Abstract
Carbon dots (CDs) with different structures were prepared by electrolysis (PE-CDs) and hydrothermal (PH-CDs) methods using proanthocyanidins as precursors. The smaller size and lower zeta potential enabled the PE-CDs treated rice seedlings to exhibit greater resistance to salt stress. The fresh weight of rice seedlings under salt stress was significantly increased by spraying CDs every other day for two weeks. PE-CDs treated group exhibited a faster electron transport rate, and the SOD activity and flavonoid content were 2.5-fold and 0.23-fold higher than those of the salt stress-treated group. Furthermore, the metabolomics and transcriptomics analysis revealed that the PsaC gene of photosystem I was significantly up-regulated under PE-CDs treatment, which accelerated electron transfer in photosystem I. The up-regulation of BX1 and IGL genes encoding indole synthesis allowed rice to enhance stress tolerance through tryptophan and benzoxazine biosynthesis pathways. These findings offer help in purposefully synthesizing CDs and boosting food production.
Keywords: Carbon dots, Proanthocyanidins, Rice seedlings, Salt stress, Metabolome, Transcriptome
Highlights
-
•
PE-CDs showed antioxidant properties, small size and high negative zeta potential.
-
•
PE-CDs could improve salt resistance of rice seedlings.
-
•
PE-CDs enhanced plant photosynthesis by up-regulating the PsaC gene of PS I.
-
•
PE-CDs enhanced antioxidant system by upregulating BX1 and IGL genes.
1. Introduction
Abiotic stresses result in significant crop losses globally each year. Nano-engineered materials can mitigate abiotic stresses and improve yield (Do Espirito Santo Pereira, Caixeta Oliveira, Fernandes Fraceto, & Santaella, 2021). However, the poor water solubility and biocompatibility, and the complexity and costly preparation process have limited their application (Chaudhary, Singh, Singh, & Rathi, 2024).
CDs, as a new type of nanomaterials, are characterized by simple preparation methods, wide sources of precursors, excellent water solubility and biocompatibility, and adjustable surface functional groups. Recent studies demonstrate its excellent ability in promoting plant growth and mitigating abiotic stress on crops (Guo et al., 2022). N-CDs alleviated the photosynthesis defect in mterf5 mutants with defective PSII function by targeting Cyt-b6f and PQs, which are located between photosystems PSII and PSI, resulting in an enhancement of photosynthetic efficiency by 24.29%, and consequently, an increase in apple soluble sugar content by 11.43% (Jing et al., 2024).
Oxidative damage due to accumulation of reactive oxygen species (ROS) in cells significantly impacts plant growth. Carboxy and amino groups on the surface of CDs have been reported to have free radical scavenging properties due to their ability to act as electron donors and acceptors (Das et al., 2014). CDs protect plants from abiotic stresses by scavenging free radicals and increasing antioxidant enzyme activity. Salvia Miltiorrhiza-derived CDs were proved to have stronger antioxidant properties than the precursors, scavenging DPPH•, O2•−, and •OH with high efficiencies of 88.9%, 95.6%, and 71.4%, respectively. This CDs was effective in mitigating oxidative damage in Italian lettuce under salt stress, with a 92.2% decrease in MDA content compared to the control (Li et al., 2021). Nitrogen and sulfur co-doped CDs were found to have 80% scavenging rate of •OH at 0.55 mg/mL. The antioxidant effect on KMnO4 was comparable to that of VC at the same concentration. The CDs could also enhance the antioxidant defense enzyme activities of tomato seedlings to enhance the drought resistance (Kou et al., 2021). Thus, having antioxidant properties is essential for CDs to mitigate abiotic stresses. In addition, the size and zeta potential of CDs are key factors because crops have size limitations on the particles that can be accessed (Wang et al., 2022). The hydrodynamic size limits for efficient nanoparticle transport in cotton and maize leaf cells have been reported to be 20 nm and 11 nm, respectively, and foliar delivery of positively charged nanoparticle in chloroplasts was more efficient (Hu et al., 2020). However, there are limited reports on the kinetics of CDs uptake by plants, the relationship between the structure of CDs and their mitigation of abiotic stresses in crops is not well understood at present. The molecular mechanisms of how CDs mitigate abiotic stresses in crops have been reported. Application of CDs in soil resulted in up-regulation of GmNRT, GmAMT, GmLB, and GmAQP gene expression in drought-stressed soybean roots, enhancing N transport and water uptake, which in turn promoted soybean growth and nutritional quality (Ji et al., 2023). PNDs enhanced drought tolerance in maize by regulating photosynthesis-related genes and synthesis genes of signaling molecules (Wang et al., 2022). However, there is a lack of in-depth studies on the role of CDs in alleviating salt stresses in rice seedlings at molecular level.
In this study, CDs were prepared by hydrothermal and electrolytic methods, respectively, using proanthocyanidins (PCs) (natural substances with strong antioxidant properties) as precursors, and the resulting CDs inherited the antioxidant properties of the precursors and possessed the ability to alleviate salt stress. In addition, the size and zeta potential of CDs were different due to the different preparation methods. The enhanced effects of two CDs on salt stress tolerance in rice were compared, and the stress mitigation abilities of CDs structures on antioxidant defense system, photosynthesis, osmoregulation, and nutrient uptake in rice seedlings were explored. The underlying mechanism responsible for the enhanced salt stress resistance was elucidated by metabolomics and transcriptomics analysis. This will provide a theoretical foundation for the structural design of functional CDs to improve crop resistance, yield and alleviate food shortage. Furthermore, novel target genes for CDs to alleviate salt stress in rice seedlings were also identified, revealing a new mechanism for CDs to alleviate plant stress.
2. Material and methods
2.1. Synthesis and characterization of CDs
PCs with a purity of 95% derived from pine bark extract were supplied by Guangdong Tianzi Natural Inc. Anhydrous ethanol was purchased from Aladdin Industries, Inc. PCs were used as precursors to prepare CDs by hydrothermal and electrolytic methods, respectively. Namely, 0.6 g PCs was mixed with 15 mL anhydrous ethanol and 45 mL water and sonicated for 30 min, then transferred into a Teflon-lined reaction kettle and placed in an oven at 200 °C/10 h, followed by filtration through filter paper and then 0.22 μm filter membrane, dialyzed with a dialysis bag (MW = 1000 Da, Solarbio, China) for 8 h, and lyophilized to obtain solid CDs. The specific step of preparing CDs by electrolysis involved weighing 1 g PCs and adding them to a beaker containing 300 mL of water. Two parallel graphite rods were used as electrodes, and a DC voltage of 60 V was applied at room temperature while magnetic stirring was conducted until one electrode was completely electrolyzed. The resulting solution was filtered, dialyzed in a dialysis bag (MW = 1000 Da, Solarbio, China) for 8 h, and then freeze-dried.
The morphology of the CDs was observed using an FEI Tecnai 12 transmission electron microscope (TEM). Functional groups on the surface of CDs were detected using a Nicolet Avatar 360 FTIR spectrophotometer. Structural characterization of the CDs was demonstrated using an Xpert Pro MPD X-ray diffractometer (XRD). A Renishaw Evolution device with a 532 nm laser as the excitation source was used to determine the Raman spectra of the CDs. A laser particle size analyzer (Zetasizer Nano ZSE) was used to determine the zeta potential. XPS analysis was also carried out using a Thermo SCIENTIFIC Nexsa spectrometer, with the source of ionizing radiation being AlKα radiation (1486.68 eV). A pass energy of 200 eV was used for the measurement spectra and 50 eV for the high-resolution spectra. The 1,1-Diphenyl-2-picrylhydrazyl (DPPH) scavenging activity, superoxide anion radical (O2•-) scavenging activity, and potassium permanganate (KMnO4) reduction rate were used to assess the antioxidant qualities of CDs. After combining 100 μL of CDs solution with an equal amount of 1 mM KMnO4 or 100 μg/mL DPPH, the mixture's absorbance at 515 or 517 nm was measured after half an hour in the absence of light. The control solution was the one without CDs. The following formula was used to compute the KMnO4 reduction rate or DPPH scavenging activity: (A1-A2) /A1 × 100% (1), where A1 and A2 stand for the absorbance values at 515 nm or 517 nm for the mixture of the control group and CDs treatment group with varied concentrations, respectively. Nitrotetrazolium blue chloride (NBT) was used to measure the O2•- scavenging activity of CDs. First, a 0.05 M phosphate buffer (pH = 7.8) was used to prepare all of the reagents. Next, to create mother liquor (ready for use), 30 mL of buffer solution, 6 mL of 130 mM methionine solution, 6 mL of 750 μM NBT, 6 mL of 100 μM EDTA-Na2, 6 mL of 20 μM riboflavin, and 5 mL of distilled water were combined. Mother liquor was combined with CDs solution at varying concentrations in an equal volume; the CDs-free mixture served as the control. After 30 min of treatment at 4000 Lux, the mixture's absorbance value was measured at 560 nm. Eq. (1) was utilized to calculate the O2•- scavenging activity.
2.2. Cultivation of rice and salt stress treatment
Rice seeds (‘Huahang 31’) were disinfected with 75% ethanol and later germinated under darkness by laying flat in germination trays containing Hoagland's nutrient solution. Three days later, the germinated trays were transferred to the greenhouse for incubation (22–30 °C, 70% relative humidity, 400 μmol m−2·s−1 light intensity with a 16 h light/8 h dark regime). Seven days later, homogeneous rice seedlings were transplanted into pots containing nutrient solution with 12 seedlings per pot. On the second day after transplanting, the nutrient solution was replaced with a nutrient solution containing 50 mmol NaCl and CDs solution was sprayed to ensure that all leaves were covered with droplets and there was no runoff. Each pot of rice seedlings was sprayed with 5 mL of CDs solution each time. Each treatment was replicated six times and ultra-pure water was sprayed as a control. The foliar spray was conducted every two days for two weeks. Based on the pre-experiment (Detailed data was shown in SI Fig. S1), the optimal concentration of CDs under salt stress was used as the concentration of CDs for each group of foliar spray. That is, PH-CDs were 10 μg/mL, PE-CDs were 30 μg/mL. The group without salt stress treatment was recorded as Non—S, the salt stress treatment group sprayed water was recorded as S, and the salt stress treatment group sprayed optimal concentration of PH-CDs and PE-CDs was recorded as PH-CDs and PE-CDs. The top two leaves of rice seedlings from each treatment were collected and their height, fresh weight, and chlorophyll content were measured.
Photosynthetic parameters of rice seedlings were tested by chlorophyll fluorometry (Phyto = PAM ED, Walz, Germany). Prior to the test, all rice seedlings were kept in darkness for the whole night in order to achieve equilibrium in their photosynthetic processes. The test leaf, which was the second leaf from the top of the rice seedlings, was cut, covered with damp gauze, and put on a platform. The effective radiation and light intensities that were measured were 0, 1, 21, 36, 56, 81, 111, 146, 231, 336, 461, 611, and 801 μmol∙m−2∙s−1. Ten test sites were chosen for every rice leaf, and six leaves were evaluated for each treatment.
2.3. Regulation of antioxidant defense system in salt-stressed rice seedlings by CDs
Determination of antioxidant defense system indicators in rice seedlings according to the method described in the literature (Li et al., 2020). Using the nitrogen blue tetrazolium (NBT) photoreduction method, the superoxide dismutase (SOD) activity was measured and expressed as the change in absorbance value at 560 nm. The guaiacol method was used to measure the peroxidase (POD) activity. A UV–Vis spectrophotometer was used to track the rate of increase in absorbance at 470 nm during a 3-min period. Using the test group without CDs as control. The rate of hydrogen peroxide breakdown was measured using the absorbance at 240 nm after two minutes to identify the presence of catalase (CAT) activity. The forinthenol method was used to determine the total plant phenol content, whereas the aluminum chloride colorimetric method was used to assess the flavonoid content. The standard curve of polyphenol content was plotted with the absorbance change under the gradient gallic acid concentration (y = 8.4366× + 0.0767, R2 = 0.9966), and the standard curve of flavonoid content was plotted with the absorbance change under the gradient rutin concentration (y = 0.352× + 0.0395, R2 = 0.9997). Using the thiobarbituric acid method, the amount of Malondialdehyde (MDA) in rice was measured in order to determine the degree of peroxidation.
2.4. Regulation of osmoregulatory substances by CDs in salt-stressed rice seedlings
The colorimetric method was used to determine the content of betaine, free proline and soluble protein by using Rays salt, ninhydrin and Thomas Brilliant Blue, respectively. The content of soluble sugar was determined by the method using anthranilone sulfate. The standard curve was plotted according to the absorbance values of the standard solutions of proline (y = 0.0279× - 0.0091, R2 = 0.9901) and betaine (y = 0.0318× + 0.0442, R2 = 0.9937) at different concentrations, and the concentration of the samples was calculated from the standard curve. For soluble sugar and soluble protein determination, standard curves were plotted with glucose standard solution (y = 0.004× - 0.0203, R2 = 0.991) and bovine serum albumin standard solution (y = 0.0067× + 0.0451, R2 = 0.993), respectively.
2.5. Regulation of mineral nutrition by CDs in salt-stressed rice seedlings
Determination of total nitrogen content of plants was performed by sulfuric acid‑hydrogen peroxide digestion, Kjeldahl method. The molybdenum antimony anti-colorimetric method was used to determine the total phosphorus content of plants. The contents of potassium (K), calcium (Ca), magnesium (Mg), manganese (Mn), iron (Fe), zinc (Zn) and copper (Cu) in rice seedlings were determined by flame atomic absorption spectrophotometry (Z-2300, Hitachi, Japan). Every eight samples, a quality control sample was done to assess stability.
2.6. Metabolomic profiling
The harvested rice seedling leaves were ground into powder under the protection of liquid nitrogen, and then vacuum freeze-dried for 24 h. 0.2000 g of the sample powder was weighed and dissolved in 1.2 mL of 70% ice methanol (4 °C) solution, and then extracted by ultrasonic extraction for 30 min, centrifuged at 12000 rpm for 10 min, and filtered through a 0.22 μm filter to obtain the supernatant. After that, the supernatant was examined using an Accela UHPLC system that was fitted with a heated electrospray ionization source and an LTQ-Orbitrap Velos mass spectrometer (Thermo Scientific, Bremen, Germany). Both positive and negative ion source modes were used for data gathering. Utilizing a Shim-pack Scepter column (2.1 × 150 mm, 1.9 μm Shimadzu, Japan), 45 °C was the column temperature and 0.3 mL/min was the flow rate. A (0.1% formic acid in water) and B (0.1% formic acid in acetonitrile) were present in the mobile phase of positive ion modes. A (5 mM ammonium acetate in water) and B (5 mM ammonium acetate in acetonitrile) were included in the negative ion mode mobile phase. The gradient elution conditions were 0-1 min, 2% B; 1–7 min, 2–40% B; 7–11 min, 40 to 95% B; 11–12.5 min, 95% B; 12.5–13.5 min, 95–2% B; 13–15 min, 2% B. The arbitrary unit of sheath gas was maintained at 60. Thirty arbitrary units of aux gas were maintained. For positive ionization mode, the spray voltage was 4 KV, while for negative ionization mode, it was −3.50 KV. The capillary temperature was maintained at 380 °C, while the probe heater temperature was maintained at 350 °C. The range of mass was 70–1050 m/z. Every ten samples, a quality control sample is done to assess stability. Following data transformation and analysis using the open-source program MS-DIAL (version 4.92, Japan), the data were filtered, standardized, and then subjected to multivariate analysis using MetaboAnalyst 5.0 for orthogonal partial least-squares discriminant analysis (OPLS-DA). The rationale behind the split was attributed to the variable significance in projection (VIP) value exceeding 1.5.
2.7. Transcriptomic profiling
The rice seedlings were removed after 2 weeks treatment and immediately placed in liquid nitrogen, preserved in dry ice and shipped to Shanghai Parsonage for reference genome transcriptome sequencing on an Illumina sequencer. The raw data were obtained and the samples were sequenced online. The 3′ endband splice sequences were eliminated using Fastp (0.22.0), and the reads that had an average quality score below Q20 were eliminated to create the clean data. High quality analyses were then performed using the clean data. NCBI provided the reference genome database. Fragments Per Kilo bases per Million fragments (FPKM) was used to standardize the expression. With DESeq (vl.38.3), a differential expression analysis between comparison groups was carried out. The regulation of the differentially expressed genes was as follows: significance P-value<0.05, expression difference fold |log2FoldChange (FC)| > 1. The GO and KEGG pathway enrichment analyses were carried out with topGO (v2.50.0) and clusterProfiler (v4.6.0) software, respectively, with a focus on GO terms and KEGG pathways with P-value<0.05.
2.8. Statistical analysis
All experiments were conducted three duplicates for each treatment. And the results were reported as mean ± standard deviation. The data were analyzed by ANOVA and LSD (P < 0.05) using SPSS statistical software.
3. Results and discussion
3.1. Characterization of CDs
PH-CDs was composed of PCs molecules polymerized to form macromolecules, and then carbonized to form CDs (Fig. 1A). The formation of PE-CDs may be the grafting of the decomposed PCs molecules in the electrolyte on the surface of the carbon core exfoliated from the carbon rod (Fig. 1B). Fig. 1C and D showed the TEM of CDs. PH-CDs and PE-CDs appeared as spherical nanoparticles with lattice spacing of 0.21 ± 0.025 nm and 0.34 ± 0.026 nm, and average particle sizes of 9.78 ± 2.45 nm and 4.87 ± 0.65 nm, respectively. The size of PE-CDs was smaller, which was in agreement with other reports that CDs prepared by electrolysis have dimensions of 2 to 6 nm (Zhang et al., 2019). CDs have capability to penetrate plant tissues through roots or aerial organs and tissues (e.g. cuticle, stomata, stomata). The uptake and transport must cross a series of chemical and physiological barriers that govern the size exclusion limit (Wang, Lombi, Zhao, & Kopittke, 2016). We suspect that the smaller size and enhanced affinity facilitates the movement of CDs into and out of plant tissues for their function.
Fig. 1.
Schematic of the synthesis of (A) PH-CDs and (B) PE-CDs. TEM images of PH-CDs (C) and PE-CDs (D). (E) FTIR of CDs compared with PCs. XPS (F), Raman spectrum (G) and Zeta potential (H) of CDs.
Subsequently, the surface functional groups of CDs were characterized by FTIR. As shown in Fig. 1E, compared with PCs, the PH-CDs exhibited fewer FTIR peaks, showing a -OH stretching vibration peak at 3450 cm−1, a -C=O peak at 1650 cm−1, and a faint -C-C peak at 1050 nm−1. The FTIR spectra of the two CDs were similar. XPS spectra showed that PH-CDs and PE-CDs were composed of C and O (Fig. 1F), the high-resolution C 1 s spectra (Fig. S2) confirmed the existence of C O, C = C/C-C, C—O, and the high-resolution O 1 s spectra (Fig. S2) confirmed the existence of C—O and C O (Yao et al., 2022). In the high-resolution O 1 s spectrum, the area corresponding to C O unit moiety in PH-CDs was larger than that in PE-CDs. However, the area associated with C—O unit moiety was lower in PH-CDs than in PE-CDs, indicating that the electrolysis method introduces more oxygen atoms. PE-CDs also contained more oxygen (34%) than PH-CDs (31%). In the Raman spectrum (Fig. 1G), the D band located at 1351 cm−1 was attributed to the functional groups on the surface of the CDs, indicating sp3 hybridized disordered carbon. The G band located at 1580 cm−1 represented the sp2 carbon structure, which was related to the graphite morphology. The ID/IG indicated the contribution of disordered surface states to the structure. The ID/IG was 0.35 for PH-CDs and 0.83 for PE-CDs, indicating more functional group modifications on the surface of PE-CDs with more oxygen-containing functional groups. The findings from Raman spectrum were consistent with those obtained from XPS.
The XRD patterns of the CDs were shown in Fig. S3, revealing two diffraction peaks at 24.5° and 42°, corresponding to the (002) and (100) crystal planes of graphite, respectively (Li et al., 2021). The zeta potential of the CDs was shown in Fig. 1H. Compared to PH-CDs, the zeta potential of PE-CDs was notably lower at −35.60 mV. The surface of PE-CDs prepared by electrolysis was rich in carboxyl and hydroxyl groups, as well as grafted with PCs-breaking groups. These hydrophilic groups contributed to the formation of high negative zeta potential. It has been reported that positively or negatively charged nanoparticles with zeta potentials higher than 20 or 30 mV were more likely to be taken up by plant cell or chloroplast membranes, whereas more neutral nanomaterials were unable to penetrate plant lipid bilayers (Hu et al., 2020). The surface of plant leaves is formed by a waxy layer of cuticles containing nano-scale (∼2 nm) hydrophilic pores and micron-scale stomata(Lv, Christie, & Zhang, 2019). Nanomaterials are mainly delivered to plants through the cuticle and stomata. Furthermore, inside the leaf, the cell wall is a biological barrier with hydrophobicity and hydrophilicity, with a reported pore size of <13 nm (Albersheim, Darvill, Roberts, Sederoff, & Staehelin, 2010) and a non-uniformly distributed negative charge (Fritz, 2007). A higher absolute value of the Zeta potential enhances the penetration of CDs into the chloroplasts through the plant cells (Wang et al., 2022).
3.2. The antioxidant properties of CDs
The antioxidant properties of the CDs were characterized by the KMnO4 degradation rate, DPPH scavenging activity and O2•- scavenging activity, as shown in Fig. S4. PH-CDs showed the highest antioxidant activity, followed by PE-CDs. At a concentration of 50 μg/mL, the KMnO4 degradation rate and O2•- scavenging activity of PH-CDs were 2.34 times and 4.94 times greater than that of ascorbic acid (VC), respectively. The exact structural type of the CDs and the type of free radical are significantly influence their antioxidant properties. Three general methods exist for scavenging free radicals: electron transfer, hydrogen transfer, and production of free radical adducts at the carbon site of SP2 (Gázquez, Cedillo, & Vela, 2007). When it happens, radical scavenging can be the result of one or more of the aforementioned actions working together. The abundant hydroxyl, carboxyl, and amino groups on the CDs surface promoted the occurrence of hydrogen transfer pathways. Unpaired electrons on the surface of CDs can be delocalized either by resonance within the aromatic domain or by chemical bond rearrangement. The oxidation level of PE-CDs was higher than PH-CDs, resulting in more C-OH and C O groups than C-O-C groups. Therefore, PH-CDs exhibited the stronger antioxidant properties. PH-CDs and PE-CDs inherited the abundant phenolic hydroxyl groups of the precursor and thus possessed antioxidant capacity. DPPH was a stable N-centered radical with its nitrogen having two lone pair electrons surrounded by three benzene rings, it readily accepted the hydrogen radical and forms a stable complex (Ionita, 2021). The effect of the CDs on DPPH radical scavenging was not as good as that of VC, indicating that the CDs had a poor hydrogen supply capacity. This finding is consistent with previous studies by other researchers (Ruiz, Yate, García, Cabanero, & Grande, 2017).
3.3. CDs promoted the growth and development of salt-stressed rice seedlings
As shown in Fig. 2A-D, CDs treatments significantly enhanced the salt stress resistance of rice seedlings, leading to an increase in the height of salt-stressed seedlings. The fresh weight of PE-CDs treatments was significantly higher by 28.87% than that of Non—S, while the chlorophyll content was not significantly different from that of Non—S. The PH-CDs treatments also enhanced the fresh weight and chlorophyll content of rice seedlings, but were slightly inferior to that of PE-CDs. PH-CDs showed the strongest antioxidant activity, but PE-CDs showed the best effect in alleviating salt stress in rice seedlings. The possible reasons are that PE-CDs were smaller and more electronegative, facilitating their entry into plant cells through the pores on the leaf surface when sprayed. As shown in the Fig. 2E-G, with the increase of photosynthetic effective radiation (PAR) in rice leaves, the CDs treatment group exhibited higher relative electron transfer rate (ETR), actual photosynthetic efficiency (Y(II)) and photochemical burst capacity (qP) compared with the S group, where the ETR of the PE-CDs group was higher than that the PH-CDs.
Fig. 2.
Growth status of rice at seedling stage under different CDs treatments. Photos (A), height (B), fresh weight (C), and chlorophyll content (C) of rice seedlings under different treatments. Photosynthesis parameters include (E) ETR, (F) Y(II), and (G) qP. The group without salt stress treatment was recorded as Non—S, the salt stress treatment group sprayed water was recorded as S, and the salt stress treatment group sprayed optimal concentration of PH-CDs and PE-CDs was recorded as PH-CDs and PE-CDs. The error bars were standard deviations (n ≥ 6). Different letters marked indicate significant differences between treatments (p < 0.05).
3.4. Spraying CDs increased the ability of antioxidant defense system in rice seedlings under salt stress
As shown in Fig. S5. After two weeks of salt stress, the activities of SOD and CAT, and the contents of total polyphenols and flavonoids in rice seedlings (S) were significantly lower than those in Non—S. Upon the treatment with CDs, these parameters showed a significant increase, indicating the potential of CDs to enhance the antioxidant defense system of rice seedlings under salt stress. POD activity was significantly increased after salt stress compared with Non—S, whereas CDs treatment reduced this increase, with PE-CDs treatment showing the greatest reduction. The activities of scavenging enzymes in plants increased under mild and moderate stress. As the duration of stress increased, the enzyme activities decreased to different degrees. The activities of SOD and CAT decreased earlier, and the activity of POD decreased later.
Different results were reported due to different durations of salt stress exposure. For example, after 3 days of salt stress treatment, the SOD activity of rice seedlings was notably higher than that of the untreated group, and the enzyme activity was significantly increased by the co-treatment of exogenous calcium and salt stress (Rahman, Nahar, Hasanuzzaman, & Fujita, 2016). At 3 days of salt stress treatment, the stress response of rice seedlings was still ongoing, and the stress stimulus produced more SOD. Our results showed that rice seedlings had lower SOD activity than the untreated group after 2 weeks of salt stress, probably because by this time the excess ROS in the seedlings had been cleared by the defense system or the salt-stressed seedlings were in a dying state. In addition, different parts of rice exhibit varied responses to salt stress. For example, after germination, the change trend of CAT content in root and shoot was opposite after salt stress treatment for 14 days (Mekawy, Abdelaziz, & Ueda, 2018). That was, compared with the control, CAT activity was significantly increased in root and decreased in shoot after salt stress treatment. The trend of CAT activity in the shoot of rice seedlings was consistent with our results. The trends of total flavonoids and total polyphenols in our results were consistent with other reports (Rahman et al., 2023).
Oxidative stress caused by salt stress leaded to lipid peroxidation in rice seedlings, as indicated by MDA content. As shown in Fig. S5F, the MDA content increased by 2 times after salt stress treatment compared with normal growing rice seedlings. MDA content was significantly decreased by 62.22% and 55.34% after treatment with PE-CDs and PH-CDs, respectively. These results indicated that rice seedlings subjected to CDs treatment suffered only slight oxidative damage. Similar results were reported by others (Basit et al., 2023).
3.5. Effect of CDs on osmotic regulatory substances in salt-stressed rice seedlings
Under high salt, cells synthesize e.g. proline, betaine, soluble sugars, soluble proteins, etc. as osmotic regulators to induce water transport across the membrane in a direction favorable to cell growth. The content of osmotic regulators is low under normal growth conditions and increases under stress. The amount of proline content reflected, to some extent, the degree of plant stress. As shown in Fig. S6A, the proline content significantly increased under salt stress, whereas it decreased by 33.25% and 12.54% after PH-CDs and PE-CDs treatments, respectively, indicating a lower stress degree. The results for betaine content were similar to those for proline. Soluble sugars are the main products of photosynthesis in higher plants and are involved in osmoregulation when plants are subjected to salt stress, and their content will increase (Boriboonkaset et al., 2013). Salt stress stimulated rice to produce more soluble sugars, and the spraying of PH-CDs could alleviate the stress degree, so the soluble sugar content was lower than S, while PE-CDs could stimulate plants to produce more soluble sugars to maintain and balance the osmotic pressure of rice cells when the stress persisted. Previous studies reported that rice varieties with higher salt tolerance showed a greater increase in soluble sugar content after salt stress (Lu et al., 2017). The soluble protein content of rice seedling under salt stress was significantly higher in the CDs-treated group compared to the S group, indicating that CDs treatments promoted rice seedlings to produce more soluble proteins and maintain osmotic pressure. The results were consistent with previous study (Huang et al., 2023).
3.6. Effect of CDs on mineral nutrition of salt-stressed rice seedlings
Excess salt in soil induces osmotic stress in plants by reducing the water potential that limits water uptake, while excess uptake of Na+ and Cl− leads to ionic stress by affecting the uptake and distribution of essential elements, thereby interfering with various metabolic processes.
The results in Fig. S7 indicate that salt stress significantly reduced the content of most mineral elements (Ca, Fe, N, Cu, K, Mg) in rice seedlings. A similar report indicated that salt stress reduced trace element concentrations (Fe2+, K+, Mg2+) in rice roots and shoots (Farooq et al., 2022). The uptake of the above elements was increased by CDs treatment. Differently, Zn content was elevated under salt stress and decreased after CDs treatment, as previously reported (Villora, Moreno, Pulgar, & Romero, 2000). Fe is a cofactor of heme and methemoglobin and plays an important role in electron transfer in respiration, and the content of active Fe was positively correlated with the activities of POD and CAT (Mehrabanjoubani et al., 2019). Interestingly, under salt stress, spraying CDs resulted in a significant increase in Fe uptake by rice seedlings. Fe content was increased by 36.39% and 29.29% in PH- CDs and PE-CDs treated groups, respectively, compared to Non—S. The POD and CAT activities of CDs-treated rice seedlings increased under salt stress, which may be related to the increase in elemental Fe content. According to the results, CDs treatment plays an important role in alleviating the uptake of mineral elements under salt stress.
3.7. Changes of metabolic substances in CDs-treated rice seedlings
The growth-promoting effects of PE-CDs on rice under salt stress have been demonstrated through physiological and biochemical perspectives. To further understand the molecular mechanism, we conducted non-targeted metabolomic analysis. First, supervised multivariate analysis (partial least squares discriminant analysis, PLS-DA) was conducted on metabolomic data from normal-growing (Non—S), salt-stressed (S), and PE-CDs-treated rice seedlings under salt stress (PE-CDs). As shown in Fig. S8, there was a clear separation between three groups, indicating that the metabolite profiles of rice seedlings changed under different treatments. Fig. 3A displayed the key contributing metabolites that contributed to the separation in the PLS-DA analysis. The top 15 ranked metabolites with VIP > 1.5 mainly included flavonoids, nucleic acids, amino acids, organic acids, sugars, fatty acids, and other compounds. The metabolites were screened by P < 0.05 and |log2Fold Change| ≥ 1, as shown in Table S1.
Fig. 3.
Changes in metabolites of rice seedlings in different treatments. (A) VIP scores of responsible metabolites leading to isolation. (B) Metabolic pathway enrichment analysis of metabolites in rice seedlings under different treatments. Bubble size indicated the pathway impacts, and bubble color depth indicated the P value.
Nucleic acid-based metabolites were significantly up-regulated under S treatment compared to Non—S, but this up-regulation was reduced with PE-CDs treatment. It indicated that the degradation of DNA and RNA in rice seedling tissues was accelerated under salt stress, but this effect was mitigated by PE-CDs. The same pattern was also observed for proline and L-phenylalanine, which are metabolites of amino acids. The results were consistent with the previous physiological index. L-Aspartic acid plays a crucial role in protein synthesis and is also involved in nucleotide formation, as well as being an important component of the tricarboxylic acid cycle and glycolysis (Han et al., 2021). L-Aspartic acid was significantly down-regulated under salt stress compared with Non—S. It indicated that many biosynthetic processes in rice seedlings were enhanced under salt stress, and more L-Aspartic acid was involved in the synthesis, resulting in a significant down-regulation, whereas PE-CDs treatment was significantly up-regulated compared with S, which again proved that the PE-CDs treatment mitigated the salt stress degree. Polyphenols and flavonoids play a crucial role in scavenging ROS. After treatment with S, several flavonoid metabolites were significantly down-regulated, indicating that salt stress disrupted the redox balance of rice seedlings and caused oxidative damage, whereas after PE-CDs treatment, these flavonoids were up-regulated, suggesting that PE-CDs played a positive role in maintaining the redox balance and reducing oxidative damage. This finding aligns with the previously determined trends in total polyphenol and total flavonoid contents. Citric acid and malic acid, important substances in the tricarboxylic acid cycle, were significantly down-regulated in salt-stressed seedlings, indicating that salt stress caused damage and affected the physiological and metabolic processes of seedlings. In contrast, there was no difference between PE-CDs and Non—S, indicating that PE-CDs alleviated this injury, which was consistent with the growth indexes (height, fresh weight, etc.) measured in the previous period. The accumulation of D-Raffinose was reported to be positively correlated with plant drought tolerance (Li, Zhang, et al., 2020). D-Raffinose showed significant up-regulation under salt stress, indicating a strong stress response. However, after treatment with PE-CDs, D-Raffinose was significantly down-regulated compared to S, suggesting that PE-CDs alleviated the degree of salt stress. A similar trend was observed in 16-Hydroxyhexadecanoic acid, a key substance in plant keratin synthesis (Bian et al., 2022).
The analysis of enriched metabolic pathways identified six pathways that were screened at P < 0.05 (Fig. 3B), namely: Cutin, suberine and wax biosynthesis, arginine biosynthesis, aminoacyl-tRNA biosynthesis, citrate cycle (TCA cycle), carbon fixation in photosynthetic organisms, and biosynthesis of unsaturated fatty acids. Cutin, suberine, and wax biosynthesis are linked to promoting plant growth (Ge et al., 2022). By integrating the metabolome with pre-physiological and biochemical indices, we discovered that PE-CDs can improve the antioxidant defense system in plants, remove ROS, protect photosynthesis, and alleviate ongoing salt stress.
3.8. Changes of transcriptional gene in CDs-treated rice seedlings
Metabolomics analysis identified molecular change events under salt stress that were alleviated by PE-CDs. These changes partially explain the improved stress tolerance by spraying PE-CDs. However, genes related to photosynthesis and the defense system were still absent. We then conducted transcriptomics analysis on rice seedlings to gain a more comprehensive understanding of how PE-CDs enhance stress tolerance.
The PCA results (Fig. S9A) demonstrated a distinct separation of each treatment group, indicating significant changes in transcriptome genes in both salt-stressed rice seedlings sprayed with PE-CDs and those without. Differentially expressed genes (DEGs) were counted and the screening conditions: expression difference fold |log2 Fold Change| > 1, P-value <0.05. As shown in Fig. S9B—D, compared with Non—S, there were 3773 DEGs in S, while after PE-CDs treatment, the number of DEGs decreased to 2630. The number of down-regulated genes (719) was greater than the number of up-regulated genes (392) after PE-CDs treatment compared to S. GO enrichment analysis of DEGs (Fig. S10) revealed that compared to Non—S, the up-regulated genes in S were significantly enriched in oxidoreductase activity, defense response, toxin metabolic process, secondary metabolic process and other terms. The down-regulated genes were significantly enriched under the terms related to photosynthesis. In contrast, the top 20 terms in the group sprayed with PE-CDs under salt stress were significantly up-regulated, including oxidoreductase activity, transcription factor activity, defense response, etc.
The KEGG database pathway analysis was performed for DEGs (Fig. S11). Compared to Non—S, S treatment significantly down-regulated genes in photosynthesis-related pathways. Compared to Non—S, the DEGs in PE-CDs treatment were enriched in metabolism of terpenoids and polyketides, biosynthesis of other secondary metabolites, carbohydrate, lipid, amino acids metabolic pathways, and signal transduction pathways.
PsaC is an important constitutive subunit of photosystem I and is involved in electron transfer in ferredoxin (Yang et al., 2017). Photosystem I is high in iron and is significantly affected by iron deficiency, and studies have shown that PsaC is reduced substantially in iron deficiency (Yadavalli, Neelam, Rao, Reddy, & Subramanyam, 2012). Results (Fig. 4A) showed that the PsaC gene expression was significantly up-regulated in PE-CDs treatment compared with S. Combined with the physiological results, this indicated that the PE-CDs treatment accelerated the ETR in the photosystem, significantly increased the uptake of Fe, elevated the chlorophyll content, and enhanced photosynthesis under salt stress in the rice seedlings, thereby alleviating the salt stress and promoting the growth of rice. The BX1 and IGL genes encode enzymes that convert indole-3-glycerolphosphate to indole, a volatile defense signaling compound and a precursor for the biosynthesis of tryptophan and defense-related benzoxazinoid (Richter et al., 2021). The BX1 and IGL genes expression were significantly up-regulated in PE-CDs compared to S. It suggested that PE-CDs treatment enhanced the rice defense system by up-regulating this gene and releasing defense signals while synthesizing large amounts of tryptophan. The JAZ protein negatively regulates the production of jasmonic acid, which is an important signaling molecule for abiotic stress, inducing costly defense responses. Increased rice investment in defense leads to reduced growth, so jasmonic acid-induced responses are down-regulated when defense is not required (Wasternack, 2017). As shown in Fig. 4A, the gene encoding the JAZ protein was significantly up-regulated in the PE-CDs compared to S, leading to a decrease in the synthesis of jasmonic acid, which in turn led to a decrease in jasmonic acid-induced investment in defense, and an increase in the amount of rice growth. It indicated that PE-CDs treatment reduced the salt stress degree and regulated defense investment and rice seedlings growth through the jasmonic acid signaling pathway.
Fig. 4.
Transcriptome analysis of rice seedlings under different treatments. (A) The relative expression levels of upregulated genes. (B) DEGs associated with Phenylpropanoid biosynthesis. Red and green colors represented genes up- and down-regulated, respectively, after treatment with PE-CDs compared to S. The embedded heatmap showed the expression levels of the relevant DEGs. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Lignin and flavonoids are products of the phenylpropanoid metabolic pathway, which affects plant clearance of ROS and tolerance to abiotic stresses (Chen, Su, Zhang, Zhan, & Zeng, 2020). As shown in Fig. 4B, HCT4 genes and peroxidase-related (Per) genes were up-regulated after PE-CDs treatment compared with S treatment, indicating that these genes played a positive role in alleviating salt stress in rice. And 4CL genes were down-regulated in this pathway, which is consistent with the reported results (Xu et al., 2021). Diterpenoid biosynthesis has been reported to contribute to the tolerance of rice seedlings to salinity stress (Li, Ma, Tai, Qiu, & Yang, 2020). An analysis of the pathway was shown in SI.
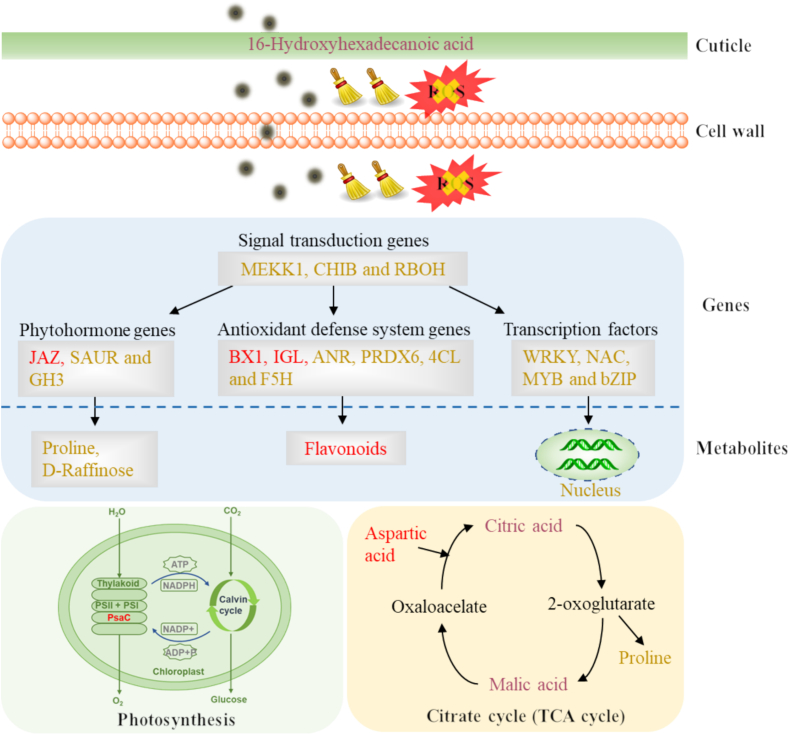
Phytohormone signaling networks regulate defense responses to help crops resist stresses. MEKK1, CHIB and RBOH genes are stress responsive genes in the MAPK signaling pathway, which play an important role in stress tolerance. Transcription factors are signaling endpoints that repress or activate the expression of target genes under different stresses. Detailed analysis on phytohormone signaling networks and transcription factors was shown in SI. Fig. 5 illustrated the possible molecular mechanisms of PE-CDs in alleviating salt stress in rice seedlings.
Fig. 5.
Schematic diagram of the molecular mechanism for PE-CDs mitigated salt stress in rice seedlings. Red color represents up-regulated genes or metabolites. Gold font represents genes or metabolites that are up-regulated compared to Non-S and down-regulated compared to S. Purple font represents no significant difference in PE-CDs treatment compared to Non—S. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Conclusion
The CDs prepared by both methods inherited the antioxidant properties of the precursors. The smaller size and lower zeta potential of PE-CDs were important factors contributing to their effectiveness. The electron donor-acceptor characteristics of PE-CDs enhanced the photosynthetic electron transport in photosynthesis. Furthermore, the photosynthesis of rice was promoted by up-regulation of the PsaC gene expression of PSI, and the defense system was enhanced by up-regulation of the BX1 and IGL genes expression. PE-CDs effectively mitigated the salt stress in rice seedlings, leading to the restoration of their fresh weight and chlorophyll content to the normal. The spraying of PE-CDs can help rice grow in salty soils, thereby expanding the global agricultural area and promoting environmental sustainability. However, field trials and the impact of CDs on the entire life cycle of crops also need further research.
CRediT authorship contribution statement
Baoyan Guo: Writing – review & editing, Writing – original draft, Validation, Investigation, Formal analysis, Data curation, Conceptualization. Fengqiong Chen: Writing – original draft, Software, Methodology, Formal analysis, Data curation, Conceptualization. Guo Liu: Writing – review & editing, Validation, Software, Resources, Methodology, Investigation, Data curation. Wentao Li: Validation, Methodology, Formal analysis, Data curation. Wei Li: Writing – original draft, Visualization, Software, Conceptualization. Jianle Zhuang: Writing – original draft, Visualization, Supervision, Project administration, Methodology, Formal analysis, Conceptualization. Xuejie Zhang: Software, Methodology, Investigation, Formal analysis. Lashuang Wang: Writing – original draft, Resources. Bingfu Lei: Writing – review & editing, Supervision, Project administration, Funding acquisition. Chaofan Hu: Writing – review & editing, Writing – original draft, Supervision. Yingliang Liu: Writing – review & editing, Writing – original draft, Visualization, Supervision, Resources, Project administration, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 12174119 and 52172142), Science and Technology Program of Guangzhou, China (Grant No. 202103000059).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101422.
Contributor Information
Bingfu Lei, Email: tleibf@scau.edu.cn.
Chaofan Hu, Email: thucf@scau.edu.cn.
Yingliang Liu, Email: tliuyl@scau.edu.cn.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- Albersheim P., Darvill A., Roberts K., Sederoff R., Staehelin A. Garland Science, Taylor and Francis Group, LLC; New York: 2010. Plant cell walls: From chemistry to biology. [Google Scholar]
- Basit F., Ulhassan Z., Mou Q., Nazir M.M., Hu J., Hu W.…Guan Y. Seed priming with nitric oxide and/or spermine mitigate the chromium toxicity in rice (Oryza sativa) seedlings by improving the carbon-assimilation and minimising the oxidative damages. Functional Plant Biology. 2023;50:121–135. doi: 10.1071/FP21268. [DOI] [PubMed] [Google Scholar]
- Bian X., Yao L., Si E., Meng Y., Li B., Ma X., Yang K., Lai Y., Shang X., Li C., Wang J., Wang H. Characterization of glossy spike mutants and identification of candidate genes regulating cuticular wax synthesis in barley (Hordeum vulgare L.) International Journal of Molecular Sciences. 2022;23:13025. doi: 10.3390/ijms232113025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boriboonkaset T., Theerawitaya C., Yamada N., Pichakum A., Supaibulwatana K., Cha-um S., Takabe T., Kirdmanee C. Regulation of some carbohydrate metabolism-related genes, starch and soluble sugar contents, photosynthetic activities and yield attributes of two contrasting rice genotypes subjected to salt stress. Protoplasma. 2013;250:1157–1167. doi: 10.1007/s00709-013-0496-9. [DOI] [PubMed] [Google Scholar]
- Chaudhary M., Singh P., Singh G.P., Rathi B. Structural features of carbon dots and their agricultural potential. ACS Omega. 2024;9:4166–4185. doi: 10.1021/acsomega.3c04638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Su W., Zhang H., Zhan Y., Zeng F. Fraxinus mandshurica 4-coumarate-CoA ligase 2 enhances drought and osmotic stress tolerance of tobacco by increasing coniferyl alcohol content. Plant Physiology and Biochemistry. 2020;155:697–708. doi: 10.1016/j.plaphy.2020.08.031. [DOI] [PubMed] [Google Scholar]
- Das B., Dadhich P., Pal P., Srivas P.K., Bankoti K., Dhara S. Carbon nanodots from date molasses: New nanolights for the in vitro scavenging of reactive oxygen species. Journal of Materials Chemistry B. 2014;2:6839–6847. doi: 10.1039/C4TB01020E. [DOI] [PubMed] [Google Scholar]
- Do Espirito Santo Pereira A., Caixeta Oliveira H., Fernandes Fraceto L., Santaella C. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials. 2021;11:267. doi: 10.3390/nano11020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq M., Asif S., Jang Y., Park J., Zhao D., Kim E., Kim K. Effect of different salts on nutrients uptake, gene expression, antioxidant, and growth pattern of selected rice genotypes. Frontiers in Plant Science. 2022;13 doi: 10.3389/fpls.2022.895282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz E. Measurement of cation exchange capacity (CEC) of plant cell walls by X-ray microanalysis (EDX) in the transmission electron microscope. Microscopy and Microanalysis. 2007;13:233–244. doi: 10.1017/S1431927607070420. [DOI] [PubMed] [Google Scholar]
- Gázquez J.L., Cedillo A., Vela A. Electrodonating and electroaccepting powers. Journal of Physical Chemistry A. 2007;111:1966–1970. doi: 10.1021/jp065459f. [DOI] [PubMed] [Google Scholar]
- Ge S., Qin K., Ding S., Yang J., Jiang L., Qin Y., Wang R. Gas chromatography–mass spectrometry metabolite analysis combined with transcriptomic and proteomic provide new insights into revealing cuticle formation during pepper development. Journal of Agricultural and Food Chemistry. 2022;70:12383–12397. doi: 10.1021/acs.jafc.2c04522. [DOI] [PubMed] [Google Scholar]
- Guo B., Liu G., Li W., Hu C., Lei B., Zhuang J., Zheng M., Liu Y. The role of carbon dots in the life cycle of crops. Industrial Crops and Products. 2022;187 doi: 10.1016/j.indcrop.2022.115427. [DOI] [Google Scholar]
- Han M., Zhang C., Suglo P., Sun S., Wang M., Su T. L-aspartate: An essential metabolite for plant growth and stress acclimation. Molecules. 2021;26:1887. doi: 10.3390/molecules26071887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P., An J., Faulkner M.M., Wu H., Li Z., Tian X., Giraldo J.P. Nanoparticle charge and size control foliar delivery efficiency to plant cells and organelles. ACS Nano. 2020;14:7970–7986. doi: 10.1021/acsnano.9b09178. [DOI] [PubMed] [Google Scholar]
- Huang X., Zheng D., Feng N., Huang A., Zhang R., Meng F., Jie Y., Mu B., Mu D., Zhou H. Effects of prohexadione calcium spraying during the booting stage on panicle traits, yield, and related physiological characteristics of rice under salt stress. Peerj. 2023;11 doi: 10.7717/peerj.14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionita P. The chemistry of DPPH· free radical and congeners. International Journal of Molecular Sciences. 2021;22:1545. doi: 10.3390/ijms22041545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y., Yue L., Cao X., Chen F., Li J., Zhang J., Wang C., Wang Z., Xing B. Carbon dots promoted soybean photosynthesis and amino acid biosynthesis under drought stress: Reactive oxygen species scavenging and nitrogen metabolism. Science of the Total Environment. 2023;856 doi: 10.1016/j.scitotenv.2022.159125. [DOI] [PubMed] [Google Scholar]
- Jing X., Liu Y., Liu X., Zhang Y., Wang G., Yang F., Zhang Y., Chang D., Zhang Z., You C., Zhang S., Wang X. Enhanced photosynthetic efficiency by nitrogen-doped carbon dots via plastoquinone-involved electron transfer in apple. Horticulture Research. 2024;11:1–12. doi: 10.1093/hr/uhae016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou E., Yao Y., Yang X., Song S., Li W., Kang Y.…Lei B. Regulation mechanisms of carbon dots in the development of lettuce and tomato. ACS Sustainable Chemistry & Engineering. 2021;9:944–953. doi: 10.1021/acssuschemeng.0c08308. [DOI] [Google Scholar]
- Li Q., Ma C., Tai H., Qiu H., Yang A. Comparative transcriptome analysis of two rice genotypes differing in their tolerance to saline-alkaline stress. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Zhang Y., Liu Y., Li X., Hao G., Han Q.…Zhao T. Raffinose synthase enhances drought tolerance through raffinose synthesis or galactinol hydrolysis in maize and Arabidopsis plants. Journal of Biological Chemistry. 2020;295:8064–8077. doi: 10.1074/jbc.RA120.013948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li W., Yang X., Kang Y., Zhang H., Liu Y., Lei B. Salvia miltiorrhiza-derived carbon dots as scavengers of reactive oxygen species for reducing oxidative damage of plants. ACS Applied Nano Materials. 2021;4:113–120. doi: 10.1021/acsanm.0c02419. [DOI] [Google Scholar]
- Lu N., Yan L., Zheng C., Yin H., Guo S., Xie X. Effects of salt stress on growth and agronomic traits of Yanfeng 47 and Yanjing 456. Crops. 2017;180:106–111. doi: 10.16035/j.issn.1001-7283.2017.05.018. [DOI] [Google Scholar]
- Lv J., Christie P., Zhang S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: Recent advances and methodological challenges. Environmental Science: Nano. 2019;6:41–59. doi: 10.1039/C8EN00645H. [DOI] [Google Scholar]
- Mehrabanjoubani P., Abdolzadeh A., Sadeghipour H.R., Aghdasi M., Bagherieh Najjar M.B., Barzegargolchini B. Silicon increases cell wall thickening and lignification in rice (Oryza sativa) root tip under excess Fe nutrition. Plant Physiology and Biochemistry. 2019;144:264–273. doi: 10.1016/j.plaphy.2019.09.047. [DOI] [PubMed] [Google Scholar]
- Mekawy A.M.M., Abdelaziz M.N., Ueda A. Apigenin pretreatment enhances growth and salinity tolerance of rice seedlings. Plant Physiology and Biochemistry. 2018;130:94–104. doi: 10.1016/j.plaphy.2018.06.036. [DOI] [PubMed] [Google Scholar]
- Rahman A., Alam M.U., Hossain M.S., Mahmud J.A., Nahar K., Fujita M., Hasanuzzaman M. Exogenous gallic acid confers salt tolerance in rice seedlings: Modulation of ion homeostasis, osmoregulation, antioxidant defense, and methylglyoxal detoxification systems. Agronomy. 2023;13:16. doi: 10.3390/agronomy13010016. [DOI] [Google Scholar]
- Rahman A., Nahar K., Hasanuzzaman M., Fujita M. Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Frontiers in Plant Science. 2016;7:609. doi: 10.3389/fpls.2016.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A., Powell A.F., Mirzaei M., Wang L.J., Movahed N., Miller J.K.…Jander G. Indole-3-glycerolphosphate synthase, a branchpoint for the biosynthesis of tryptophan, indole, and benzoxazinoids in maize. The Plant Journal. 2021;106:245–257. doi: 10.1111/tpj.15163. [DOI] [PubMed] [Google Scholar]
- Ruiz V., Yate L., García I., Cabanero G., Grande H. Tuning the antioxidant activity of graphene quantum dots: Protective nanomaterials against dye decoloration. Carbon. 2017;116:366–374. doi: 10.1016/j.carbon.2017.01.090. [DOI] [Google Scholar]
- Villora G., Moreno D.A., Pulgar G., Romero L. Yield improvement in zucchini under salt stress: determining micronutrient balance. Scientia Horticulturae. 2000;86:175–183. doi: 10.1016/S0304-4238(00)00149-7. [DOI] [Google Scholar]
- Wang C., Yang H., Yue L., Sun W., Chen F., Cao X.…Xing B. Physiological and molecular level understanding of advanced carbon dots to enhance maize drought tolerance: Modulation of photosynthesis and signaling molecules. Environmental Science: Nano. 2022;9:3821–3832. doi: 10.1039/D2EN00176D. [DOI] [Google Scholar]
- Wang P., Lombi E., Zhao F., Kopittke P.M. Nanotechnology: A new opportunity in plant sciences. Trends in Plant Science. 2016;21:699–712. doi: 10.1016/j.tplants.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Wasternack C. A plant’s balance of growth and defense – Revisited. New Phytologist. 2017;215:1291–1294. doi: 10.1111/nph.14720. [DOI] [PubMed] [Google Scholar]
- Xu C., Wei L., Huang S., Yang C., Wang Y., Yuan H., Xu Q., Zhang W., Wang M., Zeng X., Luo J. Drought resistance in Qingke involves a reprogramming of the phenylpropanoid pathway and UDP-glucosyltransferase regulation of abiotic stress tolerance targeting flavonoid biosynthesis. Journal of Agricultural and Food Chemistry. 2021;69:3992–4005. doi: 10.1021/acs.jafc.0c07810. [DOI] [PubMed] [Google Scholar]
- Yadavalli V., Neelam S., Rao A.S.V.C., Reddy A.R., Subramanyam R. Differential degradation of photosystem I subunits under iron deficiency in rice. Journal of Plant Physiology. 2012;169:753–759. doi: 10.1016/j.jplph.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Yang H., Li P., Zhang A., Wen X., Zhang L., Lu C. Tetratricopeptide repeat protein Pyg7 is essential for photosystem I assembly by interacting with PsaC in Arabidopsis. The Plant Journal. 2017;91:950–961. doi: 10.1111/tpj.13618. [DOI] [PubMed] [Google Scholar]
- Yao L., Zhao M., Luo Q., Zhang Y., Liu T., Yang Z.…Zeng K. Carbon quantum dots-based nanozyme from coffee induces cancer cell ferroptosis to activate antitumor immunity. ACS Nano. 2022;16:9228–9239. doi: 10.1021/acsnano.2c01619. [DOI] [PubMed] [Google Scholar]
- Zhang M., Wang H., Liu P., Song Y., Huang H., Shao M., Liu Y., Li H., Kang Z. Biotoxicity of degradable carbon dots towards microalgae Chlorella vulgaris. Environmental Science: Nano. 2019;6:3316–3323. doi: 10.1039/C9EN00829B. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.