Summary
Background
Leptomeningeal carcinomatosis (LMC), the metastatic spread of cancer to the leptomeninges, is a rare complication and has a dismal prognosis. Due to limited data available on LMC from India, we conducted a country-wise audit of LMC across 15 centres in India.
Methods
The current study conducted in 2020, was a retrospective, multicentric audit of adult patients (aged ≥18 years) with diagnosis of LMC and who received treatment during 2010–2020. Baseline characteristics, details related to previous treatments, cancer sites, LMC diagnosis, treatment pattern and overall survival (OS) were collected. Descriptive statistics were performed, and Kaplan Meier analysis was performed for the estimation of OS.
Findings
Among the patients diagnosed with LMC (n = 84), diagnosis was confirmed in 52 patients (61.9%) and ‘probable’ in 32 (38.1%) patients. The three most common cause of malignancy were non-small cell lung cancer (NSCLC), breast cancer and gastrointestinal cancer with 45 (53.6%), 22 (26.1%) and 9 (10.7%) patients respectively. Intrathecal therapy was offered in 33 patients (39.3%). The most common intrathecal agent was methotrexate in 23 patients (27.4%). The median OS was 90 days (95% CI 48–128). Among tested variables, intrathecal therapy administration (hazard ratio [HR] = 0.36, 95% CI 0.19–0.68) and primary in lung (HR = 0.43, 95% CI 0.23–0.83) had a favourable impact on OS.
Interpretation
Prognosis with leptomeningeal carcinomatosis is poor with a significant burden of morbidity and mortality in India. This data aims to highlight the current outcomes and facilitate further research on LMC.
Funding
None.
Keywords: Leptomeningeal carcinomatosis, Intrathecal, Outcomes, Pattern of care, LMIC
Research in context.
Evidence before this study
We searched PubMed with the terms “Leptomeningeal disease” OR “Leptomeningeal carcinomatosis OR Leptomeningeal metastasis” for articles published from database inception to and August 31, 2022, in English. We tried to include more recent publications as references.
Added value of this study
Data on the prognosis and treatment of Leptomeningeal carcinomatosis is present across literature with varying results however data from low-middle income countries (LMIC) remains scarce. Outcomes in leptomeningeal carcinomatosis remain dismal and this necessitates further studies and trials on novel modes of therapy. However, to do so, we must first accrue robust data on prognosis and outcomes with current therapy for leptomeningeal carcinomatosis in LMICs. This is the aim of our study.
Implications of all the available evidence
Outcomes in leptomeningeal carcinomatosis remain dismal. Nearly 33% of patients received best supportive care. Despite being part of treatment guidelines, only 39% of patients were able to receive intrathecal therapy in our patient population. Intrathecal therapy was associated with improved outcomes in patients with leptomeningeal carcinomatosis. Lung cancer as primary had the better outcomes among patients with leptomeningeal carcinomatosis.
Introduction
Leptomeningeal carcinomatosis (LMC) is a rare and potentially lethal complication of cancer that occurs when cancer cells spread to the membranes surrounding the brain and spinal cord, known as the leptomeninges.1,2 The cancer cells can spread to the leptomeninges through the bloodstream or via direct extension from a nearby tumour. They are resistant to most chemotherapy options due to this being a ‘sanctuary’ site where they are protected by the limited filtration across the blood brain barrier.
LMC can cause a range of neurological symptoms, such as headache, nausea, vomiting, seizures, altered sensorium, memory loss and difficulty speaking or walking. The non-specificity of these symptoms could initially be mistaken for other conditions, making LMC challenging to diagnose. If left untreated, LMC can lead to severe and potentially life-threatening complications. Diagnosis of LMC is typically made using cerebrospinal fluid (CSF) analysis and imaging modalities.
LMC is observed in approximately 10% of patients with solid tumour cancer.3 Breast cancer, lung cancer and melanoma are three malignancies commonly associated with LMC.4,5 With recent developments in systemic therapies, there is a significant improvement in the extracranial control of the above three mentioned malignancies.6, 7, 8, 9, 10 As a result of this improvement in extracranial control, there is an increase in relapse seen in sanctuary sites such as the central nervous system.11 In general, there is an increase in incidence of both brain parenchymal lesions as well as LMC. It is hypothesised that in addition to improvement in systemic control and prolonged survival, a combination of other factors like improved imaging techniques and a lower threshold for initiating diagnostic work-up have led to increase the incidence and prevalence of LMC. Median time between diagnosis of systemic cancer and diagnosis of LMC is around 1–2 years. Developments in management of leptomeningeal metastasis of solid tumours majorly include systemic chemotherapy,12 intrathecal therapy and irradiation.13 However, impact of these management modalities on prognosis currently varies widely. LMC has a dismal prognosis and hence there is a need for improvement in outcomes. Till now, only few randomised trials were conducted and previous observational studies are mostly retrospective. Also, very limited literature is available from India and other low-income and middle-income countries (LMIC). The applicability of retrospective studies’ from high-income countries in LMIC is questionable and warrants further review. The advances in systemic treatment in solid tumours that have led to improved outcomes have become inaccessible to a large proportion of patients in India and other LMICs. We aimed to understand the current prevalence of LMC and management in India. Hence, we did a retrospective country-wide analysis to determine the pattern of care and outcomes in solid tumour patients with LMC.
Methods
A multicentric retrospective analysis was conducted across 15 centres in India.
The study was conducted after Ethics Committee clearance and in accordance with the standards of Declaration of Helsinki, International Council for Harmonisation (ICH)–Good Clinical Practice (GCP), and the Indian Council of Medical Research (ICMR). The study was conceptualised in December 2019 and the data collection was done in January to March 2020. The study was presented at the American Society of Clinical Oncology (ASCO) annual conference of 2021. The study adhered to Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines.14 The flowchart is depicted in Fig. 1.
Fig. 1.
STROBE flowchart for this study.
Patient selection
Adult patients (age > or = 18 years) with LMC, treated between January 2010 and December 2019 were selected for this analysis. The diagnosis of leptomeningeal carcinomatosis was per European Association of Neuro-Oncology (EANO)15 guidelines as ‘confirmed’, ‘probable’ or ‘possible’. The definition of ‘confirmed’ LMC was if there was the presence of positive cerebrospinal fluid (CSF) cytology. In the absence of positive CSF cytology, if there was a presence of typical MRI features of leptomeningeal carcinomatosis with typical clinical features, it was labelled as ‘probable’ LMC. A diagnosis of ‘possible’ LMC was made if the CSF cytology was negative with absence of typical features on MRI but with the presence of typical clinical features. Patients with LMC due to leukaemia were excluded.
Data collection
The data was collected on a predefined data collection sheet which was shared with all investigators. The data collected was of baseline characteristics at the time of diagnosis of LMC, previous treatment records, disease status with molecular details, the pattern of care, the treatment offered, response and overall survival.
Outcomes
Overall survival was defined as the time in days from date of diagnosis of LMC to date of death or date of last follow up whichever was applicable.
Data analysis
Descriptive statistics were used for data analysis and statistical software SPSS version 26 (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp) and RStudio version 1.4.1106 (RStudio Team (2021). RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA URL http://www.rstudio.com/.) were used. Medians with range were provided for continuous variables while percentages with 95% CI were provided for non-continuous variables. Imaging Response was assessed by Response Assessment in Neuro-Oncology (RANO). Response Evaluation Criteria in Solid Tumors (RECIST) was used to assess response the primary and the type of assessment was local. Agresti-Coull Interval Method was used for the calculation of 95% CI. Overall survival was estimated using Kaplan Meier analysis. Cox regression analysis was performed to identify factors impacting survival. A p-value of 0.05 was considered statistically significant. The assumptions of Cox regression analysis were checked before performing the analysis and were met. Cox proportional hazard model was constructed, and the proportional hazard assumption was tested using Schoenfeld residuals. The median follow-up was calculated using Reverse Kaplan–Meier method.
Results
Baseline characteristics
Eighty-four patients were included in the present study. The baseline characteristics, tumour details and previous treatment details are shown in Table 1. The number of lumbar punctures required to get positive CSF cytology was 1 in 44 (52.4%), 2 in 6 (7.1%) and 3 in 2 (2.4%). In 32 (38.1%) patients the CSF cytology was either not done or was negative. The commonest site of primary leading to LMC was lung in 46 patients (54.8%). The number of lumbar punctures required to get positive CSF cytology was 1 in 44 (52.4%), 2 in 6 (7.1%) and 3 in 2 (2.4%). In 32 (38.1%) patients the CSF cytology was either not done or was negative. It was equivocal in 2 patients which was considered as negative. Fifty-three patients (73.1%) had cerebrospinal MRI at baseline. Out of these 33 (39.3%) had only cerebral MRI while the rest 20 (23.8%) had cerebrospinal. Furthermore, Ommaya reservoirs were not used in any of the patients. Four patients (16.7%) had hydrocephalus at diagnosis of Leptomeningeal metastasis. Nineteen patients (16.7%) had received WBRT.
Table 1.
Baseline details of the participants.
| Variable | Value (N = 84) |
|---|---|
| Age in years-No (%) | |
| Median (Range) | 52.5 (21–75) |
| Non-Elderly | 61 (72.6) |
| Elderlyb | 23 (27.4) |
| Gender-No (%) | |
| Male | 38 (45.2) |
| Female | 46 (54.8) |
| ECOG PS-No (%) | |
| 1 | 41 (48.8) |
| 2 | 28 (33.3) |
| 3 | 9 (10.7) |
| 4 | 6 (7.1) |
| Disease site-No (%) | |
| Breast | 22 (26.2) |
| Lung (NSCLC) | 45 (53.6) |
| Lung (SCLC) | 1 (1.2) |
| Gastrointestinal | 9 (10.7) |
| Others | 7 (8.3) |
| NSCLC-Molecular types-No (%)a | |
| EGFR mutation | 36 (42.9) |
| ALK rearrangement | 3 (3.6) |
| No mutation | 7 (8.3) |
| Breast cancer-Molecular types-No (%)a | |
| TNBC | 11 (13.1) |
| ER/PR+ & Her-2 negative | 9 (10.7) |
| ER/PR- & Her-2 positive | 1 (1.2) |
| ER/PR- & Her-2 positive | 1 (1.2) |
| Number prior therapies-No (%) | |
| Nil | 2 (2.4) |
| 1 | 43 (51.2) |
| 2 | 20 (23.8) |
| 3 | 15 (17.9) |
| ≥4 | 4 (4.8) |
| LMC diagnosis-No (%) | |
| Confirmed | 52 (61.9) |
| Probable | 32 (38.1) |
| Extracranial disease status at diagnosis-No (%) | |
| Progressive disease | 39 (46.4) |
| Stable disease | 31 (36.9) |
| Partial response | 8 (9.5) |
| Complete response | 5 (6) |
| Missing data | 1 (1.2) |
ECOG PS- Eastern Cooperative Oncology Group Performance Status. NSCLC- Non small cell lung cancer, SCLC–small cell lung cancer, EGFR-epidermal growth factor receptor, ALK- Anaplastic lymphoma kinase, ER-Estrogen receptor, PR-Progesterone receptor, Her-2-human epidermal growth factor receptor 2, LMC-Leptomeningeal carcinomatosis.
Restrict to 22 patients with breast cancer and 45 patients with NSCLC.
‘Elderly’ was defined as age >60 years.
All patients had clinical symptoms of LMC such as headache, nausea and vomiting, seizure, altered sensorium and difficulty with walking or speech. Leptomeningeal metastasis was discovered at the time of diagnosis in 9 patients (11%) and within 30 days in 12 patients (14%). The median time-period between metastatic diagnosis and LMC diagnosis was 360 days (range 0–1800). The median OS from diagnosis of metastatic disease was 587 days (95% CI 472–702).
Treatment pattern
The treatment pattern administered is shown in Table 2. The two most common treatment algorithms chosen were best supportive care and intrathecal with systemic therapy in 24 (28.6%) patients each. In EGFR mutated tumours, osimertinib was used in 1 and afatinib in 1 patient, respectively. Rest all EGFR mutated patients were exposed to only first-generation tyrosine kinase inhibitors. Similarly, in ALK-rearranged patients, none of the patients had exposure to 3rd generation ALK inhibitors. None of these patients had received immunotherapy. Intrathecal therapy was offered in 33 patients (39.3%). The most common intrathecal agent was methotrexate in 23 patients (27.4%).
Table 2.
Table depicting pattern of treatment.
| Variable | Value |
|---|---|
| Treatment pattern-No (%) | |
| Best supportive care only | 24 (28.6) |
| Systemic therapy only | 17 (20.2) |
| Intrathecal + systemic therapy | 24 (28.6) |
| Intrathecal only | 9 (10.7) |
| Radiation only | 10 (11.9) |
| Radiation-No (%) | |
| Yes | 18a(21.4) |
| No | 66 (78.6) |
| Radiation type-No (%) | |
| Focal | 15 (17.9) |
| CSI | 3 (3.6) |
| Systemic therapy-No (%) | |
| Targeted | 21 (25) |
| Chemotherapy | 16 (19) |
| Chemotherapy + targeted | 4 (4.8) |
| Type of intrathecal therapy-No (%) | |
| Methotrexate | 23 (27.4) |
| Triple | 10 (11.9) |
| Duration of therapy | |
| Median (Range) | 4 (1–14 weeks) |
Triple–methotrexate, AraC (cytosine arabinoside) and hydrocortisone. CSI- Craniospinal irradiation.
In 8 patients it was given along with systemic therapy and in 10 it was administered as the sole therapy.
Response
The response assessment with respect to cerebrospinal fluid (CSF) studies and radiological response is shown in Table 3. The main reasons that follow-up CSF cytology analysis or MRI analysis was not available were progression or death prior to the first follow-up assessment: progression and death before 2 months were observed in the majority of patients. The 30 days and 60 days OS were 73.5% (95% 62.6–81.7) and 56.9% (95% CI 45.3–66.9).
Table 3.
Response assessment details.
| Variable | Value- No (%) |
|---|---|
| CSF response | |
| Not assessed | 59 (70.2) |
| CSF negative | 13 (15.5) |
| CSF positive | 11 (13.1) |
| Missing data | 1 (1.2) |
| MRI response | |
| Not assessed | 67 (79.8) |
| Complete response | 1 (1.2) |
| Partial response | 6 (7.1) |
| Stable disease | 6 (7.1) |
| Progressive disease | 3 (3.6) |
| Missing data | 1 (1.2) |
CSF-cerebrospinal fluid.
MRI-Magnetic resonance imaging.
Overall survival
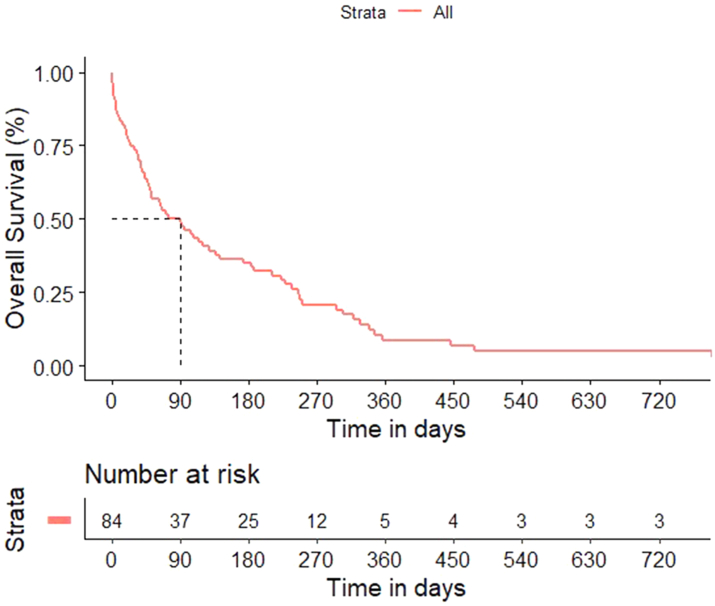
The median follow-up was 763 days (95% CI 316–1211). There were 70 deaths, and the estimated median OS was 90 days (95% CI 48–128) (Fig. 2). The 1-year and 2 years OS were 8.7% (95% CI 3.3–17.3) and 5.2% (95% CI 1.4–12.9%) respectively. The results of multivariate analysis are shown in Table 4. The median OS in breast cancer was 571 days (95% CI 335–807), that in NSCLC was 647 days (95% CI 561.0–733.0) and in other sites was 317 days (95% CI 102–533).
Fig. 2.
Overall survival curve.
Table 4.
Table depicting factors influencing overall survival.
| Variable | Median OS in days (95% CI) | Univariate Hazard ratio | Log rank p value | Multivariate Hazard ratio | 95% CI of Hazard ratio | p-value |
|---|---|---|---|---|---|---|
| Age | ||||||
| Age | Not applicablea | 0.99 | Not applicablea | 1.00 | 0.97–1.02 | 0.764 |
| Gender | ||||||
| Male | 64 (34–223) | 0.97 | 0.98 | 1.14 | 0.68–1.91 | 0.630 |
| Female | 104 (43–136) | Reference | ||||
| ECOG PS | ||||||
| 1 | 61 (38–186) | 0.93 | 0.46 | 0.66 | 0.37–1.18 | 0.159 |
| 2–4 | 104 (43–142) | Reference | ||||
| Site | ||||||
| Lung | 90 (47–227) | 0.78 | 0.69 | 0.54 | 0.29–1.014 | 0.055 |
| Non-Lung | 70 (34–128) | Reference | ||||
| Extracranial disease status | ||||||
| Progressive disease | 64 (29–172) | 1.13 | 0.012 | 1.22 | 0.72–2.03 | 0.467 |
| Non-progressive disease | 107 (43–183) | Reference | ||||
| LMC diagnosis | ||||||
| Confirm | 74 (38–142) | 0.81 | 1.000 | 0.43 | 0.23–0.81 | 0.009 |
| Probable | 94 (43–223) | Reference | ||||
| IT received | ||||||
| Yes | 142 (70–245) | 0.69 | 0.13 | 0.35 | 0.19–0.66 | 0.001 |
| No | 51 (29–94) | Reference | ||||
LMC-Leptomeningeal carcinomatosis, ECOG PS- Eastern Cooperative Oncology Group Performance Status, IT-Intrathecal.
Continuous variable. Elderly = Age >60 years.
Discussion
To our knowledge, this is one of the first multicentric data collection efforts in LMC from any LMIC across the globe. The data throws light on multiple aspects of LMC in LMIC which were previously unknown. Melanoma is not one of the commonest malignancies associated with LMC in India. It is possibly a reflection of the lower incidence of melanoma in tropical countries where it is not even within the top 10 commonest malignancies as per GLOBOCAN data.16 The commonest three malignancies associated with LMC in our present study were non-small cell lung cancer, breast cancer and gastrointestinal malignancies. The corresponding sites in western literature from the Americas region are melanoma, breast cancer and non-small cell lung cancer.17, 18, 19, 20 Thus suggesting different demography of LMC as opposed to that reported from western literature. Even in other studies reported from the Indian subcontinent the predominant disease is either non small cell lung cancer or breast cancer. In a LMC experience reported from Kochi (State of Kerala), the commonest site leading to LMC was breast (45%) followed by lung cancer (35%).21 Other large experiences from India (>1100 patients) by Patil and colleagues22 and Abraham and colleagues,23 were in lung cancer and breast cancer respectively.
A significant proportion of patients (>50%) have presented in ECOG PS 2–4 state. This reflects the pattern of practice of Indian oncologists where limited scanning of the brain and CSF axis is performed due to constraints of resources. Multiple guidelines, including National comprehensive cancer network (NCCN), suggest that imaging of the brain needs to be performed in NSCLC stage IV.24 However brain imaging is rarely done due to less accessibility to MRI and its associated cost. Further, this information often does not influence the treatment decisions as irrespective of presence or absence of central nervous system (CNS) involvement, CNS penetrating tyrosine kinase inhibitor (TKI) cannot be selected due to their prohibitively high cost. Hence multiple oncologists choose to scan the neural axis or do a CSF examination only when the patient is symptomatic. This is reflected also in the fact that all patients in the current study already had CNS symptoms. Similar experience was reported by Abraham and colleagues in breast cancer23 where nearly all patients were symptomatic with headache (47%), vomiting (47%), diplopia (20%), and seizure (20%) being the most common symptoms. In a large experience from prospective studies in NSCLC, the symptoms of leptomeningeal disease were symptoms of altered higher mental functions (48.4%) or seizures and Headache and dizziness (32.2%).22 Similar experience was published from Kochi, where the commonest presenting features were headache, vomiting, loss of consciousness, cranial nerve palsies and seizures.21
The treatment pattern suggested that a significant proportion of patients were offered the best supportive care in our study. This is in line with multiple guidelines suggesting that poor PS patients or patients with uncontrolled progressive disease should be offered the best supportive care.25 The survival in this group of patients is dismal as seen in our study where the median OS in patients with Eastern Cooperative Oncology Group Performance Status 3–4 (ECOG PS 3–4) was 29 days and in patients with the extracranial progressive disease was 64 days. Hence it is imperative that these patients are diagnosed early in the disease course. Probably, following guidelines and doing imaging to detect CNS involvement might lead to improvement in outcomes however that presents its own challenges in LMICs. While prevalence of leptomeningeal carcinomatosis has been seen to be around 3–7% with solid tumours, its incidence is often 15–20% on autopsy suggesting a large number of these are being missed owing to delayed imaging and/or poor survival before CNS symptoms become evident.
Intrathecal therapy (IT) is one of the recommended therapies for LMC.26, 27, 28 Multiple established guidelines recommend it. However, the utility of IT is tested mainly in two trials for breast cancer. One study was negative for OS benefit however had its own limitations while the second study was able to show beneficial effect on progression-free survival (PFS) in LMC in breast cancer.29, 30, 31 Such randomized data is not available for lung cancer. However, recent large retrospective analysis suggests that giving IT probably won't improve outcomes, at least in driver mutated NSCLC.32, 33, 34, 35 However, our results suggest that IT improves overall survival. This might be a result of the limited exposure to 3rd generation tyrosine kinase inhibitors (TKIs) seen in our patients. Third generation TKIs have CNS penetration ability36 and might not need IT. However first-generation TKIs have limited CNS penetration and the addition of intrathecal therapy might improve outcomes. The utility of triple IT was seen in a study reported from Kochi.21 Symptomatic improvement was noted in 70% of patients and the 6 month-OS was 38%. However the median PFS was a dismal of 2.0 months only. Dismal median OS of 2.0 months was reported by Patil et al. in a prospective study of NSCLC who had developed LMC. In that study there was trend towards improved outcomes in patients who were treated with third generation TKI with a median OS of 245 days (95% CI: 215.48–274.52) versus 52 days (95% CI: 22.62–81.38) in favour of third generation TKI.22 Similar dismal median OS of 3 months was reported in breast cancer patients from Thiruvananthapuram (State of Kerala).23
This data indicates that despite a substantial proportion of patients having targetable driver mutations, outcomes of LMC remain dismal in LMIC.37,38 This suggests that the current treatment landscape in LMICs is insufficient to adequately treat leptomeningeal carcinomatosis as outcomes have remained low even in those with targetable mutations. There is a need for the development of new treatment options like CNS-penetrating immunotherapy acknowledging that the evidence of existing treatment modalities like radiotherapy (RT) does suggest that it improves outcomes however this is scant and unsubstantiated at this point in time.
Another important facet is the cost-effectiveness of early screening modalities to screen for and identify LMC before symptoms with solid tumours in LMICs. A high percentage of LMC is seen only at autopsy compared the reported numbers with a definitive diagnosis coupled with the poor prognosis from leptomeningeal carcinomatosis suggests that early, widespread and periodic screening would very likely play a role in lowering mortality from it. However, on the other hand, the high-cost burden of this screening via imaging modalities coupled with the barrier of financial access to effective CNS-penetrating therapy raises the question of the feasibility of this strategy in LMICs. The optimal strategy lies somewhere on this spectrum where cheaper, more easily accessible screening strategies with good sensitivity would allow a smaller number to require the resource-burdening confirmatory test of high specificity to allow the most fertile situation for shared decision making of treatment between patient and doctor.
The strength of the current study is the multicentric real-world data across multiple primary tumours. The study also had few limitations, viz. treatment algorithms were heterogeneous and very few patients had exposure to 3rd generation TKIs. Unfortunately, we have not collected data on progression-free survival. The data generation for the current study was possible because of wide collaboration between clinicians at different centres. Thus, clinicians could have had differing protocols for follow-up procedures and timing. Due to this, we did not gather data on progression-free survival (PFS) as we could not maintain uniformity for reliable PFS data.
LMC has a grave prognosis and nearly one-third of the patients are treated with the best supportive care due to dismal prognosis. Intrathecal therapy, though part of guidelines, is administered in only 39% of patients with leptomeningeal carcinomatosis. IT therapy and lung primary are associated with relatively improved outcomes. This data can serve as a benchmark for further improvement to facilitate studies for further improving the outcomes.
Contributors
Conceptualisation, study design, literature search, data acquisition, data analysis, statistical analysis, manuscript preparation, writing—review and editing: all authors. Guarantor: AK.
Data sharing statement
De-identified data may be shared on a case-by-case basis upon reasonable requests to the corresponding author for a period of 5 years.
Declaration of interests
Authors declare no conflicts of interest.
Acknowledgements
None.
References
- 1.Remon J., Le Rhun E., Besse B. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: a continuing challenge in the personalized treatment era. Cancer Treat Rev. 2017;53:128–137. doi: 10.1016/j.ctrv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlain M.C. Leptomeningeal metastasis. Semin Neurol. 2010;30(3):236–244. doi: 10.1055/s-0030-1255220. [DOI] [PubMed] [Google Scholar]
- 3.Le Rhun E., Preusser M., van den Bent M., Andratschke N., Weller M. How we treat patients with leptomeningeal metastases. ESMO Open. 2019;4(Suppl 2) doi: 10.1136/esmoopen-2019-000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang N., Bertalan M.S., Brastianos P.K. Leptomeningeal metastasis from systemic cancer: review and update on management. Cancer. 2018;124(1):21–35. doi: 10.1002/cncr.30911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glitza I.C., Rohlfs M., Guha-Thakurta N., et al. Retrospective review of metastatic melanoma patients with leptomeningeal disease treated with intrathecal interleukin-2. ESMO Open. 2018;3(1) doi: 10.1136/esmoopen-2017-000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pellerino A., Brastianos P.K., Rudà R., Soffietti R. Leptomeningeal metastases from solid Tumors: recent advances in diagnosis and molecular approaches. Cancers. 2021;13:2888. doi: 10.3390/cancers13122888. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhun E.L., Le Rhun E., Rudà R., Riccardo R., Weller M. BMET-18. Diagnosis and treatment patterns for patients with leptomeningeal metastasis from solid tumors across europe. Neuro Oncol. 2016;18:vi30. doi: 10.1093/neuonc/now212.118. Available from: [DOI] [PubMed] [Google Scholar]
- 8.Hendriks L.E.L., Subramaniam D.S., Dingemans A.M.C. Frontiers Media SA; 2019. Central nervous system metastases in lung cancer patients: from prevention to diagnosis and treatment; p. 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz M., Fleisher M., Pentsova E.I. Cerebrospinal fluid circulating tumor cells for diagnosis, response evaluation, and molecular profiling of leptomeningeal metastases from solid tumors. Cancer Biomark. 2022:283–296. doi: 10.1016/b978-0-12-824302-2.00007-2. Available from: [DOI] [Google Scholar]
- 10.Prakadan S.M., Alvarez-Breckenridge C.A., Markson S.C., et al. Genomic and transcriptomic correlates of immunotherapy response within the tumor microenvironment of leptomeningeal metastases. Nat Commun. 2021;12(1):5955. doi: 10.1038/s41467-021-25860-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamba N., Wen P.Y., Aizer A.A. Epidemiology of brain metastases and leptomeningeal disease. Neuro Oncol. 2021;23(9):1447–1456. doi: 10.1093/neuonc/noab101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naidoo J., Schreck K.C., Fu W., et al. Pembrolizumab for patients with leptomeningeal metastasis from solid tumors: efficacy, safety, and cerebrospinal fluid biomarkers. J Immunother Cancer. 2021;9(8) doi: 10.1136/jitc-2021-002473. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero D. Craniospinal irradiation improves leptomeningeal metastasis control. Nat Rev Clin Oncol. 2022;19(9):567. doi: 10.1038/s41571-022-00669-3. [DOI] [PubMed] [Google Scholar]
- 14.von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 15.Le Rhun E., Guckenberger M., Smits M., et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol. 2021;32(11):1332–1347. doi: 10.1016/j.annonc.2021.07.016. [DOI] [PubMed] [Google Scholar]
- 16.356-India-fact-sheets.pdf. Globocon India Ca. https://gco.iarc.fr/today/data/factsheets/populations/356-india-fact-sheets.pdf Available from:
- 17.Thakkar J.P., Kumthekar P., Dixit K.S., Stupp R., Lukas R.V. Leptomeningeal metastasis from solid tumors. J Neurol Sci. 2020;411 doi: 10.1016/j.jns.2020.116706. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe J., Mitsuya K., Nakamoto S., et al. Leptomeningeal metastasis in ER + HER2- advanced breast cancer patients: a review of the cases in a single institute over a 15-year period. Breast Cancer Res Treat. 2021;189(1):225–236. doi: 10.1007/s10549-021-06246-z. [DOI] [PubMed] [Google Scholar]
- 19.Lu Z.Q., Cai J., Wang X., et al. Osimertinib combined with bevacizumab for leptomeningeal metastasis from EGFR-mutation non-small cell lung cancer: a phase II single-arm prospective clinical trial. Thorac Cancer. 2021;12(2):172–180. doi: 10.1111/1759-7714.13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozcan G., Singh M., Vredenburgh J.J. Leptomeningeal metastasis from non-small cell lung cancer and current landscape of treatments. Clin Cancer Res. 2022;29:11. doi: 10.1158/1078-0432.CCR-22-1585. Available from: [DOI] [PubMed] [Google Scholar]
- 21.Srinivasalu V.K., Subramaniam N., Philip A., Jose W., Pavithran K. Triple intrathecal chemotherapy for leptomeningeal carcinomatosis in solid tumors: treatment outcomes, response and their determinants. Indian J Cancer. 2021;58:84. doi: 10.4103/ijc.IJC_730_18. Available from: [DOI] [PubMed] [Google Scholar]
- 22.Patil V., Noronha V., Vallathol D.H., et al. Leptomeningeal metastasis from non-small cell lung cancer- a post-hoc analysis from four randomised clinical trials. Ecancermedicalscience. 2022;16:1414. doi: 10.3332/ecancer.2022.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abraham A.A., Annop T.M., Rona Joseph P., Vasudevan A., Kumar B.S. Clinical outcome of neoplastic meningitis associated with breast cancer. J Neurosci Rural Pract. 2022;13(1):108–113. doi: 10.1055/s-0041-1741505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guidelines Detail NCCN NSCLC NCCN. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf [cited 2022 Jul 4]. Available from:
- 25.Waki F., Ando M., Takashima A., et al. Prognostic factors and clinical outcomes in patients with leptomeningeal metastasis from solid tumors. J Neuro Oncol. 2009;93(2):205–212. doi: 10.1007/s11060-008-9758-3. [DOI] [PubMed] [Google Scholar]
- 26.Morris P.G., Reiner A.S., Szenberg O.R., et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol. 2012;7(2):382–385. doi: 10.1097/JTO.0b013e3182398e4f. [DOI] [PubMed] [Google Scholar]
- 27.Beauchesne P. Intrathecal chemotherapy for treatment of leptomeningeal dissemination of metastatic tumours. Lancet Oncol. 2010;11(9):871–879. doi: 10.1016/S1470-2045(10)70034-6. [DOI] [PubMed] [Google Scholar]
- 28.Berg S.L., Chamberlain M.C. Current treatment of leptomeningeal metastases: systemic chemotherapy, intrathecal chemotherapy and symptom management. Cancer Treat Res. 2005;125:121–146. doi: 10.1007/0-387-24199-x_8. Available from: [DOI] [PubMed] [Google Scholar]
- 29.Carausu M., Carton M., Darlix A., et al. Breast cancer patients treated with intrathecal therapy for leptomeningeal metastases in a large real-life database. ESMO Open. 2021;6(3) doi: 10.1016/j.esmoop.2021.100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaeckle K.A., Phuphanich S., Bent M.J., et al. Intrathecal treatment of neoplastic meningitis due to breast cancer with a slow-release formulation of cytarabine. Br J Cancer. 2001;84(2):157–163. doi: 10.1054/bjoc.2000.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker N. Leptomeningeal metastasis in a patient with triple-negative breast cancer: a case report and literature review. 2019. Available from: [DOI] [Google Scholar]
- 32.Alexander M., Lin E., Cheng H. Leptomeningeal metastases in non-small cell lung cancer: optimal systemic management in NSCLC with and without driver mutations. Curr Treat Options Oncol. 2020;21 doi: 10.1007/s11864-020-00759-3. Available from: [DOI] [PubMed] [Google Scholar]
- 33.Xu Q., Chen X., Qian D., et al. Treatment and prognostic analysis of patients with leptomeningeal metastases from non-small cell lung cancer. Thorac Cancer. 2015;6:407–412. doi: 10.1111/1759-7714.12188. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng H., Dean Hosgood H., Deng L., et al. Survival disparities in black patients with EGFR-mutated non–small-cell lung cancer. Clin Lung Cancer. 2020;21:177–185. doi: 10.1016/j.cllc.2019.07.003. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park S., Lee M.H., Seong M., et al. A phase II, multicenter, two cohort study of 160 mg osimertinib in EGFR T790M-positive non-small-cell lung cancer patients with brain metastases or leptomeningeal disease who progressed on prior EGFR TKI therapy. Ann Oncol. 2020;31(10):1397–1404. doi: 10.1016/j.annonc.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Zhao J., Chen M., Zhong W., et al. Cerebrospinal fluid concentrations of gefitinib in patients with lung adenocarcinoma. Clin Lung Cancer. 2013;14(2):188–193. doi: 10.1016/j.cllc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Liao B.C., Lee J.H., Lin C.C., et al. Epidermal growth factor receptor tyrosine kinase inhibitors for non-small-cell lung cancer patients with leptomeningeal carcinomatosis. J Thorac Oncol. 2015;10(12):1754–1761. doi: 10.1097/JTO.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 38.Patil V.M., Noronha V., Joshi A., et al. Phase III study of gefitinib or pemetrexed with carboplatin in EGFR-mutated advanced lung adenocarcinoma. ESMO Open. 2017;2(1) doi: 10.1136/esmoopen-2017-000168. [DOI] [PMC free article] [PubMed] [Google Scholar]