Abstract
Introduction
The human cerebellum emerges as a posterior brain structure integrating neural networks for sensorimotor, cognitive, and emotional processing across the lifespan. Developmental studies of the cerebellar anatomy and function are scant. We examine age-dependent MRI morphometry of the anterior cerebellar vermis, lobules I-V and posterior neocortical lobules VI-VII and their relationship to sensorimotor and cognitive functions.
Methods
Typically developing children (TDC; n=38; age 9–15) and healthy adults (HAC; n=31; 18–40) participated in high-resolution MRI. Rigorous anatomically informed morphometry of the vermis lobules I-V and VI-VII and total brain volume (TBV) employed manual segmentation computer-assisted FreeSurfer Image Analysis Program [http://surfer.nmr.mgh.harvard.edu]. The neuropsychological scores (WASI-II) were normalized and related to volumes of anterior, posterior vermis, and TBV.
Results
TBVs were age independent. Volumes of I-V and VI-VII were significantly reduced in TDC. The ratio of VI-VII to I-V (∼60%) was stable across age-groups; I-V correlated with visual-spatial-motor skills; VI-VII with verbal, visual-abstract and FSIQ.
Conclusions
In TDC neither anterior I-V nor posterior VI-VII vermis attained adult volumes. The "inverted U" developmental trajectory of gray matter peaking in adolescence does not explain this finding. The hypothesis of protracted development of oligodendrocyte/myelination is suggested as a contributor to TDC's lower cerebellar vermis volumes.
Keywords: Typically developing children, MRI morphometry, Cerebellar vermis, Sensorimotor and cognitive functions, Hypothesis of protracted development of oligodendrocyte/myelination
1. Introduction
The remarkable structural richness of the human cerebellum portends its functional complexity. Consisting of the midline cerebellar vermis and lateral hemispheres covered with a three-layered cortical mantle, the cerebellum is ten times smaller than the cerebrum; yet, it is estimated to contain over 60–80% of the total neurons in the human cerebrum (reported at 86 billions) and an even larger fraction of glial cells (Herculano-Houzel, 2012, Kandel et al., 2021). A recent original report on a computationally reconstructed cerebellar surface down to the level of the smallest folds, showed that the cerebellar cortex has close to 80% of the surface area of the cerebral cortex (Sereno et al., 2020). Phylogenetically the anatomical organization of the whole cerebellum involves three lobes nesting ten distinct lobules, with the anterior lobes I-V, the posterior lobules VI-IX (Crus I, Crus II, VIII, VIIA and VIIB), and the phylogenetically oldest archicerebellar flocculonodular lobule X. The classification of cerebellar lobules based on phylogenetic development and varying embryological geneses originates in seminal studies by Ingvar (1918) and Dow (1942). The authors suggested that the term neocerebellum is reserved for those cerebellar lobules which in the mammals are dominated by cortico-pontine connections. Indeed, later works on anatomical morphology of the cerebellum displayed a highly refined system of recurrent circuits from the cerebral cortex through the relay in the pontine nuclei, cerebellum, thalamus and back to the cerebral cortex (CPCTC pathway) (Itō, 1990, Middleton and Strick, 1994, Pandya and Kuypers, 1969, Pandya and Yeterian, 1984; J. D. Schmahmann and Pandya, 1997; J. Schmahmann and Pandya, 2009). Trans-neuronal virus tracing studies in primates showed that these circuits are organized in a regular series of parallel closed loops where a region of the cerebellum connects reciprocally with specific regions of neocortex such as the dorsal prefrontal, premotor, posterior parietal, and temporal that are defined as anatomical substrates of complex cognitive functions (Bostan et al., 2013, Dum and Strick, 2013).
Since the cognitive and sensorimotor functions in humans display a pattern of clear developmental stages the important question is whether these stages of cognitive development are interconnected with the structural developmental trajectory of the cerebellum. Developmental studies of the cerebellar structure/function are scant, despite that the cerebellum is one of the first brain structures to begin differentiation of cells in the prenatal stage and one of the last to mature in ontogeny and thus, it has a prolonged exposure to epigenetic risk (Triulzi et al., 2005). Consequently, cerebellar damage in early ontogeny causes broad, long-term neurological, emotional, and cognitive deficits resistant to rehabilitation (Davis et al., 2010, Keller et al., 2003). The examination of age differences in the postnatal morphology of cerebellar lobules and their functional associations is an important line of inquiry about early markers of neurodevelopmental disorders. Gaining such insight may open new concepts in designing effective treatment and preventative tools (Stoodley, 2016; ten Donkelaar et al., 2023).
For over a century now, empirical evidence is accumulating on the critical influence of early sensorimotor experience on both cognitive development, anatomy, and functions of the cerebellar vermis and hemispheres. The core evidence came from clinical and lesion studies (Babinski, 1899, Dow and Anderson, 1942, Dow and Moruzzi, 1958, Lalonde and Botez, 1986), sensorimotor deprivation (Blakemore and Van Sluyters, 1975, Harlow and Zimmermann, 1959, Hubel and Wiesel, 1970) and environmental enrichment studies (M. C. Diamond et al., 1967, Diamond et al., 1972; Greenough et al., 1986; Hebb, 1949). Lesions in the anterior cerebellar vermis and fastigial nucleus were reported to be deleterious to both spatial orientation, navigation and visuomotor coordination (Joyal et al., 1996) because of their reciprocal connections with the vestibular system (Walberg, 1972), the superior colliculus (P. Brodal and Bjaalie, 1992) and the lateral geniculate nucleus of thalamus (Graybiel, 1974). Lesions of the lateral/posterior neocerebellar vermis and hemispheres, affecting the output of the dentate nucleus, were shown to suppress visual learning and increase impulsive responses (Dow and Moruzzi, 1958, Itō, 2012a). Most of the deprivation and enrichment studies were conducted in young animals and in the visual modality (M. C. Diamond et al., 1972; Floeter and Greenough, 1979; Itō, 1984; Pysh and Weiss, 1979). Infant monkeys and rodents raised in environmentally enriched cages with various stimuli, social partners, and visual sensorimotor challenges showed an increase in the number of synaptic connections in the dendritic fields of the cerebellum; the thickness of the occipital/parietal cortex was thinner as compared to animals raised in social impoverished environments (Floeter and Greenough, 1979, Juraska et al., 1985).
These findings were substantiated by structural and functional neuroimaging studies (fMRI, PET MEG, EEG) in young human subjects showing consistent association of the posterior neocerebellar vermis (lobules VI-VII) and dentate nucleus with the dorsal and medial prefrontal cortex, premotor and inferior parietal regions during performance on tasks assessing attentional flexibility, visual-spatial working memory, language, emotions, and social cognition (Ciesielski et al., 1994, Courchesne et al., 1989, Keren-Happuch et al., 2014, Schmahmann and Pandya, 1989, Stoodley and Schmahmann, 2010, Strick et al., 2009, Van Overwalle et al., 2014, Van Overwalle et al., 2020, Venkatasubramanian et al., 2008). The proficiency of cognitive functions and increased neural tissue volume in the cerebellar subdivisions were positively correlated, with some exceptions in studies of aging (Paul et al., 2009). The studies with adults on sensory-visual-motor tasks displayed a correlative relationship of the anterior cerebellum lobules I-V, and rarely VIII, with the motor cerebral cortex, inferior parietal/occipital, medial prefrontal and posterior cingulate gyri (Itō, 2008 for review; Leiner et al., 1989; Salmi et al., 2010; Schmahmann, 1997; Stoodley and Schmahmann, 2010). fMRI resting state studies in adults added support to the cerebellar-thalamic-cerebral network connectivity (Buckner et al., 2011, Habas et al., 2009). The latter showing connections between the right vermis lobule VIIB and lobule IX with thalamus and the posterior cingulate, temporoparietal junction, and medial temporal lobes, all components of the default mode network, reported to govern the self-resting state, self-reflection, and ruminations (Buckner, 2013).
Several MRI morphometry studies of the cerebellum in children that presented data with significant conceptual and methodological internal validity, concluded a similar pattern of cerebellar-cerebral cortex correlations between the cerebellar volume and sensory or cognitive performance proficiency. Gilmore and collaborators (Knickmeyer et al., 2008) examined structural brain development in ninety-eight healthy children aged 0–2 years, a sample age rarely addressed by MRI morphometry. Tissue segmentation (gray, non-myelinated and myelinated white matter, CSF) and computer-assisted morphometry showed that, in the first year, a striking increase in the volume of the cerebellum (240%) was found, driven mainly by gray matter changes. The increase in total brain volume was 100% and, in the hippocampus, only 13%. The high correlation between the cerebellar and cerebral growth (Spearman rs = 0.94) adds to the argument for exceptionally influential cerebral-cerebellar engagement in the first two years of a child's life. This engagement is fundamentally important for the development of sensorimotor functions, motor coordination, and thus for the development of social cognition and linguistic skills (Kagan et al., 2005, Piaget, 1962, Piaget and Cook, 1952, Thach, 1996). Knickmeyer et al., (2008) discussed that neurogenesis and neuronal migration are unlikely to account for the volume increase, because the total cortical neuron numbers in years 0–1–2 are not significantly different (Shankle et al., 1999). The authors hypothesize that while the gray matter increase may reflect changes in density of neuropil (dendrites, axons, glia) and the total cerebral and cerebellar volume may be more likely influenced by white matter and suggest myelination by oligodendrocytes that develop well into adulthood (Benes et al., 1994, Lenroot and Giedd, 2006, Yakovlev and Lecours, 1967). Consistent with the above, investigations of the distributed cortical-cortical and cortical-cerebellar networks in neuroimaging (MRI, fMRI, MEG) and studies of language and speech development opened an important area of cerebellar inquiry (Murdoch, 2010). Although cortical networks and cerebellar contribution to processing linguistic tasks have been discussed in adults (Kuhl and Damasio, 2012, Kuhl and Rivera-Gaxiola, 2008), the brain areas involved during the period of language acquisition are still not clear. A whole brain MRI morphometry study by Kuhl and collaborators (Deniz Can et al., 2013) examined the local concentration of the gray and white matter in 7 month old infants as a predictor of language development at age 12 months, using a receptive and expressive language development scale by Mullen (1995). Brain regions of gray-matter (right cerebellum and right hippocampus) and white-matter (right cerebellum) were positively and strongly associated with infants’ receptive language ability at 12 months. Examining cerebellar structure as an early marker of developmental progress in linguistic skills presents a promising hypothesis for future research.
The developmental cerebellar morphometric studies are limited by lacking longitudinal data. One of the rare longitudinal studies quantified total cerebellar volume and 11 subdivisions (anterior, superior, posterior lobes, and corpus medullare of white matter, and others) using multiple MRI scans collected with 2 year intervals from females and males aged 5–24 (Tiemeier et al., 2010). The goal was to identify typical neurodevelopmental trajectories of significant morphological changes within the cerebellum. This study used manual subregion morphometric parcellation of left and right cerebellar hemispheres (per Larsell and Jansen, 1967 mapping) by two independent raters. Measures of total cerebellum volume revealed an inverted U-shape course, with a peak volume at approximately 12 years of age for females and 15.5 years of age for males. Subregion volume analyses showed that the vermis matured linearly, with larger volume in males across age. There were no significant age/volume effects in the individual vermal lobules. Positive associations were found between all three vermal lobule volumes (e.g., anterior, superior, and inferior), independent of age and sex. The Tiemeier et al., (2010) study provides evidence of significant cerebellar volumetric changes during development, with more phylogenetically younger areas showing protracted maturation and marked by sex-dependent differences. Furthermore, gray matter integrity, as measured in adolescent children (mean age 11.9) by computer-segmented morphometric volume, of the posterior cerebellar lobe has been associated with cognitive performance of vocabulary, reading, working memory, and set-shifting (Moore et al., 2017). Receptive vocabulary aptitude exhibited a positive relationship with gray matter volume in the vermal lobules of VIIIA, VIIIB, IX, lobule VI. Additionally, the greater volume in bilateral lobules VIIB, VIIIA, VIIIB, and left lobule VI were correlated with higher reading scores. Performance on working memory and set-shifting tasks showed a similar positive association of gray matter volume in the right Crus I, Crus II, VIIB, and left Crus II, bilateral VIIB, VIIIA, respectively. These strong correlational relationships between specific cerebellar regional morphometry and cognitive functions emphasized the foundational role of the cerebellum in adolescent functioning (Moore et al., 2017).
Mostly consistent with the above short literature review on the developing cerebellum structure and correlations with cognitive functions, are also reports on clinical empirical evidence of neurodevelopmental cognitive and affective syndromes involving the cerebellum in children. Findings revealed prevalence of cognitive, emotional, and sensorimotor deficits displayed in disorders associated with hypoplasia of the posterior neocerebellar vermis (lobules VI-VII), but less severe deficits in the phylogenetically earlier maturing anterior cerebellar vermis (lobules I-V) (Ciesielski et al., 1997, Courchesne et al., 1989, Schmahmann, 1997, for review). Injury to the cerebellum in children, such as irradiation insult resulting in molecular changes and reduction of postsynaptic spines in the posterior/lateral and midline cerebellum (with dentate output), were found to be associated with deficits in attention, learning, and memory (Ciesielski et al., 1994, Lesnik et al., 1998). A review of developmental disorders and the cerebellum showed lesions of the anterior vermal lobules I-V impeding motor performance, while those of posterior lobules VI-X correlated with reduced cognitive flexibility, visual-spatial working memory, verbal fluency, and emotional discord (Stoodley, 2016). Of note, the integration of cortical sensory and motor areas extends to occipital-parietal visual and temporal auditory networks (Cappe et al., 2009, Ciesielski et al., 2021, Mesulam, 1998). The sensorimotor skills are foundational for complex cognitive functioning, thus connections between the sensorimotor system span across networks serving attentional, language, executive, default mode, and socioemotional networks (Figley et al., 2017, Lewis and Van Essen, 2000, Luna et al., 2001, Uddin et al., 2011). While the major local cortical nodes of the sensorimotor system are reported to mature earlier in childhood (anterior vermis), the interconnection with other long range neural circuits subserving cognitive functions may show protracted development (Baum et al., 2020, Edde et al., 2021, Quairiaux et al., 2011, Rohr et al., 2018, Zielinski et al., 2010). Thus, we may expect dynamic developmental changes in structural relationships between two components of the cerebellar vermis: the phylogenetically earlier maturing anterior lobules I-V and the posterior neocerebellar lobules VI-VII.
This study’s focus is on typically developing children ages 9–15 years. We collected measurements of MRI anatomical morphometry of the cerebellar vermis and examined its relationship to a child's proficiency in sensorimotor and cognitive functions. Since the anterior lobules of the cerebellum develop phylogenetically earlier (Ingvar, 1918, Larsell, 1934, Leiner et al., 1986; J. D. Schmahmann and Pandya, 1995), we predict that the anterior vermis will show a significant relationship to the sensorimotor components of a visual-spatial-cognitive task, such as the Block Design (BD) of WASI-II. The BD evaluates visual-spatial perception, visual-motor coordination, error monitoring, and visual-spatial working memory. One would also predict a strong affiliation between cognitive functioning and the posterior cerebellar lobules, due to their connectivity with the parietal and prefrontal cortical circuits and visual-spatial cognition. Our additional inquiry is about the developmental progress in interactions between the older lobules I–V, and the neocerebellar lobules VI–VII, as a hypothetical sensitive developmental indicator.
We aim to answer three major questions: Is there an age-dependent difference in the anatomical MRI morphometry of the anterior phylogenetically earlier developing vermal lobules I-V as compared to the posterior neocerebellar vermal lobules VI-VII? Will the ratio of morphometry between the neocerebellar (posterior) vermis to anterior vermis change with age, and therefore, could it serve as a sensitive marker of cerebellar vermis ontogenetic maturation? Will the age-dependent changes in morphometry, if any, be correlated with visual-spatial proficiency and/or intellectual functioning, as found in the prior studies of adults and adolescents? We expect that the structural and functional correlative findings in children would more closely resemble the adult pattern for the earlier-developing anterior cerebellum, vermal lobules I-V, but be more discordant for the neocerebellar lobules VI-VII. This prediction is in line with converging evidence of protracted maturation of the neocerebellum in correspondence with the closely interconnected late-maturing prefrontal and temporal cortices (Itō, 2012a, Leiner et al., 1986, Leiner et al., 1989, Tiemeier et al., 2010).
2. Methods
2.1. Rationale
The aim of this study is to examine which of the two prime subdivisions of the cerebellum, the anterior vermis lobules I-V and the posterior/inferior neocerebellar vermis lobules VI-VII vary in progress of maturational anatomical and functional status in childhood as compared to young adulthood. Towards this aim three sets of age-dependent measures were acquired: (i) MRI volumetry of the anterior cerebellar vermis lobules I-V, and the neocerebellar posterior/inferior lobules VI-VII in reference to total brain volume (TBV); (ii) The volume ratio of neocerebellar posterior cerebellum to the phylogenetically earlier-developing anterior lobules; and (iii) Relationship of child proficiency on neuropsychological tests (i.e., the visual-spatial task targeting sensorimotor and cognitive skills and the crystalized verbal memory) to the morphometry of the anterior and neocerebellar vermis.
2.2. Participants
Typically developing children (TDC; n=38; ages 9–15) and healthy adult controls (HAC; n=31; ages 18–30) were recruited in the Southwest community of New Mexico and the north Massachusetts and Boston area between 2016 and 2021. Five of the 38 recruited pediatric participants were excluded due to lower MR image quality and related to its low inter/intra-rater reliability <90% resulting in a final sample of 33 TDC. A clinical interview with child's parents covered prenatal, perinatal, and postnatal periods with an emphasis on developmental milestones in psychomotor, visual-spatial and language skills. Among exclusion criteria were sensory visual/auditory uncorrected deficits; individual and family history of DSM diagnosis; premature birth, TBI, and history of severe systemic disease (cancer, diabetes, hepatitis, TB, immune system disorders). Study consent forms for HAC, the parental consent and assent for TDC were approved by the IRB Committee of the Massachusetts General Hospital, Ethics Committee at the University of New Mexico, and the Advarra Institutional Review Board in accordance with the Declaration of Helsinki. Both age samples were highly compatible for further statistical analyses. There were no significant differences in group distribution of the gender, handedness (tested using Pearson’s Chi-Square), Full scale IQ (WASI-II) or socioeconomic status (i.e., father’s education) which were assessed by independent samples t-tests. Table 1 provides demographics of the participants.
Table 1.
Children and Adults Demographics*.
| Age Groups | TDC (M, SD) | HAC (M, SD) |
|---|---|---|
| N | 33 | 31 |
| Age | 11.84 (1.68) | 23.94 (2.05) |
| Gender (% female) | 51.5% | 61.3% |
| Dominant Hand (% right) | 77.4% | 90.3% |
| Father Education: SES status | 15.40 (4.90) | 16.07 (3.05) |
| FSIQ WASI-II | 109.64 (14.67) | 114.65 (6.89) |
None of the demographics showed statistically significant group differences except age. Gender (x2(1) = 0.621, p=0.431) and handedness (x2(2) = 3.451, p=0.178) were equally matched across HAC and TDC, as were Full Scale IQ (F=2.992, p=0.089) and father’s education as an indicator of social economic status (F=1.581, p=0.214). TDC: Typically Developing Children; HAC: Healthy Adult Controls.
2.3. Structural Magnetic Resonance Imaging (MRI)
2.3.1. Data acquisition
High-resolution structural MR scans were obtained at the MGH/MIT/HMS Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, using a Siemens Sonata, (Siemens AG, Erlangen, Germany) for about 30% of pediatric sample and the entire adult sample with a 32-channel head coil. Imaging for the morphometric analysis was done with a 3D inversion recovery with a fast-low flip angle gradient echo sequence (MP-RAGE scans), providing 128 sagittal slices, 1.33 mm slice-thickness, with TR between inversion pulses 2730 ms; TR/TE/flip angle/TI: 2730 ms /3.44 ms/7 degrees/1000 ms; acquisition matrix of 256·192·128; square FOV of 256 mm; NEX 1; and two MP-RAGEs, each 8 min 46 s. These acquisition parameters were empirically optimized to increase gray/white and gray/cerebrospinal fluid contrast. Obtaining a single image with high contrast-to-noise, required for each participant two separate MP-RAGE acquisitions. The rest of the pediatric MRI scans from the DevCog cohort (https://devcog.mrn.org/) were obtained using 3 T Siemens Trio (Erlangen, Germany) with a 32-channel head coil. To create a single image with high contrast-to-noise, two T1-weighted MP-RAGE scans were registered for each participant, then motion-corrected and averaged to create a single, high signal-to-noise ratio image. The high-resolution structural scans of the whole brain were acquired using a 3D RF-spoiled magnetization with rapid gradient echo (MP-RAGE) sequence (TR = 2530 ms; TE = 3.39 ms; FA = 7°; FOV = 256 mm; 176 in-plane sagittal slices; voxel size = 1.33 × 1 × 1.33 mm). The quality of images in all samples was high and comparable.
2.3.2. MRI data analysis
Reconstruction of cortical and volumetric segmentation were performed using the FreeSurfer image analysis program (http://surfer.nmr.mgh.harvard.edu/; Dale et al., 1999; Fischl et al., 2002, Fischl et al., 2004; Fischl et al., 1999; Fischl and Dale, 2000; Reuter et al., 2012). The 3D structural scans were used to construct models of each individual brain. Cross-subject statistics were generated in a cortical surface-based coordinate system (Dale et al., 1999, Dale and Sereno, 1993, Fischl et al., 1999). The FreeSurfer processing sequence of acquired MR images included: (1) motion correction and averaging (Reuter et al., 2012) of two high-resolution volumetric T1-weighted images; (2) removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Ségonne et al., 2004); (3) transformation to Talairach space (Fischl et al., 2004); (4) intensity normalization (Sled et al., 1998); (5) tessellation of the gray matter/white matter boundary and automated topology correction (Fischl et al., 2001, Ségonne et al., 2007) following intensity gradients to optimally localize the gray/white and gray/pial matter, the segmentation of the subcortical white matter and deep gray matter structures (Dale et al., 1999, Fischl et al., 1999).
2.3.3. Cerebellar morphometry manual tracing protocol computer assisted by FreeSurfer algorithm
FreeSurfer morphometric procedures have shown significant test-retest reliability across scanners and field strengths (Han et al., 2006, Reuter et al., 2012), thus our approach was well-supported. Rigorous morphometric measurement of the cerebellar vermis lobules I-V and VI-VII was performed using a manual segmentation protocol assisted by computerized formula of FreeSurfer (Fischl, Sereno, Tootell, et al., 1999). The obtained cerebellar volume included gray and white matter. Our protocol of manual tracing was designed to ensure rigorous measurements of the intricate cerebellar folia, which are often a significant challenge to automated segmentation protocols. The cerebellum was measured by two independent raters well-trained in cerebellar anatomy. The raters were naïve to the subject's age, gender, and race/ethnicity. Inter- and intra-rater reliability were greater than 90% and 94%, respectively. Five cases of pediatric brain measurements were excluded due to a lower scan quality and inter- or intra-rater reliability below 90%. The final TDC sample included 33 children; and HAC sample 31 adults.
The morphometric protocol was based on anatomical atlases and published anatomical studies of the cerebellar vermis morphometry (Ciesielski et al., 1997, Courchesne et al., 1989, Lesnik et al., 1998, Nolte, 2008; J. D. Schmahmann et al., 1999, Schmahmann et al., 2000). Measurements of anterior vermis lobules I-V (lingula, central lobule, culmen) and posterior lobules VI-VII (declive, folium, tuber) were performed on the midsagittal image that most clearly showed the cerebral aqueduct, and the longest, uninterrupted primary and pre-pyramidal fissures. The vermal measurements were obtained by tracing the boundaries of lobules I-V bordered inferiorly by the primary fissure and lobules VI-VII bordered inferiorly by the pre-pyramidal fissure. The volume of each slice was in mm3 (slice thickness 1 mm). The manual tracing began and ended in the floor of the fourth ventricle for each set of three measurements of the I-V and VI-VII lobules. Two sets of measurements of sagittal plane images were obtained for each evaluated brain. The planimetry of two lateral 1 mm thick slices to the right and left from the midsagittal plane was additionally measured to increase data validity. To obtain an optimal representative measures of individual morphometry the three sagittal values were summed and averaged for each MRI brain. The topographic markers of the cerebellar vermis manual tracing are illustrated in Fig. 1A & B.
Fig. 1.
High resolution midsagittal magnetic resonance images (MRI) displaying cerebellar regions of interest (ROIs) for morphometric tracing in a10-year-old female (1A: sagittal plane; 1 C: coronal plane) and 23-year-old female (1B: sagittal plane and 1D: coronal plane). ROIs in sagittal plane included anterior Lobules I-V that are separated from the posterior Lobules VI -VII by [1] – Fissure Prima. The Lobules VI-VII are separated from Lobule VIII by [2] – Fissure Prepyramidalis. ROIs in coronal plane (C and D) illustrate one of the 12 slices across the brain from the posterior to the anterior pole. The image 1 C (TDC) and 1D (HAC) illustrate the right hemisphere tracing included into the Total Brain Volume (TBV), with exclusion of structures of posterior fossa and the cerebellum.
A ratio of the volume for vermal lobules VI-VII to lobules I-V was also calculated. We expected that this ratio may be age-dependent and therefore may serve as an anatomical marker of the differential maturation in the anterior and posterior/inferior cerebellum. Since it is not known whether the phylogenetically later-developing lobules VI-VII still increase tissue volume during adolescence (ages 9–15), or whether they are already engaged in the process of developmental pruning, tracking the ratio of VI-VII to I-V in TDC in comparison to HAC may turn out to be a valuable marker of typical neurodevelopment.
Estimating total brain volume (TBV) provided a standard baseline for cross age-group comparison of morphometric measures for any individual brain regions. At the age of 5–6 head circumference stabilizes and permits a reliable acquisition of MRI signals and later morphometry across all age groups (Caviness et al., 1996, Giedd et al., 1999, Reiss et al., 1996, Sowell et al., 2004). Moreover, until about age 8–10 children’s cerebral cortical tissue thickness increases, but after this time-point developmental pruning becomes the normative developmental course (Ciesielski et al., 2019, Shaw et al., 2008). The differential developmental time-course for the cerebellar regions has not yet been determined. It was therefore necessary to determine the total brain volume for each age group. The following protocol to estimate total brain volume (TBV) in mm3 was used. The first anterior slice where brain tissue initially was determined, and the second slice posterior to this point was used as the first TBV slice. Morphometric tracing included 11 individual slices/brain. These were apart from each other ranging 12–13 slices for individual brains. The last posterior plane where the brain tissue could be identified was measured and the second slice anterior to this point was used as the last measure point for each individual TBV. The cerebellum, and all posterior fossa structures were excluded from TBV measurements. The TBV measures were evaluated for interrater reliability with >90% concordance and averaged across raters.
2.4. Neuropsychological assessment
The Wechsler Abbreviated Scale of Intelligence-II (WASI-II; Wechsler, 2011) a brief intelligence assessment tool standardized and normalized for individuals ages 6–90 years, was administered as a component of comprehensive battery of tests evaluating attention, perception, sensory visual/motor coordination, visual working memory, visual and verbal concept formation, and verbal memory. The four core subtests of WASI-II, Vocabulary, Similarities, Block Design and Matrix Reasoning were administered in one session lasting on average 60–90 minutes, with frequent breaks to retain participants’ interest in task performance. In the present report we use data from two WASI-II Subtests: Block Design subtest (based on an original Kohs, 1920) and Vocabulary Subtest. Block Design assesses sensory visual/motor processing, visual-spatial perception and concept formation, and visual motor coordination, in restricted time. Participants are asked to recreate two-dimensional designs from patterns in a stimulus booklet using blocks with red, white, and half red/white blocks. Each test section has time-limits, with extra-credit points for faster rate of performance. The WASI-II Vocabulary Subtest measures early acquired and crystalized verbal knowledge of the meaning of words using expressive speech. Both subtest's scores were normalized for age and the results were used to examine the correlative relationship between cerebellar vermis morphometry and visual-spatial-motor skills and verbal memory expressive motor speech.
2.5. Statistical analyses
2.5.1. MRI morphometry of the cerebellar vermis and its relationship to neuropsychological skills
Three statistical applications were employed to answer the questions raised in this study. To examine age-dependent changes in the total brain volume (TBV) and in the anatomy of the cerebellar vermis, specifically lobules I-V and VI-VII, an independent samples t-test was conducted on differences in morphometric measurements between children (TDC; 9–15 yrs) and the healthy adult controls (HAC; 18–30 yrs). Further, Spearman rs correlations were conducted within the TDC group to investigate the relationship between the anatomical volume of the anterior vermis (I-V) and the neocerebellar posterior lobules (VI-VII) to neuropsychological performance proficiency. The Spearman rs values were obtained between the morphometry volume of the cerebellar lobules I-V and VI-VII with: (i) the Full-Scale Intelligence Quotient (FSIQ), (ii) Block Design WASI-II Subtest evaluating visual-spatial perception, construction and working memory, and (iii) Vocabulary WASI-II Subtest evaluating crystalized verbal memory and motor speech. Finally, based on our prior observations of the cerebellar vermis morphometry in pediatric samples (Ciesielski et al., 1997) and the current measurements we found the ratio of the volume of VI-VII to I-V lobules relatively constant (∼60%). The % ratio of VI-VII volume to I-V was calculated for each participant; the group differences were examined.
3. Results
3.1. Age-related changes in MRI cerebellar vermis morphometry
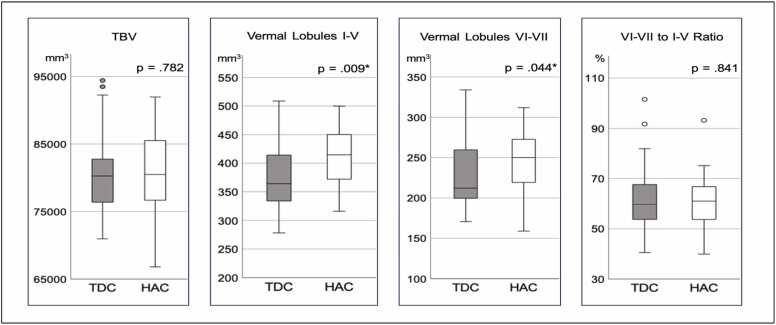
as shown in Table 2 and Fig. 2 the total brain volume (TBV) is not statistically significantly different between children and adults [TDC vs. HAC, t-statistic= −0.278; p=0.782; Cohen’s d=-0.070]. The result provides validation to our measurements of the specific cerebellar regions in both groups, which vary in size independently of TBV. In contrast, statistically significant age-related differences were found in morphometry of the two cerebellar vermal regions of interest. Both lobules I-V and VI-VII, displayed significantly lower volumetric values in TDC compared to healthy adult controls [TDC<HAC; I-V: t-statistic= −2.691, p=0.009, Cohen’s d=-0.673; VI-VII: t-statistic= −2.060, p=0.044, Cohen’s d=-0.515].
Table 2.
Differences in MRI morphometry of the Cerebellar Vermis between Adolescents and Adults.
|
MRI Morphometry |
Independent Samples T-Testtstatistic | pvalue | Mean Difference (SE) | Cohen’sd |
|---|---|---|---|---|
| TDC vs. HAC in mm3 | ||||
|
Lobules I-V 376.09 vs. 412.80 |
-2.691* | 0.009 | -36.710 (13.641) | -0.673 |
|
Lobules VI-VII 227.80 vs. 249.48 |
-2.060* | 0.044 | -21.681 (10.526) | -0.515 |
|
Ratio Lobules VI-VII to I-V 61.59% vs. 60.97% |
0.202 | 0.841 | 0.00617 (0.031) | 0.050 |
|
Total Brain Volume (TBV) 80285.55 vs. 80711.10 |
-0.278 | 0.782 | -425.551 (1529.409) | -0.070 |
TDC= Typically Developing Children; HAC = Healthy Adult Controls; SE = Standard Error
significant effect at p<0.05
Fig. 2.
Box-plot distribution of age-group contrasts in TBV and cerebellar vermis morphometry. Legend: TBV=Total Brain Volume; TDC = Typically Developing Children; HAC=Healthy Adult Controls * Significant effect at p<0.05.
3.2. Ratio of MRI volumetric measurements of lobules VI-VII to I-V
In contrast to our expectations of increasing ratio of lobules VI-VII to I-V in the mature adult brain due to dynamically increasing volume and connections of neocerebellar VI-VII with associative parietal and frontal cortices, the ratio of VI-VII to I-V lobules remained constant across 9–15 years old children and adults [p = 0.841] and reached in both groups' values oscillating ∼60% (see Table 2). This finding may suggest that the volume in both vermal regions in children ages 9–15 achieved the developmental plateau, or that the volumes of each of the two cerebellar vermis lobules, I-V and VI-VII, are still increasing with age, but the ratio may remain constant as a signature of typical development. Since the volume of both regions of interest, I-V and VI-VII, are significantly lower in children than adults, the latter explanation would be more feasible.
3.3. Correlations of MRI morphometry with FSIQ and neuropsychological measures
Table 3A shows the results of Spearman rs correlations between the morphometry of the cerebellar vermis regions and the FSIQ and between volumetric values and the results of the performance on Block Design and Vocabulary Subtests of WASI-II. The morphometry of lobules VI-VII was significantly positively correlated with FSIQ in children [TDC: VI-VII to FSIQ, rs=0.422, p=0.014]. This relationship did not extend to lobules I-V [TDC: rs==0.068, p=0.707]. Also, as we predicted, the larger volumetric values of the cerebellar vermal lobules I-V were found to be significantly positively correlated with higher accuracy in sensory/motor and visual-spatial-construction abilities as tested by the Block Design (subtest of WASI-II) [I-V: rs=0.367, p=0.035]. With a marginally lower confidence interval (CI), the volume of VI-VII lobules was also correlated with performance on Block Design subtest. Spearman rs correlations between the morphometry of the cerebellar (I-V) vermis and the Vocabulary subtest (WASI-II) were not significant (rs=0.097, p=0.605). On the other hand, correlational values between the VI-VII volume and scores on Vocabulary were approaching statistical significance (rs=0.343, p=0.059). These correlational findings are not accounted for by age and was verified through a partial correlation analysis including age. The statistical significances of age-corrected partial correlations (Table 3B) were consistent with the Spearman correlation results, and additionally corroborated a significant correlation between Vocabulary performance and volume of lobules VI-VII (r=0.385, p=0.039).
Table 3A.
Spearman rs for Vermal Volumes and Neuropsychological Skills in TDC.
| Vermis MRI & Neuropsychological Measurements | Spearman rs | p value | CI |
|---|---|---|---|
| FSIQ x Lobules I-V FSIQ x Lobules VI -VII |
0.068 0.422* |
0.707 0.014 |
[-0.292 – 0.411] [0.082 – 0.675] |
| Block Design x Lobules I-V Block Design x Lobules VI-VII |
0.367* 0.332 |
0.035 0.059 |
[0.017 – 0.637] [-0.023 – 0.613] |
| Vocabulary x Lobules I-V Vocabulary x Lobules VI-VII |
0.097 0.343 |
0.605 0.059 |
[-0.267 – 0.436] [-0.013 – 0.622] |
Legend: TDC= Typically Developing Children; CI = Confidence Interval. * significant effect at p<0.05
Spearman rs correlations: significant = 0.30; moderate = 0.40; strong = 0.70.
FSIQ = Full Scale Intelligence Quotient; Block Design and Vocabulary = WASI-II Subtests
Table 3B.
Partial Correlation Controlling for Age, Vermal Volume and Neuropsychological Skills in TDC.
| Vermis MRI & Neuropsychological Measurements | Partial correlation | p value |
|---|---|---|
| FSIQ x Lobules I-V FSIQ x Lobules VI -VII |
0.285 0.395* |
0.134 0.034 |
| Block Design x Lobules I-V Block Design x Lobules VI-VII |
0.391* 0.442* |
0.036 0.016 |
| Vocabulary x Lobules I-V Vocabulary x Lobules VI-VII |
0.189 0.385 |
0.325 0.039 |
4. Discussion
Three questions were raised about the normative pattern of age-related changes in anatomical and functional characteristics of the cerebellar vermis. First, do the age-dependent changes in MRI morphometry of the anatomical volume vary between the anterior, phylogenetically older lobules I-V, and the inferior/posterior neocerebellar lobules VI-VII in 9–15-year-old children? If yes, this would suggest that the cortical tissue volume in the phylogenetically older anterior vermis in children attain the adult-like maturational volume earlier while the neocerebellar lobules VI-VII would remain subject to protracted maturational reformation. Second, will the % ratio of the MRI volume of the later maturing lobules VI-VII to lobules I-V be higher in adults than in children and increasing with age? Third, will the functional specificity of the phylogenetically older anterior cerebellar vermis (I-V) be primarily associated with sensorimotor functions (visual processing translated to motor response), while the posterior/inferior neocerebellar vermis (VI-VII) be predominantly linked to proficiency in conceptual visual-spatial and verbal processes engaging the associative parietal and prefrontal circuits? Following the latter, we hypothesized that the volume of the cerebellar vermis VI-VII will more readily display significant correlations with the intellectual scores (FSIQ) in children. Our rigorous MRI morphometry protocol for the cerebellar vermis and neuropsychological measures lend only partial support to the above predictions.
4.1. Age-group differences in the cerebellar vermis volume indicate its protracted developmental course
In accordance with the notion that “ontogeny recapitulates phylogeny,” we expected that the phylogenetically earlier maturing anterior cerebellar vermis I-V (Leiner et al., 1989) will stabilize its volume and sensorimotor functions earlier (than lobules VI-VII), and thus may not be significantly different from the adult brain. Lobules I-V are closely interconnected with the vestibular system, superior colliculi, and with thalamus in the feedback loop of the CPCTC pathway (Badke D’Andrea et al., 2023). During the first year of life, lobules I-V in contrast to VI-VII were reported to dynamically increase in volume (Knickmeyer et al., 2008, Tau and Peterson, 2010). Consistently, the anatomical connections of the anterior lobules I-V and fastigial nucleus to the cortical frontal eye fields and the earlier maturing primary frontal motor cortex were reported to develop dynamically, thus playing an essential role in early development of sensorimotor skills, eye movement control and motor responsiveness (P. Brodal and Bjaalie, 1992; Graybiel, 1974; Lalonde, 1997; Walberg, 1972). Synchronization of cerebellar-cortical sensorimotor, default mode, and executive control networks has shown stronger functional MRI coherence in children ages 8–10 compared to infants but also to adults, indicating the critical role of the cerebellar changes across these maturational phases of early adolescence (Kipping et al., 2017).
In accordance with the literature the current data shows that although the total brain volumes (TBV) in child and adult participants were not significantly different, the volume of the anterior vermis was lower in children, remaining in the dynamic process of neural tissue growth. The difference between child vs. adult in volume of I-V lobules appears similar in magnitude to lobules VI-VII, which are topographically connected with the late maturing frontal, parietal, and temporal association cortices subserving higher cognitive functions (Bunge and Wright, 2007, Casey et al., 2008, Ciesielski et al., 2019; A. Diamond and Goldman-Rakic, 1989; Giedd et al., 2008; Gogtay et al., 2004; Heck et al., 2023; Luna and Sweeney, 2004; Sowell et al., 2001). These maturational changes include increases in integrity of connectivity within and between major networks, the cortical-basal ganglia-cerebellar loops and the CPCTC pathway (Habas et al., 2019, Heck et al., 2023). This functional plasticity reflects reorganization of neural circuitry first in increased synaptic communication and neural volume, and consecutively in synaptic pruning, essential for acquisition of higher cognitive mental flexibility and affective control (Buckner, 2013, Casey et al., 2008, Feinberg and Campbell, 2010, Hibi and Shimizu, 2012, Knickmeyer et al., 2008, Webster et al., 2011). The frontal and parietal cortex, major cortical destinations of lobules VI-VII (Balsters et al., 2010, Leiner et al., 1986), remain in the active process of maturational changes until adulthood (Gogtay et al., 2004, Sowell et al., 2001, Toga et al., 2006). Our findings of protracted maturation of the posterior cerebellar vermis are in line with findings of other studies reporting continued growth of the posterior cerebellum until late adolescence (Leiner et al., 1989, Tiemeier et al., 2010).
The question remains: what mechanism underlies the concomitant prolonged maturation of the cerebellar volume and circuitry in both the phylogenetically early (I-V) and neocerebellar (VI-VII) lobules of the cerebellar vermis? According to a conceptual review of the cerebellar and basal ganglia studies, there is strong evidence about the convergent processing of the cerebellar signal with the neocerebellum and basal ganglia on the level of thalamus, before they reach the selected associative cortical networks (Buckner, 2013, Hintzen et al., 2018). The role of the thalamus, a major filtering substation for interactive signal processing, needs to be examined morphologically and in the connectivity domain for the anterior and neocerebellum across the developmental trajectory (Wiestler et al., 2011). Supportive data comes from recent fMRI studies of resting state connectivity within the CPCTC pathway in children aged 7–12 and adults 19–40 (Badke D’Andrea et al., 2023). The connectivity between the ventral thalamus and the somatic-sensory-motor facial cortical networks was stronger in children as compared to adults (Badke D’Andrea et al., 2023). It is tempting to speculate that the maturation of the structure and connectivity of the anterior (I-V) and neocerebellar posterior vermis (VI-VII) advances jointly during coherent interactions on the level of the thalamus within the CPCTC pathway.
The framework of cognitive development suggested by our findings is consistent with the model proposed by earlier reports (A. Diamond and Goldman-Rakic, 1989; Luna et al., 2001, Luna et al., 2004, Luna et al., 2010; Rubia et al., 2007). fMRI studies using the anti-saccade task showed that flexible modulation of behavioral and cognitive acts may not be fully developed until late adolescence to adulthood (Luna et al., 2001, Luna et al., 2004). These findings suggested that age-related improvements in inhibitory control are determined not only by structural and functional properties of individual cortical brain regions but most of all by integration of within and between-network communication. Among them are the frontal-striatal-cerebellar-thalamic cortical circuits and the cingulo-opercular network (insula, anterior PFC, and thalamus) that contribute to effective selective inhibition of interference and to retention of control state, respectively (Crone et al., 2006, Rubia et al., 2006).
An increase in the myelin sheath, the fatty tissue covering axons and speeding the transmission of electrical signals across connecting neural fibers (Yakovlev and Lecours, 1967), is one of the primary markers of CNS maturation. Myelin contributes to an increase in cognitive proficiency and self-control. Such progress is seen not only in age-related improvements in structural white matter connectivity (Hagmann et al., 2010), but also in functional integration of resting state and task-related regional synchronization of brain oscillatory activity (Ciesielski et al., 2010, Fair et al., 2009, Uhlhaas et al., 2009). This view suggests that developmental improvements are supported by changes in the cortical-subcortical integration of action (Goldman and Rosvold, 1972). The current findings emphasize the role of the cerebellum in protracted maturation of cognitive skills, providing evidence about the anterior, somatosensory (I-V), and posterior, cognitive/emotional (VI-VII), vermis interaction during structural and functional developmental trajectory.
4.2. The volume ratio of vermal lobules VI-VII to I-V as a hypothetical signature of normative cognitive development
We found a consistent ratio of the lobules VI-VII to I-V falls around ∼60% in healthy children and adults. Based on our current and earlier studies of manual-computer assisted morphometry of the cerebellar vermis (Ciesielski et al., 1994, Ciesielski et al., 1997, Ciesielski et al., 2019) and on reports from other laboratories (Courchesne et al., 1994), we speculate that this stable ∼60% ratio of VI-VII to I-V lobules across adults and children may serve as a generally useful normative estimate of healthy cerebellar vermis development. The cerebellar empirical measures from healthy and ASD samples in studies by Courchesne et al., 1988, Courchesne et al., 1994 are highly consistent with ours and thus with our hypothesis (the morphometry protocol was identical in both laboratories). Rigorous verification of the ∼60% normative ratio requires longitudinal normative and clinical studies, as well as MRI morphometry measures of the complex cerebellar lobules. If verified, such a marker would be an important tool for assessing typically developing children and those displaying symptoms of vermal dysplasia, such as found in ASD, ADHD and BFRBs (Berquin et al., 1998, Ciesielski et al., 1997, Keuthen et al., 2007). A similar aim is explored by identifying developmental molecular numerical markers. These studies show that the cerebellum, with its unique structural and functional topography, depends on the precise developmental coordinates of molecular and cellular blueprint programs (Aldinger et al., 2021, Carter et al., 2018, Peng et al., 2019, Wizeman et al., 2019). The systematic mapping of the cellular pattern of spatial expression of genes guides the identification of developmental markers for early timepoints of structural cerebellar development (Leto et al., 2016, Morales and Hatten, 2006, Ramirez et al., 2022, Tam et al., 2021). Our robust normative ∼60% ratio of VI-VII to I-V, if verified, may serve a role of a simple estimate of changes in the cerebellar structure at different time-points of development. More research is necessary to verify the consistency of the volumetric ratio of the posterior-to-anterior cerebellar vermis.
4.3. Developmental model of neuropsychological skills related to cerebellar vermis morphometry
The newborn child's cerebellum is only 25% of its adult size, yet it contains the blueprint "software" needed to integrate environmental experience with developing motor, cognitive, and emotional skills from infancy (Amso and Johnson, 2005) into adulthood (Aldinger et al., 2021). Although mature cerebellar functional neuroanatomy in adults is relatively well described, our insight into the development of vermal subregions and their association with neurobehavioral skills is limited. We do know, however, that cerebellar remodeling during childhood and adolescence at both functional and anatomical levels is concomitant with increases in integrity of cortical and sub-cortical structures in volume and connectivity, and these are concurrent with an increase in neurobehavioral skills (Balsters et al., 2010).
Consistently, we observed a marked positive correlation between the morphometric volume of I-V and high performance on Block Design subtest, although this task is not targeting visual-spatial sensorimotor skills only, the effect was strong (rs=0.367, p=0.035; see Table 3). The anterior cerebellar vermis and the fastigial nucleus are linked to the spinal cord, vestibular system, and motor cortical regions, providing neural circuit transforming sensorimotor information into a motor response (Eccles et al., 1966, Itō, 1986, Snider and Eldred, 1952). In agreement, abnormalities of the anterior cerebellar lobe with central sensorimotor representations were reported to lead to motor dysmetria (hypermetria; Babinski, 1899) with reduced motor inhibitory control and difficulty in judging distance and controlling the movement of a reaching hand. Our current finding also showed the volume of the neocerebellar vermis, lobules VI-VII, with a trend to positive correlation with proficiency on Block Design (rs=0.332, p =0.059; see Table 3). Block Design subtest is targeting visual-spatial perception and organization, working memory, visual-motor planning, and coordination (Royer, 1977). This correlative trend is consistent with multiple studies and clinical data linking the posterior vermis VI-VII lobules with cognitive and emotional deficits including dysmetria of thought and emotion (Brossard-Racine et al., 2015, Guell et al., 2018, Leiner et al., 1986, Magielse et al., 2022, Molinari and Leggio, 2007, Schmahmann et al., 2019).
The current data showed a positive correlative trend between the posterior vermal lobules VI-VII and the performance on WASI-II Vocabulary subtest; the larger the volume of the VI-VII neocerebellar vermal lobules the more accurate is the recall of verbal crystalized information about different meaning of words, and more effective is verbal narrative. Several neuroimaging studies (fMRI and PET) on differential activation of the cerebellum to motor and silent speech (articulation/phonation) and implicit/explicit memory retrieval, showed predominant activation of the right lateral/posterior cerebellar hemisphere to the cognitive components of speech (verbal response selection) with coherent activation of the anterior cingulate and the left prefrontal cortex (Buckner et al., 1995, Petersen et al., 1988, Petersen et al., 1989, Raichle et al., 1994). The PET activation to automatic speech was recorded in the anterior cerebellum and insular cortex (Petersen et al., 1989). Other neuroimaging studies differentiating cerebellar activation to motor and cognitive components of speech showed significant increase in hemodynamic response in the right cerebellum and left motor cortex to the motor articulation component of speech rather than cognitive content (Ackermann et al., 1998). Recent modeling studies on language and the cerebellum emphasized that to recall and narrate one needs to activate the "feed-forward predictive networks," a proposed universal function of the cerebellum in cognitive tasks (Sokolov et al., 2017). Our data is consistent with such notion. The significant correlation between the volume of the vermal lobules VI-VII and performance on visual-spatial cognitive task and memory/narrative tasks reveals the universality of the service that the cerebellum provides to cognitive processes. This topic has been recently addressed in the concept of the Universal Cerebellar Transform (J. D. Schmahmann et al., 2019) and another theoretical concept of the cerebellum as a coordinator of communication in the brain through modulating coherence of regional oscillations (McAfee et al., 2022).
The WASI Full Scale Intelligence Quotients (FSIQ), FSIQs were related to the volumes of the posterior vermal lobules. Considering the composite character of FSIQ we did not expect to find significant correlations between FSIQ and the vermal lobules’ volume. Indeed, our results showed no significant relationship between the volume of anterior vermis (I-V) and FSIQ. However, the volume of the neocerebellar posterior lobules VI-VII was significantly positively correlated with FSIQ, such that, the larger the neocerebellar vermal lobules VI-VII, the higher FSIQ, and therefore better proficiency in various cognitive abilities, in particular visual-spatial skills (Botez et al., 1989, Itō, 2012b, Magielse et al., 2022, Moore et al., 2017, Paradiso et al., 1997). IQ scores were previously reported to be positively correlated with volume of the posterior cerebellum (Hogan et al., 2011). The studies of the cerebellar morphometry and intellectual skills in schizophrenia, ADHD, and bipolar disorder provided complementary evidence of significant correlations between the reduced tissue volume in the neocerebellum (lobules VI-VII) (Lippmann et al., 1982, Loeber et al., 2001) or increased volume (Levitt et al., 1999) with reduced cognitive functioning. The possible confound with neuroleptic-use on the cerebellar tissue loss and lower FSIQ, was verified by studies in neuroleptic-naive patients with schizophrenia; the significant correlation between reduced posterior vermis and the general intellectual decline persisted providing additional support for the relationship between the posterior cerebellar volume and FSIQ (Ichimiya et al., 2001).
Our findings showed that the complex visual-spatial task may be engaging both anterior and posterior neocerebellar vermis to support visual mental processing for action. Abnormalities of the posterior cerebellar vermis have been found in a variety of psychiatric disorders manifesting reduced cognitive inhibitory control, significant cognitive dysmetria of thought and emotion, and social-emotional dysfunctions such as in autism spectrum disorders, psychotic disorders, body-focused repetitive behaviors, mood disorders and addiction (Andreasen and Pierson, 2008, Brady et al., 2019, Jacobsen et al., 1997, Keuthen et al., 2007, Lupo et al., 2019, Miquel et al., 2016, Murakami et al., 1989; J. D. Schmahmann et al., 2019; Wabnegger and Schienle, 2019). Pathological changes in the cerebellum are suggested to be transdiagnostic across mental disorders with a high predictive value for diagnosis in children and adolescents (Moberget and Ivry, 2019, Vanes and Dolan, 2021). Our current data from children ages 9–15 indicate that the structural and functional individual modularity of the cerebellar vermis has already been defined at this age for the visual-spatial and verbal cognitive functions. Thus, neither the neural structure or functional modularity of the anterior, phylogenetically older lobules I-V and posterior, neocerebellar lobules VI-VII reached the full adult status in adolescent children, and thus both are still in a dynamic course of structural and functional maturation. This raises a question what other brain processes are contributing to this prolonged maturation of volume in the adolescent cerebellum.
The relationships we report between cognitive task performance and larger volume in specific cerebellar regions is consistent with imaging studies in typical adolescents and with the reduced cognitive skills in patients with gray matter cerebellar atrophy. Consistently, the positive correlation between the cerebellar gray matter volume in the posterior cerebellum (vermis lobules VI-VII) was reported with higher scores on verbal memory and set shifting in adolescents (Moore et al., 2017). To similar conclusion came the longitudinal study reporting the peak volume for the cerebellum at 11.8 years in females, and 15.6 years in males, time windows that are followed by the gray cells pruning (Tiemeier et al., 2010). Thus, the normative time for gray matter highest density in the adolescent cerebellum is consistent with the age of our typically developing participants, yet they display still immature, lower volume of the cerebellar vermal lobules than adults. One may speculate that the maturational processes contributing to our findings involves the protracted growth of oligodendrocytes/myelination, density of neuropil, and growing arbor of axons and dendrites. A similar explanation of contrasts in cerebellar volumes has been offered earlier (Knickmeyer et al., 2008, Mechelli et al., 2005, Tiemeier et al., 2010).
5. Limitations and future studies
The scarcity of neuroimaging developmental studies reporting quantitative morphology of the cerebellar vermis and hemispheres may reflect specific methodologic challenges of cerebellar measurements, such as highly convoluted foliated surface, easily missed anatomic demarcations due to unstable gray/white-matter contrasts in distinct subdivisions of lobules. Due to these challenges the fully automatic computerized algorithms for cerebellar morphometric evaluation are prone to distortions. Our rigorous computer assisted manual protocol was strictly controlled by the anatomical demarcations, and by rules for keeping the intra-rater and between-rater reliability above 90%. Gathering of morphometry measurements using this protocol took approximately 2 years of almost daily work to accumulate the sample discussed in the present study. The high rigor of our protocol resulted in smaller sample sizes, that limit exploration of other variables such age and gender differences, or multiple neuropsychological variables. The validity of our data relies on replicability by our own new studies and anatomical morphometry studies in other laboratories. We appreciate that such comparisons need to be treated with some caution, as the protocols of morphometry across different laboratories vary. A recent report on a novel computationally reconstructed cerebellar surface down to the level of the smallest folds raises hope for attaining a more time-efficient and more accurate computerized morphometry of the cerebellum (Sereno et al., 2020).
The high validity of data was secured by rigorous selection of participants using clinical interviews and neuropsychological assessment tools. Review of each neuropsychological data set was conducted by the same clinical neuropsychologist to exclude the cases with pathological profiles. Moreover, since the youngest children-participants were over 6 years old, the age-related gray/white brain tissue contrasts did not influence brain tissue segmentation or registration of brain coordinates during the FreeSurfer planimetric measurements. Verifying our hypothesis that the white matter volume of oligodendrocytes/myelination due to its protracted developmental trajectory until the fourth decade of life (Amlien et al., 2016; Petanjek et al., 2011) may elucidate the current finding of lower volume of the cerebellar vermis in adolescents, we plan to conduct rigorous measurements in children of the central white matter, and other individually identifiable white matter branches of the cerebellum. The current findings need to be submitted to scrutiny of replication on larger samples across broader age groups before the structure/function relationship and the immaturity of the cerebellar vermis could be considered as a predictive developmental marker and guidance for diagnostic or preventive efforts.
6. Concluding remarks
Recent decades mark a golden age for reevaluation of the human cerebellum as a critically important posterior brain structure integrating neural networks that cohere motor, cognitive, emotional, and social skills with environmental learning from early childhood into maturity. Cerebellar anatomical and cellular organization is reported to follow the timed blueprint (Aldinger et al., 2021, Dahmane and Ruiz Altaba, 1999, Hibi and Shimizu, 2012, Leto et al., 2016, Rakic and Sidman, 1970), according to which the cerebellum is the first to differentiate in the neonatal brain, but one of the last to mature (Triulzi et al., 2005). It has been shown that gray matter matures first and white matter grows far into adulthood (Knickmeyer et al., 2008). Although the functional anatomy of the cerebellum was methodically studied in adults, understanding of the developmental trajectory of the cerebellum, and in particular the cerebellar vermis, is still limited with few recent updates (Amore et al., 2021, Can et al., 2013, Romero et al., 2021). The current data from children aged 9–15 show that the cerebellar vermis, despite homogeneous molecular and macro-architecture, presents specific functional differentiation between the anterior sensorimotor and posterior neocerebellar lobules; but neither of these achieved the adult maturational volume. Additionally, as the correlative data suggest, both appear to be engaged in an interactive communication when a task is performed. Cytoarchitectural uniformity of the cerebellar vermis, as well as the cerebral-cerebellar loops distributed across the cerebellar vermis and hemispheres (Itō, 1990), serve the role of a coordinator with the rest of the cortical networks connecting through multiple cortical pathways in the pontine nuclei. Molecular and functional neuroimaging studies showed that the formation and strengthening of both sensorimotor and cognitive networks for functional cortical-cerebellum connectivity require the engagement of the CPCTC pathway with a central role of the thalamus (Habas et al., 2019, McAfee et al., 2022).
Given the cerebellum's significance in coordinating cortical-cerebellar circuits, the protracted maturation of oligodendrocytes responsible for extended axonal myelination until the fourth decade may be the prime mechanism in the development of cerebellar vermis volume in adolescents. The present data suggests that, in future developmental studies of the structural and functional complexity of the cerebellar vermis, examination of the late maturing white matter, oligodendrocyte/myelination, may become a valuable line of inquiry (Lesnik et al., 1998, Knickmeyer et al., 2008, Tiemeier et al., 2010).
CRediT authorship contribution statement
Yu-Ping Wang: Validation, Resources, Funding acquisition. Vince D. Calhoun: Resources, Funding acquisition. Douglas N. Greve: Software, Methodology. Isabel Solis: Investigation, Data curation. Tony W. Wilson: Validation, Funding acquisition. Hussein Al Azzawi: Validation, Software, Methodology. Ryan Anderson: Visualization, Investigation. Kristina T. Rewin Ciesielski: Writing – review & editing, Writing – original draft, Supervision, Project administration, Funding acquisition, Formal analysis, Conceptualization. Julia M. Stephen: Resources, Investigation, Funding acquisition, Formal analysis, Data curation. Elizabeth A. Hodgdon: Writing – review & editing, Visualization, Investigation, Formal analysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. KRC
Acknowledgements
MRI data acquisition for this study was funded in part by the NSF Grant #1539067 to VDC, JMS, Y-PW & TWW (MPI) 2015–2021; by the National Institute for Biomedical Imaging and Bioengineering (P41EB015896) and by Nancy Lurie Marks Family Foundation, Boston 2016–2018 to KTRC. We express our recognition to Moriah Stern, MS for conducting a pilot study in our Laboratory that helped to refine the morphometry protocol.
Data availability
Data will be made available on request.
References
- Ackermann H., Wildgruber D., Daum I., Grodd W. Does the cerebellum contribute to cognitive aspects of speech production? A functional magnetic resonance imaging (fMRI) study in humans. Neurosci. Lett. 1998;247(2):187–190. doi: 10.1016/S0304-3940(98)00328-0. [DOI] [PubMed] [Google Scholar]
- Aldinger K.A., Thomson Z., Phelps I.G., Haldipur P., Deng M., Timms A.E., Hirano M., Santpere G., Roco C., Rosenberg A.B., Lorente-Galdos B., Gulden F.O., O’Day D., Overman L.M., Lisgo S.N., Alexandre P., Sestan N., Doherty D., Dobyns W.B.…Millen K.J. Spatial and cell type transcriptional landscape of human cerebellar development. Nat. Neurosci. 2021;24(8):8. doi: 10.1038/s41593-021-00872-y. (Article) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amore G., Spoto G., Ieni A., Vetri L., Quatrosi G., Di Rosa G., Nicotera A.G. A Focus on the Cerebellum: From Embryogenesis to an Age-Related Clinical Perspective. Front. Syst. Neurosci. 2021;15 doi: 10.3389/fnsys.2021.646052. 〈https://www.frontiersin.org/articles/10.3389/fnsys.2021.646052〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amso D., Johnson S.P. Selection and inhibition in infancy: Evidence from the spatial negative priming paradigm. Cognition. 2005;95(2):B27–B36. doi: 10.1016/j.cognition.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Andreasen N.C., Pierson R. The role of the cerebellum in schizophrenia. Biol. Psychiatry. 2008;64(2):81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babinski J. De l’asynergie cérébelleuse. Rev. Neurol. 1899;7:806–816. [Google Scholar]
- Badke D’Andrea C., Marek S., Van A.N., Miller R.L., Earl E.A., Stewart S.B., Dosenbach N.U.F., Schlaggar B.L., Laumann T.O., Fair D.A., Gordon E.M., Greene D.J. Thalamo-cortical and cerebello-cortical functional connectivity in development. Cereb. Cortex. 2023;33(15):9250–9262. doi: 10.1093/cercor/bhad198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsters J.H., Cussans E., Diedrichsen J., Phillips K.A., Preuss T.M., Rilling J.K., Ramnani N. Evolution of the cerebellar cortex: The selective expansion of prefrontal-projecting cerebellar lobules. NeuroImage. 2010;49(3):2045–2052. doi: 10.1016/j.neuroimage.2009.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum G.L., Cui Z., Roalf D.R., Ciric R., Betzel R.F., Larsen B., Cieslak M., Cook P.A., Xia C.H., Moore T.M., Ruparel K., Oathes D.J., Alexander-Bloch A.F., Shinohara R.T., Raznahan A., Gur R.E., Gur R.C., Bassett D.S., Satterthwaite T.D. Development of structure–function coupling in human brain networks during youth. Proc. Natl. Acad. Sci. 2020;117(1):771–778. doi: 10.1073/pnas.1912034117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes F.M., Turtle M., Khan Y., Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch. Gen. Psychiatry. 1994;51(6):477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Berquin P.C., Giedd J.N., Jacobsen L.K., Hamburger S.D., Krain A.L., Rapoport J.L., Castellanos F.X. Cerebellum in attention-deficit hyperactivity disorder: A morphometric MRI study. Neurology. 1998;50(4):1087–1093. doi: 10.1212/wnl.50.4.1087. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R.C. Innate and environmental factors in the development of the kitten’s visual cortex. J. Physiol. 1975;248(3):663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan A.C., Dum R.P., Strick P.L. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn. Sci. 2013;17(5):241–254. doi: 10.1016/j.tics.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botez M.I., Botez T., Elie R., Attig E. Role of the cerebellum in complex human behavior. Ital. J. Neurol. Sci. 1989;10(3):291–300. doi: 10.1007/BF02333774. [DOI] [PubMed] [Google Scholar]
- Brady R.O., Gonsalvez I., Lee I., Öngür D., Seidman L.J., Schmahmann J.D., Eack S.M., Keshavan M.S., Pascual-Leone A., Halko M.A. Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. Am. J. Psychiatry. 2019;176(7):512–520. doi: 10.1176/appi.ajp.2018.18040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodal P., Bjaalie J.G. Organization of the pontine nuclei. Neurosci. Res. 1992;13(2):83–118. doi: 10.1016/0168-0102(92)90092-Q. [DOI] [PubMed] [Google Scholar]
- Brossard-Racine M., du Plessis A.J., Limperopoulos C. Developmental cerebellar cognitive affective syndrome in ex-preterm survivors following cerebellar injury. Cerebellum. 2015;14(2):151–164. doi: 10.1007/s12311-014-0597-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80(3):807–815. doi: 10.1016/j.neuron.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Raichle M.E., Petersen S.E. Dissociation of human prefrontal cortical areas across different speech production tasks and gender groups. J. Neurophysiol. 1995;74(5):2163–2173. doi: 10.1152/jn.1995.74.5.2163. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Krienen F.M., Castellanos A., Diaz J.C., Yeo B.T.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge S.A., Wright S.B. Neurodevelopmental changes in working memory and cognitive control. Curr. Opin. Neurobiol. 2007;17(2):243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Can D.D., Richards T., Kuhl P.K. Early gray-matter and white-matter concentration in infancy predict later language skills: A whole brain voxel-based morphometry study. Brain Lang. 2013;124(1):34–44. doi: 10.1016/j.bandl.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappe C., Morel A., Barone P., Rouiller E.M. The thalamocortical projection systems in primate: An anatomical support for multisensory and sensorimotor interplay. Cereb. Cortex. 2009;19(9):2025–2037. doi: 10.1093/cercor/bhn228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R.A., Bihannic L., Rosencrance C., Hadley J.L., Tong Y., Phoenix T.N., Natarajan S., Easton J., Northcott P.A., Gawad C. A single-cell transcriptional atlas of the developing murine cerebellum. Curr. Biol. 2018;28(18):2910–2920. doi: 10.1016/j.cub.2018.07.062. e2. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Getz S., Galvan A. The adolescent brain. Dev. Rev. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness V.S., Jr, Kennedy D.N., Richelme C., Rademacher J., Filipek P.A. The human brain age 7–11 years: A volumetric analysis based on magnetic resonance images. Cereb. Cortex. 1996;6(5):726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Ciesielski K.T., Yanofsky R., Ludwig R.N., Hill D.E., Hart B.L., Astur R.S., Snyder T. Hypoplasia of the cerebellar vermis and cognitive deficits in survivors of childhood leukemia. Arch. Neurol. 1994;51(10):985–993. doi: 10.1001/archneur.1994.00540220031012. [DOI] [PubMed] [Google Scholar]
- Ciesielski K.T., Harris R.J., Hart B.L., Pabst H.F. Cerebellar hypoplasia and frontal lobe cognitive deficits in disorders of early childhood. Neuropsychologia. 1997;35(5):643–655. doi: 10.1016/S0028-3932(96)00119-4. [DOI] [PubMed] [Google Scholar]
- Ciesielski K.T., Ahlfors S.P., Bedrick E.J., Kerwin A.A., Hämäläinen M.S. Top-down control of MEG alpha-band activity in children performing Categorical N-Back Task. Neuropsychologia. 2010;48(12):3573–3579. doi: 10.1016/j.neuropsychologia.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielski K.T.R., Stern M.E., Diamond A., Khan S., Busa E.A., Goldsmith T.E., van der Kouwe A., Fischl B., Rosen B.R. Maturational changes in human dorsal and ventral visual networks. Cereb. Cortex. 2019;29(12):5131–5149. doi: 10.1093/cercor/bhz053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielski K.T.R., Bouchard C., Solis I., Coffman B.A., Tofighi D., Pesko J.C. Posterior brain sensorimotor recruitment for inhibition of delayed responses in children. Exp. Brain Res. 2021;239(11):3221–3242. doi: 10.1007/s00221-021-06191-9. [DOI] [PubMed] [Google Scholar]
- Courchesne E., Yeung-Courchesne R., Hesselink J.R., Jernigan T.L. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N. Engl. J. Med. 1988;318(21):1349–1354. doi: 10.1056/NEJM198805263182102. [DOI] [PubMed] [Google Scholar]
- Courchesne E., Press G.A., Murakami J., Berthoty D., Grafe M., Wiley C.A., Hesselink J.R. The cerebellum in sagittal plane—anatomic-MR correlation: 1. The vermis. Am. J. Neuroradiol. 1989;10(4):659–665. doi: 10.2214/ajr.153.4.829. [DOI] [PubMed] [Google Scholar]
- Courchesne E., Saitoh O., Townsend J., Yeung-Courchesne R., Press G., Lincoln A., Haas R., Schreibman L. Cerebellar hypoplasia and hyperplasia in infantile autism. Lancet. 1994;343(8888):63–64. doi: 10.1016/S0140-6736(94)90923-7. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Wendelken C., Donohue S.E., Bunge S.A. Neural Evidence for Dissociable Components of Task-switching. Cereb. Cortex. 2006;16(4):475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- Dahmane N., Ruiz Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Dev. (Camb., Engl. ) 1999;126(14):3089–3100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Sereno M.I. Improved localizadon of cortical activity by combining EEG and MEG with mri cortical surface reconstruction: A linear approach. J. Cogn. Neurosci. 1993;5(2):162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davis E.E., Pitchford N.J., Jaspan T., McArthur D., Walker D. Development of cognitive and motor function following cerebellar tumour injury sustained in early childhood. Cortex. 2010;46(7):919–932. doi: 10.1016/j.cortex.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Deniz Can D., Richards T., Kuhl P.K. Early gray-matter and white-matter concentration in infancy predict later language skills: A whole brain voxel-based morphometry study. Brain Lang. 2013;124(1):34–44. doi: 10.1016/j.bandl.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A., Goldman-Rakic P.S. Comparison of human infants and rhesus monkeys on Piaget’s AB task: Evidence for dependence on dorsolateral prefrontal cortex. Exp. Brain Res. 1989;74(1):24–40. doi: 10.1007/BF00248277. [DOI] [PubMed] [Google Scholar]
- Diamond M.C., Lindner B., Raymond A. Extensive cortical depth measurements and neuron size increases in the cortex of environmentally enriched rats. J. Comp. Neurol. 1967;131(3):357–364. doi: 10.1002/cne.901310305. [DOI] [Google Scholar]
- Diamond M.C., Rosenzweig M.R., Bennett E.L., Lindner B., Lyon L. Effects of environmental enrichment and impoverishment on rat cerebral cortex. J. Neurobiol. 1972;3(1):47–64. doi: 10.1002/neu.480030105. [DOI] [PubMed] [Google Scholar]
- ten Donkelaar H.J., den Dunnen W.F.A., Lammens M., Wesseling P., Willemsen M., Hori A. In: Clinical Neuroembryology: Development and Developmental Disorders of the Human Central Nervous System. ten Donkelaar H.J., Lammens M., Hori A., editors. Springer International Publishing; 2023. Development and developmental disorders of the human cerebellum; pp. 523–593. [DOI] [Google Scholar]
- Dow R.S. Cerebellar action potentials in response to stimulation of the cerebral cortex in monkeys and cats. J. Neurophysiol. 1942;5(2):121–136. [Google Scholar]
- Dow R.S., Anderson R. Cerebellar action potentials in response to stimulation of proprioceptors and exteroceptors in the rat. J. Neurophysiol. 1942;5(5):363–371. [Google Scholar]
- Dow R.S., Moruzzi G. U of Minnesota Press; 1958. The Physiology and Pathology of the Cerebellum. [Google Scholar]
- Dum R.P., Strick P.L. Transneuronal tracing with neurotropic viruses reveals network macroarchitecture. Curr. Opin. Neurobiol. 2013;23(2):245–249. doi: 10.1016/j.conb.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J.C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J. Physiol. 1966;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edde M., Leroux G., Altena E., Chanraud S. Functional brain connectivity changes across the human life span: From fetal development to old age. J. Neurosci. Res. 2021;99(1):236–262. doi: 10.1002/jnr.24669. [DOI] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Power J.D., Dosenbach N.U.F., Church J.A., Miezin F.M., Schlaggar B.L., Petersen S.E. Functional brain networks develop from a “local to distributed” organization. PLoS Comput. Biol. 2009;5(5) doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I., Campbell I.G. Sleep EEG changes during adolescence: An index of a fundamental brain reorganization. Brain Cogn. 2010;72(1):56–65. doi: 10.1016/j.bandc.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Figley T.D., Mortazavi Moghadam B., Bhullar N., Kornelsen J., Courtney S.M., Figley C.R. Probabilistic white matter atlases of human auditory, basal ganglia, language, precuneus, sensorimotor, visual and visuospatial networks. Front. Hum. Neurosci. 2017;11 doi: 10.3389/fnhum.2017.00306. 〈https://www.frontiersin.org/articles/10.3389/fnhum.2017.00306〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Tootell R.B.H., Dale A.M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis: II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B., Liu A., Dale A.M. Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans. Med. Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., Van Der Kouwe A., Killiany R., Kennedy D., Klaveness S. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A., Destrieux C., Halgren E., Ségonne F., Salat D.H., Busa E., Seidman L.J., Goldstein J., Kennedy D., Caviness V., Makris N., Rosen B., Dale A.M. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Floeter M.K., Greenough W.T. Cerebellar plasticity: Modification of purkinje cell structure by differential rearing in monkeys. Science. 1979;206(4415):227–229. doi: 10.1126/science.113873. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: A longitudinal MRI study. Nat. Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Lenroot R.K., Shaw P., Lalonde F., Celano M., White S., Tossell J., Addington A., Gogtay N. Growth Factors and Psychiatric Disorders. John Wiley & Sons, Ltd; 2008. Trajectories of anatomic brain development as a phenotype; pp. 101–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. USA. 2004;101(21):8174. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman P.S., Rosvold H.E. The effects of selective caudate lesions in infant and juvenile rhesus monkeys. Brain Res. 1972;43(1):53–66. doi: 10.1016/0006-8993(72)90274-0. [DOI] [PubMed] [Google Scholar]
- Graybiel A.M. Visuo-cerebellar and cerebello-visual connections involving the ventral lateral geniculate nucleus. Exp. Brain Res. 1974;20(3):303–306. doi: 10.1007/BF00238320. [DOI] [PubMed] [Google Scholar]
- Greenough W.T., McDonald J.W., Parnisari R.M., Camel J.E. Environmental conditions modulate degeneration and new dendrite growth in cerebellum of senescent rats. Brain Res. 1986;380(1):136–143. doi: 10.1016/0006-8993(86)91437-X. [DOI] [PubMed] [Google Scholar]
- Guell X., Gabrieli J.D.E., Schmahmann J.D. Triple representation of language, working memory, social and emotion processing in the cerebellum: Convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. NeuroImage. 2018;172:437–449. doi: 10.1016/j.neuroimage.2018.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C., Kamdar N., Nguyen D., Prater K., Beckmann C.F., Menon V., Greicius M.D. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 2009;29(26):8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C., Manto M., Cabaraux P. The cerebellar thalamus. Cerebellum. 2019;18(3):635–648. doi: 10.1007/s12311-019-01019-3. [DOI] [PubMed] [Google Scholar]
- Hagmann P., Sporns O., Madan N., Cammoun L., Pienaar R., Wedeen V.J., Meuli R., Thiran J.-P., Grant P.E. White matter maturation reshapes structural connectivity in the late developing human brain. Proc. Natl. Acad. Sci. 2010;107(44):19067–19072. doi: 10.1073/pnas.1009073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Jovicich J., Salat D., van der Kouwe A., Quinn B., Czanner S., Busa E., Pacheco J., Albert M., Killiany R., Maguire P., Rosas D., Makris N., Dale A., Dickerson B., Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32(1):180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Harlow H.F., Zimmermann R.R. Affectional response in the infant monkey. Science. 1959;130(3373):421–432. doi: 10.1126/science.130.3373.421. [DOI] [PubMed] [Google Scholar]
- Hebb D.O. John Wiley & Sons Inc; 1949. The Organization of Behavior: A Neuropsychological Theory. [Google Scholar]
- Heck D.H., Fox M.B., Correia Chapman B., McAfee S.S., Liu Y. Cerebellar control of thalamocortical circuits for cognitive function: A review of pathways and a proposed mechanism. Front. Syst. Neurosci. 2023;17 doi: 10.3389/fnsys.2023.1126508. 〈https://www.frontiersin.org/articles/10.3389/fnsys.2023.1126508〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S. The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proc. Natl. Acad. Sci. 2012;109(supplement_1):10661–10668. doi: 10.1073/pnas.1201895109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi M., Shimizu T. Development of the cerebellum and cerebellar neural circuits. Dev. Neurobiol. 2012;72(3):282–301. doi: 10.1002/dneu.20875. [DOI] [PubMed] [Google Scholar]
- Hintzen A., Pelzer E.A., Tittgemeyer M. Thalamic interactions of cerebellum and basal ganglia. Brain Struct. Funct. 2018;223(2):569–587. doi: 10.1007/s00429-017-1584-y. [DOI] [PubMed] [Google Scholar]
- Hogan M.J., Staff R.T., Bunting B.P., Murray A.D., Ahearn T.S., Deary I.J., Whalley L.J. Cerebellar brain volume accounts for variance in cognitive performance in older adults. Cortex. 2011;47(4):441–450. doi: 10.1016/j.cortex.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Hubel D.H., Wiesel T.N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J. Physiol. 1970;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimiya T., Okubo Y., Suhara T., Sudo Y. Reduced volume of the cerebellar vermis in neuroleptic-naive schizophrenia. Biol. Psychiatry. 2001;49(1):20–27. doi: 10.1016/S0006-3223(00)01081-7. [DOI] [PubMed] [Google Scholar]
- Ingvar S. De erven F. Bohn; 1918. Zur phylo-und ontogenese des kleinhirns nebst ein versuch zu einheitlicher erklärung der zerebellaren funktion und lokalisation: Erste mitteilung über das kleinhirn. [Google Scholar]
- Itō M. Raven Press; 1984. The Cerebellum and Neural Control. [Google Scholar]
- Itō M. Neural systems controlling movement. Trends Neurosci. 1986;9(10):515–518. doi: 10.1016/0166-2236(86)90163-3. [DOI] [Google Scholar]
- Itō M. A new physiological concept on cerebellum. Rev. Neurol. 1990;146(10):564–569. [PubMed] [Google Scholar]
- Itō M. Control of mental activities by internal models in the cerebellum. Nat. Rev. Neurosci. 2008;9(4):304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- Itō M. In: The cerebellum: Brain for an implicit self. Itō M., editor. FT Press; 2012. Voluntary Eye Movement. [Google Scholar]
- Itō M. FT Press; 2012. The Cerebellum: Brain for an Implicit Self. [Google Scholar]
- Jacobsen L.K., Giedd J.N., Berquin P.C., Krain A.L., Hamburger S.D., Kumra S., Rapoport J.L. Quantitative morphology of the cerebellum and fourth ventricle in childhood-onset schizophrenia. Am. J. Psychiatry. 1997;154(12):1663–1669. doi: 10.1176/ajp.154.12.1663. [DOI] [PubMed] [Google Scholar]
- Joyal C.C., Meyer C., Jacquart G., Mahler P., Caston J., Lalonde R. Effects of midline and lateral cerebellar lesions on motor coordination and spatial orientation. Brain Res. 1996;739(1):1–11. doi: 10.1016/S0006-8993(96)00333-2. [DOI] [PubMed] [Google Scholar]
- Juraska J.M., Fitch J.M., Henderson C., Rivers N. Sex differences in the dendritic branching of dentate granule cells following differential experience. Brain Res. 1985;333(1):73–80. doi: 10.1016/0006-8993(85)90125-8. [DOI] [PubMed] [Google Scholar]
- Kagan J., Herschkowitz N., Snarey J., Ousley O. The neural foundations of developmental milestones. PsycCRITIQUES. 2005;50(48) [Google Scholar]
- Kandel E., Koester J.D., Mack S.H., Siegelbaum S. Principles of Neural Science, Sixth Edition. 6th edition. McGraw Hill / Medical; 2021. [Google Scholar]
- Keller A., Castellanos F.X., Vaituzis A.C., Jeffries N.O., Giedd J.N., Rapoport J.L. Progressive Loss of Cerebellar Volume in Childhood-Onset Schizophrenia. Am. J. Psychiatry. 2003;160(1):128–133. doi: 10.1176/appi.ajp.160.1.128. [DOI] [PubMed] [Google Scholar]
- Keren-Happuch E., Chen S.A., Ho M.R., Desmond J.E. A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum. Brain Mapp. 2014;35(2):593–615. doi: 10.1002/hbm.22194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuthen N.J., Makris N., Schlerf J.E., Martis B., Savage C.R., McMullin K., Seidman L.J., Schmahmann J.D., Kennedy D.N., Hodge S.M., Rauch S.L. Evidence for reduced cerebellar volumes in trichotillomania. Biol. Psychiatry. 2007;61(3):374–381. doi: 10.1016/j.biopsych.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Kipping J.A., Tuan T.A., Fortier M.V., Qiu A. Asynchronous development of cerebellar, cerebello-cortical, and cortico-cortical functional networks in infancy, childhood, and adulthood. Cereb. Cortex. 2017;27(11):5170–5184. doi: 10.1093/cercor/bhw298. [DOI] [PubMed] [Google Scholar]
- Knickmeyer R.C., Gouttard S., Kang C., Evans D., Wilber K., Smith J.K., Hamer R.M., Lin W., Gerig G., Gilmore J.H. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 2008;28(47):12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohs S.C. The block-design tests. J. Exp. Psychol. 1920;3(5):357–376. doi: 10.1037/h0074466. [DOI] [Google Scholar]
- Kuhl P., Damasio A. In: Principles of Neural Science. Fifth Edition. Kandel E., Schwartz J., Jessell T., Siegelbaum S., Hudspeth A.J., editors. McGraw-Hill Education; 2012. Language. (1–Book, Section) [Google Scholar]
- Kuhl P., Rivera-Gaxiola M. Vol. 31. 2008. Neural substrates of language acquisition; pp. 511–534. (Annu. Rev. Neurosci.). [DOI] [PubMed] [Google Scholar]
- Lalonde R. In: Schmahmann J.D., editor. Vol. 41. Academic Press; 1997. Visuospatial abilities; pp. 191–215. (International Review of Neurobiology). [DOI] [PubMed] [Google Scholar]
- Lalonde R., Botez M.I. Navigational deficits in weaver mutant mice. Brain Res. 1986;398(1):175–177. doi: 10.1016/0006-8993(86)91264-3. [DOI] [PubMed] [Google Scholar]
- Larsell O. Morphogenesis and evolution of the cerebellum. Arch. Neurol. Psychiatry. 1934;31(2):373–395. doi: 10.1001/archneurpsyc.1934.02250020161008. [DOI] [Google Scholar]
- Larsell O., Jansen J. University of Minnesota Press; 1967. The Comparative Anatomy and Histology of the Cerebellum. [Google Scholar]
- Leiner H.C., Leiner A.L., Dow R.S. Does the cerebellum contribute to mental skills? Behav. Neurosci. 1986;100(4):443–454. doi: 10.1037//0735-7044.100.4.443. [DOI] [PubMed] [Google Scholar]
- Leiner H.C., Leiner A.L., Dow R.S. Reappraising the cerebellum: What does the hindbrain contribute to the forebrain? Behav. Neurosci. 1989;103(5):998–1008. doi: 10.1037/0735-7044.103.5.998. [DOI] [PubMed] [Google Scholar]
- Lenroot R.K., Giedd J.N. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lesnik P.G., Ciesielski K.T., Hart B.L., Benzel E.C., Sanders J.A. Evidence for cerebellar-frontal subsystem changes in children treated with intrathecal chemotherapy for leukemia: Enhanced data analysis using an effect size model. Arch. Neurol. 1998;55(12):1561–1568. doi: 10.1001/archneur.55.12.1561. [DOI] [PubMed] [Google Scholar]
- Leto K., Hawkes R., Consalez G.G. In: Essentials of Cerebellum and Cerebellar Disorders: A Primer For Graduate Students. Gruol D.L., Koibuchi N., Manto M., Molinari M., Schmahmann J.D., Shen Y., editors. Springer International Publishing; 2016. Cerebellar neurogenesis; pp. 127–135. [DOI] [Google Scholar]
- Levitt J.J., McCarley R.W., Nestor P.G., Petrescu C., Donnino R., Hirayasu Y., Kikinis R., Jolesz F.A., Shenton M.E. Vol. 156. 1999. Quantitative Volumetric MRI Study of the Cerebellum and Vermis in Schizophrenia: Clinical and Cognitive Correlates; pp. 1105–1107. (American Journal of Psychiatry). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J.W., Van Essen D.C. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J. Comp. Neurol. 2000;428(1):112–137. doi: 10.1002/1096-9861(20001204)428:1<112::AID-CNE8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Lippmann S., Manshadi M., Baldwin H., Drasin G., Rice J., Alrajeh S. Cerebellar vermis dimensions on computerized tomographic scans of schizophrenic and bipolar patients. Am. J. Psychiatry. 1982;139(5):667–668. doi: 10.1176/ajp.139.5.667. [DOI] [PubMed] [Google Scholar]
- Loeber R.T., Cintron C.M.B., Yurgelun-Todd D.A. Morphometry of individual cerebellar lobules in schizophrenia. Am. J. Psychiatry. 2001;158(6):952–954. doi: 10.1176/appi.ajp.158.6.952. [DOI] [PubMed] [Google Scholar]
- Luna B., Sweeney J.A. The emergence of collaborative brain function: fMRI studies of the development of response inhibition. Ann. N. Y. Acad. Sci. 2004;1021(1):296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Luna B., Thulborn K.R., Munoz D.P., Merriam E.P., Garver K.E., Minshew N.J., Keshavan M.S., Genovese C.R., Eddy W.F., Sweeney J.A. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Luna B., Garver K.E., Urban T.A., Lazar N.A., Sweeney J.A. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B., Padmanabhan A., O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72(1):101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo M., Siciliano L., Leggio M. From cerebellar alterations to mood disorders: A systematic review. Neurosci. Biobehav. Rev. 2019;103:21–28. doi: 10.1016/j.neubiorev.2019.06.008. [DOI] [PubMed] [Google Scholar]
- Magielse N., Heuer K., Toro R., Schutter D.J.L.G., Valk S.L. A comparative perspective on the cerebello-cerebral system and its link to cognition. Cerebellum. 2022 doi: 10.1007/s12311-022-01495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfee S.S., Liu Y., Sillitoe R.V., Heck D.H. Cerebellar Coordination of Neuronal Communication in Cerebral Cortex. Front. Syst. Neurosci. 2022;15 doi: 10.3389/fnsys.2021.781527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A., Price C.J., Friston K.J., Ashburner J. Voxel-Based Morphometry of the Human Brain: Methods and Applications. Curr. Med. Imaging Rev. 2005;1(2):105–113. doi: 10.2174/1573405054038726. [DOI] [Google Scholar]
- Mesulam M.M. From sensation to cognition. Brain. 1998;121(6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Middleton F.A., Strick P.L. Anatomical Evidence for Cerebellar and Basal Ganglia Involvement in Higher Cognitive Function. Science. 1994;266(5184):458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Miquel M., Vazquez-Sanroman D., Carbo-Gas M., Gil-Miravet I., Sanchis-Segura C., Carulli D., Manzo J., Coria-Avila G.A. Have we been ignoring the elephant in the room? Seven arguments for considering the cerebellum as part of addiction circuitry. Neurosci. Biobehav. Rev. 2016;60:1–11. doi: 10.1016/j.neubiorev.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Moberget T., Ivry R.B. Prediction, psychosis, and the cerebellum. Biol. Psychiatry.: Cogn. Neurosci. Neuroimaging. 2019;4(9):820–831. doi: 10.1016/j.bpsc.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M., Leggio M.G. Cerebellar information processing and visuospatial functions. Cerebellum. 2007;6(3):214–220. doi: 10.1080/14734220701230870. [DOI] [PubMed] [Google Scholar]
- Moore D.M., D’Mello A.M., McGrath L.M., Stoodley C.J. The developmental relationship between specific cognitive domains and grey matter in the cerebellum. Dev. Cogn. Neurosci. 2017;24:1–11. doi: 10.1016/j.dcn.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales D., Hatten M.E. Molecular markers of neuronal progenitors in the embryonic cerebellar anlage. J. Neurosci. 2006;26(47):12226–12236. doi: 10.1523/JNEUROSCI.3493-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E.M. American Guidance Service, Inc; 1995. Mullen Scales of Early Learning. [Google Scholar]
- Murakami J.W., Courchesne E., Press G.A., Yeung-Courchesne R., Hesselink J.R. Reduced cerebellar hemisphere size and its relationship to vermal hypoplasia in autism. Arch. Neurol. 1989;46(6):689–694. doi: 10.1001/archneur.1989.00520420111032. [DOI] [PubMed] [Google Scholar]
- Murdoch B.E. The cerebellum and language: Historical perspective and review. Cortex. 2010;46(7):858–868. doi: 10.1016/j.cortex.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Nolte J. The Human Brain: An Introduction to its Functional Anatomy. 6th edition. Mosby; 2008. [Google Scholar]
- Pandya D.N., Kuypers H.G.J.M. Cortico-cortical connections in the rhesus monkey. Brain Res. 1969;13(1):13–36. doi: 10.1016/0006-8993(69)90141-3. [DOI] [PubMed] [Google Scholar]
- Pandya D.N., Yeterian E.H. Proposed neural circuitry for spatial memory in the primate brain. Neuropsychologia. 1984;22(2):109–122. doi: 10.1016/0028-3932(84)90055-1. [DOI] [PubMed] [Google Scholar]
- Paradiso S., Andreasen N.C., O’Leary D.S., Arndt S., Robinson R.G. Cerebellar size and cognition: Correlations with iq, verbal memory and motor dexterity. Cogn. Behav. Neurol. 1997;10(1):1. [PubMed] [Google Scholar]
- Paul R., Grieve S.M., Chaudary B., Gordon N., Lawrence J., Cooper N., Clark C.R., Kukla M., Mulligan R., Gordon E. Relative contributions of the cerebellar vermis and prefrontal lobe volumes on cognitive function across the adult lifespan. Neurobiol. Aging. 2009;30(3):457–465. doi: 10.1016/j.neurobiolaging.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Peng J., Sheng A., Xiao Q., Shen L., Ju X.-C., Zhang M., He S.-T., Wu C., Luo Z.-G. Single-cell transcriptomes reveal molecular specializations of neuronal cell types in the developing cerebellum. J. Mol. Cell Biol. 2019;11(8):636–648. doi: 10.1093/jmcb/mjy089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S.E., Fox P.T., Posner M.I., Mintun M., Raichle M.E. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331(6157) doi: 10.1038/331585a0. Article 6157. [DOI] [PubMed] [Google Scholar]
- Petersen S.E., Fox P.T., Posner M.I., Mintun M., Raichle M.E. Positron emission tomographic studies of the processing of singe words. J. Cogn. Neurosci. 1989;1(2):153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- Piaget J. The Relation of Affectivity to Intelligence in the Mental Development of the Child. Bull. Menn. Clin. 1962;26(3):129–137. [PubMed] [Google Scholar]
- Piaget J., Cook M. Vol. 8. International Universities Press; New York: 1952. (The origins of intelligence in children). [Google Scholar]
- Pysh J.J., Weiss G.M. Exercise during development induces an increase in purkinje cell dendritic tree size. Science. 1979;206(4415):230–232. doi: 10.1126/science.482938. [DOI] [PubMed] [Google Scholar]
- Quairiaux C., Mégevand P., Kiss J.Z., Michel C.M. Functional development of large-scale sensorimotor cortical networks in the brain. J. Neurosci. 2011;31(26):9574–9584. doi: 10.1523/JNEUROSCI.5995-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., Fiez J.A., Videen T.O., MacLeod A.-M.K., Pardo J.V., Fox P.T., Petersen S.E. Practice-related changes in human brain functional anatomy during nonmotor learning. Cereb. Cortex. 1994;4(1):8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- Rakic P., Sidman R.L. Histogenesis of cortical layers in human cerebellum, particularly the lamina dissecans. J. Comp. Neurol. 1970;139(4):473–500. doi: 10.1002/cne.901390407. [DOI] [PubMed] [Google Scholar]
- Ramirez M., Wu J., Liu M., Wu D., Weeden D., Goldowitz D. The cerebellar gene database: A collective database of genes critical for cerebellar development. Cerebellum. 2022;21(4):606–614. doi: 10.1007/s12311-022-01445-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss A.L., Abrams M.T., Singer H.S., Ross J.L., Denckla M.B. Brain development, gender and IQ in children: A volumetric imaging study. Brain. 1996;119(5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr C.S., Arora A., Cho I.Y.K., Katlariwala P., Dimond D., Dewey D., Bray S. Functional network integration and attention skills in young children. Dev. Cogn. Neurosci. 2018;30:200–211. doi: 10.1016/j.dcn.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero J.E., Coupe P., Lanuza E., Catheline G., Manjón J.V., Initiative A.D.N. Toward a unified analysis of cerebellum maturation and aging across the entire lifespan: A MRI analysis. Hum. Brain Mapp. 2021;42(5):1287–1303. doi: 10.1002/hbm.25293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer F.L. Information processing in the Block Design Task. Intelligence. 1977;1:32–50. doi: 10.1007/BF03039311. [DOI] [Google Scholar]
- Rubia K., Smith A.B., Woolley J., Nosarti C., Heyman I., Taylor E., Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum. Brain Mapp. 2006;27(12):973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Taylor E., Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum. Brain Mapp. 2007;28(11):1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi J., Pallesen K.J., Neuvonen T., Brattico E., Korvenoja A., Salonen O., Carlson S. Cognitive and motor loops of the human cerebro-cerebellar system. J. Cogn. Neurosci. 2010;22(11):2663–2676. doi: 10.1162/jocn.2009.21382. [DOI] [PubMed] [Google Scholar]
- Schmahmann J., Pandya D. Fiber Pathways of the Brain. 1st edition. Oxford University Press; 2009. [Google Scholar]
- Schmahmann J.D., editor. The Cerebellum and Cognition. Academic Press; 1997. [Google Scholar]
- Schmahmann J.D., Pandya D.N. Anatomical investigation of projections to the basis pontis from posterior parietal association cortices in rhesus monkey. J. Comp. Neurol. 1989;289(1):53–73. doi: 10.1002/cne.902890105. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Pandya D.N. Prefrontal cortex projections to the basilar pons in rhesus monkey: Implications for the cerebellar contribution to higher function. Neurosci. Lett. 1995;199(3):175–178. doi: 10.1016/0304-3940(95)12056-A. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Pandya D.N. The cerebrocerebellar system. Int. Rev. Neurobiol. 1997;41:31–60. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Doyon J., McDonald D., Holmes C., Lavoie K., Hurwitz A.S., Kabani N., Toga A., Evans A., Petrides M. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. NeuroImage. 1999;10(3):233–260. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Doyon J., Petrides M., Evans A.C., Toga A.W. Academic Press; 2000. MRI atlas of the human cerebellum. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Guell X., Stoodley C.J., Halko M.A. The theory and neuroscience of cerebellar cognition. Annu. Rev. Neurosci. 2019;42(1):337–364. doi: 10.1146/annurev-neuro-070918-050258. [DOI] [PubMed] [Google Scholar]
- Ségonne F., Dale A.M., Busa E., Glessner M., Salat D., Hahn H.K., Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Ségonne F., Pacheco J., Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans. Med. Imaging. 2007;26(4):518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Sereno M.I., Diedrichsen J., Tachrount M., Testa-Silva G., d’Arceuil H., De Zeeuw C. The human cerebellum has almost 80% of the surface area of the neocortex. Proc. Natl. Acad. Sci. 2020;117(32):19538–19543. doi: 10.1073/pnas.2002896117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankle W.R., Rafii M.S., Landing B.H., Fallon J.H. Approximate Doubling of Numbers of Neurons in Postnatal Human Cerebral Cortex and in 35 Specific Cytoarchitectural Areas from Birth to 72 Months. Pediatr. Dev. Pathol. 1999;2(3):244–259. doi: 10.1007/s100249900120. [DOI] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Greenstein D., Clasen L., Evans A., Rapoport J.L., Giedd J.N., Wise S.P. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Snider R.S., Eldred E. Cerebro-cerebellar relationships in the monkey. J. Neurophysiol. 1952;15(1):27–40. doi: 10.1152/jn.1952.15.1.27. [DOI] [PubMed] [Google Scholar]
- Sokolov A.A., Miall R.C., Ivry R.B. The cerebellum: Adaptive prediction for movement and cognition. Trends Cogn. Sci. 2017;21(5):313–332. doi: 10.1016/j.tics.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Tessner K.D., Toga A.W. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J. Neurosci. 2001;21(22):8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Leonard C.M., Welcome S.E., Kan E., Toga A.W. Longitudinal mapping of cortical thickness and brain growth in normal children. J. Neurosci. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J. The cerebellum and neurodevelopmental disorders. Cerebellum. 2016;15(1):34–37. doi: 10.1007/s12311-015-0715-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick P.L., Dum R.P., Fiez J.A. Cerebellum and Nonmotor Function. Annu. Rev. Neurosci. 2009;32(1):413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Tam W.Y., Wang X., Cheng A.S.K., Cheung K.-K. In search of molecular markers for cerebellar neurons. Int. J. Mol. Sci. 2021;22(4) doi: 10.3390/ijms22041850. Article 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tau G.Z., Peterson B.S. Normal development of brain circuits. Neuropsychopharmacology. 2010;35(1):147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach W.T. On the specific role of the cerebellum in motor learning and cognition: Clues from PET activation and lesion studies in man. Behav. Brain Sci. 1996;19(3):411–433. doi: 10.1017/S0140525×00081504. [DOI] [Google Scholar]
- Tiemeier H., Lenroot R.K., Greenstein D.K., Tran L., Pierson R., Giedd J.N. Cerebellum development during childhood and adolescence: A longitudinal morphometric MRI study. NeuroImage. 2010;49(1):63–70. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga A.W., Thompson P.M., Sowell E.R. Mapping brain maturation. Trends Neurosci. 2006;29(3):148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triulzi F., Parazzini C., Righini A. MRI of fetal and neonatal cerebellar development. Recent Adv. Imaging Fetus Newborn. 2005;10(5):411–420. doi: 10.1016/j.siny.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K.S., Ryali S., Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J. Neurosci. 2011;31(50):18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P.J., Roux F., Singer W., Haenschel C., Sireteanu R., Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc. Natl. Acad. Sci. 2009;106(24):9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F., Baetens K., Mariën P., Vandekerckhove M. Social cognition and the cerebellum: A meta-analysis of over 350 fMRI studies. NeuroImage. 2014;86:554–572. doi: 10.1016/j.neuroimage.2013.09.033. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F., Manto M., Cattaneo Z., Clausi S., Ferrari C., Gabrieli J.D.E., Guell X., Heleven E., Lupo M., Ma Q., Michelutti M., Olivito G., Pu M., Rice L.C., Schmahmann J.D., Siciliano L., Sokolov A.A., Stoodley C.J., van Dun K.…Leggio M. Consensus paper: Cerebellum and social cognition. Cerebellum. 2020;19(6):833–868. doi: 10.1007/s12311-020-01155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanes L.D., Dolan R.J. Transdiagnostic neuroimaging markers of psychiatric risk: A narrative review. NeuroImage: Clin. 2021;30 doi: 10.1016/j.nicl.2021.102634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubramanian G., Jayakumar P.N., Gangadhar B.N., Keshavan M.S. Neuroanatomical correlates of neurological soft signs in antipsychotic-naive schizophrenia. Psychiatry Res.: Neuroimaging. 2008;164(3):215–222. doi: 10.1016/j.pscychresns.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Wabnegger A., Schienle A. The role of the cerebellum in skin-picking disorder. Cerebellum. 2019;18(1):91–98. doi: 10.1007/s12311-018-0957-y. (psyh) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walberg F. In: Brodal A., Pompeiano O., editors. Vol. 37. Elsevier; 1972. Descending and reticular relations to the vestibular nuclei: Anatomy; pp. 585–588. (Progress in Brain Research). [DOI] [PubMed] [Google Scholar]
- Webster M.J., Elashoff M., Weickert C.S. Molecular evidence that cortical synaptic growth predominates during the first decade of life in humans. Int. J. Dev. Neurosci. 2011;29(3):225–236. doi: 10.1016/j.ijdevneu.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Pearson; 2011. Wechsler abbreviated scale of intelligence—Second Edition. [Google Scholar]
- Wiestler T., McGonigle D.J., Diedrichsen J. Integration of sensory and motor representations of single fingers in the human cerebellum. J. Neurophysiol. 2011;105(6):3042–3053. doi: 10.1152/jn.00106.2011. [DOI] [PubMed] [Google Scholar]
- Wizeman J.W., Guo Q., Wilion E.M., Li J.Y. Specification of diverse cell types during early neurogenesis of the mouse cerebellum. eLife. 2019;8 doi: 10.7554/eLife.42388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev P.I., Lecours A. In: Regional Development of the Brain in Early Life. Minkowski A., editor. Blackwell; Oxford: 1967. The myelogenetic cycles of regional maturation of the brain; pp. 3–70. [Google Scholar]
- Zielinski B.A., Gennatas E.D., Zhou J., Seeley W.W. Network-level structural covariance in the developing brain. Proc. Natl. Acad. Sci. 2010;107(42):18191–18196. doi: 10.1073/pnas.1003109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.