Abstract
Persistent HGF/Met signaling drives tumor growth and dissemination. Proteoglycans within the tumor microenvironment might control HGF availability and signaling by affecting its accessibility to Met (HGF receptor), likely defining whether acute or sustained HGF/Met signaling cues take place. Given that betaglycan (BG, also known as type III TGFβ receptor or TGFBR3), a multi-faceted proteoglycan TGFβ co-receptor, can be found within the tumor microenvironment, we addressed its hypothetical role in oncogenic HGF signaling. We found that HGF/Met promotes lung cancer and endothelial cells migration via PI3K and mTOR. This effect was enhanced by recombinant soluble betaglycan (solBG) via a mechanism attributable to its glycosaminoglycan chains, as a mutant without them did not modulate HGF effects. Moreover, soluble betaglycan extended the effect of HGF-induced phosphorylation of Met, Akt, and Erk, and membrane recruitment of the RhoGEF P-Rex1. Data-mining analysis of lung cancer patient datasets revealed a significant correlation between high MET receptor, HGF, and PREX1 expression and reduced patient survival. Soluble betaglycan showed biochemical interaction with HGF and, together, they increased tumor growth in immunocompetent mice. In conclusion, the oncogenic properties of the HGF/Met pathway are enhanced and sustained by GAG-containing soluble betaglycan.
Keywords: TGFBR3, Betaglycan, HGF, Glycosaminoglycans, RhoGEF, P-Rex1, Cancer cell migration
1. Introduction
Aberrant signaling by growth factor receptors plays a major role in cancer progression. Besides the oncogenic effects of mutant and overexpressed receptors, the availability of growth factors within the tumor microenvironment influences non-cell autonomous oncogenic signaling and represent therapeutic opportunities, as conceptually proven by clinically useful therapies against growth factors [1,2]. The tumor microenvironment contains a variable mixture of growth factors, chemotactic agonists, cytokines, and proteins shedded from membrane-bound precursors, among others, resulting from the combined secretome of cancer and stromal cells, implying that tumor growth and dissemination are under the constant influence of the integrated action of multiple agonists and extracellular glycoproteins and proteoglycans that affect growth factor availability [[3], [4], [5], [6], [7]]. Tumor-derived factors activate receptor tyrosine kinases (such as receptors for HGF, VEGF, and EGF, among others), serine-threonine kinases (as TGFβ receptors), and receptors coupled to heterotrimeric G proteins (including CXCR4 and CCR2, among many others) [7,8]. Their combined actions promote tumor growth and dissemination, with the support of stromal cells. Relevant signaling pathways leading to cell shape adjustments during tumor growth and dissemination are triggered by Rho GTPases [7], molecular switches directly turned on by RhoGEFs, which are multidomain effectors that integrate chemotactic signaling cues [9,10]. Growth factors and chemokines such as HGF [11], heregulin [12], and SDF-1/CXCL12 [13,14], activate Rho GTPases and chemotactic RhoGEFs as P-Rex1, a Rac guanine nucleotide exchange factor, whose overexpression enhances the metastatic potential of melanoma [15], breast [16,17], and prostate cancers [14].
The type III TGFβ receptor (TGFBR3), also known as betaglycan (BG), is a proteoglycan that exerts paradoxical roles in cancer, mainly preventing, but in some cases promoting, tumor growth and metastasis [18]. The outcome seems to be determined by its existence as a membrane-tethered co-receptor and as a shedded soluble form. In the first case, it potentiates TGFβ signaling by the type 1 and 2 receptors (TβRI and TβRII); while in the second, the soluble BG (solBG) form has an opposite TGFβ antagonist effect [19]. This duality has been invoked to explain how BG could act to enhance or prevent both the tumor-suppressive and oncogenic actions of TGFβ [18]. BG core protein directly binds diverse members of the TGFβ family, presenting them to their cognate serine/threonine kinase receptors [20,21]. However, the soluble BG, i.e. the shedded BG ectodomain, retains its TGFβ binding properties, but instead of presenting, it sequesters the factor [19]. This TGFβ antagonizing capacity (ligand-trap) of solBG has been proved of therapeutical value in many TGFβ-caused animal models of disease, including cancer [[22], [23], [24]]. The recombinant soluble BG used in those studies was devoid of the glycosaminoglycans (GAGs) of the endogenous TGFBR3 [25]. These GAG chains, heparan and chondroitin sulfate of diverse sizes, confer BG functional properties beyond the binding specificity of its core protein [26]. Glycosaminoglycans are complex anionic carbohydrates, postranslationally attached to specific serines in proteoglycans, endowing them with heterogeneous molecular weight, among other properties. GAGs bind growth factors and chemokines serving as co-receptors that enhance their cellular actions, presumably by improving their binding to their signaling receptors [27]. The case of bFGF is paradigmatic for such interactions and has been extensively studied [28]. HGF is another case, soluble heparin and other glycosaminoglycans are known to bind HGF, enhancing Met signaling [29,30].
In this work, we hypothesized that soluble BG regulates HGF signaling and pro-oncogenic effects in a GAG-dependent manner. Therefore, we employed a GAG-containing form of soluble BG, to demonstrate that the chemotactic and growth promoting actions of HGF are enhanced by solBG’s GAG chains.
2. Material and methods
2.1. Cell lines
Lung cancer cells (LAP0297) and porcine endothelial cells (PAE) were maintained in DMEM (Merck, D7777) supplemented with 10 % FBS (BY PRODUCTOS, 90020500) and 1 % antibiotic-antimycotic (Gibco, 15240-062) at 5 % CO2 and 37 °C. LAP0297 cells were kindly donated by Dr. Peigen Huang from the Department of Radiation Oncology, Massachusetts General Hospital, Harvard Medical School, Boston, MA [31]. Recombinant rat soluble betaglycans were prepared by transient transfection of HEK cells as described [32]. The cDNAs used to produce GAG-containing (solBG-WT) or GAG-absent (solBG-AA) forms of solBG were described as LS (Δ783-853) and LS gag− (Δ783-853), respectively [19].
2.2. Agonists and inhibitors
Cells were stimulated, as indicated in figure legends, with different agonists, as hepatocyte growth factor (HGF, 10 ng/mL, R&D Systems, 294-HGN), lysophosphatidic acid (LPA, 5 μM, Biomol, LP-100), SDF-1α/CXCL12 (50 ng/mL, PeproTech, 300-28A), interleukin-8 (IL-8, 3 nM, Sigma-Aldrich I1645), PGE2 (1 μM, Sigma, P5640), epidermal growth factor (EGF, 10 ng/mL, Gibco, 13,247–051), vascular endothelial growth factor (VEGF165, 100 ng/mL, Calbiochem, PF074), basic fibroblast growth factor (bFGF, 25 ng/mL, R&D Systems, 234-FSE/CF), platelet-derived growth factor (PDGF, 100 ng/mL, Sigma-Aldrich, P3326), sphingosine 1-phosphate (S1P, 1 μM, Sigma-Aldrich, S9666), and insulin (100 nM, Sigma, I-5500). For affinity labeling, carrier-free HGF (R&D Systems, 294-HGN025/CF) and TGFβ2 (Ciba-Geigy AG (Basel, Switzerland), were iodinated as described in Cheifetz et al. [33]. Cellular signaling inhibitors were PF-04217903 (Met receptor inhibitor (Met-i), Sigma-Aldrich, catalog SML0263), rapamycin (mTOR inhibitor, Sigma-Aldrich, 553210), wortmannin (PI3K inhibitor, 300 nM, Calbiochem, 681675).
2.3. Western blot
Cell lysates, protein pulldowns, and immunoprecipitated proteins were resolved by SDS-PAGE gels at 20–30 mAmps and transferred to Immobilon-P membranes (Millipore, catalog no. IPV00010) at 320 mAmps for 4 h. Antibodies were from the following sources: Sigma (Flag M2, F3165; FLAG, poly-Histidine, H-1029; P-Rex1, HPA001927; AKT1, P2482); Santa Cruz Biotechnology (GST, sc-138; GFP, sc-9996; ERK2, sc-154; phospho-AKT1/2/3 Ser473, sc-7985-R); Transduction Laboratories (Rac1, 610651), Cell Signaling (P-Rex1, 13168S; phospho-ERK1/2 T202/Y204, 9101; Met, cat 3127; anti-phospho-Met (Y1234/1235), cat. 3126. anti-BG rabbit polyclonal [21], and KPL (anti-mouse, 074–1802; anti-rabbit, 074–1516).
2.4. Cell migration assays
Cancer and endothelial cells were seeded on 0.02 % gelatin in 12 well plates. Confluent monolayers were incubated in serum-free DMEM, 24 h for cancer cells and 12 h for endothelial cells. Mitomycin C (12 μM, Sigma-Aldrich, catalog M0440) was added 2 h before cell monolayers were wounded in the middle with a pipette tip. Cells were washed with PBS, and stimulated with HGF, soluble BG, or both together in serum-free DMEM. Inhibitors for the Met receptor (Met-i), PI3K (wortmannin, 300 nM), or mTOR (rapamycin, 10 ng/mL) were preincubated 2 h before wounding and during cell migration. After 18 h, cells were fixed with methanol, stained with crystal violet, washed with PBS, and photographed.
2.5. Mice and tumor model
FVB male mice (6–8 weeks of age) were inoculated with 106 LAP0297 cells suspended in 100 μL Matrigel (4.5 mg/mL, BD Bioscience, diluted in DMEM), as previously described [34,35]. As indicated in the respective figure legend, in some cases, cell suspensions in matrigel were supplemented with HGF (10 ng/100 μL), solBG (1 μg/100 μL), or both, and kept at room temperature for 1 h before being injected subcutaneously in the dorsal region of mice. We used the cell suspension in matrigel to minimize the treatments diffusion away from the cells once they were implanted into the mouse. Tumors were measured with a caliper and their volume was calculated with the equation: width × length2 × π/6. All procedures were approved by UPEAL-Cinvestav Ethical Committee (protocols 33–13 and 0205–16).
2.6. Affinity labeling and dot-blot-overlay
Recombinant His6-tagged, solBGs (WT or AA) was incubated with 125I-HGF or 125I-TGFβ2 in PBS/Triton (0.05 %) for 3 h on a rocking platform. Protein complexes were incubated with Talon beads 30 μL/1 h, followed by three washes, before crosslinking with disuccinimidyl suberate (DSS, 60 μg/mL final concentration), for 15 min. The crosslinking was stopped with 10 mM Tris-HCl pH 7.4, containing 1 mM EDTA. Beads were washed and bound radioactivity was counted in a Cobra II auto-gamma (Packard). Then, the immunoprecipitated complexes were separated in SDS-PAGE and revealed by phosphor screening. For dot-blots, WT- and AA-solBGs were immobilized on PVDF membranes using a dot-blot chamber for 15 min, albumin was used as control. Vacuum was applied to aspirate the solBG preparations and each well was washed three times with PBS, blocked with 5 % milk in TBS-T for 1 h, followed by three washes with TBS-T. The labeled factors, 125I-HGF (40,000 cpms) or 125I-TGFβ2 (20,000 cpms) were incubated for 6 h at 4 °C on a rocking platform, then washed six times with TBS-T. Membranes were exposed on appropriate Phosphor screens (Exposure Cassette, Molecular Dynamics) for 2 days and revealed in a Typhoon FLA 7000 counter (General Electric).
2.7. Pulldowns of active P-Rex1 and Rac GTPase
Active P-Rex1 and Rac were isolated with recombinant GST-Rac G15A and GST-PAK-CRIB respectively, as previously described [11,34].
2.8. Membrane fractionation
Cytoplasmic and membrane fractionation was done essentially as previously described [35]. In brief, cells were scraped with cold PBS containing protease and phosphatase inhibitors, and subsequently subjected to three cycles of freezing (liquid nitrogen) and thawing (37 °C) to break the cells. A fraction was separated as total cell lysate. Afterwards, lysates were centrifuged at 1000–1400 rpm, for 10 min, the supernatant was transferred to new tubes to discard cell debris and again centrifuged at 13,000 rpm, for 10 min. The pellet, containing membranes, was solubilized with buffer containing Triton X-100. Total cell lysates, the second supernatant containing mainly cytosolic components, and the membrane fraction were analyzed by western blot.
2.9. Immunoprecipitation
Cell lysates were incubated with anti-Met antibodies at 4 °C, overnight, on a rocking platform. Immunocomplexes were isolated with G protein-sepharose (30 μL, 3 h), washed three times with lysis buffer and revealed by western blot using phospho-Met (Y1234/1235) and Met antibodies.
2.10. Statistical analysis
Cell migration assays and western blot densitometries were analyzed with the ImageJ software. Each figure presents representative results of at least three independent experiments. Data were statistically analyzed with Sigma Plot 11 using, as indicated in figure legends, t-test, or one-way ANOVA, followed by Dunnet or Tukey test. Tumor growth was analyzed by two-way ANOVA of repeated measurements, followed by Tukey test. Results were plotted in the GraphPad Prism 5 software. A statistically significant difference was considered for values of p < 0.05.
3. Results
3.1. HGF stimulates lung cancer cell migration via Met/PI3K/mTOR pathway
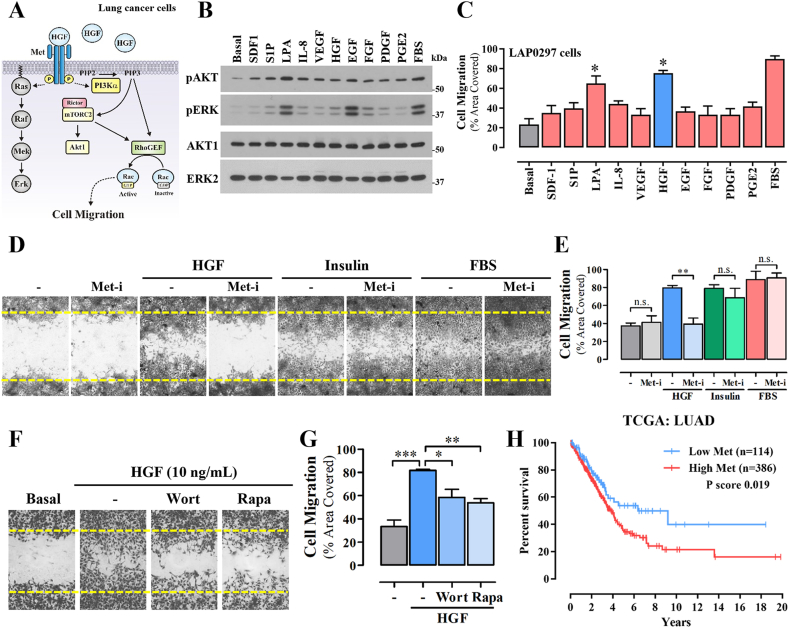
To address the hypothetical role of solBG on HGF signaling in lung cancer and endothelial cells we first characterized whether this growth factor promotes cell migration through a PI3K-dependent pathway (Fig. 1A). We initially analyzed the response of LAP0297 lung cancer cells to diverse stimuli, known to be relevant within the tumor microenvironment, assessed by the activation of Akt and Erk (Fig. 1B) and cell migration (Fig. 1C). These agonists act on GPCRs (SDF-1, S1P, LPA, IL-8, and PGE2) and receptor tyrosine kinases (VEGF, HGF, EGF, FGF, and PDGF). As shown in Fig. 1B, at 5 min, LPA and EGF were the most effective activators of Erk and Akt, while in migration experiments, HGF was the most potent agonist (Fig. 1C), followed by LPA, confirming that signaling by receptor tyrosine kinases and G protein-coupled receptors drive LAP0297 lung cancer cell migration [36]. In the case of LPA, we recently demonstrated the critical role of RhoGEF17 [37]. Although the effect of HGF on Erk was discreet at 5 min, further experiments showed that this effect was more persistent in the presence of solBG (as shown in Fig. 5D). As expected, HGF-induced cell migration was driven by Met receptors, sensitive to PF-04217903, a specific inhibitor Met tyrosine kinase (Met-i, Fig. 1D and E). Furthermore, Met-i did not affect cell migration induced by insulin or fetal bovine serum (Fig. 1D and E). Lung cancer cell migration induced by HGF was decreased by inhibition of PI3K and mTOR (wortmannin 300 nM and rapamycin 100 ng/mL, respectively). Akt phosphorylation at Ser 473 (Fig. 1, Fig. 5C) was indicative of the activation of mTORC2 [38], which is known as the complex linked to cell migration via the activation of the RacGEF P-Rex1 [39], whose activation is shown in Fig. 4. Altogether, these results indicate that HGF promotes LAP0297 lung cancer cell migration via the Met/PI3K/mTOR pathway.
Fig. 1.
HGF stimulates lung cancer cell migration via Met/PI3K/mTOR pathway. A) Putative signaling pathway activated by HGF/Met to promote lung cancer cell migration via PI3K/mTOR. B) Activation Akt and Erk in LAP0297 lung cancer cells stimulated for 5 min with different agonists (at the concentrations indicated in methods), acting on G protein-coupled receptors and tyrosine kinase receptors, known to be relevant within the tumor microenvironment. Total cell lysates were analyzed by western blot using antibodies against the phosphorylated active kinases (upper two panels) and antibodies detecting them irrespective of their phosphorylation status (lower two panels). The result is representative of 3 independent experiments. C) Agonist-driven LAP0297 lung cancer cell migration. Serum-starved confluent monolayers of LAP0297 cells, grown in twelve well plates, were wounded with a pipet tip and stimulated with the indicated agonists to migrate for 18 h towards the wounded area. Cell migration was quantitated by comparing the area covered by migrating cells, with respect to non-stimulated cells (Basal). Cells stimulated with fetal bovine serum (FBS) were used as positive control. Data represent the mean ± SEM of 3 independent experiments. *p < 0.05 (vs Basal), t-test. D) LAP0297 lung cancer cell migration stimulated with HGF (10 ng/mL), Insulin (10 nM) or FBS (10 %) was analyzed in the absence or presence of 1 μM PF-04217903 (Met receptor inhibitor, Met-i). E) Data represents the mean ± SEM of 4 independent experiments. **p < 0.0014, t-test. F) Effect of wortmannin (300 nM, Wort) and rapamycin (10 ng/mL, Rapa) on HGF-dependent migration of LAP0297 lung cancer cells. Inhibitors were preincubated 2h before and during the migration assays. G) Data represent the mean ± SEM of 3 independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA followed of Tukey. H) Kaplan-Meier plot showing the statistical correlation between MET expression and overall survival of lung cancer patients of the LUAD TCGA datasets analyzed at the https://www.proteinatlas.org/platform. Note bene: an uncropped version of the original Fig. 1B is shown in Supplementary Fig. 5S.
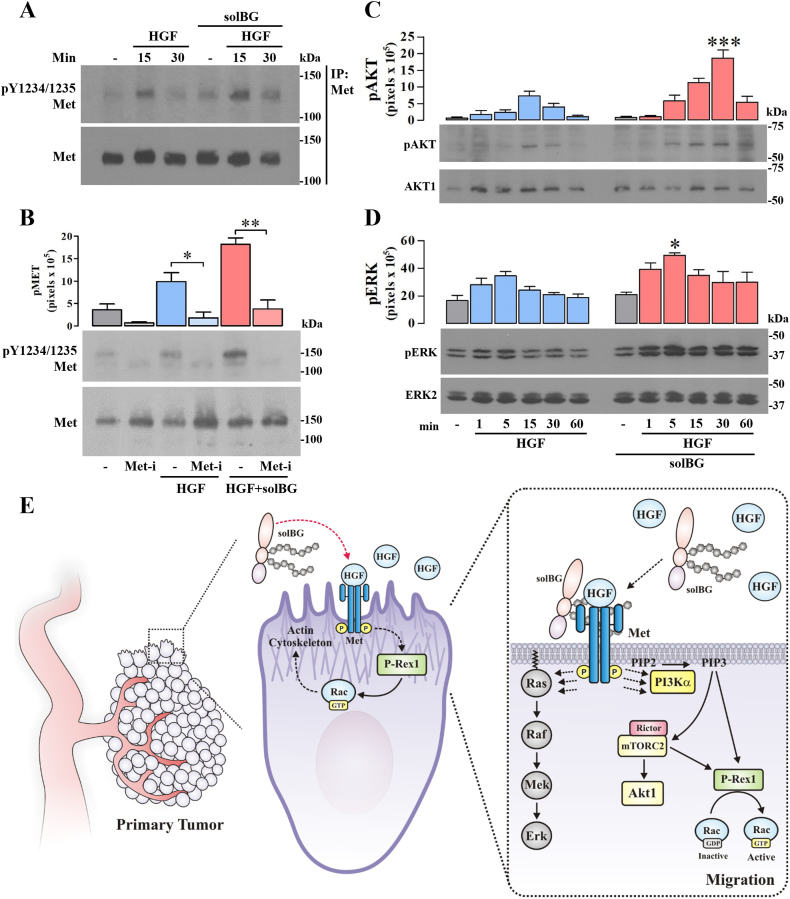
Fig. 5.
Betaglycan enhances HGF-dependent Met signaling intensity and duration. A) Effect of soluble BG on HGF-dependent phosphorylation of Met. Serum-starved confluent LAP0297 lung cancer cells were stimulated with HGF in the presence or absence of soluble BG and the activation of Met was detected by its phosphorylation at Y1234/Y1235 in immunoprecipitated Met, analyzed by western blot. Antibodies detecting Met, irrespective of its phosphorylation, were used to confirm the immunoprecipitation efficiency. The result is representative of 3 independent experiments. B) Soluble BG enhances the tyrosine kinase activity of Met stimulated by HGF. The effect of soluble BG as an enhancer of HGF-dependent activation of Met was addressed in LAP0297 cells preincubated with PF-04217903, a specific inhibitor of Met tyrosine kinase (Met-i). Met phosphorylation and the levels of total Met were detected by western blot in total cell lysates. Graph represents mean ± SEM of 3 independent experiments, *p < 0.05, **p < 0.01, t-test. C-D) Soluble BG enhances the intensity and duration of HGF signaling to Akt (C) and Erk (D). Serum-starved LAP0297 lung cancer cells were stimulated with HGF with or without soluble BG for the times indicated in (D). Activation and expression of Akt and Erk were detected by western blot in total cell lysates using antibodies against the phosphorylated and total proteins, respectively. Graphs represent mean ± SEM of 3 independent experiments, *p < 0.05 (HGF vs HGF + solBG, at the indicated time point), one-way ANOVA followed of Tukey test. E) Schematic representation of the positive effect of soluble BG on HGF-dependent tumor growth, cell migration and signaling by Met via PI3K/mTOR/P-Rex1/Rac pathway in lung cancer cells. Note bene: uncropped versions of the original Fig. 5A,B,C,D, are shown in Supplementary Fig. 5S.
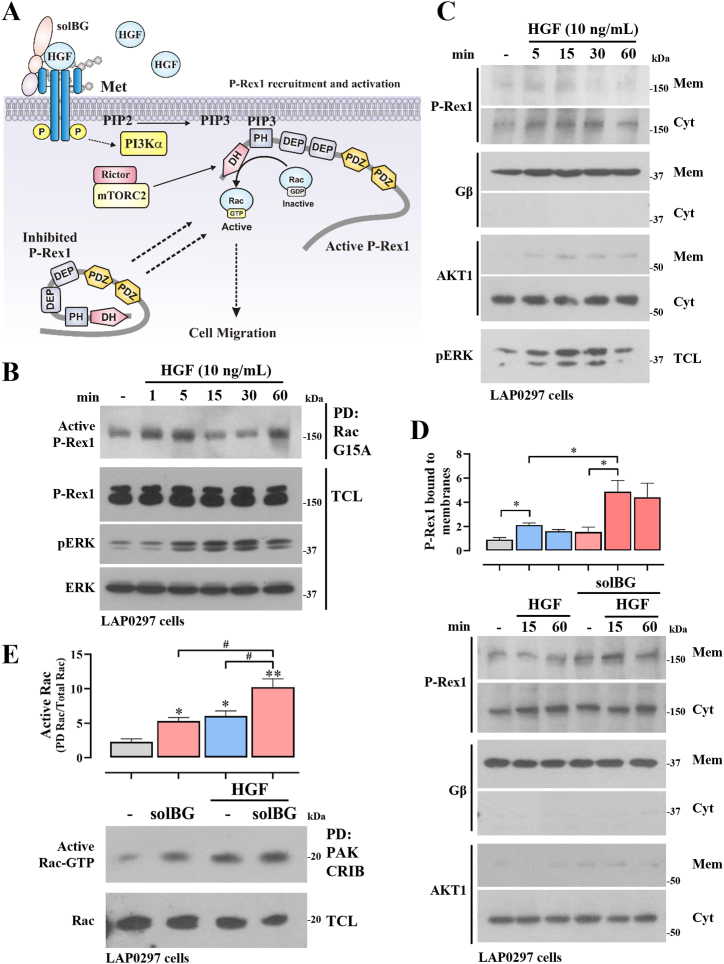
Fig. 4.
Betaglycan enhances HGF-dependent recruitment of P-Rex1 to the membrane fraction and Rac activation. A) Putative mechanism by which soluble BG enhances the effect of HGF on lung cancer cell migration via sustained recruitment and activation of P-Rex1, a Rac guanine nucleotide exchange factor. B) Time course of P-Rex1 activation in LAP0297 lung cancer cells responding to HGF. Serum-starved cells were stimulated with 10 ng/ml HGF and active P-Rex1 was isolated by pulldown with recombinant GST-Rac G15A and detected by western blot in pulldowns and, to verify expression, in total cell lysates (TCL). Expression and activation of Erk in TCL served as internal control. The result is representative of 3 independent experiments. C) Recruitment of P-Rex1 to the membrane fraction of HGF-stimulated LAP0297 lung cancer cells. Cells were stimulated for the indicated times. Membrane and cytosolic enriched fractions were prepared by centrifugation as described in methods. Gβ, Akt, and pERK were detected by western blot and served as controls. The result is representative of 3 experiments. D) Effect of soluble BG on HGF-induced P-Rex1 recruitment to the membrane fraction. The amount of P-Rex1 recruited to the membrane fraction of cells stimulated with HGF in the presence or absence of soluble BG was determined by western blot and normalized to the levels of P-Rex1 in the membrane fraction of non-stimulated cells (−). Gβ and AKT1, detected by western blot in membrane and cytosolic fractions, served as controls. Graph represents mean ± SEM of 3 independent experiments, *p < 0.05, t-test. E) Effect of solBG on Rac activation by HGF in LAP0297 lung cancer cells. Serum-starved cells were stimulated with HGF, solBG, or both, as indicated, for 15 min. Active Rac was isolated by pulldown with recombinant GST-CRIB (the effector domain of Rac at the N-terminal region of PAK). Active and total Rac was detected by western blot. Graph represents mean ± SEM of 3 independent experiments, *p < 0.05, t-test. Note bene: uncropped versions of the original Fig. 4B,C,D,E, are shown in Supplementary Fig. 5S.
3.2. High expression of components of the HGF/Met/P-Rex1/Rac pathway correlate with shorter survival of lung cancer patients
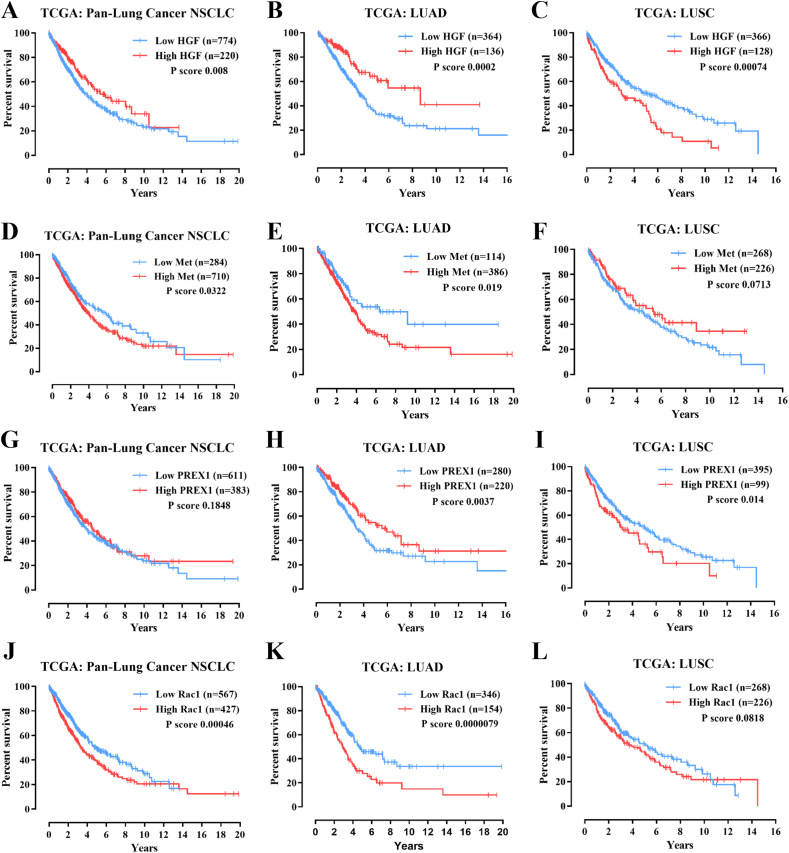
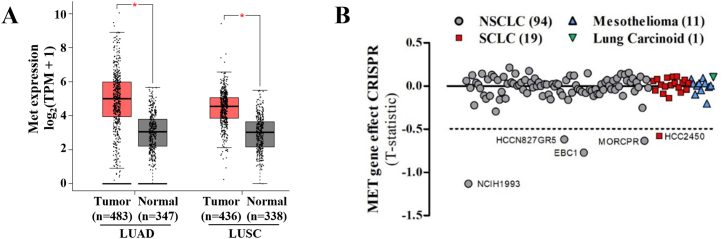
Elevated expression and genetic alterations of diverse signaling pathways, including growth factor receptor tyrosine kinases, TGFβ signaling, PI3K, and small GTPases and their regulators have been linked to cancer progression and shorter patient survival [9,40,41]. To address whether Met expression correlates with shorter survival of lung cancer patients, we analyzed the TCGA datasets in The Human Protein Atlas (https://www.proteinatlas.org/) and cBioportal (https://www.cbioportal.org/) platforms. Consistent with a negative clinical impact, high expression of MET in patients with non-small cell lung cancer, NSCLC (Fig. 1H), particularly in lung adenocarcinoma (LUAD, Fig. S1E), but not in patients with lung squamous cell carcinoma (LUSC, Fig. S1F), significantly correlated with shorter survival of lung cancer patients. In addition, the high expression of HGF, PREX1, correlated with poor prognostic in LUSC (Fig. S1A-C and Fig. S1G-I), meanwhile RAC1 in LUAD (Fig. S1J-L). In addition, according to GTEx databases http://gepia.cancer-pku.cn/), Met receptors were significantly more expressed in tumors compared to normal tissues of lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) patients (Fig. S2A). Furthermore, genome-wide loss-of-function screening datasets, analyzed at the DEPMAP platform (https://depmap.org/portal/depmap/), revealed the Met was essential in various lung cancer cell lines (Fig. S2B). The cell lines shown below −0.5 statistic value show high sensitivity to the CRISPR of the MET gene, evidencing the essentiality of this receptor in lung cancer lines. These analyses suggest that HGF/Met/P-Rex1/Rac signaling pathway plays a significant role in lung cancer progression, which might be exacerbated by extracellular factors controlling HGF availability and effect on Met receptors.
3.3. Soluble BG enhances HGF-induced migration of lung cancer and endothelial cells
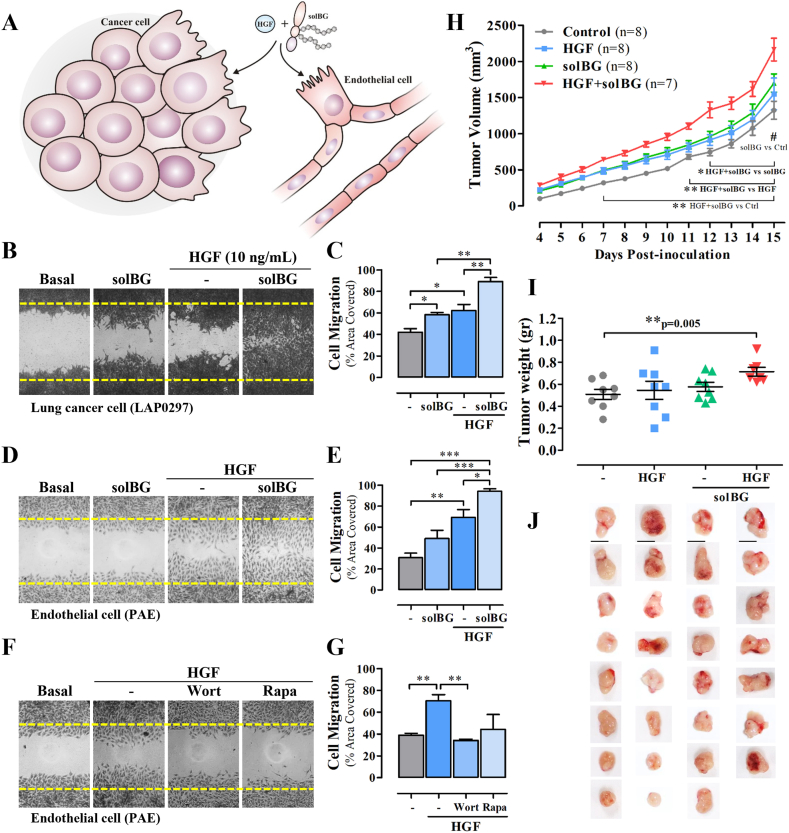
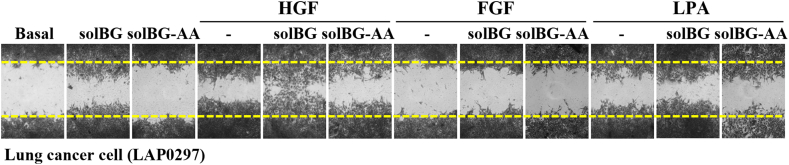
We hypothesized that solBG might enhance HGF signaling, as it contains glycosaminoglycans known to facilitate HGF signaling via Met [30,[42], [43], [44], [45]]. Therefore, we investigated whether solBG affected HGF-driven cancer and endothelial cell migration (Fig. 2A). Using LAP0297 lung cancer cells and PAE endothelial cells, we evaluated the migratory effect of HGF, alone or in combination with a GAG-containing recombinant solBG. Importantly, for this work, solBG was produced in HEK293 cells, therefore, it is endowed with GAG chains, differing in this regard from the baculoviral solBG that has been extensively used before [25]. We found that solBG by itself induces cell migration but significantly increased the effect produced by HGF in both cell types, lung cancer cells (Fig. 2B and C), and endothelial cells (Fig. 2D and E). In contrast, solBG did not affect cell migration induced by LPA or FGF (Fig. S3), suggesting a specific action on HGF signaling. This is consistent with the results of preliminary experiments in endothelial cells showing that solBG potentiates the effect of HGF on ERK and AKT activation, but not the effect of other agonists (not shown). As in the case of lung cancer cells (Fig. 1F), HGF-driven endothelial cell migration required PI3K and mTOR signaling, as indicated by the inhibitory effect of wortmannin and rapamycin (Fig. 2F and G).
Fig. 2.
Soluble Betaglycan enhances HGF-induced migration of lung cancer and endothelial cells and tumor growth in immunocompetent mice. A) Hypothetical role of soluble Betaglycan (solBG) as a promoter of lung cancer and endothelial cell migration stimulated by HGF. B-E) Effect of solBG on cell migration of LAP0297 lung cancer cells (B and C) and PAE endothelial cells (D and E). Results are representative of 3–4 independent experiments. Data represent the mean ± SEM of 3 (C) and 4 (E) independent experiments, *p < 0.05, **p < 0.01, ***p < 0.001. F) Effect of wortmannin (Wort) and rapamycin (Rapa) on PAE endothelial cell migration. The result is representative of 3 independent experiments. G) Data represent the mean ± SEM of 3 experiments, **p < 0.01, t-test. H) Effect of HGF and solBG on lung cancer tumor growth in immunocompetent mice. FVB mice were inoculated with 106 LAP0297 cells suspended in Matrigel with [10 ng/100 μL] HGF, [1 μg/100 μL] solBG, or both. Tumor volume was monitored for two weeks. Graph represents tumor size from 7 to 8 mice by group, *p < 0.05 (HGF + solBG vs solBG), **p < 0.01 (HGF + solBG vs HGF), ***p < 0.001 (HGF + solBG vs Control), #p < 0.05 (solBG vs Control), two-way repeated measures ANOVA followed of Holm Sidak test. I) The weight of excised tumors (shown in J) was determined on day 15th. Graph represents data of tumor from 7 to 8 mice by group, **p = 0.005, t-test.
3.4. BG co-inoculated with HGF enhances tumor growth
Given the positive effect of solBG on the cellular effects of HGF, we hypothesized that a tumor microenvironment containing solBG combined with HGF would increase tumor growth. To address this possibility, syngenic LAP0297 lung cancer cells were suspended in Matrigel with with solBG and HGF, then inoculated in FVB immunocompetent mice, and their combined effect was compared with the effect of only solBG, HGF or vehicle. Tumor growth was monitored for two weeks. Consistent with a protumoral effect of the combined action of solBG and HGF, bigger tumors grew under the combined effect of solBG and HGF, showing a statistically significant increase from day 7–15 (Fig. 2H). At the end of the experiment, tumors grown with solBG plus HGF reached more weight than all other conditions (Fig. 2I). The macroscopic appearance of excised tumors is shown in Fig. 2J.
3.5. solBG’s GAGs enhance HGF-driven lung cancer cell migration via Met
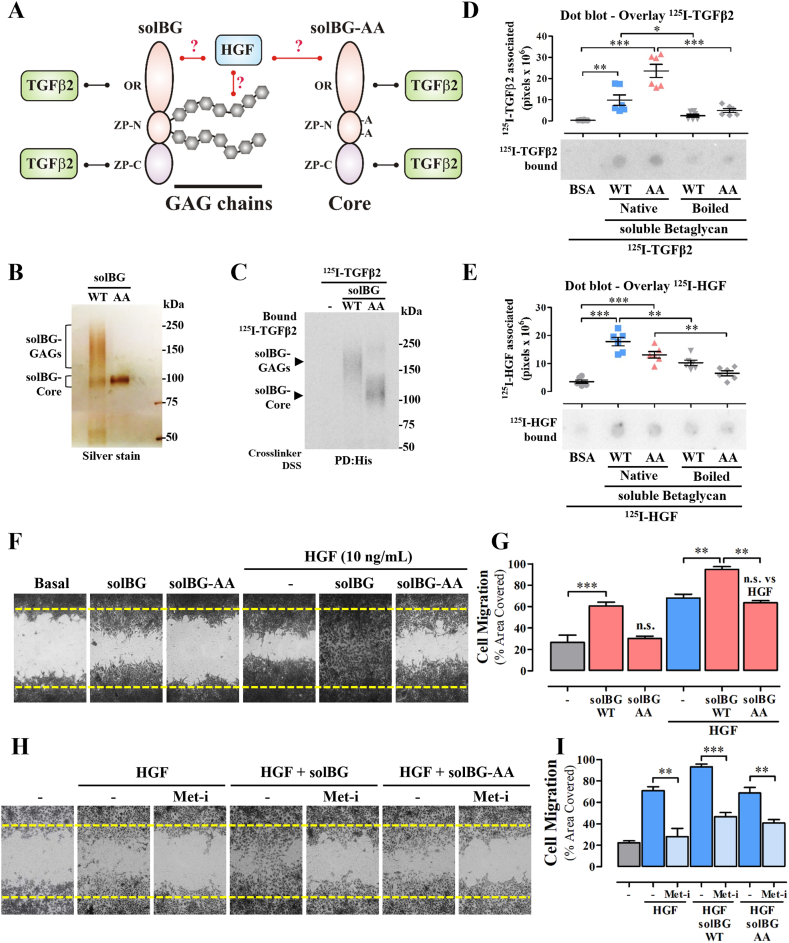
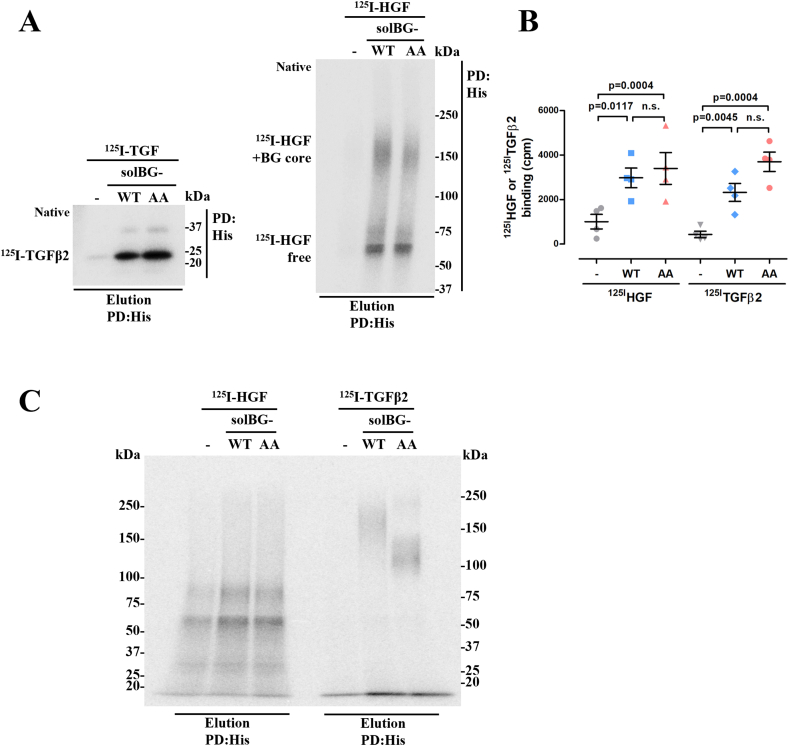
The mechanism by which solBG enhanced HGF-dependent cell migration and their combined action in tumor growth was hypothetically mediated by a biochemical interaction between solBG and HGF, likely involving the participation of the solBG’s GAG chains. In contrast, the well characterized role of BG as a TGFβ coreceptor [19] is directly attributable to the solBG core protein (Fig. 3A). To investigate whether there is a biochemical interaction between solBG and HGF, and the potential role played by solBG’s GAGs (Fig. 3A), we prepared a GAG-deficient solBG (hereby named solBG-AA to distinguish it from the solBG-WT). This solBG-AA was obtained from HEK293 cells transfected with a solBG cDNA with two Ser→Ala point mutations in the ZP-N region known to be the anchor sites for GAGs [19] (Fig. 3A). As expected, solBG-WT and solBG-AA differed in their electrophoretic mobility (Fig. 3B). Being the difference attributable to the covalently bound GAGs in the wild-type solBG, detected as a high molecular weight broad smear in the silver-stained gel in contrast to the sharp band corresponding to the core protein lacking GAGs, observed in the mutant solBG-AA (Fig. 3B). We then analyzed the potential biochemical interaction of WT and GAGs-deficient solBGs with HGF, in comparison with the well-established interaction with TGFβ2. As expected, WT and mutant solBG were both able to interact with 125I-labeled TGFβ2, revealed by pulldown of the crosslinked complex (Fig. 3, Fig. 4S), indicating that both recombinant solBGs had proper functional conformation. However, no significant crosslinking between soluble BG and HGF was detectable by this method, maybe because conventional crosslinkers do not perform efficiently on glycosaminoglycans, as shown in Fig. 4S. Considering that perhaps a solid phase was required, as it would be expected from the functional effect of solBG on HGF signaling, likely occurring at the plasma membrane, and perhaps involving the HGF receptor Met, we used immobilized solBG to address its potential interaction with HGF in dot blot assays. Compared to the binding of TGFβ, which showed a significant interaction with native solBG (both WT and AA), but not with denatured solBG (Fig. 3D), in the case of HGF, we detected a significant interaction with native wild type solBG (Fig. 3E), which was decreased with denatured (boiled) solBG, and further reduced with GAGs-free denatured solBG (Fig. 3E). Intriguingly, GAGs-free native solBG-AA exhibited a significant interaction with HGF (Fig. 3E, third spot). These results indicated that not only the solBG GAGs are important for the interaction with HGF, but also the native conformation of the immobilized core protein plays a relevant role, likely providing, in wild type solBG, a spatial orientation to the GAG chains suitable for the best interaction of HGF, as it was the most effective HGF binding partner (Fig. 3E). However, it must be kept in mind that the functional interaction between HGF and solBG that enhances HGF-dependent effects is likely influenced by additional molecular elements that are not present in the in vitro system with purified proteins. Thus, the biochemical interaction suggested by the dot-blot approach is limited and raises important questions that warrant future studies focusing on the structural basis and dynamics of the interaction between HGF and solBG, as well as the role played by the Met receptor and potentially other molecules involved in the functional interaction needed to increase cell migration and tumor growth.
Fig. 3.
Betaglycan GAGs enhance HGF-driven lung cancer cell migration via Met. A) Structural organization of soluble BG showing the hypothetical interaction of HGF with the GAG chains, absent in the solBG-AA mutant. The interaction with TGFβ2 is known to occur with the core protein [19]. B) Electrophoretic analysis of wild type and GAGs-deficient solBG. Soluble BG was isolated from the culture media of HEK293 cells engineered to secrete fully posttranslationally modified solBG (solBG WT) or GAGs-deficient mutant solBG (solBG AA). C) Pulldown analysis of the interaction between soluble BG and TGFβ2. Recombinant wild-type and GAGs-deficient solBG (WT and AA) were incubated with 125I-labeled TGFβ2, crosslinked, subjected to pulldown analysis followed by SDS_PAGE and autoradiography. D-E) Dot blot analysis of immobilized solBG incubated with 125I-labeled TGFβ2 (D) or 125I-labeled HGF (E). Recombinant wild type or GAGs-deficient solBG (WT and AA) in their native form or denatured (Boiled) were immobilized by dot blotting on PVDF membranes, blocked with 5 % milk/TBS-T and incubated with 125I-labeled TGFβ2 (D) or HGF (E). Bovine serum albumin (BSA) was used as negative control. Graphs represent mean ± SEM of 6 independent experiments *p < 0.05, **p < 0.01, ***p < 0.001, t-test. F) Effect of GAGs-deficient solBG on lung cancer cell migration stimulated by HGF. LAP0297 lung cancer cells were subjected to migration analysis in the presence of HGF, solBG, or GAGs-deficient solBG (solBG-AA), as indicated in the Figure. Area covered by migrating cells, compared to basal migration, was analyzed after 18 h. At the end of the experiment, cells were fixed and stained with crystal violet. Results are representative of 3 independent experiments. G) Quantitative analysis of LAP0297 cell migration stimulated with HGF, solBG or GAGs-deficient solBG (solBG-AA), alone or combined, as indicated. Graph represents mean ± SEM of 3 independent experiments, **p < 0.01, ***p < 0.001, t-test. H) Inhibition of Met prevents the migratory effect of HGF alone or combined with solBG. LAP0297 cells were preincubated with 1 μM, PF-04217903 (Met receptor inhibitor, Met-i) for 2 h and during the migration period (18 h). Cell migration was stimulated with HGF in the presence or not of solBG or GAGs-deficient solBG as indicated in the figure. Area covered by migrating cells was compared to the basal migration shown in the first picture. The result is representative of 3 experiments. I) Quantitative analysis of LAP0297 cell migration promoted by HGF and solBG (wild type and GAGs-deficient AA mutant), addressing with PF-04217903 (Met receptor inhibitor, Met-i) the participation of Met tyrosine kinase. Graph represents mean ± SEM of 3 independent experiments, **p < 0.01, ***p < 0.001, t-test. Note bene: uncropped versions of the original Fig. 3B,C,D,E, are shown in Supplementary Fig. 5S.
The functional role of solBG GAG chains on HGF-driven lung cancer cell migration was assayed by comparing the effect of solBG-WT with the one caused by solBG-AA, the mutant lacking GAGs. Consistent with a critical role attributable to BG’s GAGs, the positive effect of solBG-WT, enhancing cell migration caused by HGF, was lost when solBG-AA was used (Fig. 3F and G). To confirm that HGF-driven cell migration was mediated by Met receptors, cells were incubated before and during the migration assays with PF-04217903, a specific Met inhibitor (Met-i). The migratory effect of HGF, either alone or combined with solBG, was blocked when Met receptor was inhibited (Fig. 3H and I), further confirming that the positive effect of solBG’s GAG chains on HGF signaling was mediated by Met.
3.6. solBG enhances membrane recruitment of P-Rex1 stimulated by HGF
Given that Met is a potent activator of cell migration guided through the spatiotemporal activation of the Rac GTPase [46,47], and HGF activates Rac in a P-Rex1-dependent manner [11], we addressed whether this pathway was active in LAP0297 lung cancer cells and investigated whether P-Rex1 recruitment to the plasma membrane, induced by HGF, was enhanced by soluble BG (Fig. 4A). Consistent with this possibility, HGF activated P-Rex1 (Fig. 4B), which was isolated with recombinant GST-Rac G15A beads, a nucleotide-free GTPase with high affinity for active RacGEFs [48]. In addition, HGF promoted transient recruitment of P-Rex1 to the membrane fraction (Fig. 4C), which was more pronounced when solBG was combined with HGF (Fig. 4D), coinciding with a higher effect on Rac activation, as indicated by pulldown with GST-PAK-CRIB (Fig. 4E).
3.7. Soluble BG enhances the activation of Met by HGF, promoting persistent signaling
To directly address whether Met was more effectively activated by HGF in the presence of solBG, we stimulated LAP0297 lung cancer cells with HGF alone or combined with solBG-WT, for 15 and 30 min, and immunoprecipitated Met. The phosphorylation status of the active receptor was revealed with antibodies detecting pY1234/Y1235 within the catalytic tyrosine kinase domain of Met [46]. The combination of HGF with solBG increased the active state of Met receptor (Fig. 5A). Met phosphorylation promoted by HGF alone or combined with solBG was due to its own tyrosine kinase catalytic activity, as demonstrated by the inhibitory effect of its specific inhibitor (Fig. 5B). Given that all the previously analyzed effects of HGF were more prominent in the presence of solBG, we analyzed whether Met signaling to Akt and Erk were more effective and persistent in cells stimulated with HGF combined with solBG. Time course experiments revealed that both pAkt (Fig. 5C, phosphor-AKT (Ser473), the mTORC2 phosphorylation site) and pErk (Fig. 5D) reached higher levels and were more persistent when HGF was applied together with solBG.
4. Discussion
HGF and its receptor, the tyrosine kinase Met, have been recognized as drivers of tumor growth and metastatic dissemination of various cancer types [46,[49], [50], [51]]. Although their potential as drug targets is supported by preclinical studies, and targeted therapies based on antibodies and tyrosine kinase inhibitors are in clinical trials, very limited therapeutic options have been approved so far [46,49,51]. Combined therapies, based on the identification of vulnerable patients that maintain high and persistent HGF signaling due to actionable molecular coadjuvants, might contribute to the design of effective personalized therapies. Given its existence as a soluble proteoglycan within the tumor microenvironment, resulting from ectodomain shedding [18,52], and the functional role of GAGs controlling growth factor availability [26], we tested the hypothetical role of soluble BG, either containing or lacking GAGs, in the signaling effects of HGF, known as a prometastatic factor within the tumor microenvironment [46]. Our results, graphically presented in the model shown in Fig. 5E, are consistent with the idea that increased tumor growth and augmented lung cancer and endothelial cell migration are linked to the positive effect of soluble BG, which increased HGF signaling leading to persistent phosphorylation of Met and activation of its downstream pathways, including PI3K/Akt/P-Rex1/Rac and Erk, relevant in cell migration and proliferation. Since PI3K/Akt and Erk pathways were simultaneously activated by HGF and their functional crosstalk at different levels has been reported to play a role in cancer progression, particularly in the process of metastatic dissemination [53,54], we cannot rule out that some crosstalk between these signaling pathways might be involved in the functional effects of enhanced HGF signaling by betaglycan. Our findings are consistent with previous studies showing that HGF is a potent inducer of lung cancer cell migration [36]. In addition, in breast cancer cells, HGF induces Rac activation through P-Rex1 [11]. Therefore, aiming towards the long-term goal of achieving efficient personalized therapies, better effects of anti-HGF/Met therapies in cancer patients might be achieved by selecting those cases in which soluble BG is absent. In other cases, inhibition of the BG/HGF interphase or inhibition of BG posttranslational modifications can be explored as complementary alternatives.
BG plays a complex role in cancer progression. BG expression regulation has been involved in the progression or suppression of diverse malignancies by diverse mechanisms [[55], [56], [57], [58], [59]]. This duality has been evident since the pioneering works by the groups of Arteaga and Moses showed that TGFBR3 promotes tumor activity of human breast cancer cells [57,60]. Later, the notion that this duality could be ascribed to the opposing roles of membrane and soluble BG on the TGFβ modulation, led to the idea that solBG performs as a tumor suppressor. However other works have cast doubts over that simplistic view. The work by Burghardt et al. is relevant since they showed that glioblastoma cells expressing soluble BG grow larger tumors and reduce survival times when xenografted to nude mice [61]. Recombinant solBG has been used in preclinical models as a decoy receptor to prevent cancer progression due to aberrant TGFβ signaling linked to immunosuppression and mesenchymal transition [18,62]. Our present work reveals that in addition to this role, soluble BG acts also as a promoter of cancer progression via its GAG chains. Furthermore, this role unlikely is limited to HGF, as other factors, such as FGF and Wnt3a are bound by BG GAG chains [26,63]. This possibility is consistent with the marginal effect of solBG which shows some stimulatory effects on its own, perhaps acting on cell-produced HGF and likely other cell-secreted factors functionally linked to the effect of SolBG on autocrine stimulation, as indicated by the marginal effect remaining in the presence of the Met-inhibitor. Our present work provides a new and relevant facet to TGFBR3 role in cancer, namely, that its GAG status must be considered when analyzing its tumor-promoting or suppressing activities.
Our results also indicate that the use of solBG as a decoy receptor should be limited to situations where HGF is absent or plays no major role in tumor growth and dissemination. In cases in which solBG has been considered a potential therapeutic molecule to target the TGFβ pathway, higher therapeutic precision would be achieved with the GAGs-free core protein, with the idea to prevent potential TGFβ-independent effects, including those on HGF and other, previously documented, such as interactions through BG GAGs with bFGF and Wnt3a [26,63].
Our results generate new routes of research on the therapeutic potential of HGF/Met pathway and the combined effect of solBG. Given that we found that the positive effect of solBG on HGF signaling depends on Met tyrosine kinase activity, the potential assembly of a trimeric complex between solBG, HGF, and Met warrants further investigations. In addition, to understand the basis of persistent HGF/Met signaling promoted by solBG, future studies shall completely characterize the biochemical interaction of HGF and solBG. Also, determining how solBG or its GAGs affects the Met trafficking and degradation is important, as it has been mechanistically linked to the downregulation of this pathway [64].
CRediT authorship contribution statement
Rodolfo Daniel Cervantes-Villagrana: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Valentín Mendoza: Project administration, Investigation. Cynthia S. Hinck: Investigation. Rosa Luz de la Fuente-León: Investigation. Andrew P. Hinck: Resources, Conceptualization. Guadalupe Reyes-Cruz: Supervision, Resources. José Vázquez-Prado: Writing – review & editing, Supervision, Resources, Conceptualization. Fernando López-Casillas: Writing – review & editing, Supervision, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Consejo Nacional de Humanidades, Ciencia y Tecnología de México (CONAHCyT) FORDECYT-PRONACES grant 1794 (to G.R–C.) and 319283 (to J.V–P.). Work at F.L-C.‘s laboratory was supported by PAPIIT- DGAPA-UNAM’s grant # 204620. R.D.C–V. was supported by UNAM Posdoctoral Program (DGAPA-UNAM). Technical assistance was provided by Estanislao Escobar-Islas, Yazmin Torres-Santos, David Pérez-Rangel, and Jaime Estrada-Trejo.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e30520.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
figs1.
figs2.
figs3.
figs4.
References
- 1.Attwood M.M., et al. Soluble ligands as drug targets. Nat. Rev. Drug Discov. 2020;19(10):695–710. doi: 10.1038/s41573-020-0078-4. [DOI] [PubMed] [Google Scholar]
- 2.Weiss F., Lauffenburger D., Friedl P. Towards targeting of shared mechanisms of cancer metastasis and therapy resistance. Nat. Rev. Cancer. 2022;22(3):157–173. doi: 10.1038/s41568-021-00427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartolini B., et al. Heparan sulfate in the tumor microenvironment. Adv. Exp. Med. Biol. 2020;1245:147–161. doi: 10.1007/978-3-030-40146-7_7. [DOI] [PubMed] [Google Scholar]
- 4.Cox T.R. The matrix in cancer. Nat. Rev. Cancer. 2021;21(4):217–238. doi: 10.1038/s41568-020-00329-7. [DOI] [PubMed] [Google Scholar]
- 5.Jin C., et al. Decoding the spermatogonial stem cell niche under physiological and recovery conditions in adult mice and humans. Sci. Adv. 2023;9(31):eabq3173. doi: 10.1126/sciadv.abq3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19(11):1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vázquez-Prado J., et al. Gβγ pathways in cell polarity and migration linked to oncogenic GPCR signaling: potential relevance in tumor microenvironment. Mol. Pharmacol. 2016;90(5):573–586. doi: 10.1124/mol.116.105338. [DOI] [PubMed] [Google Scholar]
- 8.Cervantes-Villagrana R.D., et al. Tumor-induced neurogenesis and immune evasion as targets of innovative anti-cancer therapies. Signal Transduct. Targeted Ther. 2020;5(1):99. doi: 10.1038/s41392-020-0205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cervantes-Villagrana R.D., García-Jiménez I., Vázquez-Prado J. Guanine nucleotide exchange factors for Rho GTPases (RhoGEFs) as oncogenic effectors and strategic therapeutic targets in metastatic cancer. Cell. Signal. 2023;109 doi: 10.1016/j.cellsig.2023.110749. [DOI] [PubMed] [Google Scholar]
- 10.Lawson C.D., Ridley A.J. Rho GTPase signaling complexes in cell migration and invasion. J. Cell Biol. 2018;217(2):447–457. doi: 10.1083/jcb.201612069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cervantes-Villagrana R.D., et al. Gβγ signaling to the chemotactic effector P-REX1 and mammalian cell migration is directly regulated by Gα(q) and Gα(13) proteins. J. Biol. Chem. 2019;294(2):531–546. doi: 10.1074/jbc.RA118.006254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sosa M.S., et al. Identification of the Rac-GEF P-Rex1 as an essential mediator of ErbB signaling in breast cancer. Mol. Cell. 2010;40(6):877–892. doi: 10.1016/j.molcel.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carretero-Ortega J., et al. Phosphatidylinositol 3,4,5-triphosphate-dependent Rac exchanger 1 (P-Rex-1), a guanine nucleotide exchange factor for Rac, mediates angiogenic responses to stromal cell-derived factor-1/chemokine stromal cell derived factor-1 (SDF-1/CXCL-12) linked to Rac activation, endothelial cell migration, and in vitro angiogenesis. Mol. Pharmacol. 2010;77(3):435–442. doi: 10.1124/mol.109.060400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin J., et al. Upregulation of PIP3-dependent Rac exchanger 1 (P-Rex1) promotes prostate cancer metastasis. Oncogene. 2009;28(16):1853–1863. doi: 10.1038/onc.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindsay C.R., et al. P-Rex1 is required for efficient melanoblast migration and melanoma metastasis. Nat. Commun. 2011;2:555. doi: 10.1038/ncomms1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clements M.E., Johnson R.W. PREX1 drives spontaneous bone dissemination of ER+ breast cancer cells. Oncogene. 2020;39(6):1318–1334. doi: 10.1038/s41388-019-1064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srijakotre N., et al. PtdIns(3,4,5)P(3)-dependent Rac exchanger 1 (P-Rex1) promotes mammary tumor initiation and metastasis. Proc. Natl. Acad. Sci. U. S. A. 2020;117(45):28056–28067. doi: 10.1073/pnas.2006445117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pawlak J.B., Blobe G.C. TGF-β superfamily co-receptors in cancer. Dev. Dynam. 2022;251(1):137–163. doi: 10.1002/dvdy.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.López-Casillas F., et al. Betaglycan can act as a dual modulator of TGF-beta access to signaling receptors: mapping of ligand binding and GAG attachment sites. J. Cell Biol. 1994;124(4):557–568. doi: 10.1083/jcb.124.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S.K., Henen M.A., Hinck A.P. Structural biology of betaglycan and endoglin, membrane-bound co-receptors of the TGF-beta family. Exp Biol Med (Maywood) 2019;244(17):1547–1558. doi: 10.1177/1535370219881160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.López-Casillas F., Wrana J.L., Massagué J. Betaglycan presents ligand to the TGF beta signaling receptor. Cell. 1993;73(7):1435–1444. doi: 10.1016/0092-8674(93)90368-z. [DOI] [PubMed] [Google Scholar]
- 22.Bandyopadhyay A., et al. Systemic administration of a soluble betaglycan suppresses tumor growth, angiogenesis, and matrix metalloproteinase-9 expression in a human xenograft model of prostate cancer. Prostate. 2005;63(1):81–90. doi: 10.1002/pros.20166. [DOI] [PubMed] [Google Scholar]
- 23.Hernández-Pando R., et al. A combination of a transforming growth factor-beta antagonist and an inhibitor of cyclooxygenase is an effective treatment for murine pulmonary tuberculosis. Clin. Exp. Immunol. 2006;144(2):264–272. doi: 10.1111/j.1365-2249.2006.03049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juárez P., et al. Soluble betaglycan reduces renal damage progression in db/db mice. Am. J. Physiol. Ren. Physiol. 2007;292(1):F321–F329. doi: 10.1152/ajprenal.00264.2006. [DOI] [PubMed] [Google Scholar]
- 25.Vilchis-Landeros M.M., et al. Recombinant soluble betaglycan is a potent and isoform-selective transforming growth factor-beta neutralizing agent. Biochem. J. 2001;355(Pt 1):215–222. doi: 10.1042/0264-6021:3550215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andres J.L., et al. Binding of two growth factor families to separate domains of the proteoglycan betaglycan. J. Biol. Chem. 1992;267(9):5927–5930. [PubMed] [Google Scholar]
- 27.Hassan N., et al. Cell-surface heparan sulfate proteoglycans as multifunctional integrators of signaling in cancer. Cell. Signal. 2021;77 doi: 10.1016/j.cellsig.2020.109822. [DOI] [PubMed] [Google Scholar]
- 28.Pomin V.H. Paradigms in the structural biology of the mitogenic ternary complex FGF:FGFR:heparin. Biochimie. 2016;127:214–226. doi: 10.1016/j.biochi.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Cao T., et al. Isolation and characterization of a Chinese hamster ovary heparan sulfate cell mutant defective in both met receptor binding and hepatocyte growth factor NK1/met signaling. Cell. Physiol. Biochem. 2018;48(4):1480–1491. doi: 10.1159/000492258. [DOI] [PubMed] [Google Scholar]
- 30.Sakata H., et al. Heparin binding and oligomerization of hepatocyte growth factor/scatter factor isoforms. Heparan sulfate glycosaminoglycan requirement for Met binding and signaling. J. Biol. Chem. 1997;272(14):9457–9463. doi: 10.1074/jbc.272.14.9457. [DOI] [PubMed] [Google Scholar]
- 31.Huang P., et al. Histopathologic findings and establishment of novel tumor lines from spontaneous tumors in FVB/N mice. Comp. Med. 2008;58(3):253–263. [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S.K., et al. Structural adaptation in its orphan domain engenders betaglycan with an alternate mode of growth factor binding relative to endoglin. Structure. 2019;27(9):1427–1442.e4. doi: 10.1016/j.str.2019.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheifetz S., et al. Distinct transforming growth factor-beta (TGF-beta) receptor subsets as determinants of cellular responsiveness to three TGF-beta isoforms. J. Biol. Chem. 1990;265(33):20533–20538. [PubMed] [Google Scholar]
- 34.Chávez-Vargas L., et al. Protein kinase A (PKA) type I interacts with P-Rex1, a rac guanine nucleotide exchange factor: EFFECT on PKA localization and P-Rex1 signaling. J. Biol. Chem. 2016;291(12):6182–6199. doi: 10.1074/jbc.M115.712216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castillo-Kauil A., et al. Gα(s) directly drives PDZ-RhoGEF signaling to Cdc42. J. Biol. Chem. 2020;295(50):16920–16928. doi: 10.1074/jbc.AC120.015204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cervantes-Villagrana R.D., et al. Protumoral bone marrow-derived cells migrate via Gβγ-dependent signaling pathways and exhibit a complex repertoire of RhoGEFs. J Cell Commun Signal. 2019;13(2):179–191. doi: 10.1007/s12079-018-00502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.García-Jiménez I., et al. Gβγ mediates activation of Rho guanine nucleotide exchange factor ARHGEF17 that promotes metastatic lung cancer progression. J. Biol. Chem. 2022;298(1) doi: 10.1016/j.jbc.2021.101440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarbassov D.D., et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 39.Hernández-Negrete I., et al. P-Rex1 links mammalian target of rapamycin signaling to Rac activation and cell migration. J. Biol. Chem. 2007;282(32):23708–23715. doi: 10.1074/jbc.M703771200. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen B., et al. Genomic characterization of metastatic patterns from prospective clinical sequencing of 25,000 patients. Cell. 2022;185(3):563–575.e11. doi: 10.1016/j.cell.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez-Vega F., et al. Oncogenic signaling pathways in the cancer genome Atlas. Cell. 2018;173(2):321–337.e10. doi: 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kemp L.E., Mulloy B., Gherardi E. Signalling by HGF/SF and Met: the role of heparan sulphate co-receptors. Biochem. Soc. Trans. 2006;34(Pt 3):414–417. doi: 10.1042/BST0340414. [DOI] [PubMed] [Google Scholar]
- 43.Lyon M., Deakin J.A., Gallagher J.T. The mode of action of heparan and dermatan sulfates in the regulation of hepatocyte growth factor/scatter factor. J. Biol. Chem. 2002;277(2):1040–1046. doi: 10.1074/jbc.M107506200. [DOI] [PubMed] [Google Scholar]
- 44.Rubin J.S., et al. Dissociation of heparan sulfate and receptor binding domains of hepatocyte growth factor reveals that heparan sulfate-c-met interaction facilitates signaling. J. Biol. Chem. 2001;276(35):32977–32983. doi: 10.1074/jbc.M105486200. [DOI] [PubMed] [Google Scholar]
- 45.Zioncheck T.F., et al. Sulfated oligosaccharides promote hepatocyte growth factor association and govern its mitogenic activity. J. Biol. Chem. 1995;270(28):16871–16878. doi: 10.1074/jbc.270.28.16871. [DOI] [PubMed] [Google Scholar]
- 46.Comoglio P.M., Trusolino L., Boccaccio C. Known and novel roles of the MET oncogene in cancer: a coherent approach to targeted therapy. Nat. Rev. Cancer. 2018;18(6):341–358. doi: 10.1038/s41568-018-0002-y. [DOI] [PubMed] [Google Scholar]
- 47.Ménard L., Parker P.J., Kermorgant S. Receptor tyrosine kinase c-Met controls the cytoskeleton from different endosomes via different pathways. Nat. Commun. 2014;5:3907. doi: 10.1038/ncomms4907. [DOI] [PubMed] [Google Scholar]
- 48.García-Mata R., et al. Analysis of activated GAPs and GEFs in cell lysates. Methods Enzymol. 2006;406:425–437. doi: 10.1016/S0076-6879(06)06031-9. [DOI] [PubMed] [Google Scholar]
- 49.Fu J., et al. HGF/c-MET pathway in cancer: from molecular characterization to clinical evidence. Oncogene. 2021;40(28):4625–4651. doi: 10.1038/s41388-021-01863-w. [DOI] [PubMed] [Google Scholar]
- 50.Sim W.J., et al. c-Met activation leads to the establishment of a TGFβ-receptor regulatory network in bladder cancer progression. Nat. Commun. 2019;10(1):4349. doi: 10.1038/s41467-019-12241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y., et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol. Cancer. 2018;17(1):45. doi: 10.1186/s12943-018-0796-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elderbroom J.L., et al. Ectodomain shedding of TβRIII is required for TβRIII-mediated suppression of TGF-β signaling and breast cancer migration and invasion. Mol. Biol. Cell. 2014;25(16):2320–2332. doi: 10.1091/mbc.E13-09-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao Z., et al. AKT and ERK dual inhibitors: the way forward? Cancer Lett. 2019;459:30–40. doi: 10.1016/j.canlet.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 54.Lin S., et al. Myeloid-derived suppressor cells promote lung cancer metastasis by CCL11 to activate ERK and AKT signaling and induce epithelial-mesenchymal transition in tumor cells. Oncogene. 2021;40(8):1476–1489. doi: 10.1038/s41388-020-01605-4. [DOI] [PubMed] [Google Scholar]
- 55.Gong Q., et al. CUL4B enhances the malignant phenotype of esophageal squamous cell carcinoma by suppressing TGFBR3 expression. Biochem. Biophys. Res. Commun. 2023;676:58–65. doi: 10.1016/j.bbrc.2023.07.037. [DOI] [PubMed] [Google Scholar]
- 56.Hou X., et al. HELLS, a chromatin remodeler is highly expressed in pancreatic cancer and downregulation of it impairs tumor growth and sensitizes to cisplatin by reexpressing the tumor suppressor TGFBR3. Cancer Med. 2021;10(1):350–364. doi: 10.1002/cam4.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jovanović B., et al. Transforming growth factor beta receptor type III is a tumor promoter in mesenchymal-stem like triple negative breast cancer. Breast Cancer Res. 2014;16(4):R69. doi: 10.1186/bcr3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang N., et al. Inhibiting microRNA-424 in bone marrow mesenchymal stem cells-derived exosomes suppresses tumor growth in colorectal cancer by upregulating TGFBR3. Arch. Biochem. Biophys. 2021;709 doi: 10.1016/j.abb.2021.108965. [DOI] [PubMed] [Google Scholar]
- 59.Zhang X., et al. TGFBR3 is an independent unfavourable prognostic marker in oesophageal squamous cell cancer and is positively correlated with Ki-67. Int. J. Exp. Pathol. 2020;101(6):223–229. doi: 10.1111/iep.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Criswell T.L., et al. Knockdown of the transforming growth factor-beta type III receptor impairs motility and invasion of metastatic cancer cells. Cancer Res. 2008;68(18):7304–7312. doi: 10.1158/0008-5472.CAN-07-6777. [DOI] [PubMed] [Google Scholar]
- 61.Burghardt I., et al. A tumor-promoting role for soluble TβRIII in glioblastoma. Mol. Cell. Biochem. 2021;476(8):2963–2973. doi: 10.1007/s11010-021-04128-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang J.J., et al. Increased type III TGF-β receptor shedding decreases tumorigenesis through induction of epithelial-to-mesenchymal transition. Oncogene. 2019;38(18):3402–3414. doi: 10.1038/s41388-018-0672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jenkins L.M., et al. Altering the proteoglycan state of transforming growth factor β type III receptor (TβRIII)/Betaglycan modulates canonical Wnt/β-Catenin signaling. J. Biol. Chem. 2016;291(49):25716–25728. doi: 10.1074/jbc.M116.748624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bell E.S., et al. LC3C-Mediated autophagy selectively regulates the met RTK and HGF-stimulated migration and invasion. Cell Rep. 2019;29(12):4053–4068.e6. doi: 10.1016/j.celrep.2019.11.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.