Summary
Background
People who inject drugs are at increased risk of both HIV and hepatitis C virus (HCV) infections but face barriers to testing and engagement in care. Assisted partner services are effective in locating people with HIV but are understudied among people who inject drugs. We assessed whether assisted partner services could be used to find, test for HIV and HCV infections, and link to care the partners of people who inject drugs in Kenya.
Methods
In this prospective study at eight sites offering harm-reduction services in Kenya, we enrolled people aged 18 years or older who inject drugs and were living with HIV (index participants) between Feb 27, 2018, and Nov 1, 2021. Index participants provided information about their sexual and injecting partners (ie, anyone with whom they had had sexual intercourse or injected drugs in the previous 3 years), and then community-embedded peer educators located partners and referred them for enrolment in the study (partner participants). All participants underwent testing for HCV infection, and partner participants also underwent HIV testing. Index and partner participants with HIV but who were not on antiretroviral therapy (ART) were linked with treatment services, and those positive for HCV were linked to treatment with direct-acting antivirals. We calculated the number of index participants whom we needed to interview to identify partner participants with HIV and HCV infection.
Findings
We enrolled 989 people living with HIV who inject drugs, who mentioned 4705 sexual or injecting partners. Of these 4705 partners, we enrolled 4597 participants, corresponding to 3323 unique individuals. 597 (18%) partner participants had HIV, of whom 506 (85%) already knew their status. 358 (71%) of those who knew they were HIV positive were virally suppressed. 393 (12%) partner participants were HCV antibody positive, 213 (54%) of whom had viraemia and 104 (26%) of whom knew their antibody status. 1·66 (95% CI 1·53–1·80) index participants had to be interviewed to identify a partner with HIV, and 4·24 (3·75–4·85) had to be interviewed to find a partner living with HIV who was unaware of their HIV status, not on ART, or not virally suppressed. To find a partner seropositive for HCV who did not know their antibody status, 3·47 (3·11–3·91) index participants needed to be interviewed. Among the 331 index and partner participants living with HIV who were not on ART at enrolment, 238 (72%) were taking ART at 6-month follow-up. No adverse events were attributed to study procedures.
Interpretation
Use of assisted partner services among people with HIV who inject drugs was safe and identified partners with HIV and HCV infections. Assisted partner services was associated with increased uptake of ART for both index participants and partners.
Funding
US National Institutes of Health.
Introduction
Although substantial progress has been made towards reducing the global incidence of HIV, many countries are not on track to meet the UNAIDS 95–95-95 targets by 2030.1 The gap between the target and reality is largest for the first 95—to ensure that 95% of people with HIV are aware of their status. An estimated 5·5 million individuals do not know that they have HIV, and making these people aware of their HIV status is essential to achieve the other two UNAIDS targets of sustained treatment with antiretroviral therapy (ART) and viral suppression.1–3 People who inject drugs (PWID) are marginalised in sub-Saharan Africa, where drug use is highly stigmatised, and face substantial challenges in accessing HIV testing services, engaging in HIV care, and adhering to ART.4–7 Barriers to care for PWID include socioeconomic vulnerability, criminalisation (eg, of the use or sale of drugs and of sex work), stigmatisation, harassment, violence, and denial of care in health-care settings.8 In Kenya, HIV prevalence among PWID is estimated at 18·3%, compared with a prevalence of 3·7% in the general population.9,10
PWID are also at increased risk of hepatitis C virus (HCV) infection compared with those who do not inject drugs.11,12 Co-infection with HIV and HCV is associated with significantly higher morbidity and mortality than infection with either virus alone.13,14 In Kenya, the prevalence of HCV infection is estimated to be between 0·2% and 4% nationally,15,16 but 13–22% among PWID.17,18 PWID experience barriers to diagnosis of HCV infection and linkage to treatment services (including direct-acting antiviral therapy).8,19
Assisted partner services are an evidenced-based strategy whereby health-care providers elicit the sexual or injecting partners of people living with HIV with the aim of offering HIV testing and care to these partners. Also known as index testing, assisted partner services have been used successfully in general populations to test for HIV and link to care the partners of people newly diagnosed with HIV.20–22 In a cluster-randomised trial22 in Kenya (in which 477 partners were enrolled and tested from 1119 index participants), one new HIV infection was identified for every six-to-seven index participants interviewed and one in three partners tested had HIV and was unaware of their status. Few studies have assessed the use of assisted partner services among PWID or other key populations in sub-Saharan Africa, and very few have included testing for HCV.23
In this study, we assessed whether integrating assisted partner services into sites providing harm reduction services could help to identify and engage in testing and care the sexual and injecting partners of PWID who are at high risk of HIV or HCV infection, thereby overcoming the barriers to testing among PWID and their partners.
Methods
Study design and participants
In this prospective cohort study, we assessed whether assisted partner services could be used to identify, test, and link to care HIV-positive and HCV-positive partners of PWID living with HIV in Kenya. Participants were recruited from Feb 27, 2018, to Nov 1, 2021. Data collection continued until Jan 28, 2022. The study was done at eight sites operated by organisations offering harm-reduction services, including needle and syringe programmes and sites for methadone services, that were clustered in two regions in Kenya: the Nairobi metropolitan area (Nairobi City county) and the North coastal region (Mombasa and Kilifi counties).
This study included two primary groups: index participants and the sexual and injecting partners of index participants. Index participants were people living with HIV aged 18 years or older who had injected drugs in the past year and were primarily identified through their engagement with harm-reduction services and programmes. Potential index participants were excluded if they were considered at high risk of intimate partner violence—ie, if they had experienced intimate partner violence (defined as an episode of sexual, physical, or threatened violence perpetrated by someone with whom the victim was in a sexual relationship) in the past month. Partner participants were individuals aged 18 years or older with whom index participants had had sexual intercourse over the previous 3 years (ie, sexual partners) and individuals with whom index participants had injected drugs over the previous 3 years, irrespective of whether they had shared needles (ie, injecting partners). Partner participants could be both sexual and injecting partners, partners named by more than one index participant could be enrolled more than once, and partners who met the eligibility criteria could also be enrolled as index participants.
This study was approved by the Human Subject Division of the University of Washington Internal Review Board (STUDY00001536) and by the Kenyatta National Hospital and University of Nairobi Ethics and Research Committee (KNH-ERC/R/195, P265/05/2017). All participants provided informed consent. The study protocol has previously been published.24
Procedures
Index participants were identified by clinicians at each site through review of routine clinical data or through routine harm-reduction outreach efforts undertaken at each site by peer educators (people who formerly injected drugs trained to offer outreach and harm-reduction services to PWID). Potential index participants were referred to study staff in a private room for screening and enrolment. Iris scanning with iRespond was used to biometrically confirm that each participant was a unique individual. If eligible, study staff administered a structured questionnaire to index participants using Open Data Kit software to collect demographic data, and information about sexual behaviours (including about transactional sex, defined as ever either giving or receiving money or material goods for sex), injecting behaviours, HIV testing and history, and HCV testing and history. Index participants additionally underwent rapid testing for HCV using either SD Bioline (Abbott, Chicago, IL) or OraQuick (OraQuick, Bethlehem, PA) HCV antibody test kits. We also drew 20 mL of blood for molecular testing of HIV and HCV.
Index participants were asked to list all sexual and injecting partners in the previous 3 years, and completed a short questionnaire about each partner, in which they described the relationship and provided contact information. Study staff then worked with trained community-embedded peer educators25,26 at each study site to attempt to contact the partner, either by phone or in person. To protect the identity of the index participant, peer educators were not told which index was linked to which partner. Partners were informed that they had been mentioned as a possible participant in a research study, and that they could present to a research site to participate if they wished to. If the partner was not interested in participating, study staff would then mention the potential HIV exposure and urge the partner to get tested. Partners interested in participating came to study sites for enrolment, and were often accompanied by peer educators. After biometric identification and a short screening questionnaire, partners completed an enrolment questionnaire administered by study staff (which collected similar information to that collected for index participants). Partner participants underwent rapid testing for HIV (as per Kenya’s national HIV testing guidelines27) and HCV (as per index participants). We drew an additional 20 mL of blood from partner participants who tested positive for either HIV or HCV for HIV viral load testing or HCV PCR testing, or both.
Blood samples from both index and partner participants were collected in ethylene diamine tetra acetic acid vacutainers and transported within 4 h of collection to the University of Nairobi (Nairobi, Kenya), or to the Malindi Sub-county Hospital (Kilifi, Kenya). Dried blood spots were prepared from the whole blood and plasma aliquots were prepared after centrifugation and stored in −80°C freezers. Samples were shipped on dry ice to the KwaZulu-Natal Research Innovation and Sequencing Platform laboratory (University of KwaZulu-Natal, Durban, South Africa) for HIV and HCV viral load testing with Abbott M2000 (Abbott, Chicago, IL, USA).
All index participants and any partner participants who tested positive for either HIV or HCV antibodies were invited to complete a 6-month follow-up visit, which involved a questionnaire about testing and engagement in care for both HIV and HCV infections, and about intimate partner violence experienced. Additionally, any participant reporting moderate risk (ie, having ever experienced intimate partner violence or reporting a fear of intimate partner violence as a result of study participation) or high risk of intimate partner violence at baseline completed follow-ups for intimate partner violence at weeks 1, 2, 4, 6, and 16, at which a short questionnaire was administered about threatened, sexual, or physical violence experienced since enrolment. Participants reporting violence were linked to violence counselling and care. Peer educators located participants who were due for 6-month follow-up visits and intimate partner violence visits and brought them to the study sites. All participants received KSh400 (about the equivalent of US$3·50) compensation for their time and transportation for attending the enrolment visit and each follow-up.
Statistical analysis
We summarised sociodemographic and behavioural characteristics of index and partner participants with medians and IQRs for continuous variables and proportions for categorical variables. The primary outcome of this analysis was the incremental number needed to interview (NNTI). NNTI was defined as the number of index patients who needed to undergo assisted partner services to identify one additional partner with the outcome of interest, and was calculated by dividing the number of index participants interviewed by the number of unique partner participants fitting outcome criteria.We calculated the NNTI to identify partner participants with HIV (including previously diagnosed infections), undiagnosed HIV, previously diagnosed HIV who were not on ART or whose HIV was not virally suppressed (which we defined as a viral load of fewer than 1000 copies of HIV per mL), seropositive for HCV antibodies (including those who had previously tested positive for HCV and been treated for HCV infection), seropositive for HCV who did not know their antibody status and had not been treated, and with active HCV infections. Partner participants named by more than one index participant were counted once for each enrolment in which they fit the NNTI outcome criteria. Other outcomes assessed were uptake of ART and instances of intimate partner violence (safety assessment) at 6 months. 95% CIs for NNTIs were calculated using a bootstrap of the average number of partners of each category named per index. The interclass correlation coefficient was calculated across the eight primary study sites. Analyses were done in Stata (version 14.2).
Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
We enrolled 989 index participants (table 1). Violence (including but not limited to intimate partner violence) was common among index participants even after excluding those who had experienced intimate partner violence in the past month: 338 (34%) reported physical violence and 87 (9%) reported sexual violence in the past year (table 1; appendix 1 p 1). 68 (7%) index participants reported sharing needles and 106 (11%) reported sharing injection equipment in the last month, 436 (44%) reported not using condoms during their last sexual intercourse, and 760 (77%) had ever engaged in transactional sex. Index participants had an HCV antibody prevalence of 16%. 73 (7%) were PCR positive for HCV, and 27 (3%) had previously taken direct acting antiviral agents to treat HCV infection. 898 (91%) index partners were already aware of their HIV status and 855 (86%) were enrolled in HIV care. 800 (89%) of the 898 who were aware of their HIV status were on ART, and 800 (94%) of the 855 enrolled in HIV care were taking ART. 535 (85%) of the 627 index participants taking ART for whom viral load data were available were virally suppressed at baseline.
Table 1:
Characteristics of index participants
| Index participants (n=989) | |
|---|---|
|
| |
| Age, years | 37 (31–42) |
| Sex | |
| Female | 485 (49%) |
| Male | 504 (51%) |
| Marital status | |
| Married | 220 (22%) |
| Unmarried | 769 (78%) |
| Region | |
| Nairobi | 532 (54%) |
| Coast | 457 (46%) |
| Violence in past year | |
| Physical | 338 (34%) |
| Sexual | 87 (9%) |
| Threatened | 185 (19%) |
| Injection history and risk behaviours | |
| Needle sharing in past month | 68 (7%) |
| Equipment sharing in past month | 106 (11%) |
| Time injecting drugs, years | 5 (2–7) |
| Monthly frequency of injecting | 60 (30–90) |
| Sexual encounters in past month | 2(0–10) |
| Lifetime sexual partners | 15 (6–50) |
| Condom use during last sexual intercourse* | 553 (56%) |
| Transactional sex, ever† | 760 (77%) |
| On methadone | 228 (23%) |
| HIV and HCV data | |
| HCV antibody positive | 162 (16%) |
| HCV viraemia | 73 (7%) |
| Previously treated with direct-acting antivirals | 27 (3%) |
| Engaged in HIV care | 855 (86%) |
| On antiretroviral therapy | 800(81%) |
| Viral suppression of HIV | 535 (68%)‡ |
| Partners | |
| Also enrolled as partner§ | 502 (51%) |
| Number of partners mentioned | |
| 1–5 | 723 (73%) |
| 6–10 | 233 (24%) |
| >10 | 33 (3%) |
Data are n (%) or median (IQR). Index participants were people who inject drugs and were living with HIV. HCV=hepatitis C virus.
Defined as vaginal, oral, or anal penetrative sex.
Defined as giving or receiving money or material goods in exchange for sex.
Data were unavailable for 201 participants, so the denominator for this percentage calculation was 788.
Also named as partners and thus represented again in table 2.
Index participants mentioned 4705 partners (3410 injecting only partners, 713 sexual only partners, and 528 sexual and injecting partners; data about partner type were missing for five partners), an average of 4·76 partners per index (figure 1). Overall, 4597 partner participants were enrolled: 3365 injecting only partners, 713 sexual only partners, and 519 sexual and injecting partners. 502 partners mentioned were already or subsequently enrolled as index participants. The 4588 partners enrolled represented 3323 unique individuals (partners could be enrolled each time they were mentioned). 2706 (81%) individual partner participants were enrolled once, 340 (10%) were enrolled twice, 133 (4%) were enrolled three times, and 144 (4%) were enrolled four or more times. Median time from getting a partner’s contact information to the partner participant undergoing HIV and HCV testing was 8 days (IQR 3–24) for injecting partners and 15 days (4–65) for sexual partners.
Figure 1: Flowchart of enrolment of partners mentioned by index participants and tested for HIV and HCV.
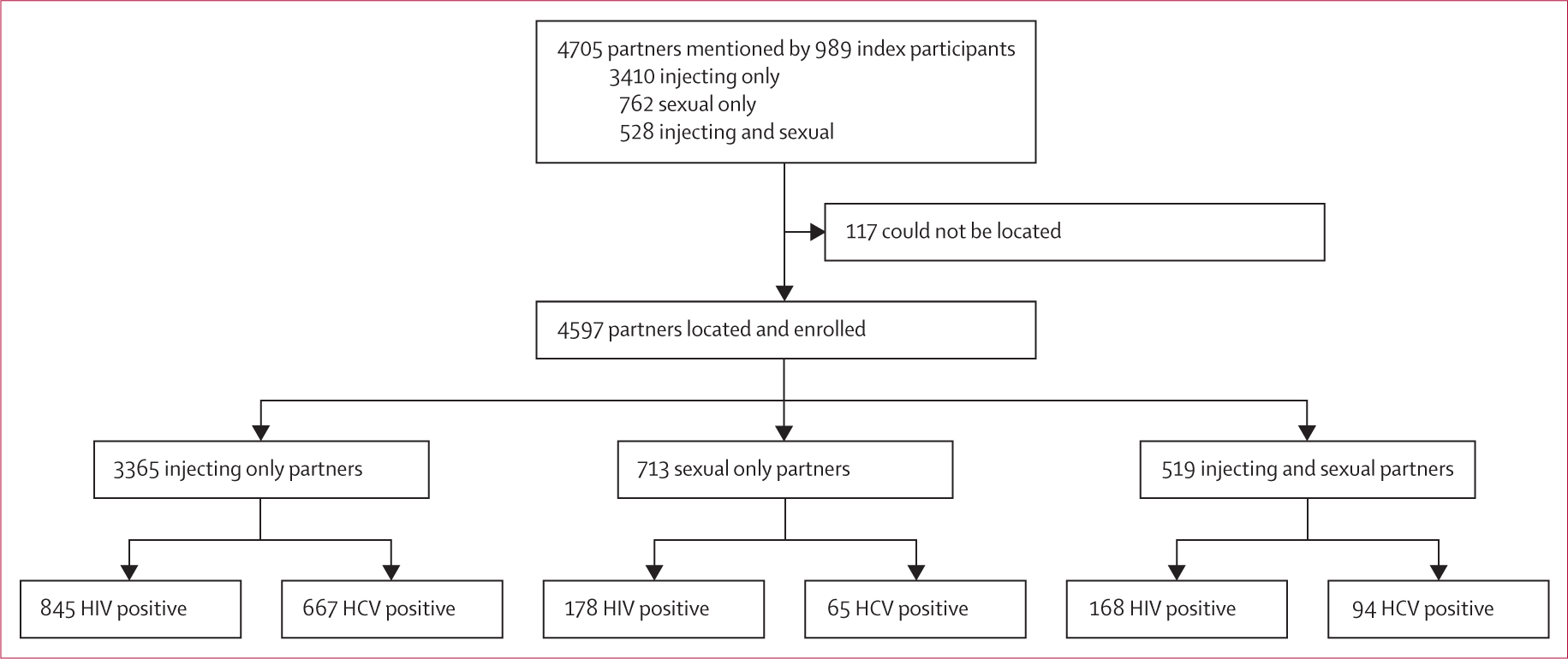
Partner participants could be enrolled more than once if they were mentioned by more than one index participant. HCV=hepatitis C virus.
Of the 3323 partner participants enrolled, 1412 (42%) reported physical or sexual violence in the past year, perpetrated by intimate partners, community members, law enforcement, and others (table 2; appendix 1 p 1). 2758 (83%) partner participants had ever used drugs and 2482 (75%) had ever injected drugs. 2267 (68%) partner participants reported previous transactional sex and 1410 (42%) reporting using a condom in their most recent sexual encounter.
Table 2:
Partner characteristics (unique individuals)
| Partner participants (n=3323) | |
|---|---|
|
| |
| Age, years | 33 (27–39) |
| Sex | |
| Female | 975 (29%) |
| Male | 2348 (71%) |
| Marital status* | |
| Married | 862 (26%) |
| Unmarried | 2457 (74%) |
| Region | |
| Nairobi | 1721 (52%) |
| Coast | 1602 (48%) |
| Violence in past year | |
| Physical | 1169 (35%) |
| Sexual | 243 (7%) |
| Threatened | 770 (23%) |
| Relationship to index participant† | |
| Injecting partners | 2342 (70%) |
| Sexual partners‡ | 597 (18%) |
| Injecting and sexual partners | 382 (11%) |
| Sexual partner’s relationship to index (reported by index)§ | |
| Spouse | 229 (18%) |
| Girlfriend or boyfriend | 724 (56%) |
| Casual sexual partner | 133 (10%) |
| Index paid partner for sex | 163 (13%) |
| Partner paid index for sex | 43 (3%) |
| Days from naming by index to HIV and HCV testing | |
| Injecting partners | 8 (3–24) |
| Sexual partners | 15 (4–65) |
| Injecting and sexual partners | 9 (3–29) |
| Risk behaviours | |
| Use of any drugs, ever | 2758(83%) |
| Injection drug use, ever | 2482 (75%) |
| Sexual encounters in past month | 2 (0–7) |
| Lifetime sexual partners | 10 (5–23) |
| Condom use during last sexual intercourse¶ | 1410 (42%) |
| Transactional sex, ever|| | 2267 (68%) |
| HIV and HCV data | |
| HCV antibody positive | 393 (12%) |
| HCV viraemia | 213 (6%) |
| Previously treated with direct-acting antivirals | 52 (2%) |
| HIV positive | 597 (18%) |
| Enrolled in HIV care | 480 (14%) |
| On antiretroviral therapy | 455 (14%) |
| Viral suppression of HIV | 358 (70%)** |
Data are n (%) or median (IQR).
Data were missing for four participants.
Data were missing for two participants.
57% of people named as sexual partners had injected drugs (43% had injected in the last month).
The denominator for these percentage calculations is 1292 (sexual partners named by multiple indexes are counted more than once).
Defined as vaginal, oral, or anal penetrative sex.
Defined as giving or receiving money or material goods in exchange for sex.
The denominator for this percentage calculation is 512 (of the 597 partners with HIV, viral load data were unavailable for 85).
597 (18%) partners were HIV positive (figure 2A). Thus, the NNTI to identify a partner participant with HIV was 1·66 (95% CI 1·53–1·80; table 3). 91 (15%) partner participants were newly testing positive for HIV, corresponding to an NNTI of 10·87 (8·83–13·93) to identify a partner living with HIV who was unaware of their positive status. Among the 506 partners with HIV who already knew their HIV status, 455 (90%) were on ART, and 358 (71%) were virally suppressed. 358 (85%) of the 421 partners who were aware of their status and for whom we have viral load data were virally suppressed. The NNTI to find a partner living with HIV who was either unaware of their status, not on ART, or on ART but not virally suppressed was 4·24 (3·75–4·85).
Figure 2: Partner enrolment HIV (A) and hepatitis C virus (B) cascades.
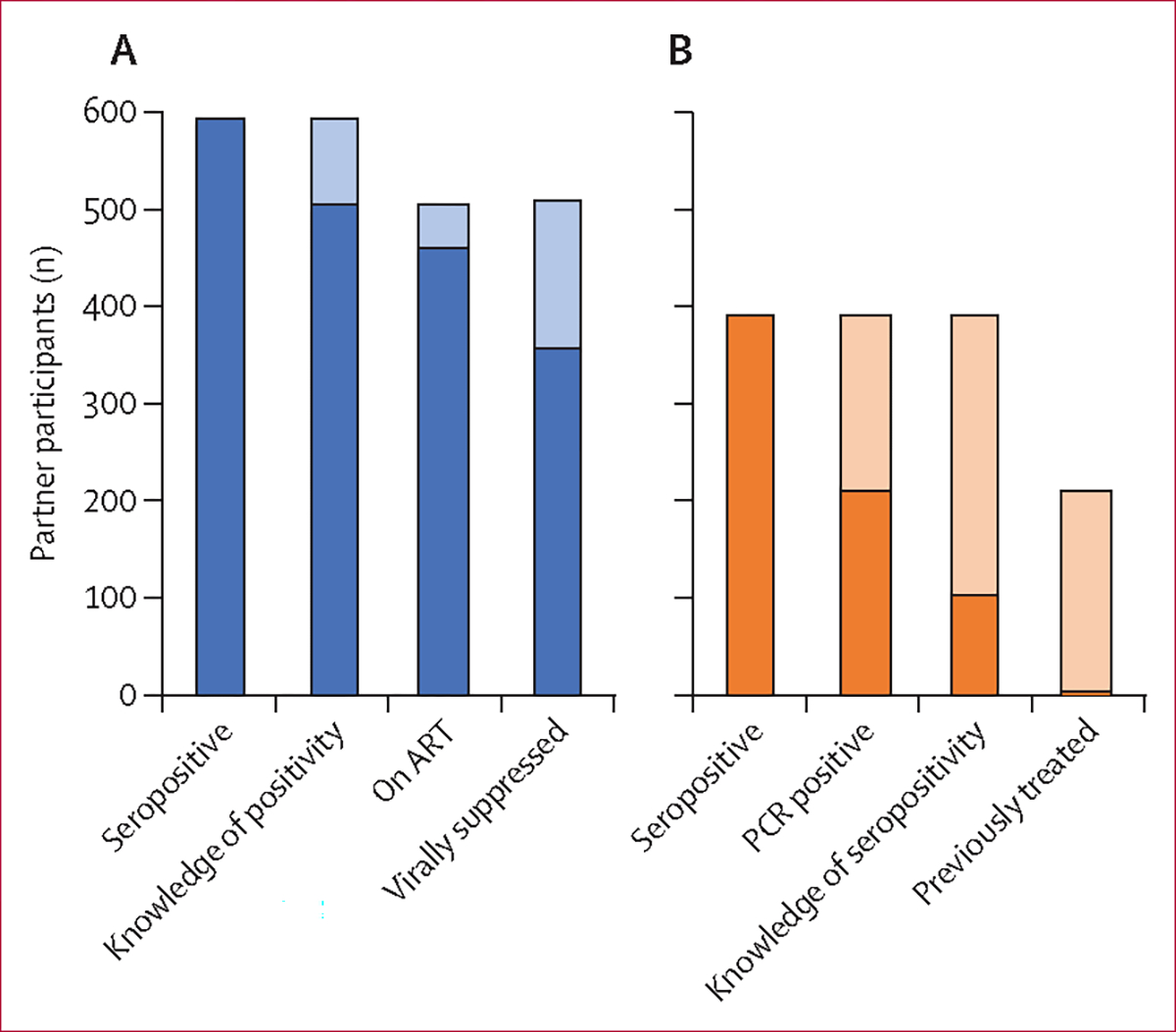
N=3312 (some participants were excluded because of incomplete data). Shaded regions represent the loss of participants between steps in the care cascade.
Table 3:
Number needed to interview (index participants) to identify partners living with HIV
| Number needed to interview (95% CI; interclass correlation coefficient) | |
|---|---|
|
| |
| Overall | |
| Any partner with HIV | 1·66 (1·53–1·80; 0·03) |
| HIV-positive partner unaware of HIV status | 10·87 (8·83–13·93; 0·02) |
| HIV-positive partner unaware of HIV status, not on ART, and not virally suppressed | 4·24 (3·75–4·85; 0·01) |
| Injecting partners | |
| Any partner with HIV | 2·65 (2·38–2·97; <0·005) |
| HIV-positive partner unaware of HIV status, not on ART, and not virally suppressed | 6·87 (5·82–8·31; <0·005) |
| Sexual partners | |
| Any partner with HIV | 7·79 (6·55–9·51; 0·07) |
| HIV-positive partner unaware of HIV status, not on ART, and not virally suppressed | 17·98 (13·74–24·14; 0·05) |
95% CIs were calculated from a non-parametric bootstrap, whereas the interclass correlation coefficient was calculated across eight study sites. ART=antiretroviral therapy
Among 2342 injecting only partner participants, 373 (16%) had HIV (NNTI 2·65 [95% CI 2·38–2·97]). Among these 373 injecting partners with HIV, 45 (12%) were unaware of their status, 32 (9%) were not on ART, and 83 (22%) were not virally suppressed. The NNTI to identify an injecting partner with HIV who was unaware of their HIV-positive status, not on ART, or not virally suppressed was 6·87 (5·82–8·31). 127 (20%) of 597 sexual only partner participants had HIV (NNTI 7·79 [6·55–9·51]). 26 (20%) of 127 were unaware of their HIV-positive status, 4 (3%) were not on ART, and 20 (16%) were on ART but not virally suppressed. The NNTI to find a sexual only partner living with HIV who was unaware of their HIV status, not on ART, or not virally suppressed was 17·98 (13·74–24·14). Among 382 sexual and injecting partners, 96 (25%) had HIV, of whom 17 (18%) were unaware of their status. Among the 79 sexual and injecting partners living with HIV who were aware of their status, eight (10%) were not on ART, and 11 (14%) were on ART but not virally suppressed.
393 (12%) partner participants were positive for HCV antibodies, resulting in an NNTI of 2·52 (95% CI 2·28–2·80; appendix 1 p 2). 213 (54%) of these 393 participants were PCR positive for HCV, and 104 (26%) knew their HCV antibody-positive status. Only four partner participants had previously been treated with direct-acting antivirals—ie, only 4% of those who knew their antibody status. The NNTI to identify a partner seropositive for HCV who did not know their antibody status and had not been treated was 3·47 (3·11–3·91), whereas that to identify a partner with HCV viraemia was 4·69 (4·07–5·46). Among the 2342 injecting only partners, 301 (13%) were HCV antibody positive, of whom 164 (54%) had HCV viraemia and 81 (27%) were aware of their antibody status. 39 (7%) of the 597 sexual only partners were seropositive for HCV, of whom 22 (56%) had viraemia, and nine (23%) were aware of their antibody status. Among the 382 sexual and injecting partners, 53 (14%) were antibody positive, of whom 25 (47%) had viraemia, and 14 (26%) were aware of their status. Data for the outcomes of HCV treatment will be reported in a separate paper.
Of 989 index participants, 874 (88%) completed 6-month follow-up visits. Of the 115 who did not complete follow-up visits, 94 (82%) were lost to follow-up, 20 (17%) died, and one (1%) withdrew. Among the 20 index participants who died, reported causes of death were complications of HIV (n=6), tuberculosis (n=3), overdose (n=1), other medical problems (n=4) and unknown (n=6). 749 (86%) of the 871 partner participants who were positive for either HIV or HCV antibodies attended 6-month follow-up visits. Of the 122 who did not complete follow-up visits, 104 (85%) were lost to follow-up and 18 (15%) died. Reported causes of death were trauma (n=4), overdose (n=3), complications of HIV (n=2), tuberculosis (n=1), other medical problems (n=4), and unknown (n=4).
Among 331 participants (189 index participants and 142 partner participants) who were not taking ART at enrolment, 238 (72%) reported being on ART at 6-month follow-up visits (table 4). 800 (81%) of 989 index participants were taking ART at enrolment, of whom 696 (87%) were still taking ART at the 6-month follow-up visit. Of the 189 index participants not on ART at enrollment, 136 (72%) reported taking ART at the 6-month follow-up visit. Among 597 partner participants with HIV, 455 (76%) were taking ART at enrolment, of whom 402 (88%) were still taking ART at 6 months. Of the 142 partner participants with HIV who were not on ART at enrolment, 102 (72%) reported taking ART by the 6-month follow-up visit.
Table 4:
ART status at enrolment and at the 6-month follow-up visit for indexes and partners with HIV
| ART status at enrolment | 6-month follow-up visit* |
|||
|---|---|---|---|---|
| Not on ART | On ART | Lost to follow-up | ||
|
| ||||
| Indexes (n=989) | ||||
| Not on ART | 189 (19%) | 25 (13%) | 136 (72%) | 28 (15%) |
| On ART | 800 (81%) | 17 (2%) | 696 (87%) | 87 (11%) |
| Partners (n=597) | ||||
| Not on ART | 142 (24%) | 17 (12%) | 102 (72%) | 23 (16%) |
| On ART | 455 (76%) | 9 (2%) | 402 (88%) | 44 (10%) |
ART=antiretroviral therapy.
At baseline, 303 (31%) of 989 index participants and 901 (27%) of 3323 partner participants reported intimate partner violence during the past year, and 73 (2%) of partner participants reported intimate partner violence in the month before enrolment. 5393 intimate partner violence follow-up visits were attempted, and 3920 (73%) went ahead. During these visits, 33 (1%) individuals reported physical intimate partner violence, 44 (1%) reported emotional intimate partner violence, and 15 (<1%) reported sexual intimate partner violence. None of the reported instances were attributed to study procedures. At 6-month follow up visits, no participants reported any study-related adverse events.
Discussion
In this study, we successfully used assisted partner services to identify and test for HIV and HCV infection the partners of PWID living with HIV. We used a novel approach in which community-embedded peer educators identified and brought the sexual and injecting partners of PWID to sites offering harm reduction and assisted partner services.24 Offering assisted partner services to four PWID with HIV resulted in the identification of one individual with unknown or poorly controlled HIV who would benefit from being tested, linked to care, or counselled. Our study showed that a large proportion of participants who were not on ART at enrolment were on treatment at 6-month follow-up, suggesting successful linkage to care. Furthermore, assisted partner services did not result in any instances of intimate partner violence or other adverse outcomes for participants, despite high baseline levels of violence.28 The absence of negative social consequences could suggest that strict adherence to ethical standards for assisted partner services (including consent and maintaining of the confidentiality of the index participant) is key to the acceptability and success of such services.29
In a previous study22 of assisted partner services in Kenya, 1183 (67%) of 1760 partners contacted agreed to testing and 5·1–7·9 index participants needed to be interviewed to identify a partner with HIV. In our study, uptake of assisted partner services was high among index participants and partners: 98% of partners mentioned by index participants were located and all contacted partners underwent testing for both HIV and HCV. We believe that the high frequency of partner location and testing was due to the involvement of community-embedded peer educators, who had long-standing relationships and detailed knowledge about local communities of PWID.25
Overall, the NNTI to identify a partner participant with HIV was 1·66, which is lower than that in previous studies in the general population.22 Analyses of the characteristics of index participants that were associated with a lower NNTI have been reported previously.30 This low NNTI could reflect the large number of partners mentioned by each index participant: whereas an average of 4·76 partners were named by each index participant in our study, between one and three partners were typically named in previous studies20,22 done in general settings. This high number of partners named could have resulted from the inclusion of injecting partners in addition to sexual partners of index participants. The HIV testing yield among the partners of PWID (15%) was higher than the prevalence in most studies done in general populations in sub-Saharan Africa.1 We were surprised that 85% of the partner participants with HIV were already aware of their status. In a previous study9 only 67% of PWID with HIV in Kenya were aware of their status. Penetration of HIV testing was thus reasonably high in this key population. The NNTI to identify a partner unaware of their HIV status was, therefore, high (10·87), although when including those who were diagnosed but not virally suppressed (an important population for outreach), that NNTI fell to 4·14.
Overall, the proportion of partner participants with viral suppression was low (70% of those with HIV who knew their status). The proportion of both index and partner participants on ART was higher at 6 months than at enrolment, suggesting that assisted partner services could help to support linkage to, and engagement in, care, in addition to testing. Assisted partner services could thus play an important role in connecting key populations and their partners—who collectively have reduced access to care and experience stigma—to services, including pre-exposure prophylaxis or ART.
Assisted partner services have been used frequently to increase diagnosis of HIV and many other sexually transmitted infections,20–23,31 but it has rarely been used to test partners for viral hepatitis infections. Although we did not deploy assisted partner services specifically for HCV infection, use of assisted partner services in PWID living with HIV identified HCV antibody-positive partners, most of whom were unaware of their status and untreated. Furthermore, the low NNTI to find a partner with HCV suggests that assisted partner services could be efficient at HCV case-finding for partners of PWID living with HIV. In the era of increasing availability of direct-acting antivirals, finding and testing people at high risk of HCV infection is imperative to eliminate HCV.32
Our study had several limitations. First, although we report the results of case-finding for HCV infection, we did not target assisted partner services to HCV-positive PWID. Our ability to report on possible applications of assisted partner services for HCV infection is thus limited, but we provide preliminary data for possible outcomes. Second, because we allowed index participants to enrol as partners and vice versa, we might have oversampled some members of the population who had many connections and were part of large networks of PWID. This limitation also applies with regard to partners who were enrolled more than once. Although this approach might complicate interpretation of our data, it also approximates real-world situations when assisted partner services are established within harm-reduction organisations: multiple enrolments of individuals is probable. Additionally, the overall proportion of individuals who were enrolled more than once was small. Third, we used convenience sampling for initial recruitment of index participants, so although substantial effort was made to also reach individuals outside clinics, we have oversampled people who engage with harm-reduction services and thus our results may not be generalisable to all PWID. Fourth, we excluded index participants with a high risk of intimate partner violence, which could result in an underestimation of the risk of intimate partner violence associated with assisted partner services. Finally, our follow-up was limited to index participants and partner participants with HIV or HCV infections, so we do not have follow-up data for other partner participants. Furthermore, the 6-month follow up data we collected was limited in scope—eg, we did not measure HIV viral load at 6 months.
In summary, assisted partner services efficaciously identified the sexual and injecting partners of PWID living with HIV who were in need of HIV and HCV testing and linkage to treatment and care. We believe that use of community-embedded peer educators and leveraging pre-existing harm-reduction structures were crucial to our success and these approaches should be considered for similar future assisted partner service programmes. Expansion of assisted partner services to include HCV testing showed that assisted partner services can be leveraged and tailored to provide specific services for key populations.
Supplementary Material
Research in context.
Evidence before this study
People who inject drugs experience a disproportionate burden of HIV and hepatitis C virus (HCV) infection and require tailored services to address barriers to testing and engagement in care. Previous research, including in Kenya, suggests that assisted partner services are an effective approach to identification of HIV infections among the sexual partners of people newly diagnosed with HIV, and linkage to care in general populations. Some findings also suggest that assisted partner services are safe and effective in populations of people who inject drugs, and WHO recommends use of assisted partner services in this population. However, few studies of the use of assisted partner services to identify HIV among the sexual and injecting partners of people who inject drugs have been done in sub-Saharan Africa, and we were not aware of any studies in which HCV infections were tested for or identified. We searched PubMed with the term “people who inject drugs” coupled with either “partner services,” “index testing,” or “partner notification” for articles published in any language up to Nov 20, 2023. Of the 15 articles identified, only one detailed a study done in sub-Saharan Africa in a population other than our study cohort. Previously published reports from our study cohort include a preliminary report on the associations between index characteristics and partners with unsuppressed HIV infections. No research article identified mentioned HCV in the context of partner services for people who inject drugs.
Added value of this study
Deployment of assisted partner services in collaboration with community-embedded peer educators was safe and efficaciously identified people with HIV and HCV infection among the partners of people who inject drugs in Kenya. Although most partners with HIV already knew their status, many were not engaged in care or virally suppressed, and assisted partner services supported linkage to and engagement with care services.
Implications of all the available evidence
Assisted partner services can be safely used to identify and link to care HIV-positive and HCV-positive partners of people who inject drugs. Harm-reduction programmes and other organisations working with people who inject drugs should consider involving peer educators in anonymosed assisted partner service programmes.
Acknowledgments
This work was supported by the US National Institutes of Health (grant R01 DA043409). NL-B was supported by a National Institutes of Health diversity supplement through the National Institute of Drug Abuse (R01 DA043409-S1). LM and SM received support from Fogarty International Center (D43 TW009580).
Footnotes
Equitable partnership declaration
The authors of this paper have submitted an equitable partnership declaration (appendix 2). This statement allows researchers to describe how their work engages with researchers, communities, and environments in the countries of study. This statement is part of The Lancet Global Health’s broader goal to decolonise global health.
Declaration of interests
AM-W has served as a consultant for WHO and has received financial support from Gilead Sciences. BLG has received financial support from Gilead and has served as an expert witness for the Los Angeles Unified School District. JS has served as a consultant for Gilead, Premera Blue Cross, Guidepoint, and Novo Nordisk, and as an expert witness for the Washington State Medical Commission. All other authors declare no competing interests.
Contributor Information
Aliza Monroe-Wise, Department of Global Health, University of Washington, Seattle, WA, USA.
Loice Mbogo, University of Washington Global Assistance Program—Kenya, Nairobi, Kenya.
Betsy Sambai, University of Washington Global Assistance Program—Kenya, Nairobi, Kenya.
Natasha Ludwig-Barron, Department of Global Health, University of Washington, Seattle, WA, USA.
Brandon L Guthrie, Department of Global Health, University of Washington, Seattle, WA, USA; Department of Epidemiology, University of Washington, Seattle, WA, USA.
David Bukusi, Kenyatta National Hospital, Nairobi, Kenya.
Bhavna H Chohan, Department of Global Health, University of Washington, Seattle, WA, USA; Kenya Medical Research Institute, Nairobi, Kenya.
Sarah Masyuko, Department of Global Health, University of Washington, Seattle, WA, USA; Kenya National AIDS and STI Control Programme, Nairobi, Kenya.
John Scott, Department of Medicine, University of Washington, Seattle, WA, USA.
Emily Juma, University of Washington Global Assistance Program—Kenya, Nairobi, Kenya.
Paul Macharia, Strathmore University, Nairobi, Kenya.
Hanley Kingston, Department of Global Health, University of Washington, Seattle, WA, USA.
William Sinkele, Support for Addictions Prevention and Treatment in Africa, Nairobi, Kenya.
Esther Gitau, Support for Addictions Prevention and Treatment in Africa, Nairobi, Kenya.
Rose Bosire, Kenya Medical Research Institute, Nairobi, Kenya.
Helgar Musyoki, Kenya National AIDS and STI Control Programme, Nairobi, Kenya.
Joshua Herbeck, Department of Global Health, University of Washington, Seattle, WA, USA.
Carey Farquhar, Department of Global Health, University of Washington, Seattle, WA, USA; Department of Epidemiology, University of Washington, Seattle, WA, USA; Department of Medicine, University of Washington, Seattle, WA, USA.
Data sharing
Data from this study, including de-identified individual participant data and data dictionaries, will be shared by authors upon request to researchers who provide a methodologically sound proposal. Proposals should be directed to the corresponding author; to access the data, researchers will need to sign a data-access agreement. Statistical analysis plans might also be requested.
References
- 1.UNAIDS. UNAIDS data 2021. 2021. Available from: https://www.unaids.org/sites/default/files/media_asset/JC3032_AIDS_Data_book_2021_En.pdf (accessed June 15, 2022).
- 2.Levi J, Raymond A, Pozniak A, Vernazza P, Kohler P, Hill A. Can the UNAIDS 90–90-90 target be achieved? A systematic analysis of national HIV treatment cascades. BMJ Glob Health 2016; 1: e000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staveteig S, Croft TN, Kampa KT, Head SK. Reaching the ‘first 90’: gaps in coverage of HIV testing among people living with HIV in 16 African countries. PLoS One 2017; 12: e0186316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Githuka G, Hladik W, Mwalili S, et al. Populations at increased risk for HIV infection in Kenya: results from a national population-based household survey, 2012. J Acquir Immune Defic Syndr 2014; 66 (suppl 1): S46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perelman J, Rosado R, Ferro A, Aguiar P. Linkage to HIV care and its determinants in the late HAART era: a systematic review and meta-analysis. AIDS Care 2018; 30: 672–87. [DOI] [PubMed] [Google Scholar]
- 6.LaMonaca K, Dumchev K, Dvoriak S, Azbel L, Morozova O, Altice FL. HIV, drug injection, and harm reduction trends in eastern Europe and central Asia: implications for international and domestic policy. Curr Psychiatry Rep 2019; 21: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larney S, Peacock A, Leung J, et al. Global, regional, and country-level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: a systematic review. Lancet Glob Health 2017; 5: e1208–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heath AJ, Kerr T, Ti L, et al. Healthcare avoidance by people who inject drugs in Bangkok, Thailand. J Public Health 2016; 38: e301–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurth AE, Cleland CM, Des Jarlais DC, et al. HIV Prevalence, estimated incidence, and risk behaviors among people who inject drugs in Kenya. J Acquir Immune Defic Syndr 2015; 70: 420–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UNAIDS. UNAIDS country: Kenya. 2023. http://www.unaids.org/en/regionscountries/countries/kenya (accessed Nov 13, 2023).
- 11.Grebely J, Larney S, Peacock A, et al. Global, regional, and country-level estimates of hepatitis C infection among people who have recently injected drugs. Addiction 2019; 114: 150–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health 2017; 5: e1192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petty LA, Steinbeck JL, Pursell K, Jensen DM. Human immunodeficiency virus and coinfection with hepatitis B and C. Infect Dis Clin North Am 2014; 28: 477–99. [DOI] [PubMed] [Google Scholar]
- 14.Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS 2008; 22: 1979–91. [DOI] [PubMed] [Google Scholar]
- 15.Bartonjo G, Oundo J, Ng’ang’a Z. Prevalence and associated risk factors of transfusion transmissible infections among blood donors at Regional Blood Transfusion Center Nakuru and Tenwek Mission Hospital, Kenya. Pan Afr Med J 2019; 34: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loarec A, Carnimeo V, Molfino L, et al. Extremely low hepatitis C prevalence among HIV co-infected individuals in four countries in sub-Saharan Africa. AIDS 2019; 33: 353–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akiyama MJ, Cleland CM, Lizcano JA, Cherutich P, Kurth AE. Prevalence, estimated incidence, risk behaviours, and genotypic distribution of hepatitis C virus among people who inject drugs accessing harm-reduction services in Kenya: a retrospective cohort study. Lancet Infect Dis 2019; 19: 1255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muasya T, Lore W, Yano K, et al. Prevalence of hepatitis C virus and its genotypes among a cohort of drug users in Kenya. East Afr Med J 2008; 85: 318–25. [DOI] [PubMed] [Google Scholar]
- 19.Molinaro S, Resce G, Alberti A, et al. Barriers to effective management of hepatitis C virus in people who inject drugs: evidence from outpatient clinics. Drug Alcohol Rev 2019; 38: 644–55. [DOI] [PubMed] [Google Scholar]
- 20.Dalal S, Johnson C, Fonner V, et al. Improving HIV test uptake and case finding with assisted partner notification services. AIDS 2017; 31: 1867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahabuka C, Plotkin M, Christensen A, et al. Addressing the first 90: a highly effective partner notification approach reaches previously undiagnosed sexual partners in Tanzania. AIDS Behav 2017; 21: 2551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cherutich P, Golden MR, Wamuti B, et al. Assisted partner services for HIV in Kenya: a cluster randomised controlled trial. Lancet HIV 2017; 4: e74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy JA, Fox SE. The outreach-assisted model of partner notification with IDUs. Public Health Rep 1998; 113 (suppl 1): 160–69. [PMC free article] [PubMed] [Google Scholar]
- 24.Monroe-Wise A, Mbogo L, Guthrie B, et al. Peer-mediated HIV assisted partner services to identify and link to care HIV-positive and HCV-positive people who inject drugs: a cohort study protocol. BMJ Open 2021; 11: e041083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ludwig-Barron NT, Guthrie BL, Mbogo L, et al. Barriers and facilitators of HIV and hepatitis C care among people who inject drugs in Nairobi, Kenya: a qualitative study with peer educators. Harm Reduct J 2021; 18: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masese LN, Ludwig-Barron NT, Mbogo L, et al. Occupational roles and risks of community-embedded peer educators providing HIV, hepatitis C and harm reduction services to persons who inject drugs in Nairobi, Kenya. PLoS One 2022; 17: e0278210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National AIDS & STI Control Program. The Kenya HIV testing services guidelines. Nairobi: Kenyan Ministry of Health, 2015. [Google Scholar]
- 28.Aung SWKH, Kingston H, Mbogo LW, et al. Prevalence and correlates of violence among sexual and injecting partners of people who inject drugs living with HIV in Kenya: a cross-sectional study. Harm Reduct J 2023; 20: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayala G, Bahati M, Balan E, et al. Partner notification: a community viewpoint. J Int AIDS Soc 2019; 22 (suppl 3): e25291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tseng AS, Sambai B, Monroe-Wise A, et al. Assisted partner services for people who inject drugs: index characteristics associated with untreated HIV in partners. J Acquir Immune Defic Syndr 2022; 91: 269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hogben M Partner notification for sexually transmitted diseases. Clin Infect Dis 2007; 44 (suppl 3): S160–74. [DOI] [PubMed] [Google Scholar]
- 32.Heath K, Hill A. WHO hepatitis C elimination targets: the global equity challenge. Lancet Gastroenterol Hepatol 2024; 9: 286–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this study, including de-identified individual participant data and data dictionaries, will be shared by authors upon request to researchers who provide a methodologically sound proposal. Proposals should be directed to the corresponding author; to access the data, researchers will need to sign a data-access agreement. Statistical analysis plans might also be requested.


