ABSTRACT
BACKGROUND:
Migraines are a common comorbidity and source of disability in patients with chronic inflammatory diseases like multiple sclerosis (MS). Recently, therapeutic agents for episodic and chronic migraine known as calcitonin gene-related peptide (CGRP) inhibitors have shown to effectively control migraine attacks and improve quality of life in the general population. This study explored the use of these novel agents in individuals with comorbid MS.
METHODS:
This was a retrospective, population-based cohort study at the University of South Florida's neurology clinic; it evaluated individuals with both MS and migraine.
RESULTS:
A total of 27 individuals with MS and chronic or episodic migraine who received treatment with a CGRP monoclonal antibody were identified. Of these, 63% reported a reduction in their migraine frequency of greater than 75%. Concurrent use of a disease-modifying therapy (DMT) for MS occurred in 82% of patients, and in 37% of these, the DMT used was also a monoclonal antibody. Adverse effects from CGRP monoclonal antibodies were mild and occurred in only 11% of patients, and no patient experienced worsening of their MS symptoms during cotreatment over the duration of the study.
CONCLUSIONS:
Our study showed a significant reduction in migraine frequency and a favorable adverse event profile for individuals with comorbid MS who took CGRP monoclonal antibodies and experienced no worsening of MS symptoms. In individuals with MS, CGRP monoclonal antibodies seem to be a safe and effective therapy for episodic or chronic migraine.
Keywords: multiple sclerosis, migraine, CGRP monoclonal antibodies, migraine prophylaxis, migraine prevention treatment
An enigmatic disorder to treat, migraines can result in severe impairment to sufferers due to symptoms such as pain, difficulty concentrating, and neurological deficits.1-3 Migraines are thought to arise from nociceptive signals of the trigeminal ganglia, with the neurons of the ganglia releasing vasoactive and inflammatory mediators that elicit pain.1 One of the vasoactive peptides is calcitonin gene–related peptide (CGRP), a 37-amino acid molecule that dilates blood vessels; its presence is markedly increased in individuals with migraines.3-6 During migraine attacks, CGRPs are found throughout the trigeminal ganglia, cortex, and various nociceptive pathways, promoting pain signaling.3-8
Therapies that inhibit CGRP signaling can improve migraine symptoms during the attack and also serve as pro-phylaxis.3 Some of these therapies are small-molecule inhibitors of the CGRP receptor, including olcegepant, telcagepant, atogepant, ubrogepant, and zavegepant. In addition, monoclonal antibodies counteract the effects of migraine, including eptinezumab, erenumab, fremanezumab, and galcane-zumab.2-4,6,7,9 Erenumab specifically targets the CGRP receptor while the other monoclonal antibodies bind to CGRP itself to antagonize the vasodilatory effects. Eptinezumab, erenumab, fremanezumab, and galcanezumab are increasingly used for long-term treatment of migraines and have been shown to significantly reduce the number of migraine days.2-4,7,10
Anti-CGRP therapy may be beneficial in individuals with comorbid migraines and multiple sclerosis (MS). Some studies have suggested that migraine is a risk factor for developing MS.7,11,12 Headaches have been estimated to occur in approximately two-thirds of individuals with MS11-14 and are associated with a higher prevalence of pain-related MS symptoms such as temporomandibular joint pain, muscle spasms, restless legs syndrome, occipital and trigeminal neuralgia, and Lhermitte sign.15 These MS symptoms can be managed with anti-inflammatory and neuropathic pain agents, but control of the disease itself has been achieved with the expansion of disease-modifying therapy (DMT) options over the past few decades.12,16 Unfortunately, some DMTs, especially interferon beta, can exacerbate headaches.12
Few studies explore how CGRP monoclonal antibodies interact with DMTs, whether their use worsens or improves MS symptoms or, alternatively, whether CGRP monoclonal antibodies improve migraines in individuals with MS.10,17 Because they may produce both pro- and anti-inflammatory effects and experiments have shown that they reduce microglial-mediated autoimmune reactions,7,18 CGRP monoclonal antibodies may play a role in reducing autoimmune inflammation in the central nervous system and thus may be beneficial for individuals with MS.10,12 One series of case studies demonstrated that the use of CGRP monoclonal antibodies for migraines in individuals with MS was associated with only a few adverse effects (AEs): urinary tract infections, sinus infections, and upper respiratory tract infections.17
Because CGRP itself has some anti-inflammatory effects, its antagonism may be associated with AEs such as rashes, hepatitis, and drug antibody formation in patients with autoimmune conditions.4,5,7,19-21 Due to the potential benefits of anti-CGRP therapy in individuals with MS described previously, as well as the possibility of these AEs, we wanted to study the efficacy of CGRP monoclonal antibodies in individuals with comorbid migraines and to see whether those who take CGRP monoclonal antibodies for their migraines experience any AEs as a result.
METHODS
Cohort Selection
We conducted a retrospective medical record review at an academic MS center in Tampa, Florida. Individuals with both MS and chronic, episodic, or unspecified migraine who were 18 years of age or older and who had received treatment with a CGRP monoclonal antibody were included (N = 27). Patients whose treatment duration was less than 4 months were excluded. This study collected data from June 2018 to January 2022. Approval for this study was granted by the institutional review board of the University of South Florida.
Patients with diagnoses of MS and migraine were identified using the International Classification of Diseases, Tenth Revision codes listed in the individuals’ electronic medical records. Data analysis was limited to active patients of the MS study institution and therefore only included notes taken during encounters that were accessible through that institution's health record system.
Primary Outcomes and Data Collection
The primary outcomes were migraine frequency prior to and following the use of CGRP monoclonal antibodies and AEs the participants experienced while on CGRP monoclonal antibodies. Headache reduction was quantified as less than 50% reduction in frequency, between 51% and 74% reduction in frequency, or greater than 75% reduction in frequency.
For each participant visit encounter between June 2018 and January 2022, we extracted MS diagnosis, migraine subtype based on the third edition of the International Classification of Headache Disorders, current use of DMT, type of DMT and CGRP monoclonal antibody, duration of DMT and CGRP monoclonal antibody use, reduction in headache frequency, AEs, other previously trialed CGRP monoclonal antibodies, age, sex, and MS symptoms. This study did not ascertain subtype of MS, as subtype was not typically listed within patients’ charts due to the note templates used for each visit encounter.
Simple statistics were calculated including means and population proportions. To determine any statistically significant difference between the proportions, χ2 analysis was performed. Because of the small sample size, a Fisher exact test was also used to further analyze the data.
RESULTS
The cohort of 27 (88.9% female) with MS and either chronic (88.9%), episodic (7.4%), or unspecified (3.7%) migraine who received treatment with a CGRP monoclonal antibody (ie, fremanezumab, eptinezumab, erenumab, or galca-nezumab; FIGURE) had a mean age of 45.6 years. Of the participants, 82% were cotreated with a DMT for their MS (ie, ocrelizumab, glatiramer acetate, siponimod, cladribine, natalizumab, fingolimod, interferon beta-1a, alemtuzumab, dimethyl fumarate, or teriflunomide; see TABLE 1).
FIGURE.
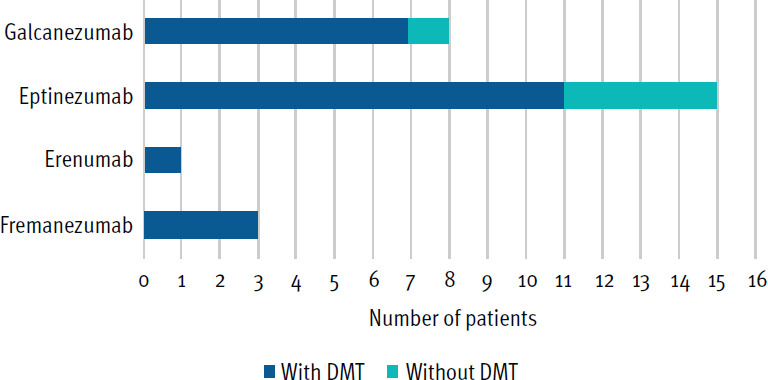
Use of Calcitonin Gene-Related Peptide Monoclonal Antibody by Type
DMT, disease-modifying therapy
TABLE 1.
Subtypes of Disease-Modifying Treatment Used
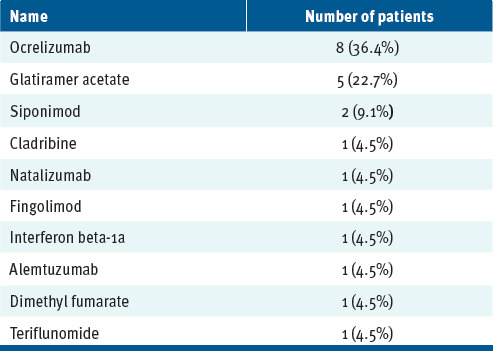
| Name | Number of patients |
|---|---|
| Ocrelizumab | 8 (36.4%) |
| Glatiramer acetate | 5 (22.7%) |
| Siponimod | 2 (9.1%) |
| Cladribine | 1 (4.5%) |
| Natalizumab | 1 (4.5%) |
| Fingolimod | 1 (4.5%) |
| Interferon beta-1a | 1 (4.5%) |
| Alemtuzumab | 1 (4.5%) |
| Dimethyl fumarate | 1 (4.5%) |
| Teriflunomide | 1 (4.5%) |
The average duration of therapy by CGRP monoclonal antibody subtype was 10 months for fremanezumab, 9 months for eptinezumab, 26 months for erenumab, and 13.5 months for galcanezumab (TABLE 2). Over the period of the study, the longest duration of treatment with any CGRP monoclonal antibody was 42 months.
TABLE 2.
Duration of Calcitonin Gene-Related Peptide Treatment
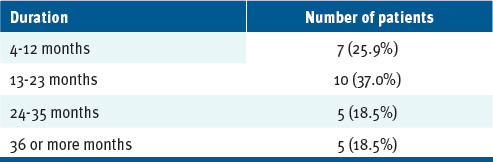
| Duration | Number of patients |
|---|---|
| 4-12 months | 7 (25.9%) |
| 13-23 months | 10 (37.0%) |
| 24-35 months | 5 (18.5%) |
| 36 or more months | 5 (18.5%) |
Eleven percent reported mild AEs (ie, muscle spasms, constipation, headache) from CGRP monoclonal antibodies. No one demonstrated worsening of their MS symptoms during cotreatment with DMT and CGRP monoclonal antibody. Of those that reported a reduction in headache frequency (n = 25, 92.6% of participants), 7.4% reported between 50% and 75% reduction in headache frequency, while 22.22% reported less than 50% reduction in headache frequency. In total, 63% reported a greater than 75% reduction in migraine frequency. There was no significant difference in reduction of headache frequency between participants who were on dual monoclonal antibody therapy (eg, erenumab plus ocrelizumab) versus those who were not, and this was true for each level of reduction in headache frequency (χ2 [2, n = 29] = 0.31, P = .86). Even when separating into only 2 groups of level of reduction (< 50% and > 50%), there was still no statistically significant difference between participants who were on dual monoclonal antibody therapy versus those who were not (χ2 [1, n = 29] = 0.068, P = .79). Analysis via Fisher's exact test also resulted in a nonstatistically significant difference between participants who were on dual monoclonal antibody therapy versus those who were not (P = 1.0).
DISCUSSION AND CONCLUSIONS
In a cohort of 27 participants with MS, use of CGRP monoclonal antibodies for migraine prophylaxis was associated with an improvement of migraine symptoms in the form of marked headache reduction for the majority. These findings are in line with the current literature that describes the ability of these medications to improve headaches,3,4,7,10 but more specifically, results show that CGRP monoclonal antibodies can be efficacious in individuals with comorbid MS.
More importantly, this study expanded on the previous letter by Gonzales-Martinez et al to further demonstrate that the CGRP monoclonal antibodies (eg, fremanezumab, eptinezumab, erenumab, galcanezumab) can be safely used in individuals with comorbid MS.17 Participants in this study often were cotreated for migraine while simultaneously receiving a variety of DMTs for MS, and the MS symptoms of the individuals on comorbid DMT and CGRP monoclonal antibodies did not get worse. Furthermore, study participants experienced limited AEs (eg, muscle spasms, constipation, worsened headache), but did not experience any rashes, elevations in liver enzymes suggesting autoimmune hepatitis, drug antibody formation, or other autoimmune-associated reactions to the CGRP monoclonal antibodies, contrary to previous reports.21
This study had a few major limitations, including being underpowered. In addition, MS subtypes (eg, RRMS, PPMS) were not ascertained. Most participants had no documented MS exacerbations, which means their MS may not have been active enough to influence the safety of CGRP monoclonal antibody use. We studied CGRP monoclonal antibodies in this population, but not small-molecule inhibitors or gepants, because at the time data was collected, they had not been available on the market for very long. This study is also constrained by limited long-term follow up, with the longest duration of treatment with CGRP monoclonal antibodies being only 42 months. As such, further exploration should aim to address whether long-term use of CGRP monoclonal antibodies has any significant effect on the MS disease course, especially among subtypes. In summary, future research utilizing a cross-sectional or randomized controlled methodology should be pursued to further establish the safety and efficacy of anti-CGRP therapy (including monoclonal antibodies and gepants) in individuals with comorbid migraines and MS.
PRACTICE POINTS
Anti-calcitonin gene-related peptide (CGRP) monoclonal antibody therapy serves as a potent migraine treatment.
Use of CGRP monoclonal antibodies was associated with decreased migraine frequency in individuals with comorbid multiple sclerosis (MS) in this cohort of 27 patients.
Concurrent use of disease-modifying therapy (DMT) for MS and treatment of migraines with CGRP monoclonal antibodies was not associated with worsening of MS symptoms.
Further research, including randomized controlled trials, is needed to provide a more specific safety profile for concurrent use of DMT and anti-CGRP therapy in patients with MS and migraines
Footnotes
PRIOR PRESENTATION: This data was previously presented at the 2022 Consortium of Multiple Sclerosis Centers Annual Meeting; June 2, 2022; National Harbor, Maryland.
FINANCIAL DISCLOSURES: Dr Maldonado receives grant support from Genentech for research study. Dr Robertson has received grant support from Anokion; Atara Biotherapeutics; Biogen; CorEvitas; EMD Serono; Genentech; GW Pharmaceuticals; Janssen; Mallinckrodt; MedDay; Novartis; Patient-Centered Outcomes Research Institute; PRIME Education, LLC; Sanofi; and TG Therapeutics. Dr Robertson has received consulting fees from Alexion, Biogen, Bristol Myers Squibb, EMD Serono, Genentech, Greenwich Biosciences, Horizon, Janssen, Mallinckrodt, Novartis, Sanofi, and TG Therapeutics. Dr Robertson has received honoraria or speaker fees from Alexion; Biogen; Bristol Myers Squibb; EMD Serono; Genentech; Horizon; Janssen; PRIME Education, LLC; Sanofi; and TG Therapeutics. The remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ashina M. Migraine. N Engl J Med. 2020;383(19):1866–1876. doi: 10.1056/NEJMra1915327. [DOI] [PubMed] [Google Scholar]
- 2.Peters GL. Migraine overview and summary of current and emerging treatment options. Am J Manag Care. 2019;25(suppl 2):S23–S34. [PubMed] [Google Scholar]
- 3.Ray JC, Kapoor M, Stark RJ, et al. Calcitonin gene related peptide in migraine: current therapeutics, future implications and potential off-target effects. J Neurol Neurosurg Psychiatry. 2021;92(12):1325–1334. doi: 10.1136/jnnp-2020-324674. [DOI] [PubMed] [Google Scholar]
- 4.Tepper SJ. History and review of anti-calcitonin gene-related peptide (CGRP) therapies: from translational research to treatment. Headache. 2018;58(suppl 3):238–275. doi: 10.1111/head.13379. [DOI] [PubMed] [Google Scholar]
- 5.Assas MB. Anti-migraine agents from an immunological point of view. J Transl Med. 2021;19(1):341. doi: 10.1186/s12967-021-02988-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang CC, Schwedt TJ. Calcitonin gene-related peptide (CGRP)-targeted therapies as preventive and acute treatments for migraine-the monoclonal antibodies and gepants. Prog Brain Res. 2020;255:143–170. doi: 10.1016/bs.pbr.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Balcziak LK, Russo AF. Dural immune cells, CGRP, and migraine. Front Neurol. 2022;13:874193. doi: 10.3389/fneur.2022.874193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messlinger K. The big CGRP flood - sources, sinks and signalling sites in the trigeminovascular system. J Headache Pain. 2018;19(1):22. doi: 10.1186/s10194-018-0848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holland PR, Goadsby PJ. Targeted CGRP small molecule antagonists for acute migraine therapy. Neurotherapeutics. 2018;15(2):304–312. doi: 10.1007/s13311-018-0617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Husain F, Pardo G, Rabadi M. Headache and its management in patients with multiple sclerosis. Curr Treat Options Neurol. 2018;20(4):10. doi: 10.1007/s11940-018-0495-4. [DOI] [PubMed] [Google Scholar]
- 11.Elliott DG. Migraine in multiple sclerosis. Int Rev Neurobiol. 2007;79:281–302. doi: 10.1016/S0074-7742(07)79012-8. [DOI] [PubMed] [Google Scholar]
- 12.Gelfand AA, Gelfand JM, Goadsby PJ. Migraine and multiple sclerosis: epidemiology and approach to treatment. Mult Scler Relat Disord. 2013;2(2):73–79. doi: 10.1016/j.msard.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Fragoso YD, Adoni T, Alves-Leon SV, et al. Migraine in 746 patients with multiple sclerosis. Arq Neuropsiquiatr. 2019;77(9):617–621. doi: 10.1590/0004-282X20190100. [DOI] [PubMed] [Google Scholar]
- 14.Sahai-Srivastava S, Wang SL, Ugurlu C, Amezcua L. Headaches in multiple sclerosis: cross-sectional study of a multiethnic population. Clin Neurol Neurosurg. 2016;143:71–75. doi: 10.1016/j.clineuro.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Kister I, Caminero AB, Monteith TS, et al. Migraine is comorbid with multiple sclerosis and associated with a more symptomatic MS course. J Headache Pain. 2010;11(5):417–425. doi: 10.1007/s10194-010-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: a review. JAMA. 2021;325(8):765–779. doi: 10.1001/jama.2020.26858. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Martinez A, Bose G, Chitnis T. Anti-CGRP therapies for migraine in multiple sclerosis patients. Mult Scler. 2022;28(13):2149–2150. doi: 10.1177/13524585221096353. [DOI] [PubMed] [Google Scholar]
- 18.Sardi C, Zambusi L, Finardi A, et al. Involvement of calcitonin gene-related peptide and receptor component protein in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2014;271(1-2):18–29. doi: 10.1016/j.jneuroim.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borkum JM. CGRP and brain functioning: cautions for migraine treatment. Headache. 2019;59(8):1339–1357. doi: 10.1111/head.13591. [DOI] [PubMed] [Google Scholar]
- 20.Cohen JM, Ning X, Kessler Y, et al. Immunogenicity of biologic therapies for migraine: a review of current evidence. J Headache Pain. 2021;22(1):3. doi: 10.1186/s10194-020-01211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray JC, Allen P, Bacsi A, et al. Inflammatory complications of CGRP monoclonal antibodies: a case series. J Headache Pain. 2021;22(1):121. doi: 10.1186/s10194-021-01330-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


