Abstract
Diabetic heart disease (DHD) is a serious complication in patients with diabetes. Despite numerous studies on the pathogenic mechanisms and therapeutic targets of DHD, effective means of prevention and treatment are still lacking. The pathogenic mechanisms of DHD include cardiac inflammation, insulin resistance, myocardial fibrosis, and oxidative stress. Macrophages, the primary cells of the human innate immune system, contribute significantly to these pathological processes, playing an important role in human disease and health. Therefore, drugs targeting macrophages hold great promise for the treatment of DHD. In this review, we examine how macrophages contribute to the development of DHD and which drugs could potentially be used to target macrophages in the treatment of DHD.
Keywords: Diabetic heart disease, Diabetic cardiomyopathy, Macrophages, Drug therapy
Introduction
Diabetes mellitus (DM) is a widespread condition with costly treatment. In 2045, DM is projected to affect 783 million individuals worldwide and incur an annual economic expense of 1054 billion USD [1]. Type 2 DM (T2DM) accounts for 90–95% of all diabetes cases [2], and people with diabetes have more than twice the risk of developing heart failure (HF) than do people without diabetes [3, 4]. DM and its complications are a serious threat to human health and have become a global public health problem [5]. The pathologic mechanisms of different diabetic complications have a high degree of commonality at the vascular level.
One of the most common forms of diabetic heart disease (DHD) is diabetic cardiomyopathy (DCM). Although researchers have devoted significant resources toward developing a treatment for DCM, only a handful of possibilities have emerged, such as using sodium-glucose cotransporter 2 inhibitors (SGLT2is) [6], glucagon-like peptide 1 receptor agonists (GLP1RAs) [7], and metformin [8]. Unfortunately, these treatments have not significantly improved patient prognosis. A central pathogenic mechanism of DHD is the inflammatory response, which exacerbates oxidative stress and endoplasmic reticulum stress [9]. Macrophages, which execute the inflammatory response, influence the course of DHD through the secretion of various inflammatory factors [10]. Therefore, a drug therapy targeting macrophages may be a promising new direction for improved DHD treatment.
Type 1 DM (T1DM) and T2DM, the two primary forms of DM, have different pathogenic mechanisms. T1DM is mainly caused by the autoimmune destruction of pancreatic β-cells and occurs in younger patients, whereas T2DM is caused by insulin resistance and relative insulin deficiency and is usually seen in older and overweight patients. Both T1DM and T2DM can induce DHD and impaired cardiac function. They share some clinical presentations (e.g., diastolic dysfunction, systolic dysfunction, HF) and pathogenic mechanisms (e.g., cardiac hypertrophy and fibrosis, oxidative stress, inflammation, metabolic dysfunction) [11]. Patients with T2DM are more likely to experience ventricular diastolic dysfunction compared to those with T1DM [12]. Although macrophages are involved in DHD, their specific roles in T1DM- and T2DM-induced DHD and possible ways of therapeutic modulation have not been well studied. Common clinical pharmacological treatments, such as with SGLT2is and GLP1RAs, have not been studied in cardiovascular outcomes trials in patients with T1DM, despite studies showing that these treatments can lower hemoglobin A1c (HbA1c) levels, reduce body weight, facilitate glucose control, and reduce insulin dose requirements [13–15]. In the present review, we discuss the role of macrophages in DHD and their pharmacological targeting, with a focus on T2DM-related DHD.
Overview of macrophages: origin, polarization, and function
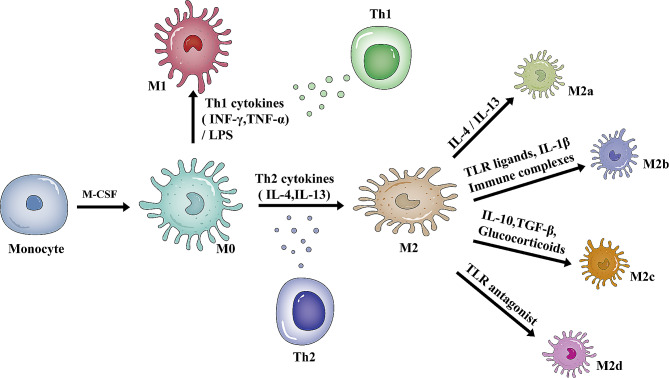
Macrophages are evolutionarily conserved innate immune cells that play an essential role in human health and disease [16]. Macrophages are heterogeneous, with phenotypes and functions that are regulated by the surrounding microenvironment. Macrophage polarization is the process by which macrophages show specific phenotypic and functional responses upon microenvironmental stimulation and signaling [17]. M0 macrophages are induced by macrophage colony-stimulating factor (M-CSF) to differentiate from monocytes and represent the initial state of macrophage polarization [18]. Depending on the type of stimulus and the pattern and functional properties of surface molecules and secreted cytokines, M0 macrophages polarize into one of two main subpopulations: M1 and M2 macrophages [19]. An illustration of macrophage polarization is shown in Fig. 1. M1 macrophages have potent anti-microbial and anti-tumor activities and participate in the clearance of pathogens by activating nicotinamide adenine dinucleotide phosphate oxidase (NOX) and producing reactive oxygen species (ROS) during injury or infection [20]. Alternately activated or M2 macrophages, which have anti-inflammatory and immunomodulatory effects, are polarized by T helper 2 (Th2) cytokines interleukin-4 (IL-4) and IL-13 [21–23]. M2 macrophages have a strong phagocytic ability, can remove dead cells, promote tissue repair and wound healing, and have pro-angiogenic and profibrotic properties [24].
Fig. 1.
A schematic representation of macrophage polarization. Monocytes can be differentiated into M0 macrophages in response to M-CSF stimulation. M0 macrophages can be polarized into M1 macrophages in response to LPS or Th1 cytokines (INF-γ, TNF-α). M0 macrophages are polarized into M2 macrophages in response to Th2 cytokines (IL-4, IL-13). Depending on the stimuli, M2 macrophages are subdivided into M2a, M2b, M2c, and M2d macrophages. Abbreviations: M-CSF: macrophage colony-stimulating factor, INF-γ: interferon-γ, TNF-α: tumor necrosis factor-α, LPS: lipopolysaccharide, IL: interleukin, TGF-β: transforming growth factor-β, TLR: toll-like receptor
M1 and M2 macrophages are not two distinct cell populations [25] but are present in a spectrum of different functional states, including pro-inflammatory and anti-inflammatory phenotypes of the cells [26]. The concept of M1/M2 is based on several in vitro studies in which macrophages were stimulated in culture with a defined set of factors [27]. Compared to in vivo situations, macrophages cultured outside their native tissue microenvironment have significant differences in their polarization and function [28]. Given the complexity of M1/M2 polarization [27], the functional and stimulant-based understanding of macrophages in the context of DHD warrants further exploration.
The role of macrophages in diabetic heart disease
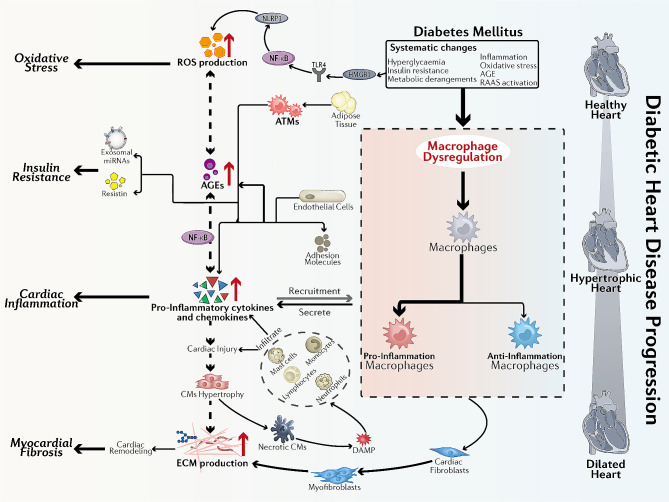
According to the currently accepted pathophysiologic mechanisms of DHD [29–32], insulin resistance and metabolic derangements induce hyperglycemic environments in patients with DM [33]. As the disease progresses, the microenvironment promotes inflammatory responses, oxidative stress, advanced glycation end product (AGE) production, and renin-angiotensin-aldosterone system (RAAS) activation. These pathophysiologic abnormalities promote cardiac stiffness, hypertrophy, and fibrosis, leading to diastolic dysfunction, systolic dysfunction, and HF. Below, we discuss the role of macrophages in these pathophysiologic mechanisms, as illustrated in Fig. 2.
Fig. 2.
Schematic diagram of the mechanisms involved in the development of DHD by macrophages. The hyperglycemic environment in diabetic patients induces CM injury, and necrotic CMs activate mast cells by releasing DAMPs, which induce the secretion of TNF-α and IL-1β, leading to the activation of ECs. Activated ECs induce monocytes to differentiate into CCR2+ macrophages and CCR2-macrophages; the former create an inflammatory environment and remove necrotic tissue, while the latter release IL-10 and TGF-β1, which activate fibroblasts to differentiate into myofibroblasts and produce ECM and collagen for tissue repair. The hyperglycemic environment also directly activates ECs, which secrete cytokines and chemokines that induce macrophage polarization to the M1 phenotype. In turn, M1 macrophages secrete inflammatory factors that promote cardiac inflammatory responses and insulin resistance. In addition, a hyperglycemic environment leads to increased production of AGEs, and the overproduction of AGEs promotes ROS production. The hyperglycemic environment can also act on HMGB1, gradually activating their downstream reactions, leading to increased ROS generation, and exacerbating oxidative stress. Furthermore, ATMs can secrete miRNA-containing exosomes that act on insulin-target cells to develop insulin resistance. Abbreviations: CM: cardiomyocyte, DAMP: danger-associated molecular pattern, TNF-α: tumor necrosis factor-α, IL: interleukin, EC: endothelial cell, TGF-β1: transforming growth factor-β1, ECM: extracellular matrix, AGE: advanced glycation end product, ROS: reactive oxygen species, HMGB1: high mobility group box 1, ATM: adipose tissue macrophage, NF-κB: nuclear factor kappa-B, NLRP3: NLR family pyrin domain-containing protein 3, TLR4: toll-like receptor 4
Cardiac inflammation
DM is characterized by hyperglycemia and insulin resistance, which promote an inflammatory state in the body [9]. The inflammatory response is a critical mechanism by which the heart responds to injury and undergoes adaptive remodeling [34]. The hyperglycemic environment in patients with DM affects protein glycation and the production of AGEs, which activate the nuclear factor kappa B (NFκB) pathway in macrophages. This activation, in turn, induces the production of inflammatory cytokines, leading to the activation of macrophages toward an inflammatory phenotype [35–37]. The dysregulation of macrophages between pro-inflammatory and anti-inflammatory phenotypes promotes excessive inflammation and cardiac injury [38]. In individuals with diabetes, macrophages secrete cytokines and chemokines that play a role in developing cardiomyocyte hypertrophy and extracellular matrix (ECM) remodeling. Stimulated by prolonged hyperglycemia, pro-inflammatory macrophages and lymphocytes accumulate and infiltrate into the heart, promoting the secretion of cytokines, such as tumor necrosis factor (TNF), IL-6, IL-1β, interferon-γ (INF-γ), and transforming growth factor-β (TGF-β), which can induce or exacerbate cardiac injury, leading to further adverse remodeling and myocardial fibrosis [39–42].
High mobility group box 1 (HMGB1) is a nuclear chromatin protein that interacts with nucleosomes, transcription factors, and histones to promote the transcription of many genes associated with inflammatory processes [43, 44]. More precisely, in a hyperglycemic environment, HMGB1 can activate toll-like receptor 4 (TLR4) [45]. TLR4 is expressed in cardiac inflammatory cells, cardiac fibroblasts, and cardiomyocytes [46], and its role in mediating inflammatory signaling in the development of DHD has been demonstrated in animal models [47–50].
Insulin resistance
Inflammation can lead to the development of insulin resistance. Studies have shown that inhibiting inflammatory response pathways can greatly enhance insulin sensitivity in both animals and humans [51, 52]. TNF-α and monocyte chemoattractant protein 1 (MCP1) play important roles in this process. TNF-α is involved in insulin resistance by activating inflammatory kinases, including c-Jun N-terminal kinase (JNK) [53] and inhibitor kappa B kinase (IKK) [54]. Previous studies have shown that increased TNF-α and macrophage levels in the adipose tissue (AT) of obese mice and humans are associated with insulin resistance [55–57]. Macrophages, especially adipose tissue macrophages (ATMs), which are a significant source of pro-inflammatory cytokines, contribute to reduced insulin sensitivity in a paracrine and potentially endocrine manner [58]. Reduction of ATMs or TNF-α by their depletion or ablation of MCP1 or its receptor in mice was associated with improved insulin sensitivity [59–61]. In addition, hepatic insulin resistance is observed with increased TNF-α expression in AT when macrophages are recruited to AT by the overexpression of MCP1. Moreover, knockout of MCP1 protected against high-fat-diet (HFD)-induced insulin resistance [60].
ATMs can also affect insulin sensitivity and glucose homeostasis via non-inflammatory pathways. Obesity triggers lysosome biogenesis, resulting in lipid catabolism and buildup of ATMs, specifically of CD11c+ macrophages [62] or Trem2+ macrophages [63]. Deficiency of Trem2 in mice on HFD reduces lipid accumulation in Trem2+ ATMs. These macrophages contribute to aggravated glucose intolerance [63]. Consistently, lipid storage in ATMs can regulate systemic glucose homeostasis and insulin sensitivity [64].
Myocardial fibrosis
Cardiac fibrosis is an unavoidable consequence of chronic myocardial injury and is characterized by the accumulation of ECM proteins in the cardiac interstitium [65]. Intracellular signaling and crosstalk between resident cardiac macrophages and other cells play a crucial role in the onset, propagation, and progression of cardiac inflammation, tissue remodeling, and myocardial fibrosis [66]. The two main types of resident cardiac macrophages are C-C motif chemokine receptor 2 (CCR2)+ and CCR2- macrophages. CCR2+ macrophages maintain their numbers primarily through monocyte recruitment and are mainly involved in pro-inflammatory responses, whereas CCR2- macrophages are primarily embryonic in origin and play a role in promoting angiogenesis and tissue repair [67]. Early in myocardial injury, cardiomyocytes undergo necrosis and release endogenous danger-associated molecular patterns (DAMPs). DAMPs activate mast cells to release pro-inflammatory cytokines, such as TNF-α and IL-1β, as well as chemokines, which activate endothelial cells and recruit activated CCR2+ monocytes and neutrophils. CCR2+ monocytes differentiate into macrophages at the site of injury and work with neutrophils to create an inflammatory environment and remove necrotic tissue [68]. In contrast to CCR2 + macrophage function, CCR2- macrophages are activated in response to myocardial injury and release IL-10 and TGF-β1 [65], which activate fibroblasts to differentiate into myofibroblasts that produce ECM and collagen for tissue repair. However, cardiomyocyte death and increased cardiac inflammation lead to enhanced activation of myofibroblasts and uncontrolled production of ECM and collagen, which accumulate in damaged cardiac tissue and eventually lead to myocardial fibrosis [69, 70].
In addition, the imbalance of M1 and M2 macrophages may be an important factor in promoting myocardial fibrosis. In the context of DHD, AGEs have the ability to direct macrophages toward a pro-inflammatory M1 phenotype, which leads to myocardial injury and, ultimately, myocardial fibrosis [71].
Oxidative stress
Oxidative stress occurs from an imbalance in the generation of free radicals and antioxidants [72]. The overproduction of ROS is believed to be a central mechanism of cardiac inflammation and remodeling [73, 74] and contributes to oxidative stress in both the early and late stages of DCM [75, 76]. In a hyperglycemic environment, HMGB1 can activate TLR4 [45]. Activation of TLR4 leads to the activation of NFκB and the NLR family pyrin domain-containing protein 3 (NLRP3) inflammasome, which recruits procaspase-1 [77, 78]. Activated caspase-1 serves as an enhancer of multiple pro-inflammatory pathways involving NFκB, chemokines, and ROS. An NFκB positive-feedback loop further increases NLRP3 inflammasome assembly and procaspase-1 activation, which stimulates additional ROS production [78].
Another key enzyme in ROS production is NOX [79]. In the diabetic heart, hyperglycemia leads to aberrant activation of mitochondrial NOX, which exacerbates ROS production [80]. In addition, macrophages under hyperglycemic conditions have reduced glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity, leading to the increased formation of AGEs and their precursors [81]. AGEs can be released extracellularly and bind to AGE receptors (RAGEs) in an autocrine and paracrine manner, resulting in increased ROS production [82]. The interaction between AGEs and RAGEs also promotes M1 polarization by inducing the secretion of IL-6 and TNF-α, which further exacerbates ROS production [83].
Trained immunity
Both T1DM and T2DM are characterized by hyperglycemia, and the primary focus of treatment is to lower blood glucose levels. However, some studies have found that the risk of cardiovascular complications persists in some diabetic patients even after glucose-lowering therapy [84]. This phenomenon is referred to as a “legacy effect” or “metabolic memory” [85]. Innate myeloid cells, such as macrophages and monocytes, have the potential for enhanced responsiveness to secondary stimulation, a phenomenon referred to as “trained immunity” [86]. Trained immunity is the process by which innate immune cells undergo long-term functional reprogramming after brief exposure to a stimulus, which persists after removing that stimulus [87]. The “legacy effect” may be related to this “trained immunity” of macrophages. Edgar et al. [88] found that bone marrow-derived macrophages (BMDMs) cultured in a high-glucose environment had increased glycolysis and enhanced polarization toward the M1 phenotype. The BMDMs isolated from diabetic mice, when cultured at physiological glucose concentrations, had persistent pro-inflammatory status despite normalization of the external glucose concentration. Therefore, therapies that target macrophage-trained immunity may offer a potential approach to treating DHD.
Pharmacological targeting of macrophages in DHD
DHD includes both coronary artery disease (CAD) and DCM. Therapeutic strategies for CAD are well established, whereas therapeutic measures for DCM, in sharp contrast, are lacking. The plasticity and adaptability of macrophages in response to various stimuli make them attractive targets for pharmacotherapy. Next, we will summarize several drugs that modulate macrophage function and have the potential to treat DHD.
Sodium-glucose cotransporter 2 inhibitors (SGLT2is)
Sodium-glucose cotransporters (SGLTs) promote the reabsorption of blood glucose after glomerular filtration in the proximal tubules of the kidney [89]. Two types of SGLT have been identified: SGLT1 and SGLT2. SGLT2 regulates about 90% of glucose reabsorption, with the remainder accomplished by SGLT1 [90].
SGLT2is selectively block SGLT2 activity on the proximal renal tubule, leading to the removal of excess glucose in the urine and ultimately resulting in lower blood glucose levels. Additionally, SGLT2is reduce glycosylated proteins, improve insulin sensitivity, and enhance beta cell function [91]. SGLT2is were first introduced as medications for managing diabetes. They were also shown to provide benefits, such as reducing body weight and decreasing blood pressure [92]. More critically, treatment with SGLT2is has been shown to reduce the risk of cardiovascular disease in patients with T2DM when compared with placebo treatment [93, 94]. There is much evidence to suggest that SGLT2is may have the potential to reduce inflammatory responses [95–98], myocardial fibrosis [95], insulin resistance [99, 100], and oxidative stress [101, 102], making them a promising treatment option for DHD. SGLT2is-related clinical studies are shown in Table 1; SGLT2is-related preclinical studies are shown in Table 2.
Table 1.
Clinical studies of SGLT2is for the treatment of DM or DM-related diseases
| Medicine | Generic Name | First Author | Year | Disease | Model | Findings | Ref. |
|---|---|---|---|---|---|---|---|
| SGLT2is | Dapagliflozin | Kato, E.T. | 2019 | T2DM/HFrEF | Human | Dapagliflozin reduced cardiovascular and all-cause mortality in patients with high rates of HFrEF and in patients with T2DM | [93] |
| Furtado, R.H.M. | 2019 | T2DM/MI | Human | Dapagliflozin reduced risk of MACE and cardiovascular death/hospitalization for HF in patients with T2DM and prior MI | [94] | ||
| McMurray, J.J.V. | 2019 | HFrEF | Human | Dapagliflozin reduced risk of worsening HF or death from cardiovascular disease in patients with HErEF | [103] | ||
| Wiviott, S.D. | 2019 | T2DM | Human | Dapagliflozin reduced cardiovascular mortality and hospitalization for HF in patients with T2DM | [104] | ||
| Empagliflozin | Zinman, B. | 2015 | T2DM | Human | Empagliflozin reduced incidence of major composite cardiovascular outcomes and death from any cause in patients with T2DM, at high risk for cardiovascular events | [105] | |
| Iannantuoni, F. | 2019 | T2DM | Human | Empagliflozin reduced inflammation and oxidative stress in patients with T2DM | [96] | ||
| Canagliflozin | Neal, B. | 2017 | T2DM | Human | Canagliflozin reduced incidence of cardiovascular and renal events in patients with T2DM | [106] |
HFrEF heart failure with reduced ejection fraction, MI myocardial infarction, MACE major adverse cardiovascular events
Table 2.
Preclinical studies of SGLT2is for the treatment of DM or DM-related diseases
| Medicine | Generic Name | First Author | Year | Disease | Model | Findings | Ref. |
|---|---|---|---|---|---|---|---|
| SGLT2is | Dapagliflozin | Lee, T.M. | 2017 | MI | Rat | Dapagliflozin attenuated myocardial fibrosis in rats after MI | [95] |
| Terami, N. | 2014 | T2DM | Mouse | Dapagliflozin significantly reduced macrophage infiltration and expression of inflammation and oxidative stress genes in the kidneys of T2DM mice | [101] | ||
| Ye, Y. | 2017 | DCM | Mouse | Dapagliflozin inhibited Nlrp3/ASC inflammasome activation and attenuated DCM in T2DM mice | [107] | ||
| Empagliflozin | Xu, L. | 2017 | Obesity | Mouse | Empagliflozin improved insulin sensitivity | [99] | |
| Oelze, M. | 2014 | T1DM | Rat | Empagliflozin ameliorated oxidative stress and inflammatory response in T1DM rats | [102] | ||
| Kern, M. | 2016 | DM | Mouse | Empagliflozin improved insulin sensitivity in DM mice | [108] | ||
| Habibi, J. | 2017 | DM | Mouse | Empagliflozin improved cardiac diastolic function in a female mouse model of DM | [109] |
MI myocardial infarction, ASC apoptosis-associated speck-like protein containing a CARD
Glucagon-like peptide 1 receptor agonists (GLP1RAs)
Glucagon-like peptide 1 (GLP-1) is produced by the posttranslational proteolytic cleavage of proglucagon protein [110]. GLP-1 can enhance insulin secretion in a glucose-dependent manner by activating the GLP-1 receptor (GLP1R) that is highly expressed on islet β cells [111]. GLP1RAs, the drugs used to treat patients with diabetes, also provide benefits for patients with cardiovascular disease. Obesity and diabetes are both risk factors for cardiovascular disease. According to a study by Lazzaroni et al. [112], GLP1RAs significantly reduced body weight in patients with T2DM. A systematic review and meta-analysis of cardiovascular outcome trials of GLP1RAs showed that GLP1RA treatment reduced major adverse cardiovascular events (MACE) by 12% and reduced all-cause mortality by 12% [113]. Furthermore, in patients treated with GLP1RAs, HF admission rates were reduced by 9% [113]. However, in another meta-analysis, GLP1RAs had no significant effect on HF admission rates [114]. Thus, although the benefit of GLP1RAs for patients with cardiovascular disease has been established, the benefits are less certain for patients with HF. Because advanced DHD is characterized by HF [32, 115], GLP1RAs may have little effect on patients with advanced DHD. However, whether GLP1RAs are effective in treating patients with early DHD or preventing the development of DHD warrants further investigation.
Preclinical studies have shown that GLP1RAs have the potential to treat DHD. The GLP1RA liraglutide increases the myocardial glucose oxidation rate and alleviates DCM in C57BL/6J mice [116]. Furthermore, liraglutide-treated rats with DCM showed reduced inflammation, myocardial fibrosis, and apoptosis [117]. Researchers showed that using the GLP1RA exenatide to treat mice with DCM significantly improved serum B-type natriuretic peptide, myocardial fibrosis, myocardial lipid deposition, and echocardiographic parameters [7]. Other researchers showed that treatment with the novel oral GLP1RA oral hypoglycemic peptide 2 (OHP2) prevents DCM in rats by alleviating cardiac lipotoxicity-induced mitochondrial dysfunction [118].
GLP1RAs treatment of DCM may be closely related to their interactions with macrophages. Under pathological stress, macrophages contribute to an excessive inflammatory response, which leads to insulin resistance and diabetes. GLP1RAs can attenuate macrophage infiltration and inhibit the expression of IL-1β, IL-6, and TNF-α [119]. In addition, GLP1RAs can inhibit macrophage polarization to the M1 pro-inflammatory phenotype [120] and promote macrophage polarization to the M2 anti-inflammatory phenotype [121, 122]. The large amount of pro-inflammatory cytokines and chemokines in adipose tissue is a key factor contributing to insulin resistance in patients with T2DM [60, 123, 124]. GLP1RAs inhibit inflammatory mediators in adipose tissue and contribute to improved insulin sensitivity [125]. Moreover, GLP1RAs demonstrate a direct protective effect on the development of diabetes-associated myocardial fibrosis and diastolic dysfunction [126]. Taken together, GLP1RAs contribute to relieving DHD by modulating macrophage function. GLP1RAs-related clinical studies are shown in Table 3; GLP1RAs-related preclinical studies are shown in Table 4.
Table 3.
Clinical studies of GLP1RAs for the treatment of DM or DM-related diseases
| Medicine | Generic Name | First Author | Year | Disease | Model | Findings | Ref. |
|---|---|---|---|---|---|---|---|
| GLP1RAs | Dulaglutide | Gerstein, H.C. | 2020 | T2DM | Human | Long-term dulaglutide use might reduce clinically relevant ischemic stroke in people with T2DM | [127] |
| Tuttolomondo, A. | 2021 | T2DM | Human | Positive effects on arterial stiffness and endothelial function indicators in patients with T2DM receiving conventional therapy with daily subcutaneous injections of 1.5 mg dulaglutide | [128] | ||
| Liraglutide | Marso, S.P. | 2016 | T2DM | Human | The composite endpoint of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke was significantly lower in T2DM patients at high cardiovascular risk | [129] | |
| Semaglutide | Husain, M. | 2019 | T2DM | Human | The cardiovascular risk profile of patients with T2DM taking oral semaglutide was not worse than those taking placebo | [130] | |
| Strain, W.D. | 2022 | T2DM | Human | Semaglutide treatment reduced stroke risk in patients with T2DM and higher cardiovascular risk compared with placebo treatment | [131] | ||
| Efpeglenatide | Gerstein, H.C. | 2021 | T2DM | Human | Patients with T2DM who received weekly subcutaneous doses of 4 or 6 mg of efpeglenatide had a lower risk of cardiovascular events than those on placebo | [132] | |
| Albiglutide | Hernandez, A.F. | 2018 | T2DM | Human | In patients with T2DM and cardiovascular disease, albiglutide was superior to placebo with respect to major adverse cardiovascular events | [133] |
Table 4.
Preclinical studies of GLP1RAs for the treatment of DM or DM-related diseases
| Medicine | Generic Name | First Author | Year | Disease | Model | Findings | Ref. |
|---|---|---|---|---|---|---|---|
| GLP1RAs | Liraglutide | Almutairi, M. | 2021 | DCM | Mouse | Liraglutide increased myocardial glucose oxidation and mitigated DCM | [116] |
| Trang, N.N. | 2021 | DCM | Rat | Liraglutide reduced inflammation, myocardial fibrosis, and apoptosis in DCM rats | [117] | ||
| Exenatide | Fang, P. | 2023 | DCM | Mouse | Exenatide significantly improved serum BNP, myocardial fibrosis, myocardial lipid deposition, and echocardiographic parameters in DCM mice | [7] | |
| OHP2 | Qian, P. | 2020 | DCM | Rat | OHP2 prevented DCM by alleviating cardiac lipotoxicity-induced mitochondrial dysfunction | [118] | |
| Recombinant adenovirus producing GLP-1 | Lee, Y.S. | 2012 | DM/ Obesity | Mouse | Recombinant adenovirus producing GLP-1 improved insulin sensitivity | [125] | |
| Exendin-4 | Tate, M. | 2016 | DM | Mouse | Exendin-4 had a protective effect on the development of diabetes-related myocardial fibrosis and diastolic dysfunction | [126] |
BNP brain natriuretic peptide, OHP2 oral hypoglycemic peptide 2
Metformin
Metformin is a first-line medication for treating patients with T2DM [134] and works by decreasing the production of glucose in the liver and activating the adenosine monophosphate-activated protein kinase (AMPK). Because metformin activates AMPK, it may also induce the regression of myocardial hypertrophy [31]. In addition, metformin enhances insulin sensitivity by increasing the activity of insulin receptor tyrosine kinase, thereby promoting glycogen synthesis and improving the recruitment and activity of the glucose transporter 4 (GLUT4) [135]. In a mouse model of transverse aortic constriction (TAC)-induced HF, treatment with metformin attenuated myocardial fibrosis by inhibiting the TGFβ1-Smad3 signaling pathway [136]. In addition, clinical studies have shown that metformin treatment reduced the incidence of HF in patients with diabetes [137, 138]. Collectively, this evidence suggests that metformin has potential for use in the treatment of DHD.
Studies have shown that metformin provides benefits by its effects on macrophages. In human macrophages, treatment with metformin selectively inhibited the differentiation of human monocytes into pro-inflammatory M1 macrophages [139]. In addition, LPS stimulated M2 macrophages to produce ROS that are harmful to surrounding tissues, a process that was inhibited by the addition of metformin [139]. Interestingly, metformin reduces oxidative stress and inflammatory responses by inhibiting the differentiation of human monocytes into M1 macrophages and limiting macrophage ROS production through the activation of AMPK [139]. Metformin can also exert anti-inflammatory effects by modulating the AMPK/mTOR signaling pathway to inhibit activation of the NLRP3 inflammasome and favor macrophage polarization toward the M2 phenotype [140]. These findings suggest that metformin acts as a cardioprotective and anti-inflammatory agent by stimulating AMPK/autophagy and thus inhibiting the NLRP3 inflammasome, which is closely associated with macrophages in DHD [141].
HMGB1 is released by necrotic cells and is a potential target for the development of anti-inflammatory therapies [142]. Metformin was found to significantly reduce the inflammatory response of LPS-stimulated macrophages in mice (in vivo) and in RAW 264.7 cells (in vitro) by inhibiting HMGB1 secretion [143]. Guo et al. [144] showed that metformin alleviated olanzapine-induced insulin resistance by inhibiting macrophage infiltration and polarization-mediated inflammatory responses in white adipose tissue of rat epididymis. Cortés et al. [145] found that the anti-inflammatory and inhibitory effects of metformin on ROS were dependent on the expression of the plasticity factor ZEB1 in macrophages. Therefore, metformin may potentially be used to treat DHD. Metformin-related clinical studies are shown in Table 5; metformin-related preclinical studies are shown in Table 6.
Table 5.
Clinical studies of metformin for the treatment of DM or DM-related diseases
| Medicine | First Author | Year | Disease | Model | Findings | Ref. |
|---|---|---|---|---|---|---|
| Metformin | van der Aa, M.P. | 2016 | Obesity | Human | Long-term treatment of obese and insulin-resistant adolescents with metformin stabilized BMI and reduced insulin resistance compared with placebo | [134] |
| Hippisley-Cox, J. | 2016 | T2DM | Human | Combination therapy with metformin and gliptins reduced risk of HF in patients with T2DM | [137] | |
| Panagiotopoulou, O. | 2020 | Obesity | Human | Children of obese mothers who were exposed prenatally to metformin compared with those who were exposed to placebo had lower central hemodynamic and cardiac diastolic indices | [146] | |
| Timmons, J.G. | 2021 | T1DM | Human | Metformin reduced carotid intima-media thickness in never-smoking patients with T1DM | [147] | |
| Eurich, D.T. | 2013 | DM/HF | Human | Metformin was safe for treating patients with DM combined with HF and did not increase the risk of lactic acidosis | [148] | |
| Bhansali, S. | 2020 | T2DM | Human | Metformin upregulated mitochondrial autophagy in patients with T2DM, resulting in improved mitochondrial morphology and function independent of its glucose-lowering effect | [149] |
BMI body mass index
Table 6.
Preclinical studies of metformin for the treatment of DM or DM-related diseases
| Medicine | First Author | Year | Disease | Model | Findings | Ref. |
|---|---|---|---|---|---|---|
| Metformin | Xiao, H. | 2010 | HF | Mouse | Metformin attenuated myocardial fibrosis by inhibiting the TGFβ1-Smad3 signaling pathway | [136] |
| Nassif, R.M. | 2022 | Inflammation | Mouse | Metformin inhibited ROS production by activating AMPK | [139] | |
| Yang, F. | 2019 | DCM | Mouse | Metformin activated AMPK, improving autophagy by inhibiting the mTOR pathway and alleviating focal death in DCM | [150] | |
| Tsoyi, K. | 2011 | Endotoxemia | Mouse | Metformin improved survival in a mouse model of lethal endotoxemia by inhibiting HMGB1 release | [151] |
mTOR mammalian target of rapamycin
Renin-angiotensin-aldosterone system inhibitors (RAASis)
Activation of the RAAS in patients with diabetes leads to inflammation, cardiac fibrosis, and oxidative stress, all of which contribute to cardiac remodeling and can be reversed or prevented by RAAS blockade [152, 153]. RAASis include angiotensin-converting enzyme inhibitors (ACEis), angiotensin II receptor blockers (ARBs), renin inhibitors, and mineralocorticoid receptor antagonists (MRAs) [154]. ACEis reduce cardiovascular disease incidence and all-cause mortality and increase cellular insulin sensitivity in patients with diabetes [155]. In a rat model of ischemic cardiomyopathy, the ARB valsartan attenuates TLR activity, inhibits NFκB activity, and reduces circulating cytokine levels [156]. Candesartan, another ARB, ameliorates abnormal local calcium release from cardiomyocytes in the atrial tissue of rats with DCM [157]. Renin inhibitors, such as aliskiren, improve left ventricular hypertrophy and end-systolic volume in patients with diabetes [158, 159]. These studies suggest that RAASis have the potential to treat DHD. The activation of RAAS contributes to the infiltration of macrophages, and the antagonistic macrophage mineralocorticoid receptors have a significant protective effect on cardiovascular remodeling [160]. Activated macrophages produce angiotensin-converting enzyme (ACE), which induces local expression of angiotensin II (Ang II). The ability of ACEis to reduce left ventricular mass and ameliorate myocardial fibrosis suggests a direct link between macrophages, macrophage-derived ACE, myofibroblasts, and left ventricular remodeling [161]. Studies have shown that aldosterone induces galectin-3 expression in macrophages and vascular endothelial cells that leads to vascular and cardiac fibrosis, implying a correlation between RAAS and macrophages [162]. In addition, adipose-infiltrating macrophages have been shown to secrete pro-inflammatory cytokines, such as IL-6 and TNF-α, which trigger activation of the RAAS [163]. In summary, a positive-feedback mechanism may exist between macrophages and the RAAS, leading to mutual activation; therefore, RAASis may indirectly inhibit macrophage hyperactivation, resulting in a therapeutic effect on DHD. RAASis-related clinical studies are shown in Table 7; RAASis-related preclinical studies are shown in Table 8.
Table 7.
Clinical studies of RAASis for the treatment of DM or DM-related diseases
| Medicine | Generic Name | First Author | Year | Disease | Model | Findings | Ref. |
|---|---|---|---|---|---|---|---|
| RAASis | Aliskiren, Losartan | Solomon, S.D. | 2009 | Hypertension | Human | Aliskiren and losartan attenuated myocardial end-organ damage effectively | [158] |
| Aliskiren | Shah, A.M. | 2012 | DM/MI | Human | Aliskiren improved left ventricular hypertrophy and end-systolic volume in patients with DM | [159] | |
| Captopril | Hansson, L. | 1999 | Hypertension/DM | Human | Captopril reduced the propensity to develop T2DM by 11% in hypertensive patients | [164] | |
| Ramipril | Yusuf, S. | 2000 | Hypertension/DM | Human | Ramipril reduced the propensity to develop T2DM by 34% in hypertensive patients | [165] | |
| ARBs | Lambers Heerspink, H.J. | 2012 | T2DM | Human | Moderation of dietary sodium potentiated the renal and cardiovascular protective effects of ARBs | [166] |
Table 8.
Preclinical studies of RAASis for the treatment of DM or DM-related diseases
| Medicine | Generic Name | First Author | Year | Disease | Model | Findings | Ref. |
|---|---|---|---|---|---|---|---|
| RAASis | Lisinopril | Fiordaliso, F. | 2006 | DM | Rat | Lisinopril reduced cardiovascular oxidative stress in DM rats | [152] |
| Olmesartan | Matsusaka, H. | 2006 | DM/MI | Mouse | Olmesartan improved left ventricular remodeling and failure after MI in DM mice | [153] | |
| Valsartan | Yang, J. | 2009 | MIRI | Rat | Valsartan prevented myocardial ischemia-reperfusion injury in rats via TLR4/NF-κB signaling pathway | [156] | |
| Candesartan | Yaras, N. | 2007 | DCM | Rat | Candesartan protected cardiomyocytes from adverse effects associated with abnormal Ca2+ release mechanisms in DCM rats | [157] |
MIRI myocardial ischemia-reperfusion injury
β2-adrenergic receptor agonists (β2ARAs)
G protein–coupled receptors (GPCRs) are important proteins that mediate most cellular responses to external stimuli [167]. Adrenergic receptors (ARs), belonging to the GPCR family, contain both α and β subtypes. β ARs can be further subdivided into β1, β2, and β3 ARs, which are located on the surface of effector cells. β2AR has been extensively studied and is found predominantly in human smooth muscle, where it regulates a variety of physiologic processes [168, 169]. Many β2ARAs have been developed. Because β2ARAs induce relaxation of airway smooth muscle, they are used to treat various respiratory diseases, particularly asthma and chronic obstructive pulmonary disease (COPD) [170]. However, many potential uses for β2ARAs remain to be explored, such as in the treatment of DHD.
In a screening of 1040 compounds with anti-inflammatory effects in rat bone marrow macrophages, researchers identified β2ARA as the most potent compound in inhibiting NFκB-dependent pro-inflammatory TNF-α production by macrophages [171]. Subsequently, they found that β2ARAs inhibited TNF-α production in peripheral blood mononuclear cells of streptozotocin (STZ)-induced diabetic rats [171]. To elucidate the mechanism, they exposed human monocytic leukemia cells and bone marrow macrophages to a high-glucose environment. High glucose reduced the expression of β-arrestin2, a negative regulator of NFκB activation, and its interaction with IκBα, subsequently enhancing the phosphorylation of IκBα and the activation of NFκB. The addition of β2ARAs enhanced the expression of β-arrestin2 and its interaction with IκBα, which led to the downregulation of NFκB. siRNA specific for β-arrestin2 reversed the β2ARA-mediated inhibition of NFκB activation and inflammatory cytokine production. In addition, Zucker diabetic fatty (ZDF) rats treated with the β2ARA salbutamol for 12 weeks showed attenuated monocyte activation, as well as pro-inflammatory and profibrotic responses in the kidneys and heart [172]. Several other studies [171, 173] have shown beneficial effects of β2ARAs in treating DM and its complications. Notably, inhibiting macrophage activation and cardiomyopathy progression with β2ARAs only occurs under hyperglycemic conditions and not normoglycemic conditions [174]. In summary, β2ARAs may be promising drugs for treating DHD. β2ARAs-related clinical studies are shown in Table 9; β2ARAs-related preclinical studies are shown in Table 10.
Table 9.
Clinical studies of β2ARAs for the treatment of DM or DM-related diseases
| Medicine | Generic Name | First Author | Year | Disease | Model | Findings | Ref. |
|---|---|---|---|---|---|---|---|
| β2ARAs | Zinterol | Kaumann, A. | 1999 | HF | Human | Zinterol improved cardiac diastolic function | [175] |
| Caveolin-3 | Gong, J. | 2020 | DM/MIRI | Human | Caveolin-3 protected diabetic hearts against I/R damage through the β2AR, cAMP/PKA, and BDNF/TrkB signaling pathways | [176] |
BDNF brain-derived neurotrophic factor, cAMP cyclic adenosine monophosphate, I/R ischemia reperfusion, PKA protein kinase A, TrkB tropomyosin-related kinase receptor type B
Table 10.
Preclinical studies of β2ARAs in DM or DM-related diseases
| Medicine | Target/Drug Name | First Author | Year | Disease | Model | Findings | Ref. |
|---|---|---|---|---|---|---|---|
| β2ARAs | β2ARA | Noh, H. | 2017 | DM | Rat | β2ARA attenuated monocyte activation and pro-inflammatory and pro-fibrotic responses in the kidney and heart | [172] |
| Thalidomide | Zhang, H. | 2018 | DM | Rat | Thalidomide may have therapeutic potential for diabetic kidney injury through an anti-inflammatory pathway | [173] | |
| Leukocyte-expressed β2AR | Grisanti, L.A. | 2016 | MI | Rat | Leukocyte-expressed β2AR played an important role in regulating the early inflammatory repair response to acute myocardial injury by promoting cardiac leukocyte infiltration | [174] | |
| Clenbuterol | van Beek, S.M.M. | 2021 | T2DM | Mouse | Clenbuterol improved whole-body glucose homeostasis | [177] |
Potential therapeutic strategy
Targeting MicroRNAs (MiRNAs)
MicroRNAs (miRNAs) are small RNA molecules that are important regulators of different cellular processes. MiRNAs control gene expression at the post-transcriptional level by disassembling or inhibiting the translation of target messenger RNAs (mRNAs) by binding to their 3’-untranslated region (3’UTR) [178].
MiRNAs play important roles in many aspects of macrophage biology, particularly in immune cells, such as monocytes and macrophages [179]. They regulate polarization, differentiation, inflammation, and phagocytosis [180]. For example, miR-720 and miR-127 promote M1 macrophage polarization and suppress M2 polarization by targeting GATA binding protein 3 (GATA3) and B-cell lymphoma 6 (BCL6), respectively, which are critical for M2 polarization [181, 182]. MiR-155 promotes M1 polarization. Both gain-of-function and loss-of-function studies in vivo have shown that miR-155 is necessary for the typical development of the macrophage inflammatory state [183]. Abdominal macrophages that overexpress miR-146a showed an increase in M2-type marker genes (e.g., cluster of differentiation 206 (CD206), arginase 1 (ARG1), C-C motif chemokine ligand 22 (CCL22), and CCL17) and a decrease in M1-type phenotypic markers (e.g., inducible nitric oxide synthase, IL-12, IL-6, TNF, and CD86) [184], indicating that miR-146a may have anti-inflammatory effects.
MiRNAs have been shown to be involved in the process of developing DCM. Myocyte enhancer factor 2 C (MEF2C) is a key transcription factor in promoting cardiomyocyte hypertrophy [185]. In mice and rats with DCM, both miR-133a and miR-373 are involved in MEF2C signaling, leading to cardiomyocyte hypertrophy and mediating cardiac fibrosis through the activation of the p300 gene [185, 186]. In addition, miR-208a [187] and miR-451 [188] are involved in cardiomyocyte hypertrophy. Liu et al. [189] showed that miR-21 levels were significantly elevated in cardiac fibroblasts treated with high glucose, leading to increased collagen synthesis and elevated phosphorylated p38 mitogen-activated protein kinase (MAPK). In addition, inhibiting miR-21 by blocking the activation of the p38 signaling pathway reduced fibrosis, suggesting that miR-21 plays a critical role in DCM. Moreover, miRNAs are associated with oxidative stress in rats with DCM. The significant downregulation of miR-499, miR-1, and miR-133 was observed in high glucose–treated cardiomyocytes, which was reversed by treatment with the antioxidant N-acetylcysteine, suggesting that the downregulation of these miRNAs in the diabetic heart is caused by oxidative stress [190].
Perhaps more importantly, miRNAs modulate macrophage polarization that occurs during the development of DCM. Wang and colleagues showed that miR-657 regulates inflammation and insulin resistance in patients with diabetes by targeting the FAM46C gene to promote macrophage polarization toward M1 [191]. A study discovered that miR-223, a significant regulator of macrophage polarization, suppresses macrophage activation toward a pro-inflammatory phenotype by inhibiting Pknox1. This prevents high-fat-diet-induced adipose tissue inflammatory response and systemic insulin resistance in mice [192]. Thus, miRNAs may control the inflammatory response and insulin resistance by regulating macrophage polarization. Furthermore, miRNAs may be involved in the pathogenesis of DCM by regulating genes related to cardiomyocyte hypertrophy, oxidative stress, and cardiac fibrosis. Together, these findings suggest that targeting miRNAs may be a novel therapeutic strategy for the treatment of DCM. MicroRNAs-related preclinical studies are shown in Table 11.
Table 11.
Preclinical studies of microRNAs in DM or DM-related cardiovascular diseases
| MicroRNAs | First Author | Year | Disease | Model | Findings | Ref. |
|---|---|---|---|---|---|---|
| MiR-471-3p | Liu, G. | 2021 | DCM | Mouse | The development of DCM was related to AGE-induced macrophage polarized to M1 type through a mechanism involving the miR-471-3p/SIRT1 pathway | [71] |
| MiR-155 | Fitzsimons, S. | 2020 | Atherosclerosis | Mouse | MiR-155 suppressed anti-inflammatory signaling in macrophages, and it has potential as a prognostic indicator and a therapeutic target | [193] |
| Jia, C. | 2017 | DCM | Mouse | Therapeutically reducing miR155 in macrophages by AuNP can serve as a promising strategy in improving cardiac function | [194] | |
| MiR-21/99a/146b/378a, MiR-33 | Phu, T.A. | 2022 | DM | Mouse | THP1-IL4-exo polarized primary macrophages to an anti-inflammatory phenotype and reprogramed their energy metabolism by increasing levels of miR-21/99a/146b/378a while reducing miR-33 | [195] |
| MiR-99a/146b/378a | Bouchareychas, L. | 2020 | Atherosclerosis | Mouse | BMDM-exo contain anti-inflammatory microRNA-99a/146b/378a. These exosomal microRNAs suppressed inflammation by targeting NF-κB and TNF-α signaling and fostering M2 polarization in recipient macrophages | [196] |
| MiR-27-3p | Li, J. | 2023 | T2DM | Mouse | Inactivation of miR-27-3p induced by M1 exosomes prevented T2DM development in high-fat-diet-fed mice | [197] |
| MiR-330-5p | Sun, J. | 2018 | T2DM | Mouse | MiR-330-5p/Tim-3 axis downregulated insulin resistance in diabetes, probably through enhancing the M2 polarization of macrophage | [198] |
| MiR-32 | Cao, J. | 2022 | T2DM, Vascular Calcification | Mouse | Extracellular vesicle miR-32 derived from macrophage promoted arterial calcification in mice with T2DM via inhibiting VSMC autophagy | [199] |
| MiR-92a | Chang, Y. | 2019 | Atherosclerosis | Mouse | Extracellular miR-92a can be transported to macrophages through extracellular vesicles to regulate KLF4 levels, thus leading to the atheroprone phenotypes of macrophage | [200] |
| MiR-130b | Zhang, M. | 2016 | T2DM | Mouse | MiR-130b was a novel regulator of macrophage polarization and contributed to adipose tissue inflammation and insulin tolerance | [201] |
| MiR-126 | Suresh Babu, S. | 2016 | DM | Mouse | In vitro efferocytosis of ACM was impaired in macrophages from diabetic mice, and miR-126 overexpression rescued diabetes-induced impairment in efferocytosis of ACM | [202] |
ACM apoptotic cardiomyocytes, AuNP gold nanoparticle, BMDM-exo exosomes produced by naive bone marrow-derived macrophages, KLF4 Krüppel-like factor 4, SIRT1 silent information regulator 1, THP1-IL4-exo human THP-1 macrophages exposed to IL-4 as a source of exosomes, Tim-3 T cell immunoglobulin domain and mucin domain-3, VSMC vascular smooth muscle cell
Melatonin
Melatonin, an endogenous indoleamine hormone with potential free radical scavenging ability, is synthesized and secreted by the pineal gland in mammals and is primarily involved in physiological activities associated with the light-dark cycle [203]. Because of their wide distribution in the body, melatonin receptors exhibit a variety of biological activities beyond antioxidant activity, such as anti-inflammatory effects [204] and regulation of insulin secretion [205]. Fiorina et al. [206] showed that changes in melatonin secretion are related to the immune status of the body. In addition, melatonin may protect against cardiac complications in patients with DM by attenuating apoptotic pathways and addressing the inflammatory response and ROS burden by promoting macrophage polarization toward an anti-inflammatory state [207]. Further studies on melatonin-targeted regulation of macrophages in DHD should be explored.
Conclusion
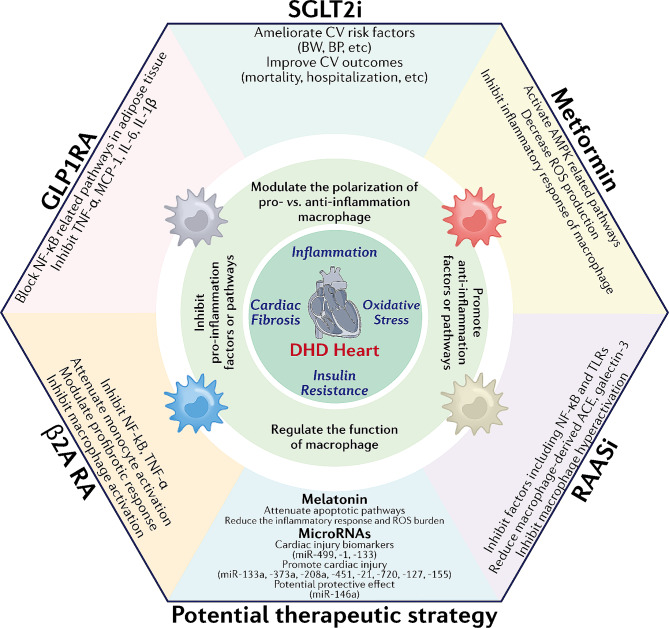
DHD is a serious complication of DM, posing a significant global health burden. Decades have passed since the discovery of DHD, but limited progress has been made in elucidating the mechanisms and identifying the therapeutic targets of DHD. Macrophages are crucial immune cells that play an indispensable role in maintaining normal physiological functions and resisting diseases. However, their role in the development of DHD has rarely been studied. In this review, we emphasized the significant role of macrophages in the pathogenesis of DHD. Macrophages play a major role in the development of DHD through mechanisms that primarily cause cardiac inflammation, insulin resistance, myocardial fibrosis, and oxidative stress. Additionally, the body’s hyperglycemic environment induces macrophage-trained immunity, which further contributes to the development of DHD. Finally, we present five classes of drugs that may have therapeutic effects on DHD by modulating macrophage function: SGLT2is, GLP1RAs, metformin, RAASis, and β2ARAs. Additionally, miRNAs may be a novel therapeutic for treating DHD, capable of targeting and modulating macrophages. In summary (Fig. 3), macrophages hold great promise as therapeutic targets for DHD and have opened an exciting avenue for developing novel treatments for DHD.
Fig. 3.
Summary schematic. Inflammation, myocardial fibrosis, insulin resistance, and oxidative stress are the four core mechanisms leading to DHD, and macrophages may play an essential role in these mechanisms. Five types of drugs—SGLT2is, GLP1RAs, metformin, RAASis, and β2ARAs—may have therapeutic potential by regulating macrophages. MiRNAs, which also regulate macrophages, may be involved in the pathogenesis of DHD and may also be potential targets for the treatment of DHD. In addition, melatonin is a promising research direction for treating DHD. Abbreviations: ACE: angiotensin-converting enzyme, AMPK: adenosine monophosphate-activated protein kinase, BW: body weight, BP: blood pressure, CV: cardiovascular, IL: interleukin, MCP-1: monocyte chemoattractant protein-1, NF-κB: nuclear factor kappa-B, ROS: reactive oxygen species, TLR: toll-like receptor, TNF-α: tumor necrosis factor-α
Acknowledgements
Nicole Stancel, PhD, ELS(D), and Shiladitya Sengupta, PhD, of the Department of Scientific Publications at The Texas Heart Institute contributed to the editing of this review article.
Abbreviations
- ACE
Angiotensin-converting enzyme
- ACEi
Angiotensin-converting enzyme inhibitor
- AGE
Advanced glycation end product
- AMPK
Adenosine monophosphate-activated protein kinase
- Ang
Angiotensin
- AR
Adrenergic receptor
- ARA
Adrenergic receptor agonist
- ARB
Angiotensin II receptor blocker
- ARG
Arginase
- ATM
Adipose tissue macrophage
- BCL
B-cell lymphoma
- BMDM
Bone marrow-derived macrophage
- CAD
Coronary artery disease
- CCR
C-C motif chemokine receptor
- CCL
C-C motif chemokine ligand
- CD
Cluster of differentiation
- COPD
Chronic obstructive pulmonary disease
- DAMP
Danger-associated molecular pattern
- DCM
Diabetic cardiomyopathy
- DHD
Diabetic heart disease
- DM
Diabetes mellitus
- ECM
Extracellular matrix
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- GLP1RA
Glucagon-like peptide 1 receptor agonist
- GLUT
Glucose transporter
- GPCR
G protein–coupled receptor
- HbA1c
Hemoglobin A1c
- HF
Heart failure
- HFrEF
Heart failure with reduced ejection fraction
- HMGB
High mobility group box
- IKK
Inhibitor kappa B kinase
- IL
Interleukin
- INF
Interferon
- JNK
c-Jun N-terminal kinase
- MACE
Major adverse cardiovascular event
- MAPK
Mitogen-activated protein kinase
- MCP
Monocyte chemoattractant protein
- M-CSF
Macrophage colony-stimulating factor
- MEF
Myocyte enhancer factor
- MRA
Mineralocorticoid receptor antagonist
- NFκB
Nuclear factor kappa B
- NLRP
NLR family pyrin domain-containing protein
- NOX
Nicotinamide adenine dinucleotide phosphate oxidase
- OHP
Oral hypoglycemic peptide
- RAAS
Renin-angiotensin-aldosterone system
- ROS
Reactive oxygen species
- SGLT
Sodium-glucose cotransporter
- TAC
Transverse aortic constriction
- Th
T helper
- TLR
Toll-like receptor
- TNF
Tumor necrosis factor
- 3'UTR
3'-untranslated region
- ZDF
Zucker diabetic fatty
Author contributions
C.Y.Z., A.S., and Y.K.S. conceived and designed the review; C.Y.Z. and Y.K.S. drafted and wrote the manuscript; A.S., S.M.S., Q.L., P.Y.L., and H.Y.C. reviewed and revised the manuscript; C.Y.Z., C.Z.L., Z.H., and Y.M.M. composed the figures and tables. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Special Foundation Projects of Joint Applied Basic Research of Yunnan Provincial Department of Science and Technology with Kunming Medical University, grant number 202301AY070001-119; the Natural Science Foundation, grant number 82270372; the Program Innovative Research Team in Science and Technology in Kunming Medical University, grant number 202405AS350014; and the Yunnan Health Training Project of High-Level Talents, grant number H-2019052.
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this article was revised: The errors in Tables 3 and 7 have been corrected.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chaoyue Zhang, Yunke Shi and Changzhi Liu contributed equally to this work.
Change history
6/29/2024
A Correction to this paper has been published: 10.1186/s12933-024-02300-4
Contributor Information
Ao Shi, Email: m1701040@sgul.ac.uk.
Hongyan Cai, Email: hyflykm@sina.com.
References
- 1.Sun H, et al. Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Standards of medical care in diabetes–2014 Diabetes Care, 2014. 37 Suppl 1: pp. S14-80. [DOI] [PubMed]
- 3.Nichols GA, et al. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes Care. 2001;24(9):1614–9. doi: 10.2337/diacare.24.9.1614. [DOI] [PubMed] [Google Scholar]
- 4.Dei Cas A, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail. 2015;3(2):136–45. doi: 10.1016/j.jchf.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Harding JL, et al. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62(1):3–16. doi: 10.1007/s00125-018-4711-2. [DOI] [PubMed] [Google Scholar]
- 6.Dasari D, et al. Canagliflozin protects diabetic cardiomyopathy by mitigating fibrosis and preserving the myocardial integrity with improved mitochondrial function. Eur J Pharmacol. 2023;949:175720. doi: 10.1016/j.ejphar.2023.175720. [DOI] [PubMed] [Google Scholar]
- 7.Fang P, et al. Glucagon-like peptide-1 receptor agonist protects against diabetic cardiomyopathy by modulating microRNA-29b-3p/SLMAP. Drug Des Devel Ther. 2023;17:791–806. doi: 10.2147/DDDT.S400249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Z, et al. Metformin ameliorates Diabetic Cardiomyopathy by activating the PK2/PKR pathway. Front Physiol. 2020;11:425. doi: 10.3389/fphys.2020.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritchie RH, Abel ED. Basic Mech Diabet Heart Disease Circ Res. 2020;126(11):1501–25. doi: 10.1161/CIRCRESAHA.120.315913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phang RJ, et al. Cellular interplay between cardiomyocytes and non-myocytes in diabetic cardiomyopathy. Cardiovasc Res. 2023;119(3):668–90. doi: 10.1093/cvr/cvac049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prakoso D, et al. Current landscape of preclinical models of diabetic cardiomyopathy. Trends Pharmacol Sci. 2022;43(11):940–56. doi: 10.1016/j.tips.2022.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Astorri E, et al. Left ventricular function in insulin-dependent and in non-insulin-dependent diabetic patients: radionuclide assessment. Cardiology. 1997;88(2):152–5. doi: 10.1159/000177322. [DOI] [PubMed] [Google Scholar]
- 13.Yamada T, et al. Sodium-glucose co-transporter-2 inhibitors as add-on therapy to insulin for type 1 diabetes mellitus: systematic review and meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2018;20(7):1755–61. doi: 10.1111/dom.13260. [DOI] [PubMed] [Google Scholar]
- 14.Mathieu C, et al. Efficacy and safety of Liraglutide added to Insulin Treatment in type 1 diabetes: the ADJUNCT ONE Treat-To-Target Randomized Trial. Diabetes Care. 2016;39(10):1702–10. doi: 10.2337/dc16-0691. [DOI] [PubMed] [Google Scholar]
- 15.Marx N et al. 2023 ESC guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023;44(39):4043–140. [DOI] [PubMed]
- 16.Park MD, et al. Macrophages in health and disease. Cell. 2022;185(23):4259–79. doi: 10.1016/j.cell.2022.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–95. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wculek SK, et al. Metabolism of tissue macrophages in homeostasis and pathology. Cell Mol Immunol. 2022;19(3):384–408. doi: 10.1038/s41423-021-00791-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cassetta L, Cassol E, Poli G. Macrophage polarization in health and disease. ScientificWorldJournal. 2011;11:2391–402. doi: 10.1100/2011/213962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bashir S, et al. Macrophage polarization: the link between inflammation and related diseases. Inflamm Res. 2016;65(1):1–11. doi: 10.1007/s00011-015-0874-1. [DOI] [PubMed] [Google Scholar]
- 21.Peng C, Li Z, Yu X. The role of pancreatic infiltrating Innate Immune cells in Acute Pancreatitis. Int J Med Sci. 2021;18(2):534–45. doi: 10.7150/ijms.51618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterling KG et al. Mucosal immunity and the Gut-Microbiota-Brain-Axis in Neuroimmune Disease. Int J Mol Sci, 2022. 23(21). [DOI] [PMC free article] [PubMed]
- 23.Hu Z, et al. Depletion of macrophages with clodronate liposomes partially attenuates renal fibrosis on AKI-CKD transition. Ren Fail. 2023;45(1):2149412. doi: 10.1080/0886022X.2022.2149412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braga TT, Agudelo JS, Camara NO. Macrophages during the fibrotic process: M2 as friend and Foe. Front Immunol. 2015;6:602. doi: 10.3389/fimmu.2015.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mills CD, et al. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164(12):6166–73. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 26.Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15:123–47. doi: 10.1146/annurev-pathmechdis-012418-012718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nahrendorf M, Swirski FK. Abandoning M1/M2 for a network model of macrophage function. Circ Res. 2016;119(3):414–7. doi: 10.1161/CIRCRESAHA.116.309194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gosselin D, et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159(6):1327–40. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan Y, et al. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat Rev Cardiol. 2020;17(9):585–607. doi: 10.1038/s41569-020-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia G, Hill MA, Sowers JR. Diabetic Cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122(4):624–38. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seferović PM, Paulus WJ. Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J. 2015;36(27):1718–27. doi: 10.1093/eurheartj/ehv134. [DOI] [PubMed] [Google Scholar]
- 32.Marwick TH, et al. Implications of underlying mechanisms for the Recognition and Management of Diabetic Cardiomyopathy. J Am Coll Cardiol. 2018;71(3):339–51. doi: 10.1016/j.jacc.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Lin HT et al. (1)H nuclear magnetic resonance (NMR)-Based cerebrospinal fluid and plasma metabolomic analysis in type 2 Diabetic patients and Risk Prediction for Diabetic Microangiopathy. J Clin Med, 2019. 8(6). [DOI] [PMC free article] [PubMed]
- 34.DeBerge M, et al. Macrophages in Heart failure with reduced versus preserved ejection fraction. Trends Mol Med. 2019;25(4):328–40. doi: 10.1016/j.molmed.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin X, et al. Advanced Glycation End products enhance macrophages polarization into M1 phenotype through activating RAGE/NF-κB pathway. Biomed Res Int. 2015;2015:p732450. doi: 10.1155/2015/732450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishra PK, et al. Diabetic Cardiomyopathy: an immunometabolic perspective. Front Endocrinol (Lausanne) 2017;8:72. doi: 10.3389/fendo.2017.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe R et al. Glucose metabolism controls disease-specific signatures of macrophage effector functions. JCI Insight, 2018. 3(20). [DOI] [PMC free article] [PubMed]
- 38.Mouton AJ, et al. Obesity, hypertension, and Cardiac Dysfunction: Novel roles of Immunometabolism in Macrophage activation and inflammation. Circ Res. 2020;126(6):789–806. doi: 10.1161/CIRCRESAHA.119.312321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dinh W, et al. Elevated plasma levels of TNF-alpha and interleukin-6 in patients with diastolic dysfunction and glucose metabolism disorders. Cardiovasc Diabetol. 2009;8:58. doi: 10.1186/1475-2840-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masters SL, Latz E, O’Neill LA. The inflammasome in atherosclerosis and type 2 diabetes. Sci Transl Med. 2011;3(81):81ps17. doi: 10.1126/scitranslmed.3001902. [DOI] [PubMed] [Google Scholar]
- 41.Biernacka A, et al. Smad3 Signaling promotes Fibrosis while preserving Cardiac and aortic geometry in obese Diabetic mice. Circ Heart Fail. 2015;8(4):788–98. doi: 10.1161/CIRCHEARTFAILURE.114.001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bajpai A, Tilley DG. The role of leukocytes in Diabetic Cardiomyopathy. Front Physiol. 2018;9:1547. doi: 10.3389/fphys.2018.01547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kokkola R, et al. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol. 2005;61(1):1–9. doi: 10.1111/j.0300-9475.2005.01534.x. [DOI] [PubMed] [Google Scholar]
- 44.Volz HC, et al. The role of HMGB1/RAGE in inflammatory cardiomyopathy. Semin Thromb Hemost. 2010;36(2):185–94. doi: 10.1055/s-0030-1251503. [DOI] [PubMed] [Google Scholar]
- 45.Tang SCW, Yiu WH. Innate immunity in diabetic kidney disease. Nat Rev Nephrol. 2020;16(4):206–22. doi: 10.1038/s41581-019-0234-4. [DOI] [PubMed] [Google Scholar]
- 46.Frantz S, et al. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104(3):271–80. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, et al. Prevention of hyperglycemia-induced myocardial apoptosis by gene silencing of toll-like receptor-4. J Transl Med. 2010;8:133. doi: 10.1186/1479-5876-8-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong B, et al. TLR4 regulates cardiac lipid accumulation and diabetic heart disease in the nonobese diabetic mouse model of type 1 diabetes. Am J Physiol Heart Circ Physiol. 2012;303(6):H732–42. doi: 10.1152/ajpheart.00948.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tao A, et al. Cardiomyocyte-fibroblast interaction contributes to diabetic cardiomyopathy in mice: role of HMGB1/TLR4/IL-33 axis. Biochim Biophys Acta. 2015;1852(10 Pt A):2075–85. doi: 10.1016/j.bbadis.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, et al. Saturated palmitic acid induces myocardial inflammatory injuries through direct binding to TLR4 accessory protein MD2. Nat Commun. 2017;8:13997. doi: 10.1038/ncomms13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118(9):2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115(5):1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aguirre V, et al. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of ser(307) J Biol Chem. 2000;275(12):9047–54. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 54.Gao Z, et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277(50):48115–21. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 55.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 56.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 59.Patsouris D, et al. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8(4):301–9. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanda H, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116(6):1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uysal KT, et al. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389(6651):610–4. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 62.Xu X, et al. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18(6):816–30. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jaitin DA, et al. Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent manner. Cell. 2019;178(3):686–e69814. doi: 10.1016/j.cell.2019.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aouadi M, et al. Lipid storage by adipose tissue macrophages regulates systemic glucose tolerance. Am J Physiol Endocrinol Metab. 2014;307(4):E374–83. doi: 10.1152/ajpendo.00187.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lafuse WP, Wozniak DJ, Rajaram MVS. Role of Cardiac macrophages on Cardiac inflammation, fibrosis and tissue repair. Cells, 2020. 10(1). [DOI] [PMC free article] [PubMed]
- 66.Hulsmans M, Sam F, Nahrendorf M. Monocyte and macrophage contributions to cardiac remodeling. J Mol Cell Cardiol. 2016;93:149–55. doi: 10.1016/j.yjmcc.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dick SA, et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat Immunol. 2019;20(1):29–39. doi: 10.1038/s41590-018-0272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wagner MJ, Khan M, Mohsin S. Healing the broken heart; the Immunomodulatory effects of Stem Cell Therapy. Front Immunol. 2020;11:639. doi: 10.3389/fimmu.2020.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Horckmans M, et al. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J. 2017;38(3):187–97. doi: 10.1093/eurheartj/ehw002. [DOI] [PubMed] [Google Scholar]
- 70.Frati G, et al. An overview of the inflammatory signalling mechanisms in the myocardium underlying the development of diabetic cardiomyopathy. Cardiovasc Res. 2017;113(4):378–88. doi: 10.1093/cvr/cvx011. [DOI] [PubMed] [Google Scholar]
- 71.Liu G, et al. The effect of mir-471-3p on macrophage polarization in the development of diabetic cardiomyopathy. Life Sci. 2021;268:118989. doi: 10.1016/j.lfs.2020.118989. [DOI] [PubMed] [Google Scholar]
- 72.Sharifi-Rad M, et al. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of Chronic diseases. Front Physiol. 2020;11:694. doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cai L, Kang YJ. Oxidative stress and diabetic cardiomyopathy: a brief review. Cardiovasc Toxicol. 2001;1(3):181–93. doi: 10.1385/ct:1:3:181. [DOI] [PubMed] [Google Scholar]
- 74.Wilson AJ, et al. Reactive oxygen species signalling in the diabetic heart: emerging prospect for therapeutic targeting. Heart. 2018;104(4):293–9. doi: 10.1136/heartjnl-2017-311448. [DOI] [PubMed] [Google Scholar]
- 75.Nishikawa T, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 76.Cai L, et al. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes. 2005;54(6):1829–37. doi: 10.2337/diabetes.54.6.1829. [DOI] [PubMed] [Google Scholar]
- 77.Zhu HZ, et al. Xiaoyaosan exerts therapeutic effects on the Colon of Chronic Restraint stress model rats via the regulation of Immunoinflammatory Activation Induced by the TLR4/NLRP3 Inflammasome Signaling Pathway. Evid Based Complement Alternat Med. 2021;2021:p6673538. doi: 10.1155/2021/6673538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pal PB, et al. Aldose Reductase mediates NLRP3 inflammasome-initiated Innate Immune Response in Hyperglycemia-Induced Thp1 monocytes and male mice. Endocrinology. 2017;158(10):3661–75. doi: 10.1210/en.2017-00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peng ML, et al. Signaling pathways related to oxidative stress in Diabetic Cardiomyopathy. Front Endocrinol (Lausanne) 2022;13:907757. doi: 10.3389/fendo.2022.907757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 82.Yan SD, et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269(13):9889–97. [PubMed] [Google Scholar]
- 83.Rendra E, et al. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology. 2019;224(2):242–53. doi: 10.1016/j.imbio.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 84.Gerstein HC, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818–28. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chalmers J, Cooper ME. UKPDS and the legacy effect. N Engl J Med. 2008;359(15):1618–20. doi: 10.1056/NEJMe0807625. [DOI] [PubMed] [Google Scholar]
- 86.Wang T, et al. Influenza-trained mucosal-resident alveolar macrophages confer long-term antitumor immunity in the lungs. Nat Immunol. 2023;24(3):423–38. doi: 10.1038/s41590-023-01428-x. [DOI] [PubMed] [Google Scholar]
- 87.Netea MG, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20(6):375–88. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Edgar L, et al. Hyperglycemia induces trained immunity in macrophages and their precursors and promotes atherosclerosis. Circulation. 2021;144(12):961–82. doi: 10.1161/CIRCULATIONAHA.120.046464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wood IS, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr. 2003;89(1):3–9. doi: 10.1079/BJN2002763. [DOI] [PubMed] [Google Scholar]
- 90.Xie L, et al. Emerging roles of Sodium glucose cotransporter 2 (SGLT-2) inhibitors in Diabetic Cardiovascular diseases: focusing on immunity, inflammation and metabolism. Front Pharmacol. 2022;13:836849. doi: 10.3389/fphar.2022.836849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee SH, Park SY, Choi CS. Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J. 2022;46(1):15–37. doi: 10.4093/dmj.2021.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shaffner J, et al. Therapeutic targeting of SGLT2: a new era in the treatment of Diabetes and Diabetic kidney disease. Front Endocrinol (Lausanne) 2021;12:749010. doi: 10.3389/fendo.2021.749010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kato ET, et al. Effect of Dapagliflozin on Heart failure and mortality in type 2 diabetes Mellitus. Circulation. 2019;139(22):2528–36. doi: 10.1161/CIRCULATIONAHA.119.040130. [DOI] [PubMed] [Google Scholar]
- 94.Furtado RHM, et al. Dapagliflozin and Cardiovascular outcomes in patients with type 2 diabetes Mellitus and previous myocardial infarction. Circulation. 2019;139(22):2516–27. doi: 10.1161/CIRCULATIONAHA.119.039996. [DOI] [PubMed] [Google Scholar]
- 95.Lee TM, Chang NC, Lin SZ. Dapagliflozin, a selective SGLT2 inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med. 2017;104:298–310. doi: 10.1016/j.freeradbiomed.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 96.Iannantuoni F et al. The SGLT2 inhibitor Empagliflozin ameliorates the Inflammatory Profile in type 2 Diabetic patients and promotes an antioxidant response in leukocytes. J Clin Med, 2019. 8(11). [DOI] [PMC free article] [PubMed]
- 97.Kim SR, et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun. 2020;11(1):2127. doi: 10.1038/s41467-020-15983-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu Q, et al. Dapagliflozin protects against chronic heart failure in mice by inhibiting macrophage-mediated inflammation, independent of SGLT2. Cell Rep Med. 2023;4(12):101334. doi: 10.1016/j.xcrm.2023.101334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu L, et al. SGLT2 inhibition by Empagliflozin Promotes Fat Utilization and Browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in Diet-induced obese mice. EBioMedicine. 2017;20:137–49. doi: 10.1016/j.ebiom.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu L, Ota T. Emerging roles of SGLT2 inhibitors in obesity and insulin resistance: focus on fat browning and macrophage polarization. Adipocyte. 2018;7(2):121–8. doi: 10.1080/21623945.2017.1413516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Terami N, et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS ONE. 2014;9(6):e100777. doi: 10.1371/journal.pone.0100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oelze M, et al. The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the Streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity. PLoS ONE. 2014;9(11):e112394. doi: 10.1371/journal.pone.0112394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McMurray JJV, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 104.Wiviott SD, et al. Dapagliflozin and Cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 105.Zinman B, et al. Empagliflozin, Cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 106.Neal B, et al. Canagliflozin and Cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 107.Ye Y, et al. SGLT-2 inhibition with Dapagliflozin reduces the activation of the Nlrp3/ASC inflammasome and attenuates the Development of Diabetic Cardiomyopathy in mice with type 2 diabetes. Further augmentation of the effects with Saxagliptin, a DPP4 inhibitor. Cardiovasc Drugs Ther. 2017;31(2):119–32. doi: 10.1007/s10557-017-6725-2. [DOI] [PubMed] [Google Scholar]
- 108.Kern M, et al. The SGLT2 inhibitor empagliflozin improves insulin sensitivity in db/db mice both as monotherapy and in combination with linagliptin. Metabolism. 2016;65(2):114–23. doi: 10.1016/j.metabol.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 109.Habibi J, et al. Sodium glucose transporter 2 (SGLT2) inhibition with empagliflozin improves cardiac diastolic function in a female rodent model of diabetes. Cardiovasc Diabetol. 2017;16(1):9. doi: 10.1186/s12933-016-0489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Orskov C, et al. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes. 1994;43(4):535–9. doi: 10.2337/diab.43.4.535. [DOI] [PubMed] [Google Scholar]
- 111.MacDonald PE, et al. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002;51(Suppl 3):S434–42. doi: 10.2337/diabetes.51.2007.s434. [DOI] [PubMed] [Google Scholar]
- 112.Lazzaroni E, et al. Anti-diabetic drugs and weight loss in patients with type 2 diabetes. Pharmacol Res. 2021;171:105782. doi: 10.1016/j.phrs.2021.105782. [DOI] [PubMed] [Google Scholar]
- 113.Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–85. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 114.Bethel MA, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6(2):105–13. doi: 10.1016/S2213-8587(17)30412-6. [DOI] [PubMed] [Google Scholar]
- 115.Pop-Busui R, et al. Heart failure: an underappreciated complication of diabetes. A Consensus Report of the American Diabetes Association. Diabetes Care. 2022;45(7):1670–90. doi: 10.2337/dci22-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Almutairi M, et al. The GLP-1 receptor agonist Liraglutide increases myocardial glucose oxidation Rates via Indirect mechanisms and mitigates Experimental Diabetic Cardiomyopathy. Can J Cardiol. 2021;37(1):140–50. doi: 10.1016/j.cjca.2020.02.098. [DOI] [PubMed] [Google Scholar]
- 117.Trang NN et al. Empagliflozin and Liraglutide differentially modulate Cardiac Metabolism in Diabetic Cardiomyopathy in rats. Int J Mol Sci, 2021. 22(3). [DOI] [PMC free article] [PubMed]
- 118.Qian P, et al. A novel oral glucagon-like peptide 1 receptor agonist protects against diabetic cardiomyopathy via alleviating cardiac lipotoxicity induced mitochondria dysfunction. Biochem Pharmacol. 2020;182:114209. doi: 10.1016/j.bcp.2020.114209. [DOI] [PubMed] [Google Scholar]
- 119.Ma X, et al. GLP-1 receptor agonists (GLP-1RAs): cardiovascular actions and therapeutic potential. Int J Biol Sci. 2021;17(8):2050–68. doi: 10.7150/ijbs.59965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wan S, Sun H. Glucagon-like peptide-1 modulates RAW264.7 macrophage polarization by interfering with the JNK/STAT3 signaling pathway. Exp Ther Med. 2019;17(5):3573–9. doi: 10.3892/etm.2019.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang N, et al. Exendin-4 induces bone marrow stromal cells Migration through Bone Marrow-Derived macrophages polarization via PKA-STAT3 signaling pathway. Cell Physiol Biochem. 2017;44(5):1696–714. doi: 10.1159/000485776. [DOI] [PubMed] [Google Scholar]
- 122.Yang L, et al. Effect of GLP-1/GLP-1R on the polarization of macrophages in the occurrence and development of atherosclerosis. Mediators Inflamm. 2021;2021:p5568159. doi: 10.1155/2021/5568159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Weisberg SP, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116(1):115–24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Winer S, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15(8):921–9. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee YS, et al. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia. 2012;55(9):2456–68. doi: 10.1007/s00125-012-2592-3. [DOI] [PubMed] [Google Scholar]
- 126.Tate M, et al. Exendin-4 attenuates adverse cardiac remodelling in streptozocin-induced diabetes via specific actions on infiltrating macrophages. Basic Res Cardiol. 2016;111(1):1. doi: 10.1007/s00395-015-0518-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gerstein HC, et al. The effect of dulaglutide on stroke: an exploratory analysis of the REWIND trial. Lancet Diabetes Endocrinol. 2020;8(2):106–14. doi: 10.1016/S2213-8587(19)30423-1. [DOI] [PubMed] [Google Scholar]
- 128.Tuttolomondo A, et al. Efficacy of dulaglutide on vascular health indexes in subjects with type 2 diabetes: a randomized trial. Cardiovasc Diabetol. 2021;20(1):1. doi: 10.1186/s12933-020-01183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Marso SP, et al. Liraglutide and Cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Husain M, et al. Oral Semaglutide and Cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–51. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 131.Strain WD, et al. Effects of Semaglutide on Stroke subtypes in type 2 diabetes: Post Hoc Analysis of the Randomized SUSTAIN 6 and PIONEER 6. Stroke. 2022;53(9):2749–57. doi: 10.1161/STROKEAHA.121.037775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gerstein HC, et al. Cardiovascular and renal outcomes with Efpeglenatide in Type 2 diabetes. N Engl J Med. 2021;385(10):896–907. doi: 10.1056/NEJMoa2108269. [DOI] [PubMed] [Google Scholar]
- 133.Hernandez AF, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–29. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 134.van der Aa MP, et al. Long-term treatment with metformin in obese, insulin-resistant adolescents: results of a randomized double-blinded placebo-controlled trial. Nutr Diabetes. 2016;6(8):e228. doi: 10.1038/nutd.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Giannarelli R, et al. Reducing insulin resistance with metformin: the evidence today. Diabetes Metab. 2003;29(4 Pt 2):s628–35. doi: 10.1016/s1262-3636(03)72785-2. [DOI] [PubMed] [Google Scholar]
- 136.Xiao H, et al. Metformin attenuates cardiac fibrosis by inhibiting the TGFbeta1-Smad3 signalling pathway. Cardiovasc Res. 2010;87(3):504–13. doi: 10.1093/cvr/cvq066. [DOI] [PubMed] [Google Scholar]
- 137.Hippisley-Cox J, Coupland C. Diabetes treatments and risk of heart failure, cardiovascular disease, and all cause mortality: cohort study in primary care. BMJ. 2016;354:i3477. doi: 10.1136/bmj.i3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Crowley MJ, et al. Clinical outcomes of Metformin Use in populations with chronic kidney disease, congestive heart failure, or Chronic Liver Disease: a systematic review. Ann Intern Med. 2017;166(3):191–200. doi: 10.7326/M16-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nassif RM et al. Metformin inhibits ROS production by human M2 macrophages via the activation of AMPK. Biomedicines, 2022. 10(2). [DOI] [PMC free article] [PubMed]
- 140.Sánchez ML et al. Polymers and Bioactive compounds with a macrophage modulation effect for the Rational Design of Hydrogels for skin regeneration. Pharmaceutics, 2023. 15(6). [DOI] [PMC free article] [PubMed]
- 141.Das B et al. Promise of the NLRP3 inflammasome inhibitors in in vivo Disease models. Molecules, 2021. 26(16). [DOI] [PMC free article] [PubMed]
- 142.Horiuchi T, et al. Metformin directly binds the alarmin HMGB1 and inhibits its proinflammatory activity. J Biol Chem. 2017;292(20):8436–46. doi: 10.1074/jbc.M116.769380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Feng X, et al. Metformin, macrophage dysfunction and atherosclerosis. Front Immunol. 2021;12:682853. doi: 10.3389/fimmu.2021.682853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Guo C, Liu J, Li H. Metformin ameliorates olanzapine-induced insulin resistance via suppressing macrophage infiltration and inflammatory responses in rats. Biomed Pharmacother. 2021;133:110912. doi: 10.1016/j.biopha.2020.110912. [DOI] [PubMed] [Google Scholar]
- 145.Cortés M, et al. Inflammatory macrophages reprogram to immunosuppression by reducing mitochondrial translation. Nat Commun. 2023;14(1):7471. doi: 10.1038/s41467-023-42277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Panagiotopoulou O, et al. Metformin use in obese mothers is associated with improved cardiovascular profile in the offspring. Am J Obstet Gynecol. 2020;223(2):e2461–24610. doi: 10.1016/j.ajog.2020.01.054. [DOI] [PubMed] [Google Scholar]
- 147.Timmons JG, et al. Metformin and carotid intima-media thickness in never-smokers with type 1 diabetes: the REMOVAL trial. Diabetes Obes Metab. 2021;23(6):1371–8. doi: 10.1111/dom.14350. [DOI] [PubMed] [Google Scholar]
- 148.Eurich DT, et al. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail. 2013;6(3):395–402. doi: 10.1161/CIRCHEARTFAILURE.112.000162. [DOI] [PubMed] [Google Scholar]
- 149.Bhansali S, et al. Metformin upregulates mitophagy in patients with T2DM: a randomized placebo-controlled study. J Cell Mol Med. 2020;24(5):2832–46. doi: 10.1111/jcmm.14834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang F, et al. Metformin inhibits the NLRP3 Inflammasome via AMPK/mTOR-dependent effects in Diabetic Cardiomyopathy. Int J Biol Sci. 2019;15(5):1010–9. doi: 10.7150/ijbs.29680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tsoyi K, et al. Metformin inhibits HMGB1 release in LPS-treated RAW 264.7 cells and increases survival rate of endotoxaemic mice. Br J Pharmacol. 2011;162(7):1498–508. doi: 10.1111/j.1476-5381.2010.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fiordaliso F, et al. Cardiovascular oxidative stress is reduced by an ACE inhibitor in a rat model of streptozotocin-induced diabetes. Life Sci. 2006;79(2):121–9. doi: 10.1016/j.lfs.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 153.Matsusaka H, et al. Angiotensin II type 1 receptor blocker attenuates exacerbated left ventricular remodeling and failure in diabetes-associated myocardial infarction. J Cardiovasc Pharmacol. 2006;48(3):95–102. doi: 10.1097/01.fjc.0000245405.41317.60. [DOI] [PubMed] [Google Scholar]
- 154.Wichter T, et al. Pregnancy in arrhythmogenic cardiomyopathy. Herzschrittmacherther Elektrophysiol. 2021;32(2):186–98. doi: 10.1007/s00399-021-00770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37(4):1053–9. doi: 10.1161/01.hyp.37.4.1053. [DOI] [PubMed] [Google Scholar]
- 156.Yang J, et al. Valsartan preconditioning protects against myocardial ischemia-reperfusion injury through TLR4/NF-kappaB signaling pathway. Mol Cell Biochem. 2009;330(1–2):39–46. doi: 10.1007/s11010-009-0098-1. [DOI] [PubMed] [Google Scholar]
- 157.Yaras N, et al. Restoration of diabetes-induced abnormal local Ca2 + release in cardiomyocytes by angiotensin II receptor blockade. Am J Physiol Heart Circ Physiol. 2007;292(2):H912–20. doi: 10.1152/ajpheart.00824.2006. [DOI] [PubMed] [Google Scholar]
- 158.Solomon SD, et al. Effect of the direct renin inhibitor aliskiren, the angiotensin receptor blocker losartan, or both on left ventricular mass in patients with hypertension and left ventricular hypertrophy. Circulation. 2009;119(4):530–7. doi: 10.1161/CIRCULATIONAHA.108.826214. [DOI] [PubMed] [Google Scholar]
- 159.Shah AM, et al. Left ventricular systolic and diastolic function, remodelling, and clinical outcomes among patients with diabetes following myocardial infarction and the influence of direct renin inhibition with aliskiren. Eur J Heart Fail. 2012;14(2):185–92. doi: 10.1093/eurjhf/hfr125. [DOI] [PubMed] [Google Scholar]
- 160.Nagase M, et al. Salt-induced nephropathy in obese spontaneously hypertensive rats via paradoxical activation of the mineralocorticoid receptor: role of oxidative stress. Hypertension. 2007;50(5):877–83. doi: 10.1161/HYPERTENSIONAHA.107.091058. [DOI] [PubMed] [Google Scholar]
- 161.Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol. 2008;130(2):147–58. doi: 10.1016/j.ijcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mansour AA, et al. The interplay of galectins-1, -3, and– 9 in the immune-inflammatory response underlying cardiovascular and metabolic disease. Cardiovasc Diabetol. 2022;21(1):253. doi: 10.1186/s12933-022-01690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Camici M, et al. Obesity-related glomerulopathy and podocyte injury: a mini review. Front Biosci (Elite Ed) 2012;4(3):1058–70. doi: 10.2741/e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hansson L, et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet. 1999;353(9153):611–6. doi: 10.1016/s0140-6736(98)05012-0. [DOI] [PubMed] [Google Scholar]
- 165.Yusuf S, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342(3):145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 166.Lambers Heerspink HJ, et al. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int. 2012;82(3):330–7. doi: 10.1038/ki.2012.74. [DOI] [PubMed] [Google Scholar]
- 167.Lefkowitz RJ. A brief history of G-protein coupled receptors (Nobel lecture) Angew Chem Int Ed Engl. 2013;52(25):6366–78. doi: 10.1002/anie.201301924. [DOI] [PubMed] [Google Scholar]
- 168.Milligan G, Svoboda P, Brown CM. Why are there so many adrenoceptor subtypes? Biochem Pharmacol. 1994;48(6):1059–71. doi: 10.1016/0006-2952(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 169.Taylor MR. Pharmacogenetics of the human beta-adrenergic receptors. Pharmacogenomics J. 2007;7(1):29–37. doi: 10.1038/sj.tpj.6500393. [DOI] [PubMed] [Google Scholar]
- 170.Xing G, et al. Recent progress in the development of β2 adrenergic receptor agonists: a patent review (2015–2020) Expert Opin Ther Pat. 2021;31(3):239–46. doi: 10.1080/13543776.2021.1865312. [DOI] [PubMed] [Google Scholar]
- 171.Ortega E, Gálvez I, Martín-Cordero L. Adrenergic regulation of macrophage-mediated Innate/Inflammatory responses in obesity and Exercise in this Condition: role of β2 adrenergic receptors. Endocr Metab Immune Disord Drug Targets. 2019;19(8):1089–99. doi: 10.2174/1871530319666190206124520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Noh H, et al. Beta 2-adrenergic receptor agonists are novel regulators of macrophage activation in diabetic renal and cardiovascular complications. Kidney Int. 2017;92(1):101–13. doi: 10.1016/j.kint.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang H, et al. Renal-protective effect of thalidomide in streptozotocin-induced diabetic rats through anti-inflammatory pathway. Drug Des Devel Ther. 2018;12:89–98. doi: 10.2147/DDDT.S149298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Grisanti LA, et al. β2-Adrenergic receptor-dependent chemokine receptor 2 expression regulates leukocyte recruitment to the heart following acute injury. Proc Natl Acad Sci U S A. 2016;113(52):15126–31. doi: 10.1073/pnas.1611023114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kaumann A, et al. Activation of beta2-adrenergic receptors hastens relaxation and mediates phosphorylation of phospholamban, troponin I, and C-protein in ventricular myocardium from patients with terminal heart failure. Circulation. 1999;99(1):65–72. doi: 10.1161/01.cir.99.1.65. [DOI] [PubMed] [Google Scholar]
- 176.Gong J, et al. Caveolin-3 protects diabetic hearts from acute myocardial infarction/reperfusion injury through β2AR, cAMP/PKA, and BDNF/TrkB signaling pathways. Aging. 2020;12(14):14300–13. doi: 10.18632/aging.103469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.van Beek SMM, et al. Prolonged β(2)-adrenergic agonist treatment improves glucose homeostasis in diet-induced obese UCP1(-/-) mice. Am J Physiol Endocrinol Metab. 2021;320(3):E619–28. doi: 10.1152/ajpendo.00324.2020. [DOI] [PubMed] [Google Scholar]
- 178.Mahtal N, et al. MicroRNAs in kidney injury and disease. Nat Rev Nephrol. 2022;18(10):643–62. doi: 10.1038/s41581-022-00608-6. [DOI] [PubMed] [Google Scholar]
- 179.Guo X, et al. miR-335 negatively regulates osteosarcoma stem cell-like properties by targeting POU5F1. Cancer Cell Int. 2017;17:29. doi: 10.1186/s12935-017-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Duroux-Richard I, Apparailly F, Khoury M. Mitochondrial MicroRNAs contribute to Macrophage Immune functions including differentiation, polarization, and activation. Front Physiol. 2021;12:738140. doi: 10.3389/fphys.2021.738140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhong Y, Yi C. MicroRNA-720 suppresses M2 macrophage polarization by targeting GATA3. Biosci Rep, 2016. 36(4). [DOI] [PMC free article] [PubMed]
- 182.Ying H, et al. MiR-127 modulates macrophage polarization and promotes lung inflammation and injury by activating the JNK pathway. J Immunol. 2015;194(3):1239–51. doi: 10.4049/jimmunol.1402088. [DOI] [PubMed] [Google Scholar]
- 183.Nazari-Jahantigh M, et al. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest. 2012;122(11):4190–202. doi: 10.1172/JCI61716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li D, et al. MiR-146a modulates macrophage polarization in systemic juvenile idiopathic arthritis by targeting INHBA. Mol Immunol. 2016;77:205–12. doi: 10.1016/j.molimm.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 185.Muñoz JP, et al. The transcription factor MEF2C mediates cardiomyocyte hypertrophy induced by IGF-1 signaling. Biochem Biophys Res Commun. 2009;388(1):155–60. doi: 10.1016/j.bbrc.2009.07.147. [DOI] [PubMed] [Google Scholar]
- 186.Ruiz MA, Chakrabarti S. MicroRNAs: the underlying mediators of pathogenetic processes in vascular complications of diabetes. Can J Diabetes. 2013;37(5):339–44. doi: 10.1016/j.jcjd.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 187.Moore A, et al. Rapid onset of cardiomyopathy in STZ-induced female diabetic mice involves the downregulation of pro-survival Pim-1. Cardiovasc Diabetol. 2014;13:68. doi: 10.1186/1475-2840-13-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kuwabara Y, et al. MicroRNA-451 exacerbates lipotoxicity in cardiac myocytes and high-fat diet-induced cardiac hypertrophy in mice through suppression of the LKB1/AMPK pathway. Circ Res. 2015;116(2):279–88. doi: 10.1161/CIRCRESAHA.116.304707. [DOI] [PubMed] [Google Scholar]
- 189.Liu S, et al. Micro-RNA 21Targets dual specific phosphatase 8 to promote collagen synthesis in high glucose-treated primary cardiac fibroblasts. Can J Cardiol. 2014;30(12):1689–99. doi: 10.1016/j.cjca.2014.07.747. [DOI] [PubMed] [Google Scholar]
- 190.Yildirim SS, et al. Relationship between downregulation of miRNAs and increase of oxidative stress in the development of diabetic cardiac dysfunction: junctin as a target protein of miR-1. Cell Biochem Biophys. 2013;67(3):1397–408. doi: 10.1007/s12013-013-9672-y. [DOI] [PubMed] [Google Scholar]
- 191.Wang P, et al. miR-657 promotes macrophage polarization toward M1 by targeting FAM46C in gestational diabetes Mellitus. Mediators Inflamm. 2019;2019:p4851214. doi: 10.1155/2019/4851214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhuang G, et al. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation. 2012;125(23):2892–903. doi: 10.1161/CIRCULATIONAHA.111.087817. [DOI] [PubMed] [Google Scholar]
- 193.Fitzsimons S, et al. microRNA-155 is decreased during atherosclerosis regression and is increased in urinary extracellular vesicles during atherosclerosis progression. Front Immunol. 2020;11:576516. doi: 10.3389/fimmu.2020.576516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jia C, et al. Gold nanoparticle-based miR155 antagonist macrophage delivery restores the cardiac function in ovariectomized diabetic mouse model. Int J Nanomed. 2017;12:4963–79. doi: 10.2147/IJN.S138400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Phu TA, et al. IL-4 polarized human macrophage exosomes control cardiometabolic inflammation and diabetes in obesity. Mol Ther. 2022;30(6):2274–97. doi: 10.1016/j.ymthe.2022.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bouchareychas L, et al. Macrophage exosomes resolve atherosclerosis by regulating hematopoiesis and inflammation via MicroRNA Cargo. Cell Rep. 2020;32(2):107881. doi: 10.1016/j.celrep.2020.107881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li JM, et al. Lipotoxicity-polarised macrophage-derived exosomes regulate mitochondrial fitness through Miro1-mediated mitophagy inhibition and contribute to type 2 diabetes development in mice. Diabetologia. 2023;66(12):2368–86. doi: 10.1007/s00125-023-05992-7. [DOI] [PubMed] [Google Scholar]
- 198.Sun J, et al. miR-330-5p/Tim-3 axis regulates macrophage M2 polarization and insulin resistance in diabetes mice. Mol Immunol. 2018;95:107–13. doi: 10.1016/j.molimm.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 199.Cao J, et al. Extracellular vesicle miR-32 derived from macrophage promotes arterial calcification in mice with type 2 diabetes via inhibiting VSMC autophagy. J Transl Med. 2022;20(1):307. doi: 10.1186/s12967-022-03502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chang YJ, et al. Extracellular MicroRNA-92a mediates endothelial cell-macrophage communication. Arterioscler Thromb Vasc Biol. 2019;39(12):2492–504. doi: 10.1161/ATVBAHA.119.312707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang M, et al. MiR-130b promotes obesity associated adipose tissue inflammation and insulin resistance in diabetes mice through alleviating M2 macrophage polarization via repression of PPAR-γ. Immunol Lett. 2016;180:1–8. doi: 10.1016/j.imlet.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 202.Suresh Babu S, et al. MicroRNA-126 overexpression rescues diabetes-induced impairment in efferocytosis of apoptotic cardiomyocytes. Sci Rep. 2016;6:36207. doi: 10.1038/srep36207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Acuña-Castroviejo D, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71(16):2997–3025. doi: 10.1007/s00018-014-1579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nabavi SM, et al. Anti-inflammatory effects of melatonin: a mechanistic review. Crit Rev Food Sci Nutr. 2019;59(sup1):S4–16. doi: 10.1080/10408398.2018.1487927. [DOI] [PubMed] [Google Scholar]
- 205.Stebelová K, Herichová I, Zeman M. Diabetes induces changes in melatonin concentrations in peripheral tissues of rat. Neuro Endocrinol Lett. 2007;28(2):159–65. [PubMed] [Google Scholar]
- 206.Fiorina P, et al. Impaired nocturnal melatonin excretion and changes of immunological status in ischaemic stroke patients. Lancet. 1996;347(9002):692–3. doi: 10.1016/s0140-6736(96)91246-5. [DOI] [PubMed] [Google Scholar]
- 207.Maity J, et al. Melatonin ameliorates myocardial infarction in obese diabetic individuals: the possible involvement of macrophage apoptotic factors. J Pineal Res. 2023;74(2):e12847. doi: 10.1111/jpi.12847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.