Abstract
Advancements in comprehending myelodysplastic neoplasms (MDS) have unfolded significantly in recent years, elucidating a myriad of cellular and molecular underpinnings integral to disease progression. While molecular inclusions into prognostic models have substantively advanced risk stratification, recent revelations have emphasized the pivotal role of immune dysregulation within the bone marrow milieu during MDS evolution. Nonetheless, immunotherapy for MDS has not experienced breakthroughs seen in other malignancies, partly attributable to the absence of an immune classification that could stratify patients toward optimally targeted immunotherapeutic approaches. A pivotal obstacle to establishing “immune classes” among MDS patients is the absence of validated accepted immune panels suitable for routine application in clinical laboratories. In response, we formed International Integrative Innovative Immunology for MDS (i4MDS), a consortium of multidisciplinary experts, and created the following recommendations for standardized methodologies to monitor immune responses in MDS. A central goal of i4MDS is the development of an immune score that could be incorporated into current clinical risk stratification models. This position paper first consolidates current knowledge on MDS immunology. Subsequently, in collaboration with clinical and laboratory specialists, we introduce flow cytometry panels and cytokine assays, meticulously devised for clinical laboratories, aiming to monitor the immune status of MDS patients, evaluating both immune fitness and identifying potential immune “risk factors.” By amalgamating this immunological characterization data and molecular data, we aim to enhance patient stratification, identify predictive markers for treatment responsiveness, and accelerate the development of systems immunology tools and innovative immunotherapies.
INTRODUCTION
Myelodysplastic neoplasms (MDS) are a heterogeneous group of clonal hematopoietic stem and progenitor cell (HSPC) disorders arising in the bone marrow (BM), 1 characterized by ineffective hematopoiesis, dysplasia, peripheral cytopenias, and an increased risk of transformation to acute myeloid leukemia (AML). 2 , 3 MDS evolve from clonal HSPC outgrowth with the acquisition of genetic lesions and the implementation of genetic alterations into prognostic scoring systems has improved MDS risk stratification. 4 , 5 , 6
In addition to cell‐intrinsic lesions, increasing evidence suggests that immune disruption and tumoral BM microenvironment alterations occur during MDS pathogenesis. 7 , 8 , 9 , 10
The association between inflammation and MDS 11 is reinforced by their linkage to an autoimmune disorder, present in 10%–20% of MDS patients. 12 , 13 , 14 MDS‐associated autoimmune disorders are typically refractory to immunosuppressive agents and difficult to manage, 12 , 15 and are associated with adverse impact on both patients' quality of life and clinical outcome. 16 While immunomodulatory therapies may lead to a sustained response in some patients, universally accepted biomarkers that predict such a response are not yet established. 17 , 18 , 19
Despite growing evidence highlighting the role of immune dysregulation in the pathogenesis and prognosis of MDS, 20 , 21 immune therapies remain underutilized in MDS management. Additionally, current diagnostic workups and prognostic models do not typically assess host immunity. 5 , 22
The International Integrative Innovative Immunology for MDS (i4MDS) initiative, supported by the European Hematology Association (EHA), is a consortium of clinician scientists, biologists, and physicians actively engaged in MDS patient research and clinical care. We envision that incorporating immunological profiling alongside molecular data could enhance patient stratification, uncover predictive treatment response biomarkers, and steer the development of innovative immunotherapies.
In this position paper, we review the main immune cell dysregulation during the disease course and propose comprehensive guidelines to harmonize and routinely implement immune assessment in MDS.
METHODS
Since its establishment in 2023, the i4MDS consortium has grown to encompass 27 centers across 10 countries, selected for their experience in the field of immunology in MDS.
The consortium has conducted several meetings to assess existing literature on immune dysregulation in MDS and to formulate recommendations for the monitoring of immune cells and cytokines.
As an initial step, i4MDS conducted a comprehensive literature search, including articles published between 1999 and 2023. This search focused on keywords such as “Myelodysplastic Syndromes” in conjunction with the name of specific immune cell types, including “Cytotoxic T lymphocytes (CTL),” “Regulatory T (Treg) cells,” “T helper (Th) cells,” “Gamma‐delta (γδ) T cells,” “B lymphocytes (Ly),” “Natural Killer (NK) cells,” “Dendritic cells (DC),” “Monocytes,” “Macrophages,” “Myeloid‐derived suppressor cells (MDSC),” “Mesenchymal Stem cells (MSC),” and “Cytokines.” Articles selected for the review of each immune cell type were included if they explored variations in cell number, phenotype, and/or function among MDS patients (Figure 1).
Figure 1.
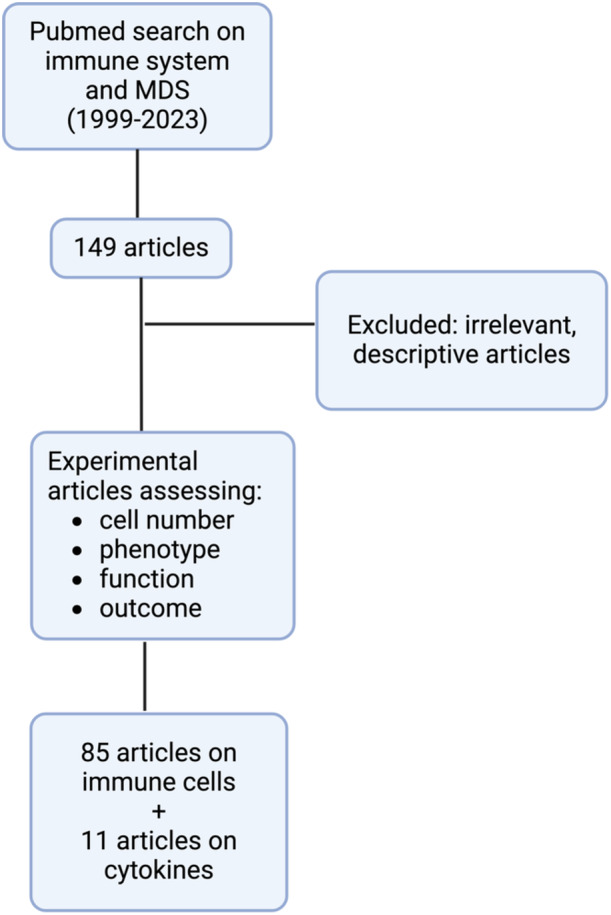
Flow diagram for literature review regarding immune cell type alterations in myelodysplastic neoplasms.
Second, i4MDS conducted a survey involving eleven hemato‐immunological clinical centers with expertise in the field: Leipzig University Hospital (Germany), MLL Munich Leukemia Laboratory (Germany), The National Heart, Lung and Blood Institute (US), Rigshospitalet, University of Copenhagen (Denmark), UMC of Amsterdam (Netherlands), University Hospital of Dresden Carl Gustav Carus (Germany), Vall d'Hebron Hospital (Spain), HMDS of Leeds (UK), Saint‐Louis Hospital of Paris (France), and Cochin Hospital of Paris (France). The aim was to identify a core set of immune cell markers for clinical flow cytometry and clinically relevant cytokines for routine assessment in clinical care. The proposed recommendations underwent an iterative panel review process aimed at achieving consensus.
IMMUNE CELL DYSREGULATIONS IN MYELODYSPLASTIC NEOPLASMS
Low‐risk (LR) MDS are characterized by a “proinflammatory state” with a higher prevalence of effector cells (e.g., Th17 and CTL) 8 , 23 whereas high‐risk (HR) MDS are dominated by an “immunosuppressive state” with increased regulatory T cells (Tregs). 7 We highlight the complex modifications in cell repertoire that occur during MDS progression (Figure 2 and Table 1). Additionally, as malignant stem cells can differentiate into mature immune cells, MDS‐related genetic lesions transmitted to downstream myeloid and lymphoid progeny can trigger aberrant inflammatory signaling and contribute to maturation defects and immune dysregulation. 106 , 107
Figure 2.
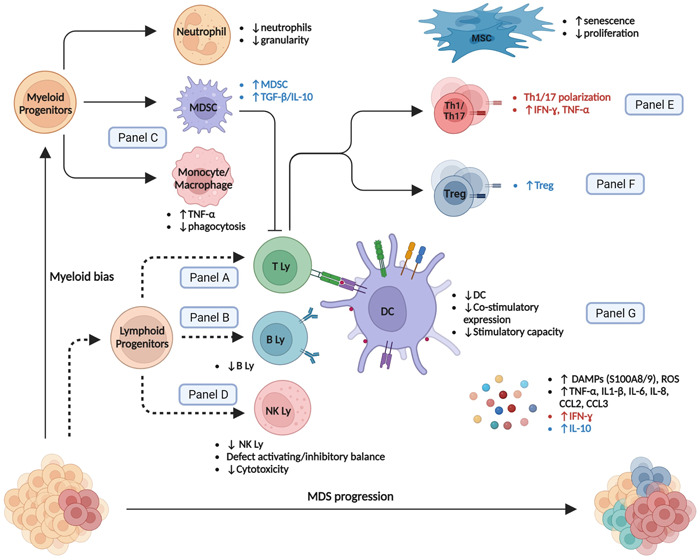
Immune and bone marrow environment changes during the MDS course. Main alterations in low‐risk, high‐risk, or all MDS patients are specified in red, blue, and black, respectively. DC, dendritic cell; IL, interleukin; Ly, lymphocyte; MDS, myelodysplastic neoplasms; MDSC, myeloid‐derived suppressor cell; MSC, mesenchymal stromal cell; NK, natural killer; Th, Helper CD4+ T cell; Treg, regulatory T cell. Dashed lines represent impaired differentiation.
Table 1.
Summary of major innate and adaptive immune cell dysregulation in MDS
| Cell type | Number | Phenotype | Function | Outcome | Other |
|---|---|---|---|---|---|
| Neutrophils |
↓ Neutrophils 24 ↓ CD54highCD181low subset, especially in HR‐MDS 7 |
↓ CD43 25 and GM‐CSF receptor expression 26 (PB) |
↓ Phagocytosis 7 ↓ Bactericidal and fungicidal activities 7 |
Neutropenia correlated with reduced LFS and OS 22 , 27 | ↓CD54highCD181low correlated with response to decitabine 28 (PB) |
| Natural killer cells (NK cells) |
↓ Total NK 29 , 30 , 31 (PB + BM) ↑ CD56bright/↓ CD56dim NK Cells ratio 32 (PB + BM) |
↓ NKG2D, DNAM1, NKp30, NKp46 (activation) 32 , 33 , 34 , 35 (PB + BM) ↑ TIGIT (inhibitory) 36 (PB) ↓ Perforine/Granzyme B (lytic granules 29 (PB) ↓ KIR (inhibitory/licensing) 32 (PB + BM) |
↓ CD56dim correlated with reduced OS 40 (BM) KIR haplotype correlated with reduced LFS and reduced OS 41 ↓ cytotoxicity correlated with reduced OS 42 (PB) |
NK cells derived from MDS clone 38 (PB) ↓ function associated with higher IPSS, abnormal karyotype, excess blasts, and age‐adjusted BM hypercellularity 37 (PB + BM) ↓ activating receptor expression correlated with elevated BM blasts 33 (BM) |
|
|
Dendritic cells (DC) |
↓ Total DC 21 , 43 , 44 , 45 (PB) ↓ BM DC progenitors 21 (PB) ↓ Myeloid cDC 46 (BM) ↓ pDC 46 (BM) |
↓ CD1a, CD54, CD80 and MHC class II 44 (PB) ↓ HLA‐DR, CD11c, CD80 and CD86 costimulatory molecules expression 47 (PB) |
↓ Allostimulatory capacity 44 , 45 , 48 , 49 (PB + BM) ↓ Endocytosis 45 (PB) ↑ IL‐10 secretion 49 (PB) |
↓ CD141High cDC correlated with reduced OS 21 (PB) | DC derived from MDS clone 43 , 44 , 45 (PB) |
| MDSC | ↑ in HR‐MDS 10 , 50 (PB + BM) |
↑ Production of S100A9 51 (BM) ↑ IL‐10/IL‐12 and TGF‐β/TNF‐α ratio in HR‐MDS 52 (BM) |
↑ TGF‐β/TNF‐α ratio correlated with increased blast % 52 (BM) | Expansion driven by S100A‐CD33 interaction 50 (BM) | |
| Monocytes/macrophages |
↑ Total, 53 notably in HR‐MDS 54 (BM) ↓ Classical monocytes 55 (PB) |
↑ CD40, 56 HLA‐DR expression 57 (PB + BM) ↑ Thrombomodulin expression in LR‐MDS 58 (PB) ↓ CD206 and SIRPα expression 53 (PB) |
↑ TGF‐β 54 and TNF‐α 56 , 59 production (PB + BM) ↓ Phagocytic function 53 (PB) ↑ Apoptotic index 59 (BM) |
↑ BM monocytes correlated with adverse prognosis 60 (BM) ↑ CD300E correlated with higher MDS risk and adverse LFS 60 (BM) |
|
| T lymphocytes | ↑ Surface expression of activation markers (HLA‐DR+, CD57+, CD28−, CD62L−) 61 (PB + BM) | T clonality restriction, 3 , 62 loss of clonal T‐cell populations in responders to ATG‐based treatment 3 | |||
| Cytotoxic T cells (CTL) |
↑ Effector CTL (CD57+) 63 , 64 , 65 (PB + BM) ↓ Total CTL 66 (PB) |
Exhausted (expression of TIM3, CD39, PD1, CTLA4) 32 , 52 , 66 , 67 (PB + BM) WT‐1 specific cytotoxic T cells have a unique phenotype with coexpression of CD39 and CXCR4 68 (PB + BM) Contracted TCR‐V β repertoire (clonal/oligoclonal) 64 , 69 (PB + BM) |
↓ Cytotoxicity 65 , 66 , 70 (PB + BM) Effector CTL inhibits bone marrow hematopoiesis 71 (BM) ↑ Tc1/Tc2 ratio 72 (PB) |
In patients with abnormal karyotype deprivation of effector T cells increased the proportion of abnormal cells 71 (BM) | |
| T regulatory cells (Treg) | In LR‐MDS dysfunctional and impaired BM homing, in HR‐MDS they retain their function and migratory capacity 74 (PB + BM) |
↑ Treg in LR‐MDS correlated with adverse prognosis 79 (PB + BM) ↑ CD4+ Treg correlated with adverse prognosis 7 (PB) In LR‐MDS Treg subset CD4(+)FOXP3(+)CD25(+)CD127(low)CD45RA(−) CD27(−) (effector memory Treg) correlated with adverse prognosis 80 (PB) ↑ Naïve Treg/induced Treg ratio correlated with adverse prognosis 81 (BM) |
↓ In patients responding to treatment (induction cht) 74 (PB + BM) | ||
|
T helper cells (Th) |
↑ Th1/Th2 ratio especially in LR‐MDS 72 , 82 (PB + BM) ↔ Th1/Th2 ratio 83 (PB) ↑ Th17 in LR‐MDS 8 , 24 , 84 , 85 (PB + BM) ↑ Th22 higher in HR‐MDS 24 (PB + BM) |
↑ CD40L expression 56 (PB + BM) |
High Th1 correlated with levels of INF‐gamma/TNF‐α and apoptotic index of nucleated cells 82 (BM) ↑ Th17 function in LR‐MDS 84 (PB + BM) |
↑ IL‐17 expression associated with more severe anemia 81 (BM) Th17 positive relation with normal karyotype, ANC, and Hb concentration; negative relation with morphologic blast count 84 (PB + BM) |
|
| Gamma‐delta T cells | ↓ Total gamma delta T cells 86 (PB) | ↔ Function 86 (PB) | Abnormalities in the Vdelta receptor repertoire 87 , 88 (PB) | ||
| B cells | ↓ Increase in apoptosis 89 and reduced progenitors in LR‐MDS 90 (BM) | ↓ B progenitors correlated with adverse prognosis 79 (PB + BM) | |||
| MSC | ↓ Proliferation 91 , 92 , 93 and increased senescence 93 , 94 , 95 (BM) |
↔ Phenotype 96 (BM) Altered expression of osteopontin, Jagged1, Kit‐ligand and angiopoietin 91 (BM) |
↓ Supporting hematopoiesis function 97 , 98 (BM) ↓ Osteogenic differentiation 91 , 93 , 95 , 99 , 100 , 101 , 102 (BM) ↑ Inhibition of DC differentiation and function in HR‐MDS 103 (BM) ↑ Production of IL‐6 and TNF‐α 59 (BM) ↑ TGF‐β, apoptosis, and immunosuppression in HR‐MDS 97 (BM) |
Abnormal karyotype 104 , 105 (BM) |
Abbreviations: BM, bone marrow; HR, high risk; IPSS, international prognostic scoring system; KIR, killer‐cell immunoglobulin‐like receptor; LFS, leukemia‐free survival; LR, low‐risk; MDS, myelodysplastic neoplasms; NK, natural killer; OS, overall survival; p/cDC, plasmacytoid (pDC)/conventional myeloid (cDC); PB, peripheral blood.
CHANGES IN INNATE IMMUNITY
Neutrophils
Functional deficits, including reduced phagocytosis 108 and decreased bactericidal and fungicidal activities, 109 have been described in MDS neutrophils. Moreover, neutrophils have been found to display an aberrant phenotype with reduced expression of GM‐CSF receptor 26 and CD43 adhesion molecule, 25 correlating with defective chemotaxis mostly in HR‐MDS. 110 A recent study demonstrated a higher frequency of low‐granule neutrophils in individuals with clonal hematopoiesis related to TET2 mutations. 111 TET2‐inactivated neutrophils had more compact neutrophil extracellular traps (NET) and decreased flow cytometer (FCM) median side scatter (SSC)‐assessed granularity. Consistently, decreased granularity has also been reported in MDS patients. 112 , 113
Natural killer (NK) cells
MDS are characterized by a decrease in NK cells, mostly the mature cytotoxic CD56dim subset, and numbers inversely correlate with disease severity. 29 , 30 , 32 MDS NK cells further show a “less activated” phenotype, reduction of lytic perforin/granzyme granules, and licensing NK Killer Ig‐Like Receptors (KIRs) molecule expression. 29 , 32 , 37 These phenotypic alterations further correlate with reduced tumor necrosis factor‐α (TNF‐α) and interferon‐γ (IFN‐γ) secretion following interleukin‐2 (IL‐2) stimulation, 29 , 33 , 38 defective NK in vitro degranulation, 33 and reduced cytotoxicity capacities, 33 , 37 , 38 which have been associated with adverse prognosis. 42 A recent study correlated the presence of TET2 mutations with reduced KIR expression and impaired NK functions, which were restored with hypomethylating agent (HMA) therapy. 114
Dendritic cells (DC)
In MDS, all BM DC subsets show significantly lower frequencies ex vivo or following in vitro generation from DC precursors. 43 , 46 , 48 , 115 MDS patients also have reduced numbers of DC progenitors, 21 and the reduction in DC further correlates with higher blast percentage and IPSS risk. 46 Additionally, CD141high conventional myeloid DC numbers are decreased in MDS patients and correlate with worse overall survival (OS). 21 Functionally, MDS DCs have reduced T‐cell priming capacities, with clear T helper 1 skewing, 48 together with a reduction of their endocytic ability. 43 These functional defects are accompanied by a reduction of HLA‐DR, CD11c, and costimulatory molecule expression after activation. 47 , 49
Myeloid‐derived suppressor cells (MDSC)
The fundamental MDSC function is to secrete immunomodulatory molecules (arginase‐1, indoleamine 2,3‐dioxygenase, NADPH‐oxidase‐2), which suppresses T‐lymphocyte proliferation while transforming growth factor‐β (TGF‐β) and IL‐10 promote NK‐cell energy and the development of suppressive Treg. 116 , 117
HR‐MDS patients have increased MDSC numbers that correlate with increased Treg levels. 10 Consistently, levels of TGF‐β and IL‐10 are elevated in HR‐MDS patients. 52 MDSC from MDS patients also display an aberrant chemokine receptor profile with increased CXCR4 and CX3CR1 expression. 118
Monocytes and macrophages
While an increase in classical monocytes ≥94% 119 is highly suggestive of chronic myelomonocytic leukemia, 120 monocyte subset composition in the context of MDS remains controversial. Velegraki et al. reported a higher proportion of CD14+/CD16+ intermediate monocytes in MDS patients, 55 while Talati et al. reported increased CD14+/CD16− classical monocytes in SF3B1‐mutated MDS. 62 Further studies may help elucidate the role of monocyte repartition in MDS and its distinction from chronic myelomonocytic leukaemia, especially in light of the most recent international classifications. 2 , 3
In MDS, macrophages exhibit an altered phenotype characterized by reduced levels of CD206 and SIRPα, 53 along with heightened expression of CD68, CD86, and CD163. 121 Functionally, Meers et al. demonstrated an increase in the CD40 (on monocytes)/CD40L (on T Lymphocytes) axis in MDS patients, resulting in elevated TNF‐α secretion by monocytes and subsequent hematopoiesis suppression. 56 Furthermore, MDS monocytes display impaired ability to differentiate in macrophages and reduced in vitro phagocytic abilities. 53 , 55
CHANGES IN ADAPTIVE IMMUNITY
T cells
The current body of literature strongly indicates the presence of clonal or oligoclonal T‐cell expansion as a distinctive feature in MDS. 122 , 123 These observations support the hypothesis of a dominant clonal T lymphocyte population suppressing hematopoietic precursors, ultimately leading to BM failure.
In one of the largest studies conducted on T cells in MDS patients, 61 the numbers of peripheral blood (PB) CD4+ and CD8+ T cells in MDS patients did not significantly differ from those in age‐matched healthy individuals. However, there appeared to be a reduction in CD8+ T cells in patients with high‐risk (HR) disease. Additionally, the authors verified that most T cells exhibited an activated phenotype, both in PB and BM. This activation was characterized by heightened expression of HLA‐DR and CD57, coupled with diminished expression of CD28 and CD62L. Importantly, the presence of this activated phenotype did not appear to influence symptoms, prognosis, or risk of disease progression. 61 Furthermore, T cells derived from MDS patients exhibited elevated expression of chemokine receptors CCR3, CCR5, and CX3CR1, while showing reduced expression of CCR7. This pattern suggests that T cells in MDS tend to possess a more mature chemokine receptor profile. 124 Hypocellular MDS seem to be characterized by clonal T and NK cell expansions, respectively in HR and LR MDS. 125
Cytotoxic CD8+ T cells
Multiple studies have consistently depicted a cytotoxic environment in low and intermediate‐risk MDS. This is evidenced by several factors, including the expansion of mature effector CTLs (CD8+, CD28−, CD57+), heightened expression of granzyme B and perforin, alterations of TCR‐Vβ repertoire, and reduced frequencies of Tregs when compared to healthy individuals. 63 , 64 , 65 This augmented cytotoxicity has a dual impact: first, it suppresses BM hematopoiesis and contributes to cytopenias; second, CTLs exhibit heightened activity against MDS cells, underscoring their role in restraining the malignant clone. 71
With the progression of MDS alterations in the immune microenvironment hinder effective anti‐tumor responses. Yang et al. revealed that PD‐1 and its two ligands, PD‐L1 and PD‐L2, along with CTLA‐4, were aberrantly upregulated in BM cells from patients with myeloid disorders. This upregulation predisposes individuals to an exhausted CTL phenotype, allowing the malignant clone to evade immune surveillance. 67 This finding aligns with another study that reported reduced cytotoxic capacity in high‐risk MDS patients. 65
CD4+ T helper cells (Th)
In MDS, there is an elevation in the Th1/Th2 ratio, characterized by a Th1 bias in patients with LR‐MDS. Two studies have demonstrated a correlation between Th1 bias and the presence of proapoptotic cytokines, such as IFN‐γ and TNF‐α. This suggests that Th1 cells may contribute to nucleated cell apoptosis, and therefore BM failure, by overproducing proapoptotic cytokines. 72 , 82
Th17 cells represent a distinct subset of CD4+ T cells, characterized by the production of IL‐17. Kordasti et al. found a significantly elevated Th17 population in LR‐MDS compared to HR‐MDS and controls, together with elevated proinflammatory cytokines and a higher apoptotic index. 8 Similarly, Zhang et al. showed elevated levels of IL‐17/IL‐17R as well as increased levels of the potentially pathogenic cytokines IFN‐γ and TNF‐α in BM supernatants. Li et al. found a positive correlation between the percentage of Th17 cells, neutrophils, and hemoglobin levels, and a negative correlation with morphologic blast percentage, as well as a significantly lower Th17 frequency in unfavorable karyotype groups. The authors speculate that Th17 may have a protective role in MDS that contributes to malignant clone inhibition as well as sustaining normal hematopoiesis. 84
Th22 cells, which mainly secrete IL‐22, TNF‐α, and IL‐13, are markedly elevated in MDS compared with healthy donors, especially in HR‐MDS. 24 There may be a role of Th22 cells in the immune evasion of MDS clone(s) and disease progression. However, their role may be more complex. Current studies on Th22 in autoimmunity and cancer show a potential biphasic activity, with both inflammatory and immunosuppressive activity, based on the focal microenvironment. 24
Regulatory T cells (Tregs)
In HR‐MDS, Tregs are increased compared to LR‐MDS and controls. 7 , 73 , 126 , 127 The expansion is typically polyclonal and involves a predominantly naïve subset. 7 High levels of Tregs have been significantly correlated with MDS with excess blasts, high IPSS score, and disease progression. 7 In early‐stage disease, published results are inconclusive. Some studies show normal or near‐normal Treg frequencies, 7 whereas others show an increased number compared to healthy controls. 77 , 78 This may indicate that even LR disease can harbor a dysregulation of immune tolerance, which can negatively affect OS. 79 Kotsianidis et al. showed that early and advanced‐stage diseases are associated with differential Treg activity. 74 In early‐stage MDS, Tregs' anti‐inflammatory potential is compromised by a suppressed CXCL12/CXCR4 axis due to CXCR4 downregulation and impaired BM homing. As a result, the authors showed a reduction of Tregs in the BM microenvironment, which could be a significant mechanism supporting the autoimmunity and BM failure observed in LR‐MDS. In late disease stages, Tregs retain their function and homing and can expand locally and systemically. 74 In both early and late‐stage disease Tregs show an inverse correlation with the numbers of Th17 and CTL, 8 , 75 which could permit the emergence of the autoimmune responses observed in the context of LR‐MDS.
B lymphocytes
Literature on the role of B cells in MDS pathogenesis is limited. Meers et al. showed that MDS patients had lower B cell counts compared to age‐matched controls. 61 B‐cell progenitors are reduced in MDS patients compared with healthy controls, 90 and BM B cells display increased apoptosis. The increase of B progenitors has been shown to correlate with adverse prognosis in LR‐MDS. 79
CHANGES IN BONE MARROW MICROENVIRONMENT
Smoldering inflammation, particularly driven by NLRP3 inflammasomes and damage‐associated molecular pattern (DAMP) molecules (e.g., S100A8/A9) via Toll‐like receptors (TLR), is a main feature in MDS. 128 , 129 Constitutively activated TLR‐signaling with downstream mitogen‐activated protein kinase (MAPK) and nuclear factor kappa B (NF‐κB) activation further induces the release of proinflammatory cytokines propagating the vicious inflammatory loop, increasing pyroptosis and consequently cytopenias. 129 TLR activation promotes the secretion of several proinflammatory cytokines, including IL‐1, IL‐6, IL‐8, TNF‐α, INF‐γ, and GM‐CSF, and leads to the priming of inflammasome components and ASC (apoptosis‐associated speck‐like protein containing a caspase‐recruitment domain) protein aggregation into large cytoplasmic aggregates (ASC specks). These large complexes act as adaptors to promote pro‐caspase1 activity leading to the conversion of pro‐IL1β and pro‐IL18 into their active form, further amplifying the inflammatory loop. 129 As a result, pyroptosis, a proinflammatory lytic form of cell death, participates in ineffective hematopoiesis.
Kornblau et al. found several upregulated (CSF3, IL‐1RA, IL‐8, IL‐12, IL‐15, and CXCL10) and downregulated (IL‐4, IL‐6, IL‐7, IL‐10, IFN‐γ, CCL3, PDGF‐BB) cytokines in PB from MDS patients compared to age‐matched controls 130 (Table 2). This points toward a profound cytokine dysregulation in MDS patients, which differs according to the MDS risk group. Kordasti et al. reported higher serum levels of proinflammatory molecules, including IL‐7, IL‐12, RANTES, and IFN‐γ in LR‐MDS patients, whereas HR‐MDS showed higher levels of suppressive cytokines IL‐10 and soluble IL2R. 8 Feng et al. also observed differential cytokine profiles according to IPSS risk stratification, with decreased levels of CCL5, CXCL5, CD40L, VEGF, and EGF in HR‐MDS compared to LR‐MDS. 131
Table 2.
Summary of major cytokine alterations in MDS.
| Proinflammatory | Anti‐inflammatory | Growth factors | Outcome | |
|---|---|---|---|---|
| Cytokines |
In all MDS: ↑ IL‐1RA, IL‐15, TNFα, IL‐6, 130 , 131 IL‐8, 130 , 132 CCL2, CXCL10 132 ; ↓ CCL3, 130 CCL4 131 (PB + BM) In LR‐MDS: ↑ IL‐7, IL‐12, CCL5, INF‐γ, 8 IL‐17 8 , 133 (PB + BM) In HR‐MDS: ↓ IL‐18 70 (BM) |
In all MDS: ↑ TGF‐β, 130 IL‐27 132 ; ↓ IL‐4 130 (PB + BM) In HR‐MDS: ↑ IL‐10, soluble IL‐2R 8 (PB) |
In all MDS: ↑ G‐CSF 131 (PB) ↑ VEGFA 130 (PB) |
↑ TGF‐β correlated with higher BM blasts % 54 (BM) ↑ TNF‐α correlated with higher BM blasts %, adverse prognosis 134 (PB) ↑ IL‐6, IL‐7, and CXCL10 correlated with adverse prognosis 135 (PB) |
Abbreviations: BM, bone marrow; PB, peripheral blood.
Interestingly, some studies reported a correlation between cytokine levels with clinical outcomes. Tsimberidou et al. found that higher TNF‐α levels (>10 pg/mL) were associated with lower rates of responses and significantly lower event‐free survival and OS. 134 Additionally, increased serum CXCL10, IL‐7, and IL‐6 levels were independently associated with adverse OS in a retrospective series of 79 patients. 135
Mesenchymal stromal cells (MSC)
Numerous reports found that MDS MSC exhibit functional defects in vitro, 91 such as increased senescence, a decline in proliferation ability, 136 , 137 and a decreased osteogenic potential. 91 , 138 , 139 Perhaps most importantly, MDS MSC showed altered capacities to sustain HSPC maintenance both in MDS animal models 140 and in in vitro cocultures. 91 This could be partly attributed to the reduced expression of essential HSPC ligands, including CXCL12, ANGPT1, and KITL, 141 coupled with higher production of the proinflammatory cytokines IL‐6 and TNF‐α. 59
There is very little data regarding the dysregulation of other stromal and vascular niche cells in the context of MDS, however, BM stromal fibroblasts of MDS patients have also shown altered cytokine expression, including IL‐6, TNF‐α, IFN‐y, and TGF‐β. 142
In summary, MDS is characterized by a multitude of intricated and interconnected dysregulations within cellular immunity and the BM microenvironment. These dysregulations exhibit heterogeneity across MDS subtypes, genetic mutations, and disease stages. This diversity underscores the importance of establishing standardized FCM panels and cytokine measurements for in‐depth exploration of immunity in MDS and for risk stratification (Figure 3).
Figure 3.
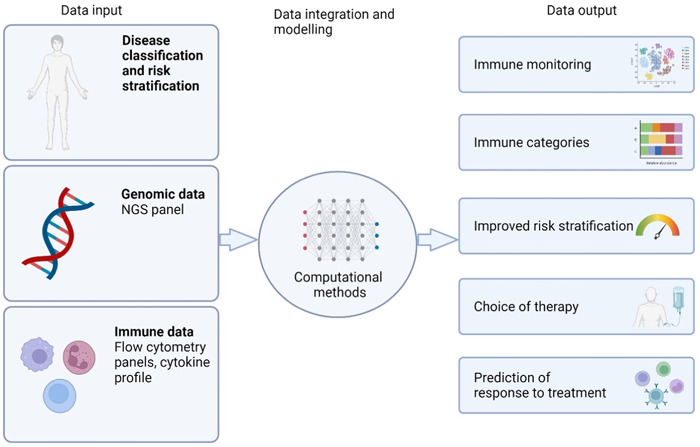
Multiomics modeling pipeline to integrate the assessment of the immune signature in myelodysplastic neoplasms.
RECOMMENDATIONS FOR IMMUNE PROFILING IN MDS PATIENTS
Guided by current knowledge and expert consensus, the i4MDS consortium presents the following recommendations as an initial step toward standardizing the assessment of immune parameters in patients with MDS.
All patients, whether they are under evaluation due to suspicion of MDS or have a confirmed diagnosis, should be requested to provide prescreening consent. This consent allows for the collection of the necessary samples for local or centralized immune profiling and the ongoing storage of research samples for future evaluations. Besides patients with suspected/diagnosed MDS, patients who are currently undergoing evaluation for persistent unexplained cytopenias (Hb <13 g/dL in men and <12 g/dL in women, ANC <1.8 × 109, PLT <150 × 109) as well as those who have already received a diagnosis of immune aplastic anemia, clonal cytopenia of undetermined significance (CCUS), VEXAS syndrome, or idiopathic cytopenia of undetermined significance (ICUS) are considered eligible for participation (Figure 4). When possible, patients will be part of registries where central diagnosis screening is not prohibited.
Figure 4.
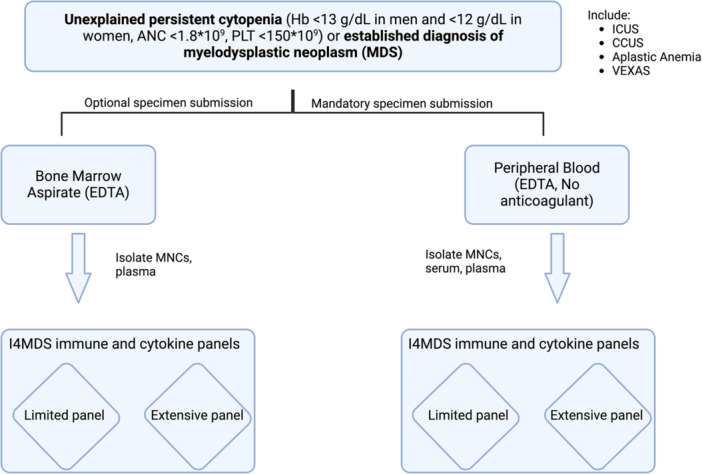
Patients screening for immune signature evaluation. ANC, absolute neutrophile count; CCUS, clonal cytopenia of unknown significance; Hb, hemoglobin; ICUS, idiopathic cytopenia of unknown significance; MNC, mononuclear cells; PLT, platelets.
Concomitant clinical data to be collected should include coexisting conditions (especially autoinflammatory and autoimmune disorders) and immunomodulatory drugs (especially systemic steroids >10 mg/day). Laboratory data should include CRP and autoimmunity‐related tests if indicated and available. Molecular sequencing (standard MDS NGS panels) should also be performed in all patients, to complement both molecular and immune data.
We recommend the collection of PB and, if feasible, BM aspirates to monitor the immune signature. Notably, significant variations in the innate and adaptive cell composition and status between peripheral and BM samples can provide valuable insights into the mechanisms underlying defective immune surveillance. Aspirates should be performed at the time of diagnosis and before initiating any new therapies.
We strongly encourage sequential assessments in accordance with institutional guidelines to monitor the dynamic evolution of the immune response during treatment. This is particularly crucial for patients undergoing treatment with hypomethylating agents (HMA) for a minimum of three cycles, given the potential immunomodulatory properties of this therapy.
When feasible, a portion of the PB and BM mononuclear cells and serum samples should be biobanked to enable more advanced multiparameter technologies, such as cytometry by time of flight or spectral flow cytometry, which can facilitate more in‐depth immune profiling. Thus, the collection of these important immunological and clinical/demographic data will enable advanced analyses to answer outstanding research questions to improve the experience and outcomes of patients with MDS.
IMMUNE CELL MONITORING USING FLOW CYTOMETRY
Table 3 summarizes the primary antigens the i4MDS panel recommends for the identification, characterization, and functional assessment of immune cells during clinical care. For each type of immune cell lineage, we have devised a suggested panel along with optional markers. The selection of these additional markers should be guided by local protocols, equipment availability, available resources, and the source of aspirates.
Table 3.
Flow cytometry panel recommendations.
| Panel | Immune subsets | Recommended markers | Optional (immune) | Optional (malignant) |
|---|---|---|---|---|
| A | T cell | CD45, CD45RA, CD3, CD4, CD8, CD5, CD7, CD16/56, CD27, TCRγδ | HLADR, CD38, CD95, CD62L, CD45RO, PD1/CTLA4/TIM3 | TRBC1, CD26 |
| B | B cell | CD45, CD20, CD19, CD10, CD27, CD38, IgD, IgM, CD25, CD22 | CD305, CD185 | kappa, lambda, CD5, CD23 |
| C | Monocyte/MDSC | CD45, CD14, CD16, CD64, CD300e, CD56, HLADR, CD11b, CD33, CD15, Lin | SLAN, CD141, CD45RO | CD34, CD13 |
| D | NK cell | CD7, CD56, CD8, CD94, CD57, CD161, CD16, CD3, KIR3DL1/DL2, CD45RO | DNAM1, NKG2D, NKp30, NKp46, KIR2DL2 | |
| E | T cell subset − 1 | CD3, CD4, CD8, CD19, CD25, CD127, CXCR5, CXCR3, CCR4, CCR6 | CD95, CD28, CCR7 | |
| F | T cell subset − 2 | CD45RA, CD3, CD4, CD25, CD127, CD194 (CCR4), CD95, CD28, CCR7, CD8 | FOXP3, CXCR5, CXCR3, CCR6, CD45RO | |
| G | Dendritic cells | CD45, Lin, CD123, CD88, HLADR, CD5, CD11C, CD141, CD163, CD11B, CD14 | CD1c, CD303, CD11b. CD45RO |
Note: Each panel explores a specific immune subset with a set of core markers and additional extra‐markers to explore their status and function (immune) or known to be dysregulated in the context of cancer (malignant).
This approach has been designed to facilitate effective and routine immune cell assessment in clinical laboratories, particularly in secondary and tertiary centers.
In the case of BM, hemodilution should be prevented, if possible, by using the first aspirate pull. Samples can be collected in ethylenediaminetetraacetic acid tubes and processed up to 72 h after collection. Nevertheless, if high dimensional flow cytometry is to be performed, we would generally recommend citrate collection tubes as they allow both genomic and flow cytometry analysis from the same sample. We recommend ammonium chloride (homemade or commercially available) lysis of mature RBC before antibody staining and suspension in paraformaldehyde for data acquisition. 143
The panels of marker combinations in Table 3 have been constructed based on 10 colors FCM, CD45 hematopoietic marker, and a viability stain. 132 Optional markers are proposed for exploring cell type‐specific functions (immune‐related) or those known or suspected to be dysregulated in the context of MDS (malignancy‐related).
In the case of BM aspirates, we also recommend the addition of a combination that includes CD34 and other appropriate markers to distinguish hematopoietic stem and progenitor cells (HSPC). Based on the current level of evidence regarding alterations in cell types in MDS (as outlined in Table 3), we strongly recommend performing, at a minimum, the T‐ and B‐cell panels. The choice to implement additional panels may be influenced by the intended treatment strategy.
We designed specific tubes for T‐cell monitoring (Panel A) and set a core panel of markers to distinguish cytotoxic, Th, and Tregs, as well as their naïve/memory phenotype (Panels E and F). For both Th (CXCR3, CCR4, CCR6, and CXCR5) subsets and Treg (CD25, CD127), cell‐surface identifying markers were preferred given their good correlation with intracellular staining and to facilitate routine implementation. 144 Panel B explores B cell isotypes, activation status, and repartition. NK cell panel D discriminates CD56bright/dim NK subsets and major activating/inhibitory/licensing NK receptors known to be dysregulated in MDS. Myeloid panel C investigates monocyte (classical, nonclassical, and intermediate) subsets along with M‐MDSC and PMN‐MDSC based on CD14/15 differential expression, while panel G analyzes plasmacytoid and myeloid DC subsets (Figure 5).
Figure 5.
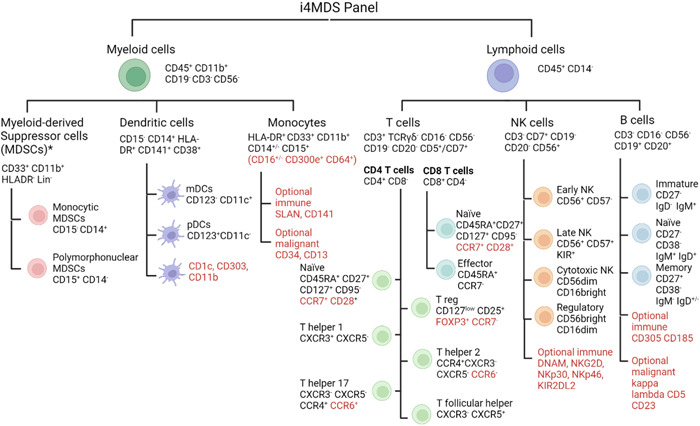
Cell markers to identify cell subtypes from the International Integrative Innovative Immunology for MDS panel (optional markers in red). *Functional assay is necessary for definite identification of myeloid‐derived suppressor cells.
Neutrophils can be individualized based on FSC/SSC scales and assessed for SSC‐based granularity. Given the lack of data regarding the immune impact of red cell nucleated progenitors and megakaryocytes in MDS, we do not recommend their monitoring in routine workup of MDS immune profiling.
CYTOKINE MONITORING IN MDS PATIENTS
Cytokine detection has classically used enzyme‐linked immunosorbent assays (ELISA), whose routine implementation has been limited by the analysis of one analyte at a time, and a relatively large sample volume to ensure accurate detection. 145 Multiplex immunoassays overcome the limitations of single‐analyte ELISA allowing simultaneous analysis of multiple cytokines with a reduced sample volume. While routine implementation of cytokine analysis is not yet widespread, we feel these techniques will play an important role in the therapeutic management of many inflammatory diseases, including MDS.
Serum cytokine levels have been shown to robustly reflect BM cytokine concentration 8 and there is high agreement between serum and plasma concentrations. 146 Peripheral blood serum (5 mL dry tube) should be the preferred source for cytokine analysis, although we also encourage BM supernatant biobanking. Lastly, it is crucial to exercise diligence regarding the patient's clinical condition to prevent sample collection during concurrent inflammatory or infectious episodes that could confound results. Additionally, any ongoing treatments, particularly those involving growth factors, immunomodulators, or novel inflammasome inhibitors, should be documented and reported, as they have the potential to impact result interpretation.
Table 4 summarizes the recommended cytokine panel, ranked in priority order established based on their potential role in disease genesis and their reported altered expression in MDS patients. Multiplex assays may allow simultaneous analysis of both pro‐inflammatory (IFN‐γ, TNF‐α, IL‐1β, IL‐1RA, IL‐2, IL‐2R, IL‐6, IL‐7, IL‐8, IL‐12, VEGF, GM‐CSF, CCL2, CCL3, CCL4, CCL5, CXCL10) and anti‐inflammatory (IL‐4, IL‐10, TGF‐β) molecules known to be dysregulated in the context of MDS. We also suggest additional markers: IL‐5 and IL‐15 for their broad proinflammatory roles in granulocyte stimulation, NK and T cell activation and proliferation, respectively; CXCL9 and CXCL12 for their important function in immune cell migration toward inflamed and BM tissue, respectively.
Table 4.
Cytokines monitoring panel recommendations.
| Cytokine panel | Recommended Markers (ranked in priority order) | Optional markers |
|---|---|---|
| Cytokines | IFN‐γ, IL‐6, IL‐8, IL‐1RA, IL‐7, IL‐12, CCL5, IL‐10, IL‐2, IL‐2R, TNF‐α, IL‐17, IL‐1β, TGF‐β, VEGF, GM‐CSF, CXCL10, IL‐4, CCL2, CCL3, CCL4, CXCR4 | IL‐5, IL‐15, CXCL9, CXCL12, CXCR1, CXCR2 |
DISCUSSION AND FUTURE DIRECTIONS
In this work, we offer recommendations to establish a structured framework for evaluating immune cells and monitoring cytokine changes at the time of diagnosis and throughout the course of the disease to standardize the immune profiling of MDS patients. Currently, manual gating and expert analysis remain the conventional practices, but we anticipate that automated gating and cell identification algorithms, currently in development or used in research studies, 147 will likely find their place in clinical practice in the near future.
Looking ahead, more sophisticated technologies, such as mass cytometry or spectral flow cytometry, could become readily available. These advancements have the potential to streamline the monitoring process, potentially reducing the need for multiple tubes and enhancing the efficiency of immune profiling.
We envision the integration of comprehensive immunological data with existing genomic analyses through the setup of a big data consortium. Through advanced statistical analysis, this could lead to improved risk stratification, identification of new prognostic biomarkers and patient subsets most likely to benefit from immunomodulatory treatments, including allogeneic hematopoietic stem cell transplantation and emerging immunotherapies.
AUTHOR CONTRIBUTIONS
Shahram Kordasti, Uwe Platzbecker, Catherine Cargo, Matteo G. Della Porta, and Lionel Adès designed the study and immune panels and supervised the project. Cristina A. Tentori and Lin P. Zhao performed the research, contributed to the panel design, and wrote the manuscript. Benedetta Tinterri, Kathryn E. Strange, Katharina Zoldan, Konstantinos Dimopoulos, Xingmin Feng, Elena Riva, Benjamin Lim, Yannick Simoni, Vidhya Murthy, Antonella Poloni, Eric Padron, Bruno A. Cardoso, Michael Cross, Susann Winter, Aida Santaolalla, Bhavisha A. Patel, Emma M. Groarke, Daniel H. Wiseman, Katy Jones, Lauren Jamieson, Charles Manogaran, Naval Daver, Laura Gallur, Wendy Ingram, P. Brent Ferrell, Katja Sockel, Nicolas Dulphy, Nicolas Chapuis, Anne S. Kubasch, Astrid M. Olsnes, Austin Kulasekararaj, Hugues De Lavellade, Wolfgang Kern, Mieke Van Hemelrijck, Dominique Bonnet, Theresia M. Westers, Sylvie Freeman, Uta Oelschlaegel, David Valcarcel, Marco G. Raddi, Kirsten Grønbæk, Michaela Fontenay, Sanam Loghavi, Valeria Santini, Antonio M. Almeida, Jonathan M. Irish, David A. Sallman, Neal S. Young, AH, and Arjan A. van de Loosdrecht contributed to panel design and critically reviewed the manuscript.
CONFLICT OF INTEREST STATEMENT
Shahram Kordasti: Novartis: Advisory Board, Speakers bureau, Alexion: Speakers bureau, Beckman Coulter: Speakers bureau, MorphoSys: Research Support (none is related to this publication). Wolfgang Kern declares part‐ownership of MLL Munich Leukemia Laboratory. Austin Kulasekararaj: Research support (to institution): Celgene/BMS and Novartis. Speaker's fees: Alexion/AstraZeneca, Akari, Apellis, Celgene/BMS, Novartis, Pfizer, Ra Pharma/UCB, Roche, SOBI. Scientific advisory board: Alexion/Astra Zeneca, Apellis, Amgen, Agios, Biocryst, Celgene/BMS, Novartis, Pfizer, Regeneron, Roche, SOBI, Janssen, Samsung and Novo Nordisk (none is related to this publication).
FUNDING
This work was supported by the CRUK City of London Centre Award [CTRQQR‐2021/100004] at KCL. C. A. T. is supported by SIE—Società Italiana di Ematologia and the Association “Amici di Beat Leukemia Dr. Alessandro Cevenini ONLUS” (www.beat-leukemia.org). M. G. D. P. is supported by European Union‐Next Generation EU‐NRRP M6C2 (Investment 2.1 Enhancement and strengthening of biomedical research in the NHS—Project PNRR‐MAD‐2022‐12376695); AIRC Foundation (Associazione Italiana per la Ricerca contro il Cancro, Milan Italy—Project #22053); 5 × 1000 MYNERVA (Myeloid Neoplasms Research Venture AIRC‐project #21267). V. S. is supported by AIRC project IG 26537‐2021.
Contributor Information
Lionel Adès, Email: lionel.ades@aphp.fr.
Shahram Kordasti, Email: shahram.kordasti@kcl.ac.uk.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Pubmed at https://pubmed.ncbi.nlm.nih.gov/.
REFERENCES
- 1. Greenplate AR, Johnson DB, Ferrell PB, Irish JM. Systems immune monitoring in cancer therapy. Eur J Cancer. 2016;61:77‐84. 10.1016/j.ejca.2016.03.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7):1703‐1719. 10.1038/s41375-022-01613-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arber DA, Orazi A, Hasserjian RP, et al. International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140(11):1200‐1228. 10.1182/blood.2022015850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kewan T, Bahaj W, Durmaz A, et al. Validation of the molecular international prognostic scoring system in patients with myelodysplastic syndromes. Blood. 2023;141(14):1768‐1772. 10.1182/blood.2022018896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sauta E, Robin M, Bersanelli M, et al. Real‐world validation of molecular international prognostic scoring system for myelodysplastic syndromes. J Clin Oncol. 2023;41(15):2827‐2842. 10.1200/JCO.22.01784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernard E, Tuechler H, Greenberg PL, et al. Molecular international prognostic scoring system for myelodysplastic syndromes. NEJM Evid. 2022;1(7):EVIDoa2200008. 10.1056/EVIDoa2200008 [DOI] [PubMed] [Google Scholar]
- 7. Kordasti SY, Ingram W, Hayden J, et al. CD4+CD25high Foxp3+ regulatory T cells in myelodysplastic syndrome (MDS). Blood. 2007;110(3):847‐850. 10.1182/blood-2007-01-067546 [DOI] [PubMed] [Google Scholar]
- 8. Kordasti SY, Afzali B, Lim Z, et al. IL‐17‐producing CD4+ T cells, pro‐inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br J Haematol. 2009;145(1):64‐72. 10.1111/j.1365-2141.2009.07593.x [DOI] [PubMed] [Google Scholar]
- 9. Winter S, Shoaie S, Kordasti S, Platzbecker U. Integrating the “Immunome” in the stratification of myelodysplastic syndromes and future clinical trial design. J Clin Oncol. 2020;38(15):1723‐1735. 10.1200/JCO.19.01823 [DOI] [PubMed] [Google Scholar]
- 10. Kittang AO, Kordasti S, Sand KE, et al. Expansion of myeloid‐derived suppressor cells correlates with number of T regulatory cells and disease progression in myelodysplastic syndrome. Oncoimmunology. 2016;5(2):e1062208. 10.1080/2162402X.2015.1062208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hecker JS, Hartmann L, Rivière J, et al. CHIP and hips: clonal hematopoiesis is common in patients undergoing hip arthroplasty and is associated with autoimmune disease. Blood. 2021;138(18):1727‐1732. 10.1182/blood.2020010163 [DOI] [PubMed] [Google Scholar]
- 12. Mekinian A, Grignano E, Braun T, et al. Systemic inflammatory and autoimmune manifestations associated with myelodysplastic syndromes and chronic myelomonocytic leukaemia: a French multicentre retrospective study. Rheumatology. 2016;55(2):291‐300. 10.1093/rheumatology/kev294 [DOI] [PubMed] [Google Scholar]
- 13. Zhao LP, Boy M, Azoulay C, et al. Genomic landscape of MDS/CMML associated with systemic inflammatory and autoimmune disease. Leukemia. 2021;35(9):2720‐2724. 10.1038/s41375-021-01152-1 [DOI] [PubMed] [Google Scholar]
- 14. Gutierrez‐Rodrigues F, Kusne Y, Fernandez J, et al. Spectrum of clonal hematopoiesis in VEXAS syndrome. Blood J. 2023;142:244‐259. 10.1182/blood.2022018774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mekinian A, Zhao LP, Chevret S, et al. A Phase II prospective trial of azacitidine in steroid‐dependent or refractory systemic autoimmune/inflammatory disorders and VEXAS syndrome associated with MDS and CMML. Leukemia. 2022;36(11):2739‐2742. 10.1038/s41375-022-01698-8 [DOI] [PubMed] [Google Scholar]
- 16. Montoro J, Gallur L, Merchán B, et al. Autoimmune disorders are common in myelodysplastic syndrome patients and confer an adverse impact on outcomes. Ann Hematol. 2018;97(8):1349‐1356. 10.1007/s00277-018-3302-0 [DOI] [PubMed] [Google Scholar]
- 17. Fenaux P, Giagounidis A, Selleslag D, et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion‐dependent patients with low‐/intermediate‐1‐risk myelodysplastic syndromes with del5q. Blood. 2011;118(14):3765‐3776. 10.1182/blood-2011-01-330126 [DOI] [PubMed] [Google Scholar]
- 18. Passweg JR, Giagounidis AAN, Simcock M, et al. Immunosuppressive therapy for patients with myelodysplastic syndrome: a prospective randomized multicenter phase III trial comparing antithymocyte globulin plus cyclosporine with best supportive care—SAKK 33/99. J Clin Oncol. 2011;29(3):303‐309. 10.1200/JCO.2010.31.2686 [DOI] [PubMed] [Google Scholar]
- 19. Sloand EM, Olnes MJ, Shenoy A, et al. Alemtuzumab treatment of intermediate‐1 myelodysplasia patients is associated with sustained improvement in blood counts and cytogenetic remissions. J Clin Oncol. 2010;28(35):5166‐5173. 10.1200/JCO.2010.29.7010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Silzle T, Blum S, Schuler E, et al. Lymphopenia at diagnosis is highly prevalent in myelodysplastic syndromes and has an independent negative prognostic value in IPSS‐R‐low‐risk patients. Blood Cancer J. 2019;9(8):63. 10.1038/s41408-019-0223-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Srivastava P, Tzetzo SL, Gomez EC, et al. Inhibition of LSD1 in MDS progenitors restores differentiation of CD141Hi conventional dendritic cells. Leukemia. 2020;34(9):2460‐2472. 10.1038/s41375-020-0765-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454‐2465. 10.1182/BLOOD-2012-03-420489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schneider M, Rolfs C, Trumpp M, et al. Activation of distinct inflammatory pathways in subgroups of LR‐MDS. Leukemia. 2023;37(8):1709‐1718. 10.1038/s41375-023-01949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shao L, Zhang L, Hou Y, et al. Th22 cells as well as Th17 cells expand differentially in patients with early‐stage and late‐stage myelodysplastic syndrome. PLoS One. 2012;7(12):e51339. 10.1371/journal.pone.0051339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kyriakou D, Alexandrakis MG, Kyriakou ES, et al. Reduced CD43 expression on the neutrophils of MDS patients correlates with an activated phenotype of these cells. Int J Hematol. 2001;73(4):483‐491. 10.1007/BF02994011 [DOI] [PubMed] [Google Scholar]
- 26. Shikama Y, Shichishima T, Ohto H, Jubinsky PT. Neutrophil‐specific reduction in the expression of granulocyte‐macrophage colony‐stimulating factor receptor subunits in myelodysplastic syndromes. Br J Haematol. 2000;111(3):863‐872. 10.1111/j.1365-2141.2000.02398.x [DOI] [PubMed] [Google Scholar]
- 27. Cordoba I, Gonzalez‐Porras JR, Such E, et al. The degree of neutropenia has a prognostic impact in low risk myelodysplastic syndrome. Leuk Res. 2012;36(3):287‐292. 10.1016/j.leukres.2011.10.025 [DOI] [PubMed] [Google Scholar]
- 28. Yang L, Li H, Liu Y, et al. Increased circulating of CD54highCD181low neutrophils in myelodysplastic syndrome. Front Oncol. 2021;10:585216. 10.3389/fonc.2020.585216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hejazi M, Manser AR, Frobel J, et al. Impaired cytotoxicity associated with defective natural killer cell differentiation in myelodysplastic syndromes. Haematologica. 2015;100(5):643‐652. 10.3324/haematol.2014.118679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gleason MK, Ross JA, Warlick ED, et al. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood. 2014;123(19):3016‐3026. 10.1182/blood-2013-10-533398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang W, Xie X, Mi H, et al. Abnormal populations and functions of natural killer cells in patients with myelodysplastic syndromes. Oncol Lett. 2018;15:5497‐5504. 10.3892/ol.2018.8062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Montes P, Bernal M, Campo LN, et al. Tumor genetic alterations and features of the immune microenvironment drive myelodysplastic syndrome escape and progression. Cancer Immunol Immunother. 2019;68(12):2015‐2027. 10.1007/s00262-019-02420-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carlsten M, Baumann BC, Simonsson M, et al. Reduced DNAM‐1 expression on bone marrow NK cells associated with impaired killing of CD34+ blasts in myelodysplastic syndrome. Leukemia. 2010;24(9):1607‐1616. 10.1038/leu.2010.149 [DOI] [PubMed] [Google Scholar]
- 34. Molero Yordi A, Peralta S, Gallur L, et al. Bone marrow microenvironment changes in myelodysplastic neoplasms and its relationship with clonal hematopoiesis and disease progression. Blood. 2022;140(Suppl 1):4024‐4025. 10.1182/blood-2022-163188 [DOI] [Google Scholar]
- 35. Epling‐Burnette PK, Bai F, Painter JS, et al. Reduced natural killer (NK) function associated with high‐risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood. 2007;109(11):4816‐4824. 10.1182/blood-2006-07-035519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meng F, Li L, Lu F, et al. Overexpression of TIGIT in NK and T cells contributes to tumor immune escape in myelodysplastic syndromes. Front Oncol. 2020;10:1595. 10.3389/fonc.2020.01595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Epling‐Burnette PK, Painter JS, Rollison DE, et al. Prevalence and clinical association of clonal T‐cell expansions in myelodysplastic syndrome. Leukemia. 2007;21(4):659‐667. 10.1038/sj.leu.2404590 [DOI] [PubMed] [Google Scholar]
- 38. Kiladjian JJ, Bourgeois E, Lobe I, et al. Cytolytic function and survival of natural killer cells are severely altered in myelodysplastic syndromes. Leukemia. 2006;20(3):463‐470. 10.1038/sj.leu.2404080 [DOI] [PubMed] [Google Scholar]
- 39. Cianga VA, Campos Catafal L, Cianga P, et al. Natural killer cell subpopulations and inhibitory receptor dynamics in myelodysplastic syndromes and acute myeloid leukemia. Front Immunol. 2021;12:665541. 10.3389/fimmu.2021.665541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aggarwal N, Swerdlow SH, TenEyck SP, Boyiadzis M, Felgar RE. Natural killer cell (NK) subsets and NK‐like T‐cell populations in acute myeloid leukemias and myelodysplastic syndromes. Cytom Part B: Clin Cytom. 2016;90(4):349‐357. 10.1002/cyto.b.21349 [DOI] [PubMed] [Google Scholar]
- 41. Stringaris K, Marin D, Barrett AJ, et al. KIR gene haplotype: an independent predictor of clinical outcome in MDS patients. Blood. 2016;128(24):2819‐2823. 10.1182/blood-2016-05-713099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tsirogianni M, Grigoriou E, Kapsimalli V, et al. Natural killer cell cytotoxicity is a predictor of outcome for patients with high‐risk myelodysplastic syndrome and oligoblastic acute myeloid leukemia treated with azacytidine. Leuk Lymphoma. 2019;60(10):2457‐2463. 10.1080/10428194.2019.1581935 [DOI] [PubMed] [Google Scholar]
- 43. Micheva I, Thanopoulou E, Michalopoulou S, et al. Impaired generation of bone marrow CD34‐derived dendritic cells with low peripheral blood subsets in patients with myelodysplastic syndrome. Br J Haematol. 2004;126(6):806‐814. 10.1111/j.1365-2141.2004.05132.x [DOI] [PubMed] [Google Scholar]
- 44. Matteo Rigolin G, Howard J, Buggins A, et al. Phenotypic and functional characteristics of monocyte‐derived dendritic cells from patients with myelodysplastic syndromes. Br J Haematol. 1999;107(4):844‐850. 10.1046/j.1365-2141.1999.01781.x [DOI] [PubMed] [Google Scholar]
- 45. Micheva I, Thanopoulou E, Michalopoulou S, et al. Defective tumor necrosis factor alpha‐induced maturation of monocyte‐derived dendritic cells in patients with myelodysplastic syndromes. Clin Immunol. 2004;113(3):310‐317. 10.1016/j.clim.2004.08.007 [DOI] [PubMed] [Google Scholar]
- 46. Saft L, Björklund E, Berg E, Hellström‐Lindberg E, Porwit A. Bone marrow dendritic cells are reduced in patients with high‐risk myelodysplastic syndromes. Leuk Res. 2013;37(3):266‐273. 10.1016/j.leukres.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 47. Davison GM, Novitzky N, Abdulla R. Monocyte‐derived dendritic cells have reduced expression of co‐stimulatory molecules but are able to stimulate autologous T‐cells in patients with MDS. Hematol Oncol Stem Cell Ther. 2013;6(2):49‐57. 10.1016/j.hemonc.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 48. van Leeuwen‐Kerkhoff N, Westers TM, Poddighe PJ, et al. Reduced frequencies and functional impairment of dendritic cell subsets and non‐classical monocytes in myelodysplastic syndromes. Haematologica. 2021;107(3):655‐667. 10.3324/haematol.2020.268136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ma L, Ceuppens J, Kasran A, Delforge M, Boogaerts M, Vandenberghe P. Immature and mature monocyte‐derived dendritic cells in myelodysplastic syndromes of subtypes refractory anemia or refractory anemia with ringed sideroblasts display an altered cytokine profile. Leuk Res. 2007;31(10):1373‐1382. 10.1016/j.leukres.2006.11.007 [DOI] [PubMed] [Google Scholar]
- 50. Rai R, Bigenwald C, Feld J, et al. Aberrant proportion of monocyte subsets in bone marrow of high‐risk myelodysplastic syndrome patients. Blood. 2022;140(Suppl 1):11432‐11433. 10.1182/blood-2022-170922 [DOI] [Google Scholar]
- 51. Cheng P, Eksioglu EA, Chen X, et al. S100A9‐induced overexpression of PD‐1/PD‐L1 contributes to ineffective hematopoiesis in myelodysplastic syndromes. Leukemia. 2019;33(8):2034‐2046. 10.1038/s41375-019-0397-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Han D, Tao J, Fu R, Shao Z. Myeloid‐derived suppressor cell cytokine secretion as prognostic factor in myelodysplastic syndromes. Innate Immun. 2020;26(8):703‐715. 10.1177/1753425920961157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Han Y, Wang H, Shao Z. Monocyte‐derived macrophages are impaired in myelodysplastic syndrome. J Immunol Res. 2016;2016:1‐7. 10.1155/2016/5479013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Allampallam K, Shetty V, Mundle S, et al. Biological significance of proliferation, apoptosis, cytokines, and monocyte/macrophage cells in bone marrow biopsies of 145 patients with myelodysplastic syndrome. Int J Hematol. 2002;75(3):289‐297. 10.1007/BF02982044 [DOI] [PubMed] [Google Scholar]
- 55. Velegraki M, Papakonstantinou N, Kalaitzaki L, et al. Increased proportion and altered properties of intermediate monocytes in the peripheral blood of patients with lower risk Myelodysplastic Syndrome. Blood Cells Mol Dis. 2021;86:102507. 10.1016/j.bcmd.2020.102507 [DOI] [PubMed] [Google Scholar]
- 56. Meers S, Kasran A, Boon L, et al. Monocytes are activated in patients with myelodysplastic syndromes and can contribute to bone marrow failure through CD40–CD40L interactions with T helper cells. Leukemia. 2007;21(12):2411‐2419. 10.1038/sj.leu.2404940 [DOI] [PubMed] [Google Scholar]
- 57. Pollyea DA, Hedin BR, O'Connor BP, Alper S. Monocyte function in patients with myelodysplastic syndrome. J Leukoc Biol. 2018;104(3):641‐647. 10.1002/JLB.5AB1017-419RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van Leeuwen‐Kerkhoff N, Westers TM, Poddighe PJ, de Gruijl TD, Kordasti S, van de Loosdrecht AA. Thrombomodulin‐expressing monocytes are associated with low‐risk features in myelodysplastic syndromes and dampen excessive immune activation. Haematologica. 2020;105(4):961‐971. 10.3324/haematol.2019.219303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Flores‐Figueroa E, Gutiérrez‐Espíndola G, Montesinos JJ, Arana‐Trejo R, Mayani H. In vitro characterization of hematopoietic microenvironment cells from patients with myelodysplastic syndrome. Leuk Res. 2002;26(7):677‐686. 10.1016/S0145-2126(01)00193-X [DOI] [PubMed] [Google Scholar]
- 60. Li L, Yu S, Hu X, et al. Immunophenotypic changes of monocytes in myelodysplastic syndrome and clinical significance. Clin Exp Med. 2022;23:787‐801. 10.1007/s10238-022-00856-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Meers S, Vandenberghe P, Boogaerts M, Verhoef G, Delforge M. The clinical significance of activated lymphocytes in patients with myelodysplastic syndromes: a single centre study of 131 patients. Leuk Res. 2008;32(7):1026‐1035. 10.1016/j.leukres.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 62. Talati C, Zhang L, Shaheen G, et al. Monocyte subset analysis accurately distinguishes CMML from MDS and is associated with a favorable MDS prognosis. Blood. 2017;129(13):1881‐1883. 10.1182/blood-2016-12-753210 [DOI] [PubMed] [Google Scholar]
- 63. Kook H, Zeng W, Guibin C, Kirby M, Young NS, Maciejewski JP. Increased cytotoxic T cells with effector phenotype in aplastic anemia and myelodysplasia. Exp Hematol. 2001;29(11):1270‐1277. 10.1016/S0301-472X(01)00736-6 [DOI] [PubMed] [Google Scholar]
- 64. Chamuleau MED, Westers TM, van Dreunen L, et al. Immune‐mediated autologous cytotoxicity against hematopoietic precursor cells in patients with myelodysplastic syndrome. Haematologica. 2009;94(4):496‐506. 10.3324/haematol.13612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sand K, Theorell J, Bruserud Ø, Bryceson YT, Kittang AO. Reduced potency of cytotoxic T lymphocytes from patients with high‐risk myelodysplastic syndromes. Cancer Immunol Immunother. 2016;65(9):1135‐1147. 10.1007/s00262-016-1865-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tao J, Li L, Wang Y, Fu R, Wang H, Shao Z. Increased TIM3+CD8+T cells in Myelodysplastic Syndrome patients displayed less perforin and granzyme B secretion and higher CD95 expression. Leuk Res. 2016;51:49‐55. 10.1016/j.leukres.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 67. Yang H, Bueso‐Ramos C, DiNardo C, et al. Expression of PD‐L1, PD‐L2, PD‐1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28(6):1280‐1288. 10.1038/leu.2013.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Suwabe T, Shibasaki Y, Sato H, et al. WT1‐specific CD8+ cytotoxic T cells with the capacity for antigen‐specific expansion accumulate in the bone marrow in MDS. Int J Hematol. 2021;113(5):723‐734. 10.1007/s12185-021-03083-0 [DOI] [PubMed] [Google Scholar]
- 69. Fozza C, Contini S, Galleu A, et al. Patients with myelodysplastic syndromes display several T‐cell expansions, which are mostly polyclonal in the CD4+ subset and oligoclonal in the CD8+ subset. Exp Hematol. 2009;37(8):947‐955. 10.1016/j.exphem.2009.04.009 [DOI] [PubMed] [Google Scholar]
- 70. Wang T, Ran N, Li N, et al. IL‐18 and IL‐18 binding protein are related to disease severity of myelodysplastic syndromes. Blood. 2022;140(Suppl 1):12297. 10.1182/blood-2022-168782 [DOI] [Google Scholar]
- 71. Zheng Z, Qianqiao Z, Qi H, Feng X, Chunkang C, Xiao L. In vitro deprivation of CD8+CD57+T cells promotes the malignant growth of bone marrow colony cells in patients with lower‐risk myelodysplastic syndrome. Exp Hematol. 2010;38(8):677‐684. 10.1016/j.exphem.2010.04.002 [DOI] [PubMed] [Google Scholar]
- 72. Tsuda H, Yamasaki H. Type I and type II T‐cell profiles in aplastic anemia and refractory anemia. Am J Hematol. 2000;64(4):271‐274. [DOI] [PubMed] [Google Scholar]
- 73. Alfinito F, Sica M, Luciano L, et al. Immune dysregulation and dyserythropoiesis in the myelodysplastic syndromes. Br J Haematol. 2010;148(1):90‐98. 10.1111/j.1365-2141.2009.07921.x [DOI] [PubMed] [Google Scholar]
- 74. Kotsianidis I, Bouchliou I, Nakou E, et al. Kinetics, function and bone marrow trafficking of CD4+CD25+FOXP3+ regulatory T cells in myelodysplastic syndromes (MDS). Leukemia. 2009;23(3):510‐518. 10.1038/leu.2008.333 [DOI] [PubMed] [Google Scholar]
- 75. Leone S, Rubino V, Palatucci AT, et al. Bone marrow CD3+ CD56+ regulatory T lymphocytes (TR3−56 cells) are inversely associated with activation and expansion of bone marrow cytotoxic T cells in IPSS‐R very‐low/low‐risk MDS patients. Eur J Haematol. 2022;109(4):398‐405. 10.1111/ejh.13822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bouchliou I, Miltiades P, Nakou E, et al. Th17 and Foxp3+ T regulatory cell dynamics and distribution in myelodysplastic syndromes. Clin Immunol. 2011;139(3):350‐359. 10.1016/j.clim.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 77. Hamdi W, Ogawara H, Handa H, Tsukamoto N, Nojima Y, Murakami H. Clinical significance of regulatory T cells in patients with myelodysplastic syndrome. Eur J Haematol. 2009;82(3):201‐207. 10.1111/j.1600-0609.2008.01182.x [DOI] [PubMed] [Google Scholar]
- 78. Fozza C, Longu F, Contini S, et al. Patients with early‐stage myelodysplastic syndromes show increased frequency of CD4+CD25high+CD127low regulatory T cells. Acta Haematol. 2012;128(3):178‐182. 10.1159/000339498 [DOI] [PubMed] [Google Scholar]
- 79. Kahn JD, Chamuleau MED, Westers TM, et al. Regulatory T cells and progenitor B cells are independent prognostic predictors in lower‐risk myelodysplastic syndromes. Haematologica. 2015;100(6):e220‐e222. 10.3324/haematol.2014.116657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mailloux AW, Sugimori C, Komrokji RS, et al. Expansion of effector memory regulatory T cells represents a novel prognostic factor in lower risk myelodysplastic syndrome. J Immunol. 2012;189(6):3198‐3208. 10.4049/jimmunol.1200602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang X, Yang X, Wang C, Huang L, Zhang Y, Wei J. High expression of plasma IL‐1β levels and transition of regulatory T‐cell subsets correlate with disease progression in myelodysplastic syndrome. Blood. 2022;140(Suppl 1):9761‐9762. 10.1182/blood-2022-165179 [DOI] [Google Scholar]
- 82. Wu L, Li X, Chang C, Ying S, He Q, Pu Q. Deviation of type I and type II T cells and its negative effect on hematopoiesis in myelodysplastic syndrome. Int J Lab Hematol. 2008;30(5):390‐399. 10.1111/j.1751-553X.2007.00970.x [DOI] [PubMed] [Google Scholar]
- 83. Hamdi W, Ogawara H, Handa H, Tsukamoto N, Murakami H. Clinical significance of Th1/Th2 ratio in patients with myelodysplastic syndrome. Int J Lab Hematol. 2009;31(6):630‐638. 10.1111/j.1751-553X.2008.01090.x [DOI] [PubMed] [Google Scholar]
- 84. Li J, Yue L, Wang H, et al. Th17 cells exhibit antitumor effects in MDS possibly through augmenting functions of CD8+ T cells. J Immunol Res. 2016;2016:1‐14. 10.1155/2016/9404705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhang Z, Li X, Guo J, et al. Interleukin‐17 enhances the production of interferon‐γ and tumour necrosis factor‐α by bone marrow T lymphocytes from patients with lower‐risk myelodysplastic syndromes. Eur J Haematol. 2013;90(5):375‐384. 10.1111/ejh.12074 [DOI] [PubMed] [Google Scholar]
- 86. Kiladjian JJ, Visentin G, Viey E, et al. Activation of cytotoxic T‐cell receptor T lymphocytes in response to specific stimulation in myelodysplastic syndromes. Haematologica. 2008;93(3):381‐389. 10.3324/haematol.11812 [DOI] [PubMed] [Google Scholar]
- 87. Geng S, Weng J, Chen S, et al. Abnormalities in the T cell receptor Vδ repertoire and Foxp3 expression in refractory anemia with ringed sideroblasts. DNA Cell Biol. 2015;34(9):588‐595. 10.1089/dna.2015.2925 [DOI] [PubMed] [Google Scholar]
- 88. Geng S, Weng J, Du X, et al. Comparison of the distribution and clonal expansion features of the T‐cell γδ repertoire in myelodysplastic syndrome‐RAEB and RAEB with progression to AML. DNA Cell Biol. 2012;31(10):1563‐1570. 10.1089/dna.2012.1769 [DOI] [PubMed] [Google Scholar]
- 89. Amin HM, Jilani I, Estey EH, et al. Increased apoptosis in bone marrow B lymphocytes but not T lymphocytes in myelodysplastic syndrome. Blood. 2003;102(5):1866‐1868. 10.1182/blood-2003-01-0221 [DOI] [PubMed] [Google Scholar]
- 90. Sternberg A. Evidence for reduced B‐cell progenitors in early (low‐risk) myelodysplastic syndrome. Blood. 2005;106(9):2982‐2991. 10.1182/blood-2005-04-1543 [DOI] [PubMed] [Google Scholar]
- 91. Geyh S, Öz S, Cadeddu RP, et al. Insufficient stromal support in MDS results from molecular and functional deficits of mesenchymal stromal cells. Leukemia. 2013;27(9):1841‐1851. 10.1038/leu.2013.193 [DOI] [PubMed] [Google Scholar]
- 92. Aanei CM, Flandrin P, Zugun Eloae F, et al. Intrinsic growth deficiencies of mesenchymal stromal cells in myelodysplastic syndromes. Stem Cells Dev. 2012;21(10):1604‐1615. 10.1089/scd.2011.0390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Choi H, Kim Y, Kang D, et al. Common and different alterations of bone marrow mesenchymal stromal cells in myelodysplastic syndrome and multiple myeloma. Cell Proliferation. 2020;53(5):e12819. 10.1111/cpr.12819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhao Y, Wu D, Fei C, et al. Down‐regulation of Dicer1 promotes cellular senescence and decreases the differentiation and stem cell‐supporting capacities of mesenchymal stromal cells in patients with myelodysplastic syndrome. Haematologica. 2015;100(2):194‐204. 10.3324/haematol.2014.109769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pang Y, Deng C, Geng S, et al. Premature exhaustion of mesenchymal stromal cells from myelodysplastic syndrome patients. Am J Transl Res. 2017;9(7):3462‐3468. [PMC free article] [PubMed] [Google Scholar]
- 96. Han Q, Sun Z, Liu L, et al. Impairment in immuno‐modulatory function of Flk1+CD31−CD34− MSCs from MDS‐RA patients. Leuk Res. 2007;31(11):1469‐1478. 10.1016/j.leukres.2006.12.016 [DOI] [PubMed] [Google Scholar]
- 97. Zhao Z, Wang Z, Li Q, Li W, You Y, Zou P. The different immunoregulatory functions of mesenchymal stem cells in patients with low‐risk or high‐risk myelodysplastic syndromes. PLoS One. 2012;7(9):e45675. 10.1371/journal.pone.0045675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhao ZG, Xu W, Yu HP, et al. Functional characteristics of mesenchymal stem cells derived from bone marrow of patients with myelodysplastic syndromes. Cancer Lett. 2012;317(2):136‐143. 10.1016/j.canlet.2011.08.030 [DOI] [PubMed] [Google Scholar]
- 99. Fei C, Zhao Y, Gu S, et al. Impaired osteogenic differentiation of mesenchymal stem cells derived from bone marrow of patients with lower‐risk myelodysplastic syndromes. Tumor Biol. 2014;35(5):4307‐4316. 10.1007/s13277-013-1565-6 [DOI] [PubMed] [Google Scholar]
- 100. Wu Y, Aanei CM, Kesr S, Picot T, Guyotat D, Campos Catafal L. Impaired expression of focal adhesion kinase in mesenchymal stromal cells from low‐risk myelodysplastic syndrome patients. Front Oncol. 2017;7:164. 10.3389/fonc.2017.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Corradi G, Baldazzi C, Očadlíková D, et al. Mesenchymal stromal cells from myelodysplastic and acute myeloid leukemia patients display in vitro reduced proliferative potential and similar capacity to support leukemia cell survival. Stem Cell Res Ther. 2018;9(1):271. 10.1186/s13287-018-1013-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hayashi Y, Kawabata KC, Tanaka Y, et al. MDS cells impair osteolineage differentiation of MSCs via extracellular vesicles to suppress normal hematopoiesis. Cell Rep. 2022;39(6):110805. 10.1016/j.celrep.2022.110805 [DOI] [PubMed] [Google Scholar]
- 103. Wang YH, Hou HA, Lin CC, et al. A CIBERSORTx‐based immune cell scoring system could independently predict the prognosis of patients with myelodysplastic syndromes. Blood Adv. 2021;5(22):4535‐4548. 10.1182/bloodadvances.2021005141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Klaus M, Stavroulaki E, Kastrinaki MC, et al. Reserves, functional, immunoregulatory, and cytogenetic properties of bone marrow mesenchymal stem cells in patients with myelodysplastic syndromes. Stem Cells Dev. 2010;19(7):1043‐1054. 10.1089/scd.2009.0286 [DOI] [PubMed] [Google Scholar]
- 105. Flores‐Figueroa E, Montesinos JJ, Flores‐Guzmán P, et al. Functional analysis of myelodysplastic syndromes‐derived mesenchymal stem cells. Leuk Res. 2008;32(9):1407‐1416. 10.1016/j.leukres.2008.02.013 [DOI] [PubMed] [Google Scholar]
- 106. Buscarlet M, Provost S, Zada YF, et al. Lineage restriction analyses in CHIP indicate myeloid bias for TET2 and multipotent stem cell origin for DNMT3A. Blood. 2018;132(3):277‐280. 10.1182/blood-2018-01-829937 [DOI] [PubMed] [Google Scholar]
- 107. Gopal A, Ibrahim R, Fuller M, et al. TIRAP drives myelosuppression through an Ifnγ‐Hmgb1 axis that disrupts the endothelial niche in mice. J Exp Med. 2022;219(3):e20200731. 10.1084/jem.20200731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Prodan M, Tulissi P, Perticarari S, et al. Flow cytometric assay for the evaluation of phagocytosis and oxidative burst of polymorphonuclear leukocytes and monocytes in myelodysplastic disorders. Haematologica. 1995;80(3):212‐218. [PubMed] [Google Scholar]
- 109. Fianchi L, Leone G, Posteraro B, et al. Impaired bactericidal and fungicidal activities of neutrophils in patients with myelodysplastic syndrome. Leuk Res. 2012;36(3):331‐333. 10.1016/j.leukres.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 110. Moretti S, Lanza F, Spisani S, et al. Neutrophils from patients with myelodysplastic syndromes: relationship between impairment of granular contents, complement receptors, functional activities and disease status. Leuk Lymphoma. 1994;13(5‐6):471‐477. 10.3109/10428199409049637 [DOI] [PubMed] [Google Scholar]
- 111. Huerga Encabo H, Aramburu IV, Garcia‐Albornoz M, et al. Loss of TET2 in human hematopoietic stem cells alters the development and function of neutrophils. Cell Stem Cell. 2023;30(6):781‐799. 10.1016/j.stem.2023.05.004 [DOI] [PubMed] [Google Scholar]
- 112. Schmidt CS, Aranda Lopez P, Dopheide JF, et al. Phenotypic and functional characterization of neutrophils and monocytes from patients with myelodysplastic syndrome by flow cytometry. Cell Immunol. 2016;308:19‐26. 10.1016/j.cellimm.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 113. Rusanova E, Simon‐Lopez R. Side scatter level of main populations in peripheral blood using flow cytometry as a tool for the screening/detection of dysplasia and its utility in the diagnosis of myelodysplastic syndromes. Blood. 2008;112(11):5091. 10.1182/blood.V112.11.5091.5091 [DOI] [Google Scholar]
- 114. Boy M, Bisio V, Zhao LP, et al. Myelodysplastic syndrome associated TET2 mutations affect NK cell function and genome methylation. Nat Commun. 2023;14(1):588. 10.1038/s41467-023-36193-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Meyerson HJ, Osei E, Schweitzer K, et al. CD1c(+) myeloid dendritic cells in myeloid neoplasia. Cytom Part B Clin Cytom. 2016;90(4):337‐348. 10.1002/cyto.b.21332 [DOI] [PubMed] [Google Scholar]
- 116. Bizymi N, Bjelica S, Kittang AO, et al. Myeloid‐derived suppressor cells in hematologic diseases: promising biomarkers and treatment targets. Hemasphere. 2019;3(1):e168. 10.1097/HS9.0000000000000168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Talmadge JE, Gabrilovich DI. History of myeloid‐derived suppressor cells. Nat Rev Cancer. 2013;13(10):739‐752. 10.1038/nrc3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chen X, Eksioglu EA, Zhou J, et al. Induction of myelodysplasia by myeloid‐derived suppressor cells. J Clin Invest. 2013;123(11):4595‐4611. 10.1172/JCI67580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wong KL, Tai JJY, Wong WC, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118(5):e16‐e31. 10.1182/blood-2010-12-326355 [DOI] [PubMed] [Google Scholar]
- 120. Selimoglu‐Buet D, Wagner‐Ballon O, Saada V, et al. Characteristic repartition of monocyte subsets as a diagnostic signature of chronic myelomonocytic leukemia. Blood. 2015;125(23):3618‐3626. 10.1182/blood-2015-01-620781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yang F, Wu Z, Yang D, Zhang X, Zhang X, Xu Y. Characteristics of macrophages from myelodysplastic syndrome microenvironment. Exp Cell Res. 2021;408(1):112837. 10.1016/j.yexcr.2021.112837 [DOI] [PubMed] [Google Scholar]
- 122. Epperson DE, Nakamura R, Saunthararajah Y, Melenhorst J, Barrett AJ. Oligoclonal T cell expansion in myelodysplastic syndrome: evidence for an autoimmune process. Leuk Res. 2001;25(12):1075‐1083. 10.1016/S0145-2126(01)00083-2 [DOI] [PubMed] [Google Scholar]
- 123. Kochenderfer JN, Kobayashi S, Wieder ED, Su C, Molldrem JJ. Loss of T‐lymphocyte clonal dominance in patients with myelodysplastic syndrome responsive to immunosuppression. Blood. 2002;100(10):3639‐3645. 10.1182/blood-2002-01-0155 [DOI] [PubMed] [Google Scholar]
- 124. Sand KE, Rye KP, Mannsåker B, Bruserud Ø, Kittang AO. Expression patterns of chemokine receptors on circulating T cells from myelodysplastic syndrome patients. Oncoimmunology. 2013;2(2):e23138. 10.4161/onci.23138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Calabretto G, Attardi E, Teramo A, et al. Hypocellular myelodysplastic syndromes (h‐MDS): from clinical description to immunological characterization in the Italian multi‐center experience. Leukemia. 2022;36(7):1947‐1950. 10.1038/s41375-022-01592-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Coats T, Smith A, Mourikis TP, Irish JM, Kordasti S, Mufti GJ. Mass cytometry reveals PD1 upregulation is an early step in MDS disease progression. Blood. 2016;128(22):4296. 10.1182/blood.V128.22.4296.4296 [DOI] [Google Scholar]
- 127. Giovazzino A, Leone S, Rubino V, et al. Reduced regulatory T cells (Treg) in bone marrow preferentially associate with the expansion of cytotoxic T lymphocytes in low‐risk MDS patients. Br J Haematol. 2019;185(2):357‐360. 10.1111/bjh.15496 [DOI] [PubMed] [Google Scholar]
- 128. Simard JC, Cesaro A, Chapeton‐Montes J, et al. S100A8 and S100A9 induce cytokine expression and regulate the NLRP3 inflammasome via ROS‐dependent activation of NF‐κB1. PLoS One. 2013;8(8):e72138. 10.1371/journal.pone.0072138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Basiorka AA, McGraw KL, Eksioglu EA, et al. The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood. 2016;128(25):2960‐2975. 10.1182/blood-2016-07-730556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kornblau SM, McCue D, Singh N, Chen W, Estrov Z, Coombes KR. Recurrent expression signatures of cytokines and chemokines are present and are independently prognostic in acute myelogenous leukemia and myelodysplasia. Blood. 2010;116(20):4251‐4261. 10.1182/blood-2010-01-262071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Feng X, Scheinberg P, Wu CO, et al. Cytokine signature profiles in acquired aplastic anemia and myelodysplastic syndromes. Haematologica. 2011;96(4):602‐606. 10.3324/haematol.2010.030536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Moudra A, Hubackova S, Machalova V, et al. Dynamic alterations of bone marrow cytokine landscape of myelodysplastic syndromes patients treated with 5‐azacytidine. Oncoimmunology. 2016;5(10):e1183860. 10.1080/2162402X.2016.1183860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Aref S, Khaled N, Al Gilany AH, Ayed M, Abouzeid T, Attia D. Impact of bone marrow natural killer cells (NK); soluble TNF‐α and IL‐32 levels in myelodysplastic syndrome patients. Asian Pacific J Cancer Prev. 2020;21(10):2949‐2953. 10.31557/APJCP.2020.21.10.2949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Tsimberidou AM, Estey E, Wen S, et al. The prognostic significance of cytokine levels in newly diagnosed acute myeloid leukemia and high‐risk myelodysplastic syndromes. Cancer. 2008;113(7):1605‐1613. 10.1002/cncr.23785 [DOI] [PubMed] [Google Scholar]
- 135. Pardanani A, Finke C, Lasho TL, et al. IPSS‐independent prognostic value of plasma CXCL10, IL‐7 and IL‐6 levels in myelodysplastic syndromes. Leukemia. 2012;26(4):693‐699. 10.1038/leu.2011.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Falconi G, Fabiani E, Fianchi L, et al. Impairment of PI3K/AKT and WNT/β‐catenin pathways in bone marrow mesenchymal stem cells isolated from patients with myelodysplastic syndromes. Exp Hematol. 2016;44(1):75‐83. 10.1016/j.exphem.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 137. Pavlaki K, Pontikoglou CG, Demetriadou A, et al. Impaired proliferative potential of bone marrow mesenchymal stromal cells in patients with myelodysplastic syndromes is associated with abnormal WNT signaling pathway. Stem Cells Dev. 2014;23(14):1568‐1581. 10.1089/scd.2013.0283 [DOI] [PubMed] [Google Scholar]
- 138. Fei C, Zhao Y, Guo J, Gu S, Li X, Chang C. Senescence of bone marrow mesenchymal stromal cells is accompanied by activation of p53/p21 pathway in myelodysplastic syndromes. Eur J Haematol. 2014;93(6):476‐486. 10.1111/ejh.12385 [DOI] [PubMed] [Google Scholar]
- 139. Ferrer RA, Wobus M, List C, et al. Mesenchymal stromal cells from patients with myelodyplastic syndrome display distinct functional alterations that are modulated by lenalidomide. Haematologica. 2013;98(11):1677‐1685. 10.3324/haematol.2013.083972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Balderman SR, Li AJ, Hoffman CM, et al. Targeting of the bone marrow microenvironment improves outcome in a murine model of myelodysplastic syndrome. Blood. 2016;127(5):616‐625. 10.1182/blood-2015-06-653113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Poon Z, Dighe N, Venkatesan SS, et al. Bone marrow MSCs in MDS: contribution towards dysfunctional hematopoiesis and potential targets for disease response to hypomethylating therapy. Leukemia. 2019;33(6):1487‐1500. 10.1038/s41375-018-0310-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kitagawa M, Saito I, Kuwata T, et al. Overexpression of tumor necrosis factor (TNF)‐α and interferon (IFN)‐γ by bone marrow cells from patients with myelodysplastic syndromes. Leukemia. 1997;11(12):2049‐2054. 10.1038/sj.leu.2400844 [DOI] [PubMed] [Google Scholar]
- 143. van der Velden VHJ, Preijers F, Johansson U, et al. Flow cytometric analysis of myelodysplasia: pre‐analytical and technical issues‐Recommendations from the European LeukemiaNet. Cytometry Part B Clin Cytom. 2023;104(1):15‐26. 10.1002/cyto.b.22046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Mahnke YD, Beddall MH, Roederer M. OMIP‐015: human regulatory and activated T‐cells without intracellular staining. Cytometry, Part A. 2013;83A(2):179‐181. 10.1002/cyto.a.22230 [DOI] [PubMed] [Google Scholar]
- 145. Yu X, Scott D, Dikici E, Joel S, Deo S, Daunert S. Multiplexing cytokine analysis: towards reducing sample volume needs in clinical diagnostics. Analyst (Lond). 2019;144(10):3250‐3259. 10.1039/c9an00297a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Gruen ME, Messenger KM, Thomson AE, et al. A comparison of serum and plasma cytokine values using a multiplexed assay in cats. Vet Immunol Immunopathol. 2016;182:69‐73. 10.1016/j.vetimm.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Verschoor CP, Lelic A, Bramson JL, Bowdish DME. An introduction to automated flow cytometry gating tools and their implementation. Front Immunol. 2015;6:380. 10.3389/fimmu.2015.00380 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in Pubmed at https://pubmed.ncbi.nlm.nih.gov/.


