Learning objectives.
By reading this article, you should be able to:
-
•
Discuss the technological progress that facilitated the development of perioperative three-dimensional transoesophageal echocardiography (3D-TOE).
-
•
Illustrate the technical principles that govern the process of 3D image generation in echocardiography.
-
•
Describe the technical limitations of 3D-TOE.
-
•
Anticipate the future developments of 3D echocardiography and their clinical impact.
Key points.
-
•
Three-dimensional transoesophageal echocardiography (3D-TOE) is a mature technology with multiple perioperative applications.
-
•
Three-dimensional imaging uses volume-based rather than sector-based scanning (i.e. two-dimensional imaging).
-
•
Three-dimensional image generation relies on the acquisition of a dataset made of multiple two-dimensional ‘slices’ of a predefined volume by using matrix array transducers.
-
•
Processing of the dataset is a critical multistep process that allows the analytical interrogation and the display of the structure of interest in 3D.
Background
Since its introduction into clinical practice by Matsumoto, over 40 yrs ago, transoesophageal echocardiography (TOE) has advanced constantly, becoming an essential part of perioperative care.1 Currently, TOE has a role in all open-heart, elective coronary artery bypass graft (CABG) and thoracic aorta surgical procedures.2, 3, 4
Three-dimensional (3D) TOE has developed and matured as a technology over the past 30 yrs, offering high-quality real-time (RT) imaging. The contemporary technology has become easier to use often with single-button acquisition and streamlined workflows. It can present large datasets with better temporal resolution and is capable of advanced on-line multiplane display. In addition, quantitative assessment of cardiac structures is possible through semi-automated parametric models. Clinically, 3D-TOE contributes actively to perioperative monitoring and diagnosis, informing surgical decision-making and detecting complications with proven utility in interventional cardiology and cardiac surgery.
This article aims to provide an up-to-date review of the basic science of 3D-TOE imaging highlighting the technical requirements, limitations, challenges and future developments. In a forthcoming accompanying article we address the clinical applications of 3D-TOE. The term ‘3D’ characterises both still images and video clips, although from a physics concept, the latter represents 4D technology with the fourth dimension representing time.
Three-dimensional imaging evolution
The idea of 3D echocardiographic imaging originated in the 1960s, but a technological lag delayed any meaningful results until 1974, when Dekker developed the first transthoracic echocardiography (TTE) 3D system.5 A mechanical articulated arm capable of measuring spatial probe displacement recorded multiple 2D images by free-hand movement.
Historically, 3D image generation used off-line post-processing reconstruction techniques of 2D images obtained by either linear sequential multiplane scanning or gated (ECG or respiratory) acquisition of different cut-planes (Supplementary Fig. S1).6 Here, gating refers to ECG (i.e. R–R interval) or respiratory cycle monitoring with image acquisition during certain moments of the cycle, allowing for consistency (i.e. recording of a structure from multiple angles/position, at the same cycle phase over sequential cycles) and lack of motion artefacts. The raw 2D images were obtained by using either TTE freehand scanning, TTE/TOE mechanically driven transducers or multiplane TOE probes (capable of electronically rotated 2D imaging acquisition over a 180° arc).7 In 1991, there was sequential linear acquisition in a TEE probe, called the ‘lobster tail’, where a motorised mechanism in the probe handle activated a linear array of crystals, recording multiple parallel 2D ECG/respiration gated sectors at 1 mm increments.8 A TEE probe using rotational scanning was an alternative proposed 2 yrs later. The probe remained in a fixed position while the imaging plane rotated by pivoting around a fixed axis similar to the transducer of modern TOE probes.8 Motion artefacts, gating adequacy and the number of 2D images affected the 3D image quality.
The next developments in RT3D ultrasound (US) systems occurred 20 yrs later.7,9 The fundamental challenge was the physical limitation of the fixed speed of sound in tissues. Overcoming this required the development of a transducer capable of volumetric acquisition and computing capacity to process a large amount of data, to present in an understandable 3D format for timely interpretation. This obliged engineering improvements in computational power and US probe design and technology.
Von Ramm pioneered RT3D acquisition in the early 1990s by developing a matrix TTE transducer with 256 non-simultaneous firing (sparse) piezoelectric crystals capable of acquiring a 60°×60° pyramid volume in one heartbeat.7,9 These first-generation RT3D probes had poor spatial and temporal resolution, with image display of 2D cut planes from the original 3D dataset rather than a volume rendered 3D image. In 2006, the first RT3D-TOE probe became available.8
Contemporary RT3D-TOE systems have benefited from the technological advances that enabled miniaturisation of transducer crystals and circuitry. The availability of more powerful central processing units (CPUs), graphic processing units, digital signal processors and other essential components fuelled the transition from hardware to software-based architectures. A contemporary US machine generates RT3D images by relying on a powerful computer with multicore CPU architecture capable of larger memory and processing speed, which backs up a complex software package that performs tasks historically executed by analogue hardware. The current 3D-TOE systems can achieve volume rates two to three times higher (i.e. >60 Hz when using multi-beat acquisition) than their predecessors, with significantly improved spatial resolution.10,11
Three-dimensional probe technology
The major difference between 2D and 3D-TOE imaging is that the latter uses volume-based rather than sector-based scanning. In 2D sector-based scanning, the transducer contains up to 128 piezoelectric crystals arranged in a single row, capable of acquiring and displaying one 2D image (slice) at a time. The modern 3D transducers have a similar footprint with a matrix design (i.e. x-y distribution) containing >2500–3000 fully sampled crystals, capable of generating a sound wave that is steerable in multiple directions (Fig. 1).13, 14, 15
Fig 1.
Transoesophageal echocardiography transducers. (A) A comparison between a 64-crystal linear array in a 2D TOE probe and (B) 2500 crystals in a matrix array 3D TOE probe. Images courtesy of Michael Corrin. (C) Microscopy image displaying the piezoelectric crystals in a matrix array compared with a human hair (with permission).7 (D) A schematic representation of the matrix array transducer shows the arrangement of thousands of piezoelectric elements in the azimuthal and elevation directions (with permission).12 Electronic activation of individual piezoelectric elements allows the acquisition of a volumetric dataset.
Beamforming is a signal processing technique where electrical signals are manipulated to generate directionally/spatially oriented US beams by the piezoelectric crystals. In addition, the beamformer handles the electrical signals (and their transmission to the US system) occurring in the crystals as a result of the returning echoes, also.16 In modern 3D-TOE systems, beamforming occurs both in the probe as fine (micro) beamforming and on the US machine computer system as coarse beamforming. This approach ensures a small probe and thin cable with less power consumption.15 There is a complex proprietary algorithm for each vendor that processes the emitting and the returning US signal.
In addition, by activating only certain lines of crystals, it is possible to have a simultaneous display of multiple 2D images in real time (i.e. biplane for two images and triplane for three images) (Fig. 2). Modern 3D-TOE probes operate at 2–8 MHz frequency and can perform all the functions of a 2D-TOE probe, including M-mode, spectral and colour flow Doppler (CFD) interrogation and speckle tracking.
Fig 2.
Multiplane imaging. The 3D matrix array probe permits simultaneous multiplane 2D imaging. (A) Illustration of the heart showing triplane imaging with the three 2D planes at 0°, 60° and 120°. (B) The corresponding three 2D images from the mid-oesophageal 0°, 60° and 120° appear simultaneously in a single display. Courtesy of Toronto General Hospital Department of Anaesthesia, Perioperative Interactive Education website (https://pie.med.utoronto.ca). (C) Biplane imaging (xPlane) with independent plane control shows two 2D images on a single display. One plane is fixed (the base view, left image) while the secondary plane (right image) can be adjusted (by moving the blue triangular marker in the left image to the right or left) so that it can show multiple orthogonal slices of the ventricle. Although in this example the secondary plane is orthogonal, its angle can be tilted between 0° and 180° in relation to the base plane.
3D Image generation
Ultrasound imaging relies on emitting acoustic pulses through tissue, followed by recording and processing the returning echoes. The goal is to represent accurately the anatomy and movement of structures. Thus, 3D image formation follows four steps: data acquisition, data storage, data processing and image display.
Data acquisition
Processing of the returning raw US signal based on timing and amplitude creates a 3D dataset of the sampled volume. Current technology presents all 3D datasets as RT3D images that move on the display as the probe moves.
The ideal 3D dataset should have a high spatial resolution to represent anatomy and temporal resolution for movement in a large volume, although in practice, these factors compete, as shown in the equation below. Temporal resolution for 3D echo is actually the volume rate in volumes per second (VPS), although frame rate (FR) often appears on the display. The spatial (number of scan lines) and temporal resolution (volume rate) are inversely proportional to the 3D volume size.
Three-dimensional datasets are acquired as pyramidal volumes, with the apex at the probe, and the base most distant. The speed of sound in tissues is a major challenge for 3D imaging. As this is relatively constant (1540 m s−1), for the same depth, a 3D volume requires more scanning time than a 2D slice of the same depth, resulting in a lower volume rate. For example, scanning a volume of 60°×60° with a depth of 16 cm would achieve a volume rate of ∼1.3 Hz.15 In contrast, at 12 cm depth, a 2D image with 90 scan lines can achieve a 62 Hz FR.14
Limiting the size of the pyramid improves temporal resolution. To avoid interference, a US pulse needs to travel the depth of the 3D volume twice before the next one can be sent. For a 10 cm depth, the pulse requires 130 μs for this round trip, resulting in a maximum pulse repetition frequency (PRF) of 7.7 Hz. If the depth is halved, a maximum PRF of 15.4 Hz can be achieved. However, decreasing the depth beyond a certain limit could cause excess reverberation and distortion of the image if the PRF is not adjusted.15 Hence, the US system automatically limits the PRF when the depth is set below a certain limit.15 A high FR is essential for accurately depicting fast moving objects in the heart.
Modern 3D US systems use electronic non-operator-controlled features such as parallel beamforming and operator-controlled features such as multi-beat ECG-gated acquisition (Supplementary Fig. S2) and adjustable sampling volume to increase the volume rate.15
Parallel beamforming (Supplementary Fig. S3) increases the volume rate by sending one wide US beam, followed by using multiple crystals to receive the reflected sound wave in parallel. This increases the volume rate by a factor equal to the number of scan lines received.16 This technique has limits as the decrease in the signal-to-noise ratio reduces the overall spatial resolution.16
ECG gating to the R wave over multiple beats and stitching together each volume creates large 3D datasets with a better temporal resolution. An alternative is to use the high-volume rate (HVR) imaging mode, which by using interpolation (see below) permits single beat capture (often without an ECG) improving temporal resolution at the expense of spatial resolution (decrease in line density).
3D acquisition modes
There are four different acquisition modes (Table 1, Fig. 3) with vendor-specific names that generate a pyramidal volume defined by three dimensions: axial plane (x-plane), azimuth or lateral plane (y-plane) and elevational plane (z-plane). All modes appear in real time, are easily obtainable, and can be a single beat or ECG gated acquisition. The 3D volume size can vary in dimensions using adjustable sizing to suit the operator's needs. Choosing the mode depends on the user's need and intention, with a compromise between volume size (spatial resolution) and temporal resolution.
Table 1.
Standard 3D acquisition modes. Modified from Vegas and colleagues11 and Vieira and Ronderos.17 IAS, interatrial septum; IVS, interventricular septum; LAA, left atrial appendage; LV, left ventricle; ROI, region of interest; RV, right ventricle.
| Live | Zoom | Full volume | CFD | |
|---|---|---|---|---|
| Dimension | 30° × 60°× depth | 20° × 20° × 90° × 90° × adjustable height | 90° × 90° × depth | Depends on the acquisition mode |
| Volume | Small size | Mid size | Large size | Variable |
| RT | Yes | Yes | Yes | Yes |
| FR | 20–30 Hz | 5–20 Hz | 20–50 Hz | < 20 Hz |
| TR | Good | Low | Good | Low |
|
SR |
Mid |
High |
Low |
Low |
|
Features | ||||
| Live mode | Used to:
It is not useful to assess anatomic relationships or for quantification. |
|||
| Zoom mode | Consider as an extension of 3D live mode. It displays a magnified subsection containing the ROI. Image acquisition is a multi-step process:
Due to a limited pyramid size less useful to assess anatomic relationships. |
|||
| Full volume | Due to large pyramid size it has poor SR and TR when used in RT (ie. single beat). Often used as multi-beat acquisition. Useful to quantify LV/RV function and whole heart visualization. Narrow near field – poor SR of the structures close to the probe. |
|||
| CFD | It decreases the temporal resolution of the primary mode. The Nyquist limit should be adjusted according to the expected flow velocity. It gives information on blood flow velocity and volume allowing a more sensitive quantification of regurgitation areas and volumes for example. |
|||
Fig 3.
Three-dimensional imaging acquisition modes. A 3D structure is displayed with an illustration of the 3D volume in relation to the heart for (A–C). (A) A live mode acquisition of aortic valve. (B) Zoom mode acquisition of the mitral valve. (C) Full volume acquisition of the left ventricle. (D) Colour flow Doppler imposed over the mitral and tricuspid valves, depicting in blue the blood inflow. Courtesy of Toronto General Hospital Department of Anaesthesia, Perioperative Interactive Education website (https://pie.med.utoronto.ca).
Data storage and processing
Data storage is an important step in the workflow of displaying a 3D image. After optimisation, there is temporary storage of the predefined 3D dataset volume on the random access memory to enable the automatic processes for 3D volume reconstruction: conversion, interpolation and segmentation. During conversion, there is a translation of scanned raw data points into an x-y-z Cartesian system, establishing their position in space. Interpolation mathematically compensates for the potential missing points to generate a full 3D volume made of voxels. The voxel is the 3D correspondent of a pixel, which is the smallest square unit of a 2D digital image for individual display. Hence, a voxel is a 'cubic pixel’, which contains the location and physical characteristic of data points in space. The voxel size is inversely proportional to the 3D image resolution. Smaller voxels contribute to better 3D spatial resolution as less interpolation is necessary. Segmentation uses software filtering to differentiate cardiac tissue from blood based on echo density and eliminates data outside the set thresholds. These steps take place in real time while scanning and moving the probe.
Data display
Rendering is a graphics term for generating an image from a dataset (2D or 3D) of multiple spatial elements. Graphic algorithms simulate effects on the summation of voxels, making a 3D image appear on a 2D display. The main rendering formats of a 3D dataset are wire-frame, surface and volume rendering (Supplementary Table S1).
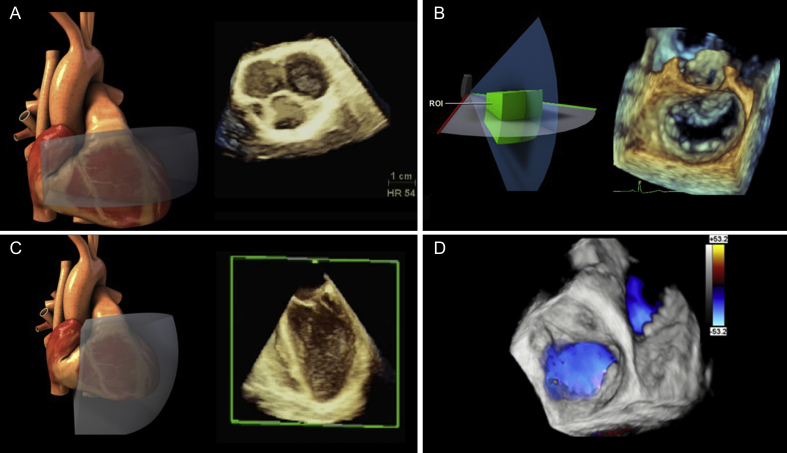
Conventional 3D rendering uses shading techniques to encode voxels based on their distance, grey-level gradient, and texture to generate a display of cardiac structures in 3D. Image enhancement is a vendor-specific software-based process that augments the 3D image, facilitating additional visual details to improve 3D perception (Supplementary Table S1). One simple technique, called chroma mapping, imposes a specific colour scheme on to 3D images enhancing certain structures (e.g. tumour and thrombi) (Supplementary Fig. S4). More advanced software can add a virtual light source to provide 3D photorealistic rendering that simulates the true texture of cardiac tissue. (e.g. TrueVue; Phillips Medical Systems, MA, USA and FlexiLight; GE HealthCare, IL, USA) (Supplementary Fig. S5).18 This significantly improves the depth perception and the ability to show abnormal anatomic structures.17,19 In addition, transparency/translucency (GlassVue; Phillips Medical Systems) is another computer-generated effect, meant to improve the diagnostic power of a 3D dataset, particularly when combined with CFD for detecting abnormal blood flow (Supplementary Fig. S5).
Post-processing
Once the 3D image appears on the display, it can be stored on the US machine for further processing (‘post-processing’). Quantitative analysis of the stored 3D dataset may occur online on the US machine or offline at a workstation. All modern 3D US machines have fully or semi-automated software packages that perform advanced interrogation and data extraction from the 3D dataset. Off-line third party software may provide more detailed analysis intended for future intervention planning. Post-processing is crucial to unlocking the information available for clinical applications. Common concepts and terminology relevant to 3D dataset processing appear below.
Guidelines for display
Guidelines exist for standardising the manipulation of 3D datasets and the display of cardiac structures three-dimensionally (see forthcoming Part 2 of this article).13 This is mainly relevant for cardiac valves, which often shows the complete valve ‘en face’ from perspectives that are above or below the valve. Rotation of the 3D dataset can orientate the valve to show the surgeon's perspective. Including a portion of a surrounding structure, such as the left atrial appendage (LAA) or aortic valve (AoV), can help quickly and reliably orientate a 3D dataset. The 2020 American Society of Echocardiography/Society of Cardiovascular Anaesthesiologists and the Society of Thoracic Surgeons guidelines for surgical decision-making in the operating room, though primarily based on 2D echo, suggest complimentary 3D images in certain situations, such as left ventricular assist device (LVAD) and valvular surgeries.3 The part 2 paper presents a detailed discussion of RT3D TEE clinical applications and available guidelines.
Cropping and slicing
Cropping (occasionally called slicing) is a processing step that enhances visualisation of the desired cardiac region of interest (ROI) in the original 3D dataset. Cropping can occur before or after dataset acquisition. A dataset cropped before storing improves temporal and spatial resolution but cannot be ‘uncropped’, as the redundant data of the original volume has been removed. Without cropping, a rendered 3D dataset appears as a solid pyramid. Cropping ‘breaks open’ this pyramid, removing irrelevant regions, by using planes (i.e. arbitrary crop), boxes (X, Y, Z orthogonal planes), auto or semi-automated options (depending on vendor) (Supplementary Table S2). It is advisable to first orientate the structure on the display using adjacent structures in the dataset because it is possible to lose spatial orientation after cropping the surrounding structures. Newer software includes presets that automatically show the mitral valve (MV), AoV and LAA from mid-oesophageal two-chamber or four-chamber captures. Another option using vendor-specific software permits multiple predefined parallel slices through the dataset. The display is similar to computed tomography (CT) scan cuts with multiple 2D images on a single screen and is very useful for evaluating left ventricular function and the MV (Supplementary Fig. S1 online video).
Multi-planar reconstruction and measurements
A major drawback in 3D imaging is the inability to perform accurate measurements directly on 3D datasets. Parametric models and multi-planar reconstruction (MPR) of 3D datasets overcome this limitation. Multi-planar reconstruction shows certain regions of the dataset based on three 2D planes (coronal, sagittal and transverse) (Supplementary Fig. S6). The planes can move independently, and are aligned through a portion of the image allowing interrogation of any ROI (e.g. valve leaflet or annulus length, area of vena contracta). Although time-consuming, using MPR is flexible by allowing slice by slice (tomographic) control of data points from a stored 3D dataset. Volume rendered images can also appear alongside the MPR reconstructions derived from the original dataset.
Newer enhancements of live MPR automation combine cropping, MPR alignment and tissue edge detection in 3D display, which is time-saving and important in echo-guided structural heart interventions.20
Valve modelling and analysis
Vendor-specific semi-automated software can analyse 3D datasets to provide static and dynamic 3D parametric models for the ventricles, MV and AoV (see in part 2 paper). The models provide robust data that can better assess pathology and aid the surgeon in surgical planning. Most software is available on the US machines permitting timely processing of the data, though stand-alone third party software can also provide off-line analysis. Part 2 of this series will describe the clinical applications of these models.
Three-dimensional image optimisation
Good 3D dataset acquisition (Fig. 4) starts with a good 2D image with optimised gain, compression, brightness, depth and centring of the ROI perpendicular to the US beam. The overall 2D gain and the 2D compression setting should be in the mid-range, with a slight increase in the time gain compensation (TGC). Two important determinants of the 3D image quality are the line density (the number of lines per unit of volume) for spatial resolution and transducer frequency for temporal and spatial resolution. Ultrasound frequency (f) and wavelength (λ) are determinants of the speed of sound (c) (i.e. c=f×λ). For a standard soft tissue US propagation speed of 1540 m s−1, a 5 MHz probe will achieve a wavelength of ∼0.3 mm. The axial resolution, specifically, is ∼1.5 times the system's wavelength. By increasing the probe frequency to 10 MHz, the resultant wavelength will be 0.15 mm, thus improving the overall spatial resolution. As probes capable of higher operating frequencies are generating smaller wavelengths (i.e. shorter pulse repetition intervals), they achieve a higher PRF, leading to better temporal resolution. The echocardiographer can adjust the size of the acquisition volume and change the transducer frequency by selecting predefined options (i.e. control the frequency bandwidth) of (vendor-specific) penetration (low frequency), general (mid frequency), resolution (high frequency).
Fig 4.
Three-dimensional dataset acquisition workflow.
Analogous to 2D imaging adjusting 3D gain, 3D brightness and 3D compression can alter the appearance of the rendered 3D dataset (Supplementary Fig. S7). Adjusting the 3D gain will affect the depth, size, thickness and location of structures imaged with 3D. Specific 3D processing options include smoothing for filtering noise, colourisation using predefined chroma maps and vision that alter spatial filtering and depth perception. Image optimisation in post-processing (i.e. on a stored dataset) is possible for gain, brightness and smoothing. It can compensate for a low- or high-power gain but not for a poorly optimised TGC gain.13
Three-dimensional artefacts
Of note, most artefacts visible on a 2D image will appear on the corresponding 3D image.21 Le and colleagues published a thorough review of 2D artefacts in echocardiography and potential solutions.22 Specifically, to 3D artefacts, Faletra and colleagues discussed this topic extensively offering explanation and solutions (Supplementary Table S3).21 Multi-beat acquisition is prone to ‘stitching’ artefacts. Here, the system registers the sub-volumes improperly in time from variations in the R–R interval of the ECG related to arrythmia or diathermy interference, or in space from significant probe movement. Gating artefacts are most prominent when the sweeping acquisition is perpendicular to the reference plane.13 During intraoperative 3D-TOE, temporarily discontinuing mechanical ventilation can suppress gating artefacts from breathing. Dropout artefact appears as a lack of tissue resulting from a poorly centred structure, shadowing from surrounding structures, insufficient gain or US beam attenuation. These can cause the misdiagnosis of holes in structures. Increasing the gain or imaging perpendicular to the structure can resolve these artefacts. Excessive 3D gain appears as brown speckling on the 3D image, decreasing resolution, depth perception and obscuring structures.
Technical limitations
As with 2D echocardiography, image quality with adequate acoustic windows requires patient cooperation with minimal arrhythmias, breathing and movement to avoid artefacts. Spatial resolution relies on good 2D image quality by imaging perpendicular to structures and acquiring small datasets needing less interpolation. Poor spatial resolution prevents automatic endocardial border delineation for automated volume quantification. Poor temporal resolution with large volume datasets and CFD may require multi-beat acquisitions, which are limited by stitching artefacts. Low temporal resolution reduces the number of images available for assessment, which is a significant limitation when quantifying flow or performing chamber quantification.
There is a learning curve to mastering 3D imaging. Time constraints and the burden on the intraoperative workflow negatively affect the image quality. Practice guidelines require a full 2D examination, with a focused 3D exam on the ROI.23 The reprieve from technical advancement is that workflow has improved 3D image quality, user interface and the addition of semi-automation or pre-programmed presets for MV and left ventricular chambers requiring minimal clicks for baseline image acquisition.24
Future developments
Cardiac 5D echocardiography, pioneered by Samsung Medison (Seoul, South Korea), is a volumetric echocardiography concept that heavily relies on automatic software processing to deliver sharp high-definition live images.25 Currently available for transabdominal fetal medicine clinical applications, it is expected to be integrated to TOE. The fifth dimension in this concept represents the full automation of data processing to produce higher standard images than the current 3D technology.
Three-dimensional ultrafast imaging relies on progress in the computation power of graphic processing units. Instead of using focused beams that create one scan line at a time, ultrafast imaging uses multiplane waves at various angles that reconstruct one full image in one emission. The typical US systems have 2D imaging FRs of 50–200 Hz, whereas an ultrafast 2D system can achieve 20,000 Hz. By comparison with a standard 3D system which samples up to a few tens of volumes per second, an ultrafast system can sample 2325 volumes per second at a depth of 12 cm.26,27 The implications in clinical practice are that one 3D ultrafast acquisition could depict the anatomy of all four cardiac chambers. This can facilitate quantification of ventricular wall motion in all segments, strain and ejection fraction (EF). Also, flow quantification by pulse, colour and tissue Doppler can occur for each voxel, allowing the simultaneous assessment of all four valves. In addition, this technique has less angle dependency, reducing out-of-plane motion.28
Automation and artificial intelligence (AI) have made considerable progress in healthcare, with multiple clinical applications, and will mark the future of 3D echocardiography.27 Current 2D AI applications are facilitating fewer human–machine interactions during measurements by using automatic algorithms for functional (e.g. strain/speckle tracking, EF, regional wall movement etc), linear, Doppler and complex modelling. Similar improvements are expected to become more common in RT3D echocardiography. Automated tools for chamber quantification and valve modelling are already available. Artificial intelligence in 3D echo imaging, dobutamine stress echo and myocardial perfusion could help the diagnosis and assessment of coronary artery disease.27 The recent approval by the Federal Drug Administration (FDA) in the United States of an AI software that acquires diagnostic quality 2D-TTE images, opens the door to integration with TOE.29 Such a system could facilitate acquisition of diagnostic quality 3D datasets by the healthcare provider with less 3D-TOE experience by assisting with image acquisition, automatised settings and processing, identification of target structures, diagnosis, reporting and prognosis.30 In addition, from an education perspective, AI integration could expand the abilities of current 3D-TOE simulators by providing training feedback and assessment of competencies.
As transducer technology evolves towards higher volume rates, 3D echo datasets could be a source for 3D printing and virtual reality/holography, instead of CT and MRI datasets.27,31 Although small feasibility studies have confirmed the clinical role and safety of RT3D-TOE-derived stereoscopic images, this technology is still nascent and relies on proprietary third party hardware/software and the RT3D US system.31 Automation, AI and machine learning could make this technique more accessible, allowing immersive interaction with the structures visualised for diagnosis and procedural planning, in the busy environment of the cardiac surgery theatre.
Conclusions
Overall, 3D-TOE is a highly attractive imaging technique that augments standard 2D echocardiography with clear indications and benefits. The current technology allows RT online visualisation of every cardiac structure, significantly aiding the examination of anatomy and surgical planning. The recent developments in 3D ultrafast and 5D imaging may simplify the overall workflow, making 3D-TOE more accessible and bring new diagnostic capabilities to the operating theatre environment. In the future the power of AI may be harnessed for automation of virtual reality and holography, facilitating closer interaction with the cardiac structures.
Declaration of interests
The authors declare that they have no conflicts of interest.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Catalin Iulian Efrimescu MD FCAI is a fellow in cardiovascular anaesthesia and intensive care at Toronto General Hospital.
William Ng MMed FANZCA FRCPC is a consultant at Toronto General and SickKids Hospitals and assistant professor of anaesthesia at the University of Toronto. His research interests include population database research in paediatric and adult congenital heart disease patients and clinical perioperative echocardiography.
Annette Vegas MD FRCPC FASE is a consultant at Toronto General Hospital and professor of anaesthesia at the University of Toronto. She is the director of perioperative echocardiography at Toronto General Hospital and a member of the EACTAIC Echo Subspecialty Committee. She is the author of multiple papers and books on echocardiography and a well-recognised educator in this field.
Matrix codes: 1A03, 2A04, 3G00
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjae.2024.03.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Matsumoto M., Oka Y., Strom J., et al. Application of transesophageal echocardiography to continuous intraoperative monitoring of left ventricular performance. Am J Cardiol. 1980;46:95–105. doi: 10.1016/0002-9149(80)90611-6. [DOI] [PubMed] [Google Scholar]
- 2.American Society of Anesthesiologists, Society of Cardiovascular Anesthesiologists Task Force on Transesophageal E Practice guidelines for perioperative transesophageal echocardiography. An updated report by the American Society of Anesthesiologists and the Society of Cardiovascular Anesthesiologists task Force on Transesophageal Echocardiography. Anesthesiology. 2010;112:1084–1096. doi: 10.1097/ALN.0b013e3181c51e90. [DOI] [PubMed] [Google Scholar]
- 3.Nicoara A., Skubas N., Ad N., et al. Guidelines for the use of transesophageal echocardiography to assist with surgical decision-making in the operating room: a surgery-based approach: from the American Society of Echocardiography in collaboration with the Society of Cardiovascular Anesthesiologists and the Society of Thoracic Surgeons. J Am Soc Echocardiogr. 2020;33:692–734. doi: 10.1016/j.echo.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Metkus T.S., Thibault D., Grant M.C., et al. Transesophageal echocardiography in patients undergoing coronary artery bypass graft surgery. J Am Coll Cardiol. 2021;78:112–122. doi: 10.1016/j.jacc.2021.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dekker D.L., Piziali R.L., Dong E., Jr. A system for ultrasonically imaging the human heart in three dimensions. Comput Biomed Res. 1974;7:544–553. doi: 10.1016/0010-4809(74)90031-7. [DOI] [PubMed] [Google Scholar]
- 6.Mor-Avi V., Mumm B., Lang R.M. In: Textbook of Three-Dimensional Echocardiography. Badano L., Lang R.M., Muraru D., editors. Springer Nature; Cham, Switzerland: 2019. The evolution of three-dimensional echocardiography: from the initial concept to real-time imaging. [Google Scholar]
- 7.Hung J., Lang R., Flachskampf F., et al. 3D echocardiography: a review of the current status and future directions. J Am Soc Echocardiogr. 2007;20:213–233. doi: 10.1016/j.echo.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Muraru D., Badano L.P. In: Manual of 3D Echocardiography. Rojo E.C., Fernandez-Golfin C., Zamorano J.L., editors. Springer International Publishing; Cham, Switzerland: 2017. Physical and technical aspects and overview of 3D- echocardiography. [Google Scholar]
- 9.Sheikh K., Smith S.W., von Ramm O., et al. Real-time, three-dimensional echocardiography: feasibility and initial use. Echocardiography. 1991;8:119–125. doi: 10.1111/j.1540-8175.1991.tb01409.x. [DOI] [PubMed] [Google Scholar]
- 10.Handke M., Heinrichs G., Moser U., et al. Transesophageal real-time three-dimensional echocardiography methods and initial in vitro and human in vivo studies. J Am Coll Cardiol. 2006;48:2070–2076. doi: 10.1016/j.jacc.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Vegas A., Meineri M., Jerath A. Springer Science+Business Media; New York, USA; 2012. Real-time three dimensional transesophageal echocardiography A step-by-step guide. [Google Scholar]
- 12.Lee W., Roh Y. Ultrasonic transducers for medical diagnostic imaging. Biomed Eng Lett. 2017;7:91–97. doi: 10.1007/s13534-017-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang R.M., Badano L.P., Tsang W., et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging. 2012;13:1–46. doi: 10.1093/ehjci/jer316. [DOI] [PubMed] [Google Scholar]
- 14.Vegas A. In: A Practical Approach to Transesophageal Echochardiography. 4th Edn. Perrino A.C., Reeves S.T., editors. Wolters Kluwer; Philadelphia: 2020. 3D TEE imaging; pp. 545–559. [Google Scholar]
- 15.Rabben S.I. In: Textbook of Real-Time Three Dimensional Echocardiography. Badano L.P., Lang R.M., Zamorano J.L., editors. Springer-Verlag; London: 2011. Technical principles of transthoracic three-dimensional echocardiography. [Google Scholar]
- 16.Muraru D., Badano L.P. In: Textbook of Three-Dimensional Echocardiography. Badano L.P. R.M. Lang, Muraru D., editors. Springer Nature; Cham, Switzerland: 2019. Physics and technical principles of three-dimensional echocardiography. [Google Scholar]
- 17.Vieira M.L.C., Ronderos R.E. In: Textbook of Three-Dimensional Echocardiograph. Badano, R.M.Lang L.P., Muraru D., editors. Springer Nature; Cham, Switzerland; 2019. Technical principles of transesophageal three-dimensional echocardiography. [Google Scholar]
- 18.Chan JSK and Lee A, Philips Ultrasound White Paper: Photorealistic imaging enhances 3D echocardiography in structural interventional cardiology, Koninklijke Philips N.V., Available from: https://www.philips.com/c-dam/b2bhc/master/landing-pages/echocardiography/expert-perspectives/epiq-elite-photorealistic-imaging.pdf (Accessed 15 June 2023).
- 19.Karagodin I., Addetia K., Singh A., et al. Improved delineation of cardiac pathology using a novel three-dimensional echocardiographic tissue transparency tool. J Am Soc Echocardiogr. 2020;33:1316–1323. doi: 10.1016/j.echo.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GE. Technical Publications Vivid E9 Version 112 User Manual GA092907 English Rev 01. 2012:5-41–5-52. General Electric Co. [Google Scholar]
- 21.Faletra F.F., Ramamurthi A., Dequarti M.C., et al. Artifacts in three-dimensional transesophageal echocardiography. J Am Soc Echocardiogr. 2014;27:453–462. doi: 10.1016/j.echo.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Le H.T., Hangiandreou N., Timmerman R., et al. Imaging artifacts in echocardiography. Anesth Analg. 2016;122:633–646. doi: 10.1213/ANE.0000000000001085. [DOI] [PubMed] [Google Scholar]
- 23.Hahn R.T., Abraham T., Adams M.S., et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2013;26:921–964. doi: 10.1016/j.echo.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Bosch J. In: Practice of Clinical Echocardiography. Otto C.M., editor. Elsevier; Philadelphia: 2017. Digital image processing and automated image analysis in echocardiography; pp. 166–181. [Google Scholar]
- 25.Weichert J. Samsung Medison Co.; 2019. Samsung White Paper: Value of 5D Heart ColorTM Tool in routine use for semiautomated evaluation of fetal cardiac structures.https://images.samsung.com/is/content/samsung/p5/pl/ultrasonograf/wiedza/white-paper/white_paper_5D_Heart_Color_HERA_W10_Germany_2019_PC_vers.pdf Available from: [Google Scholar]
- 26.Provost J., Papadacci C., Arango J.E., et al. 3D ultrafast ultrasound imaging in vivo. Phys Med Biol. 2014;59:L1–L13. doi: 10.1088/0031-9155/59/19/L1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang R.M., Addetia K., Narang A., et al. 3-Dimensional echocardiography: latest developments and future directions. JACC Cardiovasc Imaging. 2018;11:1854–1878. doi: 10.1016/j.jcmg.2018.06.024. [DOI] [PubMed] [Google Scholar]
- 28.Dave J.K., Mc Donald M.E., Mehrotra P., et al. Recent technological advancements in cardiac ultrasound imaging. Ultrasonics. 2018;84:329–340. doi: 10.1016/j.ultras.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voelker R. Cardiac ultrasound uses artificial intelligence to produce images. JAMA. 2020;323:1034. doi: 10.1001/jama.2020.2547. [DOI] [PubMed] [Google Scholar]
- 30.Tseng A.S., Lopez-Jimenez F., Pellikka P.A. Future guidelines for artificial intelligence in echocardiography. J Am Soc Echocardiogr. 2022;35:878–882. doi: 10.1016/j.echo.2022.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Bruckheimer E., Rotschild C., Dagan T., et al. Computer-generated real-time digital holography: first time use in clinical medical imaging. Eur Heart J Cardiovasc Imaging. 2016;17:845–849. doi: 10.1093/ehjci/jew087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.