Abstract
Although sevoflurane is generally considered safe, reports suggest that sevoflurane may cause postoperative liver injury more frequently than previously believed. Therefore, we aimed to compare the incidence of clinically significant postoperative liver injury following non-cardiac surgery between patients who underwent sevoflurane anesthesia and propofol-based total intravenous anesthesia. We retrospectively reviewed adult surgical patients from January 2010 to September 2022 who underwent general anesthesia in our center using sevoflurane or propofol over 3 h. After 1:1 propensity score matching, the incidence of postoperative liver injury was compared between the two groups. Out of 58,300 patients reviewed, 44,345 patients were included in the analysis. After propensity score matching, 7767 patients were included in each group. The incidence of postoperative liver injury was 1.4% in the sevoflurane group, which was similar to that in the propofol group (1.6%; p = 0.432). Comparison of the severity of postoperative alanine aminotransferase elevation showed that the incidence of borderline and mild elevation was higher in the sevoflurane group, but there was no difference in the incidence of moderate and severe elevation. In conclusion, sevoflurane anesthesia over 3 h was not associated with a higher incidence of clinically significant postoperative liver injury compared to propofol anesthesia.
Keywords: Inhalational anesthetics, Liver injury, Non-cardiac surgery, Propofol, Sevoflurane
Subject terms: Hepatitis, Liver diseases, Outcomes research
Introduction
Acute hepatic injury has long been considered a potential complication of general anesthesia and surgery1–3. Perioperative hypoxia, hypoperfusion, and hepatotoxic drugs are associated with postoperative liver injury (PLI), but the prevalence and etiology of PLI remains poorly understood. Although the impact of PLI on postoperative complications and prognosis remains unclear, multiple studies in general populations indicate that elevated serum alanine aminotransferase (ALT) levels are associated with increased morbidity and mortality4–6. Therefore, we believe it is important for anesthesiologists to understand the potential hepatotoxicity of the drugs they use, weigh the risks and benefits, and strive to avoid drug-induced PLI.
Halogenated inhalational anesthetics are among the most commonly used agents worldwide for maintenance of general anesthesia. Although generally considered to be safe and effective, they are known to be potentially hepatotoxic7,8. Halothane hepatitis, once a major drug-induced cause of acute liver failure, is believed to be caused by a hypersensitivity reaction to halothane metabolites9. Halothane is metabolized in the liver through cytochrome P450-mediated oxidative processes, resulting in the formation of metabolites that include trifluoroacetylate hepatic proteins10. These proteins have been identified as triggers for an autoimmune response that leads to PLI11. Modern inhalational anesthetics such as isoflurane, desflurane and especially sevoflurane undergo this metabolic process to a much lesser extent, and as a result, are less hepatotoxic10. Nevertheless, even with the use of these modern anesthetics, there have been documented cases of severe PLI following anesthesia, and some studies also suggest that inhalational anesthetics-induced PLI may be more common than was previously known11–18.
Hence, we conducted a large single-center retrospective study to determine whether using sevoflurane is a potential risk factor for PLI. The primary outcome was the incidence of PLI, defined as ALT levels elevated by more than 5 times the upper limit of the normal range (ULN) according to the American College of Gastroenterology guidelines, in patients who received either sevoflurane anesthesia or propofol-based total intravenous anesthesia (TIVA)19. We hypothesized that exposure to sevoflurane for more than 3 h would increase the incidence of PLI compared to propofol-based TIVA. In addition, we attempted to compare the severity of postoperative ALT elevation between the two groups.
Methods
This was a large, single-center retrospective study approved by the Institutional Review Board of Samsung Medical Center, Seoul, South Korea (IRB no. SMC 2022-08-083) and conducted in accordance with the Declaration of Helsinki. The electronic records of adult patients who underwent general anesthesia for more than 3 h using either sevoflurane or propofol between January 2010 and September 2022 at Samsung Medical Center were reviewed. Since the data was extracted from the Clinical Data Warehouse Darwin-C of Samsung Medical Center, a system in which all the personal information of the patients is removed before medical data extraction, individual informed consents were waived by the Institutional Review Board. Patients who underwent cardiac, transplantation, or hepato-biliary-pancreatic surgeries such as liver resection and pancreaticoduodenectomy were excluded. Those with preoperative ALT values of more than 2 times the ULN (normal range of serum ALT in our institution: male 0–41 U·l−1; female 0–33 U·l−1) or no postoperative ALT values, those with missing values for the covariates used in the propensity score matching, and those in whom both sevoflurane and propofol were used for the maintenance of general anesthesia were also excluded from the study. In addition, pregnant patients, and patients with a history of general anesthesia within 21 days before or after the surgery were excluded. The following baseline patient characteristics were collected: age, sex, pregnancy, body mass index (BMI), american society of anesthesiologists (ASA) physical status, the presence of underlying diseases (alcohol consumption, smoking, chronic hepatitis B or C, liver cirrhosis, diabetes mellitus, hypertension, dyslipidemia, cerebrovascular accident, chronic obstructive pulmonary disease, coronary artery disease, congestive heart failure), and preoperative laboratory results (serum ALT, albumin, hemoglobin, and creatinine). The duration of anesthesia, type of surgery, emergency surgery, intraoperative use of vasoactive drugs (intravenous ephedrine > 5 mg or phenylephrine > 100 mcg), intraoperative vasoactive inotropic scores (VIS), the number of intraoperative packed red blood cell (RBC) or fresh frozen plasma (FFP) transfusions, and the number of hypotension episodes during surgery defined as the incidence of mean arterial pressure < 65 mmHg recorded in our electronic vital sign sheets (recorded in 5 min intervals) were extracted. Data regarding the use of common hepatotoxic drugs that are frequently used in surgical patients were extracted separately for the preoperative (defined as within 24 h before surgery), intraoperative, and the postoperative periods: steroids, acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), antibiotics (amoxicillin/clavulanate, isoniazid, trimethoprim/sulfamethoxazole, fluoroquinolones, macrolides, nitrofurantoin, and minocycline) and antiepileptics (phenytoin, carbamazepine, lamotrigine, and valproate)2. The following postoperative data were collected: serum ALT values and use of inotropes/vasopressors (dopamine, dobutamine, milrinone, norepinephrine, epinephrine, vasopressin).
The primary outcome of the study was the difference in the incidence of PLI between patients who received sevoflurane and those who received propofol-based TIVA for the maintenance of general anesthesia. A patient was classified as having PLI if any postoperative serum ALT value checked within 21 days after surgery was higher than 5 times the ULN. Since the ULN in our institution is 41 U·l-1 for men and 33 U·l-1 for women, the cut-off value for PLI was above 205 U·l−1 and 165 U·l−1 respectively. The secondary outcome was the severity of postoperative ALT elevation, as defined by the American College of Gastroenterology guidelines (maximal postoperative ALT value classified into the following categories: normal ≤ ULN; borderline ≤ 2× ULN; mild ≤ 5× ULN; moderate ≤ 15× ULN; severe > 15× ULN19) between the two groups.
Continuous variables such as age, BMI, preoperative laboratory results (serum ALT, albumin, hemoglobin, creatinine), duration of anesthesia, VIS, the number of intraoperative RBC and FFP transfusions, and the duration of hypotension episodes were presented as median [interquartile range] and compared using the Wilcoxon rank-sum test. Categorical variables such as sex, ASA physical status, emergency surgery, underlying diseases (alcohol consumption, smoking, chronic hepatitis B or C, liver cirrhosis, diabetes mellitus, hypertension, dyslipidemia, cerebrovascular accident, chronic obstructive pulmonary disease, coronary artery disease, congestive heart failure), intraoperative use of vasoactive agents, preoperative and postoperative medications (steroids, acetaminophen, NSAIDs, antibiotics, antiepileptics), intraoperative medications (steroids, acetaminophen, NSAIDs), postoperative use of inotropes/vasopressors, and surgical risk (classified based on the European Society of Anaesthesiology guidelines on non-cardiac surgery) were described as numbers (%) and compared using the χ2 test or Fisher’s exact test20.
To reduce bias due to confounding variables, we used propensity score matching between the sevoflurane and the propofol group. The propensity score for receiving sevoflurane (the exposure of interest) was estimated using a multivariable logistic regression model. All variables in Tables 1and 2 were used for matching, with sex as an exact matching variable since the ALT cutoff value for the diagnosis of PLI is different between males and females. A 1:1 matching with a caliper of 0.025 using the nearest neighbor method was applied. Covariate balance was assessed using standardized mean difference, with a value under 0.1 considered acceptable. After matching, the primary outcome was assessed using univariable logistic regression. The severity of postoperative ALT elevation between the two groups was compared using multinomial logistic regression. All analyses were performed using R 3.6.1 (R Development Core Team, Vienna, Austria) or SPSS (version 27, Chicago, IL, USA). A two-sided alpha of 0.05 was used for all statistical tests.
Table 1.
Baseline characteristics in the sevoflurane and propofol groups. Values are expressed as mean (standard deviation), median [inter-quartile range], or number (%).
| Variables | Overall patients | Matched patients | ||||||
|---|---|---|---|---|---|---|---|---|
| Sevoflurane (n = 31,168) | Propofol (n = 13,177) | p-value | SMD | Sevoflurane (n = 7767) | Propofol (n = 7767) | p-value | SMD | |
| Age (year) | 59.1 (13.7) | 55.2 (14.0) | < 0.001 | 0.28 | 56.9 (14.7) | 56.7 (14.0) | 0.318 | 0.074 |
| Sex, male | 19,810 (63.6) | 5684 (43.1) | < 0.001 | 0.418 | 3865 (49.8) | 3865 (49.8) | > 0.999 | < 0.001 |
| BMI (kg/m2) | 24.3 (3.5) | 24.5 (3.7) | 0.001 | 0.048 | 24.5 (3.7) | 24.5 (3.6) | 0.988 | < 0.001 |
| ASA physical status | < 0.001 | 0.081 | 0.518 | 0.029 | ||||
| 1 | 7093 (22.8) | 3163 (24.0) | 1803 (23.2) | 1836 (23.6) | ||||
| 2 | 20,137 (64.6) | 8654 (65.7) | 5000 (64.4) | 5035 (64.8) | ||||
| 3 | 3729 (12.0) | 1255 (9.5) | 885 (11.4) | 829 (10.7) | ||||
| 4 | 195 (0.6) | 97 (0.7) | 74 (1.0) | 63 (0.8) | ||||
| 5 | 14 (0.0) | 8 (0.1) | 5 (0.1) | 4 (0.1) | ||||
| Alcohol | 6429 (20.6) | 2787 (21.2) | 0.219 | 0.013 | 1616 (20.8) | 1675 (21.6) | 0.255 | 0.019 |
| Smoking | 2709 (8.7) | 1214 (9.2) | 0.08 | 0.018 | 711 (9.2) | 728 (9.4) | 0.42 | 0.008 |
| Chronic hepatitis B | 1006 (3.2) | 486 (3.67) | 0.015 | 0.025 | 284 (3.7) | 285 (3.7) | > 0.999 | 0.001 |
| Chronic hepatitis C | 350 (1.1) | 150 (1.1) | 0.927 | 0.001 | 99 (1.3) | 94 (1.2) | 0.772 | 0.006 |
| Liver cirrhosis | 426 (1.4) | 91 (0.7) | < 0.001 | 0.067 | 80 (1.0) | 70 (0.9) | 0.46 | 0.013 |
| Diabetes mellitus | 4472 (14.3) | 1549 (11.8) | < 0.001 | 0.077 | 1023 (13.2) | 1002 (12.9) | 0.634 | 0.008 |
| Hypertension | 9583 (30.7) | 3747 (28.4) | < 0.001 | 0.05 | 2446 (31.5) | 2333 (30.0) | 0.052 | 0.032 |
| Dyslipidemia | 1717 (5.5) | 989 (7.5) | < 0.001 | 0.081 | 560 (7.2) | 537 (6.9) | 0.491 | 0.012 |
| CVA | 713 (2.3) | 374 (2.8) | < 0.001 | 0.035 | 225 (2.9) | 219 (2.8) | 0.732 | 0.005 |
| COPD | 975 (3.1) | 181 (1.4) | < 0.001 | 0.118 | 147 (1.9) | 160 (2.1) | 0.489 | 0.012 |
| CAD | 1057 (3.4) | 285 (2.2) | < 0.001 | 0.075 | 218 (2.8) | 210 (2.7) | 0.732 | 0.006 |
| CHF | 97 (0.3) | 28 (0.2) | 0.09 | 0.019 | 27 (0.3) | 20 (0.3) | 0.381 | 0.016 |
| Preoperative | ||||||||
| Creatinine (mg/dL) | 0.86 (0.42) | 0.78 (0.35) | < 0.001 | 0.164 | 0.82 (0.32) | 0.82 (0.42) | > 0.99 | 0.006 |
| ALT (U/L) | 18 [13–25] | 18 [13–26] | < 0.001 | 0.048 | 18 [13–25] | 18 [13–26] | 0.456 | 0.014 |
| Albumin (g/dL) | 4.4 [4.2–4.6] | 4.4 [4.2–4.6] | 0.348 | 0.023 | 4.4 [4.2–4.6] | 4.4 [4.2–4.6] | 0.283 | 0.019 |
| Hemoglobin (g/dL) | 13.3 [12.3–14.4] | 13.5 [12.2–14.7] | < 0.001 | 0.055 | 13.3 [12.2–14.5] | 13.4 [12.3–14.5] | 0.201 | 0.011 |
| Steroid use | 1927 (6.2) | 4687 (35.6) | < 0.001 | 0.776 | 1496 (19.3) | 1570 (20.2) | 0.141 | 0.024 |
| Acetaminophen use | 1247 (4.0) | 960 (7.3) | < 0.001 | 0.143 | 517 (6.7) | 515 (6.6) | 0.974 | 0.001 |
| NSAIDs use | 962 (3.1) | 698 (5.3) | < 0.001 | 0.11 | 352 (4.5) | 366 (4.7) | 0.619 | 0.009 |
| Antibiotics use | 355 (1.1) | 252 (1.9) | < 0.001 | 0.063 | 119 (1.5) | 119 (1.5) | > 0.99 | < 0.001 |
| Antiepileptics use | 185 (0.6) | 709 (5.4) | < 0.001 | 0.284 | 148 (1.9) | 164 (2.1) | 0.391 | 0.015 |
ASA american society of anesthesiologists, ALT alanine aminotransferase, BMI body mass index, CAD coronary artery disease, COPD chronic obstructive pulmonary disease, CVA cerebrovascular accident, NSAID nonsteroidal anti-inflammatory drug, SMD standardized mean difference.
Table 2.
Intraoperative and postoperative variables in the sevoflurane and propofol groups.
| Variables | Overall patients | Matched patients | ||||||
|---|---|---|---|---|---|---|---|---|
| Sevoflurane (n = 31,168) | Propofol (n = 13,177) | p-value | SMD | Sevoflurane (n = 7767) | Propofol (n = 7767) | p-value | SMD | |
| Emergency surgery | 1443 (4.6) | 841 (6.4) | < 0.001 | 0.077 | 499 (6.4) | 483 (6.2) | 0.621 | 0.008 |
| Surgical risk | < 0.001 | 0.449 | 0.014 | 0.047 | ||||
| 1 | 4073 (13.1) | 865 (6.6) | 778 (10.0) | 696 (9.0) | ||||
| 2 | 24,687 (79.2) | 12,246 (92.9) | 6901 (88.9) | 7005 (90.2) | ||||
| 3 | 2408 (7.7) | 66 (0.5) | 88 (1.1) | 66 (0.9) | ||||
| Duration of anesthesia (h) | 4.5 (1.6) | 5.3 (1.8) | < 0.001 | 0.431 | 4.36 (0.44) | 4.36 (0.40) | 0.431 | 0.015 |
| Vasoactive agent use | 15,734 (48.7) | 4,638 (33.7) | < 0.001 | 0.037 | 3114 (40.1) | 3011 (38.8) | 0.139 | 0.027 |
| VIS | 0.29 (4.03) | 0.14 (1.38) | 0.016 | 0.306 | 0.19 (1.61) | 0.17 (1.54) | 0.568 | 0.014 |
| Intraoperative | ||||||||
| RBC (unit) | 0.21 (3.69) | 0.39 (3.19) | < 0.001 | 0.052 | 0.35 (4.89) | 0.36 (3.86) | 0.863 | 0.003 |
| FFP (unit) | 0.02 (0.32) | 0.07 (0.70) | < 0.001 | 0.076 | 0.05 (0.46) | 0.05 (0.55) | 0.568 | 0.009 |
| Hypotension (min) | 5 [0–20] | 5 [0–20] | 0.988 | 0.004 | 5 [0–25] | 5 [0–20] | 0.185 | 0.023 |
| Steroid use | 2002 (6.4) | 832 (6.3) | 0.683 | 0.004 | 554 (7.1) | 511 (6.6) | 0.182 | 0.022 |
| Acetaminophen use | 5624 (18.0) | 929 (7.1) | < 0.001 | 0.337 | 842 (10.8) | 823 (10.6) | 0.641 | 0.008 |
| NSAIDs use | 1907 (6.1) | 261 (2.0) | < 0.001 | 0.211 | 227 (2.9) | 218 (2.8) | 0.7 | 0.007 |
| Postoperative | ||||||||
| Steroid use | 3397 (10.9) | 7868 (59.7) | < 0.001 | 1.188 | 2843 (36.6) | 2803 (36.1) | 0.294 | 0.017 |
| Acetaminophen use | 16,854 (54.1) | 10,003 (75.9) | < 0.001 | 0.47 | 5683 (73.2) | 5600 (72.1) | 0.14 | 0.024 |
| NSAIDs use | 9906 (31.8) | 7965 (60.4) | < 0.001 | 0.6 | 3633 (46.8) | 3647 (47.0) | 0.834 | 0.004 |
| Antibiotics use | 1908 (6.1) | 1544 (11.7) | < 0.001 | 0.185 | 658 (8.5) | 642 (8.3) | 0.664 | 0.007 |
| Antiepileptics use | 260 (0.8) | 785 (6.0) | < 0.001 | 0.286 | 187 (2.4) | 199 (2.6) | .0.871 | 0.01 |
| Inotropic/vasopressor | 556 (1.8) | 190 (1.4) | 0.012 | 0.027 | 120 (1.5) | 114 (1.5) | 0.742 | 0.006 |
FFP fresh frozen plasma, RBC red blood cell, NSAID nonsteroidal anti-inflammatory drug, SMD standardized mean difference, VIS vasoactive inotropic score.
Values are expressed as mean (standard deviation), median [inter-quartile range], or number (%). VIS = dopamine dose (mcg/kg/min) + dobutamine dose (mcg/kg/min) + 100∙epinephrine dose (mcg/kg/min) + 10∙milrinone dose (mcg/kg/min) + 10,000∙vasopressin dose (unit/kg/min) + 100∙norepinephrine dose (mcg/kg/min).
Results
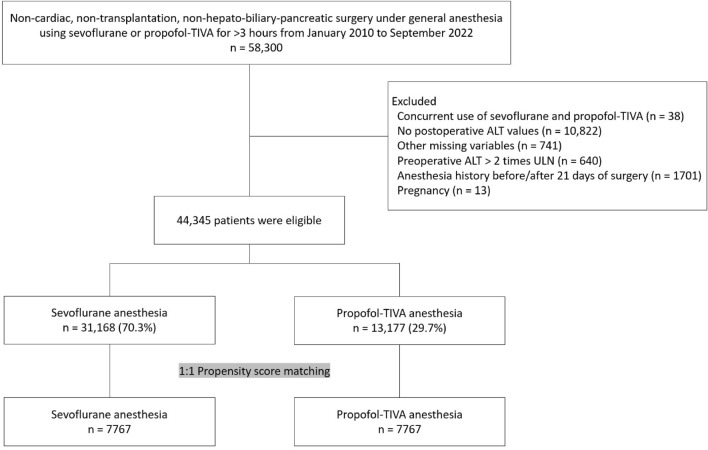
A total of 58,300 patients underwent non-cardiac, non-transplantation, non-hepato-biliary-pancreatic surgery under general anesthesia using sevoflurane or propofol-based TIVA for more than 3 h between January 2010 to September 2022. After excluding pregnant patients, patients who underwent general anesthesia within 21 days before or after the surgery, those who received both sevoflurane and propofol-based TIVA for maintenance of anesthesia, those with missing data, and those with preoperative ALT values higher than 2 times the ULN, 44,345 patients were eligible for the study (Fig. 1). Of these, 31,168 (70.3%) received sevoflurane while 13,177 (29.7%) received propofol for maintenance of general anesthesia. After 1:1 propensity score matching with sex as an exact matching variable, 7767 patients were included in each group. The baseline characteristics and intra/postoperative variables of the overall and matched patients are as shown in Tables 1, 2. Discrepancies of the variables between the two groups were well balanced after matching, with standardized mean differences smaller than 0.1.
Figure 1.
Flowchart for study population selection. ALT alanine aminotransferase, TIVA total intravenous anesthesia, ULN upper limit of normal range.
Out of all the patients, 739 (1.7%) developed PLI. Among these, 501 (1.6%) were in the sevoflurane group and 238 (1.8%) were in the propofol group (p = 0.138). After 1:1 propensity score matching, the incidence of PLI was 1.4% (112/7767) in the sevoflurane group, which was similar to the incidence in the propofol group (1.6%, 124/7767) (p = 0.432) (Table 3).
Table 3.
Postoperative liver injury and the characteristics of postoperative serum alanine aminotransferase elevation in the sevoflurane and propofol groups. Values are expressed as median [inter-quartile range] or number (%).
| Overall patients | Matched patients | |||||
|---|---|---|---|---|---|---|
| Sevoflurane (n = 31,168) | Propofol (n = 13,177) | p-value | Sevoflurane (n = 7767) | Propofol (n = 7767) | p-value | |
| Postoperative liver injury (> 5X ULN) | 501 (1.6) | 238 (1.8) | 0.138 | 112 (1.4) | 124 (1.6) | 0.432 |
| Severity of postoperative ALT elevation | ||||||
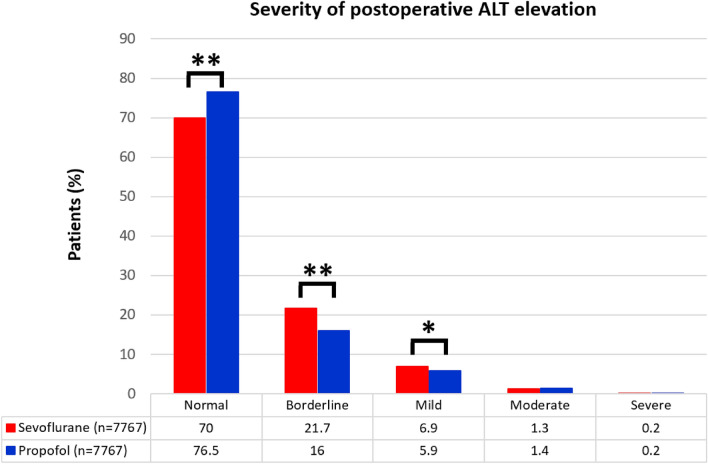
| Normal | 21,468 (68.9) | 9639 (73.2) | < 0.001 | 5436 (70.0) | 5941 (76.5) | < 0.001 |
| Borderline elevation (≤ 2X ULN) | 6794 (21.8) | 2427 (18.4) | < 0.001 | 1687 (21.7) | 1246 (16.0) | < 0.001 |
| Mild elevation (≤ 5X ULN) | 2405 (7.7) | 873 (6.6) | < 0.001 | 532 (6.9) | 456 (5.9) | 0.014 |
| Moderate elevation (≤ 15X ULN) | 415 (1.3) | 212 (1.6) | 0.026 | 97 (1.3) | 109 (1.4) | 0.44 |
| Severe elevation (> 15X ULN) | 86 (0.3) | 26 (0.2) | 0.124 | 15 (0.2) | 15 (0.2) | > 0.99 |
| Time to maximal ALT value (days) | 2 [0–7] | 4 [1–7] | < 0.001 | 3 [1–7] | 3 [0–7] | 0.027 |
ALT alanine aminotransferase, ULN upper limit of the normal range.
Comparison of the severity of postoperative ALT elevation between the two groups revealed that using sevoflurane was associated with a higher risk of developing borderline or mild elevation than using propofol (OR 1.480; 95% CI 1.363–1.606; p < 0.001 and OR 1.275; 95% CI 1.119–1.453; p < 0.001, respectively). However, there were no significant differences between the two groups in terms of patients with moderate or severe elevations (OR 0.973; 95% CI 0.738–1.282; p = 0.844 and OR1.093; 95% CI 0.534–2.238; p = 0.808, respectively) (Table 3 , Fig. 2).
Figure 2.
Comparison of the severity of postoperative ALT elevation between the sevoflurane and the propofol group. ALT alanine aminotransferase. *p < 0.05; **p < 0.001.
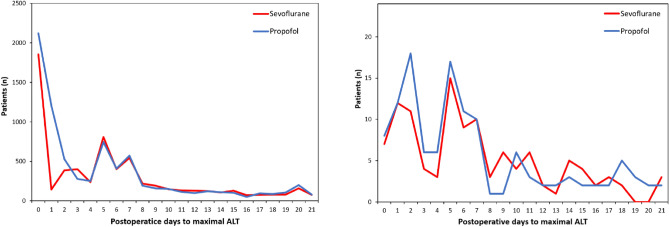
In the matched cohort, the maximal postoperative ALT values were primarily observed within POD 7. For patients who developed PLI, the maximal ALT values were distributed with a double peak of similar size at POD 0 to 2 and POD 5 to 7 (Fig. 3).
Figure 3.
The time to maximal ALT value within postoperative 21 days in the propensity score-matched cohort (left) and in patients with PLI (right). ALT alanine aminotransferase, PLI postoperative liver injury.
Discussion
In this study, using sevoflurane for maintenance of general anesthesia was not associated with PLI when compared with using propofol. Although an increased risk of borderline and mild elevation (1× to 5× ULN) of postoperative ALT values was observed in the sevoflurane group, the incidence of moderate and severe ALT elevation (> 5× ULN) did not differ significantly between the two groups. These findings suggest that the use of sevoflurane is unlikely to exhibit clinically significant hepatotoxic properties.
There are various factors that could contribute to PLI. Hypoperfusion of the liver is one of the common causes of postoperative ALT elevation21. Also, certain classes of antibiotics, antiepileptics and NSAIDs are well-known for their hepatotoxic properties2,3. Therefore, we included them as variables in our propensity score matching process, thereby ensuring balance between the sevoflurane and propofol groups.
A study by Oh et al. demonstrated that the use of sevoflurane for maintenance of anesthesia resulted in higher postoperative liver enzyme values compared to the use of propofol in patients with elevated preoperative serum liver enzymes15. However, in the study, some important factors that may cause PLI such as the use of hepatotoxic drugs in the postoperative period were not considered. Also, ALT values only within 72 h after surgery were collected. In our study, ALT values up to 21 days postoperatively were collected since PLI due to sevoflurane usually results in ALT elevation within 2 to 21 days after surgery11,22.
Two small studies investigated the incidence of PLI in trauma and surgical patients after the administration of modern inhalational anesthetics12,16. To identify possible inhalational anesthetics-induced PLI, these studies used the Council for International Organizations of Medical Sciences / Roussel Uclaf Causality Assessment Method (CIOMS/RUCAM) scoring system, which has been validated as a method of determining the likelihood of a drug as the causative agent of PLI. The studies reported that the incidence of PLI possibly caused by inhalational anesthetics was between 3 and 4.1%, which is somewhat higher than the results of our study. Although the CIOMS/RUCAM score was used to rule out inhalational anesthetics-irrelevant cases of PLI, it may have been insufficient to completely exclude them. Also, in these studies, the authors used a rather broad CIOMS/RUCAM score range for the diagnosis of possible inhalational anesthetics-induced PLI. Therefore, true inhalational anesthetics-induced PLI cases may be less than what these two studies have reported.
We considered it reasonable to compare sevoflurane with propofol to assess the potential hepatotoxicity of sevoflurane. Although propofol infusion syndrome, a potentially fatal complication that may occur after prolonged administration of high doses (typically exceeding 48 h at > 5 mg·kg−1·hr −1), is a known cause of acute liver injury, propofol is generally considered safe in terms of hepatotoxicity when administered in conventional doses during surgery23,24. Moreover, research has demonstrated that patients with pre-existing liver diseases can safely receive TIVA with propofol25.
We used serum ALT values for the diagnosis of PLI because inhalational anesthetics-induced PLI is known to be mainly hepatocellular, which is characterized by a rise in serum ALT levels11,22,26. The ALT value threshold for PLI in this study was determined based on the definitions of ALT elevation in the American College of Gastroenterology guidelines. According to the guidelines, ALT values higher than 5 times the ULN are advised to undergo immediate evaluation of the liver2,19.
For patients who developed PLI, the time point of maximal postoperative ALT values showed a double-peak distribution in both groups. Since PLI due to hepatic ischemia typically results in an immediate increase in serum ALT value that peaks within 48 h, the first peak is likely to be mainly due to liver hypoperfusion during or immediately after surgery21. In contrast, the second peak may have been caused by the hepatotoxicity of intraoperative or postoperative drugs including anesthetics. The majority of maximal ALT values were observed within 7 days after surgery, and therefore patients with a higher risk of developing PLI may benefit from closer monitoring during this period.
This study has several limitations. First, because of its retrospective design, the data may have been biased or inaccurate. We attempted to minimize these risks by using large data and performing propensity score matching with a small caliper. However, since liver enzyme elevation can be caused by various factors, there may have been hidden factors missed out in the propensity scoring. Second, we categorized all surgical procedures as low, intermediate, or high surgical risk, but a more detailed categorization may have reduced bias. Although we excluded surgeries that can directly injure the liver, it is still possible that procedures involving manipulation of the liver or alteration of hepatic blood flow were included. These procedures could potentially act as confounding variables when assessing anesthesia-related PLI. Third, since preoperative serum ALT value was defined as the most recent value within 6 months before surgery, some patients may have had undiagnosed liver injury that developed during the time interval between the last serum ALT test and surgery. Finally, postoperative ALT values were checked not daily, but depending on the physician’s decision. Therefore, it is necessary to consider this aspect when interpreting the postoperative trends of ALT changes. However, since tracking ALT values daily for an extended period after surgery is impractical in real clinical practice, we believe that the results of our analysis may provide useful information for clinicians to reference.
In conclusion, our study suggests that sevoflurane anesthesia over 3 h has minimal hepatotoxic properties comparable to propofol-based TIVA. Prospective studies are needed to confirm our findings, and further research is necessary to determine long-term prognosis.
Author contributions
D.K.R. and M.P. contributed equally as co-first authors in data acquisition and interpretation, statistics, and manuscript drafting. S.W. helped in data acquisition and figure generation. H.S.C. and J.M. contributed to the initial conceptualization of the study, data interpretation, and revision of the manuscript. All authors have approved the final version of the manuscript.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Dae Kyun Ryu and MiHye Park.
References
- 1.Little Jr DM, Wetstone HJ. Anesthesia and the liver. Anesthesiology. 1964;25:815–853. doi: 10.1097/00000542-196411000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani NP, et al. ACG clinical guideline: Diagnosis and management of idiosyncratic drug-induced liver injury. Am. J. Gastroenterol. 2021;116:878–898. doi: 10.14309/ajg.0000000000001259. [DOI] [PubMed] [Google Scholar]
- 3.Hoofnagle JH, Bjornsson ES. Drug-induced liver injury-types and phenotypes. N. Engl. J. Med. 2019;381:264–273. doi: 10.1056/NEJMra1816149. [DOI] [PubMed] [Google Scholar]
- 4.Kim HC, et al. Normal serum aminotransferase concentration and risk of mortality from liver diseases: Prospective cohort study. BMJ. 2004;328:983. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee TH, Kim WR, Benson JT, Therneau TM, Melton LJ., 3rd Serum aminotransferase activity and mortality risk in a United States community. Hepatology. 2008;47:880–887. doi: 10.1002/hep.22090. [DOI] [PubMed] [Google Scholar]
- 6.Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology. 2009;136(2):477–485 e411. doi: 10.1053/j.gastro.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 7.Bunker JP. Final report of the national halothane study. Anesthesiology. 1968;29:231–232. doi: 10.1097/00000542-196803000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Safari S, Motavaf M, Seyed Siamdoust SA, Alavian SM. Hepatotoxicity of halogenated inhalational anesthetics. Iran. Red Crescent Med. J. 2014;16:e20153. doi: 10.5812/ircmj.20153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ray DC, Drummond GB. Halothane hepatitis. Br. J. Anaesth. 1991;67:84–99. doi: 10.1093/bja/67.1.84. [DOI] [PubMed] [Google Scholar]
- 10.Njoku D, et al. Biotransformation of halothane, enflurane, isoflurane, and desflurane to trifluoroacetylated liver proteins: Association between protein acylation and hepatic injury. Anesth. Analg. 1997;84:173–178. doi: 10.1097/00000539-199701000-00031. [DOI] [PubMed] [Google Scholar]
- 11.Martin JL. Volatile anesthetics and liver injury: A clinical update or what every anesthesiologist should know. Can. J. Anaesth. 2005;52:125–129. doi: 10.1007/BF03027715. [DOI] [PubMed] [Google Scholar]
- 12.Bishop B, et al. A prospective study of the incidence of drug-induced liver injury by the modern volatile anaesthetics sevoflurane and desflurane. Aliment. Pharmacol. Ther. 2019;49:940–951. doi: 10.1111/apt.15168. [DOI] [PubMed] [Google Scholar]
- 13.Singhal S, Gray T, Guzman G, Verma A, Anand K. Sevoflurane hepatotoxicity: A case report of sevoflurane hepatic necrosis and review of the literature. Am. J. Ther. 2010;17:219–222. doi: 10.1097/MJT.0b013e318197eacb. [DOI] [PubMed] [Google Scholar]
- 14.Turillazzi E, D'Errico S, Neri M, Riezzo I, Fineschi V. A fatal case of fulminant hepatic necrosis following sevoflurane anesthesia. Toxicol. Pathol. 2007;35:840–845. doi: 10.1080/01926230701584148. [DOI] [PubMed] [Google Scholar]
- 15.Oh SK, Lim BG, Kim YS, Kim SS. Comparison of the postoperative liver function between total intravenous anesthesia and inhalation anesthesia in patients with preoperatively elevated liver transaminase levels: A retrospective cohort study. Ther. Clin. Risk Manag. 2020;16:223–232. doi: 10.2147/TCRM.S248441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin J, et al. Drug-induced hepatotoxicity: Incidence of abnormal liver function tests consistent with volatile anaesthetic hepatitis in trauma patients. Liver Int. 2014;34:576–582. doi: 10.1111/liv.12278. [DOI] [PubMed] [Google Scholar]
- 17.Martin JL, et al. Fatal hepatitis associated with isoflurane exposure and CYP2A6 autoantibodies. Anesthesiology. 2001;95:551–553. doi: 10.1097/00000542-200108000-00043. [DOI] [PubMed] [Google Scholar]
- 18.Berghaus TM, Baron A, Geier A, Lamerz R, Paumgartner G. Hepatotoxicity following desflurane anesthesia. Hepatology. 1999;29:613–614. doi: 10.1002/hep.510290211. [DOI] [PubMed] [Google Scholar]
- 19.Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: Evaluation of abnormal liver chemistries. Am. J. Gastroenterol. 2017;112:18–35. doi: 10.1038/ajg.2016.517. [DOI] [PubMed] [Google Scholar]
- 20.Kristensen SD, et al. 2014 ESC/ESA guidelines on non-cardiac surgery: Cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: Cardiovascular assessment and management of the European society of cardiology (ESC) and the European society of anaesthesiology (ESA) Eur. Heart J. 2014;35:2383–2431. doi: 10.1093/eurheartj/ehu282. [DOI] [PubMed] [Google Scholar]
- 21.Henrion J. Hypoxic hepatitis. Liver Int. 2012;32:1039–1052. doi: 10.1111/j.1478-3231.2011.02655.x. [DOI] [PubMed] [Google Scholar]
- 22.LiverTox: Clinical and Research Information on Drug-Induced Liver injury: National Institute of Diabetes and Digestive and Kidney Diseases. Sevoflurane. Updated January 1, 2018. https://www.ncbi.nlm.nih.gov/books/NBK548737/
- 23.LiverTox: Clinical and Research Information On Drug-Induced Liver Injury: National Institute of Diabetes and Digestive and Kidney Diseases. Propofol. Updated July 10, 2020. https://www.ncbi.nlm.nih.gov/books/NBK547909/
- 24.Hemphill S, McMenamin L, Bellamy MC, Hopkins PM. Propofol infusion syndrome: A structured literature review and analysis of published case reports. Br. J. Anaesth. 2019;122:448–459. doi: 10.1016/j.bja.2018.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Servin F, et al. Pharmacokinetics and protein binding of propofol in patients with cirrhosis. Anesthesiology. 1988;69:887–891. doi: 10.1097/00000542-198812000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Aithal GP, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin. Pharmacol. Ther. 2011;89:806–815. doi: 10.1038/clpt.2011.58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.