Abstract
Background
Malaria elimination in Senegal requires accurate diagnosis of all Plasmodium species. Plasmodium falciparum is the most prevalent species in Senegal, although Plasmodium malariae, Plasmodium ovale, and recently Plasmodium vivax have also been reported. Nonetheless, most malaria control tools, such as Histidine Rich Protein 2 rapid diagnosis test (PfHRP2-RDT,) can only diagnose P. falciparum. Thus, PfHRP2-RDT misses non-falciparum species and P. falciparum infections that fall below the limit of detection. These limitations can be addressed using highly sensitive Next Generation Sequencing (NGS). This study assesses the burden of the four different Plasmodium species in western and eastern regions of Senegal using targeted PCR amplicon sequencing.
Methods
Three thousand samples from symptomatic and asymptomatic individuals in 2021 from three sites in Senegal (Sessene, Diourbel region; Parcelles Assainies, Kaolack region; Gabou, Tambacounda region) were collected. All samples were tested using PfHRP2-RDT and photoinduced electron transfer polymerase chain reaction (PET-PCR), which detects all Plasmodium species. Targeted sequencing of the nuclear 18S rRNA and the mitochondrial cytochrome B genes was performed on PET-PCR positive samples.
Results
Malaria prevalence by PfHRP2-RDT showed 9.4% (94/1000) and 0.2% (2/1000) in Diourbel (DBL) and Kaolack (KL), respectively. In Tambacounda (TAM) patients who had malaria symptoms and had a negative PfHRP2-RDT were enrolled. The PET-PCR had a positivity rate of 23.5% (295/1255) overall. The PET-PCR positivity rate was 37.6%, 12.3%, and 22.8% in Diourbel, Kaolack, and Tambacounda, respectively. Successful sequencing of 121/295 positive samples detected P. falciparum (93%), P. vivax (2.6%), P. malariae (4.4%), and P. ovale wallikeri (0.9%). Plasmodium vivax was co-identified with P. falciparum in thirteen samples. Sequencing also detected two PfHRP2-RDT-negative mono-infections of P. vivax in Tambacounda and Kaolack.
Conclusion
The findings demonstrate the circulation of P. vivax in western and eastern Senegal, highlighting the need for improved malaria control strategies and accurate diagnostic tools to better understand the prevalence of non-falciparum species countrywide.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12936-024-04932-z.
Keywords: Plasmodium vivax, Diagnosis, Targeted sequencing, Senegal
Background
Malaria surveillance has been integrated as a core intervention in all malaria-endemic countries by the World Health Organization (WHO) since 2018 [1]. Senegal has made substantial efforts in malaria control through the National Malaria Control Programme (NMCP) using surveillance data to implement control strategies. Most detection strategies for malaria surveillance in peripheral health facilities (health posts) where most cases are diagnosed use Plasmodium falciparum Histidine Rich Protein 2 rapid diagnosis tests (PfHRP2-RDTs), which are specific for P. falciparum. However, the PfHRP2-RDT tests could miss low density Plasmodium infections and non-falciparum species usually responsible for asymptomatic infection [2]. Similarly, home-based management of malaria in high-transmission areas of the country only targets symptomatic individuals diagnosed by PfHRP2-RDTs. These interventions do not capture these types of infections in the health facilities and the communities leading to inappropriate strategies.
For elimination and eradication purposes, there is a need for accurate diagnosis of all Plasmodium species. Although P. falciparum is the most prevalent species in sub Saharan Africa, Plasmodium malariae, Plasmodium ovale (P. ovale curtisi and P. ovale wallikeri) and recently Plasmodium vivax have been reported [3, 4]. Plasmodium vivax and P. ovale have hypnozoites that could be responsible for relapses years after the first infection [5, 6] and P. malariae presents chronic infection with long parasite carriage. This chronicity requires complementary strategies to detect and treat these infections, thus the real burden of the non-falciparum species needs to be assessed. This could be achieved by using appropriate tools and strategies for better detection and identification of all Plasmodium spp responsible for infection.
Using highly sensitive tools such as Next Generation Sequencing (NGS) could overcome the limit of detection of standard malaria diagnosis tools that can miss low density parasitaemia which is often the case in asymptomatic malaria infection at the community level. Sequencing allows the detection of both P. falciparum and non-falciparum species for all levels of parasitaemia and to capture additional cases that are missed by the current surveillance system. The availability of more accurate data could help reveal the true burden of malaria and inform the adjustment of interventions for malaria control, elimination, and ultimately eradication.
Here, a targeted sequencing approach was used to amplify regions of two genes, the small subunit ribosomal gene of the 18S rRNA (ssu) and the mitochondrial cytochrome B (cytb). This enabled assessment of the burden of Plasmodium species among asymptomatic and symptomatic subjects in the community and febrile individuals with a negative HRP2-based RDT in western and eastern areas in Senegal during the transmission season of 2020–2021.
Methods
Study sites
Patients were recruited during the malaria transmission season of 2020–2021 in three sites across Senegal; Diourbel located in the West, Kaolack in the west central and Tambacounda in the East of Senegal. In Diourbel the participants were recruited in the areas of Sessène, Kaolack in Parcelles Assainies, and Tambacounda in the site of Gabou situated in the department of Bakel. According to 2021 data of the National Malaria Control Programme (NMCP), malaria prevalence was intermediate in Diourbel and high in Kaolack and Tambacounda. In Diourbel and Kaolack the peak of malaria transmission starts in October/November and continues until January/February. In 2021 malaria incidence was reported to be 147, 28 and 9 cases per 1000 inhabitants in Tambacounda, Kaolack and Diourbel, respectively. Diourbel has a desert climate with an average temperature of 28.9 °C and average rainfall of 516 mm. In Kaolack, the average temperature is 28.6 °C and the average rainfall is 678.5 mm. In Tambacounda, rainfall totals 735 mm per year and the average temperature is 29.4 °C (Météo Senegal,) [7–9].
Patient recruitment and sample collection
In Diourbel and Kaolack a community recruitment was conducted and included symptomatic and asymptomatic individuals of all ages. In Tambacounda, febrile patients who had a negative P. falciparum HRP2-based rapid diagnosis test were included in the study. Sample collection was carried out in December 2020 in Tambacounda and in January 2021 in Diourbel and Kaolack. For each participant, a RDT was performed, and blood was spotted on Whatman filter paper and dried at room temperature. Epidemiological data were recorded, including age, sex, address, symptoms, and history of recent travel.
Plasmodium infection/malaria diagnosis
Plasmodium infection was assessed during enrolment using HRP2-based RDT (SD BIOLINE Malaria P. falciparum Ag Test/HRP-II). Further, a Photoinduced Electron Transfer PCR (PET-PCR), and a nested PCR were performed. Parasite DNA was extracted from filter paper samples using QIAamp DNA Mini kit (Qiagen, QIAGEN, USA) according to the manufacturer’s instructions. Molecular identification of Plasmodium species was carried out using the photo-induced electron transfer (PET)-PCR assay [10] on an Applied Biosystems™ 7500 Fast Dx Real-Time PCR Instrument (Applied BioSystems). For each experimental run both a negative (no template) and a positive control sample for each species were included. Plasmodium genus and P. falciparum PET-PCR were performed in a multiplex format as previously described [10] and the other species, P. malariae, P. vivax, and P. ovale PET-PCR reactions were run separately. All PCR reactions were conducted in a 20 μl reaction mixture containing, 5 μl of template DNA, 10 μl of 2× TaqMan Environmental Master-Mix 2.0 (Applied BioSystems), and 250 μM of each forward and reverse primers of each species. Cycling conditions were as follows: initial hot start at 95 °C for 15 min, followed by 45 cycles of denaturation at 95 °C for 20 s and annealing at 60 °C for 40 s. Samples with a cycle threshold (CT) of 40 or less were considered as positive [10, 11].
Amplicon sequencing
Positive samples with PET-PCR were sequenced with the NGS platform, positive controls for each species were also sequenced. The amplicon sequencing for species and genotype identification was performed for the small subunit ribosomal of the 18S rRNA gene (ssu) and the mitochondrial cytochrome b (cytb) using previously published primers [12].
All primers were 5′-fused to universal tail sequences. The amplification was carried out according to a previously published protocol [12], the amplicons from each gene were pooled for one sample. The amplicons were purified using SPRI select beads according to standard procedures (Beckman Coulter, Life sciences). Amplicon size and purity were determined using a Tapestation. Barcode Index and Illumina sequencing adapters were attached using The Nextera® XT Index kit. The samples were then quantified, normalized and sequenced using an Illumina Miseq sequencer. Samples were sequenced in three pools as 2 × 250 bp paired-end reads.
Analysis of amplicon sequencing reads
Paired-end reads in fastq format were assessed to remove failed samples. Samples with 75 percent of reads with lengths below 100 nt were excluded from subsequent analysis. Reads were mapped to a reference database containing sequences of the ssu and cytb gene for five Plasmodium spp obtained from Genbank (Additional file 1: Table S1), using the bwa-mem aligner [13].
Mapped reads with five or more soft-clipped bases were removed from the alignments using samclip (https://github.com/tseemann/samclip) as were reads with greater than four mismatches to the reference sequences. Finally, high-quality alignments (MAPQ of 60+) were counted for each sample and a positive identification of a species was recorded where at least 10 reads for any amplicon were identified (Fig. 1).
Fig. 1.
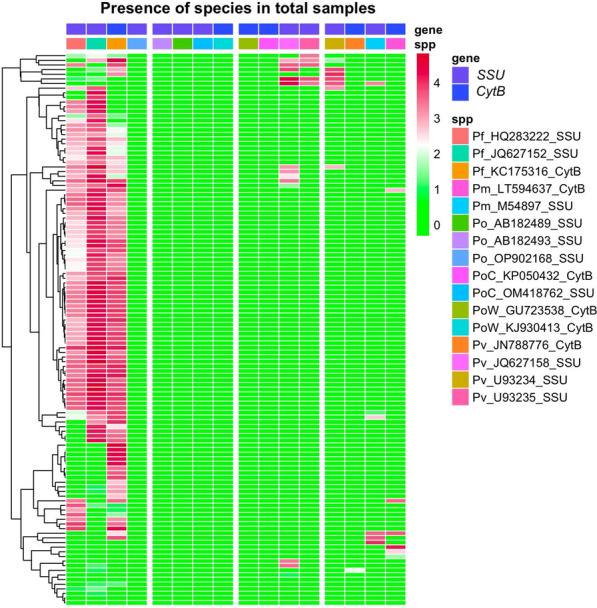
HYPERLINK "sps:id::fig1||locator::gr1||MediaObject::0" Heatmap summarizing the number of reads mapping to each reference sequence (columns) per sample (rows). Read counts were transformed to log10 pseudo-counts (read count + 0.5). Larger read counts imply stronger evidence of an identification. Pv: Plasmodium vivax, Pm: Plasmodium malariae, Pf: Plasmodium falciparum, Po: Plasmodium ovale, PoC: Plasmodium ovale curtisi, PoW: Plasmodium ovale wallikeri
Results
Plasmodium genus infection characteristics
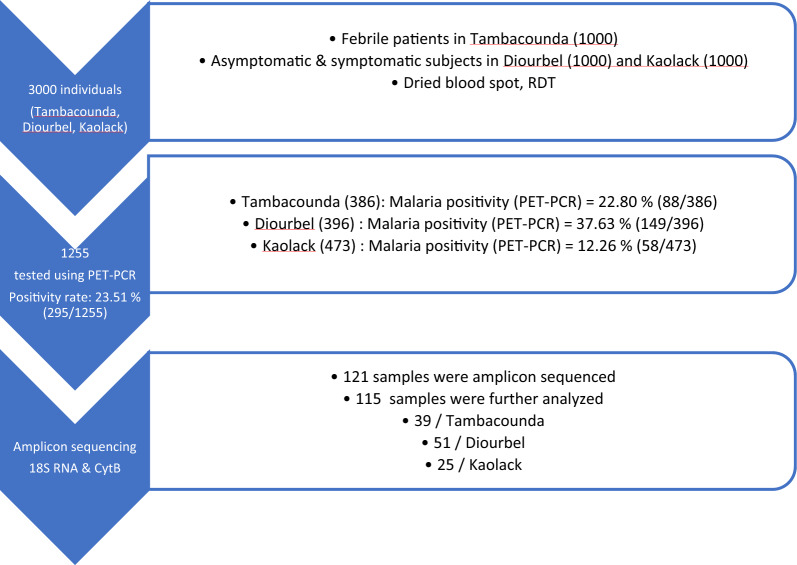
During the malaria transmission season in 2020 a total of 3000 samples were collected in three different sites Kaolack, Diourbel and Gabou towards the end of malaria transmission season. In the Tambacounda region, 1000 patients with a negative RDT were recruited. In the Kaolack and Diourbel regions, 1000 consenting individuals (247 had symptoms; 8 in Diourbel and 239 in Kaolack) have been recruited in the Sessene and Parcelles Assainies neighbourhoods, respectively.
The PfHRP2-based RDT had a positivity of 4.8% overall; 9.4% (94/1000) and 0.2% (2/1000), respectively in Diourbel and Kaolack. In Tambacounda, all patients had a negative PfHRP2-based RDT.
Among the 3000 samples collected, 396 from Diourbel, 473 from Kaolack and 386 from Tambacounda were randomly selected and a PET PCR for Plasmodium spp was performed (Fig. 2). With the PET-PCR, a positivity rate of 23.51% (295/1255) was found. According to the PET-PCR, Plasmodium spp infection in Diourbel (Sessene) and Kaolack (Parcelles Assainies) was 37.63% (149/396) and 12.26% (58/473), respectively. In Tambacounda (Gabou) where febrile patients with PfHRP2-RDT negative were recruited, malaria positivity was 22.80% (88/386) (Fig. 2).
Fig. 2.
Flow chart of sample processing. From the 3000 samples collected; 396; 473 and 386, respectively from Diourbel, Kalolack and Tambacounda were tested with PET-PCR. 121 PET-PCR positive samples were sequenced
Plasmodium species identification
Species identification was performed using amplicon deep sequencing of the ssu of the 18S rRNA (nucleus) and the cytb (mitochondrion) genes. A total of 121 samples were sequenced; six samples were excluded due to poor quality sequencing data. The remaining 115 samples were further analysed. Of these 115 samples, 51 were taken from Diourbel, 39 from Tambacounda and 25 from Kaolack (Fig. 2). NGS amplicon sequencing identified all four Plasmodium species: P. falciparum, P. vivax, P. malariae and P. ovale wallikeri. P. falciparum represented respectively 44% (51/115), 22% (25/115) and 34% of the infections in Diourbel, Kaolack and Tambacounda (Fig. 3, Additional file 1: Table S2). Non-falciparum infections (mono and mixed infections) were 23.5% (27/115) overall; 7.8% (9/115) in Diourbel; 6.1% (7/115) in Kaolack and 9.6% (11/115) in Tambacounda. Non-falciparum mono-infections represented 6.1% (7/115) of the infections. Plasmodium malariae represented 3.5% (4/115) of mono-infection, P. vivax 2.6% (3/115) and P. ovale wallikeri 0.836% (1/115) (Additional file 1: Table S2). Mixed infections represented 17.39% (20/115) overall; 6.97% (8/115); 3.48% (4/115) and 6.97% (8/115), respectively, in Diourbel, Kaolack and Tambacounda (Fig. 3, Additional file 1: Table S2).
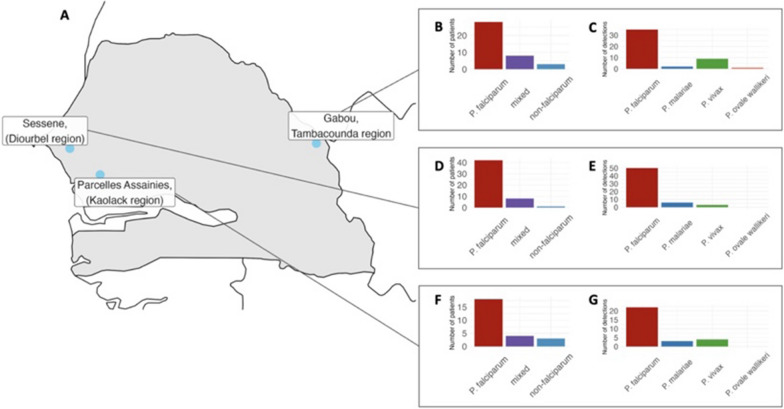
Fig. 3.
Infections with Plasmodium species across three sites in Senegal. A Map of Senegal (area in grey) showing the location of three sites where samples were collected (blue dots). B Barplot summarising the number of patients in the Tambacounda (Gabou) region that were found to be infected with either P. falciparum alone, a mixture of P. falciparum and another species (mixed) or with Plasmodium species other than falciparum (non-falciparum). C Barplot summarising the total number of detections of Plasmodium species in patient samples collected in the Tambacounda region. D Barplot summarizing the number of patients in the Diourbel region with single or mixed Plasmodium infections, details as for panel B. E Barplot summarizing the total number of species detected in samples collected from the Diourbel region, details as for panel C. F Barplot summarising the number of patients in the Kaolack region with single or mixed Plasmodium infections, details as for panel B. G Barplot summarizing the total number of species detected in samples collected from the Kaolack region, details as for panel C
In terms of identification, some infections were identified by either the ssu or the cytb gene. Among the eight non-falciparum mono-infections, three were identified with the ssu gene and five with the cytb (Additional file 1: Table S3).
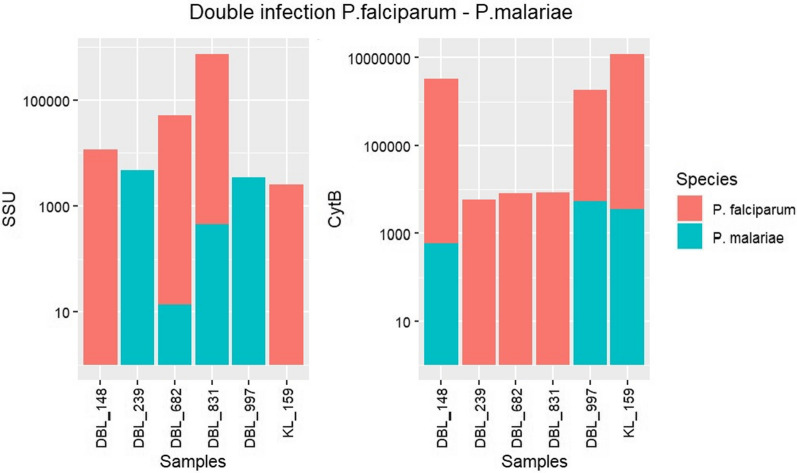
For the mixed infection of P. falciparum and P. malariae, the ssu gene failed to identify two P. malariae infections (DL_148 and KL_159) which were identified with the cytb gene and for DBL_682 the number of reads for the cytb was very low (14 reads). The cytb gene could not identify three P. malariae infections (Fig. 4, Additional file 1: Tables S4 and S6).
Fig. 4.
Barplot summarizing the number of ssu and cytb reads aligned to P. falciparum and P. malariae ssu and cytb genes in samples with mixed infections. Green represents the number of reads for P. malariae, and red the number of reads for P. falciparum. The association is represented by a bar with two colours (green and red). Due to the plot low number of reads for some samples (DBL_148; DBL_239; KL_159; DBL_682; DBL_831) the association is not shown in the bars
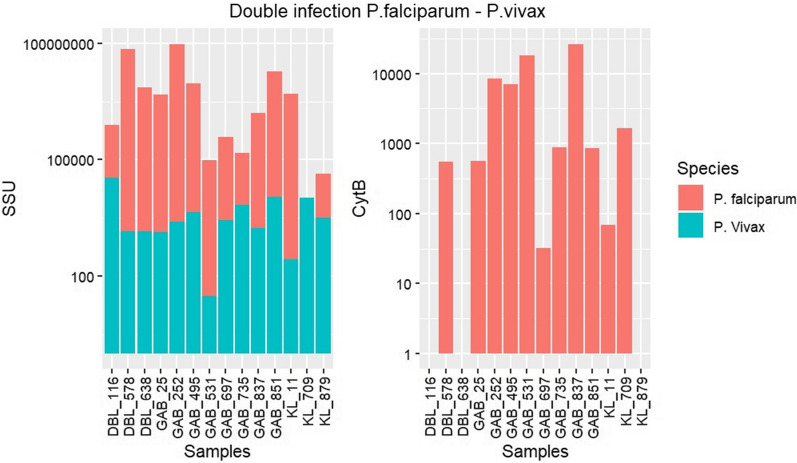
In mixed infections Pf/Pv; P. vivax was identified only with the ssu gene; three P. falciparum infections could not be detected using the cytb gene. Only one P. falciparum infection was not detected with the ssu gene (Fig. 5, Additional file 1: Tables S5 and S6).
Fig. 5.
Barplot summarizing the number of reads aligned to P. falciparum and P. vivax ssu and cytb genes in samples with mixed infections. Green represents the number of reads for P. vivax, and red the number of reads for P. falciparum. The association is represented by a bar with two colours (green and red). Due to the low number of reads for some samples the cytb gene, the association is not shown in the bars
Distribution of Plasmodium variants and species
As shown in Fig. 3, P. falciparum was responsible for 93.0% (mono- and mixed infections with other species) of the infections among which mono-infections represented 76.5% (88/115) and distributed as follows; 37.5% (42/115) in Diourbel, 27.0% in Tambacounda (31/115) and 18.3% in Kaolack (21/115). Multi-infections of P. falciparum with other species represented 16.5% (19/115). Plasmodium falciparum was present, respectively with P. malariae (Pf/Pm) and P. vivax (Pf/Pv) in 5.2% (6/115) and 11.3% (13/115) of the infections. No co-infection with P. ovale was found.
Sequencing of the ssu gene of P. falciparum was used to determine the extent of strain variation across the samples. This analysis showed six groups (Fig. 6). The green and orange groups contain the highest number of P. falciparum variants. The orange group contains variants from the three study sites: Diourbel (17), Kaolack (06) and Tambacounda (07). The green group is composed of 13 from Diourbel; 11 from Tambacounda and three from Kaolack. The yellow group is represented by Diourbel (03) and Tambacounda (04). The Red group is composed of five variants from Diourbel and one from Tambacounda. The pink group contains three strains: two from Diourbel and one from Tambacounda (Fig. 6). The nucleotide sequences that differentiate the groups are presented in Additional file 1: Table S6. The groups are distributed across all sites; no variant is specific to one site.
Fig. 6.
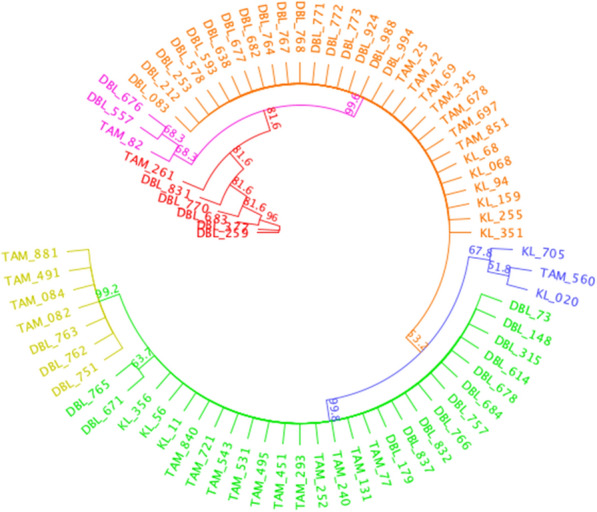
Phylogenetic tree comparing P. falciparum 18S rRNA gene sequences. P. falciparum strains are divided into 6 branches represented by different colours. The green branch comprises twenty-seven (27) variants; thirteen (13) from Diourbel, eleven (11) from Tambacounda and only three (03) variants from Kaolack. DBL, KL, TAM associated with numbers represent sample identifiers
Plasmodium vivax was responsible for 13.04% (15/115) of the infections; 11.30% (13/115) polyinfections and 1.74% (2/115) mono-infections (Additional file 1: Table S2). The prevalence of P. vivax was 6.96% (8/115) in Tambacounda; 3.48% (4/115) in Kaolack and 2.61% (3/115) in Diourbel. Mono-infection was found once in Tambacounda and Kaolack. Plasmodium malariae and P. vivax were associated in 1 sample and identified with the ssu gene, and the patient was a 26-year-old female from Tambacounda. The number of reads for the ssu gene was 25,098 for P. vivax and 1884 for P. malariae.
Two of the three individuals infected with P. vivax presented symptoms of headaches and were from Kaolack and Tambacounda. All mixed infections with P. falciparum presented symptoms except four (03 from Diourbel and 01 from Kaolack).
The phylogenetic tree of P. vivax derived from the ssu gene is composed of seven (07) branches (Fig. 7). The nucleotides differentiating the strains are listed in Additional file 1: Table S6. The black branch includes two strains from Diourbel and Kaolack. The green and the yellow branches include each one variant from Tambacounda. The orange, red and blue branches contain each two variants; respectively from Kaolack, Diourbel and Tambacounda. The purple branch consists of seven variants: five from Tambacounda and two from Kaolack (Additional file 1: Table S6).
Fig. 7.
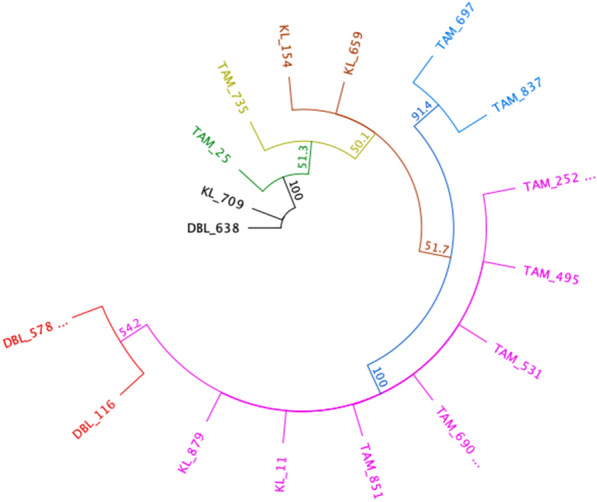
Phylogenetic tree comparing P. vivax 18S rRNA gene sequences. P. vivax strains are divided into seven branches, each represented by a different colour. The violet branch contains more variants, with seven (07) from Tambacounda and two (02) from Kaolack. The other branches are made up of two variants, and two branches have only one variant. DBL, KL, TAM associated with numbers represent sample identifiers
Plasmodium malariae was responsible for 9.56% of the infections (11/115), among which 3.47% (4/115) were mono-infections and 6.09% (0/115) were poly-infections (Additional file 1: Table S2). Plasmodium malariae prevalence in Diourbel was 5.22% (6/115); only one infection was a mono-infection. In Kaolack, P. malariae prevalence was 2.61% (3/115), two poly-infections and one mono-infection. In Tambacounda, P. malariae was responsible for two infections; one mono-infection and one associated with P. vivax (Fig. 3). There were three different variants of P. malariae among the samples. (Additional file 1: Table S6). Plasmodium ovale wallikeri was found in only one mono-infection in Tambacounda.
Infections with P. malariae mono and mixed infection Pf/Pm was found in both adults and children, characteristics of patients are presented in Additional file 1: Tables S3 and S4.
Discussion
An accurate estimation of the prevalence of malaria and the causative species is needed to achieve its elimination. The aim of this study was to determine the frequency of Plasmodium species in different endemic sites in Senegal towards the end of the malaria transmission season. The study population included asymptomatic and symptomatic individuals sampled in the community in Diourbel (Sessene) and Kaolack (Parcelles Assainies) and symptomatic patients with a negative PfHRP2-RDT test at the health post in Tambacounda.
Using a genus-specific PET-PCR, that identifies all Plasmodium species, followed by targeted deep amplicon sequencing of the ssu and the cytb genes to distinguish the different species, we detected P. falciparum, P. vivax, and P. malariae in Senegal, with a rare detection of P. ovale wallikeri. However, the genes used to identify the Plasmodium species are not discriminative enough to allow for the detection of variants or the multiplicity of infections. Although circulation of P. vivax in southern and northern Senegal [14–18] has been debated, here, P. vivax was identified in eastern (Tambacounda, Bakel) and central (Diourbel and Kaolack) regions of Senegal. In sub-Saharan Africa several studies have reported P. vivax, including in countries bordering Senegal, e.g. Mali and Mauritania, [19–22]. Most P. vivax infections (7/13) in this study were identified in Tambacounda, where the samples were collected in the health post of Gabou (Bakel district) near the border with Kayes in Mali where P. vivax has been observed [23]. However, differences in patient recruitment methods between sites; community sampling from Kaolack and Diourbel and symptomatic sampling of patients in Tambacounda, do not allow a direct comparison of prevalence. However, this strategy includes two population groups likely to be infected by non-falciparum Plasmodium that are often not considered in NMCP diagnostic and surveillance strategies.
Despite the evidence of the circulation of P. vivax in sub-Saharan Africa, most control and elimination strategies focus on P. falciparum. P. vivax produces hypnozoites that cannot be detected using current diagnostic methods used in endemic areas nor treated with artemisinin-based combination therapy and, therefore, will maintain the transmission. Beside P. vivax, P. malariae was the third most prevalent species and only one infection with P. ovale wallikeri was identified. A few studies have reported the circulation of P. malariae and P. ovale in symptomatic individuals in Senegal, P. malariae being responsible for acute renal failure [17, 24–27]. It has been reported that P. malariae and P. ovale are responsible for severe and persistent cases of malaria [28, 29]. Thus, those undiagnosed, and untreated cases of malaria can develop into severe malaria, leading to hospitalization and possibly death.
The prevalence of non-falciparum species was higher than expected both in symptomatic patients and asymptomatic individuals. With current control methods, there is a decrease in malaria caused by P. falciparum, and this could lead to an increase in malaria caused by other species that are neglected [30, 31]. This could be explained by the interaction of the species within the host as the presence of one species at a parasite density sufficient to trigger treatment seeking restricts the ability of previous lower density infections of the other species from persisting [31].
It has been shown that P. falciparum genetic diversity is a useful metric to estimate malaria transmission in endemic areas [30, 31]. In this study, the non-falciparum species identified also show a genetic diversity of the ssu gene suggesting a high frequency of transmission of these species in the areas studied. The genetic diversity is high in areas where transmission is high and low in regions implementing effective control strategies [32–36]. These results are based mainly on P. falciparum, but application of these approaches could be valuable for the non-falciparum species estimating the intensity of their transmission in endemic areas.
The positivity rate using PET-PCR was much higher than with RDTs for Plasmodium spp. infection, which is due to the higher sensitivity of the molecular techniques and to the detection of the non-falciparum species which were missed by PfHRP2-RDT. In Tambacounda, situated in the red zone where malaria transmission is the highest in the country, a high proportion of the infections was missed with the PfHRP2-RDT among febrile patients [37]. Guidelines for the biological diagnosis of malaria in Senegal are the use of PfHRP2-RDTs in health posts receiving most of malaria cases, as well as consultation departments [38]. Microscopy is available in health centres and hospital laboratories and the LAMP technique in used in the northern zone, which is in the process of eliminating malaria [38]. In Senegal, control strategies are directed against P. falciparum which is thought to be responsible for 99% of malaria cases based on data from health facilities and home-based management where symptomatic cases are diagnosed using PfHRP2-RDTs that detect only P. falciparum. In addition, the non-falciparum species may be more difficult to treat because of relapses [5, 6].
Current diagnosis strategy leads to an underestimation of non-falciparum species, which are reported in some health facilities using other diagnostic methods or during research studies. Thus, there is an urgent need to establish the prevalence of all species of Plasmodium circulating in the country to inform a better management of these undiagnosed malaria cases in health facilities that rely on only PfHRP2-RDTs for diagnosis. Thus, there is a need for sensitive diagnostic tools that can detect all Plasmodium species circulating in the country for a better management of malaria and for elimination purposes.
Asymptomatic Plasmodium infection should be investigated for determining the real burden and implement adequate strategies for elimination purposes. However, currently in Senegal no strategy targets the asymptomatic infections. In this study a high proportion of infections detected in individuals recruited in the community were asymptomatic. Most of these infections were not detected with the PfHRP2-RDT as they are often characterized by a low density of parasites that often cannot be detected by microscopy or by the RDTs used in endemic areas for malaria diagnosis [39, 40]. The prevalence of Plasmodium infection was much higher in Diourbel than Kaolack, which is in line with the NMCP data from 2021 malaria incidence [37]. These infections not seen in health facilities would, therefore, go undetected and untreated. In addition, asymptomatic low-density infections due to non-falciparum species, which go undiagnosed, could become chronic and produce gametocytes likely to infect mosquitoes and contribute to the maintenance of malaria transmission [40–42].
NMCP malaria stratification in Senegal is based on PfHRP2-RDTs positivity among febrile patients attending health facilities and symptomatic cases diagnosed with home-based malaria management by the home care provider. While this strategy is less costly it does not consider low density Plasmodium infection, asymptomatic cases, malaria caused by P. falciparum with HRP2/3 deletion or malaria due to non-falciparum species [43–45]. To overcome these challenges there is a need to include the screening of asymptomatic Plasmodium infections and the use of more sensitive diagnostic tools that can detect and identify all Plasmodium species circulating in the country.
Supplementary Information
Additional file 1: Table S1. Reference sequences of Plasmodium species used for read mapping. Table S2. Parasites species composition by site. Table S3. Non-falciparum monoinfection distribution by site. Table S4. Characteristics of mixed P. falciparum and P. malariae infections. Table S5. Characteristics of mixed P. falciparum and P. vivax infections. Table S6. Nucleotide differences between Plasmodium species based on the 18S rRNA gene.
Abbreviations
- PfHRP2-RDT
Histidine Rich Protein 2 rapid diagnosis test
- NGS
Next Generation Sequencing
- DBL
Diourbel
- KL
Kaolack
- TAM
Tambacounda
- WHO
World Health Organization
- NMCP
National Malaria Control Programme
- Ssu
Small subunit ribosomal gene of the 18S rRNA
- Cytb
Mitochondrial cytochrome B
- PET-PCR
Photo-induced electron transfer polymerase chain reaction
Author contributions
ASB: conceptualized and coordinated; JL, DSN; DAN: designed; TN, AG: collected data; BN, JC, MS, DC: Analysed and interpreted; ASB: wrote the first draft together with JL, DSN, JC and DC: revised the manuscript. All authors approved the final version.
Funding
Open Access funding provided by The Francis Crick Institute. This work was supported by the Crick African Network which receives its funding from the UK’s Global Challenges Research Fund (MR/P028071/1), and by the Francis Crick Institute which receives its core funding from Cancer Research UK (CC2079, CC0199), the UK Medical Research Council (CC2079, CC0199), and the Wellcome Trust (CC2079, CC0199). For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Availability of data and materials
All data generated or analysed during this study are included in this article and its Additional files.
Declarations
Ethics approval and consent to participate
This work was approved by the CNERS of the Ministry of Health in Senegal Protocol SEN19/49.
Consent for publication
Not applicable.
Competing interests
None declared.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO . Global technical strategy for malaria 2016–2030, 2021 update. Geneva: World Health Organization; 2021. [Google Scholar]
- 2.Stresman G, Whittaker C, Slater HC, Bousema T, Cook J. Quantifying Plasmodium falciparum infections clustering within households to inform household-based intervention strategies for malaria control programs: an observational study and meta-analysis from 41 malaria-endemic countries. PLoS Med. 2020;17:e1003370. doi: 10.1371/journal.pmed.1003370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doderer-Lang C, Atchade PS, Meckert L, Haar E, Perrotey S, Filisetti D, et al. The ears of the African elephant: unexpected high seroprevalence of Plasmodium ovale and Plasmodium malariae in healthy populations in Western Africa. Malar J. 2014;13:240. doi: 10.1186/1475-2875-13-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, Temperley WH, et al. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis. 2010;4:e774. doi: 10.1371/journal.pntd.0000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White MT, Karl S, Battle KE, Hay SI, Mueller I, Ghani AC. Modelling the contribution of the hypnozoite reservoir to Plasmodium vivax transmission. ELife. 2014;3:e04692. doi: 10.7554/eLife.04692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson LJ, Wampfler R, Betuela I, Karl S, White MT, Li Wai Suen CSN, et al. Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomized placebo controlled trial and mathematical model. PLoS Med. 2015;12:e1001891. doi: 10.1371/journal.pmed.1001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.https://planificateur.a-contresens.net/afrique/senegal/fatick/diourbel_sine/2252307.html
- 8.https://planificateur.a-contresens.net/afrique/senegal/kaolack/kaolack/2250805.html
- 9.https://planificateur.a-contresens.net/afrique/senegal/tambacounda/tambacounda/2244991.html
- 10.Lucchi NW, Karell MA, Journel I, Rogier E, Goldman I, Ljolje D, et al. PETPCR method for the molecular detection of malaria parasites in a national malaria surveillance study in Haiti, 2011. Malar J. 2014;13:462. doi: 10.1186/1475-2875-13-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lima de GFMC, Lucchi NW, Silva-Flannery L, Macedo-de-Oliveira A, Hristov AD, Inoue J, et al. Still searching for a suitable molecular test to detect hidden Plasmodium infection: a proposal for blood donor screening in Brazil. PLoS One. 2016;11:e0150391. doi: 10.1371/journal.pone.0150391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lalremruata A, Jeyaraj S, Engleitner T, Joanny F, Lang A, Bélard S, et al. Species and genotype diversity of Plasmodium in malaria patients from Gabon analysed by next generation sequencing. Malar J. 2017;16:398. doi: 10.1186/s12936-017-2044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.arXiv:1303.3997v2 [q-bio.GN] 10.48550/arXiv.1303.3997
- 14.Niang M, Sane R, Sow A, Sadio BD, Chy S, Legrand E, et al. Asymptomatic Plasmodium vivax infections among Duffy-negative population in Kedougou, Senegal. Trop Med Health. 2018;46:45. doi: 10.1186/s41182-018-0128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niang M, Diop F, Niang O, Sadio BD, Sow A, Faye O, et al. Unexpected high circulation of Plasmodium vivax in asymptomatic children from Kédougou, southeastern Senegal. Malar J. 2017;16:497. [DOI] [PMC free article] [PubMed]
- 16.Niang M, Diop F, Niang O, Sadio BD, Sow A, Faye O, et al. Unexpected high circulation of Plasmodium vivax in asymptomatic children from Kédougou, southeastern Senegal. Malar J. 2017;16:497. doi: 10.1186/s12936-017-2146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniels RF, Deme AB, Gomis JF, Dieye B, Durfee K, Thwing JI, et al. Evidence of non-Plasmodium falciparum malaria infection in Kédougou, Sénégal. Malar J. 2017;16:9. doi: 10.1186/s12936-016-1661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Badiane AS, Ndiaye T, Thiaw AB, Deme AB, Diallo MA, Seck MC, et al. High prevalence of asymptomatic Plasmodium infection in Bandafassi, South-East Senegal. Malar J. 2021;20:218. doi: 10.1186/s12936-021-03746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seck MC, Thwing J, Badiane AS, Rogier E, Fall FB, Ndiaye PI, et al. Analysis of anti-Plasmodium IgG profiles among Fulani nomadic pastoralists in northern Senegal to assess malaria exposure. Malar J. 2020;19:15. doi: 10.1186/s12936-020-3114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baird JK. African Plasmodium vivax malaria improbably rare or benign. Trends Parasitol. 2022;38:683–696. doi: 10.1016/j.pt.2022.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Twohig KA, Pfeffer DA, Baird JK, Price RN, Zimmerman PA, Hay SI, et al. Growing evidence of Plasmodium vivax across malaria-endemic Africa. PLoS Negl Trop Dis. 2019;13:e0007140. doi: 10.1371/journal.pntd.0007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ba H, Duffy CW, Ahouidi AD, Deh YB, Diallo MY, Tandia A, et al. Widespread distribution of Plasmodium vivax malaria in Mauritania on the interface of the Maghreb and West Africa. Malar J. 2016;15:80. doi: 10.1186/s12936-016-1118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernabeu M, Gomez-Perez GP, Sissoko S, Niambélé MB, Haibala AA, Sanz A, et al. Plasmodium vivax malaria in Mali: a study from three different regions. Malar J. 2012;11:405. doi: 10.1186/1475-2875-11-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niangaly A, Karthigayan G, Amed O, Coulibaly D, Sá JM, Adams M, et al. Plasmodium vivax Infections over 3 Years in Duffy Blood Group Negative Malians in Bandiagara. Mali. Am J Trop Med Hyg. 2017;97:744–752. doi: 10.4269/ajtmh.17-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diallo MA, Badiane AS, Diongue K, Deme A, Lucchi NW, Gaye M, et al. Non-falciparum malaria in Dakar: a confirmed case of Plasmodium ovale wallikeri infection. Malar J. 2016;15:429. doi: 10.1186/s12936-016-1485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badiane AS, Diongue K, Diallo S, Ndongo AA, Diedhiou CK, Deme AB, et al. Acute kidney injury associated with Plasmodium malariae infection. Malar J. 2014;13:226. doi: 10.1186/1475-2875-13-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diallo MA, Diongue K, Diagne G, Seck MC, Ndiaye M, Dièye B, et al. Le paludisme à Plasmodium ovale wallikeri et Plasmodium ovale curtisi au Sénégal en 2016 [Plasmodium ovale wallikeri and Plasmodium ovale curtisi Malaria in Senegal in 2016] Bull Soc Pathol Exot. 2017;110:286–290. doi: 10.1007/s13149-017-0578-6. [DOI] [PubMed] [Google Scholar]
- 28.Kotepui M, Kotepui KU, Milanez GD, Msangkay FR. Global prevalence and mortality of severe Plasmodium malariae infection: a systematic review and meta-analysis. Malar J. 2020;19:274. doi: 10.1186/s12936-020-03344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roucher C, Rogier C, Sokhna C, Tall A, Trape JF. A 20-year longitudinal study of Plasmodium ovale and Plasmodium malariae prevalence and morbidity in a West African population. PLoS ONE. 2014;9:e87169. doi: 10.1371/journal.pone.0087169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chenet SM, Schneider KA, Villegas L, Escalante AA. Local population structure of Plasmodium: impact on malaria control and elimination. Malar J. 2012;11:412. doi: 10.1186/1475-2875-11-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nkhoma SC, Nair S, Al-Saai S, Ashley E, McGready R, Phyo AP, et al. Population genetic correlates of declining transmission in a human pathogen. Mol Ecol. 2013;22:273. doi: 10.1111/mec.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akala HM, Watson OJ, Mitei KK, Juma DW, Verity R, Ingasia LA, et al. Plasmodium interspecies interactions during a period of increasing prevalence of Plasmodium ovale in symptomatic individuals seeking treatment: an observational study. Lancet Microbe. 2021;2:e141. doi: 10.1016/S2666-5247(21)00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor SM, Messina JP, Hand CC, Juliano JJ, Muwonga J, Tshefu AK, et al. Molecular malaria epidemiology: mapping and burden estimates for the Democratic Republic of the Congo, 2007. PLoS ONE. 2011;6:e16420. doi: 10.1371/journal.pone.0016420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Black J, Hommel M, Snounou G, Pinder M. Mixed infections with Plasmodium falciparum and P malariae and fever in malaria. Lancet. 1994;343:1095. doi: 10.1016/S0140-6736(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 35.Daniels R, Chang HH, Sene PD, Park DC, Neafsey DE, Schaffner SF, et al. Genetic surveillance detects both clonal and epidemic transmission of malaria following enhanced intervention in Senegal. PLoS ONE. 2013;8:e60780. doi: 10.1371/journal.pone.0060780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffmann EH, da Silveira LA, Tonhosolo R, Pereira FJ, Ribeiro WL, Tonon AP, et al. Geographical patterns of allelic diversity in the Plasmodium falciparum malaria-vaccine candidate, merozoite surface protein-2. Ann Trop Med Parasitol. 2001;95:117. doi: 10.1080/00034983.2001.11813622. [DOI] [PubMed] [Google Scholar]
- 37.Cowell AN, Valdivia HO, Bishop DK, Winzeler EA. Exploration of Plasmodium vivax transmission dynamics and recurrent infections in the Peruvian Amazon using whole genome sequencing. Genome Med. 2018;4(10):52. doi: 10.1186/s13073-018-0563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bulletin épidémiologique annuel 2021 du paludisme au Sénégal National Malaria Control Programme
- 39.Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol. 2014;12:833–840. doi: 10.1038/nrmicro3364. [DOI] [PubMed] [Google Scholar]
- 40.Cheng Q, Cunningham J, Gatton ML. Systematic review of sub-microscopic P. vivax infections: prevalence and determining factors. PLoS Negl Trop Dis. 2015;9:e3413. doi: 10.1371/journal.pntd.0003413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coalson JE, Walldorf JA, Cohee LM, Ismail MD, Mathanga D, Cordy RJ, et al. High prevalence of Plasmodium falciparum gametocyte infections in school-age children using molecular detection: patterns and predictors of risk from a cross-sectional study in southern Malawi. Malar J. 2016;15:527. doi: 10.1186/s12936-016-1587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohee L, Laufer M. Tackling malaria transmission in sub-Saharan Africa. Lancet Glob Health. 2018;6:e598. doi: 10.1016/S2214-109X(18)30197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Touray AO, Mobegi VA, Wamunyokoli F, Butungi H, Herren JK. Prevalence of asymptomatic P. falciparum gametocyte carriage among school children in Mbita, Western Kenya and assessment of the association between gametocyte density, multiplicity of infection and mosquito infection prevalence. Wellcome Open Res. 2021;5:259. doi: 10.12688/wellcomeopenres.16299.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brenier-Pinchart MP, Pinel C, Croisonnier A, Brion JP, Faure O, Ponard D. Diagnosis of malaria in non-endemic countries by the Parasight-F test. Am J Trop Med Hyg. 2000;63:150. doi: 10.4269/ajtmh.2000.63.150. [DOI] [PubMed] [Google Scholar]
- 45.Gamboa D, Ho MF, Bendezu J, Torres K, Chiodini PL, Barnwell JW, et al. A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3: implications for malaria rapid diagnostic tests. PLoSONE. 2010;5:e8091. doi: 10.1371/journal.pone.0008091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Reference sequences of Plasmodium species used for read mapping. Table S2. Parasites species composition by site. Table S3. Non-falciparum monoinfection distribution by site. Table S4. Characteristics of mixed P. falciparum and P. malariae infections. Table S5. Characteristics of mixed P. falciparum and P. vivax infections. Table S6. Nucleotide differences between Plasmodium species based on the 18S rRNA gene.
Data Availability Statement
All data generated or analysed during this study are included in this article and its Additional files.