Abstract
The unique aromas of mutton stem from the chemical reactions between the characteristic precursors during cooking. This study aimed to establish the relationship of volatile compounds and aroma precursors (protein, fat, free amino acids and fatty acids) in lamb from different breeds and muscle types. Hong lamb was characterized by greater tenderness and water holding capacity, higher polyunsaturated fatty acids and higher essential/non-essential amino acids in comparison with Hu lamb. Aldehydes, such as heptanal, hexanal, octanal and nonanal were higher in Hong-ST compared with Hu-ST. Principal component analysis (PCA) showed that aroma precursors were closely related to volatile components of cooked lamb. Discriminant analysis results showed that precursors and volatile compounds could be used to identify the breeds and muscle types of lamb. These findings revealed the contributors of lamb aroma and might help understand the regulatory mechanism of aroma in lamb from different breeds and muscle types.
Keywords: Lamb breed, Muscle type, Volatile compounds, Aroma precursors
Highlights
-
•
A novel sheep source, Pishan Hong lamb was developed and characterized.
-
•
Hong lamb had lower IMF but higher PUFA proportions than Hu lamb.
-
•
Aroma precursors were closely related to volatile components of cooked mutton.
-
•
Lamb breeds could be discriminated by precursors and volatile compounds.
1. Introduction
Lamb accounts for a large proportion of the meat market and is an important source of protein for human. China is competent in lamb production and consumption, with the national mutton output in 2022 estimated to be approximately 5.25 million metric tons. Chinese customers have a significant demand for lamb with improved nutritional organoleptic qualities due to economic growth (Wang et al., 2023). Therefore, breed evaluation trials are necessary for the efficient development of genetic lamb resources in order to fulfill the increasing expectations of consumers (Shackelford, Leymaster, Wheeler, & Koohmaraie, 2012). Xinjiang province is an important pillar in Chinese husbandry due to the region's advantageous geographical location. Numerous new lamb genotypes have lately been gestated here (Dawut & Tian, 2021). Pishan is located in southern Xinjiang province with unique geographical location and natural pasture, making it exceptional from the ordinaries. Hong lamb is an excellent breed of lamb bred in Pishan county. Hong lamb has the characteristics of drought resistance, cold resistance and rough feeding resistance. In 2023, Pishan Hong lamb was officially approved by the National livestock and Poultry Genetic Resources Committee. Although public interests in Hong lamb have developed due to its superior meat quality and sensory characteristics, few studies have been conducted to characterize its quality, nutritional functions and flavors. Hu lamb is a unique breed in Taihu Lake Plain in China, which has been listed in the national animal genetic resources protection list. Hu lamb is widely favored by farmers because of its strong adaptability, fast growth and high lambing rate. Hu lamb has many similar traits compared with Hong lamb. At present, there are few reports about the identification and characterization of Hong lamb and Hu lamb.
Aroma is one of the important factors that determine the sensory characteristics and commodity attributes of mutton (Liu et al., 2022). Protein, fat, amino acids and fatty acids in raw meat have been shown to be precursors to the aroma of cooked meat (Watkins, Frank, Singh, Young, & Warner, 2013). Hexanal and nonanal generated by these precursors through lipid oxidation, degradation and Maillard reaction are crucial odorants in cooked mutton (Liu et al., 2022). Previous studies reported that aroma compounds were associated with linoleic oxidation and intramuscular fat content (Benet et al., 2016). Li et al. (2022) reported that 10 odorants in cooked Hu lamb were derived from lipid oxidation. Higher n-3 polyunsaturated fatty acids (PUFAs) would also influence the lipid oxidation during cooking, which determines the generation of off-flavors. As a result, these precursors are critical for the aroma compounds of lamb meat. These precursors compositions vary according to numerous factors, especially breed and muscle type are as the most important intrinsic factors (Zhao et al., 2022). Thus, it is of great significance to study the flavor substance and sources of different breeds and muscle types of mutton.
Currently, the volatile compounds of mutton related to different processing techniques have been fully analyzed (Liu et al., 2022; Qi et al., 2022). Aldehydes and alcohols, such as hexanal, octanal, nonanal and 1-octen-3-ol were the major aroma compounds in meat products (Zhao et al., 2022). The breakdown of linoleic acid and α-linolenic acid was essential for the formation of aldehydes and alcohols. Lipid hydrolysis generate free fatty acids, which were further oxidized to generate hexanal and nonanal (Wu et al., 2023). Additionally, 3-Methylbutanal, generated by the Maillard reaction, has been reported as a major contributor to the aroma of mutton (Wu et al., 2023). Aroma precursors were mainly affected by breeds and processing technologies (Liu et al., 2022). There were studies to distinguish volatile compounds and identify the volatiles as markers (Zhao et al., 2023, Zhao et al., 2023). However, the aroma precursors of lamb and the sources of typical volatile have rarely been studied.
In the study, quality traits, aroma precursors and volatile compounds of mutton from different breeds (Hong lamb and Hu lamb) and muscle types (Longissimus thoracis et lumborum and Semitendinosus) were compared. It is hypothesized that aroma precursors of different breeds and muscle types of lamb affect volatile compounds in cooked lamb. The objectives of this study were to (1) analyze the aroma precursors of Hong lamb and Hu lamb; (2) characterize the volatile flavor compounds of cooked mutton; (3) clarify the relations of aroma precursors and volatile flavor compounds.
2. Materials and methods
All animal procedures performed in this study were approved by the Xinjiang Uygur Autonomous Region Animal Husbandry and Veterinary Bureau (Any65322301).
2.1. Sample collection and preparation
Hong lamb (short-tailed, n = 36, male, slaughter age of 180 days, slaughter weight of 47.5 ± 2.5 kg) and Hu lamb (fat-tailed, n = 36, male, slaughter age of 180 days, slaughter weight of 47.5 ± 2.5 kg) were randomly selected from Xinjiang Muyangren Animal Husbandry Technology Co. LTD (Pishan, Xinjiang). Hu lamb was the most common lamb breed in China, and it was used as a comparison group in our study. Hong and Hu lamb were raised in the same Muyangren Animal Husbandry farm and treated with the same diet. After weaning, the lamb was indoor-fed with 500 g of concentrate per day and 500 g of hay until the slaughtering (180 days of age). The whole experiment was divided into 6 batches with 6 lamb per batch. The same breeds of lamb of each batch came from the same pen. Before slaughtering, the lamb was fasted for 12 h. The lamb was slaughtered by the slaughterhouse staff according to the standard procedures of a commercial slaughterhouse. After slaughtering, the carcass was stored at 0–4 °C for 24 h. Longissimus thoracis et lumborum (LTL) muscle and Semitendinosus (ST) muscles were removed from both halves of the carcass. Raw lamb samples were transported to the laboratory with vacuum-sealed packages at −80 °C. The lamb samples were thawed at 4 °C, then visible fat and connective issues were removed. Half of the raw muscles were minced and conserved in vacuum-sealed packages for meat quality (pH, color parameters) and chemical components analysis. The remaining half raw muscles were cooked for shear force, cooking loss and volatile compounds analysis. Regarding to cooking procedure, approximately 200 g of raw lamb samples (4 × 4 × 5 cm3) were boiled with 1% NaCl and in 150% water. A thermocouple was used to monitor the temperature at the center of the sample. After the core temperature reached 75.0 ± 0.5 °C, then it was kept for 30 min. After cooking, half of the cooked mutton was used to determine shear force and cooking loss. The remaining half cooked mutton was diced into cubes measuring 1.0 × 1.0 × 1.0 cm3 in size, pulverized in liquid nitrogen and stored in vacuum-sealed packages at −80 °C until using for measurement of volatile compounds.
2.2. Meat quality analysis
2.2.1. pH value, color parameters
All parameters were measured six times for each raw lamb muscle. The pH value of raw lamb samples (after 24 h slaughtering) was measured using a calibrated pH meter (Testo 205, Testo SE & Co, KGaA, Neustadt, Germany). The color was evaluated on the surface of a 1 cm thick slice with a color meter (Chroma Meter CR-400, Osaka, Japan) using an 8 mm diameter aperture, illumonant D 65, and 2° standard observer. In addition, the color device was calibrated using a white tile (C: Y = 93.6, x = 0.3130, y = 0.3193), and the values on the surface of each slice were measured. Results were expressed as L⁎ (lightness), a⁎ (redness), and b⁎ (yellowness).
2.2.2. Shear force and cooking loss
Lamb samples were cooked to a core temperature of 75 °C, then kept for 30 min. The cooked lamb samples were sliced perpendicular to the fibers to a size of 1 × 2 × 4 cm3. The shear force of the sample was measured using a TAXT2 texture analyzer (Stable Micro Systems, Godalming, UK) with a knife blade (code HDP/BS, Stable Micro Systems). The cross-head speed was 2 mm/s with a distance of 30 mm.
Cooking loss was determined according to the method reported by Song et al. (2021). Samples were wiped to remove moisture from their surface and weighed back to determine the final weight. The percentage of cooking loss was calculated by this equation:
2.3. Proximate compositions
Proximate compositions, including moisture, intramuscular fat (IMF) and protein, were determined according to the procedures reported by Si et al. (2022) with minor adjustments. Before testing, raw lamb samples were thawed at 4 °C for 24 h. The moisture was measured based on the Method for determination of moisture content in foods in GB 5009.3–2016 (China). The protein content was determined based on the Method for determination of protein content in foods in GB 5009.5–2016 (China) by using the Kjeldahl method. The IMF was determined based on the Method for determination of fat content in foods in GB 5009.6–2016 (China) by conducting the Soxhlet extraction technique.
2.4. Free amino acids (FAA)
FAA compositions were analyzed using an amino acid analyzer (L8900, HITACHI, Tokyo, Japan) in accordance with Zhao et al., 2023, Zhao et al., 2023 with minor modification. Two grams of raw lamb meat samples were weighed and homogenized 30 s with 20 mL of 0.02 mol/L diluted hydrochloric acid. After ultrasonic extraction for 5 min, the samples were centrifuged at 4000 ×g for 10 min at 4 °C. The supernatant was diluted to 50 mL with 0.02 mol/L hydrochloric acid. The supernatant (2 mL) was mixed with 7% sulfosalicylic acid solution (v/v) of 2 mL, the mixture was centrifuge at 12,000 ×g for 10 min at 4 °C. The supernatant was filtered by 0.22 μm microporous membrane.
2.5. Fatty acids
Briefly, the total lipids of raw lamb muscles were extracted by the mixture of chloroform and methanol. To determine the compositions of fatty acid, gas chromatography was utilized. The extracted lipid was mixed with 8 mL of 2% methanol-NaOH, and heated at 80 °C until it dissolved. Then, 7 mL of 15% BF3 was combined with the mixture, and it was immersed back in an 80 °C water bath for 2 min. After cooling to room temperature, 20 mL of hexane was added to the system and shaken for 2 min before the NaCl solution (saturated) was added. The system consisted of three stages, and the third phase was extracted for analysis. The gas chromatograph apparatus (7890B, Agilent, Palo Alto, CA, USA) was equipped with flame-ionization detection (FID) detector (7890, Agilent Intuvo, CA, USA) and the column (100 m × 0.25 mm × 0.2 μm; CD-2560, Agilent Technologies, CA, USA). The chromatographic model was heated to 150 °C for 5 min, then increased to 240 °C at a rate of 4 °C/min for 28.5 min. The detector temperature was 260 °C, the column inlet temperature was 250 °C and 50:1 was the split ratio. Fatty acid concentrations were calculated by the chromatographic peak area (Wu, Zhan, Tang, Li, & Duan, 2022). The results were expressed as percentages of each fatty acid of total fatty acids.
2.6. Volatile compounds
2.6.1. Headspace solid-phase microextraction (HS-SPME)
The volatile compounds of cooked lamb muscles were extracted by headspace solid-phase microextraction (HS-SPME). According to Zhang, Zhang, Liu, Zhao, and Luo (2020) with minor modification, 2 g of cooked lamb sample was placed into a 20 mL glass sampling vial with adding 1 μL 2-methyl-3-heptanone (0.408 μmol/L, in methanol) as internal standard. The sample was heated at 60 °C for 20 min for equilibrium. Subsequently, the 50/30 μm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS; 2 cm) coating fiber (Supelco, Inc., Bellefonte, PA, USA) was exposed to the vial for 40 min to extract the volatile compounds of lamb meat.
2.6.2. Gas chromatography-mass spectrometry (GC–MS)
A gas chromatograph-mass spectrometer (GC–MS, QP 2010 ultra, Shimadzu, Tokyo, Japan) was applied for volatile compound identification as modified by Xiang et al. (2021). The compound separation was conducted on a DB-WAX column (30 m × 0.25 mm × 0.25 μm; Agilent Technologies, CA, USA). The carrier was helium with a flow rate of 1 mL/min. The temperature program was at an initial temperature of 40 °C for 3 min, followed by a ramp of 5 °C/min to 90 °C, then rising to 230 °C at a rate of 8 °C/min and holding for 10 min. The mass spectrometer was operated in electron ionization mode, with an ionising voltage of 70 eV, a source temperature of 230 °C, and a scan range of 35 to 500 m/z.
The volatile compounds were identified by comparing the mass spectra with spectra from NIST 17 Mass Spectral Database and calculating the retention index (RX). The n-alkane (C7-C26) concentration was 250 μg/mL.
2.7. Statistical analysis
The results were summarized as mean values ± standard deviation (SD). SPSS software 22.0 (IBM, Armonk, NY, USA) was used to perform full factorial ANOVA with the general linear model (GLM) for investigating the effects of breed and muscle on all characteristics. Breeds and muscle types as fixed factors, batch as random factors. Significant differences between breeds or muscle types were conducted by t-test that had a significance level of P value <0.05.
3. Results
3.1. Meat quality
The meat quality characteristics of ST and LTL muscles for Hu and Hong lamb are shown in Table 1. The pH values at 24 h after slaughtering ranged from 5.65 to 5.88, with Hong-LTL muscle having the highest pH value. Breed had no significant effect on pH24, only the values in ST muscles were slightly lower than those in LTL muscles. Besides, regarding the color index, the influence of breed and muscle type on the L⁎ (lightness) were not significant. The Hong-ST muscle displayed significantly (P < 0.05) higher a⁎ (redness, 23.83 ± 4.46) and b⁎ (yellowness, 19.72 ± 3.78) values compared to other muscle samples, indicating its intense reddish and yellowish coloration.
Table 1.
Meat quality of Semitendinosus (ST) and Longissimus thoracis et lumborum (LTL) muscles for Hu and Hong lamb.
| Traits | Breed (B) and muscle (M) |
Significance (P value) |
|||||
|---|---|---|---|---|---|---|---|
| ST |
LTL |
||||||
| Hu | Hong | Hu | Hong | PBreed | PMuscle | PInteraction | |
| pH24 | a,x5.71 ± 0.06 | a,y5.65 ± 0.05 | a,x5.75 ± 0.15 | a,x5.88 ± 0.15 | ns | ** | *** |
| Cooking loss (%) | a,x36.26 ± 1.39 | a,x37.41 ± 1.48 | b,y28.65 ± 0.12 | b,y31.69 ± 1.96 | ns | *** | *** |
| Shear force (N) | a,x45.10 ± 10.87 | a,x32.89 ± 5.72 | b,y36.61 ± 8.45 | b,y25.51 ± 4.57 | *** | * | ns |
| Color parameters | |||||||
| L⁎ | a,x39.51 ± 2.04 | a,x43.96 ± 4.50 | a,x41.47 ± 3.36 | a,x39.65 ± 3.32 | ns | ns | ns |
| a⁎ | b,x15.32 ± 3.21 | a,x23.83 ± 4.46 | a,x14.03 ± 1.91 | a,y13.87 ± 2.51 | *** | *** | *** |
| b⁎ | b,x13.48 ± 2.10 | a,x19.72 ± 3.78 | a,x13.48 ± 1.89 | a,y11.93 ± 2.37 | ** | *** | *** |
a,bThe variables with different letters indicate differences between breed in same muscle type; x,yThe variables with different letters indicate differences between muscle type in same breed. ***, P < 0.001; **, P < 0.01; *, P < 0.05; ns, not significant.
In addition, drip loss and shear force had similar tendencies, which demonstrated significant (P < 0.05) differences between breeds and muscle types. The drip loss ranged from 1.79 to 9.33%, and shear force varied from 25.51 to 45.10 N. Hong lamb had lower drip loss and shear force compared to Hu lamb, especially the Hong-LTL revealed the lowest values, indicating the tenderness and water-holding capacity (WHC) of Hong lamb were better.
3.2. Chemical compositions
The chemical compositions including moisture content, intramuscular fat (IMF), protein content and total cholesterol content in different muscles for Hu and Hong lambs are shown in Table 2. Breed had significant (P < 0.05) effects on proximate compositions, with Hong lamb having lower water, whereas higher protein content. The breed variations for moisture and protein contents were mainly reflected in ST muscles. The highest protein content (22.75 ± 0.36%) was displayed in the Hong-ST muscle. However, the effect of muscle type was not obvious for these parameters in our study.
Table 2.
The main effects of lamb breeds (Hu, Hong) and muscle types (ST, LTL) on chemical compositions.
| Chemical compositions | Breed |
PBreed | Muscle types |
PMuscle | PInteraction | ||
|---|---|---|---|---|---|---|---|
| Hu | Hong | ST | LTL | ||||
| Moisture (%) | a75.58 ± 0.11 | b75.09 ± 0.12 | * | 75.48 ± 0.12 | 75.19 ± 0.11 | NS | NS |
| IMF (%) | 2.29 ± 0.22 | 1.97 ± 0.22 | NS | 2.17 ± 0.20 | 2.09 ± 0.24 | NS | NS |
| Protein (%) | b21.33 ± 0.16 | a21.91 ± 0.21 | * | x21.95 ± 0.15 | y21.29 ± 0.22 | * | NS |
Data were expressed as means ± standard errors. a,bThe variables with different letters indicate differences between breed; x,yThe variables with different letters indicate differences between muscle types. NS, not significant, ***, P < 0.001; **, P < 0.010; *, P < 0.050.
3.3. Free amino acids (FAAs)
Table 3 shows the free amino acids (FAAs) contents in ST and LTL muscles of Hong and Hu lambs. Thr was the most abundant amino acid in lamb muscles, ranging from 73.98 to 78.28 mg/100 g, followed by His (37.22–46.59 mg/100 g), Asp (28.92–51.04 mg/100 g), Arg (28.30–47.74 mg/100 g), Glu (18.92–28.86 mg/100 g) and Ser (7.06–38.33 mg/100 g). Breed had no effect on most free amino acids except for Thr, Leu and Lys. Muscle type had a significant effect on all free amino acids (P < 0.05). All amino acids were categorized as essential amino acids (EAAs) and non-essential amino acids (NAAs). Thr and His were the main EAAs in lamb, and Ser as well as Asp were the main NAAs. The EAAs/NAAs ratio for lamb muscles ranged from 0.83 to 0.90 in our study.
Table 3.
The main effects of lamb breeds (Hu, Hong) and muscle types (ST, LTL) on free amino acid compositions.
| Free amino acids (mg/100 g) | Breed |
PBreed | Muscle types |
PMuscle | PInteraction | |||
|---|---|---|---|---|---|---|---|---|
| Hu | Hong | ST | LTL | |||||
| EAAs | Thr | 73.98 ± 1.91 | 78.28 ± 4.09 | ** | 78.14 ± 4.46 | 74.12 ± 1.29 | ** | *** |
| Val | 1.72 ± 0.91 | 2.05 ± 1.27 | NS | 2.95 ± 0.48 | 0.82 ± 0.12 | *** | ** | |
| Met | 1.62 ± 1.18 | 2.16 ± 1.61 | NS | 3.27 ± 0.55 | 0.51 ± 0.07 | *** | *** | |
| Ile | 4.25 ± 1.55 | 4.79 ± 1.64 | NS | 2.97 ± 0.40 | 6.07 ± 0.51 | *** | NS | |
| Leu | 4.11 ± 0.93 | 4.93 ± 0.63 | * | 5.24 ± 0.32 | 3.79 ± 0.66 | *** | ** | |
| Phe | 2.77 ± 1.60 | 3.47 ± 1.26 | NS | 4.52 ± 0.45 | 1.72 ± 0.52 | *** | * | |
| Lys | 4.41 ± 0.44 | 5.03 ± 0.43 | ** | 5.02 ± 0.49 | 4.42 ± 0.38 | ** | NS | |
| His | 41.11 ± 4.30 | 42.7 ± 5.20 | NS | 46.59 ± 1.54 | 37.22 ± 0.81 | *** | * | |
| Total EAAs | 133.97 ± 7.84 | 143.40 ± 12.60 | NS | 148.70 ± 7.46 | 128.67 ± 2.91 | *** | *** | |
| NAAs | Asp | 39.11 ± 11.19 | 40.85 ± 11.17 | NS | 51.04 ± 1.48 | 28.92 ± 2.19 | *** | NS |
| Ser | 22.26 ± 15.42 | 23.12 ± 15.86 | NS | 38.33 ± 0.79 | 7.06 ± 0.38 | *** | * | |
| Glu | 23.31 ± 5.85 | 24.47 ± 5.34 | NS | 28.86 ± 3.70 | 18.92 ± 0.43 | *** | NS | |
| Pro | 3.40 ± 1.54 | 3.63 ± 1.65 | NS | 1.94 ± 0.16 | 5.09 ± 0.36 | *** | NS | |
| Gly | 6.22 ± 2.77 | 6.92 ± 3.19 | NS | 3.62 ± 0.26 | 9.52 ± 0.76 | *** | * | |
| Ala | 19.70 ± 2.60 | 21.01 ± 3.16 | NS | 23.21 ± 1.05 | 17.51 ± 0.48 | *** | * | |
| Tyr | 6.26 ± 1.56 | 7.03 ± 1.91 | NS | 4.94 ± 0.43 | 8.34 ± 0.65 | *** | * | |
| Arg | 37.07 ± 10.59 | 38.97 ± 9.46 | NS | 28.30 ± 3.4 | 47.74 ± 1.68 | *** | NS | |
| Total NAAs | 157.32 ± 18.91 | 166.01 ± 19.58 | NS | 180.23 ± 8.24 | 143.10 ± 4.58 | *** | NS | |
| EAAs/NAAs | 0.86 ± 0.06 | 0.87 ± 0.03 | NS | 0.83 ± 0.03 | 0.90 ± 0.02 | *** | NS | |
Data were expressed as means ± standard errors. EAAs, essential amino acids; NAAs, non-essential amino acids. a,bThe variables with different letters indicate differences between breed; x,yThe variables with different letters indicate differences between muscle types. NS, not significant, ***, P < 0.001; **, P < 0.010; *, P < 0.050.
3.4. Fatty acids (FAs)
Among the fatty acids (FAs) assayed in different muscles of Hong and Hu lamb (Table 4), total monounsaturated fatty acids (MUFAs) are the most dominant proportion (43.78–45.53%), followed by total saturated fatty acids (SFAs) and polyunsaturated fatty acids (PUFAs). Breed had effect on SFAs and PUFAs. Hong lamb had lower SFAs and higher PUFAs compared to Hu lamb, and the ST muscles had lower PUFAs compared to LTL muscles.
Table 4.
The main effects of lamb breeds (Hu, Hong) and muscle types (ST, LTL) on fatty acid compositions.
| Fatty acids (% of total fatty acids) | Breed |
PBreed | Muscle types |
PMuscle | PInteraction | |||
|---|---|---|---|---|---|---|---|---|
| Hu | Hong | ST | LTL | |||||
| SFAs | C14:0 | 2.18 ± 0.07 | 2.07 ± 0.07 | NS | 2.19 ± 0.07 | 2.05 ± 0.08 | NS | NS |
| C15:0 | a0.66 ± 0.06 | b0.42 ± 0.06 | ** | 0.58 ± 0.05 | 0.50 ± 0.07 | NS | NS | |
| C16:0 | 22.47 ± 0.18 | 22.43 ± 0.18 | NS | x22.98 ± 0.16 | y21.91 ± 0.20 | *** | ** | |
| C17:0 | a2.61 ± 0.20 | b1.65 ± 0.20 | ** | 2.15 ± 0.18 | 2.11 ± 0.22 | NS | NS | |
| C18:0 | b12.79 ± 0.21 | a13.97 ± 0.21 | ** | y12.59 ± 0.19 | x14.18 ± 0.23 | *** | NS | |
| C23:0 | a3.17 ± 0.14 | b2.66 ± 0.14 | * | x3.27 ± 0.13 | y2.56 ± 0.16 | ** | ** | |
| Total SFAs | 43.88 ± 0.40 | 43.20 ± 0.40 | NS | 43.76 ± 0.36 | 43.32 ± 0.44 | NS | NS | |
| MUFAs | C16:1 | 2.28 ± 0.07 | 2.23 ± 0.07 | NS | 2.27 ± 0.07 | 2.24 ± 0.08 | NS | NS |
| C17:1 | a1.77 ± 0.07 | b1.20 ± 0.07 | *** | 1.54 ± 0.07 | 1.43 ± 0.08 | NS | NS | |
| C18:1n9t | a4.76 ± 0.25 | b3.70 ± 0.25 | * | 4.53 ± 0.23 | 3.93 ± 0.28 | NS | NS | |
| C18:1n9c | 35.85 ± 0.89 | 35.58 ± 0.89 | NS | 36.93 ± 0.80 | 36.50 ± 0.98 | NS | NS | |
| Total MUFAs | 44.66 ± 0.72 | 44.72 ± 0.72 | NS | 45.27 ± 0.64 | 44.10 ± 0.79 | NS | NS | |
| PUFAs | C18:2n6c | 11.17 ± 0.42 | 11.00 ± 0.42 | NS | 10.80 ± 0.38 | 11.37 ± 0.46 | NS | NS |
| C18:3n3 | 0.14 ± 0.03 | 0.04 ± 0.03 | NS | 0.08 ± 0.03 | 0.10 ± 0.04 | NS | NS | |
| C20:3n6 | 0.15 ± 0.03 | 0.15 ± 0.03 | NS | y0.09 ± 0.03 | x0.21 ± 0.04 | * | ** | |
| C20:4n6 | N.D. | a0.98 ± 0.01 | *** | N.D. | x0.98 ± 0.01 | *** | *** | |
| Total PUFAs | 11.46 ± 0.37 | 12.17 ± 0.37 | NS | y10.97 ± 0.33 | x12.67 ± 0.41 | ** | NS | |
| Total USFAs | 56.11 ± 0.41 | 56.89 ± 0.41 | NS | 56.24 ± 0.37 | 56.77 ± 0.45 | NS | NS | |
| PUFAs/SFAs | 0.26 ± 0.01 | 0.27 ± 0.01 | NS | y0.24 ± 0.01 | x0.29 ± 0.01 | ** | NS | |
Data were expressed as means ± standard errors. SFAs, saturated fatty acids; MUFAs, monounsaturated fatty acids; PUFAs, polyunsaturated fatty acids; USFAs, unsaturated fatty acids. N.D., not detected. a,bThe variables with different letters indicate differences between breed; x,yThe variables with different letters indicate differences between muscle types. NS, not significant. ***, P < 0.001; **, P < 0.010; *, P < 0.050.
In SFAs, palmitic acid (C16:0) and stearic acid (C18:0) were the most abundant ones in lamb muscles. Hong lamb had significantly (P < 0.05) higher C18:0 in LTL muscles. Moreover, the MUFAs were not significantly different between breed and muscle type. Oleic acid (C18:1n9c) accounted for the largest part of MUFAs, ranging from 35.70% to 37.85% of total fatty acids. Linoleic acid (C18:2n6c) was the most abundant PUFAs in lamb muscles, whereas no effect of breed and muscle type of this PUFA was found in our research. Linoleic acid was the only PUFAs in Hong-ST muscle. For other PUFAs in Hong-LTL muscle, cis-8,11,14-Eicosatrienoic acid (C20:3n6) showed the highest percentages and arachidonic acid (C20:4n6) was only identified in it. However, lower α-linolenic acid (C18:3n3) was found in Hong lamb compared to Hu lamb. Furthermore, the PUFAs/SFAs (P/S) ratio of lamb samples ranged from 0.24 to 0.30. No significant differences were observed for this ratio between breed and muscle type.
3.5. Volatile compounds
The volatile compounds are identified in ST and LTL muscles of boiled Hong and Hu lamb muscles by gas chromatography–mass spectrometry (GC–MS), as shown in Table 5. These volatile compounds were categorized into five groups, including 18 aldehydes, 9 alcohols, 4 acids, 3 ketones and 1 furan. Under the influence of breed and muscle type, the majority of volatile compounds were significantly different (P < 0.05).
Table 5.
Volatile compounds of Longissimus thoracis et lumborum (LTL) and Semitendinosus (ST) muscles for Hu and Hong lamb.
| Volatile compounds | RI | 1RI | Concentrations (μg/kg) |
2Threshold (μg/kg) | 3Odor description | Significance (P value) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Breed (B) and muscle (M) | |||||||||||
| ST |
LTL |
||||||||||
| Hu | Hong | Hu | Hong | B | M | B × M | |||||
| Aldehydes (18) | |||||||||||
| Hexanal | 1079 | 1081 | b,y177.3 ± 13.4 | a,y430.6 ± 48.6 | a,x516.4 ± 54.7 | a,x545.5 ± 76.7 | 5 | Green, fresh, grass | *** | *** | *** |
| Heptanal | 1154 | 1175 | b,y684.4 ± 77.1 | a,x7186. ± 1102.1 | a,x3173.1 ± 303.0 | b,y2229.5 ± 354.1 | 2.8 | Fatty, aldehyde | *** | *** | *** |
| Octanal | 1268 | 1287 | b,y224.8 ± 16.1 | a,x803.9 ± 92.4 | a,x702.2 ± 109.0 | b,y528.2 ± 105.7 | 0.59 | Citrus, floral, lemon | *** | ** | *** |
| Nonanal | 1398 | 1392 | b,y850.5 ± 350.8 | a,x2596.5 ± 279.4 | a,x1346.8 ± 770.0 | b,y1171.7 ± 241.8 | 1.1 | Citrus, floral, green | *** | ** | *** |
| Decanal | 1493 | 1494 | b,y217.3 ± 15.5 | a,x770.5 ± 76.2 | a,x1198.6 ± 277.8 | b,y522.2 ± 118.1 | 3 | Fatty, rancid, meaty | ** | *** | *** |
| Dodecanal | 1715 | 1708 | a,x239.8 ± 40.6 | N.D. | N.D. | N.D. | 0.13–0.29 | Lily, fatty, citrus | – | – | – |
| Tridecanal | 1825 | 1812 | b,y33.1 ± 7.4 | a,x99.7 ± 7.8 | a,x133.3 ± 29.4 | b,y55.1 ± 15.2 | 10 | Fatty, sweet | ns | ** | *** |
| Tetradecanal | 1907 | 1914 | b,y76.0 ± 23.3 | a,x397.6 ± 126.0 | a,x320.6 ± 60.6 | b,x248.1 ± 85.7 | 110 | Roasted, fried | ** | ns | ** |
| Pentadecanal | 2015 | 2025 | b,y307.6 ± 33.0 | a,x1391.8 ± 144.6 | a,x812.5 ± 160.2 | a,y999.9 ± 296.6 | 1000 | Fresh | *** | ns | *** |
| 4-Methoxy-benzaldehyde | 2024 | 2016 | b,y40.8 ± 3.4 | a,x84.9 ± 13.9 | a,x103.1 ± 14.6 | b,y50.4 ± 12.9 | 123.23 | Fruity | ns | * | *** |
| (E)-2-Heptanal | 1305 | 1331 | N.D. | a,x29.1 ± 8.1 | N.D. | N.D. | 40 | Fatty | – | – | – |
| (E)-2-Nonenal | 1528 | 1517 | a,y85.1 ± 28.3 | a,x68.8 ± 13.7 | a,x143.5 ± 53.2 | b,y48.2 ± 8.7 | 0.19 | Fatty, paper | *** | ns | ** |
| (E)-4-Decenal | 1449 | 1442 | a,y16.3 ± 2.0 | N.D. | a,x27.8 ± 5.4 | b,x19.9 ± 1.8 | 0.004 | Fruity, sweet, heavy | *** | *** | – |
| (E)-2-Octenal | 1410 | 1408 | b,y39.8 ± 12.8 | a,x137.2 ± 17.3 | a,x140.7 ± 59.6 | b,y71.2 ± 16.2 | 3 | Green, floral | * | * | ns |
| (E,E)-2,4-Decadienal | 1793 | 1811 | N.D. | a,x244.7 ± 38.8 | a,x332.8 ± 19.8 | b,x219.3 ± 55.7 | 0.027 | Plastic, tailing odor | *** | ns | – |
| (E)-2-Undecenal | 1743 | 1747 | b,y123.0 ± 3.5 | a,x412.9 ± 59.6 | a,x352.8 ± 63.7 | b,y264.7 ± 61.1 | 0.78 | Orange, green | *** | * | *** |
| 2-Butyl-2-octenal | 1685 | 1659 | a,x167.6 ± 46.5 | b,x101.6 ± 16.8 | N.D. | a,x120.4 ± 33.6 | 20 | Cured ham, meaty | ns | *** | – |
| 2-Methylbutanal | 888 | 910 | N.D. | N.D. | N.D. | a,x49.2 ± 14.3 | 1.0 | chocolate, bitter | – | – | – |
| Alcohols (9) | |||||||||||
| 1-Pentanol | 1274 | 1245 | b,y78.6 ± 13.1 | a,x356.6 ± 71.7 | a,x264.6 ± 90.7 | b,y198.0 ± 40.2 | 150 | Green, fruity | *** | ns | *** |
| 1-Hexanol | 1376 | 1353 | b,y37.7 ± 6.4 | a,x93.2 ± 22.7 | a,x86.4 ± 44.9 | b,y51.1 ± 12.2 | 5.6 | Green | ns | ns | ns |
| 1-Octen-3-ol | 1461 | 1443 | b,y290.3 ± 65.2 | a,y844.0 ± 126.1 | a,x1026.5 ± 336.9 | a,x1267.6 ± 92.1 | 1.5 | Mushroom | *** | * | *** |
| 1-Heptanol | 1477 | 1465 | b,y56.4 ± 16.9 | a,x135.2 ± 19.6 | a,x137.1 ± 49.1 | b,y86.8 ± 15.4 | 5.4 | Floral | ns | ns | ns |
| 1-Octanol | 1578 | 1565 | b,y86.5 ± 10.2 | a,x237.6 ± 19.1 | a,x318.9 ± 88.5 | b,y150.9 ± 41.1 | 120 | Fatty | ns | ns | *** |
| Pentadecanol | 2003 | 2023 | a,y81.6 ± 21.2 | N.D. | a,x286.9 ± 41.3 | b,x187.6 ± 68.9 | N.A. | N.A. | ** | *** | – |
| (E)-2-Octen-1-ol | 1620 | 1611 | b,y58.3 ± 18.8 | a,x362.1 ± 41.8 | a,x276.3 ± 24.7 | a,y205.6 ± 106.1 | 3 | Fruity, green apple | *** | ns | *** |
| Benzyl alcohol | 1892 | 1872 | a,y43.0 ± 34.7 | N.D. | a,x161.5 ± 94.4 | a,x76.0 ± 17.7 | 5.1 | Grass | *** | *** | – |
| Linalool | 1546 | 1522 | a,x3.9 ± 2.6 | N.D. | N.D. | N.D. | 6 | Aniseed, floral | – | – | – |
| Acids (4) | |||||||||||
| Acetic acid | 1439 | 1435 | b,y35.8 ± 5.3 | a,x52.0 ± 4.9 | a,x59.1 ± 6.2 | a,x58.5 ± 8.8 | 16,000 | Sour, vinegar | * | *** | * |
| Butanoic acid | 1624 | 1630 | a,x15.5 ± 2.3 | N.D. | N.D. | a,x59.3 ± 31.5 | 5000 | Sweet, rancid | – | – | – |
| Hexanoic acid | 1842 | 1855 | b,y129.3 ± 22.8 | a,x302.5 ± 66.0 | a,x401.6 ± 55.0 | a,x348.4 ± 87.1 | 3000 | Vinegar, green | ns | *** | ** |
| Nonanoic acid | 2177 | 2169 | b,y42.0 ± 19.6 | a,x83.0 ± 32.8 | a,x143.6 ± 34.8 | a,x104.6 ± 35.4 | 3000 | Green, fat | ns | ns | ns |
| Ketones (3) | |||||||||||
| 2,3-Octanedione | 1309 | 1321 | b,y245.8 ± 48.8 | a,x2920.1 ± 602.6 | a,x1128.3 ± 270.4 | b,y723.8 ± 196.4 | 12 | Oxidized fat, fungi | *** | *** | *** |
| (E,Z)-3,5-Octadien-2-one | 1593 | 1578 | N.D. | N.D. | N.D. | a,x27.1 ± 3.4 | 150 | Milky, candy | – | – | – |
| Acetophenone | 1627 | 1643 | b,y72.6 ± 9.8 | a,x125.0 ± 24.8 | a,x139.2 ± 25.9 | a,x98.6 ± 21.0 | 60 | Mothballs | ns | ns | ns |
| Furans (1) | |||||||||||
| 2-Pentylfuran | 1209 | 1224 | b,y52.0 ± 8.1 | a,x495.6 ± 70.1 | a,x168.1 ± 42.5 | b,y171.7 ± 39.9 | 6 | Buttery, green bean | ** | *** | *** |
N.D., not detected. N.A., not available. a,bThe variables with different letters indicate differences between breed in same muscle type; x,yThe variables with different letters indicate differences between muscle type in same breed. ***, P < 0.001; **, P < 0.01; *, P < 0.05; ns, not significant. 1Retention index of volatile compounds in literature was mainly obtained from the online database: (http://www.flavornet.org). 2, 3 Odor thresholds (in water) and odor descriptions were mainly gathered from the following literature and online database: (Sohail et al., 2022; http://www.odour.org.uk).
Aldehydes were the primary volatile compounds of cooked lamb. Heptanal was the most abundant one that conferred fatty note. Hong-ST had the highest heptanal concentration (7186.10 ± 1102.10 μg/kg). However, in LTL muscles, heptanal was not significant between breed. Similar with heptanal, the concentrations of other saturated aldehydes, such as hexanal, octanal and nonanal, were highest in Hong-ST and lowest in Hu-ST muscles. On the other hand, the unsaturated aldehydes were unique for different lamb muscles. For instance, (E)-2-heptanal was unique in Hong-ST muscle (29.10 ± 8.10 μg/kg) presenting fatty aroma, while (E)-4-decenal was absent. 2-Methylbutanl was only identified in the Hong-LTL muscle.
Although alcohols were identified in lamb samples, the thresholds of alcohols were relatively high. 1-Octen-3-ol was found in the highest concentration in Hong-LTL muscle, and linalool was absent in Hong lamb. Besides, benzyl alcohol was lower in Hong lambs compared to Hu lambs, and was not detected in Hong-ST muscle. Moreover, only three ketones were found in cooked lamb in our research, including 2,3-octanedione, (E,Z)-3,5-octadien-2-one and acetophenone. 2,3-Octanedione had extremely higher concentrations in lamb and presented a very low threshold. Despite the fact that (E, Z)-3,5-octadien-2-one was only detected in Hong-LTL muscle, the concentration was rather low when compared to the odor threshold. Acetophenone was not significant among lamb samples. Additionally, 2-pentylfuran was found highest concentration in Hong-ST muscle which was characterized by its buttery aroma. Furthermore, four acids were detected in lamb muscle, including acetic acid, butanoic acid, hexanoic acid and nonanoic acid. Hexanoic acid accounted for the highest concentration in lamb muscles (129.30–401.60 μg/kg), with LTL muscles containing significantly higher contents than those in ST muscles. Similar with hexanoic acid, higher levels of acetic acid were found in LTL muscles. No significant effect of breed and muscle type for nonanoic acid was revealed. Butanoic acid was only identified in Hu-ST and Hong-LTL muscles.
4. Discussions
4.1. Meat quality of lamb
Quality requirements and chemical compositions determination are necessary for customers to accept a new lamb meat. These variables are impacted by genetics, age, gender, and the environment (Zhao et al., 2022). In our study, Hong and Hu lambs were grown in the same environments and under the identical treatments, and their slaughter ages as well as genders were the same controlled. Therefore, the breed- and muscle type-related differences accounted for the majority of the variabilities. In general, pH24 is the most basic indicator of meat quality. Due to the lack of oxygen after slaughtering, glycolysis activity would occur in the carcass, resulting in the conversion of glycogen into lactic acid and a decrease in pH that would reach its lowest value within 18–24 h (Barrasso et al., 2022). Typically, the pH24 range does not exceed 6.0, thus verifying the quality of lamb muscles in our study (Costa et al., 2018). The pH24 did no differ between Hong and Hu lambs, one probable explanation is that lambs were treated in the same way prior to slaughter, resulting in a similar glycolysis rate. However, the pH24 values of the ST muscles were lower, most likely due to different fiber types. The LTL muscle of lamb contains abundant type I fibers (slow-oxidative fiber), but the ST muscle contains more type II (fast-twitch glycolytic) fibers (Şirin et al., 2017). Glycolytic fibers often have a high glycolysis rate, which may explain why ST muscles have lower pH24 values.
In addition, it has been established that Hong lamb is of higher quality due to its lower cooking loss and shear force, indicating that Hong lamb is superior in terms of water-holding capacity (WHC) and tenderness, respectively. These two variables are mainly influenced by the muscle structures and protein compositions. In raw muscles, myofibrils contain the majority of the water. Following slaughtering, the intact cytoskeleton protein linkages in myofibrils would cause the muscle cell to contract, thereby releasing water (Hughes, Oiseth, Purslow, & Warner, 2014). As a result, increased cytoskeleton protein breakdown would improve the WHC of meat. Accordingly, the WHC is consistent with tenderness because water is the primary plasticizer, and an increase in water loss would result in higher meat hardness and toughness (Hughes et al., 2014). Therefore, our findings corroborate the breed advantage of Hong lamb in terms of quality, and the LTL muscle in Hong lamb is more appropriate for cooking due to its greater palatability.
Meat color is another important trait used by customers to assess the quality of meat. Customers prefer meat with higher a⁎ and b⁎ values, thus Hong-ST muscle is more visibly appealing. Typically, the red color of meat is mostly determined by the concentration of myoglobin (Gan et al., 2019). Greater lipid deposition surrounding the myofibrils might promote oxidative instability, resulting in accelerated color deterioration (Renerre & Labas, 1987). Hence, in our study, the a⁎ values of Hu lamb muscles were greater than Hong-LTL muscle as a result of a comparatively higher IMF. However, although the IMF in Hong-ST muscle was relatively lower, the redness and yellowness of Hong-ST were significantly higher than other muscle samples. It may be due to the highest Fe concentration. Fe is intimately linked to the synthesis of myoglobin and hemoglobin (Eguchi & Saltman, 1984). As a result, a higher Fe content may influence the reddest hue of Hong-ST muscle, making it more appealing.
4.2. Aroma precursors of lamb
Regarding the aroma precursors such as protein, IMF, amino acids and fatty acids, they play crucial roles as taste compounds and in the generation of aroma compounds during cooking. Moreover, their compositions and concentrations are essential for animal and human health and have a substantial impact on the acceptability of lamb meat.
The significant variations in IMF deposits might be attributable to their breeds. Hong lamb is a breed of short-tailed lamb, whereas Hu lamb is a breed of fat-tailed lamb. Many studies have investigated that short-tailed accumulate less IMF and more protein than fat-tailed lamb (Zhang et al., 2020). Therefore, Hong lamb with its lower IMF and higher protein content could be regarded as a superior source of lamb. Although proximate compositions of Hong and Hu lambs are diverse, it is interesting to note that the deposition capabilities of different muscle types in Hong lamb also vary. In our study, Hong-ST muscle seems to accumulate more protein, but the lipid deposition potential of Hong-LTL muscle appears lower than Hu-LTL. Although IMF has been demonstrated to play a role in the eating quality that might influence the flavor of Hong-LTL muscle (Hopkins, Hegarty, Walker, & Pethick, 2006). Summarily, in terms of meat quality parameters, Hong lamb is a superior source of lamb due to its superior quality and chemical compositions. Different muscles in Hong lamb exhibit different characteristics, especially LTL muscle is more tender and possesses a higher water-holding capacity, which contributes to its juiciness and tenderness after cooking and provides palatability contribution that makes Hong-LTL muscle more acceptable for consumers. On the other hand, the ST muscle is visually appealing and rich in proteins, demonstrating the visible and nutritional contributions of Hong lamb. Amino acids are basic units of protein. The amino acid compositions of our study are consistent with those of prior studies about Hu lamb (Madruga, Dantas, Queiroz, Brasil, & Ishihara, 2013; Zhang et al., 2020; Zhao et al., 2022). Among these, umami amino acids and essential amino acids (EAAs) are vital for consumers. The content of FAA for Hong lamb was slightly higher than that of Hu lamb, indicating that Hong lamb had better nutritional functions. Sulfur-containing amino acids are important flavor precursors. Higher Met levels in ST muscles may contribute to the production of flavor substances such as hydrogen sulfide (Zhao et al., 2023, Zhao et al., 2023). During heating, free amino acids are degraded, resulting in the formation of aldehydes, sulfur-containing compounds, and nitrogen-containing compounds such as benzaldehyde, dimethyl trisulfide, and dimethyl pyrazine (Liu, Li, Zhang, Hamid, et al., 2023; Zhang et al., 2020). In terms of EAAs, the ratio of EAAs/NEAAs is an essential indicator for the nutritional value of meat. The FAO/WHO recommends that this ratio exceed 0.6. In our study, the EAAs/NAAs values for Hong lamb varied from 0.83 to 0.90, which is much higher than the level, confirming the ideal protein standards of Hong lamb. Glutamic acid is the main flavor component of raw and cooked lamb, and it produces umami. Therefore, ST muscle has greater Glutamic than LTL muscle. Making ST muscle more umami, tasty and preferred by consumers.
Fatty acids are crucial lipid precursors for the production of volatile compounds that provide species-aromas for cooked lamb. Meanwhile, the proportions of fatty acids are essential for human health. Hong lamb had a lower proportion of SFAs and a larger proportion of PUFAs compared to Hu lamb, the most likely cause could be breed differences in rumen metabolism and metabolic rate (Sinclair et al., 2005). The Lower SFA and PUFA in Hong lamb might be associated with lower IMF content (Choi et al., 2016). It could explain why Hong lamb (relatively lower IMF) contains lower SFAs and higher PUFAs. Normally, the PUFAs/SFAs (P/S) ratio could determine the nutritional quality of meat, and the optimal level is 0.4 or above (Wood et al., 2008). In our study, the ratio for Hong lamb is below the target, possibly because the hydrogenating action of rumen microorganisms in ruminant would reduce this ratio, thus resulting in ruminants not reaching to the optimal level (Kaić, Mioč, Kasap, & Potočnik, 2016). However, compared to the ratio of Hu lamb reported in other study (PUFA/SFA ratio was 0.12) (Zhang et al., 2020), our study had relatively higher level (PUFA/SFA ratio was 0.28). Besides, compared to Hu lamb in our study, higher proportions of PUFAs were found in Hong lamb. It would be beneficial to human health and provide more specific aromas for cooked Hong lamb since PUFAs are easily oxidized during thermal cooking (Tanimoto, Kitabayashi, Fukusima, Sugiyama, & Hashimoto, 2015).
Linoleic acid is one of the key PUFAs that could produce volatile compounds such as hexanal, heptanal and 2-pentylfuran (Domínguez et al., 2019). These three volatile compounds have lower thresholds and highest concentrations in cooked Hong-ST muscle, which could impart strong fatty, grass and buttery aromas for Hong-ST muscle compared with cooked Hu lamb. Linoleic acid is the only PUFA for Hong-ST muscle, although there is no difference in linoleic acid between Hong and Hu lamb. It is because linoleic acid is solely obtained from the diet, and two lambs are fed in the same diet (Wood et al., 2008). However, the unique lipidomic molecule would decompose and degrade during cooking, releasing more free fatty acids (e.g., linoleic acid), then generating volatiles (Liu et al., 2022). Higher Fe content in Hong-ST would promote these lipid degradation and oxidation processes (Zhou et al., 2022). Less oxidative fibers in ST muscle also display lower antioxidative capacity in cooking (Benjamin, Tharcilla, & Hopkins, 2020). Therefore, it might explain why Hong-ST has strong aromas than other muscle samples, especially the breed-varied in volatile compounds are more obviously reflected in ST muscle of two lambs. Furthermore, Hong-ST has a greater proportion of oleic acid in raw muscle, resulting in higher octanal and nonanal in cooked meat (Sohail et al., 2022). Citrus and floral aromas are provided by these two chemicals.
4.3. Flavor components of cooked mutton
In terms of the Hong-LTL muscle, the concentrations of most volatiles of it do not differ significantly from those in Hu-LTL muscle except for 1-octen-3-ol. It displays the largest amount in Hong-LTL muscle, which may possibly derive from its characteristic fatty acid, C20:4n6 (Zhang et al., 2020). Higher levels of 1-octen-3-ol may impart a mushroom aroma to cooked Hong-LTL muscle. Besides, some unique volatile compounds are generated in this muscle. For instance, (E, Z)-3,5-octadien-2-one is exclusively found in Hong-LTL muscle, and its formation is linked to linolenic acid (Liu et al., 2022). Another characteristic compound, 2-methylbutanal, is generated via Strecker degradation of isoleucine and exhibits almond and chocolate aromas, which might be associated to the excellent capacity of hydrolyzing myofibrillar protein in Hong-LTL, thus releasing larger levels of peptides (Liu et al., 2022; Zhou et al., 2022). Moreover, 2-butyl-2-octenal is only identified in LTL muscle of Hong lamb, suggesting that it is a muscle-varied compound. It is synthesized from hexanal by crotonization (Cheng, Huynh-Ba, Blank, & Robert, 2008). Hong-LTL muscle exhibits less hexanal but more 2-butyl-2-octenal in comparison to Hong-ST, indicating the rapid crotonization reaction. Faster protein hydrolysis could accelerate this crotonization reaction, which might be the reason why Hong-LTL is unique in 2-butyl-2-octenal (Cheng, Huynh-Ba, Blank, & Robert, 2008). Furthermore, although acids are found in lamb muscles, while their thresholds are very high, so they hardly contribute to lamb aromas. It has been reported that 4-methyl-butanoic acid and 4-ethyl-butanoic acid are responsible for the unpleased mutton aromas (Nixon, Wong, Johnson, & Birch, 1979). These two compounds are not found in our research. It possible because consumers are not preferred this aroma, and the breed generation has been improved in recent years.
4.4. Lamb grouping and associations
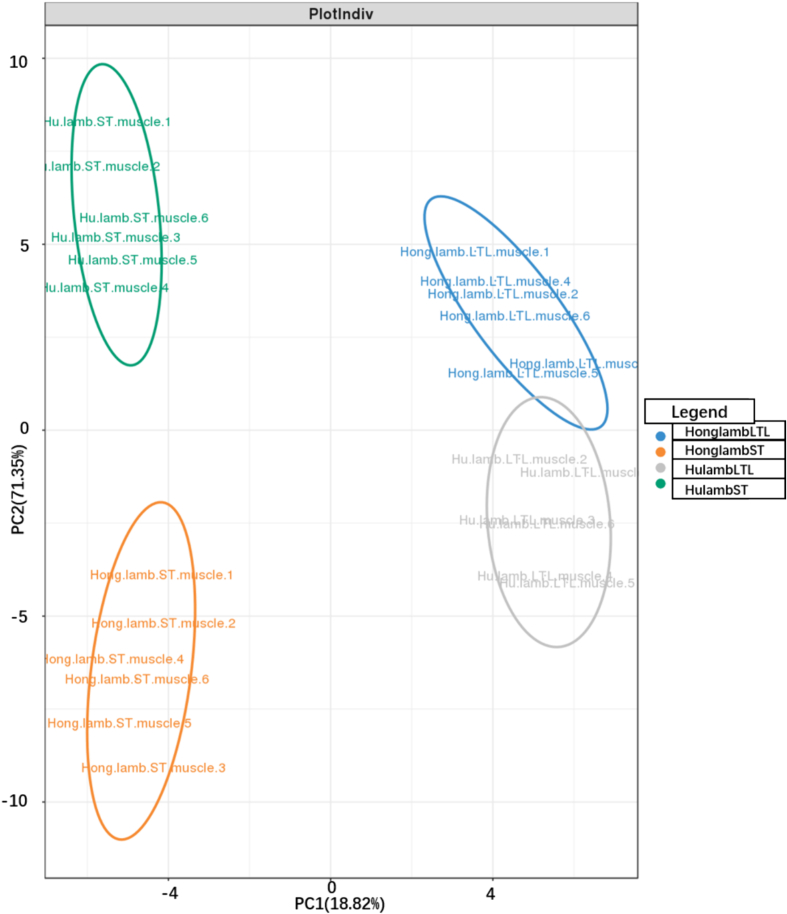
As shown in Fig. 1, principal component analysis (PCA) was utilized to analyze the relationship between aroma precursors and volatile components of cooked mutton. Partial Least Squares Discriminant Analysis (PLS-DA) was then performed based on the volatile compounds and aroma precursors to identify the breeds and muscle types of lamb. Fig. 1 displays the scores and loading plots of PCA based on aroma precursors and volatile compounds, respectively. The results indicated almost no overlap among the four groups, suggesting that the differences observed in PCA were pronounced. The different muscles of same breeds were distributed in distinct locations, and a significant difference was observed between Hu lamb and Hong lamb within the ST muscle. However, there were no significant differences in PCA results between Hu lamb and Hong lamb within the LTL muscle. The distinction between breeds was more pronounced in the ST muscle. The loading plots of PCA revealed a significant correlation between volatile components and fatty acids such as C14: 0, C15: 0, C17: 0, C18: ln9t, C18: 3n3. Thr, Val, Met, Lys and His showed associations with aldehydes such as hexanal, heptanal, octanal, (E)-2-nonenal and decanal. Alcohols such as 1-Octen-3-ol, linalool and (E)-2-Octen-1-ol were closely related to most free amino acids. This phenomenon indicated that occurrence of the Maillard reaction and Strecker degradation reaction during the cooking of mutton (Liu et al., 2023; Zhao et al., 2023, Zhao et al., 2023).
Fig. 1.
Multivariate analysis including principal component analysis (PCA) based on volatile compounds and precursor composition of the mutton.
Additionally, the results of PLS-DA based on volatile compounds and aroma precursors in different breeds and muscle types are presented in Fig. 2. The meat samples from the four groups were distributed in distinct locations, indicating clear distinctions. The LTL muscles of Hong lamb exhibited the most significant differences from the other groups, with their location far removed from the others. In summary, the volatile compounds in cooked mutton and aroma precursors in raw meat could effectively characterize and differentiate between different breeds and muscle types of lamb.
Fig. 2.
Partial least squares discriminant analysis (PLS-DA) based on volatile compounds and precursor composition of mutton.
5. Conclusion
This study revealed that Pishan Hong lamb was a promising genetic resource due to its exceptional quality and organoleptic features. Compared to Hu lamb, Hong lamb exhibited greater tenderness and water-holding capacity (WHC), larger PUFA proportions and the characteristics of unique volatile compounds after cooking. After cooking, the LTL muscle of Hong lamb was characterized as the most tender and juicy, making it the most appetizing. However, the ST muscle of Hong lamb was assessed as having an imparted strong fatty, fresh and buttery aroma, whose organoleptic properties were excellent. The characteristic precursors may have promoted chemical reactions during cooking, resulting in the typical aroma of cooked lamb. Multivariate analysis results indicated that the different lamb breeds and muscle types could be distinguished based on these precursors and volatile compounds. These results were useful for identifying and characterizing breeds and muscle types of lambs. They could provide vital data for the development of lamb in Xinjiang province and enhance the lamb production in China.
CRediT authorship contribution statement
Yu Song: Writing – review & editing, Writing – original draft, Formal analysis, Conceptualization. Laiyu Zhao: Writing – original draft, Investigation, Data curation. Ping Yang: Writing – review & editing, Validation. Feng Huang: Project administration, Funding acquisition. Yun Wu: Funding acquisition, Conceptualization. Chunhui Zhang: Writing – review & editing, Resources, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Technical System of Livestock Industry in Xinjiang (XJARS-XM-08), National Key R & D Program of China (2021YFD2100103), a key personnel training project of agriculture, rural areas in Xinjiang Uygur Autonomous Region (2022SNGGGCC039) and the Key scientific and technological projects of Xinjiang production and Construction Corps (2020AB012).
Data availability
Data will be made available on request.
References
- Barrasso R., Ceci E., Tufarelli V., Casalino G., Luposella F., Fustinoni F., Dimuccio M.M., Bozzo G. Religious slaughtering: Implications on pH and temperature of bovine carcasses. Saudi Journal of Biological Sciences. 2022;29:2396–2401. doi: 10.1016/j.sjbs.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benet I., Dolors Guardia M., Ibanez C., Sola J., Arnau J., Roura E. Low intramuscular fat (but high in PUFA) content in cooked cured pork ham decreased Maillard reaction volatiles and pleasing aroma attributes. Food Chemistry. 2016;196:76–82. doi: 10.1016/j.foodchem.2015.09.026. [DOI] [PubMed] [Google Scholar]
- Benjamin W.B.H., Tharcilla I.R.C.A., Hopkins D.L. The effect of fibre orientation, measurement interval and muscle on lamb meat drip loss values. Meat Science. 2020;161 doi: 10.1016/j.meatsci.2019.107959. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Huynh-Ba T., Blank I., Robert F. Temporal changes in aroma release of Longjing tea infusion: interaction of volatile and nonvolatile tea components and formation of 2-butyl-2-octenal upon aging. Journal of Agricultural and food Chemistry. 2008;56:2160–2169. doi: 10.1021/jf073132l. [DOI] [PubMed] [Google Scholar]
- Choi Y.S., Lee J.K., Jung J.T., Jung Y.C., Jung J.H., Jung M.O.…Choi J.S. Comparison of meat quality and fatty acid composition of longissimus muscles from purebred pigs and three-way crossbred LYD pigs. Korean Journal for Food Science of Animal Resources. 2016;36:689–696. doi: 10.5851/kosfa.2016.36.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J.B., Oliveira R.L., Silva T.M., Barbosa A.M., Borja M.S., de Pellegrini C.B.…Bezerra L.R. Fatty acid, physicochemical composition and sensory attributes of meat from lambs fed diets containing licuri cake. PLoS One. 2018;13 doi: 10.1371/journal.pone.0206863. e0206863-e0206863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawut A., Tian Y. Competitiveness of Xinjiang’s mutton industry based on diamond model. PLoS One. 2021;16 doi: 10.1371/journal.pone.0257669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez R., Pateiro M., Gagaoua M., Barba F.J., Zhang W., Lorenzo J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants. 2019;8:429. doi: 10.3390/antiox8100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi L.A., Saltman P. The aerobic reduction of Fe(III) complexes by hemoglobin and myoglobin. Journal of Biological Chemistry. 1984;259:14337–14338. doi: 10.1016/S0021-9258(17)42599-3. [DOI] [PubMed] [Google Scholar]
- Gan M., Shen L., Fan Y., Guo Z., Liu B., Chen L.…Zhu L. High altitude adaptability and meat quality in Tibetan pigs: A reference for local pork processing and genetic improvement. Animals-Basel. 2019;9 doi: 10.3390/ani9121080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins D.L., Hegarty R.S., Walker P.J., Pethick D.W. Relationship between animal age, intramuscular fat, cooking loss, pH, shear force and eating quality of aged meat from lamb. Australian Journal of Agricultural and Resource Economics. 2006;46:879–884. doi: 10.1071/EA05311. [DOI] [Google Scholar]
- Hughes J.M., Oiseth S.K., Purslow P.P., Warner R.D. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Science. 2014;98:520–532. doi: 10.1016/j.meatsci.2014.05.022. [DOI] [PubMed] [Google Scholar]
- Kaić A., Mioč B., Kasap A., Potočnik K. Meta-analysis of intramuscular fatty acid composition of Mediterranean lambs. Archiv fur Tierzucht-archives of animal breeding. 2016;59:1–8. doi: 10.5194/aab-59-1-2016. [DOI] [Google Scholar]
- Li J., Yang Y., Tang C., Yue S., Zhao Q., Li F., Zhang J. Changes in lipids and aroma compounds in intramuscular fat from Hu lamb. Food Chemistry. 2022;383 doi: 10.1016/j.foodchem.2022.132611. [DOI] [PubMed] [Google Scholar]
- Liu H., Hui T., Fang F., Li S.S., Wang Z.Y., Zhang D.Q. The formation of key aroma compounds in roasted mutton during the traditional charcoal process. Meat Science. 2022;184 doi: 10.1016/j.meatsci.2021.108689. [DOI] [PubMed] [Google Scholar]
- Liu H., Li J.Y., Zhang D.Q., Hamid N.…Gong H.S. The effect of thermal times of circulating non-fried roast technique on the formation of (non)volatile compounds in roasted mutton by multi-chromatography techniques and heat transfer analysis. Food Research International. 2023;174 doi: 10.1016/j.foodres.2023.113567. [DOI] [PubMed] [Google Scholar]
- Madruga M., Dantas I., Queiroz A., Brasil L., Ishihara Y. Volatiles and water- and fat-soluble precursors of Saanen goat and cross Suffolk lamb flavour. Molecules. 2013;18:2150–2165. doi: 10.3390/molecules18022150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon L.N., Wong E., Johnson C.B., Birch E.J. Nonacidic constituents of volatiles from cooked mutton. Journal of Agricultural and Food Chemistry. 1979;27:355–359. doi: 10.1021/jf60222a044. [DOI] [Google Scholar]
- Qi S.S., Zhan P., Tian H.L., Wang P., Ma X.P., Li K.X. Effects of thyme (Thymus vulgaris L.) addition on the volatile compounds of mutton broth during boiling. Food Science and Human Wellness. 2022;11:305–315. doi: 10.1016/j.fshw.2021.11.025. [DOI] [Google Scholar]
- Renerre M., Labas R. Biochemical factors influencing metmyoglobin formation in beef muscles. Meat Science. 1987;19:151–165. doi: 10.1016/0309-1740(87)90020-9. [DOI] [PubMed] [Google Scholar]
- Shackelford S.D., Leymaster K.A., Wheeler T.L., Koohmaraie M. Effects of breed of sire on carcass composition and sensory traits of lamb. Journal of Animal Science. 2012;90:4131–4139. doi: 10.2527/jas.2012-5219. [DOI] [PubMed] [Google Scholar]
- Si R., Na Q., Wu D., Wu X., Ming L., Ji R. Effects of age and muscle type on the chemical composition and quality characteristics of Bactrian camel (Camelus bactrianus) meat. Foods. 2022;11 doi: 10.3390/foods11071021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair L.A., Cooper S.L., Chikunya S., Wilkinson R.G., Hallett K.G., Enser M., Wood J.D. Biohydrogenation of n-3 polyunsaturated fatty acids in the rumen and their effects on microbial metabolism and plasma fatty acid concentrations in lamb. Animal Science. 2005;81:239–248. doi: 10.1079/ASC50040239. [DOI] [Google Scholar]
- Şirin E., Aksoy Y., Uğurlu M., Çiçek Ü., Önenç A., Ulutaş Z., Şen U., Kuran M. The relationship between muscle fiber characteristics and some meat quality parameters in Turkish native lamb. Small Ruminant Research. 2017;150:46–51. doi: 10.1016/j.smallrumres.2017.03.012. [DOI] [Google Scholar]
- Sohail A., Al-Dalali S., Wang J., Xie J., Shakoor A., Asimi S., Shah H., Patil P. Aroma compounds identified in cooked meat: A review. Food Research International. 2022;157 doi: 10.1016/j.foodres.2022.111385. [DOI] [PubMed] [Google Scholar]
- Song Y., Huang F., Li X., Han D., Zhao L., Liang H.…Zhang C. Water status evolution of pork blocks at different cooking procedures: A two-dimensional LF-NMR T1-T2 relaxation study. Food Research International. 2021;148 doi: 10.1016/j.foodres.2021.110614. [DOI] [PubMed] [Google Scholar]
- Tanimoto S., Kitabayashi K., Fukusima C., Sugiyama S., Hashimoto T. Effect of storage period before reheating on the volatile compound composition and lipid oxidation of steamed meat of yellowtail Seriola quinqueradiata. Fisheries Science. 2015;81:1145–1155. doi: 10.1007/s12562-015-0921-4. [DOI] [Google Scholar]
- Wang L.W., Su S.F., Zhao J., He X.L., Fu S.Y.…Liu Y.B. Effects of dietary oat supplementation on carcass traits, muscle metabolites, amino acid profiles, and its association with meat quality of small-tail Han lamb. Food Chemistry. 2023;411 doi: 10.1016/j.foodchem.2023.135456. [DOI] [PubMed] [Google Scholar]
- Watkins P.J., Frank D., Singh T.K., Young O.A., Warner R.D. Lamb meat flavor and the effect of different feeding systems: A review. Journal of Agricultural and Food Chemistry. 2013;61:3561–3579. doi: 10.1021/jf303768e. [DOI] [PubMed] [Google Scholar]
- Wood J.D., Enser M., Fisher A.V., Nute G.R., Sheard P.R., Richardson R.I., Hughes S.I., Whittington F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Science. 2008;78:343–358. doi: 10.1016/j.meatsci.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Wu T.L., Wang P., Zhang Y.Y., Zhan P., Zhao Y., Tian H.L., He W.Y. Identification of muttony-related compounds in cooked mutton tallows and their flavor intensities subjected to phenolic extract from thyme (Thymus vulgaris L.) Food Chemistry. 2023;427 doi: 10.1016/j.foodchem.2023.136666. [DOI] [PubMed] [Google Scholar]
- Wu W., Zhan J., Tang X., Li T., Duan S. Characterization and identification of pork flavor compounds and their precursors in Chinese indigenous pig breeds by volatile profiling and multivariate analysis. Food Chemistry. 2022;385:132543. doi: 10.1016/j.foodchem.2022.132543. [DOI] [PubMed] [Google Scholar]
- Xiang C., Li S., Liu H., Liang C., Fang F., Zhang D., Wang Z. Impact of chilling rate on the evolution of volatile and non-volatile compounds in raw lamb meat during refrigeration. Foods. 2021;10 doi: 10.3390/foods10112792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Zhang H., Liu M., Zhao X., Luo H. Effect of breed on the volatile compound precursors and odor profile attributes of lamb meat. Foods. 2020;9:1178. doi: 10.3390/foods9091178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Li F., Zhang X., Zhang D., Li X., Zhang, Y…Wang, W. Integrative analysis of transcriptomics and proteomics of longissimus thoracis of the Hu lamb compared with the Dorper lamb. Meat Science. 2022;193 doi: 10.1016/j.meatsci.2022.108930. [DOI] [PubMed] [Google Scholar]
- Zhao L., Zhang H., Huang F., Liu H., Wang T., Zhang C. Authenticating Tibetan pork in China by tracing the species and geographical features based on stable isotopic and multi-elemental fingerprints. Food Control. 2023;145 doi: 10.1016/j.foodcont.2022.109411. [DOI] [Google Scholar]
- Zhao L.Y., Erasmus S., Yang P., Huang F., Zhang C.H., Ruth S.V. Establishing the relations of characteristic aroma precursors and volatile compounds for authenticating Tibetan pork. Food Chemistry. 2023;427 doi: 10.1016/j.foodchem.2023.136717. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Zhang Y., Xin R., Huang X., Li Y., Dong X.…Qin L. Metal ion-mediated pro-oxidative reactions of different lipid molecules: Revealed by nontargeted lipidomic approaches. Journal of Agricultural and Food Chemistry. 2022;70:10284–10295. doi: 10.1021/acs.jafc.2c02402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.