Abstract
Background
Plasma biomarkers of Alzheimer’s disease (AD) pathology, neurodegeneration, and neuroinflammation are ideally suited for secondary prevention programs in self-sufficient persons at-risk of dementia. Plasma biomarkers have been shown to be highly correlated with traditional imaging biomarkers. However, their comparative predictive value versus traditional AD biomarkers is still unclear in cognitively unimpaired (CU) subjects and with mild cognitive impairment (MCI).
Methods
Plasma (Aβ42/40, p-tau181, p-tau231, NfL, and GFAP) and neuroimaging (hippocampal volume, centiloid of amyloid-PET, and tau-SUVR of tau-PET) biomarkers were assessed at baseline in 218 non-demented subjects (CU = 140; MCI = 78) from the Geneva Memory Center. Global cognition (MMSE) was evaluated at baseline and at follow-ups up to 5.7 years. We used linear mixed-effects models and Cox proportional-hazards regression to assess the association between biomarkers and cognitive decline. Lastly, sample size calculations using the linear mixed-effects models were performed on subjects positive for amyloid-PET combined with tau-PET and plasma biomarker positivity.
Results
Cognitive decline was significantly predicted in MCI by baseline plasma NfL (β=-0.55), GFAP (β=-0.36), hippocampal volume (β = 0.44), centiloid (β=-0.38), and tau-SUVR (β=-0.66) (all p < 0.05). Subgroup analysis with amyloid-positive MCI participants also showed that only NfL and GFAP were the only significant predictors of cognitive decline among plasma biomarkers. Overall, NfL and tau-SUVR showed the highest prognostic values (hazard ratios of 7.3 and 5.9). Lastly, we demonstrated that adding NfL to the inclusion criteria could reduce the sample sizes of future AD clinical trials by up to one-fourth in subjects with amyloid-PET positivity or by half in subjects with amyloid-PET and tau-PET positivity.
Conclusions
Plasma NfL and GFAP predict cognitive decline in a similar manner to traditional imaging techniques in amyloid-positive MCI patients. Hence, even though they are non-specific biomarkers of AD, both can be implemented in memory clinic workups as important prognostic biomarkers. Likewise, future clinical trials might employ plasma biomarkers as additional inclusion criteria to stratify patients at higher risk of cognitive decline to reduce sample sizes and enhance effectiveness.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13195-024-01478-9.
Introduction
Alzheimer’s disease (AD) pathology is defined by the accumulation of amyloid plaques (A), tau neurofibrillary tangles (T), and neurodegeneration (N) [1]. These biomarkers can be assessed using magnetic resonance imaging (MRI), positron emission tomography (PET), or cerebrospinal fluid (CSF). However, all of these are either expensive or invasive techniques even though they are accurate in measuring the AD biomarkers. Recently, plasma biomarkers proved to be a promising tool to identify AD pathology and track disease progression, finding of great importance due to their accessibility, affordability, and acceptability. For instance, plasma Aβ42/40, phosphorylated tau at threonine 181 (p-tau181), 231 (p-tau231), and neurofilament light chain (NfL) are associated respectively with ATN model measures [2–4]. Thus, implementing plasma biomarkers in the diagnostic workup could spare a significant number of expensive traditional exams to patients and improve the cost effectiveness of health services [5].
Traditional neuroimaging biomarkers have been shown to identify non-demented subjects at high risk for cognitive decline. The amyloid load evaluated by PET or CSF can predict cognitive decline in cognitively unimpaired (CU) subjects [6] and subjects with mild cognitive impairment (MCI) [7]. Likewise, hippocampal volume also significantly predicted the progression to dementia in MCI subjects [8]. Nonetheless, when compared to amyloid and hippocampal volume, tau PET was the strongest predictor of cognitive decline in the AD continuum, from CU to AD [9, 10].
In line with the evidence from neuroimaging, plasma biomarkers were found to be able to predict cognitive decline in CU and MCI. Baseline plasma Aβ42/40 and NfL concentrations were able to predict cognitive decline in a CU research population [11]. Likewise, Cullen and colleagues [12] found similar results for plasma Aβ42/40, NfL, and p-tau217. P-tau181 was also found to be efficient in predicting cognitive changes and grey matter changes in MCI and CU subjects, whereas NfL predicted cognitive deterioration only in cognitively impaired subjects [4, 13]. Further, p-tau231, a plasma marker which increases early with Aβ dysmetabolism [14], has been shown to predict increases in Aβ PET signal over 3 years [14]. In MCI subjects, baseline p-tau181 and NfL combined showed the best predictor model regarding cognitive decline, whilst Aβ42/40 was not predictive [15]. A post-mortem study showed similar findings, namely that p-tau181, p-tau231, and NfL levels were significant predictors of a steeper cognitive decline, while no significant result was found for Aβ42/40 [16]. Another study revealed similar effects for levels of p-tau181, NfL, and glial fibrillary acidic protein (GFAP); however, the Aβ42/40 was once again not a significant predictor of cognitive deterioration [17].
Overall, recent evidence suggests the potential of plasma biomarkers in the identification of at-risk subjects. However, the utility of these biomarkers in predicting cognitive changes might vary according to several factors including the cognitive status. For instance, baseline Aβ42/40 is useful for identifying at-risk CU subjects, although this is not so evident in MCI [13]. Furthermore, p-tau231 and GFAP also demonstrated their utility, even though evidence is scarce.
However, despite the literature dealing with neuroimaging and plasma biomarkers predicting cognitive changes over time, both biomarkers were mostly tested independently, and little is known about the relative predictive power when comparing them directly. Therefore, this study is intended to: (i) test the association between baseline plasma and neuroimaging biomarkers from the ATN model with cognitive decline in non-demented individuals; (ii) measure the prognostic value of plasma and neuroimaging biomarkers in cognitive decline, and (iii) calculate how adding plasma biomarkers to inclusion criteria could decrease the sample sizes in preventive AD clinical trials.
Methods
Participants
The study selected subjects from the Geneva Memory Center (GMC) cohort. All patients underwent diagnostic workup including clinical and neuropsychological evaluations, as well as biomarker assessment either as part of the diagnostic workup itself or in the context of clinical research projects [18]. Non-demented participants with plasma collection within 1.5 years from the last global cognition test and at least one follow-up exam at the GMC were enrolled. For the CU group, we considered all subjects without any cognitive impairment, including worried well, and subjective cognitive decline, whereas MCI participants were included based on objective clinical and cognitive diagnostic criteria [19]. All participants signed an informed consent form prior to enrollment in the study. The Geneva Ethics Committee approved the study (PB_2016 − 01346 and 2020_00403).
Biomarkers collection, analyses, and assessment
Plasma biomarkers
Plasma was collected in EDTA tubes, kept for two hours at room temperature prior to centrifugation (1700 g, 15 min), aliquoted as 500uL in 1.2mL polypropylene tubes, and maintained at -80 °C in the local biobank of the Memory Center of Geneva University Hospitals. Aliquots were sent under secure conditions to the Clinical Neurochemistry Laboratory, University of Gothenburg (Sweden), where they were analyzed. The concentrations of plasma Aβ42, Aβ40, GFAP, and NfL were determined on an HD-X Automated Immunoassay Analyzer using commercially available Single molecule array (Simoa) Assay Kits in accordance with the recommendations from the manufacturer (Quanterix, Billerica, MA; https://www.quanterix.com/simoa-technology/). Alternately, the levels of p-tau181 [20] and p-tau231 [4] were measured using homebrew Simoa assays developed at the Clinical Neurochemistry Laboratory, University of Gothenburg (Sweden).
Neuroimaging biomarkers
The amyloid-PET images were acquired using 18 F-florbetapir or 18 F-flutemetamol tracers, while tau-PET images were acquired using 18 F-Flortaucipir using a protocol previously described in detail here [21]. Briefly, 18 F-florbetapir images were acquired 50 min after injection of 200 MBq during 15 min; 18 F-flutemetamol images were acquired 90 min after injection of 150 MBq during 20 min; and 18 F-flortaucipir images were acquired 75 min after injection of 180 MBq during 30 min. Acquisitions were obtained on Siemens Biograph and Biograph Vision scanners (Siemens, Washington, DC), reconstructed using a 3D OSEM iterative reconstruction, corrected for randoms, dead time, normalization, scatter, attenuation, and sensitivity [21]. An in-house pipeline based on SPM12 (Wellcome Department of Cognitive Neurology, London, UK) was used for the PET images processing [21]. Considering that we used two different amyloid-PET tracers, SUVR was converted to the centiloid scale following guidelines from the Global Alzheimer’s Association Interactive Network (GAAIN) [22]. The tau-PET global tau standardized uptake value ratio (SUVR) was computed as the average across parahippocampal gyrus, amygdala, mid-occipital cortex, and inferior temporal cortex [23].
The hippocampal volume was extracted from structural 3T MRI images. The left and right hippocampal volumes were averaged and normalized according to the total intracranial volume. The extraction was performed in FreeSurfer (version7.0–recon-all; https://surfer.nmr.mgh.harvard.edu).
ATN-C measures
Plasma and neuroimaging biomarkers were included as surrogates of the ATN model. Amyloid measures were plasma Aβ42/40 and the centiloid (A), tau was evaluated with plasma p-tau181 and p-tau231 and the global SUVR (T), while neurodegeneration was assessed by NfL in plasma and the hippocampal volume (N). In addition, plasma GFAP refers to inflammatory processes (I). Our main outcome was the global score of Mini-Mental State Examination (MMSE) at baseline and consequent follow-ups (C).
Statistical analysis
The differences of baseline demographics, clinical, cognitive, and biomarkers between CU and MCI cohorts were evaluated by Mann-Whitney test for continuous variables and a Chi-squared test for categorical variables. Moreover, to evaluate if the plasma biomarkers were affected by co-morbidities [24], the medical history of cardiovascular diseases, hypertension, hypercholesterolemia, and diabetes was considered. For that, the differences in each plasma biomarker between the groups with and without co-morbidity were performed independently for the CU and MCI groups. This analysis was performed using the Wilcoxon rank sum test, while the correlation between creatinine and plasma biomarkers was assessed using Pearson’s correlation.
Our first aim to test how baseline plasma and neuroimaging biomarkers are associated with cognitive decline over time was tested through multiple linear mixed-effects (LME) models. The MMSE score was the dependent variable, whereas plasma and neuroimaging biomarkers were independent variables for CU and MCI separately. Univariate and multivariate models, corrected by age, sex, and education, were run using each biomarker alone and altogether, respectively. The models had the following structure: MMSE ~ age + sex + education + time * biomarker. An additional multivariate model was performed only with the significant predictors of cognitive decline detected in the total multivariate LME model. The model fit was evaluated using the Akaike information criterion (AIC) and all the models comprised a random intercept and a random slope of time for each subject. In addition, a subgroup analysis was performed on amyloid-PET positive (Amy+) MCI and CU subjects independently based on the visual assessment by an expert in nuclear medicine. In order to ensure a standardized comparison of all biomarkers, the levels of plasma biomarkers were transformed on the logarithmic scale prior to calculation of the z-score, whereas neuroimaging biomarkers were only z-scored (sensitivity analysis with raw values in Table S1 in Supplementary Materials).
The prognostic value of the groups positive to each plasma and neuroimaging biomarker in cognitive decline was evaluated using Cox proportional-hazards (CPH) regression models. Thus, subjects with values greater than the 90% percentile of the distribution in the CU subjects formed the positive group for each biomarker. On the other hand, cognitive decline was dichotomized in each subject if the observed decline was higher than the expected decline found by Schneider and colleagues [25] based on the follow-up duration and subject’s age. In this manner, we categorized each participant as either a “decliner” or “stable” based on the anticipated changes observed in MMSE. The same method was already implemented in a recent study investigating the predictive effect of tau and hypometabolism in cognitive decline in the same cohort [26]. Kaplan-Meier survival curves were plotted to estimate the probability of “cognitive decline” occurring over time different groups, categorized by the presence or absence of each biomarker.
Lastly, a sample size calculation was conducted to assess the potential reduction in sample sizes for future AD clinical trials by including plasma biomarker positivity as part of the inclusion criteria. Therefore, in line with the most recent clinical trials [27, 28], we calculated sample sizes considering Amy + MCI subjects combined with tau-PET and plasma biomarkers positivity identified in the CPH regression models. The number of subjects per arm was calculated aiming for the detection of cognitive decline slowing between 20% and 50% [29]. The power analysis only comprised plasma biomarkers that predicted significant cognitive decline in LME models, and it was based on previous research for mixed models [30]. For that, we considered the random slopes, inter-subject variability, and the residual error of variance from LME models that only included participants that were positive for each combination of positive biomarkers. The calculation considered a clinical trial with a duration of two years and annual assessments with a statistical power of 90% and an alpha level of 5%. The formula for calculating the sample size can be found in the Supplementary Materials [30]. At last, the number of participants to screen for preventive AD clinical trials was estimated considering the positivity rate for each biomarker and the sample size previously calculated. All analyses were performed in R (Version4.2.0).
Results
Participants
A total of 218 participants (57% females, mean age = 67.7 [SD = 8.6]) had plasma and cognitive evaluations divided by cognitive stage, namely 140 CU and 78 MCI. However, the subsample with baseline neuroimaging biomarkers was slightly lower, namely a total of 146 (CU = 75, MCI = 71) for hippocampal volume, 105 (CU = 36, MCI = 69) for amyloid-PET, and 84 (CU = 26, MCI = 58) for tau-PET. The average time (in months) between the plasma collection and the baseline MMSE evaluation was 4.3 ± 3.7 for CU and 3.6 ± 3.7 for MCI subjects. Additionally, the baseline plasma and neuroimaging biomarkers were separated by 0.6 ± 5.5 (CU = 0.05 ± 5.4, MCI = 1.4 ± 5.5) for structural MRI, 3.1 ± 6.3 (CU = 5.9 ± 5, MCI = 1.7 ± 6.5) for amy-PET, and 4.4 ± 7.1 (CU = 6.4 ± 5.3, MCI = 3.5 ± 7.6) for tau-PET. The average interval of cognitive evaluation follow-up in our cohort was 30.5 ± 13.9 months. Specifically, it was 30.3 ± 14.2 months for CU participants and 31.1 ± 13.5 for those with MCI. There were no statistically significant differences observed in all plasma biomarkers in CU and MCI between the subgroups with and without cardiovascular disease, hypercholesterolemia, hypertension, and diabetes (p > 0.05; Figure S1 in Supplementary Materials). The only exception was GFAP, which was significantly increased in CU participants with hypercholesterolemia (p = 0.01) and a marginally significant decrease in MCI participants with diabetes (p = 0.5; Figure S1 in Supplementary Materials). The mean and standard deviation of baseline variables is displayed in Table 1.
Table 1.
Baseline characteristics of the study cohort by cognitive stage. Table denotes mean ± standard deviation for continuous variables and frequency (percentage) for dichotomous variables
| Variables | CU (n = 140) | MCI (n = 78) | Total sample | p | ||
|---|---|---|---|---|---|---|
| Demographics | Mean ± SD | n | Mean ± SD | n | N | |
| Age, years | 65 ± 8 | 140 | 72 ± 7.7 | 78 | 218 | < 0.001 |
| Gender, female | 89 (64%) | 140 | 36 (46%) | 78 | 218 | 0.018 |
| Education, years | 16 ± 4 | 140 | 14 ± 4 | 78 | 218 | 0.002 |
| MMSE | 28.6 ± 1.4 | 140 | 26 ± 2.4 | 78 | 218 | < 0.001 |
| APOE carriers | 36 (28%) | 136 | 38 (49%) | 68 | 204 | < 0.001 |
| Imaging | Mean ± SD | n | Mean ± SD | n | N | |
| Hippocampal volume | 7838 ± 788 | 75 | 6983 ± 996 | 71 | 146 | < 0.001 |
| Amyloid PET positivity | 9 (25%) | 36 | 39 (57%) | 77 | 113 | 0.008 |
| Amyloid Centiloid | 14.5 ± 32.8 | 35 | 41.7 ± 42.8 | 68 | 103 | 0.002 |
| Tau PET positivity | 1 (3%) | 36 | 24 (35%) | 69 | 105 | 0.001 |
| Tau Global SUVR | 1.16 ± 0.14 | 23 | 1.32 ± 0.26 | 57 | 80 | 0.014 |
| Plasma Biomarkers | Mean ± SD | n | Mean ± SD | n | N | |
| Aβ42/Aβ40 | 0.064 ± 0.013 | 140 | 0.059 ± 0.013 | 78 | 218 | 0.009 |
| p-tau181 | 13.2 ± 7 | 140 | 19.9 ± 10 | 78 | 218 | < 0.001 |
| p-tau231 | 7.8 ± 4.1 | 140 | 12.2 ± 6.1 | 78 | 218 | < 0.001 |
| NfL | 15.8 ± 7.9 | 140 | 20.9 ± 9.3 | 78 | 218 | < 0.001 |
| GFAP | 113 ± 55 | 140 | 187 ± 113 | 78 | 218 | < 0.001 |
Linear mixed effect models
Univariate models
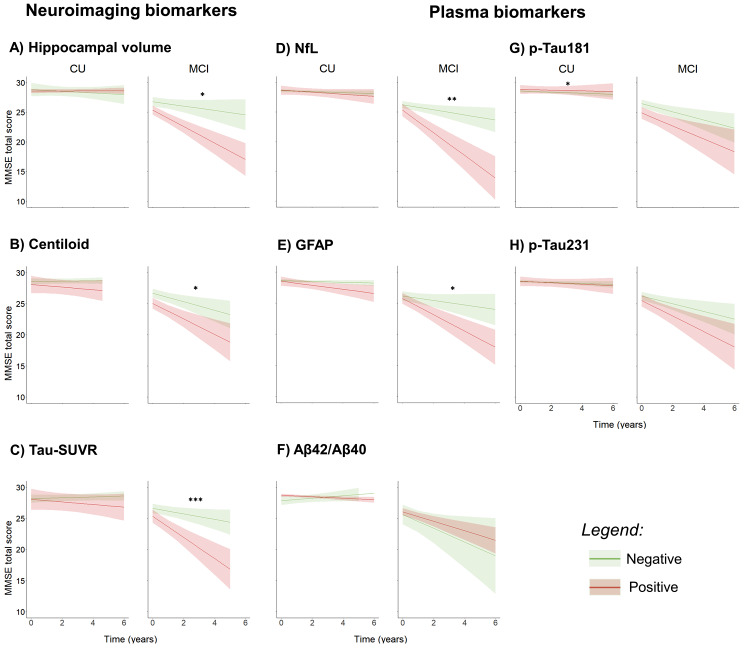
The univariate LME model in CU subjects revealed that only p-tau181 had significant interaction between the baseline level and time (β = 0.12, p = 0.02). The other biomarker models revealed no significant effect in the CU subjects (Table S2 in Supplementary Materials). In MCI, LME models revealed that cognitive decline was significantly predicted by NfL (β=-0.43, p = 0.006), GFAP (β=-0.38, p = 0.015), and a trend toward significance was observed in Aβ42/40 (β = 0.34, p = 0.051). Likewise, neuroimaging biomarkers significantly predicted cognitive decline, namely hippocampal volume (β = 0.44, p = 0.01), centiloid (β=-0.38, p = 0.04), and tau-SUVR (β=-0.66, p < 0.001). P-tau181 and p-tau231 values did not reveal a significant prediction of cognitive decline (Fig. 1; Table 2). Subgroup analysis in MCI patients positive to amyloid showed that GFAP (β=-1.32, p = 0.016) and NfL (β=-1.84, p < 0.001) significantly predicted cognitive decline, whilst for other biomarkers no significant interactions were found. All biomarkers revealed no significant results for MCI subjects negative to amyloid. In the Amy + CU group, the LME models also did not reveal any significant results in any biomarker.
Fig. 1.
Linear mixed model prediction in both CU and MCI subset based on baseline neuroimaging and plasma biomarkers. Biomarker positivity in MCI and CU was defined based on the 90% percentile of the distribution in the CU. *** p < 0.001; ** p < 0.01; * p < 0.05
Table 2.
Linear mixed models of plasma and neuroimaging biomarkers predicting MMSE score change in MCI. The first columns represent the univariate model with each biomarker modelled individually, and the last columns represent the model comprising all the biomarkers. All the models included age, sex, and years of education as fixed factors
| Predictors | Univariate model with one biomarker | Multivariate model with all biomarkers (AIC = 621) | Multivariate model with significant predictors (AIC = 715) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | Std. Error | p | AIC | Estimate | Std. Error | p | Estimate | Std. Error | p | |
| Plasma biomarkers | ||||||||||
| p-Tau181 | -0.15 | 0.17 | 0.38 | 944 | 0.59 | 0.32 | 0.06 | |||
| p-Tau231 | -0.19 | 0.29 | 0.28 | 957 | -0.15 | 0.38 | 0.69 | |||
| Aβ42/Aβ40 | 0.34 | 0.18 | 0.05 | 1002 | -0.12 | 0.22 | 0.59 | |||
| GFAP | -0.38 | 0.16 | 0.01 | 996 | 0.18 | 0.29 | 0.53 | |||
| NfL | -0.43 | 0.16 | 0.006 | 1000 | -0.8 | 0.31 | 0.009 | -0.42 | 0.17 | 0.02 |
| Neuroimaging biomarkers | ||||||||||
| Hippocampal volume | 0.44 | 0.18 | 0.01 | 921 | 0.17 | 0.25 | 0.49 | |||
| Centiloid | -0.38 | 0.19 | 0.04 | 874 | -0.06 | 0.3 | 0.84 | |||
| Tau-SUVR | -0.66 | 0.19 | 0.001 | 715 | -0.71 | 0.31 | 0.02 | -0.55 | 0.17 | 0.002 |
Multivariate models
The multivariate model in CU subjects revealed a marginally significant effect of p-tau181 (β = 0.29, p = 0.08). No other significant results were found in CU subjects. In MCI, the total LME model revealed that only NfL (β=-0.8, p = 0.009) and tau-SUVR (β=-0.71, p = 0.02) significantly predicted cognitive decline (Fig. 1; Table 2). Additionally, the multivariate LME comprising only the significant biomarkers in MCI, revealed similar results than univariate models, namely tau-SUVR (β=-0.55, p = 0.002) had a slightly larger coefficient than NfL (β=-0.42, p = 0.02). The model fit of the multivariate model comprising all the plasma and neuroimaging biomarkers (AIC = 621) was higher than all univariate models (AIC for each biomarker in Table 2), and also than the multivariate with significant predictors (AIC = 715).
Cox proportional-hazards regression model
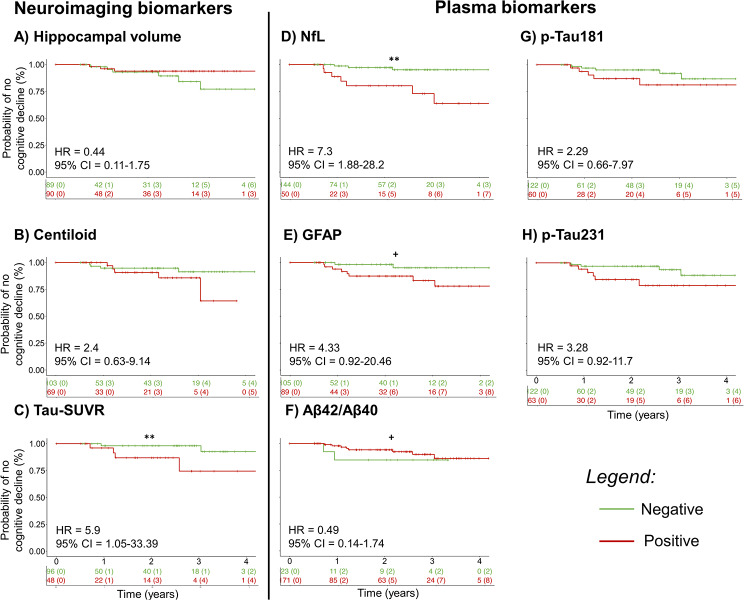
The categorization method labeled a total of 11 cognitive decliners, namely 10 of them in the MCI group and one amongst the CU. All biomarkers had non-significant effects in CU subjects, which can be explained by the low number of cognitive decliners.
CPH analysis revealed that baseline NfL (HR = 7.28, 95%CI = 1.88–28.2, p = 0.004) and tau-SUVR (HR = 5.9, 95%CI = 1.05–33.39, p = 0.04) were a significant predictor of converting to “cognitive decliner” in MCI. In line with this, baseline GFAP (HR = 4.33, 95%CI = 0.92–20.46, p = 0.06), and p-tau231 (HR = 3.28, 95%CI = 0.92–11.7, p = 0.07) had a trend toward significance. At last, no significant effect was detected in baseline p-tau181 (HR = 2.29, 95%CI = 0.66–7.97, p = 0.19), Aβ42/40 (HR = 0.49,95%CI = 0.14–1.74, p = 0.46), hippocampal volume (HR = 0.44, 95%CI = 0.11–1.75, p = 0.24), and centiloid (HR = 2.4, 95%CI = 0.63–9.14, p = 0.2) (Fig. 2).
Fig. 2.
Kaplan-Meier Survival Curve plot showing the survival probability of cognitive decline in MCI over time considering the positivity in plasma and neuroimaging biomarkers. ** p < 0.01; * p < 0.05; + p < 0.1
Sample size calculation for future AD clinical trials
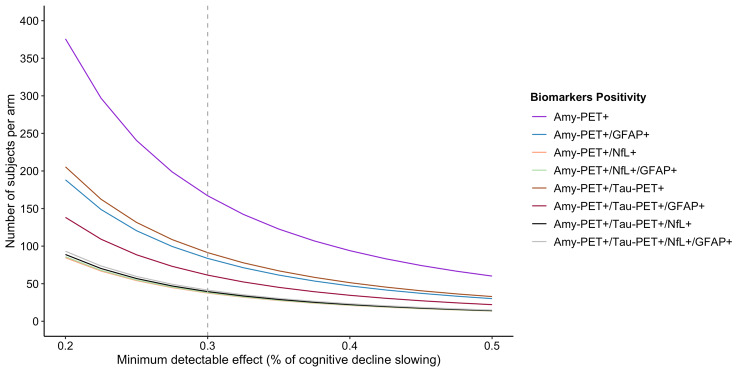
A clinical trial with Amy + MCI participants aiming a reduction of 30% of cognitive decline will need 164 participants per arm. If we additionally consider GFAP+, the sample size will reduce two-fold and 84 participants per arm will be needed. On the other hand, if we consider Amy+/ NfL + subjects the sample size will be reduced by a factor of 4 because the estimation is 38 participants per arm. Lastly, if we consider both NfL+/ GFAP+, the results will be very similar to considering only NfL+. Moreover, the number of participants needed to screen varies among the biomarkers. For instance, a preventive trial studying a reduction of 30% of cognitive decline in MCI participants will need to screen 167 Amy + subjects per arm. If we add GFAP + to the inclusion criteria, we will need to screen 142 subjects per arm and 106 subjects per arm if we consider NfL+.
Considering a trial enrolling Amy+/Tau + participants to detect a 30% slowing of cognitive decline, the study will need 100 participants per arm. In the case of adding GFAP+, the sample size will decrease to 64 participants per arm, whereas the inclusion of NfL + will decrease the sample size to 39 participants per arm. Likewise, the number of subjects to screen will decrease, namely, the Amy+/Tau + participants needed to screen will be 204 per arm, whereas the inclusion of GFAP + in screening will decrease this number to 185 and NfL + to 139. All the results for other percentages of cognitive decline are reported in Table 3; Fig. 3.
Table 3.
Number of participants to enroll and screen for future AD clinical trials. The sample size calculation was based on the LME models for subjects positive to amyloid-PET combined with tau-PET and/or plasma biomarkers positivity. The number of participants per arm was estimated for several percentages of cognitive decline slowing (measured as MMSE points/year) and the number of participants to screen were also calculated based on the positivity rate for each biomarker
| Positivity based on | Linear mixed effect models |
Number of participants per arm according to the % of cognitive decline slowing (number of participants to screen) | ||||||
|---|---|---|---|---|---|---|---|---|
| N out of 39 amy-positive MCI participants | Inter-subject variability of random slope | Residual variance | Random slope (cognitive decline) | 20% | 30% | 40% | 50% | |
| Amy-PET | 39 (100%) | 0.68 | 2.02 | -1.07 | 375 (375) | 167 (167) | 94 (94) | 60 (60) |
| Amy-PET/NfL | 14 (36%) | 0.55 | 1.92 | -2.14 | 85 (236) | 38 (106) | 21 (58) | 14 (39) |
| Amy-PET/GFAP | 23 (59%) | 0.61 | 1.97 | -1.47 | 188 (319) | 84 (142) | 47 (80) | 30 (51) |
| Amy-PET/NfL/GFAP | 12 (31%) | 0.56 | 1.94 | -2.13 | 86 (277) | 38 (123) | 22 (71) | 14 (45) |
| Amy-PET/Tau-PET | 19 (49%) | 0.68 | 1.9 | -1.36 | 226 (461) | 100 (204) | 56 (114) | 36 (100) |
| Amy-PET/Tau-PET/NfL | 11 (28%) | 0.51 | 1.92 | -2.05 | 89 (317) | 39 (139) | 22 (79) | 14 (50) |
| Tau-PET/GFAP | 13 (33%) | 0.57 | 1.98 | -1.69 | 138 (418) | 61 (185) | 35 (106) | 22 (67) |
| Tau-PET/NfL/GFAP | 10 (26%) | 0.52 | 1.94 | -2.02 | 93 (358) | 41 (158) | 23 (88) | 15 (58) |
Fig. 3.
Number of amyloid positive MCI subjects per arm for preventive clinical trials with different percentages of cognitive decline slowing considering the positivity of plasma biomarkers in subjects positive to Amy-PET only or combined with Tau-PET
Discussion
This study suggests that plasma GFAP and NfL levels, and neuroimaging biomarkers (i.e., centiloid, tau-SUVR, hippocampal volume) can predict cognitive decline in MCI subjects. Specifically, the most accurate neuroimaging and plasma biomarkers for predicting cognitive deterioration were NfL and tau-SUVR. These results were also supported by the prognostic values suggesting a higher likelihood of cognitive decline in subjects positive to NfL and global tau-SUVR. Finally, we showed that evaluating NfL and GFAP levels in future AD clinical trials could significantly reduce the number of subjects to enroll.
In line with our results, NfL has been suggested as a strong predictor of cognitive changes among plasma biomarkers, such as Aβ42/40 [11, 13, 15] or total tau [31]. In fact, our results also suggest a higher predictive power for NfL when compared to p-tau181 and p-tau231. Previous literature demonstrated that increased NfL levels were associated with N measures (i.e., brain atrophy, hypometabolism) [32], which might explain a stronger relationship with the cognitive outcome according to the ATN model. Our findings also showed that GFAP was able to predict a decrease in global cognition, even though of a smaller extend than NfL. However, recent works found similar effects between both biomarkers [17], thus, the inferiority of GFAP comparing to NfL in the predictive effect of long-term cognitive deterioration is still not clear. This might be explained by the fact that plasma GFAP might represent an earlier marker than NfL in the AD continuum, and it has been suggested to mediate the relationship between amyloid and tau pathologies in preclinical AD subjects [33]. Nonetheless, this is of particular interest because both NfL and GFAP were shown to be increased also in non-AD neurodegenerative disorders [13, 34] such as frontotemporal dementia [35]. Thus, even though NfL and GFAP are biomarkers non-specific to AD, the cognitive decline predicted by both can be explained by their association with neurodegeneration and neuroinflammation, respectively [13, 36]. Likewise, our subgroup analysis in amyloid positive subjects also suggests that both biomarkers can be used to the prognosis of AD progression. Overall, both biomarkers may play a significant role in memory clinic samples since multiple etiologies may be contributing to cognitive deterioration.
In contrary to both previously mentioned biomarkers, plasma Aβ42/40 has failed to predict cognitive decline in CU and MCI subjects [13, 15–17]. Previous literature has suggested that plasma Aβ42/40 is mostly increasing during the preclinical AD stage [37], while NfL and GFAP levels increase along the symptomatic stages of AD [2]. Nonetheless, our findings reveal a trend towards significance (i.e., p < 0.1), thus a smaller predictive power of Aβ42/40 when compared to NfL and GFAP. For p-tau181 and p-tau231, we did not observe the expected predictive effect in cognition found in previous studies with CU [13] and MCI subjects [15]. Intriguingly, we found a small negative association between p-tau181 and cognitive decline in CU subjects. This finding highlights the fact that p-tau181 is a more sensitive biomarker in later disease stages [16]. It is important to point out that despite the presence of a statistically significant effect in CU, the differences detected between the first and the last MMSE evaluation were very small in magnitude (Fig. 1). A possible explanation is that the MMSE test may not be sensitive enough to detect subtle changes in the CU population, leading to increased variability (see Limitations section). Moreover, p-tau181 levels could be influenced by other health factors such as chronic kidney disease [24], which were not considered in this study but commonly observed in memory clinic populations (for other co-morbidities analysis see Figure S1 in Supplementary Materials). A recent longitudinal study has shown minimal changes of p-tau181 and p-tau231 in Aβ-positive CU and MCI individuals, despite large baseline changes [38] This change is better represented by p-tau217 which was associated with atrophy and cognitive measure over 8-years [38]. In fact, both p-tau181 and p-tau231 have demonstrated inferior diagnostic accuracy in identifying amyloid and tau in comparison with p-tau217 [39].
Our results are also in line with previous literature suggesting that tau-SUVR was found to be the strongest predictor among neuroimaging biomarkers of cognitive deterioration [29]. Moreover, previous studies also showed that tau-SUVR was the best predictor when compared to CSF biomarkers (i.e., Aβ42, p-tau181, t-tau) [9]. Although in our study neuroimaging biomarkers such as centiloid and hippocampal volume also revealed significant predictions of cognitive changes, their predictive effect was not as strong as NfL and tau-SUVR. The prognostic values estimated in CPH models also suggested NfL and tau-SUVR as the strongest predictors of cognitive decline. Given that measures of tau and neurodegeneration are substantially related with cognitive outcome, these findings are consistent with the ATN model [1].
Lastly, our findings are also important in future preventive AD clinical trials because they allow a proper identification of the target population [40]. We demonstrated that identifying NfL + in the group of Amy + subjects would result in a fourfold reduction in sample size, whereas if we identified GFAP + we could reduce it by twofold. The addition of NfL + to the inclusion criteria of trials enrolling subjects positive for amy-PET and tau-PET can also reduce the sample size by half. The decrease in sample size is a result of include NfL and GFAP in the inclusion criteria, as these markers are indicative of a higher probability of cognitive decline. Additionally, considering the significant reduction of subjects to enroll by the implementation of plasma biomarkers, we also demonstrated that is possible to reduce the number of subjects to screen. Our findings are in line with previous sample size calculations using other neurodegeneration measures for AD clinical trials [41]. Thus, the use of plasma biomarkers of neurodegeneration and neuroinflammation to identify Amy + subjects with a higher likelihood of declining cognitively should be considered a good research practice in future clinical trials, allowing a more efficient use of resources.
Limitations
In terms of limitations, this study was not able to confirm the predictive effect in CU subjects already demonstrated in the literature [11–13, 42]. The lower sample size and the lack of long-term follow-ups might explain this result. Firstly, our CU cohort was composed of 140 participants, whereas previous studies had sample sizes ranging from 150 to 564 participants. Additionally, the maximum follow-up in our CU cohort was 5.7 years and the median was 2.2 years, only 53 subjects had follow-up later than 3-years. Lastly, MMSE might not be the most suitable neuropsychological test in CU participants given its low sensitivity in this population, which might benefit with other neuropsychological composites such as Preclinical Alzheimer Cognitive Composite [43]. Overall, the assessment of long-term effects in CU subjects has been highly influenced by these factors given that the decline in this population is not as steep as in the MCI stage.
Furthermore, the positivity in MCI subjects was estimated based on data from the CU subjects, considering the lack of approved cut-offs. As a result, the calculation of the statistical power analysis was influenced by the characteristics of our cohort. Therefore, future research should test for inter-individual differences in genotype or vascular factors in the prediction of cognitive decline. Nonetheless, our findings in sample size calculation were in line with the LME analysis, which did not consider biomarker positivity. Likewise, our comparison between plasma and neuroimaging biomarkers is limited by the fact that the population size is not identical for all analyses. We also acknowledge a vast array of p-tau immunoassays are described in the literature, with different properties, which might have influenced our result [44].
Conclusion
This study suggests that plasma NfL and GFAP could be cost-effective prognostic biomarkers in the AD continuum. Although both biomarkers are not specific for AD pathology, they can assume an important role in the prediction of cognitive decline in amyloid-positive subjects. Specifically, NfL showed a similar result to tau-SUVR and was superior when compared to other plasma and neuroimaging biomarkers. Our findings were based on a unique memory clinic sample but suggesting their applicability to improve the diagnostic workup. In addition, the implementation of plasma biomarkers can help decreasing the samples sizes of AD clinical trials.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The Clinical Research Center, at Geneva University Hospital and Faculty of Medicine provides valuable support for regulatory submissions and data management, and the Biobank at Geneva University Hospital for biofluid processing and storage. The authors thank Avid Radiopharmaceuticals Inc. for providing the 18 F-Flortaucipir tracer without being involved in the data analysis or interpretation.
Author contributions
AJM performed the data wrangling and analysis, drafted the first version of the study, and revised the following versions critically for important intellectual content.FR, AL, and GBF contributed to draft the structure of the present work and revised the first version of the work critically for important intellectual content.All authors had full access to all the data in the study, substantially contributed to the interpretation of data for the present work, revised the work critically for important intellectual content, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
The Centre de la mémoire is funded by the following private donors under the supervision of the Private Foundation of Geneva University Hospitals: A.P.R.A. - Association Suisse pour la Recherche sur la Maladie d’Alzheimer, Genève; Fondation Segré, Genève; Race Against Dementia Foundation, London, UK; Fondation Child Care, Genève; Fondation Edmond J. Safra, Genève; Fondation Minkoff, Genève; Fondazione Agusta, Lugano; McCall Macbain Foundation, Canada; Nicole et René Keller, Genève; Fondation AETAS, Genève. Competitive research projects have been funded by: H2020 (projects n. 667375), Innovative Medicines Initiative (IMI contract n. 115736 and 115952), IMI2, Swiss National Science Foundation (projects n.320030_182772 and n. 320030_169876), VELUX Foundation. Augusto J. Mendes and Federica Ribaldi is supported by the Swiss National Science Foundation (projects n.320030_182772). VG was supported by the Swiss National Science Foundation (projects 320030_169876, 320030_185028 and IZSEZ0_188355), by the Velux foundation (project 1123), by the Schmidheiny foundation, the Boninchi foundation and by the Aetas foundation. Henrik Zetterberg is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2022 − 01018 and #2019–02397), the European Union’s Horizon Europe research and innovation programme under grant agreement No 101053962, Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809–2016862), the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C, and #ADSF-21-831377-C), the Bluefield Project, the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022-0270), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), the European Union Joint Programme – Neurodegenerative Disease Research (JPND2021-00694), the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre, and the UK Dementia Research Institute at UCL (UKDRI-1003).
Open access funding provided by University of Geneva
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study was approved by Geneva Ethics Committee (PB_2016 − 01346 and 2020_00403). All participants signed an informed consent form prior to enrollment in the study.
Consent for publication
All authors consent to the publication of this study.
Author access to data
All authors had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
GBF has received unrestricted grants and support for event organisation from ROCHE Pharmaceuticals; OM Pharma; EISAI Pharmaceuticals; Biogen Pharmaceuticals. VG received grants and speaker fees through her institution from Siemens Healthineers, GE Healthcare, Novo Nordisk and Janssen. HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). The other authors have nothing to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jack CR, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology [Internet]. 2016 [cited 2023 May 9];87:539–47. https://n.neurology.org/content/87/5/539. [DOI] [PMC free article] [PubMed]
- 2.Mattsson N, Andreasson U, Zetterberg H, Blennow K, Weiner MW, Aisen P et al. Association of Plasma Neurofilament Light With Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol [Internet]. 2017 [cited 2023 May 9];74:557–66. https://pubmed.ncbi.nlm.nih.gov/28346578/. [DOI] [PMC free article] [PubMed]
- 3.Janelidze S, Mattsson N, Palmqvist S, Smith R, Beach TG, Serrano GE et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med [Internet]. 2020 [cited 2023 May 9];26:379–86. https://pubmed.ncbi.nlm.nih.gov/32123385/. [DOI] [PubMed]
- 4.Ashton NJ, Pascoal TA, Karikari TK, Benedet AL, Lantero-Rodriguez J, Brinkmalm G et al. Plasma p-tau231: a new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol [Internet]. 2021 [cited 2023 Jun 29];141:709. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8043944/. [DOI] [PMC free article] [PubMed]
- 5.Altomare D, Stampacchia S, Ribaldi F, Tomczyk S, Chevalier C, Poulain G et al. Plasma biomarkers for Alzheimer’s disease: a field-test in a memory clinic. J Neurol Neurosurg Psychiatry [Internet]. 2023 [cited 2023 May 9];jnnp-2022-330619. https://pubmed.ncbi.nlm.nih.gov/37012066/. [DOI] [PMC free article] [PubMed]
- 6.Donohue MC, Sperling RA, Petersen R, Sun CK, Weiner M, Aisen PS. Association Between Elevated Brain Amyloid and Subsequent Cognitive Decline Among Cognitively Normal Persons. JAMA [Internet]. 2017 [cited 2023 May 9];317:2305–16. https://pubmed.ncbi.nlm.nih.gov/28609533/. [DOI] [PMC free article] [PubMed]
- 7.Dickerson BC, Wolk DA. Biomarker-based prediction of progression in MCI: Comparison of AD signature and hippocampal volume with spinal fluid amyloid-β and tau. Front Aging Neurosci [Internet]. 2013 [cited 2023 May 9];5. https://pubmed.ncbi.nlm.nih.gov/24130528/. [DOI] [PMC free article] [PubMed]
- 8.Apostolova LG, Dutton RA, Dinov ID, Hayashi KM, Toga AW, Cummings JL et al. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol [Internet]. 2006 [cited 2023 May 9];63:693–9. https://pubmed.ncbi.nlm.nih.gov/16682538/. [DOI] [PubMed]
- 9.Bucci M, Chiotis K, Nordberg A. Alzheimer’s disease profiled by fluid and imaging markers: tau PET best predicts cognitive decline. Mol Psychiatry [Internet]. 2021 [cited 2023 May 9];26:5888–98. https://pubmed.ncbi.nlm.nih.gov/34593971/. [DOI] [PMC free article] [PubMed]
- 10.Peretti DE, Ribaldi F, Scheffler M, Chicherio C, Frisoni GB, Garibotto V. Prognostic value of imaging-based ATN profiles in a memory clinic cohort. Eur J Nucl Med Mol Imaging [Internet]. 2023 [cited 2023 Jul 4]; https://pubmed.ncbi.nlm.nih.gov/37358619/. [DOI] [PMC free article] [PubMed]
- 11.Aschenbrenner AJ, Li Y, Henson RL, Volluz K, Hassenstab J, Verghese P et al. Comparison of plasma and CSF biomarkers in predicting cognitive decline. Ann Clin Transl Neurol [Internet]. 2022 [cited 2023 May 9];9:1739–51. https://pubmed.ncbi.nlm.nih.gov/36183195/. [DOI] [PMC free article] [PubMed]
- 12.Cullen NC, Leuzy A, Janelidze S, Palmqvist S, Svenningsson AL, Stomrud E et al. Plasma biomarkers of Alzheimer’s disease improve prediction of cognitive decline in cognitively unimpaired elderly populations. Nat Commun [Internet]. 2021 [cited 2023 May 9];12. https://pubmed.ncbi.nlm.nih.gov/34117234/. [DOI] [PMC free article] [PubMed]
- 13.Moscoso A, Grothe MJ, Ashton NJ, Karikari TK, Lantero Rodríguez J, Snellman A et al. Longitudinal Associations of Blood Phosphorylated Tau181 and Neurofilament Light Chain With Neurodegeneration in Alzheimer Disease. JAMA Neurol [Internet]. 2021 [cited 2023 May 9];78:396–406. https://pubmed.ncbi.nlm.nih.gov/33427873/. [DOI] [PMC free article] [PubMed]
- 14.Milà-Alomà M, Ashton NJ, Shekari M, Salvadó G, Ortiz-Romero P, Montoliu-Gaya L et al. Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer’s disease. Nat Med [Internet]. 2022 [cited 2023 Jun 29];28:1797–801. https://pubmed.ncbi.nlm.nih.gov/35953717/. [DOI] [PMC free article] [PubMed]
- 15.Cullen NC, Leuzy A, Palmqvist S, Janelidze S, Stomrud E, Pesini P et al. Individualized prognosis of cognitive decline and dementia in mild cognitive impairment based on plasma biomarker combinations. Nat Aging [Internet]. 2021 [cited 2023 May 9];1:114–23. https://pubmed.ncbi.nlm.nih.gov/37117993/. [DOI] [PubMed]
- 16.Smirnov DS, Ashton NJ, Blennow K, Zetterberg H, Simrén J, Lantero-Rodriguez J et al. Plasma biomarkers for Alzheimer’s Disease in relation to neuropathology and cognitive change. Acta Neuropathol [Internet]. 2022 [cited 2023 May 9];143:487–503. https://pubmed.ncbi.nlm.nih.gov/35195758/. [DOI] [PMC free article] [PubMed]
- 17.Saunders TS, Pozzolo FE, Heslegrave A, King D, McGeachan RI, Spires-Jones MP et al. Predictive blood biomarkers and brain changes associated with age-related cognitive decline. Brain Commun [Internet]. 2023 [cited 2023 May 9];5. https://academic.oup.com/braincomms/advance-article/doi/10.1093/braincomms/fcad113/7109761. [DOI] [PMC free article] [PubMed]
- 18.Ribaldi F, Chicherio C, Altomare D, Martins M, Tomczyk S, Jelescu I et al. Brain connectivity and metacognition in persons with subjective cognitive decline (COSCODE): rationale and study design. Alzheimers Res Ther [Internet]. 2021 [cited 2023 May 9];13. https://pubmed.ncbi.nlm.nih.gov/34034799/. [DOI] [PMC free article] [PubMed]
- 19.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia [Internet]. 2011 [cited 2023 Jun 29];7:270–9. https://onlinelibrary.wiley.com/doi/full/10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed]
- 20.Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol [Internet]. 2020 [cited 2023 May 9];19:422–33. https://pubmed.ncbi.nlm.nih.gov/32333900/. [DOI] [PubMed]
- 21.Dodich A, Mendes A, Assal F, Chicherio C, Rakotomiaramanana B, Andryszak P et al. The A/T/N model applied through imaging biomarkers in a memory clinic. Eur J Nucl Med Mol Imaging [Internet]. 2020 [cited 2023 May 9];47:247–55. https://pubmed.ncbi.nlm.nih.gov/31792573/. [DOI] [PubMed]
- 22.Klunk WE, Koeppe RA, Price JC, Benzinger TL, Devous MD, Jagust WJ et al. The Centiloid Project: Standardizing quantitative amyloid plaque estimation by PET. Alzheimer’s & Dementia [Internet]. 2015 [cited 2023 Jun 5];11:1–15.e4. https://onlinelibrary.wiley.com/doi/full/10.1016/j.jalz.2014.07.003. [DOI] [PMC free article] [PubMed]
- 23.Mishra S, Gordon BA, Su Y, Christensen J, Friedrichsen K, Jackson K et al. AV-1451 PET imaging of tau pathology in preclinical Alzheimer disease: Defining a summary measure. Neuroimage [Internet]. 2017 [cited 2023 May 9];161:171–8. https://pubmed.ncbi.nlm.nih.gov/28756238/. [DOI] [PMC free article] [PubMed]
- 24.Mielke MM, Dage JL, Frank RD, Algeciras-Schimnich A, Knopman DS, Lowe VJ et al. Performance of plasma phosphorylated tau 181 and 217 in the community. Nat Med [Internet]. 2022 [cited 2023 Jun 15];28:1398–405. https://pubmed.ncbi.nlm.nih.gov/35618838/. [DOI] [PMC free article] [PubMed]
- 25.Schneider LS, Kennedy RE, Wang G, Cutter GR. Differences in Alzheimer disease clinical trial outcomes based on age of the participants. Neurology [Internet]. 2015 [cited 2023 May 9];84:1121–7. https://pubmed.ncbi.nlm.nih.gov/25681452/. [DOI] [PMC free article] [PubMed]
- 26.Boccalini C, Ribaldi F, Hristovska I, Arnone A, Peretti DE, Mu L et al. The impact of tau deposition and hypometabolism on cognitive impairment and longitudinal cognitive decline. Alzheimers Dement [Internet]. 2024 [cited 2024 Mar 12];20:221–33. https://pubmed.ncbi.nlm.nih.gov/37555516/. [DOI] [PMC free article] [PubMed]
- 27.Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks JD et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA [Internet]. 2023 [cited 2024 Jan 26];330:512–27. https://pubmed.ncbi.nlm.nih.gov/37459141/. [DOI] [PMC free article] [PubMed]
- 28.van CH CJS, RJ PA, C B et al. C, M G,. Lecanemab in Early Alzheimer’s Disease. N Engl J Med [Internet]. 2023 [cited 2024 Jan 26];388:142–3. https://pubmed.ncbi.nlm.nih.gov/36449413/.
- 29.Aschenbrenner AJ, Gordon BA, Benzinger TLS, Morris JC, Hassenstab JJ. Influence of tau PET, amyloid PET, and hippocampal volume on cognition in Alzheimer disease. Neurology [Internet]. 2018 [cited 2023 May 10];91:e859–66. https://pubmed.ncbi.nlm.nih.gov/30068637/. [DOI] [PMC free article] [PubMed]
- 30.Ard MC, Edland SD. Power calculations for clinical trials in Alzheimer’s disease. J Alzheimers Dis [Internet]. 2011 [cited 2023 May 9];26 Suppl 3:369–77. https://pubmed.ncbi.nlm.nih.gov/21971476/. [DOI] [PMC free article] [PubMed]
- 31.Marks JD, Syrjanen JA, Graff-Radford J, Petersen RC, Machulda MM, Campbell MR et al. Comparison of plasma neurofilament light and total tau as neurodegeneration markers: associations with cognitive and neuroimaging outcomes. Alzheimers Res Ther [Internet]. 2021 [cited 2023 May 10];13:1–14. https://alzres.biomedcentral.com/articles/10.1186/s13195-021-00944-y. [DOI] [PMC free article] [PubMed]
- 32.Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association Between Longitudinal Plasma Neurofilament Light and Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurol [Internet]. 2019 [cited 2023 May 10];76:791–9. https://pubmed.ncbi.nlm.nih.gov/31009028/. [DOI] [PMC free article] [PubMed]
- 33.Bellaver B, Povala G, Ferreira L, Pedro Ferrari-Souza PC, Leffa J, Lussier DT et al. FZ,. Astrocyte reactivity influences amyloid-β effects on tau pathology in preclinical Alzheimer’s disease. Nat Med [Internet]. 2023 [cited 2023 Jun 21]; 10.1038/s41591-023-02380-x. [DOI] [PMC free article] [PubMed]
- 34.Ashton NJ, Janelidze S, Al Khleifat A, van der Leuzy A, Karikari TK et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat Commun [Internet]. 2021 [cited 2023 Jun 29];12. https://pubmed.ncbi.nlm.nih.gov/34099648/. [DOI] [PMC free article] [PubMed]
- 35.Zhu N, Santos-Santos M, Illán-Gala I, Montal V, Estellés T, Barroeta I et al. Plasma glial fibrillary acidic protein and neurofilament light chain for the diagnostic and prognostic evaluation of frontotemporal dementia. Transl Neurodegener [Internet]. 2021 [cited 2023 May 10];10. https://pubmed.ncbi.nlm.nih.gov/34893073/. [DOI] [PMC free article] [PubMed]
- 36.Pichet Binette A, Palmqvist S, Bali D, Farrar G, Buckley CJ, Wolk DA et al. Combining plasma phospho-tau and accessible measures to evaluate progression to Alzheimer’s dementia in mild cognitive impairment patients. Alzheimers Res Ther [Internet]. 2022 [cited 2023 May 10];14. https://pubmed.ncbi.nlm.nih.gov/35351181/. [DOI] [PMC free article] [PubMed]
- 37.Palmqvist S, Janelidze S, Stomrud E, Zetterberg H, Karl J, Zink K et al. Performance of Fully Automated Plasma Assays as Screening Tests for Alzheimer Disease-Related β-Amyloid Status. JAMA Neurol [Internet]. 2019 [cited 2023 May 10];76:1060–9. https://pubmed.ncbi.nlm.nih.gov/31233127/. [DOI] [PMC free article] [PubMed]
- 38.Ashton NJ, Janelidze S, Mattsson-Carlgren N, Binette AP, Strandberg O, Brum WS et al. Differential roles of Aβ42/40, p-tau231 and p-tau217 for Alzheimer’s trial selection and disease monitoring. Nature Medicine 2022 28:12 [Internet]. 2022 [cited 2023 Jul 26];28:2555–62. https://www.nature.com/articles/s41591-022-02074-w. [DOI] [PMC free article] [PubMed]
- 39.Mendes AJ, Ribaldi F, Lathuiliere A, Ashton NJ, Janelidze S, Zetterberg H et al. Head-to-head study of diagnostic accuracy of plasma and cerebrospinal fluid p-tau217 versus p-tau181 and p-tau231 in a memory clinic cohort. J Neurol [Internet]. 2024 [cited 2024 Jan 26]; https://pubmed.ncbi.nlm.nih.gov/38195896/. [DOI] [PMC free article] [PubMed]
- 40.Angioni D, Hansson O, Bateman RJ, Rabe C, Toloue M, Braunstein JB et al. Can We Use Blood Biomarkers as Entry Criteria and for Monitoring Drug Treatment Effects in Clinical Trials? A Report from the EU/US CTAD Task Force. J Prev Alzheimers Dis [Internet]. 2023 [cited 2023 Jun 21]; https://link.springer.com/article/10.14283/jpad.2023.68. [DOI] [PubMed]
- 41.Mayblyum DV, Becker JA, Jacobs HIL, Buckley RF, Schultz AP, Sepulcre J et al. Comparing PET and MRI Biomarkers Predicting Cognitive Decline in Preclinical Alzheimer Disease. Neurology [Internet]. 2021 [cited 2023 May 10];96:E2933–43. https://pubmed.ncbi.nlm.nih.gov/33952655/. [DOI] [PMC free article] [PubMed]
- 42.Mattsson-Carlgren N, Salvadó G, Ashton NJ, Tideman P, Stomrud E, Zetterberg H et al. Prediction of Longitudinal Cognitive Decline in Preclinical Alzheimer Disease Using Plasma Biomarkers. JAMA Neurol [Internet]. 2023 [cited 2023 May 9];80. https://pubmed.ncbi.nlm.nih.gov/36745413/. [DOI] [PMC free article] [PubMed]
- 43.Donohue MC, Sperling RA, Salmon DP, Rentz DM, Raman R, Thomas RG et al. The Preclinical Alzheimer Cognitive Composite: Measuring Amyloid-Related Decline. JAMA Neurol [Internet]. 2014 [cited 2024 Mar 13];71:961. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4439182/. [DOI] [PMC free article] [PubMed]
- 44.Ashton NJ, Puig-Pijoan A, Milà-Alomà M, Fernández-Lebrero A, García-Escobar G, González-Ortiz F et al. Plasma and CSF biomarkers in a memory clinic: Head-to-head comparison of phosphorylated tau immunoassays. Alzheimers Dement [Internet]. 2023 [cited 2023 Jun 29];19. https://pubmed.ncbi.nlm.nih.gov/36370462/. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.