Summary
Human pluripotent stem cell (hPSC) cultures are prone to genetic drift, because cells that have acquired specific genetic abnormalities experience a selective advantage in vitro. These abnormalities are highly recurrent in hPSC lines worldwide, but their functional consequences in differentiating cells are scarcely described. In this work, we show that the loss of chromosome 18q impairs neuroectoderm commitment and that downregulation of SALL3, a gene located in the common 18q loss region, is responsible for this failed neuroectodermal differentiation. Knockdown of SALL3 in control lines impaired differentiation in a manner similar to the loss of 18q, and transgenic overexpression of SALL3 in hESCs with 18q loss rescued the differentiation capacity of the cells. Finally, we show that loss of 18q and downregulation of SALL3 leads to changes in the expression of genes involved in pathways regulating pluripotency and differentiation, suggesting that these cells are in an altered state of pluripotency.
Keywords: SALL3, 18q loss, differentiation bias, neuroectoderm, human embryonic stem cells, human pluripotent stem cells
Highlights
-
•
hPSC cultures worldwide recurrently acquire losses of chromosome 18q
-
•
Loss of 18q results in impaired neuroectodermal commitment
-
•
Downregulation of SALL3, located in 18q, is responsible for this impairment
-
•
Downregulation of SALL3 deregulates pluripotency and differentiation pathways
hPSC cultures commonly acquire recurrent chromosomal abnormalities, among which is loss of chromosome 18q. Lei and colleagues show that this aneuploidy impedes neuroectoderm commitment due to decreased expression of SALL3, a gene located in the common region of deletion. SALL3 deregulation alters pluripotency and differentiation-associated gene expression. Its knockdown mimics 18q loss, and its overexpression rescues neuroectoderm differentiation.
Introduction
Human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs), are self-renewing cells that can give rise to any cell type originating from any of the three embryonic germ layers. This makes hPSCs an attractive resource for in vitro disease modeling, developmental biology research, drug discovery, and cell transplantation therapy. A substantial number of clinical trials are under way using hPSC-derived cell products, including for the treatment of age-related macular degeneration, spinal cord injury, and type 1 diabetes (Kobold et al., 2020; Yamanaka, 2020). An important hurdle for both safe clinical translation and the reliable use of hPSCs as in vitro research models is the occurrence of cell culture drift due to the acquisition of genetic abnormalities (Andrews et al., 2022). A subset of these genetic aberrations is highly recurrent, and these aberrations are found in hPSC lines worldwide. These recurrent changes vary in size from single-nucleotide point mutations to large chromosome structural variants, the most common being gains of chromosomes 1q, 12p, 17, 20, and X and losses of 10p, 18q and 22p, as well as mutations in TP53 (Amps et al., 2011; Merkle et al., 2017, 2022). Other genetic changes include epigenetic variations, including erosion of X chromosome inactivation (Bar and Benvenisty, 2019; Geens et al., 2016), mutations in the mitochondrial genome (Van Haute et al., 2013; Zambelli et al., 2018), and an array of other point mutations and structural variants spread throughout the genome (Avior et al., 2019; Merkle et al., 2022).
These recurrent genetic changes arise from the pool of common variations in hPSC cultures via different cell competition mechanisms. hPSCs are prone to replication stress, leading to DNA damage, which in turn is a source of de novo genetic variation (Halliwell et al., 2020; Jacobs et al., 2014). For instance, up to 20% of cells in an hESC culture carry de novo structural variants, but only a minority of them have the potential to confer a selective advantage to the cells (Jacobs et al., 2014, 2016; Keller et al., 2019), leading to the mutant cells rapidly outcompeting their genetically balanced counterparts (Avery et al., 2013; Nguyen et al., 2014; Olariu et al., 2010; Price et al., 2021). The exact traits that the different chromosomal abnormalities confer on undifferentiated cells, as well as the specific driver genes of these traits, are only well established for the gain of 20q11.21 (Spits et al., 2008). This abnormality confers a decreased sensitivity to apoptosis-inducing events due to increased expression of the gene BCL2L1, located in the minimal gained region (Amps et al., 2011; Avery et al., 2013; Nguyen et al., 2014). For gains of 12p, it is thought that NANOG drives at least part of the growth advantage of the cells (Ben-David et al., 2014), and cells with a complex karyotype carrying all of the most common abnormalities (gains of 1, 12, 17 and 20q) can outcompete the other cells by corralling and mechanical compression (Price et al., 2021).
An important concern about these genetic variants is whether and how they alter the differentiation capacity of hPSCs and potentially prime differentiated cells for malignant transformation (Andrews et al., 2022; Keller et al., 2018). The gain of 1q is common in cancers, particularly lung adenocarcinoma, breast invasive carcinoma, and liver hepatocellular carcinoma (Taylor et al., 2018), and appears to confer a growth advantage to cells during differentiation from hESCs to neural precursors (Varela et al., 2012). Moreover, variants in 1q21.1 can alter neurodevelopmental trajectories upon hiPSC differentiation, with the deletion of 1q21.1 accelerating neuronal production and its duplication delaying the transition from neural progenitor cell to neuron (Chapman et al., 2022). Gains in chromosome 12 are frequently found in testicular germ cell tumors (Baker et al., 2007). hPSCs with trisomy 12 display a reduced tendency toward spontaneous differentiation (Ben-David et al., 2014). The highly recurrent gain of 20q11.21 impairs the neuroectodermal lineage commitment of hPSCs (Jo et al., 2020; Markouli et al., 2019), and hESCs with 20q11.1q11.2 amplification have a reduced propensity to differentiate down the hematopoietic lineage, maintain more immature phenotypes along the neural differentiation trajectory, and generate teratomas with foci of undifferentiated cells (Werbowetski-Ogilvie et al., 2009). The gain of chromosome 17 is common in neuroblastomas, testicular germ cell tumors, and breast cancers (Baker et al., 2007), and hPSC lines with a gain of chromosome 17 show altered differentiation patterns in embryoid bodies (Fazeli et al., 2011).
Deletions of chromosome 18q are one of the rarer recurrent structural chromosomal abnormalities in hPSCs. This deletion was first reported as a single event by Maitra et al. in 2005 (Maitra et al., 2005), and our group later found 18q deletions in three different hESC lines at relatively early passages, always as part of a derivative chromosome 18 (Spits et al., 2008). A large study by Amps et al. in 2011 revealed 5 instances of this deletion in hESC (Amps et al., 2011), and WiCell reported that 4% of the 7,300 hPSC cultures evaluated over nearly 8 years carried an 18q deletion (WiCell Cytogenetics Lab, https://www.wicell.org/media.acux/29102c0e-e88e-426b-ab7d-bac4c2a9ec6a). Chromosome 18q loss is common in cancers, especially gastrointestinal tract cancers (Taylor et al., 2018), and is linked to several disorders, including congenital malformations, developmental delays, and intellectual disability (Hogendorf et al., 2021). However, the impact of 18q loss on the functional properties of hPSCs is unknown. Therefore, the aim of this work was to examine the functional effects of 18q deletions during hPSC differentiation into the three embryonic germ layers and to determine the molecular mechanisms involved.
Results
The minimal common region of 18q loss spans 14 genes expressed in undifferentiated hESCs and includes SALL3
We initially identified 18q losses in the hESC lines Vrije Universiteit Brussel (VUB)04 and VUB26, in the form of a derivative chromosome 18. VUB04 presented a deletion at 18q21.2qter and a duplication at 5q14.2qter, and VUB26 showed the minimal 18q loss region (18q23qter) and a duplication at 7q33qter (Figure S1; Table S1) (Spits et al., 2008). These two hESC lines were not used in the present work because they further genetically drifted and acquired gain of 1q and 20q11.21, but their analysis helped to narrow down the common 18q deletion region. In the present study, we used two other hESC lines bearing derivative chromosomes 18 involving a loss of 18q losses (hESCdel18q), VUB14del18q, and VUB13del18q, as well as three chromosomally balanced lines (hESCWT [wild type]), VUB14WT, and VUB04WT and VUB03WT, which served as controls for VUB13del18q since VUB13WT was lost (details on the karyotypes of the lines and their characterization are shown in Figure S2; Table S1). We used shallow genome sequencing to confirm the karyotypes before starting the experiments, and all of the lines were routinely inspected, with qPCR assays targeting recurrent chromosomal abnormalities (1q, 12p, 20q11.21, and 17q) to confirm their genomic stability for the duration of the different experiments.
The 18q losses exhibited a common loss region from base pairs 75,773,285 to 80,373,285, spanning 37 loci. Bulk RNA sequencing (RNA-seq) of undifferentiated hESCs indicated that 14 genes within this region are expressed in undifferentiated hESCs, with counts per million greater than one in at least two samples (Figure S1A). Of these coding genes, ADNP2, SALL3, and TXNL4A had the highest expression and showed decreased transcript levels in mutant cells. ADNP2 and TXNL4A have no known function in hPSC. ADNP2 is predicted to be a transcription factor, and its silencing increases oxidative stress-mediated cell death (Kushnir et al., 2008). TXNL4A is a component of the U5 small ribonucleoprotein particle, which is involved in pre-mRNA splicing and is associated with Burn-McKeown syndrome (Wood et al., 2022). SALL3 was more promising as a candidate driver gene because it has previously been reported to regulate the differentiation propensity of hiPSC lines (Kuroda et al., 2019). Kuroda et al. showed that hiPSC lines expressing high levels of SALL3 differentiated preferentially into ectoderm, whereas hiPSC lines expressing lower levels of SALL3 tended to differentiate into mesoderm and endoderm (Kuroda et al., 2019). It has also been shown that SALL3 interacts with the Mediator complex in neural stem cells (Quevedo et al., 2019) and is related to the development of the nervous system (Ott et al., 1996). Considering these previous findings, we hypothesized that the decreased expression of SALL3 because of a loss of one copy of the gene could alter the differentiation capacity of hESCsdel18q.
hESCs with 18q loss show impaired neuroectoderm differentiation
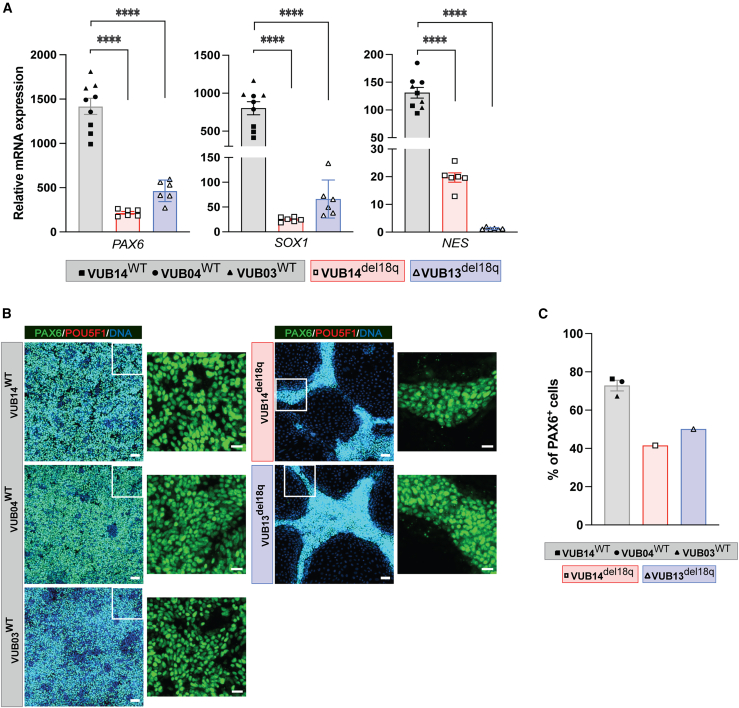
As a first step, we investigated the effect of 18q deletion on hESC ectoderm lineage commitment. hESCWT and hESCdel18q were subjected to neuroectoderm differentiation for 8 days using LDN193189 (LDN), SB431542 (SB) and retinoic acid (RA) (Douvaras and Fossati, 2015) (Figure S1B). We measured the mRNA levels of different neuroectoderm markers to evaluate neuroectoderm differentiation efficiency and the expression of the undifferentiated state markers NANOG and POUF51 (Figures 1A and S3A). VUB13del18q and VUB14del18q had significantly lower mRNA levels of PAX6, NES, and SOX1, compared to the levels in hESCWT (for all genes and both lines, p ≤ 0.0001, unpaired t test), indicating a decreased neuroectodermal differentiation efficiency in hESCdel18q. POU5F1 and NANOG mRNA expression levels were almost undetectable for all differentiated cells (Figure S3A). We also evaluated the differentiation of hESCWT and hESCdel18q cells by immunostaining (Figures 1B and 1C). We observed a lower percentage of PAX6+ cells in differentiated hESCdel18q than in differentiated hESCWT cells (70% in hESCWT vs. 50% in VUB13del18q and 41% in VUB14del18q; Figure 1C), which was consistent with the decrease in the levels of PAX6 mRNA. Taken together, these results show that hESCdel18q differentiation into neuroectoderm is impaired, and rather than to remain undifferentiated state, they misspecify.
Figure 1.
hESCs with 18q loss show impaired neuroectoderm differentiation
(A) Relative mRNA expression for neuroectoderm markers. Data are shown as means ± SEMs. Each data point refers to an independent differentiation experiment, and ∗, ∗∗, ∗∗∗, and ∗∗∗∗ represent statistical significance between samples at 5%, 1%, 0.1%, and 0.01%, respectively (unpaired t test).
(B) Immunostaining in mutant and control lines. Scale bars (original images), 50 μm, scale bars (magnification images), 20 μm.
(C) Percentages of PAX6+ cells in the immunostainings shown in (B).
hESCdel18q readily differentiates into mesendoderm derivatives but shows abnormal cardiomyocyte progenitor differentiation
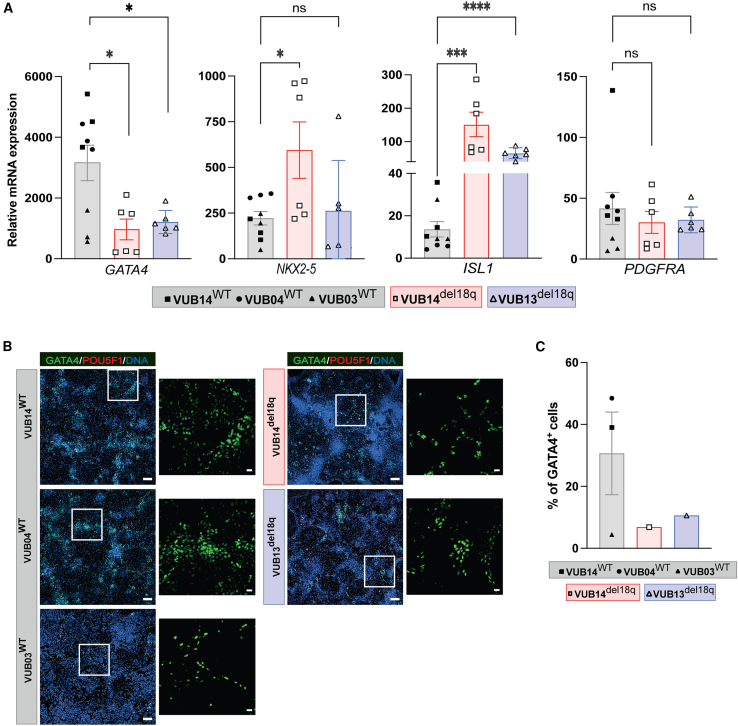
We further investigated the impact of 18q deletion on the mesendoderm differentiation capacity of hESCs by differentiating hESCWT and hESCdel18q into, in this case, cardiac progenitors and hepatoblasts. Thus, we induced the differentiation of hESCWT and hESCdel18q into mesoderm using a 5-day cardiac progenitor induction protocol described previously (Lin and Zou, 2020) (Figure S1B). We evaluated the mRNA levels of the cardiac progenitor markers GATA4, ISL1, NKX2-5, and PDGFRA (Figure 2A). hESCdel18q showed lower levels of GATA4 mRNA (p = 0.02 for VUB13del18q and p = 0.01 VUB14del18q, unpaired t test; Figure 2A). hESCdel18q lines expressed ISL1 at 15-fold higher levels, on average, than hESCWT lines (p < 0.0001 for VUB13del18q and p = 0.0005 VUB14del18q, unpaired t test; Figure 2A). We found no significant or consistent difference in the expression levels of NKX2-5 or PDGFRA between the control and mutant cardiac progenitor groups (p = 0.01 for NKX2-5 VUB14del18q, others not significant, unpaired t test; Figure 2A). We also evaluated the proportion of differentiated cardiac progenitor and undifferentiated cells by immunostaining for GATA4 and POU5F1. The percentage of GATA4+ cells was overall lower in hESCWT than in hESCdel18q, whereas the cells were POU5F1− (Figures 2B and 2C). Taken together, and bearing in mind the temporal expression of these markers during cardiac differentiation (Doyle et al., 2015; Zhang et al., 2019), the results suggest that hESCdel18q may experience differentiation delays or arrest, reaching an ISL1high GATA4low stage at day 5 but remaining less mature than their hESCWT counterparts.
Figure 2.
hESCdel18q cells show abnormal cardiac progenitor differentiation
(A) Relative mRNA expression for cardiac progenitor markers. Data are shown as means ± SEMs. Each data point refers to an independent differentiation experiment, and ∗, ∗∗, ∗∗∗, and ∗∗∗∗ represent statistical significance between samples at 5%, 1%, 0.1%, and 0.01% respectively (unpaired t test). ns, not significant.
(B) Immunostaining for GATA4 and POU5F1 in mutant and control lines. Scale bars (original images), 100 μm, and scale bars (magnification images), 20 μm.
(C) Percentage of GATA4+ cells in the immunostainings shown in (B).
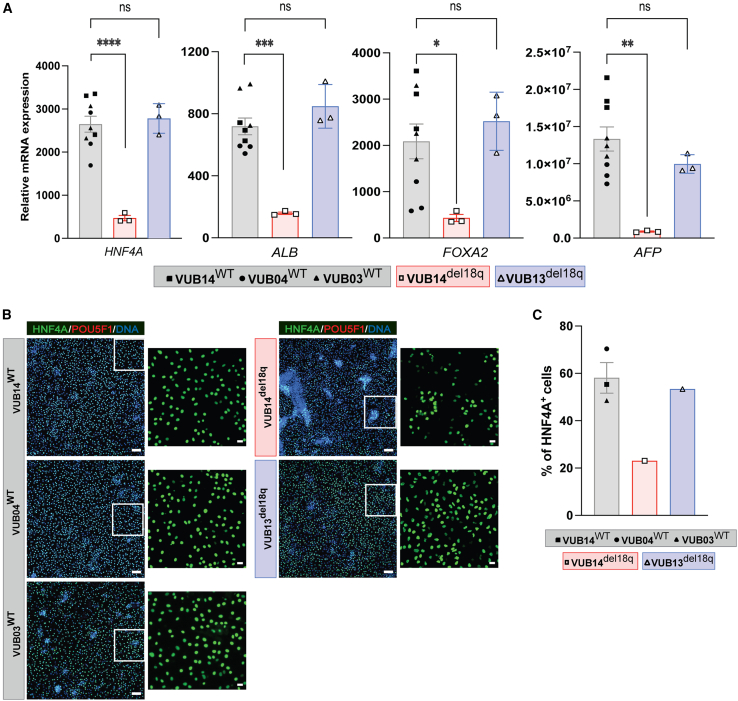
Next, we differentiated hESCWT and hESCdel18q into hepatoblasts by applying the modified differentiation protocol for 8 days, as described previously (Boon et al., 2020) (Figure S1B). We measured the expression levels of the hepatoblast markers HNF4A, AFP, ALB, and FOXA2 (Figure 3A). We found no consistent difference in the mRNA expression levels of HNF4A, ALB, FOXA2, and AFP between hESCWT and hESCdel18q (not significant for VUB13del18q, p < 0.0001, p = 0.0002, p = 0.03, and p = 0.0016, respectively, for VUB14del18q, unpaired t test; Figure 3A). We further evaluated hepatoblast differentiation by immunostaining for HNF4A and found that the percentages of HNF4A+ cells in hESCdel18q cells were in line with the mRNA expression. VUB13del18q showed results similar to those in WT cells (58% in hESCWT vs. 53% in VUB13del18q), whereas VUB14del18q had 23% positive cells (Figures 3B and 3C).
Figure 3.
hESCdel18q and their genetically balanced counterparts differentiate equally well into hepatoblasts
(A) Relative mRNA expression of hepatoblast markers. Data are shown as means ± SEMs. Each data point refers to an independent differentiation experiment, and ∗, ∗∗, ∗∗∗, and ∗∗∗∗ represent statistical significance between samples at 5%, 1%, 0.1%, and 0.01%, respectively (unpaired t test).
(B) Immunostaining for HNF4A and POU5F1 in mutant and control lines. Scale bars (original images), 100 μm, and scale bars (magnification images), 20 μm.
(C) Percentage of HNF4A+ cells in the immunostainings shown in (B).
For both mesodermal and endodermal lineage commitment, all hESCWT and hESCdel18q lines displayed low mRNA and protein levels of undifferentiated state markers (Figures S3B and S3C), indicating a loss of pluripotency for all cell lines during differentiation. Overall, our results indicate that hESCWT and hESCdel18q differentiate into definitive mesoderm and endoderm and that there may be a delay or impairment in the progression of hESCdel18q toward the cardiac progenitor stage. The differences in gene expression observed during hepatoblast differentiation are likely due to between-line variation in differentiation propensity rather than to the 18q deletion itself.
Downregulation of SALL3 impairs neuroectoderm differentiation but does not affect differentiation into mesoderm and endoderm
We next examined SALL3 mRNA expression levels in undifferentiated cells and found that SALL3 expression was significantly lower in hESCdel18q than in hESCWT (Figures S1A and S3D), supporting the notion that SALL3 could be a key gene in the altered differentiation capacity of hESCsdel18q. We first generated SALL3 knockdown (KD) lines from three hESCWT lines (VUB02WT, VUB03WT, and VUB04WT) by transducing a lentiviral vector containing short hairpin RNA (shRNA) targeting the SALL3 transcript (hESCWT_SALL3KD) or a nontargeting shRNA as a control (hESCWT−NT) (Figure S3E). Next, we generated hESCdel18q with stable overexpression (OE) of SALL3 (hESCdel18q_SALL3OE) by transducing VUB13del18q and VUB14del18q with the SALL3 lentiviral vector, and we verified the OE by measuring SALL3 mRNA levels, which were significantly increased in VUB13del18q_SALL3OE (3-fold) and VUB14del18q_SA LL3OE (5-fold) compared to controls (Figure S3F).
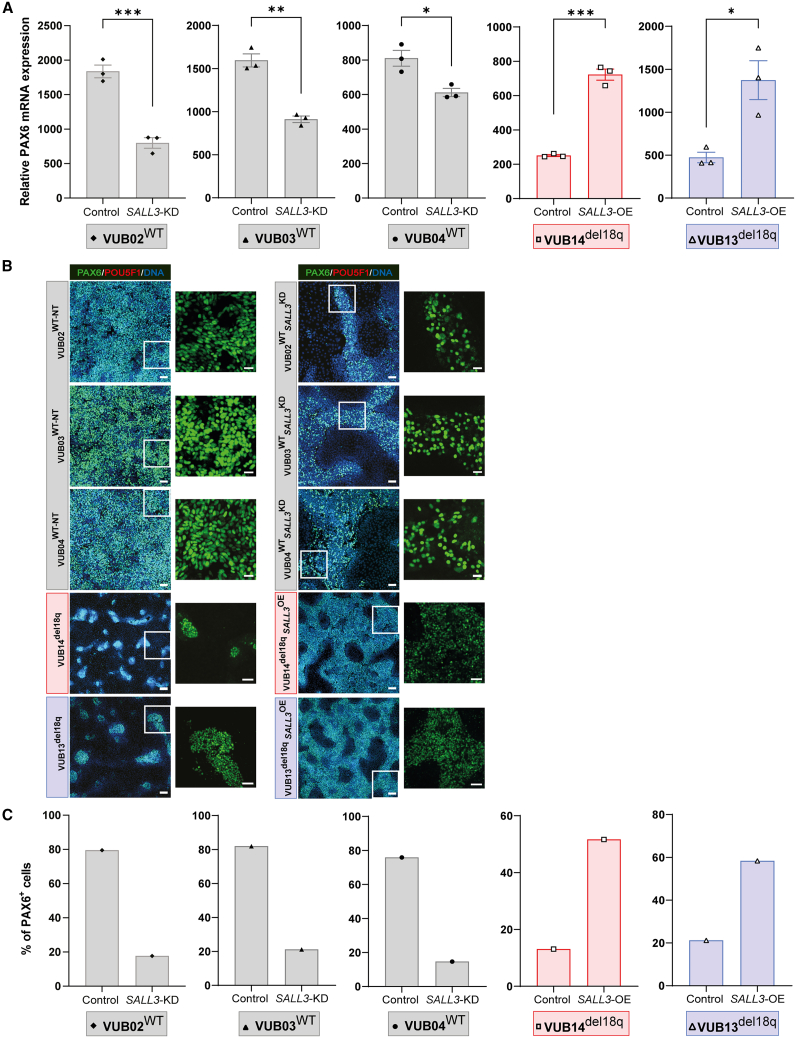
To investigate the role of SALL3 in regulating hESC differentiation propensity, we first induced neuroectodermal differentiation in hESCWT_SALL3KD, hESCdel18q_SALL3OE, and the corresponding control cells. All three hESCWT_SALL3KD lines had lower levels of all neuroectodermal (NE) markers than nontarget controls (Figures 4A, S4A, and S4B). PAX6 protein levels were also reduced in hESCWT_SALL3KD (Figures 4B and 4C), with only 20% of the cells expressing PAX6, compared to 80% of PAX6+ cells in the control group (Figures 4B and 4C). Our results show that SALL3 suppression in hESCWT recapitulates the impaired NE differentiation seen in hESCdel18q lines. In contrast, hESCdel18q_SALL3OE cells efficiently differentiated into neuroectoderm cells, accompanied by a significant increase in the mRNA levels of PAX6, SOX1, and NES (Figures 4A, S4A, and S4B); moreover, hESCdel18q_SALL3OE cultures included more PAX6+ cells than hESCdel18q cultures (60% vs. 20%, respectively) (Figures 4B and 4C). These results indicate that exongenous SALL3 expression can rescue the impairment of ectoderm differentiation caused by 18q loss.
Figure 4.
Downregulation of SALL3 drives the impaired neuroectoderm differentiation of hESCs with 18q loss
(A) Relative mRNA expression for PAX6. Data are shown as means ± SEMs. Each data point refers to an independent differentiation experiment, and ∗, ∗∗, ∗∗∗, and ∗∗∗∗ represent statistical significance between samples at 5%, 1%, 0.1%, and 0.01%, respectively (unpaired t test).
(B) Immunostaining for PAX6 and POU5F1 in mutant and control lines. Scale bars (KD groups), 50 μm, and magnification 20 μm; scale bars (OE groups), 100 μm, and magnification, 50 μm.
(C) Percentage of PAX6+ cells in the immunostainings shown in (B).
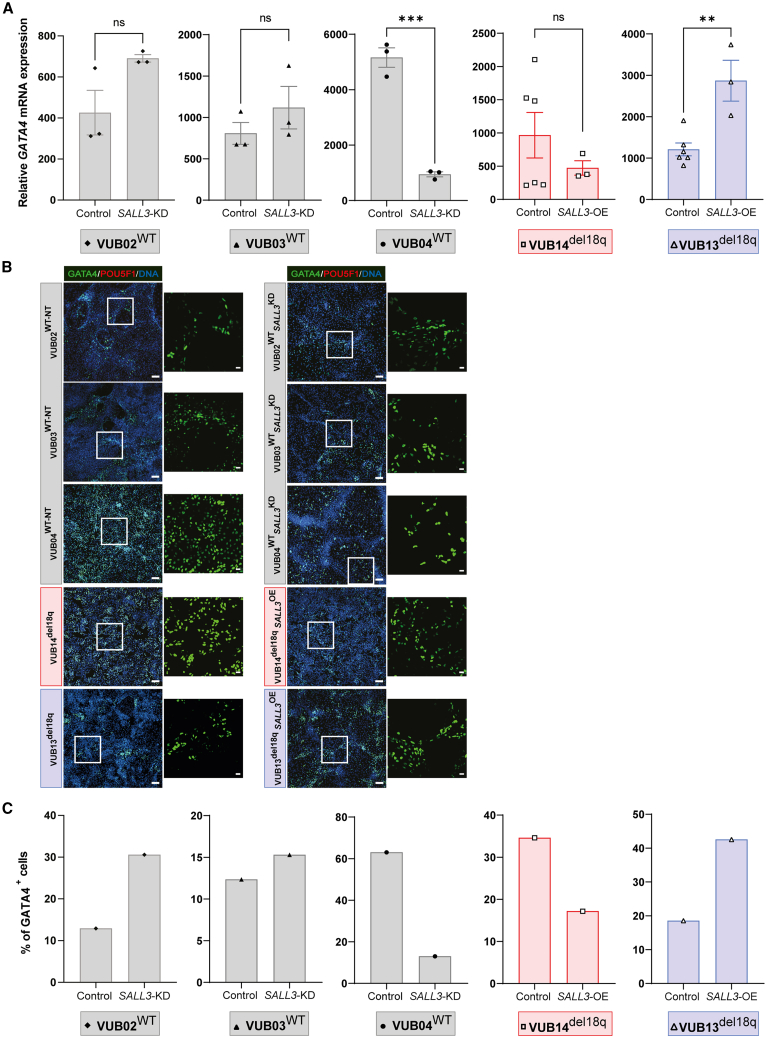
Next, we induced the differentiation of the different hESC lines into cardiac progenitors. The three hESCWT_SALL3KD lines differentiated inconsistently toward mesoderm fates. VUB04WT_SALL3KD showed lower levels of GATA4 (Figure 5A; p = 0.0003, unpaired t test), whereas compared to the controls, VUB03WT_SALL3KD and VUB02WT_SALL3KD showed no differences in GATA4 expression (Figure 5A). In addition, the percentage of GATA4+ cells detected by immunostaining in hESCWT_SALL3KD followed the same pattern, consistent with the mRNA levels of each line (Figures 5B and 5C). The hESCdel18q_SALL3OE cells exhibited variable marker profiles, with increases in GATA4 expression at both the mRNA (Figure 5A; p = 0.004, unpaired t test) and protein levels for VUB13del18q_SALL3OE, but no difference in GATA4 mRNA levels (Figure 5A; p = 0.3622, unpaired t test) and a slight decrease in GATA4 protein levels in VUB14del18q_SALL3OE (Figures 5B and 5C). Similarly, the mRNA expression levels of other markers, NKX2-5, ISL1, and PDGFRA, showed no consistent trend in the hESCWT_SALL3KD groups and exhibited no consistent differences in hESCsdel18q and hESCdel18qSALL3OE (Figures S5A–S5C).
Figure 5.
Changes in SALL3 expression do not regulate cardiac progenitor differentiation
(A) Relative mRNA expression for GATA4. Data are shown as means ± SEMs. Each data point refers to an independent differentiation experiment, and ∗, ∗∗, ∗∗∗, and ∗∗∗∗ represent statistical significance between samples at 5%, 1%, 0.1%, and 0.01%, respectively (unpaired t test).
(B) Immunostaining for GATA4 and POU5F1 in mutant and control lines. Scale bars (original images), 100 μm, and scale bars (magnification images), 20 μm. (C) Percentage of GATA4+ cells in the immunostainings shown in (B).
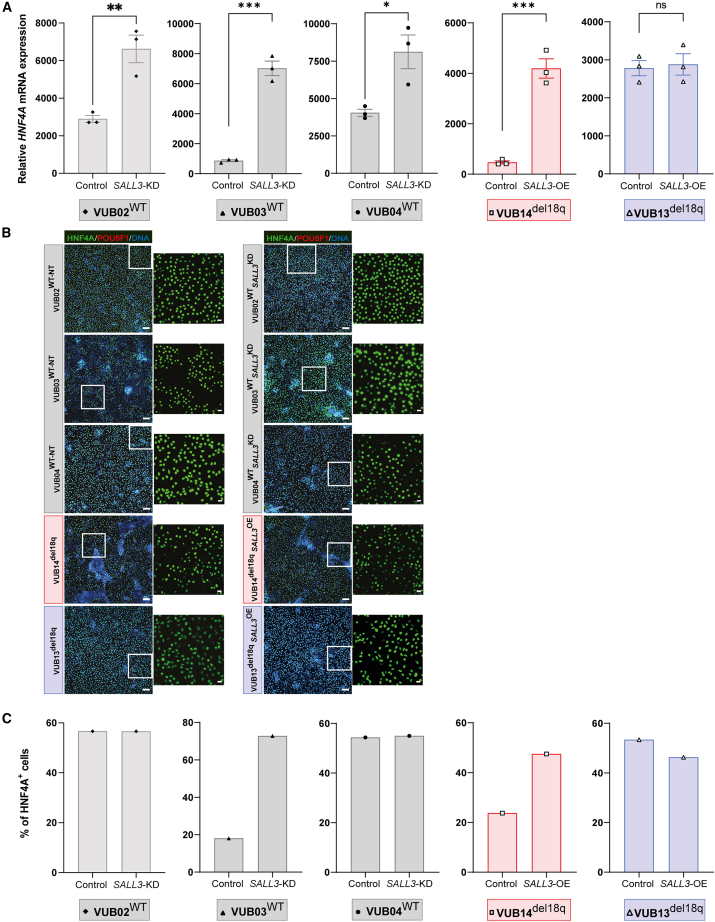
When hESCs were differentiated into hepatoblast, SALL3 downregulation in hESCWT resulted in increased HNF4A, ALB, and FOXA2 mRNA expression (Figures 6A, S6B, and S6C). The percentage of HNF4A+ cells was higher in VUB03WT_SALL3KD cells, but not in VUB04WT_SALL3KD and VUB02WT_SALL3KD cells compared to controls (Figures 6B and 6C). The mRNA expression of another marker, AFP, also did not show consistent changes (Figure S6A). Upon the OE of SALL3, the differentiation profiles into hepatoblast did not show the expected mirroring effect. The mRNA expression of all of the hepatoblast markers HNF4A, ALB, AFP, and FOXA2 increased in VUB14del18q_SALL3OE cells, consistent with the changes observed in the HNF4A protein level, but this same effect was not observed in VUB13del18q_SALL3OE cells (Figures 6 and S6).
Figure 6.
Changes in SALL3 expression do not consistently affect hepatoblast differentiation
(A) Relative mRNA expression of HNF4A. Data are shown as means ± SEMs. Each data point refers to an independent differentiation experiment, and ∗, ∗∗, ∗∗∗, and ∗∗∗∗ represent statistical significance between samples at 5%, 1%, 0.1%, and 0.01%, respectively (unpaired t test).
(B) Immunostaining for HNF4A and POU5F1 in mutant and control lines. Scale bars (original images), 100 μm, and scale bars (magnification images), 20 μm.
(C) Percentage of HNF4A+ cells.
Overall, these results suggest that the effect of SALL3 on mesoderm and endoderm differentiation may be line specific and is not the reason for the delayed progression seen during cardiac differentiation in hESCs18q.
Downregulation of SALL3 and loss of 18q result in the deregulation of genes in pathways associated with pluripotency and differentiation
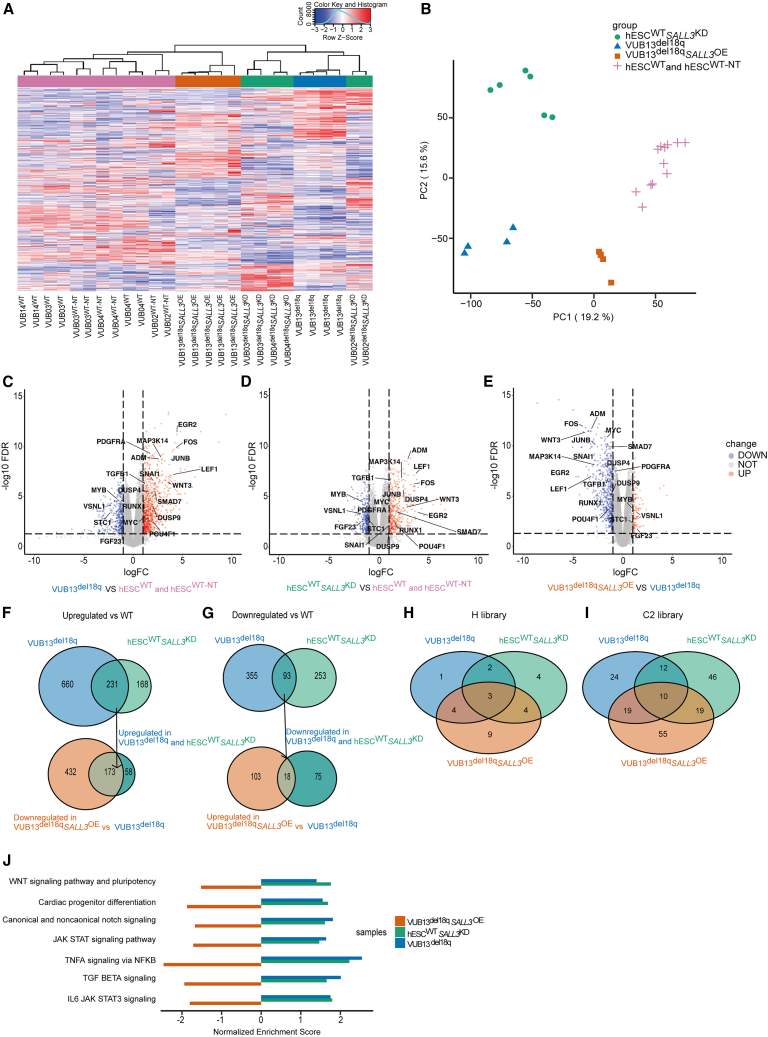
To gain deeper insight into the effect of SALL3 downregulation on the global transcriptomic profile of cells with 18q loss, we carried out bulk RNA-seq of hESCWT−NT (N = 6), hESCWT_SALL3KD (N = 6), hESCWT (N = 6), VUB13del18q (N = 4), and VUB13del18q_SALL3OE (N = 5) cells. To estimate the similarity among the samples, we generated a distance clustering heatmap with global row scaling (Figure 7A) and performed principal-component analysis (PCA) (Figure 7B). The heatmap shows that although VUB13del18q and hESCWT_SALL3KD cluster together, they cluster apart from the WT cell lines and from VUB13del18q_SALL3OE. The samples in this last group clustered more closely to the WT cell lines than to their unmodified VUB13del18q (Figure 7A). This pattern was also reflected in the first dimension of the PCA (Figure 7B), in which hESCWT, hESCWT−NT, and VUB13del18q_SALL3OE clustered more closely with one another than with VUB13del18q and hESCWT_SALL3KD. These results suggest that the downregulation of SALL3 in WT cells is sufficient to alter the transcriptome such that its profile is closer to that of an hESC line with a loss of 18q, and SALL3 OE in hESCdel18q can restore the transcriptome to a near-WT state. Taken together, these findings support our hypothesis that the differences between hESCWT and hESCdel18q are driven mostly by the downregulation of SALL3 due to the loss of one copy of this gene. For this reason, we pooled the hESCWT−NT with the untreated hESCWT for further analysis.
Figure 7.
Downregulation of SALL3 and loss of 18q result in the deregulation of genes in pathways associated with pluripotency and differentiation
(A and B) Unsupervised clustering heatmap (A) and PCA (B) of the coding genes with a count per million >1 in at least 2 samples.
(C–E) Volcano plots of differential gene expression analysis for VUB13del18q vs. hESCWT and hESCWT−NT. The labeled genes are the top 20 commonly deregulated genes across the 3 comparisons (C), hESCWT_SALL3KD vs. hESCWT and hESCWT−NT (D), and VUB13del18q_SALL3OE vs. VUB13del18q (E), with a cutoff value of |log2fold change|> 1 and FDR < 0.05.
(F) Venn diagrams show the genes downregulated in VUB13del18q_SALL3OE and upregulated in VUB13del18q and hESCWT_SALL3KD.
(G) Venn diagrams showing the genes upregulated in VUB13del18q_SALL3OE and downregulated in VUB13del18q and hESCWT_SALL3KD.
(H) Venn diagrams of the pathways in the H library common among VUB13del18q, hESCWT_SALL3KD, VUB13del18q_SALL3OE, and hESCWT.
(I) Venn diagrams of the pathways in the C2 library common among VUB13del18q, hESCWT_SALL3KD, VUB13del18q_SALL3OE, and hESCWT.
(J) Pathways commonly deregulated among the different samples are associated with pluripotency and differentiation.
Next, we carried out differential gene expression analysis to gain further insight into which genes and pathways form the basis of the differences between hESCWT and hESCdel18q and which of these are driven by SALL3. Figures 7C–7E show volcano plots of the differentially expressed genes in the different groups. We considered this differential expression to be significant at a |log2fold change| > 1.0 and false discovery rate (FDR) < 0.05. VUB13del18q shows 891 and 448 genes that are significantly upregulated and downregulated in hESCdel18q, respectively, compared to the WT cells (Figure 7C). Downregulation of SALL3 in WT cells led to the differential expression of 745 genes, 399 of which were upregulated and 346 of which were downregulated (Figure 7D). OE of SALL3 in VUB13del18q resulted in the upregulation of 121 genes and the downregulation of 605 genes (Figure 7E).
To elucidate which of the transcriptional differences between hESCWT and hESCdel18q are mediated by SALL3, we investigated the overlaps in up- and downregulated genes across conditions. First, we found that 231 and 93 genes were commonly up- and downregulated, respectively, between VUB13del18q and hESCWT_SALL3KD, representing 26% of the differentially expressed genes in hESCs with an 18q deletion (Figures 7F and 7G). Second, we compared these subsets of genes to the genes with altered gene expression in hESCdel18q upon OE of SALL3. We compared the genes that were upregulated by SALL3 KD and by loss of 18q to those downregulated by OE of SALL3 in hESC18q, and vice versa (Figures 7F and 7G). Of the 231 upregulated genes shared by the VUB13del18q and hESCWT_SALL3KD groups, 173 genes showed increased expression in VUB13del18q_SALL3OE (Figure 7F). In addition, 18 of the 93 downregulated genes shared by VUB13del18q and hESCWT_SALL3KD displayed increased expression in VUB13del18qSALL3OE (Figure 7G). This further refined the gene set to a core of 191 genes that are the most strongly regulated by SALL3 in hESCs, both by the loss of a copy of the gene itself and by the modulation of its expression (Table S2). The other differences in gene expression are likely associated with the loss of other genes in 18q or with the OE of genes in the duplicated regions of chromosomes 5 and 7, which are part of the derivative chromosome 18 in hESCdel18q.
Finally, to identify potential molecular targets and elucidate the underlying functional mechanisms that contribute to the observed impairment of differentiation capacity in hESCdel18q, we analyzed the differential gene expression of the three groups using gene set enrichment analysis and the MSigDB database. Specifically, we focused on the Kyoto Encyclopedia of Genes and Genomes and WikiPathways databases within the C2 library and the pathways of the H library. We filtered the significant pathways based on a normalized enrichment score |NES| > 1, a p < 0.05, and the proportion of leading-edge genes accounting for >30% of the entire gene set involved in the pathway.
Figures 7H and 7I show Venn diagrams of the overlap between significantly enriched pathways in the C2 and H libraries, respectively, for each of the three groups. The full list can be found in Table S3. In total, we found 13 pathways that overlapped among the 3 groups, all 13 of which were positively enriched in both VUB13del18q and hESCWT_SALL3KD and negatively enriched in VUB13del18q_SALL3OE (Figure 7J shows the cell-type-relevant pathways; the whole set can be found in Table S4). Because of the critical role that these pathways play in pluripotency maintenance and differentiation, we analyzed the expression of pluripotency-associated genes, and we found that NANOG, POU5F1, LIN28, SOX2, PODXL, SUSD2, MYC, FOXD3, and DPPA3 are overexpressed in VUB13del18q, with all but DPPA3 and POU5F1 also being overexpressed upon SALL3KD and downregulated by transgenic SALL3 OE in VUB13del18q (Figure S7A).
Discussion
In this study, we examined the repercussions of the loss of chromosome 18q on the differentiation capacity of hESCs. For this, we used an early-stage differentiation approach to generate lineage-specific cell types representing the three germ layers: neuroectoderm, hepatoblast, and cardiac progenitors. Our in vitro lineage commitment studies indicated that the deletion of 18q in hESCs impaired neuroectodermal differentiation and delayed cardiac progenitor differentiation, whereas no consistent differences were observed in the commitment toward hepatoblasts. It is important to bear in mind that we used a set of three or four markers to establish the cell identity after differentiation, precluding an in-depth analysis of the impact of the mutation on the further maturation and functionality of the obtained cells.
To study the mechanistic basis for these changes in differentiation, we looked at the genes located in the minimal region of loss. We found that decreased SALL3 expression due to the loss of one copy of the gene was sufficient to result in the observed decreased neuroectoderm differentiation, but not to modulate cardiac and hepatoblast differentiation. In this sense, our results are only partially aligned with those obtained by Kuroda et al. (2019). These authors found that downregulating SALL3 resulted not only in decreased neuroectoderm differentiation, similar to our results, but also in increased cardiac progenitor differentiation. Although we can only speculate about the reasons for these differences, it is likely that the genetic background of the cell lines plays an important role (Kilpinen et al., 2017). Also, given that SALL3 has been reported to be a modulator of DNMT3A and DNMT3B activity (Kuroda et al., 2019; Shikauchi et al., 2009), the preexisting epigenetic marks in each of the lines, particularly histone modifications, may influence the recruitment of de novo methyl transferases to methylate the DNA (Baubec et al., 2015; Weinberg et al., 2019). Furthermore, it is possible that other genes located in 18q, or in the gain regions of chromosomes 5 and 7, cause cell-line-specific effects.
In line with this reasoning, the gene expression analysis revealed that only part of the divergence between hESCs with 18q loss and their chromosomally normal isogenic counterparts was related to the differential expression of SALL3. Interestingly, the core set of deregulated genes was associated with pathways involved in the maintenance of and exit from the undifferentiated pluripotent state, and key regulators of pluripotency were both upregulated in hESCs18q and regulated by SALL3 expression. For instance, transforming growth factor β (TGF-β) signaling is key to the maintenance of the primed pluripotent state (Weinberger et al., 2016). TGF-β and bone morphogenetic protein 4 (BMP4) signaling are also core genes in regulating the balance between neuroectoderm differentiation and mesendoderm in both humans and mice (Park, 2011). Notch activation mediates TGF-β signaling during hESC and mesenchymal stem cell differentiation into smooth muscle cells (Kurpinski et al., 2010), and its inhibition supports naive state consolidation in rodent models (Weinberger et al., 2016). The cytokine tumor necrosis factor α has been shown to negatively regulate the differentiation of various cell types, including cardiomyocytes (Hamid et al., 2016) and embryoid bodies (Wuu et al., 1998). In addition, nuclear factor-κB inhibition has been found to mediate naive pluripotency in mice (Dutta et al., 2011). Overall, these results suggest that downregulation of SALL3 due to the loss of 18q alters undifferentiated-state maintenance in hESCs by affecting pluripotency-associated pathways, and these changes have profound effects on the differentiation capacity of the cells.
With hPSCs steadily moving into clinical trials (Kobold et al., 2020) and being broadly used as a cell source for in vitro modeling of, for instance, developmental processes and diseases, determining the impact of recurrent genetic abnormalities is critical (Andrews et al., 2022; Keller et al., 2018). Work from our group and others is beginning to generate a detailed picture showing how these genetic abnormalities affect differentiation in a cell lineage-specific manner. For instance, 20q11.21 gain impairs neuroectoderm commitment without affecting mesendoderm induction (Jo et al., 2020; Markouli et al., 2019), and recently, it has been shown that cells with an isochromosome 20q are not able to survive retinal pigmented epithelial cell differentiation and display overall disruptions in the ability to correctly differentiate (Vitillo et al., 2023). In this work, we show that 18q loss specifically impairs neuroectoderm commitment and appears to delay cardiac differentiation. Taken together, these results highlight the importance of the genetic screening of hPSC cultures to ensure that these abnormalities do not pass unnoticed. In a research setting, chromosomal abnormalities could lead to confounding effects that decrease the reliability and reproducibility of the work, and in a clinical setting, they could lead at best to decreased therapeutic efficacy and at worst to tumorigenesis (Andrews et al., 2022; Keller et al., 2018; Yamanaka, 2020).
In conclusion, in this study, we have characterized the differentiation capacity of hESCs with 18q loss, one of the recurrent, albeit less common, genetic abnormalities found in hPSC cultures. We found that these cells are characterized by abnormal differentiation into cardiac progenitors and an impaired capacity for neuroectoderm commitment, the latter driven by the loss of one copy of SALL3. This gene is an inhibitor of DNMT3B, and its downregulation results in changes in the expression of genes involved in the maintenance of pluripotency and in hESC differentiation. Further research will be needed to assess whether other cell-type-specific effects of this abnormality exist and may be revealed by longer differentiation protocols, beyond the progenitor stage, as well as the potential consequences of 18q loss for oncogenic potential.
Experimental procedures
Resource availability
Lead contact
Further information and requests for resources should be directed to the corresponding author, Claudia Spits (claudia.spits@vub.be).
Materials availability
All VUB stem cell lines in this study, including the genetically abnormal sublines and genetically modified lines, are available upon request and after signing a materials transfer agreement.
Data and code availability
Raw sequencing data of human samples are considered personal data by the General Data Protection Regulation of the European Union (Regulation (EU) 2016/679), because SNPs can be extracted from the reads and cannot be shared publicly. The data can be obtained from the corresponding author upon reasonable request and after signing a data use agreement. The RNA-seq counts per million tables are provided in the Data S1, which allow for downstream gene expression analysis. The data supporting all of the figures in this paper can be found at the Open Science Framework repository: https://osf.io/hpaxc/.
Ethics statement
For all parts of this study, the design and conduct complied with all of the relevant regulations regarding the use of human materials, and all of them were approved by the local ethical committee of the University Hospital UZ Brussel and the Vrije Universiteit Brussel (file no. B.U.N. 1432020000284). All of the patients donating embryos to derive hESC lines gave written consent.
hESC maintenance and passaging
All of the hESC lines in this study were derived in-house in the past. The details on the derivation and results of the characterization, including tests for pluripotency, were reported previously (Mateizel et al., 2006; 2010) and can be also found at the Open Science Framework repository: https://osf.io/esmz8/. The lines are registered in the EU hPSC registry (https://hpscreg.eu/) and available upon request. All of the experiments in this study have been carried out on cells that belong to the same working cell bank, each of which tested for genetic content by shallow whole-genome sequencing and for mycoplasma. No cells were used beyond five passages after being drawn from the working bank.
The hESCs were maintained in NutriStem hESC XF medium (NS medium; Biological Industries) with 100 U/mL penicillin/streptomycin (Thermo Fisher Scientific) in a 37°C incubator with 5% CO2 on Biolaminin 521-coated dishes (Biolamina). The culture medium was changed daily. The cells were passaged as single cells using TrypLE Express (Thermo Fisher Scientific) and split when reaching 70%–90% confluence. The medium was supplemented with 10 μM rho kinase inhibitor Y-27632 (Tocris) for the first 24 h after passaging.
Copy-number variant (CNV) analysis
The genetic content of the hESCs was assessed through shallow whole-genome sequencing by the BRIGHTcore of UZ Brussels, Belgium, as previously described (Bayindir et al., 2015). We also conducted CNV analysis using quantitative real-time PCR at regular intervals, particularly before and after performing lentiviral transduction and starting differentiation. DNA was extracted with a DNeasy Blood and Tissue Kit (Qiagen) according to the manufacturers’ protocol. qPCR was performed with the copy-number assays RNaseP (Thermo Fisher Scientific) as a reference and KIF14, NANOG, NMT1, and ID1 (Thermo Scientific) covering the 1q, 12p, 17q, and 20q regions, respectively. The reaction systems were prepared by mixing TaqMan 2× Mastermix Plus–Low ROX (Eurogentec) and the TaqMan assays together with the DNA samples. qPCR was performed on a ViiA7 thermocycler (Thermo Fisher Scientific), and Applied Biosystems Copy Caller version 2.1 was used to analyze the CNVs.
Total RNA isolation, cDNA synthesis, and quantitative real-time PCR for gene expression analysis
Total RNA was isolated using RNeasy Mini and Micro kits (Qiagen) following the manufacturer’s guidelines, including on-column DNase I treatment. mRNA was reverse transcribed into biotinylated cDNA using the First-Strand cDNA Synthesis Kit (Cytiva) with the NotI-d(T)18 primer. Quantitative real-time PCR was carried out using TaqMan mRNA expression assays (Thermo Fisher Scientific, listed in Table S5) and TaqMan 2× Mastermix Plus–Low ROX (Eurogentec) on a ViiA 7 thermocycler (Thermo Fisher Scientific) using the standard settings provided by the manufacturer. The relative expression was determined by the comparative Ct method, and GUSB was used as the housekeeping gene.
Immunostaining
Differentiated cells were fixed in a solution of PBS containing 3.7% formaldehyde (Sigma-Aldrich) for 15 min, permeabilized in 0.1% Triton X-100 for 10 min (Sigma-Aldrich) and blocked with 10% fetal bovine serum (FBS; Thermo Fisher Scientific) for 1 h at room temperature (RT). Primary antibodies were diluted in a 10% FBS (Thermo Fisher Scientific) blocking solution and incubated overnight at 4°C. Alexa 488 or Alexa 594 conjugated secondary antibodies (diluted in 10% FBS) and Hoescht (1:1,000 dilution, ThermoFisher Scientific) were applied for 1–2 h at RT. Confocal images were acquired with an LSM800 confocal microscope (Carl Zeiss). For quantification, the positive cells were counted and compared to the number of Hoescht-stained nuclei to determine the percent positivity using ZEN 2 (blue edition) imaging software. The areas are randomly selected based on the Hoescht channel, and the positive cells are quantified by calculating the ratio of total positive cells to the total number of cells within those areas. The numbers of cells quantified for each image can be found in Table S6. The lists with antibodies can be found in Table S7.
In vitro differentiation of iPSCs
The neuroectoderm differentiation was based on Douvaras and Fossati (2015). In brief, 90% confluent hESCs were subjected to neural induction for 8 days using 100 nM RA (Sigma-Aldrich), 10 μM SB431542 (Tocris), and 250 nM LDN193189 (STEMCELL Technologies). The induction of cardiac progenitor differentiation was based on Lin and Zou (2020). hESCs at 80%–90% confluence were treated with 5 mM CHIR99021 (Tocris) for 24 h, after which the cells were cultured in medium with 0.6 U/mL heparin (Sigma-Aldrich) for 24 h. Subsequently, the medium was supplemented with 0.6 U/mL heparin and 3 mM IWP2 (Tocris) for another 3 days. The 8-day depatoblast differentiation was based on Boon et al. (2020). Differentiation was initiated at 40%–50% confluency, and the hESCs were treated with with 50 ng/mL activin A (STEMCELL Technologies), 50 ng/mL WNT3A (PeproTech), and 6 μL/mL DMSO (Sigma-Aldrich) for 48 h. The cells were incubated for an additional 48 h in the same medium without WNT3A. Then, the medium was changed to contain 50 ng/mL BMP4 (STEMCELL Technologies) and 6 μL/mL DMSO for the following 4 days. More details on the differentiation and the media composition can be found in the supplemental information.
Generation of SALL3 KD and OE cell lines
hESCWT_SALL3KD cells were generated by infecting hESCWT with lentiviral particles expressing SALL3-targeted shRNAs (SigmaMISSION shRNA targeting set TRCN0000019754, TRCN0000417790) or control shRNA plasmid. hESCdel18q_SALL3OE cells were generated by infecting hESCdel18q with lentiviral particles containing the pLVSIN-EF1α puromycin vector expressing SALL3 (a gift from Yoji Sato, Division of Cell-Based Therapeutic Products, National Institute of Health Sciences, Japan).
RNA-seq
RNA-seq library preparation was performed using QuantSeq 3′ mRNA-Seq Library Prep Kits (Lexogen) following Illumina protocols. Sequencing was performed on a high-throughput Illumina NextSeq 500 flow cell. On average, 13.9 × 106 ± 7.1 × 106 paired-end reads per sample were uniquely mapped, with an average coverage per base of 101 paired reads. Details on the bioinformatic processing can be found in the supplemental information.
Statistics
All of the differentiation experiments were carried out in at least triplicate (n ≥ 3). All of the data are presented as the mean ± SEM. Statistical evaluation of the differences between two groups was performed using unpaired two-tailed t tests in GraphPad Prism9 software, with p < 0.05 determined to indicate significance.
Acknowledgments
The authors thank Yoji Sato from the Division of Cell-Based Therapeutic Products, National Institutes of Health Sciences in Japan, for kindly sharing the lentiviral construct for SALL3 OE. Y.L. is a predoctoral fellow supported by the China Scholarship Council, and M.R., C.J., N.K. and E.C.d.D. are predoctoral fellows supported by the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (FWO). This research was supported by the FWO (Fonds voor Wetenschappelijk Onderzoek – Vlaanderen; grant no. 1506617N) and the Methusalem Grant (to K.S., Vrije Universiteit Brussel).
Author contributions
Y.L. carried out all of the experiments and bioinformatics analysis unless stated otherwise and co-wrote the manuscript. D.A.D. cowrote the manuscript. N.K. packaged the lentivirus and assisted in transduction. E.C.d.D. assisted with the bioinformatics analysis. M.R. assisted in the microscopy and cell counting. C.J. and M.G. assisted with the cell culture. K.S. proofread the paper. C.S. cowrote the manuscript and designed and supervised the experimental work.
Declaration of interests
The authors declare no competing interests.
Published: March 28, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2024.03.001.
Supplemental information
References
- International Stem Cell Initiative. Amps K., Andrews P.W., Anyfantis G., Armstrong L., Avery S., Baharvand H., Baker J., Baker D., Munoz M.B., et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat. Biotechnol. 2011;29:1132–1144. doi: 10.1038/nbt.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P.W., Barbaric I., Benvenisty N., Draper J.S., Ludwig T., Merkle F.T., Sato Y., Spits C., Stacey G.N., Wang H., Pera M.F. The consequences of recurrent genetic and epigenetic variants in human pluripotent stem cells. Cell Stem Cell. 2022;29:1624–1636. doi: 10.1016/j.stem.2022.11.006. [DOI] [PubMed] [Google Scholar]
- Avery S., Hirst A.J., Baker D., Lim C.Y., Alagaratnam S., Skotheim R.I., Lothe R.A., Pera M.F., Colman A., Robson P., et al. BCL-XL Mediates the Strong Selective Advantage of a 20q11.21 Amplification Commonly Found in Human Embryonic Stem Cell Cultures. Stem Cell Rep. 2013;1:379–386. doi: 10.1016/j.stemcr.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avior Y., Eggan K., Benvenisty N. Cancer-Related Mutations Identified in Primed and Naive Human Pluripotent Stem Cells. Cell Stem Cell. 2019;25:456–461. doi: 10.1016/j.stem.2019.09.001. [DOI] [PubMed] [Google Scholar]
- Baker D.E.C., Harrison N.J., Maltby E., Smith K., Moore H.D., Shaw P.J., Heath P.R., Holden H., Andrews P.W. Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat. Biotechnol. 2007;25:207–215. doi: 10.1038/nbt1285. [DOI] [PubMed] [Google Scholar]
- Bar S., Benvenisty N. Epigenetic aberrations in human pluripotent stem cells. EMBO J. 2019;38 doi: 10.15252/embj.2018101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baubec T., Colombo D.F., Wirbelauer C., Schmidt J., Burger L., Krebs A.R., Akalin A., Schübeler D. Genomic profiling of DNA methyltransferases reveals a role for DNMT3B in genic methylation. Nature. 2015;520:243–247. doi: 10.1038/nature14176. [DOI] [PubMed] [Google Scholar]
- Bayindir B., Dehaspe L., Brison N., Brady P., Ardui S., Kammoun M., Van Der Veken L., Lichtenbelt K., Van Den Bogaert K., Van Houdt J., et al. Noninvasive prenatal testing using a novel analysis pipeline to screen for all autosomal fetal aneuploidies improves pregnancy management. Eur. J. Hum. Genet. 2015;23:1286–1293. doi: 10.1038/ejhg.2014.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-David U., Arad G., Weissbein U., Mandefro B., Maimon A., Golan-Lev T., Narwani K., Clark A.T., Andrews P.W., Benvenisty N., Carlos Biancotti J. Aneuploidy induces profound changes in gene expression, proliferation and tumorigenicity of human pluripotent stem cells. Nat. Commun. 2014;5:4825. doi: 10.1038/ncomms5825. [DOI] [PubMed] [Google Scholar]
- Boon R., Kumar M., Tricot T., Elia I., Ordovas L., Jacobs F., One J., De Smedt J., Eelen G., Bird M., et al. Amino acid levels determine metabolism and CYP450 function of hepatocytes and hepatoma cell lines. Nat. Commun. 2020;11:1393. doi: 10.1038/s41467-020-15058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman G., Alsaqati M., Lunn S., Singh T., Linden S.C., Linden D.E.J., van den Bree M.B.M., Ziller M., Owen M.J., Hall J., et al. Using induced pluripotent stem cells to investigate human neuronal phenotypes in 1q21.1 deletion and duplication syndrome. Mol. Psychiatry. 2022;27:819–830. doi: 10.1038/s41380-021-01182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douvaras P., Fossati V. Generation and isolation of oligodendrocyte progenitor cells from human pluripotent stem cells. Nat. Protoc. 2015;10:1143–1154. doi: 10.1038/nprot.2015.075. [DOI] [PubMed] [Google Scholar]
- Doyle M.J., Lohr J.L., Chapman C.S., Koyano-Nakagawa N., Garry M.G., Garry D.J. Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes as a Model for Heart Development and Congenital Heart Disease. Stem Cell Rev. Rep. 2015;11:710–727. doi: 10.1007/s12015-015-9596-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D., Ray S., Home P., Larson M., Wolfe M.W., Paul S. Self Renewal vs. Lineage Commitment of Embryonic Stem Cells: Protein Kinase C Signaling Shifts the Balance. Stem Cell. 2011;29:618–628. doi: 10.1002/stem.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli A., Liew C.-G., Matin M.M., Elliott S., Jeanmeure L.F.C., Wright P.C., Moore H., Andrews P.W. Altered patterns of differentiation in karyotypically abnormal human embryonic stem cells. Int. J. Dev. Biol. 2011;55:175–180. doi: 10.1387/ijdb.103177af. [DOI] [PubMed] [Google Scholar]
- Geens M., Seriola A., Barbé L., Santalo J., Veiga A., Dée K., Van Haute L., Sermon K., Spits C. Female human pluripotent stem cells rapidly lose X chromosome inactivation marks and progress to a skewed methylation pattern during culture. Mol. Hum. Reprod. 2016;22:285–298. doi: 10.1093/molehr/gaw004. [DOI] [PubMed] [Google Scholar]
- Halliwell J.A., Frith T.J.R., Laing O., Price C.J., Bower O.J., Stavish D., Gokhale P.J., Hewitt Z., El-Khamisy S.F., Barbaric I., Andrews P.W. Nucleosides Rescue Replication-Mediated Genome Instability of Human Pluripotent Stem Cells. Stem Cell Rep. 2020;14:1009–1017. doi: 10.1016/j.stemcr.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid T., Xu Y., Ismahil M.A., Li Q., Jones S.P., Bhatnagar A., Bolli R., Prabhu S.D. TNF receptor signaling inhibits cardiomyogenic differentiation of cardiac stem cells and promotes a neuroadrenergic-like fate. Am. J. Physiol. Heart Circ. Physiol. 2016;311:H1189–H1201. doi: 10.1152/ajpheart.00904.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haute L., Spits C., Geens M., Seneca S., Sermon K. Human embryonic stem cells commonly display large mitochondrial DNA deletions. Nat. Biotechnol. 2013;31:20–23. doi: 10.1038/nbt.2473. [DOI] [PubMed] [Google Scholar]
- Hogendorf A., Zieliński M., Constantinou M., Śmigiel R., Wierzba J., Wyka K., Wędrychowicz A., Jakubiuk-Tomaszuk A., Budzynska E., Piotrowicz M., et al. Immune Dysregulation in Patients With Chromosome 18q Deletions—Searching for Putative Loci for Autoimmunity and Immunodeficiency. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.742834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs K., Mertzanidou A., Geens M., Nguyen H.T., Staessen C., Spits C. Low-grade chromosomal mosaicism in human somatic and embryonic stem cell populations. Nat. Commun. 2014;5:4227. doi: 10.1038/ncomms5227. [DOI] [PubMed] [Google Scholar]
- Jacobs K., Zambelli F., Mertzanidou A., Smolders I., Geens M., Nguyen H.T., Barbé L., Sermon K., Spits C. Higher-Density Culture in Human Embryonic Stem Cells Results in DNA Damage and Genome Instability. Stem Cell Rep. 2016;6:330–341. doi: 10.1016/j.stemcr.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo H.Y., Lee Y., Ahn H., Han H.J., Kwon A., Kim B.Y., Ha H.Y., Kim S.C., Kim J.H., Kim Y.O., et al. Functional in vivo and in vitro effects of 20q11.21 genetic aberrations on hPSC differentiation. Sci. Rep. 2020;10:18582. doi: 10.1038/s41598-020-75657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A., Dziedzicka D., Zambelli F., Markouli C., Sermon K., Spits C., Geens M. Genetic and epigenetic factors which modulate differentiation propensity in human pluripotent stem cells. Hum. Reprod. Update. 2018;24:162–175. doi: 10.1093/humupd/dmx042. [DOI] [PubMed] [Google Scholar]
- Keller A., Tilleman L., Dziedzicka D., Zambelli F., Sermon K., Van Nieuwerburgh F., Spits C., Geens M. Uncovering low-level mosaicism in human embryonic stem cells using high throughput single cell shallow sequencing. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-51314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpinen H., Goncalves A., Leha A., Afzal V., Alasoo K., Ashford S., Bala S., Bensaddek D., Casale F.P., Culley O.J., et al. Common genetic variation drives molecular heterogeneity in human iPSCs. Nature. 2017;546:370–375. doi: 10.1038/nature22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobold S., Guhr A., Mah N., Bultjer N., Seltmann S., Seiler Wulczyn A.E.M., Stacey G., Jie H., Liu W., Löser P., Kurtz A. A Manually Curated Database on Clinical Studies Involving Cell Products Derived from Human Pluripotent Stem Cells. Stem Cell Rep. 2020;15:546–555. doi: 10.1016/j.stemcr.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda T., Yasuda S., Tachi S., Matsuyama S., Kusakawa S., Tano K., Miura T., Matsuyama A., Sato Y. SALL3 expression balance underlies lineage biases in human induced pluripotent stem cell differentiation. Nat. Commun. 2019;10:2175. doi: 10.1038/s41467-019-09511-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurpinski K., Lam H., Chu J., Wang A., Kim A., Tsay E., Agrawal S., Schaffer D.V., Li S. Transforming Growth Factor-β and Notch Signaling Mediate Stem Cell Differentiation into Smooth Muscle Cells. Stem Cell. 2010;28:734–742. doi: 10.1002/stem.319. [DOI] [PubMed] [Google Scholar]
- Kushnir M., Dresner E., Mandel S., Gozes I. Silencing of the ADNP-family member, ADNP2, results in changes in cellular viability under oxidative stress. J. Neurochem. 2008;105:537–545. doi: 10.1111/j.1471-4159.2007.05173.x. [DOI] [PubMed] [Google Scholar]
- Lin Y., Zou J. Differentiation of Cardiomyocytes from Human Pluripotent Stem Cells in Fully Chemically Defined Conditions. STAR Protoc. 2020;1 doi: 10.1016/j.xpro.2020.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra A., Arking D.E., Shivapurkar N., Ikeda M., Stastny V., Kassauei K., Sui G., Cutler D.J., Liu Y., Brimble S.N., et al. Genomic alterations in cultured human embryonic stem cells. Nat. Genet. 2005;37:1099–1103. doi: 10.1038/ng1631. [DOI] [PubMed] [Google Scholar]
- Markouli C., Couvreu De Deckersberg E., Regin M., Nguyen H.T., Zambelli F., Keller A., Dziedzicka D., De Kock J., Tilleman L., Van Nieuwerburgh F., et al. Gain of 20q11.21 in Human Pluripotent Stem Cells Impairs TGF-β-Dependent Neuroectodermal Commitment. Stem Cell Rep. 2019;13:163–176. doi: 10.1016/j.stemcr.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateizel I., De Temmerman N., Ullmann U., Cauffman G., Sermon K., Van de Velde H., De Rycke M., Degreef E., Devroey P., Liebaers I., Van Steirteghem A. Derivation of human embryonic stem cell lines from embryos obtained after IVF and after PGD for monogenic disorders. Hum. Reprod. 2006;21:503–511. doi: 10.1093/humrep/dei345. [DOI] [PubMed] [Google Scholar]
- Mateizel I., Spits C., De Rycke M., Liebaers I., Sermon K. Derivation, culture, and characterization of VUB hESC lines. In Vitro Cell. Dev. Biol. Anim. 2010;46:300–308. doi: 10.1007/s11626-010-9284-4. [DOI] [PubMed] [Google Scholar]
- Merkle F.T., Ghosh S., Kamitaki N., Mitchell J., Avior Y., Mello C., Kashin S., Mekhoubad S., Ilic D., Charlton M., et al. Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations. Nature. 2017;545:229–233. doi: 10.1038/nature22312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle F.T., Ghosh S., Genovese G., Handsaker R.E., Kashin S., Meyer D., Karczewski K.J., O’Dushlaine C., Pato C., Pato M., et al. Whole-genome analysis of human embryonic stem cells enables rational line selection based on genetic variation. Cell Stem Cell. 2022;29:472–486.e7. doi: 10.1016/j.stem.2022.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H.T., Geens M., Mertzanidou A., Jacobs K., Heirman C., Breckpot K., Spits C. Gain of 20q11.21 in human embryonic stem cells improves cell survival by increased expression of Bcl-xL. Mol. Hum. Reprod. 2014;20:168–177. doi: 10.1093/molehr/gat077. [DOI] [PubMed] [Google Scholar]
- Olariu V., Harrison N.J., Coca D., Gokhale P.J., Baker D., Billings S., Kadirkamanathan V., Andrews P.W. Modeling the evolution of culture-adapted human embryonic stem cells. Stem Cell Res. 2010;4:50–56. doi: 10.1016/j.scr.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Ott T., Kaestner K.H., Monaghan A.P., Schütz G. The mouse homolog of the region specific homeotic gene spalt of Drosophila is expressed in the developing nervous system and in mesoderm-derived structures. Mech. Dev. 1996;56:117–128. doi: 10.1016/0925-4773(96)00516-3. [DOI] [PubMed] [Google Scholar]
- Park K.S. TGF-beta Family Signaling in Embryonic Stem Cells. Int. J. Stem Cells. 2011;4:18–23. doi: 10.15283/ijsc.2011.4.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C.J., Stavish D., Gokhale P.J., Stevenson B.A., Sargeant S., Lacey J., Rodriguez T.A., Barbaric I. Genetically variant human pluripotent stem cells selectively eliminate wild-type counterparts through YAP-mediated cell competition. Dev. Cell. 2021;56:2455–2470.e10. doi: 10.1016/j.devcel.2021.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo M., Meert L., Dekker M.R., Dekkers D.H.W., Brandsma J.H., van den Berg D.L.C., Ozgür Z., van IJcken W.F.J., Demmers J., Fornerod M., et al. Mediator complex interaction partners organize the transcriptional network that defines neural stem cells. Nat. Commun. 2019;10:2669. doi: 10.1038/s41467-019-10502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikauchi Y., Saiura A., Kubo T., Niwa Y., Yamamoto J., Murase Y., Yoshikawa H. SALL3 Interacts with DNMT3A and Shows the Ability To Inhibit CpG Island Methylation in Hepatocellular Carcinoma. Mol. Cell Biol. 2009;29:1944–1958. doi: 10.1128/MCB.00840-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits C., Mateizel I., Geens M., Mertzanidou A., Staessen C., Vandeskelde Y., Van Der Elst J., Liebaers I., Sermon K. Recurrent chromosomal abnormalities in human embryonic stem cells. Nat. Biotechnol. 2008;26:1361–1363. doi: 10.1038/nbt.1510. [DOI] [PubMed] [Google Scholar]
- Taylor A.M., Shih J., Ha G., Gao G.F., Zhang X., Berger A.C., Schumacher S.E., Wang C., Hu H., Liu J., et al. Genomic and Functional Approaches to Understanding Cancer Aneuploidy. Cancer Cell. 2018;33:676–689.e3. doi: 10.1016/j.ccell.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela C., Denis J.A., Polentes J., Feyeux M., Aubert S., Champon B., Piétu G., Peschanski M., Lefort N. Recurrent genomic instability of chromosome 1q in neural derivatives of human embryonic stem cells. J. Clin. Invest. 2012;122:569–574. doi: 10.1172/JCI46268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitillo L., Anjum F., Hewitt Z., Stavish D., Laing O., Baker D., Barbaric I., Coffey P. The isochromosome 20q abnormality of pluripotent cells interrupts germ layer differentiation. Stem Cell Rep. 2023;18:782–797. doi: 10.1016/j.stemcr.2023.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg D.N., Papillon-Cavanagh S., Chen H., Yue Y., Chen X., Rajagopalan K.N., Horth C., McGuire J.T., Xu X., Nikbakht H., et al. The histone mark H3K36me2 recruits DNMT3A and shapes the intergenic DNA methylation landscape. Nature. 2019;573:281–286. doi: 10.1038/s41586-019-1534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger L., Ayyash M., Novershtern N., Hanna J.H. Dynamic stem cell states: naive to primed pluripotency in rodents and humans. Nat. Rev. Mol. Cell Biol. 2016;17:155–169. doi: 10.1038/nrm.2015.28. [DOI] [PubMed] [Google Scholar]
- Werbowetski-Ogilvie T.E., Bossé M., Stewart M., Schnerch A., Ramos-Mejia V., Rouleau A., Wynder T., Smith M.J., Dingwall S., Carter T., et al. Characterization of human embryonic stem cells with features of neoplastic progression. Nat. Biotechnol. 2009;27:91–97. doi: 10.1038/nbt.1516. [DOI] [PubMed] [Google Scholar]
- Wood K.A., Ellingford J.M., Thomas H.B., Genomics UK Research Consortium. Douzgou S., Beaman G.M., Hobson E., O’Keefe R.T., O'Keefe R.T., Newman W.G. Expanding the genotypic spectrum of TXNL4A variants in Burn-McKeown syndrome. Clin. Genet. 2022;101:255–259. doi: 10.1111/cge.14082. [DOI] [PubMed] [Google Scholar]
- Wuu Y.D., Pampfer S., Vanderheyden I., Lee K.H., De Hertogh R. Impact of tumor necrosis factor alpha on mouse embryonic stem cells. Biol. Reprod. 1998;58:1416–1424. doi: 10.1095/biolreprod58.6.1416. [DOI] [PubMed] [Google Scholar]
- Yamanaka S. Pluripotent Stem Cell-Based Cell Therapy—Promise and Challenges. Cell Stem Cell. 2020;27:523–531. doi: 10.1016/j.stem.2020.09.014. [DOI] [PubMed] [Google Scholar]
- Zambelli F., Mertens J., Dziedzicka D., Sterckx J., Markouli C., Keller A., Tropel P., Jung L., Viville S., Van de Velde H., et al. Random Mutagenesis, Clonal Events, and Embryonic or Somatic Origin Determine the mtDNA Variant Type and Load in Human Pluripotent Stem Cells. Stem Cell Rep. 2018;11:102–114. doi: 10.1016/j.stemcr.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Tao R., Campbell K.F., Carvalho J.L., Ruiz E.C., Kim G.C., Schmuck E.G., Raval A.N., da Rocha A.M., Herron T.J., et al. Functional cardiac fibroblasts derived from human pluripotent stem cells via second heart field progenitors. Nat. Commun. 2019;10:2238. doi: 10.1038/s41467-019-09831-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequencing data of human samples are considered personal data by the General Data Protection Regulation of the European Union (Regulation (EU) 2016/679), because SNPs can be extracted from the reads and cannot be shared publicly. The data can be obtained from the corresponding author upon reasonable request and after signing a data use agreement. The RNA-seq counts per million tables are provided in the Data S1, which allow for downstream gene expression analysis. The data supporting all of the figures in this paper can be found at the Open Science Framework repository: https://osf.io/hpaxc/.