Key Teaching Points.
-
•
Cilostazol was ineffective in preventing arrhythmic events in the patient.
-
•
Quinidine suspension provoked an arrhythmic storm, which was treated with the implantable cardioverter-defibrillator.
-
•
Quinidine was successful in suppressing malignant arrhythmias in Brugada syndrome (BrS).
-
•
Two variants in TTN and DSP are proposed in association with BrS.
-
•
Cilostazol in BrS must be used with caution because of resistant arrhythmic events.
Introduction
Brugada syndrome (BrS) is a disease with a high risk of developing ventricular arrhythmias leading to sudden cardiac death. Although an implantable cardioverter-defibrillator (ICD) is the first treatment option for symptomatic BrS, as it terminates malignant arrhythmias, it does not prevent arrhythmic events.1 Therefore, in subjects with an ICD who present with a ventricular tachycardia / ventricular fibrillation (VF) episode, a beneficial effect of long-term management with drugs like quinidine and cilostazol has been described.2 Herein, we present the long-term follow-up of a patient with BrS for whom cilostazol had no clinical benefit and who developed an arrhythmic storm after quinidine suspension.
Case report
A 45-year-old man was admitted to the Emergency Department owing to 15 ICD shocks over 24 hours. ICD interrogation revealed multiple VF episodes appropriately terminated. This patient was initially reported in 2012.3 The present report represents a 16-year follow-up. He received an ICD placement in May 2008 (in another hospital) after an episode of sudden cardiac arrest and was discharged without pharmacological treatment. He was first evaluated at our institution 1 month later (June 2008). Physical examination, echocardiogram, and treadmill exercise testing were all normal. The 12-lead electrocardiogram (ECG) revealed sinus bradycardia with a PR interval of 200 ms and an ECG type I pattern of BrS more evident on high precordial leads. Figure 1 exhibits “high precordial leads” from the case index as described by our group: leads V1–V3 are placed in the right intercostal space, just lateral to the sternum, and, in reality, correspond to right second, third, and fourth intercostal spaces (Figure 1).4 This patient had a history of 3 first-degree relatives deceased by sudden cardiac death (Supplemental Figure 1), so all current relatives were cited for further clinical analysis. None of them had relevant clinical findings, nor an ECG compatible with BrS. Genetic testing of the proband (case index) was performed in 2013 using next-generation sequencing, including a panel of 39 genes associated with familial arrhythmias (sponsored by Sistemas Genómicos, Valencia, Spain). Two variants of uncertain significance were identified: variant c.4372C>G (p.Arg1459Gly) in exon 23b of the DSP gene (encoding desmoplakin) and variant c.49460G>A (p.Arg16487Gln) in exon 327 of the TTN gene (encoding titin), both confirmed by Sanger sequencing (additional bioinformatic information is included in Supplemental Table 1). A cascade screening was not possible.
Figure 1.
High precordial leads: V1–V3, corresponding to right second, third, and fourth intercostal spaces, from the index case.
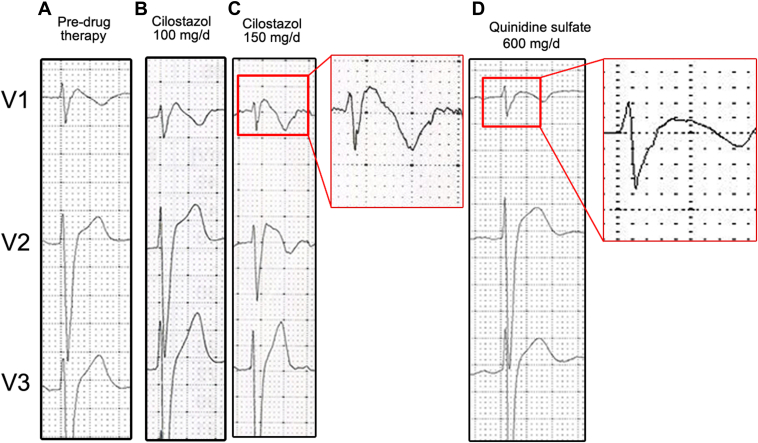
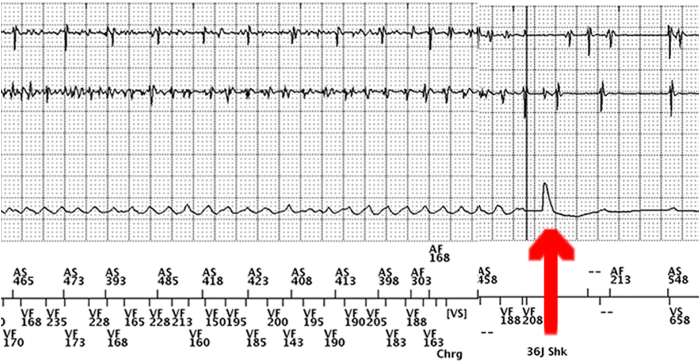
Six months after ICD implantation, the patient experienced 6 appropriate shocks during sleep. A 12-lead ECG showed J-point elevation in the right precordial leads (V1 = 5 mm; V2 = 6 mm). A complete neurological examination was normal. As quinidine is not commercially available in our country, and there were a few cases in the literature indicating that cilostazol could be beneficial, oral cilostazol (available for the treatment of arterial peripheral disease) was started at an initial dose of 100 mg per day (50 mg twice a day), which was later increased to 150 mg daily after 8 days of therapy. Regardless of cilostazol therapy, and in the absence of any other potential trigger (eg, fever, electrolyte abnormalities, alcohol intake, etc), the patient still experienced an episode of spontaneous VF, successfully terminated by the ICD, and no significant changes were observed in the ECG under cilostazol (Figure 2). Cilostazol was replaced by quinidine sulfate 300 mg twice per day, and no further arrhythmias occurred during a long-term follow-up (15 years) until November 2, 2022, when the patient arrived at the Emergency Department, presenting multiple appropriate ICD shocks owing to VF episodes (Figure 3). The patient noted that he had to stop the treatment because he could not obtain quinidine from any source. During an estimated period of 2 months, the patient only took the prescribed dose of the drug on days he felt slightly symptomatic (ie, mild palpitations, exertion, dizziness). It was not until he completely ran out of quinidine and did not take the drug for multiple consecutive days that the electrical storm presented, denoting the on-and-off phenomenon typically seen with quinidine.
Figure 2.
Precordial leads of index case before and after drug therapy. A: Pre–drug therapy. B: Under 100 mg/d of cilostazol. C: Under 150 mg/d of cilostazol. Close-up: Note the lack of a significant effect on ST-segment elevation or J point under cilostazol compared to image A. D: One week after 600 mg/d of quinidine sulfate. Close-up: Note the normalization of the ST/J point electrocardiogram morphology and disappearance of the type 1 Brugada syndrome pattern.
Figure 3.
Implantable cardioverter-defibrillator (ICD) electrogram during the arrhythmic storm event after quinidine discontinuation showed ventricular fibrillation successfully terminated by a 36 joule ICD shock (red arrow).
Discussion
Herein, we describe a patient with (1) a lack of clinical benefit to cilostazol therapy; (2) a successful therapeutic response to quinidine, with immediate suppression of the arrhythmogenic triggers (premature ventricular contractions); and (3) an “on-and-off” phenomenon manifested with arrhythmic storm appropriately treated with the ICD, associated with the suspension of medication.5,6
Chronic pharmacological treatment of BrS has been described with multiple drugs, including quinidine, bepridil, and cilostazol, to prevent arrhythmic events.2 In the presented case, it was decided to give low doses of quinidine (600 mg/day) after the first episode of the arrhythmic storm 14 years ago owing to a lack of response to cilostazol at a dose of 150 mg (the patient developed an episode of VF under treatment with this drug). After receiving quinidine treatment, the patient was symptom-free for several years. However, after discontinuing the medication for several months, he experienced an arrhythmic storm. Since quinidine is not available in the Mexican market and its importation fees are costly, many patients with BrS in the country face similar risks. This case underscores the relevance of ensuring local access to quinidine.
To obtain information on the efficacy of quinidine as a pharmacological agent to prevent arrhythmic events in patients with BrS after failed cilostazol therapy, an in-depth literature review was done. Multiple search engines were used (ie, PubMed, Google Scholar, Scopus, Scielo, and EMBASE), and the following inclusion criteria were established: (1) diagnosis of BrS (with baseline or evoked Brugada pattern I, II, or III), (2) absence of any structural heart disease (demonstrated by physical examination and/or imaging studies), and (3) presence of arrhythmic events anytime following the placement of an ICD. Five patients were identified. Supplemental Table 2 is a pooled analysis of the case reports as mentioned above.
In the literature reviewed, patients 1 and 2 (Supplemental Table 2) reported malignant arrhythmia suppression after initiation of monotherapy treatment with quinidine doses of 900–1000 mg/day.7,8 It is worth mentioning that quinidine and quinine are structurally similar to tryptophan, and quinidine may act as a competitive antagonist at the different enzymatic tryptophan binding sites, causing a decreased conversion of tryptophan into serotonin. As stated by Shenthar and colleagues,8 a tryptophan-rich diet is recommended in patients undergoing quinidine treatment to prevent cinchonism-related symptoms. Patient 3 needed triple therapy (cilostazol 100 mg/day, quinidine sulfate 600 mg/day, and diltiazem 90 mg/day) to suppress all forms of malignant arrhythmias successfully,9 although it is worth mentioning that clinical benefit in this patient solely with quinidine as monotherapy might have been achieved by equaling the dosage used for patients 1 and 2. Patient 4 presented similar circumstances as the patient outlined in this case report. He demonstrated an excellent response against the development of ventricular arrhythmias with quinidine as monotherapy. However, owing to an insufficient supply of this drug, he was then switched to a regime of cilostazol and bepridil, but episodes of VF recurred.10 Only 1 patient (patient 5) reported drug failure with both quinidine and cilostazol, although escalating the dosage with quinidine to achieve therapeutic effects was not attempted. Instead, a radiofrequency catheter ablation procedure was performed to completely stop the malignant arrhythmias. Twenty-nine months after ablation, the patient remained free of events.11 Concerning patient 4, catheter ablation was also performed owing to lack of quinidine (cases 4 and 5 from Japan). Fourteen months postablation, the patient was asymptomatic. Difficulties in obtaining quinidine were identified in Japan, India, Mexico, and Canada.
Regarding the genetic aspect of BrS, approximately 44 genes associated with BrS and its subtypes have been described. SCN5A is the most common gene affected, encompassing most cases described in the medical literature. On the other hand, DSP and TTN genes have also been associated with BrS, representing just a small fraction (<1%) of cases. The potential involvement of these 2 genes, mutated in this case, is discussed below.12
In this case report, a genetic panel of 39 genes was obtained after the clinical diagnosis, finding 2 variants of uncertain significance in DSP and TTN, respectively. Both genes were recently stated to be in association with BrS in a cohort of patients from China.13 However, with the information available today, there is not enough evidence to classify the variants as pathogenic or likely pathogenic. This DSP gene encodes a protein that anchors intermediate filaments to desmosomal plaques. The change p.Arg1458Gly is located in a moderately conserved residue (Grantham distance: 125 [0–215]), and its potential participation in heart rhythm disorders is controversial. On the other hand, variant c.49460G>A (p.Arg16487Gln), located in the TTN gene, encodes a large, abundant protein in striated muscle; the amino acids implied in the change p.Arg16487Gln have slight physicochemical differences (Grantham distance: 43 [0–215]). This variant has not been reported before as a single nucleotide variant or as the cause of a disease. Owing to the need to accumulate evidence in heart rhythm disorders, the information obtained is included for the diffusion of knowledge because BrS is predominantly autosomal dominant, and different inheritance patterns cannot be defined in this case.
A clinically relevant finding obtained from this case report is the lack of efficacy of cilostazol. This finding opposes what is stated in recent guidelines, as it is still considered a viable alternative for quinidine as pharmacological therapy for BrS.3,8 The apparent limited response to cilostazol and successful control with quinidine might be associated with the mutations and genes affected in this case (DSP and TTN) and contrast with a report including human-induced pluripotent stem cell–derived cardiomyocytes where it was found that cilostazol inhibited transient outward potassium current and reduced arrhythmic activity in 2 patients with a heterozygous mutation in SCN5A (a target of quinidine).14 This discrepancy could arise from the fact that quinidine acts through a different mechanism of action, primarily blocking rapid sodium channels (ie, SCN1A-SCN10A) and affecting calcium transport across cellular membranes. In contrast, cilostazol acts through inhibition of PDE3 (phosphodiesterase 3), causing suppression of transient outward potassium Ito. As stated by network analysis of protein interaction (Supplemental Figure 2), DSP and TTN have a more intricate interaction with fast sodium channels when compared to PDE3 (effective in some cases).14 Further functional studies are needed to gain a deeper understanding of these clinical distinctions.
To the best of our knowledge, there have been few case reports in the medical literature reporting cilostazol-resistant arrhythmic events in patients with BrS but who are not genotyped. In addition, monotherapy with cilostazol tends to cause symptomatic palpitations and impels the use of a secondary agent (eg, bepridil or other calcium channel blockers) to avoid this adverse effect. We consider that the role of cilostazol in the prophylaxis of arrhythmic events in BrS must be re-evaluated.
It is relevant to note that the reason for the suspension of pharmacological therapy with quinidine was its lack of availability in our country. The patient arranged to acquire the medication through fellow patients, until a shortage occurred. Also, although a few importer companies exist, the prices were too high for this patient and he could not afford to pay for it. This led him to discontinue the treatment, with the subsequent risk to his life. This situation is also informed in other countries where quinidine availability is scarce.5,6
Conclusion
We present a case of BrS in which cilostazol was ineffective in preventing arrhythmic events. On the contrary, an excellent clinical response to quinidine, both short-term and long-term, was demonstrated.
Disclosures
The authors have no conflicts to disclose.
Acknowledgments
To Sistemas Genómicos (Valencia, Spain) for the sponsored genetic testing provided for this patient. ATS would like to thank CONACYT for its support (CVU 382646).
Funding Sources
The Mexican National Council for Science and Technology (CONACYT) partially funded this work through Research Project Grant 845144 (CF 2019-376).
Appendix. Supplementary Data
Supplemental Figure 1.
The pedigree of the proband’s family. SCD = sudden cardiac death. P=proband
Supplemental Figure 2.
Network analysis of protein interaction between DSP, TTN, and proteins associated with cilostazol (PDE3)) and quinidine (SCN alpha and beta subunits) treatment.
References
- 1.Belhassen B., Viskin S., Antzelevitch C. The Brugada syndrome: is an implantable cardioverter defibrillator the only therapeutic option? Pacing Clin Electrophysiol. 2002;25:1634–1640. doi: 10.1046/j.1460-9592.2002.01634.x. [DOI] [PubMed] [Google Scholar]
- 2.Giustetto C., Cerrato N., Dusi V., Angelini F., De Ferrari G., Gaita F. The Brugada syndrome: pharmacological therapy. Eur Heart J Suppl. 2023;25:C32–C37. doi: 10.1093/eurheartjsupp/suad036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Márquez M.F., Bonny A., Hernández-Castillo E., et al. Long-term efficacy of low doses of quinidine on malignant arrhythmias in Brugada syndrome with an implantable cardioverter-defibrillator: a case series and literature review. Heart Rhythm. 2012;9:1995–2000. doi: 10.1016/j.hrthm.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 4.Manzano-Cabada J., Reyes-Quintero Á.E., Chávez-Gutiérrez C.A., et al. Diagnostic challenges of Brugada Syndrome in pediatric patients. J Electrocardiol. 2020;60:72–76. doi: 10.1016/j.jelectrocard.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Viskin S., Antzelevitch C., Márquez M.F., Belhassen B. Quinidine: a valuable medication joins the list of ‘endangered species.’. Europace. 2007;9:1105–1106. doi: 10.1093/europace/eum181. [DOI] [PubMed] [Google Scholar]
- 6.Malhi N., Cheung C.C., Deif B., et al. Challenge and impact of quinidine access in sudden death syndromes: a national experience. JACC Clin Electrophysiol. 2019;5:376–382. doi: 10.1016/j.jacep.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Abud A., Bagattin D., Goyeneche R., Becker C. Failure of cilostazol in the prevention of ventricular fibrillation in a patient with Brugada syndrome. J Cardiovasc Electrophysiol. 2006;17:210–212. doi: 10.1111/j.1540-8167.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 8.Shenthar J., Chakali S.S., Acharya D., Parvez J., Banavalikar B. Oral quinine sulfate for the treatment of electrical storm and prevention of recurrent shocks in Brugada syndrome after failed cilostazol therapy. HeartRhythm Case Rep. 2017;3:470–474. doi: 10.1016/j.hrcr.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehrotra S., Juneja R., Naik N., Pavri B.B. Successful use of quinine in the treatment of electrical storm in a child with Brugada syndrome. J Cardiovasc Electrophysiol. 2011;22:594–597. doi: 10.1111/j.1540-8167.2010.01907.x. [DOI] [PubMed] [Google Scholar]
- 10.Shako D., Nagase S., Nakajima K., Aiba T., Shinohara T., Kusano K. Global epicardial J wave with unipolar recording in both ventricles in a case of Brugada syndrome; masked early repolarization syndrome type 3. HeartRhythm Case Rep. 2023;9:910–913. doi: 10.1016/j.hrcr.2023.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakagawa E., Takagi M., Hiroaki T., Minoru Y. Successful radiofrequency catheter ablation for electrical storm of ventricular fibrillation in a patient with Brugada syndrome. Circ J. 2008;72:1025–1029. doi: 10.1253/circj.72.1025. [DOI] [PubMed] [Google Scholar]
- 12.Brugada R, Campuzano O, Sarquella-Brugada G, et al. Brugada syndrome. March 31, 2005 [Updated August 25, 2022]. In: Adam MP, Everman DB, Mirzaa GM, et al, eds. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2023. [PubMed]
- 13.Wang L.-L., Chen Y.-H., Sun Y., et al. Genetic profile and clinical characteristics of Brugada syndrome in the Chinese population. J Cardiovasc Dev Dis. 2022;9:369. doi: 10.3390/jcdd9110369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Y., Su J., Wang X., et al. Patient-specific iPSC-derived cardiomyocytes reveal variable phenotypic severity of Brugada syndrome. EBioMedicine. 2023;95 doi: 10.1016/j.ebiom.2023.104741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.