Summary
Background
Antimicrobial resistance (AMR) has escalated to pandemic levels, posing a significant global health threat. This study examines the patterns and trends of AMR in Bloodstream Infections (BSIs) across India, aiming to inform better surveillance and intervention strategies.
Methods
Six-year data from 21 tertiary care centers in the Indian Council of Medical Research's AMR Surveillance Network (IAMRSN) were retrospectively analyzed to estimate cluster-robust trends in resistance. Time-series analysis was used to discern lead/lag relationships between antibiotic pairs and the directional influence of resistance in community and hospital-acquired BSIs(CA/HA BSIs). A data-driven Bayesian network ensemble averaged over 301 bootstrap samples was modelled to uncover systemic associations between AMR and Sustainable Development Goals (SDGs).
Findings
Our findings indicate significant (p < 0.001) monthly increases in Imipenem and Meropenem resistance for Klebsiella, E. coli, and Acinetobacter BSIs. Importantly, Carbapenem resistance in HA-BSIs preceded that in CA-BSIs for Klebsiella and Acinetobacter (p < 0.05). At a national level, Cefotaxime resistance emerged as a potential early indicator for emerging Carbapenem resistance, proposing a novel surveillance marker. In Klebsiella BSIs, states with higher achievement of SDG3 goals showed lower Imipenem resistance. A model-based AMR scorecard is introduced for focused interventions and continuous monitoring.
Interpretation
The identified spatiotemporal trends and drug resistance associations offer critical insights for AMR surveillance aligning with WHO GLASS standards.The escalation of carbapenem resistance in BSIs demands vigilant monitoring and may be crucial for achieving SDGs by 2030. Implementing the proposed framework for data-driven evidence can help nations achieve proactive AMR surveillance.
Funding
No specific funding was received for this analysis.
Keywords: BSI, AMR, Carbapenems, Indicator-antibiotic, SDG3
Research in context.
Evidence before this study
We searched PubMed for publications from India in English from January 1, 2000, to December 31, 2022, with the terms (“India”) AND (“AMR” OR “Antimicrobial Resistance”) AND (“BSI” OR “blood stream infection”), and reviewed titles and abstracts to identify relevant articles on antimicrobial resistance in BSIs from India. Our exploration unveiled an increasing concern over AMR in BSIs, but we found a gap in nationwide, multicentric analyses exploring AMR trends and their correlation with Sustainable Development Goals (SDGs), as well as an absence of empirical evidence on cross-resistance patterns from routine surveillance data.
Added value of this study
This research significantly advances our understanding of AMR in India by offering fresh insights. Leveraging data from the non-publicly accessible Indian Council of Medical Research Antimicrobial Resistance Surveillance Network (IAMRSN), we retrospectively analyzed 81,265 records from 21 tertiary care centers, employing both statistical and machine learning methodologies. Our research is the first to situate AMR within the context of Sustainable Development Goals (SDGs), revealing a strong association between higher antibiotic resistance and lower SDG achievements. We also introduce the concept of “indicator antibiotics” such as Cefotaxime, whose resistance patterns can serve as early warning indicators for resistance to other critical antibiotics like Carbapenems. Additionally, we propose a model-derived AMR scorecard to facilitate targeted interventions and continuous monitoring of AMR in BSIs. The national and international relevance of our findings provides a strategic foundation for stakeholders aiming to refine AMR surveillance and policy-making.
Implications of all the available evidence
Our study accentuates the urgency for regionalized treatment guidelines and AMR policies in India tailored for different demographics, including age and gender. Such policies should incorporate local resistance trends, inter-correlations, indicator antibiotics, and the influence of SDG metrics on AMR. The AMR scorecard introduced by our study emerges as a pivotal tool for hospitals, enabling them to effectively augment their AMR surveillance capabilities. This approach facilitates a more nuanced understanding of AMR dynamics and empowers healthcare providers with actionable intelligence for combating AMR more efficiently. Our findings serve as a valuable resource for crafting effective AMR treatment guidelines, stewardship protocols, and infection control practices across various Indian regions. Given the absence of exhaustive, nationwide data from both community and hospital settings in India, the current study offers an empirical foundation that could steer research, policy, and funding toward effective solutions.
Introduction
AMR represents a global health crisis, with an estimated 4.95 million deaths in 2019 and projections suggesting up to 10 million annual deaths by 2050.1, 2, 3 Low- and middle-income countries (LMICs) are particularly vulnerable to AMR, a situation exacerbated by high rates of infectious diseases, increased antibiotic use, and the COVID-19 pandemic.4,5 Without appropriate interventions, AMR could undermine the Sustainable Development Goals(SDGs), potentially plunging millions into extreme poverty by 2030.6, 7, 8 India is among the worst hit, with growing resistance to key and last-resort antibiotics.9, 10, 11, 12
Bloodstream infections (BSIs) significantly impact global health and economics.13 In 2017, there were about 49 million sepsis cases worldwide, leading to 11 million deaths, 41% of which affected children under 5.14 In India, the burden is particularly high, with an estimated caseload of 11 million and a mortality rate of 3 million.14 A recent study found that 56% of cases in 35 Indian ICUs were sepsis-related, with 45% attributable to multi-drug resistant organisms.15 Although multiple studies have noted resistance to critical antibiotics, a gap remains in longitudinal, nationwide data on trends and associations of AMR in BSIs.16,17 This study aims to fill a critical gap in nationwide AMR surveillance by constructing time series and machine learning models for utilising a six-year dataset from the Indian Council of Medical Research AMR Surveillance Network (IAMRSN). We hypothesised that temporal patterns in IAMRSN data can provide data-driven, actionable evidence and early warning signals for proactive surveillance of AMR. Our objectives were to quantify local and national trends of AMR in BSIs, capture early warning signals, create locally actionable scorecards and uncover systemic associations between AMR and Sustainable Development Goals (SDGs) across India. This study can enable the development of targeted treatment guidelines and policies across India and other countries adhering to WHO GLASS standards of AMR surveillance data.
Methods
Study design and data source
The study utilized retrospective data from the ICMR AMR Surveillance Network (IAMRSN), established in 2013. This expansive network comprises nodal centers (NCs) dedicated to specific pathogen groups critical for AMR monitoring: Enterobacteriaceae, Gram-negative non-fermenters, pathogens causing Enteric fever, Diarrhoeagenic bacteria, Gram-positive organisms (including Staphylococci and Enterococci), and Fungal pathogens. Standardized data collection commenced with four NCs and has since expanded to 21 centers, incorporating additional regional centers across India. The sites under the IAMRSN are chosen based on criteria like population size and sample volume.18 The data collection covered a period from January 1, 2017, to December 31, 2022. The dataset includes de-identified patient demographic details, sample characteristics, and standardized antimicrobial susceptibility testing (AMST) results. The laboratories perform microbiological tests on clinical isolates adhering to standard operating procedures (SOPs), regularly updated and available on the ICMR website. The data management system captures quantitative data like Disk Diffusion and Minimum Inhibitory Concentrations, which are interpreted following the Clinical Laboratory Standards Institute (CLSI) guidelines to ensure consistency and reliability across India. Quality assurance was paramount, with internal and external quality controls, including participation in the National External Quality Assurance Systems (EQAS), ensuring the highest data integrity.18 These rigorous quality control measures validated the reliability of the retrospective data analyzed.
The inclusion criteria for the isolates were (i) identification of the following organisms in the isolate (Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca, Klebsiella species, Acinetobacter baumanii, Acinetobacter calcoaceticus, Acinetobacter baumaii-calcoaceticus complex, Acinetobacter species, Staphylococcus aureus, Coagulase-negative Staphylococci (CoNS), Staphylococcus species, Pseudomonas aeruginosa, Enterococcus faecalis, Enterococcus faecium, and Enterococcus species) and (ii) availability of state and district information for the isolate. We used univariate and multivariate time-series modelling to investigate cluster-robust trends and associations of AMR in BSIs in India and contextualised our findings within the framework of state-level Sustainable Development Goals (SDGs) using a data-driven Bayesian network model. The SDGs, a universal set of 17 interconnected goals established by the United Nations in 2015 as part of the 2030 Agenda for Sustainable Development, are being implemented in India under the oversight of NITI Aayog. The data pertaining to the SDG ranking of states was taken from the SDG India Baseline Report 2018.19 This study focused on analysing monthly aggregated data and did not attempt to re-identify the patients.
Descriptive analysis
The isolation percentage of an organism was calculated as:
where “n” represents the count of isolates for the organism and “N” represents the count of total positive cultures.
The rate of antimicrobial resistance (AMR) was calculated as:
where “R” represents the count of resistant isolates, “I” represents the count of intermediate isolates and “S” count of susceptible isolates. AMR rates in terms of R% have been used for the study.
Multi-drug resistance (MDR) was defined if resistance was observed to three or more antibiotic classes. MDR percentage was calculated as
For age-related analysis, the following age groups were considered: (i) Neonate- Less than one month, (ii) Paediatric- 1 month to 18 years (iii) Reproductive-greater than 18–49 years (iv) Middle-greater than 49–70 years (v) Elderly-greater than 70 years.
National trends of resistance
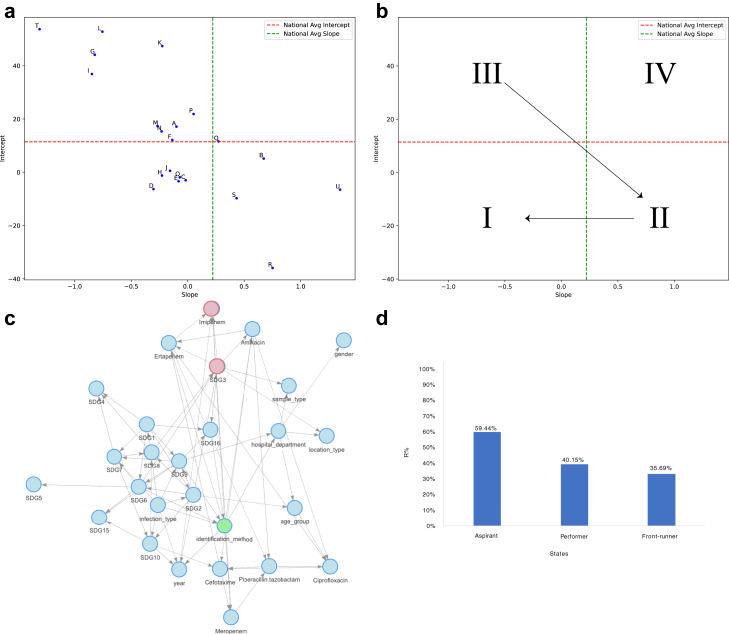
Monthly AMR rates for each antibiotic and organism were aggregated to generate univariate and multivariate time series. Robust confidence intervals (95%) were calculated using Bias-Corrected and Accelerated (BCa) bootstrapping with a rolling window of 3 months for plotting the six-year trends for every microorganism–antibiotic combination. The linear component of the time series was extracted using time series decomposition based on moving averages with a frequency of 12 (for monthly data). National estimates for resistance percentage were derived utilizing a linear regression model, with the dependent variable being resistance percentage and the independent variables being time, hospital, and their interaction effects. To mitigate potential biases introduced by clustering of data within hospitals, heteroskedasticity and autocorrelation in temporal patterns, cluster-robust estimates of significance and standard errors were calculated using the sandwich package20 in R. Further, the intercepts and slope of each hospital were studied with respect to national slope and intercept to generate an AMR Scorecard for the hospitals to monitor their resistance. To identify the linear relationship between two slopes and intercepts (continuous variables) obtained from the linear model for each hospital, Pearson's correlation21 coefficient was calculated for each drug–bug combination. Data were further stratified based on gender, zones, age groups, and community and hospital-acquired infections and analyzed separately using a simple linear regression modelling approach to mitigate potential biases arising from different AMR rates in these subsets.
Lead/lag analysis for indicator antibiotics and CA-BSI/HA-BSI associations
Minimal pre-processing of time series was done to avoid statistical artefacts. Time series with overall missing values for resistance rates of more than 20% were discarded. Time series that satisfied the criteria of less than four consecutive missing values were linearly interpolated. Stationarity of the interpolated time series was examined using the Augmented Dickey–Fuller test.22 First- or second-order differencing was applied to address non-stationarity in the series (refer to Supplementary Tables S2 and S3). Cross-correlations were computed for the transformed, stationary AMR rates at lags of 0–15 months and depicted graphically. Similarly, a lead/lag analysis was conducted for AMR rates in community-acquired and hospital-acquired bloodstream infections (CA-BSI, HA-BSI).
Local patterns of AMR and sustainable development goals
The states were stratified into zones to assess the geographical distribution of antimicrobial resistance (AMR), as outlined in Table 1. The data from individuals residing within the state of testing were analysed with the 2018 SDG India Baseline report indicators for the respective state. We extracted a subset of data to examine geographical relationships with matching patient and hospital zones. Unsupervised hierarchical clustering and visualization techniques were then employed to uncover clusters within these zones. Continuous variables were discretized using k-means clustering, a machine learning algorithm that partitions n observations into k clusters, where each observation belongs to the cluster with the nearest mean. Since it is cumbersome to incorporate all possible confounding factors in pairwise associations, a probabilistic graphical model that decomposed the joint probability into conditional dependencies in a Bayesian network structure was learned from the data. Learning the conditional dependency structure of the network is an optimization problem, which was addressed using the hill climbing algorithm and Akaike scoring criterion implemented in the wiseR23 package. AIC was used to select the simplest model that balances the goodness of fit and model complexity to adequately explain the data trends. For robust inference, 301 BN instances were learned using bootstrapping to obtain a consensus network using ensemble-averaging and majority-voting criteria. The network model was queried for inferring the change in conditional probabilities of a node upon fixing a connected node using the Exact Inference algorithm implemented in wiseR. Specifically, the covariates used in the model for E. coli were:
-
•
identification_method: Method used for identification of organism
-
•
collection_date: Date of sample collection
-
•
year: Year of sample collection
-
•
hospital_department: Hospital Department
-
•
hosp_location_type: Location within the hospital (OPD/Ward/ICU)
-
•
infection_type: Type of infection (Community/Hospital acquired)
-
•
Gender: Gender of the patient
-
•
Amikacin: Antibiotic tested
-
•
Cefotaxime: Antibiotic tested
-
•
Ciprofloxacin: Antibiotic tested
-
•
Ertapenem: Antibiotic tested
-
•
Imipenem: Antibiotic tested
-
•
Meropenem: Antibiotic tested
-
•
Nitrofurantoin: Antibiotic tested
-
•
Piperacillin.tazobactam: Antibiotic tested
-
•
Trimethoprim.sulfamethoxazole: Antibiotic tested
-
•
covid: If the patient was affected by COVID19 or not (0/1)
-
•
SDG1: No Poverty
-
•
SDG2: Zero Hunger
-
•
SDG3: Good Health and Well-being
-
•
SDG4: Quality Education
-
•
SDG5: Gender Equality
-
•
SDG6: Clean Water and Sanitation
-
•
SDG7: Affordable and Clean Energy
-
•
SDG8: Decent Work and Economic Growth
-
•
SDG9: Industry, Innovation, and Infrastructure
-
•
SDG10: Reduced Inequalities
-
•
SDG11: Sustainable Cities and Communities
-
•
SDG12: Responsible Consumption and Production
-
•
SDG13: Climate Action
-
•
SDG14: Life Below Water
-
•
SDG15: Life on Land
-
•
SDG16: Peace, Justice, and Strong Institutions
-
•
SDG17: Partnerships for the Goals
Table 1.
Distribution of isolates across gender, zones, and overall antibiotic resistance patterns.
| E. coli (%) | Klebsiella (%) | Acinetobacter (%) | Staphylococcus (%) | P. aeruginosa (%) | Enterococcus (%) | |
|---|---|---|---|---|---|---|
| Total | ||||||
| Total Isolates | 16,570/81,265 (20.39%) | 16,486/81,265 (20.29%) | 11,934/81,265 (14.69%) | 23,717/81,265 (29.18%) | 5755/81,265 (7.08%) | 6803/81,265 (8.37%) |
| Gender | ||||||
| Males (50376; 61.98%) | 9211/50,376 (18.28%) | 10,581/50,376 (21.0%) | 7611/50,376 (15.11%) | 15078/50,376 (29.93%) | 3639/50,376 (7.22%) | 4256/50,376 (8.45%) |
| Females (30867; 37.98%) | 7353/30,867 (23.82%) | 5902/30,867 (19.12%) | 4320/30,867 (14.0%) | 8632/30,867 (27.97%) | 2116/30,867 (6.86%) | 2544/30,867 (8.24%) |
| Transgender (22; 0.03%) | 6/22 (27.27%) | 3/22 (13.64%) | 3/22 (13.64%) | 7/22 (31.82%) | 0/22 (0.0%) | 3/22 (13.64%) |
| Zonea | ||||||
| North Zone (28430; 34.98%) | 3615/28,430 (12.72%) | 5711/28,430 (20.09%) | 4355/28,430 (15.32%) | 10744/28,430 (37.79%) | 1645/28,430 (5.79%) | 2360/28,430 (8.3%) |
| South Zone (27137; 33.39%) | 7742/27,137 (28.53%) | 5303/27,137 (19.54%) | 3396/27,137 (12.51%) | 6130/27,137 (22.59%) | 2048/27,137 (7.55%) | 2518/27,137 (9.28%) |
| East Zone (8529; 10.49%) | 2011/8529 (23.58%) | 1880/8529 (22.04%) | 1066/8529 (12.5%) | 2139/8529 (25.08%) | 741/8529 (8.69%) | 692/8529 (8.11%) |
| West Zone (12406; 15.26%) | 2492/12,406 (20.09%) | 2701/12,406 (21.77%) | 2054/12,406 (16.56%) | 3336/12,406 (26.89%) | 1059/12,406 (8.54%) | 764/12,406 (6.16%) |
| Central Zone (1850; 2.27%) | 304/1850 (16.43%) | 387/1850 (20.92%) | 418/1850 (22.59%) | 431/1850 (23.3%) | 117/1850 (6.32%) | 193/1850 (10.43%) |
| North-east Zone (2913; 3.58%) | 406/2913 (13.94%) | 504/2913 (17.3%) | 645/2913 (22.14%) | 937/2913 (32.17%) | 145/2913 (4.98%) | 276/2913 (9.47%) |
| MDR (%) | ||||||
| Isolates resistant to 3 or more antibiotic classes | 3938/16,570 (23.77%) | 9915/16,486 (60.14%) | 9062/11,934 (75.94%) | 12,133/23,717 (51.16%) | 1698/5755 (29.50%) | 813/6803 (11.95%) |
| Average resistance percentageover the entire study duration | ||||||
| Amikacin | 2898/16,464 (17.6) | 8799/16,356 (53.8) | 7529/10,764 (69.95) | NA | 1728/5696 (30.34) | NA |
| Cefepime | NA | NA | 8990/11455 (78.48) | NA | NA | NA |
| Cefotaxime | 11,096/14,048 (78.99) | 10,770/13,592 (79.24) | NA | NA | NA | NA |
| Cefoxitin | NA | NA | NA | 12,765/19,843 (64.33) | NA | NA |
| Ceftazidime | NA | NA | 8963/11,191 (80.09) | NA | 1881/5568 (33.78) | NA |
| Ciprofloxacin | 11,581/15,230 (76.04) | 10,312/15,116 (68.22) | NA | 11483/19,336 (59.39) | 1800/4942 (36.42) | NA |
| Clindamycin | NA | NA | NA | 9722/23,090 (42.1) | NA | NA |
| Colistin | NA | NA | 180/4446 (4.05) | NA | NA | NA |
| Ertapenem | 3016/12119 (24.89) | 7224/11,850 (60.96) | NA | NA | NA | NA |
| Erythromycin | NA | NA | NA | 15054/22,523 (66.84) | NA | NA |
| Imipenem | 3200/13,248 (24.15) | 7766/14,637 (53.06) | 8600/11,510 (74.72) | NA | 1757/5093 (34.5) | NA |
| Levofloxacin | NA | NA | 6592/10135 (65.04) | NA | NA | NA |
| Linezolid | NA | NA | NA | 291/21,918 (1.33) | NA | 500/6471 (7.73) |
| Meropenem | 3484/15,735 (22.14) | 9024/15,327 (58.88) | 8340/11,629 (71.72) | NA | 1733/5524 (31.37) | NA |
| Minocycline | NA | NA | 2900/9656 (30.03) | NA | NA | NA |
| Piperacillin-tazobactam | 5647/16,205 (34.85) | 10,439/16,169 (64.56) | 8601/11,646 (73.85) | NA | 1282/5536 (23.16) | NA |
| Teicoplanin | NA | NA | NA | NA | NA | 1044/6293 (16.59) |
| Tetracycline | NA | NA | NA | 2994/16,822 (17.8) | NA | NA |
| Trimethoprim-sulfamethoxazole | NA | NA | NA | 7931/19,223 (41.26) | NA | NA |
| Vancomycin | NA | NA | NA | 118/13,290 (0.89) | NA | 1262/6475 (19.49) |
| Ampicillin | NA | NA | NA | NA | NA | 3829/5822 (65.77) |
| Gentamicin HL | NA | NA | NA | NA | NA | 3001/5200 (57.71) |
NA Not applicable to gram-positive or gram-negative organisms.
North zone includes Himachal Pradesh, Punjab, Uttarakhand, Uttar Pradesh, Haryana, Ladakh, Chandigarh, Delhi, Jammu and Kashmir; South zone includes Andhra Pradesh, Karnataka, Kerala, Telangana, Tamil Nadu, Andaman and Nicobar, Lakshadweep, Puducherry; East zone includes Bihar, Odisha, Jharkhand, West Bengal; West zone includes Rajasthan, Gujarat, Goa, Maharashtra, Dadra and Nagar Haveli, Daman and Diu; Central zone includes Madhya Pradesh, Chhattisgarh; North-east zone includes Assam, Sikkim, Nagaland, Meghalaya, Manipur, Mizoram, Tripura, Arunachal Pradesh.
The SDG column contained the values for each state classified (Achievers- 100; Front Runner- 65–99; Performer- 50–64; Aspirant- 0–49) based on their specific goal achievement. The SDGs showing no variation in our data were removed before model development.
Patient consent and ethics approval
This study has been approved by the Ethics Committee of the Indraprastha Institute of Information Technology Delhi (IIITD-IRB/ER/11/2022/2). This study utilized the routinely collected IAMRSN data. The access to anonymized IAMRSN data was provided by ICMR. Each hospital in the Indian Council of Medical Research Antimicrobial Resistance network (IAMRSN) obtained its own ethics approvals for the collection of the surveillance data.
Role of the funding source
No funding was received specifically for this analysis.
Results
Primary analysis
The Indian Council of Medical Research (ICMR) database contained 511,224 isolates collected from January 1, 2017, to December 31, 2022. A total of 100,908 isolates were associated with bloodstream infections (from both peripheral and central catheters), of which 81,265 met the specified inclusion and exclusion criteria across 21 centres in 14 states/UTs (Fig. 1, Supplementary Figure S1). The available patient records were analysed separately for 8786 CA-BSI and 12,079 HA-BSI. Due to inconsistencies in reported date of birth, age groups could be assigned to 78183 records.
Fig. 1.
Overview of the study. Harmonized data from 21 ICMR AMR surveillance network (IAMRSN) centres were analysed for (a) six-year seasonality and linear trends, (b) indicator antibiotics (c) lead/lag associations between resistance in community and hospital-acquired infections (CA-BSI/HA-BSI), (d) spatial clusters of AMR, and (e) Bayesian network model for quantifying the associations between SDGs and AMR.
Descriptive analysis (Table 1, Supplementary Table S1) revealed geographical variations. E. coli BSIs were found to be more prevalent in isolates from Southern India, while Staphylococcus was more common in the North. Acinetobacter showed higher prevalence in the Central and North East zones. In contrast, Klebsiella, P. aeruginosa, and Enterococcus showed a more uniform distribution across the regions. A high rate of Multi-drug resistance was observed in Klebsiella, Acinetobacter, and Staphylococcus isolates.
The study observed high resistance to Cephalosporins and Fluoroquinolones in E. coli and Klebsiella isolates, with additional resistance to Carbapenems observed in the latter. Acinetobacter isolates exhibited significant resistance to all tested antibiotics except for Minocycline. Staphylococcus isolates demonstrated high resistance levels to Cefoxitin, Ciprofloxacin, and Erythromycin. P. aeruginosa isolates exhibited uniform resistance across all tested antimicrobials. Enterococcus demonstrated high resistance to Ampicillin and High-level Gentamicin, while its resistance to Linezolid was comparatively low. Comparative plots depict differences in baseline resistance levels and trends for the same antibiotic across different organisms (Supplementary Figures S9–S14).
National trends of resistance
The modelling of monthly resistance rates for each pathogen revealed significant linear trends for each drug–bug combination. These trends were observed to be broadly consistent across diverse categories such as age groups, geographical regions, infection types, and genders (Table 2). Detailed descriptions of these trends follow.
Table 2.
Resistance trends in (a) gram-negative (b) gram-positive organisms and antibiotics.
| (a) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Antibiotics | E. coli |
Klebsiella |
P. aeruginosa |
Acinetobacter |
||||
| Increasing resistance | Decreasing resistance | Increasing resistance | Decreasing resistance | Increasing resistance | Decreasing resistance | Increasing resistance | Decreasing resistance | |
| Amikacin | National, Male, Female, Middle, South, West, CA-BSI, HA-BSI | North | CA-BSI, HA-BSI, North-east | National, Male, Neonate, Paediatric, Middle, North, West | Male, Female, Paediatric, Reproductive, Middle, North, South, West, HA-BSI | Male, Reproductive, Middle, South, West, CA-BSI, HA-BSI | North-east | |
| Cefepime | NA | NA | NA | NA | National | North, West | National, Male, Female, Reproductive, Middle, South, Central, CA-BSI, HA-BSI | Neonate, North, East |
| Cefotaxime | Female, Middle, Elderly, CA-BSI, HA-BSI | National, Male, Paediatric, North | Neonate, Reproductive, Elderly, West, CA-BSI, HA-BSI | National, Middle, Paediatric, North-east | NA | NA | NA | NA |
| Ceftazidime | NA | NA | NA | NA | National, Male, Female, paediatric, Reproductive, Middle, North, South, HA-BSI | West | National, Male, Female, Neonate, Paediatric, Reproductive, Middle, South, West, Central, North-east, CA-BSI, HA-BSI | NA |
| Ciprofloxacin | National, Female, Neonate, Reproductive, Middle, South, West, Central, CA-BSI, HA-BSI | Paediatric, North | Male, Female, Paediatric, Reproductive, Middle, Elderly, North, South, East, West, Central, North-east, CA-BSI, HA-BSI | – | Female, Middle, South, West, HA-BSI | NA | NA | |
| Ertapenem | National, Middle, South, West, CA-BSI, HA-BSI | North | North, South, East, CA-BSI, HA-BSI | National, Female, Neonate, Middle, West, Central | NA | NA | NA | NA |
| Imipenem | National, Male, Female, Paediatric, Reproductive, Middle, Elderly, North, South, CA-BSI, HA-BSI | West | National, Male, Female, Paediatric, Reproductive, Middle, Elderly, North, South, North-east, CA-BSI, HA-BSI | West | Male, Female, Paediatric, Reproductive, North, HA-BSI | West | National, Male, Female, Neonate, Paediatric, Reproductive, Middle, Elderly, North, South, West, Central, CA-BSI, HA-BSI | North-east |
| Levofloxacin | NA | NA | NA | NA | Male, Female, Reproductive, Middle, North | West | Male, Female, Reproductive, Middle, Elderly, North, South, East, Central, CA-BSI, HA-BSI | National, Neonate |
| Meropenem | National, Male, Female, Neonate, Reproductive, Middle, Elderly, South, East, West, CA-BSI, HA-BSI | North | National, Male, Female, Paediatric, Reproductive, Middle, Elderly, North, South, East, Central, North-east, CA-BSI, HA-BSI | West | National, Male, Female, Reproductive, South, HA-BSI | North | National, Male, Female, Neonate, Paediatric, Reproductive, Middle, Elderly, North, South, West, Central, CA-BSI, HA-BSI | East, North-east |
| Piperacillin-tazobactam | National, Male, Female, Neonate, Reproductive,Middle, Elderly, North, South, West, North-east, CA-BSI, HA-BSI | East | National, Male, Female, Paediatric, Reproductive, Middle, Elderly, North, South, East, North-east, CA-BSI, HA-BSI | National, Male, Female, Reproductive, South, HA-BSI | Middle, North | National, Male, Female, Paediatric, Reproductive, Middle, Elderly, North, South, East, West, Central, CA-BSI, HA-BSI | Neonate, North-east | |
| Gentamicin | NA | NA | NA | NA | National, Male, Female, Middle, North, South, West | |||
| Minocycline | NA | NA | NA | NA | NA | NA | West, HA-BSI | National, Male, Female, Paediatric, Reproductive, Middle, Elderly, North |
| (b) | ||||
|---|---|---|---|---|
| Antibiotics | Enterococcus |
Staphylococcus |
||
| Increasing resistance | Decreasing resistance | Increasing resistance | Decreasing resistance | |
| Ampicillin | National, Male, Female, Paediatric, Reproductive, Middle, Elderly, North east, CA-BSI, HA-BSI | Neonate | NA | NA |
| Cefoxitin | NA | NA | Male, Female, Neonate, Paediatric, Reproductive, Middle, Elderly, North, North-east, CA-BSI | National, East, West, HA-BSI |
| Ciprofloxacin | NA | NA | National, Male, Female, Paediatric, Reproductive, Middle, North, South, East, Central, HA-BSI | Neonate, West |
| Clindamycin | NA | NA | National, Male, Female, Paediatric, Reproductive, Middle, Elderly, North, South, East, North-east, CA-BSI, HA-BSI | |
| Erythromycin | NA | NA | National, Male, Female, Reproductive, Middle, Elderly, North, South, East, CA-BSI, HA-BSI | Neonate, North-east |
| Linezolid | National | – | – | National, Male |
| Teicoplanin | Middle, North | – | NA | NA |
| Tetracycline | NA | NA | National, Male, Female, Paediatric, Reproductive, Middle, Elderly, North, South, CA-BSI, HA-BSI | West |
| Trimethoprim-sulfamethoxazole | NA | NA | East | Male, Female, Neonate, Paediatric, Reproductive, Middle, North, South, West, HA-BSI |
| Vancomycin | Male, Middle | Paediatric, North | NA | NA |
| Gentamicin HL | – | National, Male, Female, Neonate, Paediatric, Middle, Reproductive, Elderly, North, South, East, West, North-east, CA-BSI, HA-BSI | NA | NA |
–Data not available.
Exact trend values based on significance levels are available in Supplementary Tables S4–S9.
E. coli
A significant increasing national monthly trend in resistance was identified for multiple drugs: Imipenem at 0.22% (p < 10−20), Piperacillin-Tazobactam at 0.22% (p < 10−16), Ciprofloxacin at 0.18% (p < 10−9), Meropenem at 0.15% (p < 10−8), Ertapenem at 0.09% (p < 10−9) and Amikacin at 0.06% (p < 10−32). In contrast, a significant decreasing trend in resistance was observed for Cefotaxime at 0.17% (p < 10−16) (Supplementary Table S4; Supplementary Figures S2 and S8).
Klebsiella
A significant increasing national monthly trend in resistance was identified for multiple drugs: Imipenem at 0.24% (p < 0.001), Piperacillin-Tazobactam at 0.17% (p < 10−18) and Meropenem at 0.17% (p < 0.01). In contrast, a significant decreasing trend in resistance was observed for Cefotaxime at 0.25% (p < 10−16), Ertapenem at 0.09% (p < 10−16) and Amikacin at 0.04% (p < 0.05) (Supplementary Table S5; Supplementary Figures S3 and S8).
Acinetobacter
A significant increasing national monthly trend in resistance was identified for multiple drugs: Meropenem at 0.43% (p < 10−16), Imipenem at 0.21% (p < 10−16), Piperacillin-Tazobactam at 0.17% (p < 10−16), Cefepime at 0.13% (p < 10−16) and Ceftazidime at 0.08% (p < 10−16). In contrast, a significant decreasing trend in resistance was observed for Minocycline at 0.16% (p < 10−05) and Levofloxacin at 0.04% (p < 0.05) (Supplementary Table S6; Supplementary Figures S4 and S8).
P. aeruginosa
A significant increasing national monthly trend in resistance was identified for multiple drugs: Meropenem at 0.18% (p < 10−16), Ceftazidime at 0.13% (p < 10−5), Piperacillin-Tazobactam at 0.09% (p < 10−16) and Cefepime at 0.08% (p < 10−16). In contrast, a significant decreasing trend in resistance was observed for Gentamicin at 0.06% (p < 10−16) (Supplementary Table S7; Supplementary Figures S5 and S8).
Staphylococcus
A significant increasing national monthly trend in resistance was identified for multiple drugs: Erythromycin at 0.33% (p < 10−16), Ciprofloxacin at 0.29% (p < 10−16), Tetracycline at 0.21% (p < 10−16) and Clindamycin at 0.15% (p < 10−5). In contrast, a significant decreasing trend in resistance was observed for Linezolid at 0.03% (p < 10−16) and Cefoxitin at 0.073% (p < 0.001) (Supplementary Table S8; Supplementary Figures S6 and S8).
Enterococcus
A significant increasing national monthly trend in resistance was identified for multiple drugs: Linezolid at 0.19% (p < 10−5) and Ampicillin at 0.10% (p < 0.0001). In contrast, a significant decreasing trend in resistance was observed for Gentamicin HL at 0.18% (p < 0.0001) (Supplementary Table S9; Supplementary Figures S7 and S8).
Temporal associations of resistance in community and hospital acquired infections
This analysis was designed to investigate and quantify the temporal dynamics of resistance patterns in community-acquired (CA) and hospital-acquired (HA) bloodstream infections (BSIs). We hypothesised that resistance patterns observed in one setting might precede similar patterns in the other, thereby providing insights into the possible transmission of resistance from one context to another. Our analysis revealed significant associations in CA and HA BSIs (all p < 0.05). For E. coli BSIs, Amikacin and Piperacillin-tazobactam resistance in HA-BSI led resistance in CA-BSIs (Supplementary Figure S15). As anticipated, a leading association between hospitals and the community is evident in the case of Klebsiella and Acinetobacter species. Notably, a correlation is observed for the following antimicrobial agents: Amikacin, Cefotaxime, Ciprofloxacin, Imipenem, and Meropenem in Klebsiella species, and Ceftazidime, Imipenem, and Meropenem in Acinetobacter species (Supplementary Figure S16 and S17). However, in Staphylococcus, Ciprofloxacin and Erythromycin resistance in HA-BSI led resistance in CA-BSI by 3–4 months in the former and 10 months in the latter. In contrast, Clindamycin resistance in CA-BSI led to HA-BSI by 1–2 months (Supplementary Figure S18).
Indicator antibiotics as potential early warning signals for surveillance
The lead/lag analysis required minimum pre-processing for the antibiotic time series as most of the time series achieved stationarity with the maximum first-order differencing operation. The antibiotic-wise order of difference is provided in Supplementary Table S1. Lead/lag cross-correlation analyses between antibiotic pairs revealed significant associations that can be used as early warning signals for critically important antimicrobials such as Carbapenems (all p < 0.05).
For E. coli, Cefotaxime was a strong leading indicator for Imipenem and Meropenem resistance (Supplementary Figure S19), with a lead time of 9–10 months. Additionally, Ciprofloxacin and Piperacillin-tazobactam were strong leading indicators for Imipenem resistance with a lead time of 0–1 months in the case of Klebsiella BSIs (Supplementary Figure S20). Ceftazidime and Cefepime were also identified as leading indicators for Meropenem resistance in the case of Acinetobacter BSIs (Supplementary Figure S21).
Local patterns of AMR and sustainable development goals performance
In our analysis, 91.4% of the patients were tested within their zone of residence, and 70.8% were tested within their state of residence. These subsets were used for extracting zonal clusters and associations with the SDG performance of states.
E. coli
For single drug resistance, antibiotic classes formed two distinct clusters of resistance. The Central and North zones showed very high resistance to all antibiotics. The South zone generally had the lowest antibiotic resistance except for Cefotaxime and Ciprofloxacin. In MDR isolates, the Central and North-east zones showed higher resistance to all antibiotics except Amikacin in the former and Ciprofloxacin in the latter. The East zone had lower resistance except for Ciprofloxacin and Ertapenem (Supplementary Figure S22). Most importantly, the North zone had the highest monthly rate of increase in Imipenem resistance (0.33%) compared to the West zone (0.11%). Increasing resistance rates for Imipenem, Meropenem and Piperacillin-tazobactam were observed for the North, South, East, West and Central zones (Supplementary Table S6).
Klebsiella
For single drug resistance (Supplementary Figure S23), the North and Central zones depict high antibiotic resistance. The South zone had generally low resistance. The Central and East zones had a high resistance to Colistin, whereas the Central and West zones had a high resistance to Fosfomycin. In MDR isolates, the East zone showed lower resistance excepting Ciprofloxacin and Ertapenem.
Acinetobacter
For single drug resistance (Supplementary Figure S24), the North zone showed high resistance to all antibiotics except Colistin. The northeast zone generally had the lowest resistance all antibiotics except levofloxacin. In MDR isolates, the resistance level in North, South, Central and West zones is mostly high. The East zone had lower resistance except for Levofloxacin, Colistin and Ceftazidime. Most importantly, the North zone had the highest monthly rate of increase in Imipenem resistance (0.33%) compared to the West zone (0.11%). Increasing resistance rates for Imipenem, Meropenem and Piperacillin-tazobactam were observed for the North, South, East, West and Central zones (Supplementary Table S4).
P. aeruginosa
The North zone had high resistance, whereas the East and South zones had low resistance for all antibiotics (Supplementary Figure S25a). In MDR isolates, the North-east zone had low resistance to most antibiotics (Supplementary Figure S25b).
Staphylococcus
For single drug resistance, the South zone generally had high resistance to all antibiotics except Ciprofloxacin. The North zone had generally low resistance for all antibiotics except Linezolid and Vancomycin, which was also consistent across MDR isolates (Supplementary Figure S26).
Enterococcus
Antibiotic classes form two main clusters for single drug resistance (Supplementary Figure S27). The North zone had generally high resistance to all antibiotics except Gentamicin HL and Ampicillin. The East zone had high resistance for Gentamicin HL and Ampicillin. The North-east zone had a generally low resistance for all antibiotics. In MDR isolates, high resistance to Ampicillin and Vancomycin was seen across all zones except North-east in the former and West in the latter.
The findings of zonal patterns were then modelled and quantified in the context of SDGs in a data-driven Bayesian network model. For Klebsiella BSIs, an association was observed between Imipenem, Amikacin, and Ertapenem resistance and SDG3 (Good Health and Well-being) (Fig. 2). Further exploration into the variables of SDG3 revealed an association of Amikacin resistance with annual notification of Tb cases, maternal mortality and under-5 mortality. While for E. coli BSIs, an association was observed between Ciprofloxacin resistance and SDG3. Quantification of association using the exact inference algorithm revealed the high resistance percentage in aspirant states (84.57%) as compared with the performer (74.17%) and front-runner states (72.1%). Further exploration into the variables of SDG3 revealed an association of Ciprofloxacin resistance with annual notification of Tb cases.
Fig. 2.
The figure contains representative plots for a) slope and intercept for each hospital depicting variability in hospital-wise resistance rates over time relative to national benchmarks. Results are derived from linear regression analysis incorporating time, hospital, and their interaction effects to model resistance percentage dynamics. b) The AMR Scorecard displays hospitals' positions across four quadrants based on national averages for slope (rate of resistance increase) and intercept (initial resistance levels). Quadrant Irepresents hospitals with favourable AMR profiles (low initial resistance and low rate of increase), while Quadrants II, III, and IVidentify hospitals needing targeted interventions to manage increasing resistance rates, high baseline resistance, or both, respectively c) Bayesian Network model for Klebsiella, highlighting the significant association between Imipenem resistance and SDG3 d) Categorization of states based on their Sustainable Development Goals Index reveals higher resistance rates in aspirant states, underscoring the need for focused healthcare improvements in these regions.
Discussion
This study is the first to offer a nationwide framework for identifying antimicrobial resistance (AMR) trends, associations and mitigation approaches. Given the paucity of large-scale, multicentric data on this pressing public health issue, these findings are critically important.
The AMR prevalence in our study aligns with smaller, single-centric studies from India, thus affirming the validity of our findings and highlighting the need for ongoing, comprehensive monitoring of AMR at a national level.15, 16, 17,24,25 Our study estimated national AMR trends using a linear regression model, adjusting for data clustering by tertiary care centres. This model accounted for variations in resistance levels and changes over time within each hospital, adjusting for both the potential heteroskedasticity and auto-correlation within hospital clusters through cluster-robust p values. We also obtained a negative correlation value between the slope and intercept for different drug–bug combinations, suggesting ongoing efforts to contain resistance in these hospitals (Supplementary Tables S10–S15). We have also experimented with mixed effects models to handle random effects at the hospital level. However, we did not report these results because this approach could not fully address autocorrelation in time series data and failed to provide p-values for random effects, limiting our ability to assess the significance of these effects in explaining AMR variability.
Trend analysis revealed high resistance rates with increasing trends in critically important antimicrobials like Imipenem for Acinetobacter, Klebsiella and E. coli infections. However, decreasing resistance trends for Amikacin in Klebsiella BSIs and Minocycline in Acinetobacter BSIs were also observed. Although we do not have the consumption data, this may indicate a decrease in the use of these antibiotics. Our results are generally consistent with Karuna et al.’s16 findings on 693 BSI-positive patients from 10 secondary care centres and Mathur et al.’s25 findings on 2622 HA-BSIs from tertiary care centres on the relative prevalence of pathogens in Bloodstream infections in India.
We identified pronounced variations in AMR prevalence when stratified by age and geographic location, illuminating the multifaceted terrain of resistance patterns throughout the country. We found notable regional variations in Imipenem resistance, with some areas exhibiting increasing resistance and others showing declining trends. For instance, resistance rates in Klebsiella varied significantly, from a 0.42% increase to a 0.17% decrease. Similar regional disparities were observed in E. coli, Acinetobacter, and Pseudomonas aeruginosa.
Further segregation of the data into CA-BSI and HA-BSIs suggested strong temporal associations between the two settings. As anticipated, a leading association between hospitals and the community is evident in the case of Klebsiella and Acinetobacter species. Cefotaxime resistance was identified as a leading signal for Carbapenem resistance in E. coli. While these results are identified from a data-driven analysis, understanding the underlying biological mechanisms is crucial for effective antibiotic stewardship. Designing data-driven indicators will be an important step towards the design of time-relevant and contextualized stewardship policies as their associations will change, especially if stewardship is implemented effectively. For example, cefotaxime and imipenem belong to the beta-lactam class of antibiotics and work by targeting the same bacterial process of cell wall synthesis.26 Similarly, cefotaxime use may exert selective pressure in carbapenems through genetically linked or co-selected resistance exhibited within beta-lactams. Further, overlap in ecological niches of microbes can facilitate the transmission of resistance genes between bacterial populations, potentially increasing imipenem resistance over time. However, the biological hypotheses underpinning temporal associations need to be evaluated further and are outside the scope of this study.
The growing resistance to existing antibiotics amidst the shrinking pipeline of newer drugs is a serious threat to attaining the SDG target by 2030. The recent incorporation of two AMR-specific indicators into the SDGs—namely, the rates of E. coli resistance to third-generation cephalosporins and the prevalence of MRSA—emphasizes the urgency to monitor these metrics27 concurrently. Existing research suggests a direct correlation between antimicrobial resistance (AMR) and SDG 3, focusing on Good Health and Well-being. Moreover, indirect relationships have been proposed between AMR and other Sustainable Development Goals, including SDG 1 (No Poverty), SDG 2 (Zero Hunger), and SDG 8 (Decent Work and Economic Growth).6 Our data-driven Bayesian network model highlights the need to contextualize AMR within the broader SDG framework. Specifically, we found associations between SDG 3 and resistance patterns for Amikacin, Imipenem, and Ertapenem in Klebsiella and Ciprofloxacin in E. coli. This emphasizes that AMR and sustainable development are intrinsically linked and cannot be addressed without considering the other.
Our study is pioneering in quantifying the relationship between state-level SDG performance and AMR in India. While our model does not establish causality, it benefits from temporal data, with SDG indicators from 2018 serving as the backdrop for our AMR study period from 2017 to 2022.
While our study has notable strengths, such as being one of the largest multisite analyses in India, it is not without limitations. The data comes solely from tertiary care centres, which may not fully represent broader community trends. To mitigate this, we conducted separate analyses for community-acquired and hospital-acquired bloodstream infections (BSIs), and found consistent results across both settings. These centres in 14 state/UTs across the country cater to people from across the states. We used the patient's state of residence instead of the centre location for descriptive analysis to get a nationwide estimate. However, for state/zone-wise analysis, a subset of records with the same patient and hospital state/zone were analysed. Another concern is the absence of reliable antibiotic consumption data from India. We addressed this by analysing longitudinal resistance trends for each organism and antibiotic, assuming that usage patterns remained stable between 2017 and 2022. Additionally, although our Bayesian network model adjusts for potential confounders, it cannot rule out the influence of unknown factors on AMR rates.
We see similar patterns of trends and associations for Carbapenems in Urinary Tract Infections in the same datasets (Unpublished work), thus making our approach generalizable to AMR surveillance nationwide. Further, since our framework is based on interoperable data that adhere to WHO GLASS standards, it is extensible to other LMICs and hospitals following the same. Our future work will involve the deployment of the modelling framework and scorecards to improve the AMR surveillance network throughout the IAMRSN network, potentially providing early warning signals. Additionally, we plan to explore the specific impact of the COVID-19 pandemic and delve deeper into molecular associations of AMR.
Our study is a benchmark analysis of AMR trends in BSIs across India. Our findings underscore the urgency of the escalating AMR crisis and its implications for broader development goals. This necessitates immediate and targeted interventions, including further research, increased funding, and the formulation of effective local policies for AMR containment.
Contributors
JK has performed data pre-processing. JK and TS were involved in formal analysis and visualized the results. JK, TS and HS interpreted the data and wrote the manuscript. All authors have read and approved the manuscript.
Data sharing statement
While individual patient data from the IAMRSN is not publicly accessible, summarized annual reports are available on the IAMRSN website.28 Requests for complete datasets should be directed to Dr Kamini Walia, Program Officer (AMR), ICMR and are subject to approval.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
The authors declare that they have no competing interests.
Acknowledgements
We sincerely thank the Indian Council of Medical Research (ICMR) for providing the antimicrobial resistance (AMR) surveillance data compiled by IAMRSN, which was indispensable for this analysis. We thank the research staff in the ICMR AMR Surveillance Network for their immense contribution in data collection. We acknowledge the Delhi Research Implementation & Innovation (DRIIV) supported by the Principal Scientific Advisor Office, Prn.SA/Delhi/Hub/2018(C) and Delhi Knowledge Development Foundation (DKDF) Centre of Excellence in Healthcare (CoEH) CEM-06 at IIIT-Delhi.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lansea.2024.100412.
Appendix A. Supplementary data
References
- 1.World Health Organization Antimicrobial resistance. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
- 2.Cassini A., Högberg L.D., Plachouras D., et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European economic area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19:56–66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Review on antimicrobial resistance, O'Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. 2014. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf Available from: [Google Scholar]
- 4.Knight G.M., Glover R.E., McQuaid C.F., et al. Antimicrobial resistance and COVID-19: intersections and implications. eLife. 2021;10 doi: 10.7554/eLife.64139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sulis G., Sayood S., Gandra S. Antimicrobial resistance in low- and middle-income countries: current status and future directions. Expert Rev Anti Infect Ther. 2022;20(2):147–160. doi: 10.1080/14787210.2021.1951705. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization, Food and Agriculture Organization of the United Nations World organisation for animal health. Antimicrobial resistance and the United nations sustainable development cooperation framework: guidance for United nations country teams. 2021. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf Available from:
- 7.Tayler L on behalf of the AMR Tripartite and UNEP . World Health Organization; 2021. Antimicrobial resistance - tripartite update - Spring 2021.https://www.who.int/publications/m/item/antimicrobial-resistance-tripartite-update-spring-2021 Available from: [Google Scholar]
- 8.International Institute for Sustainable Development (IISD) Antimicrobial resistance threatens development, SDGs: tripartite report. SDG Knowledge Hub. https://sdg.iisd.org/news/antimicrobial-resistance-threatens-development-sdgs-tripartite-report/ Available from:
- 9.Antimicrobial Resistance Collaborators Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manesh A., Varghese G.M. CENDRIC investigators and collaborators. Rising antimicrobial resistance: an evolving epidemic in a pandemic. Lancet Microbe. 2021;2(9):e419–e420. doi: 10.1016/S2666-5247(21)00173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Periasamy H., Gnanamani A. Polymyxins resistance among gram-negative pathogens in India. Lancet Infect Dis. 2020;20(12):1362–1363. doi: 10.1016/S1473-3099(20)30855-0. [DOI] [PubMed] [Google Scholar]
- 12.Ranjalkar J., Chandy S.J. India's national action plan for antimicrobial resistance – an overview of the context, status, and way ahead. J Family Med Prim Care. 2019;8(6):1828–1834. doi: 10.4103/jfmpc.jfmpc_275_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santella B., Folliero V., Pirofalo G.M., et al. Sepsis-A retrospective cohort study of bloodstream infections. Antibiotics (Basel) 2020;9(12):851. doi: 10.3390/antibiotics9120851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudd K.E., Johnson S.C., Agesa K.M., et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond N.E., Kumar A., Kaur P., et al. Estimates of sepsis prevalence and outcomes in adult patients in the ICU in India: a cross-sectional study. Chest. 2022;161(6):1543–1554. doi: 10.1016/j.chest.2021.12.673. [DOI] [PubMed] [Google Scholar]
- 16.Karuna T., Gupta A., Vyas A., et al. Changing trends in antimicrobial susceptibility patterns of bloodstream infection (BSI) in secondary care hospitals of India. Cureus. 2023;15(4) doi: 10.7759/cureus.37800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson J., Robinson M.L., Rajput U.C., et al. High burden of bloodstream infections associated with antimicrobial resistance and mortality in the neonatal intensive care unit in Pune, India. Clin Infect Dis. 2021;73(2):271–280. doi: 10.1093/cid/ciaa554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walia K., Madhumathi J., Veeraraghavan B., et al. Establishing antimicrobial resistance surveillance & research network in India: journey so far. Indian J Med Res. 2019;149(2):164–179. doi: 10.4103/ijmr.IJMR_226_18.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NITI Aayog . NITI Aayog; New Delhi: 2018 Dec. SDG India index: baseline report 2018.http://niti.gov Available from: [Google Scholar]
- 20.Zeileis A., Köll S., Graham N. Various versatile variances: an object-oriented implementation of clustered covariances in R. J Stat Softw. 2020;95(1) doi: 10.18637/jss.v095.i01. [DOI] [Google Scholar]
- 21.Benesty J., Chen J., Huang Y., Cohen I. Noise reduction in speech processing. Springer; 2009. Pearson correlation coefficient; pp. 37–40. [Google Scholar]
- 22.RDocumentation. adf.test function. https://www.rdocumentation.org/packages/aTSA/versions/3.1.2/topics/adf.test Available from:
- 23.Sethi T., Maheshwari S. wiseR: a shiny application for end-to-end Bayesian decision network analysis and web-deployment. 2018. https://arxiv.org/ftp/arxiv/papers/2108/2108.07046.pdf Available from:
- 24.Khurana S., Bhardwaj N., Kumari M., Malhotra R., Mathur P. Prevalence, etiology, and antibiotic resistance profiles of bacterial bloodstream infections in a tertiary care hospital in Northern India: a 4-year study. J Lab Physicians. 2018;10:426–431. doi: 10.4103/JLP.JLP_78_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathur P., Malpiedi P., Walia K., et al. Health-care-associated bloodstream and urinary tract infections in a network of hospitals in India: a multicentre, hospital-based, prospective surveillance study. Lancet Glob Health. 2022;10(9):e1317–e1325. doi: 10.1016/S2214-109X(22)00274-1. [DOI] [PubMed] [Google Scholar]
- 26.Bush K., Bradford P.A. β-Lactams and β-Lactamase inhibitors: an overview. Cold Spring Harb Perspect Med. 2016;6(8) doi: 10.1101/cshperspect.a025247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization Sustainable development goals (SDGs) AMR indicator. https://www.who.int/data/gho/data/themes/topics/global-antimicrobial-resistance-surveillance-system-glass/sustainable-development-goals-amr-indicator Available from:
- 28.Indian Council of Medical Research (ICMR) AMR ICMR data. https://iamrsn.icmr.org.in/index.php/resources/amr-icmr-data Available from: [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.