Abstract
Global climate change-related water deficit negatively affect the growth, development and yield performance of multiple cereal crops, including durum wheat. Therefore, the improvement of water-deficit stress tolerance in durum wheat varieties in arid and semiarid areas has become imperative for food security. Herein, we evaluated the water deficiency resilience potential of two marker-free transgenic durum wheat lines (AlSAP-lines: K9.3 and K21.3) under well-watered and water-deficit stress conditions at both physiological and agronomic levels. These two lines overexpressed the AlSAP gene, isolated from the halophyte grass Aeluropus littoralis, encoding a stress-associated zinc finger protein containing the A20/AN1 domains. Under well-watered conditions, the wild-type (WT) and both AlSAP-lines displayed comparable performance concerning all the evaluated parameters. Ectopic transgene expression exerted no adverse effects on growth and yield performance of the durum wheat plants. Under water-deficit conditions, no significant differences in the plant height, leaf number, spike length, and spikelet number were observed between AlSAP-lines and WT plants. However, compared to WT, the AlSAP-lines exhibited greater dry matter production, greater flag leaf area, improved net photosynthetic rate, stomatal conductance, and water use efficiency. Notably, the AlSAP-lines displayed 25 % higher grain yield (GY) than the WT plants under water-deficit conditions. The RT-qPCR-based selected stress-related gene (TdDREB1, TdLEA, TdAPX1, and TdBlt101-2) expression analyses indicated stress-related genes enhancement in AlSAP-durum wheat plants under both well-watered and water-deficit conditions, potentially related to the water-deficit resilience. Collectively, our findings support that the ectopic AlSAP expression in durum wheat lines enhances water-deficit resilience ability, thereby potentially compensate for the GY loss in arid and semi-arid regions.
Keywords: Stress-associated protein, Aeluropus littoralis, Marker-free genetically engineered durum wheat, Water deficiency, Multivariate analysis
1. Introduction
Water deficit is one of the most common environmental stresses affecting crop production worldwide [1]. In recent years, approximately 40 million tons of durum wheat (Triticum turgidum subsp. durum Desf.) were produced, accounting for 5 % of the total global wheat crop. Water deficit and extreme temperature are the primary stresses limiting the cultivation of durum wheat in the Mediterranean basin, and their occurrence during flowering, pollination, and grain-filling can lead to a drop in yields. Therefore, it is crucial to develop durum wheat lines that are able to withstand future climatic changes.
Water deficit stress tolerance is a complex polygenic trait impeding dissection of its overall molecular and physiological mechanisms. Compared to conventional long-term breeding programs, the transgenic approach offers an attractive and effective solution for engineering abiotic stress tolerance. Numerous abiotic stress-related genes from a variety of organisms have been inserted into wheat via genetic transformation. Wheat water-deficit has previously been enhanced using late embryogenesis-abundant (LEA) protein-encoding genes [2]; dehydration-responsive element-binding (DREB), nitrogen assimilation control (NAC), and homeodomain-leucine zipper (HD-Zip) protein transcription factors [[3], [4], [5], [6]]; ethylene-response factor families [7]; and the aldo–keto reductase family [8]. The majority of these studies have focused on bread wheat, while considerably less progress has been made in the development of genetically modified durum wheat. Indeed, no transgenic drought-tolerant wheat has been approved for the market yet [9,10]. Because of the complex genetic characteristics of wheat, the literature data considering genetic modification is very poor in comparison to that of other economic crops.
The adaptation of plants to water deficit conditions requires transcriptional changes to the expression of key genes associated with molecular and physiological changes that allow for better tolerance to harmful conditions [10,11]. In general, plants’ abiotic stress response is initiated by signal molecules involved in signal transduction, resulting in the activation of key stress-related genes as well as changes in the cellular expression machinery, biochemical response, and physiological response [10,12]. Therefore, this disturbance at the physiological, biochemical, and metabolic levels leads to losses in crops. The plants that have a greater ability to absorb water and minerals, to regulate stomatal function and osmotic adaptation, to maintain and modify cell wall integrity, are more effective to deal with harsh environmental conditions [11,13,14].
Over the past decade, the stress-associated protein (SAP) gene family has taken a wide ampleness, multiple members have been identified, and their roles in abiotic stress tolerance have been confirmed [15]. Several members of the SAP family have been isolated from apple [16], cucumber [17], maize [18], rice [[19], [20], [21], [22]], soybean [23], and wheat [24,25] as well as from Aeluropus littoralis [26], Sorghum bicolor [27], Lobularia maritima [28], Populus trichocarpa [29], Tamarix hispida [30], and Vitis amurensis [31]. The potential of these genes to improve tolerance to abiotic stress has been validated in model plants under in vitro and greenhouse conditions.
In previous research, our group successfully isolated the AlSAP gene from Aeluropus littoralis [26]. This gene has been characterized in tobacco [26] and rice [32] under in vitro and greenhouse conditions. Recently, the potential for water deficiency tolerance in AlSAP-overexpressing rice lines has been tested in the field, with AlSAP-transgenic lines showing an increased ability to maintain productivity under stressful conditions compared to non-transgenic plants [33]. Previously, two marker-free transgenic lines of durum wheat var. Karim overexpressing the AlSAP gene, K9.3 and K21.3, were found to have appreciable levels of tolerance to abiotic stress under in vitro and greenhouse conditions [34]. In the present study, the water deficiency stress tolerance potential of K9.3 and K21.3 lines under on-farm conditions was evaluated.
2. Materials and methods
2.1. Plant materials
The Tunisian wild-type durum wheat cultivar Karim and the two T3 homozygous genetically engineered durum wheat lines (K9.3 and K21.3) expressing low and high levels of AlSAP, respectively, used in this study were provided by Dr. Afif Hassairi. These two AlSAP-genetically engineered lines were previously described by Ben Saad et al. [34].
2.2. Growth conditions and water-deficit treatment
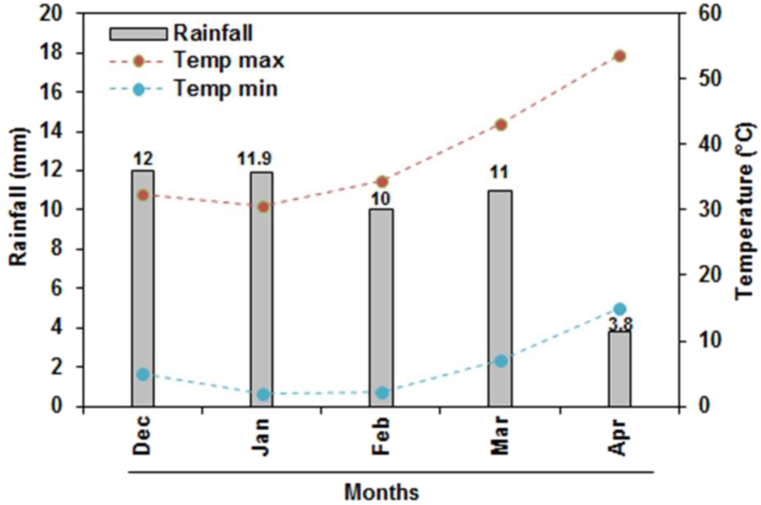
The on-farm experiment was carried out at King Saud University Dirab Agricultural Research protected Station (24°25′35.7″N, 46°34′11.0″E) in a split-plot design in a randomized complete block (the main plots were subjected to two irrigation regimes, and the subplots were linked to the three genotypes). The experiment was conducted on sandy loam soil (pH 7.9 and 7.8 g kg−1 organic matter content) with 0.220 and 0.101 m3 m−3 as field water capacity and wilting point, respectively. The climatological data collected during the growing period was illustrated in Fig. 1.
Fig. 1.
Climatological data at the experimental site for the durum wheat growing season.
The genetically engineered and wild-type seeds were sown at a rate of 150 kg ha−1 on the 15th of December in 10 rows/plots (plot dimensions: 1.5 m × 4 m in width and length, respectively). The experiment was conducted with three replications and two treatments. The ETc was calculated using the crop coefficient (Kc) of durum wheat and the daily reference evapotranspiration (ETo) [35]. A limited water regime was imposed 4 weeks after sowing and the water was administered at the eight Zadoks growth stages (ZS) [36].
2.3. Physiological plant traits measurements
2.3.1. Leaf water status parameters
Green leaves from ten plants randomly selected per plot were harvested and used to determine the leaf water content (LWC) and the relative water content (RWC) following the formulas previously described by Al-Ashkar et al. [37].
The canopy temperature (CT) was assessed using a thermometer (Therma CAM SC 3000 infrared camera, FLIR System Inc., North Billerica, MA, USA)
2.3.2. Leaf parameters
At the ripening stage (ZS 90), ten plants were randomly selected from each plot to determine the flag leaf area (FLA), total leaf area per plant (TLA), and leaf area index (LAI). ImageJ software was used to estimate the FLA and TLA.
2.3.3. Gas exchange measurements
Gas exchange measurements were conducted using a Li-6400 portable photosynthesis system (LI-COR, Inc., Lincoln, NE, USA). The following parameters: photosynthesis rate (Pn), transpiration rate (E), stomatal conductance (Gs), leaf equivalent water thickness (LEWT), water use efficiency (WUE), intrinsic water use efficiency (WUEi), stomatal limitation value (Ls), and intracellular CO2 concentration (Ci) were recorded on the flag leaves during the stage of floret development, specifically between 10:00 a.m. and 11:00 a.m.
2.3.4. Agronomic and physiological features
Several agronomic parameters were estimated at the ripening stage (ZS 90) using ten plants randomly selected from each plot. These agronomic parameters included plant height (PH), leaf number (LN), stem dry weight (SDW), leaf dry weight (LDW), total dry weight (TDW), spike length (SL), number of spikelets (NS), number of seeds per spike (NSS), thousand kernel weight (TKW), and grain yield (GY).
2.4. RNA extraction and quantitative real-time PCR assay
Total RNA was collected from frozen leaves using the E.Z.N.A Kit (Omega Bio-Tek). For RT-qPCR, one μg of total RNA per sample was employed to synthesize first-strand cDNA using a QuantiTect RT-Kit (Qiagen). The transcript accumulation of the selected stress-related genes, including dehydration-responsive element binding transcription factor (TaDREB1), plasma membrane protein (TdBlt101-2), late embryogenesis abundant (LEA) protein (TdLEA), and ascorbate peroxidase 1 gene (TdAPX1), was monitored via RT-qPCR reactions as previously outlined by Ben Romdhane et al. [38]. Relative expression was determined by the comparative threshold cycle (2−ΔΔCT) method, using the actin gene (TdActin) as the internal control (Supplementary Table S1).
2.5. Statistical analysis
All the data were subjected to two-way ANOVA analysis using XLSTAT statistical package (Addinsoft, New York, USA). Differences for each treatment × genotype combination were estimated using Duncan's multiple range test (p < 0.05). Stepwise multiple linear regressions (SMLRA) were used to identify the best traits and their contributions to grain yield.
3. Results
3.1. Evaluation of growth and physiological performance
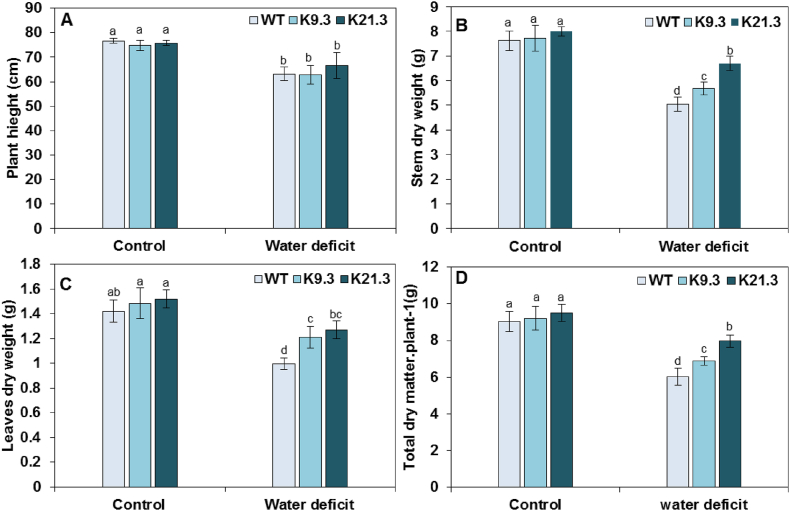
To assess the water-deficit stress effects on the growth of durum wheat plants, several parameters were monitored under normal and water-deficit conditions for the AlSAP-lines and WT plants. Under control conditions (Fig. 2A), no significant differences in plant height (PH) were recorded between the AlSAP-durum wheat lines and the WT plants. However, a reduction of PH was noticed for all the evaluated durum wheat genotypes under water-deficit stress conditions, which was estimated to 18 %, 16 %, and 12 % for the WT, K9.3, and K21.3 lines, respectively, with no significant difference (Fig. 2A).
Fig. 2.
Influence of water-deficit stress on plant high (A), stem dry weight (B), leaf dry weight (C), and total plant dry matter in AlSAP-lines (K9.3 and K21.3) and WT durum wheat plants. Bars represent the means ± SE of three replicates. Bars designated by different letters for each treatment × genotype combination are significantly different (p ≤ 0.05) according to Duncan's multiple range test.
Under well-watered control conditions, no significant differences in SDW, LDW, and TDW were recorded for the AlSAP-durum wheat lines compared to the WT plants. However, both AlSAP-lines have displayed greater SDW, LDW, and TDW than did the WT plants under water-deficit conditions (Fig. 2B—D). The WT plants appeared more affected by water-deficit stress, and their dry weight decreased by 30–33 % compared to those recorded under well-watered control conditions. Compared to the WT plants, K9.3 lines showed a significantly lower decrease (25 %) in the LDW. On the other hand, the K21.3 line showed the most significant performance under water-deficit conditions, as illustrated by the lowest decrease in SDW, LDW and TDW (Fig. 2B—D).
3.2. Leaf area parameters
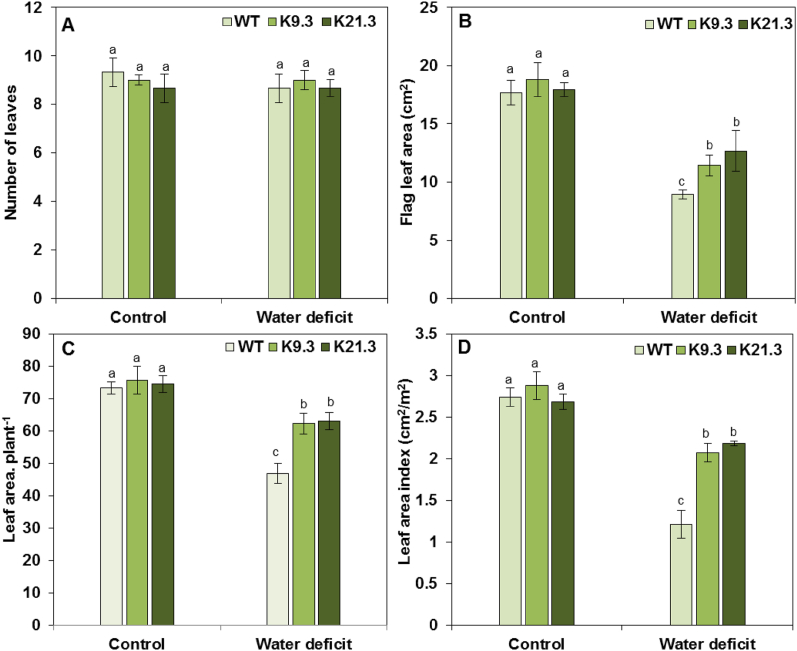
Under well-watered and water-deficit conditions, four leaf-related parameters, leaf number (LN), flag leaf area (FLA), total leaf area (TLA), and leaf area index (LAI), were monitored in the AlSAP-lines and WT plants (Fig. 3A—D). There were no significant differences in any of the leaf-related parameters assessed under normal conditions between the AlSAP-durum wheat lines and WT plants (Fig. 3A—D). Under water-deficit conditions, low and non-significant (P < 0.05) decrease in LN was noticed for all the evaluated genotypes compared with their counterparts under well-watered control conditions (Fig. 3A). The FLA was reduced in the all evaluated genotypes due to water-deficit stress, by 50 %, 39 %, and 29 % for the WT plants, and the K9.3, and K21.3 AlSAP-lines, respectively, compared to their counterparts under well-watered control conditions (Fig. 3B). Similarly, the plant leaf area (PLA) and leaf area index (LAI) significantly decreased under water-deficit stress conditions for the all evaluated genotypes (Fig. 3C—D). Interestingly, the PLA and LAI measurements of the WT plants decreased by 62 % and 56 %, respectively. However, compared with their counterparts under well-watered control conditions, the K21.3 and K9.3 AlSAP-plants maintained high ability to maintain their PLA and LAI parameters under water-deficit stress conditions despite reductions of 15–17 % and 18–28 %, respectively (Fig. 3C—D).
Fig. 3.
Effect of water-deficit stress on leaf number (A), flag leaf area (B), total leaf area (C), and leaf area index (D) of WT plants and AlSAP-genetically engineered durum wheat lines (K9.3 and K21.3). Bars represent the means ± SE of three replicates. Bars designated by different letters for each treatment × genotype combination are significantly different (p ≤ 0.05) according to Duncan's multiple range test.
3.3. Leaf water status parameters
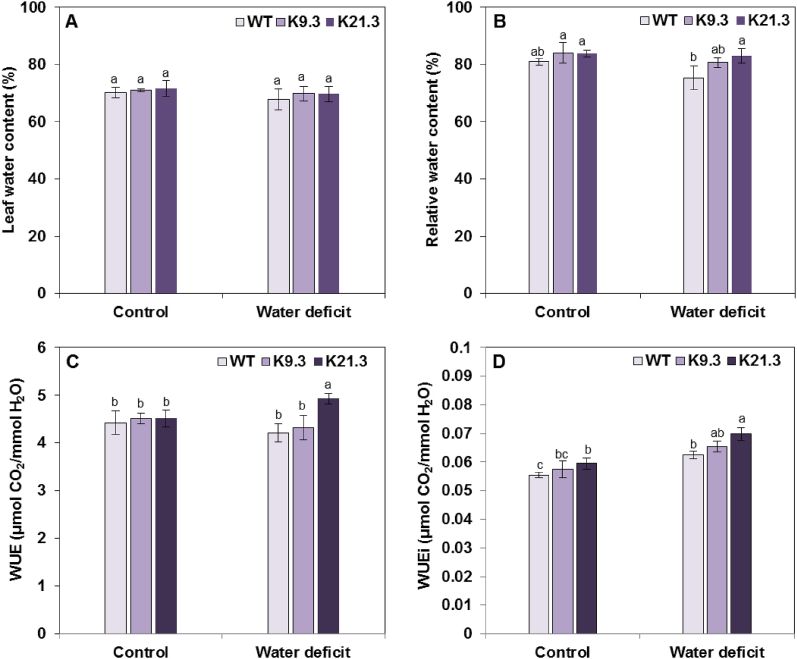
As shown in Fig. 4, the LWC of all tested genotypes slightly decreased under water-deficit stress conditions compared to that registered under well-watered control conditions. The decrease in LWC was estimated to 3.53 %, 2.71 %, and 1.71 % for the WT, K9.3, and K21.3 durum wheat plants, respectively, compared to the records registered under well-watered control conditions; however, these decreases in LWC were still not statistically significant (P < 0.05) (Fig. 4A). In the same trends, the estimated RWC values were not significantly (P < 0.05) affected in any of the tested genotypes under water-deficit stress conditions compared to those registered under well-watered control conditions (Fig. 4B). Interestingly, compared with those of the WT and K9.3 plants, the WUE and WUEi of the K21.3 plants under water-deficit conditions were significantly greater (Fig. 4B—D). In addition, the CTs of the WT plants and the AlSAP-durum wheat lines were similar under well-watered control conditions (Fig. S1). However, under water deficit conditions, the CT increased by 7.3 °C, 2.1 °C, and 1.9 °C in the WT, K9.3, and K21.3 plants, respectively, compared to their counterparts under well-watered control conditions (Fig. S1).
Fig. 4.
Effect of water-deficit stress on leaf water status parameters of WT plants and AlSAP-durum wheat lines (K9.3 and K21.3). Influence of water-deficit stress on LWC (A), RWC (B), WUE (C), and WUEi (D) in AlSAP-engineered lines and WT durum wheat plants. Bars represent the means ± SE of three replicates. Bars designated by different letters for each treatment × genotype combination are significantly different (p ≤ 0.05) according to Duncan's multiple range test.
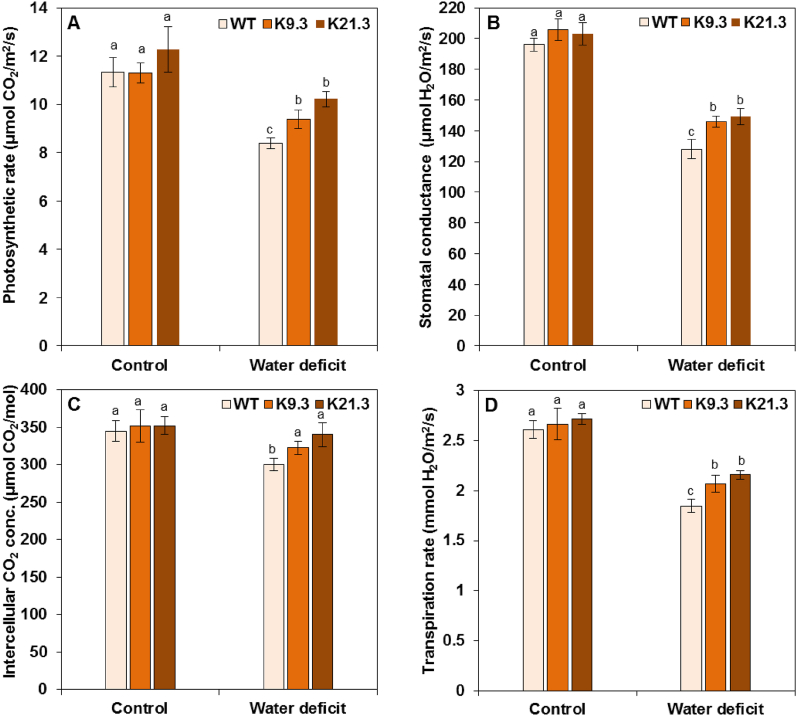
3.4. Leaf gas exchange
The leaf gas exchange rate was monitored through photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci), and transpiration rate (E) measurements (Fig. 5A—D). There were no significant differences in any of the abovementioned leaf gas exchange measurements under normal conditions between the AlSAP-lines and the WT plants (Fig. 5A—D). Under water deficit stress conditions, the Pn, Gs, Ci, and E of the AlSAP-durum wheat lines were greater than those of the WT plants. Compared with those in the WT plants, Pn, Gs, Ci, and E in the K21.3 plants under water deficit stress conditions were 21.8 %, 16.6 %, 13.3 %, and 16.8 %, respectively (Fig. 5A—D). Together, these data suggest that the expression of AlSAP gene in the K9.3 and K21.3 durum wheat lines could enhance their gas exchange capacities under water-deficit conditions.
Fig. 5.
Effect of water-deficit stress on leaf gas exchange parameters of WT plants and AlSAP-durum wheat lines (K9.3 and K21.3). Bars represent the means ± SE of three replicates. Bars designated by different letters for each treatment × genotype combination are significantly different (p ≤ 0.05) according to Duncan's multiple range test.
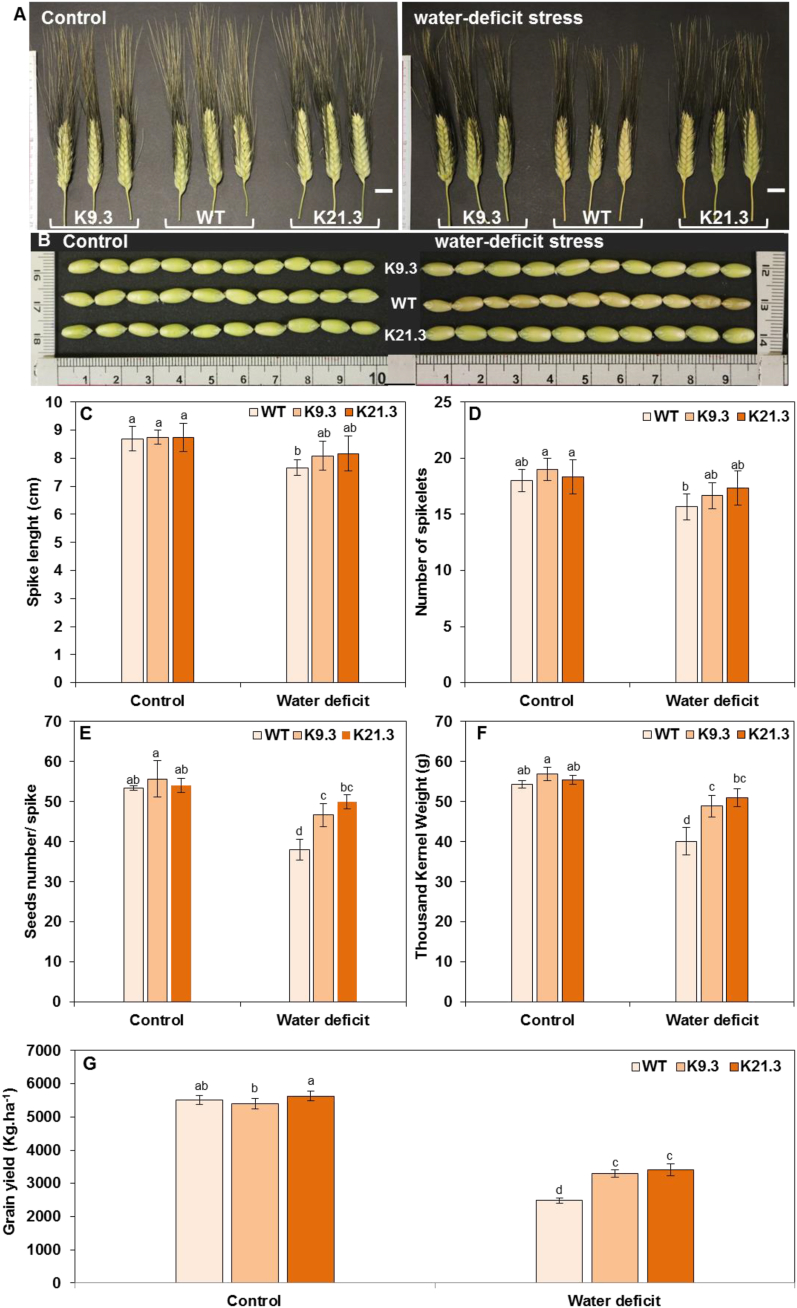
3.5. Yield and yield component parameters
The yield parameters were monitored through spike length (SL), number of spikelet (NS), number of seeds per spike (NSS), thousand-kernel weight (TKW), and grain yield (GY) measurements. As shown in Fig. 6A—D, no significant differences in spike length (SL), and number of spikelet (NS) were revealed for K9.3 and K21.3 durum wheat lines compared to WT plants under both well-watered control and water-deficit conditions. In the same trends, there were no significant differences in the number of seeds per spike (NSS) and thousand-kernel weight (TKW) between the AlSAP-durum wheat lines and the WT plants under well-watered control conditions (Fig. 6E and F). However, the NSS and TKW of the K9.3 and K21.3 durum wheat lines were 22.7–31.57 % and 21.96–27.18 % greater, respectively, than those of the WT plants under water-deficit conditions (Fig. 6E and F). Compared to the well-watered control conditions, the K9.3 and K21.3 durum wheat lines showed 16.16 % and 7.4 % decreases, respectively, in their NSS and 8.09 % and 8.83 % decrease respectively in their TKW under water-deficit stress conditions. However, the NSS and TKW of the WT plants decreased by 28.7 % and 26.22 %, respectively, under these conditions (Fig. 6E and F). The revealed high performance of the AlSAP-durum wheat lines under water deficit stress conditions in terms of greater number of seeds per spike and greater thousand kernel weight was associated with greater grain yield (GY) than that of the WT plants under such conditions (Fig. 6G).Thus, compared to that under well-watered control conditions, the GY under water-deficit stress conditions decreased by 55 %, 39.4 %, and 38.8 % for the WT, K9.3 and K21.3 plants, respectively (Fig. 6G). Interestingly, the GY of durum wheat plants overexpressing the AlSAP gene (K9.3 and K21.3 lines) was 25 % greater than that of WT plants under water-deficit stress conditions (Fig. 6G).
Fig. 6.
Effect of water-deficit stress on yield and yield component parameters of WT plants and AlSAP-genetically engineered durum wheat lines (K9.3 and K21.3). Data in B, C, D, E, and F represent the means ± SE of three replicates. Bars designated by different letters for each treatment × genotype combination are significantly different (p ≤ 0.05) according to Duncan's multiple range test.
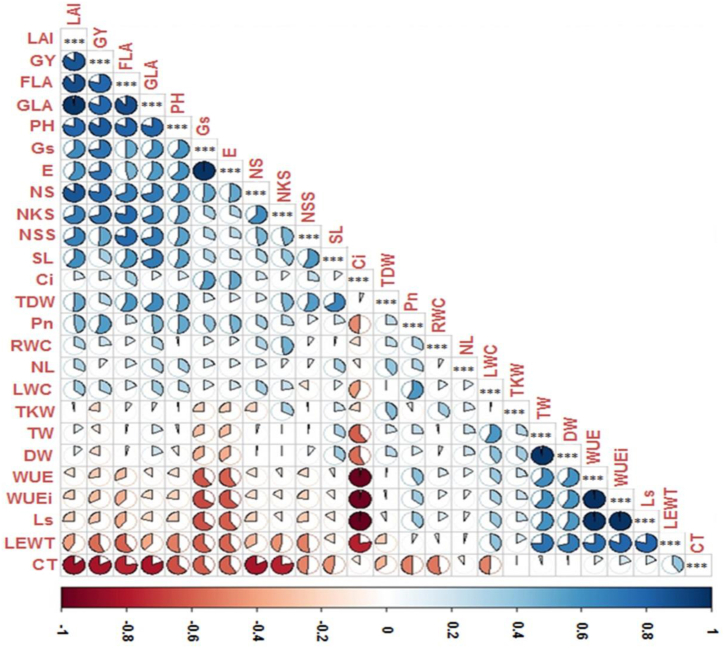
3.6. Identification of traits associated with water-deficit responses in WT and AlSAP durum wheat
The analysis of the relationships between the variables and the GY revealed high positive correlation values for LAI and GLA (0.971) and high negative correlation values for CT and GY (-0.827). The GY showed highly significant positive correlation with nine variables, namely, NS, PH, FLA, GLA, LAI, NKS, Pn, Gs, and E, and highly significant negative correlation with both the CT and LEWT variables (Fig. 7).
Fig. 7.
Correlation among all evaluated traits under water-deficit stress conditions.
To determine the most closely-related traits and the level of their impact on the performance of each evaluated genotype, a regression analysis was performed using 29 evaluated traits as interpreted variables and grain yield as the dependent variable. The stepwise regression analysis revealed significant associations between the evaluated parameters and the grain yield (P > 0.0001). Interestingly, the CT and LEWT traits significantly contributed (R2 = 0.953) with 0.822 and 0.131, respectively, to the grain yield of the WT durum wheat plants. However, the Gs and TKW traits were significant contributors (R2 = 0.870), with values of 0.750 and 0.120, respectively, to the grain yield of the K21.3 AlSAP-durum wheat lines. The mean of the predicted regression GY value ranged from 3.865 to 4.552, the error value ranged from −0.010 to 0.536, the relative error value ranged from −0.005 to 0.162, and the evaluation accuracy (%) ranged from 81.70 to 96.60, with an average value of 91.163 (Table S2).
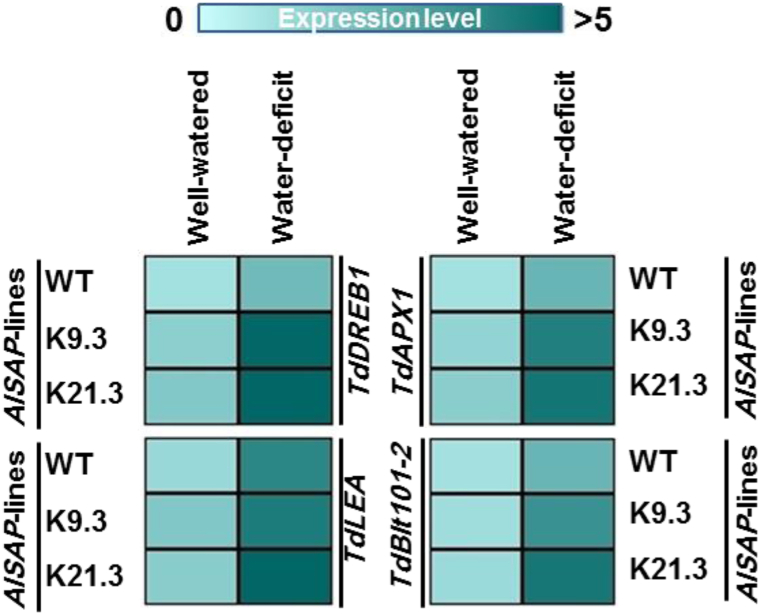
3.7. Molecular responses of WT and AlSAP-durum wheat plants to water deficit
The analysis of the expression levels of four selected stress-related genes (TdDREB1, TdLEA, TdAPX1, and TdBlt101-2) in the leaves of WT and AlSAP-durum wheat revealed a positive deregulation of the selected stress-related genes in AlSAP-durum wheat lines even under well-watered and water-deficit conditions compared to their WT counterparts. The positively deregulated stress-related genes are involved in the regulation of the expression of water scarcity-responsive genes (TdDREB1), maintenance of membrane integrity, and regulation of water balance (TdLEA and TdBlt101-2), detoxification of harmful hydrogen peroxide species and protection of plant cells from oxidative damage, which enhance the ability of plants to cope with stress conditions. Consequently, the integration of the AlSAP gene in the durum wheat genome was able to modulate stress-related genes expression and potentially prime AlSAP-engineered plants to cope with water-deficit conditions (Fig. 8).
Fig. 8.
Expression pattern of stress-related genes (TdDREB1, TdAPX1, TdLEA, and TdBlt101-2) in WT and AlSAP-durum wheat lines under well-watered and water-deficit regimes.
4. Discussion
Aggravated climate changes pose considerable risks to food security and negatively affect the global agricultural production of many crucial cereal crops, such as wheat, barley, rice, maize and etc. [39]. Reduced plant productivity due to water deficiency is a major concern for durum wheat farmers, and the development of high-yielding durum wheat cultivars is a primary goal of breeding programs. Therefore, the development of plant varieties with effective agronomic and physiological traits that enable them to withstand abiotic stress (drought, salinity, heat, and cold) has become imperative in arid and semiarid areas.
Water-deficit tolerance is a complicated trait resulting from the contributions of several putative interdependences, including water status parameters, leaf surface area, photosynthetic performance, root characteristics, plant biomass, number of spikes, and kernel features, which are interesting traits for water-deficit tolerance evaluation [[40], [41], [42]]. Maintaining an optimal balance between the water received by roots and the water lost by leaves is a crucial aspect of plant water-deficit tolerance [11,13]. During the vegetative stage in wheat, water deficit restricts leaf elongation, leaf number, leaf area, and increases leaf senescence [40,43,44]. Additionally, water deficit stress during the generative stage in wheat drastically affects the fertilization and grain yield [43]. Therefore, the generation and selection of cultivars that possess efficient water use mechanisms can be considered as approach to improve the yield of durum wheat under water-deficit stress conditions. Previously, Ben Saad et al. [26] isolated the AlSAP gene from the halophyte grass Aeluropus littoralis and functionally characterized it in tobacco, rice and durum wheat under in vitro and greenhouse conditions [26,32,34]. Recently, Ghneim-Herrera et al. [33] reported that, compared with non-genetically engineered plants, AlSAP-overexpressing rice lines exhibited marked water deficiency tolerance under field conditions, and an increased ability to maintain productivity under such stressful conditions. Two marker-free genetically modified lines of durum wheat var Karim overexpressing the AlSAP gene, K9.3 and K21.3, were previously found to have appreciable tolerance to salinity and dehydration stress under in vitro and greenhouse conditions [34]. In this study, we evaluated the water deficiency stress tolerance potential of K9.3 and K21.3 lines under on-farm conditions through morpho-physiological approach.
Under well-watered conditions, our analysis revealed no significant differences (p < 0.05) between the K9.3 and K21.3 AlSAP-lines and the WT plants for any of the evaluated parameters, including PH, NL, FLA, LWC, RWC, WUE, Pn, Gs, E, NS, LS, and GY. These results suggest that the overexpression of AlSAP gene has no detrimental effects on the growth and productivity of genetically engineered durum wheat plants, which is in line with previous findings recorded under in vitro and greenhouse conditions reported by Ben Saad et al. [34]. However, the stem‒ and leaf dry weight and total dry weight of WT, K9.3 and K21.3 plants subjected to water-deficit stress were significantly lower than those of the well-watered control plants. Furthermore, the plant heights of both the WT and AlSAP-engineered durum wheat plants were similar under the water-deficit treatment. The ability to maintain high dry weight (stem and leaves) was more pronounced in K21.3 lines, which express high levels of the AlSAP gene compared to K9.3 lines. These findings suggest that the expression of the AlSAP gene in durum wheat improved the potential of AlSAP-engineered lines to maintain their growth and dry matter under water-deficit conditions. Our results are in line with those reported by Ben Saad et al. [34], who demonstrated that AlSAP wheat lines exhibited improved biomass production under osmotic stress conditions. In rice, AlSAP expression has been found to increase the accumulation of green biomass under drought conditions [33]. Several A20/AN1-zinc-finger domain containing proteins characterized in wheat, rice, maize, and sugarcane were found to confer drought and osmotic stress tolerance as well as an improved biomass and grain yield in transgenic Arabidopsis, rice, and tobacco plants [15,[45], [46], [47]].
Leaves, especially flag leaves, are vital sources of carbohydrates. These carbohydrates produced via gas exchange and photosynthesis processes can directly influence wheat grain filling and yield [48]. Despite the similar number of plant leaves under well-watered and water-deficit conditions, the plant leaf area, and particularly the flag leaf area, were markedly affected by water deficiency. However, both the K9.3 and K21.3 AlSAP-lines maintained their leaf area indices and, in particular, their flag leaf area, which was 27–40 % greater than that of the WT plants under water-deficit conditions. Subsequently, these leaf characteristics of the AlSAP-genetically engineered durum wheat lines had direct effects on the gas exchange rate, water-use efficiency index, grain filling, and yield. Thus, our results indicated that the K9.3 and K21.3 durum wheat lines had greater gas exchange parameters in terms of their photosynthetic rate, stomatal conductance, transpiration rate, and intracellular CO2 concentration under water-deficit conditions than did their WT counterparts. Additionally, although it was more pronounced in the K21.3 lines that highly expressed the AlSAP gene, the AlSAP-overexpressing durum wheat lines exhibited a better water-use efficiency than did their WT counterparts under water deficit conditions. In rice, AlSAP-overexpressing plants showed enhanced transpiration efficiency and maintained higher CO2 concentrations than did Nipponbare control plants when subjected to severe drought stress [32]. Similarly, Ghneim-Herrera et al. [33] recorded significant differences in the photosynthetic parameters, leaf water potential, and antioxidant accumulation in the flag leaves of AlSAP-rice lines that were subjected to drought at the early tillering stage during greenhouse dry-down experiments. In contrast, under open-field conditions and drought stress applications during the flowering stage, Ghneim-Herrera et al. [33] did not observe significant differences in the aforementioned traits of AlSAP-rice lines compared to their Nipponbare counterpart plants.
There was a strong correlation between the leaf gas exchange rate and leaf CT [49]. Thus, CT is a useful indicator of plant water status, as well as the effectiveness of the transpiration process in protecting from warm-up of the leaves [37]. The K9.3 and K21.3 lines exhibited lower CT than did the WT plants under water-deficit conditions, which was probably a result of the enhanced transpiration rate in AlSAP-lines under such conditions. Previously, Gonzalez-Ribot et al. [50] reported that the morphological and physiological traits are strongly associated with the yield performance of durum wheat plants. Thus, our findings revealed that no significant differences in terms of SN, SL, TKW, and GY between the WT plants and the AlSAP-durum wheat lines under well-watered conditions. However, these aforementioned traits decreased under water-deficit conditions for the WT plants and even the AlSAP-durum wheat lines. The effect of water-deficit stress was very pronounced in WT plants, with 13, 28, 26, and 55 % decreases in their SN, NSS, TKW, and GY, respectively, compared to their records under well-watered conditions. Interestingly, although the decline by 7, 8, 8, and 39 % in the SN, NSS, TKW, and GY records, respectively, of the K21.3 AlSAP-line due to water-deficit stress, the AlSAP-durum wheat line was able to maintain a 25 % surplus of GY compared to WT plants under such conditions. These findings suggest that the expression of AlSAP gene in durum wheat lines enhances their ability to maintain flag leaf area index, WUE index, and gas exchange rate under water-deficit conditions, which positively impacts their performance in terms of NSS, TKW, and GY.
The significant correlations between evaluated traits denote overlap with others [51], but relying on these correlations alone is insufficient, and for greater accuracy, we used other statistical analyses, such as multiple regression [stepwise multiple linear regression (SMLRA)]. The SMLR results (Table S2) denoted that the traits TKW, Gs, TW, LEWT, and CT were only directly related to the GY trait, which varied according to genotype, but at least it will relate to one trait [51,52]. Therefore, we realized that TKW, Gs, TW, LEWT, and CT are reliable traits for forecasting yield performance (Table S2). As shown by the equations of the model (GY, Table S2), certain traits might display a positive correlation, whereas others may exhibit a negative correlation. To compute the plant growth and development performance, the TKW, Gs, TW, LEWT, and CT traits were the most effective traits [53,54]. The CT trait is closely related to with several photosynthetic processes involved in plant drought stress responses [55,56], and it is a robust physiological trait that should be taken into consideration in wheat breeding programs [[55], [56], [57]]. The genotype that has a high capacity to maintain low CT and gas exchange is extremely coveted due to its leaf-cooling response under stress [52,58,59].
5. Conclusions
In summary, this study constitutes the first on-farm evaluation of AlSAP-genetically engineered durum wheat lines (K9.3 and K21.3). The expression of AlSAP gene in marker-free genetically engineered durum wheat lines penalized neither yield nor plant growth under well-watered conditions and was associated with increased GY under water-deficit conditions. This performance is linked to a greater vegetative biomass, leaf area, photosynthetic rate, and water-use efficiency index under water-deficit stress. These findings are promising for the assessment of abiotic stress tolerance potential under open-field conditions and the integration of these AlSAP lines in durum wheat water-deficit tolerance breeding programs.
Data availability statement
All data is contained within the article or supplementary material.
CRediT authorship contribution statement
Walid Ben Romdhane: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Ibrahim Al-Ashkar: Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Abdullah Ibrahim: Investigation, Formal analysis, Data curation. Mohammed Sallam: Investigation, Formal analysis, Data curation. Abdullah Al-Doss: Writing – review & editing, Validation, Supervision, Formal analysis, Conceptualization. Afif Hassairi: Writing – review & editing, Validation, Supervision, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors extend their appreciation to the researchers supporting project number (RSP2024R298), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e30933.
Contributor Information
Walid Ben Romdhane, Email: wromdhane@ksu.edu.sa.
Ibrahim Al-Ashkar, Email: ialashkar@ksu.edu.sa.
Afif Hassairi, Email: ahassairi@ksu.edu.sa.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Fahad S., Bajwa A.A., Nazir U., Anjum S.A., Farooq A., Zohaib A., Sadia S., Nasim W., Adkins S., Saud S., Ihsan M.Z., Alharby H., Wu C., Wang D., Huang J. Crop production under drought and heat stress: plant responses and management options. Front. Plant Sci. 2017;8:1147. doi: 10.3389/fpls.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samtani H., Sharma A., Khurana P. Overexpression of HVA1 enhances drought and heat stress tolerance in Triticum aestivum doubled haploid plants. Cells. 2022;11(5) doi: 10.3390/cells11050912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y., Chen M., Guo J., Wang Y., Min D., Jiang Q., Ji H., Huang C., Wei W., Xu H., Chen X., Li L., Xu Z., Cheng X., Wang C., Wang C., Ma Y. Overexpression of soybean DREB1 enhances drought stress tolerance of transgenic wheat in the field. J. Exp. Bot. 2020;71(6):1842–1857. doi: 10.1093/jxb/erz569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J., Gong Y., Gao Y., Zhou Y.B., Chen M., Xu Z.S., Guo C.H., Ma Y.Z. Positively regulates drought tolerance and ABA responses in wheat (L.) Crop J. 2021;9(4):785–793. [Google Scholar]
- 5.Yang Y., Luang S., Harris J., Riboni M., Li Y., Bazanova N., Hrmova M., Haefele S., Kovalchuk N., Lopato S. Overexpression of the class I homeodomain transcription factor TaHDZipI-5 increases drought and frost tolerance in transgenic wheat. Plant Biotechnol. J. 2018;16(6):1227–1240. doi: 10.1111/pbi.12865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez F.G., Capella M., Ribichich K.F., Curin F., Giacomelli J.I., Ayala F., Watson G., Otegui M.E., Chan R.L. Field-grown transgenic wheat expressing the sunflower gene HaHB4 significantly outyields the wild type. J. Exp. Bot. 2019;70(5):1669–1681. doi: 10.1093/jxb/erz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rong W., Qi L., Wang A., Ye X., Du L., Liang H., Xin Z., Zhang Z. The ERF transcription factor TaERF3 promotes tolerance to salt and drought stresses in wheat. Plant Biotechnol. J. 2014;12(4):468–479. doi: 10.1111/pbi.12153. [DOI] [PubMed] [Google Scholar]
- 8.Fehér-Juhász E., Majer P., Sass L., Lantos C., Csiszár J., Turóczy Z., Mihály R., Mai A., Horváth G.V., Vass I., Dudits D., Pauk J. Phenotyping shows improved physiological traits and seed yield of transgenic wheat plants expressing the alfalfa aldose reductase under permanent drought stress. Acta Physiol. Plant. 2014;36(3):663–673. [Google Scholar]
- 9.Araus J.L., Serret M.D., Lopes M.S. Transgenic solutions to increase yield and stability in wheat: shining hope or flash in the pan? J. Exp. Bot. 2019;70(5):1419–1424. doi: 10.1093/jxb/erz077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan S., Anwar S., Yu S., Sun M., Yang Z., Gao Z.Q. Development of drought-tolerant transgenic wheat: achievements and limitations. Int. J. Mol. Sci. 2019;20(13):3350. doi: 10.3390/ijms20133350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukarram M., Choudhary S., Kurjak D., Petek A., Khan M.M.A. Drought: sensing, signalling, effects and tolerance in higher plants. Physiol Plant. 2021;172(2):1291–1300. doi: 10.1111/ppl.13423. [DOI] [PubMed] [Google Scholar]
- 12.Ben Romdhane W., Al-Doss A., Hassairi A. The newly assembled chloroplast genome of Aeluropus littoralis: molecular feature characterization and phylogenetic analysis with related species. Sci. Rep. 2024;14(1):6472. doi: 10.1038/s41598-024-57141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilyas M., Nisar M., Khan N., Hazrat A., Khan A.H., Hayat K., Fahad S., Khan A., Ullah A. Drought tolerance strategies in plants: a mechanistic approach. J. Plant Growth Regul. 2021;40(3):926–944. [Google Scholar]
- 14.Guizani A., Askri H., Amenta M.L., Defez R., Babay E., Bianco C., Rapana N., Finetti-Sialer M., Gharbi F. Drought responsiveness in six wheat genotypes: identification of stress resistance indicators. Front. Plant Sci. 2023;14 doi: 10.3389/fpls.2023.1232583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giri J., Dansana P.K., Kothari K.S., Sharma G., Vij S., Tyagi A.K. SAPs as novel regulators of abiotic stress response in plants. Bioessays. 2013;35(7):639–648. doi: 10.1002/bies.201200181. [DOI] [PubMed] [Google Scholar]
- 16.Dong Q., Duan D., Zhao S., Xu B., Luo J., Wang Q., Huang D., Liu C., Li C., Gong X., Mao K., Ma F. Genome-wide analysis and cloning of the apple stress-associated protein gene family reveals MdSAP15, which confers tolerance to drought and osmotic stresses in transgenic Arabidopsis. Int. J. Mol. Sci. 2018;19(9):2478. doi: 10.3390/ijms19092478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai W., Zhou Y., Pan R., Liao L., He J., Liu H., Yang Y., Liu S. Identification and expression analysis of stress-associated proteins (SAPs) containing A20/AN1 zinc finger in cucumber. Plants. 2020;9(3):400. doi: 10.3390/plants9030400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xuan N., Jin Y., Zhang H.W., Xie Y.H., Liu Y.J., Wang G.Y. A putative maize zinc-finger protein gene, ZmAN13, participates in abiotic stress response. Plant Cell Tiss Org. 2011;107(1):101–112. [Google Scholar]
- 19.Vij S., Tyagi A.K. Genome-wide analysis of the stress associated protein (SAP) gene family containing A20/AN1 zinc-finger(s) in rice and their phylogenetic relationship with Arabidopsis. MGG. 2006;276(6):565–575. doi: 10.1007/s00438-006-0165-1. [DOI] [PubMed] [Google Scholar]
- 20.Kothari K.S., Dansana P.K., Giri J., Tyagi A.K. Rice stress associated protein 1 (OsSAP1) interacts with aminotransferase (OsAMTR1) and pathogenesis-related 1a protein (OsSCP) and regulates abiotic stress responses. Front. Plant Sci. 2016;7:1057. doi: 10.3389/fpls.2016.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanneganti V., Gupta A.K. Overexpression of OsiSAP8, a member of stress associated protein (SAP) gene family of rice confers tolerance to salt, drought and cold stress in transgenic tobacco and rice. Plant Mol. Biol. 2008;66(5):445–462. doi: 10.1007/s11103-007-9284-2. [DOI] [PubMed] [Google Scholar]
- 22.Wang F., Coe R.A., Karki S., Wanchana S., Thakur V., Henry A., Lin H.C., Huang J., Peng S., Quick W.P. Overexpression of OsSAP16 regulates photosynthesis and the expression of a broad range of stress response genes in rice (Oryza sativa L.) PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0157244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X.Z., Zheng W.J., Cao X.Y., Cui X.Y., Zhao S.P., Yu T.F., Chen J., Zhou Y.B., Chen M., Chai S.C., Xu Z.S., Ma Y.Z. Genomic analysis of stress associated proteins in soybean and the role of GmSAP16 in abiotic stress responses in Arabidopsis and soybean. Front. Plant Sci. 2019;10(1453):1453. doi: 10.3389/fpls.2019.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li W., Wang Y., Li R., Chang X., Yuan X., Jing R. Cloning and characterization of TaSAP7-A, a member of the stress-associated protein tamily in common wheat. Front. Plant Sci. 2021;12(340) doi: 10.3389/fpls.2021.609351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Q.F., Mao X.G., Wang Y.X., Wang J.Y., Xi Y.J., Jing R.L. A wheat gene TaSAP17-D encoding an AN1/AN1 zinc finger protein improves salt stress tolerance in transgenic Arabidopsis. J Integr Agr. 2018;17(3):507–516. [Google Scholar]
- 26.Ben Saad R., Zouari N., Ben Ramdhan W., Azaza J., Meynard D., Guiderdoni E., Hassairi A. Improved drought and salt stress tolerance in transgenic tobacco overexpressing a novel A20/AN1 zinc-finger "AlSAP" gene isolated from the halophyte grass Aeluropus littoralis. Plant Mol. Biol. 2010;72(1–2):171–190. doi: 10.1007/s11103-009-9560-4. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y.H., Zhang L.R., Zhang L.L., Xing T., Peng J.Z., Sun S.L., Chen G., Wang X.J. A novel stress-associated protein SbSAP14 from Sorghum bicolor confers tolerance to salt stress in transgenic rice. Mol Breeding. 2013;32(2):437–449. [Google Scholar]
- 28.Ben Saad R., Farhat-Khemekhem A., Ben Halima N., Ben Hamed K., Brini F., Saibi W. The LmSAP gene isolated from the halotolerant Lobularia maritima improves salt and ionic tolerance in transgenic tobacco lines. Funct. Plant Biol. 2018;45(3):378–391. doi: 10.1071/FP17202. [DOI] [PubMed] [Google Scholar]
- 29.Li J., Sun P., Xia Y., Zheng G., Sun J., Jia H. A stress-associated protein, PtSAP13, from Populus trichocarpa provides tolerance to salt stress. Int. J. Mol. Sci. 2019;20(22):5782. doi: 10.3390/ijms20225782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao X., Wang R., Zhang Y., Li Y., Yue Y., Zhou T., Wang C. Comprehensive analysis of the stress associated protein (SAP) gene family in Tamarix hispida and the function of ThSAP6 in salt tolerance. Plant Physiol Biochem. 2021;165:1–9. doi: 10.1016/j.plaphy.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Shu X., Ding L., Gu B., Zhang H.J., Guan P.Y., Zhang J.X. A stress associated protein from Chinese wild Vitis amurensis, VaSAP15, enhances the cold tolerance of transgenic grapes. Sci Hortic-Amsterdam. 2021;285 [Google Scholar]
- 32.Ben Saad R., Fabre D., Mieulet D., Meynard D., Dingkuhn M., Al-Doss A., Guiderdoni E., Hassairi A. Expression of the Aeluropus littoralis AlSAP gene in rice confers broad tolerance to abiotic stresses through maintenance of photosynthesis. Plant Cell Environ. 2012;35(3):626–643. doi: 10.1111/j.1365-3040.2011.02441.x. [DOI] [PubMed] [Google Scholar]
- 33.Ghneim-Herrera T., Selvaraj M.G., Meynard D., Fabre D., Pena A., Ben Romdhane W., Ben Saad R., Ogawa S., Rebolledo M.C., Ishitani M., Tohme J., Al-Doss A., Guiderdoni E., Hassairi A. Expression of the Aeluropus littoralis AlSAP gene enhances rice yield under field drought at the reproductive stage. Front. Plant Sci. 2017;8(994):994. doi: 10.3389/fpls.2017.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ben Saad R., Ben Ramdhan W., Zouari N., Azaza J., Mieulet D., Guiderdoni E., Ellouz R., Hassairi A. Marker-free transgenic durum wheat cv. Karim expressing the AlSAP gene exhibits a high level of tolerance to salinity and dehydration stresses. Mol Breeding. 2012;30(1):521–533. [Google Scholar]
- 35.Allen R.G., Pereira L.S., Raes D., Smith M. Crop evapotranspiration-guidelines for computing crop water requirements-FAO Irrigation and drainage paper 56. Fao, Rome. 1998;300(9) D05109. [Google Scholar]
- 36.Zadoks J.C., Chang T.T., Konzak C.F. A decimal code for the growth stages of cereals. Weed Res. 1974;14(6):415–421. [Google Scholar]
- 37.Al-Ashkar I., Ben Romdhane W., El-Said R.A., Ghazy A., Attia K., Al-Doss A. Agro-physiologic responses and stress-related gene expression of four doubled haploid wheat lines under salinity stress conditions. Biology. 2021;10(1):56. doi: 10.3390/biology10010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ben Romdhane W., Ben Saad R., Meynard D., Zouari N., Tarroum M., Ali A., Droc G., Périn C., Morel J.-B., Fki L. Expression of an A20/AN1 stress-associated protein from Aeluropus littoralis in rice deregulates stress-related genes. J. Plant Growth Regul. 2022:1–15. [Google Scholar]
- 39.Anderson R., Bayer P.E., Edwards D. Climate change and the need for agricultural adaptation. Curr. Opin. Plant Biol. 2020;56:197–202. doi: 10.1016/j.pbi.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Pour-Aboughadareh A., Omidi M., Naghavi M.R., Etminan A., Mehrabi A.A., Poczai P., Bayat H. Effect of water deficit stress on seedling biomass and physio-chemical characteristics in different species of wheat possessing the D genome. Agronomy-Basel. 2019;9(9):522. [Google Scholar]
- 41.Morsy S.M., Elbasyoni I.S., Abdallah A.M., Baenziger P.S. Imposing water deficit on modern and wild wheat collections to identify drought‐resilient genotypes. J. Agron. Crop Sci. 2022;208(4):427–440. [Google Scholar]
- 42.Dwivedi S.K., Arora A., Singh V.P., Singh G.P. Induction of water deficit tolerance in wheat due to exogenous application of plant growth regulators: membrane stability, water relations and photosynthesis. Photosynthetica. 2018;56(2):478–486. [Google Scholar]
- 43.le Roux M.L., Burger N.F.V., Vlok M., Kunert K.J., Cullis C.A., Botha A.M. Wheat line "RYNO3936" is associated with delayed water stress-induced leaf senescence and rapid water-deficit stress recovery. Front. Plant Sci. 2020;11:1053. doi: 10.3389/fpls.2020.01053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashraf M. Stress-induced changes in wheat grain composition and quality. Crit. Rev. Food Sci. Nutr. 2014;54(12):1576–1583. doi: 10.1080/10408398.2011.644354. [DOI] [PubMed] [Google Scholar]
- 45.Li X.J., Wu Y.L., Yang B.P., Wang J.G., Zhang S.Z., Zhang M.Q. Function analysis of sugarcane A20/AN1 zinc-finger protein gene ShSAP1 in transgenic tobacco. Crop Sci. 2014;54(6):2724–2734. [Google Scholar]
- 46.Zhang N., Yin Y., Liu X., Tong S., Xing J., Zhang Y., Pudake R.N., Izquierdo E.M., Peng H., Xin M., Hu Z., Ni Z., Sun Q., Yao Y. The E3 ligase TaSAP5 alters drought stress responses by promoting the degradation of DRIP proteins. Plant physiology. 2017;175(4):1878–1892. doi: 10.1104/pp.17.01319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Priya M., Dhanker O.P., Siddique K.H.M., HanumanthaRao B., Nair R.M., Pandey S., Singh S., Varshney R.K., Prasad P.V.V., Nayyar H. Drought and heat stress-related proteins: an update about their functional relevance in imparting stress tolerance in agricultural crops. Theor. Appl. Genet. 2019;132(6):1607–1638. doi: 10.1007/s00122-019-03331-2. [DOI] [PubMed] [Google Scholar]
- 48.Racz I., Hiriscau D., Berindean I., Kadar R., Muntean E., Tritean N., Russu F., Ona A., Muntean L. The influence of flag leaf removal and its characteristics on main yield components and yield quality indices on wheat. Agronomy-Basel. 2022;12(10):2545. [Google Scholar]
- 49.Fischer R.A., Rees D., Sayre K.D., Lu Z.M., Condon A.G., Saavedra A.L. Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci. 1998;38(6):1467–1475. [Google Scholar]
- 50.Gonzalez-Ribot G., Opazo M., Silva P., Acevedo E. Traits explaining durum wheat (Triticum turgidum L. spp. Durum) yield in dry chilean mediterranean environments. Front. Plant Sci. 2017;8:1781. doi: 10.3389/fpls.2017.01781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Ashkar I., Alotaibi M., Refay Y., Ghazy A., Zakri A., Al-Doss A. Selection criteria for high-yielding and early-flowering bread wheat hybrids under heat stress. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0236351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Ashkar I., Sallam M., Ghazy A., Ibrahim A., Alotaibi M., Ullah N., Al-Doss A. Agro-physiological indices and multidimensional analyses for detecting heat tolerance in wheat genotypes. Agronomy. 2023;13(1):154. [Google Scholar]
- 53.Lopes M.S., Reynolds M.P. Stay-green in spring wheat can be determined by spectral reflectance measurements (normalized difference vegetation index) independently from phenology. J. Exp. Bot. 2012;63(10):3789–3798. doi: 10.1093/jxb/ers071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Hendawy S., Al-Suhaibani N., Al-Ashkar I., Alotaibi M., Tahir M.U., Solieman T., Hassan W.M. Combining genetic analysis and multivariate modeling to evaluate spectral reflectance indices as indirect selection tools in wheat breeding under water deficit stress conditions. Remote Sens-Basel. 2020;12(9):1480. [Google Scholar]
- 55.Rebetzke G.J., Rattey A.R., Farquhar G.D., Richards R.A., Condon B. Genomic regions for canopy temperature and their genetic association with stomatal conductance and grain yield in wheat. 2012;40(1):14–33. doi: 10.1071/FP12184. [DOI] [PubMed] [Google Scholar]
- 56.Gautam A., Prasad S.V.S., Jajoo A., Ambati D. Canopy temperature as a selection parameter for grain yield and its components in durum wheat under terminal heat stress in late sown conditions. Agr Res. 2015;4(3):238–244. [Google Scholar]
- 57.Reynolds M., Manes Y., Izanloo A., Langridge P. Phenotyping approaches for physiological breeding and gene discovery in wheat. Ann. Appl. Biol. 2009;155(3):309–320. [Google Scholar]
- 58.Bahar B., Yildirim M., Barutcular C., Genc I. Effect of canopy temperature depression on grain yield and yield components in bread and durum wheat. Not Bot Horti Agrobo. 2008;36(1):34–37. [Google Scholar]
- 59.Mason R.E., Singh R.P. Considerations when deploying canopy temperature to select high yielding wheat breeding lines under drought and heat stress. Agronomy. 2014;4(2):191–201. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data is contained within the article or supplementary material.