Abstract
Environmental pollution is escalating due to rapid global development that often prioritizes human needs over planetary health. Despite global efforts to mitigate legacy pollutants, the continuous introduction of new substances remains a major threat to both people and the planet. In response, global initiatives are focusing on risk assessment and regulation of emerging contaminants, as demonstrated by the ongoing efforts to establish the UN’s Intergovernmental Science-Policy Panel on Chemicals, Waste, and Pollution Prevention. This review identifies the sources and impacts of emerging contaminants on planetary health, emphasizing the importance of adopting a One Health approach. Strategies for monitoring and addressing these pollutants are discussed, underscoring the need for robust and socially equitable environmental policies at both regional and international levels. Urgent actions are needed to transition toward sustainable pollution management practices to safeguard our planet for future generations.
Graphical abstract
Public summary
-
•
The global economy stimulates the continuous production and release of new chemical and biological agents that challenge global health and sustainability.
-
•
Integrating green and benign-by-design principles into production processes is crucial for eliminating hazardous materials from global supply chains.
-
•
Emerging environmental management practices are essential for environmental restoration and promoting global health and sustainability.
-
•
This review explores the sources of emerging contaminants and their impact on planetary health, with an emphasis on source control and remediation strategies.
-
•
Adopting a One Health approach through interdisciplinary collaboration is essential for addressing emerging contaminants and their complex impacts.
Introduction
Before the Industrial Revolution, naturally occurring pathogens, including bacteria, fungi, and viruses, were the primary contaminants of concern, presenting threats to both human and ecosystem health.1 However, industrialization brought about significant changes in pollution patterns, introducing new contaminants into the environment such as heavy metals, industrial chemicals, and particulate matter. With the onset of the Anthropocene, humans have increasingly depleted natural resources and developed new chemical molecules, or novel entities, in pursuit of global development, resulting in waste streams transgressing planetary boundaries, disrupting natural ecosystems,2,3 and inducing changes in agricultural practices, which led to the evolution of wild-type pathogens.4 Various geogenic chemicals, encompassing metal(loid)s and other hazardous substances, are consistently discharged into the environment through diverse anthropogenic activities such as mining, mineral processing, energy production, construction, and agriculture.5
Beyond geogenic chemicals, the production of synthetic chemicals has surged since the mid-twentieth century, marking what is often referred to as the second chemical revolution (i.e., unprecedented development and use of novel synthetic chemicals).6 This surge is shown by the rapid growth of the Chemical Abstract Service Registry, which grew from 20 million in 2002 to over 204 million by 2023, suggesting an addition of nearly 15,000 new chemicals daily.7 Moreover, there has been a significant rise in efforts to genetically modify microorganisms.8,9 While synthetic chemicals and genetically engineered microorganisms have contributed positively to human well-being by facilitating the development of new drugs and advanced materials and enhancing agricultural productivity, concerns have been raised over their risks to public health and the environment. Persson et al.10 recently highlighted that humanity has exceeded the planetary boundary, or safe operating space, for anthropogenic chemicals, as the rate of chemical production outpaces the rate of hazard assessments and the establishment of regulatory measures. Similarly, Bernhardt et al.11 argued that synthetic chemicals are agents of global change.
Emerging contaminants (ECs), also referred to as contaminants of emerging concern (CECs), are newly identified synthetic or naturally occurring chemicals or biological agents that are detected in the environment and potentially hazardous or recently determined to be hazardous to humans and ecosystems. The risks associated with these contaminants are not fully understood. They may include pharmaceuticals and personal care products (PPCPs), per- and poly-fluoroalkyl substances (PFAS), emerging pathogens, cyanotoxins and other natural toxins, pesticides, industrial chemicals, micro/nano plastics, nanomaterials, antibiotic resistance genes (ARGs), and other exogenous substances that are found in the environment but are not yet well understood in terms of their impacts on humans and natural ecosystems.12,13,14 These contaminants can enter the environment through various pathways, such as industrial discharge, agricultural runoff, and improper waste disposal, leading to air, water, soil, and food contamination. They can become part of complex mixtures of chemical pollutants and biological hazards.7 Furthermore, these ECs have the potential to undergo additional transformation and long-range transport, creating unforeseen and uncharacterized chemicals and causing chemical pollution in areas distant from the source.15
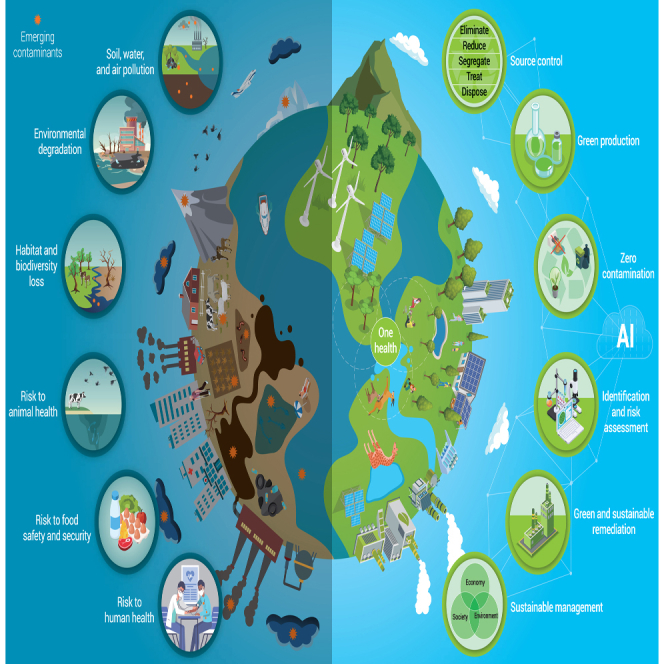
Pollution continues to pose a significant global threat, resulting in millions of premature deaths annually16,17 and widespread environmental degradation.5 Concurrently, thousands of species are facing extinction. These alarming challenges underscore the pressing need for comprehensive strategies to address the interconnected environmental and human health issues.18 Adopting a One Health perspective recognizes the interconnectedness of human health, animal health, and the environment, emphasizing the need for collaborative efforts to address EC issues. By leveraging expertise from various fields such as medicine, veterinary science, environmental science, and public health, integrated approaches will reduce risks linked to ECs and enhance the well-being of all organisms. In addition, it is timely to integrate an Indigenous world-view as the One Health concept is deeply embedded in their wider relationships with the environment. Indigenous people consider that all animate and inanimate, physical and metaphysical phenomena are connected through elaborate and complex webs of relationships.19 While focusing on ECs is crucial, dealing with existing legacy pollutants is equally important. Innovative approaches such as green chemistry, machine learning, and interdisciplinary cooperation are essential to overcome these challenges. Moreover, educational reforms are crucial to preparing future generations to effectively address environmental and health crises.20
In this review, we provide a holistic perspective on ECs, which are recognized as significant threats to human health and the sustainability of ecosystems. Through the One Health approach lens, we acknowledge the intricate connections among the health of people, animals, plants, and our shared environment. Our focus encompasses the production, utilization, and dissemination of ECs in everyday life, emphasizing their potential adverse effects, whether encountered individually or with other pollutants. These effects span various environments, affecting human health and the well-being of animals, plants, and microorganisms. We investigate methods for detecting and analyzing ECs, critically assess regulatory frameworks and policies, and propose innovative solutions to reduce their detrimental impacts on human and environmental health. By adopting the One Health approach, we underscore the necessity for a collaborative, multisectoral, and transdisciplinary response to effectively address challenges posed by ECs and to promote a sustainable and healthy future for all forms of life.
Historical perspective of ECs
Since the mid-twentieth century, the global socio-economic landscape has undergone a profound transformation, marked by a surge in industrial activity and technological advancement. This period has seen a dramatic rise in the extraction and utilization of natural resources, particularly critical minerals and petrochemicals, which are indispensable for expanding industrial sectors and the broader modernization process. The repercussions of this intensified resource exploitation have been far reaching, leading to modifications in geochemical cycles and the distribution of metals.21 Moreover, this era has been characterized by the synthesis, use, and release of novel chemical compounds, many of which persist in the environment and have the potential to accumulate biologically, thus emerging as new environmental contaminants.22
The toxicity of metal(loid)s, such as lead, mercury, cadmium, arsenic, cobalt, and chromium, as well as organic pollutants such as dichlorodiphenyltrichloroethane (DDT) and polychlorinated biphenyls (PCBs), has long been recognized.5 Some of these pollutants have been banned or had limits imposed on their use due to their adverse environmental and health effects, prompting efforts to regulate their concentrations in water, soils, and other environmental media.23 Although much is understood about legacy contaminants, ongoing advances in analytical technology and toxicology continue to reveal new risks to human health and the environment posed by ECs, enabling a better understanding of the sources, persistence, bioaccumulation potential, mobility, and toxicity of such contaminants.
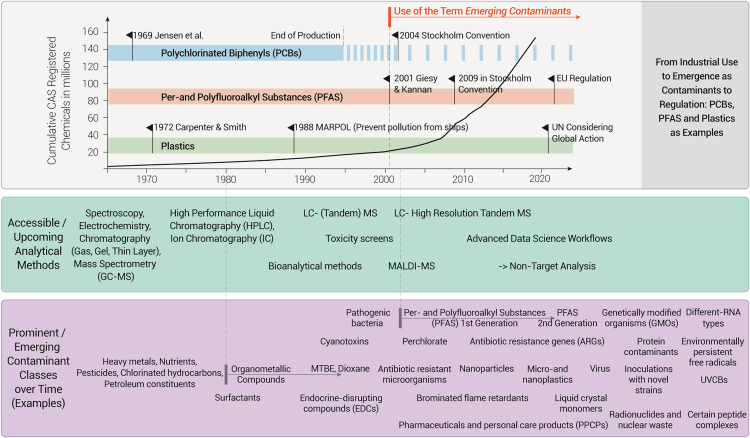
The increasing focus on environmental pollution has led to the identification of substances that have transitioned from being celebrated as beneficial chemicals to contaminants of significant concern. Examples of such evolving contaminants include plastics and their by-products, atrazine, triphenyl phosphate, tungsten, PFAS, chlorofluorocarbons, neonicotinoids, glyphosate, and many others (Table 1). This evolution is attributed to improved detection capabilities for inorganic and organic contaminants at trace levels and a better understanding of their wider ecosystem and health effects (Figure 1).
Table 1.
List of prominent ECs categorized based on their current attention and potential concern
 |
The table categorizes ECs into three groups: those currently in the spotlight (highlighted in blue), those with potential concern but less current attention (highlighted in purple), and contaminants of the past that are now emerging with renewed concern. Some ECs have been identified for control by various environmental regulatory agencies, including the Ministry of Ecology and Environment of the People’s Republic of China,32 the European Union,29 and the US EPA.37 It is important to note that this table provides only a selection of examples for each category, and there are many more ECs within each group.29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49
Figure 1.
The evolution of ECs in relation to the advances in the detection and tracking of potentially toxic chemicals in the environment or biological systems, even at trace levels
Since the early 2000s, the term ECs has been used to describe the discovery of new pollutant classes. Polychlorinated biphenyls (PCBs), per- and polyfluoroalkyl substances (PFAS), and plastics exemplify problematic substances that were in use for decades (gray bars) but emerged as contaminants (pins) and were regulated and discontinued (faded-out shadow) with different lag times. Arrows in the lower panel indicate ECs that originated as replacements for other pollutants.
Nowadays, significant attention has been devoted to addressing a wide array of ECs, which nowadays extends beyond newly introduced substances to include CECs, which have been present for some time but have recently garnered attention due to their potential impacts. As of February 2024, the US Environmental Protection Agency (EPA) Toxic Substances Control Act (TSCA) Chemical Substance Inventory contains 86,741 potentially hazardous chemicals, with 42,293 currently commercially active.24 Additionally, as a network of reference laboratories, research centres and related organisations for monitoring of emerging environmental substances, the NORMAN has identified over 700 of the most discussed ECs.25 Further, Wang et al.1 identified that over 350,000 chemicals and chemical mixtures have been registered for commercial use around the world. The continuous expansion of these inventories is expected due to the ongoing discovery of new substances and increased scrutiny of existing ones. Herein, a One Health approach is particularly relevant to the assessment and management of ECs.26,27,28 In the subsequent sections, we will focus on prominent emerging contaminants categorized based on their current attention and potential concern (Table 1).
Production, use, and environmental release of ECs
Production and use of ECs
Over the last century, global population growth, fueled by industrialization and urbanization, has spurred increased demand for consumer goods. Consequently, industries producing these goods, such as pharmaceuticals, household products, and plastics, have expanded significantly.50 The extensive use and improper disposal of these products have led to their omnipresence in the natural environment, causing continuous contamination with potentially harmful chemicals from diverse sources.51 Taking plastics as an example, their global production has surged to 460 million tons (Mt) in 2019 from 234 Mt in 2000, resulting in a doubling of plastic waste generation over the past two decades.52 This increase in plastic production and consumption has contributed to the proliferation of micro/nano-plastics in various ecosystems. While microplastics (MPs) only account for 12% of plastic waste in the natural environment, they are of significant concern because of their potential long-term impacts on ecosystems and organisms.52,53 Over time, larger plastic particles can break down into micro/nano-plastics through mechanical action and biological fragmentation (including microbial degradation and grind by metazoa during ingestion), leading to the continuous accumulation of these particles.54,55,56 This pollution is considered irreversible due to the lasting environmental impact long after the elimination of plastic emission sources.56
PPCPs represent one of the largest groups of ECs, encompassing a wide array of compounds with diverse chemical and physical properties. These substances are commonly used in daily life for various purposes, including human and animal healthcare. With over 50,000 different types of PPCPs currently produced and approximately 30 million metric tons used globally, the prevalence of these compounds may be increasing annually.57 Pharmaceuticals as the main components of PPCPs include numerous types of drugs and their metabolites, such as antibiotics (for both humans and livestock), hormones, non-steroidal anti-inflammatory drugs, anticancer drugs, antiepileptics, antidepressants, and β-blockers.58 Among these biologically active substances, antibiotics have emerged as the most commonly reported PPCPs over the past few decades,59,60 with the global consumption rate increasing from 9.8 to 14.3 defined daily doses per 1,000 population per day between 2000 and 2018.61 The increasing prevalence of associated ARGs is a well-documented health concern and is now recognized as a prominent global threat to public health.62 Besides pharmaceuticals, PPCPs also encompass various chemicals in body lotions, disinfectants, eye care, hair care, handwash, insect repellent, lipsticks, moisturizers, fragrances, shampoo, soaps, sunscreen creams, and plasticizers used in product packaging and lining63 and PFAS compounds added to cosmetics.64
Over time, advancements in knowledge and analytical methods have led to the detection of risks associated with various chemicals. During the recent COVID-19 pandemic, 64.7% of respondents never disinfected their hands using sanitizers before the COVID-19 outbreak, but 91.0% disinfect their hands at least twice per day after the COVID-19 outbreak.65 Therefore, particular attention has been paid to biocides found in disinfectants.66 With the implementation of advanced analytical instruments such as high-resolution mass spectrometry (HRMS) and artificial intelligence (AI) techniques, the potential risks posed by a broader range of PPCPs are expected to be uncovered PPCPs in the future. After production and application, PPCPs are primarily introduced to the environment directly or indirectly through the discharge of raw sewage or treated effluents of various quality from wastewater treatment, animal husbandry, animal manure, or municipal treatment plant sludge as fertilizer, and landfill leachate.67,68,69 In fact, the presence of PPCPs in surface water has become an indicator of an urbanizing water cycle.70
Engineered nanoparticles (ENPs), one of the most typical ECs, were included in the list of ECs by EPA in 2010.71 ENPs such as carbon nanoparticles (NPs),72 TiO2 NPs,73 and hydroxyapatite74 are widely incorporated in a diverse range of consumable goods, including commercial cosmetics, sporting goods, sunscreen, and toothpaste. In terms of global production, SiO2 NPs and TiO2 NPs were the largest, followed by AlOx NPs, CeO2 NPs, FeOx NPs, and ZnO NPs, carbon nanotubes (CNTs) (100–1,000 tons per year [t year−1] in 2010) and AgO NPs (55 t year−1 in 2010).75 The increasing application of ENPs in consumer products has caused their increased occurrence in the natural ecosystems.76
Pathways for environmental release of ECs
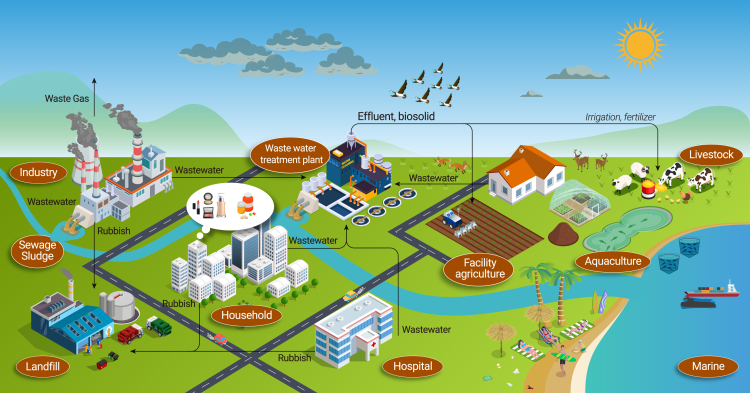
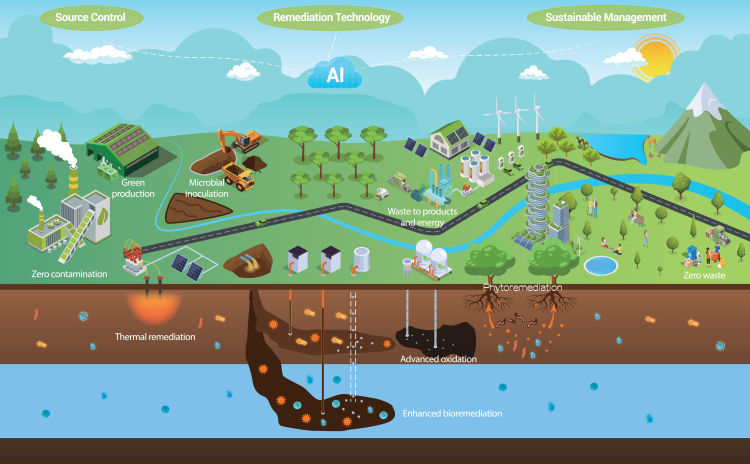
To better understand and address ECs and their harmful impacts, it is crucial to thoroughly analyze the characteristics of these substances, how they are released into the environment, and how they can affect living organisms. For example, a number of big research questions were identified by Boxall et al. to understand the risks of PPCPs in the environment, and, more recently, a synthesis of progress toward answering most of these questions was provided, within which a number of timely research needs remain unanswered.77 We can work toward a more sustainable approach by using innovative technologies to identify these contaminants, eliminate their sources, and apply green chemistry principles for designing safer chemicals.78 This comprehensive understanding of problematic substances and their pathways of exposure is essential for developing effective strategies.79,80 Figure 2 illustrates the release processes and potential pathways of emerging pollutants in different environmental compartments.
Figure 2.
Schematic illustration of the multifaceted pathways of EC production, utilization, and environmental release
Sectors such as industries, agriculture, households, hospitals, and wastewater treatment plants all contribute to the distribution of these contaminants. From industrial processes to agricultural practices and everyday household activities to medical and treatment facilities to effluent discharges, these sources collectively disseminate ECs into the environment.
In both urban and rural areas, sources of ECs can be categorized as point source discharges from wastewater treatment plants (WWTPs), which include effluents from domestic, industrial, and hospital sectors and non-point sources such as stormwater runoff from agriculture (including livestock and aquaculture) and urban areas.81,82,83,84 Additionally, ECs may originate from household products and leachates from landfills, among other sources. Conventional WWTPs were not explicitly designed to remove ECs effectively. As a result, many contaminants have been reported in treated effluents at concentrations typically ranging from ng/L to μg/L.80,85,86 The continuous discharge of ECs in these effluents challenges existing global chemical management approaches that identify chemicals as persistent using cutoff values87 because effective exposure duration increases when introduction rates from sewage or effluent discharge exceed the rate of degradation.88 Efficient treatment of wastewater containing various chemical contaminants and pathogenic microorganisms remains a significant challenge in environmental engineering,89 particularly in low- and middle-income countries.
Food production has significantly increased in recent years to meet the growing global demand. As a result, agricultural activities have become common contributors to releasing emerging pollutants into the environment.53,90,91 This is often linked to the discharge of agrochemicals,83 antibiotic residue from livestock wastes,91 microplastic debris resulting from the extensive use of plastic mulching film,53 and pathogens introduced through the application of livestock manure or WWTP biosolids as fertilizer.92 Without significant alterations to existing practices, new pollutants produced by intensive farming are expected to continue accumulating in soils, potentially polluting nearby water bodies through surface runoff and infiltration.83 Additionally, there is a risk for these pollutants to enter the atmosphere through agricultural spray drift or volatilization following pesticide application.93
Beyond the discharge of effluents from WWTPs and agricultural activities, leachate from landfills, where household wastes are deposited, constitutes a significant source of emerging pollutants in terrestrial ecosystems (Figure 2).79,94,95,96,97 PPCPs, endocrine-disrupting chemicals, and ARGs have been detected in untreated landfill leachate at concentrations ranging from ng/L to μg/L,95,98 with some concentrations surpassing proposed safe thresholds for the emergence of antibiotic resistance.99,100 When these potentially toxic leachates seep out or overflow into water bodies, they can adversely affect aquatic organisms.97 The construction industry is a significant environmental concern as it generates various contaminants, including construction and demolition waste, fly ash, plastic waste, and dust, during construction.101,102 These pollutants can potentially affect the living conditions of nearby residents and construction workers.103 However, the environmental fate of emerging pollutants associated with building sites remains largely unknown, and the application of new building materials that are being developed, such as engineered living materials,104 could also exacerbate this problem.
Particulate contaminants, such as ultrafine particles, micro(nano)plastics, and ENPs, may be released into the atmosphere through processes including volatilization, aerosol formation, and diffusive exchange.105,106 These airborne pollutants could further be transported to surrounding or remote areas through dry or wet deposition or wind events.105,107 These particles could also carry other PPCPs and move to a remote area. Fernandez et al.108 found that polycyclic aromatic hydrocarbons (PAHs), PCBs, and polybrominated diphenyl ethers are present in remote high-mountain European lakes, indicating a long-range atmospheric movement of such pollutants from urban to remote areas with the help of aerosol particles. Meteorological factors, including temperature, precipitation, wind speed, and boundary-layer mixing, play vital roles in affecting the migration behavior of airborne pollutants.109 Atmospheric compartments, mainly consisting of outdoor and ambient air, atmospheric fallout, and suspended or street/road dust, have become the transport medium of airborne contaminants and a point source of emerging pollutants in terrestrial and aquatic ecosystems.107,110
NPs can be formed by anthropogenic activities such as combustion in cooking, vehicles, thermal power plants, aircraft engines, chemical manufacturing, ore refining, smelting, and welding.111 There are three potential entry points for NPs into the environment over their lifespan: (1) during the manufacture of raw materials and nano-enabled goods, (2) during use, and (3) after disposal of items containing NPs (waste treatment).112 Life-cycle estimates indicate that the majority of NP emissions occur during the use stage and after disposal in landfills.113 However, emissions during manufacture account for less than 2% of the total output.114 ENPs may be released directly or indirectly into the environment via a built environmental system such as WWTPs or waste disposal facilities. As for direct ENP emission, ENPs can act as fertilizers to remediate soil, control the release of plant growth-regulating substances, detect pathogenic bacteria, and control plant diseases and pests. Potential secondary emissions may occur through various pathways, including the discharge from WWTPs, the utilization of biosolids as soil amendments, or leachates from landfill sites. These engineered systems play a pivotal role in dictating the destiny of ENPs, influencing whether they are discharged as effluent or incorporated into biosolids, and determining their state (whether they remain bare, coated, or undergo chemical or physical transformations).115 Sun et al.116 reported that, in the European Union in 2014, the sinks of TiO2 NPs, ZnO NPs, AgO NPs, and CNTs were mainly landfills (7,000 t year−1), sediments (7,600 t year−1), and soils (8,400 t year−1). The predominant emission pathway of TiO2 NPs and ZnO occurs via wastewater and ultimately accumulates in sewage. CNTs and AgO NPs are primarily discharged into the environment during their manufacturing and application processes, where they are subsequently deposited in landfill sites.
Additionally, concerns have been raised about the environmental and human health risks of emerging protein contaminants such as proteinaceous infectious particles (prions).117,118 Prions are misfolded forms (PrPSc) of normal cellular prion proteins (PrPC) that are capable of self-templating (thus their infectivity), and various prion strains can cause fatal neurodegenerative diseases in various hosts, such as Creutzfeldt-Jakob disease in humans, bovine spongiform encephalopathy in cattle, and chronic wasting disease (CWD) in cervids. Take CWD prions as an example: once released into the environment, they can attach to soil particles, remaining as long-term sources of contamination.119 Subsequently, these prions may be absorbed by plants from the soil and then transferred to animals through the food chain. Consequently, the presence and spread of these prions in the environment are frequently linked to the movement patterns and breeding practices of infected animals.
In summary, ECs could, directly and indirectly, enter the environment from various sources, such as industrial and agricultural operations; mining and construction activities; oil and chemical leaks; diffuse sources such as stormwater drains, roads, and parking areas, and wastewater treatment systems (Figure 2)51,120; and the use of a wide range of consumer products. ECs in soil or landfills can also seep into adjacent groundwater.121,122 River networks and wind can transport these pollutants from residential, industrial, and agricultural areas to remote regions and eventually into marine environments.51 Understanding the environmental release processes and transformation pathways of ECs is pivotal for evaluating their potential ecological impacts and for developing efficient mitigation and remediation strategies.
Advances in the detection and analysis of ECs
The development of new analytical techniques and technologies has significantly enhanced the detection and analysis of ECs. This progress has bolstered our capability to extract, quantify, and detect ECs in environmental samples. Mass spectrometry (MS) and bioanalytical techniques have been particularly effective in analyzing emerging organic contaminants.123 Furthermore, electrochemical detection methods, with a focus on green technology, have emerged to measure ECs, especially pharmaceuticals.124 These innovations have played a crucial role in elucidating the sources, classification, fate, and transport of ECs and in the development of treatment technologies for their removal.125
Sampling and analytical methods
Advanced sampling and separation
Recent global initiatives are reshaping the future of analytical chemistry, focusing on sustainable technologies. This impact is particularly evident in methodologies for sampling and sample preparation to detect and characterize ECs. Among these advancements is the solid-phase microextraction (SPME) chemical biopsy approach, which offers a flexible format for high-throughput quantification of ECs.126 Enhanced by matrix-compatible thin film coatings and balanced coverage phenomena, SPME effectively eliminates matrix effects and extracts a wide range of compounds with diverse physicochemical properties. It is effective not only with gas chromatography (GC)-MS and liquid chromatography (LC)-MS but also with direct MS coupling, showing versatility and effectiveness in analysis.127,128,129 Extraction techniques for ECs have evolved to enable on-site sampling using thin films, either through spot130 or time-weighted average sampling methods.131 In vivo sampling, employing a small-needle format, allows for the direct assessment of exposome effects in response to environmental pollution at the sampling site.132 These designed probes conduct non-exhaustive sampling over longer periods, accumulating sufficient analytes for sensitive detection via chromatography or MS. Additionally, a filter-incorporated needle-trap device facilitates the simultaneous determination of free and particle-bound pollutants in a single step when combined with SPME and measured directly with GC-MS. Portable GC-MS instruments enable gas sampling for on-site analysis.133 These advancements promise to enhance environmental protection efforts by generating large volumes of scientific data using simple, cost-effective, and sustainable analytical instrumentation. Moreover, these tools facilitate the untargeted characterization of samples, thereby aiding in the discovery of new compounds, including ECs.
Apart from mass spectrometric detection, chromatographic separation is also crucial in analyzing ECs. LC or GC is typically coupled to MS for analysis. However, very polar fractions are a problem for both. Being nonvolatile, they cannot be analyzed by GC or retained by the stationary phase of LC. Alternative chromatographic separation methods are being explored to close this gap. For example, a recent study combined supercritical fluid chromatography (SFC) with HRMS to identify unknown disinfection by-products in drinking water.134 Hydrophilic interaction chromatography (HILIC) is also commonly employed in orthogonal analysis to analyze polar compounds. For example, HILIC-HRMS was applied in disinfected water analysis, leading to the identification of a new class of polar disinfection by-products (DBPs)—halomethanesulfonic acids.135,136 An alternative that has emerged in recent years is an extra separation dimension (i.e., ion mobility spectrometry [IMS]) hyphenated to the conventional GC- or LC-MS systems. IMS is a rapid gas-phase separation technique that separates ions based on their size, shape, and charge. IMS is particularly useful for the separation of isomeric analytes or coeluting matrix components. The collision cross section (CCS) values provided by IMS analysis supplement the common identification parameters such as retention time (RT) and mass-to-charge ratio (m/z) for the screening and structural elucidation of ECs. The inclusion of IMS in non-targeted analysis significantly improves confidence in the elucidation of unknown chemical structures. For instance, ion mobility-MS (IM-MS) has been used to analyze ECs in human urine samples.137 In another example, a non-targeted LC-IM-MS analysis of emerging PFAS in aqueous film-forming foams used CCS to enhance confidence in identifying unknown chemical structures and improve specificity in suspect screening.138
Advanced MS
MS is among the most applied techniques for the analysis of ECs. HRMS instruments, such as time-of-flight (TOF) and Orbitrap mass spectrometers, offer high mass accuracy and resolution that are critical for identifying ECs through structural elucidation (see Table S1). More recently, HRMS has been applied in identifying transformation products and metabolites of ECs139 and in the non-targeted analysis/suspect screening of ECs.140 HRMS has revealed many new ECs in the environment and elucidated their transformation products and metabolites. Compared with other analytical techniques, the capability to conduct non-targeted analysis is an invaluable advantage of HRMS in ECs’ analysis. HRMS enables the integration of non-targeted analysis with bioassays and in chemico methods to identify bioactive and toxic chemicals in a sample. This combined approach enables the precise identification and broad capture of bioactive/toxic chemicals.141 For instance, an estrogen receptor α (Erα) protein affinity assay combined with HRMS has been applied to identify Erα-active compounds in source and drinking water samples from major rivers in China.142 In combination with effect-directed analyses, ultrahigh-resolution MS (i.e., Fourier transform ion cyclotron resonance MS) was adopted to identify the toxicity drivers of unknown disinfection by-products in chlorinated and chloraminated drinking waters.143 In addition to in vitro bioassays, in chemico methods based on key chemical reactions (i.e., molecular initiating events) have also been applied to identify and measure the toxicities of environmental samples.144 The combination of in vitro and in chemico assays with non-targeted chemical analysis represents a novel, more effective approach to identifying the bioactive/toxic contaminants in our environment.145,146
Other advanced analytical chemistry techniques
Nuclear magnetic resonance (NMR) spectroscopy is an advanced method for characterizing the chemistry of environmental samples.147 NMR has several advantages for the discovery of contaminants, potential transformation products, and characterizing the reactivity of contaminants over other techniques. The primary advantage is that structural elucidation can be performed without an authentic standard because the molecular profile from different NMR experiments can be used for complete structural elucidation. Another advantage is that NMR can leverage different nuclei to explore the structure of different metals and organic contaminants and their interactions with environmental and biological media. However, NMR is less sensitive than the previously described MS techniques, which can result in higher sample needs for characterization. NMR is also less accessible than other instruments, which has created a barrier in the broader application of this powerful and versatile technique for characterizing metals and contaminants and their impacts on both environmental and human health.
Electron paramagnetic resonance (EPR) can be used to detect environmentally persistent free radical (EPFR) signals without the need to capture reagents, unlike common short-lived free radicals. However, the presence of particles or colloids associated with EPFRs, along with the co-existence of paramagnetic components such as transition metals in the matrices and varying environmental conditions such as humidity and temperature, can significantly interfere with EPR detection.147,148 The interference of components makes it impractical to separate them, as they likely contribute to the formation of EPFRs. Additionally, the diverse chemical structures of EPFRs pose a challenge to their identification. Researchers have categorized EPFR types based on g values and bandwidth, referring to them as oxygen centered and/or carbon centered. However, studies have shown that both parent chemicals and their degradation by-products contribute to EPFR formation, potentially playing simultaneous roles.149 The reactivity of EPFRs varies with their structures, yet attributing signals to specific structures or quantifying the contributions of different structures remains elusive.
Suspected-target and non-target screening approaches
The number of anthropogenic chemicals has grown beyond our capacity to study them using traditional environmental monitoring approaches that rely upon the development of targeted analytical methods tailor-made to individual chemicals.150 This challenge drives the need to develop suspect and non-targeted screening (NTS) methodologies to identify ECs in complex environmental and biological media.141 The past three decades have witnessed the development of a wide range of HRMS instruments that are capable of resolving hundreds or even thousands of chemical compounds (M) by measuring the m/z of their corresponding (quasi)molecular ions (e.g., M·+, [M + H]+, and [M − H]−) with sub-part-per-million (<1 ppm) accuracy. The following sections provide a brief primer on the methodologies employed in the NTS of ECs.
Suspect screening
Modern HRMS can gather both m/z and CCS data for numerous compounds within a sample. However, sorting through these data and differentiating between ECs and the matrix is akin to finding a needle in a haystack. Comparison of experimentally obtained mass spectra with those compiled in spectral libraries (e.g., the National Institute of Standards and Technology [NIST] Mass Spectral Library) has been a time-honored approach to identifying an unknown.151,152 One drawback of spectral library searching is the finite size of the library, which may not contain (bio)transformation products, by-products, or proprietary compounds whose authentic standards may not be readily available.153 Another challenge is the reproducibility of collision-induced dissociation spectra, which vary between laboratories depending on the instrument and experimental conditions. Suspected screening practitioners increasingly rely on structure databases (e.g., PubChem, CompTox Chemicals Dashboard),154 which are orders of magnitude larger than spectral libraries. Current suspect screening methods involve the creation of a list of structures whose computed/predicted properties are then compared with those obtained by experiment. However, the database’s structural form does not always match the chemical structure observed by HRMS.155 The experimental measurements are compiled using a peak-picking algorithm, the choice of which may influence the reliability and reproducibility of results.156 The analyst is also cautioned that no single instrumental method is capable of detecting all chemical compounds and that each step of the analysis could remove compounds present in the sample.157,158 This is particularly relevant when a large suspect list consists of compounds with a wide range of properties. For example, an instrumental method suitable for the analysis of anionic PFAS may not be appropriate for emerging brominated flame retardants. Black et al.157 have highlighted the urgent need to develop predictive methods to assess which compounds will be detectable using a given set of experimental and instrumental conditions. The identity of a compound cannot be confirmed by its mass alone. This is why in silico (i.e., computer modeling) methods are essential to predicting the dissociation of compounds on the suspect list, their chromatographic RT, and CCS to assist in differentiating similar compounds. The application of harmonized values, such as the unified retention time index (RTI), is also utilized in several wide screening workflows in Europe.159,160 With the help of RTIs, the number of false positives can be reduced in the first screening step from suspect screening and non-target screening workflows. Quantum chemical161 and machine-learning-based methods162 are capable of predicting ion ratios but at greater computational cost. Chromatographic RT163 and CCS164 can also be predicted using machine learning models.
Nontargeted screening
A disadvantage of suspect screening is the fact that it requires prior knowledge of the occurrence of impurities and transformation products that are often unknown. Consequently, these compounds are absent from structure libraries, leaving the analyst with the unenviable task of answering the question, “What organic compounds are present in the environment that should not be there?” without knowing their structure(s) beforehand. Consequently, the analyst must identify the structures of the compounds detected in an NTS experiment using first-principles interpretation of their mass spectra. However, this is currently impractical for all compounds detected, which number in the thousands. Therefore, practitioners of NTS have developed a range of experimental and computational strategies to prioritize mass spectra for structure elucidation. Environmental risk assessment efforts have shown that >60% of compounds with the potential to persist in the environment and bioaccumulate contain the elements chlorine, bromine, or fluorine.165 Their mass spectra also display characteristics unique to the presence of halogens, and NTS strategies to identify ECs have largely focused on halogenated compounds.166,167,168,169,170,171 Emerging PFAS are more challenging to recognize since 19F is a single stable isotope. However, a previous study has shown that isotopic ratios (i.e., 13C/12C) can still be used to discover PFAS, which are characterized by having fewer carbon atoms than other non-fluorinated compounds with the same molecular weight.170 Recently, Zweigle et al.172 have exploited this characteristic to develop a novel approach to PFAS discovery that involves plotting the mass defect normalized to the number of carbons (MD/C) vs. mass normalized to the number of carbon atoms (m/C). Cl-, Br-, and F-containing compounds can also be revealed using ion mobility because halogenated compounds are characterized by relatively small CCS compared to their molecular weight.173,174 However, the most common approach to the discovery of unknown pollutants involves monitoring a fragment ion that is common to an entire class of pollutants. Machine learning is increasingly being used to guide NTS. Methods that predict a spectrum from a structure, such as competitive fragmentation modeling identification (CFM-ID)162 are becoming more mature. However, the reverse problem of predicting a structure from a spectrum has yet to be solved. Boiko et al.175 have recently reported on an automated tool that can assign elemental compositions in an unbiased, unconstrained way. It is anticipated that further growth in the areas of machine learning and AI will eventually enable true, unsupervised NTS.176
Advanced bioanalysis
Bioanalytical techniques
While chemical analysis-based methodologies offer significant advantages, such as low detection limits, excellent accuracy, and good selectivity for monitoring ECs, the steady growth in the development of biosensors, also known as bioanalytical tools,177 for environmental analysis cannot be overlooked. This growth is largely attributable to their superior capabilities in rapid, specific analysis and real-time monitoring. Biosensors, which are analytical devices that combine a biological recognition element with a transducer,178 have been developed to detect various ECs. Detectable ECs include antibiotics,179 pesticides,180 bisphenol A,181,182 and microplastics.183 Biosensors effectively detect ECs in environmental samples184 as well as in foodstuffs and biological samples,185,186,187,188 particularly within an effects-directed analysis framework.189
Recent advancements in biosensor technology have seen the introduction of novel biological recognition elements, such as aptamers, in sensor development. Aptamer-based biosensors, or aptasensors, have emerged as robust and powerful analytical tools for the detection of ECs. This is largely because of their high specificity for small molecules, low fabrication cost, design flexibility, and high stability. For example, specific aptamers have been developed to detect chloramphenicol in honey and enrofloxacin in sewage water.190,191 The possibility of incorporating advanced engineered nanomaterials, such as carbon-based nanomaterials, metal-organic frameworks, and noble metal NPs, into biosensor systems is being explored.192 With their good electrical conductivity, nanoscale size, and compatibility with biological molecules, these nanomaterials could significantly enhance biosensor performance. Indeed, nanomaterials have been found to increase biosensor sensitivities and lower the limit of detection by several orders of magnitude.193
Advanced analytical techniques for biological contaminants
Recent advancements in the detection of biological ECs, such as pathogens, ARGs, and functional genes associated with the biosynthesis of cyanobacterial toxins, have been facilitated by high-throughput quantitative polymerase chain reaction (qPCR) and next-generation sequencing-based methods.194,195,196 A comprehensive study recently outlined the advantages and disadvantages of these methods, including classical cultivation-based techniques, for ARG detection.197 One of the significant benefits of sequencing methods is their ability to identify a wide range of pathogens or ARGs across diverse microorganisms present in samples.198 Despite their high-throughput nature, the sensitivity of these methods relies heavily on the effectiveness of the analysis pipelines.199 In recent years, computational tools have played a pivotal role in enhancing pathogen surveillance. Notably, the development of a comprehensive pathogen database has empowered the multiple bacterial pathogen detection (MBPD) pipeline to achieve holistic habitat surveillance and coinfections of pathogenic bacteria.200 Moreover, advancements in understanding the genomic signatures of pathogens through deep-learning approaches, such as DCiPatho, have enabled highly accurate identification of pathogens on a genomic scale.201 Despite the strides made in pathogen detection through sequencing methods, monitoring the environmental dissemination of high-risk ARGs, particularly originating from pathogen hosts, remains challenging and requires novel tools.
The analytical methods for cyanobacterial toxins include biological (mouse bioassay), biochemical (enzyme-linked immunosorbent assay, protein phosphatase inhibition assay), chemical (high-pressure liquid chromatography [HPLC];, LC-MS, high-performance capillary electrophoresis, thin-layer chromatography, and GC), and molecular biological (conventional PCR, qPCR, biosensor method).202 The chemical method is the most researched and well established and is by far the most commonly used.
Distribution and fate of ECs
Emerging organic contaminants
Terrestrial ecosystems face numerous challenges arising from introducing and accumulating a range of potentially toxic organic substances (Figure 3). Synthetic and naturally occurring emerging organic contaminants (EOCs) are widespread across diverse environmental settings. Despite often existing in low concentrations, these EOCs can exert significant and enduring effects, prompting extensive research into their distribution and fate in recent years. EOCs originate from various sources, including industrial waste, agricultural runoff, and household products. They can be categorized based on their chemical properties and sources, with subsequent subsections discussing some of the most prevalent types.
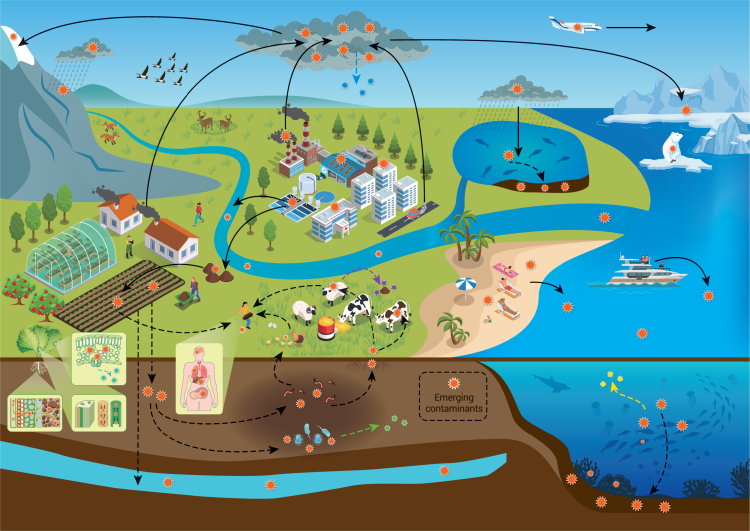
Figure 3.
Pathways through which ECs enter the environment and their subsequent fate
ECs can originate from various sources, such as industrial discharges, agricultural runoff, and wastewater effluents. Once released, ECs can undergo transformation processes such as degradation, volatilization, and bioaccumulation, influencing their distribution across different environmental compartments, including water bodies, soils, and the atmosphere.
Pharmaceuticals and personal care products
PPCPs represent substances utilized for personal health or cosmetic purposes that can find their way into the environment through multiple pathways, including excretion post consumption.203 Among PPCPs, pharmaceuticals, especially antibiotics, raise significant concerns due to their widespread use and potential environmental impact. Antibiotics, primarily administered orally for bacterial infection treatment in humans and animals, undergo enzyme-mediated metabolism before excretion, involving phase I and II processes.204 Phase I metabolism involves oxidation, reduction, and hydrolysis, transforming parent compounds into various metabolites, while phase II metabolism entails conjugation with molecules such as glucuronic acid or sulfate, further altering their chemical structure. Consequently, resulting metabolites may enter the environment at higher concentrations than their parent compounds due to these metabolic processes.205 Some pharmaceuticals resist biochemical transformation during metabolism and are excreted unchanged, entering the environment in multiple forms.206 Understanding these metabolic pathways is pivotal for identifying the diverse forms of pharmaceuticals in the environment and assessing their potential ecological and human health impacts.
Pharmaceuticals and other PPCPs enter the environment through various pathways, such as wastewater discharge from sewage treatment plants and animal farms, excretion from humans and animals, and improper disposal practices.206 Despite efforts in wastewater treatment, PPCPs are not effectively targeted for removal, often persisting due to treatment conditions.207 National surveys, such as one conducted in the United States, have shown that final effluents from WWTPs receiving discharges from PPCP manufacturers may contain concentrations of PPCPs 10–1,000 times higher than those typically found in WWTPs without such inputs.208 This trend was also observed globally, particularly for commonly used PPCPs such as antibiotics.206 Advanced analytical techniques have enabled the detection of PPCPs in sewage, groundwater, surface waters, drinking water, soil, and aquatic organisms across numerous countries, even at low concentrations.209,210,211 For instance, a comprehensive survey conducted in 2015 identified over 600 different pharmaceutical substances and their transformation products across more than 70 countries on all continents.212
Once in the environment, PPCPs undergo various processes determining their fate. Some PPCPs can degrade over time through microbial action, although the rate of biodegradation varies based on the compound’s chemical structure. PPCPs can also adsorb onto soil particles or sediment in water bodies, influencing their mobility and bioavailability. Aquatic organisms, such as fish, mollusks, and algae, can take up PPCPs from water through direct exposure or diet. It was evident that log Dow, rather than log KOW (n-octanol-water partition coefficient), is a better indicator of their bioaccumulation and trophic magnification for a marine food web.213 However, the apparent volume of distribution represents a promising proportionality constant to understanding the bioaccumulation of ionizable chemicals.214 Once in the water bodies, most PPCPs remain in the water phase because of their hydrophilic nature, such as sulfonamide antibiotics, whereas some hydrophobic ones (e.g., estrogens) might sorb to sediments or be accumulated by organisms.215 The presence of antibiotic residues in the environment might increase the risk of antibiotic resistance dissemination in environmental settings and consequently transfer to the human microbiome. Terrestrial organisms, including plants and insects, can also be exposed to PPCPs through the contaminated soil. Antibiotics are usually the most abundant PPCPs in plants originating from soils that were amended with biosolids and animal manure applications. For plants, hydrophobic compounds may partition into lipids and will be predominantly retained by roots, while most hydrophilic compounds will move to the xylem (in equilibrium with the water).216 Further studies are needed to understand the bioaccumulation of ionizable PPCPs in aquatic and terrestrial organisms.217
Cyanotoxins and other algae toxins
Risks of toxins produced during harmful blooms of algae, cyanobacteria, and other organisms represent a classic One Health topic (www.cdc.gov/habs/ohhabs.html). Cyanobacterial blooms stimulated by multiple factors, such as global warming and eutrophication of water bodies, have led to a significant increase in the frequency, distribution range, intensity, and duration of cyanobacterial blooms, thus further exacerbating the risk of algal toxin poisoning.218,219 Cyanotoxins can be classified into three groups based on their chemical structure: cyclic peptide, alkaloid, and lipopolysaccharide (LPS). Depending on the mode of toxicity to animals, toxins can be classified as hepatotoxic cyclic peptide toxins (represented as microcystin and nodularin), neurotoxic alkaloidal toxins (anatoxin, saxitoxin), cytotoxic alkaloidal toxins (cylindrospermopsin), dermatologic toxicity of alkaloidal toxins (aplysiatoxin; lyngbyatoxin), irritant toxins (LPS), and some other biologically active substances.220 Globally, microcystin-LR is the most common cyanotoxin in freshwater, brackish water, and marine habitats.221 Lakes and reservoirs differ in morphology and trophic status, which can affect the dispersal and distribution of cyanotoxins.222 At the same time, cyanotoxins are subject to transport and diffusion at the sediment-water interface, with different types of sediments exhibiting different adsorption capacities.223 Notably, algae, cyanotoxins, and toxins present in a variety of freshwater, marine, soil, and terrestrial species can be wind driven to float in the air and transported over greater distances.224 Moreover, cyanotoxins in the atmosphere may, under certain conditions, settle on the ground or in water bodies and affect the surrounding environments.225 The accumulation of cyanotoxins involves a complex process of gradual accumulation and transfer in ecosystems. The process can be manifested primarily through the cascading of cyanotoxins through the food chain and their progressive enrichment in organisms. For example, fish and shellfish, organisms that consume food rich in cyanobacterial toxins, accumulate the toxin in their tissues, resulting in a gradual buildup of cyanotoxins in the upper levels of the food chain.226
Emerging inorganic contaminants
Engineered nanoparticles
ENPs that accumulate in the environment will undergo a series of physical, chemical, and biological processes such as chemical transformation, aggregation, and dissolution. The interplay between these processes and the ENP transport ultimately determines the potential fate of ENPs.227 The chemical transformation process mainly includes the dissolution and sulfidation of ENPs. In a series of studies, it has been found that the dissolution of NPs is triggered by particle-inherent factors (e.g., surface coating, particle size, shape, and aggregation state) and environmental parameters such as solution pH, dissolved organic carbon, and temperature.228 Thereinto, the most commonly occurring passivation process, that is, the sulfidation of NPs, makes their surface appear to be almost inert, thus affecting the reactivity.
The colloidal stability of ENPs is a crucial factor that influences their fate and environmental effects.229 The homo-aggregation (interactions between the same ENPs) of NPs is positively correlated with the NP concentrations. The aggregation characteristics are often explained by the classical Derjaguin-Landau-Verwey-Overbeek theory. Owing to the low predicted ambient concentrations of ENPs (e.g., in the range of pg/L to low μg/L for surface water), homo-aggregation is less likely to happen and is affected by ionic strength. The aggregation rate of NPs increases with the surrounding medium’s ionic strength, and multivalent cations are more efficient than monovalent cations.230,231 However, heteroaggregation of ENPs with mineral particles is more common in natural environments,232 which ultimately affects the environmental fate of ENPs and their risk to ecosystems and organisms.233 The majority of the studies on ENP transport in porous media used water-saturated artificial columns often packed with quartz sand, while only a few involved natural soils.234 Key environmental factors controlling ENP transport processes are solution ionic composition, pH, and natural organic matter (NOM) chemistry, while the degree of water saturation in porous media such as soils is an additional physical factor. The impact of ionic composition, NOM, and solution pH on the NP fate is similar in aquatic systems and saturated and unsaturated porous media. For plants, an increasing number of studies related these factors to plant uptake. For instance, size-exclusion limits that range from <10 nm to the uptake of cells exceeding 20 nm for the uptake of leaves and can reach 100 nm in exceptional cases.235,236,237 Assimilation of elements from larger particles is possible if they dissolve, while low zeta potentials usually favor direct particle uptake.
Radionuclides and nuclear wastes
Whether released from nuclear power plants, medical facilities, or sites where radioactive material was improperly disposed of, radionuclides pose considerable challenges to environmental quality and human well-being.238 Radionuclides undergo radioactive decay, emitting radiation over time.238 Nuclear wastes threaten ecosystem health. Strict regulations govern the handling and disposal of nuclear waste to prevent environmental contamination.239,240 Consideration should extend beyond physical and chemical interactions to encompass biological uptake and long-term ecological consequences of radionuclides and nuclear waste.
Biological contaminants
Pathogenic bacteria
The intricate interplay between pathogenic bacteria and various environmental sources, particularly in agricultural settings, underscores the complexity of this challenge.241,242 Agricultural soils are often underestimated as reservoirs of human and animal pathogens and can give rise to a spectrum of diseases affecting air, water, and food.243 For example, bacterial species such as Bacillus anthracis, Vibrio cholera, and Burkholderia pseudomallei have the potential to cause severe infection and, in some cases, death through direct contact.244,245 Foodborne pathogens such as Escherichia coli O157:H7 and Salmonella enterica can also enter the food chain, triggering epidemics with severe health consequences.246,247
Antibiotic-resistant bacteria and resistance genes
Antibiotics and antibiotic-resistant bacteria (ARBs) carrying ARGs have existed for hundreds of thousands of years before the discovery of antibiotics by humans.248,249 However, the industrialization and widespread use of antibiotics in both human and animal populations have exerted unprecedented selective pressure on bacteria across various interconnected niches, including human, animal, and environmental microbiomes. This has led to the accelerated development of antibiotic resistance traits within these communities on a global scale.250,251 Thus, anthropogenic activities could increase the emergence of ARBs, their resistance genes, and their dissemination between the human, animal, and environmental compartments, aggravating the existing antibiotic resistance crisis.252 For example, the extensive use of antibiotics and the intensive agricultural practices prevalent in modern farming have transformed soil ecosystems into potential reservoirs of pathogens and ARGs.253 Within this soil environment, the biopollutome emerges as a complex network of pathogens and ARGs, creating a prevalent threat to ecosystems.254 Although multiple barriers restrict the flow of both bacteria and genes, pathogens recurrently acquire new resistance factors from other species, thereby reducing our ability to prevent and treat bacterial infections,62 which demands urgent and effective measures to control the formation and dissemination of ARB.
Antibiotic resistance has been referred to as a silent pandemic and has emerged as a significant concern in the realm of biological ECs.255 Hence, the increasing number of antibiotic-resistant microbes poses threats to human health. Over the last decade, ARGs have been detected in all habitats, including the natural environment and human industrial habitats.256 Anthropogenic activities play a key role in selecting genes from environmental and cellular sources, facilitating their subsequent co-option to confer antibiotic resistance. With increasing human activities, microorganisms and their genetic material move more often between humans, animals, and the environment, which collectively increases opportunities for the transmission and evolution of ARGs.256,257,258 Once these drug-resistant genes are transferred to human-associated pathogenic bacteria, such as Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species (ESKAPE) pathogens and plant pathogens, it may further exacerbate the clinical pathogenic risks.259 These pathogens are not only present in the bodies of humans and animals but can also enter the water through excretions such as feces, urine, and saliva and spread through respiratory secretions into the air. The threat posed by pathogenic bacteria also presents a significant challenge within the One Health framework.252 Over the past two decades, infectious diseases have been accountable for approximately 15 million global deaths annually.260 Meanwhile, plant diseases contribute to the loss of up to 30% of global food production each year.261
ARB and ARGs originating from human activities are recognized as emerging biological contaminants that can potentially affect environmental ecosystems.262,263 Apart from antibiotics, a range of non-antibiotic pollutants such as heavy metals, disinfectants, biocides, and non-antibiotic drugs can alter bacterial behavior and contribute to the development of antibiotic resistance.264,265,266,267,268 Furthermore, ARB and ARGs can disseminate back to the human and animal microbiomes269 through food ingestion, drinking water, and direct contact while swimming in contaminated water and while in contact with contaminated crops, thus creating a loop between the human, animal, and environmental microbiomes. Nevertheless, future research should provide quantitative information about the dissemination routes of ARB and ARGs from the environment to the human microbiome by considering human exposure and the probability of successful colonization of the human microbiome by these biological pollutants. There is an urgent need to move from descriptive, qualitative, or semi-quantitative research to quantitative risk assessments of the drivers of antibiotic resistance proliferation in the environment and its dissemination to the human microbiome.270
Viruses
Among microorganisms, viruses are most prone to becoming emerging pathogens because they can infect their hosts and adapt to new environments through mutation, genetic recombination, and reassortment.271 The pathogenicity of many bacteria is due to the virulence factors they carry encoded by lysogenic phages.272 Soil plays a significant role in the distribution and transmission of viruses in natural environments.273 Research indicates that viruses can survive in soil for varying durations depending on factors such as temperature, moisture content, pH, and the presence of an envelope. Enveloped viruses such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can survive for up to 90 days in soils with 10% moisture content.274 Additionally, enteric viruses can persist on surfaces such as door handles, banisters, and food, contributing to their transmission.244 The abundance of viruses in soil is higher in environments with high organic matter and moisture content.274 Changes in soil moisture levels can affect the composition and activity of soil DNA and RNA viruses, potentially affecting soil ecology.275 Understanding how viruses interact with soil is crucial for assessing their environmental impact and potential transmission pathways. Because the size of the host is tens to thousands of times larger than the size of the virus, viruses are more flexible than bacteria in terms of transport and dispersal by animals, wind, or rain.276 Influenza viruses,277 hepatitis A viruses, coronaviruses,251,278 and others, can survive in the soil for a long time, leading to human exposure.
Protein contaminants
Prions and Bt proteins are considered two important classes of emerging protein contaminants. Prion proteins can bind to soils and suspend in water, thus persisting in the environment for years and serving as a significant environmental reservoir for disease propagation.44,45,46,47 Our understanding of the fate and transport of prion proteins in the environment is very limited. Using sand or soil columns, previous studies found that recPrP and purified PrPSc had limited mobility, where the migration of recPrP was smaller than 1 cm in the quartz sand column and purified recPrP was primarily retained near the point of contamination in soil columns.279,280 Bt proteins were found to persist in soils for 2 months, 180 days, and up to 234 days, respectively, and were found to have the potential to be transported through the landscape by sediments and crop residue debris in surface runoff.281,282,283 Nonetheless, significant knowledge gaps remain in understanding the fate, transport, and environmental risks of protein contaminants (e.g., prions and Bt proteins).
Microplastics and nanoplastics
As one of the world’s most prominent emerging pollutants, microplastics are ubiquitously distributed across the atmosphere, pedosphere, hydrosphere, and biosphere. Micro- and nanoplastics (MNPs) could be widely detected in the terrestrial ecosystem and human body.284 Microplastic fragmentation by rotifers in aquatic ecosystems has been reported to contribute to global nanoplastic pollution.55 Plastic particles enter the environment from ubiquitous sources, posing a potential threat to aquatic organisms, soil, the atmosphere, and human health.285,286 Atmospheric microplastics are found in both indoor and outdoor air. Indoors, concentrations in residential homes can be as high as 1.96 ×104 particles m−2 day−1), while in schools, they can be as low as 6.20 ×103 particles m−2 day−1), and in dormitories (9.9 ×103 particles m−2 day−1)) they are 5.5 times higher than in offices (1.8 ×103 particles m−2 day−1)). The abundance of MPs in outdoor air showed regional differences, with higher abundance of MPs in urban air than in rural air, and higher levels of MPs in cities in northern China than in southern cities.287 Some studies have shown that atmospheric deposition of MPs ranges from 0.5 to 1,357 MP m−2 day−1 (outdoors) and 475 to 19,600 MP m−2 day−1 (indoors). During deposition, microplastics can utilize plant stomata (20–40 μm long and 5–10 μm wide), with 20–200 nm of microplastics accumulating in the stomatal lumen and passing through the stomata into leaf tissue. Research has validated the capability of polystyrene (PS) nanoplastics to infiltrate leaves and migrate to plant roots, demonstrating their ability to penetrate plant leaves through foliar exposure.288 Within the phloem, nanoplastics can travel alongside bulk water or sap, a process influenced by sap’s composition and flow rate within the stem. Furthermore, the downward movement of nanoplastics within vascular tissues requires traversal through various physiological barriers, including intercellular plasmodesmata, vesicles, and conductive cells.289 Consequently, the continuous aggregation of nanoplastics could potentially obstruct the vascular system, impeding the downward translocation of smaller nanoplastics.289 The average abundance of microplastics in fish in the oceans was 3.5 ± 0.8 particles/stripe, but, in highly polluted waters, in contrast, oysters had the highest abundance of 99.9 particles/individual.290
MNPs accumulate in many organisms in the environment, which leads to food chain pollution affecting the life and health of all organisms in the food chain. MNPs are not easy to degrade after being ingested by animals so they accumulate continuously in the body. For example, research demonstrated that mice administered 0.2- to ∼0.3-μm PS particles at a concentration of 250 µg.μL−1 via gavage exhibited absorption of plastic particles into various organs including the blood, liver, brain, spleen, testis, bladder, and others through the intestinal tract, leading to multi-organ toxicity.291 MNPs can be detected in human feces, which indicates that the intake of MNPs is high.292 After MNPs enter the gastrointestinal tract through food, the undigested MNPs are excreted with feces, but smaller MNPs will enter the systemic circulation. Some studies have found that there are MNPs in human blood, so MNPs may be transported to various organs through blood, but the mechanism of MNPs entering the blood circulation is still unclear and needs further study.293 The maximum particle diameter of MNPs taken up by organisms is determined by the morphology of species' feeding and digestive organs.294 MNPs mainly enter the respiratory and gastrointestinal tracts and can then be transferred to other secondary organs according to their size and shape.
Liquid crystal monomers
Liquid crystal monomers (LCMs) are a class of synthesized organic chemicals that are key materials for liquid crystal displays (LCDs), which can undergo phase transitions between liquid and solid states at specific temperatures. LCMs are typically diphenyl-based compounds that contain functional groups such as cyano, fluorine, chlorine, or bromine.295 The production output of LCMs for LCD panels is approximately 500 t year−1.296 However, the environmental release of LCMs during the use and dismantling of waste LCDs is a concern, and global estimates range from 1.07 to 107 kg/year.297 Numerous studies have indicated the widespread presence of LCMs in the environment, and projections suggest a significant increase in their prevalence in the near future.49 These LCMs exhibit environmental persistence, long-range migratory capabilities, and potentially harmful impacts on various species.298 Consequently, LCMs have gained attention as ECs because of their distinctive properties, including persistence, bioaccumulation, toxicity, and extensive environmental distribution.297
LCMs have been found in various environmental matrices, indicating their widespread distribution and potential exposure risk to organisms. Air is considered a significant transport medium for LCMs, allowing their migration from e-waste recycling sites to the surrounding environment. Investigations into waste LCD panel dismantling revealed atmospheric concentrations of LCMs at 68,800–385,000 pg/m3.299 LCMs have also been observed in indoor and outdoor dust, sediment, landfill leachate, sewage sludge, and soil samples. LCMs median levels in dust collected across China ranged from 41.6 to 171 ng/g,300 depending on the sampling region. LCM concentrations in urban soils from different functional zones ranged from 0.774 to 12.9 ng/g dry weight (dw).301 In biota samples, LCMs were found in wild aquatic invertebrates and fishes.302 LCMs were also detected in the hands, forehead skin wipes, and serum of e-waste dismantling workers.303 The LCM concentrations in the serum samples of the occupational workers were significantly higher than those in the reference serum samples, indicating a high exposure risk in the occupational population.175 These studies have provided direct evidence of LCMs in the environment, indicating their widespread pollution and highlighting the importance of understanding their distribution and fate.
Environmentally persistent free radical signals
Unlike traditional free radicals with lifetimes spanning milliseconds and microseconds, EPFRs are stabilized on or in specific particles, with lifetimes extending beyond days and even months. EPFRs exhibit stability and ubiquity in various environmental matrices such as atmospheric particulates, soil, biochar, and microplastics.304,305 Their presence is potentially implicated in diverse environmental and biological processes. Notably, EPFRs have been observed to mediate the generation of a significant amount of reactive oxygen species (ROS),306 recognized for their involvement in chemical degradation307 and the induction of oxidative stress, which can adversely affect organisms, leading to DNA damage and diseases such as lung and cardiovascular diseases.308 Ongoing research is addressing various aspects of EPFRs, each presenting substantial challenges.
Risks of ECs to planetary health
Environmental quality implications
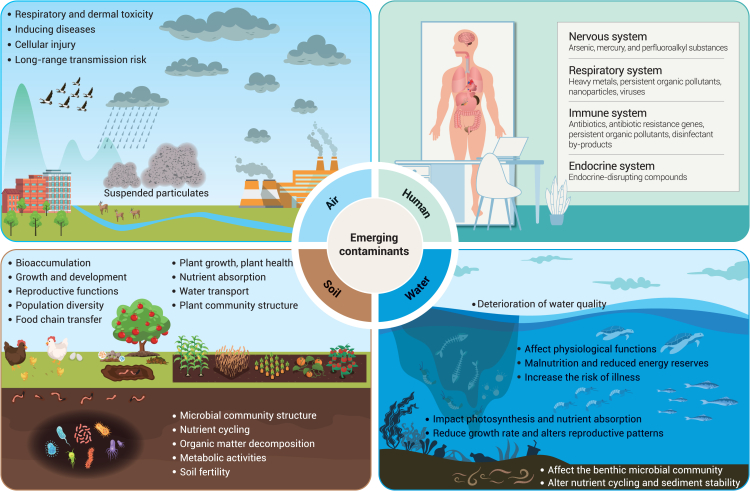
ECs present substantial risks to planetary health by disrupting ecosystems, endangering wildlife, and posing threats to human well-being.309 These contaminants exhibit characteristics such as persistence, bioaccumulation, and mobility, potentially forming enduring environmental footprints that jeopardize ecosystems.310 They can persist in the environment for extended periods without degradation, leading to bioaccumulation in organisms and the subsequent risk of reaching harmful concentrations. Many ECs demonstrate ecotoxicity, posing threats to aquatic life, plants, and other organisms; for instance, pharmaceuticals such as antibiotics and hormones can disrupt the endocrine systems of terrestrial and aquatic species, causing reproductive and developmental impairments.311 In natural settings, ecosystems often face mixtures of ECs rather than isolated substances, with interactions between these compounds potentially resulting in synergistic or antagonistic effects that amplify ecological risks.7 Moreover, some ECs, such as plastics and microplastics, can serve as carriers for other contaminants, facilitating their accumulation in aquatic organisms and potential entry into the food chain.312,313 Understanding the movement of ECs through the environment is vital for assessing their risks; factors such as volatility, solubility, and adsorption to soil particles influence contaminant transport through air, water, and soil, affecting their distribution and exposure pathways. Investigating the effects of ECs on soil, water, and air ecosystems is crucial for comprehensively evaluating their environmental implications. Here we consider some environmental quality implications of ECs in atmospheric, terrestrial, and aquatic systems.
Soil ecosystems
ECs, such as PPCPs, pesticides, and industrial chemicals, have been increasingly detected in soil environments worldwide.314 These contaminants threaten soil organisms, including bacteria, fungi, earthworms, insects, and plants. Exposure to ECs can adversely affect soil organisms, disrupting their physiological functions, reproductive capabilities, behavior, and overall health (Figure 4). Among these, PFAS and MPs have garnered much attention for their potential to alter the composition and functionality of soil bacteria and fungi.315,316,317,318 Studies indicate that exposure to such ECs can lead to shifts in microbial community structures,319,320 affecting the abundance and diversity of key microorganisms involved in nutrient cycling and organic matter decomposition.321,322,323 On the other hand, exposure to ECs can lead to the selection of bacteria and fungi that can catabolize these pollutants.324 Cyanotoxins can enter the soil through runoff and rainfall leaching. Accumulation of cyanotoxins in the soils can adversely affect plant health, animal health, microorganisms, and consequently soil health.325 Furthermore, cyanotoxins affect aerobic microbial communities at the sediment-water column interface, which may affect nitrogen transformation.326 The repercussions of these disruptions extend beyond the immediate microbial community, with potential consequences for soil health and ecosystem functioning. The metabolic activities of soil microorganisms, essential for maintaining soil fertility, are particularly vulnerable to ECs.327,328 The interference with microbial functions can hinder nutrient cycling processes, leading to imbalances in the availability of essential elements for retaining soil productivity.329,330 Additionally, the disruption of microbial communities may compromise the soil’s ability to resist pathogens and maintain resilience in the face of environmental stressors and climate change.331,332 The relationships between soil microorganisms and ECs necessitate further research to unravel the mechanisms underlying these effects and develop strategies for mitigating their impact on soil health.
Figure 4.
Interconnected negative impacts of ECs on human health, air quality, water systems, and soil ecosystems
The effects of ECs on plants reverberate through the entire ecosystem, influencing the structure and dynamics of plant communities.333,334,335 While the contaminants encompass a broad range, their overarching impact on plant health remains a common theme. ECs in soils can impede plant growth and development, posing challenges to individual species and the overall biodiversity of plant communities. One notable consequence is the alteration of nutrient uptake mechanisms in plants. ECs such as ENPs, PFAS, and MPs have been shown to interfere with the physiological processes that govern nutrient absorption.335,336,337 This disruption can lead to nutrient deficiencies, compromising the health and vigor of plant populations. Furthermore, contaminants may accumulate in plant tissues, potentially entering the food chain and posing risks to organisms feeding on contaminated plants (including human beings).338,339 Water transport mechanisms within plants are also vulnerable to the presence of ECs in soils. Certain contaminants can impede the movement of water through plant tissues. This disruption can cause reduced growth, altered reproductive patterns, and overall compromised resilience in plant communities.340,341,342 As we strive to understand the broader implications of ECs on soil plants, exploring the connections between soil, plants, and the myriad ECs that shape their interactions becomes imperative.
The impact of ECs on soil animals encompasses a wide range of organisms such as protozoa, earthworms, nematodes, and arthropods. The broad category of ECs, including but not limited to PFAS, persistent organic pollutants (POPs), and microplastics, have been reported to influence the ecological dynamics of soil animals, with cascading effects on the entire soil food web.343,344,345 Bioaccumulation is a common phenomenon observed in soil-dwelling organisms exposed to ECs. Contaminants accumulate in the tissues of these organisms, leading to elevated concentrations that can disrupt physiological functions and compromise overall health. This bioaccumulation introduces complexities to soil food webs, potentially affecting higher trophic levels that rely on soil animals for sustenance.346,347 Furthermore, soil animals can also act as carriers, leading to the migration of ECs.348,349 Nevertheless, the toxicity mechanisms of ECs in soil animals remain poorly understood. Ongoing research efforts should aim to elucidate how ECs affect soil animal populations, paving the way for informed conservation and management strategies to safeguard soil biodiversity.
In summary, the effects and impacts of ECs on soil ecosystems are complex and multifaceted. The overarching influence of these contaminants on soil microorganisms, plants, and animals underscores the need for comprehensive research to unravel the complex web of interactions within soil ecosystems. By examining the broader category of ECs without fixating on specific types, scientists can better comprehend the interconnected challenges posed by these pollutants. Continued investigation is essential to inform sustainable soil management practices that mitigate the adverse effects of ECs and preserve the health and functionality of soil ecosystems.
Aquatic systems
As a vital component of various ecosystems, the aquatic environment faces increasing challenges due to the presence of diverse ECs. For example, endocrine-disrupting compounds (EDCs) in water bodies can affect aquatic ecosystems.350 These compounds interfere with the endocrine systems of aquatic organisms, leading to disruptions in reproductive, developmental, and physiological processes.350,351 Recent studies highlighted the widespread occurrence of EDCs in water bodies, emphasizing their potential to disrupt the health of fish and amphibians.350,351,352,353 The bioaccumulation of EDCs in aquatic organisms underscores the need for continuous monitoring and regulatory measures to mitigate their impact. POPs, including PCBs, PFAS, and organochlorine pesticides, have long-lasting effects on water and sediment quality.354,355 Bioaccumulation of POPs in fatty tissues of aquatic organisms poses ecological risks.355 As noted above, PPCPs also enter water bodies through various pathways, raising concerns about their potential impact on aquatic organisms.215,356,357 Cyanotoxins provide a competitive advantage for cyanobacteria and drastically reduce the populations of certain species in aquatic ecosystems, upsetting the ecological balance.358,359 At the same time, cyanotoxins can cause water pollution problems and directly threaten drinking water quality.360 Cyanotoxins may drastically reduce the populations of some species in water bodies, upsetting the original ecological balance. Secondly, the toxic effects of cyanotoxins may also affect the variety and abundance of microorganisms in water bodies, thereby interfering with aquatic ecological processes. In some locations at some times, cyanotoxins and other toxins produced by harmful algae blooms can represent the greatest EC water quality threat to public health and ecosystems.361,362
To allow for ENP-tailored risk assessment, the developers and regulators must know the most important parameters governing the behavior and toxicity of ENPs. Engineering nanomaterial wastes in the environment are not easy to degrade and will accumulate and remain in the soil and higher plants through transport, which is bound to have a significant impact on the growth of higher plants.363 The biological effects of ENPs on higher plants can directly affect ecosystems' health, stability, and sustainable development.363 On the one hand, the presence of ENPs (such as TiO2 NPs, ZnO NPs, Fe3O4 NPs, and CNTs) can have a catalytic interaction on plant growth by increasing root activity, increasing water absorption, enhancing photosynthesis, or improving rhizosphere soil microbial communities and increasing metabolic enzyme activity. Several recent reviews have discussed ENP accumulation in terrestrial plants, which can induce physiological and biochemical responses in plants.364,365,366 Cao et al.367 documented impacts on carbon fixation and water use efficiency during photosynthesis in response to CeO2 NP exposure, which may indirectly influence soil organisms via the effect on soil moisture. On the other hand, ENPs (such as ZnO NPs, AgO NPs, CuO NPs, and CeO2 NPs) may be potentially harmful to biota via reducing seed germination, generating ROS, enhancing membrane permeability, inhibiting antioxidant enzyme activity, or damaging root hairs through physical friction. Mechanism and mode of toxicity vary among ENPs.368 Oxidative stress is a frequently reported phenomenon.369 At present, research on the biological effects of ENPs mainly includes the mechanism of toxicity of ENPs to plants under different conditions and the role of ENPs in environmental systems from the perspective of organisms. Although the research has enhanced the theoretical value of nanobiology effects and toxicity research to a certain extent, there are often many contradictions in related research results. This is due to differences in the physical and chemical properties of ENPs themselves (such as composition, shape, surface coating, and charge), or differences in culture substrates, treatment methods, and plant species, resulting in different stability and biocompatibility in the environment. This then affects the interaction between ENPs and plants.370 ENPs accumulated in plants may also spread through the food chain to higher trophic organisms, causing certain ecological risks.
In recent years, several independent studies have shown that AgNPs exhibit certain cellular or systemic toxicity to cells and body systems under both in vitro and in vivo conditions.371,372 Ag+ mainly exerts cellular/bacterial toxicity through the following toxicological mechanisms: (1) interfere with the normal Na+ and K+ ion channels on the cell membrane, resulting in the imbalance of the membrane potential inside and outside the cell,373 or bind to the sulfhydryl-containing (-SH) proteins on the cell membrane to inactivate them, destroy the barrier function and material exchange function of the cell membrane, and directly lead to cell necrosis373; (2) enter the cytoplasm of cells, interact with sulfhydryl-containing proteins, and destroy the protein structure, resulting in the inactivation of biologically active enzymes, the imbalance of intracellular REDOX reaction, and the generation of a large number of ROS leading to cell damage.374 Nano-copper, a prominent metal nanomaterial, finds widespread use across various domains.375,376 However, concerns have been raised regarding the significant harm nano-coppers can pose to human health and environmental safety. The liver is the main organ that is influenced by nanomaterials because it is the main organ involved in the metabolism of CuNPs.377 Tang et al.378 also found that the liver was the target organ for the accumulation of copper NPs through gavage. Lei et al.379 found that CuNPs could significantly increase triglyceride and phospholipid levels in the body through NMR technology and pattern recognition methods. Oral administration of CuNPs can cause hepatomegaly, hepatocyte necrosis, and hepatic insufficiency in rats and mice.380 In addition, Cu exposure can also produce significant toxic effects on the kidney, spleen, nerve, and gastrointestinal tract.379,381,382,383
Biological contaminants influence the microbial composition of sediments. The introduction of ARGs and drug-resistant bacteria into sediments can alter the balance of microbial communities, potentially affecting nutrient cycling, sediment stability, and other crucial ecological processes.384 Viruses and pathogenic bacteria contribute to microbial contamination, affecting water quality in aquatic environments.385,386 Elevated microbial loads can compromise the safety of water for both aquatic life and human consumption, leading to the spread of waterborne diseases and affecting overall ecosystem health.387,388 Biological contaminants can also be toxic to aquatic plants. Viruses, pathogenic bacteria, and other biological agents may induce stress on plants, affecting their uptake of nutrients, growth rates, and overall health.388,389 These effects can lead to changes in the abundance and distribution of aquatic vegetation. Aquatic plants can serve as vectors for transmitting pathogenic bacteria and viruses.390,391 The presence of drug-resistant bacteria in plant tissues may contribute to the dissemination of antibiotic resistance in aquatic environments.391,392 This transmission pathway can have cascading effects on the health of associated aquatic fauna. Aquatic fauna are susceptible to infections caused by biological contaminants. Viruses, pathogenic bacteria, and antibiotic-resistant organisms can compromise the immune systems of aquatic organisms, increasing their vulnerability to diseases.393 This heightened disease susceptibility may lead to declines in the population of aquatic organisms and disruptions in the ecological balance of aquatic ecosystems.394,395,396 Emerging biological contaminants can affect the reproduction and development of aquatic animals.394,395,396 Genetically modified organisms (GMOs) and RNA-based technologies, such as RNAi, can introduce novel genetic material into aquatic ecosystems. RNAi and other biological contaminants may interfere with the normal reproductive processes of aquatic organisms, potentially leading to reduced reproductive success, developmental abnormalities, and altered population dynamics.395,397
Microplastics act as carriers of various pollutants, such as PCBs, PAHs, and heavy metal(loid)s.398 These contaminants can leach from the surface of microplastics, leading to chemical contamination of water and sediments.399 This process introduces a new dimension of pollution to aquatic environments, affecting the overall quality of these habitats.400 Microplastics can also negatively affect the physiology of aquatic plants, affecting processes such as photosynthesis and nutrient uptake.401,402 This can lead to reduced growth rates, altered reproductive patterns, and diminished aquatic plant health.401,402 Moreover, microplastics are often mistaken for food by aquatic organisms, leading to ingestion at various trophic levels.403 This ingestion can cause physical harm, including internal injuries, blockages, and interference with digestive processes. The presence of microplastics in the digestive tracts of aquatic animals can also lead to malnutrition and reduced energy reserves.404,405,406 The toxicological consequences of microplastic-associated contaminants include disruption of endocrine systems, suppression of the immune system, and increased susceptibility to diseases. These effects can have profound implications for the health and survival of aquatic fauna.407,408
Other ECs, such as liquid crystals, oil spills, prions, and a class of ECs called unknown or variable composition, complex reaction products, or biological materials (UVCBs), can also introduce hazardous substances into aquatic environments. The discharge of liquid crystal contaminants can disrupt water quality and affect the health of aquatic organisms.409 The presence of these compounds may alter nutrient cycling and cause ecological imbalances.409 Organometals, such as organomercury and organotin compounds, exhibit high toxicity to aquatic organisms. These contaminants can interfere with cellular functions, impair reproduction, and cause behavioral changes in fish and invertebrates.410,411 Accumulation of organometals in sediments may have long-term implications for benthic communities. Oil spills and organic solvents, including methyl tert-butyl ether (MTBE), can contaminate habitats.411,412 These contaminants can form slicks on the water surface, affect light penetration, and reduce oxygen exchange. The effects include the smothering of aquatic vegetation and disruption of feeding behaviors in aquatic animals.411,412 Prions associated with neurodegenerative diseases can enter aquatic environments through various pathways.413 The presence of prions may pose a risk to the health of aquatic animals, potentially leading to neurological disorders. The effects on fish and other aquatic organisms are not fully understood but warrant further investigation.413,414 The UVCBs may also have unpredictable impacts on water and sediment quality and the health of aquatic plants and animals.415 Therefore, much research is needed to understand the specific effects of individual UVCBs.
Air quality
While the Industrial Revolution was a great success in technology, society, and services, it also introduced a significant quantity of harmful pollutants into the atmosphere.416 These air pollutants can penetrate the respiratory system through inhalation. Meanwhile, for certain compounds, direct air-to-skin dermal uptake is comparable to the inhalation intake, imposing a significant burden on human health.417,418,419,420,421,422,423,424
Numerous animal and human studies have shown that exposure to air pollutants, including ECs (e.g., PAHs, perfluoroalkyl sulfonate, organophosphorus ester, polybrominated diphenyl ethers [PBDEs], and paraben), can contribute to respiratory,425 dermal,426 cardiovascular,427 and immune disease428 and mortality.429 Early exposure to these pollutants in humans tends to trigger and exacerbate multiple diseases in their later life.428,430 It is confirmed that these air pollutants contribute to the production of ROS in mitochondria, cell membranes, and endoplasmic reticulum, ultimately leading to cell injury and adverse outcomes.431,432 Furthermore, these airborne pollutants can greatly affect ecology and human health because of their long-range transport, persistence, and toxicity.105 Importantly, complex airborne ECs can cause unpredictable toxic effects and health risks by interfering with transport, metabolism, and bioavailability after entering the human body.433 For example, triphenyl phosphate levels on the skin surface of e-waste dismantlers were negatively correlated with the levels of three thyroid hormones used to evaluate thyroid function.434 Human exposure to pollutants from coking contamination, including aromatic compounds mixture, metabolites of PAHs and their derivatives, chlorophenols, and nitrophenols, could increase DNA damage and lipid peroxidation, which is associated with increased disease risks.200,201 Unexpectedly, residents near coking plants faced a 1.4-times higher risk due to coking contamination.201 In addition, concentrations of ECs in the atmosphere reached thousands of picograms per cubic meter in emission sources or urban air.105 The presence of ECs also appeared in remote areas, such as the Arctic region, because of their persistence and long-range atmospheric transport.435,436
Notably, bioaerosol is another air pollutant of concern, which is a subset of atmospheric particles composed of bacteria, fungi, viruses, and their products, ranging in size from 0.001 nm to 100 μm. Cyanotoxins may enter the atmosphere in the form of aerosols and spread further afield, posing a potential threat to atmospheric safety and contributing to the ecological risk of “air eutrophication.”437,438 Bioaerosol is commonly released into the atmosphere from soil, water, vegetation, animals (including humans), composting, sewage treatment plants, landfills, farms, and healthcare sites.439,440,441,442 Due to the diffusion of plant pollen, spores, and reproductive units of microorganisms, bioaerosols can be transported over long distances across geographical barriers,443 posing a high public health risk. The occasional epidemiological spread of bioaerosol components can be highly disruptive to societies and economies, as demonstrated by the COVID-19 global pandemic.444 A previous study summarized the size-dependent particle deposition law of bioaerosols in different areas of the respiratory tract, showing that particles with a particle size larger than 0.5 μm mainly deposit in the head airway through natural sedimentation and impact, while particles with a particle size smaller than 0.5 μm can reach the lower respiratory tract through further diffusion.445 It further leads to health complications, such as allergic reactions, infectious diseases, acute toxic effects, respiratory diseases, neurological effects, and toxic reactions to cancer and non-specific symptoms.446
Risks to human health
Various ECs stemming from sources such as industrial discharges, agricultural runoff, and improper waste disposal can permeate the soil, water bodies, and the air, establishing intricate exposure pathways for wildlife and humans.447 These substances may enter the human body through various exposure routes, including ingesting contaminated water or food, inhaling air pollutants, and dermal contact with contaminated surfaces.448,449,450 Their persistent nature, mobility, and potential to accumulate in the environment heighten the risks of exposure, intensifying their impact on health.449,451 We recognize the diverse literature that has examined the public health effects of ECs, and, instead of providing a comprehensive critical review, here we aim to highlight some of the related efforts in this fast-moving area of basic and translational research.
The onset of various omic approaches, including genomics, proteomics, transcriptomics, and metabolomics, has enabled the detection of molecular-level perturbations due to environmental exposure and become increasingly used to investigate how environmental contaminants alter the biological function of organisms.452,453 Often, these findings are integrated within the development and application of adverse outcome pathways, which are chemically agnostic conceptual models that link molecular initiation events to higher levels of biological responses of relevance to chemical risk assessment.454
Because the experimental design of metabolomic approaches is highly versatile, it can be applied to study multiple scenarios with various environmental conditions and different organisms, as well as complex contaminant mixtures and wastewater effluents.452,455,456 Many studies have demonstrated the high utility of metabolomic approaches to rapidly detect fundamental shifts in organism function for a host of environmental model organisms and demonstrated how these approaches can complement traditional toxicity indicators.107,181,457,458,459 Despite widespread concern, understanding the human health risks and toxic mechanisms remains challenging because of their dynamic nature, complex compositions, and interactions of contaminants and their mixtures, which present difficulties for conventional monitoring and modeling frameworks.449 Nonetheless, evidence, although generally not well established, has suggested that exposures to ECs are associated with the following diseases.
Antibiotic resistance and infectious diseases
One of the pressing and increasing health threats posed by ECs is the rise of antibiotic resistance. PPCPs (e.g., antibacterial creams and ointments), when improperly used, disposed of, or inadequately treated, could contribute to the development of ARBs.242,460,461 This outcome poses a significant threat to public health as conventional treatments become less effective, leading to an increased prevalence of infectious diseases.461 For example, symptoms of infectious diseases, particularly those related to airway infections (e.g., lung infections), were much more common among individuals with compromised health or chronic conditions who used antibacterial medications.462 It has also been suggested that antibiotic resistance could amplify the mortality risks during pandemics of bacterial diseases, including tuberculosis and cholera, and even viral diseases, particularly in the case of influenza, where a significant proportion of deaths often is caused by bacterial pneumonia coinfections.463
Endocrine disruption and reproductive disorders
Endocrine-disrupting chemicals, such as bisphenol A (BPA) and phthalates in plastics, represent a class of ECs that mimic or interfere with the endocrine hormones, often acting as agonists or antagonists. Endocrine-disrupting chemicals primarily target the female reproductive system. They can increase the risk of various reproductive disorders, including fertility issues, developmental abnormalities, and hormone-sensitive cancers (e.g., breast cancer).464,465,466 For instance, individuals with polycystic ovarian syndrome (PCOS), a condition affecting nearly 10% of women of childbearing age with unclear etiology, have been found to have higher BPA in their serum, urine, and follicular fluid compared to those without PCOS, suggesting that BPA exposure is an important contributor to the pathogenesis of PCOS.464
Cardiopulmonary diseases
Airborne particulates can carry various ECs, including heavy metals (metalloids), POPs, NPs, and even viruses.467 The respiratory and cardiovascular systems become the primary targets, with potential consequences ranging from irritations (e.g., coughing) to chronic cardiopulmonary diseases (e.g., hypertension and chronic obstructive pulmonary diseases).468,469 A recent meta-analysis comprising 13 studies showed that higher exposure levels of PFAS, especially for perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), and perfluorononanoic acid, were significantly associated with a higher risk of hypertension.470 Notably, particulates with smaller sizes are much more harmful than larger particles because of the longer residence time and greater capacity for deeper penetration in the respiratory tract.106,471 Therefore, the airborne fine particulates could further amplify the health risks of the ECs contained. For instance, the interaction between airborne fine particles and viruses, such as H1N1, has been shown to extend viral distribution and aggravate respiratory tract infection.467
Neurotoxicity
Substantial evidence suggests that certain ECs, such as heavy metal(loid)s (e.g., arsenic and mercury), cyanotoxins,472 and POPs (e.g., perfluoroalkyl compounds), possess neurotoxic properties.473,474 Chronic exposure to these substances is associated with an increased risk of neurological disorders, including cognitive impairments, developmental delays, and neurodegenerative diseases.475,476 Even at low concentrations, these substances could exhibit great and long-lasting neurotoxicity.477 Early-life exposures are identified as a critical causal factor for the later development of Alzheimer’s and Parkinson’s diseases.478 Regions contaminated with PFAS in the drinking water exhibited a 33% higher mortality from Alzheimer’s disease compared with uncontaminated areas.479
Immune system impacts and allergic reactions
ECs may influence the immune system, potentially leading to compromised immunity or triggering allergic reactions. Studies have reported that these substances can affect the activation and survival of immune cells, potentially contributing to allergic rhinitis and other allergic responses.480 For example, epidemiological studies have demonstrated the immunosuppressive effects of PFAS on pediatric vaccination and other immune-related responses for both children and adults (e.g., diminished antibodies after vaccinations, increased risk of asthma).477,481,482 A most recent study showed that prenatal exposure to PFOS and PFOA increased the risk of non-atopic asthma at the age of 6 years by up to 2-fold.483
The description of EPFR reactivity and risks
Recent research has focused on understanding the properties and potential hazards of EPFR-containing particles. These particles have been found to display significant reactivity and toxicity, which is a cause for concern.484,485 As a result, it is crucial to establish parameters to describe their reactivities to better understand their potential impact on human health and the environment. One potential parameter to describe the reactivity of EPFR-containing particles is the intensity of EPFR signals. However, this approach has limitations, as the detected EPFR signals are associated with various structures with different reactivities.486 Additionally, the captured ROS may not fully explain the reactivity of EPFRs, as their reactivity may occur through direct contact with target reactants without the generation of ROS, and the instantly captured ROS signals may not represent the reactivity of long-lasting EPFRs.487 Further research is necessary to develop a proper parameter that correlates with the reactivity of EPFRs, which differs from the detected EPR signals, to evaluate their environmental implications accurately. Additionally, researchers should consider that EPFRs coexist with other chemical components, such as the parent chemicals, their degradation by-products, and reactive inorganic particles. The impacts of these coexisting components should be considered when identifying the reactivities or risks of EPFRs.
The reactivities of EPFRs can lead to both adverse and beneficial effects, making their manipulation highly context dependent. When EPFRs have detrimental environmental impacts, efforts should be made to mitigate or eliminate them. Conversely, if EPFRs play a positive role in pollution control, their influence should be enhanced and utilized, as seen in applications such as biochar for organic contaminant degradation.488 Although EPFR formation has been studied in various processes,489,490 understanding the preferred or unpreferred conditions for EPFR formation and quantitative descriptions of their generation and decay kinetics remains limited. EPFRs differ from common contaminants, being highly dynamic and composed of various structures, necessitating studies on their environmental behavior and risks and the development of standardized experimental protocols and standard reference samples.
In summary, the health risks associated with ECs will continue to be a major public health concern. More high-quality evidence and comprehensive strategies are urgently needed to better understand and mitigate their health effects. This requires interdisciplinary efforts, from establishing standardized contamination and public health surveillance systems to employing advanced epidemiological and molecular modeling and implementing evidence-based strategies. As we navigate this complex terrain, prioritizing research, regulatory measures, and public awareness will be paramount to curbing the adverse health effects of ECs and ensuring a healthier future for all.
Model-based assessment of fate and toxicological risks of ECs
Modeling migration and environmental impacts of ECs
The development of mathematical models to understand the migration and impacts of ECs in water, soil, and air ecosystems is a current focal point in environmental pollution research.491 These models serve as valuable complements to monitoring networks, enriching our comprehension of EC sources, distributions, and life cycles. They also offer insights into the influencing mechanisms and environmental factors shaping EC dynamics. By facilitating comprehensive risk assessments for both human health and ecosystems, EC models play a pivotal role in providing early warnings, projecting outcomes under future climate scenarios, and evaluating the efficacy of remediation technologies.
Quantitative structure-activity relationships (QSARs), one class of numerically analytical models that are developed highlighting the intrinsic correlations with or dependency on a pool of topologically, spectrally, and physicochemically interpretable structural information, can be used as an alternative approach to unravel the toxicologically relevant or environmental influencing mechanism and the structural requirements for transfer, migration, and toxicity of ECs. Furthermore, QSARs developed using advanced statistical methods, such as machine-learning techniques, along with comprehensive datasets encompassing not only structural descriptors but also environmental factors, can effectively predict the environmental fate of ECs, including volatilization, photodegradation, and bioaccumulation..492,493 Although QSARs were classically applied to the virtue screening of novel effective drugs for human health, the application of QSARs in environmental research arouses new vitality and greatly facilitates understanding the cause for the variance of toxicology and behavior of pollutants and even provides basic data guiding risk management and remediation administration. Nevertheless, the development of QSAR models is typically hindered by several limitations. These include a scarcity of experimental training data, issues related to over-fitting and noise in statistical techniques, and a lack of consideration for environmental factors. These environmental factors play a crucial role in influencing the transport, precipitation, adsorption, and desorption processes in environmental matrices. On the contrary, the stability, reliability, and predictability of QSARs would be enhanced if meta-learning big data were involved in development.494 The integration of environmental and structural factors of ECs is likely to aggravate the uncertainty of QSARs because they are hardly accommodated with the significant correlation in one model, whereas it is of particular interest for augmentation of the QSAR applicability domain. Given the numerous limitations of QSARs, high uncertainty or application factors are applied to QSAR modeling outputs during early tiers of risk assessment.
Various modeling approaches have been developed to study the transport and impacts of ECs, including fate and transport models, multimedia models, and pharmacokinetic models. Fate and transport models simulate the movement and transformation of pollutants in different environmental compartments, such as air, water, soil, and biota.495 Multimedia models integrate the fate and transport processes across multiple compartments to assess the overall environmental behavior of pollutants on regional to global scales.490 Pharmacokinetic models focus on the uptake, distribution, metabolism, and elimination of pollutants within organisms. These models draw from the findings of laboratory and field experiments to represent the physicochemical, mineralogical, and hydraulic properties of ECs, adsorption-desorption, chemical/biological transformation, and their retention in and exchange across environmental compartments.
Numerical models that integrate multiple components, multiphase flow, and multiple reaction mechanisms have become the mainstream for simulating ECs in soil-groundwater systems. Notable examples include TMVOC,496 TOUGHREACT,497 RT3D,498 PFLOTRAN,499 and PHT3D.500 For ECs in ecosystems, bioaccumulation models have been developed to integrate ecological principles, dynamic processes, and complex environmental conditions to describe and predict contaminants accumulation and migration processes within ecosystems, such as CalTOX501 and KABAM.502 However, due to the complex toxic mechanisms and biological effects involved in the transport processes of ECs in organisms,150 there is currently a lack of universal, process-based models for the migration of ECs in ecosystems.
Research efforts have increasingly focused on exploring the potential of atmospheric transport as a significant mechanism for redistributing ECs across various environmental compartments on both regional and global scales. To study this phenomenon, scientists have developed trajectory models as well as regional and global three-dimensional chemical transport models. These models aim to simulate the transport and evolution of a wide array of ECs, including microplastics, POPs, PFOS, and PAHs. For instance, certain POPs undergo long-range atmospheric transport, leading to their subsequent deposition onto the Earth’s surface and potential re-emission.503 This process, commonly referred to as "hopping," facilitates the rapid transport of POPs to northern high latitudes at rates approximately 10 times faster than in tropical regions.495 Studies also reveal that, despite global reductions in PAH emissions in recent decades, the concentrations of airborne PAHs in the Arctic region have not shown a significant decline because of the offset from increased volatilization from surfaces (e.g., ocean, snow, ice, permafrost, and soil) because of climate warming.504
Despite recent progress, important challenges remain in modeling ECs to understand their fates and impacts. The scarcity of observations is a key limiting factor in evaluating the models of most ECs, which calls for the design of multi-scale observation networks guided by models. Additionally, in the case of many ECs, there is still a lack of comprehensive understanding of transport and fate processes and toxicology within and across environmental compartments. In particular, researchers have increasingly highlighted the complex impacts of multi-pollutant interactions. Finally, the framework to represent ECs through different environment compartments may see a revolution catalyzed by the rapid development of Earth-system models.
Advancing evaluation and management of ECs through AI
In recent years, there has been a significant increase in the use of machine learning to understand and predict the chemical reactivity, toxicity, transport, and remediation of environmental contaminants.505 Among the various environmental contaminants being explored by these computational methods, PFAS have garnered particular scientific attention.506,507 The majority of machine-learning studies on PFAS have focused on supervised learning techniques, with only a handful of studies using unsupervised learning approaches. Within the former, the Wong group carried out the first machine-learning study on PFAS to predict and rationalize carbon-fluorine (C–F) bond dissociation energies to aid in their efficient treatment/removal.508 Using random forest, least absolute shrinkage and selection operator regression, and feedforward neural networks, accurate predictions for C–F bond dissociation energies within chemical accuracy of the PFAS reference data were obtained (deviations less than 0.70 kcal/mol). In addition, this pioneering study demonstrated the efficiency of the machine-learning approach, which required less than 10 min to train the data and less than a second to predict a new compound’s C–F bond dissociation energy.
Within the area of unsupervised machine learning, new unsupervised/semi-supervised machine-learning models have been created to automatically predict the bioactivities of PFAS in various human biological targets, including enzymes, genes, proteins, and cell lines.509 The semi-supervised metric learning models were used to predict the bioactivity of PFAS found in the recent Organisation of Economic Co-operation and Development (OECD) report list, which contains 4730 PFAS used in a broad range of industries and consumers. Other studies have also used machine learning to predict the bioconcentration of organic contaminants by plants, the ecotoxicity of chemicals, and the dissipation of organic contaminants in plants.510,511 Together, these studies highlight the capabilities of machine learning to understand the reactivity of PFAS, which other researchers can leverage to predict and screen other environmental contaminants.
AI is poised to revolutionize pollution control at the source, sustainable remediation of contaminated sites, and the implementation of sustainable management practices to prevent contamination (Figure 5). Through the utilization of AI technologies, such as machine learning and deep learning, significant progress can be achieved in addressing environmental challenges.176 AI can improve the efficiency and effectiveness of pollution-control measures by analyzing intricate datasets, forecasting contaminant behavior, and refining remediation strategies.512 Furthermore, AI can play a pivotal role in monitoring air and water quality, pinpointing pollution sources, and predicting the dispersion of pollutants to enable prompt and targeted remediation actions. In addition, AI-driven digital simulations and digital twins can replicate environmental scenarios, assess remediation approaches, and monitor the success of mitigation efforts to enhance decision making and resource allocation in pollution management.513 Overall, AI serves as a potent tool for enhancing environmental sustainability by offering data-driven insights, optimizing remediation endeavors, and advocating proactive measures to safeguard the environment.
Figure 5.
Strategies for controlling ECs encompass various measures, including pollution control at the source, sustainable remediation to clean up contaminated sites, and sustainable management practices to prevent contamination
Global efforts to control ECs
Pollution prevention
The increasing recognition of ECs has led to global efforts to devise efficient strategies for their prevention, detection, and remediation (Figure 5). Governments worldwide have initiated policies to encourage industries and economic sectors to reduce source pollution by changing their production processes, operations, and material usage. For instance, the European Union has implemented a series of policies and regulations to ensure the protection of the environment and reduce pollution. One of the key components of this environmental framework is the Integrated Pollution Prevention and Control (IPPC) directive, which came into effect in 2008 to prevent and reduce pollution from industrial operations.514 This directive applies to various sectors, including energy, mining, and manufacturing, and requires industries to adopt best available techniques (BATs) to reduce emissions and waste generation.515 The IPPC directive complements other regulations such as the Registration, Evaluation, Authorisation, and Restriction of Chemicals (REACH), the Zero Pollution Action Plan, and the “polluter-pays” principle.516 These regulations hold industries and businesses accountable for their environmental impact, promote sustainable practices, and ensure the long-term health and well-being of people and ecosystems. Similarly, the United States Congress enacted the Pollution Prevention Act (PPA) to promote industry pollution prevention and reduction efforts. The act aimed to encourage businesses to adopt practices that would minimize or eliminate pollution at the source through changes in production processes, operation methods, and the use of raw materials.
To balance economic development and environmental stewardship, the Chinese government has implemented diverse laws and regulations to address pollution and enhance the country’s environmental conditions.517 The Environmental Protection Law (EPL), initially enacted in 1989 and revised in 2014, stands as a cornerstone of legislation governing environmental protection in China.518 Beyond the EPL, the country has introduced specific laws and regulations focusing on distinct facets of environmental protection, encompassing the Water Pollution Prevention and Control Law, Air Pollution Prevention and Control Law, Soil Pollution Prevention and Control Law, and recently the Action Plan for Controlling Emerging Contaminants in 2022 issued by the state council. Several other countries have also enacted numerous regulations to prevent pollution and minimize environmental contamination across different industries. The United Nations Environment Programme has reported a 38-fold surge in environmental legislation implemented from 1972 to 2019.519 As of 2017, 176 countries possess legislative frameworks for the environment, and 150 countries have incorporated environmental protection or the entitlement to a healthy environment in their constitutions.520 Additionally, 164 countries have instituted government-level entities tasked with overseeing environmental protection.
Despite these advancements, enforcing these laws faces challenges, as weak enforcement is a global trend exacerbating environmental threats. The difficulties in enforcing global environmental law stem from the reluctance of individual states and the lack of effective enforcement mechanisms on the international level.521 While there has been a rise in cooperative international efforts to protect the environment, the enforcement of these laws remains a common issue. The disparity in environmental protection legislation among high-, middle-, and low-income countries may result in outsourcing production-linked emissions to low-income countries. In some developing nations, the execution and enforcement of pollution control policies are hindered by underfunded and politically weak government bodies responsible for implementation, which hampers effective enforcement.522 The focus on economic growth over environmental protection during the transition phase has resulted in inconsistent and incoherent environmental laws and regulations.523 Additionally, weak property rights, poor access to credit, and limited technology choices distort the costs of improvements to environmental quality, further hindering effective pollution control.524 The inadequacy of current pollution-prevention measures necessitates more concrete actions to address pollution on a global scale. Several strategies can be implemented to achieve this, including enforcing regulatory frameworks, adopting sustainable practices, promoting technological innovation, and engaging the public actively. These multifaceted approaches are essential for reducing pollution levels and ensuring the preservation of the environment for future generations. Herein, green chemistry presents particularly important opportunities for innovation and pollution prevention as we strive to achieve the UN’s Sustainable Development Goals.525 However, as identified by Erythropel et al.,78 two green chemistry principles of particular relevance to ECs, design benign chemicals (principle 4) and design for degradation (principle 10), have received relatively little attention, and thus represent timely research opportunities for pollution prevention.
Pollution remediation technologies
Many remediation technologies have been developed to tackle the urgent global problem of the environmental accumulation of anthropogenic pollutants.526 These technologies are vital in cleaning up contaminated sites and restoring them to environmentally acceptable conditions. Remediation methods span a spectrum of approaches, including physical techniques such as excavation, soil vapor extraction, and chemical and biological treatments designed to degrade or immobilize contaminants in both soil and water.527 The choice of a specific environmental remediation method is contingent on the type and extent of contamination, with each method having its own set of advantages and disadvantages.
Physical remediation techniques employ various processes and technologies to extract pollutants from the soil, restoring its usability. These encompass physical engineering measures, soil heat-treatment technology, and adsorption technology.528 Soil heat technology, conventionally used for pollutant removal via soil-heating-induced volatilization, also emerges as an alternative for enhancing soil conditions.529,530 Additionally, adsorption, a conventional physical remediation method, relies significantly on the robust adsorptive capacities of activated carbon and biochar materials.531,532 The large specific surface area, porous structure, and various forms of activated carbon enable efficient absorption of a broad spectrum of pollutants.533 Biochar, recognized as an environmentally friendly material, not only plays a pivotal role in alleviating soil contamination but also enhances the properties of degraded soil, serving as an ideal habitat for beneficial microbes.534,535 Ultrasonic waves are effective in destroying the structure of algae cells through the mechanical vibration effect.536 Additionally, physical methods such as manual salvage or mechanical algae removal equipment can be used to prevent the accumulation of cyanobacteria. Furthermore, the photocatalytic degradation of cyanotoxins can be achieved through the use of ultraviolet or visible-light irradiation.537
The handling of environmental pollutants such as agricultural film and other plastic waste could be effectively addressed through physical recycling methods followed by the reuse of processed materials.538,539 Physical recycling involves systematically sorting, cleaning, shredding, and melting of plastic waste to create new raw materials. These materials find application in producing diverse items such as fertilizer bags, garbage bags, and agricultural recycling water pipes.540 This approach not only mitigates the environmental repercussions of plastic pollution by diverting waste from landfills but also contributes to diminishing the need to produce new plastic. However, it is essential to acknowledge that mechanical recycling processes, including sorting, grinding, washing, drying, and re-granulation, may introduce pollution, such as volatile organic compounds and microplastic emissions.541 The economic viability of the recycling technology is also a major consideration.
In the field of chemical remediation, emphasis is placed on altering and diminishing the mobility and harmfulness of environmental contaminants through a range of chemical methods. Such techniques encompass photolysis, Fenton reactions, photocatalytic processes, and electrochemical remediation strategies.542 Photolysis leverages light radiation to decompose contaminants in soil, water, or air.543 When contaminants are exposed to light radiation, the energy from the light can initiate chemical reactions that break down the contaminants into less harmful substances. Photolysis is particularly effective for degrading organic compounds, such as industrial chemicals, that are difficult to remove using other methods.544
Fenton technology is a chemical remediation method that uses the oxidation of iron ions (Fe2+) in the presence of hydrogen peroxide to generate hydroxyl radicals.545 These hydroxyl radicals are highly reactive and effectively oxidize pollutants, transforming them into less toxic substances. This process has been widely studied and applied to treat various types of contaminated water and soil.546 Photocatalysis is another commonly used chemical remediation method that involves using catalysts to produce hydroxyl radicals, which then facilitate the rapid oxidation and decomposition of pollutants.547 This method has shown promise in treating organic pollutants and has been extensively researched for its potential applications in environmental remediation.548,549 Electric remediation, also known as electrokinetic remediation, uses direct electric current to remove organic and inorganic contaminants from contaminated soils550 by enriching contaminants to either the cathode or anode zone through electroosmosis, electromigration, and electrophoresis under an electric field.551 This technology is considered environmentally friendly and can be used to migrate and remove pollutants from the soil and sediment matrix.
While physical and chemical remediation methods have played crucial roles in combatting environmental contamination, they come with inherent limitations, including the necessity for advanced infrastructure, skilled personnel, high processing costs, increased reagent requirements, and the potential generation of secondary pollutants. For instance, in situ chemical oxidation is considered a rapid and effective means of eliminating organic pollutants from contaminated areas.552,553 However, it is expensive and can yield undesirable harmful oxidation by-products, further harming the environment. Additionally, potent oxidizing agents pose substantial health risks to those handling them, underscoring the ongoing need for research and innovation in developing more sustainable and efficient remediation strategies.554
Bioremediation is a remediation approach that uses a biological system, such as bacteria, fungi, microalgae, or plants, to eliminate or neutralize pollutants from a contaminated site.555 This method is considered cost-effective because of the relatively low cost of implementing and maintaining bioremediation systems compared with other remediation techniques.556,557 Additionally, bioremediation is viewed as an eco-friendly approach because it relies on natural processes and does not involve the use of harsh chemicals that may further harm the environment. Furthermore, bioremediation is socially acceptable as it aligns with the growing emphasis on sustainable and environmentally conscious practices.554 While bioremediation is a promising approach for managing pollutants in the environment, its full potential has yet to be realized because of several challenges associated with its implementation in natural environments.558 One of the primary challenges is the poor colonization and performance of inoculated microbes in natural environments. When introduced into contaminated sites, these microbes may struggle to survive and effectively degrade pollutants due to competition with native microorganisms, limited availability of nutrients (including trace concentration of micropollutants well below KM),559,560 and adverse environmental conditions.561 Additionally, the use of plants in bioremediation can be time consuming, as they require sufficient time to grow and establish themselves before they can effectively remove pollutants from the environment. Furthermore, high concentrations of mixed pollutants in contaminated sites can inhibit the growth of both plants and microbes, limiting their ability to remediate the environment.562 The environmental heterogeneity of contaminated sites also poses a challenge, as different areas within a site may have varying levels and types of contamination, requiring a tailored approach for effective remediation. To address these challenges, researchers have proposed integrating plants, adsorbents (such as biochar), and microbes into a single system for remediating contaminated sites.554,563 This integrated approach aims to leverage the complementary abilities of plants, adsorbents, and microbes to enhance the overall remediation process. By combining these elements, researchers seek to create a synergistic system that can more effectively mitigate the challenges associated with bioremediation and improve its overall performance in diverse environmental settings. Microbiome management is also an interesting development perspective in bioremediation.564
Sustainable management strategies
In addressing the challenges of ECs, sustainable management plays a pivotal role in their control and governance. Emphasis should be directed to advancing technologies for the management of ECs and undertaking critical research on environmental risk assessment and management of toxic and hazardous chemicals. Further research on the ecological and environmental harm mechanisms of ECs should be accelerated, and investments should be made in research on new theories and technologies for sustainable management strategies related to ECs. An environmental risk management information system for chemical substances should be established, and a platform for calculating toxicology and exposure prediction of chemical substances should be built. The early assessment and identification of key pollutants are essential for efficient control. Besides, innovation and education in green and sustainable chemistry, technology, and engineering can promote the generation of greener and more sustainable products and processes.565,566
Enterprises associated with emerging pollutants should actively implement their primary responsibility; increasing national and corporate investment in scientific research is imperative for effective governance of emerging pollutants. In recognizing that scientific research is fundamental to decision making in pollution control, sustained efforts are needed to enhance technological input. This effort involves understanding potential emerging pollutants' origins, trends, hazards, and control technologies. Scientific decision making facilitates precise and effective pollution-control measures.
Actively engaging in international cooperation is crucial, especially in cases where comprehensive research information is lacking. Utilizing global expertise and experiences in scientific research and management accelerates the screening and environmental risk control of emerging pollutants. Simultaneously, mechanisms for fund allocation are established, drawing insights from international conventions to support pollution-control initiatives at international, national, regional, and corporate levels.
Rigorous adherence to national and local requirements for the governance and sustainable control of ECs is required. Administrative departments should strengthen the supervision of the production, processing, use, import, and export of prohibited or restricted toxic and harmful chemical substances and their related products and scientifically and sustainably manage new pollutants from the source. Those comprehensive sustainable management strategies encompassing technological innovation, ecological understanding, and corporate responsibility aim to address the multifaceted challenges posed by ECs in a sustainable manner.
Management and education
Regulatory measures and policies
The increasing global production and use of chemicals in a widening range of applications and products requires a strict hazard assessment and management to protect public health and the environment. Regulatory measures and policies, therefore, play a key role in managing the production, use, and disposal of chemicals to minimize potential harm. These measures aim to strike a balance between industrial innovation and the search for environmentally safe chemicals to protect the health of organisms at all biological scales.451,567
A cornerstone of chemicals management is national regulation such as the European REACH and the assessment schemes of, for instance, the US EPA or the Chinese Ministry of Ecology and Environment that require manufacturers of chemicals to carry out comprehensive safety studies before placing their products on the market. However, regulatory efforts are not effective or equitable without effective implementation and enforcement of such policies. On an international scale, corresponding frameworks, in which scientific experts assess data on chemicals for potential hazards, exposure levels, bioaccumulation, and toxicity, include the Basel (on hazardous waste), Rotterdam (on information on exported hazardous substances), Stockholm on POPs, and Minamata (on mercury) conventions. The Globally Harmonized System of Classification and Labelling of Chemicals is a prime example of an international effort at the UN level to standardize management and assessment practices. Internationally accepted tools for testing, evaluating, and managing chemicals have been developed by the OECD and its members. Outside the OECD, the Inter-Organization Programme for the Sound Management of Chemicals provides comprehensive support to emerging economies and developing countries, where new chemical industries and consumer markets rapidly develop, but often with limited infrastructure and capacity for proper management of chemicals and waste.
One of the 17 Sustainable Development Goals (SDGs), launched by the UN General Assembly in 2015, addresses the sound management of chemicals and all wastes throughout their life cycles and decreasing their release into air, water, and land. However, more effort is needed to achieve the goal of preventing significant adverse effects of chemical pollution on human health and the environment, as stated in the United Nations Environment Programme’s Global Chemicals Outlook II.568 It is well documented that chemical pollution causes a wide range of damages to human and ecosystem health at local, regional, and global scales.5 Among other factors, pollution is responsible for global biodiversity loss,165,569 human diseases,17,570 soil and water degradation,571,572,573 stratospheric ozone depletion,574 and climate change.575
Policymakers need to balance economic, social, and environmental arguments when deciding on measures for the sound management of chemicals. Where there is evidence of environmental impact and harm from exposure to, e.g., endocrine-disrupting chemicals, PFAS, and many other chemicals, regulators may impose restrictions, bans, or set limits on emissions and discharges into the environment. These measures are often based on scientific evidence and aim to protect vulnerable populations and ecosystems. Here, the precautionary principle is an important strategy that requires taking preventive action in the face of uncertainty about potential harm. Where scientific evidence is inconclusive, regulators should opt for a cautious approach and impose restrictions until further research clarifies potential risks.
Efforts to improve the handling of chemicals go beyond their production and application stages to include properly disposing of waste and recycling products containing dangerous substances. While progress has been made in many areas, there is an urgent need for a more consistent alignment of all actors on this common goal of chemical safety. International cooperation is therefore essential to address the global nature of pollution by chemicals and waste. Recently, scientists asked for the establishment of an overarching international body to facilitate and foster broad bidirectional science-policy interactions on chemicals and waste.576 Such a science-policy panel (SPP) must address chemical pollution’s multifaceted and heterogeneous impacts that often show dynamic development. The scope of this new SPP goes beyond the remit of the above-mentioned existing bodies because their scopes and mandates are limited to certain chemicals, geographical areas, or jurisdictions. Rather, the SPP needs to work on the large array of “chemicals of emerging concern” and novel waste streams, besides the well-described legacy pollutants, trying to avoid “analysis paralysis” (the inability of decision making by overanalysis or overthinking).577 The SPP must establish and enforce a strict conflict-of-interest policy.578 In particular, experts with a conflict of interest connected to a financial or material gain would pose a high risk of conflicting and/or incompatible outcomes or delayed implementation of solutions in the decision-making process and should not be allowed to participate in the core work of the SPP, but they may still participate and contribute as observers. Independent audits should be established to verify compliance with conflict-of-interest provisions to recommend corrective action if necessary and ensure that the outputs of SPPs are transparent, impartial, credible, and scientifically robust.
The new SPP, currently prepared by the United Nations Environment Programme (UNEP) Open-ended Working Group, is expected to strengthen these efforts by recognizing the interconnectedness of global chemical trade and pollution. Through regulatory measures, society can harness the benefits of chemicals while minimizing the adverse effects of hazardous chemicals.
Public awareness and education
Public awareness and education initiatives are instrumental in engaging individuals and communities in the efforts to address emerging pollutants. By increasing public knowledge and understanding of emerging pollutants, their sources, and potential impacts, we can promote responsible behavior and encourage individuals to make informed choices that contribute to pollution prevention. There is a need to conduct public education through educational campaigns, workshops, and outreach programs on the scientific aspects of ECs, guiding the public in developing a scientific awareness of the environmental risks associated with ECs and fostering a commitment to green consumption principles. Those can empower individuals to adopt environmentally friendly practices and support sustainable behaviors. For example, drug take back events for unused medicines (i.e. www.dea.gov/takebackday), which can build up residences even after expiration dates, represent effective education and outreach strategies that empower people to contribute to pollution prevention by reducing the common practice of disposing unused pharmaceuticals in sewage systems, and thereby decreasing environmental introduction concentrations of these ECs.598,599,600,601 Meanwhile, drawing inspiration from existing international conventions, the control of emerging pollutants is executed in accordance with international law. Besides, leveraging international conventions becomes pivotal as it refines its regulatory framework and establishes a robust governance system for emerging pollutants. Collaboratively with the global community, environmental risk identification, assessment, and control of chemicals are conducted. This not only realizes commitment to controlling emerging pollutants but also fosters global initiatives for pollution control, propelling the green development of the global chemical industry and contributing to worldwide environmental governance. To actively engage in international environmental agreements concerning ECs and participate in global initiatives for managing these contaminants is essential. By actively contributing to international conventions and actions related to ECs, a positive impact can be made on global environmental governance.
Some lessons learned
The systematic discovery of new contaminants has traditionally been a common pursuit in Environmental Sciences. Compound classes that were initially considered safe and inert (e.g., chlorinated hydrocarbons in the old times, PFAS at present) turned out to be prominent contaminants as more comprehensive evidence emerged.579 At the same time, the number of chemicals registered by the Chemical Abstract Service is increasing exponentially (Figure 1), augmenting the likelihood of adverse effects and reinforcing efforts to recognize potential pollutants of tomorrow early on.150 As illustrated in Figure 1, over the years, many relevant chemicals, pathogens, and (nano)particles have been discovered. They subsequently became the subject of in-depth fate and remediation studies before being the equivalent of “usual suspects” and making their way into regulation and routine monitoring efforts. While the term ECs is an ephemeral classification, a review of the last decades can highlight the drivers that make chemicals emerge and illustrate the time span between emergence and further action.
One important driver of discoveries is analytical innovation, as illustrated in Figure 1. Biannual reviews on water analysis and ECs in the journal Analytical Chemistry are a telling record of how access to new methodologies has been instrumental in bringing new contaminants to the radar. As exemplified in Fishman and Erdmann,580 water analysis in the early 1970s was dominated by spectroscopy, electrochemistry, MS, thin layer, and GC and focused on inorganic species, petroleum hydrocarbons, and persistent organochlorides. Twenty years later, a broader suite of organic compounds had become accessible by dedicated sample extraction, HPLC, GC-MS, and the advent of biochemical methods.581,582 In the early 2000s, the introduction of matrix-assisted laser desorption-ionization (MALDI)-MS made fingerprinting of bacteria possible, and the introduction of LC-MS revolutionized routine monitoring of organic compounds such as PPCPs. At that time, the term ECs came up.583 Today, 20 years later, high-resolution mass spectrometers and advanced data processing have catalyzed non-target screening for organic compounds, bringing to our attention a broad contaminant range, including PFAS and inadvertent transformation products.209
Another driver of emerging concern is situations in which chemicals are not necessarily new but occur in such quantities that they can no longer be overlooked. Hence, the general public feels urged to address them according to the precautionary principle, even though analytical methods are yet to be established for some of them. Examples are engineered microparticles and NPs, microplastics,584 or hydraulic-fracturing chemicals in unconventional gas exploration.585 Well-known chemicals may also become of emerging concern at the moment that they are subject to stricter drinking water standards, such as perchlorate586 or PFAS.587 The emergence of new diseases, such as during the SARS-CoV-2 pandemic, can finally drive the installation of entirely new monitoring efforts, such as screening wastewater for COVID variants.588
Environmental science can make particularly important contributions if it succeeds in discovering problematic transformation products as ECs that would otherwise remain overlooked. Examples are disinfection by-products such as bromate during water treatment.589 A particularly visible case is N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (6PPD)-quinone, a highly toxic ozonation product of the tire additive 6PPD, which has led to an enigmatic acute mortality of coho salmon in the US Pacific Northwest.139,590
In particularly notorious cases, chemicals emerge as contaminants after they were introduced to replace other, regulated ones. Examples are MTBE, or 1,4-dioxane, which were introduced in lieu of tetraethyl lead to boost octane numbers in gasoline,591 or the second generation of PFAS, which have replaced the first one of PFAS or PFOA only to be recognized to be equally problematic.592
Future directions and challenges
Achieving sustainable development remains a lofty goal rather than a concrete reality without unified global endeavors to mitigate and prevent environmental pollution. While regulations have been implemented to address legacy contaminants, many unregulated chemicals and biological entities continue to be released into the environment. Moreover, enforcement and implementation of regulations for existing pollutants are inconsistent or lacking in many regions globally, posing significant threats to public health, biodiversity, and ecosystem services. The escalating presence of ECs in the environment raises apprehensions regarding their enduring and unforeseen impacts on ecosystems, water quality, and human welfare. This comprehensive review thoroughly examined the sources, behavior, pathways, and fate of ECs in the environment from various perspectives. Additionally, we explored the impacts of these contaminants on planetary health, encompassing humans, animals, and their interconnected environments, all within the framework of One Health. Notwithstanding the extensive insights into ECs presented in this review, substantial challenges persist within the current global development systems, hindering effective efforts to mitigate the impact of environmental pollution on planetary health.
-
(1)
Chemicals are crucial in modern society, and their production is increasing. However, regulating their production and use is challenging because of the global development framework. When a regulated chemical is phased out, it is often replaced with another, potentially causing new or different types of environmental or human health impacts. This process requires a balance between the benefits of synthetic chemicals and the potential risks. With thousands of new synthetic chemicals entering the environment, many not thoroughly tested, there is a need to intensify research on ECs and create a comprehensive public database detailing their sources and environmental behavior. We must advance adverse outcome pathways454 and cross-species extrapolation approaches593,594 to understand chemical attributes that target particularly susceptible species and advance the precision of environmental assessments for ECs.595 Doing so promises to inform chemical substitutions in commerce without regrets596 and the sustainable molecular design of less hazardous substances.597
-
(2)
Each year, households and workplaces contribute significantly to environmental contamination by releasing harmful chemicals through various everyday products, including toothpaste, shampoo, body creams, cleaning agents, and plastic bags. The lack of transparency from companies regarding the ingredients and quantities used in their products complicates the identification of the contaminants people may be exposed to and the potential health risks associated with them. PFAS serve as a prime example of such chemicals. Despite being in commercial use since the 1940s, their toxicity was not widely recognized until the late 1990s. Some companies were aware of the potential toxicity of PFAS but continued to incorporate them into their products. This scenario highlights the importance of transparency in chemical manufacturing processes and underscores the necessity for comprehensive testing of chemicals before their incorporation into consumer goods.
-
(3)
Efforts to combat environmental pollution face persistent challenges, including the complexity of pollutants, inadequate technological solutions, and difficulties in implementing comprehensive environmental policies. Chemical remediation techniques, often preferred, paradoxically result in greater environmental impacts than the pollution they aim to remediate. While biological methods such as bioremediation and phytoremediation offer eco-friendly alternatives, they are less efficient and more time consuming. The introduction of lab-grown or engineered microorganisms during bioremediation also carries the risk of disrupting natural ecosystems and causing unforeseen impacts. Addressing these challenges requires intensified research on innovative remediation options to effectively control pollution, enhance environmental health, and maximize ecological sustainability. Tailored remediation strategies, considering specific site conditions and contaminant characteristics, need to be developed to navigate these complex challenges. Simply stated, we need to advance green and sustainable chemistry and green engineering to realize more sustainable pollution prevention in the future.
-
(4)
The intricate interplay between environmental pollution and climate change and other factors of global environmental change presents a formidable challenge that cannot be tackled in isolation. These environmental issues are interconnected and can amplify each other, resulting in profound consequences for ecosystems, human health, and the planet at large. Recognizing the interlinked nature of these challenges is imperative for formulating sustainable solutions that safeguard ecosystems, human well-being, and the prospects of future generations. Hence, there is a pressing need for integrated approaches that concurrently tackle global environmental change, underpinned by science-based policies and collaborative endeavors. This holistic strategy is imperative for steering the world toward a more resilient and sustainable future.
In summary, the continuous generation and utilization of new products contribute to the introduction of ECs into the environment. To confront this challenge effectively, comprehensive research is imperative to understand the sources and potential repercussions of these pollutants on human health, ecosystems, and animals in agriculture, embracing the One Health. Furthermore, evaluating how these contaminants interact with various environmental factors, both living and non-living, is crucial within our ever-changing environments. Leveraging advancements in analytical techniques and AI is indispensable for monitoring these emerging environmental pollutants and predicting their behavior within intricate environmental systems. Additionally, careful consideration of the potential risks stemming from advancements in material production across diverse domains, including biotechnology and nanotechnology, is vital for fostering the responsible development of materials for environmental purposes. Addressing environmental pollution demands a paradigm shift in our lifestyles, advocating for policies geared toward minimizing contaminants and implementing coordinated efforts to tackle existing pollutants through global cooperation. This collective endeavor is vital for safeguarding the health and sustainability of our planet for the benefit of both current and future generations, aligning with the principles of One Health.
Acknowledgments
This work was funded by the National Key Research and Development Program of China (2020YFC1807000), the Strategic Priority Research Program of the Chinese Academy of Sciences (no. XDA28030501), the National Natural Science Foundation of China (41991333, 41977137, 42090060), the International Atomic Energy Agency Research Project (D15022), the Youth Innovation Promotion Association of Chinese Academy of Sciences (2011225 [Fang Wang], Y201859 [H. Wang], 2013201 [J. Su], 2021309 [Y. Song], Y2022084 [M. Ye]), Chinese Academy of Sciences President’s International Fellowship Initiative (2020DC0005, 2022DC0001, 2024DC0009), the Institute of Soil Science, Chinese Academy of Sciences (ISSAS2419), the Research Group Linkage project from Alexander von Humboldt foundation, the Center for Health Impacts of Agriculture (CHIA) of Michigan State University, and the URI STEEP Superfund Center (grant # P42ES027706). Fang Wang was partly supported by the fellowship of Alexander von Humboldt for experienced researchers, and Shennong Young Talents of the Ministry of Agriculture and Rural Affairs, China (SNYCQN006-2022). J.P. and T.R.S. were supported by the Canada Research Chair program. B.W.B. was supported by a Royal Society of New Zealand Catalyst International Leaders fellowship. K.K.B. was supported by Innovation Fund Denmark and the European Commission Horizon 2020 financed under the ERA-NET Aquatic Pollutants Joint Transnational Call (REWA, GA no. 869178). S.A.H. was partly supported by a grant from the National Institute of Environmental Health Sciences, National Institutes of Health grant number P42ES04911-29 (Project 4). T.R.S. thanks CESAM by FCT/MCTES (UIDP/50017/2020+UIDB/50017/2020+LA/P/0094/2020). All authors express their gratitude to Shu Tao, Martin Scheringer, and Jay Gan for their valuable contributions to the conceptualization and writing of the manuscript.
Author contributions
Fang Wang, L.X., B.W.B., W.P., D.E.H., X.J., D.B., T.Z., Y.L., L.Z., X.L., J.C., Z.C., R.N., Q.S., J.P., Y.G.Z., J.D.H., M.C.R., Fengchang Wu, G.Y., W.A., and J.M.T. conceived, organized, and revised the manuscript, and wrote the abstract, introduction, and future directions and challenges. M.E., B.X., and Y.W. wrote the section about the historical perspective of ECs. C.G., F.C., R.L., N.C., M.L., and H.Q. wrote the section about the production, use, and environmental release of ECs. K.S.Y.L., M.J.S., K.J.J., J.C.F.L., and Y.H. wrote a section about advances in the detection and analysis of ECs. Y.G., X.C., C.Q., G.Y., C.C., Y.S., Z.W., G.J., Y.L., Q.Y., E.T., W.Z., T.M.V., C.S.C., G.S., H.L., B.P., Y.Y., M.T., H.W., and Z.W. wrote the section about distribution and fate of ECs. D.H., S.X.C., A.E.N., T.A., Y.G., D.Z., Y.G., T.Z., Y.P., C.G., J.G., J.S., M.Y., T.R.S., H.H., S.A.H., and M.V. wrote the section about risks of ECs to planetary health. X.Z., T.M.F., H.S., J.W., B.M.W., C.G., and W.Z. wrote the section about model-based assessment of fate and toxicological risks of ECs. H.S., Y.G.Z., J.S., J.D.H., P.C., Y.Y., F.S., and F.B. wrote the section about global efforts to control ECs. A.S., D.S.A., H.L., K.K.B., L.A.T., Q.B., and M.Z. wrote the section about management and education. M.E. wrote the section about some lessons learned. Y.F. and Y.W. organized and revised references. All authors discussed and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Published Online: March 13, 2024
Footnotes
It can be found online at https://doi.org/10.1016/j.xinn.2024.100612.
Contributor Information
Fang Wang, Email: wangfang@issas.ac.cn.
Lizhong Zhu, Email: zlz@zju.edu.cn.
Wulf Amelung, Email: wulf.amelung@uni-bonn.de.
Yong-guan Zhu, Email: ygzhu@iue.ac.cn.
James M. Tiedje, Email: tiedjej@msu.edu.
Lead contact website
Fang Wang: https://people.ucas.edu.cn/∼wangfang?language=en.
James M. Tiedje: https://www.canr.msu.edu/people/james_m_tiedje.
Supplemental information
References
- 1.Wang Z., Walker G.W., Muir D.C.G., et al. Toward a Global Understanding of Chemical Pollution: A First Comprehensive Analysis of National and Regional Chemical Inventories. Environ. Sci. Technol. 2020;54:2575–2584. doi: 10.1021/acs.est.9b06379. [DOI] [PubMed] [Google Scholar]
- 2.Matlin S.A., Cornell S.E., Krief A., et al. Chemistry must respond to the crisis of transgression of planetary boundaries. Chem. Sci. 2022;13:11710–11720. doi: 10.1039/d2sc03603g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steffen W., Richardson K., Rockström J., et al. Planetary boundaries: Guiding human development on a changing planet. Science. 2015;347 doi: 10.1126/science.1259855. [DOI] [PubMed] [Google Scholar]
- 4.Lebarbenchon C., Brown S.P., Poulin R., et al. Evolution of pathogens in a man-made world. Mol. Ecol. 2008;17:475–484. doi: 10.1111/j.1365-294X.2007.03375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naidu R., Biswas B., Willett I.R., et al. Chemical pollution: A growing peril and potential catastrophic risk to humanity. Environ. Int. 2021;156 doi: 10.1016/j.envint.2021.106616. [DOI] [PubMed] [Google Scholar]
- 6.Calvo-Flores F.G., Isac-García J., Dobado J.A. John Wiley & Sons; 2018. Emerging Pollutants: Origin, Structure, and Properties. [Google Scholar]
- 7.Escher B.I., Stapleton H.M., Schymanski E.L. Tracking complex mixtures of chemicals in our changing environment. Science. 2020;367:388–392. doi: 10.1126/science.aay6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanlon P., Sewalt V. GEMs: genetically engineered microorganisms and the regulatory oversight of their uses in modern food production. Crit. Rev. Food Sci. Nutr. 2021;61:959–970. doi: 10.1080/10408398.2020.1749026. [DOI] [PubMed] [Google Scholar]
- 9.Rafeeq H., Afsheen N., Rafique S., et al. Genetically engineered microorganisms for environmental remediation. Chemosphere. 2023;310 doi: 10.1016/j.chemosphere.2022.136751. [DOI] [PubMed] [Google Scholar]
- 10.Persson L., Carney Almroth B.M., Collins C.D., et al. Outside the safe operating space of the planetary boundary for novel entities. Environ. Sci. Technol. 2022;56:1510–1521. doi: 10.1021/acs.est.1c04158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernhardt E.S., Rosi E.J., Gessner M.O. Synthetic chemicals as agents of global change. Front. Ecol. Environ. 2017;15:84–90. [Google Scholar]
- 12.Clark S.J., Segall M.D., Pickard C.J., et al. First principles methods using CASTEP. Z. für Kristallogr. - Cryst. Mater. 2005;220:567–570. [Google Scholar]
- 13.Cousins I.T., Johansson J.H., Salter M.E., et al. Outside the safe operating space of a new planetary boundary for per- and polyfluoroalkyl substances (PFAS) Environ. Sci. Technol. 2022;56:11172–11179. doi: 10.1021/acs.est.2c02765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puri M., Gandhi K., Kumar M.S. Emerging environmental contaminants: A global perspective on policies and regulations. J. Environ. Manag. 2023;332 doi: 10.1016/j.jenvman.2023.117344. [DOI] [PubMed] [Google Scholar]
- 15.Machado K.C., Grassi M.T., Vidal C., et al. A preliminary nationwide survey of the presence of emerging contaminants in drinking and source waters in Brazil. Sci. Total Environ. 2016;572:138–146. doi: 10.1016/j.scitotenv.2016.07.210. [DOI] [PubMed] [Google Scholar]
- 16.Landrigan P.J., Fuller R., Hu H., et al. Pollution and global health–an agenda for prevention. Environ. Health Perspect. 2018;126 doi: 10.1289/EHP3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuller R., Landrigan P.J., Balakrishnan K., et al. Pollution and health: a progress update. Lancet Planet. Health. 2022;6:e535–e547. doi: 10.1016/S2542-5196(22)00090-0. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y., Gasevic D., Wen B., et al. Association between air pollution and telomere length: A study of 471,808 UK Biobank participants. Innovat. Med. 2023;1 doi: 10.59717/j.xinn-med.2023.100017. [DOI] [Google Scholar]
- 19.Ataria J.M., Murphy M., McGregor D., et al. Orienting the sustainable management of chemicals and waste toward indigenous knowledge. Environ. Sci. Technol. 2023;57:10901–10903. doi: 10.1021/acs.est.3c04600. [DOI] [PubMed] [Google Scholar]
- 20.Gao P. Chasing "emerging" contaminants: An endless journey toward environmental health. Environ. Sci. Technol. 2024;58:1790–1792. doi: 10.1021/acs.est.3c10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borch T., Kretzschmar R., Kappler A., et al. Biogeochemical redox processes and their impact on contaminant dynamics. Environ. Sci. Technol. 2010;44:15–23. doi: 10.1021/es9026248. [DOI] [PubMed] [Google Scholar]
- 22.Gibson G., Cundy A., Kafwamfwa N., et al. Old" and "new" contaminants and their management: learning from the past, looking to the future. Environ. Geochem. Health. 2023;45:1091–1105. doi: 10.1007/s10653-021-01042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taheran M., Naghdi M., Brar S.K., et al. Emerging contaminants: here today, there tomorrow. Environ. Nanotechnol. Monit. Manag. 2018;10:122–126. [Google Scholar]
- 24.Us Epa, O. 2015. Chemicals under the Toxic Substances Control Act (TSCA) [Google Scholar]
- 25.NORMAN NORMAN Network group. 2024. http://www.norman-network.net/?q=node
- 26.Brack W., Barcelo Culleres D., Boxall A.B.A., et al. One planet: One health. A call to support the initiative on a global science–policy body on chemicals and waste. Environ. Sci. Eur. 2022;34 doi: 10.1186/s12302-022-00602-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogunseitan O.A. One health and the environment: From conceptual framework to implementation science. Environment. 2022;64:11–21. [Google Scholar]
- 28.Ogunseitan O.A. Chemicals management approach to sustainable development of materials. MRS Bull. 2023;48:368–374. [Google Scholar]
- 29.Union O.J.o.t.E. 2015. COMMISSION IMPLEMENTING DECISION (EU) 2015/495 of 20 March 2015 Establishing a Watch List of Substances for Union-wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council (Notified under Document C(2015) p. 1756. [Google Scholar]
- 30.Canasius K., Kanangire A.-S.M., Dida G.O., et al. UNESCO-IHP International Initiative on Water Quality (IIWQ) Emerging Pollutants in Wastewater Reuse in Developing Countries Case Studies on Emerging Pollutants in Water and Wastewater. 2023. A Systematic Review of Effects of Emerging pollutants on Human Health and Livelihoods of Population living in the Lake Victoria Basin of Kenya. [Google Scholar]
- 31.Weiss M.C., Wang L., Sargis R.M. Hormonal injustice: Environmental toxicants as drivers of endocrine health disparities. Endocrinol Metab. Clin. N. Am. 2023;52:719–736. doi: 10.1016/j.ecl.2023.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ministry of Ecology and Environment of People’s Republic of China. 2023. List of new pollutants under key control.https://www.mee.gov.cn/xxgk2018/xxgk/xxgk02/202212/W020221230613338823204.pdf [Google Scholar]
- 33.Murnyak G., Vandenberg J., Yaroschak P.J., et al. Emerging contaminants: Presentations at the 2009 toxicology and risk assessment conference. Toxicol. Appl. Pharmacol. 2011;254:167–169. doi: 10.1016/j.taap.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 34.Sauvé S., Desrosiers M. A review of what is an emerging contaminant. Chem. Cent. J. 2014;8:15. doi: 10.1186/1752-153X-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrovic M., Eljarrat E., Lopez De Alda M.J., et al. Endocrine disrupting compounds and other emerging contaminants in the environment:: A survey on new monitoring strategies and occurrence data. Anal. Bioanal. Chem. 2004;378:549–562. doi: 10.1007/s00216-003-2184-7. [DOI] [PubMed] [Google Scholar]
- 36.Naidu R., Wong M.H. Contaminants of emerging concern Foreword. Sci. Total Environ. 2013;463–464:1077–1078. doi: 10.1016/j.scitotenv.2013.05.085. [DOI] [PubMed] [Google Scholar]
- 37.Agency, U.S.E.P. Drinking Water Bipartisan Infrastructure Law (BIL) Emerging Contaminants (EC) Funding Options. 2023. DWSRF Emerging Contaminants and Examples. [Google Scholar]
- 38.Rathi B.S., Kumar P.S., Show P.L. A review on effective removal of emerging contaminants from aquatic systems: Current trends and scope for further research. J. Hazard Mater. 2021;409(20) doi: 10.1016/j.jhazmat.2020.124413. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez-Maldonado A.J., Blaney L. Advances in analysis, treatment technologies, and environmental fate of emerging contaminants. J. Hazard Mater. 2015;282:1. doi: 10.1016/j.jhazmat.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 40.López-Mahía P., Muniategui S., Prada-Rodríguez D., et al. In: Encyclopedia of Analytical Science. Second Edition. Worsfold P., Townshend A., Poole C., editors. Elsevier; 2005. SURFACTANTS AND DETERGENTS; pp. 554–561. [Google Scholar]
- 41.Ahearne J.F. Radioactive waste: The size of the problem. Phys. Today. 1997;50:24–29. [Google Scholar]
- 42.Rysz M., Alvarez P.J.J. Amplification and attenuation of tetracycline resistance in soil bacteria: aquifer column experiments. Water Res. 2004;38:3705–3712. doi: 10.1016/j.watres.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 43.Fuhrman J.A. Marine viruses and their biogeochemical and ecological effects. Nature. 1999;399:541–548. doi: 10.1038/21119. [DOI] [PubMed] [Google Scholar]
- 44.Saunders S.E., Bartz J.C., Bartelt-Hunt S.L. Prion protein adsorption to soil in a competitive matrix is slow and reduced. Environ. Sci. Technol. 2009;43:7728–7733. doi: 10.1021/es901385t. [DOI] [PubMed] [Google Scholar]
- 45.Saunders S.E., Bartz J.C., Bartelt-Hunt S.L. Influence of prion strain on prion protein adsorption to soil in a competitive matrix. Environ. Sci. Technol. 2009;43:5242–5248. doi: 10.1021/es900502f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson C.J., Phillips K.E., Schramm P.T., et al. Prions adhere to soil minerals and remain infectious. PLoS Pathog. 2006;2 doi: 10.1371/journal.ppat.0020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nichols T.A., Pulford B., Wyckoff A.C., et al. Detection of protease-resistant cervid prion protein in water from a CWD-endemic area. Prion. 2009;3:171–183. doi: 10.4161/pri.3.3.9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson R.C., Olsen Y., Mitchell R.P., et al. Lost at sea: Where is all the plastic? Science. 2004;304:838. doi: 10.1126/science.1094559. [DOI] [PubMed] [Google Scholar]
- 49.Su H., Shi S., Zhu M., et al. Persistent, bioaccumulative, and toxic properties of liquid crystal monomers and their detection in indoor residential dust. Proc. Natl. Acad. Sci. USA. 2019;116:26450–26458. doi: 10.1073/pnas.1915322116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson C., Bell S.J. Linking emerging contaminants to production and consumption practices. WIREs Water. 2022;10 [Google Scholar]
- 51.Tong X., Mohapatra S., Zhang J., et al. Source, fate, transport and modelling of selected emerging contaminants in the aquatic environment: Current status and future perspectives. Water Res. 2022;217 doi: 10.1016/j.watres.2022.118418. [DOI] [PubMed] [Google Scholar]
- 52.OECD Global Plastics Outlook. 2022. https://www.oecd-ilibrary.org/content/publication/de747aef-en
- 53.Kumar M., Xiong X., He M., et al. Microplastics as pollutants in agricultural soils. Environ. Pollut. 2020;265 doi: 10.1016/j.envpol.2020.114980. [DOI] [PubMed] [Google Scholar]
- 54.Martínez-Orgániz Á., Crespo-Barrera P.M., Becerril-Bravo J.E., et al. Pollutants of emerging concern in tourist beaches of Guerrero, Mexico: A first approach to sources. Mar. Pollut. Bull. 2023;192 doi: 10.1016/j.marpolbul.2023.114989. [DOI] [PubMed] [Google Scholar]
- 55.Zhao J., Lan R., Wang Z., et al. Microplastic fragmentation by rotifers in aquatic ecosystems contributes to global nanoplastic pollution. Nat. Nanotechnol. 2024;19:406–414. doi: 10.1038/s41565-023-01534-9. [DOI] [PubMed] [Google Scholar]
- 56.MacLeod M., Arp H.P.H., Tekman M.B., et al. The global threat from plastic pollution. Science. 2021;373:61–65. doi: 10.1126/science.abg5433. [DOI] [PubMed] [Google Scholar]
- 57.Liu N., Jin X., Feng C., et al. Ecological risk assessment of fifty pharmaceuticals and personal care products (PPCPs) in Chinese surface waters: A proposed multiple-level system. Environ. Int. 2020;136(11) doi: 10.1016/j.envint.2019.105454. [DOI] [PubMed] [Google Scholar]
- 58.Wilkinson J.L., Boxall A.B.A., Kolpin D.W., et al. Pharmaceutical pollution of the world’s rivers. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2113947119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berglund F., Ebmeyer S., Kristiansson E., et al. Evidence for wastewaters as environments where mobile antibiotic resistance genes emerge. Commun. Biol. 2023;6:321. doi: 10.1038/s42003-023-04676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manaia C.M., Aga D.S., Cytryn E., et al. The complex Interplay between antibiotic resistance and pharmaceutical and personal care products in the environment. Environ. Toxicol. Chem. 2024;43:637–652. doi: 10.1002/etc.5555. [DOI] [PubMed] [Google Scholar]
- 61.Browne A.J., Chipeta M.G., Haines-Woodhouse G., et al. Global antibiotic consumption and usage in humans, 2000–18: a spatial modelling study. Lancet Planet. Health. 2021;5:e893–e904. doi: 10.1016/S2542-5196(21)00280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Larsson D.G.J., Flach C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022;20:257–269. doi: 10.1038/s41579-021-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chakraborty A., Adhikary S., Bhattacharya S., et al. Pharmaceuticals and Personal Care Products as Emerging Environmental Contaminants: Prevalence, Toxicity, and Remedial Approaches. ACS Chem. Health Saf. 2023;30:362–388. [Google Scholar]
- 64.Whitehead H.D., Venier M., Wu Y., et al. Fluorinated compounds in North American cosmetics. Environ. Sci. Technol. Lett. 2021;8:538–544. [Google Scholar]
- 65.Guo J., Liao M., He B., et al. Impact of the COVID-19 pandemic on household disinfectant consumption behaviors and related environmental concerns: A questionnaire-based survey in China. J. Environ. Chem. Eng. 2021;9 doi: 10.1016/j.jece.2021.106168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu J., Guo J. Disinfection spreads antimicrobial resistance. Science. 2021;371:474. doi: 10.1126/science.abg4380. [DOI] [PubMed] [Google Scholar]
- 67.Carpenter C.M.G., Helbling D.E. Widespread micropollutant monitoring in the hudson river estuary reveals spatiotemporal micropollutant clusters and their sources. Environ. Sci. Technol. 2018;52:6187–6196. doi: 10.1021/acs.est.8b00945. [DOI] [PubMed] [Google Scholar]
- 68.Carpenter C.M.G., Wong L.Y.J., Johnson C.A., et al. Fall creek monitoring station: Highly resolved temporal sampling to prioritize the identification of nontarget micropollutants in a small stream. Environ. Sci. Technol. 2019;53:77–87. doi: 10.1021/acs.est.8b05320. [DOI] [PubMed] [Google Scholar]
- 69.Hu J., Li S., Zhang W., et al. Animal production predominantly contributes to antibiotic profiles in the Yangtze River. Water Res. 2023;242 doi: 10.1016/j.watres.2023.120214. [DOI] [PubMed] [Google Scholar]
- 70.Brooks B.W. Fish on prozac (and zoloft): Ten years later. Aquat. Toxicol. 2014;151:61–67. doi: 10.1016/j.aquatox.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 71.Li X., He F., Wang Z., et al. Roadmap of environmental health research on emerging contaminants: Inspiration from the studies on engineered nanomaterials. Eeh. 2022;1:181–197. doi: 10.1016/j.eehl.2022.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Volder M.F.L., Tawfick S.H., Baughman R.H., et al. Carbon nanotubes: Present and future commercial applications. Science. 2013;339:535–539. doi: 10.1126/science.1222453. [DOI] [PubMed] [Google Scholar]
- 73.Weir A., Westerhoff P., Fabricius L., et al. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012;46:2242–2250. doi: 10.1021/es204168d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sadat-Shojai M., Atai M., Nodehi A., et al. Hydroxyapatite nanorods as novel fillers for improving the properties of dental adhesives: Synthesis and application. Dent. Mater. 2010;26:471–482. doi: 10.1016/j.dental.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 75.Piccinno F., Gottschalk F., Seeger S., et al. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanoparticle Res. 2012;14:1109. [Google Scholar]
- 76.Bathi J.R., Moazeni F., Upadhyayula V.K.K., et al. Behavior of engineered nanoparticles in aquatic environmental samples: Current status and challenges. Sci. Total Environ. 2021;793 doi: 10.1016/j.scitotenv.2021.148560. [DOI] [PubMed] [Google Scholar]
- 77.Boxall A.B.A., Brooks B.W. Pharmaceuticals and personal care products in the environment: what progress has been made in addressing the big research questions? Environ. Toxicol. Chem. 2024;43:481–487. doi: 10.1002/etc.5827. [DOI] [PubMed] [Google Scholar]
- 78.Erythropel H.C., Zimmerman J.B., de Winter T.M., et al. The Green ChemisTREE: 20 years after taking root with the 12 principles. Green Chem. 2018;20:1929–1961. [Google Scholar]
- 79.Naidu R., Arias Espana V.A., Liu Y., et al. Emerging contaminants in the environment: Risk-based analysis for better management. Chemosphere. 2016;154:350–357. doi: 10.1016/j.chemosphere.2016.03.068. [DOI] [PubMed] [Google Scholar]
- 80.Tran N.H., Reinhard M., Gin K.Y.-H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018;133:182–207. doi: 10.1016/j.watres.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 81.Aguilar-Aguilar A., de León-Martínez L.D., Forgionny A., et al. A systematic review on the current situation of emerging pollutants in Mexico: A perspective on policies, regulation, detection, and elimination in water and wastewater. Sci. Total Environ. 2023;905 doi: 10.1016/j.scitotenv.2023.167426. [DOI] [PubMed] [Google Scholar]
- 82.du Plessis A. Persistent degradation: Global water quality challenges and required actions. One Earth. 2022;5:129–131. [Google Scholar]
- 83.Morin-Crini N., Lichtfouse E., Liu G., et al. Worldwide cases of water pollution by emerging contaminants: a review. Environ. Chem. Lett. 2022;20:2311–2338. [Google Scholar]
- 84.Parida V.K., Saidulu D., Majumder A., et al. Emerging contaminants in wastewater: A critical review on occurrence, existing legislations, risk assessment, and sustainable treatment alternatives. J. Environ. Chem. Eng. 2021;9 [Google Scholar]
- 85.Ramos S., Homem V., Alves A., et al. A review of organic UV-filters in wastewater treatment plants. Environ. Int. 2016;86:24–44. doi: 10.1016/j.envint.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 86.Subedi B., Balakrishna K., Sinha R.K., et al. Mass loading and removal of pharmaceuticals and personal care products, including psychoactive and illicit drugs and artificial sweeteners, in five sewage treatment plants in India. J. Environ. Chem. Eng. 2015;3:2882–2891. [Google Scholar]
- 87.Brooks B.W., Huggett D.B., Boxall A.B.A. Pharmaceuticals and personal care products: research needs for the next decade. Environ. Toxicol. Chem. 2009;28:2469–2472. doi: 10.1897/09-325.1. [DOI] [PubMed] [Google Scholar]
- 88.Ankley G.T., Brooks B.W., Huggett D.B., et al. Repeating History: Pharmaceuticals in the Environment. Environ. Sci. Technol. 2007;41:8211–8217. doi: 10.1021/es072658j. [DOI] [PubMed] [Google Scholar]
- 89.Majumder A., Gupta A.K., Ghosal P.S., et al. A review on hospital wastewater treatment: A special emphasis on occurrence and removal of pharmaceutically active compounds, resistant microorganisms, and SARS-CoV-2. J. Environ. Chem. Eng. 2021;9 doi: 10.1016/j.jece.2020.104812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Evans A.E., Mateo-Sagasta J., Qadir M., et al. Agricultural water pollution: key knowledge gaps and research needs. Curr. Opin. Environ. Sustain. 2019;36:20–27. [Google Scholar]
- 91.Nguyen M.-K., Lin C., Nguyen H.-L., et al. Occurrence, fate, and potential risk of pharmaceutical pollutants in agriculture: Challenges and environmentally friendly solutions. Sci. Total Environ. 2023;899 doi: 10.1016/j.scitotenv.2023.165323. [DOI] [PubMed] [Google Scholar]
- 92.Buta M., Hubeny J., Zieliński W., et al. Sewage sludge in agriculture – the effects of selected chemical pollutants and emerging genetic resistance determinants on the quality of soil and crops – a review. Ecotoxicol. Environ. Saf. 2021;214 doi: 10.1016/j.ecoenv.2021.112070. [DOI] [PubMed] [Google Scholar]
- 93.Wilkinson J., Hooda P.S., Barker J., et al. Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field. Environ. Pollut. 2017;231:954–970. doi: 10.1016/j.envpol.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 94.Eggen T., Moeder M., Arukwe A. Municipal landfill leachates: A significant source for new and emerging pollutants. Sci. Total Environ. 2010;408:5147–5157. doi: 10.1016/j.scitotenv.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 95.Qi C., Huang J., Wang B., et al. Contaminants of emerging concern in landfill leachate in China: A review. Emerging Contam. 2018;4:1–10. [Google Scholar]
- 96.Qian Y., Hu P., Lang-Yona N., et al. Global landfill leachate characteristics: Occurrences and abundances of environmental contaminants and the microbiome. J. Hazard Mater. 2024;461 doi: 10.1016/j.jhazmat.2023.132446. [DOI] [PubMed] [Google Scholar]
- 97.Rogers E.R., Zalesny R.S., Lin C.-H. A systematic approach for prioritizing landfill pollutants based on toxicity: Applications and opportunities. J. Environ. Manag. 2021;284 doi: 10.1016/j.jenvman.2021.112031. [DOI] [PubMed] [Google Scholar]
- 98.Yi X., Tran N.H., Yin T., et al. Removal of selected PPCPs, EDCs, and antibiotic resistance genes in landfill leachate by a full-scale constructed wetlands system. Water Res. 2017;121:46–60. doi: 10.1016/j.watres.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 99.Chung S.S., Zheng J.S., Burket S.R., et al. Select antibiotics in leachate from closed and active landfills exceed thresholds for antibiotic resistance development. Environ. Int. 2018;115:89–96. doi: 10.1016/j.envint.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 100.Bengtsson-Palme J., Larsson D.G.J. Concentrations of antibiotics predicted to select for resistant bacteria: Proposed limits for environmental regulation. Environ. Int. 2016;86:140–149. doi: 10.1016/j.envint.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 101.Hong J., Kang H., An J., et al. Towards environmental sustainability in the local community: Future insights for managing the hazardous pollutants at construction sites. J. Hazard Mater. 2021;403 doi: 10.1016/j.jhazmat.2020.123804. [DOI] [PubMed] [Google Scholar]
- 102.Zhong B., Guo J., Zhang L., et al. A blockchain-based framework for on-site construction environmental monitoring: Proof of concept. Build. Environ. 2022;217 [Google Scholar]
- 103.Kang H., Sung S., Hong J., et al. Development of a real-time automated monitoring system for managing the hazardous environmental pollutants at the construction site. J. Hazard Mater. 2021;402 doi: 10.1016/j.jhazmat.2020.123483. [DOI] [PubMed] [Google Scholar]
- 104.McBee R.M., Lucht M., Mukhitov N., et al. Engineering living and regenerative fungal–bacterial biocomposite structures. Nat. Mater. 2022;21:471–478. doi: 10.1038/s41563-021-01123-y. [DOI] [PubMed] [Google Scholar]
- 105.Barroso P.J., Santos J.L., Martín J., et al. Emerging contaminants in the atmosphere: Analysis, occurrence and future challenges. Crit. Rev. Environ. Sci. Technol. 2019;49:104–171. [Google Scholar]
- 106.Enyoh C.E., Verla A.W., Qingyue W., et al. An overview of emerging pollutants in air: Method of analysis and potential public health concern from human environmental exposure. Trends Environ. Anal. 2020;28 [Google Scholar]
- 107.Yang X., Gong J., Zhang X., et al. Evaluation of the combined toxicity of multi-walled carbon nanotubes and cadmium on earthworms in soil using multi-level biomarkers. Ecotoxicol. Environ. Saf. 2021;221 doi: 10.1016/j.ecoenv.2021.112441. [DOI] [PubMed] [Google Scholar]
- 108.Fernandez P., van Drooge B.L., Arellano L., et al. Atmospheric deposition of semivolatile organic pollutants in European high mountains: Sources, settling and chemical degradation. Sci. Total Environ. 2021;784 doi: 10.1016/j.scitotenv.2021.147099. [DOI] [PubMed] [Google Scholar]
- 109.Sridharan S., Kumar M., Singh L., et al. Microplastics as an emerging source of particulate air pollution: A critical review. J. Hazard Mater. 2021;418 doi: 10.1016/j.jhazmat.2021.126245. [DOI] [PubMed] [Google Scholar]
- 110.Mbachu O., Jenkins G., Pratt C., et al. A new contaminant superhighway? A review of sources, measurement techniques and fate of atmospheric microplastics. Water. Air. Soil. Pollut. 2020;231:85. [Google Scholar]
- 111.Jeevanandam J., Barhoum A., Chan Y.S., et al. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018;9:1050–1074. doi: 10.3762/bjnano.9.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gottschalk F., Sun T., Nowack B. Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 2013;181:287–300. doi: 10.1016/j.envpol.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 113.Keller A.A., McFerran S., Lazareva A., et al. Global life cycle releases of engineered nanomaterials. J. Nanoparticle Res. 2013;15:1692. [Google Scholar]
- 114.Gottschalk F., Nowack B. The release of engineered nanomaterials to the environment. J. Environ. Monit. 2011;13:1145–1155. doi: 10.1039/c0em00547a. [DOI] [PubMed] [Google Scholar]
- 115.Zuin S., Gaiani M., Ferrari A., et al. Leaching of nanoparticles from experimental water-borne paints under laboratory test conditions. J. Nanoparticle Res. 2013;16:2185. [Google Scholar]
- 116.Sun T.Y., Bornhöft N.A., Hungerbühler K., et al. Dynamic probabilistic modeling of environmental emissions of engineered nanomaterials. Environ. Sci. Technol. 2016;50:4701–4711. doi: 10.1021/acs.est.5b05828. [DOI] [PubMed] [Google Scholar]
- 117.Saunders S.E., Bartelt-Hunt S.L., Bartz J.C. Prions in the environment. Prion. 2008;2:162–169. doi: 10.4161/pri.2.4.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stanley B., Prusiner Nobel lecture: Prions. Proc. Natl. Acad. Sci. USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Smith C.B., Booth C.J., Pedersen J.A. Fate of prions in soil: A review. J. Environ. Qual. 2011;40:449–461. doi: 10.2134/jeq2010.0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pal A., Gin K.Y.-H., Lin A.Y.-C., et al. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci. Total Environ. 2010;408:6062–6069. doi: 10.1016/j.scitotenv.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 121.Gogoi A., Mazumder P., Tyagi V.K., et al. Occurrence and fate of emerging contaminants in water environment: A review. Groundwater for Sustainable Development. 2018;6:169–180. [Google Scholar]
- 122.Pradhan B., Chand S., Chand S., et al. Emerging groundwater contaminants: A comprehensive review on their health hazards and remediation technologies. Groundwater for Sustainable Development. 2023;20 [Google Scholar]
- 123.Pérez-Fernández V., Mainero Rocca L., Tomai P., et al. Recent advancements and future trends in environmental analysis: Sample preparation, liquid chromatography and mass spectrometry. Anal. Chim. Acta. 2017;983:9–41. doi: 10.1016/j.aca.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 124.Hassan M.H., Khan R., Andreescu S. Advances in electrochemical detection methods for measuring contaminants of emerging concerns. Electrochem. Sci. Adv. 2022;2 [Google Scholar]
- 125.Shahid M.K., Kashif A., Fuwad A., et al. Current advances in treatment technologies for removal of emerging contaminants from water – A critical review. Coord. Chem. Rev. 2021;442 [Google Scholar]
- 126.Bojko B., Looby N., Olkowicz M., et al. Solid phase microextraction chemical biopsy tool for monitoring of doxorubicin residue during in vivo lung chemo-perfusion. J. Pharm. Anal. 2021;11:37–47. doi: 10.1016/j.jpha.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reyes-Garcés N., Gionfriddo E., Gómez-Ríos G.A., et al. Advances in solid phase microextraction and perspective on future directions. Anal. Chem. 2018;90:302–360. doi: 10.1021/acs.analchem.7b04502. [DOI] [PubMed] [Google Scholar]
- 128.Zhou W., Wieczorek M.N., Javanmardi H., et al. Direct solid-phase microextraction-mass spectrometry facilitates rapid analysis and green analytical chemistry. Trac-trend. Anal. Chem. 2023;166 [Google Scholar]
- 129.Zhou W., Pawliszyn J. Perspective on SPME-MS: Green and high-performance methods for rapid screening. Anal. Chim. Acta. 2024;1291 doi: 10.1016/j.aca.2024.342244. [DOI] [PubMed] [Google Scholar]
- 130.Murtada K., Pawliszyn J. Protocol for the development of TFME-GC methods for analyzing multiclass organic constituents in water samples. Green Analytical Chemistry. 2022;2 [Google Scholar]
- 131.Ahmadi F., Sparham C., Boyacı E., et al. Time weighted average concentration monitoring based on thin film solid phase microextraction. Environ. Sci. Technol. 2017;51:3929–3937. doi: 10.1021/acs.est.6b06465. [DOI] [PubMed] [Google Scholar]
- 132.Yu M., Roszkowska A., Pawliszyn J. In vivo solid-phase microextraction and applications in environmental sciences. ACS Environ. Au. 2022;2:30–41. doi: 10.1021/acsenvironau.1c00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zeinali S., Khalilzadeh M., Pawliszyn J. The evolution of needle-trap devices with focus on aerosol investigations. Trac-trend. Anal. Chem. 2022;153 [Google Scholar]
- 134.Nihemaiti M., Icker M., Seiwert B., et al. Revisiting Disinfection Byproducts with Supercritical Fluid Chromatography-High Resolution-Mass Spectrometry: Identification of Novel Halogenated Sulfonic Acids in Disinfected Drinking Water. Environ. Sci. Technol. 2023;57:3527–3537. doi: 10.1021/acs.est.2c05536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zahn D., Frömel T., Knepper T.P. Halogenated methanesulfonic acids: A new class of organic micropollutants in the water cycle. Water Res. 2016;101:292–299. doi: 10.1016/j.watres.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 136.Zahn D., Meusinger R., Frömel T., et al. Halomethanesulfonic acids—A new class of polar disinfection byproducts: Standard synthesis, occurrence, and indirect assessment of mitigation options. Environ. Sci. Technol. 2019;53:8994–9002. doi: 10.1021/acs.est.9b03016. [DOI] [PubMed] [Google Scholar]
- 137.Belova L., Caballero-Casero N., van Nuijs A.L.N., et al. Ion mobility-high-resolution mass spectrometry (IM-HRMS) for the analysis of contaminants of emerging concern (CECs): database compilation and application to urine samples. Anal. Chem. 2021;93:6428–6436. doi: 10.1021/acs.analchem.1c00142. [DOI] [PubMed] [Google Scholar]
- 138.Luo Y.-S., Aly N.A., McCord J., et al. Rapid characterization of emerging per-and polyfluoroalkyl substances in aqueous film-forming foams using ion mobility spectrometry–mass spectrometry. Environ. Sci. Technol. 2020;54:15024–15034. doi: 10.1021/acs.est.0c04798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tian Z., Zhao H., Peter K.T., et al. A ubiquitous tire rubber–derived chemical induces acute mortality in coho salmon. Science. 2021;371:185–189. doi: 10.1126/science.abd6951. [DOI] [PubMed] [Google Scholar]
- 140.Liu Q., Li L., Zhang X., et al. Uncovering global-scale risks from commercial chemicals in air. Nature. 2021;600:456–461. doi: 10.1038/s41586-021-04134-6. [DOI] [PubMed] [Google Scholar]
- 141.Hollender J., Schymanski E.L., Singer H.P., et al. Nontarget screening with high resolution mass spectrometry in the environment: Ready to go? Environ. Sci. Technol. 2017;51:11505–11512. doi: 10.1021/acs.est.7b02184. [DOI] [PubMed] [Google Scholar]
- 142.Li Q., Wang L., Jia Y., et al. Nontargeted analysis reveals a broad range of bioactive pollutants in drinking water by estrogen receptor affinity–mass spectrometry. Environ. Sci. Technol. 2023;57:21327–21336. doi: 10.1021/acs.est.3c05060. [DOI] [PubMed] [Google Scholar]
- 143.Dong H., Cuthbertson A.A., Plewa M.J., et al. Unravelling high-molecular-weight DBP toxicity drivers in chlorinated and chloraminated drinking water: Effect-directed analysis of molecular weight fractions. Environ. Sci. Technol. 2023;57:18788–18800. doi: 10.1021/acs.est.3c00771. [DOI] [PubMed] [Google Scholar]
- 144.Yeung K., Moore N., Sun J., et al. Thiol reactome: A nontargeted strategy to precisely identify thiol reactive drinking water disinfection byproducts. Environ. Sci. Technol. 2023;57:18722–18734. doi: 10.1021/acs.est.2c05486. [DOI] [PubMed] [Google Scholar]
- 145.Prasse C. Reactivity-directed analysis–a novel approach for the identification of toxic organic electrophiles in drinking water. Environ. Sci-proc. Imp. 2021;23:48–65. doi: 10.1039/d0em00471e. [DOI] [PubMed] [Google Scholar]
- 146.Tian Z., McMinn M.H., Fang M. Effect-directed analysis and beyond: how to find causal environmental toxicants. Exposome. 2023;3 [Google Scholar]
- 147.Simpson A.J., McNally D.J., Simpson M.J. NMR spectroscopy in environmental research: From molecular interactions to global processes. Prog. Nucl. Magn. Reson. Spectrosc. 2011;58:97–175. doi: 10.1016/j.pnmrs.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 148.Li H., Pan B., Liao S., et al. Formation of environmentally persistent free radicals as the mechanism for reduced catechol degradation on hematite-silica surface under UV irradiation. Environ. Pollut. 2014;188:153–158. doi: 10.1016/j.envpol.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 149.Yi P., Chen Q., Li H., et al. A comparative study on the formation of environmentally persistent free radicals (EPFRs) on hematite and goethite: contribution of various catechol degradation byproducts. Environ. Sci. Technol. 2019;53:13713–13719. doi: 10.1021/acs.est.9b04444. [DOI] [PubMed] [Google Scholar]
- 150.Muir D.C.G., Getzinger G.J., McBride M., et al. How many chemicals in commerce have been analyzed in environmental media? A 50 year bibliometric analysis. Environ. Sci. Technol. 2023;57:9119–9129. doi: 10.1021/acs.est.2c09353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McLafferty F.W., Turecek F. University science books; 1993. Interpretation of Mass Spectra. [Google Scholar]
- 152.Holmes J.L., Aubry C., Mayer P.M. CRC press; 2006. Assigning Structures to Ions in Mass Spectrometry. [Google Scholar]
- 153.Böcker S. Searching molecular structure databases using tandem MS data: are we there yet? Curr. Opin. Chem. Biol. 2017;36:1–6. doi: 10.1016/j.cbpa.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 154.McEachran A.D., Sobus J.R., Williams A.J. Identifying known unknowns using the US EPA’s CompTox chemistry dashboard. Anal. Bioanal. Chem. 2017;409:1729–1735. doi: 10.1007/s00216-016-0139-z. [DOI] [PubMed] [Google Scholar]
- 155.McEachran A.D., Mansouri K., Grulke C., et al. MS-Ready” structures for non-targeted high-resolution mass spectrometry screening studies. J. Cheminf. 2018;10:45. doi: 10.1186/s13321-018-0299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schulze B., Heffernan A.L., Samanipour S., et al. Is nontarget analysis ready for regulatory application? Influence of peak-picking algorithms on data analysis. Anal. Chem. 2023;95:18361–18369. doi: 10.1021/acs.analchem.3c03003. [DOI] [PubMed] [Google Scholar]
- 157.Black G., Lowe C., Anumol T., et al. Exploring chemical space in non-targeted analysis: A proposed ChemSpace tool. Anal. Bioanal. Chem. 2023;415:35–44. doi: 10.1007/s00216-022-04434-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hulleman T., Turkina V., O’Brien J.W., et al. Critical assessment of the chemical space covered by LC–HRMS non-targeted analysis. Environ. Sci. Technol. 2023;57:14101–14112. doi: 10.1021/acs.est.3c03606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Aalizadeh R., Alygizakis N.A., Schymanski E.L., et al. Development and application of liquid chromatographic retention time indices in HRMS-based suspect and nontarget screening. Anal. Chem. 2021;93:11601–11611. doi: 10.1021/acs.analchem.1c02348. [DOI] [PubMed] [Google Scholar]
- 160.Alygizakis N., Lestremau F., Gago-Ferrero P., et al. Towards a harmonized identification scoring system in LC-HRMS/MS based non-target screening (NTS) of emerging contaminants. Trac-trend. Anal. Chem. 2023;159 [Google Scholar]
- 161.Koopman J., Grimme S. From QCEIMS to QCxMS: A tool to routinely calculate CID mass spectra using molecular dynamics. J. Am. Soc. Mass Spectrom. 2021;32:1735–1751. doi: 10.1021/jasms.1c00098. [DOI] [PubMed] [Google Scholar]
- 162.Wang F., Liigand J., Tian S., et al. CFM-ID 4.0: more accurate ESI-MS/MS spectral prediction and compound identification. Anal. Chem. 2021;93:11692–11700. doi: 10.1021/acs.analchem.1c01465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bouwmeester R., Martens L., Degroeve S. Comprehensive and empirical evaluation of machine learning algorithms for small molecule LC retention time prediction. Anal. Chem. 2019;91:3694–3703. doi: 10.1021/acs.analchem.8b05820. [DOI] [PubMed] [Google Scholar]
- 164.Zhang H., Luo M., Wang H., et al. AllCCS2: Curation of ion mobility collision cross-section atlas for small molecules using comprehensive molecular representations. Anal. Chem. 2023;95:13913–13921. doi: 10.1021/acs.analchem.3c02267. [DOI] [PubMed] [Google Scholar]
- 165.Mueller L.K., Ågerstrand M., Backhaus T., et al. Policy options to account for multiple chemical pollutants threatening biodiversity. Environ. Sci, Adv. 2023;2:151–161. [Google Scholar]
- 166.Ieda T., Hashimoto S. GC× GC and computational strategies for detecting and analyzing environmental contaminants. Trac-trend. Anal. Chem. 2023;165 [Google Scholar]
- 167.Jobst K.J., Shen L., Reiner E.J., et al. The use of mass defect plots for the identification of (novel) halogenated contaminants in the environment. Anal. Bioanal. Chem. 2013;405:3289–3297. doi: 10.1007/s00216-013-6735-2. [DOI] [PubMed] [Google Scholar]
- 168.Léon A., Cariou R., Hutinet S., et al. HaloSeeker 1.0: A user-friendly software to highlight halogenated chemicals in nontargeted high-resolution mass spectrometry data sets. Anal. Chem. 2019;91:3500–3507. doi: 10.1021/acs.analchem.8b05103. [DOI] [PubMed] [Google Scholar]
- 169.Koelmel J.P., Paige M.K., Aristizabal-Henao J.J., et al. Toward comprehensive per-and polyfluoroalkyl substances annotation using fluoromatch software and intelligent high-resolution tandem mass spectrometry acquisition. Anal. Chem. 2020;92:11186–11194. doi: 10.1021/acs.analchem.0c01591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang X., Di Lorenzo R.A., Helm P.A., et al. Compositional space: a guide for environmental chemists on the identification of persistent and bioaccumulative organics using mass spectrometry. Environ. Int. 2019;132 doi: 10.1016/j.envint.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Steeves K., Cahill L.S., Jobst K.J. Emerging perfluoroalkyl substances in environmental waters revealed by non-targeted screening. Curr. Opin. Environ. Sci. Health. 2024;37 [Google Scholar]
- 172.Zweigle J., Bugsel B., Zwiener C. Efficient PFAS prioritization in non-target HRMS data: Systematic evaluation of the novel MD/C-m/C approach. Anal. Bioanal. Chem. 2023;415:1791–1801. doi: 10.1007/s00216-023-04601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.MacNeil A., Li X., Amiri R., et al. Gas chromatography-(cyclic) ion mobility mass spectrometry: A novel platform for the discovery of unknown per-/polyfluoroalkyl substances. Anal. Chem. 2022;94:11096–11103. doi: 10.1021/acs.analchem.2c02325. [DOI] [PubMed] [Google Scholar]
- 174.Foster M., Rainey M., Watson C., et al. Uncovering pfas and other xenobiotics in the dark metabolome using ion mobility spectrometry, mass defect analysis, and machine learning. Environ. Sci. Technol. 2022;56:9133–9143. doi: 10.1021/acs.est.2c00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Boiko D.A., Kozlov K.S., Burykina J.V., et al. Fully automated unconstrained analysis of high-resolution mass spectrometry data with machine learning. J. Am. Chem. Soc. 2022;144:14590–14606. doi: 10.1021/jacs.2c03631. [DOI] [PubMed] [Google Scholar]
- 176.Xu Y., Liu X., Cao X., et al. Artificial intelligence: A powerful paradigm for scientific research. Innovation. 2021;2 doi: 10.1016/j.xinn.2021.100179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Escher B., Neale P., Leusch F. IWA Publishing; 2021. Bioanalytical Tools in Water Quality Assessment. [Google Scholar]
- 178.Saxena K., Chauhan N., Jain U. Advances in diagnosis of Helicobacter pylori through biosensors: Point of care devices. Anal. Biochem. 2021;630 doi: 10.1016/j.ab.2021.114325. [DOI] [PubMed] [Google Scholar]
- 179.Zhou C., Zou H., Sun C., et al. Recent advances in biosensors for antibiotic detection: Selectivity and signal amplification with nanomaterials. Food Chem. 2021;361 doi: 10.1016/j.foodchem.2021.130109. [DOI] [PubMed] [Google Scholar]
- 180.Tahirbegi I.B., Ehgartner J., Sulzer P., et al. Fast pesticide detection inside microfluidic device with integrated optical pH, oxygen sensors and algal fluorescence. Biosens. Bioelectron. 2017;88:188–195. doi: 10.1016/j.bios.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 181.Gao Y., Wang L., Zhang X., et al. Similarities and differences among the responses to three chlorinated organophosphate esters in earthworm: Evidences from biomarkers, transcriptomics and metabolomics. Sci. Total Environ. 2022;815 doi: 10.1016/j.scitotenv.2021.152853. [DOI] [PubMed] [Google Scholar]
- 182.Tsekeli T.R., Sebokolodi T.I., Sipuka D.S., et al. A poly (propylene imine) dendrimer–carbon nanofiber based aptasensor for bisphenol A in water. J. Electroanal. Chem. 2021;901 [Google Scholar]
- 183.Tang Y., Clark T.J., Yoon J.-Y. Receptor-based detection of microplastics and nanoplastics: Current and future. Biosens. Bioelectron. 2023;115361 doi: 10.1016/j.bios.2023.115361. [DOI] [PubMed] [Google Scholar]
- 184.Haigh-Flórez D., de la Hera C., Costas E., et al. Microalgae dual-head biosensors for selective detection of herbicides with fiber-optic luminescent O2 transduction. Biosens. Bioelectron. 2014;54:484–491. doi: 10.1016/j.bios.2013.10.062. [DOI] [PubMed] [Google Scholar]
- 185.Hejji L., Azzouz A., Kukkar D., et al. Recent advancements in nanomaterials-based aptasensors for the detection of emerging contaminants in foodstuffs. Trac-trend. Anal. Chem. 2023;117194 [Google Scholar]
- 186.Sarkar D.J., Behera B.K., Parida P.K., et al. Aptamer-based NanoBioSensors for seafood safety. Biosens. Bioelectron. 2023;219 doi: 10.1016/j.bios.2022.114771. [DOI] [PubMed] [Google Scholar]
- 187.Sanli S., Moulahoum H., Ugurlu O., et al. Screen printed electrode-based biosensor functionalized with magnetic cobalt/single-chain antibody fragments for cocaine biosensing in different matrices. Talanta. 2020;217 doi: 10.1016/j.talanta.2020.121111. [DOI] [PubMed] [Google Scholar]
- 188.Prossner K.M., Vadas G.G., Harvey E., et al. A novel antibody-based biosensor method for the rapid measurement of PAH contamination in oysters. Environ. Technol. Innov. 2022;28 doi: 10.1016/j.eti.2022.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Neale P.A., Escher B.I., de Baat M.L., et al. Effect-based trigger values are essential for the uptake of effect-based methods in water safety planning. 2023;42:714–726. doi: 10.1002/etc.5544. [DOI] [PubMed] [Google Scholar]
- 190.Zhou C., Sun C., Zou H., et al. Plasma colorimetric aptasensor for the detection of chloramphenicol in honey based on cage Au@AuNPs and cascade hybridization chain reaction. Food Chem. 2022;377 doi: 10.1016/j.foodchem.2021.132031. [DOI] [PubMed] [Google Scholar]
- 191.Dong J., Xu L., Dang S., et al. A sensitive photoelectrochemical aptasensor for enrofloxacin detection based on plasmon-sensitized bismuth-rich bismuth oxyhalide. Talanta. 2022;246 doi: 10.1016/j.talanta.2022.123515. [DOI] [PubMed] [Google Scholar]
- 192.Liu X., Verma G., Chen Z., et al. Metal-organic framework nanocrystal-derived hollow porous materials: Synthetic strategies and emerging applications. Innovation. 2022;3 doi: 10.1016/j.xinn.2022.100281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Malhotra B.D., Ali M.A. Nanomaterials in biosensors: Fundamentals and applications. Nanomaterials for biosensors. 2018;1 [Google Scholar]
- 194.Karkman A., Do T.T., Walsh F., et al. Antibiotic-resistance genes in waste water. Trends Microbiol. 2018;26:220–228. doi: 10.1016/j.tim.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 195.Stedtfeld R.D., Guo X., Stedtfeld T.M., et al. Primer set 2.0 for highly parallel qPCR array targeting antibiotic resistance genes and mobile genetic elements. FEMS Microbiol. Ecol. 2018;94 doi: 10.1093/femsec/fiy130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xie S.-T., Ding L.-J., Huang F.-Y., et al. VFG-Chip: A high-throughput qPCR microarray for profiling virulence factor genes from the environment. Environ. Int. 2023;172 doi: 10.1016/j.envint.2023.107761. [DOI] [PubMed] [Google Scholar]
- 197.Liguori K., Keenum I., Davis B.C., et al. Antimicrobial resistance monitoring of water environments: A framework for standardized methods and quality control. Environ. Sci. Technol. 2022;56:9149–9160. doi: 10.1021/acs.est.1c08918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.He X., Zhang N., Cao W., et al. Application progress of high-throughput sequencing in ocular diseases. J. Clin. Med. 2022;11:3485. doi: 10.3390/jcm11123485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Han D., Li Z., Li R., et al. mNGS in clinical microbiology laboratories: on the road to maturity. Crit. Rev. Microbiol. 2019;45:668–685. doi: 10.1080/1040841X.2019.1681933. [DOI] [PubMed] [Google Scholar]
- 200.Yang X., Jiang G., Zhang Y., et al. MBPD: A multiple bacterial pathogen detection pipeline for One Health practices. iMeta. 2023;2 doi: 10.1002/imt2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jiang G., Zhang J., Zhang Y., et al. DCiPatho: deep cross-fusion networks for genome scale identification of pathogens. Briefings Bioinf. 2023;24 doi: 10.1093/bib/bbad194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Massey I.Y., Wu P., Wei J., et al. A mini-review on detection methods of microcystins. Toxins. 2020;12:641. doi: 10.3390/toxins12100641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chen Y., Li M., Gao W., et al. Occurrence and risks of pharmaceuticals, personal care products, and endocrine-disrupting compounds in Chinese surface waters. J. Environ. Sci. 2023 doi: 10.1016/j.jes.2023.10.011. [DOI] [PubMed] [Google Scholar]
- 204.Berkner S., Thierbach C. Biodegradability and transformation of human pharmaceutical active ingredients in environmentally relevant test systems. Environ. Sci. Pollut. Res. Int. 2014;21:9461–9467. doi: 10.1007/s11356-013-1868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Monteiro S.C., Boxall A.B.A. Occurrence and fate of human pharmaceuticals in the environment. Rev. Environ. Contam. Toxicol. 2010;202:53–154. doi: 10.1007/978-1-4419-1157-5_2. [DOI] [PubMed] [Google Scholar]
- 206.Adeleye A.S., Xue J., Zhao Y., et al. Abundance, fate, and effects of pharmaceuticals and personal care products in aquatic environments. J. Hazard Mater. 2022;424 doi: 10.1016/j.jhazmat.2021.127284. [DOI] [PubMed] [Google Scholar]
- 207.Liu H.-Q., Lam J.C.W., Li W.-W., et al. Spatial distribution and removal performance of pharmaceuticals in municipal wastewater treatment plants in China. Sci. Total Environ. 2017;586:1162–1169. doi: 10.1016/j.scitotenv.2017.02.107. [DOI] [PubMed] [Google Scholar]
- 208.Phillips P.J., Smith S.G., Kolpin D.W., et al. Pharmaceutical formulation facilities as sources of opioids and other pharmaceuticals to wastewater treatment plant effluents. Environ. Sci. Technol. 2010;44:4910–4916. doi: 10.1021/es100356f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Richardson S.D., Ternes T.A. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2022;94:382–416. doi: 10.1021/acs.analchem.1c04640. [DOI] [PubMed] [Google Scholar]
- 210.Aydın S., Ulvi A., Bedük F., et al. Pharmaceutical residues in digested sewage sludge: Occurrence, seasonal variation and risk assessment for soil. Sci. Total Environ. 2022;817 doi: 10.1016/j.scitotenv.2021.152864. [DOI] [PubMed] [Google Scholar]
- 211.Wu W., Ma M., Hu Y., et al. The fate and impacts of pharmaceuticals and personal care products and microbes in agricultural soils with long term irrigation with reclaimed water. Agric. Water Manag. 2021;251 [Google Scholar]
- 212.aus der Beek T., Weber F.-A., Bergmann A., et al. Pharmaceuticals in the environment—Global occurrences and perspectives. 2016;35:823–835. doi: 10.1002/etc.3339. [DOI] [PubMed] [Google Scholar]
- 213.Guo X., Lv M., Song L., et al. Occurrence, distribution, and trophic transfer of pharmaceuticals and personal care products in the bohai Sea. Environ. Sci. Technol. 2023;57:21823–21834. doi: 10.1021/acs.est.3c06522. [DOI] [PubMed] [Google Scholar]
- 214.Zhang L., Brooks B.W., Liu F. Human apparent volume of distribution predicts bioaccumulation of ionizable organic chemicals in zebrafish embryos. Environ. Sci. Technol. 2022;56:11547–11558. doi: 10.1021/acs.est.2c03421. [DOI] [PubMed] [Google Scholar]
- 215.Chaves M.d.J.S., Kulzer J., Pujol de Lima P.d.R., et al. Updated knowledge, partitioning and ecological risk of pharmaceuticals and personal care products in global aquatic environments. Environ. Sci-proc. Imp. 2022;24:1982–2008. doi: 10.1039/d2em00132b. [DOI] [PubMed] [Google Scholar]
- 216.Bartrons M., Peñuelas J. Pharmaceuticals and personal-care products in plants. Trends Plant Sci. 2017;22:194–203. doi: 10.1016/j.tplants.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 217.Carter L.J., Armitage J.M., Brooks B.W., et al. Predicting the accumulation of ionizable pharmaceuticals and personal care products in aquatic and terrestrial organisms. Environ. Toxicol. Chem. 2024;43:502–512. doi: 10.1002/etc.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhang Y., Vo Duy S., Munoz G., et al. Phytotoxic effects of microcystins, anatoxin-a and cylindrospermopsin to aquatic plants: A meta-analysis. Sci. Total Environ. 2022;810(10) doi: 10.1016/j.scitotenv.2021.152104. [DOI] [PubMed] [Google Scholar]
- 219.Huisman J., Codd G.A., Paerl H.W., et al. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018;16:471–483. doi: 10.1038/s41579-018-0040-1. [DOI] [PubMed] [Google Scholar]
- 220.Xie P. Biological mechanisms driving the seasonal changes in the internal loading of phosphorus in shallow lakes. Sci. China Earth Sci. 2006;49:14–27. [Google Scholar]
- 221.Massey I.Y., Al Osman M., Yang F. An overview on cyanobacterial blooms and toxins production: their occurrence and influencing factors. Toxin Rev. 2022;41:326–346. [Google Scholar]
- 222.Wood S.A., Maier M.Y., Puddick J., et al. Trophic state and geographic gradients influence planktonic cyanobacterial diversity and distribution in New Zealand lakes. FEMS Microbiol. Ecol. 2017;93(13):234. doi: 10.1093/femsec/fiw234. [DOI] [PubMed] [Google Scholar]
- 223.Liu T., Mazmouz R., Pearson L.A., et al. Mutagenesis of the microcystin tailoring and transport proteins in a heterologous cyanotoxin expression system. ACS Synth. Biol. 2019;8:1187–1194. doi: 10.1021/acssynbio.9b00068. [DOI] [PubMed] [Google Scholar]
- 224.Wiśniewska K., Lewandowska A.U., Śliwińska-Wilczewska S. The importance of cyanobacteria and microalgae present in aerosols to human health and the environment - Review study. Environ. Int. 2019;131(11) doi: 10.1016/j.envint.2019.104964. [DOI] [PubMed] [Google Scholar]
- 225.Wiśniewska K., Śliwińska-Wilczewska S., Savoie M., et al. Quantitative and qualitative variability of airborne cyanobacteria and microalgae and their toxins in the coastal zone of the Baltic Sea. Sci. Total Environ. 2022;826(10) doi: 10.1016/j.scitotenv.2022.154152. [DOI] [PubMed] [Google Scholar]
- 226.Ferrao A.D., Kozlowsky-Suzuki B. Cyanotoxins: Bioaccumulation and effects on aquatic animals. Mar. Drugs. 2011;9:2729–2772. doi: 10.3390/md9122729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Peijnenburg W.J.G.M., Baalousha M., Chen J., et al. A review of the properties and processes determining the fate of engineered nanomaterials in the aquatic environment. Crit. Rev. Environ. Sci. Technol. 2015;45:2084–2134. [Google Scholar]
- 228.Bundschuh M., Filser J., Lüderwald S., et al. Nanoparticles in the environment: where do we come from, where do we go to? Environ. Sci. Eur. 2018;30:6. doi: 10.1186/s12302-018-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lowry G.V., Gregory K.B., Apte S.C., et al. Transformations of nanomaterials in the environment. Environ. Sci. Technol. 2012;46:6893–6899. doi: 10.1021/es300839e. [DOI] [PubMed] [Google Scholar]
- 230.Adam V., Loyaux-Lawniczak S., Labille J., et al. Aggregation behaviour of TiO2 nanoparticles in natural river water. J. Nanoparticle Res. 2016;18:13. [Google Scholar]
- 231.Baalousha M., Nur Y., Römer I., et al. Effect of monovalent and divalent cations, anions and fulvic acid on aggregation of citrate-coated silver nanoparticles. Sci. Total Environ. 2013;454–455:119–131. doi: 10.1016/j.scitotenv.2013.02.093. [DOI] [PubMed] [Google Scholar]
- 232.Zhao J., Liu F., Wang Z., et al. Heteroaggregation of graphene oxide with minerals in aqueous phase. Environ. Sci. Technol. 2015;49:2849–2857. doi: 10.1021/es505605w. [DOI] [PubMed] [Google Scholar]
- 233.Zhao J., Lin M., Wang Z., et al. Engineered nanomaterials in the environment: Are they safe? Crit. Rev. Environ. Sci. Technol. 2021;51:1443–1478. [Google Scholar]
- 234.Solovitch N., Labille J., Rose J., et al. Concurrent aggregation and deposition of TiO2 nanoparticles in a sandy porous media. Environ. Sci. Technol. 2010;44:4897–4902. doi: 10.1021/es1000819. [DOI] [PubMed] [Google Scholar]
- 235.Eichert T., Kurtz A., Steiner U., et al. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol. Plantarum. 2008;134:151–160. doi: 10.1111/j.1399-3054.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- 236.Wang P., Lombi E., Zhao F.J., et al. Nanotechnology: A New Opportunity in Plant Sciences. Trends Plant Sci. 2016;21:699–712. doi: 10.1016/j.tplants.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 237.Jia Y., Klumpp E., Bol R., et al. Uptake of metallic nanoparticles containing essential (Cu, Zn and Fe) and non-essential (Ag, Ce and Ti) elements by crops: A meta-analysis. Crit. Rev. Environ. Sci. Technol. 2023;53:1512–1533. [Google Scholar]
- 238.Santhanabharathi B., Pradhoshini K.P., Suhail Ahmed M., et al. Source, fate and transfer of primordial radionuclides as potential contaminants in environmental matrices of high and low background radiation areas–a critical review. Int. J. Environ. Anal. Chem. 2023:1–27. [Google Scholar]
- 239.Natarajan V., Karunanidhi M., Raja B. A critical review on radioactive waste management through biological techniques. Environ. Sci. Pollut. Res. 2020;27:29812–29823. doi: 10.1007/s11356-020-08404-0. [DOI] [PubMed] [Google Scholar]
- 240.Shan G., Ding Z. Emergency management of nuclear-contaminated water discharged into the ocean for marine environment security. Sustainable Horizons. 2024;10 [Google Scholar]
- 241.Banerjee S., van der Heijden M.G.A. Soil microbiomes and one health. Nat. Rev. Microbiol. 2023;21:6–20. doi: 10.1038/s41579-022-00779-w. [DOI] [PubMed] [Google Scholar]
- 242.Zhang S., Yang H., Wang M., et al. Immunomodulatory biomaterials against bacterial infections: Progress, challenges, and future perspectives. Innovation. 2023;4 doi: 10.1016/j.xinn.2023.100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Singh B.K., Yan Z.-Z., Whittaker M., et al. Soil microbiomes must be explicitly included in One Health policy. Nat. Microbiol. 2023;1–6 doi: 10.1038/s41564-023-01386-y. [DOI] [PubMed] [Google Scholar]
- 244.Steffan J.J., Derby J.A., Brevik E.C. Soil pathogens that may potentially cause pandemics, including severe acute respiratory syndrome (SARS) coronaviruses. Curr. Opin. Environ. Sci. Health. 2020;17:35–40. doi: 10.1016/j.coesh.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Limmathurotsakul D., Golding N., Dance D.A.B., et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 2016;1:15008. doi: 10.1038/nmicrobiol.2015.8. [DOI] [PubMed] [Google Scholar]
- 246.Gonzalez-Martin C., Teigell-Perez N., Valladares B., et al. The global dispersion of pathogenic microorganisms by dust storms and its relevance to agriculture. Adv. Agron. 2014;127:1–41. [Google Scholar]
- 247.Scott R.A., Thilmony R., Harden L.A., et al. Escherichia coli O157: H7 converts plant-derived choline to glycine betaine for osmoprotection during pre-and post-harvest colonization of injured lettuce leaves. Front. Microbiol. 2017;8:2436. doi: 10.3389/fmicb.2017.02436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.D'Costa V.M., King C.E., Kalan L., et al. Antibiotic resistance is ancient. Nature. 2011;477:457–461. doi: 10.1038/nature10388. [DOI] [PubMed] [Google Scholar]
- 249.Waglechner N., Culp E.J., Wright G.D. Ancient antibiotics, ancient resistance. EcoSal Plus. 2021;9 doi: 10.1128/ecosalplus.ESP-0027-2020. eESP-0027-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Levy S.B., Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat. Med. 2004;10:S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 251.Zhu L., Chen H., Cai Z. Zoonotic attack: An underestimated threat of SARS-CoV-2? Innovation. 2022;3 doi: 10.1016/j.xinn.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Fu Y., Dou Q., Smalla K., et al. Gut microbiota research nexus: One Health relationship between human, animal, and environmental resistomes. mLife. 2023;2:350–364. doi: 10.1002/mlf2.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zheng D., Yin G., Liu M., et al. Global biogeography and projection of soil antibiotic resistance genes. Sci. Adv. 2022;8(12) doi: 10.1126/sciadv.abq8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wang B., Xu J., Wang Y., et al. Tackling soil ARG-Carrying pathogens with Global-Scale metagenomics. Adv. Sci. 2023;10:e2301980. doi: 10.1002/advs.202301980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Mah T.F. Giving antibiotics an assist. Science. 2021;372:1153. doi: 10.1126/science.abj3062. [DOI] [PubMed] [Google Scholar]
- 256.Zhang Z., Zhang Q., Wang T., et al. Assessment of global health risk of antibiotic resistance genes. Nat. Commun. 2022;13(11):1553. doi: 10.1038/s41467-022-29283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Danko D., Bezdan D., Afshin E.E., et al. A global metagenomic map of urban microbiomes and antimicrobial resistance. Cell. 2021;184:3376–3393.e17. doi: 10.1016/j.cell.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Larsson D.G.J., Gaze W.H., Laxminarayan R., et al. AMR, One Health and the environment. Nat. Microbiol. 2023;8:754–755. doi: 10.1038/s41564-023-01351-9. [DOI] [PubMed] [Google Scholar]
- 259.Zhang A.N., Gaston J.M., Dai C.L., et al. An omics-based framework for assessing the health risk of antimicrobial resistance genes. Nat. Commun. 2021;12(11):4765. doi: 10.1038/s41467-021-25096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Cowie B.C., Dore G.J. The perpetual challenge of infectious diseases. N. Engl. J. Med. 2012;367:89–90. doi: 10.1056/NEJMc1204960. [DOI] [PubMed] [Google Scholar]
- 261.Savary S., Willocquet L., Pethybridge S.J., et al. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019;3:430–439. doi: 10.1038/s41559-018-0793-y. [DOI] [PubMed] [Google Scholar]
- 262.Pruden A., Pei R., Storteboom H., et al. Antibiotic resistance genes as emerging contaminants: Studies in northern colorado. Environ. Sci. Technol. 2006;40:7445–7450. doi: 10.1021/es060413l. [DOI] [PubMed] [Google Scholar]
- 263.Karkman A., Pärnänen K., Larsson D.G.J. Fecal pollution can explain antibiotic resistance gene abundances in anthropogenically impacted environments. Nat. Commun. 2019;10:80. doi: 10.1038/s41467-018-07992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Lu J., Jin M., Nguyen S.H., et al. Non-antibiotic antimicrobial triclosan induces multiple antibiotic resistance through genetic mutation. Environ. Int. 2018;118:257–265. doi: 10.1016/j.envint.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 265.Lu J., Wang Y., Jin M., et al. Both silver ions and silver nanoparticles facilitate the horizontal transfer of plasmid-mediated antibiotic resistance genes. Water Res. 2020;169 doi: 10.1016/j.watres.2019.115229. [DOI] [PubMed] [Google Scholar]
- 266.Wang Y., Lu J., Mao L., et al. Antiepileptic drug carbamazepine promotes horizontal transfer of plasmid-borne multi-antibiotic resistance genes within and across bacterial genera. ISME J. 2019;13:509–522. doi: 10.1038/s41396-018-0275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Luo Y., Liu C., Wang Y., et al. Occurrence, distribution and their correlation with different parameters of antibiotics and antibiotic resistance genes in lakes of China: A review. Mar. Pollut. Bull. 2023;193(12) doi: 10.1016/j.marpolbul.2023.115189. [DOI] [PubMed] [Google Scholar]
- 268.Pál C., Papp B., Lázár V. Collateral sensitivity of antibiotic-resistant microbes. Trends Microbiol. 2015;23:401–407. doi: 10.1016/j.tim.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Vaz-Moreira I., Nunes O.C., Manaia C.M. Bacterial diversity and antibiotic resistance in water habitats: searching the links with the human microbiome. FEMS Microbiol. Rev. 2014;38:761–778. doi: 10.1111/1574-6976.12062. [DOI] [PubMed] [Google Scholar]
- 270.Larsson D.G.J., Flach C.F., Laxminarayan R. Sewage surveillance of antibiotic resistance holds both opportunities and challenges. Nat. Rev. Microbiol. 2023;21:213–214. doi: 10.1038/s41579-022-00835-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Du P., Gao G.F., Wang Q. The mysterious origins of the Omicron variant of SARS-CoV-2. Innovation. 2022;3 doi: 10.1016/j.xinn.2022.100206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Jansson J.K., Wu R. Soil viral diversity, ecology and climate change. Nat. Rev. Microbiol. 2023;21:296–311. doi: 10.1038/s41579-022-00811-z. [DOI] [PubMed] [Google Scholar]
- 273.David Walter W., Walsh D.P., Farnsworth M.L., et al. Soil clay content underlies prion infection odds. Nat. Commun. 2011;2:200. doi: 10.1038/ncomms1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Anand U., Bianco F., Suresh S., et al. SARS-CoV-2 and other viruses in soil: An environmental outlook. Environ. Res. 2021;198 doi: 10.1016/j.envres.2021.111297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wu R., Davison M.R., Gao Y., et al. Moisture modulates soil reservoirs of active DNA and RNA viruses. Commun. Biol. 2021;4:992. doi: 10.1038/s42003-021-02514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Prosser D.J., Teitelbaum C.S., Yin S., et al. Climate change impacts on bird migration and highly pathogenic avian influenza. Nat. Microbiol. 2023;8:2223–2225. doi: 10.1038/s41564-023-01538-0. [DOI] [PubMed] [Google Scholar]
- 277.Wood J.P., Choi Y.W., Chappie D.J., et al. Environmental persistence of a highly pathogenic avian influenza (H5N1) virus. Environ. Sci. Technol. 2010;44:7515–7520. doi: 10.1021/es1016153. [DOI] [PubMed] [Google Scholar]
- 278.Carlson C.J., Chipperfield J.D., Benito B.M., et al. Species distribution models are inappropriate for COVID-19. Nat. Ecol. Evol. 2020;4:770–771. doi: 10.1038/s41559-020-1212-8. [DOI] [PubMed] [Google Scholar]
- 279.Cooke C.M., Shaw G. Fate of prions in soil: Longevity and migration of recPrP in soil columns. Soil Biol. Biochem. 2007;39:1181–1191. [Google Scholar]
- 280.Jacobson K.H., Lee S., Somerville R.A., et al. Transport of the pathogenic prion protein through soils. J. Environ. Qual. 2010;39:1145–1152. doi: 10.2134/jeq2009.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Strain K.E., Lydy M.J. The fate and transport of the Cry1Ab protein in an agricultural field and laboratory aquatic microcosms. Chemosphere. 2015;132:94–100. doi: 10.1016/j.chemosphere.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 282.Feng Y., Ling L., Fan H., et al. Effects of temperature, water content and pH on degradation of Cry1Ab protein released from Bt corn straw in soil. Soil Biol. Biochem. 2011;43:1600–1606. [Google Scholar]
- 283.Tapp H., Stotzky G. Persistence of the insecticidal toxin from Bacillus thuringiensis subsp. kurstaki in soil. Soil Biol. Biochem. 1998;30:471–476. [Google Scholar]
- 284.Jiang B., Kauffman A.E., Li L., et al. Health impacts of environmental contamination of micro- and nanoplastics: a review. Environ. Health Prev. Med. 2020;25:29. doi: 10.1186/s12199-020-00870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.McIlwraith H.K., Kim J., Helm P., et al. Evidence of microplastic translocation in wild-caught fish and implications for microplastic accumulation dynamics in food webs. Environ. Sci. Technol. 2021;55:12372–12382. doi: 10.1021/acs.est.1c02922. [DOI] [PubMed] [Google Scholar]
- 286.Piehl S., Leibner A., Löder M.G.J., et al. Identification and quantification of macro- and microplastics on an agricultural farmland. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-36172-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Feng Y., Tu C., Li R., et al. A systematic review of the impacts of exposure to micro- and nano-plastics on human tissue accumulation and health. Eeh. 2023;2:195–207. doi: 10.1016/j.eehl.2023.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Wang Y., Xiang L., Wang F., et al. Positively charged microplastics induce strong lettuce stress responses from physiological, transcriptomic, and metabolomic perspectives. Environ. Sci. Technol. 2022;56:16907–16918. doi: 10.1021/acs.est.2c06054. [DOI] [PubMed] [Google Scholar]
- 289.Sun H., Lei C., Xu J., et al. Foliar uptake and leaf-to-root translocation of nanoplastics with different coating charge in maize plants. J. Hazard Mater. 2021;416 doi: 10.1016/j.jhazmat.2021.125854. [DOI] [PubMed] [Google Scholar]
- 290.Wang Y., Xiang L., Amelung W., et al. Micro- and nanoplastics in soil ecosystems: Analytical methods, fate, and effects. Trac-trend. Anal. Chem. 2023;169 [Google Scholar]
- 291.Im C., Kim H., Zaheer J., et al. PET tracing of biodistribution for orally administered 64Cu-Labeled polystyrene in mice. J. Nucl. Med. 2022;63:461–467. doi: 10.2967/jnumed.120.256982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Zhang N., Li Y.B., He H.R., et al. You are what you eat: Microplastics in the feces of young men living in Beijing. Sci. Total Environ. 2021;767 doi: 10.1016/j.scitotenv.2020.144345. [DOI] [PubMed] [Google Scholar]
- 293.Leslie H.A., van Velzen M.J.M., Brandsma S.H., et al. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022;163 doi: 10.1016/j.envint.2022.107199. [DOI] [PubMed] [Google Scholar]
- 294.Lambert S., Scherer C., Wagner M. Ecotoxicity testing of microplastics: Considering the heterogeneity of physicochemical properties. Integrated Environ. Assess. Manag. 2017;13:470–475. doi: 10.1002/ieam.1901. [DOI] [PubMed] [Google Scholar]
- 295.Li J., Su G., Letcher R.J., et al. Liquid crystal monomers (LCMs): A new generation of persistent bioaccumulative and toxic (PBT) compounds? Environ. Sci. Technol. 2018;52:5005–5006. doi: 10.1021/acs.est.8b01636. [DOI] [PubMed] [Google Scholar]
- 296.Zhang L., Wu B., Chen Y., et al. Treatment of liquid crystals and recycling indium for stripping product gained by mechanical stripping process from waste liquid crystal display panels. J. Clean. Prod. 2017;162:1472–1481. [Google Scholar]
- 297.Liang X., Xie R., Zhu C., et al. Comprehensive identification of liquid crystal monomers-biphenyls, cyanobiphenyls, fluorinated biphenyls, and their analogues-in waste LCD panels and the first estimate of their global release into the environment. Environ. Sci. Technol. 2021;55:12424–12436. doi: 10.1021/acs.est.1c03901. [DOI] [PubMed] [Google Scholar]
- 298.Feng J.J., Sun X.F., Zeng E.Y. Predicted health and environmental hazards of liquid crystal materials via quantitative structure-property relationship modeling. J. Hazard Mater. 2023;446 doi: 10.1016/j.jhazmat.2022.130592. [DOI] [PubMed] [Google Scholar]
- 299.Shen M., Feng Z., Liang X., et al. Release and gas-particle partitioning behavior of liquid crystal monomers during the dismantling of waste liquid crystal display panels in e-waste recycling facilities. Environ. Sci. Technol. 2022;56:3106–3116. doi: 10.1021/acs.est.1c07394. [DOI] [PubMed] [Google Scholar]
- 300.Zhang S., Yang M., Li Y., et al. Occurrence, distribution, and human exposure of emerging liquid crystal monomers (LCMs) in Indoor and outdoor dust: A nationwide study. Environ. Int. 2022;164:107295. doi: 10.1016/j.envint.2022.107295. [DOI] [PubMed] [Google Scholar]
- 301.Li R., Ren K., Su H., et al. Target and suspect analysis of liquid crystal monomers in soil from different urban functional zones. Sci. Total Environ. 2023;854 doi: 10.1016/j.scitotenv.2022.158408. [DOI] [PubMed] [Google Scholar]
- 302.Wang J., Nan J., Li M., et al. First Evidence of Contamination in Aquatic Organisms with Organic Light-Emitting Materials. Environ. Sci. Technol. Lett. 2022;9:739–746. [Google Scholar]
- 303.Cheng Z., Shi Q., Wang Y., et al. Electronic-waste-driven pollution of liquid crystal monomers: Environmental occurrence and human exposure in recycling industrial parks. Environ. Sci. Technol. 2022;56:2248–2257. doi: 10.1021/acs.est.1c04621. [DOI] [PubMed] [Google Scholar]
- 304.Yang L., Liu G., Zheng M., et al. Highly elevated levels and particle-size distributions of environmentally persistent free radicals in haze-associated atmosphere. Environ. Sci. Technol. 2017;51:7936–7944. doi: 10.1021/acs.est.7b01929. [DOI] [PubMed] [Google Scholar]
- 305.Zhu K., Jia H., Sun Y., et al. Long-term phototransformation of microplastics under simulated sunlight irradiation in aquatic environments: Roles of reactive oxygen species. Water Res. 2020;173 doi: 10.1016/j.watres.2020.115564. [DOI] [PubMed] [Google Scholar]
- 306.Kelley M.A., Hebert V.Y., Thibeaux T.M., et al. Model combustion-generated particulate matter containing persistent free radicals redox cycle to produce reactive oxygen species. Chem. Res. Toxicol. 2013;26:1862–1871. doi: 10.1021/tx400227s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Yang J., Pan B., Li H., et al. Degradation of p-Nitrophenol on Biochars: Role of Persistent Free Radicals. Environ. Sci. Technol. 2016;50:694–700. doi: 10.1021/acs.est.5b04042. [DOI] [PubMed] [Google Scholar]
- 308.Mahne S., Chuang G.C., Pankey E., et al. Environmentally persistent free radicals decrease cardiac function and increase pulmonary artery pressure. Am. J. Physiol-Heartc. 2012;303:1135–1142. doi: 10.1152/ajpheart.00545.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Pereira L.C., de Souza A.O., Bernardes M.F.F., et al. A perspective on the potential risks of emerging contaminants to human and environmental health. Environ. Sci. Pollut. Res. 2015;22:13800–13823. doi: 10.1007/s11356-015-4896-6. [DOI] [PubMed] [Google Scholar]
- 310.Yadav D., Rangabhashiyam S., Verma P., et al. Environmental and health impacts of contaminants of emerging concerns: Recent treatment challenges and approaches. Chemosphere. 2021;272 doi: 10.1016/j.chemosphere.2020.129492. [DOI] [PubMed] [Google Scholar]
- 311.de Rezende A.T., Mounteer A.H. Ecological risk assessment of pharmaceuticals and endocrine disrupting compounds in Brazilian surface waters. Environ. Pollut. 2023;338 doi: 10.1016/j.envpol.2023.122628. [DOI] [PubMed] [Google Scholar]
- 312.Kinigopoulou V., Pashalidis I., Kalderis D., et al. Microplastics as carriers of inorganic and organic contaminants in the environment: A review of recent progress. J. Mol. Liq. 2022;350 [Google Scholar]
- 313.Wang J., Guo X., Xue J. Biofilm-developed microplastics as vectors of pollutants in aquatic environments. Environ. Sci. Technol. 2021;55:12780–12790. doi: 10.1021/acs.est.1c04466. [DOI] [PubMed] [Google Scholar]
- 314.Snow D.D., Cassada D.A., Larsen M.L., et al. Detection, occurrence and fate of emerging contaminants in agricultural environments. Water Environ. Res. 2017;89:897–920. doi: 10.2175/106143017X15023776270160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Wang L., Bank M.S., Rinklebe J., et al. Plastic-rock complexes as hotspots for microplastic generation. Environ. Sci. Technol. 2023;57:7009–7017. doi: 10.1021/acs.est.3c00662. [DOI] [PubMed] [Google Scholar]
- 316.Riveros G., Soria R., Villafuerte A., et al. Effects of low-density polyethylene and polyamide microplastics on the microbiological and chemical characteristics of an Andisol. Soil Use Manag. 2023;40 [Google Scholar]
- 317.Bolan N., Sarkar B., Yan Y., et al. Remediation of poly- and perfluoroalkyl substances (PFAS) contaminated soils – To mobilize or to immobilize or to degrade? J. Hazard Mater. 2021;401 doi: 10.1016/j.jhazmat.2020.123892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Ren X., Zeng G., Tang L., et al. Sorption, transport and biodegradation – An insight into bioavailability of persistent organic pollutants in soil. Sci. Total Environ. 2018;610–611:1154–1163. doi: 10.1016/j.scitotenv.2017.08.089. [DOI] [PubMed] [Google Scholar]
- 319.Wang L., Hou D. Plastistone: An emerging type of sedimentary rock. Earth Sci. Rev. 2023;247 [Google Scholar]
- 320.Jiang L., Luo C., Zhang D., et al. Biphenyl-Metabolizing microbial community and a functional operon revealed in e-waste-contaminated soil. Environ. Sci. Technol. 2018;52:8558–8567. doi: 10.1021/acs.est.7b06647. [DOI] [PubMed] [Google Scholar]
- 321.Wang C., Wang L., Ok Y.S., et al. Soil plastisphere: Exploration methods, influencing factors, and ecological insights. J. Hazard Mater. 2022;430 doi: 10.1016/j.jhazmat.2022.128503. [DOI] [PubMed] [Google Scholar]
- 322.Ma Y., Yang K., Yu H., et al. Effects and mechanism of microplastics on organic carbon and nitrogen cycling in agricultural soil: A review. Soil Use Manag. 2023;40 [Google Scholar]
- 323.Pagel H., Poll C., Ingwersen J., et al. Modeling coupled pesticide degradation and organic matter turnover: From gene abundance to process rates. Soil Biol. Biochem. 2016;103:349–364. [Google Scholar]
- 324.Wang S., Wu X., Guo R., et al. Long-term field study on fate, transformation, and vertical transport of tetrabromobisphenol a in soil–plant systems. Environ. Sci. Technol. 2021;55:4607–4615. doi: 10.1021/acs.est.0c04021. [DOI] [PubMed] [Google Scholar]
- 325.Bouaïcha N., Corbel S. Cyanobacterial toxins emerging contaminants in soils: a review of sources, fate and impacts on ecosystems, plants and animal and human health. InTech Rijeka. 2016;21:105–126. [Google Scholar]
- 326.Li H., Hollstein M., Podder A., et al. Cyanotoxin impact on microbial-mediated nitrogen transformations at the interface of sediment-water column in surface water bodies. Environ. Pollut. 2020;266 doi: 10.1016/j.envpol.2020.115283. [DOI] [PubMed] [Google Scholar]
- 327.Tang Y., Li G., Iqbal B., et al. Soil nutrient levels regulate the effect of soil microplastic contamination on microbial element metabolism and carbon use efficiency. Ecotoxicol. Environ. Saf. 2023;267 doi: 10.1016/j.ecoenv.2023.115640. [DOI] [PubMed] [Google Scholar]
- 328.Tran N.H., Urase T., Ngo H.H., et al. Insight into metabolic and cometabolic activities of autotrophic and heterotrophic microorganisms in the biodegradation of emerging trace organic contaminants. Bioresour. Technol. 2013;146:721–731. doi: 10.1016/j.biortech.2013.07.083. [DOI] [PubMed] [Google Scholar]
- 329.Khan A., Jie Z., Wang J., et al. Ecological risks of microplastics contamination with green solutions and future perspectives. Sci. Total Environ. 2023;899 doi: 10.1016/j.scitotenv.2023.165688. [DOI] [PubMed] [Google Scholar]
- 330.Xiang L., Harindintwali J.D., Wang F., et al. Manure- and straw-derived biochars reduce the ecological risk of PBDE and promote nitrogen cycling by shaping microbiomes in PBDE-contaminated soil. Chemosphere. 2023;312 doi: 10.1016/j.chemosphere.2022.137262. [DOI] [PubMed] [Google Scholar]
- 331.Hou D., Al-Tabbaa A., O’Connor D., et al. Sustainable remediation and redevelopment of brownfield sites. Nat. Rev. Earth Environ. 2023;4:271–286. [Google Scholar]
- 332.Sunyer-Caldú A., Sepúlveda-Ruiz P., Salgot M., et al. Reclaimed water in agriculture: A plot-scale study assessing crop uptake of emerging contaminants and pathogens. J. Environ. Chem. Eng. 2022;10 [Google Scholar]
- 333.Zhou P., Wang L., Gao J., et al. Nanoplastic–plant interaction and implications for soil health. Soil Use Manag. 2023;39:13–42. [Google Scholar]
- 334.Pullagurala V.L.R., Rawat S., Adisa I.O., et al. Plant uptake and translocation of contaminants of emerging concern in soil. Sci. Total Environ. 2018;636:1585–1596. doi: 10.1016/j.scitotenv.2018.04.375. [DOI] [PubMed] [Google Scholar]
- 335.Rizwan M., Ali S., Rehman M.Z.U., et al. Effects of nanoparticles on trace element uptake and toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2021;221 doi: 10.1016/j.ecoenv.2021.112437. [DOI] [PubMed] [Google Scholar]
- 336.Moreno-Jiménez E., Leifheit E.F., Plaza C., et al. Effects of microplastics on crop nutrition in fertile soils and interaction with arbuscular mycorrhizal fungi. J. Sustain. Agri. Env. 2022;1:66–72. [Google Scholar]
- 337.Jiao X., Shi Q., Gan J. Uptake, accumulation and metabolism of PFASs in plants and health perspectives: A critical review. Crit. Rev. Environ. Sci. Technol. 2021;51:2745–2776. [Google Scholar]
- 338.Wang C., Gu X., Dong R., et al. Natural solar irradiation produces fluorescent and biodegradable nanoplastics. Environ. Sci. Technol. 2023;57:6626–6635. doi: 10.1021/acs.est.2c07537. [DOI] [PubMed] [Google Scholar]
- 339.Lesmeister L., Lange F.T., Breuer J., et al. Extending the knowledge about PFAS bioaccumulation factors for agricultural plants – A review. Sci. Total Environ. 2021;766 doi: 10.1016/j.scitotenv.2020.142640. [DOI] [PubMed] [Google Scholar]
- 340.Wang F., Feng X., Liu Y., et al. Micro(nano)plastics and terrestrial plants: Up-to-date knowledge on uptake, translocation, and phytotoxicity. Resour. Conserv. Recycl. 2022;185 [Google Scholar]
- 341.Martínez-Fernández D., Barroso D., Komárek M. Root water transport of Helianthus annuus L. under iron oxide nanoparticle exposure. Environ. Sci. Pollut. Res. 2016;23:1732–1741. doi: 10.1007/s11356-015-5423-5. [DOI] [PubMed] [Google Scholar]
- 342.Khalid N., Aqeel M., Noman A. Microplastics could be a threat to plants in terrestrial systems directly or indirectly. Environ. Pollut. 2020;267 doi: 10.1016/j.envpol.2020.115653. [DOI] [PubMed] [Google Scholar]
- 343.Dummett I., Sturrock C.J., Stroud J.L. Monitoring the effects of pesticide pellets to address farmers' concerns on soil fauna, specifically earthworms. Soil Use Manag. 2023;39:1235–1244. [Google Scholar]
- 344.Su P., Wang J., Zhang D., et al. Hierarchical and cascading changes in the functional traits of soil animals induced by microplastics: A meta-analysis. J. Hazard Mater. 2022;440 [Google Scholar]
- 345.Burkhard L.P., Votava L.K. Review of per- and polyfluoroalkyl substances (PFAS) bioaccumulation in earthworms. Environ. Adv. 2022;11:100335. doi: 10.1016/j.envadv.2022.100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Hopkins K.E., McKinney M.A., Saini A., et al. Characterizing the movement of per- and polyfluoroalkyl substances in an avian aquatic-terrestrial food web. Environ. Sci. Technol. 2023;57:20249–20260. doi: 10.1021/acs.est.3c06944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Okeke E.S., Okoye C.O., Atakpa E.O., et al. Microplastics in agroecosystems-impacts on ecosystem functions and food chain. Resour. Conserv. Recycl. 2022;177 [Google Scholar]
- 348.Wang J., Coffin S., Schlenk D., et al. Accumulation of HOCs via precontaminated microplastics by earthworm eisenia fetida in soil. Environ. Sci. Technol. 2020;54:11220–11229. doi: 10.1021/acs.est.0c02922. [DOI] [PubMed] [Google Scholar]
- 349.Sobhani Z., Fang C., Naidu R., et al. Microplastics as a vector of toxic chemicals in soil: Enhanced uptake of perfluorooctane sulfonate and perfluorooctanoic acid by earthworms through sorption and reproductive toxicity. Environ. Technol. Innov. 2021;22 [Google Scholar]
- 350.Carnevali O., Santangeli S., Forner-Piquer I., et al. Endocrine-disrupting chemicals in aquatic environment: What are the risks for fish gametes? Fish Physiol. Biochem. 2018;44:1561–1576. doi: 10.1007/s10695-018-0507-z. [DOI] [PubMed] [Google Scholar]
- 351.You H.H., Song G. Review of endocrine disruptors on male and female reproductive systems. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021;244 doi: 10.1016/j.cbpc.2021.109002. [DOI] [PubMed] [Google Scholar]
- 352.Celino-Brady F.T., Lerner D.T., Seale A.P. Experimental approaches for characterizing the endocrine-disrupting effects of environmental chemicals in fish. Front. Endocrinol. 2020;11 doi: 10.3389/fendo.2020.619361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Langston W.J. Endocrine disruption and altered sexual development in aquatic organisms: an invertebrate perspective. J. Mar. Biol. Assoc. U. K. 2020;100:495–515. [Google Scholar]
- 354.Hagemann L., Buchty-Lemke M., Maaß A.L., et al. Potential hotspots of persistent organic pollutants in alluvial sediments of the meandering Wurm River, Germany. J. Soils Sediments. 2020;20:1034–1045. [Google Scholar]
- 355.Krithiga T., Sathish S., Renita A.A., et al. Persistent organic pollutants in water resources: Fate, occurrence, characterization and risk analysis. Sci. Total Environ. 2022;831 doi: 10.1016/j.scitotenv.2022.154808. [DOI] [PubMed] [Google Scholar]
- 356.Corcoll N., Acuña V., Barceló D., et al. Pollution-induced community tolerance to non-steroidal anti-inflammatory drugs (NSAIDs) in fluvial biofilm communities affected by WWTP effluents. Chemosphere. 2014;112:185–193. doi: 10.1016/j.chemosphere.2014.03.128. [DOI] [PubMed] [Google Scholar]
- 357.Osuoha J.O., Anyanwu B.O., Ejileugha C. Pharmaceuticals and personal care products as emerging contaminants: Need for combined treatment strategy. J. Hazard. Mater. Adv. 2023;9 [Google Scholar]
- 358.Chia M.A., Kramer B.J., Jankowiak J.G., et al. The individual and combined effects of the cyanotoxins, anatoxin-a and microcystin-LR, on the growth, toxin production, and nitrogen fixation of prokaryotic and eukaryotic algae. Toxins. 2019;11:43. doi: 10.3390/toxins11010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Holland A., Kinnear S. Interpreting the possible ecological role (s) of cyanotoxins: compounds for competitive advantage and/or physiological aide? Mar. Drugs. 2013;11:2239–2258. doi: 10.3390/md11072239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Bhatt P., Engel B.A., Reuhs M., et al. Cyanophage technology in removal of cyanobacteria mediated harmful algal blooms: A novel and eco-friendly method. Chemosphere. 2023;315 doi: 10.1016/j.chemosphere.2023.137769. [DOI] [PubMed] [Google Scholar]
- 361.Brooks B.W., Lazorchak J.M., Howard M.D.A., et al. In some places, in some cases, and at some times, harmful algal blooms are the greatest threat to inland water quality. Environ. Toxicol. Chem. 2017;36:1125–1127. doi: 10.1002/etc.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Brooks B.W., Lazorchak J.M., Howard M.D.A., et al. Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems? Environ. Toxicol. Chem. 2016;35:6–13. doi: 10.1002/etc.3220. [DOI] [PubMed] [Google Scholar]
- 363.Rico C.M., Majumdar S., Duarte-Gardea M., et al. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011;59:3485–3498. doi: 10.1021/jf104517j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Du W., Tan W., Peralta-Videa J.R., et al. Interaction of metal oxide nanoparticles with higher terrestrial plants: Physiological and biochemical aspects. Plant Physiol. Biochem. 2017;110:210–225. doi: 10.1016/j.plaphy.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 365.Marslin G., Sheeba C.J., Franklin G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 2017;8 doi: 10.3389/fpls.2017.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Tripathi D.K., Chauhan D.K., Singh S. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Biochem. 2017;110:2–12. doi: 10.1016/j.plaphy.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 367.Cao Z., Stowers C., Rossi L., et al. Physiological effects of cerium oxide nanoparticles on the photosynthesis and water use efficiency of soybean (Glycine max (L.) Merr.) Environ. Sci.: Nano. 2017;4:1086–1094. [Google Scholar]
- 368.von Moos N., Slaveykova V.I. Oxidative stress induced by inorganic nanoparticles in bacteria and aquatic microalgae - state of the art and knowledge gaps. Nanotoxicology. 2014;8:605–630. doi: 10.3109/17435390.2013.809810. [DOI] [PubMed] [Google Scholar]
- 369.Mwaanga P., Carraway E.R., van den Hurk P. The induction of biochemical changes in Daphnia magna by CuO and ZnO nanoparticles. Aquat. Toxicol. 2014;150:201–209. doi: 10.1016/j.aquatox.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 370.Zuverza-Mena N., Martínez-Fernández D., Du W., et al. Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses-A review. Plant Physiol. Biochem. 2017;110:236–264. doi: 10.1016/j.plaphy.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 371.Ahamed M., AlSalhi M.S., Siddiqui M.K.J. Silver nanoparticle applications and human health. Clin. Chim. Acta. 2010;411:1841–1848. doi: 10.1016/j.cca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 372.Dhawan A., Sharma V. Toxicity assessment of nanomaterials: Methods and challenges. Anal. Bioanal. Chem. 2010;398:589–605. doi: 10.1007/s00216-010-3996-x. [DOI] [PubMed] [Google Scholar]
- 373.Sun C., Yin N., Wen R., et al. Silver nanoparticles induced neurotoxicity through oxidative stress in rat cerebral astrocytes is distinct from the effects of silver ions. Neurotoxicology. 2016;52:210–221. doi: 10.1016/j.neuro.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 374.Sharma S.S., Dietz K.J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009;14:43–50. doi: 10.1016/j.tplants.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 375.Hu Q., Fang Z., Ge J., et al. Nanotechnology for cardiovascular diseases. Innovation. 2022;3 doi: 10.1016/j.xinn.2022.100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Zhao W., Yan Y., Chen X., et al. Combining printing and nanoparticle assembly: Methodology and application of nanoparticle patterning. Innovation. 2022;3 doi: 10.1016/j.xinn.2022.100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Jani P., Halbert G.W., Langridge J., et al. Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J. Pharm. Pharmacol. 1990;42:821–826. doi: 10.1111/j.2042-7158.1990.tb07033.x. [DOI] [PubMed] [Google Scholar]
- 378.Tang H., Xu M., Zhou X., et al. Acute toxicity and biodistribution of different sized copper nano-particles in rats after oral administration. Mat. SCI. Eng C-Mater. 2018;93:649–663. doi: 10.1016/j.msec.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 379.Lei R., Wu C., Yang B., et al. Integrated metabolomic analysis of the nano-sized copper particle-induced hepatotoxicity and nephrotoxicity in rats: A rapid in vivo screening method for nanotoxicity. Toxicol. Appl. Pharmacol. 2008;232:292–301. doi: 10.1016/j.taap.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 380.Manna P., Ghosh M., Ghosh J., et al. Contribution of nano-copper particles to in vivo liver dysfunction and cellular damage: Role of IκBα/NF-κB, MAPKs and mitochondrial signal. Nanotoxicology. 2012;6:1–21. doi: 10.3109/17435390.2011.552124. [DOI] [PubMed] [Google Scholar]
- 381.Meng H., Chen Z., Xing G.M., et al. Ultrahigh reactivity provokes nanotoxicity: Explanation of oral toxicity of nano-copper particles. Toxicol. Lett. 2007;175:102–110. doi: 10.1016/j.toxlet.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 382.Xu P.J., Xu J., Liu S.C., et al. In vitro toxicity of nanosized copper particles in PC12 cells induced by oxidative stress. J. Nanoparticle Res. 2012;14:906. [Google Scholar]
- 383.Zhou X., Zhao L., Luo J., et al. The toxic effects and mechanisms of nano-Cu on the spleen of rats. Int. J. Mol. Sci. 2019;20:1469. doi: 10.3390/ijms20061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Kong M., Zhang Y., Ma Y., et al. Antibiotics and antibiotic resistance change bacterial community compositions in marine sediments. Environ. Res. 2024;244 doi: 10.1016/j.envres.2023.118005. [DOI] [PubMed] [Google Scholar]
- 385.Fong T.-T., Lipp E.K. Enteric viruses of humans and animals in aquatic environments: Health risks, detection, and potential water quality assessment tools. Microbiol. Mol. Biol. Rev. 2005;69:357–371. doi: 10.1128/MMBR.69.2.357-371.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Pandey P.K., Kass P.H., Soupir M.L., et al. Contamination of water resources by pathogenic bacteria. Amb. Express. 2014;4:1–16. doi: 10.1186/s13568-014-0051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Leclerc H., Schwartzbrod L., Dei-Cas E. Microbial agents associated with waterborne diseases. Crit. Rev. Microbiol. 2002;28:371–409. doi: 10.1080/1040-840291046768. [DOI] [PubMed] [Google Scholar]
- 388.López M.M., Llop P., Olmos A., et al. Are molecular tools solving the challenges posed by detection of plant pathogenic bacteria and viruses? Curr. Issues Mol. Biol. 2009;11:13–46. [PubMed] [Google Scholar]
- 389.Ashbolt N.J. Microbial contamination of drinking water and human health from community water systems. Curr. Environ. Health Rep. 2015;2:95–106. doi: 10.1007/s40572-014-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Mehle N., Ravnikar M. Plant viruses in aqueous environment–survival, water mediated transmission and detection. Water Res. 2012;46:4902–4917. doi: 10.1016/j.watres.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 391.Sime-Ngando T. Environmental bacteriophages: viruses of microbes in aquatic ecosystems. Front. Microbiol. 2014;5:355. doi: 10.3389/fmicb.2014.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Yuan X., Lv Z., Zhang Z., et al. A Review of Antibiotics, Antibiotic Resistant Bacteria, and Resistance Genes in Aquaculture: Occurrence, Contamination, and Transmission. Toxics. 2023;11:420. doi: 10.3390/toxics11050420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Pipe R.K., Coles J.A. Environmental contaminants influencing immunefunction in marine bivalve molluscs. Fish Shellfish Immunol. 1995;5:581–595. [Google Scholar]
- 394.Balbi T., Auguste M., Ciacci C., et al. Immunological responses of marine bivalves to contaminant exposure: Contribution of the-omics approach. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.618726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Mishra R.K., Mentha S.S., Misra Y., et al. Emerging pollutants of severe environmental concern in water and wastewater: A comprehensive review on current developments and future research. Water-Energy Nexus. 2023;6:74–95. [Google Scholar]
- 396.Sun J., Khattak W.A., Abbas A., et al. Ecological adaptability of invasive weeds under environmental pollutants: A review. Environ. Exp. Bot. 2023;105492 [Google Scholar]
- 397.Kim H.J., Koedrith P., Seo Y.R. Ecotoxicogenomic approaches for understanding molecular mechanisms of environmental chemical toxicity using aquatic invertebrate, Daphnia model organism. Int. J. Mol. Sci. 2015;16:12261–12287. doi: 10.3390/ijms160612261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Barletta M., Lima A.R.A., Costa M.F. Distribution, sources and consequences of nutrients, persistent organic pollutants, metals and microplastics in South American estuaries. Sci. Total Environ. 2019;651:1199–1218. doi: 10.1016/j.scitotenv.2018.09.276. [DOI] [PubMed] [Google Scholar]
- 399.Ahmed M.B., Rahman M.S., Alom J., et al. Microplastic particles in the aquatic environment: A systematic review. Sci. Total Environ. 2021;775 doi: 10.1016/j.scitotenv.2021.145793. [DOI] [PubMed] [Google Scholar]
- 400.Li C., Gillings M.R., Zhang C., et al. Ecology and risks of the global plastisphere as a newly expanding microbial habitat. Innovation. 2024;5 doi: 10.1016/j.xinn.2023.100543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Ceschin S., Mariani F., Di Lernia D., et al. Effects of microplastic contamination on the aquatic plant Lemna minuta (least duckweed) Plants. 2023;12:207. doi: 10.3390/plants12010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Ge J., Li H., Liu P., et al. Review of the toxic effect of microplastics on terrestrial and aquatic plants. Sci. Total Environ. 2021;791 doi: 10.1016/j.scitotenv.2021.148333. [DOI] [PubMed] [Google Scholar]
- 403.Egbeocha C.O., Malek S., Emenike C.U., et al. Feasting on microplastics: ingestion by and effects on marine organisms. Aquat. Biol. 2018;27:93–106. [Google Scholar]
- 404.Rakib M.R.J., Sarker A., Ram K., et al. Microplastic toxicity in aquatic organisms and aquatic ecosystems: a review. Water Air Soil Pollut. 2023;234:52. [Google Scholar]
- 405.Alak G., Uçar A., Parlak V., et al. Identification, characterisation of microplastic and their effects on aquatic organisms. Chem. Ecol. 2022;38:967–987. [Google Scholar]
- 406.Harmon S.M., Chen Q., Ma C., et al. Microplastic Contamination in Aquatic Environments. Elsevier; 2024. The effects of microplastic pollution on aquatic organisms; pp. 355–379. [Google Scholar]
- 407.Sreelakshmi T., Chitra K. Microplastics contamination in the environment: An ecotoxicological concern. Intern. J. Zool. Invest. 2021;7:230–258. [Google Scholar]
- 408.Ašmonaitė G. 2019. Microplastics in the Aquatic Environment. [Google Scholar]
- 409.He W., Cui Y., Yang H., et al. Aquatic toxicity, ecological effects, human exposure pathways and health risk assessment of liquid crystal monomers. J. Hazard Mater. 2024;461 doi: 10.1016/j.jhazmat.2023.132681. [DOI] [PubMed] [Google Scholar]
- 410.Gojkovic Z., Simansky S., Sanabria A., et al. Interaction of Naturally Occurring Phytoplankton with the Biogeochemical Cycling of Mercury in Aquatic Environments and Its Effects on Global Hg Pollution and Public Health. Microorganisms. 2023;11:2034. doi: 10.3390/microorganisms11082034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Li Z., Yu D., Wangbao G., et al. Aquatic ecotoxicology and water quality criteria of three organotin compounds: a review. Nat. Environ. Pollut. Technol. 2019;18:217–224. [Google Scholar]
- 412.Bashir I., Lone F.A., Bhat R.A., et al. Concerns and threats of contamination on aquatic ecosystems. Bioremediation and biotechnology: sustainable approaches to pollution degradation. 2020:1–26. [Google Scholar]
- 413.Maluquer de Motes C., Cano M.J., Torres J.M., et al. Detection and survival of prion agents in aquatic environments. Water Res. 2008;42:2465–2472. doi: 10.1016/j.watres.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 414.Rickard L.N., Houston C.L., McGreavy B., et al. Fish prisons and bluehouses: Perceived risks and benefits of land-based aquaculture in four US communities. Environ. Commun. 2023;17:930–946. [Google Scholar]
- 415.Lai A., Clark A.M., Escher B.I., et al. The next frontier of environmental unknowns: substances of unknown or variable composition, complex reaction products, or biological materials (UVCBs) Environ. Sci. Technol. 2022;56:7448–7466. doi: 10.1021/acs.est.2c00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Huang T., Liu M., Xing R., et al. Threat of air pollution in the cleanest plateau. Innovation. 2023;4 doi: 10.1016/j.xinn.2023.100390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Manisalidis I., Stavropoulou E., Stavropoulos A., et al. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health. 2020;8 doi: 10.3389/fpubh.2020.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Wu C.-C., Bao L.-J., Tao S., et al. Dermal Uptake from Airborne Organics as an Important Route of Human Exposure to E-Waste Combustion Fumes. Environ. Sci. Technol. 2016;50:6599–6605. doi: 10.1021/acs.est.5b05952. [DOI] [PubMed] [Google Scholar]
- 419.Garrido J.A., Parthasarathy S., Moschet C., et al. Exposure Assessment For Air-To-Skin Uptake of Semivolatile Organic Compounds (SVOCs) Indoors. Environ. Sci. Technol. 2019;53:1608–1616. doi: 10.1021/acs.est.8b05123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Lao J.-Y., Xie S.-Y., Wu C.-C., et al. Importance of Dermal Absorption of Polycyclic Aromatic Hydrocarbons Derived from Barbecue Fumes. Environ. Sci. Technol. 2018;52:8330–8338. doi: 10.1021/acs.est.8b01689. [DOI] [PubMed] [Google Scholar]
- 421.Cai J., Shen Y., Meng X., et al. Association of developmental coordination disorder with early-life exposure to fine particulate matter in Chinese preschoolers. Innovation. 2023;4:100347. doi: 10.1016/j.xinn.2022.100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Zhang Q., Wang H., Zhu X., et al. Air pollution may increase the sleep apnea severity: A nationwide analysis of smart device-based monitoring. Innovation. 2023;4:100528. doi: 10.1016/j.xinn.2023.100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Xu H., Liu Y., Wang Q., et al. The disease burden related to time-weighted PM2.5 exposure in China and the potential health benefits of the national standards for indoor air quality: A modeling study. Sustainable Horizons. 2024;9 [Google Scholar]
- 424.Khan A.A., Kumar P., Gulia S., et al. A critical review of managing air pollution through airshed approach. Sustainable Horizons. 2024;9 [Google Scholar]
- 425.He F., Wang N., Yu X., et al. GATA3/long noncoding RNA MHC-R regulates the immune activity of dendritic cells in chronic obstructive pulmonary disease induced by air pollution particulate matter. J. Hazard Mater. 2022;438 doi: 10.1016/j.jhazmat.2022.129459. [DOI] [PubMed] [Google Scholar]
- 426.Pan T.-L., Wang P.-W., Aljuffali I.A., et al. The impact of urban particulate pollution on skin barrier function and the subsequent drug absorption. J. Dermatol. Sci. 2015;78:51–60. doi: 10.1016/j.jdermsci.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 427.Al-Kindi S.G., Brook R.D., Biswal S., et al. Environmental determinants of cardiovascular disease: Lessons learned from air pollution. Nat. Rev. Cardiol. 2020;17:656–672. doi: 10.1038/s41569-020-0371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Wang M.J., Li Q.Q., Hou M.F., et al. Inactivation of common airborne antigens by perfluoroalkyl chemicals modulates early life asthma. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2011957118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Fischer P.H., Marra M., Ameling C.B., et al. Air Pollution and Mortality in Seven Million Adults: The Dutch Environmental Longitudinal Study (DUELS) Environ. Health Perspect. 2015;123:697–704. doi: 10.1289/ehp.1408254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Shimpi P.C., More V.R., Paranjpe M., et al. Hepatic Lipid Accumulation and Nrf2 Expression following Perinatal and Peripubertal Exposure to Bisphenol A in a Mouse Model of Nonalcoholic Liver Disease. Environ. Health Perspect. 2017;125 doi: 10.1289/EHP664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Ghio A.J., Carraway M.S., Madden M.C. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J. Toxicol. Environ. Health B Crit. Rev. 2012;15:1–21. doi: 10.1080/10937404.2012.632359. [DOI] [PubMed] [Google Scholar]
- 432.Zhang Q., Meng X., Shi S., et al. Overview of particulate air pollution and human health in China: Evidence, challenges, and opportunities. Innovation. 2022;3 doi: 10.1016/j.xinn.2022.100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Zhang Y., Liu M., Peng B., et al. Impact of Mixture Effects between Emerging Organic Contaminants on Cytotoxicity: A Systems Biological Understanding of Synergism between Tris(1,3-dichloro-2-propyl)phosphate and Triphenyl Phosphate. Environ. Sci. Technol. 2020;54:10722–10734. doi: 10.1021/acs.est.0c02188. [DOI] [PubMed] [Google Scholar]
- 434.Tang J., Ma S., Hu X., et al. Handwipes as indicators to assess organophosphate flame retardants exposure and thyroid hormone effects in e-waste dismantlers. J. Hazard Mater. 2023;443 doi: 10.1016/j.jhazmat.2022.130248. [DOI] [PubMed] [Google Scholar]
- 435.Shoeib M., Harner T., Vlahos P. Perfluorinated Chemicals in the Arctic Atmosphere. Environ. Sci. Technol. 2006;40:7577–7583. doi: 10.1021/es0618999. [DOI] [PubMed] [Google Scholar]
- 436.Wallington T.J., Hurley M.D., Xia J., et al. Formation of C7F15COOH (PFOA) and Other Perfluorocarboxylic Acids during the Atmospheric Oxidation of 8:2 Fluorotelomer Alcohol. Environ. Sci. Technol. 2006;40:924–930. doi: 10.1021/es051858x. [DOI] [PubMed] [Google Scholar]
- 437.Sun Y.-F., Guo Y., Xu C., et al. Will “Air Eutrophication” Increase the Risk of Ecological Threat to Public Health? Environ. Sci. Technol. 2023;57:10512–10520. doi: 10.1021/acs.est.3c01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Wang H., Xu C., Liu Y., et al. From unusual suspect to serial killer: Cyanotoxins boosted by climate change may jeopardize megafauna. Innovation. 2021;2 doi: 10.1016/j.xinn.2021.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Rossi G.L., Corsico A., Roggeri A., et al. Human health and air conditioning systems. G. Ital. Med. Lav. 1991;13:51–54. [PubMed] [Google Scholar]
- 440.Stockwell R.E., Ballard E.L., O'Rourke P., et al. Indoor hospital air and the impact of ventilation on bioaerosols: a systematic review. J. Hosp. Infect. 2019;103:175–184. doi: 10.1016/j.jhin.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 441.Han Y., Li L., Wang Y., et al. Composition, dispersion, and health risks of bioaerosols in wastewater treatment plants: A review. Front. Environ. Sci. Eng. 2020;15:38. [Google Scholar]
- 442.Rai S., Singh D.K., Kumar A. Microbial, environmental and anthropogenic factors influencing the indoor microbiome of the built environment. J. Basic Microbiol. 2021;61:267–292. doi: 10.1002/jobm.202000575. [DOI] [PubMed] [Google Scholar]
- 443.Després V.R., Huffman J.A., Burrows S.M., et al. Primary biological aerosol particles in the atmosphere: a review. Tellus B. 2012;64 [Google Scholar]
- 444.Leung N.H.L. Transmissibility and transmission of respiratory viruses. Nat. Rev. Microbiol. 2021;19:528–545. doi: 10.1038/s41579-021-00535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Fröhlich-Nowoisky J., Kampf C.J., Weber B., et al. Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016;182:346–376. [Google Scholar]
- 446.King M.D., Lacey R.E., Pak H., et al. Assays and enumeration of bioaerosols-traditional approaches to modern practices. Aerosol Sci. Technol. 2020;54:611–633. [Google Scholar]
- 447.Fang M., Hu L., Chen D., et al. Exposome in human health: Utopia or wonderland? Innovation. 2021;2 doi: 10.1016/j.xinn.2021.100172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Picó Y., Barceló D. Microplastics and other emerging contaminants in the environment after COVID-19 pandemic: The need of global reconnaissance studies. Curr. Opin. Environ. Sci. Health. 2023;33 doi: 10.1016/j.coesh.2023.100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Lei M., Zhang L., Lei J., et al. Overview of Emerging Contaminants and Associated Human Health Effects. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/404796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Abhijith K.V., Kumar P., Omidvarborna H., et al. Improving air pollution awareness of the general public through citizen science approach. Sustainable Horizons. 2024;10 [Google Scholar]
- 451.Wang F., Harindintwali J.D., Yuan Z., et al. Technologies and perspectives for achieving carbon neutrality. Innovation. 2021;2 doi: 10.1016/j.xinn.2021.100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Kim H.M., Kang J.S. Metabolomic Studies for the Evaluation of Toxicity Induced by Environmental Toxicants on Model Organisms. Metabolites. 2021;11:485. doi: 10.3390/metabo11080485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Zhang L.-J., Qian L., Ding L.-Y., et al. Ecological and toxicological assessments of anthropogenic contaminants based on environmental metabolomics. Environ. Sci. Ecotechnol. 2021;5 doi: 10.1016/j.ese.2021.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Ankley G.T., Bennett R.S., Erickson R.J., et al. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010;29:730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- 455.Kovacevic V., Simpson M.J. Environmental Metabolomics. Elsevier; 2020. Fundamentals of environmental metabolomics; pp. 1–33. [Google Scholar]
- 456.Viant M.R. Applications of metabolomics to the environmental sciences. Metabolomics. 2009;5:1–2. [Google Scholar]
- 457.Dumas T., Courant F., Fenet H., et al. Environmental metabolomics promises and achievements in the field of aquatic ecotoxicology: viewed through the pharmaceutical lens. Metabolites. 2022;12:186. doi: 10.3390/metabo12020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Labine L.M., Pereira E.A.O., Kleywegt S., et al. Environmental metabolomics uncovers oxidative stress, amino acid dysregulation, and energy impairment in Daphnia magna with exposure to industrial effluents. Environ. Res. 2023;234 doi: 10.1016/j.envres.2023.116512. [DOI] [PubMed] [Google Scholar]
- 459.Tang R., Lan P., Ding C., et al. A new perspective on the toxicity of arsenic-contaminated soil: Tandem mass tag proteomics and metabolomics in earthworms. J. Hazard Mater. 2020;398 doi: 10.1016/j.jhazmat.2020.122825. [DOI] [PubMed] [Google Scholar]
- 460.Anwar M., Iqbal Q., Saleem F. Improper disposal of unused antibiotics: An often overlooked driver of antimicrobial resistance. Expert Rev. Anti Infect. Ther. 2020;18:697–699. doi: 10.1080/14787210.2020.1754797. [DOI] [PubMed] [Google Scholar]
- 461.Polianciuc S.I., Gurzău A.E., Kiss B., et al. Antibiotics in the environment: causes and consequences. Med. Pharm. Rep. 2020;93:231–240. doi: 10.15386/mpr-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Caioni G., Benedetti E., Perugini M., et al. Personal care products as a contributing factor to antimicrobial resistance: Current state and novel approach to investigation. Antibiotics (Basel) 2023;12 doi: 10.3390/antibiotics12040724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Yang J., Gong Y., Zhang C., et al. Co-existence and co-infection of influenza A viruses and coronaviruses: Public health challenges. Innovation. 2022;3 doi: 10.1016/j.xinn.2022.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Laws M.J., Neff A.M., Brehm E., et al. Endocrine disrupting chemicals and reproductive disorders in women, men, and animal models. Adv. Pharmacol. 2021;92:151–190. doi: 10.1016/bs.apha.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Cantonwine D.E., Hauser R., Meeker J.D. Bisphenol a and human reproductive health. Expet Rev. Obstet. Gynecol. 2013;8 doi: 10.1586/17474108.2013.811939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Matuszczak E., Komarowska M.D., Debek W., et al. The Impact of Bisphenol A on Fertility, Reproductive System, and Development: A Review of the Literature. Internet J. Endocrinol. 2019;2019 doi: 10.1155/2019/4068717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Dong Z., Ma J., Qiu J., et al. Airborne fine particles drive H1N1 viruses deep into the lower respiratory tract and distant organs. Sci. Adv. 2023;9 doi: 10.1126/sciadv.adf2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Sun Y., Wang M., Wang Y., et al. Exposure to airborne PM2.5 chemical exposome increases heart rate of middle- and old-aged populations. Innovat. Med. 2023;1 doi: 10.59717/j.xinn-med.2023.100042. [DOI] [Google Scholar]
- 469.Qi J., Wang Y., Wang L., et al. The modification effect of ozone pollution on the associations between heat wave and cardiovascular mortality. Innovat. Med. 2023;1 doi: 10.59717/j.xinn-med.2023.100043. [DOI] [Google Scholar]
- 470.Pan K., Xu J., Long X., et al. The relationship between perfluoroalkyl substances and hypertension: A systematic review and meta-analysis. Environ. Res. 2023;232 doi: 10.1016/j.envres.2023.116362. [DOI] [PubMed] [Google Scholar]
- 471.Schraufnagel D.E. The health effects of ultrafine particles. Exp. Mol. Med. 2020;52:311–317. doi: 10.1038/s12276-020-0403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Stewart I., Webb P.M., Schluter P.J., et al. Epidemiology of recreational exposure to freshwater cyanobacteria–an international prospective cohort study. BMC Publ. Health. 2006;6:1–11. doi: 10.1186/1471-2458-6-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Kothapalli C.R. Differential impact of heavy metals on neurotoxicity during development and in aging central nervous system. Current Opinion in Toxicology. 2021;26:33–38. [Google Scholar]
- 474.Park S.K., Ding N., Han D. Perfluoroalkyl substances and cognitive function in older adults: Should we consider non-monotonic dose-responses and chronic kidney disease? Environ. Res. 2021;192 doi: 10.1016/j.envres.2020.110346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Chen P., Miah M., Aschner M. Metals and Neurodegeneration. F1000Research. 2016;5 doi: 10.12688/f1000research.7431.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Kodavanti P.R.S. Neurotoxicity of persistent organic pollutants: possible mode(s) of action and further considerations. Dose Response. 2006;3:273–305. doi: 10.2203/dose-response.003.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Lee Y.J. Potential health effects of emerging environmental contaminants perfluoroalkyl compounds. Yeungnam Univ. J. Med. 2018;35:156–164. doi: 10.12701/yujm.2018.35.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Li J., Wang Y., Steenland K., et al. Long-term effects of PM2.5 components on incident dementia in the northeastern United States. Innovation. 2022;3 doi: 10.1016/j.xinn.2022.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Mastrantonio M., Bai E., Uccelli R., et al. Drinking water contamination from perfluoroalkyl substances (PFAS): an ecological mortality study in the Veneto Region, Italy. Eur. J. Publ. Health. 2018;28:180–185. doi: 10.1093/eurpub/ckx066. [DOI] [PubMed] [Google Scholar]
- 480.Sollome J., Fry R.C. In: Systems Biology in Toxicology and Environmental Health. Fry R.C., editor. Academic Press; 2015. Chapter 7 - Environmental Contaminants and the Immune System: A Systems Perspective; pp. 171–186. [Google Scholar]
- 481.von Holst H., Nayak P., Dembek Z., et al. Perfluoroalkyl substances exposure and immunity, allergic response, infection, and asthma in children: review of epidemiologic studies. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Grandjean P., Andersen E.W., Budtz-Jørgensen E., et al. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 2012;307:391–397. doi: 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Sevelsted A., Pedersen C.T., Gürdeniz G., et al. Exposures to perfluoroalkyl substances and asthma phenotypes in childhood: an investigation of the COPSAC2010 cohort. EBioMedicine. 2023;94 doi: 10.1016/j.ebiom.2023.104699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Lieke T., Zhang X.C., Steinberg C.E.W., et al. Overlooked Risks of Biochars: Persistent Free Radicals trigger Neurotoxicity in Caenorhabditis elegans. Environ. Sci. Technol. 2018;52:7981–7987. doi: 10.1021/acs.est.8b01338. [DOI] [PubMed] [Google Scholar]
- 485.Li H., Li H., Zuo N., et al. Direct toxicity of environmentally persistent free radicals to nematode Caenorhabditis elegans after excluding the concomitant chemicals. Sci. Total Environ. 2022;839 doi: 10.1016/j.scitotenv.2022.156226. [DOI] [PubMed] [Google Scholar]
- 486.Zhao J., Zhang Y., Chu G. Reactivity of aged biochars to the degradation of adsorbed p-nitrophenol: Role of intensity and species of persistent free radicals. Chemosphere. 2023;344 doi: 10.1016/j.chemosphere.2023.140362. [DOI] [PubMed] [Google Scholar]
- 487.Yang J., Pignatello J.J., Pan B., et al. Degradation of p-Nitrophenol by Lignin and Cellulose Chars: H2O2 Mediated Reaction and Direct Reaction with the Char. Environ. Sci. Technol. 2017;51:8972–8980. doi: 10.1021/acs.est.7b01087. [DOI] [PubMed] [Google Scholar]
- 488.Fang G., Liu C., Gao J., et al. Manipulation of Persistent Free Radicals in Biochar To Activate Persulfate for Contaminant Degradation. Environ. Sci. Technol. 2015;49:5645–5653. doi: 10.1021/es5061512. [DOI] [PubMed] [Google Scholar]
- 489.Tao W.M., Duan W.Y., Liu C.B., et al. Formation of persistent free radicals in biochar derived from rice straw based on a detailed analysis of pyrolysis kinetics. Sci. Total Environ. 2020;715 doi: 10.1016/j.scitotenv.2020.136575. [DOI] [PubMed] [Google Scholar]
- 490.Liu Y., Yang F., Liu S., et al. Molecular characteristics of microalgal extracellular polymeric substances were different among phyla and correlated with the extracellular persistent free radicals. Sci. Total Environ. 2023;857 doi: 10.1016/j.scitotenv.2022.159704. [DOI] [PubMed] [Google Scholar]
- 491.Arneth A., Mercado L., Kattge J., et al. Future challenges of representing land-processes in studies on land-atmosphere interactions. Biogeosciences. 2012;9:3587–3599. [Google Scholar]
- 492.Dracheva E., Norinder U., Rydén P., et al. In silico identification of potential thyroid hormone system disruptors among chemicals in human serum and chemicals with a high exposure index. Environ. Sci. Technol. 2022;56:8363–8372. doi: 10.1021/acs.est.1c07762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Xiong Y., Shi Q., Smith A., et al. Methylation and Demethylation of Emerging Contaminants Changed Bioaccumulation and Acute Toxicity in Daphnia magna. Environ. Sci. Technol. 2023;57:15213–15222. doi: 10.1021/acs.est.3c03242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Schlender T., Viljanen M., van Rijn J.N., et al. The bigger fish: A comparison of meta-Learning QSAR models on mow-resourced aquatic toxicity regression tasks. Environ. Sci. Technol. 2023;57:17818–17830. doi: 10.1021/acs.est.3c00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Wania F., Mackay D. Tracking the distribution of persistent organic pollutants. Environ. Sci. Technol. 1996;30:390A–396A. doi: 10.1021/es962399q. [DOI] [PubMed] [Google Scholar]
- 496.Pruess K., Battistelli A. Lawrence Berkeley National Laboratory; 2005. TMVOC, a Numerical Simulator for Three-phase Non-isothermal Flows of Multicomponent Hydrocarbon Mixtures in Saturated-Unsaturated Heterogeneous Media. [Google Scholar]
- 497.Xu T., Sonnenthal E., Spycher N., et al. Vol. 32. Lawrence Berkeley National Laboratory; 2004. pp. 145–165. (TOURGHREACT: A Simulation Program for Non-isothermal Multiphasereactive Geochemical Transport in Variably Saturated Geologicmedia). [Google Scholar]
- 498.Clement T.P. Pacific Northwest National Lab.(PNNL); 1999. A Modular Computer Code for Simulating Reactive Multi-Species Transport in 3-dimensional Groundwater Systems. [Google Scholar]
- 499.Hammond G.E., Lichtner P.C., Mills R.T. Evaluating the performance of parallel subsurface simulators: An illustrative example with PFLOTRAN. Water Resour. Res. 2014;50:208–228. doi: 10.1002/2012WR013483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Prommer H., Barry D.A., Zheng C. MODFLOW/MT3DMS based reactive multi-component transport modeling. Groundwater. 2003;41:247–257. doi: 10.1111/j.1745-6584.2003.tb02588.x. [DOI] [PubMed] [Google Scholar]
- 501.Mckone T., Enoch K. Lawrence Berkeley National Laboratory; 2002. CalTOX (Registered Trademark), A Multimedia Total Exposure Model Spreadsheet User's Guide. Version 4.0(Beta) [Google Scholar]
- 502.USEPA KABAM version 1.0 user's guide and technical documentation. 2009. https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/kabam-version-10-users-guide-and-technical
- 503.Mackay W.D. Global fractionation and cold condensation of low volatility organochlorine compounds in polar regions. Ambio. 1993;22:10–18. [Google Scholar]
- 504.Yu Y., Katsoyiannis A., Bohlin-Nizzetto P., et al. Polycyclic aromatic hydrocarbons not declining in arctic air despite global emission reduction. Environ. Sci. Technol. 2019;53:2375–2382. doi: 10.1021/acs.est.8b05353. [DOI] [PubMed] [Google Scholar]
- 505.Zhong S., Zhang K., Bagheri M., et al. Machine learning: New ideas and tools in environmental science and engineering. Environ. Sci. Technol. 2021;55:12741–12754. doi: 10.1021/acs.est.1c01339. [DOI] [PubMed] [Google Scholar]
- 506.Yamijala S.S.R.K.C., Shinde R., Wong B.M. Real-time degradation dynamics of hydrated per- and polyfluoroalkyl substances (PFASs) in the presence of excess electrons. Phys. Chem. Chem. Phys. 2020;22:6804–6808. doi: 10.1039/c9cp06797c. [DOI] [PubMed] [Google Scholar]
- 507.Biswas S., Yamijala S.S.R.K.C., Wong B.M. Degradation of per- and polyfluoroalkyl substances with hydrated electrons: A new mechanism from first-principles calculations. Environ. Sci. Technol. 2022;56:8167–8175. doi: 10.1021/acs.est.2c01469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Raza A., Bardhan S., Xu L., et al. A machine learning approach for predicting defluorination of per- and polyfluoroalkyl substances (PFAS) for their efficient treatment and removal. Environ. Sci. Technol. Lett. 2019;6:624–629. [Google Scholar]
- 509.Kwon H., Ali Z.A., Wong B.M. Harnessing semi-supervised machine learning to automatically predict bioactivities of per- and polyfluoroalkyl substances (PFASs) Environ. Sci. Technol. Lett. 2023;10:1017–1022. doi: 10.1021/acs.estlett.2c00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Gao F., Shen Y., Sallach J.B., et al. Predicting crop root concentration factors of organic contaminants with machine learning models. J. Hazard Mater. 2022;424 doi: 10.1016/j.jhazmat.2021.127437. [DOI] [PubMed] [Google Scholar]
- 511.Watts M. pesticide action network north america; 2012. Chlorpyrifos as a Possible Global POP. [Google Scholar]
- 512.Xu Y., Wang F., An Z., et al. Artificial intelligence for science-bridging data to wisdom. Innovation. 2023;4 doi: 10.1016/j.xinn.2023.100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Wang Z., Zhang J., Hua P., et al. Filling in missing pieces in the co-development of artificial intelligence and environmental science. The Innovation Geoscience. 2023;1 doi: 10.59717/j.xinn-geo.2023.100007. [DOI] [Google Scholar]
- 514.Peralonso M.J.R. 2024. Integrated Pollution Prevention and Control (IPPC) [Google Scholar]
- 515.Giner-Santonja G., Vázquez Calvo V., Rodríguez Lepe G. Application of AHP and corrective factors for the determination of best available techniques and emission limit values at installation level: A case study in four cement installations. Sci. Total Environ. 2019;660:834–840. doi: 10.1016/j.scitotenv.2018.12.473. [DOI] [PubMed] [Google Scholar]
- 516.Aragão A. In: Encyclopedia of Contemporary Constitutionalism. Cremades J., Hermida C., editors. Springer International Publishing; 2020. Polluter-pays principle; pp. 1–24. [Google Scholar]
- 517.Islam M.Z., Wang S. Exploring the unique characteristics of environmental sustainability in China: Navigating future challenges. Chin J Popul Resour. 2023;21:37–42. [Google Scholar]
- 518.He X. In the name of legitimacy and efficiency: Evaluating China’s legal reform on EIA. J. Environ. Law. 2020;32:441–469. [Google Scholar]
- 519.Kumar R. The United Nations and global environmental governance. Strat. Anal. 2020;44:479–489. [Google Scholar]
- 520.Kipāne A., Vilks A. Legal framework for environmental protection in the context of sustainable development. Eur. J. Sustain. Dev. 2022;11:169. [Google Scholar]
- 521.Bodansky D., van Asselt H. Oxford University Press; 2024. The Art and Craft of International Environmental Law. [Google Scholar]
- 522.Meng F., Chen Z., Wu J. How are anti-air pollution policies implemented? A network analysis of campaign-style enforcement in China. Sustainability. 2019;11:340. [Google Scholar]
- 523.Gupta S., Saksena S., Baris O.F. Environmental enforcement and compliance in developing countries: Evidence from India. World Dev. 2019;117:313–327. [Google Scholar]
- 524.Zhang K., Li Y., Qi Y., et al. Can green credit policy improve environmental quality? Evidence from China. J. Environ. Manag. 2021;298 doi: 10.1016/j.jenvman.2021.113445. [DOI] [PubMed] [Google Scholar]
- 525.Lou Y., Xu L., Gan N., et al. Chemically recyclable polyesters from CO2, H2, and 1,3-butadiene. Innovation. 2022;3 doi: 10.1016/j.xinn.2022.100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Singh R., Ram K. Environmental Degradation: Challenges and Strategies for Mitigation. Springer; 2022. Environmental Remediation Technologies; pp. 211–225. [Google Scholar]
- 527.Hamadani H., Rashid S.M., Rehman M.U., et al. 2020. Global Scenario of Remediation Techniques to Combat Environmental Pollution. [Google Scholar]
- 528.Baldissarelli D., Vargas G., Korf E., et al. Remediation of soils contaminated by pesticides using physicochemical processes: a brief review. Planta Daninha. 2019;37 [Google Scholar]
- 529.Vidonish J.E., Zygourakis K., Masiello C.A., et al. Thermal treatment of hydrocarbon-impacted soils: A review of technology innovation for sustainable remediation. Engineering. 2016;2:426–437. [Google Scholar]
- 530.Zhao C., Dong Y., Feng Y., et al. Thermal desorption for remediation of contaminated soil: A review. Chemosphere. 2019;221:841–855. doi: 10.1016/j.chemosphere.2019.01.079. [DOI] [PubMed] [Google Scholar]
- 531.Dehghani M.H., Ahmadi S., Ghosh S., et al. Recent advances on sustainable adsorbents for the remediation of noxious pollutants from water and wastewater: A critical review. Arab. J. Chem. 2023;16 [Google Scholar]
- 532.Liu X., Pang H., Liu X., et al. Orderly porous covalent organic frameworks-based materials: Superior adsorbents for pollutants removal from aqueous solutions. Innovation. 2021;2 doi: 10.1016/j.xinn.2021.100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Liu Y., Liu X., Dong W., et al. Efficient adsorption of sulfamethazine onto modified activated carbon: A plausible adsorption mechanism. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-12805-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Blenis N., Hue N., Maaz T.M., et al. Biochar production, modification, and Its uses in soil remediation: A review. Sustainability. 2023;15:3442. [Google Scholar]
- 535.Mielke K.C., Mendes K.F., Sousa R., et al. Degradation process of herbicides in biochar-amended soils: impact on persistence and remediation. Biodegradation Technology of Organic and Inorganic Pollutants. 2022;1:1–22. [Google Scholar]
- 536.Dehghani M.H. Removal of cyanobacterial and algal cells from water by ultrasonic waves—A review. J. Mol. Liq. 2016;222:1109–1114. [Google Scholar]
- 537.Khadgi N., Upreti A.R. Photocatalytic degradation of microcystin-LR by visible light active and magnetic, ZnFe2O4-Ag/rGO nanocomposite and toxicity assessment of the intermediates. Chemosphere. 2019;221:441–451. doi: 10.1016/j.chemosphere.2019.01.046. [DOI] [PubMed] [Google Scholar]
- 538.Picuno P. Innovative material and improved technical design for a sustainable exploitation of agricultural plastic film. Polym. Plast. Technol. Eng. 2014;53:1000–1011. [Google Scholar]
- 539.Picuno P., Sica C., Laviano R., et al. Experimental tests and technical characteristics of regenerated films from agricultural plastics. Polym. Degrad. Stabil. 2012;97:1654–1661. [Google Scholar]
- 540.Civancik-Uslu D., Nhu T.T., Van Gorp B., et al. Moving from linear to circular household plastic packaging in Belgium: Prospective life cycle assessment of mechanical and thermochemical recycling. Resour. Conserv. Recycl. 2021;171 [Google Scholar]
- 541.Süß M., Fischer J. Microplastic occurrence during the pre-treatment of polypropylene in a simulated washing process for mechanical recycling. Monatsh. Chem. 2023 doi: 10.1007/s00706-023-03135-7. [DOI] [Google Scholar]
- 542.Soriano A., Schaefer C., Urtiaga A. Enhanced treatment of perfluoroalkyl acids in groundwater by membrane separation and electrochemical oxidation. Chemical Engineering Journal Advances. 2020;4 [Google Scholar]
- 543.Curran W.S., Loux M.M., Liebl R.A., et al. Photolysis of imidazolinone herbicides in aqueous solution and on soil. Weed Sci. 1992;40:143–148. [Google Scholar]
- 544.Carena L., Fabbri D., Passananti M., et al. The role of direct photolysis in the photodegradation of the herbicide bentazone in natural surface waters. Chemosphere. 2020;246 doi: 10.1016/j.chemosphere.2019.125705. [DOI] [PubMed] [Google Scholar]
- 545.Ribeiro J.P., Nunes M.I. Recent trends and developments in Fenton processes for industrial wastewater treatment–A critical review. Environ. Res. 2021;197 doi: 10.1016/j.envres.2021.110957. [DOI] [PubMed] [Google Scholar]
- 546.Usman M., Jellali S., Anastopoulos I., et al. Fenton oxidation for soil remediation: A critical review of observations in historically contaminated soils. J. Hazard Mater. 2022;424 doi: 10.1016/j.jhazmat.2021.127670. [DOI] [PubMed] [Google Scholar]
- 547.Ahmad K., Ghatak H.R., Ahuja S. A review on photocatalytic remediation of environmental pollutants and H2 production through water splitting: a sustainable approach. Environ. Technol. Innov. 2020;19 [Google Scholar]
- 548.McCullagh C., Skillen N., Adams M., et al. Photocatalytic reactors for environmental remediation: a review. J. Chem. Technol. Biotechnol. 2011;86:1002–1017. [Google Scholar]
- 549.Mohammed A.M., Aziz F., Mohtar S.S., et al. A review of research trends on the usage of photocatalysis for wastewater treatment: bibliometric analysis. Sust. Wat. Resour. Man. 2023;9:88. doi: 10.1007/s40899-023-00868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Felter S.P., Zhang X., Thompson C. Butylated hydroxyanisole: Carcinogenic food additive to be avoided or harmless antioxidant important to protect food supply? Regul. Toxicol. Pharmacol. 2021;121 doi: 10.1016/j.yrtph.2021.104887. [DOI] [PubMed] [Google Scholar]
- 551.Cameselle C. Encyclopedia of Applied Electrochemistry. Springer; 2014. Electrokinetic transport in soil remediation; pp. 725–731. [Google Scholar]
- 552.Suanon F., Tang L., Sheng H., et al. Organochlorine pesticides contaminated soil decontamination using TritonX-100-enhanced advanced oxidation under electrokinetic remediation. J. Hazard Mater. 2020;393 doi: 10.1016/j.jhazmat.2020.122388. [DOI] [PubMed] [Google Scholar]
- 553.Rosas J., Vicente F., Santos A., et al. Soil remediation using soil washing followed by Fenton oxidation. Chem. Eng. J. 2013;220:125–132. [Google Scholar]
- 554.Xiang L., Harindintwali J.D., Wang F., et al. Integrating biochar, bacteria, and plants for sustainable remediation of soils contaminated with organic pollutants. Environ. Sci. Technol. 2022;56:16546–16566. doi: 10.1021/acs.est.2c02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Huang Y., Wen L., Zhang L., et al. Community-integrated multi-omics facilitates the isolation of an organohalide dehalogenation microorganism. Innovation. 2023;4 doi: 10.1016/j.xinn.2022.100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Line M., Garland C., Crowley M. Evaluation of landfarm remediation of hydrocarbon-contaminated soil at the inveresk railyard, Launceston, Australia. Waste Manag. 1996;16:567–570. [Google Scholar]
- 557.Watanabe M.E. Phytoremediation on the brink of commericialization. Environ. Sci. Technol. 1997;31:182A–186A. doi: 10.1021/es972219s. [DOI] [PubMed] [Google Scholar]
- 558.Borchert E., Hammerschmidt K., Hentschel U., et al. Enhancing microbial pollutant degradation by integrating eco-evolutionary principles with environmental biotechnology. Trends Microbiol. 2021;29:908–918. doi: 10.1016/j.tim.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 559.Sun F., Mellage A., Gharasoo M., et al. Mass-transfer-limited biodegradation at low concentrations—evidence from reactive transport modeling of isotope profiles in a bench-scale aquifer. Environ. Sci. Technol. 2021;55:7386–7397. doi: 10.1021/acs.est.0c08566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Kundu K., Marozava S., Ehrl B., et al. Defining lower limits of biodegradation: atrazine degradation regulated by mass transfer and maintenance demand in Arthrobacter aurescens TC1. ISME J. 2019;13:2236–2251. doi: 10.1038/s41396-019-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Radwan S.S., Al-Mailem D.M., Kansour M.K. Bioaugmentation failed to enhance oil bioremediation in three soil samples from three different continents. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-56099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Harindintwali J.D., Dou Q., Wen X., et al. Physiological and transcriptomic changes drive robust responses in Paenarthrobacter sp. AT5 to co-exposure of sulfamethoxazole and atrazine. J. Hazard Mater. 2024;462 doi: 10.1016/j.jhazmat.2023.132795. [DOI] [PubMed] [Google Scholar]
- 563.Harindintwali J.D., Zhou J., Yang W., et al. Biochar-bacteria-plant partnerships: Eco-solutions for tackling heavy metal pollution. Ecotoxicol. Environ. Saf. 2020;204 doi: 10.1016/j.ecoenv.2020.111020. [DOI] [PubMed] [Google Scholar]
- 564.Kour D., Kaur T., Devi R., et al. Beneficial microbiomes for bioremediation of diverse contaminated environments for environmental sustainability: present status and future challenges. Environ. Sci. Pollut. Res. 2021;28:24917–24939. doi: 10.1007/s11356-021-13252-7. [DOI] [PubMed] [Google Scholar]
- 565.Kümmerer K., Clark J. Green and sustainable chemistry. Sustain. Sci.: An Introduction. 2016:43–59. [Google Scholar]
- 566.Constable D.J.C. Green and sustainable chemistry–The case for a systems-based, interdisciplinary approach. iScience. 2021;24 doi: 10.1016/j.isci.2021.103489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Wang Y., Yu G. Ecosystem quality-based management and the development of a new eco-friendly economy. Innovation. 2023;4 doi: 10.1016/j.xinn.2023.100491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Anonymous. (2019). Global Chemicals Outlook II - from Legacies to Innovative Solutions: Implementing the 2030 Agenda for Sustainable Development – Synthesis Report (UN Environment Programme 2019)
- 569.Sigmund G., Ågerstrand M., Antonelli A., et al. Addressing chemical pollution in biodiversity research. Global Change Biol. 2023;29:3240–3255. doi: 10.1111/gcb.16689. [DOI] [PubMed] [Google Scholar]
- 570.Landrigan P.J., Fuller R., Acosta N.J., et al. The Lancet Commission on pollution and health. The lancet. 2018;391:462–512. doi: 10.1016/S0140-6736(17)32345-0. [DOI] [PubMed] [Google Scholar]
- 571.Oginah S.A., Posthuma L., Maltby L., et al. Linking freshwater ecotoxicity to damage on ecosystem services in life cycle assessment. Environ. Int. 2023;171 doi: 10.1016/j.envint.2022.107705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Backhaus T., Snape J., Lazorchak J. The impact of chemical pollution on biodiversity and ecosystem services: the need for an improved understanding. Integrated Environ. Assess. Manag. 2012;8:575–576. doi: 10.1002/ieam.1353. [DOI] [PubMed] [Google Scholar]
- 573.Beaumelle L., Thouvenot L., Hines J., et al. Soil fauna diversity and chemical stressors: A review of knowledge gaps and roadmap for future research. Ecography. 2021;44:845–859. [Google Scholar]
- 574.Tang X., Wilson S.R., Solomon K.R., et al. Changes in air quality and tropospheric composition due to depletion of stratospheric ozone and interactions with climate. Photochem. Photobiol. Sci. 2011;10:280–291. doi: 10.1039/c0pp90039g. [DOI] [PubMed] [Google Scholar]
- 575.Isaksen I.S.A., Granier C., Myhre G., et al. Atmospheric composition change: Climate-chemistry interactions. Atmos. Environ. X. 2009;43:5138–5192. [Google Scholar]
- 576.Wang Z., Altenburger R., Backhaus T., et al. We need a global science-policy body on chemicals and waste. Science. 2021;371:774–776. doi: 10.1126/science.abe9090. [DOI] [PubMed] [Google Scholar]
- 577.Ågerstrand M., Arinaitwe K., Backhaus T., et al. Key principles for the intergovernmental science–policy panel on chemicals and waste. Environ. Sci. Technol. 2023;57:2205–2208. doi: 10.1021/acs.est.2c08283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Schäffer A., Groh K.J., Sigmund G., et al. Conflicts of interest in the assessment of chemicals, waste, and pollution. Environ. Sci. Technol. 2023;57:19066–19077. doi: 10.1021/acs.est.3c04213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Budtz-Jorgensen E., Grandjean P. Application of benchmark analysis for mixed contaminant exposures: Mutual adjustment of perfluoroalkylate substances associated with immunotoxicity. PLoS One. 2018;13 doi: 10.1371/journal.pone.0205388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Fishman M.J., Erdmann D.E. Water Analysis. Anal. Chem. 1973;45:361–403. doi: 10.1021/ac00256a001. [DOI] [PubMed] [Google Scholar]
- 581.MacCarthy P., Klusman R.W., Cowling S.W., et al. Water analysis. Anal. Chem. 1993;65:244–292. doi: 10.1021/ac00012a014. [DOI] [PubMed] [Google Scholar]
- 582.Clement R.E., Koester C.J., Eiceman G.A. Environmental analysis. Anal. Chem. 1993;65:85–116. doi: 10.1021/ac00108a012. [DOI] [PubMed] [Google Scholar]
- 583.Koester C.J., Simonich S.L., Esser B.K. Environmental Analysis. Anal. Chem. 2003;75:2813–2829. [Google Scholar]
- 584.Ivleva N.P. Chemical analysis of microplastics and nanoplastics: Challenges, advanced methods, and perspectives. Chem. Rev. 2021;121:11886–11936. doi: 10.1021/acs.chemrev.1c00178. [DOI] [PubMed] [Google Scholar]
- 585.Hoelzer K., Sumner A.J., Karatum O., et al. Indications of transformation products from hydraulic fracturing additives in shale-gas wastewater. Environ. Sci. Technol. 2016;50:8036–8048. doi: 10.1021/acs.est.6b00430. [DOI] [PubMed] [Google Scholar]
- 586.Kucharzyk K.H., Crawford R.L., Cosens B., et al. Development of drinking water standards for perchlorate in the United States. J. Environ. Manag. 2009;91:303–310. doi: 10.1016/j.jenvman.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 587.Braun J.M. Enhancing regulations to reduce exposure to PFAS - Federal action on "Forever Chemicals. N. Engl. J. Med. 2023;1924–1926 doi: 10.1056/NEJMp2303333. [DOI] [PubMed] [Google Scholar]
- 588.Maryam S., Ul Haq I., Yahya G., et al. COVID-19 surveillance in wastewater: An epidemiological tool for the monitoring of SARS-CoV-2. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.978643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.von Gunten U. Oxidation processes in water treatment: are we on track? Environ. Sci. Technol. 2018;52:5062–5075. doi: 10.1021/acs.est.8b00586. [DOI] [PubMed] [Google Scholar]
- 590.Tian Z., Gonzalez M., Rideout C.A., et al. 6PPD-quinone: Revised toxicity assessment and quantification with a commercial standard. Environ. Sci. Technol. Lett. 2022;9:140–146. [Google Scholar]
- 591.Grisham J. Cutting back MTBE. Chem. Eng. News Archive. 1999;77:5. [Google Scholar]
- 592.Sedlak D. Fool Me Once. Environ. Sci. Technol. 2016;50:7937–7938. doi: 10.1021/acs.est.6b03367. [DOI] [PubMed] [Google Scholar]
- 593.LaLone C.A., Blatz D.J., Jensen M.A., et al. From protein sequence to structure: The next frontier in cross-species extrapolation for chemical safety evaluations. Environ. Toxicol. Chem. 2023;42:463–474. doi: 10.1002/etc.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Margiotta-Casaluci L., Owen S.F., Winter M.J. Cross-species extrapolation of biological data to guide the environmental safety assessment of pharmaceuticals—The state of the art and future priorities. Environ. Toxicol. Chem. 2024;43:513–525. doi: 10.1002/etc.5634. [DOI] [PubMed] [Google Scholar]
- 595.Brooks B.W., van den Berg S., Dreier D.A., et al. Towards precision ecotoxicology: Leveraging evolutionary conservation of pharmaceutical and personal care product targets to understand adverse outcomes across species and life stages. Environ. Toxicol. Chem. 2024;43:526–536. doi: 10.1002/etc.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Zimmerman J.B., Anastas P.T. Toward substitution with no regrets. Science. 2015;347:1198–1199. doi: 10.1126/science.aaa0812. [DOI] [PubMed] [Google Scholar]
- 597.Coish P., Brooks B.W., Gallagher E.P., et al. Current status and future challenges in molecular design for reduced hazard. ACS Sustainable Chem. Eng. 2016;4:5900–5906. [Google Scholar]
- 598.Persson M., Sabelström E., Gunnarsson B. Handling of unused prescription drugs — knowledge, behaviour and attitude among Swedish people. Environ. Int. 2009;35:771–774. doi: 10.1016/j.envint.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 599.Lim-Wavde K., Kauffman R.J., Dawson G.S. Household informedness and policy analytics for the collection and recycling of household hazardous waste in California. Resour. Conserv. Recycl. 2017;120:88–107. [Google Scholar]
- 600.Chung S.S., Brooks B.W. Identifying household pharmaceutical waste characteristics and population behaviors in one of the most densely populated global cities. Resour. Conserv. Recycl. 2019;140:267–277. [Google Scholar]
- 601.Lago N.C., Auler M.S., Fleith de Medeiros J., et al. Promoting unused medicine pro-environmental disposal: Characterization of consumer behavior and strategic propositions. Environ. Dev. 2022;44:100770. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.