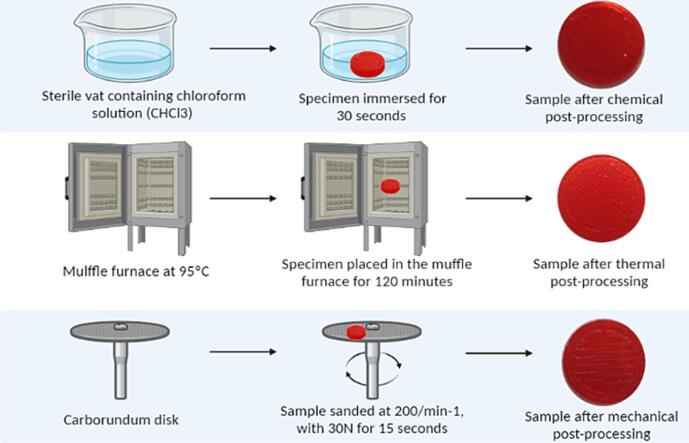
Graphical abstract
Keywords: Post-processing, Additive manufacturing, Fused filament fabrication, Polylactic acid, Microbial adhesion
Abstract
Introduction
Post-processing (PP) is performed to improve the surface, which can favor microbial adhesion and consequent pathological manifestations that impair the indication of polylactic acid (PLA) obtained by fused filament fabrication (FFF) for biomedical applications. This aims to evaluate the influence of chemical, thermal, and mechanical PP on the adhesion of Streptococcus mutants and Candida albicans, roughness, and wettability of the PLA obtained by FFF with and without thermal aging.
Methods
The specimens were designed in the 3D modeling program and printed. The chemical PP was performed by immersion in chloroform, the thermal by the annealing method, and the mechanical by polishing. Thermal aging was performed by alternating the temperature from 5 °C to 55 °C with 5000 cycles. Colony-forming unit (CFU/mL) counting was performed on dual-species biofilm of C. albicans and S. mutans. Roughness was analyzed by rugosimeter and wettability by the sessile drop technique. Data were verified for normality using the Shapiro-Wilk test, two-way ANOVA (α = 0.05) applied for CFU and wettability, and Kruskal-Wallis (α = 0.05) for roughness.
Results
Chemical, thermal, and mechanical PP methods showed no influence on CFU/mL of C. albicans (p = 0.296) and S. mutans (p = 0.055). Thermal aging did not influence microbial adhesion. Chemical PP had lower roughness, which had increased after aging. Wettability of the mechanical PP was lower.
Conclusions
Post-processing techniques, do not present an influence on the adhesion of S. mutans and C. albicans in PLA obtained by FFF, chemical PP reduced roughness, and mechanical reduced wettability. Thermal aging did not alter the microbial adhesion and altered the roughness and wettability.
1. Introduction
Chemical, thermal, and mechanical post-processing (PP) aims to refine the surface of printed objects (Mu et al., 2020, Singh et al., 2019, Valerga et al., 2019, Valerga Puerta et al., 2021). Chemical PP is easy, economical, and performed by exposure to chemical vapors or immersion in solutions such as ethyl acetate (C4H8O2), tetrahydrofuran (C4H8O), dichloromethane (CH2Cl2), and chloroform (CHCl3) (Mu et al., 2020, Valerga Puerta et al., 2021); The mechanical method consists in passing an abrasive ball or disc over the surface of the printed object to smooth the peaks and valleys caused by the printing process. Among the methods are manual sanding, abrasive flow machine, abrasive milling, sandblasting, and vibratory finishing (Mu et al., 2020, Valerga et al., 2019); The thermal is based on the annealing technique by inserting the specimens in furnaces for a predetermined time between the glass transition and the melting temperature of the polymers (Singh et al., 2019). This temperature increase reduces polymer viscosity and promotes microstructural reorganization with the filling of gaps by the increased fluidity of the polymer, reduction of valleys, increased adhesion between printed layers, and crystallinity (Amza et al., 2021) (see Fig. 1).
Fig. 1.
Graphical abstract of the post-processing methods.
The Fused Filament Fabrication (FFF) technique fabricates objects with higher roughness and porosity (Kessler et al., 2020, Valvez et al., 2021, Verbeeten et al., 2020) and operates by extrusion of filaments from thermoplastic materials such as polylactic acid (PLA) (Al Rashid and Koç, 2021, Kessler et al., 2020). The FFF 3D printer is composed of a feeding material control mechanism, heating chamber, and nozzle (Al Rashid and Koç, 2021, Verbeeten et al., 2020). The filament moves through the heating chamber until it reaches the semisolid phase; in the extruder the material is melted and deposited on the build platform, layer by layer, until the final object is obtained (Kessler et al., 2020).
The surface characteristics promoted by the FFF technique derive from the reduction in adhesion strength between printed polymer layers by increasing temperature and cooling cycle intervals, leading to residual thermal stress that promotes voids and pores. This phenomenon called “anisotropic behavior” compromises the mechanical properties of tensile strength, flexure, hardness, and compression and increases roughness (Kessler et al., 2020, Valvez et al., 2021, Verbeeten et al., 2020). To reduce these effects, it is possible to use pre-processing methods such as printing parameters: angle, layer thickness, printing speed (Mu et al., 2020, Singh et al., 2019, Valerga et al., 2019, Valerga Puerta et al., 2021), and PP (Kessler et al., 2020, Singh et al., 2019).
PLA is a semi-crystalline polymer derived from bacterial fermentation of dextrose from natural sources and has advantageous mechanical and biological properties, biocompatibility, non-toxic to human cells, and biodegradable (Abdul Samat et al., 2021, Casalini et al., 2019, Chen et al., 2019), characteristics that favor its indications in the biomedical field such as for the production of implants, suture threads, and scaffolds for tissue engineering (Abdul Samat et al., 2021, Al Rashid and Koç, 2021, Casalini et al., 2019, Chen et al., 2019).
Campos et al. (2022) developed attachments for retaining overdentures with PLA using the FFF method and observed excellent retention, hardness, and compression properties. However, attachments are subject to microbial contamination because they are placed in an area that is difficult to clean, which can promote microbial adhesion and subsequent oral and systemic diseases (de Campos et al., 2023, de Campos and dos Reis, 2023, de Campos and dos Reis, 2023, de Campos et al., 2023).
PLA attachments by FFF have a high surface roughness, which can favor the adhesion of microorganisms since rougher surfaces tend to promote higher biofilm accumulation (Alp et al., n.d.; Batak et al., 2021; de Campos et al., 2023, de Campos and dos Reis, 2023, Mei et al., 2011, Vidakis et al., 2020), due to the increase of the surface area or being associated with hydrophobicity, electrostatic interactions, and Van Der Waals forces that benefit microbial adhesion (Batak et al., 2021; Vidakis et al., 2020). Surface roughness has a major influence on microbial adhesion after the period of biofilm establishment because the microbes can find the best spaces to adhere, such as cracks and pores (Mei et al., 2011).
The primary colonizers of biofilm, the Streptococci, among them Streptococcus mutans, adhere to surfaces by electrostatic interactions (Schubert et al., 2021, Song et al., 2015). The oral biofilm is multi-species and this encourages the adhesion of the fungus Candida albicans, which attempts to adhere to materials in biofilms previously formed by bacteria and is associated with oral and systemic candidiasis and prosthetic stomatitis, a condition that most affects denture wearers (Busscher et al., 2010, Schubert et al., 2021, Song et al., 2015).
The evaluation of material behavior over time is relevant to assess the relative durability, time of applicability and use in various medical applications without compromising the patient or the functionality of the designed prosthetic component (Barrasa et al., 2021, Bechtel et al., 2022, Bergaliyeva et al., 2022). It can be performed by the thermal aging technique, which consists of the complete immersion of the sample in water at a given temperature and time, to simulate the use term (Barrasa et al., 2021, Bechtel et al., 2022, Bergaliyeva et al., 2022). The rough, porous surface and the biodegradability of PLA, accelerate aging leading to reduced mechanical performance and altered surface, which may favor the growth of microbial adhesion (Barrasa et al., 2021, Bechtel et al., 2022, Bergaliyeva et al., 2022, Fernández-Grajera et al., 2022).
Considering the medical and dental application of PLA obtained by FFF, the reduction of biofilm accumulation is paramount to prevent contamination, and infections, and improves hygiene to overcome the surface characteristics that favor microbial adhesion (de Campos et al., 2023, de Campos and dos Reis, 2023). This study aimed to evaluate the behavior of PLA obtained by FFF against dual-species biofilm, roughness, and wettability with chemical, thermal, and mechanical PP, with and without thermal aging. This study presents a null hypothesis that the different PP methods and aging have no influence on microbial adhesion, on the roughness and wettability of PLA obtained by additive manufacturing (AM).
2. Material and methods
2.1. Three-dimensional modeling
The specimens were elaborated and designed in the three-dimensional modeling program (Rhinoceros®) in the dimensions of 9Ø2mm. After this process, the file with the design was converted to “STL” format for further reading in the FlashForge Adventurer 3 3D printer (FlashForge 3D Technology Co., LTD, Zhejiang™).
2.2. Printing parameters
The printing parameters were followed according to the manufacturer of the polylactic acid (PLA) filament used (FlashForge™). The building direction was 0°, speed 50 mm/s, layer thickness 0.16 mm, extrusion temperature 210 °C, platform temperature 50 °C, and 0.4 mm extruder nozzle diameter. These parameters were transferred to the FlashPrint software that promoted the connection between the virtual model and the physical sample print.
2.3. Specimen printing
The specimen to be printed was selected and the 3D printer performed the printing of the objects with PLA filament (FlashForge™) in red coloration by the FFF method. A total of 128 specimens were printed, divided into eight groups, represented in (Table 1).
Table 1.
Specimen group and description. Groups present in the study were divided into with and without thermal aging and subdivided by the type of post-processing performed, chemical, thermal or mechanical, and the control group (no post-processing).
| Aging | Post-processing method |
|---|---|
| Without aging | Control: no post-processing |
| Chemical post-processing | |
| Thermal Post Processing | |
| Mechanical post-processing | |
| With aging | Control: no post-processing |
| Chemical post-processing | |
| Thermal Post Processing | |
| Mechanical post-processing | |
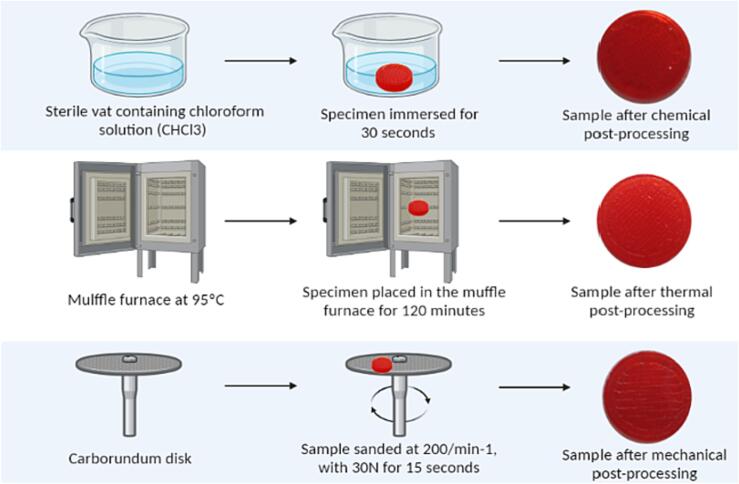
2.4. Post-processing (PP)
2.4.1. Chemical
The samples were immersed in a sterile vat containing chloroform solution (CHCl3) for 30 s. After this period, the samples were removed from the solution and dried at room temperature.
2.4.2. Thermal
It was performed using the “thermal annealing” method, which consists in raising the temperature of the samples to 95 °C for 120 min in a temperature-controlled muffle oven. After this period the samples were cooled at room temperature.
2.4.3. Mechanical
The samples were polished with an aluminum oxide composite carborundum disc (Dentorium®) at a speed of 200/min−1, with a torque of 30 N for 15 s. The disc and the entire sample surface were in complete contact for uniform polishing of the entire sample.
2.4.4. Thermal aging
Thermal aging was performed in a thermocycler (MSCT- 3Plus; Marcelo Nucci- ME; São Carlos, SP, Brazil), by alternating the temperature from 5 °C to 55 °C in distilled water, with 30 s in each temperature and 10 s of transition, 1 cycle/min, and 5,000 cycles were performed, corresponding to 6 months of clinical use.
2.5. Microbial adhesion analysis
2.5.1. Dual-species biofilm formation
Samples were sterilized with hydrogen peroxide plasma (Sterrad) and distributed (n = 8) in 24-well plates (Kasvi - GuangZhou Jet Bio-filtration Co. LTD). Microbial adhesion was performed with a dual-species biofilm composed of C. albicans (ATCC 10231) and S. mutans (ATCC 25175). The inoculum was prepared in a Brain Heart Infusion Broth (BHI) culture medium (Conda S.A Laboratory, Spain). The standardization of the inoculum was measured by optical density (OD625nm) in a spectrophotometer (Multiskan GO, Thermo Scientific Multiskan® Spectrum, Waltham, MA, USA) with absorbance between 0.08 and 0.100 and the yeasts were counted in a Neubauer chamber (HBG, Gießen, HE, Germany).
Each well of the 24-well plate containing the specimens received 1.5 mL of BHI culture medium inoculated with 106 CFU/mL of S. mutans and 106 CFU/mL of C. albicans and was incubated at 37 °C and 750 rpm of agitation in a bacteriological oven (CienLab, Campinas, SP, Brazil) for 1 h and 30 min for biofilm adherence. After this period, the specimens were washed with 2 mL of 0.085 % saline solution, and 1.5 mL of sterile BHI culture medium was added. The plates were incubated at 37 °C and 750 rpm for 48 h in a bacteriological oven.
2.5.2. Colony forming units (CFU)
The colony-forming units per milliliter (CFU/mL) were evaluated for the quantification of viable cells. For seeding, specimens were transferred to test tubes, placed in an ultrasonic bath for 20 min, and shaken individually. A 25 μL aliquot of the solution without dilution was used, and subsequently, a 25 μL aliquot was transferred to a microtube containing 250 μL of 0.85 % saline solution to obtain serial dilutions of 100 to 10−3, each microorganism was seeded in Petri dishes with specific agar, being Sabouraud Dextrose Agar for C. albicans and BHI Agar supplemented with nystatin for S. mutans.
Then, the plates were incubated at 37 °C for 24 h in a bacteriological oven, with S. mutans in microaerophile. The CFU number was counted and recorded. For the calculation, it was considered the dilution in which the number of CFU varies from 30 to 300 colonies and with the following formula:
| CFU/mL = (number of colonies × 10n) /q |
where n is the absolute value of the dilution, and q is the amount in mL, of each dilution to seed the plates. In the present study, q = 0.025.
2.6. Surface roughness
Surface roughness was assessed using the SJ. 201P rugosimeter (Mitutoyo Corporantion, Japan) in which the equipment's analyzer tip traveled 8 mm. Three measurements were taken per sample (n = 10) in the direction of its largest diameter.
2.7. Wettability
Wettability was assessed by the sessile drop method with distilled water. A 50 μL drop was deposited on each surface at constant room temperature. A time of 60 s was allowed for the partial stabilization of the water drop in contact with the substrate to obtain a measurement according to the variation of the mean angle of contact (θ) (n = 3).
2.8. Statistical analysis
Data were verified for normality by the Shapiro-Wilk test and two-way ANOVA was applied for CFU and wettability (α = 0.05). The Kruskal-Wallis non-parametric analysis (α = 0.05) was applied to the roughness data.
3. Results
3.1. Colony forming units (CFU)
The chemical, thermal, and mechanical PP (p = 0.296) and thermal aging (p = 0.563) methods showed no influence on the CFU/mL of C. albicans (Table 2).
Table 2.
Mean and standard deviation of colony forming units per milliliter (CFU/mL) for Candida albicans.
| Aging | Candida albicans | ||||
|---|---|---|---|---|---|
| Post-processing methods |
p-value | ||||
| Control | Mechanical | Thermal | Chemical | ||
| With | 5,99 (0,57) | 6,18 (0,53) | 5,81 (0,37) | 5,88 (0,45) | 0,296 |
| Without | 5,98 (0,33) | 6,14 (0,34) | 6,16 (0,37) | 5,84 (0,41) | |
| p-value | 0,563 | ||||
For S. mutans, no influence of chemical, thermal and mechanical PP methods (p = 0.055) and thermal aging (p = 0.813) was observed on CFU/mL (Table 3).
Table 3.
Mean and standard deviation of colony forming units per milliliter (CFU/mL) for Streptococcus mutans.
| Aging | Streptococcus mutans | ||||
|---|---|---|---|---|---|
| Post-processing methods |
p-value | ||||
| Control | Mechanical | Thermal | Chemical | ||
| With | 6,55 (0,76) | 6,61 (0,68) | 6,15 (0,51) | 5,93 (0,54) | 0,055 |
| Without | 6,44 (0,58) | 6,48 (0,63) | 6,39 (0,34) | 6,06 (0,59) | |
| p-value | 0, 813 | ||||
3.2. Surface roughness
For roughness, chemical PP showed lower roughness with aging, statistically different from control (p = 0.001) and thermal PP (p = 0.001). For the groups without aging, chemical PP generated lower roughness, statistically different from the control (p < 0.001) and thermal PP (p = 0.001); mechanical PP was different from the control group (p = 0.003). Thermal aging reduced the roughness of the control (p < 0.001) and thermal PP (p = 0.004) groups (Table 4).
Table 4.
Median and confidence interval (95 %) of roughness values with and without thermal aging.
| Post-processing methods | With Aging | Without Aging | p-value |
|---|---|---|---|
| Control | 4,23 [3,66; 4,73]A | 11,06 [8,98;12,49]A | <0,001 |
| Mechanical | 2,98 [2,34;4,55]B | 3,80 [3,32;4,70]BC | 0,123 |
| Thermal | 3,49 [3,22;5,39]A | 6,7 [5,42;7,85]AC | 0,004 |
| Chemical | 1,18 [0,97;2,11]B | 1,15 [1,03;1,64]B | 0,631 |
Identical letters indicate statistical similarity in the column.
3.3. Wettability
For wettability, in the groups with thermal aging, mechanical PP showed lower wettability, statistically different from the control group (p = 0.001) and chemical PP (p < 0.001). In the groups without thermal aging, mechanical PP generated lower wettability, statistically different from the control group (p < 0.001), thermal PP (p = 0.002), and chemical PP (p < 0.001). Thermal aging led to a significant increase in wettability in the control group (p < 0.001) (Table 5).
Table 5.
Mean and standard deviation of contact angle values with and without thermocycling.
| Post-processing methods | With Aging | Without Aging | p-value |
|---|---|---|---|
| Control | 44,26 ± 3,28AC | 54,57 ± 1,88A | <0,001 |
| Mechanical | 35,19 ± 1,48B | 31,65 ± 1,21C | 0,071 |
| Thermal | 40,39 ± 0,35AB | 40,04 ± 2,80B | 0,851 |
| Chemical | 46,15 ± 2,85C | 49,73 ± 2,49A | 0,069 |
Identical letters indicate statistical similarity in the column.
4. Discussion
This study evaluated the influence of chemical, thermal, and mechanical PP on the adhesion of dual-species biofilm, roughness, and wettability of PLA obtained by AM by the FFF method with and without thermal aging. It was observed that the PP did not reduce the adhesion of C. albicans and S. mutans and altered roughness and wettability. The null hypothesis was partially accepted since PP did not alter microbial adhesion, which can be explained by the fact that adhesion on surfaces is influenced by factors related to the material, such as roughness, porosity, microorganism, virulence factors, pH, pili, and in both the surface free energy and hydrophobicity (Batak et al., n.d., de Bruijn et al., 2021, Hall et al., 2021, Kreve et al, n.d.), and altered roughness and wettability, which may be related to topographical changes in the surface of the material.
In this study, PP methods were similar to those explored by (Bhandari et al., 2019, de Bruijn et al., 2021, Singh et al., 2019, Valerga et al., 2019, Valerga Puerta et al., 2021), however, these did not present evaluation of microbial adhesion associated with different PP methods. In the literature, there is a gap in this subject associated with the FFF technique; Dai et al. (2022) performed mechanical PP on resins for the base of prosthesis obtained by the digital light processing method (DLP) and observed a reduction in microbial adhesion (p = 0.0047) (Dai et al., 2022). The difference between the material and the printing technique makes comparisons with the results obtained in this study impossible, which showed no statistical differences between the control group and the PP techniques for the adhesion of S. mutans (p = 0.055) and C. albicans (p = 0.296).
S. mutans is a gram-positive, acidogenic bacterium, present in the oral cavity and able to survive in the bloodstream and cause, although rare, cardiac valve and pulmonary artery damage (Bhandari et al., 2019, Dai et al., 2022, Schubert et al., 2021). The adhesion process of S. mutans is linked to the expression of three genes (brpA, gbpB, comDE) that are responsible for the production of extracellular polysaccharides (EPS) promoting adhesion to surfaces (Dai et al., 2022), which is favored in pre-initiated biofilms by presenting co-aggregating characteristic and acidic environments (Bhandari et al., 2019, Dai et al., 2022).
C. albicans, is an opportunistic fungus present in the normal microbiota commonly found on mucosal membranes and can cause diseases, especially in immunosuppressed individuals, such as oral and systemic candidiasis, and prosthetic stomatitis in denture wearers (Krzyściak et al., 2014, Schubert et al., 2021). Its adhesion occurs by adhesins and because it is dimorphic, the initial adhesion occurs in its ovoid form and later passes to the form of hyphae (Krzyściak et al., 2014). The surface-associated factors such as roughness, hydrophobicity, and surface-free energy may favor the adhesion of S. mutans and C. albicans (Dai et al., 2022, Krzyściak et al., 2014, Schubert et al., 2021). Schubert et al (2021) observed that the adhesion of both microorganisms was not related to surface roughness but concluded that hydrophobicity is influential in promoting higher surface adhesion strength (Schubert et al., 2021).
Rough surfaces present a favorable environment for the initial adhesion of microorganisms because they present a larger contact area and mechanical retention, but Ra values lower than 0.2 μm do not influence microbial adhesion (Batak et al., n.d., de Bruijn et al., 2021, Hall et al., 2021, Kreve et al, n.d.). One of the major challenges of AM is the increased surface roughness. This creates favorable surface conditions for the adhesion of microorganisms (de Bruijn et al., 2021, Martin et al., 2021, Mu et al., 2020, Singh et al., 2019, Vidakis et al., 2020). In this study, the surface roughness of the control group was 11.3 μm without aging and 4.23 μm with aging, which proves the high roughness of the material obtained by the FFF method. Chemical, thermal, and mechanical PP techniques aim at reducing surface roughness (de Bruijn et al., 2021, Singh et al., 2019, Valerga et al., 2019, Valerga Puerta et al., 2021).
The chemical processing method reduced the roughness compared to the control and thermal groups, data also reported by Valerga et al. (2019), where PLA samples with chemical PP of immersion in CHCl3 showed a 97 % reduction in roughness (Valerga et al., 2019). This reduction can be explained by the loss of the volatile solvent from the surface of the material, which acquires a viscous appearance, and under the influence of gravity and surface tension, the irregularities were reduced, and the smoothness of the surface was increased (Castro-Casado, 2021, Valerga Puerta et al., 2021). Nevertheless, this reduction in surface roughness did not influence microbial adhesion, since it still showed values much higher than 0.2 μm, which may explain the lack of microbial reduction.
The mechanical PP acts from the removal by cutting or pressing the peaks on the surface of the specimens (de Bruijn et al., 2021, Valerga et al., 2019), which can contribute to a reduction in surface roughness, as observed in this study, where the group with mechanical PP was different after aging from the control group (p = 0.003) with lower roughness. Puerta et al. (2021) observed that mechanical PP reduced Ra surface roughness by 77 %, reducing the peaks on the surface of the samples (Valerga Puerta et al., 2021). Bruijm et al. (2021) concluded that the application of a standard force of 400 N influenced the reduction of roughness and smoothness up to 1 mm, which can be achieved in the internal layers (de Bruijn et al., 2021). The disadvantage of this method is that it is only used for objects with simple geometries because complex geometries do not fit the shape of the different types of mechanical PP, which would change their initial shape and make its use unfeasible. However, as with the chemical PP group, the roughness values were higher than 0.2 μm, which may explain the lack of reduction in microbial adhesion.
Annealing the sample is a technique for performing thermal PP, which reduces the residual thermal stresses between layers from the printing process and promotes the viscoelastic relaxation of the polymer with a consequent filling of the voids between layers and pores with reduced surface roughness (Gao et al., 2020, Singh et al., 2019). In this study, thermal PP did not reduce surface roughness statistically significantly, since this method increases the degree of crystallinity when performed at temperatures above the glass transition temperature (Tg) ranging from 55 to 65 °C, which can alter the mechanical properties such as flexural strength and roughness (Fernández-Grajera et al., 2022, Meng et al., 2012).
Hydrophobicity and surface-free energy are related to microbial adhesion through nonspecific bonding (Kreve et al, n.d., Schubert et al., 2021). Zhao et al. (2004) and Wassman et al. (2017) report that there is no consensus on which surface, hydrophobic or hydrophilic better inhibits microbial adhesion (Wassmann et al., 2017, Zhao et al., 2004), however, Kreve and Reis (2021) report that hydrophobic surfaces, such as titanium alloys, have a higher affinity for microbial adhesion (Kreve et al, n.d.). Waasman et al. (2017) further report that hydrophobic microorganisms such as S. mutans and C. albicans have a higher affinity to adhere to surfaces with similar properties (Wassmann et al., 2017).
PLA has a hydrophobic surface, with a contact angle (CA) of approximately 80°, which promotes a greater affinity for adhesion of equally hydrophobic microorganisms (Casalini et al., 2019, Hall et al., 2021). This CA can be influenced by the printing parameters inherent in the protocol applied for each experiment since the literature reports the influence of the following on the physical and mechanical properties of PLA (Brounstein et al., 2021, de Campos and dos Reis, 2023, de Campos et al., 2023, García Plaza et al., 2019). Vidakis et al. (2020) observed in the printed PLA specimens that the CA is altered depending on the roughness of the surface and the thickness of the layers, with results for 100 μm layers being 84.1° and 300 μm being 78.6° (Vidakis et al., 2020). In this study, mechanical PP reduced the CA of PLA, however, there was no correlation with microbial reduction, as observed by Dai et al. (2022) in which the reduction of CA made the surface more hydrophilic and reduced microbial adhesion in acrylic resin specimens printed with mechanical PP (Dai et al., 2022). What may differentiate the results of this study from those of Dai et al. (2022) is the type of material and processing techniques, as microbial adhesion depends on other factors such as roughness, porosity, and film thickness (Dai et al., 2022).
Despite the gap in the literature, a reduction of microorganisms on the surface of PLA samples obtained by AM is associated with the modification of the material, Brounstein, Yeager, and Labouriou (2021) incorporated into PLA titanium dioxide (TiO2) and Zinc Oxide (ZnO) and observed a reduction in the adhesion of microorganisms in the modified groups compared to pure PLA, with better results for the PLA\ZnO group (Brounstein et al., 2021). Vidakis et al. (2021) modified PLA with silicon dioxide (SiO2) at concentrations of 0.5 %, 1 %, 2 %, and 4 % and demonstrated zones of inhibition of Staphylococcus aureus and Escherichia coli mainly at the higher concentrations and for S. aureus the zone of inhibition was larger than that observed for E. coli (Vidakis et al., 2021). Despite the gap in the literature, a reduction of microorganisms on the surface of PLA samples obtained by AM is associated with the modification of the material, Brounstein, Yeager, and Labouriou (2021) [42] incorporated into PLA titanium dioxide (TiO2) and Zinc Oxide (ZnO) and observed a reduction in the adhesion of microorganisms in the modified groups compared to pure PLA, with better results for the PLA\ZnO group [42]. Vidakis et al. (2021) [43] modified PLA with silicon dioxide (SiO2) at concentrations of 0.5 %, 1 %, 2 %, and 4 % and demonstrated zones of inhibition of Staphylococcus aureus and Escherichia coli mainly at the higher concentrations and for S. aureus the zone of inhibition was larger than that observed for E. coli [43].
Fernández-Grajera et al. (2022) evaluated the influence of aging on microbial adhesion of PLA incorporated with cetyltrimethylammonium bromide (CTAB) and magnesium and observed that aging increased microbial adhesion on the surface after 3 months (p > 0.05) and further reported that there was no influence of aging on the hydrophobicity of the material (Fernández-Grajera et al., 2022), which differs from this study since aging reduced the control group's CA and surface roughness. Despite not having performed PP techniques, the results differ from those found in this study in which the adhesion of C. albicans (p = 0.563) and S. mutans (p = 0.813) did not change as a function of 6-month aging. The non-increase of these microorganisms even after aging shows that although there were no statistical differences between the control group and the different types of PP, the result was positive, especially when considering the clinical use of printed PLA as prosthetic components or dental prostheses, which will be subject to these differences in temperatures, different microorganisms, and forces during feeding in the oral cavity. However, clinical studies are needed to prove this hypothesis and evaluate these results more complexly.
Studies did not evaluate the reduction of microorganisms associated to PP methods with pure PLA, as was the objective of this study, which hinders the discussion of the results. Although this study has shown that there were no statistical differences in the adhesion of microorganisms under different types of PP, this opens up a point for discussion. Thus, despite the limitations of this study, one notes the relevance in the area of implementing in vitro techniques and assays to better explain microbial adhesion mechanisms and PP techniques.
5. Conclusion
The chemical, thermal, and mechanical PP techniques do not influence the adhesion of S. mutans and C. albicans. Chemical and mechanical PP reduce the surface roughness and mechanical PP reduces the hydrophobicity of the PLA obtained by AM using the FFF method. Thermal aging did not alter microbial adhesion of the PP methods evaluated and improved the surface roughness and hydrophobicity of the control group.
Ethical statement
It was not necessary Ethical statement for animals or people because this is an in vitro study.
The place of research
Ribeirão Preto Dental School (FORP-USP), University of São Paulo, São Paulo, Brazil e foi adicionado na title page.
Funding
None.
CRediT authorship contribution statement
Izabela Ferreira: Writing – review & editing, Conceptualization, Formal analysis, Investigation, Methodology. Murilo Rodrigues de Campos: Conceptualization, Formal analysis, Investigation, Methodology. Beatriz Danieletto Sahm: Methodology, Formal analysis, Investigation. Mariana Lima da Costa Valente: Writing – review & editing, Conceptualization, Formal analysis, Investigation, Methodology. José Augusto Marcondes Agnelli: Conceptualization, Writing – review & editing. Andréa Cândido dos Reis: Conceptualization, Methodology, Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank the laboratories of the School of Dentistry of Ribeirão Preto, FORP-USP.
References
- Abdul Samat A., Abdul Hamid Z.A., Jaafar M., Yahaya B.H. Mechanical properties and in vitro evaluation of thermoplastic polyurethane and polylactic acid blend for fabrication of 3d filaments for tracheal tissue engineering. Polymers (basel) 2021;13 doi: 10.3390/polym13183087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Rashid A., Koç M. Fused filament fabrication process: A review of numerical simulation techniques. Polymers (basel) 2021;13 doi: 10.3390/polym13203534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alp, G., Johnston, W.M., Yilmaz, B., n.d. Optical properties and surface roughness of prepolymerized poly(methyl methacrylate) denture base materials. [DOI] [PubMed]
- Amza C.G., Zapciu A., Constantin G., Baciu F., Vasile M.I. Enhancing mechanical properties of polymer 3D printed parts. Polymers (basel) 2021;13:1–18. doi: 10.3390/polym13040562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrasa J.O., Ferrández-Montero A., Ferrari B., Pastor J.Y. Characterisation and modelling of pla filaments and evolution with time. Polymers (basel) 2021;13 doi: 10.3390/polym13172899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batak, B., Çakmak, G., Johnston, W.M., Yilmaz, B., n.d. Surface roughness of high-performance polymers used for fixed implant-supported prostheses. [DOI] [PubMed]
- Bechtel S., Schweitzer R., Frey M., Busch R., Herrmann H.G. Material extrusion of structural polymer-aluminum joints—examining shear strength, wetting, polymer melt rheology and aging. Materials. 2022;15 doi: 10.3390/ma15093120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergaliyeva S., Sales D.L., Delgado F.J., Bolegenova S., Molina S.I. Effect of thermal and hydrothermal accelerated aging on 3D printed polylactic acid. Polymers (basel) 2022;14 doi: 10.3390/polym14235256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari S., Lopez-Anido R.A., Gardner D.J. Enhancing the interlayer tensile strength of 3D printed short carbon fiber reinforced PETG and PLA composites via annealing. Addit. Manuf. 2019;30 doi: 10.1016/j.addma.2019.100922. [DOI] [Google Scholar]
- Brounstein Z., Yeager C.M., Labouriau A. Development of antimicrobial PLA composites for fused filament fabrication. Polymers (basel) 2021;13:1–18. doi: 10.3390/polym13040580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busscher H.J., Rinastiti M., Siswomihardjo W., Van Der Mei H.C. Biofilm formation on dental restorative and implant materials. J. Dent. Res. 2010 doi: 10.1177/0022034510368644. [DOI] [PubMed] [Google Scholar]
- Casalini T., Rossi F., Castrovinci A., Perale G. A Perspective on polylactic acid-based polymers use for nanoparticles synthesis and applications. Front. Bioeng. Biotechnol. 2019 doi: 10.3389/fbioe.2019.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Casado D. Chemical treatments to enhance surface quality of FFF manufactured parts: a systematic review. Prog. Add. Manuf. 2021 doi: 10.1007/s40964-020-00163-1. [DOI] [Google Scholar]
- Chen X., Gao C., Jiang J., Wu Y., Zhu P., Chen G. 3D printed porous PLA/nHA composite scaffolds with enhanced osteogenesis and osteoconductivityin vivo for bone regeneration. Biomed. Mater. (bristol) 2019;14 doi: 10.1088/1748-605X/ab388d. [DOI] [PubMed] [Google Scholar]
- Dai J., Luo K., Spintzyk S., Unkovskiy A., Li P., Xu S., Fernandez P.K. Post-processing of DLP-printed denture base polymer: Impact of a protective coating on the surface characteristics, flexural properties, cytotoxicity, and microbial adhesion. Dent. Mater. 2022;38:2062–2072. doi: 10.1016/j.dental.2022.11.008. [DOI] [PubMed] [Google Scholar]
- de Bruijn A.C., Gómez-Gras G., Pérez M.A. A comparative analysis of chemical, thermal, and mechanical post-process of fused filament fabricated polyetherimide parts for surface quality enhancement. Materials. 2021;14 doi: 10.3390/ma14195880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Campos M.R., dos Reis A.C. Effect of post-processing on the mechanical properties of polymers printed by the fused filament fabrication method used as prosthodontic materials and dental biomaterials: a systematic review. Polym. Bull. 2023 doi: 10.1007/s00289-023-04816-3. [DOI] [Google Scholar]
- de Campos M.R., Kreve S., da Silva G.G., da Costa Valente M.L., dos Reis A.C. Mechanical and microstructural analysis of a new model of attachments for overdentures retained by mini-implants obtained by 3D printing with three different polymers. Polym. Bull. 2023 doi: 10.1007/s00289-023-04871-w. [DOI] [Google Scholar]
- Fernández-Grajera M., Gallardo-Moreno A.M., Luque-Agudo V., González-Martín M.L., Hierro-Oliva M. Bacterial response to the surface aging of PLA matrices loaded with active compounds. Polymers (basel) 2022;14 doi: 10.3390/polym14224976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P., Fan B., Yu X., Liu W., Wu J., Shi L., Yang D., Tan L., Wan P., Hao Y., Li S., Hou W., Yang K., Li X., Guo Z. Biofunctional magnesium coated Ti6Al4V scaffold enhances osteogenesis and angiogenesis in vitro and in vivo for orthopedic application. Bioact. Mater. 2020;5:680–693. doi: 10.1016/j.bioactmat.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García Plaza E., Núñez López P.J., Caminero Torija M.Á., Chacón Muñoz J.M. Analysis of PLA geometric properties processed by FFF additive manufacturing: Effects of process parameters and plate-extruder precision motion. Polymers (basel) 2019;11 doi: 10.3390/polym11101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D.C., Palmer P., Ji H.F., Ehrlich G.D., Król J.E. Bacterial biofilm growth on 3D-printed materials. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.646303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A., Hickel R., Reymus M. 3D printing in dentistry-state of the art. Oper. Dent. 2020;45:30–40. doi: 10.2341/18-229-L. [DOI] [PubMed] [Google Scholar]
- Kreve, S., Cândido, A., Reis, D., n.d. Influence of the electrostatic condition of the titanium surface on bacterial adhesion: A systematic review. [DOI] [PubMed]
- Krzyściak W., Jurczak A., Kościelniak D., Bystrowska B., Skalniak A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014 doi: 10.1007/s10096-013-1993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H., Kavanagh K., Velasco-Torrijos T. Targeting adhesion in fungal pathogen Candida albicans. Future Med. Chem. 2021 doi: 10.4155/fmc-2020-0052. [DOI] [PubMed] [Google Scholar]
- Mei L., Busscher H.J., Van Der Mei H.C., Ren Y. Influence of surface roughness on streptococcal adhesion forces to composite resins. Dent. Mater. 2011;27:770–778. doi: 10.1016/j.dental.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Meng B., Deng J., Liu Q., Wu Z., Yang W. Transparent and ductile poly(lactic acid)/poly(butyl acrylate) (PBA) blends: Structure and properties. Eur. Polym. J. 2012;48:127–135. doi: 10.1016/j.eurpolymj.2011.10.009. [DOI] [Google Scholar]
- Mu M., Ou C.Y., Wang J., Liu Y. Surface modification of prototypes in fused filament fabrication using chemical vapour smoothing. Addit. Manuf. 2020;31 doi: 10.1016/j.addma.2019.100972. [DOI] [Google Scholar]
- Schubert A., Bürgers R., Baum F., Kurbad O., Wassmann T. Influence of the manufacturing method on the adhesion of candida albicans and streptococcus mutans to oral splint resins. Polymers (basel) 2021;13 doi: 10.3390/polym13101534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Singh M., Prakash C., Gupta M.K., Mia M., Singh R. Optimization and reliability analysis to improve surface quality and mechanical characteristics of heat-treated fused filament fabricated parts. Int. J. Adv. Manuf. Technol. 2019;102:1521–1536. doi: 10.1007/s00170-018-03276-8. [DOI] [Google Scholar]
- Song F., Koo H., Ren D. Effects of material properties on bacterial adhesion and biofilm formation. J. Dent. Res. 2015 doi: 10.1177/0022034515587690. [DOI] [PubMed] [Google Scholar]
- Valerga A.P., Batista M., Fernandez-Vidal S.R., Gamez A.J. Impact of chemical post-processing in fused deposition modelling (FDM) on polylactic acid (PLA) surface quality and structure. Polymers (basel) 2019;11 doi: 10.3390/polym11030566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerga Puerta A.P., Lopez-Castro J.D., Ojeda López A., Fernández Vidal S.R. On improving the surface finish of 3D printing polylactic acid parts by corundum blasting. Rapid Prototyp. J. 2021;27:1398–1407. doi: 10.1108/RPJ-05-2021-0105. [DOI] [Google Scholar]
- Valvez S., Reis P.N.B., Susmel L., Berto F. Fused filament fabrication-4d-printed shape memory polymers: A review. Polymers (basel) 2021 doi: 10.3390/polym13050701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeeten W.M.H., Lorenzo-Bañuelos M., Arribas-Subiñas P.J. Anisotropic rate-dependent mechanical behavior of Poly(Lactic Acid) processed by material extrusion additive manufacturing. Addit. Manuf. 2020;31 doi: 10.1016/j.addma.2019.100968. [DOI] [Google Scholar]
- Vidakis N., Petousis M., Velidakis E., Liebscher M., Tzounis L. Three-dimensional printed antimicrobial objects of polylactic acid (PLA)-silver nanoparticle nanocomposite filaments produced by an in-situ reduction reactive melt mixing process. Biomimetics. 2020;5 doi: 10.3390/BIOMIMETICS5030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidakis N., Petousis M., Velidakis E., Mountakis N., Tzounis L., Liebscher M., Grammatikos S.A. Enhanced mechanical, thermal and antimicrobial properties of additively manufactured polylactic acid with optimized nano silica content. Nanomaterials. 2021;11 doi: 10.3390/nano11041012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassmann T., Kreis S., Behr M., Buergers R. The influence of surface texture and wettability on initial bacterial adhesion on titanium and zirconium oxide dental implants. Int. J. Implant Dent. 2017;3 doi: 10.1186/s40729-017-0093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Wang S., Müller-Steinhagen H. Tailored surface free energy of membrane diffusers to minimize microbial adhesion. Appl. Surf. Sci. 2004;230:371–378. doi: 10.1016/j.apsusc.2004.02.052. [DOI] [Google Scholar]