Abstract
Introduction
Mesenchymal stem cells (MSCs) are increasingly used for intra-articular injections in the treatment of knee osteoarthritis. The aim of this study was to use scanning electron microscopy (SEM) to compare the morphological characteristics of synovial and adipose MSCs.
Methods
Synovium and adipose tissues were concurrently harvested from eight patients with knee osteoarthritis. Suspensions of both synovial and adipose MSCs were examined to identify the presence of microspikes. In addition to this study, the MSC suspensions in four patients were applied to abraded porcine cartilage discs and observed 10 s, 10 min, and 1 h later.
Results
The median percentage of cells exhibiting microspikes was 14% for synovial MSC suspensions and 13% for adipose MSC suspensions; this difference was not statistically significant (n = 8). No notable differences were detected in the number of adherent cells or in the proportion of cells displaying microspikes or pseudopodia. Strong correlations were found between the proportion of cells with pseudopodia and the number of attached cells for both synovial (r = 0.92, n = 12) and adipose (r = 0.86, n = 12) MSCs, with no significant difference in the correlation coefficients between the two groups.
Conclusion
SEM analysis revealed no obvious differences in morphological characteristics during MSC adhesion to cartilage for either synovial or adipose MSCs.
Keywords: Mesenchymal stem cell, Synovium, Adipose tissue, Knee, Osteoarthritis, Scanning electron microscopy
1. Introduction
Osteoarthritis of the knee is a degenerative disease that occurs mainly with aging. Its main features are knee pain and impairment of walking function due to the loss of the cartilage matrix [1]. Conservative treatment includes exercise therapy, analgesic medications, and intra-articular injections of hyaluronic acid or steroids [2]. Recently, interest has been growing in intra-articular injection of mesenchymal stem cells (MSCs) as a treatment for knee osteoarthritis [3]. One source of these MSCs is the knee synovium [4].
The intra-articular injection of synovial MSCs has been shown to refurbish the knee cartilage, as analyzed by three-dimensional MRI [5], with a resulting improvement in patient-oriented assessments of pain and other symptoms. In a rat model of osteoarthritis, the majority of the injected synovial MSCs adhered to the synovium, resulting in increased gene expression related to lubrication, cartilage matrix production, and anti-inflammation [6,7]. This response suggests that these cells are involved in the generation of medicinal signaling [8].
Scanning electron microscopy (SEM) observation of synovial MSCs reveals characteristic structures, such as microspikes, blebs, and pseudopodia. Each of these structures has a general biological role. Microspikes have a reported involvement in migration, sensing, and cell–cell adhesion [9]. Blebs are associated with cell movement, cell division, and programmed cell death [10,11]. Pseudopodia play roles in cell motility [12] and particle capture [13]. We previously reported that synovial MSCs possess microspikes that interact with collagen fibers on the surface of degenerated cartilage to aid in adhesion to early-phase cartilage. Pseudopodia subsequently play a role in this adhesion process [14]. These findings suggest a possible mechanism whereby injected MSCs directly adhere to and restore degenerated cartilage.
MSCs derived from adipose tissues are increasingly used for intra-articular injections in the treatment of knee osteoarthritis [15], primarily because adipose tissues can be harvested from subcutaneous fat and are readily obtainable from elderly patients. However, the ultrastructure of adipose MSCs and their role in adhesion to cartilage tissue remain unclear. The purpose of this study was to use SEM to compare the ultrastructure of adipose MSCs and synovial MSCs, as the latter have undergone more extensive analysis. By focusing on microspikes and pseudopodia, we hope to gain insight into how effectively adipose MSCs adhere to degenerated knee cartilage and their potential for repairing knee cartilage degeneration.
2. Methods
2.1. Isolation of human synovial and adipose MSCs
The procedures involving human cells were performed in accordance with the standards of the Declaration of Helsinki (1989) and were approved by the Medical Research Ethics Committee of Tokyo Medical and Dental University. Written informed consent was obtained from all the study subjects.
Human synovium and adipose tissue were harvested from the knees of eight osteoarthritis patients (4 males and 4 females, aged 60–80 years, with a median age of 70 years) during total knee arthroplasty operations. The synovium was harvested from the femoral side at the suprapatellar pouch, and adipose tissue was obtained from the subcutaneous layer under the knee skin incision [16].
The synovium and adipose tissue were minced and digested in a solution of 3 mg/mL collagenase (Sigma-Aldrich, St Louis, MO, USA) for 3 h at 37 °C, and the digest was filtered through a 70 μm cell strainer (Greiner Bio-One GmbH, Frickenhausen, Germany). The obtained nucleated cells were cultured in a growth medium consisting of α-MEM (Thermo Fisher Scientific, Rockford, IL, USA), 1% antibiotic–antimycotic (Thermo Fisher Scientific), and 10% fetal bovine serum (Thermo Fisher Scientific) for 14 days at 37 °C in 5% CO2. The resulting human synovial MSCs were harvested at passage 0 and stocked in 95% growth medium and 5% dimethyl sulfoxide (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan). Stocked MSCs at passage 0 were thawed and cultured for 14 days in growth medium in a 145 cm2 dish at a cell density of 500 MSCs/cm2. The MSCs were detached with trypsin–EDTA (Thermo Fisher Scientific), and suspended in phosphate buffered saline (PBS) for use in the analyses.
2.2. Scanning electron microscopy (SEM)
The floating cells were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer for 2 h and washed overnight at 4 °C in the same buffer. The cells were then adhered onto MAS-coated slide glass (Matsunami Glass Ind., Ltd., Osaka, Japan). The cells that adhered to cartilage were also fixed and washed in the same manner. The cells were post-fixed with 1% OsO4 buffered with 0.1 M phosphate buffer for 2 h, dehydrated in a graded series of ethanol, and then dried in a critical point drying apparatus (HCP-2; Hitachi, Tokyo, Japan) with liquid CO2. The specimens were spatter-coated with platinum and examined using scanning electron microscopy (S-4500; Hitachi, Tokyo, Japan) [17,18].
2.3. Morphological analysis of human MSCs in suspension
Separate cultures of MSCs were prepared from the synovium and adipose tissue collected from each donor. Synovial MSCs and adipose MSCs in suspension were observed by SEM across a sample of 50 cells, and the proportion of cells with microspikes or blebs was calculated. The results from the eight different donors were presented individually.
The surfaces of MSCs were characterized according to previous reports by Adams et al. [19] and Rajaraman et al. [20]. Microspike-positive cells were defined as cells that contained at least three microspikes. Bleb-positive cells were defined as cells that showed the largest area of blebs.
2.4. Abraded porcine cartilage
Fresh porcine knees from 6-month-old animals were purchased from Shibaura Zoki Co., Ltd. (Tokyo, Japan), and the femoral bones were excised. The femoral groove cartilage with subchondral bone was hollowed out into a cylindrical shape (diameter: 8 mm, height: 5 mm) with a hole saw. The surface of the cartilage was abraded with an air drill to reproduce a 50% partial-thickness injury [14].
2.5. Analysis of adhesion of human MSCs onto abraded porcine cartilage
A cell suspension containing 1 × 106 synovial MSCs or adipose MSCs in 50 μL PBS was placed on the disc-shaped porcine cartilage. MSCs on the cartilage were viewed immediately after placement and at 10 s, 10 min, 1 h, and 24 h after the placement after dipping the cartilage 10 times in 950 μL PBS. For each donor, three pieces of cartilage were observed by SEM at each time point, with a field of view for each observation of 320 μm by 380 μm. For each piece of cartilage, nine fields of view were observed at 10 s and 10 min, and five fields of view were observed at 1 h. The average cell number per field of view was calculated for each donor, and the results were presented for each of the four donors individually [14].
2.6. Evaluation of microspikes and pseudopodia
MSCs containing microspikes and pseudopodia were quantified from the SEM images of 50 randomly selected MSCs from each donor. For each donor, three pieces of cartilage were observed at each time point. The field of view for each observation was 320 μm × 380 μm. For each piece of cartilage, nine fields of view were observed at 10 s and 10 min, and five fields of view were observed at 1 h. Initially, the center of the cartilage was observed. Subsequently, a field of view was moved up, down, left, or right at regular intervals according to the SEM scale. The average number of cells with microspikes or pseudopodia per field of view was calculated for each donor, and the results were presented for each of the four donors individually [14].”
2.7. Statistical analysis
To compare the proportion of cells with microspikes or blebs between the synovial MSC and adipose MSC suspensions, a test was performed with a significance level set at 0.05 using the BellCurve software for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan). The correlation between the proportion of cells with pseudopodia and the attached cell numbers was analyzed, and correlation coefficients of 0.00–0.19 were considered “very weak,” 0.20–0.39 as “weak,” 0.40–0.59 as “moderate,” 0.60–0.79 as “strong,” and 0.80–1.0 as “very strong” [17]. The correlation coefficients of synovial and adipose MSCs were compared by assessing the 95% confidence intervals for both sets of coefficients. Graphs were created using GraphPad Prism 10 (GraphPad Software, Boston, MA, USA), and the results for each individual donor were plotted in the same color. Comparisons between the synovial and adipose MSCs regarding adhesion of MSCs, microspikes in the adhered MSCs, and pseudopodia in the adhered MSCs were analyzed using three cartilage discs per donor. However, statistical tests were not performed because the number of donors was limited to four.
3. Results
3.1. Morphology of synovial MSCs and adipose MSCs in suspension
Both human synovial MSCs and adipose MSCs in suspension displayed cells with microspikes, cells with blebs, and cells with neither feature (Fig. 1A). The median percentage of cells with microspikes was 14% in synovial MSCs and 13% in adipose MSCs, and this difference was not statistically significant (n = 8) (Fig. 1A). Similarly, the median percentage of cells with blebs was 44% for synovial MSCs and 39% for adipose MSCs, and again, the difference was not statistically significant (n = 8).
Fig. 1.
Morphology of synovial MSCs and adipose MSCs in suspension. (A) Representative SEM images showing human synovial MSCs and adipose MSCs with microspikes, blebs, or neither microspikes nor blebs. (B) Proportion of cells with microspikes or blebs in suspensions of synovial MSCs and adipose MSCs. MSCs were isolated from synovium and adipose tissue collected from the same donor. Synovial MSCs and adipose MSCs in suspension were observed by SEM across a sample of 50 cells, and the proportion of cells with microspikes or blebs was calculated. Results from eight different donors are presented individually. N.S. indicates no significant difference as determined by the sign test (n = 8).
3.2. Adhesion of synovial MSCs and adipose MSCs onto cartilage
SEM images of non-abraded porcine cartilage showed a smooth surface, whereas the surface of the abraded cartilage was undulating, with a periodicity of approximately 50 μm (Fig. 2A). Human synovial MSCs and adipose MSCs placed on abraded porcine cartilage began to adhere as early as 10 s, and the numbers of adherent cells increased with time, according to assessments at 10 min, 1 h, and 24 h (Fig. 2B). The median number of cells per field was 4 for both synovial and adipose MSCs after 10 s, 15 cells for synovial MSCs and 13 cells for adipose MSCs after 10 min, and 175 cells for synovial MSCs and 143 cells for adipose MSCs after 1 h, indicating no obvious differences in the capacity for adhesion between the two MSC types (Fig. 2C). After 24 h, the cells adhering to the cartilage overlapped to such an extent that counting individual adherent cells was not feasible.
Fig. 2.
Adhesion of synovial MSCs and adipose MSCs onto cartilage. (A) Non-abraded porcine cartilage and abraded cartilage. (B) Representative SEM images of human synovial MSCs and adipose MSCs during adhesion evaluations onto abraded porcine cartilage. (C) Numbers of cells attached onto an abraded porcine cartilage after placement of synovial MSCs or adipose MSCs. For each donor, three pieces of cartilage were observed at each time point. The field of view for each observation was 320 μm by 380 μm. For each piece of cartilage, nine fields of view were observed at 10 s and 10 min, and five fields of view at 1 h. The average per field of view was calculated for each donor, and the results were presented for each of the four donors individually.
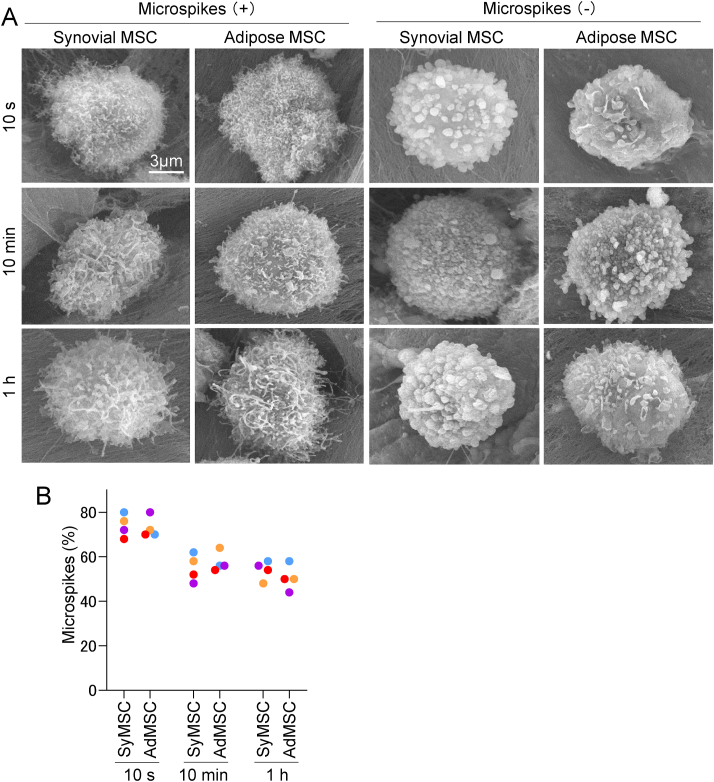
3.3. Microspikes in adhered synovial and adipose MSCs
Synovial MSCs and adipose MSCs with microspikes were observed at 10 s, 10 min, and 1 h after placing the suspension on the abraded porcine cartilage (Fig. 3A). Cells without microspikes were also observed at these time points. The median percentages of cells with microspikes were 74% for synovial MSCs and 71% for adipose MSCs after 10 s, 55% for synovial MSCs and 56% for adipose MSCs after 10 min, and 55% for synovial MSCs and 50% for adipose MSCs after 1 h, with no statistically significant differences between the two types (Fig. 3B). Further magnified images of synovial and adipose MSCs attached to porcine cartilage at 10 min revealed that, in cells with microspikes, the microspikes were caught on the collagen fibers. By contrast, in cells without microspikes, their round projections (blebs) were caught on the collagen fibers (Fig. 4).
Fig. 3.
Analysis of microspikes present in adhered synovial and adipose MSCs. (A) Representative images of cells with/without microspikes adhered onto an abraded porcine cartilage. (B) Proportions of cells with microspikes. For each donor, three pieces of cartilage were observed at each time point. The field of view for each observation was 320 μm by 380 μm. For each piece of cartilage, nine fields of view were observed at 10 s and 10 min, and five fields of view at 1 h. The average per field of view was calculated for each donor, and the results were presented for each of the four donors individually.
Fig. 4.
Representative higher magnification images of synovial and adipose MSCs with/without microspikes adhered onto an abraded porcine cartilage. Observations were performed at 10 min.
3.4. Pseudopodia in adhered synovial and adipose MSCs
No synovial MSCs or adipose MSCs with pseudopodia were observed at 10 s, but pseudopodia were observed in both MSC types at 10 min and 1 h after placing the suspensions on abraded porcine cartilage (Fig. 5A). MSCs without pseudopodia were also observed at all three time points. The median percentages of cells with pseudopodia were 0% for both synovial and adipose MSCs after 10 s, 7% for both MSC types after 10 min, and 51% for synovial MSCs and 50% for adipose MSCs after 1 h, with no statistically significant differences evident between the two MSC types (Fig. 5B). The proportion of cells with pseudopodia and the number of attached cells showed very strong correlations for both the synovial MSCs (r = 0.92, p < 0.001, n = 12) and the adipose MSCs (r = 0.86, p < 0.001, n = 12) (Fig. 5C). The 95% confidence intervals for the correlation coefficients were 0.73–0.98 for synovial MSCs and 0.56–0.96 for adipose MSCs, but the differences between the two cell types were not statistically significant.
Fig. 5.
Analysis of pseudopodia present in adhered synovial and adipose MSCs. (A) Representative images of cells with/without pseudopodia adhered onto an abraded porcine cartilage. (B) Proportions of cells with pseudopodia. For each donor, three pieces of cartilage were observed at each time point. The field of view for each observation was 320 μm by 380 μm. For each piece of cartilage, nine fields of view were observed at 10 s and 10 min, and five fields of view at 1 h. The average per field of view was calculated for each donor, and the results were presented for each of the four donors individually. (C) Relationship between the proportion of MSCs with pseudopodia and the attached cell number for synovial MSCs and adipose MSCs. The correlation coefficient (r) and p values are shown (n = 12).
4. Discussion
We used SEM to compare the changes in the morphological characteristics of synovial and adipose MSCs during their adhesion to cartilage.
We found no significant differences in the percentage of cells exhibiting microspikes and blebs between the two types of MSCs, nor did we find any notable differences in the numbers of adherent cells or in the proportions of adherent cells displaying microspikes and pseudopodia. The SEM analysis revealed no obvious differences in the changes in the morphological characteristics of synovial or adipose MSCs during their adhesion to cartilage.
Microspikes are slender cytoplasmic projections that play roles in migration, sensing, and cell–cell adhesion [9]. We previously demonstrated that microspikes can catch onto collagen fibers on the surfaces of degenerated cartilage [14] and menisci [21], and this entrapment aids in the initial cell adhesion. Our current study showed that suspensions of synovial MSCs and adipose MSCs had an equal proportion of cells with microspikes, and that these proportions remained similar for both cell types during their attachment to cartilage. This suggests that the number of cells capable of attaching was the same for both cell types, and that the attachment results over time were similarly consistent.
Blebs are small, irregular bulges on the cell membrane that arise due to cytoskeleton detachment [22]. Blebs are involved in several processes, including cell movement, cell division, and programmed cell death [10,11]. The presence of blebs in MSCs has been previously reported following transmission electron microscopy observations of bone marrow MSCs [23], and light microscopy observations have revealed ruffling [24]. In the present study, we found an equal proportion of cells with blebs in suspensions of both synovial and adipose MSCs. This uniformity in bleb presence across different MSC types suggests a shared mechanism in their physiological behaviors, irrespective of their tissue origin.
Pseudopodia are temporary arm-like extensions of a cell's cytoplasm and plasma membrane and are primarily involved in cell motility [12]. They also play a significant role in the capture of particles, a function highlighted in macrophage research [13]. Here, we observed that both synovial and adipose MSC showed strong correlations between the presence of pseudopodia and the numbers of cells that adhered to cartilage. This observation indicates that pseudopodia can facilitate the attachment of MSCs to cartilage, indicating a potential mechanism for their adherence.
SEM was chosen as the imaging method for this study because it has a unique ability to visualize cell surface morphology. Compared to confocal microscopy, SEM offers a much larger depth of field, allowing us to observe and compare the adhesion of synovial and adipose MSCs across a broad area of the cartilage surface. SEM also excels at capturing intricate details of cellular protrusions, such as microspikes and blebs, thereby providing valuable insights into how these MSCs interact with the underlying collagen fibers.
The mode of action of synovial MSCs in relation to cartilage regeneration was previously examined in our rat OA model study [6]. Cell tracking assays showed that the majority of the injected MSCs migrated to the synovium and that the cells maintained their MSC properties without differentiating into other lineages. Species-specific gene analysis of the gene expression changes in the human synovial MSCs that migrated to the rat synovium indicated that exogenous synovial MSCs acted as anti-inflammatory agents through TSG-6 expression [25], as lubrication agents by PRG-4 expression [26], and as facilitators of cartilage matrix synthesis by BMP expression [27]. Our SEM studies also revealed that synovial MSCs adhered to degenerated cartilage by interactions occurring between the microspikes/pseudopodia and collagen fibers of the cartilage surface. These findings suggest that injected MSCs directly adhere to and restore degenerated cartilage by a similar mode of action to that observed in the synovium. However, although our current SEM study shows a similar morphology for adipose MSCs adhering to degenerated cartilage, the mode of action of adipose MSCs for treatment via intraarticular injection into OA knees will likely differ from that of synovial MSCs at the molecular biological level, as the gene expression profile and properties, including chondrogenesis potential, differ between synovial and adipose MSCs [4,28].
We propose three limitations for the present study. First, we sourced adipose MSCs from subcutaneous fat in the knee rather than from the abdomen. Adipose MSCs are typically derived from abdominal fat, and differences in MSC characteristics between knee and abdominal subcutaneous fat [29,30] might lead to variations in MSC properties. Second, the method of placing the MSC suspension on porcine abraded cartilage does not entirely replicate the process of injecting MSCs into osteoarthritic knees due to differences in the surface properties of human osteoarthritis cartilage and experimentally abraded porcine cartilage. Key factors, such as the presence of joint fluid containing hyaluronic acid and joint movement, are also not replicated in ex vivo abraded cartilage experiments. Third, the study's small sample size of four limits the generalizability of our findings regarding the number of adherent cells and the proportion of cells with microspikes and pseudopodia. Although our initial hypothesis was that the synovial and adipose MSC suspensions would differ in their proportions of cells with microspikes, our results (n = 8) did not support this hypothesis and instead supported that both MSC types shared similar adherence characteristics. For this reason, the study was concluded after confirming the equivalence in these aspects with the given sample size.
Author contributions
Conceptualization, Y.F., K.E., and I.S.
Methodology, Y.F., K.E., and I.S.
Software, Y.F., K.E., and I.S.
Validation, Y.F., K.E., and I.S.
Formal Analysis, Y.F., M.T., and I.S.
Investigation, Y.F., K.E., T.T., and Y.S.
Resources, Y.F., K.E., N.O., Y.N., and H.K.
Data Curation, Y.F., K.E., and I.S.
Writing – Original Draft Preparation, Y.F. and I.S.
Writing – Review & Editing, Y.F. and I.S.
Visualization, Y.F. and I.S.
Supervision, I.S.
Project Administration, I.S.
Funding Acquisition, I.S.
Funding
This research was funded by the Japan Agency for Medical Research and Development, grant number JP22bk0104148 to I.S.
Institutional review board statement
The procedures involving human cells were performed in accordance with the standards of the Declaration of Helsinki (1989) and approved by the Medical Research Ethics Committee of Tokyo Medical and Dental University (protocol code:M2017-142-08 and date of approval:27 September 2022).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Data availability statement
The data presented in this study are available on request from the corresponding author.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Ellen Roider for English editing.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Yusuke Fuchioka, Email: fuchioka.yusuke@tmd.ac.jp.
Kentaro Endo, Email: endo.arm@tmd.ac.jp.
Yuriko Sakamaki, Email: sakamaki.bioa@tmd.ac.jp.
Takahiro Tanimoto, Email: tanimoto.arm@tmd.ac.jp.
Nobutake Ozeki, Email: ozeki.arm@tmd.ac.jp.
Yusuke Nakagawa, Email: ynakagawa.orj@tmd.ac.jp.
Hideyuki Koga, Email: koga.orj@tmd.ac.jp.
Makoto Tomita, Email: tomita.ds.makoto@gmail.com.
Ichiro Sekiya, Email: sekiya.arm@tmd.ac.jp.
References
- 1.Sharma L. Osteoarthritis of the knee. N Engl J Med. 2021;384:51–59. doi: 10.1056/NEJMcp1903768. [DOI] [PubMed] [Google Scholar]
- 2.Bert J.M., Endres N.K., Tucker C.J., Davey A.P. The conservative treatment of osteoarthritis of the knee. Orthopedics. 2018;41:256–260. doi: 10.3928/01477447-20180828-08. [DOI] [PubMed] [Google Scholar]
- 3.Shoukrie S.I., Venugopal S., Dhanoa R.K., Selvaraj R., Selvamani T.Y., Zahra A., et al. Safety and efficacy of injecting mesenchymal stem cells into a human knee joint to treat osteoarthritis: a systematic Review. Cureus. 2022;14 doi: 10.7759/cureus.24823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakaguchi Y., Sekiya I., Yagishita K., Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 5.Sekiya I., Katano H., Mizuno M., Koga H., Masumoto J., Tomita M., Ozeki N. Alterations in cartilage quantification before and after injections of mesenchymal stem cells into osteoarthritic knees. Sci Rep. 2021;11 doi: 10.1038/s41598-021-93462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozeki N., Muneta T., Koga H., Nakagawa Y., Mizuno M., Tsuji K., et al. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthritis Cartilage. 2016;24:1061–1070. doi: 10.1016/j.joca.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Sekiya I., Katano H., Ozeki N. Characteristics of MSCs in synovial fluid and mode of action of intra-articular injections of synovial MSCs in knee osteoarthritis. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22062838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caplan A.I., Hariri R. Body management: mesenchymal stem cells control the internal regenerator. Stem Cells Transl Med. 2015;4:695–701. doi: 10.5966/sctm.2014-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.SenGupta S., Parent C.A., Bear J.E. The principles of directed cell migration. Nat Rev Mol Cell Biol. 2021;22:529–547. doi: 10.1038/s41580-021-00366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang C., Hui T.H., Wei X., Shao X., Lin Y. A combined experimental and theoretical investigation on cellular blebbing. Sci Rep. 2017;7 doi: 10.1038/s41598-017-16825-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weems A.D., Welf E.S., Driscoll M.K., Zhou F.Y., Mazloom-Farsibaf H., Chang B.J., et al. Blebs promote cell survival by assembling oncogenic signalling hubs. Nature. 2023;615:517–525. doi: 10.1038/s41586-023-05758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi S., Bhagwat A.M., Al Mismar R., Goswami N., Ben Hamidane H., Sun L., Graumann J. Proteomic profiling of human cancer pseudopodia for the identification of anti-metastatic drug candidates. Sci Rep. 2018;8:5858. doi: 10.1038/s41598-018-24256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horsthemke M., Bachg A.C., Groll K., Moyzio S., Müther B., Hemkemeyer S.A., et al. Multiple roles of filopodial dynamics in particle capture and phagocytosis and phenotypes of Cdc42 and Myo10 deletion. J Biol Chem. 2017;292:7258–7273. doi: 10.1074/jbc.M116.766923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanimoto T., Endo K., Sakamaki Y., Ozeki N., Katano H., Mizuno M., et al. Human synovial mesenchymal stem cells show time-dependent morphological changes and increased adhesion to degenerated porcine cartilage. Sci Rep. 2022;12 doi: 10.1038/s41598-022-20386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J.S., Shim D.W., Kang K.Y., Chae D.S., Lee W.S. Method categorization of stem cell therapy for degenerative osteoarthritis of the knee: a Review. Int J Mol Sci. 2021;22 doi: 10.3390/ijms222413323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mochizuki T., Muneta T., Sakaguchi Y., Nimura A., Yokoyama A., Koga H., Sekiya I. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006;54:843–853. doi: 10.1002/art.21651. [DOI] [PubMed] [Google Scholar]
- 17.Fujii S., Endo K., Ozeki N., Sakamaki Y., Kohno Y., Mizuno M., et al. Comparison of adhesion of thawed and cultured synovial mesenchymal stem cells to the porcine meniscus and the relevance of cell surface microspikes. BMC Mol Cell Biol. 2022;23:53. doi: 10.1186/s12860-022-00456-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yagi M., Mizuno M., Fujisawa R., Katano H., Endo K., Ozeki N., et al. Optimal pore size of honeycomb polylactic acid films for in vitro cartilage formation by synovial mesenchymal stem cells. Stem Cell Int. 2021;2021 doi: 10.1155/2021/9239728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams J.C. Cell-matrix contact structures. Cell Mol Life Sci. 2001;58:371–392. doi: 10.1007/PL00000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajaraman R., Rounds D.E., Yen S.P., Rembaum A. A scanning electron microscope study of cell adhesion and spreading in vitro. Exp Cell Res. 1974;88:327–339. doi: 10.1016/0014-4827(74)90248-1. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki S., Mizuno M., Sakamaki Y., Mimata A., Endo K., Kohno Y., et al. Morphological changes in synovial mesenchymal stem cells during their adhesion to the meniscus. Lab Invest. 2020;100:916–927. doi: 10.1038/s41374-020-0421-8. [DOI] [PubMed] [Google Scholar]
- 22.Strychalski W., Guy R.D. A computational model of bleb formation. Math Med Biol. 2013;30:115–130. doi: 10.1093/imammb/dqr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teo G.S., Ankrum J.A., Martinelli R., Boetto S.E., Simms K., Sciuto T.E., et al. Mesenchymal stem cells transmigrate between and directly through tumor necrosis factor-α-activated endothelial cells via both leukocyte-like and novel mechanisms. Stem Cell. 2012;30:2472–2486. doi: 10.1002/stem.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X., Tang P., Guo F., Zhang M., Yan Y., Huang M., et al. mDia1 and Cdc42 regulate activin B-induced migration of bone marrow-derived mesenchymal stromal cells. Stem Cell. 2019;37:150–162. doi: 10.1002/stem.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee R.H., Pulin A.A., Seo M.J., Kota D.J., Ylostalo J., Larson B.L., et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhee D.K., Marcelino J., Baker M., Gong Y., Smits P., Lefebvre V., et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115:622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sekiya I., Larson B.L., Vuoristo J.T., Reger R.L., Prockop D.J. Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 2005;320:269–276. doi: 10.1007/s00441-004-1075-3. [DOI] [PubMed] [Google Scholar]
- 28.Segawa Y., Muneta T., Makino H., Nimura A., Mochizuki T., Ju Y.J., et al. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J Orthop Res. 2009;27:435–441. doi: 10.1002/jor.20786. [DOI] [PubMed] [Google Scholar]
- 29.Karpe F., Pinnick K.E. Biology of upper-body and lower-body adipose tissue--link to whole-body phenotypes. Nat Rev Endocrinol. 2015;11:90–100. doi: 10.1038/nrendo.2014.185. [DOI] [PubMed] [Google Scholar]
- 30.Hill J.H., Solt C., Foster M.T. Obesity associated disease risk: the role of inherent differences and location of adipose depots. Horm Mol Biol Clin Invest. 2018;33 doi: 10.1515/hmbci-2018-0012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.