Abstract
In contrast to the simian immunodeficiency virus SIVmac239, which replicates poorly in rhesus monkey alveolar macrophages, a variant with nine amino acid changes in envelope (SIVmac239/316E) replicates efficiently and to high titer in these same cells. We examined levels of viral DNA, RNA, antigen, and infectious virus to identify the nature of the block to SIVmac239 replication in these cells. Low levels of viral antigen (0.1 to 1.0 ng of p27 per ml) and infectious virus (100 to 1,000 infectious units per ml) were produced in the supernatant 1 to 4 days after SIVmac239 infection, but these levels did not increase subsequently. SIVmac239 DNA was synthesized in these macrophage cultures during the initial 24 h after infection, but the levels did not increase subsequently. Quantitation of the numbers of infectious cells in cultures over time and the results of experiments in which cells were reexposed to SIVmac239 after the initial exposure indicated that only a small proportion of cells were susceptible to SIVmac239 infection in these alveolar macrophage cultures and that the vast majority (>95%) of cells were refractory to SIVmac239 infection. In contrast to the results with SIVmac239, the levels of viral antigen, infectious virus, and viral DNA increased exponentially 2 to 7 days after infection by SIVmac239/316E, reaching levels greater than 100 ng of p27 per ml and 100,000 infectious units per ml. Since SIVmac239/316E has previously been described as a virus capable of infecting cells in a relatively CD4-independent fashion, we examined the levels of CD4 expression on the surface of fresh and cultured alveolar macrophages from rhesus monkeys. The levels of CD4 expression were extremely low, below the limit of detection by flow cytometry, on greater than 99% of the macrophages. CCR5+ cells were profoundly depleted only from alveolar macrophage cultures infected with SIVmac239/316E. High concentrations of an antibody to CD4 delayed but did not block replication of SIVmac239/316E. The results suggest that the adaptation of SIVmac316 to efficient replication in alveolar macrophages results from its ability to infect these cells in a CD4-independent fashion or in a CD4-dependent fashion even at extremely low levels of surface CD4 expression. Since resident macrophages in brains and lungs of humans also express little or no CD4, our findings predict the presence of human immunodeficiency virus type 1 that is relatively CD4 independent in the lung and brain compartments of infected people.
The primate lentiviruses human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) infect CD4+ T lymphocytes and macrophages as major target cells (4, 8, 21, 23, 31, 32, 36, 39, 54). The gene for the viral envelope (Env) proteins determines the capability of virus to infect these cells (24, 43, 45, 61). Specific regions in Env play important roles in binding of virus to receptors and in the subsequent entry of virus into cells via fusion between the viral membrane and the cell membrane of target cells (25, 62, 65). Because CD4 and chemokine receptors in the target cells are utilized by primate lentiviruses as the primary receptor and coreceptors, respectively (7, 10, 11, 13–15, 19, 20, 22, 29, 37, 38, 40, 57), sequence variation in Env proteins can influence the interaction of the virus with these receptors and coreceptors which can result in changes in the cell tropism of the virus. During HIV type 1 (HIV-1) infection, the emergence of T-cell-tropic viruses from macrophagetropic viruses has been primarily ascribed to a change from use of the coreceptor CCR5 to use of CXCR4 (9, 59). CCR5 and GPR15/BOB have been found to be the principal coreceptors of the SIVmac strains characterized to date (11, 15, 19, 29, 40). CD4-independent virus entry has been confirmed for particular isolates of SIVmac (15, 41) and HIV-2 (18, 51). Thus, Env of SIV determines macrophage tropism, relative CD4 dependence, and coreceptor usage (16, 58).
We have been studying the mechanism underlying the restricted replication of SIVmac239 in alveolar tissue macrophages (42, 43, 54). Variant viruses that are highly competent for replication in alveolar macrophages emerge in approximately 30% of the monkeys that develop AIDS following infection with SIVmac239 (12; R. C. Desrosiers and D. J. Ringler, unpublished data). Analyses of variant viruses have demonstrated that four amino acid changes (V to M at residue 67, K to E at residue 176, G to R at residue 382, and K to T at residue 573) in Env are primarily responsible for fully productive infection of the variant viruses in tissue macrophage cultures as well as for disease manifestations associated with SIV infection of tissue macrophages (12, 30, 43). Three of these changes, at residues 67, 176, and 382 (MER), are sufficient to confer high replicative capacity. Although the V3 region of SIVmac can determine cell tropism (28), none of these sequence changes that affect high replicative capacity to SIVmac239 in tissue macrophages reside in V3. Supporting these results, entry of SIVmac239 into macrophages was found to be similar to that of derivatives with these sequence changes (42). However, the mechanisms which restrict SIVmac239 replication in tissue macrophages and allow sequence variants to replicate vigorously remain to be elucidated.
Here we show that SIVmac239 infection of alveolar macrophage cultures is restricted to a small portion of the total cell population. The ability of variant viruses to replicate vigorously in alveolar macrophages appears to be related to their ability to infect in a relatively CD4-independent fashion or at extremely low CD4 densities on the cell surface.
We used SIVmac239 with wild-type nef (27) and the recombinant virus with env of SIVmac316 (called SIVmac239/316E) (42, 43) as shown in Fig. 1A. Virus stocks were prepared as described elsewhere (27, 42–44). Virus stocks used to quantify newly synthesized viral DNA were subjected to DNase digestion. There was no significant difference in 50% tissue culture infective dose per microgram of p27 antigen between the two stocks. Macrophages were obtained from bronchoalveolar lavage (BAL) specimens from healthy, mature rhesus monkeys that were serologically negative for SIV, type D retrovirus, and foamy virus. For most experiments, lymphocytes and nonadherent cells were removed by extensive pipetting and exchange of medium 2 to 3 h after incubation of cells in a CO2 incubator. More than 95% of the cells were confirmed to be macrophages as previously documented (54). For the data shown in Fig. 5 and 6, the total cell population from BAL fluid was used. For the data shown in Fig. 1 to 4 and the tables, cells were infected 2 days after plating.
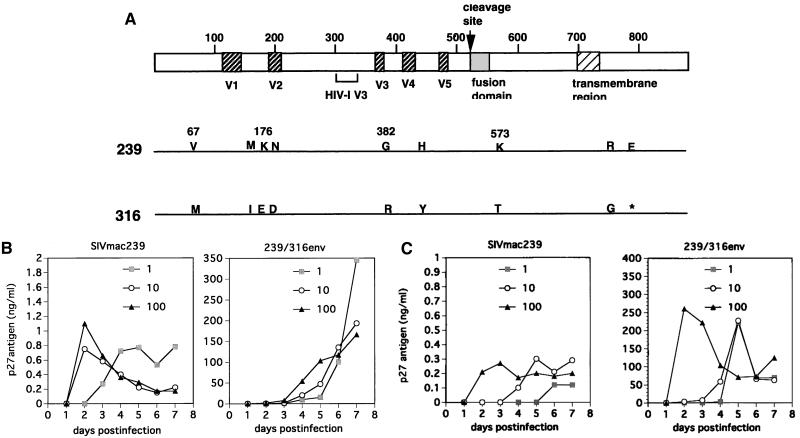
FIG. 1.
(A) Nine amino acid substitutions in Env between SIVmac239 (239) and SIVmac239/316E (316). Four amino acids (residues 67, 176, 382, and 573) are primarily responsible for the higher replicative capacity of SIVmac239/316E than of SIVmac239 in macrophages. V1 to V5 refer to variable regions in Env (5). Locations of sequences corresponding to HIV-1 V3 and the stop codon (∗) are indicated. (B and C) SIV replication in macrophages monitored by p27gag antigen concentration in culture supernatant. Here, 2 × 105 macrophages were infected with 1, 10, or 100 ng of p27gag antigen. Culture medium was collected daily, followed by a wash with Hanks balanced salt solution and replacement with fresh medium. Representative results are shown for two different monkey donors. Kinetics of SIV growth varies with the source of macrophage preparations.
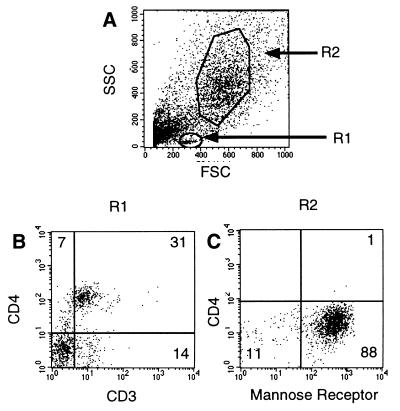
FIG. 5.
Expression of CD4 on lymphocytes and macrophages isolated via BAL as determined by flow cytometry. A lymphocyte region (R1) and a macrophage region (R2) were established using FSC/SSC (forward scatter/side scatter) in the same sample (A). (B and C) Expression of CD4 and CD3 on cells in the lymphocyte gate (B) and expression of CD4 and the mannose receptor on alveolar macrophages (C). Quadrants were established on matched isotype controls using the same gating strategy. Antibodies used for immunophenotyping of rhesus BAL fluid included anti-CD3 (6G12; kindly provided by J. Wong, Massachusetts General Hospital) (1), anti-CD4 (OKT4; American Type Culture Collection), anti-CD8 (Leu-2a; Becton Dickinson), and anti-mannose receptor (Pharmingen, Carpinteria, Calif.). Cells were stained according to standard protocols (4). Four-color flow cytometry analysis of the cells was performed using a FACS Vantage (Becton Dickinson).
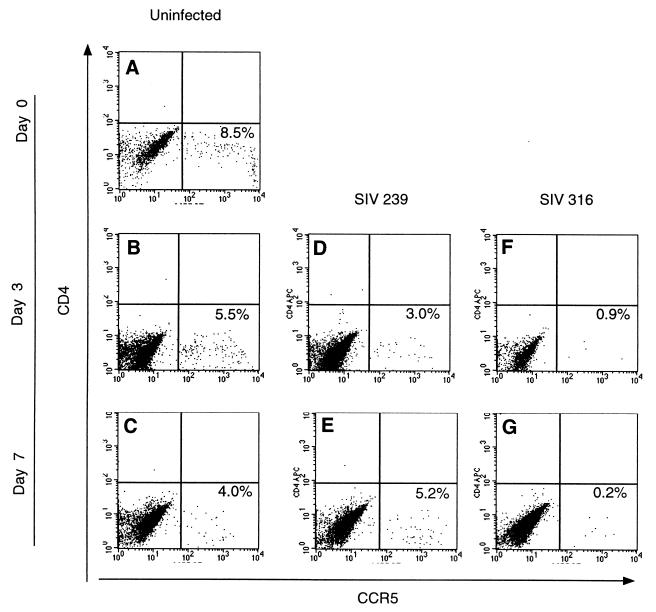
FIG. 6.
CCR5 expression on uninfected and SIV-infected BAL samples. The surface expression of CD4 and CCR5 on BAL samples was determined by flow cytometry on baseline samples (A), as well as on uninfected cells after 3 days (B) and 7 days (C) in culture. (D and E) Analysis of BAL samples from the same donor infected with SIV239; (F and G) samples infected with SIVmac239/316E. Quadrants were established with matched isotype controls.
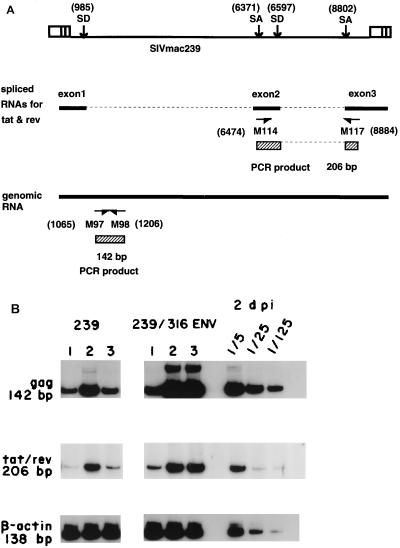
FIG. 4.
Viral RNA in macrophages infected with SIVmac239 or SIVmac239/316E. (A) Primers used for RNA PCR. A 142-bp gag sequence was amplified with primers M97 and M98; 206 bp corresponding to doubly spliced RNA for tat and rev was amplified with primers M114 and M117 (46). SD, splicing donor site; SA, splicing acceptor site. Numbers in parentheses are nucleotide numbers of SIVmac239 (52). (B) Total cell RNA was prepared from the macrophages infected with a high dose of SIV (100 ng of p27 antigen) at 1, 2, and 3 dpi. Expression of viral RNAs for gag and tat/rev was examined by reverse transcription-PCR using cDNA synthesized with random hexamer. β-Actin RNA was amplified as a control cellular RNA. Comparable results were obtained in six independent experiments.
Production of virus from infected cell cultures was monitored by measuring the levels of p27gag antigen in cell-free culture supernatants. Production of SIVmac239 generally did not exceed 1 ng of p27 per ml from these tissue macrophage cultures. Figure 1B and C show the results of independent analyses with BAL cultures from two different rhesus monkeys. Peak Gag antigen production resulting from infection by SIVmac239/316E was 100 to 1,000 times higher than what was observed with SIVmac239 (Fig. 1B and C), consistent with our prior results (42, 43). Increasing the multiplicity of infection (MOI) over a 100-fold range did not appear to affect the peak height of production of either virus, but it did appear to affect the kinetics of appearance of p27 antigen for both viruses (Fig. 1B and C). Virus production peaked much earlier with SIVmac239 than with SIVmac239/316E (Fig. 1B and C). The titers of infectious virus produced early after infection paralleled the p27 antigen measurements (Fig. 2).
FIG. 2.
Infectious titer of SIV in supernatants of macrophage cultures infected with SIVmac239 (239) or SIVmac239/316E (316). Here, 2 × 105 macrophages were infected with a high dose of SIV (100 ng of p27 antigen). Infectious SIV titer (50% tissue culture infective dose [TCID50]) of culture supernatant collected at 24-h intervals (0 to 0.5, 0.5 to 1.5, 1.5 to 2.5, and 2.5 to 3.5 days) was determined by endpoint dilution. Infectivity of diluted culture supernatant was determined by using CEMx174 cells.
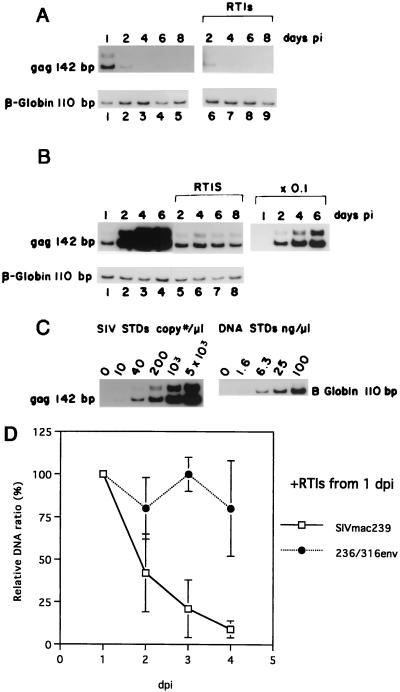
Although replication of SIVmac239 is severely restricted in alveolar macrophages, synthesis of viral DNA by reverse transcription was previously found to be normal or near normal for 24 h of observation after exposure of cells to SIVmac239 (42, 43). We extended these earlier findings by extending the period of observation for up to 8 days. As was observed previously, levels of newly synthesized viral DNA at 1 day postinfection (dpi) were comparable between SIVmac239 and SIVmac239/316E (Fig. 3A to C). After 1 dpi, however, the patterns diverged dramatically. Levels of viral DNA in macrophages infected with SIVmac239/316E increased dramatically between 1 and 6 dpi concomitant with the spread of the infection through the culture (see also infectious center assays described below). Levels of viral DNA did not increase when dissemination of infection was blocked by reverse transcriptase inhibitors (RTIs) (Fig. 3B). Levels of viral DNA in macrophages infected with SIVmac239 decreased dramatically after 1 day (Fig. 3A). The pattern of decreasing SIVmac239 DNA levels occurred similarly with or without the addition of RTIs at 24 h postinfection (p.i.). These differences in viral DNA kinetics were observed in four of four independent experiments (Fig. 3D). These results suggested instability of SIVmac239 DNA in infected cells or a loss of infected cells from the cultures by cell death. They also indicated a failure of SIVmac239 to spread through the cultures.
FIG. 3.
(A and B) Viral DNA in macrophages infected with SIVmac239 (A) and SIVmac239/316E (B); (C) standards (STDs) for SIV DNA and cell DNA; (D) viral DNA relative to DNA amount at 1 dpi in macrophages with RTIs. Mean values of four experiments and standard deviations are shown. Here, 2 × 105 macrophages were infected with a high dose of virus stock (100 ng of p27 antigen). Subsequently, the cells were maintained in the presence or absence of RTIs, which were supplemented 24 h p.i. Total cell DNA was prepared at 1, 2, 4, 6, and 8 dpi and subjected to PCR analyses with primers for Gag or β-globin.
We next examined the numbers of infected cells over time in these macrophage cultures. The proportion of infected cells varied with the macrophage preparation (Table 1). Whereas 25 and 1.6% of cells were infected with SIVmac239/316E at 1 dpi with two independent macrophage preparations, 3.1 and 0.9% of the macrophages were infected with SIVmac239 at the same time in the same two macrophage preparations (Table 1). By 3 dpi, most of the cells in the culture were infected by SIVmac239/316E. However, the numbers of cells infected with SIVmac239 did not increase over the same period; in fact, they decreased (Table 2). These results are consistent with a small proportion of cells susceptible to SIVmac239 and a failure of SIVmac239 to spread to the vast majority of cells in the cultures.
TABLE 1.
Proportion of infected cells by infectious center assay
| Expta | dpi | % of macrophages producing infectious SIV
|
|
|---|---|---|---|
| SIVmac239 | SIVmac239/316E | ||
| A | 1 | 3.1 | 25 |
| 2 | 1.3 | 67 | |
| 3 | 0.5 | 100 | |
| B | 1 | 0.9 | 1.6 |
| 2 | 0.7 | 1 | |
| 3 | 0.4 | Not done | |
In experiments A and B, 2 × 105 and 105 cells, respectively, were infected with SIV equivalent to 100 ng of p27gag.
TABLE 2.
Effect of reexposure with SIVmac239 on viral DNA levels in macrophages
| Expta | Day | dpi | SIV DNA copy no./ 100 cells | Ratio, SIV DNA level/ level at 1 dpi |
|---|---|---|---|---|
| A | 1 | 1 | 140 | 1.0 |
| 2 | 2 | 61 | 0.44 | |
| 3 | 3 | 27 | 0.19 | |
| 4 | 4 | 16 | 0.11 | |
| 5 | 5 | 11 | 0.08 | |
| 5 | 5 + 1 | 15 | 0.11 | |
| 5 | 1 | 190 | 1.4 | |
| B | 1 | 1 | 13.0 | 1.0 |
| 2 | 2 | 3.4 | 0.26 | |
| 3 | 3 | 2.3 | 0.18 | |
| 4 | 4 | 0.7 | 0.05 | |
| 4 | 4 + 1 | 2.3 | 0.18 |
See the footnote to Table 1.
We also examined the susceptibility of cells to reexposure to SIVmac239 subsequent to the initial round of infection by SIVmac239. Aliquots of the macrophages for each of these experiments were exposed to SIVmac239 on day 0 (2 days after plating), and identical aliquots were left unexposed. Levels of viral DNA were measured at days 1, 2, 3, 4, and 5 (Table 2, experiment A). On day 5, cells (both previously exposed and unexposed) were again given SIVmac239, and levels of viral DNA were measured 1 day later. The results indicated that on day 5, SIVmac239 was able to infect a susceptible population of cells in the cultures that had not been previously exposed to virus, but few or no susceptible cells remained in the cultures that had been previously exposed to SIVmac239. An independent test gave similar results (Table 2, experiment B). When SIVmac239/316E was used as the second infecting virus strain, it was able to establish infection in an uncompromised fashion (data not shown). These results are consistent with the above findings indicating that only a small minority of cells are susceptible to SIVmac239 in these alveolar macrophage cultures.
We analyzed levels of viral RNA by RNA PCR with the two sets of primers shown in Fig. 4A. RNAs for gag and tat/rev were analyzed. In SIVmac239/316E-infected cells, viral RNA levels increased from days 1 to 2 and remained high 2 and 3 dpi during the spreading infection (Fig. 4B). In SIVmac239-infected cells, levels of viral RNA increased until 2 dpi and then decreased (Fig. 4B), consistent with the viral DNA analysis. Viral RNA levels at 2 days after infection with SIVmac239/316E were approximately five times higher than those observed with SIVmac239 (Fig. 3B), consistent with slightly increased levels of SIVmac239/316E DNA at 1 dpi.
Flow cytometric analysis of BAL specimens revealed two populations of cells as shown in Fig. 5, alveolar macrophages (R2) and a minor population of lymphocytes (R1). The phenotype of these cells was confirmed by demonstrating that cells within R1, the lymphocyte gate, were predominantly CD3+ CD4+ or CD3+ CD4−, consistent with helper or cytotoxic lymphocyte populations, respectively. The larger cells were shown to express the mannose receptor, consistent with a population of macrophages (33, 50). However, more than 99% of these macrophages did not express CD4 detectably on their surface, as determined by flow cytometry. Using multiple replicate analyses of BAL samples from three rhesus monkeys, the frequency of CD4+ macrophages was 0.24% ± 0.2%. The SIV coreceptor CCR5 was shown to be expressed on a percentage of the CD4-negative alveolar macrophages (Fig. 6). BAL samples were divided into three groups: uninfected, infected with SIVmac239, and infected with SIVmac239/316E. BAL samples infected with SIVmac239/316E demonstrated a profound loss of CCR5+ cells as early as 3 dpi. This observation suggests that CCR5+ CD4− cells are the principal early targets of SIVmac239/316E in these alveolar macrophage cultures.
The antibodies for these flow cytometric determinations have been used repeatedly by our laboratories and the laboratories of others to identify CD4 and CCR5 on the surface of rhesus monkey cells with good sensitivity (2, 34, 35, 48, 49, 53, 55, 66). For the present experiments, we used appropriate CD4/CCR5-positive and -negative controls to establish gating and sensitivity. The level of CD4 on BAL that we report here is markedly reduced compared to rhesus monkey peripheral blood monocytes/macrophages. In fact, we have routinely used the same OKT4 monoclonal antibody and flow cytometry methods to routinely identify CD4 on the surface of blood monocytes from rhesus monkeys (56). Thus, the macrophages from BAL fluid are indeed significantly different from monocytes/macrophages from peripheral blood in the level of surface CD4 expression.
Finally, we examined the ability of monoclonal antibody to CD4 to block replication of SIVmac239/316E. High concentration of the 19thy5D7 monoclonal antibody significantly slowed the kinetics of virus appearance, but the CD4 blockage was not highly effective at inhibiting viral replication (Fig. 7). These results are consistent with previous testing in which the same monoclonal antibody inhibited but did not completely block the early appearance of newly synthesized SIVmac239/316E DNA after exposure of virus to alveolar macrophage cultures (42).
FIG. 7.
Inhibition of SIVmac239/316E replication in alveolar macrophages by monoclonal antibody against CD4. Macrophages (2 × 105) were pretreated at 37°C for 30 min with medium containing human immunoglobulin G (0.5 mg/ml) and no antibody (■) or a monoclonal antibody against CD4 (19thy5D7; kindly provided by S. Schlossman, Dana-Farber Cancer Institute) serially diluted 1:10 (0.1 mg/ml; ○), 1:25 (□), 1:50 (⧫), 1:100 (▴), or 1:200 (●). Cells were then infected with SIVmac239/316E (10 ng of p27 antigen) for 2 h. The cells were washed with Hanks balanced salt solution and then maintained in medium with the same concentration of antibody as was used for the pretreatment. p27gag antigen level in the culture supernatant collected at 3-day intervals was determined with a commercial p27gag antigen assay kit (Coulter).
The vast majority of lung macrophages from BAL samples appear to be refractory to infection by SIVmac239. The establishment of early infection by SIVmac239 in these alveolar macrophage cultures appears to be due to a small population of susceptible cells beyond which SIVmac239 cannot spread significantly. Although we have not shown directly what these susceptible cells are, it is likely that they are the small fraction of CD4+ macrophages in these cultures. Consistent with this, entry of SIVmac239 into the early susceptible population and viral DNA synthesis were effectively blocked by antibody to CD4 (42). Some of these susceptible cells could very well express levels of CD4 below our ability to detect them by flow cytometry. Thus, the block to replication of SIVmac239 in alveolar macrophages appears to relate to the absence of sufficient CD4 on the surface of the vast majority of these cells.
It is not entirely clear to what extent the decay in SIVmac239 DNA levels is actually due to DNA instability versus the die-off of productively infected cells. The decay in numbers of infectious cells over time (Table 1), the inability to get expected levels of SIVmac239 DNA at day 4 or 5 after an initial exposure to SIVmac239 (Table 2), and the die-off of CCR5-positive cells infected with SIVmac239/316E all argue that die-off of the small numbers of SIVmac239-infected cells is likely to be a major factor in the loss of SIVmac239 DNA over time. However, in addition to major limitations in the amount of CD4 needed to allow SIV239 entry into the vast majority of alveolar macrophages, intracellular blocks may also exist.
About 30 to 50% of monkeys that die with AIDS from SIVmac239 infection have marked, characteristic tissue lesions in brain and/or lung (12). The infected cell type that vastly predominates in the pathologic lesions in these tissues is the macrophage. The appearance of SIV encephalitis and giant cell pneumonia in monkeys is uniformly associated with the evolution of virus variants with high replicative capacity for tissue macrophages not only at the New England Regional Primate Research Center with strain SIVmac239 (12, 30, 43) but also at other research centers (3). Strains with specific sequence changes that allow high replicative capacity in macrophages have been characterized as able to infect cells in a fashion that is less dependent, or independent, of the presence of CD4 (15–17, 41, 58).
Our studies indicate that the ability of SIVmac239/316E and similar strains to replicate efficiently in cultured tissue macrophages appears to relate to the low or absent levels of CD4 on these cells and the decreased dependence of these strains on CD4. Thus, SIVmac239/316E may be able to replicate in tissue macrophages lacking detectable CD4 because of an ability to infect independent of this receptor. Evidence has also been presented that SIVmac239/316E has a higher affinity for CD4 than SIVmac239 (58; R. E. Means and R. C. Desrosiers, unpublished data). Furthermore, strains of SIV that have been described as “CD4 independent” usually, but not always, infect much better with CD4 present on the surface of the cell than in its absence (15–17, 41, 58). Thus, the increased affinity of SIVmac239/316E for CD4 may allow infection of tissue macrophages with an extremely low density of CD4 (below our ability to detect by flow cytometry) in a CD4-dependent fashion. It is important to note that these properties, and the sequence changes that are required to bring them about, are not an unusual feature of one SIV isolate or one monkey but a quite uniform feature of virtually all monkeys that die with SIV encephalitis or giant cell pneumonia (3, 12, 15, 30, 43, 54).
Our results clearly predict the evolution of HIV-1 strains with the properties of relative CD4 independence in the brains and lungs of at least some people with tissue-specific HIV-1 disease manifestations. The dearth of CD4 on the surface of human alveolar macrophages, similar to rhesus monkey alveolar macrophages, has been reported (37, 64). Resident macrophages in brains of both monkeys and humans are also largely low for CD4 expression (63), and the same sorts of SIV envelope sequence changes that allow high replicative capacity in lung macrophages occur in the brain (3, 30). It is not clear if analyses have been done appropriately to determine whether such changes or properties occur with some regularity at a terminal stage of brain or lung disease in humans (6, 22, 26, 37, 47, 60).
Acknowledgments
We thank Prabhat Sehgal for help with lung lavages, Ken Williams for helpful advice, and H. Yoshikura for critical comments.
This work was supported by grants from the Ministry of Health and Welfare and the Science and Technology Agency of Japan, the Health Sciences Foundation and Organization for Pharmaceutical Safety and Research in Japan, and the Public Health Service (AI 25328 and RR00168).
REFERENCES
- 1.Akridge R E, Oyafuso L K, Reed S G. IL-10 is induced during HIV-1 infection and is capable of decreasing viral replication in human macrophages. J Immunol. 1994;153:5782–5782. [PubMed] [Google Scholar]
- 2.Alexander L, Veazey R S, Czajak S, DeMaria M, Rosenzweig M, Lackner A A, Desrosiers R C, Sasseville V G. Recombinant simian immunodeficiency virus expressing green fluorescent protein identifies infected cells in rhesus monkeys. AIDS Res Hum Retrovir. 1999;15:11–21. doi: 10.1089/088922299311664. [DOI] [PubMed] [Google Scholar]
- 3.Anderson M G, Hauer D, Sharma D P, Joag S V, Narayan O, Zink M C, Clements J E. Analysis of envelope changes acquired by SIVmac239 during neuroadaption in rhesus macaques. Virology. 1993;195:616–626. doi: 10.1006/viro.1993.1413. [DOI] [PubMed] [Google Scholar]
- 4.Brinkmann R, Schwinn A, Muller J, Stahl-Hennig C, Coulibaly C, Hunsmann G, Czub S, Rethwilm A, Dorries R, ter Meulen V. In vitro and in vivo infection of rhesus monkey microglial cells by simian immunodeficiency virus. Virology. 1993;195:561–568. doi: 10.1006/viro.1993.1407. [DOI] [PubMed] [Google Scholar]
- 5.Burns D P W, Desrosiers R C. Selection of genetic variants of simian immunodeficiency virus in persistently infected rhesus monkeys. J Virol. 1991;65:1843–1854. doi: 10.1128/jvi.65.4.1843-1854.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan S Y, Speck R F, Power C, Gaffen S L, Chesebro B, Goldsmith M A. V3 recombinants indicate a central role for CCR5 as a coreceptor in tissue infection by human immunodeficiency virus type 1. J Virol. 1999;73:2350–2358. doi: 10.1128/jvi.73.3.2350-2358.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng-Mayer C, Liu R, Landau N R, Stamatatos L. Macrophage tropism of human immunodeficiency virus type 1 and utilization of the CC-CKR5 coreceptor. J Virol. 1997;71:1657–1661. doi: 10.1128/jvi.71.2.1657-1661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collman R, Hassan N F, Walker R, Godfrey B, Cutilli J, Hastings J C, Friedman H, Douglas S D, Nathanson N. Infection of monocyte-derived macrophages with human immunodeficiency virus type 1 (HIV-1). Monocyte-tropic and lymphocyte-tropic strains of HIV-1 show distinctive patterns of replication in a panel of cell types. J Exp Med. 1989;170:1149–1163. doi: 10.1084/jem.170.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 11.Deng H K, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 12.Desrosiers R C, Hansen-Moosa A, Mori K, Bouvier D P, King N W, Daniel M D, Ringler D J. Macrophage-tropic variants of SIV are associated with specific AIDS-related lesions but are not essential for the development of AIDS. Am J Pathol. 1991;139:29–35. [PMC free article] [PubMed] [Google Scholar]
- 13.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusion and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 14.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 15.Edinger A L, Amedee A, Miller K, Doranz B J, Endres M, Sharron M, Samson M, Lu Z H, Clements J E, Murphey-Corb M, Peiper S C, Parmentier M, Broder C C, Doms R W. Differential utilization of CCR5 by macrophage and T cell tropic simian immunodeficiency virus strains. Proc Natl Acad Sci USA. 1997;94:4005–4010. doi: 10.1073/pnas.94.8.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edinger A L, Blanpain C, Kunstman K J, Wolinsky S M, Parmentier M, Doms R W. Functional dissection of CCR5 coreceptor function through the use of CD4-independent simian immunodeficiency virus strains. J Virol. 1999;73:4062–4073. doi: 10.1128/jvi.73.5.4062-4073.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edinger A L, Mankowski J L, Doranz B J, Margulies B J, Lee B, Rucker J, Sharron M, Hoffman T L, Berson J F, Zink M C, Hirsch V M, Clements J E, Doms R W. CD4-independent, CCR5-dependent infection of brain capillary endothelial cells by a neurovirulent simian immunodeficiency virus strain. Proc Natl Acad Sci USA. 1997;94:14742–14747. doi: 10.1073/pnas.94.26.14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endres M J, Clapham P R, Marsh M, Ahuja M, Turner J D, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 19.Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 21.Gartner S, Markovits P, Markovitz D M, Kaplan M H, Gallo R C, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 22.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 23.Ho D D, Rota T R, Schooley R T, Kaplan J C, Allan J D, Groopman J E, Resnick L, Felsenstein D, Andrews C A, Hirsch M S. Isolation of HTLV-III from cerebrospinal fluid and neural tissues of patients with neurologic syndromes related to the acquired immunodeficiency syndrome. N Engl J Med. 1985;313:1493–1497. doi: 10.1056/NEJM198512123132401. [DOI] [PubMed] [Google Scholar]
- 24.Hwang S S, Boyle T J, Lyerly H K, Cullen B R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991;253:71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- 25.Jones P L, Korte T, Blumenthal R. Conformational changes in cell surface HIV-1 envelope glycoproteins are triggered by cooperation between cell surface CD4 and co-receptors. J Biol Chem. 1998;273:404–409. doi: 10.1074/jbc.273.1.404. [DOI] [PubMed] [Google Scholar]
- 26.Kabat D, Kozak S L, Wehrly K, Chesebro B. Differences in CD4 dependence for infectivity of laboratory-adapted and primary patient isolates of human immunodeficiency virus type 1. J Virol. 1994;68:2570–2577. doi: 10.1128/jvi.68.4.2570-2577.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kestler H W, III, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for the development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 28.Kirchhoff F, Mori K, Desrosiers R C. The V3 domain is a determinant of simian immunodeficiency virus cell tropism. J Virol. 1994;68:3682–3692. doi: 10.1128/jvi.68.6.3682-3692.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirchhoff F, Pohlmann S, Hamacher M, Means R E, Kraus T, Uberla K, Di Marzio P. Simian immunodeficiency virus variants with differential T-cell and macrophage tropism use CCR5 and an unidentified cofactor expressed in CEMx174 cells for efficient entry. J Virol. 1997;71:6509–6516. doi: 10.1128/jvi.71.9.6509-6516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kodama T, Mori K, Kawahara T, Ringler D J, Desrosiers R C. Analysis of simian immunodeficiency virus sequence variation in tissues of rhesus macaques with simian AIDS. J Virol. 1993;67:6522–6534. doi: 10.1128/jvi.67.11.6522-6534.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koenig S, Gendelman H E, Orenstein J M, Dal Canto M C, Pezeshkpour G H, Yungbluth M, Janotta F, Aksamit A, Martin M A, Fauci A S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 32.Koyanagi Y, Miles S, Mitsuyasu R T, Merrill J E, Vinters H V, Chen I S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 33.Koziel H, Eichbaum Q, Kruskal B A, Pinkston P, Rogers R A, Armstrong M Y, Richards F F, Rose R M, Ezekowitz R A. Reduced binding and phagocytosis of Pneumocystis carinii by alveolar macrophages from persons infected with HIV-1 correlates with mannose receptor downregulation. J Clin Investig. 1998;102:1332–1344. doi: 10.1172/JCI560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehner T, Wang Y, Cranage M, Tao L, Mitchell E, Bravery C, Doyle C, Pratt K, Hall G, Dennis M, Villinger L, Bergmeier L. Up-regulation of beta-chemokines and down-modulation of CCR5 co-receptors inhibit simian immunodeficiency virus transmission in non-human primates. Immunology. 2000;99:569–577. doi: 10.1046/j.1365-2567.2000.00993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehner T, Wang Y, Doyle C, Tao L, Bergmeier A, Mitchell E, Bogers W M, Heeney J, Kelly C G. Induction of inhibitory antibodies to the CCR5 chemokine receptor and their complementary role in preventing SIV infection in macaques. Eur J Immunol. 1999;29:2427–2435. doi: 10.1002/(SICI)1521-4141(199908)29:08<2427::AID-IMMU2427>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 36.Levy J A, Shimabukuro J, Hollander H, Mills J, Kaminsky L. Isolation of AIDS-associated retroviruses from cerebrospinal fluid and brain of patients with neurological symptoms. Lancet. 1985;2:586–588. [PubMed] [Google Scholar]
- 37.Lewin S R, Sonza S, Irving L B, McDonald C F, Mills J, Crowe S M. Surface CD4 is critical to in vitro HIV infection of human alveolar macrophages. AIDS Res Hum Retrovir. 1996;12:877–883. doi: 10.1089/aid.1996.12.877. [DOI] [PubMed] [Google Scholar]
- 38.Liao F, Alkhatib G, Peden K W, Sharma G, Berger E A, Farber J M. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandell C P, Jain N C, Miller C J, Dandekar S. Bone marrow monocyte/macrophages are an early cellular target of pathogenic and nonpathogenic isolates of simian immunodeficiency virus (SIVmac) in rhesus macaques. Lab Investig. 1995;72:323–333. [PubMed] [Google Scholar]
- 40.Marcon L, Choe H, Martin K A, Farzan M, Ponath P D, Wu L, Newman W, Gerard N, Gerard C, Sodroski J. Utilization of C-C chemokine receptor 5 by the envelope glycoproteins of a pathogenic simian immunodeficiency virus, SIVmac239. J Virol. 1997;71:2522–2527. doi: 10.1128/jvi.71.3.2522-2527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin K A, Wyatt R, Farzan M, Choe H, Marcon L, Desjardins E, Robinson J, Sodroski J, Gerard C, Gerard N P. CD4-independent binding of SIV gp120 to rhesus CCR5. Science. 1997;278:1470–1473. doi: 10.1126/science.278.5342.1470. [DOI] [PubMed] [Google Scholar]
- 42.Mori K, Ringler D J, Desrosiers R C. Restricted replication of simian immunodeficiency virus strain 239 in macrophages is determined by env but is not due to restricted entry. J Virol. 1993;67:2807–2814. doi: 10.1128/jvi.67.5.2807-2814.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mori K, Ringler D J, Kodama T, Desrosiers R C. Complex determinants of macrophage tropism in env of simian immunodeficiency virus. J Virol. 1992;66:2067–2075. doi: 10.1128/jvi.66.4.2067-2075.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naidu Y M, Kestler III H W, Li Y, Butler C V, Silva D P, Schmidt D K, Troup C D, Sehgal P K, Sonigo P, Daniel M D, Desrosiers R C. Characterization of infectious molecular clones of simian immunodeficiency virus (SIVmac) and human immunodeficiency virus type 2: persistent infection of rhesus monkeys with molecularly cloned SIVmac. J Virol. 1988;62:4691–4696. doi: 10.1128/jvi.62.12.4691-4696.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Brien W A, Koyanagi Y, Namazie A, Zhao J-Q, Diagne A, Idler K, Zack J A, Chen I S Y. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990;348:69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- 46.Park I W, Steen R, Li Y. Characterization of multiple mRNA species of simian immunodeficiency virus from macaques in a CD4+ lymphoid cell line. J Virol. 1991;65:2987–2992. doi: 10.1128/jvi.65.6.2987-2992.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Platt E J, Wehrly K, Kuhmann S E, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pohlmann S, Lee B, Meister S, Krumbiegel M, Leslie G, Doms R W, Kirchhoff F. Simian immunodeficiency virus utilizes human and sooty mangabey but not rhesus macaque STRL33 for efficient entry. J Virol. 2000;74:5075–5082. doi: 10.1128/jvi.74.11.5075-5082.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pohlmann S, Stolte N, Munch J, Ten Haaft P, Heeney J L, Stahl-Hennig C, Kirchhoff F. Co-receptor usage of BOB/GPR15 in addition to CCR5 has no significant effect on replication of simian immunodeficiency virus in vivo. J Infect Dis. 1999;180:1494–1502. doi: 10.1086/315097. [DOI] [PubMed] [Google Scholar]
- 50.Pontow S E, Kery V, Stahl P D. Mannose receptor. Int Rev Cytol. 1992;137B:221–244. doi: 10.1016/s0074-7696(08)62606-6. [DOI] [PubMed] [Google Scholar]
- 51.Reeves J D, Hibbitts S, Simmons G, McKnight A, Azevedo-Pereira J M, Moniz-Pereira J, Clapham P R. Primary human immunodeficiency virus type 2 (HIV-2) isolates infect CD4-negative cells via CCR5 and CXCR4: comparison with HIV-1 and simian immunodeficiency virus and relevance to cell tropism in vivo. J Virol. 1999;73:7795–7804. doi: 10.1128/jvi.73.9.7795-7804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Regier D A, Desrosiers R C. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res Hum Retrovir. 1990;6:1221–1231. doi: 10.1089/aid.1990.6.1221. [DOI] [PubMed] [Google Scholar]
- 53.Reimann K A, Waite B C, Lee-Parritz D E, Lin W, Uchanska-Ziegler B, O'Connell M J, Letvin N L. Use of human leukocyte-specific monoclonal antibodies for clinically immunophenotyping lymphocytes of rhesus monkeys. Cytometry. 1994;17:102–108. doi: 10.1002/cyto.990170113. [DOI] [PubMed] [Google Scholar]
- 54.Ringler D J, Wyand M S, Walsh D G, MacKey J J, Sehgal P K, Daniel M D, Desrosiers R C, King N W. The productive infection of alveolar macrophages by simian immunodeficiency virus. J Med Primatol. 1989;18:217–226. [PubMed] [Google Scholar]
- 55.Rosenzweig M, MacVittie T J, Harper D, Hempel D, Glickman R L, Johnson R P, Farese A M, Whiting-Theobald N, Linton G F, Yamasaki G, Jordan C T, Malech H L. Efficient and durable gene marking of hematopoietic progenitor cells in nonhuman primates after nonablative conditioning. Blood. 1999;94:2271–2286. [PubMed] [Google Scholar]
- 56.Rosenzweig M, Marks D F, Hempel D, Heusch M, Kraus G, Wong-Staal F, Johnson R P. Intracellular immunization of rhesus CD34+ hematopoietic progenitor cells with a hairpin ribozyme protects T cells and macrophages from simian immunodeficiency virus infection. Blood. 1997;90:4822–4831. [PubMed] [Google Scholar]
- 57.Rucker J, Samson M, Doranz B J, Libert F, Berson J F, Yi Y, Smyth R J, Collman R G, Broder C C, Vassart G, Doms R W, Parmentier M. Regions in beta-chemokine receptors CCR5 and CCR2b that determine HIV-1 cofactor specificity. Cell. 1996;87:437–446. doi: 10.1016/s0092-8674(00)81364-1. [DOI] [PubMed] [Google Scholar]
- 58.Schenten D, Marcon L, Karlsson G B, Parolin C, Kodama T, Gerard N, Sodroski J. Effects of soluble CD4 on simian immunodeficiency virus infection of CD4-positive and CD4-negative cells. J Virol. 1999;73:5373–5380. doi: 10.1128/jvi.73.7.5373-5380.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, de Goede R E, van Steenwijk R P, Lange J M, Schattenkerk J K, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shieh J T, Albright A V, Sharron M, Gartner S, Strizki J, Doms R W, Gonzalez-Scarano F. Chemokine receptor utilization by human immunodeficiency virus type 1 isolates that replicate in microglia. J Virol. 1998;72:4243–4249. doi: 10.1128/jvi.72.5.4243-4249.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shioda T, Levy J A, Cheng-Mayer C. Macrophage and T cell-line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature. 1991;349:167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- 62.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 63.Williams K, Bar-Or A, Ulvestad E, Olivier A, Antel J P, Yong V W. Biology of adult human microglia in culture: comparisons with peripheral blood monocytes and astrocytes. J Neuropathol Exp Neurol. 1992;51:538–549. doi: 10.1097/00005072-199209000-00009. [DOI] [PubMed] [Google Scholar]
- 64.Worgall S, Connor R, Kaner R J, Fenamore E, Sheridan K, Singh R, Crystal R G. Expression and use of human immunodeficiency virus type 1 coreceptors by human alveolar macrophages. J Virol. 1999;73:5865–5874. doi: 10.1128/jvi.73.7.5865-5874.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 66.Zhang H, Hannon G J, Beach D. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 1994;8:1750–1758. doi: 10.1101/gad.8.15.1750. [DOI] [PubMed] [Google Scholar]