Abstract
Study Design.
Retrospective cohort.
Objective.
To describe our technique for and evaluate the time demand, radiation exposure and outcomes of skin-anchored intraoperative three-dimensional navigation (ION) in minimally invasive (MIS) lumbar surgery, and to compare these parameters to 2D fluoroscopy for MI-TLIF.
Summary of Background Data.
Limited visualization of anatomic landmarks and narrow access corridor in MIS procedures result in greater reliance on image guidance. Although two-dimensional fluoroscopy has historically been used, ION is gaining traction.
Methods.
Patients who underwent MIS lumbar microdiscectomy, laminectomy, or MI-TLIF using skin-anchored ION and MI-TLIF by the same surgeon using 2D fluoroscopy were selected. Operative variables, radiation exposure, and short-term outcomes of all procedures were summarized. Time-demand and radiation exposure of fluoroscopy and ION for MI-TLIF were compared.
Results.
Of the 326 patients included, 232 were in the ION cohort (92 microdiscectomies, 65 laminectomies, and 75 MI-TLIFs) and 94 in the MI-TLIF using 2D fluoroscopy cohort. Time for ION setup and image acquisition was a median of 22 to 24 minutes. Total fluoroscopy time was a median of 10 seconds for microdiscectomy, 9 for laminectomy, and 26 for MI-TLIF. Radiation dose was a median of 15.2 mGy for microdiscectomy, 16.6 for laminectomy, and 44.6 for MI-TLIF, of this, 93%, 95%, and 37% for microdiscectomy, laminectomy, and MI-TLIF, respectively were for ION image acquisition, with the rest attributable to the procedure. There were no wrong-level surgeries. Compared with fluoroscopy, ION for MI-TLIF resulted in lower operative times (92 vs. 108 min, P<0.0001), fluoroscopy time (26 vs. 144 s, P<0.0001), and radiation dose (44.6 vs. 63.1 mGy, P=0.002), with equivalent time-demand and length of stay. ION lowered the radiation dose by 29% for patients and 55% for operating room personnel.
Conclusion.
Skin-anchored ION does not increase time-demand compared with fluoroscopy, is feasible, safe and accurate, and results in low radiation exposure.
Keywords: 3D image guidance, feasibility, fluoroscopy, intraoperative navigation, laminectomy, lumbar, microdiscectomy, minimally invasive surgery (MIS), radiation exposure, skin-anchored tracker
Limited visualization of anatomic landmarks and a narrow access corridor in minimally invasive (MIS) procedures results in greater reliance on image guidance during surgery. Although 2-dimensional fluoroscopy has historically been most commonly employed for this,1 intraoperative 3-dimensional navigation (ION) is gaining traction given the potential for improved accuracy and reduced radiation exposure.2–10
ION for spine surgery typically involves bone- or skin-anchored trackers to provide anatomic reference points and the use of imaging to create a three-dimensional spinal “map.” The navigation system then actively tracks the position of precalibrated instruments relative to reference points, thus providing real-time visualization of instrumentation on a 3D map of the patient’s anatomy. ION is thought to provide better visualization, improve accuracy, and enable less invasive procedures. However, ION is also associated with considerable upfront costs, intraoperative time-demand, and unknown impact with regards to radiation exposure.
Numerous studies have evaluated the accuracy of screw placement with ION.7–9,11 However, there are few reports on other applications of ION such as surgical decompression and tumor resection.3 Furthermore, a majority of the current literature is focused on navigation systems that rely on K-wires and bone-anchored trackers,2,7,11–16 which have a number of drawbacks. To our knowledge, the only published study on the use of skin-anchored ION is a small series of 45 patients in whom ION was used for pedicle screw placement following lumbar interbody fusion.8 Thus, there is a paucity of literature on the technical feasibility and outcomes of ION using skin-anchored trackers for spinal surgery.8
The purposes of this study were to 1) describe our technique for 3D ION using a skin-anchored tracker in MIS lumbar spinal surgery; 2) to evaluate the time demand, radiation exposure, and outcomes of single-level MIS lumbar decompression and fusion surgeries performed using this technique; and 3) to compare the time-demand, radiation exposure, and outcomes of skin-anchored 3D ION to conventional 2D fluoroscopy in single-level minimally invasive transforaminal lumbar interbody fusion (MI-TLIF).
METHODS
Institutional review board (IRB) approval was obtained for this study.
Study Design and Population
A retrospective review of prospectively collected data from a single-surgeon surgical database was performed. The surgeon completed a residency in Orthopedic Surgery followed by a fellowship in Spine surgery, and has been in practice at high-volume academic centers for 11 years, performing over 300 surgeries per year. For over 85% of cases the first assist was a spine fellow; in the remaining case it was an Orthopedic surgery resident.
Consecutive patients who underwent single-level MIS lumbar microdiscectomy, laminectomy, or MI-TLIF using skin-anchored ION between April 2017 and January 2019 were selected. In addition, consecutive patients who underwent single-level MI-TLIF by the same surgeon using 2D fluoroscopy between 2011 and 2016 were selected for comparison. All the procedures were performed using a tubular retractor system and an operating microscope. Patients with a history of trauma, and those with a fracture or neoplasm, were excluded from this study.
Type of image guidance used (ION vs. fluoroscopy) was not based on surgeon selection; the operating surgeon switched from fluoroscopy to ION when the latter technology became available resulting in prospective cohorts of consecutive patients for each modality, thereby minimizing selection bias. Additionally, all included cases were performed after the operating surgeon was past his own learning curve.
Surgical Technique
Intraoperative 3D Navigation Cohort
ION was performed using SpineMask Tracker, SpineMap 3D Software and NAV3i Platform (Stryker Corp). The SpineMask is a rectangular skin-adhesive stereotactic tracker that is available as a sterile-packaged, singleuse, disposable, battery-powered device. It is placed on the patient’s thoracolumbar region after preparing and draping the incision site. It utilizes light-emitting diode (LED) technology for tracking and automatically integrates with the SpineMap software. The SpineMap software is compatible with a variety of intraoperative imaging devices for automatic registration and allows for intraoperative virtual K-wire and pedicle screw planning. Continuous electrophysiologic neuromonitoring was used for all cases.
Indications for Skin-anchored 3D ION
The SpineMask tracker is best suited for percutaneous procedures and procedures that require small incisions because large vertical incisions can result in skin tension or movement, which may change the location of the reference point and thus alter the spinal map,3 resulting in decreased accuracy.8
Operating Room (OR) Setup
OR setup includes the navigation system, which consists of a tracking camera and a monitor to display the spinal map, and a 3D C-arm for image acquisition.
The navigation system is placed at the cranial end of the patient.
When the c-arm is not in use, it is steriley draped and placed in a lateral position distal to the operative area and brought into the operative field when required.
In addition, MIS typically involves the use of an operating microscope, which is placed on the opposite side of the room as the C-arm. This ensures that the C-arm can be brought into the surgical field when the surgeon is under the microscope.
The neuromonitoring unit is typically placed at the cranial end of the patient, on the opposite side of the navigation system.
Precalibrated or manually calibrated instruments can be used with the navigation system. The mGenuis Tracker (Stryker Corp, Kalamazoo, MI) is a navigation rotational adaptor that can be used to manually calibrate instruments. Instruments can be validated and initialized prior to the patient being brought into the OR. Once the instruments are validated, they can be kept on the backtable until the procedure begins. If an additional instrument is needed during the case, it can be initialized and calibrated at any point without disrupting the workflow.
Steps for Navigation Setup and 3D Image Acquisition
All procedures are performed with the patient under general anesthesia and with endotracheal intubation.
After induction, the patient is placed in the prone position on a radiolucent table (Jackson Table with a Wilson Frame).
Sterile prep and drape are applied from the cervicothoracic junction to the sacrococcygeal junction. The SpineMask is placed. Once the SpineMask is “captured” by the navigation unit, AP, and lateral images centered on the operative level are obtained using the Ziehm C-arm in order to confirm that the 3D study will be centered on the area of interest.
- The SpineMask tracker is applied to the surgical site by removing the protective backing and applying the adhesive around the skin markings. It is important to ensure that the tracker adheres smoothly and is flush with the skin.
- For decompressions, the SpineMask tracker is placed over the target level(s), i.e., the surgeon is working inside the mask.
- For lumbar fusion procedures, the SpineMask tracker is placed cranial to the surgical site, typically over the thoracic spine, i.e., the surgeon is working outside the mask.
- The SpineMask is registered with the SpineMap Software and Navigation System. This can be done by one of two methods.
- Automatic intraoperative mask registration (AIM): The C-arm is used to acquire an anteroposterior (AP) and lateral image to ensure that the surgical area of interest and at least 10 of the 31 LEDs are captured within the 3D reconstruction. These two-dimensional fluoroscopic images will also be used as the iso-center for the 3D spin. In order to ensure a clear translation during 3D image acquisition, a collision check is performed; during this the C arm will mimic the scanning pathway that will be followed while acquiring the 3D scan to check that the pathway is not obstructed.
- Stryker’s trackerless automatic registration (STAR): Similar to the AIM method, AP and lateral 2D fluoroscopic images are used to localize the surgical area of interest, which will serve as the iso-center for the 3D spin. Once this is complete, a collision check is performed. The SpineMask patient tracker is then registered by ensuring that 28 of the 31 LEDs are visible to the navigation camera. Following this, a precalibrated instrument is used to digitize registration points on the flat panel of the C-arm. Once an acceptable registration accuracy of < 2 mm has been achieved, the 3D scan can be performed. This is the preferred method of registration for the operating surgeon and was used for all cases included in the current study.
The C-arm is used to obtain a 3D image. To begin scanning, all personnel stand behind a protective lead shield, resulting in no radiation exposure to OR personnel during ION image acquisition. Therefore no lead apron is necessary. Duration of the scan is approximately 48 seconds. However, due to pulsed radiography technology, the radiation exposure is approximately equal to 8 to 9 seconds of fluoroscopy.
When the scan is complete, the image is automatically transferred to the Stryker NAV3i navigation system as a reconstructed 3D dataset.
The site of incision and its proper trajectory are identified with a calibrated pointer.
After incision, the accuracy of the 3D spinal map must be confirmed; this is done by touching known landmarks with a calibrated instrument and ensuring that it is reflected correctly on the map. Landmarks should be checked periodically during the procedure.
Once the 3D spinal map has been accurately obtained, the surgical steps are similar to those of MIS procedures17–20 described in the literature. Our operating room setup and basic steps of the workflow are outlined in the supplemental digital content, http://links.lww.com/BRS/B491 and have recently been published.21
Data Collection
Patient demographics, comorbidities, operative factors, and peri-operative data were obtained from review of electronic medical records.
Navigation-related Time Demand
Operative time was defined as the time from incision to the end of the surgical procedure. Time for ION setup was measured as the time from the completion of anesthesia induction to the start of the procedure. This accounts for patient positioning, preparing, and draping the surgical site, placing the tracker and acquiring a 3D image. Total time-demand for the ION cohort was calculated as the sum of time for ION setup and operative time; for the fluoroscopy cohort total time demand was assumed to be the same as the operative time because the use of fluoroscopy does not necessitate any additional steps prior to the start of the surgical procedure.
Radiation Exposure
Fluoroscopy time (in seconds) and radiation dose (in milligray, i.e., mGy) were obtained from the picture archiving and communication system (PACS). Total fluoroscopy time and radiation dose were recorded. For cases that utilized ION, fluoroscopy time and radiation dose for were divided into that for ION image capture and that for the procedure.
To assess radiation exposure to the patient, total fluoroscopy time and total radiation dose were analyzed. Since OR personnel are not exposed to radiation during the ION spin, radiation exposure to the surgeon and OR personnel was assessed as the fluoroscopy time and radiation dose for the procedure alone.
Statistical Analysis
Operative variables, radiation exposure, and short-term outcomes were summarized using descriptive statistics. Categorical variables were summarized as “number (percentage),” normally and non-normally distributed continuous variables were summarized as “mean± standard deviation” and “median [interquartile range]” respectively.
In patients who underwent MI-TLIF, demographics, operative factors, and radiation exposure of 2D fluoroscopy and ION were compared using chi-square test for categorical and t test for continuous variables.
Statistical significance was set as P < 0.05. All analyses were performed using the IBM Statistical Package for the Social Sciences (SPSS) version 22 (IBM Corp, Armonk, NY).
RESULTS
Of the 326 patients included, 232 were in the ION cohort (92 microdiscectomies, 65 laminectomies, and 75 MI-TLIFs) and 94 were in the fluoroscopy comparison cohort. All were single-level procedures. Demographics are presented in Table 1, and operative data in Table 2.
TABLE 1.
Demographics
| Intraoperative Navigation | Fluoroscopy | P Value | |||
|---|---|---|---|---|---|
| Microdiscectomy | Laminectomy | MI-TLIF | MI-TLIF | ION Versus Fluoro for MI-TLIF | |
| Number of cases (n) | 92 | 65 | 75 | 94 | |
| Age (yr) | 44.77 ± 15.27 | 63.56 ± 14.93 | 57.86 ± 12.94 | 56.83 ± 14.59 | 0.632 |
| Sex | 0.877 | ||||
| Male | 53 (57.6%) | 35 (53.8%) | 35 (46.7%) | 48 (51.1%) | |
| Female | 39 (42.4%) | 30 (46.2%) | 40 (53.3%) | 46 (48.9%) | |
| Body mass index (BMI, kg/m2) | 26.78 ± 5.25 | 28.20 ± 6.81 | 27.17 ± 5.18 | 27.46 ± 5.40 | 0.732 |
| American Society of Anesthesiologists (ASA) Class | 0.002 | ||||
| 1 | 26 (28.3%) | 6 (9.2%) | 9 (12.3%) | 16 (17.2%) | |
| 2 | 64 (69.6) | 55 (84.6%) | 60 (82.2%) | 53 (57.0%) | |
| 3 | 2 (2.2%) | 4 (6.2%) | 4 (5.5%) | 22 (23.7%) | |
| 4 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (2.2%) | |
TABLE 2.
Operative Factors
| Intraoperative Navigation | Fluoroscopy | P Value | |||
|---|---|---|---|---|---|
| Microdiscectomy | Laminectomy | MI-TLIF | MI-TLIF | ION Versus Fluoro for MI-TLIF | |
| Level of surgery | 0.614 | ||||
| L2-L3 | 4 (4.3%) | 0 (0.0%) | 0 (0.0%) | ||
| L3-L4 | 15 (16.3%) | 5 (7.7%) | 1 (1.3%) | 1 (1.1%) | |
| L4-L5 | 35 (38.0%) | 49 (75.4%) | 53 (70.7%) | 60 (63.8%) | |
| L5-S1 | 38 (41.3%) | 11 (16.9%) | 21 (28.0%) | 33 (35.1%) | |
| Estimated blood loss (EBL, mL) | 25 [25–25] | 25 [25–25] | 25 [25–50] | 50 [30–100] | 0.007 |
| Length of hospital stay (hours) | 8.47 [7.64–10.18] | 8.83 [7.48– 11.78] | 31.03 [27.57–49.42] | 1 [1–2] | 0.259 |
Time Demand, Radiation Exposure, and Outcomes of Skin-anchored ION
As seen in Table 3 and Figure 1, operative time was a median of 42 minutes for microdiscectomy, 50 minutes for laminectomy, and 92 minutes for MI-TLIF.
TABLE 3.
Time Demand
| Intraoperative Navigation | Fluoroscopy | P Value | |||
|---|---|---|---|---|---|
| Microdiscectomy | Laminectomy | MI-TLIF | MI-TLIF | ION Versus Fluoro for MI-TLIF | |
| Time for navigation setup and image acquisition (min) | 22 [19.5–25] | 23 [21–26] | 24 [21–28] | – | – |
| Operative time (min) | 42 [35–54] | 50 [39–58] | 92 [77–101] | 108 [95–124.5] | <0.0001 |
| Total time demand (min) | 65.5 [58–77] | 77 [66–84] | 113 [100–130] | 108 [95–124.5] | 0.044 |
Figure 1.
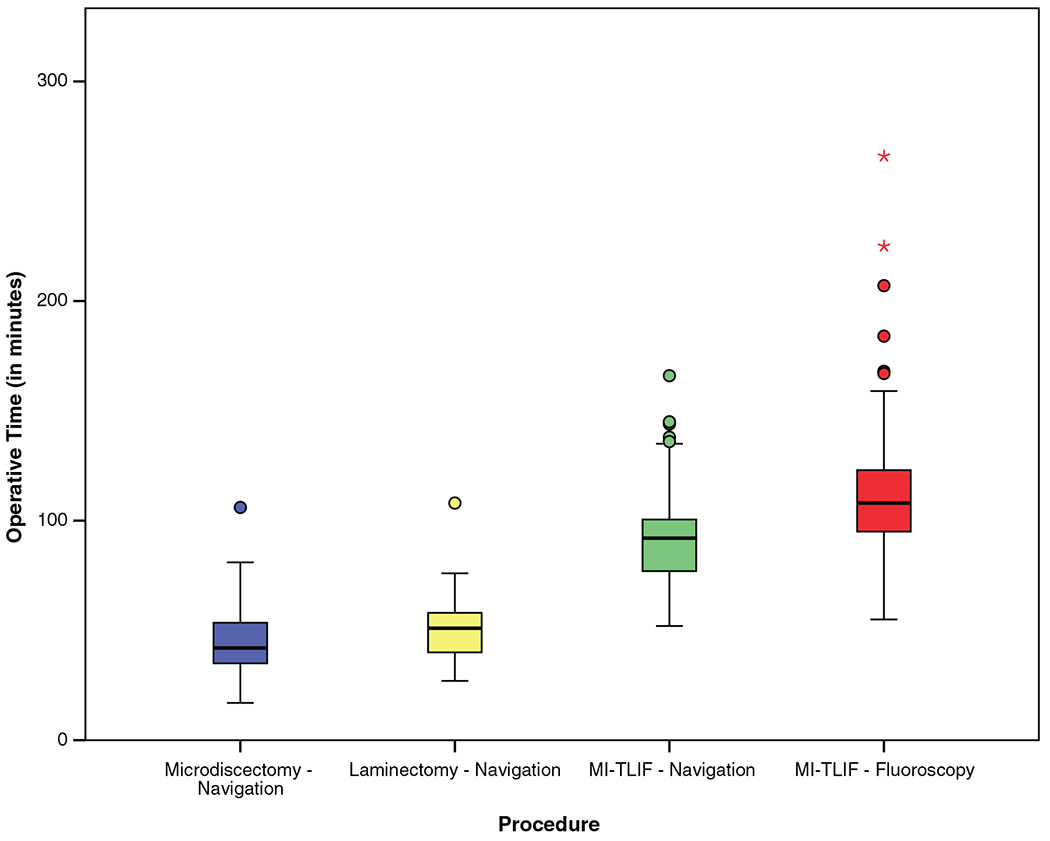
Operative time.
Time Demand for ION Setup and Image Acquisition
As seen in Figure 2 and Table 3, time for ION setup was a median of 22, 23, and 24 minutes for microdiscectomy, laminectomy, and MI-TLIF, respectively. This accounts for patient positioning, preparing, and draping the incision site, placing the tracker and acquiring a 3D image.
Figure 2.
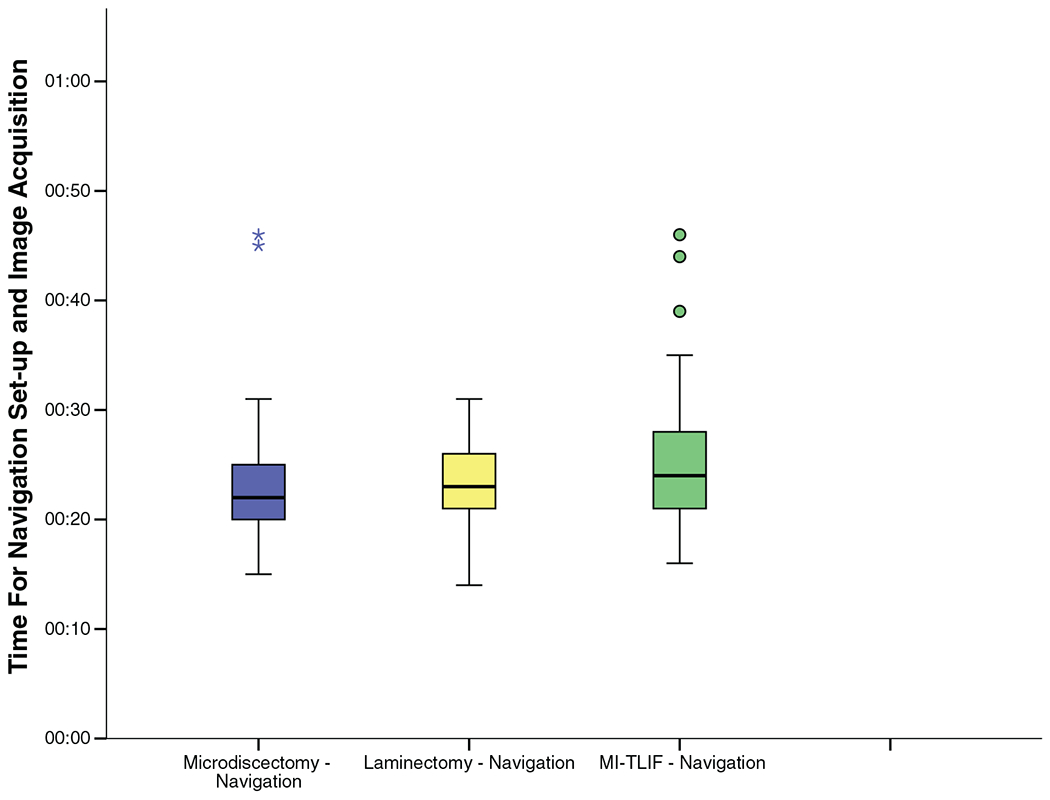
Time required for navigation setup and image acquisition for the intraoperative navigation cohort.
Radiation Exposure to the Patient
Figure 3 and Table 4 show that total fluoroscopy time was a median of 10 seconds for microdiscectomy, 9 for laminectomy, and 26 for MI-TLIF. As seen in Figure 4, of the total fluoroscopy time, about 9 seconds was required for image acquisition for ION, while the remaining was for the surgical procedure (median of 0, 0, and 17 s for microdiscectomy, laminectomy, and MI-TLIF respectively).
Figure 3.
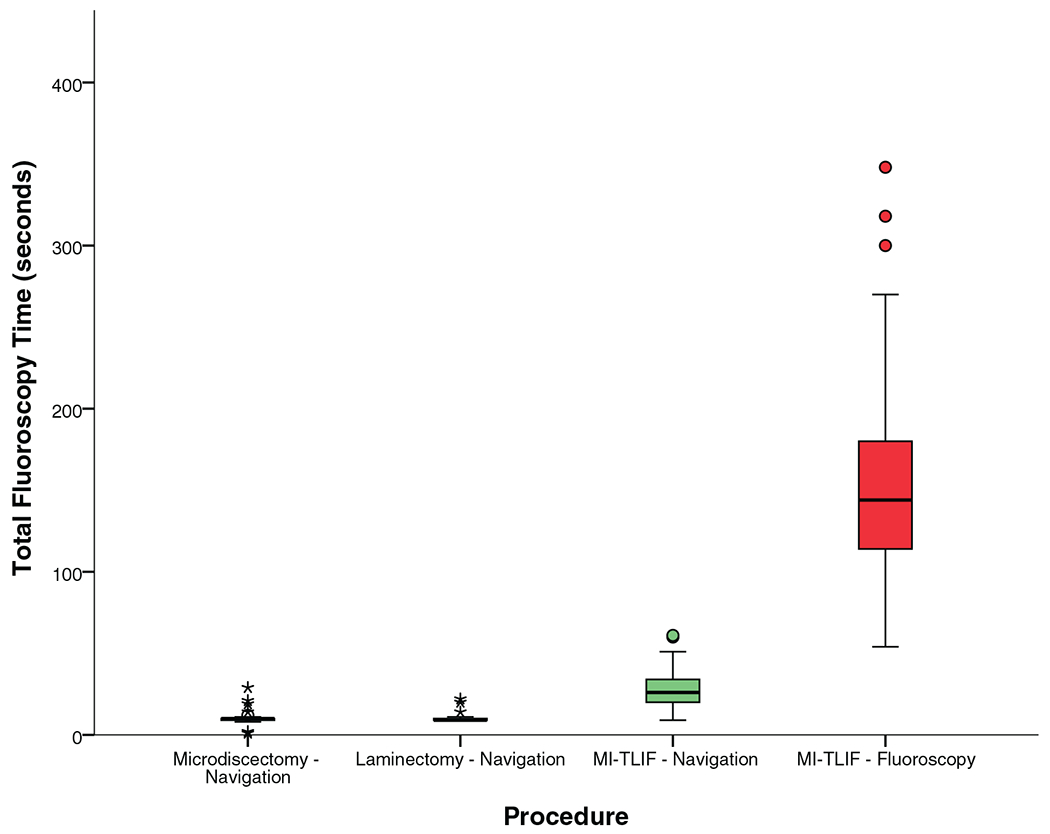
Fluoroscopy time to the patient.
TABLE 4.
Radiation Exposure
| Intraoperative Navigation | Fluoroscopy | P Value | |||
|---|---|---|---|---|---|
| Microdiscectomy | Laminectomy | MI-TLIF | MI-TLIF | ION Versus Fluoro for MI-TLIF | |
| Radiation exposure to the patient | |||||
| Fluoroscopy time (s) | |||||
| Navigation spin | 9 [9–9] | 9 [9–9] | 9 [9–9] | – | |
| Procedure | 0 [0–1] | 0 [0–1] | 17 [11–23.75] | – | |
| Total | 10 [9–10] | 9 [9–10] | 26 [20–34] | 144 [114–180] | <0.0001 |
| Radiation dose | |||||
| Navigation spin (%) | 93 [90–99] | 95 [91–98] | 37 [23–58] | – | |
| Procedure (%) | 7 [1–10] | 5 [2–9] | 63 [42–77] | – | |
| Total (mGy) | 15.6 [9.4–25.2] | 16.6 [10.85–27.2] | 44.6 [35.6–64] | 63.1 [49.1–83.25] | 0.002 |
| Radiation exposure to operating room personnel | |||||
| Fluoroscopy time (s) | 0 [0–1] | 0 [0–0] | 17 [11–23.75] | 144 [114–180] | <0.0001 |
| Radiation dose (mGy) | 0.88 [0.24–1.58] | 0.89 [0.36–1.37] | 28.34 [17.74–45.03] | 63.1 [49.1–83.25] | <0.0001 |
Figure 4.
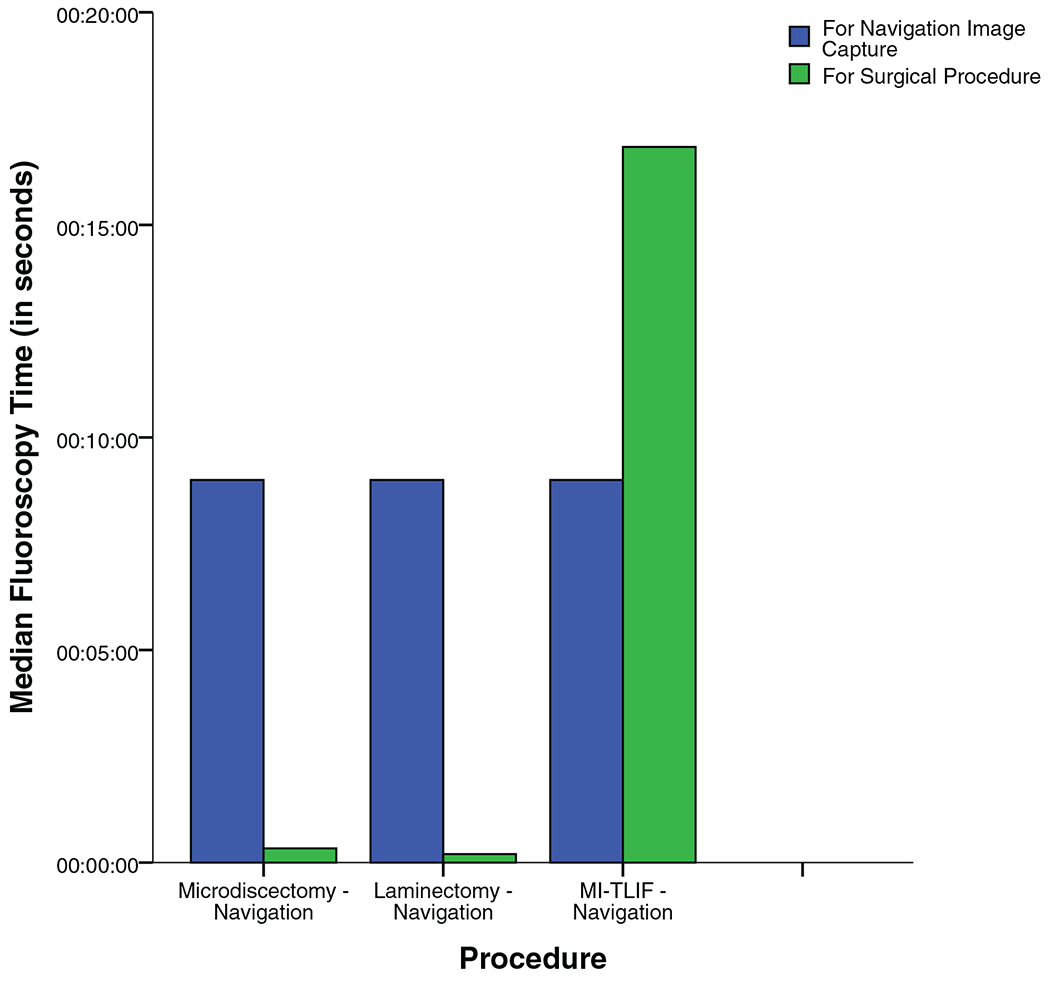
Median fluoroscopy time for image acquisition and the surgical procedure for the intraoperative navigation cohort.
As seen in Figure 5, fluoroscopy time for ION image acquisition was about 9 seconds (range: 7–11) for 96.6% of patients, indicating that only one spin was required. A total of six patients (one microdiscectomy and five MI-TLIFs) required a second spin. The need for a verification ION spin was not related to the learning curve.
Figure 5.
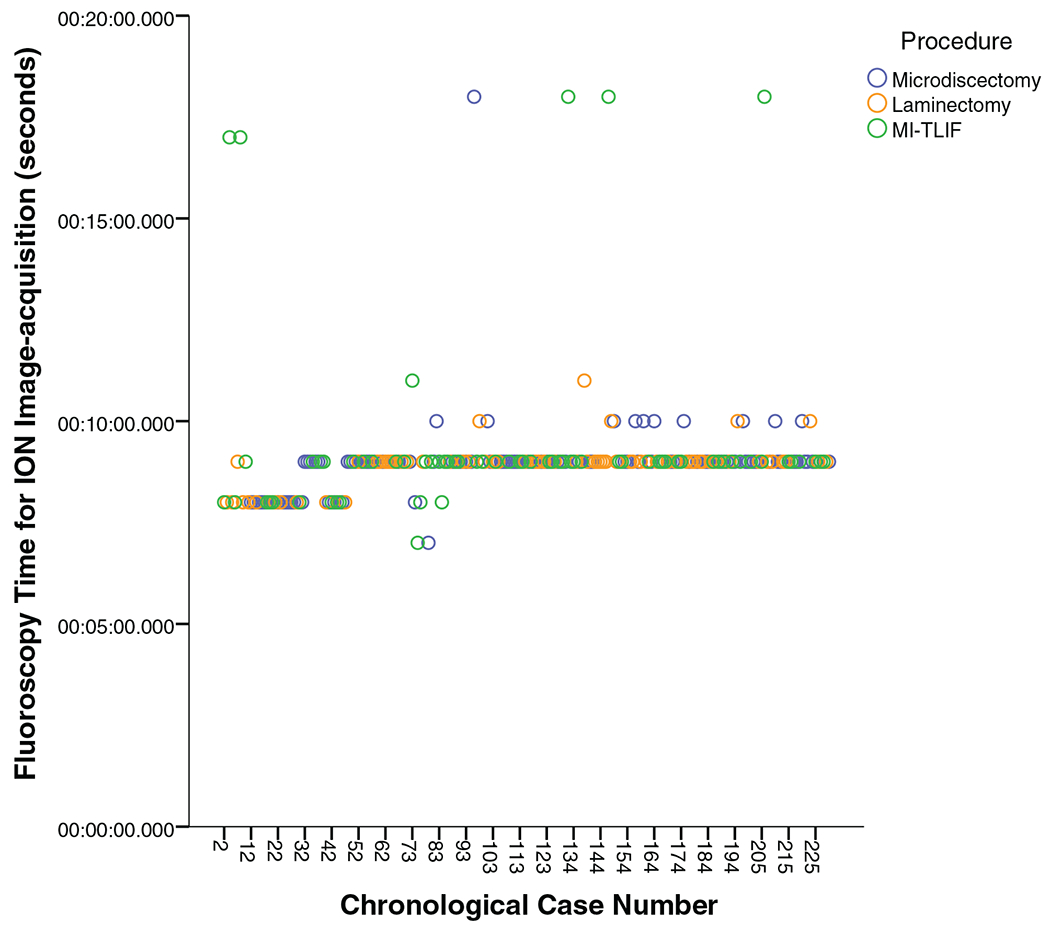
Fluoroscopy time for navigation image acquisition (note: patients who had a fluoroscopy time for ION image acquisition ranging from 7 to 11 s required only one intraoperative spin. Patients who had a fluoroscopy time for ION image acquisition of 17–18 s required second verification spin intraoperatively). ION indicates intraoperative three-dimensional navigation.
Figure 6 and Table 4 show that radiation dose to the patient was a median of 15.2 mGy for microdiscectomy, 16.6 mGy for laminectomy, and 44.6 mGy for MI-TLIF.
Figure 6.
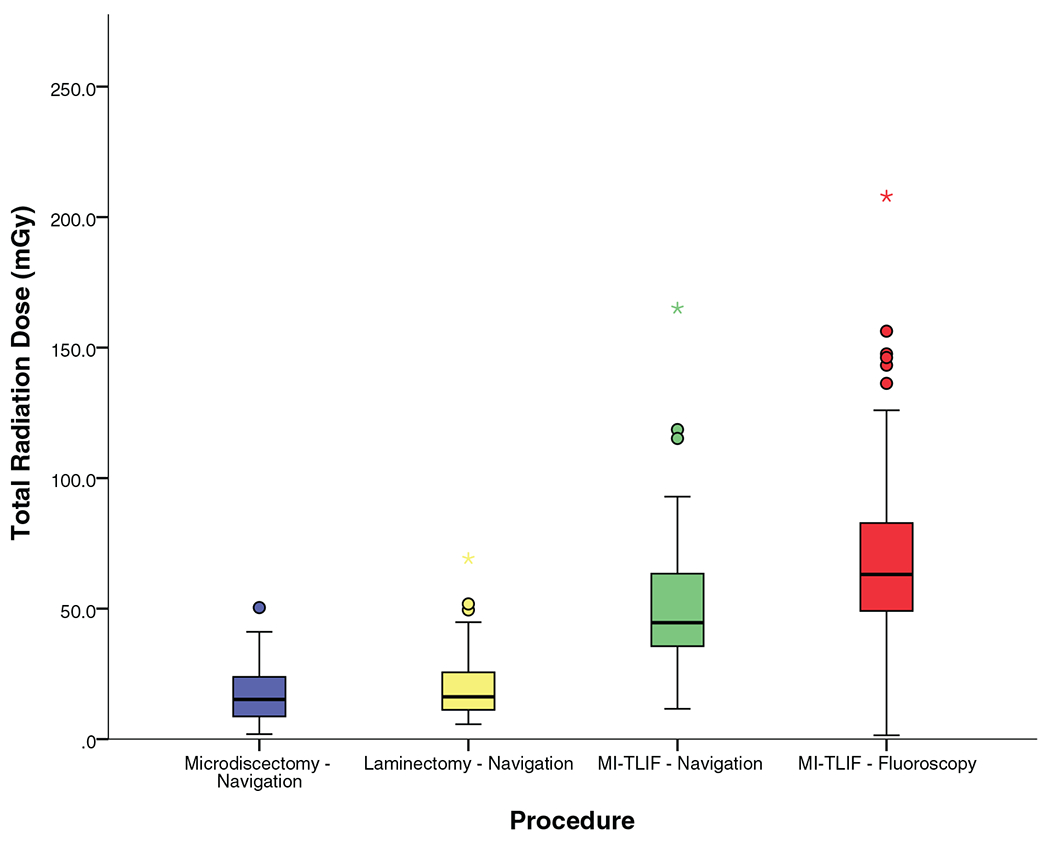
Radiation dose to the patient.
Radiation Exposure OR Personnel
Since all OR personnel are behind a protective shield during ION image acquisition, the radiation exposure was minimal for microdiscectomy and laminectomy procedures (< 1 mGy). For MI-TLIF, the median radiation exposure to OR personnel was fluoroscopy time of 17 seconds and radiation dose of 28.34 mGy. This is shown in Table 4, and Figures 7 and 8.
Figure 7.
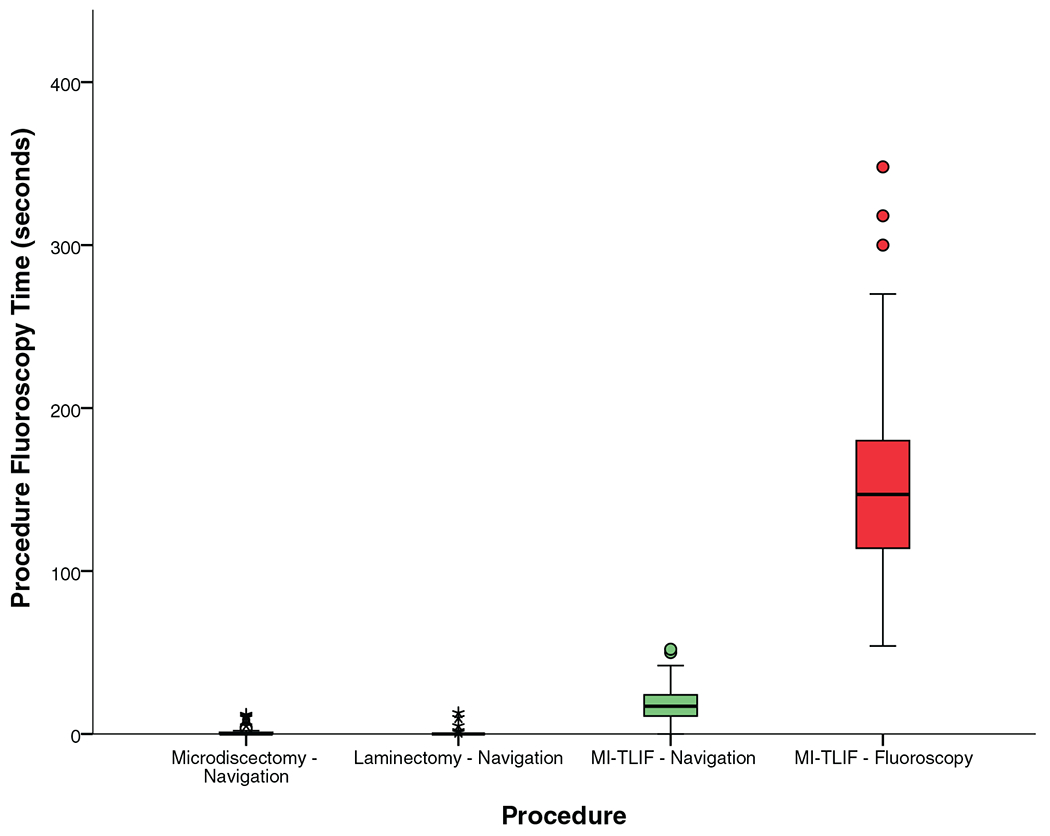
Fluoroscopy time to the operating surgeon and other operating room personnel.
Figure 8.
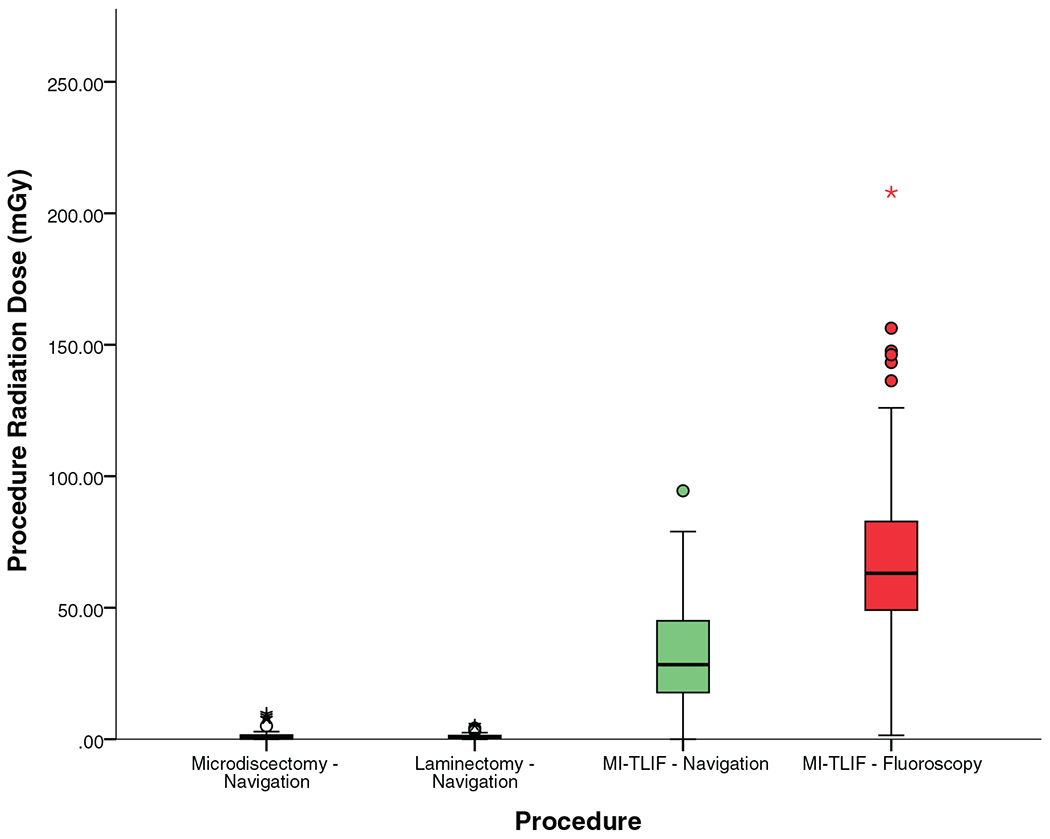
Radiation dose to the operating surgeon and other operating room personnel.
As seen in Table 5, the only reported intraoperative complication was durotomy, occurring in one laminectomy procedure, and the most common in-hospital complication was urinary retention requiring catheterization, seen 0%, 3.1%, and 6.7% of microdiscectomies, laminectomies, and MI-TLIFs respectively. There were no wrong-level surgeries.
TABLE 5.
Perioperative Outcomes of the Intraoperative Navigation Cohort
| Microdiscectomy | Laminectomy | MI-TLIF | |
|---|---|---|---|
| Number of patients requiring a repeat navigation spine | 1 (1.1%) | 0 (0.0%) | 5 (6.7%) |
| Intraoperative complication | 0 (0.0%) | 1 (1.5%) | 0 (0.0%) |
| Wrong level surgery | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Durotomy | 0 (0.0%) | 1 (1.5%) | 0 (0.0%) |
| In-hospital complications | 2 (2.2%) | 3 (4.6%) | 10 (13.3%) |
| Incision-site edema | 0 (0.0%) | 0 (0.0%) | 1 (1.3%) |
| Urinary retention requiring catheterization | 0 (0.0%) | 2 (3.1%) | 5 (6.7%) |
| Urinary tract infection | 1 (1.1%) | 0 (0.0%) | 0 (0.0%) |
| Nausea and vomiting | 0 (0.0%) | 0 (0.0%) | 2 (2.7%) |
| Fever | 0 (0.0%) | 0 (0.0%) | 1 (1.3%) |
| Cardiac arrhythmia | 0 (0.0%) | 1 (1.5%) | 0 (0.0%) |
| Respiratory depression | 0 (0.0%) | 0 (0.0%) | 1 (1.3%) |
| Postoperative sensory or motor deficit | 1 (1.1%) | 0 (0.0%) | 1 (1.3%) |
Skin-anchored 3D ION Versus 2D Fluoroscopy in MI-TLIF
Demographics
As seen in Table 1, there were no significant differences in demographics or level of surgery. Although both groups had a comparable percentage of patients with diabetes (P = 0.160) and hypertension (P = 0.241), the fluoroscopy cohort had a greater percentage of patients with American Society of Anesthesiologists (ASA) class 3 and 4 (P = 0.002).
Operative Data
As seen in Tables 2 and 3 and Figure 1, ION resulted in significantly shorter operative times (median 92 vs. 108 min, P<0.0001) and less blood loss (median 25 vs. 50 mL, P=0.007), with an equivalent length of stay (median 1 d for both, P = 0.259).
As seen in Table 3 and Figure 9, although ION required additional time for image acquisition, the total time demand (image acquisition + surgical procedure) was only slightly greater than that of procedures using fluoroscopy alone (median 113 vs. 108 min, P = 0.044). Though statistically significant, the time demand for ION includes time for patient positioning, and preparing and draping the incision site, which is not accounted for in the fluoroscopy cohort. It is likely that accounting for this would result in equivalent time demand.
Figure 9.
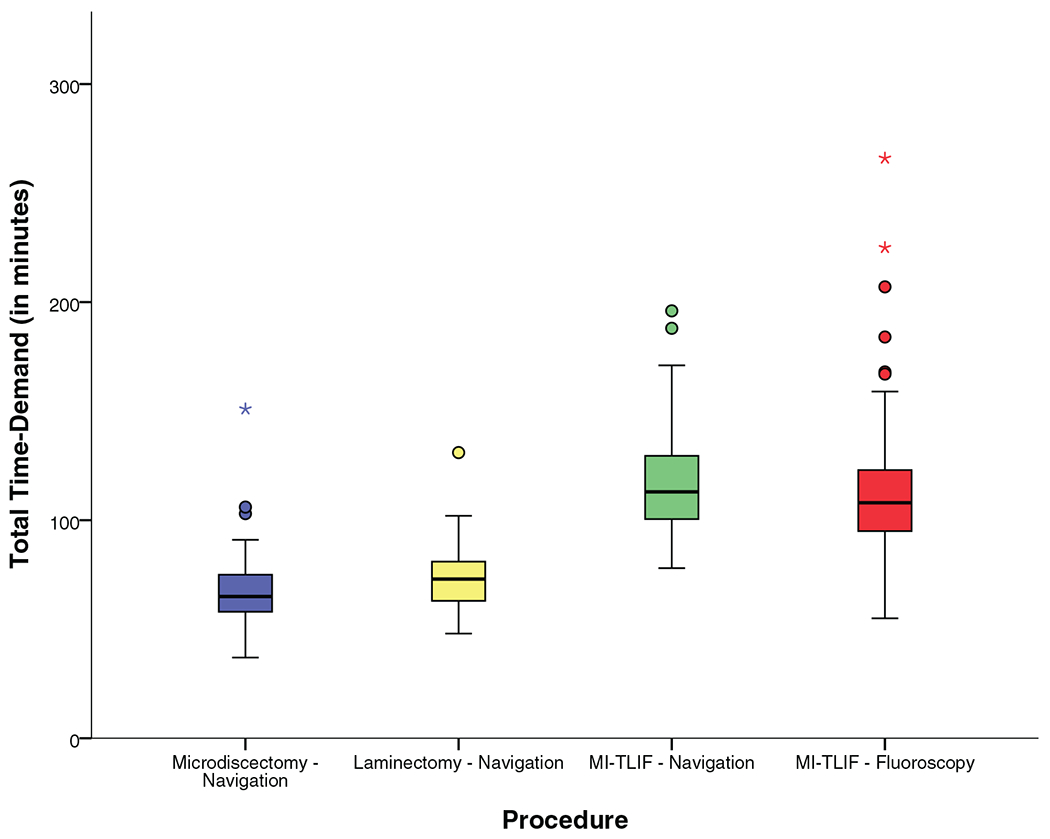
Total time demand.
Radiation Exposure to the Patient
ION resulted in significantly reduced fluoroscopy time (median 26 s vs. 144 s for fluoroscopy, P<0.0001) and lower radiation dose (median 44.6 mGy vs. 63.1 mGy for fluoroscopy, P = 0.002) to patients, resulting in a 29% reduction in the median radiation dose as demonstrated in Table 4, and Figures 3 and 6.
Radiation Exposure OR Personnel
Similarly, the use of ION also reduced the fluoroscopy time (median 17 vs. 144 s for fluoroscopy, P < 0.0001) and lower radiation dose (median 28.34 vs. 63.1 mGy for fluoroscopy, P < 0.0001) to OR personnel, resulting in a 55% reduction in the median radiation dose as seen in Table 4, and Figures 7 and 8.
DISCUSSION
Background and Rationale
While fluoroscopy has historically been most commonly employed for intraoperative image guidance, technological advances have provided computing ability to generate 3-dimenional anatomical maps that can be used. ION has shown promise in MIS surgery wherein limited visualization of anatomic landmarks and a narrow access corridor result in greater reliance on image guidance, and in scoliosis surgery wherein abnormal anatomy can make spinal instrumentation challenging.
The most commonly used navigation systems utilize bone-anchored trackers,2,7,11–16 which have a number of drawbacks.3,8 Bone-anchored trackers typically require attachment of reference clamps to bony landmarks such as spinous processes, which requires additional dissection and greater iatrogenic injury. Additionally, these reference clamps can obstruct the surgical field and move during the procedure, thus necessitating a new scan.
In order to mitigate these concerns, noninvasive skin-anchored trackers that do not require additional dissection, and are less likely to obstruct or move during the procedure have been introduced. To our knowledge, the only published study on skin-anchored ION is a small series of 45 patients in whom ION was used for pedicle screw placement following lumbar interbody fusion.8 Thus, there is a paucity of literature on skin-anchored ION for spinal surgery.
Time Demand, Radiation Exposure, and Outcomes of Skin-anchored ION
Numerous studies have described bone-anchored ION for pedicle screw placement and have reported on accuracy, radiation exposure, and operative time. Additionally, some studies have compared these parameters to conventional techniques including freehand screw placement and 2D fluoroscopy. A majority of these studies have reported overall benefit of ION in terms of increased accuracy and fewer malpositioned screws, but have found varying results with regard to operative time and radiation exposure.2,7,11–16
The current evidence for skin-anchored ION is limited.1,8 The prospective series of 45 patients by Malham and Parker,8 which described the placement of 204 screws in patients undergoing lumbar interbody fusion using the SpineMask Tracker, reported a mean fluoroscopic time of 141 seconds and radiation dose of 246.7 mGy, which is much higher than the median fluoroscopic time of 26 seconds and radiation dose of 44.6 mGy seen in our MI-TLIF cohort. Although the reason for this difference is not clearly apparent, it may be attributable to a number of factors including: 1) surgical procedure—this study included all types of interbody fusion whereas our study was limited to MI-TLIF; 2) surgical technique—our study was limited to TLIF performed using MIS techniques; 3) number of levels—our study was limited to single-level procedures, whereas the above study included multilevel procedures; 4) number of 3D spins—the above study reported a median of two spins per case, whereas a majority of our patients required only one spin; and 5) type of image-acquisition system.
In our study, we also analyzed the time demand for ION setup, which has often been cited as a deterrent to adoption of these technologies. In our study, this was found to be a median of 23 minutes, with over 70% of cases being set up in ≤25 minutes.
For decompressions, a majority of the radiation exposure was attributable to image acquisition for ION, whereas for MI-TLIF this was ≤50%. This finding is not unexpected given that in decompression surgeries image guidance is primarily required for anatomic localization, whereas in fusions it is also used to guide placement of instrumentation.
Since operating room personnel and surgeon are not exposed to radiation during ION image acquisition, the radiation dose for microdiscectomy and laminectomy procedures was minimal (<1 mGy). In the context of increased dependence on image guidance for MIS procedures, minimization of radiation dose using ION provides a valuable opportunity to decrease occupational exposure to ionizing radiation and thus reduces the associated risks.
Skin-anchored 3D ION Versus 2D Fluoroscopy in MI-TLIF
Although a number of studies have compared ION to 2D fluoroscopy for pedicle screw placement, all studies utilizing ION employed a bone-anchored tracker. As a result, there are currently no published studies directly comparing skin-anchored ION to conventional fluoroscopy. The results of our study showed that ION was advantageous in terms of shorter operative times, less blood loss, reduced radiation exposure compared with fluoroscopy.
Although ION required additional time for image acquisition prior to the start of the surgery, the total time demand of ION was only slightly greater than that of fluoroscopy. Though this difference was statistically significant, this difference is likely not clinically significant because the median difference in time demand was 5 minutes. Furthermore, the total time demand for ION included time for patient positioning, and preparing and draping the incision site, would also be performed in the fluoroscopy cohort but were not accounted for when calculating total time demand. It is likely that accounting for this would result in equivalent time demand.
ION decreased operative time by a median of 15 minutes compared to fluoroscopy, which may be attributable to improvement in workflow with ION and decreased need to obtain fluoroscopic images to guide instrumentation. Although the difference in median blood loss was statistically significant, EBL for both cohorts was minimal due to the use of MIS techniques. Thus, it is unlikely that this difference would have been clinically relevant. This equivalency of peri-operative outcomes is further evidenced by the comparable lengths of hospital stay.
Strengths
Our study addresses gaps in the literature with regard to skin-anchored ION in terms of techniques and feasibility, time demand, and radiation exposure. To our knowledge, the current study is the largest reported series on skin-anchored tracker for ION in spine surgery. Furthermore, although spinal navigation has largely been utilized for pedicle screw placement, in this study we also report on its application for other purposes including anatomic localization, bony decompression, microdiscectomy, and iliac crest bone graft harvesting, which have not been described in the literature.
Our study was a prospective series of consecutive patients undergoing these procedures, thereby eliminating selection bias; all procedures were performed by a single experienced surgeon with consistent surgical technique thus allowing for an unbiased comparison between ION and fluoroscopy.
Limitations
Our study was limited to patients undergoing single-level surgery for degenerative spinal pathology. Thus, we are unable to comment on ION for multilevel surgeries or for complex deformity cases.
This series includes patients from when the operating surgeon started using ION and may thus be impacted by the learning curve. Although the learning curve was not analyzed, we plan to evaluate and report this in a future publication. Furthermore, the comparison cohort of fluoroscopy was also subject to a learning curve when it was first used; thus, we believe the comparison is still valid.
Although the literature has focused on accuracy of screw placement, in the current study we do not report the accuracy of screw placement. This analysis was omitted because postoperative CT scans were not available for the entire cohort and would have thus biased our report. Per our protocol, postoperative CT scans are obtained at the 1-year follow-up to assess fusion; when we obtain these, we plan to analyze and report on this. Despite this limitation, we evaluated screw placement intraoperatively using neuromonitoring for all patients, and obtained final radiographs to assess accuracy of screws in both groups. The radiation dose for this final x-ray was accounted for in the radiation dose for both cohorts.
Patient-reported outcomes were not available for all patients, and hence were not included as part of the current analysis. While functional outcomes are important, the focus of our study was on assessing the safety and feasibility, and on evaluating the time-demand and radiation exposure.
ION is associated with considerable upfront costs. However, costs were not analyzed; hence we are unable to report on this matter.
CONCLUSION
This study demonstrates the safety and feasibility, and describes the technique of this new ION modality. Our results indicate that skin-anchored ION is feasible, safe, and accurate. ION resulted in low radiation exposure, short operative times, and minimal complications. Furthermore, when compared to conventional fluoroscopy for MI-TLIF, ION was advantageous in terms of shorter operative times, less blood loss, and reduced radiation exposure, with a 29% and 55% reduction in the radiation dose to patients and OR personnel, respectively. Further research is required to understand the economics of acquisition and implementation of these new technologies, and to determine if the initial upfront costs can be justified by improved safety and performance profiles.
Key Points.
The use of intraoperative navigation using a skin-anchored navigation tracker is a feasible, safe and accurate technique that results in low radiation exposure, short operative times, and minimal complications.
For decompression surgeries, a majority of the radiation dose and fluoroscopy time to patients is attributable to image acquisition for ION.
For microdiscectomy and laminectomy procedures, the use of ION results in minimal (< 1 mGy) radiation exposure to the operating surgeon and other operating room personnel.
When compared with conventional 2D fluoroscopy for MI-TLIF, ION was advantageous in terms of shorter operative times, less blood loss, reduced fluoroscopy time, and lower total radiation exposure, resulting in a 29% reduction in radiation dose to patients and a 55% reduction in radiation dose to the operating surgeon.
Further research is required to understand the economics of acquisition and implementation of these new technologies, and to determine if the initial upfront costs can be justified by improved safety and performance profiles.
Footnotes
Level of Evidence: 3
The manuscript submitted does not contain information about medical device(s)/drug(s).
Relevant financial activities outside the submitted work: board membership, consultancy, grants, stocks, royalties, employment.
Supplemental digital content is available for this article. Direct URL citations appearing in the printed text are provided in the HTML and PDF version of this article on the journal’s Web site (www.spinejournal.com).
References
- 1.Elmi-Terander A, Nachabe R, Skulason H, et al. Feasibility and accuracy of thoracolumbar minimally invasive pedicle screw placement with augmented reality navigation technology. Spine (Phila Pa 1976) 2018;43:1018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balling H. Learning curve analysis of 3D-fluoroscopy image-guided pedicle screw insertions in lumbar single-level fusion procedures. Arch Orthop Trauma Surg 2018;138:1501–9. [DOI] [PubMed] [Google Scholar]
- 3.Overley SC, Cho SK, Mehta AI, et al. Navigation and robotics in spinal surgery: where are we now?. Clin Neurosurg 2017;80:S86–99. [DOI] [PubMed] [Google Scholar]
- 4.Johnson N. Imaging, navigation, and robotics in spine surgery. Spine (Phila Pa 1976) 2016;41:S32. [DOI] [PubMed] [Google Scholar]
- 5.Härtl R, Lam KS, Wang J, et al. Worldwide survey on the use of navigation in spine surgery. World Neurosurg 2013;79:162–72. [DOI] [PubMed] [Google Scholar]
- 6.Al-Khouja L, Shweikeh F, Pashman R, et al. Economics of image guidance and navigation in spine surgery. Surg Neurol Int 2015;6:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balling H. Time demand and radiation dose in 3D-fluoroscopy-based navigation-assisted 3D-fluoroscopy-controlled pedicle screw instrumentations. Spine (Phila Pa 1976) 2018;43:E512–9. [DOI] [PubMed] [Google Scholar]
- 8.Malham GM, Parker RM. Early experience of placing image-guided minimally invasive pedicle screws without K-wires or bone-anchored trackers. J Neurosurg Spine 2018;28:357–63. [DOI] [PubMed] [Google Scholar]
- 9.Staartjes VE, Klukowska AM, Schröder ML. Pedicle screw revision in robot-guided, navigated, and freehand thoracolumbar instrumentation: a systematic review and meta-analysis. World Neurosurg 2018;116:433–443e8. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi S, Lu Y, Mcanany S, et al. Imaging modalities in orthopaedic surgery: a narrative review. J Acad Orthop Surg 2014;22:800–9. [DOI] [PubMed] [Google Scholar]
- 11.Urbanski W, Jurasz W, Wolanczyk M, et al. Increased radiation but no benefits in pedicle screw accuracy with navigation versus a freehand technique in scoliosis surgery. Clin Orthop Relat Res 2018;476:1020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tajsic T, Patel K, Farmer R, et al. Spinal navigation for minimally invasive thoracic and lumbosacral spine fixation: implications for radiation exposure, operative time, and accuracy of pedicle screw placement. Eur Spine J 2018;27:1918–24. [DOI] [PubMed] [Google Scholar]
- 13.Lian X, Navarro-Ramirez R, Berlin C, et al. Total 3D Airo® Navigation for Minimally Invasive Transforaminal Lumbar Interbody Fusion. Biomed Res Int 2016;2016:5027340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navarro-Ramirez R, Lang G, Lian X, et al. Total navigation in spine surgery; a concise guide to eliminate fluoroscopy using a portable intraoperative computed tomography 3-dimensional navigation system. World Neurosurg 2017;100:325–35. [DOI] [PubMed] [Google Scholar]
- 15.Kleck CJ, Johnson C, Akiyama M, et al. One-step minimally invasive pedicle screw instrumentation using o-arm and stealth navigation. Clin Spine Surg 2018;31:197–202. [DOI] [PubMed] [Google Scholar]
- 16.Fomekong E, Pierrard J, Raftopoulos C. Comparative cohort study of percutaneous pedicle screw implantation without versus with navigation in patients undergoing surgery for degenerative lumbar disc disease. World Neurosurg 2018;111:e410–7. [DOI] [PubMed] [Google Scholar]
- 17.Vaishnav AS, Samuel AM, Qureshi SA. MIS discectomy and laminectomy. In: Boody B, Russo G, Vaccaro A, et al. , eds. The Fellows Manual: Techniques of Spine Surgery. Jaypee Brothers Medical Publishers; 2019: In press. [Google Scholar]
- 18.Kumar A, Merrill RK, Overley SC, et al. Radiation exposure in minimally invasive transforaminal lumbar interbody fusion: the effect of the learning curve. Int J Spine Surg 2019;13:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaishnav AS, Saville P, McAnany S, et al. Retrospective review of immediate restoration of lordosis in single-level minimally invasive transforaminal lumbar interbody fusion: a comparison of static and expandable interbody cages. Oper Neurosurg (Hagerstown) 2019;pii: opz240. doi: 10.1093/ons/opz240. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.Boukebir MA, Berlin CD, Navarro-Ramirez R, et al. Ten-step minimally invasive spine lumbar decompression and dural repair through tubular retractors. Oper Neurosurg 2017;13:232–44. [DOI] [PubMed] [Google Scholar]
- 21.Virk SS, Qureshi SA. Navigation in minimally invasive spine surgery. J Spine Surg 2019;5:S25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]


