Summary
Background
Cancer is one of the leading causes of morbidity and mortality in India. Clinical trials are critical for driving innovation in cancer therapy, diagnosis, and prevention. This study aims to depict the evolving landscape of cancer clinical trials in India by analysing the clinical trials registered in Clinical Trial Registry-India (CTRI).
Methods
We identified cancer trials registered in CTRI (between 2007 and 2021) using search terms adapted from the cancer types defined by the National Cancer Institute (USA). We then collated and analysed the publicly available information from CTRI (cancer subtypes, type of trial, treatment intent, type of intervention, sponsor type, recruitment countries) and used descriptive statistics to illustrate the overall as well as year-to-year trend.
Findings
In total, we identified 1988 cancer trials, the majority of which focused on treating cancer (63%) and rest of the trials aimed at optimising the operational aspects of surgery (19%), mitigating treatment-related toxicity (10.6%), or treating cancer-related symptoms (7.8%). Focusing on trials with the intent of treating cancer, we found that most were investigating solid tumours as opposed to haematological malignancies with the most prominent cancer subtypes being breast cancer (17%), head and neck cancer (9.8%), lung cancer (9.6%), and cervical cancer (6.6%). The number of trials conducted in a given cancer subtype from our analysis overall correlated to the incidence, mortality, and 5-year prevalence of the respective cancer subtype in India; however, head and neck cancer and cervical cancer were underrepresented in trials as compared with the disease burden. The most common type of intervention was investigational drugs. The most common sponsor types were global pharmaceutical industry (26%) and research institution and hospital (26%). Despite a relatively high cancer burden, the availability of cancer trials in the Northeastern states of India was limited.
Interpretation
There is a pressing need for clinical cancer research in India to be better aligned with the nation's healthcare needs and disease burden, focusing on prevalent and deadly cancers while ensuring the availability of clinical trials across geographic regions and underserved populations.
Funding
Pi Health USA, a fully owned subsidiary of BeiGene Ltd.
Keywords: Cancer, Oncology, Clinical trial, Clinical Trial Registry-India
Research in context.
Evidence before this study
Clinical Trials Registry—India (CTRI) is an online public record system for the registration of clinical trials being conducted in India. We searched Google Scholar using the terms (“clinical trial” AND “cancer” AND “India” AND “CTRI”) to find studies published since 2007—the year when CTRI was launched. From this search, we identified several studies that analysed the clinical trial landscape in India. A study by Chaturvedi and colleagues analysed all clinical trials across therapeutic areas that were registered in CTRI between 2007 and 2015, revealing that clinical trials conducted in India were not in consonance with the health care needs and that there was a significant regional disparity in clinical trial availability. Within oncology, we found two studies that specifically analysed the cancer trial landscape in India. One study by Roy and colleagues reported that for the period between 2007 and 2017, only 350 interventional cancer trials were registered in CTRI. Another study by Chakraborty and colleagues examined the open clinical trials registered in CTRI between 2012 and 2020 and reported substantial inter-state geographical disparity in access to these trials. All the studies analysed the clinical trials registered in CTRI during a limited time frame. To the best of our current knowledge, no published studies have comprehensively analysed all cancer trials registered in CTRI since the launch of the registry, and no studies have provided a detailed, year-to-year trend of important parameters (e.g., number of trials, cancer subtype, nature of treatment, sponsor type, trial location, etc.) necessary to understand the evolving clinical trial landscape in the country.
Added value of this study
To our knowledge, this is the first comprehensive overview and analysis of publicly available information on cancer clinical trials registered in CTRI on a year-to-year basis since its inception. The study provides a high-level overview of the current state and evolving trends of clinical cancer research in India. Several interesting trends emerged from the analysis, including a significant decrease in the number of newly registered cancer trials between 2013 and 2017, a gap between the high burden of head and neck cancer in India and the relative lack of clinical trials focused on such cancers, the scarcity of phase 1 trials as compared to phase 2 or phase 3 trials, and the substantial geographic disparity in cancer trial availability across the states of India. Such a landscape analysis is the first step toward identifying gaps and optimizing the use of limited resources.
Implications of all the available evidence
There is a pressing need for clinical cancer research in India to be better aligned with the nation's healthcare needs and disease burden and to focus on prevalent and deadly cancers while ensuring the availability of clinical trials across geographic regions and disadvantaged populations.
Introduction
Cancer is one of the leading causes of morbidity and mortality in India.1 WHO Global Cancer Observatory estimates that 1.32 million new cancer cases and 0.85 million cancer-related deaths occurred in India in 2020 alone.2 The burden of cancer relative to other diseases is also increasing. According to the Global Burden of Disease study, cancer caused 8.3% of the total deaths in India in 2016, which was double the contribution of cancer in 1990.3
The cancer landscape in India is unique when compared to the rest of the world. For example, the most common type of cancer in terms of incidence or mortality among males in India is lip and oral cavity cancers (104,661 cases representing 16% of all new cancer cases in 2020 among males of all ages), as compared to prostate and lung cancers in other countries.4 In addition, cancer patients in India often present with late-stage malignancies; for example, a recent report from India's National Cancer Registry Programme showed that the majority of cancer patients were diagnosed at the locally advanced stage for breast (57%), cervix uteri (60%), head and neck (67%) and stomach (51%) cancer, whereas lung cancers were most often diagnosed with distant metastases in both males (44%) and females (48%).1 Late-stage diagnoses contribute to poor treatment outcomes, which further widens the health disparities between India and the rest of the world.5
Clinical trials are critical for driving innovative anti-cancer therapies and advancing methods to detect, diagnose, or prevent cancer. Therapeutic clinical trials offer patient-level and population-level benefits by providing patients access to promising investigational anti-cancer therapies while the data obtained contributes to a greater understanding of the disease and therapy in question. Over the past few decades, India's participation in global clinical trials has grown, due in part to the size of the patient population, low operational costs, regulatory reforms, and the changing economic environment.6,7
On July 20, 2007, the Indian Council of Medical Research's National Institute of Medical Statistics launched the Clinical Trials Registry—India (CTRI), which is an online public record system for the registration of clinical trials being conducted in India.8 Initially a voluntary measure, trial registration in CTRI has been made mandatory by the Drugs Controller General (India) since June 15, 2009. The CTRI database contains important clinical trial metadata such as the health condition/problems studied, type of trial, intervention, phase of trial, primary sponsor, sites of study, countries of recruitment, target sample size, and other details. As a result, CTRI provides a useful data set to analyse the historical and ongoing clinical cancer research landscape in India.
The information available in CTRI has been analysed in a few recent studies to elucidate the current state of clinical trials in India. Chaturvedi and colleagues analysed clinical trials across therapeutic areas that were registered in CTRI between 2007 and 2015, revealing that clinical trials conducted in India were not in consonance with the country's health care needs, and there was significant regional disparity in clinical trial availability.9 In oncology, Roy and colleagues noted that for the period of 2007–2017, only 350 interventional cancer trials were registered in CTRI.10 For comparison, 2066 interventional cancer trials in USA were registered in clinicaltrials.gov in 2017 alone.11 Additionally, Chakraborty and colleagues identified substantial inter-state geographical disparities in access to cancer clinical trials through an analysis of 181 open clinical trials registered in CTRI between 2012 and 2020.12 To the best of our current knowledge, no published studies have analysed—in a comprehensive manner—all cancer clinical trials registered in CTRI since the launch of the registry, and no studies have reported detailed, year-to-year trends of important parameters (e.g., number of trials, cancer subtype, nature of treatment, etc.) necessary to understand the evolving landscape of clinical cancer research in the country.
In this study, we analysed publicly available information on all cancer clinical trials registered in CTRI on a year-to-year basis between 2007 and 2021, including the number of trials, the cancer subtype under study, the type of intervention, phase of trial, sponsor types, countries of recruitment, and sites of study. Such an analysis would help identify potential gaps, such as the discordance between the burden of specific cancers and clinical trial availability, along with geographic access to cancer clinical trials.
Methods
Data extraction strategy
Data on CTRI are publicly available. All available data were downloaded from CTRI in the form of comma-separated values files (.csv) during April 17—26, 2022. Clinical trials registered in CTRI between 2007 (the start of the registry) and December 2021 were included for analysis.
Creation of cancer clinical trials data set
Cancer clinical trials were identified using the following search terms adapted from the National Cancer Institute (NCI, USA) Cancer Types:
“cancer” “neoplasm” “neoplasia” “tumor” “oncology” “leukemia” “lymphoma” “myeloma” “carcinoma” “sarcoma” “melanoma” “mesothelioma” “glioma” “glioblastoma” “medulloblastoma” “ependymoma” “esthesioneuroblastoma” “retinoblastoma” “neuroblastoma” “papillomatosis” “paraganglioma” “pheochromocytoma” “blastoma” “metastatic” “astrocytoma” “craniopharyngioma” “cholangiocarcinoma” “chordoma” “gestational trophoblastic disease” “histiocytosis” “mycosis fungoides” “Sezary syndrome” “myelodysplastic” “rhabdomyosarcoma” “oligodendroglioma” “ependymoma” “schwannoma” “ganglioglioma”.
We identified and compiled the key search terms that would allow extraction of all cancer types based on the NCI definition. The search was based on a partial string match in the following fields: health condition/problems studied, inclusion criteria, public title of study, scientific title of study. For search terms that contained multiple words (i.e., gestational trophoblastic disease, mycosis fungoides, Sezary syndrome), we used an exact match rather than a partial string match to avoid including non-cancer studies (e.g., if using partial string match, the search term “gestational trophoblastic disease” would have identified and included studies on gestational diabetes, which is not cancer-related). The resulting cancer clinical trials data set was then manually confirmed to only include cancer-related clinical trials.
Annotating cancer subtypes
We adapted the cancer classification by GLOBOCAN to categorize the cancer subtypes in the cancer clinical trials data set according to the following stepwise approach. First, trials that have specified the International Classification of Disease (ICD, 10th version, version 2010) codes were classified according to the GLOBOCAN cancer dictionary (Supplementary Table S1). For simplicity, we grouped cancers of the lip and oral cavity, larynx, hypopharynx, oropharynx, salivary glands, nasopharynx together into “head and neck.” Second, for the remainder of the trials, we devised an algorithm to identify key terms based on information in the health condition/problems studied, scientific title, and public title to automatically classify the cancer subtypes. The key terms corresponding to each cancer subtype are listed in Supplementary Table S1 and were identified using an exact match approach. If a trial included two or more cancer subtypes, or if the cancer type was described in general terms (e.g., solid tumours, blood cancers, etc.), then we labelled these “multiple tumour types.” Third, all results were then manually checked by two researchers independently to ensure accuracy.
Annotating trial type
We categorised the trials according to CTRI definition of the type of trials: observational, interventional, Bioavailability & Bioequivalence (BA/BE), post-marketing surveillance (PMS). This information can be found under “type of trial” in the original data entered by investigators. In the minority of cases where such information was absent, we manually reviewed the scientific title for this classification. For subsequent analyses, we focused on trials of the interventional, BA/BE, or PMS type.
Annotating treatment intent
We manually reviewed the scientific title to classify each trial to one of the following four categories according to treatment intent: treating cancer, treating cancer-related symptoms (e.g., pain, fatigue, cachexia, anaemia, or other comorbidities), mitigating treatment-related toxicity (e.g., radiation therapy-induced oral mucositis, chemotherapy-induced neutropenia, chemotherapy-induced nausea and vomiting, etc.), or optimising operational aspects of surgery (such as optimising the anaesthesia/analgesia regime or other operational aspects of surgery). For subsequent analyses, we focused on trials with the intent of treating cancer.
Annotating type of intervention
For trials with the intent of treating cancer, we manually reviewed data entered by investigators under “type of study” to classify each trial to one of the following categories: drug, radiation, surgery, ayurveda, diagnostics, medical device, screening/prevention, and others. Trials which encompassed multiple modalities of interventions (e.g., chemoradiation therapy, or radiation + surgery) were labelled as “combination.” In cases where such information was absent, we manually reviewed the scientific title of the trial for this classification.
Annotating sponsor type
For trials with the intent of treating cancer, we manually reviewed data entered by investigators under “Primary sponsor: type of sponsor” to classify each trial to one of the following categories: Pharmaceutical industry-Indian, Pharmaceutical industry-Global, Contract research organization, Research institution, Research institution and hospital, Government funding agency, Government medical college, Private medical college, Private hospital/clinic, and others. We chose these categories as they were the main sponsor types according to CTRI (i.e., menu options for primary sponsor under the “Trial Search” function in CTRI). In cases where such information was absent, we labelled these as “non-specified.”
Annotating recruitment countries
We manually reviewed data entered by investigators in “countries of recruitment” to categorise each trial to one of the two regional categories: India or multinational. We noticed that for a small fraction of trials, the “countries of recruitment” only included India, but other available information suggested that the trial was multinational with India being one of the recruitment countries (e.g., target total sample size did not match target India sample size). As such, we manually checked all trials and corrected the information when needed. In the minority of cases where the target total sample size or target India sample size information was absent, we cross-checked the trial in another database (e.g., clinicaltrials.gov) to help with this classification.
Calculations and statistical methods
After filtering the CTRI data set for cancer-specific interventional, BA/BE, or PMS studies, trial records were evaluated for the following fields: type of study, phase, registration year, sources of monetary or material support, primary sponsor: type of sponsor, primary sponsor: name, recruitment countries, number of sites, study sites: site name, study sites: site address, estimated duration (year, month, day), date of first enrolment: India, date of study completion: India, date of first enrolment: global, date of study completion: global, recruitment status: global, recruitment status: India. Fields with empty or obviously erroneous entries were treated as missing values. Data were summarised using descriptive statistics.
Epidemiologic characteristics of cancer subtypes in India were obtained from GLOBOCAN 2020, including values for incidence, mortality, and 5-year prevalence. Correlations between the number of clinical trials by cancer subtype and either the incidence, mortality, or 5-year prevalence were calculated using Pearson correlation.
The number of clinical trials within each state was counted by the state listed within the site address field. The total number of clinical trials for each state was normalised to the population of that state (per 100,000 people). The 2011 census data was used to determine the population of each state as the decennial population census which was due to be performed in 2021 was postponed due to the COVID-19 pandemic. The population of Indian states Telangana and Andhra Pradesh were calculated based on the regional population data from the 2011 census (considering the geographic redistribution in 2014 that led to creation of these two states).
Clinical trials per cancer patient were calculated by taking the ratio of clinical trials per 100,000 people and the incidence rate of cancer per 100,000 people. This number was multiplied by 100,000 to calculate the total number of clinical trials per 100,000 cancer patients. State-level cancer incidence data in India was based on the Global Burden of Disease study 1990–2016.3 Incidence data was unavailable for the states of Ladakh; Andaman and Nicobar Islands; Chandigarh; Dadra and Nagar Haveli and Daman and Diu; Lakshadweep; and Puducherry.
Results
In total we identified 1988 cancer clinical trials registered in CTRI between July 2007 and December 2021, which were labelled as either “interventional” or “BA/BE” or “PMS” in the “type of trial” data field. We manually categorised each trial into one of the following four categories based on treatment intent: treating cancer, treating cancer-related symptoms, mitigating treatment-related toxicity, or optimising operational aspects of surgery. The number and proportion of cancer clinical trials in each of these four categories and trend evolution over time are depicted in Fig. 1 (a, b). Overall, most trials (n = 1251, 63%) were aimed at treating cancer, followed by a smaller fraction aimed at optimizing operational aspects of surgery (n = 371, 19%), mitigating treatment-related toxicity (n = 210, 11%), and treating cancer-related symptoms (n = 156, 7.8%). We observed a notable increase in the number of trials in all four categories since 2017, reflecting a focus on improving cancer patients’ quality of life along with developing curative treatments. For subsequent analyses, we focused on clinical trials with the intent of treating cancer.
Fig. 1.
The number and proportion of cancer clinical trials registered on CTRI categorised by the treatment intent. (a) Overall breakdown of aggregated data from 2007 to 2021. (b) Year-to-year trend.
In terms of the type of cancer addressed, we found that most trials with the intent of treating cancer were investigating solid tumours (n = 1058, 85%) as opposed to haematological malignancies (n = 163, 13%). A small fraction either did not specify the tumor types (n = 19, 1.5%) or were investigating both solid tumours and haematological malignancies (n = 11, 0.9%). The overall breakdown and year-to-year trend are depicted in Fig. 2 (a, b). We further investigated the specific cancer subtype, using the cancer dictionary by GLOBOCAN. Overall, the most prominent cancer subtypes included breast cancer (n = 216, 17%), head and neck cancer (n = 123, 9.8%), lung cancer (n = 120, 9.6%), and cervical cancer (n = 82, 6.6%). A fraction of trials targeted multiple tumour types (n = 154, 12%) (Fig. 2c). The detailed year-to-year trend is depicted in Supplementary Table S2.
Fig. 2.
The number and proportion of cancer clinical trials (with the intent of treating cancer) registered on CTRI categorised by the cancer subtype. (a, b) Overall breakdown and year-to-year trend of clinical trials on either solid tumours or haematological malignancies. (c) Breakdown of clinical trials on specific cancer subtypes based on the GLOBOCAN definition. (d–f) Correlation between the number of clinical trials with the corresponding incidence (d), mortality (e), or 5-year prevalence data (f) on specific cancer subtypes based on the GLOBOCAN 2020 India data. Dotted lines are linear regressions.
We explored the association of the number of trials with the intent of treating cancer in specific cancer subtypes with the disease burden of those cancers in India according to 2020 GLOBOCAN data. The number of trials conducted in a given cancer subtype was significantly correlated to the incidence, mortality, and 5-year prevalence of the respective cancer subtype (r = 0.87 [p < 0.0001], 0.81 [p < 0.0001], and 0.90 [p < 0.0001], respectively, Fig. 2d–f). However, discordance exists for certain cancer subtypes. For example, head and neck cancer had the highest incidence at 18% and the highest mortality at 15% but was the focus of only 9.8% (n = 123) of cancer trials. Cervical cancer was also underrepresented, at 6.6% (n = 82) of cancer trials, despite having an incidence and mortality rate of 9.4% and 9.1%, respectively. On the other hand, the representation of breast (n = 216, 17%) or lung cancer (n = 120, 9.6%) in trials was higher than its incidence (breast cancer: 14%, lung cancer: 5.5%) or mortality (breast cancer: 11%, lung cancer: 7.8%).
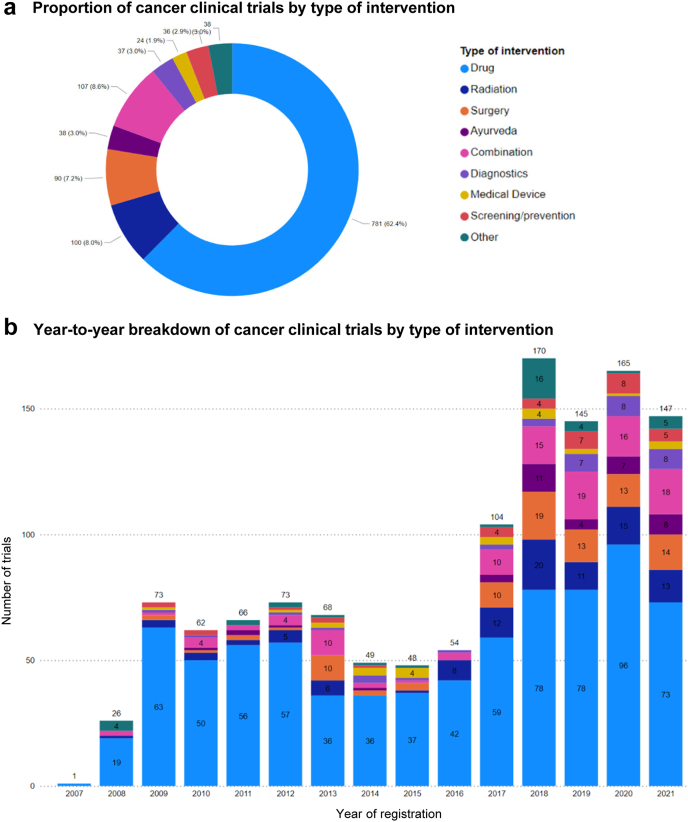
We analysed the overall pattern and year-to-year trend of the type of intervention in trials with the intent of treating cancer (Fig. 3a and b). Trials testing investigational drugs represented the majority (n = 781, 62%), followed by those testing radiation therapy (n = 100, 8.0%) or surgery (n = 90, 7.2%). A notable fraction of cancer trials involved more than one modality of intervention (n = 107, 8.6%), and such combination trials increased in numbers in recent years (2017–2021).
Fig. 3.
The number and proportion of cancer clinical trials (with the intent of treating cancer) registered on CTRI categorised by the type of intervention. (a) Overall breakdown of aggregated data from 2007 to 2021. (b) Year-to-year trend.
In terms of the distribution of trial phases among the trials with the intent of treating cancer (Fig. 4a and b), Phase 3 trials represented the largest fraction (n = 382, 31%), followed by Phase 2 trials (n = 259, 21%); Phase 1 and Phase 4 trials were less common, at 5.3% (n = 66) and 7.8% (n = 97), respectively. We note, however, that a fraction of cancer trials (n = 350, 28%) did not specify the trial phase, which was particularly noticeable in recent years (2017–2021).
Fig. 4.
The number and proportion of cancer clinical trials (with the intent of treating cancer) registered on CTRI categorised by trial phase. (a) Overall breakdown of aggregated data from 2007 to 2021. (b) Year-to-year trend.
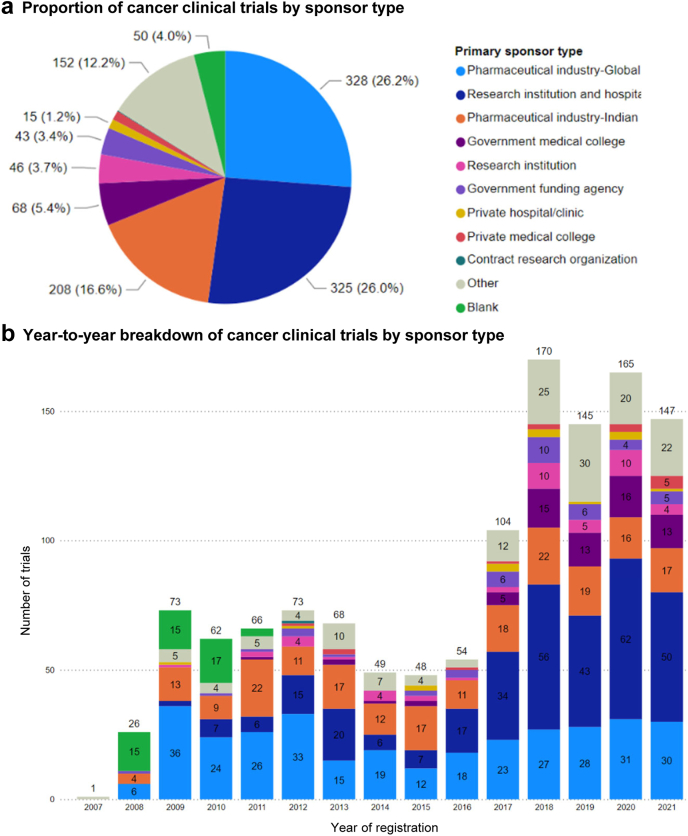
We examined the sponsorship of trials with the intent of treating cancer in India; for this analysis, we focused on the nature of the primary sponsor (Fig. 5a and b). Overall, global pharmaceutical industry (n = 328, 26%) and research institution & hospital (n = 325, 26%) were the most common sponsor types, followed by Indian pharmaceutical industry (n = 208, 17%). In the year-to-year trend, we found that the number of industry-sponsored cancer trials—including both global pharmaceutical industry-sponsored and Indian pharmaceutical industry-sponsored trials—remained largely constant (except for a dip between 2013 and 2016 for global pharmaceutical industry-sponsored trials), whereas research institution and hospital-sponsored cancer trials saw a large increase since 2017.
Fig. 5.
The number and proportion of cancer clinical trials (with the intent of treating cancer) registered on CTRI categorised by sponsor type. (a) Overall breakdown of aggregated data from 2007 to 2021. (b) Year-to-year trend.
In terms of where the trials with the intent of treating cancer were recruiting (Fig. 6a and b), we found that 22% (n = 280) of trials were multinational trials, in which India was one of the recruiting countries, whereas the rest (n = 971, 78%) were India-only trials. The year-to-year trend indicates that the growth of cancer trials in recent years was largely driven by India-only trials. The number of multinational trials sharply decreased in 2013, with only six new multinational cancer trials registered that year. While this number was slowly recovering over the past decade, it remained low or on par with pre-2013 levels. We also analysed the recruitment status of cancer trials in India. Aggregating all cancer trials from 2007 to 2021, 29% of trials are not yet recruiting, 28% of trials are open to recruitment, and 26% are marked as completed. A smaller fraction of trials was terminated (4.0%) or suspended (1.2%). When focusing specifically on recent years (2017–2021), the proportion of trials that are not yet recruiting or open to recruitment is higher, whereas the proportion of trials marked as completed is much lower (∼10%). Such data is depicted in Supplementary Figure S3.
Fig. 6.
The number and proportion of cancer clinical trials (with the intent of treating cancer) registered on CTRI categorised by recruitment country. (a) Overall breakdown of aggregated data from 2007 to 2021. (b) Year-to-year trend.
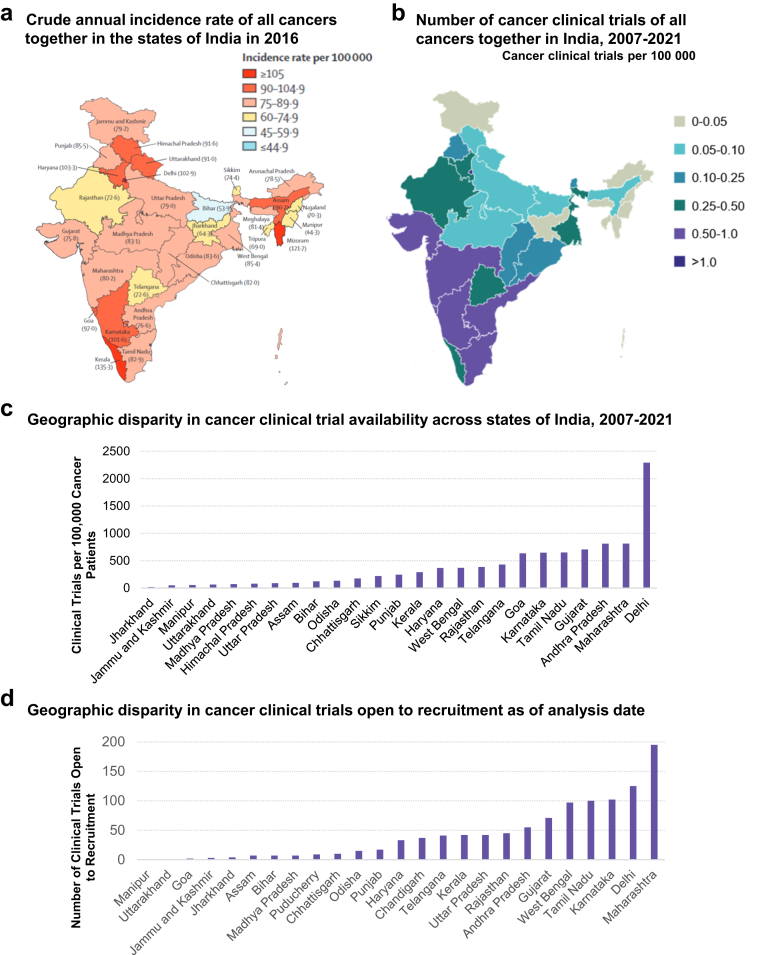
Finally, we investigated potential geographic disparities across states and regions in India by comparing cancer incidence to the number of cancer clinical trials per 100,000 people within each state (Fig. 7a and b). The Northeastern states, despite generally having higher cancer incidence rate than Southwestern states, lag well behind in the number of cancer clinical trials available per 100,000 people. We calculated the number of clinical trials per 100,000 cancer patients from the aggregate data from 2007 to 2021 to further reveal stark geographic disparities between states (Fig. 7c). Several states, including Arunachal Pradesh, Meghalaya, Mizoram, Nagaland, Tripura, Ladakh, Andaman and Nicobar Islands, Dadra and Nagar Haveli and Daman and Diu, and Lakshadweep, had no registered clinical trials available to cancer patients from 2007 to 2021. On the opposite end of the spectrum, Delhi, Maharashtra, and Andhra Pradesh led India in the availability of clinical trials for cancer patients, with over 800 clinical trials per 100,000 cancer patients available in Maharashtra and Andhra Pradesh and over 2000 clinical trials per 100,000 cancer patients available in Delhi. This geographic disparity remains evident when evaluating clinical trials currently available to cancer patients, based on the number of trials open to recruitment at present (Fig. 7d). While advancements were made in some states (e.g., West Bengal, Kerala) to better distribute cancer clinical trials across India, clinical trials continue to be sparse in the Northeastern states. In particular, Himachal Pradesh and Sikkim, which despite having clinical trials registered between 2007 and 2021, do not currently have any trials open to recruitment for cancer patients.
Fig. 7.
Geographic disparity of cancer clinical trials in the states of India. (a) Crude annual incidence rate of all cancers together in the states of India in 2016 (adapted from Lancet Oncol 2018; 19: 1289–306) (b) Density of cancer clinical trials with the intent of treating cancer in the states of India based on aggregated data from 2007 to 2021 (c) The extent of cancer clinical trial disparity for all cancers across states of India. No cancer clinical trials have been registered in Arunachal Pradesh, Meghalaya, Mizoram, Nagaland, Tripura, Ladakh, Andaman and Nicobar Islands, Dadra and Nagar Haveli and Daman and Diu, and Lakshadweep. Incidence data was unavailable for Chandigarh and Puducherry. (d) Total number of cancer clinical trials that are open to recruitment within each state as of the analysis date (April 2022). In addition to the states where there have been no clinical trials registered, there are no trials open to recruitment in Himachal Pradesh or Sikkim as of the analysis date.
Discussion
To our knowledge, this is the first comprehensive overview and analysis of the publicly available information on cancer clinical trials registered in CTRI on a year-to-year basis since its inception. Our study provides a high-level overview of the current state and evolving trends of clinical cancer research in India.
Several interesting trends were noted in our study. Our analysis revealed a significant decrease in the number of newly registered cancer clinical trials in 2013—a trend that lasted several years until 2017 (Fig. 1). One possible explanation could be the system-level sweeping regulatory reform and changes in the trial process that were put in place by the Indian central government in 2013, which imposed rigorous new requirements for conducting clinical trials in India.13 There was rapid growth of clinical research in India between 2005 and 2009, but following this research boom, there were serious allegations and media reports of unethical clinical trial practices and significant deficiencies in the clinical trial regulatory process in the country.14 As a result, the regulatory environment in India underwent drastic changes, with new and tightened regulations put in place by the Central Drugs Standard Control Organization (CDSCO) to bring transparency and accountability to the clinical trial process.14 While the new regulations were intended to protect the safety and welfare of clinical trial participants, the broad provisions and rigorous mandates may have led to varying degrees of confusion and uncertainty among researchers and sponsors in conducting clinical trials.13 As a result, there was a substantial decrease in the number of clinical trials registered in India in 2013, as observed in the current study.15,16 The Indian government has since narrowed certain provisions and addressed some of the ambiguity in the rules as part of the continued regulatory reform, which brought greater clarity to clinical trial regulations.13 From our analysis, we found that by 2017, the overall number of newly registered cancer clinical trials had recovered and surpassed pre-2013 levels (Fig. 1b). Of note, such a trend appears to be driven largely by cancer clinical trials sponsored by research institutions and hospitals, whereas those sponsored by the pharmaceutical industry (both global and Indian) have stayed close to or slightly below the pre-2013 levels (Fig. 5b). Consistent with this finding, the number of newly registered multinational cancer trials has also been slow to recover since 2013 (Fig. 6b).
In terms of the types of cancers that were addressed in clinical trials in India, we found that while overall there was a strong correlation between the burden (incidence, mortality, 5-year prevalence) and the number of clinical trials in specific cancers, certain cancers were significantly underrepresented in clinical trials. This is particularly striking for head and neck cancers, which accounted for one-sixth of all new cancer cases and one-fifth of all cancer-related deaths in 2020 but was the focus of fewer than 10% of cancer clinical trials in India (Fig. 2e–g).2 This gap between the high burden of head and neck cancer in India and the relative lack of head and neck cancer clinical trials in India highlights the potential mismatch between clinical research and the needs of the local population. More than half of head and neck cancers in the world occur in Asia, especially in India.17 Compared to high-income countries (HICs), head and neck cancers in India exhibit distinct demographic profiles, risk factors, and family and personal history.17 As such, there is an urgent need to develop evidence-based and feasible solutions to manage head and neck cancers in India. It's important to note that in India, 60–80% head and neck cancer patients present with advanced disease as compared to 40% in HICs.17 While most of the efforts usually focus on developing therapies, preventative measures to address the underlying risk factors as well as early detection through screening should not be overlooked and may improve outcomes for patients.
We found that Phase 2 and Phase 3 trials dominated the cancer clinical trial landscape in India, whereas Phase 1 trials represented only a small fraction (5.3%). This observation is consistent with the prior literature analysing clinical trials across therapeutic areas that were registered in CTRI.9,10 This is likely reflecting regulations that prohibit the conduct of Phase 1 studies of new drugs discovered or developed outside of India, limiting the potential therapeutic agents available for Phase 1 trials.18
Previous analyses of CTRI have shown geographic disparities in access to cancer clinical trials in India, with less accessible care in the northeastern states.12 Even though northeast India has a higher cancer burden relative to the rest of the country, according to the 2020 report from the Indian National Cancer Registry Programme,1 our analysis shows that cancer clinical trials are scarce in the region. Despite having some of the highest incidence rates of cancer in India, states such as Mizoram and Assam had no registered or open clinical trials available for cancer patients (Fig. 7). This gap between cancer burden and availability of cancer trials in Northeast India is consistent with prior findings, reinforcing the growing need to improves access to cancer care and innovative treatments especially among India's most vulnerable populations.12 Nationwide, major academic cancer centres, such as Tata Memorial Hospital, All India Institute of Medical Sciences, Rajiv Gandhi Cancer Institute and Research Center, have done seminal work in clinical cancer research over the past decade.19 However, more effort is needed to broaden the pool of hospitals and clinics that participate in cancer clinical trials. This would not only provide local patients with increased access to innovative treatments but would also generate insights into the unique medical comorbidities and sociodemographic variables in the region that may impact treatment success and adherence.
India accounts for nearly 20% of the global population, but only 1.5% of global clinical trials are conducted in India.20 Multiple domains have been identified as barriers to advancing clinical trials globally. These themes generally include lack of financial or personnel resources, ethical and regulatory oversight, lack of research experience and infrastructure (e.g., research-specific training, information technology), operational barriers (e.g., geography, public awareness, health literacy), and opportunity costs (e.g., competing clinical demands).21 These themes have indeed been identified in India as well.12,22
Proposed solutions span similar domains. These include harmonising research standards and agendas to align with international guidelines and increasing research education among staff, enforcement of monitoring and auditing, engagement with regulatory agencies, site involvement to consider operational feasibility, and public awareness.22,23 More specifically, several efforts are underway to address these barriers. The National Cancer Grid is a collaboration of multiple organizations and cancer centres aimed at creating management guidelines with considerations for value-based care and resource limitations, training of medical staff, and cost reductions through price negotiations.23 The International Collaboration for Research methods Development in Oncology (CReDO) is an initiative spearheaded by Tata Memorial Centre and the National Cancer Grid to provide educational workshops on training clinical staff in research methodologies related to clinical trials.24 The Apollo Hospital Based Cancer Registry has proposed standardised data collection to describe patient demographics, disease, treatments, and outcomes.23 The India Council of Medical Research National Centre for Disease Informatics operates two major national cancer registries, the Population Based and Hospital Based Cancer Registries, that are important tools for further characterising cancer epidemiology and population-level outcomes.23 Finally, considerations should be given to explore innovative digital technologies that may complement existing infrastructure to facilitate the conduct of cancer clinical trials and augment data management across a wide range of clinical settings.
Our study has several limitations. First, the analysis was limited to studies registered in CTRI. Since June 15, 2009, the CDSCO has mandated the registration of all clinical trials running in India on CTRI, including multinational trials where India is one of the recruiting countries. However, recent research has revealed that certain clinical trials were registered in ClinicalTrials.gov listing India as a recruiting country but were not registered in CTRI.25 Investigating if such a discrepancy exists for cancer clinical trials, and to what extent, could provide useful insights into potential gaps within CTRI. Second, there are important limitations to the underlying data set available from CTRI such as issues with a lack of clarity in certain classifications (e.g., “type of study”), internal inconsistencies, incomplete or non-standard information, missing data, and incomplete data.26 In particular, the lack of standardized terminology and the ambiguity in the definition of certain terms leaves much room for different interpretations; this is further exacerbated by the free-text input options which limits the ability to achieve a precise understanding of the data. Even though such nuances are unlikely to affect the overall conclusions in a major way, caution is advised when making interpretations from the data. Third, we identified cancer clinical trials from all studies registered in CTRI using a search algorithm, which is based on a list of key cancer-related terms (see more details in the Methods section). The list may not be exhaustive, resulting in the possibility of missing certain cancer trials if the description of the trial in health condition or problems studied, inclusion criteria, public title of study, or scientific title of study did not include the search terms in our algorithm. However, given the comprehensive nature of the list, we expect such cases to be rare and the impact on the overall conclusions to be minimal. Last, we used the cancer dictionary by GLOBOCAN—which is primarily based on ICD-10—to categorise the cancer subtype in each trial. Such an approach for a consistent definition was necessary to enable the subsequent analyses regarding correlation between the disease burden and the number of clinical trials for specific cancer subtypes; however, it may not reflect the categorisations used in clinical guidelines and therapeutic decision-making.
Our work here presents a comprehensive description of the current state of clinical cancer research in India. Such a landscape analysis is the first step towards identifying gaps and optimizing the use of limited resources. While much progress has been made, there remains a pressing need for clinical cancer research in India to be better aligned with the nation's healthcare needs and disease burden, focusing on prevalent and deadly cancers while ensuring availability of clinical trials across geographic regions and disadvantaged populations.
Contributors
PG, JC, and AM designed the study, with input from CS, GK, and BR. CL, MC, ZH and AP contributed to extracting, cleaning, and aggregating the data. PG, JC, ZH, MC, AM, AP, RL contributed to data analyses and interpretation. PG, AM, and AP drafted the manuscript. All authors contributed to revising the manuscript and approved the final version of the manuscript.
Data sharing statement
Data supporting the findings of the study is available from the corresponding author upon reasonable request.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
PG, JC, ZH, MC, AM, RL, CL, GK and BR are employees of Pi Health USA, which is a fully owned subsidiary of BeiGene Ltd. PG, JC, ZH, MC, AM, RL, GK and BR report having received BGNE stock grants for employment. JC is an AACR-AstraZeneca Clinical Immuno-Oncology Research Training Program grant recipient, funded from October 2020 to September 2021. JC received speaker honoraria from University of California, Davis and the Association of Northern California Oncologists. JC received support from BeiGene for attending the American Association for Cancer Research (AACR) annual meeting, the American Society of Clinical Oncology (ASCO) annual meeting, and the HLTH (health) conference in 2022. All authors report no other conflicts of interest.
Acknowledgements
The authors thank Salvatore Ferro, Bernard P. Chang, Mazen Nuwayhid, Tiffany Chen, Arunava Majumder, and Smruthi Panyam for critical review and invaluable input to the manuscript. This study is sponsored by Pi Health USA, a fully owned subsidiary of BeiGene Ltd.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lansea.2023.100323.
Contributor Information
Peng Gao, Email: kate.gao@pihealth.ai.
Bobby Y. Reddy, Email: bobby.reddy@pihealth.ai.
Appendix A. Supplementary data
References
- 1.Mathur P., Sathishkumar K., Chaturvedi M., et al. Cancer statistics, 2020: report from national cancer registry Programme, India. JCO Glob Oncol. 2020;6:1063–1075. doi: 10.1200/GO.20.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Cancer Observatory . International Agency for Research on Cancer; 2020. Cancer today. [Google Scholar]
- 3.India State-Level Disease Burden Initiative Cancer C. The burden of cancers and their variations across the states of India: the Global Burden of Disease Study 1990-2016. Lancet Oncol. 2018;19:1289–1306. doi: 10.1016/S1470-2045(18)30447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 5.Mallath M.K., Taylor D.G., Badwe R.A., et al. The growing burden of cancer in India: epidemiology and social context. Lancet Oncol. 2014;15:e205–e212. doi: 10.1016/S1470-2045(14)70115-9. [DOI] [PubMed] [Google Scholar]
- 6.Gupta Y.K., Padhy B.M. India's growing participation in global clinical trials. Trends Pharmacol Sci. 2011;32:327–329. doi: 10.1016/j.tips.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Maiti R., Raghavendra M. Clinical trials in India. Pharmacol Res. 2007;56:1–10. doi: 10.1016/j.phrs.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 8.http://ctri.nic.in/Clinicaltrials/login.php
- 9.Chaturvedi M., Gogtay N.J., Thatte U.M. Do clinical trials conducted in India match its healthcare needs? An audit of the Clinical Trials Registry of India. Perspect Clin Res. 2017;8:172–175. doi: 10.4103/2229-3485.215970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy A.M., Mathew A. Audit of cancer clinical trials in India. J Glob Oncol. 2019;5:1. doi: 10.1200/JGO.19.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.https://clinicaltrials.gov/
- 12.Chakraborty S., Mallick I., Luu H.N., et al. Geographic disparities in access to cancer clinical trials in India. Ecancermedicalscience. 2021;15:1161. doi: 10.3332/ecancer.2021.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnes M., Flaherty J., Caron M., Naqvee A., Bierer B. The evolving regulatory landscape for clinical trials in India. Food Drug Law J. 2018;73(4):601–623. https://www.jstor.org/stable/26826964 [Google Scholar]
- 14.Kondal A., Krishna G.V.M., Bansal D. Clinical trial regulations in India: progress and challenges arising from recent amendments to schedule Y of the drugs and cosmetics (D&C) act 1940 (D&C rules 1945) Pharm Med. 2016;30:1–13. doi: 10.1007/s40290-015-0127-1. [DOI] [Google Scholar]
- 15.Roy Chaudhury R., Mehta D. Regulatory developments in the conduct of clinical trials in India. Glob Health Epidemiol Genom. 2016;1:e4. doi: 10.1017/gheg.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta Y.K., Kumar B.D. Clinical trials and evolving regulatory science in India. Indian J Pharmacol. 2014;46:575–578. doi: 10.4103/0253-7613.144887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulkarni M.R. Head and neck cancer burden in India. Int J Head Neck Surg. 2013;4(1):29–35. [Google Scholar]
- 18.https://cdsco.gov.in/opencms/opencms/en/Home/
- 19.Noronha V. Making a case for cancer research in India. Cancer Res Stat Treat. 2018;1:71–74. [Google Scholar]
- 20.The L. Health in India, 2017. Lancet. 2017;389:127. doi: 10.1016/S0140-6736(17)30075-2. [DOI] [PubMed] [Google Scholar]
- 21.Unger J.M., Vaidya R., Hershman D.L., Minasian L.M., Fleury M.E. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst. 2019;111:245–255. doi: 10.1093/jnci/djy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burt T., Sharma P., Dhillon S., Manchanda M., Mittal S., Trehan N., et al. Clinical research environment in India: challenges and proposed solutions. J Clin Res Bioeth. 2014;5:1–8. doi: 10.4172/2155-9627.1000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.https://assets.ey.com/content/dam/ey-sites/ey-com/en_in/topics/health/2022/ey-making-quality-cancer-care-more-accessible-and-affordable-in-india.pdf
- 24.Ranganathan P., Chinnaswamy G., Sengar M., et al. The International Collaboration for Research methods Development in Oncology (CReDO) workshops: shaping the future of global oncology research. Lancet Oncol. 2021;22:e369–e376. doi: 10.1016/S1470-2045(21)00077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumari S., Mohan A., Saberwal G. Hidden duplicates: 10s or 100s of Indian trials, registered with ClinicalTrials.gov, have not been registered in India, as required by law. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pillamarapu M., Mohan A., Saberwal G. An analysis of deficiencies in the data of interventional drug trials registered with Clinical Trials Registry - India. Trials. 2019;20:535. doi: 10.1186/s13063-019-3592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.