Abstract
Background/Aims
Cancer stem cells (CSCs) are believed to drive tumor development and metastasis. Activin and hepatocyte growth factor (HGF) are important cytokines with the ability to induce cancer stemness. However, the effect of activin and HGF combination treatment on CSCs is still unclear.
Methods
In this study, we sequentially treated colorectal cancer cells with activin and HGF and examined CSC marker expression, self-renewal, tumorigenesis, and metastasis. The roles of forkhead box M1 (FOXM1) and sex-determining region Y-box 2 (SOX2), two stemness-related transcription factors, in activin/HGF-induced aggressive phenotype were explored.
Results
Activin and HGF treatment increased the expression of CSC markers and enhanced sphere formation in colorectal cancer cells. The tumorigenic and metastatic capacities of colorectal cancer cells were enhanced upon activin and HGF treatment. Activin and HGF treatment preferentially promoted stemness and metastasis of CD133+ subpopulations sorted from colorectal cancer cells. FOXM1 was upregulated by activin and HGF treatment, and the knockdown of FOXM1 blocked activin/HGF-induced stemness, tumorigenesis, and metastasis of colorectal cancer cells. Similarly, SOX2 was silencing impaired sphere formation of activin/HGF-treated colorectal cancers. Overexpression of SOX2 rescued the stem cell-like phenotype in FOXM1-depleted colorectal cancer cells with activin and HGF treatment. Additionally, the inhibition of FOXM1 via thiostrepton suppressed activin/HGF-induced stemness, tumorigenesis and metastasis.
Conclusions
Sequential treatment with activin and HGF promotes colorectal cancer stemness and metastasis through activation of the FOXM1/SOX2 signaling. FOXM1 could be a potential target for the treatment of colorectal cancer metastasis.
Keywords: Colorectal neoplasms, FOXM1, Neoplasm metastasis, SOX2, Stem cells
INTRODUCTION
Cancer stem cells (CSCs) have been recognized as a key player in tumor development and progression.1,2 Their capacities of self-renewal, adaptation to harsh conditions, tumorigenicity, and metastasis confer the aggressive phenotype observed in cancers.3 CSCs are highly heterogenous, and only certain subsets of CSCs are responsible for distant metastasis.4 Colorectal CSCs can be identified by cell surface markers including CD133 and CD44.5 Chemokine (C-X-C motif) receptor 4 (CXCR4) expression in primary tumors is correlated with liver metastasis and poor prognosis in colorectal cancer.6 CD133+/CXCR4+ colorectal CSCs exhibit a high metastatic capacity.7 Targeting CSCs is considered as an important anti-cancer strategy.
Forkhead box M1 (FOXM1) is a widely expressed transcription factor involved in various physiological and pathological processes.8,9 High FOXM1 expression is associated with poor prognosis of colorectal cancer patients.10 Silencing of FOXM1 decreases the proliferation and invasion of colorectal cancer cells.10 Most interestingly, dysregulation of FOXM1 has an impact on the properties of CSCs.11 Yuan et al.12 reported that FOXM1 promotes taxane resistance by modulating cancer cell stemness. Song et al.13 reported that FOXM1 can regulate the stemness and survival of colorectal cancer cells. Therefore, FOXM1 represents a pivotal factor in driving cancer stemness.
A number of cytokines including activin and hepatocyte growth factor (HGF) have been found to induce cancer stemness.14,15 Perkhofer et al.14 reported that activin signaling promotes pancreatic cancer stemness. Myofibroblasts show the ability to secret HGF to enhance stemness in gastric cancer15 and colon cancer.16 Sequential treatment with activin and HGF has been employed to induce differentiation of hepatocytes from human embryonic stem cells.17 In our previous study, we found that activin alone increased the messenger RNA and protein expression of FOXM1 through SMAD family member 2.18 HGF alone enhanced the phosphorylation of FOXM1, without altering the total protein level of FOXM1.18 Since activin and HGF have a capacity of promoting an aggressive phenotype in colorectal cancer cells,18-20 we hypothesized that activin and HGF treatment might exert a positive effect on colorectal cancer stemness through FOXM1.
In the current study, we explored the effect of sequential activin and HGF treatment on the stemness of colorectal cancer cells. In addition, the function of FOXM1 in mediating the activities of activin and HGF in colorectal cancer was determined both in vitro and in vivo.
MATERIALS AND METHODS
1. Cell culture
Colorectal cancer cell lines HCT116 and HT29 were acquired from the American Tissue Culture Collection (Manassas, VA, USA). They were cultured in Dulbecco’s modified eagle medium supplemented with 10% fetal bovine serum (Sigma Aldrich, St. Louis, MO, USA) at 37°C in a humidified incubator with 5% CO2.
2. Cell treatment
Activin and HGF were used to treat HCT116 and HT29 cells as described previously.21 Briefly, cells were grown to 70% confluence and incubated with 100 ng/mL activin A (Peprotech, Rocky Hill, NJ, USA) for 5 days, followed by 20 ng/mL HGF (Peprotech) for another 5 days. The cells were harvested and tested for gene expression, stem cell-like properties, and metastatic capacities. In some experiments, thiostrepton (MedChemExpress, Monmouth Junction, NJ, USA) was used to inhibit FOXM1 activity before activin and HGF treatment (10 μM, 48 hours).
3. Plasmids and transfection
FOXM1- and sex-determining region Y-box 2 (SOX2)-targeting short-hairpin RNA (shRNA) oligonucleotides were commercially synthesized with the sense sequences: FOXM1 shRNA, 5ʹ-CGCTACTTGACATTGGACCAA-3ʹ and SOX2 shRNA, 5ʹ-CCACCTACAGCATGTCCTA-3ʹ. The shRNA sequences were cloned into pSilencer 4.1-CMV puro vector (Thermo Fisher Scientific, Waltham, MA, USA). The SOX2-expressing plasmid was generated by inserting full-length human SOX2 cDNA into pcDNA3.1 vector. These constructs were validated by direct sequencing. Transfection of the plasmids to colorectal cancer cells was performed using Lipofectamine 3000 (Invitrogen, Waltham, MA, USA) according to the manufacturer’s instructions. For generation of stable transfectants, the transfected cells were selected with puromycin 24 hours after transfection.
4. Western blot analysis
Protein samples extracted from colorectal cancer cells after indicated treatments were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. After blocking in 5% nonfat milk, the membranes were incubated with anti-CXCR4 (1:1,000; Abcam, Cambridge, MA, USA), anti-SOX2 (1:1,000), anti-FOXM1 (1:1,000), anti-CD133 (1:1,000), anti-ALDH1 (1:1,000), anti-OCT4 (1:1,000), anti-CD44 (1:1,000; Cell Signaling Technology, Danvers, MA, USA), and anti-β-actin (1:5,000; Proteintech, Rosemont, IL, USA) antibodies overnight at 4°C. The membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies. Protein signals were detected by enhanced chemiluminescence.
5. Flow cytometry
HCT116 and HT29 cells (107 cells/sample) were incubated with PE-labeled anti-CD133 and APC-labeled anti-CXCR4 antibodies (BD Biosciences, Franklin Lakes, NJ, USA) and subjected to gene expression analysis and sorting by flow cytometry.
6. Sphere formation assay
Cells were seeded in 6-well ultra-low adherent plates (1,000 cells/well) and grown in Dulbecco’s modified eagle medium/F12 medium supplemented with 2% B27, 20 ng/mL epidermal growth factor, and 20 ng/mL basic fibroblast growth factor (Peprotech, Cranbury, NJ, USA). The number of spheres formed was counted 10 days after seeding.
7. Luciferase reporter assay
The CXCR4, FOXM1 promoter and their truncated fragments were cloned into pGL3-basic vector. All constructs were verified by sequencing. For the luciferase reporter assay, cells were plated in 24-well plates and co-transfected with luciferase reporter constructs, FOXM1 and SOX2 expressing plasmid, and pRL-TK Renilla luciferase control reporter vectors. Luciferase activity was measured 36 h after cell transfection using the Dual-Luciferase Reporter Assay system (Promega, Madison, WI, USA) according to the manufacturer’s instructions.
8. Chromatin immunoprecipitation (ChIP) assay
Cells were fixed with 1% formaldehyde for 10 minutes at room temperature. Sonicated cell lysates were subjected to immunoprecipitation using 5 μg anti-FOXM1 or anti-SOX2 (Cell Signaling Technology, Danvers, MA, USA) or isotype control IgG. Immunoprecipitated DNA was extracted and subjected to polymerase chain reaction analysis. Primers used for ChIP are listed in Table 1.
Table 1.
Primers Used in This Study
| Transcriptional factor | Promoter | Forward | Reverse |
|---|---|---|---|
| SOX2 | CXCR4 | 5'-ATGCATCTCTGTGATGGTAATACC-3' | 5'-GCTATACTTGCACATTCACAGC-3' |
| FOXM1 | 5'-AGCACTACGGTCTATTATATCC-3' | 5'-TCGGCTTTAGTTGATTTCCT-3' | |
| FOXM1 | CXCR4 | 5'-ATCCCGCTTCCCTCAAACTT-3' | 5'-ACAAACTGAAGTTTCTGGCCG-3' |
SOX2, sex-determining region Y-box 2; FOXM1, forkhead box M1.
9. Animal experiments
For in vivo tumorigenic study, male athymic BALB/c nude mice were subcutaneously injected with HCT116 cells (1×105 cells/mouse), which had received activin/HGF pretreatment or not. In some experiments, thiostrepton was administered intraperitoneally at a dose of 500 mg/kg/day. Tumor development was monitored weekly. Four weeks later, the mice were euthanized. The xenograft tumors were photographed and measured. For assessment of metastatic capacities, HCT116 cells treated with or without activin/HGF were injected into the spleen of nude mice (1×105 cells/mouse). Six weeks after cell injection, metastatic lesions in the liver were photographed and counted. All animal experiments were approved by the Institutional Animal Care and Use Committee of the Chongqing Medical University (Chongqing, China) (approval number: 2022-K377).
10. Statistical analysis
All values are expressed as the mean±standard deviation. Statistical differences were assessed using the Student t-test or one-way analysis of variance with the Tukey post-hoc test. A p<0.05 was considered statistically significant.
RESULTS
1. Activin and HGF treatment increases stemness and metastasis of colorectal cancer cells
Colorectal cancer cells were sequentially treated with activin and HGF and tested for stemness and metastasis. Western blot analysis showed that activin and HGF treatment enhanced the expression of multiple CSC markers, i.e., CD133, CD44, OCT4, SOX2, and ALDH1 (Fig. 1A). A previous study revealed that CD133+/CXCR4+ colorectal CSCs exhibit a high metastatic capacity.7 Thus, we analyzed the effect of activin and HGF on CD133 and CXCR4. Flow cytometric analysis demonstrated that the percentages of CD133+ and CXCR4+ cells were increased by activin and HGF treatment (Fig. 1B). Moreover, activin and HGF treatment potentiated the sphere formation ability of both HCT116 and HT29 cells (Fig. 1C). Meanwhile, we could find single treatment with activin or HGF can enhance the stemness of colorectal cancer cells and synergistic effect of combination of activin and HGF on stemness of colorectal cancer cells (Fig. 1C). We also evaluated the tumorigenic and metastatic capacities of colorectal cancer cells pretreated with activin and HGF in murine models. As shown in Fig. 1D-H, activin and HGF treatment promoted tumorigenesis and liver metastasis of HCT116 cells injected to nude mice. Moreover, a single treatment with activin or HGF can not significantly increase tumorigenic and metastatic capacities of colorectal cancer cells in murine models (Fig. 1D-H). However, we found synergistic effect of combination of activin and HGF on tumorigenesis and metastasis of colorectal cancer cells (Fig. 1D-H). These results indicate that activin and HGF treatment has a positive impact on colorectal cancer stemness and aggressiveness.
Fig. 1.
Activin and HGF treatment increases stemness and metastasis of colorectal cancer cells. (A) Western blot analysis of indicated proteins in HCT116 and HT29 cells treated with or without activin and HGF. (B) Flow cytometric analysis of the percentages of CD133+ and CXCR4+ cells. (C) Sphere formation assay performed in HCT116 and HT29 cells treated with or without activin and/or HGF. (D-F) Subcutaneous xenograft tumors formed from HCT116 cells with or without activin and/or HGF treatment. (G, H) Liver metastasis (arrowheads) assessed in nude mice when HCT116 cells with or without activin and/or HGF treatment were injected to the spleen. HGF, hepatocyte growth factor; FOXM1, forkhead box M1; SOX2, sex-determining region Y-box 2; NS, not significant. *p<0.05, †p<0.01, ‡p<0.001.
2. Activin and HGF treatment preferentially promotes stem cell properties and metastatic capacities of CD133+ subpopulations
Based on CD133 and CXCR4 expression, HCT116 and HT29 cells were sorted by flow cytometry into four subpopulations, i.e., CD133+CXCR4+, CD133+CXCR4–, CD133–CXCR4+, and CD133–CXCR4– (Fig. 2A and B). When cultured in ultra-low adherent plates, the CD133+CXCR4+ and CD133+CXCR4– subpopulations formed significantly more spheres than the CD133–CXCR4+ and CD133–CXCR4– subpopulations (Fig. 2C and D). Most importantly, activin and HGF treatment led to enhanced sphere formation in the CD133+ subpopulations (especially the CD133+CXCR4+ subpopulations) and, to a lesser extent, in the CD133– subpopulations. In vivo studies confirmed that the CD133+CXCR4+ and CD133+CXCR4– subpopulations had an increased ability to generate subcutaneous tumors (Fig. 2E, Supplementary Fig. 1A and B) and metastasize to the liver (Fig. 2F, Supplementary Fig. 1C). Pretreatment with activin and HGF enhanced tumorigenic and metastatic activities of the CD133+CXCR4+ and CD133+CXCR4– subpopulations (especially the CD133+CXCR4+ subpopulations).
Fig. 2.
Activin and HGF treatment preferentially promotes stem cell properties and metastatic capacities of CD133+ subpopulations. (A, B) HCT116 and HT29 cells were sorted by flow cytometry based on CD133 and CXCR4 expression. (C, D) Sphere formation assay performed in CD133+CXCR4+, CD133+CXCR4–, CD133–CXCR4+, and CD133–CXCR4– subpopulations sorted from HCT116 and HT29 cells. (E) Subcutaneous xenograft tumors formed from the CD133+CXCR4+, CD133+CXCR4–, CD133–CXCR4+, and CD133–CXCR4– subpopulations of HCT116 cells with or without activin and HGF treatment. (F) HCT116 cell subpopulations treated with activin and HGF were injected into the spleen of nude mice, and liver metastasis (arrowheads) was evaluated. HGF, hepatocyte growth factor. *p<0.05.
3. FOXM1 mediates the biological effects of activin and HGF on colorectal cancer cells
Given the essential role of FOXM1 in modulating cancer stemness,13 we checked whether activin/HGF-induced stemness in colorectal cancer cells depended on FOXM1 activity. FOXM1 was upregulated in colorectal cancer cells treated with activin and HGF (Fig. 1A). Subsequently, we knocked down FOXM1 in HCT116 and HT29 cells via delivery of FOXM1-targeting shRNAs. When endogenous FOXM1 was efficiently knocked down, a panel of CSC-related genes including SOX2, CD133, CD44, ALDH1, OCT4, and CXCR4 were downregulated in activin/HGF-treated colorectal cancer cells (Fig. 3A). Flow cytometric analysis revealed that FOXM1 knockdown resulted in a marked reduction in the percentages of the CD133+ and CXCR4+ subpopulations (Fig. 3B). Moreover, FOXM1 knockdown impaired activin/HGF-induced sphere formation from colorectal cancer CD133+CXCR4+ or CD133+CXCR4– subpopulations (Fig. 3C and D). In addition, depletion of FOXM1 attenuated activin/HGF-induced tumorigenesis (Fig. 3E, Supplementary Fig. 1D and E) and metastasis (Fig. 3F, Supplementary Fig. 1F) of CD133+CXCR4+ and CD133+CXCR4– HCT116 cells in nude mice. These results suggest that FOXM1 activity is required for activin/HGF-induced aggressive phenotype in colorectal cancer cells.
Fig. 3.
FOXM1 mediates the biological effects of activin and HGF on colorectal cancer cells. (A) Western blot analysis of the indicated proteins in colorectal cancer cells with KD of FOXM1. (B) Flow cytometric analysis revealed that FOXM1 KD reduced the percentages of the CD133+ and CXCR4+ subpopulations. Sphere formation assay performed in CD133+CXCR4+, CD133+CXCR4–, CD133–CXCR4+, and CD133–CXCR4– subpopulations sorted from FOXM1-depleted HCT116 (C) and HT29 (D) cells. Depletion of FOXM1 attenuated activin/HGF-induced tumorigenesis (E) and metastasis (arrowheads) (F) of CD133+CXCR4+ and CD133+CXCR4– HCT116 cells in nude mice. HGF, hepatocyte growth factor; FOXM1, forkhead box M1; KD, knockdown; SOX2, sex-determining region Y-box 2. *p<0.05.
4. FOXM1 regulates colorectal cancer stemness by modulating SOX2 expression
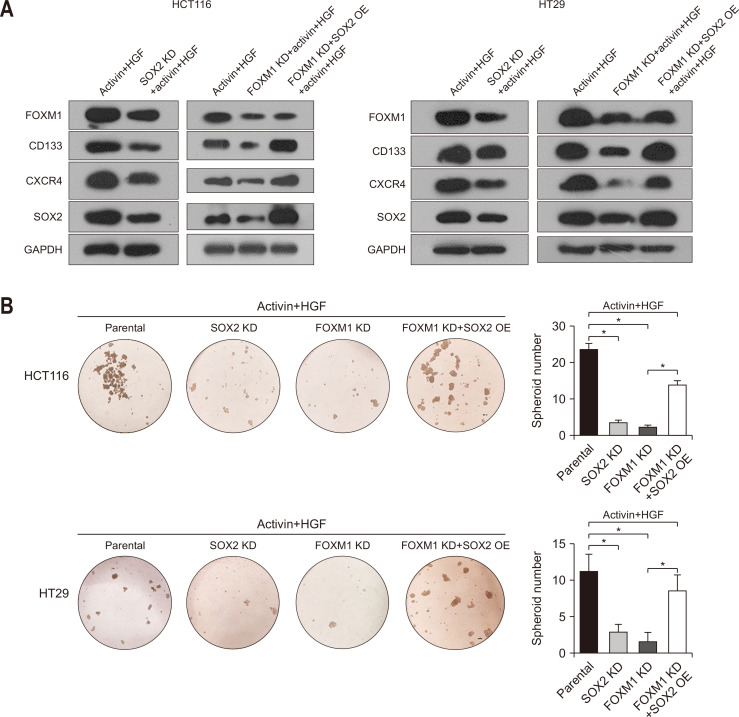
SOX2 is a key transcription factor involved in the induction of stem cell-like properties.22 Our data showed that silencing of SOX2 via specific shRNAs decreased the expression of CD133, CXCR4, and FOXM1 in HCT116 and HT29 cells exposed to activin and HGF (Fig. 4A). Consistently, depletion of SOX2 suppressed sphere formation of HCT116 and HT29 cells treated with activin and HGF (Fig. 4B). Since FOXM1 and SOX2 had a similar impact on activin/HGF-induced stemness in colorectal cancer cells (Figs 3 and 4), we speculated a possible link between FOXM1 and SOX2 in mediating activin and HGF activities. Taking into consideration that FOXM1 knockdown inhibited the expression of SOX2, we explored whether overexpression of SOX2 could rescue the stem cell-like phenotype in FOXM1-depleted colorectal cancer cells. Interestingly, enforced expression of SOX2 increased CD133 and CXCR4 expression and restored sphere formation in FOXM1-depleted HCT116 and HT29 cells upon activin and HGF treatment (Fig. 4A and B). These findings suggest the involvement of the FOXM1/SOX2 signaling cascade in activin/HGF-induced stemness in colorectal cancer cells.
Fig. 4.
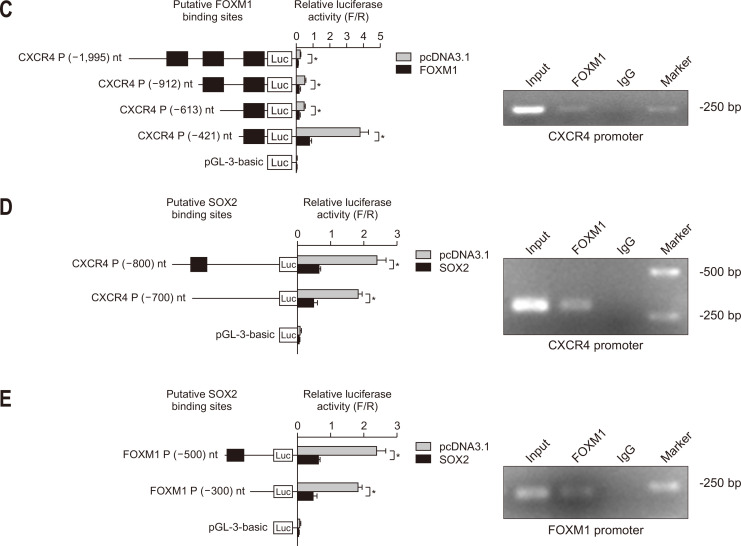
FOXM1 regulates colorectal cancer stemness by modulating SOX2 expression. (A) Western blot analysis of the indicated proteins in HCT116 and HT29 cells with SOX2 KD and the effect of SOX2 OE on protein levels in FOXM1-depleted HCT116 and HT29 cells. (B) Depletion of SOX2 suppressed sphere formation of HCT116 and HT29 cells treated with activin and HGF and enforced expression of SOX2 restored sphere formation in FOXM1-depleted HCT116 and HT29 cells upon activin and HGF treatment. (C) Luciferase reporter assays performed using the reporter constructs harboring different lengths of CXCR4 promoter fragments and ChIP assays demonstrate that FOXM1 is enriched in the promoter of CXCR4. (D) Luciferase reporter assays performed using the reporter constructs harboring different lengths of CXCR4 promoter fragments and ChIP assays demonstrate that SOX2 is enriched in the CXCR4 promoter. (E) Luciferase reporter assays performed using the reporter constructs harboring different lengths of FOXM1 promoter fragments and ChIP assays demonstrate that SOX2 is enriched in the FOXM1 promoter. FOXM1, forkhead box M1; SOX2, sex-determining region Y-box 2; KD, knockdown; OE, overexpression; HGF, hepatocyte growth factor; ChIP, chromatin immunoprecipitation; Luc, luciferase. *p<0.05.
5. The positive feedback of FOXM1 and SOX2 on CXCR4
Both FOXM1 and SOX2 are transcriptional factors. FOXM1 was reported to bind to the promoter of SOX2.23 Since FOXM1 and SOX2 regulated the expression of each other and CXCR4 (Figs 3A and 4A), we speculated that SOX2 could bind to the promoter of both CXCR4 and FOXM1 and FOXM1 could bind to the promoter of CXCR4. Jaspar was used to predict the possible bindings between transcriptional factors and promoters. FOXM1 was predicted to bind to the promoter of CXCR4 (-927/-921, -836/-830 and -177/-171). We constructed the CXCR4 promoter reporter pGL3-CXCR4 (position –1995 to +2) and its serial deletion mutant (Fig. 4C). The reporter activities of the pGL3-CXCR4 and its serial deletion mutant constructs were increased by FOXM1 overexpression. A ChIP assay in HCT116 cells using anti-FOXM1 antibody was used to validate the binding between FOXM1 and the promoter of CXCR4 (-177/-171). SOX2 was predicted to bind to the promoter of CXCR4 (-784/-774) and FOXM1 (-382/-372). We constructed the CXCR4 and FOXM1 promoter reporter pGL3-CXCR4 (position –800 to +100), pGL3-FOXM1 (position –500 to +100) and their serial deletion mutant (Fig. 4D and E). The reporter activities of the pGL3-CXCR4 and its serial deletion mutant constructs were increased by FOXM1 overexpression. ChIP assays in HCT116 cells using anti-SOX2 antibody were used to validate the binding between SOX2 and the promoter of CXCR4 (-784/-774) and FOXM1 (-382/-372). These findings suggest that the positive feedback of FOXM1 and SOX2 regulates the expression of CXCR4 in colorectal cancer cells.
6. Chemical inhibition of FOXM1 blocks activin/HGF-induced stemness and tumorigenesis of colorectal cancer cells
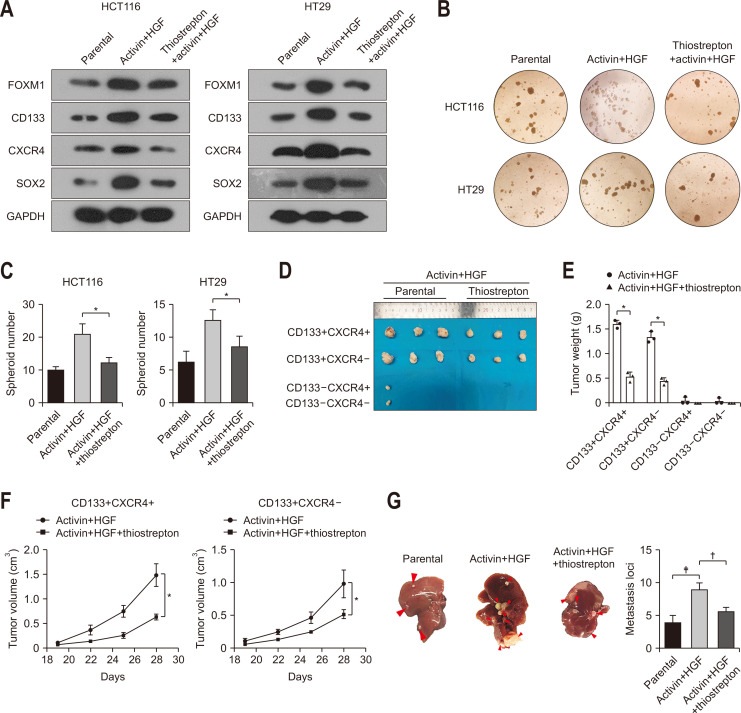
Next, we evaluated the significance of FOXM1 as a target to inhibit colorectal cancer stemness. Thiostrepton is commonly used as a chemical inhibitor of FOXM1.8 Inhibition of FOXM1 via thiostrepton attenuated the induction of CD133, CXCR4, and SOX2 in HCT116 and HT29 cells by activin and HGF treatment (Fig. 5A). Moreover, thiostrepton treatment of HCT116 and HT29 cells significantly prevented sphere formation induced by activin and HGF (Fig. 5B and C). The growth of subcutaneous tumors formed by activin/HGF-induced HCT116 cells was inhibited by thiostrepton treatment (Fig. 5D-F). Meanwhile, thiostrepton can affect metastasis of colorectal cancer cells with activin and HGF treatment (Fig. 5G).
Fig. 5.
Chemical inhibition of FOXM1 blocks activin/HGF-induced stemness, tumorigenesis and metastasis of colorectal cancer cells. (A) Western blot analysis of the indicated proteins in HCT116 and HT29 cells with or without thiostrepton treatment before exposure to activin and HGF. (B, C) Thiostrepton suppressed sphere formation of HCT116 and HT29 cells treated with activin and HGF. (D-F) Thiostrepton attenuated activin/HGF-induced tumorigenesis of CD133+CXCR4+ and CD133+CXCR4– HCT116 cells in nude mice. (G) Thiostrepton attenuated activin/HGF-induced liver metastasis (arrowheads) in HCT116 cells in nude mice. FOXM1, forkhead box M1; HGF, hepatocyte growth factor. *p<0.05, †p<0.01, ‡p<0.001.
DISCUSSION
CSCs have self-renewal and tumor-initiating capacities and thus play a central role in cancer development, metastasis, and drug resistance.1-3 Here, we show that activin and HGF are robust inducers of colorectal cancer stemness and metastasis. Sequential treatment with activin and HGF increases stem-cell properties of colorectal cancer cells in vitro. Consistently, colorectal cancer cells with pretreatment with activin and HGF become more aggressive in vivo and exhibit increased capacities to form subcutaneous xenograft tumors and liver metastases. Mechanistically, FOXM1 is induced by activin and HGF and mediates their oncogenic activities in colorectal cancer. These findings collectively suggest that activin and HGF promote colorectal cancer stemness and metastasis through induction of FOXM1.
Activin has been shown to regulate stem cell-like properties in both normal and malignant cells.24,25 Zhu et al.15 reported that HGF is secreted by myofibroblasts to induce stem cell features and tumorigenesis in gastric cancer cells. In this study, we demonstrated that activin plus HGF robustly promotes stemness in colorectal cancer cells. Moreover, the self-renewal capacity of the CD133+CXCR4+ colorectal cancer subpopulation is strikingly elevated upon activin and HGF treatment. It has been previously reported that CD133+CXCR4+ CSCs display a high metastatic property.7 Activation of CXCR4 signaling enhances colorectal cancer cell invasion.26 Consistent with these reports, colorectal cancer cells, in particular the CD133+CXCR4+ subpopulations, show increased tumorigenesis and liver metastasis after prior exposure to activin and HGF. Taken together, our data suggest that activin/HGF-mediated colorectal cancer metastasis is at least partially ascribed to enrichment of CD133+CXCR4+ subpopulations and enhancement of stemness.
A number of previous studies have indicated the importance of FOXM1 in colorectal cancer development and metastasis.10-12 Song et al.13 reported that FOXM1 is able to transactivate CD133 in colorectal cancer cells, thus enhancing cancer stemness. FOXM1-mediated stemness has also been described in other cancers such as esophageal squamous cell carcinoma27 and breast cancer.28 Our data demonstrate that FOXM1 is upregulated in colorectal cancer cells by activin and HGF. Knockdown of FOXM1 blunts activin/HGF-induced stemness and metastasis in colorectal cancer cells. These results indicate that FOXM1 signaling is involved in activin/HGF-mediated aggressive phenotype in colorectal cancer.
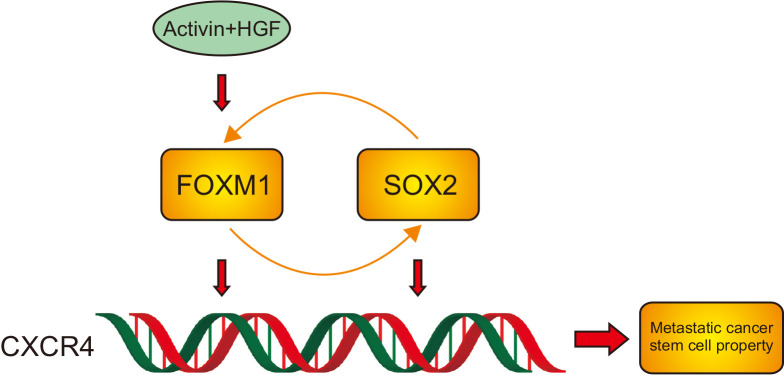
FOXM1 knockdown results in a marked decline in the expression of CSC markers including CD133, CD44, ALDH1, and OCT4. Additionally, FOXM1 knockdown suppresses the expression of SOX2 in colorectal cancer cells. SOX2 is known as a stem cell transcription factor.29-31 It has been reported that SOX2 can bind to the promoter of CD133-encoding PROM1 gene, driving its transcription.32 Zhu et al.31 reported that the SOX2-β-catenin/Beclin1 signaling pathway promotes stemness of colorectal cancer cells. Reduction of SOX2 has been shown to mediate the suppressive effect of ANGPTL1 on colorectal cancer stemness and invasion.30 Consistently, our data indicate that depletion of SOX2 blocks activin/HGF-induced stemness in colorectal cancer cells. Moreover, enforced expression of SOX2 rescued the self-renewal of FOXM1-depleted colorectal cancer cells. Additionally, chemical inhibition of FOXM1 suppresses SOX2 expression, self-renewal, and tumorigenic capacities in activin/HGF-treated colorectal cancer cells. Taken together, our data suggest that activin/HGF-induced stemness in colorectal cancer cells requires activation of the FOXM1/SOX2 signaling (Fig. 6).
Fig. 6.
A schematic model showing that activin/ hepatocyte growth factor (HGF)-induced stemness in colorectal cancer cells involves activation of the FOXM1/SOX2/CXCR4 signaling.
However, in this study, we did not validate the in vitro findings in clinical settings. More research is thus needed to determine the associations of activin and HGF with colorectal cancer stemness and metastasis. Additionally, it remains to be clarified to what extent the FOXM1/SOX2 signaling contributes to the aggressive phenotype of colorectal cancer.
In summary, our data indicate that sequential treatment with activin and HGF can enhance stemness and metastasis of colorectal cancer cells. The FOXM1/SOX2 signaling plays an important role in activin /HGF-induced stem cell properties in colorectal cancer. Targeting FOXM1 may be an efficient approach to control colorectal cancer development and metastasis.
SUPPLEMENTARY MATERIALS
Supplementary materials can be accessed at https://doi.org/10.5009/gnl220531
ACKNOWLEDGEMENTS
This work was supported by the Natural Science Foundation Project of CQ CSTC (Grant Nos. cstc2018jcyjAX0006 to J.G., cstc2021jcyj-msxmX0957 to L.D., cstc2019jcyj-msxmX0752 to J.T., CSTB2022NSCQ-MSX0137 to H.P., CSTB2022NSCQ-MSX0105 to Q.L.) and Science and Technology Research Project of Chongqing Municipal Education Commission (Grant No. KJQN202200463 to H.P.).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study concept and design: J.G., H.P. Data acquisition: H.P., T.Y., L.D., X.Y. Data analysis and interpretation: H.P., T.Y., L.D., X.Y., J.T. Drafting of the manuscript: H.P., Q.L., J.T. Critical revision of the manuscript for important intellectual content: H.P., Q.L, J.T. Statistical analysis: H.P., T.Y., X.Y. Obtained funding: J.G., H.P., L.D., Q.L., J.T. Administrative, technical, or material support; study supervision: J.G., H.P., L.D., Q.L. Approval of final manuscript: all authors.
REFERENCES
- 1.Fumagalli A, Oost KC, Kester L, et al. Plasticity of Lgr5-negative cancer cells drives metastasis in colorectal cancer. Cell Stem Cell. 2020;26:569–578. doi: 10.1016/j.stem.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujishita T, Kojima Y, Kajino-Sakamoto R, et al. The cAMP/PKA/CREB and TGFβ/SMAD4 pathways regulate stemness and metastatic potential in colorectal cancer cells. Cancer Res. 2022;82:4179–4190. doi: 10.1158/0008-5472.CAN-22-1369. [DOI] [PubMed] [Google Scholar]
- 3.Lamichhane A, Shahi Thakuri P, Singh S, et al. Therapeutic targeting of cancer stem cells prevents resistance of colorectal cancer cells to MEK inhibition. ACS Pharmacol Transl Sci. 2022;5:724–734. doi: 10.1021/acsptsci.1c00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirata A, Hatano Y, Niwa M, Hara A, Tomita H. Heterogeneity in colorectal cancer stem cells. Cancer Prev Res (Phila) 2019;12:413–420. doi: 10.1158/1940-6207.CAPR-18-0482. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Yang L, Lei W, et al. Single-cell sequencing reveals CD133+CD44--originating evolution and novel stemness related variants in human colorectal cancer. EBioMedicine. 2022;82:104125. doi: 10.1016/j.ebiom.2022.104125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J, Takeuchi H, Lam ST, et al. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–2753. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 7.Zhang SS, Han ZP, Jing YY, et al. CD133(+)CXCR4(+) colon cancer cells exhibit metastatic potential and predict poor prognosis of patients. BMC Med. 2012;10:85. doi: 10.1186/1741-7015-10-85.d4665f0ff67741f8a20a8ca692c4d91f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madhi H, Lee JS, Choi YE, et al. FOXM1 inhibition enhances the therapeutic outcome of lung cancer immunotherapy by modulating PD-L1 expression and cell proliferation. Adv Sci (Weinh) 2022;9:e2202702. doi: 10.1002/advs.202202702.a3694e30468b45b59b7a43088835829a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohata M, Imai J, Izumi T, et al. Roles of FoxM1-driven basal β-cell proliferation in maintenance of β-cell mass and glucose tolerance during adulthood. J Diabetes Investig. 2022;13:1666–1676. doi: 10.1111/jdi.13846.d05dbe7f5379427983242b019fd55228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weng W, Okugawa Y, Toden S, Toiyama Y, Kusunoki M, Goel A. FOXM1 and FOXQ1 are promising prognostic biomarkers and novel targets of tumor-suppressive mir-342 in human colorectal cancer. Clin Cancer Res. 2016;22:4947–4957. doi: 10.1158/1078-0432.CCR-16-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sher G, Masoodi T, Patil K, et al. Dysregulated FOXM1 signaling in the regulation of cancer stem cells. Semin Cancer Biol. 2022;86(Pt 3):107–121. doi: 10.1016/j.semcancer.2022.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Yuan B, Liu Y, Yu X, et al. FOXM1 contributes to taxane resistance by regulating UHRF1-controlled cancer cell stemness. Cell Death Dis. 2018;9:562. doi: 10.1038/s41419-018-0631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song IS, Jeong YJ, Jeong SH, et al. FOXM1-induced PRX3 regulates stemness and survival of colon cancer cells via maintenance of mitochondrial function. Gastroenterology. 2015;149:1006–1016. doi: 10.1053/j.gastro.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Perkhofer L, Walter K, Costa IG, et al. Tbx3 fosters pancreatic cancer growth by increased angiogenesis and activin/nodal-dependent induction of stemness. Stem Cell Res. 2016;17:367–378. doi: 10.1016/j.scr.2016.08.007.9019fa26caa448eea278bc5735935149 [DOI] [PubMed] [Google Scholar]
- 15.Zhu L, Cheng X, Shi J, et al. Crosstalk between bone marrow-derived myofibroblasts and gastric cancer cells regulates cancer stemness and promotes tumorigenesis. Oncogene. 2016;35:5388–5399. doi: 10.1038/onc.2016.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vermeulen L, De Sousa E Melo F, van der Heijden M, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 17.Hay DC, Zhao D, Fletcher J, et al. Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells. 2008;26:894–902. doi: 10.1634/stemcells.2007-0718. [DOI] [PubMed] [Google Scholar]
- 18.Peng H, Ye T, Deng L, Yang X, Jiang Z, Guo J. Sequential treatment with activin and hepatocyte growth factor induces FOXM1 to promote colorectal cancer liver metastasis. Can J Gastroenterol Hepatol. 2022;2022:8996203. doi: 10.1155/2022/8996203.7ea2cd40900443a5b06a0d97cacda86b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daitoku N, Miyamoto Y, Hiyoshi Y, et al. Activin A promotes cell proliferation, invasion and migration and predicts poor prognosis in patients with colorectal cancer. Oncol Rep. 2022;47:107. doi: 10.3892/or.2022.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joosten SP, Spaargaren M, Clevers H, Pals ST. Hepatocyte growth factor/MET and CD44 in colorectal cancer: partners in tumorigenesis and therapy resistance. Biochim Biophys Acta Rev Cancer. 2020;1874:188437. doi: 10.1016/j.bbcan.2020.188437. [DOI] [PubMed] [Google Scholar]
- 21.Takata A, Otsuka M, Kogiso T, et al. Direct differentiation of hepatic cells from human induced pluripotent stem cells using a limited number of cytokines. Hepatol Int. 2011;5:890–898. doi: 10.1007/s12072-011-9251-5. [DOI] [PubMed] [Google Scholar]
- 22.Mamun MA, Mannoor K, Cao J, Qadri F, Song X. SOX2 in cancer stemness: tumor malignancy and therapeutic potentials. J Mol Cell Biol. 2020;12:85–98. doi: 10.1093/jmcb/mjy080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H, Song Y, Qiu H, et al. Downregulation of FOXO3a by DNMT1 promotes breast cancer stem cell properties and tumorigenesis. Cell Death Differ. 2020;27:966–983. doi: 10.1038/s41418-019-0389-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ottaviani S, Stebbing J, Frampton AE, et al. TGF-β induces miR-100 and miR-125b but blocks let-7a through LIN28B controlling PDAC progression. Nat Commun. 2018;9:1845. doi: 10.1038/s41467-018-03962-x.6b26f0de8fb24a82b752ef7f07fc000b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao L, Yuan X, Sharkis SJ. Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells. Stem Cells. 2006;24:1476–1486. doi: 10.1634/stemcells.2005-0299. [DOI] [PubMed] [Google Scholar]
- 26.Wang B, Wang W, Niu W, et al. SDF-1/CXCR4 axis promotes directional migration of colorectal cancer cells through upregulation of integrin αvβ6. Carcinogenesis. 2014;35:282–291. doi: 10.1093/carcin/bgt331. [DOI] [PubMed] [Google Scholar]
- 27.Xu P, Wang L, Liu Q, et al. The abnormal expression of circ-ARAP2 promotes ESCC progression through regulating miR-761/FOXM1 axis-mediated stemness and the endothelial-mesenchymal transition. J Transl Med. 2022;20:318. doi: 10.1186/s12967-022-03507-3.924899293ad54729a877b3769afba8ca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun HL, Men JR, Liu HY, Liu MY, Zhang HS. FOXM1 facilitates breast cancer cell stemness and migration in YAP1-dependent manner. Arch Biochem Biophys. 2020;685:108349. doi: 10.1016/j.abb.2020.108349. [DOI] [PubMed] [Google Scholar]
- 29.Zhao N, Zhou L, Lu Q, et al. SOX2 maintains the stemness of retinoblastoma stem-like cells through Hippo/YAP signaling pathway. Exp Eye Res. 2022;214:108887. doi: 10.1016/j.exer.2021.108887. [DOI] [PubMed] [Google Scholar]
- 30.Chang TY, Lan KC, Chiu CY, Sheu ML, Liu SH. ANGPTL1 attenuates cancer migration, invasion, and stemness through regulating FOXO3a-mediated SOX2 expression in colorectal cancer. Clin Sci (Lond) 2022;136:657–673. doi: 10.1042/CS20220043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu Y, Huang S, Chen S, et al. SOX2 promotes chemoresistance, cancer stem cells properties, and epithelial-mesenchymal transition by β-catenin and Beclin1/autophagy signaling in colorectal cancer. Cell Death Dis. 2021;12:449. doi: 10.1038/s41419-021-03733-5.af0e54769cd24e458803227f8ea439e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iida H, Suzuki M, Goitsuka R, Ueno H. Hypoxia induces CD133 expression in human lung cancer cells by up-regulation of OCT3/4 and SOX2. Int J Oncol. 2012;40:71–79. doi: 10.3892/ijo.2011.1207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.