Abstract
Aim
Hypothermia is associated with poor prognosis in patients with sepsis. However, no studies have explored the correlation between the severity of hypothermia and prognosis.
Methods
Using data from the Japanese accidental hypothermia network registry (J‐Point registry), we examined adult patients aged ≥18 years with infectious diseases whose initial body temperature was ≤35°C from April 1, 2011 to March 31, 2016, in 12 centers. Patients were divided into three groups according to their body temperature: Tertile 1 (T1) (32.0–35.0°C), Tertile 2 (T2) (28.0–31.9°C), and Tertile 3 (T3) (<28.0°C). In‐hospital mortality was employed as a metric to assess outcomes. We conducted a multivariate logistic regression analysis to investigate the relationship between the three categories and the occurrence of in‐hospital mortality.
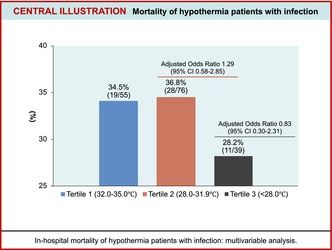
Results
A total of 572 patients were registered, and 170 eligible patients were identified. Of these patients, 55 were in T1 (32.0–35.0°C), 76 in T2 (28.0–31.9°C), and 39 in T3 (<28.0°C) groups. The overall in‐hospital mortality rate in accidental hypothermia (AH) patients with infectious diseases was 34.1%. The in‐hospital mortality rates in the T1, T2, and T3 groups were 34.5%, 36.8%, and 28.2%, respectively. The multivariable analysis demonstrated no significant differences regarding in‐hospital mortality among the three groups (T2 vs. T1, adjusted odds ratio [OR]: 1.29; 95% confidence interval [CI]: 0.58–2.89 and T3 vs. T1, adjusted OR: 0.83; 95% CI: 0.30–2.31).
Conclusion
In this multicenter retrospective observational study, hypothermia severity was not associated with in‐hospital mortality in AH patients with infectious diseases.
Keywords: accidental hypothermia, emergency department, environmental medicine, infection, mortality
This study examined 170 hypothermia patients with infectious diseases, categorizing them based on body temperature into three groups. Multivariable analysis found no significant differences between the severity of hypothermia and in‐hospital mortality.
INTRODUCTION
Infectious diseases are a major healthcare problem, impacting and burdening millions of people each year worldwide. 1 Even though outcomes have been improved by advances in understanding the underlying mechanism and its management, they are not yet satisfactorily controlled. Therefore, identifying prognostic factors and their appropriate management are important.
Infection is often accompanied by changes in body temperature, including fever and hypothermia, due to systemic inflammatory responses. While both hypothermia and fever have been discussed as possible adaptive responses to acute infections, 2 the role of hypothermia in infection remains a topic of debate due to its dual potential for both advantageous and detrimental effects. 3 Positive outcomes encompass a postponed occurrence of metabolic acidosis, decreased rates of endothelial cell apoptosis, and the suppression of a proinflammatory reaction, 4 limited bacterial growth and dissemination, protection of mitochondrial function, 5 and increased survival in mouse endotoxemia models. 6 However, previous studies have reported that suppression of inflammatory responses was harmful and that hypothermia also decreased certain inflammatory cytokines, inhibited leukocyte migration and phagocytosis, and induced persistent lymphopenia. 7 , 8 , 9 , 10 As for clinical aspects, a recent meta‐analysis demonstrated that in septic patients, hypothermia was associated with increasing mortality compared to normothermia, whereas fever was associated with decreasing mortality. 11
Several studies in animals and healthy volunteers have shown that a decrease in body temperature decreases the concentration of inflammatory cytokines. 12 , 13 , 14 The results of these studies suggest that the immune reactions are temperature‐dependent, that is, the more severe the degree of hypothermia, the more suppressed the immune response may be. Therefore, we hypothesized that, as the severity of hypothermia increases, the mortality of patients with infectious diseases may increase. Nevertheless, as far as we are aware, there has been no investigation conducted thus far into the correlation between hypothermia severity and its impact on prognosis. Therefore, using the Japanese accidental hypothermia network registry (J‐Point registry), we described the epidemiology of accidental hypothermia (AH) patients with infectious diseases and investigated whether the degree of hypothermia was associated with the prognosis of patients with infectious diseases.
METHODS
Study design, setting, and participants
This research represents a secondary examination of data obtained from the J‐Point registry, initially established as a multicenter retrospective observational study. The study methodology has been previously described. 15 In summary, the J‐Point registry consisted of eight centers designated as critical care medical centers (CCMCs) and four non‐CCMC acute care hospitals with an emergency department (ED) across the Kyoto, Osaka, and Shiga Prefectures in Japan. The median annual volume of ED visits for each participating institution was 19,651 (with an interquartile range of 13,281–27,554).
The J‐point registry retrospectively registered patients treated for AH in EDs based on the International Classification of Diseases, Tenth Revision (ICD‐10) code T68 (hypothermia) from April 1, 2011 to March 31, 2016.
This study included adult patients aged ≥18 years with AH and infectious diseases. We excluded patients whose body temperature were unknown or >35°C. Ethical approval for this study was obtained from the Ethics Committee of Kyoto Prefectural University of Medicine (ethical approval number: ERB‐C‐633‐1) and other institutions.
Data collection, quality control, and outcome measurement
The methodology for collecting data has been previously described in detail. 15 In brief, data collection was performed using a predefined uniform datasheet. Emergency physicians trained in appropriate data extraction reviewed the patient charts.
The baseline characteristics of the patients were as follows: sex, age (adult aged 18–64 years, young‐old aged 65–74 years, and old‐old aged ≥75 years 16 ), activities of daily living (ADL) before AH (independent, need some assistance, and need total assistance), location of occurrence (indoor and outdoor), mode of arrival (walk‐in and via ambulance), and past medical history (cardiovascular diseases, neurological diseases, endocrine diseases, psychiatric diseases, malignant diseases, dementia, and other).
With regard to in‐hospital‐level variables, in‐hospital data were as follows: source of infection diagnosed during initial treatment in the emergency department (pneumonia, urinary tract infection, intraabdominal infection, infection of unknown source, skin or soft‐tissue infection, central nervous system infection, multiple infection sources, and other), blood culture results, vital signs upon arrival at the hospital (body temperature, blood pressure, and heart rate), biological data (serum pH and HCO3 [mEq/L], lactate [mmol/L], sodium [mEq/L], potassium [mEq/L], glucose levels [mg/dL], white blood cell count [102/μL], and C‐reactive protein [mg/dL]), cold exposure, treatment process, and outcome. Cold exposure was determined by attending clinicians or data entry personnel. Rewarming procedures were categorized into two groups: active external/minimally invasive rewarming (warm intravenous fluids, warm blanket, forced warm air, heating pads, and warm bath) and active internal rewarming (lavage [stomach, chest, or bladder], intravascular hemodialysis, and extracorporeal membrane oxygenation). 17 Other treatment information included tracheal intubation and use of vasopressors (dopamine, dobutamine, noradrenaline, adrenaline, and vasopressin). Additionally, data on in‐hospital deaths were collected as outcome measures.
Statistical analysis
Based on the common classification of the severity of AH, the patients were divided into three groups based on body temperature: Tertile 1 (T1) (32.0–35.0°C), Tertile 2 (T2) (28.0–31.9°C), and Tertile 3 (T3) (<28.0°C). 17 Patient characteristics, in‐hospital information, and outcomes among the three groups were evaluated via the Kruskal–Wallis test for continuous variables and the chi‐squared test for categorical variables. The association of each body temperature category with in‐hospital death was assessed using multivariate logistic regression analysis, and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were calculated.
In the multivariable analysis, we selected covariates that are associated with clinical outcomes based on previous studies, including sex (men and women), age category (18–64 years, 65–74 years, and ≥ 75 years), unstable hemodynamic status (systolic blood pressure < 90 mmHg) (yes or no), positive blood culture (yes or no), and number of comorbidities in the medical history (none, one, and multiple). In addition, to examine the mortality rate of AH changes depending on the presence or absence of infectious complications, a multivariate analysis was conducted using sex, age categories, unstable hemodynamics, and the number of comorbidities as covariates.
All P‐values were two‐sided, and the significance level was set at p < 0.05. The analyses were performed using Stata/MP version 17 (StataCorp, College Station, TX, USA).
RESULTS
Among 572 patients registered in the J‐point registry, we excluded 13 patients with body temperature > 35°C, 14 patients with unknown body temperature, and eight patients aged <18 years. There were 191, 228, and 118 patients in the T1, T2, and T3 groups, respectively, who were classified based on body temperature. We then excluded 367 patients without infectious diseases from the study. The proportion of AH patients with infectious diseases was 31.6%. Finally, 170 patients were included in our analysis, with 55, 76, and 39 patients in the T1, T2, and T3 groups, respectively (Figure 1).
FIGURE 1.
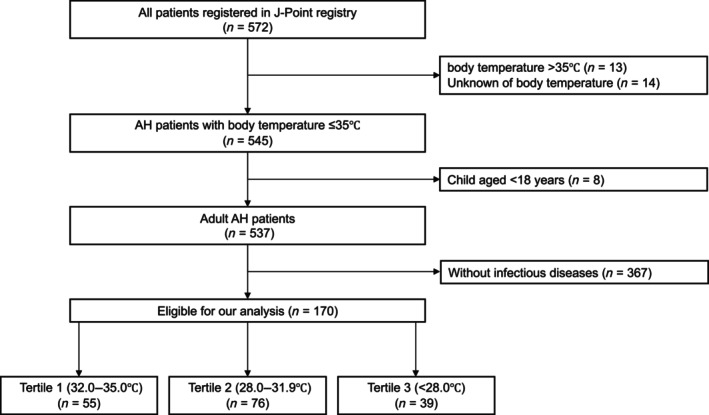
Patient flow of this study. AH, accidental hypothermia; J‐Point, Japanese accidental hypothermia network.
Table 1 shows patient characteristics according to body temperature. Approximately half of the patients were men, with a median age of around 80 years. AH with infectious diseases was most frequently documented in winter, particularly in the T3 group. In all groups, approximately 60% of the patients could independently perform an ADL, and most patients came to each ED by ambulance.
TABLE 1.
Patient characteristics according to body temperature category.
| All patients | Tertile 1 | Tertile 2 | Tertile 3 | P values a | |||||
|---|---|---|---|---|---|---|---|---|---|
| (32.0–35°C) | (28.0–31.9°C) | (<28.0°C) | |||||||
| (n = 170) | (n = 55) | (n = 76) | (n = 39) | ||||||
| Men | 88 | (51.8) | 30 | (54.5) | 34 | (44.7) | 24 | (61.5) | 0.22 |
| Age, years, median (IQR) | 81 | (70.0–87.0) | 83 | (78.0–87.0) | 83 | (71.5–89.0) | 77 | (64.0–86.0) | 0.085 |
| Age category | |||||||||
| Adults aged 18–64 years | 28 | (16.5) | 5 | (9.1) | 13 | (17.1) | 10 | (25.6) |
0.14 |
| Young‐Old aged 65–74 years | 26 | (15.3) | 7 | (12.7) | 11 | (14.5) | 8 | (20.5) | |
| Old‐Old aged ≥75 years | 116 | (68.2) | 43 | (78.2) | 52 | (68.4) | 21 | (53.8) | |
| Season | |||||||||
| Spring (3–5 month) | 37 | (21.8) | 14 | (25.5) | 19 | (25.0) | 4 | (10.3) |
0.016 |
| Summer (6–8 month) | 7 | (4.1) | 4 | (7.3) | 2 | (2.6) | 1 | (2.6) | |
| Autumn (9–11 month) | 18 | (10.6) | 10 | (18.2) | 7 | (9.2) | 1 | (2.6) | |
| Winter (12–2 month) | 108 | (63.5) | 27 | (49.1) | 48 | (63.2) | 33 | (84.6) | |
| Activities of daily living | |||||||||
| Independent | 108 | (63.5) | 31 | (56.4) | 51 | (67.1) | 26 | (66.7) |
0.45 |
| Needing some assistance | 42 | (24.7) | 14 | (25.5) | 17 | (22.4) | 11 | (28.2) | |
| Needing total assistance | 19 | (11.2) | 9 | (16.4) | 8 | (10.5) | 2 | (5.1) | |
| Unknown | 1 | (0.6) | 1 | (1.8) | 0 | (0.0) | 0 | (0.0) | |
| Location | |||||||||
| Indoor | 150 | (88.2) | 52 | (94.5) | 69 | (90.8) | 29 | (74.4) |
0.013 |
| Outdoor | 20 | (11.8) | 3 | (5.5) | 7 | (9.2) | 10 | (25.6) | |
| Mode of arrival | |||||||||
| Ambulance | 158 | (92.9) | 50 | (90.9) | 70 | (92.1) | 38 | (97.4) |
0.51 |
| Walk‐in | 12 | (7.1) | 5 | (9.1) | 6 | (7.9) | 1 | (2.6) | |
| Past medical history | |||||||||
| Cardiovascular diseases | 74 | (43.5) | 29 | (52.7) | 36 | (47.4) | 9 | (23.1) | 0.010 |
| Neurological diseases | 42 | (24.7) | 15 | (27.3) | 22 | (28.9) | 5 | (12.8) | 0.14 |
| Endocrine diseases | 35 | (20.6) | 14 | (25.5) | 16 | (21.1) | 5 | (12.8) | 0.32 |
| Psychiatric diseases | 35 | (20.6) | 7 | (12.7) | 19 | (25.0) | 9 | (23.1) | 0.22 |
| Malignant diseases | 18 | (10.6) | 8 | (14.5) | 8 | (10.5) | 2 | (5.1) | 0.36 |
| Dementia | 38 | (22.4) | 18 | (32.7) | 13 | (17.1) | 7 | (17.9) | 0.094 |
| Other | 30 | (17.6) | 7 | (12.7) | 17 | (22.4) | 6 | (15.4) | 0.35 |
| Unknown | 1 | (0.6) | 1 | (1.8) | 0 | (0.0) | 0 | (0.0) | 0.55 |
Note: Values are expressed as numbers (percentages) unless indicated otherwise.
Comparisons between the three groups were evaluated with Kruskal–Wallis test for numeric variables and χ 2 test for categorical variables.
Pneumonia was the most frequently noted source of infection in all groups. The T3 group had a higher likelihood of positive blood cultures and unstable vital signs. The proportion of cold exposure in the T1, T2, and T3 groups was 41.8% (23/55), 75.0% (57/76), and 89.7% (35/39), respectively, and was significantly higher in the T3 group. Moreover, patients in the T3 group were more likely to receive aggressive treatments such as active internal rewarming procedures, endotracheal intubation, and vasopressor use, and to be hospitalized in the intensive care unit (Table 2, Table S1).
TABLE 2.
In‐hospital data and outcome.
| All patients | Tertile 1 | Tertile 2 | Tertile 3 | P values a | |||||
|---|---|---|---|---|---|---|---|---|---|
| (32.0–35°C) | (28.0–31.9°C) | (<28.0°C) | |||||||
| (n = 170) | (n = 55) | (n = 76) | (n = 39) | ||||||
| In‐hospital data | |||||||||
| Source of infection | |||||||||
| Pneumonia | 73 | (42.9) | 22 | (40.0) | 35 | (46.1) | 16 | (41.0) | 0.31 |
| Urinary tract infection | 31 | (18.2) | 11 | (20.0) | 15 | (19.7) | 5 | (12.8) | |
| Intraabdominal infection | 12 | (7.1) | 5 | (9.1) | 5 | (6.6) | 2 | (5.1) | |
| Skin or soft‐tissue infection | 9 | (5.3) | 3 | (5.5) | 1 | (1.3) | 5 | (12.8) | |
| Central nervous system infection | 1 | (0.6) | 0 | (0.0) | 1 | (1.3) | 0 | (0.0) | |
| Other | 20 | (11.8) | 7 | (12.7) | 8 | (10.5) | 5 | (12.8) | |
| Infection of unknown source | 14 | (8.2) | 6 | (10.9) | 7 | (9.2) | 1 | (2.6) | |
| Multiple infection source | 10 | (5.9) | 1 | (1.8) | 4 | (5.3) | 5 | (12.8) | |
| Unstable hemodynamic status | 59 | (34.7) | 15 | (31.3) | 20 | (31.7) | 24 | (40.7) | 0.59 |
| Cardiac arrest | 5 | (2.9) | 0 | (0.0) | 1 | (1.3) | 4 | (10.3) | |
| Heart rate, median (IQR) | 65 | (46–83) | 71 | (59–85) | 65 | (55–88) | 52 | (40–75) | <0.001 |
| Glasgow Coma scale, median (IQR) | 10.0 | (6.0–13.0) | 11.5 | (7.0–14.0) | 10.0 | (7.0–13.0) | 7 | (3.0–10.0) | <0.001 |
| Biological data | |||||||||
| Serum pH, median (IQR) | 7.30 | (7.23–7.35) | 7.35 | (7.29–7.39) | 7.30 | (7.15–7.34) | 7.28 | (7.21–7.32) | <0.001 |
| Serum HCO3 (mEq/L), median (IQR) | 19.9 | (13.8–25.4) | 22.3 | (15.2–25.5) | 19.7 | (12.3–26.3) | 18 | (14.8–23.2) | 0.69 |
| Serum lactate (mmol/L), median (IQR) | 2.4 | (1.3–5.7) | 2.0 | (1.0–4.2) | 3.1 | (1.3–7.1) | 3.2 | (1.5–6.0) | 0.080 |
| Serum sodium (mEq/L), median (IQR) | 140.0 | (135–143) | 139.0 | (135–144) | 140.0 | (135–143) | 140 | (135–144) | 0.81 |
| Serum potassium (mEq/L), median (IQR) | 4.3 | (3.7–4.7) | 4.3 | (3.7–4.6) | 4.2 | (3.7–4.7) | 4.3 | (3.7–4.9) | 0.96 |
| Serum glucose (mg/dL), median (IQR) | 123.0 | (85.0–199.0) | 112 | (84.5–153.5) | 122 | (82.5–223.5) | 132 | (100.0–225.0) | 0.18 |
| White blood cell count, median (IQR) | 92.0 | (57.0–138.0) | 105.8 | (80.0–138.0) | 102.0 | (52.2–145.0) | 75.0 | (44.0–109.0) | 0.054 |
| CRP, median (IQR) | 4.3 | (1.3–12.3) | 3.9 | (0.6–10.8) | 4.6 | (1.6–14.9) | 5 | (1.3–8.0) | 0.23 |
| Blood culture positive | 33 | (19.5) | 7 | (12.7) | 15 | (20.0) | 11 | (28.2) | 0.18 |
| SOFA score, median (IQR) b | 5 | (3.0–7.0) | 4.0 | (3.0–6.0) | 5.0 | (3.0–7.0) | 6.0 | (4.0–9.0) | 0.46 |
| Respiratory system | 0 | (0.0–1.0) | 0 | (0.0–1.0) | 0 | (0.0–0.0) | 0 | (0.0–1.0) | 0.49 |
| Coagulation | 0 | (0.0–1.0) | 0 | (0.0–1.0) | 0 | (0.0–1.0) | 0 | (0.0–1.0) | 0.84 |
| Liver | 0 | (0.0–0.0) | 0 | (0.0–0.0) | 0 | (0.0–0.0) | 0 | (0.0–0.0) | 0.87 |
| Cardiovascular system | 0 | (0.0–1.0) | 0 | (0.0–0.0) | 0 | (0.0–1.0) | 0 | (0.0–1.0) | 0.49 |
| Central nervous system | 3 | (2.0–4.0) | 2 | (1.0–4.0) | 3 | (2.0–3.0) | 4 | (3.0–4.0) | 0.034 |
| Renal function | 1 | (0.0–2.0) | 0 | (0.0–2.0) | 1 | (0.0–2.0) | 0.5 | (0.0–1.0) | 0.42 |
| Vasopressors | 46 | (27.1) | 8 | (14.5) | 23 | (30.3) | 15 | (38.5) | 0.021 |
| Dopamine | 18 | (10.6) | 5 | (9.1) | 9 | (11.8) | 4 | (10.3) | 0.95 |
| Dobutamine | 7 | (4.1) | 1 | (1.8) | 4 | (5.3) | 2 | (5.1) | 0.61 |
| Noradrenaline | 31 | (18.2) | 4 | (7.3) | 16 | (21.1) | 11 | (28.2) | 0,02 |
| Adrenaline | 5 | (2.9) | 1 | (1.8) | 1 | (1.3) | 3 | (7.7) | 0.15 |
| Vasopressin | 1 | (0.6) | 1 | (1.8) | 0 | (0.0) | 0 | (0.0) | 0.55 |
| Emergent transvenous cardiac pacing | 2 | (1.2) | 1 | (1.8) | 0 | (0.0) | 1 | (2.6) | 0.30 |
| Admission ward | |||||||||
| No admission | 4 | (2.4) | 2 | (3.6) | 1 | (1.3) | 1 | (2.6) | 0.062 |
| General ward | 74 | (43.5) | 30 | (54.5) | 33 | (43.4) | 11 | (28.2) | |
| Intensive care unit | 92 | (54.1) | 23 | (41.8) | 42 | (55.3) | 27 | (69.2) | |
| Outcome | |||||||||
| Rewarming success | 141 | (82.9) | 43 | (78.2) | 64 | (84.2) | 34 | (87.2) | 0.53 |
| Intensive care unit days (IQR) b | 3 | (2.0–7.5) | 3 | (2.0–5.0) | 4 | (2.0–7.0) | 6 | (2.0–10.0) | 0.11 |
| Mortality days (IQR) | 7.5 | (2.0–21.0) | 17 | (2.0–48.0) | 7.5 | (3.0–17.5) | 2.0 | (1.0–7.0) | 0.037 |
| In‐hospital death | 58 | (34.1) | 19 | (34.5) | 28 | (36.8) | 11 | (28.2) | 0.68 |
Note: Values are expressed as numbers (percentages) unless indicated otherwise.
Abbreviation: SOFA, sequential organ failure assessment.
Comparisons between the three groups were evaluated with Kruskal–Wallis test for numeric variables and χ 2 test for categorical variables.
Calculated with patients admitted to intensive care unit.
The overall in‐hospital mortality rate for AH patients with infections was 34.1% (58/170 patients), significantly higher than the 20% (73/365 patients) observed in patients without infections (Table 2, Table S2). The in‐hospital mortality rates in the T1, T2, and T3 groups were 34.5% (19/55), 36.8% (28/76), and 28.2% (11/39), respectively.
The multivariable analysis demonstrated no significant differences regarding in‐hospital mortality among the three groups (T2 vs. T1, adjusted OR: 1.29; 95% CI: 0.58–2.89 and T3 vs. T1, adjusted OR: 0.83; 95% CI: 0.30–2.31). The multivariable analysis showed a significant difference in in‐hospital mortality between the old‐old group and adult group (adjusted OR: 4.32; 95% CI: 1.39–13.45). Unstable hemodynamic status was independently associated with in‐hospital mortality (adjusted OR: 2.30; 95% CI: 1.13–4.69). Multivariate analysis showed no significant differences in blood culture results or the number of medical histories with respect to in‐hospital mortality (Figure 2). In the multivariate analysis, the presence of concomitant infection was independently associated with an increased risk of in‐hospital mortality (adjusted OR: 1.88, 95% CI: 1.22–2.89). In the mild and moderate hypothermia groups, a trend of higher mortality rates was noted among patients with infections, whereas no such trend was observed in the severe group (Table S2).
FIGURE 2.
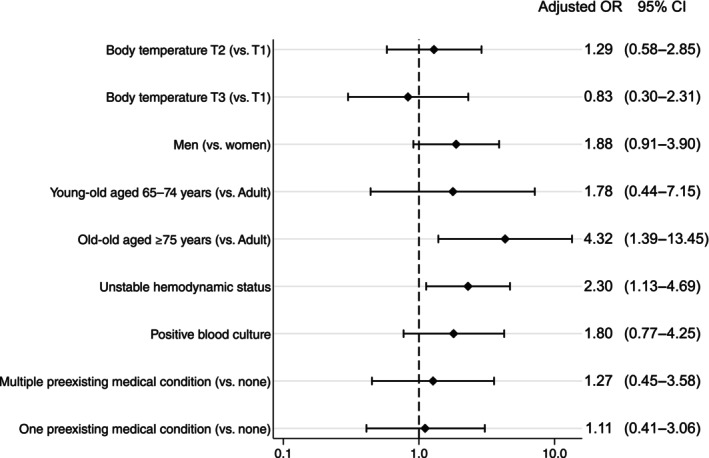
Multivariate logistic regression analysis demonstrated that the factors associated with in‐hospital death were age ≥75 years, and unstable hemodynamic status. T1, 32.0–35.0°C; T2, 28.0–31.9°C; T3, <28.0°C.
DISCUSSION
In this multicenter retrospective observational study, we found that approximately 30% of AH patients had concomitant infections. The overall in‐hospital mortality rate in AH patients with infectious diseases was 34.1%. While age and unstable hemodynamic status were associated with in‐hospital mortality, the multivariable analysis showed no significant difference in in‐hospital mortality among the three groups, and our hypothesis that the severity of hypothermia was correlated with mortality in patients with infectious diseases was not supported. A growing body of literature suggests that patients with sepsis and hypothermia have an increased mortality compared with those without hypothermia. 11 , 18 , 19 Nevertheless, as far as our awareness extends, this is the inaugural multicenter investigation exploring the association between the hypothermia severity and clinical outcomes.
In this study, several potential explanations exist for the absence of a discernible correlation between the severity of hypothermia and in‐hospital mortality. First, our results suggest that unstable hemodynamic status may have had a greater impact than body temperature. Increased severity of hypothermia can have significant effects on circulatory dynamics, including bradycardia, decreased cardiac output, and vasoconstriction. 20 The patients in the severe hypothermia group may have had a more severely impaired hemodynamic status in this study. Second, there may not have been an association between the severity of concomitant infection and hypothermia. Generally, infectious diseases alone do not lead to severe hypothermia. In this study, the proportion of patients in the severe hypothermia group who were exposed to cold was approximately 90%. Therefore, patients with severe hypothermia in this study may have been more affected by external factors, such as cold exposure, rather than by the infection itself. Third, almost all the patients in this study initiated rewarming as soon as they arrived at the ED, with the goal of achieving normothermia. Therefore, hypothermia may not have had a significant effect on the prognosis.
In this study, approximately 30% of AH patients had concomitant infections. To the best of our knowledge, this is the first study to report this epidemiological information. Importantly, this study showed that complications of infectious disease in hypothermia further increase in‐hospital mortality by approximately 15%. In cases of mild and moderate hypothermia, the higher mortality trend observed in patients with infection might be predominantly due to the effects of the infection rather than hypothermia itself. However, the absence of such a trend in severe hypothermia cases might be due to the more pronounced effects of hypothermia itself, which lead to an increase in complications including abnormalities in vital signs, life‐threatening arrhythmias, and organ failure. Early diagnosis of infectious diseases in AH patients is often difficult. Clinicians should always have a high suspicion and start treatment, including antimicrobials, when treating AH patients. Further studies to develop a high‐quality prediction model that enables the early detection of infectious complications are warranted. Given the high mortality, it is important for policymakers to improve measures to prevent the onset of hypothermia.
Our study had several limitations. First, we identified study participants using the ICD‐10 code and there is a possibility that we overlooked patients with a body temperature of ≤35°C. In the ED, body temperature is commonly measured as a vital sign; however, this carries the risk of selection bias. Second, the lack of uniformity in the definitions and diagnostic methods for infectious diseases poses a significant limitation to this study, leading a risk of bias due to misclassification. Given the retrospective nature of our research, obtaining additional information to standardize these aspects was not feasible. It is crucial for future research to aim at standardizing the definitions and diagnostic methods for infectious diseases in order to address this challenge. Third, we could not collect sufficient information about the cause of mortality. Since out of 170 people, 141 successfully rewarmed, and the success rate was close to 80% in all groups, they did not die from hypothermia. However, it remains unknown how many died from infectious diseases. Fourth, this study does not definitively establish the causal relationship between infections and hypothermia. In this study, infections were diagnosed during initial treatment in the emergency department. However, as pointed out in previous studies on hypothermia, 21 , 22 determining whether infections are the cause or a consequence of hypothermia was challenging. Furthermore, it is important to note that this database does not include the severity of the infectious diseases themselves. This exclusion may influence the interpretation of the relationship between infection and hypothermia severity. Fifth, in this study, there was a high proportion of elderly patients. Among the elderly, there may have been a preference to avoid aggressive treatments, including intubation, which could have influenced the results. 23 Unfortunately, our study does not have information regarding this aspect. Sixth, the main results should be considered underpowered because this registry had a small number of hypothermic patients with infectious disease. Furthermore, for this reason, we added only five covariates for adjustment and other confounding factors for influencing outcomes may exist. Finally, this study was purely observational in nature. While we made every effort to account for potential confounding factors, it is important to acknowledge the possibility of residual confounding when assessing the relationship between AH severity and outcomes. The findings of this research merely underscore the potential link or connection between the hypothermia severity and in‐hospital mortality.
CONCLUSION
In this study, hypothermia severity was not associated with mortality in patients with infectious diseases. While the presence of infections increased mortality, when comparing based on the presence of infections, there was a tendency for higher mortality in the mild and moderate groups in patients with infections. However, no such trend was observed in the severe group. Further prospective studies are required to confirm these findings.
CONFLICT OF INTEREST STATEMENT
YO has received a research grant from the ZOLL Foundation and overseas scholarships from the Japan Society for Promotion of Science, the FUKUDA Foundation for Medical Technology, and the International Medical Research Foundation. These organizations have no role in conducting this study.
ETHICS STATEMENT
Approval of the Research Protocol: The Ethics Committee of each institution (Kyoto Prefectural University of Medicine, Saiseikai Senri Hospital, Japanese Red Cross Kyoto Daiichi Hospital, Rakuwa‐kai Otowa Hospital, Japanese Red Cross Society Kyoto Daini Red Cross Hospital, Uji‐Tokushukai Medical Center, North Medical Center, Kyoto Medical Center, Saiseikai Shiga Hospital, Kyoto Min‐iren Chuo Hospital, Yodogawa Christian Hospital, and Fukuchiyama City Hospital) approved this study protocol.
Informed Consent: Because the study was conducted retrospectively and personal data were deidentified, all committees exempted the requirement for informed consent.
Registry and the Registration no. of the Study/trial: N/A.
Animal Studies: N/A.
Supporting information
Table S1
Table S2
ACKNOWLEDGMENTS
We express our profound gratitude to all members of the J‐Point registry group for their valuable contribution.
Shiozumi T, Miyamoto Y, Morita S, Ehara N, Miyamae N, Okada Y, et al. Association between the severity of hypothermia and in‐hospital mortality in patients with infectious diseases: The J‐Point registry. Acute Med Surg. 2024;11:e964. 10.1002/ams2.964
DATA AVAILABILITY STATEMENT
None.
REFERENCES
- 1. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49:e1063–e1143. [DOI] [PubMed] [Google Scholar]
- 2. Romanovsky AA, Székely M. Fever and hypothermia: two adaptive thermoregulatory responses to systemic inflammation. Med Hypotheses. 1998;50:219–226. [DOI] [PubMed] [Google Scholar]
- 3. Ding W, Shen Y, Li Q, Jiang S, Shen H. Therapeutic mild hypothermia improves early outcomes in rats subjected to severe sepsis. Life Sci. 2018;199:1–9. [DOI] [PubMed] [Google Scholar]
- 4. Léon K, Pichavant‐Rafini K, Ollivier H, L'Her E. Effect of induced mild hypothermia on acid‐base balance during experimental acute sepsis in rats. Ther Hypothermia Temp Manag. 2015;5:163–170. [DOI] [PubMed] [Google Scholar]
- 5. Beurskens CJP, Aslami H, Kuipers MT, Horn J, Vroom MB, van Kuilenburg ABP, et al. Induced hypothermia is protective in a rat model of pneumococcal pneumonia associated with increased adenosine triphosphate availability and turnover. Crit Care Med. 2012;40:919–926. [DOI] [PubMed] [Google Scholar]
- 6. Li XS, Liu L, Jin YL, Luo FF, Li L, Zhu J, et al. Accompanying mild hypothermia significantly improved the prognosis of septic mice than artificial mild hypothermia. Am J Emerg Med. 2015;33:1651–1658. [DOI] [PubMed] [Google Scholar]
- 7. Kimura A, Sakurada S, Ohkuni H, Todome Y, Kurata K. Moderate hypothermia delays proinflammatory cytokine production of human peripheral blood mononuclear cells. Crit Care Med. 2002;30:1499–1502. [DOI] [PubMed] [Google Scholar]
- 8. Tong G, Krauss A, Mochner J, Wollersheim S, Soltani P, Berger F, et al. Deep hypothermia therapy attenuates LPS‐induced microglia neuroinflammation via the STAT3 pathway. Neuroscience. 2017;358:201–210. [DOI] [PubMed] [Google Scholar]
- 9. Salman B, Bessler A, Beilin D. Hypothermia affects the phagocytic activity of rat peritoneal macrophages: hypothermia and macrophage function. Acta Physiol Scand. 2000;168:431–436. [DOI] [PubMed] [Google Scholar]
- 10. Drewry AM, Fuller BM, Skrupky LP, Hotchkiss RS. The presence of hypothermia within 24 hours of sepsis diagnosis predicts persistent lymphopenia. Crit Care Med. 2015;43:1165–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kushimoto S, Gando S, Saitoh D, Mayumi T, Ogura H, Fujishima S, et al. The impact of body temperature abnormalities on the disease severity and outcome in patients with severe sepsis: an analysis from a multicenter, prospective survey of severe sepsis. Crit Care. 2013;17:R271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Inoue H, Harada K, Narimatsu E, Uemura S, Aisaka W, Bunya N, et al. Pathophysiologic mechanisms of hypothermia‐induced pancreatic injury in a rat model of body surface cooling. Pancreas. 2021;50:235–242. [DOI] [PubMed] [Google Scholar]
- 13. Hildebrand F, van Griensven M, Giannoudis P, et al. Effects of hypothermia and re‐warming on the inflammatory response in a murine multiple hit model of trauma. Cytokine. 2005;31:382–393. [DOI] [PubMed] [Google Scholar]
- 14. Chaban V, de Boer E, McAdam KE, Vaage J, Mollnes TE, Nilsson PH, et al. Escherichia coli‐induced inflammatory responses are temperature‐dependent in human whole blood ex vivo. Mol Immunol. 2023;157:70–77. [DOI] [PubMed] [Google Scholar]
- 15. Matsuyama T, Morita S, Ehara N, Miyamae N, Okada Y, Jo T, et al. Characteristics and outcomes of accidental hypothermia in Japan: the J‐Point registry. Emerg Med J. 2018;35:659–666. [DOI] [PubMed] [Google Scholar]
- 16. Digital Agency . Law concerning the Assurance of Medical Care for the elderly. Tokyo: e‐Gov Law Search [cited 7 Feb 2024]. Available from: https://elaws.e‐gov.go.jp/document?lawid=357AC0000000080
- 17. Brown DJA, Brugger H, Boyd J, Paal P. Accidental hypothermia. N Engl J Med. 2012;367:1930–1938. [DOI] [PubMed] [Google Scholar]
- 18. Thomas‐Rüddel DO, Hoffmann P, Schwarzkopf D, Scheer C, Bach F, Komann M, et al. Fever and hypothermia represent two populations of sepsis patients and are associated with outside temperature. Crit Care. 2021;25:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rumbus Z, Matics R, Hegyi P, Zsiboras C, Szabo I, Illes A, et al. Fever is associated with reduced, hypothermia with increased mortality in septic patients: a meta‐analysis of clinical trials. PLoS One. 2017;12:e0170152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zafren K, Giesbrecht GG, Danzl DF, Brugger H, Sagalyn EB, Walpoth B, et al. Wilderness medical society practice guidelines for the out‐of‐hospital evaluation and treatment of accidental hypothermia: 2014 update. Wilderness Environ Med. 2014;25:S66–S85. [DOI] [PubMed] [Google Scholar]
- 21. Snijders BMG, Roos MJ, Keijsers CJPW. Incidences of underlying causes of hypothermia in older patients in the emergency department: a systematic review. Eur Geriatr Med. 2023;14:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baisse A, Parreau S, Dumonteil S, Organista A, Alais M, Ouradou V, et al. Unexplained hypothermia is associated with bacterial infection in the emergency department. Am J Emerg Med. 2023;71:134–138. [DOI] [PubMed] [Google Scholar]
- 23. Cabinet Office, Government of Japan . White Paper on Aging Society 2013. Tokyo: Cabinet Office, Government of Japan [cited 7 Feb 2024]. Available from: https://www8.cao.go.jp/kourei/whitepaper/w‐2013/zenbun/s1_2_3_04.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Data Availability Statement
None.


