Summary
Background
Semaglutide demonstrated inferior weight loss responses in patients with type 2 diabetes (T2D) compared to patients with obesity without T2D. The individualized metabolic surgery (IMS) score was validated to predict T2D remission after bariatric surgery. The parameters of the IMS are HbA1c (<7%), insulin use, T2D medications and T2D duration. We aim to assess weight loss outcomes of semaglutide based on IMS score in patients with obesity and T2D.
Methods
This is a retrospective multicentered cohort study of patients with T2D and BMI≥ 27 kg/m2 taking ≥1 mg of semaglutide recruited from January 2020 to December 2022. We excluded patients with a history of bariatric surgery or taking other anti-obesity medications. IMS was calculated at baseline and patients weight change was recorded at baseline, 3, 6, 9 and 12 months. IMS was classified as mild (0–24.9 points), moderate (25–94.9 points), and severe (95–180 points). Analysis was performed based on IMS score quartiles and combination of Mild-Moderate vs Severe categories. We performed mixed linear regression models including age, sex, and baseline weight to assess associations between IMS categories with total body weight loss percentage (TBWL%).
Findings
We included 297 patients (42% female, mean age 62 ± 12 years) in the analysis. At 12 months, there was a stepwise decrease in weight loss outcomes when comparing patients by IMS quartiles (LS mean TBWL%± SE): 8.8 ± 0.8% vs 6.9 ± 0.8% vs 5.7 ± 0.9% vs 5.0 ± 0.8%. In the mixed linear model, patients in the mild-moderate category achieved significantly superior weight loss outcomes (LS mean TBWL± SE: −8.3 ± 0.7%) than patients in the severe category (−5.5 ± 0.6%; difference: −2.9, 95% CI: −5.2 to −0.5, p = 0.006) at 12 months. There was no significant difference in glycemic improvement regardless of IMS severity at baseline.
Interpretation
In our cohort, lower IMS severity was associated with more weight loss in patients with obesity and T2D. Further studies are needed to understand T2D severity and its effect on semaglutide outcomes.
Funding
Beyond payment to the research staff by Mayo Clinic, this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Keywords: GLP-1 receptor agonists, Individualized metabolic surgery score, Diabetes, Obesity, Semaglutide
Research in context.
Evidence before this study
We searched PubMed since inception to June 1st, 2023, for original research articles assessing weight loss outcomes with semaglutide in patients with or without diabetes using the following search terms: ‘semaglutide’; ‘obesity’; ‘diabetes’; ‘weight loss’ with no language restriction. Our search demonstrated that semaglutide led to inferior weight loss outcomes in patients with diabetes compared to patients without diabetes in clinical trials and real-world studies. We identified the individualized metabolic severity (IMS) score as a promising surrogate for diabetes severity. No studies have been conducted to assess semaglutide weight loss outcomes based on diabetes severity as assessed by a surrogate clinical score.
Added value of this study
Weight loss outcomes of semaglutide are associated with diabetes severity based on the individualized metabolic surgery score. Lower severity score was associated with more weight loss in patients with obesity and diabetes.
Implications of all the available evidence
These results underscore that diabetes severity, as assessed by a surrogate clinical score, can help predict the weight loss outcomes achieved by semaglutide in patients with diabetes. The application of this score could permit a personalized approach for selection of antiobesity medications and help define realistic goals in terms of weight loss.
Introduction
Obesity is a chronic and multifactorial disease that affects over 1.9 billion individuals, representing about 39% of the world's population.1 This disease is associated with multiple comorbidities including type 2 diabetes (T2D), hypertension, hyperlipidemia, non-alcoholic fatty liver, and obstructive sleep apnea.2 In fact, obesity represents one of the most common risk factors for T2D.3 A total of 80–90% of patients with T2D are reported to have overweight or obesity.4 Both diseases are associated with multiple serious diseases including cardiovascular complications (e.g., coronary artery disease and microvascular disease) which results in remarkable morbidity and mortality rates.5
Multiple interventions have been established to enhance weight loss including lifestyle changes and diets,6 anti-obesity medications (AOMs),7 and bariatric procedures.8 Within FDA-approved anti-obesity medications, semaglutide has been shown to result in the highest total body weight loss percentage (TBWL%) of around 14.9% in randomized clinical trials with patients without T2D.7 However, in patients with T2D, this medication demonstrates an inferior TBWL% of 9.6% in 68 weeks of follow-up.9 This difference in effectiveness is also replicated in real-world trials.10
Although multiple hypotheses have been formulated to explain this variation in weight loss outcomes, there are limited data on parameters that influence these outcomes. For instance, in the Look AHEAD trial, the use of insulin and other medications for T2D was associated with inferior weight loss outcomes in response to an intensive lifestyle intervention.11 The individualized metabolic surgery (IMS) score is a scoring system that was constructed and validated to establish an evidence-based selection of bariatric surgery based on T2D severity. It includes four parameters including glycemic control (HbA1c <7%), insulin use, number of T2D medications, and duration of T2D. Eventually, patients with T2D have IMS scores between 0 and 180 and fall within 3 categories of T2D severities: mild (0–24.9), moderate (25–94.9), and severe (95–180).12 Although the IMS score is built for patients undergoing bariatric surgery, its components are not surgery-specific and can be obtained for all patients with T2D. In fact, the aim of this score is to classify patients with different T2D severity. We hypothesized that patients with more severe T2D may achieve inferior weight loss outcome in response to semaglutide. In this study, we aim to assess weight loss outcomes of semaglutide based on IMS score in patients with T2D.
Methods
Patient selection
We performed a multicentered retrospective cohort study of patients with T2D and receiving weekly subcutaneous injections of semaglutide. We included patients from the Mayo Clinic Hospitals in Minnesota, Arizona and Florida, and all affiliated hospitals in the Mayo Clinic Health System. We used a timeframe between January 2020 till December 2022 for patients starting semaglutide for the purposes of T2D or obesity treatment. We included patients with body-mass index (BMI) ≥ 27 kg/m2 and taking ≥1 mg of semaglutide (i.e., 1, 1.7, 2, 2.4 mg) to select patients who were able to tolerate the medication and advance to the higher doses. We excluded patients receiving other anti-obesity medications, with diagnosed active malignancy, with pregnancy, and with history of bariatric surgery.
Ethics
The Mayo Clinic institutional review board approved the study and waived the need for informed consent owing to its minimal-risk nature and use of de-identified data (IRB: 17-001068).
Data collection
We used the electronic medical records (EMR) to collect baseline demographic and clinical data on our patients. We abstracted information pertaining to IMS parameters (i.e., number of T2D medications, insulin use, duration of T2D, and glycemic control [HbA1c <7%]). We confirmed semaglutide start date from clinical notes and communications between patients and their physicians. Patients’ body weights were abstracted from EMR at baseline, 3, 6, 9, and 12 months. We calculated TBWL% according to the following formula: TBWL% = 100 × ([Weight at Baseline Visit − Weight at Follow-up Visit]/Weight at Baseline Visit).
Score calculation
We calculated the score for each of our patients using the following score calculator: https://riskcalc.org/Metabolic_Surgery_Score/ at the start date of semaglutide. In this score, we added the clinical information pertaining to each of the following four parameters: number of T2D medications (0–5), insulin use (yes/no), duration of T2D (0–40 years), and glycemic control (HbA1c <7%). The score calculation will result in a score between 0 and 180, reflecting the severity of T2D.
Study end points
In this study, our primary end point was to assess weight loss outcomes based on IMS score quartiles at 12 months after semaglutide start date. Secondary end points included: a) evaluating weight loss in patients with mild-moderate compared to severe categories, b) evaluating weight loss outcomes based on each of the 4 IMS parameter independently, and c) assessing change in fasting glucose and HbA1c based on IMS severities.
Statistics
For our primary endpoint, we conducted a mixed linear model using TBWL% as the dependent variable. In this model, individual patients were included as a random effect and the IMS score quartile, the data timepoint for weight collection (3, 6, 9, and 12 months) and the interaction between data timepoint and IMS score severity as fixed effects. Additional fixed covariates included sex, age, baseline weight, and binary indicator variables for the presence of hypertension, dyslipidemia, anxiety, and depression. We used Restricted Maximum Likelihood (REML) to fit the model. We used Tukey's HSD tests to estimate pairwise differences at 12 months among the IMS quartiles. We did not use data imputation for missing data. We used a similar mixed linear model for a post-hoc analyses after dichotomizing IMS categories into mild-moderate and severe. We also conducted separate multivariate linear regression models after adjusting for the same covariates to evaluate each IMS parameter as a predictor of weight loss response at 12 months and pairwise comparisons with Least Square Means Student's t test or Tukey's HSD as appropriate. In addition, we performed 2 sample independent t-test for continuous variables and chi squared test for categorical variables. In addition, we conducted a stepwise linear regression model to assess for weight loss outcomes at 12 months using the previously described covariates and individual parameters included in the IMS with forward feature selection using the minimum Bayesian Information Criterion (BIC) as the stopping rule. We conducted separate logistic regression analyses with use of insulin as an endpoint and the other IMS parameters as variables to assess for correlation among parameters. For all analysis, statistical significance was set at 2-sided p < 0.05. We used JMP Pro, version 17 (SAS Institute Inc) to perform the statistical analysis. All baseline data were normally distributed and are summarized as mean and standard deviation (SD). This study followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline (Supplementary Material).
Role of the funding source
Beyond payment to the research staff by Mayo Clinic, this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Results
Participant selection
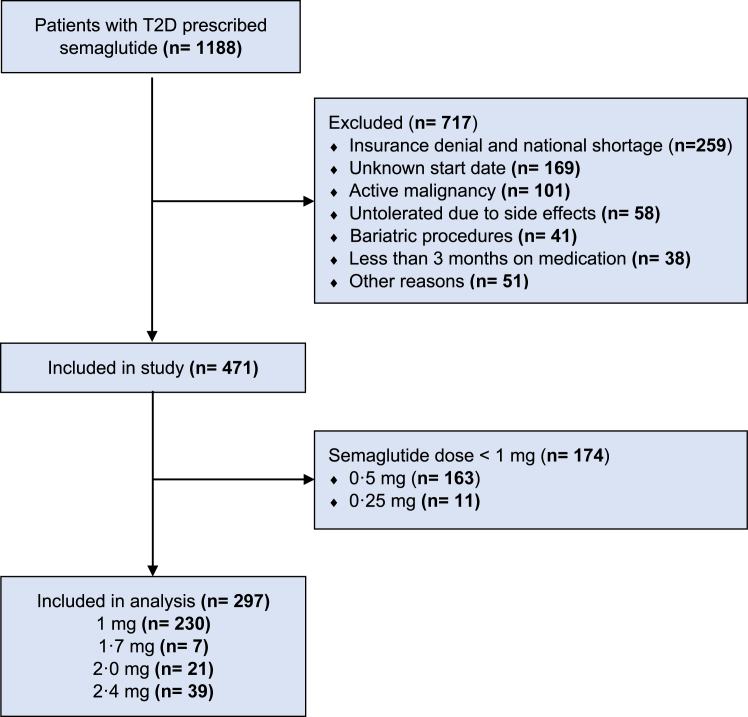
In our cohort, we had a total of 1188 semaglutide prescription for patients with T2DM and with overweight or obesity. We excluded 717 patients for multiple reasons including insurance denial and national shortage (n = 259), unknown medication start date (n = 169), and presence of an active malignancy (n = 101) (Table 1S). Out of the remaining 471 patients, 174 did not reach a dose of 1 mg or higher. Hence, a total of 297 patients with T2DM, overweight/obesity, and taking subcutaneous semaglutide (≥1 mg) were included in the analysis (Fig. 1).
Fig. 1.
Flowchart of the study.
Baseline characteristics
In this study, 297 patients (124 female [42%], mean age [SD] 61.5 ± 12 years, white 279 [94%], BMI 38.6 ± 8 kg/m2) were included in the study. Out of the 297 patients, 14 patients (5%) were in the Mild IMS severity, 97 (33%) in the Moderate, and 186 (63%) in the Severe category. There was no significant difference in demographic and anthropometric data except for age where patients in the severe category (63.6 ± 10 years) were older than the mild (57.7 ± 12 years) and moderate (57.8 ± 15 years) severities (p < 0.001). The most common comorbidities were dyslipidemia (87%) and hypertension (83%), which were significantly more prevalent in the severe category (p < 0.001). Detailed information on the IMS parameters and score derivatives are presented in Table 1.
Table 1.
Distribution of demographical, clinical, obesity comorbidities, and Individualized Metabolic Surgery (IMS) parameters among the mild, moderate, and severe categories of IMS score.
| Demographics | All patients | Mild | Moderate | Severe | p-value |
|---|---|---|---|---|---|
| N (%) | 297 (100) | 14 (5) | 97 (33) | 186 (63) | |
| Age, years (SD) | 61.5 (12) | 57.7 (12) | 57.8 (15) | 63.6 (10) | <0.001 |
| Sex, Female (%) | 124 (42) | 6 (43) | 49 (51) | 69 (37) | 0.09 |
| Race, White (%) | 279 (94) | 13 (93) | 89 (92) | 177 (95) | 0.30 |
| Clinical Parameters (SD) | |||||
| Weight, kg | 115.8 (27) | 111.1 (23) | 114.2 (27) | 117.0 (27) | 0.57 |
| Height, m | 1.73 (0.1) | 1.73 (0.1) | 1.71 (0.1) | 1.74 (0.1) | 0.15 |
| BMI, kg/m2 | 38.6 (8) | 36.9 (5) | 38.9 (9) | 38.7 (8) | 0.71 |
| Baseline HbA1c, % | 8.1 (1.6) | 6.4 (0.5) | 7.7 (0.2) | 8.5 (0.1) | <0.001 |
| Baseline fasting blood glucose, mg/dL | 178.9 (71.8) | 133.5 (20.5) | 169.4 (8.0) | 186.5 (5.6) | 0.02 |
| Obesity Comorbidities (%) | |||||
| Hypertension | 246 (83) | 10 (71) | 68 (70) | 168 (90) | <0.001 |
| Dyslipidemia | 258 (87) | 8 (57) | 78 (80) | 172 (93) | <0.001 |
| GERD | 136 (46) | 5 (36) | 46 (47) | 85 (45) | 0.71 |
| NAFLD | 76 (26) | 2 (14) | 21 (22) | 53 (29) | 0.28 |
| OSA | 177 (60) | 7 (50) | 58 (60) | 112 (60) | 0.75 |
| Anxiety | 70 (24) | 1 (7) | 31 (32) | 38 (20) | 0.03 |
| MDD | 88 (30) | 1 (7) | 36 (37) | 51 (27) | 0.04 |
| IMS Parameters | |||||
| T2D duration, years (SD) | 13.0 (8) | 1.9 (4) | 7.3 (4) | 16.9 (7) | <0.001 |
| On Insulin, yes (%) | 162 (55) | 0 (0) | 16 (17) | 146 (79) | <0.001 |
| HbA1c < 7% (%) | 89 (30) | 12 (86) | 48 (50) | 29 (16) | <0.001 |
| T2D medications (SD) | 2.6 (1) | 1.2 (0.4) | 2 (0.7) | 3 (0.7) | <0.001 |
| Mean Score (SD) | 102 (36) | 18 (3) | 71 (18) | 124 (18) | <0.001 |
Bold means p < 0.05.
Abbreviations used: BMI, Body mass index; GERD, Gastroesophageal reflux disease; NAFLD: Non-alcoholic fatty liver disease; OSA, Obstructive sleep apnea; MDD: Major depressive disorder; HbA1c: Glycated hemoglobin; T2D: Type-2 diabetes mellitus.
Weight loss outcomes
IMS quartiles
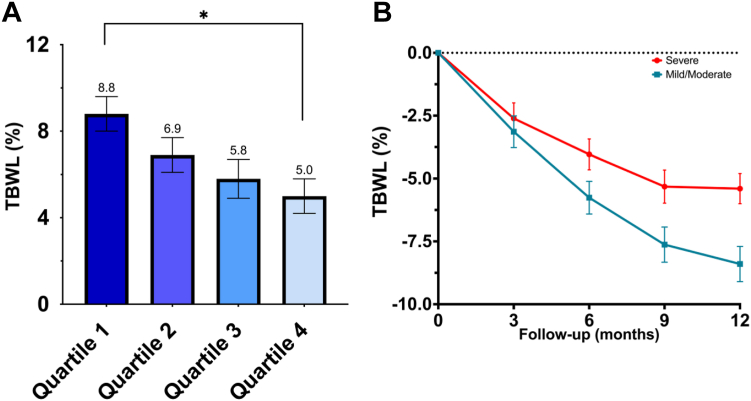
Based on IMS quartiles, 75 patients were included in the 1st quartile (12–78), 73 patients in the 2nd quartile (79–107), 78 patients in the 3rd quartile (108–129), and 71 patients in the 4th quartile (130–172) (Table 2S). At 12 months, there was a stepwise decrease in weight loss outcomes when comparing patients by IMS quartiles (LS mean TBWL%± SE): 8.8 ± 0.8% vs 6.9 ± 0.8% vs 5.7 ± 0.9% vs 5.0 ± 0.8% (Fig. 2A). There was only a statistically significant difference when comparing patients in Q1 vs. Q4 at 12 months (difference: −3.7, 95% CI: −7.4 to −0.1, p = 0.04). The detailed mixed linear model estimates and pairwise comparisons at each timepoint are presented in the Supplementary Material (Table 3S).
Fig. 2.
A. Total body weight loss percent (TBWL%) at 12 months between Individualized Metabolic Surgery (IMS) quartiles and B. TBWL% at 3, 6, 9, and 12 months in patients with Mild/Moderate vs Severe IMS categories. For both panels, mean and standard error of the mean (whiskers) are portrayed. ∗ = p < 0.05.
Combined mild-moderate vs severe IMS
Due to the small sample size and strict cut-offs in the mild category, we combined patients in the mild and moderate categories. In this new classification, 111 had a score between 0 and 95 (Mild-Moderate) and 186 had a score >95 (Severe). In the mixed linear model, patients in the mild-moderate category achieved significantly superior weight loss outcomes (LS mean TBWL± SE: −8.3 ± 0.7%) than patients in the severe category (−5.5 ± 0.6%; difference: −2.9, 95% CI: −5.2 to −0.5, p = 0.006) at 12 months (Fig. 2B). Detailed estimates for the mixed linear model and pairwise comparisons at all timepoints are presented in the Supplementary Material (Table 4S).
Independent effect of IMS parameters
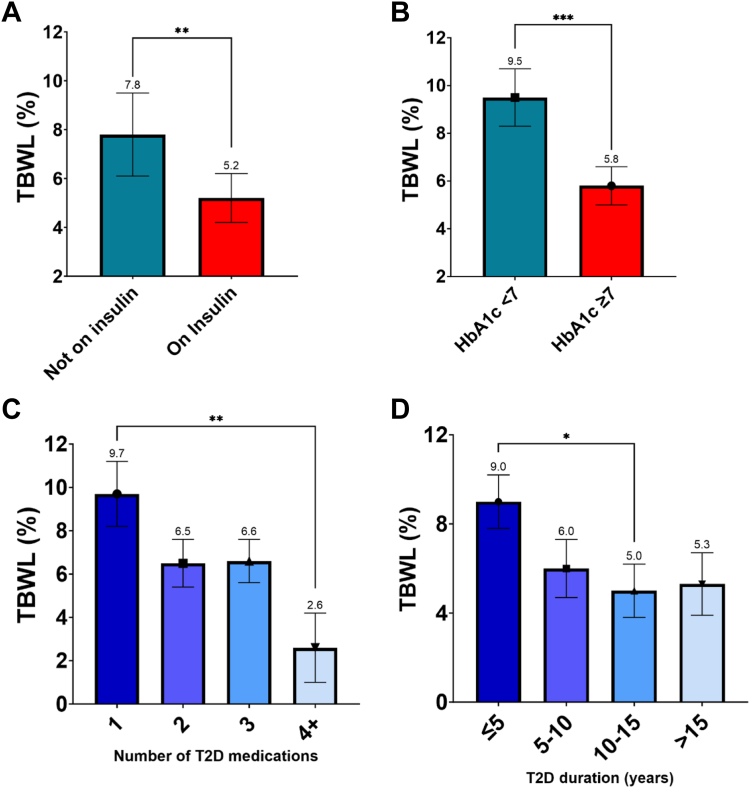
We evaluated weight loss outcomes of all IMS parameters at 12 months after adjusting for multiple covariates (Fig. 3). Patients on insulin had a lower TBWL (LS mean TBWL% ± SE) of 5.3 ± 1.0% when compared to 7.7 ± 0.9% in patients not on insulin (difference: −2.5, 95% CI: −4.4 to −0.6, p = 0.01). Patients with HbA1c ≥ 7 had lower TBWL of 5.8 ± 0.8% compared to 9.4 ± 1.2% in patients with HbA1c < 7 (difference: −3.6, 95% CI: −5.8 to −1.4, p = 0.002). Patients on one T2D medication had a TBWL of 9.7 ± 1.5% (n = 16) vs on 2 medications: 6.5 ± 1.0% (n = 55) vs on 3 medications: 6.6 ± 1.0% (n = 64) vs on ≥4 medications: 2.6 ± 1.6% (n = 15). Pairwise comparison demonstrated significant difference in TBWL% between patients on 1 T2D medication compared to ≥4 (difference: −7.1, 95% CI: −12.4 to −1.8, p = 0.004). Patients with a duration of T2D ≤ 5 years had a TBWL of 9.0 ± 1.2% (n = 32) vs 5–10 years: 6.0 ± 1.3% (n = 28) vs 10–15 years: 5.0 ± 1.2% (n = 36) vs > 15 years: 5.3 ± 1.1% (n = 54) (p = 0.004). Pairwise comparison demonstrated significant difference in TBWL% between patients with ≤5 years of T2D compared to those with 10–15 years (difference: −4.0, 95% CI: −8.0 to −0.04, p = 0.047). In the stepwise linear regression model to predict TBWL% at 12 months, using sex, depression, and use of insulin as variables, resulted in the best fit for the model (Table 5S). In regression models for TBWL% at 12 months including sex, age, baseline weight, and presence of hypertension, dyslipidemia and anxiety as covariates, the addition of use of insulin as a variable resulted in an adjusted R2 of 0.16, as compared to an adjusted R2 of 0.20 when adding the IMS numerical score as a variable (Table 6S). We conducted 3 separate logistic regression analyses with use of insulin as the endpoint and each IMS parameter as a variable). The resulting R2 (U) were 0.04 for HbA1C%, 0.17 for number of T2D medications, and 0.17 for T2D duration. T2D outcomes based on IMS severity categories.
Fig. 3.
A. Total body weight loss percentage (TBWL%) at 12 months in patient with/without insulin use, B. with HbA1c ≥ 7 vs HbA1c < 7, C. different number of type 2 diabetes (T2D) medications, and D. different duration of T2D. Least square (LS) means obtained from the linear regression models are presented in all panels. Whiskers represent standard error. For panel A and B, groups were compared with LS means Student's t tests. For panel C and D, we conducted pairwise comparisons using LS means Tukey's HSD tests. ∗ = p < 0.05, ∗∗ = p < 0.01, ∗∗∗ = p < 0.001. Non-significant pairwise comparisons are not shown.
In our cohort, multivariate linear regression models assessing for the change in fasting blood glucose and HbA1C from baseline to last follow-up demonstrated no significant difference between patients with different IMS severities, however, patients in the moderate category had numerically superior improvements in both glycemic parameters (Table 7S).
Discussion
To the best of our knowledge, this is the first multicentered study to assess the weight loss outcomes of semaglutide based on an innovative scoring system for diabetes severity in patients with T2D and overweight/obesity. In this study, we show that the weight loss outcomes of semaglutide, with doses of 1 mg or higher, decrease with an increased T2D severity. Although the TBWL% is associated with IMS score, the change in T2D parameters (i.e., fasting blood glucose and HbA1c) are not significantly affected by T2D severity. These results may contribute to a better understanding of the parameters that are associated with weight loss achieved by semaglutide in patients with T2D.
Multiple studies have demonstrated that patients with T2D experience inferior weight loss outcomes with semaglutide.7,9,10 This has been attributed to multiple factors, some are well-studied, while other are yet to be established. For instance, Overgaard et al. previously described that among patients with T2D, females and patients with lower HbA1c at baseline had superior weight loss outcomes in response to subcutaneous and oral semaglutide, with no apparent effect of concomitant medications, including insulin. In addition, they demonstrated that baseline weight affects the semaglutide exposure, with higher baseline weight resulting in lower exposure of circulating drug.13 In the IMS model, four parameters have been assessed to reflect T2D severity and remission after bariatric surgery.12 In this study, we hypothesized that the severity of T2D, represented by each of the IMS parameters, may reflect the weight loss outcomes associated with semaglutide.
One of the most commonly studied parameters that affect body weight in patients with T2D is the concomitant diabetes medications which may impede weight loss interventions and possibly result in weight gain. In fact, insulin, sulfonylureas, thiazolidinediones, and meglitinides are T2D medications that are known to result in weight gain.14 For example, in a systematic review and meta-analysis including 14,250 patients, insulin was associated with a dose-dependent mean body gain of 4.3 ± 2.74 kg upon a mean follow-up of 27.7 weeks.15 Similarly, we show a weight loss difference of 2.3% between patients on insulin compared to those not on this medication. This increase in body weight and/or hindrance for weight loss may be due a cumulative effect of several factors including the conservation of ingested calories with a better regulated glycemic level below the renal excretion threshold, inhibition of lipolysis and protein catabolism, stimulation of lipogenesis, impairment of the anorectic signals to the arcuate nucleus, and increased carbohydrate consumption to avoid hypoglycemia.14 This is supported by our observation that subjects with higher HgbA1c (>7%) had a similar benefit in glycemic control but experienced lower TBWL.
In addition, a higher baseline level of HbA1c, longer duration of T2D, and greater number of diabetes medications were associated with inferior weight loss outcomes in our study. This may be attributed to a more severe T2D, which is also associated with other complications such as microvascular complications, cardiovascular comorbidities (i.e., hypertension, peripheral artery disease) and other diseases that affect the body weight. For instance, patients with advanced T2D (i.e., less controlled [e.g., HbA1c > 7, longer duration]) are at higher risk of developing peripheral artery disease16 which may limit the activity level compared to patients without this disease.17 A limited activity level would contribute to a lower energy expenditure, resulting in a positive energy balance which ultimately causes weight gain.18 In addition, our study demonstrates that patients with higher IMS score (e.g., severe category) are older in age, which may also be influenced by a lower activity level and resting energy metabolism.19 This finding is expected as patients older in age are more likely to have a greater duration of T2D.
Although each of the multivariate linear regression models showed an association between each of the IMS parameters independently with weight loss outcomes of semaglutide, a stepwise model showed that use of insulin was the most informative factor to predict TBWL% at 12 months. The use of the numerical IMS score to predict TBWL% at 12 months led to an increase in the explained variance by 4% when compared to use of insulin. Importantly, both T2D duration and number of T2D medications were strongly correlated with use of insulin. This might be a result of collinearity among the parameters included in the IMS, however, having a scoring model that combines all these factors and establishes a unique score which is associated with weight loss outcomes of semaglutide can be of important value for clinical practice to set expectations in terms of weight loss. Although insulin use seems to be the most correlated variable with weight loss response, we have shown by the quartile IMS analysis that there is a stepwise decrease in weight loss with higher IMS score. This difference in weight loss cannot be simply explained by use of insulin alone. Hence, we believe that using the IMS score can improve the clinical practice by providing a comprehensive tool for physicians and patients to set expectations in terms of weight loss outcomes. For example, in patients using insulin, a greater duration of T2D may reflect an inferior weight loss compared to patients with a shorter duration of T2D. Importantly, it is essential to point out that the T2D outcomes (i.e., change in fasting blood glucose and HbA1c) are similar between all IMS severity scores. In fact, all patients with T2D benefit similarly from the effect of semaglutide on the glucose homeostasis regardless of T2D severity at baseline. This indicates that semaglutide is an effective treatment for all patients with diabetes, with enhanced weight loss effect in a specific group of people.
Our study has multiple strengths that would add to the aim of establishing predictors of response to AOMs (e.g., semaglutide). This study is the first of its nature to present a well-developed and validated score that potentially predicts weight loss outcomes in patient with T2D. The inclusion of a large sample size from different centers in the US add generalizability and power to our results.
There are several limitations to our current study. First, given the nature of the retrospective data collection, we had limited ability to abstract data on all the IMS parameters. Second, the majority of our patients were White with a higher proportion of male patients as opposed to most RCTs, limiting the ability to generalize the results to other external populations. Third, we had a significant decrease in the number of patients compared to our original cohort due to multiple reasons. Fourth, we have a small sample size in the mild IMS category, which make our analysis by quartile division a better analysis method to test our hypothesis. Importantly, we had a significant proportion of missing weight data at different timepoints, however, we believe that by conducting mixed linear models with repeated measures for the dependent variable we were able to better address for data missing at random.20 Finally, there is an increased susceptibility to coding errors and inaccurate documentation from EMR.
In this study, we show that IMS scoring system for T2D severity is associated with weight loss outcomes of semaglutide. A lower IMS severity reflects better weight loss outcomes in patients with obesity and T2D when taking semaglutide. Importantly, the inferior weight loss outcomes in patients with severe T2D must not hinder the use of this medication in this population. In fact, semaglutide is associated with significant cardiovascular and comorbidity improvement in patients with obesity including patients with severe T2D. Our findings could help clinicians set informed expectations for weight loss outcomes in patients with severe T2D taking semaglutide, however, it is likely that the cardiometabolic benefits associated with semaglutide treatment in this population far exceed the effect on weight loss. It is essential to apply this scoring model to previous and future randomized clinical trials to test its efficacy in predicting weight loss outcomes of semaglutide and other glucagon-like peptide-1 receptors agonists. In addition, mechanistic studies are needed to further understand the effect of T2D on weight loss outcomes of semaglutide.
Contributors
Wissam Ghusn: Conceptualization (equal), Data curation (lead). Formal Analysis (equal), Investigation (equal), Methodology (equal), Visualization (equal), Writing—Original Draft Preparation (lead), Writing—Review and Editing (equal). Diego Anazco: Conceptualization (equal), Data curation (equal). Formal Analysis (equal), Investigation (equal), Methodology (equal), Visualization (equal), Writing—Original Draft Preparation (equal), Writing—Review and Editing (equal). Wissam Ghusn and Diego Anazco contributed equally to the manuscript. Sima Fansa: Data curation (equal), Writing—Review and Editing (equal). Elif Tama: Data curation (equal), Writing—Review and Editing (equal). Lizeth Cifuentes: Data curation (equal), Writing—Review and Editing (equal). Khushboo Gala: Data curation (equal), Writing—Review and Editing (equal). Gerardo Calderon: Data curation (equal), Writing—Review and Editing (equal). Maria L. Collazo-Clavell: Supervision (equal), Writing—Review and Editing (equal). Maria D. Hurtado: Supervision (equal), Writing—Review and Editing (equal). Andres Acosta: Conceptualization (equal), Investigation (lead), Project Administration (lead), Resources (lead), Supervision (lead), Writing—Review and Editing (lead). Andres Acosta and Wissam Ghusn had access and verified the data underlying the data reported in the manuscript. All authors read and approved the final version of the manuscript.
Data sharing statement
The investigators will share deidentified data that underlies the results reported in this article after deidentification upon request by bona fide researchers who provide a methodologically appropriate proposal. Proposals should be directed to acosta.andres@mayo.edu. To gain access, data requestors will need to sign a data access agreement.
Declaration of interests
Dr Andres Acosta, and Mayo Clinic hold equity in Phenomix Sciences Inc. and are inventors of intellectual property licensed to Phenomix Sciences Inc. Dr Andres Acosta served as a consultant for Rhythm Pharmaceuticals, General Mills, Amgen, Bausch Health, RareStone; has contracts with Vivus Inc, Satiogen Pharmaceutical, and Rhythm Pharmaceutical.
Acknowledgements
Dr. Acosta is supported by the NIH (NIH K23-DK114460) and Mayo Clinic institutional grants.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102625.
Appendix A. Supplementary data
References
- 1.Chooi Y.C., Ding C., Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Apovian C.M. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22(7 Suppl):s176–s185. [PubMed] [Google Scholar]
- 3.Must A., Spadano J., Coakley E.H., Field A.E., Colditz G., Dietz W.H. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 4.Nianogo R.A., Arah O.A. Forecasting obesity and type 2 diabetes incidence and burden: the ViLA-obesity simulation model. Front Public Health. 2022;646 doi: 10.3389/fpubh.2022.818816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powell-Wiley T.M., Poirier P., Burke L.E., et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143(21):e984–e1010. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cifuentes L., Ghusn W., Feris F., et al. Phenotype tailored lifestyle intervention on weight loss and cardiometabolic risk factors in adults with obesity: a single-centre, non-randomised, proof-of-concept study. eClinicalMedicine. 2023;58 doi: 10.1016/j.eclinm.2023.101923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilding J.P.H., Batterham R.L., Calanna S., et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 8.Ghusn W., Ikemiya K., Al Annan K., et al. Diabetes mellitus remission in patients with BMI > 50 kg/m(2) after bariatric surgeries: a real-world multi-centered study. Obes Surg. 2023;33:1838. doi: 10.1007/s11695-023-06622-2. [DOI] [PubMed] [Google Scholar]
- 9.Davies M., Færch L., Jeppesen O.K., et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971–984. doi: 10.1016/S0140-6736(21)00213-0. [DOI] [PubMed] [Google Scholar]
- 10.Ghusn W., De la Rosa A., Sacoto D., et al. Weight loss outcomes associated with semaglutide treatment for patients with overweight or obesity. JAMA Netw Open. 2022;5(9):e2231982. doi: 10.1001/jamanetworkopen.2022.31982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wadden T.A., West D.S., Neiberg R.H., et al. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity. 2009;17(4):713–722. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aminian A., Brethauer S.A., Andalib A., et al. Individualized metabolic surgery score: procedure selection based on diabetes severity. Ann Surg. 2017;266(4):650–657. doi: 10.1097/SLA.0000000000002407. [DOI] [PubMed] [Google Scholar]
- 13.Overgaard R.V., Hertz C.L., Ingwersen S.H., Navarria A., Drucker D.J. Levels of circulating semaglutide determine reductions in HbA1c and body weight in people with type 2 diabetes. Cell Rep Med. 2021;2(9) doi: 10.1016/j.xcrm.2021.100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghusn W., Hurtado M.D., Acosta A. Weight-centric treatment of type 2 diabetes mellitus. Obesity Pillars. 2022;4 doi: 10.1016/j.obpill.2022.100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pontiroli A.E., Miele L., Morabito A. Increase of body weight during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta-analysis. Diabetes Obes Metab. 2011;13(11):1008–1019. doi: 10.1111/j.1463-1326.2011.01433.x. [DOI] [PubMed] [Google Scholar]
- 16.Marso S.P., Hiatt W.R. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47(5):921–929. doi: 10.1016/j.jacc.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 17.Gerage A.M., Correia M.A., Oliveira P.M.L., et al. Physical activity levels in peripheral artery disease patients. Arq Bras Cardiol. 2019;113(3):410–416. doi: 10.5935/abc.20190142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill J.O., Wyatt H.R., Peters J.C. Energy balance and obesity. Circulation. 2012;126(1):126–132. doi: 10.1161/CIRCULATIONAHA.111.087213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts S.B., Rosenberg I. Nutrition and aging: changes in the regulation of energy metabolism with aging. Physiol Rev. 2006;86(2):651–667. doi: 10.1152/physrev.00019.2005. [DOI] [PubMed] [Google Scholar]
- 20.Faria R., Gomes M., Epstein D., White I.R. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. Pharmacoeconomics. 2014;32(12):1157–1170. doi: 10.1007/s40273-014-0193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.