Abstract
Sucrose is an important storage and transport sugar of plants and an energy source for many phytopathogenic bacteria. To analyze regulation and biochemistry of sucrose metabolism of the fire blight pathogen Erwinia amylovora, a chromosomal fragment which enabled Escherichia coli to utilize sucrose as sole carbon source was cloned. By transposon mutagenesis, the scr regulon of E. amylovora was tagged, and its nucleotide sequence was determined. Five open reading frames, with the genes scrK, scrY, scrA, scrB, and scrR, had high homology to genes of the scr regulons from Klebsiella pneumoniae and plasmid pUR400. scrB and scrR of E. amylovora were fused to a histidine tag and to the maltose-binding protein (MalE) of E. coli, respectively. ScrB (53 kDa) catalyzed the hydrolysis of sucrose with a Km of 125 mM. Binding of a MalE-ScrR fusion protein to an scrYAB promoter fragment was shown by gel mobility shifts. This complex dissociated in the presence of fructose but not after addition of sucrose. Expression of the scr regulon was studied with an scrYAB promoter-green fluorescent protein gene fusion and measured by flow cytometry and spectrofluorometry. The operon was affected by catabolite repression and induced by sucrose or fructose. The level of gene induction correlated to the sucrose concentration in plant tissue, as shown by flow cytometry. Sucrose mutants created by site-directed mutagenesis did not produce significant fire blight symptoms on apple seedlings, indicating the importance of sucrose metabolism for colonization of host plants by E. amylovora.
The gram-negative bacterium Erwinia amylovora causes fire blight of apple, pear, and other rosaceous plants. Pathogenecity depends on the ability to produce the exopolysaccharide amylovoran (10, 13), to elicit a hypersensitive response on non-host plants (6, 8), and to metabolize sorbitol of the host plants (1). Rosaceous plants contain sorbitol and sucrose as storage and transport carbohydrates. The distribution of these carbohydrates is dependent on environmental conditions, species, and plant tissue (28, 52). The highest concentration of sucrose was found in the nectaries of host plants (14), which are assumed to be the main entry site for the pathogen when the pathogen is distributed by insects.
Sucrose is utilized by some but not all bacteria extracellularily or intracellularily. E. amylovora can metabolize sucrose via the secreted levansucrase, which polymerizes the homopolysaccharide levan and releases glucose from sucrose (27, 29), but also by uptake and intracellular metabolism.
The sucrose-utilizing system of enteric bacteria has been studied in Klebsiella pneumoniae and in some isolates of Escherichia coli and Salmonella spp. (45, 49). In E. coli and Salmonella spp., the conjugative plasmid pUR400 confers the ability to utilize sucrose (54), whereas the scr regulon of K. pneumoniae is located on the chromosome (42, 49). In these bacteria, the uptake of sucrose is mediated via the phosphoenolpyruvate-dependent carbohydrate:phosphotransferase system (PTS), yielding sucrose 6-phosphate, which is cleaved by an intracellular hydrolase into glucose 6-phosphate and fructose (45, 49). The scr regulons of K. pneumoniae and pUR400 consist of four structural genes: scrK codes for an ATP-dependent fructokinase (5), scrY codes for a sucrose-specific porin of the outer membrane (30), scrA codes for enzyme IIscr of the PTS, and scrB codes for an intracellular β-d-fructofuranoside fructohydrolase (EC 3.2.1.26), which cleaves sucrose 6-phosphate into β-d-fructose and α-d-glucose 6-phosphate (51). The regulon is controlled by the negative regulator ScrR (34) and is induced in medium containing sucrose, fructose, or raffinose (45, 46).
In this work, we cloned, sequenced, and characterized the scr regulon of E. amylovora. The regulation of sucrose metabolism was studied by gel shift assays and promoter-green fluorescent protein gene (gfp) fusions analyzed by flow cytometry also with bacteria extracted from plant tissue. Mutants carrying mutations in the scr genes were nonvirulent.
(A preliminary report has been published as a proceedings contribution [15].)
MATERIALS AND METHODS
Strains, plasmids, and oligonucleotides.
Table 1 lists the strains, plasmids, and oligonucleotides used in the experiments.
TABLE 1.
Stains, plasmids, oligonucleotides, and phage used
| Strain, plasmid, oligonucleotide, or phage | Properties | Source or reference |
|---|---|---|
| E. coli | ||
| GI698 | F−lacIq lacPL8 ampC::PtrpcI | Invitrogen |
| S17-1 | thi pro hsdR hsdM+ recA tra+ | 47 |
| E. amylovora | ||
| Ea1/79 | Wild-type strain isolated in 1979 in Germany | 26 |
| Ea7/74 | Wild-type strain isolated in 1974 in Germany | 26 |
| Ea7/74-S1 | Ea7/74 scrY::Tn5seq1, Kmr | This work |
| Ea1/79-S3 | Ea7/74 scrA::Tn5seq1, Kmr | This work |
| PD494Sm | Levan-deficient strain | 12 |
| PD494-S1 | PD494Sm scrY::Tn5seq1, Kmr | This work |
| Ea7/74-LS7 | Ea7/74 with Tn5 insertion in lsc, Kmr | 27 |
| Plasmids | ||
| pSU18 | P15A replicon, lacZ′, Cmr, 2.3-kb polylinker pUC18 | 7 |
| pGFPmut2 | ColE1 ori, 3.9 kb, Apr, pKEN2 derivative | 20 |
| pFG30 | 0.7-kb BamHI-PstI fragment from pGFPmut2 in pBlueSK+, 3.7 kb, Apr | Fangchery Gonga |
| pUC18 | ColE1 replicon, lacZ′, Apr, 2.7 kb | 55 |
| pMAL-c2 | Apr, expression vector | New England Biolabs |
| pQE31 | ColE1 replicon, Apr, (His)6 tag | Qiagen |
| pVK100 | IncP, mob+ cos, Tcr, Kmr | 35 |
| pSU18-Y1 | 0.25-kb PCR product with primers 3342 and 3341 from pSCR109 in pSU18, XbaI and SalI sites introduced, scrYAB promoter, Cmr | This work |
| pSU18-Y1gfp | 0.7-kb XbaI-Asp718 fragment (with gfp) from pFG30 in pSU18-Y1, Cmr | This work |
| pSU18-Y1gfpR | 1.2-kb PCR product with primers 3366 and 3367 from pSCR141 in pSU18-Y1gfp, EcoRV and Asp718 sites introduced, scrR+, Cmr | This work |
| pSCR100 | Chromosomal 20-kb HindIII fragment from E. amylovora in pVK100, scrK+Y+A+B+R+ | This work |
| pSCR107 | 1.8-kb BamHI fragment from pSCR100 in pUC18, scrA′ scrB′, Apr | This work |
| pSCR109 | 3.9-kb BamHI fragment from pSCR100 in pUC18, scrK+ scrY+ scrA′, Apr | This work |
| pSCR141 | 4.1-kb HindIII-EcoRI fragment from pSCR100 in pUC18, scrB′ scrR+, Apr | This work |
| pQE31scrB | 1.5-kb PCR product with primers 3584 and 3523 from pSCR100 in pQE31, SphI and HindIII sites introduced, scrB+, Apr | This work |
| pQE31scrR | 1-kb PCR product with primers 3583 and 3506 from pSCR141 in pQE31, BamHI and HindIII sites introduced, scrR+, Apr | This work |
| pMalscrR | 1-kb PCR product with primers 3583 and 3506 from pSCR141 in pMal-c2, BamHI and HindIII sites introduced, scrR+, Apr | This work |
| Oligonucleotidesb | ||
| 3506 | GCGAAGCTTTCACATGCCATTGCCGAAAGG (HindIII site) | This work |
| 3523 | GCGAAGCTTATTAGGCGCGATGGATCGTAG (HindIII site) | This work |
| 3341 | CGCGTCGACAGCCAGTAGCAGACATT (SalI site) | This work |
| 3342 | CGCTCTAGAAACGCTTTCCCAGGTAATG (XbaI site) | This work |
| 3366 | CGCGATATCCTGTGACAGTTCCAGCATTGAGA (EcoRV site) | This work |
| 3367 | CGCGGTACCAGTCCTGCTTGTGTCGTTAATTG (Asp718 site) | This work |
| 3583 | GCGGGATCCACTAAAAACAAACGTATTACCATTAAC (BamHI site) | This work |
| 3584 | CGCGCATGCGAGCGAAGCCCATTTGCTGAAAC (SphI site) | This work |
| Bacteriophage | ||
| λ::Tn5seq | Tn5, Kmr, λ b221cI857, Pam80, Kmr, promoter sequences from SP6 and T7 | 39 |
Ohio State University, Columbus, Ohio.
The site introduced by the underlined bases is indicated in parentheses.
Mutagenesis and screening of scr mutants.
Transposon insertions in scr genes were detected after infection of Escherichia coli S17-1(pSCR100) with phage λ::Tn5seq as white colonies on MacConkey agar (Gibco-BRL) with sucrose in the presence of kanamycin. Plasmids pSCR100-1 and pSCR100-3 were transferred into E. amylovora as described by Bernhard et al. (13). Plasmid pPH1JI, which is incompatible with pSCR100-1 and pSCR100-3, was then conjugated into a mutant, and cells were selected for resistance to chloramphenicol, kanamycin, and streptomycin to screen for bacteria with a transposon insertion in the chromosome.
Enzyme assays.
Sucrose hydrolase activity was determined in 1 ml of 100 mM phosphate buffer (pH 7.0) with 200 mM sucrose incubated at 28°C for 30 min. The reaction was stopped by boiling for 1 min. After removal of denatured protein, the glucose was determined with 10-μl aliquots added to 1 ml of 10 mM phosphate buffer–0.05% sodium azide with peroxidase (1 U), glucose oxidase (10 U), and 1 mg of ABTS (2,2-azino-di-3-ethyl-benzthiazolinsulfonate) (Boehringer, Mannheim, Germany). The reaction was complete after 30 min at 28°C, and the absorption was determined at 436 nm.
Protein purification and analysis.
(His)6 tag fusions were expressed in E. coli strain GI698 after growth at 37°C to an optical density at 600 nm (OD600) of 0.5. The gene was induced with 1 mM IPTG (isopropylthiogalactopyranoside) for 4 h at 28°C. The fused histidine residues of recombinant proteins were bound to an Ni-nitrilotriacetic acid matrix (Qiagen). The native protein (H-ScrB) was eluted with 250 mM imidazole, whereas the denaturated repressor (H-ScrR) was eluted with buffer D (8 M urea, 0.1 M NaH2PO4 [pH 5.9], 0.01 M Tris-HCl). The purification of the proteins was done with the QIAexpress system according to the protocol of the manufacturer.
For expression and purification of maltose-binding protein (MBP)-ScrR, E. coli strain DH5α(pMalscrR) was grown in Luria-Bertani (LB) medium with 0.2% glucose to an OD600 of 0.5. Expression of the malE-scrR fusion was induced with 0.5 mM IPTG for 3 h at 37°C. Cells were harvested, washed, and resuspended in column buffer (20 mM Tris-HCl [pH 7.4], 200 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol) and sonicated. The cell debris was pelleted by centrifugation (13,000 × g), and the supernatant was mixed with the amylose resin (1:1) and incubated at 4°C for 30 min. After extensive washing with column buffer, the bound protein was eluted with column buffer supplemented with 10 mM maltose.
Protein concentrations were determined by the method of Lowry (37) with bovine serum albumin as the standard. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed in 10% polyacrylamide gels (36).
Sequencing.
Sequencing was done with the automated laser fluorescent DNA sequencer (A.L.F. Express; Pharmacia Biotec). Initial sequences were obtained with fluorescent universal primers. Nucleotide sequences were determined by primer walking using unlabeled primers synthesized with an oligonucleotide synthesizer (Beckman), after brief incorporation of fluorescein-labeled dATP followed by a chase with unlabeled dATP. Nucleotide sequences were determined with plasmids pSCR107, pSCR109, and pSCR141 in both strands of the inserts. Computer analysis was done with databases for similarities to DNA and protein sequences using the programs BLAST (3; http://www3.ncbi.nlm.nih.gov /Entrez/), IDENTIFY (41; http://dna.stanford.edu/identify/), and additional programs from the BCM-Launcher (47; http://www.hgsc.bcm.tmc.edu/SearchLauncher/).
Media and virulence assays.
MM2 medium has been described (9, 11, 18). Sorbitol (1%) was replaced with other carbohydrates when indicated. DNA manipulations followed standard procedures (44).
Leaf tips of young apple seedlings (cv. Golden Delicious) were cut with scissors and inoculated with a toothpick dipped into an overnight culture of a gfp-labeled E. amylovora strain. Migration of bacteria was evaluated in a fluorescence microscope (Zeiss Axiovert, type 135) under fluorescein isothiocyanate conditions after 5 days of incubation in a growth chamber. Virulence on pears was visually determined from ooze formation at 1 week after inoculation of 0.5-cm-thick slices in a petri dish. The assays were done with at least three plant specimens for each strain.
Cytometric and fluorometric analyses.
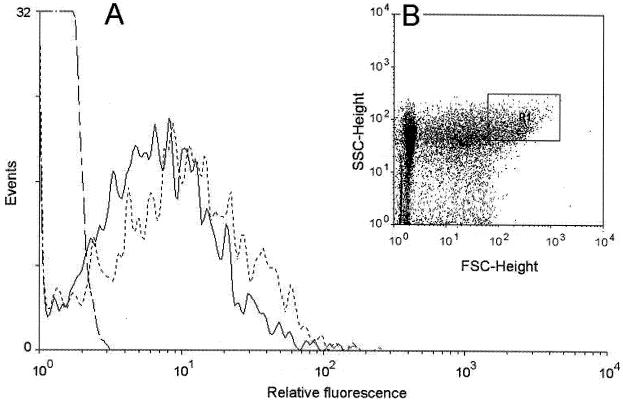
For analysis by flow cytometry and spectrofluorometry, bacteria were grown for 16 h in LB medium with 0.5% carbohydrate, centrifuged, and washed in phosphate-buffered saline (PBS) (44). The bacterial pellet was suspended in 0.5 ml of PBS for determination of fluorescence. Flow cytometry was carried out in a FACScan system (Becton Dickinson). Illumination was done with a 15-mW, 488-nm argon laser, and emission light was detected by a 530 ± 30-nm band pass filter. Photomultiplier voltages were kept constant in a given series of experiments. The fluorescence detector was set at a photomultiplier tube voltage of 546 V. Forward scatter was collected by a photomultiplier tube set at 100, and sideward scatter was collected at a photomultiplier tube set at 400 V. Data were collected for 104 particles per sample and analyzed with the program WinMDI 2.7 (http://facs.scripps.edu /software.html). Bacteria and other particles were separated by their different light-scattering properties (32), which are a complex function of their size, shape, and refractive indices (2, 40, 43). The region R1 (see Fig. 4) was defined to measure bacterial fluorescence on the light-scattering plots. LB cultures of Ea1/79(pSU18-Y1gfpR), LB medium, and extracts from uninfected plant tissue were compared to define an area where more than 95% of the particles were recovered as bacterial signals. For evaluation of R1, at least 1,000 particles were measured. An SPF-500 spectrofluorometer (American Instrument Company) was used to measure bacterial fluorescence at an excitation wavelength of 488 nm and an emission wavelength of 510 nm.
FIG. 4.
Flow cytometry of Ea1/79(pSU18-Y1gfpR) isolated from immature pears. (A) Comparison of the relative fluorescence of Ea1/79 (—·—) (background signals), Ea1/79(pSU18-Y1gfpR) isolated from immature pears (——), and Ea1/79(pSU18-Y1gfpR) isolated from immature pears which were soaked in 0.2% sucrose solution before inoculation (–––). Mean fluorescence values: Ea1/79(pSU18-Y1gfpR), 6.55; Ea1/79(pSU18-Y1gfpR) plus 0.2% sucrose, 8.87; Ea1/79, 1.26 (background). (B) Dot plot of forward (FSC) (x axis) and side (SSC) (y axis) scatter of the particles isolated from immature pears infected with E. amylovora. For measurement of bacterial fluorescence, region R1 was defined for optimal recovery of bacteria (95%).
DNA mobility shift assay.
The scrYAB promoter fragment was amplified by PCR using primers 3341 and 3342. About 0.5 pmol of the promoter fragment was incubated with protein MBP-ScrR (37 to 50 pmol) for 30 min at 30°C in 20 μl of DNA-protein binding buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 10 mM dithiothreitol, 0.1 mM EDTA, 0.05 μg of λ DNA per μl, and 0.5 μg of bovine serum albumin per μl). Before incubation, 10 mM sugar was added if indicated. Protein binding to DNA was assayed by electrophoresis in a native 5% polyacrylamide gel. After electrophoresis, the gel was stained with a 1:104 dilution of SYBR Green I (Biozym) in Tris-Borate-EDTA buffer for 30 min. The gel was analyzed with a Fluor-S Multi-Imager from Bio-Rad.
RESULTS
Identification and analysis of the scr regulon from E. amylovora.
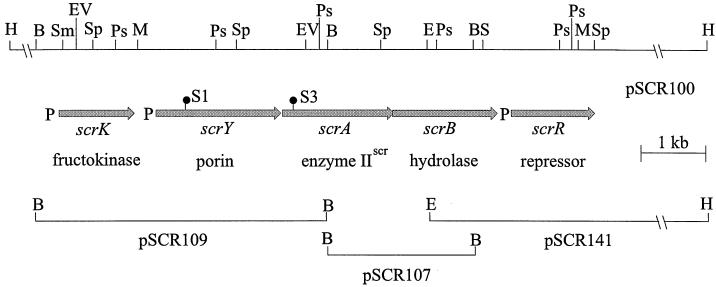
Cosmid pSCR100 (Fig. 1) was isolated from a genomic library of E. amylovora (10) obtained after HindIII digestion. Cells of E. coli strain S17-1 with the library were screened for the ability to utilize sucrose as a carbon source on MacConkey agar plates with sucrose and tetracycline and to grow on minimal medium with 1% sucrose. Cosmid pSCR100 contained a 20-kb HindIII fragment, and the scr regulon of E. amylovora was localized by transposon mutagenesis of S17-1(pSCR100) using phage λ::Tn5seq and screening on 1% sucrose, tetracycline, and kanamycin. From 1,100 transposon mutants, 2 colonies with an scr-negative phenotype were found, and cosmids pSCR100-1 and pSCR100-3 were isolated from white colonies with different insertions of Tn5seq in the scr regulon of pSCR100. By subcloning after digestion with various restriction endonucleases, the transposon insertions were localized in a 10-kb region of pSCR100.
FIG. 1.
Schematic map of the scr regulon of E. amylovora and the plasmids constructed for sequencing. The insertions S1 and S3 of Tn5seq are marked by ●. P, putative promoter; B, BamHI; E, EcoRI; EV, EcoRV; H, HindIII; M, MluI; Ps, PstI; S, SalI; Sm, SmaI; Sp, SphI.
Restriction fragments from the scr regulon of E. amylovora, which was located by transposon mutagenesis, were subcloned from pSCR100 into pUC18 and sequenced in both strands by primer walking. The cloned fragments and a summary of the mapping data are shown in Fig. 1. By computer analysis of the obtained sequence of 6.9 kb (accession number AJ250722), five open reading frames (ORFs) (encoding proteins of 308, 514, 457, 469, and 342 amino acids) with putative ribosome-binding sites were found. The sequenced ORFs showed 67 to 88% similarity to ORFs of the scr regulon from K. pneumoniae and the mobilizable plasmid pUR400. The corresponding genes were named scrK, scrY, scrA, scrB, and scrR in analogy to other scr regulons. In the region preceding scrK, a 6-bp palindrome (TAAACC/CGTTTA) was detected, similar to the palindrome TAAACC/CCTTTA identified as the binding site of the repressor ScrR in pUR400 and K. pneumoniae (5). The genes scrY, scrA, and scrB are organized in an operon structure with an intergenic space of 18 nucleotides between scrY and scrA and an overlap of scrA and scrB (ATGA), which has also been described for related genes and may indicate translational coupling (42). The nucleotide sequences in front of scrK, scrY, and scrR are less conserved than the structural genes. The 266-bp intergenic region between scrK and scrY showed the palindrome AACC/GGTT. As for pUR400 (34), the possible promoter region for scrR overlaps the end of scrB.
The insertion sites of Tn5seq in pSCR100-1 and pSCR100-3 were also determined by sequence reactions with T7 and SP6 primers, which bind to the asymmetric ends of Tn5seq. In pSCR100-1, the transposon was inserted into scrY, whereas in pSCR100-3 the insertion was in scrA (Fig. 1).
The deduced amino acid sequence of ScrK showed 67% similarity to that of the ATP-dependent fructokinase of pUR400 (5), with a typical kinase consensus sequence motif ([AG]-G-x(0,1)-[GAP]-x-N-x-[STA]-x(6)-[GS]-x(9)-G) in the N terminus of the protein. The motif is defined by the distance of specified amino acids, one amino acid of a list [in brackets] or the indicated number of any amino acid (x).
ScrY showed 76% similarity to ScrY of pUR400, a sucrose-specific porin of the outer membrane (30, 46). ScrA shows high similarity (88%) to enzyme IIscr (EIIscr) of the PTS system from K. pneumoniae and pUR400 (51). A consensus sequence of the EIIB cysteine phosphorylation region (N-[LIVMFY]-x(5)-C-x-T-R-[LIVMF]-x-[LIVMF]-x-[LIVM]-x-[DQ]) was identified in the N terminus of ScrA. ScrB of E. amylovora has 66% similarity with the β-d-fructofuranoside fructohydrolase (EC 3.2.1.26) encoded by pUR400 (46, 51). A consensus sequence of the catalytic active domain (H-x(2)-P-x(4)-[LIVM]-N-D-P-N-G) of invertases was found in the N terminus of the protein. ScrR of E. amylovora shows 83% similarity to the regulator protein of the scr regulon of pUR400. The proteins share a well defined N-terminal helix-turn-helix DNA-binding motif, characteristic of many repressors of the LacI/GalR family (53).
Cloning of scrB and purification and enzymatic activity of the sucrose hydrolase from E. amylovora.
The gene scrB of E. amylovora was amplified from plasmid pSCR100 by PCR with primers 3584 and 3523, creating SphI and HindIII sites at the ends of the 1.5-kb PCR fragment. The amplified fragment with scrB was digested and inserted into pQE31 to create pQE31scrB by fusion of scrB with the (His)6 tag sequence of the vector. The plasmid was transformed into E. coli strain GI698. The protein was expressed and purified as described in Materials and Methods. After elution of the native gene product from an Ni column, a major band of 53 kDa was detected by SDS-PAGE, corresponding in size to ScrB as deduced from the encoding ORF.
The purified sucrose hydrolase cleaved sucrose with an optimal enzymatic activity of 18,300 U/mg of homogeneous enzyme in 100 mM phosphate buffer (pH 7.0) and 200 mM sucrose at 28°C. The Michaelis-Menten constant of the sucrose hydrolase for cleavage of sucrose was 125 mM (data not shown). After freezing the enzyme in 10% glycerol at −80°C, no significant (<1%) loss of enzymatic activity was detected after 1 month. Enzymatic activity decreased at ionic strengths higher than 200 mM or lower than 50 mM sodium phosphate. The temperature optimum of the hydrolase was between 18 and 28°C. The maximum enzymatic activity was recovered at pH 7.0, whereas a pH shift to 5.6 or 8.6 caused loss of the activity.
Gene cloning, purification, and characterization of the repressor ScrR from E. amylovora.
The gene scrR of E. amylovora was amplified by PCR with primers 3583 and 3506 as a 1-kb fragment. The amplified scrR was digested with BamHI and HindIII and inserted into pQE31 to create pQE31scrR, expressed in strain GI698. Purification of the gene product was done under denaturing conditions due to low solubility of the fusion protein. The purified probe was analyzed by SDS-PAGE and showed a dominant band of 38 kDa, corresponding to the size deduced from the ORF. Several attempts to renature the protein after purification were unsuccessful.
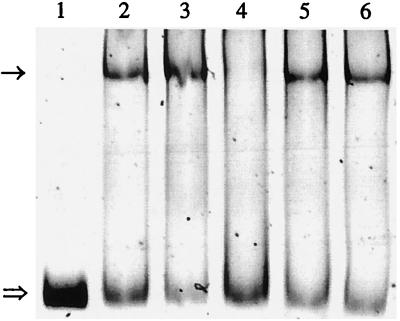
Insolubility after overexpression in E. coli has been described for other ScrR repressors (33). However, a fusion of the repressor to E. coli MBP could yield a soluble protein. Therefore, the 1-kb BamHI-HindIII fragment from pQE31scrR was cloned into pMa1-c2 to create pMalscrR. After expression in E. coli, the MBP-ScrR protein was purified by its affinity to an amylose resin. In order to show that scrR codes for a regulatory protein which binds to the scrYAB promoter region, a gel mobility shift assay with the 278-bp promoter fragment (PscrYAB) of pSU18-Y1 was carried out. The promoter fragment was shifted after addition of MBP-ScrR but was not affected by the presence of λ DNA (Fig. 2). To identify the intracellular inducer, fructose, sucrose, and glucose were tested for their ability to release bound ScrR from the promoter fragment. Fructose but not sucrose or glucose dissociated the repressor-promoter complex (Fig. 2), suggesting that fructose is the intracellular inducer of the scr operon.
FIG. 2.
DNA mobility shift assay with promoter fragment PscrYAB and protein MBP-ScrR. Lane 1, no added protein; 2, 30 pmol of MBP-ScrR; 3, 50 pmol of MBP-ScrR; 4, 50 pmol of MBP-ScrR and 10 mM fructose; 5, 50 pmol of MBP-ScrR and 10 mM sucrose; 6, 50 pmol of MBP-ScrR and 10 mM glucose. The arrow indicates the promoter fragment with the bound 81-kDa protein MBP-ScrR. The double arrow indicates the unbound fragment.
Regulation of scrYAB in E. amylovora.
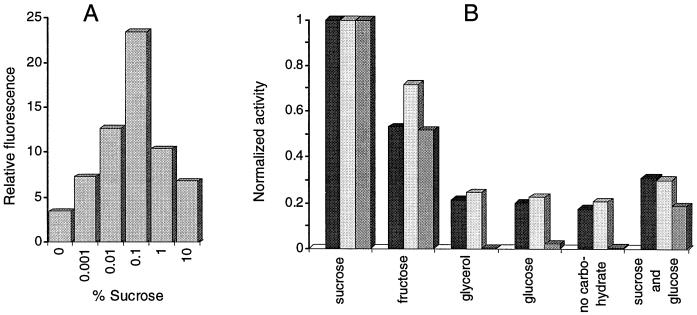
To study transcriptional regulation of the scrYAB operon of E. amylovora, the putative promoter-operator region preceding the operon was amplified as a 278-bp PCR fragment with primers 3342 and 3341. The fragment was inserted into vector pSU18 via the restriction sites SalI and XbaI. In the resulting plasmid (pSU18-Y1), the orientation of the putative promoter PscrYAB is directed against the lac promoter of the vector. To fuse the promoterless gfp gene with PscrYAB, a 700-bp XbaI-SalI fragment from pFG30 was subcloned into pSU18-Y1, yielding pSU18-Y1gfp. Constitutive expression of the reporter gene in Ea1/79(pSU18-Y1gfp) without the repressor gene cloned on the plasmid was assayed by fluorometry and flow cytometry in LB medium with and without sucrose. In order to increase the intracellular level of the repressor, a 1.2-kb fragment containing scrR was amplified with primers 3366 and 3367 by PCR. The primers introduced EcoRV and Asp718 restriction sites into pSU18-Y1gfp. The plasmid created, pSU18-Y1gfpR, was transferred into Ea1/79, and expression of the gfp gene in LB cultures with various carbohydrates was determined (Fig. 3). A high induction of the transcriptional fusion was found for 0.1% sucrose, with less effect of lower or higher concentrations (Fig. 3A). Fructose also induced the reporter fusion to some extent (Fig. 3B). Catabolite repression was observed for growth with glucose in addition to sucrose. For the levan-deficient mutant Ea7/74-LS7(pSU18-Y1gfpR), expression of the reporter gene was increased twofold compared to expression in strain Ea1/79(pSU18-Y1gfpR). Crude protein extracts of Ea7/74-LS7 cultures grown in LB with 0.5% sucrose, fructose, glucose, glycerol, or sucrose plus glucose were tested for sucrose hydrolase activity. The induction of enzyme activities by the different carbohydrates correlated with the induction of the gfp gene measured by flow cytometry and spectrofluorometry with Ea7/74-LS7(pSU18-Y1gfpR) or Ea1/79(pSU18-Y1gfpR) (Fig. 3).
FIG. 3.
Induction of the scrYAB promoter fragment in Ea1/79(pSU18-Y1gfpR). (A) Expression dependent on sucrose concentration. Fluorescence measurements were done by flow cytometry. (B) Expression of the reporter gene fusion in the presence of various sugars and expression of the sucrose hydrolase activity of Ea7/74-LS7 grown in LB medium with 0.5% of each carbohydrate. The expression of the gfp reporter gene was determined by flow cytometry (dark bars) and spectrofluorometry (light bars). The sucrose hydrolase activity is shown in an intermediate bar color. Values for sucrose were set at 1.
To measure regulation of PscrYAB in plant tissue, immature pears were used directly or soaked with 200 μl of 0.2% sucrose solution and inoculated with Ea1/79(pSU18-Y1gfpR). Four-week-old apple seedlings and cotoneaster flowers were also inoculated with Ea1/79(pSU18-Y1gfpR). For the isolation of bacteria, a crude extract from infected plant tissue was filtered and analyzed by flow cytometry. The mean fluorescence of bacteria inoculated into untreated immature pear slices was 6.55 after 2 days, while the fluorescence was increased to 8.55 after soaking the pears with 0.2% sucrose solution 1 day before inoculation (Fig. 4). Ea1/79(pSU18-Y1gfpR) isolated from the first two leaves of apple seedlings 5 days after inoculation produced a fluorescence value of 2.7; conversely, the fluorescence of the bacteria increased to a value of 6.9 when they were isolated from stem tissue.
Site-directed mutagenesis of the scr regulon in E. amylovora.
Plasmid pSCR100-1 was transferred into E. amylovora strain Ea7/74 and the levan-deficient strain PD494, and pSCR100-3 was transformed into strain Ea1/79. Homolog recombination events were screened after conjugation of plasmid pPH1JI, which is incompatible with RP4-derived plasmids. Mutants with site-specific recombination of the transposon were white on MacConkey plates with sucrose and unable to grow on solidified MM2 medium with sucrose. The mutants with transposon insertions in scrY (pSCR100-1) and scrA (pSCR100-3) were named Ea7/74-S1, PD494-S1, and Ea1/79-S3, accordingly. Complementation of the mutants was verified with pSCR100 on MacConkey agar plates with sucrose. Their level of levan synthesis was the same as for the parent strains.
Growth features of scr mutants in culture.
The growth properties of the scr mutants on various carbon sources were compared with those of their parent strains. No difference in the growth kinetics was observed in LB medium or minimal medium with glucose or sorbitol. In contrast to the parent strains, there was no growth of the scr mutants in minimal medium with 1% sucrose after 24 h. In LB medium with sucrose (5 to 40%, wt/wt), the growth of the mutants was reduced fourfold compared to the wild type, which is able to use sucrose as a carbon source, whereas the mutants must rely on the ingredients of the LB medium. For sucrose concentrations higher than 40%, the mutants and the wild-type strains were unable to grow, as shown for the levan-deficient strain PD494Sm and corresponding sucrose mutant PD494-S1 (Fig. 5), limiting E. amylovora to propagation in extreme sugar concentrations independent of their genetic features.
FIG. 5.
Growth of PD494Sm (light bars) and the sucrose mutant PD494-S1 (dark bars) in LB medium. The sucrose concentrations in the medium are indicated. Strain PD494Sm was used to avoid a slight interference by levan in determination of the cell density.
Virulence of E. amylovora scr mutants.
In virulence assays on slices of immature pears, the E. amylovora wild-type strains and the scr mutants produced similar amounts of ooze. The low content of sucrose in those pears (G. Geier and K. Geider, unpublished) is probably compensated for by other carbon sources, such as sorbitol, fructose, and glucose, or organic acids.
When inoculated into leaves of apple seedlings, wild-type strains of E. amylovora caused necrosis and sometimes even wilt. In contrast, the sucrose mutants Ea7/74-S1, PD494-S1, and Ea1/79-S3 showed strongly reduced symptoms in these assays. To confirm the inability of the mutants to colonize leaf tissue, the strains were labeled with plasmid pfdC1Z′-gfp in order to trace them via the fluorescence of the GFP (16). The parent strains labeled with gfp showed the expected migration in the veins of apple leaves at day 5 after inoculation. Conversely, the labeled mutant strains did not move from the narrow zone of inoculation at the leaf tip. Sucrose, which cannot be utilized by scr mutants, is thus an important energy source for E. amylovora to colonize the tissue of the fire blight host plants.
DISCUSSION
For colonization of plants by E. amylovora, the causative agent of fire blight, not only the sorbitol (1) but also the sucrose metabolism of the bacteria is important. These carbohydrates vary among species and within parts of a plant (14, 28, 50). Sucrose is high in the nectaries of flowers (14), a main entry site for the pathogen. High sucrose concentrations usually inhibit bacterial growth, but E. amylovora can grow even in 40% sucrose (38). Its inability to grow in 50% sucrose restricts propagation of the pathogen in an environment with extreme sugar concentrations.
The genes involved in sucrose metabolism of E. amylovora showed significant homology to the scr genes of Salmonella enterica serovar Typhimurium with pUR400 and of K. pneumoniae (45, 49). Conserved amino acid motifs in the N terminus of scr proteins of E. amylovora indicated biological function related to the enzymes from pUR400, K. pneumoniae, and other proteins in the family. The overlapping stop codon of scrA and start codon of scrB (ATGA) support transcriptional and translational coupling of the two genes, as described for the scr genes of other species (42, 51).
A transcriptional fusion of the promoter PscrYAB with gfp in the presence of scrR (pSU18-Y1gfpR) and expression studies of ScrB indicated regulation of the promoter activity from a region preceding scrYAB. Computer analysis of PscrYAB predicted binding domains for the regulator ScrR and a cyclic AMP-cyclic AMP receptor protein complex. The promoter was not only regulated by ScrR, but also induced by sucrose and fructose and suppressed by glucose. Gel mobility shift assays with the 300-bp DNA fragment preceding scrY and the repressor showed that ScrR binds to this region and that fructose and not sucrose is the molecular inducer of scrYAB transcription, as in pUR400 and K. pneumoniae. The scr repressors of pUR400 and K. pneumoniae interact with a helix-turn-helix with the operator palindrome TAAACC/GGTTTA preceding scrY and scrK and bind to fructose or fructose 1-phosphate but not to sucrose (34). A part of this sequence (AACC/GGTT) was found in front of scrY and is possibly the operator in the scr regulon of E. amylovora. Based on a possible leucine zipper motif at the C terminus of ScrR and the observation that the palindrome is present twice in the PscrYAB region of K. pneumoniae, Jahreis and Lengeler (34) proposed binding of ScrR as a tetramer to the operator. This is not supported by E. amylovora, with one palindrome in this region and without a leucine zipper motif in ScrR.
An influence of host cells on the promoter activity of a pathogen has been measured in Mycobacterium smegmatis (23), Bartonella henselae (22), Bacillus cereus (24), and Listeria monocytogenes (17) by the combination of flow cytometry and the gfp reporter gene as a marker. gfp expression in E. amylovora was also analyzed by flow cytometry. Fluorescence of bacteria from stem sections was increased twofold compared to bacteria from young leaves of apple seedlings. This increased fluorescence can be explained by the relatively low sucrose concentration in young leaves and the high concentration in stem tissue (28). The highest in vitro induction was found for sucrose concentrations between 0.01 and 0.8%, comparable to concentrations in the xylem of apple plants. Other parts of the plants can contain much higher concentrations of the sugar (14, 25, 31). In LB medium with sucrose concentrations higher than 0.8%, the activity of PscrYAB was reduced. Low fluorescence of the bacteria from cotoneaster flowers (unpublished data) can be explained by the high sucrose concentration in the nectaries, which also caused reduced fluorescence in LB medium.
Reduced virulence of sorbitol (1) and sucrose mutants could be due to a low level of nutrients in xylem vessels, requiring access to sucrose and sorbitol for colonization of the host plant by E. amylovora. The scr mutants Ea7/74-S1, PD494-S1, and Ea1/79-S3 did not grow in minimal medium with sucrose as the sole carbon source, independent of levan synthesis.
Levan may provide fast protection of E. amylovora against plant defense mechanisms (27, 29). Levansucrase could reduce the induction of the scr regulon by decreasing the sucrose concentration and providing glucose for catabolite repression shown for the levan mutant Ea7/74-LS7(pSU18-Y1gfpR) with increased fluorescence in the presence of sucrose compared with the wild type. Intracellular sucrose metabolism and extracellular levan formation can depend on a single enzyme, as in SacB of Bacillus subtilis (19). At high sucrose concentrations (>30 mM), sucrose is cleaved by extracellular SacB to form levan, and at low sucrose concentrations (≤1 mM) it is hydrolyzed in the cells. The sacB gene is regulated by sucrose via an antitermination process (21). Expression of the levansucrase gene of E. amylovora is not induced by sucrose (27), but is regulated by LsrA, encoded in the hrp region (56). Unlike the nitrogen-fixing bacterium Acetobacter diazotrophicus, which depends on an extracellular levansucrase to use sucrose as a carbon source (4), E. amylovora thus encodes two enzymes to metabolize sucrose (ScrB and Lsc). The external release of glucose did not substitute for a deficiency in sucrose metabolism, since the defect in scr mutants of E. amylovora cannot be suppressed by secreted levansucrase. Sucrose metabolism via the scr regulon of E. amylovora is thus strictly required for pathogenicity.
REFERENCES
- 1.Aldridge P, Metzger M, Geider K. Genetics of sorbitol metabolism in Erwinia amylovora and its influence on bacterial virulence. Mol Gen Genet. 1997;256:611–619. doi: 10.1007/s004380050609. [DOI] [PubMed] [Google Scholar]
- 2.Allman R, Hann A C, Manchee R, Lloyd D. Characterization of bacteria by multiparameter flow cytometry. J Appl Bacteriol. 1992;73:438–444. doi: 10.1111/j.1365-2672.1992.tb05001.x. [DOI] [PubMed] [Google Scholar]
- 3.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tools. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez B, Martínez-Drets G. Metabolic characterization of Acetobacter diazotrophicus. Can J Microbiol. 1995;41:918–924. [Google Scholar]
- 5.Aulkemeyer P, Ebner R, Heilenmann G, Jahreis K, Schmid K, Wrieden S, Lengeler J W. Molecular analysis of two fructokinases involved in sucrose metabolism of enteric bacteria. Mol Microbiol. 1991;5:2913–2922. doi: 10.1111/j.1365-2958.1991.tb01851.x. [DOI] [PubMed] [Google Scholar]
- 6.Barny M A, Guinebretiere M H, Marcais B, Coissac E, Paulin J P, Laurent J. Cloning of a large gene cluster involved in Erwinia amylovora CFBP1430 virulence. Mol Microbiol. 1990;4:777–786. doi: 10.1111/j.1365-2958.1990.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 7.Bartolome B, Jubete Y, Martinez E, de la Cruz F. Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBR322 and its derivatives. Gene. 1991;102:75–78. doi: 10.1016/0378-1119(91)90541-i. [DOI] [PubMed] [Google Scholar]
- 8.Bauer D W, Beer S V. Further characterization of an hrp gene cluster of Erwinia amylovora. Mol Plant-Microbe Interact. 1991;4:493–499. [Google Scholar]
- 9.Bellemann P, Bereswill S, Berger S, Geider K. Visualization of capsule formation by Erwinia amylovora and assays to determine amylovoran synthesis. Int J Biol Macromol. 1994;16:290–296. doi: 10.1016/0141-8130(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 10.Bellemann P, Geider K. Localization of transposon insertions in pathogenicity mutants of Erwinia amylovora and their biochemical characterization. J Gen Microbiol. 1992;138:931–940. doi: 10.1099/00221287-138-5-931. [DOI] [PubMed] [Google Scholar]
- 11.Bennet R A, Billing E. Capsulation and virulence in Erwinia amylovora. Ann Appl Biol. 1978;89:41–45. [Google Scholar]
- 12.Bereswill S, Jock S, Aldridge P, Janse J D, Geider K. Molecular characterisation of natural Erwinia amylovora strains deficient in levan synthesis. Physiol Mol Plant Pathol. 1997;51:215–225. [Google Scholar]
- 13.Bernhard F, Coplin D L, Geider K. A gene cluster for amylovoran synthesis in Erwinia amylovora: characterization and relationship to cps genes in Erwinia stewartii. Mol Gen Genet. 1993;239:158–168. doi: 10.1007/BF00281614. [DOI] [PubMed] [Google Scholar]
- 14.Bieleski R L, Redgwell R J. Sorbitol metabolism in nectaries from flowers of Rosaceae. Aust J Plant Physiol. 1980;7:15–25. [Google Scholar]
- 15.Bogs J, Geider K. Migration and gene regulation of Erwinia amylovora in host plants assayed with the green fluorescent protein. Proceedings of the Eighth International Workshop on Fire Blight, 1998. Acta Hortic. 1999;489:365–369. [Google Scholar]
- 16.Bogs J, Bruchmüller I, Erbar E, Geider K. Colonization of host plants by the fire blight pathogen Erwinia amylovora marked with genes for bioluminescence and fluorescence. Phytopathology. 1998;5:416–421. doi: 10.1094/PHYTO.1998.88.5.416. [DOI] [PubMed] [Google Scholar]
- 17.Bubert A, Sokolovic Z, Chun S K, Papatheodorou L, Simm A, Goebel W. Differential expression of the Listeria monocytogenes virulence genes in mammalian host cells. Mol Gen Genet. 1999;261:323–336. doi: 10.1007/pl00008633. [DOI] [PubMed] [Google Scholar]
- 18.Bugert P, Geider K. Molecular analysis of the ams operon required for exopolysaccharide synthesis of Erwinia amylovora. Mol Microbiol. 1995;15:917–933. doi: 10.1111/j.1365-2958.1995.tb02361.x. [DOI] [PubMed] [Google Scholar]
- 19.Chambert R, Petit-Glatron M-F. Polymerase and hydrolase activities of Bacillus subtilis levansucrase can be separately modulated by site-directed mutagenesis. Biochem J. 1991;279:35–41. doi: 10.1042/bj2790035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cormack B P, Valdivia R H, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 21.Crutz A-M, Steinmetz M, Aymerich S, Richter R, Le Coq D. Induction of levansucrase in Bacillus subtilis: an antitermination mechanism negatively controlled by the phosphotransferase system. J Bacteriol. 1990;172:1043–1050. doi: 10.1128/jb.172.2.1043-1050.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dehio M, Knorre A, Lanz C, Dehio C. Construction of versatile high-level expression vectors for Bartonella henselae and the use of green fluorescent protein as new expression marker. Gene. 1998;215:223–229. doi: 10.1016/s0378-1119(98)00319-9. [DOI] [PubMed] [Google Scholar]
- 23.Dhandayuthapani S, Via L E, Thomas C A, Horowitz P M, Deretic D, Deretic V. Green fluorescent protein as a marker for gene expression and cell biology of mycobacterial interactions with macrophages. Mol Microbiol. 1995;17:901–912. doi: 10.1111/j.1365-2958.1995.mmi_17050901.x. [DOI] [PubMed] [Google Scholar]
- 24.Dunn A K, Handelsman J. A vector for promoter trapping in Bacillus cereus. Gene. 1999;226:297–305. doi: 10.1016/s0378-1119(98)00544-7. [DOI] [PubMed] [Google Scholar]
- 25.Escobar Gutierrez A J, Gaudillere J P. Distribution, metabolisme et rôle du sorbitol chez les plantes superieures. Synth Agron. 1996;16:281–298. [Google Scholar]
- 26.Falkenstein H, Bellemann P, Walter S, Zeller W, Geider K. Identification of Erwinia amylovora, the fireblight pathogen, by colony hybridization with DNA from plasmid pEA29. Appl Environ Microbiol. 1988;54:2798–2802. doi: 10.1128/aem.54.11.2798-2802.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geier G, Geider K. Characterization and influence on virulence of the levansucrase gene from the fireblight pathogen Erwinia amylovora. Phys Mol Plant Pathol. 1993;42:387–404. [Google Scholar]
- 28.Grant C R, Rees T. Sorbitol metabolism by apple seedlings. Phytochemistry. 1981;20:1505–1511. [Google Scholar]
- 29.Gross M, Geier G, Rudolph K, Geider K. Levan and levansucrase synthesized by the fireblight pathogen Erwinia amylovora. Physiol Mol Plant Pathol. 1992;40:371–381. [Google Scholar]
- 30.Hardesty C B, Ferran C, DiRienzo J M. Plasmid-mediated sucrose metabolism in Escherichia coli: characterization of scrY, the structural gene for a phosphoenolpyruvate-dependent sucrose phosphotransferase systems outer membrane porin. J Bacteriol. 1991;173:449–456. doi: 10.1128/jb.173.2.449-456.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawker J S, Jenner C F, Niemietz C M. Sugar metabolism and compartmentation. Aust J Plant Physiol. 1991;18:227–237. [Google Scholar]
- 32.Hechard Y, Jayat C, Letellier F, Julien R, Cenatiempo Y, Ratinaud M H. On-line visualization of competitive antagonistic bacteria. Appl Environ Microbiol. 1992;58:3784–3786. doi: 10.1128/aem.58.11.3784-3786.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiratsuka K, Wang B, Sato Y, Kuramitsu H. Regulation of sucrose-6-phosphate hydrolase activity in Streptococcus mutans: characterization of the scrR gene. Infect Immun. 1998;66:3736–3743. doi: 10.1128/iai.66.8.3736-3743.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jahreis K, Lengeler J W. Molecular analysis of two ScrR repressors and of a ScrR-FruR hybrid repressor for sucrose and fructose specific regulons from enteric bacteria. Mol Microbiol. 1993;9:195–209. doi: 10.1111/j.1365-2958.1993.tb01681.x. [DOI] [PubMed] [Google Scholar]
- 35.Knauf V C, Nester E W. Wide-host-range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982;8:45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- 36.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 37.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 38.Miller T D, Schroth M N. Monitoring the epiphytic population of Erwinia amylovora on pear with a selective medium. Phytopathology. 1972;62:1175–1182. [Google Scholar]
- 39.Nag D K, Huang H V, Berg D E. Bidirectional chain-termination sequencing: transposon Tn5seq1 as a mobile source of primer sites. Gene. 1988;64:135–145. doi: 10.1016/0378-1119(88)90487-8. [DOI] [PubMed] [Google Scholar]
- 40.Nebe-von Caron G, Badley R A. Viability assessment of bacteria in mixed populations using flow cytometry. J Microsc. 1995;179:55–66. [Google Scholar]
- 41.Nevill-Manning G C, Wu T, Brutlag D L. Highly specific protein sequence motifs for genome analysis. Proc Natl Acad Sci USA. 1998;95:5865–5871. doi: 10.1073/pnas.95.11.5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Postma P W, Lengeler J W, Jacobson G R. Phosphoenolpyruvate: carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993;57:543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salzman G C, Wilder M E, Jett J H. Light scattering with stream-in-air flow system. Histochem Cytochem. 1979;27:264. doi: 10.1177/27.1.374583. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 45.Schmid K, Schupfner M, Schmitt R. Plasmid-mediated uptake and metabolism of sucrose by Escherichia coli K-12. J Bacteriol. 1982;151:68–76. doi: 10.1128/jb.151.1.68-76.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmid K, Ebner R, Altenbuchner J, Schmitt R, Lengeler J W. Plasmid-mediated sucrose metabolism in Escherichia coli K12: mapping of the scr genes of pUR400. Mol Microbiol. 1988;2:1–8. doi: 10.1111/j.1365-2958.1988.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 47.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 48.Smith R F, Wiese B A, Wojzynski M K, Davison D B, Worley K C. BCM search launcher—an integrated interface to molecular biology data base search and analysis services available on the World Wide Web. Genome Res. 1996;6:454–462. doi: 10.1101/gr.6.5.454. [DOI] [PubMed] [Google Scholar]
- 49.Sprenger G A, Lengeler J W. Analysis of sucrose catabolism in Klebsiella pneumoniae and Scr+ derivates of Escherichia coli K-12. J Gen Microbiol. 1988;134:1635–1644. doi: 10.1099/00221287-134-6-1635. [DOI] [PubMed] [Google Scholar]
- 50.Suleman P, Steiner P W. Relationship between sorbitol and solute potential in apple shoots relative to fire blight symptom development after infection by Erwinia amylovora. Phytopathology. 1994;84:1244–1250. [Google Scholar]
- 51.Titgemeyer F, Jahreis K, Ebner R, Lengeler J W. Molecular analysis of the scrA and scrB genes from Klebsiella pneumoniae and plasmid pUR400, which encode the sucrose transport protein enzyme II-Scr of the phosphotransferase system and a sucrose-6-phosphate invertase. Mol Gen Genet. 1996;250:197–206. doi: 10.1007/BF02174179. [DOI] [PubMed] [Google Scholar]
- 52.Wallart R A. Distribution of sorbitol in Rosaceae. Phytochemistry. 1980;19:2603–2610. [Google Scholar]
- 53.Weikert M J, Adhya S. A family of bacterial regulators homologous to gal and lac repressors. J Biol Chem. 1992;267:15869–15874. [PubMed] [Google Scholar]
- 54.Wohlhieter J A, Lazere J R, Snellings N R, Johnson E M, Synenki R M, Baron L S. Characterization of transmissible genetic elements from sucrose-fermenting Salmonella strains. J Bacteriol. 1975;122:401–406. doi: 10.1128/jb.122.2.401-406.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Geider K. Molecular analysis of the rlsA gene regulating levan production by the fireblight pathogen Erwinia amylovora. Phys Mol Plant Pathol. 1999;54:187–201. [Google Scholar]