Abstract
Objective
To evaluate the changes in lesion depth and mineral density of resin infiltration-treated white spot lesions against a simulated oral environment using thermal and acidic challenges in vitro.
Materials and methods
Two enamel slabs were prepared from each buccal surface of permanent human premolars, for a total of 56 slabs. Artificial white spot lesions were induced. One specimen was treated with resin infiltration, while the other was used as an untreated control. A micro-CT was used to assess the lesion depth and mineral density of each specimen. Subsequently, all specimens were subjected to 10,000 cycles of thermocycling and pH cycling for 10 days before being re-evaluated using the micro-CT. Lesion depth and mineral density were examined and compared between before and after aging procedures within each group by the paired sample t-test. The independent samples t-test was utilized to compare lesion depth progression and percentage change of mineral density between groups.
Results
After aging, there was both a significant lesion depth progression and a mineral loss in the control and resin infiltration groups. Mean lesion depth progression was 132.88 ± 4.18 µm for the control group and 52.31 ± 4.16 µm for resin infiltration group. Percentage mineral density loss as a percentage for the control and resin infiltration groups were 16.1 ± 0.64 % and 8.83 ± 0.30 %, respectively. The resin infiltration group demonstrated a significantly lower mean lesion depth progression and percentage changes in mineral loss compared to the control group.
Conclusions
The lesion depth and mineral density changes in the resin infiltrated-treated group were lower than untreated white spot lesions after aging procedures using thermal and acidic challenges.
Clinical significance
Resin infiltration is a promising approach to inhibit the progression of white spot lesions related to the initial stage of dental caries.
Keywords: Micro-computed tomographic analysis, Mineral density change, Resin infiltration, White spot lesion
1. Introduction
Dental caries is a multifactorial disease caused by an imbalance in the demineralization and remineralization processes with net mineral loss (Frencken et al., 2012, Sturdevant et al., 2019). Enamel decalcification is an early sign of dental caries that appear as non-cavitated chalky white areas. If demineralization continues over time, degradation of tooth surface integrity will subsequently occur, which can be irreversible (Denis et al., 2013). Early detection, progression halting, and remineralization promotion are key components in safeguarding lesions from progressing to an advanced stage (Frencken et al., 2012).
A conventional treatment for white spot lesions involves non-invasive approaches using remineralizing agents and behavior modifications. While these methods have proven successful in arresting most carious lesions, their efficacies are contingent upon strong patient cooperation and time-consuming. Moreover, remineralization mostly occurs at superficial level, often leaving behind residual microporosities at the subsurface level, resulting in the whitish appearance (Wang et al., 2012). In response to this aesthetic challenge, resin infiltration was introduced (Knösel et al., 2019). This technique involves creating a diffusion barrier using low-viscosity light-cure resins with a refractive index close to sound enamel. Not only occlusion of microporosities through the lesion body is achieved, the improvement of aesthetic appearance is also obtained (Dorri et al., 2015, Klaisiri and J., Thamrongananskul, N., Sriamporn, T., & Krajangta, N. , 2020). The resin used in this technique mainly consists of triethylene glycol dimethacrylate (TEGDMA), which has high penetration coefficient. However, it contains a high water absorption, making it more susceptible to deterioration in the oral environment (Polak-Kowalska and Pels, 2019). Furthermore, challenges in the intraoral environment, such as pH fluctuations, temperature changes, and moisture, probably affect the restorative material's properties and the resin-hydroxyapatite bonds, leading to the restoration degradation (Amaral et al., 2007). Yet, further investigations are required to thoroughly assess the effectiveness of this approach.
Thus, this study aimed to evaluate the lesion depth and mineral density changes of resin infiltration-treated white spot lesions in a simulated oral environment using thermocycling and pH cycling procedures.
2. Materials and methods
2.1. Specimen preparation
Twenty-eight human premolars, extracted for orthodontic treatment reasons, were used in the study upon informed consent from subjects. Teeth were kept at room temperature in a 0.1 % thymol solution within 2 months after extraction (Aydın et al., 2015). All teeth were cleaned and examined under a stereomicroscope at 20X magnification (SZ 61, Olympus, Japan). Those without restoration, caries, cracks, white spot lesions, and other enamel defects were included. Fig. 1 depicts the flowchart with the experimental design and procedures.
Fig. 1.
depicts flow chart of the experimental procedures.
Specimens were prepared by polishing buccal surfaces with a 600-grit silicon carbide paper using an automatic polishing device (MINITECH 233, PRESI, France) at 100 rpm for 45 s. From each buccal surface, two enamel slabs (2 × 2 × 3 mm3) were prepared, totaling 56 slabs. Each slab was fixed in a silicone mold (15-mm in diameter by 15-mm in thickness), filled with epoxy resin, and allowed to cure for 24 h. Throughout the non-treatment periods, the specimens were stored in 100 % humidity at 37 °C.
2.2. Artificial caries induction
Artificial white spot lesions were induced by immersion of specimens into a demineralizing solution for 4 days at 37 OC in an incubator shaker. Composition of the demineralizing solution was 2.2 mM CaCl2, 2.2 mM KH2PO4, and 0.05 M acetic acid, pH adjusted with 1 M KOH to be 4.4 (Manosubsak et al., 2019, Rana et al., 2007). After demineralization, the specimens were rinsed with deionized water for 30 s. Two slabs from each of 28 premolars were randomly assigned to the test group for resin infiltration treatment, while the control group received no treatment.
2.3. Resin infiltration procedure
The resin infiltration material’s composition (Icon, DMG, Germany) is detailed in Table 1. Following the manufacturer’s instruction, a 15 % hydrochloric acid (Icon-Etch, DMG, Germany) was applied onto the artificial caries surface for 2 min using a microbrush, water-rinsed for 30 s and air-dried for 10 s. Subsequently, a 99 % ethanol (Icon-Dry, DMG, Germany) was applied for 30 s and air-dried. A resin infiltrant (Icon-Infiltrant, DMG, Germany) was then applied for 3 min, excess removed, and light-cured (DemiTM LED light-curing system, Kerr, USA) for 40 s with an irradiance of 1,000 mW/cm2 at 1 mm distance. An infiltrant was re-applied for another minute, excess removed, and light-cured. Surface polishing employed fine and superfine aluminum oxide discs (Sof-Lex, 3 M ESPE, USA) using a slow-speed handpiece with intermittent brushing movement and light pressure, involving 10 strokes per disc and water-rinsing between sequences (Almulhim et al., 2021, Venturini et al., 2006).
Table 1.
Composition of resin infiltration material according to the manufacturer’s information.
| Product (Trade name) | Manufacturer | Composition |
|---|---|---|
| Resin infiltration material (Icon) Lot number: 261397 | DMG, Germany | Icon-etch: 15% hydrochloric acid, pyrogenic silicic acid, surface-active substance Icon-Dry: 99% ethanol Icon-Infiltration: TEGDMA-base resin, initiators, additives |
TEGDMA: Triethylene glycol dimethacrylate.
2.4. Aging procedures
To simulate the normal oral environment in terms of temperature and acid fluctuation, specimens were subjected to a consistent sequence of laboratory procedures. Initially, the specimens were thermocycled for 10,000 cycles (Thermo Cycling Unit, KMITL, Thailand) between 5 °C and 55 °C with a 30-second dwell period. Subsequently, a 10-day pH cycling was performed in a 37 °C incubator. Each cycle involved 3-hour demineralization, 2-hour remineralization, 3-hour demineralization, and 15-hour remineralization (Ozgul et al., 2015). The composition of demineralizing solution was 2.2 mM CaCl2, 2.2 mM KH2PO4, and 0.05 M acetic acid, pH adjusted with 1 M KOH to be 4.4. The remineralization solution composed of 1.5 mM CaCl2, 0.9 mM NaH2PO4, 0.15 M KCl, pH adjusted to 7 using 1 M KOH (Ozgul et al., 2015). The solutions were replaced for fresh ones for each cycle. The specimens were rinsed with distilled water for 30 s before changing to another solution.
2.5. Micro-computed tomographic (micro-CT) analysis
To assess the changes in lesion depth and mineral density of resin infiltration-treated white spot lesions, a micro-CT apparatus (µCT 35, Scanco Medical, Bassersdorf, Switzerland) was used to measure the lesion depth and mineral density before and after aging. The lowest part of each slab served as a reference point. Positioning of the specimens was carefully maintained to ensure that they were in the same orientation through the experiment procedures. Scanning parameters were set at 70 kVp voltage, 114 µA current, and 9 µm pixel size. High-resolution mode (2048 × 2048 pixels) with a 0.5-mm thick aluminum filter was used. The micro-CT was calibrated employing a set of hydroxyapatite phantom standards. The scanning results were reconstructed and analyzed using the SCANCO (Scanco, Bruttisellen, Switzerland) and ImageJ softwares (version 1.8.0, NIH, Bethesda, USA). The cross-sectional images showing the lesion area of each specimen were randomly selected for 10 images (Zhao et al., 2020). Three regions in each image were assessed. The depth of the lesion was identified based on the grayscale value corresponding to 95 % of sound enamel (Liu et al., 2012). Mineral density (mgHA/cm3) was calculated at the center (150 × 150 pixels) for 30 slides through the lesion depth using volumetric measurements from the SCANCO software.
The mean values of lesion depth progression and percentage change of mineral density were calculated. The percentage change of mineral density was calculated as follows:
3. Statistical analysis
Statistical analysis was run using the IBM SPSS version 26.0 (IBM, Chicago, USA). The Shapiro-Wilks test was utilized to confirm normal data distribution for all outcomes. Lesion depth and mineral density between before and after aging within each group were assessed by the paired sample t-test. Lesion depth progression and percentage change of mineral density between groups were assessed by the independent samples t-test at a significance level of 0.05.
4. Results
Table 2 displays the mean lesion depth before and after aging and the lesion progression. The resin-infiltrated group exhibited significantly lower mean lesion depth progression than the control group (p < 0.001). Mean mineral density and percentage change, are presented on Table 3. There was a meaningful difference before and after aging in both groups, and the resin-infiltrated group exhibited significantly lower mineral loss compared to control group (p < 0.001).
Table 2.
Mean lesion depth and mean lesion depth progression in the control and resin infiltration groups (mean ± SD).
| Groups | Mean lesion depth (µm) ± SD | Within-group p Value |
Mean lesion depth progression ± SD | Between-group p Value |
|
|---|---|---|---|---|---|
| Before aging | After aging | ||||
| Control group | 254.88 ± 21.07A | 387.75 ± 20.94B | <0.001 | 132.88 ± 4.18a | <0.001 |
| Resin infiltration group | 188.38 ± 17.42C | 240.69 ± 17.48D | 52.31 ± 4.16b | ||
Different capital letters indicate statistically significant differences in the same row (between before and after aging in the same group) (p < 0.05).
Different lower-case letters indicate statistically significant differences in the column (between resin infiltration group and control group) (p < 0.05).
Table 3.
Mean mineral density and mean percentage change in mineral density loss in the control and resin infiltration groups (mean ± SD).
| Groups | Mean mineral density (mgHA/cm3) ± SD |
Within-group p Value |
Mean % mineral loss ± SD | Between-group p Value |
|
|---|---|---|---|---|---|
| Before aging | After aging | ||||
| Control group | 1,889.52 ± 65.48A | 1,585.68 ± 65.2B | <0.001 | 16.1 ± 0.64a | <0.001 |
| Resin infiltration group | 1,948.71 ± 48.23C | 1,776.78 ± 47.7D | 8.83 ± 0.30b | ||
Different capital letters indicate statistically significant differences in the same row (between before and after aging in the same group) (p < 0.05).
Different lower-case letters indicate statistically significant differences in the column (between resin infiltration group and control group) (p < 0.05).
mgHA: Milligrams hydroxyapatite.
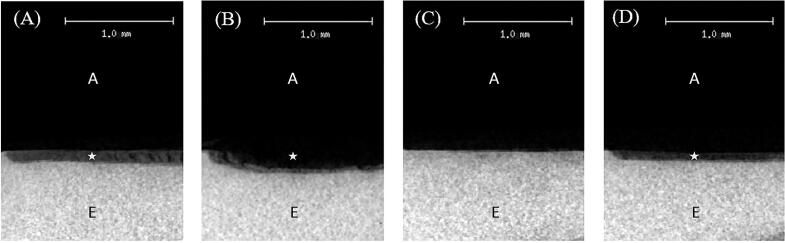
Fig. 2 illustrates the radiolucency and radiopacity of both groups before and after aging, as analyzed by a micro-CT. Although the radiopacity of lesion in resin-infiltrated group after aging (Fig. 2D) showed lower density than that of before aging (Fig. 2C), both groups demonstrated higher density than the control group (Fig. 2A and B). However, the radiopacity observed in all groups at before and after aging remained lower than that of the underlying sound enamel.
Fig. 2.
Representative 2D micro-CT images of the control group before (A) and after aging (B), and the resin infiltration group before (C) and after aging (D). Micro-CT images show demineralized area (star-shaped area) and sound enamel (E). The letter A is an area of air. Picture C shows no star-shaped area because the boundary of demineralized area could not be specified.
5. Discussion
This study evaluated the lesion depth and mineral density changes of resin infiltration-treated white spot lesions against a simulated oral environment using thermal and pH changes. The findings exhibited significant differences in lesion depth and mineral loss between the resin-infiltrated group before and after aging. Moreover, following the thermal and acidic challenges, the resin-infiltrated group presented lower lesion depth and percentage of mineral changes compared to the control untreated group. Therefore, both null hypotheses were rejected.
The micro-CT was chosen to evaluate the changes in lesion depth and mineral density because it has shown to be an effective and non-destructive approach to produce high-resolution images, allowing specimens to be examined again after aging (Kuhn et al., 1990). It provided excellent specificity in identifying enamel lesion in comparison to the gold standard methods of histology and transverse microradiography (Hamba et al., 2012, Oliveira et al., 2020). However, there have been certain drawbacks that limit its utility, including expensive cost, time-consuming scanning and reconstruction, and challenges associated with the management of large data volumes (Erpaçal et al., 2019).
Polishing surface of resin infiltration was suggested in order to reduce flaws and roughness, simultaneously increasing surface microhardness (Esteves-Oliveira et al., 2022). Roughness of resin infiltration on artificial enamel caries was reported to be lowest comparing to other non-invasive treatment (Yazkan and Ermis, 2018). Smoothness of material probably attenuates plaque accumulation, improves surface integrity, and contributes to increase material longevity. Consequently, the protocol in our study utilized the polishing method that exhibited the proper results in accordance with an aforementioned study (Esteves-Oliveira et al., 2022).
Our study found that a single session of resin infiltration significantly curtailed white spot lesion progression compared to the control group. An in vitro study conducted by Ozgul et al. (2015) similarly indicated that the lesion progression of the resin-infiltrated group following pH cycling was lower than that of the non-treated group, although this disparity did not reach statistical significance. These results align with earlier clinical investigations endorsing the effectiveness of resin infiltration in impeding the advancement of non-cavitated carious lesions. Paris et al. (2020) demonstrated a substantial difference, with only 9 % of infiltrated lesions exhibited caries progression compared to 45 % of control lesions. Moreover, Arslan and Kaplan (2020) highlighted the superior efficacy of resin infiltration in preventing the progression of non-cavitated carious lesions over a 1-year period.
The success in inhibiting further demineralization by resin infiltration may be influenced by complete resin infiltration through the lesion which occurred by capillary forces (Klaisiri and J., Thamrongananskul, N., Sriamporn, T., & Krajangta, N. , 2020, Paris et al., 2013). Resin infiltration aimed to create a diffusion barrier within the lesion by occluding microporosities through the lesion body with low-viscosity light-cured resins having a refractive index close to sound enamel (Dorri et al., 2015). Resin infiltration could envelop enamel crystallites and form an enamel hybrid layer, making infiltrated lesions became more acid-resistant and potentially preventing further demineralization (Klaisiri and J., Thamrongananskul, N., Sriamporn, T., & Krajangta, N. , 2020, Paris et al., 2020, Perdigão, 2020). However, a systematic review found that resin penetration depth was 65.39 % in white spot lesions (Soveral et al., 2021), as seen in our micro-CT image (Fig. 2C). The polymeric chain formation also did not occur throughout the entire lesion, as it depended on the enamel characteristics; therefore, infiltrated lesions may be prone to deterioration as a result of incomplete resin infiltration (Belli et al., 2011, Soveral et al., 2021).
Inducing artificial white spot lesions probably created lesions different from natural ones. This process might yield non-cavitated lesions that lack the mineralized surface layer. This artificial lesion might be sensitive to a 15 % hydrochloric acid, applied on surface lesion to remove such a mineralized layer (Paris et al., 2013). The aggressive nature of the acid applied to the artificial lesion might have implications for resin infiltration, potentially limiting its ability to complete all the porosities within the lesion and leading to incomplete resin infiltration, as depicted in Fig. 2C. The distinctions between artificial and natural lesions, particularly in terms of the mineralized surface layer and the aggressive nature of the acid treatment, shed light on potential limitations or challenges in the resin infiltration process when applied to artificially induced lesions.
A low-viscosity light-cured resin utilized in our treatment, TEGDMA, is known for its high-water absorption properties (Chen et al., 2019, Polak-Kowalska and Pels, 2019). Besides, the potential impact of lower filler quantities contributes to increased polymerization shrinkage (Polak-Kowalska and Pels, 2019). The mechanical properties of the vulnerable resin, which underwent the dual aging procedures employed in our study, may be affected. Thermocycling, based on hydrolysis and thermal expansion principles (Amaral et al., 2007), could cause crack propagation and gaps along the bonded interface, allowing fluids to pass through causing hydrolysis at the interface (Amaral et al., 2007, Deng et al., 2014). A combination challenge with pH-cycling, imitating mineral loss and gain during caries formation (Amaechi, 2019, Lei et al., 2016), may contribute to enamel demineralization at restoration margins, resulting in increased gap formation and fluid flow through the interfaces (Amaral et al., 2007). Such dual procedures in our study potentially led to the observed meaningful increases in lesion progression and percentage of mineral changes in the resin-infiltrated group after aging (Table 2, Table 3).
Given the array of proposed remineralization techniques over time, it is imperative for future studies to delve into their potential and efficacy, particularly in clinical settings. Comparisons with classical techniques such as fluoride application are also warranted. Furthermore, there is a need for qualitative evaluations utilizing scanning electron microscopy (SEM) techniques. Introducing color-tagged fluorescence to the resin infiltrant could aid in identifying degradation sites. Additionally, incorporating microbial aging procedures would be beneficial for further investigation since bacterial toxins may further deteriorate the sensitive resin-based material and increase risks of degradation. These approaches have the potential to enhance outcomes significantly. Ultimately, clinical studies with long-term follow-ups are necessary to definitively establish the efficacy of these techniques.
6. Conclusion
This study has shown that changes in lesion depth and mineral density during simulated aging processes in resin infiltration-treated lesions are lower than untreated white spot lesions. Resin infiltration is a promising method of inhibiting the progression of white spot lesions related to initial dental caries.
Ethical approval and informed consent
Research protocol was approved by the Human Research Ethics Committee of the Faculty of Dentistry, Chulalongkorn University (HREC-DCU 2022-052).
CRediT authorship contribution statement
Vongnart Predapramote: Investigation, Writing-original draft. Yanee Tantilertanant: Writing – review & editing, Validation. Sirivimol Srisawasdi: Supervision, Writing – review & editing, Validation.
Acknowledgements
The authors would like to thank the Dental Material Science Research Center and Oral Biology Research Center, the Faculty of Dentistry, Chulalongkorn University for their hospitality. The authors appreciate Assist. Prof. Dr. Soranun Chantarangsu for her guidance with statistical consultation. Dr. Joao Ferreira provided assistance in the editing of this manuscript. We received with financial assistance for expenses by the Faculty of Dentistry, Chulalongkorn University. No external funding was obtained for this research.
References
- Almulhim K., Khan A.S., Alabdulghani H., Albasarah S., Al-Dulaijan Y., Al-Qarni F.D. Effect of ageing process and brushing on color stability and surface roughness of treated white spot lesions: an in vitro analysis. Clin. Cosmet. Investig. Dent. 2021;13:413–419. doi: 10.2147/ccide.S334633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaechi B.T. Protocols to study dental caries in vitro: pH cycling models. Methods Mol. Biol. 2019;1922:379–392. doi: 10.1007/978-1-4939-9012-2_34. [DOI] [PubMed] [Google Scholar]
- Amaral F.L., Colucci V., Palma-Dibb R.G., Corona S.A. Assessment of in vitro methods used to promote adhesive interface degradation: a critical review. J. Esthet. Restor. Dent. 2007;19(6):340–354. doi: 10.1111/j.1708-8240.2007.00134.x. [DOI] [PubMed] [Google Scholar]
- Arslan S., Kaplan M.H. The effect of resin infiltration on the progression of proximal caries lesions: a randomized clinical trial. Med. Princ. Pract. 2020;29(3):238–243. doi: 10.1159/000503053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydın B., Pamir T., Baltaci A., Orman M.N., Turk T. Effect of storage solutions on microhardness of crown enamel and dentin. European Journal of Dentistry. 2015;9(2):262–266. doi: 10.4103/1305-7456.156848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belli R., Rahiotis C., Schubert E.W., Baratieri L.N., Petschelt A., Lohbauer U. Wear and morphology of infiltrated white spot lesions. J. Dent. 2011;39(5):376–385. doi: 10.1016/j.jdent.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Chen M., Li J.Z., Zuo Q.L., Liu C., Jiang H., Du M.Q. Accelerated aging effects on color, microhardness and microstructure of ICON resin infiltration. Eur. Rev. Med. Pharmacol. Sci. 2019;23(18):7722–7731. doi: 10.26355/eurrev_201909_18981. [DOI] [PubMed] [Google Scholar]
- Deng D., Yang H., Guo J., Chen X., Zhang W., Huang C. Effects of different artificial ageing methods on the degradation of adhesive-dentine interfaces. J. Dent. 2014;42(12):1577–1585. doi: 10.1016/j.jdent.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Denis M., Atlan A., Vennat E., Tirlet G., Attal J.P. White defects on enamel: diagnosis and anatomopathology: two essential factors for proper treatment (part 1) Int. Orthod. 2013;11(2):139–165. doi: 10.1016/j.ortho.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Dorri, M., Dunne, S. M., Walsh, T., & Schwendicke, F. (2015). Micro-invasive interventions for managing proximal dental decay in primary and permanent teeth. The Cochrane database of systematic reviews(11). doi:10.1002/14651858.CD010431.pub2. [DOI] [PMC free article] [PubMed]
- Erpaçal B., Adıgüzel Ö., Cangül S. The use of micro-computed tomography in dental applications. International Dental Research. 2019;9(2):78–91. doi: 10.5577/intdentres.2019.vol9.no2.7. [DOI] [Google Scholar]
- Esteves-Oliveira M., Passos V.F., Russi T., Fernandes A.R.R., Terto C.N.N., Mendonça J.S., Lima J.P.M. Randomized in situ evaluation of surface polishing protocols on the caries-protective effect of resin infiltrant. Sci. Rep. 2022;12(1):20648. doi: 10.1038/s41598-022-25091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frencken J.E., Peters M.C., Manton D.J., Leal S.C., Gordan V.V., Eden E. Minimal intervention dentistry for managing dental caries - a review: report of a FDI task group. Int. Dent. J. 2012;62(5):223–243. doi: 10.1111/idj.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamba H., Nikaido T., Sadr A., Nakashima S., Tagami J. Enamel lesion parameter correlations between polychromatic micro-CT and TMR. J. Dent. Res. 2012;91(6):586–591. doi: 10.1177/0022034512444127. [DOI] [PubMed] [Google Scholar]
- Klaisiri A.R.J., Thamrongananskul N., Sriamporn T., Krajangta N. Management of Initial Carious Lesion by resin infiltration technique. Srinakharinwirot University Dental Journal. 2020;13:65–76. [Google Scholar]
- Knösel M., Eckstein A., Helms H.J. Long-term follow-up of camouflage effects following resin infiltration of post orthodontic white-spot lesions in vivo. Angle Orthod. 2019;89(1):33–39. doi: 10.2319/052118-383.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J.L., Goldstein S.A., Feldkamp L.A., Goulet R.W., Jesion G. Evaluation of a microcomputed tomography system to study trabecular bone structure. J. Orthop. Res. 1990;8(6):833–842. doi: 10.1002/jor.1100080608. [DOI] [PubMed] [Google Scholar]
- Lei C., Jiyao L., Hockin H.K.X., Xuedong Z. In: Dental Caries: Principles and Management. Xuedong Z., editor. Springer, Berlin Heidelberg; Berlin, Heidelberg: 2016. Demineralization and remineralization; pp. 71–83. [Google Scholar]
- Liu B.Y., Lo E.C., Li C.M. Effect of silver and fluoride ions on enamel demineralization: a quantitative study using micro-computed tomography. Aust. Dent. J. 2012;57(1):65–70. doi: 10.1111/j.1834-7819.2011.01641.x. [DOI] [PubMed] [Google Scholar]
- Manosubsak N., Sukarawan W., Sriarj W. The remineralization quality by fluoridated dentifrice on artificial incipient caries lesion. Mahidol Dental Journal. 2019;39(1):53–62. [Google Scholar]
- Oliveira L.B., Massignan C., Oenning A.C., Rovaris K., Bolan M., Porporatti A.L., De Luca Canto G. Validity of micro-CT for in vitro caries detection: a systematic review and meta-analysis. Dentomaxillofacial Radiology. 2020;49(7) doi: 10.1259/dmfr.20190347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgul B.M., Orhan K., Oz F.T. Micro-computed tomographic analysis of progression of artificial enamel lesions in primary and permanent teeth after resin infiltration. J. Oral Sci. 2015;57(3):177–183. doi: 10.2334/josnusd.57.177. [DOI] [PubMed] [Google Scholar]
- Paris S., Soviero V.M., Schuch M., Meyer-Lueckel H. Pretreatment of natural caries lesions affects penetration depth of infiltrants in vitro. Clin. Oral Invest. 2013;17(9):2085–2089. doi: 10.1007/s00784-012-0909-8. [DOI] [PubMed] [Google Scholar]
- Paris S., Bitter K., Krois J., Meyer-Lueckel H. Seven-year-efficacy of proximal caries infiltration - randomized clinical trial. J. Dent. 2020;93 doi: 10.1016/j.jdent.2020.103277. [DOI] [PubMed] [Google Scholar]
- Perdigão J. Resin infiltration of enamel white spot lesions: an ultramorphological analysis. J. Esthet. Restor. Dent. 2020;32(3):317–324. doi: 10.1111/jerd.12550. [DOI] [PubMed] [Google Scholar]
- Polak-Kowalska K., Pels E. In vitro and in vivo assessment of enamel colour stability in teeth treated with low-viscosity resin infiltration–a literature review. Journal of Stomatology. 2019;72(3):135–141. [Google Scholar]
- Rana R., Itthagarun A., King N.M. Effects of dentifrices on artificial caries like lesions: an in vitro pH cycling study. Int. Dent. J. 2007;57(4):243–248. doi: 10.1111/j.1875-595x.2007.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Soveral M., Machado V., Botelho J., Mendes J.J., Manso C. Effect of resin infiltration on enamel: a systematic review and meta-analysis. Journal of Functional Biomaterials. 2021;12(3):48. doi: 10.3390/jfb12030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturdevant, C. M., Ritter, A. V., Boushell, L. W., & Walter, R. (2019). Sturdevant's art and science of operative dentistry (7th ed.): St. Louis, Missouri: Elsevier Inc.
- Venturini D., Cenci M.S., Demarco F.F., Camacho G.B., Powers J.M. Effect of polishing techniques and time on surface roughness, hardness and microleakage of resin composite restorations. Oper. Dent. 2006;31(1):11–17. doi: 10.2341/04-155. [DOI] [PubMed] [Google Scholar]
- Wang J.X., Yan Y., Wang X.J. Clinical evaluation of remineralization potential of casein phosphopeptide amorphous calcium phosphate nanocomplexes for enamel decalcification in orthodontics. Chin Med J (Engl) 2012;125(22):4018–4021. [PubMed] [Google Scholar]
- Yazkan B., Ermis R.B. Effect of resin infiltration and microabrasion on the microhardness, surface roughness and morphology of incipient carious lesions. Acta Odontol. Scand. 2018;76(7):473–481. doi: 10.1080/00016357.2018.1437217. [DOI] [PubMed] [Google Scholar]
- Zhao I.S., Yin I.X., Mei M.L., Lo E.C.M., Tang J., Li Q., Chu C.H. Remineralising dentine caries using sodium fluoride with silver nanoparticles: an in vitro study. Int. J. Nanomed. 2020;15:2829–2839. doi: 10.2147/ijn.S247550. [DOI] [PMC free article] [PubMed] [Google Scholar]