Abstract
Background/Aims
Recent studies indicate that probiotics, which have attracted attention as a treatment for irritable bowel syndrome, affect intestinal homeostasis. In this study, we investigated whether Zygosaccharomyces sapae (strain I-6), a probiotic yeast isolated from miso (a traditional Japanese fermented food), could improve irritable bowel syndrome symptoms.
Methods
Male Wistar rats were exposed to water avoidance stress (WAS). The number of defecations during WAS and the visceral hypersensitivity before and after WAS were evaluated using colorectal distension. Tight junction changes were assessed by Western blotting. Some rats were fed with strain I-6 or β-glucan from strain I-6. Changes in the intestinal microbiota were analyzed. The effect of fecal microbiota transplantation after WAS was evaluated similarly. Caco-2 cells were stimulated with interleukin-1β and tight junction changes were investigated after coculture with strain I-6.
Results
The increased number of stool pellets and visceral hypersensitivity induced by WAS were suppressed by administering strain I-6. The decrease in tight junction protein occludin by WAS was reversed by the administration of strain I-6. β-Glucan from strain I-6 also suppressed those changes induced by WAS. In the rat intestinal microbiota, treatment with strain I-6 altered the β-diversity and induced changes in bacterial occupancy. Upon fecal microbiota transplantation, some symptoms caused by WAS were ameliorated.
Conclusions
These results suggest that traditional fermented foods such as miso in Japan are valuable sources of probiotic yeast candidates, which may be useful for preventing and treating stress-induced visceral hypersensitivity.
Keywords: Irritable bowel syndrome, Yeasts, Fermented foods, Tight junction proteins, Gut microbiota
INTRODUCTION
The intestinal epithelial barrier is one of the most important factors in protecting gut homeostasis from continuous exposure to microbial pathogens and antigens, and is simultaneously responsible for nutrient and water uptake.1 Many previous studies have demonstrated that patients with irritable bowel syndrome (IBS) show dysfunction of the intestinal epithelial barrier and gut microbiota, which have been linked with visceral hypersensitivity and pain.2-6 Several studies have suggested mast cells as crucial effector cells of stress-induced stimulation in the intestine, which release multifunctional mediators such as proteases and histamine, leading to increase visceral hyper sensitivity.7 A recent study has shown that increases in epithelial permeability and mast cell activation by psychological stress were stronger in the small intestine than in the colon in the rats, suggesting importance of small intestinal injury in the stress-induced visceral hypersensitivity.8 Tight junctions (TJs) are essential for intestinal barrier function and regulate the paracellular permeability of materials.9 TJs are composed of more than 50 proteins including occludin family members which are transmembrane proteins.10
Recently, probiotics have been attracting increasing attention as a treatment for IBS, as many previous studies have shown that probiotics influence intestinal homeostasis, such as intestinal barrier functions, through TJ complex regulation.11-14 Traditional Japanese fermented foods contain not only eubacteria but also yeasts classified as fungi. In addition to probiotic strains belonging to eubacteria, yeasts have also been used as probiotics for treating IBS.12 However, few studies have examined the effects of yeasts from traditional Japanese fermented foods on IBS.
Miso, a fermented soybean product made from rice or barley malt and salt, is one of the most popular traditional Japanese fermented foods and seasonings. The fermentation process of miso is known to include many species of yeasts and other microorganisms.15 Miso has been reported to have positive effects in several human health conditions.16 We previously isolated a probiotic yeast strain, Zygosaccharomyces sapae (strain I-6) from miso and reported that it could attenuate colitis in a murine model.17 However, the effect of this strain on the IBS model has not been investigated.
In this study, we examined the effects of strain I-6 and its β-glucan on intestinal barrier dysfunction, visceral hypersensitivity in response to colorectal distension, and gut microbiota modulation in a rat model of IBS induced by water avoidance stress (WAS). To investigate the effect of strain I-6 on modulating the barrier functions of intestinal epithelial cells in vitro, we used a human intestinal epithelial cell line (Caco-2) and focused on the involvement of Dectin-1 and Toll-like receptor 2 (TLR2) on the cell surface in the expression of the TJ protein occludin.18
MATERIALS AND METHODS
1. Animals and probiotic yeast cells
Four-week-old male Wistar rats were purchased from Japan SLC Inc. (Shizuoka, Japan). Rats were housed in plastic cages with normal 12-hour light-dark cycles at a temperature of 23℃ to 24℃ with 55% humidity. The rats were fed a standard laboratory diet (CLEA Japan, Tokyo, Japan) and had free access to food and water. Laboratory animals were used in accordance with the guidelines of the “Methods and Welfare Considerations in Behavioral Research with Animals: report of a National Institutes of Health Workshop” published by the US National Institutes of Health (Bethesda, MD, USA) along the guidelines of the animal facility at the National Defense Medical College in Japan. All experimental protocols were approved by the Animal Research Committee of the National Defense Medical College (No. 18002).
Strain I-6 was obtained from our laboratory collection.17 Living strain I-6 cells were fed to the rats ad libitum from a water bottle at a concentration of 5×106 colony-forming units per milliliter. We also extracted β-glucan from strain I-6, as previously described.19 Similar to the cells, strain I-6 β-glucans were fed to the rats ad libitum from a water bottle at a concentration of 600 mg/L.
2. Animal experimental protocols
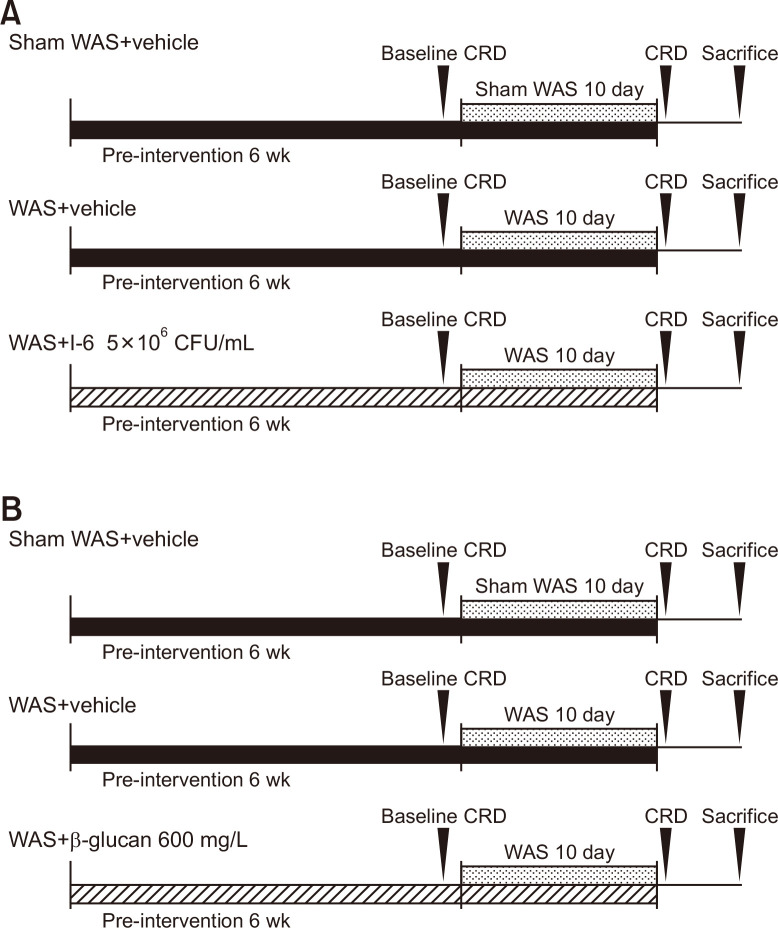
Rats were divided into three groups: sham WAS+vehicle group, WAS+vehicle group, and WAS+I-6 or β-glucan group. The animals were given water with or without strain I-6 or β-glucan for 6 weeks as a pre-intervention and allowed to drink throughout the experiment. After pre-intervention, chronic WAS in rats was used as a model of IBS (Fig. 1).20,21 The WAS protocol has been described in previous reports.22 Briefly, WAS rats were placed on a glass platform (10×10 cm) in the middle of a box (46.5×37×24 cm) filled with water (23℃) to 1 cm below the height of the platform for 60 minutes per day. The rats were placed on the platform, sham WAS rats were placed in a box without water. We counted the number of stool pellets while rats were receiving WAS for 60 minutes.
Fig. 1.
Experimental design. (A) Protocol for comparison with I-6. (B) Protocol for comparison with β-glucan. WAS, water avoidance stress; CRD, colorectal distension; CFU, colony-forming units.
3. Evaluation of visceral hypersensitivity
Visceral hypersensitivity was evaluated using colorectal distension, as previously described with a barostatometer (Distender Series IIR; G&J Electronics Inc., Toronto, ON, Canada).23 Rats were placed in an acrylic case, and a collapsible balloon was inserted 4 cm beyond the anus of each rat under anesthesia. Balloon pressure was increased at a constant rate of 1 mm Hg/s, and the threshold of the abdominal withdrawal reflex (AWR) was measured three times; the mean value was taken as the threshold AWR value. We performed colorectal distension on the day before the start of WAS (day 0) and on the day after the end of the WAS (day 11). the changes in AWR on day 11 from day 0 (baseline) were calculated and compared among groups.
4. Effect of fecal microbiota transplantation (FMT) on visceral hypersensitivity
Four-week-old male Wistar rats were purchased and bred as previously described. Rats were given water with strain I-6 at a concentration of 5×106 colony-forming units per milliliter for 6 weeks, followed by collection of fresh stools. For the control groups, water was given (n=2) for free drinking. The stool samples were diluted 10-fold with phosphate-buffered saline.
Seven-week-old male Wistar rats were purchased and divided into the control group (WAS+control stool) and the I-6 group (WAS+I-6 stool) (n=6). Rats were anesthetized using isoflurane and 1 mL of stool was transgastrically administered twice daily during the WAS treatment. Visceral hypersensitivity was compared between the two groups.
5. Effect of I-6 on cell lines in vitro
Caco-2 was obtained from Riken BRC (Tsukuba, Japan) and maintained in Dulbecco’s modified Eagle’s medium containing 15% fetal bovine serum, 1% nonessential amino acids, and 1% penicillin-streptomycin solution at 37℃ and 5% CO2. The number of passages was approximately 20–40 and 1×106 cells were seeded in 6-well plates and used after 21 days with medium changes every 2 days. Cells were stimulated with recombinant interleukin-1β (IL-1β; 10 ng/mL) (Proteintech Group, Inc., Rosemont, IL, USA) at 6 hours before cell harvest. In some experiments, cells were pretreated with strain I-6 (80 µg/mL) at 48 hours before cell harvest. In some experiments, anti-Dectin-1 antibody (0.2 ng/mL) (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and anti-TLR2 antibody (0.2 ng/mL) (Abcam, Cambridge, UK) were added 30 minutes before treatment with strain I-6.
6. Western blot analysis
Ileal end tissues and Caco-2 cells were homogenized in 0.5% sarcosylic acid-containing protease inhibitor (Roche Diagnostics K.K., Tokyo, Japan) and phosphatase inhibitor (Roche Diagnostics K.K.). The samples were shaken every 5 minutes for 30 minutes and then centrifuged for 5 minutes at 14,000 rpm at 4℃. The protein concentration was assessed using a DC protein assay kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). A total of 25 µg of proteins were separated in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis for 45 minutes at 200 V using Electrophoresis Power Supply EPS 1001 (Amersham Pharmacia Biotech, GE Healthcare, Brooklyn, NY, USA). Then it was transferred onto polyvinylidene difluoride membranes for 30 minutes at 12 V using BP-312 (BIO CRAFT, Tokyo, Japan). Membranes were blocked with 5% skim milk or 3% bovine serum albumin dissolved in phosphate buffered saline with tween 20 and incubated with the primary antibodies to occludin (Abcam) (1:1,000) and β-actin (Abcam) (1:4,000) at 4℃ overnight. After washing, the blots were incubated with anti-rabbit IgG HRP-linked antibody (Cell Signaling Technology, Inc., Danvers, MA, USA) (1:4,000) for 1 hour at room temperature. Protein bands were detected using Clarity Western ECL Substrate (Bio-Rad Laboratories Inc.). Blot images were electronically scanned using ChemiDoc Touch (Bio-Rad Laboratories Inc.).
7. High-throughput sequencing of the gut microbiota
Stool samples were collected from sham WAS+vehicle group, sham WAS+I-6 group, WAS+vehicle group and WAS+I-6 group after completion of WAS protocol and stored at –80℃. Bacterial DNA was extracted from stool samples using the QIAamp Fast DNA stool mini kit (Qiagen, Valencia, CA, USA). The variable region V3-4 of the 16S rRNA was amplified twice by PCR using specified primers and Nextera XT Index Kit (Illumina, San Diego, CA, USA). The PCR products were purified by AMPure XP beads (Beckman Coulter Genomics, Brea, CA, USA). Library quantification, normalization, and pooling were performed according to the 16S Metagenomic Sequencing Library Preparation (Illumina). The size and quality of the pooled libraries were ascertained using MultiNA (Shimadzu Corp., Kyoto, Japan). Following NaOH denaturation, the libraries were loaded into the MiSeq reagent kit V3 and sequenced on a MiSeq instrument (Illumina). The sequence data were analyzed using the Quantitative Insights Into Microbial Ecology 2 tool.24
8. Statistical analysis
Comparisons among groups were undertaken using one-way analysis of variance followed by the Tukey-Kramer post hoc test, as appropriate. Comparison of the relative abundance of intestinal microflora was made using the Kruskal-Wallis test followed by the Steel-Dwass post hoc test. A p-value of <0.05 was used to indicate statistical significance. All analyses were performed using JMP Pro, version 14, 3. 0 (SAS Institute Inc., Cary, NC, USA).
RESULTS
1. Probiotic yeast strain I-6 ameliorates symptoms in WAS-treated rats
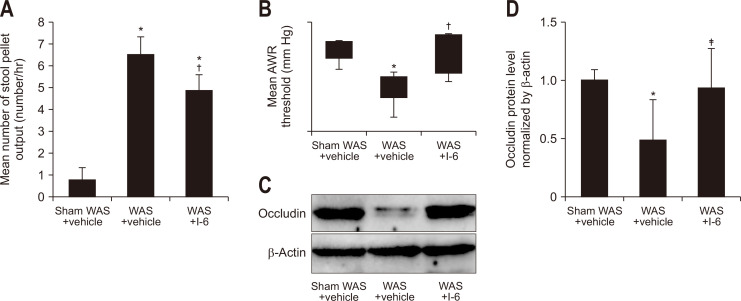
WAS treatment significantly increased the number of stool pellet output per hour compared to sham WAS treatment. The WAS with strain I-6 group significantly suppressed increase in the number of stool pellets output compared to the WAS with vehicle group (Fig. 2A). In addition, WAS treatment significantly decreased the AWR threshold compared to sham WAS treatment. The WAS with I-6 group significantly suppressed the decrease in the AWR threshold compared to the WAS with vehicle group (Fig. 2B).
Fig. 2.
Strain I-6 showed the attenuation of effects of water avoidance stress (WAS). (A, B) The rats were divided into the sham WAS+vehicle group (n=8), the WAS+vehicle group (n=10), the WAS+I-6 group (n=8). (A) Effect of oral administration of strain I-6 on the number of stool pellets in WAS-treated rats. (B) Effect of oral administration of strain I-6 on the abdominal withdrawal reflex threshold in WAS-treated rats. (C) Effect of oral administration of strain I-6 on the expression of occludin in ileal tissue. Representative Western blotting of occludin is shown in the upper blot. Equal loading of the crude protein levels was confirmed using antibodies for β-actin, as shown in the lower blot. (D) Quantitative analysis of occludin protein. The detected bands were quantified using ChemiDoc Touch, Bio-Rad Laboratories, Inc., CA, USA. The occludin expression in the sham WAS+vehicle group was assigned a value of 1, and results are expressed as means±SDs (n=7, 8, 6). The rats were divided into the same three groups. *p<0.01 compared with the sham WAS+vehicle group; †p<0.01 compared with the WAS+vehicle group; ‡p<0.05 compared with the WAS+vehicle group.
2. Probiotic yeast strain I-6 ameliorates intestinal barrier function in WAS-treated rats
Increased intestinal permeability through a decrease in the TJ protein occludin results in altered intestinal permeability in IBS.25-27 The Western blotting analysis showed that the expression of occludin was decreased in the WAS with vehicle group compared to the sham WAS with vehicle group (Fig. 2C and D). This reduction was significantly inhibited by the addition of I-6 to the WAS treatment (Fig. 2C).
3. β-Glucan extracted from the probiotic yeast strain I-6 also ameliorates symptoms in WAS-treated rats
Furthermore, we investigated whether β-glucan extracted from the cell bodies of I-6 altered stress-induced visceral hypersensitivity, as many previous studies have shown that β-glucan is a major structural component of various yeast cells and is a potent biological agent for human and rodent experimental models.25-28
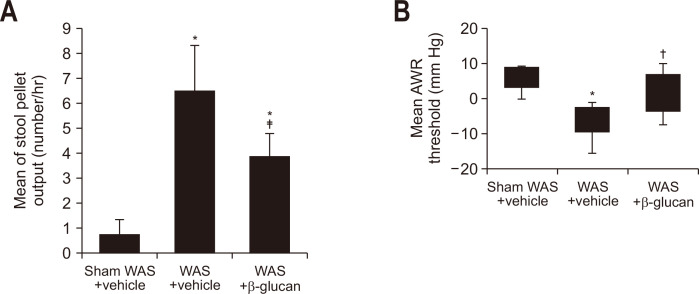
There was a significant increase in the number of stool pellets in the WAS with vehicle group compared to the sham WAS with vehicle group (Fig. 3A), but this was significantly lower in the WAS with β-glucan group compared to the WAS with vehicle group (Fig. 3A). Further, a decrease in the AWR threshold value was observed in the WAS with vehicle group compared to the sham WAS with vehicle group; these reductions were alleviated in the WAS with β-glucan group (Fig. 3B).
Fig. 3.
β-Glucan extracted from strain I-6 demonstrated the attenuation of effects of water avoidance stress (WAS). (A) Effect of oral administration of β-glucan on the number of stool pellets in WAS-treated rats. (B) Effect of oral administration of β-glucan on the abdominal withdrawal reflex threshold in WAS-treated rats. The rats were divided into a sham WAS+vehicle group (n=8), a WAS+vehicle group (n=10), and a WAS+β-glucan group (n=8). Results are expressed as means±SDs. The sham WAS+vehicle group and the WAS+vehicle group are the same as those in Fig. 2. *p<0.01 compared with the sham WAS+vehicle group; †p<0.05, ‡p<0.01 compared with the WAS+vehicle group.
4. Administration of probiotic yeast strain I-6 induced changes in the gut microbiota of rats
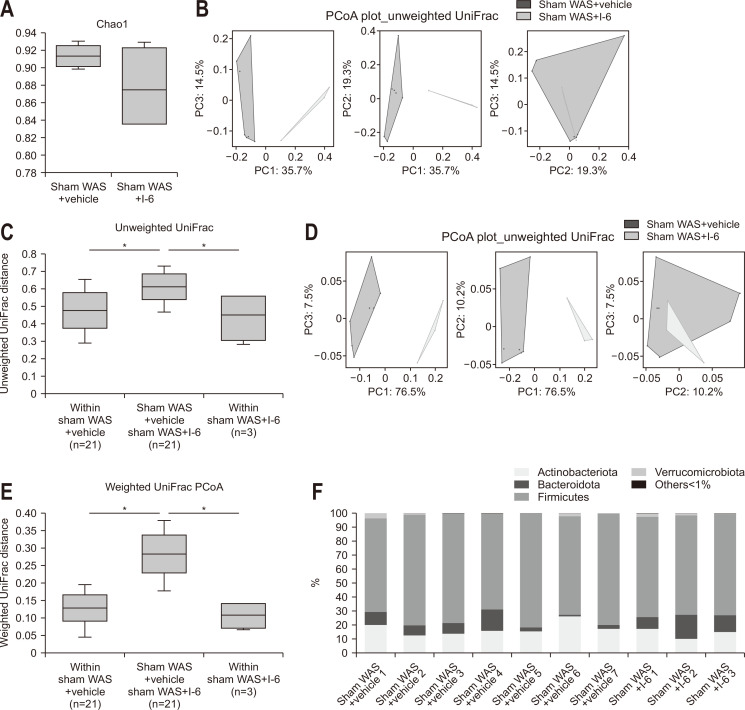
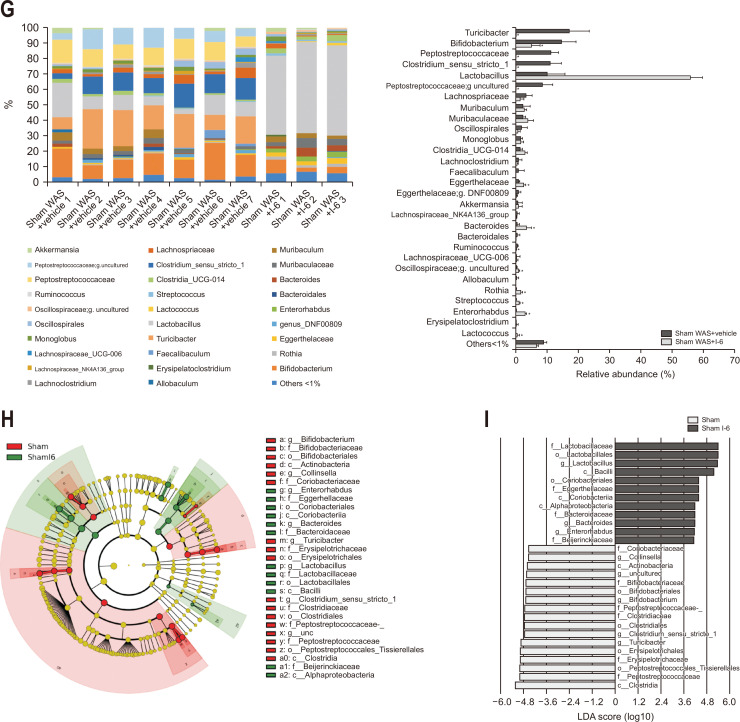
Analysis of the collected stool pellets showed no difference in α-diversity (Fig. 4A). In contrast, principal coordinate analysis for β-diversity indicated that the sham WAS+I-6 group had a distinctly different distribution (Fig. 4B and D) and showed significant difference by permutational multivariate analysis of variance analysis (Fig. 4C and E). The mean UniFrac distance analysis (unweighted) showed that the mean UniFrac distance between the sham WAS+vehicle group and the sham WAS+I-6 group was significantly higher than that within the sham WAS+vehicle group and within the sham WAS+I-6 group (Fig. 4B and C). Further analysis (weighted) showed that the mean UniFrac distance between the sham WAS+vehicle group and the sham WAS+I-6 group was significantly higher than that within the sham WAS+vehicle group and the sham WAS+I-6 group (Fig. 4D and E).
Fig. 4.
Strain I-6 treatment altered the β-diversity and intestinal bacterial abundance in rats. (A) Effect of strain I-6 on α-diversity. (B) Effect of strain I-6 on β-diversity, unweighted Unifrac principal coordinate analysis (PCoA) plot. (C) Quantification and comparison of the effect of strain I-6 on β-diversity, weighted Unifrac PCoA plot. (D) Effect of strain I-6 on β-diversity, weighted Unifrac PCoA plot. (E) Quantification and comparison of the effect of strain I-6 on β-diversity, weighted Unifrac PCoA plot. (F, G) Effect of strain I-6 on the relative intestinal bacterial abundance, phylum level and gene level. Linear discriminant analysis (LDA) effect size (LEfSe) was used to calculate the taxa that best discriminated between the sham water avoidance stress (WAS)+vehicle group and the sham WAS+I-6 group. (H) Expressed in a cladogram, taxa that reached a linear discriminant analysis score (log10) of >2.0 are highlighted and labeled accordingly. (I) LDA score>4.0 at taxonomic levels. The rats were divided into a sham WAS+vehicle group (n=7) and a sham WAS+I-6 group (n=3). Results are expressed as means±SDs. *p<0.01 compared with the sham WAS+vehicle group.
The composition of the gut microbiota was also analyzed. At the phylum level, there was no significant difference between the two groups in the five phyla with more than 1% abundance (Fig. 4F). At the genus level, in 29 genera with more than 1% abundance, the sham WAS+vehicle group showed significant changes in the abundance of 13 species (Fig. 4G). We applied LEfSe analysis to explore the taxa that best discriminated bacterial populations between the two groups. In the sham WAS+I-6 group, we found a pronounced deposition of the Lactobacillaceae and a high linear discriminant analysis ascore of this taxa (Fig. 4H and I).
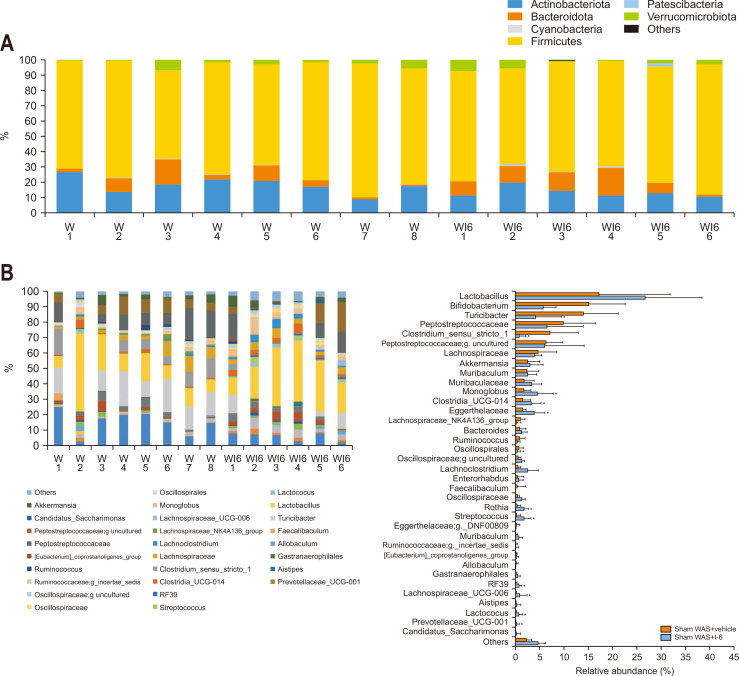
5. Probiotic yeast I-6 induced changes in the gut microbiota of WAS-treated rats
The composition of the gut microbiota was also analyzed in stress-received animals. At the phylum level, there was no significant difference between the two groups in the five phyla with more than 1% abundance (Fig. 5A). At the genus level, in 37 genera with more than 1% abundance, the WAS+I-6 group showed significant changes in the abundance of 14 species (Fig. 5B).
Fig. 5.
Strain I-6 treatment altered the intestinal bacterial abundance in stressed rats. (A, B) Effect of strain I-6 on the relative intestinal bacterial abundance, phylum level and gene level. The rats were divided into a water avoidance stress (WAS)+vehicle group (n=8) and a WAS+I-6 group (n=6). Results are expressed as means±SDs. *p<0.05, †p<0.01 compared with the WAS+vehicle group.
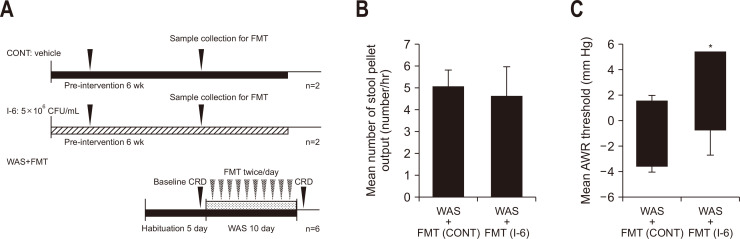
6. FMT from rats treated with strain I-6 ameliorated some of the IBS symptoms
To investigate whether changes in the gut microbiota are involved in the pathophysiology of the ameliorating effect of strain I-6 on stress-induced visceral hypersensitivity, we investigated whether amelioration of stress-induced visceral hypersensitivity in WAS-treated rats was transferrable by FMT from I-6 treated animals. We then compared the number of stool pellets and AWR of the control group with that of the I-6 group (Fig. 6A). There was no significant difference in the number of stool pellets (Fig. 6B). The AWR threshold value of the I-6 group was significantly higher than that of the control group (Fig. 6C).
Fig. 6.
Fecal microbiota transplantation (FMT) demonstrated a partial attenuation of the effects of water avoidance stress (WAS). (A) Experimental design. (B) Effect of FMT on the number of stool pellets in WAS-treated rats. (C) Effect of FMT on the abdominal withdrawal reflex (AWR) threshold in WAS-treated rats. The rats were divided into a control group (n=6) and an I-6 group (n=6). Results are expressed as means±SDs. CONT, control group; CFU, colony-forming units; CRD, colorectal distension. *p<0.05 compared with the control group.
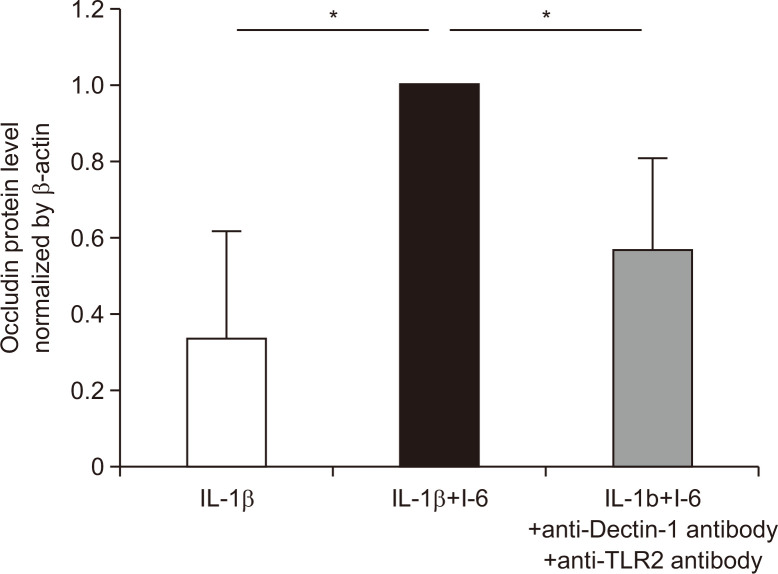
7. Strain I-6 prevented a decrease in TJ protein in vitro through Dectin-1 and TLR2
Finally, we investigated the mechanism of I-6-induced modification of occludin expression in Caco-2 cells in vitro. It was increased in the IL-1β+I-6 group compared with that in the IL-1β group, and these increases were significantly prevented in the IL-1β+I-6+anti-Dectin-1 antibody and anti-TLR2 antibody groups (Fig. 7).
Fig. 7.
Probiotic yeast (strain I-6) isolated from miso suppressed the decrease of occludin protein expression in Caco-2 cells under interleukin-1β (IL-1β) treatment. Quantitative analysis of occludin protein in the IL-1β group, IL-1β+I-6 group and IL-1β+I-6 + neutralizing antibody group (anti-Dectin-1 and anti-Toll-like receptor 2 [TLR2]). The detected bands were quantified using ChemiDoc Touch, Bio-Rad Laboratories, Inc., CA, USA. The occludin expression in the IL-1β+I-6 group was assigned a value of 1, and results are expressed as means±SDs (n=6, 14, 8). *p<0.01 compared with the IL-1β+ I-6 group.
DISCUSSION
Here, we showed that oral administration of I-6 and β-glucan of strain I-6 ameliorated the symptoms in a rat model of IBS. The magnitude of visceral hypersensitivity and number of stool pellets were significantly smaller in the WAS with I-6 group compared with those in the WAS group. Additionally, stress-induced reduction of occludin expression in the WAS group was significantly ameliorated by I-6 treatment (Fig. 2). A previous study has reported that IBS symptoms are accompanied with an increase in intestinal permeability in a rat model of IBS, and that occludin is decreased during this process.5 Furthermore, increased intestinal permeability through a decrease in TJ proteins such as occludin is one of the main pathogenic features of human IBS, and its improvement is an important target of IBS treatment.2,4,13 These findings suggest that I-6 ameliorated the symptoms in a rat model of IBS by recovering intestinal permeability through increased occludin expression. Saccharomyces boulardii, a conventional probiotic yeast, improved intestinal dysmotility and dysfunction in an animal model of IBS, but its mechanism of action remains unknown.29,30 However, the major mechanism of action of previously reported probiotics classified in eubacteria (such as strains of the genus Lactobacillus or Bifidobacterium), for improving visceral hypersensitivity, involves the action of metabolites produced by living bacterial cells, such as neurotransmitters and neuroactive substances including γ-aminobutyric acid and serotonin, on sensitive nerve endings in the gut mucosa.31 However, our data demonstrate that β-glucan from strain I-6 could also improve the symptoms (Fig. 3). These findings suggest that the cell bodies of strain I-6, but not the metabolites from I-6, are responsible for the improvement of symptoms in a rat model of IBS. However, because physiological dynamics including digestion and absorption of β-glucan were not taken into account in our in vitro experiments, pathophysiology of β-glucan from I-6 should be examined by using in vivo study in future.
We then investigated whether strain I-6 directly increased the expression of occludin in intestinal epithelial cells in vitro using IL-1β exposed Caco-2 cells. Our results demonstrated the direct effect of I-6 on epithelial TJ protein expression and indicated that this effect was mediated by both Dectin-1 and TLR2 on the surface of Caco-2 cells (Fig. 7). Although the whole yeast cell body and their β-glucans are recognized by cell surface receptors, Dectin-1 and TLR2, are expressed on innate immune cells such as macrophages and neutrophils;17,32 Dectin-1 and TLR2 are also regularly expressed on intestinal epithelial cells, and TJ functions are reported to be regulated by these receptors.33,34 A previous study showed that certain probiotic yeasts, S. boulardii, increased the expression of TJ proteins, including occludin, in an animal experimental colitis model in vivo.35 Based on these findings, our work is the first to indicate that the cell bodies of I-6, classified as probiotic yeasts, strongly increase occludin expression in intestinal epithelial cells in vitro via both Dectin-1 and TLR2.
Although the effect of I-6 on the rat IBS model has been explained by increasing intestinal permeability, we also investigated the effects of oral administration of strain I-6 on gut microbiota, because dysbiosis is involved in the pathogenesis of IBS, and a systematic review or meta-analysis showed the usefulness of probiotic therapy in the treatment of IBS.36,37 Surprisingly, high-throughput sequencing of the gut microbiota showed significant changes in β-diversity after oral administration of strain I-6 (Fig. 4B-E). In the genus-level composition of the gut microbiota, Lactobacillus spp. was increased significantly after I-6 treatment (Fig. 4G-I). There have been frequent reports of decreased Lactobacillus spp. in the stools of IBS patients.38-40 It has also been reported that β-glucan increases Lactobacillus spp.41,42 In addition, probiotic strains, such as Lactobacillus spp., have been reported to be useful in IBS.43,44 Recently it has been reported that intestinal transit time changes gut microbiota by washing out microbiota of slow growth potential.45 Thus, effect of changes of microbiota by I-6 could be caused just secondary to improvement of IBS symptoms. In this study, to determine whether changes of microbiota by strain I-6 were responsible for ameliorating effect on stress-induced visceral hypersensitivity or it was just secondary to improvement, we investigated protective effect of I-6 on stress-induced visceral hypersensitivity were transferrable by FMT from I-6 treated animals. Although the number of stool pellets between the control group and the I-6 group was not significantly different, the magnitude of the AWR threshold was significantly lower in the I-6 group than that in the control group, suggesting that the effect of I-6 on stress-induced visceral hypersensitivity is partly mediated by altered gut microbiota (Fig. 6). Recently, yeast β-glucan has been reported to exert strong probiotic activity similar to digestive fiber because it is difficult to hydrolyze in the stomach or small intestine and to be metabolized by gut microbiota in the colon.46
In conclusion, Z. sapae strain I-6 isolated from miso is remarkable in its ability to induce improvement of stress-induced visceral hypersensitivity in rats through two modes of action: direct modulation of occludin expression in epithelial cells and probiotic activity.
ACKNOWLEDGEMENTS
This research was supported by grants from the National Defense Medical College.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study concept and design: N.S., Y.O., R.H. Data acquisition: N.S., Y.O., A.T., S.I., R.T., S.N., A.M., K.I., Y.H., K.H., A.W., Y.A., M.H., C.K., S.K., K.T., R.H. Data analysis and interpretation: N.S., Y.O., R.H. Drafting of the manuscript: N.S. Critical revision of the manuscript for important intellectual content: N.S., Y.O., R.H. Statistical analysis: N.S., Y.O., R.H. Administrative, technical, or material support; study supervision: Y.O., R.H. Approval of final manuscript: all authors.
REFERENCES
- 1.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piche T, Barbara G, Aubert P, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196–201. doi: 10.1136/gut.2007.140806. [DOI] [PubMed] [Google Scholar]
- 3.Long Y, Du L, Kim JJ, et al. MLCK-mediated intestinal permeability promotes immune activation and visceral hypersensitivity in PI-IBS mice. Neurogastroenterol Motil. 2018;30:e13348. doi: 10.1111/nmo.13348. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Q, Verne ML, Fields JZ, et al. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut. 2019;68:996–1002. doi: 10.1136/gutjnl-2017-315136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu D, Gao J, Gillilland M, et al. Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology. 2014;146:484–496. doi: 10.1053/j.gastro.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattarai Y, Muniz Pedrogo DA, Kashyap PC. Irritable bowel syndrome: a gut microbiota-related disorder? Am J Physiol Gastrointest Liver Physiol. 2017;312:G52–G62. doi: 10.1152/ajpgi.00338.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014. doi: 10.1038/nrdp.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Song J, Bai T, Qian W, Hou XH. Stress induces more serious barrier dysfunction in follicle-associated epithelium than villus epithelium involving mast cells and protease-activated receptor-2. Sci Rep. 2017;7:4950. doi: 10.1038/s41598-017-05064-y.0c9a008b549e4cd69c64bcff5e42a7fa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner JR, Buschmann MM, Romero-Calvo I, Sailer A, Shen L. The role of molecular remodeling in differential regulation of tight junction permeability. Semin Cell Dev Biol. 2014;36:204–212. doi: 10.1016/j.semcdb.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 11.Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, Gómez-Llorente C, Gil A. Probiotic mechanisms of action. Ann Nutr Metab. 2012;61:160–174. doi: 10.1159/000342079. [DOI] [PubMed] [Google Scholar]
- 12.Rodiño-Janeiro BK, Vicario M, Alonso-Cotoner C, Pascua-García R, Santos J. A review of microbiota and irritable bowel syndrome: future in therapies. Adv Ther. 2018;35:289–310. doi: 10.1007/s12325-018-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ait-Belgnaoui A, Payard I, Rolland C, et al. Bifidobacterium longum and Lactobacillus helveticus synergistically suppress stress-related visceral hypersensitivity through hypothalamic-pituitary-adrenal axis modulation. J Neurogastroenterol Motil. 2018;24:138–146. doi: 10.5056/jnm16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ait-Belgnaoui A, Han W, Lamine F, et al. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: a possible action through interaction with epithelial cell cytoskeleton contraction. Gut. 2006;55:1090–1094. doi: 10.1136/gut.2005.084194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murooka Y, Yamshita M. Traditional healthful fermented products of Japan. J Ind Microbiol Biotechnol. 2008;35:791–798. doi: 10.1007/s10295-008-0362-5. [DOI] [PubMed] [Google Scholar]
- 16.Ito K. Review of the health benefits of habitual consumption of miso soup: focus on the effects on sympathetic nerve activity, blood pressure, and heart rate. Environ Health Prev Med. 2020;25:45. doi: 10.1186/s12199-020-00883-4.97f9cbf0365d496d8887c737c022fafa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okada Y, Tsuzuki Y, Sugihara N, et al. Novel probiotic yeast from Miso promotes regulatory dendritic cell IL-10 production and attenuates DSS-induced colitis in mice. J Gastroenterol. 2021;56:829–842. doi: 10.1007/s00535-021-01804-0. [DOI] [PubMed] [Google Scholar]
- 18.Amoozadeh Y, Anwer S, Dan Q, et al. Cell confluence regulates claudin-2 expression: possible role for ZO-1 and Rac. Am J Physiol Cell Physiol. 2018;314:C366–C378. doi: 10.1152/ajpcell.00234.2017. [DOI] [PubMed] [Google Scholar]
- 19.Kusmiati, Dhewantara FX. Cholesterol-lowering effect of beta glucan extracted from Saccharomyces cerevisiae in rats. Sci Pharm. 2016;84:153–165. doi: 10.3797/scipharm.ISP.2015.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vannucchi MG, Evangelista S. Experimental models of irritable bowel syndrome and the role of the enteric neurotransmission. J Clin Med. 2018;7:4. doi: 10.3390/jcm7010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Da Silva S, Robbe-Masselot C, Ait-Belgnaoui A, et al. Stress disrupts intestinal mucus barrier in rats via mucin O-glycosylation shift: prevention by a probiotic treatment. Am J Physiol Gastrointest Liver Physiol. 2014;307:G420–G429. doi: 10.1152/ajpgi.00290.2013. [DOI] [PubMed] [Google Scholar]
- 22.Bradesi S, Schwetz I, Ennes HS, et al. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–G53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 23.Musty RE, Jordan MP, Lenox RH. Criterion for learned helplessness in the rat: a redefinition. Pharmacol Biochem Behav. 1990;36:739–744. doi: 10.1016/0091-3057(90)90070-X. [DOI] [PubMed] [Google Scholar]
- 24.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 26.Reid DM, Gow NA, Brown GD. Pattern recognition: recent insights from Dectin-1. Curr Opin Immunol. 2009;21:30–37. doi: 10.1016/j.coi.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Seviour R. Medicinal importance of fungal beta-(1-->3), (1-->6)-glucans. Mycol Res. 2007;111(Pt 6):635–652. doi: 10.1016/j.mycres.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Vannucci L, Krizan J, Sima P, et al. Immunostimulatory properties and antitumor activities of glucans (review) Int J Oncol. 2013;43:357–364. doi: 10.3892/ijo.2013.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.West C, Stanisz AM, Wong A, Kunze WA. Effects of Saccharomyces cerevisiae or boulardii yeasts on acute stress induced intestinal dysmotility. World J Gastroenterol. 2016;22:10532–10544. doi: 10.3748/wjg.v22.i48.10532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brun P, Scarpa M, Marchiori C, et al. Saccharomyces boulardii CNCM I-745 supplementation reduces gastrointestinal dysfunction in an animal model of IBS. PLoS One. 2017;12:e0181863. doi: 10.1371/journal.pone.0181863.dd085a2cfca944b2931d4a95dfc9bc14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Distrutti E, Monaldi L, Ricci P, Fiorucci S. Gut microbiota role in irritable bowel syndrome: new therapeutic strategies. World J Gastroenterol. 2016;22:2219–2241. doi: 10.3748/wjg.v22.i7.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hino S, Nishimura N, Matsuda T, Morita T. Intestinal absorption of β-glucans and their effect on the immune system [Preprint] 2020. [cited 2022 Dec 18]. Available from: https://doi.org/10.20944/preprints202012.0250.v1 . [DOI]
- 33.Lin N, Xu LF, Sun M. The protective effect of trefoil factor 3 on the intestinal tight junction barrier is mediated by toll-like receptor 2 via a PI3K/Akt dependent mechanism. Biochem Biophys Res Commun. 2013;440:143–149. doi: 10.1016/j.bbrc.2013.09.049. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda Y, Adachi Y, Ishii T, Miura N, Tamura H, Ohno N. Dissociation of Toll-like receptor 2-mediated innate immune response to Zymosan by organic solvent-treatment without loss of Dectin-1 reactivity. Biol Pharm Bull. 2008;31:13–18. doi: 10.1248/bpb.31.13. [DOI] [PubMed] [Google Scholar]
- 35.Dong JP, Zheng Y, Wu T, He Q, Teng GG, Wang HH. Protective effect of Saccharomyces boulardii on intestinal mucosal barrier of dextran sodium sulfate-induced colitis in mice. Chin Med J (Engl) 2019;132:1951–1958. doi: 10.1097/CM9.0000000000000364.1e2b0d3cab3b401288eb787547e7772c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lacy BE. Review article: an analysis of safety profiles of treatments for diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:817–830. doi: 10.1111/apt.14948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shapiro J, Bernica J, Hernaez R. Risk of bias analysis of systematic reviews of probiotics for treatment of irritable bowel syndrome. Clin Gastroenterol Hepatol. 2019;17:784–785. doi: 10.1016/j.cgh.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Balsari A, Ceccarelli A, Dubini F, Fesce E, Poli G. The fecal microbial population in the irritable bowel syndrome. Microbiologica. 1982;5:185–194. doi: 10.18578/bnfc.694550661. [DOI] [PubMed] [Google Scholar]
- 39.Malinen E, Rinttilä T, Kajander K, et al. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373–382. doi: 10.1111/j.1572-0241.2005.40312.x. [DOI] [PubMed] [Google Scholar]
- 40.Chassard C, Dapoigny M, Scott KP, et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment Pharmacol Ther. 2012;35:828–838. doi: 10.1111/j.1365-2036.2012.05007.x. [DOI] [PubMed] [Google Scholar]
- 41.Russo P, López P, Capozzi V, et al. Beta-glucans improve growth, viability and colonization of probiotic microorganisms. Int J Mol Sci. 2012;13:6026–6039. doi: 10.3390/ijms13056026.41c3d9aaa5804457b8910009da07acce [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JM, Jang WJ, Lee EW, Kong IS. β-glucooligosaccharides derived from barley β-glucan promote growth of lactic acid bacteria and enhance nisin Z secretion by Lactococcus lactis. LWT. 2020;122:109014. doi: 10.1016/j.lwt.2020.109014. [DOI] [Google Scholar]
- 43.Nobaek S, Johansson ML, Molin G, Ahrné S, Jeppsson B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1231–1238. doi: 10.1111/j.1572-0241.2000.02015.x. [DOI] [PubMed] [Google Scholar]
- 44.Ducrotté P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol. 2012;18:4012–4018. doi: 10.3748/wjg.v18.i30.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vandeputte D, Falony G, Vieira-Silva S, Tito RY, Joossens M, Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. 2016;65:57–62. doi: 10.1136/gutjnl-2015-309618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Chen G, Li X, Zheng F, Zeng X. Yeast β-glucan, a potential prebiotic, showed a similar probiotic activity to inulin. Food Funct. 2020;11:10386–10396. doi: 10.1039/D0FO02224A. [DOI] [PubMed] [Google Scholar]