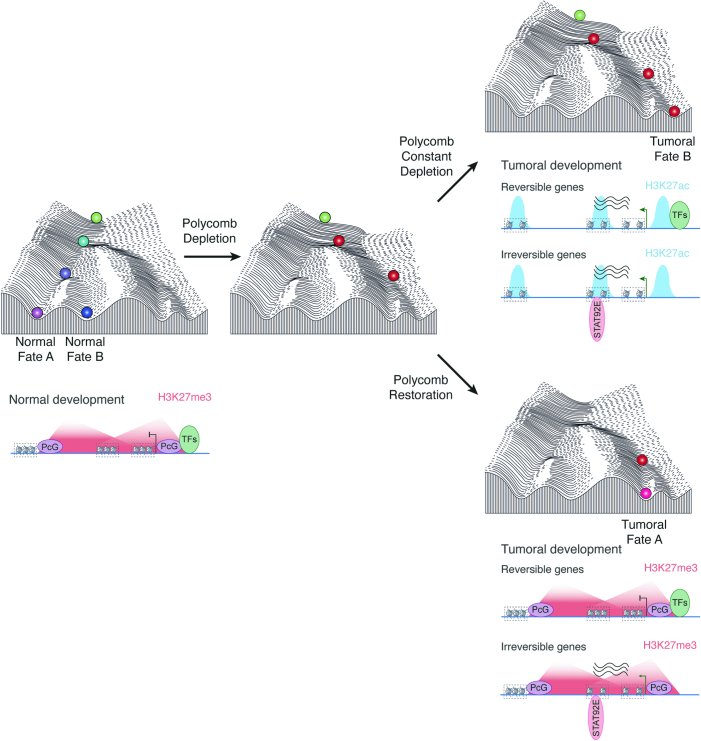
Extended Data Fig. 8. A model explaining the emergence of epigenetically initiated cancers.
The model is based on the well-known Waddington landscape depicting a marble rolling down a slope with multiple choices of trajectories that depend on the hills and valleys encountered on their path. This scheme is a metaphor for the multiple possible cell fates that can arise from a single cell representing the zygote and is frequently used to signify that epigenetic inheritance contributes to the stable transmission of cell fates, once they are determined by intrinsic and extrinsic signals. In the context of this work, we posit that Polycomb components contribute to shaping the landscape and allow for multiple normal cell fates to be established and transmitted through the developmental process. In normal development, the cells (in green) at the top of the hill will move down during differentiation in order to acquire normal fates (left panel). Upon depletion of a Polycomb component, such as the PRC1 subunits PH or PSC, the landscape is modified (center panel). If depletion is stably maintained, the modified landscape forces cells to take a path that is both aberrant and intrinsically stable, inducing cancer formation through loss of cell differentiation, loss of cell polarity and sustained proliferation (upper right panel). If Polycomb protein levels are restored, the landscape returns to its original shape. However, if restoration of the landscape occurs after cells have already chosen an aberrant route (represented by the marble in the middle of the landscape), they will no longer be able to find the healthy trajectory and will be obliged to choose from a limited set of possibilities in a diseased cell space. This may ultimately lead to the maintenance of tumour phenotypes. In addition to the Waddington landscape panels, gene panels are added, representing a putative molecular explanation for the phenomenon described here. The chromatin and functional state of reversible and irreversible genes are shown in each condition. In a physiological condition (left), both categories of genes are bound by Polycomb components and are decorated by repressive histone marks, such as H3K27me3. Upon depletion of Polycomb components such as PH, both the Polycomb complexes and their histone marks are lost and Polycomb target genes acquire active histone mark such as H3K27ac and become transcribed. At irreversible genes, transcriptional activation is dependent on the JAK–STAT signaling pathway transcription factor STAT92E (top right). Upon PRC1 re-establishment, the repressive mark and PH binding is globally recovered. However, chromatin stays open at specific sites that regulate irreversible genes, in which a DNA motif bound by the main JAK–STAT effector STAT92E is enriched. STAT92E target genes include proliferation components and zfh1, which encodes a transcription factor that represses transcription of a set of genes involved in cell differentiation. The combined, self-sustaining induction of cell proliferation and loss of differentiation induces tumorigenesis even after restoration of normal levels of Polycomb proteins on their target chromatin (bottom right, see also Fig. 5g).
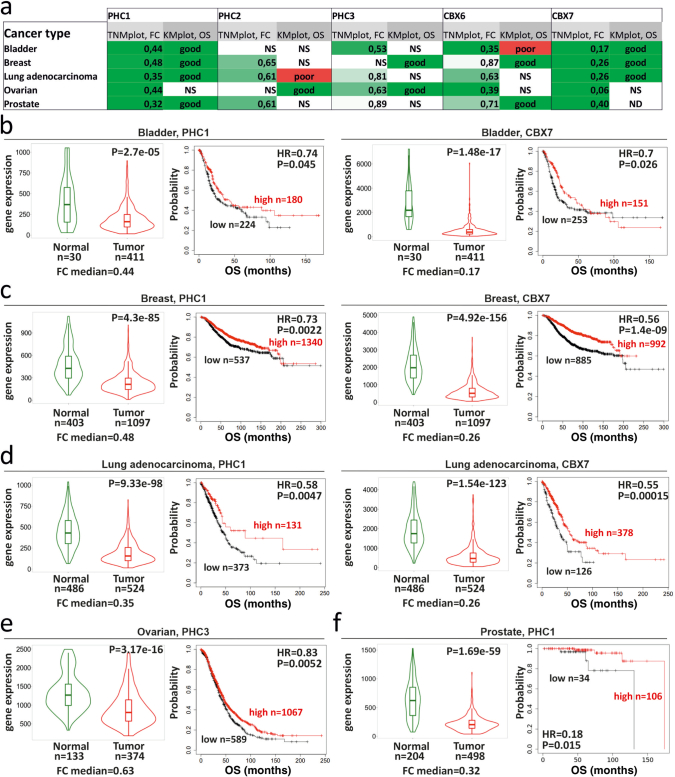
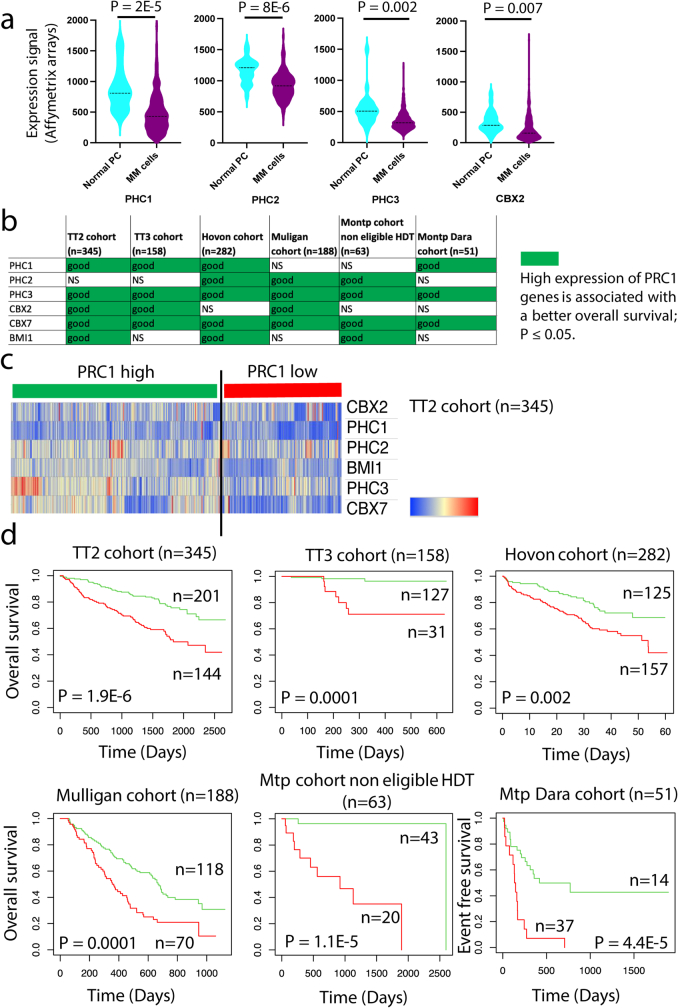
Previous work showed that self-sustaining alternative cell states can be triggered by transient perturbations in a sensitized Drosophila system56, as well as in immortalized breast cells57 or other cultured cells58, including neural progenitor cells subjected to transient inhibition of the PRC2 complex59. PRC2 impairment in mouse striatal neurons induces progressive neurodegeneration by triggering a self-sustaining transcription derailment programme over time60. Furthermore, knock-out or transient chemical inhibition of PRC2 also led cells to enter a quasi-mesenchymal state that depends on ZEB1, the mouse homologue of fly zfh1, which is highly metastatic and associated with poor patient survival53. Therefore, epigenetic events might play a major role at early stages of oncogenesis or during tumour progression in some mammalian cancers61. Our survey of a large database of different types of solid cancer (Extended Data Fig. 9) as well as of data from several cohorts of patients with multiple myeloma (Extended Data Fig. 10) indicates that low expression levels of genes encoding canonical PRC1 subunits is associated with poor patient prognosis, consistent with a putative suppressive role for PRC1 in these tumour types. Future work might address the role of epigenetic perturbations in these tumours and in other physiological processes.
Extended Data Fig. 9. Examples of the tumour suppressive role of canonical PRC1 core subunits in different cancer types.
a- Clinical correlations for PRC1 in selected cancer types. Differential gene expression (TNMplot) and clinical prognosis Kaplan-Meier plot (KMplot) results are given for PHC1, PHC2, PHC3, CBX6, CBX7 genes. TNMplot columns represent the differential gene expression analysis in tumour and matched normal tissues, which was performed using the https://tnmplot.com/ online tool. FC median: Fold change median. Statistical significance was calculated using a two-sided Mann-Whitney U test with a significance level of 0.01. NS – non-significant Mann–Whitney p-value. Green boxes indicate that gene expression is significantly lower in tumour tissues. KMplot columns show the analysis of correlation between overall survival (OS) and levels of gene expression. KMplot analysis was performed using the https://kmplot.com/ online tool. Statistical significance was calculated by a two-sided Cox regression test with a significance level of 0.05. NS – non-significant logrank p-values. Green boxes (“Good”) indicate cases in which high expression of PRC1 genes in tumours is associated with a better overall patient survival. b- Clinical prognosis for PHC1 (left) and CBX7 (right) in bladder cancer. For each gene the TNMplot (Violin plots, left panels) and KM plots (right panels) are shown. c- Clinical prognosis of PHC1 (left) and CBX7 (right) in breast cancer. For each gene the TNMplot (Violin plots, left panels) and KM plots (right panels) are shown. d- Clinical prognosis of PHC1 (left) and CBX7 (right) in lung adenocarcinoma. For each gene the TNMplot (Violin plots, left panels) and KM plots (right panels) are shown. e–f Clinical prognosis of PHC3 (e) in ovarian and PHC1 (f) in prostate cancer. For each gene the TNMplot (Violin plots, left panels) and KM plots (right panels) are shown. The Violin plots display the range of values from the minimum to the maximum value, with the box representing the values from the first quartile to the third quartile. The median is indicated by the thick line in the center, and the width of the plot, or density, reflects the frequency of the samples. In KMplots, the cohort with low gene expression level is coloured black and the cohort with high gene expression is coloured red. HR: hazard ratio.
Extended Data Fig. 10. Tumour suppressive role of core PRC1 subunits in Multiple Myeloma.
a- PHC1, PHC2, PHC3 and CBX2 gene expression is significantly downregulated in malignant plasma cells (PCs) from patients with Multiple Myeloma (MM cells) compared to normal bone marrow PCs. Affymetrix U133 P gene expression profiles of purified bone marrow PC from 22 healthy donors and purified myeloma PCs from 345 previously untreated patients were compared using publicly available data (Gene Expression Omnibus, accession number GSE2658) from the University of Arkansas for Medical Sciences (UAMS, Little Rock, AR). Statistical difference was assayed using a two-sided Student t test. b- Prognostic value of core PRC1 components in MM. The prognostic value of PHC1, PHC2, PHC3, CBX2, CBX7, and BMI1 gene expression was analyzed in 6 independent cohorts of patients with MM using the Maxstat R function and Kaplan Meier survival curves as previously described. Low expression of PHC1, PHC2, PHC3, CBX2, CBX7 and BMI1 was associated with significantly shorter overall survival in at least three independent cohorts of MM patients out of the six studied (green colour). The six cohorts included gene expression data of purified MM cells from the TT2, TT3 (accession number E-TABM- 1138; GSE2658), and Hovon (accession number GSE19784) cohorts (345, 158 and 282 newly-diagnosed MM patients treated by high-dose melphalan and autologous hematopoietic stem cell transplantation); the Mulligan cohort (188 patients at relapse treated by proteasome inhibitor in monotherapy; GSE9782); the Mtp cohort non eligible to HDT (63 newly-diagnosed MM patients non eligible to high-dose melphalan and autologous hematopoietic stem cell transplantation) and the Mtp Dara cohort (51 patients at relapse treated by anti-CD38 monoclonal antibody (Daratumumab). c- The prognostic information of PHC1, PHC2, PHC3, CBX2, CBX7 and BMI1 genes was combined. Patients of the TT2 cohort (n = 345) were ranked according to the increased value of the calculated score and a cluster was defined. d- In the TT2 cohort, a maximum difference in overall survival was obtained, using the Maxstat R package, splitting patients into high-risk for 144 patients with the lowest expression of PRC1 genes and low-risk group for the 201 patients with higher PRC1 gene expression. Using the same parameter of the TT2 training cohort, we validated the association between low expression of PRC1 genes and a poor outcome in five other independent cohorts of patients with MM.
Methods
Drosophila strains and genetics
Flies were raised on a standard cornmeal yeast extract medium at 25 °C unless otherwise indicated. Fly lines and crosses performed to deplete PRC1 subunits or to perform control experiments were generated from stocks provided by the Bloomington Drosophila Stock Center (BL) and the Vienna Drosophila Resource Center (VDRC), as indicated below for each experiment. The work with transgenic strains of Drosophila was performed under the ethical approval no. n6906C2 of the Ministère de l’Enseignement Supérieur, de la Recherche et de l’Innovation, issued on 8 April 2020.
For KD experiments of PRC1 subunits and generation of EICs, Gal80ts was used to control the temporal ph or Psc/Su(z)2 down-regulation by switching the temperature from 18 to 29 °C. KDs are generated in the larval EDs using the ey-FLP system. The rationale of the reversible KD system is the following: ph-RNAi, as well as the GFP marker, are under control of UAS sequences. Cells expressing ey-FLP (in pink in Fig. 1a) induce FLP-out of a transcriptional stop (located between two FRT sites and indicated in orange in Fig. 1a) in EDs, leading to expression of act-Gal4 (in light blue in Fig. 1a). tub-Gal80ts (in purple in Fig. 1a) encodes a ubiquitously expressed, temperature-sensitive Gal4 repressor. At restrictive temperature (29 °C), Gal80ts is inactivated. Gal4 activates UAS sequences, expressing ph-RNAi and GFP (as readout of ph-KD).
To perform KDs, flies were reared and crossed at 18 °C to inhibit Gal4 activity. A total of 80 virgin females were crossed with 20 males for each genotype and experiment. In all conditions (no, constant or transient KDs), flies were allowed to lay eggs at 18 °C for 4 h to synchronize embryonic and larval stages. As the timing of Drosophila development is temperature dependent, we adapted the timing for each KD condition to carry out phenotypic and molecular analyses at comparable developmental times. The genotypes of the flies on which we carried out the different KDs are listed below.
For ph-KD: ey-FLP, Act-gal4 (FRT.CD2 STOP) (BL#64095); TubGal80ts (BL#7019); UAS-ph-RNAi (VDRC#50028)/UAS-GFP (BL#64095).
For Psc-Su(z)2-KD: ey-FLP, Act-gal4 (FRT.CD2 STOP) (BL#64095); UAS-Psc-Su(z)2 RNAi (BL#38261, VDRC#100096); TubGal80ts (BL#7018)/UAS-GFP (BL#64095).
For control white-KD: ey-FLP, Act-gal4 (FRT.CD2 STOP) (BL#64095); TubGal80ts (BL#7019); UAS-w-RNAi (BL#33623)/UAS-GFP (BL#64095).
All dissections were performed on female larvae at the L3 stage. For the no ph-KD (no depletion), flies were kept at 18 °C throughout development and dissected 10 days AEL. For the constant ph-KD (constant depletion), flies were kept at 29 °C throughout development and dissected 5 days AEL. For the larval depletion (from L1 to L3) flies were kept at 18 °C for 48 h and shifted at 29 °C until dissection 5 days AEL. For the transient ph-KD at the L1 stage, flies were kept at 18 °C for 48 h, then shifted at 29 °C for 24 h and returned to 18 °C until dissection 9 or 11 days AEL. For the transient ph-KD at the L2 stage, flies were kept at 18 °C for 96 h, shifted at 29 °C for 24 h and returned to 18 °C until dissection 8 days AEL. For the transient ph-KD at the L3 early stage, flies were kept at 18 °C for 120 h, shifted at 29 °C for 24 h and returned to 18 °C until dissection 8 days AEL. For the transient ph-KD at the L3 late stage, flies were kept at 18 °C for 168 h, shifted at 29 °C for 24 h and returned to 18 °C until dissection 8 days AEL. For the transient Psc-Su(z)2-KD at the L1 stage, flies were kept at 18 °C for 48 h, shifted at 29 °C for 48 h and returned to 18 °C until dissection 8 days AEL. For all conditions, a minimum of three biological replicates was performed. For each replicate, 150 discs were scored in PH depletions and more than 30 discs were scored for PSC depletions. Constant and transient depletions of PH (PH-d and PH-p) or PSC-SU(Z)2 generated tumours in 100% of dissected tissues.
To assess viability, we measured adult hatching rate. For this purpose, after 4 h of egg laying, we applied the treatments described above to produce ph-KD at the desired times. The vials were maintained at 18 °C and the number of pupae was counted for each condition. The adult hatching rate was calculated by dividing the number of male and female adults hatched from pupae by the number of pupae.
For the zfh1-RNAi and Stat92E-RNAi rescue experiments under constant ph-KD, ey-FLP, Act5C-gal4 (FRT.CD2 STOP); + ; UAS-GFP (BL#64095) females were crossed with males of various genotypes. For negative control experiments, females were crossed with UAS-gfp-RNAi (BL#9331); UAS-w-RNAi (BL#33623) males. To confirm that the zfh1-RNAi and Stat92E-RNAi do not induce any significant change in the eye development we crossed female to UAS-zfh1-RNAi (VDRC#103205); UAS-w-RNAi (BL#33623) and UAS-Stat92E-RNAi (VDRC#43866); UAS-w-RNAi (BL#33623) males. Positive control experiments were conducted by crossing females with UAS-gfp-RNAi (BL#9331); UAS-ph-RNAi (VDRC#50028) males. For the rescue condition we crossed females to UAS-zfh1-RNAi (VDRC#103205); UAS-ph-RNAi (VDRC#50028) and UAS-Stat92E-RNAi (VDRC#43866); UAS-ph-RNAi (VDRC#50028) males. This systematic breeding strategy facilitated the investigation of the specific roles of zfh1 and Stat92E genes under constant ph-KD conditions.
Flies were reared and crossed at 18 °C and tumours were scored in the progeny reared at 18 °C. Note that in this genetic background there is no Gal80ts and therefore the KDs are obtained independently of the temperature. In the case of the ph-KD positive control, a tumour phenotype with 100% penetrance was observed in the progeny.
For the zfh1-RNAi and Stat92E-RNAi rescue experiments under transient ph-KD, ey-FLP, Act5C-gal4 (FRT.CD2 STOP) (BL#64095); + ; TubGal80ts (BL#7018)/TM6BTb females were crossed with males of various genotypes. For negative control experiments, females were crossed with UAS-gfp-RNAi (BL#9331); UAS-w-RNAi (BL#33623) males. To confirm that the zfh1-RNAi and Stat92E-RNAi do not induce any significant change in the eye development, we crossed female to UAS-zfh1-RNAi (VDRC#103205); UAS-w-RNAi (BL#33623) and UAS-Stat92E-RNAi (VDRC#43866); UAS-w-RNAi (BL#33623) males. Positive control experiments were conducted by crossing females with UAS-gfp-RNAi (BL#9331); UAS-ph-RNAi (VDRC#50028) males. For the rescue condition we crossed females to UAS-zfh1-RNAi (VDRC#103205); UAS-ph-RNAi (VDRC#50028) and UAS-Stat92E-RNAi (VDRC#43866); UAS-ph-RNAi (VDRC#50028) males. This systematic breeding strategy facilitated the investigation of the specific roles of the zfh1 and Stat92E genes under transient ph-KD conditions.
Flies were reared and crossed at 18 °C and flies were allowed to lay eggs overnight at 18 °C. For transient depletion, flies were kept at 18 °C for 48 h, then shifted at 29 °C for 24 h and returned to 18 °C until dissection 10 days AEL.
Allografts were performed according to the protocol described previously62. The following fly line was used: ey-FLP (BL#5580), Ubi-p63E(FRT.STOP)Stinger (BL#32249); Tub-Gal80ts (BL#7019); Act5C-Gal4(FRT.CD2), UAS-RFP (BL#30558)/UAS-ph-RNAi (VDRC#50028). Briefly, GFP-positive EDs from no-ph-KD, constant ph-KD or transient ph-KD L3 female larvae were dissected in PBS, cut into small pieces and injected into the abdomen of adult female hosts (BL#23650). The whole experiment was performed at 18 °C to avoid reactivation of ph-RNAi expression. To score tumour progression in allografts, flies were imaged every 2 days using Leica MZ FLIII to verify GFP as a readout of tumour growth. Tumours were dissected and re-injected when the host abdomen was fully GFP. Injected Drosophila pictures were taken using Ximea USB 3.1 Gen1 camera with a Sony CMOS-xiCAll sensor.
Immunostaining procedures
EDs from L3 female larvae were dissected at room temperature in 1× PBS and fixed in 4% formaldehyde for 20 min. Tissues were permeabilized for 1 h in 1× PBS + 0.5% Triton X-100 on a rotating wheel. Permeabilized tissues were blocked for 1 h in 3% BSA PBTr (1× PBS + 0.1% Triton X-100), and incubated O/N on a rotating wheel at 4 °C with primary antibodies diluted in PBTr + 1% BSA. For double-strand break staining, larvae were dissected at room temperature in 1× PBS, fixed in 4% paraformaldehyde for 30 min and primary antibodies were incubated for 2 h at room temperature. The following primary antibodies were used: goat anti-PH63 (1:500), mouse anti-ELAV (1:1,000, DSHB, catalogue no. 9F8A9), mouse anti-ABD-B (1:1,000, DSHS, catalogue no. 1A2E9), chicken anti-GFP (1:500, Invitrogen, catalogue no. A10262), rabbit anti-ZFH1 (ref. 49) (1:2,000) and rabbit anti-histone H2AvD pS137 (1:500, Rockland, catalogue no. 600-401-914). Then, samples were washed in PBTr three times before adding secondary antibodies in PBTr for 2 h at room temperature on a rotating wheel. The following secondary antibodies were used: donkey anti-goat Alexa Fluor 555 (1:1,000, Invitrogen, catalogue no. A-21432), donkey anti-mouse Alexa Fluor 647 (1:1,000, Invitrogen, catalogue no. A-31571), donkey anti-chicken (1:1,000, Clinisciences, catalogue no. 703-546-155), donkey anti-rabbit Alexa Fluor 555 (1:1,000, Invitrogen, catalogue no. A-31572), donkey anti-rabbit Alexa Fluor 488 (1:1,000, Invitrogen, catalogue no. A-21206). F-actin was stained by adding rhodamine phalloidin Alexa Fluor 555 (1:1,000, Invitrogen, catalogue no. R415) or Alexa Fluor 488 (1:1,000, Invitrogen, catalogue no. A12379). Tissues were washed three times in PBTr. DAPI (4,6-diamidino-2-phenylindole) staining was performed at a final concentration of 1 µg ml−1 for 15 min. Then discs were washed in PBTr and mounted in Vectashield medium (Eurobio Scientific, catalogue no. H-1000-10) or ProLong Gold antifade agent (Life Technologies, P36930). Image acquisition was performed using a Leica SP8-UV confocal microscope. ED areas were measured using Fiji64 by drawing contour lines around the DAPI-labelled tissue and measuring their surface. A minimum of 30 EDs was considered to measure average ED areas in each condition. Images for quantification of double-strand break foci were taken with a DeltaVision deconvolution microscope using a ×60 oil immersion objective and a CoolSNAP HQ2 camera. Images were processed using Deconvolution through SoftWoRx v.6.0. All experiments were performed in biological duplicates.
EdU staining
EdU experiments were performed using Click-iT Plus EdU Alexa fluor 555 Imaging kit (Invitrogen, catalogue no. C10638). The EDs of L3 female larvae were dissected at room temperature in Schneider medium. Then, EdU incorporation was performed for 15 min with 25 µM EdU solution on a rotating wheel at room temperature. After washing with PBS, tissues were fixed in 4% formaldehyde 30 min and washed three times with PBS. The imaginal discs were permeabilized for 1 h in 1× PBS + 0.5% Triton X-100 on a rotating wheel then blocked for 1 h in 1× PBS + 0.1% Triton X-100 + 3% BSA. EdU detection was performed according to the manufacturer’s instructions for 30 min on a rotating wheel at room temperature away from light. Next, 500 µl of Click-iT reaction cocktail were prepared per tube containing 20 EDs. After 1× PBS + 0.1% Triton wash DAPI staining was performed at a final concentration of 1 µg ml−1 for 15 min. Tissues were washed in 1× PBS + 0.1% Triton and discs were mounted in Vectashield medium. Image acquisition was performed using a Leica SP8-UV confocal microscope. Images of EdU stained EDs shown in Supplementary Videos were acquired using a Zeiss LSM980 Airyscan microscope in 4Y modality. Airyscan images of EdU stained EDs were processed with ZEN (v.3.6 Blue Edition, Zeiss) using default settings. Videos were created using Imaris (v.10.1, Oxford Instruments). All experiments were performed in biological duplicates.
Analysis of chromosomal abnormalities
Chromosome preparation and FISH were performed as previously described65,66. EDs from L3 stage larvae were dissected in 0.7% NaCl solution and incubated in Colchicine solution (3 ml of 0.7% NaCl + 100 µl of 10−3 M Colchicine) for 1 h at room temperature away from light. EDs were incubated in 0.5% sodium acetate for 7 min, followed by fixation (freshly prepared 2.5% PFA in 45% acetic acid) for 4 min on coverslip. EDs were pressed onto poly-lysine coated slides using manual force and snap frozen in liquid nitrogen. Slides were washed in 100% ethanol for 5 min, air dried and stained with fluorescence in situ hybridization (FISH) probes for AACAC, AATAT and 359 base pair (bp) repeats as previously described65. Probe sequences are: 5′-6-FAM-(AACAC)7, 5′-Cy3-TTTTCCAAATTTCGGTCATCAAATAATCAT and 5′-Cy5-(AATAT)6. FISH staining was used to help identify chromosomes in rearranged conditions. Microscopy acquisition was performed on a DeltaVision deconvolution microscope using a ×60 oil immersion objective and a CoolSNAP HQ2 camera. Images were processed for Deconvolution using SoftWoRx v.6.0.
Damage induction by X-ray exposure
L3 early-stage female larvae were transferred into a petri dish containing standard food medium, and were exposed to 5 Gy of X-rays using a Precision X-RAD iR160 irradiator. After irradiation, larval heads were dissected at indicated timepoints at room temperature in 1× PBS and fixed in 4% paraformaldehyde for 30 min before immunostaining. Microscopy and image analysis were performed as described above.
RT–qPCR experiments
L3 female larvae were dissected in Schneider medium on ice. Total RNA was extracted from EDs using TRIzol reagent. RNA purification was performed using the RNA Clean & Concentrator kit (Zymo Research, catalogue no. R1015). Reverse transcription was performed using Maxima First Strand complementary DNA synthesis kit (Invitrogen, catalogue no. K1642). Quantitative PCR (qPCR) was performed using LightCycler 480 SYBR Green I Master Mix (Roche, catalogue no. 04707516001). qPCR with reverse transcription (RT–qPCR) experiments were analysed using LightCycler and GraphPad Prism software. All experiments were performed in biological triplicates.
RNA-seq experiments
L3 female larvae were dissected in Schneider medium on ice. Total RNA was extracted from EDs using TRIzol reagent. RNA purification was performed using the RNA Clean & Concentrator kit (Zymo Research, catalogue no. R1015). Finally, poly-A RNA selection, library preparation and Illumina sequencing (20 M paired-end reads, 150 nt) were performed by Novogene (https://en.novogene.com/). All experiments were performed in triplicates.
gDNA sequencing
gDNA was isolated using QIAamp DNA Micro Kit (Qiagen) following the manufacturer’s instructions. For each biological replicate, roughly 70 EDs from wandering female larvae were dissected. In total, we sequenced four biological replicates for control samples (no ph-KD condition, that is, larvae of the crosses used for transient depletion that were reared at constant permissive temperature of 18 °C). Furthermore, 12 tumour samples were sequenced, that is, two biological replicates for six different depletion conditions as follows: (1) constant ph-KD; (2) transient ph-KD d9; (3) transient ph-KD d11; (4) early L3 ph-KD, 24 h recovery; (5) early L3 ph-KD, 96 h recovery and (6) early L3 ph-KD, 144 h recovery. All these conditions result in tumour formation. The gDNAs of all samples were processed for library preparation by Novogene (https://en.novogene.com/). Briefly, gDNA was fragmented to an average size of roughly 350 bp and then processed for DNA library preparation according to the manufacturer’s (Illumina) paired-end protocols. Sequencing was performed using the Illumina Novaseq 6000 platform to generate 150 bp paired-end reads with a coverage of at least ten times for 99% of the genome.
Western blot
Roughly 150 EDs were dissected in Schneider medium on ice per replicate. To collect sufficient material, EDs were dissected in batches, snap frozen in liquid nitrogen and stored at −80 °C. Discs were homogenized with a Tenbroeck directly in radioimmunoprecipitation assay lysis buffer (50 mM Tris pH 7.5, 150 mM NaCl, 1% NP40, 0.5% Na-deoxycholate, 0.1% SDS, 2× protease inhibitor) and incubated on ice for 10 min. If necessary, a second round of mechanical dissociation was performed. Samples were centrifuged for 10 min at 10,000g at 4 °C and the supernatant was transferred to a fresh tube. Proteins were quantified using BCA protein assay and 10 µg were used per gel lane, before 40 min of migration at 200 V in MES 20× migration buffer and 1 h of transfer (1 A). Membranes were blocked for 1 h in PBS + 0.2% Tween + 10% milk powder at room temperature, incubated O/N with primary antibodies in PBS + 0.2% Tween at 4 °C on a shaker and washed in PBS + 0.2% Tween. The following primary antibodies were used: rabbit anti-PH (1:200), rabbit anti-zfh1 (ref. 49) (1:2,000), mouse anti-beta tubulin (1:5,000, DSHB, catalogue no. AA12.1). HRP-conjugated secondary antibodies were incubated with the membrane for 2 h at room temperature. The following secondary antibodies were used: goat antirabbit (1:15,000, Sigma, catalogue no. A0545), rabbit antimouse (1:15,000, Sigma, catalogue no. A9044). Membranes were washed in PBS + 0.2% Tween and revealed using Super Signal West Dura kit (Pierce) and Chemidoc Bio-Rad. Western blots were analysed using ImageLab software v.6.1 from Bio-Rad. The full-size raw blot images are provided in the Supplementary Fig. 1.
ChIP–seq experiments
ChIP–seq on L3 EDs were performed as described previously41, with minor modifications, and 400 EDs were used per replicate. If necessary, several dissection and/or collection batches were frozen in liquid nitrogen and stored at −80 °C to collect sufficient material. Chromatin was sonicated using a Bioruptor Pico (Diagenode) for 10 min (30 s on, 30 s off). PH antibodies67 were diluted 1:100 for immunoprecipitation. After decrosslinking, DNA was purified using MicroChIP DiaPure columns from Diagenode. DNA libraries for sequencing were prepared using the NEBNext Ultra II DNA Library Prep Kit for Illumina. Sequencing (paired-end sequencing 150 bp, roughly 4 Gb per sample) was performed by Novogene (https://en.novogene.com/). All experiments were performed in biological duplicates.
CUT&RUN experiments
CUT&RUN experiments were performed as described by Kami Ahmad in protocols.io (10.17504/protocols.io.umfeu3n) with minor modifications. We dissected 50 EDs in Schneider medium, centrifuged them for 3 min at 700g and washed them twice with wash+ buffer before adding concanavalin A-coated beads. MNase digestion (pAG-MNase Enzyme from Cell Signaling) was performed for 30 min on ice. After ProteinaseK digestion, DNA was recovered using SPRIselect beads and eluted in 50 μl of Tris-EDTA. DNA libraries for sequencing were prepared using the NEBNext Ultra II DNA Library Prep Kit for Illumina. Sequencing (paired-end sequencing 150 bp, roughly 2 Gb per sample) was performed by Novogene (https://en.novogene.com/). The following antibodies were used: H3K27me3 (1:100, Active Motif, catalogue no. 39155), H3K27Ac (1:100, Active Motif, catalogue no. 39133), H2AK118Ub (1:100, Cell Signaling, catalogue no. 8240). All experiments were performed in biological duplicates.
ATAC-Seq experiments
ATAC-Seq experiments were performed using the ATAC-Seq kit from Diagenode (catalogue no. C01080002). Ten EDs were used as starting material for each replicate and condition. Tagmentated DNA was amplified by PCR using 13 cycles and the purified DNA libraries were sequenced (paired-end sequencing 150 bp, roughly 2 Gb per sample) by Novogene (https://en.novogene.com/). All experiments were performed in biological duplicates.
Statistics and reproducibility
ChIP–seq, CUT&RUN and ATAC-Seq were performed in duplicates, following Encode’s standards (https://www.encodeproject.org/chip-seq/transcription_factor/#standards; https://www.encodeproject.org/atac-seq/#standards). RNA-seq were performed in triplicates, following Encode’s recommendations (https://www.encodeproject.org/data-standards/rna-seq/long-rnas/).
In general, immunostaining experiments were performed in biological duplicates. Each biological replicate was obtained from independent genetic crosses. The only exception was the phospho-H2AV staining shown in Fig. 1j and Extended Data Fig. 2c, which was performed once, but scoring tissues that came from six independent genetic crosses. For sample sizes of immunostaining experiments, see the sheet named ‘All IF sample numbers’ in Supplementary Table 6. For transcriptomic, RT–qPCR and western blot analysis, experiments were performed in biological triplicates. ATAC-Seq, CUT&RUN, ChIP–seq and immunostaining experiments were performed in biological duplicates. Each biological replicate was obtained from independent genetic crosses.
For experiments presented in Figs. 1 and 5, as well as Extended Data Figs. 1, 2, 3, 5 and 6, involving genetic crosses with different lines and in different conditions, followed by tissue area measurements and immunofluorescence, two independent biological replicates were performed with similar results. Measured areas and the number of tissues analysed in imaging are reported in Supplementary Table 6.
Allograft experiments were performed in two independent biological replicates. In the first replicate, one starting tumour obtained on constant PH depletion and one tumour obtained from transient PH depletion were used. In the second replicate, two constant PH depletion and two transient PH depletion tumours were injected. Results were similar for both replicates. The total number of injected host flies is reported in the graphs of the Extended Data Fig. 7b,c.
Bioinformatic analyses on Drosophila datasets
All in-house bioinformatic analyses were performed in R v.3.6.3 (https://www.R-project.org/). Computations on genomic coordinate files and downstream computations were conducted using the data.table R package (data.table: Extension of ‘data.frame’. https://r-datatable.com, https://Rdatatable.gitlab.io/data.table, https://github.com/Rdatatable/data.table, v.1.14.2). In all relevant panels of figures and Extended Data figures, box plots depict the median (line), upper and lower quartiles (box) ±1.5× interquartile range (whiskers) and outliers are not shown. For each relevant panel, the statistical test that was used is specified in the caption: NS denotes not significant (P > 0.05), *P < 5 × 10−2, **P < 1 × 10−2, ***P < 1 × 10−3, ****P < 1 × 10−5.
gDNA processing and mapping of somatic variants
gDNA variant calling was performed by Novogene (https://en.novogene.com/). Briefly, base calling was performed using Illumina pipeline CASAVA v.1.8.2, and subjected to quality control using fastp with the following parameters: -g -q 5 -u 50 -n 15 -l 150 --min_trim_length 10 --overlap_diff_limit 1--overlap_diff_percent_limit 10. Then, sequencing reads were aligned to the dm6 version of the Drosophila genome using Burrows–Wheeler aligner with default parameters and duplicate reads were removed using samtools and PICARD (http://picard.sourceforge.net). Raw SNP and InDel sets were called using GATK with the following parameters: --gcpHMM 10 -stand_emit_conf 10 -stand_call_conf 30. Then, SNPs were filtered using the following criteria: SNP QD < 2, FS > 60, MQ < 30, HaplotypeScore > 13, MappingQualityRankSum < −12.5, ReadPosRankSum < −8. For INDEL variants, the following criteria were used: QD < 2, FS > 200, ReadPosRankSum < −20. UCSC known genes were used for gene and region annotations. Finally, the variants were compared to a batch-matched control sample (no ph-KD), in the search for bona fide SNVs and InDels using the MuTect2 module of the GATK package. Only SNVs and InDels variants that passed Mutect2 filtering (FILTER = “PASS”) were considered for downstream analyses. Structural variants and CNVs were detected using breakdancer (https://github.com/genome/breakdancer) and CNVnator (https://github.com/abyzovlab/CNVnator) software packages, respectively.
Then, called variants were imported in R for downstream analyses. When looking at the fraction of tumour samples that contained a given alteration (Fig. 1h), we only retained SNVs or InDels with an allelic fraction greater than 0.2, structural variants that were supported by at least five reads and CNVs with an allelic fraction bigger than 1.5 (duplication) or smaller than 0.66 (deletion).
RNA-seq processing and differential analysis
After initial quality checks of the newly generated data using fastqc (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/), the paired-end reads were aligned to a custom index consisting of the dm6 version of the Drosophila genome together with GFP, EGFP and mRFP1 sequences, using the align function from the Rsubread R package68 (v.2.0.1) with the following parameters: maxMismatches = 6, unique = TRUE. Next, aligned reads were counted for each D. melanogaster transcript (dmel_r6.36 annotation) using the featureCounts function from the Rsubread R package (v.2.0.1, isPairedEnd = TRUE) and differential expression analysis was performed using the DESeq2 R package69 (v.1.26.0, design = ~replicate + condition). The tables corresponding to the different comparisons are available in Supplementary Tables 1, 4 and 5.
For the differential analysis of the transcriptomes after no ph-KD (control), constant and transient ph-KD, each ph-RNAi sample was compared to temperature-matched w-RNAi controls (Fig. 2a and Extended Data Fig. 8b). DESeq2 outputs are available in Supplementary Tables 1 and 5. For the differential analysis of the transcriptomes after zfh1+w-KD, Stat92E+w-KD, gfp+ph-KD, zfh1+ph-KD and Stat92E+ph-KD, all were compared to temperature-matched gfp+w-KD (Supplementary Table 4).
Clustering of differentially expressed genes
For the clustering, we selected the genes that were differentially expressed (Padj < 0.05 and |log2fold2 fold change | >1) after constant or transient ph-KD (d9 or d11 AEL). In addition, we only considered the genes that did not show significant changes after no ph-KD (control). Then, log2 fold change values were clipped at the 5th and 95th percentiles and clustered using the supersom function from the kohonen R package70 (v.3.0.10). As day 9 and day 11 transient ph-KD yielded substantially similar transcriptomes, a two-layer self-organizing map was trained (layer 1, constant ph-KD; layer 2, D9 and D11 transient ph-KD) with similar weights for the two layers, using a 3 × 2 grid (topology = hexagonal, toroidal = TRUE). Clustering output is available in Supplementary Table 2.
CUT&RUN, ChIP–seq and ATAC-Seq processing, peak calling and differential analysis
After initial quality checks of the newly generated data using fastqc, the reads were aligned to the dm6 version of the Drosophila genome using bowtie 2 (ref. 71, v.2.3.5.1) with the following parameters: --local --very-sensitive-local --no-unal --no-mixed --no-discordant --phred33 -I 10 -X 700, and low mapping quality reads were discarded using samtools72 (-q 30, v.1.10, using htslib v.1.10.2-3).
PH, H3K27me3, H3K27Ac, H2AK118Ub and ATAC-Seq peaks and/or domains were called for each replicate separately and on merged reads using macs2 (ref. 73, v.2.2.7.1) with the following parameters: --keep-dup 1 -g dm -f BAMPE -B --SPMR. For PH ChIP–seq, the input sample was used as control. For H3K27me3, H3K27Ac and H2AK118Ub CUT&RUN, the IgG sample was used as control. Only peaks detected in both replicates (enrichment greater than 0 AND q value less than 0.05) and using merged replicates (enrichment greater than 2 AND q < 0.01) were retained for further analyses, after being merged with a minimum gap size of 250 bp for narrow peaks (PH, H3K27Ac and ATAC-Seq) and 2.5 kb for broad marks (H3K27me3 and H2AK118Ub). The macs2 bedgraph files were used for visualization purposes.
For the differential analysis of H3K27me3, H3K27Ac, H2AK118Ub CUT&RUN and ATAC-Seq, peaks and/or domains were first merged across all conditions (maximum gap of 250 bp for H3K27Ac and ATAC-Seq peaks; 2.5 kb for H3K27me3 and H2AK118Ub domains) and overlapping reads were counted using the featureCounts function from the Rsubread R package (v.2.0.1, isPairedEnd = TRUE). Differential analysis was then performed using the DESeq2 R package (v.1.26.0, size factors, total number of aligned reads; design, ~replicate + condition). The same procedure was used for the differential analysis of ATAC-Seq peaks between zfh1+ph-KD and gfp+ph-KD.
Clustering of differentially accessible ATAC-Seq peaks
For the clustering of ATAC-Seq peaks, we only considered the peaks showing a significant difference (Padj < 1 × 10−3 and |log2 fold change| >1) after constant or transient ph-KD (day 11 AEL) and with a minimum log10 base mean of 1.25 to avoid noisy peaks. The log2 fold change values were clipped at the 5th and 95th percentiles and clustered using the supersom function from the kohonen R package70 (v.3.0.10) using a four-layer self-organizing map (layer 1, log2fold change constant ph-KD; layer 2, log2fold change transient ph-KD; layer 3, Padj constant ph-KD; layer 4, Padj transient ph-KD) with similar weights for the four layers, using a 1 × 3 grid (topology = hexagonal, toroidal = TRUE). Full clustering output is available in Supplementary Table 3.
Classification of PcG target genes and peaks-to-gene assignment
To define PcG target genes, we defined a clean set of H3K27me3 domains in the control (no ph-KD) condition by removing artefactual splits due to sequencing gaps (github), resulting in 241 domains. Then, only the genes for which at least 50% of the gene body was overlapping with a H3K27me3 domain were considered as direct PcG target. When relevant, only irreversible, reversible and unaffected genes that were direct PcG targets when considered (Fig. 3). PcG target gene assignment is available in Supplementary Table 2 (PcG_bound and class columns).
To assess whether a gene was overlapping a H3K27me3 domain or a H3K27Ac peak in a given condition, we used different criteria. For H3K27me3 (Fig. 3b), only the genes for which at least 50% of the gene body was overlapping a confident H3K27me3 domain (‘CUT&RUN, ChIP–seq and ATAC-Seq processing, peak calling and differential analysis’ section above) were considered as hits. For H3K27Ac (Fig. 3c), only the genes containing a confident peak (‘CUT&RUN, ChIP–seq and ATAC-Seq processing, peak calling and differential analysis’ section above) in the gene body or up to 2.5 kb upstream of the TSS were considered as hits.
To assign PH peaks (Extended Data Fig. 6e) or ATAC-Seq peaks (Fig. 4b), peaks were assigned to the closest TSS with a maximum genomic separation of 25 kb (peaks that were located further away were not considered).
Gene Ontology terms enrichment
Gene Ontology terms associated with the genes of interest and a background set of genes, consisting of all the genes that passed DESeq2 initial filters, were retrieved using the AnnnotationDbi R package (https://bioconductor.org/packages/AnnotationDbi.html, v.1.48.0). For each Gene Ontology term, over-representation was assessed using a one-sided Fisher’s exact test (alternative = ‘greater’). Obtained P values were corrected for multiple testing using false discovery rate (FDR).
Motif enrichment
To search for DNA binding motifs enriched at each ATAC-Seq cluster, we used the centre of corresponding peaks ±250 bp (500 bp total). Resulting regions were analysed with the i-cisTarget online tool74, using v.6.0 of the position weight matrix database (consisting of 24,453 position weight matrics). Only top scoring motifs with a normalized enrichment score greater than 5.5 and a rank less than 50 were considered (Fig. 4e).
To search for motifs associated with increased or decreased accessibility after constant or transient ph-KD, we used a collection of non-redundant transcription factor motifs75 and counted their occurrences across all ATAC-Seq peaks ±250 bp, using the matchMotifs function from the motifmatch R package (v.1.18.0; 10.18129/B9.bioc.motifmatchr) with the following parameters: Pcutoff = 5 × 10−4, bg = ‘genome’, genome = ‘dm6’. Of note, only motifs associated with a Drosophila transcription factor gene that passed initial DESeq2 initial filters were considered. Then, we fitted two LASSO regressions using the cv.glmnet and the glmnet functions from the glmnet package in R (v.4.1.4), with the following parameter: lambdas = 10seq(2, −3, by = −0.1), standardize, TRUE; nfolds, 5), aiming at predicting log2 fold changes after constant or transient ph-KD. The top 25 motifs with the strongest |s0| coefficients in any of the two models were used to train two linear models to predict log2 fold changes after transient or constant ph-KD. Only the motifs with a significant coefficient in at least one of the two linear models (P < 1 × 10−5) were considered (Fig. 4f).
Analysis of human solid tumours
The differential gene expression analysis was carried out by using a Mann–Whitney test and the TNMplot database, which contains transcriptome-level RNA-seq data for different tumour samples from The Cancer Genome Atlas (TCGA) and The Genotype-Tissue Expression (GTEx) repositories76.
The survival analysis was carried out using the Pan-Cancer (Bladder, Lung adenocarcinoma and Rectum adenocarcinoma) or gene array (Breast, Ovarian and Prostate) datasets77,78 of the online tool www.kmplot.com (accessed on 22 December 2022). The Pan-Cancer dataset is based on TCGA data generated using the Illumina HiSeq 2000 platform with survival information derived from the published sources79. The gene-array samples were obtained using Affymetrix HGU133A and HGU133plus2 gene chips. The samples were MAS5 normalized and the mean expression in each sample was scaled to 1,000. The most reliable probe sets to represent single genes were identified usNAiing JetSet80.
In the survival analysis, each cut-off value between the lower and upper quartiles of expression was analysed by Cox proportional hazards regression and FDR was computed to correct for multiple hypothesis testing. Then, the best performing cut-off was used when drawing the Kaplan–Meier survival plots that were generated to visualize the survival differences. Hazard rates with 95% confidence intervals were computed to numerically assess the survival time difference between the two cohorts. The statistical analysis was performed in the R statistical environment (www.r-project.org). The analysis results for single genes can be validated using the platforms at www.kmplot.com and www.tnmplot.com.
Analysis of cohorts of patients with multiple myeloma
For gene expression profiling data from patients with multiple myeloma, we used six cohorts that included Affymetrix gene expression data (HGU133plus2) of purified multiple myeloma cells from the TT2 (ref. 81) (Gene Expression Omnibus, accession number GSE2658), TT3 (ref. 82) (accession number E-TABM-1138 accession number GSE4583) and Hovon83 (accession number GSE19784) cohorts (345, 158 and 282 newly diagnosed patients with multiple myeloma who were treated with high-dose melphalan and autologous haematopoietic stem cell transplantation); the Mulligan cohort84 (188 patients at relapse treated by proteasome inhibitor in monotherapy); the Mtp cohort non-eligible for HDT85 (63 newly diagnosed patients with multiple myeloma who were not eligible for high-dose melphalan and autologous haematopoietic stem cell transplantation) and the Mtp Dara cohort85,86 (51 patients at relapse treated by anti-CD38 monoclonal antibody (Daratumumab)). Gene expression data were normalized with the MAS5 algorithm and processing of the data was performed using the webtool genomicscape (http://www.genomicscape.com), as done previously87,88, using the R environment (www.r-project.org). The prognostic values of PHC1, PHC2, PHC3, CBX2, CBX7 and BMI1 gene expression was investigated using the Maxstat R function and Kaplan–Meier survival curves as previously described89. The differential gene expression analysis between normal bone marrow plasma cells from healthy donors and multiple myeloma cells from patients was carried out by using the Mann–Whitney test. The prognostic value of PHC1, PHC2, PHC3, CBX2, CBX7 and BMI1 genes was combined using our previously published methodology89 (sum of the Cox b coefficients of each of the six genes, weighted by ±1 if the patient’s multiple myeloma cell signal for a given gene is above or below the probe set Maxstat value of the gene). Clustering was performed using the Morpheus software (https://software.broadinstitute.org/morpheus) and violin plots using GraphPad Prism software (http://www.graphpad.com/scientific-software/prism/).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-024-07328-w.
Supplementary information
This file contains full descriptions for Supplementary Tables 1–6 and Supplementary Videos 1–2 (Tables and Videos supplied separately), and Supplementary Fig. 1, which contains the raw western blot data corresponding to Fig. 1b and Extended Data Fig. 1e.
Differential analyses and FPKMs of no ph-KD, transient ph-KD and constant ph-KD (fly line with conditional GFP expression).
Clustering of differentially expressed genes, PcG binding and recovery status.
Clustering of differentially accessible ATAC-Seq peaks.
Differential analyses and FPKMs of gfp-KD, zfh1-KD and Stat92E-KD transcriptomes in combination with w-KD (control) or ph-KD.
Differential analyses and FPKMs of no ph-KD, transient ph-KD and constant ph-KD (fly line with constitutive GFP expression).
Tissue area measurements and number of tissues analysed in immunofluorescence experiments.
EdU incorporation in a control early L3 ED.
EdU incorporation on a 24 h ph-KD in an early L3 ED, followed by 24 h of recovery.
Acknowledgements
We thank Montpellier Resources Imagerie facility as well as the Drosophila facilty (both affiliated to BioCampus University of Montpellier, CNRS, INSERM, Montpellier, France). We thank A.-M. Popmihaylova for help with immunostaining of Drosophila tissues. We thank J. Drouin for discussions and advice on the manuscript. We thank E. Soler for discussions on the function of the ZEB1 protein in cancer. V.P. was supported by the EpiGenMed cluster of Excellence funding (Programme d’Investissements d’Avenir of the French Ministry of Higher Education and Research) and by la Ligue Nationale Contre le Cancer. V.L. was supported by the EpiGenMed cluster of Excellence funding (PIA of the French Ministry of Higher Education and Research). A.-M.M. was supported by the University of Montpellier and a grant from the Fondation ARC (contract no. 216574, acronym ‘Epicancer’). B.S. was supported by INSERM. G.C. was supported by CNRS. I.C. was supported by National Institutes of Health grant no. R01GM117376 and National Science Foundation Career no. 1751197. Research in the G.C. laboratory was supported by grants from the European Research Council (Advanced Grant 3DEpi), the European CHROMDESIGN ITN project (Marie Skłodowska-Curie grant agreement no. 813327), the European E-RARE NEURO DISEASES grant ‘IMPACT’, by the Agence Nationale de la Recherche (PLASMADIFF3D, grant no. ANR-18-CE15-0010), by the Fondation pour la Recherche Médicale (grant no. EQU202303016), by the MSD Avenir Foundation ((Project GENE-IGH) and by the French National Cancer Institute (INCa, PIT-MM grant no. INCA-PLBIO18-362). M.E. was supported by RSF grant no. 20-74-10099.
Extended data figures and tables
Author contributions
V.L., V.P., A.-M.M. and G.C. initiated and led the project. V.P., L.F. and V.L. performed genetic experiments. V.P. performed immunostaining, molecular biology and genomic experiments. V.L. and M.D.S. performed computational analysis of genomic datasets. V.P. and A.-M.M. performed allograft experiments. B.S. performed ChIP–seq, ATAC-Seq and CUT&RUN experiments. D.N. helped with EdU imaging. M.E., B.G. and D.C. performed computational analysis of different tumour types. J.M. performed computational analysis of multiple myeloma samples. C.C.R. performed irradiation experiments and N.L.B. performed karyotyping under the guidance of I.C. V.L., V.P., A.-M.M. and G.C. wrote the manuscript. All the authors discussed the data and reviewed the manuscript.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The NGS datasets generated in this study were made publicly available in the Gene Expression Omnibus (accession number GSE222193). A UCSC browser to visualize the data is available at http://genome-euro.ucsc.edu/s/cavalli/EpiCancer.
Code availability
All custom scripts that were generated for this study were made publicly available at https://github.com/vloubiere/Parreno_Loubiere_2023.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: V. Parreno, V. Loubiere
Contributor Information
A.-M. Martinez, Email: anne-marie.martinez@igh.cnrs.fr
G. Cavalli, Email: giacomo.cavalli@igh.cnrs.fr
Extended data
is available for this paper at 10.1038/s41586-024-07328-w.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-024-07328-w.
References
- 1.McGranahan N, Swanton C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell. 2015;27:15–26. doi: 10.1016/j.ccell.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein B, et al. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brock A, Chang H, Huang S. Non-genetic heterogeneity—a mutation-independent driving force for the somatic evolution of tumours. Nat. Rev. Genet. 2009;10:336–342. doi: 10.1038/nrg2556. [DOI] [PubMed] [Google Scholar]
- 4.Flavahan WA, Gaskell E, Bernstein BE. Epigenetic plasticity and the hallmarks of cancer. Science. 2017;357:eaal2380. doi: 10.1126/science.aal2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marine JC, Dawson SJ, Dawson MA. Non-genetic mechanisms of therapeutic resistance in cancer. Nat. Rev. Cancer. 2020;20:743–756. doi: 10.1038/s41568-020-00302-4. [DOI] [PubMed] [Google Scholar]
- 6.Timp W, Feinberg AP. Cancer as a dysregulated epigenome allowing cellular growth advantage at the expense of the host. Nat. Rev. Cancer. 2013;13:497–510. doi: 10.1038/nrc3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teixeira VH, et al. Deciphering the genomic, epigenomic, and transcriptomic landscapes of pre-invasive lung cancer lesions. Nat. Med. 2019;25:517–525. doi: 10.1038/s41591-018-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavalli G, Heard E. Advances in epigenetics link genetics to the environment and disease. Nature. 2019;571:489–499. doi: 10.1038/s41586-019-1411-0. [DOI] [PubMed] [Google Scholar]
- 9.Waddington CH. The epigenotype. Int. J. Epidemiol. 1942;41:10–13. doi: 10.1093/ije/dyr184. [DOI] [PubMed] [Google Scholar]
- 10.Nam AS, Chaligne R, Landau DA. Integrating genetic and non-genetic determinants of cancer evolution by single-cell multi-omics. Nat. Rev. Genet. 2021;22:3–18. doi: 10.1038/s41576-020-0265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaffer SM, et al. Rare cell variability and drug-induced reprogramming as a mode of cancer drug resistance. Nature. 2017;546:431–435. doi: 10.1038/nature22794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutter C, Zenklusen JC. The Cancer Genome Atlas: creating lasting value beyond its data. Cell. 2018;173:283–285. doi: 10.1016/j.cell.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 13.Stunnenberg HG, International Human Epigenome, C. Hirst M. The International Human Epigenome Consortium: a blueprint for scientific collaboration and discovery. Cell. 2016;167:1145–1149. doi: 10.1016/j.cell.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Butera A, Melino G, Amelio I. Epigenetic ‘drivers’ of dancer. J. Mol. Biol. 2021;433:167094. doi: 10.1016/j.jmb.2021.167094. [DOI] [PubMed] [Google Scholar]
- 15.Piunti A, Shilatifard A. Epigenetic balance of gene expression by Polycomb and COMPASS families. Science. 2016;352:aad9780. doi: 10.1126/science.aad9780. [DOI] [PubMed] [Google Scholar]
- 16.Muller D, Gyorffy B. DNA methylation-based diagnostic, prognostic, and predictive biomarkers in colorectal cancer. Biochim. Biophys. Acta Rev. Cancer. 2022;1877:188722. doi: 10.1016/j.bbcan.2022.188722. [DOI] [PubMed] [Google Scholar]
- 17.Pon JR, Marra MA. Driver and passenger mutations in cancer. Annu. Rev. Pathol. 2015;10:25–50. doi: 10.1146/annurev-pathol-012414-040312. [DOI] [PubMed] [Google Scholar]
- 18.Kloetgen A, Thandapani P, Tsirigos A, Aifantis I. 3D chromosomal landscapes in hematopoiesis and immunity. Trends Immunol. 2019;40:809–824. doi: 10.1016/j.it.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cancer Genome Atlas Research N, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feinberg AP, Koldobskiy MA, Gondor A. Epigenetic modulators, modifiers and mediators in cancer aetiology and progression. Nat. Rev. Genet. 2016;17:284–299. doi: 10.1038/nrg.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bates SE. Epigenetic therapies for cancer. N. Engl. J. Med. 2020;383:650–663. doi: 10.1056/NEJMra1805035. [DOI] [PubMed] [Google Scholar]
- 22.Baylin, S. B. & Jones, P. A. Epigenetic determinants of cancer. Cold Spring. Harb. Perspect. Biol.10.1101/cshperspect.a019505 (2016). [DOI] [PMC free article] [PubMed]
- 23.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat. Rev. Cancer. 2004;4:143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 24.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 25.You JS, Jones PA. Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell. 2012;22:9–20. doi: 10.1016/j.ccr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alonso-Curbelo D, et al. A gene-environment-induced epigenetic program initiates tumorigenesis. Nature. 2021;590:642–648. doi: 10.1038/s41586-020-03147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vicente-Duenas C, Hauer J, Cobaleda C, Borkhardt A, Sanchez-Garcia I. Epigenetic priming in cancer initiation. Trends Cancer. 2018;4:408–417. doi: 10.1016/j.trecan.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Terekhanova NV, et al. Epigenetic regulation during cancer transitions across 11 tumour types. Nature. 2023;623:432–441. doi: 10.1038/s41586-023-06682-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makohon-Moore AP, et al. Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat. Genet. 2017;49:358–366. doi: 10.1038/ng.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald OG, et al. Epigenomic reprogramming during pancreatic cancer progression links anabolic glucose metabolism to distant metastasis. Nat. Genet. 2017;49:367–376. doi: 10.1038/ng.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fennell KA, et al. Non-genetic determinants of malignant clonal fitness at single-cell resolution. Nature. 2022;601:125–131. doi: 10.1038/s41586-021-04206-7. [DOI] [PubMed] [Google Scholar]
- 32.Mack SC, et al. Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature. 2014;506:445–450. doi: 10.1038/nature13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pascual G, et al. Dietary palmitic acid promotes a prometastatic memory via Schwann cells. Nature. 2021;599:485–490. doi: 10.1038/s41586-021-04075-0. [DOI] [PubMed] [Google Scholar]
- 34.Chatterjee A, Rodger EJ, Eccles MR. Epigenetic drivers of tumourigenesis and cancer metastasis. Semin. Cancer Biol. 2018;51:149–159. doi: 10.1016/j.semcancer.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Feinberg AP. The key role of epigenetics in human disease prevention and mitigation. N. Engl. J. Med. 2018;378:1323–1334. doi: 10.1056/NEJMra1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan HL, Morey L. Emerging roles for Polycomb-group proteins in stem cells and cancer. Trends Biochem. Sci. 2019 doi: 10.1016/j.tibs.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Parreno V, Martinez AM, Cavalli G. Mechanisms of Polycomb group protein function in cancer. Cell Res. 2022;32:231–253. doi: 10.1038/s41422-021-00606-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuettengruber B, Bourbon HM, Di Croce L, Cavalli G. Genome regulation by Polycomb and Trithorax: 70 years and counting. Cell. 2017;171:34–57. doi: 10.1016/j.cell.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Bilder D, Ong K, Hsi TC, Adiga K, Kim J. Tumour-host interactions through the lens of Drosophila. Nat. Rev. Cancer. 2021;21:687–700. doi: 10.1038/s41568-021-00387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beuchle D, Struhl G, Muller J. Polycomb group proteins and heritable silencing of Drosophila Hox genes. Development. 2001;128:993–1004. doi: 10.1242/dev.128.6.993. [DOI] [PubMed] [Google Scholar]
- 41.Loubiere V, et al. Coordinate redeployment of PRC1 proteins suppresses tumor formation during Drosophila development. Nat. Genet. 2016;48:1436–1442. doi: 10.1038/ng.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loubiere V, Papadopoulos GL, Szabo Q, Martinez AM, Cavalli G. Widespread activation of developmental gene expression characterized by PRC1-dependent chromatin looping. Sci. Adv. 2020;6:eaax4001. doi: 10.1126/sciadv.aax4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez AM, et al. Polyhomeotic has a tumor suppressor activity mediated by repression of Notch signaling. Nat. Genet. 2009;41:1076–1082. doi: 10.1038/ng.414. [DOI] [PubMed] [Google Scholar]
- 44.Classen AK, Bunker BD, Harvey KF, Vaccari T, Bilder D. A tumor suppressor activity of Drosophila Polycomb genes mediated by JAK-STAT signaling. Nat. Genet. 2009;41:1150–1155. doi: 10.1038/ng.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haag-Liautard C, et al. Direct estimation of per nucleotide and genomic deleterious mutation rates in Drosophila. Nature. 2007;445:82–85. doi: 10.1038/nature05388. [DOI] [PubMed] [Google Scholar]
- 46.Rossi F, Attolini CS, Mosquera JL, Gonzalez C. Drosophila larval brain neoplasms present tumour-type dependent genome instability. G3 Genes Genom. Genet. 2018;8:1205–1214. doi: 10.1534/g3.117.300489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sievers C, Comoglio F, Seimiya M, Merdes G, Paro R. A deterministic analysis of genome integrity during neoplastic growth in Drosophila. PLoS ONE. 2014;9:e87090. doi: 10.1371/journal.pone.0087090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beira JV, Torres J, Paro R. Signalling crosstalk during early tumorigenesis in the absence of Polycomb silencing. PLoS Genet. 2018;14:e1007187. doi: 10.1371/journal.pgen.1007187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flaherty MS, et al. chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev. Cell. 2010;18:556–568. doi: 10.1016/j.devcel.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leatherman JL, Dinardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boukhatmi H, Martins T, Pillidge Z, Kamenova T, Bray S. Notch mediates inter-tissue communication to promote tumorigenesis. Curr. Biol. 2020;30:1809–1820 e1804. doi: 10.1016/j.cub.2020.02.088. [DOI] [PubMed] [Google Scholar]
- 52.Enomoto M, Takemoto D, Igaki T. Interaction between Ras and Src clones causes interdependent tumor malignancy via Notch signaling in Drosophila. Dev. Cell. 2021;56:2223–2236 e2225. doi: 10.1016/j.devcel.2021.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, et al. Genome-wide CRISPR screen identifies PRC2 and KMT2D-COMPASS as regulators of distinct EMT trajectories that contribute differentially to metastasis. Nat. Cell Biol. 2022;24:554–564. doi: 10.1038/s41556-022-00877-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uhlirova M, Bohmann D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 2006;25:5294–5304. doi: 10.1038/sj.emboj.7601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hou XS, Perrimon N. The JAK-STAT pathway in Drosophila. Trends Genet. 1997;13:105–110. doi: 10.1016/S0168-9525(97)01006-8. [DOI] [PubMed] [Google Scholar]
- 56.Pinal N, Martin M, Medina I, Morata G. Short-term activation of the Jun N-terminal kinase pathway in apoptosis-deficient cells of Drosophila induces tumorigenesis. Nat. Commun. 2018;9:1541. doi: 10.1038/s41467-018-04000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reizel Y, et al. FoxA-dependent demethylation of DNA initiates epigenetic memory of cellular identity. Dev. Cell. 2021;56:602–612 e604. doi: 10.1016/j.devcel.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holoch D, et al. A cis-acting mechanism mediates transcriptional memory at Polycomb target genes in mammals. Nat. Genet. 2021;53:1686–1697. doi: 10.1038/s41588-021-00964-2. [DOI] [PubMed] [Google Scholar]
- 60.von Schimmelmann M, et al. Polycomb repressive complex 2 (PRC2) silences genes responsible for neurodegeneration. Nat. Neurosci. 2016;19:1321–1330. doi: 10.1038/nn.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jaffe LF. Epigenetic theories of cancer initiation. Adv Cancer Res. 2003;90:209–230. doi: 10.1016/S0065-230X(03)90007-8. [DOI] [PubMed] [Google Scholar]
- 62.Rossi F, Gonzalez C. Studying tumor growth in Drosophila using the tissue allograft method. Nat. Protoc. 2015;10:1525–1534. doi: 10.1038/nprot.2015.096. [DOI] [PubMed] [Google Scholar]
- 63.Grimaud C, et al. RNAi components are required for nuclear clustering of polycomb group response elements. Cell. 2006;124:957–971. doi: 10.1016/j.cell.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 64.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Larracuente, A. M. & Ferree, P. M. Simple method for fluorescence DNA in situ hybridization to squashed chromosomes. J. Vis. Exp.10.3791/52288 (2015). [DOI] [PMC free article] [PubMed]
- 66.Ryu T, et al. Heterochromatic breaks move to the nuclear periphery to continue recombinational repair. Nat. Cell Biol. 2015;17:1401–1411. doi: 10.1038/ncb3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schuettengruber B, et al. Functional anatomy of polycomb and trithorax chromatin landscapes in Drosophila embryos. PLoS Biol. 2009;7:e13. doi: 10.1371/journal.pbio.1000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liao Y, Smyth GK, Shi W. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. 2019;47:e47. doi: 10.1093/nar/gkz114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wehrens R, Kruisselbrink J. Flexible self-organizing maps in kohonen 3.0. J. Stat. Softw. 2018;87:1–18. doi: 10.18637/jss.v087.i07. [DOI] [Google Scholar]
- 71.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Danecek P, et al. Twelve years of SAMtools and BCFtools. Gigascience. 2021;10:giab008. doi: 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herrmann H, et al. Delineation of target expression profiles in CD34+/CD38− and CD34+/CD38+ stem and progenitor cells in AML and CML. Blood Adv. 2020;4:5118–5132. doi: 10.1182/bloodadvances.2020001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Almeida BP, Reiter F, Pagani M, Stark A. DeepSTARR predicts enhancer activity from DNA sequence and enables the de novo design of synthetic enhancers. Nat. Genet. 2022;54:613–624. doi: 10.1038/s41588-022-01048-5. [DOI] [PubMed] [Google Scholar]
- 76.Bartha A, Gyorffy B. TNMplot.com: a web tool for the comparison of gene expression in normal, tumor and metastatic tissues. Int. J. Mol. Sci. 2021;22:2622. doi: 10.3390/ijms22052622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gyorffy B. Survival analysis across the entire transcriptome identifies biomarkers with the highest prognostic power in breast cancer. Comput. Struct. Biotechnol. J. 2021;19:4101–4109. doi: 10.1016/j.csbj.2021.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lanczky A, Gyorffy B. Web-based survival analysis tool tailored for medical research (KMplot): development and implementation. J. Med. Internet Res. 2021;23:e27633. doi: 10.2196/27633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nagy A, Munkacsy G, Gyorffy B. Pancancer survival analysis of cancer hallmark genes. Sci. Rep. 2021;11:6047. doi: 10.1038/s41598-021-84787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Q, Birkbak NJ, Gyorffy B, Szallasi Z, Eklund AC. Jetset: selecting the optimal microarray probe set to represent a gene. BMC Bioinf. 2011;12:474. doi: 10.1186/1471-2105-12-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barlogie B, et al. Total therapy 2 without thalidomide in comparison with total therapy 1: role of intensified induction and posttransplantation consolidation therapies. Blood. 2006;107:2633–2638. doi: 10.1182/blood-2005-10-4084. [DOI] [PubMed] [Google Scholar]
- 82.Pineda-Roman M, et al. VTD combination therapy with bortezomib-thalidomide-dexamethasone is highly effective in advanced and refractory multiple myeloma. Leukemia. 2008;22:1419–1427. doi: 10.1038/leu.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuiper R, et al. A gene expression signature for high-risk multiple myeloma. Leukemia. 2012;26:2406–2413. doi: 10.1038/leu.2012.127. [DOI] [PubMed] [Google Scholar]
- 84.Mulligan G, et al. Gene expression profiling and correlation with outcome in clinical trials of the proteasome inhibitor bortezomib. Blood. 2007;109:3177–3188. doi: 10.1182/blood-2006-09-044974. [DOI] [PubMed] [Google Scholar]
- 85.Ovejero S, et al. The BLM helicase is a new therapeutic target in multiple myeloma involved in replication stress survival and drug resistance. Front. Immunol. 2022;13:983181. doi: 10.3389/fimmu.2022.983181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chemlal D, et al. EZH2 targeting induces CD38 upregulation and response to anti-CD38 immunotherapies in multiple myeloma. Leukemia. 2023;37:1925–1928. doi: 10.1038/s41375-023-01983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kassambara A, Moreaux J. Analysis of global gene expression profiles. Methods Mol. Biol. 2018;1792:157–166. doi: 10.1007/978-1-4939-7865-6_11. [DOI] [PubMed] [Google Scholar]
- 88.Kassambara A, et al. GenomicScape: an easy-to-use web tool for gene expression data analysis. Application to investigate the molecular events in the differentiation of B cells into plasma cells. PLoS Comput. Biol. 2015;11:e1004077. doi: 10.1371/journal.pcbi.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alaterre E, et al. Comprehensive characterization of the epigenetic landscape in multiple myeloma. Theranostics. 2022;12:1715–1729. doi: 10.7150/thno.54453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains full descriptions for Supplementary Tables 1–6 and Supplementary Videos 1–2 (Tables and Videos supplied separately), and Supplementary Fig. 1, which contains the raw western blot data corresponding to Fig. 1b and Extended Data Fig. 1e.
Differential analyses and FPKMs of no ph-KD, transient ph-KD and constant ph-KD (fly line with conditional GFP expression).
Clustering of differentially expressed genes, PcG binding and recovery status.
Clustering of differentially accessible ATAC-Seq peaks.
Differential analyses and FPKMs of gfp-KD, zfh1-KD and Stat92E-KD transcriptomes in combination with w-KD (control) or ph-KD.
Differential analyses and FPKMs of no ph-KD, transient ph-KD and constant ph-KD (fly line with constitutive GFP expression).
Tissue area measurements and number of tissues analysed in immunofluorescence experiments.
EdU incorporation in a control early L3 ED.
EdU incorporation on a 24 h ph-KD in an early L3 ED, followed by 24 h of recovery.
Data Availability Statement
The NGS datasets generated in this study were made publicly available in the Gene Expression Omnibus (accession number GSE222193). A UCSC browser to visualize the data is available at http://genome-euro.ucsc.edu/s/cavalli/EpiCancer.
All custom scripts that were generated for this study were made publicly available at https://github.com/vloubiere/Parreno_Loubiere_2023.