Abstract
Meningiomas are the most common primary intracranial tumors and can be associated with significant morbidity and mortality. Radiologists, neurosurgeons, neuro-oncologists, and radiation oncologists rely on brain MRI for diagnosis, treatment planning, and longitudinal treatment monitoring. However, automated, objective, and quantitative tools for non-invasive assessment of meningiomas on multi-sequence MR images are not available. Here we present the BraTS Pre-operative Meningioma Dataset, as the largest multi-institutional expert annotated multilabel meningioma multi-sequence MR image dataset to date. This dataset includes 1,141 multi-sequence MR images from six sites, each with four structural MRI sequences (T2-, T2/FLAIR-, pre-contrast T1-, and post-contrast T1-weighted) accompanied by expert manually refined segmentations of three distinct meningioma sub-compartments: enhancing tumor, non-enhancing tumor, and surrounding non-enhancing T2/FLAIR hyperintensity. Basic demographic data are provided including age at time of initial imaging, sex, and CNS WHO grade. The goal of releasing this dataset is to facilitate the development of automated computational methods for meningioma segmentation and expedite their incorporation into clinical practice, ultimately targeting improvement in the care of meningioma patients.
Subject terms: Translational research, CNS cancer, Brain, Databases, Brain imaging
Background & Summary
Meningiomas are the most common primary intracranial tumor in adults and can result in significant morbidity and mortality for affected patients1,2. Most meningiomas (∼80%) are fifth edition CNS World Health Organization (WHO) grade 1 benign tumors and are typically well controlled with observation, surgical resection, and/or radiation therapy3,4. However, higher grade meningiomas (CNS WHO grades 2 and 3) are associated with significantly higher morbidity and mortality rates and often recur despite optimal management3,5. Currently there is no reliable noninvasive method for identifying meningioma CNS WHO grade, assessing aggressiveness, or predicting recurrence and survival. Traditional MRI features used by clinicians to guide treatment strategy, such as meningioma size, or degree of surrounding edema, may not represent CNS WHO grade or expected clinical course2. As such, there is a need for improved radiographic assessment of meningiomas, which can help guide patient-specific treatment strategies.
Automated tumor segmentation on brain magnetic resonance imaging (MRI) has matured into a clinically viable tool that can provide objective assessments of tumor volume and can assist in surgical planning, radiotherapy planning, and treatment response assessment. However, to date, most brain tumor segmentation studies have focused exclusively on gliomas, despite the fact that meningiomas are more common, accounting for over a third of all intracranial tumors6–8. Meningiomas, while typically more circumscribed than gliomas, provide additional technical challenges for segmentation given their extra-axial location and propensity for skull-base involvement2. In addition, unlike other intracranial tumors, meningiomas are commonly diagnosed by imaging alone, which increases the importance of MRI for treatment planning.
The BraTS organization has conducted large scale international automated segmentation challenges focused on gliomas since 20127,8. The initial 2012 BraTS glioma dataset consisted of 35 training and 15 testing cases. Each case consisted of co-registered multi-sequence pre- and postcontrast MR images with associated manually annotated tumor sub-compartment labels7. Recently, the 2021 BraTS glioma challenge described a multi-society effort across RSNA, ASNR, and MICCAI, that resulted in a dataset of >2,000 cases split across 1251 training cases, 219 validation cases, and 570 testing cases9. Due to the successful historic efforts focused on automated segmentation of glioma7,8, in 2023, the BraTS organization decided to compliment the adult glioma segmentation with a cluster of challenges. This cluster includes a dedicated meningioma segmentation challenge, the “Brain Tumor Segmentation Challenge 2023: Intracranial Meningioma”10.
Here we present the BraTS Pre-operative Meningioma Dataset, which is the largest known publicly available multi-institutional dataset of meningioma multi-sequence MR images to date11,12. The BraTS Pre-operative Meningioma Dataset includes 1,141 publicly available, pre-processed, multi-sequence MR images from six different academic medical centers with manually annotated sub-compartment tumor labels, basic demographic data, and CNS WHO grade when available. The purpose of this dataset is to facilitate the development of automated multi-compartment brain MRI segmentation algorithms for intracranial meningiomas. Segmentation algorithms developed using these data will allow objective assessment of tumor volume for surgical and radiotherapy planning and will serve as a starting point for future studies focused on identifying meningioma CNS WHO grade, assessing aggressiveness, and predicting risk of recurrence based on MRI findings alone. This manuscript describes the data collection, curation, and segmentation process for the BraTS Pre-operative Meningioma Dataset.
Methods
Study population
The study population consisted of adult patients diagnosed with intracranial meningioma of any CNS WHO grade or subtype either by imaging or histopathology following resection or biopsy4. Participants were retrospectively identified from six different academic medical centers: Duke University, Yale University, Thomas Jefferson University, University of California San Francisco, University of Missouri, and University of Pennsylvania. The specific case inclusion methods (pathologic, clinical/radiologic, or both) and case collection methods (i.e., random, consecutive) were chosen by each participating site independently, often on the basis of pre-existing curated datasets. Strict requirements on participant inclusion were not imposed to reduce barriers to data sharing. All participating sites had institutional review board (IRB) approval. A waiver for informed consent was provided by each institution’s respective IRB. All image data were anonymized, and faces were digitally removed to prevent facial reconstruction.
Imaging data
Imaging data included pre-operative and pre-treatment multi-sequence MR images of the brain with corresponding expert annotated tumor sub-compartments. Multi-sequence MR images included pre-contrast T1-weighted, post-contrast T1-weighted, T2-weighted, and T2/FLAIR-weighted imaging. Exclusion criteria included lack of visible tumor on the skull-stripped MRI or the presence of any intracranial tumor that was not radiographically or pathologically consistent with meningioma (including cases of neurofibromatosis type 2 with intracranial schwannomas). Imaging parameters including field strength, echo/repetition time, slice resolution, and slice thickness varied considerably between and within sites and documentation of these variables were not required for data contribution, with the intention of reducing barriers to data sharing. The naming convention used for the anonymized case IDs was “BraTS-MEN-00XXX-00N”. In this format, “XXX” represents a unique identifier for each patient, and “00 N” indicates the interval case number of the respective pre-operative study for that particular patient. For example, if a patient underwent three pre-operative MRI studies that were included in the dataset, these would be labeled as “−001” for the first chronological case, “−002” for the second case, and “−003” for the third case, respectively, following the unique patient identifier “00XXX”. This system ensures that each case is distinctly identified, not just by the patient to whom it belongs but also by the order in which the studies were performed.
Data splits
A total of 1,424 individual MRI exams from 1,344 different patients were included in the final BraTS Pre-operative Meningioma Dataset. These were divided into a training set (1,000/1,424, 70%), a validation set (141/1,424, 10%), and a private hold-out testing set (283/1,424, 20%). Splits were random but stratified by site and by patient such that interval multi-sequence MR images from each individual patient, as denoted by “00 N”, were assigned to a single data split. Training and validation data are being made publicly available, as part of this manuscript, while the testing data is private to allow unbiased ongoing evaluation of new segmentation methods through the synapse.org platform13.
Clinical data
Clinical-pathologic information including patient age at the time of imaging, sex, and CNS WHO grade if available, were obtained from the respective electronic medical records at each institution. The age range was 14–96 years (including private testing set data) and 14–96 years in the publicly available data. The male to female ratio was 398:1,007 (including private testing set data) and 313:816 in the publicly available data. CNS WHO grade was available in 1,010 of the 1,424 total cases (including private testing set data) and in 800 of the 1,141 publicly available cases. Aggregate clinical-pathologic case-level data are provided in Table 1 (including private testing set data). Individual case-level data for the publicly available training and validation cases are freely provided on the Synapse data repository14.
Table 1.
Basic clinical and demographic data for the BraTS Pre-operative Meningioma Dataset cohort including the private test set data for cases with available patient demographic data.
| Total | Training Set | Validation Set | Testing Set | Age (Median; Min-Max) | Male: Female | CNS WHO Grade 1 | CNS WHO Grade 2 | CNS WHO Grade 3 | |
|---|---|---|---|---|---|---|---|---|---|
| All Sites | 1,424 | 1000 (70%) | 141 (10%) | 283 (20%) | 61 (14–96) | 398: 1,007 | 754 | 227 | 29 |
| DUKE | 452 | 315 (70%) | 46 (10%) | 91 (20%) | 65 (19–96) | 115: 337 | 115 | 24 | 2 |
| JEFF | 338 | 236 (70%) | 34 (10%) | 68 (20%) | 60 (19–90) | 114: 224 | 292 | 37 | 9 |
| YALE | 230 | 160 (70%) | 23 (10%) | 47 (20%) | 57 (20–92) | 69: 161 | 157 | 71 | 2 |
| MISS | 181 | 132 (73%) | 16 (9%) | 33 (18%) | 64 (14–89) | 38: 143 | 171 | 10 | 0 |
| UCSF | 180 | 126 (70%) | 18 (10%) | 35 (19%) | 58 (15–88) | 41: 119 | 19 | 41 | 16 |
| PENN | 44 | 31 (70%) | 4 (9%) | 9 (20%) | 59 (21–87) | 21: 23 | 0 | 44 | 0 |
Site abbreviations are as follows: DUKE (Duke University); JEFF (Thomas Jefferson University); YALE (Yale University); MISS (Missouri University); UCSF (University of California San Francisco); PENN (University of Pennsylvania).
Image data pre-processing
All MRI data underwent standardized image pre-processing steps including conversion from Digital Imaging and Communications in Medicine (DICOM) format to Neuroimaging Informatics Technology Initiative (NIfTI) format, co-registration of individual image series to a canonical anatomical brain (i.e., the SRI24 atlas15 space), including uniform 1 mm3 isotropic resampling, and automated skull-stripping using a deep convolutional neural network approach16. These image pre-processing steps were implemented in the open-source and publicly available Federated Tumor Segmentation (FeTS) tool17, which is the same tool that facilitated the largest brain glioblastoma study to date18. It should be noted that meningioma can extend through the skull and/or skull-base foramina and that any extra-cranial portions of tumors were implicitly excluded by the skull-stripping process2. Despite this limitation, skull-stripping was included in the pre-processing to preserve patient anonymity (by preventing potential face reconstruction) and to ensure consistency with the other BraTS 2023 challenges.
Defining meningioma sub-compartments on MRI
A key aspect of the BraTS Meningioma Pre-operative Dataset is the subdivision of the different tumor compartments that are visible on MRI sequences. The specific target volume delineation for intracranial meningioma MRI appearances and sub-compartments have been previously described19–21. In 2022, the Association des Neuro-oncologues d’Expression Francaise (ANOCEF) outlined consensus guidelines for meningioma gross tumor volume after 20 experts from 17 radiotherapy centers participated in a three round modified Delphi consensus21,22. The ANOCEF committee defined the enhancing gross tumor to include MRI T1 contrast-enhancing lesions, thickened meninges, and directly invaded bone21. This includes en-plaque meningioma and “dural tail” involvement, defined as thickening and enhancement of the dura infiltrating away from the lesion (Fig. 1)23,24. Non-enhancing tumor components include areas of mineralization or ossification typically with low signal intensity on T2-weighted imaging24 and cystic components with uniform low-signal intensity on T1-weighted imaging and high signal intensity on T2-weighted imaging24. Peritumoral edema, which appears as non-enhancing parenchymal T2/FLAIR signal hyperintensity surrounding the tumor, is present in 60% of meningioma cases and may be localized or extensive25.
Fig. 1.
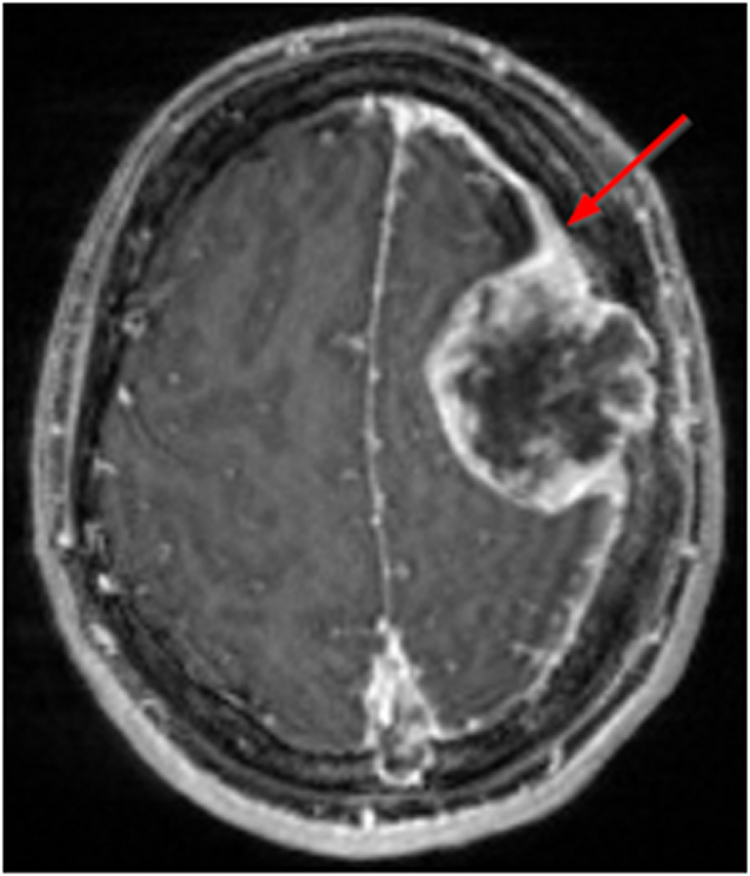
Example of an intracranial meningioma with a dural tail (red arrow) as shown on T1-weighted post-contrast MRI.
Based on this prior work and others, we defined three distinct and non-overlapping tumor sub-compartments (Fig. 2)19–21. These include “enhancing tumor”, “non-enhancing tumor core”, and surrounding non-enhancing T2/FLAIR hyperintensity (SNFH). The enhancing tumor label included all contrast enhancing meningioma, focally thickened meninges (including dural tail), as well as en-plaque meningiomas. This label approximated the compartment of active, viable tumor, which would typically be targeted by radiotherapy. The non-enhancing tumor core label included all calcification, hyperostosis, necrosis, degeneration, cystic areas, and any other atypical non-enhancing tumor findings. This label along with the enhancing tumor label (together comprising the “tumor core”) corresponded to the portion of tumor related imaging abnormality that would typically be removed in a gross total resection. The SNFH label included the entire extent of tumor-related T2/FLAIR hyperintensity surrounding the tumor core. This label was distinct from the other labels in that it was composed entirely of brain parenchyma and was not expected to contain any tumor cells, but rather represented irritated, inflamed, and/or edematous brain tissue resulting from the adjacent tumor. Importantly, non-tumor-related brain parenchymal T2/FLAIR signal abnormality, commonly related to chronic microvascular ischemic white matter changes (e.g. leukoaraiosis) or other vascular pathology, was not included in the SNFH label.
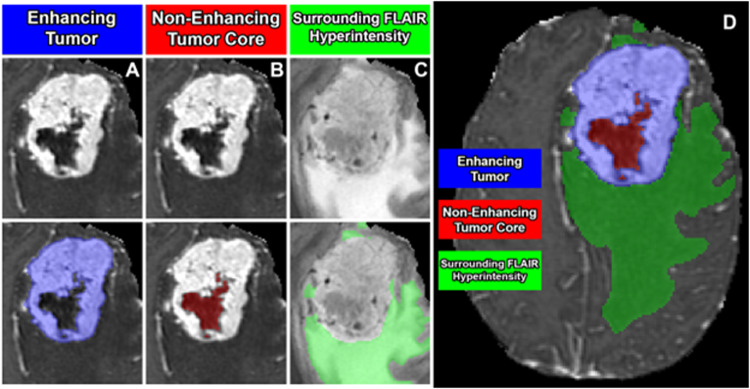
Fig. 2.
Meningioma sub-compartments considered in the BraTS Pre-operative Meningioma Dataset. Image panels A-C denote the different tumor sub-compartments included in manual annotations; (A) enhancing tumor (blue) visible on a T1-weighted post-contrast image; (B) the non-enhancing tumor core (red) visible on a T1-weighted post-contrast image; (C) the surrounding FLAIR hyperintensity (green) visible on a T2/FLAIR-weighted image; (D) combined segmentations generating the final tumor sub-compartment labels provided in the BraTS Pre-operative Meningioma Dataset.
Automated meningioma pre-segmentation
Prior to manual correction, a deep convolutional neural network-based automated segmentation model was used for automated multi-compartment pre-segmentation. This model, implemented in nnU-Net (version 1) (https://github.com/MIC-DKFZ/nnUNet/tree/nnunetv1) was initially trained on a sample of 73 manually labeled studies from a single participating institution (UCSF). Of note, this initial sample consisted entirely of meningiomas that subsequently underwent surgical resection, which may bias the model to poorer performance for non-surgical meningiomas. During the manual correction phase of the challenge preparation, the automated segmentation algorithm was periodically retrained using additional manually corrected cases from other participating sites, including sites that contributed non-surgical meningioma cases. The purpose of iteratively retraining the model with new data was to improve its generalizability to different MRI appearances of meningioma and reduce pre-segmentation bias. Model weights for each of the different meningioma pre-segmentation models are publicly available at (https://github.com/ecalabr/nnUNet_models).
Common errors of automated meningioma pre-segmentation
Based on a subjective review of pre-segmented meningioma cases by dataset organizers, a set of commonly encountered automated segmentation errors were identified. The following list of commonly encountered errors was provided to dataset annotators in an effort to reduced inter-rater variability:
A thin rim of erroneously assigned SNFH label immediately surrounding smaller meningiomas without any true associated SNFH (Fig. 3a).
Incomplete or absent segmentation of small convexity meningiomas composed entirely of enhancing tumor, particularly when more than 1 meningioma was included in the field of view (Fig. 3b)
Improper assignment or incomplete segmentation of non-enhancing tumor compartments, including exophytic hyperostosis, cystic spaces, and areas of intrinsic T1 hyperintensity, which were sometimes erroneously labeled as enhancing tumor or SNFH rather than non-enhancing tumor core (Fig. 3c)
Inclusion of non-tumor-related brain parenchymal T2/FLAIR signal abnormality, most commonly chronic microvascular ischemic white matter changes (e.g. leukoaraiosis) within the SNFH label (Fig. 3d).
Fig. 3.
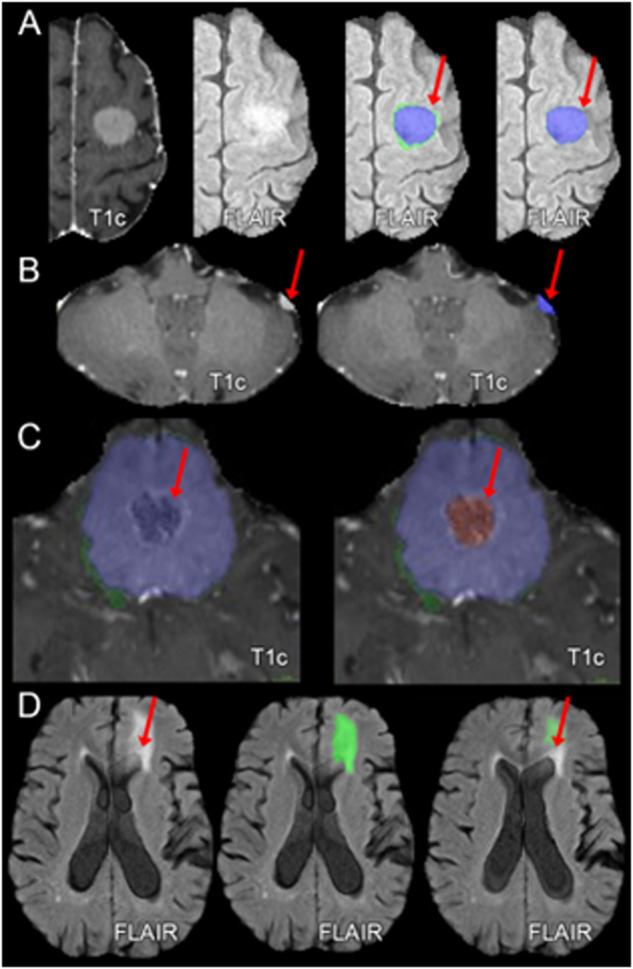
Examples of common errors of automated meningioma segmentation. (A) Erroneously marked a thin rim of edema that does not exist; (B) Missed small convexity meningioma; (C) Improper classification of non-enhancing tumor; (D) Tumor-related edema adjacent to presumed microvascular ischemic periventricular white matter FLAIR abnormalities. T1c: T1-weighted post-contrast imaging; FLAIR: T2-weighted fluid attenuated inversion recovery imaging.
Examples of each of these errors of automated meningioma segmentation are provided in Fig. 3.
Manual meningioma segmentation refinement process
For each meningioma case, manual review and refinement of pre-segmented labels was performed by individual volunteer “annotators” recruited from the ASNR society with widely varying experience levels spanning from medical students to fellowship-trained neuroradiologists. Subsequently, manually corrected annotations were reviewed by a single board-certified neuroradiologist “approver” (author EC). Manual corrections were performed using ITK-SNAP, a free, open-source, multi-platform software application used to segment structures in 3D biomedical images26. Annotators were provided with each of the following: 1) basic instruction on using ITK-SNAP for meningioma segmentation, 2) written descriptions of the composition of each tumor sub-compartment, and 3) a list (with examples) of common pre-segmentation errors to identify and address (similar to Fig. 3). In cases where manually corrected segmentations were deemed inaccurate, they were returned to the annotator pool for further corrections and re-review. This process was repeated until the segmentations were deemed accurate.
Data Records
The BraTS Meningioma Pre-operative Dataset training (1,000/1,424, 70%) and validation (141/1,424, 10%) data are publicly available on Synapse14. The testing dataset (283/1,424, 20%) will be kept private for the foreseeable future to allow for the unbiased assessment of future segmentation algorithms. The “Meningioma supplementary clinical data and imaging parameters for training and validation sets.xlsx” file on the Synapse data repository describes the case level clinical patient data and the image parameters for the training and validation cases14. The supplementary file “Meningioma Dataset Access Steps” provides step by step instructions on how to access the data.
Technical Validation
Patient clinical and demographic data
All clinical characteristics of the subjects included in the BraTS meningioma collection were obtained from clinical records from each respective academic institution without specific disclosure of the data collection method from each institution. This approach was taken to encourage data contribution. Clinical data included patient sex and age at time of the diagnosis, as well as CNS WHO grade. Specific information regarding patient pre-operative treatment was not included as part of data collection. No additional validation of the raw clinical data was conducted in the BraTS meningioma collection.
Image pre-processing and skull stripping
All pre-processing steps were manually reviewed by a fellowship-trained neuroradiologist (author EC) to ensure proper co-registration to the SRI24 atlas space, image quality, adequate skull stripping, presence of an intracranial meningioma, and absence of a non-meningioma intracranial tumor. Any pre-processing errors were manually corrected before inclusion in the dataset. For a majority of exams, original, unprocessed x and y image resolution and slice thickness were available and are included on the Synapse data repository14. Additional original image metadata was either not available, not approved for public release by governing data use agreements, or intentionally witheld to prevent BraTS challenge participants from fingerprinting exams from specific sites. However, data regarding site of origin for each exam is available and can be shared by request to the corresponding author on a case-by-case basis.
Meningioma segmentations
All manually corrected meningioma segmentations were manually reviewed by a board-certified neuroradiologist “approver” (author EC) following the established annotator/approval model used in prior BraTS challenges7. In cases where the approver identified an inaccurate or incomplete segmentation, the case was returned to a different annotator for further refinement with notes indicating remaining issues. This process was repeated, if necessary, until the segmentations were deemed accurate.
Supplementary information
Acknowledgements
We are grateful to everyone who contributed to the development and review of the tumor volume labels including volunteer annotators/approvers from the American Society of Neuroradiology (ASNR). Spyridon Bakas and Ujjwal Baid conducted part of the work reported in this manuscript at their current affiliation, as well as while they were affiliated with the Center for Biomedical Image Computing and Analytics (CBICA), the Department of Radiology, and the Department of Pathology and Laboratory Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA 19104, USA. Research reported in this publication was partly supported by the National Institutes of Health (NIH), under award numbers U24CA279629, U01CA242871, NCI K08CA256045, and NCI/ITCR U01CA242871. The content of this publication is solely the responsibility of the authors and does not represent the official views of the NIH.
Author contributions
Conceptualization (D.L., O.K., R.M., P.N., A.S.R., N.T., T.A., U.B., J.K., S.P., Y.D., F.H., A.J., C.K., F.K., B.M., N.M., S.M., J.R., Y.V., J.V., C.W., P.W., Z.J.R., Ma.A., Mi.A., A.F., A.N., A.R., S.B., E.C.), Methodology (D.L., R.M., U.B., S.B., J.R., Y.V., J.V., Ma.A., A.R., E.C.), Software (D.L., U.B., S.B., E.C.), Validation (J.R., E.C.), Formal analysis (D.L., E.C.), Resources (D.L., U.B., S.B., M.A., E.C.), Data Curation (D.L., O.K., R.B., A.K., J.K., S.F., K.L., R.M., M.P., P.N., A.S.R., N.T., Ma.A., Mi.A., A.F., A.N., A.R., E.C.), Data Annotation (D.L., E.C., N.S., D.D., K.N., D.W., A.H., J.F., Y.B., L.D., E.S., M.T., E.O., A.H., M.K., L.S., B.T., M.B., S.A., A.G., D.W., A.M., I.S., N.Y., J.M.S., R.H., M.M., A.A., K.W., T.R., D.M., A.O., AA.T., Y.S., S.F., D.K., M.P., M.H. A.S.R.), Writing - Original Draft (D.L., E.C.), Writing - Review & Editing (D.L., O.K., R.M., P.N., A.S.R., N.T., T.A., U.B., F.K., B.M., S.P., Y.D., D.G., F.H., A.J., C.K., N.M., N.S., S.M., J.R., Y.V., J.V., C.W., P.W., Z.J.R., Ma.A., Mi.A., A.F., A.N., A.R., S.B., E.C.), Supervision (Ma.A., Mi.A., A.F., A.N., A.R., S.B., E.C.), Project administration (D.L., E.C.), Funding acquisition (E.C., S.B., U.B.).
Code availability
In line with the scientific data principles of findability, accessibility, Interoperability, and reusability27, the tools used throughout the generation of these data are publicly available. Specifically, we used the FeTS toolkit [FeTS] to perform all pre-processing steps, including co-registration, and skull stripping, which is publicly available at (https://fets-ai.github.io/Front-End/)17. The nnU-Net model as used for initial pre-automated segmentation is publicly available at (https://github.com/ecalabr/nnUNet_models).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Dominic LaBella, Omaditya Khanna, Shan McBurney-Lin, Ryan Mclean, Pierre Nedelec, Arif S. Rashid, Nourel hoda Tahon.
These authors jointly supervised this work: Michelle Alonso-Basanta, Javier Villanueva-Meyer, Andreas M. Rauschecker, Ayman Nada, Mariam Aboian, Adam Flanders, Spyridon Bakas, Evan Calabrese.
Supplementary information
The online version contains supplementary material available at 10.1038/s41597-024-03350-9.
References
- 1.Ogasawara C, Philbrick BD, Adamson DC. Meningioma: A Review of Epidemiology, Pathology, Diagnosis, Treatment, and Future Directions. Biomedicines. 2021;9:319. doi: 10.3390/biomedicines9030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huntoon, K., Toland, A. M. S. & Dahiya, S. Meningioma: A Review of Clinicopathological and Molecular Aspects. Front Oncol10, (2020). [DOI] [PMC free article] [PubMed]
- 3.Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM. Meningioma Grading. Am J Surg Pathol. 1997;21:1455–1465. doi: 10.1097/00000478-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Louis DN, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perry A, Scheithauer BW, Stafford SL, Lohse CM, Wollan PC. ‘Malignancy’ in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer. 1999;85:2046–56. doi: 10.1002/(sici)1097-0142(19990501)85:9<2046::aid-cncr23>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 6.Lin D, et al. Trends in intracranial meningioma incidence in the United States, 2004‐2015. Cancer Med. 2019;8:6458–6467. doi: 10.1002/cam4.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menze BH, et al. The Multimodal Brain Tumor Image Segmentation Benchmark (BRATS) IEEE Trans Med Imaging. 2015;34:1993–2024. doi: 10.1109/TMI.2014.2377694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakas S, et al. Advancing The Cancer Genome Atlas glioma MRI collections with expert segmentation labels and radiomic features. Sci Data. 2017;4:170117. doi: 10.1038/sdata.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakas, S. BraTS 2023 Glioma Challenge. in (Vancouver, 2023).
- 10.LaBella, D. et al. The ASNR-MICCAI Brain Tumor Segmentation (BraTS) Challenge 2023: Intracranial Meningioma. ArXiv (2023).
- 11.Clark K, et al. The Cancer Imaging Archive (TCIA): Maintaining and Operating a Public Information Repository. J Digit Imaging. 2013;26:1045–1057. doi: 10.1007/s10278-013-9622-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vassantachart A, et al. Automatic differentiation of Grade I and II meningiomas on magnetic resonance image using an asymmetric convolutional neural network. Sci Rep. 2022;12:3806. doi: 10.1038/s41598-022-07859-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Synapse: Brain Tumor Segmentation (BraTS) Cluster of Challenges. https://www.synapse.org/#!Synapse:syn51156910/wiki/.
- 14.Calabrese, E. & LaBella, D. BraTS Meningioma Dataset. Synapse10.7303/syn51514106 (2023).
- 15.Rohlfing T, Zahr NM, Sullivan EV, Pfefferbaum A. The SRI24 multichannel atlas of normal adult human brain structure. Hum Brain Mapp. 2010;31:798–819. doi: 10.1002/hbm.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thakur S, et al. Brain extraction on MRI scans in presence of diffuse glioma: Multi-institutional performance evaluation of deep learning methods and robust modality-agnostic training. Neuroimage. 2020;220:117081. doi: 10.1016/j.neuroimage.2020.117081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pati S, et al. The federated tumor segmentation (FeTS) tool: an open-source solution to further solid tumor research. Phys Med Biol. 2022;67:204002. doi: 10.1088/1361-6560/ac9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pati S, et al. Federated learning enables big data for rare cancer boundary detection. Nat Commun. 2022;13:7346. doi: 10.1038/s41467-022-33407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers L, et al. Intermediate-risk meningioma: initial outcomes from NRG Oncology RTOG 0539. J Neurosurg. 2018;129:35–47. doi: 10.3171/2016.11.JNS161170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers CL, et al. High-risk Meningioma: Initial Outcomes From NRG Oncology/RTOG 0539. Int J Radiat Oncol Biol Phys. 2020;106:790–799. doi: 10.1016/j.ijrobp.2019.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martz N, et al. ANOCEF Consensus Guideline on Target Volume Delineation for Meningiomas Radiotherapy. International Journal of Radiation Oncology*Biology*Physics. 2022;114:e46. doi: 10.1016/j.ijrobp.2022.07.775. [DOI] [Google Scholar]
- 22.Nasa P, Jain R, Juneja D. Delphi methodology in healthcare research: How to decide its appropriateness. World J Methodol. 2021;11:116–129. doi: 10.5662/wjm.v11.i4.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Leary S, Adams WM, Parrish RW, Mukonoweshuro W. Atypical imaging appearances of intracranial meningiomas. Clin Radiol. 2007;62:10–17. doi: 10.1016/j.crad.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Watts J, et al. Magnetic resonance imaging of meningiomas: a pictorial review. Insights Imaging. 2014;5:113–122. doi: 10.1007/s13244-013-0302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bitzer M, et al. Angiogenesis and Brain Oedema in Intracranial Meningiomas: Influence of Vascular Endothelial Growth Factor. Acta Neurochir (Wien) 1998;140:333–340. doi: 10.1007/s007010050106. [DOI] [PubMed] [Google Scholar]
- 26.Yushkevich PA, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson MD, et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data. 2016;3:160018. doi: 10.1038/sdata.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
In line with the scientific data principles of findability, accessibility, Interoperability, and reusability27, the tools used throughout the generation of these data are publicly available. Specifically, we used the FeTS toolkit [FeTS] to perform all pre-processing steps, including co-registration, and skull stripping, which is publicly available at (https://fets-ai.github.io/Front-End/)17. The nnU-Net model as used for initial pre-automated segmentation is publicly available at (https://github.com/ecalabr/nnUNet_models).